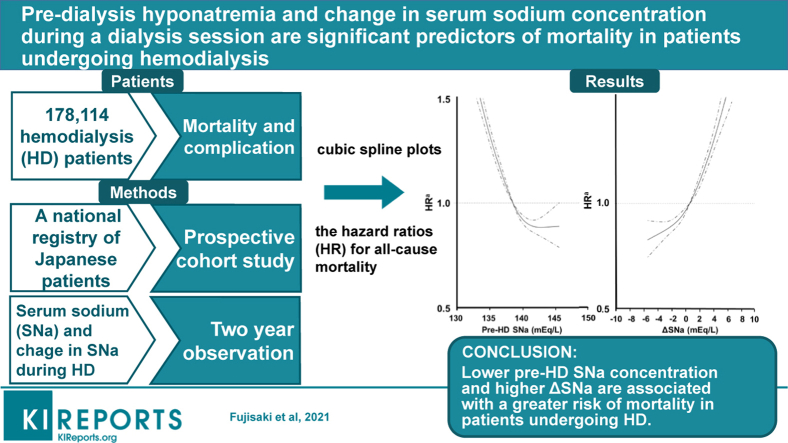
Abstract
Background
Previous studies have shown that hyponatremia is associated with greater mortality in hemodialysis (HD) patients. However, there have been few reports regarding the importance of the change in serum sodium (SNa) concentration (ΔSNa) during dialysis sessions. To investigate the relationships of pre-dialysis hyponatremia and ΔSNa during a dialysis session with mortality, we analyzed data from a national registry of Japanese patients with end-stage kidney disease.
Methods
We identified 178,114 patients in the database who were undergoing HD 3 times weekly. The study outcome was 2-year all-cause mortality, and the baseline SNa concentrations were categorized into quintiles. We evaluated the relationships of SNa concentration and ΔSNa with mortality using Cox proportional hazards models.
Results
During a 2-year follow-up period, 25,928 patients died. Each 1-mEq/l reduction in pre-HD SNa concentration was associated with a cumulatively greater risk of all-cause mortality (hazard ratio [HR], 1.05; 95% confidence interval [CI], 1.05–1.06). In contrast, a larger ΔSNa was associated with higher all-cause mortality (HR for a 1-mEq/l increase in ΔSNa, 1.02; 95% CI 1.01–1.02). The combination of low pre-HD SNa concentration and large ΔSNa was also associated with higher mortality (HR 1.09; 95% CI 1.05–1.13). Participants with the lowest SNa concentration (≤136 mEq/L) and the highest ΔSNa (>4 mEq/L) showed higher mortality than those with an intermediate pre-HD SNa concentration (137–140 mEq/L) and the lowest ΔSNa (≤2 mEq/L).
Conclusions
Lower pre-HD SNa concentration and higher ΔSNa are associated with a greater risk of mortality in patients undergoing HD.
Keywords: hemodialysis, hyponatremia, mortality, prospective cohort
Graphical abstract
See Commentary on Page 248
SNa plays an important role in the maintenance of the osmotic equilibrium in the extracellular space and determines the distribution of water between the intracellular and extracellular compartments. Previous studies have shown that HD patients with hyponatremia have a higher mortality rate,1, 2, 3, 4, 5 and indeed hyponatremia is a relatively common electrolyte disorder in dialysis patients. Recently, several studies have shown that low SNa concentration in HD patients is an important factor in their survival,6,7 and consistent associations have been shown between low baseline SNa and higher mortality. In contrast, Rhee et al.7 have reported a U-shaped relationship between pre-dialysis SNa and all-cause mortality in HD patients. However, low SNa before HD may be the result of the interplay of a number of risk factors for dysnatremia, including substantial inter-dialytic weight gain, poor nutritional status and low food intake, and comorbidities predisposing to excess thirst, that are also individually associated with a greater risk of mortality. However, hyponatremia may have a direct effect to increase mortality.2 Although the brain seems to be the main target for hyponatremia, especially in acute hyponatremia, other organs, for example the lungs and bones, also may be affected by hyponatremia.8 Furthermore, it has been reported that patients with both pneumonia and hyponatremia are more likely to die.9
However, few studies have characterized the change in SNa concentration (ΔSNa: post-HD SNa − pre-HD SNa) during an HD session. Although Hecking et al.6 reported an association between predialysis SNa and dialysate sodium concentration, ΔSNa was not calculated. Therefore, we aimed to obtain pre- and post-HD SNa concentrations from a national registry of patients with end-stage kidney disease in Japan, and to determine the relationships of SNa and ΔSNa during HD with mortality.
Methods
Database and Patient Selection
The data were extracted from the Japanese Society for Dialysis Therapy Renal Data Registry, which has previously been described in detail.10 Briefly, the Japanese Society for Dialysis Therapy has conducted annual questionnaire-based surveys of dialysis facilities throughout Japan since 1968, with questionnaires being filled out by medical staff in each facility. Because the response rate has exceeded 95% every year, the database covers nearly all the patients undergoing dialysis in Japan. Because SNa concentration data were only collected during 2008, the datasets for 2008, 2009, and 2010 were combined and analyzed in the present study. We included patients who (i) were aged ≥18 years, (ii) had undergone in-center hemodialysis 3 times a week, and (iii) had SNa data recorded pre- and post-HD. The study protocol was approved by the Ethics Committee of the Japanese Society for Dialysis Therapy (JSDT approval number 20).
Baseline Covariates
Data were collected regarding demographics (age, sex, body mass index), the primary kidney disease (diabetic or nondiabetic), the duration of HD, the number of hours of HD per session, and the pre- and post-HD body weight. In addition, predialysis albumin, urea nitrogen, pre-and post-SNa, calcium, phosphate, total cholesterol, C-reactive protein, and hemoglobin were measured. Furthermore, normalized protein catabolic rate (nPCR), a marker of dialysis adequacy (Kt/V), was calculated, the presence of cardiovascular disease (myocardial infarction, cerebral infarction, cerebral hemorrhage, or amputation of an extremity) was recorded, and the dialysate sodium concentration was measured. Dialysis doses were measured using the single-pool Kt/V method and nPCR was calculated using Shinzato’s formula.11
Study Outcomes
The main outcome measure was time to all-cause mortality during the 2-year observation period. As a secondary outcome, we also evaluated the time to the development of cardiovascular disease, defined as the onset of myocardial infarction, cerebral infarction, or cerebral hemorrhage, or amputation of an extremity.
Statistical Analysis
Data are presented as numbers (percentages) for categorical variables, and as means (SDs) for continuous variables with a normal distribution or medians (interquartile ranges) for those with a skewed distribution. The distributions of baseline characteristics, stratified according to SNa quintile, were compared using trend analysis. We calculated the change in SNa concentration (ΔSNa: post-HD SNa − pre-HD SNa) during an HD session.
Cox proportional hazards models were constructed to calculate the HR and 95% CI for all-cause, cardiovascular-related, infectious, and other mortality, in which the lowest quintiles were used as the reference groups.
We constructed 3 multivariate models: model 1, which was adjusted for age, sex, duration of HD, and number of hours of HD treatment per session; model 2, which was adjusted for the variables adjusted for in model 1, plus the hemoglobin, calcium, phosphate, and total cholesterol concentrations, ΔBW% ([pre-HD body weight − post-HD body weight] / pre-HD body weight × 100), ΔSNa, and dialysate concentration; and model 3, which was adjusted for the variables adjusted for in model 2, plus the concentrations of total cholesterol, albumin, and C-reactive protein, nPCR, and a past history of myocardial infarction, brain infarction, brain hemorrhage, amputation of an extremity, or hip fracture. We also evaluated the possibility of a continuous, nonlinear relationship between the SNa concentration and mortality using a fully adjusted, restricted cubic spline (RCS) model with 4 knots. We also examined the association of SNa concentration and ΔSNa with the onset of cerebral infarction and lower limb amputation, using a logistic regression model. Logistic regression models were constructed to calculate the odds ratio and 95% CI for cerebral infarction and lower limb amputation.
All the reported P values are 2-sided and P < 0.05 was considered to represent statistical significance. Statistical analysis was performed using JMP Statistics version 11.0 (SAS Institute, Tokyo, Japan) and the SAS software package version 9.2 (SAS Institute, Cary, NC).
Results
Study Participants
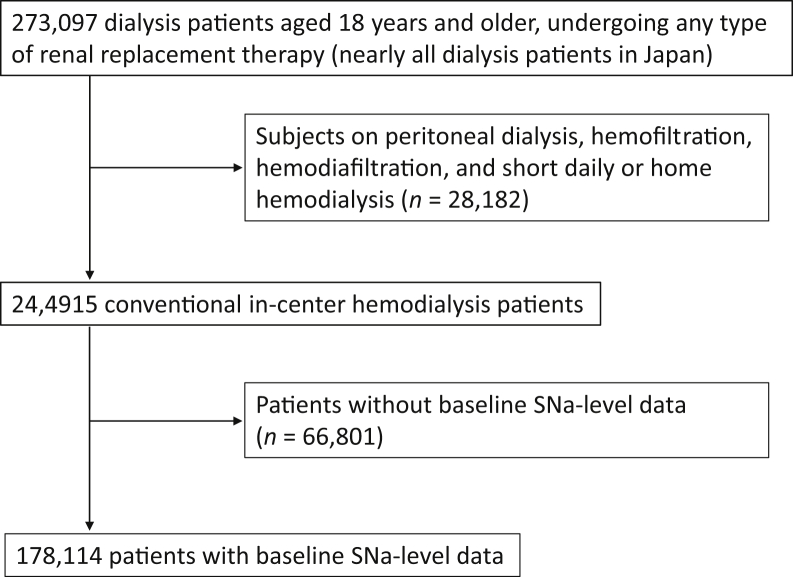
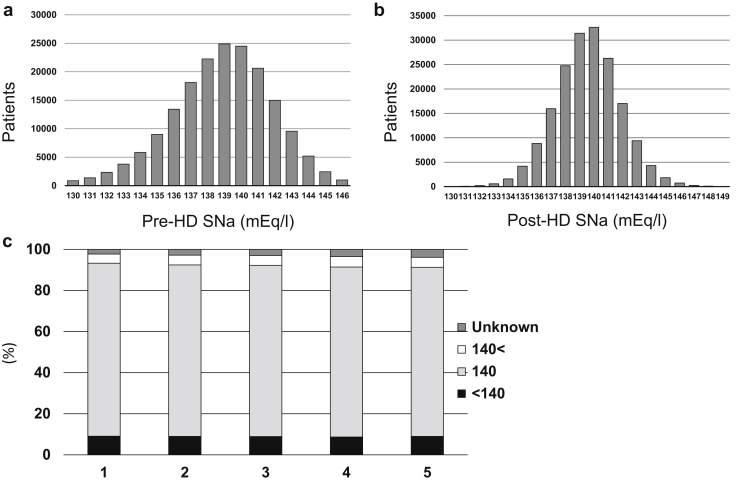
The study enrollment process is summarized in Figure 1. Of the total of 273,097 dialysis patients, those on renal replacement therapy other than HD conducted in a facility (n = 28,182) were excluded. Of the remaining 244,915 HD patients, baseline pre- and post-HD SNa data were available for 178,114 (66,801 were excluded.). The mean (SD) pre- and post-HD SNa concentrations of the participants were 139.0 (2.9) mEq/l and 139.6 (2.1) mEq/l (Figure 2a and b). The baseline characteristics of the participants according to quintiles of SNa concentration are summarized in Table 1. Lower SNa concentration was significantly associated with older age, lower albumin, calcium, phosphate, nPCR, and hemoglobin concentrations, higher C-reactive protein, prior history of cardiovascular disease, amputation of an extremity, and hip fracture.
Figure 1.
Patient selection flow chart. SNa, serum sodium concentration.
Figure 2.
Distribution of baseline serum sodium concentrations (SNa). (a) Serum sodium concentration before a hemodialysis (HD) session (pre-HD SNa). (b) Serum sodium concentration after an HD session (post-HD SNa). (c) Relationship between dialysate sodium concentration and pre-dialysis serum sodium concentration. Distribution of dialysate sodium concentration according to the mean predialysis serum sodium (Pre-HD SNa) category. Categories: 1, SNa ≤ 136 mEq/l; 2, 136 < SNa ≤ 138 mEq/l; 3, 138 < SNa ≤ 140 mEq/l; 4, 140 < SNa ≤ 141 mEq/l; 5, 141 mEq/l < SNa.
Table 1.
Baseline characteristics of the participants
| Characteristics | Total n = 178,114 | 1 SNa≤136 n = 34,588 | 2 136<SNa≤138 n = 39,968 |
3 138<SNa≤140 n = 49,076 |
4 140<SNa≤141 n = 20,537 |
5 141<SNa n = 33,945 |
P |
|---|---|---|---|---|---|---|---|
| Male/female | |||||||
| Age (years) | 65.2 ± 12.5 | 66.51 ± 12.76 | 65.04 ± 12.66 | 64.78 ± 12.42 | 64.93 ± 12.24 | 65.04 ± 12.03 | <0.001 |
| HD vintage (years) | 7.2 ± 7.2 | 6.42 ± 6.43 | 7.04 ± 6.90 | 7.30 ± 7.23 | 7.60 ± 7.45 | 7.99 ± 7.79 | <0.001 |
| HD time (min) | 235.7 ± 31.6 | 234.89 ± 32.85 | 236.57 ± 31.88 | 236.06 ± 30.48 | 235.72 ± 31.04 | 235.23 ± 30.52 | 0.299 |
| Pre-HD Hb (g/dl) | 10.4 ± 1.3 | 10.39 ± 1.37 | 10.45 ± 1.28 | 10.40 ± 1.24 | 10.37 ± 1.22 | 10.30 ± 1.23 | <0.001 |
| Pre-HD Alb (g/dl) | 3.7 ± 0.4 | 3.59 ± 0.48 | 3.69 ± 0.43 | 3.72 ± 0.41 | 3.73 ± 0.41 | 3.72 ± 0.42 | <0.001 |
| Pre-HD Ca (mg/dl) | 9.0 ± 0.8 | 8.91 ± 0.86 | 8.98 ± 0.83 | 8.97 ± 0.82 | 8.98 ± 0.81 | 8.96 ± 0.81 | <0.001 |
| Pre-HD P (mg/dl) | 5.3 ± 1.4 | 5.22 ± 1.57 | 5.33 ± 1.47 | 5.32 ± 1.41 | 5.29 ± 1.38 | 5.25 ± 1.38 | 0.0082 |
| Pre-HD T-Chol (mg/dl) | 153.2 ± 37.0 | 150.86 ± 37.69 | 152.91 ± 37.14 | 153.80 ± 36.74 | 154.57 ± 36.70 | 154.41 ± 36.15 | <0.001 |
| Pre-HD CRP (mg/dl) | 0.5 ± 1.6 | 0.84 ± 2.20 | 0.56 ± 1.64 | 0.47 ± 1.30 | 0.43 ± 1.35 | 0.40 ± 1.18 | <0.001 |
| Kt/V (single pool) | 1.4 ± 0.3 | 1.38 ± 0.31 | 1.39 ± 0.30 | 1.39 ± 0.30 | 1.39 ± 0.31 | 1.39 ± 0.30 | <0.001 |
| nPCR | 0.87 ± 0.18 | 0.88 ± 0.19 | 0.88 ± 0.18 | 0.88 ± 0.18 | 0.87 ± 0.18 | 0.86 ± 0.18 | <0.001 |
| ΔBW% | 4.18 ± 11.07 | 4.41 ± 13.29 | 4.36 ± 6.18 | 4.18 ± 8.90 | 3.94 ± 15.56 | 3.86 ± 12.51 | <0.001 |
| Past history | |||||||
| MI (%) | 5230 (2.9) | 1012 (2.9) | 1262 (2.5) | 1406 (2.9) | 592 (2.9) | 958 (2.8) | <0.001 |
| BI (%) | 21898 (12.3) | 5440 (15.7) | 4911 (9.8) | 5579 (11.4) | 2269 (11.0) | 3699 (10.9) | <0.001 |
| BH (%) | 7840 (4.4) | 1730 (5.0) | 1520 (4.4) | 1823 (3.6) | 699 (3.4) | 1222 (3.6) | <0.001 |
| Amputation (%) | 4297 (2.4) | 1312 (3.8) | 971 (1.9) | 1042 (2.1) | 386 (1.9) | 586 (1.7) | <0.001 |
| Hip fracture (%) | 3844 (2.6) | 1105 (3.2) | 827 (1.7) | 929 (1.9) | 391 (1.9) | 592 (1.7) | <0.001 |
Alb, serum albumin; BH, brain hemorrhage; BI, brain infarction; BW, body weigh; Ca, calcium; CRP, C-reactive protein; CVD, cardiovascular disease; Hb, hemoglobin; HD, hemodialysis; MI, myocardial infarction; nPCR, normal protein catabolic rate; P, phosphate; SNa, serum sodium; T.Chol. total cholesterol.
Values are presented as means ± SDs.
The Relationship Between Dialysate Sodium Concentration and Pre-HD SNa
A dialysate sodium concentration of 140 mEq/l was used in 148,388 patients (85.9%), whereas 8462 patients (4.9%) were dialyzed with dialysate containing >140 mEq/l sodium and 15,879 patients (8.9%) were dialyzed with dialysate containing <140 mEq/l sodium. In addition, the dialysate concentration was unknown for 5385 patients. The dialysate sodium concentration used was distributed nearly identically in all 5 of the mean SNa categories, with very similar percentages of patients having a dialysate sodium concentration of <140 mEq/l, exactly 140 mEq/l, or >140 mEq/l (Figure 2c). However, >80% of the participants had a dialysate sodium concentration of exactly 140 mEq/l.
Risk of Mortality and Complications Associated With Pre-HD SNa Concentration
After 2 years, a total of 25,928 deaths had occurred, of which 11,347 (43.8%) had been attributed to cardiovascular disease, 4789 (18.5%) to infectious disease, and 9792 (37.8%) to other diseases. A crude analysis showed a significant L-shaped relationship between SNa quintile and the study outcomes (Table 2).
Table 2.
Cox proportional hazards models for the rate of all-cause and cause specific mortality
| Serum sodium category | Unadjusted |
Adjusted |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
Model 3 |
||||||||||
| HR | 95% Cl | P | HR | 95% Cl | P | HR | 95% Cl | P | HR | 95% Cl | P | |
| All-cause | ||||||||||||
| 1 | 2.04 | 1.95–2.13 | <.0001 | 1.90 | 1.82–1.99 | <.0001 | 1.71 | 1.61–1.82 | <.0001 | 1.44 | 1.34–1.56 | <.0001 |
| 2 | 1.26 | 1.20–1.32 | <.0001 | 1.26 | 1.20–1.32 | <.0001 | 1.20 | 1.13–1.27 | <.0001 | 1.14 | 1.06–1.22 | 0.0004 |
| 3 | 1.07 | 1.02–1.12 | 0.0064 | 1.08 | 1.03–1.13 | 0.002 | 1.06 | 1.00–1.12 | 0.03 | 1.05 | 0.98–1.12 | 0.21 |
| 4 | Ref. | - | - | Ref. | - | Ref. | - | - | Ref. | - | - | |
| 5 | 0.98 | 0.93–1.03 | 0.45 | 0.98 | 0.93–1.03 | 0.34 | 1.00 | 0.94–1.06 | 0.96 | 1.00 | 0.93–1.08 | 0.99 |
| CVD | ||||||||||||
| 1 | 1.07 | 1.05–1.09 | <.0001 | 1.91 | 1.78–2.05 | <.0001 | 1.61 | 1.47–1.76 | <.0001 | 1.42 | 1.27–1.59 | <.0001 |
| 2 | 1.02 | 1.00–1.04 | 0.048 | 1.25 | 1.17–1.35 | <.0001 | 1.12 | 1.03–1.22 | 0.01 | 1.09 | 0.98–1.21 | 0.12 |
| 3 | 1.00 | 0.99–1.02 | 0.60 | 1.08 | 1.01–1.16 | 0.03 | 1.03 | 0.95–1.12 | 0.41 | 1.03 | 0.93–1.15 | 0.53 |
| 4 | Ref. | - | - | Ref. | - | Ref. | - | - | Ref. | - | - | |
| 5 | 1.00 | 0.98–1.02 | 0.92 | 0.94 | 0.87–1.02 | 0.15 | 0.96 | 0.88–1.05 | 0.40 | 1.00 | 0.89–1,12 | 0.99 |
| Infection | ||||||||||||
| 1 | 2.45 | 2.19–2.73 | <.0001 | 2.25 | 2.02–2.51 | <.0001 | 2.29 | 1.98–2.64 | <.0001 | 1.90 | 1.58–2.28 | <.0001 |
| 2 | 1.40 | 1.25–1.57 | <.0001 | 1.39 | 1.24–1.55 | <.0001 | 1.41 | 1.23–1.62 | <.0001 | 1.38 | 1.16–1.64 | 0.002 |
| 3 | 1.08 | 0.96–1.21 | 0.18 | 1.09 | 0.97–1.22 | 0.13 | 1.11 | 0.97–1.27 | 0.11 | 1.14 | 0.96–1.35 | 0.12 |
| 4 | Ref. | - | - | Ref. | - | Ref. | - | - | Ref. | - | - | |
| 5 | 1.06 | 0.93–1.19 | 0.38 | 1.04 | 0.92–1.18 | 0.48 | 1.07 | 0.93–1.24 | 0.33 | 1.05 | 0.87–1.26 | 0.63 |
| Others | ||||||||||||
| 1 | 1.87 | 1.74–2.02 | <.0001 | 1.75 | 1.62–1.88 | <.0001 | 1.07 | 1.05–1.10 | <.0001 | 1.27 | 1.12–1.44 | 0.0001 |
| 2 | 1.22 | 1.13–1.31 | <.0001 | 1.21 | 1.12–1.31 | <.0001 | 1.02 | 1.00–1.04 | 0.13 | 1.09 | 0.97–1.22 | 0.14 |
| 3 | 1.06 | 0.98–1.14 | 0.13 | 1.07 | 0.99–1.15 | 0.08 | 0.66 | 0.99–1.02 | 0.66 | 1.01 | 0.91–1.13 | 0.82 |
| 4 | Ref. | - | - | Ref. | - | Ref. | - | - | Ref. | - | - | |
| 5 | 0.99 | 0.91–1.07 | 0.77 | 0.98 | 0.90–1.06 | 0.65 | 1.00 | 0.98–1.02 | 0.90 | 0.98 | 0.87–1.11 | 0.80 |
CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio.
Model 1was adjusted for age, sex, duration of hemodialysis, and the length of a dialysis session.
Model 2 was adjusted for Model 1 variables + (hemoglobin, calcium, phosphate, ΔBW% ([pre-HD body weight − post-HD body weight] / pre-HD body weight × 100), change in sodium during dialysis, dialysate sodium concentration, and Kt/V).
Model 3 was adjusted for Model 2 variables + (total cholesterol, albumin, C-reactive protein, and normal protein catabolic rate) + (past history of myocardial infarction, brain infarction, brain hemorrhage, amputation of an extremity, and hip fracture).
Values are presented as means ± SDs.
Serum sodium categories: 1, SNa ≤136 mEq/l; 2, 136< SNa ≤138 mEq/l; 3, 138< SNa ≤140 mEq/l; 4, 140< SNa ≤ 141 mEq/l; 5, 141 mEq/l < SNa.
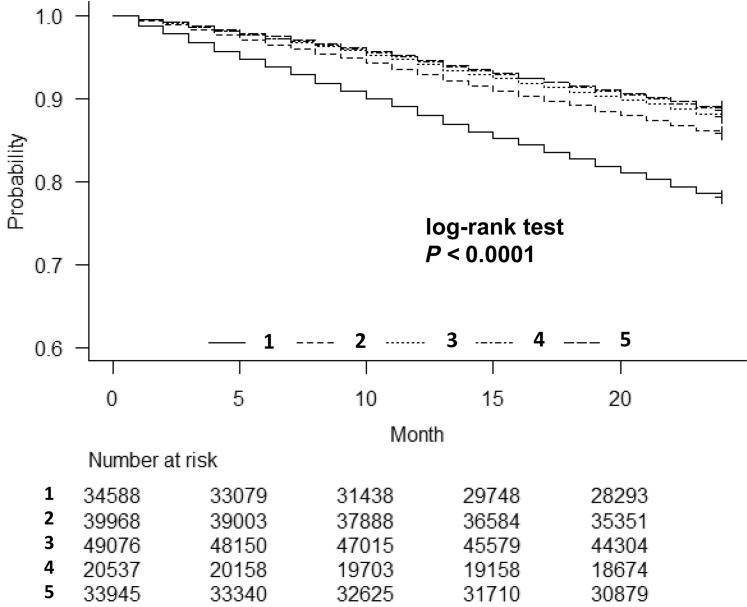
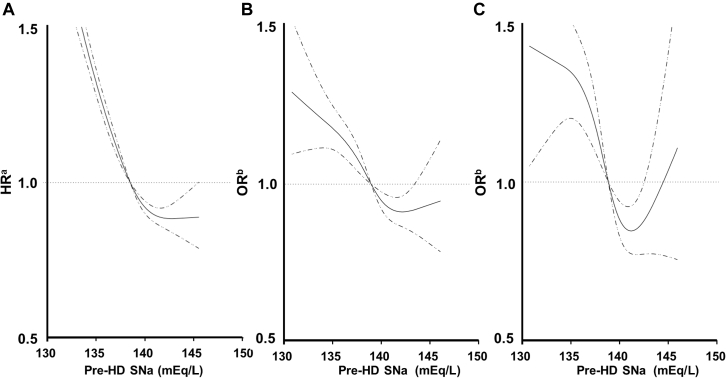
Unadjusted Kaplan-Meier curves for overall survival are shown in Figure 3. These associations were somewhat attenuated after adjustment for clinically relevant demographic factors, but they remained statistically significant (the fully adjusted HR [95% CI] of the lowest quintiles was 1.44 [1.34–1.56], P < 0.0001, for all-cause mortality;, 1.42 [1.27–1.59], P < 0.0001, for cardiovascular mortality; and 1.90 [1.58–2.28], P < 0.0001 for mortality related to infection; Table 2). The continuous, fully adjusted association between the SNa concentration and all-cause mortality was similar. Multivariable-adjusted RCS revealed that low Pre-HD SNa was associated with a higher HR for all-cause mortality (Figure. 4a). To determine the influence of low SNa on the risk of coronary heart disease, cerebral hemorrhage, cerebral infarction, amputation of a lower extremity, and hip fracture, we used multivariable-adjusted RCS, which showed that as SNa decreased, the odds ratios for cerebral infarction (Figure 4b), lower limb amputation (Figure 4c), and hip fracture (Supplementary Figure S3A) also increased.
Figure 3.
Kaplan–Meier plots for survival rates in the 5 groups during the 2-year follow-up period: univariate analysis. Categories: 1, SNa ≤ 136 mEq/l; 2, 136 < SNa ≤ 138 mEq/l; 3, 138 < SNa ≤ 140 mEq/l; 4, 140 < SNa ≤ 141 mEq/l; 5, 141 mEq/l < SNa.
Figure 4.
Multivariable-adjusted restricted cubic spline plots of (a) the hazard ratios (HR) for all-cause mortality according to serum sodium concentration before an HD session (pre-HD SNa). Multivariable-adjusted restricted cubic spline plots of the odds ratios (OR) for (b) cerebral infarction and (c) lower limb amputation according to pre-HD SNa. The solid line represents the HR and OR, and the dotted line represents the 95% confidence interval. The multivariable-adjusted model (a) Cox model and (b) logistic model was adjusted for age, sex, duration of HD, length of an HD session, dialysate sodium, history of CVD, Hb, Alb, ΔSNa, Ca, P, T-Chol, Kt/V, CRP, nPCR, ΔBW%. Alb, serum albumin; BW, body weight; Ca, calcium; CRP, C-reactive protein; Hb, hemoglobin; HD, hemodialysis; P, phosphate; nPCR, normal protein catabolic rate; T.Chol., total cholesterol.
Risk of Mortality and Complications Associated With ΔSNa
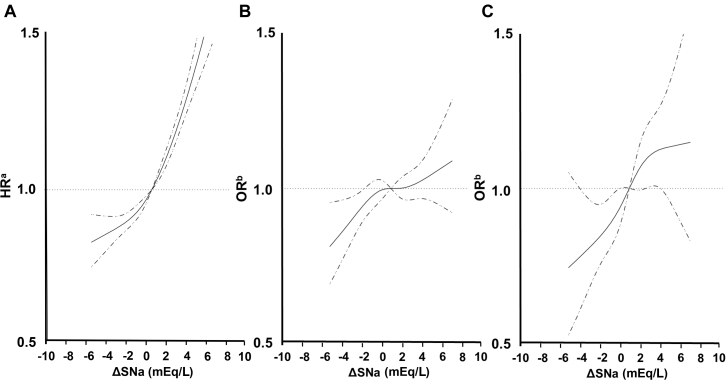
Multivariable-adjusted RCS revealed that as ΔSNa increased, so did the HR for all-cause mortality (Figure 5a) and the odds ratio for cerebral infarction (Figure 5b), lower limb amputation (Figure 5c), and hip fracture (Supplementary Figure S3B). However, such a relationship was not identified for coronary artery disease (Supplementary Figure S1B) in multivariable-adjusted RCS.
Figure 5.
Multivariable-adjusted restricted cubic spline plots of (a) the hazard ratios (HR) for all-cause mortality according to post-dialysis serum sodium concentration (SNa)– pre-dialysis SNa (ΔSNa). Multivariable-adjusted restricted cubic spline plots of the odds ratios (OR) for (b) cerebral infarction and (c) lower limb amputation according to ΔSNa. The solid line represents the HR and OR, and the dotted line represents the 95% confidence interval. The multivariable-adjusted model (a) Cox model and (b) logistic model was adjusted for age, sex, duration of HD, length of an HD session, dialysate sodium, history of cardiovascular disease (CVD), Hb, Alb, SNa, Ca, P, T-Chol, Kt/V, CRP, nPCR, and ΔBW%. Alb, serum albumin; BW, body weight; Ca, calcium; CRP, C-reactive protein; Hb, hemoglobin; HD, hemodialysis; P, phosphate; nPCR, normal protein catabolic rate; T.Chol., total cholesterol.
Risk of Mortality Associated With Pre-HD SNa by ΔSNa Level
The combination of low pre-HD SNa concentration and large ΔSNa was also associated with higher mortality (HR 1.09; 95% CI 1.05–1.13). Participants with the lowest SNa concentration (≤136 mEq/l) and the highest ΔSNa (>4 mEq/l) showed higher mortality than those with an intermediate pre-HD SNa concentration (137–140 mEq/l) and the lowest ΔSNa (≤2 mEq/l) (Table 3).
Table 3.
Adjusted mortality risk associated with pre-HD SNa by ΔSNa level
| Serum sodium category | ΔSNa category |
||
|---|---|---|---|
| 0<ΔSNa≤2 |
2<ΔSNa≤4 |
4<ΔSNa |
|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| SNa≤136 | - (N = 0) | 1.046 (0.998–1.096) | 1.089a (1.053–1.126) |
| 136<SNa≤140 | 1 (reference) | 1.007 (0.975–1.040) | 1.014 (0.981–1.048) |
| 140<SNa | 0.987 (0.954–1.021) | 0.995 (0.962–1.030) | 0.991 (0.939–1.046) |
Abbreviations: CI, confidence interval; CVD, cardiovascular disease; HD, hemodialysis; HR, hazard ratio; SNa, serum sodium.
Cox model adjusted for age, sex, HD vintage, HD time, dialysate sodium, history of cardiovascular disease, hemoglobin, albumin, calcium, phosphate, total cholesterol, Kt/V, C-reactive protein, normal protein catabolic rate, ΔBW% ([pre-HD body weight − post-HD body weight] / pre-HD body weight × 100).
< 0.0001
Discussion
The present results indicated a relationship between better survival in HD patients and SNa > 136 mEq/l. The major finding of this study is that high ΔSNa is a significant and independent predictor of mortality in HD patients. To the best of our knowledge, this is the first published study to demonstrate an adverse effect of hyponatremia and fluctuations in SNa concentration during a dialysis session. We also found significant associations between higher ΔSNa and stroke and amputation of an extremity. The principal strength of the study is that we have demonstrated a dose-response relationship between serum sodium and prognosis using a very large cohort of patients undergoing HD.
Several possible mechanisms may explain the greater mortality in patients with hyponatremia.2 First, acute and chronic hyponatremia with progressive cerebral edema could contribute to mortality. Second, hyponatremia is also a marker of the severity of the underlying disease. Several studies have found that hyponatremia occurs alongside markers of the progression of heart failure.12,13 Third, severe underlying disease causes chronic hyponatremia and contributes to higher mortality, and hyponatremia further increases the risk of mortality. These mechanisms are consistent with the findings of recent studies that hyponatremia is associated with mortality.1,14,15 In addition, when the present data were adjusted for nutritional status (serum albumin, nPCR) and body weight gain (ΔBW%), the association between hyponatremia and all-cause mortality remained. Furthermore, hyponatremia predisposes to infections, fractures, and cognitive dysfunction, which affect the prognosis of HD patients.
Recent reports have suggested that hyponatremia increases the risk of infections,15 especially tuberculosis16 and pneumonia,9 and associations between hyponatremia and greater mortality from these diseases have also been identified. The mechanism involves mucous membrane edema that develops due to extracellular hypotonicity and osmosis into the intracellular space, which compromises the microbial barrier function of the mucosa in the respiratory, gastrointestinal, and urinary tracts.15 In HD patients with hyponatremia, bone turnover abnormalities are also universal, and chronic hyponatremia has recently been reported to be a risk factor for osteoporosis.8 In addition, hyponatremia leads to cognitive dysfunction,17,18 disequilibrium, gait disturbances, falls, and bone fractures,19 all of which have a substantial influence on the prognosis of HD patients. The present data show independent associations between hyponatremia and high risks of death from infectious disease, hip fracture, and amputation of a lower extremity. However, the mechanisms linking hyponatremia with prognosis in these patients remain unclear.
We also studied the significance of fluctuations in SNa concentration during HD (ΔSNa) and investigated the relationship between ΔSNa during HD and mortality. To date, there have been few similar studies. One previous study20 showed that higher pre-HD SNa variability, indicated by measurements of mean (SD) SNa, mean variability, and mean directional range, was associated with greater all-cause mortality. Specifically, the mean directional range of SNa, representing the difference between the minimum and maximum values during 2 years of follow-up, was associated with mortality. The strength of the present study is that we measured the change in SNa between pre-HD and post-HD samples, finding that the larger the ΔSNa, the higher the all-cause mortality, and incidences of cerebral infarction, amputation of a lower extremity, and hip fracture. A larger ΔSNa may cause organ damage, but the detailed mechanism involved remains to be determined. We also found that pre-hemodialysis hypernatremia was not a mortality risk in this study. In the DOPPS Study,6 the effect of hypernatremia on mortality could not be ascertained. However, a U-shaped association between serum sodium and mortality has been observed in incident HD patients.7
In the central nervous system, an overly rapid correction of serum sodium can lead to osmotic demyelination syndrome, and chronic hyponatremia causes water to enter astrocytes in larger quantities.21 Osmotic demyelination syndrome is usually caused by overly rapid correction of serum sodium levels in patients with chronic hyponatremia, and involves demyelination, especially of the central basis pontis (pontine myelinolysis). The reasons why hyponatremia predisposes to other central nervous system disorders are unknown, but the present findings show a significant association between high ΔSNa and stroke.
The major strength of our data is that they were derived from a large national cohort, and by analyzing data from various regions of Japan, we have been able to show that SNa and ΔSNa are independent risk factors for all-cause, cardiovascular, and infectious disease-related mortality. From the results of this study, changes in plasma sodium concentration from pre- to post-dialysis may lead to organ damage. However, several limitations of the study should be acknowledged. First, the study was observational in nature, which means we cannot draw conclusions regarding cause and effect regarding the relationship between ΔSNa and mortality. Second, plasma glucose concentrations were not available for all the participants; plasma glucose influences plasma osmolality, particularly in diabetic patients, and would affect SNa and ΔSNa.22 Third, we did not have exact data regarding interdialytic weight gain and dry weight data; therefore, we could not determine the influence of interdialytic water retention. Instead, we used water removal amount per body weight (ΔBW%) to adjust the analyses. Fourth, this study did not have the data that patients were taking the drugs. We did not consider the possible effect of pharmacological interference. Fifth, in this study, the exact cause of hyponatremia could not be identified; however, a previous study23 reported that malnutrition and inflammation were associated with a higher likelihood of hyponatremia (sodium < 135 mEq/l), whereas fluid overload was associated with a lower likelihood of hyponatremia. Sixth, ΔSNa in this study reflects the same tonicity change for HD patients. As a result, this sodium gradient may affect an HD patient’s prognosis and illness. In this regard, a new hemodialysis machine with the aim to achieve no sodium gradient in HD and online HDF treatments was tested in the form of a prospective clinical trial.24, 25, 26 Advances in these devices, automated sodium and tonicity management tool embedded in hemodialysis machine, have the potential to solve the problem in the future. Finally, this study used data from 2008 to 2010 in Japan; therefore, applying our findings to current dialysis patients may be inappropriate. In addition, baseline variables in this study were derived only from the 2008 database and were based on a single measurement.
In conclusion, we have shown that low pre-HD SNa concentration and high ΔSNa are associated with a greater risk of death in HD patients. This finding may have implications for the management of dialysis in patients with hyponatremia; however, further study of these associations and the mechanisms involved is required.
Disclosures
All the authors declared no competing interests.
Acknowledgments
The authors thank the Committee of the Japanese Society for Dialysis Therapy (JSDT) for permission to use their data. The opinions reflected in this manuscript are those of the authors alone and do not reflect the official position of JSDT.
The authors express heartfelt appreciation to the Japanese Society for Dialysis Therapy, the principal investigators of all the prefectures, and all of the personnel and patients at the institutions participating in this survey. Dr. Wada, from the Department of Nephrology, Kitasaito Hospital, Asahikawa, Japan, collected the data and prepared the dataset. All authors approved the final version of the manuscript. The contents and opinions in this paper are those of the authors only and do not reflect those of the Japanese Society for Dialysis Therapy. We also thank Mark Cleasby, PhD, from the Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Part of this study was presented at the 51st Annual Meeting of the American Society of Nephrology (San Diego, CA, 2018).
Footnotes
Figure S1. Multivariable-adjusted restricted cubic spline plots of the odds ratios (OR) for coronary heart disease according to (A) serum sodium concentration before an HD session (Pre-HD SNa) and (B) post-dialysis SNa − pre-dialysis SNa (ΔSNa).
Figure S2. Multivariable-adjusted restricted cubic spline plots of the odds ratios (OR) for cerebral hemorrhage according to (A) serum sodium concentration before an HD session (Pre-HD SNa) and (B) post-dialysis SNa − pre-dialysis SNa (ΔSNa).
Figure S3. Multivariable-adjusted restricted cubic spline plots of the odds ratios (OR) for hip fracture according to (A) serum sodium concentration before an HD session (Pre-HD SNa) and (B) post-dialysis SNa − pre-dialysis SNa (ΔSNa).
Supplementary Material
Figure S1. Multivariable-adjusted restricted cubic spline plots of the odds ratios (OR) for coronary heart disease according to (A) serum sodium concentration before an HD session (Pre-HD SNa) and (B) post-dialysis SNa − pre-dialysis SNa (ΔSNa).
Figure S2. Multivariable-adjusted restricted cubic spline plots of the odds ratios (OR) for cerebral hemorrhage according to (A) serum sodium concentration before an HD session (Pre-HD SNa) and (B) post-dialysis SNa − pre-dialysis SNa (ΔSNa).
Figure S3. Multivariable-adjusted restricted cubic spline plots of the odds ratios (OR) for hip fracture according to (A) serum sodium concentration before an HD session (Pre-HD SNa) and (B) post-dialysis SNa − pre-dialysis SNa (ΔSNa).
References
- 1.Berl T. An elderly patient with chronic hyponatremia. Clin J Am Soc Nephrol. 2013;8:469–475. doi: 10.2215/CJN.03100312. [DOI] [PubMed] [Google Scholar]
- 2.Hoorn E.J., Zietse R. Hyponatremia and mortality: moving beyond associations. Am J Kidney Dis. 2013;62:139–149. doi: 10.1053/j.ajkd.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 3.Iseki K., Uehara H., Nishime K. Impact of the initial levels of laboratory variables on survival in chronic dialysis patients. Am J Kidney Dis. 1996;28:541–548. doi: 10.1016/s0272-6386(96)90465-5. [DOI] [PubMed] [Google Scholar]
- 4.Chiu D.Y., Kalra P.A., Sinha S., Green D. Association of serum sodium levels with all-cause and cardiovascular mortality in chronic kidney disease: results from a prospective observational study. Nephrology (Carlton) 2016;21:476–482. doi: 10.1111/nep.12634. [DOI] [PubMed] [Google Scholar]
- 5.Nigwekar S.U., Wenger J., Thadhani R., Bhan I. Hyponatremia, mineral metabolism, and mortality in incident maintenance hemodialysis patients: a cohort study. Am J Kidney Dis. 2013;62:755–762. doi: 10.1053/j.ajkd.2013.02.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hecking M., Karaboyas A., Saran R. Predialysis serum sodium level, dialysate sodium, and mortality in maintenance hemodialysis patients: The Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2012;59:238–248. doi: 10.1053/j.ajkd.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Rhee C.M., Ravel V.A., Ayus J.C. Pre-dialysis serum sodium and mortality in a national incident hemodialysis cohort. Nephrol Dial Transplant. 2016;31:992–1001. doi: 10.1093/ndt/gfv341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ayus J.C., Negri A.L., Kalantar-Zadeh K., Moritz M.L. Is chronic hyponatremia a novel risk factor for hip fracture in the elderly? Nephrol Dial Transplant. 2012;27:3725–3731. doi: 10.1093/ndt/gfs412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nair V., Niederman M.S., Masani N., Fishbane S. Hyponatremia in community-acquired pneumonia. Am J Nephrol. 2007;27:184–190. doi: 10.1159/000100866. [DOI] [PubMed] [Google Scholar]
- 10.Nakai S., Suzuki K., Masakane I. An overview of regular dialysis treatment in Japan (as of 31 December 2008) Ther Apher Dial. 2010;14:505–540. doi: 10.1111/j.1744-9987.2010.00893.x. [DOI] [PubMed] [Google Scholar]
- 11.Shinzato T., Nakai S., Fujita Y. Determination of Kt/V and protein catabolic rate using pre- and postdialysis blood urea nitrogen concentrations. Nephron. 1994;67:280–290. doi: 10.1159/000187980. [DOI] [PubMed] [Google Scholar]
- 12.Gheorghiade M., Abraham W.T., Albert N.M. Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: An analysis from the OPTIMIZE-HF registry. Eur Heart J. 2007;28:980–988. doi: 10.1093/eurheartj/ehl542. [DOI] [PubMed] [Google Scholar]
- 13.Hiki M., Kasai T., Yatsu S. Relationship between serum sodium level within the low-normal range on admission and long-term clinical outcomes in patients with acute decompensated heart failure. Int Heart J. 2018;59:1052–1058. doi: 10.1536/ihj.17-524. [DOI] [PubMed] [Google Scholar]
- 14.Kovesdy C.P., Lott E.H., Lu J.L. Hyponatremia, hypernatremia, and mortality in patients with chronic kidney disease with and without congestive heart failure. Circulation. 2012;125:677–684. doi: 10.1161/CIRCULATIONAHA.111.065391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mandai S., Kuwahara M., Kasagi Y. Lower serum sodium level predicts higher risk of infection-related hospitalization in maintenance hemodialysis patients: an observational cohort study. BMC Nephrol. 2013;14:276. doi: 10.1186/1471-2369-14-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roca B., Tornador N., Tornador E. Presentation and outcome of tuberculous meningitis in adults in the province of Castellon, Spain: a retrospective study. Epidemiol Infect. 2008;136:1455–1462. doi: 10.1017/S0950268807000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shavit L., Mikeladze I., Torem C., Slotki I. Mild hyponatremia is associated with functional and cognitive decline in chronic hemodialysis patients. Clin Nephrol. 2014;82:313–319. doi: 10.5414/CN108335. [DOI] [PubMed] [Google Scholar]
- 18.Xu R., Pi H., Xiong Z. Article hyponatremia and cognitive impairment in patients treated with peritoneal dialysis. Clin J Am Soc Nephrol. 2015;10:1806–1813. doi: 10.2215/CJN.02240215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ayus J.C., Fuentes N.A., Negri A.L. Mild prolonged chronic hyponatremia and risk of hip fracture in the elderly. Nephrol Dial Transplant. 2016;31:1662–1669. doi: 10.1093/ndt/gfw029. [DOI] [PubMed] [Google Scholar]
- 20.Ye X., Kooman J.P., van der Sande F.M. Increased mortality associated with higher pre-dialysis serum sodium variability : results of the International MONitoring Dialysis Outcome Initiative. Am J Nephrol. 2019;49:1–10. doi: 10.1159/000495354. [DOI] [PubMed] [Google Scholar]
- 21.Gankam Kengne F., Decaux G. Hyponatremia and the brain. Kidney Int Rep. 2017;3:24–35. doi: 10.1016/j.ekir.2017.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Penne E.L., Thijssen S., Raimann J.G. Correction of serum sodium for glucose concentration in hemodialysis patients with poor glucose control. Diabetes Care. 2010;33:e91. doi: 10.2337/dc10-0557. [DOI] [PubMed] [Google Scholar]
- 23.Dekker M.J., Marcelli D., Canaud B. Unraveling the relationship between mortality, hyponatremia, inflammation and malnutrition in hemodialysis patients: results from the international MONDO initiative. Eur J Clin Nutr. 2016;70:779–784. doi: 10.1038/ejcn.2016.49. [DOI] [PubMed] [Google Scholar]
- 24.Ponce P., Pinto B., Wojke R. Evaluation of intradialytic sodium shifts during sodium controlled hemodialysis. Int J Artif Organs. 2020;43:620–624. doi: 10.1177/0391398820903055. [DOI] [PubMed] [Google Scholar]
- 25.Kuhlmann U., Maierhofer A., Canaud B. Zero diffusive sodium balance in hemodialysis provided by an algorithm-based electrolyte balancing controller: a proof of principle clinical study. Artif Organs. 2019;43:150–158. doi: 10.1111/aor.13328. [DOI] [PubMed] [Google Scholar]
- 26.Ságová M., Wojke R., Maierhofer A. Automated individualization of dialysate sodium concentration reduces intradialytic plasma sodium changes in hemodialysis. Artif Organs. 2019;43:1002–1013. doi: 10.1111/aor.13463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.