Abstract
Proton (1H) magnetic resonance spectroscopy provides a non-invasive and quantitative measure of brain metabolites. Traumatic brain injury impacts cerebral metabolism and a number of research groups have successfully used this technique as a biomarker of injury and/or outcome in both pediatric and adult TBI populations. However, this technique is underutilized, with studies being performed primarily at centers with access to MR research support. In this paper we present a technical introduction to the acquisition and analysis of in vivo 1H magnetic resonance spectroscopy and review 1H magnetic resonance spectroscopy findings in different injury populations. In addition, we propose a basic 1H magnetic resonance spectroscopy data acquisition scheme (Supplemental Information) that can be added to any imaging protocol, regardless of clinical magnetic resonance platform. We outline a number of considerations for study design as a way of encouraging the use of 1H magnetic resonance spectroscopy in the study of traumatic brain injury, as well as recommendations to improve data harmonization across groups already using this technique.
Keywords: Magnetic resonance spectroscopy, Trauma, Brain injury, Concussion
Introduction
In vivo 1H magnetic resonance spectroscopy (MRS) provides a non-invasive method to measure brain metabolites. Since abnormal metabolism is part of the pathophysiologic cascade following traumatic brain injury (TBI), MRS has been utilized to identify metabolite changes in regions of injury, to define the extent of pathology, and as a biomarker of outcome. The extent of metabolite change differs depending on the mechanism of injury, injury severity, the region studied, and the specific acquisition technique used. However inconsistent findings, especially with milder injuries, are likely magnified due to the limited number of studies, small sample sizes, duration between injury and MRS study (72 h – several years), as well as the underlying heterogeneity of the condition and premorbid influences. To facilitate the expanded use of MRS in TBI and to improve data harmonization, the ENIGMA Brain Injury MRS subgroup (http://enigma.ini.usc.edu/ongoing/enigma-tbi/enigma-mrs/) has collaborated to define and to encourage the use of standardized data acquisition and analysis protocols that can be used on all clinical MR platforms.
This paper is divided into three sections. First, we introduce the neurometabolites quantified by proton MRS, current acquisition methods, and the basics of spectral analysis. In the second section, the reader is given an overview of the current state of MRS as applied to TBI including the potential of MRS as a biomarker of injury and its role in outcome prediction. Lastly, we present a number of important considerations for MRS study design, recommendations and opportunities for data harmonization; we also propose a basic data acquisition scheme for TBI studies.
An introduction to the MR spectrum
In contrast to MR images, an MR spectrum consists of peaks or resonances that represent the signal intensity (amplitude) received from protons in a given metabolite as a function of their frequency (i.e., position on the chemical shift axis). Each functional group of a metabolite resonates at a frequency dependent on its chemical structure. The difference in frequency between a metabolite and a known reference standard is its chemical shift and is typically expressed as parts per million (ppm), which is a magnetic field-independent frequency scale. Each peak is proportional to the concentration of that metabolite in a specified volume of interest. In vivo MRS focuses on protons in the 1–5 ppm chemical shift range (Fig. 1) that are present at high enough concentrations (>0.5 mmol/L). Several brain metabolites can be measured, with differing information content obtained with short (20–40 ms), intermediate or long (135–270 ms) echo times (TE).
Fig. 1.
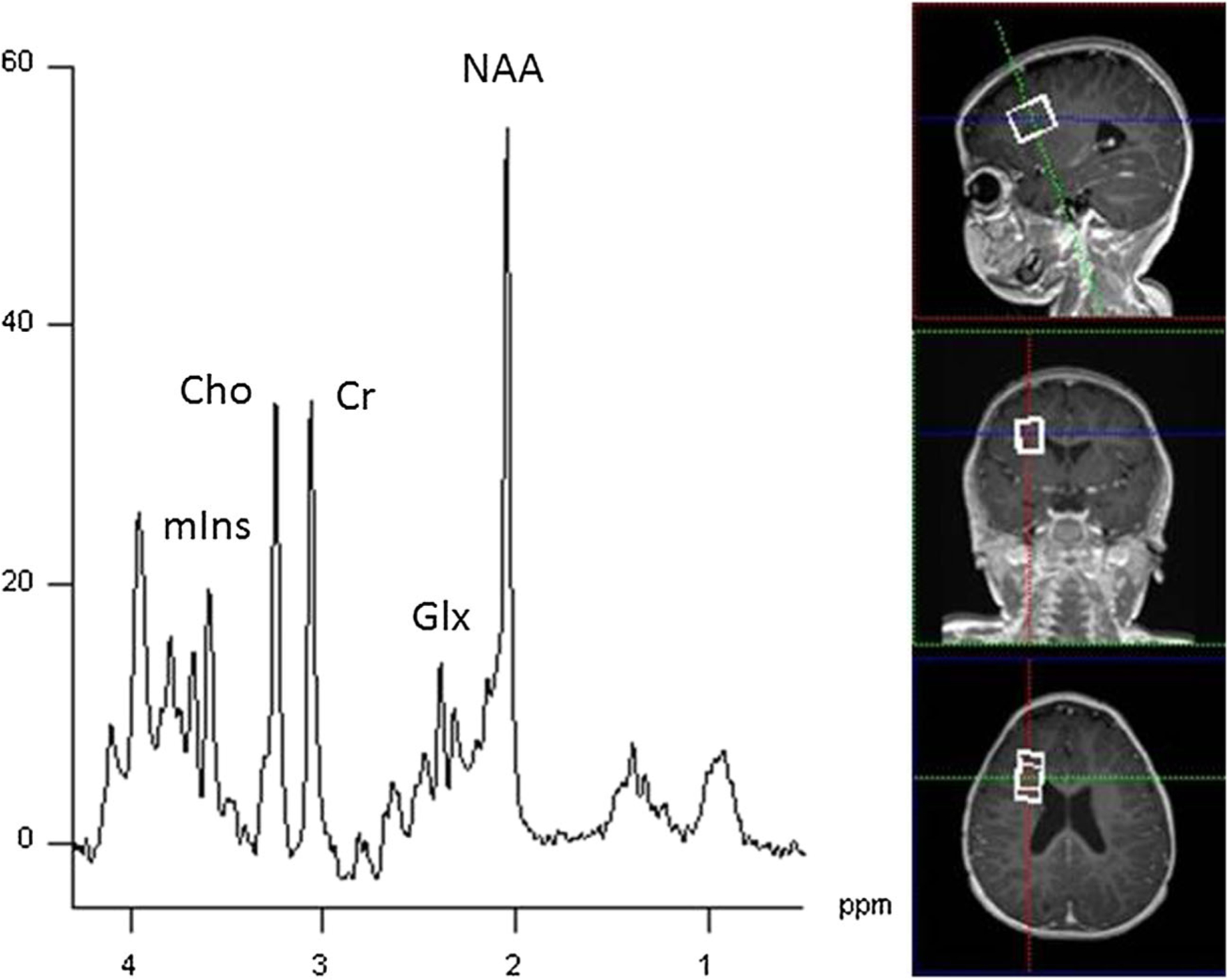
Single voxel spectroscopy (SVS PRESS; TR/TE = 3000/30 ms; 3.0 T) collected from a 20 × 20 × 20 mm3 voxel the right frontal white matter of a 10 month old showing commonly observed neurometabolites. NAA (N-acetylaspartate), Glx (overlapping glutamate and glutamine), Cr (creatine), Cho (choline), and mI (myo-inositol)
The most commonly measured brain metabolites in proton MRS and their chemical shift values can be seen in Fig. 1, taken from a single voxel (8 cm3) region in the frontal white matter and summarized in Table 1. N-acetylaspartate (2.01 ppm) is often reported as total or tNAA, representing NAA and N-acetylaspartylglutamate (NAAG; 2.04 ppm) as it is challenging to separate NAA and NAAG signals completely. NAA is an amino acid synthesized in neuronal mitochondria - an indicator of neuronal energy metabolism - and is considered a marker of neuronal health as it decreases with neuronal loss or dysfunction (Moffett et al., 2007). NAA is also considered a marker of neuronal density due to its high concentration in healthy neurons, although this interpretation is overly simplified as NAA is also an osmolyte and may be a water pump that extracts water from neurons. Additionally, NAA is a source of acetate for myelin lipogenesis and is thought to act as a reservoir for glutamate synthesis in the Krebs cycle (Clark et al., 2006). NAA levels increase dramatically from birth and plateau at 2–3 years of age (Kreis et al., 2002). During development NAA reflects active myelination and is observed early in the thalamus and later in parieto-occipital and periventricular white matter (Cady et al., 1996; Huppi & Inder, 2001).
Table 1.
Summary of Brain Metabolites in TBI
| Metabolite | Chemical shift (ppm) | Comment |
|---|---|---|
| Lipid | 0.9–1.5 | Not usually visible in MRS unless released by pathological process such as trauma (Ashwal et al., 1997). |
| Lactate (Lac) | 1.3 | End product of anaerobic glycolysis. Indicator of hypoxia and impairment of perfusion (Ross et al., 1998). |
| N-acetyl-aspartate (NAA) | 2.0 | Synthesized in neurons. Marker of neuronal viability. Reduced with brain injury (Moffett et al., 2007). |
| Glutamate/Glutamine (Glx) | 2.2–2.5 | Glutamate is the primary excitatory neurotransmitter in the brain. Glutamine is found in astrocytes. Glx may be predictive of outcome after severe TBI and associated with immunoexcitotoxicity or secondary dysfunction (Shutter et al., 2004). |
| Choline (Cho) | 3.2 | Membrane marker. Elevated in cases of high membrane turnover. May indicate diffuse axonal injury (Andrew A. Maudsley et al., 2017) or continued repair at chronic time points in combination with normalized NAA levels (Babikian et al., 2018). |
| Creatine (Cr) | 3.0 | Found in metabolic active tissues, used in energy storage and transfer. Used as an internal standard for other metabolites (e.g. NAA/Cr, Cho/Cr). May be altered by TBI (Yeo et al., 2011). |
| Myo-inositol (mI) | 3.5 | Astrocyte marker and osmolyte. Involved in the metabolism of phosphatidyl inositol (a membrane phospholipid) - expected to increase after TBI due to membrane damage (Kierans et al., 2014). May also be a glial marker. |
Total creatine (Cr; 3.03 ppm), comprised of phosphocreatine (PCr) and its precursor free Cr are markers for intact brain energy metabolism. The Cr signal has been commonly used as an internal standard for proton MRS based on the assumption that the Cr-phosphocreatine equilibrium provides a stable concentration of Cr. However, Cr concentrations are known to change with age (increasing from birth until 2–3 years of age; (Pouwels et al., 1999) as well as with pathologic conditions including TBI (Gasparovic et al., 2009). The total choline peak (Cho, 3.20 ppm) consists primarily of phosphoryl and glycerophosphoryl choline (PCh and GPC, respectively) and is considered a marker for membrane synthesis or repair, inflammation or demyelination. Cho levels are elevated at birth and decrease rapidly with maturation (Barker et al., 2010).
Metabolites with multiple associated (multiplet) resonances are optimally detected using short TE MRS acquisitions. This includes glutamate (Glu), an excitatory neurotransmitter, which can be released into the extracellular space after brain injury and plays a major role in excitotoxic neuronal death (Alessandri et al., 1999; Bullock et al., 1998). Its resonance overlaps with the amino acid glutamine (Gln) and the two are therefore often reported together as Glx (2.05–2.5 ppm and 3.7 ppm). At higher field strengths (≥ 3 T) the two metabolites are better resolved and may be measured separately, though at 3 T Glx is still often reported. Myoinositol (mI; 3.56 ppm) is an organic osmolyte located in astrocytes that increases as a result of glial proliferation (Brand et al., 1993). Myo-inositol levels are high at birth and decrease rapidly with brain maturation leveling off by approximately 2–3 years of age (Pouwels et al., 1999). Lactate (Lac; 1.31 ppm) accumulates as a result of glycolysis in conditions of decreased oxidative metabolism and is often poorly resolved in normal brain short TE MRS as the threshold for detection is within the 0.5–1 mmol range (Barker et al., 2010). While the presence of Lac in a MR spectrum has historically been considered pathological, resulting from an increase in anaerobic glycolysis, signal to noise ratio (SNR) improvements with higher field strengths have resulted in the routine appearance of small Lac peaks in otherwise normal MRS (Tomiyasu et al., 2016).
Metabolite levels also vary by region and tissue type (Baker et al., 2008; Frahm et al., 1989) and change rapidly as the brain develops (Kreis et al., 2002). In general, metabolite changes associated with brain maturation (increasing NAA and lower Cho and mI levels) occur rapidly in the first year of life (Holshouser et al., 1997) and continue to a lesser degree through adolescence and early adulthood (Horská et al., 2002; McLean et al., 2000), before declining with normal aging (Eylers et al., 2016). As a result of differences in metabolites across the brain and lifespan, the proper interpretation of spectroscopy data typically requires comparison to “control” data sets acquired from the same brain region, using the same parameters, in age-matched control subjects. If normative data from the literature is used, care must be taken to appreciate the effects of technical differences such as acquisition methods and pulse profiles, scanner field strength, and tissue composition. Other possible considerations include data processing methods, error corrections, and artifacts.
MRS pulse sequences and analysis methods
A number of localization techniques can be used to acquire proton MRS and the selection of sequence and parameters are key factors in determining the outcome of the study. Following TBI both single voxel spectroscopy (SVS) and multivoxel MR spectroscopic imaging (MRSI) techniques have been used. The following sections discuss these localization techniques, their specific advantages, disadvantages, and analysis.
Single voxel spectroscopy (SVS)
The two conventional localization schemes for SVS that are available on all commercial scanners are Point RESolved Single voxel (PRESS) and Stimulated Echo Acquisition Mode (STEAM). The most widely used localization scheme is PRESS (Bottomley, 1987). While both sequences apply orthogonal gradients with selective pulses for the voxel localization, a PRESS acquisition results in approximately twice the SNR compared to STEAM, although this is at the expense of having to use a slightly longer TE. Both methods are known to exhibit localization inaccuracies known as the chemical shift displacement (CSD) error, which is worse for PRESS acquisitions particularly at high field strengths. As a result, modifications of the PRESS technique such as semi-LASER (Scheenen et al., 2008) to reduce the CSD error and shorten the TE, have been validated at 3 T (Bednařík et al., 2015; Deelchand et al., 2018; Terpstra et al., 2016; Wijnen et al., 2010) and are now available on some clinical systems. For a detailed review of the PRESS sequence and its modifications, readers are directed to the review by (Yahya and Fallone 2007).
Single voxel localization methods such as PRESS and STEAM can readily quantify NAA, Cr, Cho, mI, Lac and Glx. However, other metabolites of interest to study after TBI, such as gamma-aminobutyric acid (GABA) and glutathione are more challenging to reliably quantify due to lower concentrations and/or overlapping resonances. The use of spectral editing methods, such as J-difference editing, MEGA (Mescher et al., 1998) or BASING (Star-Lack et al., 1997) - which separates the molecules of interest from stronger, overlying signals of more concentrated metabolites - have been developed and used at specialized imaging centers. A comprehensive review of editing approaches for brain metabolites may be found in (Harris et al., 2017).
The disadvantage to using SVS is the relatively large voxel size (typically 8 cm3) needed to obtain enough signal from the metabolites of interest. The result is that the voxel generally contains cerebrospinal fluid (CSF), which contains no observable metabolites, as well as a mixture of white matter and gray matter (see Fig. 1). The CSF fraction can be accounted for with tissue-voxel composition correction by segmenting the voxel into its white and gray matter and CSF, and scaling the metabolite level based on the volume fraction of CSF (fCSF) using the formula Ccorr = Cmeas/(1-fCSF), where Cmeas is the measured metabolite level and Ccorr is the corrected metabolite level. We recommend more comprehensive tissue corrections that additionally correct for differences in water and metabolite signal relaxations (Gasparovic et al. 2006 and 2018, discussed below). An additional challenge is accounting for differences in metabolite concentration between white matter and gray matter. On average, NAA and Cr concentrations are higher in gray matter compared to white matter while Cho concentrations are lower in gray matter (Wang & Li, 1998). While this too can be included in the tissue-volume correction (Harris et al., 2015), an alternative is to include the tissue fractions as a covariate in the statistical analysis (i.e., a factor in an ANOVA model) although at the expense of statistical power. As most MRS studies have small sample sizes, mathematical corrections are generally preferred.
MR spectroscopic imaging (MRSI)
As it can provide “images” of metabolite distributions, multi-voxel MRS is also known as MR spectroscopic imaging (MRSI; Fig. 2). The metabolic images are typically obtained with spatial resolutions in the range of ~1 cm3 for studies ≤3 T to smaller volumes at higher field strengths (Hangel et al., 2018), and may be acquired over a single (2D MRSI) or multiple (3D MRSI) brain slices. The obvious advantage of the multi-voxel approach is the simultaneous acquisition of data over multiple regions, although local B0 variations limit the volume over which spectra can be obtained with adequate quality. Volumetric MRSI methods may sample as much as 80% of the brain volume (Sabati et al., 2015), although with considerable variation between subjects and scanners. MRSI is of benefit for the following types of studies: (i) identification of multi-focal injury; (ii) identification of global injury (with 3D MRSI); (iii) exploratory analyses, in which a large number of brain regions are investigated; (iv) testing of research hypotheses formulated after data acquisition, e.g., examining regions implicated by new literature, or those functionally or structurally related to a newly identified injury site. A second, often overlooked, advantage of MRSI is the ability to separate white and gray matter components using post-processing methods. This is often done by linear regression (Hetherington et al., 1996; Tal et al., 2012) or spectral decomposition methods (Goryawala et al., 2018), which combine information from multiple voxels with knowledge of the relative tissue content in each voxel. Linear regression can be applied in any number of (n ≥ 2) voxels to investigate small regions such as the hippocampus (<10 voxels; (Meyer et al., 2016), larger areas such as 2D slabs (McLean et al., 2000; Gasparovic et al., 2011), in entire brain lobes (Maudsley et al., 2009), and/or in global brain white matter/gray matter metabolism (100’ s of voxels; Tal et al., 2012). It is especially useful for studying cortical gray matter, which due to its tortuous shape, always incurs voxel partial volume effects when examined with SVS.
Fig. 2.
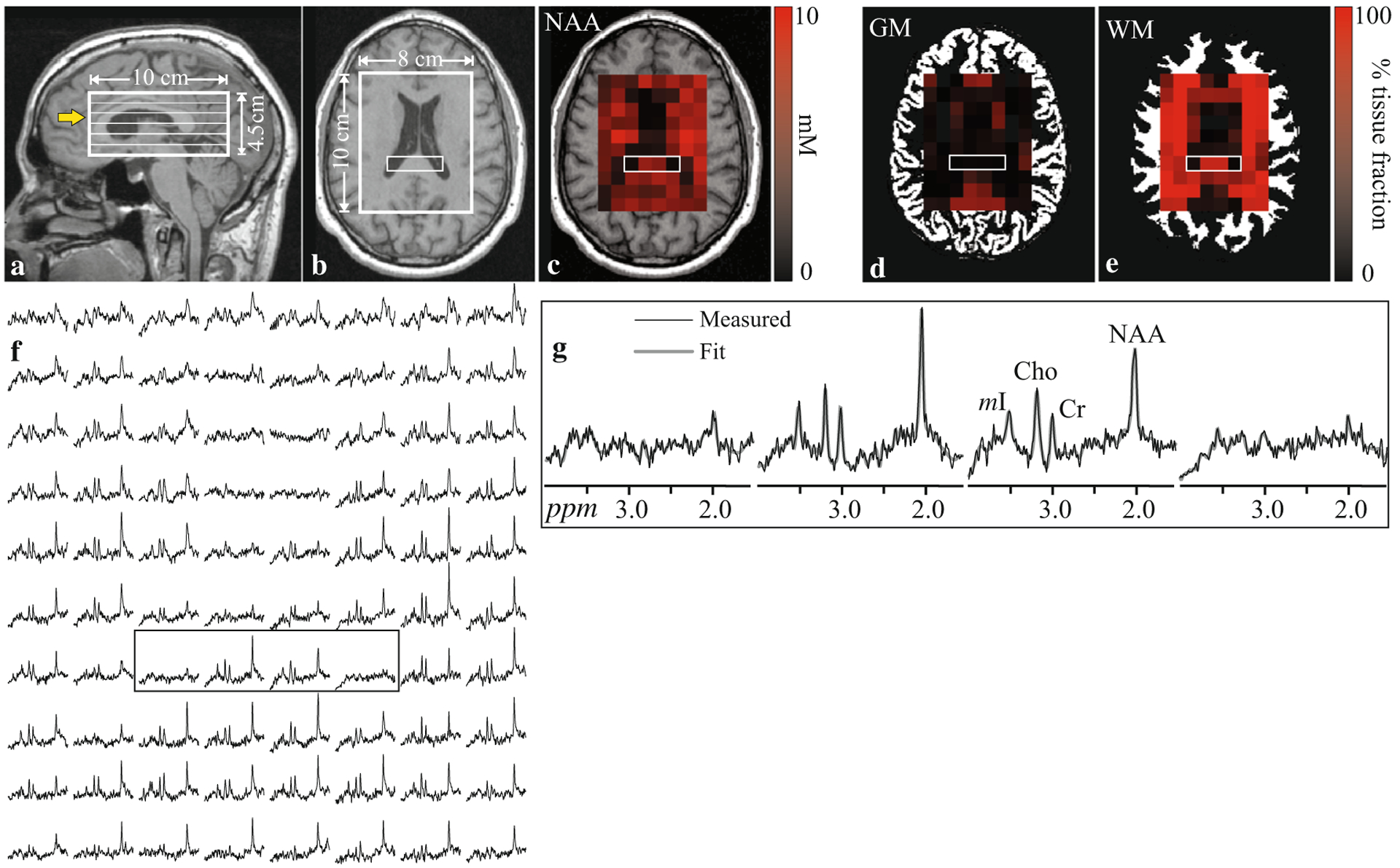
Sagittal (a), T1-weighted MRI of a patient’s head showing the location and geometry of the spectroscopy volume of interest (VOI; white frame) and the six 0.75 cm thick individual spectroscopic slices within the VOI. The yellow arrow indicates the slice shown in (b). A metabolite map overlaid on the T1-weighted image shows each voxel’s NAA concentration as a gradient from black (lowest) to red (highest) (c). Similarly each voxel’s tissue gray matter (d) and white matter (e) fractions are shown as gradient from black to red overlaid on the tissue segmentation maps. The spectral map from the VOI is shown in (f). The four spectra bound by the rectangular box are shown in detail in (g). Note the two outer spectra have no metabolite signal, because they correspond to voxels within the lateral ventricles (b). The two inner spectra originate from the splenium and correspondingly contain no gray matter (d). While they contain equal white matter fraction of about ~100% (e), the NAA signal (g) and concentration (c) are lower in one of the voxels. This patient had postconcussive symptoms and was part of a group-level analysis, which found lower global white matter NAA levels in patients compared to controls (Kirov et al., 2013a, b)
There are caveats to the above MRSI advantages relative to SVS methods. First, the smaller voxel size comes at a cost of lower SNR, which translates to reduced sensitivity to detect changes. Second, MRSI studies generally take longer to acquire (on the order of 12–18 min). Therefore, there is a higher potential for patient movement, which can affect not only data quality, but also the co-localization of the spectral matrix with the volumetric information. Third, quality control is more challenging compared to SVS as some spectra may display artifacts such as incomplete water suppression and lipid contamination. Finally, because of the effects of limited spatial sampling, each voxel contains signal from adjacent voxels, a phenomenon called “voxel bleed,” which can be remedied by avoiding acquisitions with low resolution spectral matrices, or by increasing the voxel size using a post-processing filter.
In 2D MRSI, a rectangular volume is placed at the desired transverse brain section, covering as much of the brain while avoiding the scalp lipids. 3D MRSI offers improved spatial sampling, with multiple slices allowing for the inclusion of more brain volume and structures. This can enable the study of commonly injured white matter tracts (Mandl et al., 2012) and areas remote from structural MRI-observed injury. PRESS with phase-encoding is generally used for 2D MRSI, and in principle can be extended to 3D. However, scan times become prohibitively long for full brain coverage and therefore rapid spatial-spectral sampling methods are being increasingly used for this purpose (Vidya Shankar et al., 2019). A difficulty for obtaining full-brain coverage, including sampling of cortical surface regions, is signal contamination from the strong extracranial lipid regions. Multiple approaches can be used to minimize this effect, including frequency-selective saturation, inversion-nulling (Ebel et al., 2003), and the use of higher spatial resolutions (Hangel et al., 2018) and constrained reconstruction methods (Lam et al., 2016). Additionally, the high likelihood of sampling regions with inadequate spectral quality places greater demands on robust spectral analysis and quality control. For these reasons, fully whole-brain 3D MRSI methods are not currently supported by the instrument vendors.
MRS analysis methods
All clinical scanners provide spectral processing packages that can be used for visual interpretation and a basic quantification of metabolite integrals (area under the peak). In addition, there are offline post processing tools which are commercially available (LCmodel; (Provencher, 1993) or freely available as research packages such as jMRUI (Jabłoński et al., 2017; Naressi et al., 2001), TARQUIN (Wilson et al., 2011), VeSPA (scion.duhs.duke.edu), and FID-A (https://www.opensourceimaging.org/project/fid-a-advanced-processing-and-simulation-of-mr-spectroscopy/). Many of these tools use sets of simulated or empirically measured spectra of known metabolites, macromolecules and lipid signals, known as a basis set, that are then parametrically fit to the data with modeling of the baseline and line shape variations. Basis sets may be acquired experimentally using phantoms of known concentrations or simulated from known parameters (Young et al., 1998) with the addition of macromolecules and lipid signals to the basis set improving the quantification of short TE data sets (Seeger et al., 2003). These tools not only provide quantitative metabolite levels and ratios but also provide a numerical estimate of the model fit. For example, Cramer-Rao lower bounds (CRLBs) are widely used in the literature and reflect the estimate of the error of the concentration measurement as influenced by SNR, linewidth, and signal overlap. In most publications, a CRLB <20% is often used as the cut off for a reliable measurement, although this is an arbitrary guideline and should be used with caution - particularly in TBI studies where spectra with low SNR due to injury could be arbitrarily eliminated (Kreis 2016).
When reporting spectral findings, the integral of all measured resonances for each metabolite peak is normalized by the number of protons contributing to the signal and scaled using a reference signal. The use of metabolite ratios, typically to Cr, has been widely used. However, more recently this approach has been discouraged, as Cr levels are not static, as noted above. Alternatively, semi-quantitative MRS can be obtained by scaling using the unsuppressed water signal from the same volume of interest (VOI), typically acquired in a separate acquisition commonly called a “reference” scan. The results are presented in institutional units, which can be used for comparisons to data obtained with the identical measurement parameters in identical VOIs with similar tissue concentrations. To report values that are closer to an absolute (molar or molal) concentration, the ratio of each metabolite to the water signal acquired from the same volume of interest is used and the different water and metabolite relaxation times (T1 and T2) effects and water visibility are taken into account during the quantification procedures (Gasparovic et al., 2006; Gasparovic et al., 2018; Poole et al., 2014). Another method, called “phantom replacement”, quantifies absolute amounts using as reference the metabolite signals from a separate scan of a phantom with known concentrations (Jansen et al., 2006). In this case, the relaxation values of the metabolites in the phantom should be known. In single voxel studies, the T1 and T2 effects can be minimized using longer repetition times (TR) and short echo times (TE). However, this is at the expense of a more complicated baseline due to the presence of short T2-macromolecular signals and longer scan durations with increasing TR. For larger multivoxel MR spectroscopic imaging data sets, normalization or scaling to the internal water signal represents a practical alternative to absolute concentrations.
The data analysis methods used in most SVS and MRSI TBI studies have been based on sampling preselected brain regions known to be susceptible to injury, with comparison to results from the same region in normal control subjects. For MRSI studies, multiple individual voxel results can be averaged over a region of interest (ROI) to improve SNR (Davitz et al., 2019; Johnson et al., 2012a, b; Kirov et al., 2007; Sours et al., 2015), and ROI analysis methods can be automated using spatial normalization that matches each metabolite image to a brain atlas (Maudsley et al., 2009; Spurny et al., 2019). Also widely used in TBI studies are global MRSI approaches, which obtain average concentrations over large 2D or 3D volumes (Cohen et al., 2007; Gasparovic et al., 2009; Govind et al., 2010; Kirov et al., 2013a, b; Mayer et al., 2015; Yeo et al., 2006). Since it is well-known that TBI injury is multi-focal (Biasca & Maxwell, 2007; Bigler, 2013; Browne et al., 2011; Johnson et al., 2013), averaging over a large area of diffuse injury would be expected to yield better sensitivity to injury compared to examining a smaller region (due to the higher SNR in a larger volume). Indeed, it was recently shown that when studying NAA decreases in white matter, global linear regression had better sensitivity for discriminating patients from controls when compared with regional voxel averaging (Davitz et al., 2019). Such global approaches also eliminate bias in choosing what particular (smaller) region(s) should be interrogated.
Analysis of MRSI data may also be carried out using voxel-based analysis (VBA) methods to create maps of abnormal metabolism (Maudsley et al., 2015). For analysis of individual subject data, a comparison can be made with the mean and standard deviation of a group of normal control subjects, for example to create a z-score map, and differences between groups can similarly be made using standard statistical tests (Fig. 3). VBA methods are best implemented following spatial normalization, so that comparisons are made for the same brain region and should account for the relative gray- and white-matter content in each voxel, as well as for individual metabolite maps, and for CSF volume fraction. VBA maps have the advantage that normal spatial and tissue variations are accounted for, and the result directly highlights abnormal tissue regions. Nonetheless, this approach remains difficult to integrate into clinical protocols due to the requirement for strict standardization. Prior to performing any of the above analyses, spectral quality tests must be applied and voxels not meeting these requirements should be excluded.
Fig. 3.
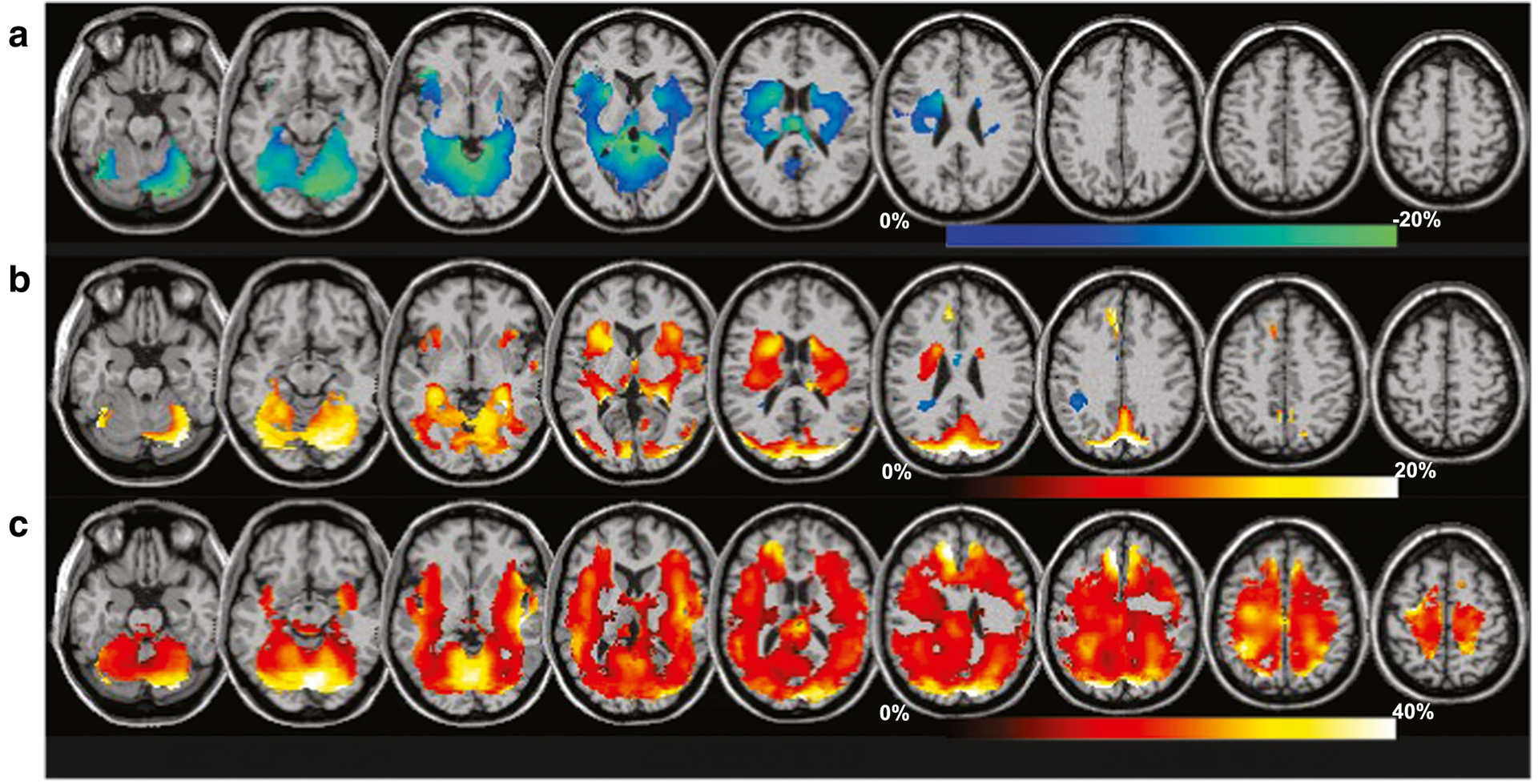
Percent differences of the group mean metabolite measures relative to control values for a group with moderate TBI (mean GCS 12.9, range 6 to 15). Regions with significant differences, for p < 0.05, are shown as a color overlay superimposed on the spatial reference magnetic resonance imaging. Results are shown for N-acetyl-aspartate/creatine (a), creatine (b), and choline (c), with the corresponding color scales indicated for each. Data was acquired at 3 T using a volumetric MRSI sequence with spatial sampling of 50×50×18 points over 280×280×180 mm3 and TE = 70 ms (modified from Maudsley et al. 2015)
Machine learning (ML) or artificial intelligence approaches for analysis of MRS data are still very much in their nascent stages but show great promise in potentially extracting novel factors within MRS data that go beyond the traditional analytical approaches. For example, the conventional fitting of MRS data as described earlier could potentially be improved, particularly in situations where SNR is low or the spectra contain artifacts (Das et al., 2017). Alternatively, ML methods may be effective in removing artifacts (Kyathanahally et al., 2018). Another potential application of ML methods in MRS of TBI may be to identify patterns in differences in brain metabolites. For example, in military-related TBI one such confound can be the comorbidity of post-traumatic stress disorder (PTSD). Pattern classification algorithms can be applied in different cohorts of soldiers with mTBI, PTSD, co-morbid mTBI and PTSD, and controls to identify potential biomarkers that can classify these groups (Mariano et al., 2017). Alternatively, the metabolite concentrations themselves can be used as the model input as applied in distinguishing between chronic repetitive sports-related TBI and controls (Louis et al., 2017). Thus far, the application of ML methods in MRS of TBI is preliminary, as robust classification can only be achieved using large datasets and most MRS studies have used relatively small cohort sizes. With uniformity in MRS methods, multisite studies that allow for combined datasets will facilitate the magnitude of data necessary for ML algorithms to be effective. Endeavors such as the ENIGMA groups or data repositories such as FITBIR will be necessary to achieve this goal.
MRS findings following TBI
MRS as a biomarker of injury
Standard clinical neuroimaging methods such as computed tomography (CT) and conventional MRI are the primary clinical neuroimaging methods for lesion detection after TBI and continue to provide diagnostic and prognostic information, particularly for patients with more severe TBI (Jeter et al., 2013; Mayer et al., 2018). Additionally, advanced MRI techniques such as volumetric MRI analysis, susceptibility-weighted imaging (microhemorrhages), diffusion tensor imaging (DTI) and tractography (white matter injury), functional MRI (network connectivity), and perfusion imaging (blood flow or volume) have produced sets of imaging biomarkers used to predict neurological and/or neuropsychological outcome across all severities of brain injury (Hunter et al., 2012; Kou et al., 2010). However, the exquisite sensitivity of MRS to noninvasively measure key neurometabolites provides a unique window into the brain’s response to injury above and beyond clinical symptoms, as well as beyond structural and functional changes observed on more conventional imaging.
Combining MRS in multimodal imaging studies can also be valuable as each modality provides information that can be complementary. For example, combining MRS with diffusion MRI can provide additional insight into alterations in white matter health and can lead to more accurate clinical outcome prediction (Dennis et al., 2018). Moreover, MRS biomarkers (i.e., metabolite levels or ratios) often show abnormalities in the injured brain even when conventional neuroimaging appears normal (Garnett et al., 2000; Holshouser et al., 2005; Kirov et al., 2013a, b). Following TBI, decreases in brain NAA, whether regional or whole-brain, have consistently been associated with outcomes following moderate to severe TBI (Friedman et al., 1998; Garnett et al., 2000; Holshouser et al., 2005; Kirov et al., 2013a, b; Maudsley et al., 2017). Longitudinal studies have shown that NAA levels are dynamic after injury - NAA remains low in patients with poor recovery suggesting neuronal loss (Fig. 4a), and recovering in patients with good outcomes suggesting recovery of mitochondrial function and/or neuronal repair (Garnett et al., 2000; Holshouser et al., 2019; Signoretti et al., 2002). Several studies have correlated reduced NAA levels after injury to long-term memory or attention deficits (Babikian et al., 2006; Holshouser et al., 2019) and combined neuropsychological function measures (Friedman et al., 1998; Govind et al., 2010; Maudsley et al., 2015; Babikian et al., 2018; Dennis et al., 2018). Increasing levels of Cho (Maudsley et al., 2017), mI (Ashwal et al., 2004), and Glx (Ashwal et al., 2004; Garnett et al., 2001; Gasparovic et al., 2009; Shutter et al., 2004) have also been associated with poor neurological outcome. The presence of Lac and lipids after severe TBI has been reported in patients with the worst outcomes (Ashwal et al., 1997; Panigrahy et al., 2010). All told, there is mounting evidence that MRS provides unique biomarkers of injury, which improves injury detection and prognosis and may be more sensitive following milder injury than imaging alone as detailed below under the heading of sports-related TBI.
Fig. 4:

MRSI volume of interest (VOI) overlaid onto a T2 weighted MR image of a 3 month old NAT patient who presented with an initial GCS score of 3, retinal hemorrhages and diffuse hypoxic-ischemic injury (a). MRSI (PRESS; TR/TE = 3000/144 ms; 1.5 T) was acquired 1 day after injury. (b). Spectral map shows a diffuse decrease of NAA and large inverted Lac peaks (doublet at 1.33 ppm; B). The Lac peaks are inverted as a result of the intermediate echo time. Spectrum from the right parieto-occipital white matter shows markedly decreased NAA and presence of Lac (c)
The following factors complicate generalizations about MRS findings following TBI: the use of metabolite ratios and differences in (i) regions of interest (ROI); (ii) sensitivity of the measurement (i.e., SNR, partial volume); (iii) age of the studied population; (iv) injury severity and type of TBI within the patient pool; and (v) duration of time between injury and MRS study. Given the heterogeneity of injury and variability in acquisition methods and sampled locations it is perhaps not surprising that findings across studies are not always consistent even when patients are examined at similar post-TBI periods, e.g., George et al. (George et al., 2014) reported reduced Cho/Cr in the thalamus, whereas increased (Govind et al., 2010) and normal (Yeo et al., 2011) white matter Cho have been reported in subacute TBI. Nevertheless, the consensus is that NAA declines, both in acute and post-acute patients, with a secondary finding of increased Cho (Gardner et al., 2014; Kirov et al., 2018; Lin et al., 2012). These abnormalities seem to increase with injury severity as assessed by the Glasgow Coma Scale (GCS), post-traumatic amnesia (PTA) and MRI findings (Garnett et al., 2000; Govind et al., 2010), and in general, white matter may be more affected than gray (Govind et al., 2010; Yeo et al., 2011). In studies including mild injuries, the interpretation of cross-sectional studies may also be hampered by a recruitment bias and prior injury in acute versus post-acute studies. The latter studies are likely to enroll patients from a physician’s office, thereby biasing their sample towards patients with worse outcome; while the former would tend to recruit all patients sustaining TBI, most of whom will recover. Indeed, serial studies in the acute stage of mild TBI show normalizations of abnormalities (Vagnozzi et al., 2010, 2013, 2008; Yeo et al., 2011), including the recovery of NAA/Cr (Vagnozzi et al., 2010, 2008). In contrast, NAA/Cr declines over time in cohorts of moderate and severe TBI (Garnett et al., 2000), where fewer people will proceed to good recovery. Another aspect to consider in relation to outcome is that Cr and Cho abnormalities likely represent different processes in acute and post-acute TBI stages (Lin et al., 2012). This is important, because initial findings may reflect reversible processes, while later measurements may reflect a longer term mechanism responsible for clinical impairment.
TBI in the pediatric population also defies generalization as there are several distinct types of injury that are associated with different ages within this population: non-accidental trauma (NAT; usually 2 years of age and under), auto versus pedestrian accidents (school age), and concussions/sports injuries and motor vehicle accidents (adolescence). In addition, neuropsychological testing becomes problematic when trying to include a large age span in a pediatric cohort since testing varies by age and cannot always be directly compared. In research studies, many of these challenges are mitigated by recruiting age-matched controls, an issue discussed below and in the companion piece in this special issue focused on pediatric moderate/severe TBI (Dennis et al., 2019).
MRS in outcome prediction
MRS is not only a tool to assess the presence and extent of an injury (as in acute studies), but it can also be used as an early predictor of future outcomes. When applied post-acutely, it can identify ongoing metabolic abnormalities that underlie persistent functional deficits. MRS studies have been used for clinical outcome prediction in both pediatric and adult populations, reviewed below separately for mild versus more severe injuries.
In children with moderate/severe TBI, acute MRS studies have shown reductions in NAA and elevations in Cho. These abnormalities correlate with later neurologic and neuropsychological outcome (Ashwal et al., 2000; Babikian et al., 2006), with reduced NAA in subcortical regions strongly predicting poor long-term cognitive outcomes (Babikian et al., 2006). Post-acute or chronic evaluations have shown similar neurometabolic alterations (Yeo et al., 2006), with correlations observed with neuropsychological and behavioral functioning (Babikian et al., 2010; Parry et al., 2004; Walz, Cecil, Wade, & Michaud, 2008), and continued abnormalities in subgroups of patients correlate with poorer trajectories of outcome (Babikian et al., 2018). Similar to children with msTBI, adult patients with TBI of varying injury severities have shown widespread alterations in whole brain Cho and/or NAA that are correlated with injury severity, symptomatology or neuropsychological functioning (Govindaraju et al., 2004; Govind et al., 2010; Kirov et al., 2013a, b; Maudsley et al., 2015) as well as with gross neurologic outcomes (Glasgow Outcome Scale; GOS) at 3 months post injury (Marino et al., 2007). Post-acute or longer measurements of decreased NAA/Cho in the basal ganglia have also correlated with neuropsychological measures of psychomotor speed, motor scanning and attention (Ariza et al., 2004).
The prognostic utility of MRS in milder injuries (including concussions) has been primarily focused on youth and sports related injuries. In two studies of MRS in pediatric populations, serial spectra were collected at multiple time points (72 h or less, 14 days, and after symptom recovery), and no metabolic abnormalities were observed, with symptom resolution and cognitive functioning normalizing by day 14 (Maugans, Farley, Altaye, Leach, & Cecil, 2012). On the other hand, in post-acute measurements of a persistently symptomatic group, reductions in NAA/Cr and NAA/Cho were observed (Bartnik-Olson et al., 2014). In adult athletes, decreased NAA/Cr ratios observed through 30 days post injury, with a modest recovery by day 15, were reported, although self-report of symptoms resolved by day 3 (Vagnozzi et al., 2008). In another study, reduced Cr in the right dorsolateral prefrontal cortex was correlated with structural and functional imaging markers that seemingly underlie persistent postconcussive symptoms (Dean et al., 2015). Further, higher levels of white matter Cr in the early subacute period (<1 month) were predictive of executive function difficulties and emotional distress (Gasparovic et al., 2009). A later sub-acute (> 1 month) and chronic (> 6 months) assessment identified decreases in Cho/Cr in the thalamus and centrum semiovale, which correlated with cognitive measures of recognition and memory (George et al., 2014). Taken together, in both pediatric and adult studies, and across all severity ranges, when there are MRS abnormalities observed on acute studies, these abnormalities have predictive utility for not only gross neurologic outcomes but also deficits in neurocognitive functions. MRS examinations beyond the acute period have also shown utility in explaining the pathobiology of persistent cognitive and other behavioral deficits (Bartnik-Olson et al., 2014). Optimally, models to assess TBI will incorporate biomarkers from multiple sources including several neuroimaging modalities, MRS, and blood biomarkers (Kawata et al., 2018), all provided at the optimal time point after injury to enhance prognostic information, treatment decisions, and patient outcome (Manivannan et al., 2018). For example, blood biomarkers, CT and conventional MR are useful early after injury, whereas metabolite levels from MRS or other advanced imaging techniques such as DTI may provide better prognostic information measured subacutely.
Distinct injury mechanisms
TBI can result from a number of different mechanisms. In the sections below the application of MRS in non-accidental trauma (NAT) in children, sports-related concussion, and military TBI are reviewed. While these injury mechanisms share some common pathological features (e.g., metabolic dysfunction, white matter injury) with other forms of accidental trauma, they have biomechanically distinct features that influence their MRS signature and outcome.
Non-accidental trauma (NAT)
Neurotrauma caused by child abuse is commonly termed NAT (non-accidental trauma). NAT features complex injury mechanisms resulting from multiple types of axonal injury that can occur in the same brain including hypoxic-ischemic injury designated as vascular axonal injury and diffuse traumatic axonal injury resulting from acceleration-deceleration forces (i.e., shaken baby) (Dolinak & Reichard, 2006; Gill et al., 2009). Inflicted trauma to the brain is reported as the leading cause of death in children younger than 2 years of age. However, non-fatal injuries can leave the victim with a lifetime of neurologic deficits (Keenan et al., 2003; Theodore et al., 2005). Skeletal radiographs and CT serve as initial imaging approaches to the suspected victim of abusive trauma, as osseous injuries are second only to skin wounds as signs of child maltreatment (Pfeiffer et al., 2018). A follow-up MRI is often used to evaluate for the presence of suspected bridging vein thrombosis in patients with subdural hematoma, microhemorrhages, ischemic injury, or ligamentous neck injury, and can increase the specificity for the diagnosis of NAT (Colbert et al., 2010; Foerster et al., 2009; Hahnemann et al., 2015; Jacob et al., 2016; Suh et al., 2001).
MRS is also useful for evaluating brain injury after NAT and provides insights into brain neurometabolism that differ from accidental TBI. Since victims of abuse are usually younger compared to children who suffer accidental TBI; normal spectral changes associated with brain maturation need to be taken into account. NAT injuries also tend to be repetitive and not isolated events, further complicating the pathobiology if subsequent injuries occur during recovery. An immature brain may explain why the degree of neurological damage suffered in children after NAT is often greater than the apparent severity of trauma (Haseler et al., 1997). In addition, infants and young children have less mature airway protective reflexes thereby placing them at higher risk of respiratory failure as reflected in the higher rates of intubation after NAT compared to accidental TBI (Ichord et al., 2007). The presence of Lac in the brain spectrum is often attributed to impaired aerobic glycolysis and is both a specific and sensitive marker of hypoxic-ischemic injury (HII). In addition, Lac accumulation has been related to multiple pathophysiological changes associated with TBI including: excessive release of glutamate, disordered mitochondrial and oxidative metabolism and systemic responses to trauma (Hovda et al., 1992; Krishnappa et al., 1999; Prasad et al., 1994). Lac is found more often in brain spectra of children after NAT and in combination with reduced NAA is associated with poor outcome (Fig. 5; Aaen et al., 2010; Ashwal et al., 1997; Haseler et al., 1997; Makoroff et al., 2005). As in accidental TBI, reduced NAA is a key MRS finding after NAT since it reflects neuronal injury or dysfunction resulting from both traumatic and hypoxic-ischemic injury and as a result is more predictive of long-term outcome than lactate presence alone. As shown in one MRSI study, reductions in NAA/Cr ratios were more predictive than clinical findings such as retinal hemorrhages or lactate presence alone for long-term outcome prediction after NAT (Aaen et al., 2010).
Fig. 5.
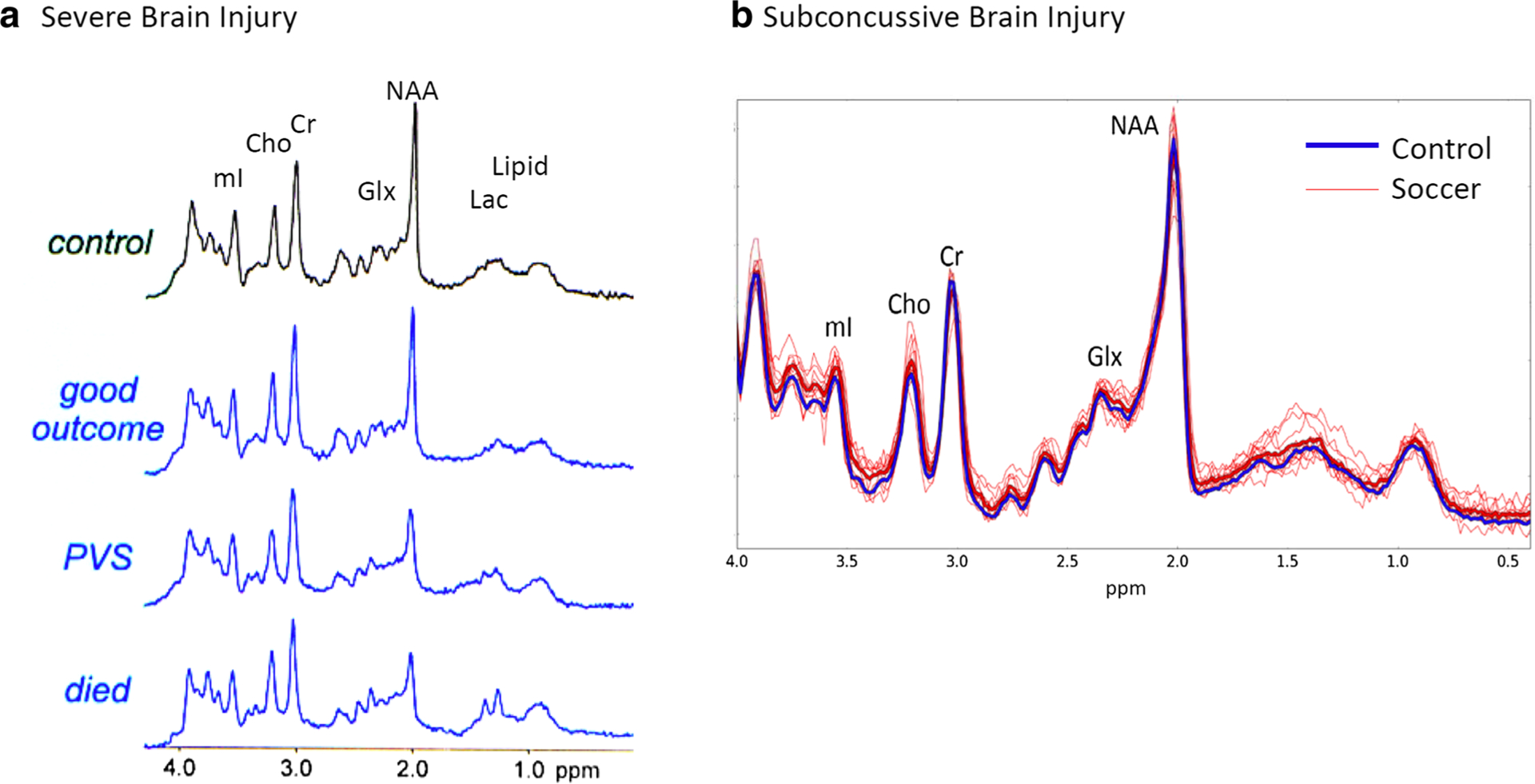
a. MRS is predictive of outcome in severe TBI (Mountford et al., 2010). Representative spectra acquired in healthy controls (top) and patients with severe traumatic brain injury based on outcome. The second from the top shows spectra with good outcome. Third from the top shows a patient in a persistent vegetative state (PVS) and the bottom spectrum shows a patient who died. Note the decrease in NAA, increase in lactate and glutamate/glutamine with greater severity of outcome. All spectra were acquired at 1.5 T using STEAM (TE = 30 ms) in the posterior cingulate. b. MRS is sensitive to subconcussive brain trauma (Koerte et al. 2015). Retired professional soccer players with no history of concussion, with the only exposure to repetitive head impacts due to heading of the soccer ball, and athletes with no exposure to head impacts were scanned using PRESS (TE = 30 ms) in the posterior cingulate area at 3 T. In heavy blue is the averaged spectrum of all controls and in red are the individual soccer players. There was a significant increase in choline and myo-inositol found in the soccer players showing spectroscopic changes due to subconcussive impacts
Sports-related TBI
With over 3.8 million concussions occurring in the United States alone, sports-related head injury is one of the leading causes of TBI (Langlois et al., 2006). Unlike single incidences of mild TBI discussed earlier, sports-related TBI are of a more repetitive nature with multiple head impacts over the course of months and years. For example, an average high school football player receives 652 hits to the head that exceed 15 G’s of force per season (Broglio et al., 2011). Similarly, longer and repeated deployments have exposed soldiers to repeated blasts (Eskridge et al., 2012). This is especially concerning as exposure to repetitive brain impacts can lead to the development of chronic traumatic encephalopathy (CTE), a neurodegenerative brain disorder that is marked by an accumulation of phosphorylated tau and leads to cognitive, psychological, memory, and mood problems later in life (McKee et al., 2009). CTE, a confirmed diagnosis only at post-mortem, has been identified in both athletes and soldiers, and has been shown to be evident even in repetitive subconcussive impacts (Stern et al., 2013). MRS is well suited for examining the effects of these potential impacts, even at a subconcussive level given its high sensitivity to injury (Fig. 4B; Alosco et al., 2019; Chamard et al., 2013; Poole et al., 2014, 2015). The recent decade has seen an increased interest in using MRS for the evaluation of sports-related head injury in the acute, subacute, and chronic settings of concussions.
As described earlier, the studies of sports-related injury by Vagnozzi et al. (Vagnozzi et al., 2010, 2013, 2008) were seminal in describing reductions of NAA/Cr ratios post-concussion and subsequent recovery. Similar findings of reductions in NAA/Cr in the motor and dorsolateral prefrontal cortex have also been shown along with changes in Glu with the use of short-echo MRS (Henry et al., 2010, 2011). Using 3D MRSI, another study also found reductions in NAA in the corpus callosum one to three weeks post injury (Johnson et al., 2012a, b). In that same time frame, Friedman et al. (2017; Friedman et al., 2017) used MEGA-PRESS to examine GABA levels and found increased GABA in the frontal lobe but decreased in the posterior cingulate in children with sports-related concussion. Other studies have also used MEGA-PRESS to access collegiate athlete’s mid-season but did not find changes in GABA (Tremblay et al., 2014; Lefebvre et al., 2018). Manning et al. (Manning et al., 2017) examined male hockey players within 24–72 h of injury and 3 months post-concussion and found decreased Cho in the prefrontal white matter only at the 3 month time point.
In a pediatric population of athletes, Bartnik-Olson et al. (Bartnik-Olson et al., 2014) used 3D MRSI and reported decreases in NAA/Cr and Cho in the corpus callosum and in parietal white matter at 2–12 months post injury. Also at the subacute stage, namely 6 months to one year post-injury, there is also gender differences reported. For example, Henry et al. (Henry et al., 2011) found increased mI in the motor cortex in male athletes whereas Charmard et al. (Chamard et al., 2013) found decreased amounts of ml in both the motor cortex and hippocampus.
Other studies focused upon subconcussive injury by utilizing a study design where athletes were scanned prior to the start of the season and then again at the end of the season. In these studies, athletes were not necessarily concussed at this time period, indicating that any exposure to head injury would likely be at a subconcussive level. Poole et al. (Poole et al., 2014) studied the dorsolateral prefrontal cortex (DLPC) and primary motor cortex at pre-, mid-, and postseason time points and found that relative to non-contract sports controls, football players exhibited decreased Cr and mI in the DLPFC and Glx, Cr, Cho in the motor cortex. A follow up study including head impact telemetry measures showed that in the DLPFC, the number of 60 G hits inversely correlated with Cr and mI concentrations, and hit metrics predicted NAA and Cho changes. In the motor cortex, duration of exposure was the strongest predictor of Glx and that it correlated negatively with number of hits, and Cr positively correlated with total number of hits experienced in the previous week. Chamard et al. (Chamard et al., 2013) also found significant decreases in NAA/Cr in the corpus callosum over the course of a season in female ice hockey players without a history of concussion, but not in male ice hockey players.
Given the concern with CTE and its impact on retired professional athletes, several studies have focused on the effects of years of repetitive head injury by examining retired athletes and controls. De Beaumont et al. (De Beaumont et al., 2013) showed reduced Glu in the motor cortex in former collegiate athletes. Lin et al. (Lin et al., 2015), however, showed increased Glu but in the posterior cingulate, as well as increases in Cho in a preliminary study of retired professional athletes. This study was then followed up in a larger cohort of retired NFL athletes where the primary finding was a decrease in NAA in the parietal white matter and reductions of Cr (Alosco et al., 2019). In a study of Australian-rules rugby players, the most significant finding was a reduction on glutathione and mI in the posterior cingulate (Gardner et al., 2014). Chronic exposure to subconcussive injuries has also been explored in a study of retired professional soccer players who never reported a concussion during their career but were exposed to subconcussive impacts to the head through heading of the soccer ball (Koerte et al., 2015). This study found increases in Cho and mI in the posterior cingulate in athletes with a history of heading compared to those that did not (Fig.4 b). The increase in mI also correlated with the self-reported number of headings of the soccer ball.
MRS studies of sports-related head injury are consistent with other types of brain injury with reductions in NAA, increases in choline and glutamate, and reductions in creatine. The changes observed in studies of subconcussive injury demonstrate the sensitivity of MRS measures. In addition, they also provide insight into potential mechanisms of injury such as neuroinflammation. While there appears to be a cumulative effect of repetitive injuries, the patterns observed are not as simple as cumulative changes in brain metabolites. While NAA changes are found, it is not as pronounced as would have been expected. Changes in mI point to other changes that occur in the brain, perhaps to the impact on glial cells in addition to the neuronal injury reflected in NAA reductions (Kierans et al. 2014; Ashwal et al. 2004). As the literature grows in this area of research, MRS will play an important role in providing insight into the underlying pathophysiological changes of repetitive brain injury.
Military TBI
There has been a surprising paucity of studies of military-related TBI. Hetherington et al. (Hetherington et al., 2014) examined 25 veterans at least 1 year after exposure to 1 or more blasts and compared them to age and gender matched healthy controls with no history of brain injury at 7 T. The results of the study showed that veterans had significantly decreased NAA/Cr and NAA/Cho ratios (possible increased Cho) similar to sports-related head injury. One of the interesting findings, particularly for a military cohort, was there was no significant difference between TBI subjects with and without post-traumatic stress, anxiety, depression, or alcohol dependence, which are the major comorbidities in military TBI. This is surprising given that MRS studies in military subjects with PTSD have shown changes in hippocampus such as decreased NAA and changes in Cho (Brown et al., 2003; Freeman et al., 1998; Kimbrell et al., 2005; Schuff et al., 2001) that may have shown a compounding effect. Mariano et al. (Mariano et al. 2017), examined several different cohorts of military veterans with and without both mTBI and PTSD and found differences in the regions of the spectrum including Glu, GABA, Cr, and Lac. Classifiers based on these features exhibited correct classification rates of 80% or better in cross-validation demonstrating that MRS has the potential to be used to differentiate between the different conditions.
Considerations for study design, multisite studies, and recommendations
The following sections outline a number of important considerations when incorporating MRS into TBI studies. In addition, opportunities for multisite MRS studies and the role of the ENIGMA consortium are also discussed. Finally, we leave the reader with a recommended MRS protocol that can be incorporated into clinical and research neuroimaging protocols, providing an additional biomarker of injury, repair/recovery, and/or prognosis.
Choice of brain region
A variety of factors determine the choice of investigated region. Chief among them are the hypothesis, level of available technical expertise, investigators’ own experience and interpretation of the supporting literature, and the question driving the application of spectroscopy. Below we list the most commonly studied ROIs, by acquisition type, i.e., SVS and MRSI.
The most common SVS voxel placement reflects the fact that preclinical and postmortem studies have identified white matter diffuse axonal injury as the hallmark site of TBI injury (Su & Bell, 2015). Most often, the targeted regions have been frontal/prefrontal white matter (Maugans et al., 2012; Schranz et al., 2018; Sivák et al., 2014; Vagnozzi et al., 2010, 2008; Walz et al., 2008; Dhandapani et al. 2014), splenium (Cecil et al., 1998; Gasparovic et al., 2009; Johnson et al., 2012a, b) and genu (Johnson et al., 2012a, b) of the corpus callosum. SVS voxel placement in gray matter is also common, with most studies focusing on the cingulate gyrus (Koerte et al., 2015; Lin et al., 2012; Louis et al., 2017), dorsolateral prefrontal cortex (Dean et al., 2015; Henry et al., 2010; Poole et al., 2014; Bari et al., 2019) and primary motor cortex (Tremblay et al., 2014). Two-dimensional spectral matrices are usually positioned to include prominent white matter regions, often either at the level of the corpus callosum, or slightly superior at the level of the centrum semiovale and corona radiata tracts (Gasparovic et al., 2009; Yeo et al., 2011; Lawrence et al., 2019; Mamere et al., 2009; Narayana et al., 2015; Sarmento et al., 2009); or through the temporal lobe for the study of the hippocampus (Hetherington et al., 2014; Kontos et al., 2017). Three-dimensional volumes are usually centered around the corpus callosum to cover these regions and the deep gray matter (George et al., 2014; Kirov et al., 2007; Kirov et al., 2013a, b; Sours et al., 2015; Holshouser et al., 2019; Sours et al., 2015), while whole-brain coverage techniques by default cover most of the supratentorial brain (Govind et al., 2010; Maudsley et al., 2015; Widerström-Noga et al., 2016; Maudsley et al., 2017; Babikian et al., 2018; Dennis et al., 2018). While there is no literature consensus on which exact brain region(s) to study with either SVS or MRSI, recent evidence suggests that for maximum sensitivity to white matter injury, location is less important than the sensitivity of the method (Davitz et al., 2019). Specifically, it was found that NAA deficits in patients would have been detected in white matter regions, as long as the MRS technique yielded coefficients of variation of less than 8%, a feat achievable with large single-voxels, or global MRSI approaches, such as linear regression over 2D or 3D volumes (Davitz et al., 2019).
Quantitation methods: ratios vs. absolute quantification
As discussed earlier, results can be obtained either as metabolic ratios (e.g., NAA/Cr) or as individual metabolites following normalization in the form of institutional or biochemical units (e.g., millimoles per kilogram of wet weight). A decision between the two requires knowledge of the mean and standard deviation of both the numerator and denominator of the proposed ratio (Hoch et al., 2017). This information can be obtained from a previous study, which used absolute quantification (Hoch et al., 2017). Therefore, we suggest that exploratory studies be carried out with absolute quantification (water referencing or phantom replacement), so that these values can be obtained before a decision is made regarding whether to use ratios. When considering literature values in TBI as the basis for this decision, the referenced and the planned study need to be matched in as many technical parameters as possible. In addition, comparisons should take into account patient age, sex, time from injury, studied region, and relative gray- and white matter tissue volume.
Control subjects
The definition of control subjects must also be considered. While controls are defined as un-affected individuals, prior exposure to trauma more generally may impact outcomes. Similarly, consequences of injury such as not being able to participate in normal daily activities can impact outcomes. Therefore, some studies have opted to use an orthopedic injury (OI) cohort as the control group, as it shares traumatic event exposure and the associated recovery, can be recruited using a similar recruitment pathway (i.e., presentation in emergency rooms), as well as other factors that are non-specific to brain injury but mediate recovery (Yeates et al., 2017, 2009). However, this practice has been called into question as some studies have shown no clear advantage over community controls (Beauchamp et al., 2017; Mathias et al., 2013). Additional considerations should be taken into account when acquiring MRS in children and adolescents after TBI. One of the more important considerations is the necessity to compare metabolite levels or ratios to normal age-matched control data as metabolite concentrations differ with developmental age (see above). In addition, control studies are not as easy to obtain in a pediatric population, particularly in younger children who may require sedation. As a result, some MRS studies use normative data acquired from children undergoing MRI for medical but non-neurological reasons or when the MRI study was interpreted as normal by a radiologist.
Time after injury and injury severity
Early MRS studies have combined TBI patients of all severities with widely variable time post injury intervals (from hours to years) into single analyses. We now appreciate that there are dynamic changes in the acute and sub-acute periods following potentially all severity ranges, and potentially ongoing changes at later time points with more severe injuries. Thus controlling for some of the variation in evolving brain chemistry over time by grouping patients into defined time-post injury intervals is critical to avoid additional noise to a study population that is already quite heterogeneous to begin with. Similar to the above reasons, separating uncomplicated mild injuries from more severe injuries will also assist to further reduce confounds in the data, as the nature and/or the time course of biochemical changes sensitive to MRS may be variable in these two groups.
Choice of outcome measures
MRS can be useful as both a diagnostic tool as well as a prognostic measure of long-term real-world outcomes. It is important to consider and to incorporate the NIH Common Data Elements (CDE) recommendation in MRS studies that are designed to address its utility as a prognostic tool (Duhaime et al., 2012; Haacke et al., 2010). While the Glasgow Outcome Scale Extended (GOSE) is the only CDE core outcome measure for TBI, our recommendation is to avoid using only gross outcome measures, unless the population studied is severely injured. Rather, focusing on the following outcome categories will provide a broad clinical and real-world perspective on functional outcomes for all age groups, injury types, and most injury severity categories: 1) overall intellectual skills (shortened screening tools included), 2) some composite measure of cognitive efficiency from standardized measures, for example from the NIH Toolbox (e.g., processing speed, attention/concentration, executive functioning) and memory (e.g., encoding), 3) measure of quality of life (NIH Toolbox or other), 4) day-to-day adaptive skills, 5) psychiatric morbidity (e.g., depression, anxiety), and 6) return to previous activities. Additional areas of interest may be considered depending on the targeted focus of the study, such as sleep, ADHD, academic functioning, and vocational functioning. In all of the above cognitive, academic, and psychiatric outcome assessments, premorbid functional levels are critical to establish via records, interviews, or formal questionnaires.
Multisite studies and ENIGMA
While concussion is a highly prevalent condition, recruitment is often challenging. In addition to typical difficulties of recruiting research participation and study compliance, the heterogeneity of concussion and study populations means that researchers are forced to decide between either accepting a high degree of heterogeneity in the sample (i.e., large age range, including prior history of head injury, various presentations times after injury, etc.) or risking a very small sample size when limiting population variance. While these challenges still exist, including multiple sites can be a solution to increasing the sample size while maintaining some degree of homogeneity in participant demographics.
Multisite MRS studies, however, have their own challenges. In addition to different analysis approaches, each of the three main vendors have made different decisions regarding the trade-offs in developing pulse sequences. Even with the well-established PRESS sequence, each of the vendors has chosen different parameters that impact the quality and localization of the PRESS signal. The result is that quantified metabolite levels are not directly comparable between different scanner vendors. There has been some effort to develop universal sequences that have the exact same excitation and refocusing pulses across all vendors, for example with the MRSI sequence (Sabati et al., 2015), and a recent consensus report recommends a semi-LASER acquisition for SVS measurements (Wilson et al., 2019). However, these are currently not provided by the instrument manufacturers and they require access to research pulse-sequences.
Additional challenges to maintaining uniformity of data acquisition include training of technicians, data quality control, and maintenance of instrumentation performance. A simple voxel replacement study shows the challenges in voxel placement replication without the additional challenges of different technicians and visualization software packages (Bai et al., 2017). One approach is to limit possible variance by not allowing rotations beyond placement on an AC-PC aligned T1-weighted image, although this limits the localization of information. However, an additional advantage of studying white matter, as in TBI, is that misregistration errors are less costly as partial volume errors would be limited to different white matter regions, and not to different tissue type or CSF.
When developing multisite studies, one option is to use human phantoms and scan the same individuals at all sites to at least be able to quantify and understand the differences in metabolite quantification. This approach was recently used in a 5-site child and adolescent concussion study (Yeates et al., 2017). Nonetheless, at the present time, MRS findings from multisite studies have been limited (Vagnozzi et al., 2010), although a number of ongoing studies are acquiring MRS. One such study is the Diagnostics, Imaging, and Genetics Network for the Objective Study and Evaluation of Chronic Traumatic Encephalopathy (DIAGNOSE-CTE; http://diagnosecte.com/) funded by the National Institutes of Health. This study is currently enrolling former NFL and collegiate football players and healthy controls across four sites (Boston University/Harvard Medical School, New York University, Mayo Clinic, and Cleveland Clinic) and it includes not only MRS but also neurological and psychological evaluation, structural and functional MRI, blood and CSF samples, and PET scans with the goal of identifying multimodal biomarkers of CTE that will allow for in-life diagnosis and treatment monitoring. Of further note, the Veteran’s Administration has the TRACTS study (Translational Research Center for TBI and Stress Disorders; https://www.boston.va.gov/research/Translational_Research_Center_for_TBI_and_Stress_Disorders_TRACTS.asp), which focuses on military veterans where MRS data is also being collected. The Chronic Effects of Neurotrauma Consortium (https://www.limbic-cenc.org/), funded by the Department of Defense, has also collected some MRS data in several of their sites, but findings have yet to be published.
ENIGMA
The ENIGMA (Enhancing NeuroImaging Genetics through Meta-Analysis) Brain Injury group was formed in the fall of 2016 with the overarching goal of creating a collaborative framework to address TBI-related questions and objectives. At present there are six subgroups representing specific clinical conditions (pediatric moderate/severe TBI, military TBI and concussion, sports-related head injury, adult moderate/severe TBI, acute emergency department mild TBI, and intimate partner violence) and two subgroups focusing on emerging imaging methodologies (MRS and arterial spin labeling). The immediate goals of the ENIGMA Brain Injury working groups are to conduct analyses on multisite datasets to look for robust effects across samples in addition to raising new hypotheses. Specific details about the goals and approach of the ENIGMA consortium and the individual subgroups can be found in companion articles in this issue.
Consensus/recommendations and an ENIGMA approach
The foregoing discussion illustrates that MRS is sensitive to brain metabolic changes that occur in some specific TBI contexts in some specific brain regions. However the discussion also illustrates that TBI is clinically heterogeneous and that existing research studies involving MRS have included small numbers of human subjects and only a few specific subtypes of the TBI. Using an ENIGMA approach could potentially enhance knowledge by extending studies to larger numbers of TBI patients having a wide variety of different TBI subtypes at a variety of times following TBI. In addition, an ENIGMA design could provide normative MRS data over a range of subject ages, gender, race, etc. which would be helpful in definitively defining the level of metabolic abnormality that is exhibited in TBI.
The foregoing discussion also illustrates that MRS technology is continuing to evolve. Frequently, the goal of many studies that use MRS to evaluate a specific brain condition is to demonstrate that some new form of MRS technology is sensitive to the brain condition. While this is useful for propelling technology development forward, use of such new technologies is not necessarily optimal for an ENIGMA design that is to be deployed across many centers because not all centers have access to new MRS technology or the expertise to implement it. For this reason it seems prudent to propose an ENIGMA design that focuses on a simple, easy, fast, low cost MRS technology that could be used in a very large number of subjects including those who are patients and are having their TBI evaluated for routine clinical purposes by MRI. The simplest, fastest, most widely available form of MRS technology is single voxel MRS. It is available as a product on all commercial MRI scanners and is frequently used in routine clinical contexts such as brain cancer, epilepsy, developmental disorders and TBI (in some centers). If single voxel MRS is considered as a candidate for an ENIGMA-inspired MRS study of TBI, it is important to consider that not all brain regions can be evaluated and there should be consistency regarding which brain region is sampled. Here it is noteworthy that many studies that have used endpoints derived from imaging, neuropsychology and electrophysiology have suggested that although the initial physical impact experienced by the brain can be heterogeneous in terms of magnitude and direction of the impact vector, a central feature of TBI seems to be white matter abnormality. Perhaps the best example of this is the fact that a variety of different TBI situations lead to diffuse axonal injury in the large white matter tracts of the brain. For this reason we recommend that MRS studies use a single frontal matter white matter region across subjects for an initial ENIGMA study.
Based on the reasoning given above the minimal technical recommendations for an ENIGMA MRS study of TBI are as follows:
Field Strength: 3.0 T
MRI Manufacturer: Any (though needs to be documented)
Pulse sequence: Although a recent consensus report recommends a semi-LASER acquisition for SVS measurements (Wilson et al., 2019), this sequence is not widely available across all platforms. As such, at this time we recommend a water-suppressed PRESS SVS sequence using the manufacturer’s commercially available standard pulse sequence software.
TR/TE/number of averages (NAV): 2000/minimum (30–35 ms)/128.
Selected volume size and location: minimum of 6 cm3 placed in the left frontal white matter (Fig. 6), avoiding gray matter as much as possible. In case of an MRI-visible abnormality (e.g. micro-hemorrhage) within this region, voxel can be placed at the same location in the right hemisphere. A number of studies report significant findings in the basal ganglia/thalamus (Holshouser et al., 2019; Kierans et al., 2014) or supraventricular gray matter (anterior or posterior cingulate gyrus; (Lin et al., 2012; Yeo et al., 2011), thus an additional voxel placed in gray matter may have added benefit depending on the study design.
Other instructions: Perform an identical non-water-suppressed acquisition (matched TR and TE) with at least 8 averages for quantification.
Ancillary MRI measurements: Provide any standard T1w, FLAIR and SWI studies that are done for clinical purposes in the same study as the MRS acquisition.
Fig. 6.
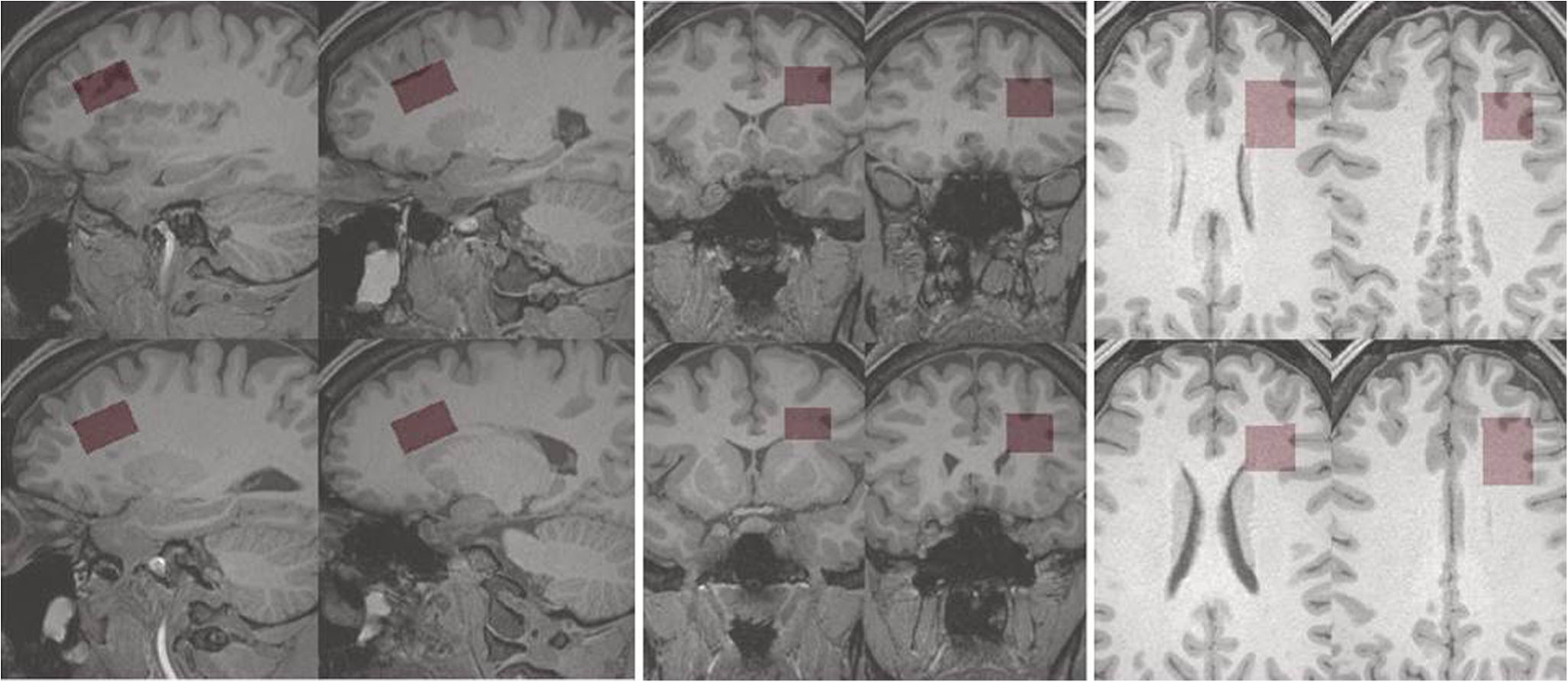
Recommended frontal white matter MRS voxel location. The recommended location, angulation and dimensions of a frontal white in matter MRS voxel is shown in red highlight, overlaid on grayscale sections through a T1-weighted 3-dimensional MR volume image. Displays of the voxel on four representative sagittal, axial and coronal sections of the T1-weighted volume image are provided. The voxel’s dimensions are 2.0 × 2.5 × 1.5 cm = 7.5 cm3. Angulation and dimensions, which are expected to depend on subject’s anatomy and head position, were adjusted for this particular subject to achieve maximal white matter content and a volume as close to 8.0 cm3 as possible
These recommended parameters will produce sufficient information to determine the water-referenced white matter concentrations of about six brain metabolites in an additional time for MRS of less than 4.0 min. A more detailed step-by-step protocol can be found in the online Supplemental Information. As argued above, if this minimalist procedure could be routinely run on many TBI patients it would be possible to evaluate how sensitive MRS-based white matter neurometabolite assessment is to a variety of different subtypes and instances of TBI. Eventually such measurements could be extended to predicting how MRS-based white matter neurometabolite assessment might be used to predict clinical (neuropsychological and behavioral) outcome, and recovery. As indicated above, obtaining normative MRS data would be important. For this, the present authors suggest that ENIGMA groups who study other brain conditions consider adding the above-described MRS measurement to their studies. The recommended protocols of a short-echo single voxel spectroscopy acquisition in white matter with reference water scan would benefit ENIGMA groups such as sports-related TBI, military TBI, and intimate partner violence studies.
Summary
The goal of this paper was to provide readers with a comprehensive overview of the use of MRS in TBI and how it can be used both clinically and in future research studies. MRS is highly complementary to other MRI modalities by providing biomarkers of tissue injury such as NAA (neuronal health), choline (membrane turnover), creatine (brain energetics), glutamate (excitatory neurotransmission), and lactate (hypoxia). Current MRS methods such as single voxel spectroscopy and chemical shift imaging are clinically available across all MR manufacturers where an additional scan of 5–10 min can provide a wealth of information that can assist with diagnosis and prognosis of injury. There still remains the need for large, prospective clinical trials to confirm the clinical utility of MRS in brain injury. The considerations and guidelines provided in this paper are intended to provide clinicians with guidance on how to implement MRS in their own studies and/or clinical practice.
Supplementary Material
Acknowledgements
K99NS096116 to Dr. Dennis. R01NS097494, R21NS112853, P41EB017183, pilot grant funding from the Alzheimer’s Disease Center at NYU Langone (NIH award P30AG008051) to Dr. Kirov. R01EB016064 to Dr. Maudsley. U54 EB020403, R01 MH116147, R56 AG058854, P41 EB015922, R01 MH111671 to Dr. Thompson. R01HD061504 to Drs. Dennis, Babikian, and Alger. W81XWH-15-1-0412, U01NS093334-02, R01NS100952-01A1 to Dr. Lin.
Footnotes
Conflict of interest Dr. Alger is the owner of NeuroSpectroScopics LLC. Dr. Lin is the co-founder of BrainSpec and a consultant for Moncton MRI. Dr. Thompson received partial research support from Biogen, Inc. for research unrelated to this manuscript.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s11682-020-00330-6) contains supplementary material, which is available to authorized users.
References
- Aaen GS, Holshouser BA, Sheridan C, Colbert C, McKenney M, Kido D, & Ashwal S (2010). Magnetic resonance spectroscopy predicts outcomes for children with nonaccidental trauma. Pediatrics, 125(2), 295–303. [DOI] [PubMed] [Google Scholar]
- Alessandri B, Doppenberg E, Zauner A, Woodward J, Choi S, & Bullock R (1999). Evidence for time-dependent glutamate-mediated glycolysis in head-injured patients: A microdialysis study. Acta Neurochirurgica. Supplement, 75, 25–28. [DOI] [PubMed] [Google Scholar]
- Alosco ML, Tripodis Y, Rowland B, Chua AS, Liao H, Martin B, Jarnagin J, Chaisson CE, Pasternak O, Karmacharya S, Koerte IK, Cantu RC, Kowall NW, McKee AC, Shenton ME, Greenwald R, McClean M, Stern RA, & Lin A (2019). A magnetic resonance spectroscopy investigation in symptomatic former NFL players. Brain Imaging and Behavior. 10.1007/s11682-019-00060-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariza M, Junqué C, Mataró M, Poca MA, Bargalló N, Olondo M, & Sahuquillo J (2004). Neuropsychological correlates of basal ganglia and medial temporal lobe NAA/Cho reductions in traumatic brain injury. Archives of Neurology, 61(4), 541–544. [DOI] [PubMed] [Google Scholar]
- Ashwal S, Holshouser BA, Shu SK, Simmons PL, Perkin RM, Tomasi LG, Knierim DS, Sheridan C, Craig K, Andrews GH, & Hinshaw DB (2000). Predictive value of proton magnetic resonance spectroscopy in pediatric closed head injury. Pediatric Neurology, 23(2), 114–125. [DOI] [PubMed] [Google Scholar]
- Ashwal S, Holshouser BA, Tomasi LG, Shu S, Perkin RM, Nystrom GA, & Hinshaw DB Jr. (1997). 1H-magnetic resonance spectroscopy-determined cerebral lactate and poor neurological outcomes in children with central nervous system disease. Annals of Neurology, 41(4), 470–481. [DOI] [PubMed] [Google Scholar]
- Ashwal S, Holshouser B, Tong K, Serna T, Osterdock R, Gross M, & Kido D (2004). Proton MR spectroscopy detected glutamate/glutamine is increased in children with traumatic brain injury. Journal of Neurotrauma, 21(11), 1539–1552. [DOI] [PubMed] [Google Scholar]
- Babikian T, Alger JR, Ellis-Blied MU, Giza CC, Dennis E, Olsen A, Mink R, Babbitt C, Johnson J, Thompson PM, & Asarnow RF (2018). Whole brain magnetic resonance spectroscopic determinants of functional outcomes in Pediatric moderate/severe traumatic brain injury. Journal of Neurotrauma, 35(14), 1637–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babikian T, Freier MC, Ashwal S, Riggs ML, Burley T, & Holshouser BA (2006). MR spectroscopy: Predicting long-term neuropsychological outcome following pediatric TBI. Journal of Magnetic Resonance Imaging: JMRI, 24(4), 801–811. [DOI] [PubMed] [Google Scholar]
- Babikian T, Marion SD, Copeland S, Alger JR, O’Neill J, Cazalis F, Mink R, Giza CC, Vu JA, Hilleary SM, Kernan CL, Newman N, & Asarnow RF (2010). Metabolic levels in the corpus callosum and their structural and behavioral correlates after moderate to severe pediatric TBI. Journal of Neurotrauma, 27(3), 473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai X, Harris AD, Gong T, Puts NAJ, Wang G, Schär M, Barker PB, & Edden RAE (2017). Voxel placement precision for GABA-edited magnetic resonance spectroscopy. Open Journal of Radiology, 7(1), 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker EH, Basso G, Barker PB, Smith MA, Bonekamp D, & Horská A (2008). Regional apparent metabolite concentrations in young adult brain measured by (1)H MR spectroscopy at 3 tesla. Journal of Magnetic Resonance Imaging: JMRI, 27(3), 489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari S, Svaldi DO, Jang I, Shenk TE, Poole VN, Lee T, Dydak U, Rispoli JV, Nauman EA, & Talavage TM (2019). Dependence on subconcussive impacts of brain metabolism in collision sport athletes: An MR spectroscopic study. Brain Imaging and Behavior, 13(3), 735–749. [DOI] [PubMed] [Google Scholar]
- Barker PB, Bizzi A, De Stefano N, Gullapalli R, & Lin DDM (2010). Clinical MR spectroscopy: Techniques and applications. Cambridge University Press. [Google Scholar]
- Bartnik-Olson BL, Holshouser B, Wang H, Grube M, Tong K, Wong V, & Ashwal S (2014). Impaired neurovascular unit function contributes to persistent symptoms after concussion: A pilot study. Journal of Neurotrauma, 31(17), 1497–1506. [DOI] [PubMed] [Google Scholar]
- Beauchamp MH, Landry-Roy C, Gravel J, Beaudoin C, & Bernier A (2017). Should Young children with traumatic brain injury be compared with community or orthopedic control participants? Journal of Neurotrauma, 34(17), 2545–2552. [DOI] [PubMed] [Google Scholar]
- Bednařík P, Moheet A, Deelchand DK, Emir UE, Eberly LE, Bareš M, Seaquist ER, & Öz G (2015). Feasibility and reproducibility of neurochemical profile quantification in the human hippocampus at 3 T. NMR in Biomedicine, 28(6), 685–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biasca N, & Maxwell WL (2007). Minor traumatic brain injury in sports: A review in order to prevent neurological sequelae. Progress in Brain Research, 161, 263–291. [DOI] [PubMed] [Google Scholar]
- Bigler ED (2013). Neuroimaging biomarkers in mild traumatic brain injury (mTBI). Neuropsychology Review, 23(3), 169–209. [DOI] [PubMed] [Google Scholar]
- Bottomley PA (1987). Spatial localization in NMR spectroscopy in vivo. Annals of the New York Academy of Sciences, 508, 333–348. [DOI] [PubMed] [Google Scholar]
- Brand A, Richter-Landsberg C, & Leibfritz D (1993). Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Developmental Neuroscience, 15(3–5), 289–298. [DOI] [PubMed] [Google Scholar]
- Broglio SP, Eckner JT, Martini D, Sosnoff JJ, Kutcher JS, & Randolph C (2011). Cumulative head impact burden in high school football. Journal of Neurotrauma, 28(10), 2069–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne KD, Chen XH, Meaney DF, & Smith DH (2011). Mild traumatic brain injury and diffuse axonal injury in swine. Journal of Neurotrauma, 28(9), 1747–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S, Freeman T, Kimbrell T, Cardwell D, & Komoroski R (2003). In vivo proton magnetic resonance spectroscopy of the medial temporal lobes of former prisoners of war with and without posttraumatic stress disorder. The Journal of Neuropsychiatry and Clinical Neurosciences, 15(3), 367–370. [DOI] [PubMed] [Google Scholar]
- Bullock R, Zauner A, Woodward JJ, Myseros J, Choi SC, Ward JD, Marmarou A, & Young HF (1998). Factors affecting excitatory amino acid release following severe human head injury. Journal of Neurosurgery, 89(4), 507–518. [DOI] [PubMed] [Google Scholar]
- Cady EB, Penrice J, Amess PN, Lorek A, Wylezinska M, Aldridge RF, Franconi F, Wyatt JS, & Reynolds EO (1996). Lactate, N-acetylaspartate, choline and creatine concentrations, and spin-spin relaxation in thalamic and occipito-parietal regions of developing human brain. Magnetic Resonance in Medicine, 36(6), 878–886. [DOI] [PubMed] [Google Scholar]
- Cecil KM, Hills EC, Sandel ME, Smith DH, McIntosh TK, Mannon LJ, Sinson GP, Bagley LJ, Grossman RI, & Lenkinski RE (1998). Proton magnetic resonance spectroscopy for detection of axonal injury in the splenium of the corpus callosum of brain-injured patients. Journal of Neurosurgery, 88(5), 795–801. [DOI] [PubMed] [Google Scholar]
- Chamard E, Lassonde M, Henry L, Tremblay J, Boulanger Y, De Beaumont L, & Théoret H (2013). Neurometabolic and micro-structural alterations following a sports-related concussion in female athletes. Brain Injury: [BI], 27(9), 1038–1046. [DOI] [PubMed] [Google Scholar]
- Clark JF, Doepke A, Filosa JA, Wardle RL, Lu A, Meeker TJ, & Pyne-Geithman GJ (2006). N-acetylaspartate as a reservoir for glutamate. Medical Hypotheses, 67(3), 506–512. [DOI] [PubMed] [Google Scholar]
- Cohen BA, Inglese M, Rusinek H, Babb JS, Grossman RI, & Gonen O (2007). Proton MR spectroscopy and MRI-volumetry in mild traumatic brain injury. AJNR. American Journal of Neuroradiology, 28(5), 907–913. [PMC free article] [PubMed] [Google Scholar]
- Colbert CA, Holshouser BA, Aaen GS, Sheridan C, Oyoyo U, Kido D, & Ashwal S (2010). Value of cerebral microhemorrhages detected with susceptibility-weighted MR imaging for prediction of long-term outcome in children with nonaccidental trauma. Radiology, 256(3), 898–905. [DOI] [PubMed] [Google Scholar]
- Das D, Coello E, Schulte RF, & Menze BH (2017). Quantification of metabolites in magnetic resonance spectroscopic imaging using machine learning. In Descoteaux M, Maier-Hein L, Franz AJP, Collins D, & D. S. (Eds.), Medical Image Computing and Computer Assisted Intervention − MICCAI 2017. (Vol. 10435). Springer. [Google Scholar]
- Davitz MS, Gonen O, Tal A, Babb JS, Lui YW, & Kirov II (2019). Quantitative multivoxel proton MR spectroscopy for the identification of white matter abnormalities in mild traumatic brain injury: Comparison between regional and global analysis. Journal of Magnetic Resonance Imaging: JMRI. 10.1002/jmri.26718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean PJ, Sato JR, Vieira G, McNamara A, & Sterr A (2015). Multimodal imaging of mild traumatic brain injury and persistent post-concussion syndrome. Brain and Behavior, 5(1), 45–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Beaumont L, Tremblay S, Henry LC, Poirier J, Lassonde M, & Théoret H (2013). Motor system alterations in retired former athletes: The role of aging and concussion history. BMC Neurology, 13, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deelchand DK, Kantarci K, & Öz G (2018). Improved localization, spectral quality, and repeatability with advanced MRS methodology in the clinical setting. Magnetic Resonance in Medicine, 79(3), 1241–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis EL, Babikian T, Alger J, Rashid F, Villalon-Reina JE, Jin Y, Olsen A, Mink R, Babbitt C, Johnson J, Giza CC, Thompson PM, & Asarnow RF (2018). Magnetic resonance spectroscopy of fiber tracts in children with traumatic brain injury: A combined MRS - diffusion MRI study. Human Brain Mapping. 10.1002/hbm.24209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis EL, Caeyenberghs K, Asarnow RF, Babikian T, Bartnik-Olson B, Bigler ED, et al. (2019). Brain imaging in Young brain-injured patients: A coordinated effort towards individualized predictors from the ENIGMA Pediatric msTBI group. Brain Imaging and Behavior, Under Review(Special Issue). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhandapani S, Sharma A, Sharma K, & Das L (2014). Comparative evaluation of MRS and SPECT in prognostication of patients with mild to moderate head injury. Journal of Clinical Neuroscience : Official Journal of the Neurosurgical Society of Australasia, 21(5), 745–750. [DOI] [PubMed] [Google Scholar]
- Dolinak D, & Reichard R (2006). An overview of inflicted head injury in infants and young children, with a review of beta-amyloid precursor protein immunohistochemistry. Archives of Pathology & Laboratory Medicine, 130(5), 712–717. [DOI] [PubMed] [Google Scholar]
- Duhaime A-C, Holshouser B, Hunter JV, & Tong K (2012). Common data elements for neuroimaging of traumatic brain injury: Pediatric considerations. Journal of Neurotrauma, 29(4), 629–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebel A, Govindaraju V, & Maudsley AA (2003). Comparison of inversion recovery preparation schemes for lipid suppression in 1H MRSI of human brain. Magnetic Resonance in Medicine, 49(5), 903–908. [DOI] [PubMed] [Google Scholar]
- Eskridge SL, Macera CA, Galarneau MR, Holbrook TL, Woodruff SI, MacGregor AJ, Morton DJ, & Shaffer RA (2012). Injuries from combat explosions in Iraq: Injury type, location, and severity. Injury, 43(10), 1678–1682. [DOI] [PubMed] [Google Scholar]
- Eylers VV, Maudsley AA, Bronzlik P, Dellani PR, Lanfermann H, & Ding XQ (2016). Detection of Normal aging effects on human brain metabolite concentrations and microstructure with whole-brain MR spectroscopic imaging and quantitative MR imaging. AJNR. American Journal of Neuroradiology, 37(3), 447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foerster BR, Petrou M, Lin D, Thurnher MM, Carlson MD, Strouse PJ, & Sundgren PC (2009). Neuroimaging evaluation of non-accidental head trauma with correlation to clinical outcomes: A review of 57 cases. The Journal of Pediatrics, 154(4), 573–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frahm J, Bruhn H, Gyngell ML, Merboldt KD, Hänicke W, & Sauter R (1989). Localized proton NMR spectroscopy in different regions of the human brain in vivo. Relaxation times and concentrations of cerebral metabolites. Magnetic Resonance in Medicine, 11(1), 47–63. [DOI] [PubMed] [Google Scholar]
- Freeman TW, Cardwell D, Karson CN, & Komoroski RA (1998). In vivo proton magnetic resonance spectroscopy of the medial temporal lobes of subjects with combat-related posttraumatic stress disorder. Magnetic Resonance in Medicine, 40(1), 66–71. [DOI] [PubMed] [Google Scholar]
- Friedman SD, Brooks WM, Jung RE, Hart BL, & Yeo RA (1998). Proton MR spectroscopic findings correspond to neuropsychological function in traumatic brain injury. AJNR. American Journal of Neuroradiology, 19(10), 1879–1885. [PMC free article] [PubMed] [Google Scholar]
- Friedman SD, Poliakov AV, Budech C, Shaw DWW, Breiger D, Jinguji T, Krabak B, Coppel D, Lewis TM, Browd S, & Ojemann JG (2017). GABA alterations in pediatric sport concussion. Neurology, 89(21), 2151–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner A, Iverson GL, & Stanwell P (2014). A systematic review of proton magnetic resonance spectroscopy findings in sport-related concussion. Journal of Neurotrauma, 31(1), 1–18. [DOI] [PubMed] [Google Scholar]
- Garnett MR, Blamire AM, Rajagopalan B, Styles P, & Cadoux-Hudson TAD (2000). A magnetic resonance spectroscopy study. Brain: A Journal of Neurology, 123, 1403–1409. [DOI] [PubMed] [Google Scholar]
- Garnett MR, Corkill RG, Blamire AM, Rajagopalan B, Manners DN, Young JD, Styles P, & Cadoux-Hudson TA (2001). Altered cellular metabolism following traumatic brain injury: A magnetic resonance spectroscopy study. Journal of Neurotrauma, 18(3), 231–240. [DOI] [PubMed] [Google Scholar]
- Gasparovic C, Bedrick EJ, Mayer AR, Yeo RA, Chen H, Damaraju E, Calhoun VD, & Jung RE (2011). Test-retest reliability and reproducibility of short-echo-time spectroscopic imaging of human brain at 3T. Magnetic Resonance in Medicine, 66 (2), 324–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparovic C, Chen H, & Mullins PG (2018). Errors in 1 H-MRS estimates of brain metabolite concentrations caused by failing to take into account tissue-specific signal relaxation. NMR in Biomedicine, 31(6), e3914. [DOI] [PubMed] [Google Scholar]
- Gasparovic C, Song T, Devier D, Bockholt HJ, Caprihan A, Mullins PG, Posse S, Jung RE, & Morrison LA (2006). Use of tissue water as a concentration reference for proton spectrocopic imaging. Magnetic Resonance in Medicine, 55(6), 1219–1226. [DOI] [PubMed] [Google Scholar]
- Gasparovic C, Yeo R, Mannell M, Ling J, Elgie R, Phillips J, Doezema D, & Mayer AR (2009). Neurometabolite concentrations in gray and white matter in mild traumatic brain injury: An 1H-magnetic resonance spectroscopy study. Journal of Neurotrauma, 26(10), 1635–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George EO, Roys S, Sours C, Rosenberg J, Zhuo J, Shanmuganathan K, & Gullapalli RP (2014). Longitudinal and prognostic evaluation of mild traumatic brain injury: A 1H-magnetic resonance spectroscopy study. Journal of Neurotrauma, 31(11), 1018–1028. [DOI] [PubMed] [Google Scholar]
- Gill JR, Goldfeder LB, Armbrustmacher V, Coleman A, Mena H, & Hirsch CS (2009). Fatal head injury in children younger than 2 years in new York City and an overview of the shaken baby syndrome. Archives of Pathology & Laboratory Medicine, 133(4), 619–627. [DOI] [PubMed] [Google Scholar]
- Goryawala MZ, Sheriff S, Stoyanova R, & Maudsley AA (2018). Spectral decomposition for resolving partial volume effects in MRSI. Magnetic Resonance in Medicine, 79(6), 2886–2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindaraju V, Gauger GE, Manley GT, Ebel A, Meeker M, & Maudsley AA (2004). Volumetric proton spectroscopic imaging of mild traumatic brain injury. AJNR. American Journal of Neuroradiology, 25(5), 730–737. [PMC free article] [PubMed] [Google Scholar]
- Govind V, Gold S, Kaliannan K, Saigal G, Falcone S, Arheart KL, Harris L, Jagid J, & Maudsley AA (2010). Whole-brain proton MR spectroscopic imaging of mild-to-moderate traumatic brain injury and correlation with neuropsychological deficits. Journal of Neurotrauma, 27(3), 483–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haacke EM, Duhaime AC, Gean AD, Riedy G, Wintermark M, Mukherjee P, Brody DL, DeGraba T, Duncan TD, Elovic E, Hurley R, Latour L, Smirniotopoulos JG, & Smith DH (2010). Common data elements in radiologic imaging of traumatic brain injury. Journal of Magnetic Resonance Imaging: JMRI, 32(3), 516–543. [DOI] [PubMed] [Google Scholar]
- Hahnemann ML, Kinner S, Schweiger B, Bajanowski T, Karger B, Pfeiffer H, & Wittschieber D (2015). Imaging of bridging vein thrombosis in infants with abusive head trauma: the “Tadpole Sign.” European Radiology, 25(2), 299–305. [DOI] [PubMed] [Google Scholar]
- Hangel G, Strasser B, Považan M, Heckova E, Hingerl L, Boubela R, Gruber S, Trattnig S, & Bogner W (2018). Ultra-high resolution brain metabolite mapping at 7 T by short-TR Hadamard-encoded FID-MRSI. NeuroImage, 168, 199–210. [DOI] [PubMed] [Google Scholar]
- Harris AD, Puts NAJ, & Edden RAE (2015). Tissue correction for GABA-edited MRS: Considerations of voxel composition, tissue segmentation, and tissue relaxations. Journal of Magnetic Resonance Imaging: JMRI, 42(5), 1431–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AD, Saleh MG, & Edden RAE (2017). Edited 1 H magnetic resonance spectroscopy in vivo: Methods and metabolites. Magnetic Resonance in Medicine, 77(4), 1377–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseler LJ, Arcinue E, Danielsen ER, Bluml S, & Ross BD (1997). Evidence from proton magnetic resonance spectroscopy for a metabolic cascade of neuronal damage in shaken baby syndrome. Pediatrics, 99(1), 4–14. [DOI] [PubMed] [Google Scholar]
- Henry LC, Tremblay S, Boulanger Y, Ellemberg D, & Lassonde M (2010). Neurometabolic changes in the acute phase after sports concussions correlate with symptom severity. Journal of Neurotrauma, 27(1), 65–76. [DOI] [PubMed] [Google Scholar]
- Henry LC, Tremblay S, Leclerc S, Khiat A, Boulanger Y, Ellemberg D, & Lassonde M (2011). Metabolic changes in concussed American football players during the acute and chronic post-injury phases. BMC Neurology, 11, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington HP, Hamid H, Kulas J, Ling G, Bandak F, de Lanerolle NC, & Pan JW (2014). MRSI of the medial temporal lobe at 7 T in explosive blast mild traumatic brain injury. Magnetic Resonance in Medicine, 71(4), 1358–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington HP, Pan JW, Mason GF, Adams D, Vaughn MJ, Twieg DB, & Pohost GM (1996). Quantitative 1H spectroscopic imaging of human brain at 4.1 T using image segmentation. Magnetic Resonance in Medicine, 36(1), 21–29. [DOI] [PubMed] [Google Scholar]
- Hoch SE, Kirov II, & Tal A (2017). When are metabolic ratios superior to absolute quantification? A statistical analysis. NMR in Biomedicine, 30(7). 10.1002/nbm.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holshouser BA, Ashwal S, Luh GY, Shu S, Kahlon S, Auld KL, Tomasi LG, Perkin RM, & Hinshaw DB Jr. (1997). Proton MR spectroscopy after acute central nervous system injury: Outcome prediction in neonates, infants, and children. Radiology, 202(2), 487–496. [DOI] [PubMed] [Google Scholar]
- Holshouser BA, Tong KA, & Ashwal S (2005). Proton MR spectroscopic imaging depicts diffuse axonal injury in children with traumatic brain injury. AJNR. American Journal of Neuroradiology, 26(5), 1276–1285. [PMC free article] [PubMed] [Google Scholar]
- Holshouser B, Pivonka-Jones J, Nichols JG, Oyoyo U, Tong K, Ghosh N, & Ashwal S (2019). Longitudinal metabolite changes after traumatic brain injury: A prospective Pediatric magnetic resonance spectroscopic imaging study. Journal of Neurotrauma, 36(8), 1352–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horská A, Kaufmann WE, Brant LJ, Naidu S, Harris JC, & Barker PB (2002). In vivo quantitative proton MRSI study of brain development from childhood to adolescence: Proton MRSI in brain development. Journal of Magnetic Resonance Imaging: JMRI, 15(2), 137–143. [DOI] [PubMed] [Google Scholar]
- Hovda DA, Becker DP, & Katayama Y (1992). Secondary injury and acidosis. Journal of Neurotrauma, 9(Suppl 1), S47–S60. [PubMed] [Google Scholar]
- Hunter JV, Wilde EA, Tong KA, & Holshouser BA (2012). Emerging imaging tools for use with traumatic brain injury research. Journal of Neurotrauma, 29(4), 654–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppi PS, & Inder TE (2001). Magnetic resonance techniques in the evaluation of the perinatal brain: Recent advances and future directions. Seminars in Neonatology: SN, 6(2), 195–210. [DOI] [PubMed] [Google Scholar]
- Ichord RN, Naim M, Pollock AN, Nance ML, Margulies SS, & Christian CW (2007). Hypoxic-ischemic injury complicates inflicted and accidental traumatic brain injury in young children: The role of diffusion-weighted imaging. Journal of Neurotrauma, 24(1), 106–118. [DOI] [PubMed] [Google Scholar]
- Jabłoński M, Starčuková J, & Starčuk Z Jr. (2017). Processing tracking in jMRUI software for magnetic resonance spectra quantitation reproducibility assurance. BMC Bioinformatics, 18(1), 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob R, Cox M, Koral K, Greenwell C, Xi Y, Vinson L, Reeder K, Weprin B, Huang R, & Booth TN (2016). MR imaging of the cervical spine in nonaccidental trauma: A tertiary institution experience. AJNR. American Journal of Neuroradiology, 37(10), 1944–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen JFA, Backes WH, Nicolay K, & Kooi ME (2006). 1H MR spectroscopy of the brain: Absolute quantification of metabolites. Radiology, 240(2), 318–332. [DOI] [PubMed] [Google Scholar]
- Jeter CB, Hergenroeder GW, Hylin MJ, Redell JB, Moore AN, & Dash PK (2013). Biomarkers for the diagnosis and prognosis of mild traumatic brain injury/concussion. Journal of Neurotrauma, 30(8), 657–670. [DOI] [PubMed] [Google Scholar]
- Johnson B, Gay M, Zhang K, Neuberger T, Horovitz SG, Hallett M, Sebastianelli W, & Slobounov S (2012a). The use of magnetic resonance spectroscopy in the subacute evaluation of athletes recovering from single and multiple mild traumatic brain injury. Journal of Neurotrauma, 29(13), 2297–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B, Zhang K, Gay M, Neuberger T, Horovitz S, Hallett M, Sebastianelli W, & Slobounov S (2012b). Metabolic alterations in corpus callosum may compromise brain functional connectivity in MTBI patients: An 1H-MRS study. Neuroscience Letters, 509(1), 5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson VE, Stewart W, & Smith DH (2013). Axonal pathology in traumatic brain injury. Experimental Neurology, 246, 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawata K, Tierney R, & Langford D (2018). Blood and cerebrospinal fluid biomarkers. Handbook of Clinical Neurology, 158, 217–233. [DOI] [PubMed] [Google Scholar]
- Keenan HT, Runyan DK, Marshall SW, Nocera MA, Merten DF, & Sinal SH (2003). A population-based study of inflicted traumatic brain injury in young children. JAMA: The Journal of the American Medical Association, 290(5), 621–626. [DOI] [PubMed] [Google Scholar]
- Kierans AS, Kirov II, Gonen O, Haemer G, Nisenbaum E, Babb JS, Grossman RI, & Lui YW (2014). Myoinositol and glutamate complex neurometabolite abnormality after mild traumatic brain injury. Neurology, 82(6), 521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbrell T, Leulf C, Cardwell D, Komoroski RA, & Freeman TW (2005). Relationship of in vivo medial temporal lobe magnetic resonance spectroscopy to documented combat exposure in veterans with chronic posttraumatic stress disorder. Psychiatry Research, 140(1), 91–94. [DOI] [PubMed] [Google Scholar]
- Kirov I, Fleysher L, Babb JS, Silver JM, Grossman RI, & Gonen O (2007). Characterizing “mild” in traumatic brain injury with proton MR spectroscopy in the thalamus: Initial findings. Brain Injury: [BI], 21(11), 1147–1154. [DOI] [PubMed] [Google Scholar]
- Kirov II, Tal A, Babb JS, Lui YW, Grossman RI, & Gonen O (2013a). Diffuse axonal injury in mild traumatic brain injury: A 3D multivoxel proton MR spectroscopy study. Journal of Neurology, 260(1), 242–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirov II, Tal A, Babb JS, Reaume J, Bushnik T, Ashman TA, Flanagan S, Grossman RI, & Gonen O (2013b). Proton MR spectroscopy correlates diffuse axonal abnormalities with postconcussive symptoms in mild traumatic brain injury. Journal of Neurotrauma, 30(13), 1200–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirov II, Whitlow CT, & Zamora C (2018). Susceptibility-weighted imaging and magnetic resonance spectroscopy in concussion. Neuroimaging Clinics of North America, 28(1), 91–105. [DOI] [PubMed] [Google Scholar]
- Koerte IK, Lin AP, Muehlmann M, Merugumala S, Liao H, Starr T, Kaufmann D, Mayinger M, Steffinger D, Fisch B, Karch S, Heinen F, Ertl-Wagner B, Reiser M, Stern RA, Zafonte R, & Shenton ME (2015). Altered neurochemistry in former professional soccer players without a history of concussion. Journal of Neurotrauma, 32(17), 1287–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontos AP, Van Cott AC, Roberts J, Pan JW, Kelly MB, McAllister-Deitrick J, & Hetherington HP (2017). Clinical and magnetic resonance spectroscopic imaging findings in veterans with blast mild traumatic brain injury and post-traumatic stress disorder. Military Medicine, 182(S1), 99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kou Z, Wu Z, Tong KA, Holshouser B, Benson RR, Hu J, & Haacke EM (2010). The role of advanced MR imaging findings as biomarkers of traumatic brain injury. The Journal of Head Trauma Rehabilitation, 25(4), 267–282. [DOI] [PubMed] [Google Scholar]
- Kreis R (2016). The trouble with quality filtering based on relative Cramér-Rao lower bounds. Magnetic Resonance in Medicine, 75(1), 15–18. [DOI] [PubMed] [Google Scholar]
- Kreis R, Hofmann L, Kuhlmann B, Boesch C, Bossi E, & Hüppi PS (2002). Brain metabolite composition during early human brain development as measured by quantitative in vivo 1H magnetic resonance spectroscopy. Magnetic Resonance in Medicine, 48(6), 949–958. [DOI] [PubMed] [Google Scholar]
- Krishnappa IK, Contant CF, & Robertson CS (1999). Regional changes in cerebral extracellular glucose and lactate concentrations following severe cortical impact injury and secondary ischemia in rats. Journal of Neurotrauma, 16(3), 213–224. [DOI] [PubMed] [Google Scholar]
- Kyathanahally SP, Döring A, & Kreis R (2018). Deep learning approaches for detection and removal of ghosting artifacts in MR spectroscopy. Magnetic Resonance in Medicine, 80(3), 851–863. [DOI] [PubMed] [Google Scholar]
- Lam F, Ma C, Clifford B, Johnson CL, & Liang Z-P (2016). High-resolution (1) H-MRSI of the brain using SPICE: Data acquisition and image reconstruction. Magnetic Resonance in Medicine, 76(4), 1059–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, & Wald MM (2006). The epide-miology and impact of traumatic brain injury: A brief overview. The Journal of Head Trauma Rehabilitation, 21(5), 375–378. [DOI] [PubMed] [Google Scholar]
- Lawrence TP, Steel A, Ezra M, Speirs M, Pretorius PM, Douaud G, Sotiropoulos S, Cadoux-Hudson T, Emir UE, & Voets NL (2019). MRS and DTI evidence of progressive posterior cingulate cortex and corpus callosum injury in the hyper-acute phase after traumatic brain injury. Brain Injury: [BI], 33(7), 854–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre G, Chamard E, Proulx S, Tremblay S, Halko M, Soman S, de Guise E, Pascual-Leone A, & Théoret H (2018). Increased Myo-inositol in primary motor cortex of contact sports athletes without a history of concussion. Journal of Neurotrauma. 10.1089/neu.2017.5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AP, Liao HJ, Merugumala SK, Prabhu SP, Meehan WP 3rd, & Ross BD (2012). Metabolic imaging of mild traumatic brain injury. Brain Imaging and Behavior, 6(2), 208–223. [DOI] [PubMed] [Google Scholar]
- Lin AP, Ramadan S, Stern RA, Box HC, Nowinski CJ, Ross BD, & Mountford CE (2015). Changes in the neurochemistry of athletes with repetitive brain trauma: Preliminary results using localized correlated spectroscopy. Alzheimer’s Research & Therapy, 7(1), 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis MS, Alosco M, Rowland B, Liao H, Wang J, Koerte I, Shenton M, Stern R, Joshi A, & Lin AP (2017). Using machine learning techniques for identification of chronic traumatic encephalopathy related spectroscopic biomarkers. IEEE Applied Imagery Pattern Recognition Workshop (AIPR), 2017, 1–5. [Google Scholar]
- Mariano LJ Irvine JM Rowland B Liao H Heaton K Orlovsky I Finkelstein K & Lin AP (2017). Virtual biopsy: Distinguishing post-traumatic stress from mild traumatic brain injury using magnetic resonance spectroscopy. 2017 IEEE Applied Imagery Pattern Recognition Workshop (AIPR), Washington, DC, 2017, pp. 1–6. [Google Scholar]
- Makoroff KL, Cecil KM, Care M, & Ball WS Jr. (2005). Elevated lactate as an early marker of brain injury in inflicted traumatic brain injury. Pediatric Radiology, 35(7), 668–676. [DOI] [PubMed] [Google Scholar]
- Mamere AE, Saraiva LAL, Matos ALM, Carneiro AAO, & Santos AC (2009). Evaluation of delayed neuronal and axonal damage secondary to moderate and severe traumatic brain injury using quantitative MR imaging techniques. AJNR. American Journal of Neuroradiology, 30(5), 947–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandl RCW, van den Heuvel MP, Klomp DWJ, Boer VO, Siero JCW, Luijten PR, & Hulshoff Pol HE (2012). Tract-based magnetic resonance spectroscopy of the cingulum bundles at 7 T. Human Brain Mapping, 33(7), 1503–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manivannan S, Makwana M, Ahmed AI, & Zaben M (2018). Profiling biomarkers of traumatic axonal injury: From mouse to man. Clinical Neurology and Neurosurgery, 171, 6–20. [DOI] [PubMed] [Google Scholar]
- Manning KY, Schranz A, Bartha R, Dekaban GA, Barreira C, Brown A, Fischer L, Asem K, Doherty TJ, Fraser DD, Holmes J, & Menon RS (2017). Multiparametric MRI changes persist beyond recovery in concussed adolescent hockey players. Neurology, 89(21), 2157–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino S, Zei E, Battaglini M, Vittori C, Buscalferri A, Bramanti P, Federico A, & De Stefano N (2007). Acute metabolic brain changes following traumatic brain injury and their relevance to clinical severity and outcome. Journal of Neurology, Neurosurgery, and Psychiatry, 78(5), 501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias JL, Dennington V, Bowden SC, & Bigler ED (2013). Community versus orthopaedic controls in traumatic brain injury research: How comparable are they? Brain Injury: [BI], 27(7–8), 887–895. [DOI] [PubMed] [Google Scholar]
- Maudsley AA, Domenig C, Govind V, Darkazanli A, Studholme C, Arheart K, & Bloomer C (2009). Mapping of brain metabolite distributions by volumetric proton MR spectroscopic imaging (MRSI). Magnetic Resonance in Medicine, 61(3), 548–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maudsley AA, Govind V, Levin B, Saigal G, Harris L, & Sheriff S (2015). Distributions of magnetic resonance diffusion and spectroscopy measures with traumatic brain injury. Journal of Neurotrauma, 32(14), 1056–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maudsley AA, Govind V, Saigal G, Gold SG, Harris L, & Sheriff S (2017). Longitudinal MR spectroscopy shows altered metabolism in traumatic brain injury. Journal of Neuroimaging: Official Journal of the American Society of Neuroimaging, 27(6), 562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maugans TA, Farley C, Altaye M, Leach J, & Cecil KM (2012). Pediatric sports-related concussion produces cerebral blood flow alterations. Pediatrics, 129(1), 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Kaushal M, Dodd AB, Hanlon FM, Shaff NA, Mannix R, Master CL, Leddy JJ, Stephenson D, Wertz CJ, Suelzer EM, Arbogast KB, & Meier TB (2018). Advanced biomarkers of pediatric mild traumatic brain injury: Progress and perils. Neuroscience and Biobehavioral Reviews, 94, 149–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Ling JM, Dodd AB, Gasparovic C, Klimaj SD, & Meier TB (2015). A longitudinal assessment of structural and chemical alterations in mixed martial arts fighters. Journal of Neurotrauma, 32(22), 1759–1767. [DOI] [PubMed] [Google Scholar]
- McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE, Santini VE, Lee H-S, Kubilus CA, & Stern RA (2009). Chronic traumatic encephalopathy in athletes: Progressive tauopathy after repetitive head injury. Journal of Neuropathology and Experimental Neurology, 68(7), 709–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean MA, Woermann FG, Barker GJ, & Duncan JS (2000). Quantitative analysis of short echo time (1)H-MRSI of cerebral gray and white matter. Magnetic Resonance in Medicine, 44(3), 401–411. [DOI] [PubMed] [Google Scholar]
- Mescher M, Merkle H, Kirsch J, Garwood M, & Gruetter R (1998). Simultaneous in vivo spectral editing and water suppression. NMR in Biomedicine, 11(6), 266–272. [DOI] [PubMed] [Google Scholar]
- Meyer EJ, Kirov II, Tal A, Davitz MS, Babb JS, Lazar M, Malaspina D, & Gonen O (2016). Metabolic abnormalities in the Hippocampus of patients with schizophrenia: A 3D multivoxel MR spectroscopic imaging study at 3T. AJNR. American Journal of Neuroradiology, 37(12), 2273–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett JR, Ross B, Arun P, Madhavarao CN, & Namboodiri AMA (2007). N-Acetylaspartate in the CNS: From neurodiagnostics to neurobiology. Progress in Neurobiology, 81(2), 89–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountford CE, Stanwell P, Lin A, Ramadan S, & Ross B (2010). Neurospectroscopy: The past, present and future. Chemical Reviews, 110(5), 3060–3086. [DOI] [PubMed] [Google Scholar]
- Narayana PA, Yu X, Hasan KM, Wilde EA, Levin HS, Hunter JV, Miller ER, Patel VKS, Robertson CS, & McCarthy JJ (2015). Multi-modal MRI of mild traumatic brain injury. NeuroImage. Clinical, 7, 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naressi A, Couturier C, Castang I, de Beer R, & Graveron-Demilly D (2001). Java-based graphical user interface for MRUI, a software package for quantitation of in vivo/medical magnetic resonance spectroscopy signals. Computers in Biology and Medicine, 31(4), 269–286. [DOI] [PubMed] [Google Scholar]
- Panigrahy A, Nelson MD Jr., & Blüml S (2010). Magnetic resonance spectroscopy in pediatric neuroradiology: Clinical and research applications. Pediatric Radiology, 40(1), 3–30. [DOI] [PubMed] [Google Scholar]
- Parry L, Shores A, Rae C, Kemp A, Waugh M-C, Chaseling R, & Joy P (2004). An investigation of neuronal integrity in severe paediatric traumatic brain injury. Child Neuropsychology: A Journal on Normal and Abnormal Development in Childhood and Adolescence, 10(4), 248–261. [DOI] [PubMed] [Google Scholar]
- Pfeiffer H, Crowe L, Kemp AM, Cowley LE, Smith AS, Babl FE, & Paediatric Research in Emergency Departments International Collaborative (PREDICT). (2018). Clinical prediction rules for abusive head trauma: A systematic review. Archives of Disease in Childhood, 103(8), 776–783. [DOI] [PubMed] [Google Scholar]
- Poole VN, Abbas K, Shenk TE, Breedlove EL, Breedlove KM, Robinson ME, Leverenz LJ, Nauman EA, Talavage TM, & Dydak U (2014). MR spectroscopic evidence of brain injury in the non-diagnosed collision sport athlete. Developmental Neuropsychology, 39(6), 459–473. [DOI] [PubMed] [Google Scholar]
- Poole VN, Breedlove EL, Shenk TE, Abbas K, Robinson ME, Leverenz LJ, Nauman EA, Dydak U, & Talavage TM (2015). Sub-concussive hit characteristics predict deviant brain metabolism in football athletes. Developmental Neuropsychology, 40(1), 12–17. [DOI] [PubMed] [Google Scholar]
- Pouwels PJ, Brockmann K, Kruse B, Wilken B, Wick M, Hanefeld F, & Frahm J (1999). Regional age dependence of human brain metabolites from infancy to adulthood as detected by quantitative localized proton MRS. Pediatric Research, 46(4), 474–485. [DOI] [PubMed] [Google Scholar]
- Prasad MR, Ramaiah C, McIntosh TK, Dempsey RJ, Hipkens S, & Yurek D (1994). Regional levels of lactate and norepinephrine after experimental brain injury. Journal of Neurochemistry, 63(3), 1086–1094. [DOI] [PubMed] [Google Scholar]
- Provencher SW (1993). Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magnetic Resonance in Medicine, 30(6), 672–679. [DOI] [PubMed] [Google Scholar]
- Ross BD, Ernst T, Kreis R, Haseler LJ, Bayer S, Danielsen E, Blüml S, Shonk T, Mandigo JC, Caton W, Clark C, Jensen SW, Lehman NL, Arcinue E, Pudenz R, & Shelden CH (1998). 1H MRS in acute traumatic brain injury. Journal of Magnetic Resonance Imaging: JMRI, 8(4), 829–840. [DOI] [PubMed] [Google Scholar]
- Sabati M, Sheriff S, Gu M, Wei J, Zhu H, Barker PB, Spielman DM, Alger JR, & Maudsley AA (2015). Multivendor implementation and comparison of volumetric whole-brain echo-planar MR spectroscopic imaging. Magnetic Resonance in Medicine, 74(5), 1209–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarmento E, Moreira P, Brito C, Souza J, Jevoux C, & Bigal M (2009). Proton spectroscopy in patients with post-traumatic headache attributed to mild head injury. Headache, 49(9), 1345–1352. [DOI] [PubMed] [Google Scholar]
- Scheenen TWJ, Klomp DWJ, Wijnen JP, & Heerschap A (2008). Short echo time 1H-MRSI of the human brain at 3T with minimal chemical shift displacement errors using adiabatic refocusing pulses. Magnetic Resonance in Medicine, 59(1), 1–6. [DOI] [PubMed] [Google Scholar]
- Schranz AL, Manning KY, Dekaban GA, Fischer L, Jevremovic T, Blackney K, Barreira C, Doherty TJ, Fraser DD, Brown A, Holmes J, Menon RS, & Bartha R (2018). Reduced brain glutamine in female varsity rugby athletes after concussion and in non-concussed athletes after a season of play. Human Brain Mapping, 39(4), 1489–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuff N, Neylan TC, Lenoci MA, Du AT, Weiss DS, Marmar CR, & Weiner MW (2001). Decreased hippocampal N-acetylaspartate in the absence of atrophy in posttraumatic stress disorder. Biological Psychiatry, 50(12), 952–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger U, Klose U, Mader I, Grodd W, & Nägele T (2003). Parameterized evaluation of macromolecules and lipids in proton MR spectroscopy of brain diseases. Magnetic Resonance in Medicine, 49(1), 19–28. [DOI] [PubMed] [Google Scholar]
- Shutter L, Tong KA, & Holshouser BA (2004). Proton MRS in acute traumatic brain injury: Role for glutamate/glutamine and choline for outcome prediction. Journal of Neurotrauma, 21(12), 1693–1705. [DOI] [PubMed] [Google Scholar]
- Signoretti S, Marmarou A, Fatouros P, Hoyle R, Beaumont A, Sawauchi S, Bullock R, & Young H (2002). Application of chemical shift imaging for measurement of NAA in head injured patients. Acta Neurochirurgica. Supplement, 81, 373–375. [DOI] [PubMed] [Google Scholar]
- Sivák Š, Bittšanský M, Grossmann J, Nosál’ V, Kantorová E, Siváková J, Demková A, Hnilicová P, Dobrota D, & Kurča E (2014). Clinical correlations of proton magnetic resonance spectroscopy findings in acute phase after mild traumatic brain injury. Brain Injury: [BI], 28(3), 341–346. [DOI] [PubMed] [Google Scholar]
- Sours C, George EO, Zhuo J, Roys S, & Gullapalli RP (2015). Hyper-connectivity of the thalamus during early stages following mild traumatic brain injury. Brain Imaging and Behavior, 9(3), 550–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurny B, Heckova E, Seiger R, Moser P, Klöbl M, Vanicek T, Spies M, Bogner W, & Lanzenberger R (2019). Automated ROI-based labeling for multi-voxel magnetic resonance spectroscopy data using FreeSurfer. Frontiers in Molecular Neuroscience, 12, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Star-Lack J, Nelson SJ, Kurhanewicz J, Huang LR, & Vigneron DB (1997). Improved water and lipid suppression for 3D PRESS CSI using RF band selective inversion with gradient dephasing (BASING). Magnetic Resonance in Medicine, 38(2), 311–321. [DOI] [PubMed] [Google Scholar]
- Stern RA, Daneshvar DH, Baugh CM, Seichepine DR, Montenigro PH, Riley DO, Fritts NG, Stamm JM, Robbins CA, McHale L, Simkin I, Stein TD, Alvarez VE, Goldstein LE, Budson AE, Kowall NW, Nowinski CJ, Cantu RC, & McKee AC (2013). Clinical presentation of chronic traumatic encephalopathy. Neurology, 81(13), 1122–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su E, & Bell M (2015). Diffuse axonal injury. In Laskowitz D & Grant G (Eds.), Translational Research in traumatic brain injury. CRC Press/Taylor and Francis Group. [Google Scholar]
- Suh DY, Davis PC, Hopkins KL, Fajman NN, & Mapstone TB (2001). Nonaccidental pediatric head injury: Diffusion-weighted imaging findings. Neurosurgery, 49(2), 309–318 discussion 318–320. [DOI] [PubMed] [Google Scholar]
- Tal A, Kirov II, Grossman RI, & Gonen O (2012). The role of gray and white matter segmentation in quantitative proton MR spectroscopic imaging. NMR in Biomedicine, 25(12), 1392–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpstra M, Cheong I, Lyu T, Deelchand DK, Emir UE, Bednařík P, Eberly LE, & Öz G (2016). Test-retest reproducibility of neurochemical profiles with short-echo, single-voxel MR spectroscopy at 3T and 7T. Magnetic Resonance in Medicine, 76(4), 1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodore AD, Chang JJ, Runyan DK, Hunter WM, Bangdiwala SI, & Agans R (2005). Epidemiologic features of the physical and sexual maltreatment of children in the Carolinas. Pediatrics, 115(3), e331–e337. [DOI] [PubMed] [Google Scholar]
- Tomiyasu M, Aida N, Shibasaki J, Tachibana Y, Endo M, Nozawa K, Shimizu E, Tsuji H, & Obata T (2016). Normal lactate concentration range in the neonatal brain. Magnetic Resonance Imaging, 34(9), 1269–1273. [DOI] [PubMed] [Google Scholar]
- Tremblay S, Beaulé V, Proulx S, Tremblay S, Marjańska M, Doyon J, Lassonde M, & Théoret H (2014). Multimodal assessment of primary motor cortex integrity following sport concussion in asymptomatic athletes. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 125(7), 1371–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagnozzi R, Signoretti S, Cristofori L, Alessandrini F, Floris R, Isgrò E, Ria A, Marziali S, Zoccatelli G, Tavazzi B, Del Bolgia F, Sorge R, Broglio SP, McIntosh TK, & Lazzarino G (2010). Assessment of metabolic brain damage and recovery following mild traumatic brain injury: A multicentre, proton magnetic resonance spectroscopic study in concussed patients. Brain: A Journal of Neurology, 133(11), 3232–3242. [DOI] [PubMed] [Google Scholar]
- Vagnozzi R, Signoretti S, Floris R, Marziali S, Manara M, Amorini AM, Belli A, Di Pietro V, D urso S, Pastore FS, Lazzarino G, & Tavazzi B (2013). Decrease in N-acetylaspartate following concussion may be coupled to decrease in creatine. The Journal of Head Trauma Rehabilitation, 28(4), 284–292. [DOI] [PubMed] [Google Scholar]
- Vagnozzi R, Signoretti S, Tavazzi B, Floris R, Ludovici A, Marziali S, Tarascio G, Amorini AM, Di Pietro V, Delfini R, & Lazzarino G (2008). Temporal window of metabolic brain vulnerability to concussion: A pilot 1H-magnetic resonance spectroscopic study in concussed athletes—Part III. Neurosurgery, 62(6), 1286–1296. [DOI] [PubMed] [Google Scholar]
- Vidya Shankar R, Chang JC, Hu HH, & Kodibagkar VD (2019). Fast data acquisition techniques in magnetic resonance spectroscopic imaging. NMR in Biomedicine, 32(3), e4046. [DOI] [PubMed] [Google Scholar]
- Walz NC, Cecil KM, Wade SL, & Michaud LJ (2008). Late proton magnetic resonance spectroscopy following traumatic brain injury during early childhood: Relationship with neurobehavioral outcomes. Journal of Neurotrauma, 25(2), 94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, & Li SJ (1998). Differentiation of metabolic concentrations between gray matter and white matter of human brain by in vivo 1H magnetic resonance spectroscopy. Magnetic Resonance in Medicine, 39(1), 28–33. [DOI] [PubMed] [Google Scholar]
- Widerström-Noga E, Govind V, Adcock JP, Levin BE, & Maudsley AA (2016). Subacute pain after traumatic brain injury is associated with lower insular N-Acetylaspartate concentrations. Journal of Neurotrauma, 33(14), 1380–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnen JP, van Asten JJA, Klomp DWJ, Sjobakk TE, Gribbestad IS, Scheenen TWJ, & Heerschap A (2010). Short echo time 1H MRSI of the human brain at 3T with adiabatic slice-selective refocusing pulses; reproducibility and variance in a dual center setting. Journal of Magnetic Resonance Imaging: JMRI, 31(1), 61–70. [DOI] [PubMed] [Google Scholar]
- Wilson M, Andronesi O, Barker PB, Bartha R, Bizzi A, Bolan PJ, Brindle KM, Choi I-Y, Cudalbu C, Dydak U, Emir UE, Gonzalez RG, Gruber S, Gruetter R, Gupta RK, Heerschap A, Henning A, Hetherington HP, Huppi PS, et al. (2019). Methodological consensus on clinical proton MRS of the brain: Review and recommendations. Magnetic Resonance in Medicine, 82(2), 527–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M, Reynolds G, Kauppinen RA, Arvanitis TN, & Peet AC (2011). A constrained least-squares approach to the automated quantitation of in vivo 1H magnetic resonance spectroscopy data. Magnetic Resonance in Medicine, 65(1), 1–12. [DOI] [PubMed] [Google Scholar]
- Yahya A, & Fallone GB (2007). Incorporating homonuclear polarization transfer into PRESS for proton spectral editing: Illustration with lactate and glutathione. Journal of Magnetic Resonance, 188(1), 111–121. [DOI] [PubMed] [Google Scholar]
- Yeates KO, Beauchamp M, Craig W, Doan Q, Zemek R, Bjornson B, Gravel J, Mikrogianakis A, Goodyear B, Abdeen N, Beaulieu C, Dehaes M, Deschenes S, Harris A, Lebel C, Lamont R, Williamson T, Barlow KM, Bernier F, et al. (2017). Advancing concussion assessment in pediatrics (A-CAP): A prospective, concurrent cohort, longitudinal study of mild traumatic brain injury in children: Protocol study. BMJ Open, 7(7), e017012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeates KO, Taylor HG, Rusin J, Bangert B, Dietrich A, Nuss K, Wright M, Nagin DS, & Jones BL (2009). Longitudinal trajectories of postconcussive symptoms in children with mild traumatic brain injuries and their relationship to acute clinical status. Pediatrics, 123(3), 735–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo RA, Gasparovic C, Merideth F, Ruhl D, Doezema D, & Mayer AR (2011). A longitudinal proton magnetic resonance spectroscopy study of mild traumatic brain injury. Journal of Neurotrauma, 28(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo RA, Phillips JP, Jung RE, Brown AJ, Campbell RC, & Brooks WM (2006). Magnetic resonance spectroscopy detects brain injury and predicts cognitive functioning in children with brain injuries. Journal of Neurotrauma, 23(10), 1427–1435. [DOI] [PubMed] [Google Scholar]
- Young K, Govindaraju V, Soher BJ, & Maudsley AA (1998). Automated spectral analysis I: Formation of a priori information by spectral simulation. Magnetic Resonance in Medicine, 40(6), 812–815. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


