Abstract
Decline in biological resilience (ability to recover) is a key manifestation of aging that contributes to increase in vulnerability to death with age eventually limiting longevity even in people without major chronic diseases. Understanding the mechanisms of this decline is essential for developing efficient antiaging and pro-longevity interventions. In this paper we discuss: a) mechanisms of the decline in resilience with age, and aging components that contribute to this decline, including depletion of body reserves, imperfect repair mechanisms, and slowdown of physiological processes and responses with age; b) antiaging interventions that may improve resilience or attenuate its decline; c) biomarkers of resilience available in human and experimental studies; and d) genetic factors that could influence resilience. There are open questions about optimal anti-aging interventions that would oppose the decline in resilience along with extending longevity limits. However, the area develops quickly, and prospects are exciting.
Keywords: resilience, robustness, aging, longevity, anti-aging interventions, reserve depletion, cell repair, debris accumulation, slowdown, biomarkers, genetics of resilience
1. Introduction
Decline in biological resilience (ability to bounce back and recover) is a key manifestation of aging that contributes to increase in vulnerability to death with age in humans and other animals (Kirkland, Stout et al. 2016, Ukraintseva, Yashin et al. 2016, Hadley, Kuchel et al. 2017). Understanding its causes and regulators is of major importance for understanding the nature of aging, clarifying the complex relationships among aging, health and longevity, and developing the efficient anti-aging interventions.
In this paper we discuss the resilience and mechanisms of its decline during aging. This is a review and position paper that provides our view on what aging is, and how it can produce the decline in resilience. We propose that aging can be viewed as a combination of three universal components: (i) depletion of limited body reserves (e.g., of stem, immune, muscle, neural cells, etc.), which poses limits to recovery; (ii) slowdown of physiological processes and responses to stress/damage, which delays the recovery with age; and (iii) inherently imperfect mechanisms of cell/tissue repair and cleaning, which result in incomplete recovery and damage accumulation over time. These aging components together create the age-decline in resilience, which in turn contributes to increase in mortality risk with age eventually limiting longevity even in people without major diseases.
We also discuss anti-aging interventions that may target the above aging components and attenuate the decline in resilience along with extending lifespan; biomarkers of resilience available in experimental and human studies; and genes and pathways that can play major roles in resilience and its decline with age.
2. What is biological resilience and its indicators in human and experimental studies?
Biological resilience (also called physiological or physical resilience) can be broadly defined as the ability to bounce back, or more specifically, as the ability to quickly and completely recover after deviation from normal physiological state or damage caused by a stressor or an adverse health event (Kirkland, Stout et al. 2016, Ukraintseva, Yashin et al. 2016, Hadley, Kuchel et al. 2017, Arbeev, Ukraintseva et al. 2019). The biological resilience declines with age, which means that an older person will recover less efficiently and have lower chances of survival following the same adverse health event compared to its younger self. For example, at age 85 an average individual has higher chances of dying following an acute hip fracture than s/he would have had in 20s (Schnell, Friedman et al. 2010, Lin, Wu et al. 2014). The decline in the resilience can contribute to the increase in mortality risk with age that eventually limits longevity even in people without chronic diseases (through lower survival chances at older ages after acute events, such as fractures, flu, bleeding, wounds, etc.). Among the oldest old individuals, the level of physical resilience may also influence their chances of becoming centenarians (Galvin, Ukraintseva et al. 2020). The universal character of the age-decline in the physiological ability to recover, and its connection to the increase in mortality risk with age (a.k.a. demographic definition of aging), makes understanding of the mechanisms of the resilience decline with age a major topic of aging research (Kirkland, Stout et al. 2016, Ukraintseva, Yashin et al. 2016, Whitson, Duan-Porter et al. 2016, Hadley, Kuchel et al. 2017, Scheffer, Bolhuis et al. 2018, Schorr, Carter et al. 2018, Varadhan, Walston et al. 2018, Arbeev, Ukraintseva et al. 2019, Hsueh, Önnerfjord et al. 2019, Colón-Emeric, Pieper et al. 2020, Parker, Colόn-Emeric et al. 2020).
2.1. Resilience vs. robustness
For better understanding of the mechanisms of the resilience decline in aging, it is necessarily to first distinguish between the notions of “resilience”, as the ability to recover after deviation from normal physiological state, and “robustness”, as the ability to resist such deviation, because these concepts are sometimes used interchangeably or are combined under the umbrella of the resilience definition (Whitson, Duan-Porter et al. 2016, Hadley, Kuchel et al. 2017, Arbeev, Ukraintseva et al. 2019).
Biological robustness could be defined as the ability to resist deviation from normal physiological state and shield oneself from an adverse health event by preventing it from happening in first place, thus avoiding its destructive consequences on the body whatsoever. In terms of biomarkers, the robustness could be characterized by the speed and magnitude of deviation of a biomarker from its normal or baseline state in response to a stressor (Figure 1a).
Figure 1.
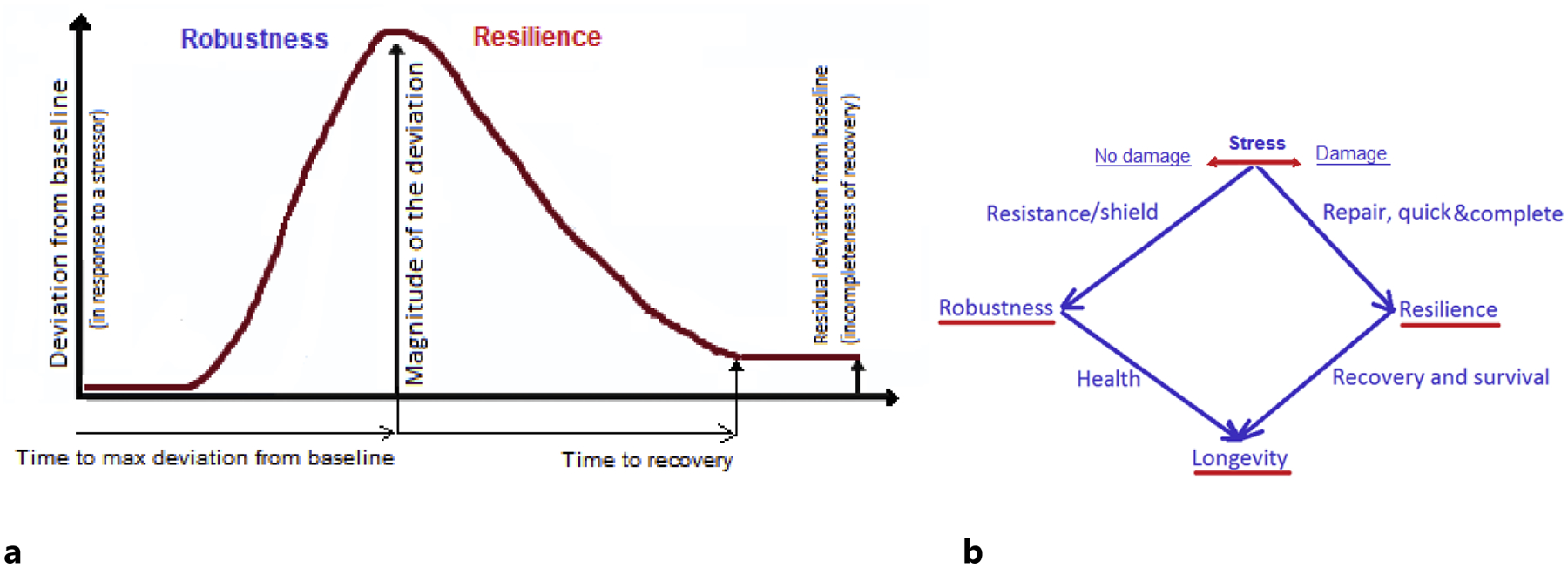
Resilience (ability to recover) vs. robustness (ability to resist deviation and avoid damage). Explanation in text.
The resilience could be characterized by the speed of recovery (time to return to baseline after the deviation), and completeness of the recovery, the latter also contributing to the allostatic load and allostatic adaptation (Yashin, Arbeev et al. 2012, Kirkland, Stout et al. 2016, Ukraintseva, Yashin et al. 2016, Arbeev, Ukraintseva et al. 2019) (Figure 1a).
A cold pressor test (CPT) that measures individual response to a physical stress (cold) (Mei, Gu et al. 2009) could be an example of test that includes physiological indicators of both resilience and robustness. The CPT first measures the increase in blood pressure (BP) after applying the cold stressor to the arm, and time to the BP peak value, which can be used as characteristics of robustness. The CPT then records the speed of returning the BP to its baseline level after withdrawal of the cold stress, and the remining excess of the BP after specified time interval, which can be used as characteristics of resilience.
In terms of diseases, induvial susceptibility to acquiring a disease, or disease risk, would characterize robustness, while chances of the recovery and survival following the disease onset would characterize resilience (Arbeev, Ukraintseva et al. 2019) (Figure 1b).
Robustness generally declines with aging, meaning that body becomes more susceptible to stressors, as it ages. E.g., skin, bones and blood vessels become less elastic and frailer with age, and easier damaged or broken. Risks of many senescence-related conditions increase towards extreme ages (e.g., old age heart failure, renal failure, elderly fractures, and general frailty) reflecting the overall decline in the robustness (Chang, Center et al. 2004, Akushevich, Kravchenko et al. 2012).
However, robustness, may also improve with age in some health domains. For instance, people could develop a better robustness to certain infections at older ages. During 2009 pandemic of H1N1 influenza, few cases occurred in people older than 65 years compared to young adults and children, potentially due to immunity they developed through the past exposure to this virus that younger people did not have (https://www.cdc.gov/h1n1flu/cdcresponse.htm). Note that on the long-term, the improvement in robustness may contribute to the decline in resilience because such improvement can happen at cost of the limited body reserves. E.g., the reserve of naïve immune cells may decrease after vaccination aiming to prevent certain disease (i.e., improve the robustness to this disease), and the lower immune reserve may limit the recovery from a future infection.
There may also be a ‘trade-off’ between male robustness and female resilience that could contribute to male–female “health-survival paradox” (better health but worse survival in men compared to women) (Oksuzyan, Juel et al. 2008). One possible reason why males are more robust (less frail and diseased) than females in middle life but become less resilient (have lower survival chances) at the oldest old ages could be that men more actively spend body reserves to maintain the higher level of robustness in their youth, and so deplete these reserves and diminish their resilience faster with age compared to females.
Also, some internal exposures can change with age in a way that may improve the robustness to certain diseases. The drop in the internal estrogens around menopause that favors the decline in the risk of female endometrial cancer afterwards could be an example (Ukraintseva and Yashin 2003, Ukraintseva, Arbeev et al. 2008). Such decline can be viewed as acquiring a better robustness to this cancer in older compared to younger women. In fact, many cancers decline in their risks at older ages (Ukraintseva and Yashin 2003, Ukraintseva, Arbeev et al. 2008, Akushevich, Kravchenko et al. 2012). One potential explanation for such a counterintuitive decline could be that aging is a combination of different processes, some of which could oppose cancer development at the oldest old ages. Indeed, slowdown in metabolism, proliferation and information processing is a major feature of aging, which may also contribute to a slower growth and postponed clinical manifestation of some cancers in advanced years of life (Ukraintseva and Yashin 2003, Anisimov, Ukraintseva et al. 2005, Ukraintseva, Yashin et al. 2016, Ukraintseva S.V. 2016), in other words, to improved robustness to these cancers. The same slowdown may impair the resilience through the deceleration of regenerative responses, slower healing and prolonged recovery time (e.g., after surgery or chemotherapy), contributing to the increase in mortality risk with age following the onsets of respective cancers (Ukraintseva and Yashin 2003, Ukraintseva, Yashin et al. 2016). That is, the same aging-related factor (e.g., the slowdown) may improve the robustness but impair the resilience to the same health condition. The ‘trade-offs’ between robustness and resilience might potentially explain why the risks of many chronic diseases (e.g., some cancers, asthma, diabetes, hypertension, arthritis, goiter) decline at older ages (80+), while the all-cause mortality risk continues to increase at the same ages (Ukraintseva and Sergeev 2000, Ukraintseva and Yashin 2001, Ukraintseva and Yashin 2003, Ukraintseva, Arbeev et al. 2010, Akushevich, Kravchenko et al. 2012, Ukraintseva, Yashin et al. 2016).
2.2. Biological vs. psychological resilience
The concept of the psychological resilience has been explored for decades, with numerous tests, practical applications and publications (Wagnild 2009, Windle, Bennett et al. 2011, Cosco, Kaushal et al. 2016). Though both biological and psychological resiliencies are similarly defined as the ‘ability to bounce back’, a closer look at their aging-changes reveals that the decline in the biological resilience with age may well coincide with the improvement in the psychological resilience, in the same individuals. This apparent confusion needs to be clarified.
The biological resilience is expected to decline rather than improve with age, as a consequence of such factors as the depletion of exhaustible body reserves (e.g., of stem, immune, neural and muscle cells), and the slower and less complete responses to stressors with age. In contrast, the psychological resilience may improve with age and sometimes be higher in older than in younger individuals, due to acquired life experiences, wisdom, psychological resistance to adversity, and the ability to percept some life events less dramatically with age. The ‘psychological resilience’ tests often capture person’s self-confidence and self-sufficiency, which might be relevant to the psychological robustness as well as to the resilience. Such personal characteristics may reflect the ability to emotionally ‘shield’ oneself from an adverse event and avoid its destructive impact in first place, as well as the ability to recover after being damaged by such event. For example, some popular psychological resilience scales (Wagnild 2009, Windle, Bennett et al. 2011, Cosco, Kaushal et al. 2016) include such items as “capacity to live with purpose, perseverance, equanimity, authenticity, and self-reliance”, “I am friends with myself”, and other questions testing the ability to regulate emotion and avoid depression. That is, ‘psychological resilience’ scales may cover both resilience and robustness, and the latter may indeed improve with age, in parallel with continuing decline in the biological resilience. This could in part explain why the psychological resilience has been shown to decrease, increase, or not change with age, depending on the study and the scale, and sometimes be higher in men than in women (Hamarat, Thompson et al. 2002, Hayman, Kerse et al. 2017). In contrast, the biological resilience generally declines with age, and is higher in women than in men, which is in line with the higher female chances of survival at the oldest old ages compared to the male chances. We have also recently shown that the longest-lived female siblings are enriched in indicators of the better psychological robustness, such as the lower level of depression, which could contribute to their exceptional longevity (Galvin, Ukraintseva et al. 2020).
2.3. Biomarkers of resilience
2.3.1. Resilience at different levels of biological organization
Biological resilience could be measured at different levels of the biological organization: cell, tissue, organ, physiological system, and the whole body (Whitson, Duan-Porter et al. 2016, Arbeev, Ukraintseva et al. 2019). For instance, the speed and quality of DNA repair would characterize the resilience at the cellular level; the ability to restore glucose levels or blood pressure back to normal after deviation caused by a stressor would characterize resilience at the level of tissue or physiological system, respectively; the ability to quickly heal a wound would be an indicator of the resilience at the organ level; and the ability to survive following an adverse health event would characterize resilience at the whole body level.
Understanding the relationships between resiliencies at the different levels of biological organization is necessarily for the proper interpretation of the associations of the resilience biomarkers with health and longevity traits. For example, restoring the resilience of an organ (e.g., bone, after the fracture) does not necessarily improves the whole-body resilience, and may even diminish it. Indeed, the process of healing the localized damage, such as elderly hip fracture, may involve not only the hip itself but multiple body systems and organs (e.g., heart and muscles), and lead to a significant depletion of the limited reserves of the aging organism. This could contribute to the excess of the all-cause mortality risk many years after the fracture itself was healed (Haentjens, Magaziner et al. 2010, Katsoulis, Benetou et al. 2017). Potentially for the same reason, people who are considered cured of a cancer (a.k.a. resilient to cancer), still show an excess in the all-cause mortality risk ten and more years since they became cancer-free (Howlader N 2019), indicating that cancer and its treatments may place significant burden on the whole-body resilience and lower it on the long-term. Given the integrated nature of body functioning, the higher order phenotypes of the whole-body resilience, such as the ability to survive following the onset of unhealthy life, may characterize person’s resilience more accurately than its cell/tissue/organ-specific biomarkers.
2.3.2. Availability of resilience biomarkers in experimental and human studies
Many biomarkers of the resilience can be found in experimental studies that measure physiological responses to various stressors in animals subjected to interventions or genetic manipulations aiming to attenuate the signs of aging and/or increase survival and longevity. For example, the ability to respond to physical stresses, such as heat, UV and oxidants, has been routinely measured (and found to be better) in C. elegans long-lived mutants (daf-2, daf-16, and age-1) with altered insulin/insulin-like growth factor-1 signaling (Johnson, Henderson et al. 2002). Impaired recovery from recurring heat stress in ras2 mutants was linked to decreased replicative lifespan in the yeast, indicating an impaired renewal of cell cycling (Shama, Kirchman et al. 1998). The resiliency to stress-induced hyperthermia was measured (and found to be better) in rats with higher baseline brain-derived neurotrophic factor (BDNF) that promotes neuronal survival (Sweeten, Sutton et al. 2020). Some studies suggested to use miRNAs (miR-1 and miR-155) involved in the regulation of BDNF expression as biomarkers of the resilience to physical stress in rats (Solich, Kuśmider et al. 2020). The resilience of skin-derived fibroblasts to the metabolic effects of low-glucose medium was used to distinguish among the cell lines from rodent species with different longevity, and showed a significant correlation with the latter (Harper, Salmon et al. 2007). Biomarkers of physical resilience in aging mice were also reviewed in (Kirkland, Stout et al. 2016).
While experimental studies of aging routinely include measures of physiological and cell responses to stressors, there is apparent deficit of similar biomarkers of the biological resilience in human studies, and especially, longitudinal measurements of such biomarkers (Mei, Gu et al. 2009, Kirkland, Stout et al. 2016, Ukraintseva, Yashin et al. 2016, Whitson, Duan-Porter et al. 2016, Hadley, Kuchel et al. 2018, Scheffer, Bolhuis et al. 2018, Colón-Emeric, Whitson et al. 2019, Schosserer, Banks et al. 2019, Wu, Li et al. 2019, Colón-Emeric, Pieper et al. 2020). To improve this situation, one should include more direct biomarkers of resilience in human aging studies, as well as exploit indirect indicators of resilience that are readily available in existing human data.
Examples of direct measures of the biological resilience that can be found in some human studies include glucose tolerance test and cold pressor test (CPT) mentioned earlier. For instance, the glucose tolerance test is included in the Framingham Heart Study (FHS) and the Baltimore Longitudinal Study of Aging (BLSA); however, it is rarely measured multiple times over decades of the observations, and almost never in the very old age, due to concerns of too much stress potentially applied to the frail individuals. An encouraging recent effort to collect and study data from one million people living in the United States and followed for at least ten years (the NIH’s “All of Us” Research Program) promises to include continuous measurements of individual physiological responses from wearable devices, allowing to construct longitudinal trajectories of the resilience markers, which would indeed be useful for aging research.
Clinical geriatrics research currently exploits at least two approaches to measuring the resilience to acute physical stressors, such as hip fracture, in the elderly. The first approach measures how quickly and completely a patient recovers (“recovery phenotype”). This approach can consider multiple outcomes simultaneously in a composite score, or identify groups of patients with similar recovery trajectories across multiple outcomes (Whitson, Duan-Porter et al. 2016, Colón-Emeric, Whitson et al. 2019, Colón-Emeric, Pieper et al. 2020). The second approach quantifies “expected recovery differential”, reflecting how patients’ actual outcomes are compared to their predicted outcome based on a population-derived model and their individual clinical characteristics at the time of the stressor (Colón-Emeric, Pieper et al. 2020, Parker, Colón-Emeric et al. 2020). E.g., self-reported pre-fracture function was a strong predictor of a high-resilience group among patients recovering after hip fracture in a one year follow-up (Colón-Emeric, Whitson et al. 2019).
2.3.3. Indirect and aggregate measures of resilience that can be constructed from human data
Indirect indicators of the resilience could be obtained using statistical/mathematical models of aging, such as the quadratic hazards model describing the relationships between the age-specific mortality risk and deviation from physiological norm due to exposures to internal or external stressors (Yashin, Arbeev et al. 2012, Yashin, Arbeev et al. 2016). This model assumes that the mortality risk is U-shaped, so that chances of dying increase with the increasing deviation from the optimal physiological state. The U-shape becomes narrower with age reflecting higher chances of dying in older compared to younger individuals with the same level of stressful exposure, meaning that older people can endure less deviation from the optimal physiological state (Yashin, Arbeev et al. 2012, Yashin, Arbeev et al. 2016). Using this model, we previously estimated parameters of the stress resistance/resilience, taking into account the life-time exposure to APOE e4 allele (genetic risk factor for both Alzheimer’s disease and all-cause mortality). We found that the APOE e4 carriers are less resilient than non-carriers because they show higher increase in total mortality risk with age following the same metabolic stress (change in blood cholesterol level) (Yashin, Arbeev et al. 2016).
Examples of aggregate measures that combine biomarkers of resilience and robustness include the composite indices of physiological dysregulation, age-associated deficits, and biological age (Kulminski, Ukraintseva et al. 2007, Yashin, Arbeev et al. 2007, Kulminski, Ukraintseva et al. 2008, Mitnitski, Song et al. 2013, Cohen, Milot et al. 2014, Arbeev, Ukraintseva et al. 2019, Dansereau, Wey et al. 2019, Arbeev, Bagley et al. 2020, Belsky, Caspi et al. 2020). For instance, Mitnitski and Rockwood developed a frailty index as the ratio of the deficits present in a person to the total number of deficits considered, and concluded that the age-associated increase in recovery time (reflecting the decline in resilience) is the main cause of the deficits accumulation during aging (Mitnitski, Song et al. 2013).
We recently suggested to use an index of physiological dysregulation (PD) as an aggregate indicator of the resilience and robustness in aging body (Arbeev, Ukraintseva et al. 2019). This composite index integrates deviations of multiple biomarkers from their baseline/normal physiological states into one estimate reflecting the loss of homeostasis in biological networks (Cohen, Milot et al. 2014, Arbeev, Ukraintseva et al. 2019, Dansereau, Wey et al. 2019). Higher PD levels signal that regulatory mechanisms are broadly compromised across multiple body domains, which may both increase person’s vulnerability to disease and reduce the ability to recover and survive. We showed that PD level increases with age and significantly influences the transition from healthy to unhealthy state (proxy for robustness), as well as survival following the onset of unhealthy life (proxy for resilience), and longevity (Arbeev, Ukraintseva et al. 2019, Arbeev, Bagley et al. 2020).
3. Mechanisms of the decline in resilience with age, and anti-aging interventions that may target these mechanisms
Biological aging is not yet fully understood, though it is apparently heterogeneous process influenced by many factors. While some of these factors could be specific for certain groups of individuals (e.g., female hormonal changes during menopause), the aging also includes ‘universal’ components that contribute to the decline in resilience and the increase in mortality risk with age in all individuals. These aging components are (Figure 2):
depletion of exhaustible body reserves with age, which limits capacity of the body to respond to stressors and recover (e.g., HSCs lose their self-renewal and regenerative potentials with age; thymus shrinks with age; muscle cells and neurons die off, etc.);
slowdown of physiological processes and responses with age, due to declines in rates of metabolism, proliferation and information processing, and potentially other factors, which can lead to a longer recovery time after stress/damage;
inherently imperfect mechanisms of cell repair and tissue cleaning, which result in incomplete recovery after damage, leading to the accumulation of senescent and malfunctioning cells, metabolic waste and residual damage in tissues over time, and to the increase in dysregulation across multiple body systems, along with the increase in the allostatic load.
Figure 2. Relationships between aging, decline in biological resilience, and longevity.
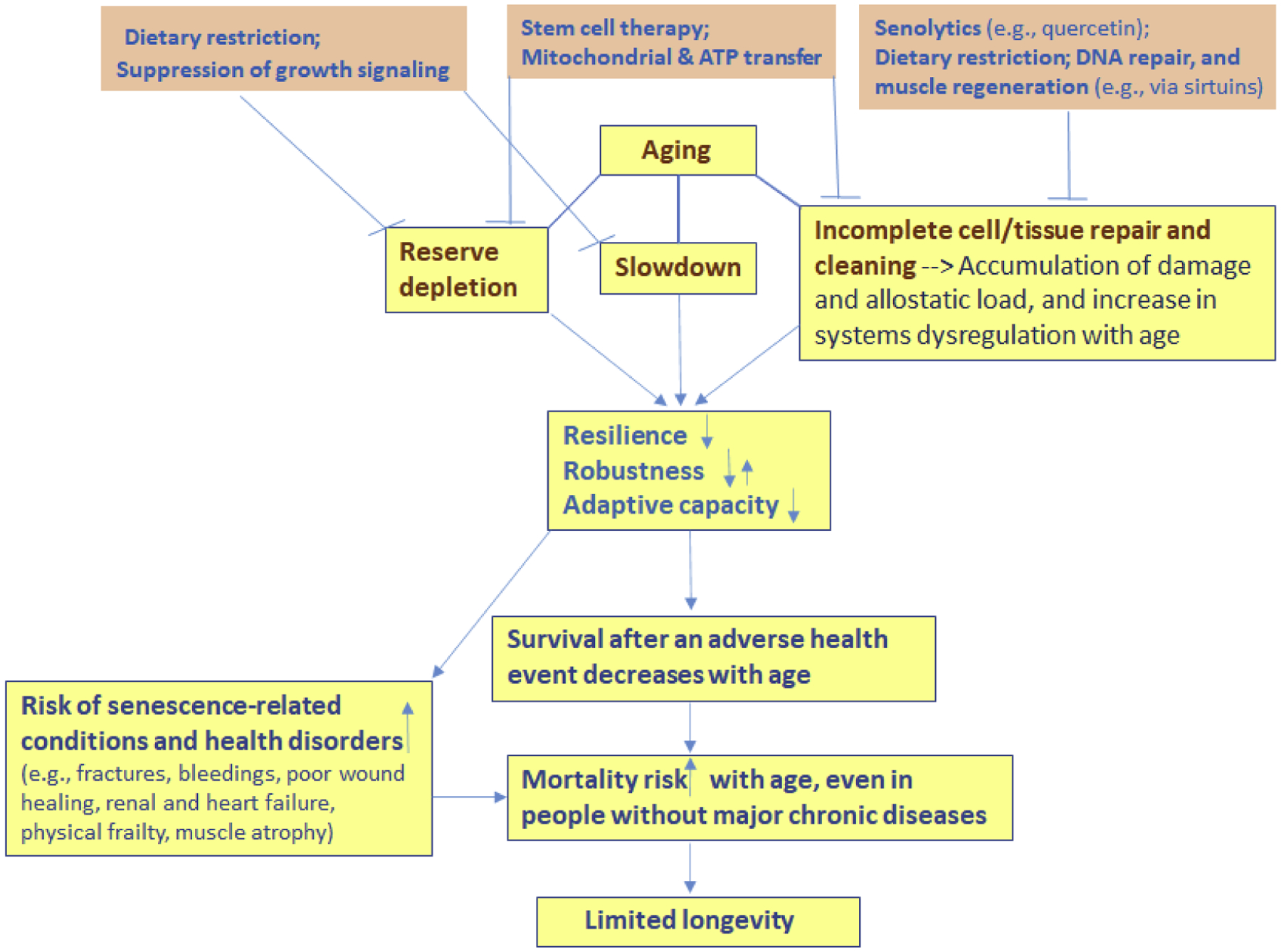
Major aging components (depletion of limited body reserves; slowdown of physiological processes and responses, and imperfect mechanisms of repair and cleaning) together contribute to the decline in the resilience (ability to recover), which in turn contributes to the increase in vulnerability to death with age, eventually limiting longevity even in people without major chronic diseases. Prospective anti-aging interventions (examples on the top) coming from experimental studies already target these aging components (see in text). For more optimal results, such interventions may need to act together, to simultaneously (i) replenish stem and other cells, (ii) improve quality of cell repair, (iii) enhance removal of senescent and malfunctioning cells, metabolic waste, and debris from tissues, (iv) decelerate the decline in speeds of metabolic, proliferative and signal processing responses to stressors, so that this decline (shown as “slowdown” on the figure) would progress slower with age.
Within this framework, the interventions aiming to attenuate the aging-related decline in resilience may have to: a) replenish reserves of the stem and other cells; b) improve the quality of DNA/cell repair; c) enhance the removal of senescent and malfunctioning cells, d) enhance the removal of metabolic waste and miscellaneous debris from tissues; and e) oppose the general slowdown of physiological processes and responses in the body, so that it would happen at a slower pace with age. Encouraging news is that many of today’s experimental anti-aging interventions already target these processes (the top of the Figure 2). Below are some notable examples:
Caloric restriction (CR), a.k.a. dietary restriction, has been shown to slow body growth and rates of aging-changes in physiological markers, decrease body temperature and plasma insulin, improve DNA repair, enhance xenobiotic detoxification, and favor the preservation of stem cell reserves (Roth, Lane et al. 2002, Anisimov, Ukraintseva et al. 2005, Bautista-Niño, Portilla-Fernandez et al. 2016, Swain, Vyjayanti et al. 2016, Garcia, Saccon et al. 2019, Ke, Firsanov et al. 2020, Li, Chen et al. 2020). Drugs and genetic modifications that inhibit the growth hormone/IGF-I axis also display some overlapping effects with those of the CR (Gesing, Bartke et al. 2011, Sun, Spong et al. 2013, Gesing, Al-Regaiey et al. 2014, Longo, Antebi et al. 2015).
Physical exercise has been shown to accelerate muscle repair, improve old muscle stem cell function, mitochondrial dynamics in older subjects, induce autophagy, slow down sarcopenia, improve myogenesis and skeletal muscle resilience to unaccustomed loading and adaptability in late life, and promote stem cell accumulation (Garg and Boppart 2016, Brett, Arjona et al. 2020, Casuso and Huertas 2020, Larrick and Mendelsohn 2020, Lavin, Perkins et al. 2020, Liang, Zeng et al. 2020).
Pharmacological interventions into aging broadly aim at improving the biological resilience. Metformin and rapamycin are two FDA-approved drugs that are being repurposed for geroprotection. The anti-hyperglycemic metformin was shown to improve proliferative potential of stem cells, promote wound healing, reduce expression of markers associated with cellular senescence, increase fasting tolerance, and induce autophagy (Storelli, Téfit et al. 2013, Barzilai, Crandall et al. 2016, Marycz, Tomaszewski et al. 2016, Fang, Yang et al. 2018, Piskovatska, Stefanyshyn et al. 2019, Song, Jiang et al. 2019, Arnold and Padilla Colón 2020). The mTOR inhibitor, rapamycin, lowered mitochondrial ROS production, increased autophagy, reduced surgery-induced T-cell exhaustion, prevented cell senescence, decreased damage accumulated with age, among other effects, though with significant heterogeneity across studies and reported adverse events (Swindell 2017, Di Francesco, Diaz-Ruiz et al. 2018, Fang, Hill et al. 2018, Qian and Liu 2018, Garcia, Saccon et al. 2019, Svatek, Ji et al. 2019, Reid, Linden et al. 2020). Senolytics, such as quercetin, are thought to produce the anti-aging effect by cleaning the body from senescent cells that have detrimental pro-aging effects on tissues. Eliminating the senescent stem cells with the senolytics improves proliferation and regenerative potential of the remaining stem cells, and may also enhance genomic stability, reduce oxidative stress, and inhibit neurodegeneration (Geng, Liu et al. 2019, Hu, Shi et al. 2020, Kirkland and Tchkonia 2020, Zhang, Yu et al. 2020). Quercetin also up-regulates SIRT1 protein expression, and the increased activity of the sirtuins (especially SIRT1 and SIRT6) has been associated with improved DNA repair, autophagy, mitochondrial biogenesis, and responses to stress and caloric restriction (Tian, Firsanov et al. 2019, Iside, Scafuro et al. 2020), i.e., with essential features of the biological resilience.
Mitochondrial transplantation therapy is a recently developed innovative strategy allowing to replenish the mitochondria in cells of a living organism and through this increase its energy production and improve capacity of the cells and whole body to respond to stressors and recover after damage (Gollihue and Rabchevsky 2017, Shin, Cowan et al. 2017, Kim, Hwang et al. 2018). Among various techniques, direct mitochondrial transfer using skin patches that are covered with microneedles filled with mitochondria (similar to those developed for insulin transfer through the skin in diabetes) might be a promising strategy for both skin and muscle rejuvenation. Similarly, a bone marrow or HSC transplantation into aging animals, as well as rejuvenating patient’s own cells by reprogramming them to pluripotency, seeks to replenish the stem cells reserves and through this restore the tissue regenerative capacity and ability to recover (Kang, Moser et al. 2020).
Diluting the plasma factors along with replenishing the albumin was shown to enhance muscle repair and hippocampal neurogenesis in old mice. Similar procedure, the therapeutic plasma exchange, elevated levels of proteins that coordinated tissue repair, promoted immune responses and rescued progenitor cell proliferation (Kang, Moser et al. 2020, Mehdipour, Skinner et al. 2020). These procedures broadly help clean up the tissues from metabolic waste and toxic factors accumulated over time, and through this improve the whole-body resilience.
Procedures such as stem cells grafting and bone marrow transplantation, as well as thymus regeneration, with thymus tissue transplants (Tajima, Pradhan et al. 2016, Majumdar and Nandi 2018, El-Kadiry and Rafei 2019), aim to replenish the reserve of immune cells or alleviate its decline, to partially restore the resilience of the immune system. Immunosenescence and inflammaging are considered major immune system changes accompanying aging (Fulop, Larbi et al. 2017, Pawelec and Gupta 2019). They could be consequences of the depletion of limited immune reserve (e.g., thymus atrophy), and slowdown of proliferative, metabolic and information processing responses of the immune and other relevant cells with age, among other factors.
Thus, many of the today’s candidate anti-aging interventions de facto aim to delay or reverse the decline in biological resilience, e.g., by replenishing the body reserves, modulating the rates of physiological processes, or improving the efficiency of cell/tissue repair and cleaning. A potential problem with using such interventions as standalone therapies is that they might produce undesirable trade-offs. For instance, an intervention that speeds up the wound healing and tissue regeneration (an anti-aging effect) by boosting metabolism and proliferation, may also lead to overspending of stem cells reserve (a pro-aging effect). An intervention such as caloric restriction may slow down the processes of depleting stem cells and accumulating debris in tissue (an anti-aging effect), while it may also potentially reduce the resilience by making the responses to stressors such as pathogens slower and healing time longer (a pro-aging effect).
We hypothesize that a potentially efficient strategy to decelerate the decline in resilience with age, while avoiding trade-offs, such as between faster recovery and overspending the reserve, could be to combine the anti-aging interventions that target different aging components (i.e., depletion of reserve, slowdown, and imperfect repairs). For instance, an intervention that speeds up the wound healing by increasing metabolism, and also promotes depletion of stem cells, could be administered together with graftings of the stem cells. Some of today’s interventions have already been tested in combinations, in the hope to synergize the beneficial effects, and offset the side effects of individual therapies. Exercise combined with caloric restriction (CR) could be an example (DiMilia, Mittman et al. 2019). Indeed, with all its benefits, the CR may favor sarcopenia in older adults, while the exercise can oppose it. The CR may low body temperature and slow physiological responses to stressors (e.g., to infections), while the exercise can boost metabolism and immune response. On the other hand, combining certain interventions (e.g., metformin with SRT1720, a sirtuin1-activator) may nullify their beneficial individual effects, or even produce a detrimental combined effect, which means that the combinations of anti-aging interventions need to be systematically evaluated (Palliyaguru, Minor et al. 2020).
One should note that the relative impacts of the different aging components (reserve depletion, slowdown, and imperfect repair/cleaning) on resilience and vulnerability to death may differ across species and strains. For instance, in one species, the cell repair could be weak but offset by large reserves of stem cells allowing to maintain the high level of the apoptosis and fast removal/replacement of the damaged cells. In another species, intracellular repair may be a major mechanism of coping with the cell damage, which would allow to save the limited reserve of stem cells. And in some other species, the enhanced clearance of the debris and metabolic waste could be a central part of the damage response, etc. If this is the case, then the effects of anti-aging interventions that increase longevity in some model organisms may not necessarily be replicated in humans. A solution, again, may be in combining the interventions addressing the different aging components and implementing them together.
4. Genes involved in resilience and its decline during aging
Which genes may influence resilience and oppose its decline with age? If to assume that such decline originates from aging components shown on Figure 2 (i.e., reserve depletion, slowdown, and imperfect repairs), then resilience-related genes could be, first of all, those that beneficially influence these components and are involved in maintaining/replenishing body reserves (e.g., of stem, muscle and neural cells); enhancing cell and tissue responses to stress and damage; improving energy production and attenuating the slowdown in rates of metabolism and information processing. Some genes (and their products) with beneficial effects on the biomarkers of stress response, recovery and survival, have been intensively studied in animal models of aging, and provide valuable source of the candidate resilience genes. Genes coding for growth hormone and IGF-1 receptors, FOXO transcription factors, target of rapamycin, p16 and sirtuins, could be examples. Also, if the age-decline in resilience is responsible for the decline in the ability to survive acute health events (e.g., fractures, flu, bleedings, stroke, etc.), contributing to the increase in the total mortality risk with age, eventually limiting lifespan, then the resilience genes could also be associated with extreme longevity, and found in respective studies. Finally, new candidate resilience genes could be proposed based on the emerging knowledge about the complex interplay among biological pathways regulating stress responses and recovery. Below we describe the sources of candidate resilience genes in more detail.
4.1. Genes featured in aging and longevity research are broadly involved in resilience
To date, hundreds of genes have been found or suggested to play a role in the aging process and influence survival and longevity of humans and model organisms (Tissenbaum 2012, Broer, Buchman et al. 2015, Bartke and Quainoo 2018, Singh, Demmitt et al. 2019) (Willcox, Donlon et al. 2008, Tacutu, Thornton et al. 2018). To take a broad picture of the biological processes related to such genes, we selected 490 genes that are available in GenAge and LongevityMap online bases of the aging and longevity genes, respectively (Tacutu, Thornton et al. 2018), and run the analysis of the enrichment of these genes with GO biological processes, using MetaCore software from Clarivate Analytics. The results (Figure 3) show that the aging and longevity-related genes are commonly involved in cell responses to stress (especially, oxidative stress), proliferation and apoptosis, which are all processes highly relevant to the biological resilience. A closer look at the most intensively studied aging/longevity genes reveals interesting differences between findings from human and experimental research.
Figure 3.
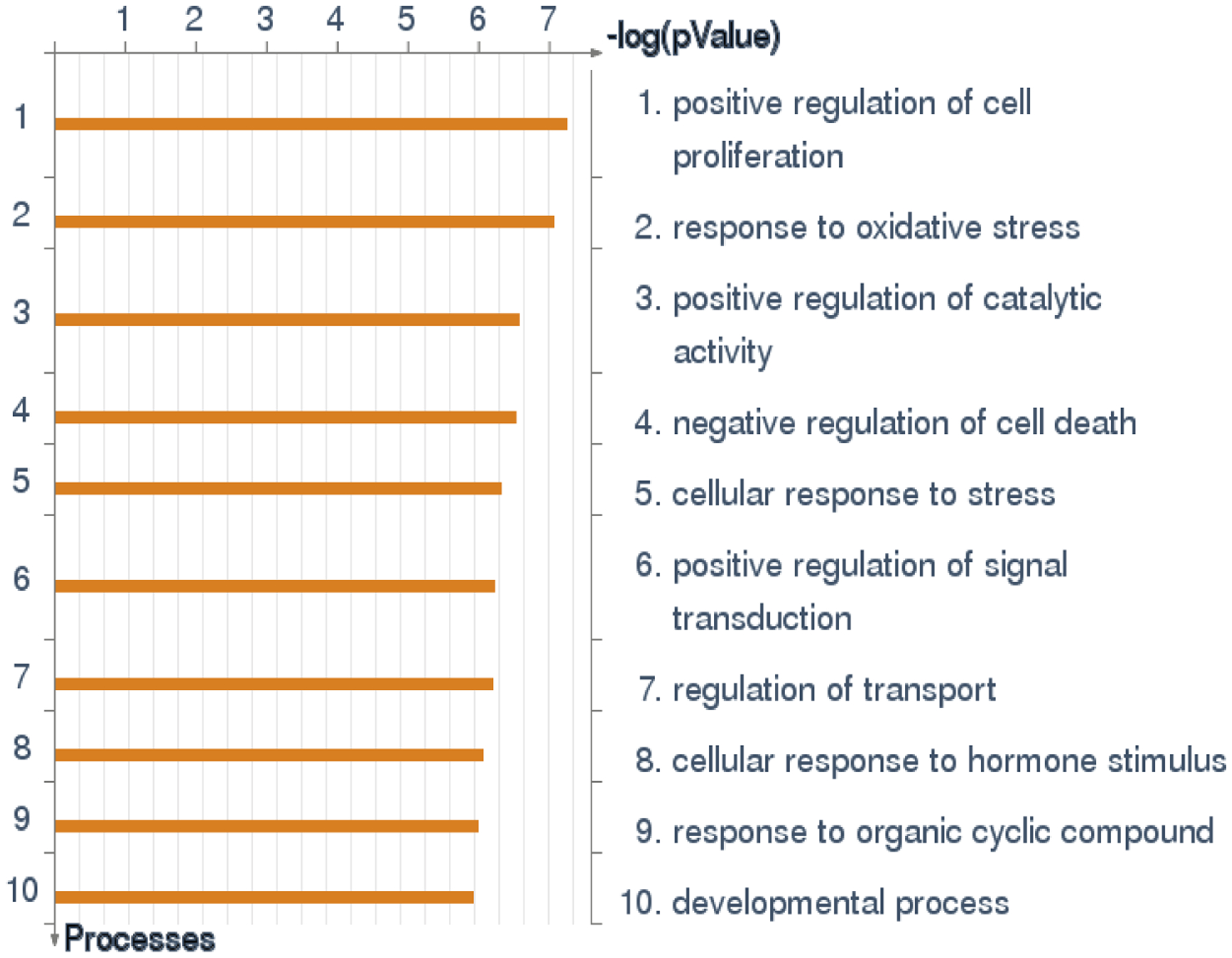
Enrichment with GO processes in the set of 490 aging and longevity-related genes from GenAge and LongevityMap bases (Tacutu, Thornton et al. 2018). The results show that these genes are commonly involved in cell responses to stress, proliferation and apoptosis (the results obtained with MetaCore software from Clarivate Analytics).
4.2. Resilience-related genes from human GWAS and clinical studies
The large human genome-wide association studies (GWAS) found several variants in APOC1/APOE/TOMM40/NECTIN2 region on chromosome 19 that have been significantly associated with reduced longevity. The APOE e4 and rs2075650 (G) minor alleles have been most consistently linked to lower chances of surviving to extreme ages (Deelen, Beekman et al. 2011, Deelen, Beekman et al. 2014, Yashin, Arbeev et al. 2018, Deelen, Evans et al. 2019), so they could be viewed as vulnerability rather than pro-longevity genes (Gerdes, Jeune et al. 2000). These same variants are also strongly associated with risk of Alzheimer’s disease in humans, and seem to play roles in brain resilience to damaging factors, such as air pollution and viral infection (Yashin, Fang et al. 2018, Haghani, Thorwald et al. 2020). Based on current knowledge, the APOE e4 can also be linked to impaired axons ability to recover after damage, which declines in both physiological aging and (exacerbated) in AD. The APOE e4 have been associated with deficient brain cholesterol transport and clearance, which may negatively affect myelin production by oligodendrocytes, and so axons de/re-myelination and maintenance (Mahley 2016, Jeong, Lee et al. 2019).
The significant pro-longevity effect was found in human GWAS for variants in FOXO3A transcription factor. FOXO3A is a member of insulin/IGF signaling pathway and a core regulator of cellular stress response that can influence cell survival, apoptosis, autophagy and DNA repair. Its associations with longevity, as well as markers of the stress response and cell resilience have been consistently shown (Tran, Brunet et al. 2002, Nygaard, Lindahl-Jacobsen et al. 2014) (Donlon, Willcox et al. 2017) (Morris, Willcox et al. 2019) (Soerensen, Nygaard et al. 2016) (Kops, Dansen et al. 2002, Warr, Binnewies et al. 2013, Broer, Buchman et al. 2015).
Recently we connected the increase in the level of physiological dysregulation (PD) in aging body with age-associated declines in resilience and robustness in humans (Arbeev, Ukraintseva et al. 2019) (see 2.3.3 above). We also conducted GWAS to find genetic factors associated with the slope of the increase in PD with age (Arbeev, Bagley et al. 2020). As result, we selected the set of genes that were significantly enriched in pathways regulating axon guidance and synaptic functioning. These genes impact the maintenance of neural circuits, synaptic plasticity, and the stabilization of the synaptic transmission and information processing throughout life (de Wit and Verhaagen 2003, Van Battum, Brignani et al. 2015, Orr, Fetter et al. 2017). Our results suggest that the decline in the ability to maintain complex neural regulatory networks may contribute to the declines in resilience and robustness during aging. Indirect support for the role of respective pathways in aging comes from the study of changes in human proteome with age (Lehallier, Gate et al. 2019). A group of ~60 proteins (‘cluster 6’) showed accelerated increase in plasma levels after the age 60, resembling the exponential increase in the mortality risk with age and suggesting the correlation of these proteins with aging process. Axon guidance, regeneration, and synaptic plasticity were significantly overrepresented among the biological functions related to these proteins.
Recent genetic studies of clinical biomarkers of the resilience also supported the role of miRNAs as potential biomarkers of responses to health-related stresses and recovery, such as response to a pharmacological treatment, and recovery after physical trauma (Hsueh, Önnerfjord et al. 2019, Lopizzo, Zonca et al. 2019). For example, two recent studies found several microRNAs linked to human cartilage regeneration (miR-21) and to clinical resilience markers of the recovery after hip fracture (miR-376a, miR-16) (Hsueh, Önnerfjord et al. 2019, Parker, Colón-Emeric et al. 2020). These microRNAs target many genes, including those involved in the axon guidance, so exact mechanisms connecting them with the decline in physical resilience are yet to be determined.
4.3. Candidate ‘resilience genes’ can be involved in the interplay between conserved aging pathways that jointly decide outcomes of cell responses to stress and damage
The most intensively studied aging-related pathways in experimental research are those that regulate outcomes of cell responses to stress and damage, especially:
IGF-1/AKT/FOXO3 signaling regulating cell survival, growth, DNA repair and apoptosis;
TP53/P21/P16 pathways regulating apoptosis, senescence and autophagy;
mTOR/S6K pathway regulating autophagy, cell survival and growth.
These pathways closely interact to decide on what will eventually happen to cell in response to a particular stressor (Figure 4, simplified) (Feng and Levine 2010).
Figure 4.
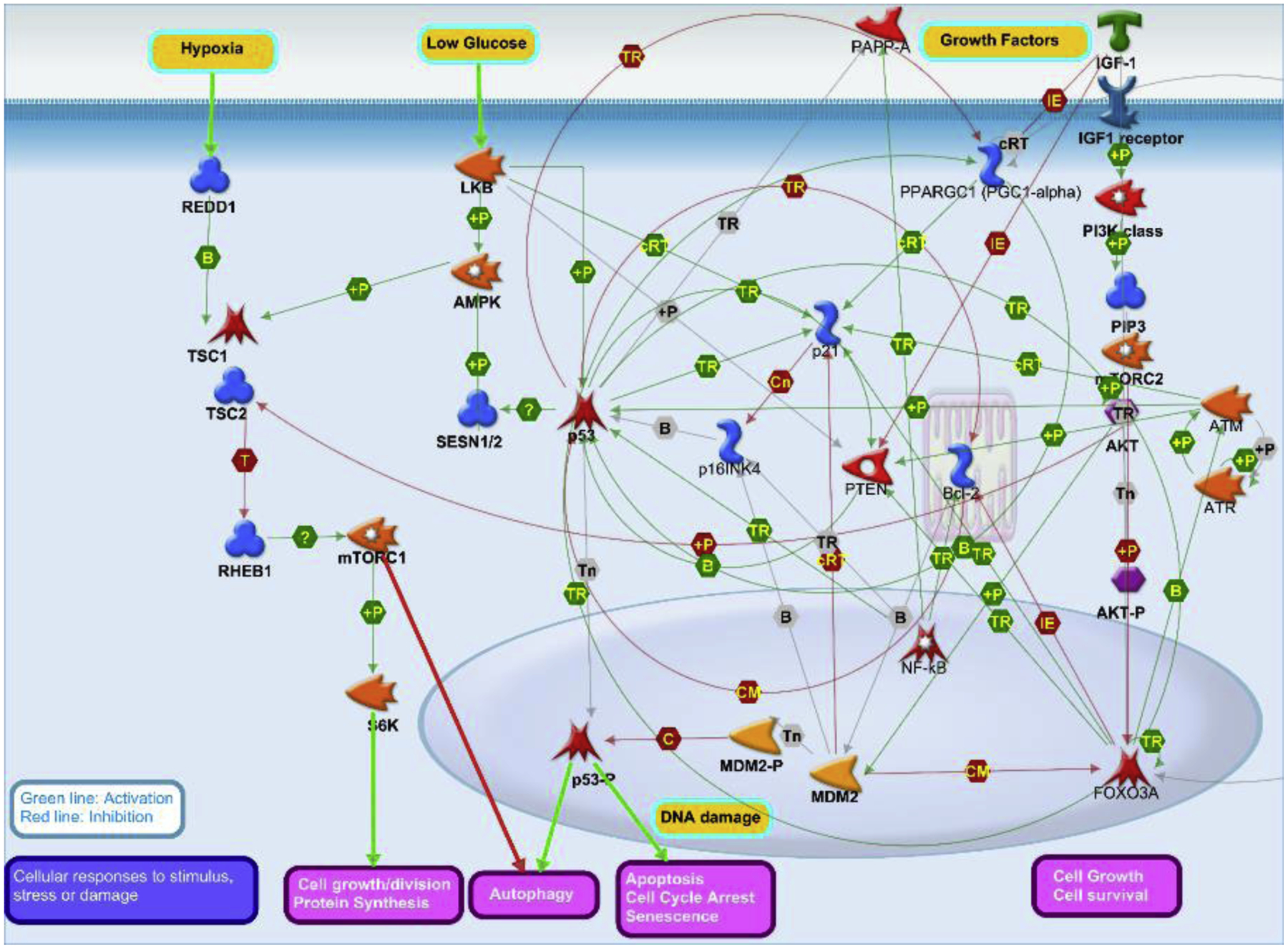
Simplified picture of the aging-related signaling pathways that have been in major focus of experimental aging research: IGF-1/AKT/FOXO3 - mediated growth/survival, TP53/P21/P16 - mediated apoptosis/senescence/autophagy, and mTOR/S6K - mediated autophagy/survival/growth. These pathways biologically interact and together decide on specific outcomes of cell responses to stress/damage, such as apoptosis, senescence, survival, growth, division, or autophagy. The choice of a particular outcome in given conditions may differ between carriers of different genotypes, which might contribute to the differences in pace of aging between individuals, species or strains (Image by Ukraintseva© 2020, using MetaCore Pathway Map Creator tool from Clarivate Analytics).
Members of these pathways and their orthologs were found to influence survival and responses to various stressors, such as heat, cold, UV, starvation and other, in model organisms including mice, worms, flies and yeast. Most of these studies focused on insulin-like growth factor-1 (IGF-1) signaling and mutations in homologs of growth hormone and IGF-1 receptors, FOXO transcription factors, target of rapamycin, p16 cell cycle inhibitor, and sirtuins (Johnson, Henderson et al. 2002, Kenyon 2010, Baker, Wijshake et al. 2011, Johnson, Sangesland et al. 2015, Uno and Nishida 2016, Bartke and Quainoo 2018, Singh, Demmitt et al. 2019). In such studies, the majority of the life-prolonging mutations increased lifespan along with improving animals ability to respond to a physical stress and recover (i.e., they improved resilience). For example, long-lived C. elegans daf-2, daf-16 and age-1 mutants have shown enhanced resilience to stresses, including heat, UV and oxidants (Johnson, Henderson et al. 2002). Based on these and similar results, it was suggested that the ability to efficiently respond to stress could be a major evolutionary determinant of longevity (Johnson, de Castro et al. 2001, Johnson, Henderson et al. 2002), though the relationship may be more complex. E.g., longevity extention may potentially be achieved through improving the robustness at cost of the resilience (see 2.1. Resilience vs. robustness).
Human candidate genes studies followed. However, majority of the genes that showed individual effects in experimental aging research have not been replicated in humans, with few exceptions such as FOXO3A (Nygaard, Lindahl-Jacobsen et al. 2014) (Zeng, Cheng et al. 2010, Soerensen, Nygaard et al. 2016, Donlon, Willcox et al. 2017, Morris, Willcox et al. 2019). A more successful pathway-based gene set approach evaluated the joint effect of SNPs in genes from relevant aging pathways on longevity in humans, and yielded significant results supporting the role of genetic variation in the IGF-1 signaling in human longevity (Deelen, Uh et al. 2013). Another study that focused on 148 candidate genes from the aging-related pathways found promising associations with human longevity of common SNPs in genes from IGF-1 signaling (e.g., IGF2R, INS) and DNA damage repair (e.g., RAD52, NTLH1, WRN) pathways (Soerensen, Dato et al. 2012). The RAD52 variants were also found in familial cases of exceptional longevity (Cash, Pita et al. 2014), which supports the role of the inherent quality of DNA repair (which characterizes cell resilience to DNA damage) in maximum longevity.
A highly conditional, context-specific character of the genetic influence on phenotypes of health, senescence and survival, as well as potential differences in the interplay among the aging-related pathways between species/strains, may play a major role in the lack of replication of individual genes found in experimental aging research (Ukraintseva, Yashin et al. 2016, Giuliani, Garagnani et al. 2018). It is important to remind that the above aging pathways (IGF-1/AKT/FOXO3A, TP53/P21/P16, and mTOR/S6K; Figure 4) work in concert to decide on outcomes of cell responses to stress/damage, such as apoptosis, senescence, survival, growth, division, or autophagy. It seems logical that genes from these pathways may influence phenotypes of resilience and longevity, as result of their interplay rather than independently. So, it may be reasonable to more actively look at the effects of genetic interactions across the conserved aging pathways on aging and longevity related traits in humans.
One should also note that while human longevity-reducing genes (in the APOE region) are predominantly involved in AD, the genes from the conserved aging pathways (Figure 4) are commonly involved in cancer and oppositely expressed in cancer and aging cells (Ukraintseva and Yashin 2003, Braeckman and Vanfleteren 2007, Feng and Levine 2010, Bartke, Westbrook et al. 2013, Anisimov 2015, Bitto, Wang et al. 2015, Tian, Firsanov et al. 2019, Zhang, Milman et al. 2020). This may potentially reflect important differences in biological mechanisms by which individual genetic factors with strong negative effect (such as APOE e4) can impair resilience and chances of reaching extreme longevity, and those factors that may (jointly) improve the resilience, which deserves further investigation. The fact that many genes from the aging pathways (Figure 4) are oppositely expressed in cancer and aging indicates that cancer could be seen (and researched) not only as a deadly disease but also as a condition resembling an aberrant local ‘rejuvenation’ in the aging body, and so, as a potential source of regenerative anti-aging therapies, e.g., involving a controlled manipulation of protooncogenes and tumor suppressors (Ukraintseva and Yashin 2003, Ukraintseva and Yashin 2005, Feng, Hu et al. 2008, Leiszter, Galamb et al. 2013).
4.4. Other potential sources of candidate ‘resilience genes’
Note that the resilience-related genes may be found not only among the abovementioned pathways regulating the outcomes of cell responses to stress and damage. The aging components shown on Figure 2 (depletion of body reserves; slowdown of physiological processes, and imperfect repairs/cleaning) all contribute to the age-decline in biological resilience. Other relevant pathways could be, e.g., those regulating renewal and maintenance of the stem cells; removal of the senescent and malfunctioning cells; removal of the metabolic waste and debris from the tissue; aging-changes in energy production and rates of metabolism, proliferation and information processing.
Studies that explore epigenetics of aging and longevity can also provide candidate genes and biomarkers relevant to resilience. For instance, methylation level of a heat-shock protein 70 (HSP70) promoter was linked to heat-stress-related epigenetic memory in individuals who are resilient to a heat stress (Kisliouk, Cramer et al. 2017). Upregulation of transcriptional repressor REST, which is a key modulator of the neuronal epigenome, was shown to facilitate neuronal resilience to stress. REST is involved in axonal growth and synaptic plasticity, and it may benefit resilience via protection against neural stem cell depletion (Mampay and Sheridan 2019). Impaired resilience to psychological stress has been linked to the age-associated epigenetic upregulation of the FKBP5 gene involved in glucocorticoid signaling and binding to the rapamycin (Sabbagh, O’Leary et al. 2014). Genome-wide variation in DNA methylation has been associated with resilience biomarkers such as ability to complete the glucocorticoid stress response through negative feedback (Taff, Campagna et al. 2019).
A recent study reported that ectopic expression of Oct4(Pou5f1), Sox2, and Klf4 genes involved in resetting the epigenome, successfully restored the youthful DNA methylation age (epigenetic biomarker of aging) of retinal ganglion neurons in old mice and mice with glaucoma, and promoted axonal regeneration after injury, along with reversion of vision loss (Lu, Brommer et al. 2020). While it remains to be determined how exactly cells store youthful epigenetic information, the epigenetic reprogramming by induction of selected genes seems to efficiently promote axons repair in CNS, which is critically important for maintaining the CNS resilience during aging. Studies of the DNA methylation age that also incorporate clinical measures of phenotypic age, found the higher epigenetic age to be associated with increased anti-viral response, and with decreased DNA damage response, based on GO terms enrichment analysis (Levine, Lu et al. 2018). Also, an intriguing observation that epigenetic age increases at a slower rate than chronological age in the oldest old (Marioni, Suderman et al. 2019), may potentially reflect a general slowdown of biological processes in the aging body, which could contribute to the slower pace of the epigenetic aging, along with continuing decline in the resilience (see also section 2.1. Resilience vs. robustness). The aging-related genetic and epigenomic changes that may influence both resistance and resilience to stressors were reviewed in (Morris, Willcox et al. 2019). Overall, studies of the genetic and epigenetic markers of biological resilience are growing and expected to yield more new findings in this area.
5. Concluding remarks
Decline in biological resilience is a key manifestation of aging. At the whole-body level, it means that the older person is able to recover after an adverse event less quickly and completely than s/he was able to do at a younger age. The decline in resilience can contribute to the increase in vulnerability to death with age even in people without major diseases, which makes it a sufficient factor limiting longevity.
Resilience (ability to recover) is not equal to robustness. The latter refers to the ability to avoid a damaging event (e.g., disease) and its destructive consequences whatsoever. The body robustness generally declines during aging; however, it may improve in some health domains, sometimes at the cost of resilience to future adverse events.
The following aging components can produce the decline in resilience: (i) depletion of exhaustible body reserves that poses limits to recovery; (ii) slowdown of physiological processes and responses that delays the recovery; and (iii) inherently imperfect mechanisms of cell/tissue repair and cleaning that lead to accumulation of damage and allostatic load contributing to progressive dysregulation of body systems with age (Figure 2).
These aging components can be seen in all aging animals, albeit their relative contributions to the decline in resilience, as well as to longevity limits, may differ across species, which could contribute to the variability of longevity and pace of aging among the species/strains. This may also be a reason why the effects of anti-aging interventions observed in lab animals are not always replicated in humans. There are open questions about relative impacts of the different aging components on the decline in resilience and the increase in mortality risk with age. However, the area develops quickly, and prospects are encouraging.
Finding the ‘optimal’ anti-aging intervention that could oppose the decline in resilience and also extend the species longevity limits remains a challenging problem. To be more efficient, the anti-aging interventions may need to target several aging components at once, e.g., help replenish body reserves, enhance cell repair and tissue cleaning, and attenuate the slowdown of metabolism, proliferation and information processing, simultaneously.
Experimental studies of aging show that genes that influence aging-related phenotypes and longevity in model organisms are commonly involved in resilience. Perhaps the most intensively studied conserved aging pathways (IGF-1/AKT/FOXO3A, TP53/P21/P16, and mTOR/S6K - mediated) work together to decide on particular cell responses to stress/damage, such as apoptosis, senescence, growth/survival or autophagy, which could play a major role in shaping the resilience and its decline with age. The fact that it takes concerted work of genes from these pathways to determine cell outcomes calls for a deeper exploration of the collective (both additive and interaction) effects of genes from respective pathways on resilience-related phenotypes.
Highlights.
Decline in biological resilience (ability to bounce back and recover) is a key manifestation of aging that contributes to increase in vulnerability to death with age limiting longevity even in people without major diseases.
Resilience is different from robustness which refers to the ability to avoid damage and its destructive consequences whatsoever. The robustness generally declines during aging; however, it may improve in some health domains, sometimes at the cost of resilience to future adverse events.
Main aging components that contribute to the decline in resilience are: (i) depletion of body reserves; (ii) slowdown of physiological processes and responses with age; and (iii) damage accumulation due to imperfect mechanisms of cell/tissue repair and cleaning.
To attenuate the age-decline in resilience, prospective anti-aging interventions may need to target all these aging components simultaneously, to avoid potential trade-offs between robustness and resilience.
Availability of direct and longitudinal biomarkers of biological resilience in human studies is limited. Indirect and aggregate indicators of the resilience available in human data should be exploited more.
Conserved aging pathways are valuable sources of candidate resilience genes. These pathways work in concert to determine outcomes of cell responses to stress/damage, playing major roles in tissue resilience. We call for a deeper exploration of the collective (additive and interaction) effects of genes from such pathways on resilience-related phenotypes.
Acknowledgements
This work was in part supported by the National Institutes of Aging/National Institutes of Health [grant numbers 1R01AG062623, 1R01AG070487, 1R01AG063971, and 2RF1AG046860].
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akushevich I, Kravchenko J, Ukraintseva S, Arbeev K and Yashin AI (2012). “Age patterns of incidence of geriatric disease in the U.S. elderly population: Medicare-based analysis.” J Am Geriatr Soc 60(2): 323–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimov VN (2015). “Conservative growth hormone/IGF-1 and mTOR signaling pathways as a target for aging and cancer prevention: do we really have an antiaging drug?” Interdiscip Top Gerontol 40: 177–188. [DOI] [PubMed] [Google Scholar]
- Anisimov VN, Ukraintseva SV, Anikin IV, Popovich IG, Zabezhinski MA, Bertsein LM, Arutjunyan AV, Ingram DK, Lane MA and Roth GS (2005). “Effects of phentermine and phenformin on biomarkers of aging in rats.” Gerontology 51(1): 19–28. [DOI] [PubMed] [Google Scholar]
- Anisimov VN, Ukraintseva SV and Yashin AI (2005). “Cancer in rodents: does it tell us about cancer in humans?” Nat Rev Cancer 5(10): 807–819. [DOI] [PubMed] [Google Scholar]
- Arbeev KG, Bagley O, Ukraintseva SV, Duan H, Kulminski AM, Stallard E, Wu D, Christensen K, Feitosa MF, Thyagarajan B, Zmuda JM and Yashin AI (2020). “Composite Measure of Physiological Dysregulation as a Predictor of Mortality: The Long Life Family Study.” Front Public Health 8: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbeev KG, Bagley O, Ukraintseva SV, Wu D, Duan H, Kulminski AM, Stallard E, Christensen K, Lee JH, Thyagarajan B, Zmuda JM and Yashin AI (2020). “Genetics of physiological dysregulation: findings from the long life family study using joint models.” Aging (Albany NY) 12(7): 5920–5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbeev KG, Ukraintseva SV, Bagley O, Zhbannikov IY, Cohen AA, Kulminski AM and Yashin AI (2019). “ “Physiological Dysregulation” as a Promising Measure of Robustness and Resilience in Studies of Aging and a New Indicator of Preclinical Disease.” J Gerontol A Biol Sci Med Sci 74(4): 462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold WD and Padilla Colón CJ (2020). “ ‘Maintaining Muscle Function Across the Lifespan: The State of Science’.” Am J Phys Med Rehabil. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL and van Deursen JM (2011). “Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders.” Nature 479(7372): 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartke A and Quainoo N (2018). “Impact of Growth Hormone-Related Mutations on Mammalian Aging.” Front Genet 9: 586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartke A, Westbrook R, Sun L and Ratajczak M (2013). “Links between growth hormone and aging.” Endokrynol Pol 64(1): 46–52. [PMC free article] [PubMed] [Google Scholar]
- Barzilai N, Crandall JP, Kritchevsky SB and Espeland MA (2016). “Metformin as a Tool to Target Aging.” Cell Metab 23(6): 1060–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista-Niño PK, Portilla-Fernandez E, Vaughan DE, Danser AH and Roks AJ (2016). “DNA Damage: A Main Determinant of Vascular Aging.” Int J Mol Sci 17(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky DW, Caspi A, Arseneault L, Baccarelli A, Corcoran DL, Gao X, Hannon E, Harrington HL, Rasmussen LJ, Houts R, Huffman K, Kraus WE, Kwon D, Mill J, Pieper CF, Prinz JA, Poulton R, Schwartz J, Sugden K, Vokonas P, Williams BS and Moffitt TE (2020). “Quantification of the pace of biological aging in humans through a blood test, the DunedinPoAm DNA methylation algorithm.” Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitto A, Wang AM, Bennett CF and Kaeberlein M (2015). “Biochemical Genetic Pathways that Modulate Aging in Multiple Species.” Cold Spring Harb Perspect Med 5(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braeckman BP and Vanfleteren JR (2007). “Genetic control of longevity in C. elegans.” Exp Gerontol 42(1–2): 90–98. [DOI] [PubMed] [Google Scholar]
- Brett JO, Arjona M, Ikeda M, Quarta M, de Morrée A, Egner IM, Perandini LA, Ishak HD, Goshayeshi A, Benjamin DI, Both P, Rodríguez-Mateo C, Betley MJ, Wyss-Coray T and Rando TA (2020). “Exercise rejuvenates quiescent skeletal muscle stem cells in old mice through restoration of Cyclin D1.” Nat Metab 2(4): 307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broer L, Buchman AS, Deelen J, Evans DS, Faul JD, Lunetta KL, Sebastiani P, Smith JA, Smith AV, Tanaka T, Yu L, Arnold AM, Aspelund T, Benjamin EJ, De Jager PL, Eirkisdottir G, Evans DA, Garcia ME, Hofman A, Kaplan RC, Kardia SL, Kiel DP, Oostra BA, Orwoll ES, Parimi N, Psaty BM, Rivadeneira F, Rotter JI, Seshadri S, Singleton A, Tiemeier H, Uitterlinden AG, Zhao W, Bandinelli S, Bennett DA, Ferrucci L, Gudnason V, Harris TB, Karasik D, Launer LJ, Perls TT, Slagboom PE, Tranah GJ, Weir DR, Newman AB, van Duijn CM and Murabito JM (2015). “GWAS of longevity in CHARGE consortium confirms APOE and FOXO3 candidacy.” J Gerontol A Biol Sci Med Sci 70(1): 110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash TP, Pita G, Domínguez O, Alonso MR, Moreno LT, Borrás C, Rodríguez-Mañas L, Santiago C, Garatachea N, Lucia A, Avellana JA, Viña J, González-Neira A and Serrano M (2014). “Exome sequencing of three cases of familial exceptional longevity.” Aging Cell 13(6): 1087–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casuso RA and Huertas JR (2020). “The emerging role of skeletal muscle mitochondrial dynamics in exercise and ageing.” Ageing Res Rev 58: 101025. [DOI] [PubMed] [Google Scholar]
- Chang KP, Center JR, Nguyen TV and Eisman JA (2004). “Incidence of hip and other osteoporotic fractures in elderly men and women: Dubbo Osteoporosis Epidemiology Study.” J Bone Miner Res 19(4): 532–536. [DOI] [PubMed] [Google Scholar]
- Cohen AA, Milot E, Li Q, Legault V, Fried LP and Ferrucci L (2014). “Cross-population validation of statistical distance as a measure of physiological dysregulation during aging.” Exp Gerontol 57: 203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colón-Emeric C, Pieper CF, Schmader KE, Sloane R, Bloom A, McClain M, Magaziner J, Huffman KM, Orwig D, Crabtree DM and Whitson HE (2020). “Two Approaches to Classifying and Quantifying Physical Resilience in Longitudinal Data.” J Gerontol A Biol Sci Med Sci 75(4): 731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colón-Emeric C, Whitson HE, Pieper CF, Sloane R, Orwig D, Huffman KM, Bettger JP, Parker D, Crabtree DM, Gruber-Baldini A and Magaziner J (2019). “Resiliency Groups Following Hip Fracture in Older Adults.” J Am Geriatr Soc 67(12): 2519–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosco TD, Kaushal A, Richards M, Kuh D and Stafford M (2016). “Resilience measurement in later life: a systematic review and psychometric analysis.” Health Qual Life Outcomes 14: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dansereau G, Wey TW, Legault V, Brunet MA, Kemnitz JW, Ferrucci L and Cohen AA (2019). “Conservation of physiological dysregulation signatures of aging across primates.” Aging Cell 18(2): e12925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit J and Verhaagen J (2003). “Role of semaphorins in the adult nervous system.” Prog Neurobiol 71(2–3): 249–267. [DOI] [PubMed] [Google Scholar]
- Deelen J, Beekman M, Uh HW, Broer L, Ayers KL, Tan Q, Kamatani Y, Bennet AM, Tamm R, Trompet S, Guðbjartsson DF, Flachsbart F, Rose G, Viktorin A, Fischer K, Nygaard M, Cordell HJ, Crocco P, van den Akker EB, Böhringer S, Helmer Q, Nelson CP, Saunders GI, Alver M, Andersen-Ranberg K, Breen ME, van der Breggen R, Caliebe A, Capri M, Cevenini E, Collerton JC, Dato S, Davies K, Ford I, Gampe J, Garagnani P, de Geus EJ, Harrow J, van Heemst D, Heijmans BT, Heinsen FA, Hottenga JJ, Hofman A, Jeune B, Jonsson PV, Lathrop M, Lechner D, Martin-Ruiz C, McNerlan SE, Mihailov E, Montesanto A, Mooijaart SP, Murphy A, Nohr EA, Paternoster L, Postmus I, Rivadeneira F, Ross OA, Salvioli S, Sattar N, Schreiber S, Stefánsson H, Stott DJ, Tiemeier H, Uitterlinden AG, Westendorp RG, Willemsen G, Samani NJ, Galan P, Sørensen TI, Boomsma DI, Jukema JW, Rea IM, Passarino G, de Craen AJ, Christensen K, Nebel A, Stefánsson K, Metspalu A, Magnusson P, Blanché H, Christiansen L, Kirkwood TB, van Duijn CM, Franceschi C, Houwing-Duistermaat JJ and Slagboom PE (2014). “Genome-wide association meta-analysis of human longevity identifies a novel locus conferring survival beyond 90 years of age.” Hum Mol Genet 23(16): 4420–4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deelen J, Beekman M, Uh HW, Helmer Q, Kuningas M, Christiansen L, Kremer D, van der Breggen R, Suchiman HE, Lakenberg N, van den Akker EB, Passtoors WM, Tiemeier H, van Heemst D, de Craen AJ, Rivadeneira F, de Geus EJ, Perola M, van der Ouderaa FJ, Gunn DA, Boomsma DI, Uitterlinden AG, Christensen K, van Duijn CM, Heijmans BT, Houwing-Duistermaat JJ, Westendorp RG and Slagboom PE (2011). “Genome-wide association study identifies a single major locus contributing to survival into old age; the APOE locus revisited.” Aging Cell 10(4): 686–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deelen J, Evans DS, Arking DE, Tesi N, Nygaard M, Liu X, Wojczynski MK, Biggs ML, van der Spek A, Atzmon G, Ware EB, Sarnowski C, Smith AV, Seppälä I, Cordell HJ, Dose J, Amin N, Arnold AM, Ayers KL, Barzilai N, Becker EJ, Beekman M, Blanché H, Christensen K, Christiansen L, Collerton JC, Cubaynes S, Cummings SR, Davies K, Debrabant B, Deleuze JF, Duncan R, Faul JD, Franceschi C, Galan P, Gudnason V, Harris TB, Huisman M, Hurme MA, Jagger C, Jansen I, Jylhä M, Kähönen M, Karasik D, Kardia SLR, Kingston A, Kirkwood TBL, Launer LJ, Lehtimäki T, Lieb W, Lyytikäinen LP, Martin-Ruiz C, Min J, Nebel A, Newman AB, Nie C, Nohr EA, Orwoll ES, Perls TT, Province MA, Psaty BM, Raitakari OT, Reinders MJT, Robine JM, Rotter JI, Sebastiani P, Smith J, Sørensen TIA, Taylor KD, Uitterlinden AG, van der Flier W, van der Lee SJ, van Duijn CM, van Heemst D, Vaupel JW, Weir D, Ye K, Zeng Y, Zheng W, Holstege H, Kiel DP, Lunetta KL, Slagboom PE and Murabito JM (2019). “A meta-analysis of genome-wide association studies identifies multiple longevity genes.” Nat Commun 10(1): 3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deelen J, Uh HW, Monajemi R, van Heemst D, Thijssen PE, Böhringer S, van den Akker EB, de Craen AJ, Rivadeneira F, Uitterlinden AG, Westendorp RG, Goeman JJ, Slagboom PE, Houwing-Duistermaat JJ and Beekman M (2013). “Gene set analysis of GWAS data for human longevity highlights the relevance of the insulin/IGF-1 signaling and telomere maintenance pathways.” Age (Dordr) 35(1): 235–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Francesco A, Diaz-Ruiz A, de Cabo R and Bernier M (2018). “Intermittent mTOR Inhibition Reverses Kidney Aging in Old Rats.” J Gerontol A Biol Sci Med Sci 73(7): 843–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMilia PR, Mittman AC and Batsis JA (2019). “Benefit-to-Risk Balance of Weight Loss Interventions in Older Adults with Obesity.” Curr Diab Rep 19(11): 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlon TA, Willcox BJ and Morris BJ (2017). “FOXO3 cell resilience gene neighborhood.” Aging (Albany NY) 9(12): 2467–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Kadiry AE and Rafei M (2019). “Restoring thymic function: Then and now.” Cytokine 120: 202–209. [DOI] [PubMed] [Google Scholar]
- Fang J, Yang J, Wu X, Zhang G, Li T, Wang X, Zhang H, Wang CC, Liu GH and Wang L (2018). “Metformin alleviates human cellular aging by upregulating the endoplasmic reticulum glutathione peroxidase 7.” Aging Cell 17(4): e12765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Hill CM, Darcy J, Reyes-Ordoñez A, Arauz E, McFadden S, Zhang C, Osland J, Gao J, Zhang T, Frank SJ, Javors MA, Yuan R, Kopchick JJ, Sun LY, Chen J and Bartke A (2018). “Effects of rapamycin on growth hormone receptor knockout mice.” Proc Natl Acad Sci U S A 115(7): E1495–e1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z, Hu W, Rajagopal G and Levine AJ (2008). “The tumor suppressor p53: cancer and aging.” Cell Cycle 7(7): 842–847. [DOI] [PubMed] [Google Scholar]
- Feng Z and Levine AJ (2010). “The regulation of energy metabolism and the IGF-1/mTOR pathways by the p53 protein.” Trends Cell Biol 20(7): 427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop T, Larbi A, Dupuis G, Le Page A, Frost EH, Cohen AA, Witkowski JM and Franceschi C (2017). “Immunosenescence and Inflamm-Aging As Two Sides of the Same Coin: Friends or Foes?” Front Immunol 8: 1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvin A, Ukraintseva S, Arbeev K, Feitosa M and Christensen K (2020). “Physical robustness and resilience among long-lived female siblings: a comparison with sporadic long-livers.” Aging (Albany NY) 12(14): 15157–15168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia DN, Saccon TD, Pradiee J, Rincón JAA, Andrade KRS, Rovani MT, Mondadori RG, Cruz LAX, Barros CC, Masternak MM, Bartke A, Mason JB and Schneider A (2019). “Effect of caloric restriction and rapamycin on ovarian aging in mice.” Geroscience 41(4): 395–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg K and Boppart MD (2016). “Influence of exercise and aging on extracellular matrix composition in the skeletal muscle stem cell niche.” J Appl Physiol (1985) 121(5): 1053–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng L, Liu Z, Wang S, Sun S, Ma S, Liu X, Chan P, Sun L, Song M, Zhang W, Liu GH and Qu J (2019). “Low-dose quercetin positively regulates mouse healthspan.” Protein Cell 10(10): 770–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes LU, Jeune B, Ranberg KA, Nybo H and Vaupel JW (2000). “Estimation of apolipoprotein E genotype-specific relative mortality risks from the distribution of genotypes in centenarians and middle-aged men: apolipoprotein E gene is a “frailty gene,” not a “longevity gene”.” Genet Epidemiol 19(3): 202–210. [DOI] [PubMed] [Google Scholar]
- Gesing A, Al-Regaiey KA, Bartke A and Masternak MM (2014). “Growth hormone abolishes beneficial effects of calorie restriction in long-lived Ames dwarf mice.” Exp Gerontol 58: 219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesing A, Bartke A, Wang F, Karbownik-Lewinska M and Masternak MM (2011). “Key regulators of mitochondrial biogenesis are increased in kidneys of growth hormone receptor knockout (GHRKO) mice.” Cell Biochem Funct 29(6): 459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani C, Garagnani P and Franceschi C (2018). “Genetics of Human Longevity Within an Eco-Evolutionary Nature-Nurture Framework.” Circ Res 123(7): 745–772. [DOI] [PubMed] [Google Scholar]
- Gollihue JL and Rabchevsky AG (2017). “Prospects for therapeutic mitochondrial transplantation.” Mitochondrion 35: 70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley EC, Kuchel GA and Newman AB (2017). “Report: NIA Workshop on Measures of Physiologic Resiliencies in Human Aging.” J Gerontol A Biol Sci Med Sci 72(7): 980–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley EC, Kuchel GA, Newman AB, Allore HG, Bartley JM, Bergeman CS, Blinov ML, Colon-Emeric CS, Dabhar FS, Dugan LL, Dutta C, Eldadah BA, Ferrucci L, Kirkland JL, Kritchevsky SB, Lipsitz LA, Nadkarni NK, Reed MJ, Schmader KE, Sierra F, Studenski SA, Varadhan R, Walston JD, Whitson HE and Yung R (2018). “Corrigendum to: Report: NIA Workshop on Measures of Physiologic Resiliencies in Human Aging.” J Gerontol A Biol Sci Med Sci 73(7): 995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haentjens P, Magaziner J, Colón-Emeric CS, Vanderschueren D, Milisen K, Velkeniers B and Boonen S (2010). “Meta-analysis: excess mortality after hip fracture among older women and men.” Ann Intern Med 152(6): 380–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghani A, Thorwald M, Morgan TE and Finch CE (2020). “The APOE gene cluster responds to air pollution factors in mice with coordinated expression of genes that differs by age in humans.” Alzheimers Dement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamarat E, Thompson D, Aysan F, Steele D, Matheny K and Simons C (2002). “Age differences in coping resources and satisfaction with life among middle-aged, young-old, and oldest-old adults.” J Genet Psychol 163(3): 360–367. [DOI] [PubMed] [Google Scholar]
- Harper JM, Salmon AB, Leiser SF, Galecki AT and Miller RA (2007). “Skin-derived fibroblasts from long-lived species are resistant to some, but not all, lethal stresses and to the mitochondrial inhibitor rotenone.” Aging Cell 6(1): 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman KJ, Kerse N and Consedine NS (2017). “Resilience in context: the special case of advanced age.” Aging Ment Health 21(6): 577–585. [DOI] [PubMed] [Google Scholar]
- Howlader N, N. A., Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds) (2019). SEER Cancer Statistics Review, 1975–2017. Bethesda, MD, National Cancer Institute. [Google Scholar]
- Hsueh MF, Önnerfjord P, Bolognesi MP, Easley ME and Kraus VB (2019). “Analysis of “old” proteins unmasks dynamic gradient of cartilage turnover in human limbs.” Sci Adv 5(10): eaax3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu T, Shi JJ, Fang J, Wang Q, Chen YB and Zhang SJ (2020). “Quercetin ameliorates diabetic encephalopathy through SIRT1/ER stress pathway in db/db mice.” Aging (Albany NY) 12(8): 7015–7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iside C, Scafuro M, Nebbioso A and Altucci L (2020). “SIRT1 Activation by Natural Phytochemicals: An Overview.” Front Pharmacol 11: 1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong W, Lee H, Cho S and Seo J (2019). “ApoE4-Induced Cholesterol Dysregulation and Its Brain Cell Type-Specific Implications in the Pathogenesis of Alzheimer’s Disease.” Mol Cells 42(11): 739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SC, Sangesland M, Kaeberlein M and Rabinovitch PS (2015). “Modulating mTOR in aging and health.” Interdiscip Top Gerontol 40: 107–127. [DOI] [PubMed] [Google Scholar]
- Johnson TE, de Castro E, Hegi de Castro S, Cypser J, Henderson S and Tedesco P (2001). “Relationship between increased longevity and stress resistance as assessed through gerontogene mutations in Caenorhabditis elegans.” Exp Gerontol 36(10): 1609–1617. [DOI] [PubMed] [Google Scholar]
- Johnson TE, Henderson S, Murakami S, de Castro E, de Castro SH, Cypser J, Rikke B, Tedesco P and Link C (2002). “Longevity genes in the nematode Caenorhabditis elegans also mediate increased resistance to stress and prevent disease.” J Inherit Metab Dis 25(3): 197–206. [DOI] [PubMed] [Google Scholar]
- Kang S, Moser VA, Svendsen CN and Goodridge HS (2020). “Rejuvenating the blood and bone marrow to slow aging-associated cognitive decline and Alzheimer’s disease.” Commun Biol 3(1): 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsoulis M, Benetou V, Karapetyan T, Feskanich D, Grodstein F, Pettersson-Kymmer U, Eriksson S, Wilsgaard T, Jørgensen L, Ahmed LA, Schöttker B, Brenner H, Bellavia A, Wolk A, Kubinova R, Stegeman B, Bobak M, Boffetta P and Trichopoulou A (2017). “Excess mortality after hip fracture in elderly persons from Europe and the USA: the CHANCES project.” J Intern Med 281(3): 300–310. [DOI] [PubMed] [Google Scholar]
- Ke Z, Firsanov D, Spencer B, Seluanov A and Gorbunova V (2020). “Short-term calorie restriction enhances DNA repair by non-homologous end joining in mice.” NPJ Aging Mech Dis 6: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon CJ (2010). “The genetics of ageing.” Nature 464(7288): 504–512. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Hwang JW, Yun CK, Lee Y and Choi YS (2018). “Delivery of exogenous mitochondria via centrifugation enhances cellular metabolic function.” Sci Rep 8(1): 3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland JL, Stout MB and Sierra F (2016). “Resilience in Aging Mice.” J Gerontol A Biol Sci Med Sci 71(11): 1407–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland JL and Tchkonia T (2020). “Senolytic drugs: from discovery to translation.” J Intern Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisliouk T, Cramer T and Meiri N (2017). “Methyl CpG level at distal part of heat-shock protein promoter HSP70 exhibits epigenetic memory for heat stress by modulating recruitment of POU2F1-associated nucleosome-remodeling deacetylase (NuRD) complex.” J Neurochem 141(3): 358–372. [DOI] [PubMed] [Google Scholar]
- Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH and Burgering BM (2002). “Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress.” Nature 419(6904): 316–321. [DOI] [PubMed] [Google Scholar]
- Kulminski A, Ukraintseva SV, Akushevich I, Arbeev KG, Land K and Yashin AI (2007). “Accelerated accumulation of health deficits as a characteristic of aging.” Exp Gerontol 42(10): 963–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulminski AM, Ukraintseva SV, Kulminskaya IV, Arbeev KG, Land K and Yashin AI (2008). “Cumulative deficits better characterize susceptibility to death in elderly people than phenotypic frailty: lessons from the Cardiovascular Health Study.” J Am Geriatr Soc 56(5): 898–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrick JW and Mendelsohn AR (2020). “Exercise Partially Rejuvenates Muscle Stem Cells.” Rejuvenation Res 23(3): 262–265. [DOI] [PubMed] [Google Scholar]
- Lavin KM, Perkins RK, Jemiolo B, Raue U, Trappe SW and Trappe TA (2020). “Effects of aging and lifelong aerobic exercise on basal and exercise-induced inflammation.” J Appl Physiol (1985) 128(1): 87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehallier B, Gate D, Schaum N, Nanasi T, Lee SE, Yousef H, Moran Losada P, Berdnik D, Keller A, Verghese J, Sathyan S, Franceschi C, Milman S, Barzilai N and Wyss-Coray T (2019). “Undulating changes in human plasma proteome profiles across the lifespan.” Nat Med 25(12): 1843–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiszter K, Galamb O, Sipos F, Krenács T, Veres G, Wichmann B, Kalmár A, Patai Á V, Tóth K, Valcz G, Molnár B and Tulassay Z (2013). “Sporadic colorectal cancer development shows rejuvenescence regarding epithelial proliferation and apoptosis.” PLoS One 8(10): e74140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ME, Lu AT, Quach A, Chen BH, Assimes TL, Bandinelli S, Hou L, Baccarelli AA, Stewart JD, Li Y, Whitsel EA, Wilson JG, Reiner AP, Aviv A, Lohman K, Liu Y, Ferrucci L and Horvath S (2018). “An epigenetic biomarker of aging for lifespan and healthspan.” Aging (Albany NY) 10(4): 573–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Chen S, Li Q, Chen L, Zhang H, Li H, Yu D, Zhang R, Niu Y, Lu S, Ye L, Zeng X, Dong G, Chen R, Aschner M, Zheng Y and Chen W (2020). “Caloric restriction attenuates C57BL/6 J mouse lung injury and extra-pulmonary toxicity induced by real ambient particulate matter exposure.” Part Fibre Toxicol 17(1): 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Zeng Z, Zhang Y and Chen N (2020). “Regulatory role of exercise-induced autophagy for sarcopenia.” Exp Gerontol 130: 110789. [DOI] [PubMed] [Google Scholar]
- Lin JC-F, Wu C-C, Lo C, Liang W-M, Cheng C-F, Wang C-B, Chang Y-J, Wu H-C and Leu T-H (2014). “Mortality and complications of hip fracture in young adults: a nationwide population-based cohort study.” BMC Musculoskeletal Disorders 15(1): 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Antebi A, Bartke A, Barzilai N, Brown-Borg HM, Caruso C, Curiel TJ, de Cabo R, Franceschi C, Gems D, Ingram DK, Johnson TE, Kennedy BK, Kenyon C, Klein S, Kopchick JJ, Lepperdinger G, Madeo F, Mirisola MG, Mitchell JR, Passarino G, Rudolph KL, Sedivy JM, Shadel GS, Sinclair DA, Spindler SR, Suh Y, Vijg J, Vinciguerra M and Fontana L (2015). “Interventions to Slow Aging in Humans: Are We Ready?” Aging Cell 14(4): 497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopizzo N, Zonca V, Cattane N, Pariante CM and Cattaneo A (2019). “miRNAs in depression vulnerability and resilience: novel targets for preventive strategies.” J Neural Transm (Vienna) 126(9): 1241–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Brommer B, Tian X, Krishnan A, Meer M, Wang C, Vera DL, Zeng Q, Yu D, Bonkowski MS, Yang JH, Zhou S, Hoffmann EM, Karg MM, Schultz MB, Kane AE, Davidsohn N, Korobkina E, Chwalek K, Rajman LA, Church GM, Hochedlinger K, Gladyshev VN, Horvath S, Levine ME, Gregory-Ksander MS, Ksander BR, He Z and Sinclair DA (2020). “Reprogramming to recover youthful epigenetic information and restore vision.” Nature 588(7836): 124–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley RW (2016). “Central Nervous System Lipoproteins: ApoE and Regulation of Cholesterol Metabolism.” Arterioscler Thromb Vasc Biol 36(7): 1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar S and Nandi D (2018). “Thymic Atrophy: Experimental Studies and Therapeutic Interventions.” Scand J Immunol 87(1): 4–14. [DOI] [PubMed] [Google Scholar]
- Mampay M and Sheridan GK (2019). “REST: An epigenetic regulator of neuronal stress responses in the young and ageing brain.” Front Neuroendocrinol 53: 100744. [DOI] [PubMed] [Google Scholar]
- Marioni RE, Suderman M, Chen BH, Horvath S, Bandinelli S, Morris T, Beck S, Ferrucci L, Pedersen NL, Relton CL, Deary IJ and Hägg S (2019). “Tracking the Epigenetic Clock Across the Human Life Course: A Meta-analysis of Longitudinal Cohort Data.” J Gerontol A Biol Sci Med Sci 74(1): 57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marycz K, Tomaszewski KA, Kornicka K, Henry BM, Wroński S, Tarasiuk J and Maredziak M (2016). “Metformin Decreases Reactive Oxygen Species, Enhances Osteogenic Properties of Adipose-Derived Multipotent Mesenchymal Stem Cells In Vitro, and Increases Bone Density In Vivo.” Oxid Med Cell Longev 2016: 9785890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehdipour M, Skinner C, Wong N, Lieb M, Liu C, Etienne J, Kato C, Kiprov D, Conboy MJ and Conboy IM (2020). “Rejuvenation of three germ layers tissues by exchanging old blood plasma with saline-albumin.” Aging (Albany NY) 12(10): 8790–8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei H, Gu D, Rice TK, Hixson JE, Chen J, Jaquish CE, Zhao Q, Chen CS, Chen JC, Gu CC, Kelly TN and He J (2009). “Heritability of blood pressure responses to cold pressor test in a Chinese population.” Am J Hypertens 22(10): 1096–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitnitski A, Song X and Rockwood K (2013). “Assessing biological aging: the origin of deficit accumulation.” Biogerontology 14(6): 709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris BJ, Willcox BJ and Donlon TA (2019). “Genetic and epigenetic regulation of human aging and longevity.” Biochim Biophys Acta Mol Basis Dis 1865(7): 1718–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygaard M, Lindahl-Jacobsen R, Soerensen M, Mengel-From J, Andersen-Ranberg K, Jeune B, Vaupel JW, Tan Q, Christiansen L and Christensen K (2014). “Birth cohort differences in the prevalence of longevity-associated variants in APOE and FOXO3A in Danish long-lived individuals.” Exp Gerontol 57: 41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oksuzyan A, Juel K, Vaupel JW and Christensen K (2008). “Men: good health and high mortality. Sex differences in health and aging.” Aging Clin Exp Res 20(2): 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr BO, Fetter RD and Davis GW (2017). “Retrograde semaphorin-plexin signalling drives homeostatic synaptic plasticity.” Nature 550(7674): 109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palliyaguru DL, Minor RK, Mitchell SJ, Palacio HH, Licata JJ, Ward TM, Abulwerdi G, Elliott P, Westphal C, Ellis JL, Sinclair DA, Price NL, Bernier M and de Cabo R (2020). “Combining a high dose of metformin with the SIRT1 activator, SRT1720, reduces lifespan in aged mice fed a high-fat diet.” J Gerontol A Biol Sci Med Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker DC, Colόn-Emeric C, Huebner JL, Chou CH, Kraus VB, Pieper CF, Sloane R, Whitson HE, Orwig D, Crabtree DM, Magaziner J, Bain JR, Muehlbauer M, Ilkayeva OR and Huffman KM (2020). “Biomarkers Associated with Physical Resilience After Hip Fracture.” J Gerontol A Biol Sci Med Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelec G and Gupta S (2019). “Editorial: Immunology of Aging.” Front Immunol 10: 1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskovatska V, Stefanyshyn N, Storey KB, Vaiserman AM and Lushchak O (2019). “Metformin as a geroprotector: experimental and clinical evidence.” Biogerontology 20(1): 33–48. [DOI] [PubMed] [Google Scholar]
- Qian M and Liu B (2018). “Pharmaceutical Intervention of Aging.” Adv Exp Med Biol 1086: 235–254. [DOI] [PubMed] [Google Scholar]
- Reid JJ, Linden MA, Peelor FF, Miller RA, Hamilton KL and Miller BF (2020). “Brain Protein Synthesis Rates in the UM-HET3 Mouse Following Treatment With Rapamycin or Rapamycin With Metformin.” J Gerontol A Biol Sci Med Sci 75(1): 40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth GS, Lane MA, Ingram DK, Mattison JA, Elahi D, Tobin JD, Muller D and Metter EJ (2002). “Biomarkers of caloric restriction may predict longevity in humans.” Science 297(5582): 811. [DOI] [PubMed] [Google Scholar]
- Sabbagh JJ, O’Leary JC 3rd, Blair LJ, Klengel T, Nordhues BA, Fontaine SN, Binder EB and Dickey CA (2014). “Age-associated epigenetic upregulation of the FKBP5 gene selectively impairs stress resiliency.” PLoS One 9(9): e107241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer M, Bolhuis JE, Borsboom D, Buchman TG, Gijzel SMW, Goulson D, Kammenga JE, Kemp B, van de Leemput IA, Levin S, Martin CM, Melis RJF, van Nes EH, Romero LM and Olde Rikkert MGM (2018). “Quantifying resilience of humans and other animals.” Proc Natl Acad Sci U S A 115(47): 11883–11890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell S, Friedman SM, Mendelson DA, Bingham KW and Kates SL (2010). “The 1-year mortality of patients treated in a hip fracture program for elders.” Geriatr Orthop Surg Rehabil 1(1): 6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorr A, Carter C and Ladiges W (2018). “The potential use of physical resilience to predict healthy aging.” Pathobiol Aging Age Relat Dis 8(1): 1403844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schosserer M, Banks G, Dogan S, Dungel P, Fernandes A, Marolt Presen D, Matheu A, Osuchowski M, Potter P, Sanfeliu C, Tuna BG, Varela-Nieto I and Bellantuono I (2019). “Modelling physical resilience in ageing mice.” Mech Ageing Dev 177: 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shama S, Kirchman PA, Jiang JC and Jazwinski SM (1998). “Role of RAS2 in recovery from chronic stress: effect on yeast life span.” Exp Cell Res 245(2): 368–378. [DOI] [PubMed] [Google Scholar]
- Shin B, Cowan DB, Emani SM, Del Nido PJ and McCully JD (2017). “Mitochondrial Transplantation in Myocardial Ischemia and Reperfusion Injury.” Adv Exp Med Biol 982: 595–619. [DOI] [PubMed] [Google Scholar]
- Singh PP, Demmitt BA, Nath RD and Brunet A (2019). “The Genetics of Aging: A Vertebrate Perspective.” Cell 177(1): 200–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soerensen M, Dato S, Tan Q, Thinggaard M, Kleindorp R, Beekman M, Jacobsen R, Suchiman HE, de Craen AJ, Westendorp RG, Schreiber S, Stevnsner T, Bohr VA, Slagboom PE, Nebel A, Vaupel JW, Christensen K, McGue M and Christiansen L (2012). “Human longevity and variation in GH/IGF-1/insulin signaling, DNA damage signaling and repair and pro/antioxidant pathway genes: cross sectional and longitudinal studies.” Exp Gerontol 47(5): 379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soerensen M, Nygaard M, Debrabant B, Mengel-From J, Dato S, Thinggaard M, Christensen K and Christiansen L (2016). “No Association between Variation in Longevity Candidate Genes and Aging-related Phenotypes in Oldest-old Danes.” Exp Gerontol 78: 57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solich J, Kuśmider M, Faron-Górecka A, Pabian P, Kolasa M, Zemła B and Dziedzicka-Wasylewska M (2020). “Serum Level of miR-1 and miR-155 as Potential Biomarkers of Stress-Resilience of NET-KO and SWR/J Mice.” Cells 9(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Jiang G, Zhang J, Guo J, Li Z, Hao K, Liu L, Cheng Z, Tong X and Dai F (2019). “Metformin prolongs lifespan through remodeling the energy distribution strategy in silkworm, Bombyx mori.” Aging (Albany NY) 11(1): 240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storelli G, Téfit M and Leulier F (2013). “Metformin, microbes, and aging.” Cell Metab 17(6): 809–811. [DOI] [PubMed] [Google Scholar]
- Sun LY, Spong A, Swindell WR, Fang Y, Hill C, Huber JA, Boehm JD, Westbrook R, Salvatori R and Bartke A (2013). “Growth hormone-releasing hormone disruption extends lifespan and regulates response to caloric restriction in mice.” Elife 2: e01098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svatek RS, Ji N, de Leon E, Mukherjee NZ, Kabra A, Hurez V, Nicolas M, Michalek JE, Javors M, Wheeler K, Sharp ZD, Livi CB, Shu ZJ, Henkes D and Curiel TJ (2019). “Rapamycin Prevents Surgery-Induced Immune Dysfunction in Patients with Bladder Cancer.” Cancer Immunol Res 7(3): 466–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain U, Vyjayanti VN, Harikrishna T, Mahipal S and Rao KS (2016). “Dietary Calorie Restriction from Adulthood Through Old Age in Rats: Improved DNA Polymerase β and DNA Gap Repair Activity in Cortical Neurons.” Neurochem Res 41(1–2): 270–277. [DOI] [PubMed] [Google Scholar]
- Sweeten BLW, Sutton AM, Wellman LL and Sanford LD (2020). “Predicting stress resilience and vulnerability: brain-derived neurotrophic factor and rapid eye movement sleep as potential biomarkers of individual stress responses.” Sleep 43(1). [DOI] [PubMed] [Google Scholar]
- Swindell WR (2017). “Meta-Analysis of 29 Experiments Evaluating the Effects of Rapamycin on Life Span in the Laboratory Mouse.” J Gerontol A Biol Sci Med Sci 72(8): 1024–1032. [DOI] [PubMed] [Google Scholar]
- Tacutu R, Thornton D, Johnson E, Budovsky A, Barardo D, Craig T, Diana E, Lehmann G, Toren D, Wang J, Fraifeld VE and de Magalhães JP (2018). “Human Ageing Genomic Resources: new and updated databases.” Nucleic Acids Res 46(D1): D1083–d1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taff CC, Campagna L and Vitousek MN (2019). “Genome-wide variation in DNA methylation is associated with stress resilience and plumage brightness in a wild bird.” Mol Ecol 28(16): 3722–3737. [DOI] [PubMed] [Google Scholar]
- Tajima A, Pradhan I, Trucco M and Fan Y (2016). “Restoration of Thymus Function with Bioengineered Thymus Organoids.” Curr Stem Cell Rep 2(2): 128–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X, Firsanov D, Zhang Z, Cheng Y, Luo L, Tombline G, Tan R, Simon M, Henderson S, Steffan J, Goldfarb A, Tam J, Zheng K, Cornwell A, Johnson A, Yang JN, Mao Z, Manta B, Dang W, Zhang Z, Vijg J, Wolfe A, Moody K, Kennedy BK, Bohmann D, Gladyshev VN, Seluanov A and Gorbunova V (2019). “SIRT6 Is Responsible for More Efficient DNA Double-Strand Break Repair in Long-Lived Species.” Cell 177(3): 622–638.e622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissenbaum HA (2012). “Genetics, life span, health span, and the aging process in Caenorhabditis elegans.” J Gerontol A Biol Sci Med Sci 67(5): 503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran H, Brunet A, Grenier JM, Datta SR, Fornace AJ Jr., DiStefano PS, Chiang LW and Greenberg ME (2002). “DNA repair pathway stimulated by the forkhead transcription factor FOXO3a through the Gadd45 protein.” Science 296(5567): 530–534. [DOI] [PubMed] [Google Scholar]
- Ukraintseva S and Yashin A (2003). “Individual Aging and Cancer Risk: How are They Related?” Demographic Research 9(8): 163–196. [Google Scholar]
- Ukraintseva S, Yashin A, Arbeev K, Kulminski A, Akushevich I, Wu D, Joshi G, Land KC and Stallard E (2016). “Puzzling role of genetic risk factors in human longevity: “risk alleles” as pro-longevity variants.” Biogerontology 17(1): 109–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukraintseva S, Yashin AI and Arbeev KG (2016). “Resilience Versus Robustness in Aging.” J Gerontol A Biol Sci Med Sci 71(11): 1533–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukraintseva SV, A. K. G., Akushevich I, Kulminski AM, Stallard E, Yashin AI (2016). Factors That May Increase Vulnerability to Cancer and Longevity in Modern Human Populations Biodemography of Aging. The Springer Series on Demographic Methods and Population Analysis. , Springer, Dordrecht: 40: 113–141. [Google Scholar]
- Ukraintseva SV, Arbeev KG, Akushevich I, Kulminski A, Arbeeva L, Culminskaya I, Akushevich L and Yashin AI (2010). “Trade-offs between cancer and other diseases: do they exist and influence longevity?” Rejuvenation Res 13(4): 387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukraintseva SV, Arbeev KG and Yashin AI (2008). “Epidemiology of hormone-associated cancers as a reflection of age.” Adv Exp Med Biol 630: 57–71. [DOI] [PubMed] [Google Scholar]
- Ukraintseva SV and Sergeev AS (2000). “[Analysis of genetic heterogeneity of bronchial asthma in relation with the age at the onset of disease].” Genetika 36(2): 266–270. [PubMed] [Google Scholar]
- Ukraintseva SV and Yashin AI (2001). “How individual age-associated changes may influence human morbidity and mortality patterns.” Mech Ageing Dev 122(13): 1447–1460. [DOI] [PubMed] [Google Scholar]
- Ukraintseva SV and Yashin AI (2003). “Opposite phenotypes of cancer and aging arise from alternative regulation of common signaling pathways.” Ann N Y Acad Sci 1010: 489–492. [DOI] [PubMed] [Google Scholar]
- Ukraintseva SV and Yashin AI (2005). “Treating cancer with embryonic stem cells: rationale comes from aging studies.” Front Biosci 10: 588–595. [DOI] [PubMed] [Google Scholar]
- Uno M and Nishida E (2016). “Lifespan-regulating genes in C. elegans.” npj Aging and Mechanisms of Disease 2(1): 16010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Battum EY, Brignani S and Pasterkamp RJ (2015). “Axon guidance proteins in neurological disorders.” Lancet Neurol 14(5): 532–546. [DOI] [PubMed] [Google Scholar]
- Varadhan R, Walston JD and Bandeen-Roche K (2018). “Can a Link Be Found Between Physical Resilience and Frailty in Older Adults by Studying Dynamical Systems?” J Am Geriatr Soc 66(8): 1455–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagnild G (2009). “A review of the Resilience Scale.” J Nurs Meas 17(2): 105–113. [DOI] [PubMed] [Google Scholar]
- Warr MR, Binnewies M, Flach J, Reynaud D, Garg T, Malhotra R, Debnath J and Passegué E (2013). “FOXO3A directs a protective autophagy program in haematopoietic stem cells.” Nature 494(7437): 323–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitson HE, Duan-Porter W, Schmader KE, Morey MC, Cohen HJ and Colón-Emeric CS (2016). “Physical Resilience in Older Adults: Systematic Review and Development of an Emerging Construct.” J Gerontol A Biol Sci Med Sci 71(4): 489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcox BJ, Donlon TA, He Q, Chen R, Grove JS, Yano K, Masaki KH, Willcox DC, Rodriguez B and Curb JD (2008). “FOXO3A genotype is strongly associated with human longevity.” Proc Natl Acad Sci U S A 105(37): 13987–13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle G, Bennett KM and Noyes J (2011). “A methodological review of resilience measurement scales.” Health Qual Life Outcomes 9: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Li YX, Marron MM, Odden MC, Newman AB and Sanders JL (2019). “Quantifying and Classifying Physical Resilience among Older Adults: The Health, Aging, and Body Composition Study.” J Gerontol A Biol Sci Med Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashin AI, Arbeev KG, Akushevich I, Kulminski A, Ukraintseva SV, Stallard E and Land KC (2012). “The quadratic hazard model for analyzing longitudinal data on aging, health, and the life span.” Phys Life Rev 9(2): 177–188; discussion 195–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashin AI, Arbeev KG, Arbeeva LS, Wu D, Akushevich I, Kovtun M, Yashkin A, Kulminski A, Culminskaya I, Stallard E, Li M and Ukraintseva SV (2016). “How the effects of aging and stresses of life are integrated in mortality rates: insights for genetic studies of human health and longevity.” Biogerontology 17(1): 89–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashin AI, Arbeev KG, Kulminski A, Akushevich I, Akushevich L and Ukraintseva SV (2007). “Cumulative index of elderly disorders and its dynamic contribution to mortality and longevity.” Rejuvenation Res 10(1): 75–86. [DOI] [PubMed] [Google Scholar]
- Yashin AI, Arbeev KG, Wu D, Arbeeva LS, Bagley O, Stallard E, Kulminski AM, Akushevich I, Fang F, Wojczynski MK, Christensen K, Newman AB, Boudreau RM, Province MA, Thielke S, Perls TT, An P, Elo I and Ukraintseva SV (2018). “Genetics of Human Longevity From Incomplete Data: New Findings From the Long Life Family Study.” J Gerontol A Biol Sci Med Sci 73(11): 1472–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashin AI, Fang F, Kovtun M, Wu D, Duan M, Arbeev K, Akushevich I, Kulminski A, Culminskaya I, Zhbannikov I, Yashkin A, Stallard E and Ukraintseva S (2018). “Hidden heterogeneity in Alzheimer’s disease: Insights from genetic association studies and other analyses.” Exp Gerontol 107: 148–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Cheng L, Chen H, Cao H, Hauser ER, Liu Y, Xiao Z, Tan Q, Tian XL and Vaupel JW (2010). “Effects of FOXO genotypes on longevity: a biodemographic analysis.” J Gerontol A Biol Sci Med Sci 65(12): 1285–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Yu K, Yang J, Xie S, Yang J and Tan L (2020). “Senolytic controls bone marrow mesenchymal stem cells fate improving bone formation.” Am J Transl Res 12(6): 3078–3088. [PMC free article] [PubMed] [Google Scholar]
- Zhang ZD, Milman S, Lin JR, Wierbowski S, Yu H, Barzilai N, Gorbunova V, Ladiges WC, Niedernhofer LJ, Suh Y, Robbins PD and Vijg J (2020). “Genetics of extreme human longevity to guide drug discovery for healthy ageing.” Nat Metab 2(8): 663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]


