Abstract
Concerns are heightened from detecting environmentally persistent man-made per- and polyfluoroalkyl substances (PFAS) in drinking water systems around the world. Many PFAS, including perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA), remain in the human body for years. Since 1999–2000, assessment of exposure to PFOS, PFOA, and other select PFAS in the U.S. general population has relied on measuring PFAS serum concentrations in participants of the National Health and Nutrition Examination Survey (NHANES). Manufacturers have replaced select chemistries (“legacy” PFAS) with PFAS with shorter biological half-lives (e.g., GenX, perfluorobutanoate [PFBA]) which may efficiently eliminate in urine. However, knowledge regarding exposure to these compounds is limited. We analyzed 2,682 urine samples for 17 legacy and alternative PFAS from 2013–2014 NHANES participants ≥6 years of age. Concentrations of some of these PFAS, measured previously in paired serum samples from the same NHANES participants, suggested universal exposure to PFOS and PFOA, and infrequent or no exposure to two short-chain PFAS, perfluorobutane sulfonate and perfluoroheptanoate. Yet, in urine, PFAS were seldom detected; the frequency of not having detectable concentrations of any of the 17 PFAS was 67.5%. Only two were detected in more than 1.5% of the population: PFBA (13.3%) and perfluorohexanoate (PFHxA, 22.6%); the 90th percentile urine concentrations were 0.1 μg/L (PFBA), and 0.3 μg/L (PFHxA). These results suggest that exposures to short-chain PFAS are infrequent or at levels below those that would result in detectable concentrations in urine. As such, these findings do not support biomonitoring of short-chain PFAS or fluorinated alternatives in the general population using urine, and highlight the importance of selecting the adequate biomonitoring matrix.
Keywords: biomonitoring, NHANES, PFAS
Introduction
Per- and polyfluoroalkyl substances (PFAS) encompass thousands of environmentally persistent man-made compounds with a wide range of chemical functionalities and variable length carbon chains containing the perfluoroalkyl moiety (CnF2n+1–) (Buck et al., 2011). PFAS have been used since the 1950s in many industrial and consumer products, including soil, stain, grease, and water resistant coatings on textiles and carpet; in the automotive, mechanical, aerospace, chemical, electrical, medical, and building/construction industries; in personal care products; in cookware non-stick coatings; and in aqueous film-forming foams (ATSDR, 2015;Dewitt, 2015).
Manufacturing of long-chain PFAS, including two of the most studied PFAS, perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA), their precursors and related compounds, changed in the United States in the 2000s (ATSDR, 2015;Dewitt, 2015). As a result, the market share of short-chain PFAS (e.g., perfluorobutane sulfonate [PFBS]) and PFAS with different chemical functional groups including perfluoroalkyl ether carboxylic acids, such as GenX (ammonium salt of 2,3,3,3,-tetrafluoro-2-(1,1,2,2,3,3,3-heptafluoropropoxy)-propanic acid), may have changed as well (Sunderland et al. 2019;Wang et al. 2019). PFBS, a replacement for PFOS, is used in consumer products including carpeting and carpeting cleaners, floor wax, and food packaging; GenX was developed to manufacture high-performance fluoropolymers without the use of PFOA (US EPA, 2018). The increased use of these other PFAS, many of which are unregulated (Pan et al., 2019), in consumer markets can result in human exposure (Sunderland et al. 2019;Wang et al. 2019).
While PFOS, PFOA and other long-chain PFAS persist in humans (ATSDR, 2015;Dewitt, 2015), short-chain PFAS (e.g., PFBS, perfluorohexanoate (PFHxA)), and other PFAS such as GenX, used to replace long-chain PFAS, have shorter elimination half-lives in animals (Gannon et al., 2016;Gannon et al., 2011;Olsen et al., 2009) and people (Nilsson et al., 2010;Olsen et al., 2009), and eliminate in urine (Gannon et al., 2016;Gannon et al., 2011;Olsen et al., 2009). Exposure to some PFAS has been related to adverse health effects (ATSDR, 2015;Dewitt, 2015;Gomis et al., 2018). Therefore, the detection of PFAS in surface water and drinking water (Gebbink et al., 2017;Heydebreck et al., 2015;Kabore et al., 2018;Pan et al., 2017;Sun et al., 2016;Wei et al., 2018) and in the blood of people from communities exposed to contaminated drinking water (Daly et al., 2018;Fromme et al., 2017;Graber et al., 2019;Hoffman et al., 2011;Holzer et al., 2009;Ingelido et al., 2018;Landsteiner et al., 2014;Stubleski et al., 2017) in many parts of the world has raised concerns about the potential health implications from human exposure to PFAS.
Exposure to long-chain PFAS in the United States is widespread as suggested by nearly universal detection of these chemicals in serum among the U.S. general population (CDC, 2019). The Centers for Disease Control and Prevention (CDC) have quantified serum concentrations of select PFAS in U.S. National Health and Nutrition Examination Survey (NHANES) participants starting with NHANES 1999–2000. Although the data suggest downward trends in exposure for several PFAS, including PFOS and PFOA, these compounds were detected even in persons born after the changes in production in the 2000s (CDC, 2019;Ye et al., 2018). By contrast, although short-chain PFAS are increasingly used to replace long-chain PFAS (Brendel et al., 2018), short-chain PFAS (e.g., PFBS, perfluoroheptanoate (PFHpA)) were seldom detected in NHANES participants, and, when detected, concentrations were rather low (CDC, 2019;Ye et al., 2018), perhaps because these PFAS have relatively short half-lives (i.e., days to weeks) and may efficiently eliminate in urine.
Information on urinary concentrations of PFAS in humans is limited, yet, it may be important to understand exposure to short-chain and alternative PFAS. To address this knowledge gap, we developed an analytical method suitable for population-based biomonitoring programs, such as NHANES, to concurrently quantify in 50 μL of urine 14 C4-C11 PFAS, and three fluorinated alternatives including GenX (Kato et al., 2018), and applied the method to obtain U.S.-nationally representative population data.
We present here the urinary concentrations of 17 PFAS in 2,682 2013–2014 NHANES participants 6 years of age and older, and evaluate the potential usefulness of urine as a biomonitoring matrix for assessment of PFAS exposure in the general population. When available, we compare these urinary concentrations to previously obtained serum concentrations from the same NHANES participants (CDC, 2019;Ye et al., 2018).
Methods
Study Population
NHANES, conducted continuously since 1999 by the National Center for Health Statistics (NCHS) at the CDC, includes direct household interviews with demographic, socioeconomic, dietary, and health-related questions, physical examinations, and collection of biological samples. Some of these samples are used to assess exposure to environmental chemicals (CDC, 2013).
For this study, we analyzed 2,682 spot urine samples collected from a random one-third subsample of 2013–2014 NHANES participants six years of age and older. The NCHS Research Ethics Review Board reviewed and approved the study protocol. To participate in the survey, all respondents gave informed written consent, parents or guardians provided written permission for participants younger than 18 years, and children 7–17 years old provided assent.
Quantification of PFAS Concentrations
The urine specimens were collected, aliquoted, and shipped on dry ice to the CDC`s National Center for Environmental Health where they were stored at −70 ⁰C until analysis. We quantified PFBS, perfluorohexane sulfonate (PFHxS), perfluoro-1-heptanesulfonate (PFHpS), linear PFOS (n-PFOS), mixture of perfluoro-5-methylheptane sulfonate (Sm-PFOS) and perfluoro-5-methylheptanoic acid (Sb-PFOA) isomers, perfluorobutanoate (PFBA), perfluoropentanoate (PFPeA), PFHxA, PFHpA, linear PFOA (n-PFOA), perfluorononanoic acid (PFNA), perfluorodecanoic acid (PFDA), perfluoroundecanoic acid (PFUnDA), 2,3,3,3,-tetrafluoro-2-(1,1,2,2,3,3,3-heptafluoropropoxy)-propanoate (HFPO-DA), dodecafluoro-3H-4,8-dioxanoate (DONA), and 9-chlorohexadecafluoro-3-oxanonane-1-sulfonate (9Cl-PF3ONS) (Table 1). The analytical method, described in detail before (Kato et al., 2018), relies on an enzymatic hydrolysis of urinary conjugates of the target biomarkers in 50 μL urine, followed by online solid phase extraction, separation by reversed phase high-performance liquid chromatography, and detection by isotope dilution-electrospray ionization tandem mass spectrometry (Supporting Information). Calibration standards, reagent blanks, and NHANES study samples were analyzed with matrix-based quality control (QC) materials (Supporting Information). The limit of detection (LOD), defined as the concentration at which a measurement has a 95% probability of being greater than zero (CDC, 2019), was calculated as three times the standard deviation obtained from five repeated measurements of low-level standards spiked onto human urine (Kato et al., 2018). The LOD was 100 parts-per trillion or ppt (i.e., 0.1 μg/L) for all analytes. The method accuracy, determined from five repeated measurements of human urine spiked with high or low concentrations of the target analytes, ranged from 93.6 to 106.2%, depending on the analyte and concentration (Kato et al., 2018). Method precision, expressed as the relative standard deviation of multiple measures of the QC materials in a two-month period, ranged from 5.6 to 10.4 %, depending on the PFAS and concentration (Kato et al., 2018).
Table 1.
Concentration ranges and estimated percentage of the U.S. general population 6 years of age and older with detectable urinary concentrations of select PFAS in urine (2013–2014 NHANES)a
| PFAS | Age group (years) | Percent detection in the U.S. population (%) | Concentration range (μg/L)b |
|---|---|---|---|
| PFBA | 6+ | 13.3 | 0.07–3.4 |
| 6–11 | 13.6 | 0.07–0.7 | |
| 12+ | 13.3 | 0.07–3.4 | |
| PFHxS | 6+ | 0.03 | 0.07–0.1 |
| 6–11 | 0 | 0.07–0.07 | |
| 12+ | 0.04 | 0.07–0.1 | |
| PFHxA | 6+ | 22.6 | 0.07–7.5 |
| 6–11 | 21.5 | 0.07–1.3 | |
| 12+ | 22.7 | 0.07–7.5 | |
| PFHpS | 6+ | 0.06 | 0.07–0.1 |
| 6–11 | 0 | 0.07–0.07 | |
| 12+ | 0.06 | 0.07–0.1 | |
| PFHpA | 6+ | 1.1 | 0.07–0.3 |
| 6–11 | 0.7 | 0.07–0.2 | |
| 12+ | 1.2 | 0.07–0.3 | |
| n-PFOS | 6+ | 0.05 | 0.07–0.6 |
| 6–11 | 0 | 0.07–0.07 | |
| 12+ | 0.05 | 0.07–0.6 | |
| Sb-PFOA | 6+ | 0.06 | 0.07–0.1 |
| 6–11 | 0.2 | 0.07–0.1 | |
| 12+ | 0.05 | 0.07–0.1 | |
| PFNA | 6+ | 0.02 | 0.07–0.1 |
| 6–11 | 0 | 0.07–0.07 | |
| 12+ | 0.02 | 0.07–0.1 | |
| GenX | 6+ | 1.2 | 0.07–0.4 |
| 6–11 | 1.5 | 0.07–0.4 | |
| 12+ | 1.2 | 0.07–0.3 |
PFBS, PFPeA, Sm-PFOS, n-PFOA, PFDA, PFUA, 9Cl-PF3ONS, and DONA were not detected in any samples.
Ranges given as minimum–maximum where minimum values below the LOD (limit of detection, 0.1 μg/L for all analytes) were replaced with 0.07 μg/L (i.e., LOD/SQR2).
In serum of 2013–2014 NHANES participants, we previously quantified concentrations of 10 of the PFAS now measured in urine in nationally representative subsamples of 639 children 3–11 years of age and of 1,993 persons 12 years of age and older (CDC, 2019;Ye et al., 2018). The 10 PFAS (PFBS and PFHpA [short-chain PFAS], and PFHxS, n-PFOS, Sm-PFOS, n-PFOA, Sb-PFOA, PFNA, PFDA, and PFUnDA [long-chain PFAS]) were quantified using analytical procedures described in detail before (Kato et al., 2011;Kato et al., 2018).
Statistical Analysis
We used Statistical Analysis System (SAS) (version 9.4; SAS Institute Inc., Cary, NC) and SUDAAN (version 13, Research Triangle Institute, Research Triangle Park, NC). All analyses incorporated sample weights and design variables to account for unequal selection probabilities caused by the complex, clustered design of NHANES and to account for oversampling certain demographic groups. For concentrations below the LOD, as recommended by NCHS, we imputed a value equal to the LOD divided by the squared root of 2 (Hornung and Reed, 1990).
For the descriptive analyses, we stratified age, self-reported in years at the last birthday, in two groups: 6–11, and ≥12 years. We also defined five race/ethnicity groups based on self-report: non-Hispanic black, non-Hispanic white, all Hispanic, Mexican American, and Asian. We calculated select percentiles (both in micrograms per liter [μg/L] and in micrograms per gram of creatinine [μg/g creatinine]) using survey sampling weights. For each analyte, we calculated the detection frequency for all samples and by age group. We did not calculate geometric means because the proportion of results <LOD was greater than 40% for all PFAS examined.
For a select group of participants (148 children 6–11 years of age, and 2,125 individuals 12 years of age and older), we previously measured in serum—collected at the same time the urine was collected—the concentrations of 10 short-chain and long-chain PFAS (but not PFAS alternatives): PFBS, PFHpA, PFHxS, n-PFOS, Sm-PFOS, n-PFOA, Sb-PFOA, PFNA, PFDA, and PFUnDA (CDC, 2019). For these participants, we compared the detection frequency of the 10 PFAS in the paired urine-serum samples.
We also calculated the proportion of the general population with detectable concentrations of up to three PFAS in urine for the matched paired urine-serum samples, as well as for the total number of urine samples.
Results
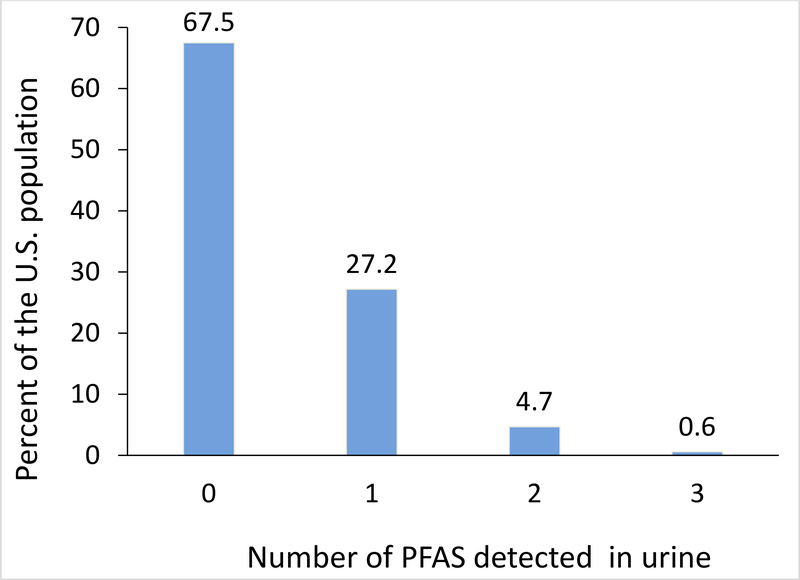
The frequency of having detectable concentrations of at least one of the 17 PFAS examined in urine of 2013–2014 NHANES participants 6 years of age and older was 27.2%, of detecting at least two was 4.7%, and of detecting three was 0.6%; the frequency of detecting none was 67.5% (Figure 1, Table S1). The detection frequency of individual PFAS in 2013–2014 NHANES participants’ urine was 22.6% (PFHxA), 13.3% (PFBA), 1.2% (GenX), 1.1% (PFHpA), <0.1% (PFHxS, PFHpS, n-PFOS, Sb-PFOA, PFNA), and 0% for the other eight PFAS (Figure S2, Table 1). Detection frequencies were similar regardless of age group (6–11 vs ≥12 years).
Figure 1.
Estimated proportion of the U.S. general population 6 years of age and older with PFAS detected in urine. Data from 2013–2014 NHANES for 17 PFAS
Concentration ranges (min–max) of the four PFAS detected most frequently in 2013–2014 NHANES participants 6 years of age and older were <LOD–7.5 μg/L (PFHxA), <LOD–3.4 μg/L (PFBA), <LOD–0.4 μg/L (GenX), <LOD–0.3 μg/L (PFHpA); ranges of all 17 PFAS for 2013–2014 NHANES participants overall and by age group are shown in Table 1. The 90th and 95th percentiles were 0.10 μg/L and 0.30 μg/L (PFBA) and 0.30 μg/L and 0.50 μg/L (PFHxA), respectively (Tables 2 and 3).
Table 2.
Select percentiles of PFHxA concentrations in urine (in μg/L and μg/g creatinine [in italic font]) for the U.S. population ≥6 years of age. Data from the National Health and Nutrition Examination Survey 2013–2014.a
| 75th percentile (95% conf. interval) |
90th percentile (95% conf. interval) |
95th percentile (95% conf. interval) |
N | |
|---|---|---|---|---|
| Total | <LOD | 0.30 (0.20, 0.40) | 0.50 (0.40, 0.60) | 2,682 |
| <LOD | 0.37 (0.32, 0.42) | 0.58 (0.44, 0.68) | 2,680 | |
| Age group | ||||
| 6–11 years | <LOD | 0.30 (0.20, 0.40) | 0.50 (0.30, 0.70) | 409 |
| <LOD | 0.37 (0.30, 0.44) | 0.58 (0.39, 0.85) | 409 | |
| 12+ years | <LOD | 0.30 (0.20, 0.40) | 0.50 (0.40, 0.60) | 2,273 |
| <LOD | 0.37 (0.32, 0.42) | 0.58 (0.44, 0.68) | 2,271 | |
| Sex | ||||
| Male | <LOD | 0.30 (0.20, 0.40) | 0.50 (0.40, 0.60) | 1,284 |
| <LOD | 0.32 (0.27, 0.39) | 0.50 (0.39, 0.64) | 1,283 | |
| Female | <LOD | 0.30 (0.20, 0.40) | 0.50 (0.40, 0.60) | 1,398 |
| <LOD | 0.41 (0.35, 0.50) | 0.61 (0.50, 0.80) | 1,397 | |
| Race | ||||
| All Hispanic | <LOD | 0.20 (0.10, 0.50) | 0.50 (0.30, 0.80) | 690 |
| <LOD | 0.35 (0.25, 0.42) | 0.48 (0.39, 0.68) | 690 | |
| Non-Hispanic white | <LOD | 0.30 (0.30, 0.40) | 0.50 (0.40, 0.60) | 987 |
| <LOD | 0.39 (0.33, 0.44) | 0.60 (0.44, 0.79) | 986 | |
| Non-Hispanic black | 0.10 (<LOD, 0.20) | 0.30 (0.20, 0.40) | 0.50 (0.40, 0.70) | 608 |
| 0.13 (<LOD, 0.18) | 0.28 (0.23, 0.37) | 0.43 (0.30, 0.59) | 608 | |
| Asian | <LOD | 0.30 (0.20, 0.40) | 0.40 (0.30, 0.60) | 287 |
| <LOD | 0.37 (0.33, 0.50) | 0.68 (0.45, 0.87) | 286 | |
| Mexican American | <LOD | 0.30 (0.10, 0.50) | 0.50 (0.20, 0.90) | 438 |
| <LOD | 0.35 (0.24, 0.44) | 0.51 (0.35, 0.77) | 438 |
<LOD means that concentrations were below the limit of detection (LOD) of 0.1 μg/L.
Table 3.
Select percentiles of PFBA concentrations in urine (in μg/L and μg/g creatinine [in italic font]) for the U.S. population ≥6 years of age. Data from the National Health and Nutrition Examination Survey 2013–2014.a
| 90th percentile (95% conf. interval) |
95th percentile (95% conf. interval) |
N | |
|---|---|---|---|
| Total | 0.10 (<LOD, 0.40) | 0.30 (0.10, 0.90) | 2,682 |
| 0.29 (<LOD, 0.42) | 0.44 (0.30, 0.70) | 2,680 | |
| Age group | |||
| 6–11 years | 0.20 (<LOD, 0.50) | 0.30 (<LOD, 0.70) | 409 |
| 0.35 (<LOD, 0.46) | 0.47 (<LOD, 0.63) | 409 | |
| 12+ years | 0.10 (<LOD, 0.40) | 0.30 (<LOD, 1.00) | 2,273 |
| 0.29 (<LOD, 0.44) | 0.44 (<LOD, 0.76) | 2,271 | |
| Sex | |||
| Male | 0.10 (<LOD, 0.50) | 0.30 (0.10, 0.70) | 1,284 |
| 0.22 (<LOD, 0.33) | 0.39 (0.22, 0.61) | 1,283 | |
| Female | 0.10 (<LOD, 0.40) | 0.30 (<LOD, 1.20) | 1,398 |
| 0.33 (<LOD, 0.50) | 0.50 (<LOD, 0.78) | 1,397 | |
| Race | |||
| All Hispanic | <LOD | 0.20 (<LOD, 0.30) | 690 |
| <LOD | 0.33 (<LOD, 0.44) | 690 | |
| Non-Hispanic white | 0.20 (<LOD, 0.60) | 0.40 (0.10, 1.20) | 987 |
| 0.35 (<LOD, 0.54) | 0.49 (0.33, 0.87) | 986 | |
| Non-Hispanic black | <LOD | 0.10 (<LOD, 0.50) | 608 |
| <LOD | 0.24 (<LOD, 0.33) | 608 | |
| Asian | 0.10 (<LOD, 0.30) | 0.20 (<LOD, 1.60) | 287 |
| 0.27 (<LOD, 0.37) | 0.37 (<LOD, 0.50) | 286 | |
| Mexican American | <LOD | 0.10 (<LOD, 0.20) | 438 |
| <LOD | 0.30 (<LOD, 0.39) | 438 |
<LOD means that concentrations were below the limit of detection (LOD) of 0.1 μg/L.
For a select group of 2013–2014 NHANES participants (N=2,273), we had concentrations in paired urine and serum, collected at the same time, of 10 PFAS: two short-chain PFAS (PFBS and PFHpA), and eight long-chain PFAS (PFHxS, n-PFOS, Sm-PFOS, n-PFOA, Sb-PFOA, PFNA, PFDA, PFUnDA). The highest detection frequency in urine was for PFHpA (1.2%) among 2013–2014 NHANES participants 12 years of age and older; the corresponding frequency in serum was 12.6% (Figure 2). PFHpA was not detected in urine of 2013–2014 NHANES children 6–11 years, but the detection frequency was 16.2% in these children’s serum. Similarly, the other short-chain PFAS, PFBS, was not detectable in urine regardless of age, but detectable in serum of 0.6% and 9.1% of 2013–2014 NHANES participants who were ≥12 years and 6–11 years of age, respectively (Figure 2). On the other hand, for long-chain PFAS, although the detection frequency in 2013–2014 NHANES participants’ serum was >98% for PFHxS, n-PFOS, Sm-PFOS, n-PFOA and PFNA, in urine, the corresponding frequency was <0.1% (Figure 2). The highest PFOS concentration in urine (n-PFOS, 0.6 μg/L) corresponded to the highest PFOS concentration in serum (n-PFOS, 1270 μg/L), in agreement with previous studies showing that concentrations of long-chain PFAS are much higher in serum than in urine (Beesoon et al., 2012;Genuis et al., 2013;Li et al., 2013;Zhang et al., 2013).
Figure 2.
Estimated proportion of the population with detectable concentrations of individual PFAS in paired 2013–2014 NHANES urine-serum samples by age group: (A) 12+ years of age (N = 2,125), and (B) 6–11 years of age (N = 148).
Discussion
We quantified urinary concentrations of 14 C4-C11 PFAS and three fluorinated PFAS alternatives in 2,682 participants 6 years of age and older from 2013–2014 NHANES. Our estimates indicate that about two-thirds (67.5%) of the U.S. general population did not have detectable urinary concentrations of any of the 17 PFAS examined. Approximately one quarter (27.2%) of the population was estimated to have detectable urinary concentrations of at least one PFAS, 4.7% of at least two PFAS, and only 0.6% of three PFAS. Furthermore, in terms of individual PFAS, the estimated detection frequencies in urine of 15 of the 17 target PFAS or fluorinated alternatives for the U.S. general population were low, in the range of 0–1.2%, with the exception of two short-chain PFAS: PFBA (13.3%) and PFHxA (22.6%). Noteworthy, although detection frequency would have been higher with a more sensitive method (e.g., LODs ≤0.01 μg/L), urinary concentrations, when detected, were low (i.e., the 90th percentiles of PFBA and PFHxA were at or around the LOD of 0.1 μg/L).
Furthermore, the 2013–2014 NHANES results in urine do not suggest extensive exposure to those 17 PFAS, including PFOS, PFOA, PFNA, and PFHxS. Yet, 2013–2014 NHANES data in serum suggest universal exposure to these four long-chain PFAS among the U.S. general population (CDC, 2019). These apparently contradictory results highlight the critical relevance of using the proper biomonitoring matrix for exposure assessment. Concentrations of biologically persistent chemicals are higher in serum than in urine (Needham et al., 2007). In agreement with this principle, the 2013–2014 NHANES data suggest that despite being detected in fewer than 0.1% of the general population’s urine, PFOS, PFOA, PFNA, and PFHxS, long-chain PFAS with elimination half-lives of years (Bartell et al., 2010;Olsen et al., 2007), were universally detected in the general population’s serum (CDC, 2019).
Also, long-chain PFAS concentrations in 2013–2014 NHANES participants 6 years of age and older were much higher in serum than in urine. For example, a serum concentration of PFOS of 1,270 μg/L, similar to median concentrations among manufacturing workers (Fu et al., 2016;Gao et al., 2015), corresponded to a urine concentration of 0.6 μg/L in this study, also similar to these manufacturing workers’ median urinary concentrations. Higher concentrations in serum compared to urine also agree with studies of children, adults and pregnant women from the general population of Canada, China, and South Korea (Genuis et al., 2013;Kim et al., 2019;Kim et al., 2014;Li et al., 2013;Zhang et al., 2015;Zhang et al., 2013), and of highly exposed populations, including occupationally-exposed Chinese workers (Fu et al., 2016;Gao et al., 2015;Zhou et al., 2014), and people accidentally exposed to PFAS through contaminated water or dust (Beesoon et al., 2012;Worley et al., 2017). Together, the 2013–2014 NHANES findings confirm that for long-chain PFAS, serum is the most appropriate biomonitoring matrix for exposure assessment regardless of the exposure type (e.g., background vs occupational). We acknowledge, however, that for experimental research and pharmacokinetic studies, using matrices other than serum (e.g., urine) may provide relevant information to better understand the biological fate of PFAS.
Of the eight short-chain and alternative PFAS examined in 2013–2014 NHANES, 13.3%, 22.6%, 1.2%, and 1.1% of the general population had detectable urinary concentrations of PFBA, PFHxA, GenX, and PFHpA, respectively. However, concentrations were low (e.g., 95th percentile was 3–5 times the LOD of 0.1 μg/L). Considering the relatively short half-life of short-chain PFAS in humans (i.e., days to ~4 weeks) (Chang et al., 2008;Olsen et al., 2009) and assuming a similar persistence for GenX in humans based on data in rodents and primates (Gannon et al., 2016), the NHANES results do not support widespread exposure to these PFAS in the general U.S. population. Occupational studies and/or studies among residents of communities exposed through drinking water can provide important information to evaluate whether exposures may be restricted to populations who live or work nearby exposure sources.
GenX, introduced as a PFOA substitute, was detected in the Cape Fear River Basin in North Carolina (Sun et al., 2016), but not in select area residents’ urine (Pritchett et al., 2019) or serum (Ahearn, 2019;Pritchett et al., 2019). One investigation involved 30 people ≥12 years of age living near a manufacturing facility and whose drinking water private wells had an average GenX concentration of 680 ppt (Pritchett et al., 2019). The other investigation included 345 individuals, 56 of them children, whose tap water contained a median GenX concentration of 50 ppt (Ahearn, 2019).
PFBA may result from industrial synthesis, metabolism, and environmental degradation of certain fluorinated chemicals (Benskin et al., 2012;Chang et al., 2008;D’Eon and Mabury, 2007). PFBA has been detected in precipitation, surface waters, water treatment facility effluents, and drinking water sources (Landsteiner et al., 2014;Llorca et al., 2017;Mak et al., 2009;Scott et al., 2006;Skutlarek et al., 2006;Wilhelm et al., 2010;Yang et al., 2012), and in the serum of people with potential exposure to PFBA through occupation and/or contaminated drinking water (Chang et al., 2008;Landsteiner et al., 2014). The fact that 96% of serum PFBA concentrations among 177 adults with plausible exposure via contaminated drinking water were <2 μg/L (Chang et al., 2008)—within ranges of the median serum concentrations in American Red Cross adult blood donors (Olsen et al., 2011) in 2000–2001 (2.3 μg/L) and 2006 (0.4 μg/L)—and its postulated 2–4 days average human serum elimination half-life (Chang et al., 2008) suggest that PFBA eliminates efficiently from the body.
PFHxA is an impurity of, and a metabolite and degradation product of fluorotelomer-based products present in the market since the 1970s (Anderson et al., 2019). PFHxA may also be used in fluorinated polymer production, aqueous firefighting foams, water/grease repellents, food/pharmaceutical packaging, and other commercial products (Anderson et al., 2019). However, because PFHxA detection in water is generally low and infrequent, drinking water might not be a PFHxA exposure route of concern for the general population (Anderson et al., 2019). In experimental studies, PFHxA doesn’t bioaccumulate in mammals (Anderson et al., 2019;Conder et al., 2008;Russell et al., 2013). Based on exposure information from professional ski wax technicians, its geometric mean elimination half-life was estimated to be 32 days (Russell et al., 2013). Compared to long-chain PFAS, PFHxA human exposure data are more limited, and concentrations much lower (e.g., serum medians range from non-detectable to 0.62 μg/L) (Anderson et al., 2019;Kim et al., 2019;Lee et al., 2017;Olsen et al., 2017;Wan et al., 2013;Zhou et al., 2016).
Together, these 2013–2014 NHANES results, investigations of populations whose drinking water was contaminated with short-chain and alternative PFAS, and occupational studies do not support recent exposure to GenX, PFBA, PFHxA among the U.S. general population, and even among individuals known to have detectable levels of these chemicals in their drinking water. Of note, even though these PFAS may eliminate relatively quickly from the human body, they will remain much longer in the environment (Ahearn, 2019;Gomis et al., 2015;Wang et al., 2015).
Two short-chain PFAS, PFHpA and PFBS, were detected more often in the 2013–2014 NHANES participants’ serum (CDC, 2019;Ye et al., 2018)) than in their urine. These PFAS have shorter biological persistence than long-chain PFAS. For example, PFBS serum elimination half-life in humans is 26 days (Olsen et al., 2009); although unknown for PFHpA (in rats, its half-life was shorter than that of PFOA (Ohmori et al., 2003)), it was proposed to be <4 weeks for PFHxA (Nilsson et al., 2010). Similar PFHpA concentration and detection patterns of paired serum/urine results (PFBS was not measured) were reported for 86 Chinese adults (22–88 years) sampled in 2010 (Zhang et al., 2013), and seven members 15–52 years of age of a Canadian family, sampled in 2008, who were exposed to higher than background concentrations of PFHxS from use of home carpet treatment products (Beesoon et al., 2012). Likewise, among 94 South Koreans 2–82 years sampled in 2014–2015, serum concentrations were higher than urine concentrations for PFHpA, although lower for PFBS (Kim et al., 2019). On the other hand, in a group of 120 South Korean children 5–13 years sampled in 2013 (Kim et al., 2014), mean concentrations of PFHpA and PFBS were higher in urine (1.35 μg/L and 0.492 μg/L) than in serum (0.312 μg/L and 0.105 μg/L), respectively. The observed discrepancies in concentration and detection patterns among the 2013–2014 NHANES and other populations may relate to differences in analytical methods and/or differences in representativeness of the examined populations. Nevertheless, in occupationally-exposed workers with concentrations considerably higher than those among the U.S. general population, serum concentrations were higher than urine concentrations for select short-chain PFAS. For example, Chinese workers from fisheries in an area close to several fluorochemical manufacturers had higher concentrations of PFHpA and PFBS in serum (median, 0.08 μg/L and 11.3 μg/L) than in urine (median, 0.0159 μg/L and 1.57 μg/L), respectively (Zhou et al., 2014). In six American workers followed for 180 days, PFBS concentrations were also higher in serum than in urine (Olsen et al., 2009). The results from these two occupational studies, and from 2013–2014 NHANES in this study suggest that serum concentrations may also be adequate for exposure assessment of short-chain PFAS with biological half-lives of several days to weeks.
Conclusions
Our results indicate that the majority of the U.S. general population in 2013–2014 did not have detectable urinary concentrations of 14 C4-C11 PFAS and three fluorinated PFAS alternatives. A small percentage of the population was estimated to have detectable—albeit relatively low and often close to the limit of detection—urinary concentrations of PFHxA (27.2%), PFBA (13.3%), PFHpA (1.1%), the fluorinated alternative GenX (1.2%), and no detectable concentrations of PFBS or the two other fluorinated alternatives. Therefore, the 2013–2014 NHANES urine PFAS data do not support widespread exposure to short-chain PFAS or fluorinated alternatives (e.g., GenX) in the U.S. general population although exposures in select populations who live or work nearby exposure sources may occur. In addition, these findings highlight challenges to assess human exposure to relatively non-biologically persistent PFAS detected in the environment at or below parts-per-trillion levels, in population-based studies.
Noteworthy, fewer than 0.1% of the U.S. general population was estimated to have detectable urinary concentrations of long-chain PFAS, including PFOS, PFOA, PFHxS, PFNA, even though 2013–2014 NHANES serum data suggested universal exposure to these legacy PFAS among the general population. These findings stress the importance of selecting the proper biomonitoring matrix. Considering that serum is the best biomonitoring matrix for long-chain PFAS, we conclude that, regardless of the exposure scenario, concentrations in serum of PFAS with biological half-lives of several days to weeks will also provide the best biomonitoring exposure assessment.
Supplementary Material
Acknowledgements:
We acknowledge John Eng for technical assistance, as well as the critical contributions of the late Xiaoyun Ye to the PFAS program until her untimely passing in 2018.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC. Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services. The authors declare no competing financial interest.
REFERENCES
- Ahearn A, 2019. A Regrettable Substitute: The Story of GenX. Environ Health Perspect 10.1289/EHP5134. [DOI] [Google Scholar]
- Anderson JK, Luz AL, Goodrum P, Durda J, 2019. Perfluorohexanoic acid toxicity, part II: Application of human health toxicity value for risk characterization. Regul. Toxicol Pharmacol. 103, 10–20. [DOI] [PubMed] [Google Scholar]
- ATSDR. 2015. Draft Toxicological Profile for Perfluoroalkyls. In: Atlanta, GA:Agency for Toxic Substances and Disease Registry; Available at: http://www.atsdr.cdc.gov/toxprofiles/tp200.pdf. [PubMed] [Google Scholar]
- Bartell SM, Calafat AM, Lyu C, Kato K, Ryan PB, Steenland K, 2010. Rate of Decline in Serum PFOA Concentrations after Granular Activated Carbon Filtration at Two Public Water Systems in Ohio and West Virginia. Environ. Health Perspect. 118, 222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesoon S, Genuis SJ, Benskin JP, Martin JW, 2012. Exceptionally High Serum Concentrations of Perfluorohexanesulfonate in a Canadian Family are Linked to Home Carpet Treatment Applications. Environ. Sci. Technol. 46, 12960–12967. [DOI] [PubMed] [Google Scholar]
- Benskin JP, Ikonomou MG, Woudneh MB, Cosgrove JR, 2012. Rapid characterization of perfluoralkyl carboxylate, sulfonate, and sulfonamide isomers by high-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 1247, 165–170. [DOI] [PubMed] [Google Scholar]
- Brendel S, Fetter E, Staude C, Vierke L, Biegel-Engler A, 2018. Short-chain perfluoroalkyl acids: environmental concerns and a regulatory strategy under REACH. Environ Sci Eur. 30, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, Jensen AA, Kannan K, Mabury SA, Van Leeuwen SPJ, 2011. Perfluoroalkyl and Polyfluoroalkyl Substances in the Environment: Terminology, Classification, and Origins. Integr. Environ. Assess. Manag. 7, 513–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. 2013. National Health and Nutrition Examination Survey: Plan and Operations, 1999–2010. In: Centers for Disease Control and Prevention; Available at: http://www.cdc.gov/nchs/data/series/sr_01/sr01_056.pdf. [Google Scholar]
- CDC. 2019. Fourth National Report on Human Exposure to Environmental Chemicals. Updated Tables, January 2019. In: Atlanta, GA:Centers for Disease Control and Prevention; National Center for Environmental Health; Division of Laboratory Sciences; Available at: http://www.cdc.gov/exposurereport. [Google Scholar]
- Chang SC, Das K, Ehresman DJ, Ellefson ME, Gorman GS, Hart JA, Noker PE, Tan YM, Lieder PH, Lau C, Olsen GW, Butenhoff JL, 2008. Comparative pharmacokinetics of perfluorobutyrate in rats, mice, monkeys, and humans and relevance to human exposure via drinking water. Toxicol. Sci. 104, 40–53. [DOI] [PubMed] [Google Scholar]
- Conder JM, Hoke RA, De Wolf W, Russell MH, Buck RC, 2008. Are PFCAs bioaccumulative? A critical review and comparison with regulatory lipophilic compounds. Environ. Sci. Technol. 42, 995–1003. [DOI] [PubMed] [Google Scholar]
- D’Eon JC, Mabury SA, 2007. Production of perfluorinated carboxylic acids (PFCAs) from the biotransformation of polyfluoroalkyl phosphate surfictants (PAPS): Exploring routes of human contamination. Environ. Sci. Technol. 41, 4799–4805. [DOI] [PubMed] [Google Scholar]
- Daly ER, Chan BP, Talbot EA, Nassif J, Bean C, Cavallo SJ, Metcalf E, Simone K, Woolf AD, 2018. Per- and polyfluoroalkyl substance (PFAS) exposure assessment in a community exposed to contaminated drinking water, New Hampshire, 2015. Int J Hyg. Environ Health 221, 569–577. [DOI] [PubMed] [Google Scholar]
- Dewitt JC, 2015. Toxicological Effects of Perfluoroalkyl and Polyfluoroalkyl Substances. Humana Press. [Google Scholar]
- Fromme H, Wockner M, Roscher E, Volkel W, 2017. ADONA and perfluoroalkylated substances in plasma samples of German blood donors living in South Germany. Int J Hyg. Environ Health 220, 455–460. [DOI] [PubMed] [Google Scholar]
- Fu J, Gao Y, Cui L, Wang T, Liang Y, Qu G, Yuan B, Wang Y, Zhang A, Jiang G, 2016. Occurrence, temporal trends, and half-lives of perfluoroalkyl acids (PFAAs) in occupational workers in China. Scientific Reports 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon SA, Fasano WJ, Mawn MP, Nabb DL, Buck RC, Buxton LW, Jepson GW, Frame SR, 2016. Absorption, distribution, metabolism, excretion, and kinetics of 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)propanoic acid ammonium salt following a single dose in rat, mouse, and cynomolgus monkey. Toxicology 340, 1–9. [DOI] [PubMed] [Google Scholar]
- Gannon SA, Johnson T, Nabb DL, Serex TL, Buck RC, Loveless SE, 2011. Absorption, distribution, metabolism, and excretion of [1-C-14]-perfluorohexanoate ([C-14]-PFHx) in rats and mice. Toxicology 283, 55–62. [DOI] [PubMed] [Google Scholar]
- Gao Y, Fu J, Cao H, Wang Y, Zhang A, Liang Y, Wang T, Zhao C, Jiang G, 2015. Differential accumulation and elimination behavior of perfluoroalkyl acid isomers in occupational workers in a manufactory in China. Environ. Sci. Technol. 49, 6953–6962. [DOI] [PubMed] [Google Scholar]
- Gebbink WA, Van Asseldonk L, Van Leeuwen SPJ, 2017. Presence of Emerging Per- and Polyfluoroalkyl Substances (PFASs) in River and Drinking Water near a Fluorochemical Production Plant in the Netherlands. Environ. Sci. Technol. 51, 11057–11065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genuis SJ, Beesoon S, Birkholz D, 2013. Biomonitoring and Elimination of Perfluorinated Compounds and Polychlorinated Biphenyls through Perspiration: Blood, Urine, and Sweat Study. ISRN. Toxicol 2013, 483832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomis MI, Vestergren R, Borg D, Cousins IT, 2018. Comparing the toxic potency in vivo of long-chain perfluoroalkyl acids and fluorinated alternatives. Environ Int. 113, 1–9. [DOI] [PubMed] [Google Scholar]
- Gomis MI, Wang Z, Scheringer M, Cousins IT, 2015. A modeling assessment of the physicochemical properties and environmental fate of emerging and novel per- and polyfluoroalkyl substances. Sci Total Environ 505, 981–991. [DOI] [PubMed] [Google Scholar]
- Graber JM, Alexander C, Laumbach RJ, Black K, Strickland PO, Georgopoulos PG, Marshall EG, Shendell DG, Alderson D, Mi Z, Mascari M, Weisel CP, 2019. Per and polyfluoroalkyl substances (PFAS) blood levels after contamination of a community water supply and comparison with 2013–2014 NHANES. J Expo. Sci Environ Epidemiol. 29, 172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydebreck F, Tang J, Xie Z, Ebinghaus R, 2015. Alternative and Legacy Perfluoroalkyl Substances: Differences between European and Chinese River/Estuary Systems. Environ. Sci. Technol. 49, 8386–8395. [DOI] [PubMed] [Google Scholar]
- Hoffman K, Webster TF, Bartell SM, Weisskopf MG, Fletcher T, Vieira VM, 2011. Private Drinking Water Wells as a Source of Exposure to Perfluorooctanoic Acid (PFOA) in Communities Surrounding a Fluoropolymer Production Facility. Environ. Health Perspect. 119, 92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer J, Goen T, Rauchfuss K, Kraft M, Angerer J, Kleeschulte P, Wilhelm M, 2009. One-year follow-up of perfluorinated compounds in plasma of German residents from Arnsberg formerly exposed to PFOA-contaminated drinking water. Int. J. Hyg. Environ. Health 212, 499–504. [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed LD, 1990. Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. Hyg. 5, 46–51. [Google Scholar]
- Ingelido AM, Abballe A, Gemma S, Dellatte E, Iacovella N, De AG, Zampaglioni F, Marra V, Miniero R, Valentini S, Russo F, Vazzoler M, Testai E, De FE, 2018. Biomonitoring of perfluorinated compounds in adults exposed to contaminated drinking water in the Veneto Region, Italy. Environ Int 110, 149–159. [DOI] [PubMed] [Google Scholar]
- Kabore HA, Vo DS, Munoz G, Meite L, Desrosiers M, Liu J, Sory TK, Sauve S, 2018. Worldwide drinking water occurrence and levels of newly-identified perfluoroalkyl and polyfluoroalkyl substances. Sci Total Environ 616–617, 1089–1100. [DOI] [PubMed] [Google Scholar]
- Kato K, Basden BJ, Needham LL, Calafat AM, 2011. Improved selectivity for the analysis of maternal serum and cord serum for polyfluoroalkyl chemicals. J. Chromatogr. A 2133–2137. [DOI] [PubMed] [Google Scholar]
- Kato K, Kalathil AA, Patel AM, Ye X, Calafat AM, 2018. Per- and polyfluoroalkyl substances and fluorinated alternatives in urine and serum by on-line solid phase extraction-liquid chromatography-tandem mass spectrometry. Chemosphere 209, 338–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Lee JH, Oh JE, 2019. Perfluoroalkyl acids in paired serum, urine, and hair samples: Correlations with demographic factors and dietary habits. Environ Pollut 248, 175–182. [DOI] [PubMed] [Google Scholar]
- Kim DH, Lee MY, Oh JE, 2014. Perfluorinated compounds in serum and urine samples from children aged 5–13 years in South Korea. Environ. Pollut. 192, 171–178. [DOI] [PubMed] [Google Scholar]
- Landsteiner A, Huset C, Williams A, Johnson J, 2014. Biomonitoring for Perfluorochemicals in a Minnesota Community With Known Drinking Water Contamination. J. Environ. Health 77, 14–19. [PubMed] [Google Scholar]
- Lee JH, Lee CK, Suh CH, Kang HS, Hong CP, Choi SN, 2017. Serum concentrations of per- and poly-fluoroalkyl substances and factors associated with exposure in the general adult population in South Korea. Int. J. Hyg. Environ. Health 220, 1046–1054. [DOI] [PubMed] [Google Scholar]
- Li JG, Guo FF, Wang YX, Zhang JL, Zhong YX, Zhao YF, Wu YN, 2013. Can nail, hair and urine be used for biomonitoring of human exposure to perfluorooctane sulfonate and perfluorooctanoic acid? Env. Int. 53, 47–52. [DOI] [PubMed] [Google Scholar]
- Llorca M, Farre M, Eljarrat E, Diaz-Cruz S, Rodriguez-Mozaz S, Wunderlin D, Barcelo D, 2017. Review of emerging contaminants in aquatic biota from Latin America: 2002–2016. Environ. Toxicol. Chem. 36, 1716–1727. [DOI] [PubMed] [Google Scholar]
- Mak YL, Taniyasu S, Yeung LWY, Lu GH, Jin L, Yang YL, Lam PKS, Kannan K, Yamashita N, 2009. Perfluorinated Compounds in Tap Water from China and Several Other Countries. Environ. Sci. Technol. 43, 4824–4829. [DOI] [PubMed] [Google Scholar]
- Needham LL, Calafat AM, Barr DB, 2007. Uses and issues of biomonitoring. Int. J. Hyg. Environ Health 210, 229–238. [DOI] [PubMed] [Google Scholar]
- Nilsson H, Karrman A, Westberg H, Rotander A, van Bavel B, Lindstrom G, 2010. A Time Trend Study of Significantly Elevated Perfluorocarboxylate Levels in Humans after Using Fluorinated Ski Wax. Environ. Sci. Technol. 44, 2150–2155. [DOI] [PubMed] [Google Scholar]
- Ohmori K, Kudo N, Katayama K, Kawashima Y, 2003. Comparison of the toxicokinetics between perfluorocarboxylic acids with different carbon chain length. Toxicology 184, 135–140. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, Zobel LR, 2007. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ. Health Perspect. 115, 1298–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, Chang SC, Noker PE, Gorman GS, Ehresman DJ, Lieder PH, Butenhoff JL, 2009. A comparison of the pharmacokinetics of perfluorobutanesulfonate (PFBS) in rats, monkeys, and humans. Toxicology 256, 65–74. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Ellefson ME, Mair DC, Church TR, Goldberg CL, Herron RM, Medhdizadehkashi Z, Nobiletti JB, Rios JA, Reagen WK, Zobel LR, 2011. Analysis of a Homologous Series of Perfluorocarboxylates from American Red Cross Adult Blood Donors, 2000–2001 and 2006. Environ. Sci. Technol. 45, 8022–8029. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Mair DC, Lange CC, Harrington LM, Church TR, Goldberg CL, Herron RM, Hanna H, Nobiletti JB, Rios JA, Reagen WK, Ley CA, 2017. Per- and polyfluoroalkyl substances (PFAS) in American Red Cross adult blood donors, 2000–2015. Environ. Res. 157, 87–95. [DOI] [PubMed] [Google Scholar]
- Pan Y, Wang J, Yeung LWY, Wei S, and Dai J 2019. Analysis of emerging per- and polyfluoroalkyl substances: Progress and current issues. Trends in Analytical Chemistry, 10.1016/j.trac.2019.04.013. [DOI] [Google Scholar]
- Pan Y, Zhang H, Cui Q, Sheng N, Yeung LWY, Guo Y, Sun Y, Dai J, 2017. First Report on the Occurrence and Bioaccumulation of Hexafluoropropylene Oxide Trimer Acid: An Emerging Concern. Environ Sci Technol. 51, 9553–9560. [DOI] [PubMed] [Google Scholar]
- Pritchett JR, Rinsky JL, Dittman B, Christensen A, Langley R, Moore Z, Fleischauer AT, Koehler K, Calafat A, Rogers R, Esters L, Jenkins R, Collins F, Conner D, and Breysse P 7–26-2019. Targeted Biomonitoring for GenX and Other Per- and Polyfluoroalkyl Substances (PFAS)—North Carolina, 2018. MMWR Morb Mortal Wkly Rep. 2019. July 26 In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell MH, Nilsson H, Buck RC, 2013. Elimination kinetics of perfluorohexanoic acid in humans and comparison with mouse, rat and monkey. Chemosphere 93, 2419–2425. [DOI] [PubMed] [Google Scholar]
- Scott BF, Spencer C, Mabury SA, Muir DCG, 2006. Poly and perfluorinated carboxylates in north American precipitation. Environ. Sci. Technol. 40, 7167–7174. [DOI] [PubMed] [Google Scholar]
- Skutlarek D, Exner M, Farber H, 2006. Perfluorinated surfactants in surface and drinking water. Environ. Sci. Pollut. Res. Int. 13, 299–307. [DOI] [PubMed] [Google Scholar]
- Stubleski J, Salihovic S, Lind PM, Lind L, Dunder L, McCleaf P, Euren K, Ahrens L, Svartengren M, van Bavel B, Karrman A, 2017. The effect of drinking water contaminated with perfluoroalkyl substances on a 10-year longitudinal trend of plasma levels in an elderly Uppsala cohort. Environ. Res. 159, 95–102. [DOI] [PubMed] [Google Scholar]
- Sun M, Arevalo E, Strynar M, Lindstrom A, Richardson M, Kearns B, Pickett A, Smith C, Knappe DRU, 2016. Legacy and Emerging Perfluoroalkyl Substances Are Important Drinking Water Contaminants in the Cape Fear River Watershed of North Carolina. Environ. Sci. Technol. Lett. 3, 415–419. [Google Scholar]
- Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG (2019) A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J Expo Sci Environ Epidemiol 29:131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US EPA. 2018. GenX and PFBS Draft Toxicity Assessments. In https://www.epa.gov/pfas/genx-and-pfbs-draft-toxicity-assessments.
- Wan HT, Leung PY, Zhao YG, Wei X, Wong MH, Wong CKC, 2013. Blood plasma concentrations of endocrine disrupting chemicals in Hong Kong populations. J. Hazard. Mater. 261, 763–769. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chang W, Wang L, Zhang Y, Zhang Y, Wang M, Wang Y, Li P. 2019. A review of sources, multimedia distribution and health risks of novel fluorinated alternatives. Ecotoxicol Environ Saf. 2019 July 4;182:109402. doi: 10.1016/j.ecoenv.2019.109402. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Wang Z, Cousins IT, Scheringer M, Hungerbuehler K, 2015. Hazard assessment of fluorinated alternatives to long-chain perfluoroalkyl acids (PFAAs) and their precursors: status quo, ongoing challenges and possible solutions. Environ Int 75, 172–179. [DOI] [PubMed] [Google Scholar]
- Wei C, Wang Q, Song X, Chen X, Fan R, Ding D, Liu Y, 2018. Distribution, source identification and health risk assessment of PFASs and two PFOS alternatives in groundwater from non-industrial areas. Ecotoxicol. Environ Saf. [DOI] [PubMed] [Google Scholar]
- Wilhelm M, Bergmann S, Dieter HH, 2010. Occurrence of perfluorinated compounds (PFCs) in drinking water of North Rhine-Westphalia, Germany and new approach to assess drinking water contamination by shorter-chained C4-C7 PFCs. Int. J. Hyg. Environ. Health 213, 224–232. [DOI] [PubMed] [Google Scholar]
- Worley RR, Moore SM, Tierney BC, Ye X, Calafat AM, Campbell S, Woudneh MB, Fisher J, 2017. Per- and polyfluoroalkyl substances in human serum and urine samples from a residentially exposed community. Env. Int. 106, 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LP, Tian SY, Zhu LY, Liu ZT, Zhang YH, 2012. Bioaccumulation and distribution of perfloroalkyl acids in seafood products from Bohai Bay, China. Environ. Toxicol. Chem. 31, 1972–1979. [DOI] [PubMed] [Google Scholar]
- Ye X, Kato K, Wong LY, Jia T, Kalathil A, Latremouille J, Calafat AM, 2018. Per- and polyfluoroalkyl substances in sera from children 3 to 11 years of age participating in the National Health and Nutrition Examination Survey 2013–2014. Int. J. Hyg. Environ Health 221, 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Sun H, Qin X, Gan Z, Kannan K, 2015. PFOS and PFOA in paired urine and blood from general adults and pregnant women: assessment of urinary elimination. Environ. Sci. Pollut. Res. Int. 22, 5572–5579. [DOI] [PubMed] [Google Scholar]
- Zhang YF, Beesoon S, Zhu LY, Martin JW, 2013. Biomonitoring of Perfluoroalkyl Acids in Human Urine and Estimates of Biological Half-Life. Environ. Sci. Technol. 47, 10619–10627. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Hu LW, Qian Z, Chang JJ, King C, Paul G, Lin S, Chen PC, Lee YL, Dong GH, 2016. Association of perfluoroalkyl substances exposure with reproductive hormone levels in adolescents: By sex status. Env. Int. 94, 189–195. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Shi YL, Vestergren R, Wang T, Liang Y, Cai YQ, 2014. Highly Elevated Serum Concentrations of Perfluoroalkyl Substances in Fishery Employees from Tangxun Lake, China. Environ. Sci. Technol. 48, 3864–3874. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.