Supplemental Digital Content is available in the text.
Keywords: acute respiratory distress syndrome, animal, mechanical ventilation, nebulizer, pulmonary surfactant
Objectives:
Effective treatment options for surfactant therapy in acute respiratory distress syndrome and coronavirus disease 2019 have not been established. To conduct preclinical studies in vitro and in vivo to evaluate efficiency, particle size, dosing, safety, and efficacy of inhaled surfactant using a breath-synchronized, nebulized delivery system in an established acute respiratory distress syndrome model.
Design:
Preclinical study.
Setting:
Research laboratory.
Subjects:
Anesthetized pigs.
Intervention:
In vitro analysis included particle size distribution and inhaled dose during simulated ventilation using a novel breath-synchronized nebulizer. Physiologic effects of inhaled aerosolized surfactant (treatment) were compared with aerosolized normal saline (control) in an adult porcine model (weight of 34.3 ± 0.6 kg) of severe acute respiratory distress syndrome (Pao2/Fio2 <100) with lung lavages and ventilator-induced lung injury during invasive ventilation.
Measurements and Main Results:
Mass median aerosol diameter was 2.8 µm. In vitro dose delivered distal to the endotracheal tube during mechanical ventilation was 85% ± 5%. Nebulizers were functional up to 20 doses of 108 mg of surfactant. Surfactant-treated animals (n = 4) exhibited rapid improvement in oxygenation with nearly full recovery of Pao2/Fio2 (~300) and end-expiratory lung volumes with nominal dose less than 30 mg/kg of surfactant, whereas control subjects (n = 3) maintained Pao2/Fio2 less than 100 over 4.5 hours with reduced end-expiratory lung volume. There was notably greater surfactant phospholipid content and lower indicators of lung inflammation and pathologic lung injury in surfactant-treated pigs than controls. There were no peridosing complications associated with nebulized surfactant, but surfactant-treated animals had progressively higher airway resistance post treatment than controls with no differences in ventilation effects between the two groups.
Conclusions:
Breath-synchronized, nebulized bovine surfactant appears to be a safe and feasible treatment option for use in coronavirus disease 2019 and other severe forms of acute respiratory distress syndrome.
The acute respiratory distress syndrome (ARDS) is characterized by severe hypoxemic respiratory failure (Pao2/Fio2 < 200) (1), radiographic evidence of diffuse bilateral alveolar infiltrates, and reduced functional residual capacity (FRC) and lung compliance (2). Although the specific mechanisms leading to these abnormalities still require elucidation, repeated cycling of the lungs with a ventilator at suboptimal FRC and/or excessive tidal volume (VT) contribute to ventilator-induced lung injury (VILI) (3). In ARDS, surfactant deficiency results from severe proinflammatory and anti-inflammatory pulmonary responses, alveolar epithelial and endothelial injury, microvascular leak, inflammatory edema, and type II pneumocyte proliferation (4, 5).
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) uses the angiotensin-converting enzyme 2 receptor to access, infect, and destroy alveoli lining and surfactant-producing type II pneumocytes (6–9). Biopsy and postmortem specimens in COVID-19 reveal diffuse alveolar damage, protein leak, hyaline membrane formation, inflammation in the alveolar walls, and desquamation of type II pneumocytes (10, 11). Radiographic evidence in COVID-19 patients includes ground glass opacities reminiscent of hyaline membrane disease, a key feature of primary surfactant-deficiency and respiratory distress syndrome (RDS) in premature infants (12, 13). Based on pathophysiologic similarities with ARDS (14) and newborn RDS, a strong rational exists for using exogenous surfactant as a potential treatment option for severe COVID-19 ARDS (9, 15, 16).
The purpose of this research was to conduct preclinical studies to address particle size, dosing, safety, and feasibility with a novel breath-synchronized nebulizer for surfactant delivery in a porcine ARDS model prior to conducting a study in subjects with COVID-19 ARDS.
METHODS
A more detailed description of all study methods can be found by accessing the online supplement (Supplemental methods, Supplemental Digital Content 2, http://links.lww.com/CCX/A502).
Nebulized Surfactant Drug and Breath-Synchronized Delivery System
A liquid bovine surfactant, SF-RI 1 (Alveofact, 108 mg/vial; Lyomark Pharma, Oberhaching, Germany), was delivered with a prototype breath-synchronized aerosol delivery system (Aerogen Pharma Corp., San Mateo, CA), using a novel photo-defined aperture plate vibrating mesh nebulizer capable of generating aerosol during inhalation (Fig. 1A).
Figure 1.
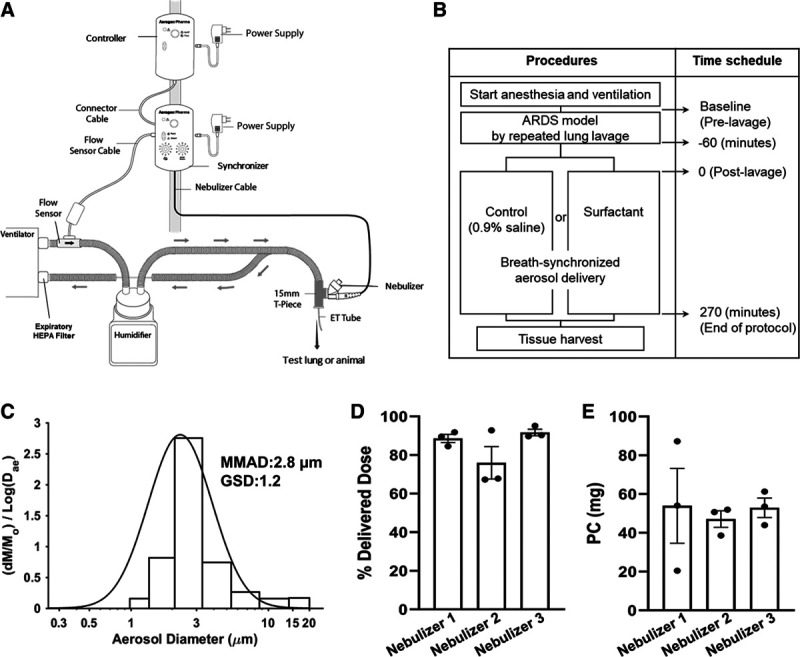
Protocol and in vitro data. Schematic of the surfactant delivery system used for test lung or animals (A) and animal study protocol procedures (B). This nebulizer produces aerosol with a novel, two-layer vibrating mesh aperture plate and prevents drug loss to the system on exhalation by synchronizing drug delivery to a ventilator breath by means of a flow change sensor placed within the inspiratory limb of the ventilator. The schematic was provided courtesy of Aerogen Pharma. Distribution of particle sizes, mass median aerosol diameter (MMAD), and geometric sd (GSD) obtained from the aerosol impactor (C); delivered lung dose based on the gravimetric mass detected on a lung model filter and referenced to the nominal surfactant dose (D); and phosphatidylcholine (PC) levels on the filter (E) following breath-synchronized nebulized surfactant with simulated mechanical ventilation of coronavirus disease 2019. Nebulizers were tested in triplicate using three different nebulizers (n = 9). Delivered surfactant dose was 85% ± 5%, and the phosphatidylcholine level was 51 ± 2 mg (~65% of nominal phosphatidylcholine level [~78 mg in 108 mg of surfactant]). Values are means ± sem. ARDS = acute respiratory distress syndrome.
In Vitro Study Design
In vitro experiments were conducted to assess feasibility and ability of the nebulizer to deliver surfactant for treatment of large animals supported by mechanical ventilation. We evaluated: 1) operational reliability, 2) aerosol particle size, and 3) delivered lung dose during mechanical ventilation.
In a fume hood, we ran three separate nebulizers in continuous output mode to evaluate durability and longevity based on the nebulizer’s ability to function reliably when nebulizing 20 doses of surfactant. The mass median aerosol diameter (MMAD) and geometric sd (GSD) of surfactant aerosol were measured using a high-performance multistage next generation impactor (NGI). Fine particle fraction (FPF) was calculated as the proportion of all particles, consisting of respirable particles less than 5.4 µm, delivered to the NGI. Delivered dose of nebulized surfactant was collected on a filter distal to the endotracheal tube during mechanical ventilation of a lung model configured with representative lung mechanics and ventilator settings previously described in COVID-19 patients (Fig. S1, Supplemental Digital Content 3, http://links.lww.com/CCX/A503) (12). Delivered lung dose was quantified by measuring gravimetric mass deposited on a filter placed at the end of the endotracheal tube and further analyzed to quantify phospholipid content based on phosphatidylcholine, a major constituent of SF-RI 1 surfactant. Particle sizing and delivered lung dose studies were each conducted in triplicate using three different nebulizers (n = 9).
In Vivo Animal Study Design
All experimental animal procedures were approved by Seattle Children’s Institutional Animal Care and Use Committee (number ACUC00604). We used a porcine model of severe ARDS, induced by repeated lavage of the lungs and VILI (17). Experiments in Yorkshire pigs (S&S Farms, Ramona, CA) were conducted to determine the impact of nebulized surfactant on Pao2/Fio2 ratio, oxygenation index (OI), ventilation efficiency index (VEI), mean pulmonary artery pressure (PAP), lung mechanics, end-expiratory lung volumes (EELV) based on electrical impedance tomography (EIT; PulmoVista 500 system; Draeger, Lubeck, Germany), and lung injury/inflammatory variables determined by histology, inflammatory lung edema, and cytokine profile (Fig. 1B).
Breath-Synchronized Surfactant Replacement for Porcine Severe ARDS Model
Following instrumentation and induction of surfactant-deficiency and lung injury, pigs were randomized to receive nebulized surfactant in dose increments (108 mg in 2.4 mL) or placebo (2.4 mL normal saline) 1 hour after stabilization of the ARDS model. Each dose was administered to end of nebulization, with a pause of 5 minutes between consecutive doses to a total of 10 doses. Blood gases, hemodynamic variables, lung mechanics, and EIT were recorded at baseline (post lavage) and every 30 minutes following initiation of nebulization.
Porcine Lung Samples
Following final dosing, animals were euthanized with euthasol (100 mg/kg), and the whole body was perfused with cold normal saline. The lungs were removed while ventilated at peak inspiratory pressure of 20 cm H2O. A bronchoalveolar lavage fluid (BALF) sample was removed from the left upper lobe using gentle suction. The left lower lobe was infused with 10% neutral formaldehyde fixative at a constant pressure of 25 cm H2O for histology in an established manner (18). The right lower lobe was frozen in liquid nitrogen and stored at –80°C, for later quantification of surfactant phosphatidylcholine and cytokine levels. The right middle lobe was used to quantify microvascular leak and inflammatory edema with the wet-to-dry weight ratio (W/D) measurement (19).
Cytokine Measurement
Plasma, BALF, and lung tissue levels of inflammatory markers were measured and analyzed in duplicate using the Eve Technologies Discovery Assay Pig Cytokine Array (Eve Technologies, Calgary, AB, Canada).
Histopathologic Evaluation
Paraffin embedded lung tissues were sectioned at 5 μm thickness, and hematoxylin and eosin (H&E) stained slides from three separate sites per animal were examined. Lung injury was reviewed and scored by a pulmonary pathologist (G.D.), blinded to group, according to a semiquantitative grading scale (Table 1) modified from that proposed by the American Thoracic Society (20) and based on other relevant histologic phenotypes recently described in cases of severe COVID-19 ARDS (21).
TABLE 1.
Lung Injury Score
| Lung injury scoring system | |||
|---|---|---|---|
| Variables | Score | ||
| A | Alveolar neutrophils | 0 | Absent |
| B | Interstitial neutrophils | 1 | Mild, localized |
| C | Capillary congestion and/or hemorrhage within alveolar spaces | 2 | Moderate, larger areas |
| D | Proteinaceous exudate/edema | 3 | Severe, ubiquitous |
| E | Hyaline membranes | ||
| F | Alveolar septal thickening with/without reactive pneumocytes | ||
| G | Lobular remodeling (smooth muscle hyperplasia, fibrosis) | ||
Statistical Analysis
All data are presented as means ± sem. Two-way analysis of variance was used for the evaluation of the effects of group and time and their interaction and both Sidak and Dunnett multiple comparisons tests were performed on these data. Other data were compared between two groups and analyzed with unpaired t tests. The criterion for significance was p value of less than 0.05 for all comparisons.
RESULTS
Studies In Vitro
The MMAD was 2.8, GSD was 1.2 (Fig. 1C), and FPF was 91% ± 6%. Delivered dose of aerosol surfactant to a filter distal to the endotracheal tube during ventilation was 85% ± 5% of the nominal dose placed into the nebulizer, determined gravimetrically (Fig. 1D). Lung model filter phosphatidylcholine levels were 51 ± 2 mg or 65% delivered dose based on nominal phosphatidylcholine levels found in each dose (Fig. 1E). The nebulizer delivery time for a single surfactant dose by the breath-synchronized nebulizer system was 12.6 ± 1.8 minutes or total aerosol output of 7.4 ± 0.5 mg/min. In delivering up to 20 doses of surfactant with three separate nebulizers, we observed no catastrophic failure or interruption of aerosol output.
Studies In Vivo
Porcine ARDS Model.
Eight pigs weighing 34.3 ± 0.6 kg were selected for the experiment, and one died from a lethal cardiac arrhythmia during pulmonary artery catheter insertion. Seven animals (Control, n = 3; Surfactant, n = 4) were analyzed in this study. Baseline data before lavage were similar between two groups on gas exchange, hemodynamics, and lung mechanics (Table 2). All animals had Pao2/Fio2 of less than 100, increased mean PAP, airway resistance, and lung elastance, and decreased lung compliance following lavage, and there were no differences in any variables between two groups at 1 hour post final lavage (pre treatment).
TABLE 2.
Baseline Data
| Pre Lavage, Mean ± sem | 1 hr Post Final Lavage(Pre Treatment), Mean ± sem | |||||
|---|---|---|---|---|---|---|
| Control (n = 3) | Surfactant (n = 4) | p | Control (n = 3) | Surfactant (n = 4) | p | |
| Body weight (kg) | 32.9 ± 0.7 | 34.9 ± 0.7 | 0.11 | — | — | — |
| pH | 7.46 ± 0.01 | 7.45 ± 0.02 | 0.61 | 7.32 ± 0.01 | 7.25 ± 0.05 | 0.30 |
| Paco2 (mm Hg) | 41 ± 3 | 40 ± 1 | 0.64 | 57 ± 4 | 58 ± 7 | 0.93 |
| Pao2/Fio2 | 527 ± 12 | 478 ± 19 | 0.10 | 83 ± 9 | 81 ± 5 | 0.86 |
| Oxygenation index | 2.2 ± 0.6 | 1.7 ± 0.1 | 0.36 | 24.5 ± 4.1 | 19.8 ± 3.6 | 0.42 |
| Ventilation efficiency index | 0.37 ± 0.02 | 0.41 ± 0.05 | 0.61 | 0.11 ± 0.02 | 0.11 ± 0.02 | 0.77 |
| Mean pulmonary arterial pressure (mm Hg) | 22 ± 2 | 20 ± 2 | 0.67 | 39 ± 4 | 40 ± 2 | 0.84 |
| Lung compliance (mL/cm H2O) | 35.1 ± 2.9 | 39.3 ± 3.6 | 0.49 | 10.6 ± 1.8 | 13.5 ± 1.1 | 0.28 |
| Respiratory system compliance (mL/cm H2O) | 28.0 ± 2.3 | 30.9 ± 3.2 | 0.53 | 8.5 ± 1.3 | 8.3 ± 1.1 | 0.92 |
| Airway Resistance (cm H2O/L/s) | 7.3 ± 0.5 | 7.6 ± 0.3 | 0.62 | 10.7 ± 1.7 | 11.9 ±1.1 | 0.55 |
| Elastance (cm H2O/mL) | 37.0 ± 3.1 | 34.8 ± 2.5 | 0.59 | 131.7 ± 11.3 | 118.0 ± 13.6 | 0.50 |
| Positive end-expiratory pressure (cm H2O) | 5 | 5 | — | 9.3 ± 1.1 | 7.5 ± 2.2 | 0.53 |
| Lavages (n) | — | — | — | 9 ± 2 | 7 ± 2 | 0.36 |
Dashes indicate not applicable.
Gas Exchange, Hemodynamics, and Lung Mechanics.
Dosing was achieved over a 4.5-hour period. After starting nebulization, the Pao2/Fio2 and OI gradually recovered, and these effects became more apparent at 150–180 minutes following initiation of the treatment (Fig. 2), whereas Pao2/Fio2 values remained consistently low (~100) in the controls. There were no differences in pH, Paco2, VEI, mean PAP, lung compliance, respiratory system compliance, and elastance between the two groups following nebulization, but airway resistance was greater in Surfactant subjects than Controls (Fig. 2 and Fig. 3A). Based on EIT measurements, relative improvement in alveolar recruitment and recovery of FRC from baseline were shown with greater ΔEELV in the Surfactant group than Controls. Improvements in ΔEELV became more evident following 90 minutes of surfactant nebulization, with distributive changes, representative of recruitment, occurring in the dorsal lung regions (dorsal ΔEELV), whereas Controls showed evidence of alveolar collapse and consolidation in dependent lung regions over time (Fig. 3B–D). There were no peridosing complications associated with either nebulized surfactant or placebo.
Figure 2.
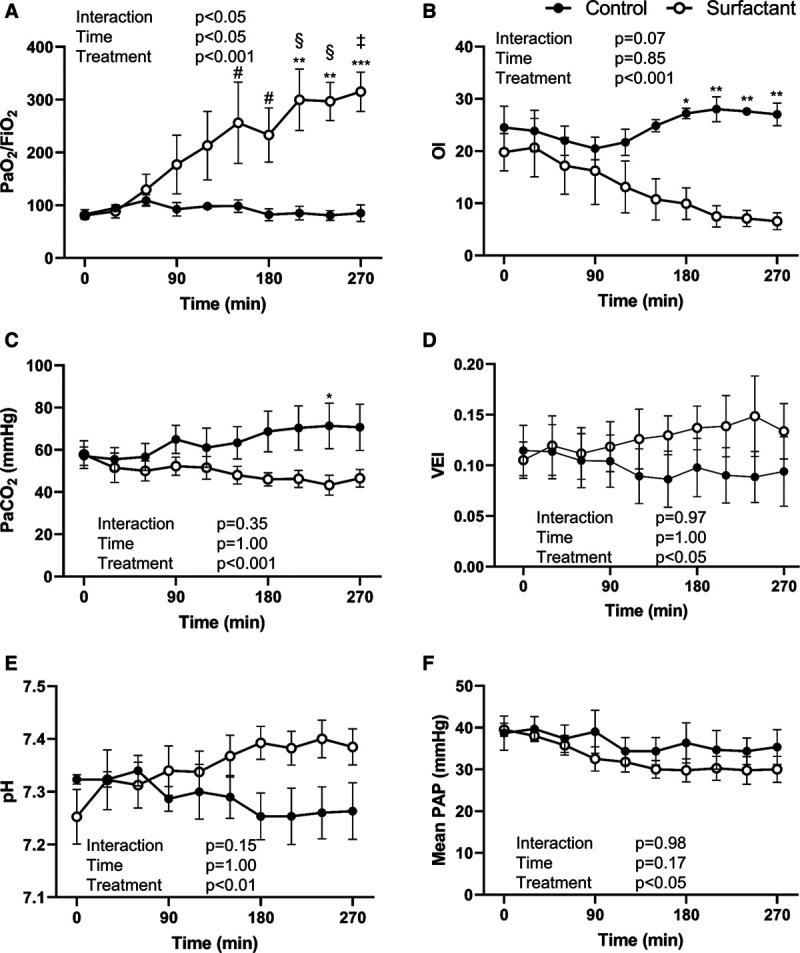
Gas exchange and hemodynamics. The changes in blood gas and hemodynamic data in the Control and the Surfactant groups during the 4.5-hr treatment period. The Surfactant group showed significant improvement in Pao2/Fio2 and oxygenation index (OI) throughout the study period. Both the Surfactant group and the Control group had no changes from baseline (time 0) in mean pulmonary artery pressure (PAP). Values are means ± sem. *p < 0.05; **p < 0.01; ***p < 0.001 compared between two groups. #p < 0.05; §p < 0.01; ‡p < 0.001 compared with 0 (at the start of the treatment) within groups.VEI = ventilation efficiency index.
Figure 3.
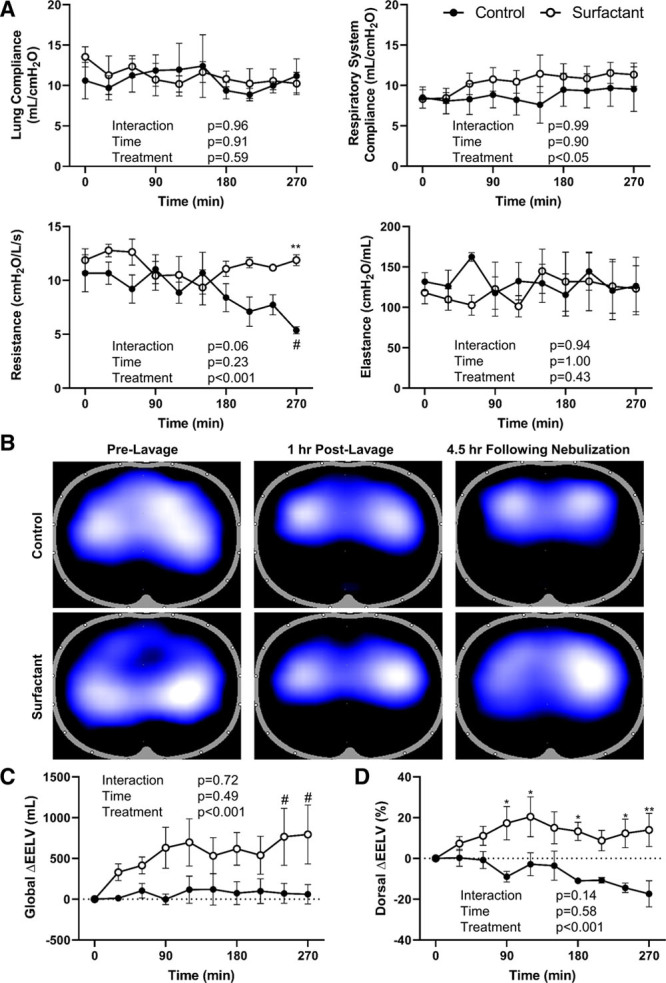
Changes in lung mechanics data in the Control and the Surfactant groups during the 4.5-hr treatment period. Both the Surfactant and the Control group had no changes from baseline (time 0) in lung or respiratory system compliance and elastance (A). Representative electrical impedance tomography changes between one Surfactant and one Control are shown pre lavage, 1 hr post lavage, and 4.5 hr following nebulization (B). The global end-expiratory lung volume (ΔEELV) (C) did not change significantly and dorsal ΔEELV (%) (D) decreased from baseline in Controls; by contrast, both ΔEELV and ΔEELV (%) improved in pigs treated with nebulized surfactant. Values are means ± sem. *p < 0.05; **p < 0.01 compared between two groups. #p < 0.05 compared with 0 (at the start of the treatment) within groups.
Histology and Lung Injury Score.
We observed the macroscopic appearance after the tissue harvest at the end of protocol. Compared with the well-inflated lungs of the surfactant-treated animals, the controls had large areas of discoloration suggestive of pulmonary collapse, edema, and hemorrhage (Fig. 4A). The W/D ratio of lung tissue was higher in the Control group than Surfactant group suggesting higher extravascular fluid accumulation with pulmonary edema or hemorrhage in the Control group (Fig. 4B). Macroscopic appearance of excised lungs showed abundant foamy liquid, assumed to be surfactant overflow, from the cut surface of lung in surfactant-treated animals, which was absent in Controls (Fig. S2A, Supplemental Digital Content 3, http://links.lww.com/CCX/A503). Surfactant content (phosphatidylcholine) recovered from lung homogenate showed greater repletion in Surfactant animals than Controls (Fig. S2B, Supplemental Digital Content 3, http://links.lww.com/CCX/A503).
Figure 4.
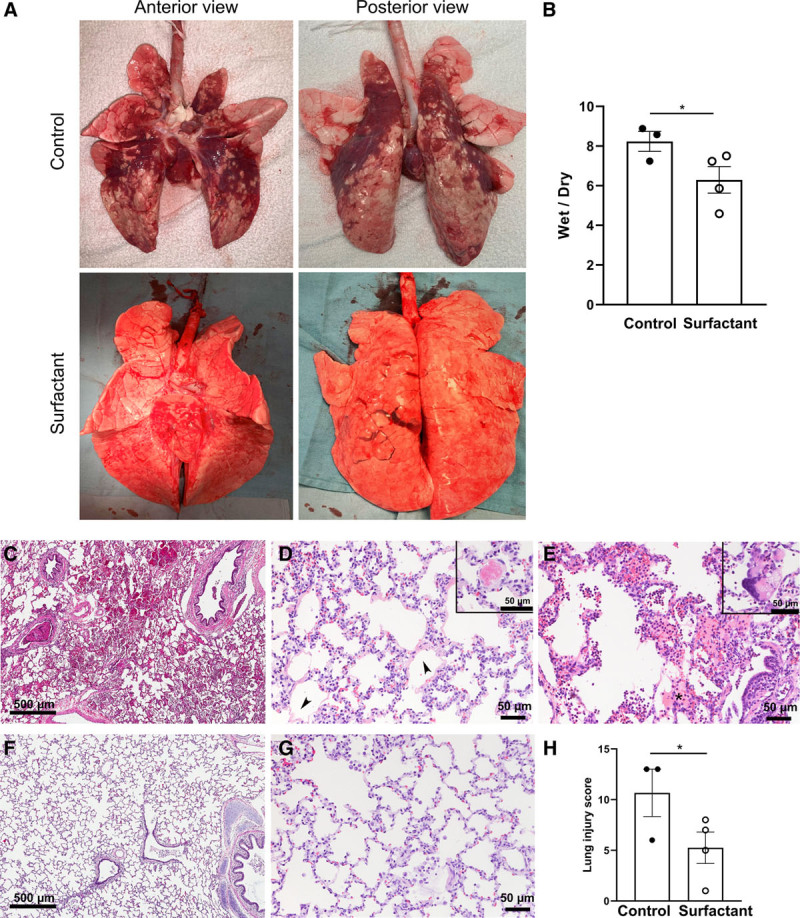
Macro- and microscopic appearance of lung tissue and cytokine levels. A, Gross appearance of the lungs is shown while being ventilated ex vivo with peak inspiratory pressures of 20 cm H2O. Compared with the well-inflated lungs of the surfactant-treated animals, the control lungs are collapsed with patchy hemorrhage. B, The wet-to-dry weight ratio was lower in Surfactant group than Control group. Representative lung histology in control (C–E) and surfactant-treated animals (F, G). C, Hemorrhage, (D) septal thickening, hyaline membranes (arrowheads), (E) neutrophilic inflammation and proteinaceous exudate (asterisk) were more prominent in the control lungs; vascular thrombi (D, inset) and multinucleated giant cells (E, inset) were occasionally seen. The alveolar architecture was generally well preserved in the surfactant-treated group with less inflammation and thin alveolar walls (F and G). H, The Lung Injury Score was lower in surfactant-treated than control animals. Values are means ± sem. *p < 0.05.
H&E staining showed that lung tissue from the Control group had obvious pathologic changes, including neutrophilic inflammation change, capillary congestion, alveolar hemorrhage, lung edema, hyaline membranes, and alveolar wall thickening (Fig. 4C–E). In contrast, lung tissues from the Surfactant group had almost normal structures and less histopathologic injury when examined under light microscopy (Fig. 4, F and G). This resulted in Control subjects having higher histological lung injury scores than surfactant-treated animals (Fig. 4H) (Table S1, Supplemental Digital Content 1, http://links.lww.com/CCX/A501).
Cytokine Measurement.
All cytokine levels in plasma, lung tissue, and BALF are provided in Supplemental Online Tables (Tables S2–S4, Supplemental Digital Content 1, http://links.lww.com/CCX/A501). Plasma data were standardized by prelavage baseline data and were reported as the change ratio from prelavage baseline data due to the variation of baseline data. The plasma cytokines interferon-gamma, interleukin (IL)-1ra, and IL-6 (Fig. S3A, Supplemental Digital Content 3, http://links.lww.com/CCX/A503) and BAL cytokine IL-1ra/IL-1ra (Fig. S3C, Supplemental Digital Content 3, http://links.lww.com/CCX/A503) were higher in Controls than surfactant-treated animals. Other cytokine variables in plasma, BAL, and lung tissue did not differ statistically, but generally lower plasma cytokine levels were found in the surfactant-treated animals than Control animals (Fig. S3, B and C, Supplemental Digital Content 3, http://links.lww.com/CCX/A503; and Tables S2–S4, Supplemental Digital Content 1, http://links.lww.com/CCX/A501).
DISCUSSION
Using a large animal model emulating ARDS and severe VILI, we demonstrated a notable positive response to inhaled surfactant aerosol. We used a surfactant dose less than 30% of that administered by bolus instillation in preterm infants with RDS. Although inhaled surfactant has long been speculated to have benefit in treatment in ARDS, there are limited data to compare delivery efficiency with an effective dose. With a label dose of 100 mg/kg for instillation in infants, similar dosing in a 70 kg adult would render inhaled surfactant impractical. To that end, we limited dosing to 10 nebulized surfactant doses, which resulted in nearly full recovery of prelung lavage values with mean Pao2/Fio2 (> 300). The rapid recovery in oxygenation observed in these surfactant-treated animals coincided with favorable recovery in EELV, which was more pronounced in the dependent lung regions. This improvement suggests that aerosol was deposited into regions of the lungs that are prone to developing consolidation. We postulate that delivery of small aerosol droplets adequately coated alveoli with surface active material reduced alveolar surface tension and produced the improvement in EELV and oxygenation. Thus, prevention of atelectasis and reduced effects from VILI following nebulized surfactant best explain why lower extravascular lung water (W/D ratio) and decreased immunologic and histologic evidence of lung injury were observed in surfactant-treated animals compared with controls.
Multiple large clinical trials in adult ARDS have failed to show significant reduction in mortality using various methods of surfactant delivery (22–25). The liquid instillation method is likely poorly distributed, with drug loss occurring in large airways and regions of normal compliance. Surfactant is then delivered inadequately to alveoli, resulting in failure in more recent trials (26). Data from both recent investigations of fluid dynamic modeling, and the original studies which demonstrated efficacy, suggest that large fluid volumes with high concentration surfactant and distal intrabronchial drug delivery are needed to maximize alveolar surfactant delivery (23, 25, 27).
Liquid bolus surfactant administration is associated with several complications, including agitation/discomfort, refractory hypoxemia, hemodynamic instability, and airway occlusion (28). Findings from adult ARDS studies have used proportionally higher surfactant doses of 250–300 mg/kg, which necessitates an instilled surfactant volume of 280–400 mL (29). Some studies were terminated as they showed adverse events in the treatment groups: liquid instillation was poorly tolerated, with airway obstruction and increased hypoxia (22, 29, 30). Instillation of liquid surfactant at these doses and volumes could potentially overwhelm the cardiopulmonary system and add to respiratory failure and VILI. We did not observe adverse events or changes in lung mechanics and gas exchange to suggest airway occlusion or any other deleterious effects associated with nebulized surfactant treatment in the current study, which we attribute to the low relative administration volume (24 mL) and small droplet size used.
Nebulized surfactant replacement therapy, as an alternative to liquid instillation, is not a novel concept. Previous studies that evaluated short-term physiologic outcomes in animals with RDS have demonstrated improved pulmonary mechanics, lung structure integrity, and reduced lung inflammation, even with minimal deposition in the lungs (31–35). Nebulized surfactant in large animal ARDS models has shown improved oxygenation (36) and reduced lung injury (37), but benefits of this therapy with antecedent nebulizers have not been established in ARDS. Based on findings from a large clinical trial, nebulized synthetic surfactant in patients with ARDS had no significant effect on oxygenation or 30-day survival (38). Early attempts at aerosolizing surfactant were likely hampered by poor clinical efficacy due the type of surfactant provided, poor nebulizer performance, and patient selection.
Prior aerosol technology has achieved poor drug deposition in the lungs; relatively large droplets, frequent clogging by viscous surfactants, and low output rates have all contributed to limited delivery efficiency (39, 40). Also, nebulizers that produce aerosolized surfactant continuously throughout the respiratory cycle have demonstrated extremely poor lung deposition with ~99% of surfactant depositional loss occurring in the expiratory limb of the ventilator tubing, Y piece, and nebulizer (33). Nebulizer technology has improved, but aerosolization may still require two to five times the dose of liquid surfactant instillation due to high expiratory drug loss. Recent studies using novel continuous output nebulizers in surfactant-deficient small animal models of RDS showed improvement in gas exchange, but only when 200–750 mg/kg nebulized surfactant was used (41, 42).
Our study emphasizes the importance of providing aerosolized surfactant using a nebulizer that generates a concentrated volume of small droplets for timed delivery in synchrony with the inspiratory phase to minimize drug loss in the expiratory limb. The choice of droplet size is critical to delivery efficiency with multiple reports suggesting that droplets exiting the endotracheal tube are generally less than 3 µm (43). The inhaled mass from our study in vitro (65–85%) was six- to eight-fold greater than values reported from previous ventilation studies that evaluated continuous output nebulization with larger aerosol medication droplets (44), which we ascribe to generation of small aerosol droplets (MMAD < 3 µm) during the initial 80% of the inspiratory cycle in combination with nebulizer positioning at the proximal airway. These findings corroborate high efficiency surfactant nebulization based on rapid improvement in oxygenation and EELV and reduced lung injury in a short amount of time using a weight-based surfactant dose approximately 1/3 (~30 mg/kg) of that previously established for neonates (100 mg/kg) (45). Also, compared with a previous report of liquid surfactant administration to large animals with a tracheal catheter or bronchoscopy (46), we used approximately one tenth of the fluid volume administered as aerosol. This advance in nebulizer technology represents an option to simply and safely treat severe ARDS or COVID-19, with potentially fewer side effects than tracheal instillation or bronchoscopic administration.
LIMITATIONS
There are some limitations in this study. We used a surfactant-deficient ARDS animal model induced by repeated lavage. Repeated pulmonary lavages in anesthetized pigs causes lung injury and surfactant deficiency resembling major aspects of human ARDS, but alveoli are generally highly recruitable (47, 48). However, we took additional measures during lavage to induce severe VILI using low PEEP and excessive VT and Fio2. Following lavage, we applied lower PEEP levels than are used clinically in severe ARDS in order to evaluate the effects of alveolar recruitment related to surfactant replacement. Although this was not an infectious model of SARS-CoV-2, there were similarities to COVID-19 reports with this model, including refractory oxygenation and ventilation impairment, pulmonary arterial hypertension, reduced compliance and FRC, histologic evidence of hyaline membrane formation and pulmonary vascular thrombi, and severe pulmonary inflammation and injury (21). The second limitation is that this was a short-term study. We confirmed rapid improvement of oxygenation and prevention of lung injury and some indicators of inflammation within 4.5 hours after starting treatment, but long-term follow-up studies to approximate administration of surfactant and the continuous treatment effect on VILI after discontinuation of surfactant administration are needed Third, animal numbers were small due to limited availability of large animals during the COVID-19 pandemic. Nonetheless, animal numbers were adequate to show the statistical difference on Pao2/Fio2, our primary outcome variable. In addition, we did not conduct a comparison with liquid bolus or bronchoscopic instillation of surfactant, in the present study. A study comparing novel approaches using bronchoscopic liquid administration to aerosolized surfactant will provide clinically important information such as the difference of peridosing side effects and the distribution of drug delivery. Last, we kept the Fio2 at 1.0 for all experiments and used an arbitrary number of ten nebulizer doses. In a clinical setting, oxygen and nebulized surfactant would be titrated based on a dose response, so it is likely subjects may require fewer doses of surfactant to achieve oxygenation goals.
CLINICAL IMPLICATIONS
Treatment approaches for COVID-19 are rapidly emerging. Nebulized surfactant may be a viable option to be used with invasive and noninvasive ventilation with COVID-19. Thus, we propose that successful completion of these studies and rapid translation to the bedside could alter the current treatment paradigm for ARDS and COVID-19 induced by SARS-CoV-2 and other respiratory viruses.
CONCLUSIONS
Breath-synchronized nebulized surfactant therapy was shown to improve oxygenation, alveolar recruitment, and prevent lung injury and inflammation at low dose administration in a porcine ARDS model. Administration of aerosol surfactant appears to be a safe and feasible therapeutic option for consideration in ventilated patients with severe forms of ARDS. Further studies in humans are recommended.
ACKNOWLEDGMENTS
We would like to thank Farhad Imam, MD, PhD, of the Bill and Melinda Gates Foundation for facilitating dialogue in the community via Microsoft Team forums. We would also like to thank Donna Dupras and Rebecca Engberg for their contributions to this science.
Supplementary Material
Footnotes
This work was performed at the Seattle Children’s Research Institute, Seattle, WA.
Drs. DiBlasi and Kajimoto contributed equally to this article.
Literature search, study design, data collection, data analysis, and article preparation was done by Drs. DiBlasi and Kajimoto. Drs. Pfeiffer and Fink provided input on the design of studies in vitro. Drs. Pfeiffer, Zimmerman, Crotwell, Malone, Ringer, and Uthamanthil assisted in acquiring measurements and data analysis, and Drs. Deutsch and Ledee provided surfactant assay results and interpretation of histology and lung injury score, respectively. Dr. Portman oversaw all aspects of this research.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
Dr. DiBlasi has served as a consultant, received research funding, and has been on the speaker’s bureau for Draeger Medical, Bunnell Medical, Vapotherm, and Vero Biotech. Dr. Fink is Chief Scientific Officer of Aerogen Pharma Corp. Draeger Medical provided the PulmoVista electrical impedance tomography unit and V500 ventilator for this study. Prototype nebulizers were provided by Aerogen Pharma Corp. Surfactant was purchased using Seattle Children’s Research Institute internal funds. The remaining authors have disclosed have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Ranieri VM, Rubenfeld GD, Thompson BT, et al. ; ARDS Definition Task Force. Acute respiratory distress syndrome: The Berlin definition. JAMA. 2012; 307:2526–2533 [DOI] [PubMed] [Google Scholar]
- 2.Panwar R, Madotto F, Laffey JG, et al. Compliance phenotypes in early acute respiratory distress syndrome before the COVID-19 pandemic. Am J Respir Crit Care Med. 2020; 202:1244–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chu EK, Whitehead T, Slutsky AS. Effects of cyclic opening and closing at low- and high-volume ventilation on bronchoalveolar lavage cytokines. Crit Care Med. 2004; 32:168–174 [DOI] [PubMed] [Google Scholar]
- 4.Adhikari N, Burns KE, Meade MO. Pharmacologic therapies for adults with acute lung injury and acute respiratory distress syndrome. Cochrane Database Syst Rev. 2004; 2004:CD004477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dushianthan A, Goss V, Cusack R, et al. Altered molecular specificity of surfactant phosphatidycholine synthesis in patients with acute respiratory distress syndrome. Respir Res. 2014; 15:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding Y, Wang H, Shen H, et al. The clinical pathology of severe acute respiratory syndrome (SARS): A report from China. J Pathol. 2003; 200:282–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet. 2020; 395:565–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mason RJ. Pathogenesis of COVID-19 from a cell biology perspective. Eur Respir J. 2020; 55:2000607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schousboe P, Wiese L, Heiring C, et al. Assessment of pulmonary surfactant in COVID-19 patients. Crit Care. 2020; 24:552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian S, Hu W, Niu L, et al. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020; 15:700–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020; 8:420–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle region - case series. N Engl J Med. 2020; 382:2012–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koumbourlis AC, Motoyama EK. Lung mechanics in COVID-19 resemble respiratory distress syndrome, not acute respiratory distress syndrome: Could surfactant be a treatment? Am J Respir Crit Care Med. 2020; 202:624–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brault C, Zerbib Y, Kontar L, et al. COVID-19 versus non-COVID-19-related acute respiratory distress syndrome: Differences and similarities. Am J Respir Crit Care Med. 2020; 202:1301–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Busani S, Dall’Ara L, Tonelli R, et al. Surfactant replacement might help recovery of low-compliance lung in severe COVID-19 pneumonia. Ther Adv Respir Dis. 2020; 14:1753466620951043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takano H. Pulmonary surfactant itself must be a strong defender against SARS-CoV-2. Med Hypotheses. 2020; 144:110020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russ M, Kronfeldt S, Boemke W, et al. Lavage-induced surfactant depletion in pigs as a model of the acute respiratory distress syndrome (ARDS). J Vis Exp. 2016; 115:e53610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thurlbeck WM. Post-mortem lung volumes. Thorax. 1979; 34:735–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang XX, Sha XL, Li YL, et al. Lung injury induced by short-term mechanical ventilation with hyperoxia and its mitigation by deferoxamine in rats. BMC Anesthesiol. 2020; 20:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matute-Bello G, Downey G, Moore BB, et al. ; Acute Lung Injury in Animals Study Group. An official American Thoracic Society workshop report: Features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol. 2011; 44:725–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bradley BT, Maioli H, Johnston R, et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington state: A case series. Lancet. 2020; 396:320–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raghavendran K, Willson D, Notter RH. Surfactant therapy for acute lung injury and acute respiratory distress syndrome. Crit Care Clin. 2011; 27:525–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richman PS, Spragg RG, Robertson B, et al. The adult respiratory distress syndrome: First trials with surfactant replacement. Eur Respir J Suppl. 1989; 3:109s–111s [PubMed] [Google Scholar]
- 24.Spragg RG, Taut FJH, Günther A, et al. Surfactant replacement therapy in ARDS. Chest. 2009; 136:321–322 [DOI] [PubMed] [Google Scholar]
- 25.Willson DF, Chess PR, Notter RH. Surfactant for pediatric acute lung injury. Pediatr Clin North Am. 2008; 55:545–575, ix [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grotberg JB, Filoche M, Willson DF, et al. Did reduced alveolar delivery of surfactant contribute to negative results in adults with acute respiratory distress syndrome? Am J Respir Crit Care Med. 2017; 195:538–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Filoche M, Tai CF, Grotberg JB. Three-dimensional model of surfactant replacement therapy. Proc Natl Acad Sci U S A. 2015; 112:9287–9292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walsh BK, Daigle B, DiBlasi RM, et al. ; American Association for Respiratory Care. AARC clinical practice guideline. Surfactant replacement therapy: 2013. Respir Care. 2013; 58:367–375 [DOI] [PubMed] [Google Scholar]
- 29.Meng SS, Chang W, Lu ZH, et al. Effect of surfactant administration on outcomes of adult patients in acute respiratory distress syndrome: A meta-analysis of randomized controlled trials. BMC Pulm Med. 2019; 19:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spragg RG, Lewis JF, Wurst W, et al. Treatment of acute respiratory distress syndrome with recombinant surfactant protein C surfactant. Am J Respir Crit Care Med. 2003; 167:1562–1566 [DOI] [PubMed] [Google Scholar]
- 31.Dijk PH, Heikamp A, Bambang Oetomo S. Surfactant nebulisation prevents the adverse effects of surfactant therapy on blood pressure and cerebral blood flow in rabbits with severe respiratory failure. Intensive Care Med. 1997; 23:1077–1081 [DOI] [PubMed] [Google Scholar]
- 32.Dijk PH, Heikamp A, Bambang Oetomo S. Surfactant nebulisation: Lung function, surfactant distribution and pulmonary blood flow distribution in lung lavaged rabbits. Intensive Care Med. 1997; 23:1070–1076 [DOI] [PubMed] [Google Scholar]
- 33.Dijk PH, Heikamp A, Piers DA, et al. Surfactant nebulisation: Safety, efficiency and influence on surface lowering properties and biochemical composition. Intensive Care Med. 1997; 23:456–462 [DOI] [PubMed] [Google Scholar]
- 34.Fok TF, al-Essa M, Dolovich M, et al. Nebulisation of surfactants in an animal model of neonatal respiratory distress. Arch Dis Child Fetal Neonatal Ed. 1998; 78:F3–F9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewis JF, Ikegami M, Jobe AH, et al. Aerosolized surfactant treatment of preterm lambs. J Appl Physiol (1985). 1991; 70:869–876 [DOI] [PubMed] [Google Scholar]
- 36.Spengler D, Winoto-Morbach S, Kupsch S, et al. Novel therapeutic roles for surfactant-inositols and -phosphatidylglycerols in a neonatal piglet ARDS model: A translational study. Am J Physiol Lung Cell Mol Physiol. 2018; 314:L32–L53 [DOI] [PubMed] [Google Scholar]
- 37.Lutz C, Carney D, Finck C, et al. Aerosolized surfactant improves pulmonary function in endotoxin-induced lung injury. Am J Respir Crit Care Med. 1998; 158:840–845 [DOI] [PubMed] [Google Scholar]
- 38.Anzueto A, Baughman RP, Guntupalli KK, et al. Aerosolized surfactant in adults with sepsis-induced acute respiratory distress syndrome. Exosurf acute respiratory distress syndrome sepsis study Group. N Engl J Med. 1996; 334:1417–1421 [DOI] [PubMed] [Google Scholar]
- 39.Mazela J, Polin RA. Aerosol delivery to ventilated newborn infants: Historical challenges and new directions. Eur J Pediatr. 2011; 170:433–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willson DF. Aerosolized surfactants, anti-inflammatory drugs, and analgesics. Respir Care. 2015; 60:774–790; discussion 790–793 [DOI] [PubMed] [Google Scholar]
- 41.Bianco F, Ricci F, Catozzi C, et al. From bench to bedside: In vitro and in vivo evaluation of a neonate-focused nebulized surfactant delivery strategy. Respir Res. 2019; 20:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lampland AL, Wolfson MR, Mazela J, et al. Aerosolized KL4 surfactant improves short-term survival and gas exchange in spontaneously breathing newborn pigs with hydrochloric acid-induced acute lung injury. Pediatr Pulmonol. 2014; 49:482–489 [DOI] [PubMed] [Google Scholar]
- 43.Fink J, Ari A. Aerosol delivery to intubated patients. Expert Opin Drug Deliv. 2013; 10:1077–1093 [DOI] [PubMed] [Google Scholar]
- 44.Ari A, Atalay OT, Harwood R, et al. Influence of nebulizer type, position, and bias flow on aerosol drug delivery in simulated pediatric and adult lung models during mechanical ventilation. Respir Care. 2010; 55:845–851 [PubMed] [Google Scholar]
- 45.Proquitté H, Dushe T, Hammer H, et al. Observational study to compare the clinical efficacy of the natural surfactants alveofact and curosurf in the treatment of respiratory distress syndrome in premature infants. Respir Med. 2007; 101:169–176 [DOI] [PubMed] [Google Scholar]
- 46.Lewis J, McCaig L, Häfner D, et al. Dosing and delivery of a recombinant surfactant in lung-injured adult sheep. Am J Respir Crit Care Med. 1999; 159:741–747 [DOI] [PubMed] [Google Scholar]
- 47.Ballard-Croft C, Wang D, Sumpter LR, et al. Large-animal models of acute respiratory distress syndrome. Ann Thorac Surg. 2012; 93:1331–1339 [DOI] [PubMed] [Google Scholar]
- 48.Otáhal M, Mlček M, Vítková I, et al. A novel experimental model of acute respiratory distress syndrome in pig. Physiol Res. 2016; 65:S643–S651 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


