Abstract
Introduction
The possibility of vertical transmission of SARS-CoV-2 from the mother to the fetus is one of the most crucial issues regarding the COVID-19 effects on pregnancy. In this study, we aimed to explore the risk of maternal-fetal transmission before 24 weeks of gestation, through analysis of abortion materials collected from PCR-positive women with pregnancy loss. To the best of our knowledge, apart from case reports, this study is the first prospective work on the vertical transmission of SARS-CoV-2 in early pregnancy.
Methods
The patients who had attended our clinic with the diagnosis of pregnancy loss before 24 weeks of gestation were screened for COVİD-19. Vertical transmission in PCR-positive women was assessed through the presence of SARS-CoV-2 RNA in fetal-placental tissues by rt-PCR test.
Results
24 of 210 (%11,4) pregnant women participating in the study had positive rt-PCR results. Placenta and curettage material samples of these PCR-positive patients were analyzed and all valid test results (21 samples) were negative for SARS CoV-2 RNA. In three cases, the rt-PCR results were invalid due to failed internal controls.
Discussion
In the literature, the possibility of intrauterine vertical transmission of SARS-CoV-2 is still controversial. The findings of the present study did not reveal any evidence of vertical transmission of SARS-CoV-2 in early pregnancy.
Keywords: SARS-CoV-2, Maternal-fetal transmission, Early pregnancy, rt-PCR, Placenta
1. Introduction
2019 coronavirus disease pandemic (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is an important concern for pregnant women and obstetricians [1]. Numerous studies highlighted the relationship between COVID-19 infection and adverse pregnancy outcomes such as preterm birth, low birth weight, high neonatal intensive care unit admission and stillbirth [2,3].
One of the most crucial questions about the effects of COVID-19 on pregnant women is whether the virus is passed from the mother to the fetus. The answer is still unclear, although some studies based on molecular, serological and histopathological tests on fetal-placental and neonatal tissues suggest that vertical transmission is possible [[4], [5], [6]].
Despite intense interest in this topic since the beginning of the pandemic, there are still some research gaps. The vast majority of the studies addressing maternal-fetal vertical transmission of SARS-CoV-2 focused on the third trimester of pregnancy. Our knowledge about the transmission in early pregnancy is very limited. This is a very delicate period where the fetus is more vulnerable and viral transmissions can potentially cause adverse effects on prenatal development. Therefore, it is important to clarify the vertical transmission in early pregnancy [7].
In this study, we aimed to investigate the risk of maternal-fetal transmission of SARS-CoV-2 in early pregnancy (<24 weeks of gestation). Since the diagnosis of COVID-19 in fetus or placenta during pregnancy requires invasive procedures, we examined the possibility of vertical transmission through abortion materials collected from PCR-positive women with pregnancy loss.
2. Material and methods
2.1. Participants
This is a prospective study investigating women with early pregnancy loss (<24 weeks of gestation) who had attended the perinatology clinic at Ankara City Hospital (Turkey) between September 1, 2020 and December 1, 2020. Since the beginning of the COVID-19 pandemic, this tertiary reference hospital serves as one of the main national pandemic centers with an extensive experience dealing with COVID-19 infected pregnant women [8].
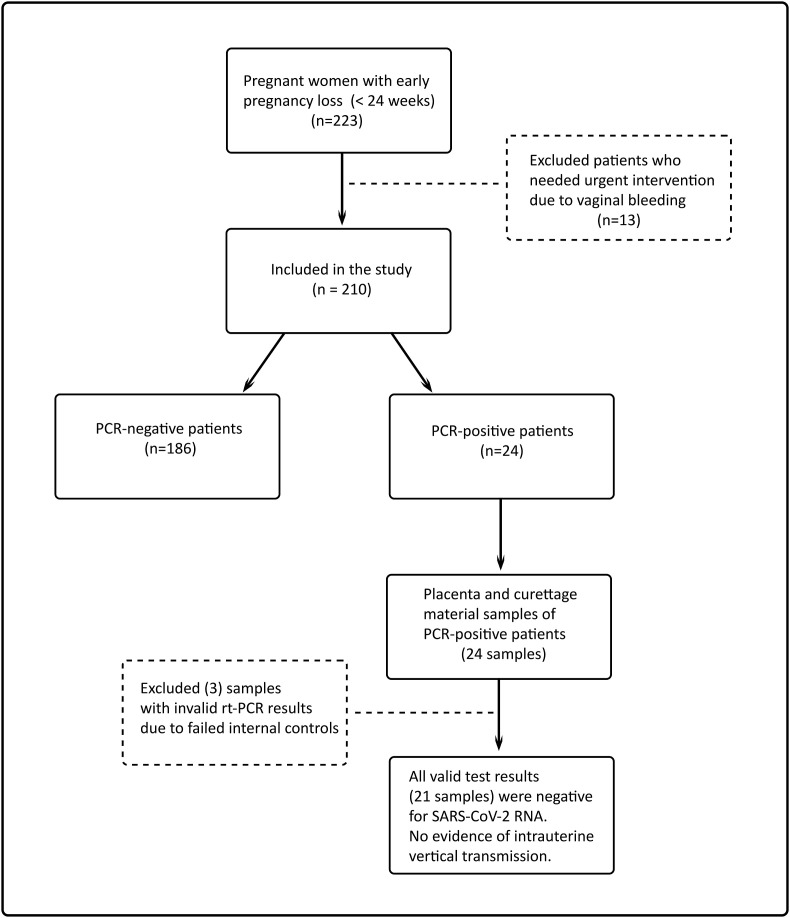
Pregnancy loss was diagnosed when observed an intrauterine empty gestational sac or an embryo or fetus without cardiac activity in the ultrasound examination [9]. During the study period, all patients with a diagnosis of pregnancy loss before 24 weeks of gestation and who hospitalized for medical or surgical abortion procedure in our center were assessed for participation to the study. 13 patients who needed emergency intervention due to vaginal bleeding were not included in the study. A total of 210 patients participated in the study (Fig. 1 ). The patients were informed about the study and the written informed consent was obtained.
Fig. 1.
Flow chart of the study participants.
All patients were screened for SARS-CoV-2 infection before medical or surgical abortion procedure. Abortion material of patients with positive results was sampled for SARS-CoV-2 RNA analysis.
2.2. Sample collection and processing methods
2.2.1. Maternal samples
Maternal samples were collected by nasopharyngeal and oropharyngeal swabs and transported to the laboratory with viral transport medium (VTM, various manufacturer) within 12 h and tested on arrival.
2.2.2. Placental samples
In pregnancies later than 12 weeks, within minutes of removal of the placenta after medically induced abortion, placental biopsy was taken from the fetal surface of the placenta. Due to the difficulty of obtaining separate placental tissue in early pregnancies, samples were taken from the abortion material consisting of decidua, trophoblastic and fetal tissue mixture obtained by aspiration curettage. During the material collection and transfer to the laboratory, care was taken to comply with the sterility rules and to prevent contamination. The samples were transferred to the hospital's molecular virology laboratory within 30–60 min after collection.
2.2.3. Tissue lysis
An approximately 2–3 mm3 of placental samples or abortion material was sectioned, and digested on a 65 °C heat block with 1000 μl of Buffer ATL (Qiagen, Hilden, Germany) and 50 μl of proteinase K (Qiagen, Hilden, Germany) for min 3 h.
2.2.4. Nucleic acid extraction
After denaturing for 10 min at 95 °C, vortex and spin processes were performed. 100 μl of this sample was then dissolved in 100 μl of Viral Nucleic Acid Extraction buffer (various manufacturers) and vortexed for 15 min before Polymerase Chain Reaction (PCR).
2.2.5. Real Time Reverse Transcriptase Polymerase Chain Reaction
SARS-CoV-2 in placental tissues and abortion material were detected by Real Time Reverse Transcriptase Polymerase Chain Reaction (rt-PCR) method targeting Orf1ab and N genes.
Real-time reverse-transcription PCR was performed by using Coronex COVID-19 rt-qPCR Detection Kit (DS Bio and Nano Technology, Ankara, Turkey) with 20 μL reaction containing 5 μL of RNA, 12,5 μL of CORONEX-Covid 19 DS Mix E (rt-qPCR Master mix) and 2,5 μL of CORONEX-Covid 19 DS PP1 (Orf1ab, N and RNP gene and primer-probe mix). Thermal cycling was performed at 48 °C for 20 min for reverse transcription, followed by 95 °C for 2 min and then 35 cycles of 95 °C for 5 s, 60 °C for 10 s, in Rotor-Gene Q device (Qiagen, Hilden, Germany). Cycle threshold (Ct) values of less than 35 were defined as positive.
2.3. Statistical analyses
Statistical analyses were carried out with IBM SPSS Statistics for Windows, version 23 (IBM Corp., Armonk, N.Y., USA). We used descriptive statistical methods. The mean and standard deviation were calculated for continuous and normally distributed variables. Categorical variables were presented as frequencies and percentages. To compare the differences between the PCR-positive and PCR negative patients' characteristics, we used Student t-test for continuous variables and Fisher's exact test for categorical variables.
2.4. Ethical approval
This study was conducted in accordance with the Declaration of Helsinki principles. It was approved by the Ethics Review Committee of Ankara City Hospital (approval number: E1-20-1222).
3. Results
Twenty-four of 210 (11,4%) pregnant women participating in the study had positive rt-PCR results for SARS-CoV-2 RNA (Fig. 1). There was no significant difference between PCR-positive and PCR-negative patients in terms of maternal age, gestational week, gravida, parity, previous abortions and pre-pregnancy body mass index. In the vast majority of the study group (181 of 210 patients, 86,2%), pregnancy loss occurred during the first trimester. The demographic characteristics were presented in Table 1 .
Table 1.
Demographic characteristics of the study group.
| COVID-19 Negative |
COVID-19 Positive |
P | |
|---|---|---|---|
| n = 186 | n = 24 | ||
| Maternal age, years, mean ± SD | 29,69 ± 6,61 | 30,58 ± 5,8 | ,528 |
| Gestational age, weeks, n (%) | ,113 | ||
| <14 | 163 (87,6) | 18 (75) | |
| ≥14 | 23 (12,4) | 6 (25) | |
| Gravida, n (%) | ,58 | ||
| 1 | 43 (23,1) | 5 (20,8) | |
| 2 | 58 (31,2) | 10 (41,7) | |
| ≥3 | 85 (45,7) | 9 (37,5) | |
| Parity, n (%) | ,744 | ||
| 0 | 57 (30,7) | 9 (37,5) | |
| 1 | 62 (33,3) | 9 (37,5) | |
| ≥2 | 67 (36) | 6 (25) | |
| Previous abortions, n (%) | ,867 | ||
| 0 | 125 (67,2) | 15 (62,5) | |
| 1 | 45 (24,2) | 7 (29,2) | |
| ≥2 | 16 (8,6) | 2 (8,3) | |
| Pre-pregnancy Body Mass Index, n (%) | ,071 | ||
| <30 | 159 (85,5) | 17 (70,8) | |
| ≥30 | 27 (14,5) | 7 (29,2) | |
| Smoking status, n (%) | ,227 | ||
| Non-smokers | 171 (91,9) | 24 (100) | |
| Smokers | 15 (8,1) | 0 | |
| Co-morbid diseases, n (%) | ,747 | ||
| Present | 161 (86,6) | 22 (91,7) | |
| Absent | 25 (13,4) | 2 (8,3) |
Among 24 PCR-positive pregnant women, 20 (83,3%) had no symptoms of COVID-19. Three patients had mild COVİD-19 disease with non-specific symptoms such as dry cough, sore throat, fatigue, diarrhea, and fewer. One patient with 19 weeks of gestation had critical COVİD-19 disease. Spontaneous abortion occurred while she was being treated in the intensive care unit, and after 4 days she died. Clinical and laboratory findings of the PCR-positive patients were presented in Table 2 .
Table 2.
Clinical and laboratory findings of the PCR-positive patients.
| Patient no. | Age, year | Gestational week | COVID-19 Status |
COVİD-19 Symptom |
Lymphocyte (103/mm3) | CRP (mg/dL) | IL-6 (pg/mL) | COVID-19 Treatment |
rt-PCR result of the abortion material |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 32 | 9 | Mild | Cough, fatigue | 1080 | 4,6 | 16,8 | Hydroxychloroquine | Negative |
| 2 | 33 | 16 | Asymptomatic | None | 1590 | 13,6 | 7,59 | None | Negative |
| 3 | 16 | 18 | Asymptomatic | None | 520 | 56 | – | None | Negative |
| 4 | 35 | 12 | Asymptomatic | None | 1250 | 9,3 | 7,79 | None | Negative |
| 5 | 34 | 8 | Asymptomatic | None | 1790 | 14,1 | 10,8 | None | Invalid |
| 6 | 38 | 10 | Mild | Diarrhea, fatigue | 1970 | 3,9 | 6,03 | Favipiravir | Negative |
| 7 | 33 | 6 | Asymptomatic | None | 790 | 103,7 | 126 | None | Negative |
| 8 | 33 | 9 | Asymptomatic | None | 1290 | 14,1 | – | None | Negative |
| 9 | 31 | 5 | Asymptomatic | None | 1700 | 11,7 | 5,8 | None | Negative |
| 10 | 27 | 19 | Critical | Respiratory and multi-organ failure | 820 | 156 | 23 | Lopinavir-ritonavir, corticosteroid, IL-1 receptor antagonist | Invalid |
| 11 | 35 | 17 | Asymptomatic | None | 1630 | 140,8 | – | None | Negative |
| 12 | 31 | 14 | Asymptomatic | None | 1180 | – | – | None | Invalid |
| 13 | 43 | 7 | Mild | Sore throat | 1400 | 32,4 | 10,2 | Hydroxychloroquine | Negative |
| 14 | 30 | 9 | Asymptomatic | None | 1030 | 33,8 | – | None | Negative |
| 15 | 28 | 7 | Asymptomatic | None | 1920 | 10,1 | – | None | Negative |
| 16 | 29 | 7 | Asymptomatic | None | 1520 | 5,7 | – | None | Negative |
| 17 | 37 | 10 | Mild | Cough, fever | 1940 | 118,6 | 6,51 | Hydroxychloroquine | Negative |
| 18 | 34 | 10 | Asymptomatic | None | 1420 | 13,8 | – | None | Negative |
| 19 | 28 | 8 | Asymptomatic | None | 2800 | 1,1 | 3,37 | None | Negative |
| 20 | 24 | 8 | Asymptomatic | None | 2130 | 3,8 | 7,02 | None | Negative |
| 21 | 28 | 9 | Asymptomatic | None | 1430 | – | – | None | Negative |
| 22 | 21 | 8 | Asymptomatic | None | 1240 | 1,6 | – | None | Negative |
| 23 | 31 | 7 | Asymptomatic | None | 1780 | 0,9 | – | None | Negative |
| 24 | 23 | 18 | Asymptomatic | None | 1110 | 24,2 | – | None | Negative |
Abortion materials of 24 pregnant women infected with COVID-19 were analyzed for SARS-CoV-2 RNA by rt-PCR test. In three cases, the rt-PCR results were invalid due to failed internal controls. All valid test results (21 samples) were negative for SARS-CoV-2 RNA. In others words, the analysis of PCR-positive patients’ abortion materials did not reveal any evidence of maternal-fetal vertical transmission of SARS-CoV-2.
4. Discussion
Questions regarding transmission of SARS-CoV-2 from mothers to fetuses during pregnancy remains pending. Since angiotensin converting enzyme 2 (ACE2) receptor, which is estimated to be the primary receptor of SARS-CoV-2, is highly expressed in maternal-fetal interface cells, there is a theoretical risk of maternal-fetal vertical transmission and subsequent adverse perinatal outcomes such as fetal malformations, early pregnancy losses and stillbirth [1]. Increasing evidences from studies that highlighting the presence of viral RNA and proteins in fetal and neonatal tissues and detection of positive serology also support the possibility of vertical transmission of SARS-CoV-2 [[10], [11], [12], [13], [14], [15]].
However, current available data from literature reviews showed that the evidence provided by the studies is poor and the vertical transmission of SARS-CoV-2 is still uncertain [[4], [5], [6]]. A meta-analysis, reviewing 38 studies, reported that viral transmission was mostly studied in neonatal nasopharyngeal swabs, and SARS-CoV-2 RNA positivity was 3,2% (22 out of 936 cases). In addition, SARS-CoV-2 RNA positivity was found to be 7,7% in placental samples (two of 26 cases), 3,6% in umbilical cord blood (one of 28 cases), and 9,7% in anal/rectal swabs (three of 31 cases), and the serological tests were positive in three of 81 cases (3.7%). In amniotic fluid samples (51 cases), no positive result was reported [16].
In the current literature, most of the data on vertical transmission are about pregnant women in third trimester, and the data regarding the transmission in early pregnancy is very limited. To the best of our knowledge, apart from case reports, the present study is the first prospective work on the vertical transmission of SARS-CoV-2 in early pregnancy. Baud et al. reported a 19-week case of second trimester fetal loss in a COVİD-19 infected woman with documented SARS-CoV-2 PCR positivity on the fetal surface of the placenta [17]. In another case report on a pregnant woman in second trimester with COVID-19 infection, Hoiser et al. demonstrated the presence of SARS-CoV-2 RNA in placental tissues [18]. In the present study, as a result of analysis on the abortion materials of twenty-one COVİD-19 infected pregnant women, we could not find any evidence to vertical transmission in early pregnancy.
It has been shown that the expression of ACE-2 and transmembrane protease serine type 2 receptors, which are used by the virus to enter the host cell, increases in placental and fetal tissues as the gestational age progresses [19]. Future studies are needed to investigate whether low receptor levels are protective for SARS-CoV-2 vertical transmission in early pregnancy.
In some studies, on pregnant women infected with SARS-CoV-2, non-pathognomonic histopathological changes such as decidual vasculopathy and intervillositis in the placenta have been reported [18,20]. These histopathological changes may be associated with fetal growth restriction, preterm delivery, miscarriage and stillbirth [20,21], and this raises concerns about COVID-19 related adverse pregnancy outcomes. Studies investigating the relationship between COVID-19 and pregnancy loss present controversial findings. While some studies suggested a higher risk of stillbirth among COVID-19 patients [3,22], others have not shown any increase in miscarriage and stillbirth during the pandemic [23,24].
In the present study, the COVİD-19 positivity rate was 11,4% among women with early pregnancy loss, and asymptomatic positive patient rate was 9,5%. In another study conducted in the same center between April and June 2020, asymptomatic positive patient rate among pregnant women was 1,4% [25]. Although it is not appropriate to make a comparison due to the fact that the two studies were not conducted in the same period, the difference in COVID 19 positivity rate between women with pregnancy loss and pregnant women is remarkable.
5. Conclusion
Previous studies reporting evidence of vertical transmission of SARS-CoV-2 mostly based on the third trimester of pregnancy. The results of this study focusing on early pregnancy do not support the possibility of intrauterine vertical transmission of SARS-CoV-2. More research is needed to reveal the risk of vertical transmission during pregnancy and whether the early pregnancy is less vulnerable.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgement
We thank to Siemens Healthineers Turkey for providing rt-PCR kits for fetal-placental samples as a gift and analyzing the samples.
References
- 1.Komine-Aizawa S., Takada K., Hayakawa S. Placental barrier against COVID-19. Placenta. 2020;99:45–49. doi: 10.1016/j.placenta.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith V., Seo D., Warty R., Payne O., Salih M., Chin K.L., Ofori-Asenso R., Krishnan S., da Silva Costa F., Vollenhoven B., Wallace E. Maternal and neonatal outcomes associated with COVID-19 infection: a systematic review. PLoS One. 2020;15(6) doi: 10.1371/journal.pone.0234187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khalil A., von Dadelszen P., Draycott T., Ugwumadu A., O'Brien P., Magee L. Change in the incidence of stillbirth and preterm delivery during the COVID-19 pandemic. J. Am. Med. Assoc. 2020;324(7):705–706. doi: 10.1001/jama.2020.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahyuddin A.P., Kanneganti A., Wong J.J.L., Dimri P.S., Su L.L., Biswas A., Illanes S.E., Mattar C.N.Z., Huang R.Y., Choolani M. Mechanisms and evidence of vertical transmission of infections in pregnancy including SARS-CoV-2s. Prenat. Diagn. 2020;40(13):1655–1670. doi: 10.1002/pd.5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juan J., Gil M.M., Rong Z., Zhang Y., Yang H., Poon L.C. Effect of coronavirus disease 2019 (COVID-19) on maternal, perinatal and neonatal outcome: systematic review. Ultrasound Obstet. Gynecol. 2020;56(1):15–27. doi: 10.1002/uog.22088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bahadur G., Homburg R., Yoong W., Singh C., Bhat M., Kotabagi P., Acharya S., Huirne J., Doreski P.A., Łukaszuk M., Muneer A. Adverse outcomes in SAR-CoV-2 (COVID-19) and SARS virus related pregnancies with probable vertical transmission. JBRA assisted reproduction. 2020;24(3):351–357. doi: 10.5935/1518-0557.20200057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Iorio R., Bianchi P., Bastianelli C., Brosens I., Benagiano G. Vertical transmission of SARS-CoV-2 infection in early pregnancy: what is the evidence? J. Matern. Fetal Neonatal Med. 2020:1–2. doi: 10.1080/14767058.2020.1825671. [DOI] [PubMed] [Google Scholar]
- 8.Sahin D., Tanacan A., Erol S.A., Anuk A.T., Yetiskin F.D.Y., Keskin H.L., Ozcan N., Ozgu-Erdinc A.S., Eyi E.G.Y., Yucel A., Tayman C., Unlu S., Dinc B., Sari E., Surel A.A., Moraloglu O.T. Updated experience of a tertiary pandemic center on 533 pregnant women with COVID-19 infection: a prospective cohort study from Turkey. Int. J. Gynaecol. Obstet. 2021;152(3):328–334. doi: 10.1002/ijgo.13460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ACOG Practice Bulletin No. 200 Early pregnancy loss. Obstet. Gynecol. 2018;132(5):e197–e207. doi: 10.1097/AOG.0000000000002899. [DOI] [PubMed] [Google Scholar]
- 10.Penfield C.A., Brubaker S.G., Limaye M.A., Lighter J., Ratner A.J., Thomas K.M., Meyer J.A., Roman A.S. Detection of severe acute respiratory syndrome coronavirus 2 in placental and fetal membrane samples. American journal of obstetrics & gynecology MFM. 2020;2(3):100133. doi: 10.1016/j.ajogmf.2020.100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patanè L., Morotti D., Giunta M.R., Sigismondi C., Piccoli M.G., Frigerio L., Mangili G., Arosio M., Cornolti G. Vertical transmission of coronavirus disease 2019: severe acute respiratory syndrome coronavirus 2 RNA on the fetal side of the placenta in pregnancies with coronavirus disease 2019-positive mothers and neonates at birth. American journal of obstetrics & gynecology MFM. 2020;2(3):100145. doi: 10.1016/j.ajogmf.2020.100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng H., Xu C., Fan J., Tang Y., Deng Q., Zhang W., Long X. Antibodies in infants born to mothers with COVID-19 pneumonia. J. Am. Med. Assoc. 2020;323(18):1848–1849. doi: 10.1001/jama.2020.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vivanti A.J., Vauloup-Fellous C., Prevot S., Zupan V., Suffee C., Do Cao J., Benachi A., De Luca D. Transplacental transmission of SARS-CoV-2 infection. Nat. Commun. 2020;11(1):3572. doi: 10.1038/s41467-020-17436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fenizia C., Biasin M., Cetin I., Vergani P., Mileto D., Spinillo A., Gismondo M.R., Perotti F., Callegari C., Mancon A., Cammarata S., Beretta I., Nebuloni M., Trabattoni D., Clerici M., Savasi V. Analysis of SARS-CoV-2 vertical transmission during pregnancy. Nat. Commun. 2020;11(1):5128. doi: 10.1038/s41467-020-18933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Facchetti F., Bugatti M., Drera E., Tripodo C., Sartori E., Cancila V., Papaccio M., Castellani R., Casola S., Boniotti M.B., Cavadini P., Lavazza A. SARS-CoV2 vertical transmission with adverse effects on the newborn revealed through integrated immunohistochemical, electron microscopy and molecular analyses of Placenta. EBioMedicine. 2020;59:102951. doi: 10.1016/j.ebiom.2020.102951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kotlyar A.M., Grechukhina O., Chen A., Popkhadze S., Grimshaw A., Tal O., Taylor H.S., Tal R. Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2021;224(1):35–53.e3. doi: 10.1016/j.ajog.2020.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baud D., Greub G., Favre G., Gengler C., Jaton K., Dubruc E., Pomar L. Second-trimester miscarriage in a pregnant woman with SARS-CoV-2 infection. J. Am. Med. Assoc. 2020;323(21):2198–2200. doi: 10.1001/jama.2020.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hosier H., Farhadian S.F., Morotti R.A., Deshmukh U., Lu-Culligan A., Campbell K.H., Yasumoto Y., Vogels C.B., Casanovas-Massana A., Vijayakumar P., Geng B., Odio C.D., Fournier J., Brito A.F., Fauver J.R., Liu F., Alpert T., Tal R., Szigeti-Buck K., Perincheri S., Larsen C., Gariepy A.M., Aguilar G., Fardelmann K.L., Harigopal M., Taylor H.S., Pettker C.M., Wyllie A.L., Cruz C.D., Ring A.M., Grubaugh N.D., Ko A.I., Horvath T.L., Iwasaki A., Reddy U.M., Lipkind H.S. SARS-CoV-2 infection of the placenta. J. Clin. Invest. 2020;130(9):4947–4953. doi: 10.1172/JCI139569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Auriti C., De Rose D.U., Tzialla C., Caforio L., Ciccia M., Manzoni P., Stronati M. Vertical transmission of SARS-CoV-2 (COVID-19): are hypotheses more than evidences? Am. J. Perinatol. 2020;37(S 02):S31–s38. doi: 10.1055/s-0040-1714346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shanes E.D., Mithal L.B., Otero S., Azad H.A., Miller E.S., Goldstein J.A. Placental pathology in COVID-19. Am. J. Clin. Pathol. 2020;154(1):23–32. doi: 10.1093/ajcp/aqaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bos M., Harris-Mostert E., van der Meeren L.E., Baelde J.J., Williams D.J., Nikkels P.G.J., Bloemenkamp K.W.M., van der Hoorn M.L.P. Clinical outcomes in chronic intervillositis of unknown etiology. Placenta. 2020;91:19–23. doi: 10.1016/j.placenta.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Kc A., Gurung R., Kinney M.V., Sunny A.K., Moinuddin M., Basnet O., Paudel P., Bhattarai P., Subedi K., Shrestha M.P., Lawn J.E., Målqvist M. Effect of the COVID-19 pandemic response on intrapartum care, stillbirth, and neonatal mortality outcomes in Nepal: a prospective observational study, the Lancet. Global health. 2020;8(10):e1273–e1281. doi: 10.1016/S2214-109X(20)30345-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cosma S., Carosso A.R., Cusato J., Borella F., Carosso M., Bovetti M., Filippini C., D’Avolio A., Ghisetti V., Di Perri G., Benedetto C. Coronavirus disease 2019 and first-trimester spontaneous abortion: a case-control study of 225 pregnant patients. Am. J. Obstet. Gynecol. 2020 doi: 10.1016/j.ajog.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stowe J., Smith H., Thurland K., Ramsay M.E., Andrews N., Ladhani S.N. Stillbirths during the COVID-19 pandemic in England, April-June 2020. J. Am. Med. Assoc. 2021;325(1):86–87. doi: 10.1001/jama.2020.21369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanacan A., Erol S.A., Turgay B., Anuk A.T., Secen E.I., Yegin G.F., Ozyer S., Kirca F., Dinc B., Unlu S., Yapar Eyi E.G., Keskin H.L., Sahin D., Surel A.A., Tekin O.M. The rate of SARS-CoV-2 positivity in asymptomatic pregnant women admitted to hospital for delivery: experience of a pandemic center in Turkey. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020;253:31–34. doi: 10.1016/j.ejogrb.2020.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]