Highlights
-
•
Peptide hormone GIP shows an extremely short half-life of 90 s in mice.
-
•
Administration of GIP yields a very low ratio of intact:total peptide.
-
•
Co-administration with DPP-4 inhibitors increases the half-life of GIP to 4 min.
-
•
We highly recommend DPP-4 inhibition in future experiments utilizing exogenous GIP.
Abbreviations: GIP, glucose-dependent insulinotropic polypeptide; GIPR, glucose-dependent insulinotropic polypeptide receptor DPP-4, dipeptidyl peptidase-4; PC1/3, prohormone convertase 1/3; RIA, radioimmunoassays; ELISA, enzyme-linked immunosorbent assay; GPCR, G protein-coupled receptor; IV, intravenous; IP, intraperitoneal; SC, subcutaneous
Keywords: GIP, DDP-4, Peptide degradation, GIP ELISA, Pharmacokinetics
Abstract
Like other peptide hormones, glucose-dependent insulinotropic polypeptide (GIP) is rapidly cleared from the circulation. Dipeptidyl peptidase-4 (DPP-4) is known to be involved. Information on the overall pharmacokinetics of GIP in rodents is, however, lacking. We investigated the pharmacokinetics of exogenous GIP after intravenous, subcutaneous and intraperitoneal injection with and without DPP-4 inhibition in conscious female C57Bl/6 mice. Secondly, we compared total and intact GIP levels measured by an in-house RIA and commercially available ELISA kits to determine the suitability of these methods for in vivo and in vitro measurements. GIP half-life following intravenous injection amounted to 93 ± 2 s, which was extended to 5 ± 0.6 min by inhibition of DPP-4. Intact GIP levels following subcutaneous and intraperitoneal GIP administration were approximately 15 % of total GIP. The area under the curve of intact GIP (GIP exposure) following GIP injection was significantly increased by DPP-4 inhibition, whereas total GIP levels remained unchanged. We found significant variation between measurements of total, but not intact GIP performed with our in-house RIA and ELISAs in samples obtained after in vivo administration of GIP. Different preanalytical sample preparation (EDTA plasma, heparin plasma, assay buffer and PBS) significantly influenced results for all ELISA kits used. Thus, in experiments involving exogenous GIP(1–42) administration in mice, it is important to consider that this will result in a very low ratio of intact:total peptide but co-administration of a DPP-4 inhibitor greatly elevates this ratio. Furthermore, for comparison of GIP levels, it is essential to maintain uniformity concerning assay methodology and sample preparation.
1. Introduction
Glucose-dependent insulinotropic polypeptide (GIP) is a 42-amino acid polypeptide hormone secreted by entero-endocrine K cells located in the proximal intestine [1,2]. GIP is generated from a precursor peptide, pro-GIP, through posttranslational cleavage by prohormone convertase 1/3 (PC1/3), resulting in the formation of the biologically active peptide GIP(1–42) [3]. Ingested nutrients represent the major stimulus for GIP release, and especially carbohydrates and fat effectively stimulate GIP release [4].
Upon release into the circulation, GIP binds to the GIP receptor (GIPR), a class B1 G protein-coupled receptor (GPCR) expressed in several tissues throughout the body (see below) [5,6]. Downstream signaling of this receptor is mainly mediated through binding of Gαs protein, resulting in activation of signaling cascades including increased concentrations of cyclic AMP (cAMP) [7]. Binding of the hormone to GIPRs expressed on β cells of the pancreas results in an enhanced but strictly glucose-dependent release of insulin from these cells. Because of its insulinotropic actions GIP(1–42) is classified as one of incretin hormones, responsible for the enhanced insulin-secretion upon oral glucose administration compared to glucose infused intravenously to reach similar plasma concentrations [8]. The GIPR is expressed in many other tissues, including white adipose tissue, the cardiovascular system, bone cells, central nervous system, the gut, lungs, leukocytes and spleen, where it may elicit multiple functions, most of which are not fully understood [5,9].
GIP(1–42) is rapidly degraded by the enzyme dipeptidyl peptidase-4 (DPP-4). This serine peptidase is expressed throughout the body both as a membrane-bound protein in vascular endothelium, in the liver, in gut and renal brush-border membranes and as a soluble protein in plasma [9,10]. DPP-4 cleaves the biologically active GIP(1–42) between the second and third amino acid at the N-terminus, resulting in the formation of GIP(3–42). This truncated peptide has no known biological activity [10], but may act as a very weak antagonist at the GIP receptor. The plasma half-life of GIP(1–42) in humans has been measured to approximately 7 min [11]. Half-life of GIP(1–42) in rodents is thought to be even shorter and in a study of anaesthetized rats, the plasma half-life was approximately 2 min [12]. More detailed information on GIP(1–42) in rodents, especially in mice, is still lacking.
Detailed knowledge on GIP(1–42) kinetics in mice is essential when setting up experiments involving exogenous GIP(1–42) administration in vivo as well as ex vivo and even more important for interpretation of data regarding concentrations of endogenous GIP and its various molecular forms, since a rapid elimination will limit the exposure of the hormones to the receptor. This study aimed to elucidate the metabolism of exogenously administered GIP(1–42) in conscious mice with and without the presence of a DPP-4 inhibitor. Furthermore, we aimed to investigate to what extent current commercially available ELISA-kits are capable of measuring both intact (GIP(1–42)) and total GIP (comprising GIP(1–42) + GIP(3–42) and possibly additional molecular forms) levels in mouse plasma.
2. Materials and methods
2.1. Mice
Animal experiments were performed with permission from the Danish Animal Experiments Inspectorate (license 2013−15-2934−00833) and the local ethical committee in accordance with the guidelines of Danish legislation governing animal experimentation (1987) and the National Institutes of Health (publication number 85−23). Female C57Bl/6Jrj mice (12 weeks old) were obtained from Janvier Labs (Saint Berthevin Cedex, France) and housed in groups of eight in individually ventilated cages with a 12 -h light cycle. The mice had ad libitum access to standard chow and water and were left to acclimatize for 1 week prior to the experimental day.
2.2. Peptide
Synthetic mouse GIP(1–42), was obtained from Caslo peptides (Lyngby, Denmark). Prior to delivery, the purity of the peptide was determined to be 98.03 %, and the correct molecular weight was ascertained by mass spectrometry. The peptide was dissolved in 0.9 % NaCl containing 0.1 % casein (solution from bovine milk – 5% in water, Sigma Aldrich, St Louis, USA) and stored at -20 °C.
2.3. Exogenous GIP injection in conscious mice
Mice (n = 6–9) were weighed and tail marked, and then fasted from 0800 h to 1300 h with access to water. At time t=-10 min mice received either 1) subcutaneous (SC) injection with 1.5 mg valine-pyrrolidide (Val-Pyr) (a gift from Novo Nordisk, Bagsværd, Denmark) in 50 u L 40 % 0.9 % NaCl + 60 % 0.04 mM phosphate buffer or 2) a SC injection with vehicle. At t = 0 min, the mice received 25 nmol/kg BW GIP through either intravenous (IV), SC or intraperitoneal (IP) injection. Blood samples (50 μL) were drawn from the retro-orbital plexus at t = 0, 1, 3, 5, 10 and 20 min using EDTA coated glass capillaries (Vitrex, Vasekær, Denmark), and immediately added to pre-chilled Eppendorf tubes (Eppendorf, Hamburg, Germany) containing 5 μL Val-Pyr (Novo Nordisk, Bagsværd, Denmark). The tubes were centrifuged (3500 g, 20 min, 4 °C) and plasma was transferred into Eppendorf tubes on dry ice and stored at -20 °C.
2.4. Radioimmunoassays
Total and intact GIP were measured by in-house radioimmunoassays (RIAs). Total GIP was measured using an antibody that targets the free C-terminal part of the hormone (code no 80,867), thereby detecting both intact GIP and N-terminally truncated forms of GIP [13]. Intact GIP immunoreactivity was determined using antiserum code no. 98,171, which reacts with the N-terminus of intact GIP [11]. Mouse GIP(1–42) was used for standard curves and prepared in 80 mM phosphate buffer containing 1% HSA, 10 mM EDTA (Titriplex, Merck Millipore, Burlington, MA, USA), 5% aprotinin (Trasylol, Nordic Group, Hoofddorp, The Netherlands) and 10μM Val-Pyr. Samples were carefully diluted to ensure that samples would fall within the standard range of the assays.
2.5. ELISA assays
Plasma samples were measured with the following ELISA kits: Millipore Rat/Mouse GIP (total) (EZRMGIP-55 K, RRID:AB_2801384, EMD Millipore, Burlington, MA, USA); Crystal Chem Mouse Active GIP (81511, Crystal Chem, Elk Grove Village, IL, USA); and Crystal Chem Mouse GIP ELISA Kit (81517, Crystal Chem, Elk Grove Village, IL, USA). Samples were carefully diluted in kit-specific assay buffer (per manufacturer’s instructions) and measured with the different ELISA kits on the same day to avoid excess freeze/thaw cycles. The manufacturer’s instructions were followed closely.
2.6. In vitro mouse GIP(1–42) measurements
Mouse GIP(1–42) was dissolved to reach concentrations of 0, 25 and 400 pM in PBS, mouse heparin-plasma (SMPH35−0050, Equitech Bio, Kerrville, TX, USA), mouse EDTA plasma and assay buffer provided by the three ELISA kits mentioned above, all containing 0.1 % casein. Mouse EDTA plasma was obtained from 5-hr fasted C57Bl/6Jrj using EDTA coated glass capillaries (Vitrex, Vasekær, Denmark). These samples were immediately measured with the three ELISA kits as per the manufacturer’s instructions.
2.7. Calculations and statistical analysis
Half-life (t½) of exogenously GIP(1–42) administered IV was calculated with the following formulas: k= (ln(Ct1)-ln(Ct2))/Δt and t½= ln(2)/k with Ct1 at t = 1 and Ct2 at t = 10 min. Apparent volume of distribution (Apparent Vd) was calculated using the following formulas: C0=Ct/e^(-k*t) and Apparent Vd= total dose/C0 and corrected for body weight. Clearance (CL) was calculated by the following formula: CL = k*Vd and corrected for body weight. Half-lives were determined from the concentration vs time curves after semi-logarithmical transformation. Results are presented as mean ± SE. Data were analyzed with 2-way ANOVA with Tukey’s multiple comparisons test and unpaired t-tests where appropriate. Statistical significance was accepted at P < 0.05.
3. Results
3.1. Pharmacokinetics of exogenous mouse GIP(1–42) in mice
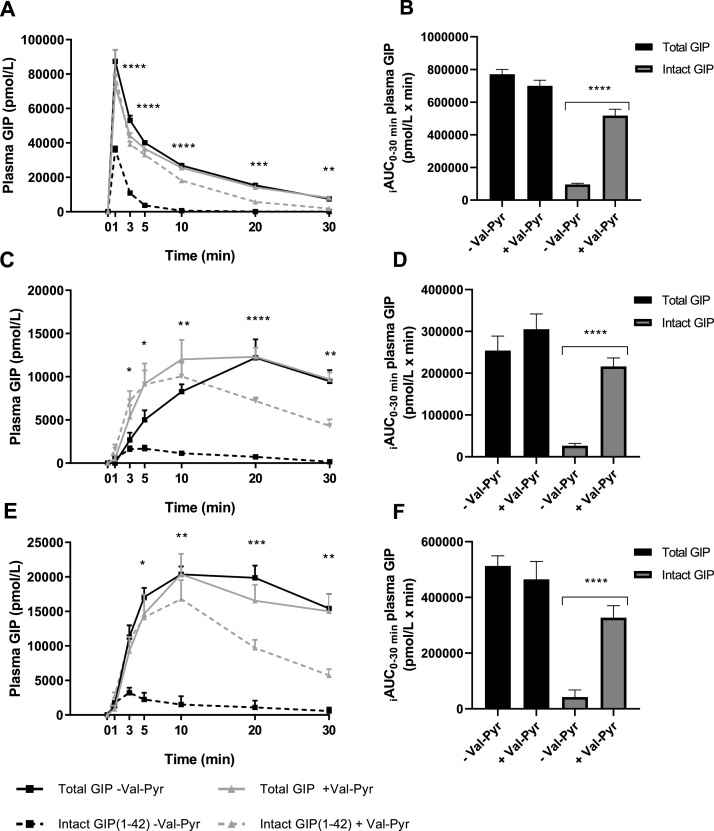
Fig. 1 show concentrations of total and intact GIP in plasma following IV (Fig. 1A), SC (Fig. 1C) and IP (Fig. 1E) injection of 25 nmol/kg BW mouse GIP(1–42) as measured by in-house RIAs. Co-administration of the DPP-4 inhibitor Val-Pyr did not significantly affect total GIP measurements after either type injection, as shown by a two-way ANOVA with Tukey’s multiple comparisons test (IV; P > 0.0629, SC; P > 0.3973, IP; P > 0.6726). It did, however, significantly elevate intact GIP concentrations between time points t = 3 and t = 30 min after IV and SC injection (P < 0.01 and P < 0.05, respectively) and between time points t = 5 and t = 30 following IP injection (P < 0.05). Exposure to intact GIP, as shown by the incremental area under the curve (iAUC), was highest following intravenous and lowest following subcutaneous administration of GIP(1–42). The apparent half-life of GIP(1–42) with and without co-administration of Val-Pyr was determined with data resulting from IV administration of GIP(1–42). We estimated the apparent half-life of intact GIP(1–42) to be 93 ± 2 s, which was extended to an apparent half-life of 5 ± 0.6 min by co-administration of Val-Pyr, a significant increase (P < 0.0001). Clearance, corrected for body weight, was reduced by Val-Pyr administration (placebo 207 ± 16 μL/min/g vs Val-Pyr co-administration 45 ± 4 16 μL/min/g, P < 0.0001). Apparent volume of distribution corrected for body weight was not altered by co-administration of Val-Pyr (placebo 0.34 ± 0.1 mL/g vs Val-Pyr co-administration 0.46 ± 0.03 mL/g, P = 0.104).
Fig. 1.
Co-administration of DPP-4 inhibitor Val-Pyr increased Intact GIP but not Total GIP plasma levels as measured with in-house radioimmunoassay. Measurements after (A), intravenous injection, (C), subcutaneous injection and (E), intraperitoneal injection of 25 nmol/kg BW exogenous mouse GIP (1-42) with and without Val-Pyr co-administration; *, statistical significance as calculated with two-way ANOVA with Tukey’s multiple comparisons test of Intact GIP(1-42) - Val-Pyr vs. Intact GIP(1-42) + Val-Pyr; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. iAUC data is shown in (B), intravenous GIP iAUC, (D), subcutaneous GIP iAUC and (F), intraperitoneal GIP iAUC; *, statistical significance as calculated with unpaired t-tests of Intact GIP(1-42) - Val-Pyr vs. Intact GIP(1-42) + Val-Pyr; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 Data are presented as mean ± SE, n = 6-9.
When comparing peaks of intact to total GIP concentrations, we find that after IV injection, intact GIP concentrations reached a mean of 41 % of total GIP concentrations without co-administration of Val-Pyr. This was increased to 110 % when mice were injected with Val-Pyr. Peaks in concentration of intact GIP following SC and IP injection without Val-Pyr co-administration were 14 % and 16 % of peak concentrations of total GIP, respectively. With co-administration of Val-Pyr, these values increased to 82 % for both administration routes, respectively. Furthermore, for all routes of administration, the iAUC for intact GIP measurements was significantly elevated upon co-administration of Val-Pyr (Fig. 1B; IV 5.4-fold increase, P < 0.0001, Fig. 1D; SC 8.1-fold increase, P < 0.0001, Fig. 1F; IP 7.8-fold increase, P < 0.0001). iAUC of intact GIP can be calculated as a percentage of iAUC of total GIP. This percentage was significantly increased for all administration routes with co-injection of Val-Pyr (IV placebo 13 ± 1% vs Val-Pyr co-administration 74 ± 3%, P < 0.0001; SC placebo 10 ± 1% vs Val-Pyr co-administration 72 ± 3%, P < 0.0001; IV placebo 9 ± 6% vs Val-Pyr co-administration 71 ± 3%, P < 0.0001).
3.2. Measurement of total and intact GIP with commercial ELISAs
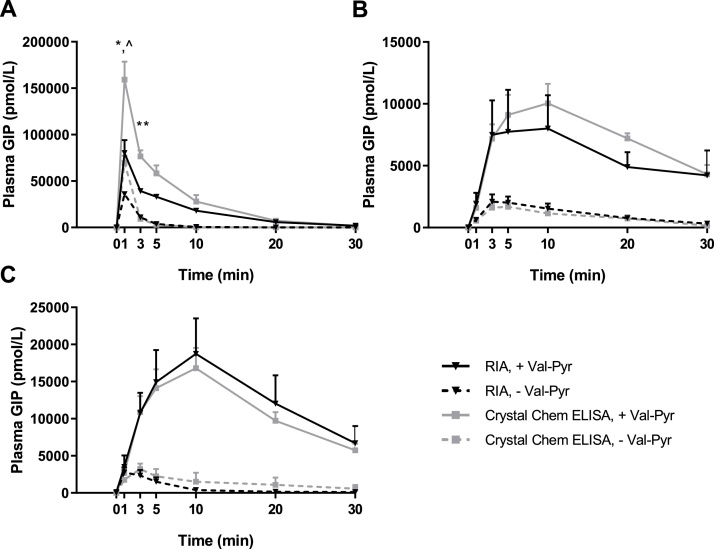
Fig. 2 shows intact GIP concentrations in the obtained plasma samples measured by in-house RIA compared to the commercially available ELISA kit from Crystal Chem (Crystal Chem Mouse Active GIP). Crystal Chem’s ELISA kit clearly measured higher intact GIP concentrations compared to our in-house RIA in samples of IV GIP injection obtained 1 and 3 min after injection (P < 0.05), whereas no significant differences were observed in samples of SC and IP GIP injection.
Fig. 2.
In-house radioimmunoassay for Intact GIP and Crystal Chem’s Intact GIP ELISA kit measured similar Intact GIP plasma concentrations following SC and IP, but not IV GIP administration. Samples were obtained following (A), intravenous, (B), subcutaneous and (C), intraperitoneal injection of 25 nmol/kg BW exogenous mouse GIP (1-42) with and without Val-Pyr co-administration; *, statistical significance of RIA, - Val-Pyr vs Crystal Chem Elisa, - Val-Pyr; *P < 0.05, **P < 0.01; ^, statistical significance of RIA, + Val-Pyr vs Crystal Chem ELISA, +Val-Pyr; ^P < 0.05 as calculated with 2-way ANOVA with Tukey’s multiple comparisons test. Data are presented as mean ± SE, n = 6-9.
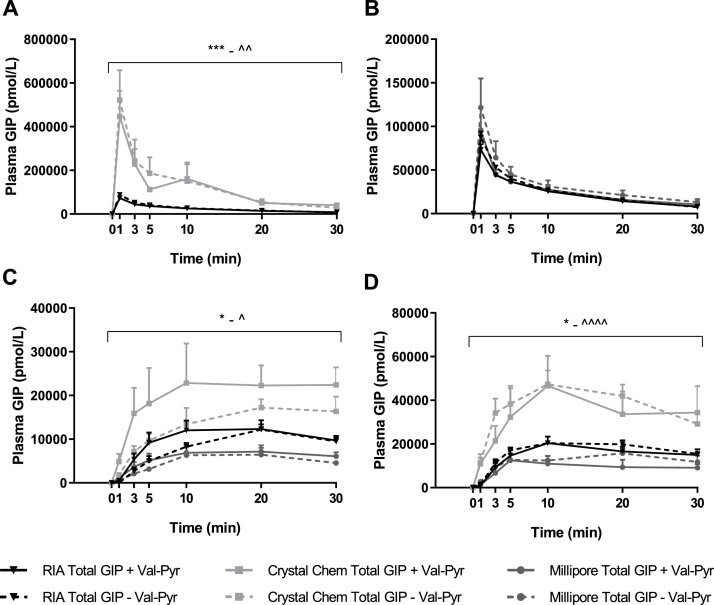
Fig. 3 shows total GIP concentrations in the obtained plasma samples measured by in-house RIA compared to the commercially available ELISA kits from Crystal Chem (Crystal Chem Mouse GIP ELISA Kit) and Millipore (Millipore Rat/Mouse GIP (total). Fig. 3A shows IV plasma samples measured by Crystal Chem’s total ELISA kit to have significantly higher concentrations of total GIP compared to concentrations obtained through RIA measurements(P < 0.01). Concentrations obtained with Millipore’s Total GIP ELISA kit showed no difference compared to concentrations obtained with RIA, as shown in Fig. 3B. Fig. 3C and 3D show that Crystal Chem’s ELISA measurements give higher concentrations of total GIP compared to RIA (SC; P < 0.05, IP; P < 0.05) whereas concentrations measured with Millipore’s ELISA are not significantly different to RIA.
Fig. 3.
Inconsistent measurements of Total plasma GIP concentrations in samples measured with in-house RIA, Crystal Chem's Total GIP ELISA kit and Millipores total GIP ELISA kit. Samples were obtained following (A and B), intravenous, (C), subcutaneous and (D), intraperitoneal injection of 25 nmol/kg BW exogenous mouse GIP (1-42) with and without Val-Pyr co-administration. (A) shows concentrations measured with RIA and Crystal Chems Total GIP ELISA kit, whereas (B) shows concentrations measured with RIA and Millipores Total GIP ELISA kit. *, statistical significance of RIA Total GIP, +Val-Pyr vs. Crystal Chem Total GIP, + Val-Pyr; *P < 0.05, ***P < 0.001; ^RIA Total GIP, - Val-Pyr vs. Crystal Chem Total GIP, - Val-Pyr; ^P < 0.05, ^^P < 0.01, ^^^^P < 0.0001 as calculated with 2-way ANOVA with Tukey’s multiple comparisons test. Data are presented as mean ± SE, n = 6-9.
3.3. In vitro mouse GIP(1–42) measurements
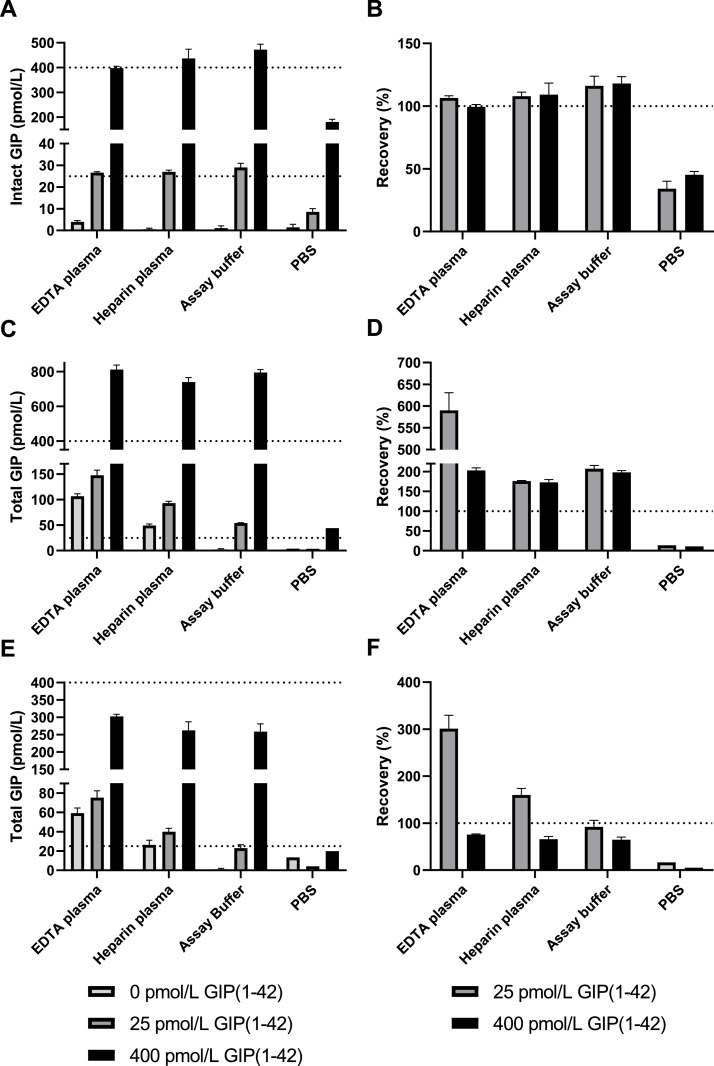
We tested accuracy of the three ELISA kits in EDTA plasma, Heparin plasma, ELISA kit assay buffer and PBS. Fig. 4 depicts both absolute measurements (Fig. 4A, C, E.) and recovery (%) (Fig. 4B, D, F). Crystal Chem’s Intact GIP ELISA was capable of accurately measuring GIP concentrations of 25 and 400 pM in both EDTA plasma, Heparin plasma and Assay buffer, with recovery rates of close to 100 %. It underestimated GIP concentrations when measuring GIP dissolved in PBS with a recovery rate of 40–50 %.
Fig. 4.
In vitro measurements of Intact and Total GIP levels show inconsistent results with varying assays and sample preparations. Measurements were performed with (A), Crystal Chem's Intact GIP ELISA kit, (C), Crystal Chem's Total GIP ELISAkit and (E), Millipores Total GIP ELISA kit. The different media contained either 0, 25 or 400 pmol/L mouse GIP(1-42). (B, D and F) show the recovery rates of these concentrations for the respective ELISA kits. Data are presented as mean ± SE, n = 4.
Total GIP concentrations were overestimated with Crystal Chem’s Total GIP ELISA kit (Fig. 4C, D). In EDTA plasma, a GIP concentration of just above 100 pM was measured in our baseline sample. In all media besides PBS, Total GIP concentrations were overestimated by 165 %–590 %.
Millipore’s Total GIP ELISA kit overestimated GIP concentrations in both EDTA and heparin plasma containing 0 and 25 pM GIP, but underestimated concentrations in those samples containing 400 pM GIP (Fig. 4E, F). Like the other ELISA kits, also this kit underestimated GIP concentrations when GIP was dissolved in PBS + 0.1 % casein.
4. Discussion
With this study, we show that in mice, the apparent half-life of exogenous mouse GIP(1–42) is approximately 90 s and that this increased significantly with co-administration of the DPP-4 inhibitor Val-Pyr. Previous studies have shown half-life of human GIP(1–42) in humans to be approximately 7 min [11], but this is the first study to show the dramatically shorter half-life of GIP(1–42) in conscious mice. Furthermore, it was shown that clearance rate was significantly reduced by Val-Pyr co-administration whilst the apparent volume of distribution was not altered. The volume of distribution was similar to what has previously been found in humans [14]. This study also provides insight into the pharmacokinetic curves of intact and total GIP concentrations following different routes of administration. Of the three routes of administration assessed, intravenous injection yields the highest exposure of intact GIP(1–42), followed by intraperitoneal injection and lastly subcutaneous injection. Furthermore, we show that both SC and IP injection of GIP(1–42) without co-administration of a DPP-4 inhibitor yields very low concentrations of the intact peptide (approximately 15 % of total GIP plasma concentrations). This is most likely due to rapid degradation of GIP(1–42) by DPP-4, hereby yielding GIP(3–42), which no longer possesses biological activity [15]. When planning studies utilizing exogenous GIP(1–42) administration in mice it must be realized that the exposure of intact GIP will be extremely brief. Our results also show that it may be possible to extend the exposure period markedly by co-administration of a DPP-4 inhibitor.
Intact GIP(1–42) levels were measured with our in-house RIA assay (98,171), which targets the N-terminus of the hormone. These results were compared to measurements performed with Crystal Chem’s Mouse Active (Intact) GIP ELISA kit. This kit specifically measures GIP(1–42) with no reported cross-reactivity to GIP(3–42). Our results show that even though GIP(1–42) concentrations are overestimated in IV samples when comparing the ELISA to our RIA, there was no difference in plasma GIP(1–42) concentrations following SC and IP GIP(1–42) injection. This was supported by our in vitro data, which showed that the ELISA kit accurately measured GIP levels of both 25 and 400 pM, which are concentrations at the low and high-end of the kit’s range. It is unclear why the kit seems to overestimate GIP levels in IV plasma, as measurements were performed after appropriate dilution. We will continue to explore this question in our laboratory.
Interestingly, the peak of intact GIP(1–42) concentrations after IV-injections with co-administration of Val-Pyr measured with our in-house RIA were slightly higher compared to the peak total GIP concentrations measured in the same samples. Most likely the antibody against the N-terminus pick up GIP fragments, for instance GIP 1–30 amide (see below) that cannot be measured by the C-terminal antibody, in addition to the intact molecule GIP [15].
Total GIP levels were compared after measurements with our in-house RIA assay (80,867) and commercially available sandwich ELISA kits. Our in-house RIA measures both intact GIP(1–42) and N-terminally truncated variants of GIP by using an antibody directed against the C-terminal of the peptide. Millipore’s Rat/Mouse GIP (total) ELISA kit reports to have a 100 % cross-reactivity with GIP(1–42) and GIP(3–42), although it is unclear where the antibodies used in this kit bind. Crystal Chem’s Mouse GIP ELISA kit claims a high specificity to GIP, but also in this case, it is not disclosed where on the peptide the antibodies used in this kit bind. When comparing to concentrations measured by RIA, Crystal Chem’s kit consistently overestimates total GIP plasma concentrations. This was confirmed with our in vitro data, where recovery rates of Total GIP measured in plasma and assay buffer spiked with GIP(1–42) exceeded 150 %. Millipore’s kit, on the other hand, measured total GIP concentrations similar to those measured with RIA in IV samples. However, concentrations of total GIP in SC and IP samples were underestimated compared to RIA measurements. In vitro data show that the kit underestimated total concentrations of GIP. Furthermore, we observed inconsistency in GIP concentrations measured between sample types (EDTA plasma, Heparin plasma, Assay buffer and PBS). Although we cannot say with absolute certainty which kit should be used to measure GIP concentrations, these data remind us that to compare measurements of GIP levels, uniformity should be maintained concerning assay and sample choice.
Throughout this study, we have focused on GIP(1–42) degradation by DPP-4. It has been suggested that rodents have higher DPP-4 activity compared to humans, which can, at least in part, explain the shorter half-life of GIP in rodents [12]. In addition, there are indications that GIP(1–42) may be degraded by other enzymes [16]. Neprilysin was proposed as a possible candidate [17]. This enzyme was demonstrated to enhance the degradation of glucagon-like peptide-1 in mice in a species-specific manner [18]. It was, however, shown that GIP is unlikely to be degraded by this enzyme [17]. Lack of accessibility to cleavage sites possibly renders it less susceptible to cleavage by neprilysin. Whether enzymes besides DPP-4 are of significant importance for GIP(1–42) degradation remains to be clarified. We found that, following DPP-4 inhibition, GIP(1–42) clearance was 45 μL/min/g bodyweight, which is higher than the glomerular filtration rate of approximately 10 μL/min/g bodyweight found in C57Bl6 mice in a separate study [19]. Renal clearance therefore cannot explain most of the clearance and this suggests that other enzymes may play a role in the rapid GIP degradation in mice. Enzymes specifically expressed in the mouse could, combined with the enhanced DPP-4 activity observed in this species [12], contribute to the shorter half-life of this peptide in mice compared to humans.
As mentioned in the introduction, GIP(1–42) is produced in the K cells from a proGIP sequence after post-translational processing with PC1/3. A subset of K cells has been found that lacks PC1/3 but expresses PC2. Post-translation processing of pro-GIP by this enzyme yields GIP(1–30), which is then amidated to form GIP(1–30NH2) [20]. GIP(1–30NH2) has been found to circulate in low concentrations in humans and to activate the GIPR in a manner similar to GIP (1–42) [20,21]. This peptide is degraded by DPP-4 to form GIP(3−30NH2), which functions as a competitive antagonist at the GIPR [6,21]. Assays that measure total GIP are often directed at the C-terminus and may therefore not pick up these truncated variations of GIP unless this assay is directed towards the central region of GIP [9,22]. One should therefore show great care when selecting an assay to measure either total or intact GIP levels, depending on the research question.
A limitation of this study is that pharmacological levels of GIP(1–42) were used to assess the pharmacokinetics of the intact peptide. Rates of degradation may differ between physiological and pharmacological plasma levels of intact GIP(1–42), although this is rarely the case. Half-life of physiological levels of intact GIP(1–42) would be difficult to measure, as the high rate of degradation would rapidly result in insufficient levels of peptide to be detected by the various detection assays.
To our knowledge, this is the first study that has investigated the pharmacokinetics of exogenously administered GIP(1–42) in mice. We show that after IV administration, the half-life of the hormone is approximately 90 s and that this can be extended significantly by co-administration of a DPP-4 inhibitor. The variation seen between different methods of measuring total and intact GIP levels in both in vivo and in vitro settings demonstrates the importance of consistency of assay and sample choice.
Data availability
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
CRediT authorship contribution statement
Geke Aline Boer: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing - original draft, Visualization, Funding acquisition. Bolette Hartmann: Conceptualization, Methodology, Resources, Writing - review & editing, Supervision, Project administration. Jens Juul Holst: Conceptualization, Methodology, Validation, Resources, Writing - review & editing, Supervision, Funding acquisition.
Declaration of Competing Interest
All authors declare to have no conflict of interest associated with their contribution to this manuscript.
Acknowledgements
We would like to thank Tabatha Emilia de A Constantini and Lene Brus Albæk for their excellent technical assistance. This work was supported by a grant from AP Møller Fonden (17-L-0366) to GAB, a grant from the NovoNordisk Foundation (NNF15OC0016574) and an ERC Advanced Grant (695069) to JJH.
Contributor Information
Geke Aline Boer, Email: alineb@sund.ku.dk.
Bolette Hartmann, Email: bhartmann@sund.ku.dk.
Jens Juul Holst, Email: jjholst@sund.ku.dk.
References
- 1.Jörnvall H., Carlquist M., Kwauk S., Otte S.C., McIntosh C.H.S., Brown J.C., Mutt V. Amino acid sequence and heterogeneity of gastric inhibitory polypeptide (GIP) FEBS Lett. 1981;123(2):205–210. doi: 10.1016/0014-5793(81)80288-8. [DOI] [PubMed] [Google Scholar]
- 2.Buffa R., Polak J.M., Pearse A.G.E., Solcia E., Grimelius L., Capella C. Identification of the intestinal cell storing gastric inhibitory peptide. Histochemistry. 1975;43(3):249–255. doi: 10.1007/BF00499706. [DOI] [PubMed] [Google Scholar]
- 3.Ugleholdt R., Poulsen M.L.H., Holst P.J., Irminger J.C., Orskov C., Pedersen J., Rosenkilde M.M., Zhu X., Steiner D.F., Holst J.J. Prohormone convertase 1/3 is essential for processing of the glucose-dependent insulinotropic polypeptide precursor. J. Biol. Chem. 2006;281(16):11050–11057. doi: 10.1074/jbc.M601203200. [DOI] [PubMed] [Google Scholar]
- 4.Knapper J.M.E., Heath A., Fletcher J.M., Morgan L.M., Marks V. GIP and GLP-1(7–36)amide secretion in response to intraduodenal infusions of nutrients in pigs, Comparative Biochemistry and Physiology Part C: pharmacology. Toxicology and Endocrinology. 1995;111(3):445–450. doi: 10.1016/0742-8413(95)00046-1. [DOI] [PubMed] [Google Scholar]
- 5.Usdin T.B., Mezey E., Button D.C., Brownstein M.J., Bonner T.I. Gastric inhibitory polypeptide receptor, a member of the secretin-vasoactive intestinal peptide receptor family, is widely distributed in peripheral organs and the brain. Endocrinology. 1993;133(6):2861–2870. doi: 10.1210/endo.133.6.8243312. [DOI] [PubMed] [Google Scholar]
- 6.Gabe M.B.N., van der Velden W.J.C., Smit F.X., Gasbjerg L.S., Rosenkilde M.M. Molecular interactions of full-length and truncated GIP peptides with the GIP receptor – a comprehensive review. Peptides. 2020;125 doi: 10.1016/j.peptides.2019.170224. [DOI] [PubMed] [Google Scholar]
- 7.Gabe M.B.N., Sparre-Ulrich A.H., Pedersen M.F., Gasbjerg L.S., Inoue A., Bräuner-Osborne H., Hartmann B., Rosenkilde M.M. Human GIP(3-30)NH(2) inhibits G protein-dependent as well as G protein-independent signaling and is selective for the GIP receptor with high-affinity binding to primate but not rodent GIP receptors. Biochem. Pharmacol. 2018;150:97–107. doi: 10.1016/j.bcp.2018.01.040. [DOI] [PubMed] [Google Scholar]
- 8.McIntyre N., Holdsworth C.D., Turner D.S. NEW INTERPRETATION OF ORAL GLUCOSE TOLERANCE. Lancet (London, England) 1964;2(7349):20–21. doi: 10.1016/s0140-6736(64)90011-x. [DOI] [PubMed] [Google Scholar]
- 9.Deacon C.F. Metabolism of GIP and the contribution of GIP to the glucose-lowering properties of DPP-4 inhibitors. Peptides. 2020;125 doi: 10.1016/j.peptides.2019.170196. [DOI] [PubMed] [Google Scholar]
- 10.Mentlein R., Gallwitz B., Schmidt W.E. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7-36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur. J. Biochem. 1993;214(3):829–835. doi: 10.1111/j.1432-1033.1993.tb17986.x. [DOI] [PubMed] [Google Scholar]
- 11.Deacon C.F., Nauck M.A., Meier J., Hücking K., Holst J.J. Degradation of endogenous and exogenous gastric inhibitory polypeptide in healthy and in type 2 diabetic subjects as revealed using a new assay for the intact peptide. J. Clin. Endocrinol. Metab. 2000;85(10):3575–3581. doi: 10.1210/jcem.85.10.6855. [DOI] [PubMed] [Google Scholar]
- 12.Kieffer T.J., McIntosh C.H., Pederson R.A. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology. 1995;136(8):3585–3596. doi: 10.1210/endo.136.8.7628397. [DOI] [PubMed] [Google Scholar]
- 13.Lindgren O., Carr R.D., Deacon C.F., Holst J.J., Pacini G., Mari A., Ahreń B. Incretin hormone and insulin responses to oral versus intravenous lipid administration in humans. J. Clin. Endocrinol. Metab. 2011;96(8):2519–2524. doi: 10.1210/jc.2011-0266. [DOI] [PubMed] [Google Scholar]
- 14.Idorn T., Knop F.K., Jørgensen M.B., Christensen M., Holst J.J., Hornum M., Feldt-Rasmussen B. Elimination and Degradation of Glucagon-like Peptide-1 and Glucose-Dependent Insulinotropic Polypeptide in Patients with End-Stage Renal Disease. J. Clin. Endocrinol. Metab. 2014;99(7):2457–2466. doi: 10.1210/jc.2013-3809. [DOI] [PubMed] [Google Scholar]
- 15.Deacon C.F., Plamboeck A., Rosenkilde M.M., Heer Jd., Holst J.J. 2006. GIP-(3–42) Does Not Antagonize Insulinotropic Effects of GIP at Physiological Concentrations; pp. E468–E475. 291(3) [DOI] [PubMed] [Google Scholar]
- 16.Hupe-Sodmann K., Göke R., Göke B., Thole H.H., Zimmermann B., Voigt K., McGregor G.P. Endoproteolysis of glucagon-like peptide (GLP)-1 (7-36) amide by ectopeptidases in RINm5F cells. Peptides. 1997;18(5):625–632. doi: 10.1016/s0196-9781(97)00123-x. [DOI] [PubMed] [Google Scholar]
- 17.Hupe-Sodmann K., McGregor G.P., Bridenbaugh R., Goke R., Goke B., Thole H., Zimmermann B., Voigt K. Characterisation of the processing by human neutral endopeptidase 24.11 of GLP-1(7-36) amide and comparison of substrate specificity of the enzyme for other glucagon-like peptides. Regul. Pept. 1995;58(3):149–156. doi: 10.1016/0167-0115(95)00063-h. [DOI] [PubMed] [Google Scholar]
- 18.Windeløv J.A., Wewer Albrechtsen N.J., Kuhre R.E., Jepsen S.L., Hornburg D., Pedersen J., Jensen E.P., Galsgaard K.D., Winther-Sørensen M., Ørgaard A., Deacon C.F., Mann M., Kissow H., Hartmann B., Holst J.J. Why is it so difficult to measure glucagon-like peptide-1 in a mouse? Diabetologia. 2017;60(10):2066–2075. doi: 10.1007/s00125-017-4347-7. [DOI] [PubMed] [Google Scholar]
- 19.Schock-Kusch D., Geraci S., Ermeling E., Shulhevich Y., Sticht C., Hesser J., Stsepankou D., Neudecker S., Pill J., Schmitt R., Melk A. Reliability of Transcutaneous Measurement of Renal Function in Various Strains of Conscious Mice. PLoS One. 2013;8(8) doi: 10.1371/journal.pone.0071519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujita Y., Asadi A., Yang G.K., Kwok Y.N., Kieffer T.J. Differential processing of pro-glucose-dependent insulinotropic polypeptide in gut, American journal of physiology. Gastrointestinal and liver physiology. 2010;298(5):G608–14. doi: 10.1152/ajpgi.00024.2010. [DOI] [PubMed] [Google Scholar]
- 21.Hansen L.S., Sparre-Ulrich A.H., Christensen M., Knop F.K., Hartmann B., Holst J.J., Rosenkilde M.M. N-terminally and C-terminally truncated forms of glucose-dependent insulinotropic polypeptide are high-affinity competitive antagonists of the human GIP receptor. Br. J. Pharmacol. 2016;173(5):826–838. doi: 10.1111/bph.13384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yanagimachi T., Fujita Y., Takeda Y., Honjo J., Sakagami H., Kitsunai H., Takiyama Y., Abiko A., Makino Y., Kieffer T.J., Haneda M. Dipeptidyl peptidase-4 inhibitor treatment induces a greater increase in plasma levels of bioactive GIP than GLP-1 in non-diabetic subjects. Mol. Metab. 2017;6(2):226–231. doi: 10.1016/j.molmet.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.