Abstract
Purpose:
Calorie restriction (CR) is an effective treatment for obesity-related liver and metabolic disease. However, CR studies in individuals without obesity are needed to see if CR could delay disease onset. Liver biomarkers indicate hepatic health and are linked to cardiometabolic disease. Our aim was to examine the effects of a two-year CR intervention on liver biomarkers in healthy individuals without obesity.
Methods:
The Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy (CALERIE) study was a two-year randomized controlled trial. Overall, 218 participants (body mass index: 25.1±1.7 kg/m2) were enrolled into control group (n=75) that ate ad libitum (AL) or a CR group (n=143) that aimed to decrease energy intake by 25%. Alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), and bilirubin were measured during the trial.
Results:
At month 24, relative to the AL group, ALP (−7±1 IU/L; P<0.01) and GGT (− 0.11 ± 0.04 log IU/L; P = 0.02) decreased and bilirubin increased (0.21±0.06 log mg/dL; P<0.01) in the CR group; no between-group differences in ALT (−1±1 IU/L; P>0.99) or AST (2±2 IU/L; P=0.68) were revealed. However, sex-by-treatment-by-time interactions (P<0.01) were observed, with CR (vs. control) inducing reduced ALT and GGT and increased AST in men only (P≤0.02).
Conclusions:
In metabolically healthy individuals without obesity, two years of CR improves several liver biomarkers, with potentially greater improvements in men. These data suggest that sustained CR may improve long-term liver and metabolic disease risk in healthy adults.
Trial registration:
Clinicaltrials.gov (NCT00427193) Registered January 2007.
Keywords: energy restriction, liver enzymes, bilirubin, cardiometabolic health, aging
1. INTRODUCTION
Aging predisposes individuals to functional and structural impairments in the liver, as well as metabolic disease [1]. The development of non-alcoholic fatty liver disease (NAFLD), the prominent cause of chronic liver disease [2] and a risk factor for cardiovascular disease [3], is multifaceted. Among accepted theories, insulin resistance triggers intrahepatic fat accumulation, with a subsequent increase in lipid intermediates further deteriorating insulin resistance [4–6]. Although the precise processes are not known, decreased adiponectin is implicated in the development of insulin resistance by promoting the progression of a pro-inflammatory milieu in the liver [7–9]. This elevation in inflammation is critical in accelerating the hepatic aging process [10].
Liver function is commonly assessed via biomarkers linked to liver health. These include liver enzymes, such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) and gamma-glutamyl transferase (GGT), and the end product of heme catabolism, bilirubin. Higher concentrations of liver enzymes are related positively to adiposity [11] and negatively to insulin sensitivity [12, 13], whereas bilirubin has been negatively associated with the former and positively with the latter [14]. Further, within healthy ranges, higher concentrations of liver enzymes and lower concentrations of bilirubin are related to increased risk of NAFLD [15, 16], cardiovascular disease [17, 18] and mortality [19, 20].
As a dietary regimen that restricts energy intake without malnutrition, calorie restriction (CR) is a lifestyle modification that improves longevity and aging in multiple species [21, 22]. Improvements in liver biomarkers after energy restriction diets have been observed in individuals with obesity [23, 24], abnormal concentrations of liver biomarkers [25] and cardiometabolic conditions [26]. Intriguingly, in one study, liver biomarker concentrations were improved in men but not in women after a four-month low-calorie diet, suggesting sex may modulate the change in liver health in response to CR [27]. However, to our knowledge, no study has examined the effect of long-term CR on liver biomarkers in metabolically healthy participants without obesity. Liver biomarker responses to CR in healthy individuals will inform whether CR can be used as a general preventive strategy for metabolic conditions. In the Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy (CALERIE) phase 1 trial, six months of CR stimulated a 9% reduction in ALT and ALP in individuals without obesity [28]. The second phase of CALERIE, which examined the effects of two years of CR on biomarkers of aging, has illustrated the positive metabolic benefits of CR [22, 29], but the effect of sustained (> 1 year) CR on levels of an array of liver biomarkers is unknown.
Therefore, the primary aim was to assess the effect of sustained CR on liver biomarkers in metabolically healthy individuals without obesity enrolled in the CALERIE phase 2 trial. We hypothesized that CR would alter concentrations of liver biomarkers in a direction consistent with improved metabolic health. Our exploratory aims were to examine if sex modulates the influence of CR on liver biomarkers and if changes in anthropometry, insulin sensitivity, and adiponectin are related to changes in liver biomarkers.
2. MATERIAL AND METHODS
The CALERIE phase 2 study was a two-year, parallel-group, randomized control trial (clinicaltrials.gov registration: NCT00427193) that was conducted at three clinical sites: Pennington Biomedical Research Center (Baton Rouge, LA, USA), Washington University School of Medicine (St Louis, MO, USA) and Tufts University (Boston, MA, USA) [30]. Duke Clinical Research Institute (Durham, NC, USA) served as the coordinating center for the study.
As detailed elsewhere [30], participants were randomly enrolled to either a CR group or an ad libitum (AL) control group. The CR group participated in an intensive behavioral intervention aimed to decrease energy intake by 25%, while the AL group was instructed to maintain habitual energy intake. All institutions received approval from their respective institutional review boards. Participants provided written informed consent before inclusion in the trial and obtained financial compensation for their participation.
2.1. Participants
Recruitment, screening processes and exclusion criteria for CALERIE phase 2 have been reported [30, 31]. In short, participants were eligible at screening if they were aged 20–50 years (men) or 20–47 years (women), had a body mass index (BMI) of 22.0–27.9 kg/m2, and completed comprehensive screening assessments. They were ineligible if they had significant medical conditions (history or clinical manifestation of cardiovascular disease or diabetes), psychiatric or behavioral problems (history or clinical manifestation of any eating disorders) or had engaged in drug or alcohol abuse in the previous two years. Women were also excluded if they were pregnant or had plans to become pregnant during the trial [30].
2.2. Study design
Before randomization, baseline testing was performed over a 6-week period and included numerous health-related assessments, as well as two 14-day back-to-back measures of doubly labelled water (DLW) to determine participants’ habitual energy intake and 25% CR prescription. Randomization was stratified by study site, sex, and BMI, with the BMI dichotomized into normal weight (22.0 ≤ BMI < 25.0 kg/m2) and overweigt (25.0 ≤ BMI < 28.0 kg/m2). Participants were allocated in a 2:1 ratio in favor of CR in each stratum, with randomization sequences produced via a permuted block randomization technique.
Details of the CR intervention have been reported elsewhere [32]. Briefly, the intervention targeted an immediate and sustained 25% decrease in energy intake relative to habitual intake, as determined at baseline [33]. No stringent requirements were enforced with respect to macronutrient intake and meal timing, and participants modified their diet as they pleased to ensure sufficient adherence to the CR regimen [30, 32]. Adherence to CR was determined at 6-month intervals by measuring the total daily energy expenditure via DLW and adjusting for changes in body composition [33]. Additionally, as detailed previously [32, 34], mathematical models that estimated weight changes during CR served as a guide for participant adherence. More specifically, weight change was measured and compared to estimated weight change during individual and group sessions that were led by study interventionists. Food records were administered to estimate percent energy from fat, carbohydrate, and protein [35].
The intervention was administered by behaviorists and nutritionists. Individual counselling sessions were delivered weekly during the first month, twice monthly until month 12, and then monthly until trial completion. To facilitate adherence, additional sessions were arranged if required and sessions were cus tomized in accord with the participants’ dietary requirements and weight targets. In addition to individual sessions, group sessions were initiated after week four and occurred twice monthly [32]. Numerous techniques were used to assist the CR group [32]. Each participant was instructed to self-monitor food intake and review this with their interventionist. This was aided by the provision of food scales, measuring cups and spoons, and portion size training. Other topics were incorporated in the intervention, including maintaining motivation, managing food cravings, managing hunger, goal setting, and social support [32]. The AL group had quarterly sessions with study investigators, but did not receive any counseling and were instructed to maintain their usual dietary intake. Both groups were provided multivitamin and calcium supplements, and neither group received an exercise prescription.
2.3. Outcomes
All outcomes reported herein were collected during assessments performed at baseline and within a 14-day window at months 6, 12, and 24.
2.3.1. Body weight, waist circumference, and percent CR
Body weight was measured using a calibrated scale (Scale-Tronix 5200, Welch Allyn). Waist circumference was measured at the minimal and umbilical points using a tape measure. Percent CR was calculated using the intake balance method [21], relying on concurrent measures of total daily energy expenditure and body composition [33]. Percent energy intake from fat, carbohydrate, and protein, were measured using six-day food diaries and analyzed with Nutrition Data System for Research (Minneapolis, MN, USA).
2.3.2. Blood biomarkers
Serum blood samples were collected in the morning following an overnight fast and analyzed in a central laboratory at the University of Vermont. Ortho Clinical Diagnostics Vitros (Rochester, NY, USA) chemistry analyzers were used to measure concentrations of ALT, AST, ALP, GGT, and bilirubin. Glucose (YSI Instruments, Fullerton, CA, USA) and insulin (Elecsys 2010, Roche Diagnostics, Indianapolis, IN, USA) were measured during an oral glucose tolerance test, with samples taken at baseline, 30 min, 60 min, 90 min and 120 min. Adiponectin was assessed with an enzyme immunoassay (R&D Systems, Minneapolis, MN, USA).
2.4. Statistical methods
Power calculations revealed that the CALERIE phase 2 trial had greater than 90% power to detect between-group differences in the two primary outcomes of the study: resting metabolic rate and core temperature [30]. The present manuscript assesses exploratory endpoints of CALERIE phase 2; hence, as done previously [21, 22], intent-to-treat analyses were performed by including all participants who started the intervention. Comparisons at baseline were made via the Wilcoxon rank-sum test. For change scores, an intent-to-treat criterion was used [21], with variables that departed from normality in data distribution histograms log-transformed. A repeated-measures analysis of covariance was performed, with treatment, time and treatment x time as independent variables, and change from baseline as the dependent variable. Baseline measures, study site, sex, and baseline BMI stratum were used as covariates. In addition, self-reported alcohol consumption was used as a covariate for the change in liver enzymes. Between-group differences at individual time points and within-group changes over time were examined by defining contrasts among the regression variables. Type-1 error was controlled using hierarchical gatekeeping (i.e. the treatment x time interaction was tested first and, if significant, between-group differences at each time were tested at α = 0.05). If nonsignificant, the treatment main effect was tested next and, if significant, between-group differences at each time point were tested at α = 0.05. In other instances, a Bonferroni correction was applied at each time point and P values were adjusted by multiplying the nominal P value by the number of comparisons. To explore the effect of sex-related variations, sex-by-treatment-by-time interactions, sex-by-treatment interactions, and main effects of sex were included in models, with the same gatekeeping strategy applied. A similar approach was performed for BMI strata.
Absolute standardized effect sizes (ES) are included to supplement key change scores, with between-group ESs calculated from pooled models and sex-specific ESs calculated from separate models for men and women. Without clinical anchors, an ES of 0.20 was considered the minimum important difference, whereas 0.5 and 0.8 were considered moderate and large, respectively [36]. Spearman correlations were determined in the CR group to investigate if the change in liver biomarkers was related to change in weight, waist circumference, insulin sensitivity (based on the Matsuda Index [37]), or change in adiponectin. Data analysis was performed using SAS, version 9.2 (SAS Institute Inc). Unless otherwise documented, data are presented as adjusted mean (±SE).
3. RESULTS
3.1. Participants
As detailed elsewhere [21] and in Fig. 1, an intent-to-treat cohort of 218 participants (143 CR, 75 AL) started the trial. For multiple reasons, 30 participants (26 CR, 4 AL) withdrew during the trial. Participants were predominantly women (69.7%) and Caucasian (77.1%), with age and BMI ranging from 20.7 to 50.8 years and 21.3 to 29.0 kg/m2, respectively, at baseline. Baseline demographic and anthropometric characteristics did not differ between groups (P ≥ 0.34; Table 1).
Fig. 1.
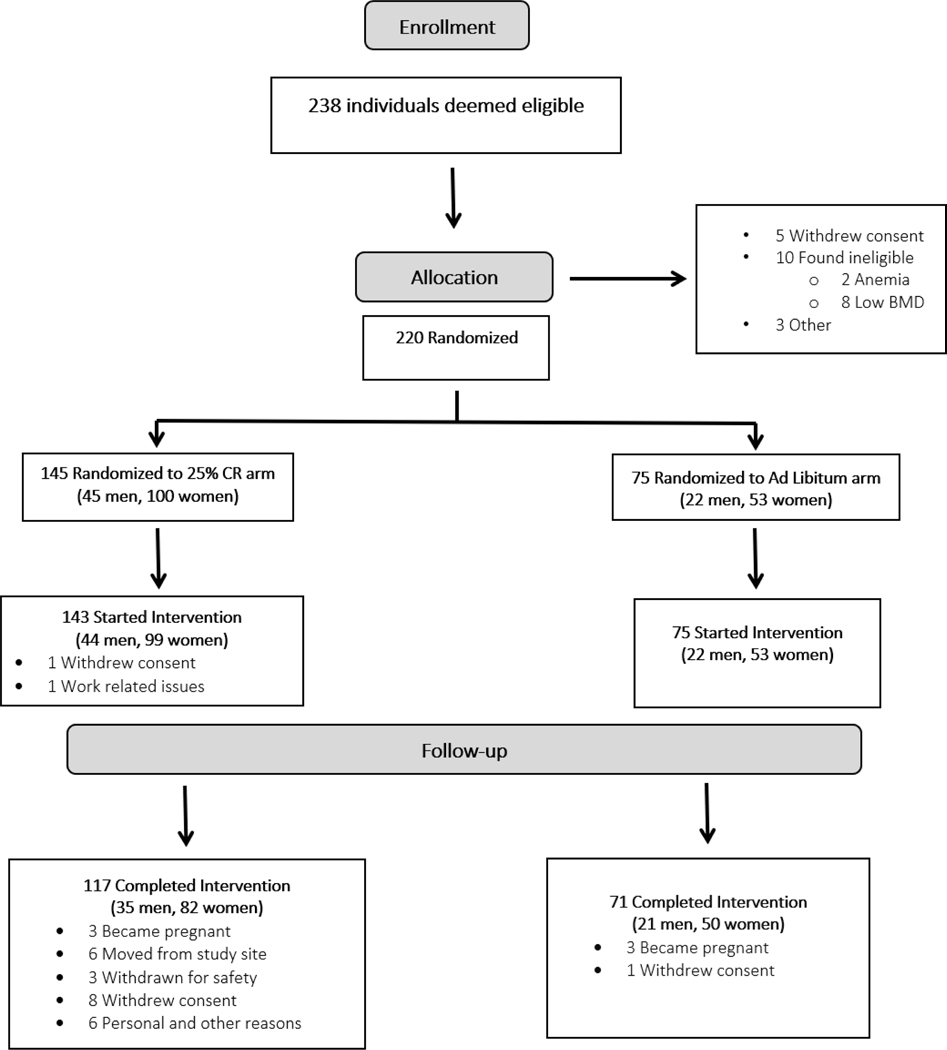
CALERIE phase 2 CONSORT diagram
Table 1.
Baseline demographic and anthropometric measures of CALERIE phase 2 participants.
| Characteristic | AL (n = 75) | CR (n = 143) |
|---|---|---|
| Age (years) | 37.9 (7.0) | 38.0 (7.3) |
| Sex | ||
| Men | 22 (29.3) | 44 (30.8) |
| Women | 53 (70.7) | 99 (69.2) |
| Race | ||
| White | 57 (76.0) | 111 (77.6) |
| African American | 11 (14.7) | 15 (10.5) |
| Othera | 7 (9.3) | 17 (11.9) |
| Height (cm) | 168.4 (8.3) | 168.9 (8.6) |
| Weight (kg) | 71.3 (8.6) | 71.8 (9.2) |
| Body mass index (kg/m2) | 25.1 (1.6) | 25.2 (1.8) |
| Body fat (%) | 33.6 (6.6) | 32.9 (6.1) |
Abbreviations: AL, ad libitum; CR, calorie restriction.
Values are mean (± SD) except for Sex and Race, which are mean (%).
Includes American Indian/Alaska Native, Asian, more than one race, and unknown.
Wilcoxon rank sum test revealed no significant differences between CR and AL groups (P ≥ 0.34).
3.2. Adherence and anthropometry
The CR group achieved an average 11.9 ± 0.7% decrease in energy intake over the 2-year intervention, whereas the AL group showed no change in energy intake (P < 0.01 versus CR group). Food records indicated that percent calories from fat decreased by 4.8% ± 0.4% and 3.4% ± 0.5% in the CR group at month 12 and month 24, respectively (P < 0.01 versus CR group). Percent calories from carbohydrate (month 12: 3.6% ± 0.5%; month 24: 2.4% ± 0.5%) and protein (month 12: 1.8% ± 0.3%; month 24: 1.1% ± 0.3%) increased in the CR group at months 12 and 24 (P ≤ 0.07 versus CR group). During the trial, men and women showed a similar change in DLW-derived energy intake (P ≥ 0.20). Food records suggested no sex-related differences in percent energy intake from carbohydrate or fat (P ≥ 0.11), yet men increased their percent energy intake from protein compared to females in response to CR (P < 0.05).
The CR group lost 7.5 ± 0.3 kg from baseline to month 24, which was greater than the AL group (P < 0.01), whose weight did not change (P < 0.01 versus CR group). The CR group likewise displayed a reduction in waist circumference at month 24 (6.1 ±, 0.4 cm; P < 0.01); no change was observed in the AL group (P < 0.01 versus CR group). Weight loss from baseline to month 24 was influenced by sex in the CR group, with men losing 1.0 ± 0.5 kg more than women (P = 0.04); however, sex did not affect change in waist circumference in the CR group (P > 0.05).
3.3. Circulating liver biomarkers
At baseline, men displayed higher concentrations of all liver biomarkers than women (P < 0.01), though no differences between the CR group and the AL group were observed (P ≥ 0.15; Table 2). Concentrations of all biomarkers were within safe ranges and remained so for the duration of the trial [38].
Table 2.
Baseline concentrations of liver-related biomarkers in CALERIE phase 2 participants.
| Men |
Women |
Overall |
||||
|---|---|---|---|---|---|---|
| AL (n = 22) | CR (n = 44) | AL (n = 53) | CR (n = 99) | AL (n = 75) | CR (n = 143) | |
| ALT (IU/L) | 26 (12) | 24 (9) | 15 (5) | 16 (6) | 18 (9) | 18 (8) |
| AST (IU/L) | 22 (6) | 22 (5) | 19 (4) | 19 (6) | 20 (5) | 20 (5) |
| ALP (IU/L) | 70 (16) | 65 (16) | 58 (15) | 57 (18) | 62 (16) | 59 (18) |
| GGT (IU/L) | 27 (19) | 24 (13) | 14 (6) | 16 (11) | 18 (13) | 18 (12) |
| Bilirubin (mg/dL) | 0.71 (0.46) | 0.62 (0.30) | 0.59 (0.42) | 0.48 (0.23) | 0.63 (0.43) | 0.52 (0.26) |
Abbreviations: AL, ad libitum; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CR, calorie restriction; GGT, gamma-glutamyl transferase.
Values are mean (± SD).
Wilcoxon rank sum test revealed significant differences between men and women for all biomarkers (P < 0.01), but no significant differences between CR and AL groups were observed (P ≥ 0.15).
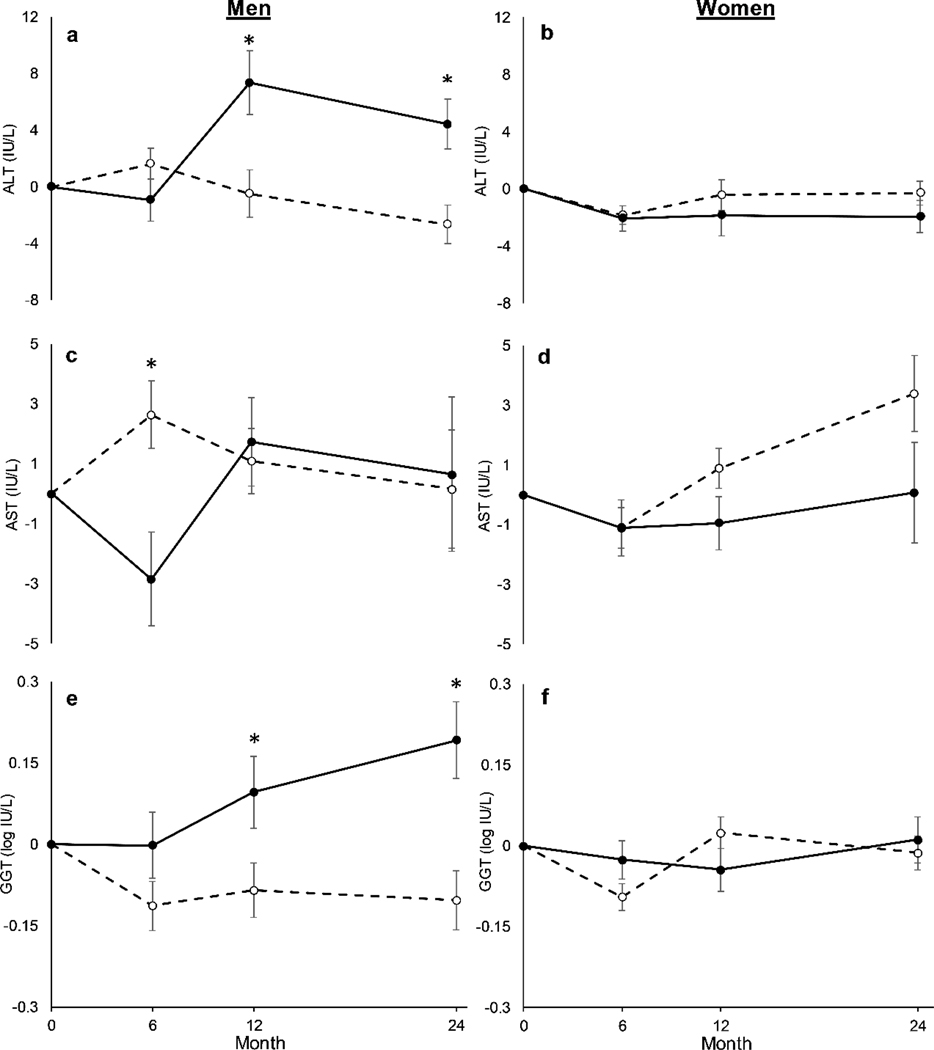
No between-group differences were observed in ALT or AST at any time point (P ≥ 0.29; Table 3), but sex-by-treatment-by-time interactions were observed (P < 0.01). Post-hoc comparisons showed that in men, the CR group demonstrated a reduction in ALT at month 12 (CR: −0 ± 2 IU/L vs. AL: 7 ± 2 IU/L; P < 0.01; ES = 0.57) and month 24 (CR: −3 ± 1 IU/L vs. AL: 4 ± 2 IU/L; P < 0.01; ES = 0.93) compared to the AL group (Fig. 2a). There were, conversely, no between-group differences in ALT in women (P ≥ 0.23; ES ≤ 0.23; Fig. 2b). In addition, men in the CR group had an increase in AST relative to the AL group at month 6 (CR: 3 ± 1 IU/L vs. AL: −3 ± 2 IU/L; P < 0.01; ES = 0.52; Fig. 2c), but no differences were observed at any other time point (P ≥ 0.71), and no between-group differences were seen in women (P ≥ 0.12; ES ≤ 0.30; Fig. 2d). Changes in ALT and AST in response to CR were not significantly altered by baseline BMI (P ≥ 0.11; data not shown).
Table 3.
Adjusted changes from baseline in liver-related biomarkers during CALERIE phase 2 at months 6, 12, and 24.
| AL (n = 75) |
CR (n = 143) |
Between-group difference |
||||
|---|---|---|---|---|---|---|
| Changea | Within P valueb | Changea | Within P valueb | P valueb | Effect sizec | |
| ALT (IU/L) | ||||||
| Month 6 | −1 (1) | 0.45 | −0 (1) | >0.99 | >0.99 | 0.14 |
| Month 12 | 2 (1) | 0.64 | 0 (1) | >0.99 | >0.99 | 0.14 |
| Month 24 | 1 (1) | >0.99 | −1 (1) | >0.99 | >0.99 | 0.14 |
| AST (IU/L) | ||||||
| Month 6 | −1 (1) | 0.69 | 1 ( 1) | 0.82 | 0.29 | 0.25 |
| Month 12 | 0 (1) | >0.99 | 2 (1)† | 0.03 | 0.76 | 0.17 |
| Month 24 | 1 (1) | >0.99 | 3 (1)† | 0.02 | 0.68 | 0.18 |
| ALP (IU/L) | ||||||
| Month 6 | −1 (1) | 0.35 | −5 (1)†* | <0. 01 | <0.01 | 0.44 |
| Month 12 | 0 (1) | >0.99 | −4 (1)†* | <001 | 0.01 | 0.46 |
| Month 24 | 2 (1) | 0.45 | −5 (1)†* | <0.01 | <0.01 | 0.73 |
| GGT (log IU/L) | ||||||
| Month 6 | −0.02 (0.03) | >0. 99 | −0.10 (0.02)†* | <0. 01 | 0.02 | 0.36 |
| Month 12 | −0.00 (0.03) | >0.99 | −0.01 (0.03) | >0.99 | 0.86 | 0.03 |
| Month 24 | 0.06 (0.04) | 0.26 | −0.04 (0.03)* | 0.39 | 0.02 | 0.35 |
| Bilirubin (log mg/dL) | ||||||
| Month 6 | 0.08 (0.04) | 0.19 | 0.17 (0.03)† | <0. 01 | 0.08 | 0.31 |
| Month 12 | −0.00 (0.05) | >0.99 | 0.11 (0.03 )†* | <0.01 | 0.04 | 0.33 |
| Month 24 | −0.06 (0.05) | 0.66 | 0.16 (0.04)†* | <0.01 | <0.01 | 0.58 |
Abbreviations: AL, ad libitum; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CR, calorie restriction; GGT, gamma-glutamyl transferase.
Values are the least-squares adjusted means (SE) obtained from analyses that used an intention-to-treat approach to determine whether change in the outcome variables differed between the CR and AL groups. Comparisons are controlled for baseline values.
All P values reflect Bonferroni corrections, truncated at >0.99 where necessary.
Values are absolute Cohen effect sizes of between-group differences.
Significant difference from baseline (P < 0.05).
Significant difference versus AL group (P < 0.05).
Fig. 2.
Adjusted changes in alanine amino transferase (ALT; a & b), aspartate aminotransferase (AST; c & d) and gamma-glutamyl transferase (GGT; e & f) in men and women during CALERIE phase 2. Dashed lines represent calorie restriction (CR), whereas solid lines represent ad libitum (AL) control group. *P < 0.05 between CR and AL groups
The CR group exhibited a reduction in ALP versus the AL group at all time points (P ≤ 0.01; Table 3), with greater overall reduction in women (main effect of sex; P = 0.03; Supplementary Fig. 1). The change in GGT was smaller in the CR group than the AL group at month 6 (P = 0.02) and month 24 (P = 0.02; Table 3). A three-way sex-by-treatment-by-time interaction was observed (P < 0.01), with GGT in the CR group lower at month 12 (CR: −0.09 ± 0.05 log IU/L vs. AL: 0.10 ± 0.07 log IU/L; P = 0.02; ES = 0.68) and month 24 (CR: 0.10 ± 0.05 log IU/L vs. AL: 0.19 ± 0.07 log IU/L; P < 0.01; ES = 1.02) compared to the AL group in men (Fig. 2e) but not women (P ≥ 0.10; ES ≤ 0.30; Fig. 2f). Further, the CR-induced decrease in GGT was greater in individuals who were overweight compared to individuals who were normal weight (BMI-by-treatment effect; P = 0.02; Supplementary Fig. 2). In the CR group, there was an increase in bilirubin at months 12 (P = 0.04) and 24 (P < 0.01) compared to the AL group (Table 3), with an overall larger elevation in men (main effect of sex; P < 0.01; Supplementary Fig. 1). Baseline BMI did not affect the CR-induced changes in ALP and bilirubin (P ≥ 0.24; data not shown).
3.4. Liver biomarkers and indices of cardiometabolic health
Spearman correlations are reported in Table 4. Weight loss was positively related to reductions in ALT and GGT in the CR group at month 24 (P < 0.01), but weight loss was not associated with changes in other outcome variables (P ≥ 0.06; Table 4). A reduction in waist circumference was associated with ALT, AST, ALP, and GGT reductions (P ≤ 0.01), but no significant relationship was observed with change in bilirubin (P = 0.24).
Table 4.
Spearman correlation coefficients between change in liver-related biomarkers and changes in weight, waist circumference, Matsuda index and adiponectin at month 24 in the calorie restriction group (n = 143).
| ALT (IU/L) |
AST (IU/L) |
ALP (IU/L) |
GGT (log IU/L) |
Bilirubin (mg/dL) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ρ | p | ρ | p | ρ | p | ρ | p | ρ | p | |
| Weight (kg) | 0.30* | <0.01 | 0.17 | 0.06 | 0.17 | 0.06 | 0.29* | <0.01 | −0.04 | 0.70 |
| Waist circumference (cm) | 0.39* | <0.01 | 0.30* | <0.01 | 0.26* | <0.01 | 0.25* | 0.01 | −0.11 | 0.24 |
| Matsuda index | −0.08 | 0.47 | −0.02 | 0.84 | −0.14 | 0.20 | −0.16 | 0.15 | 0.14 | 0.21 |
| Adiponectin (ng/mL) | −0.12 | 0.20 | −0.18 | 0.05 | −0.21* | 0.02 | −0.25* | 0.01 | −0.00 | 0.97 |
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transferase. Values are Spearmans rho (ρ) and P values from simple linear correlations.
Significant Spearman correlation (P < 0.05).
No relationships were seen between change in liver biomarkers and change in insulin sensitivity estimated (P ≥ 0.15). However, change in adiponectin was negatively related to change in ALP and GGT (P ≤ 0.02; Table 4). No other significant correlations were revealed (P ≥ 0.05; Table 4).
4. DISCUSSION
The present analysis shows that sustained CR elicited small-to-moderate improvements in multiple liver biomarkers in healthy individuals without obesity. Overall, CR reduced ALP and GGT and increased bilirubin, with larger effects seen after prolonged CR. Despite no overall differences in the changes in ALT and AST between groups, there was a sex effect, with men having reductions in ALT and GGT and increases in AST. Such changes in healthy participants who presented with healthy liver biomarker concentrations at baseline suggest that sustained CR could enhance long-term liver and metabolic health.
Improvements in liver biomarkers have been observed after energy restrictive regimens in individuals with clinically altered concentrations and/or metabolic conditions [25, 26]. We showed that, in individuals without obesity, CR over 24 months provoked a small-to-moderate reduction in ALP and GGT and a moderate increase in bilirubin. Together, these results bolster positive findings reported from CR-related studies in individuals with obesity [27] or with shorter (≤ 6 months) interventions [28]. Although participants exhibited healthy liver biomarkers at baseline, the modest improvements within normal ranges of ALP, GGT and bilirubin are not negligible since they are linearly associated with metabolic disease [16–19] and mortality [20, 39]. The modest CR-induced changes in these biomarkers therefore likely reflects benefits to liver function and cardiometabolic health, underscoring the many other health improvements demonstrated in CALERIE phase 2 [22, 29]. These changes may also imply that sustained CR in healthy adulthood may retard or prevent the development of age- and obesity-related liver and metabolic conditions.
Notable sex-specific effects on liver enzymes were observed during CR. We found CR-induced decreases in ALT in men but not women. Specifically, at month 24, men in the CR group presented a 3 IU/L decrease, whereas men in the AL group showed a 4 IU/L rise. A rise in ALT increases NAFLD risk in a dose-response manner, with a 55% decreased risk of NAFLD development in men when concentrations are 19–21 IU/L compared to 26–34 IU/L [15]. Thus, potentially, men experienced a reduced risk of NAFLD in response to sustained CR. Greater reductions in GGT in response to CR were also displayed in men compared to women. Alongside ALT, this observation is consistent with one study which showed that only men exhibited a decrease in ALT and GGT after a four-month low-calorie diet [27]. It is possible the greater reduction in weight seen in men during CALERIE phase 2 was implicated by associations between changes in enzyme and weight. Differences could also be linked to the greater percent energy intake from protein exhibited by men during CR, as consumption of this macronutrient can influence liver fat and biomarkers [40]. However, studies are needed to confirm these differences, since self-report estimates of food intake can be unreliable [41] and long-term percent CR was similar between sexes.
In addition, CR exerted sex-specific effects on AST. At month 6, men in the CR group presented a 6 IU/L increase in AST compared to the AL group, while there were no between-group differences in women. An increase in AST after short-term (≤ 6 months) dietary restriction possibly occurs due to temporary increases in portal inflammation [23, 27], yet the reasons for sexual dimorphism are unclear. Regardless, the absence of between-group differences at months 12 and 24 indicates that the rise in AST was abolished with prolonged CR. Clearly, more studies are required because sex-based variations in the hepatic pathways linked to liver disease exist, with men showing higher risk for NAFLD [42] and worse liver health independent of BMI [43]. Work in rodents likewise reports differential sex-related liver responses to CR [44], indicating that sex-specific treatment responses may need to be considered [42].
Obesity is a well-established predictor of liver enzymes [45]. In this regard, we observed positive correlations between weight change and changes in ALT and GGT. Additionally, CR-induced reductions in GGT were greater in individuals who were overweight, suggesting CR may have greater benefits for individuals with increased adiposity. However, weight change was not associated with ALP and AST, plus BMI at baseline did not moderate the effect of CR on other liver biomarkers. Waist circumference is a stronger indicator of adverse metabolic conditions than body weight or subcutaneous adiposity [46]. Indeed, individuals who display a normal BMI can develop liver and cardiovascular conditions in the presence of a large waist circumference [47, 48]. In this respect, the change in all liver enzymes was positively related to the change in waist circumference, supporting work showing that waist circumference is a stronger predictor of hepatic enzyme concentrations than weight [11, 49].
While the precise function of liver biomarkers and their role in the pathogenesis of NAFLD and cardiovascular disease is unclear, a decrease in insulin sensitivity could initiate the development of these conditions [5]. In this context, we found that the change in liver biomarkers were not related to change in insulin sensitivity. Our results are at odds with evidence reporting associations between liver biomarkers and insulin sensitivity [12, 14]. It is possible that, relative to these studies, our cohort of healthy individuals without obesity were insulin sensitive and exhibited limited variability in endpoints.
We did observe negative correlations between the change in adiponectin and the changes in GGT and ALP during CR. Several lines of evidence indicate that adiponectin impacts liver integrity: it increases glucose utilization within hepatocytes [50]; it is lower in individuals with NAFLD versus controls [7]; and it potently lowers inflammation [8, 9], which is a prominent driver of liver aging [10]. As such, the relationships we saw may suggest that the CR-induced changes in liver biomarkers are involved in decreasing the pro-inflammatory milieu and in turn metabolic disease risk [51]. We did not, however, find consistent relationships with liver biomarkers and adiponectin. Further scientific inquiry is required to elucidate the interconnections linking liver biomarkers with metabolic disease and investigate how they contribute to improved metabolic health during CR.
A key strength of the present study was that our data were yielded from a highly structured two-year randomized controlled trial containing comprehensive assessments of body composition and liver biomarkers. The trial is limited by the unequal enrolment of men and women, and differences in sample size may limit generalizability and could have influenced the power to detect sex-related variations [52]. Moreover, in spite of data from a subset of participants that suggested CR stimulated a small decrease in liver lipid content [29], it is unfortunate that we were unable to capture changes in liver fat or the microscopic structure of hepatocytes in all CALERIE phase 2 participants, given the role these biomarkers play in liver health [53]. It is also possible, given the relative infrequency of food intake measurement and the flaws associated with self-report measures [41], we did not capture reliable insights into macronutrient intake, and further work is required to test the effect of diet composition on liver biomarkers [54]. Nonetheless, we note that the primary goal of the intervention was to stimulate CR, and our long-term assessments of percent CR were determined by DLW, which is a more objective and robust estimate of free-living energy intake [33].
Taken together, in metabolically healthy individuals without obesity, a two-year CR intervention modestly improves several liver biomarkers, with potentially larger improvements in men. In agreement with evidence suggesting that CR augments healthspan, we postulate that these findings are indicative of CR’s potential in improving metabolic function and slowing age-related liver and cardiometabolic disease in young and middle-aged adults who are healthy. Follow-up studies over several years are now needed to determine if these changes alter the development of metabolic conditions.
Supplementary Material
Acknowledgements:
We are indebted to the study participants who invested over 2 years to participate in this trial.
Funding: The research was supported by the National Institute on Aging and the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (grants U01AG022132, U01AG020478, U01AG020487, and U01AG020480); National Obesity Research Center (grant P30 DK072476), sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases; and the National Institute of General Medical Sciences of the National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center (grant 1 U54 GM104940). The work was also supported by the American Heart Association (grant #20POST35210907) (J.L.D). C. H is supported by a National Institutes of Health National Research Service Award (T32 DK064584). The funder for this exploratory analysis had a role in study design and data collection, but no role in data analysis, data interpretation, or writing of the manuscript. The corresponding author had access to all the data and had final responsibility for the decision to submit for publication.
Footnotes
DECLERATIONS
Conflicts of interest/Competing interests: All authors declare no conflicts of interest relevant to the study.
Ethical approval: All CALERIE institutions received institutional review board approval. CALERIE was performed in line with the ethical standards cited in the 1964 Declaration of Helsinki and its later amendments.
Consent to participate: All participants provided written informed consent before their inclusion in the study.
Consent for publication: All authors agreed with the content of the manuscript and gave consent to submit.
Availability of data and material: Individual de-identified participant data will not be shared, but data can be freely downloaded via the CALERIE website (http://calerie.duke.edu).
Code availability: Not applicable.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Sheedfar F, Biase S Di, Koonen D, Vinciguerra M (2013) Liver diseases and aging: Friends or foes? Aging Cell 12:950–954. 10.1111/acel.12128 [DOI] [PubMed] [Google Scholar]
- 2.Younossi ZM, Koenig AB, Abdelatif D, et al. (2016) Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64:73–84. 10.1002/hep.28431 [DOI] [PubMed] [Google Scholar]
- 3.Byrne CD, Targher G (2015) NAFLD: A multisystem disease. Journal of Hepatology 62:S47–S64. 10.1016/j.jhep.2014.12.012 [DOI] [PubMed] [Google Scholar]
- 4.Kumashiro N, Erion DM, Zhang D, et al. (2011) Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proceedings of the National Academy of Sciences of the United States of America 108:16381–16385. 10.1073/pnas.1113359108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Day CP (2002) Pathogenesis of steatohepatitis. Best Practice and Research in Clinical Gastroenterology 16:663–678. 10.1053/bega.2002.0333 [DOI] [PubMed] [Google Scholar]
- 6.Taylor R (2008) Pathogenesis of type 2 diabetes: Tracing the reverse route from cure to cause. Diabetologia 51:1781–1789. 10.1007/s00125-008-1116-7 [DOI] [PubMed] [Google Scholar]
- 7.Bugianesi E, Pagotto U, Manini R, et al. (2005) Plasma Adiponectin in nonalcoholic fatty liver is related to hepatic insulin resistance and hepatic fat content, not to liver disease severity. Journal of Clinical Endocrinology and Metabolism 90:3498–3504. 10.1210/jc.2004-2240 [DOI] [PubMed] [Google Scholar]
- 8.Yoon H jin Cha BS (2014) Pathogenesis and therapeutic approaches for non-alcoholic fatty liver disease. World Journal of Hepatology 6:800–811. 10.4254/wjh.v6.i11.800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitehead JP, Richards AA, Hickman IJ, et al. (2006) Adiponectin - A key adipokine in the metabolic syndrome. Diabetes, Obesity and Metabolism 8:264–280. 10.1111/j.1463-1326.2005.00510.x [DOI] [PubMed] [Google Scholar]
- 10.Morsiani C, Bacalini MG, Santoro A, et al. (2019) The peculiar aging of human liver: A geroscience perspective within transplant context. Ageing Research Reviews 51:24–34. 10.1016/j.arr.2019.02.002 [DOI] [PubMed] [Google Scholar]
- 11.Stranges S, Dorn JM, Muti P, et al. (2004) Body fat distribution, relative weight, and liver enzyme levels: a population-based study. Hepatology 39:754–763. 10.1002/hep.20149 [DOI] [PubMed] [Google Scholar]
- 12.Bonnet F, Ducluzeau PH, Gastaldelli A, et al. (2011) Liver enzymes are associated with hepatic insulin resistance, insulin secretion, and glucagon concentration in healthy men and women. Diabetes 60:1660–1667. 10.2337/db10-1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallace TM, Utzschneider KM, Tong J, et al. (2007) Relationship of liver enzymes to insulin sensitivity and intra-abdominal fat. Diabetes Care 30:2673–2678. 10.2337/dc06-1758 [DOI] [PubMed] [Google Scholar]
- 14.Choi SH, Yun KE, Choi HJ (2013) Relationships between serum total bilirubin levels and metabolic syndrome in Korean adults. Nutrition, Metabolism and Cardiovascular Diseases 23:31–37. 10.1016/j.numecd.2011.03.001 [DOI] [PubMed] [Google Scholar]
- 15.Chang Y, Ryu S, Sung E, Jang Y (2007) Higher concentrations of alanine aminotransferase within the reference interval predict nonalcoholic fatty liver disease. Clinical Chemistry 53:686–692. 10.1373/clinchem.2006.081257 [DOI] [PubMed] [Google Scholar]
- 16.Kwak MS, Kim D, Chung GE, et al. (2012) Serum bilirubin levels are inversely associated with nonalcoholic fatty liver disease. Clinical and Molecular Hepatology 18:383–390. 10.3350/cmh.2012.18.4.383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunutsor SK, Apekey TA, Khan H (2014) Liver enzymes and risk of cardiovascular disease in the general population: A meta-analysis of prospective cohort studies. Atherosclerosis 236:7–17. 10.1016/j.atherosclerosis.2014.06.006 [DOI] [PubMed] [Google Scholar]
- 18.Novotný L, Vítek L (2003) Inverse relationship between serum bilirubin and atherosclerosis in men: A meta-analysis of published studies. Experimental Biology and Medicine 228:568–571. 10.1177/15353702-0322805-29 [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Zhang D, Huang R, et al. (2017) Gamma-glutamyltransferase and risk of cardiovascular mortality: A dose-response meta-analysis of prospective cohort studies. PLoS ONE 12:1–19. 10.1371/journal.pone.0172631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kunutsor SK, Apekey TA, Seddoh D, Walley J (2014) Liver enzymes and risk of all-cause mortality in general populations: A systematic review and meta-analysis. International Journal of Epidemiology 43:187–201. 10.1093/ije/dyt192 [DOI] [PubMed] [Google Scholar]
- 21.Ravussin E, Redman LM, Rochon J, et al. (2015) A 2-year randomized controlled trial of human caloric restriction: Feasibility and effects on predictors of health span and longevity. Journals of Gerontology - Series A Biological Sciences and Medical Sciences 70:1097–1104. 10.1093/gerona/glv057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kraus WE, Bhapkar M, Huffman KM, et al. (2019) 2 years of calorie restriction and cardiometabolic risk (CALERIE): exploratory outcomes of a multicentre, phase 2, randomised controlled trial. The Lancet Diabetes & Endocrinology 7:673–683. 10.1016/S2213-8587(19)30151-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andersen T, Gluud C, Franzmann MB, Christoffersen P (1991) Hepatic effects of dietary weight loss in morbidly obese subjects. Journal of Hepatology 12:224–229. 10.1016/0168-8278(91)90942-5 [DOI] [PubMed] [Google Scholar]
- 24.Christensen P, Bliddal H, Riecke BF, et al. (2011) Comparison of a low-energy diet and a very low-energy diet in sedentary obese individuals: a pragmatic randomized controlled trial. Clinical Obesity 1:31–40. 10.1111/j.1758-8111.2011.00006.x [DOI] [PubMed] [Google Scholar]
- 25.de Luis DA, Aller R, Izaola O, et al. (2008) Effect of a hypocaloric diet in transaminases in nonalcoholic fatty liver disease and obese patients, relation with insulin resistance. Diabetes Research and Clinical Practice 79:74–78. 10.1016/j.diabres.2007.07.015 [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto M, Iwasa M, Iwata K, et al. (2007) Restriction of dietary calories, fat and iron improves non-alcoholic fatty liver disease. Journal of Gastroenterology and Hepatology 22:498–503. 10.1111/j.1440-1746.2006.04548.x [DOI] [PubMed] [Google Scholar]
- 27.Gasteyger C, Larsen TM, Vercruysse F, Astrup A (2008) Effect of a dietary-induced weight loss on liver enzymes in obese subjects. American Journal of Clinical Nutrition 87:1141–1147. 10.1093/ajcn/87.5.1141 [DOI] [PubMed] [Google Scholar]
- 28.Larson-Meyer DE, Newcomer BR, Heilbronn LK, et al. (2008) Effect of 6-month calorie restriction and exercise on serum and liver lipids and markers of liver function. Obesity 16:1355–1362. 10.1038/oby.2008.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Most J, Gilmore LA, Smith SR, et al. (2018) Significant improvement in cardiometabolic health in healthy nonobese individuals during caloric restriction-induced weight loss and weight loss maintenance. American Journal of Physiology-Endocrinology and Metabolism 314:E396–E405. 10.1152/ajpendo.00261.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rochon J, Bales CW, Ravussin E, et al. (2011) Design and conduct of the CALERIE study: comprehensive assessment of the long-term effects of reducing intake of energy. The Journals of Gerontology: Series A Biological sciences and medical sciences 66:97–108. 10.1093/gerona/glq168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stewart TM, Bhapkar M, Das S, et al. (2013) Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy Phase 2 (CALERIE Phase 2) screening and recruitment: Methods and results. Contemporary Clinical Trials 34:10–20. 10.1016/j.cct.2012.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rickman AD, Williamson DA, Martin CK, et al. (2011) The CALERIE Study: Design and methods of an innovative 25% caloric restriction intervention. Contemporary Clinical Trials 32:874–881. 10.1016/j.cct.2011.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Racette SB, Das SK, Bhapkar M, et al. (2012) Approaches for quantifying energy intake and %calorie restriction during calorie restriction interventions in humans: the multicenter CALERIE study. AJP: Endocrinology and Metabolism 302:E441–E448. 10.1152/ajpendo.00290.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pieper C, Redman L, Racette S, et al. (2011) Development of adherence metrics for caloric restriction interventions. Clinical Trials 8:155–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meydani SN, Das SK, Pieper CF, et al. (2016) Long-term moderate calorie restriction inhibits inflammation without impairing cell-mediated immunity: A randomized controlled trial in non-obese humans. Aging 8:1416–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen J (1988) Statistical power analysis for the behavioral sciences. Lawrence Erlbaum Associates, Hillsdale, New Jersey [Google Scholar]
- 37.Matsuda M, DeFronzo RA (1999) Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 22:1462–1470. 10.2337/diacare.22.9.1462 [DOI] [PubMed] [Google Scholar]
- 38.Romashkan S V, Das SK, Villareal DT, et al. (2016) Safety of two-year caloric restriction in non-obese healthy individuals. Oncotarget 7:19124–19133. 10.18632/oncotarget.8093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horsfall LJ, Rait G, Walters K, et al. (2011) Serum bilirubin and risk of respiratory disease and death. JAMA - Journal of the American Medical Association 305:691–697. 10.1001/jama.2011.124 [DOI] [PubMed] [Google Scholar]
- 40.Drummen XM, Dorenbos E, Vreugdenhil ACE, et al. (2018) Long-term effects of increased protein intake after weight loss on intrahepatic lipid content and implications for insulin sensitivity: A preview study. American Journal of Physiology - Endocrinology and Metabolism 315:E885–E891. 10.1152/ajpendo.00162.2018 [DOI] [PubMed] [Google Scholar]
- 41.Dhurandhar N V, Schoeller D, Brown AW, et al. (2015) Energy balance measurement: when something is not better than nothing. International Journal of Obesity 39:1109–1113. 10.1038/ijo.2014.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lonardo A, Nascimbeni F, Ballestri S, et al. (2019) Sex differences in nonalcoholic fatty liver disease: state of the art and identification of research gaps. Hepatology 70:1457–1469. 10.1002/hep.30626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ter Horst KW, Gilijamse PW, De Weijer BA, et al. (2015) Sexual dimorphism in hepatic, adipose tissue, and peripheral tissue insulin sensitivity in obese humans. Frontiers in Endocrinology 6:182. 10.3389/fendo.2015.00182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kane AE, Sinclair DA, Mitchell JR, Mitchell SJ (2018) Sex differences in the response to dietary restriction in rodents. Current Opinion in Physiology 6:28–34. 10.1016/j.cophys.2018.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salvaggio A, Periti M, Miano L, et al. (1991) Body mass index and liver enzyme activity in serum. Clinical Chemistry 37:720–723 [PubMed] [Google Scholar]
- 46.Kwon H, Kim D, Kim JS (2017) Body fat distribution and the risk of incident metabolic syndrome: A longitudinal cohort study. Scientific Reports 7:10955. 10.1038/s41598-017-09723-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pang Q, Zhang JY, Song SD, et al. (2015) Central obesity and nonalcoholic fatty liver disease risk after adjusting for body mass index. World Journal of Gastroenterology 21:1650–1662. 10.3748/wjg.v21.i5.1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elffers TW, De Mutsert R, Lamb HJ, et al. (2017) Body fat distribution, in particular visceral fat, is associated with cardiometabolic risk factors in obese women. PLoS ONE 12:e0185403. 10.1371/journal.pone.0185403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chung GE, Kim D, Kwark MS, et al. (2015) Visceral adipose tissue area as an independent risk factor for elevated liver enzyme in nonalcoholic fatty liver disease. Medicine (United States) 94:e573. 10.1097/MD.0000000000000573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamauchi T, Kamon J, Minokoshi Y, et al. (2002) Adiponectin stimulates glucose utilization and fatty - acid oxidation by activating AMP-activated protein kinase. Nature Medicine 8:1288–1295. 10.1038/nm788 [DOI] [PubMed] [Google Scholar]
- 51.Dandona P, Aljada A, Chaudhuri A, et al. (2005) Metabolic syndrome: A comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circu lation 111:1448–1454. 10.1161/01.CIR.0000158483.13093.9D [DOI] [PubMed] [Google Scholar]
- 52.Cohen J (1992) Statistical power analysis. Current Directions in Psychological Science 1:1304–1312. 10.1111/1467-8721.ep10768783 [DOI] [Google Scholar]
- 53.Newsome PN, Cramb R, Davison SM, et al. (2018) Guidelines on the management of abnormal liver blood tests. Gut 67:6–19. 10.1136/gutjnl-2017-314924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eslamparast T, Tandon P, Raman M (2017) Dietary composition independent of weight loss in the management of non-alcoholic fatty liver disease. Nutrients 9:800. 10.3390/nu9080800 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.