Abstract
Dysfunction in dopaminergic neuronal systems underlie a number of neurologic and psychiatric disorders such as Parkinson’s disease, drug addiction, and schizophrenia. Dopamine systems communicate via two mechanisms, a fast “phasic” release (sub-second to second) that is related to salient stimuli and a slower “tonic” release (minutes to hours) that regulates receptor tone. Alterations in tonic levels are thought to be more critically important in enabling normal motor, cognitive, and motivational functions, and dysregulation in tonic dopamine levels are associated with neuropsychiatric disorders. Therefore, development of neurochemical recording techniques that enable rapid, selective, and quantitative measurements of changes in tonic extracellular levels are essential in determining the role of dopamine in both normal and disease states. Here, we review state-of-the-art advanced analytical techniques for in vivo detection of tonic levels, with special focus on electrochemical techniques for detection in humans.
Keywords: Dopamine, Electrochemistry, Voltammetry, Tonic dopamine, Sensors, Neurochemistry
1. Introduction
The typical human brain has been estimated to have over 100 trillion synapses. Each of these synapses has a unique chemical composition further complicated by intricate regulation of neurotransmitter release and clearance kinetics, as well as connectivity with other brain regions. Understanding neurotransmitter signaling dynamics provides insights into healthy and diseased brain activity and network regulation. For example, normal functioning of dopaminergic systems underlie learning, incentive-motivation, and reward processing [1–7]. However, dysregulation of dopamine neurotransmission is found in many disease states, most notably in neurological and psychiatric disorders such as Parkinson’s disease, drug addiction, schizophrenia, Tourette’s syndrome, and indirectly in mediating anhedonia in depression [8,9]. Given that the population of patients affected by these diseases is substantial, understanding dopamine signaling dynamics is requisite for probing and treating these diseases. In this review we describe various techniques used for in vivo tonic dopamine detection. We also evaluate their usefulness in both clinical and pre-clinical investigations. Finally, we discuss the applicability of electrochemical techniques for enhancing understanding of neurochemical changes that may arise in response to deep brain stimulation (DBS) therapy in the treatment of these diseases characterized by dopaminergic dysfunction.
Dopamine release in the brain occurs on two timescales, termed phasic and tonic [8,10–12]. Phasic dopamine neurotransmission occurs as a result of burst-firing of neurons that release dopamine rapidly into the synaptic cleft in relatively high concentrations [ 12,13 ]. Relatively slower tonic release is caused by pace-maker-like firing of neurons that leads to an extrasynaptic tonic level dopamine [8,12,14–16]. Overall dopaminergic tone has been thought to consist primarily of extrasynaptic escape of intrasynaptic dopamine molecules [8,17]. This spillover, or volume transmission, produces and enhances tonic dopamine levels that affect transporter function and synaptic plasticity [15,18,19].
Changes in tonic dopamine levels are postulated to have important functional roles in incentive-motivation and underlie behavioral flexibility [14]. For example, increases in tonic dopamine levels in the striatum and nucleus accumbens have been shown to be positively correlated with movement and motivation, respectively [20–23]. Studies have also shown that elevations in tonic dopamine levels directly relate to long-term average reward response rates [21,24]. In contrast, decreases in the tonic firing of dopaminergic neurons have been observed where expected rewards have been omitted (i.e., reward prediction error) or where aversive stimuli have been applied [3,25–28]. Omission of expected rewards, and decreased tonic dopamine levels in the striatum and nucleus accumbens, are thought to facilitate behavioral flexibility and learning of new response strategies by affecting cortico-striatal processing [29,30]. Further research has found that this may be mediated through reductions or pauses in tonic dopamine stimulation of high-affinity D2 receptors [31], which exhibit effects on prefrontal cortical inputs [32]. As such, techniques that can measure variations in tonic dopamine levels in vivo with high resolution will certainly help to clarify the functional role of dopaminergic tone and are indeed important for studying disease pathology in pre-clinical and clinical investigations.
Techniques developed to effectively quantify basal dopamine extracellular levels in the brain can be divided into three technological in vivo methodologies: (1) microdialysis, (2) imaging, and (3) electrochemical techniques. Microdialysis has been classically used for measurements of tonic levels of neurochemicals due to its ability to collect and selectively measure concentrations of a number of chemical species in the extracellular space [33]. However, its applicability in humans is limited by reliance upon external analysis techniques which are not made in real-time or easily translatable to ambulatory subjects. Furthermore, microdialysis probes are relatively large and as a result have been shown to cause inflammatory damage which may affect accurate sampling [34]. More recent advances in tonic dopamine detection have centered on imaging and voltammetric techniques. While the established clinical use and the non-invasive nature of functional MRI (fMRI) and positron emission tomography (PET) imaging are major advantages, they are currently unable to directly probe tonic dopamine levels, have poor spatial resolution (>1 mm), and are considerably less cost-effective than electrochemical techniques [35,36]. Therefore, substantial efforts have been made to develop new electrochemical methods for tonic dopamine detection due to their accessibility and enhanced spatial and temporal resolution.
Presently, electrochemical techniques for tonic dopamine concentration measurements encompass amperometry, fast-scan cyclic voltammetry (FSCV), and recently developed complex voltammetric waveform techniques. While amperometric techniques have indeed been shown to measure, albeit through simulations, tonic dopamine levels [37], their lack of chemical specificity and limited measurement lifetime have prompted a shift in focus towards modified FSCV and complex waveform techniques. Notable modifications to FSCV for tonic dopamine measurement include: kinetic uptake and diffusion modeling, waveforms that rely on charge balancing, and modified waveforms with stripping and adsorption parameters. These techniques have been successfully applied in vivo to terminal fields of rodent models and demonstrated consistency within ~100 nM extracellular concentrations of dopamine between studies [38–40]. In addition to modified FSCV techniques, a number of complex voltammetric waveform techniques have been conceived [41–43]. One notable technique uses multiple cyclic square waves with dynamic background subtraction and current modeling (termed multiple-cyclic square wave voltammetry or M-CSWV) to achieve objective and reproducible measurements of tonic levels [42,44]. The success of these various electrochemical methods, including their high spatial, temporal, and chemical resolution, provides great promise for the study of neurophysiology and the treatment of disease in humans. We foresee that the continued development of these techniques will change the way we understand and treat neurologic disease [45,46].
2. Techniques for neurochemical activity monitoring
2.1. Microdialysis
Microdialysis is a technique used to sample chemicals from the extracellular fluid [47]. A microdialysis probe consists of an inner lumen and an outer lumen with a semi-permeable membrane that allows for diffusion of molecules from the external environment into the probe. Perfusion fluid is pumped into the inner lumen, and the resultant dialysate is collected (Fig. 1). The dialysate is then purified for the analyte of interest and quantified with external analysis [47]. Microdialysis has several advantages for quantification of tonic dopamine levels, most important of which is the ability to analyze the dialysate with a variety of techniques that provide high chemical specificity (e.g., high-performance liquid chromatography followed by electrochemical detection, fluorescence, or mass spectrometry) [33,47–51]. In addition, these techniques can detect and distinguish dopamine at low nanomolar concentrations [52–54]. One particular variation of microdialysis that has been especially important for determination of tonic dopamine concentrations is termed no-net-flux calibration. This technique differs from conventional microdialysis by adding known concentrations of the neurotransmitter of interest to the perfusate. By doing so, this method accounts for neurotransmitter clearance kinetics which can confound measurements, and thus provides a means of estimating extracellular concentrations adjacent to the dialysis membrane surface [55]. Studies that have examined tonic dopamine concentration using no-net-flux calibration have found normal striatal levels to be in the 2.5–15 nM range [56–59]. In addition to its use in examining normal and disease brain physiology, microdialysis can also be applied to investigate therapeutic mechanisms. For example, a study in a rodent Parkinson’s disease model showed striatal tonic dopamine levels increased in response to subthalamic nucleus deep brain stimulation [60].
Fig. 1.
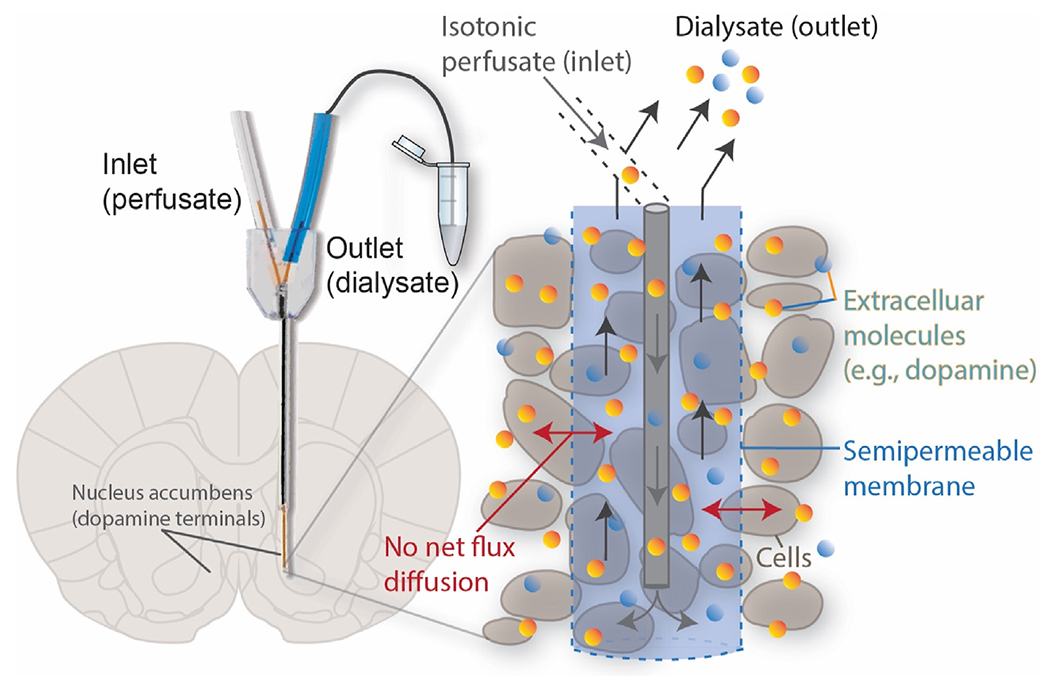
No-net-flux microdialysis to determine the extracellular concentrations of analytes in the brain. A microdialysis probe is inserted into the brain region of interest and perfused with a known concentration of analyte. When no net flux through the semipermeable membrane is established, the known perfusate concentration is equal to the extracellular concentration. Dialysate flows through the outlet and is collected for external analysis.
While microdialysis has been used to measure tonic extracellular dopamine concentrations in vivo, it has limitations for human translation. The diameter and typical length of the microdialysis probe’s sampling membrane (>200 μm and 1–2 mm, respectively) is larger than the diameter of typical neuronal cells and terminals (~10–50 μm and 0.1–1 μm, respectively) and thus, inherently causes significant tissue damage [34,61]. This damage can perturb normal neuronal function and extracellular concentration estimates may be inaccurate [34,62–64]. Thus, while microdialysis has been thought to measure absolute concentrations of neurochemicals in extracellular fluid, computer modeling has shown tissue damage affects neuronal terminal dynamics and can obstruct normal extracellular volume transmission, ultimately obfuscating concentration estimates [65]. In addition, while < 4 min sampling rates have been achieved for catecholamines [53,66–71], more commonly, 10 min sampling rates are necessary at flow rates of 0.1–3.0 μL/min. This rate allows for enough analyte to cross the membrane into the dialysate to be collected for analysis. Due to this, the interpretation of microdialysis has caused some controversy because of tissue damage from the probe [34,72] and disruption in release and reuptake mechanisms affecting neurotransmitter concentrations within the extracellular space [47,65,73]. In addition, microdialysis perfusion, dialysate collection, and detection instrumentation is not presently amenable to chronic human studies and therefore its use is limited to pre-clinical and intra-operative investigations [74,75]. Advances in microfabrication, sampling methods, and modulation of the immune response around the probe have all expanded microdialysis applications and may one day permit chronic human studies [68–70]. Furthermore, future advances may eventually enable temporal resolution capable of recording individual efflux events with high spatial resolution, which would expand the phenomena accessible for study with microdialysis [71].
3. Imaging
3.1. PET and fMRI
Positron emission tomography (PET) is a technique whereby molecular radiotracers, structurally similar to drugs that bind endogenous targets, are used to indirectly measure neurotransmission. Radioactive analogs first bind to endogenous receptors; subsequent displacement of the radiotracer by the endogenous analyte causes a measurable change in radioactivity that is dependent on the concentration of the endogenous analyte [35,76]. A number of radiotracers for dopamine receptors have been developed, both receptor agonists and antagonists [76]. These have enabled indirect measurement of tonic dopamine dynamics in disease states, during behavioral tests, and in response to pharmacological agents and electrical stimulation [77,78]. One newly reported technique utilized the radioligand [11C]raclopride to visualize sub-second dopamine dynamics [79]. These variations in the [11C]raclopride signal were used to extrapolate low frequency extracellular dopamine fluctuations and the results were validated against FSCV in vivo [80].
In addition to PET imaging, functional MRI (fMRI) has been used as a non-invasive way to measure regional metabolic activity and indirectly yield information about dopaminergic neurotransmission [81]. fMRI takes advantage of the magnetic properties of hydrogen to visualize tissues and fluids of interest. This can be applied to visualize blood flow in the brain. When hemoglobin, a ferrous component of blood that transports oxygen, is oxygenated, it is diamagnetic and opposes the applied magnetic field. When it is de-oxygenated, it is paramagnetic, and aligns with the magnetic field. Therefore, the oxygenation level of blood determines image contrast, and this is known as the blood oxygen level-dependent (BOLD) signal [82]. BOLD signals have been shown to correlate with blood flow and thus, metabolic rate. Therefore, brain areas experiencing high metabolic rates have greater BOLD signals [82]. Brain regions known for dopamine transmission (e.g., the nucleus accumbens), can be assessed with fMRI and dopamine content indirectly quantified [81]. The resolution of fMRI with BOLD contrast is typically 4 mm, which is sufficient for differentiation between dopaminergic centers in the human brain and the surrounding structures. With perturbations, for example pharmacologic drug modulators, indirect estimations of dopamine concentrations have been extrapolated [36,83,84]. Additionally, changes in regional metabolic activity can be measured when patients are undergoing behavioral tasks, taking pharmacologic agents, or receiving electrical stimulation [36]. However, it is important to note that fMRI techniques may only suggest changes in neurotransmitter concentrations due to changes in blood flow; a fundamental limitation of fMRI is the inability to definitively report changes in dopamine concentration. This limitation precludes the use of fMRI for clinical and pre-clinical studies that are aimed at determining specific chemical changes that arise in various disease states. PET and fMRI presently remain unable to provide precise and direct measurements of tonic dopamine levels, limiting their use for detailed physiological studies. In addition, the current resolution is limited to the supra-millimeter range. Furthermore, the resources and costs for imaging make this technique inaccessible [85]. Efforts are underway to address these shortcomings, including the development of fMRI coupled in real-time with PET imaging to provide an integrated approach to in vivo dopamine visualization [86–88].
Research has been conducted to develop molecular probes to better quantify dopamine levels with MRI, and two have the potential to measure tonic dopamine levels. First, is the development of a dopamine-sensitive probe derived from the bacterial cytochrome P450 BM3 [89]. This probe has a dopamine-specific ligand pocket embedded with a paramagnetic iron atom that decreases in MRI signal intensity with dopamine binding. In vivo experimentation successfully measured dopamine over other interferents with both pharmacological and electrical stimulation [89–91 ]. Despite the promise of this technique, it currently suffers from a number of technical and practical limitations: 1) it has a micromolar limit of detection, far greater than what has been demonstrated extrasynaptically, 2) it can only measure a relative percent change in dopamine concentration, 3) it has not been demonstrated to cross the blood-brain-barrier and thus requires direct infusion, and 4) the resolution is limited to the voxel size. The second recent and promising molecular imaging technique uses the paramagnetic pigment neuromelanin [92]. Neuromelanin is a product of L-Dopa and dopamine metabolism known to accumulate in the substantia nigra, giving it its characteristic dark color [93]. Binding of iron to neuromelanin forms a paramagnetic complex capable of MRI detection. In vivo application demonstrated the use of neuromelanin as an indirect correlate of dopamine concentration and values derived from this technique were successfully validated against post-mortem histology, fMRI, and PET imaging. The limitations of this technique are similar to those outlined for the dopamine-sensitive probe, which are limitations general to all molecular MRI probes. Additionally, as noted by the authors, lack of complete signal specificity and signal interferents from tissue properties can confound neuromelanin measurement, reducing the usefulness of this technique.
3.2. Biochemical and genetically encoded optical dopamine sensors
Genetically encoded sensors are presently restricted for use in pre-clinical studies, however recent advances that have the potential to achieve tonic dopamine detection in pre-clinical models warrant discussion in this review. Most genetically encoded sensors are designed to take advantage of endogenous recognition elements (e.g., a dopamine receptor, DR). This allows for sensor tunability with large linear dynamic ranges and high selectivity. Rapidly advancing capabilities in genetic manipulation of pre-clinical animal models has led to the creation of several genetically encoded dopamine sensors. A recent prominent advance was the development of dLight1 by Patriarchi and colleagues [94,95]. The dLight platform relies on the engineering of a fluorescent indicator protein fused with the dopamine receptor. When endogenous dopamine binds to the receptor-indicator, a conformational change increases fluorescence. This elegant design can be applied to several subtypes of dopamine receptors, which have Kd values that range from 4 nM to 1.6 μM. While this ratiometric technique allows for measurement of relative changes in dopamine concentration, the initial report only investigated phasic changes in dopaminergic signaling. Patriarchi et al. comment on the potential use of dLight1.4 (the D4R sensor, Kd 4 nM) for measurement of dopaminergic tone. This technology is still in early developmental stages and whether its broad applicability stretches into the realm of pre-clinical tonic dopamine detection remains to be determined.
The Genetically Encoded GPCR-Activation-Based-Dopamine(DA) (GRABDA) sensor also uses a DR-GFP chimera protein to detect dopamine using ratiometric fluorescence [96]. It was shown to monitor dopamine release in flies, fish, and mice performing complex behaviors. Two variants of the sensor demonstrated affinity to dopamine with EC50 values of 10 and 130 nM with temporal resolution of seconds. Additionally, the GRABDA sensor platform made tonic dopamine measurements in freely moving mice over the course of several minutes in response to pharmacological manipulations and optogenetic stimulation of dopamine releasing cell bodies. The GRABDA platform may be the most promising genetically encoded sensor for pre-clinical tonic dopamine detection.
Another genetically encoded sensor, DRD2-iTango2, which uses cells that express the iTango platform, allows for identification of dopamine releasing cells in vivo [97]. Cells with the iTango platform express higher levels of a blue light-induced reporter gene when in the presence of dopamine. The second generation iTango2 demonstrated enhanced temporal resolution (5 min) when compared to its predecessor and was successfully employed in behaving animals to identify neurons sensitive to dopamine in reward-based learning [97,98]. The use of iTango2 for tonic dopamine detection appears challenging at present.
Cell-based Neurotransmitter Fluorescent Engineered Reporters (CNiFERs), while not genetically encoded, are injectable cell-based calcium sensors that have been used to indirectly quantify dopamine release in vivo with temporal resolution of seconds [99]. Upon binding of endogenous dopamine to the dopamine D2 receptor expressed on the CNiFER cell, downstream calcium release results in a fluorescence resonance energy transfer (FRET) change that is proportional to the extracellular dopamine concentration. Unfortunately, among other limitations, this technique requires implantation of the cell-based CNiFERs into the brain region of interest, which is technically challenging and relatively damaging to the brain. Nonetheless, with the aid of in vivo and in vitro calibrations, CNiFERs have the potential for use in tonic dopamine detection in response to behavioral and pharmacological manipulations. In addition to CNiFERs, near-infrared fluorescent catecholamine sensors have been developed that are able to detect phasic changes in dopamine with low-micrometer spatial resolution and sub-second temporal resolution and represent an important advance in in vivo dopamine detection [100,101].
The use of genetic techniques to measure tonic dopamine in vivo is in its infancy. Genetically encoded sensors have relatively large dynamic ranges and enhanced selectivity, due to their basis in endogenous recognition elements. Unfortunately, they often suffer from low signal-to-noise ratios, which complicates their usefulness in vivo. Additionally, they often require site-specific injections of fluorescent dyes, or other elements necessary for analyte detection [102]. Finally, reliance on fluorescent and luminescent probes limits the continuous use of these sensors to the sub-hour temporal regime due to sensor photobleaching. Overall, the use of biochemical and genetically encoded sensors for tonic dopamine detection shows promise but further development is necessary to elucidate their usefulness in vivo before human translation can be considered.
4. Electrochemistry
4.1. Early attempts
4.1.1. Constant potential amperometry
In constant potential amperometry, an electrode is held at a specific DC potential to oxidize or reduce chemical species of interest [103]. Using Faraday’s Law, Q = nmF (where Q = charge passed, n = numbers of electrons, m = the moles of substance oxidized, and F = Faraday’s constant) and integration of the current generated to determine charge, the moles of the species of interest can be calculated. Its application in vivo has allowed direct measurement of neurotransmitter release in a variety of disease models and behavioral studies [104,105]. The benefit of this technique is that the sub-millisecond temporal resolution is limited only by the data acquisition rate and the mass transport rate of the analyte. In addition, the small diameter size of the electrode (<10 μm) causes minimal tissue damage and allows for single-cell recording. Studies have demonstrated this technique is highly sensitive and can achieve attomole to zeptomole quantification of neurotransmitter exocytosis from single neurons [106,107]. Venton et al. applied this technique with simulated tonic firing (asynchronous electrical stimulation) and model-derived dopamine release kinetics to monitor tonic dopamine concentrations [37]. This model determined tonic dopamine concentrations to be ~30 nM with pulled glass capillary carbon-fiber microelectrodes (CFMEs).
A major disadvantage of constant potential amperometry for tonic measurements is its chemical non-selectivity. As the electrode is held at a constant potential, any electroactive analyte that oxidizes or reduces at that potential will be detected. The low chemical specificity of this technique complicates measurements in heterogeneous solutions, such as the brain. As such, it is necessary to verify amperometric signals through administration of selective drug modulators (e.g., GBR12909 reuptake inhibitor for dopamine) or by electrical stimulation of known neuronal populations that elicit release of the neurotransmitter of interest [37]. Despite these controls, it remains difficult to resolve the true change in a select species if multiple analytes constitute the signal. Thus, this technique has limited potential for future application due to poor specificity when compared to other electrochemical techniques.
4.1.2. Chronoamperometry
Chronoamperometry is a modified amperometric technique that employs potential step waveforms to increase analyte resolution [108]. Double potential step chronoamperometry, which includes an anodic and cathodic step, is commonly used to access both oxidative and reductive properties of the analyte. The current recorded during the anodic step is proportional to the neurochemical concentration, and typically measured at a fixed time in the step (usually at the end of the step to minimize non-faradaic interference) [109,110]. The ratio of the oxidation and reduction currents is used to identify the species (e.g., dopamine: 0.55 V oxidation potential, −0.2 V reduction potential vs. Ag/AgCl) allowing for better chemical identification than with constant potential amperometry. Indeed, this technique has been successfully used to measure neurotransmitter fluctuations [111–114]. Tonic dopamine quantification was achieved by Blaha et al. who conducted measurements in the striatum over 3 weeks in rats chronically implanted with stearate-modified graphic paste recording electrodes [115]. They determined the tonic dopamine concentration to be ~85 nM and were among the first studies to demonstrate that microdialysis underestimated tonic concentrations [116]. While this technique is an improvement over constant potential amperometry for the measurement of dopamine, pharmacologic drugs that perturb dopamine concentrations are still necessary to validate measurements. Furthermore, this technique, without some form of electrode surface modification [117], is unable to delineate biologic amines from their metabolites, a capability of other voltammetric techniques (discussed henceforth). These limitations have led to its decline in use and thus chronoamperometry has limited applicability for tonic dopamine recording in animals and in humans.
4.1.3. Differential normal pulse voltammetry
In 1982, differential normal pulse voltammetry [118] was designed to combine the advantages of differential pulse voltammetry and normal pulse voltammetry [119] to measure mammalian extracellular DA concentrations. The differential pulse waveform consists of sequential pulses with increasing amplitudes, within which, there is a double potential step and equivalent return to a constant baseline potential. The current is measured immediately before the application of a pulse and at the end of the pulse. The current difference is plotted as a function of the applied potential resulting in a differential pulse voltammogram [120]. By sampling the current just before the potential is changed, the effect of the capacitive charging current can be decreased leaving the faradaic signal for analysis. Background capacitive currents are constant and thus can be subtracted out of each sampled current signal, leaving the faradaic signal for analysis. Differential pulse voltammetry can be used with myriad voltammetric waveforms as described in Osteryoung et al. [121]. Further development of this technique by Gonon et al. achieved measurements of tonic dopamine levels [122]. Use of this technique with CFMEs measured extracellular tonic dopamine concentrations to be 15–25 nM in the rat striatum and 23–40 nM in the nucleus accumbens [122,123]. Like chronoamperometry, this technique is limited by its inability to discriminate against known electroactive interferents, such as 3,4-dihydroxyphenylacetic acid (DOPAC), a principle metabolite of dopamine. In addition, it is unable to account for background drift in the capacitive current over time, which limits its use for chronic recording. Therefore, it too has limited applicability for tonic dopamine recording in the laboratory and in humans.
4.2. Modified FSCV techniques
Fast-scan cyclic voltammetry (FSCV) is a well-established electrochemical technique used to measure relative (i.e., phasic) changes in electroactive neurotransmitters [124]. Standard FSCV uses a triangular waveform applied to a recording electrode. The potential is quickly swept anodically then cathodically causing oxidation and reduction currents. For the measurement of dopamine, the potential window for these electrochemical reactions is typically −0.4 V–1.3 V and the triangle waveform is repeated every 100 ms (10 Hz) with a typical sweep rate of 400 V/s [125]. Due to the speed of the potential sweep, a large capacitive background current is generated, together with much smaller faradaic currents superimposed on the capacitive current at the electrode. To remove the background current from the signal to permit accurate measurement of the faradaic current for dopamine quantification, background subtraction is performed [126]. Traditional background subtraction precludes accurately determining tonic dopamine concentrations. One reason for this is the background current is only stable for approximately 2 min before it begins to drift in magnitude, which complicates the subtraction [125]. In addition, oxidation at the electrode surface by application of the FSCV waveform results in progressive etching of the carbon fiber, resulting in current changes and further complications [127,128]. Thus, only phasic changes in concentration over a relatively brief period of time (1–2 min) following the single initial background subtraction can be quantified using FSCV. New voltammetric techniques (vide infra) are being developed to overcome these limitations and allow for tonic dopamine measurements.
A unique modification of FSCV to measure tonic dopamine concentrations using additional background subtraction steps was proposed by Borland and Michael [17]. They used FSCV (waveform: 0.0 V to 1.0 to −0.5 V–0.0 V at a scan rate of 300 V/s and a frequency of 2.5 Hz) and a modified background subtraction to examine dopamine release in response to intra-striatal infusions of a glutamate receptor antagonist, kynurenate. To validate the chemical species, they averaged voltammograms collected over 200 s intervals and subtracted them from a baseline interval. They then compared the resultant voltammogram with an experimental dopamine voltammogram. Using this method, they were able to measure a decrease in dopamine of 2.6 ± 0.2 μM (mean ± SEM, n = 19 rats) after kynurenate infusion. However, selectivity for dopamine over metabolites such as DOPAC, a known electroactive interferent, was not addressed. Additionally, the use of background subtraction over a relatively long time period (200 s) is concerning given background currents are known to shift after approximately 120 s [125].
Charge-balancing multiple waveform fast-scan cyclic voltammetry (CBM-FSCV) builds on previous FSCV-modified waveforms for tonic neurotransmitter measurements [39]. This technique utilizes a charge-balanced waveform and two novel parameterizations to address capacitive charging current issues. The first parameter is a charge balancing waveform with a 0 V holding potential to reduce the background current variance between multiple pulses in a series. The second is a dual subtraction method whereby an initial subtraction is made in each pulse within the waveform train, and a second subtraction is made from a single reference point before dopamine is detected. This technique demonstrated sensitivity for dopamine of 85.4 ± 14.3 nA/μM and a limit of detection of 5.8 ± 0.9 nM for CFMEs in vitro. This level of detection is sufficient for measuring meaningful ranges of dopamine in vivo [129]. In addition, this technique achieved selectivity over the major electroactive interferents, DOPAC and ascorbic acid. Furthermore, in vivo studies demonstrated stable tonic recordings for 2 h with a temporal resolution of 0.1 Hz and the ability to measure both increases and decreases in dopamine when levels were experimentally modified with drug manipulation. However, this technique is limited by the ability to measure only prolonged changes in tonic dopamine levels, and not absolute levels. New techniques capable of absolute tonic measurements have limited the application of CBM-FSCV.
4.3. Modified voltammetric techniques for tonic dopamine measurements
To indirectly assess tonic dopamine levels, Chen and Budygin modified the dopamine kinetic equation first described by the Wightman group that implies a zero basal concentration [73,129]. They adjusted this model to consider theoretical uptake and experimental clearance rates of dopamine after electrical stimulation of dopaminergic neurons. This analysis was applied as a post-processing step and therefore is able to extract tonic concentration estimates using conventional FSCV waveforms. Application in vivo determined dopamine concentration values to be between 95 and 220 nM, significantly higher than classical concentration estimates determined by no-net-flux microdialysis, but in line with prior electrochemical analysis experiments and our own work [38,42,115,129,130]. This method is limited by the inability to measure absolute values. In addition, it is based on the assumption of normal uptake and clearance rates, which may be altered with stimulation, in disease states, or with pharmacological agents [131 ]. However, the advantage of this technique is its ability to estimate changes in tonic levels with FSCV data, which may be useful in both pre-clinical research and in human translation when both phasic and tonic data are desired.
Fast-scan controlled-adsorption voltammetry (FSCAV, Fig. 2) uses the principles of stripping voltammetry to determine tonic dopamine concentrations in real-time [38,132]. First, to minimize adsorption, a triangular waveform (−0.4 V–1.3 V, scan rate of 1200 V/s) is applied at 100 Hz to a CFME. Then, a constant holding potential is applied (−0.4 V) for 10 s, allowing dopamine to adsorb to the CFME surface until equilibrium is reached. Then, the same triangular waveform is re-applied, and the recorded dopamine measurement is representative of the equilibrium-state (or tonic) dopamine concentration. In vitro studies with Nafion-coated CFMEs demonstrated FSCAV was sensitive up to 3.4 ± 0.8 nM of dopamine [132]. Importantly, these studies showed selectivity over the interferents DOPAC, ascorbic acid, and pH changes. In vivo studies where FSCAV was applied in the mouse nucleus accumbens core determined tonic dopamine concentrations to be 90 ± 9 nM (mean ± SEM, n = 20 animals) [38]. In addition, stable measurements were achieved over 90 min and pharmacological drug manipulations (AMPT, GBR-12909, and pargyline, synthesis inhibitor, reuptake blocker, and monoamine oxidase inhibitor, respectively) validated the ability of the technique to detect both increases and decreases in tonic dopamine concentrations. Therefore, the sensitivity, selectivity and ability to resolve absolute dopamine concentrations make it ideal for continued pre-clinical research and ultimately human translation.
Fig. 2.
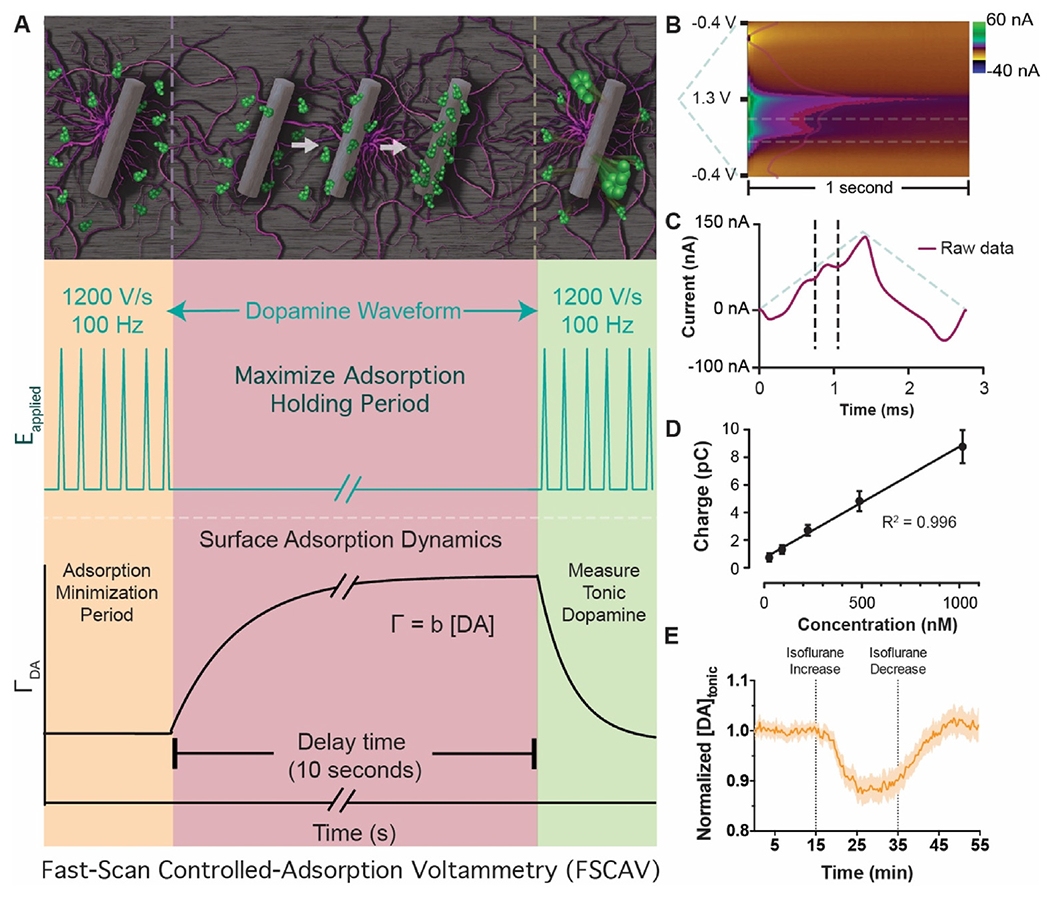
Fast-scan controlled-adsorption voltammetry. (A, Left) Adsorption of dopamine at the electrode surface is minimized during the application of the 100 Hz triangle waveform. (A, middle) The electrode is held at a negative potential for 10 s, during which time dopamine maximally adsorbs to the electrode surface and an adsorption equilibrium is reached. (A, right) The 100 Hz triangle waveform is re-applied, and information rich measurement is made that represents the equilibrium dopamine concentration (i.e., the tonic level). (B) Pseudo-color representation of recorded current and superimposed integration limits (horizontal dashed lines) typically used for dopamine quantification. (C) Current taken from the 12th scan after the holding period is used for tonic dopamine quantification. Superimposed integration limits and applied waveform are shown in dashed lines. (D) Calibration of electrodes in dopamine solutions demonstrates a linear dynamic range over several orders of magnitude. Adapted from Ref. [38] with permission from the Royal Society of Chemistry. (E) Example of tonic dopamine detection data in response to changing levels of the anesthetic isoflurane.
Convolution-based non-faradaic current removal has been developed for use with FSCV to allow tonic dopamine detection with high temporal resolution [40] and does not require significant waveform modification [133]. The major innovations include a new mode of background subtraction and an altered holding potential. In this method developed by the Wightman group, a pre-scan small-amplitude pulse is performed to elucidate non-capacitance ionic interferents of the faradaic oxidative current of dopamine. The derivative of the current response from the pulse is convolved with the waveform to generate a background prediction that is digitally subtracted out. In addition, it was found that a 0 V holding potential resulted in a more complete background subtraction by removing quinone-like species. The authors demonstrated the success of this technique and selectivity over interferents (ascorbic acid, DOPAC) both in vitro and in vivo (41 nM dopamine, n = 3 rats). Furthermore, they were able to achieve sampling rates of 1 Hz, improved over other new techniques such as FSCAV. However, this technique suffers from a lower sensitivity and poorer chemical resolution, likely due to adsorption kinetics at relatively high sampling rates. It remains to be determined if further reductions in the scan frequency resolve this confound. Nevertheless, this technique is advantageous if applications require high sampling rate over sensitivity.
Square wave voltammetry is the superposition of a traditional square wave on a staircase waveform [121]. This waveform combines the advantages of both cyclic voltammetry and pulse techniques. As multiple square wave potential changes are contained within each staircase waveform (Fig. 3A), a wealth of electrochemical information can be obtained [134]. Due to the complex design of the waveform, square wave voltammetry typically has a relatively slower scan rate in comparison to FSCV, and its application for phasic neurochemical measurement has historically been limited. However, this technique has recently gained attention for applications where temporal resolution can be traded for chemical resolution, as is the case for measuring tonic dopamine levels [135].
Fig. 3.
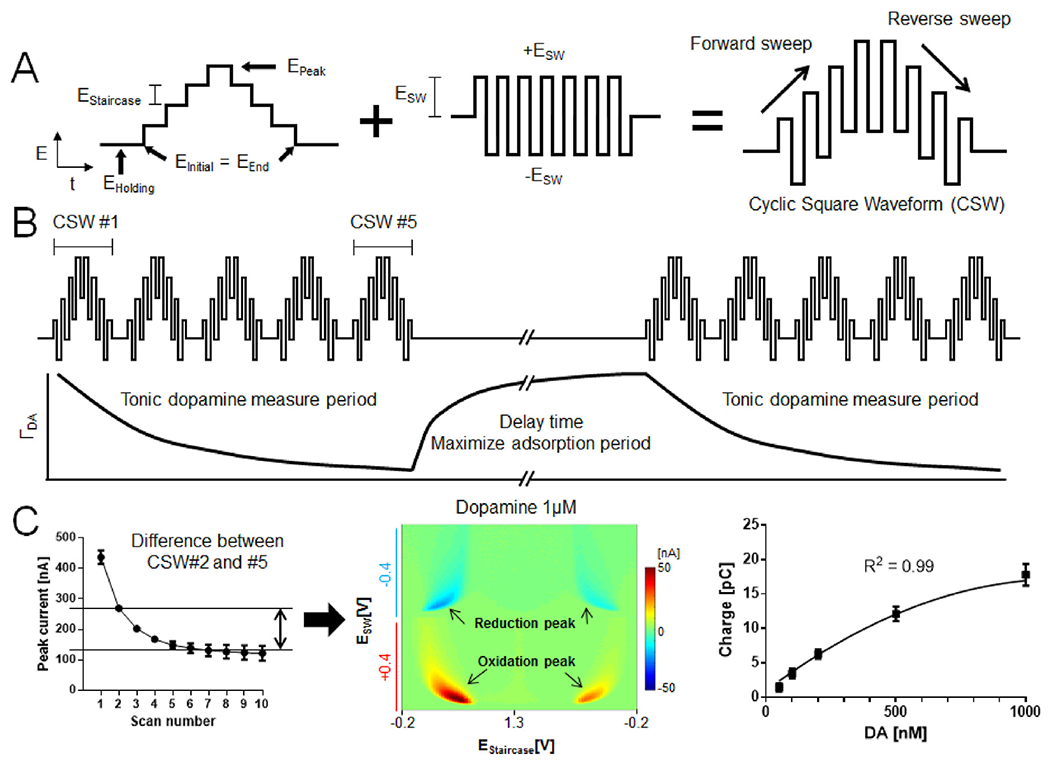
Multiple cyclic square wave voltammetry permits tonic dopamine quantification. (A) Schematic design of the cyclic square waveform. (B) Multiple cyclic square wave (CSW) applied repeatedly and dopamine adsorption time profile utilized to determine basal dopamine concentrations. (C) Peak current of dopamine (1 μM) at each CSW (left), pseudo-color plot of difference between CSW #2 and #5 (middle), M-CSWV signal correlates with tonic dopamine concentration (right; n = 4 electrodes). Adapted from Ref. [42] with permission from Elsevier.
Recently, Oh et al. developed a novel methodology using square wave voltammetry which exploits adsorption equilibrium at the CFME surface to measure basal concentrations of dopamine in vivo with high selectivity, sensitivity, and a 10 s (0.1 Hz) temporal resolution. Multiple cyclic square wave voltammetry (M-CSWV, Fig. 3) uses cyclic square wave voltammetric waveforms in conjunction with a delayed holding potential period to control dopamine adsorption to the CFME surface [42]. Dynamic background subtraction [39] and capacitive current modeling were used to eliminate large capacitive background currents, allowing basal dopamine concentrations to be measured. M-CSWV demonstrated high sensitivity (limit of detection of ~0.17 nM) and selectivity against potential electroactive interferents, including ascorbic acid, DOPAC, and pH changes. M-CSWV in preliminary in vivo studies was successfully used to measure tonic dopamine levels in rat striatum at 120 ± 18 nM (n = 7 rats). As is the case for many emerging electrochemical measurement techniques [38,42,116], this concentration is considerably higher than values obtained with no-net-flux microdialysis. In addition, pharmacological drug manipulations, which include nomifensine, pargyline, and AMPT, validated M-CSWV’s selectivity to dopamine and the ability to measure both increases and decreases in tonic levels [42,44]. Like FSCAV, M-CSWV shows considerable promise for continued pre-clinical investigation and ultimately human translation.
Another technique, recently reported by Taylor et al., utilized square wave voltammetry (SWV) for tonic dopamine recording (Fig. 4) [43]. Similar to M-CSWV, this technique has dynamic background subtraction and filtering to remove capacitive charging currents and noise. This technique was demonstrated in vitro to selectively record dopamine against electroactive interferents ascorbic acid, DOPAC, and uric acid. Additionally, in vivo studies performed with both CFMEs and gold microelectrode arrays (MEA) coated with poly (3,4-ethylenedioxythiophene (PEDOT)/carbon nanotube (CNT) [136] successfully measured tonic dopamine in the striatum (~82 nM). Using MEAs (see Section 4 below), they were able to simultaneously measure changes in tonic dopamine at multiple electrode sites simultaneously, the first demonstration of its kind. This adds considerable dimensionality for in vivo recording, and could provide a greater depth of understanding where proximal but distinct regions have different dopaminergic activity, such as the nucleus accumbens core and shell [137]. Additional studies of SWV and electrode design will need to examine biofouling and the capability for chronic monitoring. Nevertheless, this SWV technique and integration with MEA design are opportune for future development, and demonstrate that multichannel tonic recordings are on the horizon.
Fig. 4.
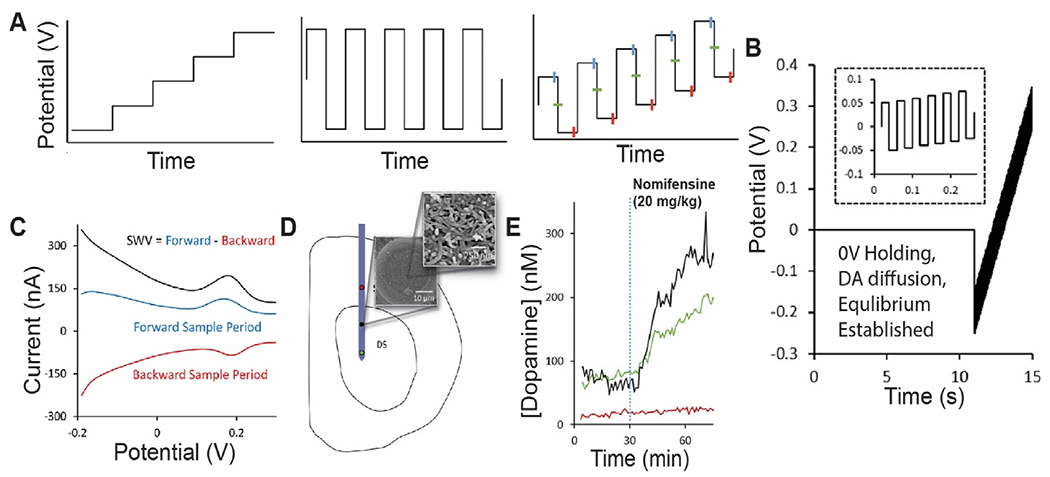
PEDOT/CNT-functionalized carbon-fiber microelectrode measurements of tonic dopamine via SWV. (A) The waveform consists of the superposition of a staircase wave and a square wave. Forward current is measured at the end of each anodic holding period, and backward current is measured at the end of each cathodic holding period. (B) An initial 0 V hold followed by a series of anodic and cathodic step and holds (zoom shown in the inset) that transverse the defined potential window is applied. (C) Representative SWV measurement of a 1 μM standard solution of dopamine reveals the SWV current (black) response to be the difference of the forward (blue) and reverse (red) current responses. (D) Schematic of microelectrode array placement in the dorsal striatum (DS) of the rat. Multiple recording sites indicated by red, black, and green circles. SEM image of CNT functionalization on electrode surface. (E) Electrodes located within the DS (green and black) showed clear, nomifensine-dependent DA detection. Adapted with permission from Ref. [43]. Copyright 2019 American Chemical Society.
A summary of techniques described in section 3 can be found in Table 1.
Table 1.
Electrochemical techniques for tonic dopamine measurement.
| Technique | Temporal resolution | Limit of detection (nM) | Reported [DA]tonic | Hardware & software | Ref. |
|---|---|---|---|---|---|
| Chronoamperometry | 1 min | 19 ± 6 | 86 ± 6 nM, rat striatum | E-chempro | [115] |
| FSCAV | 20 s | 3.7 ± 0.5 | 90 ± 9 nM, mouse NAc | NI DAQ LabVIEW | [38] |
| CBM-FSCV | 10 s | 5.8 ± 0.9 | >73 ± 5 nM,a rat striatum | NI DAQ, LabVIEW, Matlab | [39] |
| Convolution-based current removal | 1 s | <40a | 41 ± 13 nM, rat NAc | NI DAQ, LabVIEW | [133] |
| M-CSWV | 10 s | 0.17 ± 0.03 | 120 ± 18 nM, rat striatum | NI DAQ, WINCS, LabVIEW | [42] |
| SWV | 15 s | 2.03 ± 0.09 | 82 ± 6 nM, rat striatum | N/Aa, Matlab | [43] |
not reported in original manuscript.
5. Electrodes for tonic measurement
A comprehensive review of electrodes for tonic dopamine detection is outside of the scope of this review. However, we will briefly describe select prominent electrodes and surface modifications used for dopamine detection. For more detail on electrodes used for in vivo dopamine measurement please see Puthongkham & Venton [138] or Sajid et al. [139].
5.1. Carbon-fiber microelectrodes
CFMEs have been the backbone of voltammetry since their introduction in the 1970s [140]. They are recognized for their small size, adsorptive properties, stable charging currents, and moderate resistance to biofouling [141]. These CFMEs are commonly 50–200 μm in length with a diameter of <10 μm. The size permits greater spatial resolution and less tissue damage [142]. These properties make them very attractive for monitoring neurotransmission over long periods of time (hours – months) [143]. Commonly, AS-4 carbon fibers are used for dopamine detection, which have a polyacrylonitrile (PAN)-based structure. While these bare pyrolytic carbon electrodes have demonstrated robustness in their ability to detect neurotransmitters, a number of surface modifications (e.g., PEDOT and Nafion) have been employed that improve sensitivity and selectivity [43,144–158].
5.2. Diamond electrodes
Long-term application of FSCV waveforms has been found to cause CFMEs to erode and decline in sensitivity to dopamine [128,159]. This decline impedes their use for chronic in vivo monitoring, and the superior material properties of diamond electrodes (hardness, biofouling resistance) have led to their investigation as a potential solution [160,161]. Most recent advances in the field by Puthongkham et al. developed nano-diamond coated CFMEs [41]. They reported a limit of detection of 3 nM and 50% less biofouling compared to uncoated CFMEs, which is in line with previous studies by Bennet et al. and Rusinek et al. [161,162]. Despite this finding, diamond electrodes have generally suffered from lower sensitivity than CFMEs, and thus have not gained much traction in pre-clinical studies. In addition, they have yet to be tested with tonic recording techniques. Further research in material design and integration with tonic recording techniques are ultimately needed to resolve their future potential. Nonetheless, diamond electrodes are an attractive solution to electrode longevity for eventual in-human recordings.
5.3. Electrode arrays
Microelectrode arrays (MEA) permit multiplexing and increase the total amount of tissue sampled, which allows spatially resolved measurements of dopamine and other neurochemicals across brain microenvironments. This is notable as structures like the striatum have gradients of interneuron densities that may contribute to altering levels of dopamine release [163]. Carbon has been used in MEAs due to its excellent electrochemical properties and compatibility with FSCV [164–168]. In addition to carbon fibers and carbon nanotubes, conductive metals and diamond have been used for array detection of dopamine [169–172]. Recently, Taylor et al. developed a PEDOT/CNT-coated gold vertically-stacked MEA with 200 μm spacing capable of tonic dopamine detection simultaneously across multiple electrode contacts [43].
5.4. Challenges in longevity and safety
A major limitation of tonic neurochemical recording techniques is microelectrode lifetime, where signal quality has been found to degrade over time. The longest reported in vivo recording at a CFME thus far has been four months [143]. For clinical applications, most notably in closed-loop DBS, tonic recordings may well need to occur for the remaining lifespan of the patient (i.e., years). Efforts are being made in microelectrode composition to increase implantable electrode lifetime [161]. For example, diamond-based electrodes erode 90% slower and composite coatings with PEDOT/Nafion and Nafion/CNT have demonstrated decreased biofouling in vivo [43,147,161]. In addition to biofouling, safety and therapeutic benefit will need to be thoroughly addressed before long-term human use is considered. While the risks of permanently implanted recording electrodes are likely similar to those of implanted DBS electrodes and shunts (e.g., infection, hemorrhage, and cognitive decline [173]), differences in instrumentation architecture, material composition, and implantation location necessitate extensive independent study.
6. Conclusion: future perspectives for electrochemical tonic dopamine detection
Tonic neurotransmitter recording techniques hold considerable importance for understanding neural mechanisms and pathophysiology. Diseases such as Parkinson’s disease, Tourette syndrome, drug addiction, and schizophrenia are all characterized by dopaminergic dysfunction. To treat these conditions more effectively, it is vital to develop techniques that enhance understanding of dopaminergic function, particularly in disease models. To this end, microdialysis has been used substantially and successfully in pre-clinical research [174] and new efforts are being made to address issues of temporal resolution [68,175,176]. However, microdialysis collection and detection techniques currently prohibit chronic human studies [12]. In addition, the size of the dialysis probe limits the spatial resolution of study [34,61,62,177].
While microdialysis has proven useful, its present limitations encourage the further development of in vivo electrochemical techniques. The development of tonic in vivo recording techniques, such as FSCAV and M-CSWV, have for the first time allowed investigation of tonic dopamine dynamics at relatively high temporal resolution in real-time with micrometer resolution. We anticipate these techniques will gain widespread use in neurophysiological study, especially with the incorporation of multichannel arrays. Efforts are being made in our laboratories and others to develop instrumentation and software for widespread dissemination and use in animal, and eventually, human studies. Once achieved, these techniques will allow for myriad uses in probing nervous system physiology that was previously unachievable.
6.1. Human application
Measurement of tonic dopamine concentrations in humans with voltammetry is on the horizon [178]. Already, phasic dopamine, adenosine, and serotonin have been successfully recorded intraoperatively by two groups [45,161,179,180]. The same surgical procedures and electrode designs used in these human studies can be readily applied for tonic measurements, increasing the speed of translation. In contrast to microdialysis, PET, or fMRI, for example, voltammetric signals can be wirelessly sent to portable computers [181–184] permitting chronic clinical investigations in ambulatory subjects. We foresee two potential uses for in vivo human application, investigation of disease mechanisms and closed-loop deep brain stimulation (DBS) therapy.
An exciting foreseeable in-human application is for intraoperative investigation of dysfunctional neurophysiology. In Parkinson’s disease, for example, degradation of nigrostriatal dopaminergic neurons is thought to perturb striatal tonic dopamine levels [185,186]. Investigation of disease physiology has been hampered by the difficulty in creating animal models that adequately represent the human condition. Furthermore, animal models do not capture individual variation in disease characteristics, are phenotypically and genetically homogenous, and live in highly controlled environments. Therefore, the optimal setting for monitoring dopamine in these diseases lies in humans. While implantation of recording electrodes for academic objectives is fraught with ethical complication (see next section), there exists opportunity for investigation during surgical implantation of deep brain stimulation electrodes in human subjects undergoing Phase-II epilepsy monitoring and other brain neurosurgical interventions. Surgical trajectories often include the striatum, where dopaminergic dysfunction is thought to exist in many of the aforementioned diseases [187]. Therefore, intra-operative tonic recordings can be achieved in humans without added risk of patient harm, and provide unparalleled insight into disease pathophysiology. This strategy has already been employed by Kishida et al. and Agnesi et al. to measure phasic dopamine fluctuations in the human striatum with CFMEs [45,188]. With the insight gained from these studies, we foresee future experimentation in chronic tonic recording paradigms where patients are fitted with implanted state-of-the-art recording technology. To this end, new miniature recording devices are being developed that would allow for recording outside of the operating room [184,189]. For the first time, tonic neurotransmitter fluctuations can be correlated with disease symptomatology, drug therapy, and stimulation. These will inevitably power new therapeutic strategies.
One promising therapeutic strategy is to probe the effects and mechanism(s) of DBS therapy in order to develop smart systems that provide real-time control of disease symptomatology [190]. Current DBS systems are static and require manual parameter optimization (e.g., frequency, voltage, pulse width) via open-loop systems to maximize therapeutic value and minimize stimulation side effects [175]. These systems can take months, and even years in some cases, of manual adjustments before optimal control is achieved, all while patients receive sub-optimal therapy. These settings are then typically left static while efficacy diminishes either due to disease progression or DBS electrode biofouling, with limited and time-consuming adjustments made by a neurologist [175]. A smart system with biochemical feedback control could remedy this issue and improve therapy. Indeed, the development of closed-loop DBS systems are underway, and successful development of FDA-approved systems based on electrophysiologic feedback have ensued (e.g., Neuropace, Medtronic Percept) [191,192]. Thus, in a similar fashion, tonic dopamine levels, including other electroactive neurotransmitters such as serotonin, may be used as an additional and direct biomarker for closed-loop DBS systems. Efforts are underway to understand if lab-drawn conclusions on dopaminergic dysfunction can be used as a biomarker for stimulation modulation [184,193,194].
6.2. Ethical considerations
The ethical implications of neurochemical monitoring must be taken into consideration for human translation. Dopamine plays a fundamental role in shaping one’s personality, as evidenced by its role in reward and incentive-motivation. This is also apparent when one considers how dopamine receptor antagonists are the backbone of antipsychotic treatment. Therefore, recording and modulating dopamine must be examined with the best interests of the patient in mind (benevolence) [195]. Furthermore, in recording neurochemicals, we face issues of privacy. Information storage and access safeguards are crucial elements that must be addressed in counsel with the patient (justice). When considering modulating (e.g., stimulating) neurochemistry, we must ensure a patient’s autonomy is not compromised. Moreover, the idea of self-estrangement, or even creation of a new self, is evidently a problem [196]. In summary, we must be cautious of our proposals, careful and deliberative with our decisions, and engaged with the goals and wishes of the patient.
Acknowledgements
This research was supported by the NIH R01NS112176 award, The Grainger Foundation, and the National Research Foundation of Korea (NRF-2017R1A2B2006896). Training grant funding for A.E.R. was supported by NIH F31NS115202, NIH R25GM055252-23, NIH TL1TR002380-03, and NIH T32GM065841-17. Training grant funding for T.A.G. was supported by NIH T32GM062584 and NIH T32GM008804.
Footnotes
Declaration of competing interest
The authors declare no competing financial interest.
References
- [1].Pan WX, Schmidt R, Wickens JR, Hyland BI, J. Neurosci 25 (2005) 6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wise RA, Drug Alcohol Depend. 51 (1998) 13. [DOI] [PubMed] [Google Scholar]
- [3].Tobler PN, Fiorillo CD, Schultz W, Science 307 (2005) 1642. [DOI] [PubMed] [Google Scholar]
- [4].Berridge KC, Robinson TE, Brain Res Brain Res Rev 28 (1998) 309. [DOI] [PubMed] [Google Scholar]
- [5].Jay TM, Prog. Neurobiol 69 (2003) 375. [DOI] [PubMed] [Google Scholar]
- [6].Balleine BW, Delgado MR, Hikosaka O, J. Neurosci 27 (2007) 8161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].LeDoux J, Neuron 73 (2012) 653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Grace AA, Nat. Rev. Neurosci 17 (2016) 524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hyman SE, Malenka RC, Nat. Rev. Neurosci 2 (2001) 695. [DOI] [PubMed] [Google Scholar]
- [10].Grace AA, Addiction 95 (Suppl 2) (2000) S119. [DOI] [PubMed] [Google Scholar]
- [11].Dreyer JK, Herrik KF, Berg RW, Hounsgaard JD, J. Neurosci 30 (2010) 14273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Grace AA, Bunney BS, J. Neurosci 4 (1984) 2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N, Nature 482 (2012) 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Goto Y, Otani S, Grace AA, Neuropharmacology 53 (2007) 583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Keefe KA, Zigmond MJ, Abercrombie ED, J. Neural Transm. Gen. Sect 91 (1993) 223. [DOI] [PubMed] [Google Scholar]
- [16].Grace AA, Bunney BS, Brain Res. 333 (1985) 271. [DOI] [PubMed] [Google Scholar]
- [17].Borland LM, Michael AC, J. Neurochem 91 (2004) 220. [DOI] [PubMed] [Google Scholar]
- [18].Grace AA, Neuroscience 41 (1991) 1. [DOI] [PubMed] [Google Scholar]
- [19].Floresco SB, West AR, Ash B, Moore H, Grace AA, Nat. Neurosci 6 (2003) 968. [DOI] [PubMed] [Google Scholar]
- [20].Freed CR, Yamamoto BK, Science 229 (1985) 62. [DOI] [PubMed] [Google Scholar]
- [21].Hamid AA, Pettibone JR, Mabrouk OS, Hetrick VL, Schmidt R, Vander Weele CM, Kennedy RT, Aragona BJ, Berke JD, Nat. Neurosci 19 (2016) 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Howe MW, Tierney PL, Sandberg SG, Phillips PE, Graybiel AM, Nature 500 (2013) 575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM, Nature 422 (2003) 614. [DOI] [PubMed] [Google Scholar]
- [24].Niv Y, Daw ND, Joel D, Dayan P, Psychopharmacology (Berl) 191 (2007) 507. [DOI] [PubMed] [Google Scholar]
- [25].Grace AA, Bunney BS, Eur. J. Pharmacol 59 (1979) 211. [DOI] [PubMed] [Google Scholar]
- [26].Ungless MA, Magill PJ, Bolam JP, Science 303 (2004) 2040. [DOI] [PubMed] [Google Scholar]
- [27].Pezze MA, Heidbreder CA, Feldon J, Murphy CA, Neuroscience 108 (2001) 91. [DOI] [PubMed] [Google Scholar]
- [28].Dombrowski PA, Maia TV, Boschen SL, Bortolanza M, Wendler E, Schwarting RK, Brandao ML, Winn P, Blaha CD, Da Cunha C, Behav. Brain Res 241 (2013) 112. [DOI] [PubMed] [Google Scholar]
- [29].Meck WH, Benson AM, Brain Cognit. 48 (2002) 195. [DOI] [PubMed] [Google Scholar]
- [30].Goto Y, Grace AA, Nat. Neurosci 8 (2005) 805. [DOI] [PubMed] [Google Scholar]
- [31].Yapo C, Nair AG, Clement L, Castro LR, Hellgren Kotaleski J, Vincent P, J. Physiol 595 (2017) 7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Matsuda Y, Marzo A, Otani S, J. Neurosci 26 (2006) 4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chefer VI, Thompson AC, Zapata A, Shippenberg TS, Curr Protoc Neurosci Chapter 7 (2009). Unit7 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Clapp-Lilly KL, Roberts RC, Duffy LK, Irons KP, Hu Y, Drew KL, J. Neurosci. Methods 90 (1999) 129. [DOI] [PubMed] [Google Scholar]
- [35].Vaquero JJ, Kinahan P, Annu. Rev. Biomed. Eng 17 (2015) 385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bruinsma TJ, Sarma VV, Oh Y, Jang DP, Chang SY, Worrell GA, Lowe VJ, Jo HJ, Min HK, Front. Neurosci 12 (2018) 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Venton BJ, Zhang H, Garris PA, Phillips PE, Sulzer D, Wightman RM, J. Neurochem 87 (2003) 1284. [DOI] [PubMed] [Google Scholar]
- [38].Atcherley CW, Wood KM, Parent KL, Hashemi P, Heien ML, Chem Commun (Camb) 51 (2015) 2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Oh Y, Park C, Kim DH, Shin H, Kang YM, DeWaele M, Lee J, Min HK, Blaha CD, Bennet KE, Kim IY, Lee KH, Jang DP, Anal. Chem 88 (2016) 10962. [DOI] [PubMed] [Google Scholar]
- [40].Johnson JA, Rodeberg NT, Wightman RM, Anal. Chem 90 (2018) 7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Puthongkham P, Venton BJ, ACS Sens. 4 (2019) 2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Oh Y, Heien ML, Park C, Kang YM, Kim J, Boschen SL, Shin H, Cho HU, Blaha CD, Bennet KE, Lee HK, Jung SJ, Kim IY, Lee KH, Jang DP, Biosens. Bioelectron 121 (2018) 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Taylor IM, Patel NA, Freedman NC, Castagnola E, Cui XT, Anal. Chem 91 (2019) 12917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Barath AS, Rusheen AE, Rojas Cabrera JM, Price JB, Owen RL, Shin H, Jang DP, Blaha CD, Lee KH, Oh Y, Front. Neurosci 14 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kishida KT, Saez I, Lohrenz T, Witcher MR, Laxton AW, Tatter SB, White JP, Ellis TL, Phillips PE, Montague PR, Proc. Natl. Acad. Sci. U. S. A 113 (2016) 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kasasbeh A, Lee K, Bieber A, Bennet K, Chang SY, Stereotact. Funct. Neurosurg 91 (2013) 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Watson CJ, Venton BJ, Kennedy RT, Anal. Chem 78 (2006) 1391. [DOI] [PubMed] [Google Scholar]
- [48].Yoshitake T, Yoshitake S, Fujino K, Nohta H, Yamaguchi M, Kehr J, J. Neurosci. Methods 140 (2004) 163. [DOI] [PubMed] [Google Scholar]
- [49].Santos-Fandila A, Zafra-Gomez A, Barranco A, Navalon A, Rueda R, Ramirez M, Talanta 114 (2013) 79. [DOI] [PubMed] [Google Scholar]
- [50].Ji C, Li W, Ren XD, El-Kattan AF, Kozak R, Fountain S, Lepsy C, Anal. Chem 80 (2008) 9195. [DOI] [PubMed] [Google Scholar]
- [51].Greco S, Danysz W, Zivkovic A, Gross R, Stark H, Anal. Chim. Acta 771 (2013) 65. [DOI] [PubMed] [Google Scholar]
- [52].Heidbreder CA, Lacroix L, Atkins AR, Organ AJ, Murray S, West A, Shah AJ, J. Neurosci. Methods 112 (2001) 135. [DOI] [PubMed] [Google Scholar]
- [53].Gu H, Varner EL, Groskreutz SR, Michael AC, Weber SG, Anal. Chem 87 (2015) 6088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Yoshitake T, Kehr J, Todoroki K, Nohta H, Yamaguchi M, Biomed. Chromatogr 20 (2006) 267. [DOI] [PubMed] [Google Scholar]
- [55].Justice JB Jr., J. Neurosci. Methods 48 (1993) 263. [DOI] [PubMed] [Google Scholar]
- [56].Tang A, Bungay PM, Gonzales RA, J. Neurosci. Methods 126 (2003) 1. [DOI] [PubMed] [Google Scholar]
- [57].Martin-Fardon R, Sandillon F, Thibault J, Privat A, Vignon J, J. Neurosci. Methods 72 (1997) 123. [DOI] [PubMed] [Google Scholar]
- [58].Chen NH, Lai YJ, Pan WH, Neurosci. Lett 225 (1997) 197. [DOI] [PubMed] [Google Scholar]
- [59].Parsons LH, Justice JB Jr., J. Neurochem 58 (1992) 212. [DOI] [PubMed] [Google Scholar]
- [60].Bruet N, Windels F, Bertrand A, Feuerstein C, Poupard A, Savasta M, J. Neuropathol. Exp. Neurol 60 (2001) 15. [DOI] [PubMed] [Google Scholar]
- [61].Szarowski DH, Andersen MD, Retterer S, Spence AJ, Isaacson M, Craighead HG, Turner JN, Shain W, Brain Res. 983 (2003) 23. [DOI] [PubMed] [Google Scholar]
- [62].Benveniste H, Diemer NH, Acta Neuropathol. 74 (1987) 234. [DOI] [PubMed] [Google Scholar]
- [63].Di Chiara G, Carboni E, Morelli M, Cozzolino A, Tanda GL, Pinna A, Russi G, Consolo S, Neuroscience 55 (1993) 451. [DOI] [PubMed] [Google Scholar]
- [64].Blaha CD, Coury A, Phillips AG, Neuroscience 75 (1996) 543. [DOI] [PubMed] [Google Scholar]
- [65].Yang H, Peters JL, Allen C, Chern SS, Coalson RD, Michael AC, Anal. Chem 72 (2000) 2042. [DOI] [PubMed] [Google Scholar]
- [66].Ngo KT, Varner EL, Michael AC, Weber SG, ACS Chem. Neurosci 8 (2017) 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Yang H, Thompson AB, McIntosh BJ, Altieri SC, Andrews AM, ACS Chem. Neurosci 4 (2013) 790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Young AM, J. Neurosci. Methods 138 (2004) 57. [DOI] [PubMed] [Google Scholar]
- [69].Cheng J, Feenstra MG, Learn. Mem 13 (2006) 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Shou M, Ferrario CR, Schultz KN, Robinson TE, Kennedy RT, Anal. Chem 78 (2006) 6717. [DOI] [PubMed] [Google Scholar]
- [71].Kennedy RT, Curr. Opin. Chem. Biol 17 (2013) 860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Chen KC, J. Neurochem 92 (2005) 46. [DOI] [PubMed] [Google Scholar]
- [73].Chen KC, Budygin EA, J. Neurosci. Methods 164 (2007) 27. [DOI] [PubMed] [Google Scholar]
- [74].Kanthan R, Shuaib A, Griebel R, Miyashita H, Stroke 26 (1995) 870. [DOI] [PubMed] [Google Scholar]
- [75].Suominen T, Uutela P, Ketola RA, Bergquist J, Hillered L, Finel M, Zhang H, Laakso A, Kostiainen R, PloS One 8 (2013), e68007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Elsinga PH, Hatano K, Ishiwata K, Curr. Med. Chem 13 (2006) 2139. [DOI] [PubMed] [Google Scholar]
- [77].Pappata S, Dehaene S, Poline JB, Gregoire MC, Jobert A, Delforge J, Frouin V, Bottlaender M, Dolle F, Di Giamberardino L, Syrota A, Neuroimage 16 (2002) 1015. [DOI] [PubMed] [Google Scholar]
- [78].Koepp MJ, Gunn RN, Lawrence AD, Cunningham VJ, Dagher A, Jones T, Brooks DJ, Bench CJ, Grasby PM, Nature 393 (1998) 266. [DOI] [PubMed] [Google Scholar]
- [79].Ramchandani VA, Umhau J, Pavon FJ, Ruiz-Velasco V, Margas W, Sun H, Damadzic R, Eskay R, Schoor M, Thorsell A, Schwandt ML, Sommer WH, George DT, Parsons LH, Herscovitch P, Hommer D, Heilig M, Mol. Psychiatr 16 (2011) 809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Lippert RN, Cremer AL, Edwin Thanarajah S, Korn C, Jahans-Price T, Burgeno LM, Tittgemeyer M, Bruning JC, Walton ME, Backes H, Nat. Commun 10 (2019) 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D, Neuroimage 18 (2003) 263. [DOI] [PubMed] [Google Scholar]
- [82].Logothetis NK, J. Neurosci 23 (2003) 3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Jenkins BG, Neuroimage 62 (2012) 1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Ren J, Xu H, Choi JK, Jenkins BG, Chen YI, Synapse 63 (2009) 764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Buck AK, Herrmann K, Stargardt T, Dechow T, Krause BJ, Schreyogg J, J. Nucl. Med. Technol 38 (2010) 6. [DOI] [PubMed] [Google Scholar]
- [86].Sander CY, Hooker JM, Catana C, Normandin MD, Alpert NM, Knudsen GM, Vanduffel W, Rosen BR, Mandeville JB, Proc. Natl. Acad. Sci. U. S. A 110 (2013) 11169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Sander CY, Hesse S, Q. J. Nucl. Med. Mol. Imaging 61 (2017) 414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Badgaiyan RD, Prog. Brain Res 211 (2014) 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Shapiro MG, Westmeyer GG, Romero PA, Szablowski JO, Kuster B, Shah A, Otey CR, Langer R, Arnold FH, Jasanoff A, Nat. Biotechnol 28 (2010) 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Lee T, Cai LX, Lelyveld VS, Hai A, Jasanoff A, Science 344 (2014) 533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Li N, Jasanoff A, Nature 580 (2020) 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Cassidy CM, Zucca FA, Girgis RR, Baker SC, Weinstein JJ, Sharp ME, Bellei C, Valmadre A, Vanegas N, Kegeles LS, Brucato G, Jung Kang U, Sulzer D, Zecca L, Abi-Dargham A, Horga G, Proc. Natl. Acad. Sci. U. S. A 116 (2019) 5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Zecca L, Tampellini D, Gerlach M, Riederer P, Fariello RG, Sulzer D, Mol. Pathol 54 (2001) 414. [PMC free article] [PubMed] [Google Scholar]
- [94].Patriarchi T, Cho JR, Merten K, Howe MW, Marley A, Xiong WH, Folk RW, Broussard GJ, Liang R, Jang MJ, Zhong H, Dombeck D, von Zastrow M, Nimmerjahn A, Gradinaru V, Williams JT, Tian L, Science 360 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Cosme CV, Palissery GK, Lerner TN, Trends Neurosci. 41 (2018) 566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Sun F, Zeng J, Jing M, Zhou J, Feng J, Owen SF, Luo Y, Li F, Wang H, Yamaguchi T, Yong Z, Gao Y, Peng W, Wang L, Zhang S, Du J, Lin D, Xu M, Kreitzer AC, Cui G, Li Y, Cell 174 (2018) 481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Lee D, Creed M, Jung K, Stefanelli T, Wendler DJ, Oh WC, Mignocchi NL, Luscher C, Kwon HB, Nat. Methods 14 (2017) 495. [DOI] [PubMed] [Google Scholar]
- [98].Barnea G, Strapps W, Herrada G, Berman Y, Ong J, Kloss B, Axel R, Lee KJ, Proc. Natl. Acad. Sci. U. S. A 105 (2008) 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Muller A, Joseph V, Slesinger PA, Kleinfeld D, Nat. Methods 11 (2014) 1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Beyene AG, McFarlane IR, Pinals RL, Landry MP, ACS Chem. Neurosci 8 (2017) 2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Beyene AG, Delevich K, Del Bonis-O’Donnell JT, Piekarski DJ, Lin WC, Thomas AW, Yang SJ, Kosillo P, Yang D, Prounis GS, Wilbrecht L, Landry MP, Sci Adv 5 (2019), eaaw3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Wang H, Jing M, Li Y, Curr. Opin. Neurobiol 50 (2018) 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Adams RN, Anal. Chem 48 (1976) 1126A. [DOI] [PubMed] [Google Scholar]
- [104].Pothos EN, Behav. Brain Res 130 (2002) 203. [DOI] [PubMed] [Google Scholar]
- [105].Borisovska M, Bensen AL, Chong G, Westbrook GL, J. Neurosci 33 (2013) 1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Hochstetler SE, Puopolo M, Gustincich S, Raviola E, Wightman RM, Anal. Chem 72 (2000) 489. [DOI] [PubMed] [Google Scholar]
- [107].Jaffe EH, Marty A, Schulte A, Chow RH, J. Neurosci 18 (1998) 3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Michael AC, Borland LM, Electrochemical Methods for Neuroscience, CRC Press/Taylor & Francis, Boca Raton, 2007. [PubMed] [Google Scholar]
- [109].Adams RN, Prog. Neurobiol 35 (1990) 297. [DOI] [PubMed] [Google Scholar]
- [110].Blaha CD, Lane RF, Brain Res. Bull 10 (1983) 861. [DOI] [PubMed] [Google Scholar]
- [111].Gerhardt GA, Hoffman AF, J. Neurosci. Methods 109 (2001) 13. [DOI] [PubMed] [Google Scholar]
- [112].Hoffman AF, Gerhardt GA, J. Neurochem 70 (1998) 179. [DOI] [PubMed] [Google Scholar]
- [113].Miller AD, Forster GL, Yeomans JS, Blaha CD, Neuroscience 136 (2005) 531. [DOI] [PubMed] [Google Scholar]
- [114].Unger EL, Eve DJ, Perez XA, Reichenbach DK, Xu Y, Lee MK, Andrews AM, Neurobiol. Dis 21 (2006) 431. [DOI] [PubMed] [Google Scholar]
- [115].Blaha CD, Pharmacol. Biochem. Behav 55 (1996) 351. [DOI] [PubMed] [Google Scholar]
- [116].Blaha CD, Phillips AG, Behav. Pharmacol 7 (1996) 675. [PubMed] [Google Scholar]
- [117].Blaha CD, Jung ME, J. Electroanal. Chem. Interfacial Electrochem 310 (1991) 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Borman S, Anal. Chem 54 (1982) A698. [Google Scholar]
- [119].Ewing AG, Dayton MA, Wightman RM, Anal. Chem 53 (1981) 1842. [Google Scholar]
- [120].Drake KF, Van Duyne RP, J. Electroanal. Chem. Interfacial Electrochem 89 (1978) 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Osteryoung J, Osteryoung R, Anal. Chem 57 (1985) 101. [Google Scholar]
- [122].Gonon FG, Navarre F, Buda MJ, Anal. Chem 56 (1984) 573. [DOI] [PubMed] [Google Scholar]
- [123].Marcus MM, Mathe JM, Nomikos GG, Svensson TH, Neuropharmacology 40 (2001) 482. [DOI] [PubMed] [Google Scholar]
- [124].Heien ML, Johnson MA, Wightman RM, Anal. Chem 76 (2004) 5697. [DOI] [PubMed] [Google Scholar]
- [125].Heien ML, Khan AS, Ariansen JL, Cheer JF, Phillips PE, Wassum KM, Wightman RM, Proc. Natl. Acad. Sci. U. S. A 102 (2005) 10023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Howell JO, Kuhr WG, Ensman RE, Wightman RM, J. Electroanal. Chem. Interfacial Electrochem 209 (1986) 77. [Google Scholar]
- [127].Roberts JG, Moody BP, McCarty GS, Sombers LA, Langmuir 26 (2010) 9116. [DOI] [PubMed] [Google Scholar]
- [128].Keithley RB, Takmakov P, Bucher ES, Belle AM, Owesson-White CA, Park J, Wightman RM, Anal. Chem 83 (2011) 3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Owesson-White CA, Roitman MF, Sombers LA, Belle AM, Keithley RB, Peele JL, Carelli RM, Wightman RM, J. Neurochem 121 (2012) 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Kawagoe KT, Garris PA, Wiedemann DJ, Wightman RM, Neuroscience 51 (1992) 55. [DOI] [PubMed] [Google Scholar]
- [131].Vaughan RA, Foster JD, Trends Pharmacol. Sci 34 (2013) 489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Atcherley CW, Laude ND, Parent KL, Heien ML, Langmuir 29 (2013) 14885. [DOI] [PubMed] [Google Scholar]
- [133].Johnson JA, Hobbs CN, Wightman RM, Anal. Chem 89 (2017) 6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Helfrick JC Jr., Bottomley LA, Anal. Chem 81 (2009) 9041. [DOI] [PubMed] [Google Scholar]
- [135].Park C, Oh Y, Shin H, Kim J, Kang Y, Sim J, Cho HU, Lee HK, Jung SJ, Blaha CD, Bennet KE, Heien ML, Lee KH, Kim IY, Jang DP, Anal. Chem 90 (2018) 13348. [DOI] [PubMed] [Google Scholar]
- [136].Xu G, Beibei L, Cui XT, Ling L, Luo X, Sensor. Actuator. B Chem 188 (2013) 405. [Google Scholar]
- [137].Di Chiara G, Behav. Brain Res 137 (2002) 75. [DOI] [PubMed] [Google Scholar]
- [138].Puthongkham P, Venton BJ, Analyst 145 (2020) 1087–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Sajid M, Baig N, et al. , Trac. Trends Anal. Chem 111 (2019) 47–61. [Google Scholar]
- [140].L Ponchon J, Cespuglio R, Gonon F, Jouvet M, Pujol JF, Anal. Chem 51 (1979) 1483. [DOI] [PubMed] [Google Scholar]
- [141].Huffman ML, Venton BJ, Analyst 134 (2009) 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Peters JL, Miner LH, Michael AC, Sesack SR, J. Neurosci. Methods 137 (2004) 9. [DOI] [PubMed] [Google Scholar]
- [143].Clark JJ, Sandberg SG, Wanat MJ, Gan JO, Horne EA, Hart AS, Akers CA, Parker JG, Willuhn I, Martinez V, Evans SB, Stella N, Phillips PE, Nat. Methods 7 (2010) 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Heien ML, Phillips PE, Stuber GD, Seipel AT, Wightman RM, Analyst 128 (2003) 1413. [DOI] [PubMed] [Google Scholar]
- [145].Rice RJ, Pontikos NM, L McCreery R, J. Am. Chem. Soc 112 (1990) 4617. [Google Scholar]
- [146].Kovach PM, Deakin MR, Wightman RM, J. Phys. Chem 90 (1986) 4612. [Google Scholar]
- [147].Vreeland RF, Atcherley CW, Russell WS, Xie JY, Lu D, Laude ND, Porreca F, Heien ML, Anal. Chem 87 (2015) 2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Nagy G, Gerhardt GA, Oke AF, Rice ME, Adams RN, Szentirmay MN, Martin CR, Anal. Chem (1985) 85. [Google Scholar]
- [149].Witkowski A, Brajter-Toth A, Anal. Chem 64 (1992) 635. [DOI] [PubMed] [Google Scholar]
- [150].Pihel K, Walker QD, Wightman RM, Anal. Chem 68 (1996) 2084. [DOI] [PubMed] [Google Scholar]
- [151].Hsueh CC, Brajtertoth A, A. Anal. Chem 66 (1994) 2458. [Google Scholar]
- [152].Strand AM, Venton BJ, Anal. Chem 80 (2008) 3708. [DOI] [PubMed] [Google Scholar]
- [153].Yang C, Trikantzopoulos E, Jacobs CB, Venton BJ, Anal. Chim. Acta 965 (2017) 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Hocevar SB, Wang J, Deo RP, Musameh M, Ogorevc B, Electroanalysis 17 (2005) 417. [Google Scholar]
- [155].Jacobs CB, L Vickrey T, Venton BJ, Analyst 136 (2011) 3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Zhang Y, Pan Y, Su S, Zhang L, Li S, Shao M, Electroanalysis 19 (2007) 1695. [Google Scholar]
- [157].Xiao N, Venton BJ, Anal. Chem 84 (2012) 7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Jacobs CB, Ivanov IN, Nguyen MD, Zestos AG, Venton BJ, Anal. Chem 86 (2014) 5721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Takmakov P, Zachek MK, Keithley RB, Walsh PL, Donley C, McCarty GS, Wightman RM, Anal. Chem 82 (2010) 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Yoshimi K, Naya Y, Mitani N, Kato T, Inoue M, Natori S, Takahashi T, Weitemier A, Nishikawa N, McHugh T, Einaga Y, Kitazawa S, Neurosci. Res 71 (2011) 49. [DOI] [PubMed] [Google Scholar]
- [161].Bennet KE, Tomshine JR, Min HK, Manciu FS, Marsh MP, Paek SB, Settell ML, Nicolai EN, Blaha CD, Kouzani AZ, Chang SY, Lee KH, Front. Hum. Neurosci 10 (2016) 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Rusinek CA, Guo Y, Rechenberg R, Becker MF, Purcell E, Verber M, McKinney C, Li W, J. Electrochem. Soc 165 (2018) G3087. [Google Scholar]
- [163].Nakano K, Kayahara T, Tsutsumi T, Ushiro H, J. Neurol 247 (Suppl 5) (2000) V1. [DOI] [PubMed] [Google Scholar]
- [164].Zachek MK, Takmakov P, Moody B, Wightman RM, McCarty GS, Anal. Chem 81 (2009) 6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Zachek MK, Park J, Takmakov P, Wightman RM, McCarty GS, Analyst 135 (2010) 1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Zhang B, L Adams K, Luber SJ, Eves DJ, Heien ML, Ewing AG, Anal. Chem 80 (2008) 1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Zhang B, Heien ML, Santillo MF, Mellander L, Ewing AG, Anal. Chem 83 (2011) 571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Lin Y, Trouillon R, Svensson MI, Keighron JD, Cans AS, Ewing AG, Anal. Chem 84 (2012) 2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Suzuki I, Fukuda M, Shirakawa K, Jiko H, Gotoh M, Biosens. Bioelectron 49 (2013) 270. [DOI] [PubMed] [Google Scholar]
- [170].Zhang S, Song Y, Wang M, Zhang Z, Fan X, Song X, Zhuang P, Yue F, Chan P, Cai X, Biosens. Bioelectron 85 (2016) 53. [DOI] [PubMed] [Google Scholar]
- [171].Zhang S, Song Y, Wang M, Xiao G, Gao F, Li Z, Tao G, Zhuang P, Yue F, Chan P, Cai X, Microsystems & Nanoengineering, 2019. [Google Scholar]
- [172].Tomagra G, Picollo F, Battiato A, Picconi B, De Marchis S, Pasquarelli A, Olivero P, Marcantoni A, Calabresi P, Carbone E, Carabelli V, Front. Neurosci 13 (2019) 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Fenoy AJ, Simpson RK Jr., J. Neurosurg 120 (2014) 132. [DOI] [PubMed] [Google Scholar]
- [174].Stahl SM, Pradko JF, Haight BR, Modell JG, Rockett CB, Learned-Coughlin S, Prim. Care Companion J. Clin. Psychiatry 6 (2004) 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Chang CH, Grace AA, Biol. Psychiatr 76 (2014) 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Venton BJ, Robinson TE, Kennedy RT, Maren S, Eur. J. Neurosci 23 (2006) 3391. [DOI] [PubMed] [Google Scholar]
- [177].Westergren I, Nystrom B, Hamberger A, Johansson BB, J. Neurochem 64 (1995) 229. [DOI] [PubMed] [Google Scholar]
- [178].Lee KH, Biomedical Engineering Letters 1 (2011) 152. [Google Scholar]
- [179].Moran RJ, Kishida KT, Lohrenz T, Saez I, Laxton AW, Witcher MR, Tatter SB, Ellis TL, Phillips PE, Dayan P, Montague PR, Neuropsychopharmacology 43 (2018) 1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Chang S-Y, Kim I, Marsh MP, Jang DP, Hwang S-C, Van Gompel JJ, Goerss SJ, Kimble CJ, Bennet KE, Garris PA, Wireless Fast-Scan Cyclic Voltammetry to Monitor Adenosine in Patients with Essential Tremor during Deep Brain Stimulation, Elsevier, 2012, p. 760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Bledsoe JM, Kimble CJ, Covey DP, Blaha CD, Agnesi F, Mohseni P, Whitlock S, Johnson DM, Horne A, Bennet KE, Lee KH, Garris PA, J. Neurosurg 111 (2009) 712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Chang SY, Jay T, Munoz J, Kim I, Lee KH, Analyst 137 (2012) 2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Griessenauer CJ, Chang SY, Tye SJ, Kimble CJ, Bennet KE, Garris PA, Lee KH, J. Neurosurg 113 (2010) 656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Lee KH, Lujan JL, Trevathan JK, Ross EK, Bartoletta JJ, Park HO, Paek SB, Nicolai EN, Lee JH, Min HK, Kimble CJ, Blaha CD, Bennet KE, Sci. Rep 7 (2017) 46675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Lang AE, Lozano AM, N. Engl. J. Med 339 (1998) 1130. [DOI] [PubMed] [Google Scholar]
- [186].Hornykiewicz O, Neurology 51 (1998) S2. [DOI] [PubMed] [Google Scholar]
- [187].Gerfen CR, Surmeier DJ, Annu. Rev. Neurosci 34 (2011) 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Agnesi F, Tye SJ, Bledsoe JM, Griessenauer CJ, Kimble CJ, Sieck GC, Bennet KE, Garris PA, Blaha CD, Lee KH, J. Neurosurg 111 (2009) 701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Bozorgzadeh B, Schuweiler DR, Bobak MJ, Garris PA, Mohseni P, IEEE Trans Biomed Circuits Syst 10 (2016) 654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Price JB, Rusheen AE, Barath AS, Rojas Cabrera JM, Shin H, Chang SY, Kimble CJ, Bennet KE, Blaha CD, Lee KH, Oh Y, Neurosurg. Focus 49 (2020) E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Morrell MJ, R.N.S.S.i.E.S. Group, Neurology 77 (2011) 1295.21917777 [Google Scholar]
- [192].Medtronic, FDA Approves First-Of-Its-Kind Percept™ PC Neurostimulator with BrainSense™ Technology, https://newsroom.medtronic.com/news-releases/news-release-details/fda-approves-first-its-kind-percepttm-pc-neurostimulator, 2020.
- [193].Trevathan JK, Yousefi A, Park HO, Bartoletta JJ, Ludwig KA, Lee KH, Lujan JL, ACS Chem. Neurosci 8 (2017) 394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Grahn PJ, Mallory GW, Khurram OU, Berry BM, Hachmann JT, Bieber AJ, Bennet KE, Min HK, Chang SY, Lee KH, Lujan JL, Front. Neurosci 8(2014) 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Unterrainer M, Oduncu FS, Med Health Care Philos 18 (2015) 475. [DOI] [PubMed] [Google Scholar]
- [196].Gilbert F, Cook M, O’Brien T, Illes J, Sci. Eng. Ethics 25 (2019) 83. [DOI] [PMC free article] [PubMed] [Google Scholar]


