Abstract
Ca2+ flux into the mitochondrial matrix through the MCU holocomplex (MCUcx) has recently been measured quantitatively and with milliseconds resolution for the first time under physiological conditions in both heart and skeletal muscle. Additionally, the dynamic levels of Ca2+ in the mitochondrial matrix ([Ca2+]m) of cardiomyocytes were measured as it was controlled by the balance between influx of Ca2+ into the mitochondrial matrix through MCUcx and efflux through the mitochondrial Na+ / Ca2+ exchanger (NCLX). Under these conditions [Ca2+]m was shown to regulate ATP production by the mitochondria at only a few critical sites. Additional functions attributed to [Ca2+]m continue to be reported in the literature. Here we review the new findings attributed to MCUcx function and provide a framework for understanding and investigating mitochondrial Ca2+ influx features, many of which remain controversial. The properties and functions of the MCUcx subunits that constitute the holocomplex are challenging to tease apart. Such distinct subunits include EMRE, MCUR1, MICUx (i.e. MICU1, MICU2, MICU3), and the pore-forming subunits (MCUpore). Currently, the specific set of functions of each subunit remains non-quantitative and controversial. The more contentious issues are discussed in the context of the newly measured native MCUcx Ca2+ flux from heart and skeletal muscle. These MCUcx Ca2+ flux measurements have been shown to be a highly-regulated, tissue-specific with femto-Siemens Ca2+ conductances and with distinct extramitochondrial Ca2+ ([Ca2+]i) dependencies. These data from cardiac and skeletal muscle mitochondria have been examined quantitatively for their threshold [Ca2+]i levels and for hypothesized gatekeeping function and are discussed in the context of model cell (e.g. HeLa, MEF, HEK293, COS7 cells) measurements. Our new findings on MCUcx dependent matrix [Ca2+]m signaling provide a quantitative basis for on-going and new investigations of the roles of MCUcx in cardiac function ranging from metabolic fuel selection, capillary blood-flow control and the pathological activation of the mitochondrial permeability transition pore (mPTP). Additionally, this review presents the use of advanced new methods that can be readily adapted by any investigator to enable them to carry out quantitative Ca2+ measurements in mitochondria while controlling the inner mitochondrial membrane potential, ΔΨm.
Introduction
Cytosolic Ca2+ signaling is used by diverse cells to regulate a broad range of cellular, subcellular and supra-cellular activities[1, 2]. The diversity and subtlety of these signaling modalities is grand, ranging from contraction in cardiac, skeletal and smooth muscle, to neurotransmission in CNS and peripheral nerve cells, and to multiple other functions in different organs of many eukaryotes. In addition to these fairly direct actions, Ca2+ signaling entrains parallel processing that provide support and feedback for the direct actions. Under physiological conditions, it has been hypothesized for many years that Ca2+ entry into mitochondria also regulates ATP production in parallel and this ATP production replenishes the energy consumed by the molecular motors and transducers that execute the functions of the cells. Only recently, however, have the details of the regulation of ATP production by mitochondrial matrix calcium ([Ca2+]m) been identified [3]. Importantly, specific putative calcium-sensitive proteins within the matrix, hypothesized to be critical to the Ca2+ sensitivity of ATP production, have been ruled out. This was done in mitochondria from cardiac ventricular myocytes under physiological conditions while Ca2+ sensitive ATP production was measured simultaneously. There is no question that additional quantitative investigations under alternative physiological conditions, during stressful circumstances and in disease-states still need to be determined. When done this would help us characterize the full spectrum of the Ca2+ sensitivity of ATP production by mitochondria. Importantly, however, the first quantitative fluxes of Ca2+ through the mitochondrial Ca2+ uniporter complex (MCUcx) associated with this physiological activity were also measured in conjunction with the measurements of ATP production. Moreover, the regulation and function of ATP synthase itself by the voltage across the mitochondrial inner membrane, ΔΨm, was also demonstrated and characterized for the first time in a mammalian system.
Here we review the recent literature that examines the mitochondrial calcium uniporter holocomplex, MCUcx. It is formed around four pore-forming subunits (MCUpore) and these are complemented by addition components including four EMRE subunits, and several MICU subunits (from the MICUx family, possibly a MICU1 and MICU2 dimer) [4] and possibly other subunits such as MICUR1[5]. MCUcx works as the holocomplex and is the key mitochondrial Ca2+ channel that enables each mitochondrion to continuously monitor cellular activity by reporting the cytosolic [Ca2+]i level to the matrix. By this means, the mitochondria are able to respond to increased activity with enhanced ATP production (see Figs. 1 and 2 and ref. [3]). In addition, a very important new set of features of the MCUcx have been suggested amidst some controversy. One such features is the hypothesized conductance threshold for Ca2+ in the MCUcx which may be under control of one or more of the “non-pore” subunits charged with a “gatekeeping” function, MICU1 and MICU2. In this review, we discuss these matters and place them in the context of our new investigations of MCUcx [3] along with recent work by others. There are three primary “take-home” conclusions from our treatment of Ca2+ influx through MCUcx. First, it is risky to generalize by assuming that all MCU holocomplexes (MCUcx) from different tissues are the same. We [3] and others [6] show dramatic differences in the MCUcx from heart and skeletal muscle. Second, the gold-standard of function should be the intact MCUcx channels taken from native tissue. Certainly, data on MCUcx obtained from other systems such as model cell lines has informed our understanding of MCUcx function and enabled us to do an impressive array of novel experiments, but these systems have been highly engineered. It remains an important job for those of us who wish to explain physiological function and disease-dependent changes to integrate the model system data into the native tissue function. Third, to facilitate reproducibility and discussion, direct conclusion about the function of MCUcx should include quantitative measurements of the MCUcx Ca2+ flux and the electrochemical gradients driving the MCUcx Ca2+ flux.
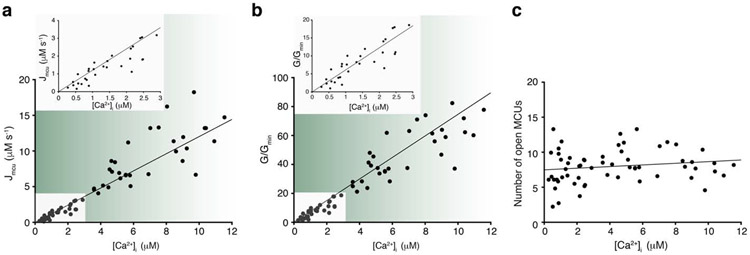
Figure 1. MCUcx flux and its regulation by [Ca2+]i in heart.
a. Measurements of the MCUcx Ca2+ influx (Jmcu) in heart mitochondria (units are scaled to liter of cytosol). Jmcu (μM s−1) is plotted as a function of measured extramitochondrial, [Ca2+]i. Each of these measurements of Jmcu were carried out along with measurements of [Ca2+]i, [Ca2+]m, and ΔΨm. The inset (top) is a zoomed-in view of the region of the plot between 0 and 3 μM [Ca2+]i. Linear least-squares fit to the filled circles is shown (slope = 1.2, going through the origin at 0, 0). b. MCUcx conductance (G) for each of 63 experiments shown in a, normalized to the minimal conductance (Gmin) of each dataset (G/Gmin). G/Gmin is plotted as a function of [Ca2+]i. Inset (top) is a zoomed-in view of the region between 0 and 3 μM [Ca2+]i. Linear least- squares fit line to the filled circles is shown (slope = 6.1, going through the origin at 0,0). c. Number of open MCUcx channels per mitochondrion plotted as a function of [Ca2+]i. Taken from b after division by the number of mitochondria per liter cytosol and dividing by the [Ca2+]i-dependent unitary conductance of MCUcx (For additional technical details see [3, 7, 9]). Linear least-squares fit to the filled circles is shown (slope = 0.116, intercept = 7.48). Panels a-c are taken with publisher permission from Wescott et. al., Nature Metabolism, 2019 [3].
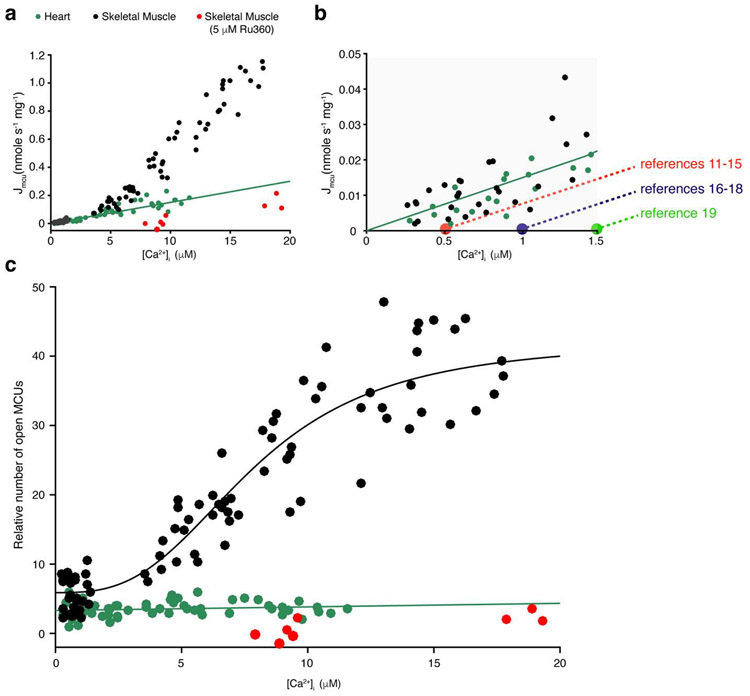
Figure 2. MCUcx and its regulation by [Ca2+]i in heart and skeletal muscle.
a. Measurement of the MCUcx Ca2+ influx (JMCU-cx) in cardiac mitochondria (green circles), skeletal muscle (black circles), and skeletal muscle with Ru360 (5 μM, red circles). For convenience on the graphs, Jmcu is used to represent JMCU-cx . Thus Jmcu is plotted as a function of measured extramitochondrial [Ca2+]i. Each of these measurements of Jmcu were carried out along with measurements of [Ca2+]i, [Ca2+]m, and ΔΨm. Linear least-squares fit to the heart mitochondria data is shown (slope = 0.015). b. Zoomed-in view of region between 0 and 3 μM of [Ca2+]i. from a. Black and green filled circles are from [3]. Overlaid dashed lines intercept the vertical axis at the [Ca2+]i levels where conduction thresholds of the MCUcx were proposed to occur. The levels of [Ca2+]i at which each threshold was proposed to be are taken from the indicated studies. The red filled circle is based on the threshold value from references [11-15], the purple from[16-18], the green from reference [19] c. Relative number of open MCUcx channels per mitochondrion plotted as a function of [Ca2+]i (For additional technical details see [3, 7, 9]). Linear least-squares fit to the heart mitochondria data is shown (slope = 0.051, intercept = 3.3). Skeletal muscle MCUcx data was fitted to a modified Hill equation yielding a K0.5 of 7.9 μM and a Hill coefficient of 2.95. Panels a-c are taken with publisher permission from Wescott et. al., Nature Metabolism, 2019 [3].
The biophysical features of MCUcx.
Electrophysiological studies showed that the MCUcx is a highly selective, low conductance Ca2+ channel [6, 7]. The single-channel conductance of the MCUcx (gmcu) was found to follow a Michaelis-Menten relationship, with a Km of 19 mM (much higher than the physiological diastolic levels found in heart of ~100 - ~300 nM) and with a maximal gmcu of ~ 6-7 pS (at 105 mM [Ca2+]i, see [7-9]), ~10,000 fold greater that that observed under physiological conditions. This relationship between the Ca2+ conductance of the MCUcx and the Ca2+ concentration is a typical biophysical feature of highly selective ion channels [10]. Typically, the conductance of an open channel increases as the availability of the conducting ion(s) increases[10]. Due to these biophysical features of the MCUcx, electrophysiological examination of MCUcx at physiological [Ca2+]i is particularly challenging. Under such conditions, the single-channel conductance of the MCUcx is low (~0.1 fS at 500 nM [Ca2+]i and ΔΨm = −160 mV, see [9]). An additional limitation is the low copy number of MCUcx channels in the inner-mitochondrial membrane of several tissues such as the heart [6]. As a result of these difficulties, to date, no electrophysiological examinations of MCUcx current have been reported over the range of [Ca2+]i of 300 nM to 10 μM, a range of particular interest, in which [Ca2+]i-dependent allosteric gating of the MCUcx is proposed to take place [11-19].
To measure Ca2+ flux (JCa) we carried out calibrated fluorescent measurements of Ca2+ to quantify Ca2+ influx through MCUcx channels even under physiological conditions [3]. These experiments replaced electrophysiological examinations of mitoplasts which are limited by low signal-to-noise characteristics at physiological [Ca2+]i. To do the quantitative measurements of MCUcx Ca2+ influx, fluorescence signals from a large number of mitochondria at physiological [Ca2+]i were carried out. By contrast, the electrophysiological recordings used patch-clamp methods in a single mitoplast (i.e. the inner-membrane product of a single mitochondrion) but at the "cost" of needing to use about 10,000 times higher [Ca2+]i. The primary cost of the physiological methods we used was the technical challenge of extracting quantitative calibrated signals (also see Table 1). However, this effort enables us to measure and account for all of the Ca2+ in all of the relevant compartments. Specifically, instead of the traditional fluorescent estimations of MCUcx activity that are derived primarily from measuring the free extramitochondrial [Ca] ([Ca2+]extra), we measured quantitatively (i.e. with full calibration) all of the Ca2+ signals in all of the relevant compartments. This includes the extra-mitochondrial Ca2+ (free-Ca2+ and Ca2+ bound to buffer) and the free Ca2+ in the mitochondrial matrix, [Ca2+]m. We also measured ΔΨm. In addition, the rates of change of [Ca2+]i and [Ca2+]m were also measured. To implement the above method, we measured [Ca2+]extra, total (i.e. the total concentration of Ca2+ outside the mitochondria) over the range of 0.3 - 5 μM. This required us to eliminate all of the "invisible" Ca2+ buffers such as EGTA. Thus, the only Ca2+ buffer that we used was the Ca2+-sensitive fluorescent indicator Fluo-4. This approach not only enabled us to measure the [Ca2+]extra, total, but it also avoided the comingling of the effects of "other" Ca2+ buffers and the Ca2+ flux through MCUcx Ca2+ channels. In our approach, the RU360-sensitive time-dependent decline of the [Ca2+]extra, total is taken as the total MCUcx Ca2+ influx (Jmcu). In addition to measuring Jmcu, quantitative measurements of ΔΨm and the free [Ca2+] inside ([Ca2+]m) and outside ([Ca2+]extra) the mitochondria were carried out. Together, Jmcu, ΔΨm, [Ca2+]m and [Ca2+]extra provide the means of estimating the physiological conductance of MCUcx (Gmcu) with the typical Hodgkin–Huxley model [i.e., Imcu = Gmcu(ΔΨm-ECa2+)]. From Gmcu that reflects the conductance of all the channels that are simultaneously open and the unitary conductance of a single open MCU (gmcu), the number of open MCUcx channels was estimated.
Table 1:
diverse methodologies to measure Ca2+ influx via MCUcx.
| General approach | Central tools |
Measures | Remarks | Recent | Related | |
|---|---|---|---|---|---|---|
| 1 | Radioactive mitochondrial Ca2+ uptake measurements | Radiolabeled 45Ca2+ | Cell/mitochondria 45Ca2+ retention | Insensitive to pH. Can be conducted over a wide range of [Ca2+] (nM to mM), low temporal resolution, cannot be combined with simultaneous measurements of other signals. Uses either isolated mitochondria or permeabilized cells. Does not quantitatively measure total JMCUcx due to dynamic binding to extramitochondrial buffers. | [4, 11, 17, 80] | [22, 81] |
| 2 | Fluorescent measurements of [Ca2+]i | Ca2+-sensitive fluorescent indicators | Change in free extra-mitochondrial [Ca2+] | High temporal resolution can be combined with simultaneous measurements of other signals. Uses either isolated mitochondria or permeabilized cells. Does not quantitatively measure total JMCUcx due to dynamic binding to extram itochondrial buffers. | [5, 11, 14, 16, 18, 26, 67, 74, 82, 83] | [84] |
| 3 | Electrophysiological measurements of IMCUcx | Patch-clamping the IMM | Whole-mitoplast or single-channel MCUcx currents | High temporal resolution. Quantitative direct measurements of MCUcx currents. Can be combined with simultaneous measurements of other fluorescent signals. Does not have the sensitivity below [Ca2+] of ~10 μM. Requires mitoplast preparation. | [6, 7, 64] | [85, 86] |
| 4 | Fluorescent measurements of total mitochondrial Ca2+ influx via MCUcx | Ca2+-sensitive and voltage-sensitive fluorescent indicators | Total MCUcx Ca2+ flux (JMCUcx), MCUcx conductance (GMCUcx). | High temporal resolution. Quantitatively measure total JMCUcx together with simultaneous quantitative measurements of [Ca2+]i, [Ca2+]m, and ΔΨm. Highly sensitivity below [Ca2+]i of ~10 μM. Carried out in isolated mitochondria but not in permeabilized cells. | [3] | [87], [88, 89] |
This approach was undertaken to separate electrochemical effects (thermodynamic) from other inherent properties of the MCUcx channel (e.g. the number of open MCUcx). While electrochemical gradients drive ion movement through the open MCUcx channel, we centered on examining how the opening of the MCUcx channel is gated allosterically by physiological levels of [Ca2+]i. These experiments were carried out with mitochondria isolated from skeletal muscle and heart muscle. In terms of extent, our measurements in mitochondria from heart and skeletal muscle were consistent with published genetic and electrophysiological evidence. Skeletal muscle mitochondria were reported to have the highest MCUcx current density [6] and express the highest copy numbers of MCUcx when compared to other mammalian tissues [20]. Heart has been reported to have one of the lowest. Furthermore, our estimation of the number of open MCUcx channels in a heart mitochondrion (i.e., 5-15) is around the range reported by electrophysiological studies (15-65 per cardiac mitochondrion [6]). Surprisingly, we found that the regulation of the number of open MCUcx channels by [Ca2+]i is tissue-dependent (See Fig. 2.). We found that the number of open MCUcx channels is regulated by [Ca2+]i in skeletal muscle but not in heart.
Ca2+ signaling in mitochondria under physiological conditions.
In heart muscle cells, the abundant intermyofibrillar mitochondria (IFM) are frequently exposed to large local elevations of cytosolic Ca2+ ([Ca2+]i). Each end of the IFM is only about 100 nm away from the release sites containing clusters of ryanodine receptors on the junctional sarcoplasmic reticulum, the intracellular sites of [Ca2+]i release. About 5,000 - 10,000 of these SR Ca2+ release sites open nearly simultaneously during each heartbeat, leading to a cell-wide transient elevation of [Ca2+]i (i.e., the [Ca2+]i transient). In the diastolic period between heartbeats, rare releases of Ca2+ from individual sites occur stochastically throughout the cell, giving rise to clearly visualizable Ca2+ sparks [21]. During a Ca2+ spark, one end of the IFM is exposed to local elevation of [Ca2+]i from about a 100 nM to as high as about 10 μM. The exposure to high levels of [Ca2+]i (i.e. μM levels) is brief, lasting only a few milliseconds. During a cell-wide [Ca2+]i transient, both ends of the IFM mitochondria are frequently exposed to similar spatiotemporal elevations of [Ca2+]i. This locally elevated [Ca2+]i can enter the mitochondrial matrix via MCUcx, an entry that is further driven by the large negative potential (ΔΨm) across the mitochondrial inner membrane of about −150 mV [3, 7, 22-25].
Recently, Ca2+ flux into the mitochondrial matrix through MCUcx has been measured quantitatively and with milliseconds resolution for the first time under physiological conditions [3] (also see Table 1). These measurements were carried out in a manner that enabled estimating the number of open MCUcx and how such number depends on [Ca2+]i. A primary goal of this approach was to test the hypothesis that the opening of the MCUcx is gated allosterically by [Ca2+]i. The tests were carried out over the physiological range of [Ca2+]i, from 0.3 to over 10 μM, a range where [Ca2+]i-dependent gating was suspected to occur. Over this range, these data from cardiac mitochondria suggest that the number of open MCUcx is relatively constant in heart and does not appear to be regulated by [Ca2+]i. These findings contradict earlier generalizations that such [Ca2+]i-dependent gating function is a broad feature of all types of MCUcx’s. Furthermore, in the physiological context of the heart, these findings suggest that [Ca2+]m is kept tightly regulated without a [Ca2+]i-dependent gating and without a threshold level for conducting Ca2+, features that have been suggested to be imparted by molecular constituents (i.e. subunits) of the MCUcx that together function as "gatekeepers" of the channel [11-19]. Importantly, the putative gatekeeping function has yet to be defined quantitatively or mechanistically in any tissue-identified and testable manner (also see discussion below) and so this should be a near-term goal in reevaluating the gatekeeping hypothesis. As suggested by prior studies, the putative purpose of gatekeeping is to limit Ca2+ entry through MCUcx. Thus, “gatekeeping” may be preventing excessively high influx and the development of ‘overload’ of [Ca2+]m. In heart, however, the constant and low number of open MCUcx in each cardiac mitochondrion (about 5-15 [3]) measured over [Ca2+]i from ~ 100 nM to ~ 10 μM appears to be sufficient to keep [Ca2+]m physiologically regulated.
How the Ca2+ influx through MCUcx together with the efflux of matrix Ca2+ is managed to prevent Ca2+ overload in the mitochondrial matrix is a topic of active research. We still know little about how the mitochondrial Na+/ Ca2+ exchanger (NCLX) dynamically regulates the levels of Ca2+ in the mitochondrial matrix ([Ca2+]m). To evaluate the dynamics of [Ca2+]m we carried out measurements in patch-clamped cardiomyocytes under physiologically relevant conditions when both MCUcx and NCLX are active to understand how [Ca2+]m is controlled by physiological [Ca2+]i signals. We found that under pronged quiescent conditions when global "diastolic" [Ca2+]i is stable around 100 nM, [Ca2+]m was static and slightly higher than [Ca2+]i. However, as soon as [Ca2+]i transients were electrically evoked, [Ca2+]m started to rise gradually and slowly, reaching relatively stable steady-state levels over the course of minutes. With additional β-adrenergic stimulation to increase the amplitude of the [Ca2+]i transients, a higher steady-state levels of [Ca2+]m were reached [3]. Thus, while cytosolic Ca2+ rises abruptly and declines sharply with each contraction-relaxation cycle, [Ca2+]m does not. Instead, the [Ca2+]m dynamics are a low-pass filter version of the [Ca2+]i signals. In a physiological context, these findings suggest that a single heartbeat-produced [Ca2+]i transient does not materially change [Ca2+]m. Instead, [Ca2+]m is controlled by the frequency and amplitudes of multiple [Ca2+]i transients, therefore tracking changes in the heart rate over time and favoring the influence of the diastolic [Ca2+]i. These measurements also show that under physiological conditions Ca2+ influx into the matrix appears to occur through MCUcx even when the diastolic [Ca2+]i is quite low. This Ca2+ influx occurs over the diastolic [Ca2+]i range of 100 nM to 300 nM and results in a measured increase in matrix [Ca2+]m from ~200 nM to ~400 nM as shown recently (Fig. 3 in Ref. [3]). These measurements were obtained at low stimulation rates (0.5 Hz) in rat ventricular myocytes and suggest a need to re-examine the hypothesis that there is a "threshold" for Ca2+ conductance through the MCUcx below which there is no Ca2+ flux. These new data are consistent with the notion that there is not a physiological threshold of the MCUcx between 100 nM and 300 nM and that this channel conducts Ca2+ at low diastolic [Ca2+]i concentrations. Data testing this hypothesis is also presented in Fig. 1 (above).
In other cell types, cytosolic Ca2+ signals are profoundly different than those in heart cells [1, 2]. The conduction of such [Ca2+]i signals through the MCUcx channels is also tissue-specific. The heart mitochondria were found to have the lowest number of MCUcx, the skeletal was found to have the highest of all tested mammalian tissues [6]. Furthermore, we recently found that regulation of the MCUcx by [Ca2+]i in heart and skeletal muscle is vastly different [3]. The number of open MCUcx channels is regulated by [Ca2+]i in skeletal muscle, in heart it is not (see Fig 2C). Furthermore, other studies suggested that the regulation of the liver MCUcx by [Ca2+]i is different than the regulation of the MCUcx in heart and skeletal muscles [19, 26]. These, and other differences that exist between the MCUcx of different tissues are important topics for future research. Better understanding of the tissue-specific MCUcx can potentially further our understanding of the MCUcx complex subunits and broadly advance our understanding of the physiological roles of mitochondrial Ca2+ signaling in different tissues.
Regulation of mitochondrial ATP production by matrix Ca2+
Over 40 years ago, Denton, McCormack and co-workers reported that increasing levels of free calcium could augment the enzymatic activity of pyruvate dehydrogenase (PDH) as well as other single enzymatic steps within the Krebs cycle. In later review articles published in the 1990s [27, 28], Denton, McCormack and co-workers surveyed the advances in our understanding of the roles of mitochondrial calcium. Their work highlighted a fundamental need for actual measurements that tested their hypothesis. Such work had yet to be done – it was clear that the net effect of physiologic [Ca2+]m on the rate of ATP production had not then been determined.
We directly address this gap in our understanding in [3]. Here we presented findings that measured how physiological calcium in the mitochondrial matrix ([Ca2+]m) regulated the rate of mitochondrial ATP production [3]. We used isolated cardiac mitochondria and measured ATP production quantitatively by calibrating the luminescence signal from luciferin/luciferase. Quantitative measurements of [Ca2+]m were done using Ca2+-sensitive fluorescent indicators that had been loaded into the mitochondrial matrix. These experiments were carried out over a wide range of [Ca2+]m levels from quiescent (resting) levels of about 100 nM to a level as high as 2.5 μM [Ca2+]m. We showed that increasing levels of [Ca2+]m elevated the rate of ATP production in a Michaelis-Menten type of relationship, with a [Ca2+]m K0.5 of around 600 nM. Saturation was reached at about 2 μM. This regulation of ATP production occurred through a [Ca2+]m-dependent modulation of pyruvate dehydrogenase and glutamate dehydrogenase activity and not through any effect of Ca2+ on ATP synthase (ETC complex V) or on electron transport chain complexes II, III or IV or on the dehydrogenases within the Krebs cycle itself. Our new findings provide direct evidence contrasting some earlier predictions on where elevated [Ca2+]m acted to stimulate ATP production [27-38].
Our findings that [Ca2+]m partly controls ATP production by the mitochondria through only a few pivotal regulatory sites is new and is contrary to the large number of earlier publications that hypothesized many [Ca2+]m-sensitive regulatory sites [27-38]. A key difference between the work by Wescott et al (2019) and previous publications is that no prior study had actually carried out time-dependent parallel measurements of [Ca2+]m and ATP production. Instead, prior studies had measured the rate of oxygen consumption as a surrogate for ATP measurements. [Ca2+]m was also not measured. Instead, the free Ca2+ present in the experimental buffer solutions was calculated using Ca2+-chelation software or other calculation modules or other means.
There is no question that additional quantitative investigations under alternative physiological conditions still need to be carried out to characterize better the full spectrum of Ca2+ sensitivity in mitochondria. Furthermore, mitochondrial calcium sensitivity likely occurs with tissue specific features that remain unclear. Such diversity would not be surprising because the features of energy consumption and the daily metabolic rates vary greatly among the major organs and tissues [39, 40]. In addition, mitochondria from different tissues metabolize different proportions of ‘food-stocks’ to fuel ATP production. Furthermore, not all tissues undergo large and acute changes in ATP consumption as do the heart and skeletal muscles. For example, the measured variations in oxygen consumption in the liver and the kidneys is rather modest, rising from the basal O2-consumption levels of the tissue to levels that are about 25 percent higher [41-48]. In contrast, in the heart, with increased adrenergic tone and increased circulatory demands, the rate of oxygen consumption can rise by 2.5-fold to upward of 4.5-fold [49-58]. Even larger increases in oxygen consumption occurs in the skeletal muscle. In this tissue, depending on the fiber type, a transition from a relaxed muscle to a physiologic tetanic contraction can lead to 6-fold or even 17-fold rise in oxygen consumption [59-62].
The character of the sensitivity of ATP production to mitochondrial calcium in different tissues and cell types remains a critical topic for future work. Equally important is the need to understand how changes in the sensitivity of [Ca2+]m-dependent ATP production may contribute to the pathogenesis of multiple diseases. Indeed, dysfunction of mitochondria related to [Ca2+]m management has been linked to the etiology of multiple degenerative diseases and diseases of aging (further reviewed in [40, 63]). Yet, how the dysfunctions of mitochondria contribute to such pathologies remain ill-defined.
The function of the subunits of MCUcx
"MCUcx Threshold".
A large number of publications, many in high impact journals (e.g. [11-19]), have suggested that there is an extramitochondrial [Ca2+] level below which there is no MCUcx Ca2+ flux into the mitochondrial matrix. This [Ca2+] level has been given the name "threshold" or "MCUcx threshold". The existence of such MCUcx threshold was first suggested by Mallilankaraman et al., 2012 who also suggested that it serves to prevents excessive mitochondrial Ca2+ loading. The threshold was suggested to occur because the MCUcx includes molecular components that act as steric plugs or "gatekeepers" of the channel [16]. Since then the concept of such a threshold has been used as a tool in determining how each of the MCUcx-subunits interacted with the MCUpore itself to better understand the entire MCUcx function. This "threshold" level has been used to "unpack" the details of the MCUcx function based on diverse indirect experiments. Despite the pivotal importance of the putative "threshold" in sorting out the molecular functioning of the MCUcx, the MCUcx Ca2+ flux and the critical [Ca2+]i level of this threshold, to date there appears to be little agreement regarding the numbers. Importantly too, the putative MCUcx threshold has not been quantitatively measured. If indeed such threshold is an important feature of MCUcx, it would need to be determined by direct measurements of the levels of [Ca2+]i and [Ca2+]M as well as ΔΨM, and by quantitative measurement of the actual influx of Ca2+ into the mitochondria through MCUcx (i.e. JMCU-cx). With this approach, thermodynamic effects and allosteric gating effects can be distinguished and measured. The first such set of such measurements were done in [3] and are shown in Fig. 1a [3], (additional details are in Table 1). As clearly shown in the inset of Fig. 1a (at high resolution), the quantitative measurement of the MCUcx threshold suggests that it does not exist. Put otherwise, the threshold in cardiac myocyte MCUcx is found at 0 nM [Ca2+]i. In skeletal muscle the MCUcx threshold is also found at 0 nM [Ca2+]i as shown in Fig. 2. In further support of these conclusions, direct quantitative electrophysiological measurements of MCUcx by Garg et al. [64] showed that there is no conductance occlusion in the absence of [Ca2+]i. Under these conditions of nominally zero [Ca2+]i, with Na+ as the permeant ion, MCUcx readily conducts Na+ in patch clamped mitoplasts. Put another way, the MCUpore subunits are not blocked in the absence of extramitochondrial Ca2+ [64]. This work further demonstrates that a conduction “threshold” is not a feature of the MCUcx in Ca2+ - free conditions. In addition, the single-channel recordings in Garg et al., show that MICU1, an auxiliary subunit of the MCUcx, does not function as a plug -- as suggested by earlier estimates [11-19]. Instead, these single-channel recordings demonstrate that MICU1 enable the channel to remain open for longer periods of time. Thus, several independent careful quantitative studies [3, 64] demonstrate that a conduction threshold is not a necessary feature of the MCUcx, that the MCUcx channel conducts Ca2+ whenever it is available, and that in some tissues allosteric regulation of MCUcx by cytosolic Ca2+ occurs. Furthermore, these findings also indicate a different action of MICU1 on MCUcx than has been reported heretofore. Importantly, a conduction block, or conduction occlusion, has not been shown to occur by any study that quantitatively examined the conductance of the MCUcx at low Ca2+ [6, 7, 65].
Figs. 1-2 provide clear evidence that the MCUcx conduction threshold is indistinguishable from 0 nM [Ca2+]i in mitochondria from cardiac ventricular myocytes and from mitochondria from fast twitch skeletal muscle. At [Ca2+]i levels between 300 nM to 3.0 μM the MCUcx flux appear to increase linearly as the concentration of the conducting Ca2+ increases. The extrapolated value of the zoomed-in linear fit at values less than 3.0 μM is 0 μM [Ca2+]i. To understand better the spread of the suggested values along with the papers in which the data were presented, Fig. 2b shows the summary of these results graphically. A dashed line with the same slope as the fit shown in the inset in Fig. 1a is used to represent each cluster of findings with "thresholds" at 0.5 μM (red) [11-15], 1.0 μM (purple) [16-18] and 1.5 μM (green) [19]. It is clear that none of the suggested "threshold" lines fit the data better than the working conclusion from experiments on cardiac and skeletal muscle mitochondria. The same value of 0 μM [Ca2+]i is estimated when there is only a modest influence of the MICUs (in mitochondria from cardiac myocytes) or an overwhelming influence of the MICUs (in mitochondria from skeletal muscle fast twitch myocytes) [66]. Importantly, all of the technologies used in [3] are standard analytic methods and simply involve measuring all Ca2+ levels and careful calibration of Ca2+, ΔΨM and pH. Using this approach, the actual influx of Ca2+ into the mitochondria through MCUcx (i.e. the (JMCU-cx) was measured. In sum, these measurements were conducted above and below 0.5 μM [Ca2+]i, which is the lowest "threshold" level proposed in the literature [11-15]. In all cases, the findings contradict the hypothesis that in mitochondria of all tissues there is a "threshold" or minimum non-zero [Ca2+]i needed to enable a measurable (JCa)MCU-cx.
The MICU's: MICU1, MICU2 and MICU3: "the MICU's".
The function of the MICU's in the MCUcx remain enigmatic. MICU1 appears to be expressed in every tissue tested [13] and mice with MICU1 knocked out are partially [19] or completely [67] peri-natal lethal. Mice that are MICU2−/− [68] develop heart disease. In contrast, knocking out the pore-forming subunit, the MCUpore itself, is not necessarily embryonic lethal when carried out on some genetic backgrounds [69, 70]. However, despite the apparent importance of understanding better the function of MICU1, the investigational results and conclusions are not yet clear and consistent. Knocking down MICU1 has been reported to increase maximum JMCU-cx for Ca2+ in one study [71] but did not change JMCU-cx in other investigations [11, 16] and blocked it in yet another study [72]. Presumably methodological differences may account for these contradictory results since they were all carried out in the same cell line (HeLa cells). It is also not yet clear what is the degree to which MICU1 is affected by Ca2+ inside the mitochondrial matrix to regulate MCUcx ([16, 65, 73, 74], further discussed in [75]). There is also the surprising result that comes from an investigation in drosophila, where flies with knockout of the MCUpore (MCUpore−/−) live but when MICU1 was also knocked out the creatures were not viable [76]. This finding raises the question differently: what is the vital function that MICU1 fulfills in the absence of the MCUpore? For us, the key approach to resolve the questions raised by these findings is to carry our quantitative experiments that measure critical variables in functional mitochondria. Thus, it remains an important job for those of us who wish to explain disease, to integrate the model system data into the native tissue function. Importantly, there are stark differences in how the MCUcx of different tissues conduct Ca2+ [6] and differences in the molecular composition of the MCUcx among tissues[26]. In addition, while MICU1 is found in all tissues tested there appear to be a unique splice variant of MICU1 with expression that is limited to only a few tissues [66]. Additionally, stress or disease or physical activity may also matter. Thus, when animals are bred with altered MCUcx, sedentary animals should be compared with stressed animals. For maximum understanding, testing exercise stress, stress of specific energy sources and stress of specific kinases and diseases are the kind of investigative approaches that are likely to significantly advance our understanding.
Gating of MCUcx
In 1979 Marco Bragadin, Tullio Pozzan, and Giovanni Felice Azzone [77] were able to measure a much larger mitochondrial Ca2+ influx than in any previous studies. These measurements were possible because they identified a key shortcoming of prior approaches. They discovered that mitochondrial Ca2+ influx rapidly degraded its own driving force, the mitochondrial membrane potential (ΔΨm), because of the large influx of positive charge carried by Ca2+. To solve this problem they used a mitochondrial "voltage clamp" using the K+ ionophore valinomycin and the K+ gradient to set ΔΨm [78]. With this approach, in isolated liver mitochondria [77], Bragadin et al., (1979) found a very large mitochondrial Ca2+ influx that continued to increase with the increasing levels of extramitochondrial Ca2+ and only begun to saturate at about 200 μM Ca2+. The observed Km was around 75 μM, more then 10 times higher than in previous studies. The sigmoidal shape of the Ca2+ influx curve and other findings led Bragadin et al., to suggest that allosteric binding cooperativity augments mitochondrial Ca2+ influx through a Ruthenium Red sensitive “multi-subunit carrier”, that came to be called the MCUcx. Notably, with this experimental approach, Bragadin et al., also discovered the importance of setting ΔΨm or quantitatively measuring ΔΨm when investigating the properties of the MCUcx. Nearly 25 years later, even tighter control of ΔΨm was achieved by Kirichok et al., 2004 using electrophysiological approach to voltage-clamp the vesicular preparation of the inner mitochondrial membrane of COS-7 cells, the mitoplast [7]. The findings of Kirichok et al., revealed that MCUcx is a Ca2+-selective ion channel, with a much higher Km than shown before (i.e. 19 mM). This work also enabled discovery of the other central role of ΔΨm on the MCUcx; it became clear that ΔΨm not only powers Ca2+ movement via the MCUcx, but that the voltage itself also tightly controls the open probability of the MCUcx channel. At ΔΨm of −80 mV the open probability was only 0.11 but it steeply rises to 0.93 at ΔΨm of −160 mV. Learning these critical lessons from Bragadin et al., and from Kirichok et al, we measured ΔΨm quantitatively along with each measurement of MCUcx flux (Jmcu). We also used valinomycin to be able to carry out Jmcu measurements at high extramitochondrial Ca2+ ([Ca2+]i). Like Bragadin et al, we did not observe saturation of Jmcu over this range of [Ca2+]i as shown in Figure 2a. We then used these measurements to assess how [Ca2+]i allosterically regulates the number of open MCUs. We found that while [Ca2+]i did not affect the number of open MCUs in heart mitochondria, in skeletal muscle [Ca2+]i allosterically increased number of open MCUs by about 8 fold, with a K0.5 of 7.9 μM. This tissue-dependent [Ca2+]i-sensitivity of the MCUcx is likely due to different molecular composition of the MCUcx in different tissues [19, 26, 66], but particularly important is the potential involvement of MICU1 as the component of the channel that interacts with [Ca2+]i [79]. Indeed, recently, Garg et al., 2020, who used a mitoplast preparation from mouse embryonic fibroblasts (MEF) to examine the gating of IMCUcx found that voltage-dependent stochastic opening and closure of the MCUcx channel still occur in the absence of MICU1 [64]. However, Garg et al. also found that in the absence of MICU1, the open probability of the MCUcx was two- to threefold lower than in the WT MCUcx that contain MICU1. This finding further supports the role of MICU1 as a critical component of the activation gate in the MCUpore itself.
The physiological role of MCUcx
An increasing number of recent studies use mice with genetically altered MCUcx channels expressed in their native tissues. These mice may have a subunit knocked out, over-expressed or mutated [18, 69, 70, 82, 83, 90, 91]. Experiments with these mice use native tissues, or cells or mitochondria, that, despite the genetic manipulations and functional consequences, still sustain the life of the mouse. Perhaps, as expected, this approach is not free of controversy [92, 93]. Nevertheless, investigating the MCUcx channels in their native environments has several fundamental advantages that make the effort worthwhile. A key advantage is that one can interpret and discuss the results in the metabolic and physiological contexts of the investigated tissue. Additionally, one can draw from the existing rich literature and have new understanding guided by past metabolic and functional investigations. Such experiments also permit one to compare the results of MCUcx experiments with those done in animals or preparations using fully functional wild-type MCUcx channels.
A broad and consistent finding of all studies is that knocking out the "pore forming subunit" of the MCUcx (i.e. the MCUpore) leads to mitochondria that are incapable of rapidly taking up Ca2+. Somewhat similar results are observed when the highly-selective blocker of MCUcx RU360 is used in experiments with the wild-type MCUcx. Unexpectedly, conditional MCUpore knockout in the adult heart and complete embryonic MCUpore knockout mice produced on certain genetic backgrounds display no apparent phenotype when these animals are sedentary [69, 70, 82, 83, 90]. Thus, in all of these mouse models, mitochondrial ATP was produced at sufficiently high rates so that the basic energetic needs of the contracting heart were met. However, when fight-or-flight responses were demanded by acute β-adrenergic stimulation, some of the models performed poorly [82, 83, 90, 91]. Further examination revealed broad transcriptional adaptation that occurs in response to life without a functional cardiac MCUcx [90]. Importantly, such adaptations that accompany genetic deletion of MCUpore include changes of mitochondrial fuel selection, with shift towards mitochondrial fatty-acid metabolism in skeletal muscle [94], as well as augmentation of cardiac fatty-acid oxidation as compensatory source of high-energy to sustain high ATP production [95]. These findings further support the suggested role of MCUcx Ca2+ flux as the signal that stimulates pyruvate-fueled ATP production by mitochondria [3]. Thus, in the absence of functional MCUcx channels, as an adaptation, other carbon ‘food-stocks’ are consumed at higher rates.
How does mPTP activation depend on MCUcx?
A huge number of reports in the literature show that prolonged or repeated exposures of mitochondria to high levels of extramitochondrial [Ca2+] underlie the opening of mitochondrial permeability transition pores (mPTP's)[96-99] and the depolarization of mitochondria. This methodological approach to studying mPTP has linked MCUcx to mPTP activation. Activation of mPTP is generally thought to be a catastrophic event but recently it has also been viewed as a reversible physiological "venting" of excessive mitochondrial Ca2+ [100, 101]. This venting hypothesis has not been tested rigorously yet and so remains open. The most striking gap in the body of mPTP work, however, it the absence of a viable hypothesis of the molecular identity of the mPTP [99, 102, 103]. The current work on this topic has led to an embroiled high-profile debate that includes the suggestion that one of the most exquisite proteins in biology, the ATP synthase, Complex V, can be transformed to take on the role of mPTP [104-107]. The ATP synthase hypothesis has been strongly disputed by John Walker, well-known for his work on the crystal structure of the ATP synthase[108-111].
A recent provocative experimental result showed that less dramatic mPTP events could be generated by means other than elevated extramitochondrial [Ca2+] and could be gently graded[112]. Furthermore, these more modest mPTP events were shown to be reversible and consistent with the activation of a single channel-like entity. The experimental tool used in these experiments was photon stress [112, 113]. Photon stress is a method that was shown to generate reactive oxygen species (ROS) that activated mPTP. That mPTP was activated in single mitochondria was supported by the rapid and reversible depolarization of a single mitochondrion by mild photon stress. That the photon stress dependent mPTP depolarization was due to elevated ROS was supported by the effective block or slowing of mPTP development by pretreatment with antioxidants. Like the earlier hypothesized source of mPTP, all causes of the mPTP event have been hypothesized to be placed in the inner mitochondrial membrane (IMM). When mPTP's are activated, the increase in the IMM permeability, also called "permeability transition", causes a loss of osmotic balance of the mitochondrion, a degradation of ΔΨm, and diminished capacity to produce ATP by the ATP synthase. MCUcx plays a major role in the Ca2+ dependent activation of mPTP because it is the primary pathway by which Ca2+ enters the mitochondrial matrix. Another experimental characteristic of the mPTP is its huge conductance, often identified as a "mega-channel" [114, 115]. For example, when activated, substances as large as the fluorescent dye calcein (623 MD) can permeate the mPTP and were shown to enter or leave the mitochondria [116]. Recent work suggests that multiple genetically-distinct complexes can each function as mPTP's. If so, then knocking one complex out may still leave other complexes capable of functioning as mPTP's and making the unique identification exquisitely difficult. Included in the list of such candidates are macromolecular complexes which are otherwise known as selective carriers such as the adenine nucleotide translocase (ANT) and also ATP synthase. These carriers are proposed to mediate or be inherently transformed into non-selective pores that function as mPTP [104-107, 117]. The molecular details remain unclear and controversial both with respect to the mechanism of mPTP activation and the molecular identity of the mPTP. Thus, out of the mix of hypotheses, perhaps the simplest is the ROS hypothesis because it can account both for photon stress and Ca2+ overload. If mitochondrial Ca2+ overload works to activate mPTP by profoundly abolishing the reducing power within the matrix, then the effect of MCUcx Ca2+ influx is to activate mPTP by increasing matrix ROS. Unfortunately, we are still not sure what the activation mechanism(s) of mPTP is/are and what the identity of the mPTP protein(s) is/are.
OVERVIEW - MCUcx: A flexible regulator of mitochondrial metabolism
MCUcx: lynchpin of metabolic feed-back regulation.
The key to the MCUcx success as a metabolic regulator of mitochondria is customizing the details of the MCUcx components to the tissue itself. It is clear, for example, that the MCUcx Ca2+ flux, JMCU-cx, in heart is very different than it is in skeletal muscle MCUcx at [Ca2+]i above 3 μM. While this difference makes sense from the known biological properties of skeletal muscle, and is implied in Fig. 2, we are only now in a position to explore how the subunit composition of MCUcx in the two tissues enable the functional differences [13, 18, 26]. There are also likely important differences in regulatory kinases, utilization of different carbon sources of energy (e.g. carbohydrate, fat, protein), and circulatory contributions to metabolism.
Novel regulation of blood flow in heart: electro-metabolic regulation -- possible role of MCUcx.
The mitochondria in heart and the structure of the tissue in heart enable the mitochondria to play a pivotal role in controlling local blood flow as well as in making ATP. Clearly one of the vexing issues in heart is matching the supply of blood along with its oxygen and sources of energy to the work-load of the ventricular myocardium. This must occur continuously and flexibly and must match the needs of the animal and the tissue. Recently, Zhao et al., (PNAS 2020) [118] described how the cytosolic [ATP] and cytosolic [ADP] in heart can influence local blood flow. How the MCUcx Ca2+ channels contribute to this process has not been systematically studied. It seems reasonable, however, to speculate that the MCUcx channels may contribute importantly to this process. If so, when a cell like a ventricular myocyte is driven by action potentials and elevated [Ca2+]i signals to produce more force (that consumes more ATP), this same Ca2+ signal increases ATP production by the mitochondria as presented quantitatively by [3], and briefly reviewed here. If the blood flow should be inadequate, to maintain ATP production, then cytosolic [ATP] will start to decrease (while [ADP] is increasing). These changes in ATP and ADP concentrations activate the KATP channels in the sarcolemmal membrane of cardiac myocytes by increasing the open probability of the KATP channels in the myocytes. The larger number of open KATP channels produce a time-averaged hyperpolarization of the cardiac myocytes. This hyperpolarization would then increase the injection of hyperpolarizing current into the cardiac capillary endothelial cells through gap junctions. The resulting relative hyperpolarization of the endothelial cells hyperpolarizes (and relaxes) contractile capillary pericytes and vascular smooth muscle cells. By this means the capillary pericytes and the small arteriole vascular smooth muscle cells relax and increase the blood flow through the arterioles and capillaries supplying the nearby ATP-depleted ventricular myocytes. This electrical network thus works instantaneously upstream to provide increased blood flow to the needy ventricular myocytes. This vascular electrical network and blood flow control mechanism, recently characterized in heart [118], also enables the removal of metabolic waste and CO2 and contributes to the relaxation of the local venous system. By this chain of events, the MCUcx Ca2+ flux into the mitochondrial matrix not only regulates ATP production by the mitochondria but also regulates blood flow locally to the ventricular myocytes.
Highlights.
Ca2+ flux through the MCUcx (holocomplex) is measured in heart and in skeletal muscle.
There is no threshold for the dependence of MCUcx Ca2+ flux on [Ca2+]i.
We discuss how matrix [Ca2+]m regulates ATP production.
We discuss how MCUcx may influence mPTP and blood flow in heart.
We discuss the utility of using valinomycin "voltage clamp" in mitochondrial studies.
Acknowledgement and Funding
This work was supported by American Heart Association grants SDG 15SDG22100002 (to L.B.); By 1R01HL142290, U01 HL116321, 1 R01 HL140934, 1R01 AR071618 (to W.J.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- [1].Clapham DE, Calcium signaling, Cell 131(6) (2007) 1047–58. [DOI] [PubMed] [Google Scholar]
- [2].Cheng H, Lederer WJ, Calcium sparks, Physiol Rev 88(4) (2008) 1491–545. [DOI] [PubMed] [Google Scholar]
- [3].Wescott AP, Kao JPY, Lederer WJ, Boyman L, Voltage-energized calcium-sensitive ATP production by mitochondria, Nature Metabolism 1(10) (2019) 975–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fan M, Zhang J, Tsai CW, Orlando BJ, Rodriguez M, Xu Y, Liao M, Tsai MF, Feng L, Structure and mechanism of the mitochondrial Ca(2+) uniporter holocomplex, Nature 582(7810) (2020) 129–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mallilankaraman K, Cardenas C, Doonan PJ, Chandramoorthy HC, Irrinki KM, Golenar T, Csordas G, Madireddi P, Yang J, Muller M, Miller R, Kolesar JE, Molgo J, Kaufman B, Hajnoczky G, Foskett JK, Madesh M, MCUR1 is an essential component of mitochondrial Ca2+ uptake that regulates cellular metabolism, Nat Cell Biol 14(12) (2012) 1336–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fieni F, Lee SB, Jan YN, Kirichok Y, Activity of the mitochondrial calcium uniporter varies greatly between tissues, Nat Commun 3 (2012) 1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kirichok Y, Krapivinsky G, Clapham DE, The mitochondrial calcium uniporter is a highly selective ion channel, Nature 427(6972) (2004) 360–4. [DOI] [PubMed] [Google Scholar]
- [8].De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R, A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter, Nature 476(7360) (2011) 336–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Williams GS, Boyman L, Chikando AC, Khairallah RJ, Lederer WJ, Mitochondrial calcium uptake, Proc Natl Acad Sci U S A 110(26) (2013) 10479–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hille B, Ionic channels of excitable membranes, 2nd ed., Sinauer Associates, Sunderland, Mass, 1992. [Google Scholar]
- [11].Csordas G, Golenar T, Seifert EL, Kamer KJ, Sancak Y, Perocchi F, Moffat C, Weaver D, de la Fuente Perez S, Bogorad R, Koteliansky V, Adijanto J, Mootha VK, Hajnoczky G, MICU1 controls both the threshold and cooperative activation of the mitochondrial Ca(2)(+) uniporter, Cell Metab 17(6) (2013) 976–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Patron M, Checchetto V, Raffaello A, Teardo E, Vecellio Reane D, Mantoan M, Granatiero V, Szabo I, De Stefani D, Rizzuto R, MICU1 and MICU2 finely tune the mitochondrial Ca2+ uniporter by exerting opposite effects on MCU activity, Mol Cell 53(5) (2014) 726–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Vecellio Reane D, Vallese F, Checchetto V, Acquasaliente L, Butera G, De Filippis V, Szabo I, Zanotti G, Rizzuto R, Raffaello A, A MICU1 Splice Variant Confers High Sensitivity to the Mitochondrial Ca2+ Uptake Machinery of Skeletal Muscle, Mol Cell 64(4) (2016) 760–773. [DOI] [PubMed] [Google Scholar]
- [14].Paillard M, Csordas G, Huang KT, Varnai P, Joseph SK, Hajnoczky G, MICU1 Interacts with the D-Ring of the MCU Pore to Control Its Ca(2+) Flux and Sensitivity to Ru360, Mol Cell 72(4) (2018) 778–785 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Debattisti V, Horn A, Singh R, Seifert EL, Hogarth MW, Mazala DA, Huang KT, Horvath R, Jaiswal JK, Hajnoczky G, Dysregulation of Mitochondrial Ca(2+) Uptake and Sarcolemma Repair Underlie Muscle Weakness and Wasting in Patients and Mice Lacking MICU1, Cell Rep 29(5) (2019) 1274–1286 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mallilankaraman K, Doonan P, Cardenas C, Chandramoorthy HC, Muller M, Miller R, Hoffman NE, Gandhirajan RK, Molgo J, Birnbaum MJ, Rothberg BS, Mak DO, Foskett JK, Madesh M, MICU1 is an essential gatekeeper for MCU-mediated mitochondrial Ca(2+) uptake that regulates cell survival, Cell 151(3) (2012) 630–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tsai MF, Phillips CB, Ranaghan M, Tsai CW, Wu Y, Willliams C, Miller C, Dual functions of a small regulatory subunit in the mitochondrial calcium uniporter complex, Elife 5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lambert JP, Luongo TS, Tomar D, Jadiya P, Gao E, Zhang X, Lucchese AM, Kolmetzky DW, Shah NS, Elrod JW, MCUB Regulates the Molecular Composition of the Mitochondrial Calcium Uniporter Channel to Limit Mitochondrial Calcium Overload During Stress, Circulation 140(21) (2019) 1720–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Liu JC, Liu J, Holmstrom KM, Menazza S, Parks RJ, Fergusson MM, Yu ZX, Springer DA, Halsey C, Liu C, Murphy E, Finkel T, MICU1 Serves as a Molecular Gatekeeper to Prevent In Vivo Mitochondrial Calcium Overload, Cell Rep 16(6) (2016) 1561–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Raffaello A, De Stefani D, Sabbadin D, Teardo E, Merli G, Picard A, Checchetto V, Moro S, Szabo I, Rizzuto R, The mitochondrial calcium uniporter is a multimer that can include a dominant-negative pore-forming subunit, EMBO J 32(17) (2013) 2362–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cheng H, Lederer WJ, Cannell MB, Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle, Science 262(5134) (1993) 740–4. [DOI] [PubMed] [Google Scholar]
- [22].Nicholls DG, Crompton M, Mitochondrial calcium transport, FEBS Lett 111(2) (1980) 261–8. [DOI] [PubMed] [Google Scholar]
- [23].De Stefani D, Rizzuto R, Pozzan T, Enjoy the Trip: Calcium in Mitochondria Back and Forth, Annu Rev Biochem 85 (2016) 161–92. [DOI] [PubMed] [Google Scholar]
- [24].Matlib MA, Zhou Z, Knight S, Ahmed S, Choi KM, Krause-Bauer J, Phillips R, Altschuld R, Katsube Y, Sperelakis N, Bers DM, Oxygen-bridged dinuclear ruthenium amine complex specifically inhibits Ca2+ uptake into mitochondria in vitro and in situ in single cardiac myocytes, J Biol Chem 273(17) (1998) 10223–31. [DOI] [PubMed] [Google Scholar]
- [25].Lu X, Ginsburg KS, Kettlewell S, Bossuyt J, Smith GL, Bers DM, Measuring local gradients of intramitochondrial [Ca(2+)] in cardiac myocytes during sarcoplasmic reticulum Ca(2+) release, Circ Res 112(3) (2013) 424–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Paillard M, Csordas G, Szanda G, Golenar T, Debattisti V, Bartok A, Wang N, Moffat C, Seifert EL, Spat A, Hajnoczky G, Tissue-Specific Mitochondrial Decoding of Cytoplasmic Ca2+ Signals Is Controlled by the Stoichiometry of MICU1/2 and MCU, Cell Rep 18(10) (2017) 2291–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].McCormack JG, Halestrap AP, Denton RM, Role of calcium ions in regulation of mammalian intramitochondrial metabolism, Physiol Rev 70(2) (1990) 391–425. [DOI] [PubMed] [Google Scholar]
- [28].McCormack JG, Denton RM, Mitochondrial Ca2+ transport and the role of intramitochondrial Ca2+ in the regulation of energy metabolism, Dev Neurosci 15(3-5) (1993) 165–73. [DOI] [PubMed] [Google Scholar]
- [29].Glancy B, Balaban RS, Role of mitochondrial Ca2+ in the regulation of cellular energetics, Biochemistry 51(14) (2012) 2959–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Covian R, Balaban RS, Cardiac mitochondrial matrix and respiratory complex protein phosphorylation, Am J Physiol Heart Circ Physiol 303(8) (2012) H940–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Covian R, French S, Kusnetz H, Balaban RS, Stimulation of oxidative phosphorylation by calcium in cardiac mitochondria is not influenced by cAMP and PKA activity, Biochim Biophys Acta 1837(12) (2014) 1913–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Williams GS, Boyman L, Lederer WJ, Mitochondrial calcium and the regulation of metabolism in the heart, J Mol Cell Cardiol 78 (2015) 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Nguyen MH, Jafri MS, Mitochondrial calcium signaling and energy metabolism, Ann N Y Acad Sci 1047 (2005) 127–37. [DOI] [PubMed] [Google Scholar]
- [34].Glancy B, Willis WT, Chess DJ, Balaban RS, Effect of calcium on the oxidative phosphorylation cascade in skeletal muscle mitochondria, Biochemistry 52(16) (2013) 2793–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Moreno-Sanchez R, Regulation of oxidative phosphorylation in mitochondria by external free Ca2+ concentrations, J Biol Chem 260(7) (1985) 4028–34. [PubMed] [Google Scholar]
- [36].Murphy AN, Kelleher JK, Fiskum G, Submicromolar Ca2+ regulates phosphorylating respiration by normal rat liver and AS-30D hepatoma mitochondria by different mechanisms, J Biol Chem 265(18) (1990) 10527–34. [PubMed] [Google Scholar]
- [37].Moreno-Sanchez R, Hogue BA, Hansford RG, Influence of NAD-linked dehydrogenase activity on flux through oxidative phosphorylation, Biochem J 268(2) (1990) 421–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Territo PR, Mootha VK, French SA, Balaban RS, Ca(2+) activation of heart mitochondrial oxidative phosphorylation: role of the F(0)/F(1)-ATPase, Am J Physiol Cell Physiol 278(2) (2000) C423–35. [DOI] [PubMed] [Google Scholar]
- [39].Wang Z, Ying Z, Bosy-Westphal A, Zhang J, Schautz B, Later W, Heymsfield SB, Muller MJ, Specific metabolic rates of major organs and tissues across adulthood: evaluation by mechanistic model of resting energy expenditure, Am J Clin Nutr 92(6) (2010) 1369–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Boyman L, Karbowski M, Lederer WJ, Regulation of Mitochondrial ATP Production: Ca(2+) Signaling and Quality Control, Trends Mol Med (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].van Dyke RW, Gollan JL, Scharschmidt BF, Oxygen consumption by rat liver: effects of taurocholate and sulfobromophthalein transport, glucagon, and cation substitution, Am J Physiol 244(5) (1983) G523–31. [DOI] [PubMed] [Google Scholar]
- [42].Bracht L, Caparroz-Assef SM, Bracht A, Bersani-Amado CA, Effect of the Combination of Ezetimibe and Simvastatin on Gluconeogenesis and Oxygen Consumption in the Rat Liver, Basic Clin Pharmacol Toxicol 118(6) (2016) 415–20. [DOI] [PubMed] [Google Scholar]
- [43].Shadrin KV, Morgulis II, Pahomova VG, Rupenko AP, Khlebopros RG, Characteristics of oxygen transport through the surface of the isolated perfused rat liver, Dokl Biochem Biophys 464 (2015) 298–300. [DOI] [PubMed] [Google Scholar]
- [44].do Nascimento GS, Constantin RP, Gilglioni EH, de Castro Ghizoni CV, Bracht A, Utsunomiya KS, Yamamoto NS, Ishii-Iwamoto EL, Constantin J, Constantin RP, The acute effects of citrus flavanones on the metabolism of glycogen and monosaccharides in the isolated perfused rat liver, Toxicol Lett 291 (2018) 158–172. [DOI] [PubMed] [Google Scholar]
- [45].de Medeiros HC, Constantin J, Ishii-Iwamoto EL, Mingatto FE, Effect of fipronil on energy metabolism in the perfused rat liver, Toxicol Lett 236(1) (2015) 34–42. [DOI] [PubMed] [Google Scholar]
- [46].Colturato CP, Constantin RP, Maeda AS Jr., Constantin RP, Yamamoto NS, Bracht A, Ishii-Iwamoto EL, Constantin J, Metabolic effects of silibinin in the rat liver, Chem Biol Interact 195(2) (2012) 119–32. [DOI] [PubMed] [Google Scholar]
- [47].Cohen JJ, Merkens LS, Peterson OW, Relation of Na+ reabsorption to utilization of O2 and lactate in the perfused rat kidney, Am J Physiol 238(5) (1980) F415–27. [DOI] [PubMed] [Google Scholar]
- [48].Silva P, Hallac R, Spokes K, Epstein FH, Relationship among gluconeogenesis, QO2, and Na+ transport in the perfused rat kidney, Am J Physiol 242(5) (1982) F508–13. [DOI] [PubMed] [Google Scholar]
- [49].From AH, Petein MA, Michurski SP, Zimmer SD, Ugurbil K, 31P-NMR studies of respiratory regulation in the intact myocardium, FEBS Lett 206(2) (1986) 257–61. [DOI] [PubMed] [Google Scholar]
- [50].From AH, Zimmer SD, Michurski SP, Mohanakrishnan P, Ulstad VK, Thoma WJ, Ugurbil K, Regulation of the oxidative phosphorylation rate in the intact cell, Biochemistry 29(15) (1990) 3731–43. [DOI] [PubMed] [Google Scholar]
- [51].Katz LA, Swain JA, Portman MA, Balaban RS, Relation between phosphate metabolites and oxygen consumption of heart in vivo, Am J Physiol 256(1 Pt 2) (1989) H265–74. [DOI] [PubMed] [Google Scholar]
- [52].Portman MA, Heineman FW, Balaban RS, Developmental changes in the relation between phosphate metabolites and oxygen consumption in the sheep heart in vivo, J Clin Invest 83(2) (1989) 456–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Zhang J, Duncker DJ, Xu Y, Zhang Y, Path G, Merkle H, Hendrich K, From AH, Bache RJ, Ugurbil K, Transmural bioenergetic responses of normal myocardium to high workstates, Am J Physiol 268(5 Pt 2) (1995) H1891–905. [DOI] [PubMed] [Google Scholar]
- [54].Elliott AC, Smith GL, Allen DG, The metabolic consequences of an increase in the frequency of stimulation in isolated ferret hearts, J Physiol 474(1) (1994) 147–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Matthews PM, Bland JL, Gadian DG, Radda GK, The steady-state rate of ATP synthesis in the perfused rat heart measured by 31P NMR saturation transfer, Biochem Biophys Res Commun 103(3) (1981) 1052–9. [DOI] [PubMed] [Google Scholar]
- [56].Massie BM, Schwartz GG, Garcia J, Wisneski JA, Weiner MW, Owens T, Myocardial metabolism during increased work states in the porcine left ventricle in vivo, Circ Res 74(1) (1994) 64–73. [DOI] [PubMed] [Google Scholar]
- [57].Schwartz GG, Greyson CR, Wisneski JA, Garcia J, Steinman S, Relation among regional O2 consumption, high-energy phosphates, and substrate uptake in porcine right ventricle, Am J Physiol 266(2 Pt 2) (1994) H521–30. [DOI] [PubMed] [Google Scholar]
- [58].Xu Y, Lu L, Zhu P, Schwartz GG, beta-adrenergic stimulation induces transient imbalance between myocardial substrate uptake and metabolism in vivo, Am J Physiol 275(6) (1998) H2181–90. [DOI] [PubMed] [Google Scholar]
- [59].Hoppeler H, Hudlicka O, Uhlmann E, Relationship between mitochondria and oxygen consumption in isolated cat muscles, J Physiol 385 (1987) 661–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ferrara PJ, Verkerke ARP, Brault JJ, Funai K, Hypothermia Decreases O2 Cost for Ex Vivo Contraction in Mouse Skeletal Muscle, Med Sci Sports Exerc 50(10) (2018) 2015–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].McAllister RM, Ogilvie RW, Terjung RL, Impact of reduced cytochrome oxidase activity on peak oxygen consumption of muscle, J Appl Physiol (1985) 69(1) (1990) 384–9. [DOI] [PubMed] [Google Scholar]
- [62].McAllister RM, Terjung RL, Acute inhibition of respiratory capacity of muscle reduces peak oxygen consumption, Am J Physiol 259(6 Pt 1) (1990) C889–96. [DOI] [PubMed] [Google Scholar]
- [63].Boyman L, Williams GS, Lederer WJ, The growing importance of mitochondrial calcium in health and disease, Proc Natl Acad Sci U S A 112(36) (2015) 11150–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Vivek Garg IP, Unsulangi Tiffany, Suzuki Junji, Milescu Lorin S., and Kirichok Yuriy, The Mechanism of MICU-Dependent Gating of the Mitochondrial Ca2+ Uniporter, bioRxiv, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Vais H, Payne R, Paudel U, Li C, Foskett JK, Coupled transmembrane mechanisms control MCU-mediated mitochondrial Ca(2+) uptake, Proc Natl Acad Sci U S A 117(35) (2020) 21731–21739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Vecellio Reane D, Vallese F, Checchetto V, Acquasaliente L, Butera G, De Filippis V, Szabo I, Zanotti G, Rizzuto R, Raffaello A, A MICU1 Splice Variant Confers High Sensitivity to the Mitochondrial Ca(2+) Uptake Machinery of Skeletal Muscle, Mol Cell 64(4) (2016) 760–773. [DOI] [PubMed] [Google Scholar]
- [67].Antony AN, Paillard M, Moffat C, Juskeviciute E, Correnti J, Bolon B, Rubin E, Csordas G, Seifert EL, Hoek JB, Hajnoczky G, MICU1 regulation of mitochondrial Ca(2+) uptake dictates survival and tissue regeneration, Nat Commun 7 (2016) 10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Bick AG, Wakimoto H, Kamer KJ, Sancak Y, Goldberger O, Axelsson A, DeLaughter DM, Gorham JM, Mootha VK, Seidman JG, Seidman CE, Cardiovascular homeostasis dependence on MICU2, a regulatory subunit of the mitochondrial calcium uniporter, Proc Natl Acad Sci U S A 114(43) (2017) E9096–E9104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Holmstrom KM, Pan X, Liu JC, Menazza S, Liu J, Nguyen TT, Pan H, Parks RJ, Anderson S, Noguchi A, Springer D, Murphy E, Finkel T, Assessment of cardiac function in mice lacking the mitochondrial calcium uniporter, J Mol Cell Cardiol 85 (2015) 178–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Pan X, Liu J, Nguyen T, Liu C, Sun J, Teng Y, Fergusson MM, Rovira II, Allen M, Springer DA, Aponte AM, Gucek M, Balaban RS, Murphy E, Finkel T, The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter, Nat Cell Biol 15(12) (2013) 1464–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Hoffman NE, Chandramoorthy HC, Shamugapriya S, Zhang X, Rajan S, Mallilankaraman K, Gandhirajan RK, Vagnozzi RJ, Ferrer LM, Sreekrishnanilayam K, Natarajaseenivasan K, Vallem S, Force T, Choi ET, Cheung JY, Madesh M, MICU1 motifs define mitochondrial calcium uniporter binding and activity, Cell Rep 5(6) (2013) 1576–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Perocchi F, Gohil VM, Girgis HS, Bao XR, McCombs JE, Palmer AE, Mootha VK, MICU1 encodes a mitochondrial EF hand protein required for Ca(2+) uptake, Nature 467(7313) (2010) 291–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Nemani N, Shanmughapriya S, Madesh M, Molecular regulation of MCU: Implications in physiology and disease, Cell Calcium 74 (2018) 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Vais H, Mallilankaraman K, Mak DD, Hoff H, Payne R, Tanis JE, Foskett JK, EMRE Is a Matrix Ca(2+) Sensor that Governs Gatekeeping of the Mitochondrial Ca(2+) Uniporter, Cell Rep 14(3) (2016) 403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Boyman L, Lederer WJ, How the mitochondrial calcium uniporter complex (MCUcx) works, Proc Natl Acad Sci U S A 117(37) (2020) 22634–22636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Tufi R, Gleeson TP, von Stockum S, Hewitt VL, Lee JJ, Terriente-Felix A, Sanchez-Martinez A, Ziviani E, Whitworth AJ, Comprehensive Genetic Characterization of Mitochondrial Ca(2+) Uniporter Components Reveals Their Different Physiological Requirements In Vivo, Cell Rep 27(5) (2019) 1541–1550 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Bragadin M, Pozzan T, Azzone GF, Kinetics of Ca2+ carrier in rat liver mitochondria, Biochemistry 18(26) (1979) 5972–8. [DOI] [PubMed] [Google Scholar]
- [78].Scarpa A, Azzone GF, The mechanism of ion translocation in mitochondria. 4. Coupling of K+ efflux with Ca2+ uptake, Eur J Biochem 12(2) (1970) 328–35. [DOI] [PubMed] [Google Scholar]
- [79].Kamer KJ, Grabarek Z, Mootha VK, High-affinity cooperative Ca(2+) binding by MICU1-MICU2 serves as an on-off switch for the uniporter, EMBO Rep 18(8) (2017) 1397–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Phillips CB, Tsai CW, Tsai MF, The conserved aspartate ring of MCU mediates MICU1 binding and regulation in the mitochondrial calcium uniporter complex, Elife 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Reed KC, Bygrave FL, A kinetic study of mitochondrial calcium transport, Eur J Biochem 55(3) (1975) 497–504. [DOI] [PubMed] [Google Scholar]
- [82].Luongo TS, Lambert JP, Yuan A, Zhang X, Gross P, Song J, Shanmughapriya S, Gao E, Jain M, Houser SR, Koch WJ, Cheung JY, Madesh M, Elrod JW, The Mitochondrial Calcium Uniporter Matches Energetic Supply with Cardiac Workload during Stress and Modulates Permeability Transition, Cell Rep 12(1) (2015) 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Kwong JQ, Lu X, Correll RN, Schwanekamp JA, Vagnozzi RJ, Sargent MA, York AJ, Zhang J, Bers DM, Molkentin JD, The Mitochondrial Calcium Uniporter Selectively Matches Metabolic Output to Acute Contractile Stress in the Heart, Cell reports 12(1) (2015) 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Wei AC, Liu T, Cortassa S, Winslow RL, O'Rourke B, Mitochondrial Ca2+ influx and efflux rates in guinea pig cardiac mitochondria: low and high affinity effects of cyclosporine A, Biochim Biophys Acta 1813(7) (2011) 1373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Garg V, Kirichok YY, Patch-Clamp Analysis of the Mitochondrial Calcium Uniporter, Methods Mol Biol 1925 (2019) 75–86. [DOI] [PubMed] [Google Scholar]
- [86].Petronilli V, Szabo I, Zoratti M, The inner mitochondrial membrane contains ion-conducting channels similar to those found in bacteria, FEBS Lett 259(1) (1989) 137–43. [DOI] [PubMed] [Google Scholar]
- [87].Boyman L, Mikhasenko H, Hiller R, Khananshvili D, Kinetic and equilibrium properties of regulatory calcium sensors of NCX1 protein, J Biol Chem 284(10) (2009) 6185–93. [DOI] [PubMed] [Google Scholar]
- [88].Scaduto RC Jr., Grotyohann LW, Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives, Biophys J 76(1 Pt 1) (1999) 469–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Eberhard M, Erne P, Analysis of calcium binding to alpha-lactalbumin using a fluorescent calcium indicator, Eur J Biochem 202(3) (1991) 1333–8. [DOI] [PubMed] [Google Scholar]
- [90].Rasmussen TP, Wu Y, Joiner ML, Koval OM, Wilson NR, Luczak ED, Wang Q, Chen B, Gao Z, Zhu Z, Wagner BA, Soto J, McCormick ML, Kutschke W, Weiss RM, Yu L, Boudreau RL, Abel ED, Zhan F, Spitz DR, Buettner GR, Song LS, Zingman LV, Anderson ME, Inhibition of MCU forces extramitochondrial adaptations governing physiological and pathological stress responses in heart, Proc Natl Acad Sci U S A 112(29) (2015) 9129–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Wu Y, Rasmussen TP, Koval OM, Joiner ML, Hall DD, Chen B, Luczak ED, Wang Q, Rokita AG, Wehrens XH, Song LS, Anderson ME, The mitochondrial uniporter controls fight or flight heart rate increases, Nat Commun 6 (2015) 6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Garbincius JF, Luongo TS, Elrod JW, The debate continues - What is the role of MCU and mitochondrial calcium uptake in the heart?, J Mol Cell Cardiol (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Liu JC, Is MCU dispensable for normal heart function?, J Mol Cell Cardiol (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Kwong JQ, Huo J, Bround MJ, Boyer JG, Schwanekamp JA, Ghazal N, Maxwell JT, Jang YC, Khuchua Z, Shi K, Bers DM, Davis J, Molkentin JD, The mitochondrial calcium uniporter underlies metabolic fuel preference in skeletal muscle, JCI Insight 3(22) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Altamimi TR, Karwi QG, Uddin GM, Fukushima A, Kwong JQ, Molkentin JD, Lopaschuk GD, Cardiac-specific deficiency of the mitochondrial calcium uniporter augments fatty acid oxidation and functional reserve, J Mol Cell Cardiol 127 (2019) 223–231. [DOI] [PubMed] [Google Scholar]
- [96].Crompton M, Virji S, Doyle V, Johnson N, Ward JM, The mitochondrial permeability transition pore, Biochem Soc Symp 66 (1999) 167–79. [DOI] [PubMed] [Google Scholar]
- [97].Carraro M, Carrer A, Urbani A, Bernardi P, Molecular nature and regulation of the mitochondrial permeability transition pore(s), drug target(s) in cardioprotection, J Mol Cell Cardiol 144 (2020) 76–86. [DOI] [PubMed] [Google Scholar]
- [98].Briston T, Selwood DL, Szabadkai G, Duchen MR, Mitochondrial Permeability Transition: A Molecular Lesion with Multiple Drug Targets, Trends Pharmacol Sci 40(1) (2019) 50–70. [DOI] [PubMed] [Google Scholar]
- [99].Halestrap AP, Richardson AP, The mitochondrial permeability transition: a current perspective on its identity and role in ischaemia/reperfusion injury, J Mol Cell Cardiol 78 (2015) 129–41. [DOI] [PubMed] [Google Scholar]
- [100].Lu X, Kwong JQ, Molkentin JD, Bers DM, Individual Cardiac Mitochondria Undergo Rare Transient Permeability Transition Pore Openings, Circ Res 118(5) (2016) 834–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Kwong JQ, Molkentin JD, Physiological and pathological roles of the mitochondrial permeability transition pore in the heart, Cell Metab 21(2) (2015) 206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Baines CP, Gutierrez-Aguilar M, The still uncertain identity of the channel-forming unit(s) of the mitochondrial permeability transition pore, Cell Calcium 73 (2018) 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Karch J, Molkentin JD, Identifying the components of the elusive mitochondrial permeability transition pore, Proc Natl Acad Sci U S A 111(29) (2014) 10396–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Alavian KN, Beutner G, Lazrove E, Sacchetti S, Park HA, Licznerski P, Li H, Nabili P, Hockensmith K, Graham M, Porter GA Jr., Jonas EA, An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore, Proc Natl Acad Sci U S A 111(29) (2014) 10580–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Alavian KN, Dworetzky SI, Bonanni L, Zhang P, Sacchetti S, Li H, Signore AP, Smith PJ, Gribkoff VK, Jonas EA, The mitochondrial complex V-associated large-conductance inner membrane current is regulated by cyclosporine and dexpramipexole, Mol Pharmacol 87(1) (2015) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Mnatsakanyan N, Jonas EA, ATP synthase c-subunit ring as the channel of mitochondrial permeability transition: Regulator of metabolism in development and degeneration, J Mol Cell Cardiol 144 (2020) 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Giorgio V, von Stockum S, Antoniel M, Fabbro A, Fogolari F, Forte M, Glick GD, Petronilli V, Zoratti M, Szabo I, Lippe G, Bernardi P, Dimers of mitochondrial ATP synthase form the permeability transition pore, Proc Natl Acad Sci U S A 110(15) (2013) 5887–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].He J, Ford HC, Carroll J, Ding S, Fearnley IM, Walker JE, Persistence of the mitochondrial permeability transition in the absence of subunit c of human ATP synthase, Proc Natl Acad Sci U S A (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].He J, Carroll J, Ding S, Fearnley IM, Walker JE, Permeability transition in human mitochondria persists in the absence of peripheral stalk subunits of ATP synthase, Proc Natl Acad Sci U S A (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Carroll J, He J, Ding S, Fearnley IM, Walker JE, Persistence of the permeability transition pore in human mitochondria devoid of an assembled ATP synthase, Proc Natl Acad Sci U S A 116(26) (2019) 12816–12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Walker JE, Carroll J, He J, Reply to Bernardi: The mitochondrial permeability transition pore and the ATP synthase, Proc Natl Acad Sci U S A 117(6) (2020) 2745–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Boyman L, Coleman AK, Zhao G, Wescott AP, Joca HC, Greiser BM, Karbowski M, Ward CW, Lederer WJ, Dynamics of the mitochondrial permeability transition pore: Transient and permanent opening events, Arch Biochem Biophys 666 (2019) 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Boyman L, Chikando AC, Williams GS, Khairallah RJ, Kettlewell S, Ward CW, Smith GL, Kao JP, Lederer WJ, Calcium movement in cardiac mitochondria, Biophys J 107(6) (2014) 1289–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Szabo I, Zoratti M, The giant channel of the inner mitochondrial membrane is inhibited by cyclosporin A, J Biol Chem 266(6) (1991) 3376–9. [PubMed] [Google Scholar]
- [115].Szabo I, Zoratti M, The mitochondrial megachannel is the permeability transition pore, J Bioenerg Biomembr 24(1) (1992) 111–7. [DOI] [PubMed] [Google Scholar]
- [116].Petronilli V, Miotto G, Canton M, Brini M, Colonna R, Bernardi P, Di Lisa F, Transient and long-lasting openings of the mitochondrial permeability transition pore can be monitored directly in intact cells by changes in mitochondrial calcein fluorescence, Biophys J 76(2) (1999) 725–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Bround MJ, Bers DM, Molkentin JD, A 20/20 view of ANT function in mitochondrial biology and necrotic cell death, J Mol Cell Cardiol (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Zhao G, Joca HC, Nelson MT, Lederer WJ, ATP- and voltage-dependent electro-metabolic signaling regulates blood flow in heart, Proc Natl Acad Sci U S A 117(13) (2020) 7461–7470. [DOI] [PMC free article] [PubMed] [Google Scholar]