Abstract
The adrenal gland is a source of sex steroid precursors, and its activity is particularly relevant during fetal development and adrenarche. Following puberty, the synthesis of androgens by the adrenal gland has been considered of little physiologic importance. Dehydroepiandrosterone (DHEA) and its sulfate, DHEAS, are the major adrenal androgen precursors, but they are biologically inactive. The second most abundant unconjugated androgen produced by the human adrenals is 11(β-hydroxyandrostenedione (11OHA4). 11-Ketotestosterone, a downstream metabolite of 11OHA4 (which is mostly produced in peripheral tissues), and its 5α-reduced product, 11-ketodihydrotestosterone, are bioactive androgens, with potencies equivalent to those of testosterone and dihydrotestosterone. These adrenal-derived androgens all share an oxygen atom on carbon 11, so we have collectively termed them 11-oxyandrogens. Over the past decade, these androgens have emerged as major components of several disorders of androgen excess, such as congenital adrenal hyperplasia, premature adrenarche and polycystic ovary syndrome, as well as in androgen-dependent tumours, such as castration-resistant prostate cancer. Moreover, in contrast to the more extensively studied, traditional androgens, circulating concentrations of 11-oxyandrogens do not demonstrate an age-dependent decline. This Review focuses on the rapidly expanding knowledge regarding the implications of 11-oxyandrogens in human physiology and disease.
The adrenal gland is a recognized source of sex steroid precursors, largely dehydroepiandrosterone (DHEA) and its sulfate, DHEAS. These steroids are particularly relevant during key developmental stages, including during fetal development and adrenarche. The adrenal contribution to the synthesis of androstenedione and testosterone, which are also produced by the gonads, has been primarily recognized in the context of several disorders of androgen excess, including congenital adrenal hyperplasia (CAH)1, premature adrenarche2,3, polycystic ovary syndrome (PCOS)4 and androgen-producing or androgen-dependent tumours5,6. The adrenals are also the principal source of a set of 11-oxygenated metabolites of androstenedione and testosterone, 11β-hydroxyandrostenedione (11OHA4) and 11β-hydroxytestosterone (11OHT). These metabolites are then converted, mostly in peripheral tissues, to 11-ketoandrostenedione (11KA4) and 11-ketotestosterone (11KT)7,8. Collectively, these 19-carbon (C19) steroids that all have an oxygen atom on carbon 11 (FIG. 1) are known as 11-oxyandrogens. Although the existence of 11-oxyandrogens has been acknowledged for several decades, exploration of their implications in human physiology and disease has been minimal. Rapidly expanding evidence suggests that 11-oxyandrogens are important hormones in human physiology, from adrenarche through to old age, as well as in several disorders of androgen excess. This Review discusses the physiology and pathology of adrenal C19 steroids, emphasizing the previously under-recognized roles of 11-oxyandrogens.
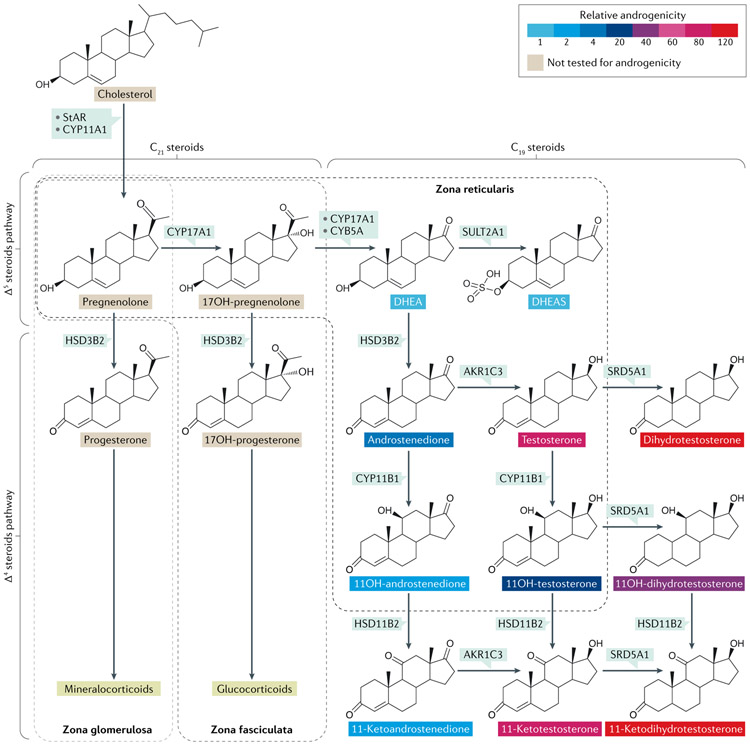
Fig. 1 ∣. Adrenal androgen synthesis.
The adrenal cortex is structured into three concentric layers, and each layer produces specific steroids: mineralocorticoids are synthesized in the outermost layer (zona glomerulosa); glucocorticoids are produced in the middle layer (zona fasciculata); and androgens are produced in the inner layer (zona reticularis). The enzyme 3β-hydroxysteroid dehydrogenase/Δ5/4-isomerase type 2 (HSD3B2) catalyses oxidation of the hydroxyl group on carbon 3 of steroids to a keto group and, simultaneously, isomerization of the double bond from the B ring (Δ5 steroids) to the A ring (Δ4 steroids). Androgens share a 19-carbon structure (C19 steroids), whereas mineralocorticoids and glucocorticoids have a 21-carbon structure (C21 steroids). The most abundant C19 steroids produced by the adrenal glands are dehydroepiandrosterone (DHEA) and its sulfate (DHEAS). The adrenal glands also produce a set of unique androgens, synthesized via the adrenal-specific enzyme, cytochrome P450 11β-hydroxylase (CYP11B1). These steroids are called 11-oxyandrogens, and 11-hydroxyandrostenedione is the most abundant. The relative androgenic potency of tested androgens is depicted based on data from Rege et al.85 and Storbeck et al.80. Testosterone and 11-ketotestosterone are potent androgens. Steroid 5α-reductases (SRD5A) convert testosterone and 11-ketotestosterone to dihydrotestosterone and 11-ketodihydrotestosterone, respectively. Dihydrotestosterone and 11-ketodihydrotestosterone are the most potent androgens. AKR1C3, aldo-keto reductase family 1 member C3 (also known as 17β-hydroxysteroid dehydrogenase type 5); CYB5A, cytochrome b5 type A; CYP11A1, cytochrome P450 cholesterol side chain cleavage; CYP17A1, cytochrome P450 17A1; OH, hydroxy; StAR, steroidogenic acute regulatory protein; SULT2A1, steroid sulfotransferase type 2A1.
Biosynthesis of adrenal androgens
Adrenal synthesis of androgens.
The adrenal cortex is the origin for three major types of steroids: mineralocorticoids, glucocorticoids and androgens. Adrenal steroid production is highly dynamic and tightly controlled by adrenocorticotropic hormone (ACTH), angiotensin II and other regulators. All adrenal steroids are synthesized de novo and immediately released, with no stored reserves. To ensure a maximum synthetic efficiency in response to regulatory signals, the adrenal cortex is structured into three different layers (also called zones), each with distinct enzymatic machineries (FIG. 1). Most adrenal androgen synthesis occurs in the innermost adrenal cortical zone, the zona reticularis.
Steroidogenesis begins with the transfer of cholesterol, the common precursor for all steroids, from the cytoplasm to the outer mitochondrial membrane, via incompletely understood mechanisms9. Tropic stimulating hormones act via second messengers to induce the acute expression and activation of the steroidogenic acute regulatory protein (StAR), which orchestrates the transfer of cholesterol to the inner mitochondrial membrane, where it is then converted to pregnenolone by the cholesterol side chain cleavage enzyme (CYP11A1)10,11. StAR’s facilitation of the movement of cholesterol to the inner mitochondrial membrane is considered the rate-limiting step in all steroidogenic tissues except the placenta9. The 37-kDa human StAR precursor protein12 is cleaved to a 30-kDa form13,14 upon entry into the mitochondria. StAR contains a StAR-related lipid transfer domain that forms a β-sheet rich sterol-binding pocket, which can host a single molecule of cholesterol15. StAR is thought to repeatedly shuttle cholesterol across the mitochondrial membrane, until it is inactivated by proteolytic cleavage. StAR is also expressed in the gonads and in many tissues that do not produce steroids (such as the nervous system or granulocytes), which suggests that it might have other cellular functions16.
Once StAR delivers cholesterol within the mitochondrion, CYP11A1 converts cholesterol to pregnenolone via three serial reactions: 20-hydroxylation, 22-hydroxylation and scission of the C20–C22 bond9,17-19. Both StAR and CYP11A1 are expressed in all adrenal cortical zones, and zone-specific repertoires of downstream enzymes determine the compartmentalized steroidogenic flux within the adrenal cortex. ACTH is the primary regulator of CYP11A1 expression20 and the synthesis of C19 steroids in the zona reticularis21.
The conversion of pregnenolone to DHEA (the initial C19 steroid) occurs in two steps, which are both mediated by cytochrome P450 17A1 (CYP17A1; also known as 17α-hydroxylase or 17,20-lyase)22. CYP17A1 first hydroxylates pregnenolone in position 17, and subsequently breaks the C17–C20 bond of 17-hydroxypregnenolone (FIG. 1). From a single gene, a 2.1-kb human CYP17A1 mRNA is translated into a 57-kDa protein common to the adrenals and gonads23,24. A type II P450 enzyme, CYP17A1, is located in the endoplasmic reticulum, where it receives electrons from NADPH via a flavoprotein called P450 oxidoreductase (POR)25. Human CYP17A1 catalyses the 17α-hydroxylation of both pregnenolone and progesterone with similar efficiencies; conversely, for the 17,20-lyase reaction, CYP17A1 yields approximately 50-fold more DHEA from 17-hydroxypregnenolone than androstenedione does from 17-hydroxyprogesterone (17OHP4)26,27. The 17,20-lyase reaction is further enhanced by the cofactor cytochrome b5 type A (CYB5A) in both the Δ4 and Δ5 pathways28-31 (FIG. 1). The mechanisms by which CYB5A modulates the 17,20-lyase reaction are incompletely understood, but CYB5A proportionately increases the coupling of NADPH consumption to product formation in vitro29,32.
CYP17A1 is essential for the synthesis of both cortisol and C19 steroids and is abundantly expressed in the zonae fasciculata and reticularis. ACTH is the primary regulator of CYP17A1 expression in both zones33,34. Growth factors, including insulin-like growth factor 1 (IGF1) and IGF2, also enhance adrenal CYP17A1 expression, whereas transforming growth factor-β inhibits CYP17A1 expression35-37. During adrenarche, the thin zona reticularis starts to expand, CYB5A expression increases and the augmented 17,20-lyase activity of CYP17A1 promotes the synthesis of C19 steroids in this innermost cortical zone38-40 (FIG. 2).
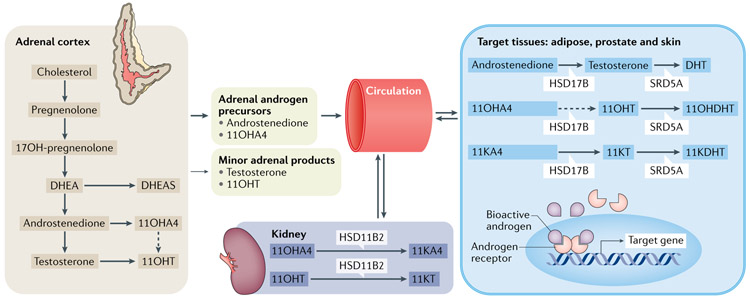
Fig. 2 ∣. Synthesis, circulation and metabolism of 11-oxyandrogens.
19-carbon (C19) steroids are synthesized in the adrenal cortex, including androgen precursors, predominantly dehydroepiandrosterone (DHEA), its sulfate (DHEAS), androstenedione and 11β-hydroxyandrostenedione (11OHA4), and smaller quantities of bioactive androgens, such as testosterone and 11β-hydroxytestosterone (11OHT).The kidney expresses 11β-hydroxysteroid dehydrogenase type 2 (HSD11B2), which converts the adrenal-derived 11OHA4 and 11OHT into 11-ketoandrostenedione (11KA4) and 11-ketotestosterone (11KT), respectively. Other target tissues, such as the adipose tissue, prostate, hair follicles and genital skin, are able to convert the adrenal androgen precursors androstenedione, 11OHA4 and 11KA4 into bioactive androgens via 17β-hydroxysteroid dehydrogenase (HSD17B) enzymes and steroid 5α-reductases (SRD5A). The bioactive androgens bind the androgen receptors, located in the cytoplasm, and the hormone-receptor complex translocates to the nucleus, where it activates several androgen-responsive genes. 11KDHT, 11-ketodihydrotestosterone; 11OHDHT, 11β-hydroxydihydrotestosterone; DHT, dihydrotestosterone; OH, hydroxy.
The nascent DHEA can be either diverted towards downstream androgens or sulfonated to DHEAS. The sulfotransferase SULT2A1 catalyses the sulfonation of the 3β-hydroxyl group of DHEA using the co-substrate 3′-phosphoadenosine 5′-phosphosulfate (PAPS)41. During fetal development, SULT2A1 utilizes DHEA and other Δ5 steroids (including pregnenolone, 17-hydroxypregnenolone and androst-5-ene-3β,17β-diol (Adiol)) as substrates. The DHEAS generated in the fetal adrenal gland is utilized in the fetal liver and placenta as the substrate for oestriol synthesis42. The expression of SULT2A1 and CYB5A increases in the adrenal gland during adrenarche39,43. As the adrenal gland begins to produce C19 steroids, SULT2A1 preferentially utilizes DHEA as a substrate. As DHEA is a precursor for androstenedione and testosterone, the conjugation of DHEA acts as a kind of trap, preventing excessive adrenal androgen production. Defects in DHEA sulfonation allow abundant production of DHEA, which is subsequently diverted to the active androgen testosterone, after it is first converted to androstenedione, by 3β-hydroxysteroid dehydrogenase/Δ5/4-isomerase type 2 (HSD3B2) in the adrenal glands or HSD3B1 in peripheral tissues44. Although defects in SULT2A1 itself have not been described, defects in the enzyme that synthesizes PAPS, the mandatory sulfate donor, compromise DHEA sulfonation44-46. In humans, PAPS synthase (PAPSS) has a ubiquitously expressed isoform, PAPSS1, and the PAPSS2 isoform is expressed in cartilage, the adrenal glands and the liver, with the latter two organs being the major sites of DHEA sulfonation46. Deficiency of PAPSS2 prevents DHEA sulfonation and leads to adrenal androgen excess, as reported in a girl with premature pubarche, advanced bone age, acne, hirsutism and secondary amenorrhoea44.
In addition to DHEA, SULT2A1 can also efficiently sulfonate pregnenolone, 17-hydroxypregenolone and Adiol; ACTH acutely stimulates the formation of pregnenolone sulfate and 17-hydroxypregenolone sulfate47. Although the intra-adrenal expression of SULT2A1 is much higher in the zona reticularis than in the zona fasciculata48, ACTH acutely stimulates the production of pregnenolone sulfate and 17-hydroxypregenolone sulfate within the zona reticularis and in the inner zona fasciculata. By contrast, the synthesis of the androgen precursors DHEAS and androstenediol sulfate occurs exclusively in the zona reticularis49. Owing to its long half-life (7–10h)50,51, which reduces its diurnal fluctuations52, measuring the levels of DHEAS has been used to assess the chronic integrity of the hypothalamic–pituitary–adrenal axis53, such as in excluding secondary adrenal insufficiency54,55 and screening for ACTH-independent hypercortisolism56,57, which are both associated with low levels of DHEAS. The use of DHEAS as a measure of integrated ACTH synthesis is limited in certain scenarios, for example in pubertal children who could be undergoing ACTH-independent adrenarche58,59 or in patients with adrenal cortical carcinomas, which can co-produce cortisol and DHEAS5.
Another key enzyme for the synthesis of all bioactive steroids, including androgens, is HSD3B2. HSD3B2 catalyses oxidation of the hydroxyl group on carbon 3 of the steroids to a keto group and, simultaneously, isomerization of the double bond from the B ring (Δ5 steroids) to the A ring (Δ4 steroids)60-62 (FIG. 1). Pregnenolone, 17-hydroxypregnenolone, DHEA and Adiol are all substrates for HSD3B2 (REFS63,64), which are irreversibly converted to progesterone, 17OHP4, androstenedione and testosterone, respectively. Of the two human isoforms65, HSD3B2 is the major isoform expressed in the adrenals and gonads66,67. Within the adrenal gland, HSD3B2 expression is abundant in the zonae glomerulosa and fasciculata, but negligible in the zona reticularis48. In contrast to the zonae glomerulosa and fasciculata, the zona reticularis expresses considerably more SULT2A1 than HSD3B2, favouring the synthesis of DHEAS rather than androstenedione and testosterone. The efficient synthesis of androgens from pregnenolone requires co-expression of CYB5A (to produce DHEA) and HSD3B2 (to convert DHEA to androstenedione); this co-expression is present in the testicular Leydig cells and the ovarian theca cells. Conversely, within the adrenal gland, the expression of HSD3B2 and CYB5A is segregated: HSD3B2 within the zonae glomerulosa and fasciculata, and CYB5A in the zona reticularis. It is believed that the synthesis of androstenedione and downstream Δ4 C19 steroids occurs at the interface between the zona fasciculata and the zona reticularis, where cells expressing HSD3B2 and CYB5A, respectively, are in close contact68. The areas of HSD3B2 and CYB5A proximity have been reported to be largest in people aged 13–20 years68.
Androstenedione is converted to testosterone by 17β-hydroxysteroid dehydrogenase (HSD17B) enzymes. In the testis, 17βHSD type 3 (17βHSD3) catalyses this reaction69. The adrenal gland expresses a much less efficient enzyme in the aldo-keto reductase (AKR) family, AKR1C3 (also known as HSD17B type 5)70, which is also found in non-steroidogenic tissues71-73. An increase in AKR1C3 transcription contributes to hyperandrogenism in PCOS74.
The canonical adrenal pathways to aldosterone, cortisol and DHEAS omit the 11-oxyandrogen pathway. Androstenedione and testosterone are both substrates for the adrenal enzyme cytochrome P450 11β-hydroxylase (CYP11B1), yielding 11OHA4 and 11OHT, respectively. CYP11B1, which also catalyses the last step in cortisol synthesis under the regulation of ACTH75, is abundantly expressed in the zonae fasciculata and reticularis of the adrenal gland48; however, in humans, CYP11B1 expression in the gonads is negligable76. Little or no 11OHA4 derives from cortisol, via CYP17A1 (REF.77). Primarily in the periphery and, to a lesser extent within the adrenal glands, 11OHA4 and 11OHT can be oxidized to 11KA4 and 11KT, respectively, by the enzyme 11β-hydroxysteroid dehydrogenase type 2 (11βHSD2). In addition, 11KT derives from the reduction of 11KA4, via AKR1C3, which is analogous to androstenedione conversion to testosterone in the periphery48.
Activation of adrenal androgens in peripheral tissues.
As noted in a previous section, the majority of C19 steroids produced by the adrenal gland consist of androgen precursors that can be activated in target tissues, such as the prostate or skin (FIG. 2). DHEA in the circulation can be converted to androstenedione by peripheral HSD3B1; subsequently, androstenedione is further metabolized to testosterone by AKR1C3 (REF.78). The abundant amounts of 11OHA4 produced by the adrenal glands can be oxidized to 11KA4 in the periphery, predominately by HSD11B2 in the kidney. 11KA4 is then converted to 11KT by AKR1C3 in other peripheral tissues expressing this enzyme, including adipose tissue79. The catalytic efficiency of AKR1C3 is in fact eightfold higher when it utilizes 11KA4 rather than androstenedione as a substrate79.
Steroid 5α-reductases (SRD5A) are known for converting testosterone to the more potent androgen dihydrotestosterone (DHT), but SRD5A can also utilize androstenedione and all of the 11-oxygenated derivatives of androstenedione and testosterone as substrates80. Two distinct isoenzymes exist in humans: type 1 (SRD5A1) is expressed in multiple tissues, including the liver and skin, whereas type 2 (SRD5A2) is found in male reproductive tissues. SRD5A2 preferentially utilizes testosterone, whereas SRD5A1 catalyses reduction of androstenedione more efficiently than testosterone81. The DHT homologue, 11-ketodihydrotestosterone (11KDHT), can be generated either from 11β-hydroxydihydrotestosterone, via 11βHSD2, or from 11-keto-5α-androstanedione, via AKR1C3 (REF.80).
Androgenic potency
Although DHEA and DHEAS are the staple adrenal androgens, their bioactivity is negligible (FIG. 1). Similarly, androstenedione and its 11-oxygenated metabolites have little biological activity82,83. By contrast, testosterone and 11KT are potent agonists of the androgen receptor (AR, encoded by NR3C4). Using cell models engineered to express the human AR and a luciferase reporter, we found that the maximum androgenic activity of 11KT approached that of testosterone, whereas that of 11OHT was more modest7,84. The induction of expression of the AR target gene was also similar for both testosterone and 11KT (REF.85). Storbeck and colleagues obtained similar results in a COS-1 cell system expressing human AR; in addition, they demonstrated that 11KDHT is equipotent to DHT80 (FIG. 1). Moreover, using two androgen-dependent prostate cancer cell lines, the same group showed that 11KT and 11KDHT bind to the human AR with similar affinities to testosterone and DHT, respectively86. In the same study, all four steroids activated the expression of AR-regulated genes and promoted cell growth86.
Circulation patterns
During human fetal development, the adrenal glands are a major source of DHEA and DHEAS, which are utilized by the placenta for oestrogen synthesis42. At birth, the adrenal glands are similar in size to the kidneys, largely due to the presence of the fetal zone, which involutes soon after birth42. Levels of DHEA and DHEAS also decline rapidly after birth, and their concentrations remain negligible until adrenarche87. Adrenarche is an endocrine phenomenon characterized by a rise in the synthesis of DHEA and DHEAS in parallel with the developmental expansion of the adrenal zona reticularis88-90. This process begins sometime in childhood and progresses gradually, prior to and independent of puberty91. The hormonal changes of adrenarche precede its clinical manifestations, including the appearance of axillary and/or pubic hair growth (pubarche), adult body odour and mild acne, and they typically initiate around age 6–8 years in both boys and girls91-95. Although a rise in the circulating concentrations of the major adrenal C19 steroids, DHEA and DHEAS, has been long associated with adrenarche, we demonstrated in a study published in 2018 that levels of the potent androgens testosterone and 11KT also rise during adrenarche, with circulating concentrations of 11KT exceeding those of testosterone in girls with normal or premature adrenarche85. The phenotypic manifestations associated with adrenarche could be attributed to the action of androgens directly released from the adrenal gland and/or to the peripheral conversion of DHEA to testosterone96-100 and of 11OHA4 to 11KT (REFS80,101) in other organs or in target tissues, such as hair follicles or genital skin.
Following adrenarche, the adrenal production of DHEA and DHEAS continues to rise gradually and peaks in the mid-20s (FIG. 3). Subsequently, levels of DHEA and DHEAS decline with ageing, such that by age 80 years, DHEA and DHEAS concentrations are only about 20% of their mid-20s peak levels102. Throughout life, DHEAS concentrations are generally higher in boys and men than in age-matched girls and women; however, the cause of this sexual dimorphism is poorly understood. Beginning with puberty, both the gonads and the adrenals contribute to the production of androstenedione and testosterone103,104, either directly or indirectly by providing androgen precursors. The contribution of the adrenal gland to the circulating levels of testosterone throughout reproductive ages is similar to that of the ovaries in women105, whereas in men the overwhelming proportion of testosterone originates from the testes. Like DHEA and DHEAS, androstenedione and testosterone decline gradually with ageing in both sexes106-112. In contrast to these traditional androgens, we have found that the circulating concentrations of 11-oxyandrogens remain fairly stable, or even show a modest up-trend in some postmenopausal women113 (FIG. 3).
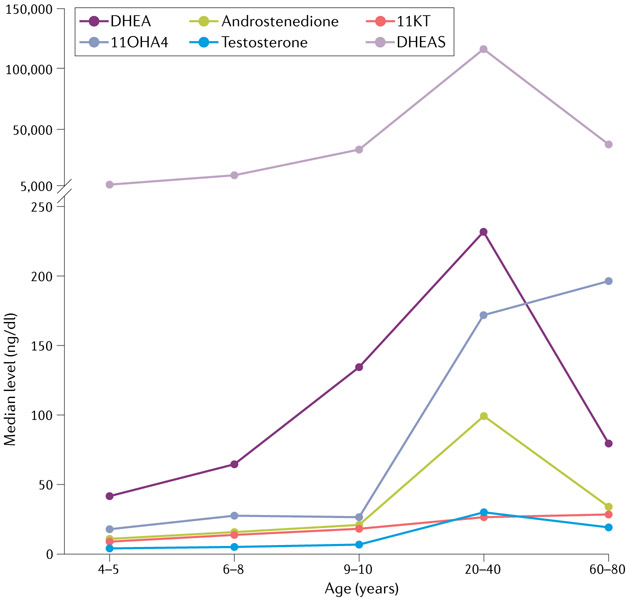
Fig. 3 ∣. Distribution of key C19 steroids across the human female lifespan.
Levels of dehydroepiandrosterone (DHEA) and its sulfate (DHEAS), the major adrenal androgen precursors, begin to increase around age 6 years, peak around the mid-20s and, subsequently, decline gradually. In women during reproductive years, androstenedione and testosterone are produced in similar amounts by both the adrenal glands and the ovaries, and their concentrations decline gradually with ageing. By contrast, levels of 11β-hydroxyandrostenedione (11OHA4) and 11-ketotestosterone (11KT) remain elevated in postmenopausal women. Data obtained from Rege et al.85 and Nanba et al.113, and expressed in medians. Total n = 283 girls and women (aged 4–5 years, n = 22; aged 6–8 years, n = 38; aged 9–10 years, n = 23; aged 20–40 years, n = 100; aged 60–80 years, n = 100).
Ageing is accompanied by an involution of the zona reticularis, which explains the concurrent decline in levels of DHEA and DHEAS114,115. Nonetheless, we have observed that the sharp zonal separation of the expression of HSD3B2 and CYB5A that is characteristic of adrenal glands in young people becomes less distinct in women aged >60 years113. Such morphological changes (that is, the sharp zonal separation becoming less distinct in women aged >60 years) would facilitate the conversion of DHEA to androstenedione and, subsequently, to downstream androgens, including 11-oxyandrogens.
Of the unconjugated C19 steroids produced in the adrenal glands, 11OHA4 is the second most abundant (with DHEA being the first) throughout most life stages (FIG. 3), and its circulating concentrations far exceed those of androstenedione, both under baseline conditions and after stimulation with cosyntropin (a synthetic form of ACTH). The predominance of 11OHA4 over androstenedione has been demonstrated both in cultured human adrenal cells116 and in serum obtained from human adrenal veins7,8. Notably, 11OHA4 is the most abundant unconjugated C19 steroid in postmenopausal women, with concentrations over twofold higher than those of DHEA113 (FIG. 3 and TABLE 1). The adrenal gland also produces smaller amounts of 11OHT, 11KA4 and 11KT, and ACTH enhances the synthesis of all four 11-oxyandrogens7,8. Peripheral concentrations of 11KT exceed those of testosterone before puberty in both sexes85. In women of reproductive age, circulating levels of 11KT and testosterone are fairly similar, whereas after menopause, the ratio of 11KT to testosterone increases again113 (FIG. 3 and TABLE 1). Considering that the androgenic potency of 11KT is similar to that of testosterone, its quantitative dominance in prepubertal children and postmenopausal women suggests that 11KT is the primary mediator of many processes previously attributed to testosterone, including adrenarche. The physiological functions of 11-oxyandrogens throughout adulthood and ageing remain to be determined. As gonadal function also undergoes an age-dependent decline in both sexes, albeit more abruptly in women than in men, the persistent production of bioactive adrenal androgens might have residual benefits on bone, muscle, the brain or other tissues and functions.
Table 1 ∣.
Concentrations of major C19 steroids in healthy individuals and those with androgen excess
| Study details | Steroid level (ng/dl) | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|
| DHEAS | DHEA | Androstenedione | Testosterone | 11OHA4 | 11KA4 | 11OHT | 11KT | ||
| 100 premenopausal women (aged 20–40 years) vs 100 postmenopausal women (aged 60–80 years) | 116,268 (71,112–206,477) vs 38,747 (22,258–53,873); P < 0.0001 | 232 (155–403) vs 79 (52–134); P < 0.0001 | 99 (76–140) vs 34 (23–51); P < 0.0001 | 30 (22–40) vs 19 (14–31); P < 0.0001 | 172 (118–261) vs 196 (141–289); P = 0.06 | 37 (26–55) vs 34 (23–46); P = 0.05 | 14 (9–23) vs 20 (13–28); P = 0.0002 | 26 (19–38) vs 28 (22–37); P = 0.3 | 110 |
| 37 girls with premature adrenarche vs 54 control girls (all aged 4–7 years) | 38,227 (23,035–57,073) vs 9,321 (4,333–17,637); P < 0.001 | 242 (162–429) vs 52.3 (32.8–89.5); P < 0.001 | 17.0 (11.4–22.8) vs 13.1 (9.2–22.4); P = 0.238 | 5.2 (4.6–5.8) vs 4.6 (3.7–5.2); P < 0.001 | 45.7 (29.3–67.5) vs 26.1 (16.7–38.9); P < 0.001 | 15.0 (12.4–22.1) vs 10.8 (8.2–13.8); P < 0.001 | 6.2 (4.9–7.4) vs 3.8 (2.6–6.1); P < 0.001 | 18.1 (12.3–22.9) vs 11.2 (8.4–16.5); P < 0.001 | 84 |
| 38 patients with 21OHD (19 females) vs 38 control individuals (19 females); all aged 3–59 years | 18,744 (7,847–64,308) vs 139,784 (58,409–186,697); P < 0.0001 | 29 (16–85) vs 175 (118–318); P < 0.0001 | 155 (72–390) vs 42 (22–63); P < 0.0001 | 80 (38–162) vs 26 (12–309); P = 0.09 | 351 (188–792) vs 118 (70–154); P < 0.0001 | 96 (58–143) vs 31 (20–42); P < 0.0001 | 59 (21–104) vs 15 (9–21); P < 0.0001 | 171 (105–366) vs 50 (29–78); P < 0.0001 | 8 |
| 114 women with PCOS vs 49 control women; all aged 18–40 years | 300,111 (203,506–451,661) vs 222,804 (125,535–351,365); P < 0.001 | 406 (300–524) vs 205 (121–340); P < 0.001 | 768 (484–1,009) vs 169 (103–264); P < 0.001 | 20 (14–29) vs 9 (6–14); P < 0.001 | 958 (508–1,444) vs 205 (148–372); P < 0.001 | 402 (255–565) vs 81 (60–114); P < 0.001 | 12 (9–15) vs 6 (3–9); P < 0.01 | 73 (54–118) vs 45 (36–54); P < 0.01 | 194 |
Data are expressed as medians (interquartile ranges) and are extracted from four publications8,83,109,193. 11KA4, 11-ketoandrostenedione; 11KT, 11-ketotestosterone; 11OHA4, 11β-hydroxyandrostenedione; 11OHT, 11β-hydroxytestosterone; 21OHD, 21-hydraxylase deficiency; DHEA, dehydroepiandrosterone; DHEAS, DHEA sulfate; PCOS, polycystic ovary syndrome.
In teleost fish, 11KT is produced in the gonads as a male-specific androgen117-121; however, in human beings and other primates, 11-oxyandrogens are of adrenal origin122. Patients with adrenal insufficiency produce negligible amounts of 11-oxyandrogens8,123,124, and 11-oxyandrogens circulate at similar concentrations in healthy men and women, including during the reproductive lifespan, when males have a high testicular testosterone output8,76,122. An important clinical implication for disorders of androgen excess is that circulating levels of 11OHA4 and 11OHT primarily reflect adrenal androgen output, with rare exceptions. One of our studies provided a comprehensive analysis of 11-oxyandrogens across 18 animal species and demonstrated that, among non-primate animals, only pigs and guinea pigs synthesize 11-oxyandrogens and therefore might serve as suitable animal models for future investigations122.
Adrenal androgens in human disorders
Congenital adrenal hyperplasia.
A group of autosomal recessive defects in genes encoding enzymes required for cortisol synthesis cause CAH. These genetic defects can lead to complete or partial enzymatic insufficiencies, resulting in the wide spectrum of clinical severity seen in this disorder. In the most severe forms of CAH, also called classic CAH, cortisol and/or aldosterone synthesis are inadequate, and patients require lifelong glucocorticoid replacement therapy. Milder, or non-classic, CAH features normal cortisol synthesis; however, this normal cortisol synthesis is achieved at the expense of elevated levels of androgens. In all CAH forms, however, the hypothalamic–pituitary axis is overactivated, in an attempt to overcome the enzymatic blockade (that is, the lack of enzymes in the cortisol and/or aldosterone synthesis pathways) and preserve cortisol synthesis. Stimulated by the ensuing increase in levels of ACTH that are occurring as a result of overactivation of the hypothalamic–pituitary axis, the steroidogenic stream favours the accessible pathways (that is, pathways with normally functioning enzymes), leading to excessive amounts of steroids that are usually only present at low levels.
Most cases of CAH are due to 21-hydroxylase (CYP21A2) deficiency. Classic 21-hydroxylase deficiency (21OHD) occurs in ~1:16,000 neonates125; non-classic 21OHD is much more common, with a prevalence of roughly 1:1,000 white people and an even higher prevalence in selected ethnicities, such as Ashkenazi Jewish people, Hispanic people and people with Mediterranean origins126.
In 21OHD (both forms), the synthesis of mineralocorticoids and glucocorticoids is obstructed, which leads to accumulation of 17OHP4, the main substrate of CYP21A2. As a result of stimulation by ACTH, 17OHP4 is diverted towards formation of adrenal androgens and androgen precursors127. Although 17-hydroxypregnenolone is the preferred substrate of the human 17,20-lyase128, the supraphysiologic concentrations of 17OHP4 in 21OHD allow some conversion of 17OHP4 to androstenedione, which can be further metabolized to testosterone by AKR1C3. Subsequently, CYP11B1 catalyses the reactions from androstenedione and testosterone to 11OHA4 and 11OHT, respectively, which are further metabolized to 11KA4 and 11KT, predominantly in the periphery.
Serum levels of 17OHP4, androstenedione and, in women, testosterone, are normally used to assess disease control and to guide treatment in patients with 21OHD; however, they are unreliable biomarkers129,130. The marked elevations in levels of 17OHP4 in the serum of patients with 21OHD fluctuate widely with time after glucocorticoid exposure, and only normalize when supraphysiological glucocorticoid doses are used, which can lead to adverse effects131. Additionally, 17OHP4, androstenedione and testosterone are also synthesized by the gonads, further confounding their clinical utility after puberty. In contrast to 17OHP4, levels of the major adrenal C19 steroids, DHEA and DHEAS, are paradoxically low in patients with classic 21OHD, even when disease control is suboptimal8,132, making them unreliable biomarkers of disease control.
Owing to their adrenal origin, 11-oxyandrogens have been proposed as biomarkers of androgen excess in 21OHD. In an early study using immunoassays, it was found that the ratio of androstenedione to 11OHA4 was lower in women with non-classic 21OHD and higher in women with chronic anovulation than in control women133. Another study found that levels of 11OHA4 were higher at baseline in women with untreated non-classic 21OHD than in unaffected control women matched for age and BMI, whereas the levels were similar after cosyntropin stimulation134. Using mass spectrometry, we have shown that not only levels of 11OHA4 but also levels of 11KA4, 11OHT and 11KT are threefold to fourfold higher in patients with classic 21OHD of both sexes during usual glucocorticoid treatment than in control individuals matched for age and sex8 (TABLE 1). A subsequent study measured the daily urinary excretion of androgen metabolites in 99 children with classic 21OHD who were receiving treatment and showed that the 11-oxyandrogen metabolite 11β-hydroxyandrosterone was the most abundant urinary androgen metabolite and the only one that was higher than in healthy children135.
In girls, women and prepubertal boys with classic 21OHD, levels of 11KT correlate directly with those of testosterone. However, it is notable that circulating concentrations of 11KT are twice as high as those of testosterone in these patients8. Conversely, levels of both 11KT and 11OHT are inversely correlated with levels of testosterone and luteinizing hormone in post-pubertal male individuals with 21OHD (REFS8,136). These findings suggest that 11KT is a better biomarker of disease control than testosterone in men, with evidence that the high concentrations of 11KT in male patients with 21OHD that is poorly controlled suppress the hypothalamic–pituitary–gonadal axis and subsequently testicular testosterone synthesis. Additionally, this inverse correlation between levels of 11KT and testosterone in sexually mature men with 21OHD demonstrates that testicular testosterone is not a source of 11KT, but rather that 11KT derives predominately from adrenal precursors.
To further explore the clinical utility of 11-oxyandrogens, we studied the association between a set of 23 steroids and clinical findings suggestive of poor 21OHD control, such as adrenal volume, testicular adrenal rest tumours (TART), bone age, menstrual irregularities and hirsutism136. In a cross-sectional study of 114 patients with classic 21OHD, we found that, along with 21-deoxycortisl, the four 11-oxyandrogens correlated best with adrenal volume and levels of ACTH136. In addition, levels of all four 11-oxyandrogens, and in particular 11OHA4, were threefold to sevenfold higher in male patients with TART than in those without TART of similar ages. Furthermore, as previously reported137, levels of testosterone were similar between patients who had 21OHD with or without TART. Levels of 11OHT and 11KT were also higher in women with 21OHD who had menstrual disturbances (such as irregular menses or amenorrhoea) and hirsutism than in those without these additional symptoms. These initial studies suggest that 11-oxyandrogens are excellent biomarkers of 21OHD control, and prospective longitudinal studies are needed to determine the dynamics of these 11-oxyandrogens in individuals with 21OHD, in particular in response to therapy.
Premature adrenarche.
Premature adrenarche is defined as the precocious appearance of pubic and/or axillary hair before age 8 years in girls and 9 years in boys138-142. Unlike precocious puberty, premature adrenarche does not manifest with the development of secondary sexual characteristics, such as breast development and testicular growth143. Along with elevated age-adjusted DHEA and DHEAS synthesis, children with premature adrenarche might have modestly advanced bone age and accelerated linear growth144,145. Steroid profiles of infants with fine genital hair showed a mild elevation in levels of DHEAS compared with healthy pre-adrenarchal children, thus indicating that pubic hair in infancy might represent an early-onset variant of premature adrenarche146. Premature adrenarche is more common in girls than boys, with a ratio of 9:1 (REF.147), and it has been reported to occur in 9.5% of African-American girls and 1.5% of white girls148. Although premature adrenarche had long been considered a benign condition, studies from the past few years have indicated that dysregulation of adrenarche predisposes to increased cardiovascular risk and increased insulin resistance later in life, including in adolescence and adulthood140,142,149. Many studies have attempted to determine the link between the early androgen excess seen in premature adrenarche and factors related to the metabolic syndrome150-153. A subgroup of girls with premature adrenarche have an increased susceptibility for developing PCOS in adult life154-158. Moreover, in some cases, early or exaggerated adrenarche is an early manifestation of PCOS in girls who progress rapidly from premature adrenarche to puberty to oligomenorrhoea and hyperandrogenism, and these patients tend to have marked insulin resistance and/or obesity153,159-162.
Although the molecular causes of premature adrenarche remain unclear, there is supporting evidence for early expansion of the zona reticularis163, elevated 17,20-lyase activity164, and increased IGF1 (REF.165) and anti-Müllerian hormone166 production being involved. Apparent cortisone reductase deficiency due to decreased 11β-hydroxysteroid dehydrogenase type 1 (11βHSD1) activity causes adrenal hyperandrogenaemia and premature pubarche, which results in symptoms similar to those seen in children with premature adrenarche167,168. Other genetic causes of premature adrenarche are apparent DHEA sulfotransferase deficiency due to PAPSS2 deficiency44, glucocorticoid resistance169 and increased androgen sensitivity owing to a CAG repeat polymorphism in the gene that encodes the androgen receptor170.
Although high serum concentrations of DHEA, DHEAS and androstenedione have been historically associated with normal and premature adrenarche2,3,93,95,140,171,172, these steroids exhibit weak androgen activity and are thereby unlikely to directly cause the clinical manifestations observed in these conditions. It should be noted, however, that DHEA, DHEAS and androstenedione act as a reservoir of precursors for production of potent androgens, including testosterone and DHT, in the periphery or target tissues173-175. Testosterone has been used as the conventional marker for androgen excess in prepubertal children, and several studies have demonstrated elevations in serum concentrations of testosterone and its urinary metabolites that are related to premature adrenarche2,3,85,93,144,171,176. Our group has highlighted the 11-oxyandrogens as major contributors to the androgen excess of premature adrenarche85. We found that premature adrenarche is characterized by marked alterations in the circulating steroid profile, including increases in levels of adrenal-derived steroid sulfates, testosterone and all of the 11-oxyandrogens, earlier than in control children. Notably, circulating concentrations of 11KT were higher than levels of testosterone in prepubertal girls and more so in girls with premature adrenarche (3.5-fold) than in age-matched reference girls (2.4-fold) (TABLE 1). This observation supports the theory that the 11-oxyandrogens, especially 11KT, contribute to the precocious androgenic phenotype of premature adrenarche.
Polycystic ovary syndrome.
PCOS is the most common ovarian disorder, affecting 5–15% of reproductive-aged women177-179. PCOS and non-classic 21OHD share several phenotypic features, including reproductive abnormalities and hyperandrogenism177,180, which makes their clinical distinction difficult. In contrast to the monogenic aetiology of 21OHD, PCOS is a mixed, heterogenic condition with poorly understood pathogenesis. The diagnosis of PCOS is based on a set of criteria, of which ovarian dysfunction and clinical and/or biochemical evidence of hyperandrogenism are included in all expert guidelines181,182. Patients with PCOS have an increased risk of metabolic abnormalities, such as obesity, insulin resistance, type 2 diabetes mellitus, cardiovascular disease and dyslipidaemia183-185. These risks are also observed in patients with 21OHD, but are as a consequence of therapies featuring supraphysiologic levels of glucocorticoid186.
Clinical and/or biochemical evidence of androgen excess is common177 but not universal182 in women with PCOS. The ovaries have been long considered the primary source of androgen excess in women with PCOS187,188. The androgen excess in women with PCOS is enhanced with gonadotropin-releasing hormone agonists189,190, and testosterone and androstenedione respond less to chronic dexamethasone suppression than in healthy women191, suggesting that the androgens predominantly originate in the ovary. Although testosterone and androstenedione are produced by both the ovaries and the adrenal glands, androstenedione has been suggested to be superior to testosterone as a marker of hyperandrogenism and metabolic risk in women with PCOS192-194. Notably, a subgroup of women with PCOS often have elevated circulating concentrations of DHEA and DHEAS4,195, and, in contrast to testosterone and androstenedione, DHEA concentrations are markedly suppressed with long-term night time dexamethasone use in these patients191. These two findings suggest that the adrenal glands have a role as contributors towards androgen excess in a subset of patients with PCOS. Moreover, along with cortisol, the response of adrenal androgens to stimulation with cosyntropin is exaggerated in patients with PCOS196,197, suggesting that hyper-reactivity of the adrenal zona reticularis to ACTH might be implicated in the aetiology of hyperandrogenism for certain patients with PCOS.
Another indication for an adrenal contribution to the pool of androgens in PCOS is the elevation of circulating levels of 11OHA4 in many of these women, which was initially demonstrated by studies using immunoassays in the early 1990s (REFS133,198). In a more recent study, levels of 11OHA4, 11KA4, 11OHT and 11KT, as well as their urinary metabolite 11β-hydroxyandrosterone, were all higher in 114 women with PCOS than in 49 healthy women199 (TABLE 1). In addition, this study found that serum concentrations of 11OHA4 and 11KA4, but not the urinary 11-oxygenated testosterone metabolites, correlated weakly with BMI, insulin levels and HOMA-IR.
Castration-resistant prostate cancer.
Prostate cancer is the second most common cancer in men globally. Prostate cancer growth is promoted by androgens, which in men are produced predominantly in the testes. Medical or surgical androgen deprivation is usually implemented when there is evidence of metastatic disease that requires systemic therapy200. Progression of disease despite androgen deprivation is referred to as castration-resistant prostate cancer (CRPC). Although circulating levels of testosterone fall dramatically after androgen deprivation therapy, intratumoural DHT synthesis continues201,202, probably from precursors produced by the adrenal glands. Suppression of adrenal-derived androgen synthesis with abiraterone acetate, a potent P450 17A1 inhibitor, and inhibition of androgen receptor signalling with enzalutamide improves survival in men with CRPC203-205, which supports the idea that CRPC is androgen dependent. Molecular alterations that promote both intratumoural androgen synthesis and androgen signalling promote cancer cell growth. For instance, upregulation of androgen receptors and of enzymes involved in androgen synthesis (such as AKR1C3, HSD3B1, HSD17B3 and SRD5A1) have been reported in CRPC tissue samples206-210. CRPC cells from patients exhibit an isoenzyme shift from SRD5A2 to SRD5A1 (REF.210). SRD5A1 preferentially utilizes androstenedione rather than testosterone as a substrate, which is funnelled to DHT via 5α-androstanedione211. Consequently, inhibiting both SRD5A1 and SRD5A2 is important to prevent cancer growth in patients with CRPC.
11OHA4 from the adrenal glands, which circulates in higher concentrations than androstenedione, is an additional substrate for potent androgen synthesis within CRPC cells from patients. The LNCaP androgen-dependent prostate cancer cell line metabolizes 11OHA4 to the potent androgens 11KT and 11KDHT, via the enzymes HSD11B2, AKR1C3 and SRD5A1 (REFS79,80). The dynamic interplay between key androgenic enzymes within prostate cancer tissue seems to favour the formation of active androgens, which are essential for tumour growth. Using in vitro human prostate cancer cell models, it was shown that the kinetics of the two HSD11B isoenzymes favour the formation of 11-keto androgens via HSD11B2 (REF.212), which is expressed in prostate cancer cells213,214. Owing to the increased expression of SRD5A1 and AKR1C3, along with downregulation of HSD17B2 (REF.215), the enzymatic milieu of prostate cancer cells favours the synthesis of the potent androgens DHT and 11KDHT. Furthermore, 11-oxyandrogens have been identified in tumour tissue from three patients with CRPC; of these, 11OHA4 was the most abundant216,217. In the same patients, the serum concentrations of 11KT and 11KDHT were considerably higher than those of testosterone and DHT, respectively.
Additional intratumoural metabolism that could contribute to cancer progression includes reduced inactivation of bioactive androgens via conjugation, such as the sulfonation or glucuronidation reactions carried out by sulfotransferase and uridine 5′-diphospho-glucuronosyltransferase (UGT) enzymes, respectively. Using in vitro androgen-dependent prostate cancer cell-line models, inactivation of 11KT and 11KDHT was found to occur considerably more slowly than that of testosterone and DHT86. Similarly, in contrast to testosterone, which conjugates to testosterone–glucuronide within 48 h in LNCaP cells, this glucuronidation was inefficient for 11KT and 11KDHT (REFS216,217). Consequently, intracellular availability of 11KT and 11KDHT is prolonged, allowing these AR agonists to activate the expression of endogenous AR-regulated genes and contribute to cancer progression86.
Conclusions
The high circulating levels of DHEAS throughout most of adulthood and the weak capacity of the zona reticularis to produce testosterone have focused most studies of adrenal androgens on the Δ5 pathway and their subsequent conversion to androstenedione and testosterone. Although the ability of the human adrenal to produce 11OHA4 has been recognized for several decades mainly as a curiosity, an explosion of observations made over the past 5 years has illuminated the profound contributions of 11-oxyandrogens to human health and disease. With the advent of clinical assays for 11-oxyandrogens, studies are underway to define the utility of their measurements in the management of disorders such as 21OHD and in the prognosis of CRPC. Additional studies to define the kinetics of 11-oxyandrogen disposition and responses to treatment will provide further insight regarding the adrenal contributions to androgen-derived implications.
Key points.
Dehydroepiandrosterone (DHEA) and its sulfate, DHEAS, are the most abundant adrenal androgen precursors, but they are biologically inactive as androgens.
The adrenal gland is the primary source of a set of 11-oxygenated 19-carbon steroids, also termed 11-oxyandrogens, which have several roles in human physiology and disease.
11-Ketotestosterone is a bioactive 11-oxyandrogen, with a potency similar to that of testosterone, and its concentrations exceed those of testosterone in prepubertal children and in postmenopausal women.
Concentrations of 11-oxyandrogens are elevated in several disorders of androgen excess, including premature adrenarche, congenital adrenal hyperplasia and polycystic ovary syndrome, and they can contribute to the progression of castration-resistant prostate cancer.
Adrenarche.
An early stage in sexual maturation specific to humans and other primates that is caused by androgen synthesis from the adrenal glands and is associated with the development of pubic hair, body odour, skin oiliness and acne.
Steroidogenesis.
The process of steroid synthesis.
Tropic.
Related to hormones that stimulate the growth and activity of target glands.
Second messengers.
Small intracellular molecules that mediate the effects of first messengers, that is, neurotransmitters and hormones.
Pubarche.
The first appearance of pubic hair at puberty.
Sulfonation.
The transfer of a sulfonate group (SO3−1) from the universal sulfonate donor 3′-phosphoadenosine 5′-phosphosulfate (PAPS) to an appropriate acceptor molecule.
Glucuronidation.
A process of steroid metabolism that consists of transfer of the glucuronic acid component of uridine diphosphate glucuronic acid to a substrate by any of several types of UDP-glucuronosyltransferase.
Acknowledgements
The authors acknowledge the support of grants from the NIH (1K08DK109116 to A.F.T., R01DK069950 and R01DK43140 to W.E.R., R01GM086596 to R.J.A.) and the University of Michigan (MCubed U064177 to A.F.T. and W.E.R.).
Footnotes
Competing interests
The authors declare no competing interests.
Peer review information
Nature Reviews Endocrinology thanks V. Papadopoulos, S. Wudy and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.White PC & Speiser PW Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocr. Rev 21, 245–291 (2000). [DOI] [PubMed] [Google Scholar]
- 2.Doberne Y, Levine LS & New MI Elevated urinary testosterone and androstanediol in precocious adrenarche. Pediatric Res. 9, 794–797 (1975). [DOI] [PubMed] [Google Scholar]
- 3.Korth-Schutz S, Levine LS & New MI Evidence for the adrenal source of androgens in precocious adrenarche. Acta Endocrinol. 82, 342–352 (1976). [DOI] [PubMed] [Google Scholar]
- 4.Azziz R, Black V, Hines GA, Fox LM & Boots LR Adrenal androgen excess in the polycystic ovary syndrome: sensitivity and responsivity of the hypothalamic–pituitary–adrenal axis. J. Clin. Endocrinol. Metab 83, 2317–2323 (1998). [DOI] [PubMed] [Google Scholar]
- 5.Else T et al. Adrenocortical carcinoma. Endocr. Rev 35, 282–326 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharifi N Minireview: androgen metabolism in castration-resistant prostate cancer. Mol. Endocrinol 27, 708–714 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rege J et al. Liquid chromatography–tandem mass spectrometry analysis of human adrenal vein 19-carbon steroids before and after ACTH stimulation. J. Clin. Endocrinol. Metab 98, 1182–1188 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turcu AF et al. Adrenal-derived 11-oxygenated 19-carbon steroids are the dominant androgens in classic 21-hydroxylase deficiency. Eur. J. Endocrinol 174, 601–609 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller WL & Auchus RJ The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev 32, 81–151 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark BJ, Wells J, King SR & Stocco DM The purification, cloning and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR). J. Biol. Chem 269, 28314–28322 (1994). [PubMed] [Google Scholar]
- 11.Stocco DM & Clark BJ Regulation of the acute production of steroids in steroidogenic cells. Endocr. Rev 17, 221–244 (1996). [DOI] [PubMed] [Google Scholar]
- 12.Sugawara T et al. Human steroidogenic acute regulatory protein: functional activity in COS-1 cells, tissue-specific expression, and mapping of the structural gene to 8p11.2 and a pseudogene to chromosome 13. Proc. Natl Acad. Sci. USA 92, 4778–4782 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Granot Z et al. Proteolysis of normal and mutated steroidogenic acute regulatory proteins in the mitochondria: the fate of unwanted proteins. Mol. Endocrinol 17, 2461–2476 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Artemenko IP, Zhao D, Hales DB, Hales KH & Jefcoate CR Mitochondrial processing of newly synthesized steroidogenic acute regulatory protein (StAR), but not total StAR, mediates cholesterol transfer to cytochrome P450 side chain cleavage enzyme in adrenal cells. J. Biol. Chem 276, 46583–46596 (2001). [DOI] [PubMed] [Google Scholar]
- 15.Tsujishita Y & Hurley JH Structure and lipid transport mechanism of a StAR-related domain. Nat. Struct. Biol 7, 408–414 (2000). [DOI] [PubMed] [Google Scholar]
- 16.Anuka E, Gal M, Stocco DM & Orly J Expression and roles of steroidogenic acute regulatory (StAR) protein in ‘non-classical’, extra-adrenal and extra-gonadal cells and tissues. Mol. Cell. Endocrinol 371, 47–61 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Koritz SB & Kumar AM On the mechanism of action of the adrenocorticotrophic hormone. The stimulation of the activity of enzymes involved in pregnenolone synthesis. J. Biol. Chem 245, 152–159 (1970). [PubMed] [Google Scholar]
- 18.Shikita M & Hall PF The stoichiometry of the conversion of cholesterol and hydroxycholesterols to pregnenolone (3β-hydroxypregn-5-en-20-one) catalysed by adrenal cytochrome P-450. Proc. Natl Acad. Sci. USA 71, 1441–1445 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimizu K, Hayano M, Gut M & Dorfman RI The transformation of 20α-hydroxcholesterol to isocaproic acid and C21 steroids. J. Biol. Chem 236, 695–699 (1961). [Google Scholar]
- 20.John ME, John MC, Boggaram V, Simpson ER & Waterman MR Transcriptional regulation of steroid hydroxylase genes by corticotropin. Proc. Natl Acad. Sci. USA 83, 4715–4719 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reiter EO, Fuldauer VG & Root AW Secretion of the adrenal androgen, dehydroepiandrosterone sulfate, during normal infancy, childhood, and adolescence, in sick infants, and in children with endocrinologic abnormalities. J. Pediatr 90, 766–770 (1977). [DOI] [PubMed] [Google Scholar]
- 22.Zuber MX, Simpson ER & Waterman MR Expression of bovine 17α-hydroxylase cytochrome P450 cDNA in non-steroidogenic (COS-1) cells. Science 234, 1258–1261 (1986). [DOI] [PubMed] [Google Scholar]
- 23.Chung BC et al. Cytochrome P450c17 (steroid 17α-hydroxylase/17,20 lyase): cloning of human adrenal and testis cDNAs indicates the same gene is expressed in both tissues. Proc. Natl Acad. Sci. USA 84, 407–411 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kagimoto M, Winter JS, Kagimoto K, Simpson ER & Waterman MR Structural characterization of normal and mutant human steroid 17α-hydroxylase genes: molecular basis of one example of combined 17α-hydroxylase/17,20 lyase deficiency. Mol. Endocrinol 2, 564–570 (1988). [DOI] [PubMed] [Google Scholar]
- 25.Yasukochi Y & Masters BS Some properties of a detergent-solubilized NADPH-cytochrome c (cytochrome P-450) reductase purified by biospecific affinity chromatography. J. Biol. Chem 251, 5337–5344 (1976). [PubMed] [Google Scholar]
- 26.Flück CE, Miller WL & Auchus RJ The 17,20-lyase activity of cytochrome P450c17 from human fetal testis favors the Δ5 steroidogenic pathway. J. Clin. Endocrinol. Metab 88, 3762–3766 (2003). [DOI] [PubMed] [Google Scholar]
- 27.Imai T, Globerman H, Gertner JM, Kagawa N & Waterman MR Expression and purification of functional human 17α-hydroxylase/17,20-lyase (P450c17) in Escherichia coli. Use of this system for study of a novel form of combined 17α-hydroxylase/17,20-lyase deficiency. J. Biol. Chem 268, 19681–19689 (1993). [PubMed] [Google Scholar]
- 28.Onoda M & Hall PF Cytochrome b5 stimulates purified testicular microsomal cytochrome P450 (C21 side-chain cleavage). Biochem. Biophys. Res. Commun 108, 454–460 (1982). [DOI] [PubMed] [Google Scholar]
- 29.Auchus RJ, Lee TC & Miller WL Cytochrome b5 augments the 17,20 lyase activity of human P450c17 without direct electron transfer. J. Biol. Chem 273, 3158–3165 (1998). [DOI] [PubMed] [Google Scholar]
- 30.Katagiri M, Kagawa N & Waterman MR The role of cytochrome b5 in the biosynthesis of androgens by human P450c17. Arch. Biochem. Biophys 317, 343–347 (1995). [DOI] [PubMed] [Google Scholar]
- 31.Lee-Robichaud P, Wright JN, Akhtar ME & Akhtar M Modulation of the activity of human 17α-hydroxylase-17,20-lyase (CYP17) by cytochrome b5: endocrinological and mechanistic implications. Biochem. J 308, 901–908 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peng HM et al. Cytochrome b5 activates the 17,20-lyase activity of human cytochrome P450 17A1 by increasing the coupling of NADPH consumption to androgen production. Biochemistry 55, 4356–4365 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simpson ER et al. Regulation of the biosynthesis of steroidogenic enzymes. J. Steroid Biochem 27, 801–805 (1987). [DOI] [PubMed] [Google Scholar]
- 34.Zuber MX, John ME, Okamura T, Simpson ER & Waterman MR Bovine adrenocortical cytochrome P-450(17α). Regulation of gene expression by ACTH and elucidation of primary sequence. J. Biol. Chem 261, 2475–2482 (1986). [PubMed] [Google Scholar]
- 35.Kristiansen SB, Endoh A, Casson PR, Buster JE & Hornsby PJ Induction of steroidogenic enzyme genes by insulin and IGF-I in cultured adult human adrenocortical cells. Steroids 62, 258–265 (1997). [DOI] [PubMed] [Google Scholar]
- 36.Lebrethon MC, Jaillard C, Naville D, Begeot M & Saez JM Effects of transforming growth factor-β1 on human adrenocortical fasciculata–reticularis cell differentiated functions. J. Clin. Endocrinol. Metab 79, 1033–1039 (1994). [DOI] [PubMed] [Google Scholar]
- 37.Penhoat A, Rainey WE, Viard I & Saez JM Regulation of adrenal cell-differentiated functions by growth factors. Hormone Res. 42, 39–43 (1994). [DOI] [PubMed] [Google Scholar]
- 38.Mapes S, Corbin CJ, Tarantal A & Conley A The primate adrenal zona reticularis is defined by expression of cytochrome b5, 17α-hydroxylase/17,20-lyase cytochrome P450 (P450c17) and NADPH-cytochrome P450 reductase (reductase) but not 3β-hydroxysteroid dehydrogenase/Δ5–4 isomerase (3β-HSD). J. Clin. Endocrinol. Metab 84, 3382–3385 (1999). [DOI] [PubMed] [Google Scholar]
- 39.Suzuki T et al. Developmental changes in steroidogenic enzymes in human postnatal adrenal cortex: immunohistochemical studies. Clin. Endocrinol 53, 739–747 (2000). [DOI] [PubMed] [Google Scholar]
- 40.Rege J et al. Age-dependent increases in adrenal cytochrome b5 and serum 5-androstenediol-3-sulfate. J. Clin. Endocrinol. Metab 101, 4585–4593 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Otterness DM et al. Human dehydroepiandrosterone sulfotransferase gene: molecular cloning and structural characterization. DNA Cell Biol. 14, 331–341 (1995). [DOI] [PubMed] [Google Scholar]
- 42.Rainey WE, Rehman KS & Carr BR The human fetal adrenal: making adrenal androgens for placental estrogens. Semin. Reprod. Med 22, 327–336 (2004). [DOI] [PubMed] [Google Scholar]
- 43.Nakamura Y, Gang HX, Suzuki T, Sasano H & Rainey WE Adrenal changes associated with adrenarche. Rev. Endocr. Metab. Disord 10, 19–26 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Noordam C et al. Inactivating PAPSS2 mutations in a patient with premature pubarche. N. Engl. J. Med 360, 2310–2318 (2009). [DOI] [PubMed] [Google Scholar]
- 45.Weinshilboum RM et al. Sulfation and sulfotransferases 1: sulfotransferase molecular biology: cDNAs and genes. FASEB J. 11, 3–14 (1997). [PubMed] [Google Scholar]
- 46.Strott CA Sulfonation and molecular action. Endocr. Rev 23, 703–732 (2002). [DOI] [PubMed] [Google Scholar]
- 47.Rege J et al. Adrenocorticotropin acutely regulates pregnenolone sulfate production by the human adrenal in vivo and in vitro. J. Clin. Endocrinol. Metab 103, 320–327 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rege J et al. Transcriptome profiling reveals differentially expressed transcripts between the human adrenal zona fasciculata and zona reticularis. J. Clin. Endocrinol. Metab 99, E518–E527 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Endoh A, Kristiansen SB, Casson PR, Buster JE & Hornsby PJ The zona reticularis is the site of biosynthesis of dehydroepiandrosterone and dehydroepiandrosterone sulfate in the adult human adrenal cortex resulting from its low expression of 3β-hydroxysteroid dehydrogenase. J. Clin. Endocrinol. Metab 81, 3558–3565 (1996). [DOI] [PubMed] [Google Scholar]
- 50.Baulieu EE Dehydroepiandrosterone (DHEA): a fountain of youth? J. Clin. Endocrinol. Metab 81, 3147–3151 (1996). [DOI] [PubMed] [Google Scholar]
- 51.Buster JE, Abraham GE, Kyle FW & Marshall JR Serum steroid levels following a large intravenous dose of a steroid sulfate precursor during the second trimester of human pregnancy. II. Pregnenolone sulfate. J. Clin. Endocrinol. Metab 38, 1038–1045 (1974). [DOI] [PubMed] [Google Scholar]
- 52.Longcope C Dehydroepiandrosterone metabolism. J. Endocrinol 150, S125–S127 (1996). [PubMed] [Google Scholar]
- 53.Fischli S et al. Dehydroepiandrosterone sulfate in the assessment of the hypothalamic–pituitary–adrenal axis. J. Clin. Endocrinol. Metab 93, 539–542 (2008). [DOI] [PubMed] [Google Scholar]
- 54.Nasrallah MP & Arafah BM The value of dehydroepiandrosterone sulfate measurements in the assessment of adrenal function. J. Clin. Endocrinol. Metab 88, 5293–5298 (2003). [DOI] [PubMed] [Google Scholar]
- 55.Sayyed Kassem L, El Sibai K, Chaiban J, Abdelmannan D & Arafah BM Measurements of serum DHEA and DHEA sulphate levels improve the accuracy of the low-dose cosyntropin test in the diagnosis of central adrenal insufficiency. J. Clin. Endocrinol. Metab 97, 3655–3662 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dennedy MC et al. Low DHEAS: a sensitive and specific test for detection of subclinical hypercortisolism in adrenal incidentalomas. J. Clin. Endocrinol. Metab 102, 786–792 (2017). [DOI] [PubMed] [Google Scholar]
- 57.Yener S, Yilmaz H, Demir T, Secil M & Comlekci A DHEAS for the prediction of subclinical Cushing’s syndrome: perplexing or advantageous? Endocrine 48, 669–676 (2015). [DOI] [PubMed] [Google Scholar]
- 58.Vaiani E et al. Central adrenal insufficiency could not be confirmed by measurement of basal serum DHEAS levels in pubertal children. Horm. Res. Paediatr 82, 332–337 (2014). [DOI] [PubMed] [Google Scholar]
- 59.Bencsik Z et al. Low dehydroepiandrosterone sulfate (DHEA-S) level is not a good predictor of hormonal activity in nonselected patients with incidentally detected adrenal tumors. J. Clin. Endocrinol. Metab 81, 1726–1729 (1996). [DOI] [PubMed] [Google Scholar]
- 60.Thomas JL, Myers RP & Strickler RC Human placental 3β-hydroxy-5-ene-steroid dehydrogenase and steroid 5–4-ene-isomerase: purification from mitochondria and kinetic profiles, biophysical characterization of the purified mitochondrial and microsomal enzymes. J. Steroid Biochem 33, 209–217 (1989). [DOI] [PubMed] [Google Scholar]
- 61.Lachance Y et al. Characterization of human 3β-hydroxysteroid dehydrogenase/Δ5–Δ4-isomerase gene and its expression in mammalian cells. J. Biol. Chem 265, 20469–20475 (1990). [PubMed] [Google Scholar]
- 62.Lorence MC, Murry BA, Trant JM & Mason JI Human 3β-hydroxysteroid dehydrogenase/Δ5–4 isomerase from placenta: expression in nonsteroidogenic cells of a protein that catalyzes the dehydrogenation/isomerization of C21 and C19 steroids. Endocrinology 126, 2493–2498 (1990). [DOI] [PubMed] [Google Scholar]
- 63.Thomas JL, Myers RP & Strickler RC Human placental 3β-hydroxy-5-ene-steroid dehydrogenase and steroid 5/4-ene-isomerase: purification from mitochondria and kinetic profiles, biophysical characterization of the purified mitochondrial and microsomal enzymes. J. Steroid Biochem 33, 209–217 (1989). [DOI] [PubMed] [Google Scholar]
- 64.Lee TC, Miller WL & Auchus RJ Medroxyprogesterone acetate and dexamethasone are competitive inhibitors of different human steroidogenic enzymes. J. Clin. Endocrinol. Metab 84, 2104–2110 (1999). [DOI] [PubMed] [Google Scholar]
- 65.Labrie F, Simard J, Luu-The V, Belanger A & Pelletier G Structure, function and tissue-specific gene expression of 3β-hydroxysteroid dehydrogenase/5-ene-4-ene isomerase enzymes in classical and peripheral intracrine steroidogenic tissues. J. Steroid Biochem. Mol. Biol 43, 805–826 (1992). [DOI] [PubMed] [Google Scholar]
- 66.Lachance Y et al. Structure of the human type II 3β-hydroxysteroid dehydrogenase/Δ5Δ–4 isomerase 3β-HSD) gene: adrenal and gonadal specificity. DNA Cell Biol. 10, 701–711 (1991). [DOI] [PubMed] [Google Scholar]
- 67.Luu The V et al. Full length cDNA structure and deduced amino acid sequence of human 3β-hydroxy-5-ene steroid dehydrogenase. Mol. Endocrinol 3, 1310–1312 (1989). [DOI] [PubMed] [Google Scholar]
- 68.Nakamura Y et al. 3βHSD and CYB5A double positive adrenocortical cells during adrenal development/aging. Endocr. Res 40, 8–13 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Geissler WM et al. Male pseudohermaphroditism caused by mutations of testicular 17β-hydroxysteroid dehydrogenase 3. Nat. Genet 7, 34–39 (1994). [DOI] [PubMed] [Google Scholar]
- 70.Nakamura Y et al. Type 5 17β-hydroxysteroid dehydrogenase (AKR1C3) contributes to testosterone production in the adrenal reticularis. J. Clin. Endocrinol. Metab 94, 2192–2198 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dufort I, Rheault P, Huang XF, Soucy P & Luu-The V Characteristics of a highly labile human type 5 17β-hydroxysteroid dehydrogenase. Endocrinology 140, 568–574 (1999). [DOI] [PubMed] [Google Scholar]
- 72.Deyashiki Y et al. Molecular cloning of two human liver 3α-hydroxysteroid/dihydrodiol dehydrogenase isoenzymes that are identical with chlordecone reductase and bile-acid binder. Biochem. J 299, 545–552 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lin HK et al. Expression and characterization of recombinant type 2 3α-hydroxysteroid dehydrogenase (HSD) from human prostate: demonstration of bifunctional 3α/17β-HSD activity and cellular distribution. Mol. Endocrinol 11, 1971–1984 (1997). [DOI] [PubMed] [Google Scholar]
- 74.Qin K, Ehrmann DA, Cox N, Refetoff S & Rosenfield RL Identification of a functional polymorphism of the human type 5 17β-hydroxysteroid dehydrogenase gene associated with polycystic ovary syndrome. J. Clin. Endocrinol. Metab 91, 270–276 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mornet E, Dupont J, Vitek A & White PC Characterization of two genes encoding human steroid 11β-hydroxylase (P-450(11)β). J. Biol. Chem 264, 20961–20967 (1989). [PubMed] [Google Scholar]
- 76.Imamichi Y et al. 11-Ketotestosterone is a major androgen produced in human gonads. J. Clin. Endocrinol. Metab 101, 3582–3591 (2016). [DOI] [PubMed] [Google Scholar]
- 77.Axelrod LR, Kraemer DC, Burdett J Jr. & Goldzieher JW Biosynthesis of 11-hydroxyandrostenedione by human and baboon adrenals. Acta Endocrinol. 72, 545–550 (1973). [DOI] [PubMed] [Google Scholar]
- 78.Penning TM, Wangtrakuldee P & Auchus RJ Structural and functional biology of aldo-keto reductase steroid-transforming enzymes. Endocr. Rev 40, 447–475 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barnard M et al. 11-Oxygenated androgen precursors are the preferred substrates for aldo-keto reductase 1C3 (AKR1C3): implications for castration resistant prostate cancer. J. Steroid Biochem. Mol. Biol 183, 192–201 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Storbeck KH et al. 11β-Hydroxydihydrotestosterone and 11-ketodihydrotestosterone, novel C19 steroids with androgenic activity: a putative role in castration resistant prostate cancer? Mol. Cell. Endocrinol 377, 135–146 (2013). [DOI] [PubMed] [Google Scholar]
- 81.Andersson S & Russell DW Structural and biochemical properties of cloned and expressed human and rat steroid 5α-reductases. Proc. Natl Acad. Sci. USA 87, 3640–3644 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rosemberg E & Dorfman RI Biological activity of 9α-fluoro-11β-hydroxy-Δ4-androstene-3,17-dione. Proc. Soc. Exp. Biol. Med 99, 336–338 (1958). [PubMed] [Google Scholar]
- 83.Dorfman RI, Rooks WH II, Jones JB & Leman JD Androgenic activity of highly purified 5α-androstane and 5α-androstan-17β-ol. J. Med. Chem 9, 930–931 (1966). [DOI] [PubMed] [Google Scholar]
- 84.Campana C et al. Development of a novel cell based androgen screening model. J. Steroid Biochem. Mol. Biol 156, 17–22 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rege J et al. 11-Ketotestosterone is the dominant circulating bioactive androgen during normal and premature adrenarche. J. Clin. Endocrinol. Metab 103, 4589–4598 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pretorius E et al. 11-Ketotestosterone and 11-ketodihydrotestosterone in castration resistant prostate cancer: potent androgens which can no longer be ignored. PLoS One 11, e0159867 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rainey WE, Carr BR, Sasano H, Suzuki T & Mason JI Dissecting human adrenal androgen production. Trends Endocrinol. Metab 13, 234–239 (2002). [DOI] [PubMed] [Google Scholar]
- 88.Cutler GB Jr. & Loriaux DL Andrenarche and its relationship to the onset of puberty. Fed. Proc 39, 2384–2390 (1980). [PubMed] [Google Scholar]
- 89.Havelock JC, Auchus RJ & Rainey WE The rise in adrenal androgen biosynthesis: adrenarche. Semin. Reprod. Med 22, 337–347 (2004). [DOI] [PubMed] [Google Scholar]
- 90.Hui XG et al. Development of the human adrenal zona reticularis: morphometric and immunohistochemical studies from birth to adolescence. J. Endocrinol 203, 241–252 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Remer T, Boye KR, Hartmann MF & Wudy SA Urinary markers of adrenarche: reference values in healthy subjects, aged 3–18 years. J. Clin. Endocrinol. Metab 90, 2015–2021 (2005). [DOI] [PubMed] [Google Scholar]
- 92.Korth-Schutz S, Levine LS & New MI Dehydroepiandrosterone sulfate (DS) levels, a rapid test for abnormal adrenal androgen secretion. J. Clin. Endocrinol. Metab 42, 1005–1013 (1976). [DOI] [PubMed] [Google Scholar]
- 93.Korth-Schutz S, Levine LS & New MI Serum androgens in normal prepubertal and pubertal children and in children with precocious adrenarche. J. Clin. Endocrinol. Metab 42, 117–124 (1976). [DOI] [PubMed] [Google Scholar]
- 94.Guran T et al. Reference values for serum dehydroepiandrosterone-sulphate in healthy children and adolescents with emphasis on the age of adrenarche and pubarche. Clin. Endocrinol 82, 712–718 (2015). [DOI] [PubMed] [Google Scholar]
- 95.Rosenfield RL & Lucky AW Acne, hirsutism, and alopecia in adolescent girls. Clinical expressions of androgen excess. Endocrinol. Metab. Clin. North Am 22, 507–532 (1993). [PubMed] [Google Scholar]
- 96.Arlt W et al. Dehydroepiandrosterone replacement in women with adrenal insufficiency. N. Engl. J. Med 341, 1013–1020 (1999). [DOI] [PubMed] [Google Scholar]
- 97.Arlt W et al. Oral dehydroepiandrosterone for adrenal androgen replacement: pharmacokinetics and peripheral conversion to androgens and estrogens in young healthy females after dexamethasone suppression. J. Clin. Endocrinol. Metab 83, 1928–1934 (1998). [DOI] [PubMed] [Google Scholar]
- 98.Ke Y et al. Serum levels of sex steroids and metabolites following 12 weeks of intravaginal 0.50% DHEA administration. J. Steroid Biochem. Mol. Biol 154, 186–196 (2015). [DOI] [PubMed] [Google Scholar]
- 99.Labrie F et al. Effect of intravaginal DHEA on serum DHEA and eleven of its metabolites in postmenopausal women. J. Steroid Biochem. Mol. Biol 111, 178–194 (2008). [DOI] [PubMed] [Google Scholar]
- 100.O’Reilly MW et al. AKR1C3-mediated adipose androgen generation drives lipotoxicity in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab 102, 3327–3339 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Swart AC & Storbeck KH 11β-Hydroxyandrostenedione: downstream metabolism by 11βHSD, 17βHSD and SRD5A produces novel substrates in familiar pathways. Mol. Cell. Endocrinol 408, 114–123 (2015). [DOI] [PubMed] [Google Scholar]
- 102.Kroboth PD, Salek FS, Pittenger AL, Fabian TJ & Frye RF DHEA and DHEA-S: a review. J. Clin. Pharmacol 39, 327–348 (1999). [DOI] [PubMed] [Google Scholar]
- 103.Kirschner MA & Bardin CW Androgen production and metabolism in normal and virilized women. Metabolism 21, 667–688 (1972). [DOI] [PubMed] [Google Scholar]
- 104.Abraham GE Ovarian and adrenal contribution to peripheral androgens during the menstrual cycle. J. Clin. Endocrinol. Metab 39, 340–346 (1974). [DOI] [PubMed] [Google Scholar]
- 105.Longcope C Adrenal and gonadal androgen secretion in normal females. Clin. Endocrinol. Metab 15, 213–228 (1986). [DOI] [PubMed] [Google Scholar]
- 106.Eisenhofer G et al. Reference intervals for plasma concentrations of adrenal steroids measured by LC-MS/MS: impact of gender, age, oral contraceptives, body mass index and blood pressure status. Clin. Chim. Acta 470, 115–124 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Elmlinger MW, Kuhnel W, Wormstall H & Doller PC Reference intervals for testosterone, androstenedione and SHBG levels in healthy females and males from birth until old age. Clin. Lab 51, 625–632 (2005). [PubMed] [Google Scholar]
- 108.Rothman MS et al. Reexamination of testosterone, dihydrotestosterone, estradiol and estrone levels across the menstrual cycle and in postmenopausal women measured by liquid chromatography–tandem mass spectrometry. Steroids 76, 177–182 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Davison SL, Bell R, Donath S, Montalto JG & Davis SR Androgen levels in adult females: changes with age, menopause, and oophorectomy. J. Clin. Endocrinol. Metab 90, 3847–3853 (2005). [DOI] [PubMed] [Google Scholar]
- 110.Haring R et al. Age-specific reference ranges for serum testosterone and androstenedione concentrations in women measured by liquid chromatography–tandem mass spectrometry. J. Clin. Endocrinol. Metab 97, 408–415 (2012). [DOI] [PubMed] [Google Scholar]
- 111.Wu FC et al. Hypothalamic–pituitary–testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European male aging study. J. Clin. Endocrinol. Metab 93, 2737–2745 (2008). [DOI] [PubMed] [Google Scholar]
- 112.Handelsman DJ et al. Age-specific population centiles for androgen status in men. Eur. J. Endocrinol 173, 809–817 (2015). [DOI] [PubMed] [Google Scholar]
- 113.Nanba AT et al. 11-Oxygenated C19 steroids do not decline with age in women. J. Clin. Endocrinol. Metab 104, 2615–2622 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Parker CR Jr. Dehydroepiandrosterone and dehydroepiandrosterone sulfate production in the human adrenal during development and aging. Steroids 64, 640–647 (1999). [DOI] [PubMed] [Google Scholar]
- 115.Parker CR Jr. et al. Effects of aging on adrenal function in the human: responsiveness and sensitivity of adrenal androgens and cortisol to adrenocorticotropin in premenopausal and postmenopausal women. J. Clin. Endocrinol. Metab 85, 48–54 (2000). [DOI] [PubMed] [Google Scholar]
- 116.Xing Y et al. The effects of ACTH on steroid metabolomic profiles in human adrenal cells. J. Endocrinol 209, 327–335 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kindler PM, Philipp DP, Gross MR & Bahr JM Serum 11-ketotestosterone and testosterone concentrations associated with reproduction in male bluegill (Lepomis macrochirus: centrarchidae). Gen. Comp. Endocrinol 75, 446–453 (1989). [DOI] [PubMed] [Google Scholar]
- 118.Feist G, Schreck CB, Fitzpatrick MS & Redding JM Sex steroid profiles of coho salmon (Oncorhynchus kisutch) during early development and sexual differentiation. Gen. Comp. Endocrinol 80, 299–313 (1990). [DOI] [PubMed] [Google Scholar]
- 119.Kobayashi M & Nakanishi T 11-Ketotestosterone induces male-type sexual behavior and gonadotropin secretion in gynogenetic crucian carp, Carassius auratus langsdorfii. Gen. Comp. Endocrinol 115, 178–187 (1999). [DOI] [PubMed] [Google Scholar]
- 120.Nagahama Y, Miura T & Kobayashi T The onset of spermatogenesis in fish. Ciba Found. Symp 182, 255–267 (1994). [DOI] [PubMed] [Google Scholar]
- 121.Miura T, Yamauchi K, Takahashi H & Nagahama Y The role of hormones in the acquisition of sperm motility in salmonid fish. J. Exp. Zool 261, 359–363 (1992). [DOI] [PubMed] [Google Scholar]
- 122.Rege J et al. Circulating 11-oxygenated androgens across species. J. Steroid Biochem. Mol. Biol 190, 242–249 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Polson DW, Reed MJ, Franks S, Scanlon MJ & James VH Serum 11β-hydroxyandrostenedione as an indicator of the source of excess androgen production in women with polycystic ovaries. J. Clin. Endocrinol. Metab 66, 946–950 (1988). [DOI] [PubMed] [Google Scholar]
- 124.Ibrahim F et al. Plasma 11β-hydroxy-4-androstene-3,17-dione: comparison of a time-resolved fluoroimmunoassay using a biotinylated tracer with a radioimmunoassay using a tritiated tracer. J. Steroid Biochem. Mol. Biol 84, 563–568 (2003). [DOI] [PubMed] [Google Scholar]
- 125.Therrell BL Newborn screening for congenital adrenal hyperplasia. Endocrinol. Metab. Clin. North. Am 30, 15–30 (2001). [DOI] [PubMed] [Google Scholar]
- 126.Speiser PW et al. High frequency of nonclassical steroid 21-hydroxylase deficiency. Am. J. Hum. Genet 37, 650–667 (1985). [PMC free article] [PubMed] [Google Scholar]
- 127.Turcu AF & Auchus RJ Adrenal steroidogenesis and congenital adrenal hyperplasia. Endocrinol. Metab. Clin. North. Am 44, 275–296 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fluck CE, Miller WL & Auchus RJ The 17,20-lyase activity of cytochrome p450c17 from human fetal testis favors the Δ5 steroidogenic pathway. J. Clin. Endocrinol. Metab 88, 3762–3766 (2003). [DOI] [PubMed] [Google Scholar]
- 129.Speiser PW et al. Disease expression and molecular genotype in congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J. Clin. Invest 90, 584–595 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Krone N, Braun A, Roscher AA, Knorr D & Schwarz HP Predicting phenotype in steroid 21-hydroxylase deficiency? Comprehensive genotyping in 155 unrelated, well defined patients from southern Germany. J. Clin. Endocrinol. Metab 85, 1059–1065 (2000). [DOI] [PubMed] [Google Scholar]
- 131.Charmandari E, Matthews DR, Johnston A, Brook CG & Hindmarsh PC Serum cortisol and 17-hydroxyprogesterone interrelation in classic 21-hydroxylase deficiency: is current replacement therapy satisfactory? J. Clin. Endocrinol. Metab 86, 4679–4685 (2001). [DOI] [PubMed] [Google Scholar]
- 132.Rezvani I, Garibaldi LR, Digeorge AM & Artman HG Disproportionate suppression of dehydroepiandrosterone sulfate (DHEAS) in treated patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Pediatr. Res 17, 131–134 (1983). [DOI] [PubMed] [Google Scholar]
- 133.Carmina E, Stanczyk FZ, Chang L, Miles RA & Lobo RA The ratio of androstenedione:11β-hydroxyandrostenedione is an important marker of adrenal androgen excess in women. Fertil. Steril 58, 148–152 (1992). [DOI] [PubMed] [Google Scholar]
- 134.Huerta R et al. 11β-Hydroxyandrostenedione and Δ5-androstenediol as markers of adrenal androgen production in patients with 21-hydroxylase-deficient nonclassic adrenal hyperplasia. Fertil. Steril 72, 996–1000 (1999). [DOI] [PubMed] [Google Scholar]
- 135.Kamrath C, Wettstaedt L, Boettcher C, Hartmann MF & Wudy SA Androgen excess is due to elevated 11-oxygenated androgens in treated children with congenital adrenal hyperplasia. J. Steroid Biochem. Mol. Biol 178, 221–228 (2018). [DOI] [PubMed] [Google Scholar]
- 136.Turcu AF et al. 11-Oxygenated androgens are biomarkers of adrenal volume and testicular adrenal rest tumors in 21-hydroxylase deficiency. J. Clin. Endocrinol. Metab 102, 2701–2710 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Claahsen-van der Grinten HL, Sweep FC, Blickman JG, Hermus AR & Otten BJ Prevalence of testicular adrenal rest tumours in male children with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Eur. J. Endocrinol 157, 339–344 (2007). [DOI] [PubMed] [Google Scholar]
- 138.Albright F, Smith PH & Fraser R A syndrome characterized by primary ovarian insufficiency and decreased stature: report of 11 cases with a digression on hormonal control of axillary and pubic hair. Am. J. Med. Sci 2014, 625–648 (1942). [Google Scholar]
- 139.Ibanez L et al. Postpubertal outcome in girls diagnosed of premature pubarche during childhood: increased frequency of functional ovarian hyperandrogenism. J. Clin. Endocrinol. Metab 76, 1599–1603 (1993). [DOI] [PubMed] [Google Scholar]
- 140.Idkowiak J et al. Premature adrenarche: novel lessons from early onset androgen excess. Eur. J. Endocrinol 165, 189–207 (2011). [DOI] [PubMed] [Google Scholar]
- 141.Talbot NB, Butler AM, Berman RA, Rodriguez PM & Maclachlan EA Excretion of 17-keto steroids by normal and abnormal children. Am. J. Dis. Child 65, 364–375 (1943). [Google Scholar]
- 142.Voutilainen R & Jaaskelainen J Premature adrenarche: etiology, clinical findings, and consequences. J. Steroid Biochem. Mol. Biol 145, 226–236 (2015). [DOI] [PubMed] [Google Scholar]
- 143.Herman-Giddens ME et al. Secondary sexual characteristics and menses in young girls seen in office practice: a study from the pediatric research in office settings network. Pediatrics 99, 505–512 (1997). [DOI] [PubMed] [Google Scholar]
- 144.Voutilainen R, Perheentupa J & Apter D Benign premature adrenarche: clinical features and serum steroid levels. Acta Paediatr. Scand 72, 707–711 (1983). [DOI] [PubMed] [Google Scholar]
- 145.Sopher AB et al. Bone age advancement in prepubertal children with obesity and premature adrenarche: possible potentiating factors. Obesity 19, 1259–1264 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kaplowitz P & Soldin SJ Steroid profiles in serum by liquid chromatography–tandem mass spectrometry in infants with genital hair. J. Pediatr. Endocrinol. Metab 20, 597–605 (2007). [DOI] [PubMed] [Google Scholar]
- 147.Novello L & Speiser PW Premature adrenarche. Pediatr. Ann 47, e7–e11 (2018). [DOI] [PubMed] [Google Scholar]
- 148.Saenger P & Dimartino-Nardi J Premature adrenarche. J. Endocrinol. Invest 24, 724–733 (2001). [DOI] [PubMed] [Google Scholar]
- 149.Celik N et al. The association between premature adrenarche and cardiovascular risk may be greater than expected. Horm. Res. Paediatr 87, 7–14 (2017). [DOI] [PubMed] [Google Scholar]
- 150.Ibanez L et al. Hyperinsulinemia and decreased insulin-like growth factor-binding protein-1 are common features in prepubertal and pubertal girls with a history of premature pubarche. J. Clin. Endocrinol. Metab 82, 2283–2288 (1997). [DOI] [PubMed] [Google Scholar]
- 151.Ibanez L, Potau N, Chacon P, Pascual C & Carrascosa A Hyperinsulinaemia, dyslipaemia and cardiovascular risk in girls with a history of premature pubarche. Diabetologia 41, 1057–1063 (1998). [DOI] [PubMed] [Google Scholar]
- 152.Mathew RP et al. Evidence of metabolic syndrome in lean children with premature pubarche at diagnosis. Metabolism 57, 733–740 (2008). [DOI] [PubMed] [Google Scholar]
- 153.Kaya G, Yavas Abali Z, Bas F, Poyrazoglu S & Darendeliler F Body mass index at the presentation of premature adrenarche is associated with components of metabolic syndrome at puberty. Eur. J. Pediatr 177, 1593–1601 (2018). [DOI] [PubMed] [Google Scholar]
- 154.Ibanez L, Potau N & Carrascosa A Insulin resistance, premature adrenarche, and a risk of the polycystic ovary syndrome (PCOS). Trends Endocrinol. Metab 9, 72–77 (1998). [DOI] [PubMed] [Google Scholar]
- 155.Kousta E Premature adrenarche leads to polycystic ovary syndrome? Long-term consequences. Ann. N. Y. Acad. Sci 1092, 148–157 (2006). [DOI] [PubMed] [Google Scholar]
- 156.Legro RS Detection of insulin resistance and its treatment in adolescents with polycystic ovary syndrome. J. Pediatr. Endocrinol. Metab 15, 1367–1378 (2002). [PubMed] [Google Scholar]
- 157.Rosenfield RL Clinical review: Identifying children at risk for polycystic ovary syndrome. J. Clin. Endocrinol. Metab 92, 787–796 (2007). [DOI] [PubMed] [Google Scholar]
- 158.Siklar Z, Ocal G, Adiyaman P, Ergur A & Berberoglu M Functional ovarian hyperandrogenism and polycystic ovary syndrome in prepubertal girls with obesity and/or premature pubarche. J. Pediatr. Endocrinol. Metab 20, 475–481 (2007). [DOI] [PubMed] [Google Scholar]
- 159.Ibanez L et al. Hyperinsulinemia in postpubertal girls with a history of premature pubarche and functional ovarian hyperandrogenism. J. Clin. Endocrinol. Metab 81, 1237–1243 (1996). [DOI] [PubMed] [Google Scholar]
- 160.Ollila MM et al. Weight gain and dyslipidemia in early adulthood associate with polycystic ovary syndrome: prospective cohort study. J. Clin. Endocrinol. Metab 101, 739–747 (2016). [DOI] [PubMed] [Google Scholar]
- 161.West S et al. Irregular menstruation and hyperandrogenaemia in adolescence are associated with polycystic ovary syndrome and infertility in later life: Northern Finland Birth Cohort 1986 study. Hum. Reprod 29, 2339–2351 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Banerjee S et al. Hormonal findings in African-American and Caribbean Hispanic girls with premature adrenarche: implications for polycystic ovarian syndrome. Pediatrics 102, E36 (1998). [DOI] [PubMed] [Google Scholar]
- 163.Toscano V et al. Changes in steroid pattern following acute and chronic adrenocorticotropin administration in premature adrenarche. J. Steroid Biochem 32, 321–326 (1989). [DOI] [PubMed] [Google Scholar]
- 164.Kim SH, Moon JY, Sasano H, Choi MH & Park MJ Body fat mass is associated with ratio of steroid metabolites reflecting 17,20-lyase activity in prepubertal girls. J. Clin. Endocrinol. Metab 101, 4653–4660 (2016). [DOI] [PubMed] [Google Scholar]
- 165.Vuguin P, Linder B, Rosenfeld RG, Saenger P & DiMartino-Nardi J The roles of insulin sensitivity, insulin-like growth factor I (IGF-I), and IGF-binding protein-1 and -3 in the hyperandrogenism of African-American and Caribbean Hispanic girls with premature adrenarche. J. Clin. Endocrinol. Metab 84, 2037–2042 (1999). [DOI] [PubMed] [Google Scholar]
- 166.Paterson WF et al. Exaggerated adrenarche in a cohort of Scottish children: clinical features and biochemistry. Clin. Endocrinol 72, 496–501 (2010). [DOI] [PubMed] [Google Scholar]
- 167.Jamieson A et al. Apparent cortisone reductase deficiency: a functional defect in 11β-hydroxysteroid dehydrogenase type 1. J. Clin. Endocrinol. Metab 84, 3570–3574 (1999). [DOI] [PubMed] [Google Scholar]
- 168.Malunowicz EM, Romer TE, Urban M & Bossowski A 11β-Hydroxysteroid dehydrogenase type 1 deficiency (‘apparent cortisone reductase deficiency’) in a 6-year-old boy. Horm. Res 59, 205–210 (2003). [DOI] [PubMed] [Google Scholar]
- 169.Charmandari E, Kino T & Chrousos GP Primary generalized familial and sporadic glucocorticoid resistance (Chrousos syndrome) and hypersensitivity. Endocr. Dev 24, 67–85 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lappalainen S, Utriainen P, Kuulasmaa T, Voutilainen R & Jaaskelainen J Androgen receptor gene CAG repeat polymorphism and X-chromosome inactivation in children with premature adrenarche. J. Clin. Endocrinol. Metab 93, 1304–1309 (2008). [DOI] [PubMed] [Google Scholar]
- 171.Likitmaskul S et al. ‘Exaggerated adrenarche’ in children presenting with premature adrenarche. Clin. Endocrinol 42, 265–272 (1995). [DOI] [PubMed] [Google Scholar]
- 172.Utriainen P, Laakso S, Liimatta J, Jaaskelainen J & Voutilainen R Premature adrenarche — a common condition with variable presentation. Horm. Res. Paediatr 83, 221–231 (2015). [DOI] [PubMed] [Google Scholar]
- 173.Kaufman FR et al. Dehydroepiandrosterone and dehydroepiandrosterone sulfate metabolism in human genital skin. Fertil. Steril 54, 251–254 (1990). [PubMed] [Google Scholar]
- 174.Pelletier G Expression of steroidogenic enzymes and sex-steroid receptors in human prostate. Best. Pract. Res. Clin. Endocrinol. Metab 22, 223–228 (2008). [DOI] [PubMed] [Google Scholar]
- 175.Rosenfield RL Hirsutism and the variable response of the pilosebaceous unit to androgen. J. Investig. Dermatol. Symp. Proc 10, 205–208 (2005). [DOI] [PubMed] [Google Scholar]
- 176.Liimatta J et al. Serum androgen bioactivity is low in children with premature adrenarche. Pediatr. Res 75, 645–650 (2014). [DOI] [PubMed] [Google Scholar]
- 177.Azziz R et al. The prevalence and features of the polycystic ovary syndrome in an unselected population. J. Clin. Endocrinol. Metab 89, 2745–2749 (2004). [DOI] [PubMed] [Google Scholar]
- 178.Fauser BC et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-sponsored 3rd PCOS Consensus Workshop Group. Fertil. Steril 97, 28–38 (2012). [DOI] [PubMed] [Google Scholar]
- 179.Lauritsen MP et al. The prevalence of polycystic ovary syndrome in a normal population according to the Rotterdam criteria versus revised criteria including anti-Mullerian hormone. Hum. Reprod 29, 791–801 (2014). [DOI] [PubMed] [Google Scholar]
- 180.Goodman NF et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome — part 1. Endocr. Pract 21, 1291–1300 (2015). [DOI] [PubMed] [Google Scholar]
- 181.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril 81, 19–25 (2004). [DOI] [PubMed] [Google Scholar]
- 182.Azziz R et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil. Steril 91, 456–488 (2009). [DOI] [PubMed] [Google Scholar]
- 183.Randeva HS et al. Cardiometabolic aspects of the polycystic ovary syndrome. Endocr. Rev 33, 812–841 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Legro RS Polycystic ovary syndrome and cardiovascular disease: a premature association? Endocr. Rev 24, 302–312 (2003). [DOI] [PubMed] [Google Scholar]
- 185.Wild RA Long-term health consequences of PCOS. Hum. Reprod. Update 8, 231–241 (2002). [DOI] [PubMed] [Google Scholar]
- 186.Han TS, Walker BR, Arlt W & Ross RJ Treatment and health outcomes in adults with congenital adrenal hyperplasia. Nat. Rev. Endocrinol 10, 115–124 (2014). [DOI] [PubMed] [Google Scholar]
- 187.Stahl NL, Teeslink CR, Beauchamps G & Greenblatt RB Serum testosterone levels in hirsute women: a comparison of adrenal, ovarian and peripheral vein values. Obstet. Gynecol 41, 650–654 (1973). [PubMed] [Google Scholar]
- 188.Stahl NL, Teeslink CR & Greenblatt RB Ovarian, adrenal, and peripheral testosterone levels in the polycystic ovary syndrome. Am. J. Obstet. Gynecol 117, 194–200 (1973). [DOI] [PubMed] [Google Scholar]
- 189.Barnes RB, Rosenfield RL, Burstein S & Ehrmann DA Pituitary–ovarian responses to nafarelin testing in the polycystic ovary syndrome. N. Engl. J. Med 320, 559–565 (1989). [DOI] [PubMed] [Google Scholar]
- 190.Ehrmann DA, Rosenfield RL, Barnes RB, Brigell DF & Sheikh Z Detection of functional ovarian hyperandrogenism in women with androgen excess. N. Engl. J. Med 327, 157–162 (1992). [DOI] [PubMed] [Google Scholar]
- 191.Lachelin GC et al. Long term effects of nightly dexamethasone administration in patients with polycystic ovarian disease. J. Clin. Endocrinol. Metab 55, 768–773 (1982). [DOI] [PubMed] [Google Scholar]
- 192.O’Reilly MW et al. Hyperandrogenemia predicts metabolic phenotype in polycystic ovary syndrome: the utility of serum androstenedione. J. Clin. Endocrinol. Metab 99, 1027–1036 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Conway G et al. The polycystic ovary syndrome: a position statement from the European Society of Endocrinology. Eur. J. Endocrinol 171, 1–29 (2014). [DOI] [PubMed] [Google Scholar]
- 194.Pasquali R et al. Defining hyperandrogenism in women with polycystic ovary syndrome: a challenging perspective. J. Clin. Endocrinol. Metab 101, 2013–2022 (2016). [DOI] [PubMed] [Google Scholar]
- 195.Hoffman DI, Klove K & Lobo RA The prevalence and significance of elevated dehydroepiandrosterone sulfate levels in anovulatory women. Fertil. Steril 42, 76–81 (1984). [DOI] [PubMed] [Google Scholar]
- 196.Lucky AW, Rosenfield RL, McGuire J, Rudy S & Helke J Adrenal androgen hyperresponsiveness to adrenocorticotropin in women with acne and/or hirsutism: adrenal enzyme defects and exaggerated adrenarche. J. Clin. Endocrinol. Metab 62, 840–848 (1986). [DOI] [PubMed] [Google Scholar]
- 197.Azziz R, Boots LR, Parker CR Jr., Bradley E Jr. & Zacur HA 11β-Hydroxylase deficiency in hyperandrogenism. Fertil. Steril 55, 733–741 (1991). [PubMed] [Google Scholar]
- 198.Stanczyk FZ, Chang L, Carmina E, Putz Z & Lobo RA Is 11β-hydroxyandrostenedione a better marker of adrenal androgen excess than dehydroepiandrosterone sulfate? Am. J. Obstet. Gynecol 165, 1837–1842 (1991). [DOI] [PubMed] [Google Scholar]
- 199.O’Reilly MW et al. 11-Oxygenated C19 steroids are the predominant androgens in polycystic ovary syndrome. J. Clin. Endocrinol. Metab 102, 840–848 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Heidenreich A et al. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur. Urol 65, 467–479 (2014). [DOI] [PubMed] [Google Scholar]
- 201.Montgomery RB et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 68, 4447–4454 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Titus MA, Schell MJ, Lih FB, Tomer KB & Mohler JL Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin. Cancer Res 11, 4653–4657 (2005). [DOI] [PubMed] [Google Scholar]
- 203.de Bono JS et al. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med 364, 1995–2005 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ryan CJ et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med 368, 138–148 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Scher HI et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med 367, 1187–1197 (2012). [DOI] [PubMed] [Google Scholar]
- 206.Pfeiffer MJ, Smit FP, Sedelaar JP & Schalken JA Steroidogenic enzymes and stem cell markers are upregulated during androgen deprivation in prostate cancer. Mol. Med 17, 657–664 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hamid AR et al. Aldo-keto reductase family 1 member C3 (AKR1C3) is a biomarker and therapeutic target for castration-resistant prostate cancer. Mol. Med 18, 1449–1455 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Chang KH et al. A gain-of-function mutation in DHT synthesis in castration-resistant prostate cancer. Cell 154, 1074–1084 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Mitsiades N et al. Distinct patterns of dysregulated expression of enzymes involved in androgen synthesis and metabolism in metastatic prostate cancer tumors. Cancer Res. 72, 6142–6152 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Titus MA et al. Steroid 5α-reductase isozymes I and II in recurrent prostate cancer. Clin. Cancer Res 11, 4365–4371 (2005). [DOI] [PubMed] [Google Scholar]
- 211.Chang KH et al. Dihydrotestosterone synthesis bypasses testosterone to drive castration-resistant prostate cancer. Proc. Natl Acad. Sci. USA 108, 13728–13733 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Gent R, du Toit T, Bloem LM & Swart AC The 11β-hydroxysteroid dehydrogenase isoforms: pivotal catalytic activities yield potent C11-oxy C19 steroids with 11βHSD2 favouring 11-ketotestosterone, 11-ketoandrostenedione and 11-ketoprogesterone biosynthesis. J. Steroid Biochem. Mol. Biol 189, 116–126 (2019). [DOI] [PubMed] [Google Scholar]
- 213.Dovio A et al. Differential expression of determinants of glucocorticoid sensitivity in androgen-dependent and androgen-independent human prostate cancer cell lines. J. Steroid Biochem. Mol. Biol 116, 29–36 (2009). [DOI] [PubMed] [Google Scholar]
- 214.Page N, Warriar N & Govindan MV 11β-Hydroxysteroid dehydrogenase and tissue specificity of androgen action in human prostate cancer cell LNCaP. J. Steroid Biochem. Mol. Biol 49, 173–181 (1994). [DOI] [PubMed] [Google Scholar]
- 215.Gao X et al. Functional silencing of HSD17B2 in prostate cancer promotes disease progression. Clin. Cancer Res 25, 1291–1301 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.du Toit T et al. Profiling adrenal 11β-hydroxyandrostenedione metabolites in prostate cancer cells, tissue and plasma: UPC2-MS/MS quantification of 11β-hydroxytestosterone, 11keto-testosterone and 11keto-dihydrotestosterone. J. Steroid Biochem. Mol. Biol 166, 54–67 (2017). [DOI] [PubMed] [Google Scholar]
- 217.du Toit T & Swart AC Inefficient UGT-conjugation of adrenal 11β-hydroxyandrostenedione metabolites highlights C11-oxy C19 steroids as the predominant androgens in prostate cancer. Mol. Cell. Endocrinol 461, 265–276 (2018). [DOI] [PubMed] [Google Scholar]