Abstract
Background:
Racial and socioeconomic disparities in breast cancer mortality persist. In Boston, MA, Black, Non-Hispanic women and Medicaid-insured individuals are 2–3 times more likely to have delays in treatment compared to White or privately insured women. While evidence-based care coordination strategies for reducing delays exist, they are not systematically implemented across healthcare settings.
Methods:
Translating Research Into Practice (TRIP) utilizes community engaged research methods to address breast cancer care delivery disparities. Four Massachusetts Clinical and Translational Science Institute (CTSI) hubs collaborated with the Boston Breast Cancer Equity Coalition (The Coalition) to implement an evidence-based care coordination intervention for Boston residents at risk for delays in breast cancer care. The Coalition used a community-driven process to define the problem of care delivery disparities, identify the target population, and develop a rigorous pragmatic approach. We chose a cluster-randomized, stepped-wedge hybrid type I effectiveness-implementation study design. The intervention implements three evidence-based strategies: patient navigation services, a shared patient registry for use across academic medical centers, and a web-based social determinants of health platform to identify and address barriers to care. Primary clinical outcomes include time to first treatment and receipt of guideline-concordant treatment, which are captured through electronic health records abstraction. We will use mixed methods to collect the secondary implementation outcomes of acceptability, adoption/penetration, fidelity, sustainability and cost.
Conclusion:
TRIP utilizes an innovative community-driven research strategy, focused on interdisciplinary collaborations, to design and implement a translational science study that aims to more efficiently integrate proven health services interventions into clinical practice.
Keywords: patient navigation, implementation science, stepped-wedge design, social determinants of health, breast cancer disparities
Introduction
Inequity in breast cancer mortality among Black, non-Hispanic women compared to White women is a well-recognized challenge. In 2014, a national study examining race-specific breast cancer mortality rates in the 50 largest U.S. cities identified increases in Black:White disparities, largely due to substantial improvements in White rates.1 Boston, Massachusetts had the fifth highest rate ratio; from 1990–1994 to 2005–2009, the Black:White breast cancer mortality rate ratio in Boston increased from 0.94 to 1.49. In response, a group of multi-sector stakeholders including the Massachusetts Cancer Registry, state and local health departments, community advocacy organizations, and academic health centers in Boston, where breast cancer patients receive care, convened to explore a community-driven response to these findings. This group formed the Boston Breast Cancer Equity Coalition (the Coalition) with the explicit goal of using diverse stakeholder perspectives to develop city-wide solutions for inequities in breast cancer outcomes.2
Early work of the Coalition explored available data to identify modifiable targets for action. Local data for 2007–2012 showed a Black:White mortality rate ratio of 1.36, as well as a Black:Hispanic mortality rate ratio of 4.60 and a Black:Asian mortality rate ratio of 5.28. From 2001–2012, this resulted in 74 excess Black deaths among women less than 65 years.3 Further analyses found that compared with White, insured breast cancer patients in Boston, Black, non-Hispanic women, and those on Medicaid were 2–3 times more likely to have delays in initiating treatment beyond 60 days, a delay associated with worse outcomes.4 During this time period, Black women in Boston received mammography screenings at the same rates, had approximately an equal likelihood of presenting with advanced disease (4–5%), and had a lower incidence of breast cancer than White women. These findings are consistent with a growing body of evidence that addressing delays in the receipt of timely breast cancer treatment is one important approach to achieving equity in cancer outcomes.5–10
Evidence-based interventions that address barriers to timely cancer treatment in at-risk communities exist, but are not implemented systematically across health systems.11 The Coalition identified three interventions most relevant to our community: 1) Patient Navigation. This patient-centered care coordination model uses lay health workers integrated into the healthcare team to reduce delays in cancer care for those with social determinants of health.12–18 2) Patient Registries. Clinical registries that span healthcare systems address health disparities by providing a means of tracking at risk populations in need of care.19, 20 3) Screening for Social Determinants of Health (SDOH). Systematic screening for SDOH that affect access to care has potential to identify patients at risk for non-adherence and improve outcomes by directing them to available resources that address their health-related social needs.21, 22 Currently, we lack implementation strategies that address the challenges in translating these findings from single clinics into public health strategies (T3-T4 implementation translation). We present here the methods for Translating Research Into Practice (TRIP), a community-engaged, cluster-randomized, stepped-wedge hybrid type I effectiveness-implementation study that aims to facilitate the transfer of this scientific evidence into everyday practice to mitigate health disparities.
Overall Approach
Our approach aims to surmount known barriers to the implementation of promising evidence-based care coordination interventions by partnering four Clinical and Translational Science Institute (CTSI) hubs that possess the necessary translational infrastructure with an active multi-stakeholder Coalition who share a common community health goal.
Our main hypothesis is that implementation of the TRIP intervention will reduce care delivery disparities in breast cancer, and that this approach could be applicable to addressing disparities in other regions and health conditions. The TRIP study has three strategic aims correlated with the design, execution, and dissemination of the intervention. These aims are:
Conduct formative work to develop and refine delivery models that will integrate the multi-component intervention (patient navigation, shared registry, platform to screen/address SDOH) across six participating academic medical centers.
Conduct a cluster-randomized, stepped wedge hybrid effectiveness-implementation trial at 6 academic medical centers in Boston caring for the largest proportions of minority, low-income women with breast cancer to assess effectiveness and implementation of the integrated intervention.
Disseminate the integrated intervention to other CTSI hubs and community-academic partnerships.
The study will be conducted in three phases, corresponding with our Specific Aims: (1) intervention development and refinement (months 1–9); (2) rigorous testing of the intervention to evaluate both clinical effectiveness (reducing treatment delays for women with breast cancer), as well as its potential for implementation in real-world clinical settings (months 9–48); and (3) widespread dissemination to the CTSI consortium and beyond (months 36–60).
Stakeholder Collaboration
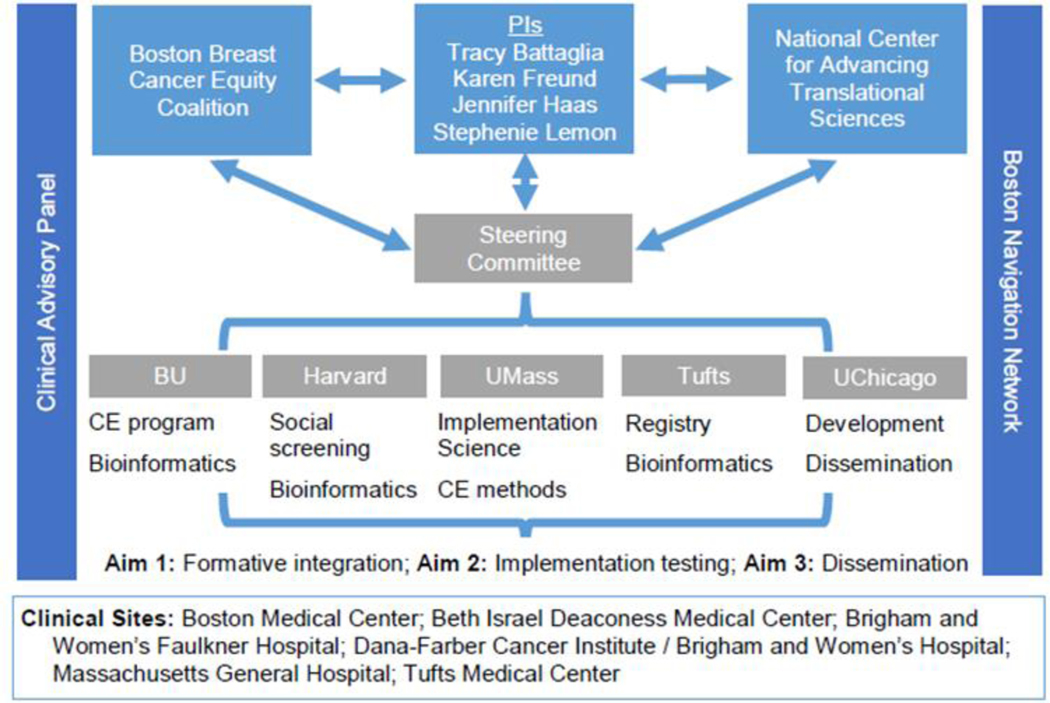
The TRIP project is a collaboration between the four Massachusetts CTSI hubs and an established community partner, the Coalition (See Organizational Chart, Figure 1). Our partnership leverages complementary strengths and builds on previous, synergistic work conducted at each CTSI hub to address long-standing regional disparities in breast cancer mortality. The Coalition informed all components of the study including organizational structure. Additionally, the Multiple-PI model in this program brings together PIs from the four Massachusetts CTSI hubs with complementary expertise. The CTSI hubs represented by our multiple PIs include Boston University’s Community Engagement Program, Tufts Medical Center’s bioinformatics program that can harness clinical data for implementation research, Harvard Catalyst’s Health Disparities Research Program, and the University of Massachusetts Center for Clinical and Translational Science’s Implementation Science program. In addition, our Steering Committee includes the Executive Director of Equal Hope, a not-for-profit organization dedicated to the fight against breast cancer inequalities in Metropolitan Chicago with representation from the Chicago CTSI programs.
Figure 1.
TRIP Organizational Chart
In addition to regularly attending quarterly Coalition meetings, two stakeholder groups support the day to day execution and dissemination of the intervention. Each stakeholder group includes active members from the Coalition.
The Clinical Advisory Panel (CAP) includes clinical leadership at each of the six study sites with representation from medical and surgical oncology. The CAP meets monthly and guides the study team on implementation strategies, facilitating local adoption, and analyzing clinical outcomes.
The Boston Patient Navigator Network is an existing network of patient navigators and their supervisors. The study team attends these quarterly meetings to provide study updates and obtain essential input on intervention components and implementation strategies.
The Steering Committee manages oversight and coordination of project management, research administration, publications and data sharing, and integration of all resources needed for the project. Steering Committee members include the PIs, the statistician, a CAP representative, a Navigator Network representative and a Chicago representative.
Experimental Design
This study is a Type 1 hybrid clinical effectiveness-implementation trial23 which aims to improve timely, quality breast cancer care among at-risk breast cancer patients through implementation of an integrated, evidence-based patient navigation intervention. Our primary outcome of clinical effectiveness will be evaluated using data abstracted from Electronic Health Records (EHR). Our secondary outcome of intervention implementation uses mixed methods to measure intervention uptake in real world clinical settings.
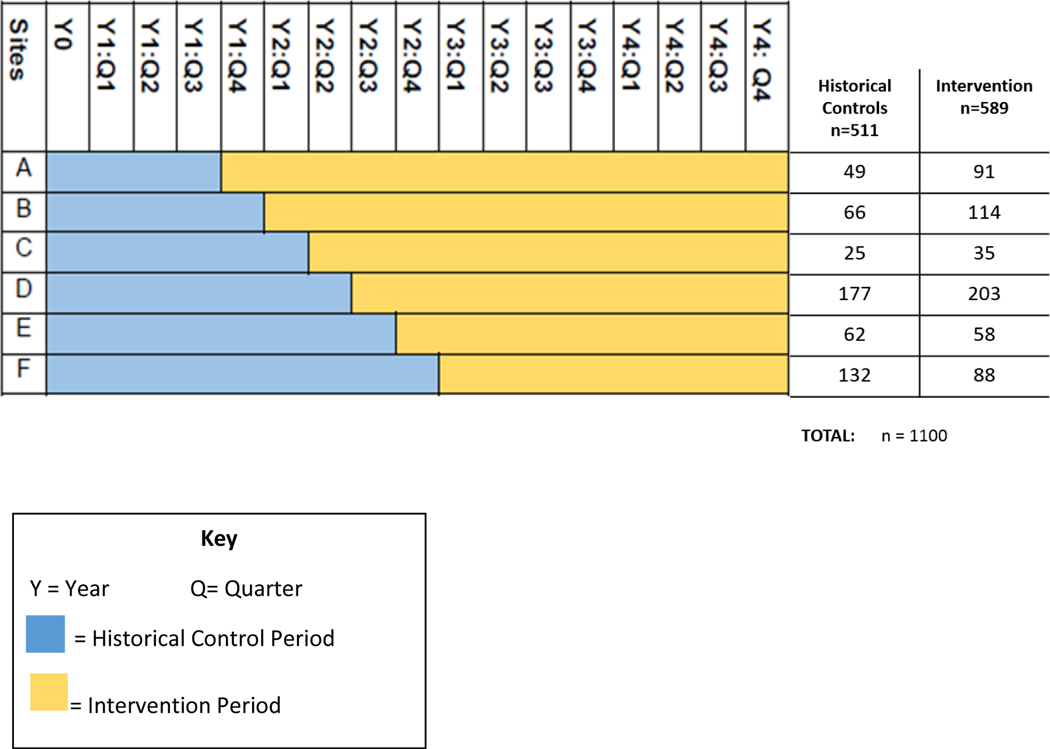
We will use a prospective, stepped wedge cluster randomized design23–26 to study implementation of the evidence-based intervention across six participating academic medical centers in Boston. In a stepped wedge design there is no randomization at the patient level, but participating sites (medical centers) are randomized with respect to the timing at which they ‘step’ or cross over from the control condition to the intervention. As pictured in Figure 2, the pragmatic stepped wedge study design involves a sequential roll-out of the intervention across the six participating sites over three-month intervals or “steps” where crossover occurs. Historical control data will be collected from each site for a minimum of 21 months and maximum of 36 months prior to intervention roll-out, depending on their assigned crossover. Sequential crossover to the intervention at participating sites will occur every three months over a 15-month period, followed by an additional 24 months of full study intervention period.
Figure 2.
Stepped-wedge study design: Schedule of events for site roll out in the intervention.
Study Setting
The six academic medical centers we have partnered with are Beth Israel Deaconess Medical Center, Boston Medical Center, Brigham and Women’s Faulkner Hospital, Dana-Farber Cancer Institute, Massachusetts General Hospital and Tufts Medical Center. These six academic medical centers have been identified by data from the Massachusetts Cancer Registry as sites that care for over 90% of women with breast cancer who are at risk for poor outcomes in Boston, including Black or Hispanic, Non-English speaking, and/or have no insurance or public health insurance. As summarized in Figure 2, we estimate accrual of 1,100 study subjects, including approximately 511 historical controls and 589 intervention subjects across the six sites.
Eligibility Criteria
The target population includes vulnerable inner-city women with risk for delay in breast cancer care. Massachusetts (MA) Cancer Registry data identified the following characteristics of Boston residents with greatest delays in breast cancer treatment: Black, Hispanic, non-English speaking, and public health insurance. All women with breast cancer diagnosed at a participating study site during the study period will be eligible for inclusion if they meet all three study criteria: 1) women greater than 18 years of age; 2) reside in the City of Boston; and 3) have one or more of the following risk factors for delays in care: Black race and/or Hispanic ethnicity, primary spoken language is not English, and/or have public insurance or uninsured status at the time of diagnosis.
Enrollment
As this is a health system level intervention, all eligible women receive the intervention. Navigator protocols include tailored workflow maps that specify how each navigator identifies newly diagnosed breast cancer cases who meet TRIP eligibility criteria within each of the six clinical sites. We expect every eligible patient to be enrolled, as the TRIP intervention will be implemented as a standard of care at each participating site.
The Intervention
The intervention includes three integrated components:
1. Patient Navigation services following standard operating procedures that are guided by the Principles of Care Management27 in collaboration with a network of navigators across the six health systems.
This includes: a) identifying women eligible for navigation services; b) identifying barriers to initiating timely cancer care services, with a particular emphasis on social barriers; c) providing assistance to address these barriers through local and regional resources; and, finally, d) tracking women over time across the participating clinical sites to ensure they complete their entire course of cancer care. A TRIP navigation protocol was designed by the study team to reflect evidence-based best practices for oncology navigation.28 The innovation here is the integrated network of navigation across regional health systems.
2. A real time patient registry that is shared across the six health systems.
The registry was built using the HIPAA compliant REDCap platform through collaboration with clinical providers, patient navigators, informatics specialists, and experts from the REDCap team at Vanderbilt University. Navigators enter basic demographic information and track screening and referrals for social determinants of health (see 3. below). Clinical information is kept to a minimum in an effort to reduce redundancy with the EHR and minimize double data entry. This navigator tool produces reports that prioritize the navigator’s caseload based on pre-determined markers of timely care (e.g. days since diagnosis) and directs them with an actionable list of patients with pending navigation needs. Some of these functions require manual manipulation of the data using SAS and uploading that data back into REDCap. The registry allows communication between navigators, specifically around patients receiving care in more than one location or transferring care between institutions, to prevent delays and gaps in care. Navigators can message each other directly through REDCap. They can also write notes about appointments and treatment received. Research staff are also able to use the registry as a monitoring tool to track navigator activity.
3. A systematic screening and referral system to identify and address SDOH needs.
At baseline and 3 months navigators conduct a systematic screening for social needs across 9 social domains including: housing insecurity, food insecurity, paying for basic utilities, family caregiving, legal, transportation, paying for treatment, education, and employment. We will partner with Aunt Bertha, a web-based social network platform, to develop a TRIP-specific screening and referral system to support navigators in connecting patients with available social services. The Aunt Bertha29 platform is an online network of thousands of verified social service programs including nonprofits and social care providers who serve the Boston communities. Navigators will work with patients to identify the most pressing domains and then identify available community services to address each domain. At each contact, the navigators check on the status of referrals and assess whether a woman would like to receive additional referrals from similar or different domains.
The planned process of rolling out the integrated intervention begins with partnering with a clinical oncology champion at each site to identify existing navigation staff and document baseline navigation workflow. We plan to use several evidence-based implementation strategies30 to promote intervention adoption into existing workflows including: stakeholder engagement, development of a standardized intervention protocol, iterative training and technical assistance on evidence-based protocol, and continuous monitoring and feedback. Once a navigator has been designated as the TRIP study navigator and completed their required trainings, they are able to start navigating patients under the TRIP protocol.28 Navigators will prospectively identify newly diagnosed breast cancer patients meeting eligibility requirements and initiate the protocol. Key protocol activities include systematic and longitudinal screening for the social determinants of health, use of a web-based platform to identify resources and initiate referrals to address social issues that might interfere with cancer care, and use of a shared patient registry to communicate with navigators from other participating study sites in the event a study subject transfers care during the intervention period.
Study Outcomes
Outcome measures.
Our study is powered on the primary clinical effectiveness outcome, time to initiation of cancer treatment. This is a continuous0020outcome defined as the number of days from diagnosis (Time 0) to treatment initiation (Time 1). Treatment initiation is defined as receipt of either surgical, radiation, or systemic therapy. Table 1 displays the specific data elements that will be used to define treatment initiation.
Table 1.
Data Elements for “Time to Initiation of Primary Breast Cancer Treatment” Calculations
| Time period | Date of |
|---|---|
| Time 0 | First definitive tissue diagnosis (i.e. biopsy) |
| Time 1 | First definitive treatment, including any of the following: |
| definitive surgical procedure (lumpectomy or mastectomy) | |
| external radiation therapy session, neoadjuvant chemotherapy infusion, prescription for neoadjuvant hormone therapy | |
Secondary clinical outcomes include select measures of Guideline Concordant or Quality cancer care, as defined jointly by the Commission on Cancer and the National Comprehensive Cancer Network.13 Specific measures of guideline concordant/quality care (yes/no) are defined in Table 2.
Table 2.
Secondary clinical outcomes: Guideline-Concordant Care Quality Measures
| Treatment Domain | CoC* Criteria for Quality Cancer Care |
|---|---|
| Radiation | Radiation therapy administered within 365 days of diagnosis for women < 70 years receiving breast conserving surgery |
| Radiation therapy administered following any mastectomy within 1 year (365 days) of diagnosis of breast cancer for women with ≥ 4 positive regional lymph nodes | |
| Chemotherapy | Combination chemotherapy administered within 120 days of diagnosis for women <70 with AJCC T1c N0 M0, or Stage II or III Estrogen Receptor and Progesterone Receptor (ER and PR) negative breast cancer |
| Hormonal | Tamoxifen or aromatase inhibitor administered within 365 days of diagnosis for women with AJCC T1c N0 M0, or Stage II or III ER and/or PR+ breast cancer. |
CoC=Commission on Cancer; https://www.facs.org/quality%20programs/cancer/ncdb/qualitymeasures
Implementation outcomes include acceptability, local adoption/penetration, fidelity to the intervention protocol, sustainability and cost of the intervention across each site.31 Acceptability will examine perceptions of the intervention components among patient navigators and other members of site navigation teams. Adoption/penetration will measure the reach and integration of TRIP and its practices throughout each institution. Fidelity is being conceptualized as navigator adherence to the standard operating procedures. Sustainability will examine the extent to which TRIP is maintained or institutionalized within the academic medical center’s ongoing operations. Finally, the cost of implementing the integrated TRIP intervention components will be captured through microcosting measurement.31–35
Data Sources
TRIP uses multiple data sources as outlined in Table 3. The EHR will serve as the data source for all socio-demographic and clinical covariates, as well as the primary and secondary clinical effectiveness outcomes. EHR data will be captured in two ways: (a) automated extraction will capture all demographic variables and some discrete clinical data; and (b) manual abstraction to collect the complex data elements required to create treatment variables.
Table 3.
Data Sources and their corresponding role in study analyses
| Source | Study Domain | Data Variables | Data Collection |
|---|---|---|---|
| EHRs | Covariates | Race/ethnicity, age, insurance status, comorbid conditions, cancer stage | Automated extraction of discrete EHR fields |
| Effectiveness (outcomes) | Time to treatment (# days) Guideline-concordant treatment (yes/no) | Manual abstraction using standardized form | |
| Registry | Implementation | Fidelity to navigation protocol. e.g. completed intake, communicated with other navigators | Aggregate reports from REDCap platform |
| SDOH Platform | Implementation | Fidelity to protocol. e.g. completed systematic screen at baseline and 3 months y/n | Aggregate reports from SDOH Screening platform |
| Navigator Interviews | Implementation | Acceptability, Adoption/Penetration | Transcripts from one on one structured interviews with TRIP Navigators |
| Navigator Observations | Implementation | Fidelity, Adoption/Penetration | Field observations of TRIP navigators in the clinical setting |
| Focus Groups | Implementation | Acceptability, Adoption/penetration, Sustainability | Transcripts from focus groups with hospital administrators and clinicians |
| Surveys | Implementation | Cost | Survey (study personnel) of costs associated with developing and implementing training, registry and SDOH screening platform; Survey of time spent on TRIP related activities (navigators and supervisor) |
The main sources of data for our implementation outcomes include: the REDCap registry, the SDOH screening platform and qualitative data collected through observations, interviews, and surveys. The REDCap registry and SDOH screening platforms will provide quantitative measures of fidelity to the intervention protocol, as documented by the navigators in their day to day activities. Navigator interviews will measure acceptability, and local adoption/penetration. Observations of navigators in the field will further assess fidelity, as well as local adoption/penetration. Focus groups with academic medical center leadership and the study’s clinical advisory panel at month 24 of the intervention period will provide data to further examine acceptability, adoption and sustainability. Finally, the use of surveys will determine the amount of time patient navigators contribute for TRIP patients as well as time spent by study personnel in the development of intervention components, which will inform our cost estimates.
Statistical Analysis of Effectiveness Outcome
Our comparative analyses between the intervention and usual care (historical control) groups will employ the intent-to-treat principle. The primary outcome is time to initiation of treatment. We will employ Cox-type proportional hazards-based models for correlated time-to-event data. These models will account for clustering by study site or the inclusion of a random intercept. We will examine the proportional hazards assumption using plots (survival, -log(log), and Schoenfeld residuals), as well as statistical tests including Kolmogorov-type supremum tests and the inclusion of interaction term of intervention group with the log of follow-up time in the model. Other potential modifiers of the effectiveness of intervention, confounders, or covariates can be added to this model as fixed effects. Although we do not expect effect modification in the study data, we will examine the potential for such effects (interaction) through the use of stratified analyses and the inclusion of interaction terms with study group in our statistical models. A priori candidate effect modifiers include race/ethnicity, cancer stage and type, patient age, and insurance status. Statistically significant interactions with intervention will be retained and the nature of heterogeneous intervention effects will be estimated using the interaction model. Fidelity data will also be linked with effectiveness outcomes in order to determine the extent to which it influences the ability of the intervention to improve patient outcomes.
We will calculate the costs of implementing the intervention, including costs associated with developing and implementing the registry and screening systems, training and materials costs, and navigator costs. Staff costs of implementing the program will be calculated by multiplying the amount of time spent by an hourly wage estimate. We will provide a range of cost estimates that users can use to guide their own implementation estimates.
Qualitative Analyses
At least two members of the study team will independently review transcripts from each of the first three interviews and focus groups to identify important concepts that emerge and create a codebook used for subsequent interviews. Then, two coders will independently code interviews for agreement analysis. Coders will meet twice monthly to review code interpretation and to discuss new codes. Disagreements about code meanings will be resolved by consensus. Thematic analysis will focus on the perceptions of acceptability, adoption/penetration and sustainability related to implementing the TRIP standard of care. After coding is finalized and consensus has been reached, codes will be reviewed to generate cross-cutting themes within each site. Similarities and differences in the themes will be compared across sites. This will allow us to identify the extent to which TRIP is seen as acceptable or having substantial reach within the health system among different sites.
Sample Size and Power
Based on Massachusetts Cancer Registry data, we expect approximately 1100 total patients over the full intervention period. With approximately 500 historical control patients, and 550 intervention patients. Sample size estimates assume 80% power and a two-sided alpha of 0.05 for cross-sectional stepped wedge studies comparing intervention to usual care in two-group statistical analyses. This method incorporates information on the number of steps used in the stepped wedge design, the number of subjects per time period, and the degree of clustering via the intra-class correlation coefficient (ICC) to compute the design effect. A sample size of 220 subjects per group in a log rank test will provide 80% power at a two-sided alpha of 0.05 to detect a difference in the proportion of subjects with treatment at time, t, of 81% in the intervention group compared to 70% in the usual care group, a level estimated from recent data from the clinical sites for the planned study. This difference yields a hazard ratio of 1.75, a clinically meaningful effect size for time-to-treatment post-diagnosis. Based on our planned number of steps, enrollment per study period, and a reasonable ICC of 0.1, the design effect is 2.29. Thus, we will need to enroll and follow at least 1,008 subjects to provide 80% power for analysis of intervention effectiveness.
Dissemination
In year 5, we will develop a web-based multi-media intervention toolkit that describes background information, procedures and protocols, intervention components and required resources that will be made available at no cost to investigators at CTSI hubs, other academic institutions, and community organizations.
Discussion
The TRIP study addresses a critical gap in the translational research enterprise, namely the transfer and application of scientific evidence that is necessary to mitigate health disparities into everyday practice. It is the first ever city wide implementation study aimed at coordinating oncology care delivery across multiple academic medical centers. TRIP includes an integrated, multi-component intervention that builds from preliminary work of the investigative team and their community partner, the Coalition. Using a community engaged approach that includes governance by multiple stakeholder teams, TRIP is designed to overcome barriers to widespread implementation and dissemination of evidence-based practices that will improve the delivery of guideline-concordant care to vulnerable populations. TRIP draws upon the principles of implementation science to understand how to systematically facilitate deployment and utilization of three evidence-based approaches into one integrated model of care to improve the quality and effectiveness of care delivery, in this case for minority and/or low-income women with breast cancer.
TRIP utilizes a stepped-wedge study cluster randomized design that is increasingly being used in the evaluation of service delivery type interventions.24 The design involves random and sequential crossover of clusters from control to intervention until all clusters are exposed. We considered several alternative designs. While the primary target of the intervention is the patient, the intervention will be delivered by navigators within a given health care system. Thus, individual or provider level randomization is not feasible. The decision to choose a randomized stepped wedge, versus a standard group-randomized trial, was chosen for two commonly reported reasons. One is that our clinical and public health partners prefer the intervention to be rolled out to all sites/navigators and to not have pure control sites. The second is that a stepped wedge design allows us to maximize our resources, spacing out the roll out of the navigator intervention and technology-based components over time. Additional benefits of this approach are the inclusion of contemporaneous controls to minimize the impact of temporal trends on outcomes and the ability to incorporate sites that differ in size.
Our scientific premise is that there are critical evidence and delivery gaps that promote disparities in the clinical outcomes of women with breast cancer. A major focus of the National Center for Advancing Translational Science within the National Institutes of Health is for the CTSI hubs to work together to address such delivery gaps and bottlenecks, with specific attention to those that affect special populations and contribute to health disparities. This translational project has several innovations:
Our collaborative approach is innovative. The engagement of communities in T4 research focused on health system clinical care delivery is necessary to implement an effective intervention and ensure broad translation to communities at risk. Using community engagement approaches in partnership with a community-led Coalition has great potential to address the known barriers to achieving information sharing and implementation of the evidence across diverse health systems. The Coalition includes stakeholders who provide leadership representative of the population and the ability to make practice improvements in real life clinical settings.
Our inclusion of all major health care systems in the geographic area is innovative. While multi-site trials are common, few are designed for systems to collaborate to address the implementation of evidence-based approaches to address the needs of an entire community that has care fragmented across so many academic medical centers and clinical sites. Our hypothesis that some of the delays in care reflect transitions between health care systems, for either structural, insurance coverage or patient preference reasons. This approach if successful, can be translated to other locations and other health care conditions where disparities in outcomes are prominent and persistent.
Likewise, our regional registry is innovative. It will provide clinical providers, practices, and healthcare systems with timely metrics on the processes of navigation. The ability to share standardized navigation data within and across health systems is innovative and necessary - as our preliminary work suggests 49% of Black women in Boston transfer their care within these health systems during their cancer treatment. This infrastructure will accelerate scientific and public health progress in monitoring disparities.
Our systematic approach to screening and intervention for SDOH is innovative. Addressing social needs that may deter women from engaging with their care or lead to poorer health status is a crucial step to addressing disparities in care. By integrating systematic screening and referral to resources to address social determinants of health, we will examine a novel, generalizable model using an electronic platform that is integrated with the provision of care.
Our study design is innovative. While each individual component of the intervention is evidence based and not innovative, the TRIP intervention unites the three components of navigation, shared patient registries, and standardized SDOH screenings in a unique and novel way. The long-term goal of this study is to make available an evidence-tested, integrated intervention that can be implemented and effective in complex delivery systems. The pragmatic stepped wedge study design will allow us to test effectiveness and implementation outcomes in the context of complex intervention delivery systems while also maintaining scientific rigor.
This study provides a model of how community-academic partnerships can drive innovations in information sharing and systems implementation to address health disparities. Results of this study will demonstrate the efficacy in a city-wide, integrated intervention model that can be applied to other regions and other health conditions. Inclusion of a specific aim devoted to widespread dissemination will make the study findings, tools and lessons learned available to the entire CTSI network and beyond.
Acknowledgements
The authors would like to acknowledge the partnership of TRIP’s stakeholders and community organizations: Boston Patient Navigator Network, Boston Breast Cancer Equity Coalition, Boston Public Health Commission: Pink & Black, Massachusetts Department of Public Health: Office of Community Health Workers; The National Center to Advance Translational Science, the Boston University Clinical and Translational Science Institute (CTSI), University of Massachusetts CTSI, the Harvard CTSI, Tufts CTSI; our partner institutions Boston Medical Center, Brigham and Women’s Faulkner Hospital, Massachusetts General Hospital, Tufts Medical Center, Beth Israel Deaconess Medical Center, and Dana Farber Cancer Institute; and finally, the TRIP Consortium and our patient navigators.
Funding
Research reported in this publication was supported by the National Center for Advancing Translational Sciences and the Office of Behavioral and Social Sciences Research of the National Institutes of Health under Award Number U01TR002070. Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under the Harvard University CTSA Award NumberUL1TR002541,Tufts University CTSA Award NumberUL1TR002544, Boston University CTSA Award Number1UL1TR001430, and University of Massachusetts CTSA Award Number UL1 TR001453-03.The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure of Funding:
• Grant Title: Translating Research into Practice: A Regional Collaborative to Reduce Disparities in Breast Cancer Care
Funding Agency: U01 TR002070, NIH/ NCATS
• Grant Title: Harvard Clinical and Translational Science Center (UL1)
Funding Agency: UL1TR000170, NIH/ NCATS
• Grant Title: Tufts Clinical and Translational Science Institute
Funding Agency: UL1TR002544, NIH/ NCATS
• Grant Title: University of Massachusetts Center for Clinical and Translational Science
Funding Agency: UL1TR001453, NIH/ NCATS
• Grant Title: Boston University Clinical and Translational Science Institute
Funding Agency: UL1TR001430, NIH/ NCATS
• Grant Title: Clinical Professorship: American Cancer Society # CRP-17-112-06-COUN
Footnotes
Conflicts of interest
Sharon Bak: The author has no competing interests to disclose.
Karen Burns White: The author has no competing interests to disclose.
Nicole Casanova: The author has no competing interests to disclose.
Jennifer S Haas: The author has no competing interests to disclose.
Stephenie C Lemon: The author has no competing interests to disclose.
Tracy A Battaglia: Chair, National Navigation Roundtable (no honorarium, travel to meetings support by the American Cancer Society); Executive Committee Member, Boston Breast Cancer Equity Coalition (volunteer, no honorarium); PI, UO1TR002070 NIH/NCATS “Translating Research into Practice” a multisite patient navigation intervention study.
Karen M Freund: Policy Task Force Chair - National Navigation Roundtable (no honorarium, travel to meetings support by the American Cancer Society); Consultant and travel to a national meeting, support by the National Minority Quality Forum.
Rachel A Freedman: Institutional funding from Eisai and Puma, no personal funding.
Ethics
This study protocol has been reviewed and approved by the Boston University Institutional Review Board (IRB), IRB# H-37314. The Boston University IRB is the Reviewing IRB for all participating institutions, as determined by SMART IRB.
Translating Research Into Practice (TRIP) Consortium:
• Beth Israel Deaconess Medical Center (Ted James MD, Susan McCauley RN, Ellen Ohrenberger RN BSN, JoEllen Ross RN BSN, Leo Magrini BS)
• Boston Breast Equity Coalition Steering Committee (Sharon Bak MPH, Magnolia Contreras MSW MBA, Susan Gershman MS MPH PhD CTR, Mark Kennedy MBA, Anne Levine MEd MBA, Erica Warner ScD MPH, Karen Burns White MS)
• Brigham and Women’s Hospital (Cheryl Clark MD ScD)
• Boston Medical Center (Bill Adams MD, Nicole Casanova BA, Katie Finn BA, Christine Gunn PhD, Naomi Ko MD, Ariel Maschke MA, Katelyn Mullikin BA, Laura Ochoa BA, Chris Shanahan MD MPH, Sam Steil BA, Tracy Battaglia MD MPH, Victoria Xiao BS)
• Boston University (Howard Cabral PhD, Clara Chen MS, Carolyn Finney, Chris Lloyd-Travaglini MPH, Stephanie Loo MSc)
• Dana Farber Cancer Institute (Rachel Freedman MD MPH, Yoscairy Raymond, Deborah Toffler MSW LCSW)
• Equal Hope (Anne Marie Murphy PhD)
• Massachusetts General Hospital (Beverly Moy MD, Jennifer Haas MD MPH, Caylin Marotta MPH, Aileen Navarrete, Sanja Percac-Lima MD PhD, Emma Whited BA, Amy Wint MS)
• Tufts Medical Center (Jack Erban MD, Karen Freund MD MPH, Will Harvey MD MSc, Danielle Krzyszczyk BA, Amy LeClair PhD MPhil, Susan Parsons MD MRP, Feng Qing Wang BS)
• University of Massachusetts Lowell; Boston University (Serena Rajabiun MA MPH PhD)
• University of Massachusetts Medical School (Stephenie Lemon PhD)
References
- 1.Flaherty. More work to do. Boston Herald; 2016. [Google Scholar]
- 2.Coalition BBCE. Our Vision. [cited 2019 November 6].
- 3.Hunt BR, Whitman S, Hurlbert MS. Increasing Black:White disparities in breast cancer mortality in the 50 largest cities in the United States. Cancer Epidemiol. 2014; 38:118–23. [DOI] [PubMed] [Google Scholar]
- 4.McLaughlin JM, Anderson RT, Ferketich AK, Seiber EE, Balkrishnan R, Paskett ED. Effect on survival of longer intervals between confirmed diagnosis and treatment initiation among low-income women with breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012; 30:4493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdel-Rahman O. Impact of timeliness of adjuvant chemotherapy and radiotherapy on the outcomes of breast cancer; a pooled analysis of three clinical trials. Breast. 2018; 38:175–80. [DOI] [PubMed] [Google Scholar]
- 6.Bleicher RJ, Ruth K, Sigurdson ER, Beck JR, Ross E, Wong YN, et al. Time to Surgery and Breast Cancer Survival in the United States. JAMA oncology. 2016; 2:330–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chavez-MacGregor M, Clarke CA, Lichtensztajn DY, Giordano SH. Delayed Initiation of Adjuvant Chemotherapy Among Patients With Breast Cancer. JAMA oncology. 2016; 2:322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong J, Esham KS, Boehm L, Karim SA, Lin M, Mao D, et al. Timeliness of Treatment Initiation in Newly Diagnosed Patients With Breast Cancer. Clin Breast Cancer. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin JJ, Egorova N, Franco R, Bickell NA. Breast Cancer: Does Type of Hospital Where You Get Surgery Affect Survival? Journal for healthcare quality : official publication of the National Association for Healthcare Quality. 2019; 41:49–58. [DOI] [PubMed] [Google Scholar]
- 10.Research AAfC. Delaying Adjuvant Chemotherapy Associated with Worse Outcomes for Patients with Triple-negative Breast Cancer. San Antonio: 2018; Available from: https://www.aacr.org/Newsroom/Pages/News-Release-Detail.aspx?ItemID=1251. [Google Scholar]
- 11.McGlynn EA, Asch SM, Adams J, Keesey J, Hicks J, DeCristofaro A, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003; 348:2635–45. [DOI] [PubMed] [Google Scholar]
- 12.Battaglia TA, Bak SM, Heeren T, Chen CA, Kalish R, Tringale S, et al. Boston Patient Navigation Research Program: the impact of navigation on time to diagnostic resolution after abnormal cancer screening. Cancer Epidemiol Biomarkers Prev. 2012; 21:1645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freund KM, Battaglia TA, Calhoun E, Dudley DJ, Fiscella K, Paskett E, et al. National Cancer Institute Patient Navigation Research Program: methods, protocol, and measures. Cancer. 2008; 113:3391–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gunn CM, Clark JA, Battaglia TA, Freund KM, Parker VA. An assessment of patient navigator activities in breast cancer patient navigation programs using a nine-principle framework. Health Serv Res. 2014; 49:1555–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ko NY, Darnell JS, Calhoun E, Freund KM, Wells KJ, Shapiro CL, et al. Can patient navigation improve receipt of recommended breast cancer care? Evidence from the National Patient Navigation Research Program. J Clin Oncol. 2014; 32:2758–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ko NY, Snyder FR, Raich PC, Paskett ED, Dudley DJ, Lee JH, et al. Racial and ethnic differences in patient navigation: Results from the Patient Navigation Research Program. Cancer. 2016; 122:2715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodday AM, Parsons SK, Snyder F, Simon MA, Llanos AA, Warren-Mears V, et al. Impact of patient navigation in eliminating economic disparities in cancer care. Cancer. 2015; 121:4025–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitley EM, Raich PC, Dudley DJ, Freund KM, Paskett ED, Patierno SR, et al. Relation of comorbidities and patient navigation with the time to diagnostic resolution after abnormal cancer screening. Cancer. 2017; 123:312–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lake ET, Staiger D, Horbar J, Kenny MJ, Patrick T, Rogowski JA. Disparities in perinatal quality outcomes for very low birth weight infants in neonatal intensive care. Health Serv Res. 2015; 50:374–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morales LS, Staiger D, Horbar JD, Carpenter J, Kenny M, Geppert J, et al. Mortality among very low-birthweight infants in hospitals serving minority populations. Am J Public Health. 2005; 95:2206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gottlieb LM, Hessler D, Long D, Laves E, Burns AR, Amaya A, et al. Effects of Social Needs Screening and In-Person Service Navigation on Child Health: A Randomized Clinical Trial. JAMA Pediatr. 2016; 170:e162521.. [DOI] [PubMed] [Google Scholar]
- 22.Page-Reeves J, Kaufman W, Bleecker M, Norris J, McCalmont K, Ianakieva V, et al. Addressing Social Determinants of Health in a Clinic Setting: The WellRx Pilot in Albuquerque, New Mexico. J Am Board Fam Med. 2016; 29:414–8. [DOI] [PubMed] [Google Scholar]
- 23.Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Medical care. 2012; 50:217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hemming K, Haines TP, Chilton PJ, Girling AJ, Lilford RJ. The stepped wedge cluster randomised trial: rationale, design, analysis, and reporting. BMJ : British Medical Journal. 2015; 350:h391.. [DOI] [PubMed] [Google Scholar]
- 25.Hussey MA, Hughes JP. Design and analysis of stepped wedge cluster randomized trials. Contemp Clin Trials. 2007; 28:182–91. [DOI] [PubMed] [Google Scholar]
- 26.Spiegelman D. Evaluating Public Health Interventions: 2. Stepping Up to Routine Public Health Evaluation With the Stepped Wedge Design. Am J Public Health. 2016; 106:453–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldsmith SB. Principles of Health Care Management: Foundations for a Changing Health Care System. Sudbury, MA: Jones and Bartlett Publishers; 2011. [Google Scholar]
- 28.Freund KM, Haas JS, Lemon SC, Burns White K, Casanova N, Dominici LS, et al. Standardized activities for lay patient navigators in breast cancer care: Recommendations from a citywide implementation study. Cancer. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aunt Bertha. Aunt Bertha, a Public Benefit Corp; [updated https://company.auntbertha.com/about/].
- 30.Powell BJ, Waltz TJ, Chinman MJ, Damschroder LJ, Smith JL, Matthieu MM, et al. A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implementation Science. 2015; 10:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Proctor E, Silmere H, Raghavan R, Hovmand P, Aarons G, Bunger A, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011; 38:65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frick KD. Microcosting quantity data collection methods. Medical care. 2009; 47:S76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neumann PJ, Sanders GD, Russell LB, Siegel JE, & Ganiats TG, eds. Cost-effectiveness in health and medicine.: Oxford University Press; 2016. [Google Scholar]
- 34.Shrestha RK, Gardner L, Marks G, Craw J, Malitz F, Giordano TP, et al. Estimating the cost of increasing retention in care for HIV-infected patients: results of the CDC/HRSA retention in care trial. Journal of acquired immune deficiency syndromes (1999). 2015; 68:345–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith MW, Barnett PG. Direct measurement of health care costs. Medical care research and review : MCRR. 2003; 60:74s–91s. [DOI] [PubMed] [Google Scholar]