Abstract
Background
Smell and taste loss are highly prevalent symptoms in coronavirus disease 2019 (COVID‐19), although few studies have employed objective measures to quantify these symptoms, especially dysgeusia. Reports of unrecognized anosmia in COVID‐19 patients suggests that self‐reported measures are insufficient for capturing patients with chemosensory dysfunction.
Objectives
The purpose of this study was to quantify the impact of recent COVID‐19 infection on chemosensory function and demonstrate the use of at‐home objective smell and taste testing in an at‐risk population of healthcare workers.
Methods
Two hundred and fifty healthcare workers were screened for possible loss of smell and taste using online surveys. Self‐administered smell and taste tests were mailed to respondents meeting criteria for elevated risk of infection, and one‐month follow‐up surveys were completed.
Results
Among subjects with prior SARS‐CoV‐2 infection, 73% reported symptoms of olfactory and/or gustatory dysfunction. Self‐reported smell and taste loss were both strong predictors of COVID‐19 positivity. Subjects with evidence of recent SARS‐CoV‐2 infection (<45 days) had significantly lower olfactory scores but equivalent gustatory scores compared to other subjects. There was a time‐dependent increase in smell scores but not in taste scores among subjects with prior infection and chemosensory symptoms. The overall infection rate was 4.4%, with 2.5% reported by PCR swab.
Conclusion
Healthcare workers with recent SARS‐CoV‐2 infection had reduced olfaction and normal gustation on self‐administered objective testing compared to those without infection. Rates of infection and chemosensory symptoms in our cohort of healthcare workers reflect those of the general public.
Keywords: Anosmia, Brief Smell Identification Test, Chemosensory dysfunction, COVID‐19, Dysgeusia, Gustation, Healthcare workers, Objective testing, Olfaction, Screening, University of Pennsylvania Smell Identification Test
INTRODUCTION
Acute olfactory and gustatory dysfunction are highly prevalent symptoms in patients infected with the novel coronavirus disease 2019 (COVID‐19). 1 , 2 , 3 , 4 , 5 , 6 , 7 Because early and otherwise asymptomatic transmission plays an important role in viral spread, identifying and characterizing early, isolated symptoms is critical for improving the screening and diagnosis of COVID‐19. Many studies have been conducted using subjective reports of acute chemosensory dysfunction, but the results of these studies may be affected by recall bias and sampling issues. Subjective measures are further complicated by the causal relationship between perceived smell and taste loss, as olfaction is a well‐established contributor to the sensory experience of “taste.” 8 , 9 While several COVID‐19 studies have employed objective, i.e. psychophysical, measures of smell function, few have similarly tested taste. 5 , 10 , 11 Of these studies, one found a deficit in taste when subjects reported whether self‐prepared solutions were sweet, sour, bitter or salty, 12 while two other studies using taste strips embedded with sweet, sour, salty, and bitter tastants did not find significant taste loss. 11 , 13 Such testing can also be used to identify individuals that are unaware of their chemosensory deficits. In two studies, Moein et al. 10 , 14 found olfactory deficits on psychophysical testing in 95%‐98% of COVID‐19 patients, while only 28% of these patients endorsed a subjective loss of smell.
More studies are needed to determine whether psychophysical smell and taste testing can identify subclinical anosmia and dysgeusia in at‐risk populations. Objective testing of both modalities may also help elucidate how the gustatory and olfactory systems are independently impacted by COVID‐19. To meet these objectives, we employed validated, self‐administered smell and taste tests to screen for chemosensory dysfunction in a population of healthcare workers at a tertiary academic medical center.
MATERIALS AND METHODS
Study design and subject recruitment
A cohort study screening for COVID‐19‐related smell and taste dysfunction of adult healthcare workers at our institution was conducted using web‐based questionnaires and self‐administered smell and taste tests, summarized in Figure 1. Participants completed a brief screening questionnaire detailing their demographic information, work environment, recent symptoms of loss of smell or taste, recent hallmark COVID‐19 symptoms (shortness of breath, cough, fever, sore throat, malaise), and prior SARS‐CoV‐2 testing and results. Participants were asked to indicate whether they had contact with someone known or suspected to have COVID‐19, in line with CDC screening criteria released at the time. Participants who believed they had been exposed and who had spent a portion of the prior two weeks in a patient‐facing clinical environment were selected to complete self‐administered smell and taste tests at home. Survey respondents who were currently pregnant, did not provide a mailing address, or provided invalid emails were excluded from the taste and smell testing. An end‐of‐study questionnaire was distributed one month following enrollment. Study data were collected and managed using research electronic data capture tools (REDCap) hosted at the University of Pennsylvania. 15 The protocol was approved by the Institutional Review Board of the University of Pennsylvania (IRB#: 842920) and the study was performed following the principles of the Declaration of Helsinki.
Figure 1.
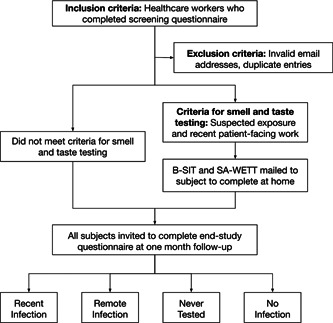
Study design.
Olfactory and gustatory tests
The Brief Smell Identification Test (B‐SIT) and the self‐administered version of the Waterless Empirical Taste Test (SA‐WETT®) were mailed, along with instructions for their self‐administration and scoring, to addresses provided by participants on the screening survey. 16 , 17
The B‐SIT is a 12‐odorant abbreviated version of the 40‐odorant University of Pennsylvania Smell Identification Test (UPSIT), which are both designed to be self‐administered. 18 The B‐SIT has been validated with a sensitivity of 63%, specificity 88%, and overall accuracy of 71% using a score of ≤ 8 when compared to the gold‐standard UPSIT. 19 This test uses microencapsulated (scratch‐and‐sniff) odorants that are released using the tip of a pencil. Four possibilities for the identity of the odorant are provided, and the subject is required to choose one of the four even if no smell is perceived. The stimuli are designed to be easily identified by most persons from a range of cultures. The total number of correct items serves as the test score.
The Brief Self‐administered Waterless Empirical Taste Test (SA‐WETT®) is comprised of 27 disposable plastic strips. 20 Each strip contains one of four concentration of either dried sucrose (0.20, 0.10, 0.05, 0.025 g/ml), citric acid (0.025, 0.05, 0.10, 0.20 g/ml), sodium chloride (0.0313, 0.0625, 0.125, 0.25 g/ml), caffeine (0.011, 0.022, 0.044, 0.088 g/ml), or monosodium glutamate (0.017, 0.034, 0.068, 0.135 g/ml). The strips are interspersed with blank strips designed to eliminate the need for water rinses. The subject is instructed to taste the strip and indicate whether a taste is perceived as sweet, sour, salty, bitter, or brothy (umami), or indicate if no taste is perceived. For both tests, participants self‐administered and scored their tests with the provided answer keys and reported the total number of correctly identified B‐SIT and SA‐WETT® items. The test‐retest reliability of the Brief Self‐Administered WETT® is 0.89; its split‐half reliability of 0.81). 20 These values are only slightly below those of the full 53‐item WETT® (respective r's = 0.92 and 0.88). 17
Evaluation of COVID‐19 status
On both the initial screening and end‐of‐study survey, subjects were asked to indicate whether they had undergone SARS‐CoV‐2 nasopharyngeal/oropharyngeal (NP/OP) PCR swab or anti‐SARS‐CoV‐2 serum antibody testing, and to provide the results of those tests. Subjects were classified into four groups: 1) "Never Tested" indicating they had never been tested by either method; 2) "No Infection" indicated by a negative swab and/or serum antibody test either prior to or during the course of the study; 3) "Recent Infection" indicated by a positive swab test <45 days prior to smell/taste testing, or a positive antibody test with symptoms <45 days prior to smell/taste testing; and 4) “Remote Infection” indicated by a positive swab ≥45 days prior to smell/taste testing, or positive antibody test with no symptoms or with symptom onset >45 days prior to smell/taste testing. The 45‐day cutoff was chosen based on prior literature reports indicating chemosensory symptoms last up to 40 days before resolution. 21 , 22
Statistical analysis
Statistical analysis and data visualization were performed using R Statistical Software. 23 , 24 Odds ratios (ORs) with 95% confidence intervals were computed via univariate logistic regression with COVID‐19 Status (Infection vs. No Infection as the dependent variables). One‐way analyses of variance (ANOVAs) were performed to determine whether mean B‐SIT and SA‐WETT® scores differed between the four COVID‐19 status groups. The Tukey post‐hoc test for multiple comparisons was performed on the one‐way ANOVAs to determine how test results differed between each individual group. A logarithmic regression curve was fit and R2 values computed for the relationship between B‐SIT/SA‐WETT® scores and the time since onset of chemosensory symptoms in subjects showing previous evidence of SARS‐CoV‐2 infection. Statistical significance was defined as P < 0.05.
RESULTS
There were 302 responses to the screening questionnaire, of which 22 entries were excluded for invalid email addresses or duplicate responses. At one‐month follow‐up, 250 subjects had completed the end‐study questionnaire (89% response rate) and were included in data analysis (Table 1). The mean times from enrollment to completion of the B‐SIT/SA‐WETT® and end‐study questionnaire were 25.8 and 34.1 days, respectively. The majority of respondents were women (81%). Many subjects reported spending time in the inpatient setting (38%), ambulatory setting (30%), and the ICU (19%). Subjects who spent their time in "other" settings were classified as patient‐facing (cardiac catheterization lab, home healthcare, thermal screening) and non‐patient facing (telehealth, administration, working from home). They constituted 6% and 16% of the study population, respectively. It should be noted that subjects were free to select multiple work environments. Self‐reported dysgeusia and anosmia/hyposmia were more prevalent (29 subjects; 11% and 7%, respectively) than other common COVID‐19 symptoms, and nearly half (48%) of subjects believed they may have been exposed, in any setting, to someone with suspected or confirmed COVID‐19.
Table 1.
Sample demographics.
| Item | Number | Percent(%) |
|---|---|---|
| Total | 250 | |
| Sex | ||
| Male | 45 | 18.0 |
| Female | 202 | 80.8 |
| Not specified | 3 | 1.2 |
| Clinical setting (may select multiple) | ||
| ER | 15 | 6.0 |
| Inpatient | 94 | 37.6 |
| ICU | 47 | 18.8 |
| OR | 14 | 5.6 |
| Ambulatory | 75 | 30.0 |
| COVID‐19 testing site | 10 | 4.0 |
| Other: patient‐facing | 16 | 6.4 |
| Other: not patient‐facing | 39 | 15.6 |
| Self‐reported symptoms | ||
| Chemosensory dysfunction | 29 | 11.6 |
| Anosmia/Hyposmia | 18 | 7.2 |
| Dysgeusia | 27 | 10.8 |
| Cough | 9 | 3.6 |
| Fever | 2 | 0.8 |
| Shortness of breath | 6 | 2.4 |
| Malaise | 8 | 3.2 |
| Sore throat | 7 | 2.8 |
| Allergic rhinitis | 73 | 29.2 |
| Chronic rhinosinusitis | 6 | 2.4 |
| Nasal congestion | 58 | 23.2 |
| Suspected exposure | 119 | 47.6 |
| COVID‐19 status | ||
| Recent infection (≤45 days) | 5 | 2.0 |
| Remote infection (>45 days) | 6 | 2.4 |
| No infection | 85 | 34.0 |
| Never tested | 154 | 61.6 |
The mean age was 40.3 (18‐72) years old, 3 missing ages.
Overall, a minority of subjects reported evidence of SARS‐CoV‐2 infection (11/250, 4.4%, median age 40.6, range 24‐68), 7 of whom tested positive by NP or OP swab, and 4 by positive serum antibody test. There were 6 subjects who had tested positive for the virus prior to enrollment in the study, and 5 (4 antibody, 1 swab) who received positive test results during the course of the study. A larger proportion tested negative via swab or antibody test (34%), and the majority (61%) never sought any COVID‐19 testing. Recent‐onset chemosensory dysfunction was reported in 73% (8/11), 4.7% (4/86), 7.8% (12/154) of subjects with evidence of prior infection, no prior infection, and no testing, respectively. In the cohort of subjects who had received COVID‐19 testing, self‐reported recent‐onset loss of taste (OR = 54.7) and smell (OR = 32.0) were strongly associated with COVID‐19 positivity (Table 2).
Table 2.
Univariate analysis of variables associated with COVID‐19 positivity.
| Odds ratio | 95% CI | P‐value | |
|---|---|---|---|
| Sudden onset dysgeusia | 54.67 | 10.36‐288.58 | <0.001* |
| Sudden onset anosmia/hyposmia | 32.00 | 6.12‐167.36 | <0.001* |
| Fever, cough, SOB, sore throat, or malaise | 8.13 | 2.10‐32.69 | 0.003* |
| Nasal congestion | 2.16 | 0.57‐8.19 | 0.26 |
| Sex, female | 5.88 | 0.33‐104.65 | 0.23 |
| Age ≥ 40 | 2.32 | 0.63‐8.51 | 0.21 |
| Hospital setting | 15.11 | 0.86‐264.94 | 0.06 |
| Elevated risk of infection | 10.48 | 1.28‐85.44 | 0.03* |
P < 0.05.
There were 128 subjects who worked in a patient‐facing setting and reported a potential exposure, meeting criteria for B‐SIT/SA‐WETT® testing. These criteria captured 10 of the 11 subjects with evidence of prior infection, including all 8 subjects who reported olfactory and/or gustatory dysfunction. Figure 2 displays the scores of the smell and taste tests as a function of time since the onset of chemosensory dysfunction. Figure 2A (B‐SIT, P = 0.02, R2 = 0.61) demonstrates a time‐dependent increase in olfactory testing scores, while Figure 2B (SA‐WETT®, P = 0.68, R2 = 0.04) shows no relationship between time and gustatory testing scores. Of subjects whose scores are plotted, 6 reported a subjective loss of smell (3 reduced, 3 absent) and all 8 reported a subjective loss of taste (5 reduced, 3 absent) prior to completing the B‐SIT/SA‐WETT®.
Figure 2.
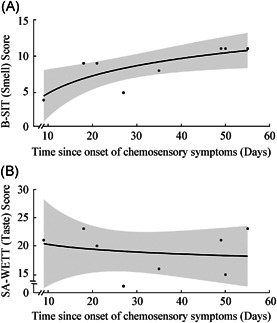
B‐SIT scores (A) and SA‐WETT® scores (B) compared to onset of chemosensory dysfunction in patients with swab‐confirmed SARS‐CoV‐2 infection or positive antibody test with COVID‐19 symptoms. Logarithmic regression model with 95% confidence interval is plotted, with (A) P = 0.02 and R2 = 0.61, and (B) P = 0.68 and R2 = 0.04.
The mean B‐SIT scores were significantly different when compared according to COVID‐19 status [F(3, 104)=14.73, P < 0.001, η2 = 0.30]. As shown in Figure 3A, the mean B‐SIT score of the Recent Infection group (7.00, 95% CI: 4.09‐9.91) was significantly lower than those of the Remote Infection (11.40, CI: 10.72‐12.08, P < 0.001), No Infection (10.92, CI: 10.46‐11.39, P < 0.001), and Never Tested (10.86, CI: 10.57‐11.16, P < 0.001) groups, which did not differ significantly from one another (all P > 0.05). Conversely, the mean total SA‐WETT® scores were not significantly influenced by COVID‐19 status [Figure 3B; F(3, 104) = 0.23, P = 0.85, η2 = 0.01]. Scores for the individual taste modalities of the SA‐WETT® did not differ significantly by COVID‐19 status (P value ranging from 0.08 to 0.95).
Figure 3.
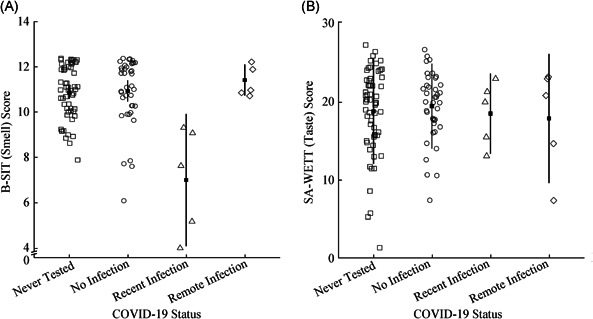
B‐SIT scores (A) and SA‐WETT® scores (B) by COVID‐19 status. One‐way ANOVA demonstrated significance for B‐SIT scores (P < 0.001) but not for SA‐WETT® scores (P = 0.85). Key: “No Infection” = negative swab or antibody test before or during study; “Recent Infection” = positive swab <45 days prior to smell/taste testing, or positive antibody test with symptoms <45 days prior to smell/taste testing; “Remote Infection” = positive swab >45 days prior to smell/taste testing, or positive antibody test with no symptoms or symptoms >45 days prior to smell/taste testing. Error bars represent 95% confidence intervals.
DISCUSSION
This study represents one of the first efforts to screen for COVID‐19‐related olfactory and gustatory dysfunction in healthcare workers, a population that may be at an increased risk of infection. We present a novel approach for the screening of chemosensory symptoms in at‐risk individuals using self‐administered smell and taste tests, which offer the benefit of reducing potential exposures. Overall, we found that 73% of subjects with prior SARS‐CoV‐2 infection reported experiencing acute olfactory and/or gustatory dysfunction, compared to 4.7% of those who tested negative for the virus and 7.8%, of those who were never tested. There was a clear deficit in olfaction among subjects with recent evidence of infection with SARS‐CoV‐2. While these subjects performed significantly worse on smell testing, their performance on all modalities of taste testing was equivalent to subjects in other groups. Many studies have reported that olfaction and gustation return, often to baseline, in patients with the virus, although the time to recovery is highly variable. 1 , 21 , 25 , 26 Similarly, we observed a time‐related increase in smell test scores among subjects with prior infection. There was no relationship between time and taste scores within the same cohort, despite all subjects reporting a subjective impairment in taste. Our findings are consistent with the recent study by Hintschich et al. 13 of COVID‐19 subjects that found an association between self‐reported smell dysfunction and psychophysical‐based deficits, an association not observed for taste.
Our findings suggest that damage to the olfactory system is a primary contributor to reports of taste loss in COVID‐19, as opposed to direct viral damage to taste receptors. If true taste deficits are, in fact, present, the mechanism by which SARS‐CoV‐2 causes such impairment is unknown. The angiotensin‐converting‐enzyme 2 (ACE2) receptor, which facilitates SARS‐CoV‐2 invasion, is expressed in the both taste buds and the olfactory neuroepithelium. 27 , 28 An inflammatory process involving cytokine release following ACE2 receptor binding has been proposed as well. 29 Given our finding that olfaction, not gustation, seems to be more impaired by COVID‐19, it seems plausible that reports of taste loss in this population are due to loss of flavor sensations secondary to olfactory system damage, in accord with the findings of Hintschich et al. 13
Notable studies by Vaira et al. 12 and Petrocelli et al. 30 employing at‐home solution‐based testing found results that contrast with ours, namely deficits in smell and taste function in some COVID‐19 patients. Importantly, Vaira et al. 31 validated their model for self‐administered testing in comparison to operator‐administered testing in a clinical environment, finding no statistically significant differences in results. We believe that the taste strip and microencapsulated delivery mechanisms used in our study and the study by Hintschich et al. 13 are equivalent or superior methodologies for evaluating chemosensory function at home, as subjects are not required to prepare solutions and are unaware of the identity of the odorant or tastant until after the test has been completed. 17 Compared to solution‐based testing, taste strip testing may also better isolate gustatory dysfunction from the influence of impaired retronasal olfaction.
Many studies exist that demonstrate the predictive value of self‐reported chemosensory symptoms and argue for its inclusion in screening criteria. 1 , 2 , 5 However, subjective self‐assessment has not been established as a reliable method for quantifying chemosensory dysfunction, largely due to the interactions between taste, smell, and chemesthesis, and the inherent variability in chemosensory perception between individuals. 32 , 33 , 34 While some studies have characterized the length and severity of smell and taste symptoms in COVID‐19 patients, there is a growing recognition in the literature of the need for psychophysical gustatory testing in this population. 3 , 5 , 7 , 10 , 22 , 35 , 36 , 37 , 38 One goal of our study was to identify subjects with subclinical chemosensory dysfunction who do not have obvious symptoms of COVID‐19. However, the low incidence of infection in our sample precluded us from such identification. Further research in a larger cohort of at‐risk persons will be needed to better demonstrate the effectiveness of olfactory and gustatory testing in COVID‐19 screening.
In the early stages of the pandemic, shortages of personal protective equipment, limited testing capacity, and lack of understanding of the virus raised concerns among healthcare workers treating COVID‐19 patients. 39 , 40 Our study revealed a perceived infection rate of 4.4%, with a 2.5% rate reported by PCR swab testing. Of subjects who received COVID‐19 testing, the positive test rate was 11.5%. As of June 27th, near the closure of our study, the overall infection rate reported by PCR swab among all residents aged 20‐74 in our home institution's county was 1.9%. Since our study did not require COVID‐19 testing of our subjects, the actual infection rate may be higher than that which we found. However, the same phenomenon exists in the general community, where the actual infection rate is likely underestimated since many asymptomatic individuals may not have sought testing. Our results therefore suggest that, in our health care population, the risk of infection is similar to that of the general public.
Overall, our data demonstrate a high rate of chemosensory dysfunction in healthcare workers, with 11.6% of the cohort reporting smell and/or taste alterations at any point during the study. This is comparable to the general population, where the prevalence of self‐reported smell loss is between 1.4% and 15.3%, most commonly due to upper respiratory infection, sinus disease, aging, and idiopathic causes. 9 , 41 , 42 Recent‐onset chemosensory symptoms were more predictive of COVID‐19 positivity than other hallmark COVID‐19 symptoms in our study population. Our data are consistent with the findings of Lan et al., 43 who found that self‐reported anosmia and ageusia were the strongest independent predictors of SARS‐CoV‐2 infection in healthcare workers, followed by fever and myalgias.
There are a few limitations to be considered in this study. A small sample size of remotely and recently infected subjects contributed to variability in B‐SIT and SA‐WETT® scores and lessens our ability to generalize findings to all healthcare workers. Furthermore, a large proportion (61%) of our cohort never sought testing for the virus, even though 12 of these non‐tested subjects reported recent‐onset smell or taste dysfunction. As a crowd‐sourced screening study, we did not require COVID‐19 testing. Such testing may have helped increase the number of recently infected subjects and clarify the etiology of the chemosensory symptoms in the non‐tested group. Due to the sampling methodology, men were underrepresented in our sample. In the general population, women tend to outperform men on both SA‐WETT® and UPSIT, although the effect sizes are relatively weak. 17 , 44 Performance on psychophysical tests is also impacted by differences in baseline chemosensory function between subjects, and additional studies employing psychophysical tests at multiple timepoints may reduce variability and further characterize anosmia and dysgeusia in COVID‐19.
CONCLUSION
Screening for recent loss of smell and taste plays an important role in preventing early transmission of SARS‐CoV‐2. While there is an increasing number of subjective reports in the literature, few have used psychophysical tools to characterize acute chemosensory dysfunction in COVID‐19. Our study demonstrates that healthcare workers with recent infection had reduced olfaction and normal gustation on psychophysical testing compared to those without infection. While there have been concerns for patient‐to‐provider transmission in the hospital setting, our data showed an infection rate and prevalence of chemosensory symptoms that is comparable to the general population. As we continue to understand the presentation and pathogenesis of COVID‐19, earlier treatment and self‐isolation will help fight against the rapid spread of this pandemic.
AUTHOR CONTRIBUTIONS
Austin Cao and Zachary Nimmo contributed equally as co‐first authors. Cao, Nimmo, Mirza, and Doty had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: All authors.
Acquisition, analysis, and interpretation of the data: Cao, Nimmo, Mirza, Doty.
Drafting of the manuscript: Cao, Nimmo, Mirza, Doty.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Cao, Nimmo, Brody.
Administrative, technical, or material support: Mirza, Cohen, Brody, Doty.
Supervision: Mirza, Doty.
FINANCIAL DISCLOSURES
None.
CONFLICT OF INTEREST DISCLOSURES
Dr. Richard Doty is a consultant to Eisai Co, Ltd, Merck Pharmaceuticals, the Michael J. Fox Foundation for Parkinson's Research, Septodont, Inc., and Johnson & Johnson. He receives royalties from Cambridge University Press, Johns Hopkins University Press, and John Wiley & Sons, Inc. He is president of, and a major shareholder in, Sensonics International, a manufacturer and distributor of smell and taste tests, including the tests used in this study. No other authors have disclosures to report.
Cao AC, Nimmo ZM, Mirza N, Cohen NA, Brody RM, Doty RL. Objective screening for olfactory and gustatory dysfunction during the COVID‐19 pandemic: a prospective study in healthcare workers using self‐administered testing. World J Otorhinolaryngol Head Neck Surg. 2022;8:249‐256. 10.1016/j.wjorl.2021.02.001
REFERENCES
- 1. Yan CH, Faraji F, Prajapati DP, Boone CE, DeConde AS. Association of chemosensory dysfunction and COVID‐19 in patients presenting with influenza‐like symptoms. Int Forum Allergy Rhinol. 2020;10:806‐813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tong JY, Wong A, Zhu D, Fastenberg JH, Tham T. The prevalence of olfactory and gustatory dysfunction in COVID‐19 patients: a systematic review and meta‐analysis. Otolaryngol Head Neck Surg. 2020;163:3‐11. [DOI] [PubMed] [Google Scholar]
- 3. Borsetto D, Hopkins C, Philips V, et al. Self‐reported alteration of sense of smell or taste in patients with COVID‐19: a systematic review and meta‐analysis on 3563 patients. Rhinology. 2020;58:430‐436. [DOI] [PubMed] [Google Scholar]
- 4. Spinato G, Fabbris C, Polesel J, et al. Alterations in smell or taste in mildly symptomatic outpatients with SARS‐CoV‐2 infection. JAMA. 2020;323:2089‐2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lechien JR, Chiesa‐Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild‐to‐moderate forms of the coronavirus disease (COVID‐19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277:2251‐2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Agyeman AA, Chin KL, Landersdorfer CB, Liew D, Ofori‐Asenso R. Smell and taste dysfunction in patients with COVID‐19: a systematic review and meta‐analysis. Mayo Clin Proc. 2020;95:1621‐1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hannum ME, Ramirez VA, Lipson SJ, et al. Objective sensory testing methods reveal a higher prevalence of olfactory loss in COVID‐19‐positive patients compared to subjective methods: a systematic review and meta‐analysis. Chem Senses. 2020;45:865‐874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Burdach KJ, Doty RL. The effects of mouth movements, swallowing, and spitting on retronasal odor perception. Physiol Behav. 1987;41:353‐356. [DOI] [PubMed] [Google Scholar]
- 9. Deems DA, Doty RL, Settle RG, et al. Smell and taste disorders, a study of 750 patients from the University of Pennsylvania Smell and Taste Center. Arch Otolaryngol Head Neck Surg. 1991;117:519‐528. [DOI] [PubMed] [Google Scholar]
- 10. Moein ST, Hashemian SM, Mansourafshar B, Khorram‐Tousi A, Tabarsi P, Doty RL. Smell dysfunction: a biomarker for COVID‐19. Int Forum Allergy Rhinol. 2020;10:944‐950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bocksberger S, Wagner W, Hummel T, et al. Temporary hyposmia in COVID‐19 patients. HNO. 2020;68:440‐443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vaira LA, Hopkins C, Salzano G, et al. Olfactory and gustatory function impairment in COVID‐19 patients: Italian objective multicenter‐study. Head Neck. 2020;42:1560‐1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hintschich CA, Wenzel JJ, Hummel T, et al. Psychophysical tests reveal impaired olfaction but preserved gustation in COVID‐19 patients. Int Forum Allergy Rhinol. 2020;10:1105‐1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moein ST, Hashemian SM, Tabarsi P, Doty RL. Prevalence and reversibility of smell dysfunction measured psychophysically in a cohort of COVID‐19 patients. Int Forum Allergy Rhinol. 2020;10:1127‐1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)‐‐a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Doty RL, Marcus A, Lee WW. Development of the 12‐item Cross‐Cultural Smell Identification Test (CC‐SIT). Laryngoscope. 1996;106:353‐356. [DOI] [PubMed] [Google Scholar]
- 17. Doty RL, Wylie C, Potter M. Validation of the Waterless Empirical Taste Test (WETT®). Behav Res Methods. Published online September 3 2020. [DOI] [PubMed] [Google Scholar]
- 18. Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav. 1984;32:489‐502. [DOI] [PubMed] [Google Scholar]
- 19. El Rassi E, Mace JC, Steele TO, et al. Sensitivity analysis and diagnostic accuracy of the Brief Smell Identification Test in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol. 2016;6:287‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Doty R. Self‐Administered Waterless Empirical Taste Test (SA‐WETT) Administration Manual. Sensonics International; 2020. [Google Scholar]
- 21. Lee Y, Min P, Lee S, Kim SW. Prevalence and duration of acute loss of smell or taste in COVID‐19 patients. J Korean Med Sci. 2020;35:e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chary E, Carsuzaa F, Trijolet J‐P, et al. Prevalence and recovery from olfactory and gustatory dysfunctions in Covid‐19 infection: a prospective multicenter study. Am J Rhinol Allergy. 2020;34:686‐693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Team RC . R: A language and environment for statistical computing. Vienna, Austria; 2013.
- 24. Wickham H. Ggplot2: elegant graphics for data analysis. Springer; 2016. [Google Scholar]
- 25. Boscolo‐Rizzo P, Borsetto D, Fabbris C, et al. Evolution of altered sense of smell or taste in patients with mildly symptomatic COVID‐19. JAMA Otolaryngol Neck Surg. 2020;146:729‐732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vaira LA, Hopkins C, Petrocelli M, et al. Smell and taste recovery in coronavirus disease 2019 patients: a 60‐day objective and prospective study. J Laryngol Otol. 2020;134:703‐709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019‐nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lozada‐Nur F, Chainani‐Wu N, Fortuna G, Sroussi H. Dysgeusia in COVID‐19: possible mechanisms and implications. Oral Surg Oral Med Oral Pathol Oral Radiol. 2020;130:344‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Petrocelli M, Ruggiero F, Baietti AM, et al. Remote psychophysical evaluation of olfactory and gustatory functions in early‐stage coronavirus disease 2019 patients: the Bologna experience of 300 cases. J Laryngol Otol. 2020;134:571‐576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vaira LA, Salzano G, Petrocelli M, Deiana G, Salzano FA, De Riu G. Validation of a self‐administered olfactory and gustatory test for the remotely evaluation of COVID‐19 patients in home quarantine. Head Neck. 2020;42:1570‐1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hummel T, Whitcroft KL, Andrews P, et al. Position paper on olfactory dysfunction. Rhinol J. 2017;54:1‐30. [DOI] [PubMed] [Google Scholar]
- 33. Whitcroft KL, Hummel T. Clinical diagnosis and current management strategies for olfactory dysfunction: a review. JAMA Otolaryngol Neck Surg. 2019;145:846‐853. [DOI] [PubMed] [Google Scholar]
- 34. Parma V, Ohla K, Veldhuizen MG, et al. More than smell‐COVID‐19 is associated with severe impairment of smell, taste, and chemesthesis. Chem Senses. 2020;45:609‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Adamczyk K, Herman M, Fraczek J, et al. Sensitivity and specifity of prediction models based on gustatory disorders in diagnosing COVID‐19 patients: a case‐control study. medRxiv. Published online June 3, 2020:2020.05.31.20118380.
- 36. Han AY, Mukdad L, Long JL, Lopez IA. Anosmia in COVID‐19: mechanisms and significance. Chem Senses. 2020;45:423‐428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ottaviano G, Carecchio M, Scarpa B, Marchese‐Ragona R. Olfactory and rhinological evaluations in SARS‐CoV‐2 patients complaining of olfactory loss. Rhinol. 2020;58:400‐401. [DOI] [PubMed] [Google Scholar]
- 38. Renaud M, Leon A, Trau G, et al. Acute smell and taste loss in outpatients: all infected with SARS‐CoV‐2? Rhinol. 2020;58:406‐409. [DOI] [PubMed] [Google Scholar]
- 39. Wang J, Zhou M, Liu F. Reasons for healthcare workers becoming infected with novel coronavirus disease 2019 (COVID‐19) in China. J Hosp Infect. 2020;105:100‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lechner M, Chandrasekharan D, Jumani K, et al. Anosmia as a presenting symptom of SARS‐CoV‐2 infection in healthcare workers – A systematic review of the literature, case series, and recommendations for clinical assessment and management. Rhinol. 2020;58:394‐399. [DOI] [PubMed] [Google Scholar]
- 41. Yang J, Pinto JM. The epidemiology of olfactory disorders. Curr Otorhinolaryngol Rep. 2016;4:130‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Desiato VM, Levy DA, Byun YJ, Nguyen SA, Soler ZM, Schlosser RJ. The prevalence of olfactory dysfunction in the general population: a systematic review and meta‐analysis. Am J Rhinol Allergy. 2020:1945892420946254. [DOI] [PubMed] [Google Scholar]
- 43. Lan FY, Filler R, Mathew S, et al. COVID‐19 symptoms predictive of healthcare workers' SARS‐CoV‐2 PCR results. PLoS One. 2020;15:e0235460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sorokowski P, Karwowski M, Misiak M, et al. Sex differences in human olfaction: a meta‐analysis. Front Psychol. 2019;10:242. [DOI] [PMC free article] [PubMed] [Google Scholar]


