Abstract
Introduction:
Aphasia is a debilitating language disorder and even mild forms of aphasia can negatively affect functional outcomes, mood, quality of life, social participation, and the ability to return to work. Language deficits after post-stroke aphasia are heterogeneous.
Areas covered:
The first part of this manuscript reviews the traditional syndrome-based classification approach as well as recent advances in aphasia classification that incorporate automatic speech recognition for aphasia classification. The second part of this manuscript reviews the behavioral approaches to aphasia treatment and recent advances such as non-invasive brain stimulation techniques and pharmacotherapy options to augment the effectiveness of behavioral therapy.
Expert opinion:
Aphasia diagnosis has largely evolved beyond the traditional approach of classifying patients into specific syndromes and instead focuses on individualized patient profiles. In the future there is a great need for more large scale randomized, double-blind, placebo-controlled clinical trials of behavioral treatments, non-invasive brain stimulation and medications to boost aphasia recovery.
Keywords: Aphasia, stroke, speech therapy, noninvasive brain stimulation, rehabilitation, medication
1. Introduction
Approximately 1/3 of people who have a stroke will be diagnosed with aphasia (1,2), which is an acquired language disorder where patients experience impairments of various aspects of their language system (i.e., phonological, morphological, semantic, syntactic, and/or pragmatic). Aphasia is not a singular disorder, and can look very different from patient to patient. Even within the same patient, symptoms associated with aphasia can change quite drastically, particularly within the first few weeks and months following a stroke. The specific profile of language impairments depends on many factors including the size and location of stroke, health background (e.g., diabetes, history of prior stroke), access to quality medical care, how quickly medical treatment was received after stroke, and the time since stroke. Furthermore, even the mildest forms of aphasia can have detrimental effects on patient’s lives including loss of employment, social isolation, depression, and lower quality of life (3–6).
1.1. World Health Organization International Classification of Functioning, Disability, and Health (WHO ICF)
The WHO ICF is a framework for describing and organizing information on functioning and disability (7). The ICF stresses health and functioning rather than disability. The ICF distinguishes between impairments in body function or structure, activity limitations (difficulties an individual may have in executing tasks/actions), and participation restrictions (difficulties participating in life situations). It is important to consider the ICF when making diagnostic and treatment decisions for individuals with stroke-based aphasia (8–10).
2. Aphasia diagnostics
2.1. Classification of aphasia
Historically aphasiologists developed several different methods of classifying different subtypes of aphasia. The most popular system is the Boston classification system, which was developed in the 1960s by Norman Geschwind, Frank Benson, Harold Goodglass, and Edith Kaplan, who updated classical descriptions of aphasia subtypes. The Boston neoclassical classification system includes eight aphasia subtypes: 1) Broca’s, 2) Transcortical Motor, 3) Global, 4) Mixed Transcortical (aka Isolation aphasia), 5) Wernicke’s, 6) Transcortical Sensory, 7) Conduction, and 8) Anomic. Each of these subtypes is characterized by a specific profile of symptoms based on fluency of verbal expression (i.e., fluent vs. non-fluent speech), language comprehension skills, and repetition abilities (see Figure 1). It should be noted that most people with aphasia will have some level of difficulty with comprehension, spontaneous speech, reading, and writing. Typically, aphasia assessment is focused on identifying areas with the most profound impairment. These classical profiles are sometimes termed cortical aphasias, and are based on an understanding of classic left hemisphere cortical language regions such as Broca’s area and Wernicke’s area. Each subtype was theorized to be associated with damage to particular cortical regions, with some potential extension into subcortical regions, although the reliability of these predictions is debated in literature (11,12). For example, Kasselimis and colleagues (11) classified 65 patients using the Boston classification system and also obtained neuroimaging in each patient. Lesions were only located in the regions predicted by the Boston system in 36.5% of cases. On the other hand, Yourganov and colleagues (12) found a high correlation between aphasia syndrome and predicted lesion location.
Figure 1.
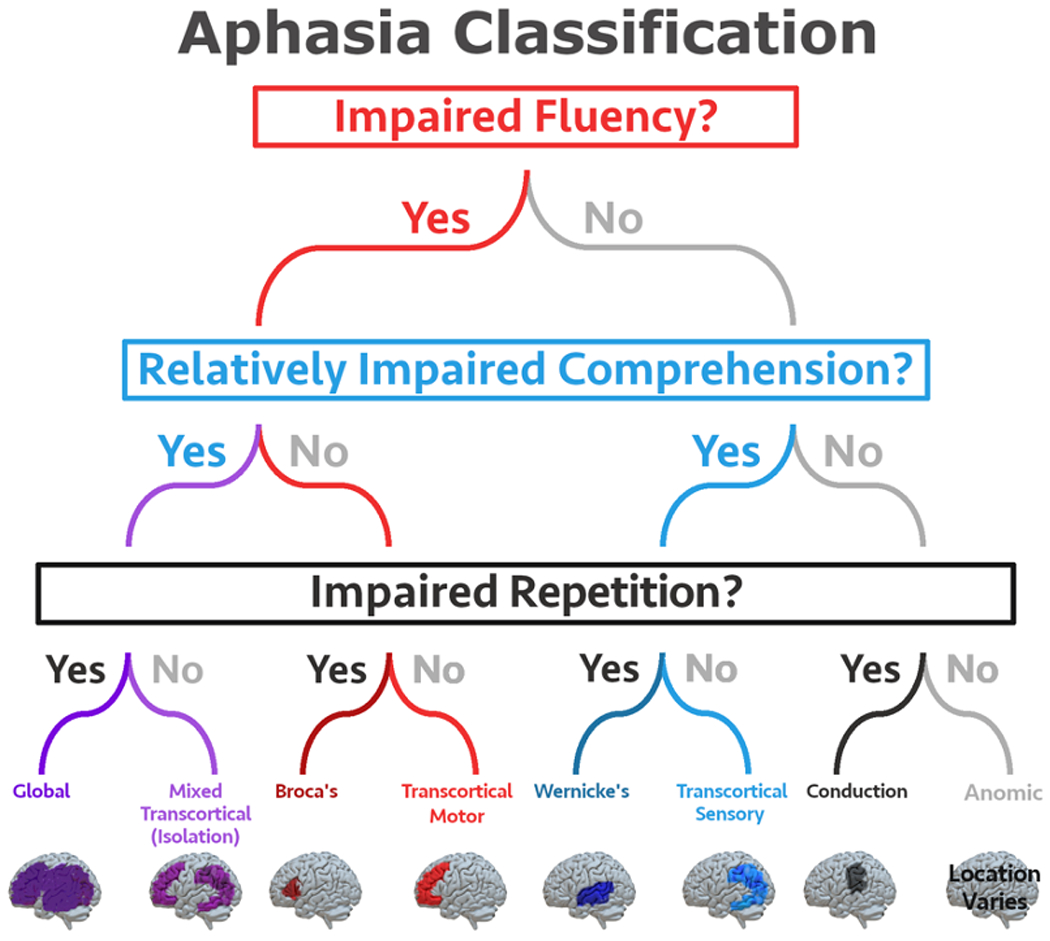
Aphasia Classification. The eight aphasia subtypes identified by the Boston neoclassical system are defined based on measures of fluency, comprehension, and repetition. Note the figure depicts lesions that are often associated with each subtype of aphasia, however there is inherent heterogeneity in stroke patients and a patient may present with a specific subtype of aphasia even if their lesion does not match the area depicted in this figure.
2.1.1. Boston aphasia syndromes
2.1.1.1. Non-fluent aphasias:
2.1.1.1.1. Broca’s aphasia
Broca’s aphasia is often termed “expressive aphasia” and is characterized by halting, effortful, non-fluent speech that has reduced phrase length, impaired melody, and diminished words per minute. Language output (both written and spoken) is agrammatic, meaning it consists mostly of content words with few, if any, function words. Repetition is typically impaired. Comprehension of single words and syntactically simpler sentences (e.g., active sentences) are often spared; comprehension of syntactically complex sentences (e.g., passive sentences) is often impaired. Individuals with Broca’s aphasia have a range of reading and writing skills. While Broca’s aphasia is associated with damage to Broca’s area, in chronic Broca’s aphasia the damage often extends into surrounding frontal lobe areas, insula, and sometimes subcortical structures (13,14). Because Broca’s area is located near the motor strip, it is also often accompanied by right hemiplegia.
2.1.1.1.2. Transcortical motor aphasia
Transcortical motor aphasia presents very similarly to Broca’s aphasia, except repetition of words and sentences is relatively preserved. More fluent speech is observed when a patient is repeating words, phrases, or sentences, compared to their spontaneous speech output. Patients have great difficulty initiating speech, and often present with echolalia (15). Transcortical motor aphasia is associated with lesions just anterior or superior to Broca’s area in the medial frontal cortex and the presupplementary motor area (15–17).
2.1.1.1.3. Global aphasia
Global aphasia is the most severe subtype of aphasia, as patients experience difficulties with all aspects of language. However, other modalities like facial expressions and gestures can be used to communicate basic needs or feelings (18). Comprehension is significantly impaired even at the single word level, and spoken output is severely limited. Spontaneous speech, naming, and repetition are often constrained to recurring utterances (e.g., “nuh, nuh, nuh”; parts of speech “I want to” etc.). Global aphasia is associated with large left hemisphere lesions affecting Broca’s area and Wernicke’s area (14,19).
2.1.1.1.4. Mixed transcortical aphasia (aka isolation aphasia)
Mixed transcortical aphasia is similar to global aphasia, except repetition skills are spared. Lesions are typically large and surround Broca’s and Wernicke’s area (watershed regions). Broca’s and Wernicke’s areas remain intact, but language recognition and production appear to be isolated from intentions generated elsewhere in the brain (20).
2.1.1.2. Fluent aphasias:
2.1.1.2.1. Wernicke’s aphasia
Wernicke’s aphasia is often called “receptive aphasia” and is characterized by fluent speech, paired with significant impairments of comprehension, naming, and repetition. Speech is fluent so the rhythm of speech is maintained, but it typically consists of jargon and is empty of meaning with a mix of sentence constructions (paragrammatism. Language output contains many paraphasias including semantic paraphasia (e.g., saying “train” for the target word “bus”) and neologisms (nonwords like “fluffertump”). Error awareness is often poor due to limited auditory comprehension, and this makes communication less effective compared to patients with Broca’s aphasia. Reading and writing are frequently significantly impaired. Wernicke’s aphasia is typically associated with damage to Wernicke’s area along with neighboring temporal and parietal regions (21).
2.1.1.2.2. Transcortical sensory aphasia
Transcortical sensory aphasia is similar to Wernicke’s aphasia, except repetition skills are intact. This type of aphasia is associated with lesions surrounding Wernicke’s area, between the areas of the brain fed by the middle cerebral artery (MCA) and the posterior cerebral artery (PCA) (18,22).
2.1.1.2.3. Conduction aphasia
Patients with conduction aphasia have fluent speech with phonemic distortions, relatively good comprehension, and mild to moderate naming deficits. Repetition skills are disproportionally impaired relative to comprehension and expression. Conduction aphasia was classically associated with damage to left arcuate fasciculus, which is a white matter tract connecting Wernicke’s and Broca’s areas (18). However, more recent research implicates temporoparietal regions (18,23).
2.1.1.2.4. Anomic aphasia
Anomic aphasia is the least severe aphasia syndrome, and is characterized by marked difficulty with naming but no other profound comprehensive and expressive deficits. Speech is fluent with the exception of intermittent pauses and hesitations resulting from word finding difficulties. Lesions can be located anywhere in the left hemisphere language network, including subcortical regions (14,16).
2.1.2. Other types of aphasia
2.1.2.1. Subcortical aphasia
More recently subcortical aphasias have also been identified, where damage is confined to subcortical areas alone. In the review of eight neoclassic syndromes it was noted that subcortical damage may accompany cortical damage. However, in subcortical aphasias, only subcortical damage is present. Subcortical aphasias are often divided into two groups: thalamic aphasia, and striato-capsular aphasia. Thalamic aphasia is characterized by severe anomia, presence of verbal paraphasias, reduced spontaneous verbal output, with variable comprehension skills (24–26). Variability in comprehension findings across studies of patients with thalamic aphasia may be due to the involvement of specific thalamic nuclei or subnuclei (25). Striato-capsular aphasia is associated with lesions in basal ganglia (head of caudate nucleus, putamen, anterior limb of the internal capsule). Researchers have attempted to define clinical syndromes associated with specific areas of basal ganglia damage (27,28), but no strong clinical consensus has been reached (16,29). Mounting evidence (29–31) suggests that basal ganglia lesions are associated with hypoperfusion in cortical areas, which in turn explains symptoms of aphasia.
2.1.2.2. Crossed aphasia
Crossed aphasia is the term used to describe aphasia that results from damage to the hemisphere non-dominant for language (in most individuals this is the right hemisphere). Crossed aphasia is rare, but appears to result from a small minority of people who have reversed asymmetry of language functions in the right hemisphere even when they are right-handed. Crossed aphasia can be a mirror image of the left hemisphere profile so each of the neoclassical syndromes discussed above could potentially occur (32,33). However, in about 40% of cases anomalous profiles also occur where the extent and site of lesion doesn’t map well to the associated symptoms (33).
2.1.2.3. Alexia and agraphia
In addition to broad language comprehension and production deficits, stroke can also cause reading and writing deficits. Alexia refers to reading deficits and agraphia refers to writing deficits. In cases of pure alexia, patients demonstrate reading impairments in the absence of any other deficits (34,35). Pure alexia is associated with simultaneous damage to 1) left occipital cortex, which causes right homonymous hemianopsia where visual information is initially processed in the right occipital cortex, and 2) splenium of the corpus callosum, which then prevents visual information in the right hemisphere from crossing over to the left hemisphere, where language is processed (36). Pure agraphia refers to cases where writing impairments are present in the absence of other difficulties (35). Spelling deficits are associated with damage to left inferior parietal cortex and left occipitotemporal cortex (37).
2.1.3. Classification considerations
2.1.3.1. Recent advances in aphasia classification
There is no perfect aphasia classification system. It is relatively common for a patient’s language impairment profile to be unclassifiable because they do not fit neatly within any of the well-defined neoclassical aphasia syndromes. Even when patients do fit within a specific profile, they may differ quite significantly from other patients who have the same syndrome classification. For example, one patient with Broca’s aphasia may also have mild-moderate reading comprehension deficits, while another does not. Additionally, a patient’s classification may change depending on the specific test battery that was used. Because of these concerns, researchers have continued to develop new approaches to aphasia classification. For example, several researchers have investigated the utility of machine learning approaches to reduce uncertainties in aphasia classification (38–40). Alternatively, some researchers advocate for moving away from a syndrome-based approach and instead focusing on a more individualized approach which aims to identify the precise points of impairment in language processing, such as semantic, phonological, or syntactic disorders (11,41,42).
2.1.3.2. Classification - time since stroke:
Another aspect of aphasia classification to consider is the time since stroke. Patients undergo a period of spontaneous recovery immediately following a stroke, where they may experience drastic improvements in language and cognitive functioning. Therefore, a patient may look very different if tested at the acute stage (within ~ 1 week of stroke) compared to the chronic stage (often defined as more than six months or one-year post-stroke). Unsurprisingly, there is significant variability across patients in terms of aphasia recovery in the months following a stroke (43). Because of the natural recovery and functional reorganization, a patient’s aphasia classification is likely to evolve rapidly over the first few days, weeks, and months following a stroke. For example, patients diagnosed with acute Broca’s aphasia may recover and be later diagnosed as having chronic anomic aphasia (44,45). Acute aphasia may resolve completely by the chronic stage of recovery (44). Typically, language impairments will be most severe at the acute stage, with the greatest period of recovery occurring within the first three months (46). However, some patients do experience decline, which can be attributed to several possible factors including the onset of vascular dementia or a lack of speech and language therapy (47,48).
2.1.4. Clinical terminology for classification
Depending on the clinical setting, speech language pathologists are often not expected to classify syndromes according to the Boston classification system. Some other common classifications include distinguishing between nonfluent and fluent aphasia. Patients may also be described as having receptive aphasia vs. expressive aphasia. Receptive aphasia refers to difficulty with language (auditory or written) comprehension, while expressive aphasia refers to difficulty with language production. Sometimes speech language pathologists will describe the relative severity of receptive and/or expressive deficits as either mild, moderate, or severe. For example, a patient may be described as having aphasia with mild receptive deficits and moderate-severe expressive deficits. However, this isn’t best practice as classifying receptive vs. expressive deficits does not provide any information about the type of receptive or expressive deficits. For example, we would expect all patients with aphasia to have expressive language deficits on some level (e.g., word finding difficulty, non-fluent speech, etc.). Thus, stating a patient has mild expressive deficits does not provide information about whether the deficits are due to word finding difficulties, or non-fluent speech or another type of deficit.
2.2. Diagnostic testing
Conducting a comprehensive assessment is vital to forming meaningful and feasible treatment goals and activities. Moreover, in light of the WHO ICF, aphasia assessment must surpass simply identifying deficits and instead aim to gain a full understanding of how deficits have restricted the patient’s daily life and social activities. It is important to first obtain an accurate case history including background information such as occupation, language and cultural background, and medical history. A comprehensive aphasia assessment includes each component of language (e.g., syntax, semantics), in every modality (comprehending and expressing spoken language, written language, and gestures). Fluency and quality of spontaneous speech should be assessed using tasks like picture description, and asking open ended questions. Naming can be assessed using confrontation naming tasks. Auditory comprehension should be assessed at several levels including single words (nouns and verbs), sentences (syntactically simple and complex), and multi-step commands. It is also important to investigate the reliability of yes/no responses to ascertain if the patient has more reliable yes/no responses with gestures vs. speech. Repetition of words, phrases, and sentences should also be assessed. It is critical to consider repetition skills relative to other language skills.
The Boston Diagnostic Aphasia Examination, 3rd edition (BDAE) (49), and the Western Aphasia Battery – Revised (WAB-R) (50) are the most common comprehensive aphasia assessments. The BDAE assesses spontaneous speech (conversational and narrative), auditory comprehension, repetition, reading, and writing. The BDAE has a short form that takes about 30-45 minutes to complete, and an extended standard form. The BDAE also includes the Boston Naming Test (BNT), which is a widely used measure of confrontation object naming. Similarly, the WAB-R assesses spoken language production and comprehension, reading and writing, praxis, and constructional and visuospatial skills. Both the BDAE and the WAB-R allow clinicians to classify patients into syndromes. However, as discussed in the “Recent Advances in Aphasia Classification” section above, a growing number of researchers and clinicians are moving away from the syndrome-based approach. Instead there is an emphasis on identifying specific deficits rather than trying to fit individual patients into a “syndrome” box.
In line with this new way of thinking, the Comprehensive Aphasia Test (CAT) (51) is a comprehensive assessment that does not assign patients to syndrome classifications. The CAT consists of an initial screening for cognitive deficits that may impact performance on the aphasia battery, a comprehensive language performance assessment (the main body of the test), and a disability questionnaire. The disability questionnaire asks the individual with aphasia to assess their degree of disability in all four language modalities, and is designed to ascertain the emotional consequences and impact that language difficulties have on their daily life.
It is important to follow up the comprehensive battery with tests designed to probe further into specific linguistic and/or cognitive deficits. For example, the Psycholinguistic Assessment of Language Processing in Aphasia (PALPA (52)), has 60 assessments that a clinician can select from depending on the specific area they want to probe in further detail. The Northwestern Assessment of Verbs and Sentences (NAVS) (53) can be used to examine the comprehension and production of action verbs and several types of canonical and noncanonical sentences, as well as the production of verb argument structure in sentence contexts. If clinicians suspect patients have syntactic deficits, or difficulties with verbs, the NAVS can offer a thorough assessment of different verb- and sentence-types. It is also important to diagnose nonlinguistic cognitive deficits (e.g., difficulties with memory, attention, executive functioning), as the presence and severity of domain-general cognitive deficits will impact treatment decisions.
Another important area to probe is functional communication. The ASHA Functional Assessment of Communication Skills for Adults (ASHA-FACS) (54) can be used to evaluate functional communication in four areas: 1) social communication, 2) communication of basic needs, 3) reading, writing and number concepts, 4) and daily planning. Some other tests of functional communication include The Amsterdam-Nijmegen Everyday Language Test (ANELT) (55), the Scenario Test (56) and the Communicative Effective Index (CETI) (57).
Activity limitations, poor functional communication, and changes to social relationships can all negatively impact quality of life in individuals with aphasia (58–60). It is important to assess quality of life in aphasia because there is a high prevalence of poor quality of life in this group (61). The Stroke and Aphasia Quality of Life Scale (SAQOL) (62) is an interview-based self-report scale that can be used to assess quality of life in aphasia.
3. Aphasia treatment
3.1. Behavioral approaches to treatment
Aphasia therapy varies across several dimensions and depends upon many factors including patient goals, specific impairments, treatment setting, and delivery model. Clinicians can take a restorative approach, where the goal is to improve deficits, or a compensatory approach, where the goal is to compensate for deficits that cannot be restored. With regards to the WHO ICF (7), restorative approaches focus on body functions/structures while compensatory approaches focus on activities/participation. Another distinction can be made between impairment-based approaches, which focus on training specific linguistic deficits, and functional approaches, which emphasize real-life treatment goals that will have value outside of the therapy room. The impairment-based approach follows a medical model, whereas a functional approach follows a patient-centered social model. Clinicians may choose to use a combined impairment-based and functional approach, which is likely to have the most positive outcome for individuals with aphasia (63).
3.1.1. Review of treatment options
Many behavioral approaches to aphasia treatment exist, and here we provide a brief overview of a portion of the numerous available options. First, community support approaches aim to help individuals with aphasia successfully participate in their community. For example, the Life Participation Approach to Aphasia (LPAA) is a general philosophy, rather than a service delivery model, that focuses on re-engagement in life. LPAA aims to empower individuals with aphasia to participate in their recovery and fully engage in daily activities of their choice. A strong support system is vital to this approach (64). Community aphasia groups can have a support model where the main goal is to provide social support to participants, or a therapy model, where the primary goal is to provide speech language therapy services. It is vital that clinicians keep the needs of each individual participant in mind. People with severe aphasia will likely require increased structure to help them get the most out of the group (65), while people with mild aphasia are more at risk for feeling like an outsider (66). Community aphasia groups offer an excellent opportunity for combating the social isolation that has devastating consequences on people with aphasia (65,67,68).
For individual therapy sessions, clinicians can choose from many different approaches depending on the goals and characteristics of their individual clients. One unique approach is Melodic Intonation Therapy (MIT). Because right hemisphere functions like singing and knowledge of melody and rhythm are often relative strengths in individuals with non-fluent aphasia, MIT capitalizes on these strengths to improve language expression. Ideal candidates for MIT have: nonfluent speech with the ability to produce some intelligible words while singing familiar songs, relatively good comprehension, impaired repetition, a good attention span and strong motivation (69). MIT consists of several levels from singing simple phrases to speaking phrases with five or more syllables (69–71). Another popular approach for expressive language deficits is Constraint-Induced Language Therapy (CILT). CILT encourages individuals with aphasia to use spoken language, and discourages the use of compensatory strategies such as writing and using gestures in place of spoken language (72). A hallmark of CILT is its intensive approach requiring massed practice (e.g., 3-hour therapy sessions at least five days a week for two weeks).
Several therapy options are available for patients with word finding deficits. Semantic feature analysis (SFA) trains patients to produce semantic information when they are struggling to produce a specific word. It is theorized to improve word retrieval by increasing semantic network activation (73,74). If a client cannot produce a target word they would be prompted to answer questions about what it is used for, what it looks like, where it is found etc. Phonological Components Analysis (PCA) treatment was modeled after the SFA approach, and asks participants to identify five phonological components related to the target word as a method for treating word finding deficits (75). Another word retrieval treatment is Verb Network Strengthening Treatment (VNeST), which focuses on promoting word retrieval at the phrase- and sentence-level. Similar to SFA, VNeST is also designed to promote semantic network activation. VNeST aims to strengthen associations between verbs and related agents and patients (76–78). The protocol involves giving the client an appropriate transitive verb (e.g., “cook”), and instructing them to produce related agents and patients (e.g., “chef cooks dinner”). Research demonstrates that VNeST leads to improved single word retrieval, as well as word retrieval in sentences (76–78).
Treatment of Underlying Forms (TUF) is a treatment approach that also focuses on sentence-level tasks. TUF is used to treat the comprehension and expression of sentences, and is designed for patients with mild – moderately severe agrammatic Broca’s aphasia (79). Patients who will most likely benefit from TUF have better word-level than sentence-level comprehension, more difficulty comprehending semantically reversible sentences than non-reversible sentences, and more difficulty comprehending non-canonical than canonical sentences. They also have more difficulty producing verbs than nouns, and more difficulty producing syntactically complex vs. simple sentences. Research indicates TUF promotes improvement on trained syntactic structures as well as generalization to untrained structures (79–81).
3.1.2. Treatment settings
Aphasia treatment can occur in many different settings, and will often look quite different from one setting to the next. An aphasia assessment typically occurs during the initial hospital stay in the days following a stroke. Patients are subsequently discharged to an acute rehabilitation unit, a nursing home or skilled nursing facility, or back to their home. Treatment may begin during the acute stay in the hospital, but it is common for patients to be transferred to a lower level of care relatively quickly, and therefore treatment may not begin until after they have been transferred. Aphasia treatment centers offer another unique treatment option. Aphasia centers are dedicated to providing resources and therapy specifically tailored for individuals with aphasia, and offer activities designed to increase participation in line with the WHO ICF model (82,83).
Recently, given the global COVID-19 pandemic, an emphasis has been placed on providing clients with therapy via telepractice. Telepractice has the added benefit of allowing speech language pathologists to reach patients who are isolated either by geography, or by physical limitations. Research shows that language therapy provided via telepractice can benefit patients (84) and can also be successfully used to provide communication partner training (85). In addition to telepractice techniques, research has shown promising results for computer-based and tablet-based therapy (86–88). Various computer programs have been developed for aphasia rehabilitation. These include communication aids such as Sentence-Shaper (Psycholinguistic Technologies, Jenkintown, PA) (89), Lingraphica (Lingraphica Inc., Princeton, NJ), and Touchspeak (Touchspeak, London, England). In addition, there are several self-delivered tablet or desktop based aphasia therapy. These include Sentactics (90), ORLA-VT (Oral Reading for Language in Aphasia (91), and Constant Therapy (87). Self-delivered language therapy can also be used to increase treatment intensity, as patients can participate in therapy tasks at home between formal language therapy sessions. Advances in technology also means that therapy can be conducted within a virtual environment. For example, EVA Park is a multi-user virtual world that will likely be available in the near future where individuals with aphasia can interact with their speech pathologist, and other individuals with aphasia (92–94). They create their own avatar and can explore EVA Park while practicing their language and communication. The COVID-19 pandemic has resulted in a push for creating more diagnostic and therapy materials, which will be beneficial to clinicians and patients even after the pandemic has resolved.
Research indicates that the benefits of neuroplasticity can extend well beyond the first year following a stroke, and therefore therapy-induced recovery can be seen even in individuals who have had chronic aphasia for several years (95–97). Therefore, even in cases where insurance has limited the total number of formal language therapy sessions, the growing virtual options may allow individuals with aphasia to access the resources they sorely need.
3.2. Pharmacological treatment
Pharmacotherapy has been used in the treatment of post-stroke aphasia for several decades now; however, there has been a recent emphasis on augmenting language skills in aphasia with pharmacological agents already approved for the treatment of other neurological and psychiatric disorders (98,99). Pharmacological interventions for aphasia are mainly designed to strengthen networks subserving language and language-related cognitive functions such as attention and memory (100). The theoretical rationale for pharmacological intervention in aphasia is based on the notion that re-establishing the activity of specific neurotransmitters that are dysfunctional, but not irretrievably damaged, brain regions may strengthen neural activity in networks mediating attention, word learning, and memory (101,102). Pharmacological agents are increasingly used alone and in combination with speech and language therapy to boost language recovery. Please see (98,99) for reviews on this topic.
Catecholaminergic, Cholinergic, Nootropic and Serotonergic drugs are the main classes of drugs investigated for the treatment of aphasia. Bromocriptine, a Catecholaminergic drug has been the most studied drug for patients with aphasia. Positive effects of Bromocriptine have been seen mainly in non-fluent chronic aphasias (103–106), but these studies were mostly case studies or open label trials. In addition, language gains were only specific to certain language subtests, but were not effective in moderate to severe cases. Randomized, double-blind controlled trials failed to replicate positive results of Bromocriptine (104,107,108). It should be noted that the use of bromocriptine is no longer recommended due to high frequency of contraindications (> 40 %) (109) and the increased risk of inducing off-target effects such as valvular heart disease (110) and painful hemidystonia in aphasic persons with hemiparesis (111).
Donepezil is the mostly commonly investigated cholinergic drug in the treatment of aphasia. Cholinergic agents specifically acetylcholinesterase inhibitors such as Donepezil, are widely used in the treatment of Alzheimer’s disease. Donepezil has been reported to result in improvement of spontaneous speech, naming and comprehension in chronic post-stroke aphasia in randomized trials, open label trials, and case studies (112–115). It also has a well-tolerated safety profile. Piracetam, the nootropic agent is another drug that is commonly used in the treatment of aphasia (101,116,117). However, studies assessing the efficacy of piracetam on post-stroke aphasia have produced inconsistent results (99). In addition, piracetam acts on the initial phase of stroke (118,119), but its beneficial effects disappear in the chronic stage (116). Memantine is an uncompetitive N-methyl-D-aspartate (NMDA) receptor antagonist. Like Piracetam, the initial enthusiasm to evaluate the potential efficacy of memantine in post-stroke aphasia was prompted by the beneficial effects obtained with this drug in language and communication among patients with Alzheimer’s disease (100). Two studies showed that memantine alone and combined with constraint-induced aphasia therapy (CIAT) improved aphasia severity (120,121).
Interest in serotonin selective reuptake inhibitors (SSRIs) after stroke has been renewed by a better knowledge of post-stroke depression. The issue of depression is important to consider in the treatment of post-stroke aphasia. There is a high rate of post-stroke depression ranging from 30-60% of stroke survivors (122,123). Furthermore, untreated depression can impede not only language performance, but also motivation to participate in SLT (124,125). The SSRI fluvoxamine has showed significant improvements in naming, reduced perseverations, and mood after a 4-week treatment compared to controls (126). A recent study showed that patients with damage to left posterior superior temporal gyrus and/or superior longitudinal fasciculus/arcuate fasciculus showed better naming outcome if they took SSRIs for 3 months after stroke [47].
It is important to keep in mind that drugs prescribed for the treatment of other diseases or stroke complications (e.g. epilepsy) can interfere with aphasia recovery [96, 97]. For example, although seizures are common following stroke, anticonvulsant drugs such as the use of topiramate and zonisamide should not be recommended as first-choice medications because they can impair attention, psychomotor speed, short-term and working memories, as well as expressive language (127,128).
3.3. Non-invasive brain stimulation
There has been an increasing interest in the use of non-invasive brain stimulation techniques (NIBS) such as transcranial direct current stimulation (tDCS) and transcranial magnetic stimulation (TMS) to enhance recovery of aphasia. Please see (129), (130) for detailed reviews. This interest stems from the growing body of evidence indicating that non-invasive brain stimulation techniques can induce long-lasting changes in neural excitability resulting in functional reorganization and improved speech and language performance.
3.3.1. Transcranial direct current stimulation (tDCS)
tDCS is usually administered via saline-soaked surface sponge electrodes attached to the scalp and connected to a direct current stimulator with low intensities (1-2 mA). tDCS can increase or decrease cortical excitability due to a shift of the resting membrane potential of the nerve cells in the brain (131). Anodal stimulation typically increases cortical excitability, whereas cathodal stimulation lowers cortical excitability. The majority of the tDCS studies in aphasia have focused on excitatory, anodal stimulation applied to the left hemisphere perilesional or ipsilesional regions (132–140). Other tDCS studies have focused on inhibitory, cathodal stimulation to the healthy right hemisphere regions to inhibit cross-hemisphere inhibition, allowing greater activation of the lesioned left hemisphere (141–143). A few studies have focused on bihemispheric tDCS, aiming at concomitantly increasing left hemisphere excitability with anodal stimulation and decreasing right hemisphere excitability with cathodal stimulation (144–146). The newest approach to neuromodulation for aphasia is targeting the right cerebellum (147,148), a region that is structurally and functionally connected to the left hemisphere language region. This is particularly well suited for patients who have large left hemisphere stroke where it may be difficult to find viable tissue to stimulate in the left hemisphere.
The results of tDCS studies in aphasia have been mostly positive indicating that tDCS can augment aphasia therapy; however, the majority of the studies are small. So far, only two tDCS studies have included more than 50 patients (135,149). The largest clinical trial of tDCS to augment naming treatment for post stroke aphasia was done by Fridriksson and colleagues (135) using a randomized, double-blind, sham-controlled design. In this study of 74 chronic stroke patients with aphasia, tDCS electrode placement was individualized for each participant based on the area of greatest activation in the left hemisphere during spoken naming pre-treatment functional magnetic resonance imaging (fMRI). Patients received 15 sessions of tDCS combined with computerized aphasia treatment. tDCS was associated with significantly greater change in number of correctly naming pictured objects compared to sham. Spielmann and colleagues (149) studied the effect of tDCS on naming outcome in 58 patients with subacute (first three months) post stroke aphasia. Using a randomized, double-blind, sham-controlled design, participants received anodal stimulation to the left inferior frontal gyrus combined with naming treatment for 10 days. The study by Spielmann and colleagues failed to show significant effects of tDCS. The authors hypothesized that an effect of tDCS might be difficult to achieve in the subacute phase, as spontaneous recovery is rather high in this phase compared with the relatively stable chronic phase.
3.3.2. Transcranial Magnetic Stimulation (TMS)
TMS is a non-invasive brain stimulation method that induces changes in neuronal firing via electromagnetic induction. Typically, a brief and strong current is delivered through a stimulation coil over the scalp, which induces a perpendicular time-varying magnetic field that penetrates the scalp without attenuation. This magnetic field will induce a weak and short-lived current at the site of stimulation (150). Repetitive TMS (rTMS) can be delivered at both high frequency (excitatory) and low frequency (inhibitory).
The majority of TMS studies have applied low-frequency rTMS to right hemisphere regions to inhibit right hemisphere activation during language-related tasks and to encourage perilesional left hemisphere activation (151–156). Other studies have focused on facilitating activation of residual left hemisphere regions (157) or facilitating activation of right hemisphere regions (155) via high frequency rTMS. Theta burst stimulation (TBS) is another type of rTMS protocol that has recently gained interest. TBS protocols induce robust, long-lasting changes in activation in much shorter time periods than are necessary for traditional rTMS (158,159).
Both low-frequency and high-frequency rTMS have been beneficial in improving language outcomes for subacute and chronic aphasia. However, the majority of studies are small. There are only a few relatively large randomized sham-controlled trials (154,160). Hu et al. showed that that both high and low frequency rTMS of the right IFG can be effective in chronic post stroke aphasia. Ren et al., results indicate that inhibitory low frequency rTMS of right IFG and right STG both lead to language improvements in subacute aphasia, demonstrating that targets beyond right IFG can be effective.
Both tDCS and TMS are promising tools for the treatment of aphasia. However, it is unclear which approach is beneficial for improving language skills. There is an increased interest in using tDCS compared to TMS because tDCS is less expensive and portable; and does not carry a risk of inducing seizures (161,162). In addition, tDCS is more easily paired with simultaneous speech therapy, making it more amenable to widespread clinical use.
3.3. Reperfusion therapies
Over the last two decades, various therapeutic approaches have been developed for treating acute ischemic stroke. Reperfusion therapies are medical treatments in acute stroke that restore blood flow either by surgical removal of a blood clot or with medications that dissolve clots. Reperfusion therapies, particularly intravenous thrombolysis with recombinant tissue plasminogen activator (rTPA), have been shown to be effective in improving language after acute ischemic stroke in the left hemisphere (163–166). For example, a recent study in patients with ischemic stroke showed significantly higher percentage of resolved aphasia in patients treated with rTPA compared to the non-treated group, and a higher percentage of global aphasia was observed in the non-treated group compared to treated patients (167). However, many stroke patients do not receive this treatment due to the narrow treatment window (<4.5 h) (168).
Carotid stenting, with or without angioplasty, is a common procedure to restore blood flow, prevent stroke, and/or improve tissue function. For example, Hillis and colleagues have shown that acute aphasia resolved after left carotid stenting associated with reperfusion of the language cortex (169). Increasing blood pressure is another approach that investigators have used to improve stroke symptoms. In a series of studies, Hillis et al. showed that restoring blood flow to specific cortical regions in the left hemisphere after acute stroke results in improved language performance (170–172).
4. Expert opinion
There are many challenges to aphasia diagnosis and treatment. One of the most fundamental challenges remains the significant gap in translating clinical research into practice. Only a small percentage of research findings will ultimately influence clinical practice, and the findings that do change clinical practice take many years to do so (173,174). There are many factors that contribute to this gap. However, the most obvious factor is that the majority of research treatment studies cannot be successfully implemented in clinical settings due to challenges such as time constraints, insurance coverage limitations, and lack of resources at clinical sites. Many research studies in aphasia find positive outcomes when they provide patients with many hours of language therapy or provide them with many hours of intensive language therapy per day (175–177), but most patients do not have access to this dosage or intensity of language therapy.
Both language therapy intensity and dosage are important considerations in aphasia treatment, yet the field has no clear definition of dosage versus intensity. The terms are sometimes used interchangeably to refer to the number of hours given in a specific period of time, or the total number of hours of therapy provided during a treatment study (178). Little research addresses the question of treatment intensity in acute/subacute recovery stages. Bakheit and colleagues (179) compared intensive treatment where therapy was delivered 5 hours per week for 12 weeks to standard non-intensive treatment of 2 hours per week for 12 weeks. No significant difference was found between groups, but the mean scores were consistently higher in the intensive group. Recent research suggests that patients do benefit from increased intensity even in the acute/subacute stages of stroke (180–182). Some researchers question how well patients will tolerate intensive therapy in the first few weeks following stroke, and have found patients are patients are more likely to drop out of high intensity treatments (179,183), however others have demonstrated that it is feasible to increase therapy intensity even in the early stages of stroke recovery (180–182).
Several studies in chronic aphasia have demonstrated that high intensity aphasia treatment is more beneficial than low intensity treatment (183,184). However, a recent review in chronic aphasia concluded that while some evidence suggests patients benefit more from intensive therapy, there is stronger evidence to suggest that patients benefit from lower intensity treatment in the chronic recovery phase (185). Pierce and colleagues (185) ultimately conclude that more evidence (i.e., randomized trials with large sample sizes) is required before it can be unequivocally stated that low vs. high intensity therapy is more likely to benefit patients with chronic aphasia. Future research must disentangle the effects of dosage and intensity, and moreover must consider real world constraints on clinical practice.
Additionally, advances in technology will allow clinicians to provide patients with therapy tools outside of the clinic room. Clinicians can provide patients with intensive homework programs provided through various tablet-based treatment apps. It is imperative for clinicians to educate and familiarize themselves with ways they can use new technology to provide their patients with the best possible treatment outcomes. Randomized trials are needed to evaluate the effect of computer or tablet delivered therapy, with or without clinician-delivered therapy, in both the clinic setting and via telerehabilitation. The COVID-19 pandemic has created an unprecedented situation that is limiting on-site clinical services. Therefore, it is critical that rehabilitation professionals and researchers find optimal ways to deliver therapy services to patients with aphasia. Offering telerehabilitation will also help expand the number of patients who can receive therapy as many patients have barriers preventing them from receiving in-person therapy.
Advances in technology will also impact diagnostic methods. Aphasia diagnosis has largely evolved beyond the traditional approach of classifying patients into specific syndromes and instead focuses on individualized patient profiles. Creating individualized patient profiles should rely on more than simply understanding the severity of comprehension and production deficits. Rather clinicians must investigate the underlying impairments that are contributing to deficits within each patient. For example, a patient may have a naming deficit that is rooted in phonological impairment and another patient may have an impaired semantic network. If clinicians take the time to discover the underlying impairment in each patient , then they will be able to provide targeted individualized therapy that will have a better likelihood of benefitting their patients.
Medications for the treatment of aphasia have had mixed success. Beneficial effects have been reported for several drugs including piracetam and donepezil when combined with language therapy. However, data on long-term benefits are limited. There is a paucity of large RCTs as the majority of the trials are small open-label trials or case studies. In addition, there is wide variation in participant characteristics (e.g., fluent vs. non-fluent aphasia), timing of intervention (acute vs. chronic), type of language therapy (e.g., auditory comprehension vs naming), therapy duration, medication dosage, and outcome measures, leading to inconsistent findings. Further, the neuroplastic changes that are taking place as a result of pharmacotherapy are still unclear. Effort should be directed to identify the optimal dosage and timing of administration of different drugs. In addition, large scale randomized controlled trials are needed to identify appropriate candidates using well-defined clinical criteria and neuroimaging techniques to understand the mechanism of neural recovery.
Non-invasive brain simulation techniques are increasing used to modulate brain plasticity and accelerate language recovery. However, it remains unclear which area of the brain (left, right, or cerebellum), and which kind of stimulation (inhibitory or excitatory) is more effective in augmenting aphasia treatment. To know which region to target with NIBS, we need a better understanding of the mechanisms underlying recovery from aphasia. Similar to pharmacotherapy, there are wide variations in experimental factors including different types of aphasia, lesion site and location, NIBS stimulation parameters, different types of language therapy, therapy duration, and different outcome measures. This presents a major challenge to interpreting the findings. Finally, there are several practical issues that need to be addressed before we can adopt tDCS in clinical settings including training of clinicians, affordability and reimbursement. It is also essential to include outcome measures that show that intervention (behavioral, brain stimulation or pharmacotherapy) makes a difference in functional communication or quality of life, rather than simply focusing on impairment-based outcome measures.
Research studies should focus on the individual factors and biomarkers that could predict NIBS and pharmacotherapy response. Blood and saliva biomarkers are good candidates given the ease of access of sample collection, the possibility of storing samples for further analyses, and the availability of commercial kits, making it easier to be replicated in clinical or research facilities. For example, Fridriksson and colleagues (186) show that the analysis of genetic polymorphisms of Brain-Derived Neurotrophic Factor (BDNF) may help to determine the response to tDCS combined with behavioral therapy in chronic stroke patients with aphasia Furthermore, novel machine learning approaches are being developed that will allow for more accurate prediction of individual outcomes and response to therapy using data from brain imaging, behavioral measures, or their combination (187–189). The goal is to reach a point where clinicians are able to easily synthesize behavioral, lesion, and genetic information using sophisticated machine learning algorithms to select individualized therapy techniques (e.g., best behavioral therapy plus the best neuromodulation approach) that will most benefit each patient.
Article highlights:
Aphasia diagnostics should expand beyond simply classifying patients by aphasia syndrome. Instead an effort should be made to determine which linguistic and cognitive mechanisms are impaired.
Comprehensive aphasia evaluation must include a thorough case history, assessments of linguistic/cognitive skills, and an appraisal of how functional communication has been affected by aphasia.
Aphasia treatment must be individualized for patients in consideration of their goals, specific strengths, and deficits.
Aphasia studies need to incorporate both impairment and functional based outcome measures. Aphasia treatment studies have largely focused on impairment-based outcomes (e.g., naming); however, an improvement of impairment level outcome naming is not always followed by an improvement of functional communication. Therefore, it is necessary that primary outcome measures incorporate changes in functional communication, behavior, and quality of life measures.
Pharmacological agents already approved for the treatment of other neurological and psychiatric disorders have been studied in patients with post-stroke aphasia. Several small case studies, open-label trials, and small randomized clinical trials show that pharmacotherapy provides benefits in stroke patients with aphasia; however, benefits are not evident for all drugs and for all aphasia severity levels.
Non-invasive brain stimulation (NIBS) technologies such as transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS) have shown promising results in case studies and clinical trials; however, these methods remain investigational for post-stroke aphasia and are not approved for clinical use.
There is wide variation in protocols including stimulation location, stimulation intensity, number of treatment sessions, outcome measures, type of aphasia treatment, and time post-stroke. Determining optimal NIBS parameters as well as the mechanisms underlying treatment improvement is critical to facilitate transition to clinical practice.
Acknowledgments
Funding
The research reported in this paper was supported by the National Institutes of Health (National Institute on Deafness and Other Communication Disorders) through award R00 DC015554. The content is solely the responsibility of the authors and does not necessarily represent the views of the National Institutes of Health.
Footnotes
Declaration of interests
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or conflict with the subject matter or materials discussed in this manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (*) or of considerable interest (**) to readers.
- 1.Engelter S, Gostynski M, Papa S, et al. Epidemiology of aphasia attributable to first ischemic stroke incidence, severity, fluency, etiology, and thrombolysis. Stroke. 2006; 37:1379–84 [DOI] [PubMed] [Google Scholar]
- 2.Flowers HL, Skoretz SA, Silver FL, et al. Poststroke Aphasia Frequency, Recovery, and Outcomes: A Systematic Review and Meta-Analysis. Archives of Physical Medicine and Rehabilitation. 2016; 97:2188–201 [DOI] [PubMed] [Google Scholar]
- 3.Baker C, Worrall L, Rose M, Hudson K, Ryan B, O’Byrne L. A systematic review of rehabilitation interventions to prevent and treat depression in post-stroke aphasia. Disability and rehabilitation. 2018; 40:1870–92 [DOI] [PubMed] [Google Scholar]
- 4.Boden-Albala B, Litwak E, Elkind MSV, Rundek T, Sacco RL. Social isolation and outcomes post stroke. Neurology. 2005; 64:1888–92 [DOI] [PubMed] [Google Scholar]; * Investigated the relationship between social isolation and outcome in stroke and found that lack of social support contributes significantly to poorer outcomes. This study highlights the importance of considering social support when working with stroke patients.
- 5.Dalemans RJP, de Witte L, Wade D, van den Heuvel W. Social participation through the eyes of people with aphasia. International Journal of Language & Communication Disorders. 2010; 45:537–50 [DOI] [PubMed] [Google Scholar]
- 6.Naess H, Hammersvik L, Skeie GO. Aphasia among Young Patients with Ischemic Stroke on Long-term Follow-up. Journal of Stroke and Cerebrovascular Diseases. 2009; 18:247–50. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization International Classification of Functioning, Disability and Health (ICF). Geneva, Switzerland: World Health Organization; 2001 [Google Scholar]
- 8.Simmons-Mackie N, Kagan A. Application of the ICF in Aphasia. Seminars in Speech and Language. 2007; 28:244–53 [DOI] [PubMed] [Google Scholar]
- 9.Wallace SJ, Worrall L, Rose T, et al. Which outcomes are most important to people with aphasia and their families? an international nominal group technique study framed within the ICF. Disability and Rehabilitation. 2017; 39:1364–79 [DOI] [PubMed] [Google Scholar]
- 10.Worrall L, Sherratt S, Rogers P, et al. What people with aphasia want: Their goals according to the ICF. Disability and Rehabilitation. 2017; 39:1364–79 [DOI] [PubMed] [Google Scholar]; * This qualitative study identified nine broad categories of goals of people with aphasia that encompass all components of the WHO ICF. Most goals centered around the Activity and Participation components.
- 11.Kasselimis DS, Simos PG, Peppas C, Evdokimidis I, Potagas C. The unbridged gap between clinical diagnosis and contemporary research on aphasia: A short discussion on the validity and clinical utility of taxonomic categories. Brain and Language. 2017;164:63–7 [DOI] [PubMed] [Google Scholar]
- 12.Yourganov G, Smith KG, Fridriksson J, Rorden C. Predicting aphasia type from brain damage measured with structural MRI. Cortex. 2015; 73:203–15 [DOI] [PMC free article] [PubMed] [Google Scholar]; * This describes largescale study in 98 patients with 5 classic aphasia profiles. The authors found that aphasia type could be predicted based on patterns of underlying neural damage, which suggests classic aphasia profiles should still be considered in some cases when classifying aphasia type.
- 13.Kang EK, Sohn HM, Han M- K, Kim W, Han TR, Paik N- J. Severity of Post-stroke Aphasia According to Aphasia Type and Lesion Location in Koreans. Journal of Korean Medical Science. 2010; 25:123–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Z- H, Zhao X- Q, Wang C- X, Chen H- Y, Zhang Y- M. Neuroanatomic correlation of the post-stroke aphasias studied with imaging. Neurological Research. 2008; 30:356–60. [DOI] [PubMed] [Google Scholar]
- 15.Crosson B, Ford A, Raymer AM. Transcortical motor aphasia In: Raymer AM, Gonzalez Rothi LJ, editors. Handbook of aphasia and language disorders Oxford University Press, New York: New York: Oxford University Press; 2017 [Google Scholar]
- 16.Alexander MP, Hillis AE. Aphasia. In: Handbook of Clinical Neurology [Internet]. Elsevier; 2008. [cited 2020 Jul 14]. p. 287–309. (Neuropsychology and Behavioral Neurology; vol. 88). Available from: http://www.sciencedirect.com/science/article/pii/S0072975207880146 [DOI] [PubMed] [Google Scholar]
- 17.Freedman M, Alexander MP, Naeser MA. Anatomic basis of transcortical motor aphasia. Neurology. 1984; 34:409–409 [DOI] [PubMed] [Google Scholar]
- 18.Davis GA. Aphasiology: Disorders and Clinical Practice. 2nd Ed Boston: Pearson; 2007 [Google Scholar]
- 19.Mazzocchi F, Vignolo LA. Localisation of Lesions in Aphasia: Clinical-CT Scan Correlations in Stroke Patients. Cortex. 1979; 15:627–53 [DOI] [PubMed] [Google Scholar]
- 20.Rapcsak SZ, Krupp LB, Rubens AB, Reim J. Mixed transcortical aphasia without anatomic isolation of the speech area. Stroke. 1990; 21:953–6 [DOI] [PubMed] [Google Scholar]
- 21.Naeser MA, Hayward RW. Lesion localization in aphasia with cranial computed tomography and the Boston Diagnostic Aphasia Exam. Neurology. 1978; 28:545–51 [DOI] [PubMed] [Google Scholar]
- 22.Alexander MP, Hiltbrunner B, Fischer RS. Distributed Anatomy of Transcortical Sensory Aphasia. Arch Neurol. 1989; 46:885–92 [DOI] [PubMed] [Google Scholar]
- 23.Hickok G, Erhard P, Kassubek J, et al. A functional magnetic resonance imaging study of the role of left posterior superior temporal gyrus in speech production: implications for the explanation of conduction aphasia. Neuroscience Letters. 2000. June 23;287(2):156–60. [DOI] [PubMed] [Google Scholar]
- 24.Benson DF, Ardila A. Aphasia, a clinical perspective New York: Oxford University Press; 1996 [Google Scholar]
- 25.Crosson B Thalamic mechanisms in language: A reconsideration based on recent findings and concepts. Brain and Language. 2013; 126:73–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raymer AM, Moberg P, Crosson B, Nadeau S, Rothi LJG. Lexical–semantic deficits in two patients with dominant thalamic infarction. Neuropsychologia. 1997; 35: 211–9. [DOI] [PubMed] [Google Scholar]
- 27.Cappa SF, Cavallotti G, Guidotti M, et al. Subcortical Aphasia: Two Clinical-CT Scan Correlation Studies. Cortex. 1983; 19:227–41 [DOI] [PubMed] [Google Scholar]
- 28.Naeser MA, Alexander MP, Helm-Estabrooks N, et al. Aphasia With Predominantly Subcortical Lesion Sites: Description of Three Capsular/Putaminal Aphasia Syndromes. Arch Neurol. 1982; 39: 2–14 [DOI] [PubMed] [Google Scholar]
- 29.Radanovic M, Mansur LL. Aphasia in vascular lesions of the basal ganglia: A comprehensive review. Brain and Language. 2017; 173:20–32 [DOI] [PubMed] [Google Scholar]
- 30.Choi JY, Lee KH, Na DL, et al. Subcortical Aphasia After Striatocapsular Infarction: Quantitative Analysis of Brain Perfusion SPECT Using Statistical Parametric Mapping and a Statistical Probabilistic Anatomic Map. J Nucl Med. 2007; 48:194–200 [PubMed] [Google Scholar]
- 31.Hillis AE, Barker PB, Wityk RJ, et al. Variability in subcortical aphasia is due to variable sites of cortical hypoperfusion. Brain and Language. 2004; 89:524–30 [DOI] [PubMed] [Google Scholar]
- 32.Alexander MP, Fischette MR, Fischer RS. Crossed aphasias can be mirror image or anomalouscase reports, review and hypothesis. Brain. 1989; 112:953–73 [DOI] [PubMed] [Google Scholar]
- 33.Mariën P, Paghera B, De Deyn PP, Vignolo LA. Adult Crossed Aphasia in Dextrals Revisited. Cortex. 2004; 40:41–74 [DOI] [PubMed] [Google Scholar]
- 34.Barton JJS. Disorders of higher visual processing In: Kennard C, Leigh RJ, editors. Handbook of Clinical Neurology [Internet]. Elsevier; 2011. [cited 2020 Jul 15]. p. 223–61. (Neuro-ophthalmology; vol. 102). Available from: http://www.sciencedirect.com/science/article/pii/B9780444529039000157 [DOI] [PubMed] [Google Scholar]
- 35.Hillis AE. Chapter 15 Cognitive processes underlying reading and writing and their neural substrates In: Handbook of Clinical Neurology [Internet]. Elsevier; 2008. [cited 2020 Jul 15]. p. 311–22. (Neuropsychology and Behavioral Neurology; vol. 88). Available from: http://www.sciencedirect.com/science/article/pii/S0072975207880158 [DOI] [PubMed] [Google Scholar]
- 36.Hillis AE. Aphasia. Neurology. 2007. July 10; 69:200–13 [DOI] [PubMed] [Google Scholar]
- 37.Rapcsak SZ, Beeson PM. Neuroanatomical correlates of spelling and writing In: The handbook of adult language disorders, 2nd ed New York, NY, US: Psychology Press; 2015. p. 87–116 [Google Scholar]
- 38.Moshtagh-Khorasani M, Akbarzadeh-T M- R, Jahangiri N, Khoobdel M. An intelligent system based on fuzzy probabilities for medical diagnosis–a study in aphasia diagnosis. Journal of research in medical sciences: the official journal of Isfahan University of Medical Sciences. 2009;14:89. [PMC free article] [PubMed] [Google Scholar]
- 39.Konstantinopoulou G, Kovas K, Hatzilygeroudis I, Prentzas J. An Approach using Certainty Factor Rules for Aphasia Diagnosis. In: 2019 10th International Conference on Information, Intelligence, Systems and Applications (IISA). IEEE; 2019. p. 1–7 [Google Scholar]
- 40.Sabahi F A novel generalized belief structure comprising unprecisiated uncertainty applied to aphasia diagnosis. Journal of biomedical informatics. 2016; 62:66–77 [DOI] [PubMed] [Google Scholar]
- 41.Marshall J Classification of aphasia: Are there benefits for practice? Aphasiology. 2010; 24:408–12 [Google Scholar]
- 42.Gordon J The fluency dimension in aphasia. Aphasiology. 1998;12:673–88 [Google Scholar]
- 43.Lazar RM, Speizer AE, Festa JR,et al. Variability in language recovery after first-time stroke. Journal of Neurology, Neurosurgery & Psychiatry. 2008; 79:530–4 [DOI] [PubMed] [Google Scholar]
- 44.Maas MB, Lev MH, Ay H, et al. The Prognosis for Aphasia in Stroke. Journal of Stroke and Cerebrovascular Diseases. 2012; 21:350–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pedersen PM, Vinter K, Olsen TS. Aphasia after Stroke: Type, Severity and Prognosis. CED. 2004; 17:35–43 [DOI] [PubMed] [Google Scholar]
- 46.El Hachioui H, Lingsma HF, van de Sandt-Koenderman ME, et al. Recovery of aphasia after stroke: a 1-year follow-up study. Journal of neurology. 2013; 260:166–71 [DOI] [PubMed] [Google Scholar]
- 47.Hillis AE, Beh YY, Sebastian R, et al. Predicting recovery in acute poststroke aphasia. Annals of Neurology. 2018; 83:612–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Long C, Sebastian R, Faria AV, Hillis AE. Longitudinal imaging of reading and naming recovery after stroke. Aphasiology. 2018; 32:839–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goodglass H, Barresi B. Boston Diagnostic Aphasia Examination - Third Edition (BDAE-3). San Antonio, TX: Pearson; 2000 [Google Scholar]
- 50.Kertesz A Western Aphasia Battery - Revised. San Antonio, TX: Harcourt Assessment, Inc.; 2007 [Google Scholar]
- 51.Swinburn K, Porter G, Howard D. Comprehensive aphasia test. Taylor & Francis; 2004 [Google Scholar]
- 52.Kay J, Lesser R, Coltheart M. The Psycholinguistic Assessment of Language Processing in Aphasia (PALPA). Hove, UK: Lawrence Erlbaum Associates; 1992 [Google Scholar]
- 53.Thompson CK. Northwestern Assessment of Verbs and Sentences (NAVS) [Internet]. 2012. [cited 2018 Apr 24]. Available from: https://www.scholars.northwestern.edu/en/publications/northwestern-assessment-of-verbs-and-sentences-navs [Google Scholar]
- 54.Frattali CM, Thompson CM, Holland AL, et al. The FACS of life ASHA FACS--a functional outcome measure for adults. ASHA. 1995; 37:40–6 [PubMed] [Google Scholar]
- 55.Blomert L, Kean ML, Koster C, Schokker J. Amsterdam-Nijmegen everyday language test: construction, reliability and validity. Aphasiology. 1994; 8:381–407 [Google Scholar]
- 56.van der Meulen I, van de Sandt-Koenderman WME, Duivenvoorden HJ, Ribbers GM. Measuring verbal and non-verbal communication in aphasia: reliability, validity, and sensitivity to change of the Scenario Test. International Journal of Language & Communication Disorders. 2010; 45:424–35 [DOI] [PubMed] [Google Scholar]
- 57.Lomas J, Pickard L, Bester S, et al. The communicative effectiveness index: Development and psychometric evaluation of a functional communication measure for adult aphasia. Journal of speech and hearing disorders. 1989; 54:113–24 [DOI] [PubMed] [Google Scholar]
- 58.Bullier B, Cassoudesalle H, Villain M, et al. New factors that affect quality of life in patients with aphasia. Annals of Physical and Rehabilitation Medicine. 2020; 63:33–7 [DOI] [PubMed] [Google Scholar]
- 59.Hilari K, Byng S. Health-related quality of life in people with severe aphasia. International journal of language & communication disorders. 2009; 44:193–205 [DOI] [PubMed] [Google Scholar]
- 60.Ross K, Wertz R. Quality of life with and without aphasia. Aphasiology. 2003; 17:355–64 [Google Scholar]
- 61.Dickey L, Kagan A, Lindsay MP, et al. Incidence and Profile of Inpatient Stroke-Induced Aphasia in Ontario, Canada. Archives of Physical Medicine and Rehabilitation. 2010; 91:196–202 [DOI] [PubMed] [Google Scholar]
- 62.Katerina Hilari, Sally Byng, Lamping Donna L, Smith Sarah C. Stroke and Aphasia Quality of Life Scale-39 (SAQOL-39). Stroke. 2003; 34:1944–50 [DOI] [PubMed] [Google Scholar]
- 63.Galletta EE, Barrett AM. Impairment and functional interventions for aphasia: having it all. Current physical medicine and rehabilitation reports. 2014; 2:114–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chapey R, Duchan JF, Elman RJ, et al. Life participation approach to aphasia: A statement of values for the future. The ASHA Leader. 2000; 5:4–6 [Google Scholar]
- 65.Lanyon L, Worrall L, Rose M. Combating social isolation for people with severe chronic aphasia through community aphasia groups: consumer views on getting it right and wrong. Aphasiology. 2018; 32:493–517 [Google Scholar]
- 66.Lanyon L, Worrall L, Rose M. Exploring participant perspectives of community aphasia group participation: from “I know where I belong now” to “Some people didn’t really fit in.” Aphasiology. 2018; 32:139–63 [Google Scholar]
- 67.Attard MC, Lanyon L, Togher L, Rose ML. Consumer perspectives on community aphasia groups: a narrative literature review in the context of psychological well-being. Aphasiology. 2015; 29:983–1019 [Google Scholar]
- 68.Attard MC, Loupis Y, Togher L, Rose ML. Experiences of people with severe aphasia and spouses attending an Interdisciplinary Community Aphasia Group. Disability and Rehabilitation. 2020; 42:1382–96 [DOI] [PubMed] [Google Scholar]
- 69.Helm-Estabrooks N, Nicholas M, Morgan A. Melodic intonation therapy. Austin, TX: Pro-Ed, Inc.; 1989 [Google Scholar]
- 70.Albert ML, Sparks RW, Helm NA. Melodic Intonation Therapy for Aphasia. Arch Neurol. 1973; 29:130–1. [DOI] [PubMed] [Google Scholar]
- 71.Helm-Estabrooks N, Albert ML, Nicholas M. Manual of aphasia and aphasia therapy. 2nd Edition. Austin, TX: Pro Ed, Inc.; 2004 [Google Scholar]
- 72.Pulvermüller F, Neininger B, Elbert T, et al. Constraint-induced therapy of chronic aphasia after stroke. Stroke. 2001; 32:1621–6 [DOI] [PubMed] [Google Scholar]
- 73.Boyle M, Coelho CA. Application of semantic feature analysis as a treatment for aphasic dysnomia. American Journal of Speech-Language Pathology. 1995; 4:94–8 [Google Scholar]
- 74.Coelho CA, McHugh RE, Boyle M. Semantic feature analysis as a treatment for aphasic dysnomia: A replication. Aphasiology. 2000;1 4:133–42 [Google Scholar]
- 75.Leonard C, Rochon E, Laird L. Treating naming impairments in aphasia: Findings from a phonological components analysis treatment. Aphasiology. 2008; 22:923–47 [Google Scholar]
- 76.Edmonds LA. A Review of Verb Network Strengthening Treatment: Theory, Methods, Results, and Clinical Implications. Topics in Language Disorders. 2016; 36:123 [Google Scholar]
- 77.Edmonds LA, Nadeau SE, Kiran S. Effect of Verb Network Strengthening Treatment (VNeST) on lexical retrieval of content words in sentences in persons with aphasia. Aphasiology. 2009; 23:402–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Edmonds LA, Mammino K, Ojeda J. Effect of Verb Network Strengthening Treatment (VNeST) in Persons With Aphasia: Extension and Replication of Previous Findings. Am J Speech Lang Pathol. 2014; 23: S312–29 [DOI] [PubMed] [Google Scholar]
- 79.Thompson CK, Shapiro L. Treating agrammatic aphasia within a linguistic framework: Treatment of Underlying Forms. Aphasiology. 2005; 19:1021–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mack JE, Thompson CK. Recovery of Online Sentence Processing in Aphasia: Eye Movement Changes Resulting From Treatment of Underlying Forms. J Speech Lang Hear Res. 2017; 60:1299–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thompson CK. Treatment of underlying forms: A linguistic specific approach for sentence production deficits in agrammatic aphasia In: Language intervention strategies in adult aphasia. Williams & Wilkins; 2001. p. 605–28 [Google Scholar]
- 82.Elman RJ. Aphasia centers and the life participation approach to aphasia. Topics in Language Disorders. 2016; 36:154–67 [Google Scholar]
- 83.Simmons-Mackie N, Holland AL. Aphasia centers in North America: a survey. Semin Speech Lang. 2011; 32:203–15 [DOI] [PubMed] [Google Scholar]
- 84.Hall N, Boisvert M, Steele R. Telepractice in the Assessment and Treatment of Individuals with Aphasia: A Systematic Review. Int J Telerehabil. 2013; 5:27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Power E, Falkenberg K, Barnes S, et al. A pilot randomized controlled trial comparing online versus face-to-face delivery of an aphasia communication partner training program for student healthcare professionals. International Journal of Language & Communication Disorders [Internet]. in press [cited 2020 Jul 21];n/a(n/a). Available from: http://onlinelibrary.wiley.com/doi/abs/10.1111/1460-6984.12556 [DOI] [PubMed] [Google Scholar]
- 86.Aftonomos LB, Steele RD, Wertz RT. Promoting recovery in chronic aphasia with an interactive technology. Archives of physical medicine and rehabilitation. 1997; 78:841–6 [DOI] [PubMed] [Google Scholar]
- 87.Des Roches CA, Balachandran I, Ascenso EM, et al. Effectiveness of an impairment-based individualized rehabilitation program using an iPad-based software platform. Frontiers in Human Neuroscience. 2015; 8:1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Godlove J, Anantha V, Advani M, et al. Comparison of therapy practice at home and in the clinic: a retrospective analysis of the constant therapy platform data set. Frontiers in Neurology. 2019;10:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Linebarger MC, Schwartz MF, Romania JR, Kohn SE, Stephens DL. Grammatical encoding in aphasia: Evidence from a “processing prosthesis.” Brain and Language. 2000; 75:416–27 [DOI] [PubMed] [Google Scholar]
- 90.Thompson CK, Choy JJ, Holland A, Cole R. Sentactics®: Computer-automated treatment of underlying forms. Aphasiology. 2010; 24:1242–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cherney LR. Oral reading for language in aphasia (ORLA): Evaluating the efficacy of computer-delivered therapy in chronic nonfluent aphasia. Topics in stroke rehabilitation. 2010;17:423–31 [DOI] [PubMed] [Google Scholar]
- 92.Carragher M, Talbot R, Devane N, Rose M, Marshall J. Delivering storytelling intervention in the virtual world of EVA Park. Aphasiology. 2018; 32:37–9 [Google Scholar]
- 93.Galliers J, Wilson S, Marshall J, et al. Experiencing EVA park, a multi-user virtual world for people with aphasia. ACM Transactions on Accessible Computing (TACCESS). 2017;10:1–24 [Google Scholar]
- 94.Marshall J, Booth T, Devane N, et al. Evaluating the benefits of aphasia intervention delivered in virtual reality: results of a quasi-randomised study. PloS one. 2016; 11:e0160381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Crosson B, Rodriguez AD, Copland D, et al. Neuroplasticity and aphasia treatments: new approaches for an old problem. Journal of Neurology, Neurosurgery & Psychiatry. 2019; 90:1147–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kurland J, Baldwin K, Tauer C. Treatment-induced neuroplasticity following intensive naming therapy in a case of chronic Wernicke’s aphasia. Aphasiology. 2010. July 20;24(6–8):737–51 [Google Scholar]
- 97.Marcotte K, Adrover-Roig D, Damien B, et al. Therapy-induced neuroplasticity in chronic aphasia. Neuropsychologia. 2012; 50:1776–86 [DOI] [PubMed] [Google Scholar]; ** This study investigated patterns of brain activation that predicted best response to treatment in a group of individuals with chronic aphasia. This study is important because it demonstrated therapy-induced neuroplastic changes in a group of individuals with chronic aphasia, and it demonstrated that specific patterns of activation were predictive of improvement.
- 98.Berthier ML. Ten key reasons for continuing research on pharmacotherapy for post-stroke aphasia. Aphasiology. 2020;1–35 [Google Scholar]
- 99.Saxena S, Hillis AE. An update on medications and noninvasive brain stimulation to augment language rehabilitation in post-stroke aphasia. Expert review of neurotherapeutics. 2017;17:1091–107 [DOI] [PubMed] [Google Scholar]
- 100.Berthier ML, Pulvermüller F, Dávila G, et al. Drug therapy of post-stroke aphasia: a review of current evidence. Neuropsychology review. 2011; 21:302. [DOI] [PubMed] [Google Scholar]
- 101.Kessler J, Thiel A, Karbe H, Heiss WD. Piracetam improves activated blood flow and facilitates rehabilitation of poststroke aphasic patients. Stroke. 2000; 31:2112–6 [DOI] [PubMed] [Google Scholar]
- 102.Berthier ML, Pulvermüller F. Neuroscience insights improve neurorehabilitation of poststroke aphasia. Nature Reviews Neurology. 2011; 7:86–97 [DOI] [PubMed] [Google Scholar]
- 103.Albert ML. Neurobiological aspects of aphasia therapy. Aphasiology. 1988; 2:215–8 [Google Scholar]
- 104.Sabe L, Leiguarda R, Starkstein SE. An open-label trial of bromocriptine in nonfluent aphasia. Neurology. 1992; 42: 1637–1638 [DOI] [PubMed] [Google Scholar]
- 105.Bragoni M, Altieri M, Di Piero V, et al. Bromocriptine and speech therapy in non-fluent chronic aphasia after stroke. Neurological Sciences. 2000; 21:19–22 [DOI] [PubMed] [Google Scholar]
- 106.Gold M, VanDam D, Silliman ER. An open-label trial of bromocriptine in nonfluent aphasia: a qualitative analysis of word storage and retrieval. Brain and Language. 2000; 74:141–56 [DOI] [PubMed] [Google Scholar]
- 107.Gupta SR, Mlcoch AG, Scolaro C, Moritz T. Bromocriptine treatment of nonfluent aphasia. Neurology. 1995; 45:2170–3 [DOI] [PubMed] [Google Scholar]
- 108.Ashtary F, Janghorbani M, Chitsaz A, et al. A randomized, double-blind trial of bromocriptine efficacy in nonfluent aphasia after stroke. Neurology. 2006. ;66:914–6 [DOI] [PubMed] [Google Scholar]
- 109.Altieri M, Di Piero V, Lenzi GL, Walker-Batson D, Curtis S, Unwin DH. Drugs and recovery: a challenge for a few? Stroke. 2002;33(4):1170–1170 [DOI] [PubMed] [Google Scholar]
- 110.Tan LC, Ng KK, Au W- L, et al. Bromocriptine use and the risk of valvular heart disease. Movement disorders. 2009; 24:344–9 [DOI] [PubMed] [Google Scholar]
- 111.Leiguarda R, Merello M, Sabe L, Starkstein S. Bromocriptine-induced dystonia in patients with aphasia and hemiparesis. Neurology. 1993; 43:2319–2319 [DOI] [PubMed] [Google Scholar]
- 112.Berthier ML, Hinojosa J, del Carmen Martín M, Fernández I. Open-label study of donepezil in chronic poststroke aphasia. Neurology. 2003; 60:1218–9 [DOI] [PubMed] [Google Scholar]
- 113.Berthier ML, Green C, Higueras C, et al. A randomized, placebo-controlled study of donepezil in poststroke aphasia. Neurology. 2006; 67:1687–9 [DOI] [PubMed] [Google Scholar]
- 114.Tsz-Ming C, Kaufer DJ. Effects of donepezil on aphasia, agnosia, and apraxia in patients with cerebrovascular lesions. J Neuropsychiatry Clin Neurosci. 2001;13:140 [Google Scholar]
- 115.Pashek GV, Bachman DL. Cognitive, linguistic and motor speech effects of donepezil hydrochloride in a patient with stroke-related aphasia and apraxia of speech. Brain and Language. 2003; 1:179–80 [Google Scholar]
- 116.Güngör L, Terzi M, Onar MK. Does long term use of piracetam improve speech disturbances due to ischemic cerebrovascular diseases? Brain and language. 2011; 117:23–7 [DOI] [PubMed] [Google Scholar]
- 117.Orgogozo J- M. Piracetam in the treatment of acute stroke. Pharmacopsychiatry. 1999; 32:25–32 [DOI] [PubMed] [Google Scholar]
- 118.De Deyn PP, De Reuck J, Deberdt W, et al. Treatment of acute ischemic stroke with piracetam. Stroke. 1997; 28:2347–52 [DOI] [PubMed] [Google Scholar]
- 119.Enderby P, Broeckx J, Hospers W, et al. Effect of piracetam on recovery and rehabilitation after stroke: a double-blind, placebo-controlled study. Clinical neuropharmacology. 1994;17:320–31 [DOI] [PubMed] [Google Scholar]
- 120.Barbancho MA, Berthier ML, Navas-Sánchez P, et al. Bilateral brain reorganization with memantine and constraint-induced aphasia therapy in chronic post-stroke aphasia: An ERP study. Brain and Language. 2015;145:1–10 [DOI] [PubMed] [Google Scholar]
- 121.Berthier ML, Green C, Lara JP, et al. Memantine and constraint-induced aphasia therapy in chronic poststroke aphasia. Annals of Neurology. 2009; 65:577–85 [DOI] [PubMed] [Google Scholar]
- 122.Van de Weg FB, Kuik DJ, Lankhorst GJ. Post-stroke depression and functional outcome: a cohort study investigating the influence of depression on functional recovery from stroke. Clinical rehabilitation. 1999; 13:268–72 [DOI] [PubMed] [Google Scholar]
- 123.Barker-Collo SL. Depression and anxiety 3 months post stroke: prevalence and correlates. Archives of Clinical Neuropsychology. 2007;22(4):519–31 [DOI] [PubMed] [Google Scholar]
- 124.Gabaldón L, Fuentes B, Frank-García A, Díez-Tejedor E. Poststroke depression: importance of its detection and treatment. Cerebrovascular Diseases. 2007; 24:181–8 [DOI] [PubMed] [Google Scholar]
- 125.Skidmore ER, Whyte EM, Holm MB, et al. Cognitive and Affective Predictors of Rehabilitation Participation After Stroke. Archives of Physical Medicine and Rehabilitation. 2010; 91:203–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tanaka Y, Albert M, Aketa S, et al. Serotonergic Therapy for Fluent Aphasia: P02. 150. Neurology. 2004;62 [Google Scholar]
- 127.Cappa SF, Ortelli P, Garibotto V, Zamboni M. Reversible nonfluent aphasia and left frontal hypoperfusion during topiramate treatment. Epilepsy & Behavior. 2007; 10:192–4 [DOI] [PubMed] [Google Scholar]
- 128.Mula M, Trimble MR, Thompson P, Sander JW. Topiramate and word-finding difficulties in patients with epilepsy. Neurology. 2003; 60:1104–7 [DOI] [PubMed] [Google Scholar]
- 129.Breining BL, Sebastian R. Neuromodulation in Post-stroke Aphasia Treatment. Curr Phys Med Rehabil Rep. 2020; 8:44–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Berube S, Hillis AE. Advances and Innovations in Aphasia Treatment Trials. Stroke. 2019; 50:2977–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Floel A, Cohen LG. Recovery of function in humans: Cortical stimulation and pharmacological treatments after stroke. Neurobiology of Disease. 2010; 37:243–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Baker JM, Rorden C, Fridriksson J. Using transcranial direct-current stimulation to treat stroke patients with aphasia. Stroke. 2010; 41:1229–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Darkow R, Martin A, Würtz A, Flöel A, Meinzer M. Transcranial direct current stimulation effects on neural processing in post-stroke aphasia. Human Brain Mapping. 2017; 38:1518–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fridriksson J, Richardson JD, Baker JM, Rorden C. Transcranial direct current stimulation improves naming reaction time in fluent aphasia: a double-blind, sham-controlled study. Stroke. 2011; 42:819–21. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This is the largest post-stroke aphasia tDCS trial using a randomized, double-blind, sham controlled design in 74 chronic patients. Participants received tDCS to the left temporal lobe region based on naming activation on the fMRI. tDCS was associated with greater change in number of correctly naming pictured objects compared to sham.
- 135.Fridriksson J, Rorden C, Elm J, Sen S, George MS, Bonilha L. Transcranial Direct Current Stimulation vs Sham Stimulation to Treat Aphasia After Stroke: A Randomized Clinical Trial. JAMA Neurol. 2018; 75:1470–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Meinzer M, Darkow R, Lindenberg R, Flöel A. Electrical stimulation of the motor cortex enhances treatment outcome in post-stroke aphasia. Brain. 2016; 139:1152–63 [DOI] [PubMed] [Google Scholar]
- 137.Wu D, Wang J, Yuan Y. Effects of transcranial direct current stimulation on naming and cortical excitability in stroke patients with aphasia. Neuroscience Letters. 2015; 589:115–20 [DOI] [PubMed] [Google Scholar]
- 138.Campana S, Caltagirone C, Marangolo P. Combining Voxel-based Lesion-symptom Mapping (VLSM) With A-tDCS Language Treatment: Predicting Outcome of Recovery in Nonfluent Chronic Aphasia. Brain Stimulation. 2015; 8:769–76 [DOI] [PubMed] [Google Scholar]
- 139.Pestalozzi MI, Di Pietro M, Martins Gaytanidis C, et al. Effects of Prefrontal Transcranial Direct Current Stimulation on Lexical Access in Chronic Poststroke Aphasia. Neurorehabil Neural Repair. 2018; 32:913–23 [DOI] [PubMed] [Google Scholar]
- 140.Branscheidt M, Hoppe J, Zwitserlood P, Liuzzi G. tDCS over the motor cortex improves lexical retrieval of action words in poststroke aphasia. Journal of Neurophysiology. 2017; 119:621–30 [DOI] [PubMed] [Google Scholar]
- 141.da Silva FR, Mac-Kay APMG, Chao JC, Santos MD dos, Gagliadi RJ. Trancranial direct current stimulation: A study on naming performance in aphasic individuals. CoDAS. 2018; 30:1–6 [DOI] [PubMed] [Google Scholar]
- 142.You DS, Kim D- Y, Chun MH, et al. Cathodal transcranial direct current stimulation of the right Wernicke’s area improves comprehension in subacute stroke patients. Brain and Language. 2011; 119:1–5 [DOI] [PubMed] [Google Scholar]
- 143.Kang EK, Kim YK, Sohn HM, et al. Improved picture naming in aphasia patients treated with cathodal tDCS to inhibit the right Broca’s homologue area. Restorative Neurology and Neuroscience. 2011; 29:141–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Feil S, Eisenhut P, Strakeljahn F, et al. Left Shifting of Language Related Activity Induced by Bihemispheric tDCS in Postacute Aphasia Following Stroke. Front Neurosci [Internet]. 2019. [cited 2020 Aug 10];13. Available from: https://www.frontiersin.org/articles/10.3389/fnins.2019.00295/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Marangolo P, Fiori V, Sabatini U, et al. Bilateral Transcranial Direct Current Stimulation Language Treatment Enhances Functional Connectivity in the Left Hemisphere: Preliminary Data from Aphasia. Journal of Cognitive Neuroscience. 2016; 28:724–38 [DOI] [PubMed] [Google Scholar]
- 146.Manenti R, Petesi M, Brambilla M, et al. Efficacy of semantic–phonological treatment combined with tDCS for verb retrieval in a patient with aphasia. Neurocase. 2015; 21:109–19 [DOI] [PubMed] [Google Scholar]
- 147.Sebastian R, Saxena S, Tsapkini K, et al. Cerebellar tDCS: a novel approach to augment language treatment post-stroke. Frontiers in human neuroscience. 2017;10:695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Marangolo P, Fiori V, Caltagirone C, Pisano F, Priori A. Transcranial cerebellar direct current stimulation enhances verb generation but not verb naming in poststroke aphasia. Journal of cognitive neuroscience. 2018; 30:188–99. [DOI] [PubMed] [Google Scholar]
- 149.Spielmann K, van de Sandt-Koenderman WME, Heijenbrok-Kal MH, Ribbers GM. Transcranial direct current stimulation does not improve language outcome in subacute poststroke aphasia. Stroke. 2018; 49:1018–20 [DOI] [PubMed] [Google Scholar]
- 150.Hartwigsen G The neurophysiology of language: Insights from non-invasive brain stimulation in the healthy human brain. Brain and language. 2015;148:81–94 [DOI] [PubMed] [Google Scholar]
- 151.Haghighi M, Mazdeh M, Ranjbar N, Seifrabie MA. Further evidence of the positive influence of repetitive transcranial magnetic stimulation on speech and language in patients with aphasia after stroke: results from a double-blind intervention with sham condition. Neuropsychobiology. 2018; 75:185–92 [DOI] [PubMed] [Google Scholar]
- 152.Yoon TH, Han SJ, Yoon TS, Kim JS, Yi TI. Therapeutic effect of repetitive magnetic stimulation combined with speech and language therapy in post-stroke non-fluent aphasia. NeuroRehabilitation. 2015; 36:107–14 [DOI] [PubMed] [Google Scholar]
- 153.Rubi-Fessen I, Hartmann A, Huber W, et al. Add-on effects of repetitive transcranial magnetic stimulation on subacute aphasia therapy: enhanced improvement of functional communication and basic linguistic skills. A randomized controlled study. Archives of physical medicine and rehabilitation. 2015; 96:1935–1944. e2. [DOI] [PubMed] [Google Scholar]
- 154.Ren C, Zhang G, Xu X, et al. The effect of rTMS over the different targets on language recovery in stroke patients with global aphasia: a randomized sham-controlled study. BioMed research international. 2019; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This relatively large (N=45) randomized, sham controlled study of subacute post stroke aphasia showed that inhibitory low frequency rTMS of right IFG and right STG both lead to language improvements, demonstrating that targets beyond right IFG can be effective.
- 155.Hara T, Abo M, Kobayashi K, et al. Effects of low-frequency repetitive transcranial magnetic stimulation combined with intensive speech therapy on cerebral blood flow in post-stroke aphasia. Translational stroke research. 2015; 6:365–74 [DOI] [PubMed] [Google Scholar]; * This study showed that pre-treatment language lateralization can be used to guide rTMS protocol design. Positive results were observed both for inhibitory low frequency rTMS of the right IFG in patients who had left lateralized language, supporting recovery, and for excitatory high frequency rTMS of the right IFG in patients who had right lateralized language, supporting compensation.
- 156.Harvey DY, Podell J, Turkeltaub PE, et al. Functional reorganization of right prefrontal cortex underlies sustained naming improvements in chronic aphasia via repetitive transcranial magnetic stimulation. Cognitive and behavioral neurology: official journal of the Society for Behavioral and Cognitive Neurology. 2017; 30:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhang H, Chen Y, Hu R, et al. rTMS treatments combined with speech training for a conduction aphasia patient: A case report with MRI study. Medicine. 2017; 96(32). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Griffis JC, Nenert R, Allendorfer JB, Szaflarski JP. Interhemispheric plasticity following intermittent theta burst stimulation in chronic poststroke aphasia. Neural plasticity. 2016;2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Vuksanović J, Jelić MB, Milanović SD, Kačar K, Konstantinović L, Filipović SR. Improvement of language functions in a chronic non-fluent post-stroke aphasic patient following bilateral sequential theta burst magnetic stimulation. Neurocase. 2015; 21:244–50. [DOI] [PubMed] [Google Scholar]
- 160.Hu X, Zhang T, Rajah GB, et al. Effects of different frequencies of repetitive transcranial magnetic stimulation in stroke patients with non-fluent aphasia: a randomized, sham-controlled study. Neurological research. 2018; 40:459–65. [DOI] [PubMed] [Google Scholar]
- 161.Turkeltaub PE. Brain Stimulation and the Role of the Right Hemisphere in Aphasia Recovery. Curr Neurol Neurosci Rep. 2015; 15:72. [DOI] [PubMed] [Google Scholar]
- 162.Holland R, Crinion J. Can tDCS enhance treatment of aphasia after stroke? Aphasiology. 2012; 26:1169–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Perler BA, Murphy K, Sternbach Y, et al. Immediate postoperative thrombolytic therapy: an aggressive strategy for neurologic salvage when cerebral thromboembolism complicates carotid endarterectomy. Journal of vascular surgery. 2000; 31:1033–7 [DOI] [PubMed] [Google Scholar]
- 164.Denier C, Flamand-Roze C, Dib F, et al. Aphasia in stroke patients: early outcome following thrombolysis. Aphasiology. 2015;29(4):442–56. [Google Scholar]
- 165.Martins IP, Fonseca J, Morgado J, et al. Language improvement one week after thrombolysis in acute stroke. Acta Neurologica Scandinavica. 2017;135:339–45. [DOI] [PubMed] [Google Scholar]
- 166.Furlanis G, Ridolfi M, Polverino P, et al. Early recovery of aphasia through thrombolysis: The significance of spontaneous speech. Journal of Stroke and Cerebrovascular Diseases. 2018; 27:1937–48 [DOI] [PubMed] [Google Scholar]
- 167.Menichelli A, Furlanis G, Sartori A, et al. Thrombolysis’ benefits on early post-stroke language recovery in aphasia patients. Journal of Clinical Neuroscience. 2019;70:92–5. [DOI] [PubMed] [Google Scholar]
- 168.Zhou Z, Lu J, Liu W- W, et al. Advances in stroke pharmacology. Pharmacology & therapeutics. 2018;191:23–42. [DOI] [PubMed] [Google Scholar]
- 169.Hillis AE, Wityk RJ, Barker PB, et al. Subcortical aphasia and neglect in acute stroke: the role of cortical hypoperfusion. Brain. 2002; 125:1094–104. [DOI] [PubMed] [Google Scholar]; * This study showed that cases of aphasia or hemispatial neglect that result from infarcts restricted to subcortical regions can be accounted for by associated cortical hypoperfusion, at least in the acute phase.
- 170.Hillis AE, Ulatowski JA, Barker PB, et al. A pilot randomized trial of induced blood pressure elevation: effects on function and focal perfusion in acute and subacute stroke. Cerebrovascular diseases. 2003;16:236–46. [DOI] [PubMed] [Google Scholar]
- 171.Hillis AE, Barker PB, Beauchamp NJ, et al. Restoring blood pressure reperfused Wernicke’s area and improved language. Neurology. 2001;56:670–2. [DOI] [PubMed] [Google Scholar]
- 172.Hillis AE, Kleinman JT, Newhart M, et al. Restoring Cerebral Blood Flow Reveals Neural Regions Critical for Naming. J Neurosci. 2006; 26:8069–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Green LW, Ottoson JM, Garcia C, Hiatt RA. Diffusion theory and knowledge dissemination, utilization, and integration in public health. Annual review of public health. 2009;30 [DOI] [PubMed] [Google Scholar]
- 174.Colditz G, Emmons KM. The promise and challenges of dissemination and implementation research. In: Brownson R, Colditz G, Proctor E, editors. Dissemination and implementation research in health: Translating science to practice. New York, NY: Oxford University Press; 2012. p. 3–22 [Google Scholar]
- 175.Bhogal SK, Teasell R, Speechley M. Intensity of aphasia therapy, impact on recovery. Stroke. 2003; 34:987–93 [DOI] [PubMed] [Google Scholar]
- 176.Cherney LR, Patterson JP, Raymer A, Frymark T, Schooling T. Evidence-based systematic review: Effects of intensity of treatment and constraint-induced language therapy for individuals with stroke-induced aphasia. Journal of Speech, Language, and Hearing Research. 2008; 51:1282–99 [DOI] [PubMed] [Google Scholar]
- 177.Cherney LR, Patterson JP, Raymer AM. Intensity of aphasia therapy: Evidence and efficacy. Current neurology and neuroscience reports. 2011;11:560. [DOI] [PubMed] [Google Scholar]; * This is a systematic review of treatment studies that directly compares conditions of higher and lower intensity treatment for aphasia
- 178.Harnish SM, Morgan J, Lundine JP, et al. Dosing of a cued picture-naming treatment for anomia. American Journal of Speech-Language Pathology. 2014; 23: S285–99 [DOI] [PubMed] [Google Scholar]
- 179.Bakheit AMO, Shaw S, Barrett L, et al. A prospective, randomized, parallel group, controlled study of the effect of intensity of speech and language therapy on early recovery from poststroke aphasia. Clin Rehabil. 2007; 21:885–94 [DOI] [PubMed] [Google Scholar]
- 180.Carpenter J, Cherney LR. Increasing aphasia treatment intensity in an acute inpatient rehabilitation programme: a feasibility study. Aphasiology. 2016; 30:542–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Godecke E, Hird K, Lalor EE, Rai T, Phillips MR. Very early poststroke aphasia therapy: a pilot randomized controlled efficacy trial. International Journal of Stroke. 2012; 7:635–44 [DOI] [PubMed] [Google Scholar]
- 182.Martins IP, Leal G, Fonseca I, et al. A randomized, rater-blinded, parallel trial of intensive speech therapy in sub-acute post-stroke aphasia: The SP-I-R-IT study. International journal of language & communication disorders. 2013; 48:421–31 [DOI] [PubMed] [Google Scholar]
- 183.Brady MC, Kelly H, Godwin J, Enderby P, Campbell P. Speech and language therapy for aphasia following stroke. Cochrane database of systematic reviews. 2016;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Breitenstein C, Grewe T, Flöel A, et al. Intensive speech and language therapy in patients with chronic aphasia after stroke: a randomised, open-label, blinded-endpoint, controlled trial in a health-care setting. The Lancet. 2017; 389:1528–38 [DOI] [PubMed] [Google Scholar]; ** This randomized, multicenter trial show that 3 weeks of intensive speech and language therapy administered under routine clinical conditions significantly improved verbal communication in patients with chronic aphasia after stroke, compared with 3 weeks deferral of intensive speech and language therapy.
- 185.Pierce JE, O’Halloran R, Menahemi-Falkov M, et al. Comparing higher and lower weekly treatment intensity for chronic aphasia: A systematic review and meta-analysis. Neuropsychological Rehabilitation. 2020;1–25 [DOI] [PubMed] [Google Scholar]
- 186.Fridriksson J, Elm J, Stark BC, et al. BDNF genotype and tDCS interaction in aphasia treatment. Brain stimulation. 2018; 11:1276–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gu Y, Bahrani M, Billot A, et al. A machine learning approach for predicting post-stroke aphasia recovery: a pilot study. In: Proceedings of the 13th ACM International Conference on PErvasive Technologies Related to Assistive Environments. 2020. p. 1–9. [Google Scholar]
- 188.Halai AD, Woollams AM, Ralph MAL. Investigating the effect of changing parameters when building prediction models for post-stroke aphasia. Nature Human Behaviour. 2020;1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rosenberg MD, Song H. Predicting post-stroke aphasia from brain imaging. Nature Human Behaviour. 2020; 4:675–6 [DOI] [PubMed] [Google Scholar]


