Abstract
Introduction
The uptake of the Kidney Disease: Improving Global Outcomes (KDIGO) 2012 chronic kidney disease (CKD) Guideline is not fully described in real-world nephrology practice across the world.
Methods
We used baseline data from the CKD Outcomes and Practice Patterns Study (2013–2017), a 4-country cohort of patients with estimated glomerular filtration rate <60 ml/min per 1.73 m2 recruited from national samples of nephrology clinics, to describe adherence to measures for monitoring and delaying CKD progression. Data were collected as in clinical practice, except laboratory measures per protocol in France.
Results
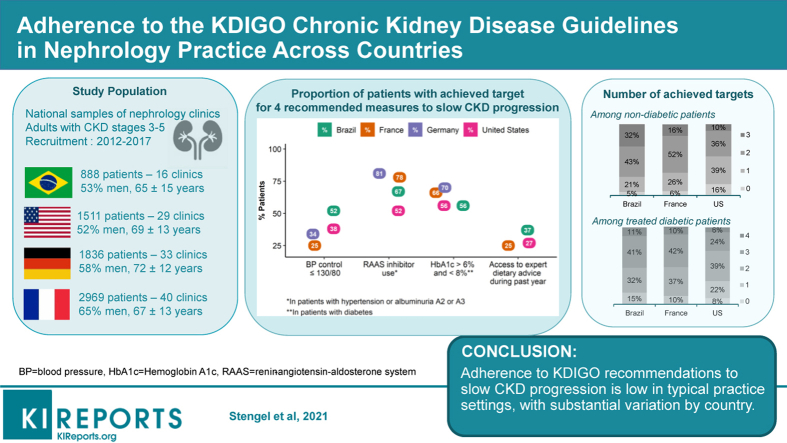
The mean age ranged from 65 years in Brazil to 72 years in Germany. Albuminuria (mostly proteinuria) was measured routinely in 36% to 43% of patients in Brazil, Germany, and the United States. Blood pressure control (≤140/90 mm Hg) ranged from 49% in France to 76% in Brazil; <40% of patients had blood pressure ≤130/80 mm Hg everywhere but Brazil (52%). More than 40% of nephrologists in Brazil reported a systolic blood pressure target ≤130 mm Hg for nondiabetic patients without proteinuria, but only 19% to 24% elsewhere. Prescription of renin-angiotensin aldosterone system inhibitors ranged from 52% in the United States to 81% in Germany. Dietary advice was more frequent for salt than protein intake; dietitian visits were uncommon. In nondiabetic patients, achievement of all 3 targets including blood pressure ≤130/80 mm Hg, renin-angiotensin aldosterone system inhibition, and dietary advice, ranged from 10% in the United States to 32% in Brazil; in treated diabetic patients, this ranged from 6% to 11% after including hemoglobin A1c target.
Conclusion
Adherence to recommendations to slow CKD progression is low in typical practice settings, and substantial variation among countries for some indicates opportunities for improvement.
Keywords: albuminuria, blood pressure control, chronic kidney disease, dietary advice, lifestyle, renin-angiotensin system inhibition
Graphical abstract
The recognition of the burden of CKD, which affects 11% to 13% of the population worldwide, has improved substantially over the past decade.1, 2, 3, 4 Risks of kidney failure, cardiovascular disease, and mortality associated with each CKD stage have been well defined.5, 6, 7, 8, 9 Improvements in the prevention of kidney failure have been slow, and the need for kidney replacement continues to rise in some, but not all, high-income countries.10,11 Although population aging and improved access to dialysis explain part of this growth, inadequate implementation of prevention measures may also play a role in the current inability to reduce the incidence of kidney failure.
In 2012, KDIGO reviewed the available evidence-based measures that are associated with slowing CKD progression in a widely disseminated clinical practice guideline.12 This guideline recommends monitoring glomerular filtration rate (GFR) and albuminuria at least annually in people with CKD to assess progression. It also provides graded recommendations or suggestions for blood pressure (BP) control, use of renin-angiotensin system inhibitors, glycemic control, lifestyle, and dietary advice. The major benefits of BP control and renin-angiotensin-aldosterone system inhibitors (RAASis) used to slow CKD progression are well established,13, 14, 15, 16 but recommended BP targets may be difficult to achieve.17, 18, 19, 20, 21, 22 In contrast, evidence is less conclusive for lifestyle and dietary recommendations including smoking cessation, achieving healthy body mass index, lowering protein and salt intake, and providing individuals with expert dietary advice.23, 24, 25, 26 To our knowledge, the extent of the adherence to these KDIGO recommendations has not been systematically evaluated.
We examined data from the Chronic Kidney Disease Outcomes and Practice Patterns Study (CKDopps), a prospective cohort study of patients with moderate and advanced CKD that is conducted among national samples of nephrology clinics in Brazil, Germany, France, and the United States.27 Our goal was to assess the current level of achievement of the KDIGO 2012 measures aimed at delaying CKD progression, summarized in Table 1.
Table 1.
Summary of KDIGO 2012a recommended measures for slowing CKD progression
| Measures | Description of measures |
|---|---|
| Blood pressure and RAS inhibition |
|
| CKD and AKI risk |
|
| Protein intake |
|
| Glycemic control |
|
| Salt intake |
|
| Lifestyle |
|
| Additional dietary advice |
|
AKI, acute kidney injury; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; KDIGO, Kidney Disease: Improving Global Outcomes; RAS, renin-angiotensin system; UAE, urinary albumin excretion.
Categories: 1, recommendation; 2, suggestion, not graded. Evidence: A, high; B, moderate; C, low; D, very low.
According to the KDIGO CKD Work Group.12
Approximate equivalents for UAE per 24 hours—expressed as protein excretion rate per 24 hours, albumin-to-creatinine ratio, protein-to-creatinine ratio, and protein reagent strip results are given in the Methods.
Methods
Participants
CKDopps is a prospective cohort study of adult patients with moderate or advanced CKD recruited from national samples of nephrology clinics in Brazil, France, Germany, and the United States between 2012 and 2017. Details of its protocol have been published.27 In brief, a national list of nephrology clinics in each country, stratified by geographic region and clinic characteristics (size and public vs. private), was assembled to serve as a sampling frame for selecting samples as nationally representative as possible. Each clinic developed a census to identify all eligible patients ≥18 years of age (with no upper limit), with an estimated GFR (eGFR) <60 ml/min per 1.73 m2 at screening, and no previous chronic dialysis or kidney transplant. Census patients who met eligibility criteria were then sequentially approached for study participation until the target enrollment was met. At each clinic, the enrollment goal was 60 patients with eGFR <30 ml/min per 1.73 m2 (CKD stage 4–5) and 20 patients with eGFR 30 to 59 ml/min per 1.73 m2 (CKD stage 3), except in France where a higher proportion of patients with CKD stage 3 were included and in Germany, where no patients with stage 5 were recruited. As of December 2017, a total of 7204 patients (from 118 clinics) with available data were included: 888 (16 clinics) in Brazil, 2969 (40 clinics) in France, 1836 (33 clinics) in Germany, and 1511 (29 clinics) in the United States. All patients signed informed consent as required by national and local ethics committee regulations.
Data Collection
CKDopps collected patient-, physician-, and clinic-level data with a common protocol and language-appropriate data collection instruments in all participating countries. In each country, clinical research associates or study nurses ensure study sites’ protocol adherence and quality of data collection. No clinical data were collected beyond those performed as part of usual care as the aim is to evaluate typical nephrology clinic practices. One exception was laboratory measurements in France, where a standard set of urine and blood tests was requested annually, with active reminders by clinical research associates (unlike in the other countries).28 Baseline clinical data were collected from medical records. These data included CKD history, past acute kidney injury (AKI) events, cardiovascular risk factors, and comorbidities. Nephrologists reported the primary cause of CKD and available kidney biopsy specimen findings. The study protocol allowed 1 outpatient BP or weight value to be recorded each month. In this analysis, we used values recorded at the enrollment visit or, if these were missing, within 3 months before or after enrollment. Prescriptions of RAASis, including angiotensin-converting enzyme inhibitors (ACEis), angiotensin receptor blockers (ARBs), direct renin inhibitors, and aldosterone receptor antagonists, were recorded along with all other prescribed medications. For CKDopps analyses, patients were classified with hypertension if so reported in the medical record or if they were prescribed antihypertensive medications, and with diabetes if so reported in the medical record, if they were prescribed glucose-lowering medication, or if they had hemoglobin A1c (HbA1c) ≥6.5% or fasting glucose ≥7.0 mmol/l or a random glucose ≥11.0 mmol/l. eGFR was calculated with the Chronic Kidney Disease Epidemiology Collaboration equation.29 Albuminuria or proteinuria, measured from spot or 24-hour urine, was used to assign patients to albuminuria (or proteinuria) categories as defined in KDIGO: (i) A1 (normal to mildly increased), albuminuria <30 (proteinuria <150) mg/g or mg/24 hours; (ii) A2 (moderately increased), 30 to 300 (150–500) mg/g or mg/24 hours; (iii) A3 (severely increased), >300 to 2200 (500–3000) mg/g or mg/24 hours; (iv) or A3 (severely increased, nephrotic syndrome), >2200 (>3000) mg/g or mg/24 hours.
When spot or 24-hour urine values were not available, dipstick values were used for grading albuminuria by including none or trace proteinuria, 1+, 2+, and ≥3+, in albuminuria categories A1, A2, A3, and A3 (nephrotic syndrome), respectively. A patient questionnaire was administered at enrollment to capture the patient experience regarding quality of life, medical care, and advice provided by their nephrologist and other providers, including dietitians. Finally, in each clinic, nephrologists were asked to complete a questionnaire about their practice patterns. In all, 215 physicians participated: 18 (from 12 of 16 clinics) in Brazil, 137 (from 38 of 40 clinics) in France, 16 (from 16 of 33 clinics) in Germany, and 44 (from 26 of 29 clinics) in the United States. The BP targets requested were systolic BP (SPB) and diastolic BP upper limits for nondiabetic CKD patients without proteinuria, and whether these targets were different for nondiabetic patients with proteinuria 30 to 300 mg/g, ≥300 mg/g, and for diabetic patients.
Studied Measures
We assessed KDIGO 2012 CKD guideline measures for monitoring and delaying CKD progression. This guideline recommends assessing albuminuria at least annually in people with CKD. Among KDIGO measures for delaying CKD progression, we studied those that were either recommended or suggested, regardless of their level of evidence: A (high), B (moderate), C (low), or D (very low). These included 12 graded recommendations or suggestions for adults, summarized in Table 1. Of note, we assessed RAASi (not renin-angiotensin system inhibitor) prescription in patients with KDIGO recommendations for use, thus excluding those without hypertension or albuminuria.
Statistical Analyses
Baseline cross-sectional data from Brazil, France, Germany, and the United States were used for the analyses. Standard descriptive statistics (means and standard deviations [SDs] or medians and interquartile ranges [IQRs] for continuous variables and frequencies for categorical variables) were used to report patient characteristics by country, and by CKD stages 3a, 3b, and 4 to 5 (i.e., eGFR 45–59, 30–44, and <30 ml/min per 1.73 m2, respectively. The prevalence of albuminuria (or proteinuria or dipstick) monitoring, defined by the report of ≥1 measurement during the 6-month period before enrollment, was compared by country and by diabetes status. Adherence to recommended measures for slowing CKD progression were compared by country and by CKD stage, as described above. When recommendations varied according to albuminuria level, measures were compared by albuminuria category: A1 versus A2+ for BP control, and A1 to A2 versus A3 according to diabetes status for RAASi use. We also developed a composite indicator to assess the number of achieved targets according to diabetes status. In nondiabetic patients, this indicator was based on 3 target measures: (i) BP control, (ii) RAAS inhibition, and (iii) dietary advice, including patient report of dietician visit or advice to reduce salt or protein intake. In diabetic patients, it was based on (i), (ii), or (iii) and an HbA1c >6% and <8%. In each patient subgroup, 3 levels of BP control were studied: ≤140/90, ≤130/80, and ≤120/80 mm Hg.
All statistical analyses were conducted with SAS software (version 9.4; SAS Institute Inc., Cary, NC).
Results
Participant Characteristics by Country
The mean age ranged from 65 years of age in Brazil to 72 years of age in Germany (Table 2). The proportion of men was higher in France and Germany than in Brazil and the United States; in the latter 2 countries, about 25% of patients were black. Most patients had CKD stages 4 to 5, except in France where a higher proportion of patients had CKD stage 3. Diabetic and hypertensive nephropathies were the most common reported CKD causes, although only a small minority had biopsy-proven diagnoses. The prevalence of obesity and diabetes was highest in the United States, and that of cardiovascular disease was highest in France and Germany. Close to 1 of 4 patients in France reported they had ever had AKI. In the other countries, the prevalence of AKI in the 6 months before enrollment was 6%. The median number of medications per patient ranged from 7 in Brazil to 11 in the United States. As CKD stage increased, patients tended to be older and to have more comorbidities and more medications prescribed (Supplementary Table S1).
Table 2.
Patient characteristics, by country
| Characteristics | Brazil | France | Germany | US |
|---|---|---|---|---|
| Patients, n | 888 | 2969 | 1836 | 1511 |
| Age, yr | 65.3 ± 14.8 | 66.9 ± 12.9 | 72.0 ± 12.4 | 68.5 ± 12.9 |
| Men, % | 53 | 65 | 58 | 52 |
| Black, % | 26 | 3 | — | 21 |
| Body mass index, kg/m2 | 27.8 ± 5.3 | 28.7 ± 5.9 | 29.2 ± 5.6 | 31.3 ± 7.0 |
| eGFR, ml/min per 1.73 m2 | 25.7 ± 11.6 | 32.2 ± 11.3 | 27.9 ± 9.9 | 26.1 ± 11.2 |
| CKD stage (eGFR in ml/min per 1.73 m2), % | ||||
| 3 (30–59) | 31 | 54 | 27 | 31 |
| 4 (15–29) | 52 | 42 | 74 | 56 |
| 5 (<15, not on dialysis)a | 18 | 4 | — | 14 |
| Years since CKD diagnosis | 2.3 [0.7–5.1] | 5.0 [2.0–10.0] | — | 3.3 [1.2–6.2] |
| Primary cause of CKD, % | ||||
| Diabetes | 35 | 20 | 29 | 36 |
| Hypertension/large vessel disease | 34 | 27 | 33 | 35 |
| Glomerulonephritis | 9 | 17 | 9 | 7 |
| Interstitial nephritis/pyelonephritis | 9 | 12 | 6 | 4 |
| Polycystic kidney disease | 4 | 6 | 4 | 2 |
| Other | 5 | 8 | 17 | 12 |
| Unknown | 5 | 10 | 2 | 3 |
| Biopsy-proven diagnosis, % | 11 | 24 | — | 14 |
| Comorbidities, % | ||||
| Obesity (≥30 kg/m2) | 33 | 36 | 40 | 52 |
| Diabetes | 48 | 43 | 48 | 59 |
| Hypertension | 97 | 91 | 96 | 96 |
| Any cardiovascular disease | 45 | 53 | 53 | 50 |
| Coronary heart disease | 22 | 25 | 29 | 30 |
| Heart failure | 15 | 13 | 14 | 16 |
| Acute kidney injury,b % | 6 | 24 | 6 | 6 |
| Laboratory measurements | ||||
| Serum uric acid, mg/dl | 6.9 ± 1.9 | 7.2 ± 2.0 | 7.6 ± 2.1 | 7.1 ± 2.1 |
| HbA1c in patients with diabetes, % | 7.2 ± 1.5 | 7.2 ± 1.2 | 7.2 ± 1.2 | 7.4 ± 1.7 |
| Medications prescribed | ||||
| No medications, % | 0.3 | 0.4 | 0.7 | 0.2 |
| No. of medications | 7 [5–10] | 8 [5–11] | 10 [7–12] | 11 [7–14] |
| Any antihypertensive medications, % | 95 | 94 | 97 | 95 |
| Antidiabetic medications, % | 38 | 36 | 34 | 43 |
CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; IQR, interquartile range; US, United States.
Results are shown as prevalence, mean ± SD or median [25th,75th percentiles].
No CKD stage 5 at inclusion in Germany.
Any acute kidney injury event at baseline or in 6-month interval before baseline in Brazil, Germany, and the United States; history of ever having acute kidney injury in France.
Albuminuria Monitoring and Category, by Diabetes Status and by Country
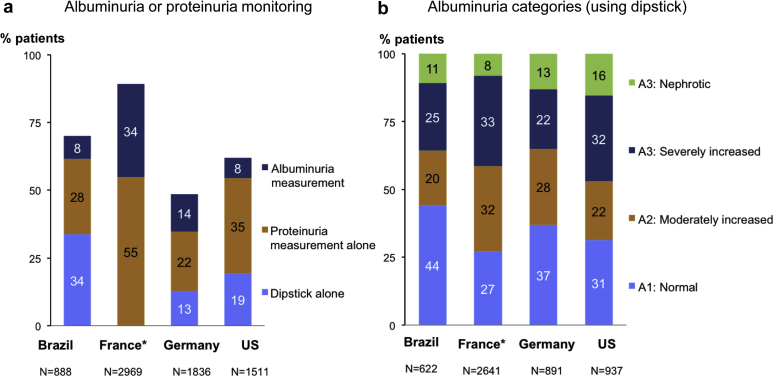
Albuminuria or proteinuria was routinely measured in fewer than half of the patients in Brazil, Germany, and the United States; the French study protocol called for measurements (Figure 1a). Proteinuria was more commonly measured than albuminuria in all countries. Dipstick proteinuria was the most frequently reported monitoring modality in Brazil. Spot urine albumin-to-creatinine, as recommended, was rarely measured and only slightly more often in patients with than without diabetes (Supplementary Table S2). The overall prevalence of CKD stage A3, based on either albuminuria or proteinuria measurements or dipstick, was 36%, 41%, 35%, and 48% in Brazil, France, Germany, and the United States, respectively (Figure 1b); it was higher in patients with (vs. without) diabetes (Supplementary Table S2).
Figure 1.
Albuminuria or proteinuria monitoring and albuminuria or equivalent categories, by country. (a) Albuminuria or proteinuria monitoring. (b) Albuminuria or equivalent categories (including dipstick). ∗Requested laboratory measurements per study protocol in France versus routine measurements in other countries. US, United States.
Blood Pressure and RAAS Inhibition, by Country
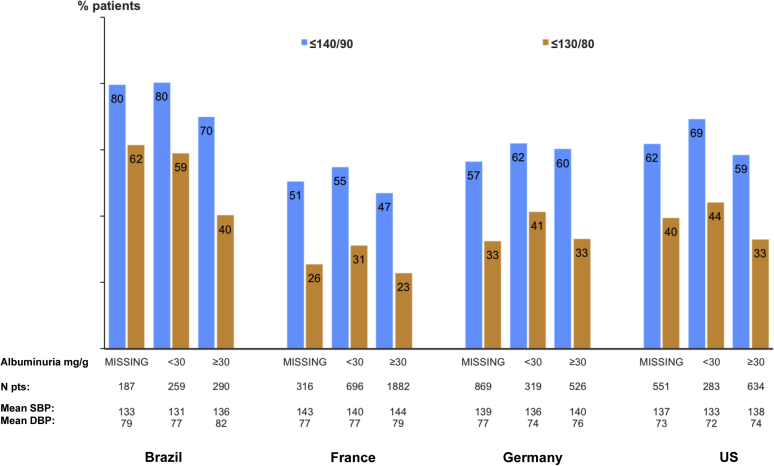
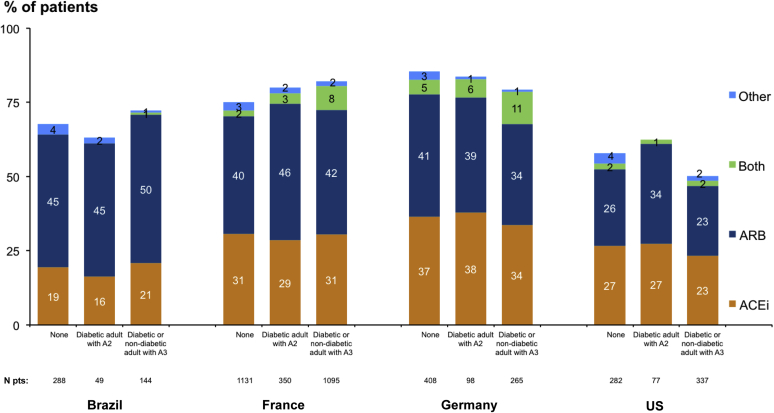
The mean SBP ranged from 133 ± 21 mm Hg to 142 ± 20 mm Hg. This was higher in Germany and France than in Brazil or the United States, and higher in patients with than without albuminuria ≥30 mg/g (Table 3, Figure 2). Mean diastolic BP ranged from 73 ± 12 mm Hg to 79 ± 12 mm Hg. The percentage of patients with diastolic BP ≤90 mm Hg was >85% in all countries. BP control ≤140/90 mm Hg ranged from 49% in France to 76% in Brazil, BP control ≤130/80 mm Hg ranged from 25% to 52%, and ≤120/80 ranged from 13% to 35%. In patients with KDIGO recommendations for use, RAASi prescription (mainly ACEis or ARBs) was 67%, 78%, 81%, and 52%, in Brazil, France, Germany, and the United States, respectively. It was lower in the United States and Brazil at CKD stages 4 to 5 but did not vary according to albuminuria category in any country (Figure 3).
Table 3.
Blood pressure control and prescription of renin-angiotensin-aldosterone system inhibitors, by country
| Characteristics | Brazil | France | Germany | US |
|---|---|---|---|---|
| BP control | ||||
| Patients with BP measurement, n | 736 | 2898 | 1714 | 1468 |
| Systolic BP, mm Hg | ||||
| Mean ± SD | 133 ± 21 | 142 ± 20 | 139 ± 20 | 137 ± 21 |
| ≤140, % | 78 | 51 | 60 | 63 |
| ≤130, % | 57 | 31 | 37 | 42 |
| ≤120, % | 36 | 14 | 19 | 22 |
| Diastolic BP, mm Hg | ||||
| Mean ± SD | 79 ± 12 | 78 ± 12 | 76 ± 11 | 73 ± 12 |
| ≤90, % | 90 | 87 | 93 | 94 |
| ≤80, % | 75 | 62 | 74 | 77 |
| Systolic/diastolic BP, mm Hg, % | ||||
| ≤140/90 | 76 | 49 | 59 | 62 |
| ≤130/80 | 52 | 25 | 34 | 38 |
| ≤120/80 | 35 | 13 | 18 | 21 |
| RAASi prescription in patients with KDIGO recommendation for usea | ||||
| Patients with data on RAASi prescription, n | 588 | 2969 | 1415 | 1102 |
| Patients with hypertension or proteinuria, n | 584 | 2878 | 1400 | 1089 |
| ACEi, % | 18 | 30 | 36 | 25 |
| ARB, % | 46 | 41 | 37 | 24 |
| Both ACEi and ARB, % | 0.2 | 5 | 5 | 1 |
| Other,b % | 2 | 2 | 3 | 2 |
| None, % | 33 | 22 | 19 | 48 |
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BP, blood pressure; KDIGO, Kidney Disease: Improving Global Outcomes; RAASi, renin-angiotensin-aldosterone system inhibitor.
KDIGO recommendations for RAASi use means having hypertension or albuminuria A2 or A3.
A prescription for a renin inhibitor or aldosterone antagonist without a concurrent prescription for an ACEi or ARB.Results are shown as prevalence or mean ± SD.
Figure 2.
Blood pressure control according to albuminuria category, by country. DBP, diastolic blood pressure; SBP, systolic blood pressure; US, United States.
Figure 3.
Renin-angiotensin-aldosterone system inhibitor prescription according to Kidney Disease: Improving Global Outcomes recommendations for use in patients with chronic kidney disease with or without diabetes, by country. A2, Albuminuria 30-300 mg/g; A3, albuminuria >300 mg/g; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blockers.
Nephrologist BP Target According to Patient Profile, by Country
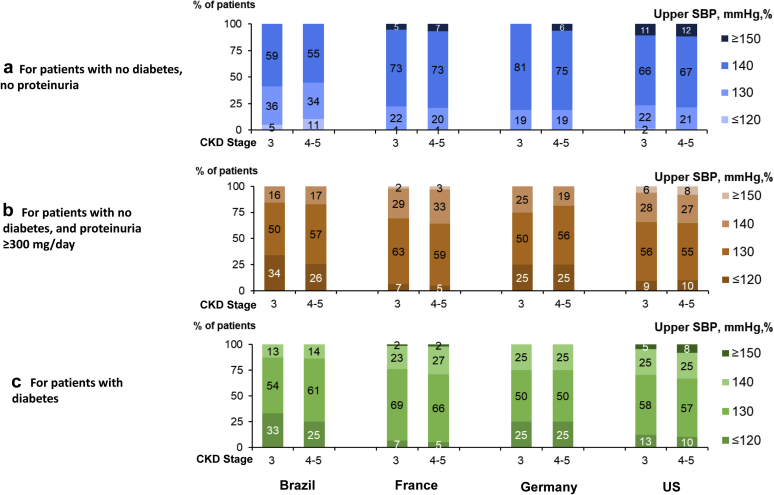
More than 40% of nephrologists in Brazil reported a target SBP ≤130 mm Hg for nondiabetic patients without proteinuria, but only 19% to 24% did so in the other countries (Figure 4). Different SBP targets for nondiabetic patients with proteinuria <300 mg/d versus without proteinuria were reported by 26% of nephrologists in Brazil, 19% in France, 31% in Germany, and 23% in the United States. However, 59%, 49%, 63%, and 34%, respectively, had different targets for nondiabetic patients with proteinuria ≥300 mg/d versus <300 mg/d. Five percent, 14%, 25%, and 9% had different SBP targets for diabetic versus nondiabetic patients with proteinuria ≥300 mg/d. When targets differed, 70% to 87% reported a SBP upper limit of 130 mm Hg or lower in diabetic and nondiabetic patients with proteinuria ≥300 mg/d; >25% reported 120 mm Hg as the upper limit for patients with diabetes in Brazil and Germany, but ≤13% in the United States and France.
Figure 4.
Nephrologists’ systolic blood pressure (SBP) target according to patient diabetes status and proteinuria level, by chronic kidney disease (CKD) stage and by country. (a) For patients with no diabetes and no proteinuria. (b) For patients with no diabetes and proteinuria ≥300 mg/d. (c) For patients with diabetes. ∗Data from the Nephrology Practice Survey. US, United States.
HbA1c Level Among Diabetic Patients by CKD Stage, by Country
Mean HbA1c in patients with diabetes was higher in the United States than in the other countries but did not vary by CKD stage (Table 2, Supplementary Table S1). A significant percentage of patients had HbA1c <6% in all 4 countries; this percentage was particularly high in Brazil (Supplementary Figure S1).
Achievement of Lifestyle and Dietary Advice Measures, by Country
Past smoking was high among patients in France and the United States, but current smoking was ≤12% in all countries (Table 4). Fairly few patients had a body mass index within the normal range. Those with CKD stages 4 to 5 more often reported having received dietary advice than those with stage 3 CKD, and this advice concerned salt more often than protein, phosphorus, or potassium intake (Supplementary Figure S2). Overall, US patients were less likely to report having received dietary advice than Brazilian or French patients. Dietitian visits during the past year were uncommon in all countries.
Table 4.
Achievement of lifestyle and dietary advice measures
| Characteristics | Brazil | France | Germany | US |
|---|---|---|---|---|
| Patients, n | 888 | 2969 | 1836 | 1511 |
| Achievement of recommendations from medical records | ||||
| Smoking, % | ||||
| Current | 7 | 12 | 5 | 9 |
| Past | 28 | 48 | — | 38 |
| Never | 65 | 40 | — | 53 |
| Body mass index 20–25 kg/m2, % | 28 | 25 | 21 | 16 |
| Receipt of advice from patient questionnairea | ||||
| Patients reporting on dietary advice, n | 505 | 2523 | 0 | 761 |
| Patients received advice to reduce protein intake, salt intake, etc, % | ||||
| Protein intake | 49 | 42 | — | 19 |
| Salt intake | 79 | 73 | — | 53 |
| Potassium intake | 46 | 47 | — | 32 |
| Phosphorus intake | 28 | 16 | — | 15 |
| Patients having seen a dietitian during past year | 37 | 25 | — | 27 |
| Quantitative dietary assessmentb from medical records, % | ||||
| With 24-h urinary urea measurement | 9 | 46 | 6 | 0.5 |
| With 24-h urinary sodium measurement | 9 | 55 | 3 | 0.7 |
US, United States.
Response rates to the patient questionnaire were 57% in Brazil, 85% in France, and 50% in the US. Patient questionnaires were not available in Germany, and only current smoking status was reported.
Routine laboratory measurements in Brazil, Germany, and the US, and requested per study protocol in France.
Number of Achieved Targets According to Diabetes Status, by Country
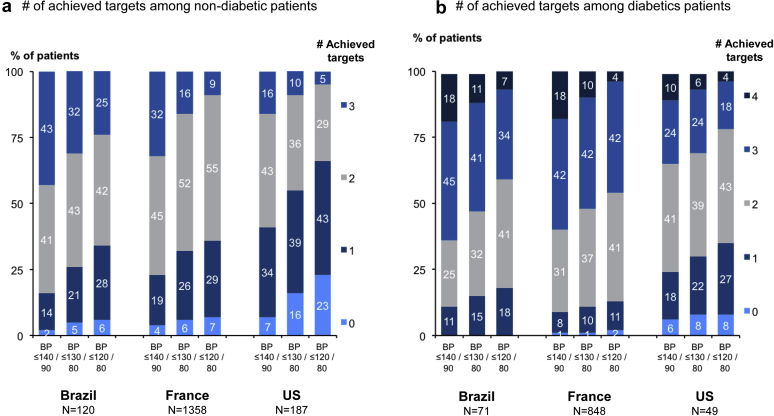
In nondiabetic patients, achievement of all 3 targets including BP control ≤130/80 mm Hg, RAAS inhibition, and dietary advice ranged from 10% in the United States to 32% in Brazil (Figure 5a); in treated diabetic patients, achievement of these targets, as well as an HbA1c >6% and <8%, ranged from 6% to 11% (Figure 5b). Achieving ≥2 targets in nondiabetic patients ranged from 46% in the United States to 75% in Brazil and achieving ≥3 targets in diabetic patients ranged from 30% in the United States to 52% in the 2 other countries. As expected, these percentages were higher for BP control ≤140/90 mm Hg, and substantially lower for BP control ≤120/80 mm Hg.
Figure 5.
Number of achieved targets according to diabetes status, by country. (a) Number of achieved targets among nondiabetic patients. (b) Number of achieved targets among patients with diabetes. BP, blood pressure; US, United States.
Discussion
Based on a comprehensive evaluation of the KDIGO 2012 guideline measures aimed at slowing CKD progression, this study shows an overall low level of adherence in current nephrology practices in countries with different epidemiologic backgrounds and health care systems. A major strength of this study is that it collects extensive data from both patients and providers during routine care in clinics that are representative of local real-world situations in respective countries. We were able to describe important variations by country in BP control and RAASi prescription, but also to reveal substantial differences in the BP levels targeted by nephrologists. We highlight the limited access to dietitians for patients not undergoing dialysis. Surprisingly, measuring the albumin-to-creatinine ratio did not appear to be standard practice in nephrology clinics. These findings have several implications for clinical practice and in the development and implementation of upcoming CKD guidelines.
Real-life assessment of guideline implementation is a key step to identifying barriers to the dissemination of clinical recommendations, and international comparisons have demonstrated their usefulness for dialysis.30, 31, 32 To our knowledge, CKDopps is the first study to report a multinational holistic evaluation of guideline-recommended measures for delaying CKD progression. The management of CKD progression aims to address a set of established risk factors, including kidney-specific and general lifestyle measures, with the greatest consensus around the importance of BP control and RAAS inhibition, and increasing attention for diet. While achieving 3 (BP control ≤130/80 mm Hg, RAAS inhibition, and dietary advice) or 4 (including HbA1c target in diabetic patients) targets was observed in <20% of them everywhere except for nondiabetic patients in Brazil, this study highlights substantial country variability in achieving ≥2 or ≥3 targets in nondiabetic or diabetic patients, indicating opportunities for improvement.
Several studies have identified poor achievement of BP control and high prevalence of apparent treatment-resistant hypertension in patients with reduced kidney function.17, 18, 19, 20,33 CKDopps confirms important country variations in BP control, and a low level of BP control ≤120/80 mm Hg everywhere except Brazil, where a lower body mass index among patients may contribute to this achievement. The worldwide assessment of uncontrolled hypertension in CKD conducted by the international network of CKD cohorts (iNET-CKD) across 4 continents (17 countries, including CKDopps countries) recently showed that country variations were only partly explained by patient demographic and clinical profiles, and demonstrated striking heterogeneity in antihypertensive prescriptions.22
With the exception of RAASis, recommended as first-line treatment for most patients with CKD, the lack of consensus about treatment strategies for second-, third-, and fourth-line antihypertensive drugs makes it difficult to disentangle the relative impact on hypertension control of prescription patterns versus clinician BP goals, or other factors, including but not limited to patient comorbidities, diet, medication adherence, and medication costs and availability.34 Interestingly, CKDopps shows that while almost all nephrologists reported an upper SBP target of 140 mm Hg for nondiabetic CKD patients without albuminuria, only 19% to 31% reported a lower SBP target for those with moderately increased proteinuria, as suggested by guidelines. In contrast, for patients with diabetes or severely increased proteinuria, more than two thirds of nephrologists reported SBP targets of ≤130 mm Hg. A SBP target ≤120 mm Hg was uncommon, but nephrologists had completed the CKDopps survey before the Systolic Blood Pressure Intervention Trial results suggested a potential survival benefit from SBP that low in patients with CKD.35 CKDopps also shows that reducing salt intake may be overlooked in these patients despite its impact on BP level in CKD.36 While most patients reported being advised to reduce salt intake, few patients were monitored for urinary sodium excretion.
A previous CKDopps study revealed large variations in RAASi prescription patterns, with apparent underuse in the United States and Brazil, even among patients with strong class-specific recommendations for diabetes, heart failure, or high albuminuria.37 Underprescription of RAASis has been identified in other countries, including Uruguay, China, and Thailand; these results contrast with their widespread use in most European countries, Canada, Japan, and South Korea.22 The use of ACEis or ARBs is controversial at eGFRs <30 ml/min per 1.73 m2, and hyperkalemia is an important contraindication.38 Underuse of RAASis in advanced-stage CKD may reflect discontinuation of treatment because of hyperkalemia or an episode of AKI, a common event over the course of CKD, as CKDopps shows. Fear of reintroducing RAASi is a likely explanation in some of these cases. The ongoing STOP ACEi trial should show the impact of discontinuation of ACEi/ARBs on eGFR in patients with advanced progressive CKD.39
As expected, diabetes was a leading cause of CKD and a major comorbidity, in general, albeit with large variations among countries. Overall, glycemic control was poor; nearly half the patients, regardless of CKD stage, exceeded the target HbA1c of approximately 7% (53 mmol/mol). The significant number of patients with low HbA1c (<6%), however, may reflect some overtreatment. The prevalence of reported hypoglycemia was high in France, especially in women with CKD and diabetes treated with insulin.40
Although lifestyle and dietary improvements may plausibly slow CKD progression, the KDIGO recommendations about lifestyle and diet and adherence to them are limited. Achievement of these recommendations in CKDopps participants was low except for smoking. In terms of nutrition advice, although most patients reported receiving advice to reduce sodium intake, fewer than half reported such advice for protein, potassium, or phosphorus intake, and only a minority had access to expert dietary advice. Greater evidence about the benefit and cost-effectiveness of this expert advice is needed to support its widespread use.
Finally, CKDopps also revealed poor adherence to the guidelines for the evaluation of albuminuria. Although KDIGO recommends assessment of “GFR and albuminuria at least annually in people with CKD, and more often for individuals at higher risk of progression and/or where measurement will impact therapeutic decisions,” CKDopps shows that this practice is far from widespread for patients with advanced CKD under nephrology care. Despite its potential inaccuracy, dipstick proteinuria appeared to be used alone in one fifth of patients in the United States and one third of patients in Brazil. When measurements were performed, proteinuria (spot or 24-hour urine) was measured 2 to 4 times more often than albuminuria, except in the United States. Patients with diabetes were monitored for proteinuria or albuminuria only slightly more often than those without. Recommendations for regular albuminuria monitoring are based on strong evidence that both GFR and albuminuria are valuable for assessing progression, and that albuminuria is more sensitive and specific than proteinuria in detecting glomerular injury.41 Nevertheless, there have been no studies evaluating the utility of more or less frequent monitoring or showing the advantage of albuminuria over proteinuria monitoring in slowing CKD progression. In addition, the availability of an equation to convert protein-to-creatinine ratio to albumin-to-creatinine ratio makes the latter easier and cheaper to estimate.42 Of note, although the protocol used in France to achieve albuminuria measurement cannot be implemented routinely, we can speculate that the use of standard protocols and laboratory test prescriptions managed by nurses may improve adherence to recommendations.
Major strengths of this study include the representativeness of nephrology clinic samples in each country, including both academic and community-based clinics where patient profiles and care may differ, as well as the extensive data collection from both patients and providers during routine clinical care. Representativeness of the studied population and data collection from routine care are indeed key elements to provide an unbiased picture of current practices that are generalizable to the population of participating country nephrology clinics. Nephrologist responses about therapeutic goals such as BP targets are unique elements often not included in other CKD cohort studies.
This study also has limitations. The cross-sectional analytic design provides a picture of clinical practices at a given time during the course of CKD but precludes any evaluation of changes between routine visits. Another limitation is the use of routine BP measurements, which may overestimate uncontrolled hypertension as compared with standardized measurements. Finally, the low response rate to the patient questionnaire may have introduced selection bias regarding data on lifestyle recommendations. The potentially greater likelihood that respondents versus nonrespondents reported receiving dietary advice and visiting dieticians might have resulted in overestimation.
In conclusion, overall adherence to the KDIGO 2012 guidelines for limiting CKD progression appears to be suboptimal in nephrology settings, where it was expected to be higher than in primary care practices.43 Substantial intercountry variations in BP control and RAAS inhibition point to areas for improvement, especially in regions where uncontrolled BP remains common or RAAS inhibition is underused. The low implementation of dietary measures to reduce the progression of CKD across participating countries is also concerning. Raising physician and patient awareness about the benefits of these measures and improving access to expert dietary advice are both necessary. The impact on clinical outcomes merits further study and will be evaluated during follow-up. Moreover, revisions of recommendations are frequently needed, and the incorporation of new targets and therapies (i.e., sodium glucose co-transporter 2 inhibitors and glucagon-like peptide 1 agonists) into clinical practice will need to be assessed in future studies.
Acknowledgements
We thank Janet Leslie, Medical Technical Writer with Arbor Research Collaborative for Health, and Jo-Ann Cahn, independent editor and translator, in revising the presentation of the researchers’ results and finalizing the manuscript.
Global support for the ongoing DOPPS Programs is provided without restriction on publications by a variety of funders. For details, see https://www.dopps.org/AboutUs/Support.aspx. In France, CKDopps is based on the CKD-REIN study funded by the Agence Nationale de la Recherche (ANR-10-COHO-0001) through the 2010 Cohortes-Investissements d’Avenir program and by the 2010 Programme Hospitalier de Recherche Clinique. CKD-REIN is also supported through a public–private partnership with Amgen, Fresenius Medical Care, and GlaxoSmithKline, since 2012, Lilly France since 2013, and Otsuka Pharmaceutical since 2015, Baxter and Merck Sharp & Dohme-Chibret (MSD France) from 2012 to 2017, Sanofi-Genzyme from 2012 to 2015, and Vifor Fresenius and AstraZeneca since 2018. In Germany, funding support for participation of German CKD clinics in CKDopps is provided by Wissenschaftliches Institut für Nephrologie of the Verband Deutsche Nierenzentren. In the United States and Brazil, support for the CKDopps Coordinating Center has been provided by Keryx.
Footnotes
Table S1. Patient characteristics, by CKD stage and country.
Table S2. Albuminuria monitoring∗ in CKD, by diabetes status and country.
Figure S1. HbA1c level (%) among patients with diabetes, by CKD stage and country.
Figure S2. Percentages of patients receiving recommended lifestyle and dietary counseling, and smoking, by CKD stage and country—data from the patient questionnaire.
Contributor Information
Bénédicte Stengel, Email: benedicte.stengel@inserm.fr.
CKDopps investigators:
Antonio Lopes, Roberto Pecoits-Filho, Christian Combe, Christian Jacquelinet, Ziad Massy, Bénédicte Stengel, Johannes Duttlinger, Danilo Fliser, Gerhard Lonnemann, Helmut Reichel, Takashi Wada, Kunihiro Yamagata, Ron Pisoni, Bruce Robinson, Viviane Calice da Silva, Ricardo Sesso, Elodie Speyer, Koichi Asahi, Junichi Hoshino, Ichiei Narita, Rachel Perlman, Friedrich Port, Nidhi Sukul, Michelle Wong, Eric Young, and Jarcy Zee
Appendix
CKDopps Steering Committee and Country Investigators
Antonio Lopes and Roberto Pecoits-Filho (Brazil); Christian Combe, Christian Jacquelinet, Ziad Massy, and Bénédicte Stengel (France); Johannes Duttlinger, Danilo Fliser, Gerhard Lonnemann, and Helmut Reichel (Germany); Takashi Wada and Kunihiro Yamagata (Japan); and Ron Pisoni and Bruce Robinson (United States).
Additional CKDopps Research Group
Viviane Calice da Silva and Ricardo Sesso (Brazil); Elodie Speyer (France); Koichi Asahi, Junichi Hoshino, and Ichiei Narita (Japan); and Rachel Perlman, Friedrich Port, Nidhi Sukul, Michelle Wong, Eric Young, and Jarcy Zee (United States).
Disclosure
All the authors declared no competing interests.
Supplementary Material
References
- 1.Hu J.R., Coresh J. The public health dimension of chronic kidney disease: what we have learnt over the past decade. Nephrol Dial Transplant. 2017;32(suppl 2):ii113–ii120. doi: 10.1093/ndt/gfw416. [DOI] [PubMed] [Google Scholar]
- 2.Jager K.J., Fraser S.D.S. The ascending rank of chronic kidney disease in the global burden of disease study. Nephrol Dial Transplant. 2017;32(suppl 2):ii121–ii128. doi: 10.1093/ndt/gfw330. [DOI] [PubMed] [Google Scholar]
- 3.Thomas B., Matsushita K., Abate K.H. Global cardiovascular and renal outcomes of reduced GFR. J Am Soc Nephrol. 2017;28:2167–2179. doi: 10.1681/ASN.2016050562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mills K.T., Xu Y., Zhang W. A systematic analysis of worldwide population-based data on the global burden of chronic kidney disease in 2010. Kidney Int. 2015;88:950–957. doi: 10.1038/ki.2015.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levey A.S., de Jong P.E., Coresh J. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80:17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 6.Gansevoort R.T., Matsushita K., van der Velde M. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int. 2011;80:93–104. doi: 10.1038/ki.2010.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Astor B.C., Matsushita K., Gansevoort R.T. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int. 2011;79:1331–1340. doi: 10.1038/ki.2010.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Velde M., Matsushita K., Coresh J. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79:1341–1352. doi: 10.1038/ki.2010.536. [DOI] [PubMed] [Google Scholar]
- 9.Grams M.E., Sang Y., Ballew S.H. A meta-analysis of the association of estimated GFR, albuminuria, age, race, and sex with acute kidney injury. Am J Kidney Dis. 2015;66:591–601. doi: 10.1053/j.ajkd.2015.02.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saran R., Robinson B., Abbott K.C. US Renal Data System 2017 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2018;71(3 suppl 1):A7. doi: 10.1053/j.ajkd.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson B.M., Akizawa T., Jager K.J. Factors affecting outcomes in patients reaching end-stage kidney disease worldwide: differences in access to renal replacement therapy, modality use, and haemodialysis practices. Lancet. 2016;388:294–306. doi: 10.1016/S0140-6736(16)30448-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 13.Xie X., Atkins E., Lv J. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet. 2016;387:435–443. doi: 10.1016/S0140-6736(15)00805-3. [DOI] [PubMed] [Google Scholar]
- 14.Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl. 2012;2:337–414. [Google Scholar]
- 15.Brenner B.M., Cooper M.E., de Zeeuw D. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 16.Ruggenenti P., Perna A., Gherardi G. Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet. 1999;354:359–364. doi: 10.1016/S0140-6736(98)10363-X. [DOI] [PubMed] [Google Scholar]
- 17.Muntner P., Anderson A., Charleston J. Hypertension awareness, treatment, and control in adults with CKD: results from the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis. 2010;55:441–451. doi: 10.1053/j.ajkd.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Konta T., Ikeda A., Ichikawa K. Blood pressure control in a Japanese population with chronic kidney disease: a baseline survey of a nationwide cohort. Am J Hypertens. 2012;25:342–347. doi: 10.1038/ajh.2011.217. [DOI] [PubMed] [Google Scholar]
- 19.Titze S., Schmid M., Kottgen A. Disease burden and risk profile in referred patients with moderate chronic kidney disease: composition of the German Chronic Kidney Disease (GCKD) cohort. Nephrol Dial Transplant. 2015;30:441–451. doi: 10.1093/ndt/gfu294. [DOI] [PubMed] [Google Scholar]
- 20.Lee S., Oh H.J., Lee E.K. Blood pressure control during chronic kidney disease progression. Am J Hypertens. 2017;30:610–616. doi: 10.1093/ajh/hpx017. [DOI] [PubMed] [Google Scholar]
- 21.Mills K.T., Bundy J.D., Kelly T.N. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation. 2016;134:441–450. doi: 10.1161/CIRCULATIONAHA.115.018912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alencar de Pinho N., Levin A., Fukagawa M. Considerable international variations in blood pressure control and antihypertensive prescription patterns in chronic kidney disease. Kidney Int. 2019;96:983–994. doi: 10.1016/j.kint.2019.04.032. [DOI] [PubMed] [Google Scholar]
- 23.Levey A.S., Greene T., Beck G.J. Dietary protein restriction and the progression of chronic renal disease: what have all of the results of the MDRD study shown? Modification of Diet in Renal Disease Study group. J Am Soc Nephrol. 1999;10:2426–2439. doi: 10.1681/ASN.V10112426. [DOI] [PubMed] [Google Scholar]
- 24.Fouque D., Laville M. Low protein diets for chronic kidney disease in non diabetic adults. Cochrane Database Syst Rev. 2009;3:CD001892. doi: 10.1002/14651858.CD001892.pub3. [DOI] [PubMed] [Google Scholar]
- 25.Metzger M., Yuan W.L., Haymann J.P. Association of a low-protein diet with slower progression of CKD. Kidney Int Rep. 2018;3:105–114. doi: 10.1016/j.ekir.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones-Burton C., Mishra S.I., Fink J.C. An in-depth review of the evidence linking dietary salt intake and progression of chronic kidney disease. Am J Nephrol. 2006;26:268–275. doi: 10.1159/000093833. [DOI] [PubMed] [Google Scholar]
- 27.Mariani L., Stengel B., Combe C. The CKD Outcomes and Practice Patterns Study (CKDopps): rationale and methods. Am J Kidney Dis. 2016;68:402–413. doi: 10.1053/j.ajkd.2016.03.414. [DOI] [PubMed] [Google Scholar]
- 28.Stengel B., Combe C., Jacquelinet C. The French Chronic Kidney Disease-Renal Epidemiology and Information Network (CKD-REIN) cohort study. Nephrol Dial Transplant. 2014;29:1500–1507. doi: 10.1093/ndt/gft388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liabeuf S., Van Stralen K.J., Caskey F. Attainment of guideline targets in EURODOPPS haemodialysis patients: are differences related to a country's healthcare expenditure and nephrologist workforce? Nephrol Dial Transplant. 2017;32:1737–1749. doi: 10.1093/ndt/gfw409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Locatelli F., Karaboyas A., Pisoni R.L. Mortality risk in patients on hemodiafiltration versus hemodialysis: a ‘real-world’ comparison from the DOPPS. Nephrol Dial Transplant. 2018;33:683–689. doi: 10.1093/ndt/gfx277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tentori F., Fuller D.S., Port F.K. The DOPPS practice monitor for US dialysis care: potential impact of recent guidelines and regulatory changes on management of mineral and bone disorder among US hemodialysis patients. Am J Kidney Dis. 2014;63:851–854. doi: 10.1053/j.ajkd.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rossignol P., Massy Z.A., Azizi M. The double challenge of resistant hypertension and chronic kidney disease. Lancet. 2015;386:1588–1598. doi: 10.1016/S0140-6736(15)00418-3. [DOI] [PubMed] [Google Scholar]
- 34.Fretheim A., Oxman A.D. International variation in prescribing antihypertensive drugs: its extent and possible explanations. BMC Health Serv Res. 2005;5:21. doi: 10.1186/1472-6963-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheung A.K., Rahman M., Reboussin D.M. Effects of intensive BP control in CKD. J Am Soc Nephrol. 2017;28:2812–2823. doi: 10.1681/ASN.2017020148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alencar de Pinho N., Kaboré J., Laville M. urinary sodium-to-potassium ratio and blood pressure in CKD. Kidney Int Rep. 2020;5:1240–1250. doi: 10.1016/j.ekir.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pecoits-Filho R.F., Fliser D., Tu C. Prescription of renin-angiotensin-aldosterone system inhibitors (RAASi) and its determinants in patients with advanced CKD under nephrologist care. J Clin Hypertens. 2019;21:991–1001. doi: 10.1111/jch.13563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weir M.R., Lakkis J.I., Jaar B. Use of renin-angiotensin system blockade in advanced CKD: an NKF-KDOQI controversies report. Am J Kidney Dis. 2018;72:873–884. doi: 10.1053/j.ajkd.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 39.Bhandari S., Ives N., Brettel E.A. Multicentre randomized controlled trial of angiotensin-converting enzyme inhibitor/angiotensin receptor blocker withdrawal in advanced renal disease: the STOP-ACEi trial. Nephrol Dial Transplant. 2016;31:255–261. doi: 10.1093/ndt/gfv346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balkau B., Metzger M., Andreelli F. Impact of sex and glucose-lowering treatments on hypoglycaemic symptoms in people with type 2 diabetes and chronic kidney disease. The French Chronic Kidney Disease - Renal Epidemiology and Information Network (CKD-REIN) Study. Diabetes Metab. 2019;45:175–183. doi: 10.1016/j.diabet.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 41.Coresh J., Heerspink H.J.L., Sang Y. Change in albuminuria and subsequent risk of end-stage kidney disease: an individual participant-level consortium meta-analysis of observational studies. Lancet Diabetes Endocrinol. 2019;7:115–127. doi: 10.1016/S2213-8587(18)30313-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sumida K., Nadkarni G.N., Grams M. Conversion of urine protein-creatinine to albumin-creatinine ratio for use in CKD risk equations. Ann Intern Med. 2020;173:426–435. doi: 10.7326/M20-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tummalapalli S.L., Powe N.R., Keyhani S. Trends in quality of care for patients with CKD in the United States. Clin J Am Soc Nephrol. 2019;14:1142–1150. doi: 10.2215/CJN.00060119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.