Abstract
This study aimed to examine how bone health changed among T2DM patients in the past decade. Continuous National Health and Nutrition Examination Survey (NHANES) data from 2005–2006 to 2013–2014 were analyzed to examine the trends of bone mineral density (BMD) and the prevalence trends of osteoporosis osteopenia among T2DM patients and non-diabetic people aged 40 years and older. The age- and BMI-adjusted mean BMD of the femur neck for the four NHANES cycles decreased linearly in both T2DM patients and non-diabetic people (both Plinear trend ≤ 0.009). Among women with T2DM, the mean BMD in 2013–2014 was significantly lower than that in 2005–2006, even after adjusting for multiple covariates. During 2005–2014, the prevalence of osteoporosis among T2DM patients and non-diabetic people increased but with no significant linear trend (both Plinear trend > 0.05), while the prevalence of osteopenia in the two populations increased linearly (both Plinear trend < 0.04). Age- and BMI-adjusted mean BMD decreased in 2013–2014 in patients with T2DM and non-diabetic people, while the prevalence of osteoporosis and osteopenia increased in both groups.
Subject terms: Diabetes, Metabolic bone disease, Epidemiology
Introduction
Osteoporosis and low bone mass (osteopenia) affect approximately 200 million people across the world1, including 54 million people in the United States2. People with these conditions are prone to have fractures. Indeed, around 158 million people were at a high fracture risk in 2010, with that number predicted to double by 20143. These related fractures will ultimately lead to many issues in those afflicted, including movement restriction, disability, and severe morbidity.
Diabetes is characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both4,5. The number of people with diabetes worldwide has risen from 108 million in 1980 to 422 million in 20146. Even more notably, type 2 diabetes mellitus (T2DM) accounts for 90–95% of all diabetes7. The prevalence of diabetes increased rapidly from 4.4 to 10.0% in the US between 1996 and 20158, and more than 30 million suffer from T2DM9.
Osteoporosis and T2DM are affected by aging and often coexist in the elderly10. T2DM affects bone metabolism and strength by influencing osteoblast and osteoclast11. The imbalance between osteoblast and osteoclast might cause osteoporosis11. As well, T2DM might affect bone quality and quantity, leading to a change in the structural properties of bone mass12. T2DM affects bone homeostasis, so related fractures are considered a result of T2DM12,13. Several studies have already reported an increased fracture risk among T2DM patients14–17. Therefore, evaluating the bone health of individuals with T2DM is essential in preventing osteoporosis and related fractures. However, to our knowledge, how the bone health of T2DM patients changed in recent years remains unclear. As BMD is the most important single predictor for osteoporotic fractures, the dual-energy X-ray absorptiometry (DXA)-based BMD has been the benchmark technique used in osteoporosis diagnosis18,19. DXA-based BMD plays a crucial role in osteoporosis/osteopenia management and fracture risk assessment. Therefore, the present study aimed to test whether people with T2DM have had an elevated or decreased BMD in the past decades and compare the results to non-diabetic people. We also examined the trends of osteoporosis and osteopenia prevalence in T2DM patients and non-diabetic people.
Methods
Data source
Data were obtained from the continuous National Health and Nutrition Examination Survey (NHANES). The survey uses a complex, multistage probability design to select a nationally representative sample of non-institutionalized civilians in the US population20. NHANES collects data through interviews and physical examination. The interview includes demographic, socioeconomic, dietary, and health-related questions, while the examination consists of medical, dental, and physiological measurements. From 2005–2006, the survey started measuring femur and spine BMD; however, the BMD was not measured for NHANES in 2011–2012. Therefore, only four cycles (2005–2006, 2007–2008, 2009–2010, and 2013–2014) were included for this study. Subjects younger than 40 years old were excluded since participants are more likely to develop T2DM if they are 40 or over21.
Human participants
NHANES study protocol was approved by the National Center for Health Statistics Research Ethics Review Board. Written informed consent was obtained for all adult participants. This secondary analysis was approved by the institutional review board at the University of Nevada, Las Vegas (#1004670). All research reported in this manuscript was performed in accordance with relevant guidelines/regulations.
Diabetes mellitus
Subjects who had a positive response to the question, “Have you ever been told by a doctor that you have diabetes?” were defined as having a diagnosed diabetes. Individuals who reported taking pills to lower blood sugar were classified as having a diagnosed T2DM as well. This classification method was used22, and other study using NHANES data adopted this method as well23. Among participants without diagnosed diabetes, individuals who had a hemoglobin A1c level of 6.5% or higher, or a fasting plasma glucose level of 126 mg/dL or higher, or a 2-h plasma glucose level of 200 mg/dL or higher5, were all classified as having “undiagnosed diabetes.” Because we could not distinguish between type 1 and type 2 diabetes based on these lab results, all of the undiagnosed diabetes in this study was assumed to be T2DM since T2DM comprises the majority (90–95%) of diabetes7. Diagnosed T2DM and undiagnosed T2DM were combined as the T2DM group in this study. People were classified as non-diabetic if they did not have diagnosed or undiagnosed diabetes.
BMD measurement
Femur neck BMD and spine BMD were measured using a Hologic QDR-4500A fan-beam densitometer during 2005–2010. Both BMDs were obtained with a Hologic Discovery model A densitometer (Hologic, Inc., Bedford, MA, USA) in 2013–2014. During 2005–2010, Hologic Discovery v12.4 and APEX v3.0 were used for analyzing the femur and spine scans, respectively. APEX v4.0 was used for the analysis in the two regions in 2013–2014. In this study, we focused on analyzing the femur neck BMD primarily because BMD at the femur neck has the highest predictive value for hip fracture, and the hip is the site of highest clinical relevance24. We used femur neck BMD over spine BMD in this study because of significant differences in BMD measures between using Discovery v12.4 during 2005–2010 and using APEX v4.0 in 2013–2014, with the exception of the femur neck25. In NHANES, DXA scans were used for the BMD measurement since the system has a number of advantages, the primary being a consensus that BMD results can be interpreted using the World Health Organization T-score definition of osteoporosis, thus having a proven ability to predict fracture risk26.
Definition for osteoporosis and osteopenia
In this study, the diagnosis of osteoporosis or osteopenia was based on T-score results. T-scores were calculated as (BMDmeasured − mean BMDreference)/SDreference. Osteoporosis was defined as a T-score of BMD ≤ − 2.5, and osteopenia was defined as − 2.5 < T-score ≤ − 127. Consistent with the International Society for Clinical Densitometry’s corresponding guidelines, the reference group for calculating these scores for the femur neck consisted of 20–29 years old non-Hispanic Caucasian women from NHANES III28. In our study, subjects who lacked valid BMD data were excluded.
Other variables
Age, sex, race/ethnicity, smoking status, physical activities, fracture history, and family history were ascertained by questionnaire. For the race/ethnicity groups, “Mexican American” and “Other Hispanic” were merged into a single group called “Hispanic,” and the remaining groups were “non-Hispanic White,” “non-Hispanic Black,” and “non-Hispanic other,” respectively. BMD-related variables, including body mass index (BMI), previous fracture29,30, smoking status31,32, physical activity33,34, and family history of osteoporosis35, were considered for analysis. BMI was derived from measured weight in kilograms, divided by the square of height in meters. Individuals who had suffered a broken or fractured hip, wrist, or spine were considered as having a previous fracture. Smoking status was categorized into smokers and non-smokers. Smokers were respondents who had smoked at least 100 cigarettes during their lifetime; otherwise, subjects were defined as non-smokers. Self-reported physical activity was categorized as “inactive” and “active.” Participants who were sedentary or only performed basic activities, which refers to light-intensity activities like standing and walking slowly, were considered to be inactive; otherwise, the individuals were classified as active36. In the present study, the participants were defined as having a family history of osteoporosis if their parent(s) ever had the disease.
Statistical analysis
Sampling weights were utilized to account for unequal selection probabilities, nonresponse, and non-coverage37. The age- and BMI-adjusted mean BMD at the femur neck, prevalence of osteoporosis and osteopenia, and corresponding 95% confidence interval in every survey cycle for both T2DM patients and non-diabetic people were calculated based on weighted data. Standard errors, which were employed to calculate 95% confidence intervals, were estimated using Taylor series linearization. The US 2000 Census was used as the standard population for age adjustment. Tests for linear trend over the four survey cycles were conducted using orthogonal contrast. Multiple linear regression was used to examine the BMD trend while holding other variables constant. The standardized coefficient from linear regression was employed to examine each variable’s relative importance in the regression model for BMD prediction. The survey cycle was included as a categorical variable in the multiple linear regression in order to determine if the mean BMD in 2013–2014 differed from previous survey cycles after adjusting for major confounders. We also conducted separate sensitivity analyses to examine the mean BMD trends across the four survey cycles in T2DM patients and non-diabetic population after adjusting for age and weight (instead of BMI) and in diagnosed and undiagnosed T2DM patients after adjusting for age and BMI. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
The number of eligible participants in this study was 11,901, and their characteristics by survey cycles are presented in Table 1. During the four survey cycles, the mean age was around 62 years for T2MD patients and 56 years for the non-diabetic population. From 2005 to 2014 mean BMI increased among T2DM patients (from 29.93 to 31.38 kg/m2), as well as among the non-diabetic population (from 27.75 to 28.27 kg/m2). In each survey cycle, more than half of T2DM patients were men, while most non-diabetic participants were women. Over the four survey cycles, the percentage of physical inactivity increased from 14.04 to 34.61% in T2DM patients and from 9.89 to 23.80% in the non-diabetic population.
Table 1.
Weighted characteristics of subjects aged 40 and older, among T2DM patients and non-diabetic population in 4 NHANES (2005–2006, 2007–2008, 2009–2010, and 2013–2014).
| Variable | Survey cycle | |||||||
|---|---|---|---|---|---|---|---|---|
| 2005–2006 | 2007–2008 | 2009–2010 | 2013–2014 | |||||
| With T2DM (N = 409) | Non-diabetic (N = 1853) | With T2DM (N = 720) | Non-diabetic (N = 2465) | With T2DM (N = 734) | Non-diabetic (N = 2619) | With T2DM (N = 674) | Non-diabetic (N = 2427) | |
| Age, mean (SD), years | 62.12 ± 0.85 | 55.51 ± 0.71 | 61.47 ± 0.51 | 55.60 ± 0.32 | 62.47 ± 0.72 | 55.84 ± 0.39 | 61.22 ± 0.71 | 56.42 ± 0.34 |
| BMI, mean (SD), kg/m2 | 29.93 ± 0.30 | 27.75 ± 0.21 | 31.32 ± 0.29 | 27.74 ± 0.12 | 31.23 ± 0.27 | 27.86 ± 0.14 | 31.38 ± 0.26 | 28.27 ± 0.19 |
| Gender, N (%) | ||||||||
| Men | 227 (53.26) | 975(48.53) | 394 (51.87) | 1209 (46.39) | 377 (52.12) | 1323 (48.08) | 371 (57.04) | 1162 (47.81) |
| Women | 182 (46.74) | 878 (51.47) | 326 (48.13) | 1256 (53.61) | 357 (47.88) | 1296 (51.92) | 303 (42.96) | 1265 (52.19) |
| Race, N (%) | ||||||||
| Hispanic | 115 (13.33) | 323 (7.05) | 208 (11.92) | 617 (9.21) | 260 (15.67) | 678 (9.76) | 192 (16.44) | 496 (10.26) |
| NH White | 166 (66.58) | 1108 (80.19) | 316 (67.67) | 1315 (76.36) | 293 (64.45) | 1411 (76.17) | 234 (61.41) | 1132 (73.44) |
| NH Black | 112 (14.60) | 356 (8.13) | 174 (14.47) | 437 (8.75) | 134 (12.04) | 418 (8.77) | 156 (13.26) | 462 (9.38) |
| NH other | 16 (5.49) | 66 (4.63) | 22 (5.94) | 96 (5.68) | 47 (7.84) | 112 (5.29) | 92 (8.88) | 337 (6.92) |
| Previous fracture, N (%) | 55 (15.79) | 272 (14.97) | 89 (13.76) | 328 (14.49) | 74 (11.22) | 306 (12.02) | 73(12.80) | 260 (12.34) |
| Smoking, N (%) | 226(52.20) | 995 (53.61) | 380 (52.21) | 1245 (48.77) | 362 (49.04) | 1287 (46.54) | 326 (50.96) | 1111(45.30) |
| Physical inactivity, N (%) | 73 (14.04) | 244 (9.89) | 310 (41.22) | 708 (22.40) | 308 (38.44) | 733 (22.57) | 226 (34.61) | 612 (23.80) |
| Family history of osteoporosis, N (%) | 28 (10.17) | 213 (14.29) | 67 (12.71) | 334 (16.74) | 61 (8.97) | 292 (14.47) | 74 (13.51) | 312 (16.84) |
Adjusted mean femur neck BMD
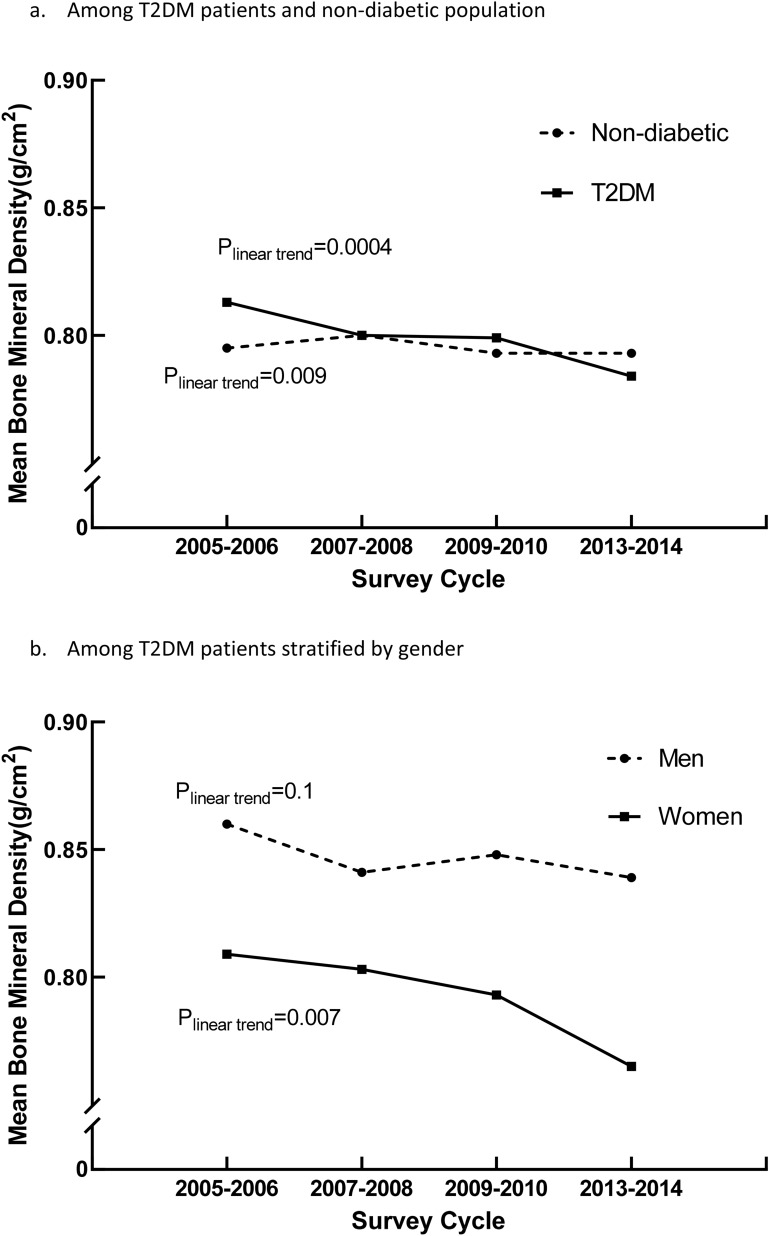
The age- and BMI-adjusted mean BMD of T2DM patients and non-diabetic people in the four survey cycles are shown in Fig. 1a. For T2DM patients, the mean BMD decreased linearly from 0.813 g/cm2 (95% CI 0.796–0.829 g/cm2) to 0.784 g/cm2 (95% CI 0.771–0.796 g/cm2) during 2005–2014 (Plinear trend = 0.004). Meanwhile, the mean BMD of non-diabetic also decreased linearly during the four survey cycles (Plinear trend = 0.0009), from 0.795 to 0.773 g/cm2.
Figure 1.
Age and body mass index-adjusted mean bone mineral density in 4 NHANES (2005–2006, 2007–2008, 2009–2010, and 2013–2014).
The results of age- and BMI- adjusted mean BMD of T2DM patients stratified by gender are presented in Fig. 1b. Among patients with T2DM, women had a lower mean BMD than men during the four survey cycles. In 2005–2014, the mean BMD of women with T2DM linearly decreased (Plinear trend = 0.007), but no significant linear trend (Plinear trend = 0.1) was observed among men with T2DM.
The age- and BMI-adjusted mean BMD of the non-diabetic population stratified by gender are presented in Supplementary Figure 1. Significant linear trends were observed for both genders during 2005–2014 (both Plinear trend ≤ 0.005). Our sensitivity analysis revealed that the results of age- and weight-adjusted mean BMD (Supplementary Figure 2) were similar to age- and BMI-adjusted mean BMD. In addition, the age- and BMI-adjusted mean BMD (Supplementary Figure 3) decreased linearly in people with diagnosed T2DM (P linear trend = 0.02), but not for people with undiagnosed T2DM (Plinear trend = 0.39) during 2005–2014.
Prevalence of osteoporosis and osteopenia
The age- and BMI-adjusted prevalence of osteoporosis and osteopenia of T2DM patients and non-diabetic people from 2005 to 2014 are presented in Table 2. The adjusted prevalence of osteoporosis among T2DM patients increased from 3.13% (95% CI 1.39–4.87%) to 6.10% (95% CI 4.47–7.75%) during 2005–2014, and the linear trend was close to being statistically significant (Plinear trend = 0.054). For non-diabetic individuals, the adjusted prevalence of osteoporosis was stable during this period (Plinear trend = 0.35). The adjusted osteopenia prevalence of T2DM patients and non-diabetic people had a significant increase in the linear trend (both Plinear trend ≤ 0.04).
Table 2.
Age- and BMI-adjusted prevalence of osteoporosis and osteopenia among T2DM patients and non-diabetic population in 4 NHANES (2005–2006, 2007–2008, 2009–2010, and 2013–2014).
| Prevalence | Survey cycle | ||||
|---|---|---|---|---|---|
| 2005–2006 | 2007–2008 | 2009–2010 | 2013–2014 | P value for linear trend | |
| Osteoporosis | |||||
| T2DM | 3.13 (1.39–4.87) | 4.50 (1.55–7.44) | 3.10 (1.75–4.44) | 6.10 (4.47–7.75) | 0.054 |
| Non diabetic | 4.03 (3.26–4.79) | 3.03 (2.30–3.74) | 3.81 (3.18–4.46) | 4.38 (3.39–5.37) | 0.35 |
| Osteopenia | |||||
| T2DM | 27.83 (21.62–34.03) | 31.14 (26.75–35.54) | 32.56 (27.15–37.97) | 35.49 (30.06–40.92) | 0.04 |
| Non diabetic | 32.23 (29.81–34.64) | 30.27 (28.14–32.41) | 32.82 (30.81–34.84) | 38.20 (35.51–40.90) | 0.0005 |
The age- and BMI-adjusted prevalence of osteoporosis and osteopenia among T2DM patients by gender are presented in Table 3. The linear trend of the osteoporosis prevalence among women approached the borderline of significance (Plinear trend = 0.08) but was non-significant for men (Plinear trend = 0.14). The osteoporosis prevalence among women with T2DM increased from 3.95% (95% CI 0–7.98%) to 10.13% (95% CI 6.06–14.21%) during the four survey cycles. For the prevalence of osteopenia among T2DM patients, a significant increase in linearly trend was observed in women (Plinear trend = 0.04), but not in men (Plinear trend = 0.35). The prevalence of osteoporosis and osteopenia in non-diabetic people by gender is presented in Supplementary Table 1. The prevalence of osteoporosis in non-diabetic men and women was stable during the four survey cycles (both Plinear trend > 0.29). However, significant linear trends in osteopenia prevalence were observed in non-diabetic men and women during the four survey cycles (both Plinear trend ≤ 0.004).
Table 3.
Age- and BMI-adjusted prevalence of osteoporosis and osteopenia among T2DM patients stratified by gender in 4 NHANES (2005–2006, 2007–2008, 2009–2010, and 2013–2014).
| Prevalence | Survey cycle | ||||
|---|---|---|---|---|---|
| 2005–2006 | 2007–2008 | 2009–2010 | 2013–2014 | P value for linear trend | |
| Osteoporosis | |||||
| Men | 0.02 (0–0.30) | 2.29 (0–4.87) | 1.31 (0–2.63) | 1.21 (0.42–2.00) | 0.14 |
| Women | 3.95 (0–7.98) | 4.95 (1.67–8.23) | 3.19 (0.81–5.57) | 10.13 (6.06–14.21) | 0.08 |
| Osteopenia | |||||
| Men | 21.34 (14.34–28.34) | 22.44 (18.05–26.82) | 20.98 (14.68–27.28) | 27.14 (19.01–35.26) | 0.35 |
| Women | 25.22 (18.82–31.62) | 31.13 (25.08–37.17) | 35.34 (27.52–43.15) | 35.42 (26.82–44.02) | 0.04 |
Multiple linear regression
For women with T2DM, older age and previous fractures were significantly associated with decreased BMD, based on the multiple linear regression (both p ≤ 0.03; Supplementary Table 2). For men with T2DM, older age, family history of osteoporosis, physical inactivity, and smoking were associated with significant BMD reduction (all p < 0.007; Supplementary Table 3). The standardized coefficient revealed that age played a more critical role than other risk factors in lowering BMD for T2DM patients of both sexes. The selected covariates explained around 35% variance of BMD at the femur neck in women and about 27% in men. The mean BMD of women with T2DM in 2013–2014 was significantly lower than in 2005–2006 (p = 0.0007; Supplementary Table 2), even after adjusting for various confounders. The results of non-diabetic individuals, both women and men, are respectively presented in Supplementary Table 4 and Table 5. For non-diabetic people, a family history of osteoporosis, age, and physical inactivity had a significant negative impact on BMD in both men and women.
Discussion
In this study, decreasing BMD trends at the femur neck were observed among T2DM patients and non-diabetic people during 2005–2014. In addition, the prevalence of osteoporosis and osteopenia among subjects with T2DM increased correspondingly in the four survey cycles. Specifically, a significant increasing linear trend in the prevalence of osteoporosis and osteopenia was observed among women with T2DM. These consistent results demonstrate that an unfavorable trend of change in bone health in T2DM patients may be occurring in the United States.
Compared to our prior study regarding the US BMD trend in 2005–201438, the present research further examined the BMD trend, osteoporosis, and osteopenia in the US's T2DM population. There is a paucity of research investigating the BMD trend and the pattern of osteoporosis and osteopenia in T2DM patients, specifically a lack of data comparing the trend between individuals with T2DM and those without diabetes. In this study, T2DM patients had a higher BMD than non-diabetic people, consistent with Dr. Oei et al.’s research16,39. Among T2DM patients, men had a higher BMD mean than women. The possible reason for this is that men have a larger bone mass than women40. Notably, the mean BMD in 2013–2014 among women with T2DM was significantly lower than the mean BMD in 2005–2006. Such a difference remained when adjusted for multiple related factors, implying proper intervention might be needed. Although such a linear trend of BMD was not observed among men in the current study, attention should be paid to men since a previous study showed that men with diabetes had a higher risk of fracture41. Our findings of non-diabetic people were consistent with a recent study conducted by Dr. Chi Chen et al. that found a declining BMD trend among adults with normal glucose regulation42. The decreasing mean BMD trend of this study demonstrates that the bone health of T2DM patients and non-diabetic people might have deteriorated significantly in recent years. In Dr. Black and colleagues’ study43, people with the lowest quartile of BMD almost had fivefold increased hip fracture risk during 25 years follow-up when compared with those with the highest quartile of BMD. Therefore, as shown in the current study, primary prevention and treatment should be conducted among the non-diabetic population with the lowest quartile BMD.
The multiple linear regression results indicated that sex, age, race, previous fracture, BMI, smoking, and physical activity are significant for T2DM patients6,8,27,29–35,44–48. Thus these related risk factors might partially contribute to the declining trend in mean BMD. The percentage of physical inactivity increased from 14.04 to 34.61% among T2DM and from 9.89 to 23.80% among non-diabetics. Given the association between physical inactivity and lower BMD34, the increased physically inactive lifestyle might also explain the decreasing BMD trends occurring during the four survey cycles. However, the decreased BMD in 2013–2014 among women with T2DM remained significant after controlling for major confounding effects in the multiple regression analysis. Thus other factors such as antidiabetic medication of T2DM patients may play a role in the observed trend49,50. For example, Dr. Monami and colleagues found an increased risk of fracture among T2DM patients who used insulin51. Patients with insulin usually have complications such as microvascular disease, which might impair the bone quality and individual balance52, thus increasing fracture risk. Additionally, Schwartz et al. reported an increased bone loss in diabetic women who had taken thiazolidinediones medication (TZD), including rosiglitazone, pioglitazone, and troglitazone49. Several studies investigated the effect of TZD on bone metabolism and found it is associated with increased adipogenesis and impaired osteoblastogenesis, which might lead to impaired bone formation and ultimately to fractures53. Other studies found that two medications, exenatide (a glucagon-like peptide 1 receptor agonist) and dapagliflozin (a sodium glucose cotransporter-2 inhibitor) increased the risk of bone fractures54,55, while dipeptidyl peptidase-4 inhibitors were associated with decreased fracture risk26,54,56. Further research is warranted to explain the overall declining BMD trend in T2DM patients.
The underlying pathogenic mechanism of bone fragility in T2DM is complex and not fully understood12. Not only decreased bone mass but also bone microstructure might contribute to bone fracture. Typically, increased cortical porosity and reduced cortical density lead to bone structure change52. Prior studies found that T2DM patients had greater cortical porosity57,58, but lower cortical bone density59. Therefore, bone resistance to mechanical stress among T2DM patients will be increased, which will result in an increased risk of fracture.
Several limitations should be acknowledged in interpreting the finding of this study. First, many of the NHANES participants were excluded from BMD measurement due to hip fractures, pregnancy, or other reasons. However, nonresponse in the examination data was accounted for by sampling weights in NHANES. Second, information about T2DM treatment, which might impact bone health, is limited in NHANES. For example, TZD is widely prescribed for the treatment of T2DM, and accumulating evidence indicates that TZD could cause bone loss and increase fracture risk in humans, specifically in women49. However, too few participants reported this information, so we cannot analyze it to yield valid results. Third, the evaluation of the trend in hemoglobin A1c among T2DM patients would be informative since several studies reported the association between poor glycemic control and increased risk of fractures60–63. However, the missing value of hemoglobin A1c from NHANES is too much (around 80%) to get an accurate evaluation. Finally, the study design of NHANES is cross-sectional, which restricts the assessment of causal relationships.
Osteoporosis and low BMD lead to fractures, which cause severe consequences for both individual patients and health care systems24. Osteoporosis-related fractures often lead to decreased quality of life, disability, and even death for patients and are also associated with $20 billion in expenses in the US64. Due to T2DM patients having normal or higher BMD, BMD-based T-score might underestimate the fracture risk of T2DM patients, and evaluation of bone health and osteoporosis diagnosis among them might be challenging52. Derived from continuous NHANES data, our findings regarding osteoporosis trends and mean BMD among T2DM patients can be used to inform public health policy and thus contribute to needed reform. Policy intervention may help to reduce risk factors associated with the downward trend of bone health inT2DM patients.
Conclusion
In summary, a decreasing age- and BMI-adjusted mean BMD trend has been observed in T2DM patients and the non-diabetic population in recent years. The unfavorable trend indicates a future downward shift in the bone health of T2DM patients, so bone health should be monitored in diabetic patients. Therefore, additional studies are warranted to understand the decreasing BMD trend among T2DM patients more thoroughly to prevent fractures and their subsequent deleterious consequences on individuals with diabetes.
Supplementary Information
Author contributions
Conceptualization, Y.X. and Q.W.; methodology, Y.X. and Q.W.; software, Y.X.; validation, Y.X. and Q.W.; formal analysis, Y.X.; investigation, Y.X. and Q.W.; resources, Q.W.; data curation, Y.X.; writing—original draft preparation, Y.X. and Q.W.; writing—review and editing, Y.X. and Q.W.; visualization, Y.X.; supervision, Q.W.; project administration, Q.W.; funding acquisition, Q.W.
Funding
The research and analysis described in the current publication were supported by a grant from the National Institute of General Medical Sciences (P20GM121325), a grant from the National Institute on Minority Health and Health Disparities of the National Institutes of Health (R15MD010475).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-83263-4.
References
- 1.Kanis, J. A. WHO technical report (University of Sheffield, 2007).
- 2.Foundation, N. O. 54 million Americans affected by osteoporosis and low bone mass. https://www.nof.org/news/54-million-americans-affected-by-osteoporosis-and-low-bone-mass/ (2014).
- 3.Odén A, McCloskey EV, Kanis JA, Harvey NC, Johansson H. Burden of high fracture probability worldwide: Secular increases 2010–2040. Osteoporos. Int. 2015;26:2243–2248. doi: 10.1007/s00198-015-3154-6. [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes A. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA. 2015;314:1021–1029. doi: 10.1001/jama.2015.10029. [DOI] [PubMed] [Google Scholar]
- 6.Kakkar R. Rising burden of diabetes-public health challenges and way out. Nepal J. Epidemiol. 2016;6:557–559. doi: 10.3126/nje.v6i2.15160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Almeida-Pititto B, et al. Type 2 diabetes in Brazil: Epidemiology and management. Diabetes Metab. Syndr. Obes. 2015;8:17–28. doi: 10.2147/DMSO.S72542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benoit SR, Hora I, Albright AL, Gregg EW. New directions in incidence and prevalence of diagnosed diabetes in the USA. BMJ Open Diabetes Res. Care. 2019;7:e000657. doi: 10.1136/bmjdrc-2019-000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rowley WR, Bezold C, Arikan Y, Byrne E, Krohe S. Diabetes 2030: Insights from yesterday, today, and future trends. Popul. Health Manag. 2017;20:6–12. doi: 10.1089/pop.2015.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paschou SΑ, et al. Type 2 diabetes and osteoporosis: A guide to optimal management. J. Clin. Endocrinol. Metab. 2017;102:3621–3634. doi: 10.1210/jc.2017-00042. [DOI] [PubMed] [Google Scholar]
- 11.Sassi F, et al. Type 2 diabetes affects bone cells precursors and bone turnover. BMC Endocr. Disord. 2018;18:55–55. doi: 10.1186/s12902-018-0283-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Napoli N, et al. The alliance of mesenchymal stem cells, bone, and diabetes. Int. J. Endocrinol. 2014;2014:690783. doi: 10.1155/2014/690783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrari SL, et al. Diagnosis and management of bone fragility in diabetes: An emerging challenge. Osteoporos. Int. 2018;29:2585–2596. doi: 10.1007/s00198-018-4650-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koh WP, et al. Diabetes and risk of hip fracture in the Singapore Chinese Health Study. Diabetes Care. 2010;33:1766–1770. doi: 10.2337/dc10-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li G, et al. Frailty and risk of fractures in patients with type 2 diabetes. Diabetes Care. 2019;42:507. doi: 10.2337/dc18-1965. [DOI] [PubMed] [Google Scholar]
- 16.Bonds DE, et al. Risk of fracture in women with type 2 diabetes: The Women's Health Initiative Observational Study. J. Clin. Endocrinol. Metab. 2006;91:3404–3410. doi: 10.1210/jc.2006-0614. [DOI] [PubMed] [Google Scholar]
- 17.Vestergaard P, Rejnmark L, Mosekilde L. Relative fracture risk in patients with diabetes mellitus, and the impact of insulin and oral antidiabetic medication on relative fracture risk. Diabetologia. 2005;48:1292–1299. doi: 10.1007/s00125-005-1786-3. [DOI] [PubMed] [Google Scholar]
- 18.Gregson CL, Hardcastle SA, Cooper C, Tobias JH. Friend or foe: High bone mineral density on routine bone density scanning, a review of causes and management. Rheumatology (Oxford) 2013;52:968–985. doi: 10.1093/rheumatology/ket007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Office of the Surgeon General (US). Bone Health and Osteoporosis: A Report of the Surgeon General (U.S. Department of Health and Human Services, Office of the Surgeon General, Rockville, 2004). [PubMed]
- 20.Chen, T. C., Clark, J., Riddles, M. K., Mohadjer, L. K., Fakhouri, T. H. I. National Health and Nutrition Examination Survey, 2015−2018: Sample design and estimation procedures. Vital Health Stat. 2184 (2020). [PubMed]
- 21.Sattar N, et al. Age at diagnosis of type 2 diabetes mellitus and associations with cardiovascular and mortality risks. Circulation. 2019;139:2228–2237. doi: 10.1161/CIRCULATIONAHA.118.037885. [DOI] [PubMed] [Google Scholar]
- 22.Liu X, Zhu T, Manojlovich M, Cohen HW, Tsilimingras D. Racial/ethnic disparity in the associations of smoking status with uncontrolled hypertension subtypes among hypertensive subjects. PLoS ONE. 2017;12:e0182807–e0182807. doi: 10.1371/journal.pone.0182807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu B, et al. Understanding CKD among patients with T2DM: Prevalence, temporal trends, and treatment patterns-NHANES 2007–2012. BMJ Open Diabetes Res. Care. 2016;4:e000154. doi: 10.1136/bmjdrc-2015-000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312:1254. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Looker AC, Sarafrazi Isfahani N, Fan B, Shepherd JA. Trends in osteoporosis and low bone mass in older US adults, 2005–2006 through 2013–2014. Osteoporos. Int. 2017;28:1979–1988. doi: 10.1007/s00198-017-3996-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blake GM, Fogelman I. The role of DXA bone density scans in the diagnosis and treatment of osteoporosis. Postgrad. Med. J. 2007;83:509–517. doi: 10.1136/pgmj.2007.057505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foundation, I. O. Understanding the results of your DXA test. https://www.iofbonehealth.org/news/understanding-results-your-dxa-test (2016).
- 28.Kelly, T. L. Bone mineral density reference databases for American men and women. J. Bone Miner. Res.5 (1990).
- 29.Kuru P, et al. Fracture history in osteoporosis: Risk factors and its effect on quality of life. Balkan Med. J. 2014;31:295–301. doi: 10.5152/balkanmedj.2014.13265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA, III, Berger M. Patients with prior fractures have an increased risk of future fractures: A summary of the literature and statistical synthesis. J. Bone Miner. Res. 2000;15:721–739. doi: 10.1359/jbmr.2000.15.4.721. [DOI] [PubMed] [Google Scholar]
- 31.Eleftheriou KI, et al. Bone structure and geometry in young men: The influence of smoking, alcohol intake and physical activity. Bone. 2013;52:17–26. doi: 10.1016/j.bone.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Mackey DC, et al. High-trauma fractures and low bone mineral density in older women and men. JAMA. 2007;298:2381–2388. doi: 10.1001/jama.298.20.2381. [DOI] [PubMed] [Google Scholar]
- 33.Carter MI, Hinton PS. Physical activity and bone health. Mo Med. 2014;111:59–64. [PMC free article] [PubMed] [Google Scholar]
- 34.(US), O. o. t. S. G. Bone health and osteoporosis: A report of the surgeon general. Assessing the Risk of Bone Disease and Fracture. (2004). [PubMed]
- 35.Orchard TS, et al. Magnesium intake, bone mineral density, and fractures: Results from the Women's Health Initiative Observational Study. Am. J. Clin. Nutr. 2014;99:926–933. doi: 10.3945/ajcn.113.067488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.National Heart Lung and Blood Institute. Clinical guidelines on the identification, Evaluation, and Treatment of Overweight and Obesity in Adults (1998). [PubMed]
- 37.Wirth KE, TchetgenTchetgen EJ. Accounting for selection bias in association studies with complex survey data. Epidemiology (Cambridge, Mass.) 2014;25:444–453. doi: 10.1097/ede.0000000000000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu Y, Wu Q. Decreasing trend of bone mineral density in US multiethnic population: Analysis of continuous NHANES 2005–2014. Osteoporos. Int. 2018;29:2437–2446. doi: 10.1007/s00198-018-4648-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oei L, et al. High bone mineral density and fracture risk in type 2 diabetes as skeletal complications of inadequate glucose control. Diabetes Care. 2013;36:1619. doi: 10.2337/dc12-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nieves JW, et al. Males have larger skeletal size and bone mass than females, despite comparable body size. J. Bone Miner. Res. 2005;20:529–535. doi: 10.1359/JBMR.041005. [DOI] [PubMed] [Google Scholar]
- 41.Napoli N, et al. Fracture risk in diabetic elderly men: The MrOS study. Diabetologia. 2014;57:2057–2065. doi: 10.1007/s00125-014-3289-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen C, et al. Trends in bone mineral density, osteoporosis, and osteopenia among U.S. adults with prediabetes, 2005–2014. Diabetes Care. 2020;43:1008. doi: 10.2337/dc19-1807. [DOI] [PubMed] [Google Scholar]
- 43.Black DM, et al. The ability of a single BMD and fracture history assessment to predict fracture over 25 years in postmenopausal women: The study of osteoporotic fractures. J. Bone Miner. Res. 2018;33:389–395. doi: 10.1002/jbmr.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harreiter J, Kautzky-Willer A. Sex and gender differences in prevention of type 2 diabetes. Front. Endocrinol. (Lausanne) 2018;9:220–220. doi: 10.3389/fendo.2018.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alva ML, Hoerger TJ, Zhang P, Gregg EW. Identifying risk for type 2 diabetes in different age cohorts: Does one size fit all? BMJ Open Diabetes Res. Care. 2017;5:e000447–e000447. doi: 10.1136/bmjdrc-2017-000447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spanakis EK, Golden SH. Race/ethnic difference in diabetes and diabetic complications. Curr. Diabetes Rep. 2013;13:814–823. doi: 10.1007/s11892-013-0421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ganz ML, et al. The association of body mass index with the risk of type 2 diabetes: A case–control study nested in an electronic health records system in the United States. Diabetol. Metab. Syndr. 2014;6:50. doi: 10.1186/1758-5996-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.U.S. Department of Health and Human Services. Bone Health and Osteoporosis: A Report of the Surgeon General (U.S. Department of Health and Human Services, Office of the Surgeon General, 2004).
- 49.Schwartz AV, et al. Thiazolidinedione use and bone loss in older diabetic adults. J. Clin. Endocrinol. Metab. 2006;91:3349–3354. doi: 10.1210/jc.2005-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sambrook P, Cooper C. Osteoporosis. Lancet. 2006;367:2010–2018. doi: 10.1016/S0140-6736(06)68891-0. [DOI] [PubMed] [Google Scholar]
- 51.Monami M, et al. Bone fractures and hypoglycemic treatment in type 2 diabetic patients: A case–control study. Diabetes Care. 2008;31:199–203. doi: 10.2337/dc07-1736. [DOI] [PubMed] [Google Scholar]
- 52.Napoli N, et al. Mechanisms of diabetes mellitus-induced bone fragility. Nat. Rev. Endocrinol. 2017;13:208–219. doi: 10.1038/nrendo.2016.153. [DOI] [PubMed] [Google Scholar]
- 53.Kawai M, Rosen CJ. PPARγ: A circadian transcription factor in adipogenesis and osteogenesis. Nat. Rev. Endocrinol. 2010;6:629–636. doi: 10.1038/nrendo.2010.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao C, Liang J, Yang Y, Yu M, Qu X. The impact of glucagon-like peptide-1 on bone metabolism and its possible mechanisms. Front. Endocrinol. (Lausanne) 2017;8:98–98. doi: 10.3389/fendo.2017.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blau JE, Taylor SI. Adverse effects of SGLT2 inhibitors on bone health. Nat. Rev. Nephrol. 2018;14:473–474. doi: 10.1038/s41581-018-0028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Monami M, Dicembrini I, Antenore A, Mannucci E. Dipeptidyl peptidase-4 inhibitors and bone fractures: A meta-analysis of randomized clinical trials. Diabetes Care. 2011;34:2474–2476. doi: 10.2337/dc11-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pritchard JM, et al. Changes in trabecular bone microarchitecture in postmenopausal women with and without type 2 diabetes: A two year longitudinal study. BMC Musculoskelet. Disord. 2013;14:114. doi: 10.1186/1471-2474-14-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pritchard JM, et al. Association of larger holes in the trabecular bone at the distal radius in postmenopausal women with type 2 diabetes mellitus compared to controls. Arthritis Care Res. (Hoboken) 2012;64:83–91. doi: 10.1002/acr.20602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ho-Pham LT, Chau PMN, Do AT, Nguyen HC, Nguyen TV. Type 2 diabetes is associated with higher trabecular bone density but lower cortical bone density: The Vietnam Osteoporosis Study. Osteoporos. Int. 2018;29:2059–2067. doi: 10.1007/s00198-018-4579-5. [DOI] [PubMed] [Google Scholar]
- 60.Conway BN, Long DM, Figaro MK, May ME. Glycemic control and fracture risk in elderly patients with diabetes. Diabetes Res. Clin. Pract. 2016;115:47–53. doi: 10.1016/j.diabres.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li CI, et al. Glycated hemoglobin level and risk of hip fracture in older people with type 2 diabetes: A competing risk analysis of Taiwan diabetes cohort study. J. Bone Miner. Res. 2015;30:1338–1346. doi: 10.1002/jbmr.2462. [DOI] [PubMed] [Google Scholar]
- 62.Schneider AL, et al. Diabetes and risk of fracture-related hospitalization: The Atherosclerosis Risk in Communities Study. Diabetes Care. 2013;36:1153–1158. doi: 10.2337/dc12-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oei L, et al. High bone mineral density and fracture risk in type 2 diabetes as skeletal complications of inadequate glucose control: The Rotterdam Study. Diabetes Care. 2013;36:1619–1628. doi: 10.2337/dc12-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Solomon DH, Patrick AR, Schousboe J, Losina E. The Potential economic benefits of improved postfracture care: A cost-effectiveness analysis of a fracture liaison service in the US Health-Care System. J. Bone Miner. Res. 2014;29:1667–1674. doi: 10.1002/jbmr.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.