Abstract
Hypertension is related to increased risk of cognitive decline in a highly age-dependent manner. However, conflicting evidence exists on the relation between age of hypertension onset and cognition. Our goal was to investigate the association between early- vs. late-onset hypertension and mid-life cognitive performance in 2946 Coronary Artery Risk Development in Young Adults study participants (mean age 55±4, 57% women). The participants underwent 9 repeat examinations, including blood pressure measurements, between 1985–1986 and 2015–2016. The participants underwent brain MRI imaging and completed Digit Symbol Substitution test (DSST), Rey Auditory Verbal Learning test (RAVLT), Stroop Interference test and the Montreal Cognitive Assessment (MoCA) to evaluate cognitive function at the Year 30 exam. We assessed the relation between age of hypertension onset and cognitive function using linear regression models adjusted for cognitive decline risk factors, including systolic blood pressure. We observed that individuals with early-onset hypertension (onset at <35 years) had 0.24±0.09, 0.22±0.10, 0.27±0.09, and 0.19±0.07 lower standardized z-scores in DSST, Stroop test, MoCA, and a composite cognitive score than participants without hypertension (p<0.05 for all). In contrast, hypertension onset at ≥35 years was not associated with cognitive function (p>0.05 for all). In a subgroup of 559 participants neither early- nor late-onset hypertension was related to macrostructural brain alterations (p>0.05 for all). Our results indicate that early-onset hypertension is a potent risk factor for midlife cognitive impairment. Thus, age of hypertension onset assessment in clinical practice could improve risk stratification of cognitive decline in hypertensive patients.
Keywords: Hypertension, age of onset, cognition, brain, risk factors, follow-up studies
Graphical Abstract
Introduction
Numerous studies have suggested strong correlations between hypertension and risk for cognitive impairment, morphologic brain changes, and dementia.1–3 However, lack of clear consensus remains on the impact of age on the relationship between hypertension and cognition-related outcomes. The persistent equipoise concerning this relationship may be related to important yet previously under-studied heterogeneity of effects.
Accumulating evidence indicates that the relation between blood pressure (BP) and cognitive function is age-dependent.4–7 Prior findings indicate that high midlife BP is associated with cognitive decline and altered brain structure later in life.8–11 Some evidence suggests that elevated BP in young adulthood could impair the cognitive capacity already in middle-aged individuals.12–15 On the other hand, high BP in elderly individuals does not seem to predict cognitive impairment, and even a protective effect has been observed.16–19 Considering the global challenge of aging populations and increasing burden of dementia,20,21 it is crucial to understand in more detail the BP-related mechanisms leading to cognitive decline.
Although the impact of elevated BP on cognition seems age-dependent, the relation between age of hypertension onset and cognitive function remains unclear. In prior studies, we have demonstrated that early-onset hypertension is an important risk factor for subclinical cardiovascular disease and cardiovascular mortality.22,23 Hypertension onset by midlife has been reported to be associated with an increased risk for dementia in women, but not in men.24 However, in another study, hypertension onset in late-life surprisingly protected against dementia.25 Therefore, more evidence on the impact of hypertension onset age besides chronological age and improved individualized approaches for estimating risk of cognitive decline are called for. In this study, we therefore aimed to further elucidate whether early- versus late-onset hypertension is related to cognitive impairment and structural brain changes already by midlife in 2946 CARDIA (Coronary Artery Risk Development in Young Adults) study participants. We hypothesized that early-onset hypertension would associate more robustly with worse cognitive function and smaller brain volumes than late-onset hypertension.
Methods
Study sample
Our study sample includes participants from the prospective CARDIA cohort study.26 All data and materials of the CARDIA study have been made publicly available at the National Institutes of Health’s Biologic Specimen and Data Repository Information Coordinating Center and can be accessed at https://biolincc.nhlbi.nih.gov/studies/cardia/. The CARDIA cohort was recruited in 1985–1986 and involved 5115 participants (mean age 25±4 years, range 18–30 years). The cohort included individuals of different ethnic backgrounds, sex, and educational levels across four study centers (Birmingham, AL; Chicago, IL; Minneapolis, MN; and Oakland, CA). We studied participants who attended the Year 30 exam of the CARDIA study in 2015–2016 (n=3358). The flow of this study is visualized in Figure S1 in the online-only Data Supplement. We included 2946 of these individuals in our study sample after excluding subjects with missing covariate or outcome data (n=412). Additionally, 599 of these participants also attended a brain MRI substudy. All study participants provided informed consent and the study protocol was approved by each centers’ institutional committee.
Exposures and covariates
The participants underwent up to nine regularly conducted follow-up examinations between 1985–1986 (Year 0 exam) and 2015–2016 (Year 30 exam). At each exam, BP was measured three times after 5 minutes of quiet rest from the right arm of all participants in sitting position by centrally trained technicians.22,26 A random zero mercury sphygmomanometer was used through the six first follow-up exams, and we calibrated the oscillometric recordings obtained with Omron model HEM907XL from the last three exams to corresponding sphygmomanometer values to attain comparable values.27 In accordance with previous studies,23 we defined hypertension onset as BP ≥140/90 mmHg or self-reported use of any antihypertensive medication on two successive exams and formed subgroups according to the participants’ age of hypertension onset (<35 years, 35–44 years, ≥45 years, and no hypertension).
Demographic characteristics, years of completed education, smoking status, amount of alcohol intake (expressed as total ethanol consumption), and use of antihypertensive agents were assessed by self-report at all exams. Sedentary behavior time was evaluated with the CARDIA Sedentary Behavior Questionnaire.28 Weight and height were measured at each exam. Body mass index (BMI) was calculated as weight (kg)/(height (cm))2. Total serum cholesterol, high-density lipoprotein cholesterol (HDL-cholesterol), and glucose levels were measured from fasting samples as previously described.26,29 We defined diabetes as serum fasting glucose of ≥7 mmol/l or use of antihyperglycemics.
Cognitive function tests
Four cognitive function tests were administered by trained and certified CARDIA technicians at the Year 30 exam (between 2015 and 2016) as previously reported.30 The Digit Symbol Substitution test (DSST) measures psychomotor speed, scored as number of correctly substituted symbols, therefore a higher score indicating better performance.31 The long-delay free recall scores from the Rey Auditory Verbal Learning test (RAVLT), counted as number of words, were used to assess verbal memory with higher score indicating better performance.32 The Stroop test measures executive function, scored as the sum of time in seconds and number of errors required to complete each subtest.33 An interference score was calculated by subtracting the subtest two scores from the subtest three score, thus higher interference score represented worse test performance. The Montreal Cognitive Assessment (MoCA) has been demonstrated as a screening tool for mild cognitive impairment, while covering for several cognitive function components, such as orientation, calculations, memory and executive function.34 Higher MoCA score indicates better global cognitive function. All test scores were also standardized into z-scores. We used inversed z-scores for Stroop test to make interpretation similar with the other tests, thus all negative z-scores indicating worse and positive z-scores indicating better performance. A composite cognitive function score was computed as the average of z-scores from DSST, RAVLT and Stroop test to assess overall cognitive function as previously described.30
Brain MRI acquisition and measures
The brain MRI substudy was performed at three study sites using 3-T MR scanners at the CARDIA Year 30 exam concurrently with the cognitive tests. Further technical and methodological details have been reported previously.35 The brain MRI images were quantitatively analyzed for total brain volume (TBV), white matter volume (WMV), grey matter volume (GMV), abnormal white matter volume (AWMV) and white matter fractional anisotropy (WMFA). TBV was computed from the sagittal 3-D T1 sequence as the sum of WMV and GMV. Total intracranial volume was estimated as the sum of TBV and cerebral spinal fluid volume. AWMV was computed from the sagittal 3-D FLAIR T1 and T2 sequences. AWMV depicts the amount of brain tissue damaged by ischemia, penumbra tissue, inflammation, or demyelination. WMFA was estimated using diffusion tensor imaging and represents the micro-structural integrity of the white matter tracts. WMFA is expressed as a scalar value, ranging from 0 (isotropy) and 1 (anisotropy). Higher fractional anisotropy is often present in highly myelinated white matter tracts, whereas lower fractional anisotropy is related to aging and neurodegenerative disorders.36 All brain-related values were also transformed into z-scores.
Statistical analyses
We studied the Year 30 examination characteristics in all participants and separately in subgroups by hypertension onset age. To compare the between-group differences in characteristics and cognitive function measures, we used chi-squared test for categorial variables and one-way analysis of variance for continuous variables. Alcohol intake was standardized into a z-score due to skewed distribution. Additionally, we compared the baseline characteristics between the study sample and the excluded participants using two-sample t-tests for the continuous variables and the X2 tests for categorial variables. We assessed the relation of hypertension onset age with cognitive function and MRI measures using linear regression models, with participants without hypertension as the reference group. We also analyzed the association with cognitive function using an alternative definition for age of hypertension onset based on only elevated BP without considering antihypertensive medication use. The minimally adjusted linear regression model was adjusted for age, sex, race, and education. The fully adjusted linear regression model was adjusted for age, sex, race, education, diabetes, BMI, smoking, alcohol intake, sedentary time, use of any antihypertensive medication and present systolic BP. All models with MRI measures as the outcome variable were also adjusted for intracranial volume. All covariates were drawn from the Year 30 exam. We also examined the associations between cognitive function and hypertension onset age in subgroups by sex and race by including an onset age x subgroup interaction term in the model. We performed all analyses using SAS, version 9.4 (SAS Institute, Cary, NC, USA). The null hypothesis was rejected for two-tailed P<0.05.
Results
Table 1 shows the characteristics of the whole study sample and by subgroups according to hypertension onset age. Participants with hypertension onset at <35 years of age (early-onset hypertension) were more likely to be male and African American than any of the other race-ethnic and sex categories. Individuals with early-onset hypertension were also more likely to be diabetic, smoke and use antihypertensive medication. Participants who were lost to follow-up or excluded were more likely to be men, black, and smokers compared to participants included in the study sample (Table S1 in the online-only Data Supplement). No other major differences were observed in the baseline characteristics between the included and excluded participants, although other statistically significant differences were also observed. Cognitive function test scores at the Year 30 exam are shown in Table 2 according to hypertension onset age. Individuals with hypertension onset <35 years of age had the lowest scores in all cognitive function tests, except for the Stroop test where the mean score was the highest. Participants with early-onset hypertension also had the smallest TBV, WMV and GMV (Table S2 in the online-only Data Supplement). There were statistically significant differences between the four groups in brain volumes (p<0.01 for all) but not in AWMV or WMFA (p>0.05 for both).
Table 1.
Characteristics of the study sample and in subgroups by hypertension onset age.
| HTN Onset Age |
||||||
|---|---|---|---|---|---|---|
| Characteristic | All | < 35 y | 35–44 y | ≥45 y | No HTN | p-value |
| N | 2946 | 112 | 277 | 426 | 2131 | |
| Age, years | 55.1 (3.6) | 55.1 (3.5) | 54.2 (3.7) | 56.6 (3.0) | 54.9 (3.6) | <0.001 |
| Age, range | 48–60 | 48–60 | 48–60 | 49–60 | 48–60 | - |
| Women, n (%) | 1689 (57.3) | 59 (52.7) | 162 (58.5) | 240 (56.3) | 1228 (57.6) | 0.71 |
| Black race, n (%) | 1373 (46.6) | 87 (77.7) | 203 (73.3) | 261 (61.3) | 822 (38.6) | <0.001 |
| Body mass index, kg/m² | 30.4 (6.8) | 33.7 (7.3) | 34.6 (7.1) | 32.7 (6.9) | 29.2 (6.3) | <0.001 |
| Current smoker, n (%) | 425 (14.4) | 23 (20.5) | 43 (15.5) | 80 (18.8) | 279 (13.1) | 0.004 |
| Total cholesterol, mmol/l | 5.0 (1.0) | 4.7 (1.0) | 4.7 (1.0) | 4.8 (1.0) | 5.1 (0.9) | <0.001 |
| HDL-cholesterol, mmol/l | 1.6 (0.5) | 1.5 (0.6) | 1.5 (0.5) | 1.5 (0.5) | 1.6 (0.5) | <0.001 |
| Systolic blood pressure, mmHg | 118 (15.1) | 125 (17.6) | 126 (17.7) | 125 (16.6) | 116 (13.2) | <0.001 |
| Diastolic blood pressure, mmHg | 72.2 (10.5) | 75.8 (11.5) | 77.4 (11.2) | 76.0 (11.1) | 70.6 (9.7) | <0.001 |
| Use of antihypertensive medication, n (%) | 943 (32.0) | 104 (92.9) | 253 (91.3) | 374 (87.8) | 212 (10.0) | <0.001 |
| Diabetes, n (%) | 385 (13.1) | 39 (34.8) | 82 (29.6) | 109 (25.6) | 155 (7.3) | <0.001 |
| Alcohol intake, ml/day (median, IQR) | 2.4 (0–14.6) | 0 (0–7.7) | 2.4 (0–12.2) | 0 (0–14.3) | 4.8 (0–15.3) | 0.07 |
| Education, years | 14.9 (1.9) | 14.1 (1.9) | 14.4 (1.9) | 14.5 (1.9) | 15.0 (1.9) | <0.001 |
| Sedentary time, h/day | 7.5 (4.2) | 9.1 (4.3) | 8.6 (4.5) | 8.0 (4.3) | 7.2 (4.0) | <0.001 |
Values are obtained from the Year 30 exam. Values are reported as mean (SD) unless stated otherwise. HTN, hypertension; y, years; IQR, interquartile range.
Table 2.
Mean unadjusted cognitive function scores at Year 30 exam according to hypertension onset age.
| HTN Onset Age |
||||||
|---|---|---|---|---|---|---|
| All | <35 y | 35–44 y | ≥45 y | No HTN | p-value | |
| N | 2946 | 112 | 277 | 426 | 2131 | - |
| RAVLT, words | 8.5 (3.4) | 6.9 (3.2) | 7.5 (3.4) | 7.7 (3.4) | 8.9 (3.4) | <0.001 |
| DSST, symbols | 68.0 (17) | 58.0 (18) | 64.0 (17) | 62.9 (16) | 70.1 (16) | <0.001 |
| Stroop test, score | 22.5 (12) | 27.5 (14) | 24.9 (13) | 25.1 (14) | 21.4 (11) | <0.001 |
| Composite cognitive score, z | 0.0 (0.7) | −0.5 (0.8) | −0.2 (0.8) | −0.2 (0.8) | 0.1 (0.7) | <0.001 |
| MoCA, score | 23.9 (3.9) | 21.2 (3.9) | 22.5 (3.9) | 23.1 (4.1) | 24.4 (3.7) | <0.001 |
Values are reported as mean (SD). Stroop interference test score equals seconds and number of errors, with higher score indicating worse performance. Composite cognitive score is calculated as the average z-scores from RAVLT, DSST and inversed Stroop test. HTN, hypertension; y, years; DSST, Digit Symbol Substitution test; RAVLT, Rey Auditory Verbal Learning test; MoCA, Montreal Cognitive Assessment.
The associations between hypertension onset age subgroups and cognitive function measures are presented in Table 3. Compared to participants without hypertension, individuals with hypertension onset at <35 years of age had 0.57±0.09, 0.71±0.09, 0.50±0.09, 0.81±0.09 and 0.60±0.07 lower unadjusted z-scores (β ± standard error) for the RAVLT, DSST, Stroop test, MoCA, and composite cognitive score in unadjusted models (p<0.001 for all), respectively. The associations between early-onset hypertension group and lower z-scores for cognitive function tests remained statistically significant in the fully adjusted model (p<0.05 for all), except for the RAVLT (p=0.22). Hypertension onset between 35 to 44 years and 45 years or over was not related to cognitive test performance (p>0.05 for all). We observed no significant sex-specific differences in the associations between hypertension onset age and cognitive function (Table S3 in the online-only Data Supplement). Similarly, even though the associations were slightly more prominent among white individuals, the trends were similar in subgroups by race (Table S4 in the online-only Data Supplement). The results were attenuated when age of hypertension onset was defined only based on elevated BP (Table S5 in the online-only Data Supplement). Moreover, hypertension onset at <35 years of age was associated with lower TBV, WMV and GMV in the unadjusted model (p<0.01 for all, Table S6 in the online-only Data Supplement). However, in the adjusted models hypertension onset age was not related to any of the brain MRI measures (p>0.05 for all).
Table 3.
Association (β±SE) of hypertension onset age with cognitive performance at Year 30.
| Age of hypertension onset | RAVLT | DSST | Stroop test | Composite cognitive score | MoCA |
|---|---|---|---|---|---|
| N | 2946 | 2946 | 2946 | 2946 | 2946 |
| Unadjusted model | |||||
| <35 years | −0.57±0.09* | −0.71±0.09* | −0.50±0.09* | −0.60±0.07* | −0.81±0.09* |
| 35–44 years | −0.40±0.06* | −0.36±0.06* | −0.29±0.06* | −0.35±0.05* | −0.46±0.06* |
| ≥45 years | −0.34±0.05* | −0.42±0.05* | −0.30±0.05* | −0.36±0.04* | −0.33±0.05* |
| No hypertension | Ref. | Ref. | Ref. | Ref. | Ref. |
| Minimally adjusted model | |||||
| <35 years | −0.21±0.08‡ | −0.34±0.08* | −0.24±0.09† | −0.26±0.06* | −0.36±0.08* |
| 35–44 years | −0.14±0.06‡ | −0.12±0.05‡ | −0.09±0.06 | −0.12±0.04† | −0.13±0.05‡ |
| ≥45 years | −0.10±0.05‡ | −0.16±0.05* | −0.12±0.05‡ | −0.13±0.03* | −0.03±0.04 |
| No hypertension | Ref. | Ref. | Ref. | Ref. | Ref. |
| Fully adjusted model | |||||
| <35 years | −0.12±0.09 | −0.24±0.09† | −0.22±0.10‡ | −0.19±0.07† | −0.27±0.09† |
| 35–44 years | −0.07±0.07 | −0.03±0.07 | −0.08±0.07 | −0.06±0.05 | −0.06±0.07 |
| ≥45 years | −0.03±0.06 | −0.07±0.06 | −0.11±0.07 | −0.07±0.04 | 0.04±0.06 |
| No hypertension | Ref. | Ref. | Ref. | Ref. | Ref. |
Values are for 1-SD difference estimate (beta) with standard error. Minimally adjusted model is adjusted for age, sex, race, and education. Fully adjusted model is adjusted for age, sex, race, education, diabetes, body mass index, smoking, alcohol intake, sedentary time, use of antihypertensive medication, and systolic blood pressure.
P<0.001
P<0.01
P<0.05. DSST, Digit Symbol Substitution test; RAVLT, Rey Auditory Verbal Learning test; MoCA, Montreal Cognitive Assessment.
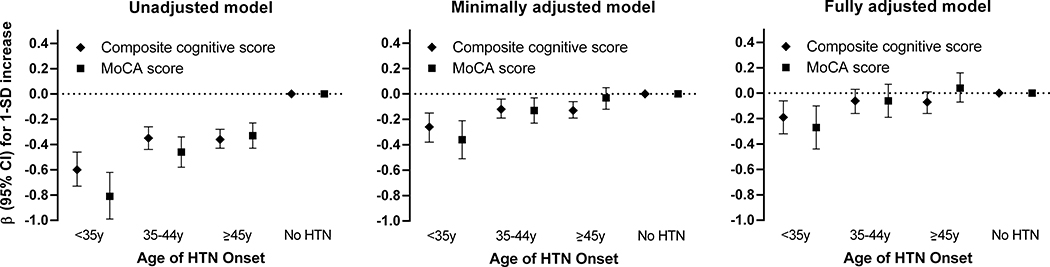
Figure 1 illustrates the relation between hypertension onset age and global cognitive function scores. Both the composite cognitive and MoCA scores were lowest in the <35 years of hypertension onset subgroup compared to the other subgroups. In fully adjusted models, early-onset hypertension was associated with 0.19 (95% CI, 0.06–0.32) and 0.27 (95% CI, 0.10–0.44) lower z-scores for composite cognitive and MoCA scores, respectively. A linear relationship with decreasing age of hypertension onset was observed for both the composite cognitive and MoCA scores (p<0.01 for trend in both). Subgroups with hypertension onset ≥35 years of age were not associated with cognitive dysfunction as measured by global cognitive function scores after adjusting for several potential contributing factors (p>0.05 for all).
Figure 1. Association between hypertension onset age and 1-SD increase in mean global cognitive function scores at Year 30.
Minimally adjusted model is adjusted for age, sex, race, and education. Fully adjusted model is adjusted for age, sex, race, education, diabetes, body mass index, smoking, alcohol intake, sedentary time, use of antihypertensive medication and systolic blood pressure. Composite cognitive score was computed as the average of the Digit Symbol Substitution test, the Rey Auditory Verbal Learning test, and the inversed Stroop interference test score. HTN, hypertension; y, year; MoCA, Montreal Cognitive Assessment.
Discussion
In this study we observed that, after adjusting for several covariates (age, sex, race, education, diabetes, BMI, smoking, alcohol intake, sedentary time, antihypertensive medication use, and present systolic BP), early-onset hypertension was associated with worse cognitive test performance than late-onset hypertension. However, age of hypertension onset was not associated with any quantified brain MRI measures. Our findings suggest that early age of hypertension onset is associated with worse cognitive function in midlife, but not with commonly assessed structural brain alterations, irrespective of the present systolic BP at the time of cognitive function assessment.
Several previous studies, including data from the CARDIA study, have demonstrated that the relation between BP and cognition is highly dependent on the individual’s age at exposure and outcome assessment.8,11,18,37,38 However, most of these former studies have not considered impact of hypertension onset age in their analyses. Results from The 90+ Study indicated that self-reported hypertension onset after 80 years is associated with lower dementia risk.25 In that study, compared to individuals without self-reported hypertension, hypertension onset between 80 to 89 years and ≥90 years was related to hazard ratios (HR) of 0.58 (95% CI 0.34–0.98) and 0.37 (95% CI 0.19–0.73) for developing all-cause dementia, respectively. In another report from the Multiphasic Health Checkups study, however, hypertension onset during mid-adulthood was associated with HR of 1.68 (95% CI 1.20–2.34) for late-life dementia in women, compared to those with stable normotension.24 The study sample included individuals with a wide age-spectrum (from 30 to 56 years), with no further analyses according to their hypertension onset age. In addition, the analyses were not adjusted for alcohol intake, sedentary time, or physical activity. The results from our study support the findings from these two studies, indicating that particularly early-onset hypertension is a risk factor for impaired midlife cognitive function, whereas late-onset hypertension is not. Considering that hypertension is a symptomless condition, distinguishing between the actual age of hypertension onset from the age at hypertension diagnosis is virtually impossible. In this study, the maximum delay between hypertension onset and hypertension diagnosis was 5 years (the time between follow-up visits) and this delay may be even longer in everyday clinical practice. The impact of early-onset hypertension on cognitive function was less prominent when an alternative definition of hypertension onset age which did not consider antihypertensive medication use was used (Table S5 in the online-only Data Supplement). However, omitting initiation of antihypertensive medication use from the definition of hypertension onset age will most likely lead to misclassification as even well-controlled hypertensive individuals have considerable residual cardiovascular disease risk. Nevertheless, given that worse cognitive performance relates to increased dementia risk,15,31 early-onset hypertension may predispose to overt dementia later in life.
Cumulative BP burden is an important contributing factor for cognitive impairment.13,14,39–43 Given the chronic nature of hypertension, it is presumable that long-term exposure is a major determinant for the adverse effects of hypertension on cognitive function. Most previous studies in this domain have used complex formulas to estimate BP exposure from repeated BP measurements during follow-up. A recent study on 191 CARDIA study participants demonstrated that higher cumulative BP was associated with worse cognitive performance and gait.15 However, the study sample was considerably smaller compared to this study and the clinical applicability of the exposure variable may be limited. Compared to other indices of cumulative BP burden, age of hypertension onset is likely to similarly represent the overall hypertension chronicity level. Yet, hypertension onset age could act as a feasible surrogate measure to assess an individual’s overall lifetime exposure to BP and risk of hypertension in offspring.23 Additionally, as onset or presence of hypertension at much older age do not seem to confer increased risk for cognitive decline,16,18,25 assessment of hypertension onset age could aid clinicians to differentiate high-risk individuals from those who are unlikely to experience cognitive impairment. This may also have important implications for hypertensive patients as adequate hypertension treatment has been suggested to slow, or even prevent, cognitive decline and development of dementia.44,45
Unlike cognitive function, the relation between long-term BP burden and structural brain changes in midlife remains unclear.5,6 In our study, we did not observe an association between hypertension onset age and TBV, WMV, GMV, AWMV or WMFA. However, most previous studies have reported morphologic brain alterations only after midlife.5,11 This is further supported by observations from the Framingham Heart Study, which demonstrated that relevant age-related differences in MRI-quantified brain volumes begin and gradually increase after the age of 50 years.46 Structural brain changes are commonly present in hypertensive individuals with dementia, although the optimal age and protocols for imaging remain disputed.47 Therefore cognitive function tests are used to detect altered cognitive function at an early age prior to manifestation of structural brain changes or overt dementia. Our results suggest that the potential harms of early-onset hypertension are detectable already in midlife prior to quantifiable structural brain changes.
The strengths of our study include a large, diverse study population with up to 30 years of regular follow-up visits. 65.7 % of individuals who participated in the baseline examination took part in examination 9. The study sample included less men, African Americans and smokers compared to individuals excluded or lost to follow-up. (Table S1 in the online-only Data Supplement) No other clinically significant differences were observed between these two groups. However, MRI was performed in a subsample, resulting in loss of statistical power for the MRI-related outcomes. Additionally, our findings may not be generalizable to the entire age spectrum as our study sample did not include elderly individuals. Another strength of our study is that we were able to reliably determine the actual age of hypertension onset in virtually all participants as the participants were young at study baseline. However, an even longer follow-up time and availability of data for incident dementia could perhaps have enabled us to provide more comprehensive answers on the relation of hypertension with cognitive function. Considering our observational study design, future studies with prospective and experimental study designs and varying study populations are needed to draw even more robust conclusions on the impact of hypertension onset age on cognition. Additionally, we had no information on history of acute stroke, which could have impacted the results. As another limitation, baseline data on cognitive function or brain MRI measurements were not available. Yet, we adjusted our analyses for several other factors that correlate with cognitive function, such as education. Moreover, we were unable to adjust the analyses for antihypertensive treatment intensity during follow-up. However, we aimed to minimize this effect by adjusting for prevalent antihypertensive mediation use.
Perspectives
Overall, our findings expand the current knowledge of adverse events related to early-onset hypertension beyond cardiovascular disease related outcomes.22,23 In effect, the timing of hypertension onset appears to impact cognitive function rather than structural brain alterations, which may reflect features specific to hypertension-related brain pathophysiology that warrant further investigation. Previous literature suggests that impaired neurophysiological function in younger individuals may precede the detected neuroanatomical changes in elderly hypertensive persons.7,48 The potential underlying mechanisms of early-onset hypertension on cognition might result from long-term exposure to high BP, or via induced early vascular aging, leading to microvascular brain changes. Thus, these initial cognitive changes related to early-onset hypertension could potentially be detected through functional brain imaging techniques such as PET imaging.49 However, extended follow-up of these individuals to later life may also reveal an association between the age of hypertension onset and macrostructural brain changes, such as decreased brain volumes. Nevertheless, assessing age of hypertension onset could offer advantages in dementia risk stratification of hypertensive individuals in clinical practice, as previous data suggests that hypertension onset age could also be defined reliably by self-report.22,25 Given the current evidence on the risks of early-onset hypertension, physicians treating high BP should be aware of the age at which their patients initially developed hypertension.
Supplementary Material
Novelty and Significance.
What Is New?
Early-onset hypertension is a strong and highly heritable risk factor for cardiovascular disease.
The association between age of hypertension onset and midlife cognition is unclear.
What Is Relevant?
Early-onset and not late-onset hypertension is associated with functional cognitive impairment in midlife.
Early- and late-onset hypertension are nor related to structural brain changes in midlife.
Summary.
Our results expand previous evidence on early-onset hypertension being an important independent risk factor for adverse events. Assessing age of hypertension onset could improve the dementia risk stratification of individuals with hypertension.
Acknowledgements
We acknowledge the important contributions of the CARDIA participants and investigators. This study was conducted using CARDIA Research Materials obtained from the NHLBI Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of the CARDIA study or the NHLBI.
Sources of funding
K. Suvila was supported by grants from the Finnish Foundation for Cardiovascular Research, the Finnish Medical Foundation, the Turku University Foundation and the University of Turku. T.J. Niiranen was funded by the Academy of Finland (grant n:o 321351), the Paavo Nurmi Foundation, the Finnish Medical Foundation, and the Emil Aaltonen Foundation. S. Cheng was supported by National Institute of Health grants R01-HL134168, R01-HL131532, R01-HL143227, and R01-HL142983. J.A.C. Lima was supported by contracts HHSN268201300025C, HHSN268201300026C, HHSN268201300027C, HHSN268201300028C, HHSN268201300029C, and HHSN268200900041C from the National Heart, Lung, and Blood Institute, the Intramural Research Program of the National Institute on Aging, and an intra-agency agreement between National Institute on Aging and National Heart, Lung, and Blood Institute (AG0005).
Footnotes
Conflicts of Interest(s)/Disclosure(s)
None.
References
- 1.Skoog I, Lernfelt B, Landahl S, Palmertz B, Andreasson LA, Nilsson L, Persson G, Odén A, Svanborg A. 15-year longitudinal study of blood pressure and dementia. Lancet. 1996; 347:1141–1145. doi: 10.1016/S0140-6736(96)90608-X [DOI] [PubMed] [Google Scholar]
- 2.Kilander L, Nyman H, Boberg M, Hansson L, Lithell H. Hypertension is related to cognitive impairment: A 20-year follow-up of 999 men. Hypertension. 1998; 31:780–786. doi: 10.1161/01.HYP.31.3.780 [DOI] [PubMed] [Google Scholar]
- 3.Novak V, Hajjar I. The relationship between blood pressure and cognitive function. Nat. Rev. Cardiol. 2010; 7:686–698. doi: 10.1038/nrcardio.2010.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005; 4:487–499. doi: 10.1016/S1474-4422(05)70141-1 [DOI] [PubMed] [Google Scholar]
- 5.Power MC, Schneider ALC, Wruck L, Griswold M, Coker LH, Alonso A, Jack CR, Knopman D, Mosley TH, Gottesman RF. Life-course blood pressure in relation to brain volumes. Alzheimer’s Dement. 2016; 12:890–899. doi: 10.1016/j.jalz.2016.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ou YN, Tan CC, Shen XN, Xu W, Hou XH, Dong Q, Tan L, Yu JT. Blood pressure and risks of cognitive impairment and dementia: A systematic review and meta-analysis of 209 prospective studies. Hypertension. 2020; 76:217–225. doi: 10.1161/HYPERTENSIONAHA.120.14993 [DOI] [PubMed] [Google Scholar]
- 7.Waldstein SR. Hypertension and neuropsychological function: A lifespan perspective. Exp Aging Res. 1995; 21:321–352. doi: 10.1080/03610739508253989 [DOI] [PubMed] [Google Scholar]
- 8.Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik RJ. The Association Between Midlife Blood Pressure Levels and Late-Life Cognitive Function: The Honolulu-Asia Aging Study. JAMA. 1995; 274:1846–1851. doi: 10.1001/jama.1995.03530230032026 [DOI] [PubMed] [Google Scholar]
- 9.Kivipelto M, Helkala EL, Laakso MP, Hänninen T, Hallikainen M, Alhainen K, Soininen H, Tuomilehto J, Nissien A. Midlife vascular risk factors and Alzheimer’s disease in later life: Longitudinal, population based study. BMJ. 2001; 322:1447–1451. doi: 10.1136/bmj.322.7300.1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGrath ER, Beiser AS, DeCarli C, Plourde KL, Vasan RS, Greenberg SM, Seshadri S. Blood pressure from mid-to late life and risk of incident dementia. Neurology. 2017; 89:2447–2454. doi: 10.1212/WNL.0000000000004741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lane CA, Barnes J, Nicholas JM, Sudre CH, Cash DM, Parker TD, Malone IB, Lu K, James S-N, Keshavan A, et al. Associations between blood pressure across adulthood and late-life brain structure and pathology in the neuroscience substudy of the 1946 British birth cohort (Insight 46): an epidemiological study. Lancet Neurol. 2019; 18:942–952. doi: 10.1016/s1474-4422(19)30228-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maillard P, Seshadri S, Beiser A, Himali JJ, Au R, Fletcher E, Carmichael O, Wolf PA, DeCarli C. Effects of systolic blood pressure on white-matter integrity in young adults in the Framingham Heart Study: A cross-sectional study. Lancet Neurol. 2012; 11:1039–1047. doi: 10.1016/S1474-4422(12)70241-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rovio SP, Pahkala K, Nevalainen J, Juonala M, Salo P, Kähönen M, Hutri-Kähönen N, Lehtimäki T, Jokinen E, Laitinen T, Taittonen L, Tossavainen P, Viikari JSA, Rinne JO, Raitakari OT. Cardiovascular Risk Factors From Childhood and Midlife Cognitive Performance: The Young Finns Study. J Am Coll Cardiol. 2017; 69:2279–2289. doi: 10.1016/j.jacc.2017.02.060 [DOI] [PubMed] [Google Scholar]
- 14.Yaffe K, Vittinghoff E, Pletcher MJ, Hoang TD, Launer LJ, Whitmer R, Coker LH, Sidney S. Early adult to midlife cardiovascular risk factors and cognitive function. Circulation. 2014; 129:1560–1567. doi: 10.1161/CIRCULATIONAHA.113.004798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahinrad S, Kurian S, Garner CR, Sedaghat S, Nemeth AJ, Moscufo N, Higgins JP, Jacobs DR, Hausdorff JM, Lloyd-Jones DM, Sorond FA. Cumulative Blood Pressure Exposure During Young Adulthood and Mobility and Cognitive Function in Midlife. Circulation. 2020; 141:712–724. doi: 10.1161/CIRCULATIONAHA.119.042502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo Z, Viitanen M, Fratiglioni L, Winblad B. Low blood pressure and dementia in elderly people: The Kungsholmen project. BMJ. 1996; 312:805–808. doi: 10.1136/bmj.312.7034.805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kritz-Silverstein D, Laughlin GA, McEvoy LK, Barrett-Connor E. Sex and Age Differences in the Association of Blood Pressure and Hypertension with Cognitive Function in the Elderly: The Rancho Bernardo Study. J Prev Alzheimer’s Dis. 2017; 4:165–173. doi: 10.14283/jpad.2017.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Euser SM, Van Bemmel T, Schram MT, Gussekloo J, Hofman A, Westendorp RGJ, Breteler MMB. The effect of age on the association between blood pressure and cognitive function later in life: Brief reports. J Am Geriatr Soc. 2009; 57:1232–1237. doi: 10.1111/j.1532-5415.2009.02264.x [DOI] [PubMed] [Google Scholar]
- 19.Li G, Rhew IC, Shofer JB, Kukull WA, Breitner JCS, Peskind E, Bowen JD, McCormick W, Teri L, Crane PK, Larson EB. Age-varying association between blood pressure and risk of dementia in those aged 65 and older: A community-based prospective cohort study. J Am Geriatr Soc. 2007; 55:1161–1167. doi: 10.1111/j.1532-5415.2007.01233.x [DOI] [PubMed] [Google Scholar]
- 20.Vos T, Abajobir AA, Abbafati C, Abbas KM, Abate KH, Abd-Allah F, Abdulle AM, Abebo TA, Abera SF, Aboyans V, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017; 390:1211–1259. doi: 10.1016/S0140-6736(17)32154-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nichols E, Szoeke CEI, Vollset SE, Abbasi N, Abd-Allah F, Abdela J, Aichour MTE, Akinyemi RO, Alahdab F, Asgedom SW, et al. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019; 18:88–106. doi: 10.1016/S1474-4422(18)30403-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suvila K, McCabe EL, Lima JAC, Aittokallio J, Yano Y, Cheng S, Niiranen TJ. Self-Reported Age of Hypertension Onset and Hypertension-Mediated Organ Damage in Middle-Aged Individuals. Am J Hypertens. 2020; 33:644–651. doi: 10.1093/ajh/hpaa055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niiranen TJ, McCabe EL, Larson MG, Henglin M, Lakdawala NK, Vasan RS, Cheng S. Heritability and risks associated with early onset hypertension: Multigenerational, prospective analysis in the Framingham Heart Study. BMJ. 2017; 357. doi: 10.1136/bmj.j1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilsanz P, Mayeda ER, Glymour MM, Quesenberry CP, Mungas DM, DeCarli C, Dean A, Whitmer RA. Female sex, early-onset hypertension, and risk of dementia. Neurology. 2017; 89:1886–1893. doi: 10.1212/WNL.0000000000004602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corrada MM, Hayden KM, Paganini-Hill A, Bullain SS, DeMoss J, Aguirre C, Brookmeyer R, Kawas CH. Age of onset of hypertension and risk of dementia in the oldest-old: The 90+ Study. Alzheimer’s Dement. 2017; 13:103–110. doi: 10.1016/j.jalz.2016.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Liu K, Savage PJ. Cardia: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988; 41:1105–1116. doi: 10.1016/0895-4356(88)90080-7 [DOI] [PubMed] [Google Scholar]
- 27.Jacobs DR, Yatsuya H, Hearst MO, Thyagarajan B, Kalhan R, Rosenberg S, Smith LJ, Barr RG, Duprez DA. Rate of decline of forced vital capacity predicts future arterial hypertension: The Coronary Artery Risk Development in Young Adults Study. Hypertension. 2012; 59:219–225. doi: 10.1161/HYPERTENSIONAHA.111.184101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gibbs BB, King WC, Davis KK, Rickman AD, Rogers RJ, Wahed A, Belle SH, Jakicic J. Objective vs. self-report sedentary behavior in overweight and obese young adults. J Phys Act Heal. 2015; 12:1551–1557. doi: 10.1123/jpah.2014-0278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim C, Siscovick DS, Sidney S, Lewis CE, Kiefe CI, Koepsell TD. Oral Contraceptive Use and Association With Glucose, Insulin, and Diabetes in young adult women: The CARDIA Study. Diabetes Care. 2002; 25:1027–1032. doi: 10.2337/diacare.25.6.1027 [DOI] [PubMed] [Google Scholar]
- 30.Mcevoy CT, Hoang T, Sidney S, Steffen LM, Jacobs DR, Shikany JM, Wilkins JT, Yaffe K. Dietary patterns during adulthood and cognitive performance in midlife: The CARDIA study. Neurology. 2019; 92:E1589–E1599. doi: 10.1212/WNL.0000000000007243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wechsler D Manual for the Wechsler Adult Intelligence Scale—Revised. Psychol Corp New York. 1981; 10:1209–1212. doi: 10.1016/0191-8869(89)90091-3 [DOI] [Google Scholar]
- 32.Rosenberg SJ, Ryan JJ, Prifitera A. Rey auditory‐verbal learning test performance of patients with and without memory impairment. J Clin Psychol. 1984; 40:785–787. doi: [DOI] [PubMed] [Google Scholar]
- 33.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935; 18:643–662. doi: 10.1037/h0054651 [DOI] [Google Scholar]
- 34.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005; 53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 35.Dougherty RJ, Moonen J, Yaffe K, Sidney S, Davatzikos C, Habes M, Launer LJ. Smoking mediates the relationship between SES and brain volume: The CARDIA study. PLoS One. 2020; 15:e0239548. doi: 10.1371/journal.pone.0239548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kochunov P, Thompson PM, Lancaster JL, Bartzokis G, Smith S, Coyle T, Royall DR, Laird A, Fox PT. Relationship between white matter fractional anisotropy and other indices of cerebral health in normal aging: Tract-based spatial statistics study of aging. Neuroimage. 2007; 35:478–487. doi: 10.1016/j.neuroimage.2006.12.021 [DOI] [PubMed] [Google Scholar]
- 37.Reis JP, Loria CM, Launer LJ, Sidney S, Liu K, Jacobs DR, Zhu N, Lloyd-Jones DM, He K, Yaffe K. Cardiovascular health through young adulthood and cognitive functioning in midlife. Ann Neurol. 2013; 73:170–179. doi: 10.1002/ana.23836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pase MP, Beiser A, Aparicio H, DeCarli C, Vasan RS, Murabito J, Seshadri S. Interarm differences in systolic blood pressure and the risk of dementia and subclinical brain injury. Alzheimer’s Dement. 2016; 12:438–445. doi: 10.1016/j.jalz.2015.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abell JG, Kivimäki M, Dugravot A, Tabak AG, Fayosse A, Shipley M, Sabia S, Singh-Manoux A. Association between systolic blood pressure and dementia in the Whitehall II cohort study: Role of age, duration, and threshold used to define hypertension. Eur Heart J. 2018; 39:3119–3125. doi: 10.1093/eurheartj/ehy288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu J, Huang Y, Chen G, Liu X, Wang Z, Cao Y, Li H, Song L, Li C, Zhao H, Chen S, Wang Y, Zhang R, Wang A, Wu S. Cumulative systolic blood pressure exposure in relation to cognitive function in middle-aged and elderly adults: A prospective, population-based study. Med (United States). 2016; 95:e5514. doi: 10.1097/MD.0000000000005514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elias PK, Elias MF, D’Agostino RB, Cupples LA, Wilson PW, Silbershatz H, Wolf PA. NIDDM and blood pressure as risk factors for poor cognitive performance: The Framingham Study. Diabetes Care. 1997; 20:1388–1395. doi: 10.2337/diacare.20.9.1388 [DOI] [PubMed] [Google Scholar]
- 42.Power MC, Tchetgen EJT, Sparrow D, Schwartz J, Weisskopf MG. Blood pressure and cognition: Factors that may account for their inconsistent association. Epidemiology. 2013; 24:886–893. doi: 10.1097/EDE.0b013e3182a7121c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walker KA, Sharrett AR, Wu A, Schneider ALC, Albert M, Lutsey PL, Bandeen-Roche K, Coresh J, Gross AL, Windham BG, Knopman DS, Power MC, Rawlings AM, Mosley TH, Gottesman RF. Association of midlife to late-life blood pressure patterns with incident dementia. JAMA. 2019; 322:535–545. doi: 10.1001/jama.2019.10575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shah K, Qureshi SU, Johnson M, Parikh N, Schulz PE, Kunik ME. Does use of antihypertensive drugs affect the incidence or progression of dementia? A systematic review. Am. J. Geriatr. Pharmacother. 2009; 7:250–261. doi: 10.1016/j.amjopharm.2009.11.001 [DOI] [PubMed] [Google Scholar]
- 45.Ding J, Davis-Plourde KL, Sedaghat S, Tully PJ, Wang W, Phillips C, Pase MP, Himali JJ, Gwen Windham B, Griswold M, et al. Antihypertensive medications and risk for incident dementia and Alzheimer’s disease: a meta-analysis of individual participant data from prospective cohort studies. Lancet Neurol. 2019; 4422:1–10. doi: 10.1016/S1474-4422(19)30393-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, Beiser A, D’Agostino R, Wolf PA. Measures of brain morphology and infarction in the framingham heart study: Establishing what is normal. Neurobiol Aging. 2005; 26:491–510. doi: 10.1016/j.neurobiolaging.2004.05.004 [DOI] [PubMed] [Google Scholar]
- 47.Knopman DS, Jack CR, Wiste HJ, Weigand SD, Vemuri P, Lowe VJ, Kantarci K, Gunter JL, Senjem ML, Mielke MM, Machulda MM, Roberts RO, Boeve BF, Jones DT, Petersen RC. Age and neurodegeneration imaging biomarkers in persons with Alzheimer disease dementia. Neurology. 2016; 87:691–698. doi: 10.1212/WNL.0000000000002979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oschwald J, Guye S, Liem F, Rast P, Willis S, Röcke C, Jäncke L, Martin M, Mérillat S. Brain structure and cognitive ability in healthy aging: A review on longitudinal correlated change. Rev Neurosci. 2019; 31:1–57. doi: 10.1515/revneuro-2018-0096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Habib M, Mak E, Gabel S, Su L, Williams G, Waldman A, Wells K, Ritchie K, Ritchie C, O’Brien JT. Functional neuroimaging findings in healthy middle-aged adults at risk of Alzheimer’s disease. Ageing Res. Rev. 2017; 36:88–104. doi: 10.1016/j.arr.2017.03.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.