Abstract
Block copolymer nanoparticles prepared via polymerization-induced self-assembly (PISA) represent an emerging class of organic Pickering emulsifiers. Such nanoparticles are readily prepared by chain-extending a soluble homopolymer precursor using a carefully selected second monomer that forms an insoluble block in the chosen solvent. As the second block grows, it undergoes phase separation that drives in situ self-assembly to form sterically stabilized nanoparticles. Conducting such PISA syntheses in aqueous solution leads to hydrophilic nanoparticles that enable the formation of oil-in-water emulsions. Alternatively, hydrophobic nanoparticles can be prepared in non-polar media (e.g., n-alkanes), which enables water-in-oil emulsions to be produced. In this review, the specific advantages of using PISA to prepare such bespoke Pickering emulsifiers are highlighted, which include fine control over particle size, copolymer morphology, and surface wettability. This has enabled various fundamental scientific questions regarding Pickering emulsions to be addressed. Moreover, block copolymer nanoparticles can be used to prepare Pickering emulsions over various length scales, with mean droplet diameters ranging from millimeters to less than 200 nm.
Introduction
At the turn of the last century, Ramsden1 and Pickering2 independently discovered that various types of particles can stabilize emulsions. Over the past two decades, seminal studies by Binks and co-workers have led to a resurgence of interest in such Pickering emulsions.3−8 This is because particulate emulsifiers offer numerous advantages over conventional surfactant or polymeric emulsifiers, including superior long-term emulsion stability and reduced foaming during homogenization.6 Consequently, Pickering emulsions have been evaluated for various applications in food manufacturing,9−11 agrochemicals,12−15 cosmetics,16,17 and pharmaceuticals.17−20
It is well known that surfactants typically adsorb and desorb from interfaces on rapid time scales.21 Unlike surfactants, colloidal particles that adsorb at oil/water or air/water interfaces are not necessarily amphiphilic.3,5−7,22,23 Nevertheless, particles are often irreversibly adsorbed at an interface if they are of sufficient size and have appropriate surface wettability.24−26 The driving force for particle adsorption is minimization of the interfacial area, which lowers the free energy of the system.6,21 The amount of energy, ΔE, required to remove a spherical particle of radius r from the oil/water interface is given by eq 1(27)
| 1 |
where γow is the oil/water interfacial tension and θw is the three-phase contact angle. Figure 1 shows how the three-phase contact angle affects the detachment energy for a 20 nm particle adsorbed at the toluene/water interface.5 The calculated energy of detachment is greatest for θw = 90° and falls rapidly on either side of this value. The contact angle is directly related to the particle wettability, which dictates the type of emulsion that is formed.6 More specifically, hydrophilic particles are preferentially wetted by the aqueous phase (θw < 90°) and hence form oil-in-water (o/w) emulsions. In contrast, hydrophobic particles (θw > 90°) produce water-in-oil (w/o) emulsions.5 In principle, using a judicious combination of hydrophilic and hydrophobic particles should enable the preparation of either water-in-oil-in-water (w/o/w) or oil-in-water-in-oil (o/w/o) Pickering double emulsions.28,29
Figure 1.
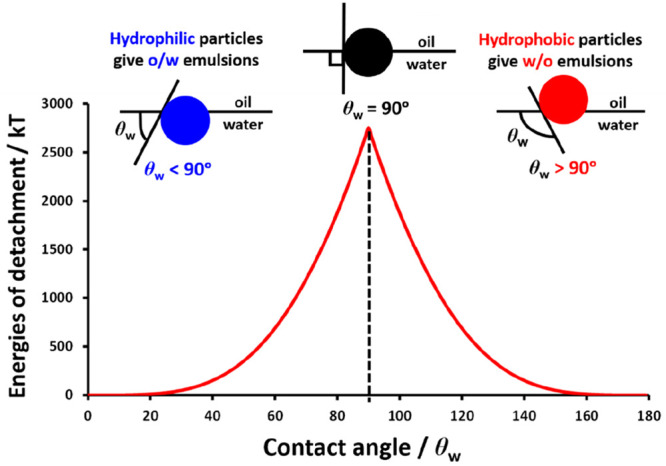
Spatial location of a spherical particle adsorbed at a planar oil–water interface for a contact angle θw measured through the aqueous phase such that θw is less than 90° (blue), equal to 90° (black), or greater than 90° (red). In general, hydrophilic particles (θw < 90°) form oil-in-water (w/o) Pickering emulsions whereas hydrophobic particles (θw > 90°) give rise to water-in-oil (w/o) Pickering emulsions. The energy of detachment versus the contact angle is shown for the specific case of a spherical nanoparticle of 10 nm radius adsorbed at a planar toluene–water interface for which γow = 0.036 N m–1.5,6
Many types of inorganic particles have been utilized as Pickering emulsifiers, including silica,3,30 titania,31,32 magnetite,33 and clay.3,4,30,31,33−37 Similarly, various organic particles such as cellulose nanorods,38−41 carbon black,42,43 carbon nanotubes,44 graphene oxide sheets,45,46 and aqueous polymer particles (e.g., latexes,22,47−54 microgels,55,56 and block copolymer nanoparticles57) have been evaluated in this context. Within the latter category, it is typically found that charge-stabilized latexes produce w/o emulsions whereas sterically stabilized latexes usually form o/w emulsions, as depicted in Figure 2.22,49 On the basis of pioneering studies by Binks and others, the use of inorganic particles to form Pickering emulsions is well understood.6,21,26,35,58−65 In the prototypical case of silica, particle wettability can be tuned by partial alkylation of the silanol surface groups5 or by adding either a cationic surfactant61,66 or an electrolyte.3,34 However, such approaches tend to produce incipient flocculation in solution, which in turn leads to the formation of relatively thick multilayers of adsorbed particles. In principle, polymer-based particles offer several advantages as Pickering emulsifiers. If they are designed to have appropriate surface wettability, then no surface modification is required and adsorption at the oil–water interface leads to the formation of well-defined monolayers.3,8,22,51,57,67−74 Moreover, surface wettability can be readily tuned by selecting an appropriate steric stabilizer block.73
Figure 2.
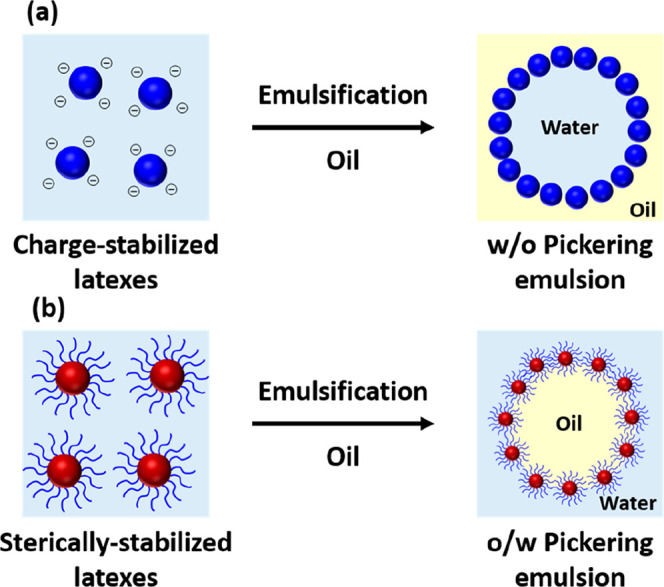
Schematic representation of the formation of (a) water-in-oil (w/o) Pickering emulsions using charge-stabilized latex particles or (b) oil-in-water (o/w) Pickering emulsions using sterically stabilized latex particles via high-shear homogenization of an aqueous dispersion of latex particles with oil.
Velev and co-workers were the first to report using latex particles as Pickering emulsifiers.47 In this case, the oil phase was 1-octanol and charge-stabilized polystyrene particles bearing either sulfate or amidine surface groups were utilized. Subsequently, Binks et al. used near-monodisperse polystyrene latex particles to stabilize w/o Pickering emulsions using cyclohexane as a model oil.22 Weitz and co-workers prepared colloidosomes using water-in-decalin Pickering emulsions stabilized by 0.7 μm poly(methyl methacrylate) latex particles coated in a layer of poly(hydroxystearic acid).48
Subsequently, Binks, Armes, and co-workers prepared a pH-sensitive polystyrene latex using a poly[2-(dimethylamino)ethyl methacrylate-block-methyl methacrylate] (PDMA-PMMA) diblock copolymer as a steric stabilizer. The cationic character of the PDMA block could be adjusted by controlling the solution pH.49 Such latex particles adsorbed onto n-hexadecane droplets when high-shear homogenization was conducted at pH 8 to produce stable Pickering emulsions. However, stable emulsions could not be obtained at pH 3 because protonation of the PDMA block led to highly hydrophilic particles that were insufficiently wetted by the oil phase. Thus, such latexes simply exhibit pH-dependent Pickering emulsifier behavior,75 as opposed to the pH-responsive behavior that was originally (and erroneously) reported.49,50 In a related study, the thermoresponsive nature of the same PDMA-PMMA-stabilized polystyrene latex particles was explored.51 Heating an o/w emulsion stabilized by such particles up to 70 °C (i.e., above the cloud point of the hydrophilic PDMA block) led to significant droplet coalescence. Moreover, w/o emulsions were obtained if the same aqueous latex and oil were separately heated to 70 °C prior to emulsification. The relatively hydrophobic nature of the flocculated particles under such conditions accounts for this phase inversion.51
Subsequently, Fujii et al. prepared lightly cross-linked poly(4-vinylpyridine)/silica nanocomposite particles for use as stimulus-responsive Pickering emulsifiers.76,77 Such particles stabilized Pickering emulsions at pH 8–9, but the addition of acid caused rapid demulsification. This is because protonation of the 4-vinylpyridine units at low pH induces particle swelling: lateral repulsion between the resulting highly swollen cationic microgel-like particles leads to their desorption from the oil–water interface. Similarly, pH-responsive Pickering emulsifiers based on polymer latexes have also been reported. For example, Morse and co-workers prepared lightly cross-linked latexes composed of either poly(2-(tert-butylamino)ethyl methacrylate) or poly(2-(diethylamino)ethyl methacrylate).78,79 Such sterically stabilized latexes act as effective Pickering emulsifiers at pH 10, but acidification resulted in rapid demulsification owing to a latex-to-microgel transition. In principle, such Pickering emulsifiers can be reused by raising the solution pH to its original value. In practice, the progressive build-up of background salt leads to a gradual reduction in the extent of microgel swelling, which effectively limits the number of pH cycles.79
Another class of stimulus-responsive Pickering emulsifiers is the poly(N-isopropylacrylamide) (PNIPAM)-based microgels originally reported by Ngai et al. and further developed by Richtering and co-workers.55,56,80,81 PNIPAM homopolymer exhibits a lower critical solution temperature (LCST) of around 32 °C.82 Thus, aqueous dispersion copolymerization of NIPAM with bis(acrylamide) cross-linker using persulfate as a free radical initiator at 70 °C affords a charge-stabilized latex, and the resulting lightly cross-linked particles exhibit a latex-to-microgel transition on cooling below this temperature.83−85 Moreover, pH-responsive PNIPAM-based microgels can be prepared by introducing methacrylic acid (MAA) as a comonomer. Ngai et al. reported the first example of a Pickering emulsifier that exhibited both pH-responsive and thermoresponsive behavior.55,80 The incorporation of MAA units within the PNIPAM-based particles led to microgel swelling on raising the solution pH. Thus, o/w emulsions stabilized by such P(NIPAM-co-MAA) microgels are stable at 25 °C and pH 9.4 but become unstable at 60 °C on lowering the pH to 6.1.55,80 This is because the adsorbed microgels shrink at the oil–water interface, thus leading to a reduction in surface coverage and hence droplet coalescence.86 In follow-up studies, Richtering and co-workers have postulated that the viscoelastic behavior of the microgel-coated interface determines the emulsion stability.56,81,87−90
The invention of living anionic polymerization by Szwarc et al.91,92 in the 1950s ultimately enabled the rational design of various examples of amphiphilic diblock copolymers such as poly(ethylene oxide)-polystyrene or poly(acrylic acid)-polystyrene. It has been well established by Eisenberg et al.93−95 and others96 that such amphiphilic diblock copolymers undergo self-assembly in aqueous solution to form spherical, worm-like, or vesicular nano-objects.93,94,96−98 Such self-assembly is enthalpically driven and depends on both χ and N, where χ is the Flory–Huggins interaction parameter and N is the overall degree of polymerization of the copolymer chains.97 However, traditional post-polymerization processing routes invariably involve an organic cosolvent such as DMF or THF, gradual addition of water over prolonged time scales, and relatively low copolymer concentrations (<1.0% w/w), which unfortunately preclude many potential commercial applications.
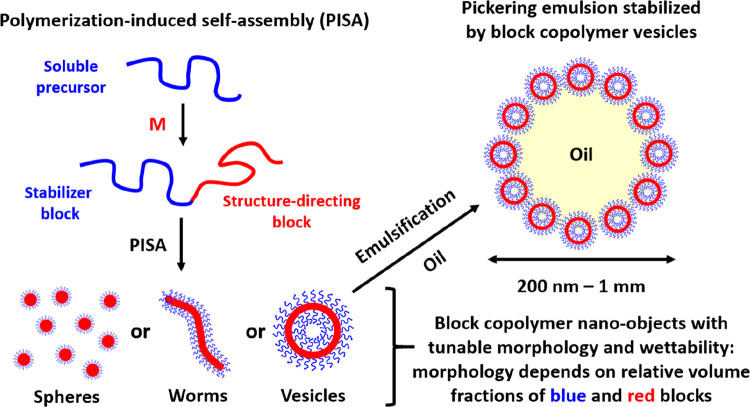
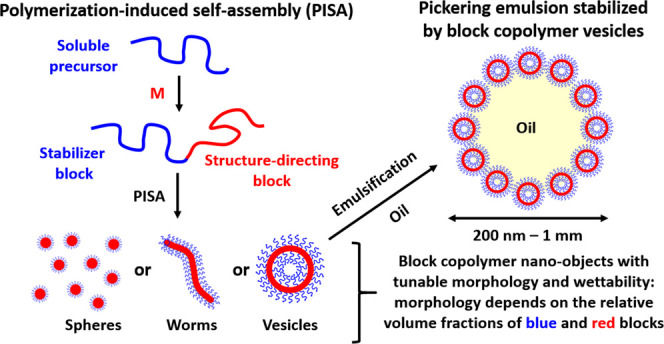
Fortunately, the development of controlled radical polymerization techniques99−101 such as reversible addition–fragmentation chain transfer (RAFT) polymerization102−105 has enabled the efficient synthesis of block copolymer nano-objects via polymerization-induced self-assembly (PISA).106−115 Importantly, RAFT polymerization is exceptionally tolerant of monomer functionality, which enables the rational design of nano-objects bearing hydroxyl, amine, or carboxylic acid groups. Moreover, such PISA syntheses can be conducted at relatively high copolymer concentrations (up to 50% w/w).116,117 In a typical protocol, a soluble homopolymer is chain-extended using an appropriate monomer in a suitable solvent such that the growing second block gradually becomes insoluble, which drives in situ self-assembly to form diblock copolymer nanoparticles, as depicted in Figure 3. Depending on the solubility of the monomer in the continuous phase, the PISA synthesis involves either dispersion or emulsion polymerization.108,118−134 Systematic variation of the relative volume fractions of the two blocks provides control over the copolymer morphology.116,135,136 Over the past decade or so, the generic nature of PISA has been demonstrated for a wide range of vinyl monomers in various solvents including water,132,137−143 polar solvents (e.g., ethanol or methanol),144−157 non-polar solvents (e.g., n-alkanes),110,158−163 ionic liquids,164 silicone oil,165,166 and supercritical CO2.167−170 Typically, pseudo-phase diagrams are constructed to enable the reproducible targeting of morphologies for a given PISA formulation.140 The basic design rules for the preparation of spheres,140,158 worms,171−175 vesicles,176−179 framboidal vesicles,72,137,180,181 and lamellae182−184 are now well-established. In many cases, the final copolymer morphology is dictated primarily by the relative volume fractions of the two blocks, as indicated by the geometric packing parameter introduced by Israelachvili and co-workers to account for surfactant self-assembly.185 For example, spheres are produced when using a relatively long soluble stabilizer block and/or working at relatively low copolymer concentrations,140,158 whereas vesicles can be obtained by targeting highly asymmetric diblock compositions (i.e., relatively long insoluble blocks) at higher copolymer concentrations.137,145 It is also well-established that worm-like particles typically occupy relatively narrow phase space between that of spheres and vesicles,162,171,172 framboidal vesicles can be produced from ABC triblock copolymers if the B and C blocks are both insoluble and enthalpically incompatible,72,186 and targeting stiff, inflexible insoluble blocks favors lamellae formation.183,184
Figure 3.
Schematic representation of polymerization-induced self-assembly (PISA), whereby a soluble blue precursor block is chain-extended using a suitable vinyl monomer to produce a red insoluble structure-directing block. Depending on the relative volume fractions of the blue and red blocks, in situ self-assembly produces either spheres, worms, or vesicles. PISA can be conducted in either water or various oils. In the case of aqueous PISA, the addition of a suitable oil followed by emulsification via high-shear homogenization leads to the formation of Pickering emulsions, as illustrated above for the case of vesicles.187
Recently, we have exploited PISA to design new block copolymer nano-objects for use as bespoke Pickering emulsifiers.71−74,117,187−195 More specifically, PISA enables the copolymer morphology and surface chemistry to be tuned by the judicious selection of the soluble stabilizer and insoluble structure-directing blocks. Such syntheses can be conducted in either water or n-alkanes to afford either hydrophilic or hydrophobic sterically stabilized nanoparticles, respectively. Such nanoparticles can be used to prepare oil-in-water,117,187,188 water-in-oil,71,189 and multiple emulsions.73,191 In particular, the versatility offered by PISA enables interesting scientific questions to be addressed in the context of Pickering emulsions. Do linear block copolymer nanoparticles survive high-shear homogenization or is their covalent stabilization required? Can we readily distinguish between these two scenarios? Can vesicles be used to stabilize Pickering emulsions? Do worms offer any advantages over spheres? Does refractive index matching enable highly transparent Pickering emulsions to be prepared? Can spheres be made sufficiently small (and stable) to enable the preparation of Pickering nanoemulsions? What is the effect of introducing minimal nanoparticle surface charge on the formation and stability of Pickering emulsions? Such research topics are discussed in the remaining sections of this feature article.
Effect of Copolymer Morphology on Pickering Emulsifier Performance
Thompson et al. reported the first example of polymer-based Pickering emulsifiers prepared via PISA.187 Linear poly(glycerol monomethacrylate)-poly(2-hydroxypropyl methacrylate) [PGMA45-PHPMA200] vesicles were prepared at 10% w/w solids using a RAFT aqueous dispersion polymerization formulation (Figure 4a). However, such non-crosslinked vesicles did not survive the high-shear homogenization conditions required for emulsification with n-dodecane. Instead, in situ dissociation occurred, and the resulting oil droplets became stabilized by individual amphiphilic PGMA45-PHPMA200 chains. This problem was confirmed using two characterization techniques. First, the volume-average oil droplet diameter determined by laser diffraction proved to be independent of the copolymer concentration (Figure 4b), whereas a strong concentration dependence is invariably observed for Pickering emulsions.58,196
Figure 4.
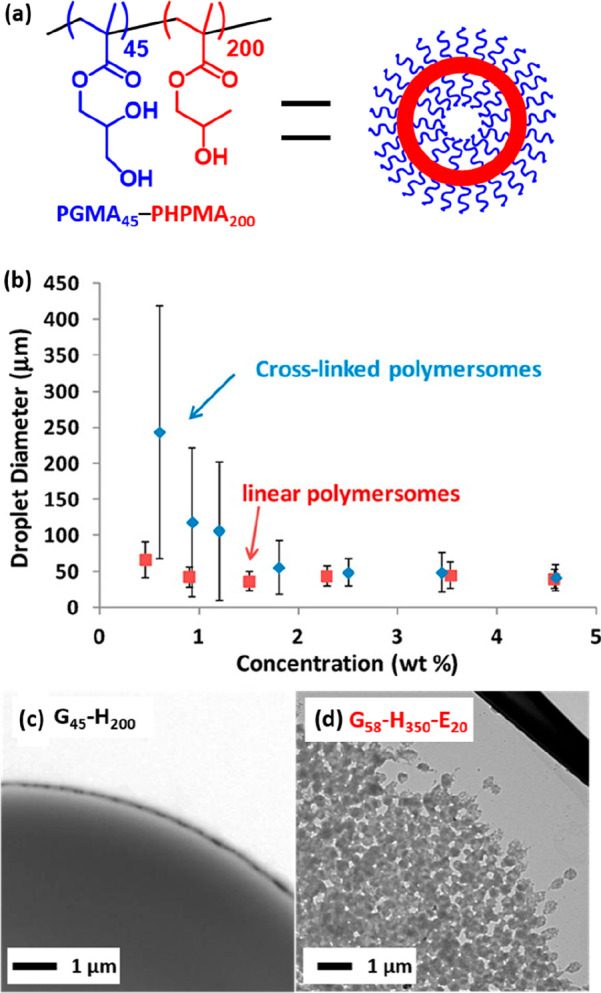
(a) Chemical structure of linear PGMA45-PHPMA200 vesicles. (b) Volume-average droplet diameter (obtained by laser diffraction) vs copolymer concentration for both linear PGMA45-PHPMA200 and cross-linked PGMA58-PHPMA350-PEGDMA20 vesicles. TEM images recorded for an individual dried, cross-linked colloidosome prepared using (c) linear PGMA45-PHPMA200 vesicles and (d) cross-linked PGMA58-PHPMA350-PEGDMA20 vesicles. Reproduced from ref (187) (copyright 2012 American Chemical Society).
Second, TEM studies of the dried oil droplets indicated a smooth, featureless morphology with no evidence of the original vesicles (Figure 4c). This study highlighted the importance of verifying the formation of genuine Pickering emulsions when using block copolymer nanoparticles. In situ vesicle dissociation was attributed to the weakly hydrophobic nature of the membrane-forming PHPMA block.197,198 In view of this problem, ethylene glycol dimethacrylate (EGDMA) was added as a third comonomer to form cross-linked vesicles, which proved to be stable when subjected to high-shear homogenization.187 In this case, the expected upturn in oil droplet diameter was observed as the vesicle concentration was lowered. Furthermore, TEM studies revealed the presence of intact vesicles at the oil/water interface (Figure 4c). Such vesicle-stabilized Pickering emulsions could be covalently stabilized by dissolving a tolylene-2,4-diisocyanate-terminated poly(propylene glycol) diisocyanate cross-linker (PPG-TDI) in the oil phase prior to homogenization, leading to the formation of so-called colloidosomes.48,187,199
Turbidimetry experiments indicated that most of the vesicles were not adsorbed at the oil/water interface and instead remained within the continuous aqueous phase. As the copolymer concentration used to prepare such Pickering emulsions was reduced from 2.5 to 0.6% w/w, the vesicle adsorption efficiency increased from 57 to 78% w/w. The relatively weak affinity of the vesicles for the oil/water interface is presumably related to their aqueous cores, which necessarily lowers the Hamaker constant and hence reduces the enthalpy of adsorption.
Subsequently, Thompson and co-workers reported that linear PGMA-PHPMA spheres and worms also underwent in situ dissociation to form soluble copolymer chains during high-shear homogenization.188 However, laser diffraction studies confirmed that this problem could be circumvented by either covalent stabilization using EGDMA cross-linker or by the addition of a sufficiently hydrophobic third block such as poly(benzyl methacrylate) (PBzMA) (Figure 5). Using the former strategy, PGMA100-PHPMA200-PEGDMA20 spheres and PGMA45-PHPMA100-PEGDMA10 worms were prepared via PISA, and their performance as putative Pickering emulsifiers for the stabilization of n-dodecane-in-water emulsions was compared.188 It is well established that worms are formed during PISA via the 1D stochastic fusion of multiple spheres.139,140,200 This is important because it means that the mean worm thickness is directly related to the dimensions of the initial spheres. Moreover, given that both types of nanoparticles utilized a hydroxyl-functional PGMA block as a steric stabilizer (Figure 4a), essentially identical surface wettabilities can be assumed. Thompson and co-workers188 argued that, for sufficiently anisotropic worms, their specific surface area, Aw, can be estimated using the relation Aw ≈ 2/ρR, where ρ is the particle density and R is the mean worm cross-sectional radius. In contrast, prior to their 1D fusion to form worms, the spheres have a specific surface area, As, given by As = 3/ρr, where r is the mean sphere radius and, to a reasonable approximation, r ≈ R. Therefore, the reduction in specific surface area (Aw/As) that occurs during the 1D fusion of multiple spheres to form a single worm is only around 33%, whereas the energy of attachment of a sufficiently anisotropic worm (L/2R > 20) composed of x spheres is estimated to be at least x times higher than the individual spherical nanoparticles. In summary, highly anisotropic diblock copolymer worms are expected to adsorb at an oil–water interface much more strongly than the corresponding precursor diblock copolymer spheres while retaining a relatively high specific surface area. Turbidimetry studies conducted on the lower aqueous phase formed after emulsion creaming indicated relatively high adsorption efficiencies (∼90%) for both spheres and worms. More importantly, the worms produced significantly finer n-dodecane droplets than the spheres. This was attributed to the highly anisotropic nature of the former nanoparticles, which allows the droplet surface to become sufficiently coated to prevent coalescence at approximately half the surface coverage. Similar observations were made by Vermant and co-workers, who found that Pickering emulsions prepared using polystyrene rods were more stable relative to those prepared using their spherical precursors.201,202 Such experiments also account for the excellent Pickering emulsion performance observed for highly anisotropic cellulose nanofibers, for which no spherical counterparts exist.38 More broadly, various groups have reported that model anisotropic particles differ fundamentally in their interfacial adsorption behavior compared to isotropic particles.54,203−205
Figure 5.
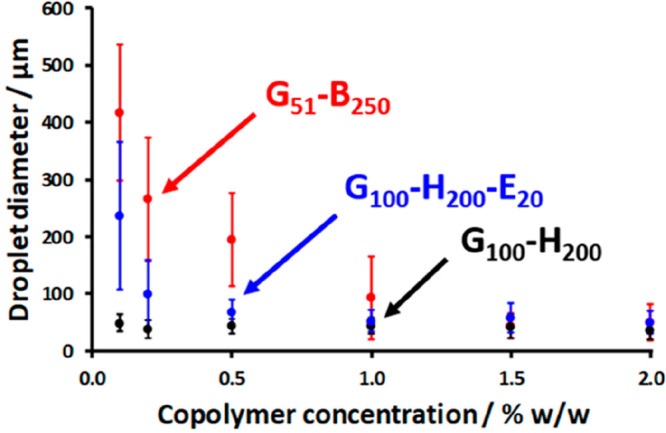
Volume-average droplet diameter versus copolymer concentration plot obtained by laser diffraction analysis of n-dodecane-in-water emulsions prepared using linear (red) PGMA51-PBzMA250, (black) PGMA100-PHPMA200 spheres, and (blue) cross-linked PGMA100-PHPMA200-PEGDMA20 spheres. Error bars represent the standard deviation for each droplet diameter rather than the experimental error. Reproduced from ref (188) (copyright 2014 Royal Society of Chemistry).
Thompson et al. also directly compared the Pickering emulsifier performance of linear hydrophobic poly(lauryl methacrylate)-poly(benzyl methacrylate) (PLMA-PBzMA) worms and spheres prepared in n-dodecane.189 For this PISA formulation, the worms are thermoresponsive and can be transformed into spheres when heated to 150 °C owing to surface plasticization of the core-forming PBzMA chains.159 Moreover, this morphological transition is effectively irreversible if it is conducted at sufficiently low copolymer concentration (e.g., ≤1.0% w/w).159 Thus the Pickering emulsifier performance of highly anisotropic PLMA16-PBzMA37 worms for the stabilization of w/o emulsions could be compared to that of chemically identical spheres for the first time. Again, significantly smaller mean droplet diameters (D) were observed for the worms when working above a certain critical copolymer mass (mp). Furthermore, the fractional droplet surface coverage, C, differed markedly for worms and spheres (Figure 6). As expected, spherical nanoparticles exhibited a constant surface coverage with copolymer concentration. In contrast, higher surface coverages were observed for worms at higher copolymer concentration. The isotropic nature of spheres means that maximum packing requires six interparticle contacts with nearest neighbors, whereas worms can form a loose packing at low concentration and a more densely packed layer at relatively high concentration. Similar observations have been made for anisotropic cellulose nanofibers.38,39 Relatively short fibers formed a densely packed layer at the oil/water interface, whereas longer fibers led to lower surface coverages with a more open 2D network.39 Small-angle X-ray scattering (SAXS) studies conducted on a worm-stabilized Pickering emulsion indicated that the mean thickness of the worm layer surrounding the water droplets is comparable to the worm cross-sectional diameter. This indicates monolayer coverage rather than multilayer formation. Finally, the thermoresponsive behavior of PLMA16-PBzMA37 worms was exploited to induce demulsification. Heating the w/o Pickering emulsion to 95 °C induced a worm-to-sphere transition, with concomitant droplet coalescence being observed owing to copolymer desorption from the oil/water interface.
Figure 6.
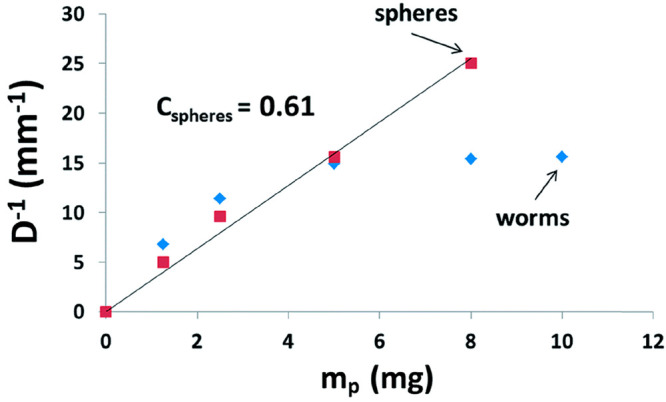
Effect of varying the copolymer particle mass mp on the mean droplet diameter D for two series of water-in-n-dodecane emulsions stabilized using PLMA16-PBzMA37 spheres (red circles) and PLMA16-PBzMA37 worms (blue squares). Note the deviation from linearity for the latter particles. Reproduced from ref (189) (copyright 2015 Royal Society of Chemistry).
Xue and co-workers compared the stability of diblock copolymer worms and spheres when such nano-objects were subjected to high-shear homogenization.206 To prepare such diblock copolymer nanoparticles, poly(N-(2-methacryloyloxy)ethyl pyrrolidone) (PNMEP53) was chain-extended by the RAFT polymerization of 2-perfluorooctylethyl methacrylate (FMA) in chloroform. The resulting PNMEP53-PFMAx block copolymers were then self-assembled to form either spheres (x = 5) or worms (x = 10) in water by traditional post-polymerization processing via a solvent switch. Oil-in-water Pickering emulsions were prepared by the high-shear homogenization of aqueous dispersions of such nanoparticles with n-dodecane. TEM and laser diffraction studies confirmed that both types of nanoparticles survived emulsification, presumably owing to the highly hydrophobic nature of the PFMA core-forming block. This study used the twisted intramolecular charge transfer state (TICT) of Nile Red to distinguish between the fluorescence of this dye dissolved in n-dodecane droplets and that within the nanoparticle cores. More specifically, the excitation and emission wavelengths for Nile Red dissolved in n-dodecane are 490–520 and 530–570 nm, respectively, whereas these bands are red-shifted to 576 and 621 nm for the dye-loaded nanoparticles.206 Thus, if Nile red was solubilized within the nanoparticles prior to emulsification, excitation at 576 nm led to significantly greater fluorescence intensity than that observed for the oil droplets, indicating that the nanoparticles were adsorbed at the oil/water interface in the form of Pickering emulsions.
The effect of varying the shear rate on the fluorescence intensity of the dye dissolved in the oil droplets (Ioil) relative to that for the dye-loaded PNMEP53-PFMA5 spheres (Ilayer) was also examined. As expected, greater shear rates led to higher Ioil/Ilayer ratios (Figure 7).206 For example, dye fluorescence originating from the oil droplets dominates at 24 000 rpm, indicating that such conditions cause in situ nanoparticle dissociation, leading to emulsion stabilization by the individual amphiphilic PNMEP53-PFMA5 diblock copolymer chains. A similar experiment was conducted using the PNMEP53-PFMA10 worms. In this case, at least some of the worms remained intact at 24 000 rpm. The authors of this study attributed this observation to the worms being less susceptible to degradation under shear than the spheres. However, it seems much more likely that the greater stability of the worms is simply the result of the higher DP of the hydrophobic PFMA block that is required to form such nano-objects.188 Although these PNMEP53-PFMAx spheres and worms were prepared by traditional postpolymerization processing, this study is clearly consistent with the observation of in situ nanoparticle dissociation reported when using linear diblock copolymer nano-objects prepared via PISA. Moreover, it confirms that such dissociation can occur even when using highly hydrophobic perfluorinated structure-directing blocks, although the mean DPs of such chains are admittedly rather low.
Figure 7.
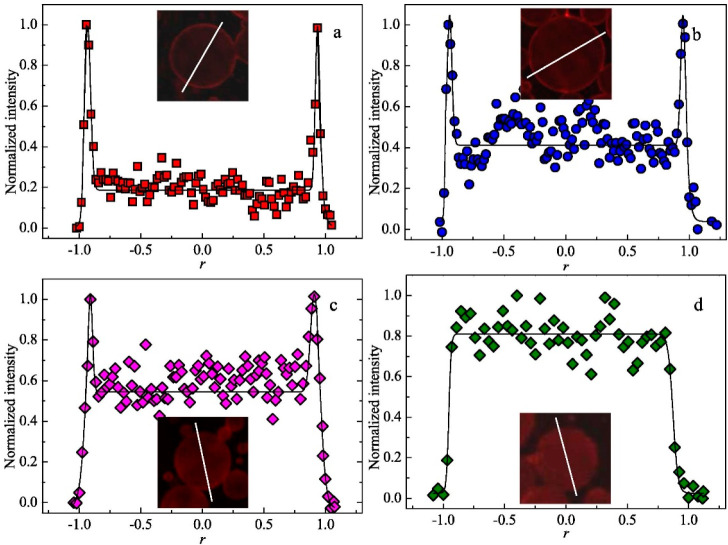
Fluorescence data recorded as a function of distance r (with data fits using both Gaussian and Boltzmann methods) obtained for an n-dodecane-in-water Pickering emulsion prepared at an oil volume fraction of 0.50 using 0.50% w/w PNMEP53-PFMA5 via high-shear homogenization at (a) 6000, (b) 12 000, (c) 18 000, or (d) 24 000 rpm, respectively. In each case, the inset confocal microscopy image shows the individual emulsion droplet, and the white line indicates the cross-sectional diameter through which the fluorescence intensity is calculated as a function of r. Reproduced from ref (206) (copyright 2020 Elsevier).
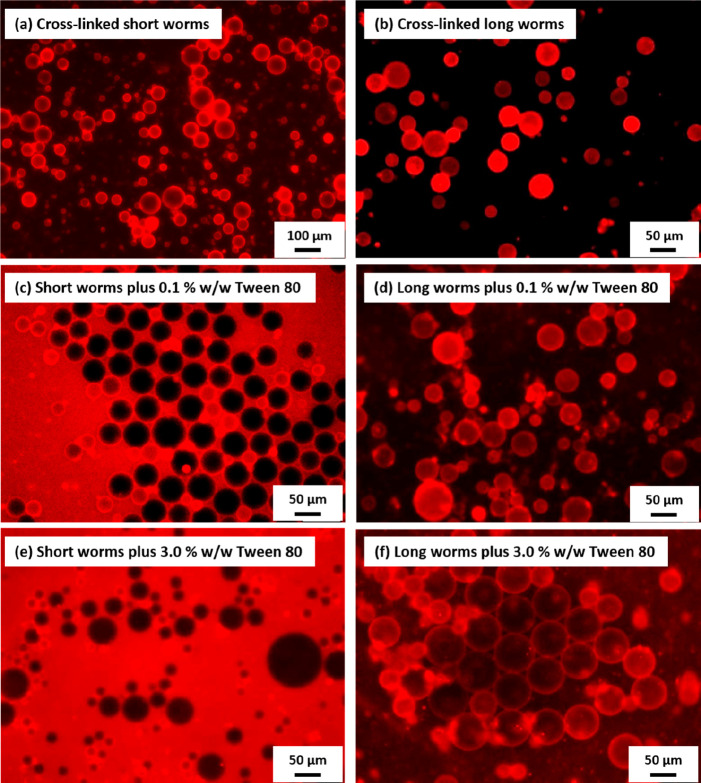
Recently, we reported the effect of nanoparticle anisotropy on the stability of an o/w Pickering emulsion in the presence of a non-ionic surfactant.194 RAFT aqueous dispersion polymerization was used to prepare epoxy-functional PGMA48-P(HPMA90-stat-GlyMA15) worms (where GlyMA denotes glycidyl methacrylate). The thermoresponsive nature of such linear precursor nanoparticles was exploited to produce two types of cross-linked worms of essentially the same copolymer composition.207 More specifically, 3-aminopropyltriethoxysilane (APTES) was utilized in a post-polymerization cross-linking protocol developed by Lovett et al.208 The primary amine group in this reagent reacts with the epoxy groups on the GlyMA units while its siloxy groups react with the secondary alcohol groups on the HPMA units to confer covalent stabilization. Either relatively long or relatively short cross-linked worms were prepared to stabilize n-dodecane-in-water Pickering emulsions, with a fluorescent label being introduced by reacting rhodamine B piperazine with a minor fraction of the epoxy groups on the GlyMA residues prior to APTES addition. This enabled fluorescence microscopy to be used to monitor the precise location of the worms before and after addition of a non-ionic surfactant (Tween 80) to each Pickering emulsion (Figure 8). A much higher surfactant concentration was required to displace long worms from the oil/water interface compared to the short worms. This is because the former nanoparticles are much more strongly adsorbed than the latter.188,189
Figure 8.
Fluorescence microscopy images obtained for emulsions prepared by the high-shear homogenization of 0.25% w/w aqueous PGMA48-P(HPMA90-stat-GlyMA15) copolymer dispersions with 50 vol % n-dodecane at 13 500 rpm for 2 min before and after the addition of either 0.1 or 3.0% w/w non-ionic surfactant (Tween 80). (a) Pickering emulsion stabilized using short PGMA48-P(HPMA90-stat-GlyMA15) cross-linked worms. (b) Pickering emulsion stabilized using long PGMA48-P(HPMA90-stat-GlyMA15) cross-linked worms. (c) Surfactant-stabilized emulsion obtained after the addition of 0.1% w/w Tween 80, which displaces the short worms initially adsorbed at the oil/water interface. (d) Pickering emulsion obtained after the addition of 0.1% w/w Tween 80, which cannot displace the long worms initially adsorbed at the oil/water interface. (e) Surfactant-stabilized emulsion obtained after the addition of 3.0% w/w Tween 80, which displaces the short worms adsorbed at the oil/water interface. (f) Mixed emulsion obtained after the addition of 3.0% w/w Tween 80, which partially displaces the long worms adsorbed at the oil/water interface. Reproduced from ref (194) (copyright 2018 American Chemical Society).
Zhang and co-workers utilized cross-linked triblock copolymer worms to prepare high-internal-phase Pickering emulsions (HIPEs) in which the volume fraction of the dispersed phase exceeded 0.74.209 Such worms were first prepared via RAFT dispersion polymerization of BzMA in ethanol using a poly(2-(dimethylamino)ethyl methacrylate) (PDMA) precursor. These linear PDMA37-PBzMA96 worms were subsequently cross-linked via chain extension using EGDMA. After transferring the covalently stabilized PDMA37-PBzMA96-PEGDMA9 worms into water, the resulting dispersion was subjected to high-shear homogenization with varying amounts of cyclohexane. Highly viscous HIPEs possessing an internal phase ranging from 0.77 to 0.84 exhibited good long-term stability. Furthermore, a remarkably low copolymer concentration (0.3%) was sufficient to stabilize a HIPE prepared at an oil volume fraction of 0.77. This remarkable observation was attributed to the dense gel network formed by the highly anisotropic worms. To prepare porous monoliths, either silica or Fe3O4 nanoparticles were added to the aqueous phase prior to homogenization to act as a co-stabilizer. After freeze-drying for 12 h, the 3D hierarchical structure survived in the form of a free-standing porous monolith. In the case of the Fe3O4 nanoparticles, such ultralight hybrid materials proved to be responsive to an applied magnetic field.209
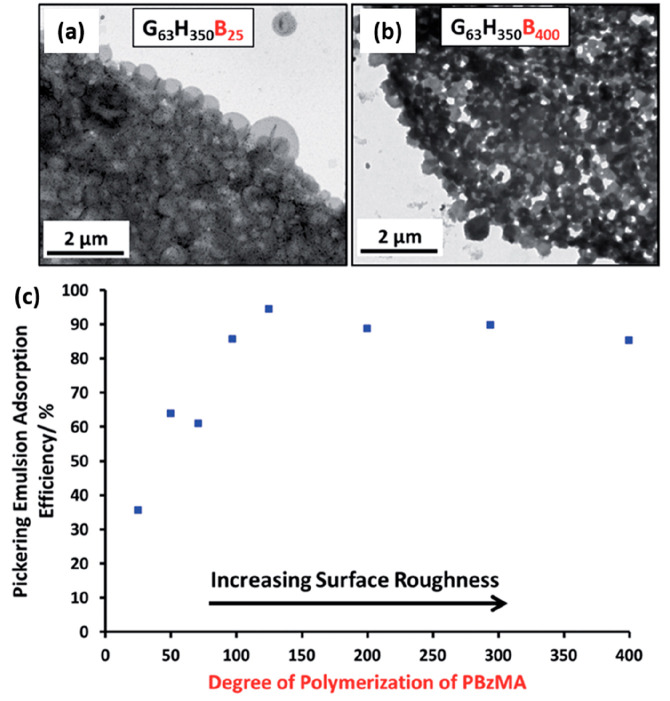
Chambon and co-workers reported that the chain extension of PGMA-PHPMA precursor vesicles using a water-immiscible monomer such as BzMA or MMA resulted in the formation of framboidal (raspberry-like) triblock copolymer vesicles via seeded RAFT aqueous emulsion polymerization.186 Subsequently, a series of PGMA63-PHPMA350-PBzMAz framboidal vesicles were evaluated by Mable et al. as putative Pickering emulsifiers.72 As expected, the PGMA63-PHPMA350 precursor vesicles did not survive the high-shear conditions required to generate Pickering emulsions. In contrast, PGMA63-PHPMA350-PBzMAz vesicles led to the formation of genuine Pickering emulsions, as confirmed by laser diffraction and TEM studies.72 Moreover, the strongly hydrophobic nature of the third PBzMA block proved to be sufficient to prevent vesicle dissociation. Turbidimetric analysis of the lower aqueous phase after emulsion creaming was again used to assess the Pickering emulsifier performance of these framboidal vesicles. Systematic variation of the DP(z) of the PBzMA block enabled their surface roughness to be tuned, which enabled the adsorption efficiency to be determined as a function of surface roughness (Figure 9c). Increasing the PBzMA DP(z) from 25 to 125 at a constant copolymer concentration led to an increase in adsorption efficiency from 36 to 94%. Furthermore, framboidal vesicles with optimal surface roughness exhibited significantly higher adsorption efficiency than that observed for non-framboidal PGMA63-PHPMA350-PEGDMA20 cross-linked vesicles (67%).187
Figure 9.
TEM images obtained for Pickering emulsions comprising n-dodecane droplets stabilized using aqueous dispersions of (a) PGMA63-PHPMA350-PBzMA25 and (b) PGMA63-PHPMA350-PBzMA400 vesicles. (c) Variation of Pickering emulsion adsorption efficiency (Aeff) against PBzMA DP for a series of PGMA63-PHPMA350-PBzMA vesicles of increasing surface roughness. Reproduced from ref (72) (copyright 2015 Royal Society of Chemistry).
Another example of framboidal vesicles was reported by Xiu and co-workers.181 In this case, PGMA-PHPMA precursor vesicles were chain-extended using GlyMA via seeded RAFT aqueous emulsion polymerization, resulting in the formation of epoxy-functional framboidal vesicles. Such framboidal vesicles were shown to be an efficient Pickering emulsifier for n-hexane-in-water emulsions, with higher PGlyMA DPs and copolymer concentrations leading to the formation of finer oil droplets.
The same research group also evaluated so-called multicompartment block copolymer nanoparticles (MBCPs) as Pickering emulsifiers.210 Such nanoparticles were prepared via photoinitiated PISA in a two-step synthesis. First, a poly(poly(ethylene glycol) methyl ether acrylate) (PPEGA) precursor was chain-extended via the RAFT aqueous dispersion polymerization of HPMA to yield well-defined spheres. Such spheres were then chain-extended using GlyMA to produce MBCP nanoparticles. The Pickering performance of the precursor PPEGA15.6-PHPMA400 spheres was compared to that of the final PPEGA15.6-PHPMA400-PGlyMAn particles, which had a distinctly framboidal morphology. There was an upturn in the mean droplet diameter at lower copolymer concentrations, indicating the formation of genuine Pickering emulsions (Figure 10). As previously discussed, PHPMA-core diblock copolymer nanoparticles typically dissociate to form individual copolymer chains during high-shear homogenization.72,187,188,194 In contrast, laser diffraction data suggested that the PPEGA15.6-PHPMA400 precursor nanoparticles survived emulsification intact.211 Increasing the DP of the PGlyMA block up to 300 led to greater surface roughness, a lower limiting copolymer concentration, and the formation of finer emulsion droplets for a given copolymer concentration.
Figure 10.
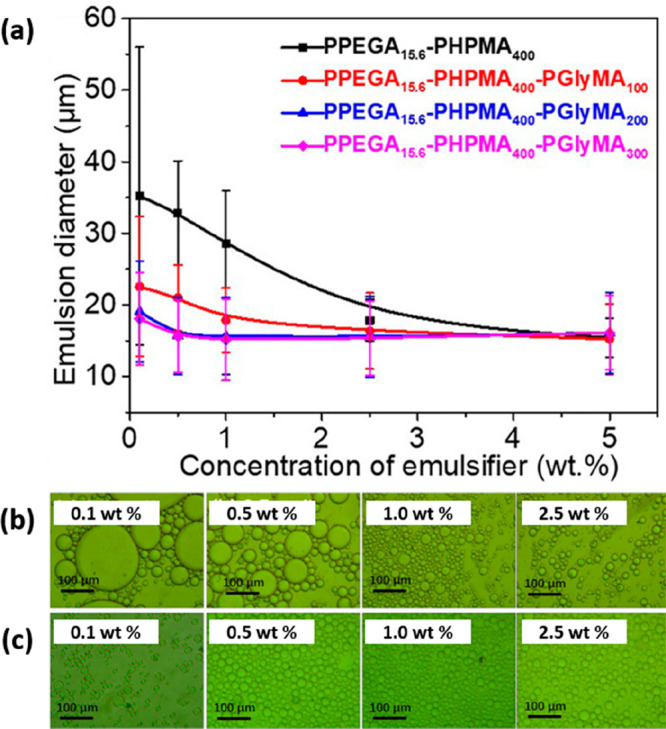
(a) Effect of varying the copolymer concentration on the mean droplet diameter of Pickering emulsions prepared using PPEGA15.6-PHPMA400-PGlyMAn multicompartment block copolymer nanoparticles (MBCPs). Optical microscopy images recorded for n-hexane-in-water emulsions stabilized using (b) PPEGA15.6-PHPMA400 precursor nanoparticles and (c) epoxy-functionalized PPEGA15.6-PHPMA400-PGlyMA300 nanoparticles at the stated copolymer concentrations. Reproduced from ref (210) (copyright 2019 American Chemical Society).
A summary of most of the block copolymer nano-objects discussed in this section and their Pickering emulsifier performance is shown in Table 1.
Table 1. Summary of Pickering Emulsions Prepared Using Block Copolymer Nanoparticles of Differing Morphologies.
| block copolymer composition | copolymer morphology | linear or cross-linked | emulsion type | genuine Pickering emulsion? | ref |
|---|---|---|---|---|---|
| PGMA45-PHPMA200 | vesicular | linear | o/w | no | (187) |
| PGMA58-PHPMA350-PEGDMA20 | vesicular | cross-linked | o/w | yes | (187) |
| PGMA63-PHPMA350-PBzMA25 | vesicular | linear | o/w | yes | (72) |
| PPEGA15.6-PHPMA400-PGlyMA300 | multicompartmental | linear | o/w | yes | (210) |
| PGMA100-PHPMA200-PEGDMA20 | spherical | cross-linked | o/w | yes | (188) |
| PGMA45-PHPMA140 | worm-like | linear | o/w | no | (188) |
| PGMA45-PHPMA100-PEGDMA10 | worm-like | cross-linked | o/w | yes | (188) |
| PGMA51-PBzMA50 | spherical | linear | o/w | yes | (188) |
| PGMA37-PHPMA60-PBzMA30 | worm-like | linear | o/w | yes | (188) |
| PNMP53-PFMA5 | spherical | linear | o/w | depends on the shear rate | (206) |
| PNMP53-PFMA10 | worm-like | linear | o/w | yes | (206) |
| PGMA48-P(HPMA90-stat-GlyMA15) | short worms | cross-linked | o/w | yes | (194) |
| PGMA48-P(HPMA90-stat-GlyMA15) | long worms | cross-linked | o/w | yes | (194) |
| PLMA16-PBzMA37 | worm-like | linear | w/o | yes | (189) |
| PLMA16-PBzMA37 | spherical | linear | w/o | yes | (189) |
Design of Pickering Emulsifiers with Tunable Surface Wettability
Using either hydrophilic or hydrophobic stabilizer blocks enables PISA to be conducted in either polar or non-polar solvents. As already noted, the chemical nature of the stabilizer block directly influences the surface wettability of such block copolymer nanoparticles and therefore dictates the type of Pickering emulsion that is formed. For example, PGMA-stabilized spheres, worms, or vesicles invariably stabilize oil-in-water emulsions.72,73,117,187,191 Clearly, the hydrophilic PGMA chains produce a three-phase particle contact angle of less than 90°. In contrast, the core-forming block has little or no influence over the surface wettability of such particles, with o/w Pickering emulsions being obtained when using either weakly hydrophobic (cross-linked) PHPMA cores187,188 or strongly hydrophobic cores such as PBzMA.117 On the other hand, using a highly hydrophobic stabilizer block such as PLMA or poly(stearyl methacrylate) (PSMA) almost invariably leads to the formation of w/o Pickering emulsions.73,74,189,212 Such nanoparticles are preferentially wetted by the oil to produce a three-phase contact angle that is greater than 90°.
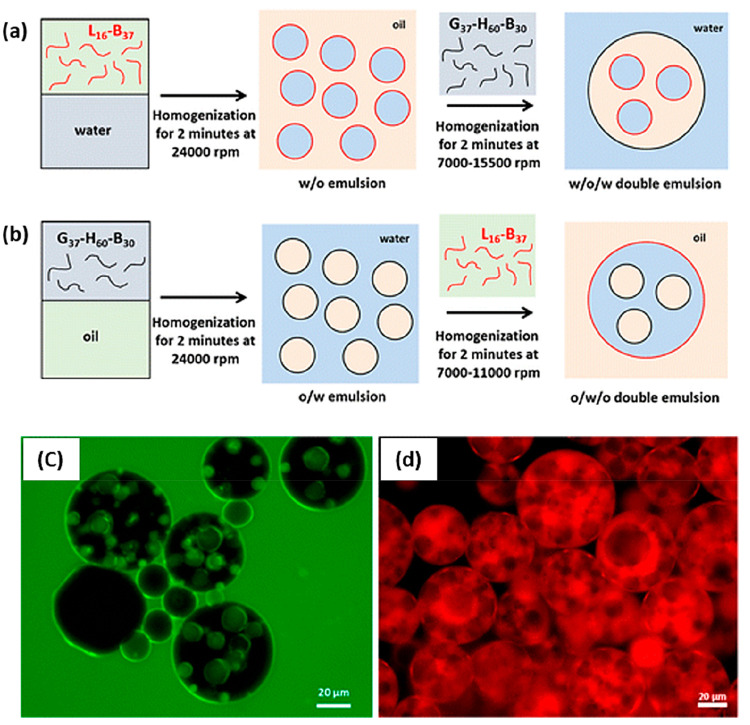
Thompson et al. used hydrophobic PLMA16-PBzMA37 worms in conjunction with hydrophilic PGMA37-PHPMA60-PBzMA30 worms to prepare Pickering double emulsions.73Figure 11 shows how either water-in-oil-in-water (w/o/w) or oil-in-water-in-oil (o/w/o) Pickering double emulsions could be obtained depending on the emulsification protocol. The former emulsions were obtained by first preparing a precursor w/o emulsion stabilized using PLMA16-PBzMA37 worms in n-dodecane. A relatively high stirring rate of 24 000 rpm was chosen to generate the smallest possible mean droplet diameter. Subsequently, this w/o emulsion was then homogenized with an equal volume of an aqueous dispersion of PGMA37-PHPMA60-PBzMA30 worms. A lower stirring rate of 7000 rpm was used in this second step to produce larger aqueous droplets and hence favor the formation of the desired w/o/w Pickering double emulsion. Similarly, o/w/o Pickering double emulsions could be prepared by first homogenizing n-dodecane to form a precursor o/w emulsion stabilized using PGMA37-PHPMA60-PBzMA30 worms, followed by its homogenization with an equal volume of n-dodecane containing PLMA16-PBzMA37 worms.
Figure 11.
Schematic representation of the preparation of (a) w/o/w double emulsions and (b) o/w/o double emulsions by the judicious combination of two types of highly anisotropic block copolymer worms as Pickering emulsifiers. Fluorescence microscopy images confirm the successful formation of w/o/w Pickering double emulsions where (c) the aqueous phase is labeled with fluorescein and (d) the n-dodecane phase is labeled with Nile Red. Reproduced from ref (73) (copyright 2015 American Chemical Society).
More recently, Rymaruk and co-workers reported that a range of poly(3-[tris(trimethylsiloxy)silyl]propyl methacrylate)-poly(benzyl methacrylate) (PSiMA-PBzMA) spheres could be prepared directly in a low-viscosity silicone oil (DM5).213 Such sterically stabilized nanoparticles were evaluated as Pickering emulsifiers for ten biosourced oils. For three of these oils, using a copolymer concentration of 2.0% w/w and a DM5 volume fraction of 0.50 led to the formation of oil-in-oil Pickering emulsions, with DM5 forming the continuous phase in each case (Figure 12a). Such emulsions remained stable for at least two months when stored at 20 °C. To improve the Pickering emulsifier performance of such PSiMA-stabilized spheres, lauryl methacrylate (LMA) was statistically copolymerized with BzMA when preparing the core-forming block. The resulting optimized PSiMA19-P(BzMA190-stat-LMA10) nanoparticles enabled the formation of stable oil-in-oil emulsions when using nine of the ten biosourced oils, as shown in Figure 12b.
Figure 12.
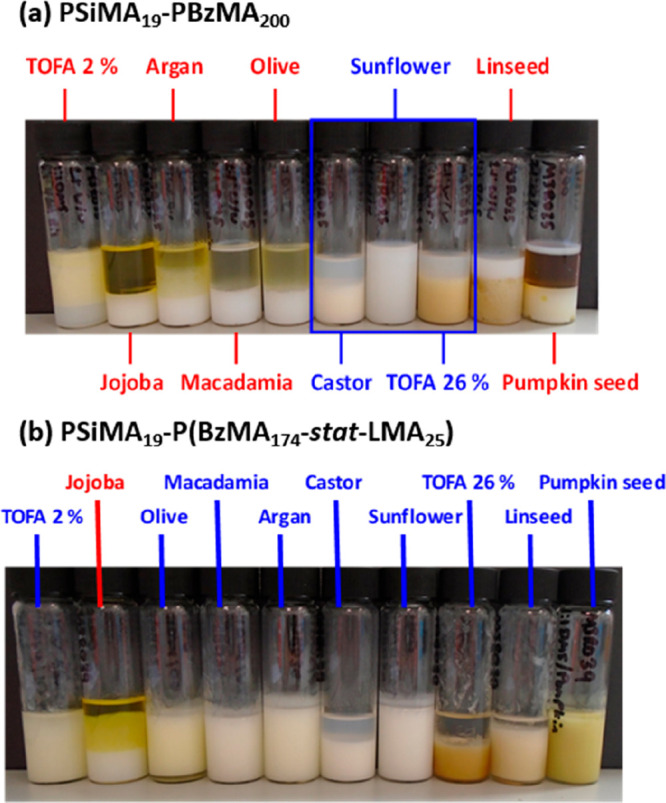
(a) Digital photograph recorded after standing for two months at 20 °C showing various biosourced oil-in-oil Pickering emulsions prepared using a 2.0% w/w dispersion of PSiMA19-PBzMA200 spheres in a silicone oil (DM5). Each biosourced oil is indicated above or below the relevant vial: emulsions that remained stable after two months are denoted in blue, whereas those that undergo (partial) phase separation on this time scale are shown in red. (b) Digital photograph of various oil-in-DM5 Pickering emulsions prepared using a 2.0% w/w dispersion of PSiMA19-P(BzMA174-stat-LMA25) spheres in DM5 recorded after storage for two months at 20 °C. In each case, the DM5 volume fraction was 0.50 and the PSiMA19-P(BzMA175-stat-LMA25) concentration was 2.0% w/w. Emulsions that remained stable after two months are indicated in blue, whereas the single jojoba oil-based emulsion that underwent phase separation over this time period is shown in red. Reproduced from ref (213) (copyright 2020 Elsevier).
This was attributed to the enhanced wettability of the nanoparticles by the biosourced oil, thus leading to stronger interfacial adsorption. This study clearly demonstrates that the chemical nature of the core-forming block can influence the surface wettability of block copolymer nanoparticles, in addition to that of the stabilizer block.
An and co-workers prepared oil-in-oil HIPEs from semifluorinated block copolymer nanoparticles.214 Spherical diblock copolymer nanoparticles were initially prepared in DMF via RAFT dispersion polymerization of heptadecafluorodecyl methacrylate (HDFDMA) using a PMMA43 precursor. Such PMMA43-PHDFDMA50 nanoparticles were transferred into DMSO and subsequently subjected to high-shear homogenization with cyclohexane (volume fraction = 0.80). This led to the formation of a highly viscous cyclohexane-in-DMSO HIPE. This is an example of a non-aqueous HIPE, which has been seldom reported.215,216 In the same study, PSMA15-PHDFDMA50 short rods were prepared via RAFT dispersion polymerization in n-dodecane. A 5% w/w dispersion of such nanoparticles was used to prepare a relatively stable DMF-in-n-dodecane Pickering emulsion by homogenization with an equivalent volume of DMF. This is a rather rare example of such an emulsion because these two solvents are usually considered to be miscible.217
In general, block copolymer nanoparticles comprising a hydrophilic stabilizer block are expected to produce o/w emulsions, while those containing a hydrophobic stabilizer block should afford w/o emulsions. However, block copolymer nanoparticles prepared by RAFT dispersion polymerization in non-polar oils comprising a relatively hydrophilic core-forming block do not appear to follow this general rule. For example, Cunningham and co-workers reported that poly(stearyl methacrylate)-poly(N-2-(methacryloyloxy)ethyl pyrrolidone) (PSMA14-PNMEP49) spheres prepared in n-dodecane could form either w/o or o/w Pickering emulsions depending on the emulsification conditions.212 Thus, o/w emulsions were obtained when conducting high-shear homogenization with an equal volume fraction of water at 3500–24 000 rpm, whereas low-shear emulsification by hand-shaking led to w/o emulsions. This unexpected result was attributed to in situ inversion of the nanoparticles during homogenization to form hydrophilic PNMEP49-PSMA14 block copolymer spheres. As expected, increasing the shear rate led to a reduction in the mean oil droplet diameter (Figure 13c). Increasing the oil volume fraction from 50 to 75% v/v prevented nanoparticle inversion and hence enabled the formation of w/o Pickering emulsions.
Figure 13.
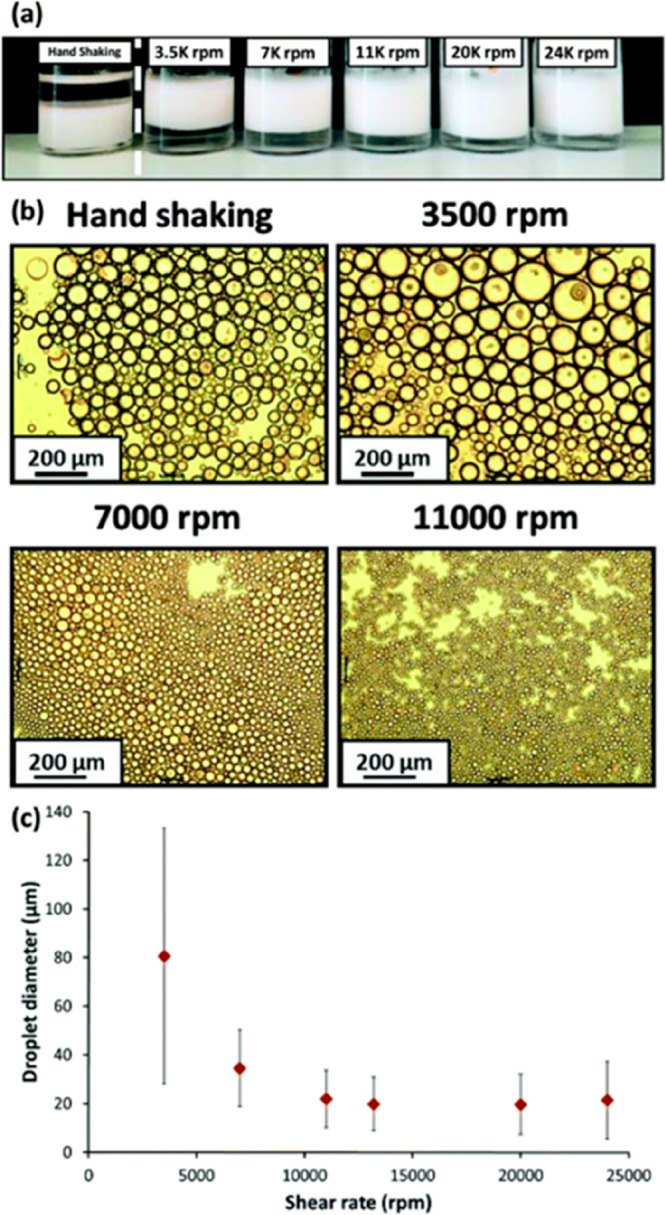
(a) Digital photographs obtained for a series of Pickering emulsions prepared using 1.0% w/w PSMA14-PNMEP49 spherical nanoparticles at various shear rates. Oil-in-water emulsions are formed in all cases, except when low-shear hand-shaking is used; this latter approach instead results in the formation of a water-in-oil emulsion. (b) Optical microscopy images recorded for the droplets prepared via hand-shaking or via homogenization at 3500, 7000, or 11 000 rpm (scale bar = 200 μm). (c) Shear rate dependence for the mean droplet diameter (as determined by laser diffraction) of Pickering emulsions prepared using PSMA14-PNMEP49 spheres as the sole emulsifier. The error bars represent the standard deviation of each volume-average droplet diameter rather than the experimental error. Reproduced from ref (212) (copyright 2016 Royal Society of Chemistry).
In a related study by György et al., the Pickering emulsifier performance of PSMA12-PHPMA50 spheres was explored.218 For this system, the relatively polar PHPMA core-forming block is not actually water-soluble, hence different emulsifier behavior was anticipated. In this case, the emulsion type depended on the water volume fraction. At relatively low water volume fractions (0.125–0.375), w/o Pickering emulsions were obtained at 1.0% w/w copolymer concentration. However, using water volume fractions of 0.50–0.75 led to the formation of a w/o/w Pickering double emulsion, as confirmed by fluorescence microscopy. Thus this is a relatively rare example of a double emulsion that can be prepared using a single copolymer composition.
Stabilization of Giant Pickering Droplets
In recent years, there have been a number of reports of particle-stabilized droplets of approximately 1 to 2 mm diameter.190,195,219−226 Such “giant” Pickering emulsions are typically prepared using capillaries and can act as model systems to provide useful insights into coalescence behavior221 and particle adsorption kinetics.195 The use of spherical latex particles to stabilize giant Pickering emulsions has been studied in some detail.220 Thompson et al. used conventional free radical polymerization to prepare PGMA-stabilized polystyrene latexes of either 135 or 905 nm diameter via aqueous emulsion or alcoholic dispersion polymerization, respectively.221 Such latexes were then used to prepare millimeter-sized n-dodecane droplets. High-speed video imaging was used to monitor the coalescence of these latex-coated droplets.219 Longer coalescence times were observed for Pickering emulsions prepared using the 902 nm latex, and either bilayer formation or a bridging monolayer occurred prior to coalescence.221 Moreover, giant colloidosomes were produced by adding an oil-soluble cross-linker (PPG-TDI) to the oil phase (sunflower oil) prior to droplet formation.221 Cross-linking for 20 min at 25 °C led to a reduction in the interfacial elasticity and prevented any droplet coalescence. In contrast, giant oil droplets coated with charge-stabilized poly(tert-butylamino)ethyl methacrylate (PTBAEMA) latex particles coalesced on close contact in the absence of any PPG-TDI cross-linker.224
Block copolymer nanoparticles prepared via PISA have also been used as emulsifiers for millimeter-sized droplets.190,195 As previously discussed, linear PGMA-PHPMA block copolymer worms are unstable with respect to nanoparticle dissociation when subjected to high-shear homogenization. However, a highly hydrophobic block (e.g., PBzMA) can be added to the nanoparticle cores to confer stability. Thus Mable et al. prepared linear PGMA-PHPMA-PBzMA triblock copolymer worms via RAFT-mediated PISA.190 Such worms were evaluated as Pickering emulsifiers for the stabilization of o/w emulsions prepared under low-shear conditions (i.e., hand-shaking). Optical microscopy and laser diffraction studies confirmed that the worms survived such emulsification conditions and adsorbed intact at the oil/water interface. Much larger millimeter-sized oil droplets were produced using low-shear hand-shaking compared to those using high-shear homogenization. In contrast to the PGMA-PHPMA-PBzMA worms, droplet diameters for emulsions prepared using PGMA-PHPMA worms remained relatively constant with increasing copolymer concentration. This indicates that such worms dissociate even during low-shear emulsification, generating individual amphiphilic diblock copolymer chains that adsorb at the oil/water interface, rather than nanoparticles.
Subsequently, Cunningham et al. used either 22 nm diameter PGMA39-PBzMA60 spheres or PGMA37-PHPMA60-PBzMA30 worms (mean worm width = 26 nm) in turn to stabilize millimeter-sized n-dodecane droplets.195 Dynamic interfacial tension measurements were conducted to assess the kinetics of adsorption for these two morphologies. In both cases, a rapid initial reduction in interfacial tension occurred within 20 s, with a more gradual but still significant reduction being observed thereafter. This provided direct evidence for nanoparticle adsorption at the oil/water interface and suggested the possibility of post-adsorption nanoparticle reorganization. The worms lowered the interfacial tension significantly more than the spheres, indicating that the former had a stronger affinity for the n-dodecane/water interface. Both spheres and worms stabilized giant Pickering droplets, but the former proved to be more effective at stabilizing the interface in the absence of any interfacial aging. This was attributed to the very high capillary pressure generated by such small nanoparticles. In contrast, the significantly larger worms required interfacial aging for at least 90 s before droplet stability was achieved owing to their slower diffusion to the interface and rearrangement after initial adsorption. Systematic variation of the copolymer concentration revealed that the worms were able to stabilize giant Pickering emulsions at lower concentrations than the equivalent 22 nm spheres. Finally, the effect of mean sphere diameter on droplet coalescence time was examined for 22, 41, 60, and 91 nm PGMA39-PBzMAx spheres (Figure 14). Stable droplets were obtained using either 22 or 41 nm spheres, but coalescence was always observed when using 60 and 91 nm spheres, even after relatively long aging times. Presumably, this reduction in droplet stability is related to the lower capillary pressure for such larger particles, since all other parameters remained constant.
Figure 14.
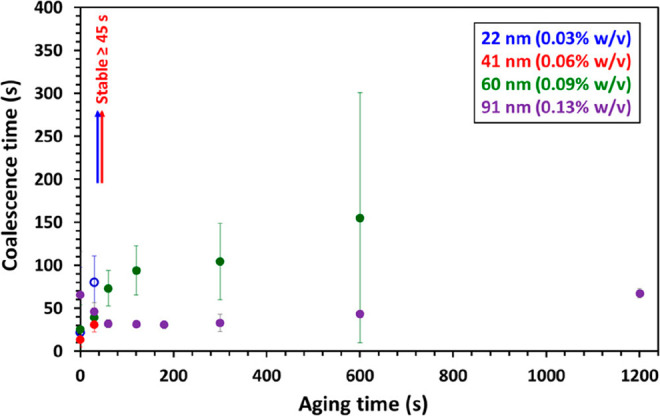
Coalescence time vs aging time plot for two n-dodecane droplets grown in the presence of dilute aqueous dispersions of PGMA39-PBzMAx spheres of varying mean diameter. Open symbols indicate the conditions for which, in some cases, droplets were stable toward coalescence for at least 30 min. Reproduced from ref (195) (copyright 2017 American Chemical Society).
Pickering Nanoemulsions
Nanoemulsions comprise oil or water droplets for which the mean droplet diameter is less than 200 nm.227,228 There are various reports of copolymer- or surfactant-stabilized nanoemulsions in the literature.229 In contrast, there have been remarkably few examples of Pickering nanoemulsions in which the droplets are solely stabilized by solid particles.192,193,230−233 No doubt one reason for the paucity of such studies is the rule-of-thumb requirement that the Pickering emulsifier should be at least 5–10 times smaller than the mean droplet diameter. However, the recent development of polymerization-induced self-assembly now enables the highly convenient synthesis of sterically stabilized diblock copolymer spheres of 20–25 nm diameter directly in the form of concentrated aqueous dispersions.117,234 In principle, such nanoparticles should constitute model Pickering emulsifiers for the stabilization of oil-in-water nanoemulsions.
For example, Thompson and co-workers chain-extended a water-soluble PGMA48 precursor via RAFT aqueous emulsion polymerization of 2,2,2-trifluoroethyl methacrylate (TFEMA) to form PGMA48-PTFEMA50 spheres of approximately 25 nm diameter,192 as previously reported by Akpinar and co-workers.234 As discussed above, the hydrophobic character of the core-forming block is of critical importance when preparing Pickering emulsions using block copolymer nanoparticles. Selecting a weakly hydrophobic block such as PHPMA usually means that the nanoparticles do not survive the high-shear homogenization conditions required for droplet formation. On the other hand, nanoparticles with highly hydrophobic core-forming blocks such as PTFEMA typically remain intact and therefore can act as genuine Pickering emulsifiers. Indeed, this criterion is particularly important for the formation of Pickering nanoemulsions because even more energy-intensive conditions are required.
Initially, a Pickering macroemulsion of approximately 40 μm diameter was prepared via high-shear homogenization of a 7.0% w/w aqueous dispersion of PGMA48-PTFEMA50 spheres with n-dodecane at 15 500 rpm. A relatively high copolymer concentration was deliberately employed during this initial stage because a large excess of non-adsorbed nanoparticles is required to stabilize the nanoemulsion generated in the second stage. Such precursor emulsions were then subjected to high-pressure microfluidization to generate much finer droplets (Figure 15). The final size of the oil droplets depended on both the applied pressure and also the number of passes through the microfluidizer. At least eight passes were required to reach the minimum mean droplet diameter of 220 nm at an applied pressure of 20 000 psi.
Figure 15.
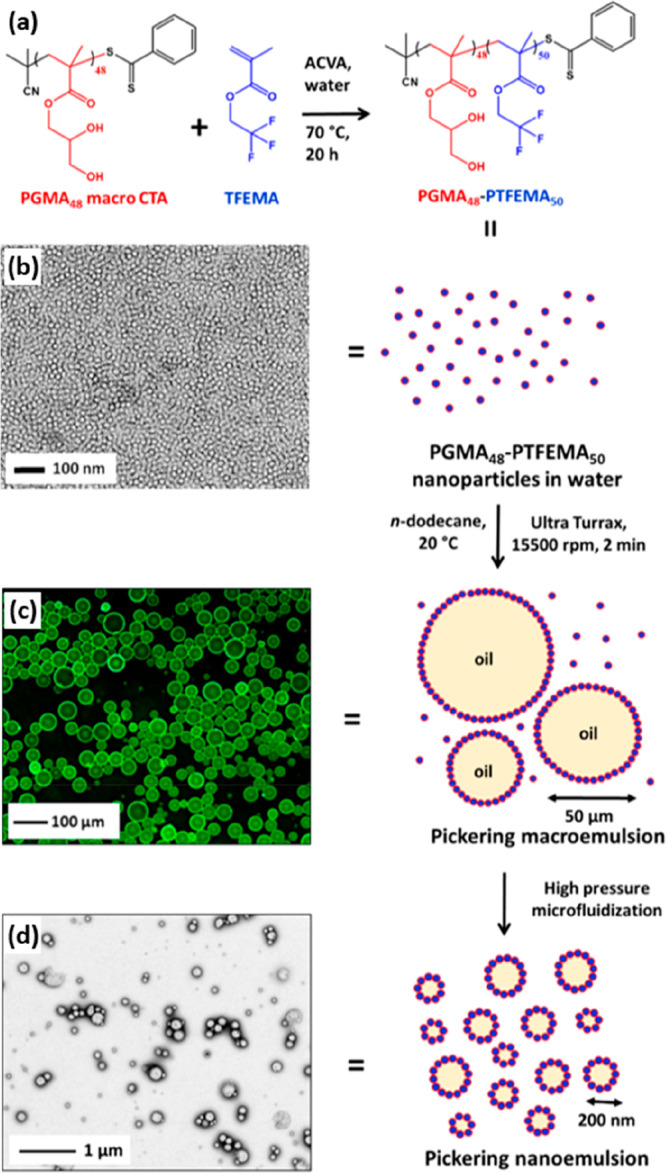
Schematic representation of the preparation of Pickering nanoemulsions. (a) Synthesis of PGMA48-PTFEMA50 nanoparticles of 25 nm diameter via RAFT emulsion polymerization of TFEMA using a PGMA48 steric stabilizer. (b) TEM image of the resulting PGMA48-PTFEMA50 nanoparticles. (c) Fluorescence micrograph of the initial Pickering macroemulsion produced when excess nanoparticles are homogenized with n-dodecane for 2.0 min at 15 500 rpm. (d) This precursor macroemulsion was then further processed using a commercial LV1 microfluidizer (Microfluidics Ltd., USA) to afford a Pickering nanoemulsion. (See the TEM image recorded after drying such droplets.) Reproduced from ref (192) (copyright 2017 American Chemical Society).
Subtracting the thickness of the adsorbed monolayer of 25 nm PGMA48-PTFEMA50 spheres indicates a mean oil droplet diameter of less than 200 nm, which lies within the range required for a genuine nanoemulsion. Moreover, such nanoparticles enabled the formation of high-internal-phase nanoemulsions at oil volume fractions of up to 0.80. However, TEM analysis of dried nanoemulsion droplets prepared at 30 000 psi revealed no evidence of the original nanoparticles. At this higher applied pressure, nanoparticle dissociation occurred and the molecularly dissolved PGMA48-PTFEMA50 copolymer chains acted as an amphiphilic polymeric surfactant. This problem could be circumvented by incorporating EGDMA as a third block: the resulting covalently stabilized PGMA48-PTFEMA45-PEGDMA5 remained intact even at an applied pressure of 30 000 psi, thus ensuring the formation of genuine Pickering emulsions under such conditions.
In a follow-up study, Thompson et al. examined the effect of varying the oil type on the long-term stability of Pickering nanoemulsions prepared using the same PGMA48-PTFEMA50 nanoparticles.193 Thus, a series of nanoemulsions prepared using four n-alkanes were prepared using an LV1 microfluidizer, and their relative long-term stabilities were assessed using analytical centrifugation.31 More specifically, a LUMiSizer instrument was employed to size the aging droplets over a six-week period (Figure 16). Significant broadening of the droplet size distribution was observed in each case, although the change in the mean droplet diameter was minimal. For the more stable nanoemulsions prepared using either n-tetradecane or n-dodecane, over 90% of the droplets remained below 1 μm after six weeks. Conversely, nanoemulsions prepared using n-octane proved to be relatively unstable, which correlates with the higher water solubility of this oil.
Figure 16.
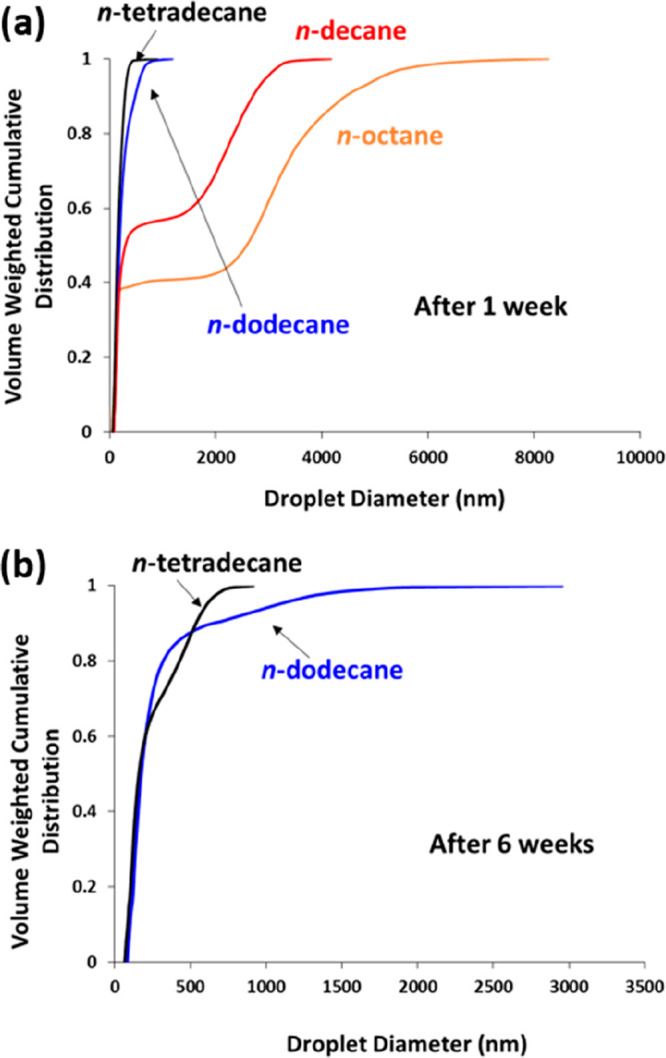
Volume-weighted cumulative size distributions determined by analytical centrifugation (LUMiSizer instrument) for a series of four n-alkane-in-water Pickering nanoemulsions: (a) after aging for one week at 20 °C and (b) after aging for six weeks at 20 °C. Significant evaporation of the more volatile n-octane and n-decane oils occurred within 1 week, so no further analysis was possible in these two cases. Reproduced from ref (193) (copyright 2018 American Chemical Society).
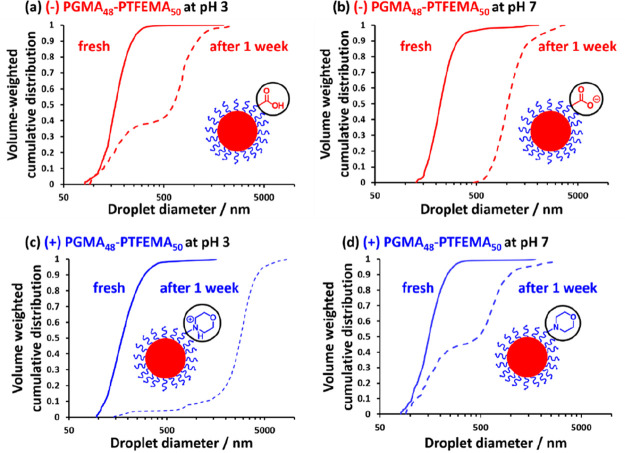
We recently explored the effect of introducing charge at the end of the steric stabilizer block on the formation and long-term stability of Pickering nanoemulsions prepared using PGMA48-PTFEMA50 nanoparticles.232 RAFT-mediated PISA enables the design of block copolymer nanoparticles with minimal surface charge by simply selecting an appropriate RAFT agent when preparing the steric stabilizer precursor. Hence PGMA chains bearing carboxylic acid, morpholine, or neutral end-groups were chain-extended by the RAFT aqueous emulsion polymerization of TFEMA. Thus ionization of the carboxylic acid group at neutral pH introduced a terminal anionic charge, whereas protonation of the tertiary amine group at low pH conferred cationic charge. Analysis of the aqueous phase after microfluidization by gel permeation chromatography using a UV detector enabled convenient quantification of the nanoparticle adsorption efficiency. Up to 90% of the neutral nanoparticles were adsorbed at the surface of the oil droplets. In contrast, introducing either anionic or cationic charge at the stabilizer chain ends significantly reduced the nanoparticle adsorption efficiency. Moreover, SAXS studies indicated that the packing efficiency of neutral nanoparticles at the oil/water interface was significantly higher than that of nanoparticles bearing charged end groups. Analytical centrifugation was used to evaluate the long-term stability of such Pickering nanoemulsions, with those prepared using nanoparticles bearing charged end-groups proving to be significantly less stable than those bearing neutral end-groups. Figure 17 shows droplet size distributions recorded for both freshly prepared and one-week-old Pickering nanoemulsions. If the adsorbed nanoparticles were in their neutral state, then the droplet size distribution became bimodal. On the other hand, if the same nanoparticles possessed charged end-groups, then larger droplets were produced on aging but the size distribution remained unimodal.
Figure 17.
Volume-weighted cumulative size distributions determined by analytical centrifugation (LUMiSizer instrument) for fresh (solid line) and aged (for one week at 20 °C, dashed line) n-dodecane-in-water Pickering nanoemulsions prepared using 7.0% w/w PGMA48-PTFEMA50 nanoparticles prepared using (a) a carboxylic acid-based RAFT agent aged at pH 3; (b) the same carboxylic acid-based RAFT agent aged at pH 7; (c) a morpholine-based RAFT agent aged at pH 3; and (d) the same morpholine-based RAFT agent aged at pH 7. Microfluidizer conditions: 20 000 psi, 10 passes. Reproduced from ref (232) (copyright 2020 American Chemical Society).
Transparent Pickering Emulsions
It is well known that emulsions usually exhibit high turbidity owing to strong light scattering by the droplet phase. However, according to Snell’s law, an emulsion should be transparent if the continuous phase and the droplet phase possess the same refractive index.235 For surfactant-stabilized emulsions, the emulsifier is far too small to cause any additional light scattering, so examples of highly transparent conventional emulsions are not uncommon.235−237 On the other hand, the design of transparent Pickering emulsions is much more challenging owing to additional light scattering arising from the adsorbed particles.71,191 In this case, the droplet phase, the continuous phase, and the Pickering emulsifier must possess precisely the same refractive index to minimize light scattering and achieve high transparency. In principle, the refractive index of block copolymer nanoparticles prepared via PISA can be tuned by simply varying the copolymer composition. Thus, such nanoparticles are strong candidates for the design of transparent emulsions. However, the refractive index of water (1.33) lies well below that of most oils. Thus, either water-soluble or water-miscible species must be added to the aqueous phase to raise its refractive index to that of the oil phase.
In an alternative approach, Thompson and co-workers reported the preparation of an almost isorefractive non-aqueous Pickering emulsion using diblock copolymer worms.71 This formulation comprised ethylene glycol-in-n-tetradecane emulsions stabilized by PLMA16-PBzMA37 worms. These two immiscible liquids were selected owing to their almost identical refractive index (∼1.43). However, the core-forming PBzMA block has a relatively high refractive index of 1.57, so such emulsions are only translucent (transmittance = 70–80%, depending on the precise wavelength of visible light) owing to weak light scattering by the adsorbed worms.
Subsequently, Rymaruk et al. demonstrated that highly transparent Pickering double emulsions could be prepared by selecting a model oil, designing suitable diblock copolymer nanoparticles, and employing an appropriate concentration of a water-soluble additive.191 Semifluorinated PTFEMA was selected as the core-forming block owing to its relatively low refractive index of 1.42, which almost perfectly matches that of n-dodecane. Thus, judicious addition of either 50.5% sucrose or 67% glycerol to an aqueous dispersion of PGMA56-PTFEMA500 nanoparticles, followed by homogenization with n-dodecane, produced a highly transparent n-dodecane-in-water Pickering emulsion, as shown in Figure 18. Moreover, complementary water-in-n-dodecane Pickering emulsions of similarly high transmittance could also be prepared by using hydrophobic PLMA39-PTFEMA800 nanoparticles synthesized via PISA in n-dodecane. Finally, combining these hydrophilic and hydrophobic nanoparticles enabled the preparation of an o/w/o Pickering double emulsion that exhibited a mean transmittance of almost 90% across the visible spectrum. This study highlights the versatility and potential offered by PISA for the rational design of bespoke Pickering emulsifiers of tunable size and surface chemistry.
Figure 18.
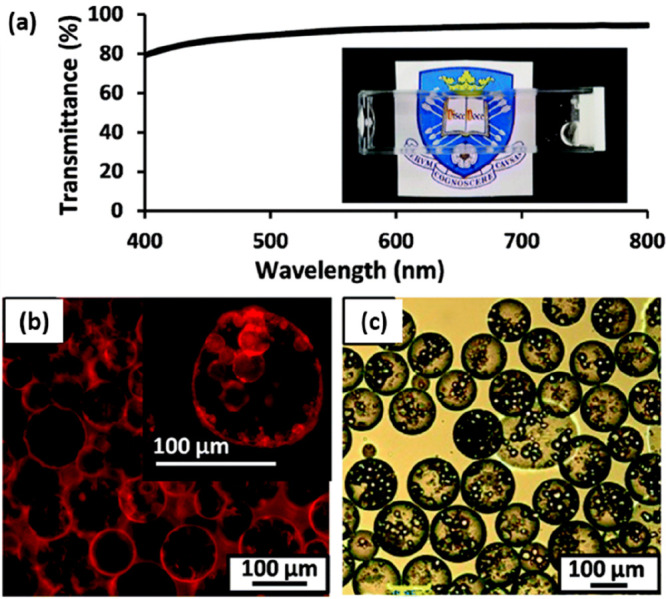
(a) Transmittance% spectrum recorded for an n-dodecane-in-50.5% aqueous sucrose-in-n-dodecane Pickering double emulsion. (Inset) Digital photograph illustrates the highly transparent nature of this refractive-index-matched emulsion. (b) Fluorescence micrograph recorded for the same Pickering double emulsion prepared with Nile Red dye dissolved in the oil phase. (c) Optical micrograph obtained for the same emulsion prepared in the absence of any sucrose (i.e., with pure water) in order to provide contrast. Reproduced from ref (191) (copyright 2016 Royal Society of Chemistry).
Conclusions and Prospect
PISA enables the facile synthesis of a wide range of block copolymer nano-objects as concentrated dispersions in either water or various oils. The particle size, copolymer morphology, and surface chemistry can be predicted by selecting appropriate steric stabilizer and structure-directing blocks and targeting the desired DPs. Many of these nano-objects can be used as model polymer-based Pickering emulsifiers to examine the effect of varying the particle size, morphology, surface roughness, and surface charge. In principle, this enables the effect of varying such parameters on the interfacial surface tension, adsorption dynamics, interparticle forces, and interfacial mechanics to be examined, although such model experimental studies are yet to be performed. In some cases, such Pickering emulsifiers may be prone to dissociate into individual amphiphilic copolymer chains during high-shear homogenization. However, this technical problem can be addressed by either covalent stabilization or the addition of a more solvophobic block such as PBzMA.72,117,187,188,194 Recently, we have reported protocols for preparing spheres, worms, and vesicles via RAFT aqueous emulsion polymerization of vinyl monomers that exhibit moderate aqueous solubility (15–20 g dm–3).238−240 Such nano-objects are expected to act as new Pickering emulsifiers that are stable toward high-shear emulsification without recourse to covalent stabilization. RAFT aqueous emulsion polymerization has also enabled the synthesis of relatively small block copolymer nanoparticles possessing highly hydrophobic cores. Such hydrophilic nanoparticles can be used to prepare model n-alkane-in-water Pickering nanoemulsions.192 This has enabled systematic studies of the effect of varying (i) the n-alkane type193 and (ii) the introduction of terminal ionic charge232 on the rate of demulsification via Ostwald ripening. In principle, using a suitably hydrophobic stabilizer block such as poly(lauryl methacrylate) or poly(stearyl methacrylate) should enable the formation of the analogous water-in-oil Pickering nanoemulsions if an n-alkane-insoluble core-forming block such as PBzMA158 or PTFEMA241 confers sufficient stability to prevent in situ degradation during microfluidization. Indeed, we have just exemplified this concept.242 Remarkably, PISA has also enabled the preparation of transparent Pickering double emulsions.191 More specifically, the refractive index of the nanoparticle emulsifier can be tuned by selecting an appropriate core-forming block to match that of the chosen oil, with the refractive index of the aqueous phase being subsequently tuned by the addition of a suitable water-soluble additive (e.g., sucrose or glycerol). Such studies highlight the rational design capability afforded by PISA for the preparation of a wide range of block copolymer nanoparticles to act as bespoke Pickering emulsifiers. This versatility augurs well for potential commercial applications of this technology.
Acknowledgments
We thank the EPSRC for a CDT PhD studentship to support S.J.H. (EP/L016281) and also an Established Career Particle Technology Fellowship (EP/R003009) for S.P.A. DSM (Geleen, The Netherlands) is acknowledged for partial support of S.J.H.’s PhD studentship and for permission to publish this work.
Biographies

Saul J. Hunter is a current PhD student working in the Armes group at the University of Sheffield. He was awarded the Haworth prize and medal when graduating at the top of his class with an MChem degree in chemistry from the same institution in 2017. His EPSRC-sponsored research project is focused on Pickering (nano)emulsions and is partially supported by DSM Research (The Netherlands). He has coauthored five publications to date, including three papers in Langmuir, and he expects to receive his PhD degree by the end of 2021.

Steven P. Armes received his BSc (1983) and PhD (1987) degrees in chemistry from the University of Bristol and worked as a postdoctoral fellow at Los Alamos National Laboratory in New Mexico (1987–1989). He accepted a lectureship at Sussex University in 1989 and was promoted to full professor in 2000. He moved to the University of Sheffield in 2004. He works at the interface of synthetic polymer chemistry and colloid science and has published more than 650 papers to date (H-index 113). His research interests include block copolymer self-assembly, polymerization-induced self-assembly, Pickering emulsions, colloidal nanocomposite particles, microgels, gels, foams, and conducting polymer particles for space science applications.
The authors declare no competing financial interest.
References
- Ramsden W. Separation of Solids in the Surface-Layers of Solutions and ‘Suspensions’ (Observations on Surface-Membranes, Bubbles, Emulsions, and Mechanical Coagulation). Preliminary Account. Proc. R. Soc. London 1903, 72, 156–164. 10.1098/rspl.1903.0034. [DOI] [Google Scholar]
- Pickering S. U. Emulsions. J. Chem. Soc., Trans. 1907, 91, 2001–2021. 10.1039/CT9079102001. [DOI] [Google Scholar]
- Binks B P.; Lumsdon S O. Stability of oil-in-water emulsions stabilised by silica particles. Phys. Chem. Chem. Phys. 1999, 1, 3007–3016. 10.1039/a902209k. [DOI] [Google Scholar]
- Ashby N. P.; Binks B. P. Pickering emulsions stabilised by Laponite clay particles. Phys. Chem. Chem. Phys. 2000, 2, 5640–5646. 10.1039/b007098j. [DOI] [Google Scholar]
- Binks B. P.; Lumsdon S. O. Influence of Particle Wettability on the Type and Stability of Surfactant-Free Emulsions. Langmuir 2000, 16, 8622–8631. 10.1021/la000189s. [DOI] [Google Scholar]
- Binks B. P. Particles as surfactants—similarities and differences. Curr. Opin. Colloid Interface Sci. 2002, 7, 21–41. 10.1016/S1359-0294(02)00008-0. [DOI] [Google Scholar]
- Aveyard R.; Binks B. P.; Clint J. H. Emulsions stabilised solely by colloidal particles. Adv. Colloid Interface Sci. 2003, 100, 503–546. 10.1016/S0001-8686(02)00069-6. [DOI] [Google Scholar]
- Binks B. P. Colloidal Particles at a Range of Fluid-Fluid Interfaces. Langmuir 2017, 33, 6947–6963. 10.1021/acs.langmuir.7b00860. [DOI] [PubMed] [Google Scholar]
- Wu J.; Shi M.; Li W.; Zhao L.; Wang Z.; Yan X.; Norde W.; Li Y. Pickering emulsions stabilized by whey protein nanoparticles prepared by thermal cross-linking. Colloids Surf., B 2015, 127, 96–104. 10.1016/j.colsurfb.2015.01.029. [DOI] [PubMed] [Google Scholar]
- Xiao J.; Li Y.; Huang Q. Recent advances on food-grade particles stabilized Pickering emulsions: Fabrication, characterization and research trends. Trends Food Sci. Technol. 2016, 55, 48–60. 10.1016/j.tifs.2016.05.010. [DOI] [Google Scholar]
- Liu F.; Tang C.-H. Soy Protein Nanoparticle Aggregates as Pickering Stabilizers for Oil-in-Water Emulsions. J. Agric. Food Chem. 2013, 61, 8888–8898. 10.1021/jf401859y. [DOI] [PubMed] [Google Scholar]
- Tang C.; Li Y.; Pun J.; Mohamed Osman A. S.; Tam K. C. Polydopamine microcapsules from cellulose nanocrystal stabilized Pickering emulsions for essential oil and pesticide encapsulation. Colloids Surf., A 2019, 570, 403–413. 10.1016/j.colsurfa.2019.03.049. [DOI] [Google Scholar]
- Fowler J.Pickering Emulsion Formulations. U.S. Patent, 0234230, 2010.
- Formstone C.; De Heer M. I.; Taylor P.; Haseldine S. J.. Herbicidal Composition Comprising Polymeric Microparticles Containing a Herbicide. WO2013034513 A2, 2013.
- Mulqueen P. J.; Taylor P.; Gittins D. I.. Microencapsulation, WO2009063257 A2, 2009.
- Marku D.; Wahlgren M.; Rayner M.; Sjoo M.; Timgren A. Characterization of starch Pickering emulsions for potential applications in topical formulations. Int. J. Pharm. 2012, 428, 1–7. 10.1016/j.ijpharm.2012.01.031. [DOI] [PubMed] [Google Scholar]
- Marto J.; Ascenso A.; Simoes S.; Almeida A. J.; Ribeiro H. M. Pickering emulsions: challenges and opportunities in topical delivery. Expert Opin. Drug Delivery 2016, 13, 1093–1107. 10.1080/17425247.2016.1182489. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Fang Z.; Chen X.; Zhang W.; Xie Y.; Chen Y.; Liu Z.; Yuan W. An Overview of Pickering Emulsions: Solid-Particle Materials, Classification, Morphology, and Applications. Front. Pharmacol. 2017, 8, 287. 10.3389/fphar.2017.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frelichowska J.; Bolzinger M.-A.; Valour J.-P.; Mouaziz H.; Pelletier J.; Chevalier Y. Pickering w/o emulsions: Drug release and topical delivery. Int. J. Pharm. 2009, 368, 7–15. 10.1016/j.ijpharm.2008.09.057. [DOI] [PubMed] [Google Scholar]
- Frelichowska J.; Bolzinger M.-A.; Pelletier J.; Valour J.-P.; Chevalier Y. Topical delivery of lipophilic drugs from o/w Pickering emulsions. Int. J. Pharm. 2009, 371, 56–63. 10.1016/j.ijpharm.2008.12.017. [DOI] [PubMed] [Google Scholar]
- Finkle P.; Draper H. D.; Hildebrand J. H. The Theory of Emulsification 1. J. Am. Chem. Soc. 1923, 45, 2780–2788. 10.1021/ja01665a002. [DOI] [Google Scholar]
- Binks B. P.; Lumsdon S. O. Pickering Emulsions Stabilized by Monodisperse Latex Particles: Effects of Particle Size. Langmuir 2001, 17, 4540–4547. 10.1021/la0103822. [DOI] [Google Scholar]
- Anjali T. G.; Basavaraj M. G. Shape-Anisotropic Colloids at Interfaces. Langmuir 2019, 35, 3–20. 10.1021/acs.langmuir.8b01139. [DOI] [PubMed] [Google Scholar]
- Wilde P. Interfaces: Their role in foam and emulsion behaviour. Curr. Opin. Colloid Interface Sci. 2000, 5, 176–181. 10.1016/S1359-0294(00)00056-X. [DOI] [Google Scholar]
- Kabalnov A. Thermodynamic and theoretical aspects of emulsions and their stability. Curr. Opin. Colloid Interface Sci. 1998, 3, 270–275. 10.1016/S1359-0294(98)80071-X. [DOI] [Google Scholar]
- Levine S.; Bowen B. D.; Partridge S. J. Stabilization of emulsions by fine particles I. Partitioning of particles between continuous phase and oil/water interface. Colloids Surf. 1989, 38, 325–343. 10.1016/0166-6622(89)80271-9. [DOI] [Google Scholar]
- Clint J. H.; Taylor S. E. Particle size and interparticle forces of overbased detergents: A Langmuir trough study. Colloids Surf. 1992, 65, 61–67. 10.1016/0166-6622(92)80175-2. [DOI] [Google Scholar]
- Garti N. Double emulsions — scope, limitations and new achievements. Colloids Surf., A 1997, 123–124, 233–246. 10.1016/S0927-7757(96)03809-5. [DOI] [Google Scholar]
- Cunha A. G.; Mougel J.-B.; Cathala B.; Berglund L. A.; Capron I. Preparation of Double Pickering Emulsions Stabilized by Chemically Tailored Nanocelluloses. Langmuir 2014, 30, 9327–9335. 10.1021/la5017577. [DOI] [PubMed] [Google Scholar]
- Binks B. P.; Lumsdon S. O. Catastrophic Phase Inversion of Water-in-Oil Emulsions Stabilized by Hydrophobic Silica. Langmuir 2000, 16, 2539–2547. 10.1021/la991081j. [DOI] [Google Scholar]
- Menner A.; Ikem V.; Salgueiro M.; Shaffer M. S. P.; Bismarck A. High internal phase emulsion templates solely stabilised by functionalised titania nanoparticles. Chem. Commun. 2007, 4274–4276. 10.1039/b708935j. [DOI] [PubMed] [Google Scholar]
- Ikem V. O.; Menner A.; Bismarck A. High-Porosity Macroporous Polymers Sythesized from Titania-Particle-Stabilized Medium and High Internal Phase Emulsions. Langmuir 2010, 26, 8836–8841. 10.1021/la9046066. [DOI] [PubMed] [Google Scholar]
- Zhou J.; Qiao X.; Binks B. P.; Sun K.; Bai M.; Li Y.; Liu Y. Magnetic Pickering Emulsions Stabilized by Fe3O4 Nanoparticles. Langmuir 2011, 27, 3308–3316. 10.1021/la1036844. [DOI] [PubMed] [Google Scholar]
- Binks B. P.; Whitby C. P. Nanoparticle silica-stabilised oil-in-water emulsions: improving emulsion stability. Colloids Surf., A 2005, 253, 105–115. 10.1016/j.colsurfa.2004.10.116. [DOI] [Google Scholar]
- Frelichowska J.; Bolzinger M.-A.; Chevalier Y. Pickering emulsions with bare silica. Colloids Surf., A 2009, 343, 70–74. 10.1016/j.colsurfa.2009.01.031. [DOI] [Google Scholar]
- Tcholakova S.; Denkov N. D.; Lips A. Comparison of solid particles, globular proteins and surfactants as emulsifiers. Phys. Chem. Chem. Phys. 2008, 10, 1608–1627. 10.1039/b715933c. [DOI] [PubMed] [Google Scholar]
- Yang F.; Liu S.; Xu J.; Lan Q.; Wei F.; Sun D. Pickering emulsions stabilized solely by layered double hydroxides particles: The effect of salt on emulsion formation and stability. J. Colloid Interface Sci. 2006, 302, 159–169. 10.1016/j.jcis.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Kalashnikova I.; Bizot H.; Cathala B.; Capron I. New Pickering Emulsions Stabilized by Bacterial Cellulose Nanocrystals. Langmuir 2011, 27, 7471–7479. 10.1021/la200971f. [DOI] [PubMed] [Google Scholar]
- Kalashnikova I.; Bizot H.; Bertoncini P.; Cathala B.; Capron I. Cellulosic nanorods of various aspect ratios for oil in water Pickering emulsions. Soft Matter 2013, 9, 952–959. 10.1039/C2SM26472B. [DOI] [Google Scholar]
- Jiménez Saelices C.; Capron I. Design of Pickering Micro- and Nanoemulsions Based on the Structural Characteristics of Nanocelluloses. Biomacromolecules 2018, 19, 460–469. 10.1021/acs.biomac.7b01564. [DOI] [PubMed] [Google Scholar]
- Capron I.; Rojas O. J.; Bordes R. Behavior of nanocelluloses at interfaces. Curr. Opin. Colloid Interface Sci. 2017, 29, 83–95. 10.1016/j.cocis.2017.04.001. [DOI] [Google Scholar]
- Saha A.; Nikova A.; Venkataraman P.; John V. T.; Bose A. Oil Emulsification Using Surface-Tunable Carbon Black Particles. ACS Appl. Mater. Interfaces 2013, 5, 3094–3100. 10.1021/am3032844. [DOI] [PubMed] [Google Scholar]
- Katepalli H.; John V. T.; Bose A. The Response of Carbon Black Stabilized Oil-in-Water Emulsions to the Addition of Surfactant Solutions. Langmuir 2013, 29, 6790–6797. 10.1021/la400037c. [DOI] [PubMed] [Google Scholar]
- Briggs N. M.; Weston J. S.; Li B.; Venkataramani D.; Aichele C. P.; Harwell J. H.; Crossley S. P. Multiwalled Carbon Nanotubes at the Interface of Pickering Emulsions. Langmuir 2015, 31, 13077–13084. 10.1021/acs.langmuir.5b03189. [DOI] [PubMed] [Google Scholar]
- He Y. Q.; Wu F.; Sun X. Y.; Li R. Q.; Guo Y. Q.; Li C. B.; Zhang L.; Xing F. B.; Wang W.; Gao J. P. Factors that Affect Pickering Emulsions Stabilized by Graphene Oxide. ACS Appl. Mater. Interfaces 2013, 5, 4843–4855. 10.1021/am400582n. [DOI] [PubMed] [Google Scholar]
- Kim J.; Cote L. J.; Kim F.; Yuan W.; Shull K. R.; Huang J. X. Graphene Oxide Sheets at Interfaces. J. Am. Chem. Soc. 2010, 132, 8180–8186. 10.1021/ja102777p. [DOI] [PubMed] [Google Scholar]
- Velev O. D.; Furusawa K.; Nagayama K. Assembly of Latex Particles by Using Emulsion Droplets as Templates. 1. Microstructured Hollow Spheres. Langmuir 1996, 12, 2374–2384. 10.1021/la9506786. [DOI] [Google Scholar]
- Dinsmore A. D.; Hsu M. F.; Nikolaides M. G.; Marquez M.; Bausch A. R.; Weitz D. A. Colloidosomes: Selectively Permeable Capsules Composed of Colloidal Particles. Science 2002, 298, 1006–1009. 10.1126/science.1074868. [DOI] [PubMed] [Google Scholar]
- Amalvy J. I.; Armes S. P.; Binks B. P.; Rodrigues J. A.; Unali G. F. Use of sterically-stabilised polystyrene latex particles as a pH-responsive particulate emulsifier to prepare surfactant-free oil-in-water emulsions. Chem. Commun. 2003, 1826–1827. 10.1039/b304967a. [DOI] [PubMed] [Google Scholar]
- Amalvy J. I.; Unali G. F.; Li Y.; Granger-Bevan S.; Armes S. P.; Binks B. P.; Rodrigues J. A.; Whitby C. P. Synthesis of Sterically Stabilized Polystyrene Latex Particles Using Cationic Block Copolymers and Macromonomers and Their Application as Stimulus-Responsive Particulate Emulsifiers for Oil-in-Water Emulsions. Langmuir 2004, 20, 4345–4354. 10.1021/la035921c. [DOI] [PubMed] [Google Scholar]
- Binks B. P.; Murakami R.; Armes S. P.; Fujii S. Temperature-Induced Inversion of Nanoparticle-Stabilized Emulsions. Angew. Chem. 2005, 117, 4873–4876. 10.1002/ange.200501073. [DOI] [PubMed] [Google Scholar]
- Binks B. P.; Murakami R.; Armes S. P.; Fujii S.; Schmid A. pH-Responsive Aqueous Foams Stabilized by Ionizable Latex Particles. Langmuir 2007, 23, 8691–8694. 10.1021/la700444a. [DOI] [PubMed] [Google Scholar]
- Fujii S.; Cai Y.; Weaver J. V. M.; Armes S. P. Syntheses of Shell Cross-Linked Micelles Using Acidic ABC Triblock Copolymers and Their Application as pH-Responsive Particulate Emulsifiers. J. Am. Chem. Soc. 2005, 127, 7304–7305. 10.1021/ja050049a. [DOI] [PubMed] [Google Scholar]
- Coertjens S.; De Dier R.; Moldenaers P.; Isa L.; Vermant J. Adsorption of Ellipsoidal Particles at Liquid-Liquid Interfaces. Langmuir 2017, 33, 2689–2697. 10.1021/acs.langmuir.6b03534. [DOI] [PubMed] [Google Scholar]
- Ngai T.; Behrens S. H.; Auweter H. Novel emulsions stabilized by pH and temperature sensitive microgels. Chem. Commun. 2005, 331–333. 10.1039/b412330a. [DOI] [PubMed] [Google Scholar]
- Brugger B.; Rosen B. A.; Richtering W. Microgels as Stimuli-Responsive Stabilizers for Emulsions. Langmuir 2008, 24, 12202–12208. 10.1021/la8015854. [DOI] [PubMed] [Google Scholar]
- Wang F.; Tang J.; Liu H.; Yu G.; Zou Y. Self-assembled polymeric micelles as amphiphilic particulate emulsifiers for controllable Pickering emulsions. Materials Chemistry Frontiers 2019, 3, 356–364. 10.1039/C8QM00540K. [DOI] [Google Scholar]
- Binks B. P.; Whitby C. P. Silica Particle-Stabilized Emulsions of Silicone Oil and Water: Aspects of Emulsification. Langmuir 2004, 20, 1130–1137. 10.1021/la0303557. [DOI] [PubMed] [Google Scholar]
- Horozov T. S.; Binks B. P. Particle-Stabilized Emulsions: A Bilayer or a Bridging Monolayer?. Angew. Chem., Int. Ed. 2006, 45, 773–776. 10.1002/anie.200503131. [DOI] [PubMed] [Google Scholar]
- Fielding L. A.; Armes S. P. Preparation of Pickering emulsions and colloidosomes using either a glycerol-functionalised silica sol or core-shell polymer/silica nanocomposite particles. J. Mater. Chem. 2012, 22, 11235–11244. 10.1039/c2jm31433a. [DOI] [Google Scholar]
- Binks B. P.; Rodrigues J. A.; Frith W. J. Synergistic Interaction in Emulsions Stabilized by a Mixture of Silica Nanoparticles and Cationic Surfactant. Langmuir 2007, 23, 3626–3636. 10.1021/la0634600. [DOI] [PubMed] [Google Scholar]
- Binks B. P.; Isa L.; Tyowua A. T. Direct Measurement of Contact Angles of Silica Particles in Relation to Double Inversion of Pickering Emulsions. Langmuir 2013, 29, 4923–4927. 10.1021/la4006899. [DOI] [PubMed] [Google Scholar]
- Fouilloux S.; Malloggi F.; Daillant J.; Thill A. Aging mechanism in model Pickering emulsion. Soft Matter 2016, 12, 900–904. 10.1039/C5SM02134K. [DOI] [PubMed] [Google Scholar]
- Liu K.; Jiang J.; Cui Z.; Binks B. P. pH-Responsive Pickering Emulsions Stabilized by Silica Nanoparticles in Combination with a Conventional Zwitterionic Surfactant. Langmuir 2017, 33, 2296–2305. 10.1021/acs.langmuir.6b04459. [DOI] [PubMed] [Google Scholar]
- Pieranski P. Two-Dimensional Interfacial Colloidal Crystals. Phys. Rev. Lett. 1980, 45, 569–572. 10.1103/PhysRevLett.45.569. [DOI] [Google Scholar]
- Binks B. P.; Rodrigues J. A. Enhanced Stabilization of Emulsions Due to Surfactant-Induced Nanoparticle Flocculation. Langmuir 2007, 23, 7436–7439. 10.1021/la700597k. [DOI] [PubMed] [Google Scholar]
- Arditty S.; Whitby C. P.; Binks B. P.; Schmitt V.; Leal-Calderon F. Some general features of limited coalescence in solid-stabilized emulsions. Eur. Phys. J. E: Soft Matter Biol. Phys. 2003, 11, 273–281. 10.1140/epje/i2003-10018-6. [DOI] [PubMed] [Google Scholar]
- Dupin D.; Armes S. P.; Connan C.; Reeve P.; Baxter S. M. How Does the Nature of the Steric Stabilizer Affect the Pickering Emulsifier Performance of Lightly Cross-Linked, Acid-Swellable Poly(2-vinylpyridine) Latexes?. Langmuir 2007, 23, 6903–6910. 10.1021/la063170j. [DOI] [PubMed] [Google Scholar]
- Guo H.; Yang D.; Yang M.; Gao Y.; Liu Y.; Li H. Dual responsive Pickering emulsions stabilized by constructed core crosslinked polymer nanoparticles via reversible covalent bonds. Soft Matter 2016, 12, 9683–9691. 10.1039/C6SM02336C. [DOI] [PubMed] [Google Scholar]
- Walsh A.; Thompson K. L.; Armes S. P.; York D. W. Polyamine-Functional Sterically Stabilized Latexes for Covalently Cross-Linkable Colloidosomes. Langmuir 2010, 26, 18039–18048. 10.1021/la103804y. [DOI] [PubMed] [Google Scholar]
- Thompson K. L.; Lane J. A.; Derry M. J.; Armes S. P. Non-aqueous Isorefractive Pickering Emulsions. Langmuir 2015, 31, 4373–4376. 10.1021/acs.langmuir.5b00630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mable C. J.; Warren N. J.; Thompson K. L.; Mykhaylyk O. O.; Armes S. P. Framboidal ABC triblock copolymer vesicles: a new class of efficient Pickering emulsifier. Chem. Sci. 2015, 6, 6179–6188. 10.1039/C5SC02346G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson K. L.; Mable C. J.; Lane J. A.; Derry M. J.; Fielding L. A.; Armes S. P. Preparation of Pickering Double Emulsions Using Block Copolymer Worms. Langmuir 2015, 31, 4137–4144. 10.1021/acs.langmuir.5b00741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzelli S. L.; Jones E. R.; Thompson K. L.; Armes S. P. Preparation of non-aqueous Pickering emulsions using anisotropic block copolymer nanoparticles. Colloid Polym. Sci. 2016, 294, 1–12. 10.1007/s00396-015-3785-3. [DOI] [Google Scholar]
- Read E. S.; Fujii S.; Amalvy J. I.; Randall D. P.; Armes S. P. Effect of Varying the Oil Phase on the Behavior of pH-Responsive Latex-Based Emulsifiers: Demulsification versus Transitional Phase Inversion. Langmuir 2005, 21, 1662–1662. 10.1021/la047603z. [DOI] [PubMed] [Google Scholar]
- Fujii S.; Read E. S.; Binks B. P.; Armes S. P. Stimulus-Responsive Emulsifiers Based on Nanocomposite Microgel Particles. Adv. Mater. 2005, 17, 1014–1018. 10.1002/adma.200401641. [DOI] [Google Scholar]
- Fujii S.; Armes S. P.; Binks B. P.; Murakami R. Stimulus-Responsive Particulate Emulsifiers Based on Lightly Cross-Linked Poly(4-vinylpyridine)-Silica Nanocomposite Microgels. Langmuir 2006, 22, 6818–6825. 10.1021/la060349l. [DOI] [PubMed] [Google Scholar]
- Morse A. J.; Dupin D.; Thompson K. L.; Armes S. P.; Ouzineb K.; Mills P.; Swart R. Novel Pickering Emulsifiers based on pH-Responsive Poly(tert-butylaminoethyl methacrylate) Latexes. Langmuir 2012, 28, 11733–11744. 10.1021/la301936k. [DOI] [PubMed] [Google Scholar]
- Morse A. J.; Armes S. P.; Thompson K. L.; Dupin D.; Fielding L. A.; Mills P.; Swart R. Novel Pickering Emulsifiers Based on pH-Responsive Poly(2-(diethylamino)ethyl methacrylate) Latexes. Langmuir 2013, 29, 5466–5475. 10.1021/la400786a. [DOI] [PubMed] [Google Scholar]
- Ngai T.; Auweter H.; Behrens S. H. Environmental Responsiveness of Microgel Particles and Particle-Stabilized Emulsions. Macromolecules 2006, 39, 8171–8177. 10.1021/ma061366k. [DOI] [Google Scholar]
- Brugger B.; Rütten S.; Phan K.-H.; Möller M.; Richtering W. The Colloidal Suprastructure of Smart Microgels at Oil-Water Interfaces. Angew. Chem., Int. Ed. 2009, 48, 3978–3981. 10.1002/anie.200900239. [DOI] [PubMed] [Google Scholar]
- Schild H. G. Poly(N-isopropylacrylamide): experiment, theory and application. Prog. Polym. Sci. 1992, 17, 163–249. 10.1016/0079-6700(92)90023-R. [DOI] [Google Scholar]
- Pelton R. H.; Chibante P. Preparation of aqueous latices with N-isopropylacrylamide. Colloids Surf. 1986, 20, 247–256. 10.1016/0166-6622(86)80274-8. [DOI] [Google Scholar]
- Pelton R. Temperature-sensitive aqueous microgels. Adv. Colloid Interface Sci. 2000, 85, 1–33. 10.1016/S0001-8686(99)00023-8. [DOI] [PubMed] [Google Scholar]
- Snowden M. J.; Chowdhry B. Z.; Vincent B.; Morris G. E. Colloidal copolymer microgels of N-isopropylacrylamide and acrylic acid: pH, ionic strength and temperature effects. J. Chem. Soc., Faraday Trans. 1996, 92, 5013–5016. 10.1039/ft9969205013. [DOI] [Google Scholar]
- Tsuji S.; Kawaguchi H. Thermosensitive Pickering Emulsion Stabilized by Poly(N-isopropylacrylamide)-Carrying Particles. Langmuir 2008, 24, 3300–3305. 10.1021/la701780g. [DOI] [PubMed] [Google Scholar]
- Brugger B.; Richtering W. Emulsions Stabilized by Stimuli-Sensitive Poly(N-isopropylacrylamide)-co-Methacrylic Acid Polymers: Microgels versus Low Molecular Weight Polymers. Langmuir 2008, 24, 7769–7777. 10.1021/la800522h. [DOI] [PubMed] [Google Scholar]
- Brugger B.; Vermant J.; Richtering W. Interfacial layers of stimuli-responsive poly-(N-isopropylacrylamide-co-methacrylicacid) (PNIPAM-co-MAA) microgels characterized by interfacial rheology and compression isotherms. Phys. Chem. Chem. Phys. 2010, 12, 14573–14578. 10.1039/c0cp01022g. [DOI] [PubMed] [Google Scholar]
- Richtering W. Responsive Emulsions Stabilized by Stimuli-Sensitive Microgels: Emulsions with Special Non-Pickering Properties. Langmuir 2012, 28, 17218–17229. 10.1021/la302331s. [DOI] [PubMed] [Google Scholar]
- Geisel K.; Isa L.; Richtering W. Unraveling the 3D Localization and Deformation of Responsive Microgels at Oil/Water Interfaces: A Step Forward in Understanding Soft Emulsion Stabilizers. Langmuir 2012, 28, 15770–15776. 10.1021/la302974j. [DOI] [PubMed] [Google Scholar]
- Szwarc M. /‘Living/’ Polymers. Nature 1956, 178, 1168–1169. 10.1038/1781168a0. [DOI] [Google Scholar]
- Szwarc M.; Levy M.; Milkovich R. Polymerization Initiated by Electron Transfer to Monomer. A New Method of Formation of Block Copolymers. J. Am. Chem. Soc. 1956, 78, 2656–2657. 10.1021/ja01592a101. [DOI] [Google Scholar]
- Gao Z.; Varshney S. K.; Wong S.; Eisenberg A. Block Copolymer “Crew-Cut” Micelles in Water. Macromolecules 1994, 27, 7923–7927. 10.1021/ma00104a058. [DOI] [Google Scholar]
- Zhang L.; Eisenberg A. Multiple Morphologies of “Crew-Cut” Aggregates of Polystyrene-b-poly(acrylic acid) Block Copolymers. Science 1995, 268, 1728–1731. 10.1126/science.268.5218.1728. [DOI] [PubMed] [Google Scholar]
- Zhang L.; Eisenberg A. Multiple Morphologies and Characteristics of “Crew-Cut” Micelle-like Aggregates of Polystyrene-b-poly(acrylic acid) Diblock Copolymers in Aqueous Solutions. J. Am. Chem. Soc. 1996, 118, 3168–3181. 10.1021/ja953709s. [DOI] [Google Scholar]
- Discher B. M.; Won Y.-Y.; Ege D. S.; Lee J. C.-M.; Bates F. S.; Discher D. E.; Hammer D. A. Polymersomes: Tough Vesicles Made from Diblock Copolymers. Science 1999, 284, 1143–1146. 10.1126/science.284.5417.1143. [DOI] [PubMed] [Google Scholar]
- Mai Y.; Eisenberg A. Self-assembly of block copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. 10.1039/c2cs35115c. [DOI] [PubMed] [Google Scholar]
- Matsen M. W.; Bates F. S. Origins of Complex Self-Assembly in Block Copolymers. Macromolecules 1996, 29, 7641–7644. 10.1021/ma960744q. [DOI] [Google Scholar]
- Patten T. E.; Matyjaszewski K. Atom Transfer Radical Polymerization and the Synthesis of Polymeric Materials. Adv. Mater. 1998, 10, 901–915. . [DOI] [Google Scholar]
- Hawker C. J.; Bosman A. W.; Harth E. New Polymer Synthesis by Nitroxide Mediated Living Radical Polymerizations. Chem. Rev. 2001, 101, 3661–3688. 10.1021/cr990119u. [DOI] [PubMed] [Google Scholar]
- Moad G.; Rizzardo E.; Thang S. H. Living Radical Polymerization by the RAFT Process. Aust. J. Chem. 2005, 58, 379–410. 10.1071/CH05072. [DOI] [Google Scholar]
- Chiefari J.; Chong Y. K.; Ercole F.; Krstina J.; Jeffery J.; Le T. P. T.; Mayadunne R. T. A.; Meijs G. F.; Moad C. L.; Moad G.; Rizzardo E.; Thang S. H. Living Free-Radical Polymerization by Reversible Addition-Fragmentation Chain Transfer: The RAFT Process. Macromolecules 1998, 31, 5559–5562. 10.1021/ma9804951. [DOI] [Google Scholar]
- Moad G.; Rizzardo E.; Thang S. H. Living Radical Polymerization by the RAFT ProcessA First Update. Aust. J. Chem. 2006, 59, 669–692. 10.1071/CH06250. [DOI] [Google Scholar]
- Moad G.; Rizzardo E.; Thang S. H. Living Radical Polymerization by the RAFT Process - A Second Update. Aust. J. Chem. 2009, 62, 1402–1472. 10.1071/CH09311. [DOI] [Google Scholar]
- Moad G.; Rizzardo E.; Thang S. H. Living Radical Polymerization by the RAFT Process - A Third Update. Aust. J. Chem. 2012, 65, 985–1076. 10.1071/CH12295. [DOI] [Google Scholar]
- Mühlebach A.; Gaynor S. G.; Matyjaszewski K. Synthesis of Amphiphilic Block Copolymers by Atom Transfer Radical Polymerization (ATRP). Macromolecules 1998, 31, 6046–6052. 10.1021/ma9804747. [DOI] [Google Scholar]
- Delaittre G.; Nicolas J.; Lefay C.; Save M.; Charleux B. Surfactant-free synthesis of amphiphilic diblock copolymer nanoparticles via nitroxide-mediated emulsion polymerization. Chem. Commun. 2005, 614–616. 10.1039/b415959d. [DOI] [PubMed] [Google Scholar]
- Ferguson C. J.; Hughes R. J.; Pham B. T. T.; Hawkett B. S.; Gilbert R. G.; Serelis A. K.; Such C. H. Effective ab Initio Emulsion Polymerization under RAFT Control. Macromolecules 2002, 35, 9243–9245. 10.1021/ma025626j. [DOI] [Google Scholar]
- Warren N. J.; Armes S. P. Polymerization-Induced Self-Assembly of Block Copolymer Nano-objects via RAFT Aqueous Dispersion Polymerization. J. Am. Chem. Soc. 2014, 136, 10174–10185. 10.1021/ja502843f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derry M. J.; Fielding L. A.; Armes S. P. Polymerization-induced self-assembly of block copolymer nanoparticles via RAFT non-aqueous dispersion polymerization. Prog. Polym. Sci. 2016, 52, 1–18. 10.1016/j.progpolymsci.2015.10.002. [DOI] [Google Scholar]
- Canning S. L.; Smith G. N.; Armes S. P. A Critical Appraisal of RAFT-Mediated Polymerization-Induced Self-Assembly. Macromolecules 2016, 49, 1985–2001. 10.1021/acs.macromol.5b02602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfold N. J. W.; Yeow J.; Boyer C.; Armes S. P. Emerging Trends in Polymerization-Induced Self-Assembly. ACS Macro Lett. 2019, 8, 1029–1054. 10.1021/acsmacrolett.9b00464. [DOI] [PubMed] [Google Scholar]
- D’Agosto F.; Rieger J.; Lansalot M. RAFT-Mediated Polymerization-Induced Self-Assembly. Angew. Chem., Int. Ed. 2020, 59, 8368. 10.1002/anie.201911758. [DOI] [PubMed] [Google Scholar]
- Rieger J. Guidelines for the Synthesis of Block Copolymer Particles of Various Morphologies by RAFT Dispersion Polymerization. Macromol. Rapid Commun. 2015, 36, 1458–1471. 10.1002/marc.201500028. [DOI] [PubMed] [Google Scholar]
- Charleux B.; Delaittre G.; Rieger J.; D’Agosto F. Polymerization-Induced Self-Assembly: From Soluble Macromolecules to Block Copolymer Nano-Objects in One Step. Macromolecules 2012, 45, 6753–6765. 10.1021/ma300713f. [DOI] [Google Scholar]
- Derry M. J.; Fielding L. A.; Armes S. P. Industrially-relevant polymerization-induced self-assembly formulations in non-polar solvents: RAFT dispersion polymerization of benzyl methacrylate. Polym. Chem. 2015, 6, 3054–3062. 10.1039/C5PY00157A. [DOI] [Google Scholar]
- Cunningham V. J.; Alswieleh A. M.; Thompson K. L.; Williams M.; Leggett G. J.; Armes S. P.; Musa O. M. Poly(glycerol monomethacrylate)-Poly(benzyl methacrylate) Diblock Copolymer Nanoparticles via RAFT Emulsion Polymerization: Synthesis, Characterization, and Interfacial Activity. Macromolecules 2014, 47, 5613–5623. 10.1021/ma501140h. [DOI] [Google Scholar]
- Prescott S. W.; Ballard M. J.; Rizzardo E.; Gilbert R. G. RAFT in Emulsion Polymerization: What Makes it Different?. Aust. J. Chem. 2002, 55, 415–424. 10.1071/CH02073. [DOI] [Google Scholar]
- Prescott S. W.; Ballard M. J.; Rizzardo E.; Gilbert R. G. Rate Optimization in Controlled Radical Emulsion Polymerization Using RAFT. Macromol. Theory Simul. 2006, 15, 70–86. 10.1002/mats.200500052. [DOI] [Google Scholar]
- Ferguson C. J.; Hughes R. J.; Nguyen D.; Pham B. T. T.; Gilbert R. G.; Serelis A. K.; Such C. H.; Hawkett B. S. Ab Initio Emulsion Polymerization by RAFT-Controlled Self-Assembly. Macromolecules 2005, 38, 2191–2204. 10.1021/ma048787r. [DOI] [Google Scholar]
- Truong N. P.; Dussert M. V.; Whittaker M. R.; Quinn J. F.; Davis T. P. Rapid synthesis of ultrahigh molecular weight and low polydispersity polystyrene diblock copolymers by RAFT-mediated emulsion polymerization. Polym. Chem. 2015, 6, 3865–3874. 10.1039/C5PY00166H. [DOI] [Google Scholar]
- Ganeva D. E.; Sprong E.; de Bruyn H.; Warr G. G.; Such C. H.; Hawkett B. S. Particle Formation in ab Initio RAFT Mediated Emulsion Polymerization Systems. Macromolecules 2007, 40, 6181–6189. 10.1021/ma070442w. [DOI] [Google Scholar]
- Chaduc I.; Crepet A.; Boyron O.; Charleux B.; D’Agosto F.; Lansalot M. Effect of the pH on the RAFT Polymerization of Acrylic Acid in Water. Application to the Synthesis of Poly(acrylic acid)-Stabilized Polystyrene Particles by RAFT Emulsion Polymerization. Macromolecules 2013, 46, 6013–6023. 10.1021/ma401070k. [DOI] [Google Scholar]
- Chaduc I.; Zhang W.; Rieger J.; Lansalot M.; D’Agosto F.; Charleux B. Amphiphilic Block Copolymers from a Direct and One-pot RAFT Synthesis in Water. Macromol. Rapid Commun. 2011, 32, 1270–1276. 10.1002/marc.201100240. [DOI] [PubMed] [Google Scholar]
- Chaduc I.; Girod M.; Antoine R.; Charleux B.; D’Agosto F.; Lansalot M. Batch Emulsion Polymerization Mediated by Poly(methacrylic acid) MacroRAFT Agents: One-Pot Synthesis of Self-Stabilized Particles. Macromolecules 2012, 45, 5881–5893. 10.1021/ma300875y. [DOI] [Google Scholar]
- Poon C. K.; Tang O.; Chen X.-M.; Pham B. T. T.; Gody G.; Pollock C. A.; Hawkett B. S.; Perrier S. Preparation of Inert Polystyrene Latex Particles as MicroRNA Delivery Vectors by Surfactant-Free RAFT Emulsion Polymerization. Biomacromolecules 2016, 17, 965–973. 10.1021/acs.biomac.5b01633. [DOI] [PubMed] [Google Scholar]
- Rieger J.; Stoffelbach F.; Bui C.; Alaimo D.; Jérôme C.; Charleux B. Amphiphilic Poly(ethylene oxide) Macromolecular RAFT Agent as a Stabilizer and Control Agent in ab Initio Batch Emulsion Polymerization. Macromolecules 2008, 41, 4065–4068. 10.1021/ma800544v. [DOI] [Google Scholar]
- Rieger J.; Zhang W.; Stoffelbach F.; Charleux B. Surfactant-Free RAFT Emulsion Polymerization Using Poly(N,N-dimethylacrylamide) Trithiocarbonate Macromolecular Chain Transfer Agents. Macromolecules 2010, 43, 6302–6310. 10.1021/ma1009269. [DOI] [Google Scholar]
- Rieger J.; Osterwinter G.; Bui C.; Stoffelbach F.; Charleux B. Surfactant-Free Controlled/Living Radical Emulsion (Co)polymerization of n-Butyl Acrylate and Methyl Methacrylate via RAFT Using Amphiphilic Poly(ethylene oxide)-Based Trithiocarbonate Chain Transfer Agents. Macromolecules 2009, 42, 5518–5525. 10.1021/ma9008803. [DOI] [Google Scholar]
- Boissé S.; Rieger J.; Pembouong G.; Beaunier P.; Charleux B. Influence of the stirring speed and CaCl2 concentration on the nano-object morphologies obtained via RAFT-mediated aqueous emulsion polymerization in the presence of a water-soluble macroRAFT agent. J. Polym. Sci., Part A: Polym. Chem. 2011, 49, 3346–3354. 10.1002/pola.24771. [DOI] [Google Scholar]
- Zhang W.; D’Agosto F.; Boyron O.; Rieger J.; Charleux B. One-Pot Synthesis of Poly(methacrylic acid-co-poly(ethylene oxide) methyl ether methacrylate)-b-polystyrene Amphiphilic Block Copolymers and Their Self-Assemblies in Water via RAFT-Mediated Radical Emulsion Polymerization. A Kinetic Study. Macromolecules 2011, 44, 7584–7593. 10.1021/ma201515n. [DOI] [Google Scholar]
- Grazon C.; Rieger J.; Sanson N.; Charleux B. Study of poly(N,N-diethylacrylamide) nanogel formation by aqueous dispersion polymerization of N,N-diethylacrylamide in the presence of poly(ethylene oxide)-b-poly(N,N-dimethylacrylamide) amphiphilic macromolecular RAFT agents. Soft Matter 2011, 7, 3482–3490. 10.1039/c0sm01181a. [DOI] [Google Scholar]
- Zhang W.; D’Agosto F.; Boyron O.; Rieger J.; Charleux B. Toward a Better Understanding of the Parameters that Lead to the Formation of Nonspherical Polystyrene Particles via RAFT-Mediated One-Pot Aqueous Emulsion Polymerization. Macromolecules 2012, 45, 4075–4084. 10.1021/ma300596f. [DOI] [Google Scholar]
- Zhang W.; D’Agosto F.; Dugas P.-Y.; Rieger J.; Charleux B. RAFT-mediated one-pot aqueous emulsion polymerization of methyl methacrylate in presence of poly(methacrylic acid-co-poly(ethylene oxide) methacrylate) trithiocarbonate macromolecular chain transfer agent. Polymer 2013, 54, 2011–2019. 10.1016/j.polymer.2012.12.028. [DOI] [Google Scholar]
- Canton I.; Warren N. J.; Chahal A.; Amps K.; Wood A.; Weightman R.; Wang E.; Moore H.; Armes S. P. Mucin-Inspired Thermoresponsive Synthetic Hydrogels Induce Stasis in Human Pluripotent Stem Cells and Human Embryos. ACS Cent. Sci. 2016, 2, 65–74. 10.1021/acscentsci.5b00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfold N. J. W.; Ning Y.; Verstraete P.; Smets J.; Armes S. P. Cross-linked cationic diblock copolymer worms are superflocculants for micrometer-sized silica particles. Chem. Sci. 2016, 7, 6894–6904. 10.1039/C6SC03732A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren N. J.; Mykhaylyk O. O.; Mahmood D.; Ryan A. J.; Armes S. P. RAFT Aqueous Dispersion Polymerization Yields Poly(ethylene glycol)-Based Diblock Copolymer Nano-Objects with Predictable Single Phase Morphologies. J. Am. Chem. Soc. 2014, 136, 1023–1033. 10.1021/ja410593n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanazs A.; Armes S. P.; Ryan A. J. Self-Assembled Block Copolymer Aggregates: From Micelles to Vesicles and their Biological Applications. Macromol. Rapid Commun. 2009, 30, 267–277. 10.1002/marc.200800713. [DOI] [PubMed] [Google Scholar]
- Blanazs A.; Madsen J.; Battaglia G.; Ryan A. J.; Armes S. P. Mechanistic Insights for Block Copolymer Morphologies: How Do Worms Form Vesicles?. J. Am. Chem. Soc. 2011, 133, 16581–16587. 10.1021/ja206301a. [DOI] [PubMed] [Google Scholar]
- Blanazs A.; Ryan A. J.; Armes S. P. Predictive Phase Diagrams for RAFT Aqueous Dispersion Polymerization: Effect of Block Copolymer Composition, Molecular Weight, and Copolymer Concentration. Macromolecules 2012, 45, 5099–5107. 10.1021/ma301059r. [DOI] [Google Scholar]
- Verber R.; Blanazs A.; Armes S. P. Rheological studies of thermo-responsive diblock copolymer worm gels. Soft Matter 2012, 8, 9915–9922. 10.1039/c2sm26156a. [DOI] [Google Scholar]
- Jiang Y.; Xu N.; Han J.; Yu Q.; Guo L.; Gao P.; Lu X.; Cai Y. The direct synthesis of interface-decorated reactive block copolymer nanoparticles via polymerisation-induced self-assembly. Polym. Chem. 2015, 6, 4955–4965. 10.1039/C5PY00656B. [DOI] [Google Scholar]
- Shen W.; Chang Y.; Liu G.; Wang H.; Cao A.; An Z. Biocompatible, Antifouling, and Thermosensitive Core-Shell Nanogels Synthesized by RAFT Aqueous Dispersion Polymerization. Macromolecules 2011, 44, 2524–2530. 10.1021/ma200074n. [DOI] [Google Scholar]
- Semsarilar M.; Jones E. R.; Blanazs A.; Armes S. P. Efficient Synthesis of Sterically-Stabilized Nano-Objects via RAFT Dispersion Polymerization of Benzyl Methacrylate in Alcoholic Media. Adv. Mater. 2012, 24, 3378–3382. 10.1002/adma.201200925. [DOI] [PubMed] [Google Scholar]
- Gonzato C.; Semsarilar M.; Jones E. R.; Li F.; Krooshof G. J. P.; Wyman P.; Mykhaylyk O. O.; Tuinier R.; Armes S. P. Rational Synthesis of Low-Polydispersity Block Copolymer Vesicles in Concentrated Solution via Polymerization-Induced Self-Assembly. J. Am. Chem. Soc. 2014, 136, 11100–11106. 10.1021/ja505406s. [DOI] [PubMed] [Google Scholar]
- Jones E. R.; Semsarilar M.; Blanazs A.; Armes S. P. Efficient Synthesis of Amine-Functional Diblock Copolymer Nanoparticles via RAFT Dispersion Polymerization of Benzyl Methacrylate in Alcoholic Media. Macromolecules 2012, 45, 5091–5098. 10.1021/ma300898e. [DOI] [Google Scholar]
- Pei Y.; Dharsana N. C.; van Hensbergen J. A.; Burford R. P.; Roth P. J.; Lowe A. B. RAFT dispersion polymerization of 3-phenylpropyl methacrylate with poly[2-(dimethylamino)ethyl methacrylate] macro-CTAs in ethanol and associated thermoreversible polymorphism. Soft Matter 2014, 10, 5787–5796. 10.1039/C4SM00729H. [DOI] [PubMed] [Google Scholar]
- Jones E. R.; Semsarilar M.; Wyman P.; Boerakker M.; Armes S. P. Addition of water to an alcoholic RAFT PISA formulation leads to faster kinetics but limits the evolution of copolymer morphology. Polym. Chem. 2016, 7, 851–859. 10.1039/C5PY01795E. [DOI] [Google Scholar]
- Gibson R. R.; Cornel E. J.; Musa O. M.; Fernyhough A.; Armes S. P. RAFT dispersion polymerisation of lauryl methacrylate in ethanol-water binary mixtures: synthesis of diblock copolymer vesicles with deformable membranes. Polym. Chem. 2020, 11, 1785–1796. 10.1039/C9PY01768B. [DOI] [Google Scholar]
- Ding Z.; Gao C.; Wang S.; Liu H.; Zhang W. Macro-RAFT agent mediated dispersion polymerization: the monomer concentration effect on the morphology of the in situ synthesized block copolymer nano-objects. Polym. Chem. 2015, 6, 8003–8011. 10.1039/C5PY01202C. [DOI] [Google Scholar]
- Zehm D.; Ratcliffe L. P. D.; Armes S. P. Synthesis of Diblock Copolymer Nanoparticles via RAFT Alcoholic Dispersion Polymerization: Effect of Block Copolymer Composition, Molecular Weight, Copolymer Concentration, and Solvent Type on the Final Particle Morphology. Macromolecules 2013, 46, 128–139. 10.1021/ma301459y. [DOI] [Google Scholar]
- Lowe A. B. RAFT alcoholic dispersion polymerization with polymerization-induced self-assembly. Polymer 2016, 106, 161–181. 10.1016/j.polymer.2016.08.082. [DOI] [Google Scholar]
- Wan W.-M.; Sun X.-L.; Pan C.-Y. Formation of Vesicular Morphologies via Polymerization Induced Self-Assembly and Re-Organization. Macromol. Rapid Commun. 2010, 31, 399–404. 10.1002/marc.200900640. [DOI] [PubMed] [Google Scholar]
- Huang C.-Q.; Pan C.-Y. Direct preparation of vesicles from one-pot RAFT dispersion polymerization. Polymer 2010, 51, 5115–5121. 10.1016/j.polymer.2010.08.056. [DOI] [Google Scholar]
- Huang C.-Q.; Wang Y.; Hong C.-Y.; Pan C.-Y. Spiropyran-Based Polymeric Vesicles: Preparation and Photochromic Properties. Macromol. Rapid Commun. 2011, 32, 1174–1179. 10.1002/marc.201100197. [DOI] [PubMed] [Google Scholar]
- He W.-D.; Sun X.-L.; Wan W.-M.; Pan C.-Y. Multiple Morphologies of PAA-b-PSt Assemblies throughout RAFT Dispersion Polymerization of Styrene with PAA Macro-CTA. Macromolecules 2011, 44, 3358–3365. 10.1021/ma2000674. [DOI] [Google Scholar]
- Karagoz B.; Boyer C.; Davis T. P. Simultaneous Polymerization-Induced Self-Assembly (PISA) and Guest Molecule Encapsulation. Macromol. Rapid Commun. 2014, 35, 417–421. 10.1002/marc.201300730. [DOI] [PubMed] [Google Scholar]
- Fielding L. A.; Derry M. J.; Ladmiral V.; Rosselgong J.; Rodrigues A. M.; Ratcliffe L. P. D.; Sugihara S.; Armes S. P. RAFT dispersion polymerization in non-polar solvents: facile production of block copolymer spheres, worms and vesicles in n-alkanes. Chem. Sci. 2013, 4, 2081–2087. 10.1039/c3sc50305d. [DOI] [Google Scholar]
- Fielding L. A.; Lane J. A.; Derry M. J.; Mykhaylyk O. O.; Armes S. P. Thermo-responsive Diblock Copolymer Worm Gels in Non-polar Solvents. J. Am. Chem. Soc. 2014, 136, 5790–5798. 10.1021/ja501756h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houillot L.; Bui C.; Save M.; Charleux B.; Farcet C.; Moire C.; Raust J.-A.; Rodriguez I. Synthesis of Well-Defined Polyacrylate Particle Dispersions in Organic Medium Using Simultaneous RAFT Polymerization and Self-Assembly of Block Copolymers. A Strong Influence of the Selected Thiocarbonylthio Chain Transfer Agent. Macromolecules 2007, 40, 6500–6509. 10.1021/ma0703249. [DOI] [Google Scholar]
- Pei Y.; Thurairajah L.; Sugita O. R.; Lowe A. B. RAFT Dispersion Polymerization in Nonpolar Media: Polymerization of 3-Phenylpropyl Methacrylate in n-Tetradecane with Poly(stearyl methacrylate) Homopolymers as Macro Chain Transfer Agents. Macromolecules 2015, 48, 236–244. 10.1021/ma502230h. [DOI] [Google Scholar]
- Lopez-Oliva A. P.; Warren N. J.; Rajkumar A.; Mykhaylyk O. O.; Derry M. J.; Doncom K. E. B.; Rymaruk M. J.; Armes S. P. Polydimethylsiloxane-Based Diblock Copolymer Nano-objects Prepared in Nonpolar Media via RAFT-Mediated Polymerization-Induced Self-Assembly. Macromolecules 2015, 48, 3547–3555. 10.1021/acs.macromol.5b00576. [DOI] [Google Scholar]
- Smith G. N.; Canning S. L.; Derry M. J.; Jones E. R.; Neal T. J.; Smith A. J. Ionic and Nonspherical Polymer Nanoparticles in Nonpolar Solvents. Macromolecules 2020, 53, 3148–3156. 10.1021/acs.macromol.0c00121. [DOI] [Google Scholar]
- Zhang Q.; Zhu S. Ionic Liquids: Versatile Media for Preparation of Vesicles from Polymerization-Induced Self-Assembly. ACS Macro Lett. 2015, 4, 755–758. 10.1021/acsmacrolett.5b00360. [DOI] [PubMed] [Google Scholar]
- Rymaruk M. J.; Hunter S. J.; O’Brien C. T.; Brown S. L.; Williams C. N.; Armes S. P. RAFT Dispersion Polymerization in Silicone Oil. Macromolecules 2019, 52, 2822–2832. 10.1021/acs.macromol.9b00129. [DOI] [Google Scholar]
- Rymaruk M. J.; O’Brien C. T.; Brown S. L.; Williams C. N.; Armes S. P. RAFT Dispersion Polymerization of Benzyl Methacrylate in Silicone Oil Using a Silicone-Based Methacrylic Stabilizer Provides Convenient Access to Spheres, Worms, and Vesicles. Macromolecules 2020, 53, 1785–1794. 10.1021/acs.macromol.9b02697. [DOI] [Google Scholar]
- Hasell T.; Thurecht K. J.; Jones R. D. W.; Brown P. D.; Howdle S. M. Novel one pot synthesis of silver nanoparticle-polymer composites by supercritical CO2 polymerisation in the presence of a RAFT agent. Chem. Commun. 2007, 3933–3935. 10.1039/b710503g. [DOI] [PubMed] [Google Scholar]
- Zong M.; Thurecht K. J.; Howdle S. M. Dispersion polymerisation in supercritical CO2 using macro-RAFT agents. Chem. Commun. 2008, 5942–5944. 10.1039/b812827h. [DOI] [PubMed] [Google Scholar]
- Dong S.; Zhao W.; Lucien F. P.; Perrier S.; Zetterlund P. B. Polymerization induced self-assembly: tuning of nano-object morphology by use of CO2. Polym. Chem. 2015, 6, 2249–2254. 10.1039/C4PY01632G. [DOI] [Google Scholar]
- Xu A.; Lu Q.; Huo Z.; Ma J.; Geng B.; Azhar U.; Zhang L.; Zhang S. Synthesis of fluorinated nanoparticles via RAFT dispersion polymerization-induced self-assembly using fluorinated macro-RAFT agents in supercritical carbon dioxide. RSC Adv. 2017, 7, 51612–51620. 10.1039/C7RA08202A. [DOI] [Google Scholar]
- Byard S. J.; Williams M.; McKenzie B. E.; Blanazs A.; Armes S. P. Preparation and Cross-Linking of All-Acrylamide Diblock Copolymer Nano-Objects via Polymerization-Induced Self-Assembly in Aqueous Solution. Macromolecules 2017, 50, 1482–1493. 10.1021/acs.macromol.6b02643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfold N. J. W.; Whatley J. R.; Armes S. P. Thermoreversible Block Copolymer Worm Gels Using Binary Mixtures of PEG Stabilizer Blocks. Macromolecules 2019, 52, 1653–1662. 10.1021/acs.macromol.8b02491. [DOI] [Google Scholar]
- Byard S. J.; O’Brien C. T.; Derry M. J.; Williams M.; Mykhaylyk O. O.; Blanazs A.; Armes S. P. Unique aqueous self-assembly behavior of a thermoresponsive diblock copolymer. Chem. Sci. 2020, 11, 396–402. 10.1039/C9SC04197D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B.; Lv X.; Zhu A.; Zheng J.; Yang Y.; An Z. Morphological Stabilization of Block Copolymer Worms Using Asymmetric Cross-Linkers during Polymerization-Induced Self-Assembly. Macromolecules 2018, 51, 2776–2784. 10.1021/acs.macromol.8b00246. [DOI] [Google Scholar]
- Zhang B.; Lv X.; An Z. Modular Monomers with Tunable Solubility: Synthesis of Highly Incompatible Block Copolymer Nano-Objects via RAFT Aqueous Dispersion Polymerization. ACS Macro Lett. 2017, 6, 224–228. 10.1021/acsmacrolett.7b00056. [DOI] [PubMed] [Google Scholar]
- Blackman L. D.; Doncom K. E. B.; Gibson M. I.; O’Reilly R. K. Comparison of photo- and thermally initiated polymerization-induced self-assembly: a lack of end group fidelity drives the formation of higher order morphologies. Polym. Chem. 2017, 8, 2860–2871. 10.1039/C7PY00407A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu L.; Zhang H.; Wang B.; Zhan Y.; Xing C.; Pan C.-Y. CO2-Responsive Nano-Objects with Assembly-Related Aggregation-Induced Emission and Tunable Morphologies. ACS Appl. Mater. Interfaces 2020, 12, 1348–1358. 10.1021/acsami.9b18792. [DOI] [PubMed] [Google Scholar]
- Varlas S.; Foster J. C.; Georgiou P. G.; Keogh R.; Husband J. T.; Williams D. S.; O’Reilly R. K. Tuning the membrane permeability of polymersome nanoreactors developed by aqueous emulsion polymerization-induced self-assembly. Nanoscale 2019, 11, 12643–12654. 10.1039/C9NR02507C. [DOI] [PubMed] [Google Scholar]
- Yao H.; Ning Y.; Jesson C. P.; He J.; Deng R.; Tian W.; Armes S. P. Using Host-Guest Chemistry to Tune the Kinetics of Morphological Transitions Undertaken by Block Copolymer Vesicles. ACS Macro Lett. 2017, 6, 1379–1385. 10.1021/acsmacrolett.7b00836. [DOI] [PubMed] [Google Scholar]
- Mable C. J.; Fielding L. A.; Derry M. J.; Mykhaylyk O. O.; Chambon P.; Armes S. P. Synthesis and pH-responsive dissociation of framboidal ABC triblock copolymer vesicles in aqueous solution. Chem. Sci. 2018, 9, 1454–1463. 10.1039/C7SC04788F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q.; Zhang Y.; Li X.; He J.; Tan J.; Zhang L. Enzyme catalysis-induced RAFT polymerization in water for the preparation of epoxy-functionalized triblock copolymer vesicles. Polym. Chem. 2018, 9, 4908–4916. 10.1039/C8PY01053F. [DOI] [Google Scholar]
- Wang X.; Zhou J.; Lv X.; Zhang B.; An Z. Temperature-Induced Morphological Transitions of Poly(dimethylacrylamide)-Poly(diacetone acrylamide) Block Copolymer Lamellae Synthesized via Aqueous Polymerization-Induced Self-Assembly. Macromolecules 2017, 50, 7222–7232. 10.1021/acs.macromol.7b01644. [DOI] [Google Scholar]
- Yang P.; Ratcliffe L. P. D.; Armes S. P. Efficient Synthesis of Poly(methacrylic acid)-block-Poly(styrene-alt-N-phenylmaleimide) Diblock Copolymer Lamellae Using RAFT Dispersion Polymerization. Macromolecules 2013, 46, 8545–8556. 10.1021/ma401797a. [DOI] [Google Scholar]
- Yang P.; Mykhaylyk O. O.; Jones E. R.; Armes S. P. RAFT Dispersion Alternating Copolymerization of Styrene with N-Phenylmaleimide: Morphology Control and Application as an Aqueous Foam Stabilizer. Macromolecules 2016, 49, 6731–6742. 10.1021/acs.macromol.6b01563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israelachvili J. N.; Mitchell D. J.; Ninham B. W. Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers. J. Chem. Soc., Faraday Trans. 2 1976, 72, 1525–1568. 10.1039/f29767201525. [DOI] [Google Scholar]
- Chambon P.; Blanazs A.; Battaglia G.; Armes S. P. Facile Synthesis of Methacrylic ABC Triblock Copolymer Vesicles by RAFT Aqueous Dispersion Polymerization. Macromolecules 2012, 45, 5081–5090. 10.1021/ma300816m. [DOI] [Google Scholar]
- Thompson K. L.; Chambon P.; Verber R.; Armes S. P. Can Polymersomes Form Colloidosomes?. J. Am. Chem. Soc. 2012, 134, 12450–12453. 10.1021/ja305789e. [DOI] [PubMed] [Google Scholar]
- Thompson K. L.; Mable C. J.; Cockram A.; Warren N. J.; Cunningham V. J.; Jones E. R.; Verber R.; Armes S. P. Are block copolymer worms more effective Pickering emulsifiers than block copolymer spheres?. Soft Matter 2014, 10, 8615–8626. 10.1039/C4SM01724B. [DOI] [PubMed] [Google Scholar]
- Thompson K. L.; Fielding L. A.; Mykhaylyk O. O.; Lane J. A.; Derry M. J.; Armes S. P. Vermicious thermo-responsive Pickering emulsifiers. Chem. Sci. 2015, 6, 4207–4214. 10.1039/C5SC00598A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mable C. J.; Thompson K. L.; Derry M. J.; Mykhaylyk O. O.; Binks B. P.; Armes S. P. ABC Triblock Copolymer Worms: Synthesis, Characterization, and Evaluation as Pickering Emulsifiers for Millimeter-Sized Droplets. Macromolecules 2016, 49, 7897–7907. 10.1021/acs.macromol.6b01729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rymaruk M. J.; Thompson K. L.; Derry M. J.; Warren N. J.; Ratcliffe L. P. D.; Williams C. N.; Brown S. L.; Armes S. P. Bespoke contrast-matched diblock copolymer nanoparticles enable the rational design of highly transparent Pickering double emulsions. Nanoscale 2016, 8, 14497–14506. 10.1039/C6NR03856E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson K. L.; Cinotti N.; Jones E. R.; Mable C. J.; Fowler P. W.; Armes S. P. Bespoke Diblock Copolymer Nanoparticles Enable the Production of Relatively Stable Oil-in-Water Pickering Nanoemulsions. Langmuir 2017, 33, 12616–12623. 10.1021/acs.langmuir.7b02267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson K. L.; Derry M. J.; Hatton F. L.; Armes S. P. Long-Term Stability of n-Alkane-in-Water Pickering Nanoemulsions: Effect of Aqueous Solubility of Droplet Phase on Ostwald Ripening. Langmuir 2018, 34, 9289–9297. 10.1021/acs.langmuir.8b01835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S. J.; Thompson K. L.; Lovett J. R.; Hatton F. L.; Derry M. J.; Lindsay C.; Taylor P.; Armes S. P. Synthesis, Characterization, and Pickering Emulsifier Performance of Anisotropic Cross-Linked Block Copolymer Worms: Effect of Aspect Ratio on Emulsion Stability in the Presence of Surfactant. Langmuir 2019, 35, 254–265. 10.1021/acs.langmuir.8b03727. [DOI] [PubMed] [Google Scholar]
- Cunningham V. J.; Giakoumatos E. C.; Ireland P. M.; Mable C. J.; Armes S. P.; Wanless E. J. Giant Pickering Droplets: Effect of Nanoparticle Size and Morphology on Stability. Langmuir 2017, 33, 7669–7679. 10.1021/acs.langmuir.7b01383. [DOI] [PubMed] [Google Scholar]
- Aveyard R.; Binks B. P.; Clint J. H. Emulsions stabilised solely by colloidal particles. Adv. Colloid Interface Sci. 2003, 100–102, 503–546. 10.1016/S0001-8686(02)00069-6. [DOI] [Google Scholar]
- Madsen J.; Armes S. P.; Lewis A. L. Preparation and Aqueous Solution Properties of New Thermoresponsive Biocompatible ABA Triblock Copolymer Gelators. Macromolecules 2006, 39, 7455–7457. 10.1021/ma062198z. [DOI] [Google Scholar]
- Madsen J.; Armes S. P.; Bertal K.; MacNeil S.; Lewis A. L. Preparation and Aqueous Solution Properties of Thermoresponsive Biocompatible AB Diblock Copolymers. Biomacromolecules 2009, 10, 1875–1887. 10.1021/bm9002915. [DOI] [PubMed] [Google Scholar]
- Thompson K. L.; Williams M.; Armes S. P. Colloidosomes: Synthesis, properties and applications. J. Colloid Interface Sci. 2015, 447, 217–228. 10.1016/j.jcis.2014.11.058. [DOI] [PubMed] [Google Scholar]
- Derry M. J.; Fielding L. A.; Warren N. J.; Mable C. J.; Smith A. J.; Mykhaylyk O. O.; Armes S. P. In situ small-angle X-ray scattering studies of sterically-stabilized diblock copolymer nanoparticles formed during polymerization-induced self-assembly in non-polar media. Chem. Sci. 2016, 7, 5078–5090. 10.1039/C6SC01243D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madivala B.; Vandebril S.; Fransaer J.; Vermant J. Exploiting particle shape in solid stabilized emulsions. Soft Matter 2009, 5, 1717–1727. 10.1039/b816680c. [DOI] [Google Scholar]
- Madivala B.; Fransaer J.; Vermant J. Self-Assembly and Rheology of Ellipsoidal Particles at Interfaces. Langmuir 2009, 25, 2718–2728. 10.1021/la803554u. [DOI] [PubMed] [Google Scholar]
- Dugyala V. R.; Anjali T. G.; Upendar S.; Mani E.; Basavaraj M. G. Nano ellipsoids at the fluid-fluid interface: effect of surface charge on adsorption, buckling and emulsification. Faraday Discuss. 2016, 186, 419–434. 10.1039/C5FD00136F. [DOI] [PubMed] [Google Scholar]
- Wang A.; Rogers W. B.; Manoharan V. N. Effects of Contact-Line Pinning on the Adsorption of Nonspherical Colloids at Liquid Interfaces. Phys. Rev. Lett. 2017, 119, 108004. 10.1103/PhysRevLett.119.108004. [DOI] [PubMed] [Google Scholar]
- Loudet J. C.; Yodh A. G.; Pouligny B. Wetting and Contact Lines of Micrometer-Sized Ellipsoids. Phys. Rev. Lett. 2006, 97, 018304. 10.1103/PhysRevLett.97.018304. [DOI] [PubMed] [Google Scholar]
- Xue Y.; Li X.; Dong J. Interfacial characteristics of block copolymer micelles stabilized Pickering emulsion by confocal laser scanning microscopy. J. Colloid Interface Sci. 2020, 563, 33–41. 10.1016/j.jcis.2019.12.016. [DOI] [PubMed] [Google Scholar]
- Blanazs A.; Verber R.; Mykhaylyk O. O.; Ryan A. J.; Heath J. Z.; Douglas C. W. I.; Armes S. P. Sterilizable Gels from Thermoresponsive Block Copolymer Worms. J. Am. Chem. Soc. 2012, 134, 9741–9748. 10.1021/ja3024059. [DOI] [PubMed] [Google Scholar]
- Lovett J. R.; Ratcliffe L. P. D.; Warren N. J.; Armes S. P.; Smallridge M. J.; Cracknell R. B.; Saunders B. R. A Robust Cross-Linking Strategy for Block Copolymer Worms Prepared via Polymerization-Induced Self-Assembly. Macromolecules 2016, 49, 2928–2941. 10.1021/acs.macromol.6b00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q.; Wang C.; Fu M.; Wang J.; Zhu S. Pickering high internal phase emulsions stabilized by worm-like polymeric nanoaggregates. Polym. Chem. 2017, 8, 5474–5480. 10.1039/C7PY00912G. [DOI] [Google Scholar]
- Zhang Y.; Yu L.; Dai X.; Zhang L.; Tan J. Structural Difference in Macro-RAFT Agents Redirects Polymerization-Induced Self-Assembly. ACS Macro Lett. 2019, 8, 1102–1109. 10.1021/acsmacrolett.9b00509. [DOI] [PubMed] [Google Scholar]
- Tan J.; Dai X.; Zhang Y.; Yu L.; Sun H.; Zhang L. Photoinitiated Polymerization-Induced Self-Assembly via Visible Light-Induced RAFT-Mediated Emulsion Polymerization. ACS Macro Lett. 2019, 8, 205–212. 10.1021/acsmacrolett.9b00007. [DOI] [PubMed] [Google Scholar]
- Cunningham V. J.; Armes S. P.; Musa O. M. Synthesis, characterisation and Pickering emulsifier performance of poly(stearyl methacrylate)-poly(N-2-(methacryloyloxy)ethyl pyrrolidone) diblock copolymer nano-objects via RAFT dispersion polymerisation in n-dodecane. Polym. Chem. 2016, 7, 1882–1891. 10.1039/C6PY00138F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rymaruk M. J.; Cunningham V. J.; Brown S. L.; Williams C. N.; Armes S. P. Oil-in-oil pickering emulsions stabilized by diblock copolymer nanoparticles. J. Colloid Interface Sci. 2020, 580, 354–364. 10.1016/j.jcis.2020.07.010. [DOI] [PubMed] [Google Scholar]
- Shen L.; Guo H.; Zheng J.; Wang X.; Yang Y.; An Z. RAFT Polymerization-Induced Self-Assembly as a Strategy for Versatile Synthesis of Semifluorinated Liquid-Crystalline Block Copolymer Nanoobjects. ACS Macro Lett. 2018, 7, 287–292. 10.1021/acsmacrolett.8b00070. [DOI] [PubMed] [Google Scholar]
- Cai D.; Thijssen J. H. T.; Clegg P. S. Making Non-aqueous High Internal Phase Pickering Emulsions: Influence of Added Polymer and Selective Drying. ACS Appl. Mater. Interfaces 2014, 6, 9214–9219. 10.1021/am501328r. [DOI] [PubMed] [Google Scholar]
- Pérez-García M. G.; Carranza A.; Puig J. E.; Pojman J. A.; del Monte F.; Luna-Bárcenas G.; Mota-Morales J. D. Porous monoliths synthesized via polymerization of styrene and divinyl benzene in nonaqueous deep-eutectic solvent-based HIPEs. RSC Adv. 2015, 5, 23255–23260. 10.1039/C5RA02374B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier B. J.; de Leon A.; Hemmingsen C.; Pentzer E. Polymerizations in oil-in-oil emulsions using 2D nanoparticle surfactants. Polym. Chem. 2018, 9, 1547–1550. 10.1039/C7PY01819C. [DOI] [Google Scholar]
- György C.; Hunter S. J.; Girou C.; Derry M. J.; Armes S. P. Synthesis of poly(stearyl methacrylate)-poly(2-hydroxypropyl methacrylate) diblock copolymer nanoparticles via RAFT dispersion polymerization of 2-hydroxypropyl methacrylate in mineral oil. Polym. Chem. 2020, 11, 4579–4590. 10.1039/D0PY00562B. [DOI] [Google Scholar]
- Ata S. Coalescence of Bubbles Covered by Particles. Langmuir 2008, 24, 6085–6091. 10.1021/la800466x. [DOI] [PubMed] [Google Scholar]
- Ata S.; Davis E. S.; Dupin D.; Armes S. P.; Wanless E. J. Direct Observation of pH-Induced Coalescence of Latex-Stabilized Bubbles Using High-Speed Video Imaging. Langmuir 2010, 26, 7865–7874. 10.1021/la904708x. [DOI] [PubMed] [Google Scholar]
- Thompson K. L.; Giakoumatos E. C.; Ata S.; Webber G. B.; Armes S. P.; Wanless E. J. Direct Observation of Giant Pickering Emulsion and Colloidosome Droplet Interaction and Stability. Langmuir 2012, 28, 16501–16511. 10.1021/la3025765. [DOI] [PubMed] [Google Scholar]
- Morse A. J.; Tan S.-Y.; Giakoumatos E. C.; Webber G. B.; Armes S. P.; Ata S.; Wanless E. J. Arrested coalescence behaviour of giant Pickering droplets and colloidosomes stabilised by poly(tert-butylaminoethyl methacrylate) latexes. Soft Matter 2014, 10, 5669–5681. 10.1039/C4SM00801D. [DOI] [PubMed] [Google Scholar]
- Ueno K.; Bournival G.; Wanless E. J.; Nakayama S.; Giakoumatos E. C.; Nakamura Y.; Fujii S. Liquid marble and water droplet interactions and stability. Soft Matter 2015, 11, 7728–7738. 10.1039/C5SM01584G. [DOI] [PubMed] [Google Scholar]
- Morse A. J.; Giakoumatos E. C.; Tan S.-Y.; Webber G. B.; Armes S. P.; Ata S.; Wanless E. J. Giant pH-responsive microgel colloidosomes: preparation, interaction dynamics and stability. Soft Matter 2016, 12, 1477–1486. 10.1039/C5SM02450A. [DOI] [PubMed] [Google Scholar]
- Cunningham V. J.; Giakoumatos E. C.; Marks M.; Armes S. P.; Wanless E. J. Effect of morphology on interactions between nanoparticle-stabilised air bubbles and oil droplets. Soft Matter 2018, 14, 3246–3253. 10.1039/C7SM02280H. [DOI] [PubMed] [Google Scholar]
- Ata S.; Pugh R. J.; Jameson G. J. The influence of interfacial ageing and temperature on the coalescence of oil droplets in water. Colloids Surf., A 2011, 374, 96–101. 10.1016/j.colsurfa.2010.11.012. [DOI] [Google Scholar]
- Solans C.; Izquierdo P.; Nolla J.; Azemar N.; Garcia-Celma M. J. Nano-emulsions. Curr. Opin. Colloid Interface Sci. 2005, 10, 102–110. 10.1016/j.cocis.2005.06.004. [DOI] [Google Scholar]
- McClements D. J. Nanoemulsions versus microemulsions: terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. 10.1039/C2SM06903B. [DOI] [Google Scholar]
- Gupta A.; Eral H. B.; Hatton T. A.; Doyle P. S. Nanoemulsions: formation, properties and applications. Soft Matter 2016, 12, 2826–2841. 10.1039/C5SM02958A. [DOI] [PubMed] [Google Scholar]
- Persson K. H.; Blute I. A.; Mira I. C.; Gustafsson J. Creation of well-defined particle stabilized oil-in-water nanoemulsions. Colloids Surf., A 2014, 459, 48–57. 10.1016/j.colsurfa.2014.06.034. [DOI] [Google Scholar]
- Sihler S.; Schrade A.; Cao Z.; Ziener U. Inverse Pickering Emulsions with Droplet Sizes below 500 nm. Langmuir 2015, 31, 10392–10401. 10.1021/acs.langmuir.5b02735. [DOI] [PubMed] [Google Scholar]
- Hunter S. J.; Penfold N. J. W.; Chan D. H.; Mykhaylyk O. O.; Armes S. P. How Do Charged End-Groups on the Steric Stabilizer Block Influence the Formation and Long-Term Stability of Pickering Nanoemulsions Prepared Using Sterically Stabilized Diblock Copolymer Nanoparticles?. Langmuir 2020, 36, 769–780. 10.1021/acs.langmuir.9b03389. [DOI] [PubMed] [Google Scholar]
- Schrade A.; Landfester K.; Ziener U. Pickering-type stabilized nanoparticles by heterophase polymerization. Chem. Soc. Rev. 2013, 42, 6823–6839. 10.1039/c3cs60100e. [DOI] [PubMed] [Google Scholar]
- Akpinar B.; Fielding L. A.; Cunningham V. J.; Ning Y.; Mykhaylyk O. O.; Fowler P. W.; Armes S. P. Determining the Effective Density and Stabilizer Layer Thickness of Sterically Stabilized Nanoparticles. Macromolecules 2016, 49, 5160–5171. 10.1021/acs.macromol.6b00987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J.; Erickson M.; Parr J. Refractive index matching and clear emulsions. J. Cosmet. Sci. 2005, 56, 253–265. [PubMed] [Google Scholar]
- Hibberd D. J.; Mackie A. R.; Moates G. K.; Penfold R.; Watson A. D.; Barker G. C. Preparation and characterisation of a novel buoyancy and refractive index matched oil-in-water emulsion. Colloids Surf., A 2007, 301, 453–461. 10.1016/j.colsurfa.2007.01.023. [DOI] [Google Scholar]
- Husband F. A.; Garrood M. J.; Mackie A. R.; Burnett G. R.; Wilde P. J. Adsorbed Protein Secondary and Tertiary Structures by Circular Dichroism and Infrared Spectroscopy with Refractive Index Matched Emulsions. J. Agric. Food Chem. 2001, 49, 859–866. 10.1021/jf000688z. [DOI] [PubMed] [Google Scholar]
- Cockram A. A.; Neal T. J.; Derry M. J.; Mykhaylyk O. O.; Williams N. S. J.; Murray M. W.; Emmett S. N.; Armes S. P. Effect of Monomer Solubility on the Evolution of Copolymer Morphology during Polymerization-Induced Self-Assembly in Aqueous Solution. Macromolecules 2017, 50, 796–802. 10.1021/acs.macromol.6b02309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotherton E. E.; Hatton F. L.; Cockram A. A.; Derry M. J.; Czajka A.; Cornel E. J.; Topham P. D.; Mykhaylyk O. O.; Armes S. P. In Situ Small-Angle X-ray Scattering Studies During Reversible Addition-Fragmentation Chain Transfer Aqueous Emulsion Polymerization. J. Am. Chem. Soc. 2019, 141, 13664–13675. 10.1021/jacs.9b06788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton F. L.; Park A. M.; Zhang Y.; Fuchs G. D.; Ober C. K.; Armes S. P. Aqueous one-pot synthesis of epoxy-functional diblock copolymer worms from a single monomer: new anisotropic scaffolds for potential charge storage applications. Polym. Chem. 2019, 10, 194–200. 10.1039/C8PY01427B. [DOI] [Google Scholar]
- Cornel E. J.; van Meurs S.; Smith T.; O’Hora P. S.; Armes S. P. In Situ Spectroscopic Studies of Highly Transparent Nanoparticle Dispersions Enable Assessment of Trithiocarbonate Chain-End Fidelity during RAFT Dispersion Polymerization in Nonpolar Media. J. Am. Chem. Soc. 2018, 140, 12980–12988. 10.1021/jacs.8b07953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter S. J.; Cornel E. J.; Mykhaylyk O. O.; Armes S. P.. Effect of salt on the formation and stability of water-in-oil Pickering nanoemulsions stabilized by diblock copolymer nanoparticles. Langmuir 2020, 36, 10.1021/acs.langmuir.0c02742. [DOI] [PMC free article] [PubMed] [Google Scholar]