Abstract
Celiac disease (CD) is an autoimmune disorder of the small intestinal mucosa in genetically susceptible subjects consuming gluten. Gluten in wheat, rye and barley is harmful for some individuals and leads to various symptoms. Research has shown that treatment with probiotics in CD patients could improve the symptoms by the gluten hydrolysis. For this purpose, different databases such as Medline, PubMed, Scopus, and Google Scholar were searched using the following keywords: Celiac disease, Wheat flour, Gluten, glutamine, Probiotic, Bifidobacterium, Lactobacillus, Enzymes, Wheat allergy, Immune system, T cells, HLA-DQ2, HLA-DQ8, Gluten-free diet, Proteolysis, α2-gliadin fragment, Gliadin, 33-mer peptide, and Zonulin. The search aimed to retrieve the articles published during 2000-2019. Today, a gluten-free diet (GFD) is the only celiac disease treatment. Biotechnological strategy based on probiotic treatment could degrade gluten. Research has shown that combination of the probiotic enzyme is more effective than single probiotic on gluten hydrolysis. The result of different studies showed that probiotic mixture has the capacity to hydrolyze a considerable concentration of the 33-mer of gliadin completely. The present study was aimed to investigate associations between the capacities of probiotics on gluten hydrolysis.
Key Words: Celiac disease, Gliadin, Gluten-free diet, Probiotics, Wheat
Introduction
Celiac disease (CD) is an intestinal malabsorption disorder, characterized by duodenal villous atrophy, caused by gluten proteins from wheat barley and rye, in people with genetic susceptibility (1,2). CD individuals may present gastrointestinal and non-gastrointestinal symptoms due to malabsorption (3,4). It is estimated that around 1% of the general population is affected by celiac disease that can affect both genders in all ages and races. Gluten-Free Diet (GFD) is the most effective treatment for these patients (5,6). Diet is the main factor in modulating the composition and function of gut microbiota, and intestinal microbiota plays a key role in determining a person's health status. In active CD patients, microbial compounds change and many studies have shown that probiotics are effective in repairing the composition of beneficial species (7). It is demonstrated that probiotics have gluten hydrolysis enzymes as an alternative or adjuvant treatment for relieving symptoms of CD and could be critical in the management of the disease (8,9). It seems that these enzymes can be used to digest and destroy gluten in patients with gluten sensitivity. The aim of this review article is to discuss characteristics of probiotics and the use of probiotics as a novel therapy for CD.
Immunotoxic components of wheat
Food allergy can be defined as an immune response for food (10). Wheat is the most common allergenic food and one of the eight most common food allergens (milk, eggs, fish, crustacean shellfish, tree nuts, peanuts, wheat, and soybean) (11). Wheat allergy is affected by immunologic responses to a range of proteins in wheat. These can be immunoglobulin E (IgE) mediated allergies, and non-IgE-mediated allergies, or a combination of both, as confirmed by other international guidelines of allergic reactions to wheat. Food allergens are usually proteins, but sometimes Heptane’s are known by allergen-specific immune cells and show specific immunologic responses (12). The main protein in wheat and some other cereals is called gluten. Gluten is obtained from cereals after the removal of the water-soluble components along with starch particles. It is the main insoluble protein from wheat, rye, and barley (Table 1) (9).
Table 1.
Protein present in cereals
| Cereals | Protein |
|---|---|
| Wheat | Gliadin |
| Barley | Hordein |
| Rye | Secalin |
| Oat | Avenin |
| Maize | Zein |
| Rice | Glutelin |
Proline and glutamine are the main gluten protein components, and enzymes in the intestine of healthy subjects can only partially digest gluten (13). In gluten-sensitive patients, the undigested protein fragments stimulate immunodominance with T-cells activation and proinflammatory interleukins. In CD patients, intestinal immune response is triggered by gluten protein and initiates the symptoms (14). One of the digestion resistant and immunodominant gluten peptides that are highly reactive to isolate celiac T-cells is α-gliadin 33-mer (15). Studies have shown that long-term use of gluten, at a rate of 10 to 50 mg per day, can damage the lining of the intestinal mucosa, by increasing the number of intrauterine lymphocytes (16,17). The U.S. Food and Drug Administration (FDA) announced that gluten free products containing no more than 20 ppm are safe for CD patients (18, 19). However, GFD is the only treatment for gluten-related disorders. Today, there are developing alternative therapies based on beneficial gut bacteria (probiotics, such as Bifidobacterium and Lactobacillus)(20). Wheat gluten in food could be removed or reduced by biotechnological strategies with probiotics that hydrolyze immunogenic gluten component.
The role of probiotics in gluten hydrolysis
Recent advances in the treatment of CD provides new and promising strategies. Therefore, other treatments have been introduced, like genetically modified gluten and gliadin, tissue transglutaminase inhibitors, zonulin inhibitors and lately probiotics (21). The FAO/WHO reports that probiotics are living microorganisms in foods or supplements that are administered in adequate amounts to improve the host’ health (21,22). Nowadays, numerous probiotics have been advised as adjuvant therapy for controlling the CD (23). A novel treatment for CD is microbial proteases which are efficient in detoxifying gluten. Clinical research and further therapeutic interventions showed that the probiotic mixture was more effective than a single strain for CD (8,24). The relative abundance of beneficial microbes by probiotics is one of the best options for CD therapy (Table 2). The most important probiotics used in foods and supplements are Bifidobacteria and Lactobacilli strains. Bifidobacterium lactis can stimulate specific antigen-specific cytotoxic T lymphocytes, natural lethal cells and macrophages (9). Probiotics can be used to destroy the epitopes before reaching the intestinal mucosa, repairing epithelial healing, or directly targeting pathological immune responses (22,25). Dipeptidyl peptidase is often used for gluten hydrolyzation. Probiotics secrete the enzyme exo-peptidase to break the end of a peptide. A report showed an association between lower intestinal dipeptidyl peptidase activity and mucosal damage in celiac patients and other malabsorption syndromes (26). One way to reduce the risk of gluten contamination of gluten-free products is to ferment the dough with lactic acid bacteria (27).
Table 2.
Mechanism of probiotics in celiac disease
| Mechanism | Possible probiotics |
|---|---|
| Enzymatic gluten degradation or pre-ingestion fermentation | VSL#3 long-lasting fermentation by Lactobacilli and fungal proteases |
| Maintenance of barrier of gastrointestinal tract | Bifidobacterium and Lactobacilli play a fundamental role |
A combination of probiotics with specific properties appears to be effective in gliadin hydrolysis than single strains (24).
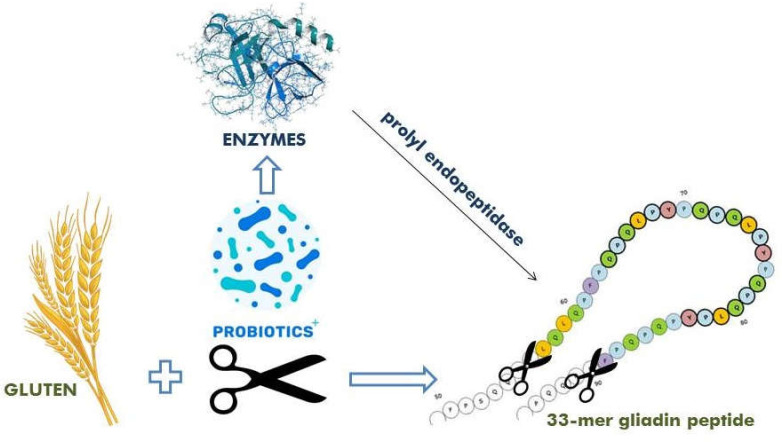
Probiotics can impress the CD by three mechanisms. The first is to digest gluten proteins into small unsafe polypeptides, removing or reducing the CD pathogenesis, hence stopping immune reactions. The second is to protect the intestinal barrier by barring the availability of immunogenic polypeptides to lamina propria. The third and the most interesting is the critical role of probiotics in the intestinal microbiota hemostasis and the regulation of the innate and adaptive immune system (20). The possible mechanism for using enzyme therapy for celiac patients is that degraded gluten proteins in the small intestine contain gliadin peptides, which are rich in proline, glutamine, and 33 mer, which can be broken down by enzymes in the gastrointestinal tract. Therefore, this can be considered as a substitute to GFD (15). Endopeptidase enzyme in probiotic could separate and hydrolyze the internal bonds of gliadin. This enzyme can digest gluten peptides into small and safe molecules, which are well tolerated in celiac patients (28). 33-mer gliadin peptide from α2 gliadin is the main immunogenic component for CD and other gluten-related diseases (15). The α-gliadin 33-mer is a digestible gluten-resistant peptide, and a structural change of 33 mer could be the first unknown cause of the disease (Fig. 1). The enzyme activity of probiotics can break down peptide bonds including proline.
Figure 1.
Hydrolysis of 33-mer peptide with exposure to probiotics and their enzymes such as prolyl-endopeptidase
Previous research about the use of probiotics for gliadin hydrolysis
In a recent randomized controlled trial in subjects with non-celiac gluten sensitivity, 37 adult patients were enrolled in the study, 12 were treated with enzyme combination and 25 with placebo. The enzyme mixture was consumed 3 times a day for a month. This study showed a statistically significant decrease in the level of IgA anti-gliadin antibodies and inflammation compared to placebo group. Only one CD patient was positive for gluten IgG after enzymatic treatment (29). Recently, food technicians have used a gluten hydrolysis strategy with a residual concentration of less than 10 ppm, which is a diet with lactobacilli and fungal proteases (29,30). In another study, the effect of bacteria on the destruction of gliadin-induced cells by probiotic bacteria (fermented and Bifidobacterium lactis) in intestinal epithelial cell culture was determined. They suggested that Bifidobacterium lactis was able to protect the epithelial cell permeability caused by gliadin. Furthermore, both probiotics were able to protect against cell ruffling and alterations in tight junctions. Also, probiotics without gliadin as a control did not show significant changes to the intestinal epithelial cells. Probiotics can improve the damage of intestinal epithelial cells caused by gluten contaminated foods and may even accelerate mucosal healing after the initiation of a GFD (31). Studies conducted in 2015 showed that the gliadin peptides may reduce by the activity of proteinase and peptidase in the gut microbiota, which in turn affect their toxicity. (26,32). Several studies demonstrated that 144 strains of 35 bacterial species can hydrolyze gluten. Most of these strains were from Firmicutes and Actinobacteria phyla that can improve CD symptoms. A total of thirty-one strains of gluten-degrading bacteria were isolated from human small intestine, 27 of which showed peptidolytic activity compared to 33 mer peptides (28,33). Researchers have developed a new method for reducing gluten allergy by making sourdough with probiotics such as Lactobacillus to cleave proline and gliadin peptides, including the 33-mr peptides (15, 33). Most of these cases are lactobacilli which can reduce the immunogenicity of the 33-mer peptide and provide a protective effect for lactobacillus in gluten hydrolysis (20). Lactobacilli need large amounts of amino acids nitrogen for suppling metabolic energy and growth. The mixtures of lactobacilli and Bifidobacteria have a complex proteolytic and lipolysis effect that can be involved in the breakdown of gluten (gliadin) and its peptides. These are used as a probiotic supplement for the treatment of celiac disease (20,34). A few other probiotic compounds are used to treat CD, including Florisia (Lactobacillus brevis, Lactobacillus plantarum, Lactobacillus salivarius, and subsp. Salicinius), Oxadrop (Lactobacillus acidophilus, B. infantis, L. brevis, and S. thermophilus), and Yovis (B. Infantis, B. breve, L. acidophilus, B. longum, L. plantarum, L. casei, L. delbrueckii subsp. Bulgaricus, Thermophilus, Streptococcus salivarius subsp , Enterococcus faecium and the most beneficial probiotic is the mixture of eight strains (VSL#3), Bifidobacterium breve, Bifidobacterium infantis, acidophilus, Lactobacillus plantarum, Lactobacillus casei, Lactobacillus delbrueckii sub sp. bulgaricus, Streptococcus thermophilus and Bifidobacterium longum, which decreased wheat sensitivity. The results showed that VSL#3 c is more effective on gliadin degradation than a single strain alone, and had a beneficial effect on the treatment of CD (24). A research by Shan et al. showed that unique 33 amino acid peptides out of 266 amino acid of α2 gliadin are resistant to hydrolyze in the gastrointestinal tract. It showed that an oral bacterial peptidase could be used to detoxify the predominantly immunosuppressive gliadin epitopes (35). A study in 2008 used the mixture of bacteria and barley-derived proteases-PEP and endoprotease B-isoform, respectively and showed that oral consumption of this mixture by celiac patients can change the gluten epitopes to non-toxic parts; therefore, they proposed that these protease enzymes may be a useful treatment and might allow CD patients to take in modest amounts of gluten in their diets (36). An earlier study showed that epithelium with exposure to probiotics in gastrointestinal tract can hydrolyze gluten, which is the one way to maintain the health of CD patients. The lactobacilli strain hydrolyzed gluten under the simulated gastrointestinal condition and the gluten content was lower than 10 ppm after six hours (8). Previous studies have shown significant alteration of intestinal permeability in Caco-2 cells when the Lactobacillus rhamnosus was added to gliadin peptides, all demonstrating the inhibitory effect on pathogenic bacteria through interaction with lymphatic tissue and villi (37).
Bifidobacterium strains such as Bifidobacterium longum and Bifidobacterium bifidum reduce the toxicity and inflammatory factors of gluten peptides. Also, they have potentials to improve CD symptoms (24,38). Various studies have shown that the intervention of probiotics may offer new treatment including (24), gluten vaccination (39), gluten tolerance and immunomodulation (40), tissue transglutaminase inhibitors (41,42), HLA-DQ2 or HLA-DQ8 blockers (43), genetically modified gluten (44,45), and glutenase supplement diet (16). These possible treatments are available for CD management but require further research (9). A new and safe treatment of celiac disease is cleaving the gliadin into small nontoxic peptides before reaching the intestinal mucosa, such as the use of oral supplements with prolyl oligopeptidase (46). A study evaluated the effect of aspergillopepsin from Aspergillus niger and dipeptidyl peptidase from Aspergillus oryzae showed that gluten hydrolyzing simulated intestinal conditions. The experiments demonstrated that the use of single peptidase alone (neither aspergillopepsin nor dipeptidyl peptidase) cannot eliminate gluten peptides and the use of enzyme combinatin can significantly reduce gluten levels (47). In a study conducted in 2015, Aspergillus niger prolylendoprotease enzyme enhanced gluten degradation in the stomach of healthy subjects (48). Many bacterial and fungal enzyme supplements can break down gluten and prolamins with their endopeptidases or proteinases (49). Nowadays, the effective treatment available for CD individuals is a forceful lifelong GFD (45). Although GFD increases the absorption of vitamins and nutrients, a gluten-free diet can prevent severe autoimmune diseases caused by celiac disease. Unfortunately, even in strict diet plans, there may be a small amount of gluten in the GFD and many people inadvertently eat gluten-containing foods (36). Also, CD patients have issues regarding availability, quality and variety of gluten-free products’ where these products are generally more expensive than their counterparts (50). Deep understanding of biochemical and molecular mechanism through probiotics that affect CD will help formulate probiotics and their optimum operational conditions such as time, temperature and concentration which enhance gluten hydrolysis create a novel therapeutic model to change the course of CD (20).
Conclusion
Celiac disease is a well-known autoimmune disorder of the last decades. Nowadays, a lifelong strict gluten-free diet (GF) is an effective treatment for CD and scientists are looking for beneficial treatments to improve the quality of life in these patients. Furthermore, in the last two decades, probiotics have been increasingly consumed for their beneficial effects. It is proposed that a combination of probiotics can completely hydrolyze the toxic and allergenic components of gluten by their enzymes, including 33 mer degradation of gliadin, and the enzymes can revolutionize the gluten-free manufacturing industry. In the future, GFD alternatives, which are the only effective therapies available, can be used as enzyme supplements as a new treatment strategy for CD instead of using a gluten-free diet.
Conflict of interests
The authors declare that they have no conflict of interest.
References
- 1.Rostami K, Marsh MN, Johnson MW, Mohaghegh H, Heal C, Holmes G, et al. ROC-king onwards: intraepithelial lymphocyte counts, distribution & role in coeliac disease mucosal interpretation. Gut. 2017;66:2080–2086. doi: 10.1136/gutjnl-2017-314297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamboj AK, Oxentenko AS. Clinical and Histologic Mimickers of Celiac Disease. Clin Transl Gastroenterol. 2017;8:e114. doi: 10.1038/ctg.2017.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ehsani-Ardakani MJ, Rostami-Nejad M, Villanacci V, Volta U, Manenti S, Caio G, et al. Gastrointestinal and Non-Gastrointestinal Presentation in Patients with Celiac Disease. Arch Iran Med. 2013;16:78–82. [PubMed] [Google Scholar]
- 4.Mustalahti K, Catassi C, Reunanen A, Fabiani E, Heier M, McMillan S, et al. The prevalence of celiac disease in Europe: results of a centralized, international mass screening project. Ann Med. 2010;42:587–95. doi: 10.3109/07853890.2010.505931. [DOI] [PubMed] [Google Scholar]
- 5.Gujral N, Freeman H, Thomson A. Celiac disease: Prevalence, diagnosis, pathogenesis and treatment. World J Gastroenterol. 2012;18:6036–59. doi: 10.3748/wjg.v18.i42.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clemente E, Efthymakis K, Carletti E, Capone V, Sperduti S, Bologna G, et al. An explorative study identifies miRNA signatures for the diagnosis of non-celiac wheat sensitivity. PLoS One. 2019;14:e0226478. doi: 10.1371/journal.pone.0226478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bascunan KA, Araya M, Roncoroni L, Doneda L, Elli L. Dietary Gluten as a Conditioning Factor of the Gut Microbiota in Celiac Disease. Am Soc for Nutr. 2019:1–15. doi: 10.1093/advances/nmz080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francavilla R, De Angelis M, Giuseppe Rizzello C, Cavallo N, Dal Bello F, Gobbettid M. Selected Probiotic Lactobacilli Have the Capacity to Hydrolyze Gluten Peptides during Simulated Gastrointestinal Digestion. Appl Environ Microbiol. 2017;83:1–12. doi: 10.1128/AEM.00376-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar J, Kumar M, Pandey R, Chauhan N. Physiopathology and Management of Gluten-Induced Celiac Disease. J Food Sci. 2017;82:270–7. doi: 10.1111/1750-3841.13612. [DOI] [PubMed] [Google Scholar]
- 10.Boyce JA, Assa’ad A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the diagnosis and management of food allergy in the United States: report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126:S1–58. doi: 10.1016/j.jaci.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hofer G, Wieser S, Bogdos MK, Gattinger P, Nakamura R, Ebisawa M, et al. Three-dimensional structure of the wheat β-amylase Tri a 17, a clinically relevant food allergen. Allergy. 2019;74:1009–13. doi: 10.1111/all.13696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cianferoni A. Wheat allergy: diagnosis and management. J Asthma Allergy. 2016;9:13–25. doi: 10.2147/JAA.S81550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helmerhorst EJ, Zamakhchari M, Schuppan D, Oppenheim FG. Discovery of a novel and rich source of gluten–degrading microbial enzymes in the oral cavity. PLoS One. 2010;5:e13264. doi: 10.1371/journal.pone.0013264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meresse B, Malamut G, Cerf-Bensussan N. Celiac Disease: An Immunological Jigsaw. Immunity. 2012;36:907–19. doi: 10.1016/j.immuni.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Schalk K, Lang CH, Wieser H, Koehler P, Anne Scherf K. Quantitation of the immunodominant 33-mer peptide from α-gliadin in wheat flours by liquid chromatography tandem mass spectrometry. Sci Rep. 2017;7:45092. doi: 10.1038/srep45092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caputo I, Lepretti M, Martucciello S, Esposito C. Enzymatic Strategies to Detoxify Gluten: Implications for Celiac Disease. Enzyme Res. 2010:1–9. doi: 10.4061/2010/174354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Catassi C, Fabiani E, Iacono G, D'Agate C, Francavilla R, Biagi F, et al. Am J Clin Nutr. 2007;85:160–6. doi: 10.1093/ajcn/85.1.160. [DOI] [PubMed] [Google Scholar]
- 18.Gilissen LJWJ, Van der Meer IM, Smulders MJM. Why Oats Are Safe and Healthy for Celiac Disease Patients. Med Sci. 2016;4:1–9. doi: 10.3390/medsci4040021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lerner BA, Phan Vo LT, Yates S, Rundle AG, Green PHR, Lebwohl B. Detection of Gluten in Gluten-Free Labeled Restaurant Food: Analysis of Crowd-Sourced Data. Am J Gastroenterol. 2019;114:792–7. doi: 10.14309/ajg.0000000000000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chibbar R, Dieleman LA. The Gut Microbiota in Celiac Disease and probiotics. Nutrients. 2019;11:2375. doi: 10.3390/nu11102375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Sousa Morae LF, Grzeskowiak LM, de Sales Teixeira TF, Gouveia Peluzio, Mdo C. Intestinal microbiota and probiotics in celiac disease. Clin Microbiol Rev. 2014;27:482–9. doi: 10.1128/CMR.00106-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Food and Agriculture Organization of the United Nations, World Health Organization. World Health Organization. 2002. Guidelines for the evaluation of probiotics in food. Geneva, Switzerland: World Health Organization; 2002. Accessed 27 April 2014. [Google Scholar]
- 23.Ghouri YA, Richards DM, Rahimi EF, Krill JT, Jelinek KA, DuPont AW. Systematic review of randomized controlled trials of probiotics, prebiotics, and synbiotics in inflammatory bowel disease. Clin Exp Gastroenterol. 2014;7:473–87. doi: 10.2147/CEG.S27530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Angelis M, Rizzelo CG, Fasano A, Clemente MG, De Simone C, Silano M, et al. VSL#3 probiotic preparation has the capacity to hydrolyze gliadin polypeptides responsible for celiac sprue. Biochim Biophys Acta. 2006;1762:80–93. doi: 10.1016/j.bbadis.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Lukic J, Chen V, Strahinic I, Begovic J, Lev-Tov H, Davis SC, et al. Probiotics or pro-healers: the role of beneficial bacteria in tissue repair. Wound Repair Regen. 2017;25:912–22. doi: 10.1111/wrr.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ash, M. The Golden Age of Gluten Free Living: New Findings, Tests and Treatments. Clinical Education; 2015. [Google Scholar]
- 27.Siddiqi RA, Singh Sogi D, Sehajpa PK. Effect of short-term sourdough fermentation on wheat protein. Cogent Food Agric. 2016;2:1132983–10. [Google Scholar]
- 28.Moreno-Amador ML, Arévalo-Rodríguez M, Durán EM, Martínez-Reyes JC, Sousa-Martín C. A new microbial gluten-degrading prolyl endopeptidase: Potential application in celiac disease to reduce gluten immunogenic peptides. PLoS One. 2019;14:e0218346. doi: 10.1371/journal.pone.0218346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Volta U, Tovoli F, Cicola R, Parisi C, Fabbri A, Piscaglia M, et al. Serological tests in gluten sensitivity (nonceliac gluten intolerance) J Clin Gastroenterol. 2012;46:680–5. doi: 10.1097/MCG.0b013e3182372541. [DOI] [PubMed] [Google Scholar]
- 30.Greco L, Gobbetti M, Auricchio R, Di Mase R, Paparo F, Di Cagno R, et al. Safety for celiac disease of baked goods made of wheat flour hydrolyzed during food processing. Clin Gastroenterol Hepatol. 2011;9:24–9. doi: 10.1016/j.cgh.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 31.Sahebekhtiari N, Nochi Z, Eslampour MA, Dabiri H, Bolfion M, Taherikalani M, et al. Characterization of Staphylococcus aureus strains isolated from raw milk of bovine subclinical mastitis in Tehran and Mashhad. Acta Microbiol Immunol Hung. 2011;58:113–21. doi: 10.1556/AMicr.58.2011.2.4. [DOI] [PubMed] [Google Scholar]
- 32.Socha P, Mickowska B, Urminska D, Kacmarova K. The use of different proteases to hydrolyze gliadins. J Microbiol Biotech Food Sci. 2015;4:101–4. [Google Scholar]
- 33.Herran AR, Perez-Andres J, Caminero A, Nistal E, Vivas S, de Morales RJM, et al. Gluten-degrading bacteria are present in human small intestine of healthy volunteers and celiac patients. Res Microbiol. 2017;168:673–84. doi: 10.1016/j.resmic.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 34.Olivares M, Laparra M, Sanz Y. Influence of Bifidobacterium longum CECT 7347 and gliadin peptides on intestinal epithelial cell proteome. J Agric Food Chem. 2012;59:7666–71. doi: 10.1021/jf201212m. [DOI] [PubMed] [Google Scholar]
- 35.Shan L, Mathews II, Khosla C. Structural and mechanistic analysis of two prolyl endopeptidases: role of interdomain dynamics in catalysis and specificity. Proc Natl Acad Sci USA. 2005;102:3599–604. doi: 10.1073/pnas.0408286102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watson P, Ding A, McMillan SA, et al. Implications of enzymatic detoxification of food gluten in coeliac disease. Gastroenterology. 2008;134:A213. [Google Scholar]
- 37.Orlando A, Linsalata M, Notarnicola M, Tutino V, Russo F. Lactobacillus GG restoration of the gliadin induced epithelial barrier disruption: the role of cellular polyamines. BMC Microbiol. 2014;14:1–12. doi: 10.1186/1471-2180-14-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cristofori F, Francavilla R, Capobianco D, Dargenio VN, Filardo S, Mastromarino P. Bacterial-Based Strategies to Hydrolyze Gluten Peptides and Protect Intestinal Mucosa. Front Immunol. 2020;11:567801. doi: 10.3389/fimmu.2020.567801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keijzer C, van der Zee R, van Eden W, Broere F. Treg inducing adjuvants for therapeutic vaccination against chronic inflammatory diseases. Front Immunol. 2013;4:1–10. doi: 10.3389/fimmu.2013.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Veeraraghavan G, Leffler DA, Kaswala DH, Mukherjee R. Celiac disease update: new therapies. Expert Rev Gastroenterol Hepato. 2015;19:913–27. doi: 10.1586/17474124.2015.1033399. [DOI] [PubMed] [Google Scholar]
- 41.Sollid LM, Jabri B. Celiac disease and transglutaminase 2: a model for post–translational modification of antigens and HLA association in the pathogenesis of autoimmune disorders. Curr Opin Immunol. 2011;23:732–8. doi: 10.1016/j.coi.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Makharia GK. Current and emerging therapy for celiac disease. Front Med. 2014;1:1–14. doi: 10.3389/fmed.2014.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim CY, Quarsten H, Bergseng E, Bergseng E, Khosla C, Sollid LM. Structural basis for HLA–DQ2–mediated presentation of gluten epitopes in celiac disease. Proc Natl Acad Sci USA. 2004;101:4175–9. doi: 10.1073/pnas.0306885101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schuppan D, Junker Y, Barisani D. Celiac disease: from pathogenesis to novel therapies. Gastroenterol. 2009;137:1912–33. doi: 10.1053/j.gastro.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 45.Stoven S, Murray JA, Marietta E. Celiac Disease: Advances in Treatment via Gluten Modification. Clin Gastroenterol Hepatol. 2012;10:859–62. doi: 10.1016/j.cgh.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stepniak D, Spaenij-Dekking L, Mitea C, Moester M, de Ru A, Baak-Pablo R, et al. Highly efficient gluten degradation with a newly identified prolyl endoprotease: implications for celiac disease. Am J Physiol Gastrointest Liver Physiol. 2006:1–35. doi: 10.1152/ajpgi.00034.2006. [DOI] [PubMed] [Google Scholar]
- 47.Ehren J, Morón B, Martin E, Bethune MT, Gray GM, Khosla C. A food-grade enzyme preparation with modest gluten detoxification properties. PLoS One. 2009;21 doi: 10.1371/journal.pone.0006313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Montserrat V, Bruins MJ, Edens L, Koning F. Influence of dietary components on Aspergillus niger prolyl endoprotease mediated gluten degradation. Food Chem. 2015;1:440–5. doi: 10.1016/j.foodchem.2014.11.053. [DOI] [PubMed] [Google Scholar]
- 49.Kaukinen K. Lindfors K. Novel Treatments for Celiac Disease: Glutenases and Beyond. Dig Dis. 2015:277–81. doi: 10.1159/000369536. [DOI] [PubMed] [Google Scholar]
- 50.Duar RM, Clark KJ, Patil PB, Hernández C, Brüning S, Burkey TE, et al. Identification and characterization of intestinal lactobacilli strains capable of degrading immunotoxic peptides present in gluten. J Appl Microbiol. 2015;118:515–27. doi: 10.1111/jam.12687. [DOI] [PubMed] [Google Scholar]