Abstract
BACKGROUND AND AIMS:
A high proportion of patients develop chronic kidney disease (CKD) after liver transplantation (LT). We aimed to develop clinical/protein models to predict future glomerular filtration rate (GFR) deterioration in this population.
APPROACH AND RESULTS:
In independent multicenter discovery (CTOT14) and single-center validation (BUMC) cohorts, we analyzed kidney injury proteins in serum/plasma samples at month 3 after LT in recipients with preserved GFR who demonstrated subsequent GFR deterioration versus preservation by year 1 and year 5 in the BUMC cohort. In CTOT14, we also examined correlations between serial protein levels and GFR over the first year. A month 3 predictive model was constructed from clinical and protein level variables using the CTOT14 cohort (n = 60). Levels of β−2 microglobulin and CD40 antigen and presence of hepatitis C virus (HCV) infection predicted early (year 1) GFR deterioration (area under the curve [AUC], 0.814). We observed excellent validation of this model (AUC, 0.801) in the BUMC cohort (n = 50) who had both early and late (year 5) GFR deterioration. At an optimal threshold, the model had the following performance characteristics in CTOT14 and BUMC, respectively: accuracy (0.75, 0.8), sensitivity (0.71, 0.67), specificity (0.78, 0.88), positive predictive value (0.74, 0.75), and negative predictive value (0.76, 0.82). In the serial CTOT14 analysis, several proteins, including β−2 microglobulin and CD40, correlated with GFR changes over the first year.
CONCLUSIONS:
We have validated a clinical/protein model (PRESERVE) that early after LT can predict future renal deterioration versus preservation with high accuracy. This model may help select recipients at higher risk for subsequent CKD for early, proactive renal sparing strategies.
Chronic kidney disease (CKD) is a significant clinical problem following liver transplantation (LT). A majority of LT recipients have stage 3 CKD by 2 years after LT and ~20% progress to ≥stage 4 within 5 years.(1−4) Moreover, recipients with renal dysfunction after LT have reduced survival, particularly if renal replacement therapy is required.(1,3) The etiology of CKD after LT is multifactorial and includes recipient factors, perioperative kidney injury, and calcineurin-inhibitor (CNI)-induced nephrotoxicity.(3,5,6)
Serum creatinine and estimated (eGFR) glomerular filtration rate (GFR) lack sensitivity and specificity and represent late manifestations of significant kidney injury, yet these remain the primary measures currently used to monitor renal function. Measured GFR (mGFR) is more precise than eGFR measures, but is not commonly performed or used to serially monitor renal function. Several studies have suggested that individual biomarkers may be useful in predicting intraoperative renal injury or in improving eGFR accuracy after LT.(7–12) For instance, cystatin C–based GFR equations are somewhat superior in estimating mGFR compared to creatinine-based estimations.(4,13–15)
Even with advances in estimating GFR, most previous investigations have focused on biomarkers that correlate with the presence of already established CKD. We previously performed such an initial exploratory study comparing a large unbiased multianalyte panel (Rules Based Medicine [RBM] DiscoveryMAP; n = 189 proteins; discovery cohort) and a renal injury panel (RBM KidneyMAP; n = 16 proteins; validation cohort) between LT recipients with and without late CKD.(10) Of these large sets of plasma proteins, only 10 had levels that were significantly higher in LT recipients with CKD, most being established renal injury proteins such as cystatin C, α−1 microglobulin, β−2 microglobulin, trefoil factor-3, and CD40. However, this profile merely demonstrates proteins present at CKD diagnosis. What would have more tangible clinical impact would be to determine whether these renal injury proteins were present before the occurrence of GFR deterioration and irreversible CKD. This could allow preclinical identification of renal injury such that early interventions (e.g., CNI withdrawal) could be instituted to avoid future CKD. As such, we performed an interim exploratory study comparing the same large unbiased discovery proteins in LT recipients with normal GFR early post-transplant who either had future renal deterioration versus continued preservation by year 5.(16) Of the large panel spanning multiple pathways, only six proteins (cystatin C, α−1 microglobulin, β−2 microglobulin, macrophage colony stimulating factor 1, CD40 antigen, and erythropoietin) were higher in patients subsequently developing CKD and all were renal injury proteins that were remarkably similar to those observed in our late CKD study.(10)
With this preliminary discovery work identifying the key proteins involved in early preclinical and late CKD, we turned our attention toward combining these proteins and clinical risk factors to construct an early predictive model to detect patients at risk for (e.g., before) deterioration of GFR. The goal was to validate a model that could be implemented in clinical practice early after LT to identify patients at need for preemptive nephroprotective strategies.
Materials and Methods
OVERVIEW
The study was designed to test the central hypothesis that a model combining preidentified blood renal injury proteins and clinical factors can detect early subclinical renal injury to predict future progression to CKD after LT. Our first aim was to analyze samples from the multicenter 1-year prospective study, NIAID CTOT14 (National Institute of Allergy and Infectious Diseases Clinical Trials in Organ Transplantation 14; NCT01672164), and a single-center 5-year biorepository cohort at Baylor University Medical Center (BUMC), to respectively discover and validate this clinical/protein model. Our second aim was to analyze serial samples collected from the CTOT14 cohort to study changes in peripheral blood protein levels during the course of GFR changes in LT recipients. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institutional review committee at each center. No donor organs were obtained from executed prisoners or other institutionalized persons. Informed consent was obtained from all enrolled patients under IRB approval.
BIOMARKER PLATFORM
The multianalyte panels (Myriad RBM, Inc., Austin, TX) are comprehensive, quantitative, validated immunoassay products measuring human proteins in both serum and plasma using Luminex Bead technology. For both cohorts, we used a targeted 16 renal injury protein panel (KidneyMAP v.1.0) given that all of the proteins of interest from our previous unbiased discovery work were within this panel.(10,16) The following proteins were included: β−2 microglobulin, cystatin C, macrophage colony stimulating factor 1, α−1 microglobulin, CD40 antigen, erythropoietin, apolipoprotein A-IV, clusterin, kidney injury molecule-1, neutrophil gelatinase-associated lipocalin, osteopontin, Tamm-Horsfall protein, tissue inhibitor of metalloproteinases 1, trefoil factor-3, vascular endothelial growth factor, and thrombomodulin.
CTOT14 MULTICENTER SERIAL COHORT
The CTOT14 study prospectively enrolled 202 LT recipients at seven U.S. centers to identify biomarkers of several LT complications. Enrollment of new recipients commenced in October 2012, and last patient visit was in December 2015. Adult LT recipients were enrolled if they had undergone primary deceased or live donor LT and excluded if they had received previous or multiorgan transplants or were human immunodeficiency virus (HIV) infected. The serial sample collection schedule post-LT was planned as follows: week 2 and month 1, 2, 3, 6, 9, 12, 15, 18, 21, and 24. We limited this analysis to year 1 given that only a small percentage of the study was followed to month 24. For all analyses, we used CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) estimated GFR for our group definitions and serial analyses.(17) All CTOT14 data were collected serially into an electronic database, MEDIDATA RAVE, managed by the Data Coordinating Center (DCC; Rho Federal Systems). Oversight by the DCC included development of the study protocol, review of clinical site visits, classification of clinical phenotypes at the sample level, validation of analyses, and manuscript review.
Given that there are no standard definitions for serial decline of GFR or progression to CKD over time, we used a definition supported by the published literature that a >10% persistent decline in GFR over a shorter time period such as 12 months can be predictive of future progression to CKD.(18,19) This definition was used to delineate the trajectory of GFR in CTOT14 given its shorter follow-up, with use of additional Kidney Disease: Improving Global Outcomes (KDIGO) CKD criteria in the longer-term BUMC cohort below. In CTOT14, we identified the subset of patients with baseline 3 months posttransplant eGFR >60 mL/min/1.73 m2 (n = 97; Supporting Fig. S1). This time point was used to allow for any recovery of perioperative kidney injury and also to match up with the month 3 BUMC collections for validation. We then applied the following criteria to define two groups: (1) Diminished GFR (CTOT14-D) was defined as >10% decline in eGFR from baseline to year 1, with the majority (≥50%) of the intervening eGFR values lower than baseline eGFR. (2) Preserved GFR (CTOT14-P) was defined as ≤10% decline in eGFR, with the majority (≥50%) of the intervening eGFR values within 10% of the baseline eGFR. These criteria were chosen to account for intermittent improvements or declines in eGFR >10% so as to define phenotypes with sustained decline versus preservation of renal function.
BUMC COHORT
Once the CTOT14 model was developed, it was validated in this independent BUMC cohort. The BUMC biorepository has been collecting blood/tissue samples and accompanying prospective clinical data from LT recipients since 1985 under an institutional review board–approved protocol that included mGFR determinations (iothalamate) and eGFR (CKD-EPI) at 3 months and annually following LT. The unique aspects are the protocol-based assessments of simultaneous eGFR and mGFR as well as longitudinal follow-up beyond 1 year. The majority (two-thirds) of the recipients included in this study were transplanted after the year 2000.
We excluded 1,324 subjects with ≥1 missing GFR values and, similar to the CTOT14 cohort, second or multiorgan transplants and HIV-infected recipients. Thus, we identified 220 LT recipients with preserved GFR (mGFR and eGFR ≥60 mL/min/1.73 m2 by CKD-EPI), normal liver tests, and sera available at month 3 (Supporting Fig. S2). The same 3- to 12-month criteria as CTOT14 were then applied, using an electronic software algorithm implemented in the BUMC data, followed by manual (J.L., S.A.) confirmation of the same group criteria as CTOT14 above. In addition, given that the BUMC cohort had longer follow-up, we continued to stratify patients by those with both 1-year deterioration and progression to more advanced CKD by year 5 (mGFR <45 mL/min/1.73 m2 or ≥KDIGO CKD stage 3b)(20) versus those who had 1-year and up to 5-year GFR preservation. This validation cohort was used to determine whether the 3-month CTOT14 model was also predictive of late renal outcomes, which may be more clinically relevant. The two groups were defined as follows: (1) diminished GFR (BUMC-D): CTOT14-D criteria above and mGFR <45 mL/min/1.73 m2 every year up to and including year 5; (2) Preserved GFR (BUMC-P): CTOT14-P criteria above and (3) mGFR >60 mL/min/1.73 m2 every year up to and including year 5. Given that the BUMC repository does not store plasma like the CTOT14 samples, we used serum samples, which was deemed acceptable by the manufacturer (Myriad RBM, Inc.)
STATISTICAL ANALYSIS
We tested for these 16 proteins in all samples collected early after LT (month 3 time point) when GFR was preserved (≥60 mL/min/1.73 m2) and samples were available. We employed Pearson’s chi-square test or Fisher’s exact test for categorical variables and t test or Wilcoxon rank-sum test for continuous variables to compare baseline demographics between the two groups. Association of protein abundance with GFR status were assessed using logistic regressions with and without the adjustment of baseline covariates. Using the CTOT14 as the discovery cohort, we developed a month 3 prediction model for GFR deterioration by year 1 based on multivariable logistic regression with step-wise variable selection and Bayesian information criterion, which started with seven key clinical and 16 multianalyte protein variables. Internal validation was performed using C-statistic with 1,000 bootstrap samples. We then fixed the prediction model and externally validated its performance in the BUMC cohort. In a subanalysis, we tested the performance of the model in the non-HCV (hepatitis C virus) cohorts.
In CTOT14, because we collected serial samples over the first transplant year, we performed an additional analysis of change of protein levels over time in relation to GFR change. This analysis began at month 1 through year 1 to give the full spectrum of serial changes in proteins over time, starting from eGFR > 60 (≥60 mL/min/1.73 m2) at month 1 and then divided by diminished or preserved eGFR by the same criteria above. For the statistical analysis, we used a generalized linear mixed-effect model to examine whether the serial protein levels over time differed between the diminished and preserved GFR groups. We also estimated the average protein change from month 1 to 12 for each individual by the slope from a linear regression model. All analyses were done using R: A language and environment for statistical computing (Version 3.3.2; R Foundation for Statistical Computing, Vienna, Austria).
Results
CLINICAL CHARACTERISTICS
Figure 1 displays the percentage change in eGFR from the month 3 baseline to year 1 in all CTOT14 subjects, divided by preserved and diminished cohorts. Table 1 displays the clinical characteristics of both the CTOT14 discovery and BUMC validation cohorts with month 3 samples, divided by those developing eGFR deterioration versus preservation. In the CTOT14 cohort, the only statistically significant difference was lower eGFR at year 1 in the CTOT14-D versus CTOT14-P group, which was expected. There was a trend toward a lower percentage of HCV infection in the CTOT14-D group. In the BUMC cohort, the BUMC-D group was also less HCV positive, older, more on late mammalian target of rapamycin (mTOR)-inhibitor therapy at year 5, and also as expected, had lower measured and estimated GFRs at years 1 and 5. Other clinical parameters were not statistically different.
FIG. 1.
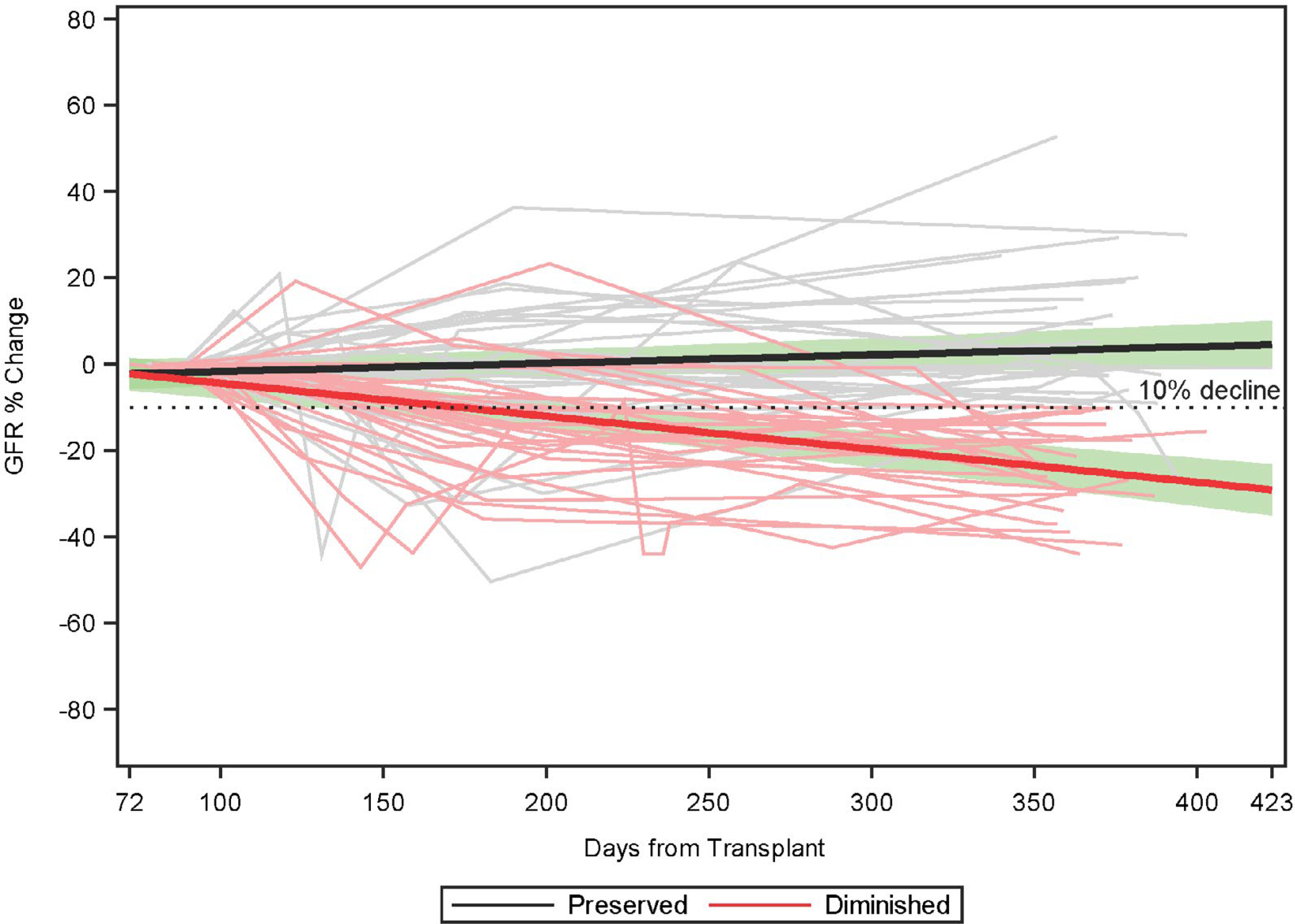
Percentage change in eGFR from baseline month 3 to year 1 in the preserved (CTOT-P) versus diminished (CTOT-D) GFR groups. The horizontal dashed line represents a 10% decline from baseline. Individual subject trajectories are shown in gray (preserved) and light red (diminished) with the mean predicted lines (black for preserved, red for diminished) overlaid. The green bands reflect the associated 95% confidence interval.
TABLE 1.
Clinical Characteristics: Discovery (CTOT14) and Validation (BUMC) Cohorts
| CTOT14-D* |
CTOT14-P* |
BUMC-D† |
BUMC-P† |
|||
|---|---|---|---|---|---|---|
| Variable | N = 28 | N = 32 | P Value | N = 18 | N = 32 | P Value |
| At LT | ||||||
| Age at enrollment (years) | 52.5 (12.2) | 54.1 (8.7) | 0.575 | 54.33 (9.3) | 44.31 (11.0) | 0.002 |
| Sex (% male) | 17 (60.7%) | 25 (78.1%) | 0.142 | 10 (55.6) | 22 (68.8) | 0.531 |
| Race (% white) | 22 (78.6%) | 27 (84.4%) | 0.562 | 17 (94.4) | 26 (81.2) | 0.386 |
| HCV as listing diagnosis‡ | 4 (14.3%) | 11 (34.4%) | 0.073 | 1 (5.6%) | 14 (43.8%) | 0.005 |
| Hypertension | 6 (21.4%) | 8 (25.0%) | 0.744 | 1 (5.6) | 2 (6.2) | >0.999 |
| Diabetes | 7 (25.0%) | 7 (21.9%) | 0.775 | 2 (11.1) | 4 (12.5) | >0.999 |
| Month 3 post-LT | ||||||
| CNI therapy, n (%) | 24 (85.7%) | 31 (96.9%) | 0.175 | 18 (100) | 32 (100) | >0.999 |
| Alanine aminotransferase (U/L) | 44.1 (77.8) | 45.5 (46.71) | 0.304 | 39.5 (24.5) | 39.5 (29.1) | 0.997 |
| Alkaline phosphatase (U/L) | 132.6 (177.1) | 139.0 (121.1) | 0.740 | 105.1 (35.2) | 90.4 (33.1) | 0.131 |
| Total bilirubin (mg/dL) | 0.9 (0.9) | 0.7 (0.89) | 0.733 | 0.54 (.31) | 0.58 (0.33) | 0.708 |
| mGFR (mL/min/1.73 m2) | n/a | n/a | 80.2 (16.74) | 82.1 (26.8) | 0.363 | |
| eGFR (mL/min/1.73 m2) | 81.2 (17.6) | 81.3 (16.23) | 0.941 | 80.2 (16.7) | 85.9 (24.5) | 0.776 |
| Year 1 post-LT | ||||||
| CNI therapy, n (%) | 22 (78.6%) | 28 (87.5%) | 0.491 | 18 (100%) | 32 (100%) | >0.999 |
| Mycophenolic acid, n (%) | 9 (32.1%) | 8 (25.0%) | 0.540 | 8 (44.4%) | 16 (50%) | 0.706 |
| mTOR-inhibitor therapy, n (%) | 4 (14.3%) | 7 (21.9%) | 0.449 | 3 (16.7%) | 1 (3.1%) | 0.127 |
| mGFR (mL/min/1.73 m2) | n/a | n/a | 54.7 (16.1) | 98.3 (20.7) | <0.001 | |
| eGFR (mL/min/1.73 m2) | 62.5 (16.7) | 84.6 (16.0) | <0.001 | 47.0 (11.6) | 91.0 (24.2) | <0.001 |
| At year 5 post-LT | n/a | n/a | ||||
| CNI therapy, n (%) | n/a | n/a | 16 (88.8%) | 32 (100%) | 0.125 | |
| Mycophenolic acid or azathioprine, n (%) | n/a | n/a | 9 (50%) | 15 (83.3%) | 0.831 | |
| mTOR-inhibitor therapy, n (%) | n/a | n/a | 6 (33.3%) | 0 (0%) | 0.0012 | |
| mGFR (mL/min/1.73 m2) | n/a | n/a | 33.9 (7.6) | 94.7 (22.7) | <0.001 | |
| eGFR (mL/min/1.73 m2) | n/a | n/a | 39.3 (14.1) | 84.6 (28.9) | <0.001 | |
Defined by GFR > 60 at month 3 and then >10% deterioration in GFR (Diminished) vs. ≤10% (Preserved) at year 1.
Defined by GFR > 60 at month 3, >10% deterioration in GFR (Diminished) vs. ≤10% (Preserved) at year 1, and then GFR < 45 (Diminished) vs. GFR ≥60 mL/min (Preserved) up to 5 years.
Two in the CTOT14-D group and 4 in the CTOT14-P group were HCV nonviremic in the study period. None of the BUMC patients were HCV nonviremic.
Abbreviation: n/a, not applicable.
PREDICTIVE MODEL GENERATION FROM THE CTOT14 DISCOVERY COHORT
Table 2 displays the month 3 plasma protein level comparison in the CTOT14 plasma discovery cohort, divided by renal outcomes at year 1. We initially performed a univariate logistic regression analysis that included the 16 renal injury proteins and seven key clinical variables from Table 1 (age, sex, race, HCV as listing diagnosis, hypertension, diabetes, and baseline month 3 eGFR) known to be clinically associated with changes in GFR (Supporting Table S1). From these data, we used a step-wise logistic regression approach with variable selection to develop a model at 3 months that was most predictive of 1-year diminished eGFR (Fig. 2A). This resulted in a model containing two proteins (β−2 microglobulin, CD40) and one clinical (HCV positivity) variable that had high prediction of eGFR deterioration (area under the curve [AUC], 0.81). We achieved similar results after internal bootstrapping (ordinary resampling method): AUC, 0.82; SD, 0.05. We named this model PRESERVE (Prediction of REnal outcomeS aftER liVEr transplant) with the formula: log it(p) = −1.1445231 +0.8616163b2 microglobulin −1.4749716cd40−2.0455445hcv+. Performance characteristics of this model are displayed in Supporting Table S2. The overall accuracy (0.75), sensitivity (0.71), specificity (0.78), positive predictive value (PPV; 0.74), and negative predictive value (NPV; 0.76) were most optimal at a probability threshold of 0.5.
TABLE 2.
Month 3 protein Level Comparison: Discovery (CTOT14) and Validation (BUMC) Cohorts
| CTOT14 (Predict 1 Year) Plasma |
BUMC (Predict 1 and 5 Year) Sera |
|||||
|---|---|---|---|---|---|---|
| Diminished | Preserved | P Value | Diminished | Preserved | P Value | |
| β-2 microglobulin (μg/mL) | 3.75 [2.58; 5.32] | 2.55 [2.20; 3.70] | 0.011 | 4.25 [3.70; 5.42] | 3.15 [2.35; 3.92] | 0.002 |
| Cystatin C (ng/mL) | 1,325 [1,222; 1,872] | 1,105 [1,028; 1,322] | 0.004 | 1,435 [1,282; 1,578] | 1,085 [896; 1,350] | 0.001 |
| Macrophage colony stimulating factor 1 (ng/mL) | 0.94 [0.66; 1.60] | 0.90 [0.66; 1.22] | 0.694 | 2.15 [1.80; 2.92] | 1.50 [0.83; 2.20] | 0.007 |
| α-1 microglobulin (μg/mL) | 23.0 [15.8; 29.0] | 18.5 [15.0; 24.0] | 0.135 | 24.0 [21.2; 29.8] | 21.0 [15.8; 24.0] | 0.007 |
| CD40 antigen (ng/mL) | 0.94 [0.72; 1.50] | 0.94 [0.76; 1.42] | 0.911 | 1.50 [1.30; 1.70] | 1.10 [0.98; 1.40] | 0.001 |
| Erythropoietin (mIU/mL) | 18.0 [14.0; 32.2] | 15.5 [9.67; 23.0] | 0.099 | 33.0 [24.0; 38.5] | 23.5 [19.8; 29.0] | 0.041 |
| Apolipoprotein A-IV (μg/mL) | 3,850 [3,640; 5,520] | 5,100 [4,052; 7,350] | 0.113 | 49.5 [38.5; 64.2] | 40.5 [33.8; 61.8] | 0.189 |
| Clusterin (μg/mL) | 256 [234; 297] | 271 [228; 349] | 0.382 | 262 [208; 283] | 212 [184; 237] | 0.027 |
| Kim-1 (ng/mL) | 0.08 [0.05; 0.12] | 0.12 [0.09; 0.23] | 0.065 | 0.10 [0.07; 0.20] | 0.08 [0.04; 0.11] | 0.067 |
| Neutrophil gelatinase-associated lipocalin (ng/mL) | 280 [177; 425] | 220 [172; 270] | 0.095 | 391 [234; 484] | 294 [223; 378] | 0.176 |
| Osteopontin (ng/mL) | 42.0 [35.5; 62.5] | 37.5 [34.0; 48.0] | 0.187 | 25.5 [17.2; 30.0] | 21.0 [11.8; 25.2] | 0.056 |
| Tamm-Horsfall protein (μg/mL) | 0.03 [0.02; 0.04] | 0.02 [0.02; 0.03] | 0.588 | 0.03 [0.03; 0.04] | 0.04 [0.03; 0.05] | 0.117 |
| Tissue inhibitor of metalloproteinases 1 (ng/mL) | 114 [84.8; 153] | 97.0 [84.8; 118] | 0.187 | 154 [137; 184] | 146 [123; 166] | 0.122 |
| Trefoil factor-3 (μg/mL) | 0.20 [0.14; 0.26] | 0.16 [0.13; 0.20] | 0.045 | 0.15 [0.12; 0.20] | 0.12 [0.10; 0.15] | 0.037 |
| Vascular endothelial growth factor (pg/mL) | 94.0 [81.5; 133] | 92.5 [75.8; 122] | 0.386 | 256 [192; 355] | 298 [188; 400] | 0.864 |
| Thrombomodulin (ng/mL) | 7.15 [5.28; 8.90] | 6.55 [4.80; 7.23] | 0.157 | 7.20 [6.70; 9.10] | 6.50 [5.15; 7.82] | 0.037 |
FIG. 2.
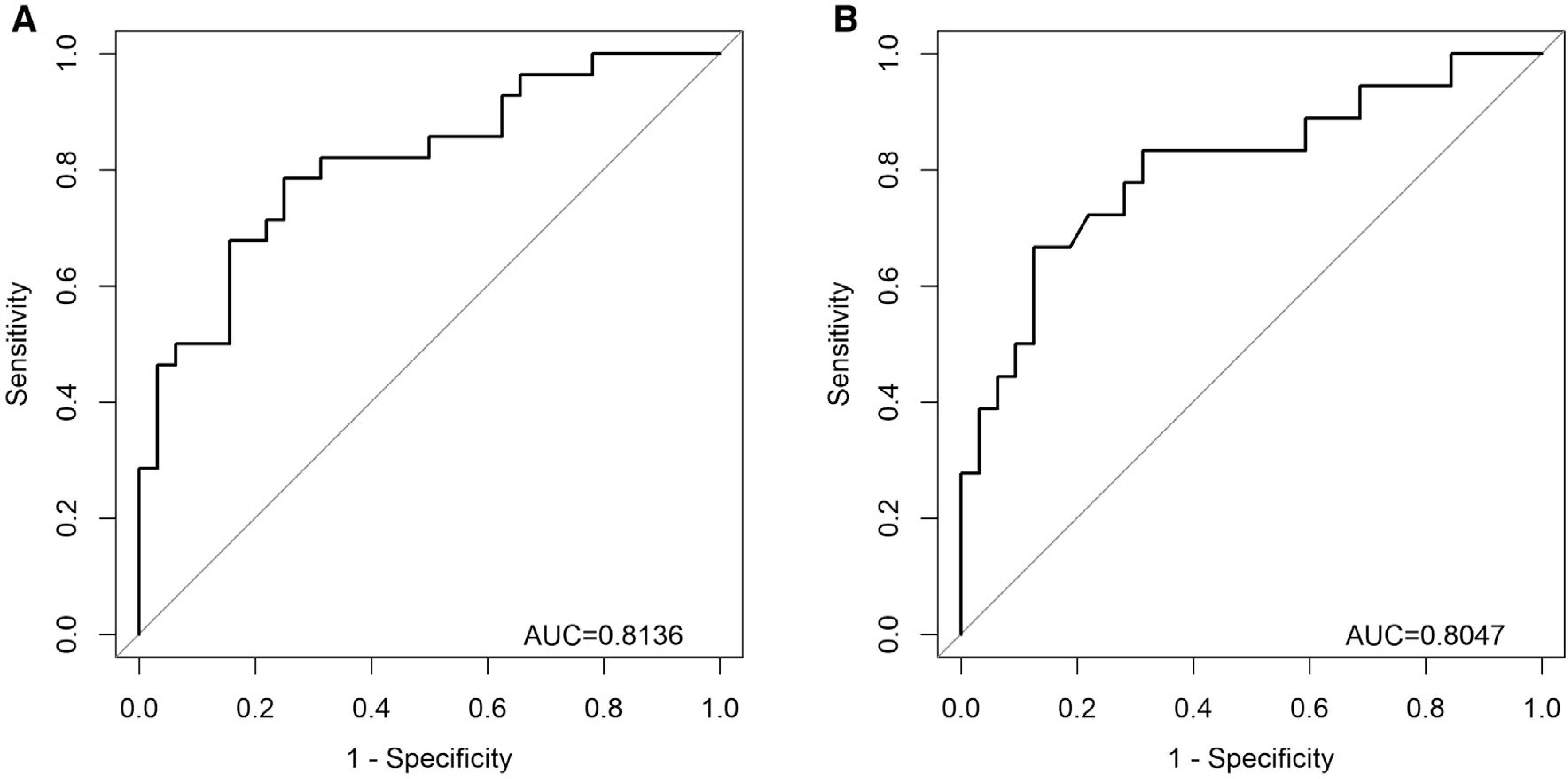
(A) Receiver operating curve for the PRESERVE step-wise logistic regression model predicting GFR deterioration at 1 year post-LT in the initial CTOT14 plasma discovery cohort. Variables included in the PRESERVE model: HCV+ (yes/no), β−2 microglobulin protein level, and CD40 protein level. (B) Receiver operating curve for the same PRESERVE step-wise logistic regression model predicting GFR deterioration at both 1 and 5 years post-LT in the BUMC serum validation cohort.
PREDICTIVE MODEL VALIDATION FROM THE BUMC COHORT
Table 2 also displays the month 3 serum protein level comparison in the BUMC validation cohort. The PRESERVE model was then tested in the BUMC cohort, to provide external independent model validation. Similar to CTOT14, the PRESERVE model had high prediction of eGFR deterioration (AUC, 0.80) at 1 and 5 years (Fig. 2B). Applying the 0.5 threshold from the PRESERVE model led to excellent performance characteristics (Supporting Table S3): accuracy (0.80), sensitivity (0.67), specificity (0.88), PPV (0.75), and NPV (0.82).
PREDICTIVE MODEL ASSESSMENT IN THE NON-HCV RECIPIENTS
Given the declining prevalence of HCV infection in LT recipients, we tested the accuracy of the PRESERVE model in the non-HCV cohorts. These were CTOT14 and BUMC recipients with no history of HCV or were HCV+, but nonviremic attributable to previous antiviral therapy. This had a lower AUC in the CTOT14 group (0.75), but in the independent external BUMC validation cohort, the AUC (0.79) was nearly equivalent to the AUC (0.80 above) of the overall cohort. In addition, at the same 0.5 threshold, the performance characteristics were similar to the overall cohort: accuracy (0.77 vs. 0.80), sensitivity (0.71 vs. 0.67), specificity (0.83 vs. 0.88), PPV (0.80 vs. 0.75), and NPV (0.75 vs. 0.82).
CTOT14: SERIAL ANALYSIS OF PROTEIN LEVEL AND GFR CHANGE FROM MONTH 1 TO 12
We also examined the trajectory of each protein level over time in relation to change in eGFR, providing support for the validity of these biomarkers and a more robust understanding of mechanisms of renal injury in this population. Serial protein and eGFR values were available only in the CTOT14 prospective study. In requiring a minimum of four samples tied to eGFR, 31 CTOT14-D and 24 CTOT14-P subjects had 306 serial proteins tested. The following proteins were higher over time in the CTOT14-D versus CTOT14-P group: α−1 microglobulin, vascular endothelial growth factor, CD40 antigen, kidney injury molecule-1, neutrophil gelatinase-associated lipocalin, tissue inhibitor of metalloproteinases-1, β−2 microglobulin, cystatin C, and trefoil factor-3 (Supporting Table S4). Tamm-Horsfall protein (uromodulin) demonstrated the opposite effect, decreasing with time in the CTOT14-D group. We then combined the groups and analyzed specifically for an association between protein levels and eGFR over time in a linear mixed model. The following varied significantly with eGFR (Fig. 3; P < 0.05): α−1 microglobulin, β−2 microglobulin, cystatin C, trefoil factor-3, thrombomodulin (negative correlation), and Tamm-Horsfall protein (positive correlation). Those that trended toward significance were apolipoprotein A-IV (0.06), vascular endothelial growth factor (0.06), CD40 (P = 0.07), and kidney injury molecule-1 (0.08).
FIG. 3.
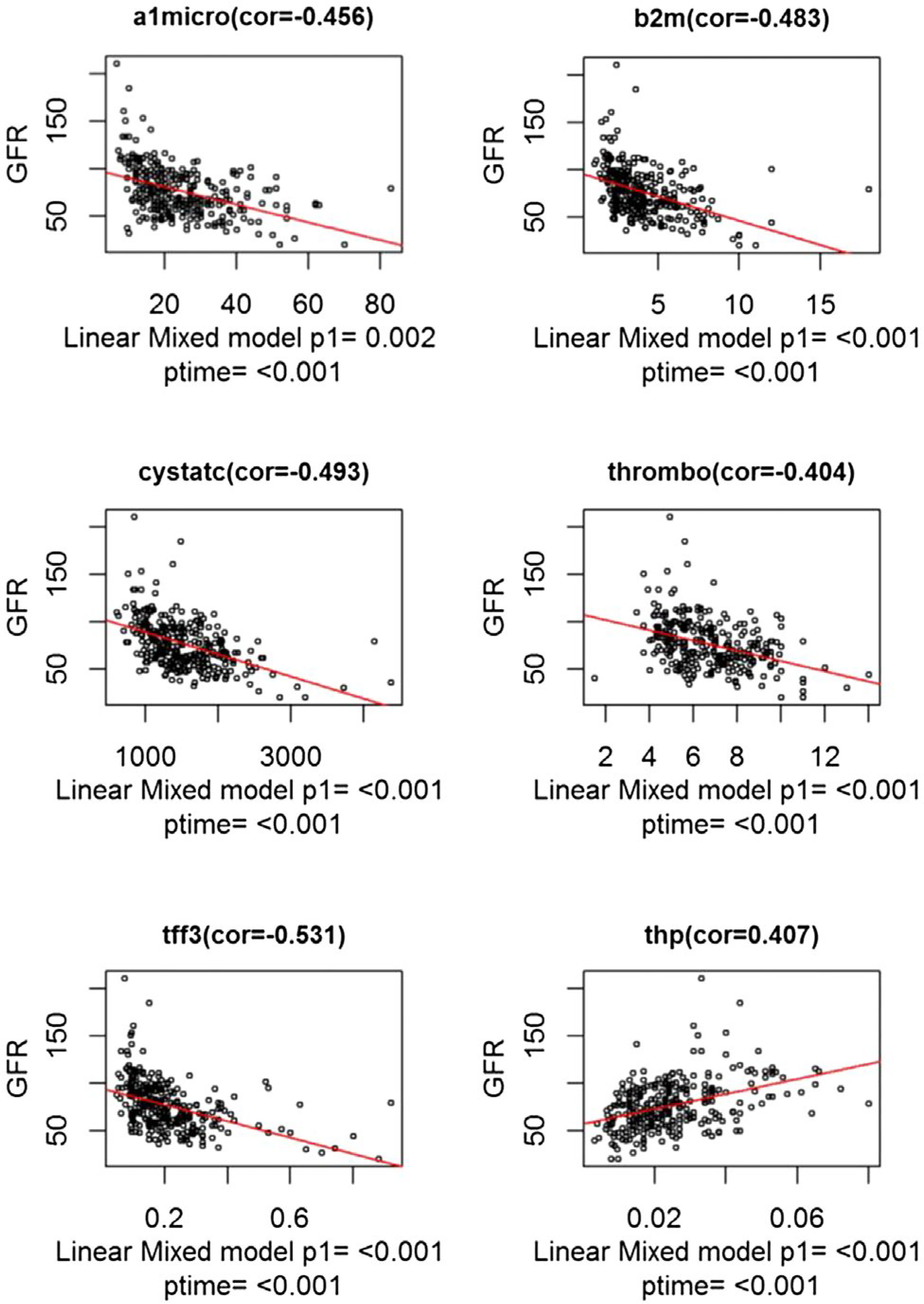
This figure highlights the specific plasma proteins that significantly correlated with serial eGFR changes over the first year following LT: CTOT14 cohort. Abbreviations: a1micro, α−1 microglobulin; b2m, β−2 microglobulin; cor, correlation; cystatc, cystatin C; tff3, trefoil factor-3; thp, Tamm-Horsfall protein; thrombo, thrombomodulin.
Discussion
Several reports of both blood and urine biomarkers have shown correlations with acute or chronic kidney injury in the general population.(11,21–25) Proteins such as albumin, kidney injury molecule-1, neutrophil gelatinase-associated lipocalin, and β−2 microglobulin, are associated with future GFR deterioration, but mainly in patients with abnormal baseline renal function. In LT recipients, causes of native kidney injury are similar to the general population, with the main difference being the use of nephrotoxic CNI therapies. High levels of cystatin C, neutrophil gelatinase-associated lipocalin, α−1 microglobulin, β−2 microglobulin, and others correlate with established CKD in liver recipients.(7–10,26–33) Given that many recipients have kidney injury at LT that does not resolve, biomarkers post-LT in this setting would be merely diagnostic, not predictive, and have little advantage over the standard creatinine-based GFR estimates. Validation of a predictive model could stratify future recipients for early, more aggressive renal preservation strategies, such as CNI minimization or withdrawal, rather than applying such strategies more broadly to all recipients where they might not be necessary and/or carry a higher risk of rejection. As an example, one previous study attempted to withdraw tacrolimus 3 months after LT in favor of everolimus.(34) That arm of the study was stopped early because of a higher rate of rejection, although those successful had significantly better GFR at 1 year than tacrolimus-maintained groups. An early predictive model could guide such efforts to identify and intervene on only those at higher risk of GFR deterioration and thus provide a personalized approach to LT management.
Our study was therefore designed to test the potential of a predictive biomarker model (PRESERVE) to identify LT recipients at risk of developing future CKD. We examined all patients with preserved renal function at 3 months after LT, but still at risk for de novo future CKD.(13) Important in our model development were the preliminary studies we performed analyzing a large set of unbiased discovery proteins in both established post-LT CKD and early after transplant before CKD. From this panel, it was clearly identified that renal injury proteins were elevated in both settings, the latter (early) being most relevant for our current model generation, given the potential to proactively identify patients at higher CKD risk. Thus, we developed the PRESERVE model from the clinical and previously identified renal injury markers in the prospective CTOT14 cohort and validated it in the independent BUMC cohort having longer follow-up, measured GFRs, and established CKD.
The final PRESERVE model included one clinical variable (HCV as the primary indication for LT) and two renal injury proteins, β−2 microglobulin and CD40 antigen. The accuracy and AUC of PRESERVE for both cohorts were high (~0.8), with a somewhat higher specificity and NPV than sensitivity and PPV, suggesting that it may be slightly better at predicting future GFR preservation over deterioration. In addition, several of these proteins increased with declining GFR in our serial analysis and were present in both the early and late LT CKD protein profile we previously reported,(10,16) supporting an evolution of protein expression preceding full CKD. The model also performed well in both plasma and serum in the two cohorts. In recent reports, we demonstrated similar findings by discovering a model using plasma samples and validating it with serum samples.(35,36) In this instance, two pretransplant proteins, tissue inhibitor of metalloproteinase 1 and osteopontin, along with age and diabetes (REVERSE model) were predictive of renal recovery within weeks following transplantation. Thus, serum and plasma appear interchangeable in both models, which can facilitate clinical applications and laboratory testing with more flexibility.
The proteins identified correlate with pathways of both native renal injury and protection, best observed in the serial analysis linking protein levels and GFR changes over time. Trefoil factor-3 and thrombomodulin have been reported as sensitive markers of early and reversible renal injury.(10,37,38) β−2 microglobulin is the light chain of major histocompatibility complex expressed on all nucleated cells and, like α−1 microglobulin and cystatin C, is filtered by the glomerulus and reabsorbed/catabolized by the proximal tubule.(39,40) Similar to our findings, a recent nontransplant study found associations between β−2 microglobulin, cystatin C, and future CKD, even in patients with baseline eGFR >60 mL/min/1.73 m2.(19) Although it is not clear whether β−2 microglobulin levels are related to decreased glomerular filtration or tissue injury itself, they appear predictive at preclinical renal injury stages regardless of the mechanism. CD40 levels also correlate with GFR changes, but may be more related to inflammation. CD4+ T cells and platelets express CD40 ligand and mediate proinflammatory events in proximal tubular epithelial cells through interaction with CD40 antigen.(41) Both CD40 and β−2 microglobulin are related to costimulation pathways and the major histocompatibility structure, respectively, providing some evidence for immune-mediated renal injury. Even with these potential pathways, without renal biopsies, we cannot determine the exact cause of GFR deterioration in our cohorts; it is likely that multiple factors in addition to CNI nephrotoxicity are involved.(13) Finally, in the serial analysis, we found a direct correlation between better GFR and plasma Tamm-Horsfall protein levels (also known as uromodulin). This fits well with previous studies showing uromodulin being protective and inversely associated with CKD.(42,43)
Interestingly, HCV as a listing diagnosis was the only clinical variable remaining in the model after regression. In both CTOT14 and BUMC cohorts, HCV was less prevalent in the patients who had diminished GFR over time, and the absence of HCV was associated with GFR deterioration in the PRESERVE model. This is somewhat surprising given that HCV infection has been shown to correlate with CKD in both general and transplant populations.(3,44) As a potential explanation, given that the study enrollment took place in eras in which patients were transplanted with active HCV viremia, GFR improvement may have been related to practices such as minimizing CNI exposure in efforts to limit HCV recurrence. This is entirely speculative given that we do not have enough serial data on immunosuppression management and drug levels to confirm this hypothesis. Another explanation could be that the non-HCV populations, particularly those transplanted for nonalcoholic steatohepatitis (NASH) with metabolic syndrome, have known high rates of CKD after LT and thus the model (absence of HCV correlating with diminished GFR) may be reflecting this risk and useful for future populations.(13)
Given that our model includes HCV as a key clinical variable, it was reassuring that it performed well in both partially treated (6 nonviremic of 15 HCV+) CTOT14 discovery and untreated viremic BUMC validation cohorts. However, the current practice is to eradicate HCV before or soon after transplantation with oral antiviral therapy, and thus LT populations are increasingly non-HCV (e.g., NASH, alcoholic cirrhosis) or nonviremic.(45–48) To ensure that the PRESERVE model would still be usable for future LT populations, we performed additional analyses testing its accuracy in the non-HCV cohorts. The PRESERVE model had nearly equivalent AUC, accuracy, and other performance characteristics (sensitivity, specificity, PPV, and NPV) in the non-HCV population. Thus, even with a dwindling HCV prevalence, we believe the model should perform well and be applicable to future recipients, both HCV and non-HCV.
Our study has limitations that need to be addressed. First, we recognize that although single-time point predictions like the PRESERVE model may be useful in clinical practice, they are not always definitive, particularly when other renal insults can occur over time. Even though month 3 likely represents a renal baseline or steady state, future studies will need to test the model serially or even earlier after LT. It is also not known whether the elevated kidney injury proteins at month 3 are related to recovery from earlier posttransplant renal injury or new-onset subclinical renal injury. We suspect the former given that there was a greater improvement in eGFR from the time of LT to month 3 in the CTOT14-D group (71.7 ± 28.8 to 81.2 ± 17.6 mL/min/1.73 m2) than CTOT14-P (79.6 ± 27.7 to 81.3 ± 16.2 mL/min/1.73 m2). Regardless of timing, this subclinical renal injury at month 3 appears to predict future deterioration. Second, our sample size was relatively small in relation to the overall cohort, and this was because we needed to identify “clean” phenotypes of patients who had distinct GFR trajectories over time, rather than testing all patients and trajectories that would limit model generation and validation. However, we acknowledge that exclusion of these patients with less pure phenotypes may reduce the generalizability of the model to the transplant population as a whole. Given that the PRESERVE model performed well in independent discovery and validation cohorts, it now needs to be more robustly tested in randomized, renal sparing interventional trials that include the larger LT population. Third, the validation cohort (BUMC) was more remotely transplanted and had differences in clinical characteristics (Table 1) compared to CTOT14. However, the primary model was developed using the more contemporary CTOT14 group, and the fact that it had similar accuracy in the BUMC group strengthens its validity and external generalizability, as it appears agnostic to era. Finally, we defined 1-year renal deterioration by >10% persistent drop in eGFR, which may be considered modest in clinical impact. However, the literature supports that this early reduction predicts continued renal deterioration,(18,19) and the PRESERVE model prediction held up in our BUMC cohort who had more substantial, clinically relevant renal dysfunction by year 5 (≥CKD stage 3b).
In summary, we have identified and validated a clinical/protein model that can stratify recipients early after LT who are risk for future GFR deterioration. Based on these findings, we anticipate that nephroprotective strategies could be targeted toward LT recipients that are predicted to progress to CKD according to the PRESERVE model. Similarly, the pre-LT REVERSE model previously reported may allow for earlier personalized approaches.(35,36) We envision testing both models in imminent randomized controlled trials at the time of and weeks after LT to ultimately use them as personalized management strategies.
Supplementary Material
Acknowledgments
Supported by the following grants: R21AI113916–01 (PI: Levitsky) and U01AI084146 (PI: Abecassis) from the National Institute of Allergy and Infectious Disease; internal funds from the Northwestern University Comprehensive Transplant Center and the Baylor Transplant Institute.
Potential conflict of interest: Dr. Klintmalm advises Veloxis and owns stock in Transplant Genomics. Dr. Levitsky has research grants and speaks for Novartis. He also advises and owns stock in Transplant Genomics. Dr. Abecassis is a co-founder and owns stock in Transplant Genomics.
Abbreviations:
- AUC
area under the curve
- BUMC
Baylor University Medical Center
- CKD-EPI
Chronic Kidney Disease Epidemiology Collaboration
- CTOT
Clinical Trials in Organ Transplantation
- CNIs
calcineurin inhibitors
- CKD
chronic kidney disease
- eGFR
estimated GFR
- GFR
glomerular filtration rate
- HCV
hepatitis C virus
- LT
liver transplantation
- mGFR
measured GFR
- mTOR
mammalian target of rapamycin
- NPV
negative predictive value
- PPV
positive predictive value
- RBM
Rules Based Medicine
Footnotes
Supporting Information
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1002/hep.30939/suppinfo.
REFERENCES
- 1).Gonwa TA, Mai ML, Melton LB, Hays SR, Goldstein RM, Levy MF, Klintmalm GB. End-stage renal disease (ESRD) after orthotopic liver transplantation (OLTX) using calcineurin-based immunotherapy: risk of development and treatment. Transplantation 2001;72:1934–1939. [DOI] [PubMed] [Google Scholar]
- 2).Velidedeoglu E, Bloom RD, Crawford MD, Desai NM, Campos L, Abt PL, et al. Early kidney dysfunction post liver transplantation predicts late chronic kidney disease. Transplantation 2004;77:553–556. [DOI] [PubMed] [Google Scholar]
- 3).Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med 2003;349:931–940. [DOI] [PubMed] [Google Scholar]
- 4).Allen AM, Kim WR, Therneau TM, Larson JJ, Heimbach JK, Rule AD. Chronic kidney disease and associated mortality after liver transplantation—a time-dependent analysis using measured glomerular filtration rate. J Hepatol 2014;61:286–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Schwarz A, Haller H, Schmitt R, Schiffer M, Koenecke C, Strassburg C, et al. Biopsy-diagnosed renal disease in patients after transplantation of other organs and tissues. Am J Transplant 2010;10:2017–2025. [DOI] [PubMed] [Google Scholar]
- 6).Kim JY, Akalin E, Dikman S, Gagliardi R, Schiano T, Bromberg J, et al. The variable pathology of kidney disease after liver transplantation. Transplantation 2010;89:215–221. [DOI] [PubMed] [Google Scholar]
- 7).Gerhardt T, Poge U, Stoffel-Wagner B, Ahrendt M, Wolff M, Spengler U, et al. Estimation of glomerular filtration rates after orthotopic liver transplantation: evaluation of cystatin C-based equations. Liver Transpl 2006;12:1667–1672. [DOI] [PubMed] [Google Scholar]
- 8).Niemann CU, Walia A, Waldman J, Davio M, Roberts JP, Hirose R, Feiner J. Acute kidney injury during liver transplantation as determined by neutrophil gelatinase-associated lipocalin. Liver Transpl 2009;15:1852–1860. [DOI] [PubMed] [Google Scholar]
- 9).Biancofiore G, Pucci L, Cerutti E, Penno G, Pardini E, Esposito M, et al. Cystatin C as a marker of renal function immediately after liver transplantation. Liver Transpl 2006;12:285–291. [DOI] [PubMed] [Google Scholar]
- 10).Levitsky J, Salomon DR, Abecassis M, Langfelder P, Horvath S, Friedewald J, et al. Clinical and plasma proteomic markers correlating with chronic kidney disease after liver transplantation. Am J Transplant 2011;11:1972–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis 2009;54:1012–1024. [DOI] [PubMed] [Google Scholar]
- 12).O’Riordan A, Johnston O, McMorrow T, Wynne K, Maguire P, Hegarty JE, et al. Identification of apolipoprotein AI as a serum biomarker of chronic kidney disease in liver transplant recipients, using proteomic techniques. Proteomics Clin Appl 2008;2:1338–1348. [DOI] [PubMed] [Google Scholar]
- 13).Levitsky J, O’Leary JG, Asrani S, Sharma P, Fung J, Wiseman A, Niemann CU. Protecting the kidney in liver transplant recipients: practice-based recommendations from the American Society of Transplantation Liver and Intestine Community of Practice. Am J Transplant 2016;16:2532–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).De Souza V, Hadj-Aissa A, Dolomanova O, Rabilloud M, Rognant N, Lemoine S, et al. Creatinine- versus cystatine C-based equations in assessing the renal function of candidates for liver transplantation with cirrhosis. Hepatology 2014;59:1522–1531. [DOI] [PubMed] [Google Scholar]
- 15).Shlipak MG, Matsushita K, Arnlov J, Inker LA, Katz R, Polkinghorne KR, et al. Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med 2013;369:932–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Levitsky J, Asrani S, Zhao L, Abecassis M, Jennings L, Klintmalm GB. Early proteomic predictors of late chronic kidney disease in liver transplant recipients. Am J Transplant 2016;16(Suppl 3): 332–333. [Google Scholar]
- 17).Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Rosansky SJ. Renal function trajectory is more important than chronic kidney disease stage for managing patients with chronic kidney disease. Am J Nephrol 2012;36:1–10. [DOI] [PubMed] [Google Scholar]
- 19).Astor BC, Shafi T, Hoogeveen RC, Matsushita K, Ballantyne CM, Inker LA, Coresh J. Novel markers of kidney function as predictors of ESRD, cardiovascular disease, and mortality in the general population. Am J Kidney Dis 2012;59:653–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int 2011;80:17–28. [DOI] [PubMed] [Google Scholar]
- 21).Lurbe E, Redon J, Kesani A, Pascual JM, Tacons J, Alvarez V, Batlle D. Increase in nocturnal blood pressure and progression to microalbuminuria in type 1 diabetes. N Engl J Med 2002;347:797–805. [DOI] [PubMed] [Google Scholar]
- 22).Hsu CY, Xie D, Waikar SS, Bonventre JV, Zhang X, Sabbisetti V, et al. Urine biomarkers of tubular injury do not improve on the clinical model predicting chronic kidney disease progression. Kidney Int 2017;91:196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Nadkarni GN, Rao V, Ismail-Beigi F, Fonseca VA, Shah SV, Simonson MS, et al. Association of urinary biomarkers of inflammation, injury, and fibrosis with renal function decline: the ACCORD Trial. Clin J Am Soc Nephrol 2016;11:1343–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Alderson HV, Ritchie JP, Pagano S, Middleton RJ, Pruijm M, Vuilleumier N, Kalra PA. The associations of blood kidney injury molecule-1 and neutrophil gelatinase-associated lipocalin with progression from CKD to ESRD. Clin J Am Soc Nephrol 2016;11:2141–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Looker HC, Colombo M, Hess S, Brosnan MJ, Farran B, Dalton RN, et al. Biomarkers of rapid chronic kidney disease progression in type 2 diabetes. Kidney Int 2015;88:888–896. [DOI] [PubMed] [Google Scholar]
- 26).Slack AJ, McPhail MJ, Ostermann M, Bruce M, Sherwood R, Musto R, et al. Predicting the development of acute kidney injury in liver cirrhosis—an analysis of glomerular filtration rate, proteinuria and kidney injury biomarkers. Aliment Pharmacol Ther 2013;37:989–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Allen AM, Kim WR, Larson JJ, Colby C, Therneau TM, Rule AD. Serum cystatin C as an indicator of renal function and mortality in liver transplant recipients. Transplantation 2015;99:1431–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Portal AJ, McPhail MJ, Bruce M, Coltart I, Slack A, Sherwood R, et al. Neutrophil gelatinase—associated lipocalin predicts acute kidney injury in patients undergoing liver transplantation. Liver Transpl 2010;16:1257–1266. [DOI] [PubMed] [Google Scholar]
- 29).Barreto R, Elia C, Sola E, Moreira R, Ariza X, Rodriguez E, et al. Urinary neutrophil gelatinase-associated lipocalin predicts kidney outcome and death in patients with cirrhosis and bacterial infections. J Hepatol 2014;61:35–42. [DOI] [PubMed] [Google Scholar]
- 30).Tsuchimoto A, Shinke H, Uesugi M, Kikuchi M, Hashimoto E, Sato T, et al. Urinary neutrophil gelatinase-associated lipocalin: a useful biomarker for tacrolimus-induced acute kidney injury in liver transplant patients. PLoS One 2014;9:e110527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Sirota JC, Walcher A, Faubel S, Jani A, McFann K, Devarajan P, et al. Urine IL-18, NGAL, IL-8 and serum IL-8 are biomarkers of acute kidney injury following liver transplantation. BMC Nephrol 2013;14:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Li Y, Zhu M, Xia Q, Wang S, Qian J, Lu R, et al. Urinary neutrophil gelatinase-associated lipocalin and L-type fatty acid binding protein as diagnostic markers of early acute kidney injury after liver transplantation. Biomarkers 2012;17:336–342. [DOI] [PubMed] [Google Scholar]
- 33).Gatta A, Amodio P, Frigo A, Merkel C, Milani L, Zuin R, Ruol A. Evaluation of renal tubular damage in liver cirrhosis by urinary enzymes and beta-2-microglobulin excretions. Eur J Clin Invest 1981;11:239–243. [DOI] [PubMed] [Google Scholar]
- 34).De Simone P, Nevens F, De Carlis L, Metselaar HJ, Beckebaum S, Saliba F, et al. Everolimus with reduced tacrolimus improves renal function in de novo liver transplant recipients: a randomized controlled trial. Am J Transplant 2012;12:3008–3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Levitsky J, Baker TB, Jie C, Ahya S, Levin M, Friedewald J, et al. Plasma protein biomarkers enhance the clinical prediction of kidney injury recovery in patients undergoing liver transplantation. Hepatology 2014;60:2017–2026. [DOI] [PubMed] [Google Scholar]
- 36).Levitsky J, Asrani SK, Abecassis M, Ruiz R, Jennings LW, Klintmalm G. External validation of a pretransplant biomarker model (REVERSE) predictive of renal recovery after liver transplantation. Hepatology 2019. April 19 10.1002/hep.30667 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 37).Yu Y, Jin H, Holder D, Ozer JS, Villarreal S, Shughrue P, et al. Urinary biomarkers trefoil factor 3 and albumin enable early detection of kidney tubular injury. Nat Biotechnol 2010;28:470–477. [DOI] [PubMed] [Google Scholar]
- 38).Kusaka M, Kuroyanagi Y, Ichino M, Sasaki H, Maruyama T, Hayakawa K, et al. Serum Tissue inhibitor of metalloproteinases 1 (TIMP-1) predicts organ recovery from delayed graft function after kidney transplantation from donors after cardiac death. Cell Transplant 2010;19:723–729. [DOI] [PubMed] [Google Scholar]
- 39).Flynn FV, Lapsley M, Sansom PA, Cohen SL. Urinary excretion of beta 2-glycoprotein-1 (apolipoprotein H) and other markers of tubular malfunction in “non-tubular” renal disease. J Clin Pathol 1992;45:561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40).Vincent C, Dennoroy L, Revillard JP. Molecular variants of beta 2-microglobulin in renal insufficiency. Biochem J 1994;298(Pt 1): 181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41).Laxmanan S, Datta D, Geehan C, Briscoe DM, Pal S. CD40: a mediator of pro- and anti-inflammatory signals in renal tubular epithelial cells. J Am Soc Nephrol 2005;16:2714–2723. [DOI] [PubMed] [Google Scholar]
- 42).Leiherer A, Muendlein A, Saely CH, Brandtner EM, Geiger K, Fraunberger P, Drexel H. The value of uromodulin as a new serum marker to predict decline in renal function. J Hypertens 2018;36:110–118. [DOI] [PubMed] [Google Scholar]
- 43).Steubl D, Block M, Herbst V, Nockher WA, Schlumberger W, Satanovskij R, et al. Plasma uromodulin correlates with kidney function and identifies early stages in chronic kidney disease patients. Medicine (Baltimore) 2016;95:e3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44).Guitard J, Ribes D, Kamar N, Muscari F, Cointault O, Lavayssiere L, et al. Predictive factors for chronic renal failure one year after orthotopic liver transplantation. Ren Fail 2006;28:419–425. [DOI] [PubMed] [Google Scholar]
- 45).Terrault NA, McCaughan GW, Curry MP, Gane E, Fagiuoli S, Fung JYY, et al. International Liver Transplantation Society consensus statement on hepatitis C management in liver transplant candidates. Transplantation 2017;101:945–955. [DOI] [PubMed] [Google Scholar]
- 46).Charlton M, Everson GT, Flamm SL, Kumar P, Landis C, Brown RS Jr., et al. Ledipasvir and sofosbuvir plus ribavirin for treatment of HCV infection in patients with advanced liver disease. Gastroenterology 2015;149:649–659. [DOI] [PubMed] [Google Scholar]
- 47).Levitsky J, Verna EC, O’Leary JG, Bzowej NH, Moonka DK, Hyland RH, et al. Perioperative ledipasvir-sofosbuvir for HCV in liver-transplant recipients. N Engl J Med 2016;375: 2106–2108. [DOI] [PubMed] [Google Scholar]
- 48).Kwo PY, Mantry PS, Coakley E, Te HS, Vargas HE, Brown R Jr., et al. An interferon-free antiviral regimen for HCV after liver transplantation. N Engl J Med 2014;371:2375–2382. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


