Abstract
Data on prognostic factors associated with outcome following resection of perihilar cholangiocarcinoma vary. We sought to define and characterize current available evidence on prognostic factors associated with perihilar cholangiocarcinoma after resection. The PubMed, Embase, and Cochrane library were systematically searched for relevant studies published before December 2019. Prognostic factors were identified from multivariate regression analyses in studies. Only high-quality studies were included (Newcastle–Ottawa Scale > 6 stars). A total of 45 studies involving 7338 patients were analyzed. The meta-analysis demonstrated that serum bilirubin levels (hazard ratio: 1.76, 95% confidence interval: 1.27–2.44), serum CA19-9 levels (hazard ratio: 1.32, 95% confidence interval: 1.05–1.65), tumor size (hazard ratio: 1.27, 95% confidence interval: 1.04–1.55), major vascular involvement (hazard ratio: 1.61, 95% confidence interval: 1.09–2.38), distance metastasis (hazard ratio: 17.60, 95% confidence interval: 2.01–154.09), perioperative blood transfusion (hazard ratio: 1.36, 95% confidence interval: 1.15–1.62), T-stage (hazard ratio: 1.96, 95% confidence interval: 1.47–2.61), lymph node metastasis (hazard ratio: 2.06, 1.83–2.31), resection margin status (hazard ratio: 2.34, 95% confidence interval: 1.89–2.89), not-well histology differentiation (hazard ratio: 2.03, 95% confidence interval: 1.69–2.44), perineural invasion (hazard ratio: 2.37, 95% confidence interval: 1.59–3.55), and lymphovascular invasion (hazard ratio: 1.41, 95% confidence interval: 1.15–1.73) were prognostic factors for poorer overall survival. Adjuvant chemotherapy (hazard ratio: 0.37, 95% confidence interval: 0.25–0.55) had a positive effect on prolonged overall survival. In addition, positive resection margin status (hazard ratio: 1.96, 95% confidence interval: 1.47–2.61) and lymph node metastasis (hazard ratio: 2.06, 95% confidence interval: 1.83–2.31) were associated with poorer disease-free survival. The prognostic factors identified in the present meta-analysis can be used to characterize patients in clinical practice and enrich prognostic tools, which could be included in future trial designs and generate hypotheses to be tested in future research to promote personalized treatment.
Keywords: disease-free survival, overall survival, perihilar cholangiocarcinoma, prognostic factors, resection
Introduction
Perihilar cholangiocarcinoma (PHC), which accounts for 60–70% of all cholangiocarcinoma,1,2 is defined as adenocarcinoma of the biliary tract originating from the second-degree bile ducts to the insertion of the cystic duct into the common bile duct.2,3 PHC has an annual incidence of 1 to 2 per 100,000 individuals in the United States.4 At diagnosis, however, most patients are ineligible for resection because of locally advanced or metastatic disease.3,5 Resection is the only potentially curative option for patients with resectable PHC and most often results in a median overall survival (OS) of only about 35–40 months.6–8
Identifying which patients have a dismal prognosis and which treatments are most likely to benefit patients would enable personalized treatment strategies and improve survival. A variety of prognostic factors are associated with outcome following curative resection of PHC, including resection margin, lymph node status, tumor-node-metastasis (TNM) stage, tumor size, tumor differentiation, perineural invasion, and adjuvant chemotherapy.9,10 However, available prognostic indexes have used different sets of factors based on a limited number of patients and consistent evidence for prognostic factors is still lacking.
This study sought to review systematically the available evidence on the survival of patients with PHC following curative-intent resection as well as analyze clinically relevant prognostic factors.
Methods
A systematic review and meta-analysis on the existing published medical literature were conducted according to the Cochrane Collaboration guidelines.11
Literature search strategy
The PubMed, Embase, and Cochrane Library were searched for studies published before December 2019 using the following terms and strategy to find the relevant studies: (“cholangiocarcinoma” or “bile duct tumor” or “perihilar cholangiocarcinoma” or “hilar cholangiocarcinoma”) AND (“resection” or “surgery” or “surgical”). The references of the included studies, relevant reviews and meta-analyses were manually screened to look for other eligible studies. Only studies written in English, regardless of which patient population was included.
Eligibility criteria
The inclusion criteria for the eligible studies were (1) studies that reported resected PHC patients; (2) information about PHC populations was provided; (3) studies reported on prognostic factors in multivariate regression analyses; (4) survival data were provided; (5) only high-quality studies were included (NOS score > 6 stars). Studies that met any of the following criteria were excluded: (1) studies on patients with intrahepatic cholangiocarcinoma or distal bile duct carcinoma; (2) studies on patients with gallbladder carcinoma; (3) recurrent PHC; (4) replicated data report from the same author, department, and institution; (5) abstracts, reviews, case reports, letters to the editor, and articles available in non-English language were excluded from analysis.
Data extraction
Two reviewers (L.L. and C.L.) independently screened the titles, abstract, and full texts of the studies and performed data extraction, and a third author (T.Y.) cross-checked the data. Any disagreement was resolved through discussion. The data extracted included the surname of the first author, country, year of publication, period of patient inclusion, number of patients, characteristic of the including patients, independent risk factors of OS, independent risk factors of disease-free survival (DFS). In addition, the number of relevant studies and patients were also calculated, which stratified by sex, age, Bismuth–Corlette classification,12 major vascular involvement, portal vein involvement, hepatic artery involvement, preoperative jaundice, preoperative biliary drainage, preoperative percutaneous transhepatic biliary drainage (PTBD), preoperative endoscopic retrograde biliary drainage (ERBD), preoperative portal vein embolism, surgical procedures, perioperative blood transfusion, TNM stage (pT1-2, pT3-4, N0, N1-2, M1 and M0), surgical margin (R0 and R1), histology differentiation, lymphovascular invasion, perineural invasion, perioperative complication, perioperative mortality, adjuvant chemotherapy, and radiation. Furthermore, prognostic factors for OS and DFS were identified using multivariate Cox regression analyses from the various studies. We extracted the available multivariate hazard ratios (HRs) with 95% confidence intervals (CIs) for further meta-analysis.
Quality assessment
The modified Newcastle–Ottawa Scale (NOS) was used to assess the quality of the non-randomized studies which were included in the meta-analysis.13 The maximum possible score was 9 stars and the minimum score was 0. The sum score >6 means a high quality. The Cochrane methodology was used to assess the “risk of bias.” The Grading of Recommendations Assessment, Development and Evaluation (GRADE) System was used to assess the quality of the evidence and the strength of the recommendations.14
Data analysis
The Review Manager (RevMan, the Cochrane Collaboration, Oxford, UK) version 5.3 was used for data pooling. The primary end-points of this meta-analysis were OS and DFS. The effect measures for the OS and DFS were expressed as HR. The pooled HR and the 95% CI of the outcomes were calculated. Statistical method of Exp(O-E)/Var was adopted to calculate pooled HR. According to the updating Cochrane handbook, random-effects model was chosen as a priority for all analyses, and then the alternative test was performed as a sensitivity test. The results of the data pooling in the meta-analysis were presented as “forest plots.” Generally, heterogeneity between the studies was assessed using the I2 statistic and chi-square (χ2) based Q-test. An I2 > 50 or p < 0.1 indicated significant heterogeneity.15 A p < 0.05 in the Z-test on pooled data was considered as a statistically significant difference. The 95% CI of the pooled ratio was provided for analysis of statistically significant, as well as the effect range estimate.
Results
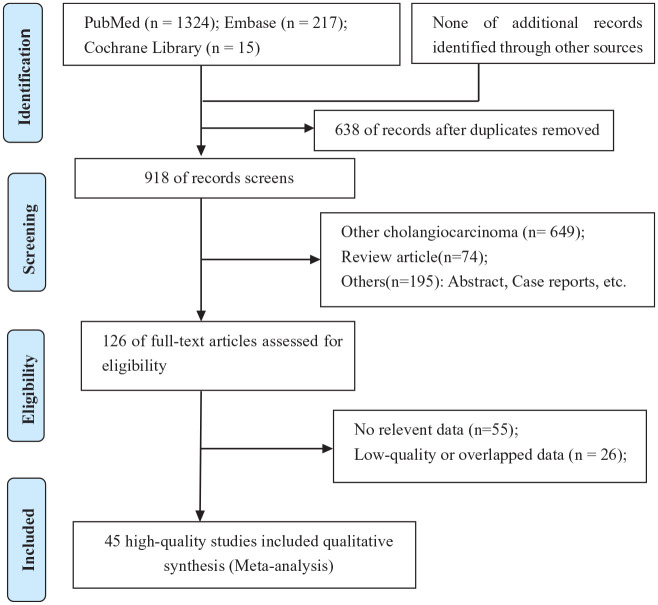
Through searches of PubMed (n = 1324), Embase (n = 217), and Cochrane library (n = 15) databases, 918 articles were identified while 638 duplicate references were excluded. After title and abstract reviewing, 792 of the 918 original articles were eliminated for failure to meet the inclusion criteria. In addition, of the remaining 126 studies, 55 were excluded after reviewing the full-text due to incomplete data; 26 studies were excluded after reviewing the full-text due to the overlapped data from a same institution or low quality (NOS score ⩽ 6 stars). Eventually, 45 retrospective studies2,7,10,12,16–56 with high quality were included in the systematic review and meta-analysis. The search and screening processes of the medical literature review are summarized in Figure 1.
Figure 1.
PRISMA flow diagram showing selection of articles for review.
Quality assessment of the included studies
Quality assessment of the included non-randomized controlled trials was evaluated based on the NOS. All of the 45 non-randomized controlled trials studies were relatively high quality with overall NOS scores ranging from 7 to 8 (Supplement Table 1).
Baseline characteristics of the included patients
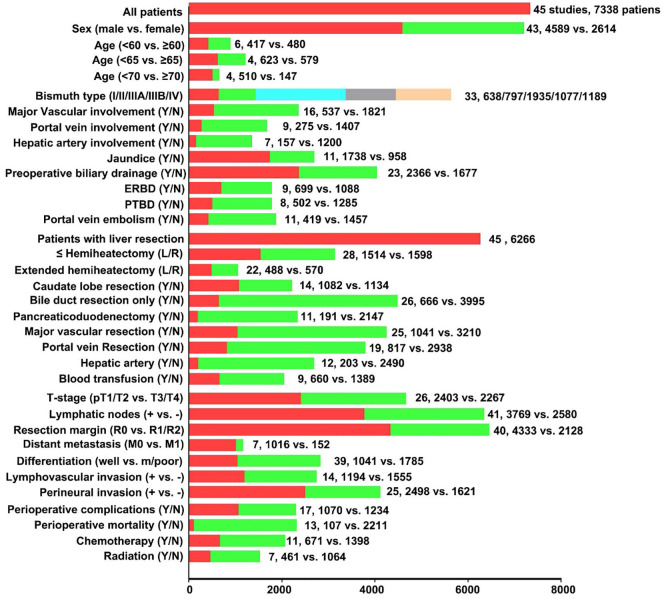
Forty-five studies2,7,10,12,16–56 that reported 7338 patients undergoing resection of PHC were published between 1996 and 2018. Fifteen studies2,12,16,18,19,21,26,31,32,39,40,43,49,50,53 were from Western countries and 28 studies7,10,17,20,22–24,27–31,33–38,41,42,44–48,51,52,54,56 were from Asia. One studies55 from Australia, and one study25 from the cooperation of Japan and United Kingdom. Four studies12,21,31,33 only included patients with Bismuth–Corlette type III or IV PHC and three studies29,38,50 only reported patients with PHC and major hepatectomy. The detailed information of the characteristics of included patients, prognosis of OS and DFS were presented in Table 1. The number of included studies and patients stratified by different characteristics were summarized in Figure 2. Furthermore, more detailed baseline characteristics of the patients in each study were shown in Table 2.
Table 1.
Characteristics of the included studies and independent risk factors.
| Study | Year | Country | Period | Patients’ character | Number | Independent risk factors of OS | Independent risk factors of DFS |
|---|---|---|---|---|---|---|---|
| Bagante and colleagues16 | 2015 | United States, Europe | 1995–2014 | PCC | 437 | N, T, CA19-9 | – |
| Morine and colleagues17 | 2011 | Japan | 1994–2008 | PCC | 22 | N, R, M | – |
| Giuliante and colleagues18 | 2016 | Italy | 1992–2007 | PCC | 175 | N, R | – |
| Hakeem and colleagues19 | 2014 | United Kingdom | 1994–2010 | PCC | 78 | N, HD, M | – |
| Hu and colleagues20 | 2016 | China | 1990–2014 | PCC | 381 | N, R, tumor size, HD, vascular invasion | – |
| Hoffmann and colleagues21 | 2015 | Germany | 2001–2012 | B-C type III/IV PCC |
60 | R, PBT | R, CLI, blood loss |
| Nakanishi and colleagues22 | 2016 | Japan | 1998–2015 | PCC | 168 | N, M | – |
| Li and colleagues23 | 2011 | China | 1990–2009 | PCC | 187 | N, R | – |
| Yan and colleagues24 | 2014 | China | 198–2007 | PCC | 131 | N, R, bilirubin | – |
| Kimura and colleagues25 | 2017 | Japan, United Kingdom | 1995–2014 | PCC | 183 | N, R, HD, PBT, MVI, PTBD | PBT, N, HD, MVI, R, biliary drainage |
| Matsuo and colleagues7 | 2012 | Japan | 1991–2008 | PCC | 157 | N, R, HD, HR | – |
| Coelen and colleagues26 | 2014 | Netherlands | 1998–2013 | PCC | 100 | HD, low skeletal and muscle mass | – |
| Sano and colleagues10 | 2007 | Japan | 1990–2004 | PCC | 99 | N, R, HD | – |
| Wang and colleagues27 | 2015 | China | 2005–2012 | PCC | 154 | N, R, tumor size | – |
| Titapun and colleagues28 | 2015 | Thailand | 2006–2011 | PCC | 153 | N, R, HD | – |
| Unno and colleagues29 | 2009 | Japan | 2001–2008 | PCC with major hepatectomy | 125 | Sex, T, R, HD | – |
| Yubin and colleagues30 | 2008 | China | 1990–2004 | PCC | 115 | N, R, M, HD | – |
| Zaydfudim and colleagues31 | 2013 | United States | 1993–2011 | B-C type III PCC | 80 | HD | – |
| Bhutiani and colleagues32 | 2018 | United States | 2000–2015 | PCC | 256 | N, LVI, chemotherapy/radiation | – |
| Cheng and colleagues33 | 2012 | China | 2001–2010 | B-C type III/IV PCC |
171 | N, R, CA19-9, HD | – |
| Chen and colleagues34 | 2016 | China | 2000–2009 | PCC | 235 | Age, N, R, CA19-9, PVI, HAI | – |
| Wang and colleagues35 | 2015 | China | 1999–2009 | PCC | 204 | N | – |
| Cai and colleagues36 | 2014 | China | 2008–2013 | PCC | 168 | N, R, CA19-9 | – |
| Kang and colleagues37 | 2016 | Korea | 1991–2010 | PCC | 403 | N, HD | – |
| Seyama and colleagues38 | 2003 | Japan | 1989–2001 | PCC with major hepatectomy | 58 | N, R, CEA | – |
| DeOliveira and colleagues2 | 2007 | United States | 1973–2004 | PCC | 281 | N, R | – |
| Silva and colleagues39 | 2005 | United Kingdom | 1992–2003 | PCC | 45 | T, R | – |
| Baton and colleagues12 | 2006 | France | 1984–2003 | B-C type III/IV PCC |
59 | Sex, N, R, M, chemotherapy | Sex, N, bilirubin, chemotherapy, R |
| Klempnauer and colleagues40 | 1996 | Germany | 1971–1995 | PCC | 137 | N | – |
| Nagino and colleagues41 | 2013 | Japan | 1977–2010 | PCC | 574 | N, R, PBT, HD, PVR/HAR | – |
| Kosuge and colleagues42 | 1999 | Japan | 1980–1997 | PCC | 65 | Sex, N, R, HD, extension to gallbladder | – |
| Neuhaus and colleagues43 | 1999 | Germany | 1988–1998 | PCC | 95 | N, R, PN, HD, PVR | – |
| Cheng and colleagues44 | 2006 | China | 1997–2002 | PCC | 75 | N, HR, radiotherapy, bilirubin | – |
| Hasegawa and colleagues45 | 2007 | Japan | 1990–2003 | PCC | 49 | N, R | – |
| Murakami and colleagues46 | 2009 | Japan | 1990–2007 | PCC | 38 | Chemotherapy | – |
| Lee and colleagues47 | 2009 | Korea | 2001–2008 | PCC | 302 | N, R, HD | – |
| Miyazaki and colleagues48 | 2006 | Japan | 1981–2004 | PCC | 161 | N, R, PVR, HAR | – |
| Buettner and colleagues49 | 2016 | United States, Europe |
1988–2014 | PCC | 407 | Age, N, PN, LVI | – |
| Chauhan and colleagues50 | 2010 | United States | 1988–2004 | PCC with major hepatectomy | 51 | N, R, complication, C-index | – |
| Chen and colleagues51 | 2009 | China | 2000–2007 | PCC | 138 | UICC stage, HD | – |
| Cho and colleagues52 | 2012 | Korea | 2000–2009 | PCC | 105 | R, bilirubin | – |
| Dumitrascu and colleagues53 | 2013 | Romania | 1996–2012 | PCC | 90 | R, CLI, chemotherapy, N-L ratio | – |
| Furusawa and colleagues54 | 2013 | Japan | 1990–2012 | PCC | 144 | N, R | – |
| Saxena and colleagues55 | 2010 | Australia | 1992–2009 | PCC | 42 | R, HD | – |
| Song and colleagues56 | 2012 | Korea | 1995–2010 | PCC | 230 | N, R, bilirubin | – |
Age (old vs young); B-C type, Bismuth–Corlette classification (type I/II/III(A/B)/IV); biliary drainage (with vs without); bilirubin (high vs low), serum bilirubin levels; blood loss (more vs less); CA19-9 (high vs low), serum CA19-9 levels; chemotherapy/radiation (with vs without); chemotherapy (without vs with), adjuvant chemotherapy; C-index (high vs low); CLI (with vs without), caudate lobe invasion; CLR (with vs without), caudate lobe resection; complication (with vs without); DFS, disease-free survival; extension to gallbladder (with vs without); HAI (with vs without), hepatic artery invasion; HAR (with vs without), hepatic artery resection; HD (moderate/ poor vs well), histological differentiation; M (+ vs −), with distance or liver metastasis; Muscle mass (low vs high); MVI (+ vs –), microvascular invasion; N (+ vs −), lymphatic nodes metastasis; N-L ratio (high vs low), neutrophil-to-lymphocyte ratio; low skeletal (low vs high); LVI (+ vs −), lymphovascular invasion; OS, overall survival; PBT (with vs without), perioperative blood transfusion; PN (+ vs −), perineural invasion; PTBD (percutaneous transhepatic biliary drainage vs endoscopic retrograde biliary drainage); PVI (with vs without), portal vein invasion; PVR (with vs without), portal vein resection; R, resection margin status (R1 or 2 vs R0); sex (male vs female); T (T3/T4 vs T1/T2), T-stage; tumor size (large vs small); UICC stage (high/low), UICC tumor stage; vascular invasion (with vs without).
Figure 2.
Number of included studies and patients stratified by different characteristics.
Table 2.
Baseline characteristics of the patients, their tumors, and long-term survival.
| Study | Number | Male | Mean CA19-9 | Size | Bismuth–Corlette Type |
T1/T2 | N0 | R0 | PN(+) | Median OS | 5-OS | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | II | IIIA | IIIB | IV | |||||||||||
| Bagante and colleagues16 | 437 | 272 | 102 | 2.5 | 67 | 70 | 216 | 84 | 49 | 208 | 296 | 356 | – | – | |
| Morine and colleagues17 | 22 | 17 | – | – | – | – | – | – | – | 15 | – | – | – | – | |
| Giuliante and colleagues18 | 175 | 100 | – | 2.7 | 2 | 30 | 133 | 10 | 43 | 105 | 143 | 126 | – | – | |
| Hakeem and colleagues19 | 78 | 45 | – | 3.0 | – | – | – | – | – | 36 | 30 | 46 | 69 | – | 26% |
| Hu and colleagues20 | 381 | 231 | 348 | – | 95 | 92 | 102 | 92 | – | – | – | – | 26 | 28% | |
| Hoffmann and colleagues21 | 60 | 37 | 251 | – | 1 | 15 | 13 | 31 | 30 | – | 23 | – | 28 | 18% | |
| Nakanishi and colleagues22 | 168 | 120 | 199 | – | – | – | – | – | 25 | – | 102 | 152 | 140 | – | – |
| Li and colleagues23 | 187 | 129 | – | – | – | – | – | – | – | – | 89 | 141 | 42 | – | 30 |
| Yan and colleagues24 | 131 | 75 | – | – | 6 | 21 | 45 | 53 | 6 | – | – | 90 | – | 35 | 22% |
| Kimura and colleagues25 | 183 | 106 | – | – | – | – | – | – | – | 107 | 104 | 112 | – | – | – |
| Matsuo and colleagues7 | 157 | – | – | 3.0 | – | – | – | – | – | – | – | 120 | – | 39 | 32% |
| Coelen and colleagues26 | 100 | 64 | 143 | – | 1 | 6 | 37 | 35 | 21 | – | 75 | 72 | 72 | 37 | – |
| Sano and colleagues10 | 99 | 69 | 101 | – | – | – | – | – | – | 40 | 52 | 58 | 32 | 34 | 38% |
| Wang and colleagues27 | 154 | 92 | – | – | – | – | – | – | – | – | 99 | 138 | – | – | – |
| Titapun and colleagues28 | 153 | 113 | – | – | 5 | 9 | 72 | 63 | 4 | – | 103 | 66 | – | 20 | 21% |
| Unno and colleagues29 | 125 | 93 | – | – | 2 | 23 | 33 | 24 | 43 | 40 | 66 | 79 | – | 27 | 35% |
| Yubin and colleagues30 | 115 | 60 | – | – | 59 | 23 | 10 | 14 | 9 | – | 83 | 92 | – | – | – |
| Zaydfudim and colleagues31 | 80 | 50 | – | – | – | – | 42 | 38 | – | 67 | 49 | 74 | – | – | – |
| Bhutiani and colleagues32 | 256 | 151 | – | 3.0 | 28 | 36 | 63 | 46 | 48 | 164 | 155 | – | 173 | – | – |
| Cheng and colleagues33 | 171 | 113 | 181 | – | – | – | – | – | – | – | 69 | 134 | 92 | – | – |
| Chen and colleagues34 | 235 | 158 | – | 2.8 | 17 | 52 | 56 | 110 | – | 159 | 172 | 208 | 119 | – | – |
| Wang and colleagues35 | 204 | 122 | – | – | 18 | 40 | 65 | 54 | 27 | – | 75 | 153 | – | – | 24% |
| Cai and colleagues36 | 168 | 96 | – | – | 18 | 18 | 12 | 30 | 90 | – | 108 | 141 | – | – | 29% |
| Kang and colleagues37 | 403 | 288 | – | – | 37 | 54 | 132 | 64 | 116 | 124 | 137 | 116 | – | 20 | 19% |
| Seyama and colleagues38 | 58 | 40 | – | – | 9 | 8 | 14 | 11 | 16 | 2 | 0 | 37 | 49 | – | – |
| DeOliveira and colleagues2 | 281 | 163 | – | – | – | – | – | – | – | – | 202 | 53 | – | 13 | 10% |
| Silva and colleagues39 | 45 | – | – | – | – | – | – | – | – | – | 20 | 23 | – | 26 | 32% |
| Baton and colleagues12 | 59 | 36 | – | – | – | – | – | – | – | – | – | 46 | 46 | 30 | 22% |
| Klempnauer and colleagues40 | 137 | 83 | – | – | 8 | 18 | 45 | 58 | 7 | 62 | 96 | 106 | 33 | 21 | 26% |
| Nagino and colleagues41 | 574 | 381 | – | – | 88 | 225 | 261 | – | 276 | 374 | 387 | – | |||
| Kosuge and colleagues42 | 65 | 50 | – | – | 4 | 14 | 8 | 31 | 8 | 27 | 58 | 34 | 50 | 28 | 26% |
| Neuhaus and colleagues43 | 95 | 50 | – | – | 6 | 8 | 27 | 29 | 25 | – | – | 58 | – | – | 22% |
| Cheng and colleagues44 | 75 | 42 | – | – | 23 | 9 | 37 | 6 | – | – | – | – | 21 | 36 | 12% |
| Hasegawa and colleagues45 | 49 | 29 | – | – | 8 | 3 | 7 | 31 | – | 32 | 36 | 28 | 30 | – | |
| Murakami and colleagues46 | 38 | 22 | – | – | 5 | 33 | – | 16 | 23 | 31 | 32 | 22 | 30% | ||
| Lee and colleagues47 | 302 | 223 | – | – | 16 | 41 | 131 | 62 | 52 | 187 | 229 | 231 | 218 | – | 33% |
| Miyazaki and colleagues48 | 161 | 102 | – | – | – | – | – | – | – | 104 | 84 | 102 | 144 | – | 30% |
| Buettner and colleagues49 | 407 | 250 | – | 2.5 | 56 | 58 | 84 | 95 | 69 | 389 | 269 | 179 | 169 | 24 | 21% |
| Chauhan and colleagues50 | 51 | 36 | – | – | – | – | – | – | – | – | 27 | 37 | 26 | – | – |
| Chen and colleagues51 | 138 | 86 | – | – | 11 | 34 | 43 | 35 | 15 | 83 | 90 | 123 | – | – | – |
| Cho and colleagues52 | 105 | 67 | 149 | – | 12 | 8 | 39 | 18 | 28 | 49 | 59 | – | 43 | 36 | 34% |
| Dumitrascu and colleagues53 | 90 | 52 | – | – | 22 | 26 | 33 | 9 | 50 | 45 | 68 | 11 | 26 | 27% | |
| Furusawa and colleagues54 | 144 | 102 | – | – | 32 | 28 | 23 | 61 | 119 | 76 | 107 | – | – | 34% | |
| Saxena and colleagues55 | 42 | 23 | – | – | 3 | 36 | 32 | 1 | 22 | 30 | 27 | 20 | – | 24% | |
| Song and colleagues56 | 230 | 151 | – | – | 68 | 127 | 125 | – | – | – | 176 | – | 39 | 33% | |
OS, overall survival; PN, perineural invasion.
Prognostic factors for OS
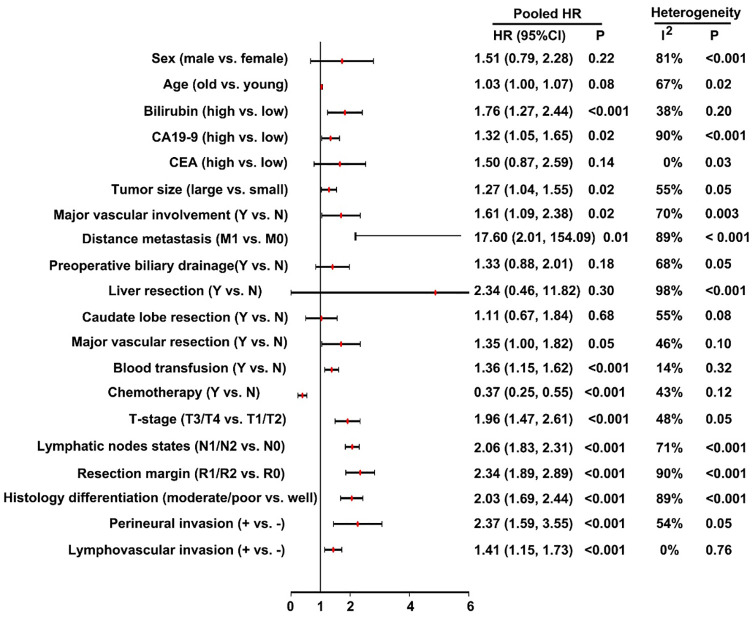
According to the systematic review, a total of 33 risk factors were investigated in multivariate regression analyses (Table 1). From these risk factors, 20 risk factors of OS were available for meta-analysis (Figure 3). Factors with clinically relevant prognostic value of OS included: preoperative serum bilirubin levels, preoperative serum CA19-9 levels, tumor size, major vascular involvement, distance metastasis, perioperative blood transfusion, T-stage, lymph node metastasis, resection margin status, not-well histology differentiation, perineural invasion and lymphovascular invasion. Adjuvant chemotherapy was a protective factor for OS. Of note, factors of sex, age, carcinoembryonic antigen (CEA), preoperative biliary drainage, with liver resection, with caudate lobe resection and with major vascular resection were not statistically associated with postoperative prognosis. Meanwhile, the heterogeneity test demonstrated some factors with high heterogeneity (I2 > 50% or p < 0.05). No significant publication bias was found in the funnel plot.
Figure 3.
Forest plots pooled the overall survival stratified by different risk factors.
Prognostic factors for DFS
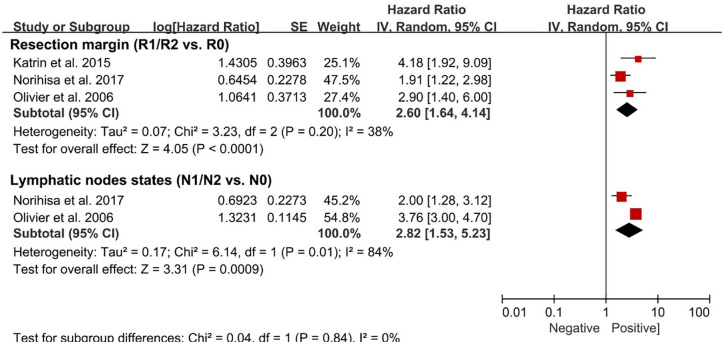
According to the systematic review, a total of 12 risk factors of DFS were investigated in multivariate regression analyses (Table 1). Among these risk factors, only two risk factors were available for meta-analysis. The clinically relevant prognostic factors associated with DFS included: positive resection margin status (HR: 1.96, 1.47–2.61) and lymph node metastasis (HR: 2.06, 1.83–2.31; Figure 4). Meanwhile, the heterogeneity test demonstrated lymph node metastasis with high heterogeneity (I2 = 84%, p = 0.01). No significant publication bias was found in the funnel plot.
Figure 4.
Forest plots pooled the disease-free survival stratified by different risk factors.
Sensitivity analysis
A sensitivity analysis was performed, in which one study at a time was removed, and the other reports analyzed to estimate whether the results changed significantly by the removal of a single study. The sensitivity analysis demonstrated that the present meta-sensitivity analysis did not suggest an undue influence of any single study.
Discussion
This meta-analysis aimed to assess the available evidence on the prognostic factors for patients with PHC following resection. To this end, 45 high-quality retrospective studies comprising 7338 patients were included in the meta-analysis. Of note, the prognostic factors with a significant effect on OS included serum bilirubin levels, serum CA19-9 levels, tumor size, major vascular involvement, distance metastasis, perioperative blood transfusion, T-stage, lymph node metastasis, resection margin status, not-well histology differentiation, perineural invasion, and lymphovascular invasion. In addition, positive resection margin status and lymph node metastasis had a negative effect on DFS.
PHC is a relatively uncommon malignancy with high mortality which is reported to occur more frequently in recent years. As the progress of preoperative management and surgical resection techniques, an enhancement of resectability rate of PHC ranging from 80% to 87% has been achieved. R0 resection has becoming a gold standard of surgical treatment of PHC. Nevertheless, the prognosis is still very poor. As described previously, the prognosis of PHC is associated with multifactors.23,47,52 To improve the survival rate of PHC postoperatively, each clinicopathological factors that can be controlled, associated with prognosis, should be miniaturized.
To our knowledge, there are only two meta-analyses that have reported the prognosis of patients with resectable PHC. In 2018, Bird and colleagues57 (included 24 studies) and Tang and colleagues58 (included 38 studies) performed a meta-analysis to only assess the clinicopathological factors associated with prognosis of patients with resectable PHC, respectively. In addition, both of these studies pooled univariable HRs and included some studies with overlapped data. Compared with the two previous meta-analyses, the current review was much more extensive as it included 45 studies comprising 7338 patients. Of note, the method of data extraction and calculation was more robust as it was an adopted HR from multivariable Cox regression analysis. In addition, in this meta-analysis, demographic characteristics, clinicopathological characteristics, surgical procedures, and perioperative treatments were systematic analyzed. Another strength of this study only included high-quality studies (NOS scores ⩾ 6 stars), and some studies with overlapped data were also excluded.
In this meta-analysis, the results demonstrated that serum bilirubin levels, perioperative blood transfusion, T-stage (T3/T4), lymphovascular invasion were independent risk factors for OS and without heterogeneity. Serum CA19-9 levels, tumor size, major vascular involvement, distance metastasis, lymph node metastasis, resection margin status, not-well histology differentiation and perineural invasion were also independent risk factors but with high heterogeneity. Meanwhile, adjuvant chemotherapy had a positive effect on OS without heterogeneity. In addition, serum CEA levels and with major vascular resection were not independent risk factors for OS and without heterogeneity. Sex, age, preoperative biliary drainage, with caudate lobe resection and with liver resection were also not independent risk factors for OS but with high heterogeneity. Furthermore, lymph node metastasis and resection margin status had a negative effect on DFS, but the former had with a significant heterogeneity. Factors with significant heterogeneity indicated that the prognostic value of this variable is yet to be defined.
Lymph node metastasis and margin status were significant prognostic factor in our meta-analysis. Previous studies have similarly reported lymph node metastasis and margin status to be significant prognostic factors for survival, along with perineural invasion and not-well tumor differentiation. PHC recurrence after surgical resection results in poor prognosis and short OS times. Positive margin status and lymph node metastasis were also found to be independent prognostic factors for the DFS. Adjuvant chemo- and/or radiation therapy has not yet been standardized. Surgical resection associated with adjuvant therapy may provide the most favorable outcome. The present meta-analysis also showed that postoperative adjuvant chemotherapy was a positive prognostic factor for PHC after curative resection. However, the difference of chemotherapy protocols and/or radiotherapy were not analyzed in-depth, because the available data were limited.
Several limitations should be considered when interpreting data from this study. Although we only selected high-quality studies, all of the included studies were predominantly retrospective in nature. As such, there may be inherent selection bias from some of the studies. The consistency and representativeness of patients included was suboptimal. This heterogeneity in the selection of patients may have led to selection bias. In addition, not all relevant factors were reported in each study and analyzed in multivariable Cox regression analysis. Finally, some prognostic factors were with significant heterogeneity. Subsequently, the results of these factors should be interpreted with caution.
In conclusion, this systematic and meta-analysis provides updated and more robust evidence on prognostic factors in resection of PHC. Prognostic factors identified in this review can be used to better characterize patients in clinical practice, guide the development of better prognostic models, and be used in future trial design as stratification factors or to be included in regression review analyses. Due to some factors with high heterogeneity, future randomized controlled trials are needed to better define the role of these factors.
Supplemental Material
Supplemental material, sj-pdf-1-cmg-10.1177_2631774521993065 for Prognostic factors of resectable perihilar cholangiocarcinoma: a systematic review and meta-analysis of high-quality studies by Lei Liang, Chao Li, Hang-Dong Jia, Yong-Kang Diao, Hao Xing, Timothy M. Pawlik, Wan Yee Lau, Feng Shen, Dong-Sheng Huang, Cheng-Wu Zhang and Tian Yang in Therapeutic Advances in Gastrointestinal Endoscopy
Footnotes
Author contributions: L.L., H.X., F.S., D.-S.H, C.-W.Z., and T.Y. conceived and designed the study. L.L. and C.L. searched the literature and extracted the data. L.L., H.-D.J., and Y.-K.D. wrote the manuscript. T.M.P. and W.Y.L. proofread the manuscript. T.Y. obtained funding. All authors approved the final version of the manuscript. The authors declare no competing financial interests.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (grant nos. 81672699 and 81972726). Approval for this study was obtained from the Ethics Committee of our hospital.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Congress publication: The abstract of this study has been presented as a poster presentation in the congress: “3rd International Advanced Liver & Pancreas Surgery Symposium—ISLS 2019” (10–12 October 2019, Istanbul, Turkey).
ORCID iDs: Lei Liang  https://orcid.org/0000-0003-3294-2978
https://orcid.org/0000-0003-3294-2978
Hang-Dong Jia  https://orcid.org/0000-0001-7638-2605
https://orcid.org/0000-0001-7638-2605
Tian Yang  https://orcid.org/0000-0002-8333-3673
https://orcid.org/0000-0002-8333-3673
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Lei Liang, Department of Hepatobiliary, Pancreatic and Minimal Invasive Surgery, Zhejiang Provincial People’s Hospital, People’s Hospital of Hangzhou Medical College, Hangzhou, China; Key Laboratory of Tumor Molecular Diagnosis and Individualized Medicine of Zhejiang Province, Hangzhou, China.
Chao Li, Department of Hepatobiliary Surgery, Eastern Hepatobiliary Surgery Hospital, The Second Military Medical University, Shanghai, China.
Hang-Dong Jia, Department of Hepatobiliary, Pancreatic and Minimal Invasive Surgery, Zhejiang Provincial People’s Hospital, People’s Hospital of Hangzhou Medical College, Hangzhou, China; Key Laboratory of Tumor Molecular Diagnosis and Individualized Medicine of Zhejiang Province, Hangzhou, China.
Yong-Kang Diao, Department of Hepatobiliary, Pancreatic and Minimal Invasive Surgery, Zhejiang Provincial People’s Hospital, People’s Hospital of Hangzhou Medical College, Hangzhou, China; Key Laboratory of Tumor Molecular Diagnosis and Individualized Medicine of Zhejiang Province, Hangzhou, China.
Hao Xing, Department of Hepatobiliary Surgery, Eastern Hepatobiliary Surgery Hospital, The Second Military Medical University, Shanghai, China.
Timothy M. Pawlik, Department of Surgery, The Ohio State University Wexner Medical Center, Columbus, OH, USA
Wan Yee Lau, Department of Hepatobiliary Surgery, Eastern Hepatobiliary Surgery Hospital, The Second Military Medical University, Shanghai, China; Prince of Wales Hospital, Faculty of Medicine, The Chinese University of Hong Kong, Shatin, Hong Kong SAR, China.
Feng Shen, Department of Hepatobiliary Surgery, Eastern Hepatobiliary Surgery Hospital, The Second Military Medical University, Shanghai, China.
Dong-Sheng Huang, Key Laboratory of Tumor Molecular Diagnosis and Individualized Medicine of Zhejiang Province, Hangzhou, China.
Cheng-Wu Zhang, Department of Hepatobiliary, Pancreatic and Minimal Invasive Surgery, Zhejiang Provincial People’s Hospital, People’s Hospital of Hangzhou Medical College, Hangzhou, China.
Tian Yang, Department of Hepatobiliary Surgery, Eastern Hepatobiliary Surgery Hospital, The Second Military Medical University, Shanghai 200438, China.
References
- 1. Nakeeb A, Pitt HA, Sohn TA, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg 1996; 224: 463–473; discussion 473–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. DeOliveira ML, Cunningham SC, Cameron JL, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg 2007; 245: 755–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet 2014; 383: 2168–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65: 5–29. [DOI] [PubMed] [Google Scholar]
- 5. Brito AF, Abrantes AM, Encarnacao JC, et al. Cholangiocarcinoma: from molecular biology to treatment. Med Oncol 2015; 32: 245. [DOI] [PubMed] [Google Scholar]
- 6. Pichlmayr R, Lamesch P, Weimann A, et al. Surgical treatment of cholangiocellular carcinoma. World J Surg 1995; 19: 83–88. [DOI] [PubMed] [Google Scholar]
- 7. Matsuo K, Rocha FG, Ito K, et al. The Blumgart preoperative staging system for hilar cholangiocarcinoma: analysis of resectability and outcomes in 380 patients. J Am Coll Surg 2012; 215: 343–355. [DOI] [PubMed] [Google Scholar]
- 8. Groot Koerkamp B, Wiggers JK, Allen PJ, et al. American Joint Committee on Cancer staging for resected perihilar cholangiocarcinoma: a comparison of the 6th and 7th editions. HPB 2014; 16: 1074–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Murakami Y, Uemura K, Sudo T, et al. Prognostic factors after surgical resection for intrahepatic, hilar, and distal cholangiocarcinoma. Ann Surg Oncol 2011; 18: 651–658. [DOI] [PubMed] [Google Scholar]
- 10. Sano T, Shimada K, Sakamoto Y, et al. Prognosis of perihilar cholangiocarcinoma: hilar bile duct cancer versus intrahepatic cholangiocarcinoma involving the hepatic hilus. Ann Surg Oncol 2008; 15: 590–599. [DOI] [PubMed] [Google Scholar]
- 11. Panic N, Leoncini E, de Belvis G, et al. Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLoS ONE 2013; 8: e83138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baton O, Azoulay D, Adam DV, et al. Major hepatectomy for hilar cholangiocarcinoma type 3 and 4: prognostic factors and longterm outcomes. J Am Coll Surg 2007; 204: 250–260. [DOI] [PubMed] [Google Scholar]
- 13. Ofek Shlomai N, Rao S, Patole S. Efficacy of interventions to improve hand hygiene compliance in neonatal units: a systematic review and meta-analysis. Eur J Clin Microbiol Infect Dis 2015; 34: 887–897. [DOI] [PubMed] [Google Scholar]
- 14. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336: 924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bagante F, Tran T, Spolverato G, et al. Perihilar cholangiocarcinoma: number of nodes examined and optimal lymph node prognostic scheme. J Am Coll Surg 2016; 222: 750–759.e752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morine Y, Shimada M, Utsunomiya T, et al. Clinical impact of lymph node dissection in surgery for peripheral-type intrahepatic cholangiocarcinoma. Surg Today 2012; 42: 147–151. [DOI] [PubMed] [Google Scholar]
- 18. Giuliante F, Ardito F, Guglielmi A, et al. Association of lymph node status with survival in patients after liver resection for hilar cholangiocarcinoma in an Italian Multicenter Analysis. JAMA Surg 2016; 151: 916–922. [DOI] [PubMed] [Google Scholar]
- 19. Hakeem AR, Marangoni G, Chapman SJ, et al. Does the extent of lymphadenectomy, number of lymph nodes, positive lymph node ratio and neutrophil-lymphocyte ratio impact surgical outcome of perihilar cholangiocarcinoma. Eur J Gastroenterol Hepatol 2014; 26: 1047–1054. [DOI] [PubMed] [Google Scholar]
- 20. Hu HJ, Mao H, Shrestha A, et al. Prognostic factors and long-term outcomes of hilar cholangiocarcinoma: a single-institution experience in China. World J Gastroenterol 2016; 22: 2601–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hoffmann K, Luible S, Goeppert B, et al. Impact of portal vein resection on oncologic long-term outcome in patients with hilar cholangiocarcinoma. Surgery 2015; 158: 1252–1260. [DOI] [PubMed] [Google Scholar]
- 22. Nakanishi Y, Tsuchikawa T, Okamura K, et al. Prognostic impact of the site of portal vein invasion in patients with surgically resected perihilar cholangiocarcinoma. Surgery 2016; 159: 1511–1519. [DOI] [PubMed] [Google Scholar]
- 23. Li H, Qin Y, Cui Y, et al. Analysis of the surgical outcome and prognostic factors for hilar cholangiocarcinoma: a Chinese experience. Dig Surg 2011; 28: 226–231. [DOI] [PubMed] [Google Scholar]
- 24. Yan Y, Lu N, Tian W, et al. Evolution of surgery for Klatskin tumor demonstrates improved outcome: a single center analysis. Tumori 2014; 100: e250–e256. [DOI] [PubMed] [Google Scholar]
- 25. Kimura N, Young AL, Toyoki Y, et al. Radical operation for hilar cholangiocarcinoma in comparable Eastern and Western centers: outcome analysis and prognostic factors. Surgery 2017; 162: 500–514. [DOI] [PubMed] [Google Scholar]
- 26. Coelen RJ, Wiggers JK, Nio CY, et al. Preoperative computed tomography assessment of skeletal muscle mass is valuable in predicting outcomes following hepatectomy for perihilar cholangiocarcinoma. HPB 2015; 17: 520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang ST, Shen SL, Peng BG, et al. Combined vascular resection and analysis of prognostic factors for hilar cholangiocarcinoma. Hepatobiliary Pancreat Dis Int 2015; 14: 626–632. [DOI] [PubMed] [Google Scholar]
- 28. Titapun A, Pugkhem A, Luvira V, et al. Outcome of curative resection for perihilar cholangiocarcinoma in Northeast Thailand. World J Gastrointest Oncol 2015; 7: 503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Unno M, Katayose Y, Rikiyama T, et al. Major hepatectomy for perihilar cholangiocarcinoma. J Hepatobiliary Pancreat Sci 2010; 17: 463–469. [DOI] [PubMed] [Google Scholar]
- 30. Yubin L, Chihua F, Zhixiang J, et al. Surgical management and prognostic factors of hilar cholangiocarcinoma: experience with 115 cases in China. Ann Surg Oncol 2008; 15: 2113–2119. [DOI] [PubMed] [Google Scholar]
- 31. Zaydfudim VM, Clark CJ, Kendrick ML, et al. Correlation of staging systems to survival in patients with resected hilar cholangiocarcinoma. Am J Surg 2013; 206: 159–165. [DOI] [PubMed] [Google Scholar]
- 32. Bhutiani N, Scoggins CR, McMasters KM, et al. The impact of caudate lobe resection on margin status and outcomes in patients with hilar cholangiocarcinoma: a multi-institutional analysis from the US Extrahepatic Biliary Malignancy Consortium. Surgery 2018; 163: 726–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cheng QB, Yi B, Wang JH, et al. Resection with total caudate lobectomy confers survival benefit in hilar cholangiocarcinoma of Bismuth type III and IV. Eur J Surg Oncol 2012; 38: 1197–1203. [DOI] [PubMed] [Google Scholar]
- 34. Chen P, Li B, Zhu Y, et al. Establishment and validation of a prognostic nomogram for patients with resectable perihilar cholangiocarcinoma. Oncotarget 2016; 7: 37319–37330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang Y, Yang H, Shen C, et al. Surgical procedure and long-term survival of hilar cholangiocarcinoma. Int J Clin Exp Med 2015; 8: 1122–1128. [PMC free article] [PubMed] [Google Scholar]
- 36. Cai WK, Lin JJ, He GH, et al. Preoperative serum CA19-9 levels is an independent prognostic factor in patients with resected hilar cholangiocarcinoma. Int J Clin Exp Pathol 2014; 7: 7890–7898. [PMC free article] [PubMed] [Google Scholar]
- 37. Kang MJ, Jang JY, Chang J, et al. Actual long-term survival outcome of 403 consecutive patients with hilar cholangiocarcinoma. World J Surg 2016; 40: 2451–2459. [DOI] [PubMed] [Google Scholar]
- 38. Seyama Y, Kubota K, Sano K, et al. Long-term outcome of extended hemihepatectomy for hilar bile duct cancer with no mortality and high survival rate. Ann Surg 2003; 238: 73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Silva MA, Tekin K, Aytekin F, et al. Surgery for hilar cholangiocarcinoma: a 10 year experience of a tertiary referral centre in the UK. Eur J Surg Oncol 2005; 31: 533–539. [DOI] [PubMed] [Google Scholar]
- 40. Klempnauer J, Ridder GJ, von Wasielewski R, et al. Resectional surgery of hilar cholangiocarcinoma: a multivariate analysis of prognostic factors. J Clin Oncol 1997; 15: 947–954. [DOI] [PubMed] [Google Scholar]
- 41. Nagino M, Ebata T, Yokoyama Y, et al. Evolution of surgical treatment for perihilar cholangiocarcinoma: a single-center 34-year review of 574 consecutive resections. Ann Surg 2013; 258: 129–140. [DOI] [PubMed] [Google Scholar]
- 42. Kosuge T, Yamamoto J, Shimada K, et al. Improved surgical results for hilar cholangiocarcinoma with procedures including major hepatic resection. Ann Surg 1999; 230: 663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Neuhaus P, Jonas S, Bechstein WO, et al. Extended resections for hilar cholangiocarcinoma. Ann Surg 1999; 230: 808–818; discussion 819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cheng Q, Luo X, Zhang B, et al. Predictive factors for prognosis of hilar cholangiocarcinoma: postresection radiotherapy improves survival. Eur J Surg Oncol 2007; 33: 202–207. [DOI] [PubMed] [Google Scholar]
- 45. Hasegawa S, Ikai I, Fujii H, et al. Surgical resection of hilar cholangiocarcinoma: analysis of survival and postoperative complications. World J Surg 2007; 31: 1256–1263. [DOI] [PubMed] [Google Scholar]
- 46. Murakami Y, Uemura K, Sudo T, et al. Gemcitabine-based adjuvant chemotherapy improves survival after aggressive surgery for hilar cholangiocarcinoma. J Gastrointest Surg 2009; 13: 1470–1479. [DOI] [PubMed] [Google Scholar]
- 47. Lee SG, Song GW, Hwang S, et al. Surgical treatment of hilar cholangiocarcinoma in the new era: the Asan experience. J Hepatobiliary Pancreat Sci 2010; 17: 476–489. [DOI] [PubMed] [Google Scholar]
- 48. Miyazaki M, Kato A, Ito H, et al. Combined vascular resection in operative resection for hilar cholangiocarcinoma: does it work or not? Surgery 2007; 141: 581–588. [DOI] [PubMed] [Google Scholar]
- 49. Buettner S, van Vugt JL, Gani F, et al. A comparison of prognostic schemes for perihilar cholangiocarcinoma. J Gastrointest Surg 2016; 20: 1716–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chauhan A, House MG, Pitt HA, et al. Post-operative morbidity results in decreased long-term survival after resection for hilar cholangiocarcinoma. HPB 2011; 13: 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chen XP, Lau WY, Huang ZY, et al. Extent of liver resection for hilar cholangiocarcinoma. Br J Surg 2009; 96: 1167–1175. [DOI] [PubMed] [Google Scholar]
- 52. Cho MS, Kim SH, Park SW, et al. Surgical outcomes and predicting factors of curative resection in patients with hilar cholangiocarcinoma: 10-year single-institution experience. J Gastrointest Surg 2012; 16: 1672–1679. [DOI] [PubMed] [Google Scholar]
- 53. Dumitrascu T, Chirita D, Ionescu M, et al. Resection for hilar cholangiocarcinoma: analysis of prognostic factors and the impact of systemic inflammation on long-term outcome. J Gastrointest Surg 2013; 17: 913–924. [DOI] [PubMed] [Google Scholar]
- 54. Furusawa N, Kobayashi A, Yokoyama T, et al. Surgical treatment of 144 cases of hilar cholangiocarcinoma without liver-related mortality. World J Surg 2014; 38: 1164–1176. [DOI] [PubMed] [Google Scholar]
- 55. Saxena A, Chua TC, Chu FC, et al. Improved outcomes after aggressive surgical resection of hilar cholangiocarcinoma: a critical analysis of recurrence and survival. Am J Surg 2011; 202: 310–320. [DOI] [PubMed] [Google Scholar]
- 56. Song SC, Choi DW, Kow AW, et al. Surgical outcomes of 230 resected hilar cholangiocarcinoma in a single centre. ANZ J Surg 2013; 83: 268–274. [DOI] [PubMed] [Google Scholar]
- 57. Bird NTE, McKenna A, Dodd J, et al. Meta-analysis of prognostic factors for overall survival in patients with resected hilar cholangiocarcinoma. Br J Surg 2018; 105: 1408–1416. [DOI] [PubMed] [Google Scholar]
- 58. Tang Z, Yang Y, Zhao Z, et al. The clinicopathological factors associated with prognosis of patients with resectable perihilar cholangiocarcinoma: a systematic review and meta-analysis. Medicine 2018; 97: e11999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-cmg-10.1177_2631774521993065 for Prognostic factors of resectable perihilar cholangiocarcinoma: a systematic review and meta-analysis of high-quality studies by Lei Liang, Chao Li, Hang-Dong Jia, Yong-Kang Diao, Hao Xing, Timothy M. Pawlik, Wan Yee Lau, Feng Shen, Dong-Sheng Huang, Cheng-Wu Zhang and Tian Yang in Therapeutic Advances in Gastrointestinal Endoscopy