Summary
Elevated frequency of Th17‐like cells expressing Toll‐like receptors (TLRs) has been recently associated with relapsing–remitting multiple sclerosis (MS) pathogenesis, a chronic inflammatory demyelinating autoimmune disease of the central nervous system. We aimed to investigate the impact of current major depressive disorder (MDD) on the behaviour of these cells following in vitro stimulation with TLR2, TLR4, TLR5 and TLR9 agonists. Here, the level of both cell proliferation and cytokine production related to Th17/Tc17 phenotypes in response to TLR2 (Pam3C) and TLR4 (LPS) ligands was significantly higher in CD4+ and CD8+ T‐cell cultures from MS/MDD patients when compared to non‐depressed patients. These cytokine levels were positively associated with neurological disabilities in patients. No difference for responsiveness to TLR5 (flagellin) and TLR9 (ODN) agonists was observed. LPS, but not Pam3C, induced significant IL‐10 release, mainly in patients without MDD. Interestingly, more intense expression of TLR2 and TLR4 on these cells was observed in MDD patients. Finally, in vitro addition of serotonin and treatment of MDD patients with selective serotonin reuptake inhibitors (SSRIs) reduced the production of Th17/Tc17‐related cytokines by CD4+ and CD8+ T cells in response to Pam3C and LPS. However, only SSRI therapy diminished the frequency and intensity of TLR2 and TLR4 expression on circulating CD4+ and CD8+ T cells. In summary, although preliminary, our findings suggest that adverse events that elevate circulating levels of TLR2 and TLR4 ligands can affect MS pathogenesis, particularly among depressed patients.
Keywords: cytokines, major depressive disorder, Multiple sclerosis, PAMP, selective serotonin reuptake inhibitors, serotonin, Tc17, Th17, TLR
The occurrence of major depressive disorder (MDD) in multiple sclerosis (MS) patients elevated the frequency of circulating CD4+ and CD8+ T‐cells expressing high number of TLR2 and TLR4 molecules able to respond to Pam3C and LPS by producing elevated levels of Th17/Tc17 cytokines. Interestingly, treatment of those patients with selective serotonin reuptake inhibitors (SSRIs) diminished both frequency and intensity of TLR2 and TLR4 expression on circulating CD4+ and CD8+ T‐cells and Th17/Tc17‐related cytokines. In contrast, the levels of IL‐10 were up‐regulated.

Abbreviations
- 5‐HT
5‐hydroxytryptamine
- BDI
Beck Depression Inventory
- CNS
central nervous system
- CPM
counts per minute
- DAMP
damage‐associated molecular pattern
- DCs
dendritic cells
- DMT
disease‐modifying therapy
- EAE
experimental autoimmune encephalomyelitis
- EDSS
Expanded Disability Status Scale
- FLA
flagellin
- HS
healthy subjects
- IDO
indoleamine 2,3‐dioxygenase
- LPS
lipopolysaccharide
- MDD
major depressive disorder
- MMPs
matrix metalloproteinases
- MS
multiple sclerosis
- ODN
oligodeoxynucleotides
- Pam3C
synthetic triacylated lipopeptide
- PAMP
pathogen‐associated molecular pattern
- PBMC
peripheral blood monocular cell
- PMA
phorbol 12‐myristate 13‐acetate
- PRR
pattern recognition receptor
- RRMS
relapsing–remitting MS
- SLE
systemic lupus erythematosus
- SSRI
selective serotonin reuptake inhibitor
- TLR
Toll‐like receptor
INTRODUCTION
Multiple sclerosis (MS) is a chronic inflammatory demyelinating autoimmune disease of the central nervous system (CNS) mainly mediated by effector T cells directed against myelin sheath antigens. 1 By affecting any area of the CNS, MS can lead to sensory, autonomic, cognitive and motor function deficits. 2 , 3 After definitive diagnosis, most patients (> 80%) present the relapsing–remitting MS (RRMS) form of the disease, characterized by repeated acute inflammatory flare‐ups followed by periods of remission. 4
Much evidence appears to suggest the involvement of different myelin‐specific T‐cell phenotypes in RRMS pathogenesis, particularly those related to Th17 cell subsets. Classically, not only high plasma levels of IL‐17A (IL‐17) have been observed in those patients during clinical relapses, but also elevated frequency of Th17 cells, which are associated with radiological activity of the disease. 5 , 6 , 7 , 8 , 9 . In addition, increased levels of IL‐23, IL‐1β and IL‐6, cytokines involved in the differentiation of encephalitogenic Th17 cells 10 , 11 , 12 , 13 , 14 , 15 , are produced by antigen‐presenting cells of MS patients. 16 , 17 , 18 . Further, IFN‐γ‐secreting Th17 cells have also been implicated in MS pathogenesis. 19 , 20 . Other cytokines, such as IL‐21, IL‐22 and GM‐CSF, are also produced by some encephalitogenic CD4+ T cells. 20 , 21 , 22 , 23 , 24 , 25 Finally, cytotoxic CD8+ T cells may not only cause oligodendrocyte death and neuronal damage through release of cytotoxic molecules, but also may potentiate brain lesions by secreting IFN‐γ (Tc‐1) or IL‐17 (Tc‐17). 26 , 27 , 28 , 29 , 30 , 31 All these cytokines can lead to oligodendrocyte apoptosis and breakdown of both the myelin sheath and blood–brain barrier by inducing local (microglia) and migrating [monocytes and dendritic cell (DCs)] phagocytes to release a broad arsenal of cytotoxic mediators, such as reactive oxygen intermediates and matrix metalloproteinases (MMPs). 32
Although the treatment of RRMS with immunomodulatory drugs referred to as disease‐modifying therapy (DMTs). 33 , the disease progression to neurodegenerative form should be influenced by environmental events, such as psychiatric disorders.
The association between MS and major depressive disorder (MDD) has been known for some time. 34 Prevalence of recurrent MDD in MS patients has been estimated at around 50%, almost three times the rate of the general population. 34 Under‐diagnosed by physicians and therefore undertreated, MDD in MS patients is the strongest determinant of impaired quality of life, sleep disturbance, fatigue and cognitive dysfunction. 34
Although MDD may be linked to advanced neurodegeneration in secondary progressive MS patients 35 , in the RRMS form, this psychiatric disorder should be linked to elevated levels of pro‐inflammatory cytokines. It is known that MDD is directly associated with elevated levels of circulating IL‐1β, IL‐6 and TNF‐α 36 and that these cytokines have been associated with fatigue 37 , the most frequent symptom for MS patients, being seen as more disabling than the physical limitations of MS. 38 Further, mood disorders in MS patients have been widely associated with an elevated risk of clinical relapse. 39
It is possible that inflammation associated with MDD involves signalling through a pattern recognition receptor (PRR), such as the Toll‐like receptor (TLR) family. Indeed, elevated TLR expression was observed in peripheral blood monocular cells (PBMCs) of MDD patients. 40 Due to their immune adjuvant properties on DCs, signalling via TLRs by molecular patterns from both pathogens (PAMPs) has been implicated in the pathogenesis of autoimmune diseases, such as MS 20 , 41 . Ligands for TLR2 (lipopeptides) and TLR4 (lipopolysaccharides)cy from pathogens can have an adverse impact on MS by increasing the production of IL‐1β, IL‐6 and IL‐23 by DCs. 42 , 43 Furthermore, elevated IL‐6 levels can damage the function of regulatory T‐cell subsets, such as the release of anti‐inflammatory cytokine IL‐10. 44 , 45 . Interestingly, elevated frequency of circulating Th17 cell subsets and Treg cells expressing functional TLR has been documented in MS patients. 20 , 46 Ferreira et al. 46 demonstrated a positive correlation between the frequency of TLR2+ and TLR4+Th17 cell subsets and the number of active brain lesions and neurological disabilities in RRMS patients.
One of the main theories of MDD suggests that this pathology is associated with a reduction in monoamine production, particularly serotonin (5‐HT, 5‐hydroxytryptamine). 47 Apart from its role in regulating mood, cognition, sleep and appetite, this neurotransmitter plays different immunomodulatory roles. 48 A recent study published by our group demonstrated the ability of 5‐HT to decrease in vitro the proportion of Th1/Tc1 and Th17/Tc17 cells while elevating the frequency of different regulatory T‐cell subsets in MS patients. 49 Although these findings are interesting, so far, no study evaluated the impact of both 5‐HT and treatment of MDD with selective serotonin reuptake inhibitors (SSRIs) on functional profile of CD4+ and CD8+ T cells expressing TLRs in response to different ligands for these PRRs.
MATERIALS AND METHODS
Patients
For our study, 50 RRMS patients, with (n = 25) or without (n = 25) major depressive disorder (MDD), were recruited from January 2017 to March 2020 from Gaffrée e Guinle University Hospital/UNIRIO (Rio de Janeiro, Brazil). Among MDD patients, the severity of depressive symptoms was determined according to the Beck Depression Inventory (BDI) (Table 1). All patients had been in clinical remission phase for at least 6 months and were undergoing disease‐modifying therapy (DMT). Patients undergoing IFN‐beta therapy were excluded as this drug has been associated with depression. 34 The impact of depression treatment on some immune parameters was investigated in 10 MS/MDD patients 6 months after therapy with selective serotonin reuptake inhibitors (SSRIs) (fluoxetine, 20 mg/day). This duration for treatment was chosen because it is sufficient for mood stabilization to take place using SSRIs. Demographic data such as gender and age at disease are shown in Table 1. Other autoimmune diseases were excluded through clinical and serological testing. Smokers were also excluded. The neurological disability status of patients was evaluated at the time of blood sampling by one of the authors (C.V.) and was scored according to the Expanded Disability Status Scale (EDSS). 50 To avoid confounding factors related to depression associated with advanced neurodegeneration, no patient recruited presented EDSS ≥6. For assays, 25 healthy subjects matched by age and gender were recruited to participate in this study. Written informed consent was obtained from each individual following a complete description of the study. The study was approved by the Ethical Committee for Research on Human Subjects of the Federal University of the State of Rio de Janeiro.
Table 1.
Demographic and clinical features of the MS patients and healthy subjects
| HS a | Patients b | ||
|---|---|---|---|
| MS | MS/MDD | ||
| No. of subjects (n) | 25 | 25 | 25 |
| Gender, female/male (n) | 15/10 | 15/10 | 15/10 |
| Age [(years), mean ±SD] | 37.8 ± 10.3 | 39 ± 13 | 36.2 ± 13.1 |
| Disease duration [(years), mean ±SD] c | NA g | 7.9 ± 5.5 | 9.3 ± 6.1 |
| EDSS [median (range)] d | NA | 3 (0‐4.5) | 4 (0‐5.5) |
| BDI f (%) | |||
| Mild | 0 | 0 | 16 |
| Moderate | 0 | 0 | 28 |
| Severe | 0 | 0 | 56 |
| Treatment time with DMTs [years (range)] e | NA | 2.7 (1–7.3) | 2.3 (1–5.8) |
Data from healthy subjects.
Data from relapsing–remitting multiple sclerosis (RRMS) with (MS/MDD) or without (MS) major depressive disorder (MDD) in remission phase. Age (years) refers to age when the blood samples were collected.
Disease duration refers to the number of years since disease onset.
EDSS, Expanded Disability Status Scale.
Severity of depressive symptoms was determined according to the Beck Depression Inventory (BDI).
Treatment time in years with disease‐modifying therapy (DMT) [natalizumab (n = 7 MS and 8 MS/MDD), dimethyl fumarate (n = 7 MS and n = 7 MS/MDD), fingolimod (n = 05 MS and n = 6 MS/MDD) and glatiramer acetate (n = 5 MS and n = 5 MS/MDD)].
Not analysed.
Cell cultures
Peripheral blood mononuclear cells (PBMCs) were separated by a Ficoll–Paque gradient. In some experiments, enriched CD4+ and CD8+ T cells were obtained via negative selection using magnetic columns according to the manufacturer's instructions (EasySepTM, StemCell Technology, Canada). The purity of CD4+ and CD8+ T cells was >98%, as measured by flow cytometry (data not shown). The PBMCs (1 × 106/ml), or purified CD4+ and CD8+ T cells (0.5 × 106/ml), were cultured in triplicate in the presence of RPMI‐1640 medium supplemented with 2 μM of L‐glutamine (GIBCO, Carlsbad, CA, USA), 10% of fetal calf serum, 20U/ml of penicillin, 20 μg/ml of streptomycin and 20 mM of HEPES buffer for 2 days at 37°C and 5% CO2. The effect of different PAMPs, all obtained from InvitroGen (San Diego, CA, USA), was evaluated after addition of agonists for TLR2 [synthetic triacylated lipopeptide (Pam3Csk4, 1 μg/ml)], TLR4 [lipopolysaccharide (LPS, 100 ng/ml) from Escherichia coli], TLR5 (flagellin (FLA, 1 µg/ml)] or TLR9 [CpG oligodeoxynucleotides (ODN M362, 1 μM/ml)]. These concentrations were chosen from a previous study conducted by Voo et al. 51 and Ferreira et al. 20 . The effect of serotonin (5‐HT) on cytokine production was evaluated after adding 200 ng/ml of 5‐HT at the beginning of the incubation period. The serotonin concentration used here was based on the study performed by Soga et al. 52 and represents the physiological brain concentration of serotonin. 53
Proliferation assay
The proliferation of CD4+ and CD8+ T cells (0.5 × 106/ml) in response to different TLR ligands was measured by [3H] thymidine incorporation, added to cultures at 4 μCi/well 8 h prior to the conclusion of the 2‐day incubation period. This duration was determined via temporal kinetics (24, 48 and 72 h) evaluating the PBMC proliferation in response to TLR ligands (data not shown). The cells were harvested in glass fibre filters in an automatic cell harvester, and radioactive incorporation was measured using a liquid scintillation counter. The results were shown as mean ±sd of counts per minute (cpm).
Flow cytometry analysis
Mouse anti‐human monoclonal antibodies (mAbs) to CD3‐PE‐Cy5.5, CD4‐FITC, CD8‐PE, CD14‐APC, TLR2‐APC, TLR4‐APC and IL‐17‐PE‐Cy7 and all isotype control antibodies were purchased from eBioscienceTM (Thermo Fischer Scientific). Whole peripheral blood of each subject was stimulated in 24‐well flat‐bottom plates (2 ml/well) with phorbol 12‐myristate 13‐acetate (PMA, 20 ng/ml; Sigma‐Aldrich) plus ionomycin (IO, 600 ng/ml; Sigma‐Aldrich) at 37 °C in a humidified 5% CO2 incubator for 4 h. For cytokine measurement optimization, brefeldin A (10 μg/ml; Sigma‐Aldrich) was also added. Briefly, whole blood cells were incubated with various combinations of mAbs for surface markers for 30 min at room temperature in the dark, according to the manufacturer's instructions. The cells were washed with PBS+2%FBS, and then, the whole blood cells were lysed with Fix/Lyse solution (eBiosciences) for 10 min at room temperature before cell permeabilization, performed by incubating cells with Cytofix/Cytoperm solution (BD Pharmingen, San Diego, CA) at 4°C for 20 min. After washing, the mAb for IL‐17‐PE‐Cy7 was added and incubated for 30 min at 4°C. The cells were acquired on Attune NxT flow cytometers (Thermo Fisher Corporation) and analysed using FlowJo. Isotype control antibodies and single‐stained samples were used to periodically check the settings and gates on the flow cytometer. After acquisition of 200,000 events, lymphocytes were gated based on forward and side scatter properties after the exclusion of dead cells, using propidium iodide [mean 3.57 (range 0.9 to 6.1%)] and doublets. Additionally, gated cells were negative for CD14 marker (Fig. S1).
Quantification of in vivo and in vitro cytokines
Plasma cytokine levels were quantified by ELISA technique using OptEIA ELISA kits (BD, Pharmingen, San Diego, CA), according to the manufacturer's instructions. Each ELISA was performed using pairs of antibodies against IL‐1β, TNF‐α, IL‐6, IL‐17, IFN‐γ and IL‐10. The reaction was revealed with streptavidin–horseradish peroxidase, using 3,3′,5,5′‐tetramethylbenzidine (TMB) as a substrate. Recombinant human cytokines, at concentrations ranging from 3.5 to 500 pg/ml, were used to construct standard curves. The in vitro cytokine production by cell cultures stimulated for 2 days with different TLR ligands was quantified by Luminex using human Th1/Th2/TH17 Cytokine 18‐Plex Panel (InvitroGen, San Diego, CA, USA) according to the manufacturer's instructions. This multiplex bead‐based enzyme‐linked immunosorbent assay was used to measure IFN‐γ, TNF‐α, GM‐CSF, IL‐1β, IL‐6, IL‐8, IL‐10, IL‐12, IL‐18, IL‐21, IL‐22, IL‐23 and IL‐17A (IL‐17) in the supernatants from TLR‐activated immune cells.
Statistical analysis
The statistical analysis was performed using Prism 8.0 software (GraphPad Software). All immunological evaluations were done triplicate in each individual, and the intra‐assay variability ranged from 8% to 17.5% (median value of 9.7%) as calculated by the software above. Comparisons between immune assays in the cell cultures from the three different groups (control group, MS and MS/MDD patients) were performed with two‐way ANOVA followed by the Tukey test for data with Gaussian distribution and the Kruskal–Wallis test followed by Dunn's test for data without Gaussian distribution. Also, the results were corrected by the Bonferroni test. The non‐parametric Mann–Whitney U‐test and Student's t‐test were applied to determine whether the two groups were statistically different for non‐parametric and parametric variables, respectively. Correlations between parametric and non‐parametric variables were investigated using Pearson's and Spearman's correlations, respectively. Significance in all experiments was at p < 0.05.
RESULTS
Major depression elevated the in vivo and in vitro levels of pro‐inflammatory cytokines in MS patients
As shown in Table 1, 50 RRMS patients were recruited to the present study, 25 with (MS/MDD) and 25 without (MS) major depressive disorder (MDD), with both groups being matched for age and gender. According to BDI, the depressive symptoms among MDD patients were stratified into mild (n = 4, 16%), moderate (n = 7, 28%) and severe (n = 14, 56%). The mean duration of the disease was similar between the two patient subgroups, as well as the EDSS score. In general, no important difference was observed concerning the DMT scheme in both subgroups [natalizumab (7 MS × 8 MS/MDD), dimethyl fumarate (7 MS × 7 MS/MDD), fingolimod (5 MS × 6 MS/MDD) and glatiramer acetate (6 MS × 4 MS/MDD)]. Also, no statistical difference was observed with regard to immunological assays among patients under different DMT schemes (data not shown). In some experiments, samples from 25 age‐ and gender‐matched healthy subjects (HS) were also used.
Here, the first immune parameter evaluated was the plasma levels of IL‐1β, IL‐6, IL‐10, IL‐17, IFN‐γ and TNF‐α in MS and MS/MDD patients. Notably, the concentrations of all cytokines were almost undetectable in the HS subjects (data not shown). Among the patients, not only IL‐1β and IL‐6 levels were significantly higher in the MS/MDD group (Figure 1A), but also their concentration was also directly correlated with neurological disabilities determined by the EDSS score (Figure 1B).
Figure 1.
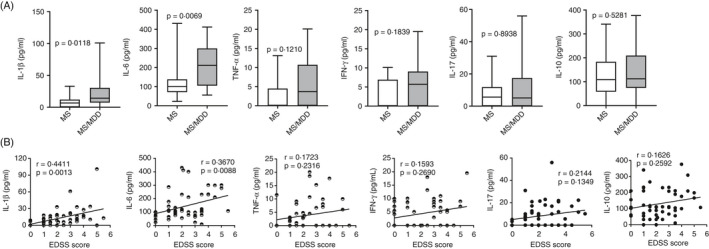
Impact of MDD on circulating levels of cytokines in patients with MS and their relationship with the degree of neurological disability. In (A), plasma of MS patients with (n = 25) or without (n = 25) MDD was submitted to ELISA for quantification of IL‐1, IL‐6, TNF‐α, IFN, IL‐17 and IL‐10 cytokines. Data are shown as mean ±SD of seven independent experiments with 3 to 4 MS and 3 to 4 MS/MDD samples per experiment. Significance was calculated by comparing MS versus MS/MDD, and thepvalues are indicated in the figure. In (B), the correlation between cytokine levels and the EDSS score at the time of blood collection is shown
Elevated IL‐1β and IL‐6 levels are classically produced by innate immune cells activated via pattern receptors, such as members of the TLR family. 54 Here, higher levels of IL‐1β (Figure 2A), IL‐6 (Figure 2B), IL‐8 (Figure 2C), IL‐18 (Figure 2D), GM‐CSF (Figure 2F), IL‐23 (Figure 2) and IL‐17 (Figure 2J) were released by PBMC cultures in MS/MDD patients than in MS ones after addition of TLR2 (Pam3C), TLR4 (LPS) and TLR5 (FLA) ligands, but not TLR9 agonist (ODN). The same pattern was observed for TNF‐α (Figure 2E) and IL‐22 (Figure 2L) production by MDD‐derived cell cultures in response to Pam3C and LPS. In general, TLR2 and TLR4 agonists were more effective in inducing cytokine responsiveness than TLR5 and TLR9 ligands. Additionally, among TLR2 and TLR4 ligands, Pam3C was more potent at enhancing IL‐17 (Figure 2J; p = 0.0009) and IL‐22 (Figure 2L; p = 0.0018) production, while LPS showed greater capacity to upregulate IL‐6 release (Figure 2B; p = 0.0152) in MS/MDD‐derived PBMC cultures (Figure 2). No difference was observed between the two subgroups, or between the different PAMPs, in terms of secretion of IL‐12 (Figure 2G), IFN‐γ (Figure 2H) and IL‐21 (Figure 2K). As expected, lower levels of all pro‐inflammatory cytokines were quantified in the supernatants from PBMC cultures from HS subjects in comparison with MS and MS/MDD patients (data not shown). Although the TLR9 ligand was less effective at inducing inflammatory cytokine production, ODN, as well as LPS, was the best PAMPs to elevate IL‐10 production. Nonetheless, the levels of this anti‐inflammatory cytokine were lower in the cell culture from MS/MDD patients (Figure 2M).
Figure 2.
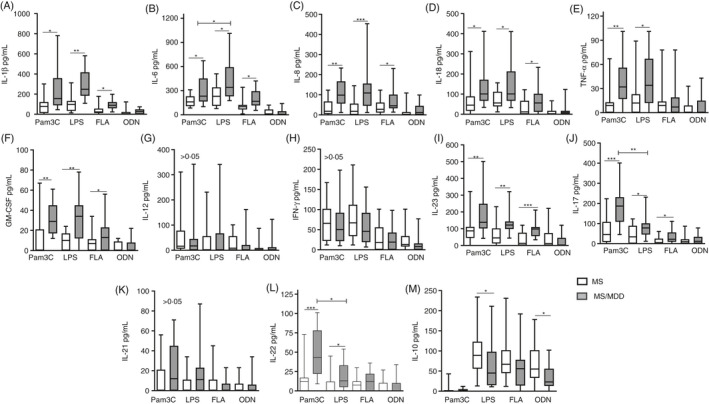
Comparative analysis of cytokine production by PBMC of patients with MS with or without MDD in response to different TLR ligands. The cytokine content in the supernatants collected from PBMC cultures (1 × 106/ml) of MS patients with (MS/MDD,n = 25) or without (MS,n = 25) MDD was maintained for 2 days in the absence or presence of lipopolysaccharide (LPS, 100 gg/ml), Pam3Csk4 (Pam3C, 1 lg/ml), flagellin (FLA, 1 µg/ml) and oligodeoxynucleotide (ODN, 1 µM/ml). The cytokine concentrations [(A) IL‐1β, (B) IL‐6, (C) IL‐18, (D) IL‐18, (E) TNF‐α, (F) GM‐CSF, (G) IL‐12, (H) IFN‐γ, (I) IL‐23, (J) IL‐17, (K) IL‐21, (L) IL‐22 and (M) IL‐10] were determined using Luminex. Data are shown as mean ±SD of seven independent experiments with 3 to 4 MS and 3 to 4 MS/MDD samples per experiment. Significance was calculated by comparing MS versus MS/MDD, and (*), (**) and (***) indicatepvalues <0.05, <0.001 and <0.0001
Serotonin down‐modulates the in vitro production of pro‐inflammatory cytokines but elevates IL‐10 release
One of the main theories of MDD pathology suggests a reduction in monoamine production, particularly serotonin (5‐HT), a neurotransmitter with immunological functions. 47 In the present study, physiological concentration of 5‐HT (200 ng/ml) reduced the production of IL‐1β (Figure 3A), IL‐6 (Figure 3B), IL‐18 (Figure 3D), IL‐23 (Figure 3G) and IL‐17 (Figure 3H) by PBMC from MS and MS/MDD patients in response to Pam3C and LPS. Only in MS/MDD patients did 5‐HT decrease the release of IL‐8 (Figure 3C) and GM‐CSF (Figure 3F). 5‐HT did not affect TNF‐α production (Figure 3E). In cell cultures from both experimental groups, while 5‐HT decreased IL‐22 production (Figure 3I) induced by Pam3C, this neurotransmitter potentiated IL‐10 (Figure 3J) release in response to LPS.
Figure 3.
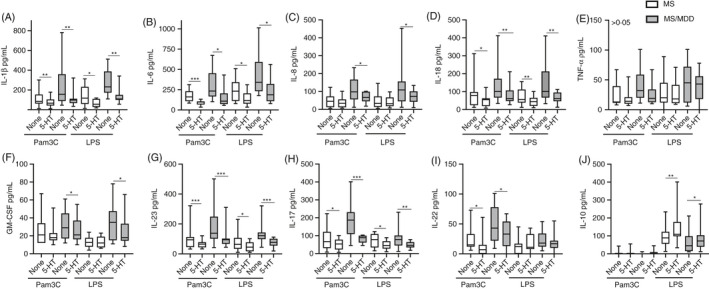
Modulatory effects of serotonin (5‐HT) on cytokine production by PBMC of patients with MS with or without MDD in response to TLR2 and TLR4 ligands. The PBMC cultures (1 × 106/ml) from MS patients with (MS/MDD,n = 25) or without (MS,n = 25) MDD were activated for 2 days with TLR2 (Pam3C, 1 µg/ml) and TLR4 (LPS, 100 ng/ml). In some cultures, 5‐HT (200 ng/ml) was added and the cytokine production [(A) IL‐1β, (B) IL‐6, (C) IL‐18, (D) IL‐18, (E) TNF‐α, (F) GM‐CSF, (G) IL‐23, (H) IL‐17, (I) IL‐22 and (J) IL‐10] assayed by Luminex. Data are shown as mean ±SD of seven independent experiments with 3 to 4 MS and 3 to 4 MS/MDD samples per experiment. Significance was calculated by comparing MS versus MS/MDD, and (*), (**) and (***) indicatepvalues <0.05, <0.001 and <0.0001
Higher TLR2 and TLR4 expression and elevated cytokine production were observed on CD4+ and CD8+ T cells from MS patients with major depression
Previous results demonstrated an elevated responsiveness of PBMC from MS/MDD patients to TLR2 and TLR4 ligands. Although TLRs are classically expressed on innate immune cells, they are also expressed on chronically activated T cells. 55 Following the gate strategy shown in Figure 4A, a higher percentage of CD4+ and CD8+ T cells capable of expressing TLR2 (Figure 4B) and TLR4 (Figure 4E) was observed in MS and MS/MDD patients than in HS. Although the proportion of these cell subsets showed no difference between the two patient subgroups, the mean fluorescence intensity (MFI) of TLR2 (Figure 4C) and TLR4 (Figure 4F) for CD4+ and CD8+ T cells was significantly higher in MS/MDD patients. In addition, the percentage of IL‐17‐secreting CD4+ and CD8+ T cells positive for TLR2 (Figure 4D) and TLR4 (Figure 4G) was also significantly higher in the cultures from MS/MDD than MS patients. As expected, the frequency of these IL‐17+ TLR+ T‐cell subsets was significantly lower in HS (Figure 4D, G).
Figure 4.
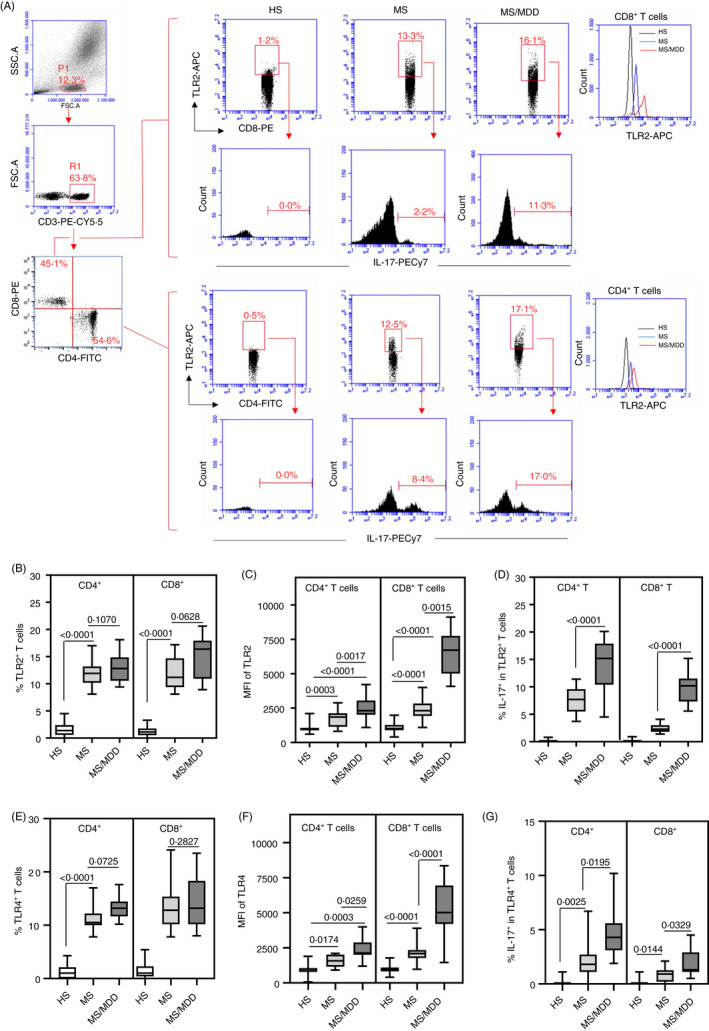
Comparison of the proportion of circulating IL‐17‐secreting CD4+and CD8+T cells expressing TLR2 and TLR4 in MS patients with or without MDD. The mean proportion of CD4+and CD8+T cells positive for TLR2 (B) and TLR4 (E), as well as MFI of TLR2 (C) and TLR4 (F) for these cells, was determined by cytometry following representative dot plots and histograms shown in panel A after acquisition of 200,000 events in samples obtained from healthy subjects (HS,n = 25) and MS patients with (MS/MDD,n = 25) or without (MS,n = 25) MDD. The mean frequency of IL‐17‐secreting CD4+and CD8+T cells among TLR2+(D) and TLR4+(G) cells was also evaluated after activation of those cells with PMA plus ionomycin. Data are shown as mean ±SD of seven independent experiments with 3 to 4 samples per experiment from each group (HS, MS and MS/MDD) (Figure2S). Significance was calculated by comparing different cell culture conditions from HS, MS and MS/MDD
In order to investigate the role of TLR ligands in directly modulating the behaviour of T lymphocytes, CD4+ and CD8+ T cells from MS patients were purified and cultured for 2 days with different TLR agonists. As demonstrated in Figure 5A, the uptake of [3H] thymidine by these lymphocytes in response to Pam3C and LPS, but not to FLA and ODN, was significantly higher in MS/MDD patients. Regarding cytokine production in response to TLR2 and TLR4 ligands, the occurrence of major depression is associated with the release of IL‐6 and IL‐17 by CD4+ (Figure 5B) and CD8+ (Figure 5C) T cells. Also, IL‐1β production by CD4+ (Figure 5B) and CD8+ (Figure 5C) T cells, as well as TNF‐α and IL‐22 release by CD4+ T cells (Figure 5B), in response to Pam3C was significantly higher in MS/MDD patients. No difference was observed for IL‐21 and IFN‐γ production. As compared to the TLR4 ligand, TLR2 agonist was a weak inducer of IL‐10, and the production of this anti‐inflammatory cytokine by LPS‐activated CD4+ (Figure 5B) and CD8+ (Figure 5C) T cells was lower in the MS/MDD group. Like PBMC cultures, 5‐HT diminished IL‐6 and IL‐17 release by both CD4+ (Figure 6A) and CD8+ (Figure 6B) T‐cell cultures from MS (O) and MS/MDD (•) patients in response to Pam3C and LPS. Moreover, this neurotransmitter upregulated IL‐10 release by LPS‐activated CD4+ T cells (Figure 6A). In these cell cultures, 5‐HT did not change the level of expression of TLR2 and TLR4 (data not shown).
Figure 5.
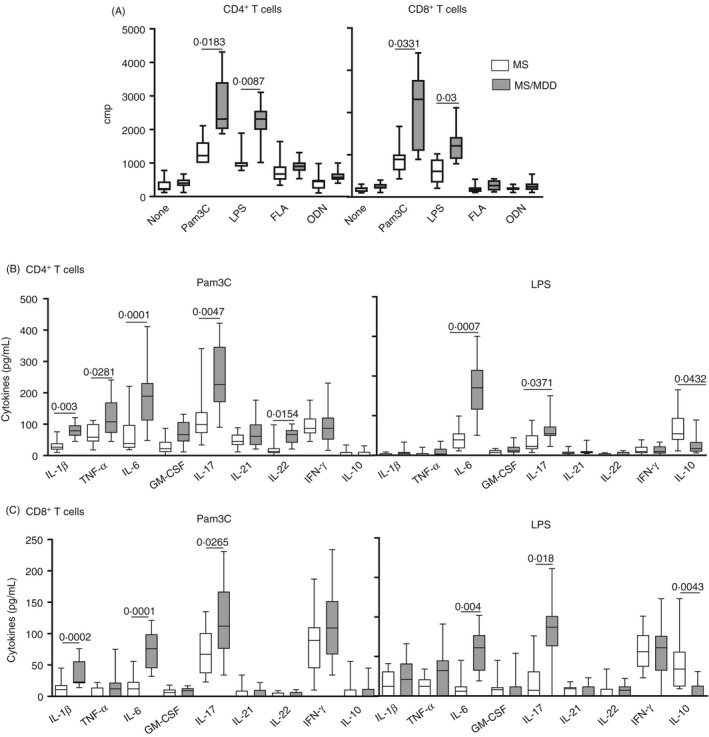
Impact of MDD on proliferative response and cytokine production by CD4+and CD8+T cells from MS patients in response to different TLRs. Purified CD4+and CD8+T cells (0.5 × 106/ml), obtained from MS patients with (MS/MDD,n = 15) or without (MS,n = 15) MDD, were maintained for 2 days in the presence of medium (none), lipopolysaccharide (LPS, 100 gg/ml), Pam3Csk4 (Pam3C, 1 lg/ml), flagellin (FLA, 1 µg/ml) or oligodeoxynucleotide (ODN, 1 µM/ml). In (A), the cell proliferation was determined by [3H]] TdR uptake. The cytokine contents in the supernatants from (B) CD4+and (C) CD8+T‐cell cultures were evaluated by Luminex. Data are shown as mean ±SD of seven independent experiments with 2 to 3 samples per experiment from MS and MS/MDD patients (FigureS3). Significance was calculated, and thepvalues are shown in the figure
Figure 6.
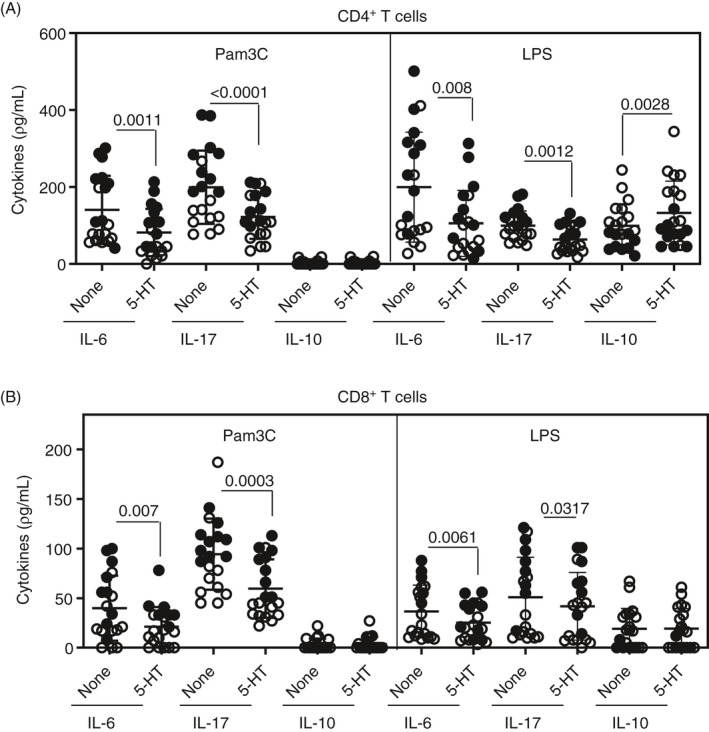
Effect of serotonin (5‐HT) on IL‐6, IL‐17 and IL‐10 produced by CD4+and CD8+T cells from MS and MS/MDD patients in response to LPS and Pam3C. Purified (A) CD4+and (B) CD8+T cells (0.5 × 106/ml), obtained from MS [n =10 (O)] and MS/MDD [n =10 (•)] patients, were maintained for 2 days in the presence LPS (100 ng/ml) or Pam3Csk4 (Pam3C, 1 µg/ml). The cytokine levels were evaluated by Luminex. Data are shown as mean ±SD of five independent experiments with 4 samples per experiment (2 MS and 2 MS/MDD patients). Significance was calculated by comparing different cell culture conditions from MS versus MS/MDD patients, and thepvalues are shown in the figure
With regard to EDSS score, a positive and significant correlation was observed between the levels of IL‐6, IFN‐γ, IL‐17 and IL‐21 produced by Pam3C‐stimulated CD4+ T cells and neurological disabilities (Table 2). The same correlation was seen between the production of IL‐6 and IL‐17 in those cultures in response to LPS. With regard to CD8+ T cells, a direct and significant correlation was observed between the EDSS score and IL‐6 levels induced by Pam3C and LPS, as well as IL‐17 concentration in response to the TLR2 ligand (Table 2). Furthermore, patients with a higher degree of neurological disability tend to have a higher frequency of cells IL‐17‐secreting (CD4+ and CD8+) T cells positives for TLR2 (p = 0.0588) e TLR4 (p = 0.0617) (data not shown). In contrast, the levels of IL‐10 by Pam3C‐activated CD4+ T cells were significantly lower in those patients with higher EDSS score (Table 2).
Table 2.
The correlation between cytokines produced by TLR‐activated CD4+ and CD8+ T cells and neurological disabilities in RRMS patients
| Pam3C | LPS | |||
|---|---|---|---|---|
| r | p | r | p | |
| CD4+ T cells | ||||
| IL−1β | 0.3986 | 0.0817 | 0.2190 | 0.3535 |
| IL−6 | 0.5633 | 0.0097 | 0.6013 | 0.0050 |
| TNF‐α | 0.3669 | 0.1116 | 0.1814 | 0.4440 |
| IFN‐γ | 0.5390 | 0.0142 | 0.3989 | 0.0814 |
| IL−17 | 0.5725 | 0.0083 | 0.6175 | 0.0037 |
| IL−21 | 0.4807 | 0.0319 | 0.3344 | 0.1496 |
| IL−22 | 0.3001 | 0.1988 | 0.3165 | 0.1740 |
| GM‐CSF | 0.3515 | 0.1285 | 0.3391 | 0.1486 |
| IL−10 | −0.5013 | 0.0243 | −0.7282 | 0.0003 |
| CD8+ T cells | ||||
| IL−1β | 0.2973 | 0.2031 | 0.2639 | 0.2608 |
| IL−6 | 0.5053 | 0.0231 | 0.6143 | 0.0040 |
| TNF‐α | 0.2165 | 0.3593 | 0.3753 | 0.1030 |
| IFN‐γ | 0.1905 | 0.4212 | 0.3783 | 0.1431 |
| IL−17 | 0.4905 | 0.0281 | 0.3970 | 0.0831 |
| IL−21 | 0.2601 | 0.2681 | 0.3166 | 0.1738 |
| IL−22 | 0.0569 | 0.8115 | 0.2410 | 0.3060 |
| GM‐CSF | 0.2686 | 0.2522 | 0.3701 | 0.1084 |
| IL−10 | −0.2538 | 0.2803 | 0.1175 | 0.6217 |
The levels of different cytokines produced by CD4+ and CD8+ T‐cell cultures maintained for 48 h in the presence of Pam3C (TLR2 agonist) and LPS (TLR4 agonist) were correlated with neurological disabilities, determined by EDSS score, in 20 RRMS patients (10 without and 10 MDD).
Bold indicate significant values after comparison (p <0.05).
The depression treatment with SSRIs affects the expression of TLR2 and TLR4, as well as cytokine production by CD4+ and CD8+ T cells
Previous results have demonstrated the ability of serotonin (5‐HT) to negatively modulate the in vitro production of various pro‐inflammatory cytokines in response to Pam3C and LPS. Our final objective was to determine the impact of depression treatment on the TLR2 and TLR4 expression and cytokine production by MS/MDD‐derived CD4+ and CD8+ T cells. Therefore, blood samples from 10 MS/MDD patients (4 moderate and 6 severe MDD) were collected just before (t0) and 6 months after the treatment with serotonin reuptake inhibitors (SSRIs). As demonstrated in Figure 7, SSRIs diminished not only the percentage of circulating CD4+ (Figure 7A, B) and CD8+ (Figure 7A, D) T cells positive for TLR2 and TLR4, but also the MFI of TLR2 and TLR4 per CD4+ (Figure 7A, C) and CD8+ (Figure 7A, E) T cells. Moreover, SSRIs decrease the production of IL‐1β, IL‐6, TNF‐α and IL‐17 by Pam3C‐ or LPS‐activated CD4+ T cells (Figure 7F). Depression treatment also attenuated the secretion of IL‐1β, IL‐6 and IL‐17 by CD8+ T cells stimulated with TLR2 and TLR4 ligands (Figure 7G). On the other hand, SSRI therapy increased the ability of LPS‐stimulated CD4+ T cells to produce IL‐10 (Figure 7F). Notably, circulating levels of IL‐1β (20.1 ± 19.7 pg/ml × 11.8 ± 10.1 pg/ml, p = 0.0211) and IL‐6 (201.6 ± 113 pg/ml × 78 ± 61 pg/ml, p = 0.0071) were also lower after MDD treatment.
Figure 7.
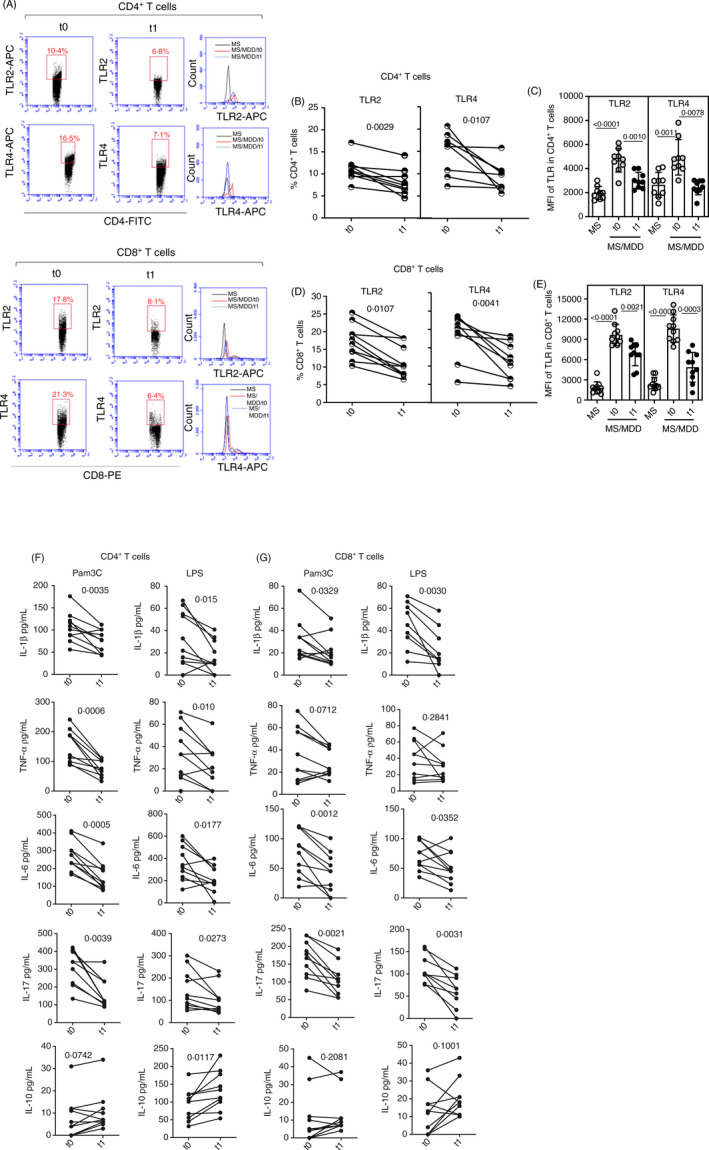
Impact of MDD treatment on TLR2 and TLR4 expression and cytokine release by CD4+and CD8+T cells from MS patients. Purified CD4+and CD8+T cells (0.5 × 106/ml), obtained from 10 MS/MDD patients just before (t0) and 6 months (t1) after treatment with SSRIs, were analysed for TRL2 and TLR4 expression by cytometry following the strategy shown in the panel A. In (B) and (D), the mean frequency of CD4+and CD8+T cells positive for TLR2 and TLR4, respectively, is shown. In (C) and (E), the intensity of TLR2 and TLR4 expression on CD4+and CD8+T cells, respectively, is shown. In (F) and (G), the cytokines released by (F) CD4+(G) and CD8+T cells after stimulation by Pam3C (1 µg/ml) and LPS (100 ng/ml) for 2 days are presented. Data are shown as mean ±SD of five independent experiments with 4 samples per experiment (2 MS and 2 MS/MDD patients). Significance was calculated by comparing different cell culture conditions from MS versus MS/MDD, and thepvalues are shown in the figure
DISCUSSION
MS is a heterogeneous multifactorial autoimmune disease influenced by environmental factors that can affect T‐cell behaviour, such as with major depressive disorder (MDD). The association between MS and depression has been known for some time 34 , and lifetime prevalence of MDD in those patients has been estimated at almost three times the rate as the general population. 34 The results presented here are original and reveal the impact of MDD in favouring the expansion of circulating encephalitogenic‐like T cells with enhanced responsiveness to mainly TLR2 and TLR4 ligands. This phenomenon should be associated with the known imbalance of cytokines in MDD subjects.
Psychological stress is associated with elevated plasma levels of pro‐inflammatory cytokines, such as IL‐1β, IL‐6 and TNF‐α 36 , and in the present study, higher plasma levels of IL‐1β and IL‐6 were dosed in MS/MDD patients as compared to just MS patients. This difference was not related to mean time of disease duration and the type of DMT therapy. Notably, all patients stated that they were taking the medication regularly.
Classically, IL‐1β and IL‐6 are produced by activated innate immune cells following activation of PRRs, such as members of TLRs, by different PAMPs. 56 While TLR5 binds to flagellin, TLR2 and TLR4 recognize lipopolysaccharides (LPS) and lipopeptides, respectively. 54 In contrast, TLR9, like other endosomal TLRs, recognizes nucleic acid structures, which serve as molecular signatures for viral and bacterial infections. 57 Upon PAMP engagement, the myeloid differentiation primary response protein 88 (MyD88), an adaptor protein associated with the majority of TLRs, except TLR3, activates nuclear factor NF‐κB, which in turn triggers signalling cascades via phosphatidylinositol‐4,5‐bisphosphate 3‐kinase/protein kinase B (PI3 K/AKT) and Ras/mitogen‐activated protein kinase (MAPK). These events promote cell survival and proliferation, as well as cytokine production. 58 Here, the occurrence of major depression elevated the production of IL‐1β, IL‐6, IL‐8, IL‐18, GM‐CSF, TNF‐α, IL‐22, IL‐23 and IL‐17 by PBMC cultures from MS patients, mainly in response to TLR2 (Pam3C) and TLR4 (LPS) ligands. Among our TLR agonists, ODN was less efficient at inducing pro‐inflammatory cytokine production in MS and MS/MDD patients. We believe that this event is not primarily related to lower TLR9 expression, as some studies have demonstrated elevated expression of this PRR on PBMCs from both MS 41 and MDD 40 , 59 subjects.
This high responsiveness to TLR2 and TLR4 agonists may contribute to MS pathogenesis, as accessory cells in PBMC, particularly PAMP‐activated DCs, may lead to a breakdown in immunological tolerance through non‐specific activation of myelin‐specific T cells. 60 , 61 Indeed, the higher production of IL‐1β, IL‐6 and IL‐23 by monocytes and DCs in response to TLR2 and TLR4 agonists should favour the induction and expansion of pathogenic Th17 cell subsets implicated in neuronal lesions, such as those able to produce IL‐17, GM‐CSF and IFN‐γ. 62 Nonetheless, functional TLRs are also expressed on MS‐derived T cells. 20 , 46 Here, in line with those studies, elevated frequency of CD4+ and CD8+ T cells expressing TLRs has been observed in MS patients as compared to healthy subjects. Although the percentage of those T‐cell subsets showed no difference between MS and MS/MDD patients, the occurrence of major depression was associated with higher density of TLR2 and TLR4 molecules on the surface of CD4+ and CD8+ T cells. Moreover, the frequency of those cells capable of producing IL‐17 was also higher among depressed patients as compared to non‐depressed ones. Due to resource limitation, it was not possible to evaluate TLR5, TLR9 and other cytokines by cytometry. These findings suggested that higher TLR2 and TLR4 expression on T cells from MS/MDD patients could explain elevated responsiveness of these cells to Pam3C and LPS and that this phenomenon might be associated with depression. Indeed, elevated expression of TLR mRNA was not only observed in PBMC from MDD, but TLR4 mRNA levels were also associated with the severity of MDD. 63 We did not observe any correlation between the level of expression of TLR2 or TLR4 with the BDI score, a scale that estimates the severity of depression symptoms, which may be because the majority of our patients presented severe depression. The ability of Pam3C and LPS in elevating both lymphoproliferation and production of some Th17/Tc‐17‐related cytokines by MS‐derived CD4+ and CD8+ T cells reveals that TLR2 and TLR4 are functional. Furthermore, higher [3H] thymidine uptake and cytokine (IL‐1β, IL‐6, IL‐17, IL‐22 and TNF‐α) production were observed in the cell cultures from MS/MDD patients when compared to MS patients. These cytokines should influence MS pathogenesis.
High IL‐17 levels have been detected in the peripheral blood and cerebrospinal fluid of patients with RRMS during clinical relapses. 9 . Further, the expression of IL‐17 has been detected in astrocytes and oligodendrocytes in areas of active MS lesions. 64 Moreover, IL‐22, produced by myelin‐specific Th17 and Th22 cells, has been associated with both clinical relapses 23 and the number of active brain lesions 65 . Along with IL‐17 and IL‐22, IL‐1β, IL‐6 and TNF‐α might contribute to demyelination by inducing the production of free radicals derived from oxygen and metalloproteinase‐9 through microglia and migrant macrophages/DCs. 26
TLRs are involved in the pathogenesis of other Th17‐mediated autoimmune diseases, such as systemic lupus erythematosus (SLE) 66 and rheumatoid arthritis 67 . In patients suffering from SLE, high expression of TLR3 to TLR9 on T cells was related to disease activity. 61 In experimental autoimmune encephalomyelitis (EAE), the murine model for MS, Farez et al. 68 demonstrated a role for TLR2 in CNS inflammation. Reynolds et al. 69 revealed that the disease was attenuated in animals deficient in TLR2+CD4+ T cells. Further, high TLR4 expression was identified on mononuclear cells found in the CNS lesions of patients with MS. 70
Although our study was performed in patients during remission phase, the plasma levels of IL‐1β and IL‐6, as well as some cytokines, such as IL‐6 and IL‐17, produced by CD4+ T cells stimulated with Pam3C and LPS were also directly correlated with neurological disabilities. Also, higher release of IL‐6, by Pam3C and LPS, and IL‐17, in response to Pam3C, was observed in CD8+ T‐cell cultures from MS patients with the highest EDSS score. No correlation between BDI score and the level of neurological impairment was observed.
Beyond their involvement in MS relapses, elevated levels of cytokines, such as IL‐6 and IL‐22, might compromise the function of CD4+ CD25high FoxP3+ regulatory T cells (Tregs) and Tr1 cells (IL‐10+FoxP3−) in those patients. 44 , 45 , 64 Elevated IL‐6 levels have also been associated with functional damage to circulating Tregs in MDD subjects. 71 Although we did not analyse classical regulatory T‐cell markers, the occurrence of MDD was associated with lower production of IL‐10 by both PBMC and CD4+ and CD8+ T cells in response to LPS and ODN, the two best inducers of these anti‐inflammatory cytokines in our system. Although Pam3C is a weak IL‐10 inducer, the production of pro‐inflammatory cytokines following TLR2 signalling may also negatively impact Tregs. In MS patients, Nyirenda et al. 46 demonstrated not only an elevated TLR2 expression on Tregs, but also signalling through this PRR inhibited, in vitro, the suppressive action of these cells. Collectively, our findings suggest that the occurrence of MDD amplifies T‐cell responsiveness to exogenous (PAMPs) or endogenous (DAMPs) TLR ligands.
Systemic infections have been associated with the risk of MS relapse and increased radiological activity, which was accompanied by high activation of myelin‐specific T cells. 72 Moreover, other sources of TLR ligands can come from the intestine of patients with gut dysbiosis that has been implicated with imbalance of Treg/Th17 towards Th17 pro‐inflammatory response and increased intestinal permeability. 73 , 74 Finally, the presence DAMPs, such as HSP70 and HMGB, could also contribute to MS. These intracellular proteins are ubiquitously expressed and act as chaperones; thus, their expression is upregulated in response to physical and psychological stress. 75 The release of HSP70 and HMGB1 induces inflammation by activating immune cells through TLR2 and TLR4. 75 In MS patients, HSP70 is strongly expressed on brain lesions where it is found forming a immunogenic complex with myelin basic protein. 76
In line with our findings, imbalances in TLR expression appear to be associated with MDD. Alterations in TLR1 to TLR‐9 expression, especially TLR3 and TLR4, have been reported in the PBMC of MDD patients. 40 , 59 Interestingly, a short period of selective serotonin reuptake inhibitor (SSRI) therapy (4 weeks) significantly decreased the expression of mRNA for all TLRs and IL‐6 in PBMC, together with significant improvement in depressive symptoms. 40 This therapy acts by elevating the availability of serotonin (5‐HT), a neurotransmitter that regulates mood, cognition, sleep and appetite. 48 Serotoninergic neurotransmission is altered in the brain of MS patients, 77 and in RRMS patients, lower availability of 5‐HT may be related to excessive IFN‐γ and TNF‐α production that induces indoleamine 2,3‐dioxygenase (IDO), depleting tryptophan, an essential amino acid for serotonin synthesis. 78 Apart from its role as a CNS neurotransmitter, the 5‐HT, through a wide array of receptors (5‐HT1‐7) and in a dose‐dependent manner, carries out different actions on immune cells. 79 , 80 Snir et al. 48 showed that TNF‐α production by LPS‐activated mononuclear cells was inhibited following addition of 5‐HT2A receptor agonist. Also, in the murine model for rheumatoid arthritis, Chabbi‐Achengli et al. 81 demonstrated the 5‐HT2A receptor signalling reduced the severity of collagen‐induced arthritis (CIA) by reducing the frequency of type II collagen‐specific Th17 cells. In humans, 5‐HT2B receptor is expressed in monocyte‐derived CD1a+DC, and signalling through this receptor reduced the production of IL‐6, IL‐8, IL‐12 and TNF‐α by these cells activated via TLR2, TLR3 and TLR7/8. 82 Moreover, Muller et al. 83 demonstrated that 5‐HT4 receptor agonists decreased IFN‐γ production by mononuclear cells from healthy subjects. Although we have not investigated the subtype of 5‐HT receptor, the physiological concentration of this neurotransmitter was able to reduce the production of many pro‐inflammatory cytokines (IL‐1β, IL‐6, IL‐18, IL‐23 and IL‐17) by Pam3C‐ and LPS‐activated PBMC cultures from MS patients with or without MDD. Also, 5‐HT decreased the release of IL‐22, IL‐8 and GM‐CSF by PBMC from MS/MDD. Similarly, 5‐HT diminished the IL‐6 and IL‐17 released by purified CD4+ and CD8+ T cells from MS with or without MDD in response to TLR2 and TLR4 ligands. By contrast, this neurotransmitter elevated IL‐10 production by LPS‐activated CD4+ T cells in MS and MS/MDD patients. These findings are in agreement with our previous study 49 demonstrating the ability of 5‐HT to downregulate the production of Th17 cytokines and elevate the functional status of Tregs and Tr‐1 cells in cultures from MS patients following activation via CD28 and TCR. These in vitro effects of 5‐HT on cytokine profiles in the present study were not associated with a reduction in TLR2 and TLR4 expression on CD4+ or CD8+ T cells (data not shown). Nonetheless, the treatment of MS/MDD patients with SSRIs was very efficient at altering not only TLR2 and TLR4 expression, but also the cytokine profile of T cells. In the present study, regardless of the small sample size, 6 months of SSRI treatment diminished both the frequency of TLR2+ and TLR4+ and the intensity of expression of these PRRs on circulating CD4+ and CD8+ T cells. Furthermore, MDD therapy also reduced the levels of IL‐1β, IL‐6 and IL‐17 produced by CD4+ and CD8+ T cells, and TNF‐α by CD4+ T cells, in response to TLR2 and TLR4 ligands. On the other hand, SSRI therapy increased the ability of LPS‐stimulated CD4+ T cells to produce IL‐10. The inability of 5‐HT to modulate TLR2 and TLR4 expression on T cells may be explained for two non‐exclusive reasons, the first, the short cell culture time (2 days); and second, the capacity of SSRI therapy to reduce the expression of these TLRs is independent of its pharmacological action in increasing the availability of this neurotransmitter.
Mood disorders in MS patients have been widely associated with an elevated risk of clinical relapse. 39 Interestingly, treatment for depression with SSRIs was associated with a reduction in disease activity. 84 In EAE, lower severity of the disease following SSRI treatment was associated with reduced T‐cell activation. 85 Therefore, it is possible that this benefit is linked to the capacity of SSRIs to reduce pro‐inflammatory cytokines, such as IL‐17, and increase IL‐10 production by T cells. 86 , 87 , 88 , 89
Some studies have found no correlation between disease severity and depression. 90 , 91 However, depression in MS is associated with severe fatigue, and this ‘phantom symptom’, as it is not detected in neurological examinations, decreases physical and/or mental energy levels and may occur while at rest, during physical activity or in association with clinical attacks. 92 . Some studies have demonstrated higher plasma levels of TNF‐α and IL‐6, as well as elevated IFN‐γ, IL‐17 and IL‐22 released by T cells from MS patients with fatigue. 37 , 93 Although the BDI score is not very appropriate for fatigue evaluation, it comprises a question on fatigue‐related symptoms. As expected, patients who reported severe fatigue had higher immune reactivity to TLR2‐ and TLR4‐related PAMPs, as well as higher production of Th17 phenotype‐related cytokines (data not shown). A complete neuropsychological evaluation of this population, including fatigue, cognition and quality of life, is now being conducted by a professor from our group, to be correlated with our data on T‐cell behaviour.
There is a possibility that adverse events that increase the levels of TLR ligands, such as intestinal dysbiosis or cell damage, can impact the course of MS by favouring the expansion of encephalitogenic T cells, and this is more evident in patients suffering from recurrent, rather than single episode of MDD. At this moment, we are seeking to determine how these ligands can alter T‐cell responses to antigens in the myelin sheath before and after SSRI therapy.
Although the study has some weaknesses, such as our small sample size and the need for a prospective study, the events described reveal an expansion of circulating Th17/Tc17‐like cells expressing high levels of functional TLR2 and TLR4 in MS patients with MDD. The capacity of exogenous serotonin, and treatment with SSRI, to attenuate imbalances in cytokine networks produced by these T cells in response to TLR2 and TLR4 agonists might be additionally evaluated in a larger sample, because the findings will impact on the follow‐up of MS patients with major depression.
FUNDING INFORMATION
This work was supported by the Fundação de Amparo à Pesquisa Carlos Chagas Filho (FAPERJ, grant number: E‐26/202.940/2017) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, grant number: 301.780/2017‐0).
AUTHOR CONTRIBUTIONS
CCV, RMA and PMS performed patient monitoring and sample collection. CAMB and JI designed wrote the paper. MCS, TMK, MOSDC, HAAO and FA performed the experiments. CAMBL and ADR analysed the data. CAMB contributed with vital reagents. All authors participated in the critical revision of the manuscript, provided important intellectual input and read and approved the final version of the manuscript.
CONFLICT OF INTEREST
All authors declare that there are no conflicts of interest.
Supporting information
Figure S1. Flow cytometry supporting information data of PBMC.
Figure S2. Percentage of CD4+ and CD8+ T cells from healthy subjects (HS) and MS patients suffering, or not (MS), from major depressive disorder (MS/MDD) able to express TLR2 (A) and TLR4 (B) in each independent experiment are shown. Also, the MFI for TLR2 (C) and TLR4 (D), as well as the proportion of TLR2+ (E) and TLR4+ (F) IL‐17‐secreting (CD4+ and CD8+) T cells, are shown per experiment. Each experiment was performed with 3 or 4 subjects for each group of subjects (HS, MS and MS/MDD).
Figure S3. Dosage of different cytokines, per experiment, on supernatants collected from purified CD4+ and CD8+ T cells from MS patients suffering, or not (MS), from major depressive disorder (MS/MDD) following addition of Pam3Csk4 (Pam3C, 1 g/mL) and LPS (100 ng/mL). We dosed IL‐1β (A), TNF‐α (B), IL‐6 (C), GM‐CSF (D), IL‐17 (E), IL‐21 (F), IL‐22 (G), IFN‐ (H), IL‐10 (I). Each experiment was performed with 2 or 3 subjects for each group of patients (MS and MS/MDD).
Senior author: Cleonice A.M. Bento.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, D.B., upon reasonable request.
REFERENCES
- 1. Lisak RP. Multiple sclerosis: evidence is immunopathogenesis. Neurology. 1980; 30:99–105. [DOI] [PubMed] [Google Scholar]
- 2. M.S.I.F., 2013 . Atlas of MS 2013: Mapping Multiple Sclerosis Around the World. Multiple Sclerosis International Federation. Available at: https://www.msif.org/wp‐content/uploads/2014/09/Atlas‐of‐MS.pdf. Accessed June 1, 2020.
- 3. Milo R, Miller A. Revised diagnostic criteria of multiple sclerosis. Autoimmun Rev. 2014; 13:518–24. [DOI] [PubMed] [Google Scholar]
- 4. Schumacher GA, Beebe G, Kibler RF, Kurland LT, Kurtzke JF, McDowell F, et al. Problems of experimental trials of therapy in multiple sclerosis: report by the panel on the evaluation of experimental trials of therapy in multiple sclerosis. Ann N Y Acad Sci. 2006; 122:552–68. [DOI] [PubMed] [Google Scholar]
- 5. Matusevicius D, Kivisäkk P, He B, Kostulas N, Özenci V, Fredrikson S, et al. Interleukin‐17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler J. 1999; 5:101–4. [DOI] [PubMed] [Google Scholar]
- 6. Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, et al. Gene‐microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002; 8:500–8. [DOI] [PubMed] [Google Scholar]
- 7. Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM, et al. Interleukin‐17 production in central nervous system‐infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Clin Pathol. 2008; 172:146–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brucklacher‐Waldert V, Stuerner K, Kolster M, Wolthausen J, Tolosa E. Phenotypical and functional characterization of T helper 17 cells in multiple sclerosis. Brain 2009; 132:3329–41. [DOI] [PubMed] [Google Scholar]
- 9. Lovett‐Racke AE, Yang Y, Racke MK. Th1 versus Th17: Are T cell cytokines relevant in multiple sclerosis? Biochimica et Biophysica Acta (BBA) ‐ Molecular Basis of Disease. 2011; 1812(2):246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL‐23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005; 201:233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, et al IL‐6 programs TH‐17 cell differentiation by promoting sequential engagement of the IL‐21 and IL‐23 pathways. Nat Immunol. 2007; 8:967–74. [DOI] [PubMed] [Google Scholar]
- 12. Gyülvészi G, Haak S, Becher B. IL‐23‐driven encephalo‐tropism and Th17 polarization during CNS‐inflammation in vivo. Eur J Immunol. 2009; 39:1864–9. [DOI] [PubMed] [Google Scholar]
- 13. McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce‐Shaikh B, Blumenschein WM, et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17–producing effector T helper cells in vivo. Nat Immunol. 2009; 10:314–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. El‐Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, et al. The encephalitogenicity of TH17 cells is dependent on IL‐1‐ and IL‐23‐induced production of the cytokine GM‐CSF. Nat Immunol. 2011; 12:568–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mufazalov IA, Schelmbauer C, Regen T, Kuschmann J, Wanke F, Gabriel LA, et al. IL‐1 signaling is critical for expansion but not generation of autoreactive GM‐CSF + Th17 cells. EMBO J. 2016; 36:102–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krei K, Fredrikson S, Fontana A, Link H. Interleukin‐6 is elevated in plasma in multiple sclerosis. J Neuroimmunol. 1991; 31:147–53. [DOI] [PubMed] [Google Scholar]
- 17. Vaknin‐Dembinsky A, Balashov K, Weiner HL. IL‐23 Is Increased in Dendritic Cells in Multiple Sclerosis and Down‐Regulation of IL‐23 by Antisense Oligos Increases Dendritic Cell IL‐10 Production. J Immunol. 2006; 176:7768–74. [DOI] [PubMed] [Google Scholar]
- 18. Shajarian M, Alsahebfosoul F, Etemadifar M, Sedaghat N, Shahbazi M, Firouzabadi FP, et al. M. IL‐23 Plasma level measurement in relapsing remitting multiple sclerosis (RRMS) patients compared to healthy subjects. Immunol Invest. 2014; 44:36–44. [DOI] [PubMed] [Google Scholar]
- 19. Kebir H, Ifergan I, Alvarez JI, Bernard M, Poirier J, Arbour N, et al. Preferential recruitment of interferon‐γ‐expressing TH17 cells in multiple sclerosis. Ann Neurol. 2009; 66:390–402. [DOI] [PubMed] [Google Scholar]
- 20. Ferreira TB, Hygino J, Wing AC, Kasahara TM, Sacramento PM, Camargo S, et al. Different interleukin‐17‐secreting Toll‐like receptor+ T‐cell subsets are associated with disease activity in multiple sclerosis. Immunology. 2017; 154:239–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xu W, li R, Dai Y, Wu A, Wang H, Cheng C, et al. IL‐22 secreting CD4+ T cells in the patients with neuromyelitis optica and multiple sclerosis. J Neuroimmunol. 2013; 261:87–91. [DOI] [PubMed] [Google Scholar]
- 22. Jia L, Wu C. The Biology and Functions of Th22 Cells. T Helper Cell Differentiation and Their Function. 2014; 841:209–30. [DOI] [PubMed] [Google Scholar]
- 23. Rolla S, Bardina V, De Mercanti S, Quaglino P, De Palma R, Gned D, et al. Th22 cells are expanded in multiple sclerosis and are resistant to IFN‐β. J Leukoc Biol. 2014; 96:1155–64. [DOI] [PubMed] [Google Scholar]
- 24. Rasouli J, Ciric B, Imitola J, Gonnella P, Hwang D, Mahajan K, et al. Expression of GM‐CSF in T cells is increased in multiple sclerosis and suppressed by IFN‐β therapy. J Immunol. 2015; 194:5085–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Restorick SM, Durant L, Kalra S, Hassan‐Smith G, Rathbone E, Douglas MR, et al. CCR6 + Th cells in the cerebrospinal fluid of persons with multiple sclerosis are dominated by pathogenic non‐classic Th1 cells and GM‐CSF‐only‐secreting Th cells. Brain Behav Immun. 2017; 64:71–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vartanian T, Li Y, Zhao M, Stefansson K. Interferon‐gamma‐induced oligodendrocyte cell death: implications for the pathogenesis of multiple sclerosis. Mol Med. 1995; 1:732–43. [PMC free article] [PubMed] [Google Scholar]
- 27. Pouly S, Becher B, Blain M, Antel JP. Interferon‐γ Modulates Human Oligodendrocyte Susceptibility To Fas‐Mediated Apoptosis. J Neuropathol Exp Neurol. 2000; 59:280–6. [DOI] [PubMed] [Google Scholar]
- 28. Bjartmar C, Wujek J, Trapp B. Axonal loss in the pathology of MS: consequences for understanding the progressive phase of the disease. J Neurol Sci. 2003; 206:165–71. [DOI] [PubMed] [Google Scholar]
- 29. Huber M, Heink S, Pagenstecher A, Reinhard K, Ritter J, Visekruna A, et al. IL‐17A secretion by CD8+ T cells supports Th17‐mediated autoimmune encephalomyelitis. J Clin Invest. 2012; 123:247–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zang YCQ, Li S, Rivera VM, Hong J, Robinson RR, Breitbach WT, et al. Increased CD8+ Cytotoxic T Cell Responses to Myelin Basic Protein in Multiple Sclerosis. J Immunol 2004; 172:5120–7. [DOI] [PubMed] [Google Scholar]
- 31. Salehi Z, Doosti R, Beheshti M, Janzamin E, Sahraian MA, Izad M. Differential frequency of CD8+ T cell subsets in multiple sclerosis patients with various clinical patterns. PLoS One 2016; 11:e0159565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ortiz GG, Pacheco‐Moisés FP, Bitzer‐Quintero OK, Ramírez‐Anguiano AC, Flores‐Alvarado LJ, Ramírez‐Ramírez V, et al. Immunology and oxidative stress in multiple sclerosis: Clinical and basic approach. Clin Dev Immunol. 2013; 2013:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Robertson D, Moreo N. Disease‐modifying therapies in multiple sclerosis: overview and treatment considerations. Fed Pract. 2016; 33:28–34. [PMC free article] [PubMed] [Google Scholar]
- 34. Palé LA, Caballero JL, Buxareu BS, Serrano PS, Solà VP. Systematic review of depression in patients with multiple sclerosis and its relationship to interferonβ treatment. Mult Scler Relat Disord 2017; 17:138–43. [DOI] [PubMed] [Google Scholar]
- 35. Hind D, Kaklamanou D, Beever D, Webster R, Lee E, Barkham M, et al. The assessment of depression in people with multiple sclerosis: a systematic review of psychometric validation studies. BMC Psychiatry. 2016; 16:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Goldsmith DR, Rapaport MH, Miller BJ. A meta‐analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. 2016; 21:1696–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Alvarenga‐Filho H, Sacramento PM, Ferreira TB, Hygino J, Abreu JEC, Carvalho SR, et al. Combined exercise training reduces fatigue and modulates the cytokine profile of T‐cells from multiple sclerosis patients in response to neuromediators. J Neuroimmunol. 2016; 293:91–9. [DOI] [PubMed] [Google Scholar]
- 38. Asano M, Berg E, Johnson K, Turpin M, Finlayson ML. A scoping review of rehabilitation interventions that reduce fatigue among adults with multiple sclerosis. Disabil Rehabil. 2014; 37:729–38. [DOI] [PubMed] [Google Scholar]
- 39. Mohr DC, Hart SL, Julian L, Cox D, Pelletier D. Association between stressful life events and exacerbation in multiple sclerosis: A meta‐analysis. BMJ. 2004; 328:731–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hou TY, Huang TL, Lin CC, Wu MK, Hung YY. Effects of selective serotonin reuptake inhibitors and serotonin‐norepinephrine reuptake inhibitors on toll‐like‐ receptors expression profiles. Neuropsychiatry. 2018; 8:243–8. [Google Scholar]
- 41. Miranda‐Hernandez S, Baxter AG. Role of toll‐like receptors in multiple sclerosis. Am J Clin Exp Immunol. 2013; 2:75–93. [PMC free article] [PubMed] [Google Scholar]
- 42. Marshak‐Rothstein A. Toll‐like receptors in systemic autoimmune disease. Nat Rev Immunol. 2006; 6:823–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Farrugia M, Baron B. The role of toll‐like receptors in autoimmune diseases through failure of the self‐recognition mechanism. Int J Inflamm. 2017; 2017:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen X, Das R, Komorowski R, Beres A, Hessner MJ, Mihara M, et al. Blockade of interleukin‐6 signaling augments regulatory T‐cell reconstitution and attenuates the severity of graft‐versus‐host disease. Blood. 2009; 114:891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ferreira TB, Hygino J, Barros PO, Teixeira B, Kasahara TM, Linhares UC, et al. Endogenous interleukin‐6 amplifies interleukin‐17 production and corticoid‐resistance in peripheral T cells from patients with multiple sclerosis. Immunology. 2014; 143:560–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nyirenda MH, Morandi E, Vinkemeier U, Constantin‐Teodosiu D, Drinkwater S, Mee M, et al. TLR2 Stimulation Regulates the Balance between Regulatory T Cell and Th17 Function: A Novel Mechanism of Reduced Regulatory T Cell Function in Multiple Sclerosis. J Immunol. 2015; 194:5761–74. [DOI] [PubMed] [Google Scholar]
- 47. Cowen PJ, Browning M. What has serotonin to do with depression? World Psychiatry. 2015; 14:158–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Snir O, Hesselberg E, Amoudruz P, Klareskog L, Zarea‐Ganji I, Catrina AI, et al. Genetic variation in the serotonin receptor gene affects immune responses in rheumatoid arthritis. Genes Immun. 2012; 14:83–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sacramento PM, Monteiro C, Dias ASO, Kasahara TM, Ferreira TB, Hygino J, et al. Serotonin decreases the production of Th1/Th17 cytokines and elevates the frequency of regulatory CD4+ T‐cell subsets in multiple sclerosis patients. Eur J Immunol. 2018; 48:1376–88. [DOI] [PubMed] [Google Scholar]
- 50. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology. 1983; 33:1444. [DOI] [PubMed] [Google Scholar]
- 51. Voo KS, Bover L, Harline ML, Weng J, Sugimoto N, Liu Y‐J. Targeting of TLRs Inhibits CD4+Regulatory T Cell Function and Activates Lymphocytes in Human Peripheral Blood Mononuclear Cells. J Immunol. 2014; 193:627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Soga F, Katoh N, Inoue T, Kishimoto S. Serotonin Activates Human Monocytes and Prevents Apoptosis. J Invest Dermatol. 2007; 127:1947–55. [DOI] [PubMed] [Google Scholar]
- 53. Fernstrom JD, Wurtman RJ. Control of brain 5‐HT content by dietary carbohydrates In: Barchas J, Usdin E, eds. Serotonin and Behavior. Philadelphia, USA: Elsevier Inc., 1973: 121–8. [Google Scholar]
- 54. Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004; 16:3–9. [DOI] [PubMed] [Google Scholar]
- 55. Mills KHG. TLR‐dependent T cell activation in autoimmunity. Nat Rev Immunol 2011; 11:807–22. [DOI] [PubMed] [Google Scholar]
- 56. Jin C, Flavell RA. Innate sensors of pathogen and stress: Linking inflammation to obesity. Journal of Allergy and Clinical Immunology. 2013; 132:287–94. [DOI] [PubMed] [Google Scholar]
- 57. Akira S, Uematsu S, Takeuchi O. Pathogen Recognition and Innate Immunity. Cell 2006; 124:783–801. [DOI] [PubMed] [Google Scholar]
- 58. Takeda K, Akira S. Toll‐like receptors in innate immunity. Int Immunol 2005; 17:1–14. [DOI] [PubMed] [Google Scholar]
- 59. Pandey GN, Rizavi HS, Ren X, Bhaumik R, Dwivedi Y. Toll‐like receptors in the depressed and suicide brain. J Psychiatr Res 2014; 53:62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hansen B, Hussain R, Lovettracke A, Thomas J, Racke M. Multiple toll‐like receptor agonists act as potent adjuvants in the induction of autoimmunity. J Neuroimmunol 2006; 172:94–103. [DOI] [PubMed] [Google Scholar]
- 61. Klonowska‐Szymczyk A, Wolska A, Robak T, Cebula‐Obrzut B, Smolewski P, Robak E. Expression of toll‐like receptors 3, 7, and 9 in peripheral blood mononuclear cells from patients with systemic lupus erythematosus. Mediators Inflamm 2014; 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Racke MK, Drew PD. Toll‐like receptors in multiple sclerosis. Toll‐Like Receptors: Roles in Infection and Neuropathology. 2009; 336:155–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hung Y‐Y, Wu M‐K, Huang T‐L, Huang KW, Huang GY‐L. Association between toll‐like receptor 4 expression and symptoms of major depressive disorder. Neuropsychiatr Dis Treat. 2015; 11:1853–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhen J, Yuan J, Fu Y, Zhu R, Wang M, Chang H, et al. IL‐22 promotes Fas expression in oligodendrocytes and inhibits FOXP3 expression in T cells by activating the NF‐κB pathway in multiple sclerosis. Mol Immunol. 2017; 82:84–93. [DOI] [PubMed] [Google Scholar]
- 65. Wing AC, Hygino J, Ferreira TB, Kasahara TM, Barros PO, Sacramento PM, et al. Interleukin‐17‐ and interleukin‐22‐secreting myelin‐specific CD4+T cells resistant to corticoids are related with active brain lesions in multiple sclerosis patients. Immunology. 2015; 147:212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Christensen SR, Shlomchik MJ. Regulation of lupus‐related autoantibody production and clinical disease by Toll‐like receptors. Semin Immunol 2007; 19:11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Thwaites R, Chamberlain G, Sacre S. Emerging Role of Endosomal Toll‐Like Receptors in Rheumatoid Arthritis. Front Immunol 2014; 5:5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Farez MF, Quintana FJ, Gandhi R, Izquierdo G, Lucas M, Weiner HL. Toll‐like receptor 2 and poly(ADP‐ribose) polymerase 1 promote central nervous system neuroinflammation in progressive EAE. Nat Immunol 2009; 10:958–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Reynolds JM, Pappu BP, Peng J, Martinez GJ, Zhang Y, Chung Y, et al. Toll‐like Receptor 2 Signaling in CD4+ T Lymphocytes Promotes T Helper 17 Responses and Regulates the Pathogenesis of Autoimmune Disease. Immunity 2010; 32:692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bsibsi M, Ravid R, Gveric D, Van Noort JM. Broad Expression of Toll‐Like Receptors in the Human Central Nervous System. J Neuropathol Exp Neurol 2002; 61:1013–21. [DOI] [PubMed] [Google Scholar]
- 71. Hodes GE, Ménard C, Russo SJ. Integrating Interleukin‐6 into depression diagnosis and treatment. Neurobiology of Stress. 2016; 4:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Correale J, Fiol M, Gilmore W. The risk of relapses in multiple sclerosis during systemic infections. Neurology 2006; 67:652–9. [DOI] [PubMed] [Google Scholar]
- 73. Kirby T, Ochoa‐Repáraz J. The Gut Microbiome in Multiple Sclerosis: A Potential Therapeutic Avenue. Medical Sciences. 2018; 6:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Opazo MC, Ortega‐Rocha EM, Coronado‐Arrázola I, Bonifaz LC, Boudin H, Neunlist M, et al. Intestinal Microbiota Influences Non‐intestinal Related Autoimmune Diseases. Front Microbiol 2018; 9:432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Franklin TC, Xu C, Duman RS. Depression and sterile inflammation: Essential role of danger associated molecular patterns. Brain Behav Immun. 2018; 72:2–13. [DOI] [PubMed] [Google Scholar]
- 76. Aquino DA, Capello E, Weisstein J, Sanders V, Lopes C, Tourtellotte WW, et al. Multiple sclerosis: altered expression of 70‐ and 27‐kDa heat shock proteins in lesions and myelin. J Neuropathol Exp Neurol. 1997; 56:664–72. [PubMed] [Google Scholar]
- 77. Hesse S, Moeller F, Petroff D, Lobsien D, Luthardt J, Regenthal R, et al. Altered serotonin transporter availability in patients with multiple sclerosis. Eur J Nucl Med Mol Imaging. 2014; 41:827–35. [DOI] [PubMed] [Google Scholar]
- 78. Robinson CM, Hale PT, Carlin JM. The Role of IFN‐γ and TNF‐α‐Responsive Regulatory Elements in the Synergistic Induction of Indoleamine Dioxygenase. J Interferon Cytokine Res. 2005; 25:20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Shajib MS, Khan WI. The role of serotonin and its receptors in activation of immune responses and inflammation. Acta Physiol. 2014; 213:561–74. [DOI] [PubMed] [Google Scholar]
- 80. Herr N, Bode C, Duerschmied D. The Effects of Serotonin in Immune Cells. Front Cardiovasc Med. 2017; 4:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Chabbi‐Achengli Y, Coman T, Collet C, Callebert J, Corcelli M, Lin H, et al. Serotonin is involved in autoimmune arthritis through Th17 immunity and bone resorption. Am J Pathol. 2016; 186:927–37. [DOI] [PubMed] [Google Scholar]
- 82. Szabo A, Gogolak P, Koncz G, Foldvari Z, Pazmandi K, Miltner N, et al. Immunomodulatory capacity of the serotonin receptor 5‐HT2B in a subset of human dendritic cells. Sci Rep 2018; 8:1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Müller T, Dürk T, Blumenthal B, Grimm M, Cicko S, Panther E, et al. 5‐Hydroxytryptamine modulates migration, cytokine and chemokine release and T‐cell priming capacity of dendritic cells In Vitro and In Vivo. PLoS One 2009; 4:e6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Foley P, Lawler A, Chandran S, Mead G. Potential disease‐modifying effects of selective serotonin reuptake inhibitors in multiple sclerosis: systematic review and meta‐analysis. J Neurol Neurosurg Psychiatry. 2014; 85:709–10. [DOI] [PubMed] [Google Scholar]
- 85. Scott CF, Cashman N, Spitler L. Experimental Allergic Encephalitis; Treatment with Drugs Which Alter CNS Serotonin Levels. Journal of Immunopharmacology. 1982; 4:153–62. [DOI] [PubMed] [Google Scholar]
- 86. Xia Z, Depierre JW, Nässberger L. Tricyclic antidepressants inhibit IL‐6, IL‐1β and TNF‐α release in human blood monocytes and IL‐2 and interferon‐γ in T cells. Immunopharmacology. 1996; 34:27–37. [DOI] [PubMed] [Google Scholar]
- 87. Kubera M, Curzytek K, Majewska‐Szczepanik M, Szczepanik M, Marcińska K, Ptak W, et al. Inhibitory effect of antidepressant drugs on contact hypersensitivity reaction. Pharmacol Rep. 2012; 64:714–22. [DOI] [PubMed] [Google Scholar]
- 88. Kubera M, Lin A‐H, Kenis G, Bosmans E, van Bockstaele D, Maes M. Anti‐inflammatory effects of antidepressants through suppression of the interferon‐γ/Interleukin‐10 production ratio. J Clin Psychopharmacol. 2001; 21:199–206. [DOI] [PubMed] [Google Scholar]
- 89. Gobin V, Van Steendam K, Fevery S, Tilleman K, Billiau AD, Denys D, et al. Fluoxetine reduces murine graft‐versus‐host disease by induction of T cell Immunosuppression. J Neuroim Pharmacol. 2013; 8:934–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Möller A, Wiedemann G, Rohde U, Backmund H, Sonntag A. Correlates of cognitive impairment and depressive mood disorder in multiple sclerosis. Acta Psychiatr Scand 1994; 89:117–21. [DOI] [PubMed] [Google Scholar]
- 91. Uccelli MM, Mohr LM, Battaglia MA, Zagami P, Mohr DC. Peer support groups in multiple sclerosis: Current effectiveness and future directions. Mult Scler J. 2004; 10:80–4. [DOI] [PubMed] [Google Scholar]
- 92. Colombo B, Annovazzi P, Comi G. Understanding fatigue in multiple sclerosis: new insights in causes and assessment. Neurol Sci. 2006; 27:s304–s306. [Google Scholar]
- 93. Yang T, Yang Y, Wang D, Li C, Qu Y, Guo J, et al. The clinical value of cytokines in chronic fatigue syndrome. J Transl Med. 2019; 17:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flow cytometry supporting information data of PBMC.
Figure S2. Percentage of CD4+ and CD8+ T cells from healthy subjects (HS) and MS patients suffering, or not (MS), from major depressive disorder (MS/MDD) able to express TLR2 (A) and TLR4 (B) in each independent experiment are shown. Also, the MFI for TLR2 (C) and TLR4 (D), as well as the proportion of TLR2+ (E) and TLR4+ (F) IL‐17‐secreting (CD4+ and CD8+) T cells, are shown per experiment. Each experiment was performed with 3 or 4 subjects for each group of subjects (HS, MS and MS/MDD).
Figure S3. Dosage of different cytokines, per experiment, on supernatants collected from purified CD4+ and CD8+ T cells from MS patients suffering, or not (MS), from major depressive disorder (MS/MDD) following addition of Pam3Csk4 (Pam3C, 1 g/mL) and LPS (100 ng/mL). We dosed IL‐1β (A), TNF‐α (B), IL‐6 (C), GM‐CSF (D), IL‐17 (E), IL‐21 (F), IL‐22 (G), IFN‐ (H), IL‐10 (I). Each experiment was performed with 2 or 3 subjects for each group of patients (MS and MS/MDD).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, D.B., upon reasonable request.


