Abstract
Oncolytic viruses (OVs) specifically infect, replicate and eventually destroy tumor cells, with no concomitant toxicity to adjacent normal cells. Furthermore, OVs can regulate tumor microenvironments and stimulate anti-tumor immune responses. Mesenchymal stem cells (MSCs) have inherent tumor tropisms and immunosuppressive functions. MSCs carrying OVs not only protect viruses from clearing by the immune system, but they also deliver the virus to tumor lesions. Equally, cytokines released by MSCs enhance anti-tumor immune responses, suggesting that MSCs carrying OVs may be considered as a promising strategy in enhancing the anti-tumor efficacies of virotherapy. In the present review, preclinical and clinical studies were evaluated and discussed, as well as the effectiveness of MSCs carrying OVs for tumor treatment.
Keywords: oncolytic virus, mesenchymal stem cells, cellular carriers, tumor tropism, immunosuppressive function, oncolytic virotherapy
1. Introduction
With the development of targeted therapies and cellular immunotherapies, such as T cell-based, natural killer (NK) cell-based and dendritic cell (DC)-based immunotherapies, the therapeutic efficacy of cancer treatment has been greatly improved (1). However, the overall remission and survival rate of patients with certain tumors has not been fundamentally addressed. In recent decades, oncolytic viruses (OVs) have generated widespread interest, and have become a major focus of interest for clinicians and scientists (2,3). These viruses include adenovirus, measles virus, reovirus, herpes simplex virus, Newcastle disease virus, vesicular stomatitis, vaccinia virus and poliovirus (4,5).
Previous preclinical and clinical studies have demonstrated that the intratumoral injection of OVs is effective, although the efficacy toward disseminated and metastatic tumors remains modest (6,7). Numerous factors can affect viral efficiency in reaching tumor tissue, including viral destruction by the immune system and viral absorption by tissues and organs (8,9). Therefore, appropriate carrier vehicles are required to deliver OVs to tumor sites in order to improve therapeutic efficacy.
In recent years, mesenchymal stem cells (MSCs) have become a promising cellular vehicle for anti-tumor drug delivery, thanks to their inherent tumor tropism (10–13). MSCs can specifically migrate to the tumor or inflammatory site. A recent review has reported that MSCs can be modified by advanced approaches to suppress tumor growth (14). Furthermore, MSCs exert immunosuppressive functions, by inhibiting NK proliferation, cytotoxicity and cytokine production (15), suppressing differentiation and function of DC (16) and inducing therefore the emergence of regulatory T cells. These features make MSCs ideal candidates for OVs delivery. In the present review, an overview of MSC loading of OVs for oncolytic virotherapy was provided. We briefly introduced MSC characteristics for OV delivery and summarized developments in the MSC oncolytic virotherapy arena.
2. Mechanisms of oncolytic virotherapy
In the last decades, great progress has been made in elucidating the molecular mechanisms of OV infection. OVs can infect target cells using low-affinity binding to sialic acid residues, from where they internalize via specific high-affinity receptors (17,18). The expression of OV strain receptors on the cell surface is a crucial factor in determining viral infection (19). However, accumulating evidence from preclinical and clinical studies has indicated that growth conditions and genetic background of tumor cells can affect cell sensitivity to OVs (19). For example, cathepsin B and cathepsin L are critical for viral shelling, which is associated with the sensitivity of tumor cells to oncolytic reoviruses; however, virus shelling is also limited by low levels of cathepsin B and cathepsin L in normal cells (20). In addition, Ras mutations can increase cell sensitization to reoviruses (21,22). Following OV infection, virus progeny replicates highly in tumor cells, eventually lysing and killing infected cells. Subsequently, tumor cell lysis releases infectious viral progeny that spreads to surrounding tumor cells, causing more tumor cells to undergo oncolysis. However, OV replication is often limited in healthy cells, thus viral clearance is rapid with minimal oncolysis (23).
With expanding OV research, virotherapy has gradually changed from direct oncolysis to virus mediated anti-tumor immunity (24,25). It has been demonstrated that the immune system serves a crucial role in oncolytic virotherapy. On the one hand, inherent and adaptive immunities control viral infections, reducing or eliminating their oncolytic potential. On the other hand, viruses can trigger anti-tumor immune responses through a variety of mechanisms. Firstly, tumor-associated antigens (TAAs) and neoantigens (TANs), which are released by tumor cells, are captured by antigen-presenting cells and are ultimately activated by tumor specific T cells in order to respond to tumor antigens (26,27). Secondly, OVs can promote immunogenic cell death by cell necrosis, immunogenic apoptosis and autophagic cell death (27–30), subsequently releasing danger-associated molecular patterns (DAMPs), including ATP and high-mobility group box 1 protein (28,31,32). In addition, virus-induced tumor cell death also leads to the release of pathogen-associated molecular patterns (PAMPs), such as nucleic acids, proteins and viral capsid components (33,34). DAMPs and PAMPs are recognized by pattern recognition receptors (PRRs) on innate immune cells, such as DC and NK cells, in turn activating NF-κB signaling and releasing type I interferon (IFN), proinflammatory cytokines and chemokines (35,36). However, these molecules promote the recruitment and activation of macrophages, NK, DC and tumor specific cytotoxic T lymphocytes to the tumor microenvironment (TME), and help reverse the immunosuppressive state of TME (32,35–38). In addition, tumor cells infected with OVs express virus-specific antigens on their surface, which facilitate their destruction by anti-viral T cells (39). Therefore, OVs can induce anti-tumor immune response, even if the virus does not effectively replicate (40).
3. The main hurdles limiting OV efficacy for virotherapy
In 2015, the US Food and Drug Administration approved Amgen's talimogene laherparepvec (T-VEC or Imlygic®) for the treatment of melanoma (41), and in December of the same year, T-VEC was approved by the European Medicines Agency for the treatment of unresectable stage IIIB/C and stage IVM1a melanoma (42). The T-VEC success has significantly promoted OV research and clinical applications, and aroused great interest in the academic and industry communities (43,44). However, in most cases, the elicited immune response limits the killing effects of OVs, the efficacy remains modest, and the ultimate therapeutic efficacy of OVs as a systemic administration reagent is limited (45–47). There are four reasons that may explain this phenomenon: i) Individuals carry anti-viral antibodies, such as anti-reovirus and anti-measles virus antibodies. After systemic administration, OVs are quickly cleared by pre-existing antibodies, which hinders OV efficacy (48,49); ii) OVs are cleared by macrophages located in the liver and spleen; iii) for solid tumors, OVs must pass through the endothelial layer to reach target cells, therefore physical barriers pose a significant challenges to viral transmission; and iv) due to interactions between OVs and antigen presenting cells, extensive anti-viral immunity, pre-existing circulating antibodies and blood factors, such as coagulation factors and complement proteins, OVs are easily cleared by the host's immune system (50). Taken together, these factors suggest that it may be difficult to determine whether enough OV particles could reach the tumor site. In the following sections of this review, current strategies for OVs loading by MSCs for anti-tumor therapy will be discussed.
4. MSC biology
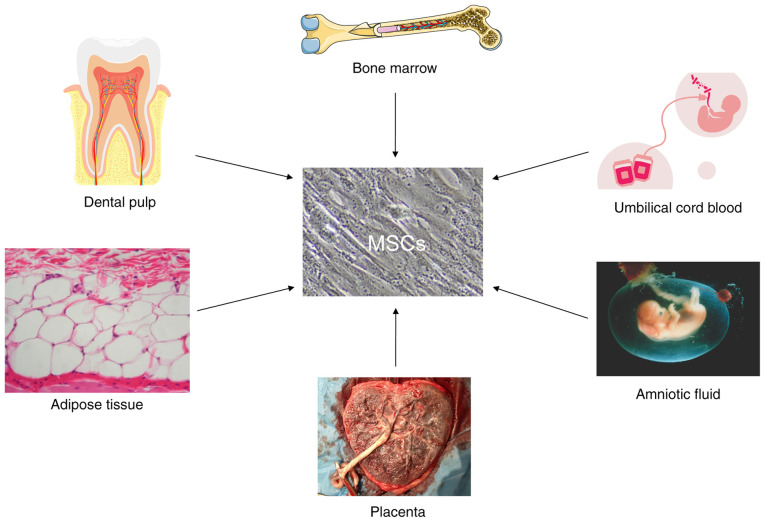
MSCs are adult stem cells derived from the mesoderm that can be isolated from various tissues, including bone marrow, adipose tissue, dental pulp, placenta, amniotic fluid, umbilical cord, Wharton's jelly and umbilical cord blood (51,52) (Fig. 1). Although MSCs derived from these tissues contain diverse background genetic lineages, they can exert intrinsic and extrinsic effects, and MSCs cultured in vitro may share common features in agreement with the International Society of Cell Therapy (ISCT) criteria established in 2006 (53). Firstly, under in vitro culture conditions, MSCs exhibit spindle-shaped or fusiform morphology. Secondly, in vitro cultured MSCs express CD73, CD90 and CD105 markers on their surface; however, they express no monocyte markers, such as HLA-DR, CD14 or CD11b, CD79α or CD19, and no hematopoietic markers, such as CD34 and CD45 (53). In addition, MSCs can differentiate into osteoblasts, adipocytes and chondroblasts following specific in vitro differentiation conditions (53). Although MSCs have the potential to express surface antigens and differentiate, other characteristics of MSCs that would support anti-tumor therapeutic interests are vital. In the following section, MSC functions, including inherent tumor tropisms, as well as the immunosuppression and paracrine characteristics of anti-tumor MSC carrying OVs will therefore be discussed.
Figure 1.
Different sources of MSCs in humans. MSCs, mesenchymal stem cells.
5. MSCs loaded with OVs-the anti-tumor story
MSC tumor tropisms facilitate OV delivery to tumor sites
MSCs undergo chemotaxis and migration to tumor lesions (54). A recent study has reported that MSCs migrate and bind to the tumor matrix and target the TME (14). At these sites, the tumor oxidation state, vascularization and tumor inflammatory status can affect MSC migration efficiency (55). Furthermore, MSCs have been demonstrated to exert positive chemotactic effects on solid tumors, such as hepatocellular carcinoma (55), breast cancer (56) and glioma (57).
MSCs migrate to damaged tissue or inflammatory sites and release simultaneous secretory cytokines (58,59). In addition to tumor cells, the TME also contains immune cells, fibroblasts, vascular endothelial cells, adipocytes and tumor stromal cells, which secrete large numbers of cytokines, such as vascular endothelial growth factor (VEGF), platelet derived growth factor (PDGF), interleukin (IL)-8, IL-6, stromal cell-derived factor-1 (SDF-1), basic fibroblast growth factor (bFGF), granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), monocyte chemoattractant protein-1 (MCP-1), hepatocyte growth factor (HGF), tumor necrosis factor-α (TNF-α), transforming growth factor-β (TGF-β), urokinase type plasminogen activator receptor, vascular cell and intercellular cell adhesion molecules (VCAM, ICAM), C-X-C motif chemokine ligand-12 (CXCL-12), C-C motif chemokine ligand-2 (CCL-2), C-C motif chemokine ligand-3 (CCL-3), C-C motif chemokine receptor 4 (CCR4) and C-X-C motif chemokine receptor 4 (CXCR4) (59–63).
Pavon et al (64) reported that human umbilical cord blood-derived MSCs express the chemokine receptors CCR2 and CXCR4, and demonstrated that MCP-1/CCL2 and SDF-1/CXC12 secreted by CD133-positive GBM cells can induce MSC migration in vitro. Furthermore, in vivo experiments confirmed that MSCs can cross the blood-brain barrier and migrate to glioblastoma tumor areas (64). In addition, Lejmi et al (63) co-cultured hepatoma cells with MSCs and demonstrated that the expression of matrix metalloproteinase-1 is significantly increased in MSCs, promoting therefore MSCs migration toward hepatoma cells. In essence, cytokines secreted by immune and tumor cells are key to inducing the chemotactic migration of MSCs and are the central theoretical tenet for MSCs as OV cellular vehicles (65,66). Therefore, when OVs are loaded onto MSCs, they exploit the inherent tumor tendency of MSCs to reach tumor sites, thereby increasing OV targeting and enhancing oncolysis.
MSC immunosuppressive functions protect OV clearance from the immune system
MSC immunological characteristics serve crucial roles in the therapeutic efficacy of MSCs loaded with OVs towards tumors. Evidence indicates that MSCs amplified in vitro do not express HLA-II or costimulatory molecules, such as CD40, CD80, CD83, CD86 and CD154 (67). Therefore, no additional immunosuppressants are required for autologous or allogeneic MSC transplantation. In addition, MSCs exert strong immunosuppressive functions. For example, MSCs produce and release a variety of soluble cytokines, including IL-6, IL-10, TGF-β, heme oxygenase-1, inducible nitric oxide synthase and indoleamine-2-dioxygenase-3 (68), which play major roles in immunosuppression. At present, MSCs are used for immunomodulation, mostly for immune rejection and autoimmune diseases, such as hematopoietic stem cell transplantation, organ transplantation, rheumatoid arthritis and systemic lupus erythematosus (69,70). However, the underlying mechanisms of MSC immunosuppressive function in vivo remain unclear.
In recent years, increasing evidence from preclinical and clinical studies has indicated that MSCs exert immunosuppressive functions by inhibiting the activity of certain types of immune cell, including T, B lymphocytes and NKs, thereby affecting monocytes, DC and macrophage function (71–74). MSCs affect the activation, proliferation, maturation, cytokine production and cytotoxic activity of innate and adaptive immune cells (68). Indeed, MSCs can reduce cytokine secretion from helper T cells, weaken the killing effects of effector T lymphocytes (75), hinder B lymphocyte differentiation and impede their ability to secrete immunoglobulin (76,77), and inhibit INF-γ secretion by NK cells and reduce their killing effects (78). In addition, MSCs prevent CD14+ monocytes and CD34+ progenitor cells from differentiating into mature DC cells (79). Importantly, MSCs promote the emergence of regulatory immune subsets, including CD8+CD28− T lymphocytes (80), CD4+CD25+FOXP3+ T lymphocytes (81), IL-10-producing B lymphocytes (82) and IL-10-producing DCs (83). Therefore, inhibiting immune cell functions and promoting the emergence of regulatory immune cell subsets, could serve positive roles in MSC immunosuppressive functions. These functions are key MSC features in protecting OVs from immune system clearance, and a guarantee to enhance OV spread and increase viral persistence (84).
MSC carriers induce systemic anti-tumor immune responses
It has been reported that MSCs promote tumorigenesis through various mechanisms, such as inhibition of local immune responses (51), stimulation of epithelial-mesenchymal transformation, inhibition of tumor cell apoptosis and promotion of angiogenesis and tumor metastasis (85). Previous studies have demonstrated that MSCs, in contrast to their tumorigenic functions, can inhibit tumor growth by inhibiting angiogenesis (86), inducing cell cycle arrest (14,87), enhancing inflammatory infiltration (88) and inhibiting proliferation-associated signaling pathways (14).
Although there is some controversy over whether MSCs inhibit or promote tumor growth, emerging evidence indicates that oncolytic adenovirus (OAD)-infected MSCs induce anti-tumor immune responses and increase leukocyte infiltration into tumor lesions (89). Similarly, Mahasa et al (10) predicted the therapeutic efficacy of MSCs loaded with OAD in a Hep3B cell tumor model using an integrated mathematical-experimental model, and demonstrated that MSCs loaded with OAD can promote tumor therapeutic efficacy. In addition, a phase I clinical trial (NCT01844661) of bone marrow-derived MSCs carrying Celyvir for the treatment of metastatic or refractory tumors was completed and reported that the combination of MSCs and Celyvir is safe (90). Following treatment with MSCs carrying Celyvir, except for the increase in the amount of oncolytic virus administered to patients, minimizing toxicities and avoiding direct tumor injections, no grades 2–5 toxicities were reported (90). However, the safety and efficacy of MSCs carrying Celyvir require further evaluation in a phase II setting.
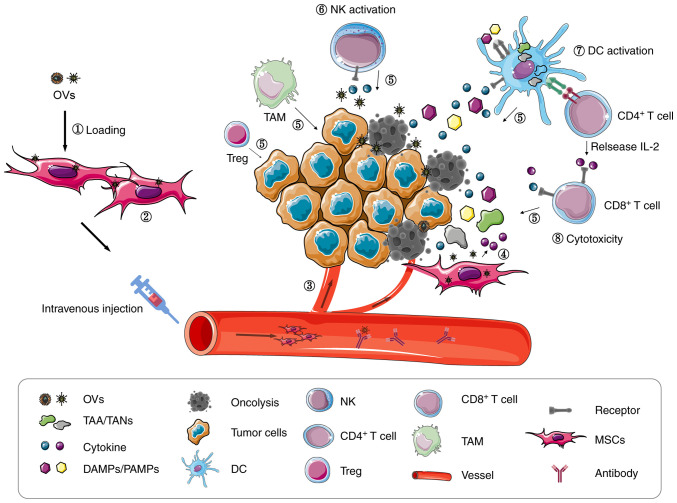
Mechanically, after MSC infection with the human OAD icovir-5 in vitro, the NF-κB signaling pathway is activated and releases large numbers of cytokines, such as IL-6, CXCL2, CXCL10 and CCL5 (91). These cytokines facilitate the migration of NK and T cells, amongst others, to the TME (Fig. 2). Indeed, 48 h following Celyvir transplantation, the levels of peripheral blood monocytes, NK cells and neutrophils are increased (89). Furthermore, the first-in-child trial of autologous MSCs infected with the human OAD icovir-5 (Celyvir) demonstrated that the number of circulating B-lymphocytes and dendritic cells is significantly higher in pediatric patients, and that CD4 and CD8 T lymphocytes are also higher in children at most time points, compared with adult cohorts (90). These preclinical data illustrate that MSCs can release cytokines that might promote anti-tumor immune responses mediated by OVs. These data are instrumental in encouraging more virotherapy preclinical and clinical studies, investigating the utility of MSCs as OV carriers for patients with advanced cancer.
Figure 2.
MSC carriers enhance anti-tumor efficacy of oncolytic virotherapy. (1) MSCs loaded with OVs. (2) MSCs provide a replication locale for OVs to produce more virus particles. (3) Tumor tropisms and immunosuppressive MSC functions facilitate precise OV targeting to tumor lesions. OVs infect tumor cells and release ‘dangerous’ signals. (4) OVs alter MSC cytokine profiles. (5) Cytokines induce immune cell migration to the TME. (6) NK activation. (7) DCs activation. (8) Tumor antigen specific T cell activation. OVs, oncolytic virus; DC, dendritic cell; MSCs, mesenchymal stem cells; DAMPs, danger-associated molecular patterns; PAMPs, Pathogen-associated molecular patterns; TAAs, tumor-associated antigens; TANs, tumor-associated neoantigens; NK, natural killer; TAM, tumor-associated macrophage; TME, tumor microenvironment.
MSCs as carriers for delivering OVs
The majority of preclinical studies indicate efficacy factors for MSCs as carriers for OV delivery (92–94). Du et al (95) used MSCs as cellular carriers for oncolytic herpes simplex virus (HSV) in order to assess efficacy in immune-deficient and immune-competent mouse melanoma metastasis models. The results demonstrated that transplanted MSCs carrying HSV could migrate to the tumor site and significantly prolong mouse survival. Furthermore, in immune-competent mice, the combination of MSC-HSV and the anti-programmed death ligand 1 (anti-PD-L1) immune checkpoint inhibitor could increase CD8+ T lymphocyte infiltration, leading to the production of IFN-γ and significant prolongation of mouse survival.
For enveloped OVs, MSCs can deliver viruses to tumor sites via hetero-cellular fusion. Ong et al (96) loaded bone marrow-derived MSCs with oncolytic measles virus, and co-cultured them with human hepatocellular carcinoma cells in vitro. The results demonstrated that syncytia number increases when MSCs carries the measles virus, which is not the case with non-enveloped virus. Furthermore, in the presence of high titer anti-measles virus antibodies, virus-infected MSCs significantly induce heterocellular formation when compared with naked virus. In addition, MSCs precisely deliver the measles virus to tumor lesions in a patient-derived hepatocellular carcinoma model (96). These results were consistent with Castleton et al (97) who reported MSC delivery of the measles virus in a model for acute lymphoblastic leukemia, suggesting that OV infected MSCs could significantly prolong survival and improve anti-tumor efficacy when compared with the naked virus.
In addition, genetic engineering improves MSC delivery efficiency, enhances viral oncolytic activity and reduces virotherapy side effects. Yoon et al (55) reported that the OAD infection capability of MSCs is enhanced after modification of the fiber domain of OADs, allowing the virus to replicate efficiently in MSCs. These MSCs infected with OADs could effectively lyse hepatocellular carcinoma cells in vitro. Importantly, following MSC-OAD transplantation, MSCs home to the tumor site, facilitating a high accumulation of virions at the site, and ultimately leading to tumor growth inhibition. In another study, Kaczorowski et al (66) deleted the anti-apoptotic gene E1B19K from OAD and inserted the cell death ligand TRAIL gene of OAD. After intravenous injection of virally infected MSCs, adenovirus capsid protein is detected in tumor xenografts established by cancer stem cell of pancreatic ductal adenocarcinoma. Similarly, following viral MSC treatment, the tumor size decreases significantly, the tumor cell proliferation-associated Ki67 and CD24 expression decreases and the tumor cell apoptosis-associated caspase-3 activity increases (66). In addition, OADs significantly increase virus release from MSCs following the deletion of the anti-apoptotic virus gene E1B19K, or the overexpression of the cell death ligand TRAIL, while MSC migration ability remains unaffected (98). These data suggest that genetic modification of OADs can induce effective oncolysis, which may represent a promising strategy for OVs in clinical applications. Similarly, MSCs as carriers for the delivery of genetically modified OVs may be considered as a useful method for improving oncolytic virotherapy efficacy (Table I)(99–106). However, MSCs can also be modified by genetic modification or preconditioned to modification in order to improve their inherent properties, such as enhanced migration, adhesion and survival, and reduced premature senescence (107). OV delivery and virotherapy efficacy may therefore be improved.
Table I.
MSCs as carriers for OV delivery.
| Author, year | Strategies | Results | (Refs.) |
|---|---|---|---|
| Yoon et al, 2019, Cancer Res | MSCs loading OADs | MSCs cells locate to the tumor site and lead to the accumulation of high virion levels in the tumor tissue, which eventually led to the inhibition of tumor growth. | (55) |
| Du et al, 2017, Proc Natl Acad Sci USA | MSCs loading OHSV | Combination of MSCs-OHSV and an anti-PD-L1 immune checkpoint inhibitor increases the number of CD8+ tumor infiltrating T lymphocytes and significantly prolonges mice survival. | (95) |
| Kazimirsky et al, 2016, Stem Cell Res Ther | MSCs loading NDV | Factors secreted by MSCs infected with virus make glioma cells sensitive to the cytotoxicity. of NDV TRAIL and NDV have synergistic effect in inducing glioma cell death. | (99) |
| Kaczorowski et al, 2016, Oncotarget | MSCs loading E1B19K deleted or TRAIL inserted OADs | After treatment, the tumor volume decreased significantly, Ki67 and CD24 expression is decreased and caspase-3 activity is increased. | (66) |
| Melen et al, 2016, Cancer Lett | MSCs loading genetically modified OADs | Clinical trials confirm the safety of MSCs loading genetically modified OADs. | (100) |
| Leoni et al, 2015, Oncotarget | MSCs loading OHSV | MSCs-OHSV significantly inhibit the brain metastasis of breast cancer in NSG mice. | (101) |
| Hoyos et al, 2015, Mol Ther | MSCs loading ICOVIR15 and Inducible Caspase 9 suicide gene (iC9) inserted OADs | MSCs loading ICOVIR15 increase the control of tumor growth and prolonge the survival of tumor-bearing mice. | (102) |
| Franco-Luzon et al, 2020, Oncotarget Morales-Molina et al, 2020, Cancers (Basel) | MSCs loading ICOVIR5 | MSCs carrying ICOVIR5 enhance anti-tumor. effects | (103,104) |
| Hammer et al, 2015, Int J Cancer | MSCs loading E1B19K deleted or TRAIL inserted OADs | This strategy increases the release of the OADs from MSCs, while MSC migration ability is not affected. | (98) |
| Ong et al, 2013, J Hepatol Castleton et al, 2014, Blood Mader et al, 2009, Clin Cancer Res | MSCs loading MV | In the presence of high titer anti-measles virus antibodies, measles virus-infected MSCs can significantly induce heterocellular formation when compared with naked virus alone. In addition, MSCs accurately deliver measles virus to tumor lesions and prolong mice survival. | (96,97,105) |
| Hai et al, 2012, Chin J Cancer | MSCs loading genetically modified OADs | MSCs carrying replicable adenovirus can significantly inhibit tumor growth in vivo. | (94) |
| Ahmed et al, 2010, Mol Ther | MSCs loading OADs | MSCs carrying OADs enhance the spread and persistence of OADs. | (84) |
| Hakkarainen et al, 2007, Hum Gene Ther | MSCs loading infectious enhanced OADs | Intravenously transplanted MSCs are mainly located in the lung, and the virus is released to advanced orthotopic breast and lung tumors to improve the efficacy. | (106) |
OADs, Oncolytic Adenovirus; OHSV, Oncolytic Herpes Simplex Virus; NDV, Newcastle Disease Virus; MV, Measles Virus; MSCs, mesenchymal stem cells; MSCs-OHSV, mesenchymal stem cells loading Oncolytic Herpes Simplex Virus.
6. Conclusions and perspectives
In summary, MSCs enhance the anti-tumor efficacy of virotherapy through numerous factors. Firstly, MSCs provide a replication location for OVs, facilitating the production of more virus particles, which is beneficial for virotherapy. Secondly, the tumor tropism and immunosuppression function of MSCs allow the virus to accurately reach the tumor site and enhance the transmission and persistence of the virus. Thirdly, oncolysis leads to the release of ‘dangerous’ signals, such as TAAs/TANs and DAMPs/PAMPs, activating local anti-tumor immune responses, and converting the TME from an immunosuppressive to an immunostimulatory environment (93,103). However, cytokines released by MSCs recruit immune cells to the TME, further enhancing the anti-tumor immune response. Therefore, MSC carriers are considered as promising cellular vehicles for OV delivery. Assuming the high quality of MSCs and appropriate conditions of MSCs loading the virus, it is worth treating malignant tumors with such therapy, which could lead to a restrain of tumor growth progression in patients. However, further investigation is required to evaluate the effects of MSC loading viruses and explore the immune regulation mechanisms of MSCs on anti-viral and anti-tumor immune responses.
In TME, cancer-associated fibroblasts, adipocytes, Tregs, mesenchymal stromal cells and tumor-associated macrophages release numerous cytokines, such as IL-10, which support immune evasion and tumor growth (108). In recent years, oncolytic virotherapy (OVT) has been demonstrated to relieve the tumor immunosuppressive environments, and enhance anti-tumor immune responses (109,110). OVs stimulate anti-tumor immune responses which in turn, enhance the efficacy of immune checkpoint inhibitors (ICIs) (111). For this reason, emerging evidence from preclinical and clinical trials has indicated that combined OVT and ICIs could improve the anti-tumor therapeutic efficacy (112–114). In view of the contribution of MSCs to the activation of immune responses in virotherapy, combined MSC loading OVs with ICIs could be considered as a major therapeutic area for future anti-tumor research.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- MSCs
mesenchymal stem cells
- OVs
oncolytic virus
- OVT
oncolytic virotherapy
- TME
tumor microenvironment
- TAAs
tumor-associated antigens
- TANs
tumor-associated neoantigens
- DAMPs
danger-associated molecular patterns
- PAMPs
Pathogen-associated molecular patterns
- OAD
oncolytic adenovirus
- NK
natural killer
- DC
dendritic cell
Funding Statement
This study was supported by the National Natural Science Foundation of China (grant no. 81871313), the Graduate Student Innovation Program in Guizhou Province [grant no. Qian Jiao He YJSCXJH (2020) 143], Key projects of Guizhou Provincial Department of Science and Technology [grant no. Qian Ke He Zhi Cheng (2020) 4Y192], the Guizhou Provincial Natural Science Foundation [grant no. (2019)5663], the Program for Top Scientifc and Technological Talents in Guizhou Province [grant no. KY (2018)049], the Guizhou Province Science and Technology Talent Platform Project [grant no. (2019)5406], the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (grant nos. 2018PT31048 and 2019PT310013) and the Special Grant for Central Government Supporting Local Science and Technology Development, Science and Technology Department of Guizhou Province [grant no. (2019)4008].
Funding
This study was supported by the National Natural Science Foundation of China (grant no. 81871313), the Graduate Student Innovation Program in Guizhou Province [grant no. Qian Jiao He YJSCXJH (2020) 143], Key projects of Guizhou Provincial Department of Science and Technology [grant no. Qian Ke He Zhi Cheng (2020) 4Y192], the Guizhou Provincial Natural Science Foundation [grant no. (2019)5663], the Program for Top Scientifc and Technological Talents in Guizhou Province [grant no. KY (2018)049], the Guizhou Province Science and Technology Talent Platform Project [grant no. (2019)5406], the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (grant nos. 2018PT31048 and 2019PT310013) and the Special Grant for Central Government Supporting Local Science and Technology Development, Science and Technology Department of Guizhou Province [grant no. (2019)4008].
Availability of data and materials
Not applicable.
Authors' contributions
XW and ZH conceived the review. XW wrote the review. ZH and XZ revised the review. XW, XZ and ZH proofread the manuscript and revised the manuscript for intellectual content. All authors read and approved the final version.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Hayes C. Cellular immunotherapies for cancer. Ir J Med Sci. 2020 Jul 1; doi: 10.1007/s11845-020-02264-w. (Epub ahead of print). doi: 10.1007/s11845-020-02264-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alard E, Butnariu AB, Grillo M, Kirkham C, Zinovkin DA, Newnham L, Macciochi J, Pranjol MZI. Advances in anti-cancer immunotherapy: Car-T cell, checkpoint inhibitors, dendritic cell vaccines, and oncolytic viruses, and emerging cellular and molecular targets. Cancers (Basel) 2020;12:1826. doi: 10.3390/cancers12071826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hemminki O, Dos Santos JM, Hemminki A. Oncolytic viruses for cancer immunotherapy. J Hematol Oncol. 2020;13:84. doi: 10.1186/s13045-020-00922-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romero D. Immunotherapy: Oncolytic viruses prime antitumour immunity. Nat Rev Clin Oncol. 2018;15:135. doi: 10.1038/nrclinonc.2018.15. [DOI] [PubMed] [Google Scholar]
- 5.Engeland CE, Bell JC. Introduction to oncolytic virotherapy. Methods Mol Biol. 2020;2058:1–6. doi: 10.1007/978-1-4939-9794-7_1. [DOI] [PubMed] [Google Scholar]
- 6.Kolb EA, Sampson V, Stabley D, Walter A, Sol-Church K, Cripe T, Hingorani P, Ahern CH, Weigel BJ, Zwiebel J, Blaney SM. A phase I trial and viral clearance study of reovirus (Reolysin) in children with relapsed or refractory extra-cranial solid tumors: A children's oncology group phase I consortium report. Pediatr Blood Cancer. 2015;62:751–758. doi: 10.1002/pbc.25464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamid O, Ismail R, Puzanov I. Intratumoral immunotherapy-update 2019. Oncologist. 2020;25:e423–e438. doi: 10.1634/theoncologist.2019-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roy DG, Bell JC, Bourgeois-Daigneault MC. Magnetic targeting of oncolytic VSV-based therapies improves infection of tumor cells in the presence of virus-specific neutralizing antibodies in vitro. Biochem Biophys Res Commun. 2020;526:641–646. doi: 10.1016/j.bbrc.2020.03.135. [DOI] [PubMed] [Google Scholar]
- 9.Schirrmacher V, van Gool S, Stuecker W. Breaking therapy resistance: An update on oncolytic newcastle disease virus for improvements of cancer therapy. Biomedicines. 2019;7:66. doi: 10.3390/biomedicines7030066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahasa KJ, de Pillis L, Ouifki R, Eladdadi A, Maini P, Yoon AR, Yun CO. Mesenchymal stem cells used as carrier cells of oncolytic adenovirus results in enhanced oncolytic virotherapy. Sci Rep. 2020;10:425. doi: 10.1038/s41598-019-57240-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hadrys A, Sochanik A, McFadden G, Jazowiecka-Rakus J. Mesenchymal stem cells as carriers for systemic delivery of oncolytic viruses. Eur J Pharmacol. 2020;874:172991. doi: 10.1016/j.ejphar.2020.172991. [DOI] [PubMed] [Google Scholar]
- 12.Naseri Z, Oskuee RK, Forouzandeh-Moghadam M, Jaafari MR. Delivery of LNA-antimiR-142-3p by mesenchymal stem cells-derived exosomes to breast cancer stem cells reduces tumorigenicity. Stem Cell Rev Rep. 2020;16:541–556. doi: 10.1007/s12015-019-09944-w. [DOI] [PubMed] [Google Scholar]
- 13.Altaner C, Altanerova U. Mesenchymal stem cell exosome-mediated prodrug gene therapy for cancer. Methods Mol Biol. 2019;1895:75–85. doi: 10.1007/978-1-4939-8922-5_6. [DOI] [PubMed] [Google Scholar]
- 14.Kostadinova M, Mourdjeva M. Potential of mesenchymal stem cells in anti-cancer therapies. Curr Stem Cell Res Ther. 2020;15:482–491. doi: 10.2174/1574888X15666200310171547. [DOI] [PubMed] [Google Scholar]
- 15.Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: Role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111:1327–1333. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- 16.Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120–4126. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- 17.Lei J, Jacobus EJ, Taverner WK, Fisher KD, Hemmi S, West K, Slater L, Lilley F, Brown A, Champion B, et al. Expression of human CD46 and trans-complementation by murine adenovirus 1 fails to allow productive infection by a group B oncolytic adenovirus in murine cancer cells. J Immunother Cancer. 2018;6:55. doi: 10.1186/s40425-018-0350-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koehler M, Aravamudhan P, Guzman-Cardozo C, Dumitru AC, Yang J, Gargiulo S, Soumillion P, Dermody TS, Alsteens D. Glycan-mediated enhancement of reovirus receptor binding. Nat Commun. 2019;10:4460. doi: 10.1038/s41467-019-12411-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phillips MB, Stuart JD, Rodriguez Stewart RM, Berry JT, Mainou BA, Boehme KW. Current understanding of reovirus oncolysis mechanisms. Oncolytic Virother. 2018;7:53–63. doi: 10.2147/OV.S143808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakurai F, Inoue S, Kaminade T, Hotani T, Katayama Y, Hosoyamada E, Terasawa Y, Tachibana M, Mizuguchi H. Cationic liposome-mediated delivery of reovirus enhances the tumor cell-killing efficiencies of reovirus in reovirus-resistant tumor cells. Int J Pharm. 2017;524:238–247. doi: 10.1016/j.ijpharm.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Mahalingam D, Goel S, Aparo S, Patel Arora S, Noronha N, Tran H, Chakrabarty R, Selvaggi G, Gutierrez A, Coffey M, et al. A phase II study of pelareorep (REOLYSIN(R)) in combination with gemcitabine for patients with advanced pancreatic adenocarcinoma. Cancers (Basel) 2018;10:160. doi: 10.3390/cancers10060160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jonker DJ, Tang PA, Kennecke H, Welch SA, Cripps MC, Asmis T, Chalchal H, Tomiak A, Lim H, Ko YJ, et al. A randomized phase II study of FOLFOX6/bevacizumab with or without pelareorep in patients with metastatic colorectal cancer: IND.210, a canadian cancer trials group trial. Clin Colorectal Cancer. 2018;17:231–239 e7. doi: 10.1016/j.clcc.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Davola ME, Mossman KL. Oncolytic viruses: How ‘lytic’ must they be for therapeutic efficacy? Oncoimmunology. 2019;8:e1581528. doi: 10.1080/2162402X.2019.1596006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo Y, Lin C, Zou Y, Ju F, Ren W, Lin Y, Wang Y, Huang X, Liu H, Yu Z, et al. Tumor-targeting oncolytic virus elicits potent immunotherapeutic vaccine responses to tumor antigens. Oncoimmunology. 2020;9:1726168. doi: 10.1080/2162402X.2020.1726168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pidelaserra-Marti G, Engeland CE. Mechanisms of measles virus oncolytic immunotherapy. Cytokine Growth Factor Rev. 2020;56:28–38. doi: 10.1016/j.cytogfr.2020.07.009. [DOI] [PubMed] [Google Scholar]
- 26.Pol JG, Bridle BW, Lichty BD. Detection of tumor antigen-specific T-cell responses after oncolytic vaccination. Methods Mol Biol. 2020;2058:191–211. doi: 10.1007/978-1-4939-9794-7_12. [DOI] [PubMed] [Google Scholar]
- 27.Keshavarz M, Solaymani-Mohammadi F, Miri SM, Ghaemi A. Oncolytic paramyxoviruses-induced autophagy; a prudent weapon for cancer therapy. J Biomed Sci. 2019;26:48. doi: 10.1186/s12929-019-0542-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bommareddy PK, Zloza A, Rabkin SD, Kaufman HL. Oncolytic virus immunotherapy induces immunogenic cell death and overcomes STING deficiency in melanoma. Oncoimmunology. 2019;8:1591875. doi: 10.1080/2162402X.2019.1591875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma J, Ramachandran M, Jin C, Quijano-Rubio C, Martikainen M, Yu D, Essand M. Characterization of virus-mediated immunogenic cancer cell death and the consequences for oncolytic virus-based immunotherapy of cancer. Cell Death Dis. 2020;11:48. doi: 10.1038/s41419-020-2236-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Shao X, Gu L, Jiang K, Wang S, Chen J, Fang J, Guo X, Yuan M, Shi J, et al. Targeting STAT3 enhances NDV-induced immunogenic cell death in prostate cancer cells. J Cell Mol Med. 2020;24:4286–4297. doi: 10.1111/jcmm.15089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shao X, Wang X, Guo X, Jiang K, Ye T, Chen J, Fang J, Gu L, Wang S, Zhang G, et al. STAT3 contributes to oncolytic newcastle disease virus-induced immunogenic cell death in melanoma cells. Front Oncol. 2019;9:436. doi: 10.3389/fonc.2019.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu Q, Rangaswamy US, Wang W, Robbins SH, Harper J, Jin H, Cheng X. Evaluation of newcastle disease virus mediated dendritic cell activation and cross-priming tumor-specific immune responses ex vivo. Int J Cancer. 2020;146:531–541. doi: 10.1002/ijc.32694. [DOI] [PubMed] [Google Scholar]
- 33.Garg AD, Agostinis P. Cell death and immunity in cancer: From danger signals to mimicry of pathogen defense responses. Immunol Rev. 2017;280:126–148. doi: 10.1111/imr.12574. [DOI] [PubMed] [Google Scholar]
- 34.Jiang H, Fueyo J. Healing after death: Antitumor immunity induced by oncolytic adenoviral therapy. Oncoimmunology. 2014;3:e947872. doi: 10.4161/21624011.2014.947872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kepp O, Senovilla L, Vitale I, Vacchelli E, Adjemian S, Agostinis P, Apetoh L, Aranda F, Barnaba V, Bloy N, et al. Consensus guidelines for the detection of immunogenic cell death. Oncoimmunology. 2014;3:e955691. doi: 10.4161/21624011.2014.955691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garg AD, Galluzzi L, Apetoh L, Baert T, Birge RB, Bravo-San Pedro JM, Breckpot K, Brough D, Chaurio R, Cirone M, et al. Molecular and translational classifications of DAMPs in immunogenic cell death. Front Immunol. 2015;6:588. doi: 10.3389/fimmu.2015.00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Das K, Urbiola C, Spiesschaert B, Mueller P, Wollmann G. Analysis of immunological treatment effects of virotherapy in tumor tissue. Methods Mol Biol. 2020;2058:155–177. doi: 10.1007/978-1-4939-9794-7_10. [DOI] [PubMed] [Google Scholar]
- 38.Reale A, Vitiello A, Conciatori V, Parolin C, Calistri A, Palu G. Perspectives on immunotherapy via oncolytic viruses. Infect Agent Cancer. 2019;14:5. doi: 10.1186/s13027-018-0218-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sobol PT, Boudreau JE, Stephenson K, Wan Y, Lichty BD, Mossman KL. Adaptive antiviral immunity is a determinant of the therapeutic success of oncolytic virotherapy. Mol Ther. 2011;19:335–344. doi: 10.1038/mt.2010.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gujar S, Pol JG, Kim Y, Lee PW, Kroemer G. Antitumor benefits of antiviral immunity: An underappreciated aspect of oncolytic virotherapies. Trends Immunol. 2018;39:209–221. doi: 10.1016/j.it.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 41.Ledford H. Cancer-fighting viruses win approval. Nature. 2015;526:622–623. doi: 10.1038/526622a. [DOI] [PubMed] [Google Scholar]
- 42.O'Donoghue C, Doepker MP, Zager JS. Talimogene laherparepvec: Overview, combination therapy and current practices. Melanoma Manag. 2016;3:267–272. doi: 10.2217/mmt-2016-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sunshine JC, Sosman J, Shetty A, Choi JN. Successful treatment of in-transit metastatic melanoma in a renal transplant patient with combination T-VEC/Imiquimod immunotherapy. J Immunother. 2020;43:149–152. doi: 10.1097/CJI.0000000000000319. [DOI] [PubMed] [Google Scholar]
- 44.Masoud SJ, Hu JB, Beasley GM, Stewart JH IV, Mosca PJ. Efficacy of talimogene laherparepvec (T-VEC) therapy in patients with in-transit melanoma metastasis decreases with increasing lesion size. Ann Surg Oncol. 2019;26:4633–4641. doi: 10.1245/s10434-019-07691-3. [DOI] [PubMed] [Google Scholar]
- 45.Howard F, Muthana M. Designer nanocarriers for navigating the systemic delivery of oncolytic viruses. Nanomedicine (Lond) 2020;15:93–110. doi: 10.2217/nnm-2019-0323. [DOI] [PubMed] [Google Scholar]
- 46.Phan M, Watson MF, Alain T, Diallo JS. Oncolytic viruses on drugs: Achieving higher therapeutic efficacy. ACS Infect Dis. 2018;4:1448–1467. doi: 10.1021/acsinfecdis.8b00144. [DOI] [PubMed] [Google Scholar]
- 47.Rosewell Shaw A, Suzuki M. Oncolytic viruses partner with T-cell therapy for solid tumor treatment. Front Immunol. 2018;9:2103. doi: 10.3389/fimmu.2018.02103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hwang CC, Igase M, Sakurai M, Haraguchi T, Tani K, Itamoto K, Shimokawa T, Nakaichi M, Nemoto Y, Noguchi S, et al. Oncolytic reovirus therapy: Pilot study in dogs with spontaneously occurring tumours. Vet Comp Oncol. 2018;16:229–238. doi: 10.1111/vco.12361. [DOI] [PubMed] [Google Scholar]
- 49.Mok DZL, Chan KR. The effects of pre-existing antibodies on live-attenuated viral vaccines. Viruses. 2020;12:520. doi: 10.3390/v12050520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harrington K, Freeman DJ, Kelly B, Harper J, Soria JC. Optimizing oncolytic virotherapy in cancer treatment. Nat Rev Drug Discov. 2019;18:689–706. doi: 10.1038/s41573-019-0029-0. [DOI] [PubMed] [Google Scholar]
- 51.Naji A, Eitoku M, Favier B, Deschaseaux F, Rouas-Freiss N, Suganuma N. Biological functions of mesenchymal stem cells and clinical implications. Cell Mol Life Sci. 2019;76:3323–3348. doi: 10.1007/s00018-019-03125-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Volarevic V, Markovic BS, Gazdic M, Volarevic A, Jovicic N, Arsenijevic N, Armstrong L, Djonov V, Lako M, Stojkovic M. Ethical and safety issues of stem cell-based therapy. Int J Med Sci. 2018;15:36–45. doi: 10.7150/ijms.21666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop DJ, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 54.Salmasi Z, Hashemi M, Mahdipour E, Nourani H, Abnous K, Ramezani M. Mesenchymal stem cells engineered by modified polyethylenimine polymer for targeted cancer gene therapy, in vitro and in vivo. Biotechnol Prog. 2020;36:e3025. doi: 10.1002/btpr.3025. [DOI] [PubMed] [Google Scholar]
- 55.Yoon AR, Hong J, Li Y, Shin HC, Lee H, Kim HS, Yun CO. Mesenchymal stem cell-mediated delivery of an oncolytic adenovirus enhances antitumor efficacy in hepatocellular carcinoma. Cancer Res. 2019;79:4503–4514. doi: 10.1158/0008-5472.CAN-18-3900. [DOI] [PubMed] [Google Scholar]
- 56.Vangala G, Imhoff FM, Squires CML, Cridge AG, Baird SK. Mesenchymal stem cell homing towards cancer cells is increased by enzyme activity of cathepsin D. Exp Cell Res. 2019;383:111494. doi: 10.1016/j.yexcr.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 57.Kwon S, Yoo KH, Sym SJ, Khang D. Mesenchymal stem cell therapy assisted by nanotechnology: A possible combinational treatment for brain tumor and central nerve regeneration. Int J Nanomedicine. 2019;14:5925–5942. doi: 10.2147/IJN.S217923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thomas JG, Parker Kerrigan BC, Hossain A, Gumin J, Shinojima N, Nwajei F, Ezhilarasan R, Love P, Sulman EP, Lang FF. Ionizing radiation augments glioma tropism of mesenchymal stem cells. J Neurosurg. 2018;128:287–295. doi: 10.3171/2016.9.JNS16278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choi SA, Lee JY, Kwon SE, Wang KC, Phi JH, Choi JW, Jin X, Lim JY, Kim H, Kim SK. Human adipose tissue-derived mesenchymal stem cells target brain tumor-initiating cells. PLoS One. 2015;10:e0129292. doi: 10.1371/journal.pone.0129292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Verdelli C, Vaira V, Corbetta S. Parathyroid tumor microenvironment. Adv Exp Med Biol. 2020;1226:37–50. doi: 10.1007/978-3-030-36214-0_3. [DOI] [PubMed] [Google Scholar]
- 61.Karagiannis K, Proklou A, Tsitoura E, Lasithiotaki I, Kalpadaki C, Moraitaki D, Sperelakis I, Kontakis G, Antoniou KM, Tzanakis N. Impaired mRNA expression of the migration related chemokine receptor CXCR4 in mesenchymal stem cells of COPD patients. Int J Inflam. 2017;2017:6089425. doi: 10.1155/2017/6089425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Armakolas A, Dimakakos A, Loukogiannaki C, Armakolas N, Antonopoulos A, Florou C, Tsioli P, Papageorgiou E, Alexandrou TP, Stathaki M, et al. IL-6 is associated to IGF-1Ec upregulation and Ec peptide secretion, from prostate tumors. Mol Med. 2018;24:6. doi: 10.1186/s10020-018-0003-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lejmi E, Perriraz N, Clement S, Morel P, Baertschiger R, Christofilopoulos P, Meier R, Bosco D, Buhler LH, Gonelle-Gispert C. Inflammatory chemokines MIP-1δ and MIP-3α are involved in the migration of multipotent mesenchymal stromal cells induced by hepatoma cells. Stem Cells Dev. 2015;24:1223–1235. doi: 10.1089/scd.2014.0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pavon LF, Sibov TT, de Souza AV, da Cruz EF, Malheiros SM, Cabral FR, de Souza JG, Boufleur P, de Oliveira DM, de Toledo SR, et al. Tropism of mesenchymal stem cell toward CD133+ stem cell of glioblastoma in vitro and promote tumor proliferation in vivo. Stem Cell Res Ther. 2018;9:310. doi: 10.1186/s13287-018-1049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ramirez M, Garcia-Castro J, Melen GJ, Gonzalez-Murillo A, Franco-Luzon L. Patient-derived mesenchymal stem cells as delivery vehicles for oncolytic virotherapy: Novel state-of-the-art technology. Oncolytic Virother. 2015;4:149–155. doi: 10.2147/OV.S66010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kaczorowski A, Hammer K, Liu L, Villhauer S, Nwaeburu C, Fan P, Zhao Z, Gladkich J, Gross W, Nettelbeck DM, Herr I. Delivery of improved oncolytic adenoviruses by mesenchymal stromal cells for elimination of tumorigenic pancreatic cancer cells. Oncotarget. 2016;7:9046–9059. doi: 10.18632/oncotarget.7031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mehler VJ, Burns C, Moore ML. Concise review: Exploring immunomodulatory features of mesenchymal stromal cells in humanized mouse models. Stem Cells. 2019;37:298–305. doi: 10.1002/stem.2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gao F, Chiu SM, Motan DA, Zhang Z, Chen L, Ji HL, Tse HF, Fu QL, Lian Q. Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death Dis. 2016;7:e2062. doi: 10.1038/cddis.2015.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abbasi-Kangevari M, Ghamari SH, Safaeinejad F, Bahrami S, Niknejad H. Potential therapeutic features of human amniotic mesenchymal stem cells in multiple sclerosis: Immunomodulation, inflammation suppression, angiogenesis promotion, oxidative stress inhibition, neurogenesis induction, MMPs regulation, and remyelination stimulation. Front Immunol. 2019;10:238. doi: 10.3389/fimmu.2019.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ma ZJ, Wang YH, Li ZG, Wang Y, Li BY, Kang HY, Wu XY. Immunosuppressive effect of exosomes from mesenchymal stromal cells in defined medium on experimental colitis. Int J Stem Cells. 2019;12:440–448. doi: 10.15283/ijsc18139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carreras-Planella L, Monguio-Tortajada M, Borras FE, Franquesa M. Immunomodulatory effect of MSC on B cells is independent of secreted extracellular vesicles. Front Immunol. 2019;10:1288. doi: 10.3389/fimmu.2019.01288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wilson A, Chee M, Butler P, Boyd AS. Isolation and characterisation of human adipose-derived stem cells. Methods Mol Biol. 2019;1899:3–13. doi: 10.1007/978-1-4939-8938-6_1. [DOI] [PubMed] [Google Scholar]
- 73.Zhang F, Wang C, Wen X, Chen Y, Mao R, Cui D, Li L, Liu J, Chen Y, Cheng J, Lu Y. Mesenchymal stem cells alleviate rat diabetic nephropathy by suppressing CD103+ DCs-mediated CD8+ T cell responses. J Cell Mol Med. 2020;24:5817–5831. doi: 10.1111/jcmm.15250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Haddad R, Saldanha-Araujo F. Mechanisms of T-cell immunosuppression by mesenchymal stromal cells: What do we know so far? Biomed Res Int. 2014;2014:216806. doi: 10.1155/2014/216806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rozenberg A, Rezk A, Boivin MN, Darlington PJ, Nyirenda M, Li R, Jalili F, Winer R, Artsy EA, Uccelli A, et al. Human mesenchymal stem cells impact Th17 and Th1 responses through a prostaglandin E2 and myeloid-dependent mechanism. Stem Cells Transl Med. 2016;5:1506–1514. doi: 10.5966/sctm.2015-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khare D, Or R, Resnick I, Barkatz C, Almogi-Hazan O, Avni B. Mesenchymal stromal cell-derived exosomes affect mRNA expression and function of B-lymphocytes. Front Immunol. 2018;9:3053. doi: 10.3389/fimmu.2018.03053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V, Uccelli A. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 78.Rezaei Kahmini F, Shahgaldi S, Moazzeni SM. Mesenchymal stem cells alter the frequency and cytokine profile of natural killer cells in abortion-prone mice. J Cell Physiol. 2020;235:7214–7223. doi: 10.1002/jcp.29620. [DOI] [PubMed] [Google Scholar]
- 79.Xu LL, Fu HX, Zhang JM, Feng FE, Wang QM, Zhu XL, Xue J, Wang CC, Chen Q, Liu X, et al. Impaired function of bone marrow mesenchymal stem cells from immune thrombocytopenia patients in inducing regulatory dendritic cell differentiation through the Notch-1/Jagged-1 signaling pathway. Stem Cells Dev. 2017;26:1648–1661. doi: 10.1089/scd.2017.0078. [DOI] [PubMed] [Google Scholar]
- 80.Liu Q, Zheng H, Chen X, Peng Y, Huang W, Li X, Li G, Xia W, Sun Q, Xiang AP. Human mesenchymal stromal cells enhance the immunomodulatory function of CD8(+)CD28(−) regulatory T cells. Cell Mol Immunol. 2015;12:708–718. doi: 10.1038/cmi.2014.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.El Omar R, Xiong Y, Dostert G, Louis H, Gentils M, Menu P, Stoltz JF, Velot E, Decot V. Immunomodulation of endothelial differentiated mesenchymal stromal cells: Impact on T and NK cells. Immunol Cell Biol. 2016;94:342–356. doi: 10.1038/icb.2015.94. [DOI] [PubMed] [Google Scholar]
- 82.Cho KA, Lee JK, Kim YH, Park M, Woo SY, Ryu KH. Mesenchymal stem cells ameliorate B-cell-mediated immune responses and increase IL-10-expressing regulatory B cells in an EBI3-dependent manner. Cell Mol Immunol. 2017;14:895–908. doi: 10.1038/cmi.2016.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu X, Qu X, Chen Y, Liao L, Cheng K, Shao C, Zenke M, Keating A, Zhao RC. Mesenchymal stem/stromal cells induce the generation of novel IL-10-dependent regulatory dendritic cells by SOCS3 activation. J Immunol. 2012;189:1182–1192. doi: 10.4049/jimmunol.1102996. [DOI] [PubMed] [Google Scholar]
- 84.Ahmed AU, Rolle CE, Tyler MA, Han Y, Sengupta S, Wainwright DA, Balyasnikova IV, Ulasov IV, Lesniak MS. Bone marrow mesenchymal stem cells loaded with an oncolytic adenovirus suppress the anti-adenoviral immune response in the cotton rat model. Mol Ther. 2010;18:1846–1856. doi: 10.1038/mt.2010.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Atiya H, Frisbie L, Pressimone C, Coffman L. Mesenchymal stem cells in the tumor microenvironment. Adv Exp Med Biol. 2020;1234:31–42. doi: 10.1007/978-3-030-37184-5_3. [DOI] [PubMed] [Google Scholar]
- 86.Cai C, Hou L, Zhang J, Zhao D, Wang Z, Hu H, He J, Guan W, Ma Y. The inhibitory effect of mesenchymal stem cells with rAd-NK4 on liver cancer. Appl Biochem Biotechnol. 2017;183:444–459. doi: 10.1007/s12010-017-2456-x. [DOI] [PubMed] [Google Scholar]
- 87.Fathi E, Sanaat Z, Farahzadi R. Mesenchymal stem cells in acute myeloid leukemia: A focus on mechanisms involved and therapeutic concepts. Blood Res. 2019;54:165–174. doi: 10.5045/br.2019.54.3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.El-Khadragy MF, Nabil HM, Hassan BN, Tohamy AA, Waaer HF, Yehia HM, Alharbi AM, Moneim AEA. Bone marrow cell therapy on 1,2-Dimethylhydrazine (DMH)-induced colon cancer in rats. Cell Physiol Biochem. 2018;45:1072–1083. doi: 10.1159/000487349. [DOI] [PubMed] [Google Scholar]
- 89.Morales-Molina A, Gambera S, Cejalvo T, Moreno R, Rodriguez-Milla MA, Perise-Barrios AJ, Garcia-Castro J. Antitumor virotherapy using syngeneic or allogeneic mesenchymal stem cell carriers induces systemic immune response and intratumoral leukocyte infiltration in mice. Cancer Immunol Immunother. 2018;67:1589–1602. doi: 10.1007/s00262-018-2220-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ruano D, Lopez-Martin JA, Moreno L, Lassaletta A, Bautista F, Andion M, Hernandez C, Gonzalez-Murillo A, Melen G, Alemany R, et al. First-in-human, first-in-child trial of autologous MSCs carrying the oncolytic virus Icovir-5 in patients with advanced tumors. Mol Ther. 2020;28:1033–1042. doi: 10.1016/j.ymthe.2020.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rincon E, Cejalvo T, Kanojia D, Alfranca A, Rodriguez-Milla MA, Gil Hoyos RA, Han Y, Zhang L, Alemany R, Lesniak MS, García-Castro J. Mesenchymal stem cell carriers enhance antitumor efficacy of oncolytic adenoviruses in an immunocompetent mouse model. Oncotarget. 2017;8:45415–45431. doi: 10.18632/oncotarget.17557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Banijamali RS, Soleimanjahi H, Soudi S, Karimi H, Abdoli A, Seyed Khorrami SM, Zandi K. Kinetics of oncolytic reovirus T3D replication and growth pattern in mesenchymal stem cells. Cell J. 2020;22:283–292. doi: 10.22074/cellj.2020.6686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Keshavarz M, Ebrahimzadeh MS, Miri SM, Dianat-Moghadam H, Ghorbanhosseini SS, Mohebbi SR, Keyvani H, Ghaemi A. Oncolytic newcastle disease virus delivered by mesenchymal stem cells-engineered system enhances the therapeutic effects altering tumor microenvironment. Virol J. 2020;17:64. doi: 10.1186/s12985-020-01326-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hai C, Jin YM, Jin WB, Han ZZ, Cui MN, Piao XZ, Shen XH, Zhang SN, Sun HH. Application of mesenchymal stem cells as a vehicle to deliver replication-competent adenovirus for treating malignant glioma. Chin J Cancer. 2012;31:233–240. doi: 10.5732/cjc.011.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Du W, Seah I, Bougazzoul O, Choi G, Meeth K, Bosenberg MW, Wakimoto H, Fisher D, Shah K. Stem cell-released oncolytic herpes simplex virus has therapeutic efficacy in brain metastatic melanomas. Proc Natl Acad Sci USA. 2017;114:E6157–E6165. doi: 10.1073/pnas.1700363114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ong HT, Federspiel MJ, Guo CM, Ooi LL, Russell SJ, Peng KW, Hui KM. Systemically delivered measles virus-infected mesenchymal stem cells can evade host immunity to inhibit liver cancer growth. J Hepatol. 2013;59:999–1006. doi: 10.1016/j.jhep.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Castleton A, Dey A, Beaton B, Patel B, Aucher A, Davis DM, Fielding AK. Human mesenchymal stromal cells deliver systemic oncolytic measles virus to treat acute lymphoblastic leukemia in the presence of humoral immunity. Blood. 2014;123:1327–1335. doi: 10.1182/blood-2013-09-528851. [DOI] [PubMed] [Google Scholar]
- 98.Hammer K, Kazcorowski A, Liu L, Behr M, Schemmer P, Herr I, Nettelbeck DM. Engineered adenoviruses combine enhanced oncolysis with improved virus production by mesenchymal stromal carrier cells. Int J Cancer. 2015;137:978–990. doi: 10.1002/ijc.29442. [DOI] [PubMed] [Google Scholar]
- 99.Kazimirsky G, Jiang W, Slavin S, Ziv-Av A, Brodie C. Mesenchymal stem cells enhance the oncolytic effect of newcastle disease virus in glioma cells and glioma stem cells via the secretion of TRAIL. Stem Cell Res Ther. 2016;7:149. doi: 10.1186/s13287-016-0414-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Melen GJ, Franco-Luzon L, Ruano D, Gonzalez-Murillo A, Alfranca A, Casco F, Lassaletta A, Alonso M, Madero L, Alemany R, et al. Influence of carrier cells on the clinical outcome of children with neuroblastoma treated with high dose of oncolytic adenovirus delivered in mesenchymal stem cells. Cancer Lett. 2016;371:161–170. doi: 10.1016/j.canlet.2015.11.036. [DOI] [PubMed] [Google Scholar]
- 101.Leoni V, Gatta V, Palladini A, Nicoletti G, Ranieri D, Dall'Ora M, Grosso V, Rossi M, Alviano F, Bonsi L, et al. Systemic delivery of HER2-retargeted oncolytic-HSV by mesenchymal stromal cells protects from lung and brain metastases. Oncotarget. 2015;6:34774–34787. doi: 10.18632/oncotarget.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hoyos V, Del Bufalo F, Yagyu S, Ando M, Dotti G, Suzuki M, Bouchier-Hayes L, Alemany R, Brenner MK. Mesenchymal stromal cells for linked delivery of oncolytic and apoptotic adenoviruses to non-small-cell lung cancers. Mol Ther. 2015;23:1497–1506. doi: 10.1038/mt.2015.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Franco-Luzon L, Gonzalez-Murillo A, Alcantara-Sanchez C, Garcia-Garcia L, Tabasi M, Huertas AL, Chesler L, Ramirez M. Systemic oncolytic adenovirus delivered in mesenchymal carrier cells modulate tumor infiltrating immune cells and tumor microenvironment in mice with neuroblastoma. Oncotarget. 2020;11:347–361. doi: 10.18632/oncotarget.27401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Morales-Molina A, Rodriguez-Milla MA, Gimenez-Sanchez A, Perise-Barrios AJ, Garcia-Castro J. Cellular virotherapy increases tumor-infiltrating lymphocytes (TIL) and decreases their PD-1+ subsets in mouse immunocompetent models. Cancers (Basel) 2020;12:1920. doi: 10.3390/cancers12071920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mader EK, Maeyama Y, Lin Y, Butler GW, Russell HM, Galanis E, Russell SJ, Dietz AB, Peng KW. Mesenchymal stem cell carriers protect oncolytic measles viruses from antibody neutralization in an orthotopic ovarian cancer therapy model. Clin Cancer Res. 2009;15:7246–7255. doi: 10.1158/1078-0432.CCR-09-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hakkarainen T, Sarkioja M, Lehenkari P, Miettinen S, Ylikomi T, Suuronen R, Desmond RA, Kanerva A, Hemminki A. Human mesenchymal stem cells lack tumor tropism but enhance the antitumor activity of oncolytic adenoviruses in orthotopic lung and breast tumors. Hum Gene Ther. 2007;18:627–641. doi: 10.1089/hum.2007.034. [DOI] [PubMed] [Google Scholar]
- 107.Ocansey DKW, Pei B, Yan Y, Qian H, Zhang X, Xu W, Mao F. Improved therapeutics of modified mesenchymal stem cells: An update. J Transl Med. 2020;18:42. doi: 10.1186/s12967-020-02234-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Najafi M, Goradel NH, Farhood B, Salehi E, Solhjoo S, Toolee H, Kharazinejad E, Mortezaee K. Tumor microenvironment: Interactions and therapy. J Cell Physiol. 2019;234:5700–5721. doi: 10.1002/jcp.27425. [DOI] [PubMed] [Google Scholar]
- 109.Oh CM, Chon HJ, Kim C. Combination immunotherapy using oncolytic virus for the treatment of advanced solid tumors. Int J Mol Sci. 2020;21:7743. doi: 10.3390/ijms21207743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sostoa J, Dutoit V, Migliorini D. Oncolytic viruses as a platform for the treatment of malignant brain tumors. Int J Mol Sci. 2020;21:7449. doi: 10.3390/ijms21207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sivanandam V, LaRocca CJ, Chen NG, Fong Y, Warner SG. Oncolytic viruses and immune checkpoint inhibition: The best of both worlds. Mol Ther Oncolytics. 2019;13:93–106. doi: 10.1016/j.omto.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Heinio C, Havunen R, Santos J, de Lint K, Cervera-Carrascon V, Kanerva A, Hemminki A. TNFα and IL2 encoding oncolytic adenovirus activates pathogen and danger-associated immunological signaling. Cells. 2020;9:798. doi: 10.3390/cells9040798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ribas A, Dummer R, Puzanov I, VanderWalde A, Andtbacka RHI, Michielin O, Olszanski AJ, Malvehy J, Cebon J, Fernandez E, et al. Oncolytic virotherapy promotes intratumoral T cell infiltration and improves Anti-PD-1 immunotherapy. Cell. 2018;174:1031–1032. doi: 10.1016/j.cell.2018.07.035. [DOI] [PubMed] [Google Scholar]
- 114.Sun L, Funchain P, Song JM, Rayman P, Tannenbaum C, Ko J, McNamara M, Marcela Diaz-Montero C, Gastman B. Talimogene laherparepvec combined with anti-PD-1 based immunotherapy for unresectable stage III–IV melanoma: A case series. J Immunother Cancer. 2018;6:36. doi: 10.1186/s40425-018-0337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.