Abstract
Study Objectives
Obstructive sleep apnea (OSA) is associated with cardiovascular and cerebrovascular morbidity. Patients with sickle cell disease (SCD) are at increased risk for both neurologic complications (NC) and OSA. However, the relationship between OSA and SCD complications is unclear. We hypothesized that there would be an association between OSA diagnosis and SCD complications.
Methods
Hospital discharge records of patients with SCD aged < 19 years were obtained for the years 1997, 2000, 2003, 2006, 2009, and 2012 from the Kid’s Inpatient Database. The primary outcome, NC, a composite of stroke, transient ischemic attack, and seizures. Secondary outcomes included acute chest syndrome (ACS), vaso-occlusive crisis, length of hospital stay, and inflation-adjusted cost of hospitalization. Multivariable regression was conducted to ascertain the association of OSA with primary and secondary outcomes. Analyses were adjusted for the use of noninvasive mechanical ventilation (NIMV) to determine its role as NC risk modifier.
Results
There were 203,705 SCD discharges included in the analysis, of which 2,820 (1.4%) and 4,447 (2.2%) also included OSA and NC diagnoses. Multivariable logistic regression indicated that OSA was associated with NC (adjusted odds ratio [OR], 1.50 [95% CI 1.02–2.21], p = 0.039) and ACS (OR, 1.34 [95% CI 1.08–1.67], p = 0.009) in children with SCD. In the multivariable analysis adjusted for NIMV, the significant association between OSA and NC was no longer observed (OR, 1.39 [95% CI 0.94–2.05], p = 0.100).
Conclusions
OSA is associated with a 50% increase of odds of NC in children with SCD in this nationwide dataset. The use of NIMV to treat OSA may modify the risk of OSA-associated NC.
Keywords: neurologic complications, obstructive sleep apnea, hospitalization, Kids’ inpatient database, sickle cell disease
Statement of Significance.
In this cross-sectional study of 203,705 children with sickle cell disease discharged in the United States from 1997 to 2012, children with obstructive sleep apnea (OSA) were at 50% increased odds of having neurologic complications (NC) as compared with those without OSA. Importantly, the use of noninvasive mechanical ventilation may modify the risk of NC.
Introduction
Sickle cell disease (SCD) is a hemoglobinopathy with a prevalence of 17 cases per 10,000 people among African Americans [1]. The point mutation in the β-globin gene results in sickle hemoglobin that is less soluble and more prone to polymerization during deoxygenation [2, 3]. This feature leads to various acute and chronic complications that impose great health and economic burden [4]. Common complications associated with SCD include acute chest syndrome (ACS), pain crisis, neurologic complications (NC; e.g. stroke and seizures), anemia, and infection [5, 6]. Stroke, for example, affects about 0.001%–0.01% of the general pediatric population in the United States [7, 8], while occurs in approximately 10% of children with hemoglobin SS under the age of 20 years [9, 10]. Seizures are considered part of NC as they can be the first manifestation of strokes in children [11, 12].
Obstructive sleep apnea (OSA) is a disorder of breathing during sleep that results in fragmented sleep and intermittent hypoxemia from recurrent partial or complete obstruction of the upper airway [13]. In children, untreated OSA results in multiple adverse health outcomes, including neurobehavioral problems and cardiovascular complications [14–16]. In adults, OSA is associated with increased risks of NC, such as stroke and transient ischemic attack (TIA) [17–19]. OSA has a prevalence of 1%–5% among children in the United States and has been reported to be higher in children with SCD [1, 14, 20]. The higher prevalence of OSA among SCD children has been partially attributed to the adenotonsillar hypertrophy as a compensatory mechanism for the loss of splenic function [21]. It has been posited that nocturnal hypoxemia from OSA would exacerbate the NC of SCD [1, 22].
Several studies have examined the association between OSA and NC in patients with SCD [23–25], and the effect of adenotonsillectomy on the reduction of NC in SCD, but the results are mixed due to small sample sizes [26, 27]. The mixed results from previous studies might be partially explained by the inability to account for confounders and the limited statistical power as NC were not as prevalent among children. Therefore, we aimed to address this issue by utilizing the Kids Inpatient Database (KID) [28], a US nationwide inpatient database to assess the association between OSA and NC in children with SCD. We hypothesized that OSA would be associated with NC among children with SCD.
Methods
Study design and population
This retrospective cohort study was conducted using the hospital discharge data from patients with SCD aged 0–18 years for the years 1997, 2000, 2003, 2006, 2009, and 2012 from the US Representative Kids’ Inpatient Database (KID) [28]. The KID is an administrative all-payer inpatient care database made of a stratified sample of pediatric discharges from all short-term, nonfederal, general, and specialty hospitals and includes sampling weights to generate national estimates of annual pediatric hospitalizations, outcomes, and healthcare costs [28]. The KID data are released every 3 years with approximately 3 million pediatric discharges in each sample from participating states [28]. As all data obtained were deidentified, IRB approval was not needed.
Hospitalized children with SCD were identified using the International Classification of Diseases, 9th revision, Clinical Modification (ICD-9-CM) code. SCD was defined based on the diagnostic codes in any diagnosis fields validated by previous studies (Supplementary Table S1) [29–31].
Measurements of variables
The following patient characteristics were included in the analyses: age, gender, race, type of insurance, median household income quartiles based on counties of residence, and admission season. Hispanic is included as part of the race instead of ethnicity per the reporting method of KID. Hospital characteristics were also included in the analyses, including hospital region, teaching status of the hospital, and hospital size depending on the region of the hospital. Comorbidities, procedures or treatments of patients ascertained using diagnostics codes validated in previous studies were also included, including OSA, sleep-disordered breathing (SDB), hypertension, obesity, congenital heart diseases, coagulopathy, types of SCD, diabetes mellitus (DM), asthma, iron-deficiency anemia (IDA), central sleep apnea (CSA), blood transfusion, cranial angiography, tonsillectomy and/or adenoidectomy (AT), and noninvasive mechanical ventilation (NIMV). Supplementary Table S1 provides the full list of ICD-9-CM codes used in the analysis.
Definition of outcomes
The primary outcome was NC. This is a composite outcome consisting of stroke (e.g. ischemic stroke and hemorrhagic stroke), TIA, and seizures. Seizures were included as a part of NC as they can be the first manifestation of strokes in children [11, 12]. Secondary outcomes included ACS, vaso-occlusive crisis (VOC), length of hospital stay in days, and inflation-adjusted cost of hospitalization. To allow for direct comparisons between years for hospital charges accounting for inflation, we converted all charges to 2012 US dollars using the average Consumer Price Index. Similarly, ICD-9-CM codes validated in previous studies were used to ascertain the abovementioned outcomes (Supplementary Table S1) [31–34].
Statistical analysis
Categorical variables were presented as counts and proportions, and the differences between groups were tested using Pearson’s χ 2 test. Continuous variables were summarized as mean with standard deviation (SD), and the differences between groups were examined using the t-test. Two-way analysis of variance was performed for seasonal variations. It was determined a priori to conduct comparisons between patients with and without OSA, and between years. For comparison between years, three subperiods were created: subperiod 1 (years 1997 and 2000), subperiod 2 (years 2003 and 2006), and subperiod 3 (years 2009 and 2012).
To determine the association between OSA and NC in children with SCD, multivariable logistic regression analyses adjusting for patient characteristics, hospital characteristics, patients’ comorbidities, and treatment received were performed. The final model was adjusted for the following variables: age categories, gender, race, hospital region, hospital size, household incomes, congenital heart disease, coagulopathy, obesity, hypertension, asthma, types of SCD, blood transfusion, and AT. Variables adjusted in the model initially were determined based on potential relevance given the literature and then were further refined using a statistical backward selection approach. Similar analyses were also performed for binary secondary outcomes, including ACS and VOC. Of note, patients in 1997 and 2000 were not included in the analyses for ACS as the ICD-9 codes for ACS (517.3) was not coined during these years. For continuous secondary outcomes, including the length of hospital stay and inflation-adjusted cost of hospitalization, multivariable linear regression analyses accounting for the abovementioned variables were also conducted. Analyses were conducted with and without the inclusion of NIMV or AT as both were considered effective treatments for OSA [14, 35]. If the association between OSA and NC altered after inclusion of the treatment for OSA (e.g. NIMV and AT), it would suggest that the treatment may modify the risk of NC associated with OSA in SCD patients. A sensitivity analysis that combined OSA with SDB was performed to further delineate the association of the big SDB umbrella with primary and secondary outcomes.
All the analyses were accounted for sampling weights provided in the KID. Data were presented consistent with Healthcare Cost and Utilization Project reporting methodologies [28]. All tests were two-sided, and a p-value of less than 0.05 was considered statistically significant. Statistical analysis was conducted using Stata 14.0 (Stata Corp, College Station, TX)
Results
Demographic and patient characteristics
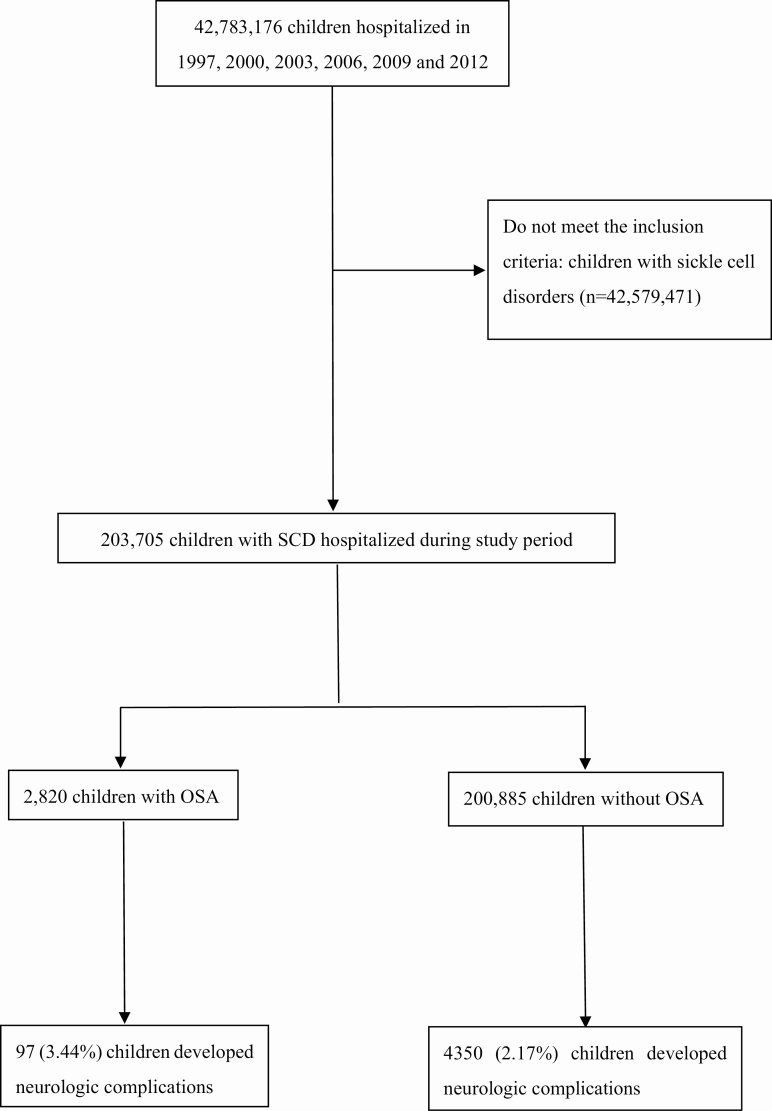
There were 203,705 SCD discharges during the study period (Figure 1). There was a total of 36,382 patient discharges in 1997, 31,218 in 2000, 35,557 in 2003, 32,613 in 2006, 34,422 in 2009, and 35,512 in 2012 among children with SCD. Among discharged children with SCD, 1.38% (n = 2,820) carried a diagnosis of OSA. Compared with children with SCD without OSA, those with OSA were older, more likely to utilize public insurance (e.g. Medicare/Medicaid), and more likely to receive care at teaching hospitals and hospitals in the western United States (Table 1). Additionally, children with OSA were more likely to have hypertension, DM, asthma, and congenital heart disease; and more likely to receive AT, transfusion, cranial angiography, and NIMV. Across the subperiods, there were notable trend changes regarding age, female sex, race, season of hospital admission, household income, hospital region, hospital size, teaching status of hospitals, hypertension, DM, IDA, congenital heart disease, OSA, blood transfusion, NIMV, and cranial angiography (Supplementary Table S2). The proportion of hospitalizations associated with NC among children with SCD across study periods were stratified by age categories and months (Supplemental Figure S1). Children aged 15–18 years old accounted for the majority of the discharges. However, there were no significant changes across months (p = 0.97).
Figure 1.
Construction of the study cohort. Schematic illustrating of the construction of the study cohort using the KID.
Table 1.
Characteristics of children with SCD stratified by obstructive sleep apnea
| Total (n = 203,705) | No OSA (n = 200,885) | OSA (n = 2,820) | P-value | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age, mean (SD) | 9.34 (0.05) | 9.33 (0.05) | 9.92 (0.17) | 0.0001 |
| Age categories | ||||
| 0–4 years, n (%) | 56,787 (27.88) | 56,359 (28.06) | 428 (15.19) | <0.0001 |
| 5–9 years, n (%) | 43,075 (21.15) | 42,147 (20.98) | 928 (32.91) | |
| 10–14 years, n (%) | 49,693 (24.39) | 48,871 (24.33) | 822 (29.17) | |
| 15–18 years, n (%) | 54,149 (26.58) | 53,508 (26.64) | 641 (22.74) | |
| Gender | ||||
| Female, n (%) | 99,400 (48.80) | 98,055 (48.81) | 1,345 (47.70) | 0.3179 |
| Obesity, n (%) | 1,103 (0.54) | 925 (0.46) | 178 (6.31) | <0.0001 |
| Race | ||||
| White, n (%) | 3,368 (1.65) | 3,313 (1.65) | 55 (1.95) | 0.4912 |
| Black, n (%) | 156,650 (76.90) | 154,415 (76.87) | 2,235 (79.27) | |
| Hispanic, n (%) | 8,886 (4.36) | 8,779 (4.37) | 107 (3.80) | |
| Asian or Pacific Islander, n (%) | 393 (0.19) | N/A | N/A | |
| Native American, n (%) | 149 (0.07) | N/A | N/A | |
| Others, n (%) | 3,519 (1.73) | 3,476 (1.73) | 43 (1.51) | |
| Admission season | ||||
| Spring (Mar–May), n (%) | 44,133 (21.67) | 43,441 (21.62) | 692 (24.56) | 0.0058 |
| Summer (Jun–Aug), n (%) | 39,377 (19.33) | 38,775 (19.30) | 602 (21.34) | |
| Fall (Sep–Nov), n (%) | 43,327 (21.27) | 42,690 (21.25) | 637 (22.58) | |
| Winter (Dec–Feb), n (%) | 45,915 (22.54) | 45,305 (22.55) | 610 (22.55) | |
| Insurance | ||||
| Public, n (%) | 118,348 (58.10) | 116,512 (58.00) | 1,836 (65.12) | 0.0001 |
| Private, n (%) | 49,117 (24.11) | 48,501 (24.14) | 616 (21.84) | |
| Others, n (%) | 36,008 (17.68) | 35,649 (17.75) | 359 (12.73) | |
| Median household income | ||||
| 0–25%, n (%) | 87,794 (43.10) | 86,586 (43.10) | 1,208 (42.83) | 0.6028 |
| 26–50%, n (%) | 49,930 (24.51) | 49,277 (24.53) | 653 (23.15) | |
| 51–75%, n (%) | 34,876 (17.12) | 34,379 (17.11) | 497 (17.63) | |
| 76–100%, n (%) | 25,752 (12.64) | 25,356 (12.62) | 395 (14.01) | |
| Hospital region | ||||
| Northeast, n (%) | 50,037 (24.56) | 49,336 (24.56) | 702 (24.89) | 0.014 |
| Midwest, n (%) | 34,828 (17.10) | 34,420 (17.13) | 408 (14.47) | |
| South, n (%) | 98,632 (48.42) | 97,333 (48.45) | 1,299 (46.06) | |
| West, n (%) | 20,208 (9.92) | 19,796 (9.85) | 411 (14.59) | |
| Hospital size | ||||
| Small, n (%) | 29,447 (14.46) | 29,081 (14.48) | 366 (12.96) | 0.0846 |
| Medium, n (%) | 41,981 (20.61) | 41,432 (20.62) | 549 (19.48) | |
| Large, n (%) | 125,497 (61.61) | 123,754 (61.60) | 1,743 (61.83) | |
| Teaching hospital, n (%) | 161,407 (79.24) | 158,985 (79.14) | 2,422 (85.89) | <0.0001 |
| Comorbidity | ||||
| Sickle cell anemia type | ||||
| SS, n (%) | 185,206 (90.92) | 182,654 (90.92) | 2,553 (90.54) | 0.9273 |
| S Beta, n (%) | 6,066 (2.98) | 5,987 (2.98) | 79 (2.81) | |
| SC, n (%) | 7,038 (3.45) | 6,929 (3.45) | 109 (3.88) | |
| Asthma, n (%) | 28,620 (14.05) | 27,340 (13.76) | 980 (34.76) | <0.0001 |
| Hypertension, n (%) | 755 (0.37) | 731 (0.36) | 24 (0.84) | 0.0030 |
| Diabetes mellitus, n (%) | 481 (0.24) | 460 (0.23) | 21 (0.74) | 0.0216 |
| Coagulopathy, n (%) | 788 (0.39) | 770 (0.38) | 18 (0.63) | 0.4098 |
| IDA, n (%) | 558 (0.27) | N/A | N/A | 0.1348 |
| Congenital heart diseases, n (%) | 1,058 (0.52) | 1,029 (0.51) | 29 (1.05) | 0.0022 |
| Treatment | ||||
| Transfusion, n (%) | 38,562 (18.93) | 37,703 (18.77) | 859 (30.48) | <0.0001 |
| Cranial angiography, n (%) | 632 (0.31) | 610 (0.30) | 21 (0.75) | 0.0065 |
| NIMV, n (%) | 977 (0.48) | 870 (0.43) | 106 (3.77) | <0.0001 |
| AT, n (%) | 1,434 (0.70) | 523 (0.26) | 911 (32.31) | <0.0001 |
| T&A, n (%) | 1,190 (0.58) | 373 (0.19) | 816 (28.95) | <0.0001 |
| Tonsillectomy, n (%) | 75 (0.04) | 46 (0.02) | 29 (1.03) | <0.0001 |
| Adenoidectomy, n (%) | 169 (0.08) | 103 (0.05) | 66 (2.34) | <0.0001 |
| Outcomes | ||||
| Neurologic complications, n (%) | 4,447 (2.18) | 4,350 (2.17) | 97 (3.45) | 0.0014 |
| Ischemic stroke, n (%) | 702 (0.34) | 688 (0.34) | 14 (0.51) | 0.4729 |
| Hemorrhagic stroke, n (%) | 137 (0.07) | N/A | N/A | |
| TIA, n (%) | 291 (0.14) | N/A | N/A | 0.8903 |
| Seizures, n (%) | 3,415 (1.68) | 3,333 (1.66) | 82 (2.91) | 0.0003 |
| ACS, n (%) | 12,108 (8.90) | 11,842 (8.85) | 266 (11.27) | 0.0028 |
| VOC, n (%) | 126,562 (62.13) | 125,268 (62.36) | 1,295 (45.92) | <0.0001 |
| Cost of hospitalization*, mean (SD) | 17,184 (465) | 17,084 (459) | 24,223 (1,409) | <0.0001 |
| Length of hospitalization (d), mean (SD) | 4.01 (0.04) | 4.01 (0.04) | 3.94 (0.15) | 0.611 |
| Mortality, n (%) | 246 (0.12) | N/A | N/A | 0.0132 |
OSA, obstructive sleep apnea; TIA, transient ischemic attack; IDA, iron deficiency; NIMV, noninvasive mechanical ventilation; ACS, acute chest syndrome; VOC, vaso-occlusion crisis; SD, standard deviation; n, sample size; d, day; N/A, not available as weighted frequency less than 10.
*Cost was adjusted to 2012 USD using an average consumer price index.
Primary outcome
There were 4,447 (2.18%) patients with NC among hospitalized children with SCD (n = 203,705). These consisted of ischemic stroke (n = 702), hemorrhagic stroke (n = 137), TIA (n = 291), and seizures (n = 3,415). Compared with the non-OSA group, the OSA group was more likely to have seizures (2.91% vs. 1.66%, p = 0.0003) but not stroke (0.51% vs. 0.34%, p = 0.4729). Compared with children without OSA, children with OSA were more likely to have NC (3.45% vs. 2.17%, p = 0.0014; Table 1).
In multivariable logistic regression models adjusted for age categories, gender, race, hospital region, hospital size, household incomes, congenital heart disease, coagulopathy, obesity, hypertension, asthma, transfusion, and AT, OSA (adjusted odds ratio [OR], 1.50 [95% CI 1.02–2.21], p = 0.039) was significantly associated with NC (Table 2). Multivariable logistic regression models were also performed to explore the impact of OSA on stroke (adjusted OR, 1.37 [95% CI 0.54–3.46], p = 0.510) and seizures (adjusted OR, 1.43 [95% CI 0.96–2.13], p = 0.077; Supplementary Table S3).
Table 2.
Association between NC and obstructive sleep apnea among children with SCD
| Neurologic complication | ||||
|---|---|---|---|---|
| NIMV not included | NIMV included | |||
| Predictors | OR (95% CI) | P-value | OR (95% CI) | P-value |
| Age | ||||
| 0–4 years | 0.51 (0.41–0.63) | <0.0001 | 0.51 (0.41–0.63) | <0.0001 |
| 5–9 years | 1.08 (0.90–1.31) | 0.408 | 1.08 (0.89–1.31) | 0.421 |
| 10–14 years | 1.13 (0.94–1.37) | 0.182 | 1.13 (0.94–1.36) | 0.193 |
| 15–18 years | Ref | Ref | ||
| Gender | ||||
| Female | 0.99 (0.85–1.15) | 0.874 | 0.99 (0.85–1.16) | 0.887 |
| Black | 0.98 (0.82–1.17) | 0.822 | 0.99 (0.83–1.18) | 0.899 |
| Insurance | ||||
| Public | Ref | Ref | ||
| Private | 0.86 (0.70–1.04) | 0.128 | 0.86 (0.71–1.05) | 0.130 |
| Others | 0.92 (0.74–1.15) | 0.459 | 0.92 (0.74–1.15) | 0.473 |
| Median household income | ||||
| 0–25% | Ref | Ref | ||
| 26–50% | 1.08 (0.87–1.34) | 0.487 | 1.08 (0.87–1.34) | 0.490 |
| 51–75% | 1.11 (0.89–1.39) | 0.346 | 1.11 (0.89–1.38) | 0.359 |
| 76–100% | 1.11 (0.89–1.41) | 0.336 | 1.12 (0.89–1.40) | 0.341 |
| Season | ||||
| Spring | Ref | Ref | ||
| Summer | 1.16 (1.03–1.31) | 0.013 | 1.16 (1.03–1.31) | 0.012 |
| Fall | 1.17 (1.02–1.33) | 0.021 | 1.17 (1.02–1.33) | 0.022 |
| Winter | 1.00 (0.89–1.13) | 0.989 | 1.00 (0.89–1.13) | 0.949 |
| Hospital region | ||||
| Northeast | Ref | Ref | ||
| Midwest | 1.11 (0.83–1.48) | 0.475 | 1.12 (0.84–1.50) | 0.424 |
| South | 0.99 (0.76–1.27) | 0.909 | 0.99 (0.77–1.28) | 0.946 |
| West | 1.17 (0.84–1.64) | 0.360 | 1.19 (0.85–1.67) | 0.310 |
| Hospital size | ||||
| Small | Ref | Ref | ||
| Medium | 1.04 (0.75–1.44) | 0.831 | 1.04 (0.75–1.45) | 0.824 |
| Large | 1.30 (0.98–1.72) | 0.067 | 1.30 (0.98–1.71) | 0.071 |
| Asthma | 1.14 (0.96–1.35) | 0.138 | 1.13 (0.95–1.34) | 0.164 |
| HTN | 2.60 (1.66–4.08) | <0.0001 | 2.58 (1.65–4.05) | <0.0001 |
| Obesity | 0.98 (0.47–2.05) | 0.957 | 0.93 (0.44–1.97) | 0.859 |
| Congenital heart diseases | 5.69 (3.53–9.19) | <0.0001 | 5.50 (3.38–8.95) | <0.0001 |
| Coagulopathy | 5.78 (3.15–10.61) | <0.0001 | 5.65 (3.06–10.45) | <0.0001 |
| SS SCA | 1.35 (1.11–1.64) | 0.003 | 1.36 (1.12–1.65) | 0.002 |
| Obstructive sleep apnea | 1.50 (1.02–2.21) | 0.039 | 1.39 (0.94–2.05) | 0.100 |
| Central sleep apnea | 17.12 (1.32–222.89) | 0.030 | 17.49 (1.30–234.88) | 0.031 |
| AT | 0.48 (0.24–0.94) | 0.032 | 0.51 (0.26–1.01) | 0.052 |
| NIMV | N/A | N/A | 2.66 (1.46–4.84) | 0.001 |
| Transfusion | 1.84 (1.57–2.16) | <0.0001 | 1.81 (1.54–2.12) | <0.0001 |
CI, confidence interval; OR, odds ratio; HTN, hypertension; IDA, iron deficiency; NIMV, noninvasive mechanical ventilation; AT, adenotonsillectomy; SCD, sickle cell disease; ACS, acute chest syndrome; VOC, vaso-occlusion crisis.
Given that intermittent hypoxemia associated with OSA could be a mechanism leading to NC that might be ameliorated by AT or NIMV, we adjusted for both treatments of OSA in the multivariable logistic regression model. The adjustment of NIMV made the initially significant association (adjusted OR, 1.50 [95% CI 1.02–2.21], p = 0.039) between OSA and NC non-significant (adjusted OR, 1.39 [95% CI 0.94–2.05], p = 0.10; Table 2), suggesting that NIMV may modify the risk of NC. However, this observation was not seen in the AT-adjusted model. The association between OSA and NC did not change significantly before (adjusted OR, 1.19 [95% CI 0.83–1.72], p = 0.34) and after (adjusted OR, 1.39 [95% CI 0.94–2.05], p = 0.10) including AT in the multivariable logistic regression model.
Secondary outcomes
There were 12,108 (9%) patients with ACS among hospitalized children with SCD (n = 136,104) from 2003 to 2012. Compared with children without OSA, children with OSA were more likely to have ACS (11.27% vs. 8.85%, p = 0.003; Table 1). There were 126,562 (62.13%) patients with VOC among hospitalized children with SCD (n = 203,705). Compared with children without OSA, children with OSA were less likely to have VOC (45.92% vs. 62.36%, p < 0.0001; Table 1). In multivariable logistic regression models adjusted for aforementioned variables, OSA was significantly associated with ACS (adjusted OR, 1.34 [95% CI 1.08–1.67], p = 0.009; Supplementary Table S4). The association became non-significant with the inclusion of NIMV (adjusted OR, 1.13 [95% CI 0.89–1.43], p = 0.319), suggesting that NIMV may modify the risk of ACS. OSA was not significantly associated with VOC on multivariable logistic regression models (adjusted OR, 0.96 [95% CI 0.81–1.15], p = 0.664), and was not affected by the inclusion of NIMV in the regression model (adjusted OR, 0.94 [95% CI 0.79–1.12], p = 0.490).
Hospital utilization and healthcare cost for SCD
Among children with SCD, the mean inflation-adjusted cost of hospitalization was 17,184 (SD, 465) US dollars, and the mean length of hospital stay was 4.01 (SD, 0.04) days. Compared with children without SCD, those with SCD have a higher inflation-adjusted cost of hospitalization (17,084 [SD, 459] vs. 24,223 [SD, 1,409], p < 0.0001) but similar length of hospitalization (4.01 [SD, 0.04] vs. 3.94 [SD, 0.15], p = 0.611; Table 1). Trend analyses were performed comparing different subperiods and showed a significantly shorter length of hospitalization (p < 0.001), and higher inflation-adjusted cost of hospitalization (p < 0.001) in the subperiod of 2009 and 2012 as compared with two other subperiods (Supplementary Table S2).
In multivariable linear regression models adjusted for aforementioned variables, OSA was significantly associated with inflation-adjusted cost (adjusted β, 5,163 [95% CI 2223–8102], p < 0.001) but not length of hospitalization (adjusted β, 0.02 [95% CI–0.28–0.33], p = 0.881; Table 3).
Table 3.
Association between secondary outcomes and obstructive sleep apnea among children with SCD
| Length of hospital stay (d) | Cost of hospitalization (USD)* | |||
|---|---|---|---|---|
| Predictors | β (95% CI) | P value | β (95% CI) | P value |
| Age | ||||
| 0–4 years | −1.39 (−1.52 to −1.25) | <0.0001 | −5060 (−5952 to −4167) | <0.0001 |
| 5–9 years | −1.23 (−1.36 to −1.10) | <0.0001 | −3404 (−4394 to −2414) | <0.0001 |
| 10–14 years | −0.71 (−0.82 to −0.60) | <.0001 | −1986 (−2773 to −1198) | <0.0001 |
| 15–18 years | Ref | Ref | ||
| Female | 0.11 (0.03 to 0.19) | 0.007 | 330 (−327 to 987) | 0.324 |
| Black | −0.06 (−0.21 to 0.08) | 0.394 | −190 (−1539 to 1159) | 0.783 |
| Insurance | ||||
| Public | Ref | Ref | ||
| Private | −0.03 (−0.14 to 0.09) | 0.649 | 1285 (283 to 2286) | 0.012 |
| Others | 0.18 (−0.02 to 0.38) | 0.083 | −877 (−2615 to 862) | 0.323 |
| Median household income | ||||
| 0–25% | Ref | Ref | ||
| 26–50% | 0.15 (0.05 to 0.24) | 0.003 | 992 (169 to 1814) | 0.018 |
| 51–75% | 0.10 (−0.03 to 0.24) | 0.142 | 1105 (82 to 2127) | 0.034 |
| 76–100% | 0.04 (−0.10 to 0.18) | 0.584 | 500 (−951 to 1952) | 0.499 |
| Season | ||||
| Spring | Ref | Ref | ||
| Summer | −0.01 (−0.10 to 0.08) | 0.839 | 1292 (595 to 1989) | <0.001 |
| Fall | 0.05 (−0.05 to 0.14) | 0.339 | 1189 (541 to 1837) | <0.001 |
| Winter | −0.02 (−0.11 to 0.07) | 0.674 | −6 (−610 to 599) | 0.985 |
| Hospital region | ||||
| Northeast | Ref | Ref | ||
| Midwest | −0.19 (−0.42 to 0.03) | 0.090 | −3134 (−6032 to 236) | 0.034 |
| South | −0.37 (−0.56 to −0.19) | <0.0001 | −5129 (−7679 to −2579) | <0.0001 |
| West | 0.19 (−0.15 to 0.54) | 0.264 | 5789 (1953 to 9626) | 0.003 |
| Hospital size | ||||
| Small | Ref | Ref | ||
| Medium | 0.13 (−0.14 to 0.40) | 0.347 | −2041 (−5799 to 1717) | 0.287 |
| Large | 0.13 (−0.10 to 0.36) | 0.281 | −1066 (−4590 to 2459) | 0.553 |
| Asthma | 0.06 (−0.05 to 0.17) | 0.262 | 2875 (1917 to 3833) | <0.0001 |
| HTN | 5.20 (3.89 to 6.50) | <0.0001 | 62462 (44915 to 80009) | <0.0001 |
| Obesity | 0.45 (0.04 to 0.86) | 0.031 | 4468 (374 to 8561) | 0.032 |
| Congenital heart diseases | 5.38 (3.82 to 6.93) | <0.0001 | 34538 (24751 to 44325) | <0.0001 |
| Coagulopathy | 3.72 (2.23 to 5.21) | <0.0001 | 47104 (28016 to 66192) | <0.0001 |
| SS SCA | 0.09 (−0.03 to 0.22) | 0.152 | −4004 (−5149 to −2859) | <0.0001 |
| Obstructive sleep apnea | 0.02 (−0.28 to 0.33) | 0.881 | 5163 (2223 to 8102) | 0.0001 |
| Central sleep apnea | −0.58 (−1.18 to 0.29) | 0.062 | 891 (−5504 to 7285) | 0.785 |
| AT | −1.21 (−1.52 to −0.90) | <0.0001 | −4379 (−6693 to −2065) | <0.0001 |
| Transfusion | 1.51 (1.31 to 1.70) | <0.0001 | 13379 (11456 to 15303) | <0.0001 |
CI, confidence interval; OR, odds ratio; HTN, hypertension; IDA, iron deficiency; NIMV, noninvasive mechanical ventilation; AT, adenotonsillectomy; SCD, sickle cell disease; ACS, acute chest syndrome; VOC, vaso-occlusion crisis; USD, US dollars; d, days.
*Cost was adjusted to 2012 USD using an average consumer price index.
The result of sensitivity analyses that combined SDB and OSA to assess the association with primary and secondary outcomes are detailed in Supplementary Tables S5 and S6. SDB was significantly associated with higher odds of NC and ACS, and longer hospital stay.
Discussion
This nationwide study showed that OSA was an independent risk factor for NC in children with SCD, and confirmed that OSA is an independent risk factor for ACS [36]. In addition, this study adds to the literature that NIMV might modify the risk of NC associated with OSA in this population. These findings highlight the public health burden of OSA on children with SCD, and possible ways to remediate the risk associated with OSA.
Several mechanisms have been proposed to explain the association between NC and OSA among children with SCD [37]. First, nocturnal hypoxemia from untreated OSA may promote polymerization of sickle hemoglobin [38–40], the adhesion of red blood cell to endothelium via binding molecules (e.g. ICAM-1 and P-selectin) [41–43], and hypercoagulable state due to platelet activation, thrombin generation, and endothelial dysfunction [44, 45]. Inflammation also plays an important role in vaso-occlusion in SCD. Inflammatory status caused by recurrent tissue ischemia from microvascular obstruction and infection due to functional asplenia could enhance vaso-occlusion as it increases the interaction of leukocytes and endothelial cell and hypercoagulable status [46–49]. Furthermore, it has been hypothesized that persistent inflammatory status and functional asplenia are associated with a higher prevalence of adenotonsillar hypertrophy in children with SCD that could lead to further nocturnal hypoxemia [21]. Additional comorbidities (e.g. hypertension) commonly seen in OSA and SCD were also identified as risk factors for NC [9]. To test the hypothesis that OSA is an independent risk factor for NC in children with SCD, it is important to account for the aforementioned contributing and confounding factors.
The findings that OSA is an independent risk factor for NC among SCD children are in agreement with the literature. In a case–control study, Katz et al. demonstrated that children with OSA and SCD were more likely to have neurologic and cardiovascular complications compared with children with SCD alone using descriptive statistics (p < 0.01) [23]. Similarly, in a survival analysis by Kirkham et al., central nervous complications were associated with nocturnal hypoxemia (<96% SaO2) on Kaplan–Meier curves (p = 0.0026), but not OSA based on polysomnography (PSG) results on Cox regression analysis (Hazard ratio 1.41 [95% CI 0.45–4.38], p = 0.55) [25]. Expanding on their findings, we not only reconfirmed the association between OSA and NC among children with SCD on descriptive statistics, but also quantified the risks of NC associated with OSA while accounting for potential confounders. Furthermore, these data showed an association between OSA and ACS, one of the secondary outcomes, in children with SCD. This finding is consistent with the results of Takahashi et al. [36]. It suggests that many SCD complications might share similar underlying mechanisms associated with nocturnal hypoxemia that could be contributed to OSA.
Ascertainment of the association between OSA and NC in children with SCD is important as the introduction of specific interventions for OSA could modify the risk of NC. An interesting finding of the current study is that patients who received AT had lower odds of NC compared with those who did not. The protective effect was also seen in previous studies. Using administrative data, Tripathi et al. suggested that AT was associated with a reduced rate of visits for cerebrovascular ischemia (e.g. stroke and TIA) in children with SCD [27]. Finch et al. also demonstrated a significant improvement of the apnea–hypopnea index and reduction in oxyhemoglobin desaturation by comparing pre- and post-adenotonsillectomy status among children with OSA and SCD [26]. However, sometimes children still suffer from persistent OSA after AT, which could explain the need of NIMV [50, 51]. Our finding that NIMV might modify the risk of NC conferred by OSA in SCD patients is crucial as it provides evidence to support the use of NIMV in this specific population. NIMV can stabilize the upper airway and favor lung recruitment, consequently improving nocturnal hypoxemia [52, 53]. Yet it is intriguing that the use of NIMV was associated with increased odds of NC. This paradoxical finding could be partially explained by reverse causation as sicker patients (e.g. those with more severe OSA) might have been more likely to receive NIMV. Further large prospective research on this topic is needed.
There were a few intriguing findings of this study. First, the OSA group was less likely to have VOC as compared with the non-OSA group (Table 1). This could result from reverse causality: As compared with SCD patients without OSA, those with OSA with frequent nocturnal hypoxemia might be more likely to receive hydroxyurea and other disease-modifying therapies (e.g. Voxelotor) to prevent VOC and other SCD complications and consequently decrease the probability of having VOC. Another explanatory factor could be hypoxic training from chronic hypoxemia leading to increased red blood cell mass and consequently oxygen-carrying capacity [54]. However, it is unclear if the increased RBC mass from hypoxia training, or chronic hypoxemia, will benefit the SCD patients as the main pathophysiology of sickle cell anemia results from the qualitative change of the hemoglobin rather from an inadequate quantity of RBC [2]. These hypotheses could not be tested using the KID administrative database which does not capture pharmacologic treatment information. To definitively answer this question, future studies are needed. Second, seizures accounted for the majority of NC seen in SCD patients, especially in the OSA group (2.91% vs. 1.66%, p = 0.0003). The observed association between OSA and seizures is pathophysiologically plausible. First of all, the intermittent nocturnal hypoxemia from OSA might lead to polymerization of sickle hemoglobin and subsequent development of SCD complications, such as seizure, established morbidity [55]. Second, OSA and seizures may mutually affect each other. Described adverse effects of antiepileptic drugs include weight gain and decreased upper airway tone, both of which may contribute to a higher prevalence of OSA [56]. Conversely, OSA may cause suboptimal epilepsy control via several mechanisms, such as sleep deprivation, cerebral hypoxemia, decreased cardiac output, and cardiac arrhythmias [56]. Several studies have shown that treatment of OSA may lead to improved control of epilepsy in the general population [57, 58]. Similar principles should be applicable to SCD patients with OSA and seizures. The association between OSA and seizures was non-significant on multivariable analysis possibly because we are examining a relatively uncommon pediatric pathology specifically among SCD patients.
As reported by others [30, 36], our data also showed elevated inflation-adjusted costs of hospitalization in children with SCD. This could be a reflection of several factors. The use of newer treatment and testing for SCD at higher costs could also partially explain the observation [36]. Other contributory factors included changes in prevalence or incidences of diseases (e.g. obesity and asthma), changes in service utilization, changes in service price as well as intensity across the study period [59]. The combinational effect of these factors could subsequently lead to a surge in the inflation-adjusted cost of hospitalization.
This study should be interpreted in the context of its strengths and limitations. Our analyses validated OSA as an independent risk factor for NC among children with SCD. Furthermore, it has shown that treatment for OSA, including AT and NIMV, could potentially modify the risk of NC associated with OSA among children with SCD. However, this study bears several limitations. A major limitation inherent to the use of administrative databases for healthcare research is the risk of misclassification bias due to miscoding. For instance, OSA status was determined using ICD-9 codes in our study instead of polysomnography (PSG), the gold standard. Additional to diagnostic inaccuracies from miscoding, the lack of PSG information prevents us from stratifying the severity of OSA for more analyses. Furthermore, the KID database does not contain detailed information regarding patients’ clinical presentation, laboratory findings, PSG, and medical treatment (e.g. hydroxyurea) either. Second, the KID database does not contain unique patient identifiers to distinguish recurrent admission or for longitudinal studies. Therefore, the significant association between NC and OSA could be potentially driven by a small population with a history of recurrent admission as repeated measurements were not appropriately adjusted. Third, differences in socioeconomic status (SES), insurance status, and access to medical care (teaching vs. non-teaching hospital) between OSA and non-OSA group may introduce confounding effects that bias the association between OSA and NC. To prevent the confounding effects from these factors, we adjust for the insurance status, SES, regions of residence, and size of the hospitals in the multivariable logistic regression to ensure the association between OSA and NC is valid. Fourth, NC, a composite of stroke, seizures, and TIA, were used as the primary outcome. This could introduce heterogeneity and make interpretation more difficult as compared with using a stroke or seizure alone. However, one should note that seizures and stroke are not mutually exclusive. This is particularly true in children as seizures can be the first manifestation of strokes [11, 12]. Those with the diagnostic codes of seizures alone without stroke might have subclinical strokes that were not captured clinically or by the ICD-9 codes. Furthermore, a study has reported that seizures and epilepsy are more common in children with SCD as compared with the general population based on a Jamaica sickle cell cohort, suggesting NC of SCD were not solely limited to cerebrovascular events [55]. Therefore, we consider it is a reasonable practice to report the composite outcome, NC, in addition to reporting stroke and seizures alone. Fifth, one should note that the KID database only allows for analyses of hospitalized patients. This results in a selective population that is generally sicker or needs hospitalization for certain treatments or procedures. Therefore, making epidemiologic estimates, such as the prevalence of OSA among children with SCD, would be undesirable as it does not include patients in outpatient settings. However, our assessment of the association between NC and OSA among children with SCD should be valid as NC would usually require hospitalization for diagnosis and treatment. Moreover, we could not ascertain whether NIMV was initiated as a result of NC, ACS, CSA, or OSA. Limited by the nature of the administrative database, the unclear temporal association between outcomes and NIMV and AT the study design prevented us from establishing causality. Prospective studies will be needed to address this issue.
Conclusion
This study summarized the epidemiology of hospitalized children with SCD using a US nationwide database, and confirmed OSA as an independent risk factor for NC among hospitalized children with SCD. Results indicated that NIMV but not AT might modify the risk of NC associated with OSA among this vulnerable population. Prospective studies are needed to validate whether the association between OSA and NC among children with SCD observed in this study is of causality, and the best OSA treatment to mitigate NC complications.
Funding
This work was supported by the National Institutes of Health K01 HL 130719 (Tapia) and K23 HL 135346 (Cielo).
Disclosure statement
Financial disclosure statement: The authors have no financial relationships relevant to this article to disclose.
Non-financial disclosure statement: The authors have no conflicts of interest relevant to this article to disclose.
Author contribution
Drs Po-Yang Tsou, Yu-Hsun Wang, and Pei-Lun Kuo had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design were carried out by Po-Yang Tsou and Ignacio Tapia. Drafting of the manuscript by Po-Yang Tsou and Ignacio Tapia. Critical revision of the manuscript for important intellectual content by Christopher Cielo, Melissa S. Xanthopoulos, Yu-Hsun Wang, Pei-Lun Kuo, and Ignacio Tapia. Statistical analysis by Po-Yang Tsou. Supervision by Ignacio Tapia.
Supplementary Material
References
- 1. Rosen CL, et al. Obstructive sleep apnea and sickle cell anemia. Pediatrics. 2014;134(2):273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schatz J, et al. Sickle cell disease as a neurodevelopmental disorder. Ment Retard Dev Disabil Res Rev. 2006;12(3):200–207. [DOI] [PubMed] [Google Scholar]
- 3. Embury SH The not-so-simple process of sickle cell vasoocclusion. Microcirculation. 2004;11(2):101–113. [DOI] [PubMed] [Google Scholar]
- 4. Kauf TL, et al. The cost of health care for children and adults with sickle cell disease. Am J Hematol. 2009;84(6):323–327. [DOI] [PubMed] [Google Scholar]
- 5. Rees DC, et al. Sickle-cell disease. Lancet. 2010;376(9757):2018–2031. [DOI] [PubMed] [Google Scholar]
- 6. Kato GJ, et al. Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev. 2007;21(1):37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khoury JC, et al. Abstract T P367: stable childhood stroke rates over 17 years: report from a population-based study. Stroke. 2015;46(suppl_1):ATP367–ATP367. [Google Scholar]
- 8. Tsze DS, et al. Pediatric stroke: a review. Emerg Med Int. 2011;2011:734506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ohene-Frempong K, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91(1):288–294. [PubMed] [Google Scholar]
- 10. Powars D, et al. The natural history of stroke in sickle cell disease. Am J Med. 1978;65(3):461–471. [DOI] [PubMed] [Google Scholar]
- 11. Zimmer JA, et al. Age-related variation in presenting signs of childhood arterial ischemic stroke. Pediatr Neurol. 2007;37(3):171–175. [DOI] [PubMed] [Google Scholar]
- 12. Mallick AA, et al. Childhood arterial ischaemic stroke incidence, presenting features, and risk factors: a prospective population-based study. Lancet Neurol. 2014;13(1):35–43. [DOI] [PubMed] [Google Scholar]
- 13. Strollo PJ Jr, et al. Obstructive sleep apnea. N Engl J Med. 1996;334(2):99–104. [DOI] [PubMed] [Google Scholar]
- 14. Marcus CL, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012;130(3):e714–e755. [DOI] [PubMed] [Google Scholar]
- 15. Gozal D, et al. Neurocognitive and behavioral morbidity in children with sleep disorders. Curr Opin Pulm Med. 2007;13(6):505–509. [DOI] [PubMed] [Google Scholar]
- 16. Marcus CL, et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. N Engl J Med. 2013;368(25):2366–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Loke YK, et al. Association of obstructive sleep apnea with risk of serious cardiovascular events: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2012;5(5):720–728. [DOI] [PubMed] [Google Scholar]
- 18. Buck J, et al. Surgery in sickle cell disease. Hematol Oncol Clin North Am. 2005;19(5):897–902, vii. [DOI] [PubMed] [Google Scholar]
- 19. Kemp JS Obstructive sleep apnea and sickle cell disease. J Pediatr Hematol Oncol. 1996;18(2):104–105. [DOI] [PubMed] [Google Scholar]
- 20. Lumeng JC, et al. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):242–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Strauss T, et al. Upper airway lymphoid tissue size in children with sickle cell disease. Chest. 2012;142(1):94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brooks LJ, et al. Does sleep-disordered breathing contribute to the clinical severity of sickle cell anemia? J Pediatr Hematol Oncol. 1996;18(2):135–139. [DOI] [PubMed] [Google Scholar]
- 23. Katz T, et al. Comorbid obstructive sleep apnea and increased risk for sickle cell disease morbidity. Sleep Breath. 2018;22(3):797–804. [DOI] [PubMed] [Google Scholar]
- 24. Needleman JP, et al. Mechanisms of nocturnal oxyhemoglobin desaturation in children and adolescents with sickle cell disease. Pediatr Pulmonol. 1999;28(6):418–422. [DOI] [PubMed] [Google Scholar]
- 25. Kirkham FJ, et al. Nocturnal hypoxaemia and central-nervous-system events in sickle-cell disease. Lancet. 2001;357(9269):1656–1659. [DOI] [PubMed] [Google Scholar]
- 26. Finch P, et al. Effects of adenotonsillectomy on polysomnographic parameters in children with sickle cell disease. Pediatr Blood Cancer. 2013;60(7):E26–E28. [DOI] [PubMed] [Google Scholar]
- 27. Tripathi A, et al. Cost-effectiveness of adenotonsillectomy in reducing obstructive sleep apnea, cerebrovascular ischemia, vaso-occlusive pain, and ACS episodes in pediatric sickle cell disease. Ann Hematol. 2011;90(2):145–150. [DOI] [PubMed] [Google Scholar]
- 28. HCUP Kids’ Inpatient Database (KID). Healthcare Cost and Utilization Project (HCUP). 1997, 2000, 2003, 2006, 2009, and 2012. Rockville, MD: Agency for Healthcare Research and Quality; www.hcup-us.ahrq.gov [Google Scholar]
- 29. Bou-Maroun LM, et al. An analysis of inpatient pediatric sickle cell disease: incidence, costs, and outcomes. Pediatr Blood Cancer. 2018;65(1):e26758. [DOI] [PubMed] [Google Scholar]
- 30. Atwood CM, et al. Blood transfusion in children with sickle cell disease undergoing tonsillectomy. Int J Pediatr Otorhinolaryngol. 2017;103:117–120. [DOI] [PubMed] [Google Scholar]
- 31. McCavit TL, et al. National trends in incidence rates of hospitalization for stroke in children with sickle cell disease. Pediatr Blood Cancer. 2013;60(5):823–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ong AA, et al. Down syndrome and pediatric obstructive sleep apnea surgery: a national cohort. Laryngoscope. 2018;128(8):1963–1969. [DOI] [PubMed] [Google Scholar]
- 33. Adil MM, et al. Transient ischemic attack requiring hospitalization of children in the United States: kids’ inpatient database 2003 to 2009. Stroke. 2014;45(3):887–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Baker C, et al. Contribution of sickle cell disease to the pediatric stroke burden among hospital discharges of African-Americans-United States, 1997–2012. Pediatr Blood Cancer. 2015;62(12):2076–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marcus CL, et al. Use of nasal continuous positive airway pressure as treatment of childhood obstructive sleep apnea. J Pediatr. 1995;127(1):88–94. [DOI] [PubMed] [Google Scholar]
- 36. Takahashi T, et al. Acute chest syndrome among children hospitalized with vaso-occlusive crisis: a nationwide study in the United States. Pediatr Blood Cancer. 2018;65(3):e26885. [DOI] [PubMed] [Google Scholar]
- 37. Gileles-Hillel A, et al. Hemoglobinopathies and sleep—the road less traveled. Sleep Med Rev. 2015;24:57–70. [DOI] [PubMed] [Google Scholar]
- 38. Chirico EN, et al. Role of oxidative stress in the pathogenesis of sickle cell disease. IUBMB Life. 2012;64(1):72–80. [DOI] [PubMed] [Google Scholar]
- 39. Christoph GW, et al. Understanding the shape of sickled red cells. Biophys J. 2005;88(2):1371–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim M, et al. Hypoxia-enhanced adhesion of red blood cells in microscale flow. Microcirculation. 2017;24(5). doi: 10.1111/micc.12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wood K, et al. Differential expression of E- and P-selectin in the microvasculature of sickle cell transgenic mice. Microcirculation. 2004;11(4):377–385. [DOI] [PubMed] [Google Scholar]
- 42. Kato GJ, et al. Levels of soluble endothelium-derived adhesion molecules in patients with sickle cell disease are associated with pulmonary hypertension, organ dysfunction, and mortality. Br J Haematol. 2005;130(6): 943–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Setty BN, et al. Vascular cell adhesion molecule-1 is involved in mediating hypoxia-induced sickle red blood cell adherence to endothelium: potential role in sickle cell disease. Blood. 1996;88(6):2311–2320. [PubMed] [Google Scholar]
- 44. Tomer A, et al. Thrombogenesis in sickle cell disease. J Lab Clin Med. 2001;137(6):398–407. [DOI] [PubMed] [Google Scholar]
- 45. Ataga KI, et al. Beta-thalassaemia and sickle cell anaemia as paradigms of hypercoagulability. Br J Haematol. 2007;139(1):3–13. [DOI] [PubMed] [Google Scholar]
- 46. Zhang D, et al. Neutrophils, platelets, and inflammatory pathways at the nexus of sickle cell disease pathophysiology. Blood. 2016;127(7):801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zennadi R, et al. Epinephrine-induced activation of LW-mediated sickle cell adhesion and vaso-occlusion in vivo. Blood. 2007;110(7):2708–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zennadi R, et al. Sickle red cells induce adhesion of lymphocytes and monocytes to endothelium. Blood. 2008;112(8):3474–3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Amer J, et al. Red blood cells, platelets and polymorphonuclear neutrophils of patients with sickle cell disease exhibit oxidative stress that can be ameliorated by antioxidants. Br J Haematol. 2006;132(1):108–113. [DOI] [PubMed] [Google Scholar]
- 50. Friedman M, et al. Updated systematic review of tonsillectomy and adenoidectomy for treatment of pediatric obstructive sleep apnea/hypopnea syndrome. Otolaryngol Head Neck Surg. 2009;140(6):800–808. [DOI] [PubMed] [Google Scholar]
- 51. Mitchell RB Adenotonsillectomy for obstructive sleep apnea in children: outcome evaluated by pre- and postoperative polysomnography. Laryngoscope. 2007;117(10):1844–1854. [DOI] [PubMed] [Google Scholar]
- 52. Sullivan CE, et al. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;1(8225):862–865. [DOI] [PubMed] [Google Scholar]
- 53. Alex CG, et al. Effects of continuous positive airway pressure on upper airway and respiratory muscle activity. J Appl Physiol (1985). 1987;62(5):2026–2030. [DOI] [PubMed] [Google Scholar]
- 54. Levine BD Intermittent hypoxic training: fact and fancy. High Alt Med Biol. 2002;3(2):177–193. [DOI] [PubMed] [Google Scholar]
- 55. Ali SB, et al. Seizures in the Jamaica cohort study of sickle cell disease. Br J Haematol. 2010;151(3):265–272. [DOI] [PubMed] [Google Scholar]
- 56. Sivathamboo S, et al. Sleep-disordered breathing in epilepsy: epidemiology, mechanisms, and treatment. Sleep. 2018;41(4). doi: 10.1093/sleep/zsy015. [DOI] [PubMed] [Google Scholar]
- 57. Malow BA, et al. Treating obstructive sleep apnea in adults with epilepsy: a randomized pilot trial. Neurology. 2008;71(8):572–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Segal E, et al. Effect of treatment of obstructive sleep apnea on seizure outcomes in children with epilepsy. Pediatr Neurol. 2012;46(6):359–362. [DOI] [PubMed] [Google Scholar]
- 59. Dieleman JL, et al. Factors associated with increases in US health care spending, 1996–2013. JAMA. 2017;318(17):1668–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.