Abstract
With the development of insulin resistance (IR), there is a compensatory increase in the plasma insulin response to offset the defect in insulin action to maintain normal glucose tolerance. The insulin response is the result of two factors: insulin secretion and metabolic clearance rate of insulin (MCRI). Subjects (104 with normal glucose tolerance [NGT], 57 with impaired glucose tolerance [IGT], and 207 with type 2 diabetes mellitus [T2DM]), divided in nonobese and obese groups, received a euglycemic insulin-clamp (40 mU/m2 ⋅ min) and an oral glucose tolerance test (OGTT) (75 g) on separate days. MCRI was calculated during the insulin-clamp performed with [3-3H]glucose and the OGTT and related to IR: peripheral (glucose uptake during the insulin clamp), hepatic (basal endogenous glucose production × fasting plasma insulin [FPI]), and adipocyte (fasting free fatty acid × FPI). MCRI during the insulin clamp was reduced in obese versus nonobese NGT (0.60 ± 0.03 vs. 0.73 ± 0.02 L/min ⋅ m2, P < 0.001), in nonobese IGT (0.62 ± 0.02, P < 0.004), and in nonobese T2DM (0.68 ± 0.02, P < 0.03). The MCRI during the insulin clamp was strongly and inversely correlated with IR (r = −0.52, P < 0.0001). During the OGTT, the MCRI was suppressed within 15–30 min in NGT and IGT subjects and remained suppressed. In contrast, suppression was minimal in T2DM. In conclusion, the development of IR in obese subjects is associated with a decline in MCRI that represents a compensatory response to maintain normal glucose tolerance but is impaired in individuals with T2DM.
Introduction
Type 2 diabetes mellitus (T2DM) is a complex metabolic disease with multiple pathophysiologic disturbances. However, in its simplest form, T2DM develops when there is an imbalance between insulin secretion and insulin resistance (IR) (1,2) and the plasma insulin response to an ingested meal or glucose is unable to compensate for the severity of IR (1–4). The relationship between the plasma insulin response and IR is curvilinear, with declining insulin sensitivity being matched proportionately by an increase in the plasma insulin concentration to maintain normal glucose tolerance (NGT) (2). With the development of T2DM, this curvilinear relationship is shifted down and to the left. This relationship is referred to as the disposition index and has been used as an index of β-cell function (2). However, the plasma insulin response is the composite of two separate variables: insulin secretion by the pancreatic β-cells (1–3) and the metabolic clearance rate of insulin (MCRI) (5,6). In normal glucose-tolerant subjects, the net result of these two distinct processes is coordinated to produce a plasma insulin response that precisely offsets the severity of IR to maintain NGT. Therefore, the disposition index is not a pure measure of β-cell function, and in some instances, the true measure of β-cell function (obtained by deconvolution of the plasma C-peptide response) can move in the opposite direction of the plasma insulin response. Thus, when individuals with T2DM are treated with pioglitazone, the disposition index (Δinsulin [I]/Δglucose [G] ÷ IR) declines, whereas insulin secretion (ΔISR [insulin secretory rate]/ΔG) and β-cell function (ΔISR/ΔG ÷ IR) increase (7). Thus, pioglitazone simultaneously increased insulin secretion (plasma C-peptide deconvolution) and augmented the MCRI (7). However, because the magnitude of increase in MCRI vastly outweighed the increase in insulin secretion, the plasma insulin response declined. Thus, if the plasma insulin concentration was used to calculate the disposition index, it erroneously would be concluded that pioglitazone treatment was associated with a decline in insulin secretion secondary to the improvement in insulin action (7), and the stimulatory effect of thiazolidinedione on insulin secretion would be missed. The important role of insulin clearance in determining the plasma insulin response and, thus, overall glucose tolerance has received recent emphasis by the observation that short-term (3-day) feeding of a high-carbohydrate/low-fat diet, independent of total energy intake, markedly reduced insulin clearance in healthy nonobese subjects. In contrast, 3 days of a high-fat/low-carbohydrate diet resulted in an increase in insulin clearance (8).
We previously showed that in NGT healthy subjects, the liver is the primary site of insulin removal throughout the physiologic range of plasma insulin concentrations (6). Hepatic insulin clearance involves insulin binding to its receptor, followed by endocytosis and subsequent intracellular degradation (9). Reduced insulin clearance in IR states has been related to total body obesity, especially visceral (10–12), diabetes (13), increased liver fat content (14,15), elevated plasma free fatty acid (FFA) (16), and IR per se (17,18). Most individuals with T2DM are obese, manifest moderate to severe hepatic and peripheral IR, and have increased liver fat (14,15). The aim of the current study was to identify metabolic conditions associated with reduced MCRI.
Research Design and Methods
Subjects
The study population comprised 368 individuals who participated in the San Antonio Metabolism (SAM) Study (4). Their clinical characteristics are shown in Table 1. Based on a 75-g oral glucose tolerance test (OGTT), there were 104 normal glucose tolerant (NGT) individuals, 57 subjects with impaired glucose tolerance (IGT), and 206 subjects with T2DM. Subjects with T2DM were drug naïve or were treated with metformin with or without sulfonylurea. By defining obesity as BMI ≥30 kg/m2, subjects were further divided into nonobese and obese groups. Of the study population, 47% of NGT, 60% of IGT, and 65% of T2DM were of Mexican American ethnicity, and the remainder were of Caucasian descent (P = NS between groups), reflecting the ethnic distribution in the San Antonio area. All subjects had normal liver, cardiopulmonary, and kidney function as determined by medical history, physical examination, screening blood tests, electrocardiogram, and urinalysis. No NGT or IGT subject was taking any medication known to affect glucose tolerance. No subject participated in any regular exercise program, and body weight was stable (±3 lb) for at least 3 months before the study in all subjects. Subjects with T2DM who were taking metformin or a sulfonylurea had their sulfonylurea stopped 3 days before the study. No subject with T2DM ever received thiazolidinedione or insulin. The study protocol was approved by The University of Texas Health Science Center Institutional Review Board.
Table 1.
Anthropometric and metabolic characteristics
| Nonobese | Obese | ||||||
|---|---|---|---|---|---|---|---|
| NGT n = 73 | IGT n = 28 | T2DM n = 97 | NGT n = 31 | IGT n = 29 | T2DM n = 110 | P value obese vs. nonobese | |
| Sex | 0.12 | ||||||
| Female | 42 | 14 | 36 | 19 | 17 | 64 | |
| Male | 31 | 14 | 61 | 12 | 12 | 46 | |
| Race/ethnicity | 0.11 | ||||||
| Caucasian | 38 | 13 | 37 | 17 | 10 | 35 | |
| Mexican American | 35 | 15 | 60 | 14 | 19 | 75 | |
| Age (years) | 40 ± 2 | 50 ± 2* | 53 ± 1* | 37 ± 2 | 41 ± 3# | 51 ± 1*§ | 0.73 |
| BMI (kg/m2) | 25.9 ± 0.3 | 27.5 ± 0.4* | 27.0 ± 0.2* | 37.0 ± 0.9# | 34.3 ± 0.6# | 35.4 ± 0.6# | <0.0001 |
| Waist (cm)† | 87.6 ± 1.5 | 98.4 ± 2.1* | 94.9 ± 1.1* | 109.7 ± 5.2 | 107.5 ± 3.1 | 109.3 ± 1. ± 2 | <0.0001 |
| HbA1c (%) | 4.9 ± 0.2 | 5.2 ± 0.4 | 7.8 ± 0.2*§ | 4.5 ± 0.4 | 4.9 ± 0.6 | 8.2 ± 0.2*§ | 0.007 |
| FPG (mg/dL) | 93 ± 1 | 104 ± 2 | 177 ± 5*,§ | 95 ± 1 | 99 ± 2 | 170 ± 4*§ | <0.02 |
| FPI (μU/mL) | 7.3 (3.1) | 10.8 (7.3)* | 12.3 (9.1)*§ | 11.8 (12.6)# | 13.4 (5.6)# | 18.4 (10.5)*§# | <0.0001 |
| F–C-peptide (ng/mL) | 1.4 ± 0.1 | 2.1 ± 0.2* | 2.2 ± 0.1* | 2.5 ± 0.3# | 2.3 ± 0.2 | 3.1 ± 0.1*§# | <0.0001 |
| F-FFA (mmol/L) | 0.62 ± 0.03 | 0.70 ± 0.05 | 0.69 ± 0.03 | 0.77 ± 0.04# | 0.75 ± 0.04 | 0.79 ± 0.02# | <0.0001 |
| HOMA-IR (mU/L × mmol/L) | 1.6 (0.6) | 2.8 (1.8)* | 5.2 (4.1)*§ | 2.5 (2.7)# | 3.3 (1.3) | 7.4 (4.3)*§# | <0.0001 |
| Rd (mg/kg ⋅ min) | 3.7 ± 0.3 | 2.9 ± 0.4* | 2.5 ± 0.1*§ | 2.3 ± 0.3# | 1.9 ± 0.2*# | 1.9 ± 0.1*§# | <0.0001 |
| Rd/SSPI (mg/kg ⋅ min/units/L) | 68 (36) | 45 (18)* | 34 (27)*§ | 31 (41)# | 31 (15)# | 25 (13)*§# | <0.0001 |
| Hepatic IR (EGP × FPI) | 13 (9) | 18 (15)* | 22 (17)* | 20 (14)# | 21 (8)# | 29 (15)*§# | <0.0001 |
| Adipose IR (FPI × F-FFA) | 4.2 (3.7) | 6.6 (5.3)* | 7.6 (6.8)* | 9.0 (11.7)# | 8.7 (5.3)# | 13.6 (7.6)*§# | <0.0001 |
| ISR-basal (pmol/min ⋅ m2) | 59 (46) | 81 (65)* | 90 (65)* | 102 (78)# | 112 (55)# | 127 (83)*§,# | <0.0001 |
| ISR-total (nmol/min ⋅ m2) | 35 (24) | 39 (22) | 20 (15)*§ | 49 (31)# | 41 (26) | 27 (21)*§# | 0.001 |
| ΔISR/ΔG ÷ IR | 47.2 (41.8) | 16.6 (8.6)* | 2.8 (3.7)*§ | 16.7 (31.0)# | 17.2 (12.4) | 2.6 (3.2)*§ | <0.0007 |
| ΔI/ΔG ÷ IR | 12.5 (10.9) | 5.1 (4.7)* | 0.9 (0.7)*§ | 9.2 (10.4) | 5.2 (2.7)* | 1.1 (0.9)*§# | 0.038 |
| MCRI clamp (L/min ⋅ m2) | 0.73 ± 0.02 | 0.62 ± 0.02* | 0.68 ± 0.02* | 0.60 ± 0.03# | 0.64 ± 0.02 | 0.67 ± 0.02* | <0.05 |
| Exogenous MCRI during clamp (L/min ⋅ m2) | 0.69 ± 0.02 | 0.56 ± 0.02* | 0.63 ± 0.02* | 0.55 ± 0.03# | 0.58 ± 0.02 | 0.61 ± 0.01* | <0.003 |
| MCRI fasting (L/min ⋅ m2) | 1.45 ± 0.1 | 1.30 ± 0.1 | 1.34 ± 0.1 | 1.29 ± 0.1 | 1.29 ± 0.1 | 1.17 ± 0.1 | 0.02 |
| MCRI OGTT (L/min ⋅ m2) | 0.91 ± 0.04 | 0.93 ± 0.08 | 1.28 ± 008*§ | 0.73 ± 0.06# | 0.76 ± 0.05 | 0.93 ± 0.04*§# | <0.0001 |
| RdIns (pmol/min ⋅ m2) | 285 ± 15 | 306 ± 23 | 185 ± 12*§ | 428 ± 38 | 386 ± 38 | 261 ± 18*§ | <0.002 |
Entries represent mean ± SEM or median (interquartile range) depending whether the variable was normally or not normally distributed. F-, fasting; FPG, fasting plasma glucose.
P < 0.05 vs. NGT.
P < 0.05 vs. IGT within nonobese and obese groups.
P < 0.05 obese vs. nonobese in each glucose tolerance group.
Data were available for 91 nonobese and 95 obese subjects.
Experimental Protocol
All metabolic tests were performed at the Clinical Research Center in the morning (∼7–8 a.m.) after a 10- to 12-h overnight fast, except water. During the OGTT, blood samples were obtained at −30, −15, 0, 30, 60, 90, and 120 min for measurement of plasma glucose, insulin, C-peptide, and FFA concentrations. On a separate day, subjects received a 120-min euglycemic insulin clamp (40 mU/m2 ⋅ min) with a 3H-tracer infusion (4). In subjects with T2DM, the plasma glucose concentration was allowed to decline to 100 mg/dL, at which level it was maintained. In NGT and IGT subjects, the plasma glucose concentration was clamped at the basal fasting level.
Analytical Techniques
Plasma glucose concentration was measured by the glucose oxidase reaction (Beckman Glucose Analyzer, Fullerton, CA). Plasma insulin and C-peptide were measured by radioimmunoassay (DPC, Los Angeles, CA), and plasma FFA was measured spectrophometrically (Wako, Neuss, Germany). Plasma [3-3H]glucose radioactivity was measured in Somogyi precipitates and used to calculate the plasma glucose-specific activity.
Calculations
Glucose and insulin areas under the OGTT curve (AUC) were calculated using the trapezoidal rule.
Glucose Fluxes
After an overnight fast, steady-state conditions prevail, and basal endogenous glucose production (EGP) equals the titrated glucose infusion rate (disintegrations per min/min) divided by the plasma titrated glucose-specific activity (disintegrations per min/mg). During the insulin clamp, nonsteady state conditions are present, and Ra and Rd were calculated with the Steele equation. Values for Rd represent those during the last 40 min of the insulin clamp.
IR
The hepatic IR index was calculated as the product of basal EGP and fasting plasma insulin (FPI) concentration (15). The adipocyte IR index was calculated as the product of the fasting plasma FFA concentration and FPI concentration (19). Peripheral tissue (primarily reflects muscle) IR was calculated as the inverse of total Rd during the last 40 min of the insulin clamp, normalized by the steady-state plasma insulin (SSPI) concentration (Per-IR = 1 ÷ [Rd/SSPI]).
Insulin Secretion
Basal and OGTT ISRs were calculated from deconvolution of the plasma C-peptide concentration curve based on the two-pool model of Van Cauter as described in Ferrannini et al. (3); for modeling analyses, we used the MLAB software (Civilized Software, Silver Spring, MD). As indices of β-cell function, we calculated the incremental ratio of AUCs of the insulin-to-glucose concentration during the OGTT and the incremental ratio of AUCs of the ISR to glucose concentration during OGTT factored by peripheral IR (ΔI/ΔG ÷ IR or ΔISR/ΔG ÷ IR) that are indexes of β-cell function.
Insulin Clearance
The MCRI during the euglycemic insulin clamp was calculated as the insulin infusion rate plus residual endogenous ISR divided by the SSPI concentration during the last 40 min of the insulin clamp (i.e., [insulin infusion rate + residual ISR] ÷ SSPI), while an estimate of the exogenous MCRI was calculated by computing (insulin infusion rate ÷ [SSPI − Ie]), where Ie is the endogenous insulin concentration during the clamp that was estimated as the FPI × C-peptide clamp/fasting C-peptide as previously described (6). During a 40 mU/min ⋅ m2 euglycemic insulin clamp, the suppression of C-peptide and endogenous insulin secretion is ∼50% of the fasting value, regardless of the state of IR (6,20–22). Because in this data set the measurement of C-peptide during the insulin clamp was not available, residual ISR during the insulin clamp was estimated as 50% of the fasting ISR, and Ie was estimated as 50% of fasting insulin according to our previous observation (6).
Insulin clearance (MCRI) during the fasting state was calculated as fasting ISR divided by FPI concentration, because at steady state RdIns = ISR. During the OGTT we used a single pool model to describe insulin kinetics, as previously proposed (23), where the input is the ISR obtained by deconvolution of the plasma C-peptide concentration curve (calculated as described above). The formulas to calculate the rate of insulin extraction RdIns(t) and insulin clearance MCRI(t) and at each time point of OGTT are given by
where VIns is the volume of distribution of insulin which was previously estimated as 141 mL/kg (24–26). To estimate temporal changes in insulin extraction and clearance, insulin concentrations were first smoothed by spline fitting using Data Curve Fit Creator (SRS1 Software, Newton Centre, MA), and dI(t)/dt was calculated from fitted data using a 5-min interval.
The total insulin extraction and clearance during the glucose challenge was calculated from Eqs. 1 and 2 as
Equation 4 is slightly different but more accurate than the formula previously used by Jung et al. (24) and Smith et al. (27) (i.e., [AUC-ISR/AUC-I] − VIns × [I120 − I0]/AUC-I), while AUC RdIns 0–120 is the same (see Supplementary Material for the explanation). The mean RdIns and MCRI during the OGTT (Table 1) were calculated from the AUC divided by time (120 min).
Statistical Analysis
All data are presented as the mean ± SEM if normally distributed or as median (interquartile range) if nonnormally distributed. Group values were compared by ANOVA and Bonferroni-Dunn post hoc analysis. Associations between variables were tested with Spearman rank correlations. All nonnormally distributed variables were Ln-transformed before comparison. The contribution of multiple factors to the MCRI was tested by multivariate analysis using mixed models with both continuous (e.g., age and BMI) and categorical (e.g., sex and race/ethnicity) variables. A P value of <0.05 was considered statistically significant.
Data and Resource Availability
The data sets analyzed during the current study are not publicly available for reasons of privacy. Anonymous data are available from the corresponding author on reasonable request.
Results
Clinical, Anthropometric, and Metabolic Characteristics
The six groups were well matched for sex and race/ethnicity (Table 1). There was an upward trend for age from NGT to IGT to T2DM for both the nonobese and obese groups. Nonobese subjects with T2DM and IGT subjects, to a lesser extent, had a higher BMI and percentage of body fat compared with the NGT group.
IR
During the postabsorptive state, the hepatic IR index and adipocyte IR index increased from NGT to IGT to T2DM (P < 0.001) within both the nonobese and the obese groups, being significantly higher in the obese versus the nonobese group (Table 1). During the euglycemic insulin clamp, peripheral insulin sensitivity (Rd/SSPI concentrations at the end of the clamp) (Table 1) in the nonobese group decreased from NGT to IGT (P < 0.001) to T2DM (P < 0.001). In the obese group, peripheral IR already was maximally manifest in NGT subjects and was similar to that in the groups with IGT and T2DM (Table 1).
Insulin Secretion/β-Cell Function
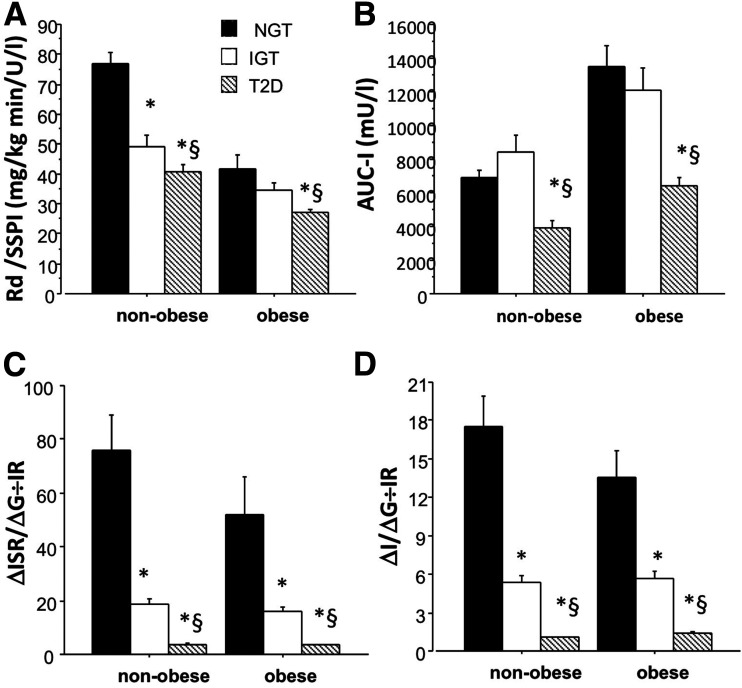
During the postabsorptive state, the basal ISR increased significantly from NGT to IGT (P < 0.001) and from IGT to T2DM (P < 0.001) within both the nonobese group and obese group (Table 1). During the OGTT, the ISR increased slightly from NGT to IGT in the nonobese group and then declined in the group with T2DM (P < 0.001) (Table 1). In the nonobese group, the ISR declined progressively from NGT to IGT to T2DM (P < 0.001) (Table 1). When the ISR was factored by the increment in plasma glucose concentration during the OGTT and then related to the measure of IR during the insulin clamp (i.e., the insulin secretion/IR or disposition index), β-cell function declined markedly from NGT to IGT (P < 0.00001) to T2DM (P < 0.00001) in both the nonobese and obese groups (Fig. 1).
Figure 1.
A: Rd factored by insulin at the end of the clamp. B: AUC of insulin during the OGTT. C: Changes in incremental insulin secretion divided by incremental glucose concentrations during OGTT factored by IR. D: Changes in incremental insulin concentrations divided by incremental glucose concentrations during OGTT factored by IR in individuals with NGT, IGT, and T2DM based on BMI (i.e., nonobese if BMI <30 kg/m2 or obese if BMI ≥30 kg/m2). *P < 0.05 T2DM vs. NGT; §P < 0.05 T2DM vs. IGT.
MCRI
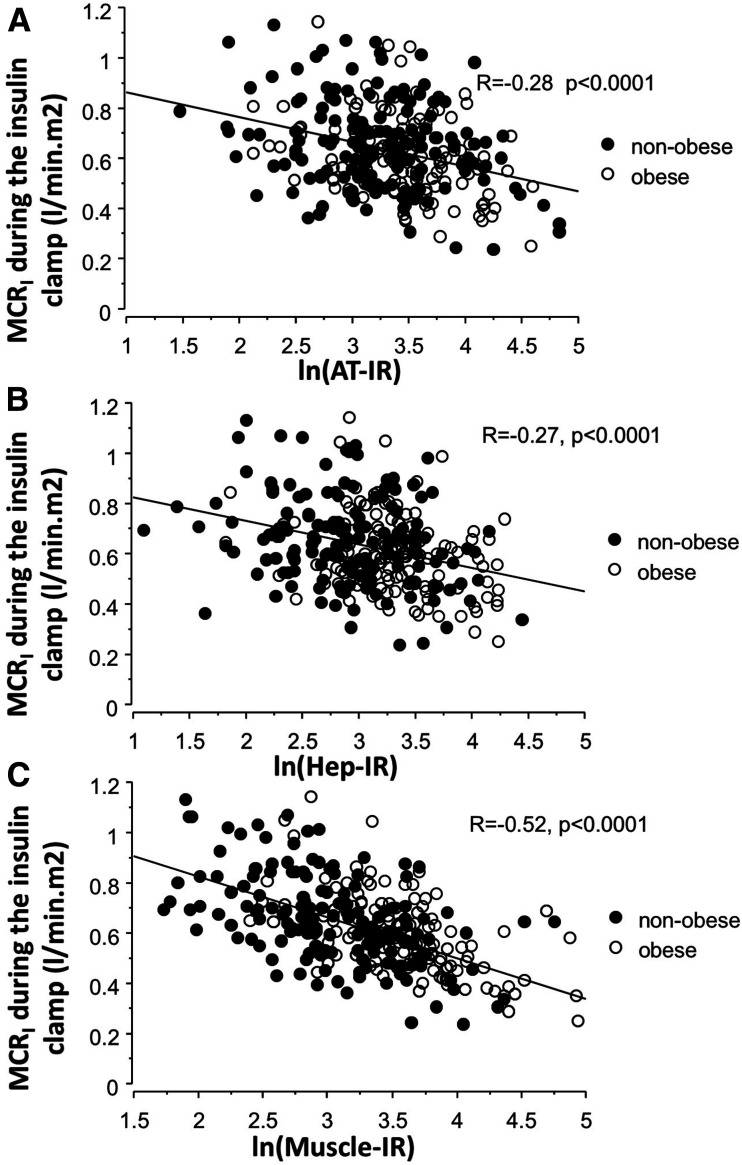
The MCRI during the euglycemic insulin clamp is shown in Table 1 for the six groups. Within the nonobese group, the clamp MCRI was decreased significantly (P < 0.01) in both the IGT and T2DM groups compared with the NGT group. In the obese NGT group, the MCRI was markedly decreased compared with the nonobese NGT group (P < 0.001) (Table 1); no further reduction in the MCRI was observed in the obese IGT or T2DM groups. The MCRI was inversely correlated with peripheral (r = −0.52), with hepatic (r = −0.27), and with adipocyte (r = −0.28) IR (all P < 0.0001) (Fig. 2). The clamp MCRI was not correlated with the fasting ISR (r = 0.03), OGTT ISR (r = 0.05), 2-h plasma glucose during the OGTT (r = −0.02), or HbA1c (r = −0.10), but it was positively correlated with β-cell function (r = 0.13 for ΔI/ΔG ÷ IR, P < 0.02 and r = 0.14 for ΔISR/ΔG ÷ IR, P < 0.009). We found that waist circumference was inversely correlated with the clamp MCRI (−0.43, P < 0.0001) but modestly with the MCRI during both the OGTT (r = −0.22, P = 0.002) and fasting state (r = −0.18, P = 0.016). Liver fat (measured by MRS in a small subgroup n = 51) was inversely correlated with the MCRI both in the fasting state (r = −0.40, P < 0.005) and during the OGTT (r = −0.387, P < 0.007) but not with the clamp MCRI.
Figure 2.
Correlation between the clamp MCRI vs. adipocyte (AT) (A), hepatic (Hep) (B), and peripheral (muscle) (C) IR in obese and nonobese subjects.
In multivariate analysis, after accounting for age, sex, and BMI, the MCRI remained independently correlated with peripheral IR (total r = −0.48, P < 0.0001), hepatic IR (r = −0.21, P < 0.0003), and adipocyte IR (r = −0.23, P < 0.0001). MCRI remained independently correlated with IR also if further adjusting for abdominal obesity (by adding waist circumference) as a covariate.
The MCRI during fasting conditions was significantly reduced in obese versus nonobese NGT subjects (P < 0.05) and was significantly reduced (P < 0.05) in both nonobese and obese patients with T2DM versus NGT nonobese and obese subjects, respectively (Table 1).
During the OGTT, the MCRI, calculated from the insulin model, which primarily reflects the liver (28), was significantly and similarly suppressed at all time points in nonobese and obese NGT and IGT subjects (Table 1). In contrast in both nonobese and obese patients with T2DM, suppression of MCRI was markedly impaired (Fig. 3). This is consistent with the increased C-peptide AUC/insulin AUC (P < 0.01–0.05) in subjects with T2DM during the OGTT (Table 1).
Figure 3.
MCRI during the OGTT in nonobese (A) and obese subjects (B) with NGT, IGT, and T2DM. Insulin concentrations during the OGTT in nonobese (C) and obese subjects (D) with NGT, IGT, and T2DM. Insulin Rd (extraction) during the OGTT in nonobese (E) and obese subjects (F) with NGT, IGT, and T2DM. ISRs during the OGTT in nonobese (G) and obese subjects (H) with NGT, IGT, and T2DM. Time in minutes is represented on the x axis. *P < 0.05 T2DM vs. NGT; §P < 0.05 T2DM vs. IGT.
Discussion
The great majority of individuals with T2DM are obese, and both obesity and diabetes are IR states (1,2). Early in the natural history of T2DM, the plasma insulin response is in‐creased. However, the relative contributions of increased ISR and decreased MCRI to the compensatory hyperinsulinemia have been poorly studied in humans. It generally is believed that the increase in plasma insulin response derives from the ability of the β-cell to read the severity of total-body IR and adjust its secretion of insulin (ΔI/ΔG) appropriately to offset the defect in insulin action (1–4). This relationship has been referred to as the disposition index (2). However, the plasma insulin response represents the sum of the β-cell ISR and the MCRI. Few studies have examined the MCRI in T2DM, and these studies have involved small numbers of subjects, with variable results ranging from normal (29,30) to decreased (13,31) to increased (18). Recently, we (7) and others (8) have shown that acute as well as chronic changes in the MCRI can have a profound effect on the plasma insulin response to a meal change. In the current study, we have examined the interrelationships between MCRI versus obesity, T2DM, and IR (in muscle/liver/adipocytes) in influencing the plasma insulin response during an OGTT.
As can be seen in Table 1, the state of glucose tolerance significantly impacted the MCRI. Thus, within the nonobese group, progression of NGT to IGT was associated with a significant decrease in the MCRI (measured during the euglycemic insulin clamp), which did not decline further with the development of overt T2DM. Obesity had an independent effect to reduce the MCRI, as manifested by the reduced MCRI in the obese NGT versus nonobese NGT groups (P < 0.01). However, the progression from obese NGT to obese IGT and obese T2DM caused no further decline in the MCRI. Thus, both the glucose tolerance status (nonobese NGT → nonobese IGT and nonobese T2DM) and obesity status (nonobese NGT to obese NGT) were associated with a reduction in the MCRI. These results are consistent with previous observations (11,18,24,32) demonstrating that obesity is associated with a significant decrease in MCRI. However, obesity is an IR state (1) that is associated with adipocyte IR (19) and with elevated plasma FFA, which have been shown to reduce the MCRI (16,33). Hepatic and visceral fat also have been shown to be related to MCRI (14,15,33). Also, MCRI in this cohort was inversely correlated to waist circumference and liver fat. Obesity and IR (measured with the euglycemic insulin clamp technique) also have been shown to be strongly related (34–36). In the current study, the MCRI and IR were strongly and inversely correlated (r = −0.52, P < 0.0001) in both the nonobese and obese groups (Fig. 2). In multivariate analysis including BMI and waist circumference, this correlation persisted unabated, indicating that IR—not obesity per se—was the causal factor related to the decreased MCRI observed during the insulin clamp.
After glucose ingestion, the contribution of tissues to the maintenance of NGT differs significantly from that after intravenous glucose/insulin administration (i.e., the euglycemic insulin clamp), with the liver playing a more predominant role in glucose disposal (1) and in insulin clearance (28). Therefore, we calculated the MCRI during the OGTT, which provides insight into the role of the liver in insulin extraction under conditions when insulin is secreted directly in the portal vein, exposing the liver to very high plasma insulin concentrations. As can be seen in Fig. 3, at all time points after glucose ingestion, the MCRI in nonobese and obese NGT and IGT subjects was significantly suppressed. In contrast, the suppression of MCRI was markedly impaired in both obese and nonobese patients with T2DM. This finding is consistent with results previously published by Bonora et al. (13) and which have been largely overlooked.
Recently, Bergman et al. (37) hypothesized that reduced hepatic insulin clearance in T2DM results in peripheral hyperinsulinemia, which in turn, exacerbates the IR, placing stress on the β-cell and contributing to β-cell failure in T2DM. We believe that the present results are more consistent with the opposing view: the decrease in hepatic extraction of insulin during the OGTT represents a compensatory response to offset the defect in insulin secretion in order to overcome IR. Recently, Smith et al. (27) evaluated insulin secretion and clearance during the OGTT in lean and obese subjects without diabetes and reported that obese subjects, especially those with nonalcoholic fatty liver disease, had higher plasma insulin concentrations, ISRs, and total hepatic extraction. They did not, however, discuss whether there was any difference in insulin clearance between obese and lean subjects during fasting nor did they mention whether there was a change in MCRI after glucose ingestion versus fasting in lean or obese subjects (27). Because the extraction (Rd) of insulin was increased in obese subjects, they argued that hyperinsulinemia in obese NGT subjects “is due to an increase in insulin secretion, not a reduction in total insulin extraction by the liver or extrahepatic tissues.” Our data indicate that in response to glucose ingestion, the ISR increases but that there is also a reduction in total insulin clearance that is independent of glucose tolerance status and the degree of obesity. Thus, an increase in ISR as well as a decrease in MCRI both contribute to the increase in plasma insulin concentration. As pointed out by Smith et al. (27), and consistent with our results, because of the marked increase in ISR in obese subjects without diabetes, which far outweighed the decrease in MCRI, insulin extraction increased. This was evident also in obese subjects with diabetes.
Insulin clearance reflects the intrinsic ability of all tissues in the body to remove insulin. Insulin clearance by nonhepatic tissues (primarily the kidney and, to a lesser extent, muscle) is constant over a wide range of plasma insulin concentrations (6,38). Thus, the reduction in insulin clearance during the OGTT primarily reflects the liver. We previously showed, using the insulin clamp in combination with renal vein catheterization, that hepatic insulin extraction is constant up to a plasma insulin concentration of ∼500 μU/mL (6). Therefore, even though the obese subjects without diabetes had a marked increase in insulin secretion, it is unlikely that the portal vein insulin concentration was >500 μU/mL and that factor(s) other than hepatic insulin saturability might explain the decrease in MCRI after glucose ingestion. However, it should be noted that in our previous study (6), only lean subjects without diabetes were studied and that the number of subjects who received the high-dose (5 and 10 mU/m2 ⋅ min) insulin clamp studies was relatively small (n = 5). Therefore, a large sample size of obese subjects, with and without diabetes, who receive a high dose in an insulin clamp is warranted to address the issue of hepatic insulin saturability in these two groups.
In both nonobese and obese IGT subjects, the MCRI declined during the OGTT (Fig. 3A and B), indicating that the intrinsic ability of the liver to remove insulin from the circulation is reduced. Further, the decline in MCRI is similar in IGT and NGT subjects, indicating that the mechanisms(s) regulating the decline in hepatic insulin clearance is (are) intact. If the normal decline in MCRI had not occurred in IGT subjects, glucose tolerance would have been impaired to an even greater extent. The rate of insulin disappearance (Rd) or extraction, which is the product of MCRI and the plasma insulin concentration, increased similarly in nonobese NGT and IGT subjects and increased, but not as much, in obese IGT versus obese NGT subjects because the plasma insulin concentration markedly increased during the OGTT. This should not be interpreted to mean that reduced hepatic MCRI is not playing an important role in maintaining glucose homeostasis.
In obese NGT subjects, the MCRI decreased similarly to that in nonobese NGT subjects (Fig. 3A and B). If this had not occurred, glucose tolerance would not have remained normal. Nonetheless, the insulin Rd (extraction) increased, albeit not as much as in obese NGT subjects, because of the marked increase in insulin secretion which was strongly correlated with the severity of IR. Whether other as-of-yet unidentified factors related to obesity contribute to the increase in insulin secretion remains to be determined.
A very different picture is observed in T2DM, where the MCRI failed to decline from baseline in nonobese subjects with diabetes and the decline was markedly impaired in obese individuals with diabetes. Thus, the normal physiologic decline in hepatic MCRI is lost in T2DM and, when combined with the defects in insulin secretion and IR, contributes to the marked deterioration of glycemic control. Again, although the insulin Rd (extraction) is increased compared with baseline in T2DM, this should not be interpreted to mean that the decrease in hepatic MCR is not an important contributor to the glucose intolerance.
Our results also help to clarify factors that influence the plasma insulin response in obese individuals with varying degrees of glucose tolerance ranging from normal to IGT to T2DM. Obese NGT compared with nonobese NGT subjects had an increase in the ISR, both during the basal state and during the OGTT as well as a decrease in the MCRI. Thus, enhanced insulin secretion and reduced MCRI both contributed to the elevated plasma insulin levels observed in obese NGT subjects. The reduction in MCRI during the OGTT primarily reflects reduced hepatic extraction since nonhepatic insulin clearance is directly dependent on the peripheral (i.e., posthepatic) insulin concentration (23,25,27,39).
The inability of the diabetic liver to normally suppress its extraction of insulin (Fig. 3) has previously been observed (13). It is very unlikely that diabetic nephropathy could have influenced the MCRI because in all subjects the plasma creatinine concentration was ≤1.4 mg/dL and decreased renal extraction of insulin is not observed until the glomerular filtration rate decreases to ∼20 mL/min (40).
In summary, the MCRI is reduced both by obesity and T2DM. When NGT, IGT, and T2DM groups are examined individually or as a whole, the MCRI is strongly and inversely related to peripheral, hepatic, and adipocyte IR. In IR states, including both obesity and diabetes, reduced MCRI represents an important mechanism that contributes to the compensatory hyperinsulinemia in an attempt to maintain normal glucose homeostasis. In response to glucose ingestion, this compensatory response in MCRI is impaired in individuals with T2DM.
Article Information
Acknowledgments. The authors thank Lorrie Albarado (University of Texas Health Science Center, San Antonio, TX) for her expert assistance in preparing the manuscript.
Funding. This study was supported in part by National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases grant DK24092 (R.A.D.) and internal funds of the Consiglio Nazionale delle Ricerche (National Research Council), project MEDIFENCE (“MEtabolic DIseases, Food and ENvironment: from CElls to humans”) grant number DSB.AD003.061.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. A.G. analyzed the data. A.G. and M.A.G. contributed to data acquisition. A.G. and R.A.D. contributed to the study design. A.G. and R.A.D. wrote the manuscript. M.A.G. contributed to the discussion. All authors drafted or revised the article and approved the final version of the manuscript. R.A.D. is the guarantor of this work, and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.13103318.
References
- 1.DeFronzo RA Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009;58:773–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet 2014;383:1068–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA β-Cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab 2005;90:493–500 [DOI] [PubMed] [Google Scholar]
- 4.Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, DeFronzo RA; San Antonio metabolism study . Beta-cell dysfunction and glucose intolerance: results from the San Antonio metabolism (SAM) study. Diabetologia 2004;47:31–39 [DOI] [PubMed] [Google Scholar]
- 5.Asare-Bediako I, Paszkiewicz RL, Kim SP, et al. Assessment of hepatic insulin extraction from in vivo surrogate methods of insulin clearance measurement. Am J Physiol Endocrinol Metab 2018;315:E605–E612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrannini E, Wahren J, Faber OK, Felig P, Binder C, DeFronzo RA. Splanchnic and renal metabolism of insulin in human subjects: a dose-response study. Am J Physiol 1983;244:E517–E527 [DOI] [PubMed] [Google Scholar]
- 7.DeFronzo RA, Tripathy D, Abdul-Ghani M, Musi N, Gastaldelli A. The disposition index does not reflect β-cell function in IGT subjects treated with pioglitazone. J Clin Endocrinol Metab 2014;99:3774–3781 [DOI] [PubMed] [Google Scholar]
- 8.Bojsen-Møller KN, Lundsgaard AM, Madsbad S, Kiens B, Holst JJ. Hepatic insulin clearance in regulation of systemic insulin concentrations—role of carbohydrate and energy availability. Diabetes 2018;67:2129–2136 [DOI] [PubMed] [Google Scholar]
- 9.Rabkin R, Ryan MP, Duckworth WC. The renal metabolism of insulin. Diabetologia 1984;27:351–357 [DOI] [PubMed] [Google Scholar]
- 10.Peiris AN, Mueller RA, Smith GA, Struve MF, Kissebah AH. Splanchnic insulin metabolism in obesity. Influence of body fat distribution. J Clin Invest 1986;78:1648–1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polonsky KS, Given BD, Hirsch L, et al. Quantitative study of insulin secretion and clearance in normal and obese subjects. J Clin Invest 1988;81:435–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee CC, Haffner SM, Wagenknecht LE, et al. Insulin clearance and the incidence of type 2 diabetes in Hispanics and African Americans: the IRAS Family Study. Diabetes Care 2013;36:901–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonora E, Zavaroni I, Coscelli C, Butturini U. Decreased hepatic insulin extraction in subjects with mild glucose intolerance. Metabolism 1983;32:438–446 [DOI] [PubMed] [Google Scholar]
- 14.Kotronen A, Vehkavaara S, Seppälä-Lindroos A, Bergholm R, Yki-Järvinen H. Effect of liver fat on insulin clearance. Am J Physiol Endocrinol Metab 2007;293:E1709–E1715 [DOI] [PubMed] [Google Scholar]
- 15.Gastaldelli A, Cusi K, Pettiti M, et al. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology 2007;133:496–506 [DOI] [PubMed] [Google Scholar]
- 16.Wiesenthal SR, Sandhu H, McCall RH, et al. Free fatty acids impair hepatic insulin extraction in vivo. Diabetes 1999;48:766–774 [DOI] [PubMed] [Google Scholar]
- 17.Jones CN, Pei D, Staris P, Polonsky KS, Chen YD, Reaven GM. Alterations in the glucose-stimulated insulin secretory dose-response curve and in insulin clearance in nondiabetic insulin-resistant individuals. J Clin Endocrinol Metab 1997;82:1834–1838 [DOI] [PubMed] [Google Scholar]
- 18.Kim SH, Reaven GM. Insulin clearance: an underappreciated modulator of plasma insulin concentration. J Investig Med 2016;64:1162–1165 [DOI] [PubMed] [Google Scholar]
- 19.Gastaldelli A, Gaggini M, DeFronzo RA. Role of adipose tissue insulin resistance in the natural history of type 2 diabetes: results from the San Antonio Metabolism Study. Diabetes 2017;66:815–822 [DOI] [PubMed] [Google Scholar]
- 20.Galderisi A, Polidori D, Weiss R, et al. Lower insulin clearance parallels a reduced insulin sensitivity in obese youths and is associated with a decline in β-cell function over time. Diabetes 2019;68:2074–2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elahi D, Nagulesparan M, Hershcopf RJ, et al. Feedback inhibition of insulin secretion by insulin: relation to the hyperinsulinemia of obesity. N Engl J Med 1982;306:1196–1202 [DOI] [PubMed] [Google Scholar]
- 22.DeFronzo RA, Binder C, Wahren J, Felig P, Ferrannini E, Faber OK. Sensitivity of insulin secretion to feedback inhibition by hyperinsulinaemia. Acta Endocrinol (Copenh) 1981;98:81–86 [DOI] [PubMed] [Google Scholar]
- 23.Campioni M, Toffolo G, Basu R, Rizza RA, Cobelli C. Minimal model assessment of hepatic insulin extraction during an oral test from standard insulin kinetic parameters. Am J Physiol Endocrinol Metab 2009;297:E941–E948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jung SH, Jung CH, Reaven GM, Kim SH. Adapting to insulin resistance in obesity: role of insulin secretion and clearance. Diabetologia 2018;61:681–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polidori DC, Bergman RN, Chung ST, Sumner AE. Hepatic and extrahepatic insulin clearance are differentially regulated: results from a novel model-based analysis of intravenous glucose tolerance data. Diabetes 2016;65:1556–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hovorka R, Powrie JK, Smith GD, Sönksen PH, Carson ER, Jones RH. Five-compartment model of insulin kinetics and its use to investigate action of chloroquine in NIDDM. Am J Physiol 1993;265:E162–E175 [DOI] [PubMed] [Google Scholar]
- 27.Smith GI, Polidori DC, Yoshino M, et al. Influence of adiposity, insulin resistance, and intrahepatic triglyceride content on insulin kinetics. J Clin Invest 2020;130:3305–3314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polonsky KS, Rubenstein AH. C-peptide as a measure of the secretion and hepatic extraction of insulin. Pitfalls and limitations. Diabetes 1984;33:486–494 [DOI] [PubMed] [Google Scholar]
- 29.Polonsky KS, Gumbiner B, Ostrega D, Griver K, Tager H, Henry RR. Alterations in immunoreactive proinsulin and insulin clearance induced by weight loss in NIDDM. Diabetes 1994;43:871–877 [DOI] [PubMed] [Google Scholar]
- 30.Baffy G, Brunt EM, Caldwell SH. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. J Hepatol 2012;56:1384–1391 [DOI] [PubMed] [Google Scholar]
- 31.Orskov H, Christensen NJ. Plasma disappearance rate of injected human insulin in juvenile diabetic, maturity-onset diabetic and nondiabetic subjects. Diabetes 1969;18:653–659 [DOI] [PubMed] [Google Scholar]
- 32.Tchernof A, Després JP. Pathophysiology of human visceral obesity: an update. Physiol Rev 2013;93:359–404 [DOI] [PubMed] [Google Scholar]
- 33.Strang BD, Bertics SJ, Grummer RR, Armentano LE. Relationship of triglyceride accumulation to insulin clearance and hormonal responsiveness in bovine hepatocytes. J Dairy Sci 1998;81:740–747 [DOI] [PubMed] [Google Scholar]
- 34.DeFronzo RA Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetologia 2010;53:1270–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeFronzo RA Insulin secretion, insulin resistance, and obesity. Int J Obes 1982;6(Suppl. 1):73–82 [PubMed] [Google Scholar]
- 36.Bogardus C, Lillioja S, Mott D, Reaven GR, Kashiwagi A, Foley JE. Relationship between obesity and maximal insulin-stimulated glucose uptake in vivo and in vitro in Pima Indians. J Clin Invest 1984;73:800–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bergman RN, Piccinini F, Kabir M, Kolka CM, Ader M. Hypothesis: role of reduced hepatic insulin clearance in the pathogenesis of type 2 diabetes. Diabetes 2019;68:1709–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asare-Bediako I, Paszkiewicz RL, Kim SP, et al. Variability of directly measured first-pass hepatic insulin extraction and its association with insulin sensitivity and plasma insulin. Diabetes 2018;67:1495–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Utzschneider KM, Kahn SE, Polidori DC. Hepatic insulin extraction in NAFLD is related to insulin resistance rather than liver fat content. J Clin Endocrinol Metab 2019;104:1855–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mak RH, DeFronzo RA. Glucose and insulin metabolism in uremia. Nephron 1992;61:377–382 [DOI] [PubMed] [Google Scholar]