Abstract
Purpose
To develop a deep learning-based method to achieve vessel segmentation and measurement on fundus images, and explore the quantitative relationships between retinal vascular characteristics and the clinical indicators of renal function.
Methods
We recruited patients with type 2 diabetes mellitus with different stages of diabetic retinopathy (DR), collecting their fundus photographs and results of renal function tests. A deep learning framework for retinal vessel segmentation and measurement was developed. The correlation between the renal function indicators and the severity of DR were explored, then the correlation coefficients between indicators of renal function and retinal vascular characteristics were analyzed.
Results
We included 418 patients (eyes) with type 2 diabetes mellitus. The albumin to creatinine ratio, blood uric acid, blood creatinine, blood albumin, and estimated glomerular filtration rate were significantly correlated with the progression of DR (P < 0.05); no correlation existed in other metrics (P > 0.05). The fractal dimension was found to significantly correlate with most of the clinical parameters of renal function (P < 0.05).
Conclusions
The albumin to creatinine ratio, blood uric acid, blood creatinine, blood albumin, and estimated glomerular filtration rate have significant correlation with the progression of moderate to proliferative DR. Through deep learning-based vessel segmentation and measurement, the fractal dimension was found to significantly correlate with most clinical parameters of renal function.
Translational Relevance
Deep learning-based vessel segmentation and measurement on color fundus photographs could explore the relationships between retinal characteristics and renal function, facilitating earlier detection and intervention of type 2 diabetes mellitus complications.
Keywords: retinal vascular metrics, renal function, type 2 diabetes mellitus, deep learning segmentation
Introduction
Type 2 diabetes mellitus (T2DM) is evolving to a major crisis for the global health system, the related disability, health care costs, and socioeconomic burdens are overwhelming and troublesome.1 The disability and mortality of T2DM are usually caused by its complications. Diabetic retinopathy (DR) and diabetic nephropathy (DN) are the major microvascular complications in patients with T2DM.2,3 Paralleling the rapid increase in the prevalence of diabetes mellitus, DR has become the leading cause of irreversible blindness worldwide, so is DN for end-stage renal disease.4,5 Thus, earlier detection of T2DM complications by proper clinical biomarkers might facilitate timely intervention and a achieve better prognosis.
The eye and kidney have several structural, developmental, and organizational similarities6; current studies suggest that DN and DR share common microvascular pathophysiologic mechanisms, including inflammation, endothelial dysfunction, oxidative stress, and thickening of the basement membrane.7 Several epidemiologic studies suggested DR and DN usually progress parallelly and share close relationship8; thus, it is reasonable to assume that the severity of DR is a predictor of renal function. Several studies were performed to explore the relationship between the severity of DR and the indicators of renal function, like the estimated glomerular filtration rate (eGFR), serum creatinine, albuminuria, and albumin to creatinine ratio (ACR), although these results were not conclusive.9-11
Because DR can be evaluated noninvasively by ocular examination and fundus photography, previous studies have investigated the retinal vascular characteristics in patients with DR and DM12,13 and applied these quantitative indicators to assess the risk of DR progression.14,15 The retinal vessel-derived metrics included vessel caliber class (e.g., the central retinal arteriolar equivalent [CRAE], central retinal venular equivalent [CRVE], and arteriole-to-venule ratio [AVR]) and vessel geometric class (e.g., fractal dimension [Df], curvature tortuosity, and branching angle).16 Retinal microvascular damages have been characterized with widen retinal venular caliber and reduced Df in DM, and been proved to be associated with the severity of DR.12–15 However, fewer studies have been conducted as yet to quantify the direct relationships between retinal vascular characteristics and the clinical markers of renal function under different severity levels of DR, although there is profound clinical significance.
A possible obstacle to such research is how to measure retinal vascular characteristics accurately. Compared with conventional qualitative evaluation of DR, quantitative analyses manipulated by ophthalmologists are more time consuming and laborious, and the manual results are usually highly variable to extract detailed measurement for retinal vessel structure. Most previous studies applied the semiautomated computer-assisted software (e.g., Singapore I Vessel Assessment, SIVA; National University of Singapore, Singapore) to achieve retinal vessel segmentation and measure the vessel-derived metrics.16 Recently, numerous retinal vessel segmentation algorithms have been proposed by using deep learning-based methods.17–19 However, most of them were developed and evaluated on public datasets, and it is still a challenging problem when we applied them to the actual collected fundus images. The difficulties are mainly caused by intensity inhomogeneity, low contrast, and variable thickness between the capillaries and major vessels on a fundus retinal image. The retinal lesions and exudates would further complicate the segmentation.20 Thus, it is necessary to make some improvements to make these vessel segmentation methods suitable for the real-world use scenarios of vessel-derived metrics measurement.
The aim of our study was to explore the quantitative relationships between retinal vascular characteristics and the clinical indicators of renal function under different severity levels of DR. We improved a deep learning-based method19 to achieve vessel segmentation on our collected fundus images, and extracted multiple retinal vessel-derived metrics, including vessel caliber class and vessel geometric class. The relationships between the estimated retinal vascular characteristics and renal function under different severity levels of DR were investigated.
Methods
This single-center, cross-sectional study was conducted between January 2017 and January 2020 in the Department of Ophthalmology of Peking Union Medical College Hospital. The study was approved by the institutional Review Board/Ethics Committee of Peking Union Medical College Hospital and conducted following the tenets of the Declaration of Helsinki. Written informed consent was obtained from each patient before treatment.
Participants
The following inclusion criteria were used: (1) confirmation of T2DM diagnosis based on the criteria of the American Diabetes Association21; (2) patients had their hospital visits for DR screening or treatment; (3) patients had detailed medical records and fundus photograph; and (4) the patients took the laboratory tests within 1 month around the shooting time of the fundus photograph. The exclusion criteria were (1) patients with uncontrolled hypertension (blood pressure of ≥160/100 mm Hg) and severe anemia (hemoglobin of ≤10.0 g/dL), which might cause similar fundus and renal changes, as in T2DM; (2) patients with coexisting nondiabetic renal diseases, such as acute renal failure, chronic glomerulonephritis, interstitial nephritis, membranous nephropathy, or IgA nephropathy; (3) pregnant subjects, patients with systemic diseases, especially those involving antineutrophil cytoplasmic antibodies, such as vasculitis, antiglomerular basement membrane disease and lupus nephritis, or patients with drug-induced kidney injury; (4) patients with type 1 diabetes mellitus; and (5) patients with poor quality images of the ocular fundus.
Clinical Examinations of Renal Function
The included indicators of renal function included eGFR, urinary microalbuminuria (UMAlb), urinary creatinine (UCr), urinary ACR (mg/g creatinine), blood urea, blood uric acid (UA), blood creatinine (Cr), and blood albumin (Alb). The eGFR was calculated from serum Cr concentration using the Chronic Kidney Disease-Epidemiology Collaboration equation.22
Ocular Examinations
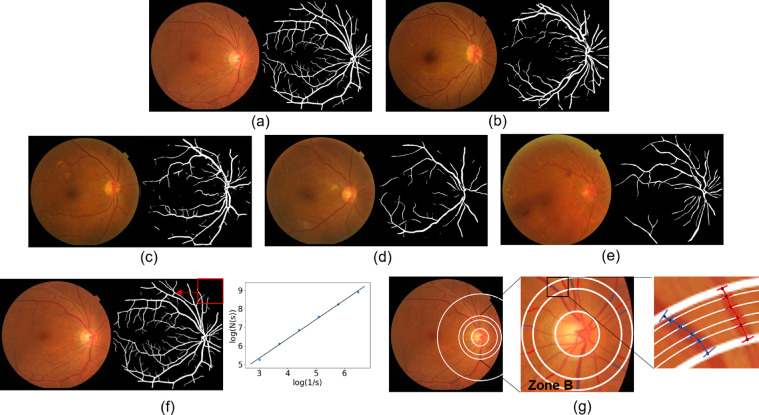
The bilateral fundus photography images were obtained at 50° by a fundus camera (CX-1, Topcon, Topcon Corporation, Tokyo, Japan) after fully dilating the pupils with 1% tropicamide, the maximal grade in any of the 7 standard photographic fields was used to define the DR levels. The severity of DR was categorized as none, mild nonproliferative DR (NPDR), moderate NPDR, severe NPDR, or proliferative DR (PDR).23 The typical lesion of NPDR includes microaneurysms, hemorrhages, venous beading, and intraretinal microvascular abnormalities. DR was classified as PDR if neovascularization, pre-retinal hemorrhages, vitreous hemorrhage, or panretinal laser photocoagulation scars were identified. The severity of DR in the worse affected eye was used for DR grading, image processing and measurement. Image quality and confounding conditions (pathology or image artifacts) that might affect measurements were recorded. The quality control criteria were as follows: (1) no severe artifact or blurring; (2) brightness that was not too dark or too light; and (3) the image field contained the entire optic disc and macula. Low-quality images were discarded. If an image was ungradable or could not be measured accurately, the other eye was used. If both eyes have poor quality images, then that patient was excluded from this study. The DR grading was made by 2 experienced retinal specialists (X.Y.Z. and W.F.Z.), disagreements were evaluated by kappa text and agreement was achieved by discussion with the corresponding author (C.Y.X.). Figure 1 illustrates the typical fundus images of healthy eye (Fig. 1a) and DR eyes at different stages (Figs. 1b–1e).
Figure 1.
Automatic segmentation and quantitative measurement of retinal vessels. (a) The retinal vessel segmentation results in healthy eyes, (b) mild DR, (c) moderate DR, (d) severe NPDR, (e) proliferative retinopathy, and (f) the quantitative calculation of Df. Each segmented mask was divided into a series of squares by sliding windows, s represents the relative value of the window width to the image width, N(s) is the number of squares that contain blood vessels. Adjust the value of s to get multiple groups (s, N(s)), Df was then defined as the gradient of logarithms of the number of squares and the size of those squares; (g) is the measurement principle diagram of vessel caliber biomarkers. Zone B is defined as an area of 0.50 to 0.75 disc diameter surrounding the optic disc, all arterioles (red lines) and venules (blue lines) coursing through zone B were measured, 6 vessel diameters were obtained at different locations for each vessel and then the Knudtson–Hubbard formula was used to calculate average retinal arteriolar and venular caliber.
Retinal Vessel Segmentation Via Deep Learning
We realized the automatic extraction of retinal vessel based on a recent published deep learning-based approach called NFN+.19 Two publicly available online datasets called DRIVE20 and STARE24 were used for model development. DRIVE contains 40 fundus images from 7 early DR and 33 normal eyes.20 STARE has 20 fundus images, 10 of which have lesions (including bleeding, hard exudation) and 10 of which are healthy fundus images.24 All fundus images have the corresponding vessel segmentation results generated by experts. To improve the performance of vessel segmentation for our collected images in clinical use scenarios, we performed a special data augmentation called vignetting mask,25 in addition to the frequently used data augmentation. Vignetting is an optical phenomenon wherein illumination falls off around the light source. We constructed different vignetting masks by multiplying two 1-dimenstional gaussian kernels and multiplied them with original image to generate fundus images with uneven illumination, which often happens in clinical fundus image acquisition. The NFN+ segmentation network was implemented with Keras in Python, using Tensorflow as a backend. We trained the model for 150 epochs on an NVIDIA Tesla P100 GPU. More details about the implementation and training protocol could be found in.19,25
To evaluate the performance of vessel segmentation methods, we selected randomly 50 fundus images from the collected dataset, with 10 fundus images of each DR stage. The corresponding vessel masks were outlined manually by 2 experienced retinal specialists (X.Y.Z. and W.F.Z.) with in-house labeling software. With the annotated ground truth, we compared the vessel segmentation results of NFN+19 with or without vignetting mask augmentation.25 The evaluation metrics included the dice coefficient (DICE) and accuracy (ACC), and DICE = 2*TP/(2*TP + FP + FN), ACC = (TP + TN)/(TP + FP + FN + TN), where TP, TN, FP and FN are the number of true positive, true negative, false positive, and false negative image pixels, respectively.
Measurement of Retinal Vascular Metrics
After the vessel segmentation mask generated by deep learning model, the artery/vein vessel classification was performed manually by 2 experienced retinal specialists (X.Y.Z. and W.F.Z.) with in-house software. Then we measured a vessel geometric class called retinal vascular Df.16 The Df is a global measure to quantify the physiologic complexity, including vascular networks. Using the box-counting method (Fig. 1f), each segmented mask was divided into a series of squares of various side lengths along the centerline tracings of retinal vessels.16 Retinal vascular Df was then defined as the gradient of logarithms of the number of boxes and the size of those boxes. The more complex the branching pattern, the greater the Df. Besides the vessel geometric class, we also measured the diameters of all arterioles and venules coursing through an area of 0.50 to 0.75 disc diameter surrounding the optic disc (zone B in Fig. 1g).26 The Knudtson–Hubbard formula was used to calculate average retinal arteriolar and venular caliber, named as CRAE and CRVE, respectively. And the AVR is the ratio of CRAE to CRVE.26
Statistical Analysis
The means and standard deviations were calculated for all continuous clinical variables. The Kolmogorov–Smirnov test was used to determine the normality of the distribution for each variable. First, one-way analysis of variance was used to determine the group effect on each clinical variable, respectively. Then the independent sample t-test was performed between healthy control and the 4 different levels of DR to determine which group comparisons reached statistical significance. We defined the different levels of statistical significant with P < 0.05, P < 0.01 and, P < 0.001. To further determine the relationships of clinical variables, we calculated the correlation coefficients between indicators of renal function (including eGFR, UMAlb, UCr, ACR, blood urea, UA, Cr, and Alb) and retinal vascular characteristics (including CRAE, CRVE, AVR, and Df). A P value of less than 0.05 was considered statistically significant, and the Bonferroni test was used to adjust the probabilities. Linear regression was also used to investigate the correlation coefficients when they reached the statistical significance. All the statistical analyses were performed by using the R statistical package (version 3.6; The R Foundation, Vienna, Austria).
Results
General Data and Vessel Segmentation Evaluation
A total of 418 patients with T2DM with available renal function examinations and fundus images from Peking Union Medical College Hospital were included in our study, including 75 subjects without DR and 343 patients with DR. At first, 422 patients with T2DM were recruited for this study. However, 4 patients with DR did not have clear photographs of both their eyes. Therefore, 418 patients were included ultimately. The baseline characteristics are summarized in Table 1. The mean patient age was 52.45 ± 14.72 years and 35% were female. The prevalence of non-DR, mild NPDR, moderate NPDR, severe NPDR, and PDR were 17.9%, 9.6%, 30.6%, 17.0%, and 24.9%, respectively (Table 1). The study population was equally distributed for sex and age among the different stages of DR (P > 0.05).
Table 1.
The Clinical Characteristics of Renal Function and Retinal Vascular Metrics in Different Grades of DR
| Characteristics | No DR (n = 75) | Mild NPDR (n = 40) | Moderate NPDR (n = 128) | Severe NPDR (n = 71) | PDR (n = 104) | Coefficient (95% CI) | P Value (ANOVA) |
|---|---|---|---|---|---|---|---|
| Age (years) | 51.77 ± 14.28 | 48.82 ± 11.13 | 54.0 ± 13.92 | 53.63 ± 13.3 | 48.71 ± 18.88 | –0.05 (–0.14 to 0.05) | 0.07 |
| Sex (male:female) | 39:36 | 24:16 | 85:43 | 51:20 | 73:31 | 0.14 (–0.04 to 0.31) | 0.06 |
| UMAlb (mg/L) | 113.75 ± 517.5 | 27.96 ± 39.85 | 559.66 ± 1349.19 | 871.16 ± 1937.81 | 3423.0 ± 13967.76 | 0.11 (–0.10 to 0.32) | 0.06 |
| UCr (mmol/L) | 11.13 ± 7.5 | 8.83 ± 6.55 | 13.14 ± 11.02 | 11.19 ± 7.77 | 11.98 ± 7.39 | 0.04 (–0.08 to 0.17) | 0.36 |
| ACR (mg/g) | 132.81 ± 631.22 | 40.18 ± 54.13 | 527.94 ± 1466.33 | 865.5 ± 2021.56) | 1278.38 ± 2100.34 | 0.26 (0.13 to 0.37) | <0.001 |
| Blood urea (mmol/L) | 6.04 ± 1.6 | 5.37 ± 1.41 | 6.96 ± 3.39 | 7.06 ± 2.89 | 17.68 ± 90.19 | 0.08 (–0.02 to 0.18) | 0.42 |
| UA (µmol /L) | 321.68 ± 85.82 | 303.56 ± 95.61 | 332.35 ± 94.22 | 325.09 ± 99.46 | 372.1 ± 142.08 | 0.15 (0.05 to 0.25) | 0.01 |
| Cr (µmol/L) | 71.24 ± 18.46 | 61.89 ± 12.52 | 82.58 ± 37.98 | 112.51 ± 160.28 | 123.31 ± 147.66 | 0.13 (0.02 to 0.23) | 0.02 |
| Alb (g/L) | 44.83 ± 4.88 | 45.17 ± 3.62 | 44.61 ± 4.56 | 42.92 ± 4.75 | 42.02 ± 6.04 | –0.21 (–0.31 to –0.11) | <0.001 |
| eGFR | 95.14 ± 18.76 | 106.75 ± 10.63 | 90.41 ± 26.06 | 87.28 ± 30.43 | 78.09 ± 33.28 | –0.20 (–0.30 to –0.10) | <0.001 |
| Df | 1.4 ± 0.04 | 1.38 ± 0.07 | 1.34 ± 0.07 | 1.27 ± 0.13 | 1.11 ± 0.19 | –0.62 (–0.68 to –0.56) | <0.001 |
| CRAE | 334.92 ± 51.82 | 330.24 ± 91.45 | 348.85 ± 84.75 | 217.69 ± 43.26 | 214.81 ± 43.97 | –0.43 (–0.56 to –0.27) | <0.001 |
| CRVE | 416.03 ± 42.97 | 418.34 ± 71.63 | 449.04 ± 73.0 | 343.0 ± 51.51 | 353.04 ± 44.6 | –0.26 (–0.42 to –0.09) | 0.004 |
| AVR | 0.82 ± 0.10 | 0.78 ± 0.11 | 0.79 ± 0.15 | 0.65 ± 0.1 | 0.62 ± 0.11 | –0.44 (–0.57 to –0.28) | <0.001 |
ACR, urinary albumin-to-creatinine ratio; Alb, albumin; ANOVA analysis of variance; Cr, creatinine.
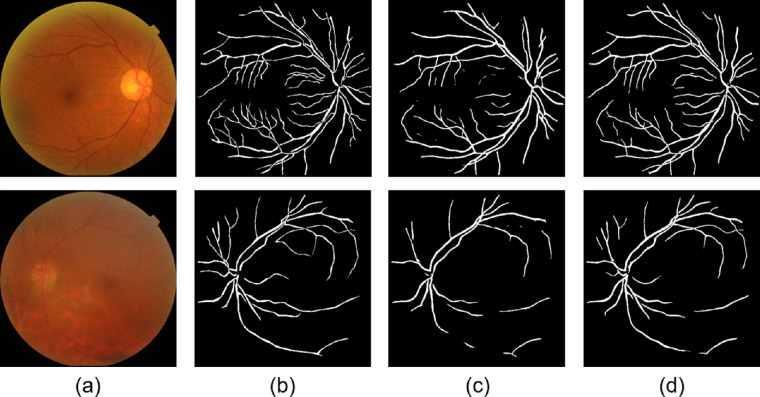
Figure 2 provides 2 examples of vessel segmentation, one of mild NPDR and another of PDR. With vignetting mask augmentation, we could find that NFN+ model achieved better segmentation performance of retinal vessels. In the testing dataset of 50 fundus images, the averaged DICE is 0.773 and ACC is 0.966 for NFN+ without vignetting mask augmentation, whereas the averaged DICE is 0.837 and ACC is 0.976 for NFN+ with vignetting mask augmentation.
Figure 2.
The vessel segmentation results of 2 example fundus images. The first row is an example of mild NPDR, and the second row is another example of PDR. (a) The original fundus images, (b) the annotated ground truth of retinal vessels, (c) the outputs of the NFN+ model without vignetting mask augmentation, and (d) the outputs of the NFN+ model with vignetting mask augmentation.
Renal Function and the Severity of DR
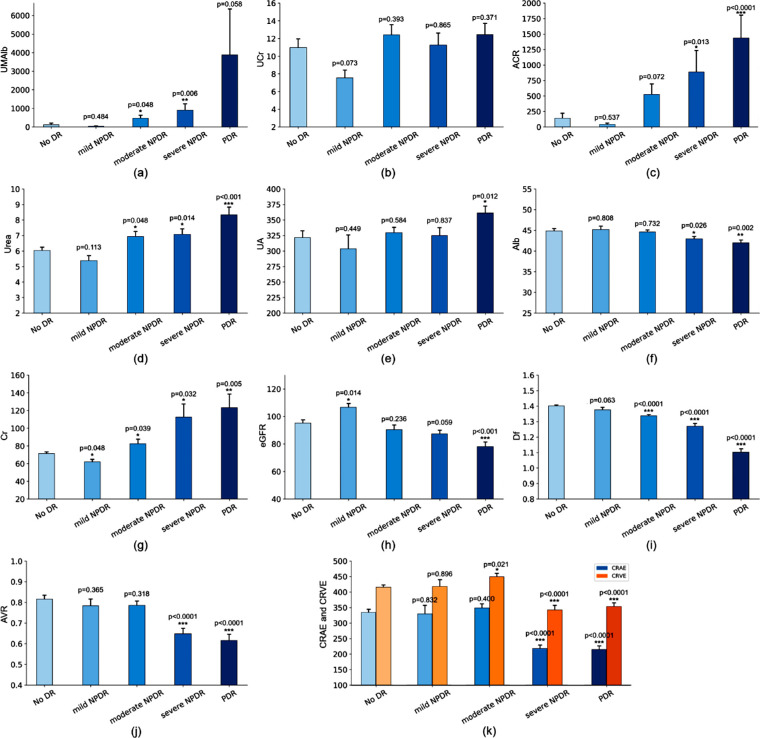
Among the included indicators of renal function (Figure 3a–k; Table 1), ACR (r = 0.26; 95% CI, 0.13–0.37; P < 0.001), UA (r = 0.15; 95% CI, 0.05–0.25; P = 0.01), Cr (r = 0.13; 95% CI, 0.02–0.23; P = 0.02), Alb (r = −0.21; 95% CI, −0.31 to −0.11; P < 0.001), and eGFR (r = −0.20; 95% CI, −0.30 to −0.10; P < 0.001) had significant correlation with the progression of DR, although no correlation existed for UMAlb (r = 0.11; 95% CI, −0.10 to 0.32; P = 0.06), UCr (r = 0.04; 95% CI, −0.08 to 0.17; P = 0.36), or blood urea (r = 0.08; 95% CI, −0.02 to 0.18; P = 0.42).
Figure 3.
The correlations of renal function indicators and retinal vascular metrics with the severity of DR: (a) UMAlb, (b) UCr, (c) ACR, (d) blood urea, (e) blood UA, (f) blood Alb, (g) blood Cr, (h) eGFR, (i) Df, (j) AVR, and (k) CRAE and CRVE. ACR, urinary albumin-to-creatinine ratio; Alb, albumin; Cr, creatinine. *P < 0.05; **P < 0.01.
For eGFR (Fig. 3h), patients with mild NPDR had a significantly higher eGFR than that of patients without DR (P = 0.014), then it decreased with the progression of DR, patients with PDR had a significant lower eGFR than that of patients without DR (P < 0.001).
For ACR and Cr, they decreased at mild NPDR, then increased significantly at severe NPDR (ACR, P = 0.013; Cr; P = 0.032) and PDR (ACR, P < 0.0001; Cr; P = 0.005), whereas Alb declined significantly at severe NPDR (P = 0.026) and PDR (P = 0.002).
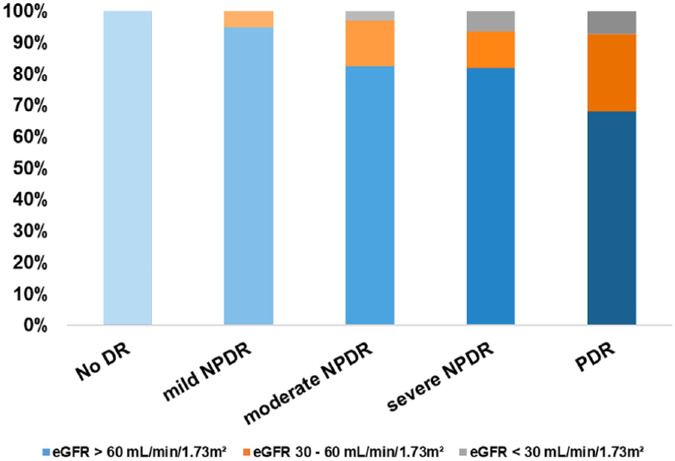
The percentages of patients with an eGFR of <30 mL/min/1.73m2 were 0%, 0%, 2.9%, 6.7%, and 7.4% in the non-DR, mild NPDR, moderate NPDR, severe NPDR, and PDR groups, respectively; the percentages of patients with an eGFR of 30 to 60mL/min/1.73 m2 were 0%, 5.3%, 14.7%, 11.7%, and 24.5%, respectively (Fig. 4).
Figure 4.
The distribution of the eGFR in different stage of DR.
Retinal Vascular Metrics and the Severity of DR
For the retinal vascular metrics, the Df decreased significantly after DR advanced to moderate NPDR (P < 0.0001) and AVR after severe NPDR (P < 0.0001). CRAE and CRVE increased at mild and moderate NPDR, then decreased significantly at severe NPDR and PDR (P < 0.0001). The detailed tendency of each metrics with the advance of DR were shown in Figure 3.
Renal Function and Retinal Vascular Metrics
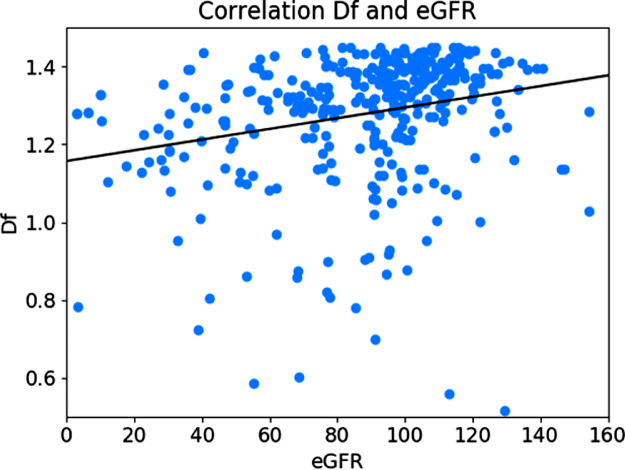
Among the correlation analysis between the renal function indicators and retinal vascular metrics (Table 2), the Df was positively correlated with eGFR (Fig. 5; r = 0.24; 95% CI, 0.14 – 0.33; P < 0.001). Additionally, UMAlb (r = −0.29; 95% CI, −0.40 to −0.17; P < 0.001), ACR (r = −0.21; 95% CI, −0.33 to −0.09; P < 0.001), and Cr (r = −0.15; 95% CI, −0.25 to −0.05; P < 0.003), Alb (r = 0.17; 95% CI; 0.07–27, P = 0.001) were also significantly correlated with the Df.
Table 2.
The Correlation Analysis Between the Renal Function Indicators and Retinal Vascular Metrics
| CRAE | CRVE | AVR | Df | |||||
|---|---|---|---|---|---|---|---|---|
| Coefficient (95% CI) | P Value | Coefficient (95% CI) | P Value | Coefficient (95% CI) | P Value | Coefficient (95% CI) | P Value | |
| UMAlb (mg/L) | 0.08 (−0.14 to 0.29) | 0.47 | 0.11 (−0.09 to 0.33) | 0.28 | 0.01 (−0.20 to 0.22) | 0.92 | −0.29 (−0.40 to −0.17) | <0.001 |
| UCr (mmol/L) | 0.11 (−0.11 to 0.31) | 0.32 | 0.00 (−0.21 to 0.21) | 0.99 | 0.16 (−0.05 to 0.36) | 0.13 | 0.09 (−0.04 to 0.217) | 0.15 |
| ACR (mg/g) | −0.01 (−0.22 to 0.20) | 0.93 | 0.10 (−0.11 to 0.31) | 0.36 | −0.10 (−0.31 to 0.11) | 0.34 | −0.21 (−0.33 to −0.09) | <0.001 |
| Blood urea (mmol/L) | −0.14 (−0.32 to 0.05) | 0.16 | −0.06 (−0.25 to 0.13) | 0.51 | −0.16 (−0.34 to 0.02) | 0.09 | −0.01 (−0.11 to 0.09) | 0.89 |
| UA (µmol/L) | 0.10 (−0.08 to 0.29) | 0.28 | 0.21 (0.02 to 0.38) | 0.03 | −0.03 (−0.22 to 0.16) | 0.73 | −0.02 (−0.13 to 0.08) | 0.63 |
| Cr (µmol/L) | −0.04 (−0.22 to 0.15) | 0.66 | 0.01 (−0.17 to 0.20) | 0.88 | −0.08 (−0.26 to 0.11) | 0.41 | −0.15 (−0.25 to −0.05) | 0.003 |
| Alb (g/L) | 0.04 (−0.15 to 0.23) | 0.66 | 0.06 (−0.14 to 0.25) | 0.56 | 0.01 (−0.17 to 0.21) | 0.88 | 0.17 (0.07 to 0.27) | 0.001 |
| eGFR | 0.12 (−0.07 to 0.30) | 0.20 | 0.11 (−0.08 to 0.29) | 0.25 | 0.11 (−0.08 to 0.29) | 0.27 | 0.24 (0.14 to 0.33) | <0.001 |
ACR, urinary albumin-to-creatinine ratio; Alb, albumin; Cr, creatinine.
Figure 5.
The correlation coefficients between eGFR and Df (P < 0.05, r = 0.24).
Discussion
Our study included 418 patients with T2DM with detailed renal function indicators and FP. Among the included indicators of renal function, ACR, UA, Cr, Alb, and eGFR were found to have a significant correlation with the progression of DR, whereas no correlation existed for UMAlb, UCr, or blood urea. Through deep learning-based vessel segmentation and measurement, we found that the DFs, CRAE, CRVE, and AVR decreased significantly with the advance from DR moderate to PDR. Among the correlation analysis between the renal function indicators and retinal vascular metrics, Df was found to significantly correlate with eGFR, UMAlb, ACR, Cr, and Alb, although no correlation existed in other metrics. However, the correlations between the renal function indicators and the progression of DR or retinal vascular metrics were not high.
DR and DN are thought to progress in a parallel manner with common microvascular pathophysiologic mechanisms; the eye and the kidney share several structural, developmental, and organizational similarities.4,5 At the early stage of DN, the eGFR increases as a result of glomerular hyperfiltration,27,28 then decreases with the progression of DN. The findings from our study are consistent with this tendency; we found that patients with T2DM with mild NPDR had a higher eGFR than patients without DR, and the eGFR then decreased with the progression of moderate to proliferative DR. Patients with PDR had a significantly lower eGFR than that of patients without DR. Current clinical guidelines, like the Kidney Disease: Improving Global Outcomes guidelines, suggested that the eGFR and ACR share the same value in the diagnosis of chronic renal disease.29,30 Our results verify that the ACR increased with the advance of DR, but demonstrated the opposite tendency with eGFR; both parameters changed dramatically when DR advance to moderate NPDR or worse. Based on these findings from our studies, around 18% of patients with moderate and severe NPDR had an impaired eGFR, and some of them even had eGFR of less than 30 mL/min/1.73 m2. For patients with PDR, 24.5% had an eGFR of 30 to 60 mL/min/1.73 m2, 7.4% had an eGFR of less than 30 mL/min/1.73 m2. Thus, patients with T2DM with mild NPDR or worse should be advised to have undergo renal function examinations to facilitate earlier detection and intervention for the renal complications. However, those metrics did not change regularly and clearly from mild to moderate DR, which might be due to not obvious changes inherently or irreversibility between these 2 stages.
To date, most studies have used semiautomated computer-assisted software to achieve retinal vessel segmentation and measure the vessel-derived metrics like CRAE, CRVE, AVR, and Df, a time-consuming and potentially inaccurate process. In this work, we achieved a deep learning-based model called NFN+ to extract vessel mask from fundus image automatically, which could significantly reduce the burden of manual or semiautomated measurement for vessel-derived metrics.19 In NFN+, the front network converts an image patch into a probabilistic retinal vessel map, and the followed network further refines the map to achieve a better postprocessing module, which contributes to represent the vessel structures implicitly. After the artery/vein vessel manual classification, we then measured the Df using the box-counting method and calculate the vessel caliber class metrics (CRAE, CRVE, and AVR) based the Knudtson–Hubbard formula with programming. As shown in Figure 3, the Df decreased significantly with the progression of DR (P < 0.05). The CRAE, CRVE, and AVR all decreased significantly as the level of DR deteriorates to severe DR or PDR. In the early diagnosis of DR, the morphologic attributes of blood vessels, especially the Df, played an essential role constructing a retinal computer-aided diagnosis system.14
Many studies have reported the relationship between DR and chronic kidney disease, although few studies had examined the association between retinal vascular metrics and renal function indicators, the conclusion remains uncertain. Some studies reported smaller retinal arterioles, larger retinal venules associated with chronic kidney disease, and narrower CRAE independently associated with higher albuminuria and impaired eGFR.10 However, other investigators insisted that whether retinal vessel calibers predict the presence of chronic kidney disease was still not clear.31–33 Through deep learning-based vessel segmentation and measurement, we found that the Df, CRAE, CRVE, and AVR were all decreased significantly with the advance of DR. Only the Df was found to significantly correlate with the renal function indicators (eGFR, UMAlb, ACR, Cr, and Alb), and no correlation existed in other retinal vascular metrics (CRAE, CRVE, and AVR). Therefore, the Df should be regarded as a retinal vascular metric with great clinical significance; it is significantly correlated not only with the severity of DR and retinal circulation, but also with renal function indicators, like the eGFR and ACR.
Our study has several limitations which can be strengthened. First, although we found some renal indicators were significantly associated with DR progression or retinal vascular metrics, the correlations were relatively low (ranging from 0.13 to 0.26), which might partly limit the clinical value of our study. However, our study revealed the retinal vascular metrics, such as the Df, was significantly correlated with the severity of DR, retinal circulation, and the renal function indicators like eGFR and ACR. All these discoveries could provide clues for further study. Second, we need verify our findings on a larger scale dataset. And furthermore, it is better to collect follow-up data to perform the longitudinal analysis along with the condition development. Third, optical coherence tomography angiography can provide a highly detailed view of the retinal microvascular morphologic features noninvasively.34 By using optical coherence tomography angiography imaging, we can examine retinal neurovascular changes in different stage of DR, which is similar to a recent study in chronic kidney disease.34
Conclusion
With deep learning-based vessel segmentation, we achieved the extraction of multiple retinal vascular metrics, including the CRAE, CRVE, and retinal vascular Df. We found that the ACR, UA, Cr, Alb, and eGFR have a significant correlation with the progression of moderate DR to proliferative DR. Additionally, the Df was found to significantly correlate with eGFR, UMAlb, ACR, CR, and Alb. We hope that such observed relationships could be used to facilitate earlier detection and intervention for the T2DM complications.
Acknowledgments
Disclosure: X. Zhao, None; Y. Liu, None; W. Zhang, None; L. Meng, None; B. Lv, None; C. Lv, None; G. Xie, None; Y. Chen, None
References
- 1. Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al.. IDF diabetes atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract . 2017; 128: 40–50. [DOI] [PubMed] [Google Scholar]
- 2. Yau JW, Rogers SL, Kawasaki R, et al.. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care . 2012; 35(3): 556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sivaprasad S, Gupta B, Crosby-Nwaobi R, Evans J.. Prevalence of diabetic retinopathy in various ethnic groups: a worldwide perspective. Surv Ophthalmol . 2012; 57(4): 347–370. [DOI] [PubMed] [Google Scholar]
- 4. Huang OS, Tay WT, Ong PG, et al.. Prevalence and determinants of undiagnosed diabetic retinopathy and vision-threatening retinopathy in a multiethnic Asian cohort: the Singapore Epidemiology of Eye Diseases (SEED) study. Br J Ophthalmol . 2015; 99(12): 1614–1621. [DOI] [PubMed] [Google Scholar]
- 5. Rodríguez-Poncelas A, Mundet-Tudurí X, Miravet-Jiménez S, et al.. Chronic kidney disease and diabetic retinopathy in patients with type 2 diabetes. PLoS One . 2016; 11(2): e0149448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Farrah TE, Dhillon B, Keane PA, Webb DJ, Dhaun N.. The eye, the kidney, and cardiovascular disease: old concepts, better tools, and new horizons. Kidney Int . 2020; 98(2): 323–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barrios C, Zierer J, Wurtz P, et al.. Circulating metabolic biomarkers of renal function in diabetic and non-diabetic populations. Sci Rep . 2018; 8(1): 15249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ahola-Olli AV, Mustelin L, Kalimeri M, et al.. Circulating metabolites and the risk of type 2 diabetes: a prospective study of 11,896 young adults from four Finnish cohorts. Diabetologia . 2019; 62(12): 2298–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McKay GJ, Paterson EN, Maxwell AP, et al.. Retinal microvascular parameters are not associated with reduced renal function in a study of individuals with type 2 diabetes. Sci Rep . 2018; 8(1): 3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yip W, Ong PG, Teo BW, et al.. Retinal vascular imaging markers and incident chronic kidney disease: a prospective cohort study. Sci Rep. 2017; 7(1): 9374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nagaoka T, Yoshida A.. Relationship between retinal blood flow and renal function in patients with type 2 diabetes and chronic kidney disease. Diabetes Care . 2013; 36(4): 957–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nguyen TT, Wang JJ, Sharrett AR, et al.. Relationship of retinal vascular caliber with diabetes and retinopathy: the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care . 2008; 31(3): 544–549. [DOI] [PubMed] [Google Scholar]
- 13. Ikram MK, Cheung CY, Lorenzi M, Klein R, Jones TL, Wong TY.. Retinal vascular caliber as a biomarker for diabetes microvascular complications. Diabetes Care . 2013; 36(3): 750–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klein R, Lee KE, Danforth L, et al.. The relationship of retinal vessel geometric characteristics to the incidence and progression of diabetic retinopathy. Ophthalmology . 2018; 125(11): 1784–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cheung CY, Sabanayagam C, Law AK, et al.. Retinal vascular geometry and 6 year incidence and progression of diabetic retinopathy. Diabetologia . 2017; 60(9): 1770–1781. [DOI] [PubMed] [Google Scholar]
- 16. Wang SB, Mitchell P, Liew G, et al.. A spectrum of retinal vasculature measures and coronary artery disease. Atherosclerosis . 2018; 268: 215–224. [DOI] [PubMed] [Google Scholar]
- 17. Liskowski P, Krawiec K.. Segmenting retinal blood vessels with deep neural networks. IEEE Trans Med Imaging . 2016; 35(11): 2369–2380. [DOI] [PubMed] [Google Scholar]
- 18. Zhao H, Li HQ, Cheng L.. Improving retinal vessel segmentation with joint local loss by matting. Pattern Recognition . 2020; 98: 107068. [Google Scholar]
- 19. Wu Y, Xia Y, Song Y, Zhang Y, Cai W.. NFN+: a novel network followed network for retinal vessel segmentation. Neural Netw . 2020; 126: 153–162. [DOI] [PubMed] [Google Scholar]
- 20. Staal J, Abramoff MD, Niemeijer M, Viergever MA, van Ginneken B.. Ridge-based vessel segmentation in color images of the retina. IEEE Trans Med Imaging . 2004; 23(4): 501–509. [DOI] [PubMed] [Google Scholar]
- 21. American Diabetes Association. Standards of medical care in diabetes-2019 abridged for primary care providers. Clin Diabetes . 2019; 37(1): 11–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D.. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med . 1999; 130(6): 461–470. [DOI] [PubMed] [Google Scholar]
- 23. Grading diabetic retinopathy from stereoscopic color fundus photographs–an extension of the modified Airlie House classification. ETDRS report number 10. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology . 1991; 98(5 Suppl): 786–806. [PubMed] [Google Scholar]
- 24. Hoover A, Kouznetsova V, Goldbaum M.. Locating blood vessels in retinal images by piecewise threshold probing of a matched filter response. IEEE Trans Med Imaging . 2000; 19(3): 203–210. [DOI] [PubMed] [Google Scholar]
- 25. Nasery V, Soundararajan KB, Galeotti J, IEEE. Learning to segment vessels from poorly illuminated fundus images. In: 2020 IEEE 17th International Symposium on Biomedical Imaging . Iowa City, Iowa, April 4–7, 2020: 1232–1236. [Google Scholar]
- 26. Niemeijer M, Xu X, Dumitrescu AV, et al. Automated measurement of the arteriolar-to-venular width ratio in digital color fundus photographs[J]. IEEE Trans Med Imaging . 2011; 30(11): 1941–1950. [DOI] [PubMed] [Google Scholar]
- 27. Chagnac A, Zingerman B, Rozen-Zvi B, Herman-Edelstein M.. Consequences of glomerular hyperfiltration: the role of physical forces in the pathogenesis of chronic kidney disease in diabetes and obesity. Nephron . 2019; 143(1): 38–42. [DOI] [PubMed] [Google Scholar]
- 28. Tonneijck L, Muskiet MH, Smits MM, et al.. Glomerular hyperfiltration in diabetes: mechanisms, clinical significance, and treatment. J Am Soc Nephrol . 2017; 28(4): 1023–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Romero-Aroca P, Baget-Bernaldiz M, Navarro-Gil R, et al.. Glomerular filtration rate and/or ratio of urine albumin to creatinine as markers for diabetic retinopathy: a ten-year follow-up study. J Diabetes Res . 2018; 2018: 5637130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kidney disease: Improving Global Outcomes Diabetes Work Group. KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int . 2020; 98(4S): S1–S115. [DOI] [PubMed] [Google Scholar]
- 31. Yau JW, Xie J, Kawasaki R, et al.. Retinal arteriolar narrowing and subsequent development of CKD Stage 3: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis . 2011; 58(1): 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sabanayagam C, Shankar A, Klein BE, et al.. Bidirectional association of retinal vessel diameters and estimated GFR decline: the Beaver Dam CKD Study. Am J Kidney Dis . 2011; 57(5): 682–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yip W, Sabanayagam C, Teo BW, et al.. Retinal microvascular abnormalities and risk of renal failure in Asian populations. PLoS One . 2015; 10(2): e0118076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhuang X, Cao D, Zeng Y, et al.. Associations between retinal microvasculature/microstructure and renal function in type 2 diabetes patients with early chronic kidney disease. Diabetes Res Clin Pract . 2020; 168: 108373. [DOI] [PubMed] [Google Scholar]