Abstract
Objectives
To determine the outcomes of chronically ventilated patients outside the setting of intensive care units.
Design
Systematic review.
Setting and participants
Studies evaluating patients on chronic invasive mechanical ventilation in different care settings.
Methods
A systematic literature search of the PubMed, Embase, Cochrane Library, CINAHL (EBSCOhost), LILACS and Scopus databases from inception to March 27, 2020. Studies reporting mortality outcomes of patients ≥18 years of age on chronic invasive mechanical ventilation in intensive care units and other care settings were eligible for inclusion.
Results
Sixty studies were included in the systematic review. Mortality rates ranged from 13.7% to 77.8% in ICUs (n = 17 studies), 7.8%–51.0% in non-ICUs including step-down units and inpatient wards (n = 26 studies), and 12.0%–91.8% in home or nursing home settings (n = 19 studies). Age was associated with mortality in all care settings. Weaning rates ranged from 10.0% to 78.2% across non-ICU studies. Studies reporting weaning as their primary outcome demonstrated higher success rates in weaning. Home care studies reported low incidences of ventilator failure. None of the studies reported ventilator malfunction as the primary cause of death.
Conclusions and implications
Mortality outcomes across various settings were disparate due to methodological and clinical heterogeneity among studies. However, there is evidence to suggest non-ICU venues of care as a comparable alternative to ICUs for stable, chronically ventilated patients, with the additional benefit of providing specialized weaning programs. By synthesizing the global data on managing chronically ventilated patients in various care settings, this study provides health care systems and providers alternative venue options for the delivery of prolonged ventilatory care in the context of limited ICU resources.
Keywords: Chronic or prolonged mechanical ventilation, Care settings, Intensive care unit, Long-term care, Step-down unit, Home mechanical ventilation
Chronic or prolonged mechanical ventilation, Care settings, Intensive care unit, Long-term care, Step-down unit, Home mechanical ventilation
1. Introduction
Advances in critical care medicine and the application of invasive mechanical ventilation in the intensive care unit (ICU) have led to improved short-term survival and mortality outcomes in acutely critically ill patients [1]. With the increase in the number of ventilator-dependent individuals, there have been concerns about equitable access to ICU resources for critically ill patients, particularly the duration of ventilatory support warranted for such patients.
Acute respiratory failure due to severe acute respiratory coronavirus 2 (SARS-CoV-2) has led to an unprecedented rise in the number of critically ill patients requiring invasive mechanical ventilation, predominantly among older adults and those with multiple comorbidities [2]. In the current scientific literature, rates of invasive mechanical ventilation range from 2% to 47% in patients infected with coronavirus 2019 (COVID-19) [3, 4, 5]. Guidelines and recommendations on the management of tracheostomy in patients requiring mechanical ventilation during the COVID-19 pandemic have been published [6]. Although the data is still emerging on the average duration of intubation among COVID-19 patients, some studies report mechanical ventilator support for a median of 18 days (IQR 9–28) to a maximum of 31 days [7, 8]. As the growing incidence of COVID-19 strains the very limits of resource capacity in intensive care units and as healthcare providers are forced to make burdening decisions regarding patient care, it is becoming increasingly important to understand whether ventilator support can be provided safely in alternate clinical settings, particularly for patients requiring longer durations of mechanical ventilation.
In the past, chronically ventilated patients have been transitioned to various alternative care settings after stabilization of a critical illness to prevent overutilization of critical care resources [9, 10]. Depending on the availability of resources such as infrastructure and technology in different regions of the world, patients are transitioned outside of the ICU to general medical or surgical units, specialized respiratory units, subacute facilities, long-term care facilities such as nursing homes (NH), or home care [1, 11]. In some instances, patients remain in the ICU for extended periods when a healthcare system is unable to provide complex care for ventilated patients in a non-ICU setting [12].
In 1998, clinical guidelines regarding the management of chronically ventilated patients outside the ICU was published where experts outlined the criteria for ICU discharge and described characteristics of potential alternative care settings. Albeit clinical outcomes of chronically ventilated patients in alternative care settings were discussed briefly, the need for comprehensive survival- and mortality-related data collection from various care settings was broadly acknowledged [11]. While many studies have since published on this topic, there are no systematic reviews examining the mortality outcomes across different care settings to date [13].
Given that some countries have yet to reach the peak of the COVID-19 pandemic, and as the risk of outbreak recurrence remains, it is critical to review and synthesize available evidence on clinical outcomes associated with chronic mechanical ventilation in care settings outside of the intensive care unit and provide healthcare providers and healthcare systems guidance on optimizing ventilatory care for patients. We aim to systematically review mortality outcomes of chronically ventilated patients across various care settings to determine the safety of chronically ventilated patients. Additionally, as older adults have been disproportionately impacted by COVID-19, we examined reported mortality outcomes of older mechanically ventilated patients in care settings outside of the ICU.
2. Methods
This systematic review was conducted and reported in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement [14].
2.1. Search strategy
An electronic search of the PubMed, Embase (Embase and Embase classic), Cochrane Library, CINAHL (EBSCOhost), LILACS, and Scopus databases, from inception to March 27, 2020, were completed. A combination of keywords and controlled vocabulary terms was used and divided into two distinct search themes. The first theme related to any medical condition requiring chronic or prolonged mechanical ventilation and included the following terms: artificial respiration, mechanical ventilation, and chronic or prolonged mechanical ventilation. Venues of care formed the second concept of our research framework and were identified with the following terminology: health care delivery, home health care, home care, chronic disease hospitals, care venues, community or long-term care, intermediate units, nursing home, chronic ventilator units, and step-down units. The search strategy from all databases is detailed in Supplementary Table S1. Reference lists of included studies were hand-searched to identify additional relevant articles.
2.2. Study selection
Two reviewers (S.M.S and G.K.S) independently reviewed all titles and abstracts. Studies were then further assessed for relevance and selected for full-text review if they met the inclusion criteria. Covidence, an online data extraction tool, was used to screen and select studies for inclusion.
2.3. Eligibility criteria
Studies were included for the following criteria: i) a majority of the study cohort were aged ≥18 years with at least 50% of the study population invasively ventilated; ii) clearly identified care setting; and iii) mortality while admitted in the specified care setting was reported. All studies using iterations of the terms ‘chronic,’ ‘prolonged,’ ‘long-term,’ or ‘dependent’ (e.g., ventilator-dependent) mechanical ventilation to describe the study population were included. The authors agreed upon this criterion to account for various ventilation lengths considered as “prolonged” which can range from as short as ≥ 24 h to as long as ≥ 14–21 days [15].
Studies were restricted to original research published in the English language. Review articles, abstracts, conference proceedings, editorials, and opinion pieces were excluded. If the reported mortality outcome was related to the investigation of a specific intervention, the study was excluded. However, weaning was an exception to this rule if it was inherent to the services offered by the care setting.
2.4. Data extraction
Standardized abstraction forms were created a priori to extract relevant information according to the research objectives. Two independent reviewers (S.M.S. and G.K.S.) conducted the data extraction. Retrieved data included the author, year of publication, geographic location, care setting, study characteristics including study design and minimum ventilation requirement of the cohort, and population characteristics including age, duration of ventilation and sample size. Additional information, including characteristics of non-ICU care settings and underlying cause of respiratory failure, were extracted for comprehensive data analysis. The mortality rate was the primary outcome measure. If mortality rate was not reported, the number of deaths in a given sample was extrapolated to determine the rate. Secondary outcomes included risk factors for mortality and other commonly reported outcomes which may reflect the safety of the care setting. Risk factors significantly associated with mortality using multivariable analysis were collected if the association was found in at least two studies. Disagreements and discrepancies between reviewers were resolved through discussion and consensus.
2.5. Assessment of methodological quality
Study quality was evaluated by two reviewers (S.M.S. and G.K.S.) using the National Institute of Health (NIH) Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies [16]. All discrepancies in study ratings were resolved in a consensus meeting. Eligible studies were not excluded based on overall study quality.
3. Results
3.1. Study characteristics
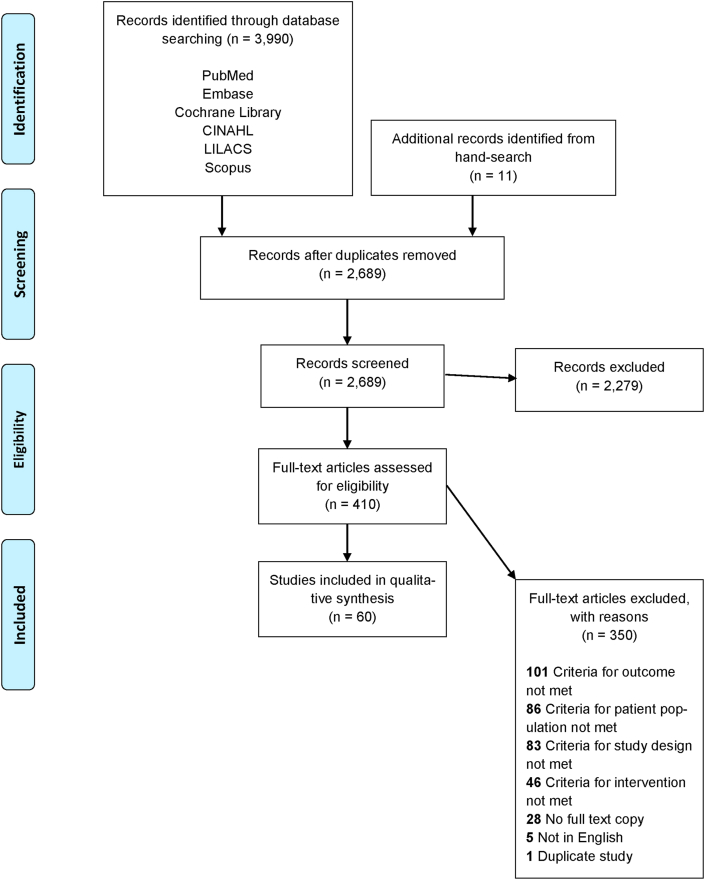
Our search yielded 3,990 titles and references from electronic databases. Eleven additional studies were included from hand searches. After duplicate studies were removed, 2,689 abstracts were screened, and 410 full-text articles were assessed for study eligibility. A total of 60 studies were included for qualitative synthesis (Figure 1). Table 1 provides an overview of the characteristics of the 60 included studies [9, 10, 12, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73]. Three major care settings were identified among selected studies: 1) ICU; 2) step-down units or wards, also referred to as ‘non-ICU’ settings; and 3) home or nursing home [11]. Studies on non-ICU settings were primarily from North America and Europe, while studies on ICU and home care were globally distributed. Among the 60 full-text articles, 28 studies reported mortality as their primary outcome measure.
Figure 1.
PRISMA flow diagram for study identification and selection.
Table 1.
Characteristics of Included Studies in this Systematic Review.
| Author, Year | Country | Study Design | Setting | Nomenclature Used to Define Study Population | Inclusion criteria - Minimum No. Days on MV | No. Subjects, n | Mortality, Primary vs. Secondary Outcome |
|---|---|---|---|---|---|---|---|
| Abitağaoğlu, 2019 [17] | Turkey | RC | ICU | Home mechanical ventilation | Failure to wean | 51 | Primary |
| Aksoy, 2019 [18] | Turkey | RC | Home | Home mechanical ventilation | 90 | 134 | Primary |
| Bagley, 1997 [19] | USA | RC | Non-ICU unit | Ventilator-dependent | Failure to wean | 278 | Secondary |
| Bigatello, 2007 [20] | USA | PC | Non-ICU unit | Prolonged mechanical ventilation | NR | 210 | Secondary |
| Björling, 2006 [21] | Sweden | RC | Home | Long-term tracheostomy | 4 years | 94 | Secondary |
| Bonnici, 2016 [22] | UK | PC | Non-ICU unit | NR | 28 | 262 | Primary |
| Carson, 1999 [23] | USA | PC | Non-ICU unit | NR | 14 | 133 | Primary |
| Carson, 2008 [24] | USA | PC | ICU | Prolonged mechanical ventilation | 21 | 200 | Primary |
| Cazzolli, 1996 [25] | USA | PC | Home/NH | Home mechanical ventilation | 1 year | 50 (25 in NH) | Primary |
| Ceriana, 2003 [26] | Italy | PC | Non-ICU unit | NR | NR | 96 | Secondary |
| Chelluri, 2003 [27] | USA | PC | ICU | Prolonged mechanical ventilation | 2 | 813 | Secondary |
| Cohen, 1993 [28] | USA | RC | ICU | Prolonged mechanical ventilation | 2 | 45 | Secondary |
| Combes, 2003 [29] | France | PC | ICU | Long-term mechanical ventilation | 14 | 347 | Primary |
| Combes, 2007 [30] | France | RC | ICU | Prolonged mechanical ventilation | 3 | 166 | Primary |
| Cordasco, 1991 [31] | USA | RC | Non-ICU unit | Long-term ventilator dependent patient | 7 | 99 | Secondary |
| Cox, 2004 [32] | USA | RC | ICU | Prolonged mechanical ventilation | 3 | 9,794 | Primary |
| Cox, 2007 [33] | USA | PC | ICU | Prolonged mechanical ventilation | 4 or 21 | 381 | Primary |
| Dasgupta, 1999 [34] | USA | PC | Non-ICU unit | Ventilator-dependent | NR | 212 | Primary |
| Fini, 2014 [35] | Italy | PC | Non-ICU unit | Long-term mechanical ventilation | NR | 47 | Primary |
| Transferred to Home | 27 | ||||||
| Transferred to NH | 10 | ||||||
| Fischer, 1982 [36] | USA | PC | Home | Respirator-dependent | Failure to wean | 29 | Secondary |
| Ghiani, 2020 [37] | Germany | RC | Non-ICU unit | Prolonged mechanical ventilation | Failure to wean | 263 | Secondary |
| Gracey 1997 [38] | USA | PC | Non-ICU unit | Prolonged mechanical ventilation | 21 | 206 | Primary |
| Guber, 2002 [39] | Israel | PC | Home | Home-ventilated patients | 30 | 25 | Secondary |
| Hannan, 2013 [40] | Australia | PC | Non-ICU unit | NR | 14 | 78 | Secondary |
| Hendin, 2019 [41] | Canada | RC | Non-ICU unit | Tracheostomy-ventilated patients | Failure to wean | 50 | Secondary |
| Hill, 2017 [42] | Canada | RC | ICU | Prolonged mechanical ventilation | 21 | 11,594 | Primary |
| Kojicic, 2011 [43] | USA | RC | ICU | Prolonged mechanical ventilation | Failure to wean∗ | 65 | Secondary |
| Krieger, 1988 [44] | USA | PC | Non-ICU unit | Long-term ventilator patients | 3 | 11 | Secondary |
| Lai, 2016 [45] | Taiwan | RC | Non-ICU unit | Prolonged mechanical ventilation | 21 | 510 | Primary |
| Latriano, 1996 [46] | USA | RC | Non-ICU unit | Prolonged mechanical ventilation | NR | 224 | Primary |
| Leroy, 2014 [47] | France | RC | ICU | Prolonged mechanical ventilation | 21 | 201 | Secondary |
| Li, 2016 [48] | China | PC | ICU | Prolonged mechanical ventilation | 21 | 157 | Secondary |
| Lin, 1996 [49] | Taiwan | PC | Home | Home mechanical ventilation | 30 | 34 | Secondary |
| Lone, 2011 [50] | UK | RC | ICU | Prolonged mechanical ventilation | 21 | 349 | Secondary |
| Mamary, 2011 [51] | USA | PC | Non-ICU unit | Prolonged mechanical ventilation | NR | 182 | Primary |
| Mok, 2016 [52] | Korea | RC | ICU | Prolonged mechanical ventilation | 21 | 184 | Secondary |
| Moss, 1993 [53] | USA | PC | Home | NR | NR | 19 | Secondary |
| Muir, 1994 [54] | France | RC | Home/NH | Home mechanical ventilation | 1 year | 253 (41 in NH) | Primary |
| Nugent, 1996 [55] | Ireland | PC | Home | Home mechanical ventilation | NR | 13 | Secondary |
| Pilcher, 2005 [56] | UK | PC | Non-ICU unit | Ventilator dependent | Failure to wean | 153 | Primary |
| Polverino 2010 [57] | Italy | RC | Non-ICU unit | Prolonged mechanical ventilation | 15 | 3,106 | Primary |
| Robson, 2003 [58] | UK | PC | ICU | NR | 21 | 161 | Secondary |
| Roh, 2019 [59] | Korea | RC | ICU | Prolonged mechanical ventilation | 21 | 302 | Secondary |
| Rose, 2012 [60] | Canada | RC | Non-ICU unit | Prolonged mechanical ventilation | 21 | 115 | Secondary |
| Rose, 2019 [61] | Canada | RC | Home/NH | Ventilator-assisted individuals | 21 | 52 (7 in NH) | Secondary |
| Saiphoklang, 2019 [62] | Thailand | PC | Home | Home mechanical ventilation | 21 | 12 | Primary |
| Salahuddin, 2005 [12] | Pakistan | PC | Home | Ventilator-dependent | NR | 11 | Primary |
| Sancho, 2011 [63] | Spain | PC | Home | Home tracheostomy mechanical ventilation | NR | 38 | Secondary |
| Scheinhorn, 1994 [64] | USA | RC | Non-ICU unit | Prolonged mechanical ventilation/Ventilator-dependent | NR | 421 | Primary |
| Transferred to Home | 26 | ||||||
| Transferred to NH | 49 | ||||||
| Scheinhorn, 2007 [65] | USA | PC | Non-ICU unit | Prolonged mechanical ventilation | NR | 1,419 | Primary |
| Schonhofer, 2002 [66] | Germany | RC | Non-ICU unit | Prolonged mechanical ventilation | 14 & failure to wean | 403 | Primary |
| Seneff, 2000 [9] | USA | RC | Non-ICU unit | Chronically ventilated patients | 21 | 1,702 | Primary |
| Acute Hospital | 432 | ||||||
| Sivak, 1982 [67] | USA | PC | Home | Home care ventilation | ≥30 | 21 | Secondary |
| Spataro, 2012 [68] | Italy | PC | Home/Acute Hospital | NR | NR | 87 (5 in Acute Hospital) | Secondary |
| Stoller, 2003 [69] | USA | PC | Non-ICU unit | Long-term mechanical ventilation | 14–21 | 162 | Primary |
| Tsara, 2015 [10] | Greece | RC | Non-ICU unit | NR | Failure to wean | 548 | Primary |
| Unroe, 2010 [70] | USA | PC | ICU | Prolonged mechanical ventilation | 4 or 21† | 126 | Secondary |
| Vitacca, 2011 [71] | Italy | PC | Home | Home mechanical ventilation | NR | 12 | Secondary |
| Wijkstra, 2003 [72] | Canada | RC | Non-ICU unit | Ventilator-dependent | Failure to wean | 50 | Secondary |
| Wolf, 1993 [73] | Israel | PC | Home | Respirator-dependent | 1 year | 13 | Secondary |
CS, cross-sectional study; NH, nursing home; NR, not reported; PC, prospective cohort study; RC, retrospective cohort study.
Inclusion criteria included failure to wean or placement of tracheostomy in anticipation of long-term ventilation.
Inclusion criteria required mechanical ventilation for at least >96 h with tracheostomy or at least >21 days without tracheostomy.
The methodological assessment of included studies is detailed in Supplementary Table S2. Fifty-three studies were determined to have a ‘fair’ or ‘good’ study quality as per the NIH Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. The remaining seven studies were deemed to be of inadequate methodological rigor but were still included in the qualitative analysis.
3.2. Patient characteristics and mortality outcomes
Patient characteristics and mortality outcomes by ICU, non-ICU, and home/NH settings are presented in Tables 2, 3, and 4. Fourteen out of 17 (82%) ICU and 21 out of 26 (81%) non-ICU studies reported a mean or median age of 60 years or older. Subjects in the home/NH study cohort tended to be younger, with only 9 out of 18 (50%) of the studies reporting a mean or median age of 60 years or older (Tables 2, 3, and 4).
Table 2.
Chronic ventilation patients cared for in the ICU and mortality outcomes, arranged by year.
| First author, Year | Inclusion criteria - Minimum No. Days on MV | Subjects, n | Age, years∗ | Total MV Duration, days∗ |
Mortality N, (%) |
|---|---|---|---|---|---|
| Cohen, 1993 [28] | 2 | 45 | 85 (3) | 24 (31) | 35 (77.8) |
| Combes, 2003 [29] | 14 | 347 | Survivors: 63 (14) Non-survivors: 67 (13) |
37 (25) | 150 (43.2) |
| Chelluri, 2003 [27] | 2 | 813 | 60 (19) | 9 (4, 16) Md (IQR) | NR (29.0) |
| Robson, 2003 [58] | 21 | 161 | 69 (21–88) Md (range) | NR | 22 (13.7) |
| Cox, 2004 [32] | 3 | 9,794 | 62 (48, 72) Md(IQR)† | NR | 2,804 (28.6)‡ |
| Cox, 2007 [33] | 4 | 267 | 66 (45, 75) Md(IQR) | 16 (10, 24) Md (IQR) | 53 (19.9)‡ |
| 21 | 114 | 66 (47, 74) Md(IQR) | 27 (23, 36) Md (IQR) | 36 (31.6)‡ | |
| Combes, 2007 [30] | 3 | 166 | 59 (16) | 35 (25) | 55 (33.1) |
| Carson, 2008 [24] | 21 | 200 | 56 (17) | 35 (26, 51) Md (IQR) | 82 (41.0)‡ |
| Unroe, 2010 [70] | 4 or 21§ | 126 | 55 (16) | 27 (18, 27) Md (IQR) | 23 (18.3)‡ |
| Lone, 2011 [50] | 21 | 349 | 60 (15) | 33 (15) | 83 (26.2)|| |
| Kojicic, 2011 [43] | Failure to wean∗∗ | 65 | 68 (49, 80) Md(IQR) | 24 (18, 37) Md (IQR) | 19 (29.2)‡ |
| Leroy, 2014 [47] | 21 | 201 | 64 (14) | 37 (20) | 83 (41.3) |
| Li, 2016 [48] | 21 | 157 | 74 (18–103) Md (range) | 51 (21–3419) Md (range)†† | 31 (19.8) |
| Mok, 2016 [52] | 21 | 184 | 65 (19–91) Md (range) | 35 (21–160) Md (range) | 85 (46.2) |
| Hill, 2017 [42] | 21 | 11,594 | NR | NR | 4,932 (42.4)‡ |
| Roh, 2019 [59] | 21 | 302 | 63 (18–89) Mn (range) | 29 (21–199) Mn (range) | 95 (31.5) |
| Abitağaoğlu, 2019 [17] | Failure to wean | 51 | 78 (8) | 41 (23) | 13 (25.5) |
NR, not reported; MV, mechanical ventilation.
Values are presented as mean (SD) unless otherwise specified.
Cox et al. included subjects from 1993 to 2002 and presented median (IQR) age of patients per period as follows: 65 (51, 73) for 1993, 65 (49, 73) for 1997, and 62 (48, 72) for 2002.
Mortality rates reported at discharge from the acute hospital. In these studies, the number of deaths explicitly occurring in the ICU was not provided separately.
Inclusion criteria required mechanical ventilation for at least >96 h with tracheostomy or at least >21 days without tracheostomy.
Denominator used to calculate the mortality rate was 317 instead of 349.
Inclusion criteria included failure to wean or placement of tracheostomy in anticipation of long-term ventilation.
Total duration of ventilation was not provided. The median (range) of ventilation for 109/157 subjects at the start of the study was reported to be 51 (21–3419). The study duration was 28 days.
Table 3.
Chronic ventilation patients cared for in non-ICU units and mortality outcomes, arranged by year.
| First author, Year | Non-ICU unit Nomenclature | Inclusion criteria - Minimum No. Days on MV |
Subjects, n | Age, years∗ | Current MV Duration, days∗ | Mortality N, (%) |
|---|---|---|---|---|---|---|
| Krieger, 1988 [44] | Non-invasive monitoring unit | 3 | 11 | 75 (11) | 12 (9) PMV: 11 (11) |
3 (27.2) |
| Cordasco, 1991 [31] | Non-ICU ventilator care service | 7, with tracheostomy† | 99 | 21–84‡ | - | 25 (25.3) |
| Scheinhorn, 1994 [64] | Regional weaning center | NR | 421 | Weaned: 68 (1) Not weaned: 71 (1) |
58 Mn PMV: 49 Mn |
116 (27.6) |
| Latriano, 1996 [46] | Non-monitor respiratory care floor | NR | 224 | 66 (17) | 49.9 (66) PMV: 23 (19) |
111 (49.6) |
| Bagley, 1997 [19] | Ventilator weaning unit | Failure to wean | 278 | 67 (21–99) Mn (range) | - | 133 (47.8) |
| Gracey, 1997 [38] | Ventilator-dependent rehabilitation unit | 21 | 206 | 65 (14) | - PMV: 58 (72) |
16 (7.8) |
| Dasgupta, 1999 [34] | Respiratory special care unit | NR | 212 | 68 (60, 73) Md (IQR) | 13 (15, 23) Md (IQR) PMV: 25 Md |
20 (9.4) |
| Carson, 1999 [23] | Long-term acute care hospital | 14 | 133 | 71 (12) | - PMV: 25 (9–123) Md (range) |
66 (49.6) |
| Seneff, 2000 [9] | Long-term acute care facility | 21 | 1,702 | 71 Mn | - | NR (51.0) |
| Schonhofer, 2002 [66] | Respiratory intensive care unit | 14 & failure to wean | 403 | 66 (59, 71) Md (IQR) | - PMV: 33 (19, 50) Md (IQR) |
98 (24.3) |
| Stoller, 2003 [69] | Respiratory special care unit | 14–21 | 162 | 65 (14) | - | 27 (16.7) |
| Ceriana, 2003 [26] | Respiratory intensive care unit | NR | 96 | 67 (12) | - PMV: 38 (15)§ |
13 (13.5) |
| Wijkstra, 2003 [72] | Chronic assisted ventilatory care unit | Failure to wean | 50 | 46 (18–76) Mn (range) | - PMV: 0–12600‡ |
11 (22.0) |
| Pilcher, 2005 [56] | Respiratory unit | Failure to wean | 153 | 62 (49, 72) Md (IQR) | - | 42 (27.5) |
| Bigatello, 2007 [20] | Acute respiratory unit | NR | 210 | 66 (52, 75) Md (IQR) | - PMV: 21 (12, 32) Md (IQR) |
30 (14.3) |
| Scheinhorn, 2007 [65] | Long-term care hospital | NR | 1,419 | 72 (18–98) Md (range) | 21 (1–365) Md (range) PMV: 25 (0–1154) Md (range) |
353 (24.9)|| |
| Polverino 2010 [57] | Respiratory intensive care unit | 15 | 3,106 | 1991–95: 78 (1) | - | 82 (9.0) |
| 1995–00: 76 (4) | - | 138 (14.0) | ||||
| 2001–05: 73 (5) | - | 184 (15.0) | ||||
| Mamary, 2011 [51] | Ventilation rehabilitation unit | NR | 182 | 64 (16) | Total MV duration∗∗ | 35 (19.2) |
| Rose, 2012 [60] | Prolonged ventilation weaning center | 21 | 115 | 70 (59, 77) Md (IQR) | - PMV: 55 (37, 89) Md (IQR) |
15 (13.0) |
| Hannan, 2013 [40] | Ventilation weaning unit | 14 | 78 | 59 (16–85) Mn (range) | - PMV: 27 (16–36) Md (range) |
8 (10.3) |
| Fini, 2014 [35] | Multidisciplinary center | NR | 47 | 62 (13)†† | - | 10 (21.3) |
| Tsara, 2015 [10] | Respiratory intermediate unit | Failure to wean | 548 | 57 (18) | - | NR (15.0) |
| Lai, 2016 [45] | Respiratory care center | 21 | 510 | 84 (3) | - | 111 (21.8) |
| Bonnici, 2016 [22] | Specialist weaning, rehabilitation, and home MV center | 28 | 262 | 64 (53, 73) | - | 38 (14.5) |
| Hendin, 2019 [41] | Medical wards | Failure to wean | 50 | 56 (19–83) Mn (range) | - | 12 (24.0) |
| Ghiani, 2020 [37] | National weaning center | Failure to wean | 263 | 71 (11) | Total MV duration∗∗ PMV: 27 (22) |
38 (14.5) |
LOS, length of stay; MV, mechanical ventilation; NR, not reported; PMV, prior duration of mechanical ventilation.
Values are presented as mean (SD) unless otherwise specified. If the range or IQR was not stated, only the mean or median was reported.
Tracheostomy must be in place for at least one week before a subject is transferred.
Subjects were presented in subcategories; the mean (range) was reported for each subcategory. For simplified data presentation, only the range of reported values has been shown.
Length of prior mechanical ventilation was only given for 40 subjects who were admitted for weaning.
Denominator used to calculate the mortality rate was 1,414 instead of 1,419.
Total duration of mechanical ventilation since initiation was 55 (42.7) days for Mamary et al. and 52.2 (28.6) days for Ghiani et al.
Age at diagnosis of Amyotrophic Lateral Sclerosis was given. The mean time from diagnosis to invasive ventilation ranged from 799.2 to 849.3 days.
Table 4.
Chronic ventilation patients cared for at home and mortality outcomes, arranged by year.
| First author, Year | Subjects, n∗ | Age, years† | Duration of HMV, months or years† | Follow-up Period, years | Mortality N, (%) |
|---|---|---|---|---|---|
| Sivak, 1982 [67] | 21 | 59 (26–81) | 2.2 (0.1–17.0) yr | NR | 5 (23.8) |
| Fischer, 1982 [36] | 29 | 61 (31–73) | 2.4 (0.1–6.8) yr‡ | 2 | 12 (41.4) |
| Wolf, 1993 [73] | 13 | 17–78§ | 6.4 (1.0–12.0) yr | NR | 2 (15.3) |
| Moss, 1993 [53] | 19 | 57 (36–78) | 1.7 (0.3–5.8) yr | 1 | 7 (36.4) |
| Muir, 1994 [54] | 253 (41 NH) | 63 (9) | Minimum 1.0 yr|| | 6–10 | 203 (84.9)∗∗ |
| Scheinhorn, 1994 [64] | 26 | NR | Minimum 3.4 (0.2) mo†† | 1 | 14 (53.9) |
| 49 NH‡‡ | NR | 45 (91.8) | |||
| Lin, 1996 [49] | 34 | 48 (21) | 10.6 (8.7) mo | 4 mo | 5 (14.7) |
| Nugent, 1996 [55] | 13 | 36 (13–67) | 0.1–12.0 yr§§ | 1 | 2 (15.4) |
| Cazzolli, 1996 [25] | 50 (25 NH) | 60 (31–89) | 1.0–14.0 yr|||| | 7 | 23 (46.0) |
| Guber, 2002 [39] | 25 | 38 (1–72) | 1.1 (0.2–2.7) yr | 2 | 3 (12.0) |
| Salahuddin, 2005 [12] | 11 | 49 (10–98) | 9.5 (3.2–15.7) mo Mn (95% CI) | 5 | 3 (27.3) |
| Björling, 2006 [21] | 27 | 17–79¶ | Survivors: 26.0 (22.0–34.0) yr Non-survivors: 14.0 (4.0–27.0) yr |
23 | 18 (66.7) |
| 67# | 18–91¶ | NR | 10 | 11 (16.4) | |
| Vitacca, 2011 [71] | 12 | 72 (6) | NR | 1 | 4 (33.3) |
| Sancho, 2011 [63] | 38 | 65 (9) | NR | 1 | 8 (21.1) |
| Spataro, 2012 [68] | 87¶¶ | 61 (47–66) Md (range) | NR | 10 | 52 (59.8) |
| Fini, 2014 [35] | 37 (10 NH) | Home: 58## NH: 69## |
NR | 12 | 19 (51.4) |
| Rose, 2019 [61] | 52 (7 NH) | 64 (45, 75) Md (IQR) | NR | 4 | 25 (48.1) |
| Saiphoklang, 2019 [62] | 12 | 72 (18) | NR | 2–7 | 5 (41.7) |
| Aksoy, 2019 [18] | 134 | 66 (54, 73) Md (IQR) | NR | 1 | 26 (19.4) |
HMV, home mechanical ventilation; NH, nursing home; NR, not reported.
Total number of subjects. For some studies which had a few of their subjects in a nursing home or long-term care facility, the number of NH participants is stated in the parenthesis.
Values are presented as mean (SD) or mean (range) unless otherwise specified.
Duration of HMV derived from how long patients were observed.
Range. No mean or median reported.
Subjects were ventilated for at least one year. This data was derived from the study's inclusion criteria.
Denominator used to calculate the mortality rate was 239.
Subjects were ventilated for a mean duration of 3.4 months in the prior care setting. Duration of ventilation at home or long-term care facility was not provided.
Outcomes for subjects cared for at home versus subjects cared for in nursing homes were reported separately.
The range of duration of HMV based on initiation date (in years) subtracted from the year the study was conducted. HMV initiation in the same year as the study was assumed to be at least one month.
Based on the survival information provided – 8 patients lived for 1–2 years after HMV initiation while among patients alive by the end of the study, three had been living with HMV for 11–14 years.
Subjects were presented in subcategories, and the median was reported for each subcategory. For simplified data presentation, the range of actual values has been demonstrated.
Two groups of patients were studied. The first cohort was patients cared for at the formal start of the unit in 1982, while the second patient cohort was cared for in 1997. For the second cohort, only adults were included in the analysis because there was a substantial number of patients less than 18 years old.
Five subjects remained in the acute hospital and were still included in the analysis.
Mean age at diagnosis of Amyotrophic Lateral Sclerosis was given. The mean time from diagnosis to invasive ventilation ranged from 799.2 to 849.3 days.
The minimum duration of ventilation of patients for inclusion in ICU studies ranged from 2 to 21 days. Most non-ICU studies required a ventilation duration of ≥14 days, except for one study [44]. While the ventilation requirements for home care/NH-based studies were not always reported, a majority of studies reported a minimum ventilation duration of 12 months (Tables 2, 3, and 4). Additionally, studied non-ICU and home/NH settings had their own criteria for admission which influenced characteristics of cohorts. Many non-ICU settings required patients to be hemodynamically stable without the need for pressors and have a tracheostomy with a ventilation requirement of FiO ≤45% and PEEP ≤8 [31, 37, 41, 46, 51, 60, 72]. Other non-ICU settings had additional requirements such as absence of multi-organ failure [66], dialysis requirement [19, 60, 66], uncontrolled infection [10, 40, 51, 60], invasive or continuous monitoring needs [34, 51, 60, 69, 72], and presence of rehabilitation potential [22, 34, 37, 38, 40, 60, 64, 65, 66]. Only Carson et al. [23] which had a mortality rate of 49.6%, had a liberal criterion accepting patients on vasopressors. Similar to many of the non-ICU settings, patients needed to be medically stable for home transfer, although specific details were not provided by any of the home care studies.
Mortality rates across different care settings ranged broadly from 13.7% to 77.8% in ICUs (Table 2), 7.8%–51.0% in non-ICUs (Table 3), and 12.0%–91.8% in home/NH settings (Table 4). Among studies reporting data from home/NH settings, studies with longer follow-up durations reported higher mortality rates.
Six studies, which specifically examined outcomes of older adults, identified an expansive range of mortality outcomes [9, 17, 28, 34, 38, 45]. Abitağaoğlu [17] and Seneff et al. [9] reported mortality rates of 9.8% and 51.0% among ventilated patients aged ≥60 years in the ICU and step-down unit, respectively. An ICU study of patients aged ≥80 years old reported one of the highest mortality rates (77.8%) [28]. In comparison, studies of octogenarians in non-ICU settings reported mortality rates of 5.3% [38], 21.8% [45], and 27% [34] but the minimum length of ventilation differed between ICU and non-ICU studies.
3.3. Risk factors for mortality by level of care
Age was the most frequently studied risk factor for mortality across all care settings. A total of 6 studies (3 ICU [29, 30, 59] and 3 non-ICU [34, 45, 51]) examined age as a risk factor for short-term, in-patient mortality. Among these studies, only 2 ICU [29, 30] studies reported an association between increasing age and higher mortality. A total of 19 studies (7 ICU [24, 29, 33, 42, 43, 47, 52], 9 non-ICU [9, 20, 23, 35, 38, 40, 51, 66, 69] and 3 home care [18, 49, 63]) examined the association between age and long-term mortality. Of these, 5 ICU [24, 29, 42, 43, 47], 7 non-ICU [9, 20, 23, 38, 40, 66, 69] and 1 home care [18] studies reported significantly higher long-term mortality in older age groups. Fifteen these studies examined mortality up to 1-year while the rest had 6 months to 5 years follow-up time [9, 35, 66, 69].
APACHE [74] and SAPS [75] scores, despite being measures of ICU mortality risk, were studied and were found to be associated with both short-term and long-term mortality in ICU [30, 43] and non-ICU studies [9, 45, 51]. Scores were obtained on admission to the studied care setting. Other risk factors associated with short- and long-term mortality in ICU studies included an immunocompromised state [29, 30], respiratory disease as a cause of admission [30, 42], low platelet counts [24, 52, 59], presence of organ dysfunction [24, 29, 30, 47, 59], and vasopressor use [24, 47, 59]. The latter three variables were collected at day 21 of mechanical ventilation. In non-ICU studies, low albumin level [45, 51] was associated with short-term mortality. In contrast, etiology of acute illness [9, 20, 51] and weaning outcome at the non-ICU setting [20, 66] were associated with long-term mortality. For home care or NH settings, only one study identified a risk factor associated with mortality – heart failure or cerebrovascular disease as an underlying diagnosis [18].
3.4. Additional outcomes by care setting
Weaning outcomes were reported in 20 out of 26 (77%) of non-ICU studies [10, 19, 20, 22, 23, 26, 31, 34, 37, 38, 40, 41, 46, 51, 56, 57, 60, 64, 65, 66]. Weaning rates, or the percent of the study subjects that achieved successful weaning, ranged from 10.0% to 78.2%. The criteria for successful weaning varied considerably across these studies. If the definition of “weaned” included patients who continue to require non-invasive ventilation (NIV), the weaning rates were between 61.3% to 78.2% [22, 40, 66]. However, these rates dropped to a range of 25.3%–59.9% if ongoing use of NIV was not a criterion for a successful wean [31, 34, 37, 56, 57, 60, 65]. Additionally, successful weaning rates varied greatly depending on the minimum duration of time a patient needed to be ventilator-independent to be labelled as “weaned.” Studies requiring ≥48 h [26, 34] or ≥7 days [19, 64, 66] of spontaneous breathing had weaning rates between 59.9%–67.5% and 32.0%–61.3%, respectively. If the weaning criteria required spontaneous breathing up to discharge [10, 20, 51, 56], weaning rates ranged between 37.9% to 68.7%. Numerous studies with weaning as the primary outcome excluded patients identified as irreversibly ventilator dependent. Such studies [20, 22, 26, 34, 37, 38, 40, 60, 65, 66] had higher rates of weaning (43.0%–78.2%) compared to studies with weaning as a secondary outcome and with liberal inclusion criteria (10.0%–68.7%) [19, 23, 31, 41, 46, 51, 56].
Ten [21, 39, 49, 54, 55, 61, 62, 63, 71, 73] out of 19 (52%) home care/NH studies reported on hospitalization and complications rates. Chronically mechanically ventilated patients in home care settings requiring at least one hospitalization ranged between 44.1% to 60.0% [49, 61, 63, 73]. However, when examined for a follow-up period of one-year, Sancho et al. [63] and Rose et al. [61] calculated a mean hospitalization per patient of only 0.82 (SD 0.98) and 1.7 (SD 2.9), respectively; and Guber et al. [39] demonstrated that patients were admitted to the hospital for an average of 3.3 days annually. Additionally, patients on home mechanical ventilation spent more than 94% of their time outside of the hospital [21] or were hospital-free for about 1.1 years [61].
Ventilator malfunction was among the cited reasons for hospitalization in 7.7% [55] to 28.6% [73] of home care patients. Other studies [54, 62] reported ventilator failure as a cause of complication but did not state whether these patients required hospitalization. For example, Muir et al. [54] identified ventilator failure as the cause of acute respiratory failure in 3.9% (4/102) of patients, while Saiphoklang and colleagues [62] reported ventilator failure as a complication in 1.9% of cases (1/53). None of the home care studies reported ventilator malfunction as a primary cause of death [12, 49, 55, 62, 73], except for Fischer et al. [36], in which ventilator failure was not ruled out as a cause of cardiac arrest in one patient. Lastly, pulmonary infection (e.g., pneumonia) was the most common medical complication in patients ventilated at home [49, 54, 62, 63, 73]. Other reported complications included tracheostomy-related problems, such as loose tracheostomy, tracheostomy stenosis/granulation tissue formation [49, 54, 62, 63], and pneumothorax [54, 62, 73].
4. Discussion
This systematic review synthesizes the global experience of managing care of chronically ventilated patients in and outside of ICU settings. In light of the ongoing COVID-19 pandemic, the identification and consideration of other care venues for delivering high-quality ventilatory support for patients is warranted, particularly in resource-strained ICU settings. We observed that while mortality outcomes within and between care settings were disparate, mortality outcomes of patients in non-ICU studies were crudely comparable to those in intensive care units. Interestingly, mortality rates due to ventilator failure were low in home care, demonstrating the future potential of home care as an alternate care venue. Older age was identified as a key risk factor for mortality across all care settings.
Our main study limitation is the considerable clinical heterogeneity among included studies. Clinical characteristics related to study populations such as age and disease etiology, as well as differences in facility staffing, admission criteria and care objectives, varied broadly among included studies. In addition, factors including total ventilation duration and illness acuity or severity inevitably contributed to disparities in mortality outcomes. Although a meta-analysis of included studies was not possible due to significant heterogeneity, the strength of this systematic review lies in the rigorous synthesis of the care and outcomes of chronically ventilated patients in different care settings across the globe.
Our most notable finding was discovering evidence that suggest safety of non-ICU settings. Three-quarters of non-ICU studies had mortality rates at par with ICU studies which particularly examined patient cohorts who were considered stable for transfer out of the ICU but remained in the unit due to lack of alternative care settings [17, 48, 50, 58]. A study by Krieger et al. [44] had a considerably low mortality rate of 27.2% compared to most ICU studies despite requiring only a minimum ventilation duration of 3 days, which meant early transfer out of the ICU. A retrospective study by Seneff et al. [9] demonstrated no significant differences in 6-month mortality rates between ventilated patients transferred to a non-ICU setting and ventilated patients who remained in an acute hospital setting. Lastly, Gracey et al. [38], which examined patient outcomes before and after the establishment of a non-ICU setting in a tertiary hospital, found improvement in survival with the availability of the venue. In addition to these outcomes, non-ICU settings provide patient-centered programs aimed at weaning and rehabilitation which are integral for patient recovery and improved clinical outcomes.
Given these findings, a non-ICU setting is thus a potential alternative setting for stable patients requiring prolonged ventilation during the COVID-19 pandemic and in the event of limited ICU resources. Newly established units or existing units such as non-ICU respiratory care units, long-term care facilities, and hospital wards can be used as care settings. In terms of staffing, nurse-to-patient ratios can range from 1:2 to 1:4, although there was one study that had a nurse-to-patient ratio of 1:6 [44]. To ensure safety, patient transfers from the ICU to a non-ICU setting should be limited to those with cardiorespiratory stability [11].
With respect to home care, increased risk for viral transmission to family members or caregivers may limit its role in the current COVID-19 pandemic. However, considering its benefits such as improved quality of life, decreased rate of hospitalization and length of stay [13], and reduced health care costs [39, 54, 73], further discussion is warranted to consider home care as a possible alternative. In our systematic review, higher mortality rates were seen among home care patient populations. However, mortality rates of home care studies should be assessed with caution as there were considerable methodological and clinical heterogeneity including variable differences in observation periods and underlying etiology, respectively. For example, five studies [21, 25, 35, 54, 68] demonstrated higher mortality rates but also had extended periods of study observation. Muir et al. [54] also had the highest mortality rate as the entirety of the study cohort had a diagnosis of COPD, a disease known to cause poor mortality outcomes [66, 76]. Additionally, comparison of home care to other care settings could not be made due to differences not only in ventilation duration but also in patient and study factors. Despite these limitations, we found that home care has only been studied head-to-head with nursing homes and has been shown to have better survival outcomes [35, 64]. Although the reason is unclear, this suggests that ventilator care, albeit daunting and seemingly complex, can safely occur outside centralized health care institutions. Indeed, our systematic review underscores that ventilator failure is rarely the cause of death for patients on home mechanical ventilation [77] and there is no increased risk of hospitalizations as a result of home mechanical ventilation [13, 21, 39, 61, 63].
Our study illustrates the safety of providing ventilatory care in settings outside the intensive care unit, more than two decades after the release of the consensus statement on “Mechanical Ventilation Beyond the Intensive Care Unit.” [11] Moving forward, we recommend future prospective studies wherein two or more venues of care are directly compared within a study to minimize confounding and selection bias. Moreover, we implore researchers to identify additional or alternative measures of mortality and/or survival to sufficiently estimate the impact of a care setting on life expectancy. Lastly, as safety is not contingent on mortality or survival outcomes, clinical outcomes such as complication rates, hospitalization rates, and quality of life should also be compared across care settings.
5. Conclusion
With the COVID-19 pandemic pressing the limits of ICU bed and ventilator capacities, critically ill patients requiring prolonged mechanical ventilation can quickly overwhelm healthcare systems. Safe and high-quality care can be delivered to ventilator-dependent patients in non-ICU settings such as long-term acute care facilities or hospital wards as these settings demonstrated mortality rates comparable to that of intensive care units. Future investigations with direct comparisons of two or more care settings may add value to the findings of this systematic review and equip health systems with alternative settings to safely care for patients on prolonged mechanical ventilation in resource-limited settings.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Donahoe M.P. Current venues of care and related costs for the chronically critically ill. Respir. Care. 2012;57(6):867–888. doi: 10.4187/respcare.01656. [DOI] [PubMed] [Google Scholar]
- 2.Bhatraju P.K., Ghassemieh B.J., Nichols M. Covid-19 in critically ill patients in the seattle region - case series. N. Engl. J. Med. 2020;382(21):2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study [published correction appears in Lancet Respir Med. 2020 Apr;8(4):e26] Lancet Respir. Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGrath B.A., Brenner M.J., Warrillow S.J. Tracheostomy in the COVID-19 era: global and multidisciplinary guidance. Lancet Respir. Med. 2020;8(7):717–725. doi: 10.1016/S2213-2600(20)30230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cummings M.J., Baldwin M.R., Abrams D. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chao T.N., Harbison S.P., Braslow B.M. Outcomes after Tracheostomy in COVID-19 Patients [published online ahead of print, 2020 Jun 11] Ann. Surg. 2020;272(3):e181–e186. doi: 10.1097/SLA.0000000000004166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seneff M.G., Wagner D., Thompson D., Honeycutt C., Silver M.R. The impact of long-term acute-care facilities on the outcome and cost of care for patients undergoing prolonged mechanical ventilation. Crit. Care Med. 2000;28(2):342–350. doi: 10.1097/00003246-200002000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Tsara V., Moisiadis N., Antoniadou M., Serasli E. Characteristics and outcome of patients with difficult weaning from mechanical ventilation: an 18 years' experience of a respiratory intermediate unit attached to a pulmonary department. Hippokratia. 2015;19(1):37–40. [PMC free article] [PubMed] [Google Scholar]
- 11.Make B.J., Hill N.S., Goldberg A.I. Mechanical ventilation beyond the intensive care unit. Report of a consensus conference of the American College of Chest Physicians. Chest. 1998;113(5 Suppl):289S–344S. doi: 10.1378/chest.113.5_supplement.289s. [DOI] [PubMed] [Google Scholar]
- 12.Salahuddin N., Haider K., Husain S.J. Outcome of home mechanical ventilation. J. Coll. Phys. Surg. Pak. 2005;15(7):387–390. [PubMed] [Google Scholar]
- 13.MacIntyre E.J., Asadi L., Mckim D.A., Bagshaw S.M. Clinical outcomes associated with home mechanical ventilation: a systematic review. Can. Respir J. 2016;2016:6547180. doi: 10.1155/2016/6547180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liberati A., Altman D.G., Tetzlaff J. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann. Intern. Med. 2009;151(4):W65–W94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 15.MacIntyre N.R., Epstein S.K., Carson S. Management of patients requiring prolonged mechanical ventilation: report of a NAMDRC consensus conference. Chest. 2005;128(6):3937–3954. doi: 10.1378/chest.128.6.3937. [DOI] [PubMed] [Google Scholar]
- 16.National Institutes of Health . 2014. Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies.https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/cohort Accessed on. [Google Scholar]
- 17.Abitağaoğlu S., Demiroluk Ö., Köksal C. Transition to home mechanical ventilation for geriatric patients with prolonged weaning. Turk Geriatri Dergisi. 2019;22:18–24. [Google Scholar]
- 18.Aksoy E., Ocaklı B. Long-term survival of patients with tracheostomy having different diseases followed up in the respiratory intensive care unit outpatient clinic: which patients are lucky? Turk. Thorac. J. 2019;20(3):182–187. doi: 10.5152/TurkThoracJ.2018.18120. Published 2019 Apr 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bagley P.H., Cooney E. A community-based regional ventilator weaning unit: development and outcomes. Chest. 1997;111(4):1024–1029. doi: 10.1378/chest.111.4.1024. [DOI] [PubMed] [Google Scholar]
- 20.Bigatello L.M., Stelfox H.T., Berra L., Schmidt U., Gettings E.M. Outcome of patients undergoing prolonged mechanical ventilation after critical illness. Crit. Care Med. 2007;35(11):2491–2497. doi: 10.1097/01.CCM.0000287589.16724.B2. [DOI] [PubMed] [Google Scholar]
- 21.Björling G., Johansson U.B., Andersson G., Schedin U., Markström A., Frostell C. A retrospective survey of outpatients with long-term tracheostomy. Acta Anaesthesiol. Scand. 2006;50(4):399–406. doi: 10.1111/j.1399-6576.2005.00939.x. [DOI] [PubMed] [Google Scholar]
- 22.Mifsud Bonnici D., Sanctuary T., Warren A. Prospective observational cohort study of patients with weaning failure admitted to a specialist weaning, rehabilitation and home mechanical ventilation centre. BMJ Open. 2016;6(3) doi: 10.1136/bmjopen-2015-010025. Published 2016 Mar 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carson S.S., Bach P.B., Brzozowski L., Leff A. Outcomes after long-term acute care. An analysis of 133 mechanically ventilated patients. Am. J. Respir. Crit. Care Med. 1999;159(5 Pt 1):1568–1573. doi: 10.1164/ajrccm.159.5.9809002. [DOI] [PubMed] [Google Scholar]
- 24.Carson S.S., Garrett J., Hanson L.C. A prognostic model for one-year mortality in patients requiring prolonged mechanical ventilation. Crit. Care Med. 2008;36(7):2061–2069. doi: 10.1097/CCM.0b013e31817b8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cazzolli P.A., Oppenheimer E.A. Home mechanical ventilation for amyotrophic lateral sclerosis: nasal compared to tracheostomy-intermittent positive pressure ventilation. J. Neurol. Sci. 1996;139(Suppl):123–128. doi: 10.1016/0022-510x(96)00099-8. [DOI] [PubMed] [Google Scholar]
- 26.Ceriana P., Delmastro M., Rampulla C., Nava S. Demographics and clinical outcomes of patients admitted to a respiratory intensive care unit located in a rehabilitation center. Respir. Care. 2003;48(7):670–676. [PubMed] [Google Scholar]
- 27.Chelluri L., Mendelsohn A.B., Belle S.H. Hospital costs in patients receiving prolonged mechanical ventilation: does age have an impact? Crit. Care Med. 2003;31(6):1746–1751. doi: 10.1097/01.CCM.0000063478.91096.7D. [DOI] [PubMed] [Google Scholar]
- 28.Cohen I.L., Lambrinos J., Fein I.A. Mechanical ventilation for the elderly patient in intensive care. Incremental changes and benefits. JAMA. 1993;269(8):1025–1029. [PubMed] [Google Scholar]
- 29.Combes A., Costa M.A., Trouillet J.L. Morbidity, mortality, and quality-of-life outcomes of patients requiring >or=14 days of mechanical ventilation. Crit. Care Med. 2003;31(5):1373–1381. doi: 10.1097/01.CCM.0000065188.87029.C3. [DOI] [PubMed] [Google Scholar]
- 30.Combes A., Luyt C.E., Nieszkowska A., Trouillet J.L., Gibert C., Chastre J. Is tracheostomy associated with better outcomes for patients requiring long-term mechanical ventilation? Crit. Care Med. 2007;35(3):802–807. doi: 10.1097/01.CCM.0000256721.60517.B1. [DOI] [PubMed] [Google Scholar]
- 31.Cordasco E.M., Jr., Sivak E.D., Perez-Trepichio A. Demographics of long-term ventilator-dependent patients outside the intensive care unit. Cleve. Clin. J. Med. 1991;58(6):505–509. doi: 10.3949/ccjm.58.6.505. [DOI] [PubMed] [Google Scholar]
- 32.Cox C.E., Carson S.S., Holmes G.M., Howard A., Carey T.S. Increase in tracheostomy for prolonged mechanical ventilation in North Carolina, 1993-2002. Crit. Care Med. 2004;32(11):2219–2226. doi: 10.1097/01.ccm.0000145232.46143.40. [DOI] [PubMed] [Google Scholar]
- 33.Cox C.E., Carson S.S., Lindquist J.H. Differences in one-year health outcomes and resource utilization by definition of prolonged mechanical ventilation: a prospective cohort study. Crit. Care. 2007;11(1):R9. doi: 10.1186/cc5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dasgupta A., Rice R., Mascha E., Litaker D., Stoller J.K. Four-year experience with a unit for long-term ventilation (respiratory special care unit) at the Cleveland Clinic Foundation. Chest. 1999;116(2):447–455. doi: 10.1378/chest.116.2.447. [DOI] [PubMed] [Google Scholar]
- 35.Fini N., Georgoulopoulou E., Vinceti M. Noninvasive and invasive ventilation and enteral nutrition for ALS in Italy. Muscle Nerve. 2014;50(4):508–516. doi: 10.1002/mus.24187. [DOI] [PubMed] [Google Scholar]
- 36.Fischer D.A., Prentice W.S. Feasibility of home care for certain respiratory-dependent restrictive or obstructive lung disease patients. Chest. 1982;82(6):739–743. doi: 10.1378/chest.82.6.739. [DOI] [PubMed] [Google Scholar]
- 37.Ghiani A., Paderewska J., Sainis A., Crispin A., Walcher S., Neurohr C. Variables predicting weaning outcome in prolonged mechanically ventilated tracheotomized patients: a retrospective study. J. Intensive Care. 2020;8(19) doi: 10.1186/s40560-020-00437-4. Published 2020 Feb 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gracey D.R., Hardy D.C., Naessens J.M., Silverstein M.D., Hubmayr R.D. The mayo ventilator-dependent rehabilitation unit: a 5-year experience. Mayo Clin. Proc. 1997;72(1):13–19. doi: 10.4065/72.1.13. [DOI] [PubMed] [Google Scholar]
- 39.Guber A., Morris E., Chen B., Israeli S. First experience with the home-care management system for respiratory patients in Israel. Isr. Med. Assoc. J. 2002;4(6):418–420. [PubMed] [Google Scholar]
- 40.Hannan L.M., Tan S., Hopkinson K. Inpatient and long-term outcomes of individuals admitted for weaning from mechanical ventilation at a specialized ventilation weaning unit. Respirology. 2013;18(1):154–160. doi: 10.1111/j.1440-1843.2012.02266.x. [DOI] [PubMed] [Google Scholar]
- 41.Hendin A., Rose L., McKim D.A. Tracheostomy ventilation outside the intensive care unit: experience of a tertiary care hospital. Can. J. Resp. Crit. Care Sleep Med. 2020;4:99–105. [Google Scholar]
- 42.Hill A.D., Fowler R.A., Burns K.E. Long-term outcomes and health care utilization after prolonged mechanical ventilation. Ann. Am. Thorac. Soc. 2017;14:355–362. doi: 10.1513/AnnalsATS.201610-792OC. [DOI] [PubMed] [Google Scholar]
- 43.Kojicic M., Li G., Ahmed A. Long-term survival in patients with tracheostomy and prolonged mechanical ventilation in Olmsted County, Minnesota. Respir. Care. 2011;56(11):1765–1770. doi: 10.4187/respcare.01096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krieger B.P., Ershowsky P., Spivack D., Thorstenson J., Sackner M.A. Initial experience with a central respiratory monitoring unit as a cost-saving alternative to the intensive care unit for Medicare patients who require long-term ventilator support. Chest. 1988;93(2):395–397. doi: 10.1378/chest.93.2.395. [DOI] [PubMed] [Google Scholar]
- 45.Lai C.C., Ko S.C., Chen C.M., Weng S.F., Tseng K.L., Cheng K.C. The outcomes and prognostic factors of the very elderly requiring prolonged mechanical ventilation in a single respiratory care center. Medicine (Baltim.) 2016;95(2):e2479. doi: 10.1097/MD.0000000000002479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Latriano B., McCauley P., Astiz M.E., Greenbaum D., Rackow E.C. Non-ICU care of hemodynamically stable mechanically ventilated patients. Chest. 1996;109(6):1591–1596. doi: 10.1378/chest.109.6.1591. [DOI] [PubMed] [Google Scholar]
- 47.Leroy G., Devos P., Lambiotte F., Thévenin D., Leroy O. One-year mortality in patients requiring prolonged mechanical ventilation: multicenter evaluation of the ProVent score. Crit. Care. 2014;18(4):R155. doi: 10.1186/cc13994. Published 2014 Jul 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li J., Zhan Q.Y., Wang C. Survey of prolonged mechanical ventilation in intensive care units in mainland China. Respir. Care. 2016;61(9):1224–1231. doi: 10.4187/respcare.04295. [DOI] [PubMed] [Google Scholar]
- 49.Lin M.C., Huang C.C., Lan R.S., Tsai Y.H. Home mechanical ventilation: investigation of 34 cases in Taiwan. Changgeng Yi Xue Za Zhi. 1996;19(1):42–49. [PubMed] [Google Scholar]
- 50.Lone N.I., Walsh T.S. Prolonged mechanical ventilation in critically ill patients: epidemiology, outcomes and modelling the potential cost consequences of establishing a regional weaning unit. Crit. Care. 2011;15(2):R102. doi: 10.1186/cc10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mamary A.J., Kondapaneni S., Vance G.B., Gaughan J.P., Martin U.J., Criner G.J. Survival in patients receiving prolonged ventilation: factors that influence outcome. Clin. Med. Insights Circulatory, Respir. Pulm. Med. 2011;5:17–26. doi: 10.4137/CCRPM.S6649. Published 2011 Apr 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mok J.H., Kim Y.H., Jeong E.S. Clinical application of the ProVent score in Korean patients requiring prolonged mechanical ventilation: a 10-year experience in a university-affiliated tertiary hospital. J. Crit. Care. 2016;33:158–162. doi: 10.1016/j.jcrc.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 53.Moss A.H., Casey P., Stocking C.B., Roos R.P., Brooks B.R., Siegler M. Home ventilation for amyotrophic lateral sclerosis patients: outcomes, costs, and patient, family, and physician attitudes. Neurology. 1993;43(2):438–443. doi: 10.1212/wnl.43.2.438. [DOI] [PubMed] [Google Scholar]
- 54.Muir J.F., Girault C., Cardinaud J.P., Polu J.M. Survival and long-term follow-up of tracheostomized patients with COPD treated by home mechanical ventilation. A multicenter French study in 259 patients. French Cooperative Study Group. Chest. 1994;106(1):201–209. doi: 10.1378/chest.106.1.201. [DOI] [PubMed] [Google Scholar]
- 55.Nugent A.M., Lyons J.D., Gleadhill I.C., MacMahon J. Home ventilation in northern Ireland. Ulster Med. J. 1996;65(1):47–50. [PMC free article] [PubMed] [Google Scholar]
- 56.Pilcher D.V., Bailey M.J., Treacher D.F., Hamid S., Williams A.J., Davidson A.C. Outcomes, cost and long term survival of patients referred to a regional weaning centre. Thorax. 2005;60(3):187–192. doi: 10.1136/thx.2004.026500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Polverino E., Nava S., Ferrer M. Patients' characterization, hospital course and clinical outcomes in five Italian respiratory intensive care units. Intensive Care Med. 2010;36(1):137–142. doi: 10.1007/s00134-009-1658-2. [DOI] [PubMed] [Google Scholar]
- 58.Robson V., Poynter J., Lawler P.G., Baudouin S.V. The need for a regional weaning centre, a one-year survey of intensive care weaning delay in the Northern Region of England. Anaesthesia. 2003;58(2):161–165. doi: 10.1046/j.1365-2044.2003.02964_1.x. [DOI] [PubMed] [Google Scholar]
- 59.Roh J., Shin M.J., Jeong E.S., Lee K. Association between medical costs and the ProVent model in patients requiring prolonged mechanical ventilation. Tuberc. Respir. Dis. 2019;82(2):166–172. doi: 10.4046/trd.2018.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rose L., Fraser I.M. Patient characteristics and outcomes of a provincial prolonged-ventilation weaning centre: a retrospective cohort study. Can. Respir J. 2012;19(3):216–220. doi: 10.1155/2012/358265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rose L., Watling L., Kohli R. Transition program for ventilator assisted individuals from acute care to home. Can. J. Resp. Crit. Care Sleep Med. 2019;3:100–105. [Google Scholar]
- 62.Saiphoklang N., Kanitsap A., Ruchiwit P., Pirompanich P., Sricharoenchai T., Cooper C.B. Patient characteristics and outcomes of a home mechanical ventilation program in a developing country. Lung India. 2019;36(3):207–211. doi: 10.4103/lungindia.lungindia_219_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sancho J., Servera E., Díaz J.L., Bañuls P., Marín J. Home tracheotomy mechanical ventilation in patients with amyotrophic lateral sclerosis: causes, complications and 1-year survival. Thorax. 2011;66(11):948–952. doi: 10.1136/thx.2011.160481. [DOI] [PubMed] [Google Scholar]
- 64.Scheinhorn D.J., Artinian B.M., Catlin J.L. Weaning from prolonged mechanical ventilation. The experience at a regional weaning center. Chest. 1994;105(2):534–539. doi: 10.1378/chest.105.2.534. [DOI] [PubMed] [Google Scholar]
- 65.Scheinhorn D.J., Hassenpflug M.S., Votto J.J. Post-ICU mechanical ventilation at 23 long-term care hospitals: a multicenter outcomes study. Chest. 2007;131(1):85–93. doi: 10.1378/chest.06-1081. [DOI] [PubMed] [Google Scholar]
- 66.Schönhofer B., Euteneuer S., Nava S., Suchi S., Köhler D. Survival of mechanically ventilated patients admitted to a specialised weaning centre. Intensive Care Med. 2002;28(7):908–916. doi: 10.1007/s00134-002-1287-5. [DOI] [PubMed] [Google Scholar]
- 67.Sivak E.D., Cordasco E.M., Gipson W.T., Stelmak K. Clinical considerations in the implementation of home care ventilation: observations in 24 patients. Cleve. Clin. Q. 1983;50(2):219–225. doi: 10.3949/ccjm.50.2.219. [DOI] [PubMed] [Google Scholar]
- 68.Spataro R., Bono V., Marchese S., La Bella V. Tracheostomy mechanical ventilation in patients with amyotrophic lateral sclerosis: clinical features and survival analysis. J. Neurol. Sci. 2012;323(1-2):66–70. doi: 10.1016/j.jns.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 69.Stoller J.K., Xu M., Mascha E., Rice R. Long-term outcomes for patients discharged from a long-term hospital-based weaning unit. Chest. 2003;124(5):1892–1899. doi: 10.1378/chest.124.5.1892. [DOI] [PubMed] [Google Scholar]
- 70.Unroe M., Kahn J.M., Carson S.S. One-year trajectories of care and resource utilization for recipients of prolonged mechanical ventilation: a cohort study. Ann. Intern. Med. 2010;153(3):167–175. doi: 10.1059/0003-4819-153-3-201008030-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vitacca M., Bianchi L., Bazza A., Clini E.M. Advanced COPD patients under home mechanical ventilation and/or long term oxygen therapy: Italian healthcare costs. Monaldi Arch. Chest Dis. 2011;75(4):207–214. doi: 10.4081/monaldi.2011.208. [DOI] [PubMed] [Google Scholar]
- 72.Wijkstra P.J., Avendaño M.A., Goldstein R.S. Inpatient chronic assisted ventilatory care: a 15-year experience. Chest. 2003;124(3):850–856. doi: 10.1378/chest.124.3.850. [DOI] [PubMed] [Google Scholar]
- 73.Wolf E., Penchas S., Werczberger A. Home care therapy for respirator-dependent patients. Isr. J. Med. Sci. 1993;29(10):617–623. [PubMed] [Google Scholar]
- 74.Knaus W.A., Draper E.A., Wagner D.P., Zimmerman J.E. Apache II: a severity of disease classification system. Crit. Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 75.Le Gall J., Lemeshow S., Saulnier F. A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 76.Chailleux E., Fauroux B., Binet F., Dautzenberg B., Polu J.M. Predictors of survival in patients receiving domiciliary oxygen therapy or mechanical ventilation. A 10-year analysis of ANTADIR Observatory. Chest. 1996;109(3):741–749. doi: 10.1378/chest.109.3.741. [DOI] [PubMed] [Google Scholar]
- 77.Srinivasan S., Doty S.M., White T.R. Frequency, causes, and outcome of home ventilator failure. Chest. 1998;114(5):1363–1367. doi: 10.1378/chest.114.5.1363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supplementary material/referenced in article.