Abstract
The aim of the current study was to evaluate the effect of sustained physiologic increase of ∼50 mg/dL in plasma glucose concentration on insulin secretion in normal glucose-tolerant (NGT) subjects. Twelve NGT subjects without family history of type 2 diabetes mellitus (T2DM; FH−) and 8 NGT with family history of T2DM (FH+) received an oral glucose tolerance test and two-step hyperglycemic clamp (100 and 300 mg/dL) followed by intravenous arginine bolus before and after 72-h glucose infusion. Fasting plasma glucose increased from 94 ± 2 to 142 ± 4 mg/dL for 72 h. First-phase insulin secretion (0–10 min) increased by 70%, while second-phase insulin secretion during the first (10–80 min) and second (90–160 min) hyperglycemic clamp steps increased by 3.8-fold and 1.9-fold, respectively, following 72 h of physiologic hyperglycemia. Insulin sensitivity during hyperglycemic clamp declined by ∼30% and ∼55% (both P < 0.05), respectively, during the first and second hyperglycemic clamp steps. Insulin secretion/insulin resistance (disposition) index declined by 60% (second clamp step) and by 62% following arginine (both P < 0.005) following 72-h glucose infusion. The effect of 72-h glucose infusion on insulin secretion and insulin sensitivity was similar in subjects with and without FH of T2DM. Following 72 h of physiologic hyperglycemia, metabolic clearance rate of insulin was markedly reduced (P < 0.01). These results demonstrate that sustained physiologic hyperglycemia for 72 h 1) increases absolute insulin secretion but impairs β-cell function, 2) causes insulin resistance, and 3) reduces metabolic clearance rate of insulin.
Introduction
β-Cell dysfunction and insulin resistance in liver and muscle represent the core pathophysiologic defects in type 2 diabetes mellitus (T2DM) (1,2). Persistent hyperglycemia has been shown to impair insulin-mediated glucose disposal in humans and to induce β-cell dysfunction in animal models of diabetes (3–6). These deleterious effects of sustained hyperglycemia have been referred to as glucotoxicity (7). Prolonged exposure of human islets to hyperglycemia decreases insulin content and impairs secretion (8,9). In humans, enhanced insulin secretion has been noted with improved glycemic control (10,11). In contrast, deterioration of insulin secretion has been shown in animal models exposed to chronic hyperglycemia. In pancreatectomized rats, physiologic hyperglycemia results in marked β-cell dysfunction, while correction of the hyperglycemia improves the defect in insulin secretion (12). A number of mechanisms have been proposed to explain the glucotoxic effect of hyperglycemia on β-cell function, including enhanced hexosamine flux, increased reactive oxygen species, decreased PDX-1 expression, and increased endoplasmic reticulum stress (13–16).
In humans, the effect of chronic hyperglycemia on β-cell function is less clear. Some studies have shown no effect (17), others have shown increased insulin secretion in response to prolonged hyperglycemia (18–20), while some studies have demonstrated a deleterious effect on β-cell function (21,22). Further, while mild hyperglycemia (8.8 mmol/L) failed to show any deleterious effect on insulin secretion, more severe hyperglycemia (12.6 mmol/L) caused marked impairment in β-cell function (22). In part, these divergent results may be explained by the variable degree of hyperglycemia, duration of hyperglycemia, methods used to assess β-cell function, and species differences (i.e., humans vs. rodents). Further, none of these previous studies accounted for the change in insulin sensitivity when evaluating changes in β-cell function following prolonged exposure to hyperglycemia.
The current study was designed to evaluate the effect of a sustained (72-h) physiologic increase (∼50 mg/dL) in plasma glucose concentration on insulin secretion, β-cell function, and insulin sensitivity in normal glucose-tolerant (NGT) subjects without family history of diabetes (FH−). Since we previously have shown that NGT individuals with family history of diabetes (FH+) are more susceptible to the deleterious effects of lipotoxicity (23), we also examined if NGT subjects with strong FH of T2DM are more susceptible to glucotoxicity than healthy subjects without FH of T2DM.
Research Design and Methods
Subjects
Clinical and laboratory characteristics of FH− and FH+ subjects are shown in Table 1. FH+ subjects had at least two first-degree relatives with T2DM. Twelve healthy NGT subjects without FH of T2DM and 8 healthy NGT subjects with FH of T2DM participated in study. No subject was taking any medication known to affect glucose metabolism. All subjects were in good general health as determined by medical history, physical examination, screening blood tests, urinalysis, and electrocardiogram. Body weight was stable (±3 pounds) in all subjects for at least 3 months prior to study, and no subject participated in an excessively heavy exercise program. All studies were performed on the Bartter Research Unit (BRU), South Texas Veterans Health Care System, Audie L. Murphy VA Hospital in San Antonio, TX. The study was approved by the Institutional Review Board of the University of Texas Health Science Center at San Antonio, TX, and all study participants signed written informed consent prior to participation. The data sets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Table 1.
Clinical and laboratory characteristics of participants
| FH− | FH+ | P value | |
|---|---|---|---|
| Number | 12 | 8 | |
| Age (years) | 50 ± 4 | 48 ± 3 | NS |
| Sex (male/female) | 9/3 | 4/4 | NS |
| BMI (kg/m2) | 26 ± 0.9 | 26 ± 0.6 | NS |
| HbA1c (mmol/mol) | 12.0 ± 0.2 | 11.9 ± 0.2 | NS |
| FPG (mg/dL) | 95 ± 2 | 93 ± 3 | NS |
| 2-h glucose (mg/dL) | 102 ± 6 | 107 ± 5 | NS |
| FPI (µU/mL) | 9 ± 1 | 11 ± 2 | NS |
| 2-h insulin (µU/mL) | 32 ± 6 | 46 ± 9 | NS |
| F-CP (ng/mL) | 2.6 ± 0.5 | 2.3 ± 0.2 | NS |
| 2-h CP (ng/mL) | 9.4 ± 1.6 | 13.5 ± 1.8 | NS |
| Matsuda index | 5.30 ± 0.7 | 3.63 ± 0.5 | 0.05 |
| ΔCP/Δglucose (0–120 min) | 0.27 ± 0.04 | 0.29 ± 0.04 | NS |
| SBP (mmHg) | 121 ± 5 | 128 ± 3 | NS |
| DBP (mmHg) | 75 ± 4 | 77 ± 3 | NS |
| Total cholesterol (mg/dL) | 161 ± 10 | 192 ± 13 | NS |
| Triglyceride (mg/dL) | 90 ± 15 | 225 ± 82 | NS |
| HDL (mg/dL) | 49 ± 2 | 42 ± 3 | NS |
| LDL (mg/dL) | 94 ± 9 | 114 ± 8 | NS |
Data are mean ± SE or n. CP, C-peptide; DBP, diastolic blood pressure; F, fasting; FPG, fasting plasma glucose; FPI, fasting plasma insulin; SBP, systolic blood pressure.
Oral Glucose Tolerance Test
All subjects received a 75-g oral glucose tolerance test (OGTT) at 8:00 a.m. following 10-h overnight fast to document normal glucose tolerance. Blood samples were drawn at −30, −15, and 0 min and every 15 min thereafter for 2 h for determination of plasma glucose, insulin, and C-peptide concentrations. Body weight and height were recorded. Total body fat content and percent body fat were determined by DXA on the day of the OGTT.
Hyperglycemic Clamp
Subjects were admitted to BRU at 6:30 a.m. on the day of study after a 10–12-h overnight fast. A catheter was placed into an antecubital vein for infusion of all test substances. A second catheter was inserted retrogradely into a vein on the dorsum of the hand, which was inserted into heated box (60°C) to obtain arterialized blood. At the start of the hyperglycemic clamp (time zero), plasma glucose concentration was acutely raised and maintained at 100 mg/dL above baseline for 80 min by a prime-variable infusion of 20% glucose (24). Plasma glucose, insulin, and C-peptide were measured every 2 min during the first 10 min and every 5–15 min for 70 min. At 80 min, plasma glucose concentration was acutely raised and maintained at 400 mg/dL for an additional 110 min. Plasma glucose, insulin, and C-peptide were measured every 2 min from 80 to 90 min and every 5–15 min from 90 to 160 min. This provided a maximum hyperglycemic stimulus to insulin secretion. At 160 min, an intravenous bolus of 5 g of arginine was given, and plasma insulin response was followed for an additional 30 min. Plasma glucose, insulin, and C-peptide were measured every 2 min for 10 min following arginine bolus injection and every 5 min for the subsequent 20 min. Combined hyperglycemia (400 mg/dL) and arginine provides a further stimulus for insulin secretion (25,26). Following completion of the hyperglycemic clamp, subjects received a meal and returned to home.
Within 3–5 days, subjects returned to BRU and received a variable glucose infusion to raise plasma glucose concentration by ∼50 mg/dL for 72 h. During the hospitalization for the 72 h, saline infusion subjects were on a standard isocaloric weight-maintaining diet consisting of 50% carbohydrates, 20% fat, and 30% protein. During the hospital admissions for the 72-h dextrose infusion, the dietary caloric intake was moderately reduced to prevent weight gain. During both hospital admissions, participants were completely ambulatory while receiving the dextrose and saline infusions.
On the morning of day 4, the glucose infusion was stopped at 6:00 a.m. and plasma glucose concentration allowed to drop spontaneously to the fasting level. This ensured that both the increment in plasma glucose concentration and the absolute plasma glucose concentration achieved during the hyperglycemic clamp were virtually identical in the studies performed before and after 72 h of glucose and saline infusion. At 8:00 a.m., the two-step hyperglycemic clamp with arginine was repeated exactly as previously described.
Calculations
The acute insulin (I0–10) response, the insulin secretory rate (ISR0–10), and glucose-potentiated arginine-stimulated insulin secretion (IArg 0–10) were calculated as the incremental area under the curve (AUC) for the first 10 min of each hyperglycemic clamp step (0–10 and 80–90 min) and following arginine (160–170 min). Second-phase insulin secretion during the two steps of the hyperglycemic clamp were calculated as the incremental AUC for insulin from 10–80 and 90–160 min. Insulin sensitivity was calculated as the mean glucose infusion rate during last 20 min (60–80 and 140–160 min) of each hyperglycemic clamp step divided by mean plasma insulin concentration during the corresponding period (M/I). The insulin secretion/insulin resistance (IS/IR) (disposition) index was calculated for the first and second phases of insulin secretion as product of: M/I × CP0–10, M/I × CP10–80, M/I × CP90–160, and M/I × CPArg 0–10, where CP = C-peptide. Since similar glucose levels were achieved during both hyperglycemic clamp steps before and after chronic glucose infusion, we did not adjust for the plasma glucose concentration during the calculation of the IS/IR index. The ISR was calculated from deconvolution of the plasma C-peptide concentration curve (27). The metabolic clearance rate of insulin (MCRI) was calculated as the ratio of the rate of insulin disappearance, RdIns, divided by the plasma insulin concentration (28). During the fasting state, RdIns = ISR so MCRI was calculated as the ratio of ISR and plasma insulin concentration (i.e., MCRI[0] = ISR[0]/I[0]). During the hyperglycemic clamp, insulin Rd was calculated, taking into account the change in plasma insulin concentration (i.e., as RdIns[t] = ISR[t] − dI[t]/dt × VIns), and insulin clearance was calculated as MCRI (t) = RdIns(t)/I(t), where VIns is the volume of distribution of insulin, which was estimated as 140 mL/kg (29–31).
Analytical Measurements
Plasma glucose concentration was determined by glucose oxidase reaction (Analox Glucose Analyzer; Beckman Coulter, Fullerton, CA). Plasma insulin and C-peptide concentrations were measured by radioimmunoassay (Coat-A-Count; Diagnostic Products Corp., Los Angeles, CA).
Sample Size Estimation
We hypothesized that the 72-h glucose infusion to create sustained physiologic hyperglycemia would reduce parameters of β-cell function and insulin sensitivity in NGT subjects by ∼25%, similar to values reported in individuals with prediabetes with impaired glucose tolerance. Based upon this assumption, we estimated that 12 NGT subjects would be required with a power of 90% and α = 0.05.
Statistical Analysis
Data are presented as mean ± SEM. Comparison of variables before and after 72 h of glucose or saline infusion was performed using a paired, two-tailed Student t test. When more than two groups were analyzed, ANOVA was used. P < 0.05 was considered statistically significant.
Data and Resource Availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Results
OGTT
FH+ subjects had slightly, but not significantly, higher fasting and 2-h plasma insulin concentrations (Table 1). FH+ subjects also had a slightly reduced Matsuda index of insulin sensitivity (3.63 ± 0.50 vs. 5.30 ± 0.70; P = 0.05), although there was no difference in indices of insulin secretion obtained from OGTT between FH+ and FH− groups (Table 1).
Chronic Glucose Infusion
Baseline fasting plasma glucose (94 ± 2 mg/dL) was increased to 140 ± 2, 142 ± 2, and 143 ± 2 mg/dL after 24, 48, and 72 h, respectively. After 72 h, the glucose infusion was discontinued and plasma glucose returned to the fasting plasma glucose level (90 ± 2 mg/dL) prior to start of hyperglycemic clamp.
Hyperglycemic Clamp Before and After 72 h of Glucose Infusion
Plasma glucose concentrations during the hyperglycemic clamp before and after 72-h glucose infusion were similar in FH− and FH+ subjects (Fig. 1A and B). The fasting plasma insulin concentration was slightly higher (P < 0.05) following 72-h glucose infusion in both FH− (14 ± 1 vs. 9 ± 1 mU/L) and FH+ (19 ± 3 vs. 13 ± 1 mU/L) (Fig. 1C and D). The fasting plasma C-peptide concentration showed a similar trend following 72-h glucose infusion in FH− (3.7 ± 0.3 vs. 2.4 ± 0.2 ng/mL) and FH+ (3.6 ± 0.2 vs. 3.0 ± 0.4 ng/mL) (both P < 0.05) subjects (Fig. 1E and F).
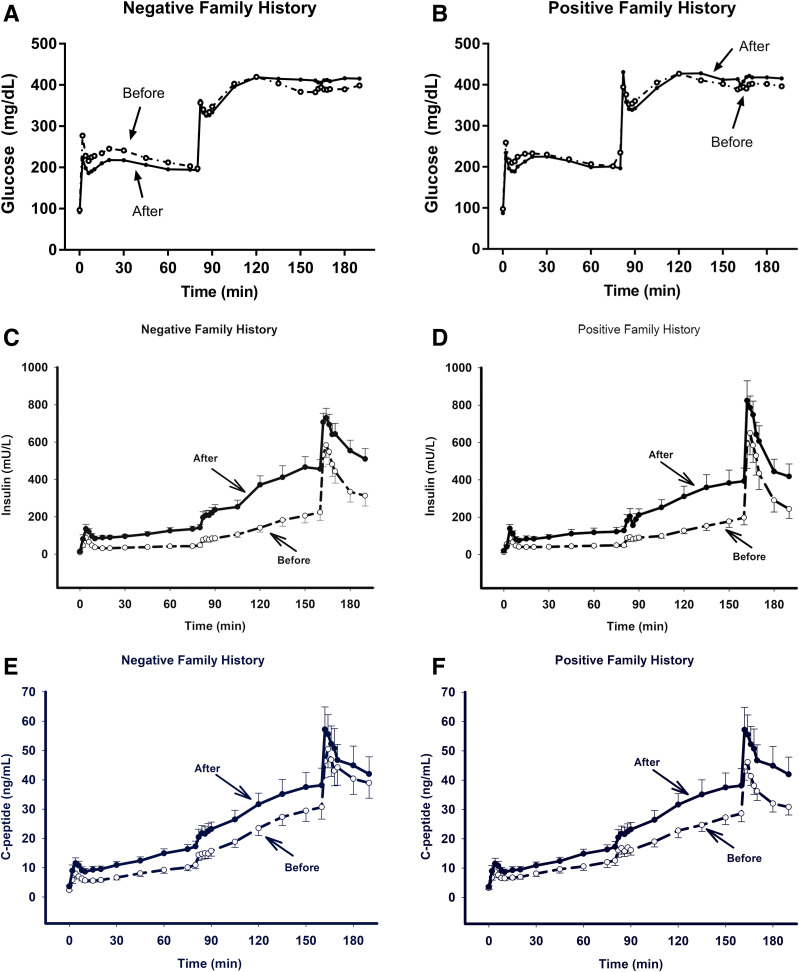
Figure 1.
Plasma glucose (A and B), insulin (C and D), and C-peptide (E and F) concentrations and ISR (G and H) during the two-step hyperglycemic clamp in FH− and FH+ subjects before and after 72-h glucose infusion. Conven., conventional.
During the hyperglycemic clamp (Figs. 1 and 2), the increment in first-phase insulin response (0–10 min) was significantly higher (P < 0.05) following 72 h of glucose infusion in both the FH− (450 ± 78 vs. 817 ± 152 mU/L · min) and FH+ (436 ± 28 vs. 722 ± 116 mU/L · min; P < 0.05) (Fig. 2A). The first-phase ISR (0–10 min) following 72-h glucose infusion paralleled the increase in plasma insulin concentration in FH− (15,403 ± 2,768 vs. 22,938 ± 3,865 pmol/min; P = 0.02) subjects, although the change was not significant in FH+ (16,051 ± 1,765 vs. 16,451 ± 2,669 pmol/min; P = NS) subjects. The increment in plasma insulin/increment in plasma glucose and increment in plasma C-peptide/increment in plasma glucose paralleled the incremental changes in plasma insulin and C-peptide concentrations since the increment in plasma glucose concentration was similar during the hyperglycemic clamps performed before and after the 72-h glucose infusion.
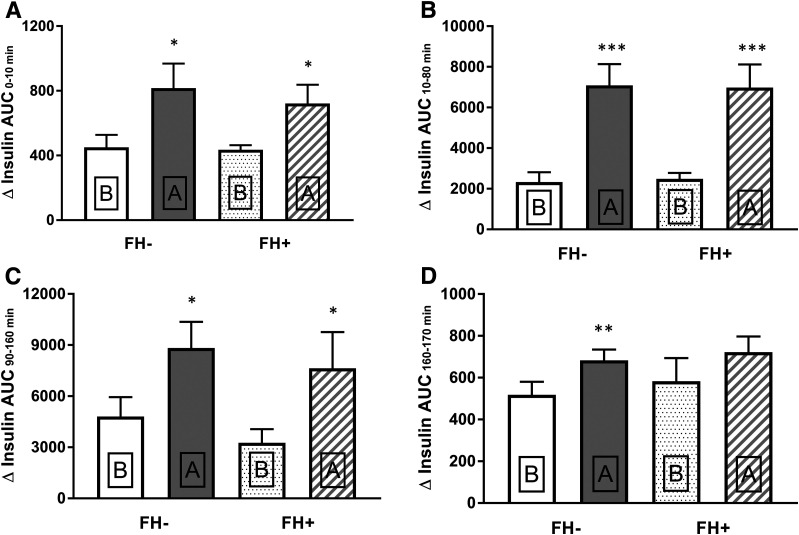
Figure 2.
First-phase (0–10 min) and second-phase (10–80 min) plasma insulin responses during the first hyperglycemic clamp step (100 mg/dL) before (B) and after (A) 72-h glucose infusion in FH− and FH+ subjects (A and B). Second-phase (90–160 min) plasma insulin response during the second hyperglycemic clamp step (300 mg/dL) (C) and glucose-potentiated arginine-stimulated plasma insulin response (160–170 min) (D) before and after 72-h glucose infusion.
The increment in second-phase insulin secretion (AUC) during first hyperglycemic clamp step (10–80 min) after 72 h of glucose infusion was significantly greater (P < 0.05) in both FH− (7,090 ± 1,047 vs. 2,327 ± 489 mU/L · min; P < 0.005) and FH+ (6,979 ± 1,298 vs. 2,483 ± 336 mU/L · min; P < 0.005) groups (Fig. 2B). During the second hyperglycemic clamp step (90–160 min), the increment in second-phase insulin secretion (AUC) also was greater in both FH− (8,823 ± 1,542 vs. 4,802 ± 1,139 mU/L · min; P < 0.005) and FH+ (7,632 ± 2,134 vs. 3,265 ± 805 mU/L · min; P = 0.02) (Fig. 2C) groups following 72 h of glucose infusion. Following 72-h glucose infusion, the plasma insulin response to arginine increased modestly (FH−) or did not change significantly (FH+) (Fig. 2D).
MCRI
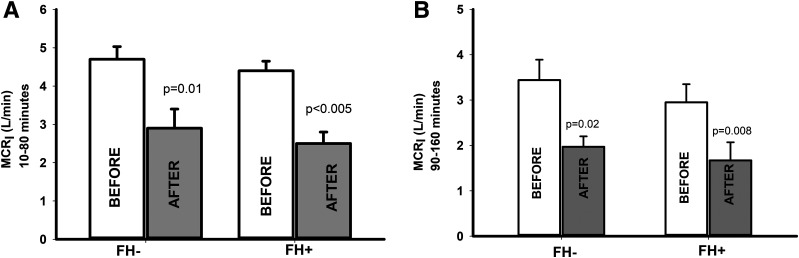
Following 72-h glucose infusion, the plasma C-peptide response during the 10–80- and 90–160-min time periods was less compared with the plasma insulin response in both FH− and FH+ groups. The MCRI during both the 10–80 and 90–160 min of the first and second hyperglycemic steps was significantly (P < 0.05) reduced in both FH− and FH+ subjects (Fig. 3).
Figure 3.
MCRI in FH− and FH+ subjects during the first (10–80 min) (A) and second (90–160 min) (B) hyperglycemic clamp steps before and after 72-h glucose infusion.
β-Cell Sensitivity to Glucose
Despite similar plasma glucose levels during the hyperglycemic clamp, insulin secretion (deconvolution of plasma C-peptide curve) was greater following chronic glucose infusion (Fig. 1G and H). The rate of increase in plasma insulin concentration in relation to the increase in plasma glucose concentration was greater following the chronic glucose infusion (Fig. 4).
Figure 4.
β-Cell sensitivity to glucose before and after 72-h glucose infusion.
Insulin Sensitivity (M/I Ratio)
Insulin sensitivity (M10–80/I10–80) during the first hyperglycemic clamp step decreased significantly following 72 h of glucose infusion in both FH− (15.9 ± 3.5 to 12.0 ± 3.0, mg/kg/min per mU/L; P = 0.02) and FH+ (16.6 ± 3.0 to 12.6 ± 2.3 mg/kg/min per mU/L; P = 0.03) subjects. The decline in insulin sensitivity was more pronounced during the second hyperglycemic clamp step (M90–160/I90–160) in both FH− (18.6 ± 4.6 to 8.1 ± 2.0; P = 0.001) and FH+ (16.7 ± 3.7 vs. 7.6 ± 1.3; P = 0.005) subjects (Fig. 5).
Figure 5.
Insulin sensitivity (M/I) in FH− and FH+ subjects before and after 72-h glucose infusion during the first (10–80 min) (A) and second (90–160 min) (B) hyperglycemic clamp steps.
IS/IR (ΔC-peptide × M/I) Index
The IS/IR (disposition) index in FH− subjects during the first hyperglycemic clamp step (10–80 min) (2,278 ± 488 to 2,397 ± 501; P = NS) did not change significantly. During the second hyperglycemic clamp step (90–160 min) (8,389 ± 1,901 to 3,555 ± 933; P = 0.009) and during arginine infusion (160–170 min) (2,796 ± 578 to 910 ± 137; P < 0.05), the IS/IR index declined significantly following 72-h glucose infusion (Fig. 6). The decline in IS/IR index in FH+ subjects paralleled that in FH− subjects (Fig. 6).
Figure 6.
IS/IR (disposition) index before and after 72-h glucose infusion in FH− and FH+ subjects during the first (10–80 min) (A) and second (90–160 min) (B) hyperglycemic clamp steps. Cpep, C-peptide.
Discussion
The major findings of the current study are that 72 h of sustained physiologic hyperglycemia (50 mg/dL) in healthy NGT subjects with or without FH of T2DM: 1) enhanced insulin secretion in absolute terms but caused a decrease in the IS/IR (disposition) index, which was most evident during the second hyperglycemic clamp step; 2) resulted in a decrease in tissue sensitivity to insulin; and 3) markedly reduced the MCRI. Unlike our previous study, which demonstrated that 12 h of lipid infusion markedly reduced β-cell function in FH+ but not in FH− subjects (23), the present results demonstrate a similar glucotoxic effect in both groups.
Controversy exists concerning the effect of prolonged hyperglycemia on β-cell function. Some studies have demonstrated that chronic hyperglycemia enhances insulin secretion (18–20,32), while some studies have shown no effect (17), or even a decrease (21,22). Multiple factors can explain these discordant results including; magnitude and duration of hyperglycemia; failure to take into account changes in MCRI; use of plasma insulin concentration rather than plasma C-peptide concentration as the index of β-cell function; and failure to account for changes in insulin sensitivity and express β-cell function as the IS/IR (disposition) index. The current study takes into account all of these variables. First, the hyperglycemic clamp was used to quantitate plasma insulin and C-peptide responses to a fixed, highly reproducible glycemic stimulus. Second, the hyperglycemic stimulus was physiologic, ∼50 mg/dL and within the range of fasting plasma glucose levels observed in patients with T2DM. Third, the duration of hyperglycemia, 72 h, was sufficiently prolonged to observe a deleterious effect on β-cell function. Fourth, measurement of plasma C-peptide concentration allowed for a more direct measure of insulin secretion. Fifth, because the hyperglycemia clamp provides a measure of insulin sensitivity, one can calculate the IS/IR (disposition) index, which provides a more precise assessment of β-cell function (24) than can be obtained from simply measuring insulin secretion. Sixth, because plasma C-peptide and insulin concentrations were simultaneously measured, one can examine the effect of chronic hyperglycemia on the MCRI. Lastly, combined hyperglycemia (400 mg/dL) plus arginine infusion provides a supraphysiologic stimulus to the β-cell response to secrete insulin.
Although the plasma C-peptide response tended to parallel the plasma insulin response (Fig. 1C and D vs. Fig. 1E and F), the magnitude of response during both hyperglycemic clamp steps was less than the insulin response. Calculation of the MCRI confirmed a marked and highly significant reduction in MCRI (Fig. 3). Thus, the increased plasma insulin response following 72 h of hyperglycemia (Fig. 1) resulted from a small insignificant increase in insulin secretion and a large significant reduction in MCRI. This observation underscores the need to use the plasma C-peptide, not the plasma insulin, response when calculating the IS/IR (disposition) index as a measure of β-cell function, especially when there are large changes in insulin sensitivity, as occurred in the current study and in our previous study with the insulin-sensitizing agent pioglitazone (33) and in insulin-resistant states (34–36). The mechanism by which sustained hyperglycemia impairs the MCRI or the organ (liver, kidney, and muscle) responsible for the decrease in MCRI is not answered by the present results. We previously demonstrated that a small physiologic increase in plasma insulin concentration from 7 to only 21 μU/mL for 72 h caused marked insulin resistance (37), similar to that observed in the current study. Since insulin degradation involves binding of insulin to its receptorvfollowed by internalization and subsequent degradation (38,39), it is possible that the hyperinsulinemia-induced insulin resistance interferes with the internalization-degradation process or inhibits insulin-degrading enzyme (40). Alternatively, hyperglycemia per se or some metabolic consequence of hyperglycemia is responsible for the decreased MCRI. Elevated plasma free fatty acids (FFAs) can reduce the MCRI (41). Since the fasting plasma FFA concentration can be expected to be markedly reduced following 72 h of hyperglycemia, this is an unlikely explanation for the decrease in MCRI. Further, since elevated plasma FFAs cause insulin resistance (23,42), while reduction of plasma FFAs enhances insulin sensitivity (43), the severity of hyperglycemia-induced insulin resistance in the current study would be underestimated. Most studies that have examined the molecular mechanisms via which hyperglycemia impairs β-cell function have used cultured β-cells in vitro or in animal models (5,44,45). In these studies, decreased insulin biosynthesis and increased β-cell apoptosis related to impaired PDX-1 activity have been identified (46). Chronic hyperglycemia also increases reactive oxygen species generation (47) and enhances hexosamine flux (48), both of which can impair insulin secretion.
In response to the first hyperglycemic clamp step, there was a small nonsignificant decrease in IS/IR index. However, during the second hyperglycemic clamp step and in response to arginine, the IS/IR index was markedly reduced (Fig. 6). To our knowledge, this is the first study in vivo in humans to document a reduction in β-cell function in response to a progressively increasing β-cell stress. These results are in agreement with an in vitro study performed with cultured human islets (13,49). In response to an acute hyperglycemic stimulus, insulin/C-peptide are secreted from a readily releasable pool (50). However, after prolonged exposure to glucose, insulin secretion is more dependent on release of recently synthesized proinsulin (51). The present results are compatible with a defect in the β-cells’ ability to respond to an acute hyperglycemic stimulus by enhanced secretion of newly formed insulin. Arginine increases insulin secretion by β-cell membrane depolarization (52) and, in combination with hyperglycemia, leads to a further increase in insulin secretion. Nonetheless, the effect of 72 h of physiologic hyperglycemia on glucose-stimulated and arginine-stimulated insulin secretion was very similar. The marked defect in arginine-stimulated insulin secretion underscores the previous suggestion that sustained hyperglycemia for only 72 h impairs β-cell insulin secretion when exposed to progressively increasing stress. The decrease in IS/IR index (disposition index) cannot be explained by reduced β-cell glucose sensitivity (Fig. 4), which, in contrast, was increased, perhaps reflecting a compensatory response to offset the decline in insulin sensitivity.
There are some limitations to the current study. While the hyperglycemic clamp remains the gold standard to evaluate the β-cell response to glucose, it does not allow one to evaluate the contribution of the incretin axis (i.e., glucagon-like peptide 1 and glucose-dependent insulinotropic polypeptide) to the hyperglycemia-induced defect in β-cell function (20). The duration of hyperglycemia, 72 h, is relatively short compared with the natural history of development of hyperglycemia in T2DM, and more prolonged hyperglycemia (i.e., weeks to months) could have produced a more pronounced β-cell defect. However, it is difficult to keep healthy NGT subjects on the Clinical Research Center (at South Texas Veterans Health Care System) for >3–4 days. Nonetheless, the present results demonstrated that 72 h of physiologic hyperglycemia is sufficient to induce significant insulin resistance. Therefore, we believe that it is reasonable to assume that the presence of hyperglycemia in patients with diabetes (which exists for much longer than 72 h) contributes, at least in part, to the development of insulin resistance and impaired β-cell function. Consistent with this, we have shown that treatment of patients with T2DM for 12 days with dapagliflozin to lower the plasma glucose concentration significantly improved both insulin sensitivity and β-cell function (53).
In summary, sustained (72-h) physiologic (50 mg/dL) hyperglycemia in NGT subjects leads to an increased plasma insulin response that results from a modest increase in insulin secretion, enhanced β-cell sensitivity to glucose, and a marked decrease in MCRI. β-Cell function, quantitated by the IS/IR index during the hyperglycemic clamp and insulin secretion in response to acute arginine administration, was significantly impaired by chronic exposure to physiologic hyperglycemia.
Article Information
Funding. This work was supported by National Institutes of Health grant DK-24092-36 (to R.A.D.). D.T.’s salary is supported by the South Texas Veterans Health Care System, Audie L. Murphy VA Hospital.
Duality of Interest. C.S.-H. is a speaker for Novo Nordisk. R.A.D. is on the advisory board of AstraZeneca, Novo Nordisk, Janssen, Boehringer Ingelheim, and Intarcia Therapeutics; received research support from Boehringer Ingelheim, AstraZeneca, Janssen, and Merck; and is on the speakers bureaus of Novo Nordisk and AstraZeneca. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. R.A.D. designed the study. A.M. and D.T. conducted the study. A.M., D.T., X.C., and R.A.D. wrote the manuscript and researched data. R.A.D. reviewed and edited the manuscript. I.V., M.A.-G., C.S.-H., and A.G. contributed to discussion and reviewed and edited the manuscript. R.A.D. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
A.M. and D.T. contributed equally to the completion of the study.
References
- 1.Defronzo RA Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009;58:773–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tripathy D, Eriksson KF, Orho-Melander M, Fredriksson J, Ahlqvist G, Groop L. Parallel manifestation of insulin resistance and beta cell decompensation is compatible with a common defect in type 2 diabetes. Diabetologia 2004;47:782–793 [DOI] [PubMed] [Google Scholar]
- 3.Dubois M, Vacher P, Roger B, et al. Glucotoxicity inhibits late steps of insulin exocytosis. Endocrinology 2007;148:1605–1614 [DOI] [PubMed] [Google Scholar]
- 4.Kuo M, Zilberfarb V, Gangneux N, Christeff N, Issad T. O-GlcNAc modification of FoxO1 increases its transcriptional activity: a role in the glucotoxicity phenomenon? Biochimie 2008;90:679–685 [DOI] [PubMed] [Google Scholar]
- 5.Leibowitz G, Yuli M, Donath MY, et al. beta-cell glucotoxicity in the Psammomys obesus model of type 2 diabetes. Diabetes 2001;50(Suppl. 1):S113–S117 [DOI] [PubMed] [Google Scholar]
- 6.Maedler K, Sergeev P, Ris F, et al. Glucose-induced β cell production of IL-1β contributes to glucotoxicity in human pancreatic islets. J Clin Invest 2017;127:1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rossetti L, Giaccari A, DeFronzo RA. Glucose toxicity. Diabetes Care 1990;13:610–630 [DOI] [PubMed] [Google Scholar]
- 8.Eizirik DL, Korbutt GS, Hellerström C. Prolonged exposure of human pancreatic islets to high glucose concentrations in vitro impairs the beta-cell function. J Clin Invest 1992;90:1263–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Alessandris C, Andreozzi F, Federici M, et al. Increased O-glycosylation of insulin signaling proteins results in their impaired activation and enhanced susceptibility to apoptosis in pancreatic beta-cells. FASEB J 2004;18:959–961 [DOI] [PubMed] [Google Scholar]
- 10.Højberg PV, Vilsbøll T, Rabøl R, et al. Four weeks of near-normalisation of blood glucose improves the insulin response to glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes. Diabetologia 2009;52:199–207 [DOI] [PubMed] [Google Scholar]
- 11.Hu Y, Li L, Xu Y, et al. Short-term intensive therapy in newly diagnosed type 2 diabetes partially restores both insulin sensitivity and β-cell function in subjects with long-term remission. Diabetes Care 2011;34:1848–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossetti L, Shulman GI, Zawalich W, DeFronzo RA. Effect of chronic hyperglycemia on in vivo insulin secretion in partially pancreatectomized rats. J Clin Invest 1987;80:1037–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maedler K, Sergeev P, Ris F, et al. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest 2002;110:851–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marchetti P, Bugliani M, Lupi R, et al. The endoplasmic reticulum in pancreatic beta cells of type 2 diabetes patients. Diabetologia 2007;50:2486–2494 [DOI] [PubMed] [Google Scholar]
- 15.Maris M, Ferreira GB, D’Hertog W, et al. High glucose induces dysfunction in insulin secretory cells by different pathways: a proteomic approach. J Proteome Res 2010;9:6274–6287 [DOI] [PubMed] [Google Scholar]
- 16.Tanaka Y, Gleason CE, Tran POT, Harmon JS, Robertson RP. Prevention of glucose toxicity in HIT-T15 cells and Zucker diabetic fatty rats by antioxidants. Proc Natl Acad Sci U S A 1999;96:10857–10862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flax H, Matthews DR, Levy JC, Coppack SW, Turner RC. No glucotoxicity after 53 hours of 6.0 mmol/l hyperglycaemia in normal man. Diabetologia 1991;34:570–575 [DOI] [PubMed] [Google Scholar]
- 18.Boden G, Chen X, Kolaczynski JW, Polansky M. Effects of prolonged hyperinsulinemia on serum leptin in normal human subjects. J Clin Invest 1997;100:1107–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Byrne MM, Sturis J, Polonsky KS. Insulin secretion and clearance during low-dose graded glucose infusion. Am J Physiol 1995;268:E21–E27 [DOI] [PubMed] [Google Scholar]
- 20.Vollmer K, Gardiwal H, Menge BA, et al. Hyperglycemia acutely lowers the postprandial excursions of glucagon-like Peptide-1 and gastric inhibitory polypeptide in humans. J Clin Endocrinol Metab 2009;94:1379–1385 [DOI] [PubMed] [Google Scholar]
- 21.Solomon TP, Knudsen SH, Karstoft K, Winding K, Holst JJ, Pedersen BK. Examining the effects of hyperglycemia on pancreatic endocrine function in humans: evidence for in vivo glucotoxicity. J Clin Endocrinol Metab 2012;97:4682–4691 [DOI] [PubMed] [Google Scholar]
- 22.Boden G, Ruiz J, Kim CJ, Chen X. Effects of prolonged glucose infusion on insulin secretion, clearance, and action in normal subjects. Am J Physiol 1996;270:E251–E258 [DOI] [PubMed] [Google Scholar]
- 23.Kashyap S, Belfort R, Gastaldelli A, et al. A sustained increase in plasma free fatty acids impairs insulin secretion in nondiabetic subjects genetically predisposed to develop type 2 diabetes. Diabetes 2003;52:2461–2474 [DOI] [PubMed] [Google Scholar]
- 24.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–E223 [DOI] [PubMed] [Google Scholar]
- 25.Larsson H, Ahrén B. Glucose-dependent arginine stimulation test for characterization of islet function: studies on reproducibility and priming effect of arginine. Diabetologia 1998;41:772–777 [DOI] [PubMed] [Google Scholar]
- 26.Ward WK, Bolgiano DC, McKnight B, Halter JB, Porte D Jr. Diminished B cell secretory capacity in patients with noninsulin-dependent diabetes mellitus. J Clin Invest 1984;74:1318–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes 1992;41:368–377 [DOI] [PubMed] [Google Scholar]
- 28.Shapiro ET, Tillil H, Miller MA, et al. Insulin secretion and clearance. Comparison after oral and intravenous glucose. Diabetes 1987;36:1365–1371 [DOI] [PubMed] [Google Scholar]
- 29.Jung SH, Jung CH, Reaven GM, Kim SH. Adapting to insulin resistance in obesity: role of insulin secretion and clearance. Diabetologia 2018;61:681–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polidori DC, Bergman RN, Chung ST, Sumner AE. Hepatic and extrahepatic insulin clearance are differentially regulated: results from a novel model-based analysis of intravenous glucose tolerance data. Diabetes 2016;65:1556–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hovorka R, Powrie JK, Smith GD, Sönksen PH, Carson ER, Jones RH. Five-compartment model of insulin kinetics and its use to investigate action of chloroquine in NIDDM. Am J Physiol 1993;265:E162–E175 [DOI] [PubMed] [Google Scholar]
- 32.Ward WK, Halter JB, Beard JC, Porte D Jr. Adaptation of B and A cell function during prolonged glucose infusion in human subjects. Am J Physiol 1984;246:E405–E411 [DOI] [PubMed] [Google Scholar]
- 33.DeFronzo RA, Tripathy D, Abdul-Ghani M, Musi N, Gastaldelli A. The disposition index does not reflect β-cell function in IGT subjects treated with pioglitazone. J Clin Endocrinol Metab 2014;99:3774–3781 [DOI] [PubMed] [Google Scholar]
- 34.Lorenzo C, Hanley AJ, Wagenknecht LE, et al. Relationship of insulin sensitivity, insulin secretion, and adiposity with insulin clearance in a multiethnic population: the insulin Resistance Atherosclerosis study. Diabetes Care 2013;36:101–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marini MA, Frontoni S, Succurro E, et al. Decreased insulin clearance in individuals with elevated 1-h post-load plasma glucose levels. PLoS One 2013;8:e77440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shah A, Holter MM, Rimawi F, et al. Insulin clearance after oral and intravenous glucose post-gastric bypass and gastric banding weight loss. Diabetes Care 2019;42:311–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Del Prato S, Leonetti F, Simonson DC, Sheehan P, Matsuda M, DeFronzo RA. Effect of sustained physiologic hyperinsulinaemia and hyperglycaemia on insulin secretion and insulin sensitivity in man. Diabetologia 1994;37:1025–1035 [DOI] [PubMed] [Google Scholar]
- 38.Duckworth WC, Hamel FG, Peavy DE. Hepatic metabolism of insulin. Am J Med 1988;85:71–76 [DOI] [PubMed] [Google Scholar]
- 39.Sato H, Terasaki T, Mizuguchi H, Okumura K, Tsuji A. Receptor-recycling model of clearance and distribution of insulin in the perfused mouse liver. Diabetologia 1991;34:613–621 [DOI] [PubMed] [Google Scholar]
- 40.Farris W, Mansourian S, Chang Y, et al. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci U S A 2003;100:4162–4167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wiesenthal SR, Sandhu H, McCall RH, et al. Free fatty acids impair hepatic insulin extraction in vivo. Diabetes 1999;48:766–774 [DOI] [PubMed] [Google Scholar]
- 42.Groop LC, Bonadonna RC, Shank M, Petrides AS, DeFronzo RA. Role of free fatty acids and insulin in determining free fatty acid and lipid oxidation in man. J Clin Invest 1991;87:83–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bajaj M, Suraamornkul S, Romanelli A, et al. Effect of a sustained reduction in plasma free fatty acid concentration on intramuscular long-chain fatty Acyl-CoAs and insulin action in type 2 diabetic patients. Diabetes 2005;54:3148–3153 [DOI] [PubMed] [Google Scholar]
- 44.Leahy JL, Bonner-Weir S, Weir GC. Beta-cell dysfunction induced by chronic hyperglycemia. Current ideas on mechanism of impaired glucose-induced insulin secretion. Diabetes Care 1992;15:442–455 [DOI] [PubMed] [Google Scholar]
- 45.Leibowitz G, Uçkaya G, Oprescu AI, Cerasi E, Gross DJ, Kaiser N. Glucose-regulated proinsulin gene expression is required for adequate insulin production during chronic glucose exposure. Endocrinology 2002;143:3214–3220 [DOI] [PubMed] [Google Scholar]
- 46.Chen F, Sha M, Wang Y, et al. Transcription factor Ets-1 links glucotoxicity to pancreatic beta cell dysfunction through inhibiting PDX-1 expression in rodent models. Diabetologia 2016;59:316–324 [DOI] [PubMed] [Google Scholar]
- 47.Gerber PA, Rutter GA. The role of oxidative stress and hypoxia in pancreatic beta-cell dysfunction in diabetes mellitus. Antioxid Redox Signal 2017;26:501–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaneto H, Xu G, Song KH, et al. Activation of the hexosamine pathway leads to deterioration of pancreatic beta-cell function through the induction of oxidative stress. J Biol Chem 2001;276:31099–31104 [DOI] [PubMed] [Google Scholar]
- 49.Federici M, Hribal M, Perego L, et al. High glucose causes apoptosis in cultured human pancreatic islets of Langerhans: a potential role for regulation of specific Bcl family genes toward an apoptotic cell death program. Diabetes 2001;50:1290–1301 [DOI] [PubMed] [Google Scholar]
- 50.Bratanova-Tochkova TK, Cheng H, Daniel S, et al. Triggering and augmentation mechanisms, granule pools, and biphasic insulin secretion. Diabetes 2002;51(Suppl. 1):S83–S90 [DOI] [PubMed] [Google Scholar]
- 51.Rorsman P, Renström E. Insulin granule dynamics in pancreatic beta cells. Diabetologia 2003;46:1029–1045 [DOI] [PubMed] [Google Scholar]
- 52.Sener A, Blachier F, Rasschaert J, Mourtada A, Malaisse-Lagae F, Malaisse WJ. Stimulus-secretion coupling of arginine-induced insulin release: comparison with lysine-induced insulin secretion. Endocrinology 1989;124:2558–2567 [DOI] [PubMed] [Google Scholar]
- 53.Shannon C, Merovci A, Xiong J, et al. Effect of chronic hyperglycemia on glucose metabolism in subjects with normal glucose tolerance. Diabetes 2018;67:2507–2517 [DOI] [PMC free article] [PubMed] [Google Scholar]