Abstract
Introduction
Patients with advanced non–dialysis-dependent chronic kidney disease (NDD-CKD) are prone to potassium (K) imbalances due to reduced kidney function. Both hypo- and hyperkalemia are associated with increased mortality; however, it is unclear if K variability before dialysis initiation is associated with outcomes after dialysis initiation.
Methods
We identified 34,167 US veterans with advanced NDD-CKD transitioning to dialysis between October 1, 2007, through March 31, 2015, who had at least 1 K measurement each year over a 3-year period before transition (3-year prelude). For each patient, a linear mixed-effects model was used to regress K over time (in years) over the 3-year prelude to derive K variability (square root of the average squared distance between the observed and estimated K). The main outcomes of interest were 6-month all-cause and cardiovascular mortality after dialysis initiation. Multivariable Cox and Fine-Gray competing risk regression adjusted for 3-year prelude K intercept, K slope (per year), demographics, smoking status, comorbidities, length of hospitalizations, body mass index, vascular access type, medications, average estimated glomerular filtration rate, and number of K measurements over the 3-year prelude were used to assess the association of K variability (expressed as quartiles) with all-cause and cardiovascular mortality, respectively.
Results
Higher prelude K variability was associated with higher multivariable-adjusted risk of all-cause mortality but not cardiovascular mortality (adjusted hazard/subhazard ratios [95% confidence interval] for highest quartile [vs. lowest] of K variability, 1.14 [1.03–1.25] and 0.99 [0.85–1.16] for all-cause and cardiovascular mortality, respectively).
Conclusion
Higher K variability is associated with higher all-cause mortality after dialysis initiation.
Keywords: chronic kidney disease, dialysis, end-stage renal disease, potassium, survival
Graphical abstract
Potassium (K) is an important body electrolyte, and plasma K levels are maintained in normal range primarily by the kidneys.1 Thus, patients with advanced NDD-CKD are prone to plasma K variability and dyskalemias (hypo- and hyperkalemia, especially the latter) because of the reduced homeostatic potential of the kidney.1,2 Additionally, the use of medications such as renin-angiotensin-aldosterone system inhibitors (RAASi) and diuretics to manage highly prevalent comorbid conditions like hypertension and cardiovascular diseases and dietary K intake in NDD-CKD patients may further contribute to increased plasma K variability.1, 2, 3, 4 Both hyperkalemia and hypokalemia are associated with an increased risk of mortality, adverse clinical outcomes, and increased economic burden in patients with NDD-CKD.5, 6, 7, 8, 9
Although the association of dyskalemias with mortality has been well studied in NDD-CKD, the relationship of plasma K variability with mortality is unclear. In 2 separate cohorts of patients undergoing peritoneal dialysis, higher K variability was associated with an increased risk of mortality,10,11 whereas in settings such as RAASi initiators with stage 3 to 5 CKD,12 intensive care unit patients,13,14 hospitalized patients with heart failure,15 and hospitalized patients with acute myocardial infarction,16 the association of K variability with mortality varied. Patients with advanced NDD-CKD transitioning to dialysis represent a unique population that might face frequent and major changes in plasma K because of reduced kidney function, a high prevalence of comorbidities, and the use of medications that affect plasma K levels; thus, they experience high mortality rates after dialysis initiation.17 Given the lack of studies in this population, we sought to assess the association between K variability before dialysis initiation with mortality after dialysis initiation. We hypothesized that higher K variability before dialysis initiation would be associated with a greater risk of mortality after initiation.
Methods
Study Population
We assessed longitudinal data from the Transition of Care in Chronic Kidney Disease, a nationally representative historic cohort of US veterans with incident end-stage renal disease transitioning to dialysis from 1 October 2007 (first dialysis transition date) through 31 March 2015 (last dialysis transition date).18, 19, 20, 21, 22 A total of 102,477 US veterans were identified from the United States Renal Data System as a source population, with a median (interquartile range) of 6.2 (2.8–9.3) years and 1.6 (0.6–2.3) years of data availability before and after dialysis initiation, respectively. An initial sample of 60,128 US veterans with nonmissing predialysis plasma K measurements recorded at any Veterans Affairs (VA) facility was identified. The final study sample included 34,167 patients with K measured at least once each year in a 3-year period before dialysis initiation (3-year prelude). The study selection criteria are shown in Supplementary Figure S1.
Exposure
The main exposure of interest was K variability. A single mixed-effects model with a random intercept and slope was estimated of K over time (years) over the 3-year prelude period for all of the patients with patient serving as the random effect. K variability was derived as the square root of the average squared distance between observed K values of each patient and the estimated (model-based) K values (expected values for each individual at the respective time points) (Supplementary Figure S2). Mixed-effects models were used to derive K variability because they account for intraindividual correlations of repeated measurements, heterogenous variability of measurements over time, and unbalanced design (i.e., repeated measurements collected at different times for each patient).23,24 Supplementary Figure S3 compares the distribution of K variability estimated from a mixed-effects regression (Supplementary Figure S3A) versus an ordinary least squares regression (as used in previous studies for estimating variability19,25; Supplementary Figure S3B) and shows the difference between K variability estimated from a mixed-effects regression and ordinary least squares regression (i.e., K variability [mixed effects] − K variability [ordinary least squares regression]; Supplementary Figure S3C). The distributions in Supplementary Figure S3 show that the estimates produced by mixed-effects regression were similar to those produced by ordinary least squares regression. K variability was categorized into quartiles as <0.31, 0.31 to <0.41, 0.41 to <0.52, and ≥0.52 mEq/l. K variability was expressed as quartiles because previous studies observed worse outcomes associated with higher K variability quartiles.10,11,13,14 Similarly, literature on the association of clinical parameter (e.g.. hemoglobin and systolic blood pressure) variability with outcomes in CKD suggests worse outcomes associated with higher variability expressed as quartiles.19,20,26
Covariates
Patient demographic characteristics and the type of vascular access at dialysis initiation was extracted from the United States Renal Data System Patient and Medical Evidence file. Data on marital and smoking status were obtained from VA records.27,28 Preexisting comorbidities at the time of dialysis initiation were identified from the VA Inpatient and Outpatient Medical SAS and the VA/Centers for Medicare and Medicaid Services databases using the International Classification of Diseases, Ninth Revision, Clinical Modification diagnostic and procedure codes and Current Procedural Terminology codes. The Charlson Comorbidity Index score was calculated using the Deyo modification for administrative data sets as a measure of comorbidity burden, and kidney disease was excluded from the algorithm.29 Data on prescribed medications were collected from both VA pharmacy dispensation records and Centers for Medicare and Medicaid Services Medicare Part D, and patients with at least 1 prescription over the 3-year prelude period were recorded as having been treated with the medication. Laboratory data over the 3-year prelude period were obtained from VA research databases as previously described.30,31 The estimated glomerular filtration rate (eGFR) was calculated by the Chronic Kidney Disease Epidemiology Collaboration using outpatient serum creatinine values.32
Outcomes
The main outcomes of interest were time to all-cause and cardiovascular mortality after dialysis initiation. The main follow-up period for mortality assessment was 6 months after dialysis initiation. Additionally, we assessed mortality over a follow-up of 1 month after dialysis initiation and during 2 to 6 months after dialysis initiation among those who survived the initial 1 month to assess whether the relationship between K variability and mortality differed among those surviving longer. Follow-up for mortality analyses started at dialysis initiation (or at 1 month after dialysis initiation for those surviving the first month), and patients were censored at loss of follow-up, the end of the respective follow-up periods (6 months or 1 month), kidney transplantation, or the last date of available follow-up (1 September 2015 and 30 July 2015 for all-cause and cardiovascular mortality, respectively), whichever occurred first. Information on all-cause mortality data, censoring events, and associated dates was obtained from VA and United States Renal Data System data sources. Cardiovascular mortality data were obtained from the United States Renal Data System.
Statistical Analysis
Patient characteristics were summarized for the entire sample and by K variability quartiles and presented as counts (percentages) for categorical variables and mean (SD) for continuous normally distributed variables or median (interquartile range [25th–75th percentile]) for continuous skewed variables. Normality was checked by visual inspection of the histogram, normal probability plot, and the quantile-quantile plot. Differences across K variability quartiles were assessed using χ2 tests for categorical data, 1-way analysis of variance for continuous normal data, and the Kruskal-Wallis test for continuous skewed data. The association between K variability and mortality was assessed using Cox proportional hazard models for all-cause mortality and Fine-Gray competing risk regression for cardiovascular mortality by treating mortality by all other causes as competing events. Models were incrementally adjusted for the following confounders based on theoretical considerations: model 1 adjusted for K intercept and K slope over the 3-year prelude estimated using the linear mixed-effects model; model 2 adjusted for model 1 plus age, sex, race, marital status, and smoking status; model 3 adjusted for model 2 plus comorbidities (diabetes, congestive heart failure [CHF], hypertension, peripheral vascular disease, cerebrovascular disease, lung disease, peptic ulcer disease, paraplegia/hemiplegia, anemia, atrial fibrillation, ischemic heart disease, liver disease, and malignancies), Charlson Comorbidity Index , cumulative length of hospitalizations, 3-year prelude average body mass index (BMI), and vascular access type; model 4 adjusted for model 3 plus medications (RAASi, Na-polystyrene sulphonate, loop diuretics, potassium-sparing diuretics, digoxin, beta blockers, calcium channel blockers, insulin, oral hypoglycemics, calcineurin inhibitors, trimethoprim, and azole antifungals); and model 5 adjusted for model 4 plus the average eGFR and the number of K measurements over the 3-year prelude period. Univariable associations of exposure/confounders with mortality were assessed in univariable survival models. However, all the confounders listed previously were used in the multivariable-adjusted models, irrespective of statistical significance in univariable analyses. We conducted subgroup analyses after categorizing patients by age, race, prevalent diabetes and CHF, the use of Na-polystyrene sulphonate and RAASi, eGFR, and the number of K measurements. Potential interactions between K variability quartiles and the selected subgroups were tested by including interaction terms. Restricted cubic spline models with 2 degrees of freedom were used to investigate nonlinearity in fully adjusted associations between log-transformed K variability and mortality. Missingness was low (race [<0.01%], marital status [0.05%], smoking status [0.07%], eGFR [0.5%], BMI [0.9%], and vascular access [8.6%]), and hence missing values were not imputed. Complete data were available for 30,703 (89.9%) patients for the main multivariable-adjusted survival model (model 5); thus, all survival models (models 1–5) were conducted using 30,703 patients. Similarly, all univariable associations were tested using the 30,703 patients.
As a sensitivity analysis, we identified a subsample of patients (n = 25,152) with at least 3 K measurements in the last year before dialysis initiation (1-year prelude). Methods similar to the main analysis were used for defining K variability quartiles (categorized as <0.30, 0.30 to <0.40, 0.40 to <0.52, and ≥0.52 mEq/l), covariates, outcomes, statistical models, and subgroup analyses; however, covariates such as K intercept and slope, cumulative length of hospitalizations, BMI, medication use, average eGFR, and K measurements were measured over the 1-year prelude period. A 2-sided P value <0.05 was used as a threshold of statistical significance for all statistical analyses. All analyses were conducted in SAS Enterprise guide v7.1 (SAS Institute, Cary, NC) and STATA/MP Version 15 (STATA Corporation, College Station, TX). The study was approved by the Institutional Review Boards of the Memphis and Long Beach VA medical centers, with exemption from informed consent.
Results
Patient Characteristics
The mean (SD) age of the sample was 67.0 (10.8) years; 98% were men, 29% were black, 77% were diabetic, and 64% had CHF. The median (25th–75th) 3-year prelude eGFR was 22.9 (17.2–32.9) ml/min/1.73 m2. Patients had a median number (25th–75th) of K measurements of 19 (8–35), with a mean (SD) 3-year prelude K intercept of 4.47 (0.42) mEq/l, K slope of 0.008 (0.14) mEq/l/yr, and median (25th–75th) K variability of 0.41 (0.31–0.52). Overall and K variability quartile-wise patient characteristics are presented in Table 1. Compared with the lowest K variability quartile (<0.31 mEq/l), those in the higher K variability quartiles were more likely to be younger, black, and current smokers; have a higher prevalence of some comorbidities (CHF, diabetes, hypertension, liver diseases, paraplegia/hemiplegia, and anemia); have longer cumulative length of hospitalization, a higher 3-year prelude mean K intercept, and more frequent K measurements but lower 3-year prelude median eGFR levels; and were more likely to be treated by medications that affect K levels (all P values <0.05).
Table 1.
Patient characteristics overall and by potassium variability quartiles over the 3-year prelude
| Characteristic | All (n = 34,167) | Potassium variability quartiles (mEq/l) |
P value | |||
|---|---|---|---|---|---|---|
| <0.31 (n = 8421) | 0.31–<0.41 (n = 8757) | 0.41–<0.52 (n = 8695) | ≥0.52 (n = 8294) | |||
| Age (yr) | 67 (10.8) | 70.4 (10.7) | 67.1 (10.7) | 65.7 (10.6) | 64.8 (10.4) | <0.0001c |
| Sex (male) | 33,495 (98) | 8304 (98.6) | 8591 (98.1) | 8489 (97.6) | 8111 (97.8) | <0.0001d |
| Race | <0.0001d | |||||
| White | 23,309 (68.2) | 6251 (74.2) | 5869 (67.0) | 5743 (66.1) | 5446 (65.7) | |
| Black | 9885 (28.9) | 1983 (23.6) | 2637 (30.1) | 2700 (31.1) | 2565 (30.9) | |
| Other | 971 (2.8) | 186 (2.2) | 251 (2.9) | 251 (2.9) | 283 (3.4) | |
| Marital status (married) | 18,846 (55.1) | 5473 (64.9) | 4832 (55.2) | 4520 (51.9) | 4021 (48.4) | <0.0001d |
| Smoking status | <0.0001d | |||||
| Current | 12,247 (35.8) | 2789 (33.1) | 2733 (31.2) | 2647 (30.4) | 2375 (28.6) | |
| Past | 11,351 (33.2) | 2440 (28.9) | 3078 (35.1) | 3288 (37.8) | 3441 (41.5) | |
| Never | 10,544 (30.8) | 3188 (37.8) | 2938 (33.5) | 2753 (31.7) | 2472 (29.8) | |
| Comorbidities | ||||||
| Congestive heart failure | 21,722 (63.6) | 5283 (62.7) | 5466 (62.4) | 5542 (63.7) | 5431 (65.5) | 0.0001d |
| Diabetes mellitus | 26,488 (77.5) | 6231 (73.9) | 6795 (77.6) | 6825 (78.5) | 6637 (80.0) | <0.0001d |
| Peripheral vascular disease | 18,099 (52.9) | 4703 (55.8) | 4483 (51.2) | 4505 (51.8) | 4408 (53.2) | <0.0001d |
| Cerebrovascular disease | 15,011 (43.9) | 3986 (47.3) | 3789 (43.3) | 3678 (42.3) | 3558 (42.9) | <0.0001d |
| Lung disease | 19,135 (56) | 4770 (56.6) | 4791 (54.7) | 4855 (55.8) | 4719 (56.9) | 0.02d |
| Peptic ulcer disease | 3702 (10.8) | 986 (11.7) | 893 (10.2) | 910 (10.5) | 913 (11.0) | 0.008d |
| Hypertension | 33,848 (99.1) | 8325 (98.8) | 8702 (99.4) | 8616 (99.1) | 8205 (98.9) | 0.002d |
| Ischemic heart disease | 22,790 (66.7) | 5803 (68.9) | 5798 (66.2) | 5764 (66.3) | 5425 (65.4) | <0.0001d |
| Malignancies | 10,651 (31.2) | 3046 (36.2) | 2711 (30.9) | 2538 (29.2) | 2356 (28.4) | <0.0001d |
| Liver disease | 6736 (19.7) | 1553 (18.4) | 1644 (18.8) | 1730 (19.9) | 1809 (21.8) | <0.0001d |
| Paraplegia/hemiplegia | 1981 (5.8) | 447 (5.3) | 464 (5.3) | 507 (5.8) | 563 (6.8) | <0.0001d |
| Anemia | 28,743 (84.1) | 6958 (82.6) | 7323 (83.6) | 7390 (84.9) | 7072 (85.3) | <0.0001d |
| Atrial fibrillation | 8318 (24.3) | 2332 (27.7) | 2073 (23.7) | 2015 (23.2) | 1898 (22.9) | <0.0001d |
| Charlson Comorbidity Index | 5 (3, 7) | 5 (3, 7) | 5 (3, 7) | 5 (3, 7) | 5 (3, 7) | <0.0001e |
| Cumulative length of hospitalization | 9 (1, 24) | 4 (0, 14) | 7 (1, 20) | 11 (3, 28) | 16 (4, 38) | <0.0001e |
| Body mass index (kg/m2) | 29.6 (6.2) | 29.9 (6.1) | 29.8 (6.1) | 29.4 (6.2) | 29.1 (6.6) | <0.0001d |
| Vascular access type | <0.0001d | |||||
| Arteriovenous fistula | 6949 (20.3) | 1659 (19.7) | 1946 (22.2) | 1823 (20.9) | 1521 (18.3) | |
| Arteriovenous graft | 805 (2.4) | 186 (2.2) | 207 (2.4) | 224 (2.6) | 188 (2.3) | |
| Catheter | 23,313 (68.2) | 5704 (67.7) | 5802 (66.3) | 5920 (68.1) | 5887 (70.9) | |
| Other | 165 (0.5) | 35 (0.4) | 39 (0.5) | 40 (0.5) | 51 (0.6) | |
| Missing | 2935 (8.6) | 837 (9.9) | 763 (8.7) | 688 (7.9) | 647 (7.8) | |
| Medications | ||||||
| RAAS inhibitors | 26,898 (78.7) | 5945 (70.6) | 6905 (78.9) | 7132 (82.0) | 6916 (83.4) | <0.0001d |
| SPS | 9003 (26.3) | 419 (4.9) | 1307 (14.9) | 2784 (32.0) | 4493 (54.2) | <0.0001d |
| Loop diuretics | 27,320 (79.9) | 5592 (66.4) | 7070 (80.7) | 7446 (85.6) | 7212 (86.9) | <0.0001d |
| K-sparing diuretics | 5620 (16.4) | 874 (10.4) | 1261 (14.4) | 1587 (18.3) | 1898 (22.9) | <0.0001d |
| Digoxin | 2447 (7.2) | 531 (6.3) | 543 (6.2) | 672 (7.7) | 701 (8.5) | <0.0001d |
| Beta blockers | 28,321 (82.9) | 6208 (73.7) | 7254 (82.8) | 7526 (86.6) | 7333 (88.4) | <0.0001d |
| Calcium channel blockers | 27,020 (79.1) | 6050 (71.8) | 7001 (79.9) | 7096 (81.6) | 6873 (82.9) | <0.0001d |
| Insulin | 18,220 (53.3) | 3217 (38.2) | 4478 (51.1) | 5206 (59.9) | 5319 (64.1) | <0.0001d |
| Oral hypoglycemics | 13,224 (38.7) | 3067 (36.4) | 3344 (38.2) | 3406 (39.2) | 3407 (41.1) | <0.0001d |
| Trimethoprim | 1859 (5.4) | 391 (4.6) | 423 (4.8) | 489 (5.6) | 556 (6.7) | <0.0001d |
| Azole antifungals | 8113 (23.7) | 1427 (16.9) | 1957 (22.4) | 2308 (26.5) | 2421 (29.2) | <0.0001d |
| Calcineurin inhibitors | 694 (2.0) | 129 (1.5) | 174 (1.9) | 202 (2.3) | 189 (2.3) | 0.0007d |
| Mannitol | 160 (0.5) | 11 (0.1) | 35 (0.4) | 57 (0.7) | 57 (0.7) | <0.0001d |
| Suxamethonium | 30 (0.1) | 1 (0.01) | 3 (0.03) | 10 (0.1) | 16 (0.2) | 0.0002d |
| NSAID | 249 (0.7) | 42 (0.5) | 56 (0.6) | 75 (0.9) | 76 (0.9) | 0.004d |
| Pentamidine | 19 (0.1) | 2 (0.02) | 0 | 8 (0.09) | 9 (0.1) | 0.005d |
| Penicillin G | 87 (0.2) | 6 (0.07) | 18 (0.2) | 29 (0.3) | 34 (0.4) | <0.0001d |
| Laboratory parametersa | ||||||
| K intercept (mEq/l) | 4.47 (0.42) | 4.38 (0.37) | 4.43 (0.40) | 4.50 (0.43) | 4.58 (0.46) | <0.0001d |
| K slope (mEq/l/yr) | 0.008 (0.14) | 0.01 (0.07) | 0.01 (0.12) | 0.005 (0.15) | 0.002 (0.18) | <0.0001d |
| Last K valueb (mEq/l) | 4.49 (0.66) | 4.42 (0.52) | 4.47 (0.59) | 4.52 (0.69) | 4.58 (0.82) | <0.0001d |
| K variability (mEq/l) | 0.41 (0.31–0.52) | 0.25 (0.19–0.28) | 0.36 (0.34–0.39) | 0.46 (0.44–0.49) | 0.60 (0.56–0.68) | <0.0001e |
| eGFR (ml/min per 1.73 m2) | 22.9 (17.2–32.9) | 23.9 (17.5–37) | 22.1 (16.6–31.6) | 22.4 (17.1–31.2) | 23.2 (17.5–32.3) | <0.0001e |
| Number of K measurements | 19 (8, 35) | 8 (5, 16) | 19 (9, 31) | 26 (13, 44) | 30 (16, 56) | <0.0001e |
eGFR, estimated glomerular filtration rate; K, potassium; NSAID, nonsteroidal anti-inflammatory drugs; RAASi, renin angiotensin-aldosterone system inhibitor; SPS, Na-polystyrene sulphonate.
Data presented as n (%), mean (SD), or median (25th–75th) unless otherwise noted.
Measured over the 3-year prelude period.
Potassium value closest to dialysis.
P value for 1-way analysis of variance.
P value for the χ2 test.
P value for the Kruskal-Wallis test.
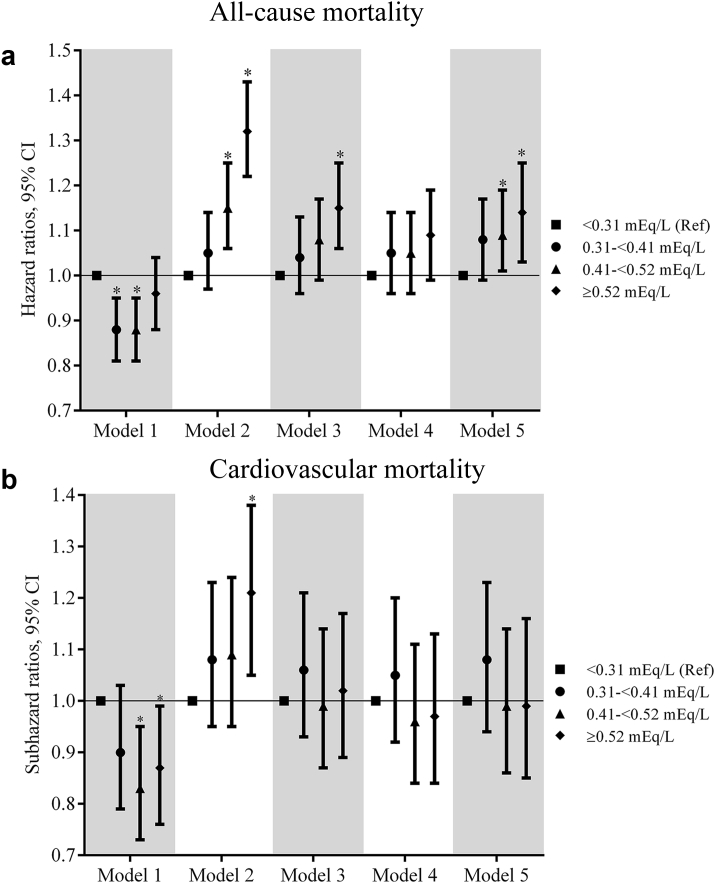
Association of K Variability Before Dialysis Initiation With All-Cause Mortality
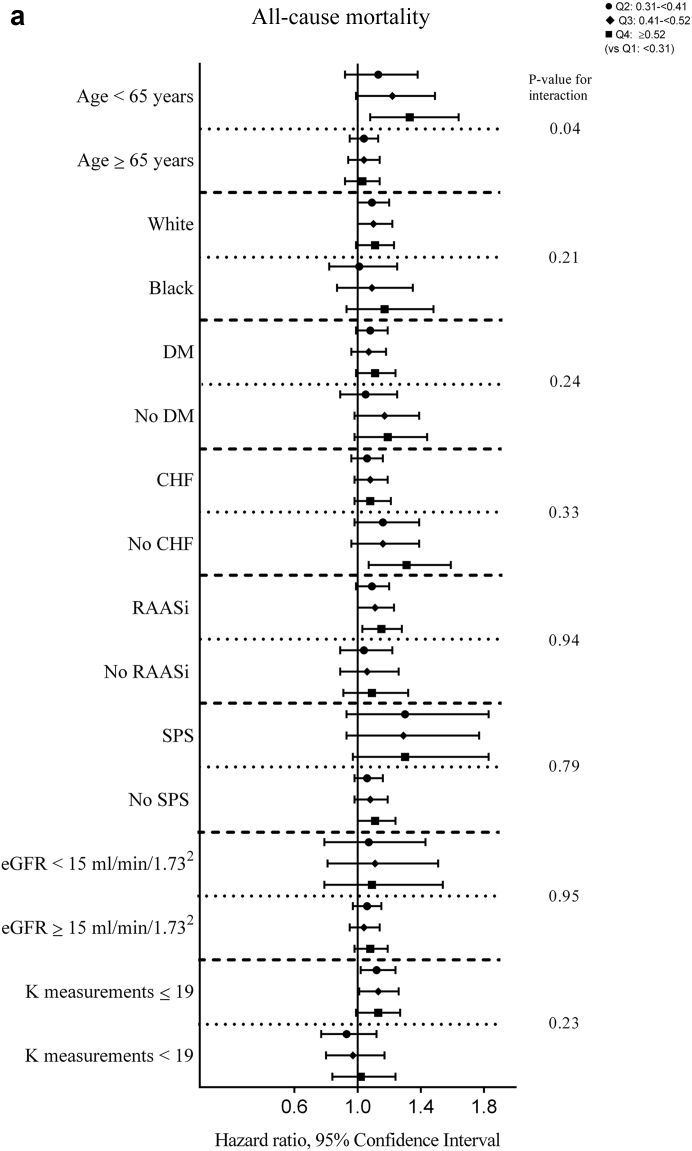
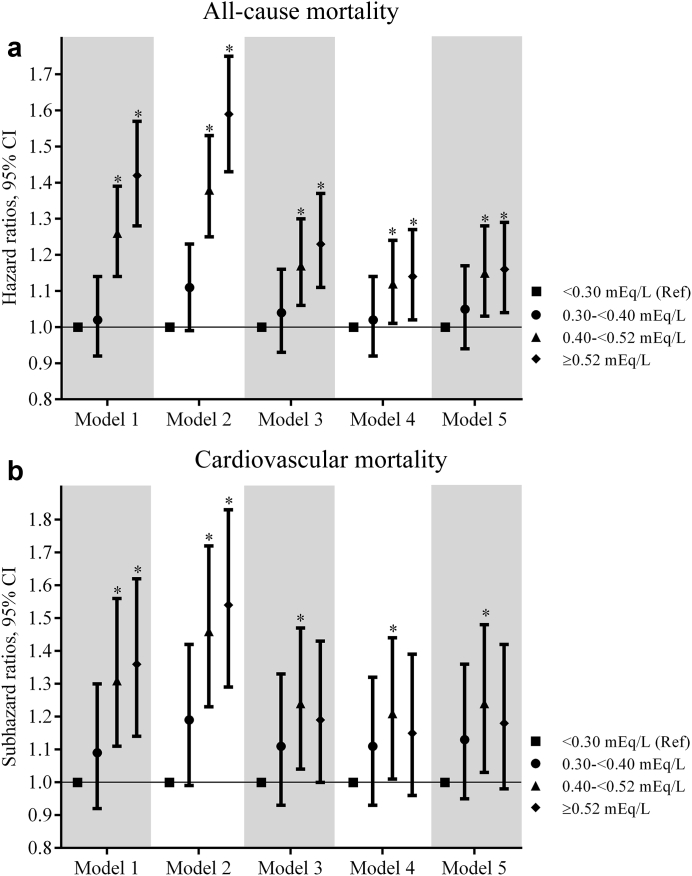
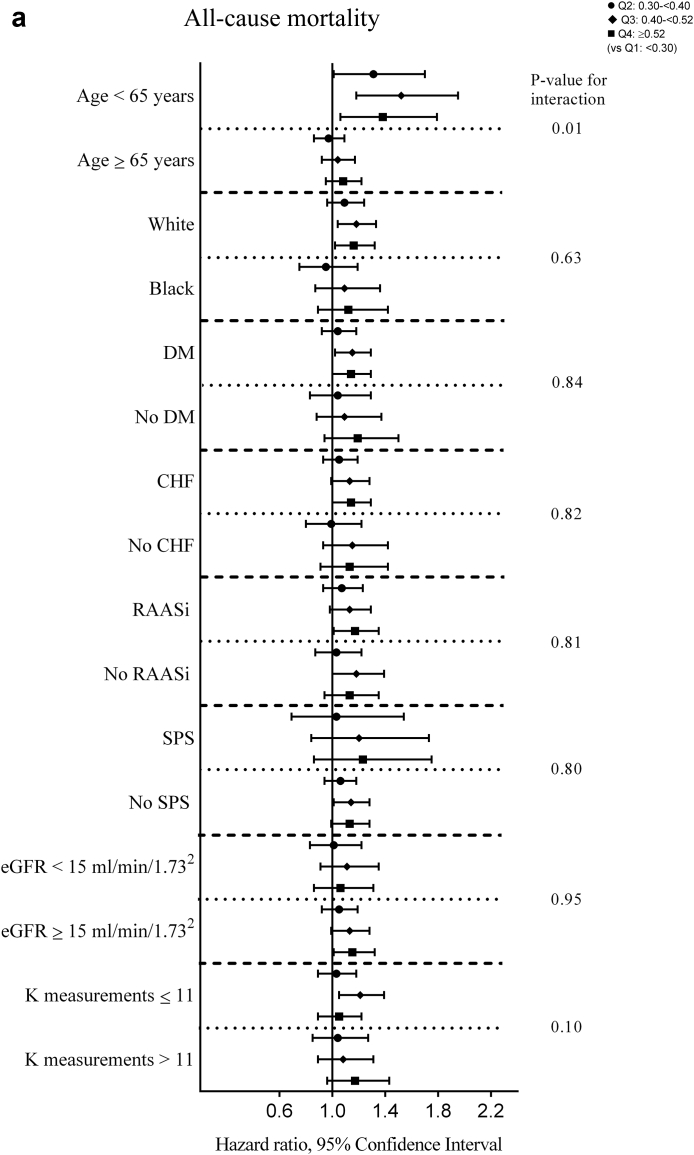
There were a total of 5362 (15.7%) all-cause deaths during the 6-month period after dialysis initiation (crude mortality rate = 345.9/1000 patient-years; 95% confidence interval [CI], 336.6–355.4). In the univariable analyses, a significantly lower all-cause mortality risk was observed for quartiles 2 and 3 versus quartile 1 (hazard ratios [HRs] [95% CI; P value] for quartiles 2 through 4 [vs. quartile 1], 0.88 (0.81-0.95; 0.001), 0.87 (0.80-0.94; 0.0007), and 0.94 (0.87-1.02; 0.15); Supplementary Table S1). Figure 1a shows the multivariable-adjusted HRs of all-cause mortality associated with each K variability quartile. In the fully adjusted multivariable model (model 5), an incrementally higher risk of death with each higher K variability quartile was observed with a significantly higher risk associated with quartile 3 and quartile 4 of K variability (adjusted HRs [95% CI, P value] for quartiles 2 through 4 [vs. quartile 1], 1.08 [0.99–1.17, 0.07], 1.09 [1.01–1.19, 0.04], and 1.14 [1.03–1.25, 0.008] in model 5; Figure 1a, Supplementary Table S2). In subgroup analyses, higher K variability was associated with higher all-cause mortality across the subgroups, with significantly greater contributions of K variability to all-cause mortality among those <65 years (vs. ≥65 years; Figure 2a, Supplementary Table S3). Higher levels of K variability appeared to be monotonically associated with higher all-cause mortality within 6 months after dialysis initiation in spline analyses (Supplementary Figure S4). K variability was not associated with mortality within 1 month after dialysis initiation; however, among those surviving the first month after dialysis initiation, the association between K variability and mortality was qualitatively similar to results observed for the total 6-month follow-up period (Supplementary Tables S4 and S5).
Figure 1.
The association of potassium variability quartiles over the 3-year prelude with 6-month (a) all-cause mortality and (b) cardiovascular mortality after dialysis initiation. The models are as follows: model 1, adjusted for potassium intercept and slope over the 3-year prelude; model 2, adjusted for variables in model 1 plus demographics (age, sex, race, marital status, and smoking status); model 3, adjusted for variables in model 2 plus comorbidities (congestive heart failure, peripheral vascular disease, cerebrovascular disease, lung disease, peptic ulcer disease, paraplegia/hemiplegia, anemia, atrial fibrillation, hypertension, ischemic heart disease, diabetes, liver disease, and malignancies), Charlson Comorbidity Index, cumulative length of hospitalizations, body mass index over the 3-year prelude period, and vascular access type; model 4, adjusted for variables in model 3 plus medications (renal-angiotensin-aldosterone system inhibitors, Na-polystyrene sulphonate, loop diuretics, potassium-sparing diuretics, digoxin, beta blockers, calcium channel blockers, insulin, oral hypoglycemics, calcineurin inhibitors, trimethoprim, and azole antifungals); and model 5, adjusted for variables in model 4 plus the average eGFR and number of potassium measurements over the 3-year prelude period. CI, confidence interval.
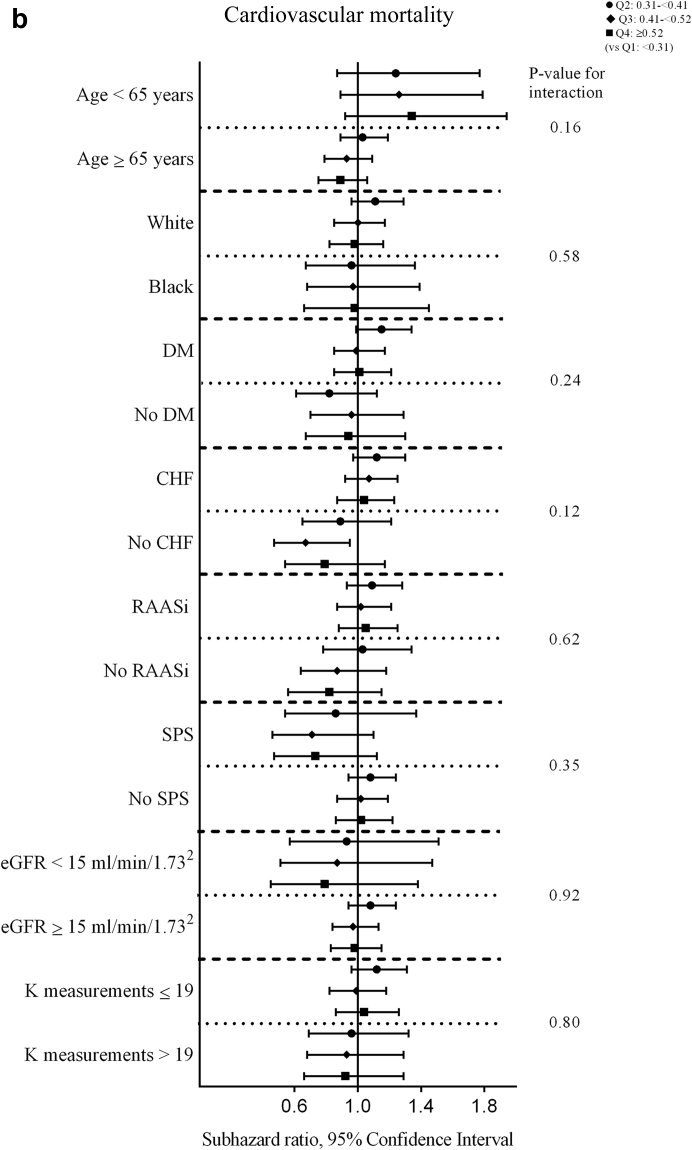
Figure 2.
Adjusted hazard/subhazard ratios (95% confidence interval) of 6-month (a) all-cause mortality and (b) cardiovascular mortality after dialysis initiation with potassium variability quartiles over the 3-year prelude in selected subgroups. The model adjusted for potassium intercept and slope over the 3-year prelude, demographics (age, sex, race, marital status, and smoking status), comorbidities (congestive heart failure, peripheral vascular disease, cerebrovascular disease, lung disease, peptic ulcer disease, paraplegia/hemiplegia, anemia, atrial fibrillation, hypertension, ischemic heart disease, diabetes, liver disease, and malignancies), Charlson Comorbidity Index, cumulative length of hospitalizations, body mass index over the 3-year prelude period, vascular access type, medications (renal-angiotensin-aldosterone system inhibitor [RAASi], Na-polystyrene sulphonate, loop diuretics, potassium sparing diuretics, digoxin, beta blockers, calcium channel blockers, insulin, oral hypoglycemics, calcineurin inhibitors, trimethoprim, azole antifungals), average estimated glomerular filtration rate (eGFR), and number of potassium measurements over the 3-year prelude period. CHF, congestive heart failure; DM, diabetes mellitus; K, potassium; SPS; Na-polystyrene sulphonate.
For the sensitivity analysis (K variability over 1-year prelude) in the univariable survival analyses, a significantly higher all-cause mortality risk was observed for quartiles 3 and 4 versus quartile 1 (HRs [95% CI, P value] for quartiles 2 through 4 [vs. quartile 1], 1.01 [0.91–1.12, 0.89], 1.22 [1.10–1.35, 0.0001], and 1.34 [1.21–1.48, <0.0001]; Supplementary Table S6). Multivariable-adjusted results (model 5) for the sensitivity analysis were qualitatively similar to the main analysis for the association of K variability with all-cause mortality for the 6-month follow-up period (adjusted HRs [95% CI, P value] for quartiles 2 through 4 [vs. quartile 1], 1.05 [0.94–1.17, 0.39], 1.15 [1.03–1.28, 0.01], and 1.16 [1.04-1.29; 0.009] in model 5; Figure 3a, Supplementary Table S7) and for the 1-month and 1- to 6-month follow-ups (Supplementary Tables S8 and S9). Subgroup analyses showed similar patterns of association as the main analysis, with significantly greater contributions of K variability quartiles to all-cause mortality within 6 months after dialysis initiation among those <65 years (Figure 4a, Supplementary Table S10). Higher levels of K variability appeared to be monotonically associated with higher all-cause mortality within 6 months after dialysis initiation in the spline analyses (Supplementary Figure S5).
Figure 3.
The association of potassium variability quartiles over the 1-year prelude with 6-month (a) all-cause mortality and (b) cardiovascular mortality after dialysis initiation. The models are as follows: model 1, adjusted for potassium intercept and slope over a 1-year prelude; model 2, adjusted for variables in model 1 plus demographics (age, sex, race, marital status, smoking status); model 3, adjusted for variables in model 2 plus comorbidities (congestive heart failure, peripheral vascular disease, cerebrovascular disease, lung disease, peptic ulcer disease, paraplegia/hemiplegia, anemia, atrial fibrillation, hypertension, ischemic heart disease, diabetes, liver disease, malignancies), Charlson comorbidity index, cumulative length of hospitalizations, and body mass index over the 1-year prelude period, and vascular access type; model 4, adjusted for variables in model 3 plus medications (renal-angiotensin-aldosterone system inhibitors, Na-polystyrene sulphonate, loop diuretics, potassium sparing diuretics, digoxin, beta blockers, calcium channel blockers, insulin, oral hypoglycemics, calcineurin inhibitors, trimethoprim, azole antifungals); and model 5, adjusted for variables in model 4 plus average estimated glomerular filtration rate and number of potassium measurements over the 1-year prelude period. CI, confidence interval.
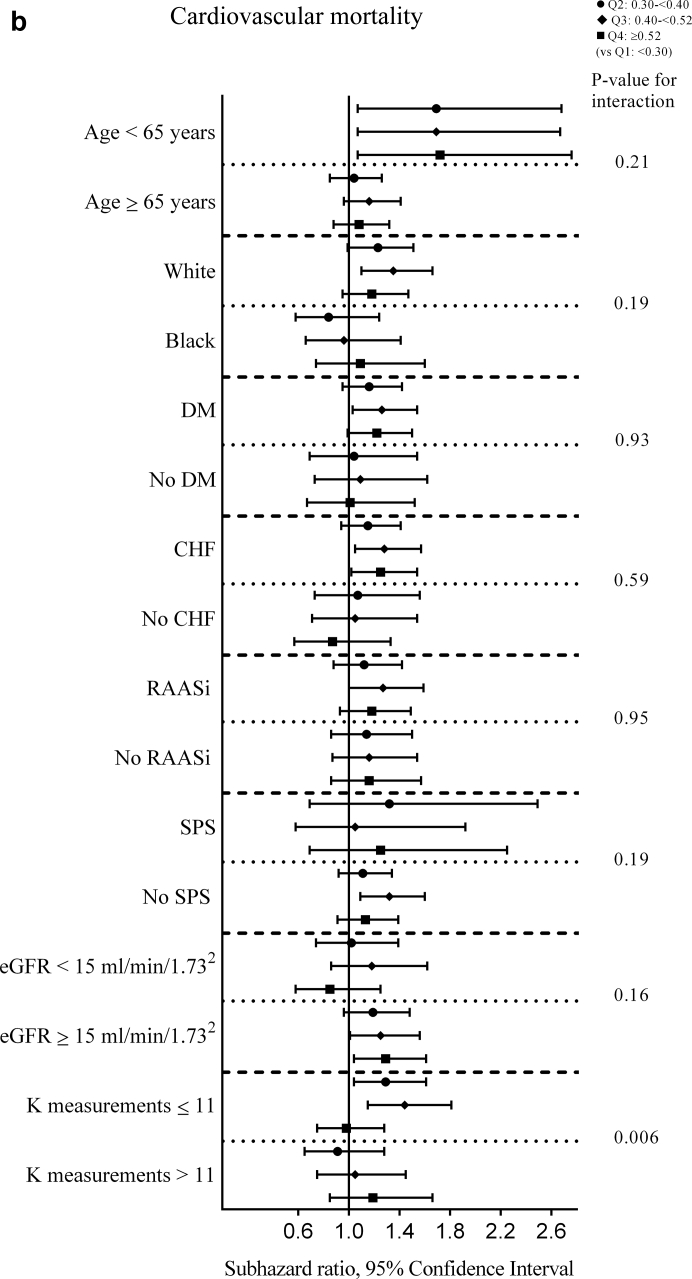
Figure 4.
Adjusted hazard/subhazard ratios (95% confidence interval) of 6-month (a) all-cause mortality and (b) cardiovascular mortality after dialysis initiation with prelude potassium variability quartiles over the 1-year prelude in selected subgroups. The model adjusted for potassium intercept and slope over the 1-year prelude, demographics (age, sex, race, marital status, and smoking status), comorbidities (congestive heart failure, peripheral vascular disease, cerebrovascular disease, lung disease, peptic ulcer disease, paraplegia/hemiplegia, anemia, atrial fibrillation, hypertension, ischemic heart disease, diabetes, liver disease, and malignancies), Charlson Comorbidity Index, cumulative length of hospitalizations, body mass index over the 1-year prelude period, vascular access type, medications (renal-angiotensin-aldosterone system inhibitor [RAASi], Na-polystyrene sulphonate, loop diuretics, potassium sparing diuretics, digoxin, beta blockers, calcium channel blockers, insulin, oral hypoglycemics, calcineurin inhibitors, trimethoprim, and azole antifungals), average estimated glomerular filtration rate (eGFR), and the number of potassium measurements over the 1-year prelude period. CHF, congestive heart failure; DM, diabetes mellitus; K, potassium; SPS, Na-polystyrene sulphonate.
Association of K Variability Before Dialysis Initiation With Cardiovascular Mortality
A total of 1915 (5.6%) cardiovascular deaths were observed during the 6-month follow-up period after dialysis initiation (crude mortality rate 124.6/1000 patient-years; 95% CI, 119.1–130.4). In the univariable analyses, a significantly lower cardiovascular mortality risk was observed for quartile 3 versus quartile 1 (subhazard ratios [SHRs] [95% CI, P value] for quartiles 2 through 4 [vs. quartile 1], 0.90 [0.79–1.03, 0.13], 0.84 [0.73–0.95, 0.008], and 0.88 [0.77–1.00, 0.88], Supplementary Table S11). Figure 1b shows the multivariable-adjusted SHRs of cardiovascular mortality associated with each K variability quartile. In the final multivariable adjusted model (model 5), there was no significant difference in the risk of death with each higher K variability quartile (adjusted SHRs [95% CI, P value] for quartiles 2 through 4 [vs. quartile 1], 1.08 [0.94–1.23, 0.29], 0.99 [0.86–1.14, 0.86], and 0.99 [0.85–1.16, 0.95] in model 5; Figure 1b, Supplementary Table S12). Subgroup analyses showed no significant interactions (Figure 2b, Supplementary Table S13). No significant association was observed between K variability treated as a continuous variable and cardiovascular mortality within 6 months after dialysis initiation in spline analysis (Supplementary Figure S6). K variability was not associated with cardiovascular mortality within 1 month after dialysis initiation (Supplementary Table S14). Among those surviving the first month after dialysis initiation, the association between K variability and mortality was qualitatively similar to results observed for the total 6-month follow-up period (Supplementary Table S15).
For the sensitivity analysis (K variability over the 1-year prelude) in the univariable analyses, a significantly higher cardiovascular mortality risk was observed for quartile 3 and 4 versus quartile 1 (SHRs [95% CI, P value] for quartiles 2 through 4 [vs. quartile 1], 1.08 [0.91–1.29, 0.39], 1.28 [1.08–1.52, 0.004], and 1.30 [1.10–1.54, 0.002], Supplementary Table S16). Multivariable-adjusted results (model 5) for the sensitivity analysis (K variability over 1-year prelude) showed a statistically significantly higher risk of cardiovascular mortality associated with quartile 3 (vs. quartile 1) and a similar but not statistically significantly higher risk for quartile 2 and quartile 4 (adjusted SHRs [95% CI, P value] for quartiles 2 through 4 [vs. quartile 1], 1.13 [0.95–1.36, 0.17], 1,24 [1.03–1.48, 0.02], and 1.18 [0.98–1.42, 0.08] in model 5; Figure 3b, Supplementary Table S17). Similar results were observed among those surviving the first month after dialysis initiation with a significantly higher risk of cardiovascular mortality associated with higher K variability (quartile 3 and 4 [vs. quartile 1], Supplementary Table S18). K variability did not have any association with 1-month cardiovascular mortality (Supplementary Table S19). Subgroup analyses showed significantly greater contributions of K variability to cardiovascular mortality among those with ≤11 K measurements (vs. >11 K measurements) over the 1-year prelude (Figure 4b, Supplementary Table S20). When treated as a continuous variable in spline analyses, K variability showed a similar association (compared with quartile assessment) with cardiovascular mortality within 6 months after dialysis initiation (Supplementary Figure S7).
Discussion
In this large nationally representative cohort of 34,167 US veterans with advanced NDD-CKD transitioning to dialysis, we observed that higher K variability before dialysis was associated with higher all-cause mortality but not cardiovascular mortality within 6 months after dialysis initiation. K variability when assessed over a 1-year prelude before dialysis initiation showed similar patterns of association with all-cause mortality. However, higher K variability quartiles over the 1-year prelude were associated with a higher risk of cardiovascular mortality within 6 months after dialysis initiation, possibly because of change in the nature of K variability (e.g., higher hyperkalemia risk), a closer follow-up period before dialysis initiation to assess K variability, and more interventions to correct dyskalemias. K variability had no association with all-cause mortality and cardiovascular mortality within 1 month of dialysis initiation, which might be due to the different causes and numbers of deaths experienced by patients within this period. Among those who survived the first month after dialysis initiation, the association of K variability with all-cause and cardiovascular mortality was similar to the associations observed over the complete 6-month follow-up period for both evaluation periods.
Our results align with certain aspects of previous studies that have assessed the association of K variability with survival outcomes, almost exclusively in peritoneal dialysis patients.10, 11, 12 Studies in peritoneal dialysis patients (cumulative N = 1243) found a higher risk of all-cause and cardiovascular mortality with higher K variability.10,11 In the only study examining patients with moderate to advanced NDD-CKD who initiated RAASi, K variability had no association with all-cause mortality.12 However, in that study, K variability was measured using only 2 K measurements (from RAASi initiation to first follow-up) within 90 days of initiation, thus lacking sufficient longitudinal evaluation. In the present study, we assessed variability using at least 3 K measurements (median [25th–75th]: 19 [8–35]), and we studied the association of K variability and mortality in an advanced NDD-CKD population transitioning to dialysis, a unique population that experiences high rates of mortality immediately after dialysis initiation.17 Thus, our study extends the current literature to this unique population and demonstrates the potential prognostic impact of K variability before dialysis initiation (especially K variability measured in the last year before dialysis initiation) on survival after dialysis initiation.
Impaired K handling by progressively impaired kidneys1,2; the common use of RAASi (hyperkalemic effect) and diuretics (hypokalemic effect) in CKD and other medications affecting plasma K levels33,34; and a high prevalence of comorbidities such as hypertension, cardiovascular disease, and diabetes4 can be hypothesized as some reasons that contribute to a high K variability in patients with NDD-CKD. Of note, in our study, an overwhelming majority of patients had hypertension, the prevalence of diabetes and CHF was higher in the higher K variability quartiles, and the use of RAASi and diuretics was high and increased with higher K variability quartiles. Plasma K levels are typically maintained within a narrow range, ensuring the maintenance of resting cell membrane potential and neuromuscular and cardiomuscular excitability.35 Given the characteristics of our population, patients could be expected to experience more frequent or more sudden plasma K fluctuations, thus inducing frequent dyskalemias (hypo-/hyperkalemias) and greater dyskalemia-associated adverse events like arrhythmias, stroke, heart attack, hypertension, and sudden cardiac deaths.6,33,34,36 The association of K variability with all-cause mortality can be potentially explained by the development of such cardiovascular events and their further downstream consequences like hypotension and myocardial ischemia, eventually increasing the risk of mortality.6,37 However, the increased all-cause mortality risk of higher K variability did not translate to an association with higher cardiovascular mortality risk in our cohort. This might be observed due to survivorship bias wherein those with higher K variability and experiencing associated cardiovascular mortality might not survive until dialysis initiation, which could not be assessed because of the nature of our cohort. Second, cardiovascular events like arrhythmias and sudden cardiac deaths are immediate effects of dyskalemias; therefore, K fluctuations may not be associated with more distant cardiovascular deaths. Another potential explanation for the observed associations is that higher K variability may be a surrogate marker of patient characteristics or processes that portend poorer prognosis. For example, frequent changes in dosage or discontinuation of pharmacologic agents affecting K levels (e.g., RAASi) might increase K variability and also lead to adverse future outcomes.38
The practical utility of our results is primarily in helping the prediction of future clinical outcomes in incident dialysis patients whose predialysis clinical and laboratory characteristics can be used to prognosticate their mortality and thus aid in decision making about best treatment modalities.39,40 K variability is not readily available/assessed in clinical practice, but the increasing use of electronic health care systems and the development of artificial intelligence-based prognostic algorithms will make its incorporation in prognostic models increasingly available. The direct clinical utility of these results is less clear. The quantification of K variability requires a potentially extended amount of time (e.g., 1–3 years in our study) with assessment of outcomes after the evaluation period. The pathophysiological effects of dyskalemias (such as sudden cardiac death) are expected to manifest acutely; hence, distant outcomes associated with higher K variability may not signal mechanistic links, and it is unclear if the correction or prevention of K fluctuations can result in better outcomes. Nevertheless, it is possible that the higher K variability detected during the evaluation period in our study is an early manifestation of subsequent dyskalemic events, which could potentially be prevented with early interventions. The plausibility of this hypothesis will need to be tested in future studies.
Limitations
Our study results need to be interpreted in light of several limitations. First, we used observational data; hence, we cannot infer causality but only associations. Second, the cohort consisted of predominantly male US veterans (98%); thus, the results may not be generalizable to women or a broader general population. Third, we used predialysis assessment of K variability in a cohort of incident dialysis patients, and, thus, survivorship bias prevents us from extending any of our conclusions to patients with advanced CKD who did not reach end-stage renal disease. Nevertheless, assessments of variability (of any kind) require the availability of serial measurements; hence, an evaluation period that is unaffected by deaths or other dropouts is often specified in studies assessing variability. Finally, because of the nature of the study, we cannot eliminate the possibility of unmeasured confounders such as a lack of data about dietary K intake.
In conclusion, a higher K variability before dialysis initiation (especially K variability measured in the last year before dialysis initiation) is associated with an increased risk of mortality in incident dialysis patients. Thus, higher K variability may serve as a clinically important prognostic marker for future clinical events.
Disclosure
CPK reports personal fees from Amgen, personal fees from Sanofi-Aventis, personal fees from Fresenius Medical Care, personal fees from Keryx, grants from Shire, personal fees from Bayer, personal fees from Abbott, personal fees from Abbvie, personal fees from Dr. Schar, personal fees from Astra-Zeneca, personal fees from Takeda, personal fees from Tricida, and personal fees from Reata outside the submitted work. KK-Z reports personal fees from Abbott, personal fees from Abbvie, personal fees from Alexion, personal fees from Amgen, personal fees from Astra-Zeneca, personal fees from Aveo, personal fees from Chugai, other from DaVita, personal fees from Fresenius Medical Services, personal fees from Genentech, personal fees from Haymarket, personal fees from Hospira, personal fees from Kabi, personal fees from Keryx, personal fees from Navartis, personal fees from Pfizer, personal fees from Relypsa, personal fees from Resverlogix, personal fees from Sandoz, personal fees from Sanofi, personal fees from Shire, personal fees from Vifor, personal fees from ZS-Pharma, personal fees from UpToDate, grants and personal fees from National Institutes of Health, personal fees from Baxter, personal fees from Dr. Schaer, personal fees from PCORI, and personal fees from Amag Pharma outside the submitted work. MZM reports personal fees from Abbvie and personal fees from CareDx outside the submitted work. All the other authors declared no competing interests.
Acknowledgments
Supported by grant 5U01DK102163 from the National Institutes of Health (NIH) to KK-Z and CPK and by resources from the US Department of Veterans Affairs (VA). The data reported here have been supplied in part by the United States Renal Data System (USRDS). Support for VA/CMS data is provided by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, VA Information Resource Center (project numbers SDR 02-237 and 98-004).
Footnotes
Table S1. Univariable Cox regression assessing association between exposure/confounders (over the 3-year prelude) and all-cause mortality within 6 months after dialysis initiation.
Table S2. The association of potassium variability quartiles over the 3-year prelude with 6-month all-cause mortality after dialysis initiation.
Table S3. Adjusted hazard (95% CI) of 6-month all-cause mortality after dialysis initiation with potassium variability quartiles over the 3-year prelude in selected subgroups all-cause mortality after dialysis initiation.
Table S4. The association of potassium variability quartiles over the 3-year prelude with 1-month all-cause mortality after dialysis initiation.
Table S5. The association of potassium variability quartiles over the 3-year prelude with all-cause mortality between 2 to 6 months among those who survived 1-month after dialysis initiation.
Table S6. Univariable Cox regression assessing association between exposure/confounders (over the 1-year prelude) and all-cause mortality within 6 months after dialysis initiation.
Table S7. The association of K variability quartiles over the 1-year prelude with 6-month all-cause mortality after dialysis initiation.
Table S8. The association of potassium variability quartiles over the 1-year prelude with 1-month all-cause mortality after dialysis initiation.
Table S9. The association of potassium variability quartiles over the 1-year prelude with all-cause mortality between 2 to 6 months among those who survived within 1 month of dialysis initiation.
Table S10. Adjusted hazard ratios (95% CI) of 6-month all-cause mortality after dialysis initiation with prelude potassium variability quartiles over the 1-year prelude in selected subgroups.
Table S11. Univariable Cox regression assessing the association between exposure/confounders (over the 3-year prelude) and cardiovascular mortality within 6 months after dialysis initiation.
Table S12. The association of potassium variability quartiles over the 3-year prelude with 6-month cardiovascular mortality after dialysis initiation.
Table S13. Adjusted subhazard ratios (95% CI) of 6-month cardiovascular mortality after dialysis initiation with potassium variability quartiles over the 3-year prelude in selected subgroups.
Table S14. The association of potassium variability quartiles over the 3-year prelude with 1-month cardiovascular mortality after dialysis initiation.
Table S15. The association of potassium variability quartiles over the 3-year prelude with cardiovascular between 2 to 6 months among those who survived within 1 month of dialysis initiation.
Table S16. Univariable Cox regression assessing the association between exposure/confounders (over the 1-year prelude) and cardiovascular mortality within 6 months after dialysis initiation.
Table S17. The association of potassium variability quartiles over the 1-year prelude with 6-month cardiovascular mortality after dialysis initiation.
Table S18. The association of potassium variability quartiles over the 3-year prelude with all-cause mortality between 2 to 6 months among those who survived 1 month after dialysis initiation.
Table S19. The association of potassium variability quartiles over the 1-year prelude with 1-month cardiovascular mortality after dialysis initiation.
Table S20. Adjusted subhazard ratios (95% CI) of 6-month cardiovascular mortality after dialysis initiation with prelude potassium variability quartiles over the 1-year prelude in selected subgroups.
Figure S1. Sample selection criteria.
Figure S2. Potassium variability calculation.
Figure S3. A histogram of (A) potassium variability estimated by linear mixed-effects regression with random intercept and slope, (B) potassium variability estimated by ordinary least squares regression, and (C) the difference between potassium variability estimated by linear mixed-effects regression and ordinary least squares regression.
Figure S4. The association of potassium variability over the 3-year prelude with 6-month all-cause mortality after dialysis initiation.
Figure S5. The association of potassium variability over the 1-year prelude with 6-month all-cause mortality after dialysis initiation.
Figure S6. The association of potassium variability over the 3-year prelude with 6-month cardiovascular mortality after dialysis initiation.
Figure S7. The association of potassium variability over the 1-year prelude with 6-month cardiovascular mortality after dialysis initiation.
Supplementary Material
References
- 1.Kovesdy C.P., Appel L.J., Grams M.E. Potassium homeostasis in health and disease: a scientific workshop cosponsored by the National Kidney Foundation and the American Society of Hypertension. Am J Kidney Dis. 2017;70:844–858. doi: 10.1053/j.ajkd.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Gilligan S., Raphael K.L. Hyperkalemia and hypokalemia in CKD: prevalence, risk factors, and clinical outcomes. Adv Chronic Kidney Dis. 2017;24:315–318. doi: 10.1053/j.ackd.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 3.McCullough P.A., Beaver T.M., Bennett-Guerrero E. Acute and chronic cardiovascular effects of hyperkalemia: new insights into prevention and clinical management. Rev Cardiovasc Med. 2014;15:11–23. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance System--United States. http://www.cdc.gov/ckd Available at:
- 5.Collins A.J., Pitt B., Reaven N. Association of serum potassium with all-cause mortality in patients with and without heart failure, chronic kidney disease, and/or diabetes. Am J Nephrol. 2017;46:213–221. doi: 10.1159/000479802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kovesdy C.P., Matsushita K., Sang Y. Serum potassium and adverse outcomes across the range of kidney function: a CKD Prognosis Consortium meta-analysis. Eur Heart J. 2018;39:1535–1542. doi: 10.1093/eurheartj/ehy100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korgaonkar S., Tilea A., Gillespie B.W. Serum potassium and outcomes in CKD: insights from the RRI-CKD cohort study. Clin J Am Soc Nephrol. 2010;5:762–769. doi: 10.2215/CJN.05850809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fitch K., Woolley J.M., Engel T., Blumen H. The clinical and economic burden of hyperkalemia on Medicare and commercial payers. Am Health Drug Benefits. 2017;10:202–210. [PMC free article] [PubMed] [Google Scholar]
- 9.Luo J., Brunelli S.M., Jensen D.E., Yang A. Association between serum potassium and outcomes in patients with reduced kidney function. Clin J Am Soc Nephrol. 2016;11:90–100. doi: 10.2215/CJN.01730215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Q., Xu F., Fan L. Serum potassium levels and its variability in incident peritoneal dialysis patients: associations with mortality. PLoS One. 2014;9 doi: 10.1371/journal.pone.0086750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li S.H., Xie J.T., Long H.B. Time-averaged serum potassium levels and its fluctuation associate with 5-year survival of peritoneal dialysis patients: two-center based study. Sci Rep. 2015;5:15743. doi: 10.1038/srep15743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garlo K.G., Bates D.W., Seger D.L. Association of changes in creatinine and potassium levels after initiation of renin angiotensin aldosterone system inhibitors with emergency department visits, hospitalizations, and mortality in individuals with chronic kidney disease. JAMA Netw Open. 2018;1 doi: 10.1001/jamanetworkopen.2018.3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hessels L., Hoekstra M., Mijzen L.J. The relationship between serum potassium, potassium variability and in-hospital mortality in critically ill patients and a before-after analysis on the impact of computer-assisted potassium control. Crit Care. 2015;19:4. doi: 10.1186/s13054-014-0720-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engelhardt L.J., Balzer F., Muller M.C. Association between potassium concentrations, variability and supplementation, and in-hospital mortality in ICU patients: a retrospective analysis. Ann Intensive Care. 2019;9:100. doi: 10.1186/s13613-019-0573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan S.S., Campia U., Chioncel O. Changes in serum potassium levels during hospitalization in patients with worsening heart failure and reduced ejection fraction (from the EVEREST trial) Am J Cardiol. 2015;115:790–796. doi: 10.1016/j.amjcard.2014.12.045. [DOI] [PubMed] [Google Scholar]
- 16.Shiyovich A., Gilutz H., Plakht Y. Potassium fluctuations are associated with inhospital mortality from acute myocardial infarction. Soroka Acute Myocardial Infarction II (SAMI-II) Project. Angiology. 2018;69:709–717. doi: 10.1177/0003319717740004. [DOI] [PubMed] [Google Scholar]
- 17.Saran R., Robinson B., Abbott K.C. US Renal Data System 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2019;73:A7–A8. doi: 10.1053/j.ajkd.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaipov A., Molnar M.Z., Potukuchi P.K. Acute kidney injury following coronary revascularization procedures in patients with advanced CKD. Nephrol Dial Transplant. 2019;34:1894–1901. doi: 10.1093/ndt/gfy178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sumida K., Diskin C.D., Molnar M.Z. Pre-end-stage renal disease hemoglobin variability predicts post-end-stage renal disease mortality in patients transitioning to dialysis. Am J Nephrol. 2017;46:397–407. doi: 10.1159/000484356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sumida K., Molnar M.Z., Potukuchi P.K. Pre-end-stage renal disease visit-to-visit systolic blood pressure variability and post-end-stage renal disease mortality in incident dialysis patients. J Hypertens. 2017;35:1816–1824. doi: 10.1097/HJH.0000000000001376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sumida K., Molnar M.Z., Potukuchi P.K. Blood pressure before initiation of maintenance dialysis and subsequent mortality. Am J Kidney Dis. 2017;70:207–217. doi: 10.1053/j.ajkd.2016.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Streja E., Kovesdy C.P., Soohoo M. Dialysis provider and outcomes among United States veterans who transition to dialysis. Clin J Am Soc Nephrol. 2018;13:1055–1062. doi: 10.2215/CJN.12951117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fitzmaurice G.M., Ravichandran C. A primer in longitudinal data analysis. Circulation. 2008;118:2005–2010. doi: 10.1161/CIRCULATIONAHA.107.714618. [DOI] [PubMed] [Google Scholar]
- 24.Singer J.D., Willett J.B. Oxford University Press; Oxford, UK: 2003. Applied Longitudinal Data Analysis, Modeling Change and Event Occurence. [Google Scholar]
- 25.Yang W., Israni R.K., Brunelli S.M. Hemoglobin variability and mortality in ESRD. J Am Soc Nephrol. 2007;18:3164–3170. doi: 10.1681/ASN.2007010058. [DOI] [PubMed] [Google Scholar]
- 26.Gosmanova E.O., Mikkelsen M.K., Molnar M.Z. Association of systolic blood pressure variability with mortality, coronary heart disease, stroke, and renal disease. J Am Coll Cardiol. 2016;68:1375–1386. doi: 10.1016/j.jacc.2016.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGinnis K.A., Brandt C.A., Skanderson M. Validating smoking data from the Veteran's Affairs Health Factors dataset, an electronic data source. Nicotine Tob Res. 2011;13:1233–1239. doi: 10.1093/ntr/ntr206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soohoo M., Moradi H., Obi Y. Statin therapy before transition to end-stage renal disease with posttransition outcomes. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.118.011869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deyo R.A., Cherkin D.C., Ciol M.A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 30.Kovesdy C.P., Norris K.C., Boulware L.E. Association of race with mortality and cardiovascular events in a large cohort of US Veterans. Circulation. 2015;132:1538–1548. doi: 10.1161/CIRCULATIONAHA.114.015124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kovesdy C.P., Alrifai A., Gosmanova E.O. Age and Outcomes Associated with BP in Patients with Incident CKD. Clin J Am Soc Nephrol. 2016;11(5):821–831. doi: 10.2215/CJN.08660815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwartz A.B. Potassium-related cardiac arrhythmias and their treatment. Angiology. 1978;29:194–205. doi: 10.1177/000331977802900302. [DOI] [PubMed] [Google Scholar]
- 34.Weiss J.N., Qu Z., Shivkumar K. Electrophysiology of hypokalemia and hyperkalemia. Circ Arrhythm Electrophysiol. 2017;10 doi: 10.1161/CIRCEP.116.004667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palmer B.F., Clegg D.J. Physiology and pathophysiology of potassium homeostasis: core curriculum 2019. Am J Kidney Dis. 2019;74:682–695. doi: 10.1053/j.ajkd.2019.03.427. [DOI] [PubMed] [Google Scholar]
- 36.Sica D.A., Struthers A.D., Cushman W.C. Importance of potassium in cardiovascular disease. J Clin Hypertens (Greenwich) 2002;4:198–206. doi: 10.1111/j.1524-6175.2002.01728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goyal A., Spertus J.A., Gosch K. Serum potassium levels and mortality in acute myocardial infarction. JAMA. 2012;307:157–164. doi: 10.1001/jama.2011.1967. [DOI] [PubMed] [Google Scholar]
- 38.Gosmanova E.O., Molnar M.Z., Naseer A. Longer predialysis ACEi/ARB utilization is associated with reduced postdialysis mortality. Am J Med. 2020;133:1065–1073.e3. doi: 10.1016/j.amjmed.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akbilgic O., Obi Y., Potukuchi P.K. Machine learning to identify dialysis patients at high death risk. Kidney Int Rep. 2019;4:1219–1229. doi: 10.1016/j.ekir.2019.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Obi Y., Nguyen D.V., Zhou H. Development and validation of prediction scores for early mortality at transition to dialysis. Mayo Clin Proc. 2018;93:1224–1235. doi: 10.1016/j.mayocp.2018.04.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.