Abstract
Women constitute half of the world’s population, yet neuroscience research does not serve the sexes equally. Fifty years of preclinical animal evidence documents the tightly-coupled relationship between our endocrine and nervous systems, yet human neuroimaging studies rarely consider how endocrine factors shape the structural and functional architecture of the human brain. Here, we quantify several blind spots in neuroimaging research, which overlooks aspects of the human condition that impact women’s health (e.g. the menstrual cycle, hormonal contraceptives, pregnancy, menopause). Next, we illuminate potential consequences of this oversight: today over 100 million women use oral hormonal contraceptives, yet relatively few investigations have systematically examined whether disrupting endogenous hormone production impacts the brain. We close by presenting a roadmap for progress, highlighting the University of California Women’s Brain Initiative which is addressing unmet needs in women’s health research.
Keywords: sex hormones, neuroimaging, birth control, women’s health
Neuroscientists have plumbed the depths of the mind and brain to extraordinary lengths, but occasionally we forget that the brain is part of a larger, integrated biological system. The brain is an endocrine organ, one whose structure and function is intimately tied to the action of neuromodulatory hormones (Taxier et al., 2020; Galea et al., 2017; Hara et al., 2015; Taylor et al., 2019; Woolley and McEwen, 1993). The brain coordinates the release of hormones from peripheral endocrine glands and, in turn, is a major target of these signaling molecules. Fifty years of accumulating evidence from animal studies documents the tightly-coupled relationship between our endocrine and nervous systems (Frick and Kim, 2018; Galea et al., 2017; Hara et al., 2015; Woolley and McEwen, 1993). Yet human neuroimaging studies rarely consider how endocrine factors shape the structural and functional architecture of the human brain (Beltz and Moser, 2019; Hampson, 2020; Jacobs and Goldstein, 2018; Pletzer and Kerschbaum, 2014; Taylor et al., 2019). Advances in brain imaging techniques over the past twenty years have given us unprecedent insight into the human brain, with rapidly expanding knowledge of the metabolic, neurochemical, neurophysiological, and morphological basis of brain function. The advent of large-scale neuroimaging databases has further advanced the field by providing datasets of 100s–1000s of participants (Casey et al., 2018; Sudlow et al., 2015; Van Essen et al., 2013). Sex differences research has benefited from these datasets, with the statistical power to identify sex differences in brain development (Kaczkurkin et al., 2018; Lenroot and Giedd, 2006) and across the adult lifespan (Lotze et al., 2019; Ritchie et al., 2018). While considering sex as a biological variable of interest (Beery and Zucker, 2011; Clayton and Collins, 2014; Wald and Wu, 2010) has become increasingly common in human neuroimaging (Ruigrok et al., 2014; Sacher et al., 2013), looking beyond sex differences to study women’s brain health is still uncommon (Galea et al., 2018; Jacobs and Goldstein, 2018; Taylor et al., 2019)
Human neuroimaging has almost entirely overlooked how the brain responds to major changes in sex hormone production (e.g. during the menstrual cycle, pregnancy, menopause, or andropause). During the average human menstrual cycle, women experience an ~8-fold increase in 17β-estradiol (the most prevalent and potent form of estrogen in mammals) and an ~80-fold increase in progesterone (Stricker et al., 2006). During pregnancy, production of sex hormones surge throughout the gestational window (Abbassi-Ghanavati et al., 2009). Later in life, women experience a steep decline in sex hormone production during the transition to menopause (Burger, 2002). For men, testosterone production shows a protracted decline beginning in the mid-30s and continuing throughout life (Fabbri et al., 2016). The field has also largely overlooked the neuronal effects of disrupting sex hormone production via common exogenous hormone manipulations (e.g. oral hormonal contraceptives, androgenic anabolic steroids, and gonadotropin releasing hormone agents) (Beltz and Moser, 2019; Cahill, 2018; Montoya and Bos, 2017; Pletzer and Kerschbaum, 2014). For example, sex hormone production is chronically suppressed in the 100 million women worldwide who use oral hormonal contraceptives (Gaspard et al., 1983; Rivera et al., 1999; Spona et al., 1996). How do these shifts in gonadal hormone production shape the brain? The human neuroimaging community has not adequately addressed these factors (Hampson, 2020). Beyond obscuring basic knowledge about the endocrine basis of brain function, this oversight places a disproportionate burden on women’s health (Feldman et al., 2019; Parekh et al., 2011).
In this Perspective, we highlight seminal findings from the animal and human literature establishing the neuroendocrine basis of brain structure and function. Next, we take stock of how often endocrine factors are considered in human brain imaging studies, revealing major blind spots in the field. Using oral hormonal contraceptives as an example, we consider the ramifications of this oversight. We close by presenting a roadmap for progress, highlighting efforts from the University of California Women’s Brain Initiative to address unmet needs in women’s health research.
1. Brief review of sex hormone action in the central nervous system
Sex steroid hormones (androgens, estrogens, progestogens) are produced primarily by the gonads and coordinate the physiological changes that occur during puberty, the menstrual cycle, pregnancy, and menopause. Within the central nervous system, estrogen and progesterone receptors are expressed widely throughout the brain (McEwen, 2002; McEwen and Alves, 1999; Rossetti et al., 2016), with enriched expression in extra-hypothalamic regions such as the hippocampus and prefrontal cortex (PFC) (Almey et al., 2015; Brinton et al., 2008). Estradiol and progesterone signaling are critical components of cell survival and plasticity, exerting excitatory and inhibitory effects that are evident across multiple spatial and temporal scales (Frick and Kim, 2018; Galea et al., 2017). Below, we highlight major discoveries from the past 20 years establishing estrogen and progesterone’s actions in higher-order cognitive regions of the brain (for comprehensive reviews of sex hormone action in memory circuitry, see Taxier et al., 2020; Frick, 2019; Frick et al., 2018; Galea et al., 2019; Hara et al., 2015).
1.1. Sex hormones regulate hippocampal and prefrontal cortex morphology across species
Animal studies offer unambiguous evidence that sex steroid hormones shape the synaptic organization of the brain, particularly within the hippocampus and PFC (Taxier et al., 2020; Frick et al., 2015; Frick and Kim, 2018; Galea et al., 2017; Hara et al., 2015; Woolley and McEwen, 1993). Rodent (Frick et al., 2015; Frick and Kim, 2018; Mahmoud et al., 2016; Woolley and McEwen, 1993) and non-human primate (Hao et al., 2003) studies have established 17β-estradiol and progesterone as powerful regulators of hippocampal morphology. At the epigenetic level, estradiol induces chromatin modifications that promote hippocampal plasticity (reviewed in Fortress and Frick, 2014). At the synaptic level, estradiol and progesterone regulate spine proliferation in the hippocampus (Hara et al., 2015). For example, dendritic spine density in CA1 neurons varies by ~30% over the 4–5-day rodent estrous cycle (Woolley et al., 1990; Woolley and McEwen, 1992). Hormone deprivation (via gonadectomy) in the rat (Gould et al., 1990; Woolley and McEwen, 1993) and African green monkey (Leranth et al., 2002) leads to a pronounced loss of spines on CA1 neurons, which is reversed by estrogen replacement (Gould et al., 1990; Leranth et al., 2002; Woolley and McEwen, 1993).
At the macroscopic level, total hippocampal volume is related to plasma estradiol levels in the meadow vole (Galea et al., 1999) and fluctuates across the estrous cycle in the mouse (Qiu et al., 2013). In-vivo magnetic resonance imaging (MRI) in mice demonstrates that estrous stage–related hippocampal volume changes are detectable within a 24-hour period (Qiu et al., 2013). In humans, hippocampal volume increases from the late-luteal/early-follicular phase to the late-follicular phase of the menstrual cycle (Lisofsky et al., 2015; Protopopescu et al., 2008). Further, recent evidence suggests that progesterone dynamically shapes medial temporal lobe morphology across a ~28-day menstrual cycle, with volumetric changes in CA2/3, parahippocampal cortex, entorhinal cortex and perirhinal cortex—effects that were blocked by progesterone suppression (Taylor et al., 2020a). During pregnancy, changes in sex hormone production during gestation modulate hippocampal plasticity in rodents (reviewed in Galea et al., 2014; Kinsley and Lambert, 2008; Workman et al., 2012) and likely mediate the transient decline in hippocampal volume observed in humans post-pregnancy (Hoekzema et al., 2017). Finally, the abrupt hormonal changes associated with surgical menopause lead to structural changes in the medial temporal lobe, including thinning of the parahippocampus/entorhinal cortex (Zeydan et al., 2019), while estradiol administration in postmenopausal women increases hippocampal volume (Albert et al., 2017). Together, these findings provide converging cross-species evidence that sex hormones induce structural changes in the hippocampus on rapid and protracted timescales.
Non-human primate studies have established similar relationships within the PFC (Hao et al., 2006; Morrison et al., 2006). In female rhesus macaques, ~50% of PFC pyramidal neurons express estrogen receptors (ER-α) and those with enriched PFC ER-α expression show stronger working memory performance (Wang et al., 2010). At the synaptic level, cyclic estradiol administration in ovariectomized rhesus macaques leads to increased spine density in PFC neurons (Hao et al., 2006) and improved working memory performance relative to estradiol-depleted controls (Rapp et al., 2003).
1.2. Sex hormones regulate memory circuitry function – evidence from human neuroimaging
Behavioral and neuroimaging studies in humans have established sex hormones’ role in the regulation of memory circuitry, with consistent effects observed within dorsolateral (BA9/46) and ventrolateral (BA44/45/47) prefrontal cortex, posterior parietal cortex (BA40/7), and medial temporal lobe regions (Duff and Hampson, 2000; Dumas et al., 2010; Hampson, 2018; Hampson and Morley, 2013; Jacobs and D’Esposito, 2011; Shanmugan and Epperson, 2014). This research builds on pioneering work from Berman et al., (1997) and Shaywitz et al., (1999), who used pharmacological blockade and hormone replacement techniques to illustrate the influence of estradiol and progesterone on regional activity in memory circuitry. Recent studies offer additional evidence that functional changes in ER-rich regions of the brain are tied to ovarian status. Menstrual cycle-stage and hormone-related effects have been observed in resting-state functional connectivity (De Bondt et al., 2015; Lisofsky et al., 2015; Petersen et al., 2014; Syan et al., 2017; Weis et al., 2019), and longitudinal studies have reported that endogenous fluctuations in estrogen and progesterone influence cortical network dynamics (Arélin et al., 2015; Pritschet et al., 2020), with prominent effects in intrinsic brain networks with hubs in PFC. Later in life, the depletion of sex hormones during the menopausal transition impacts PFC and hippocampal activity when participants engage in working memory and episodic memory tasks (Jacobs et al., 2017, 2016). In a study of midlife adults (ages 45–55), higher 17β-estradiol serum concentrations were associated with greater hippocampal modulation during an episodic memory encoding task and better memory retrieval (Jacobs et al., 2016). In a related study, the authors found that working memory (WM)–related activity in dorsolateral PFC and the hippocampus was also modulated by women’s reproductive stage. Postmenopausal women exhibited the strongest WM-related PFC activity, disinhibition of the hippocampus (which is typically suppressed as WM load increases), and greater PFC–hippocampal functional connectivity, relative to premenopausal and perimenopausal women. Further, the magnitude of regional activity and connectivity within WM circuitry was strongly related to WM performance. The findings suggest that with advancing reproductive stage there may be a shift in reliance away from PFC–parietal circuits toward PFC–hippocampal pathways for successful WM performance (Jacobs et al., 2016). In sum, research targeting the midlife menopausal transition has revealed the neurobiological consequences of neuroendocrine aging, above and beyond the more well-established effects of chronological aging (Jacobs and Goldstein, 2018; Rentz et al., 2017; Taylor et al., 2019).
An emerging theory from the human literature is that estradiol increases the efficiency of cortical circuits within the PFC. In young women performing a WM task, PFC activity is exaggerated under low estradiol conditions and reduced under high estradiol conditions (Jacobs and D’Esposito, 2011). The same pattern is observed decades later in life: as estradiol production declines over the menopausal transition, WM-dependent PFC activity becomes exaggerated despite no deficit in performance (Jacobs et al., 2017). In a recent dense-sampling study, Pritschet and colleagues (2020) applied time-lagged methods from dynamical systems analysis to reveal that day-to-day changes in estradiol enhance the global efficiency of large-scale functional networks, particularly networks with hubs in PFC (e.g. Default Mode and Frontal Control; Schaefer et al., 2018; Yeo et al., 2011). Next, the authors observed transient functional reorganization of PFC nodes coincident with peaks in serum estradiol (Mueller et al., 2020). An intriguing possibility is that this increased efficiency of cortical circuits may be mediated through dopamine signaling pathways (Almey et al., 2015; Cai and Arnsten, 1997; Duff and Hampson, 2000; Jacobs and D’Esposito, 2011; Williams and Goldman-Rakic, 1995), given estradiol’s ability to potentiate dopamine release (Becker, 1990). Note that estradiol also influences a number of other neuromodulatory pathways (Amin et al., 2005; Epperson et al., 2012).
2. Identifying blind spots in human neuroimaging
While animal studies have documented the role of sex hormones in the brain for decades, human neuroimaging research has not kept pace. Given a groundswell of evidence that sex hormones regulate the structure and function of the mammalian brain, we sought to document the frequency with which human neuroimaging studies consider endocrine factors. We approached this in two ways. First, to capture a contemporary state of the field, we analyzed every empirical human neuroimaging paper (i.e., utilizing structural or functional magnetic resonance imaging (MRI), diffusion-weighted imaging (DWI), transcranial magnetic stimulation (TMS), electroencephalogram (EEG), magnetoencephalography (MEG), or positron emission tomography (PET)) published in five leading neuroscience journals in 2018: Nature Neuroscience, Neuron, Journal of Neuroscience, NeuroImage, and Human Brain Mapping. Articles (n=1,066) were coded based on a range of women’s health factors, including whether the article mentioned participants’ menstrual cycle phase, pubertal stage, hormonal contraceptive use, pregnancy, menopause status, endocrine disorders, direct hormone assays and more (a full description of the methods are provided in Supplementary Material). These journals were selected to reflect leading ‘mainstream’ neuroscience journals, as opposed to those focused more narrowly on reproductive health/hormones per se.
Two percent (n=29) of the articles surveyed mentioned a women’s health factor (Figure 1 – top). Of those that did, 20% (n=6) used the information to exclude women (i.e. to justify conducting a male-only study); 52% (n=15) did so to characterize the general study population (e.g. reporting hormonal contraceptive use) but did not use the data further; 10% (n=3) regressed out the influence of endocrine factors (e.g., including HRT use as a covariate of no interest); and only 17% (n=5) of the subset explicitly focused some aspect of their study design or analysis on a women’s health research question. Of those who did conduct endocrine-related analyses (n=9), 55% (n=5) identified a significant effect. In short, while ~2% of brain imaging articles surveyed mentioned an endocrine factor, far fewer — less than half of one percent —investigated the relationship between a women’s health factor and the brain.
Figure 1. Women’s health factors are severely understudied in human neuroimaging research.
Top | In 2018, only 2% of neuroimaging articles published in leading neuroscience journals mentioned endocrine/women’s reproductive health factors. Of those, 20% merely did so to exclude women and justify conducting a male-only study. Less than 0.5% of articles directly studied sex hormones or endocrine-related topic. Bottom | Publication count of human neuroimaging studies from 1995–2018. The number of brain imaging articles that consider the totality of women’s reproductive health is dwarfed by other research categories, such as ‘reward processing.’
Next, a historical survey of neuroimaging papers published from 1995–2018 revealed the persistence of this oversight across all journals indexed on PubMed. Women’s health factors are severely understudied in human neuroimaging. Of the ~43,000 human neuroimaging articles published over the last 25 years, fewer than 300 were focused on women’s reproductive health (including the menstrual cycle, pregnancy, menopause, hormonal contraceptive use, and more; see Supplementary Material). Figure 1 (bottom) illustrates the magnitude of this disparity. The number of articles dedicated to understanding the neuronal effects of a broad range of women’s health factors barely registers on a plot of neuroimaging articles published over time—accounting for ~0.5% of total publications—and is dwarfed by papers on ‘reward processing’ (shown for comparison). Women constitute half of the world’s population, yet the brain imaging community rarely considers basic aspects of women’s health. Since the mid-1990s the number of human neuroimaging studies has exploded, and the number of neuroimaging studies addressing women’s health has not kept pace.
3. A spotlight on oral hormonal contraceptives
Perhaps one of the most striking illustrations of this oversight is neuroscience’s neglect with respect to one of the largest natural experiments in human history (Beltz and Moser, 2019): over the past half-century, women have used oral hormonal contraceptives without full knowledge of their influence on the central nervous system, as few rigorous human neuroimaging studies of oral hormonal contraception (OC) have been conducted. Here, we use OC to highlight the missed opportunities in our historical failure to consider the brain in its endocrine context. We close by presenting a roadmap for how to address these oversights as quickly and effectively as possible.
First introduced in the U.S. in 1960, “the pill” revolutionized women’s reproductive health and was quickly adopted as the first widespread hormonal method of birth control. By 1967, 13 million women were using the pill, by 1984 those numbers rose to 50–80 million (Knowles and Correia, 2015), and today OC is used by more than 100 million women worldwide (Christin-Maitre, 2013; Petitti, 2003). In the US alone, 10 million women currently use OC and 60 million have done so over their lifetime (Daniels et al., 2015; Daniels and Jones, 2013; Jones et al., 2013).
3.1. Oral contraception’s mechanism of action
“The pill” is sold under ~100 different brand names with more than 40 different formulations. Almost all consist of a combination of two synthetic sex hormones, ethinyl estradiol and progestin, that act predominantly on endogenous sex steroid hormone receptors (Louw-du Toit et al., 2017; Sitruk-Ware and Nath, 2013). OC has been described as “mimicking pregnancy,” though this mechanistic explanation is misleading. During pregnancy, estradiol and progesterone production increase throughout the gestational period (Abbassi-Ghanavati et al., 2009; Berg and Kuss, 1992; Schock et al., 2016; Tal et al., 2000). In contrast, oral contraceptives prevent ovulation by mimicking the negative feedback effects of estradiol and progesterone. The exogenous hormones introduced by the pill limit gonadotropin releasing hormone secretion from the hypothalamus, in turn inhibiting follicle stimulating hormone (FSH) and luteinizing hormone (LH) release by the anterior pituitary. The reduction in FSH prevents follicle growth, the mid-cycle surge in estradiol, and the LH surge that would trigger ovulation (Bronson, 1981; Jones and Lopez, 2013). By disrupting hypothalamic and pituitary hormones, OC chronically suppresses ovarian production of estradiol and progesterone. In women using OC, endogenous sex hormone concentrations are on par with or below levels observed during the early follicular phase of freely cycling women (De Bondt et al., 2013; Gaspard et al., 1983; Rapkin et al., 2006; Rivera et al., 1999; Spona et al., 1996). Some formulations of OC can suppress progesterone concentrations by 75–97% (Gaspard et al., 1983; Rapkin et al., 2006; Taylor et al., 2020a) and while hormone levels typically return to baseline within months after discontinuation of pill use, lower concentrations of endogenous sex hormones have been observed in former users of OC relative to never users years after discontinuation (Chan et al., 2008).
More than 50 years have passed since the widespread adoption of the pill, yet few studies have investigated the impact of chronic ovarian hormone suppression and synthetic hormone regimens on brain regions that are densely populated with sex hormone receptors and modulated by sex hormones. It is unclear whether long-term ovarian hormone suppression has consequences at the macroscopic level of brain morphology and function in humans, but emerging evidence from a handful of small-scale human studies raises the possibility.
3.2. Effects of oral contraceptives on human brain structure and function
Despite the significant changes in endocrine status induced by OC use, neuroscientists lack a detailed understanding of how estrogen receptor–rich brain structures like the hippocampus and PFC respond to chronic suppression of sex hormone production. Observational studies have started to lay the groundwork for understanding OC’s effects on the central nervous system. Although a comprehensive summary of the OC literature is beyond the scope of this Perspective (see the authoritative reviews by Beltz and Moser, 2019; Montoya and Bos, 2017; Pletzer and Kerschbaum, 2014), here we review notable findings across imaging modalities.
Structural MRI studies of brain morphology most consistently suggest OC-related differences in limbic regions, though inconsistent results across studies and small sample sizes limit interpretation of this literature. In current users of OC (hereafter referred to as ‘OC users’) relative to naturally cycling women, both less gray matter volume (GMV) in the amygdala, parahippocampal gyrus (Lisofsky et al., 2016; Pletzer, 2019), and ventral temporal cortex (Pletzer et al., 2010, 2015), as well as greater volume in amygdala, hippocampal and parahippocampal gyrus (Sharma et al., 2020) have been observed. Duration of OC use in former users has been associated with greater volumes in bilateral hippocampus and basal ganglia (Pletzer et al., 2019), and others have reported no influence of age of initiation (Sharma et al., 2020), previous pill use, or progestin formulation (Lisofsky et al., 2016). Weak and/or inconsistent effects have been observed in prefrontal cortex (De Bondt et al., 2013; Petersen et al., 2015; Pletzer et al., 2010).
Functional MRI studies of resting-state functional connectivity have also shown mixed results. In one study, users of OC exhibited weaker connectivity relative to naturally cycling women within two major intrinsic brain networks - Executive Control and Default-Mode (Petersen et al., 2014). However, this contradicts other reports documenting no group differences in these networks (deBondt et al., 2015), or amplified whole-brain connectivity (Pritschet et al., 2020) with OC use. Moving forward, resting-state studies with robust sample sizes or carefully characterized subject populations are critical to draw firm conclusions about OC’s impact on intrinsic brain networks.
A number of studies have probed group differences in task-evoked fMRI (Abler et al., 2013; Bonenberger et al., 2013; Pletzer et al., 2014; Rumberg et al., 2010). Evidence from a randomized, double-blind, placebo-controlled study of emotion processing found that OC users show reduced emotion-related activity in bilateral PFC relative to participants’ pre-treatment baseline assessments (Gingnell et al., 2013). Similarly, an emotional arousal study found that OC users show less amygdala activity in response to negatively arousing stimuli relative to naturally cycling controls (Petersen et al., 2015). In contrast, relative to non-users, OC users show enhanced parahippocampal and hippocampal activity during fear learning following cortisol administration (Merz et al., 2012a), and enhanced amygdala and PFC activation during extinction learning (Merz et al., 2012b). Relative to non-users, current OC users show greater working memory–related activity in the right frontal lobe when stimuli are negatively arousing images (Sharma et al. 2020), but no difference for positive or neutral stimuli. In sum, inconsistent effects of OC use have been observed across brain imaging modalities. It is difficult to determine what factors mediate these conflicting results, in part, because OC formulations, age of initiation, and duration of past and present OC use differ within and between study populations, when reported at all. These inconsistencies could also reflect that OC has little to no reliable influence, yet drawing this conclusion also necessitates a sufficiently-powered, rigorously designed study.
While the studies summarized here reflect early efforts to characterize the neuronal effects of OC use, research in this arena is nascent. Though effects of OC use are generally observed within limbic brain regions, the variety and inconsistency of these findings suggest that these effects are nuanced, and that “current user versus non-user” comparisons are insufficient. Oral contraceptive use varies across multiple dimensions (age of initiation, duration of use, hormone formulation and schedule), any of which could influence the magnitude of OC’s impact on the structural and functional architecture of the brain (Hampson, 2020). Well-powered, systematic, quasi-experimental approaches that take these factors into account are essential for making meaningful scientific progress. Below we highlight some of the most pressing questions for future research.
3.3. Formulation and regimen
There are currently more than 40 OC formulations on the market, yet few studies differentiate between them (see Engman et al., 2017; Pletzer et al., 2015, for exceptions). Most OC formulations pair a synthetic estrogen with a progestin, though progestin-only pills are available. The estrogen component can vary by type (e.g. ethinyl estradiol or mestranol) and dose (ranging from “ultra-low dose” formulations of 0.01 mg to higher doses of 0.05 mg). The progestin component also varies by type (with effects ranging from strongly anti-androgenic to strongly androgenic) and dose (0.1–3.0 mg/pill). Hormonal regimens also vary based on whether the drug dose is constant or variable across a pill pack (e.g. monophasic versus multi-phasic doses).
These variations likely alter the downstream neurobiological effects of the pill. For example, in preclinical studies, 17β-estradiol’s neuronal effects depend on whether the hormone is administered cyclically, continuously, and with or without progestin. In ovariectomized animal models, hormone replacement regimens that consist of cyclic estradiol unopposed by progesterone enhance PFC spine density (Hao et al., 2007; Tang, 2004). However, regimens containing continuous estradiol (with or without progestin) and cyclic estradiol paired with cyclic progestin fail to induce similar synaptogenic effects (Ohm et al., 2012; reviewed in Hara et al., 2015), though these effects differ by brain region (i.e., hippocampus: Gould et al., 1990; Woolley et al., 1993). Additionally, a regimen of low-dose (but not high-dose) continuous ethinyl estradiol and levonorgestrel in rats induces a decrease in tyrosine hydroxylase in the locus coeruleus and a decrease in brain-derived neurotrophic factor (BDNF) mRNA in the hippocampus (Simone et al., 2015).
Given inconsistencies in the human neuroimaging literature on OC, future studies should, at minimum, report which formulation of OC is being used in their research samples (Hampson, 2020) or restrict sample populations to women using the same oral contraceptive regimen. In either case it should be acknowledged that results may not generalize to OC use as a whole, and may be specific to those formulations under investigation.
3.4. Direct hormone assays
Serum assessments of circulating sex hormones are essential for characterizing the endocrine effects of a particular OC formulation, yet these data are rarely acquired. While it is generally assumed that OC chronically suppresses the ovarian production of estradiol and progesterone, the magnitude of suppression may vary by OC formulation. In one study of OC users (of various formulations), mean estradiol and progesterone concentrations were suppressed to levels at or below those observed in the early follicular phase of naturally cycling controls (De Bondt et al., 2013). In contrast, a hormone regimen of low-dose ethinyl estradiol (0.02 mg) and levonorgestrel (0.1 mg) had strong suppressive effects on progesterone, with serum concentrations reduced by ~97% over a 28-day period, but no detectable suppressive effect on estradiol (Figure 2). Under this low-dose OC regimen (Aubra, Afaxys Pharmaceuticals), dynamic changes in estradiol mimicked those observed under naturally cycling conditions (Taylor et al., 2020). This variability in suppression (Vandever et al., 2008) underscores the critical importance of direct hormone assays in neuroimaging studies that consider endocrine factors. Thus, assumptions of universally suppressive effects of OC use are insufficient, and it is critical to determine the downstream endocrine effects of each regimen when forming hypotheses about neuronal effects of OC use. Failing to do so will make findings uninterpretable and hinder efforts at reproducibility. For a complete picture, studies should also assess serum concentrations of exogenous hormones, i.e. those attributable to the hormone regimen itself.
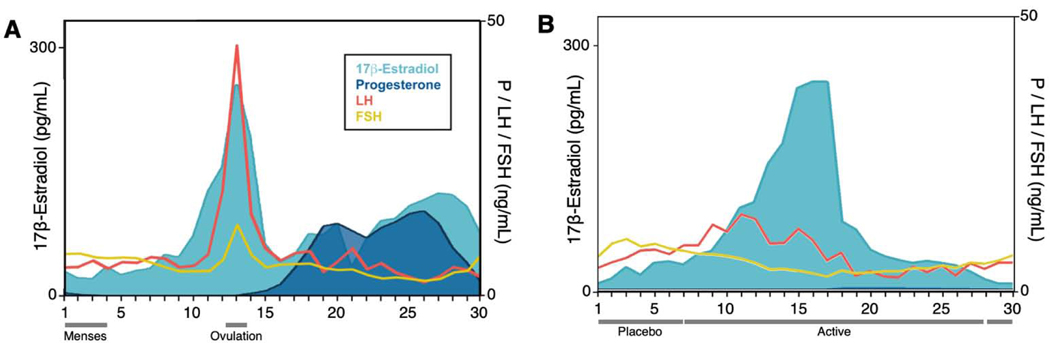
Figure 2. Endocrine profile of a woman across a menstrual cycle and on oral hormonal contraception.
A. Pituitary gonadotropins (LH, FSH) and gonadal hormones (estradiol, progesterone) across 30 days of a complete menstrual cycle. Estradiol exhibits a 8-fold increase prior to ovulation. Progesterone concentrations increase 80-fold during the luteal phase. B. Hormone concentrations during 30 days on a combined oral hormonal contraceptive (0.02 mg ethinyl estradiol, 0.1 mg levonorgestrel). In response to this OC formulation, progesterone was suppressed by 97% on average while estradiol concentrations were unmodified, representing incomplete suppression by the low-dose hormone regimen. Exogenous hormone concentrations were very low: ethinyl estradiol, M = 0.01 ng/mL; levonorgestrel, M = 0.91 ng/mL. Abbreviations: P, progesterone; LH, luteinizing hormone; FSH, follicle-stimulating hormone, Ref: Taylor et al., 2020
Finally, to fully understand the neurobiological effects of OC, we need preclinical animal studies that interrogate the extent to which ovarian hormone suppression alters hormone concentrations locally in the CNS. Rodent studies suggest some congruity between central and peripheral levels. For example, concentrations of sex hormones from serum are correlated with levels acquired from cerebral cortex and hippocampal tissue (Caruso et al., 2013) and a 4-week OC regimen of ethinyl estradiol and levonorgestrel suppressed concentrations of progesterone in the hippocampus by 65% (Porcu et al., 2012). In contrast, a recent study in marmosets reported opposing effects of an aromatase inhibitor on peripheral and central estradiol concentrations (Gervais et al., 2019). Peripheral hormone suppression could induce compensatory upregulation of hormone synthesis de novo in the brain. Preclinical studies are required to clarify the complex relationship between peripheral and central hormone levels, basic science work that will guide our mechanistic understanding of OC’s effect at the mesoscopic/macroscopic scale of human brain imaging.
3.5. Defining a control group
In the current literature, comparisons are often drawn between women currently using OC versus those not using OC. However, this comparison group conflates women who are naturally cycling now but have used OC in the past (“ever users”) with women who have never used hormonal contraceptives (“never users”). The hormonal milieu of past OC users may not be the same as women who have never used hormonal contraception, as some OC-related changes may persist on the order of months (Balogh et al., 1981; Panzer et al., 2006) or years (Chan et al., 2008) after pill discontinuation. Given our limited knowledge of long-term effects of OC use, control groups that mix “ever” and “never” users may obscure findings.
3.6. Age of initiation and duration of use
Two additional understudied factors that may shape OC’s influence on the brain are age of initiation and duration of use. Up to one-third of OC users begin OC use in early adolescence, yet we know relatively little about how hormone suppression impacts the developing brain (Cahill, 2018), though studies report higher incidences of depressive/mood effects in younger users (Mizutani et al., 2014; Skovlund et al., 2016). While the hippocampus and basal ganglia typically reach maturity in late childhood or early adolescence (Gogtay et al., 2006; Segawa, 2000), the development of the PFC is protracted, with cortical volumes stabilizing in the mid-20s (Lenroot and Giedd, 2006). The neuroendocrine changes that accompany puberty produce a second ‘window of opportunity’ or sensitive period in brain development (see Fuhrmann et al., 2015, for review).
In girls, the pubertal transition typically begins at 10–11 years of age and ends between the ages of 15 and 17. Many women begin OC use during this pubertal period. In a US study, 36% of 13–18-year-olds filled a prescription for OC (Ehrlich et al., 2011), and in a population Danish study, ~28% of 15–19-year-olds used OC (Skovlund et al., 2016). Given the early age of first exposure, OC use in adolescence has the potential to alter the organizational effects of endogenous sex hormones via chronic ovarian hormone suppression. However, to our knowledge, no large-scale prospective study has examined the impact of age of initiation and duration of OC use on neuronal development. Further, the short- and long-term effects of OC may differ. In adults, even short-term OC use is associated with gray matter volume changes (Lisofsky et al., 2016; Pletzer et al., 2015), however it is unclear whether these changes persist over time (Pletzer et al., 2019), or whether the magnitude of change tracks with total duration of use over longer timescales (e.g. years, decades).
In sum, in the interest of rigorous and reproduceable science, brain imaging studies on OC should incorporate these ‘best practices’ into study designs. This includes thoroughly characterizing participant demographics (e.g. formulation and regimen of OC used, age of initiation and duration of use, lifetime history of OC use and other hormone-based medications, and serum assessments of endogenous and exogenous sex hormones), enhancing sample sizes to allow for test-retest reliability, and prospectively designing studies with specificity around the OC formulation being studied.
4. A roadmap for the future: harnessing new methodological and technological approaches to bolster women’s health research
Beyond OC, there are many other opportunities to expand research efforts to advance knowledge on women’s health in cognitive neuroscience. Below we propose three programmatic initiatives to aid in this pursuit. We describe “Big Data” approaches, including the University of California Women’s Brain Initiative, that are beginning to address unmet areas of women’s health research at the population level. Next, we describe innovations in methodological and computational approaches in human neuroimaging that capture the dynamic properties of the endocrine system. We end with recommendations for cross-species translational studies that capitalize on emerging technologies from systems neuroscience to decipher estrogen and progesterone’s influence on populations of neurons recorded chronically at subcellular resolution. Our hope is that together these approaches generate novel discoveries about hormone action in the mammalian brain and stimulate research efforts, particularly within the human neuroimaging community.
4.1. The University of California Women’s Brain Initiative: Using ‘Big Data’ to benefit women’s health
Over the last ten years human neuroimaging has witnessed a remarkable growth in “Big Data” initiatives that are mapping the structural and functional connectome of the human brain at the population level. Large-scale, multi-site, “population neuroscience” approaches like the Human Connectome Project (HCP) (Van Essen et al., 2013) have transformed our understanding of brain organization and variability across disease states. Sister studies such as HCP-Aging (Bookheimer et al., 2019) and HCP-Development (Somerville et al., 2018) bring a lifespan perspective, while UK Biobank (Sudlow et al., 2015) merges brain phenotyping with extensive electronic health records in midlife and older adults. These initiatives offer an invaluable resource for probing fundamental questions about the human brain, yet it is striking that none were designed with women’s health in mind.
To address this, in 2019 we launched a population-based neuroimaging database dedicated specifically to strengthening women’s health research. The University of California Women’s Brain Initiative (UC-WBI) leverages the activity of the University of California’s brain imaging community. Although still in its infancy (data collection has rolled out at UC Santa Barbara and UC Berkeley, with a current n = 400), our goal is to expand to the nine UC campuses with a research-dedicated MRI facility, targeting the ~10,000 unique individuals scanned across sites each year. In addition to pooling standard MRI sequences and demographic/behavioral data, the UC-WBI provides extensive life-history data across a range of women’s health factors via a Women’s Reproductive Health History battery (e.g., the publicly available Menopause Health Questionnaire from the North American Menopause Society, at https://tinyurl.com/NAMS-MHQ).
One driving question for the UC-WBI is to leverage the population neuroimaging approach to understand how oral hormonal contraceptives impact the human brain. OC use is the kind of multifactorial problem that would benefit from a large-scale dataset that captures normal variability in OC use within the population. Using data generated from the UC-WBI database, we are investigating the association between OC use and brain morphology with respect to a person’s age of initiation, duration of use, and OC formulation, with participants matched across a broad range of demographic variables. This approach will set a new standard for OC–brain research, help define a path forward for rigorous, controlled follow-up studies, and represents one of a multitude of research questions that can be asked within the broader UC-WBI framework. Ultimately, our goal is to provide an open-access dataset that the neuroimaging community can draw upon to ask questions at the intersection of women’s health and the brain.
4.2. Dense-sampling neuroimaging studies capture the dynamic properties of the endocrine system
A central feature of the mammalian endocrine system is that hormone secretion varies over time. Circadian, infradian, and circannual rhythms are essential for sustaining many physiological processes. However, the study of brain–hormone interactions in human neuroscience relies heavily on cross-sectional designs that, by nature, cannot capture dynamic changes in hormone production. In network neuroscience, an emerging trend is to flip the cross-sectional design by densely sampling individuals over timescales of weeks, months, or years to provide greater insight into the dynamic properties of the human brain (Gordon et al., 2017; Gratton et al., 2018; Poldrack et al., 2015). Applying these dense-sampling approaches to probe brain–hormone interactions could reveal organizational principles of the functional connectome previously unknown, transforming our understanding of how hormones influence brain states.
For example, using resting-state functional MRI (rsMRI), Arélin and colleagues (2015) sampled an individual every 2–3 days across four menstrual cycles and found that progesterone was associated with increased connectivity between the hippocampus, dlPFC and sensorimotor cortex, providing evidence that inter-regional connectivity varies over the cycle. In a series of recent dense-sampling studies, we probed the dynamic properties of the brain over a complete menstrual cycle (30 consecutive days) and throughout an oral contraceptive regimen (30 consecutive days) (Pritschet et al., 2020; Taylor et al., 2020b). Using rsMRI and daily serum hormone measurements we probed the extent to which day-to-day changes in sex hormones modulate the brain’s intrinsic network architecture. Estradiol was associated with increased coherence across broad swaths of cortex while progesterone had the opposite, inhibitory effect (Pritschet et al., 2020). These effects were pronounced in network hubs populated with estrogen receptors and offer compelling evidence that sex hormones modulate widespread patterns of functional connectivity in the human brain. In a follow-up study, we applied techniques from complex systems analysis to capture the functional reorganization of canonical brain networks over time (Mueller et al., 2020). Over the menstrual cycle, brain networks displayed a high degree of network stability. One striking exception was a high degree of flexibility within the default mode network (localized to regions of the PFC) during ovulation, coincident with peaks in serum estradiol.
Next, using high-resolution imaging of the medial temporal lobe (MTL), we found that intrinsic fluctuations in progesterone across the menstrual cycle are associated with volumetric changes in CA2/3, entorhinal, perirhinal, and parahippocampal cortex. Chronic progesterone suppression induced by OC (Figure 2) eliminated these cycle-dependent effects. Building on earlier rodent findings (Gould et al. 1990; Woolley & McEwen, 1992), these results suggest that progesterone can rapidly and dynamically shape MTL morphology across the human menstrual cycle over unprecedented time-scales (Taylor et al., 2020b).
In sum, dense-sampling brain imaging studies offer a novel approach for probing the intrinsic dynamics of the human brain with high spatiotemporal resolution. Emerging results suggest that sex steroid hormones shape the functional and structural architecture of the human brain over unprecedented timescales. Moving forward, these dense-sampling approaches could be applied to brain imaging studies of other major neuroendocrine transitions, such as pubertal development or reproductive aging (e.g. menopause).
4.3. Systems Neuroscience Approaches
To fully understand hormone action in the mammalian brain, research efforts should be harmonized across rodent, non-human primate and human studies using translational and back-translational approaches. In particular, emerging technologies from systems neuroscience could be leveraged to decipher estrogen and progesterone’s influence on populations of neurons via chronic recording in awake behaving animals. Despite powerful evidence that sex steroid hormones influence spine structure and synaptic plasticity in rodents (Frick et al., 2015; Frick and Kim, 2018; Galea et al., 2017; Hara et al., 2015; Woolley and McEwen, 1993), hormonal influences on neural processing at the cellular and microcircuit level in intact animals is poorly understood. For example, an open question is whether estradiol-driven spine turnover in the hippocampus induces functional changes in hippocampal neuron activity during the performance of a cognitive task (e.g. navigation).
Historically, it has been technically difficult to chronically record neural activity from the same neurons across many sessions (e.g. over a 4–5-day rodent estrous cycle). However, recent developments in genetically encoded sensors and physiology instrumentation have greatly improved researchers’ ability to measure activity in the same neurons over long timescales. Genetically-encoded calcium indicators (Chen et al., 2013; Tian et al., 2009) combined with 2-photon imaging enable the chronic measurement of activity in large neural populations over several weeks in the hippocampus (Hainmueller and Bartos, 2018; Kaufman et al., 2020) and cortex (Driscoll et al., 2017; Huber et al., 2012; Pho et al., 2018). Moreover, genetic identification of particular cell types can indicate exactly how sex steroid hormones modulate neural microcircuitry, and has already been used effectively to investigate estrous cycle regulation of social touch (Clemens et al., 2019). This approach could be leveraged to measure changes in functional properties (e.g. hippocampal place fields) across the estrous cycle and disambiguate the specific cell types that are modulated. Measuring changes in large-scale neural activity across the estrous cycle or in response to pharmacological manipulation would offer a powerful approach for understanding how gonadal hormones influence neural responses and cognitive processing at the systems level.
5. Conclusion
Fifty years of basic science research has established a critical role for sex hormones in higher-order brain regions, including the hippocampus and prefrontal cortex. Yet, human brain imaging studies often overlook basic elements of endocrinology and women’s reproductive health. Moving forward, large-scale population-based studies, targeted dense-sampling studies, and translational research will provide novel insight into sex hormone action in the mammalian brain. Applying a women’s health lens to the study of the human brain is long overdue. Doing so may be critical for understanding basic principles of brain function and for women’s health at large.
Supplementary Material
Highlights.
Sex hormones are powerful neuromodulators that shape brain structure and function
Yet only 0.5% of neuroimaging articles consider endocrine-related health factors
This oversight obscures basic knowledge and limits progress in women’s health
100M women use oral contraception, yet we lack knowledge of its effects in the CNS
The brain imaging community should prioritize women’s health research
Acknowledgments.
Thank you to Bridget Queenan and Michael J. Goard for helpful edits and feedback. We also thank Emily Cao and Bridget Bush for assistance with data collection.
Funding. This work was supported by the Harvey L. Karp Discovery Award (CMT), the Brain and Behavior Research Foundation (EGJ), and the Hellman Family Fund (EGJ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbassi-Ghanavati M, Greer LG, Cunningham FG, 2009. Pregnancy and Laboratory Studies: A Reference Table for Clinicians. Obstet. Gynecol. 114, 1326–1331. 10.1097/AOG.0b013e3181c2bde8 [DOI] [PubMed] [Google Scholar]
- Abler B, Kumpfmüller D, Grön G, Walter M, Stingl J, Seeringer A, 2013. Neural Correlates of Erotic Stimulation under Different Levels of Female Sexual Hormones. PLOS ONE 8, e54447. 10.1371/journal.pone.0054447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert K, Hiscox J, Boyd B, Dumas J, Taylor W, Newhouse P, 2017. Estrogen enhances hippocampal gray-matter volume in young and older postmenopausal women: a prospective dose-response study. Neurobiol. Aging 56, 1–6. 10.1016/j.neurobiolaging.2017.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almey A, Milner TA, Brake WG, 2015. Estrogen receptors in the central nervous system and their implication for dopamine-dependent cognition in females. Horm. Behav. 74, 125–138. 10.1016/j.yhbeh.2015.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin Z, Canli T, Epperson CN, 2005. Effect of Estrogen-Serotonin Interactions on Mood and Cognition. Behav. Cogn. Neurosci. Rev. 4, 43–58. 10.1177/1534582305277152 [DOI] [PubMed] [Google Scholar]
- Arélin K, Mueller K, Barth C, Rekkas PV, Kratzsch J, Burmann I, Villringer A, Sacher J, 2015. Progesterone mediates brain functional connectivity changes during the menstrual cycle—a pilot resting state MRI study. Front. Neurosci. 9 10.3389/fnins.2015.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, 1990. Estrogen rapidly potentiates amphetamine-induced striatal dopamine release and rotational behavior during microdialysis. Neurosci. Lett. 118, 169–171. 10.1016/0304-3940(90)90618-J [DOI] [PubMed] [Google Scholar]
- Beery AK, Zucker I, 2011. Sex bias in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 35, 565–572. 10.1016/j.neubiorev.2010.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltz AM, Moser JS, 2019. Ovarian hormones: a long overlooked but critical contributor to cognitive brain structures and function. Ann. N. Y. Acad. Sci. 1464, 156–180. 10.1111/nyas.14255 [DOI] [PubMed] [Google Scholar]
- Berg FD, Kuss E, 1992. Serum concentration and urinary excretion of “classical” estrogens, catecholestrogens and 2-methoxyestrogens in normal human pregnancy. Arch. Gynecol. Obstet. 251, 17–27. 10.1007/BF02718274 [DOI] [PubMed] [Google Scholar]
- Berman KF, Schmidt PJ, Rubinow DR, Danaceau MA, Horn JDV, Esposito G, Ostrem JL, Weinberger DR, 1997. Modulation of cognition-specific cortical activity by gonadal steroids: A positron-emission tomography study in women. Proc. Natl. Acad. Sci. 94, 8836–8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonenberger M, Groschwitz RC, Kumpfmueller D, Groen G, Plener PL, Abler B, 2013. It’s all about money: oral contraception alters neural reward processing. NeuroReport 24, 951–955. 10.1097/WNR.0000000000000024 [DOI] [PubMed] [Google Scholar]
- Bookheimer SY, Salat DH, Terpstra M, Ances BM, Barch DM, Buckner RL, Burgess GC, Curtiss SW, Diaz-Santos M, Elam JS, Fischl B, Greve DN, Hagy HA, Harms MP, Hatch OM, Hedden T, Hodge C, Japardi KC, Kuhn TP, Ly TK, Smith SM, Somerville LH, Uğurbil K, van der Kouwe A, Van Essen D, Woods RP, Yacoub E, 2019. The Lifespan Human Connectome Project in Aging: An overview. NeuroImage 185, 335–348. 10.1016/j.neuroimage.2018.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, Morgan TE, Pike CJ, Mack WJ, Stanczyk FZ, Nilsen J, 2008. Progesterone receptors: Form and function in brain. Front. Neuroendocrinol., Rapid Actions of Steroid Hormones 29, 313–339. 10.1016/j.yfrne.2008.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson RA, 1981. Oral contraception: mechanism of action. Clin. Obstet. Gynecol. 24, 869–877. [DOI] [PubMed] [Google Scholar]
- Burger HG, 2002. Hormonal Changes in the Menopause Transition. Recent Prog. Horm. Res. 57, 257–275. 10.1210/rp.57.1.257 [DOI] [PubMed] [Google Scholar]
- Cahill L, 2018. How does hormonal contraception affect the developing human adolescent brain? Curr. Opin. Behav. Sci., Sex and Gender 23, 131–135. 10.1016/j.cobeha.2018.06.015 [DOI] [Google Scholar]
- Cai JX, Arnsten AFT, 1997. Dose-Dependent Effects of the Dopamine D1 Receptor Agonists A77636 or SKF81297 On Spatial Working Memory in Aged Monkeys. J. Pharmacol. Exp. Ther. 283, 183–189. [PubMed] [Google Scholar]
- Caruso D, Pesaresi M, Abbiati F, Calabrese D, Giatti S, Garcia-Segura LM, Melcangi RC, 2013. Comparison of plasma and cerebrospinal fluid levels of neuroactive steroids with their brain, spinal cord and peripheral nerve levels in male and female rats. Psychoneuroendocrinology 38, 2278–2290. 10.1016/j.psyneuen.2013.04.016 [DOI] [PubMed] [Google Scholar]
- Casey BJ, Cannonier T, Conley MI, Cohen AO, Barch DM, Heitzeg MM, Soules ME, Teslovich T, Dellarco DV, Garavan H, Orr CA, Wager TD, Banich MT, Speer NK, Sutherland MT, Riedel MC, Dick AS, Bjork JM, Thomas KM, Chaarani B, Mejia MH, Hagler DJ, Daniela Cornejo M, Sicat CS, Harms MP, Dosenbach NUF, Rosenberg M, Earl E, Bartsch H, Watts R, Polimeni JR, Kuperman JM, Fair DA, Dale AM, 2018. The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Dev. Cogn. Neurosci., The Adolescent Brain Cognitive Development (ABCD) Consortium: Rationale, Aims, and Assessment Strategy 32, 43–54. 10.1016/j.dcn.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan M-F, Dowsett M, Folkerd E, Wareham N, Luben R, Welch A, Bingham S, Khaw K-T, 2008. Past oral contraceptive and hormone therapy use and endogenous hormone concentrations in postmenopausal women: Menopause 15, 332–339. 10.1097/gme.0b013e31806458d9 [DOI] [PubMed] [Google Scholar]
- Chen T-W, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, Looger LL, Svoboda K, Kim DS, 2013. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300. 10.1038/nature12354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christin-Maitre S, 2013. History of oral contraceptive drugs and their use worldwide. Best Pract. Res. Clin. Endocrinol. Metab. 27, 3–12. 10.1016/j.beem.2012.11.004 [DOI] [PubMed] [Google Scholar]
- Clayton JA, Collins FS, 2014. Policy: NIH to balance sex in cell and animal studies. Nat. News 509, 282 10.1038/509282a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens AM, Lenschow C, Beed P, Li L, Sammons R, Naumann RK, Wang H, Schmitz D, Brecht M, 2019. Estrus-Cycle Regulation of Cortical Inhibition. Curr. Biol. 29, 605–615.e6. 10.1016/j.cub.2019.01.045 [DOI] [PubMed] [Google Scholar]
- Daniels K, Daugherty J, Jones J, Mosher W, 2015. Current Contraceptive Use and Variation by Selected Characteristics Among Women Aged 15–44: United States, 2011–2013. Natl. Health Stat. Rep. 1–14. https://doi.org/Retrieved from: http://europepmc.org/abstract/med/26556545 [PubMed] [Google Scholar]
- Daniels K, Jones J, 2013. Contraceptive methods women have ever used: United States, 1982–2010. US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics. [Google Scholar]
- De Bondt T, Jacquemyn Y, Van Hecke W, Sijbers J, Sunaert S, Parizel PM, 2013. Regional gray matter volume differences and sex-hormone correlations as a function of menstrual cycle phase and hormonal contraceptives use. Brain Res. 1530, 22–31. 10.1016/j.brainres.2013.07.034 [DOI] [PubMed] [Google Scholar]
- De Bondt T, Smeets D, Pullens P, Van Hecke W, Jacquemyn Y, Parizel PM, 2015. Stability of resting state networks in the female brain during hormonal changes and their relation to premenstrual symptoms. Brain Res. 1624, 275–285. 10.1016/j.brainres.2015.07.045 [DOI] [PubMed] [Google Scholar]
- Driscoll LN, Pettit NL, Minderer M, Chettih SN, Harvey CD, 2017. Dynamic Reorganization of Neuronal Activity Patterns in Parietal Cortex. Cell 170, 986–999.e16. 10.1016/j.cell.2017.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff SJ, Hampson E, 2000. A Beneficial Effect of Estrogen on Working Memory in Postmenopausal Women Taking Hormone Replacement Therapy. Horm. Behav. 38, 262–276. 10.1006/hbeh.2000.1625 [DOI] [PubMed] [Google Scholar]
- Dumas JA, Kutz AM, Naylor MR, Johnson JV, Newhouse PA, 2010. Increased memory load-related frontal activation after estradiol treatment in postmenopausal women. Horm. Behav. 58, 929–935. 10.1016/j.yhbeh.2010.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich E, Gibson T, Mark T, 2011. Trends in prescriptions for oral contraceptives among U.S. teenagers. Truven Health Anal. [Google Scholar]
- Engman J, Poromaa IS, Moby L, Wikström J, Fredrikson M, Gingnell M, 2017. Hormonal Cycle and Contraceptive Effects on Amygdala and Salience Resting-State Networks in Women with Previous Affective Side Effects on the Pill. Neuropsychopharmacology 43, 555–563. 10.1038/npp.2017.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperson CN, Amin Z, Ruparel K, Gur R, Loughead J, 2012. Interactive effects of estrogen and serotonin on brain activation during working memory and affective processing in menopausal women. Psychoneuroendocrinology 37, 372–382. 10.1016/j.psyneuen.2011.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri E, An Y, Gonzalez-Freire M, Zoli M, Maggio M, Studenski SA, Egan JM, Chia CW, Ferrucci L, 2016. Bioavailable Testosterone Linearly Declines Over A Wide Age Spectrum in Men and Women From The Baltimore Longitudinal Study of Aging. J. Gerontol. Ser. A 71, 1202–1209. 10.1093/gerona/glw021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman S, Ammar W, Lo K, Trepman E, Zuylen M van Etzioni, O., 2019. Quantifying Sex Bias in Clinical Studies at Scale With Automated Data Extraction. JAMA Netw. Open 2, e196700–e196700. 10.1001/jamanetworkopen.2019.6700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortress AM, Frick KM, 2014. Epigenetic regulation of estrogen-dependent memory. Front. Neuroendocrinol. 35, 530–549. 10.1016/j.yfrne.2014.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, 2019. Estrogens and Memory: Basic Research and Clinical Implications. Oxford University Press. [Google Scholar]
- Frick KM, Kim J, 2018. Mechanisms underlying the rapid effects of estradiol and progesterone on hippocampal memory consolidation in female rodents. Horm. Behav. 104, 100–110. 10.1016/j.yhbeh.2018.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Kim J, Tuscher JJ, Fortress AM, 2015. Sex steroid hormones matter for learning and memory: estrogenic regulation of hippocampal function in male and female rodents. Learn. Mem. 22, 472–493. 10.1101/lm.037267.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Tuscher JJ, Koss WA, Kim J, Taxier LR, 2018. Estrogenic regulation of memory consolidation: A look beyond the hippocampus, ovaries, and females. Physiol. Behav., The Proceedings of the American University Symposium on Sex Differences: from Neuroscience to the Clinic and Beyond 187, 57–66. 10.1016/j.physbeh.2017.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann D, Knoll LJ, Blakemore S-J, 2015. Adolescence as a Sensitive Period of Brain Development. Trends Cogn. Sci. 19, 558–566. 10.1016/j.tics.2015.07.008 [DOI] [PubMed] [Google Scholar]
- Galea L. a. M., Leuner B, Slattery DA, 2014. Hippocampal Plasticity during the Peripartum Period: Influence of Sex Steroids, Stress and Ageing. J. Neuroendocrinol. 26, 641–648. 10.1111/jne.12177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea LA, Qiu W, Duarte-Guterman P, 2018. Beyond sex differences: short and long-term implications of motherhood on women’s health. Curr. Opin. Physiol., Sex Differences 6, 82–88. 10.1016/j.cophys.2018.06.003 [DOI] [Google Scholar]
- Galea LAM, Choleris E, YK Albert A, McCarthy MM, Sohrabji F, 2019. The Promises and Pitfalls of Sex Difference Research. Front. Neuroendocrinol. 100817. 10.1016/j.yfrne.2019.100817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea LAM, Frick KM, Hampson E, Sohrabji F, Choleris E, 2017. Why estrogens matter for behavior and brain health. Neurosci. Biobehav. Rev, SI:IBNS-2015 76, 363–379. 10.1016/j.neubiorev.2016.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea LAM, Perrot-Sinal TS, Kavaliers M, Ossenkopp K-P, 1999. Relations of hippocampal volume and dentate gyrus width to gonadal hormone levels in male and female meadow voles. Brain Res. 821, 383–391. 10.1016/S0006-8993(99)01100-2 [DOI] [PubMed] [Google Scholar]
- Gaspard UJ, Romus MA, Gillain D, Duvivier J, Demey-Ponsart E, Franchimont P, 1983. Plasma hormone levels in women receiving new oral contraceptives containing ethinyl estradiol plus levonorgestrel or desogestrel. Contraception 27, 577–590. 10.1016/0010-7824(83)90023-9 [DOI] [PubMed] [Google Scholar]
- Gervais NJ, Remage-Healey L, Starrett JR, Pollak DJ, Mong JA, Lacreuse A, 2019. Adverse Effects of Aromatase Inhibition on the Brain and Behavior in a Nonhuman Primate. J. Neurosci. 39, 918–928. 10.1523/JNEUROSCI.0353-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Nugent TF, Herman DH, Ordonez A, Greenstein D, Hayashi KM, Clasen L, Toga AW, Giedd JN, Rapoport JL, Thompson PM, 2006. Dynamic mapping of normal human hippocampal development. Hippocampus 16, 664–672. 10.1002/hipo.20193 [DOI] [PubMed] [Google Scholar]
- Gordon EM, Laumann TO, Gilmore AW, Newbold DJ, Greene DJ, Berg JJ, Ortega M, Hoyt-Drazen C, Gratton C, Sun H, Hampton JM, Coalson RS, Nguyen AL, McDermott KB, Shimony JS, Snyder AZ, Schlaggar BL, Petersen SE, Nelson SM, Dosenbach NUF, 2017. Precision Functional Mapping of Individual Human Brains. Neuron 95, 791–807.e7. 10.1016/j.neuron.2017.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Woolley C, Frankfurt M, McEwen B, 1990. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J. Neurosci. 10, 1286–1291. 10.1523/JNEUROSCI.10-04-01286.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton C, Laumann TO, Nielsen AN, Greene DJ, Gordon EM, Gilmore AW, Nelson SM, Coalson RS, Snyder AZ, Schlaggar BL, Dosenbach NUF, Petersen SE, 2018. Functional Brain Networks Are Dominated by Stable Group and Individual Factors, Not Cognitive or Daily Variation. Neuron 98, 439–452.e5. 10.1016/j.neuron.2018.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainmueller T, Bartos M, 2018. Parallel emergence of stable and dynamic memory engrams in the hippocampus. Nature 558, 292–296. 10.1038/s41586-018-0191-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson E, 2020. A brief guide to the menstrual cycle and oral contraceptive use for researchers in behavioral endocrinology. Horm. Behav. 119, 104655. 10.1016/j.yhbeh.2019.104655 [DOI] [PubMed] [Google Scholar]
- Hampson E, 2018. Estrogens, Aging, and Working Memory. Curr. Psychiatry Rep. 20, 109 10.1007/s11920-018-0972-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson E, Morley EE, 2013. Estradiol concentrations and working memory performance in women of reproductive age. Psychoneuroendocrinology 38, 2897–2904. 10.1016/j.psyneuen.2013.07.020 [DOI] [PubMed] [Google Scholar]
- Hao J, Janssen WG, Tang Y, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, others, 2003. Estrogen increases the number of spinophilin-immunoreactive spines in the hippocampus of young and aged female rhesus monkeys. J. Comp. Neurol. 465, 540–550. [DOI] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Janssen WGM, Lou W, Lasley BL, Hof PR, Morrison JH, 2007. Interactive effects of age and estrogen on cognition and pyramidal neurons in monkey prefrontal cortex. Proc. Natl. Acad. Sci. 104, 11465–11470. 10.1073/pnas.0704757104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Leffler AE, Leffler SR, Janssen WGM, Lou W, McKay H, Roberts JA, Wearne SL, Hof PR, Morrison JH, 2006. Estrogen Alters Spine Number and Morphology in Prefrontal Cortex of Aged Female Rhesus Monkeys. J. Neurosci. 26, 2571–2578. 10.1523/JNEUROSCI.3440-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Waters EM, McEwen BS, Morrison JH, 2015. Estrogen Effects on Cognitive and Synaptic Health Over the Lifecourse. Physiol. Rev. 95, 785–807. 10.1152/physrev.00036.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekzema E, Barba-Müller E, Pozzobon C, Picado M, Lucco F, García-García D, Soliva JC, Tobeña A, Desco M, Crone EA, Ballesteros A, Carmona S, Vilarroya O, 2017. Pregnancy leads to long-lasting changes in human brain structure. Nat. Neurosci. 20, 287–296. 10.1038/nn.4458 [DOI] [PubMed] [Google Scholar]
- Huber D, Gutnisky DA, Peron S, O’Connor DH, Wiegert JS, Tian L, Oertner TG, Looger LL, Svoboda K, 2012. Multiple dynamic representations in the motor cortex during sensorimotor learning. Nature 484, 473–478. 10.1038/nature11039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs EG, D’Esposito M, 2011. Estrogen Shapes Dopamine-Dependent Cognitive Processes: Implications for Women’s Health. J. Neurosci. 31, 5286–5293. 10.1523/JNEUROSCI.6394-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs EG, Goldstein JM, 2018. The middle-aged brain: biological sex and sex hormones shape memory circuitry. Curr. Opin. Behav. Sci., Sex and Gender 23, 84–91. 10.1016/j.cobeha.2018.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs EG, Weiss B, Makris N, Whitfield-Gabrieli S, Buka SL, Klibanski A, Goldstein JM, 2017. Reorganization of Functional Networks in Verbal Working Memory Circuitry in Early Midlife: The Impact of Sex and Menopausal Status. Cereb. Cortex 27, 2857–2870. 10.1093/cercor/bhw127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs EG, Weiss BK, Makris N, Whitfield-Gabrieli S, Buka SL, Klibanski A, Goldstein JM, 2016. Impact of Sex and Menopausal Status on Episodic Memory Circuitry in Early Midlife. J. Neurosci. 36, 10163–10173. 10.1523/JNEUROSCI.0951-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J, Mosher W, Daniels K, 2013. Current contraceptive use in the united states, 2006–2010, and changes in patterns of use since 1995. [PubMed] [Google Scholar]
- Jones RE, Lopez KH, 2013. Human Reproductive Biology. Academic Press. [Google Scholar]
- Kaczkurkin AN, Raznahan A, Satterthwaite TD, 2018. Sex differences in the developing brain: insights from multimodal neuroimaging. Neuropsychopharmacology 1 10.1038/s41386-018-0111-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman AM, Geiller T, Losonczy A, 2020. A Role for the Locus Coeruleus in Hippocampal CA1 Place Cell Reorganization during Spatial Reward Learning. Neuron 105, 1018–1026.e4. 10.1016/j.neuron.2019.12.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsley CH, Lambert KG, 2008. Reproduction-Induced Neuroplasticity: Natural Behavioural and Neuronal Alterations Associated with the Production and Care of Offspring. J. Neuroendocrinol. 20, 515–525. 10.1111/j.1365-2826.2008.01667.x [DOI] [PubMed] [Google Scholar]
- Knowles J, Correia J, 2015. The Birth Control Pill - A History. Planned Parenthood Federation of America. [Google Scholar]
- Lenroot RK, Giedd JN, 2006. Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neurosci. Biobehav. Rev. 30, 718–729. 10.1016/j.neubiorev.2006.06.001 [DOI] [PubMed] [Google Scholar]
- Leranth C, Shanabrough M, Redmond DE, 2002. Gonadal hormones are responsible for maintaining the integrity of spine synapses in the CA1 hippocampal subfield of female nonhuman primates. J. Comp. Neurol. 447, 34–42. 10.1002/cne.10230 [DOI] [PubMed] [Google Scholar]
- Lisofsky N, Mårtensson J, Eckert A, Lindenberger U, Gallinat J, Kühn S, 2015. Hippocampal volume and functional connectivity changes during the female menstrual cycle. NeuroImage 118, 154–162. 10.1016/j.neuroimage.2015.06.012 [DOI] [PubMed] [Google Scholar]
- Lisofsky N, Riediger M, Gallinat J, Lindenberger U, Kühn S, 2016. Hormonal contraceptive use is associated with neural and affective changes in healthy young women. NeuroImage 134, 597–606. 10.1016/j.neuroimage.2016.04.042 [DOI] [PubMed] [Google Scholar]
- Lotze M, Domin M, Gerlach FH, Gaser C, Lueders E, Schmidt CO, Neumann N, 2019. Novel findings from 2,838 Adult Brains on Sex Differences in Gray Matter Brain Volume. Sci. Rep. 9, 1671 10.1038/s41598-018-38239-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louw-du Toit R, Perkins MS, Hapgood JP, Africander D, 2017. Comparing the androgenic and estrogenic properties of progestins used in contraception and hormone therapy. Biochem. Biophys. Res. Commun. 491, 140–146. 10.1016/j.bbrc.2017.07.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud R, Wainwright SR, Galea LAM, 2016. Sex hormones and adult hippocampal neurogenesis: Regulation, implications, and potential mechanisms. Front. Neuroendocrinol., Hormonal Regulation of Adult Neurogenesis: Implications for Disease 41, 129–152. 10.1016/j.yfrne.2016.03.002 [DOI] [PubMed] [Google Scholar]
- McEwen B, 2002. Estrogen actions throughout the brain. Recent Prog. Horm. Res. 57, 357–384. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Alves SE, 1999. Estrogen Actions in the Central Nervous System. Endocr. Rev. 20, 279–307. 10.1210/edrv.20.3.0365 [DOI] [PubMed] [Google Scholar]
- Merz C, Tabbert K, Schweckendiek J, Klucken T, Vaitl D, Stark R, Wolf OT, 2012a. Oral contraceptive usage alters the effects of cortisol on implicit fear learning. Horm. Behav. 62, 531–538. 10.1016/j.yhbeh.2012.09.001 [DOI] [PubMed] [Google Scholar]
- Merz C, Tabbert K, Schweckendiek J, Klucken T, Vaitl D, Stark R, Wolf OT, 2012b. Neuronal correlates of extinction learning are modulated by sex hormones. Soc. Cogn. Affect. Neurosci. 7, 819–830. 10.1093/scan/nsr063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani S, Noro Y, Kotera M, Goto S, 2014. Pharmacoepidemiological characterization of druginduced adverse reaction clusters towards understanding of their mechanisms. Comput. Biol. Chem., Advances in Bioinformatics: Twelfth Asia Pacific Bioinformatics Conference (APBC2014) 50, 50–59. 10.1016/j.compbiolchem.2014.01.006 [DOI] [PubMed] [Google Scholar]
- Montoya ER, Bos PA, 2017. How Oral Contraceptives Impact Social-Emotional Behavior and Brain Function. Trends Cogn. Sci. 21, 125–136. 10.1016/j.tics.2016.11.005 [DOI] [PubMed] [Google Scholar]
- Morrison JH, Brinton RD, Schmidt PJ, Gore AC, 2006. Estrogen, Menopause, and the Aging Brain: How Basic Neuroscience Can Inform Hormone Therapy in Women. J. Neurosci. 26, 10332–10348. 10.1523/JNEUROSCI.3369-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller JM, Pritschet L, Santander T, Taylor CM, Grafton ST, Jacobs EG, Carlson JM, 2020. Dynamic community detection reveals transient reorganization of functional brain networks across a female menstrual cycle. bioRxiv 2020.06.29.178152. 10.1101/2020.06.29.178152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohm DT, Bloss EB, Janssen WG, Dietz KC, Wadsworth S, Lou W, Gee NA, Lasley BL, Rapp PR, Morrison JH, 2012. Clinically Relevant Hormone Treatments Fail to Induce Spinogenesis in Prefrontal Cortex of Aged Female Rhesus Monkeys. J. Neurosci. 32, 11700–11705. 10.1523/JNEUROSCI.1881-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh A, Fadiran EO, Uhl K, Throckmorton DC, 2011. Adverse effects in women: implications for drug development and regulatory policies. Expert Rev. Clin. Pharmacol. 4, 453–466. 10.1586/ecp.11.29 [DOI] [PubMed] [Google Scholar]
- Petersen N, Kilpatrick LA, Goharzad A, Cahill L, 2014. Oral contraceptive pill use and menstrual cycle phase are associated with altered resting state functional connectivity. NeuroImage 90, 24–32. 10.1016/j.neuroimage.2013.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen N, Touroutoglou A, Andreano JM, Cahill L, 2015. Oral contraceptive pill use is associated with localized decreases in cortical thickness. Hum. Brain Mapp. 36, 2644–2654. 10.1002/hbm.22797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitti DB, 2003. Combination Estrogen–Progestin Oral Contraceptives. N. Engl. J. Med. 349, 1443–1450. 10.1056/NEJMcp030751 [DOI] [PubMed] [Google Scholar]
- Pho GN, Goard MJ, Woodson J, Crawford B, Sur M, 2018. Task-dependent representations of stimulus and choice in mouse parietal cortex. Nat. Commun. 9, 1–16. 10.1038/s41467-018-05012-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletzer B, Harris T, Hidalgo-Lopez E, 2019. Previous contraceptive treatment relates to grey matter volumes in the hippocampus and basal ganglia. Sci. Rep. 9, 11003 10.1038/s41598-019-47446-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletzer B, Kronbichler M, Aichhorn M, Bergmann J, Ladurner G, Kerschbaum HH, 2010. Menstrual cycle and hormonal contraceptive use modulate human brain structure. Brain Res. 1348, 55–62. 10.1016/j.brainres.2010.06.019 [DOI] [PubMed] [Google Scholar]
- Pletzer B, Kronbichler M, Kerschbaum H, 2015. Differential effects of androgenic and anti-androgenic progestins on fusiform and frontal gray matter volume and face recognition performance. Brain Res. 1596, 108–115. 10.1016/j.brainres.2014.11.025 [DOI] [PubMed] [Google Scholar]
- Pletzer B, Kronbichler M, Nuerk H-C, Kerschbaum H, 2014. Hormonal contraceptives masculinize brain activation patterns in the absence of behavioral changes in two numerical tasks. Brain Res. 1543, 128–142. 10.1016/j.brainres.2013.11.007 [DOI] [PubMed] [Google Scholar]
- Pletzer BA, Kerschbaum HH, 2014. 50 years of hormonal contraception—time to find out, what it does to our brain. Front. Neurosci. 8, 256 10.3389/fnins.2014.00256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Laumann TO, Koyejo O, Gregory B, Hover A, Chen M-Y, Gorgolewski KJ, Luci J, Joo SJ, Boyd RL, Hunicke-Smith S, Simpson ZB, Caven T, Sochat V, Shine JM, Gordon E, Snyder AZ, Adeyemo B, Petersen SE, Glahn DC, Reese Mckay D, Curran JE, Göring HHH, Carless MA, Blangero J, Dougherty R, Leemans A, Handwerker DA, Frick L, Marcotte EM, Mumford JA, 2015. Long-term neural and physiological phenotyping of a single human. Nat. Commun. 6, 1–15. 10.1038/ncomms9885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcu P, Mostallino MC, Sogliano C, Santoru F, Berretti R, Concas A, 2012. Long-term administration with levonorgestrel decreases allopregnanolone levels and alters GABAA receptor subunit expression and anxiety-like behavior. Pharmacol. Biochem. Behav. 102, 366–372. 10.1016/j.pbb.2012.05.011 [DOI] [PubMed] [Google Scholar]
- Pritschet L, Santander T, Taylor CM, Layher E, Yu S, Miller MB, Grafton ST, Jacobs EG, 2020. Functional reorganization of brain networks across the human menstrual cycle. NeuroImage 220, 117091. 10.1016/j.neuroimage.2020.117091 [DOI] [PubMed] [Google Scholar]
- Protopopescu X, Butler T, Pan H, Root J, Altemus M, Polanecsky M, McEwen B, Silbersweig D, Stern E, 2008. Hippocampal structural changes across the menstrual cycle. Hippocampus 18, 985–988. 10.1002/hipo.20468 [DOI] [PubMed] [Google Scholar]
- Qiu LR, Germann J, Spring S, Alm C, Vousden DA, Palmert MR, Lerch JP, 2013. Hippocampal volumes differ across the mouse estrous cycle, can change within 24hours, and associate with cognitive strategies. NeuroImage 83, 593–598. 10.1016/j.neuroimage.2013.06.074 [DOI] [PubMed] [Google Scholar]
- Rapkin AJ, Morgan M, Sogliano C, Biggio G, Concas A, 2006. Decreased neuroactive steroids induced by combined oral contraceptive pills are not associated with mood changes. Fertil. Steril. 85, 1371–1378. 10.1016/j.fertnstert.2005.10.031 [DOI] [PubMed] [Google Scholar]
- Rapp PR, Morrison JH, Roberts JA, 2003. Cyclic Estrogen Replacement Improves Cognitive Function in Aged Ovariectomized Rhesus Monkeys. J. Neurosci. 23, 5708–5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentz DM, Weiss BK, Jacobs EG, Cherkerzian S, Klibanski A, Remington A, Aizley H, Goldstein JM, 2017. Sex differences in episodic memory in early midlife: impact of reproductive aging. Menopause 24, 400–408. 10.1097/GME.0000000000000771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie SJ, Cox SR, Shen X, Lombardo MV, Reus LM, Alloza C, Harris MA, Alderson HL, Hunter S, Neilson E, Liewald DCM, Auyeung B, Whalley HC, Lawrie SM, Gale CR, Bastin ME, McIntosh AM, Deary IJ, 2018. Sex Differences in the Adult Human Brain: Evidence from 5216 UK Biobank Participants. Cereb. Cortex 28, 2959–2975. 10.1093/cercor/bhy109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera R, Yacobson I, Grimes D, 1999. The mechanism of action of hormonal contraceptives and intrauterine contraceptive devices. Am. J. Obstet. Gynecol. 181, 1263–1269. 10.1016/S0002-9378(99)70120-1 [DOI] [PubMed] [Google Scholar]
- Rossetti MF, Cambiasso MJ, Holschbach MA, Cabrera R, 2016. Oestrogens and Progestagens: Synthesis and Action in the Brain. J. Neuroendocrinol. 28 10.1111/jne.12402 [DOI] [PubMed] [Google Scholar]
- Ruigrok ANV, Salimi-Khorshidi G, Lai M-C, Baron-Cohen S, Lombardo MV, Tait RJ, Suckling J, 2014. A meta-analysis of sex differences in human brain structure. Neurosci. Biobehav. Rev. 39, 34–50. 10.1016/j.neubiorev.2013.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumberg B, Baars A, Fiebach J, Ladd ME, Forsting M, Senf W, Gizewski ER, 2010. Cycle and gender-specific cerebral activation during a verb generation task using fMRI: Comparison of women in different cycle phases, under oral contraception, and men. Neurosci. Res. 66, 366–371. 10.1016/j.neures.2009.12.011 [DOI] [PubMed] [Google Scholar]
- Sacher J, Neumann J, Okon-Singer H, Gotowiec S, Villringer A, 2013. Sexual dimorphism in the human brain: evidence from neuroimaging. Magn. Reson. Imaging 31, 366–375. 10.1016/j.mri.2012.06.007 [DOI] [PubMed] [Google Scholar]
- Schaefer A, Kong R, Gordon EM, Laumann TO, Zuo X-N, Holmes AJ, Eickhoff SB, Yeo BTT, 2018. Local-Global Parcellation of the Human Cerebral Cortex from Intrinsic Functional Connectivity MRI. Cereb. Cortex 28, 3095–3114. 10.1093/cercor/bhx179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schock H, Zeleniuch-Jacquotte A, Lundin E, Grankvist K, Lakso H-Å, Idahl A, Lehtinen M, Surcel H-M, Fortner RT, 2016. Hormone concentrations throughout uncomplicated pregnancies: a longitudinal study. BMC Pregnancy Childbirth 16, 146 10.1186/s12884-016-0937-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segawa M, 2000. Development of the nigrostriatal dopamine neuron and the pathways in the basal ganglia. Brain Dev., Segawa Supplement 22, 1–4. 10.1016/S0387-7604(00)00149-2 [DOI] [PubMed] [Google Scholar]
- Shanmugan S, Epperson CN, 2014. Estrogen and the prefrontal cortex: Towards a new understanding of estrogen’s effects on executive functions in the menopause transition. Hum. Brain Mapp. 35, 847–865. 10.1002/hbm.22218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Smith SA, Boukina N, Dordari A, Mistry A, Taylor BC, Felix N, Cameron A, Fang Z, Smith A, Ismail N, 2020. Use of the birth control pill affects stress reactivity and brain structure and function. Horm. Behav. 124, 104783. 10.1016/j.yhbeh.2020.104783 [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Pugh KR, Fulbright RK, Skudlarski P, Mencl WE, Constable RT, Naftolin F, Palter SF, Marchione KE, Katz L, Shankweiler DP, Fletcher JM, Lacadie C, Keltz M, Gore JC, 1999. Effect of Estrogen on Brain Activation Patterns in Postmenopausal Women During Working Memory Tasks. JAMA 281, 1197–1202. 10.1001/jama.281.13.1197 [DOI] [PubMed] [Google Scholar]
- Simone J, Bogue EA, Bhatti DL, Day LE, Farr NA, Grossman AM, Holmes PV, 2015. Ethinyl estradiol and levonorgestrel alter cognition and anxiety in rats concurrent with a decrease in tyrosine hydroxylase expression in the locus coeruleus and brain-derived neurotrophic factor expression in the hippocampus. Psychoneuroendocrinology 62, 265–278. 10.1016/j.psyneuen.2015.08.015 [DOI] [PubMed] [Google Scholar]
- Sitruk-Ware R, Nath A, 2013. Characteristics and metabolic effects of estrogen and progestins contained in oral contraceptive pills. Best Pract. Res. Clin. Endocrinol. Metab. 27, 13–24. 10.1016/j.beem.2012.09.004 [DOI] [PubMed] [Google Scholar]
- Skovlund CW, Mørch LS, Kessing LV, Lidegaard Ø, 2016. Association of Hormonal Contraception With Depression. JAMA Psychiatry 73, 1154–1162. 10.1001/jamapsychiatry.2016.2387 [DOI] [PubMed] [Google Scholar]
- Somerville LH, Bookheimer SY, Buckner RL, Burgess GC, Curtiss SW, Dapretto M, Stine Elam J, Gaffrey MS, Harms MP, Hodge C, Kandala S, Kastman EK, Nichols TE, Schlaggar BL, Smith SM, Thomas KM, Yacoub E, Van Essen DC, Barch DM, 2018. The Lifespan Human Connectome Project in Development: A large-scale study of brain connectivity development in 5–21 year olds. NeuroImage 183, 456–468. 10.1016/j.neuroimage.2018.08.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spona J, Elstein M, Feichtinger W, Sullivan H, Lüdicke F, Müller U, Düsterberg B, 1996. Shorter pill-free interval in combined oral contraceptives decreases follicular development. Contraception 54, 71–77. 10.1016/0010-7824(96)00137-0 [DOI] [PubMed] [Google Scholar]
- Stricker Reto, Eberhart R, Chevailler M-C, Quinn FA, Bischof P, Stricker René, 2006. Establishment of detailed reference values for luteinizing hormone, follicle stimulating hormone, estradiol, and progesterone during different phases of the menstrual cycle on the Abbott ARCHITECT® analyzer. Clin. Chem. Lab. Med. CCLM 44 10.1515/CCLM.2006.160 [DOI] [PubMed] [Google Scholar]
- Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M, Liu B, Matthews P, Ong G, Pell J, Silman A, Young A, Sprosen T, Peakman T, Collins R, 2015. UK Biobank: An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age. PLOS Med. 12, e1001779. 10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syan SK, Minuzzi L, Costescu D, Smith M, Allega OR, Coote M, Hall GBC, Frey BN, 2017. Influence of endogenous estradiol, progesterone, allopregnanolone, and dehydroepiandrosterone sulfate on brain resting state functional connectivity across the menstrual cycle. Fertil. Steril. 107, 1246–1255.e4. 10.1016/j.fertnstert.2017.03.021 [DOI] [PubMed] [Google Scholar]
- Tal R, Taylor HS, Burney RO, Mooney SB, Giudice LC, 2000. Endocrinology of Pregnancy, in: Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A, Hershman JM, Kaltsas G, Koch C, Kopp P, Korbonits M, McLachlan R, Morley JE, New M, Perreault L, Purnell J, Rebar R, Singer F, Trence DL, Vinik A, Wilson DP (Eds.), Endotext. MDText.com, Inc., South Dartmouth (MA). [Google Scholar]
- Tang Y, 2004. Estrogen Replacement Increases Spinophilin-immunoreactive Spine Number in the Prefrontal Cortex of Female Rhesus Monkeys. Cereb. Cortex 14, 215–223. 10.1093/cercor/bhg121 [DOI] [PubMed] [Google Scholar]
- Taylor CM, Pritschet L, Olsen R, Layher E, Santander T, Grafton ST, Jacobs EG, 2020a. Progesterone shapes medial temporal lobe volume across the human menstrual cycle. bioRxiv 2020.02.04.934141. 10.1101/2020.02.04.934141 [DOI] [PubMed] [Google Scholar]
- Taylor CM, Pritschet L, Olsen RK, Layher E, Santander T, Grafton ST, Jacobs EG, 2020b. Progesterone shapes medial temporal lobe volume across the human menstrual cycle. NeuroImage 220, 117125. 10.1016/j.neuroimage.2020.117125 [DOI] [PubMed] [Google Scholar]
- Taylor CM, Pritschet L, Yu S, Jacobs EG, 2019. Applying a Women’s Health Lens to the Study of the Aging Brain. Front. Hum. Neurosci. 13 10.3389/fnhum.2019.00224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taxier LR, Gross KS, & Frick KM (2020). Oestradiol as a neuromodulator of learning and memory. Nature Reviews Neuroscience, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, Bargmann CI, Jayaraman V, Svoboda K, Looger LL, 2009. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat. Methods 6, 875–881. 10.1038/nmeth.1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Smith SM, Barch DM, Behrens TEJ, Yacoub E, Ugurbil K, 2013. The WU-Minn Human Connectome Project: An overview. NeuroImage, Mapping the Connectome 80, 62–79. 10.1016/j.neuroimage.2013.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandever MA, Kuehl TJ, Sulak PJ, Witt I, Coffee A, Wincek TJ, & Reape KZ (2008). Evaluation of pituitary–ovarian axis suppression with three oral contraceptive regimens. Contraception, 77(3), 162–170. [DOI] [PubMed] [Google Scholar]
- Wald C, Wu C, 2010. Of Mice and Women: The Bias in Animal Models. Science 327, 1571–1572. 10.1126/science.327.5973.1571 [DOI] [PubMed] [Google Scholar]
- Wang ACJ, Hara Y, Janssen WGM, Rapp PR, Morrison JH, 2010. Synaptic Estrogen Receptor-α Levels in Prefrontal Cortex in Female Rhesus Monkeys and Their Correlation with Cognitive Performance. J. Neurosci. 30, 12770–12776. 10.1523/JNEUROSCI.3192-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis S, Hodgetts S, Hausmann M, 2019. Sex differences and menstrual cycle effects in cognitive and sensory resting state networks. Brain Cogn., Resting-state fMRI and Cognition 131, 66–73. 10.1016/j.bandc.2017.09.003 [DOI] [PubMed] [Google Scholar]
- Williams GV, Goldman-Rakic PS, 1995. Modulation of memory fields by dopamine Dl receptors in prefrontal cortex. Nature 376, 572–575. 10.1038/376572a0 [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS, 1990. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J. Neurosci. 10, 4035–4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS, 1993. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J. Comp. Neurol. 336, 293–306. 10.1002/cne.903360210 [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS, 1992. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat [published erratum appears in J Neurosci 1992 Oct;12(10):following table of contents]. J. Neurosci. 12, 2549–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman JL, Barha CK, Galea LAM, 2012. Endocrine substrates of cognitive and affective changes during pregnancy and postpartum. Behav. Neurosci. 126, 54–72. 10.1037/a0025538 [DOI] [PubMed] [Google Scholar]
- Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zöllei L, Polimeni JR, Fischl B, Liu H, Buckner RL, 2011. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 106, 1125–1165. 10.1152/jn.00338.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeydan B, Tosakulwong N, Schwarz CG, Senjem ML, Gunter JL, Reid RI, Rocca LG, Lesnick TG, Smith CY, Bailey KR, Lowe VJ, Roberts RO, Jack CR, Petersen RC, Miller VM, Mielke MM, Rocca WA, Kantarci K, 2019. Association of Bilateral Salpingo-Oophorectomy Before Menopause Onset With Medial Temporal Lobe Neurodegeneration. JAMA Neurol. 76, 95–100. 10.1001/jamaneurol.2018.3057 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.