Abstract
Background
The liver is the most frequent site for metastatic spread in colorectal cancer (CRC) patients, and these patients have much poorer prognosis than those without metastasis. Previous studies have shown that IgG Fc binding protein (FCGBP) plays important roles in tumorigenesis, progression, and prognosis, but its role in CRC metastasis remains unclear.
Purpose
In this study, we are aimed to explore the significance of FCGBP in liver metastatic CRC (LMCRC) patients.
Methods
We analyzed the expression of FCGBP RNA between CRC primary samples and liver metastatic samples in the Gene Expression Omnibus (GEO) database and The Cancer Genome Atlas (TCGA). Next, we assessed the expression of FCGBP protein in 135 paired primary CRC (PC) samples and LMCRC samples. Finally, we explored the relationship between the expression features and clinicopathological characteristics.
Results
The expression data of FCGBP were obtained from the GEO and TCGA databases. FCGBP RNA expression was evaluated between primary lesions (PC) and liver metastatic lesions (LM). FCGBP RNA was down-regulated in PC and LM, and especially lower in LM (p<0.001). Next, the expression of FCGBP protein was evaluated by an immunohistochemistry array in 135 paired primary tumor tissues and metastatic tissues. We found that FCGBP protein was down-regulated in primary lesions and metastatic lesions, especially in metastatic lesions. According to the immunohistochemistry score (SI), each cohort was divided into FCGBP-positive (SI=4–12) and FCGBP-negative (SI=0–3) groups. In both groups, the levels of CEA (PC group, 3.880 vs 77.049, p<0.001; LM group, 3.890 vs 14.239, p=0.008) and CA19-9 (PC group, 8.610 vs 111.700, p<0.001; LM group, 7.660 vs 19.380, p=0.037) were lower than those in the FCGBP-negative group. FCGBP positivity in the LM cohort was an independent risk factor in both overall survival (HR 1.573, 95% Cl [1.017–2.433], p=0.042) and disease-free survival (HR 1.869, 95% Cl [1.256–2.781], p=0.002).
Conclusion
This study found a relationship between FCGBP expression and clinical information of LMCRC patients, and found that FCGBP expression decreased with disease development. The expression of FCGBP in liver metastasis is associated with both the overall and progression-free survival. Our results show that FCGBP could be a promising prognostic factor for LMCRC.
Keywords: colorectal cancer, IgG Fc binding protein, liver metastasis, prognosis, biomarker
Introduction
Colorectal cancer (CRC) is one of the most common malignant diseases that threaten people’s lives worldwide. In recent years, survival rates of CRC have increased owing to earlier diagnosis with colonoscopy and improved treatment strategies. The global incidence and mortality of CRC could be higher in the next 10 years, with more than 2.2 million new cases and 1.1 million cancer deaths annually.1 According to the most recent research data published by CNCC (Chinese National Cancer Center, China), it was estimated that there were more than 376,300 new CRC cases and 191,000 CRC-related deaths in 2015.2 The treatment strategy mainly depends on the TNM staging classification.3 For metastatic CRC patients, chemotherapy and neoadjuvant chemoradiotherapy are the main therapeutic options. But the efficacy of chemotherapy is greatly limited by individual differences and drug targets. Therefore, to discover significant clinical biomarkers aiming to improve patients’ prognoses and provide clinical strategies is our top priority.
IgG Fc binding protein (FCGBP) was first discovered as an Fc portion of the IgG molecule binding site in intestinal and colonic epithelia. It binds monomeric and aggregated IgG but is immunologically distinct from already known IgG Fc receptors.4 FCGBP plays a crucial part in cell protection and anti-inflammation in tissues.5 It is a protein and an important component of mucosal immunological defenses.6 FCGBP has been reported to be implicated in ulcerative colitis, which is a chronic inflammatory disease predisposing to CRC.7 It is produced by goblet cells in the colon and secreted into the bowel lumen with mucus, which suggests that it may be involved in immune protection and inflammation in the intestine. Although the actual function of FCGBP is poorly understood, the clinical significance of FCGBP has been reported in some types of cancer. It has been reported that FCGBP is down-regulated in thyroid carcinoma.8 Down-regulation of IgG binding protein in prostate cancer was found by Gazi et al.9 FCGBP was identified as being associated with osteosarcoma metastasis and may facilitate the individual management of patients after osteosarcoma treatment.10 Yasui and Tanaka reported that compared to normal tissues, FCGBP was down-regulated in cancer tissues. Research based on the AOM/DSS chronic bowel inflammation model showed that FCGBP protein was markedly decreased in cancerous tissues.11 All of the above evidence indicated that FCGBP has been identified as a down-regulated protein in many cancers and suggests that it may play a key role in homeostasis. However, no prior studies have reported FCGBP as a biomarker in CRC, especially in metastatic CRC.
In this study, we analyzed the expression of FCGBP RNA between CRC primary samples and liver metastatic samples in the Gene Expression Omnibus (GEO) database. Then, we assessed the expression of FCGBP RNA in CRC and its prognostic significance based on The Cancer Genome Atlas (TCGA). Next, we assessed the expression of FCGBP protein in primary CRC (PC) samples and liver metastasis of CRC (LMCRC) samples. Finally, we explored the relationship between the expression features and clinicopathological characteristics.
Methods
Patients and Tissue Samples
In this study, 135 paired specimens, including normal mucosa, primary tumor, and liver metastasis tissue, were collected from CRC patients who were diagnosed with no other metastasis according to CT scans. All of the patients had undergone surgical operation from January 2006 to February 2007 and were followed up to December 2012. The diagnosis was all confirmed by the Pathology Department of Cancer Institute and Hospital and all cases could provide complete clinical information, including age, gender, location of the tumor, histological classification, TNM stage, and follow-up information. All patients were followed up regularly every 3 months until the fifth year after the resection.
FCGBP Expression Analyses in GEO and TCGA Databases
To evaluate FCGBP expression between PC and LMCRC, we summarized the expression profiling microarray data from the GEO database with accession numbers GSE41258 and GSE68468 (Affymetrix Human Genome U133A Array). The standardized FCGBP expression was divided into Normal (normal tissue), Primary, and Metastasis in these datasets . For data from GEO, the different expression genes were analyzed by the limma package in Supplementary Table 1, and for data from TCGA, the different expression genes were analyzed by UCSC XENA in Supplementary Table 2. The cohort was divided into four groups: Normal (normal mucosa), Primary, Metastasis, and Recurrence.
Tissue Microarray and Immunohistochemistry
Tissue microarrays (TMAs) were adopted after HE staining verification. Punched samples of 1 mm diameter were measured, taken from the tumor center. Different specimens collected from the same patient were placed on the same TMA.
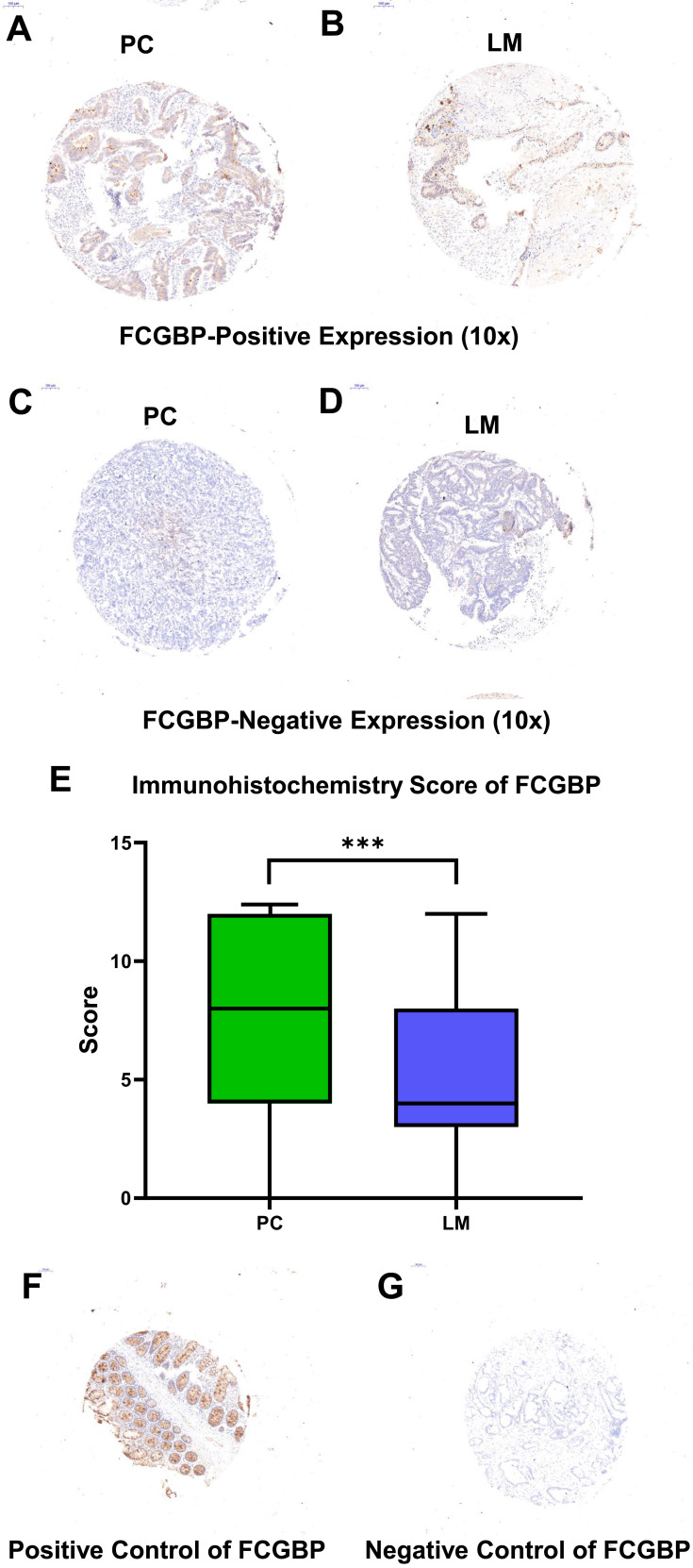
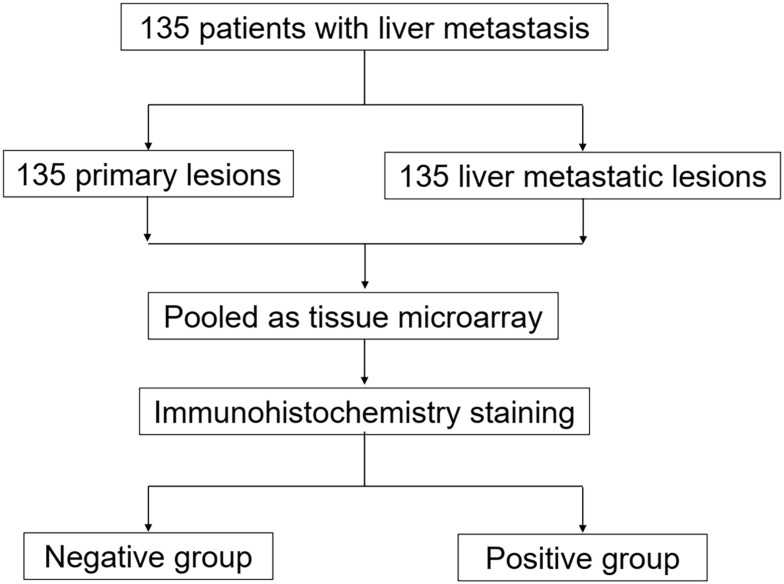
Immunohistochemical staining was performed on the TMA slides with FCGBP (#HPA003564; 1:500; Sigma-Aldrich, USAs) rabbit polyclonal antibody. The immunohistochemistry score (SI) was calculated by the staining intensity (0, negative; 1, weak; 2, moderate; 3, strong) multiplied by the positive rate of stained cells (0–5%, 0; 6–25%, 1; 26–50%, 2; 51–75%, 3; >75%, 4). In this study, SI=4–12 was defined as positive staining, while SI=0–3 was defined as negative staining. Positive rate=Positive samples/(Positive samples + Negative samples). Positive and negative controls for FCGBP immunohistochemistry are shown in Figure 1F and G. The main points of our study are illustrated as a flowchart in Figure 2.
Figure 1.
Representative immunohistochemistry staining pictures of FCGBP. High expression in CRC tissue and liver metastatic tissue (10× for A, B, SI=10) and low expression (10× for C, D, SI=1) for FCGBP protein are shown. (E) The immunohistochemistry score of FCGBP protein in PC and LM, ***p<0.001. (F, G) Positive and negative controls for FCGBP immunohistochemistry.
Figure 2.
Flowchart of the main points.
Cell Transfection, RNA Extraction, and qRT-PCR Analyses
Transfections were carried out using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s instructions. Si-RNAs (GenePharma, China) were used to knock down gene expression.
The total RNA was extracted from cells using TRIzol reagent (Invitrogen, Carlsbad, CA). Reverse-transcribed complementary DNA was synthesized with random primers or microRNA specific stem-loop primers. Subsequently, the cDNA was subjected to real-time PCR on a 7500 real-time PCR system (AB Applied Biosystems, Mannheim, Germany). GAPDH was used as an internal control. Primers and si-FCGBP sequences are listed in Supplementary Table 3.
Cell Migration Assay
The cell migration assay was performed using the Transwell chamber from BD Biosciences (San Jose, CA, USA). In brief, cells (1×105) were seeded in the upper chamber in serum-free media. The lower chamber was filled with corresponding media with 10% FBS as a chemoattractant. Cells were incubated for 24 h at 37°C, fixed in formaldehyde, and stained with crystal violet for 10 min. Cells that migrated to the bottom surface of the filter were counted as invaded cells.
Statistical Analysis
The statistical analyses were performed using a paired t-test or one-way ANOVA. Survival curves were calculated by the Kaplan–Meier method and the log-rank test was used to compare the overall survival (OS) and disease-free survival (DFS). Univariate and multivariate analyses for prognosis were applied, using the Cox proportional hazards regression model. The calculations were carried out using SPSS Statistics 23.0 or GraphPad Prism 8.0. p values less than 0.05 were considered statistically significant.
Results
Evaluation of Immunohistochemical Scores of TMAs in LMCRC Patients
The demographic characteristics of LMCRC patients in our study are summarized in Table 1. A total of 270 tumor samples were divided into two cohorts, the primary CRC tumor cohort (n=135) and liver metastasis tumor cohort (n=135). Each cohort was classified into FCGBP-negative (SI=0–3) and FCGBP-positive (SI=4–12) groups. Representative immunohistochemistry (IHC) images of FCGBP specimens are shown in Figure 1. FCGBP-positive expression (10×) in PC and LM is shown in Figure 1A and B, while negative expression (10×) in PC and LM is shown in Figure 1C and D. The expression of FCGBP protein in LM was much lower than that in PC (p<0.001), and it is shown in Figure 1E. According to the paired samples, FCGBP-negative in PC while FCGBP-positive in LM means positive conversion; FCGBP-positive in PC while FCGBP-positive in LM means negative conversion; other combinations mean no changes. Positive sample numbers and rates in the two cohorts are shown in Table 2.
Table 1.
Demographic Characteristics of LMCRC Patients
| Factor | Patients, No. |
|---|---|
| Age, median (range) (years) | 59.5 (21–78) |
| Gender | |
| Men | 83 |
| Women | 52 |
| CEA (range) | 30.65 (1.40–278.90) |
| CA19-9 (range) | 42.91 (2.98–665.90) |
| Positive nodes (range) | 3 (0–18) |
| Clinical tumor (T) classification | |
| cT2 | 5 |
| cT3 | 72 |
| cT4 | 58 |
| Clinical nodal (N) classification | |
| cN0 | 54 |
| cN1 | 46 |
| cN2 | 35 |
| Perineural invasion | |
| Yes | 45 |
| No | 90 |
| Venous invasion | |
| Yes | 65 |
| No | 70 |
| Lymphatic invasion | |
| Yes | 60 |
| No | 75 |
Table 2.
FCGBP Expression Pattern in Different Samples by IHC Staining
| Sample | Total | FCGBP-Negative | FCGBP-Positive | FCGBP Rate (%) |
|---|---|---|---|---|
| Primary tumor tissue | 135 | 17 | 118 | 87.41 |
| Liver metastasis tumor tissue | 135 | 40 | 95 | 70.37 |
| Positive conversion | 15 | |||
| Negative conversion | 38 | |||
| No changes | 82 |
FCGBP mRNA Was Down-Regulated in Colorectal Liver Metastasis in Colorectal Cancer Based on Public Databases
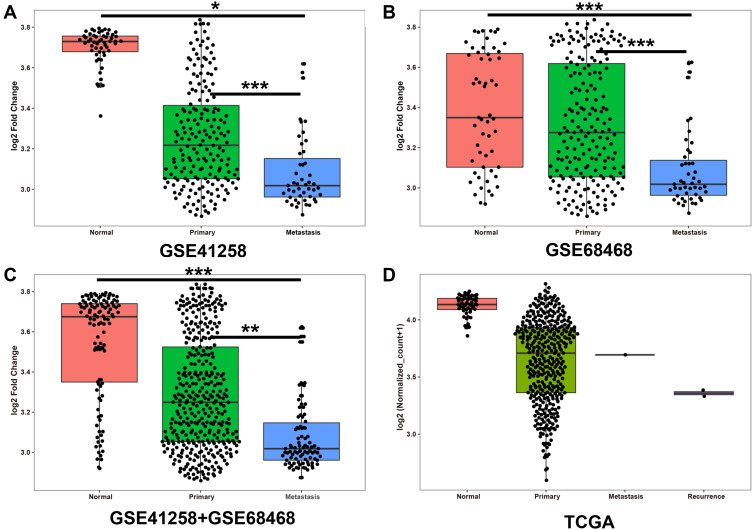
According to the results of FCGBP protein expression in LMCRC TMAs, we then evaluated FCGBP mRNA expression in normal tissues, primary tissues, and liver metastasis tissues based on two databases from GEO. As shown in Figure 3A and B, in both GES41258 and GSE68468 datasets, the FCGBP mRNA expression was decreased with the progression of the disease. If the GES41258 and GSE68468 dataset were analyzed as a whole, a similar pattern was acquired (Figure 3C).
Figure 3.
FCGBP expression pattern in normal intestinal tissue, primary tumor, liver metastasis on GEO and TCGA database, and recurrence tissue additionally in TCGA. (A) GSE41258, (B) GSE68468, and (C) GSE41258+GSE68468 were collected from GEO; (D) summarized from TCGA. * p<0.05, **p<0.01, ***p<0.001.
To further confirm FCGBP mRNA expression in CRC patients, we then assessed FCGBP mRNA expression in TCGA database. Thus, 434 CRC patients were collected and divided into four groups. Then, 51 normal tissues, 380 primary tumor tissues, one metastatic tissue, and two recurrence tissues were compared. The results were consistent with the GEO data (Figure 3D).
Relationship Between FCGBP Expression and Clinical Features in the Primary Tumor Lesions in Our Cohort
According to the results of the public data, we further analyzed FCGBP expression patterns and the clinical information including age, gender, CEA and CA19-9 values, number of positive nodes, and TN classification.
As shown in Table 3, in the primary tumor cohort, 17 patients were defined as negative and 118 as positive. According to the statistical data in the table, age (60 vs 59 years, p=0.745) and gender (p=0.066) were not significantly different between the two groups. Previous work showed that higher levels of CEA and CA19-9 were important biomarkers of CRC patients and predicted poor prognosis.23 In the FCGBP-positive group, the levels of CEA (3.880 vs 77.049, p<0.001) and CA19-9 (8.610 vs 111.700, p<0.001) were lower than in the FCGBP-negative group. The number of positive nodes was similar between the two groups (3 vs 2, p=0.145). We also assessed the T classification and N classification in the two groups. The percentage of patients in different T classifications was similar between the two groups. However, the percentage of cN0 in the FCGBP-positive group was higher (41.5% vs 29.4%) and the percentage of cN2 was lower (19.5% vs 70.6%) than those in the FCGBP-negative group (p<0.001).
Table 3.
Analyses of Relative Clinicopathological Factors and FCGBP Expression in Patients
| Factor | Patients, No. (Primary) | p-Value | Patients, No. (Metastasis) | p-Value | ||
|---|---|---|---|---|---|---|
| Negative (n=17) | Positive (n=118) | Negative (n=40) | Positive (n=95) | |||
| Age, median (range) (years) | 60 (32–72) | 59 (21–78) | 0.745 | 60.5 (21–74) | 58 (29–78) | 0.539 |
| Gender | 0.066 | 0.315 | ||||
| Men | 7 | 76 | 22 | 61 | ||
| Women | 10 | 42 | 18 | 34 | ||
| CEA (range) | 77.049 (1.40–278.90) | 3.880 (0.10–322.20) | <0.001 | 14.239 (1.36–78.90) | 3.890 (0.10–322.20) | 0.008 |
| CA19-9 (range) | 111.70 (2.98–665.90) | 8.61 (0.60–827.10) | <0.001 | 19.38 (0.60–418.70) | 7.66 (0.60–827.10) | 0.037 |
| Positive nodes | 3 (0–15) | 2 (0–18) | 0.145 | 2 (0–18) | 1 (0–10) | 0.012 |
| Clinical tumor (T) classification | 0.492 | 0.352 | ||||
| cT2 | 0 | 5 | 3 | 2 | ||
| cT3 | 9 | 63 | 21 | 51 | ||
| cT4 | 8 | 50 | 16 | 42 | ||
| Clinical nodal (N) classification | <0.001 | 0.003 | ||||
| cN0 | 5 (29.4%) | 49 (41.5%) | 8 (20%) | 46 (48.4%) | ||
| cN1 | 0 (0%) | 46 (39.0%) | 15 (37.5%) | 31 (32.6%) | ||
| cN2 | 12 (70.6%) | 23 (19.5%) | 17 (42.5%) | 18 (18.9%) | ||
| Perineural invasion | 0.359 | 0.2183 | ||||
| Yes | 4 | 41 | 10 | 35 | ||
| No | 13 | 77 | 30 | 60 | ||
| Venous invasion | 0.923 | 0.511 | ||||
| Yes | 8 | 57 | 21 | 44 | ||
| No | 9 | 61 | 19 | 51 | ||
| Lymphatic invasion | 0.417 | 0.643 | ||||
| Yes | 6 | 54 | 19 | 41 | ||
| No | 11 | 64 | 21 | 54 | ||
Relationship Between FCGBP Expression and Clinical Features in the Liver Metastasis Tumor Lesions in Our Cohort
As shown in Table 3, 40 metastatic tissues were defined as negative and 95 as positive. There was no significant difference between the two groups with respect to age (60 vs 58 years, p=0.539) and gender (p=0.315). In the FCGBP-positive group, the levels of CEA (3.890 vs 14.239, p=0.008) and CA19-9 (7.660 vs 19.380, p=0.037) were lower than those in the FCGBP-negative group. There was a significant difference between the number and patterns of positive nodes (1vs 2, p=0.012). The percentage of patients in different T classifications was nearly the same between the two groups (p=0.352). However, the percentage of cN0 in the FCGBP-positive group was higher (48.4% vs 20.0%) and the percentage of cN2 was much lower (18.9% vs 42.5%) than those in the FCGBP-negative group (p=0.003).
FCGBP-Positivity Was Associated with Suppressed Migration in Cells and Better Survival in LMCRC
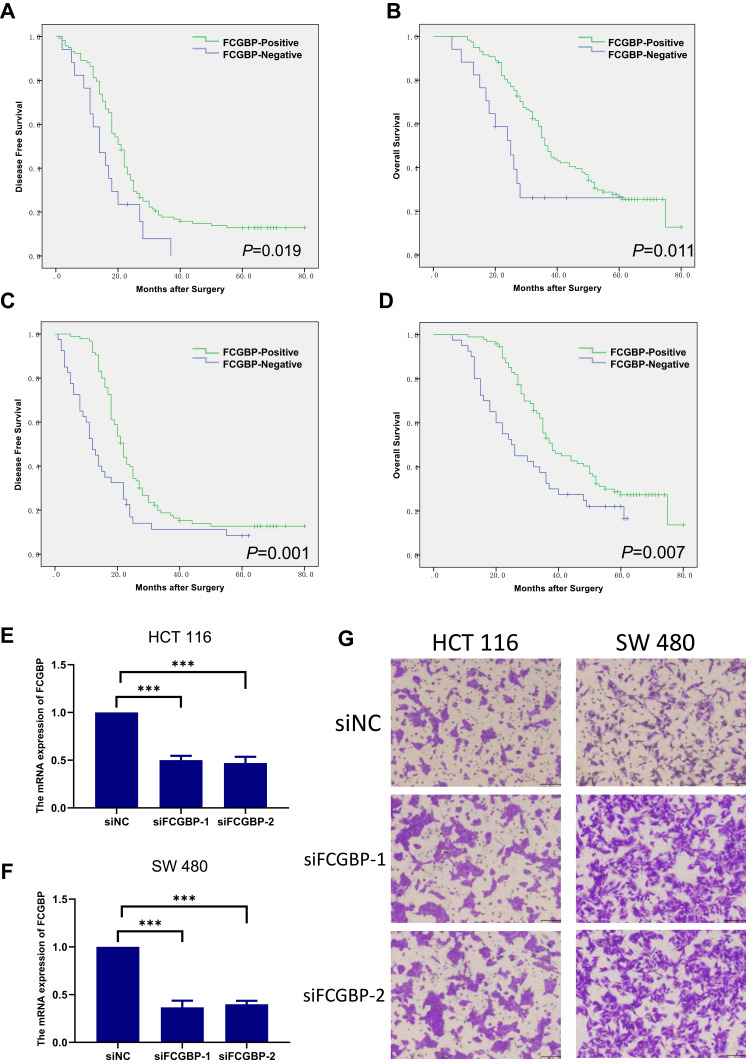
To investigate the migration ability of FCGBP in CRC cells, we chose the less highly metastatic cell lines HCT116 and SW480 and transfected them with si-FCGBP and si-NC. The validation of the knockdown efficacy of FCGBP was evaluated by qRT-PCR (Figure 4E and F). The Transwell assay showed that the migration capacities of HCT116 and SW480 were inhibited by FCGBP knockdown compared with the control group (Figure 4G).
Figure 4.
Overall survival and disease-free survival in LMCRC. (A, B) Primary tumor cohort, (C, D) liver metastasis tumor cohort. (E, F) mRNA expression of FCGBP in HCT116 and SW480 cell lines. ***p<0.001. (G) Transwell assay that evaluated the migration capacities of HCT116 and SW480 after FCGBP knockdown.
To further identify the prognostic significance of FCGBP in LMCRC patients, we analyzed the survival in the two cohorts.
In the primary tumor cohort, the FCGBP-positive group had a better OS and DFS (OS p=0.011 and DFS p=0.019; Figure 4A and B). Univariate Cox analysis demonstrated that FCGBP-positivity was a correlative factor for both OS (HR 2.223, 95% Cl [1.203–4.109], p=0.011) and DFS (HR 1.842, 95% Cl [1.082–3.137], p=0.024). Multivariate Cox analysis showed that FCGBP-positivity was an independent prognostic factor for OS (HR 2.035, 95% Cl [1.052–3.938], p=0.035) but not for DFS (HR 1.570, 95% Cl [0.884–2.787], p=0.123) (Tables 4 and 5).
Table 4.
Cox Analyses of Potential Prognostic Factors for Overall Survival in LMCRC
| Group | Comparison | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% Cl | p-Value | HR | 95% Cl | p-Value | ||
| Primary tumor cohort | FCGBP-negative vs FCGBP-positive | 2.223 | 1.203–4.109 | 0.011 | 2.035 | 1.052–3.938 | 0.035 |
| Liver metastasis tumor cohort | FCGBP-negative vs FCGBP-positive | 1.611 | 1.049–2.473 | 0.029 | 1.573 | 1.017–2.433 | 0.042 |
Table 5.
Cox Analyses of Potential Prognostic Factors for Disease-Free Survival in LMCRC
| Group | Comparison | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% Cl | p-Value | HR | 95% Cl | p-Value | ||
| Primary tumor cohort | FCGBP-negative vs FCGBP-positive | 1.842 | 1.082–3.137 | 0.024 | 1.570 | 0.884–2.787 | 0.123 |
| Liver metastasis tumor cohort | FCGBP-negative vs FCGBP-positive | 1.874 | 1.263–2.782 | 0.002 | 1.869 | 1.256–2.781 | 0.002 |
In the liver metastasis tumor cohort, the FCGBP-positive group also had a better OS and DFS (p=0.007 for OS and p=0.001 for DFS; Figure 4C and D). Multivariate Cox analysis illustrated that FCGBP-positivity was an independent prognostic factor for both OS (HR 1.573, 95% Cl [1.017–2.433], p=0.042) and DFS (HR 1.869, 95% Cl [1.256–2.781], p=0.002) (Tables 4 and 5).
Discussion
In this study, immunohistochemical staining was performed in LMCRC TMAs with FCGBP antibody. We compared the IHC score of FCGBP between primary tumor tissues and liver metastasis tissues and found that FCGBP expression was decreased with disease development. We verified the phenomenon based on TMAs in the GEO and TCGA datasets to confirm that FCGBP mRNA expression was decreased with the progression of the disease. As for clinical information, we found that CEA and CA19-9 levels were lower in the FCGBP-positive group. The percentage of cN0 in the FCGBP-positive group was higher and the percentage of cN2 was lower than that in the FCGBP-negative group. In the LM cohort, we found that FCGBP-positivity was an independent risk factor in both OS and DFS. FCGBP may be a prognostic factor for LMCRC patients.
At present, metastasis remains an important factor contributing toward the majority of cancer-associated mortalities, as metastatic cancer is often resistant to conventional therapies.12 Cell adhesion occurring in the vasculature of specific organs is an essential step in cancer metastasis, and FCGBP was significantly enriched in the function of cell adhesion.13,14 In the intestine, FCGBP protein exists in a large complex containing Muc2 and Trefoil peptide, suggesting that it may be important in the maintenance of homeostasis and integrity of the epithelium.15,16 However, reliable and sensitive methods to detect early metastasis in CRC are not currently available. Metastasis is facilitated by the cell–cell interactions between the endothelium and tumor cells in distant tissues. Previous studies found that down-regulated FCGBP was associated with many malignant diseases and it is known as a prognostic marker in a variety of cancers. Ma et al performed whole exome-sequencing analysis of 63 CRC cases, and found that with the deficiency of FCGBP, CRC developed and showed worse survival rates.17 At stage I or II CRC, FCGBP was reported to be positively associated with the prognosis of CRC.18 Onstenk et al measured CTCs of liver metastasis in CRC patients and their primary tumor tissues and found that FCGBP was down-regulated.19 Meanwhile, in other kinds of cancer, FCGBP also has positive effects on prognosis. In HPV-infected patients, FCGBP expression was up-regulated and negatively correlated with TGF-β in head and neck squamous cell carcinoma, and this was meaningful to the prognosis of patients.20 The current study indicated that FCGBP expression could be further evaluated as a biomarker for predicting survival in patients with gallbladder cancer, and FCGBP could be a promising target in the control of gallbladder cancer progression. Xiong et al found that immunohistochemical staining of FCGBP was decreased, and evaluated it as a biomarker for predicting survival in patients with gallbladder cancer. They found that NT5E was the most decreased gene, while FCGBP was the most decreased gene in the TGF-β-induced gallbladder cancer cells, which suggests that these two genes may play essential roles in epithelial–mesenchymal transition. It could be a potential target in the control of gallbladder cancer progression.21 Using the univariate Cox model and Kaplan–Meier survival curves, a correlation between FCGBP expression and OS in ovarian adenocarcinoma was observed.22
Metastasis is the main reason for death in CRC patients, especially liver metastasis in CRC, which has much poorer prognosis. Previous works found that FCGBP can be broadly evaluated as a biomarker in all stages of CRC. However, the present studyfound that FCGBP-positivity in LMCRC is linked to longer survival. Multivariate Cox analysis illustrated that FCGBP-positivity was an independent prognostic factor for OS and DFS in the liver metastasis cohort but not in the primary tumor cohort. We found that FCGBP could predict prognosis more adequately in metastatic tumors. We first reported that FCGBP can predict prognosis in LMCRC and we thought that it could be a potential biomarker for LMCRC patients. The pattern of FCGBP expression is gradually down-regulated as the cancer develops, and we speculate that FCGBP may be a tumor suppressor gene. The function of FCGBP and the mechanisms through which FCGBP is down-regulated with cancer development deserve further investigation.
Conclusions
In this study, we discovered that FCGBP expression was much lower in liver metastasis tumor tissues compared with primary tumor tissues in liver metastatic CRC patients. The expression of FCGBP in liver metastasis is associated with the overall survival and progression-free survival. In summary, FCGBP may be a potential biomarker to predict the prognosis of CRC.
Funding Statement
This study was supported by CAMS Innovation Fund for Medical Sciences (CIFMS) (2016-I2M-1-001), Beijing Science and Technology Program (D17110002617004).
Abbreviations
FCGBP, IgG Fc binding protein; CRC, colorectal cancer; LMCRC, liver metastatic colorectal cancer; GEO, Gene Expression Omnibus; PC, primary cancer lesion; LM, metastatic cancer lesion; CNCC, Chinese National Cancer Center, China; TCGA, The Cancer Genome Atlas; TMA, tissue microarray; OS, overall survival; DFS, disease-free survival; SI, immunohistochemistry score.
Data Sharing Statement
The data supporting the results of this study are available from TCGA database GEO database; clinical and follow-up information are available from the Cancer Institute and Hospital.
Ethics Approval and Informed Consent
This article was carried out in accordance with the recommendations of 7th edition of the TNM staging system, the Clinical Research Ethics Committee of Cancer Institute and Hospital, Chinese Academy of Medical Sciences, with written informed consent being obtained from all subjects. The IRB number of the study is KY-3019-037. The protocol was approved by the Clinical Research Ethics Committee of Cancer Institute and Hospital, Chinese Academy of Medical Sciences. All procedures performed in this study were in accordance with the 1964 Helsinki Declaration and its later amendments.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest.
References
- 1.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683–691. doi: 10.1136/gutjnl-2015-310912 [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 3.Zhao Z, Zou S, Guan X, et al. Apolipoprotein E overexpression is associated with tumor progression and poor survival in colorectal cancer. Front Genet. 2018;9:650. doi: 10.3389/fgene.2018.00650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kobayashi K, Blaser MJ, Brown WR. Identification of a unique IgG Fc binding site in human intestinal epithelium. J Immunol. 1989;143(8):2567–2574. [PubMed] [Google Scholar]
- 5.Selbach M, Mann M. Protein interaction screening by quantitative immunoprecipitation combined with knockdown (QUICK). Nat Methods. 2006;3(12):981–983. doi: 10.1038/nmeth972 [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi K, Ogata H, Morikawa M, et al. Distribution and partial characterisation of IgG Fc binding protein in various mucin producing cells and body fluids. Gut. 2002;51(2):169–176. doi: 10.1136/gut.51.2.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Risques RA, Lai LA, Himmetoglu C, et al. Ulcerative colitis-associated colorectal cancer arises in a field of short telomeres, senescence, and inflammation. Cancer Res. 2011;71(5):1669–1679. doi: 10.1158/0008-5472.CAN-10-1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Donovan N, Fischer A, Abdo EM, et al. Differential expression of IgG Fc binding protein (FcgammaBP) in human normal thyroid tissue, thyroid adenomas and thyroid carcinomas. J Endocrinol. 2002;174(3):517–524. doi: 10.1677/joe.0.1740517 [DOI] [PubMed] [Google Scholar]
- 9.Gazi MH, He M, Cheville JC, Young CY. Downregulation of IgG Fc binding protein (Fc gammaBP) in prostate cancer. Cancer Biol Ther. 2008;7(1):70–75. doi: 10.4161/cbt.7.1.5131 [DOI] [PubMed] [Google Scholar]
- 10.Dong S, Huo H, Mao Y, Li X, Dong L. A risk score model for the prediction of osteosarcoma metastasis. FEBS Open Bio. 2019;9(3):519–526. doi: 10.1002/2211-5463.12592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yasui Y, Tanaka T. Protein expression analysis of inflammation-related colon carcinogenesis. J Carcinog. 2009;8:10. doi: 10.4103/1477-3163.51851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Rogoff HA, Keates S, et al. Suppression of cancer relapse and metastasis by inhibiting cancer stemness. Proc Natl Acad Sci U S A. 2015;112(6):1839–1844. doi: 10.1073/pnas.1424171112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bendas G, Borsig L. Cancer cell adhesion and metastasis: selectins, integrins, and the inhibitory potential of heparins. Int J Cell Biol. 2012;2012:676731. doi: 10.1155/2012/676731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qi C, Hong L, Cheng Z, Yin Q. Identification of metastasis-associated genes in colorectal cancer using metaDE and survival analysis. Oncol Lett. 2016;11(1):568–574. doi: 10.3892/ol.2015.3956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albert TK, Laubinger W, Muller S, et al. Human intestinal TFF3 forms disulfide-linked heteromers with the mucus-associated FCGBP protein and is released by hydrogen sulfide. J Proteome Res. 2010;9(6):3108–3117. doi: 10.1021/pr100020c [DOI] [PubMed] [Google Scholar]
- 16.Johansson ME, Thomsson KA, Hansson GC. Proteomic analyses of the two mucus layers of the colon barrier reveal that their main component, the Muc2 mucin, is strongly bound to the Fcgbp protein. J Proteome Res. 2009;8(7):3549–3557. doi: 10.1021/pr9002504 [DOI] [PubMed] [Google Scholar]
- 17.Ma R, Jing C, Zhang Y, et al. The somatic mutation landscape of Chinese colorectal cancer. J Cancer. 2020;11(5):1038–1046. doi: 10.7150/jca.37017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang W, Shi J, Zhou Y, et al. Integrating proteomics and transcriptomics for the identification of potential targets in early colorectal cancer. Int J Oncol. 2019;55(2):439–450. doi: 10.3892/ijo.2019.4833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Onstenk W, Sieuwerts AM, Mostert B, et al. Molecular characteristics of circulating tumor cells resemble the liver metastasis more closely than the primary tumor in metastatic colorectal cancer. Oncotarget. 2016;7(37):59058–59069. doi: 10.18632/oncotarget.10175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Liu Y, Liu H, et al. FcGBP was upregulated by HPV infection and correlated to longer survival time of HNSCC patients. Oncotarget. 2017;8(49):86503–86514. doi: 10.18632/oncotarget.21220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiong L, Wen Y, Miao X, Yang Z. NT5E and FcGBP as key regulators of TGF-1-induced epithelial-mesenchymal transition (EMT) are associated with tumor progression and survival of patients with gallbladder cancer. Cell Tissue Res. 2014;355(2):365–374. doi: 10.1007/s00441-013-1752-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi CH, Choi JJ, Park YA, et al. Identification of differentially expressed genes according to chemosensitivity in advanced ovarian serous adenocarcinomas: expression of GRIA2 predicts better survival. Br J Cancer. 2012;107(1):91–99. doi: 10.1038/bjc.2012.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo H, Shen K, Li B, Li R, Wang Z, Xie Z. Clinical significance and diagnostic value of serum NSE, CEA, CA19-9, CA125 and CA242 levels in colorectal cancer. Oncol Lett. 2020;20(1):742–570. [DOI] [PMC free article] [PubMed] [Google Scholar]