Abstract
Renal interstitial fibrosis is the most important pathological process in chronic renal failure. Previous studies have shown that poricoic acid A (PAA), the main chemical constituent on the surface layer of the mushroom Poria cocos, has protective effects against oxidative stress and acute kidney injury. The present study aimed to investigate the potential roles of PAA on the pathological process of renal fibrosis and the associated molecular mechanism. The NRK-49F cell line was treated with transforming growth factor-β1 (TGF-β1) with or without PAA or platelet-derived growth factor C (PDGF-C). Cell Counting Kit-8 assay, western blotting and 5-ethynyl-2'-deoxyuridine immunofluorescence staining were performed to examine cell growth, protein expression and cell proliferation, respectively. Data from the present study showed that 10 µM PAA attenuated TGF-β1-induced NRK-49F cell extracellular matrix (ECM) accumulation, fibrosis formation and proliferation. Renal fibrosis with the activation of Smad3 and mitogen-activated protein kinase (MAPK) pathways were also inhibited by PAA treatment. PDGF-C reversed the inhibitory effects of PAA on TGF-β1-induced renal fibroblast proliferation and activation of the Smad3/MAPK pathway. The present study suggested that suppression of TGF-β1-induced renal fibroblast ECM accumulation, fibrosis formation and proliferation by PAA is mediated via the inhibition of the PDGF-C, Smad3 and MAPK pathways. The present findings not only revealed the potential anti-fibrotic effects of PAA on renal fibroblasts, but also provided a new insight into the prevention of fibrosis formation via regulation of the PDGF-C, Smad3 and MAPK signaling pathways.
Keywords: poricoic acid A, transforming growth factor-β1, renal fibrosis, platelet-derived growth factor C, cell proliferation
Introduction
Renal fibrosis is caused by imbalance between synthesis and degradation of the extracellular matrix (ECM) constituents, including collagen I, III and IV, as a result of various pathological factors, leading to glomerulosclerosis, tubulointerstitial fibrosis, hyalinization and sclerosis of the renal small vessels (1,2). These cellular and molecular events ultimately result in parenchymal obstruction and renal failure (3,4). Renal fibrosis can also result from a variety of other factors that are associated with kidney injury, including hypertension, inflammation, high sugar, high fat and drug damage (5). However, previous studies have shown that even if these factors are controlled effectively, including blood pressure, blood glucose and drug damage, the process of renal fibrosis remains difficult to prevent (6). Therefore, investigating the molecular mechanism underlying the occurrence and development of fibrosis, identifying therapeutic agents and target genes that can directly interfere with the fibrotic process has become an important topic of study in recent years (7-9).
The formation and progression of renal fibrosis is a complex and dynamic process that includes inflammatory cell infiltration, fibroblast activation and proliferation, ECM accumulation, tubular atrophy and microvascular degeneration (10,11). A number of genes have been reported to be involved in this process. Among them, transforming growth factor (TGF)-β1 is considered to be the most important fibrogenic factor, which initiates the occurrence and accelerates the progress of fibrosis (12). In addition, recent reports also found that a number of other factors, including platelet-derived growth factor (PDGF), Smad3, connective tissue growth factor and angiotensin II, enhanced the constituents of the ECM (13,14). Although the mechanism behind the formation and development of renal fibrosis has been extensively studied, effective treatment strategies has yet to be found (15,16). Therefore, it is of importance to explore the mechanism of renal fibrosis further to find novel potential therapeutic drugs.
Poria cocos Wolf (Polyporaceae) is a traditional Chinese medicine that has been applied for >2,000 years in China that is also widely distributed and easy to obtain because it exists in many plants (17,18). Reported medicinal properties that have been associated with Poria cocos include diuretic effects, invigorating the spleen, tranquilizes the heart and edema elimination (19-22). Poricoic acid A (PAA) is one of the main chemical constituents on the surface layer of Poria cocos Wolf. PAA has been documented to significantly alleviate oxidative stress and suppress the increase in inflammatory factors during acute kidney injury (23). Chen et al (24) reported that PAA exerted renoprotective and antifibrotic effects by inhibiting TGF-β/Smad3 and Wnt/β-catenin signaling pathways, whilst other studies have also demonstrated that PAA could suppress the renin-angiotensin system (RAS) to prevent chronic kidney disease progression (25,26). However, to the best of our knowledge, the mechanistic effects of PAA on renal fibrosis remain to be elucidated.
The present study aimed to investigate the effects of PAA on TGF-β1-induced renal fibroblast proliferation and fibrosis formation in addition to the potential mechanism underlying the actions of PAA. Information from the present study may provide a novel avenue for the treatment of fibrosis formation during the development of renal failure.
Materials and methods
Cell culture and drug treatment
NRK-49F cells (cat. no. CRL-1570; American Type Culture Collection), a rat renal interstitial fibroblast cell line (27,28), were used to investigate the effects of PAA on renal fibrosis. Cells were cultured in DMEM (cat. no. 11965-092; Gibco, Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (cat. no. SH30071.02; HyClone; GE Healthcare Life Sciences) in a 5% CO2 atmosphere at 37˚C.
To determine the optimal concentration of PAA (Shanxi Medicine company), cells were exposed to serial concentrations of PAA (1, 2, 5, 10, 15 and 20 µM) for 24 h at 37˚C in a 5%CO2 incubator. The effects of PAA on renal fibrosis were also determined using recombinant human TGF-β1 (5 ng/ml; cat. no. 240-B; R&D Systems, Inc.) in the presence or absence of recombinant PDGF-C protein (50 ng/ml; cat. no. 1687-CC; R&D Systems, Inc.) for 24 h at 37˚C in a 5% CO2 incubator.
Cell growth assay
NRK-49F cells were cultured in 96-well plates, 100 µl per well with 1x104 cells and exposed to 10 µmol PAA for 24 h. Subsequently, 30 µl CCK-8 solution (Dojindo Molecular Technologies, Inc.) was added to each well where the cells were incubated for 4 h at 37˚C. The optical density (OD) value was detected at a wavelength of 450 nm where a microplate reader (Thermo Fisher Scientific, Inc.) was used to calculate cell viability and inhibition rate. The detailed calculations are as follows: Cell viability (%)=[(As-Ab)/(Ac-Ab)]x100; inhibition rate (%)=[(Ac-As)/(Ac-Ab)] x100; As=OD value of the experimental wells; Ab=OD value of blank wells; Ac=OD value of control wells.
Western blotting
The expression of PDGF-C/Smad3/MAPK or ECM related proteins was analyzed by western blotting. Total protein was isolated from NRK-49F cells with or without PAA treatments using radioimmunoprecipitation assay (RIPA) buffer (cat. no. 89900; Thermo Fisher Scientific, Inc.). Protein levels were quantified using a BCA protein assay kit (Thermo Fisher Scientific, Inc.). A total of 30 µg protein was loaded in each lane and subsequently run on 12% SDS-PAGE gels. The separated proteins were subsequently transferred to PVDF membranes. Then, the membranes were washed three times with 0.1% Tween-20 TBS solution (TBST) and incubated for 1 h at room temperature with 5% of non-fat milk (cat. no. 1706404; Bio-rad, Laboratories, Inc.). Subsequently, the following primary antibodies were used at 1:1,000 dilution: E-cadherin (cat. no. 14472S; Cell Signaling Technology, Inc.), collagen I (cat. no. 84336S; Cell Signaling Technology, Inc.), collagen IV (cat. no. 50273S; Cell Signaling Technology, Inc.), alpha smooth muscle actin (α-SMA; cat. no. ab5694; Abcam), fibronectin (FN; cat. no. 26836S; Cell Signaling Technology, Inc.), PDGF-C (cat. no. abc1392; Sigma-Aldrich; Merck KGaA), phosphorylated (p)-Smad3 (cat. no. 9520; Cell Signaling Technology, Inc.), Smad3 (cat. no. 9523; Cell Signaling Technology, Inc.), p-ERK1/2 (cat. no. 4370; Cell Signaling Technology, Inc.), ERK1/2 (cat. no. 4695; Cell Signaling Technology, Inc.), p-p38 (cat. no. 4511; Cell Signaling Technology, Inc.), p38 (cat. no. 8690; Cell Signaling Technology, Inc.) and GAPDH (cat. no. sc-137179; Santa Cruz Biotechnology, Inc.). Following incubation with the respective primary antibodies overnight at 4˚C, the membranes were washed three times with TBST solution. Subsequently, the membranes were exposed to horseradish peroxidase-conjugated goat anti-rabbit (1:5,000; cat. no. ab6721; Abcam), goat anti-mouse (1:5,000; cat. no. ab05719; Abcam), and rabbit anti-goat (1:5,000; cat. no. ab5755; Abcam) secondary antibodies for 1 h at room temperature prior to treatment with ECL reagent (cat. no. RPN2232; GE Healthcare), using autoluminography to visualize protein bands. Protein expression was then quantified using ImageJ (version 1.48V; National Institutes of Health) with GAPDH as the loading control.
Cell proliferation assay
To assess kidney cell proliferation, 2x105 NRK-49F cells/per well in 1ml culture medium were plated onto coverslips in a 6-well plate and allowed to grow overnight at 37˚C in a 5% CO2 incubator. Cells were incubated with Alexa Fluor 555 labeled with 5-ethynyl-2'-deoxyuridine (EdU; cat. no. A10044; Invitrogen; Thermo Fisher Scientific, Inc.) at a final concentration of 10 µM for 24 h at 37˚C in 5% CO2 before being harvested and subjected to treatments in accordance with the Click-iT®EdU Alexa Fluor® 555 Imaging kit (cat. no. C10353; Invitrogen; Thermo Fisher Scientific, Inc., cat. no. C10353). All procedures were performed according to the manufacturer's instructions. Nuclei were stained with DAPI at final a concentration of 3 µM. EdU-positive cells with DAPI-labeled nuclei were counted to evaluate cell proliferation.
Statistical analysis
Data are presented as the mean ± SD. Statistical analysis was performed using GraphPad Prism 6.0 (GraphPad Software, Inc.). One-way ANOVA followed by Tukey's post hoc test was used for comparisons between multiple groups. P<0.05 was considered to indicate a statistically significant difference. All data presented are from at least three independent experiments.
Results
In vitro screening for the optimal concentration of PAA
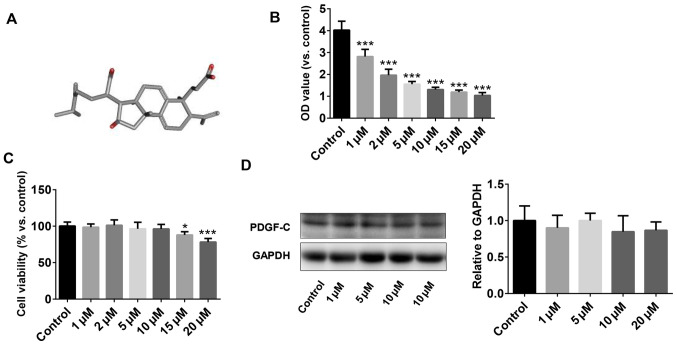
The chemical structure of PAA extracted from the epidermis of Poria cocos is shown in Fig. 1A. The effect of PAA on cell viability was measured using CCK-8 assay. To test the optimal concentration of PAA on cell growth, serial concentrations of PAA, 1-20 µM final concentration were used. The results revealed that concentrations of PAA >1 µM reduced NRK-49F cell viability in a dose-dependent manner. However, the inhibitory effect did not increase further when the concentration of PAA applied was >10 µM (Fig. 1B). The CCK-8 assay results demonstrated that PAA exerted no cytotoxic effects on NRK-49F cells at concentrations <10 µM, but cell viability was significantly reduced following treatment with 15 and 20 µM PAA (Fig. 1C). Therefore, 10 µM PAA was applied for subsequent experiments. The effects of different concentrations of PAA on PDGF-C protein expression were explored further by western blotting (Fig. 1D). PAA did not exert significant effects on PDGF-C protein expression at all concentrations tested compared with that of the control group.
Figure 1.
Effects of PAA treatment on NRK-49F cell viability. (A) The chemical structural formula of PAA. (B) Cytotoxicity of NRK-49F cells following treatment with PAA as measured using Cell Counting kit-8. (C) Cytotoxicity was measured after treatment with PAA at the indicated concentrations. (D) PDGF-C protein expression was measured by western blotting after treatment with PAA at the indicated concentrations. n=6. *P<0.05 and ***P<0.001 vs. Control. PAA, poricoic acid A; OD, optical density; PDGF-C, platelet-derived growth factor C.
PAA inhibits TGF-β1-induced ECM accumulation, fibrosis formation and proliferation by NRK-49F cells
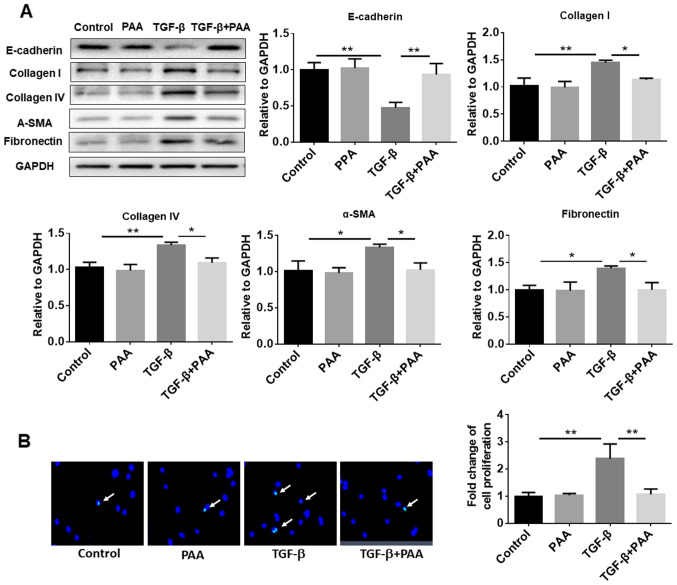
To explore the effects of PAA on TGF-β1-induced NRK-49F cell ECM accumulation and fibrosis formation, western blotting was performed to examine the expression of E-cadherin, collagen I, α-SMA, FN and collagen IV, proteins associated with ECM and fibrosis. The results showed that PAA treatment alone had no significant effect on the expression levels of these proteins compared with those of the control group. In contrast, TGF-β1 alone decreased the expression of E-cadherin and increased the expression of collagen I, α-SMA, FN and collagen IV. However, in the presence of TGF-β1, PAA significantly increased E-cadherin expression and significantly reducing those of collagen I, α-SMA, FN and collagen IV in NRK-49F cells compared with their expression in cells following TGF-b1 treatment alone (Fig. 2A). In addition, EdU staining results confirmed that the TGF-β1-induced NRK-49F cell proliferation was significantly attenuated by PAA (Fig. 2B).
Figure 2.
Effect of PAA on the expression of ECM proteins and TGF-β1-induced cell proliferation. (A) Expression of E-cadherin, collagen I, collagen IV, α-SMA and fibronectin, proteins associated with ECM, were measured by western blotting following treatment with PAA, TGF-β1 or a combination of both PAA and TGF-β1. (B) Cell proliferation was measured after TGF-β1 and/or PAA treatment using 5-ethynyl-2'-deoxyuridine staining. n=3. *P<0.05 and **P<0.01. Magnification x40. PAA, poricoic acid A; TGF-β1, transforming growth factor-β1; α-SMA, α-smooth muscle actin; ECM, extracellular matrix.
PDGF-C and Smad3/MAPK pathways are suppressed by PAA treatment in NRK-49F cells
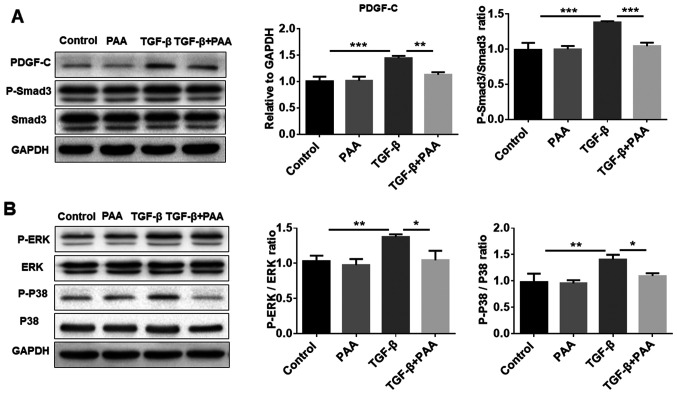
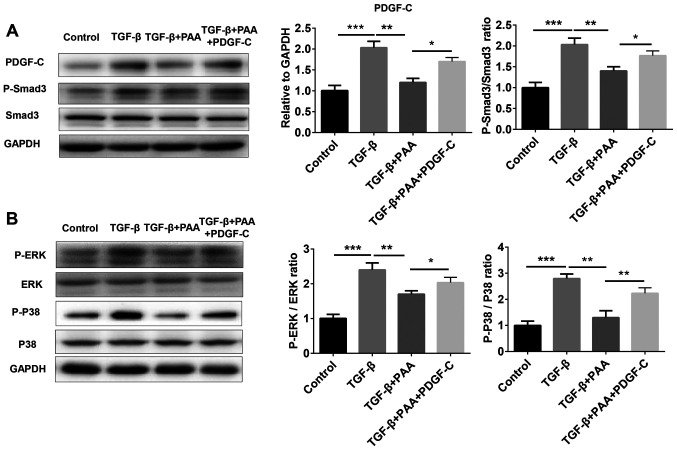
Previous studies have shown that PDGF-C serves an important role in renal fibrosis as a mediator of renal interstitial fibrosis, where the Smad3 and MAPK pathways are reported to be involved (29,30). To explore further whether PAA can regulate the PDGF-C, Smad3 and MAPK signaling pathways in NRK-49F cells, western blotting was performed to examine Smad3 and MAPK phosphorylation. The results showed that PAA alone did not significantly alter PDGF-C and p-Smad3 levels compared with those in the control group; however, PAA treatment significantly reversed TGF-β1-induced PDGF-C expression, Smad3 (Fig. 3A), ERK1/2 and P38 MAPK phosphorylation (Fig. 3B). These data suggest that PAA can inhibit TGF-β1-induced activation of the Smad and MAPK signaling pathways.
Figure 3.
Effect of PAA on PDGF-C, Smad and MAPK signaling on NRK-49F cells. (A) The expression of PDGF-C and Smad3 phosphorylation were examined by western blot analysis following treatment with PAA, TGF-β1 or a combination of PAA and TGF-β1. (B) Western blot analysis was used to evaluate the phosphorylation of proteins associated with MAPK signaling. n=3. *P<0.05, **P<0.01 and ***P<0.001. PAA, poricoic acid A; TGF-β1, transforming growth factor-β1.
PDGF-C reverses the inhibitory effects of PAA on TGF-β1-stimulated NRK-49F cells
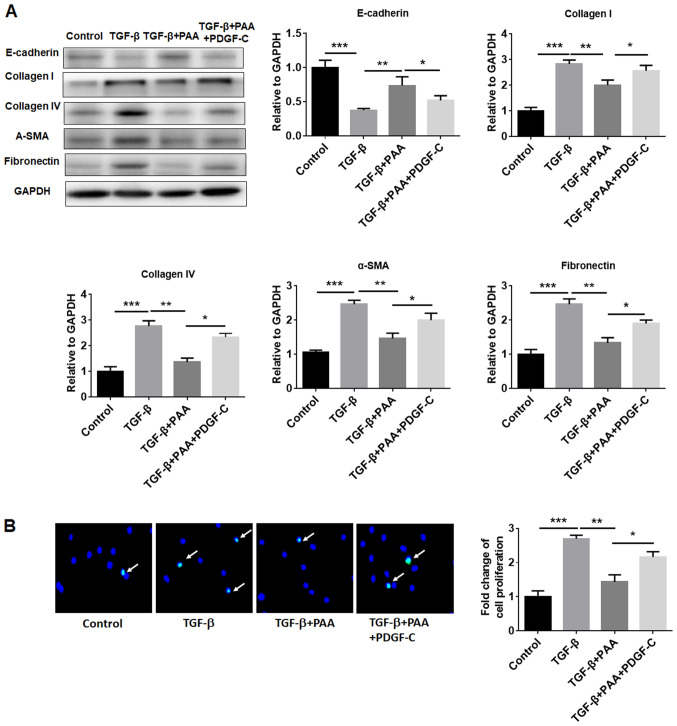
To evaluate the effects of PDGF-C on the inhibitory effects of PAA on NRK-49F cell ECM accumulation, fibrosis formation and proliferation further, western blotting and EdU immunofluorescence staining were performed. The western blotting results revealed that the inhibitory effects of PAA on TGF-β1-induced renal fibroblast ECM accumulation and fibrosis formation was significantly reversed by PDGF-C treatment compared with those observed in the TGF-β1+PAA group (Fig. 4A). PDGF-C treatment also reversed the inhibitory effects of PAA on TGF-β1-induced NRK-49F cell proliferation (Fig. 4B).
Figure 4.
PDGF-C reverses the inhibitory effects of PAA on the expression of extracellular matrix proteins and cell proliferation in NRK-49F cells. (A) Effects of PDGF-C on the expression of E-cadherin, collagen I, collagen IV, α-SMA and fibronectin in PAA- and/or TGF-β1-treated cells were examined by western blotting. (B) PDGF-C reversed the inhibitory effects of PAA on NRK-49F cell proliferation according to 5-ethynyl-2'-deoxyuridine staining. n=3. *P<0.05, **P<0.01 and ***P<0.001. Magnification x40. PAA, poricoic acid A; TGF-β1, transforming growth factor-β1; PDGF-C, platelet-derived growth factor C; α-SMA, α-smooth muscle actin.
PDGF-C treatment eliminates the inhibition of TGF-β1-stimulatedSmad3 and MAPK signaling by PAA in NRK-49F cells
To further examine whether PDGF-C is involved in the inhibitory role of PAA on the Smad3 and MAPK signaling activation induced by TGF-β1, the expression of proteins associated with the Smad3 and MAPK pathways were measured by western blotting in cells treated with or without TGF-β1, PAA and PDGF-C. The results showed that the increased expression of proteins associated with the Smad3 and MAPK pathway induced by TGF-β1 was significantly reduced after PAA treatment (Fig. 5A). By contrast, additive PDGF-C treatment significantly increased the expression of these proteins compared with that in the TGF-β1+PAA groups (Fig. 5).
Figure 5.
PDGF-C treatment reactivates TGF-β1-induced Smad and MAPK signaling in NRK-49F cells. (A) PDGF-C treatment promoted the expression of PDGF-C and phosphorylation of Smad3 in the presence of PAA. (B) PDGF-C treatment increased ERK and P38 MAPK phosphorylation in the presence of PAA. n=3. *P<0.05, **P<0.01 and ***P<0.001. PAA, poricoic acid A; TGF-β1, transforming growth factor-β1; PDGF-C, platelet-derived growth factor C; P38, p38 MAPK.
Discussion
Since renal interstitial fibrosis serves a role in the development of renal failure, the degree of fibrotic development is closely associated with the prognosis of chronic kidney disease and renal failure (31). Therefore, research on the prevention and treatment of renal interstitial fibrosis has garnered attention over recent years (32). Traditional Chinese medicine has also made significant progress in the study of renal fibrosis treatment (33). In the present study, PAA significantly inhibited TGF-β1-induced renal fibroblast proliferation, ECM production and fibrosis formation. The present results suggested that PAA serves a role in the progression of renal fibrosis.
Among the factors associated with renal interstitial fibrosis, TGF-β1 has been most extensively studied (34). A number of studies have shown that a variety of cytokines and growth factors participate in the occurrence and development of renal interstitial fibrosis, of which TGF-β1 appeared to serve the most significant role (35,36). The biological functions of TGF-β1 in kidney development include promotion of ECM accumulation and synthesis of matrix components (37). TGF-β1 has also been implicated in epithelial-mesenchymal transition, whose markers include FN, E-cadherin, vimentin and α-SMA (38). Although several clinical trials targeting TGF-β1 using anti-TGFβ1 antibodies in kidney disease as well as animal model have been performed, no significant improvement in the symptoms have been observed in either patients or mouse models (39-41). Therefore, the present study used a rat renal fibroblast cell line treated with TGF-β1 as a cell model. TGF-β1 significantly promoted fibroblast proliferation and the expression of fibrosis-related proteins. PAA alone did not alter the expression of these proteins, including collagen I, collagen IV, α-SMA, FN, PDGF-C, as well as the phosphorylation of Smad3, ERK and p38, but it could significantly suppress the expressions of these proteins induced by TGF-β1. Other previous studies suggested that PAA could suppress renal fibrosis via inhibiting the functions of TGF-β1 via the RAS/TGF-β/Smad axis (26) or the Wnt/β-catenin pathway (25). The current results showed that PAA extenuated renal cell proliferation through suppressing the Smad3/MAPK pathway. These results can be compared with those obtained in animal models, such as unilateral ureteral obstruction (42) or 5/6 nephrectomy mice (43), where alleviation of renal fibrosis was mediated through the inhibition of the TGF-β/Smad and Wnt/β-catenin signaling pathways in the kidneys or RAS. In addition, other proteins associated with renal fibrosis, including angiotensinogen, renin, angiotensin-converting enzymes and tubular angiotensin II type 1 receptors (25) can be measured in clinical patients in future studies. The results of the present study showed that the inhibitory effects of PAA on TGF-β1-induced renal fibroblast proliferation were mediated by acting the Smad3/MAPK pathway.
PDGF-C, originally identified in platelets, has a variety of documented biological functions (44). Previous studies demonstrated that PDGF-C expression in renal interstitial fibrosis was significantly increased during progression to renal fibrosis (45,46). Inhibition of PDGF-C activity can significantly inhibit the development of renal fibrosis (46,47). In the present study, although PAA alone did not affect PDGF-C expression in NRK-49F cells, PDGF-C significantly reversed the inhibitory effects of PAA in the presence of TGF-β1. By contrast, treatment with PDGF-C can partially reverse the effects of PAA in TGF-β1-induced renal fibroblasts. The present study reported that PDGF-C stimulated mesenchymal cells proliferation through binding to the PDGF-α receptor (44). The interaction between proximal tubule cells and adjacent fibroblasts is critical for renal fibrosis (48). NRK-49F cells were used in the present study since it exhibits a characteristic fibroblast spindle-like morphology that is easy to monitor in response to PDGF (27,28). These results suggested that PAA treatment inhibited the proliferation, ECM aggregation and fibrosis of renal fibroblasts by inhibiting the functions of TGF-β1. However, the inhibitory effects could be reversed by PDGF-C.
Previous studies have revealed that Smad3 and MAPK signaling pathways are involved in the development of renal fibrosis (49,50). The expression of Smad3 and MAPK is significantly activated during renal fibrosis (50,51) and the inhibition of Smad and MAPK pathways has been reported to significantly reduce renal ECM aggregation and fibrosis formation (52). The present study showed that PAA could suppress the increased upregulation of Smad and MAPK signaling induced by TGF-β1, which were reversed by the presence of PDGF-C.
In conclusion, data from the present study suggest that PAA treatment suppressed TGF-β1-induced renal fibroblast ECM accumulation, fibrosis formation and proliferation by inhibiting the PDGF-C, Smad3 and MAPK signaling pathways. PDGF-C can block the effect of PAA on TGF-β1-induced fibroblasts and promoted the activation of the Smad and MAPK pathways induced by TGF-β1. These findings revealed the potential of PAA application in protecting against the fibrosis of renal fibroblasts. The present study also provided a new insight into the prevention of fibrosis formation through regulation by PDGF-C. However, further in vivo studies are necessary to fully clarify the role of PAA in renal fibrosis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author's contributions
QL and GW conceived and designed the experiment. QL, YM and HJ performed experiments and collected and analyzed the data. QL and GW wrote the manuscript. All authors revised and approved the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Shiber S, Eliakim-Raz N, Yair M. Retroperitoneal fibrosis: Case series of five patients and review of the literature. Rev Bras Reumatol Engl Ed. 2016;56:101–104. doi: 10.1016/j.rbre.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Zhou D, Liu Y. Renal fibrosis in 2015: Understanding the mechanisms of kidney fibrosis. Nat Rev Nephrol. 2016;12:68–70. doi: 10.1038/nrneph.2015.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu C, Sun L, Xiao L, Han Y, Fu X, Xiong X, Xu X, Liu Y, Yang S, Liu F, Kanwar YS. Insights into the mechanisms involved in the expression and regulation of extracellular matrix proteins in diabetic nephropathy. Curr Med Chem. 2015;22:2858–2870. doi: 10.2174/0929867322666150625095407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang J, Yang Q, Nie Y, Guo H, Zhang F, Zhou X, Yin X. Tetrahydrobiopterin contributes to the proliferation of mesangial cells and accumulation of extracellular matrix in early-stage diabetic nephropathy. J Pharm Pharmacol. 2017;69:182–190. doi: 10.1111/jphp.12677. [DOI] [PubMed] [Google Scholar]
- 5.Klinkhammer BM, Goldschmeding R, Floege J, Boor P. Treatment of renal fibrosis-turning challenges into opportunities. Adv Chronic Kidney Dis. 2017;24:117–129. doi: 10.1053/j.ackd.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Sandner P, Stasch JP. Anti-fibrotic effects of soluble guanylate cyclase stimulators and activators: A review of the preclinical evidence. Respir Med. 2017;122 (Suppl 1):S1–S9. doi: 10.1016/j.rmed.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Cai J, Tang C, Dong Z. Mitophagy in acute kidney injury and kidney repair. Cells. 2020;9: pii(E338) doi: 10.3390/cells9020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang J, Goldschmeding R, Samarakoon R, Higgins PJ. Protein phosphatase Mg(2+)/Mn(2+) dependent-1A and PTEN deregulation in renal fibrosis: Novel mechanisms and co-dependency of expression. FASEB J. 2020;34:2641–2656. doi: 10.1096/fj.201902015RR. [DOI] [PubMed] [Google Scholar]
- 9.Marko L, Park JK, Henke N, Rong S, Balogh A, Klamer S, Bartolomaeus H, Wilck N, Ruland J, Forslund SK, et al. B-cell lymphoma/leukemia 10 (Bcl10) and angiotensin II-induced kidney injury. Cardiovasc Res. 2019;116:1059–1070. doi: 10.1093/cvr/cvz169. [DOI] [PubMed] [Google Scholar]
- 10.Li O, Ma Q, Li F, Cai GY, Chen XM, Hong Q. Progress of small ubiquitin-related modifiers in kidney diseases. Chin Med J (Engl) 2019;132:466–473. doi: 10.1097/CM9.0000000000000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Genovese F, Manresa AA, Leeming DJ, Karsdal MA, Boor P. The extracellular matrix in the kidney: A source of novel non-invasive biomarkers of kidney fibrosis? Fibrogenesis Tissue Repair. 2014;7(4) doi: 10.1186/1755-1536-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castellone MD, Laukkanen MO. TGF-beta1, WNT, and SHH signaling in tumor progression and in fibrotic diseases. Front Biosci (Schol Ed) 2017;9:31–45. doi: 10.2741/s470. [DOI] [PubMed] [Google Scholar]
- 13.van Roeyen CRC, Martin IV, Drescher A, Schuett KA, Hermert D, Raffetseder U, Otten S, Buhl EM, Braun GS, Kuppe C, et al. Identification of platelet-derived growth factor C as a mediator of both renal fibrosis and hypertension. Kidney Int. 2019;95:1103–1119. doi: 10.1016/j.kint.2018.11.031. [DOI] [PubMed] [Google Scholar]
- 14.Ghayur A, Padwal MK, Liu L, Zhang J, Margetts PJ. SMAD3-dependent and-independent pathways in glomerular injury associated with experimental glomerulonephritis. Am J Physiol Renal Physiol. 2019;317:F152–F62. doi: 10.1152/ajprenal.00406.2018. [DOI] [PubMed] [Google Scholar]
- 15.Breyer MD, Susztak K. The next generation of therapeutics for chronic kidney disease. Nat Rev Drug Discov. 2016;15:568–588. doi: 10.1038/nrd.2016.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mencke R, Olauson H, Hillebrands JL. Effects of Klotho on fibrosis and cancer: A renal focus on mechanisms and therapeutic strategies. Adv Drug Deliv Rev. 2017;121:85–100. doi: 10.1016/j.addr.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Li S, Wang Z, Gu R, Zhao Y, Huang W, Wang Z, Xiao W. A new epidioxy-tetracyclic triterpenoid from Poria cocos Wolf. Nat Prod Res. 2016;30:1712–1717. doi: 10.1080/14786419.2015.1136909. [DOI] [PubMed] [Google Scholar]
- 18.Lee SR, Lee S, Moon E, Park HJ, Park HB, Kim KH. Bioactivity-guided isolation of anti-inflammatory triterpenoids from the sclerotia of Poria cocos using LPS-stimulated Raw264.7 cells. Bioorg Chem. 2017;70:94–99. doi: 10.1016/j.bioorg.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 19.Li S, Zhang J, Li S, Liu C, Liu S, Liu Z. Extraction and separation of lactate dehydrogenase inhibitors from Poria cocos (Schw.) Wolf based on a hyphenated technique and in vitro methods. J Sep Sci. 2017;40:1773–1783. doi: 10.1002/jssc.201700054. [DOI] [PubMed] [Google Scholar]
- 20.Zhao YY, Feng YL, Du X, Xi ZH, Cheng XL, Wei F. Diuretic activity of the ethanol and aqueous extracts of the surface layer of Poria cocos in rat. J Ethnopharmacol. 2012;144:775–778. doi: 10.1016/j.jep.2012.09.033. [DOI] [PubMed] [Google Scholar]
- 21.Feng YL, Lei P, Tian T, Yin L, Chen DQ, Chen H, Mei Q, Zhao YY, Lin RC. Diuretic activity of some fractions of the epidermis of Poria cocos. J Ethnopharmacol. 2013;150:1114–1118. doi: 10.1016/j.jep.2013.10.043. [DOI] [PubMed] [Google Scholar]
- 22.Zhao YY, Feng YL, Bai X, Tan XJ, Lin RC, Mei Q. Ultra performance liquid chromatography-based metabonomic study of therapeutic effect of the surface layer of Poria cocos on adenine-induced chronic kidney disease provides new insight into anti-fibrosis mechanism. PLoS One. 2013;8(e59617) doi: 10.1371/journal.pone.0059617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen DQ, Feng YL, Chen L, Liu JR, Wang M, Vaziri ND, Zhao YY. Poricoic acid A enhances melatonin inhibition of AKI-to-CKD transition by regulating Gas6/AxlNFkappaB/Nrf2 axis. Free Radic Biol Med. 2019;134:484–497. doi: 10.1016/j.freeradbiomed.2019.01.046. [DOI] [PubMed] [Google Scholar]
- 24.Chen DQ, Cao G, Zhao H, Chen L, Yang T, Wang M, Vaziri ND, Guo Y, Zhao YY. Combined melatonin and poricoic acid A inhibits renal fibrosis through modulating the interaction of Smad3 and beta-catenin pathway in AKI-to-CKD continuum. Ther Adv Chronic Dis. 2019;10(2040622319869116) doi: 10.1177/2040622319869116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang M, Chen DQ, Chen L, Cao G, Zhao H, Liu D, Vaziri ND, Guo Y, Zhao YY. Novel inhibitors of the cellular renin-angiotensin system components, poricoic acids, target Smad3 phosphorylation and Wnt/β-catenin pathway against renal fibrosis. Br J Pharmacol. 2018;175:2689–2708. doi: 10.1111/bph.14333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang M, Chen DQ, Chen L, Liu D, Zhao H, Zhang ZH, Vaziri ND, Guo Y, Zhao YY, Cao G. Novel RAS Inhibitors Poricoic Acid ZG and Poricoic Acid ZH attenuate renal fibrosis via a Wnt/β-catenin pathway and targeted phosphorylation of smad3 Signaling. J Agric Food Chem. 2018;66:1828–1842. doi: 10.1021/acs.jafc.8b00099. [DOI] [PubMed] [Google Scholar]
- 27.Tan RJ, Zhou D, Liu Y. Signaling crosstalk between tubular epithelial cells and interstitial fibroblasts after kidney injury. Kidney Dis (Basel) 2016;2:136–144. doi: 10.1159/000446336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang N, Cunningham K, Enger MD. TGF beta elicits opposite responses in clonal subpopulations of NRK-49F cells. Exp Cell Res. 1991;196:13–19. doi: 10.1016/0014-4827(91)90450-9. [DOI] [PubMed] [Google Scholar]
- 29.Floege J, Eitner F, Alpers CE. A new look at platelet-derived growth factor in renal disease. J Am Soc Nephrol. 2008;19:12–23. doi: 10.1681/ASN.2007050532. [DOI] [PubMed] [Google Scholar]
- 30.Huang C, Day ML, Poronnik P, Pollock CA, Chen XM. Inhibition of KCa3.1 suppresses TGF-β1 induced MCP-1 expression in human proximal tubular cells through Smad3, p38 and ERK1/2 signaling pathways. Int J Biochem Cell Biol. 2014;47:1–10. doi: 10.1016/j.biocel.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 31.Liu M, Ning X, Li R, Yang Z, Yang X, Sun S, Qian Q. Signalling pathways involved in hypoxia-induced renal fibrosis. J Cell Mol Med. 2017;21:1248–1259. doi: 10.1111/jcmm.13060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waasdorp M, de Rooij DM, Florquin S, Duitman J, Spek CA. Protease-activated receptor-1 contributes to renal injury and interstitial fibrosis during chronic obstructive nephropathy. J Cell Mol Med. 2019;23:1268–1279. doi: 10.1111/jcmm.14028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu W, Lin S, Cai Q, Zhang L, Shen A, Chen Y, Chu J, Peng J. Qingxuan jiangya decoction mitigates renal interstitial fibrosis in spontaneously hypertensive rats by regulating transforming growth factor-β1/Smad signaling pathway. Evid Based Complement Alternat Med. 2017;2017(1576328) doi: 10.1155/2017/1576328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lawson JS, Liu HH, Syme HM, Purcell R, Wheeler-Jones CPD, Elliott J. The cat as a naturally occurring model of renal interstitial fibrosis: Characterisation of primary feline proximal tubular epithelial cells and comparative pro-fibrotic effects of TGF-β1. PLoS One. 2018;13(e0202577) doi: 10.1371/journal.pone.0202577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu J, Yu TT, Zhang K, Li M, Shi HJ, Meng XJ, Zhu LS, Zhu LK. HGF alleviates renal interstitial fibrosis via inhibiting the TGF-β1/SMAD pathway. Eur Rev Med Pharmacol Sci. 2018;22:7621–7627. doi: 10.26355/eurrev_201811_16378. [DOI] [PubMed] [Google Scholar]
- 36.Loeffler I. MKP2 suppresses TGF-β1-induced epithelial-to-mesenchymal transition through JNK inhibition. Clin Sci (Lond) 2019;133:545–550. doi: 10.1042/CS20180881. [DOI] [PubMed] [Google Scholar]
- 37.Okuda S, Languino LR, Ruoslahti E, Border WA. Elevated expression of transforming growth factor-beta and proteoglycan production in experimental glomerulonephritis. Possible role in expansion of the mesangial extracellular matrix. J Clin Invest. 1990;86:453–462. doi: 10.1172/JCI114731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi HI, Kim DH, Park JS, Kim IJ, Kim CS, Bae EH, Ma SK, Lee TH, Kim SW. Peroxiredoxin V (PrdxV) negatively regulates EGFR/Stat3-mediated fibrogenesis via a Cys48-dependent interaction between PrdxV and Stat3. Sci Rep. 2019;9(8751) doi: 10.1038/s41598-019-45347-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vincenti F, Fervenza FC, Campbell KN, Diaz M, Gesualdo L, Nelson P, Praga M, Radhakrishnan J, Sellin L, Singh A, et al. A phase 2, double-blind, placebo-controlled, randomized study of fresolimumab in patients with steroid-resistant primary focal segmental glomerulosclerosis. Kidney Int Rep. 2017;2:800–810. doi: 10.1016/j.ekir.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trachtman H, Fervenza FC, Gipson DS, Heering P, Jayne DR, Peters H, Rota S, Remuzzi G, Rump LC, Sellin LK, et al. A phase 1, single-dose study of fresolimumab, an anti-TGF-β antibody, in treatment-resistant primary focal segmental glomerulosclerosis. Kidney Int. 2011;79:1236–1243. doi: 10.1038/ki.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGaraughty S, Davis-Taber RA, Zhu CZ, Cole TB, Nikkel AL, Chhaya M, Doyle KJ, Olson LM, Preston GM, Grinnell CM, et al. Targeting Anti-TGF-β therapy to fibrotic kidneys with a dual specificity antibody approach. J Am Soc Nephrol. 2017;28:3616–3626. doi: 10.1681/ASN.2017010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang ZH, He JQ, Zhao YY, Chen HC, Tan NH. Asiatic acid prevents renal fibrosis in UUO rats via promoting the production of 15d-PGJ2, an endogenous ligand of PPAR-γ. Acta Pharmacol Sin. 2020;41:373–382. doi: 10.1038/s41401-019-0319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bian X, Bai Y, Su X, Zhao G, Sun G, Li D. Knockdown of periostin attenuates 5/6 nephrectomy-induced intrarenal renin-angiotensin system activation, fibrosis, and inflammation in rats. J Cell Physiol. 2019;234:22857–22873. doi: 10.1002/jcp.28849. [DOI] [PubMed] [Google Scholar]
- 44.Li X, Ponten A, Aase K, Karlsson L, Abramsson A, Uutela M, Bäckström G, Hellström M, Boström H, Li H, et al. PDGF-C is a new protease-activated ligand for the PDGF alpha-receptor. Nat Cell Biol. 2000;2:302–309. doi: 10.1038/35010579. [DOI] [PubMed] [Google Scholar]
- 45.Eitner F, Bucher E, van Roeyen C, Kunter U, Rong S, Seikrit C, Villa L, Boor P, Fredriksson L, Bäckström G, et al. PDGF-C is a proinflammatory cytokine that mediates renal interstitial fibrosis. J Am Soc Nephrol. 2008;19:281–289. doi: 10.1681/ASN.2007030290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boor P, Babickova J, Steegh F, Hautvast P, Martin IV, Djudjaj S, Nakagawa T, Ehling J, Gremse F, Bücher E, et al. Role of platelet-derived growth factor-CC in capillary rarefaction in renal fibrosis. Am J Pathol. 2015;185:2132–2142. doi: 10.1016/j.ajpath.2015.04.022. [DOI] [PubMed] [Google Scholar]
- 47.Wang Y, Abu-Asab MS, Yu CR, Tang Z, Shen D, Tuo J, Li X, Chan CC. Platelet-derived growth factor (PDGF)-C inhibits neuroretinal apoptosis in a murine model of focal retinal degeneration. Lab Invest. 2014;94:674–682. doi: 10.1038/labinvest.2014.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen CL, Chou KJ, Lee PT, Chen YS, Chang TY, Hsu CY, Huang WC, Chung HM, Fang HC. Erythropoietin suppresses epithelial to mesenchymal transition and intercepts Smad signal transduction through a MEK-dependent mechanism in pig kidney (LLC-PK1) cell lines. Exp Cell Res. 2010;316:1109–1118. doi: 10.1016/j.yexcr.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 49.Grynberg K, Ma FY, Nikolic-Paterson DJ. The JNK signaling pathway in renal fibrosis. Front Physiol. 2017;8(829) doi: 10.3389/fphys.2017.00829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu ZJ, Shu S, Li ZJ, Liu YM, Zhang RY, Zhang Y. Liuwei Dihuang pill treats diabetic nephropathy in rats by inhibiting of TGF-β/SMADS, MAPK, and NF-kB and upregulating expression of cytoglobin in renal tissues. Medicine (Baltimore) 2017;96(e5879) doi: 10.1097/MD.0000000000005879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakagawa T, Lan HY, Glushakova O, Zhu HJ, Kang DH, Schreiner GF, Böttinger EP, Johnson RJ, Sautin YY. Role of ERK1/2 and p38 mitogen-activated protein kinases in the regulation of thrombospondin-1 by TGF-beta1 in rat proximal tubular cells and mouse fibroblasts. J Am Soc Nephrol. 2005;16:899–904. doi: 10.1681/ASN.2004080689. [DOI] [PubMed] [Google Scholar]
- 52.Okano K, Hibi A, Miyaoka T, Inoue T, Sugimoto H, Tsuchiya K, Akiba T, Nitta K. Inhibitory effects of the transcription factor Ets-1 on the expression of type I collagen in TGF-β1-stimulated renal epithelial cells. Mol Cell Biochem. 2012;369:247–254. doi: 10.1007/s11010-012-1388-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.