Abstract
Patients with COVID-19 often complain of smell and taste disorders (STD). STD emerge early in the course of the disease, seem to be more common in SARS-CoV-2 infection than in other upper respiratory tract infections, and could in some cases persist for long after resolution of respiratory symptoms. Current evidence suggests that STD probably result from a loss of function of olfactory sensory neurons and taste buds, mainly caused by infection, inflammation, and subsequent dysfunction of supporting non-neuronal cells in the mucosa. However, the possible occurrence of other mechanisms leading to chemosensory dysfunction has also been hypothesized, and contrasting data have been reported regarding the direct infection of sensory neurons by SARS-CoV-2. In this mini-review, we summarize the currently available literature on pathogenesis, clinical manifestations, diagnosis, and outcomes of STD in COVID-19 and discuss possible future directions of research on this topic.
Keywords: COVID-19, Anosmia, Ageusia
1. Introduction
Clinical manifestations of COVID-19 range from mild, cold-like symptoms typically associated with respiratory tract infections, such as cough and fever, to severe pneumonia with respiratory failure [1,2]. Frequently, patients also experience smell and taste disorders (STD) [[3], [4], [5], [6], [7], [8], [9]]. These mainly consist of a decrease or loss of smell (hyposmia and anosmia) and taste (hypogeusia and ageusia); alterations in the chemesthesis-that is, the chemical sensitivity of mucosa to irritants-; and/or variations in the quality of chemosensory perception (phantosmia and parosmia).
In this mini-review, we will discuss pathogenesis and clinical implications of STD in COVID-19. Given that, to date, studies investigating olfaction disorders largely outnumber those focusing on other chemical senses, we will discuss the former with particular attention and provide a brief overview of the current literature on the latter.
2. Physiology and pathogenesis
The sense of smell results from the interactions between a volatile compound and the chemoreceptors expressed on the olfactory sensory neurons. The olfactory sensory neurons are located at the top of the nasal cavity and are surrounded by supporting cells, including sustentacular cells, microvillar cells, mucous-secreting Bowman’s glands, and stem cells. Upon activation of olfactory sensory neurons, the action potential is transmitted to the olfactory bulb and subsequently to the amygdala, the hippocampus, and the primary olfactory cortex. Alterations at any point in this pathway may lead to olfactory disorders [10].
The perception of flavors is complex and involves the senses of taste and smell as well as chemesthesis. The sense of taste requires the activation of gustatory receptors on the tongue, which receive innervation from cranial nerves VII, IX, and X and recognize the five taste modalities—that is, sweet, bitter, salty, sour, and umami. The gustatory cues, however, are combined with the sensations provided by retronasal olfaction to give rise to flavors [11]. Finally, chemesthesis contributes to perception of certain food characteristics, such as spiciness or cold, through sensitive afferents of the trigeminal nerve. As a result of the olfactory-gustatory interactions underlying flavor perception, patients often find it difficult to distinguish between ageusia or dysgeusia and olfactory disorders, and therefore smell and taste symptoms are often reported together [12].
Olfactory disorders could be distinguished into conductive and sensorineural [13]. Conductive disorders are caused by a mechanical obstacle that impedes the interactions between olfactory neurons and volatile compounds. In the context of an upper respiratory tract infection, this is due to the production of excessive mucus and/or to the swelling of the respiratory epithelium mucosa. On the other hand, sensorineural disorders result from injury of neuronal structures, most often olfactory sensory neurons, or olfactory bulbs. Olfactory disorders have been reported in infections caused by several respiratory viruses, including coronaviruses [14,15]. They usually follow the onset of respiratory symptoms and are associated with inflammatory changes in the respiratory mucosa and mucous discharge [16,17].
In contrast, COVID-19 patients usually report a loss of taste or smell without nasal congestion or discharge [18,19]. These features suggest that anosmia could possibly be the consequence of a localized impairment of airflow conduction or of a sensorineural damage.
Of interest, imaging studies in SARS-CoV-2 infected subjects have indicated a swelling and obstruction of respiratory clefts, which are the narrow passages which allow inspired air to reach the olfactory epithelium [20]. Indeed, a bilateral obstruction of respiratory clefts, detected by computed tomography and magnetic resonance imaging, has been reported in a young female patient with COVID-19 associated anosmia without rhinorrhea [20]. A larger and more recent study correlated magnetic resonance findings to objective evaluation of olfaction in 20 patients with COVID-19, observing an impaired smell detection associated with olfactory cleft obstruction in 95 % of patients; interestingly, at the 1-month follow-up, the majority of patients recovered from anosmia and resolved olfactory cleft obstruction [21].
Nevertheless, the development or persistence of anosmia after resolution of respiratory symptoms [22], as well as the report of symptoms such as phantosmia and parosmia, might be consistent with a sensorineural anosmia. The known neuroinvasive potential of other coronaviruses [23] has led to the speculation that COVID-19-related anosmia could reflect direct infection, injury, and death of neuronal cells [19].
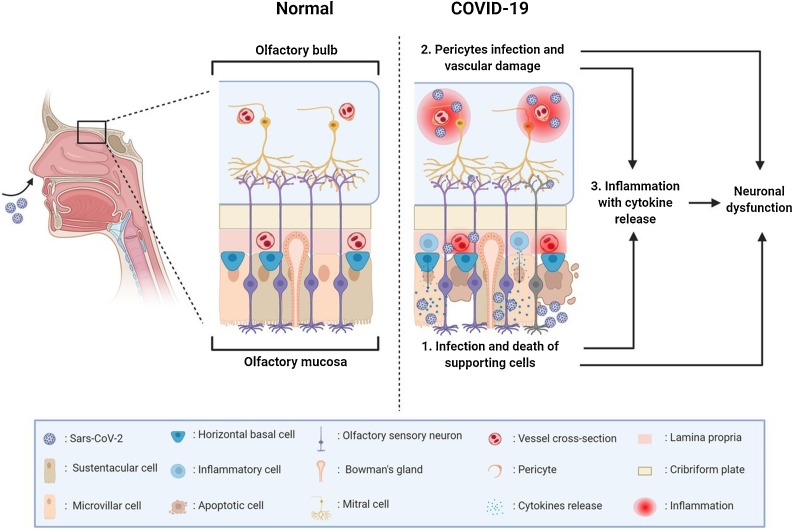
For cell infection, SARS-CoV-2 requires the binding to a surface cell receptor for the spike protein, which is identified in the angiotensin converting enzyme (ACE)-2 protein, and the proteolytic action of host’s proteases like TMPRSS2 [24,25]. Recent single-cell RNA-sequencing and immunostaining studies have demonstrated that ACE-2 is not expressed by olfactory sensory neurons and olfactory bulbs mitral cells, although it is expressed at a significant level by other supporting cells in the olfactory mucosa, including sustentacular and microvillar cells [24,26]. Viral infection of vascular pericytes (which express ACE-2) and/or immune-mediated vascular damage in both olfactory mucosa and olfactory bulb have also been hypothesized as a possible cause of olfactory impairment; indeed, a magnetic resonance microscopy study found evidence of microvascular injury in the olfactory bulbs of COVID-19 patients [27]. SARS-CoV-2 infection could thus give rise to anosmia by different, nonmutually exclusive mechanisms (Fig. 1 ) [26,28].
Fig. 1.
Possible pathogenesis of olfactory disorders in COVID-19. Olfactory disorders in COVID-19 may results from: 1) Infection and damage of supporting cells of the olfactory epithelium, leading to inflammation and alterations in local homeostasis; 2) Infection or immune-mediated damage of endothelial cells and vascular pericytes, leading to hypoperfusion and inflammation. In both cases, recruitment of inflammatory cells, cytokine release and generation of neurotoxic compounds may indirectly influence the neuronal signaling. Olfactory cleft obstruction and possibly direct infection of neuronal cells may also occur. (Created with Biorender.com).
The lack of ACE-2 expression by olfactory sensory neurons argues against their direct infection in COVID-19. However, the SARS-CoV-2 antigen has been detected in olfactory sensory neurons in a hamster model of infection [29], but intranasal SARS-CoV-2 inoculation in animal models has not been consistently associated with identification of viral antigens in brain tissue [30,31]. Of note, a recently published study on post-mortem samples revealed the co-localization of a coronavirus antigen and SARS-CoV-2 RNA in olfactory sensory neurons of patients deceased with COVID-19. Intriguingly, viral RNA was also detected in central nervous system areas not directly connected with olfactory structures, arguing for a possible SARS-CoV-2 neurotropism [32].
In summary, the currently available evidence suggests that the most likely cause of anosmia during COVID-19 is an altered function of olfactory sensory neurons, associated with the infection and death of supporting cells, microvillar cells, and vascular pericytes. However, other inflammation-mediated mechanisms, involving focal mucosal swelling and airflow obstruction could also possibly occur, and the hypothesis of a direct infection of olfactory sensory neurons deserves additional investigations.
Only limited data are available on the mechanisms involved in the pathogenesis of taste disorders in COVID-19 [33]. Single cell RNA-sequencing studies demonstrated that epithelial cells of the tongue express ACE-2 receptors at a significant level, arguing for a possible role of the buccal mucosa as an entry door for SARS-CoV-2 [34]. Of note, a study on mouse model suggested no expression of ACE-2 in taste buds but showed a considerable expression in epithelial cells of the basal region of filiform papillae [35]. Thus it could be hypothesized that, similarly to what suggested for olfactory disorders, the pathogenesis of taste disorders in COVID-19 may involve indirect damage of taste receptors through infection of epithelial cells and subsequent local inflammation.
3. Epidemiologic and clinical features
The proportion of COVID-19 subjects experiencing STD is considerable, around 41 % and 62 % according to two recent meta-analyses [36,37]. STD are usually reported within three days from the beginning of other COVID-19 manifestations [6,38] and have presented as the first symptoms in up to one quarter of the cases [39].
The possible use of STD for diagnosis of SARS-CoV-2 infection in subjects with clinical suspicion is an area of active research. Reporting STD was associated with the highest odd-ratio of SARS-CoV-2 infection in two large studies—one performed by the use of a smartphone app and involving more than two million people, and the other that prospectively followed a population of healthcare workers [40,41]. Self-reported STD in patients presenting at emergency departments with respiratory symptoms had a low sensitivity (22 %) but a high specificity (97 %) for the diagnosis of SARS-CoV-2 infection, which is similar to the sensitivity and specificity reported for a history of close contact with a confirmed COVID-19 case [4]. A recent, prospective diagnostic study which evaluated olfactory function in a large cohort of patients prior to COVID-19 testing confirmed these findings, reporting similar values of sensitivity and specificity [42].
Indeed, STD could be useful in distinguishing COVID-19 from other upper respiratory tract infections. A case-control study showed a higher prevalence of STD in COVID-19 patients (39 %) compared to an age- and sex-matched control cohort of patients with H1N1 influenza (12.5 %) [18].
Thus, investigating the presence of STD may be helpful for identifying subjects with cold-like symptoms who are likely to test positive for SARS-CoV-2 and could prompt the testing of patients reporting no symptoms of respiratory tract involvement [43].
The clinical evaluation of chemical senses alterations during COVID-19 could be challenging. Most of the studies on STD have been carried out by self-reporting questionnaires and phone interviews (i.e., subjective evaluations). These approaches, while enabling the evaluation of large-scale cohorts of patients, are associated with predictable bias. Such limitations can be overcome by using standardized tests (i.e., objective evaluations) [[44], [45], [46]], where patients are asked to recognize a number of odorants and/or foods [47]. In two different studies in which objective evaluations of STD were used, the proportion of COVID-19 patients with olfactory alterations was 73 % and 98 %, which is considerably higher than what was observed in self-reported questionnaires [5,48]. A recent meta-analysis confirmed these findings, reporting a prevalence of smell disorders of 77 % by objective assessment but of only 44 % by subjective evaluation [49].
Only few studies have explored taste and smell disorders separately, mainly due to the olfactory-gustatory interactions underlying multisensory flavor perception. Of note, in a study that investigated chemosensory perceptions, 60 % of patients reported a selective decrease in one or more specific taste modalities, most often the gustation of salty taste [50]. Further observations, possibly involving the use of objective tests to evaluate gustation, are needed to address the potential clinical interest of taste disorders in COVID-19.
While rarely used to investigate chemical senses disorders, imaging studies could show pathological findings in several patients with STD. Besides the aforementioned obstruction of respiratory clefts, brain magnetic resonance may reveal bilateral olfactory bulbs’ hyperintensity and enlargement in fluid-attenuated inversion recovery and T2 sequences. These features, which are coherent with the presence of local edema and inflammation, intriguingly disappear after the resolution of symptoms [51,52].
STD seem to not influence neither the clinical course of COVID-19 nor its severity. Although early reports suggested a milder course of COVID-19 in subjects experiencing anosmia [53], larger cross-sectional and case-control studies argued against this hypothesis, showing no differences in the rate of hospitalization or in the severity of disease between patients with and without STD [38].
The evolution and prognosis of STD in COVID-19 appears to be favorable, but the timing of resolution may vary [54]. Median duration has been reported to be around 10 days in subjects with mild COVID-19, with a complete resolution of STD in 89 % of patients after 4 weeks from diagnosis [55]. However, some observational studies have shown that a more prolonged course could be possible [22], with about one-third of subjects reporting only a partial improvement of STD 40 days after diagnosis, and a small proportion (5%) reporting no improvement. A loss of olfactory sensory neurons due to dysfunction of supporting cells, inflammation-related apoptosis, or possibly direct infection could be hypothesized in patients showing slow recovery from of STD [56]. In this case, symptom resolution would occur after recruitment of olfactory epithelium reserve stem cells. Distortions of olfaction such as parosmia or phantosmia might emerge during this period due to the immaturity of recently formed neuronal networks; however, these symptoms have been rarely reported in COVID-19 [50].
Post-viral and post-traumatic STD could influence severely the quality of life of affected subjects [57]. Few papers have explored this topic in COVID-19; a recent preprint suggested that long-term lasting alterations in chemicals senses after SARS-CoV-2 infection could have a considerable impact on daily living [58]. Unfortunately, the treatment of these conditions is challenging. Some benefit has been reported with the use of systemic and local glucocorticoids [59] and with olfactory training [60]. However, no data are available to date on the efficacy of these measures in post−COVID-19 STD.
4. Conclusions
STD are frequent in COVID-19, appear early in the course of the disease, and can be the only symptom of infection. Preliminary evidence does not support a primary role for direct infection of olfactory sensory neurons and taste buds in causing STD, suggesting that the loss of function of such neuronal structures may rather be a consequence of the infection of non-neuronal cells in the olfactory epithelium, oral mucosa, and possibly the olfactory bulb. However, the contrasting data on the penetration of SARS-CoV-2 in olfactory neurons highlight the need for further investigations.
STD detection could be useful to identify and isolate patients with suspected COVID-19, especially when the prevalence of undifferentiated upper respiratory tract infection is high (e.g., winter months). Moreover, the presence of chemosensory alterations could prompt SARS-CoV-2 testing in afebrile patients with no respiratory symptoms. The assessment of STD by objective evaluations should be encouraged in both research and clinical practice, given the substantial higher sensitivity and lower risk of bias of these methods compared to subjective evaluations. Moreover, differential assessment of taste and chemesthetic functions may also be relevant.
Defining STD pathogenesis in COVID-19 could help to elucidate a possible mechanism of SARS-CoV-2 neuroinvasion and the relationship with other central nervous system disorders during the disease. Finally, a better knowledge of the mechanisms associated with STD could help in developing new therapeutic options for subjects with long-lasting impairment of taste and olfaction.
Funding
This study was in part supported by the Italian Ministry of Health (Ricerca Corrente).
References
- 1.Zhu N., Zhang D., Wang W., et al. A novel coronavirus from patients with pneumonia in China. N. Engl. J. Med. 2019;2020(382):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hornuss D., Lange B., Schröter N., Rieg S., Kern W.V., Wagner D. Anosmia in COVID-19 patients. Clin. Microbiol. Infect. 2020 doi: 10.1016/j.cmi.2020.05.017. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7242197/. Accessed 13 September 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wee L.E., Chan Y.F.Z., Teo N.W.Y., et al. The role of self-reported olfactory and gustatory dysfunction as a screening criterion for suspected COVID-19. Eur. Arch. Otorhinolaryngol. 2020;277:2389–2390. doi: 10.1007/s00405-020-05999-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaira L.A., Deiana G., Fois A.G., et al. Objective evaluation of anosmia and ageusia in COVID-19 patients: single-center experience on 72 cases. Head Neck. 2020;42:1252–1258. doi: 10.1002/hed.26204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Speth M.M., Singer-Cornelius T., Oberle M., Gengler I., Brockmeier S.J., Sedaghat A.R. Olfactory dysfunction and sinonasal symptomatology in COVID-19: prevalence, severity, timing, and associated characteristics. Otolaryngol. Head. Neck Surg. 2020;163:114–120. doi: 10.1177/0194599820929185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huart C., Philpott C., Konstantinidis I., et al. Comparison of COVID-19 and common cold chemosensory dysfunction. Rhinology. 2020 doi: 10.4193/Rhin20.251. [DOI] [PubMed] [Google Scholar]
- 8.Giacomelli A., Pezzati L., Conti F., et al. Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross-sectional study. Clin. Infect. Dis. 2020;71:889–890. doi: 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klopfenstein T., Zahra H., Kadiane-Oussou N.J., et al. New loss of smell and taste: uncommon symptoms in COVID-19 patients on Nord Franche-Comte cluster, France. Int. J. Infect. Dis. 2020 doi: 10.1016/j.ijid.2020.08.012. Available at: http://www.sciencedirect.com/science/article/pii/S1201971220306378. Accessed 15 September 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel R.M., Pinto J.M. Olfaction: anatomy, physiology, and disease. Clin. Anat. 2014;27:54–60. doi: 10.1002/ca.22338. [DOI] [PubMed] [Google Scholar]
- 11.Simon S.A., de Araujo I.E., Gutierrez R., Nicolelis M.A.L. The neural mechanisms of gustation: a distributed processing code. Nat. Rev. Neurosci. 2006;7:890–901. doi: 10.1038/nrn2006. [DOI] [PubMed] [Google Scholar]
- 12.Landis B.N., Frasnelli J., Reden J., Lacroix J.S., Hummel T. Differences between orthonasal and retronasal olfactory functions in patients with loss of the sense of smell. Arch. Otolaryngol. Head Neck Surg. 2005;131:977–981. doi: 10.1001/archotol.131.11.977. [DOI] [PubMed] [Google Scholar]
- 13.Hummel T., Whitcroft K.L., Andrews P., et al. Position paper on olfactory dysfunction. Rhinol. Suppl. 2017;54:1–30. doi: 10.4193/Rhino16.248. [DOI] [PubMed] [Google Scholar]
- 14.Cavazzana A., Larsson M., Münch M., Hähner A., Hummel T. Postinfectious olfactory loss: a retrospective study on 791 patients. Laryngoscope. 2018;128:10–15. doi: 10.1002/lary.26606. [DOI] [PubMed] [Google Scholar]
- 15.Pellegrino R., Cooper K.W., Di Pizio A., Joseph P.V., Bhutani S., Parma V. Coronaviruses and the chemical senses: past, present, and future. Chem. Senses. 2020;45:415–422. doi: 10.1093/chemse/bjaa031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dalton P. Olfaction and anosmia in rhinosinusitis. Curr. Allergy Asthma Rep. 2004;4:230–236. doi: 10.1007/s11882-004-0031-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seiden A.M. Postviral olfactory loss. Otolaryngol. Clin. North Am. 2004;37:1159–1166. doi: 10.1016/j.otc.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Beltrán-Corbellini Á, Chico‐García J.L., Martínez‐Poles J., et al. Acute-onset smell and taste disorders in the context of COVID-19: a pilot multicentre polymerase chain reaction based case–control study. Eur. J. Neurol. 2020;27:1738–1741. doi: 10.1111/ene.14273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cocco A., Amami P., Desai A., Voza A., Ferreli F., Albanese A. Neurological features in SARS-CoV-2-infected patients with smell and taste disorder. J. Neurol. 2020 doi: 10.1007/s00415-020-10135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eliezer M., Hautefort C., Hamel A.-L., et al. Sudden and complete olfactory loss of function as a possible symptom of COVID-19. JAMA Otolaryngol. Head Neck Surg. 2020;146:674. doi: 10.1001/jamaoto.2020.0832. [DOI] [PubMed] [Google Scholar]
- 21.Eliezer M., Hamel A.-L., Houdart E., et al. Loss of smell in patients with COVID-19: MRI data reveal a transient edema of the olfactory clefts. Neurology. 2020;95:e3145–e3152. doi: 10.1212/WNL.0000000000010806. [DOI] [PubMed] [Google Scholar]
- 22.Chiesa‐Estomba C.M., Lechien J.R., Radulesco T., et al. Patterns of smell recovery in 751 patients affected by the COVID-19 outbreak. European Journal of Neurology. 2020 doi: 10.1111/ene.14440. Available at: https://onlinelibrary.wiley.com/doi/abs/10.1111/ene.14440. Accessed 13 September 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Netland J., Meyerholz D.K., Moore S., Cassell M., Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J. Virol. 2008;82:7264–7275. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen M., Shen W., Rowan N.R., et al. Elevated ACE2 expression in the olfactory neuroepithelium: implications for anosmia and upper respiratory SARS-CoV-2 entry and replication. Eur. Respir. J. 2020 doi: 10.1183/13993003.01948-2020. Available at: https://erj.ersjournals.com/content/early/2020/07/23/13993003.01948-2020. Accessed 12 September 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffmann M., Kleine-Weber H., Schroeder S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181 doi: 10.1016/j.cell.2020.02.052. 271-280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brann D.H., Tsukahara T., Weinreb C., et al. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci. Adv. 2020;6:eabc5801. doi: 10.1126/sciadv.abc5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee M.-H., Perl D.P., Nair G., et al. Microvascular injury in the brains of patients with Covid-19. N. Engl. J. Med. 2020;0 doi: 10.1056/NEJMc2033369. null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sims J.T., Krishnan V., Chang C.-Y., et al. Characterization of the cytokine storm reflects hyperinflammatory endothelial dysfunction in COVID-19. J. Allergy Clin. Immunol. 2020 doi: 10.1016/j.jaci.2020.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang A.J., AC-Y Lee, Chu H., et al. Severe acute respiratory syndrome coronavirus 2 infects and damages the mature and immature olfactory sensory neurons of hamsters. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa995. Available at: https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciaa995/5871998. Accessed 5 October 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun S.-H., Chen Q., Gu H.-J., et al. A mouse model of SARS-CoV-2 infection and pathogenesis. Cell Host Microbe. 2020;28 doi: 10.1016/j.chom.2020.05.020. 124-133.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Munster V.J., Feldmann F., Williamson B.N., et al. Respiratory disease in rhesus macaques inoculated with SARS-CoV-2. Nature. 2020;585:268–272. doi: 10.1038/s41586-020-2324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meinhardt J., Radke J., Dittmayer C., et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2020:1–8. doi: 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- 33.Cooper K.W., Brann D.H., Farruggia M.C., et al. COVID-19 and the chemical senses: supporting players take center stage. Neuron. 2020;107:219–233. doi: 10.1016/j.neuron.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu H., Zhong L., Deng J., et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci. 2020;12:1–5. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Z., Zhou J., Marshall B., Rekaya R., Ye K., Liu H.-X. SARS-CoV-2 Receptor ACE2 Is Enriched in a Subpopulation of Mouse Tongue Epithelial Cells in Nongustatory Papillae but Not in Taste Buds or Embryonic Oral Epithelium. ACS Pharmacol. Transl. Sci. 2020;3:749–758. doi: 10.1021/acsptsci.0c00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agyeman A.A., Chin K.L., Landersdorfer C.B., Liew D., Ofori-Asenso R. Smell and taste dysfunction in patients with COVID-19: a systematic review and meta-analysis. Mayo Clin. Proc. 2020;95:1621–1631. doi: 10.1016/j.mayocp.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rocke J., Hopkins C., Philpott C., Kumar N. Is loss of sense of smell a diagnostic marker in COVID-19: a Systematic Review and Meta-analysis. Clin. Otolaryngol. 2020 doi: 10.1111/coa.13620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neto D.B., Fornazieri M.A., Dib C., et al. Chemosensory dysfunction in COVID-19: prevalences, recovery rates, and clinical associations on a large brazilian sample. Otolaryngol. Head. Neck Surg. 2020 doi: 10.1177/0194599820954825. Available at: https://journals.sagepub.com/doi/10.1177/0194599820954825. Accessed 13 September 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaye R., Chang C.W.D., Kazahaya K., Brereton J., Denneny James C., III COVID-19 anosmia reporting tool: initial findings. Otolaryngol. Head. Neck Surg. 2020 doi: 10.1177/0194599820922992. Available at: https://journals.sagepub.com/doi/10.1177/0194599820922992. Accessed 12 September 2020. [DOI] [PubMed] [Google Scholar]
- 40.Menni C., Valdes A.M., Freidin M.B., et al. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat. Med. 2020;26:1037–1040. doi: 10.1038/s41591-020-0916-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iversen K., Bundgaard H., Hasselbalch R.B., et al. Risk of COVID-19 in health-care workers in Denmark: an observational cohort study. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30589-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Villerabel C., Makinson A., Jaussent A., et al. Diagnostic value of patient-reported and clinically tested olfactory dysfunction in a population screened for COVID-19. Jama Otolaryngol. Neck Surg. 2021 doi: 10.1001/jamaoto.2020.5074. Available at: https://doi.org/10.1001/jamaoto.2020.5074. Accessed 1 September 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bénézit F., Turnier P.L., Declerck C., et al. Utility of hyposmia and hypogeusia for the diagnosis of COVID-19. Lancet Infect. Dis. 2020;20:1014–1015. doi: 10.1016/S1473-3099(20)30297-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Double K.L., Rowe D.B., Hayes M., et al. Identifying the pattern of olfactory deficits in parkinson disease using the brief smell identification test. Arch. Neurol. 2003;60:545–549. doi: 10.1001/archneur.60.4.545. [DOI] [PubMed] [Google Scholar]
- 45.Saito S., Ayabe-Kanamura S., Takashima Y., et al. Development of a smell identification test using a novel stick-type odor presentation kit. Chem. Senses. 2006;31:379–391. doi: 10.1093/chemse/bjj042. [DOI] [PubMed] [Google Scholar]
- 46.Mueller C.A., Grassinger E., Naka A., Temmel A.F.P., Hummel T., Kobal G. A self-administered odor identification test procedure using the “Sniffin’ sticks”. Chem. Senses. 2006;31:595–598. doi: 10.1093/chemse/bjj064. [DOI] [PubMed] [Google Scholar]
- 47.Kobayashi M., Reiter E.R., DiNardo L.J., Costanzo R.M. A new clinical olfactory function test: cross-cultural influence. Arch. Otolaryngol. Head Neck Surg. 2007;133:331. doi: 10.1001/archotol.133.4.331. [DOI] [PubMed] [Google Scholar]
- 48.Moein S.T., Hashemian S.M., Mansourafshar B., Khorram-Tousi A., Tabarsi P., Doty R.L. Smell dysfunction: a biomarker for COVID-19. Int. Forum Allergy Rhinol. 2020;10:944–950. doi: 10.1002/alr.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hannum M.E., Ramirez V.A., Lipson S.J., et al. Objective sensory testing methods reveal a higher prevalence of olfactory loss in COVID-19–positive patients compared to subjective methods: a systematic review and meta-analysis. Chem. Senses. 2020 doi: 10.1093/chemse/bjaa064. Available at:https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7543258/Accessed 21 November 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parma V., Ohla K., Veldhuizen M.G., et al. More than Smell-COVID-19 is associated with severe impairment of smell, taste, and chemesthesis. Chem. Senses. 2020;45:609–622. doi: 10.1093/chemse/bjaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Politi Ls, Salsano E., Grimaldi M. Magnetic resonance imaging alteration of the brain in a patient with coronavirus disease 2019 (COVID-19) and anosmia. JAMA Neurol. 2020;77:1028–1029. doi: 10.1001/jamaneurol.2020.2125. [DOI] [PubMed] [Google Scholar]
- 52.Galougahi M.K., Ghorbani J., Bakhshayeshkaram M., Naeini A.S., Haseli S. Olfactory bulb magnetic resonance imaging in SARS-CoV-2-Induced anosmia: the first report. Acad. Radiol. 2020;27:892–893. doi: 10.1016/j.acra.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yan C.H., Faraji F., Prajapati D.P., Ostrander B.T., DeConde A.S. Self-reported olfactory loss associates with outpatient clinical course in COVID-19. Int. Forum Allergy Rhinol. 2020;10:821–831. doi: 10.1002/alr.22592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hopkins C., Surda P., Whitehead E., Kumar B.N. Early recovery following new onset anosmia during the COVID-19 pandemic - an observational cohort study. J. Otolaryngol. Head Neck Surg. 2020;49:26. doi: 10.1186/s40463-020-00423-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boscolo-Rizzo P., Borsetto D., Fabbris C., et al. Evolution of altered sense of smell or taste in patients with mildly symptomatic COVID-19. JAMA Otolaryngol. Head Neck Surg. 2020 doi: 10.1001/jamaoto.2020.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Welge-Lüssen A., Wolfensberger M. Olfactory disorders following upper respiratory tract infections. Adv. Otorhinolaryngol. 2006;63:125–132. doi: 10.1159/000093758. [DOI] [PubMed] [Google Scholar]
- 57.Croy I., Nordin S., Hummel T. Olfactory disorders and quality of life—an updated review. Chem. Senses. 2014;39:185–194. doi: 10.1093/chemse/bjt072. [DOI] [PubMed] [Google Scholar]
- 58.Watson D.L.B., Campbell M., Hopkins C., Smith B., Kelly C., Deary V. Altered Smell and Taste: anosmia, parosmia and the impact of long Covid-19. medRxiv. 2020 doi: 10.1371/journal.pone.0256998. 2020.11.26.20239152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seo B.S., Lee H.J., Mo J.-H., Lee C.H., Rhee C.-S., Kim J.-W. Treatment of postviral olfactory loss with glucocorticoids, Ginkgo biloba, and mometasone nasal spray. Arch. Otolaryngol. Head Neck Surg. 2009;135:1000–1004. doi: 10.1001/archoto.2009.141. [DOI] [PubMed] [Google Scholar]
- 60.Damm M., Pikart L.K., Reimann H., et al. Olfactory training is helpful in postinfectious olfactory loss: a randomized, controlled, multicenter study. Laryngoscope. 2014;124:826–831. doi: 10.1002/lary.24340. [DOI] [PubMed] [Google Scholar]