Abstract
Diabetes is a disease of insulin insufficiency, requiring many to rely on exogenous insulin with constant monitoring to avoid a fatal outcome. Islet transplantation is a recent therapy that can provide insulin independence, but the procedure is still limited by both the availability of human islets and reliable tests to assess their function. While stem cell technologies are poised to fill the shortage of transplantable cells, better methods are still needed for predicting transplantation outcome. To ensure islet quality, we propose that the next generation of islet potency tests should be biomimetic systems that match glucose stimulation dynamics and cell microenvironmental preferences and rapidly assess conditional and continuous insulin secretion with minimal manual handing. Here, we review the current approaches for islet potency testing and outline technologies and methods that can be used to arrive at a more predictive potency test that tracks islet secretory capacity in a relevant context. With the development of potency tests that can report on islet secretion dynamics in a context relevant to their intended function, islet transplantation can expand into a more widely accessible and reliable treatment option for individuals with diabetes.
Introduction
Islet transplantation has emerged as a promising treatment for the most severe cases of insulin-dependent diabetes, offering the potential for a complete reprieve from regular insulin injections. Transplanted islets augment a recipient’s ability to respond to elevated glucose levels in the bloodstream by secreting insulin. In contrast to the surgical approach of a whole pancreas transplant, islet transplantation involves a relatively risk-free infusion of islets isolated from a donor pancreas into the portal vein of a recipient’s liver. While both methods require immunosuppression, islet transplantation boasts rates of insulin independence on par with whole pancreas transplantation and confers advantages including prevention of severe hypoglycemic events and improvements in overall glucose control (1–3). Islet transplantation is now a standard of care in several countries, but it still awaits U.S. Food and Drug Administration approval (1). Most recently, after an impressive collaboration across eight U.S. sites in a phase III clinical trial, >70% of recipients achieved clinical goals after 2 years, with a stark reduction in severe hypoglycemic events and insulin dependence (4,5).
While islet transplantation is a therapy with potential, its success has been hindered by the poor availability of functional human islets. Efforts have begun in establishing standards for generating islet products (5), but human islets from cadaveric pancreata are scarce due to stringent inclusion and exclusion criteria, as well as an involved islet isolation process (6). Furthermore, the quality of these preparations continues to vary despite protracted efforts to identify trends in their function based on donor or isolation information (7,8). Initial infusions of cadaveric islet preparations that have passed current quality standards often fail to yield insulin independence (6,9). The low success rate for insulin independence necessitates subsequent dosing and represents a costly and time-consuming burden that can also leave individuals with diabetes vulnerable in the interim between infusions. Stem cell therapies have recently become available as a possible alternative source of tissue for transplantation (10,11) and are poised to address the shortage of cadaveric islets by providing an unlimited and controlled source for insulin-producing cells. However, these cell therapies are still relatively nascent. Although work has been done to improve their application to human transplantation (12–19), further development is needed for large-scale commercialization of differentiation protocols with consistent quality and proven safety and function prior to use in humans. Regardless of cell source, the inability to predict functional quality prior to product release remains a common hurdle (20).
For facilitation of the manufacturing quality control of insulin-producing cells and for informing formal medical recommendations known as clinical practice guidelines (21), there is a pressing need to develop quantifiable metrics that reliably test the function of cells in a range of physiological conditions. We argue that current potency testing fails to provide cues from the native microenvironment that regulate islet function and thus propose that the next generation of islet potency tests should be biomimetic systems that can assess conditional insulin secretion pretransplant with minimal manual handing or measurement. Matching the spatiotemporal stimuli that elicit normal islet behavior in vivo while dynamically assessing their functional potential for transplantation provides the framework for developing a more predictive potency test. This article outlines the existing standards for determining islet potency and highlights proposed methods that should supplement or replace the current complement of tests. We specifically consider the use of technologies that can measure insulin secretion in response to dynamic stimulation, with the addition of microenvironmental cues to provide a relevant context for assessing islet behavior.
Islet Procurement and Potency Testing for Good Clinical Practice
Current Practices
Clinical practice guidelines (21) have outlined accepted procedures for islet manufacture and transplantation (22). Prior to potency testing, islets are isolated from the surrounding exocrine tissue of donor pancreata (Fig. 1). The isolation process typically occurs in a cell processing center in compliance with Current Good Manufacturing Practice and Current Good Tissue Practice guidelines that stem from recommendations from the U.S. Food and Drug Administration (23). Islet isolation has been gradually refined to increase both purity and yield, leading to Clinical Islet Transplantation (CIT) Protocol CIT-07, a protocol sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases and the National Institute of Allergy and Infectious Diseases for a phase III clinical trial in North America intended to demonstrate the safety and efficacy of islet transplantation for type 1 diabetes (24). This recent multisite collaboration produced a series of protocols aimed at standardization across isolation and transplantation centers (5). The study successfully demonstrated the efficacy of islet transplantation for lowering HbA1c levels, drastically reducing the incidence of severe hypoglycemic events, and, in some cases, allowing for insulin independence (4,5). However, given the resource intensiveness and health risks of the procedure, methods that can facilitate or improve positive clinical outcomes should be considered. Despite rigorous investigation of many factors relating to isolation, identification of parameters that consistently contribute to the potency of an islet batch remains elusive (8,25,26). Most notably, a single islet infusion is often insufficient for a recipient to attain full insulin independence, thus requiring one or more subsequent infusions, along with their associated costs and risk of complications (4,5). Infusions that provide less therapeutic benefit could stem from graft failure (6) due to complicating factors such as peritransplant glycemic control, microangiopathy, advanced glycation end products, and/or autoreactive T cells (27,28). Immunosuppression, which affects islet revascularization and insulin secretion (29), is yet another factor. Still, the use of poor-quality islets that have not been effectively evaluated for function prior to transplantation may contribute to the ineffectiveness of some islet infusions. Functional potency tests are therefore a necessary component of clinical practice guidelines for islet transplantation that can screen out poorer-quality islet preparations to reduce the number of infusions needed to provide clinical benefits. Isolation of islets disrupts and even removes much of the surrounding extracellular matrix (ECM) through enzymatic digestion and mechanical separation (30), which may partially explain some of the discrepancies in function for transplantation (31,32). Generally speaking, while cadaveric islets are stripped of their endogenous surroundings, stem cell–derived products are generated without many of these native cues.
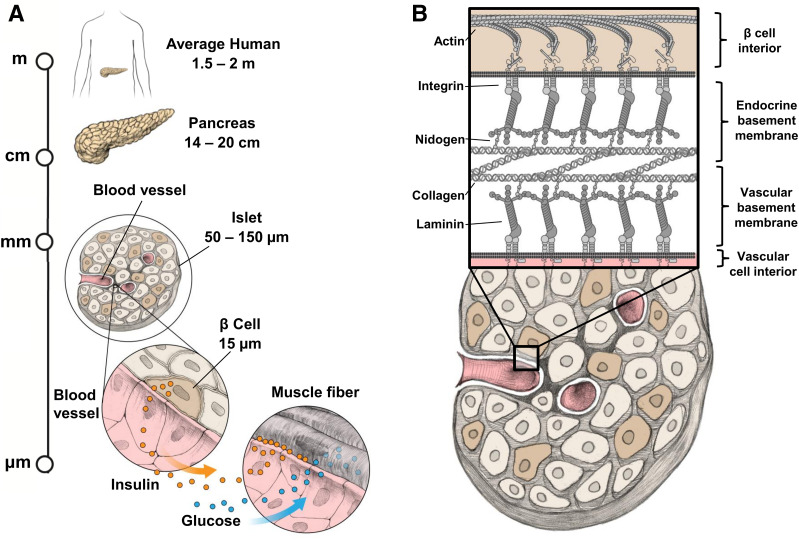
Figure 1.
Hierarchical structure of the pancreatic islet. A: Human islets reside in the pancreas as sparse “islands” of endocrine cells embedded within exocrine tissue. Blood vessels supply individual islets with glucose and collect secreted hormones to send throughout the body. The most common cell type within the islet is the β-cell, responsible for secreting insulin when glucose levels are elevated following a meal. Insulin travels with glucose in the bloodstream to cells of the body, allowing those cells to take in the glucose. B: Blood vessels penetrating into human islets form a double basement membrane with the islet cells, comprised of membrane-bound integrins that link the actin within the β-cells to a network of ECM proteins. Integrins are depicted binding laminins, which form a trimeric branching network that connects to tetrameric collagen type IV networks via bridging molecules such as nidogen.
Establishing potency tests to set islet batch quality criteria was a stated secondary objective of CIT-07 (24), and those adopted (5) were generally consistent with common practices (6,23). In current islet transplantation practice, the purity of a preparation is typically assessed with use of dithizone, which preferentially stains zinc that is highly concentrated in β-cells. Islet counts are also conducted with this stain by examining the cross-sectional areas of individual islets and converting these into islet equivalents (IEQ), a unit corresponding to the size of a 150-μm-diameter islet. The islet dose is then defined as the number of IEQ delivered to a recipient normalized by recipient’s weight, with units of IEQ/kg. Prior to transplantation, viability is also determined as a measure of membrane integrity. Stains such as propidium iodide highlight DNA exposed by dead cells lacking an intact membrane, while a secondary stain such as fluorescein diacetate will become fluorescent in viable cells with sufficient endogenous enzymatic activity. A functional test of potency often relies on quantifying the glucose-stimulated insulin secretion (GSIS), where a portion of the isolated islets are treated first with a low-glucose stimulus and then a high-glucose stimulus, and the resulting insulin secreted in each condition is measured with an ELISA. The stimulation index, which is the ratio of insulin produced in high- and low-glucose conditions, is then considered a fundamental indication of the conditional release of insulin in response to glucose. Sterility testing is also conducted for looking for growth of a range of pathogens, as well as measurement of endotoxin levels.
As shown in Fig. 2, islet product release criteria including those used in CIT-07 typically specify that the purity should be at least 30% and the viability at least 70% for use in transplantation (5). An islet dose of >5,000 IEQ/kg is the minimum recommended islet dose to achieve a noticeable clinical result (5), although in practice higher doses are often preferred (1,33). While GSIS may be conducted in a preliminary assessment of an islet preparation, insulin secretion is not used to determine whether a batch of islets is suitable for transplantation (Fig. 2). Indeed, although GSIS was performed twice per islet lot during the CIT-07 study, neither GSIS nor sterility was strictly considered a product release criterion because of how challenging it can be to complete these techniques prior to transplantation (5). As such, the GSIS conducted in the study had no bearing on selection of islet lots for transplantation. Furthermore, Ricordi et al. (5) report that two lots of islets actually failed the outlined GSIS criterion (i.e., stimulation index >1) after GSIS results were available, even though these lots had already been used for transplantation in patients. A more efficient potency test could prevent the use of islet preparations that have low functional potency in transplantation. It is also important to note that, with the exception of islet dose (34), none of the aforementioned criteria are reliable predictors of transplantation success (9,23). While it is unclear what specific circumstances led to a secondary infusion in each case, inadequate islet screening may partially explain why in the CIT-07 study an initial infusion was not sufficient to achieve insulin independence in a majority of cases (4,5). Adjustments to the clinical practice guidelines for islet transplantation must include the use of more predictive potency tests to ensure that only islet products with high functional quality are delivered to recipients.
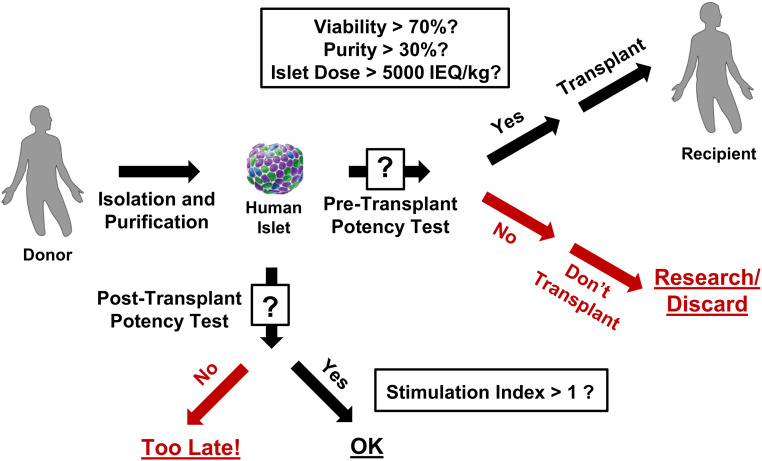
Figure 2.
Current quality control process used for islet transplantation. After isolation and purification of islets from donor pancreas, criteria for determining potency prior to transplantation into a recipient (i.e., “Pre-Transplant Potency Test”) typically include testing for viability (>70%), purity (>30%), and islet dose (>5,000 IEQ/kg). While testing of the secretion of insulin in response to glucose stimulation—measured as a ratio of insulin produced in a condition of high glucose divided by insulin produced in a condition of low glucose (target of stimulation index >1)—is sometimes also conducted, the results are not available quickly enough to be used in decision-making regarding transplantation (i.e., “Post-Transplant Potency Test”). IEQ, Islet Equivalent; kg, kilogram.
Alternative Potency Tests
In light of the poor predictive nature of the standard islet potency metrics, alternative approaches have been considered. Several proposed microscopy methods and other imaging motifs are used, with the intention of replacing or streamlining the traditional tests. Examples include digital approaches for IEQ and islet purity determination (35–37), improved methods for imaging zinc on the membrane of β-cells (38,39), implementation of Raman spectroscopy to locate regions of insulin and glucagon (40), the use of immunohistochemistry (41) or islet size (42), and a method for evaluating β-cell apoptosis (43). As a substitute for measuring insulin secretion, techniques have been explored to look at the electrophysiological activity of islets by measurement of their elicited electrical potentials (44) or calcium signaling (45) in response to glucose stimulation. Similarly, given the importance that metabolism plays in insulin secretion, other tests have begun to look at the metabolic state of the islets, with assessment of mitochondrial health (46), ADP-to-ATP ratio (47), or reactive oxygen species (48). In particular, the possible utility of either static or glucose-stimulated oxygen consumption rate (OCR) has become an intense area of focus (33,34,49–52). In the era of genomic analysis, various groups have also begun to search for unique biomarkers, identifying specific miRNA (miRNA-375, miRNA-200c) (53,54), long noncoding RNA (MALAT1) (55), or transcripts (56,57), correlated with either islet isolation quality or transplantation outcome. Multiparametric approaches have also attempted to look at a combination of some of these metrics (58,59).
A number of the studies that recommend a specific potency test have in fact compared several potency tests but often differ in their conclusions regarding predictive potential of a given test. This inconsistency across the literature likely speaks to the variability in the islet preparations (8), differences in transplant success criteria, and possibly the fact that such correlations—especially in studies with small cohorts—may be localized findings that are not reproducible. Many tests are not specific to β-cell function, or undertaking them would require significant time or resources, limiting their potential value as a potency test that can be conducted prior to transplantation. Not surprisingly, it has long been shown that the total islet dose introduced into a patient is a significant predictor of transplant success, since a higher mass of tissue is more likely to include some functional tissue (34). However, the use of islet dose as a metric can be lacking because islet mass alone does not consider the true viability or function of the tissue being introduced (34). Of the alternative potency tests that have been proposed, the most promising thus far are those that capitalize on recent transplantation trials in humans, where the OCR dose (OCR normalized to DNA content and multiplied by islet dose) and transcription levels of specific miRNAs associated with islet damage (miRNA-375, miRNA-200c) have separately been found to correlate to clinical outcomes (33,34,53). An advantage of both methods is that the measurement of either OCR dose or miRNA levels may be rapidly conducted (49,55). However, while these results represent an exciting step forward, they cannot yet predict clinical outcomes. These metrics likely do not fully capture the functional potential of islet preparations, since neither measurement was assessed in a dynamic context. Indeed, an islet’s fundamental role involves sensing dynamic glucose levels and secreting hormones as a response, so it should be imperative to understand stimulus-response coupling. Lending support to this idea, Kurian et al. (57) found that a set of human islet genes whose expression was most predictive of diabetes reversal in mice after transplantation correlated with glucose sensing and did not overlap with pathways associated with either oxygen consumption or apoptosis, indicating that damage to the islet tissue and metabolic activity alone may not offer a complete picture. A separate study also found that human islets show evidence of β-cell dedifferentiation after isolation, pointing to the need for a potency test that accounts specifically for β-cell function (32). Together, the findings suggest that for development of clinical practice guidelines with a sufficiently complete set of potency tests, unique metrics of overall islet health or basal metabolism may need to be augmented by a metric that is more specific to functional hormone release.
The Need for a Better Secretion-Based Potency Test
While it is necessary to evaluate insulin secretory ability of islets prior to transplantation, existing standards for the evaluation of islet secretory function fail to meet the efficiency and accuracy needed for better clinical practice guidelines. The transplantation of human islets into rodents has previously been considered a gold standard for assessing the secretory function of an islet preparation, with correlations drawn between positive clinical outcomes posttransplant in humans and those in both diabetic (60) and normoglycemic (61) mice. However, aside from a consideration of species differences (45,62,63), these animal models are not suitable for predictive potency testing because they take days or weeks to achieve a reliable result and can require complicated protocol coordination (9,64). As mentioned previously, static GSIS is another standard method of evaluating the islet conditional response to glucose stimulation. Unfortunately, static GSIS is typically considered a poor predictor of transplantation outcome (34). The insufficient predictive power of static GSIS may very well be due to the low time resolution, as insulin collects for as much as an hour under each condition, during which time the continuous accumulation of insulin may negatively disrupt insulin secretion (45). Perifusion methods have also been used to stimulate islets with pulses of varied glucose concentrations delivered under flow, in a variation known as dynamic GSIS (45,65). Insulin secretion from the same isolations of human islets assayed in static GSIS and dynamic GSIS systems have not been found to be correlated (8), indicating that the two techniques are indeed measuring islet function differently. Although dynamic GSIS systems are capable of sampling insulin secretion from islets down to a minute resolution (45) and would represent an improvement over static GSIS, the technique still suffers from a bottleneck in insulin quantification. Typically, islet secretions are collected into a multiwell plate, and the insulin contained in the wells is measured separately with ELISA. The lack of coupling between islet stimulation and insulin measurement represents a time burden, and the total time and effort required to achieve insulin secretion measurements are the reason that transplantation is often done before GSIS results have been finalized (5). Criticism of GSIS as it is currently practiced should also consider that this technique likely suffers from some of the highest variability of any islet quality metric. For both static and dynamic GSIS, low-glucose treatments in literature range from 1 mmol/L to 6 mmol/L (8,25), while high-glucose treatments range from 11 mmol/L to 28 mmol/L (45,48). Exposure times and temperatures at each condition are also varied.
The success of islet transplantation desperately depends on the introduction of functional islets, but current methods for testing insulin secretion are too slow or require too much effort for getting useful results prior to islet transplantation. Mirroring the steps the field has taken to rally around standards of practice for islet isolation and transplantation (5,7,66,67), every effort should be made to incorporate an in vitro potency test for measuring human islet secretory function. Inspiration for such a potency test must come from the body. Islets receive designated perfusion in the native human pancreas (68), and will receive a continuous supply of oxygen, nutrients, and glucose once infused into the portal vein following transplantation (Fig. 1A). Thus, the ability to obtain quality information in the form of secretion data will rely on the use of dynamic stimulation with integrated insulin sensing capabilities, and the relevance of the data to transplantation will depend on mimicking the physiological conditions islet cells would normally experience in vivo.
Dynamic Insulin Sensing in a Biomimetic Context
Integration of Insulin Sensors
By reliance on physical properties that can be easily sampled and digitized with available sensing technologies, promising optical and electromagnetic techniques have been developed to assess the cell-specific functional health of neurons (69–71), cardiomyocytes (72–78), and endothelial or epithelial cells (79–82), or to look at metabolic activity in a range of cell types (83–85). In contrast, detecting dynamic protein secretions in vitro—as is required for quantification of the insulin secretion of pancreatic islets—poses a unique challenge. Traditional methods for measuring protein secretion involve taking snapshots of protein and expression levels, such as with Western blotting or ELISA, for example. These techniques coordinate selective targeting of a protein of interest using antibodies and some form of visualization of the presence of the target-antibody complex for quantification. More modern proteomic and transcriptomic approaches have greatly expanded the complement of proteins or transcripts that can be assessed simultaneously to hundreds or thousands (86–89) but still cannot provide quick measurements of protein levels. In all these cases, quantification of protein levels secreted by the cells relies on removal of the medium surrounding the cells at discrete time points for subsequent analysis. A detailed temporal portrait of hormone secretions such as those required for pancreatic islets is thus limited by the sampling method used, as well as the inability to make quick, in situ measurements.
Microfluidics has become a burgeoning technology in recent decades that promises to overcome limitations posed by static culture. Microfluidic devices can mimic blood flow in delivering nutrients, oxygen, and stimuli to cells and removing waste and cellular secretions. In practice, these devices typically constitute an array of small channels and chambers (with dimensions typically <1 mm) microfabricated into polymeric or glass material and uniquely designed to achieve precise flow rates and reagent concentrations. Custom media or buffer solutions can be delivered by a series of supporting equipment such as pumps, tubing, and connectors, although several options exist for pumpless flow as well (90). Between the design of the device itself and associated instrumentation, a microfluidic device can provide a continuous and automated method for assaying biological activity with high spatial and temporal control. Dynamic delivery of chemical stimuli is an important feature of microfluidic devices, as best exemplified by studies such as those that have looked at entraining islet secretion (91–93). In addition to the advantages related to perfusion, microfluidics can also provide an inherent method of sampling secretions from cells, with secretions being continuously swept downstream in the fluid flow. A comprehensive list of microfluidic systems that have been reported for assessing islet function can be found in Table 1.
Table 1.
Comparison of microfluidic devices developed for assessing islet function
| Reference number | Material | Islet positioning | Islet source | Islet number | Parallel stimulation | Insulin detection | Measurement method | Other measurements |
|---|---|---|---|---|---|---|---|---|
| 94 | Thermoplastic/glass | Automated | Human | 16–32 | Yes | On-chip | Fluorescence anisotropy | Hypoxia |
| 144 | Glass | Manual | Human/mouse | 1 | — | On-chip | Electrophoretic immunoassay | |
| 115 | Glass | Manual | Mouse | 4 × 1 | Yes | On-chip | Electrophoretic immunoassay | |
| 95 | Glass | Manual | Mouse | 15 × 1 | Yes | On-chip | Electrophoretic immunoassay | [Ca2+]i |
| 96 | Glass | Manual | Mouse | 15 × 1 | Yes | On-chip | Electrophoretic immunoassay | [Ca2+]i NADPH |
| 93 | Glass | Manual | Mouse | 1–10 | No | On-chip | Electrophoretic immunoassay | |
| 141 | Glass | Manual | Mouse | 1 | — | On-chip | Electrophoretic immunoassay | |
| 143 | Glass | Manual | Mouse | 1 | — | On-chip | Electrophoretic immunoassay | |
| 145 | Glass | Manual | Mouse | 4–11 | No | On-chip | Electrophoretic immunoassay | Islet amyloid polypeptide |
| 202 | Glass | Manual | Mouse | 1 | — | On-chip | Electrophoretic immunoassay | (Upstream adipocyte culture) |
| 146 | Glass | Manual | Mouse | 1 | — | On-chip | Electrophoretic immunoassay | |
| 151 | Glass | Manual | Mouse | 1 | — | On-chip | Fluorescence anisotropy | |
| 150 | PDMS/glass | Manual | Mouse | 1 | — | On-chip | Homogeneous fluorescence assay | |
| 142 | Glass | Manual | Rat | 1 | — | On-chip | Electrophoretic immunoassay | |
| 97 | PDMS/glass/SU-8 | Automated | Human | Up to 225 | No | Off-chip | ELISA | Membrane permittivity, dieletric spectra |
| 98 | PDMS/glass | Automated | Human pseudoislets | 32 (pseudoislets) | No | Off-chip | ELISA | Dynamic insulin imaging, viability, IF |
| 99 | Thermoplastic | Manual | Human/mouse | 3 × 10–60 | No | Off-chip | ELISA | Viability; optogenetics |
| 100 | PDMS/glass | Automated | Human/mouse | 100 | No | Off-chip | ELISA | [Ca2+]i, mitochondrial potential |
| 101 | PDMS/glass | Automated | Human/mouse | 20 | No | Off-chip | ELISA | [Ca2+]i, mitochondrial potential |
| 148 | PDMS/glass | Manual | Mouse | 1 | — | Off-chip | Homogeneous fluorescence assay | [Ca2+]i |
| 117 | PDMS/glass | Manual | Mouse | 8 | Yes | Off-chip | ELISA | [Ca2+]i, islet volume |
| 103 | PDMS/glass | Automated | Mouse | 25–30 | No | Off-chip | ELISA | [Ca2+]i, mitochondrial potential |
| 104 | PDMS | Automated | Mouse | 2 × 20 | No | Off-chip | ELISA | [Ca2+]i, mitochondrial potential |
| 107 | PDMS | Automated | Mouse | 3 × 10 | No | Off-chip | ELISA | [Ca2+]i, mitochondrial potential, viability |
| 108 | PDMS/glass | Automated | Mouse | 3 × 4–6 | No | Off-chip | ELISA | [Ca2+]i, NADPH, endothelial cell length/area |
| 109 | PDMS | Automated | Mouse | 20 | No | Off-chip | ELISA | [Ca2+]i, mitochondrial potential |
| 102 | PDMS | Automated | Mouse | 25–30 | No | Off-chip | ELISA | [Ca2+]i, mitochondrial potential |
| 120 | PDMS/glass | Manual | Mouse | <10 | No | Off-chip | ELISA | Glycerol secretion in adipocytes (cultured separately) |
| 114 | Glass | Automated | Mouse | 50 | No | Off-Chip | ELISA | [Ca2+]i, NADPH, oxygen consumption |
| 119 | PDMS/glass | Manual | Mouse | ∼10 | No | Off-chip | Homogeneous fluorescence assay | Free fatty acid uptake in adipocytes (cultured separately) |
| 118 | PDMS/glass | Manual | Rat | 1 | — | Off-chip | RIA | Oxygen concentration (commercial flow cell) |
| 105 | PDMS/glass | Automated | Human/mouse | 300 (100 analyzed) | No | — | — | [Ca2+]i, mitochondrial potential, viability |
| 106 | PDMS | Automated | Human/rat | 100 (25 analyzed) | No | — | — | [Ca2+]i, mitochondrial potential, NADPH |
| 110 | PDMS/glass | Automated | Mouse | 5–10 | No | — | — | Electron transfer flavoprotein, NADPH, lipoamide dehydrogenase |
| 203 | PDMS/glass | Manual | Mouse | 1 | — | — | — | [Ca2+]i |
| 111 | PDMS/glass | Automated | Mouse | 1 | — | — | — | [Ca2+]i, NADPH |
| 112 | PDMS/glass | Automated | Mouse | 1 | — | — | — | Zinc secretion, [Ca2+]i |
| 113 | PDMS/glass | Automated | Mouse | 10 | No | — | — | [Ca2+]i, endothelial cell length/area |
Features of devices for measuring islet function are listed, including materials used for device fabrication, how islets are loaded and positioned in the device, the source and number of islets that fit into the device, whether individual islets within the device are stimulated in parallel, whether insulin is measured within the device (“on-chip”) or off-line (“off-chip”), the method for measuring insulin, and any additional measurements made on the device. [Ca2+]i, calcium signaling; IF, immunofluorescence; RIA, radioimmunoassay.
While there have been numerous examples of microfluidic systems created for study of islet function to date, not all would be equally suitable for screening islet potency prior to transplantation. In the design of microfluidic devices for assaying islet potency, desired features include the ability to continuously measure a metric of islet function from a group of islets for batch analysis (94–106), positioning of islets within the device with minimal manual handling (94,97,98,100–114), the ability to stimulate the islets dynamically and homogeneously (94–96,115), and the use of materials and equipment that are easily scalable (94,99). Because much islet research with use of microfluidics to date has focused on elucidating basic principles of islet physiology, several devices are configured for measuring the activity of groups of islets <10—or even individual islets—which is likely too small for batch processing of islets for functional potency. Typically, samples of 15–100 islets are assayed for conventional GSIS (8,25,26), although the actual number needed to adequately account for the diversity of islets within an islet preparation is still under debate (116). Inherent to the integration of a continuous measurement of islet function is establishing an appropriate balance of islet number and flow rate to ensure that the signal level matches the limit and range of detection for the measurement technique in use. For devices that can screen function from a larger group of islets, many routinely require manual selection and loading of islets, which can drastically increase the protocol time for islet potency testing at scale. Furthermore, a number of devices are designed with a well or chamber intended for holding a large number of islets—akin to the wells found in static culture—but this arrangement does not typically ensure homogeneous or synchronized stimulation of islets due to their random positioning within the device. This configuration may instead lead to unintended variability in results, as islets may experience heterogeneous and uncontrolled local concentrations of oxygen, glucose, or other chemical cues. Thus, a device that can automatically position a sufficient number of islets for batch testing such that they receive parallel flow is preferred. In terms of construction, many devices are made from polydimethylsiloxane (PDMS) and/or glass, which are typically not amenable for scalable manufacturing. Thermoplastic devices are more suitable to fabrication at scale, but they can suffer from issues relating to feature size and disruptions to optical signals.
With regard to determining islet potency, several devices have incorporated methods to measure metrics of islet health or function aside from insulin secretion testing, such as intracellular calcium (95,96,100–107,109,114,117), mitochondrial membrane potential (100–107,109), NADPH (96,106,114), or viability/hypoxia (94,105,107). The use of multimodal approaches to screen different facets of islet function may prove to be important in providing a more detailed understanding the complex state of islets prior to transplantation (57). Unfortunately, considering the necessity of measuring dynamic insulin secretion, many devices are fundamentally limited due to their reliance on ELISA to conduct off-line insulin measurements (97,98,100–104,107–109,114,117–120). These systems therefore suffer from the same time and effort disadvantages experienced with traditional GSIS, practically translating into the stimulation and the protein quantification steps occurring on separate days. A continuous sensor integrated downstream of islets is required to overcome the time delay in obtaining relevant secretion data prior to transplantation.
In recognition of the particular need for a continuous insulin sensor, several technologies have been developed in recent years. Some use synthetic strands of RNA or DNA, known as “aptamers,” to bind to the insulin. These approaches often use an electrochemical (121–128) or surface plasmon resonance (129,130) sensing principle, wherein insulin-targeting aptamers conjugated to various active elements are adhered to a metal surface and can alter the electromagnetic properties of the system as a result of aptamer conformation change during insulin binding (131). In some cases, the aptamers adhered to a surface do not release their target compounds once bound, which necessitates cycles of wash steps to regenerate the sensors for repeated measurements (122,132,133). Promising work has also been done without wash steps for drug compounds (128,134,135) and some proteins (138–140), though it has not yet been demonstrated for hormone secretion detection from living human islets with temporal sampling high enough for dynamic GSIS. Progress is nonetheless being made to continue improving the aptamer technology for sensing insulin (121–124,129,131)—as well as other proteins more broadly (125,126).
Another option for sensing is to instead introduce a continuously flowing source of binding molecules that mix with the secretions and possibly other reagents to allow for optical signal detection. Such methods afford the possibility of using a broader range of antibodies and binding molecules, as they do not need to balance binding and release kinetics in order to allow for continuous sensing. One example is an electrophoretic sampling immunoassay, where antibodies against insulin and other hormones have been mixed with the cellular secretions and with fluorescently labeled versions of those molecules, and the mixture is then subjected to an electric field that separates the molecules based on size (92,93,95,96,115,141–146). While incredibly precise temporal measurements can be made with this technique, electrophoretic sampling has the unfortunate downside of requiring sophisticated equipment and a special balance of salt concentrations to ensure proper molecular migration and thus may not be suitable for large-scale application. Another option for insulin detection is an antibody-based assay known as “homogeneous fluorescence” or FRET-PINCER, where two antibodies against insulin are each conjugated with a DNA strand attached to a fluorophore and undergo fluorescence resonance energy transfer (FRET) when binding to a common insulin molecule (147). The homogeneous fluorescence assay has been modified for use in a microfluidic system that relies on formation of water in oil emulsion droplets created downstream of islets (148–150). Each droplet represents a distinct time point in the dynamic secretion and prevents loss of temporal resolution as the assay reagents mix and fluoresce. Although the technology is equally impressive for its time precision (150), it also requires elaborate flow control for droplet formation that may be prohibitive for multiplexing. Another recently introduced microfluidic method that borrows from a variant of antibody-based detection uses the principle of fluorescence anisotropy (94,151). Incidentally, this technique uses the same reagents as electrophoretic detection but relies on changes in polarization of fluorescent light in response to average molecular rotation of the mixture (152). Using this sensing technique, we have produced continuous insulin secretion data that mimicked the dynamic response found with the more conventional, two-step dynamic GSIS for the same batch of human islets (94). Since antibody binding decreases rotation rate in proportion to the size of the target analyte, the main limitation of this technique is the requirement that the analyte (e.g., insulin is ∼6 kDa) be sufficiently smaller than the antibody (∼150 kDa). In addition, fluorescence anisotropy is also influenced by buffer composition. For example, measurable differences in baseline readings have been observed in low (2.8 mmol/L) versus high (20 mmol/L) glucose concentrations, requiring background subtraction for islet potency testing (94). Using fluorescence anisotropy, work has also been done to show the possible detection of other hormones aside from insulin, such as glucagon, opening up the possibility of measuring the secretions of multiple hormones (153,154).
Integrated insulin sensors offer a powerful method for probing islet cell function. The recent development of several microfluidic devices that couple dynamic stimulation with continuous insulin secretion measurements is in line with the needs of potency testing, which currently lacks an efficient means to test the functional capacity of the tissue to produce hormones. Efforts are necessary to adapt the existing technologies for quality screening demands that may include the ability to assess more islets or for running multiplexed or multiparametric assays to add to the predictive value. Regardless, a system that can combine automated islet loading, homogeneous and dynamic stimulation, and, most importantly, continuous insulin detection will be the key to offering a fast potency test for islet transplantation.
Integration of Microenvironmental Cues
Beyond dynamic stimulation and integrated insulin sensing, the addition of cues from the native islet microenvironment would increase the relevance of potency tests to islet transplantation. As with myriad other tissues (155–164), the microenvironment of human islet cells dictates their biological function (165–170). For example, interactions between the insulin-producing β-cells and the endothelial cells of the adjoining vasculature have been shown to be particularly important for mediating β-cell activity including insulin secretion (31). Human islets also have a unique double-layer basement membrane, a specialized form of ECM typically found between a tissue and adjacent endothelial cells (165) that constitutes the local microenvironment experienced by β-cells (Fig. 1B). Experiments that have aimed to quantify the composition of ECM proteins in islets have revealed an abundance of structural proteins like collagens (e.g., ColVI, ColIV, ColI) and laminins (e.g., 511, 521), as well as a variety of matricellular proteins including osteopontin (SPP1), agrin, biglycan, osteonectin (SPARC), and tenascin C (171–173). Existing data on islet matrix proteins are almost certainly incomplete, since islets compose only 2% of the pancreas by mass and are typically isolated with a blend of collagenases and additional matrix-degrading enzymes (174,175). While enzymes used during islet isolation degrade matrix proteins, transcripts from ECM genes remain intact and can help paint a more complete picture. Our own comparison of published transcript and protein expression data from isolated whole islets and purified islet cells suggests that β-cells rely on neighboring mesenchymal cells to provide most of the matrix proteins found in the basement membrane layer (170) (Fig. 3A), although β-cells do transcribe multiple ECM genes (e.g., laminin 511 and SPP1) and likely contribute to matrix elaboration.
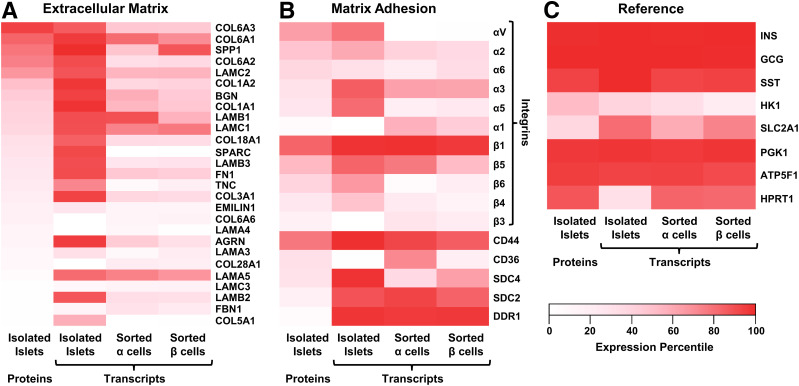
Figure 3.
Expression of ECM and matrix adhesion proteins in pancreatic islets. A–C: Heat maps of proteomics data for ECM (panel A), matrix attachment (panel B), and reference (panel C) proteins from isolated human islets (199) (first column) in comparison with transcriptomic data from either isolated human islets (200) (second column) or flow-sorted α-cells (third column) or β-cells (fourth column) (201). Each data set was percentile normalized prior to selection and plotting of the protein subsets. INS, insulin; GCG, glucagon; SST, somatostatin; HK1, hexokinase; SLC2A1, GLUT protein type 1.
When in contact with ECM, specialized proteins on the surface of cells such as integrins, syndecans, and discoidin domain receptors bind to the ECM and help to transmit information about the forces of cellular attachment—including responses to substrate stiffness—in a process known as mechanotransduction (176,177). As for matrix proteins, we similarly compared matrix attachment gene expression in islet cells (Fig. 3B). Reference proteins, such as the highly expressed islet hormones (insulin, glucagon, somatostatin), hexokinase, and the GLUT protein type 1, as well as ubiquitous housekeeping proteins (PGK1, ATP5F1, HPRT1), were also plotted (Fig. 3C). This analysis suggested that the most abundant integrin complex in islet endocrine cells is integrin α3/β1, a laminin receptor (178). The importance of laminin interactions is demonstrated by contributions to cell fate decisions during pancreatic development. While laminin engagement induces endocrine differentiation, pancreatic progenitors that attach to fibronectin via integrin α5/β1 complexes induce YAP1 transcriptional programs and form ductal tissue (179). Accordingly, blocking integrin attachment to laminin in developing islets decreases endocrine cell differentiation and survival (173,180). While different variants of integrin are expressed in different relative amounts for human and rodent islets (171), integrins in mice have been implicated in playing an important mechanistic role in initiating (181,182) and directionally polarizing (183) insulin secretion. Thus, the islet microenvironment provides necessary regulation of insulin secretion to help maintain glucose homeostasis.
It is therefore important to assess islet function in a context that mimics the signals that would normally be present from neighboring biological structures (165,170). However, establishing a proper microenvironment for in vitro systems is particularly challenging with regard to the surfaces that contact cells. Materials used to house cells in culture are typically from nonbiological sources. To address this dilemma, one may functionalize a given abiotic surface with ECM proteins that the β-cells experience in vivo (170). Matching the mechanical stiffness of the body presents an additional concern. While the healthy whole human pancreas has an elastic modulus of 1–3 kPa (184,185), and measurements of individual mouse islets have modulus values ranging from 100 Pa to 10 kPa (186), the stiffness of materials used for in vitro systems is typically on the order of 1 MPa and even as high as 1 GPa. As diabetes progresses, the exocrine pancreas and capillaries adjacent to islets stiffen (187–189), and it has been suggested that microvascular stiffening contributes to alterations in islet function (189). It is thus reasonable to suggest that the stiffer materials commonly used for culturing islet cells may provide pathological signals. The use of unmodified, nonbiological materials for culturing cells thus poses a significant chance of altering cell behavior. Indeed, the lack of physiological ECM and mechanical forces in traditional in vitro culture may partially explain why GSIS results do not correlate with transplantation outcome. This problem is compounded by the removal of native microenvironment during the isolation of donor islets, or the absence of these cues in the generation of stem cell therapies.
Improving the relevance of quality control testing for cell products—and perhaps the function of the cell products for their intended purpose—requires addition of cues that will allow for a cell product to experience conditions closer in form and function to those of native islets. Microencapsulation technologies and specialized containment devices have been developed primarily to address the immune attack of islet grafts (16–19,190–193), although these also serve a dual purpose in protecting cadaveric islets encased within from the stiff materials that would likely be used for in vitro functional testing. Such encapsulation strategies can also be tuned to provide appropriate ECM and contact forces for cells at the outermost layer of islets. In recognition of the important interplay of islets with the vasculature, microfluidic devices have also started to incorporate physiologically relevant conditions, such as utilizing endothelial cells and controlling shear forces (94,113,194,195). Combining these advances with glucose stimulation and insulin sensing will likely produce potency tests with more physiologically accurate responses, though it is also necessary to ensure that the contextual cues of endothelial cells in particular can be applied in a short enough window prior to transplantation. Other specific conditions islets experience during transplantation could also be mimicked in a potency test. For instance, it is estimated that >60% of an islet graft is destroyed by immune processes within hours of infusion, suggesting that there is a need for predicting how islets can avoid an immune response (6). Human islets embedded in a matrix derived from decellularized human pancreata elicit a reduced immune response in a humanized mouse model (196), and the same type of evaluation could be conducted in a microfluidic device, wherein immune cells would be added to simulate an inflammatory response or an immune rejection. With consideration of the posttransplantation environment, isolated islets or differentiated stem cell products could be prepared and analyzed prior to transplantation to elicit more accurate predictions of their functional potential. Accordingly, different transplantation sites (197) such as the portal vein or omental adipose tissue could be recapitulated to identify the best site for a given batch. By assessing islet function within a biomimetic microenvironment over time, the potency test may also help predict graft remodeling posttransplant or even improve the quality of batches that would otherwise fail quality control or fail to provide therapeutic benefit to patients. Our vision for an ideal potency test is depicted in Fig. 4. Such a potency test would automatically assess the islet secretion response to dynamic stimulation in a coupled system and would be poised to evaluate the increasing supply cell products from cadaveric or stem cell sources. The development of a system that recapitulates the native microenvironment of islets while sensing their secretory function is imperative for establishing an effective potency test.
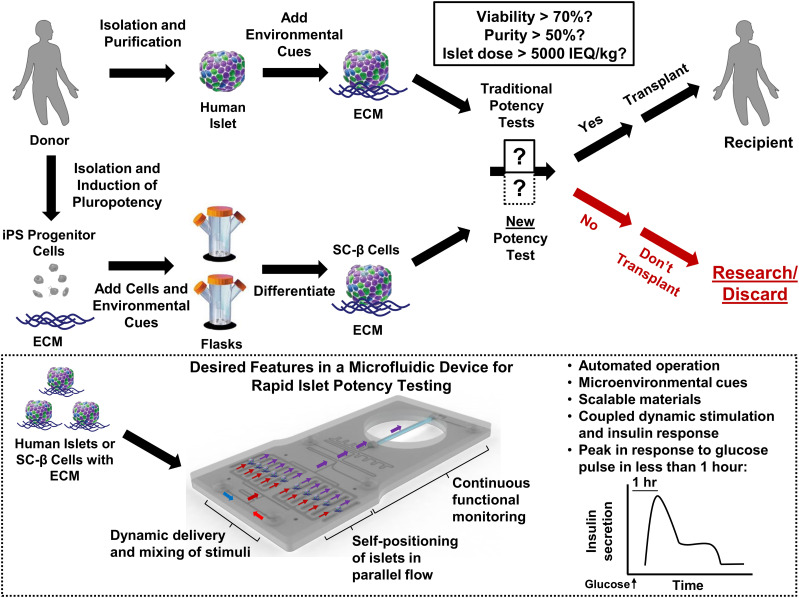
Figure 4.
Proposed paradigm for future islet transplantation. Next-generation islet transplantation can depend on either cadaveric donor islets or the use of stem cell–derived insulin-producing cells (SC-β cells), which should increase the supply of tissue. For supplementation of existing standards (solid black box) and allowing for β-cell-specific functional assessment, a new potency test (dotted black box) should be automated, be made of scalable materials, rely on coupled dynamic stimulation to and continuous insulin measurements from islets positioned in parallel, be able to measure a peak in response to glucose stimulation in <1 hr, and provide microenvironmental cues that mimic the islet conditions in vivo. Inset depicts an example of such a test that utilizes a microfluidic device designed with features that meet these criteria. hr, hour; iPS, induced pluripotent stem.
Conclusions
Reliable potency testing for use in islet transplantation clinical practice guidelines is stalled by the absence of rapid methods for sensing β-cell function. Because β-cells directly sample glucose concentration in the blood and respond by secreting insulin, a core identity of the β-cell is the dynamic coupling of glucose and insulin secretion. Integrated systems that deliver dynamic glucose stimulation and measure resultant insulin secretion continuously will lead to a more useful assessment of pretransplant islet screening. Given the importance of other secretagogues and physiological cues in inducing postprandial insulin secretion (198), it may also be useful to investigate other stimuli for insulin secretion. Also, since capillaries pass through pancreatic islets to allow for insulin to enter the bloodstream, the microenvironment that characterizes the interface between β-cells and capillaries should serve as a key inspiration. Adjusting ECM and stiffness can help donor islets recover within an environment similar to that from which they were extracted and can also improve stem cell–derived products by constructing developmental signals that they normally lack. Future potency tests for pancreatic islets will therefore rely on both continuous insulin measurements and replication of the microenvironmental cues that mimic in vivo conditions. Biomimetic potency tests that efficiently sense dynamic β-cell secretory function can dovetail with the increased availability of human islets and stem cell replacements to broaden access to a life-changing treatment for individuals with diabetes and help guarantee successful clinical outcomes.
Article Information
Acknowledgments. The authors thank Michael Rosnach, Harvard University, for illustrations and Dr. Bradley L. Hodges, Prothelia Incorporated, for helpful discussion.
Funding. This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases award UC4 DK104165.
Duality of Interest. The authors are named inventors on pending patent applications PCT US16/62693 (A.L.G., B.D.P., and K.K.P.) and PCT US18/15601 (A.L.G. and K.K.P.), filed by Harvard University. K.K.P. is a principal at KK Parker & Associates, with consulting responsibilities for several biotechnology and pharmaceutical companies. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. A.L.G. and B.D.P. analyzed the literature and drafted the manuscript with editing and guidance by K.K.P. and D.A.M.
Footnotes
A.L.G. and B.D.P. contributed equally to this work.
References
- 1.Shapiro AMJ, Pokrywczynska M, Ricordi C. Clinical pancreatic islet transplantation. Nat Rev Endocrinol 2017;13:268–277 [DOI] [PubMed] [Google Scholar]
- 2.Gruessner AC 2011 update on pancreas transplantation: comprehensive trend analysis of 25,000 cases followed up over the course of twenty-four years at the International Pancreas Transplant Registry (IPTR). Rev Diabet Stud 2011;8:6–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rickels MR, Peleckis AJ, Markmann E, et al. Long-term improvement in glucose control and counterregulation by islet transplantation for type 1 diabetes. J Clin Endocrinol Metab 2016;101:4421–4430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hering BJ, Clarke WR, Bridges ND, et al.; Clinical Islet Transplantation Consortium . Phase 3 trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care 2016;39:1230–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ricordi C, Goldstein JS, Balamurugan AN, et al. National Institutes of Health–sponsored Clinical Islet Transplantation Consortium phase 3 trial: manufacture of a complex cellular product at eight processing facilities. Diabetes 2016;65:3418–3428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gamble A, Pepper AR, Bruni A, Shapiro AMJ. The journey of islet cell transplantation and future development. Islets 2018;10:80–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hart NJ, Powers AC. Use of human islets to understand islet biology and diabetes: progress, challenges and suggestions. Diabetologia 2019;62:212–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kayton NS, Poffenberger G, Henske J, et al. Human islet preparations distributed for research exhibit a variety of insulin-secretory profiles. Am J Physiol Endocrinol Metab 2015;308:E592–E602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papas KK, Suszynski TM, Colton CK. Islet assessment for transplantation. Curr Opin Organ Transplant 2009;14:674–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pagliuca FW, Millman JR, Gürtler M, et al. Generation of functional human pancreatic β cells in vitro. Cell 2014;159:428–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kroon E, Martinson LA, Kadoya K, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol 2008;26:443–452 [DOI] [PubMed] [Google Scholar]
- 12.Millman JR, Xie C, Van Dervort A, Gürtler M, Pagliuca FW, Melton DA. Generation of stem cell-derived β-cells from patients with type 1 diabetes. Nat Commun 2016;7:11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sneddon JB, Tang Q, Stock P, et al. Stem cell therapies for treating diabetes: progress and remaining challenges. Cell Stem Cell 2018;22:810–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ribeiro D, Kvist AJ, Wittung-Stafshede P, Hicks R, Forslöw A. 3D-Models of insulin-producing β-cells: from primary islet cells to stem cell-derived islets. Stem Cell Rev Rep 2018;14:177–188 [DOI] [PubMed] [Google Scholar]
- 15.Veres A, Faust AL, Bushnell HL, et al. Charting cellular identity during human in vitro β-cell differentiation. Nature 2019;569:368–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robert T, De Mesmaeker I, Stangé GM, et al. Functional beta cell mass from device-encapsulated hESC-derived pancreatic endoderm achieving metabolic control. Stem Cell Reports 2018;10:739–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang R, Faleo G, Russ HA, et al. Nanoporous immunoprotective device for stem-cell-derived β-cell replacement therapy. ACS Nano 2017;11:7747–7757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vegas AJ, Veiseh O, Gürtler M, et al. Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat Med 2016;22:306–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agulnick AD, Ambruzs DM, Moorman MA, et al. Insulin-producing endocrine cells differentiated in vitro from human embryonic stem cells function in macroencapsulation devices in vivo. Stem Cells Transl Med 2015;4:1214–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.U.S. Food and Drug Administration Guidance for Industry: Considerations for Allogeneic Pancreatic Islet Cell Products, 2009. Accessed 9 January 2020. Available from https://www.fda.gov/media/77497/download.
- 21.Graham R, Mancher M, Miller Wolman D, Greenfield S, Steinberg E. Clinical Practice Guidelines We Can Trust. Washington, DC, National Academy of Sciences, 2011, p. 291. [PubMed] [Google Scholar]
- 22.U.S. Food and Drug Administration , 2018. E6(R2) Good Clinical Practice: Integrated Addendum to ICH E6(R1). Accessed 24 January 2020. Available from https://www.fda.gov/media/93884/download
- 23.Yamamoto T, Horiguchi A, Ito M, et al. Quality control for clinical islet transplantation: organ procurement and preservation, the islet processing facility, isolation, and potency tests. J Hepatobiliary Pancreat Surg 2009;16:131–136 [DOI] [PubMed] [Google Scholar]
- 24.National Institute of Diabetes and Digestive and Kidney Diseases , 2012. Clinical Islet Transplantation (CIT) Protocol CIT-07: Islet Transplantation in Type 1 Diabetes. Version No. 8.0, Publication No. BB-IND 9336. Accessed 24 January 2020. Available from https://repository.niddk.nih.gov/media/studies/cit-07/Protocol.pdf
- 25.Lyon J, Manning Fox JE, Spigelman AF, et al. Research-focused isolation of human islets from donors with and without diabetes at the Alberta Diabetes Institute IsletCore. Endocrinology 2016;157:560–569 [DOI] [PubMed] [Google Scholar]
- 26.Henquin JC Influence of organ donor attributes and preparation characteristics on the dynamics of insulin secretion in isolated human islets. Physiol Rep 2018;6:e13646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piemonti L, Everly MJ, Maffi P, et al. Alloantibody and autoantibody monitoring predicts islet transplantation outcome in human type 1 diabetes. Diabetes 2013;62:1656–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rickels MR, Robertson RP. Pancreatic islet transplantation in humans: recent progress and future directions. Endocr Rev 2019;40:631–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rostambeigi N, Lanza IR, Dzeja PP, et al. Unique cellular and mitochondrial defects mediate FK506-induced islet β-cell dysfunction. Transplantation 2011;91:615–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cross SE, Vaughan RH, Willcox AJ, et al. Key matrix proteins within the pancreatic islet basement membrane are differentially digested during human islet isolation. Am J Transplant 2017;17:451–461 [DOI] [PubMed] [Google Scholar]
- 31.Narayanan S, Loganathan G, Dhanasekaran M, et al. Intra-islet endothelial cell and β-cell crosstalk: implication for islet cell transplantation. World J Transplant 2017;7:117–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teo AKK, Lim CS, Cheow LF, et al. Single-cell analyses of human islet cells reveal de-differentiation signatures. Cell Death Discov 2018;4:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kitzmann JP, O’Gorman D, Kin T, et al. Islet oxygen consumption rate dose predicts insulin independence for first clinical islet allotransplants. Transplant Proc 2014;46:1985–1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papas KK, Bellin MD, Sutherland DER, et al. Islet oxygen consumption rate (OCR) dose predicts insulin independence in clinical islet autotransplantation. PLoS One 2015;10:e0134428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang LJ, Kissler HJ, Wang X, et al. Application of digital image analysis to determine pancreatic islet mass and purity in clinical islet isolation and transplantation. Cell Transplant 2015;24:1195–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang LJ, Kaufman DB. Digital image analysis to assess quantity and morphological quality of isolated pancreatic islets. Cell Transplant 2016;25:1219–1225 [DOI] [PubMed] [Google Scholar]
- 37.Buchwald P, Bernal A, Echeverri F, Tamayo-Garcia A, Linetsky E, Ricordi C. Fully automated islet cell counter (ICC) for the assessment of islet mass, purity, and size distribution by digital image analysis. Cell Transplant 2016;25:1747–1761 [DOI] [PubMed] [Google Scholar]
- 38.Khiatah B, Qi M, Wu Y, et al. Pancreatic human islets and insulin-producing cells derived from embryonic stem cells are rapidly identified by a newly developed Dithizone. Sci Rep 2019;9:9295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen M, Zhang S, Xing Y, et al. Genetically encoded, photostable indicators to image dynamic Zn2+ secretion of pancreatic islets. Anal Chem 2019;91:12212–12219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hilderink J, Otto C, Slump C, et al. Label-free detection of insulin and glucagon within human islets of Langerhans using Raman spectroscopy. PLoS One 2013;8:e78148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berkova Z, Saudek F, Girman P, et al. Combining donor characteristics with immunohistological data improves the prediction of islet isolation success. J Diabetes Res 2016;2016:4214328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jin SM, Kim KS, Lee SY, et al. Enhanced prediction of porcine islet yield and posttransplant outcome using a combination of quantitative histomorphometric parameters and flow cytometry. Cell Transplant 2010;19:299–311 [DOI] [PubMed] [Google Scholar]
- 43.Todorov I, Nair I, Avakian-Mansoorian A, et al. Quantitative assessment of β-cell apoptosis and cell composition of isolated, undisrupted human islets by laser scanning cytometry. Transplantation 2010;90:836–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lebreton F, Pirog A, Belouah I, et al. Slow potentials encode intercellular coupling and insulin demand in pancreatic beta cells. Diabetologia 2015;58:1291–1299 [DOI] [PubMed] [Google Scholar]
- 45.Cabrera O, Jacques-Silva MC, Berman DM, et al. Automated, high-throughput assays for evaluation of human pancreatic islet function. Cell Transplant 2008;16:1039–1048 [PMC free article] [PubMed] [Google Scholar]
- 46.Hanson MS, Steffen A, Danobeitia JS, Ludwig B, Fernandez LA. Flow cytometric quantification of glucose-stimulated β-cell metabolic flux can reveal impaired islet functional potency. Cell Transplant 2008;17:1337–1347 [DOI] [PubMed] [Google Scholar]
- 47.Goto M, Holgersson J, Kumagai-Braesch M, Korsgren O. The ADP/ATP ratio: a novel predictive assay for quality assessment of isolated pancreatic islets. Am J Transplant 2006;6:2483–2487 [DOI] [PubMed] [Google Scholar]
- 48.Armann B, Hanson MS, Hatch E, Steffen A, Fernandez LA. Quantification of basal and stimulated ROS levels as predictors of islet potency and function. Am J Transplant 2007;7:38–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pepper AR, Hasilo CP, Melling CWJ, et al. The islet size to oxygen consumption ratio reliably predicts reversal of diabetes posttransplant. Cell Transplant 2012;21:2797–2804 [DOI] [PubMed] [Google Scholar]
- 50.Suszynski TM, Avgoustiniatos ES, Stein SA, Falde EJ, Hammer BE, Papas KK. Assessment of tissue-engineered islet graft viability by fluorine magnetic resonance spectroscopy. Transplant Proc 2011;43:3221–3225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suszynski TM, Mueller KR, Gruessner AC, Papas KK. Metabolic profile of pancreatic acinar and islet tissue in culture. Transplant Proc 2014;46:1960–1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fraker C, Timmins MR, Guarino RD, et al. The use of the BD oxygen biosensor system to assess isolated human islets of langerhans: oxygen consumption as a potential measure of islet potency. Cell Transplant 2006;15:745–758 [DOI] [PubMed] [Google Scholar]
- 53.Saravanan PB, Kanak MA, Chang CA, et al. Islet damage during isolation as assessed by miRNAs and the correlation of miRNA levels with posttransplantation outcome in islet autotransplantation. Am J Transplant 2018;18:982–989 [DOI] [PubMed] [Google Scholar]
- 54.Yoshimatsu G, Takita M, Kanak MA, et al. MiR-375 and miR-200c as predictive biomarkers of islet isolation and transplantation in total pancreatectomy with islet autotransplantation. J Hepatobiliary Pancreat Sci 2016;23:585–594 [DOI] [PubMed] [Google Scholar]
- 55.Wong WKM, Jiang G, Sørensen AE, et al. The long noncoding RNA MALAT1 predicts human pancreatic islet isolation quality. JCI Insight 2019;5:e129299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Itoh T, Takita M, SoRelle JA, et al. Correlation of released HMGB1 levels with the degree of islet damage in mice and humans and with the outcomes of islet transplantation in mice. Cell Transplant 2012;21:1371–1381 [DOI] [PubMed] [Google Scholar]
- 57.Kurian SM, Ferreri K, Wang CH, et al. Gene expression signature predicts human islet integrity and transplant functionality in diabetic mice. PLoS One 2017;12:e0185331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qi M, Bilbao S, Forouhar E, Kandeel F, Al-Abdullah IH. Encompassing ATP, DNA, insulin, and protein content for quantification and assessment of human pancreatic islets. Cell Tissue Bank 2018;19:77–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hanson MS, Park EE, Sears ML, et al. A simplified approach to human islet quality assessment. Transplantation 2010;89:1178–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gaber AO, Fraga D, Kotb M, Lo A, Sabek O, Latif K. Human islet graft function in NOD-SCID mice predicts clinical response in islet transplant recipients. Transplant Proc 2004;36:1108–1110 [DOI] [PubMed] [Google Scholar]
- 61.Caiazzo R, Gmyr V, Kremer B, et al. Quantitative in vivo islet potency assay in normoglycemic nude mice correlates with primary graft function after clinical transplantation. Transplantation 2008;86:360–363 [DOI] [PubMed] [Google Scholar]
- 62.Rorsman P, Braun M. Regulation of insulin secretion in human pancreatic islets. Annu Rev Physiol 2013;75:155–179 [DOI] [PubMed] [Google Scholar]
- 63.Alcazar O, Buchwald P. Concentration-dependency and time profile of insulin secretion: dynamic perifusion studies with human and murine islets. Front Endocrinol (Lausanne) 2019;10:680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen W, Shu Z, Gao D, Shen AQ. Sensing and sensibility: single-islet-based quality control assay of cryopreserved pancreatic islets with functionalized hydrogel microcapsules. Adv Healthc Mater 2016;5:223–231 [DOI] [PubMed] [Google Scholar]
- 65.Song SH, Kjems L, Ritzel R, et al. Pulsatile insulin secretion by human pancreatic islets. J Clin Endocrinol Metab 2002;87:213–221 [DOI] [PubMed] [Google Scholar]
- 66.Nano R, Kerr-Conte JA, Bosco D, et al. Islets for research: nothing is perfect, but we can do better. Diabetes 2019;68:1541–1543 [DOI] [PubMed] [Google Scholar]
- 67.Brissova M, Niland JC, Cravens J, Olack B, Sowinski J, Evans-Molina C. The integrated islet distribution program answers the call for improved human islet phenotyping and reporting of human islet characteristics in research articles. Diabetes 2019;68:1363–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jansson L, Barbu A, Bodin B, et al. Pancreatic islet blood flow and its measurement. Ups J Med Sci 2016;121:81–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Enright HA, Felix SH, Fischer NO, et al. Long-term non-invasive interrogation of human dorsal root ganglion neuronal cultures on an integrated microfluidic multielectrode array platform. Analyst (Lond) 2016;141:5346–5357 [DOI] [PubMed] [Google Scholar]
- 70.Jäckel D, Bakkum DJ, Russell TL, et al. Combination of high-density microelectrode array and patch clamp recordings to enable studies of multisynaptic integration. Sci Rep 2017;7:978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tsai D, Sawyer D, Bradd A, Yuste R, Shepard KL. A very large-scale microelectrode array for cellular-resolution electrophysiology. Nat Commun 2017;8:1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lind JU, Yadid M, Perkins I, et al. Cardiac microphysiological devices with flexible thin-film sensors for higher-throughput drug screening. Lab Chip 2017;17:3692–3703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alford PW, Feinberg AW, Sheehy SP, Parker KK. Biohybrid thin films for measuring contractility in engineered cardiovascular muscle. Biomaterials 2010;31:3613–3621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grosberg A, Alford PW, McCain ML, Parker KK. Ensembles of engineered cardiac tissues for physiological and pharmacological study: heart on a chip. Lab Chip 2011;11:4165–4173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Uzel SGM, Platt RJ, Subramanian V, et al. Microfluidic device for the formation of optically excitable, three-dimensional, compartmentalized motor units. Sci Adv 2016;2:e1501429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kujala VJ, Pasqualini FS, Goss JA, Nawroth JC, Parker KK. Laminar ventricular myocardium on a microelectrode array-based chip. J Mater Chem B Mater Biol Med 2016;4:3534–3543 [DOI] [PubMed] [Google Scholar]
- 77.Pradhapan P, Kuusela J, Viik J, Aalto-Setälä K, Hyttinen J. Cardiomyocyte MEA data analysis (CardioMDA)--a novel field potential data analysis software for pluripotent stem cell derived cardiomyocytes. PLoS One 2013;8:e73637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Natarajan A, Stancescu M, Dhir V, et al. Patterned cardiomyocytes on microelectrode arrays as a functional, high information content drug screening platform. Biomaterials 2011;32:4267–4274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chung B, Kim J, Liu HW, et al. Microfluidic in vitro brain endothelial monolayer model to evaluate cell-penetrating peptides. Micro Nano Syst Lett 2019;7:13 [Google Scholar]
- 80.Xu P, Elamin E, Elizalde M, et al. Modulation of intestinal epithelial permeability by plasma from patients with Crohn’s disease in a three-dimensional cell culture model. Sci Rep 2019;9:2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Trietsch SJ, Naumovska E, Kurek D, et al. Membrane-free culture and real-time barrier integrity assessment of perfused intestinal epithelium tubes. Nat Commun 2017;8:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maoz BM, Herland A, Henry OYF, et al. Organs-on-chips with combined multi-electrode array and transepithelial electrical resistance measurement capabilities. Lab Chip 2017;17:2294–2302 [DOI] [PubMed] [Google Scholar]
- 83.McCain ML, Agarwal A, Nesmith HW, Nesmith AP, Parker KK. Micromolded gelatin hydrogels for extended culture of engineered cardiac tissues. Biomaterials 2014;35:5462–5471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Davis BN, Yen R, Prasad V, Truskey GA. Oxygen consumption in human, tissue-engineered myobundles during basal and electrical stimulation conditions. APL Bioeng 2019;3:026103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Taddeo EP, Stiles L, Sereda S, et al. Individual islet respirometry reveals functional diversity within the islet population of mice and human donors. Mol Metab 2018;16:150–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Issaq H, Veenstra T. Two-dimensional polyacrylamide gel electrophoresis (2D-PAGE): advances and perspectives. Biotechniques 2008;44:697–698, 700 [DOI] [PubMed] [Google Scholar]
- 87.Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet 2009;10:57–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nilsson T, Mann M, Aebersold R, Yates JR III, Bairoch A, Bergeron JJM. Mass spectrometry in high-throughput proteomics: ready for the big time. Nat Methods 2010;7:681–685 [DOI] [PubMed] [Google Scholar]
- 89.Rosenberg JM, Utz PJ. Protein microarrays: a new tool for the study of autoantibodies in immunodeficiency. Front Immunol 2015;6:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Narayanamurthy V, Jeroish ZE, Bhuvaneshwari KS, et al. Advances in passively driven microfluidics and lab-on-chip devices: a comprehensive literature review and patent analysis. RSC Adv 2020;10:11652–11680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dhumpa R, Truong TM, Wang X, Bertram R, Roper MG. Negative feedback synchronizes islets of Langerhans. Biophys J 2014;106:2275–2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dhumpa R, Truong TM, Wang X, Roper MG. Measurement of the entrainment window of islets of Langerhans by microfluidic delivery of a chirped glucose waveform. Integr Biol 2015;7:1061–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yi L, Wang X, Dhumpa R, Schrell AM, Mukhitov N, Roper MG. Integrated perfusion and separation systems for entrainment of insulin secretion from islets of Langerhans. Lab Chip 2015;15:823–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Glieberman AL, Pope BD, Zimmerman JF, et al. Synchronized stimulation and continuous insulin sensing in a microfluidic human Islet on a Chip designed for scalable manufacturing. Lab Chip 2019;19:2993–3010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dishinger JF, Reid KR, Kennedy RT. Quantitative monitoring of insulin secretion from single islets of Langerhans in parallel on a microfluidic chip. Anal Chem 2009;81:3119–3127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nunemaker CS, Dishinger JF, Dula SB, et al. Glucose metabolism, islet architecture, and genetic homogeneity in imprinting of [Ca2+]i and insulin rhythms in mouse islets. PLoS One 2009;4:e8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Heileman K, Daoud J, Hasilo C, Gasparrini M, Paraskevas S, Tabrizian M. Microfluidic platform for assessing pancreatic islet functionality through dielectric spectroscopy. Biomicrofluidics 2015;9:044125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zbinden A, Marzi J, Schlünder K, et al. Non-invasive marker-independent high content analysis of a microphysiological human pancreas-on-a-chip model. Matrix Biol 2019;85–86:205–220 [DOI] [PubMed] [Google Scholar]
- 99.Lenguito G, Chaimov D, Weitz JR, et al. Resealable, optically accessible, PDMS-free fluidic platform for ex vivo interrogation of pancreatic islets. Lab Chip 2017;17:772–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mohammed JS, Wang Y, Harvat TA, Oberholzer J, Eddington DT. Microfluidic device for multimodal characterization of pancreatic islets. Lab Chip 2009;9:97–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xing Y, Nourmohammadzadeh M, Elias JEM, et al. A pumpless microfluidic device driven by surface tension for pancreatic islet analysis. Biomed Microdevices 2016;18:80. [DOI] [PubMed] [Google Scholar]
- 102.Adewola AF, Lee D, Harvat T, et al. Microfluidic perifusion and imaging device for multi-parametric islet function assessment. Biomed Microdevices 2010;12:409–417 [DOI] [PubMed] [Google Scholar]
- 103.Adewola AF, Wang Y, Harvat T, Eddington DT, Lee D, Oberholzer J. A multi-parametric islet perifusion system within a microfluidic perifusion device. J Vis Exp 2010:4–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee D, Wang Y, Mendoza-Elias JE, et al. Dual microfluidic perifusion networks for concurrent islet perifusion and optical imaging. Biomed Microdevices 2012;14:7–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nourmohammadzadeh M, Xing Y, Lee JW, et al. A microfluidic array for real-time live-cell imaging of human and rodent pancreatic islets. Lab Chip 2016;16:1466–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nourmohammadzadeh M, Lo JF, Bochenek M, et al. Microfluidic array with integrated oxygenation control for real-time live-cell imaging: effect of hypoxia on physiology of microencapsulated pancreatic islets. Anal Chem 2013;85:11240–11249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang Y, Lee D, Zhang L, et al. Systematic prevention of bubble formation and accumulation for long-term culture of pancreatic islet cells in microfluidic device. Biomed Microdevices 2012;14:419–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sankar KS, Green BJ, Crocker AR, Verity JE, Altamentova SM, Rocheleau JV. Culturing pancreatic islets in microfluidic flow enhances morphology of the associated endothelial cells. PLoS One 2011;6:e24904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lo JF, Wang Y, Blake A, et al. Islet preconditioning via multimodal microfluidic modulation of intermittent hypoxia. Anal Chem 2012;84:1987–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lam AK, Silva PN, Altamentova SM, Rocheleau JV. Quantitative imaging of electron transfer flavoprotein autofluorescence reveals the dynamics of lipid partitioning in living pancreatic islets. Integr Biol 2012;4:838–846 [DOI] [PubMed] [Google Scholar]
- 111.Rocheleau JV, Walker GM, Head WS, McGuinness OP, Piston DW. Microfluidic glucose stimulation reveals limited coordination of intracellular Ca2+ activity oscillations in pancreatic islets. Proc Natl Acad Sci U S A 2004;101:12899–12903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Easley CJ, Rocheleau JV, Head WS, Piston DW. Quantitative measurement of zinc secretion from pancreatic islets with high temporal resolution using droplet-based microfluidics. Anal Chem 2009;81:9086–9095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Silva PN, Green BJ, Altamentova SM, Rocheleau JV. A microfluidic device designed to induce media flow throughout pancreatic islets while limiting shear-induced damage. Lab Chip 2013;13:4374–4384 [DOI] [PubMed] [Google Scholar]
- 114.Schulze T, Mattern K, Früh E, Hecht L, Rustenbeck I, Dietzel A. A 3D microfluidic perfusion system made from glass for multiparametric analysis of stimulus-secretioncoupling in pancreatic islets. Biomed Microdevices 2017;19:47. [DOI] [PubMed] [Google Scholar]
- 115.Dishinger JF, Kennedy RT. Serial immunoassays in parallel on a microfluidic chip for monitoring hormone secretion from living cells. Anal Chem 2007;79:947–954 [DOI] [PubMed] [Google Scholar]
- 116.Dybala MP, Hara M. Heterogeneity of the human pancreatic islet. Diabetes 2019;68:1230–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Godwin LA, Pilkerton ME, Deal KS, Wanders D, Judd RL, Easley CJ. Passively operated microfluidic device for stimulation and secretion sampling of single pancreatic islets. Anal Chem 2011;83:7166–7172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen W, Lisowski M, Khalil G, Sweet IR, Shen AQ. Microencapsulated 3-dimensional sensor for the measurement of oxygen in single isolated pancreatic islets. PLoS One 2012;7:e33070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li X, Brooks JC, Hu J, Ford KI, Easley CJ. 3D-templated, fully automated microfluidic input/output multiplexer for endocrine tissue culture and secretion sampling. Lab Chip 2017;17:341–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brooks JC, Ford KI, Holder DH, Holtan MD, Easley CJ. Macro-to-micro interfacing to microfluidic channels using 3D-printed templates: application to time-resolved secretion sampling of endocrine tissue. Analyst (Lond) 2016;141:5714–5721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hu J, Wang T, Kim J, Shannon C, Easley CJ. Quantitation of femtomolar protein levels via direct readout with the electrochemical proximity assay. J Am Chem Soc 2012;134:7066–7072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hu J, Yu Y, Brooks JC, et al. A reusable electrochemical proximity assay for highly selective, real-time protein quantitation in biological matrices. J Am Chem Soc 2014;136:8467–8474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhao Y, Xu Y, Zhang M, Xiang J, Deng C, Wu H. An electrochemical dual-signaling aptasensor for the ultrasensitive detection of insulin. Anal Biochem 2019;573:30–36 [DOI] [PubMed] [Google Scholar]
- 124.Wu Y, Midinov B, White RJ. Electrochemical aptamer-based sensor for real-time monitoring of insulin. ACS Sens 2019;4:498–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kang D, Parolo C, Sun S, Ogden NE, Dahlquist FW, Plaxco KW. Expanding the scope of protein-detecting electrochemical DNA “scaffold” sensors. ACS Sens 2018;3:1271–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kang D, Sun S, Kurnik M, Morales D, Dahlquist FW, Plaxco KW. New architecture for reagentless, protein-based electrochemical biosensors. J Am Chem Soc 2017;139:12113–12116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li H, Dauphin-Ducharme P, Ortega G, Plaxco KW. Calibration-free electrochemical biosensors supporting accurate molecular measurements directly in undiluted whole blood. J Am Chem Soc 2017;139:11207–11213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Arroyo-Currás N, Somerson J, Vieira PA, Ploense KL, Kippin TE, Plaxco KW. Real-time measurement of small molecules directly in awake, ambulatory animals. Proc Natl Acad Sci U S A 2017;114:645–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Singh V, Nerimetla R, Yang M, Krishnan S. Magnetite-quantum dot immunoarray for plasmon-coupled-fluorescence imaging of blood insulin and glycated hemoglobin. ACS Sens 2017;2:909–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cho H, Kumar S, Yang D, et al. Surface-enhanced Raman spectroscopy-based label-free insulin detection at physiological concentrations for analysis of islet performance. ACS Sens 2018;3:65–71 [DOI] [PubMed] [Google Scholar]
- 131.Singh V, Krishnan S. Electrochemical and surface plasmon insulin assays on clinical samples. Analyst (Lond) 2018;143:1544–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhou Q, Kwa T, Gao Y, Liu Y, Rahimian A, Revzin A. On-chip regeneration of aptasensors for monitoring cell secretion. Lab Chip 2014;14:276–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shin SR, Kilic T, Zhang YS, et al. Label-free and regenerative electrochemical microfluidic biosensors for continual monitoring of cell secretomes. Adv Sci (Weinh) 2017;4:1600522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ferguson BS, Hoggarth DA, Maliniak D, Ploense K, White RJ, Woodward N, et al. Real-time, aptamer-based tracking of circulating therapeutic agents in living animals. Sci Transl Med 2013;5:213ra165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chien J-C, Mage PL, Soh HT, Arbabian A An aptamer-based electrochemical-sensing implant for continuous therapeutic-drug monitoring in vivo. 2019 Symposium on VLSI Circuits. Kyoto, Japan, IEEE, 2019, pp. C312–C313. DOI: 10.23919/VLSIC.2019.8777991.
- 136.Liu G, Cao C, Ni S, Feng S, Wei H. On-chip structure-switching aptamer-modified magnetic nanobeads for the continuous monitoring of interferon-gamma ex vivo. Microsyst Nanoeng 2019;5:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liu Y, Yan J, Howland MC, Kwa T, Revzin A. Micropatterned aptasensors for continuous monitoring of cytokine release from human leukocytes. Anal Chem 2011;83:8286–8292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hao Z, Wang Z, Li Y, et al. Measurement of cytokine biomarkers using an aptamer-based affinity graphene nanosensor on a flexible substrate toward wearable applications. Nanoscale 2018;10:21681–21688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhou Q, Patel D, Kwa T, et al. Liver injury-on-a-chip: microfluidic co-cultures with integrated biosensors for monitoring liver cell signaling during injury. Lab Chip 2015;15:4467–4478 [DOI] [PubMed] [Google Scholar]
- 140.Liu Y, Liu Y, Matharu Z, Rahimian A, Revzin A. Detecting multiple cell-secreted cytokines from the same aptamer-functionalized electrode. Biosens Bioelectron 2015;64:43–50 [DOI] [PubMed] [Google Scholar]
- 141.Reid KR, Kennedy RT. Continuous operation of microfabricated electrophoresis devices for 24 hours and application to chemical monitoring of living cells. Anal Chem 2009;81:6837–6842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Schultz NM, Huang L, Kennedy RT. Capillary electrophoresis-based immunoassay to determine insulin content and insulin secretion from single islets of Langerhans. Anal Chem 1995;67:924–929 [DOI] [PubMed] [Google Scholar]
- 143.Roper MG, Shackman JG, Dahlgren GM, Kennedy RT. Microfluidic chip for continuous monitoring of hormone secretion from live cells using an electrophoresis-based immunoassay. Anal Chem 2003;75:4711–4717 [DOI] [PubMed] [Google Scholar]
- 144.Bandak B, Yi L, Roper MG. Microfluidic-enabled quantitative measurements of insulin release dynamics from single islets of Langerhans in response to 5-palmitic acid hydroxy stearic acid. Lab Chip 2018;18:2873–2882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lomasney AR, Yi L, Roper MG. Simultaneous monitoring of insulin and islet amyloid polypeptide secretion from islets of Langerhans on a microfluidic device. Anal Chem 2013;85:7919–7925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shackman JG, Dahlgren GM, Peters JL, Kennedy RT. Perfusion and chemical monitoring of living cells on a microfluidic chip. Lab Chip 2005;5:56–63 [DOI] [PubMed] [Google Scholar]
- 147.Heyduk E, Moxley MM, Salvatori A, Corbett JA, Heyduk T. Homogeneous insulin and C-peptide sensors for rapid assessment of insulin and C-peptide secretion by the islets. Diabetes 2010;59:2360–2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chen D, Du W, Liu Y, et al. The chemistrode: a droplet-based microfluidic device for stimulation and recording with high temporal, spatial, and chemical resolution. Proc Natl Acad Sci U S A 2008;105:16843–16848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Negou JT, Avila LA, Li X, Hagos TM, Easley CJ. Automated microfluidic droplet-based sample chopper for detection of small fluorescence differences using lock-in analysis. Anal Chem 2017;89:6153–6159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li X, Hu J, Easley CJ. Automated microfluidic droplet sampling with integrated, mix-and-read immunoassays to resolve endocrine tissue secretion dynamics. Lab Chip 2018;18:2926–2935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Schrell AM, Mukhitov N, Yi L, Adablah JE, Menezes J, Roper MG. Online fluorescence anisotropy immunoassay for monitoring insulin secretion from islets of Langerhans. Anal Methods 2017;9:38–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jameson DM, Ross JA. Fluorescence polarization/anisotropy in diagnostics and imaging. Chem Rev 2010;110:2685–2708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Schrell AM, Mukhitov N, Roper MG. Multiplexing fluorescence anisotropy using frequency encoding. Anal Chem 2016;88:7910–7915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Leng W, Evans K, Roper MG. A microfluidic platform integrating pressure-driven and electroosmotic-driven flow with inline filters for affinity separations. Anal Methods 2019;11:5768–5775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bray MA, Sheehy SP, Parker KK. Sarcomere alignment is regulated by myocyte shape. Cell Motil Cytoskeleton 2008;65:641–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Alford PW, Nesmith AP, Seywerd JN, Grosberg A, Parker KK. Vascular smooth muscle contractility depends on cell shape. Integr Biol 2011;3:1063–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kuo PL, Lee H, Bray MA, et al. Myocyte shape regulates lateral registry of sarcomeres and contractility. Am J Pathol 2012;181:2030–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ye GJC, Aratyn-Schaus Y, Nesmith AP, Pasqualini FS, Alford PW, Parker KK. The contractile strength of vascular smooth muscle myocytes is shape dependent. Integr Biol (Camb) 2014;6:152–163 [DOI] [PubMed] [Google Scholar]
- 159.McCain ML, Yuan H, Pasqualini FS, Campbell PH, Parker KK. Matrix elasticity regulates the optimal cardiac myocyte shape for contractility. Am J Physiol Heart Circ Physiol 2014;306:H1525–H1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rohr S, Schölly DM, Kléber AG. Patterned growth of neonatal rat heart cells in culture. Morphological and electrophysiological characterization. Circ Res 1991;68:114–130 [DOI] [PubMed] [Google Scholar]
- 161.Czöndör K, Garcia M, Argento A, et al. Micropatterned substrates coated with neuronal adhesion molecules for high-content study of synapse formation. Nat Commun 2013;4:2252. [DOI] [PubMed] [Google Scholar]
- 162.García-Parra P, Cavaliere F, Maroto M, et al. Modeling neural differentiation on micropatterned substrates coated with neural matrix components. Front Cell Neurosci 2012;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Micropatterned surfaces for control of cell shape, position, and function. Biotechnol Prog 1998;14:356–363 [DOI] [PubMed] [Google Scholar]
- 164.Dike LE, Chen CS, Mrksich M, Tien J, Whitesides GM, Ingber DE. Geometric control of switching between growth, apoptosis, and differentiation during angiogenesis using micropatterned substrates. In Vitro Cell Dev Biol Anim 1999;35:441–448 [DOI] [PubMed] [Google Scholar]
- 165.Virtanen I, Banerjee M, Palgi J, et al. Blood vessels of human islets of Langerhans are surrounded by a double basement membrane. Diabetologia 2008;51:1181–1191 [DOI] [PubMed] [Google Scholar]
- 166.Kaido T, Yebra M, Cirulli V, Montgomery AM. Regulation of human β-cell adhesion, motility, and insulin secretion by collagen IV and its receptor α1β1. J Biol Chem 2004;279:53762–53769 [DOI] [PubMed] [Google Scholar]
- 167.Kaido T, Yebra M, Cirulli V, Rhodes C, Diaferia G, Montgomery AM. Impact of defined matrix interactions on insulin production by cultured human β-cells: effect on insulin content, secretion, and gene transcription. Diabetes 2006;55:2723–2729 [DOI] [PubMed] [Google Scholar]
- 168.Otonkoski T, Banerjee M, Korsgren O, Thornell LE, Virtanen I. Unique basement membrane structure of human pancreatic islets: implications for β-cell growth and differentiation. Diabetes Obes Metab 2008;10(Suppl. 4):119–127 [DOI] [PubMed] [Google Scholar]
- 169.Beattie GM, Rubin JS, Mally MI, Otonkoski T, Hayek A. Regulation of proliferation and differentiation of human fetal pancreatic islet cells by extracellular matrix, hepatocyte growth factor, and cell-cell contact. Diabetes 1996;45:1223–1228 [DOI] [PubMed] [Google Scholar]
- 170.Townsend SE, Gannon M. Extracellular matrix-associated factors play critical roles in regulating pancreatic β-cell proliferation and survival. Endocrinology 2019;160:1885–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Arous C, Wehrle-Haller B. Role and impact of the extracellular matrix on integrin-mediated pancreatic β-cell functions. Biol Cell 2017;109:223–237 [DOI] [PubMed] [Google Scholar]
- 172.Gaetani R, Aude S, DeMaddalena LL, et al. Evaluation of different decellularization protocols on the generation of pancreas-derived hydrogels. Tissue Eng Part C Methods 2018;24:697–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Aamodt KI, Powers AC. Signals in the pancreatic islet microenvironment influence β-cell proliferation. Diabetes Obes Metab 2017;19(Suppl. 1):124–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.McShane P, Sutton R, Gray DW, Morris PJ. Protease activity in pancreatic islet isolation by enzymatic digestion. Diabetes 1989;38(Suppl. 1):126–128 [DOI] [PubMed] [Google Scholar]
- 175.Brandhorst D, Brandhorst H, Johnson PRV. Enzyme development for human islet isolation: five decades of progress or stagnation? Rev Diabet Stud 2017;14:22–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Nyitray CE, Chavez MG, Desai TA. Compliant 3D microenvironment improves β-cell cluster insulin expression through mechanosensing and β-catenin signaling. Tissue Eng Part A 2014;20:1888–1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Galli A, Algerta M, Marciani P, et al. Shaping pancreatic β-cell differentiation and functioning: the influence of mechanotransduction. Cells 2020;9:413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hohenester E Structural biology of laminins. Essays Biochem 2019;63:285–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mamidi A, Prawiro C, Seymour PA, et al. Mechanosignalling via integrins directs fate decisions of pancreatic progenitors. Nature 2018;564:114–118 [DOI] [PubMed] [Google Scholar]
- 180.Saleem S, Li J, Yee S-P, Fellows GF, Goodyer CG, Wang R. beta1 integrin/FAK/ERK signalling pathway is essential for human fetal islet cell differentiation and survival. J Pathol 2009;219:182–192 [DOI] [PubMed] [Google Scholar]
- 181.Rondas D, Tomas A, Halban PA. Focal adhesion remodeling is crucial for glucose-stimulated insulin secretion and involves activation of focal adhesion kinase and paxillin. Diabetes 2011;60:1146–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Rondas D, Tomas A, Soto-Ribeiro M, Wehrle-Haller B, Halban PA. Novel mechanistic link between focal adhesion remodeling and glucose-stimulated insulin secretion. J Biol Chem 2012;287:2423–2436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gan WJ, Do OH, Cottle L, et al. Local integrin activation in pancreatic β cells targets insulin secretion to the vasculature. Cell Rep 2018;24:2819–2826.e3 [DOI] [PubMed] [Google Scholar]
- 184.Ji R, Li J, Yin Z, et al. Pancreatic stiffness response to an oral glucose load in obese adults measured by magnetic resonance elastography. Magn Reson Imaging 2018;51:113–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Shi Y, Cang L, Zhang X, et al. The use of magnetic resonance elastography in differentiating autoimmune pancreatitis from pancreatic ductal adenocarcinoma: a preliminary study. Eur J Radiol 2018;108:13–20 [DOI] [PubMed] [Google Scholar]
- 186.Nagy N, de la Zerda A, Kaber G, et al. Hyaluronan content governs tissue stiffness in pancreatic islet inflammation. J Biol Chem 2018;293:567–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mohapatra S, Majumder S, Smyrk TC, et al. Diabetes mellitus is associated with an exocrine pancreatopathy: conclusions from a review of literature. Pancreas 2016;45:1104–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Cameron JD, Cruickshank JK. Glucose, insulin, diabetes and mechanisms of arterial dysfunction. Clin Exp Pharmacol Physiol 2007;34:677–682 [DOI] [PubMed] [Google Scholar]
- 189.Pollak OJ Human pancreatic atherosclerosis. Ann N Y Acad Sci 1968;149:928–939 [DOI] [PubMed] [Google Scholar]
- 190.Korsgren O Islet encapsulation: physiological possibilities and limitations. Diabetes 2017;66:1748–1754 [DOI] [PubMed] [Google Scholar]
- 191.Alagpulinsa DA, Cao JJL, Driscoll RK, et al. Alginate-microencapsulation of human stem cell-derived β cells with CXCL12 prolongs their survival and function in immunocompetent mice without systemic immunosuppression. Am J Transplant 2019;19:1930–1940 [DOI] [PubMed] [Google Scholar]
- 192.Bochenek MA, Veiseh O, Vegas AJ, et al. Alginate encapsulation as long-term immune protection of allogeneic pancreatic islet cells transplanted into the omental bursa of macaques. Nat Biomed Eng 2018;2:810–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Motté E, Szepessy E, Suenens K, et al.; Beta Cell Therapy Consortium EU-FP7 . Composition and function of macroencapsulated human embryonic stem cell-derived implants: comparison with clinical human islet cell grafts. Am J Physiol Endocrinol Metab 2014;307:E838–E846 [DOI] [PubMed] [Google Scholar]
- 194.Jun Y, Lee J, Choi S, et al. In vivo-mimicking microfluidic perfusion culture of pancreatic islet spheroids. Sci Adv 2019;5:eaax4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Bowles AC, Ishahak MM, Glover SJ, Correa D, Agarwal A. Evaluating vascularization of heterotopic islet constructs for type 1 diabetes using an in vitro platform. Integr Biol (Camb) 2019;11:331–341 [DOI] [PubMed] [Google Scholar]
- 196.Sackett SD, Tremmel DM, Ma F, et al. Extracellular matrix scaffold and hydrogel derived from decellularized and delipidized human pancreas. Sci Rep 2018;8:10452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Liljebäck H, Espes D, Carlsson P-O. Unsurpassed intrahepatic islet engraftment - the quest for new sites for beta cell replacement. Cell Med 2019;11:2155179019857662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mann E, Bellin MD. Secretion of insulin in response to diet and hormones. Pancreapedia 2016;1:1–16 [Google Scholar]
- 199.Schrimpe-Rutledge AC, Fontès G, Gritsenko MA, et al. Discovery of novel glucose-regulated proteins in isolated human pancreatic islets using LC-MS/MS-based proteomics. J Proteome Res 2012;11:3520–3532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Eizirik DL, Sammeth M, Bouckenooghe T, et al. The human pancreatic islet transcriptome: expression of candidate genes for type 1 diabetes and the impact of pro-inflammatory cytokines. PLoS Genet 2012;8:e1002552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Blodgett DM, Nowosielska A, Afik S, et al. Novel observations from next-generation RNA sequencing of highly purified human adult and fetal islet cell subsets. Diabetes 2015;64:3172–3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lu S, Dugan CE, Kennedy RT. Microfluidic chip with integrated electrophoretic immunoassay for investigating cell-cell interactions. Anal Chem 2018;90:5171–5178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhang X, Grimley A, Bertram R, Roper MG. Microfluidic system for generation of sinusoidal glucose waveforms for entrainment of islets of Langerhans. Anal Chem 2010;82:6704–6711 [DOI] [PMC free article] [PubMed] [Google Scholar]