Abstract
The solute carrier 8 (SLC8) family of sodium-calcium exchangers (NCXs) functions as an essential regulatory system that couples opposite fluxes of sodium and calcium ions across plasmalemmal membranes. NCXs, thereby, play key roles in maintaining an ion homeostasis that preserves cellular integrity. Hence, alterations in NCX expression and regulation have been found to lead to ionic imbalances that are often associated with intracellular calcium overload and cell death. On the other hand, intracellular calcium has been identified as a key driver for a multitude of downstream signaling events that are crucial for proper functioning of biological systems, thus highlighting the need for a tightly controlled balance. In the CNS, NCXs have been primarily characterized in the context of synaptic transmission and ischemic brain damage. However, a much broader picture is emerging. NCXs are expressed by virtually all cells of the CNS including oligodendrocytes (OLGs), the cells that generate the myelin sheath. With a growing appreciation of dynamic calcium signals in OLGs, NCXs are becoming increasingly recognized for their crucial roles in shaping OLG function under both physiological and pathophysiological conditions. In order to provide a current update, this review focuses on the importance of NCXs in cells of the OLG lineage. More specifically, it provides a brief introduction into plasmalemmal NCXs and their modes of activity, and it discusses the roles of OLG expressed NCXs in regulating CNS myelination and in contributing to CNS pathologies associated with detrimental effects on OLG lineage cells.
Keywords: oligodendrocyte, myelin, sodium-calcium exchange, ion homeostasis, signaling
Introduction
Signaling mediated by the divalent cation calcium is of critical importance for proper functioning of the central nervous system (CNS) [1]. More precisely, spatially and temporally well-coordinated dynamics of intracellular calcium signals act on diverse downstream targets, They, thereby, engage in a multitude of functions ranging from cellular metabolism and gene expression to cell migration and differentiation in both CNS neurons [2–4] and CNS glial cells, i.e. astrocytes [5, 6], microglia [7, 8], and oligodendrocytes (OLGs) [9–13]. On the other hand, abnormalities in calcium signaling are thought to underlie many different neurological and neurodegenerative diseases [14–19]. Thus, there is a critical need for maintaining a well-balanced calcium homeostasis to ensure faultless CNS function. This need is addressed by molecular players that control the movement of calcium ions across membranes and are represented by numerous types of calcium channels and transporters, including the solute carrier 8 (SLC8) family of sodium-calcium exchangers (NCXs).
Historically, the phenomenon of sodium-calcium exchange across the plasma membrane was first described to occur in squid axons and the mammalian heart but it has since been recognized to apply to most mammalian cell types [20–24]. In the CNS, the activity of NCXs has probably been best characterized as a counter transport system that is present in astrocytes and neurons, where it plays important physiological roles by regulating synaptic transmission and plasticity [6, 25–29]. Under pathophysiological conditions, changes in the expression and function of NCXs in astrocytes, neurons and microglia have been associated with neurodegeneration and neuroinflammation [30–36]. Thus, NCXs are increasingly recognized as a critical regulatory system controlling CNS function. Despite a growing knowledge base, however, the roles of NCXs in glial cells belonging to the OLG lineage are only starting to emerge.
This review is focused on providing an update on the current knowledge on plasmalemmal NCXs in OLGs, the myelinating cells of the CNS. In more detail, it presents a brief overview on the role of plasmalemmal NCXs in maintaining calcium and sodium homeostasis. In addition, it provides a detailed discussion on the functional involvement of OLG expressed NCXs in regulating OLG differentiation and myelination, and in contributing to the pathophysiology in a variety of CNS diseases affecting cells of the OLG lineage.
Sodium-calcium exchangers of the SLC8 family: a brief overview
The SLC8 family of NCXs represents one branch of the much larger calcium-cation antiporter (CaCA) superfamily. Collectively, CaCA family members play critical roles in regulating cellular calcium homeostasis by facilitating the efflux of calcium against its electrochemical gradient and in counter exchange for other cations. In the case of NCXs, one calcium ion is counter exchanged by three sodium ions [37–40]. In mammals, NCXs are encoded by three SLC8 genes: SLC8A1 (NCX1) [41], SLC8A2 (NCX2) [42], and SLC8A3 (NCX3) [43]. Interestingly, a fourth family member, NCX4 encoded by SLC8A4, has been found present in teleost, amphibian, and reptilian species; in mammals and birds, however, it has secondarily and independently been lost [44–46]. Recent evidence further suggests that the long-wanted mitochondrial sodium-calcium exchanger is encoded by the SLC8B1 gene giving rise to the protein product NCLX [47–49]. This review is focused on the three mammalian SLC8A-derived NCXs, for which in the following the NCX nomenclature will be used for both genes and proteins, and uppercase letters will be used independent of the referenced species.
The three distinct mammalian NCXs share a highly conserved overall structure and characteristic transport functions [50]. More specifically, current structural models predict that mammalian NCXs are composed of ten transmembrane helices (TM1–10) and a long cytosolic f-loop positioned between TM5 and TM6 (Fig. 1) [51]. The transmembrane helices are arranged in two pseudo-symmetrical halves (TM1–5 and TM6–10), which form a tightly packed core domain. Notably, TM2–3 and TM7–8 contain two highly conserved homologous sequence motifs, the α1 and α2-repeat; these α repeats form a pocket, including twelve ion-coordinating residues (four in TM2 and TM7, and two in TM3 and TM8) and four ion binding sites, that is responsible for ion recognition and translocation [51–54]. This structure and the presence of two distinct passageways for separate access of sodium and calcium ions to their respective central binding sites [51–54] allows an alternating-access model of secondary active transport; in other words, they allow consecutive exposure of ligand binding domains at opposite sides of the membrane and, upon each ion-binding event, a conversion between two major conformational states, an inward-facing and an outward-facing state [55, 56]. The directionality of NCX-mediated ion exchange is reversible and depends on the relative concentrations of calcium and sodium ions as well as the membrane potential. Thus, NCXs can operate in a forward (calcium efflux) and reverse (calcium influx) mode both of which are thought to exert important physiological functions [20, 57]. NCX activity is regulated through a tandem of calcium-binding domains, CBD1 and CBD2, which are located within the cytosolic f-loop [58–62]. The CBD1 domain functions as the primary high-affinity allosteric calcium sensor [63, 64], while the CBD2 domain of NCX1 and NCX3 (but not NCX2) is subject to alternative splicing, thereby conferring different kinetic properties to individual NCX splice variants [61, 65–69]. Based on these features, increased intracellular calcium levels activate the forward mode, while the reverse mode is favored in the presence of increased intracellular sodium concentrations and a positive membrane potential [20]. In addition, increased cytosolic sodium levels have been shown to transiently inactivate NCX1 and NCX3 (but not NCX2) via interaction of sodium with a site that is located outside of the CBD domains and has been referred to as the eXchanger Inhibitory Peptide (XIP) autoinhibitory region [47, 63, 66, 70, 71]. Such inactivation can prevent entry of a toxic amount of calcium through reverse mode operation. Interestingly, sodium-mediated inactivation can be relieved by calcium-binding to CBD2 splice variants containing one of the mutually exclusive alternatively spliced exons, namely exon A in NCX1 and exon B in NCX3; these splice variants are expressed preferentially in excitable cells [44, 61, 66, 70, 72–74]. From a functional point of view, it is notable that isoforms and splice variants such as NCX2 and NCX3-AC, which exhibit low sensitivity to sodium-dependent inactivation, are capable to retain forward mode activity even during high amplitude and prolonged sodium transients [75, 76]. Apart from calcium and sodium, NCX activity has been found to be regulated allosterically by non-transported ion species (protons and other monovalent cations), phosphatidylinositol bisphosphate and other acidic phospholipids, and an interacting fatty-acid binding protein referred to as soluble cytosolic regulatory protein (SCRP) or regulatory protein of the squid nerve sodium calcium exchanger (ReP1-NCXSQ) [77–79].
Fig. 1. Topology model for sodium calcium exchangers of the SLCA8 family.
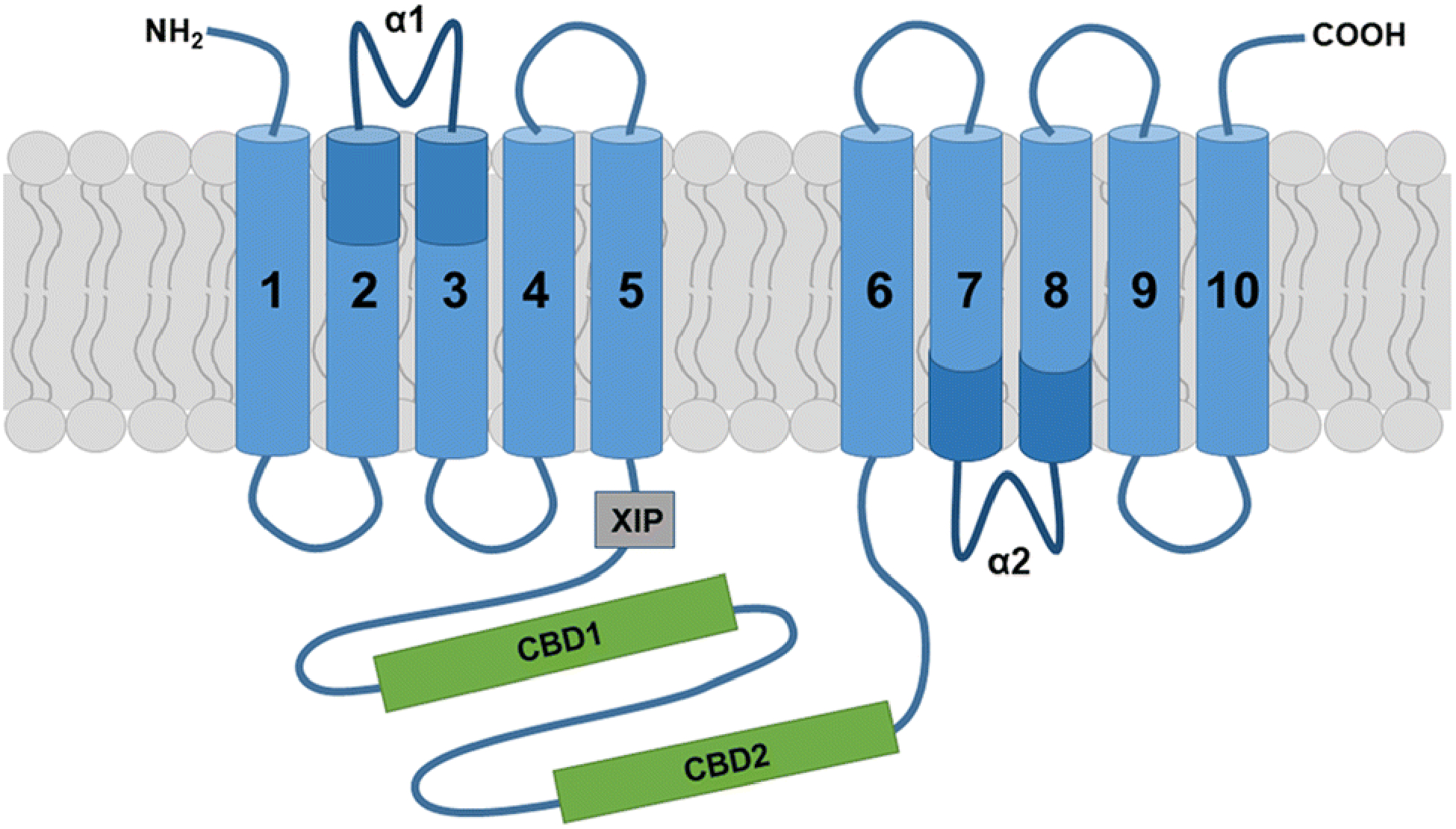
The current model for NCX topology proposes five transmembrane helices in both the first and second hydrophobic cluster. The two conserved α-repeats (α1 and α2) contain ion-coordinating amino acid residues that are involved in ion transport activities. The cytosolic f-loop encompasses the two calcium binding domains CBD1 and CBD2, and the exchanger inhibitory peptide (XIP) site, which is present in NCX1 and NCX3 and has been implicated in sodium-dependent inactivation.
Due to their different regulatory properties, one mechanism of tissue- and/or cell type-specific calcium homeostasis can be achieved by the differential expression of specific NCX genes and, in the case of NCX1 and NCX3, their individual splice variants [57, 75, 80–82]. In this context, NCX2 and NCX3 are present primarily in brain and skeletal muscle, whereas NCX1 is found more universally distributed [41–43, 83, 84]. Cardiac and skeletal muscle cells express predominantly one NCX isoform/splice variant while several isoform/splice variants coexist in neurons and glial cells, possibly allowing, especially in the latter, a parallel increase in both calcium and sodium ions [75, 83]. Hence, calcium and sodium signaling can be tightly linked through the activities of NCXs, a phenomenon that has been best characterized for astrocytes and their regulatory roles in synaptic transmission [6, 26, 75, 85]. Furthermore, different tissues, i.e. heart, kidney and brain, have been found to express NCX1 via three alternate promoters, resulting in independent transcriptional regulation in the absence of changes in protein structure or function [86–89]. In this context, the brain NCX1 promoter represents a ubiquitous GC-rich TATA-less promoter that gives rise to the majority of the NCX1 transcripts found in the brain, possibly through binding at its AP-2 binding site, but it is also active elsewhere [86].
Taken together, plasmalemmal NCXs function as unique transporter systems that control intracellular calcium homeostasis and that can directly couple the transfer of calcium and sodium ions across membranes. Cell- and tissue-specific characteristics of calcium-sodium exchange are achieved through the different regulatory properties of the individual NCXs and their alternatively spliced variants as well as through specific NCX1 promoter use. Hence, NCXs critically contribute to the tight control of calcium and sodium signaling events that is necessary to maintain physiological conditions within the CNS [6, 75, 90].
Sodium-calcium exchangers in oligodendrocytes
Oligodendrocytes (OLGs) are specialized cells of the CNS that generate the axon enwrapping myelin sheath, which enables rapid and efficient saltatory conduction and provides metabolic axonal support [91]. During development, cells of the OLG lineage originate as bipolar and migratory OLG progenitor cells (OPCs), which undergo a stepwise lineage progression by maturing first into premyelinating immature OLGs that extend a complex process network and then into mature OLGs that produce and maintain the myelin sheath [92, 93]. Next to extensive alterations in morphology, precisely regulated changes in gene expression patterns mark each of the individual stages of the OLG lineage [94–97].
The first characterization of NCXs in cells of the OLG lineage dates back to a study published by Quednau et al. [83], which revealed, using reverse transcriptase-polymerase chain reaction (RT-PCR), that all three mammalian genes are expressed in primary cultures of differentiating OLGs. Regarding the presence of alternatively spliced variants, both exon A and B containing mRNA transcripts were detected for NCX1 but only exon B containing ones were found for NCX3. Subsequent investigations confirmed these results but they also suggested that while NCX2 is the most highly expressed NCX gene in the brain (except the brain stem), its levels may be relatively low in OLGs [98, 99]. When assessing the distribution of NCX1 on myelinated axons in vivo in the adult rodent CNS, positive results were obtained for the optic nerve and spinal cord but not the corpus callosum, hence indicating regional heterogeneity pertaining the expression of the different NCX genes in mature OLGs [100]. Markedly, a developmental switch in NCX gene expression was observed in primary cultures of cortical OLG lineage cells in which NCX1 was found to be expressed predominantly at the progenitor stage while NCX3 expression was seen upregulated once cells started to differentiate [101, 102]. Consistent with the view that NCX3 represents the main contributor to sodium-calcium exchange in cortical OLGs, NCX3-targeted gene silencing was found to reduce overall NCX activity (forward and reverse mode) by about 80% [101]. Given the expression of NCX1 in the adult optic nerve and spinal cord, it appears, however, that the developmental downregulation of NCX1 may be specific to cortical OLG lineage cells and aimed at generating regional differences in the characteristics of sodium-calcium exchange within differentiating and mature OLGs.
Functionally, it has been proposed that NCX1 activity in OPCs contributes to the regulation of OPC migration, possibly via a mechanism that is induced by signaling through gamma-aminobutyric acid (GABA) receptors and subsequent elevation in intracellular sodium levels triggering calcium influx (and compensatory sodium efflux) through reverse operation of the exchanger (Fig. 3A) [99]. This pathway has been characterized in OPCs using hippocampal slices [99], and calcium signaling has been shown to contribute to the regulation of OPC migration [9, 11, 103, 104]. However, GABA-stimulated OPC migration events have not yet been assessed in detail in vivo [105], and they may be specific to OPCs leaving the postnatal subventricular zone (SVZ) [99].
Fig. 3. Proposed physiological roles of NCX activities in cells of the OLG lineage.
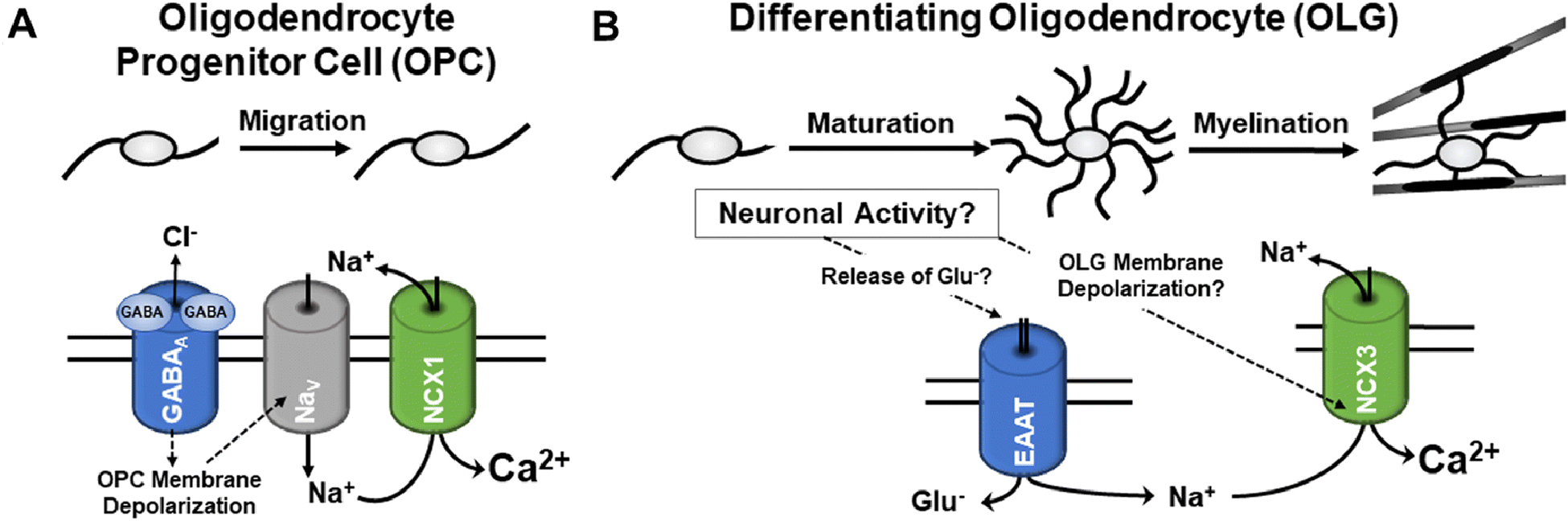
A: For OPCs located in the postnatal subventricular zone (SVZ), activation of GABAA receptors has been reported to induce a chemotactic migration response that is mediated by calcium influx via reverse mode NCX1 activity triggered by sodium influx through non-inactivating sodium channels (Nav) after GABA-induced membrane depolarization [99]. B: For differentiating and myelinating OLGs, a differentiation promoting role emerges for NCX3 [101, 102]. Thus far, two neuronal activity-induced pathways have been proposed. First, OLG membrane depolarization-induced reverse mode NCX activity has been implicated in promoting the syntheses of the myelin protein myelin basic protein (MBP) and the processes of active myelination [106, 107]. Second, activation of sodium-dependent glutamate transporters (EAAT), presumably through glutamate release from electrically active axons, was found to promote the morphological maturation of differentiating OLGs by a molecular mechanism involving reverse mode NCX activity and an increase in intracellular calcium concentration (Fig. 2) [109, 110].
In differentiating and myelinating OLGs, NCX3 activity has been implicated in promoting OLG maturation [101, 102] and the onset of local synthesis of myelin basic protein (MBP) [106]. As an underlying mechanism for the latter, it has been proposed that neuronal activity, presumably through an increase in extracellular potassium concentration, leads to local increases in intracellular sodium levels and changes in membrane potential; these changes drive an influx of calcium via reverse mode NCX3 activity, thereby triggering the onset local MBP translation in the vicinity of electrically active axons [106, 107]. Consistent with a prominent role of NCX3 in regulating the appearance of myelin proteins, protein levels for MBP and 2’,3’-cyclic-nucleotide 3’-phosphodiesterase (CNP) were found to be reduced in the spinal cords of NCX3 knockout mice [101]. It is of note that the CNS myelin mRNA pool has recently been found to be much larger than previously recognized [108]. Specifically, it was found to include mRNAs, such as PLP1 and CNP, which have traditionally been associated with a localization solely to the OLG soma. These findings suggest that locally regulated translation may affect a broader range of myelin-related transcripts, thus providing a new level of complexity to the regulation of myelination in general and via the activity of NCXs.
In our own studies, we identified a signaling mechanism that promotes the morphological maturation of OLGs, i.e. process outgrowth and branching, via activation of OLG expressed sodium-dependent glutamate transporters and subsequent increase in intracellular calcium concentrations [109, 110]. Based on studies done in astrocytes, the observed influx in calcium was proposed to be mediated by reverse mode NCX activity [109, 111]. To address this hypothesis experimentally, primary cultures of differentiating OLGs were treated, prior to stimulating sodium-dependent glutamate transporters via application of D-aspartate (D-Asp), with the selective NCX inhibitor SN6 that blocks preferentially reverse mode operation [112, 113]. D-Asp was used in these experiments as glutamate-equivalent transporter substrate since it has been described to not stimulate non-NMDA ionotropic and metabotropic glutamate receptors [114, 115] and to not be metabolized by glutamine synthetase [116]. As shown in Fig. 2, D-Asp treatment increased process outgrowth and the OLG’s process network area to an extent similar to the one reported previously [109]. Importantly, this effect could be effectively blocked by pre-incubation with SN6, thus demonstrating an activation of NCX reverse mode activity downstream of glutamate transporter activation. In support of in vivo relevance of this pathway, recent studies demonstrated that D-Asp treatment can stimulate in vivo remyelination by promoting OLG maturation via a pathway that involves, among others, the activation of glutamate transporters and the sodium-calcium exchanger NCX3 [117]. Considering that glutamate is released along at least a subset of unmyelinated and electrically active axons [118–120], the glutamate transporter-NCX pathway may, similar to the extracellular potassium-OLG membrane depolarization pathway [106, 107], contribute to the adaptive program of electrical activity-dependent myelination (Fig. 3B) [121–124]. Future studies are, however, needed to determine the in vivo contribution of either pathway to developmental myelination and myelin repair and to establish the extent of crosstalk and interaction, beyond an involvement of NCX reverse mode activity, between these two pathways.
Fig. 2. Inhibition of the reverse mode of Na+/Ca2+ exchange attenuates glutamate transporter stimulated morphological maturation of oligodendrocytes.
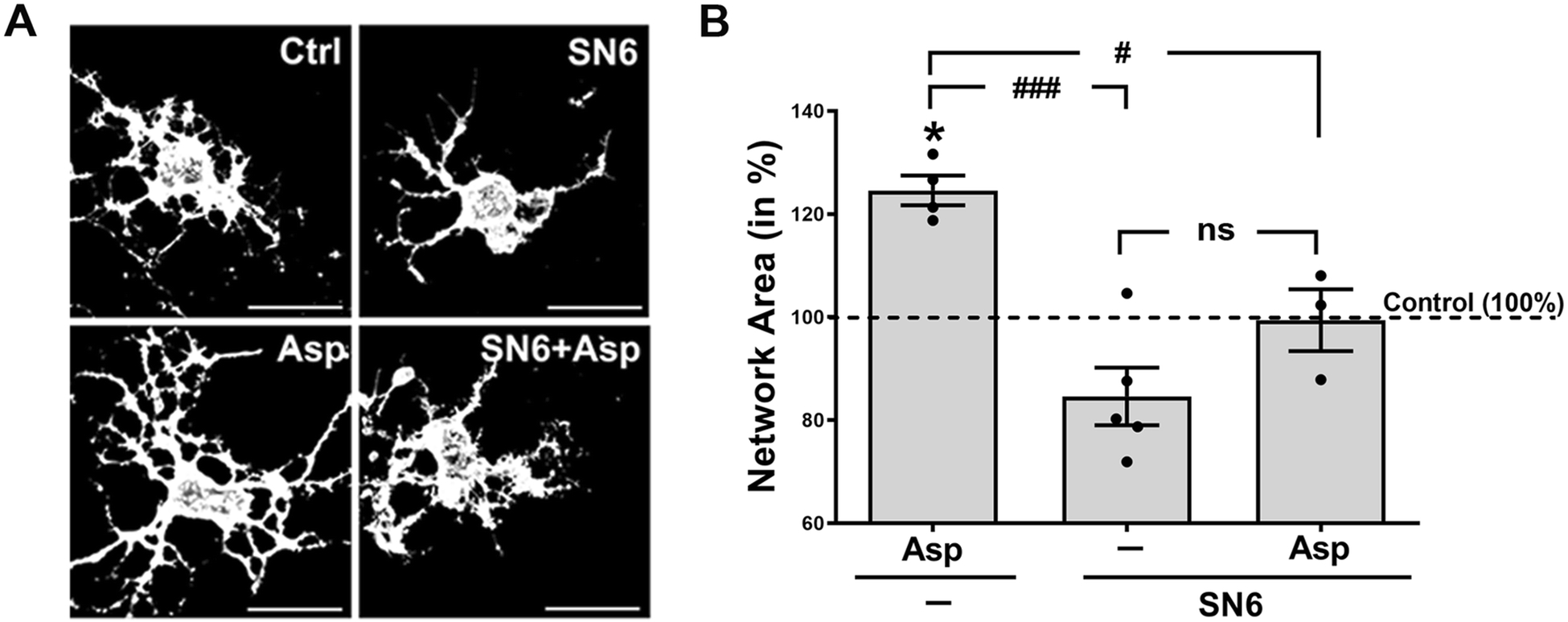
Cells were treated for 6h as indicated. Asp (D-Asp, 100 μM), SN6 (a selective inhibitor of Na+/Ca2+ exchange (reverse mode), 10 μM). A: Representative images of differentiating oligodendrocytes after immunostaining with O4 hybridoma supernatants. Scale bars: 20 μm. B: Graph depicting network areas. The mean values for control (non-treated) cells were set to 100% (dotted horizonal line) and experimental values were calculated accordingly. Dots represent individual experiments, horizontal lines indicate means, error bars are depicted as SEM. *p≤0.05 (compared to control; one sample t-test); #p≤0.05, ###p≤0.001, nsnot significant (ANOVA, Tukey’s multiple comparison test)
Collectively, it becomes apparent that sodium-calcium exchange is critically involved in the regulation of various physiological processes related to CNS myelination. In OPCs, it is primarily NCX1 that is involved in controlling cell migration in vitro and possibly from the postnatal SVZ into the brain parenchyma. At more mature stages of the OLG lineage, NCX3 emerges as the primary NCX gene in OLGs in which sodium-calcium exchange has been implicated in signaling pathways promoting maturation. Remarkably, all these physiological roles have been shown mediated by well-controlled reverse mode NCX activities leading to calcium influx.
Potential contributions of oligodendrocyte-expressed sodium-calcium exchangers to CNS disease
Premyelinating OLGs have been shown to be particularly vulnerable to hypoxic-ischemic injury, a process that has been implicated in brain injury in premature infants [125]. Interestingly, oxygen-glucose deprivation (OGD) as a model of ischemic damage was found to lead to a downregulation of NCX3 expression in premyelinating OLGs; attenuation of this downregulation was shown to prevent calcium overload, an effect that was attributed to NCX3 forward mode operation (Fig. 4B), thereby improving OLG viability and maturation [126]. Similar observations were made under conditions of lead (Pb) poisoning, whereby Pb is thought to enhance the generation of reactive oxygen species, to reduce the antioxidant defense system of cells and to decrease the expression of calcium extrusion proteins [127, 128]. Thus, under conditions of hypoxic-ischemic injury and oxidative stress, NCX3 activity appears to exert protective functions by ameliorating injury to premyelinating OLGs. Such a beneficial role for NCX3 is supported by studies investigating ischemic preconditioning as a mechanism by which a brief non-injurious episode of ischemia protects the brain from a subsequent lethal insult. This protective effect has, at least in part, been attributed to an upregulation of NCX1 and NCX3 [30, 129, 130]. Interestingly, in addition to beneficial forward mode NCX avtivity, reverse mode operation has been implicated in counteracting ischemia-induced injury processes by dampeming intracellular sodium overload and promoting calcium refilling into the endoplasmic reticulum (Fig. 4B) [129, 131]. Undoubtedly, further research will be neceesary to more precisely define the contribtions of NCX activities to the pathology seen upon hypoxic-ischemic injury to the brain.
Fig. 4. Proposed pathophysiological roles of NCX activities in cells of the OLG lineage.
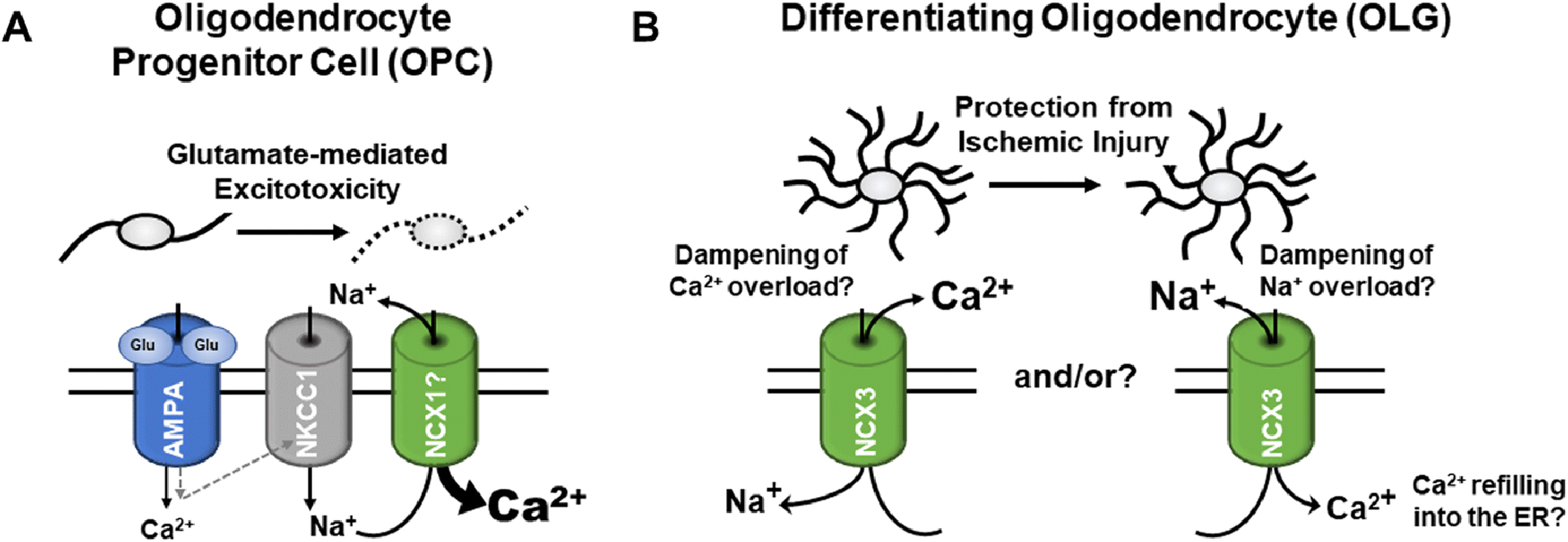
A: For OPCs, overactivation of AMPA receptors has been associated with glutamate-mediated excitotoxic injury; more specifically, a pathway involving the sodium-potassium-chloride co-transporter 1 (NKCC1) and the reverse mode of NCX activity has recently been described to lead to calcium overload and OPC injury upon prolonged AMPA receptor activation [132]. B: For differentiating OLGs a protective role of NCX3 activity is emerging in the context of ischemic-hypoxic injury and oxidative stress; possible beneficial effects of both forward (left) [126] and reverse mode activity have been proposed [129, 131].
Somewhat different from the above proposed protective roles, reverse mode NCX activity has, during glutamate-mediated excitotoxicity, been proposed to trigger calcium overload and to thereby compromise mitochondrial function and OLG viability [132]. In OPCs, such pathological reverse mode NCX activity has been shown to be triggered by activation of AMPA (alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptors and subsequent sodium influx via the sodium-potassium-chloride co-transporter 1 (NKCC1) (Fig. 4A) [132]. Increased levels of glutamate, possibly triggering pathways of glutamate-mediated excitotoxicity, have been associated with OLG and myelin damage in a number of human CNS pathologies and their rodent animal models; these include the major demyelinating disease in human, Multiple Sclerosis (MS) [133–135], hypoxia/ischemia-mediated neonatal and adult white matter damage [136, 137], and spinal cord injury [75, 138, 139]. For human adult OLGs, however, it has been reported that the expression of AMPA receptor subunits is low and that they are resistant to excitotoxic injury [140]. On the other hand, AMPA receptor-mediated calcium signaling in rodents has been described to be transiently enhanced in perinatally-derived OPCs and immature OLGs [141, 142], thus pointing toward a developmental window of vulnerability in both rodents and humans that may be altered under inflammatory conditions associated with CNS injury.
The origin of cells for glioblastoma multiforme (GBM), the most lethal primary neoplasm in the CNS, has been proposed to be represented, next to neural stem cells, by OPCs [143]. Given the vulnerability of OPCs to reverse mode NCX activity-induced cell death (see above) and evidence for NCX expression by glioma cells [144, 145], selective blockade of the forward mode of NCX activity has, in analogy to studies done in other cancer cell types [146], been explored as a strategy to suppress GBM growth [147]. Remarkably, inhibition of forward mode NCX activity was found to suppress tumor growth of established glioma cell lines both in vitro and in vivo. Treatment of astrocytes under the same conditions was found to not affect cell viability, possibly due to differences in NCX gene expression patterns. Effects on other CNS cells, importantly neurons and cells of the OLG lineage, however, were not assessed in these studies.
In summary, alterations in NCX expression and/or activity in both the forward and reverse mode have been implicated in contributing to a variety of CNS pathologies. Currently, however, knowledge about the exact contributions of either mode of activity, individual NCX genes or alternatively spliced variants is limited, thus complicating the design of well-targeted therapeutic approaches.
Conclusions
Cells of the OLG lineage have been reported to express all three of the mammalian NCX genes. However, NCX3 is beginning to emerge as the most prominent NCX gene in differentiating and mature OLGs, where it has been identified to play crucial roles in regulating OLG maturation and myelin sheath formation [101, 106, 107, 148]. NCX1, on the other hand, appears to be primarily operative during OPC migration [99]. Despite an increasing awareness of NCX function in OLGs, knowledge still remains limited. For example, increasing evidence has revealed diversity and potential heterogeneity within the OLG lineage [149–152]. In this regard, NCX2 may be enriched in a subtype of mature OLGs possibly involved in synaptic activity [149]. Such potential regional differences, however, have not been investigated in detail. In addition, potential diversity in sodium-calcium exchanger kinetics, due to the expression of different NCX genes and/or splice variants, has not been fully explored. Notably, most of the functional roles of NCXs in OLGs have been associated with changes in intracellular calcium concentrations. In contrast, little is known about potential roles of intracellular sodium transients in OLGs, despite the known coupling of calcium and sodium ion fluxes and crosslinking between calcium and sodium signaling [75].
In addition, to their involvement in developmental processes, NCXs expressed by OLG lineage cells have been implicated in contributing to pathophysiological mechanisms under a number of neurological disorder conditions. Thus, characterizing NCX activity may provide insight into novel therapeutic approaches. Thus far, therapeutically targeting NCX activity has shown promise in patients with coronary heart disease [153]. In addition, NCXs have been proposed as druggable targets for the treatment of cerebral ischemia [31, 154] and brain tumors [147]. However, it is becoming increasingly clear that NCXs play diverse roles under various pathological conditions and compared to physiological processes. Thus, a deeper understanding of the functional roles of NCXs under both physiological and pathophysiological conditions as well as within the diverse cell types of the CNS, including OLGs, is needed to be able to specifically target individual CNS disease promoting processes involving NCXs.
Acknowledgments
The authors are supported by grants from the National Institute of Health (B.F.), the National Multiple Sclerosis Society (B.F.) and the Commonwealth Health Research Board (B.F.).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Compliance with Ethical Standards
Conflict of interest: The authors declare that they have no potential conflicts.
References
- 1.Kawamoto EM, Vivar C, Camandola S (2012) Physiology and pathology of calcium signaling in the brain. Front Pharmacol 3:61 10.3389/fphar.2012.00061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brini M, Calì T, Ottolini D, Carafoli E (2014) Neuronal calcium signaling: function and dysfunction. Cell Mol Life Sci 71:2787–814. 10.1007/s00018-013-1550-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horigane S-I, Ozawa Y, Yamada H, Takemoto-Kimura S (2019) Calcium signalling: a key regulator of neuronal migration. J Biochem 165:401–409. 10.1093/jb/mvz012 [DOI] [PubMed] [Google Scholar]
- 4.Toth AB, Shum AK, Prakriya M (2016) Regulation of neurogenesis by calcium signaling. Cell Calcium 59:124–34. 10.1016/j.ceca.2016.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shigetomi E, Patel S, Khakh BS (2016) Probing the Complexities of Astrocyte Calcium Signaling. Trends Cell Biol 26:300–312. 10.1016/j.tcb.2016.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verkhratsky A, Untiet V, Rose CR (2019) Ionic signalling in astroglia beyond calcium. J Physiol. 10.1113/JP277478 [DOI] [PubMed] [Google Scholar]
- 7.Färber K, Kettenmann H (2006) Functional role of calcium signals for microglial function. Glia 54:656–65. 10.1002/glia.20412 [DOI] [PubMed] [Google Scholar]
- 8.Brawek B, Garaschuk O (2013) Microglial calcium signaling in the adult, aged and diseased brain. Cell Calcium 53:159–69. 10.1016/j.ceca.2012.12.003 [DOI] [PubMed] [Google Scholar]
- 9.Zhang M, Liu Y, Wu S, Zhao X (2019) Ca2+ Signaling in Oligodendrocyte Development. Cell Mol Neurobiol 39:1071–1080. 10.1007/s10571-019-00705-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pitman KA, Young KM (2016) Activity-dependent calcium signalling in oligodendrocyte generation. Int J Biochem Cell Biol 77:30–34. 10.1016/j.biocel.2016.05.018 [DOI] [PubMed] [Google Scholar]
- 11.Butt AM (2006) Neurotransmitter-mediated calcium signalling in oligodendrocyte physiology and pathology. Glia 54:666–675. 10.1002/glia.20424 [DOI] [PubMed] [Google Scholar]
- 12.Soliven B (2001) Calcium Signalling in Cells of Oligodendroglial Lineage. Microsc Res Tech 15:672–679. [DOI] [PubMed] [Google Scholar]
- 13.Cheli VT, Santiago González DA, Spreuer V, Paez PM (2015) Voltage-gated Ca++ entry promotes oligodendrocyte progenitor cell maturation and myelination in vitro. Exp Neurol 265:69–83. 10.1016/j.expneurol.2014.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burgoyne RD, Helassa N, McCue HV, Haynes LP (2019) Calcium Sensors in Neuronal Function and Dysfunction. Cold Spring Harb Perspect Biol 11(5). 10.1101/cshperspect.a035154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schrank S, Barrington N, Stutzmann GE (2019) Calcium-Handling Defects and Neurodegenerative Disease. Cold Spring Harb Perspect Biol. 10.1101/cshperspect.a035212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berridge MJ (2014) Calcium signalling and psychiatric disease: bipolar disorder and schizophrenia. Cell Tissue Res 357:477–92. 10.1007/s00441-014-1806-z [DOI] [PubMed] [Google Scholar]
- 17.Shigetomi E, Saito K, Sano F, Koizumi S (2019) Aberrant Calcium Signals in Reactive Astrocytes: A Key Process in Neurological Disorders. Int J Mol Sci 20 10.3390/ijms20040996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verkhratsky A, Rodríguez-Arellano JJ, Parpura V, Zorec R (2017) Astroglial calcium signalling in Alzheimer’s disease. Biochem Biophys Res Commun 483:1005–1012. 10.1016/j.bbrc.2016.08.088 [DOI] [PubMed] [Google Scholar]
- 19.Nedergaard M, Rodríguez JJ, Verkhratsky A (2010) Glial calcium and diseases of the nervous system. Cell Calcium 47:140–9. 10.1016/j.ceca.2009.11.010 [DOI] [PubMed] [Google Scholar]
- 20.Blaustein MP, Lederer WJ (1999) Sodium/Calcium Exchange: Its Physiological Implications. Physiol Rev 79:763–854. [DOI] [PubMed] [Google Scholar]
- 21.DiPolo Reinaldo; Beauge L (1987) Plasma membrane mechanisms for intracellular calcium regulation in squid axons. Acta Physiol Pharmacol Latinoam 37:437–444. [PubMed] [Google Scholar]
- 22.Khananshvili D (1990) Distinction between the two basic mechanisms of cation transport in the cardiac Na(+)−Ca2+ exchange system. Biochemistry 29:2437–42. 10.1021/bi00462a001 [DOI] [PubMed] [Google Scholar]
- 23.Reeves JP, Condrescu M, Chernaya G, Gardner JP (1994) Na+/Ca2+ antiport in the mammalian heart. J Exp Biol 196:375–388. [DOI] [PubMed] [Google Scholar]
- 24.Baker PF, Blaustein MP, Hodgkin AL, Steinhardt RA (1969) The influence of calcium on sodium efflux in squid axons. J Physiol 200:431–58. 10.1113/jphysiol.1969.sp008702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reyes RC, Verkhratsky A, Parpura V (2012) Plasmalemmal Na+/Ca2+ exchanger modulates Ca2+-dependent exocytotic release of glutamate from rat cortical astrocytes. ASN Neuro 4 10.1042/AN20110059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parpura V, Sekler I, Fern R (2016) Plasmalemmal and mitochondrial Na(+) −Ca(2+) exchange in neuroglia. Glia 64:1646–54. 10.1002/glia.22975 [DOI] [PubMed] [Google Scholar]
- 27.Roome CJ, Power EM, Empson RM (2013) Transient reversal of the sodium/calcium exchanger boosts presynaptic calcium and synaptic transmission at a cerebellar synapse. J Neurophysiol 109:1669–80. 10.1152/jn.00854.2012 [DOI] [PubMed] [Google Scholar]
- 28.Jeon D, Yang Y-M, Jeong M-J, et al. (2003) Enhanced learning and memory in mice lacking Na+/Ca2+ exchanger 2. Neuron 38:965–76. 10.1016/s0896-6273(03)00334-9 [DOI] [PubMed] [Google Scholar]
- 29.Molinaro P, Viggiano D, Nisticò R, et al. (2011) Na+ −Ca2+ exchanger (NCX3) knock-out mice display an impairment in hippocampal long-term potentiation and spatial learning and memory. J Neurosci 31:7312–21. 10.1523/JNEUROSCI.6296-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molinaro P, Cuomo O, Pignataro G, et al. (2008) Targeted disruption of Na+/Ca2+ exchanger 3 (NCX3) gene leads to a worsening of ischemic brain damage. J Neurosci 28:1179–84. 10.1523/JNEUROSCI.4671-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pignataro G, Sirabella R, Anzilotti S, et al. (2014) Does Na+/Ca2+ exchanger, NCX, represent a new druggable target in stroke intervention? Transl Stroke Res 5:145–55. 10.1007/s12975-013-0308-8 [DOI] [PubMed] [Google Scholar]
- 32.Jeffs GJ, Meloni BP, Sokolow S, et al. (2008) NCX3 knockout mice exhibit increased hippocampal CA1 and CA2 neuronal damage compared to wild-type mice following global cerebral ischemia. Exp Neurol 210:268–273. 10.1016/j.expneurol.2007.10.013 [DOI] [PubMed] [Google Scholar]
- 33.Sirabella R, Sisalli MJ, Costa G, et al. (2018) NCX1 and NCX3 as potential factors contributing to neurodegeneration and neuroinflammation in the A53T transgenic mouse model of Parkinson’s Disease. Cell Death Dis 9:725 10.1038/s41419-018-0775-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takuma K, Ago Y, Matsuda T (2013) The glial sodium-calcium exchanger: a new target for nitric oxide-mediated cellular toxicity. Curr Protein Pept Sci 14:43–50. [DOI] [PubMed] [Google Scholar]
- 35.Noda M, Ifuku M, Mori Y, Verkhratsky A (2013) Calcium influx through reversed NCX controls migration of microglia In: Advances in Experimental Medicine and Biology. Springer, Boston, MA, pp 289–294 [DOI] [PubMed] [Google Scholar]
- 36.Boscia F, Gala R, Pannaccione A, et al. (2009) NCX1 expression and functional activity increase in microglia invading the infarct core. Stroke 40:3608–17. 10.1161/STROKEAHA.109.557439 [DOI] [PubMed] [Google Scholar]
- 37.Khananshvili D (2013) The SLC8 gene family of sodium-calcium exchangers (NCX) - structure, function, and regulation in health and disease. Mol Aspects Med 34:220–35. 10.1016/j.mam.2012.07.003 [DOI] [PubMed] [Google Scholar]
- 38.Lytton J (2007) Na + /Ca 2+ exchangers: three mammalian gene families control Ca 2+ transport. Biochem J 406:365–382. 10.1042/BJ20070619 [DOI] [PubMed] [Google Scholar]
- 39.Carafoli E (1987) Intracellular calcium homeostasis. Annu Rev Biochem 56:395–433. 10.1146/annurev.bi.56.070187.002143 [DOI] [PubMed] [Google Scholar]
- 40.Philipson KD, Nicoll DA (2000) Sodium-Calcium Exchanger: A Molecular Perspective. Annu Rev Physiol 62:111–133. 10.1146/annurev.physiol.62.1.111 [DOI] [PubMed] [Google Scholar]
- 41.Nicoll D, Longoni S, Philipson K (1990) Molecular cloning and functional expression of the cardiac sarcolemmal Na(+)−Ca2+ exchanger. Science 250:562–565. 10.1126/science.1700476 [DOI] [PubMed] [Google Scholar]
- 42.Li Z, Matsuokas S, Hryshkob LV, et al. (1994) Cloning of the NCX2 isoform of the plasma membrane Na+−Ca2+ exchanger. J Biol Chem 269:17434–17439. [PubMed] [Google Scholar]
- 43.Nicoll DA, Quednau BD, Qui Z, et al. (1996) Cloning of a third mammalian Na+−Ca2+ exchanger, NCX3. J Biol Chem 271:24914–21. 10.1074/jbc.271.40.24914 [DOI] [PubMed] [Google Scholar]
- 44.On C, Marshall CR, Perry SF, et al. (2009) Characterization of zebrafish (Danio rerio) NCX4: a novel NCX with distinct electrophysiological properties. Am J Physiol Physiol 296:C173–C181. 10.1152/ajpcell.00455.2008 [DOI] [PubMed] [Google Scholar]
- 45.On C, Marshall CR, Chen N, et al. (2008) Gene structure evolution of the Na+−Ca2+ exchanger (NCX) family. BMC Evol Biol 8:127 10.1186/1471-2148-8-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marshall CR, Fox JA, Butland SL, et al. (2005) Phylogeny of Na+/Ca2+ exchanger (NCX) genes from genomic data identifies new gene duplications and a new family member in fish species. Physiol Genomics 21:161–73. 10.1152/physiolgenomics.00286.2004 [DOI] [PubMed] [Google Scholar]
- 47.Boyman L, Williams GSB, Khananshvili D, et al. (2013) NCLX: The mitochondrial sodium calcium exchanger. J. Mol. Cell. Cardiol 59:205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palty R, Silverman WF, Hershfinkel M, et al. (2010) NCLX is an essential component of mitochondrial Na+/Ca 2+ exchange. Proc Natl Acad Sci U S A 107:436–441. 10.1073/pnas.0908099107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palty R, Sekler I (2012) The mitochondrial Na+/Ca2+ exchanger. Cell Calcium 52:9–15 [DOI] [PubMed] [Google Scholar]
- 50.Linck B, Qiu Z, He Z, et al. (1998) Functional comparison of the three isoforms of the Na+/Ca2+ exchanger (NCX1, NCX2, NCX3). Am J Physiol 274:C415–23. 10.1152/ajpcell.1998.274.2.C415 [DOI] [PubMed] [Google Scholar]
- 51.Ren X, Philipson KD (2013) The topology of the cardiac Na+/Ca2+ exchanger, NCX1. J Mol Cell Cardiol 57:68–71. 10.1016/j.yjmcc.2013.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liao J, Li H, Zeng W, et al. (2012) Structural insight into the ion-exchange mechanism of the sodium/calcium exchanger. Science 335:686–90. 10.1126/science.1215759 [DOI] [PubMed] [Google Scholar]
- 53.Liao J, Marinelli F, Lee C, et al. (2016) Mechanism of extracellular ion exchange and binding-site occlusion in a sodium/calcium exchanger. Nat Struct Mol Biol 23:590–599. 10.1038/nsmb.3230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Giladi M, Shor R, Lisnyansky M, Khananshvili D (2016) Structure-functional basis of ion transport in sodium–calcium exchanger (NCX) proteins. Int. J. Mol. Sci 17: 1949 10.3390/ijms17111949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jardetzky O (1966) Simple allosteric model for membrane pumps. Nature 211:969–970. 10.1038/211969a0 [DOI] [PubMed] [Google Scholar]
- 56.Forrest LR, Krämer R, Ziegler C (2011) The structural basis of secondary active transport mechanisms. Biochim Biophys Acta 1807:167–188. 10.1016/j.bbabio.2010.10.014 [DOI] [PubMed] [Google Scholar]
- 57.Khananshvili D (2014) Sodium-calcium exchangers (NCX): molecular hallmarks underlying the tissue-specific and systemic functions. Pflugers Arch 466:43–60. 10.1007/s00424-013-1405-y [DOI] [PubMed] [Google Scholar]
- 58.Giladi M, Hiller R, Hirsch JA, Khananshvili D (2013) Population shift underlies Ca2+-induced regulatory transitions in the sodium-calcium exchanger (NCX). J Biol Chem 288:23141–23149. 10.1074/jbc.M113.471698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ottolia M, Nicoll DA, John S, Philipson KD (2010) Interactions between Ca2+ binding domains of the Na +−Ca2+ exchanger and secondary regulation. Channels 4: 159–162. 10.4161/chan.4.3.11386 [DOI] [PubMed] [Google Scholar]
- 60.Giladi M, Lee SY, Ariely Y, et al. (2017) Structure-based dynamic arrays in regulatory domains of sodium-calcium exchanger (NCX) isoforms. Sci Rep 7:993 10.1038/s41598-017-01102-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hilge M, Aelen J, Vuister GW (2006) Ca2+ Regulation in the Na+/Ca2+ Exchanger Involves Two Markedly Different Ca2+ Sensors. Mol Cell 22:15–25. 10.1016/j.molcel.2006.03.008 [DOI] [PubMed] [Google Scholar]
- 62.Besserer GM, Ottolia M, Nicoll DA, et al. (2007) The second Ca2+-binding domain of the Na+−Ca 2+ exchanger is essential for regulation: Crystal structures and mutational analysis. Proc Natl Acad Sci U S A 104:18467–18472. 10.1073/pnas.0707417104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hilgemann DW, Matsuoka S, Nagel GA, Collins A (1992) Steady-state and dynamic properties of cardiac sodium-calcium exchange. Sodium-dependent inactivation. J Gen Physiol 100:905–932. 10.1085/jgp.100.6.905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hilge M (2013) Ca(2+) regulation in the Na(+)/Ca (2+) exchanger features a dual electrostatic switch mechanism. Adv Exp Med Biol 961:27–33. 10.1007/978-1-4614-4756-6_3 [DOI] [PubMed] [Google Scholar]
- 65.Hilge M (2012) Ca2+ regulation of ion transport in the Na+/Ca2+ exchanger. J Biol Chem 287:31641–31649. 10.1074/jbc.R112.353573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tal I, Kozlovsky T, Brisker D, et al. (2016) Kinetic and equilibrium properties of regulatory Ca2+-binding domains in sodium–calcium exchangers 2 and 3. Cell Calcium 59:181–188. 10.1016/J.CECA.2016.01.008 [DOI] [PubMed] [Google Scholar]
- 67.Steinman L, Altmann D, Sansom D, et al. (1996) Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system. Cell 85:299–302. 10.1016/S0092-8674(00)81107-1 [DOI] [PubMed] [Google Scholar]
- 68.Lee SY, Giladi M, Bohbot H, et al. (2016) Structure-dynamic basis of splicing-dependent regulation in tissue-specific variants of the sodium-calcium exchanger. FASEB J 30:1356–1366. 10.1096/fj.15-282251 [DOI] [PubMed] [Google Scholar]
- 69.Giladi M, Bohbot H, Buki T, et al. (2012) Dynamic features of allosteric Ca2+ sensor in tissue-specific NCX variants. Cell Calcium 51:478–485. 10.1016/j.ceca.2012.04.007 [DOI] [PubMed] [Google Scholar]
- 70.Matsuoka S, Nicoll DA, He Z, Philipson KD (1997) Regulation of cardiac Na(+)−Ca2+ exchanger by the endogenous XIP region. J Gen Physiol 109:273–286. 10.1085/jgp.109.2.273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li Z, Nicoll DA, Collins A, et al. (1991) Identification of a peptide inhibitor of the cardiac sarcolemmal Na(+)−Ca2+ exchanger. J Biol Chem 266:1014–1020 [PubMed] [Google Scholar]
- 72.Giladi M, Tal I, Khananshvili D (2016) Structural Features of Ion Transport and Allosteric Regulation in Sodium-Calcium Exchanger (NCX) Proteins. Front Physiol 7:30 10.3389/fphys.2016.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hilgemann DW, Collins A, Matsuoka S (1992) Steady-state and dynamic properties of cardiac sodium-calcium exchange. Secondary modulation by cytoplasmic calcium and ATP. J Gen Physiol 100:933–961. 10.1085/jgp.100.6.933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chernysh O, Condrescu M, Reeves JP (2008) Sodium-dependent inactivation of sodium/calcium exchange in transfected Chinese hamster ovary cells. Am J Physiol Cell Physiol 295:C872–882. 10.1152/ajpcell.00221.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Verkhratsky A, Trebak M, Perocchi F, et al. (2018) Crosslink between calcium and sodium signalling. Exp Physiol 103:157–169. 10.1113/EP086534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Michel LYM, Verkaart S, Koopman WJH, et al. (2014) Function and regulation of the Na+−Ca2+ exchanger NCX3 splice variants in brain and skeletal muscle. J Biol Chem 289:11293–11303. 10.1074/jbc.M113.529388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.DiPolo R, Beaugé L (2006) Sodium/calcium exchanger: Influence of metabolic regulation on ion carrier interactions. Physiol Rev 86:155–203 [DOI] [PubMed] [Google Scholar]
- 78.Berberián G, Bollo M, Montich G, et al. (2009) A novel lipid binding protein is a factor required for MgATP stimulation of the squid nerve Na+/Ca2+ exchanger. Biochim Biophys Acta - Biomembr 1788:1255–1262. 10.1016/j.bbamem.2008.12.016 [DOI] [PubMed] [Google Scholar]
- 79.Cousido-Siah A, Ayoub D, Berberián G, et al. (2012) Structural and functional studies of ReP1-NCXSQ, a protein regulating the squid nerve Na+/Ca2+ exchanger. Acta Crystallogr D Biol Crystallogr 68:1098–107. 10.1107/S090744491202094X [DOI] [PubMed] [Google Scholar]
- 80.Lariccia V, Amoroso S (2018) Calcium- and ATP-dependent regulation of Na/Ca exchange function in BHK cells: Comparison of NCX1 and NCX3 exchangers. Cell Calcium 73:95–103. 10.1016/j.ceca.2018.04.007 [DOI] [PubMed] [Google Scholar]
- 81.Dyck C, Omelchenko A, Elias CL, et al. (1999) Ionic Regulatory Properties of Brain and Kidney Splice Variants of the NCX1 Na-Ca 2 Exchanger. J Gen Physiol 114:701–711. 10.1085/jgp.114.5.701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Matsuoka S (2004) Forefront of Na+/Ca2+ exchanger studies: Regulation kinetics of Na+/Ca2+ exchangers. J Pharmacol Sci 96:12–14. [DOI] [PubMed] [Google Scholar]
- 83.Quednau BD, Nicoll D, Philipson KD (1997) Tissue specificity and alternative splicing of the Na+/Ca2+ exchanger isoforms NCXl, NCX2, and NCX3 in rat. Am J Physiol 272:C1250–1261. 10.1152/ajpcell.1997.272.4.C1250 [DOI] [PubMed] [Google Scholar]
- 84.Nakasaki Y, Iwamoto T, Hanada H, et al. (1993) Cloning of the rat aortic smooth muscle Na+/Ca2+ exchanger and tissue-specific expression of isoforms1. J Biochem 114:528–534. 10.1093/oxfordjournals.jbchem.a124211 [DOI] [PubMed] [Google Scholar]
- 85.Rose CR, Ransom BR (1996) Mechanisms of H+ and Na+ changes induced by glutamate, kainate, and D-aspartate in rat hippocampal astrocytes. J Neurosci 16:5393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nicholas SB, Yang W, Lee SL, et al. (1998) Alternative promoters and cardiac muscle cell-specific expression of the Na+/Ca2+ exchanger gene. Am J Physiol - Hear Circ Physiol 274:H217–H232. 10.1152/ajpheart.1998.274.1.H217 [DOI] [PubMed] [Google Scholar]
- 87.Barnes KV, Cheng G, Dawson MM, Menick DR (1997) Cloning of cardiac, kidney, and brain promoters of the feline ncx1 gene. J Biol Chem 272:11510–11517. [PubMed] [Google Scholar]
- 88.Koban MU, Brugh SA, Riordon DR, et al. (2001) A distant upstream region of the rat multipartite Na(+)−Ca(2+) exchanger NCX1 gene promoter is sufficient to confer cardiac-specific expression. Mech Dev 109:267–79. 10.1016/s0925-4773(01)00548-2 [DOI] [PubMed] [Google Scholar]
- 89.Lee S-L, Yu ASL, Lytton J (1994) Tissue-specific Expression of Na+−Ca2+ exchanger Isoforms. J Biol Chem 269:14849–14852. [PubMed] [Google Scholar]
- 90.Yu X-M, Groveman RB, Fang X-Q, Lin S-X (2010) The role of intracellular sodium (Na+) in the regulation of calcium (Ca2+)-mediated signaling and toxicity. Health (Irvine Calif) 02:8–15. 10.4236/health.2010.21002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stadelmann C, Timmler S, Barrantes-Freer A, Simons M (2019) Myelin in the Central Nervous System: Structure, Function, and Pathology. Physiol Rev 99:1381–1431. 10.1152/physrev.00031.2018 [DOI] [PubMed] [Google Scholar]
- 92.Pfeiffer S, Warrington AE, Bansal R (1993) The oligodendrocyte and its many cellular processes. Trends Cell Biol 3:191–197. 10.1016/0962-8924(93)90213-K [DOI] [PubMed] [Google Scholar]
- 93.Michalski JP, Kothary R (2015) Oligodendrocytes in a nutshell. Front Cell Neurosci 9:340 10.3389/fncel.2015.00340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Elbaz B, Popko B (2019) Molecular Control of Oligodendrocyte Development. Trends Neurosci 42:263–277. 10.1016/j.tins.2019.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tiane A, Schepers M, Rombaut B, et al. (2019) From OPC to Oligodendrocyte: An Epigenetic Journey. Cells 8 10.3390/cells8101236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sock E, Wegner M (2019) Transcriptional control of myelination and remyelination. Glia 67:2153–2165. 10.1002/glia.23636 [DOI] [PubMed] [Google Scholar]
- 97.Wheeler NA, Fuss B (2016) Extracellular cues influencing oligodendrocyte differentiation and (re)myelination. Exp Neurol 283:512–30. 10.1016/j.expneurol.2016.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yu L, Colvin RA (1997) Regional differences in expression of transcripts for Na+/Ca2+ exchanger isoforms in rat brain. Mol Brain Res 50:285–292. 10.1016/S0169-328X(97)00202-7 [DOI] [PubMed] [Google Scholar]
- 99.Tong XP, Li XY, Zhou B, et al. (2009) Ca2+ signaling evoked by activation of Na+ channels and Na+/Ca2+ exchangers is required for GABA-induced NG2 cell migration. J Cell Biol 186:113–128. 10.1083/jcb.200811071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Steffensen I, Waxman SG, Mills L, Stys PK (1997) Immunolocalization of the Na+ −Ca2+ exchanger in mammalian myelinated axons. Brain Res 776:1–9. 10.1016/S0006-8993(97)00868-8 [DOI] [PubMed] [Google Scholar]
- 101.Boscia F, D’avanzo C, Pannaccione A, et al. (2012) Silencing or knocking out the Na+/Ca2+ exchanger-3 (NCX3) impairs oligodendrocyte differentiation. Cell Death Differ 19:562–572. 10.1038/cdd.2011.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Boscia F, D’Avanzo C, Pannaccione A, et al. (2013) New Roles of NCX in Glial Cells: Activation of Microglia in Ischemia and Differentiation of Oligodendrocytes. Adv Exp Med Biol 961:307–316. 10.1007/978-1-4614-4756-6_26 [DOI] [PubMed] [Google Scholar]
- 103.Simpson PB, Armstrong RC (1999) Intracellular signals and cytoskeletal elements involved in oligodendrocyte progenitor migration. Glia 26:22–35. [PubMed] [Google Scholar]
- 104.Paez PM, Fulton D, Colwell CS, Campagnoni AT (2009) Voltage-operated Ca2+ and Na+ channels in the oligodendrocyte lineage. J Neurosci Res 87:3259–3266. 10.1002/jnr.21938 [DOI] [PubMed] [Google Scholar]
- 105.Habermacher C, Angulo MC, Benamer N (2019) Glutamate versus GABA in neuron-oligodendroglia communication. Glia 67:2092–2106. 10.1002/glia.23618 [DOI] [PubMed] [Google Scholar]
- 106.Friess M, Hammann J, Unichenko P, et al. (2016) Intracellular ion signaling influences myelin basic protein synthesis in oligodendrocyte precursor cells. Cell Calcium 60:322–330. 10.1016/j.ceca.2016.06.009 [DOI] [PubMed] [Google Scholar]
- 107.Hammann J, Bassetti D, White R, et al. (2018) α2 isoform of Na +,K + -ATPase via Na +,Ca 2+ exchanger modulates myelin basic protein synthesis in oligodendrocyte lineage cells in vitro. Cell Calcium 73:1–10. 10.1016/j.ceca.2018.03.003 [DOI] [PubMed] [Google Scholar]
- 108.Thakurela S, Garding A, Jung RB, et al. (2016) The transcriptome of mouse central nervous system myelin. Sci Rep 6:25828 10.1038/srep25828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Martinez-Lozada Z, Waggener CT, Kim K, et al. (2014) Activation of sodium-dependent glutamate transporters regulates the morphological aspects of oligodendrocyte maturation via signaling through calcium/calmodulin-dependent kinase IIβ’s actin-binding/-stabilizing domain. Glia 62:1543–1558. 10.1002/glia.22699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Suárez-Pozos E, Thomason EJ, Fuss B (2019) Glutamate Transporters: Expression and Function in Oligodendrocytes. Neurochem Res. 10.1007/s11064-018-02708-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Martínez-Lozada Z, Hernández-Kelly LC, Aguilera J, et al. (2011) Signaling through EAAT-1/GLAST in cultured Bergmann glia cells. Neurochem Int 59:871–879. 10.1016/j.neuint.2011.07.015 [DOI] [PubMed] [Google Scholar]
- 112.Iwamoto T, Kita S, Shigekawa M (2002) [Functional analysis of Na+/Ca2+ exchanger using novel drugs and genetically engineered mice]. Nihon Yakurigaku Zasshi 120:91P–93P [PubMed] [Google Scholar]
- 113.Iwamoto T, Inoue Y, Ito K, et al. (2004) The exchanger inhibitory peptide region-dependent inhibition of Na +/Ca2+ exchange by SN-6 [2- [4-(4-nitrobenzyloxy)benzyl]-thiazolidine-4-carboxylic acid ethyl ester], a novel benzyloxyphenyl derivative. Mol Pharmacol 66:45–55. 10.1124/mol.66.1.45 [DOI] [PubMed] [Google Scholar]
- 114.Gong XQ, Frandsen A, Lu WY, et al. (2005) D-aspartate and NMDA, but not L-aspartate, block AMPA receptors in rat hippocampal neurons. Br J Pharmacol 145:449–459. 10.1038/sj.bjp.0706199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sugiyama H, Ito I, Watanabe M (1989) Glutamate receptor subtypes may be classified into two major categories: a study on Xenopus oocytes injected with rat brain mRNA. Neuron 3:129–132. 10.1016/0896-6273(89)90121-9 [DOI] [PubMed] [Google Scholar]
- 116.Bender AS, Woodbury DM, White HS (1997) The rapid L- and D-aspartate uptake in cultured astrocytes. Neurochem Res 22:721–726. 10.1023/a:1027358211472 [DOI] [PubMed] [Google Scholar]
- 117.de Rosa V, Secondo A, Pannaccione A, et al. (2019) D-Aspartate treatment attenuates myelin damage and stimulates myelin repair. EMBO Mol Med 11:e9278 10.15252/emmm.201809278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kukley M, Capetillo-Zarate E, Dietrich D (2007) Vesicular glutamate release from axons in white matter. Nat Neurosci 10:311–20. 10.1038/nn1850 [DOI] [PubMed] [Google Scholar]
- 119.Ziskin JL, Nishiyama A, Rubio M, et al. (2007) Vesicular release of glutamate from unmyelinated axons in white matter. Nat Neurosci 10:321–330. 10.1038/nn1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wake H, Lee PR, Fields RD (2011) Control of Local Protein Synthesis… Background. Science 333:1647–1652. 10.1126/science.1206998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Foster AY, Bujalka H, Emery B (2019) Axoglial interactions in myelin plasticity: Evaluating the relationship between neuronal activity and oligodendrocyte dynamics. Glia 67:2038–2049. 10.1002/glia.23629 [DOI] [PubMed] [Google Scholar]
- 122.Monje M (2018) Myelin Plasticity and Nervous System Function. Annu Rev Neurosci 41:61–76. 10.1146/annurev-neuro-080317-061853 [DOI] [PubMed] [Google Scholar]
- 123.Chorghay Z, Káradóttir RT, Ruthazer ES (2018) White Matter Plasticity Keeps the Brain in Tune: Axons Conduct While Glia Wrap. Front Cell Neurosci 12:428 10.3389/fncel.2018.00428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bechler ME, Swire M, Ffrench-Constant C (2018) Intrinsic and adaptive myelination-A sequential mechanism for smart wiring in the brain. Dev Neurobiol 78:68–79. 10.1002/dneu.22518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Back SA, Luo NL, Borenstein NS, et al. (2001) Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci 21:1302–1312. 10.1523/jneurosci.21-04-01302.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cai Q, Ma T, Tian Y, et al. (2018) Catalpol inhibits ischemia-induced premyelinating oligodendrocyte damage through regulation of intercellular calcium homeostasis via Na+/Ca2+ exchanger 3. Int J Mol Sci 19 10.3390/ijms19071925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ma T, Wu X, Cai Q, et al. (2015) Lead Poisoning Disturbs Oligodendrocytes Differentiation Involved in Decreased Expression of NCX3 Inducing Intracellular Calcium Overload. Int J Mol Sci 16: 19096–19110. 10.3390/ijms160819096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Deng W, McKinnon RD, Poretz RD (2001) Lead Exposure Delays the Differentiation of Oligodendroglial Progenitors in Vitro. Toxicol Appl Pharmacol 174:235–244. 10.1006/TAAP.2001.9219 [DOI] [PubMed] [Google Scholar]
- 129.Pignataro G, Cuomo O, Vinciguerra A, et al. (2013) NCX as a key player in the neuroprotection exerted by ischemic preconditioning and postconditioning. In: Advances in Experimental Medicine and Biology. Adv Exp Med Miol 961:223–240. 10.1007/978-1-4614-4756-6_19 [DOI] [PubMed] [Google Scholar]
- 130.Pignataro G, Gala R, Cuomo O, et al. (2004) Two sodium/calcium exchanger gene products, NCX1 and NCX3, play a major role in the development of permanent focal cerebral ischemia. Stroke 35:2566–2570. 10.1161/01.STR.0000143730.29964.93 [DOI] [PubMed] [Google Scholar]
- 131.Gerkau NJ, Rakers C, Durry S, et al. (2018) Reverse NCX attenuates cellular sodium loading in metabolically compromised cortex. Cereb Cortex 28:4264–4280. 10.1093/cercor/bhx280 [DOI] [PubMed] [Google Scholar]
- 132.Chen H, Kintner DB, Jones M, et al. (2007) AMPA-mediated excitotoxicity in oligodendrocytes: Role for Na + −K + −Cl − co-transport and reversal of Na + /Ca 2+ exchanger. J Neurochem 102:1783–1795. 10.1111/j.1471-4159.2007.04638.x [DOI] [PubMed] [Google Scholar]
- 133.Stover JF, Pleines UE, Morganti-Kossmann MC, et al. (1997) Neurotransmitters in cerebrospinal fluid reflect pathological activity. Eur J Clin Invest 27:1038–1043. 10.1046/j.1365-2362.1997.2250774.x [DOI] [PubMed] [Google Scholar]
- 134.Macrez R, Stys PK, Vivien D, et al. (2016) Mechanisms of glutamate toxicity in multiple sclerosis: biomarker and therapeutic opportunities. Lancet Neurol 15:1089–1102. 10.1016/S1474-4422(16)30165-X [DOI] [PubMed] [Google Scholar]
- 135.Newcombe J, Uddin A, Dove R, et al. (2008) Glutamate receptor expression in multiple sclerosis lesions. Brain Pathol 18:52–61. 10.1111/j.1750-3639.2007.00101.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Follett PL, Deng W, Dai W, et al. (2004) Glutamate receptor-mediated oligodendrocyte toxicity in periventricular leukomalacia: a protective role for topiramate. J Neurosci 24:4412–4420. 10.1523/JNEUROSCI.0477-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.McCracken E, Fowler JH, Dewar D, et al. (2002) Grey matter and white matter ischemic damage is reduced by the competitive AMPA receptor antagonist, SPD 502. J Cereb Blood Flow Metab 22:1090–1097. 10.1097/00004647-200209000-00006 [DOI] [PubMed] [Google Scholar]
- 138.Park E, Velumian AA, Fehlings MG (2004) The role of excitotoxicity in secondary mechanisms of spinal cord injury: A review with an emphasis on the implications for white matter degeneration. J Neurotrauma 21:754–774. 10.1089/0897715041269641 [DOI] [PubMed] [Google Scholar]
- 139.Rosenberg LJ, Teng YD, Wrathall JR (1999) 2,3-Dihydroxy-6-nitro-7-sulfamoyl-benzo(f)quinoxaline reduces glial loss and acute white matter pathology after experimental spinal cord contusion. J Neurosci 19:464–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wosik K, Ruffini F, Almazan G, et al. (2004) Resistance of human adult oligodendrocytes to AMPA/kainate receptor-mediated glutamate injury. Brain 127:2636–2648. 10.1093/brain/awh302 [DOI] [PubMed] [Google Scholar]
- 141.Itoh T, Beesley J, Itoh A, et al. (2002) AMPA glutamate receptor-mediated calcium signaling is transiently enhanced during development of oligodendrocytes. J Neurochem 81:390–402. 10.1046/j.1471-4159.2002.00866.x [DOI] [PubMed] [Google Scholar]
- 142.Ceprian M, Fulton D (2019) Glial Cell AMPA Receptors in Nervous System Health, Injury and Disease. Int J Mol Sci 20 10.3390/ijms20102450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Fan X, Xiong Y, Wang Y (2019) A reignited debate over the cell(s) of origin for glioblastoma and its clinical implications. Front Med 13:531–539. 10.1007/s11684-019-0700-1 [DOI] [PubMed] [Google Scholar]
- 144.Hsu LS, Chou WY, Chueh SH (1995) Evidence for a Na+/Ca2+ exchanger in neuroblastoma x glioma hybrid NG108–15 cells. Biochem J 309:445–452. 10.1042/bj3090445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Amoroso S, De Maio M, Russo GM, et al. (1997) Pharmacological evidence that the activation of the Na(+)−Ca2+ exchanger protects C6 glioma cells during chemical hypoxia. Br J Pharmacol 121:303–309. 10.1038/sj.bjp.0701092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rodrigues T, Estevez GNN, Tersariol ILDS (2019) Na+/Ca2+ exchangers: Unexploited opportunities for cancer therapy? Biochem Pharmacol 163:357–361. 10.1016/j.bcp.2019.02.032 [DOI] [PubMed] [Google Scholar]
- 147.Hu H-J, Wang S-S, Wang Y-X, et al. (2019) Blockade of the forward Na+ /Ca2+ exchanger suppresses the growth of glioblastoma cells through Ca2+ -mediated cell death. Br J Pharmacol 176:2691–2707. 10.1111/bph.14692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Boscia F, D’Avanzo C, Pannaccione A, et al. (2013) New roles of NCX in glial cells: Activation of microglia in ischemia and differentiation of oligodendrocytes. Adv Exp Med Biol 961:307–316. 10.1007/978-1-4614-4756-6_26 [DOI] [PubMed] [Google Scholar]
- 149.Marques S, Zeisel A, Codeluppi S, et al. (2016) Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science 352:1326–1329. 10.1126/science.aaf6463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Foerster S, Hill MFE, Franklin RJM (2019) Diversity in the oligodendrocyte lineage: Plasticity or heterogeneity? Glia 67:1797–1805. 10.1002/glia.23607 [DOI] [PubMed] [Google Scholar]
- 151.Spitzer SO, Sitnikov S, Kamen Y, et al. (2019) Oligodendrocyte Progenitor Cells Become Regionally Diverse and Heterogeneous with Age. Neuron 101:459–471.e5. 10.1016/j.neuron.2018.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jäkel S, Agirre E, Mendanha Falcão A, et al. (2019) Altered human oligodendrocyte heterogeneity in multiple sclerosis. Nature 566:543–547. 10.1038/s41586-019-0903-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Watanabe Y (2019) Cardiac Na+/Ca2+ exchange stimulators among cardioprotective drugs. J Physiol Sci. 10.1007/s12576-019-00721-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Song S, Luo L, Sun B, Sun D (2019) Roles of glial ion transporters in brain diseases. Glia. 10.1002/glia.23699 [DOI] [PMC free article] [PubMed] [Google Scholar]


