Abstract
Introduction
Maintaining a stable dry state is critical for long-term preservation of live biomaterials at suprazero temperatures. The objective of the study was to characterize the effect of moisture content on DNA integrity within the germinal vesicle (GV) of feline oocytes following dehydration and storage at 22–24 °C.
Methods
Using microwave-assisted drying, conditions that led to a predictable and stable moisture content in trehalose solutions were determined. To explore moisture content stability during storage, trehalose samples were dried for 15 min and stored in glass vials at 11 or 43% RH for 8 weeks. To examine whether this condition allowed proper storage of GVs, permeabilized cat oocytes were incubated in trehalose for 10 min and dried for 15 or 30 min. Oocytes then were rehydrated to assess DNA integrity either directly after drying or after 8 weeks of storage in an 11% RH environment. Raman spectroscopy was used to identify the states of dried samples during storage.
Results
Moisture content was stable during the storage period. There was no significant difference in DNA integrity between fresh and dried samples without storage. After 8 weeks of storage, DNA integrity was maintained in GVs dried for 30 min. Samples dried for 15 min and stored were compromised, suggesting crystallization of the preservation matrix during storage. Biostabilization was optimal when samples were directly processed to moisture contents consistent with storage in the glassy state.
Conclusion
Microwave-assisted drying processing and storage conditions were optimized to ensure stable long-term storage of structural and functional properties of genetic resources.
Electronic supplementary material
The online version of this article (10.1007/s12195-020-00635-y) contains supplementary material, which is available to authorized users.
Keywords: Dry preservation, Germinal vesicles, Microwave-assisted drying, Moisture sorption isotherm, Amorphous
Introduction
Since the first successful offspring born from frozen-thawed human oocytes in 1986,11 oocyte preservation has attracted increasing attention as a fertility preservation option for patients who are experiencing infertility due to cancer treatment and/or age-related fertility decline. However, the large cell size, significant water content, and sensitivity of microtubular spindles to non-physiological conditions increase the difficulty of mature oocyte preservation compared to other cell types. Alternatively, immature oocytes at the germinal vesicle (GV) stage lack a distinct meiotic spindle and contain nuclear envelope-enclosed genetic materials, making them more tolerant to certain preservation manipulations than their mature (metaphase II) counterparts.14 Preservation, recovery, and subsequent transfer of GVs into enucleated conspecific cytoplast has become a viable option to rescue the genome from an incompetent or subpar oocyte.13,35
Cryopreservation is the standard method for preserving oocytes. However, cryopreserved biomaterials need to be maintained at ultralow temperatures within liquid nitrogen-charged containers, which leads to high cost and logistical challenges for both transportation and storage. Dry preservation may provide a simpler and more affordable approach for long-term storage. Mimicking anhydrobiotic organisms in nature, dry preservation involves introduction of a disaccharide sugar, which can transform to a glassy state under low humidity conditions and facilitate the reduction of molecule mobility.1,21 Trehalose is the most common disaccharide used in dry preservation because of its superior protective capacity, often attributed to its ability to form a glassy state with a high glass transition temperature (Tg, the temperature at which the properties of the material change from liquid-like to solid-like), thus allowing storage at ambient temperatures.12,15
Moisture removal techniques previously developed for mammalian cells including air-drying,23 vacuum drying,22 spray drying,30 spin drying,9 and freeze-drying,16 each with its challenges in obtaining uniformly dried samples. We previously demonstrated that the use of microwave-assisted drying enhances the diffusion and evaporation of water from the interior of the sample via the delivery of small pulses of energy to yield faster and more predictable drying rates and uniformly dried samples, while avoiding thermal injury to the cells.7,8,20 Recently our group has demonstrated that microwave-assisted drying can be used to rapidly and reproducibly dehydrate GV oocytes to moisture contents that enable ambient storage in a glassy state.20 In order to prevent reversion of the metastable glass into a crystallized form that might compromise long-term storage success, it is critical that samples are packaged in a way that enables an appropriate moisture content to be maintained during storage. Trehalose that was introduced into oocytes by forming large pores with α-hemolysin was found to protect DNA integrity during drying, storage, and rehydration. However, the wide range of environmental relative humidity (RH) resulted in high variation in the end moisture contents. In addition, the Dri-shield bags that were used to contain dried GVs were found to absorb additional moisture, which might have caused DNA fragmentation.18
The objective of the study was to investigate the stability of moisture content and trehalose glass state and further characterize the effect of moisture content on DNA integrity within cat GVs, with improved moisture control during drying and ambient storage. First, a drying chamber was set up to achieve a constant RH of 11% to mitigate moisture fluctuation during microwave-assisted dehydration. Second, dehydrated samples were stored in glass vials with constant RH. Our goal was to confirm optimal processing and storage conditions for long-term biobanking of genome resources.
Materials and Methods
Assessment of Microwave-Assisted Desorption Kinetics and Temperature Excursions During Drying
In previous feline gamete work, trehalose (Pfanstiehl, Inc., USA) in Tris–EDTA (TE; Sigma-Aldrich, MO) buffer solution was used as the carrier glass-forming preservative solution.20,23 To prescribe specific drying end-points for storage studies, a reference drying curve was established for a 40 μL droplet of this trehalose/TE solution at an initial concentration of 1.1 M. Heat-assisted drying was conducted using a customized microwave (CEM SAM 255, Matthews, NC) operating at 20% microwave power. The end moisture content was determined at 5 min intervals of dehydration processing for up to 50 min, in a controlled RH of 11.0 ± 0.6%, which was achieved by flowing dried air into a chamber and monitoring with a temperature and RH logger (HH314A, Omega, CT). Karl Fisher (KF) titration (V20S, Mettler-Toledo, Columbus, OH) was used to determine the water content of samples. The mass of moisture in each sample was calculated by multiplying the weight percent moisture content by the mass of a 40 μL of 1.1 M trehalose/TE droplet (determined by average weight of 10 replicate samples). The anhydrous mass of 10 replicates was used to determine an average dry weight for 40 μL of 1.1 M trehalose/TE solution. The moisture content was expressed as grams of H2O per gram of dry weight (g H2O/g DW).
The maximum temperatures of samples attained during microwave processing were estimated using Thermax™ irreversible temperature sensitive labels (LCRHallcrest, Glenview, IL) that indicated temperature thresholds at 29, 34, 37, 40, 42, and 44 °C. Each label was adhered to the top of a polyethylene syringe filter holder (Millipore, Billerica, MA), on which a trehalose-loaded glass fiber filter was placed. Eight holders were then placed in a custom turntable for drying. A color change from silver white to black indicated that the temperature threshold had been reached.
While the drying solutions in this study contained 10 mM Tris and 1 mM EDTA, the Tg of the solution was not likely to be altered significantly by such a small mass contribution of salt. The Tg at each drying point was thus estimated by using a Gordon–Taylor fit for trehalose–water data according to:
| 1 |
where x is the mass fraction of water, k = 5.2, Tg,Tre (Tg of trehalose) is 100 °C, and Tg,w (Tg of water) is – 135 °C.12
Determination of the Desorption Isotherm for Trehalose/TE Solution
To construct a desorption isotherm of trehalose/TE solution, desiccators consisting of different saturated salt solutions were utilized to generate stable RH conditions. The RH was measured using a HH314A humidity meter (Omega, CT). There was a difference in the theoretical 24 and the measured RH value of the salt solution (Supplemental Table 1). Droplets of 1.1 M trehalose buffer solutions (40 μL each) were placed on glass fiber filters, transferred into desiccators on top of the sample holders, and kept at ambient temperature (23 °C). Water loss from the droplets was determined by periodically measuring sample mass until three serial measurements of equal value were obtained (14–21 days). At least three replicates of samples were used at each RH and data were expressed as mean ± standard deviation (SD). The experimental desorption data were fitted, using Matlab software (Mathworks, MA), to the Guggenheim–Anderson–de Boer (GAB) equation given by:
| 2 |
where aw is water activity, M is the equilibrium moisture content, M0 is monolayer of desorbed water, C is the Guggenheim constant and K is a constant that corrects for the properties of multilayer molecules with respect to the bulk liquid.5
Moisture Content Stability of Dehydrated Trehalose/TE Solution Stored in Glass Vials
To determine the expected moisture content variability of samples stored in a given humidity, trehalose/TE solutions were dried, stored, and monitored for moisture content over time. Samples of 1.1 M trehalose/TE solution were dried for 15 min in 11% RH and then directly placed into 5-mL serum tubing vials (Wheaton™, Fisher Scientific, Suwanee, GA) that were pre-equilibrated with either 11% RH air in the dry chamber or with 43% RH air in the environmental chamber (Caron, OH). Vials were sealed with rubber stoppers and crimped with aluminum caps to avoid moisture leakage. Samples were stored at 4 °C or 23 °C for 0 or 2 days or 1, 2, 4, or 8 weeks. Moisture contents were assessed at the end of each storage period. Data (n = 9) were pooled to determine the overall moisture content of each treatment group.
Oocyte Collection
Ovaries from domestic cats were harvested from a local spay and neuter clinic after routine ovariohysterectomy. Fresh ovaries were placed immediately in phosphate-buffered saline (PBS) supplemented with 100 IU/mL of penicillin and 100 IU/mL of streptomycin (Mediatech, Inc., Manassas, VA) at 4 °C and then transported on ice within 2 h to the laboratory. Cumulus–oocyte-complexes (COCs) were collected from antral follicles by repeatedly slicing the ovaries in handling medium [HEPES-buffered minimum essential medium (H-MEM; Gibco Laboratories, Grand Island, NY) supplemented with 2.0 mM l-glutamine 1.0 mM sodium pyruvate, 100 IU/mL penicillin, 100 IU/mL streptomycin and 4 mg/mL bovine serum albumin (BSA; Sigma-Aldrich)]. Each COC was classified according to the standard quality criteria described by Wood and Wildt.38 Only grade I (uniformly dark cytoplasm, ≥ 5 layers of tightly compacted cumulus cells) and grade II (uniformly dark cytoplasm, < 5 cell layers) COCs were selected and then denuded of cumulus cells by exposure to 0.2% hyaluronidase (Sigma-Aldrich) for 15 min at 38 °C, followed by vortexing and rinsing with handling medium. Some denuded oocytes from each batch served as the fresh control. These were fixed in 4% paraformaldehyde overnight and then transferred to 70% ethanol before the assessment of DNA integrity. The others were immediately processed by microwave-assisted drying.
Microwave-Assisted Drying, Storage, and Rehydration of Oocytes
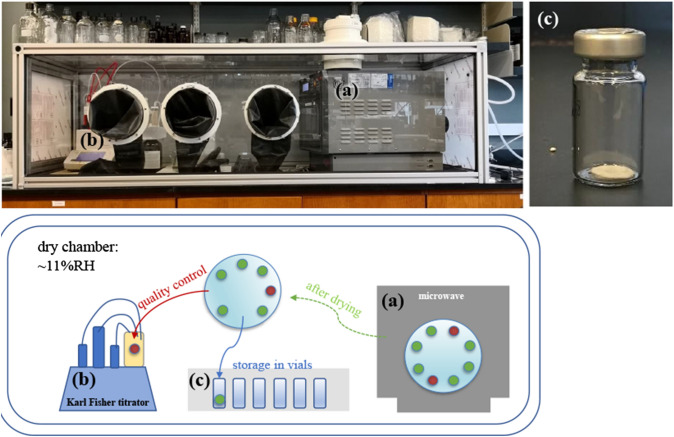
To allow trehalose loading, the cell membranes of oocytes were permeabilized by exposure to 10 μg/mL hemolysin for 15 min at 38 °C. After rinsing with handling medium, oocytes were immersed in 1.1 M trehalose/TE solution for 10 min at room temperature and then immediately dehydrated. A volume of 40 μL trehalose/TE solution along with 5–7 of oocytes was deposited on glass fiber filters and microwave processed in a controlled RH of 11.0 ± 0.6%. Within each batch, six filter samples with oocytes were randomly placed on the turntable (green in Fig. 1). Three samples were taken out of the microwave after 15 min and either directly rehydrated or stored in glass vials for 8 weeks at 11% RH at ambient temperature (22–24 °C). Remaining samples were processed for an additional 15 min for a total of 30 min drying. At each time point, one sample was used for quality control purposes to evaluate the moisture content (red in Fig. 1).
Figure 1.
Work station (top) and sample workflow (bottom) for processing oocytes for storage in vials with in a controlled RH chamber. Within each batch three samples of dried oocytes (green) were taken out of the microwave (a) after 15 min and either directly rehydrated or stored in glass vials (c). Remaining samples were processed for an additional 15 min. At each time point one sample was used for quality control purposes to evaluate the moisture content (red) with a Karl Fisher titrator (b).
Rehydration was performed by exposure to 500 μL of handling medium (omitting BSA) for 30 min. Recovered oocytes were fixed in 4% paraformaldehyde overnight and then transferred to 70% ethanol before the assessment of DNA integrity.
Assessment of DNA Integrity
DNA fragmentation was detected by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay using an in situ cell death detection kit (Roche Applied Science, Indianapolis, IN). After rinsing off the ethanol, oocytes were permeabilized with 0.5% Triton X100 for 30 min at room temperature before exposure to TUNEL reaction mixture for 1 h at 38 °C. Oocytes were then washed three times in PBS and mounted on slides with Vectashield containing DAPI (Vector Labs, Inc., Burlingame, CA). Each slide was examined under an epifluorescence microscope (Olympus BX41; Olympus Corporation, Melville, NY) using SPOT software 5.0 (Diagnostic Instruments, Inc., Sterling Heights, MI). TUNEL positive/negative oocytes were classified based on the presence/absence of green fluorescence in GVs.20
Raman Spectroscopy
Raman spectroscopy was used to distinguish the amorphous state of dried samples from the crystallized state. Dried samples embedded in the filter paper are indiscernible by eye, thus samples were also dried on the surface of glass coverslips to correlate visualize appearances with Raman spectra. Control amorphous trehalose samples were prepared as follows: a 40 μL of 1.1 M trehalose/TE solution was dropped on the coverslip (or on the filter paper) and then heated in an oven at 125 °C for 48 h. The amorphous samples were transported for Raman testing within 2 h in a container with dry silica bead desiccant to prevent moisture absorption from the ambient environment. Control crystalline trehalose samples were obtained by placing amorphous trehalose samples in 75% RH and allowing for visually observable crystals to form. Three replicates were performed using the same procedure.
The measurements were conducted with a Horiba Xplora confocal Raman microscope with a 1200 g/mm grating. A 785 nm laser was used as the excitation source. Data were taken using a 40 × (NA = 0.65) microscope lens. The excitation density is estimated as D = P/A, where A is the area determined by diffraction limited spot size. The laser power P was measured at the exit of the microscope lens (about 15 mW of full power). Hole size was set to be 100 μm. All measurements were performed at room temperature. For each Raman spectrum, its acquisition time was 10 s and averaged 5 times. On each sample at least three spectra were taken.
Experimental Design and Statistical Analysis
Experiment 1 was conducted to determine the microwave-assisted desorption kinetics of a 40 μL droplet of 1.1 M trehalose/TE solution in a controlled 11% RH environment in order to predict moisture content at a given drying time. The moisture content achieved was then compared to moisture levels of samples that were slowly equilibrated over saturated lithium chloride (LiCl) solution (RH = 12%). The Tg values of dried samples were predicted from their moisture contents by the Gordon–Taylor equation, and then further used to evaluate storage conditions. Experiment 2 established a moisture sorption isotherm to identify expected equilibrium moisture contents for prescribed RH levels. Trehalose/TE samples that were dried for 15 min were stored in glass vials at 4 and 23 °C at 11% RH and 43% RH for 8 weeks to test moisture content stability during storage. Based on these findings, experiment 3 was conducted to examine the influences of 15-min and 30-min microwave-drying (8 batches) and 8-week storage (14 batches) at ambient temperature (23 °C) on the DNA integrity of GV oocytes by TUNEL assays. Raman spectroscopy was used to identify the states of dried samples during storage. Data from all replicates were expressed as mean ± SD. One-way ANOVA and Kruskal–Wallis ANOVA were used for statistical analysis to compare treatment groups. Differences were considered significant at p < 0.05.
Results
Microwave-Assisted Drying Kinetics
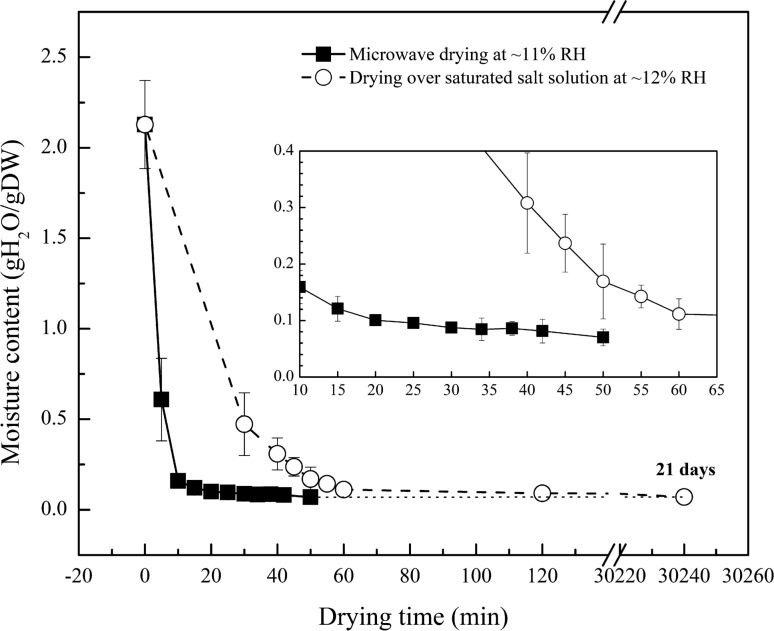
To prescribe appropriate drying times for the GVs, a microwave-assisted drying curve was established for a 1.1 M trehalose/TE solution dried in an 11% RH environment. The average moisture content rapidly decreased from 2.1 ± 0.2 to 0.087 ± 0.007 g H2O/g DW over the first 30 min, while no further significant decrease (p > 0.05) was observed between 30 and 50 min (Fig. 2). In comparison, a similar moisture content was achieved after several hours of drying over a saturated LiCl solution, which had a RH level of approximately 12% (Fig. 2). Moreover, the highest temperature reached during microwave drying was between 42 and 44 °C by the 15 min drying point. No further increase in temperature was noted for longer drying times.
Figure 2.
Comparison of moisture levels of microwave processed samples (black squares) to samples that were slowly equilibrated over saturated lithium chloride solution (white circles). Inset represents close-up view of low moisture content region.
Desorption Isotherm
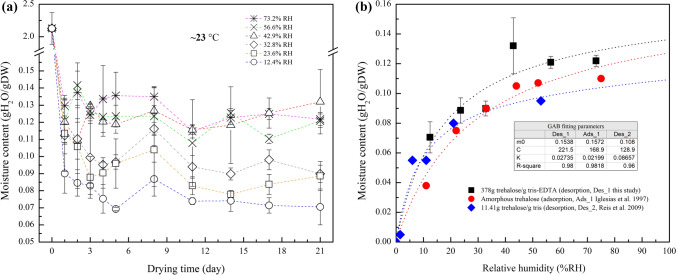
To better understand the effect of RH on the moisture content of trehalose glasses, the kinetics of water loss from trehalose/TE solutions at various RH levels were investigated (Fig. 3a). The water desorption isotherm determined over saturated salt solutions at 23 °C is represented in Fig. 3b, and parallels that of published trehalose sorption isotherms created using similar methodologies.25,29,41 Data fits were obtained with the GAB model. At RH levels of 32.8% and below, samples achieved moisture contents below the level typically associated with crystalline trehalose dihydrate (0.105 g H2O/g DW), suggesting that samples were likely in the amorphous state. No significant difference in moisture contents was observed among samples equilibrated at 42.9% and above, and the isotherm reaches a plateau (0.12–0.13 g H2O/g DW) consistent with the formation of trehalose dihydrate. Direct visual confirmation of crystallization was challenging to observe due to the nature of the glass fiber paper. Based on these results, storage at RH levels below 42.9% will likely be necessary to maintain a metastable amorphous glassy condition at 23 °C.
Figure 3.
(a) Kinetics of water loss at 23 °C from trehalose buffer solutions over saturated salt solutions for a range of fixed humidity conditions and (b) water desorption or adsorption isotherms of trehalose solutions at 23 °C.
Storage Stability of Trehalose/TE Solution
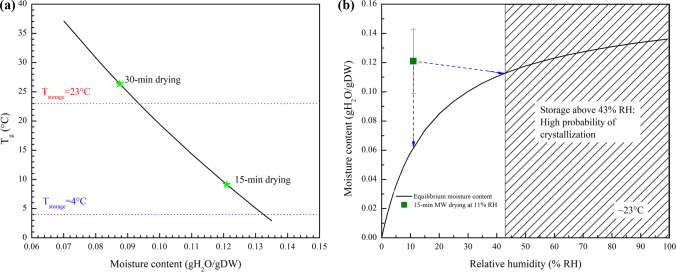
The calculated Tg provides insight into a suitable condition for storage (Fig. 4a). The moisture level achieved after 15 min of drying was predicted to yield a Tg of 9 °C, suggesting that samples maintained at this moisture level should be stored below 9 °C in order to retain the glassy state. Conversely, samples dried for 30 min yielded moisture contents that corresponded to a Tg of 26.3 °C, which should provide for stable storage at room temperature (23 °C), if the biologic can tolerate drying to this level.
Figure 4.
(a) Tg values at different moisture contents were predicted by the Gordon–Taylor equation. The Tg values (green stars) produced by drying for 15 min and 30 min were higher than general cold storage temperature (4 °C, blue dotted line) and room temperature (23 °C, red dotted line), respectively. (b) Moisture content data from samples processed by microwave for 15 min (green square) are overlaid on the fitting curve (black line) obtained by the GAB model. The dotted lines (blue) represent the possible change in equilibrated moisture content during storage at 11 and 43% RH.
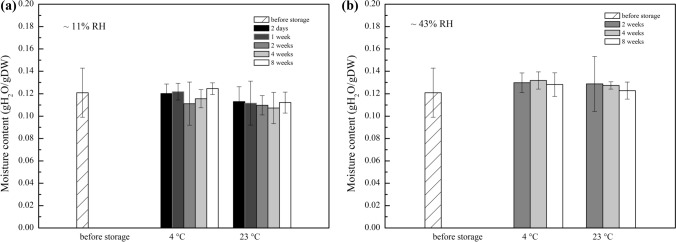
Microwave-assisted drying enables rapid dehydration to desired moisture levels. Samples that were processed with microwave heating for 50 min converged on the values achieved in 21 days when drying over a saturated salt solution (0.07 g H2O/g DW; Fig. 2). However, over-processing with heating can cause damage to biological samples.20,37 We proposed a two-step drying strategy, using dynamic drying as a first step, followed by ‘in-vial’ final equilibration of moisture content as a finishing step, to avoid over-processing. Samples were dried to 0.121 ± 0.022 g H2O/g DW in 11% RH with microwave processing and then stored at 11 and 43% RH at both 4 and 23 °C. All samples exhibited statistically insignificant drying over the 8-week period (Fig. 5).
Figure 5.
Moisture contents of trehalose buffer solutions that were dried for 15 min and then stored in glass vials with ~ 11% RH air (a) and ~ 43% RH air (b) for up to 8 weeks at 4 °C or ambient temperature (23 °C). There was no significant difference in the moisture content of samples before storage and for each period during the 8-week storage period, for both relative humidity conditions (p > 0.05). Data was expressed as mean ± SD, n = 9.
Impact of Microwave-Assisted Drying and Storage on Recovery and DNA Integrity of Oocytes
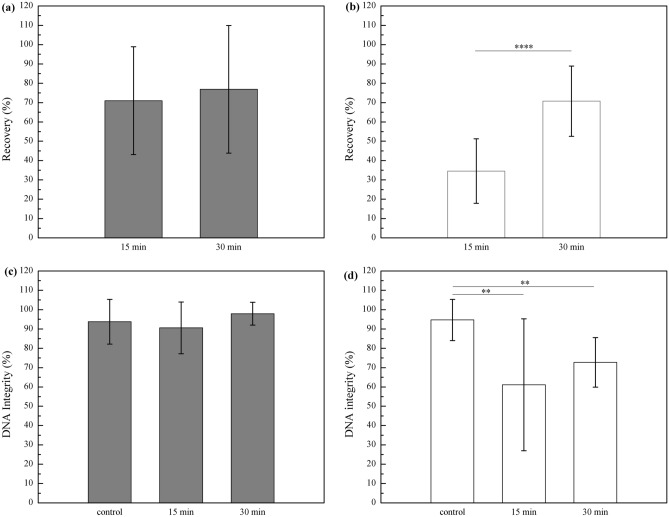
Next, the effect of select processing and storage conditions on the DNA integrity of oocytes was pursued. There was no significant difference in the percentage of oocytes recovered from filters after rehydration between samples dried for 15 and 30 min without storage (Fig. 6a), while the recovery rate of the 15 min drying group dramatically decreased (p < 0.05) to 34.5 ± 16.7% following 8 weeks of storage (Fig. 6b). TUNEL analysis showed no significant difference in DNA integrity between fresh samples (n = 31) and samples recovered directly after microwave-assisted drying (n = 65 for 15 min drying, n = 62 for 30 min) (p > 0.05; Fig. 6c). After 8-week storage, 61.1 ± 5.3 and 72.7 ± 12.8% of recovered oocytes in the 15- (n = 47) and 30-min (n = 113) dried groups, respectively, had intact DNA, which was lower (p < 0.05) than control samples (97.9 ± 5.9%; Fig. 6d).
Figure 6.
Percentage of recovered oocytes relative to the total number of treated oocytes after microwave-assisted drying (a) and after 8-week storage in 11% RH air at ambient temperatures (b) and percentage of oocytes with intact DNA relative to the total number of recovered oocytes after microwave-assisted drying (c) and after 8-week storage in 11% RH at ambient temperatures (d). Data was expressed as mean ± standard deviation (SD). One-way and Kruskal–Wallis ANOVA was used for statistical analysis, **p < 0.01, ****p < 0.0001.
Determining the State of Dried Samples During Storage
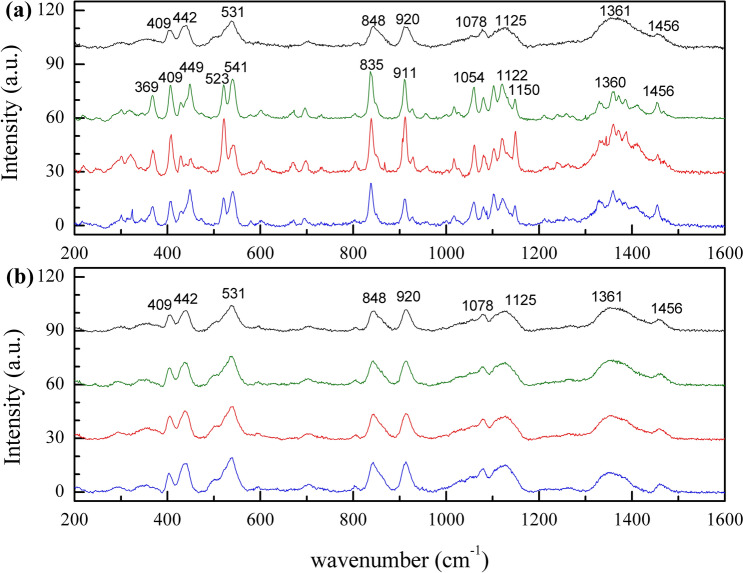
We suspected that loss of GVs was due to inadequate suppression of molecular mobility and transition from amorphous into crystalline trehalose. Raman spectroscopy was conducted to identify the states of samples. Consistent with previous studies,36 differences in Raman spectra of crystalline and amorphous trehalose both on the coverslips and on the filter papers were observed (Supplemental Fig. 1). Crystalline trehalose exhibited sharp and narrow bands while the amorphous bands were broadened. Because of their structure, the system of crystals is more conformationally ordered than in the amorphous state.10,27 This disordered state of amorphous molecules corresponds to a strong broadening of the Raman mode.
Spectra from samples dried for 15 min and 30 min (Fig. 7) suggest that these samples were in the amorphous state before storage. After 2 weeks of storage, the samples dried for 15 min exhibited a crystalline band pattern, whereas samples dried for 30 min maintained their amorphous spectra until 8 weeks. In Fig. 7a, amorphous samples (dried for 15 min before storage) can be distinguished from crystalline samples after storage in the fingerprint region from 300 to 1500 cm−1. In the regions from 1000 to 1200 cm−1 and from 1300 to 1500 cm−1, the peaks in amorphous samples (black) are broader and poorly resolved compared to those in crystalline trehalose (green, red, blue) assigned to C–O stretching and/or C–OH side group deformation. The clear shifts of the intense bands can be observed from 920 to 911 cm−1 and from 848 to 835 cm−1, which are associated with conformational deformation of the glycosidic linkage in trehalose, while the bands in the region from 500 to 550 cm−1 are associated with exocyclic deformations.10,17,40 These results suggest that samples that were dried for only 15 min have a moisture content and molecular mobility that resulted in crystallization after storage for 2 weeks at 23 °C, while the samples dried for 30 min were adequately dry to suppress molecular mobility and maintain an amorphous state for at least 8 weeks.
Figure 7.
Raman spectra of samples dried for 15 min (a) and for 30 min (b) on filter papers before storage (black) and after 2- (green), 4- (red) and 8- (blue) week storage.
Discussion
Microwave-assisted processing for 30 min in a 11% RH environment was determined to yield samples that demonstrated high recovery and DNA integrity after processing and storage in glass vials for up to 8 weeks at room temperature, whereas samples processed for 15 min resulted in poor recovery after storage.
Different drying methods produce a range of different drying rates, and often yield products with spatially different moisture distributions. In general, passive droplet drying mechanisms lead to a spatially non-uniform distribution of the solid phases.18,19 During passive drying, the higher rates of evaporation on the surface result in extremely steep concentration gradients, yielding a glassy skin on the surface of the droplet. This glassy skin provides mechanical resistance that indirectly affects evaporation by delaying and sometimes stopping movement of water from the center of droplets. The presence of a wet center and dry peripheral region can lead to unpredictable and unstable storage of desiccated samples. Our previous work demonstrated the feasibility for successful microwave-assisted dehydration of the mammalian GV via the delivery of small pulses of energy. In general, evaporative cooling slows the evaporation rate, thus these pulses serve to increase the temperature of the sample modestly to overcome this slow-down in drying.20
Although heating during drying can favorably enhance both the process and product, temperature excursions in excess of physiologic temperatures can induce thermal damage to cells. Therefore it is important to ensure that thermal excursions are not exceeding physiologically tolerable levels during microwave-assisted drying. In previous studies, the temperature of samples was measured with a hand-held infrared sensor immediately after the drying was terminated and the microwave door was opened, and thus did not represent a measure of the maximum sample temperature during drying.8,31 In the current study, temperature indicators were used to monitor peak temperatures attained near the sample during microwave-assisted drying. Because the indicators are not directly embedded in the sample, they remain an estimate of peak temperature. The heated trehalose-buffer samples in this study were estimated to attain peak temperatures between 40 and 42 °C during microwaving for periods between 15 and 30 min, which modestly exceeds the physiological temperature in cats (38.5 °C).
By analyzing desorption kinetics, we demonstrated that microwave-assisted drying of samples could accelerate the attainment of equilibrium moisture values. Using a chamber with controlled 11% RH, we were able to achieve a moisture content of 0.11 g H2O/g DW in 15 min, halving the time required in an uncontrolled RH environment.20 In the current study, desiccated house air was used to establish the 11% RH environmental, but in the future other levels could be achieved by using mixtures of dry and moist air.
Moisture adsorption/desorption isotherms describe the relationship between the equilibrium moisture content and the RH at constant temperature and pressure, and are important for predicting moisture changes that may occur during storage.26 Many different models with a range of fitting parameters, have been developed to relate sorption behavior to the microstructures of different types of samples.2,6,26,32–34 The three-parameter GAB, considered an extension of the Brunauer–Emmett–Teller (BET) model, has been found to predict the equilibrium moisture content of many materials with greater accuracy than other two-parameter equations.28 This equation was used to model the data in the current study to better understand the effect of sample characteristics on processing parameters, and consequently the ultimate functionality of the preserved biologic.
The advantage of drying on glass fiber filters is that the surface tension is broken by reducing the force of cohesion and adhesion and the droplet dispenses into the filter paper. This changes adsorption from multilayer to monolayer, facilitating the rapid removal of water from samples during drying. The desorption isotherm is thus characteristic of the combined matrix of sugar composition and glass fiber paper. One concern is that the porous structure may exhibit a hysteresis effect, which will result in higher equilibrium water content of some desorbed samples than that of sorbed ones at a given humidity level and temperature.3,39 As shown in Fig. 3b, the desorption isotherm determined in this study was consistent with the desorption isotherm32 and the adsorption isotherm for amorphous trehalose previously reported,25 suggesting that hysteresis effects may not be significant.
The conditions that lead to crystallization of trehalose in stored composition is of great interest in this study. At lower RH, water is strongly bound and is not available for chemical reactions or as a plasticizer. However, when RH increases, more water molecules become free to function as a plasticizer to facilitate the glassy to crystalline phase transition.5,25 The sorption curve obtained in our analysis showed that the equilibrated moisture content at 42.9% RH (0.11 g H2O/g DW) is above the level required to form trehalose dihydrate (0.105 g H2O/g DW) and the corresponding Tg is below room temperature. It is well known that the crystallization of amorphous trehalose is a time-dependent phenomenon that will occur as a consequence of holding the system above its Tg. Crystallization of trehalose is thus probable for compositions held at this moisture level, in agreement with previous studies of adsorption of water in amorphous trehalose.25
Despite a preponderance of studies on different dynamic drying techniques, little attention has been given to moisture control during packaging and long-term storage of dehydrated cells. Previous work from the food and pharmaceutical sciences show that the shelf-life is dependent on aw and Tg of the product.34 The aw, which equilibrates to the RH of the water in the environment surrounding the sample, describes the degree of “boundness” of water in a sample. Boundness will affect the availability of water to participate in physical, chemical and microbiological reactions,4 including the degradative reactions that lead to loss of biological function of the product. In addition, samples should be stored below the Tg in order to maintain preserved samples in a low mobility glassy state. In the case of the disaccharide-based preservative, samples can transition from an amorphous state to a crystalline state 25 when stored above Tg, potentially damaging the structure and functionality of incorporated cells and molecules.
The current study aimed to develop both a drying process to achieve prescribed moisture levels and a storage methodology to retain an appropriate RH level for extended storage. As shown in Fig. 4b, after 15 min of drying at 11% RH, the moisture level of the sample was close to the equilibrium level in a 43% environment. It was hypothesized that the sample would continue to dry in the 11% environment, but would experience minimal changes in a 43% environment. However, minimal change in moisture content was observed in all cases. There was a modest but insignificant change in the average of moisture content from 0.12 to 0.11 g H2O/g DW when stored at 23 °C. This decrease might have come from water evaporated from the samples into the headspace above the samples. The evaporated water could in turn elevate the RH in the 9 mL glass vials from 11% to near 43% to reach a new equilibrium and prevent further moisture loss in the samples. Further studies on sampling and analysis of the headspace moisture content would be needed to confirm this hypothesis.
Our results indicated that microwave-assisted drying for 15 min at 11% RH produced a moisture level that enables 4 °C storage, whereas 30 min of drying was needed to create a moisture level facilitating stable storage at ambient temperature. At both levels, the recovery rates after processing were above 70%. It is not clear if sample losses were due to direct physical damage or are an artifact of sample handling. It is promising that the recovered oocytes exhibited no additional DNA damage, consistent with our previous studies.20 In other words, if samples are recovered from the filter paper, the specimens are of high quality from the standpoint of DNA integrity.
When samples are dried and then stored, significant differences emerge between samples that were processed for 15 and 30 min. The recovery rate of samples processed for 30 min did not change before or after 8-week storage, but the DNA integrity in those samples moderately decreased from 98 to 73%. In contrast, fewer than half of the 15-min dried oocytes were able to be recovered after 8-week storage. Raman analysis confirmed that drying for 15 min produced samples with a moisture content and molecular mobility that resulted in crystallization after storage for 2 weeks at 23 °C. Those crystals could disrupt the structure of dried oocytes and cause a drastic reduction in recovery rate. This may also explain the lower DNA integrity observed in these samples compared to the 30 min processed counterparts. It was thus concluded that the best approach was to directly process samples to moisture contents consistent with storage in the glassy state, as finishing drying ‘in-vial’ was difficult to achieve. In the current work 30 min of drying in 11% RH was sufficient to yield intact GVs with high DNA integrity.
In conclusion, microwave-assisted drying processing and storage conditions were optimized in this study to ensure stable long-term storage of functionally viable genetic resources. A moisture sorption isotherm was established to enable prediction of moisture changes that could occur during storage and thus affect the shelf-life stability. Over 70% of the recovered GVs that were dried for 30 min and then stored in vials at ambient temperatures for 8 weeks at 11% RH (expected to be in a glassy state) had intact DNA. Further studies focusing on enhancing tolerance to lower moisture contents could enable stable storage of GVs at potentially higher temperatures while maintaining DNA integrity and cellular functionality.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
Research reported in this publication was supported by the Office of the Director, National Institutes Of Health under Award Number R01OD023139. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors are indebted to Dr. Alida Kinney and staff at the Cabarrus Spay and Neuter Clinic for generously facilitating the transfer of resources from consenting pet-owners to UNC Charlotte. We also thank Sidney Richards for technical assistance.
Conflict of interest
Shangping Wang, Pei-Chih Lee, Amanda Elsayed, Fan Zhang, Yong Zhang, Pierre Comizzoli, and Gloria D. Elliott declare that they have no conflicts of interest.
Ethical Approval
The study did not require the approval of an Animal Care and Use Committee because cat ovaries were collected at local veterinary clinics as byproducts from owner-requested routine ovario-hysterectomies.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Acker JP, Lu XM, Young V, Cheley S, Bayley H, Fowler A, Toner M. Measurement of trehalose loading of mammalian cells porated with a metal-actuated switchable pore. Biotechnol. Bioeng. 2003;82:525–532. doi: 10.1002/bit.10599. [DOI] [PubMed] [Google Scholar]
- 2.Aktas T, Ulger P, Daglioglu F, Sahin FH. Sorption isotherms and net isosteric heat of sorption for plum osmotically pre-treated with trehalose and sucrose solutions. Bulg. J. Agric. Sci. 2014;20:8. [Google Scholar]
- 3.Al Hodali, R. Numerical simulation of an agricultural foodstuffs drying unit using solar energy and adsorption process. PhD Thesis, Universite Libre de Bruxelles, Belgium 1997.
- 4.Al-Muhtaseb AH, McMinn WAM, Magee TRA. Water sorption isotherms of starch powders—Part 1: mathematical description of experimental data. J. Food Eng. 2004;61:297–307. doi: 10.1016/S0260-8774(03)00133-X. [DOI] [Google Scholar]
- 5.Andrade RD, Lemus R, Perez CE. Models of sorption isotherms for food: uses and limitations. Vitae-Rev. Fac. Quimica Farm. 2011;18:324–333. [Google Scholar]
- 6.Basu S, Shivhare US, Mujumdar AS. Models for sorption isotherms for foods: a review. Dry. Technol. 2006;24:917–930. doi: 10.1080/07373930600775979. [DOI] [Google Scholar]
- 7.Cellemme SL, Van Vorst M, Paramore E, Elliott GD. Advancing microwave technology for dehydration processing of biologics. Biopreserv. Biobank. 2013;11:278–284. doi: 10.1089/bio.2013.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakraborty N, Biswas D, Parker W, Moyer P, Elliott GD. A role for microwave processing in the dry preservation of mammalian cells. Biotechnol. Bioeng. 2008;100:782–796. doi: 10.1002/bit.21801. [DOI] [PubMed] [Google Scholar]
- 9.Chakraborty N, Menze MA, Malsam J, Aksan A, Hand SC, Toner M. Cryopreservation of spin-dried mammalian cells. PLoS ONE. 2011;6:e24916–e24916. doi: 10.1371/journal.pone.0024916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chakravarty P, Bhardwaj SP, King L, Suryanarayanan R. Monitoring phase transformations in intact tablets of trehalose by FT-Raman spectroscopy. AAPS PharmSciTech. 2009;10:1420–1426. doi: 10.1208/s12249-009-9337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen C. Pregnancy after human oocyte cryopreservation. Lancet. 1986;1:884–886. doi: 10.1016/S0140-6736(86)90989-X. [DOI] [PubMed] [Google Scholar]
- 12.Chen T, Fowler A, Toner M. Literature review: supplemented phase diagram of the trehalose–water binary mixture. Cryobiology. 2000;40:277–282. doi: 10.1006/cryo.2000.2244. [DOI] [PubMed] [Google Scholar]
- 13.Comizzoli P, Pukazhenthi BS, Wildt DE. The competence of germinal vesicle oocytes is unrelated to nuclear chromatin configuration and strictly depends on cytoplasmic quantity and quality in the cat model. Hum. Reprod. 2011;26:2165–2177. doi: 10.1093/humrep/der176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Comizzoli P, Wildt DE, Pukazhenthi BS. Impact of anisosmotic conditions on structural and functional integrity of cumulus–oocyte complexes at the germinal vesicle stage in the domestic cat. Mol. Reprod. Dev. 2008;75:345–354. doi: 10.1002/mrd.20769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crowe JH, Carpenter JF, Crowe LM. The role of vitrification in anhydrobiosis. Annu. Rev. Physiol. 1998;60:73–103. doi: 10.1146/annurev.physiol.60.1.73. [DOI] [PubMed] [Google Scholar]
- 16.Dang-Nguyen TQ, Nguyen HT, Nguyen MT, Somfai T, Noguchi J, Kaneko H, Kikuchi K. Maturation ability after transfer of freeze-dried germinal vesicles from porcine oocytes. Anim. Sci. J. 2018;89:1253–1260. doi: 10.1111/asj.13067. [DOI] [PubMed] [Google Scholar]
- 17.De Gelder J, De Gussem K, Vandenabeele P, Moens L. Reference database of Raman spectra of biological molecules. J. Raman Spectrosc. 2007;38:1133–1147. doi: 10.1002/jrs.1734. [DOI] [Google Scholar]
- 18.Deegan RD, Bakajin O, Dupont TF, Huber G, Nagel SR, Witten TA. Capillary flow as the cause of ring stains from dried liquid drops. Nature. 1997;389:827–829. doi: 10.1038/39827. [DOI] [Google Scholar]
- 19.Deegan RD, Bakajin O, Dupont TF, Huber G, Nagel SR, Witten TA. Contact line deposits in an evaporating drop. Phys. Rev. E. 2000;62:756–765. doi: 10.1103/PhysRevE.62.756. [DOI] [PubMed] [Google Scholar]
- 20.Elliott GD, Lee P-C, Paramore E, Van Vorst M, Comizzoli P. Resilience of oocyte germinal vesicles to microwave-assisted drying in the domestic cat model. Biopreserv. Biobank. 2015;13:164–171. doi: 10.1089/bio.2014.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elliott GD, Wang S, Fuller BJ. Cryoprotectants: a review of the actions and applications of cryoprotective solutes that modulate cell recovery from ultra-low temperatures. Cryobiology. 2017;76:74–91. doi: 10.1016/j.cryobiol.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Feng HY, Wu LJ, Xu A, Hu BR, Hei TK, Yu ZL. Survival of mammalian cells under high vacuum condition for ion bombardment. Cryobiology. 2004;49:241–249. doi: 10.1016/j.cryobiol.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Graves-Herring, J. E., D. E. Wildt, and P. Comizzoli. Retention of structure and function of the cat germinal vesicle after air-drying and storage at suprazero temperature1. Biol. Reprod. 88:139, 131–137–139, 131–137, 2013. [DOI] [PMC free article] [PubMed]
- 24.Greenspan L. Humidity fixed-points of binary saturated aqueous-solutions. J. Res. Natl Bur. Stand. A. 1977;81:89–96. doi: 10.6028/jres.081A.011. [DOI] [Google Scholar]
- 25.Iglesias HA, Chirife J, Buera MP. Adsorption isotherm of amorphous trehalose. J. Sci. Food Agric. 1997;75:183–186. doi: 10.1002/(SICI)1097-0010(199710)75:2<183::AID-JSFA860>3.0.CO;2-T. [DOI] [Google Scholar]
- 26.Kaymak-Ertekin F, Gedik A. Sorption isotherms and isosteric heat of sorption for grapes, apricots, apples and potatoes. Lebensm.-Wiss. Technol. Food Sci. Technol. 2004;37:429–438. doi: 10.1016/j.lwt.2003.10.012. [DOI] [Google Scholar]
- 27.Kotula AP, Snyder CR, Migler KB. Determining conformational order and crystallinity in polycaprolactone via Raman spectroscopy. Polymer. 2017;117:1–10. doi: 10.1016/j.polymer.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lomauro CJ, Bakshi AS, Labuza TP. Evaluation of food moisture sorption isotherm equations. 1. Fruit, vegetable and meat-products. Lebensm.-Wiss. Technol. 1985;18:111–117. [Google Scholar]
- 29.Mazzobre MF, Longinotti MP, Corti HR, Buera MP. Effect of salts on the properties of aqueous sugar systems, in relation to biomaterial stabilization. 1. Water sorption behavior and ice crystallization/melting. Cryobiology. 2001;43:199–210. doi: 10.1006/cryo.2001.2345. [DOI] [PubMed] [Google Scholar]
- 30.Millqvist-Fureby A, Malmsten M, Bergenstahl B. Spray-drying of trypsin—surface characterisation and activity preservation. Int. J. Pharm. 1999;188:243–253. doi: 10.1016/S0378-5173(99)00226-4. [DOI] [PubMed] [Google Scholar]
- 31.Patrick JL, Elliott GD, Comizzoli P. Structural integrity and developmental potential of spermatozoa following microwave-assisted drying in the domestic cat model. Theriogenology. 2017;103:36–43. doi: 10.1016/j.theriogenology.2017.07.037. [DOI] [PubMed] [Google Scholar]
- 32.Reis J, Sitaula R, Bhowmick S. Water activity and glass transition temperatures of disaccharide based buffers for desiccation preservation of biologics. J. Biomed. Sci. Eng. 2009;02:594–605. doi: 10.4236/jbise.2009.28086. [DOI] [Google Scholar]
- 33.Saad A, Touati B, Draoui B, Tabti B, Abdenebi A, Benaceur S. Mathematical modeling of moisture sorption isotherms and determination of isosteric heats of sorption of Ziziphus leaves. Model. Simul. Eng. 2014;2014:8. [Google Scholar]
- 34.Sitaula R, Bhowmick S. Moisture sorption characteristics and thermophysical properties of trehalose–PBS mixtures. Cryobiology. 2006;52:369–385. doi: 10.1016/j.cryobiol.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 35.Takeuchi T, Neri QV, Palermo GD. Construction and fertilization of reconstituted human oocytes. Reprod. Biomed. Online. 2005;11:309–318. doi: 10.1016/S1472-6483(10)60838-3. [DOI] [PubMed] [Google Scholar]
- 36.Tuschel D. Molecular spectroscopy workbench why are the Raman spectra of crystalline and amorphous solids different? Spectroscopy. 2017;32:26–33. [Google Scholar]
- 37.Weng L, Song W, Jacobs DJ, Elliott GD. Molecular insights into water vapor absorption by aqueous lithium bromide and lithium bromide/sodium formate solutions. Appl. Therm. Eng. 2016;102:125–133. doi: 10.1016/j.applthermaleng.2016.03.153. [DOI] [Google Scholar]
- 38.Wood TC, Wildt DE. Effect of the quality of the cumulus–oocyte complex in the domestic cat on the ability of oocytes to mature, fertilize and develop into blastocysts in vitro. J. Reprod. Fertil. 1997;110:355–360. doi: 10.1530/jrf.0.1100355. [DOI] [PubMed] [Google Scholar]
- 39.Yazdani M, Sazandehchi P, Azizi M, Ghobadi P. Moisture sorption isotherms and isosteric heat for pistachio. Eur. Food Res. Technol. 2006;223:577–584. doi: 10.1007/s00217-006-0256-6. [DOI] [Google Scholar]
- 40.Yu G, Yap YR, Pollock K, Hubel A. Characterizing intracellular ice formation of lymphoblasts using low-temperature Raman spectroscopy. Biophys. J. 2017;112:2653–2663. doi: 10.1016/j.bpj.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang J, Zografi G. The relationship between “BET”- and “Free volume”-derived parameters for water vapor absorption into amorphous solids. J. Pharm. Sci. 2000;89:1063–1072. doi: 10.1002/1520-6017(200008)89:8<1063::AID-JPS11>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.