Abstract
The nutritional status at diagnosis, as well as weight loss during chemotherapy, are important factors for morbidity and mortality in cancer patients. They might also influence outcomes in patients with acute myeloid leukemia (AML) receiving allogeneic hematopoietic stem cell transplantation (HSCT). We evaluated the body mass index (BMI) at diagnosis, prior to HSCT, and the BMI difference (ΔBMI = BMIHSCT–BMIdiagnosis) in 662 AML patients undergoing allogeneic HSCT. Patients being obese at AML diagnosis had significantly higher nonrelapse mortality (NRM) and shorter overall survival (OS) after HSCT, but no distinct cumulative incidence of relapse than nonobese patients. Weight loss during chemotherapy (ΔBMI > –2) was a strong predictor for higher NRM and shorter OS in univariate and multivariate analyses. These results were observed across all European LeukemiaNet (ELN) 2017 risk groups but especially in patients with favorable or intermediate ELN2017 risk and patients transplanted in morphologic complete remission. Only in patients being obese at AML diagnosis, weight loss did not result in adverse outcomes. ΔBMI > –2 represents a strong, independent, and modifiable risk factor for AML patients treated with HSCT. Nutritional monitoring and supplementation during disease course might improve patients’ outcomes.
Introduction
Overweight and obesity represent global health problems with an increasing prevalence, which nearly tripled since 1975. In 2016 more than 1.9 billion adults were overweight and over 650 million were obese.1 Defined by a body mass index (BMI) of ≥ 25 kg/m2 and ≥ 30 kg/m2, overweight and obesity have been generally associated with a higher all-cause mortality,2 especially due to associated cardiovascular or metabolic comorbidities.3 In cancer patients, data remains inconsistent according to the diagnosed neoplasm with a higher relative risk of mortality with increasing BMI in adenocarcinoma of the esophagus, endometrial cancer, or kidney cancer but lower cancer-related mortality for higher BMI in squamous cell carcinoma of the esophagus or lung cancer.4,5 Also, in the context of hematologic neoplasm treated with allogeneic hematopoietic stem cell transplantation (HSCT), the prognostic relevance of the BMI remains controversial. Sorror et al6 included a BMI of ≥35 kg/m2 prior to HSCT as one risk factor into the widely used hematopoietic cell transplantation comorbidity index (HCT-CI), which predicts higher nonrelapse mortality (NRM) and shorter overall survival (OS) in patients undergoing HSCT. In contrast, other studies showed no impact on outcomes7,8 or an increased risk of transplant-related mortality and shorter OS for underweight patients prior to HSCT.9-11 Only weight loss during allogeneic HSCT was consistently associated with worse survival.12 Also, in patients newly diagnosed with acute myeloid leukemia (AML), data on the prognostic impact of the nutritional status at diagnosis remain inconsistent. Some studies reported higher complete remission (CR) rates, lower incidences of resistant disease, and longer OS for overweight or obese patients receiving chemotherapy, concluding that obesity alone should be no argument against the application of intensive therapies.13,14 In contrast, other analyses did not observe distinct outcomes according to BMI at AML diagnosis.15,16
Allogeneic HSCT represents the consolidation treatment with the highest chance of sustained remissions and is usually applied in intermediate and high-risk individuals with AML.17,18 Clinical data on the prognostic impact of BMI in AML with regard to the applied postremission therapy, especially an allogeneic HSCT, are lacking. Since AML patients often suffer from weight loss during intensive chemotherapy, we speculated that not only the nutritional status at diagnosis or prior to the start of HSCT conditioning regimen but also weight changes from AML diagnosis to HSCT might be relevant parameters to predict outcomes. Importantly, weight loss during therapy might be preventable by adequate nutritional support and, thus, could represent an easily modifiable patient-related risk factor. Therefore, the main objectives of the here presented study were to evaluate the prognostic impact of the BMI at diagnosis and HSCT as well as BMI changes between diagnosis and HSCT in AML patients undergoing allogeneic HSCT.
Methods
Patients and treatment
We analyzed 662 AML patients receiving an allogeneic HSCT at a median age of 59.4 years (range 16.3-74.9 y) between July 1998 and December 2019 at the University Hospital Leipzig. Median time from diagnosis to HSCT was 4.6 months (range 0.2-103.3 mo). Conditioning regimens were either myeloablative (MAC, n = 170, 26%), of reduced intensity (n = 98, 15%), or nonmyeloablative (n = 394, 60%). All patients received granulocyte colony-stimulating factor-stimulated peripheral blood stem cells as graft source. Stem cell donors were human leukocyte antigen (HLA) matched related (n = 130, 20%), haploidentical related (n = 12, 2%), HLA matched unrelated (n = 394, 59%), or unrelated and had at least 1 HLA mismatch (n = 126, 19%). Prior to allogeneic HSCT, patients received age-dependent standard cytarabine-based chemotherapy protocols. Details on the applied chemotherapies and conditioning regimens are given in the Supplementary Information (http://links.lww.com/HS/A131). Further patients’ characteristics are shown in Table 1 and Supplementary Table S1 (http://links.lww.com/HS/A131). Patients’ comorbidities were assessed by the HCT-CI.6 Written informed consent was obtained from all patients in accordance with the Declaration of Helsinki. Median follow-up after HSCT was 3.1 years for patients alive.
Table 1.
Clinical and Genetic Characteristics for Patients According to BMI Difference Between Diagnosis and Allogeneic HSCT (ΔBMI > –2 vs ≤ –2) in AML Patients Receiving Allogeneic HSCT With BMI at Both Timepoints Available (n = 369).
| Characteristics | All Patients, n = 369 | ΔBMI ≤ –2, n = 212 | ΔBMI > –2, n = 157 | P |
|---|---|---|---|---|
| Sex, n (%) | 0.53 | |||
| Male | 194 | 115 (54) | 79 (50) | |
| Female | 175 | 97 (46) | 78 (50) | |
| BMI at diagnosis, n (%) | <0.001 | |||
| < 25 kg/m2 | 142 | 116 (55) | 26 (17) | |
| 25-29.9 kg/m2 | 156 | 76 (36) | 80 (51) | |
| ≥ 30 kg/m2 | 71 | 20 (9) | 51 (32) | |
| Disease origin, n (%) | 0.001 | |||
| Secondary | 139 | 95 (45) | 44 (28) | |
| De novo | 230 | 117 (55) | 113 (72) | |
| Hemoglobin, g/dL | 0.58 | |||
| Median | 8.9 | 8.9 | 8.9 | |
| Range | 3.2-15.7 | 4.5-14.7 | 3.2-15.7 | |
| Platelet count, × 109/L | 0.40 | |||
| Median | 65 | 64 | 65 | |
| Range | 2-501 | 2-517 | 2-501 | |
| WBC, × 109/L | 0.71 | |||
| Median | 5.7 | 5.7 | 5.7 | |
| Range | 0.1-385 | 0.1-385 | 0.5-366 | |
| Blood blasts, % | 0.20 | |||
| Median | 18 | 17 | 20.5 | |
| Range | 0-98 | 0-97 | 0-98 | |
| BM blasts, % | 0.005 | |||
| Median | 50 | 45.6 | 55 | |
| Range | 0-95 | 0-95 | 3-95 | |
| Normal karyotype, n (%) | 0.83 | |||
| Absent | 211 | 121 (58) | 90 (60) | |
| Present | 146 | 86 (42) | 60 (40) | |
| ELN2017 genetic risk group, n (%) | 0.73 | |||
| Favorable | 83 | 44 (25) | 39 (29) | |
| Intermediate | 98 | 57 (33) | 41 (31) | |
| Adverse | 128 | 74 (42) | 54 (40) | |
| Age at HSCT, y | 0.001 | |||
| Median | 61.0 | 58.9 | 62.5 | |
| Range | 16.3-76.8 | 16.3-74.9 | 20.0-76.8 | |
| BMI at HSCT, n (%) | 0.91 | |||
| < 25 kg/m2 | 204 | 115 (54) | 89 (57) | |
| 25-29.9 kg/m2 | 132 | 78 (37) | 54 (34) | |
| ≥ 30 kg/m2 | 33 | 19 (9) | 14 (9) | |
| Time from diagnosis to HSCT, d | 0.93 | |||
| Median | 120 | 118 | 123 | |
| Range | 7-2504 | 7-2248 | 41-2504 | |
| ECOG performance status at HSCT, n (%) | 0.98 | |||
| 0 | 29 | 16 (8) | 13 (8) | |
| 1 | 175 | 99 (48) | 76 (49) | |
| 2 | 133 | 78 (38) | 55 (36) | |
| 3 | 24 | 14 (7) | 10 (6) | |
| HCT-CI score, n (%) | 0.19 | |||
| 0 | 137 | 86 (44) | 51 (37) | |
| 1/2 | 90 | 55 (28) | 35 (26) | |
| ≥ 3 | 105 | 54 (28) | 51 (37) | |
| Conditioning regimens, n (%) | 0.05 | |||
| Nonmyeloablative | 218 | 117 (55) | 101 (64) | |
| Reduced intensity | 57 | 31 (15) | 26 (17) | |
| Myeloablative | 94 | 64 (30) | 30 (19) | |
| Remission status at HSCT, n (%) | 0.99 | |||
| First CR/CRi | 162 | 150 (71) | 112 (71) | |
| Second CR/CRi | 43 | 25 (12) | 18 (11) | |
| No CR/CRi | 64 | 37 (17) | 27 (18) |
AML = acute myeloid leukemia; BM = bone marrow; BMI = body mass index; CR = complete remission; CRi = complete remission with incomplete peripheral recovery; ECOG = Eastern Cooperative Oncology Group; ELN = European LeukemiaNet; HCT-CI = hematopoietic cell transplantation comorbidity index; HSCT = hematopoietic stem cell transplantation; WBC = white blood count.
Evaluation of BMI
The BMI at diagnosis (n = 381) and up to 28 days prior to the start of HSCT conditioning regimens (n = 650) were evaluated by dividing the patient’s weight at either timepoint in kilogram through the square of the patient’s height in meters. According to the World Health Organization (WHO) classification, patients were classified to be under-/normal weight (BMI < 25 kg/m2), overweight (BMI 25-29.9 kg/m2), or obese (BMI ≥ 30 kg/m2). The BMI difference (ΔBMI, n = 369) was calculated as BMI at diagnosis subtracted from the BMI at the time of HSCT. For ΔBMI, a cut-point of –2 was determined applying the R package “OptimalCutpoints” and divided patients according to their incidence of death after HSCT into 2 groups with unchanged/increased BMI (ΔBMI ≤ –2, 57%) and decreased BMI (ΔBMI > –2, 43%).
Cytogenetics, molecular marker, and measurable residual disease
Diagnostic cytogenetic analyses were performed centrally using standard techniques of banding and in situ hybridization. The mutation status of the CCAAT/enhancer-binding protein alpha (CEBPA), nucleophosmin 1 (NPM1) gene and the tyrosine kinase domain of FMS-related tyrosine kinase 3 (FLT3-TKD) as well as the presence or absence of an internal tandem duplications in the FLT3 gene (FLT3-ITD) were evaluated as previously described.19 For patients with material available, the mutation status of 54 genes included in the TruSight Myeloid Sequencing Panel (Illumina) was evaluated at diagnosis as previously described.20,21 Patients were grouped according to the European LeukemiaNet (ELN) 2017 risk classification.17 Determination of the pre-HSCT measurable residual disease (MRD) status was performed as previously described.22-24
Definition of clinical endpoints and statistical analyses
All statistical analyses were performed using the R statistical software platform (version 3.4.3).25 OS was calculated from HSCT until death from any cause. The competing risks cumulative incidence of relapse (CIR) and NRM were calculated from HSCT to relapse or death, respectively, using the Fine and Gray method.26 Associations with baseline clinical, demographic, and molecular features were compared using the Kruskal-Wallis test and Fisher exact tests for continuous and categorical variables, respectively. OS was calculated using the Kaplan-Meier method and groups were compared using the log-rank test. Multivariate analyses are described in the Supplementary Information (http://links.lww.com/HS/A131).
Results
BMI at diagnosis and prior to HSCT
The median BMI at AML diagnosis was significantly higher than prior to HSCT (median 25.8 versus 24.7 kg/m2, P < 0.001). According to WHO classification, at diagnosis versus prior to HSCT, 39% versus 53% of patients were under-/normal weight, 42% versus 35% of patients were overweight, and 20% versus 12% of patients were obese (Figure 1). At diagnosis and prior to HSCT, there was a higher incidence of female patients in the obese and under-/normal weight patient cohort while the overweight patient cohort harbored a higher incidence of male patients (Supplementary Tables S2 and S3, http://links.lww.com/HS/A131). Patients being overweight or obese at diagnosis (P = 0.01 and P = 0.02, respectively) or prior to HSCT (P = 0.006 and P = 0.02, respectively) were older than patients being under-/normal weight. The infused numbers of CD34+ and CD3+ cells per kg body weight were lower with increasing BMI (P = 0.03 and P = 0.003, respectively, Supplementary Figure S1, http://links.lww.com/HS/A131).
Figure 1.
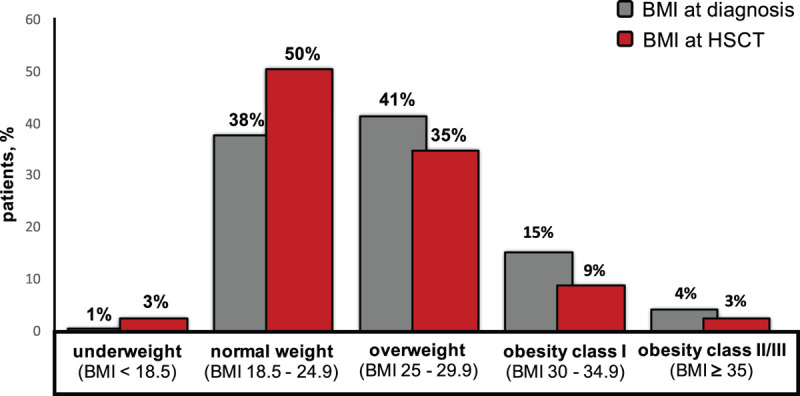
Distribution of BMI categories according to the WHO classification at AML diagnosis (gray bars) and prior to allogeneic HSCT (red bars). AML = acute myeloid leukemia; BMI = body mass index; HSCT = hematopoietic stem cell transplantation; WHO = World Health Organization.
Obese patients at diagnosis had similar CIR (P = 0.37, Supplementary Figure 2A, http://links.lww.com/HS/A131), but significantly higher NRM (P = 0.05, Figure 2A) and shorter OS (P = 0.004, Figure 2B) than overweight or under-/normal weight patients. In contrast, despite an optical separation of the NRM (Figure 2C) and OS (Figure 2D) curves, no significant prognostic impact was found for BMI prior to HSCT (CIR, P = 0.46 [Supplementary Figure S2B, http://links.lww.com/HS/A131]; NRM, P = 0.15; and OS, P = 0.10). Outcomes according to the BMI at diagnosis and prior to HSCT within the distinct WHO BMI categories are shown in Supplementary Figure S3 (http://links.lww.com/HS/A131).
Figure 2.
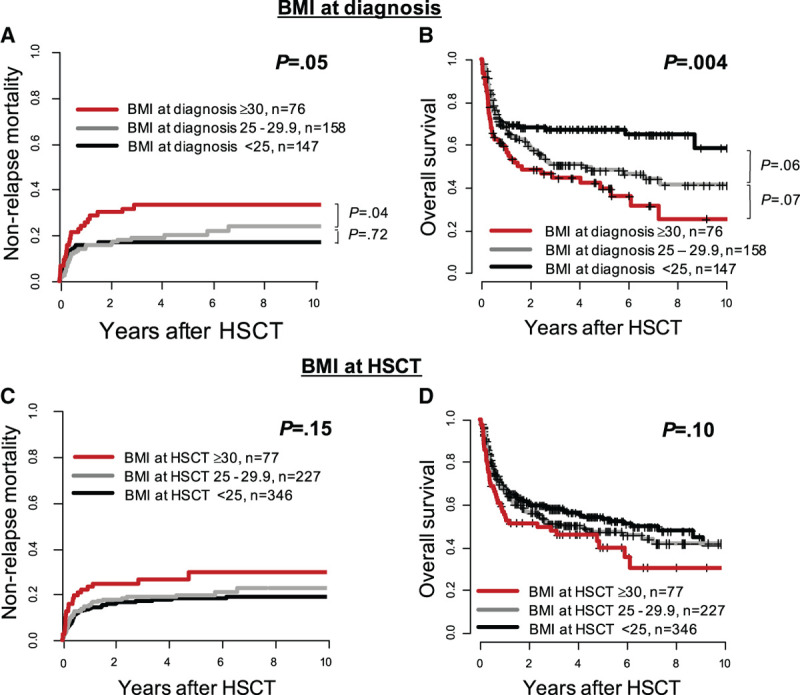
Outcome according to BMI at diagnosis and prior to HSCT (< 25 kg/m2 vs 25-29.9 kg/m2 vs ≥ 30 kg/m2) in AML patients receiving allogeneic HSCT. (A), Nonrelapse mortality and (B) overall survival according to BMI at diagnosis (n = 381). (C), Nonrelapse mortality and (D) overall survival according to BMI prior to HSCT (n = 650). AML = acute myeloid leukemia; BMI = body mass index; HSCT = hematopoietic stem cell transplantation.
Characteristics and outcomes of AML patients according to BMI change between diagnosis and HSCT
Patients with ΔBMI > –2 were older (P = 0.001), had a higher BMI at diagnosis (P < 0.001), and were more likely to have de novo AML (P = 0.001, Table 1). They were also more likely to be DNMT3A mutated (P = 0.05). In contrast, both groups did not vary regarding the ELN2017 risk at diagnosis (P = 0.73), the HCT-CI score (P = 0.19) and Eastern Cooperative Oncology Group (ECOG) performance status prior to HSCT (P = 0.96), time from diagnosis to HSCT (P = 0.93) or their pre-HSCT MRD (P = 0.88) or morphologic remission status at HSCT (P = 0.99).
Weight loss (ΔBMI > –2) between diagnosis and HSCT was a strong predictor for higher NRM (P = 0.006, Figure 3A) and shorter OS (P < 0.001, Figure 3B) while CIR was similar in both groups (P = 0.40, Supplementary Figure 2C, http://links.lww.com/HS/A131). The causes of death in remission did not differ significantly between both groups (P = 0.59) and are described in detail in the Supplementary Information (http://links.lww.com/HS/A131). In multivariate analyses, ΔBMI > –2 remained significant for higher NRM (hazard ratio, 1.23; P = 0.008) after adjustment for donor type and for shorter OS (odds ratio, 0.82; P = 0.001) after adjustment for ELN2017 risk, age, and remission status at HSCT (Figure 4).
Figure 3.
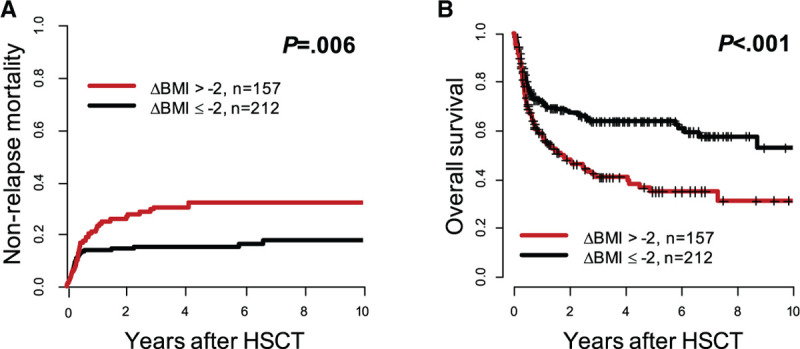
Outcome according to BMI difference between diagnosis and allogeneic HSCT (ΔBMI > –2 vs ≤ –2) in AML patients receiving allogeneic HSCT (n = 369). (A) Nonrelapse mortality and (B) overall survival. AML = acute myeloid leukemia; BMI = body mass index; HSCT = hematopoietic stem cell transplantation.
Figure 4.
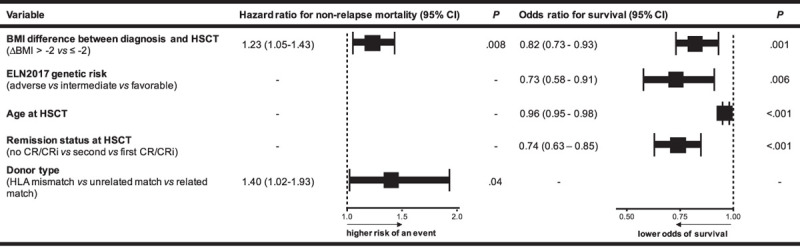
Multivariate analyses for outcomes according to BMI difference between diagnosis and allogeneic HSCT and other prognostic variables. Forest plot showing hazard ratios and odds ratios from logistic regression models for nonrelapse mortality and OS, respectively. Variables considered in the models were those significant at α = 0.10 in univariable analyses. For nonrelapse mortality endpoint, variables considered were BM blast count at diagnosis, disease origin (de novo vs secondary AML), BMI at diagnosis (< 25 kg/m2 vs 25 to < 30 kg/m2 vs ≥ 30 kg/m2), age at HSCT, BMI change (ΔBMI > –2 vs ≤ –2), and donor type (HLA mismatch vs matched unrelated vs matched related). For OS endpoint, variables considered were platelet count at diagnosis, BM blasts at diagnosis, disease origin (de novo vs secondary AML), BMI at diagnosis (< 25 kg/m2 vs 25 to < 30 kg/m2 vs ≥ 30 kg/m2), ELN2017 genetic risk group, remission status at HSCT (no CR/CRi vs second CR/CRi vs first CR/CRi), HCT-CI score (0 vs 1/2 vs 3), BMI at HSCT (< 25 kg/m2 vs 25 to <30 kg/m2 vs ≥ 30 kg/m2), BMI change (ΔBMI > –2 vs ≤ –2), and donor type (HLA mismatch vs matched unrelated vs matched related). AML = acute myeloid leukemia; BM = bone marrow; BMI = body mass index; CI = confidence interval; CR = complete remission; CRi = complete remission with incomplete peripheral recovery; ELN = European LeukemiaNet; HCT-CI = hematopoietic cell transplantation comorbidity index; HLA = human leukocyte antigen; HSCT = hematopoietic stem cell transplantation; OS = overall survival.
Subgroup analyses for BMI change between diagnosis and HSCT
Analyzing the 3 ELN2017 risk groups separately (Figure 5), the prognostic impact of ΔBMI > –2 was particularly seen in ELN2017 favorable- and intermediate-risk patients demonstrating a higher NRM (P = 0.09 and P = 0.02) and shorter OS (P = 0.2 and P = 0.002). However, no significant impact was observed in ELN2017 adverse-risk patients (NRM, P = 0.41; OS, P = 0.20). ΔBMI > –2 was also a significant prognostic factor for higher NRM and shorter OS in patients transplanted in morphologic remission (P = 0.01 and P < 0.001, respectively) but—despite the separation of outcome curves—did not significantly impact NRM (P = 0.15) or OS (P = 0.10) in the particularly high-risk population of patients transplanted with active disease (Supplementary Figure S4, http://links.lww.com/HS/A131). When we analyzed the prognostic impact of weight changes depending on the BMI category at diagnosis, we observed that weight loss (ΔBMI > –2) was of prognostic significance in under-/normal weight (NRM, P = 0.09; OS, P = 0.007) and overweight (NRM, P = 0.10; OS, P = 0.09), but not in obese patients at diagnosis (NRM, P = 0.81; OS P = 0.70; Supplementary Figure S5, http://links.lww.com/HS/A131). Again, weight change between diagnosis and HSCT did not impact CIR in any of the analyzed subgroups.
Figure 5.
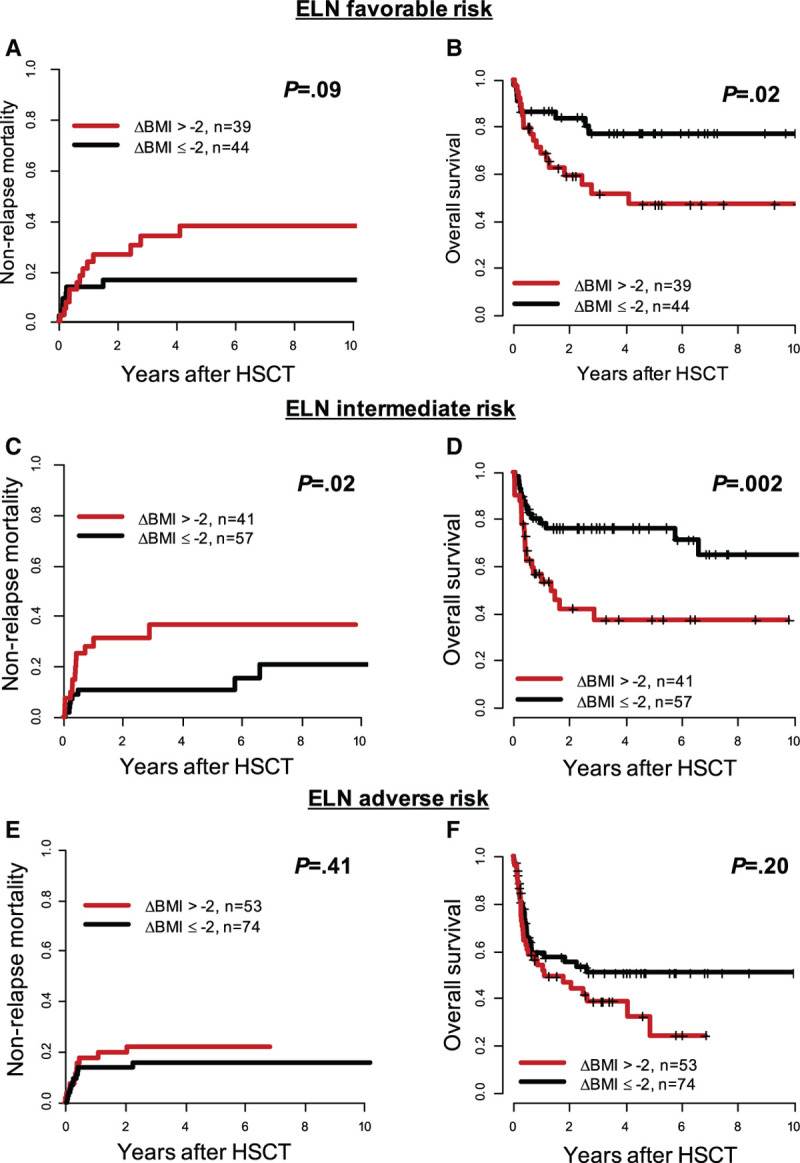
Outcome according to BMI difference between diagnosis and allogeneic HSCT (ΔBMI > –2 vs ≤ –2) within the 3 ELN2017 genetic risk groups in AML patients receiving allogeneic HSCT (n = 369). (A), Nonrelapse mortality and (B) overall survival in ELN2017 favorable-risk patients (n = 83). (C), Nonrelapse mortality and (D) overall survival in ELN2017 intermediate-risk patients (n = 98). (E), Nonrelapse mortality and (F) overall survival in ELN2017 adverse-risk patients (n = 127). AML = acute myeloid leukemia; BMI = body mass index; ELN = European LeukemiaNet; HSCT = hematopoietic stem cell transplantation.
Discussion
Analyzing AML patients undergoing induction chemotherapy, previous retrospective studies indicated either beneficial outcomes with higher CR rates and better OS or comparable outcomes for obese compared with nonobese individuals.14,15,27 These findings were partly explained by the recommendations against reduction of chemotherapy dosages in obese patients,28 leading to absolute higher chemotherapy dosages and a suggested consecutive higher effectiveness of chemotherapy. In the here analyzed HSCT treated patient cohort, obesity at AML diagnosis was associated with significantly higher NRM and shorter OS but similar CIR, which was also observed stepwise according to the 5 WHO categories (underweight: BMI < 18.5 kg/m2, normal weight: BMI 18.5-24.9 kg/m2, overweight: BMI 25-29.9 kg/m2, obesity grade 1: BMI 30-34.9 kg/m2, and obesity grade 2/3: BMI ≥ 35 kg/m2; Supplementary Figure 3, http://links.lww.com/HS/A131). In contrast to induction and consolidation chemotherapy alone, higher previous exposure to cytotoxic substances prior to HSCT may lead to higher HSCT-related mortality, which could explain the observed differences between the applied postremission therapy settings.
The common assumption that obesity prior to HSCT represents a risk factor for post-HSCT mortality6 was already attenuated by a variety of studies which reported no or even beneficial outcome impacts for overweight or obese patients with hematologic malignancies, including AML, undergoing HSCT.7,9-11,13 Our study stands in line with these findings as—despite an optical separation of the NRM and OS curves—the BMI prior to HSCT did not significantly correlate with patients’ outcomes (Figure 2).
Especially weight loss during chemotherapy—depicted as a ΔBMI > –2—presented a strong and independent risk factor for adverse outcomes after HSCT. While CIR was not significantly different, patients suffering weight loss had a significantly higher NRM and shorter OS, which was also seen independently from other prognostic factors in multivariate analyses. AML-related risk factors such as ELN2017 genetic risk or the remission status prior to HSCT did not differ between both groups, indicating that they did not influence weight changes in our patient cohort (Table 1). Previously, one analysis in patients with myelodysplastic syndrome showed a correlation of high-risk disease with weight loss prior to HSCT and, subsequently, higher relapse risk and shorter OS after HSCT.29 Regarding AML, a Japanese study group showed that weight reduction between diagnosis and HSCT in AML patients significantly associated with higher NRM and shorter OS in 184 AML patients.13 The authors concluded that patients suffering weight loss have an inferior general condition and, therefore, more often develop infections and graft-versus-host disease (GvHD), representing the main causes for the higher NRM. While we observed a higher mortality in patients suffering weight loss, the causes of death did not differ significantly from patients maintaining their weight (Supplementary Table 1, http://links.lww.com/HS/A131). However, compared with our study, the study of Ando et al13 was characterized by a younger age with consecutively more intensive conditioning regimens (MAC in 78% of patients) and bone marrow as main graft source. Furthermore, the distribution of the WHO defined BMI subgroups differed from our study with a higher proportion of patients classified as under-/normal weight at diagnosis (73% versus 39%), and a lower proportion classified as overweight or obese (6% versus 20% and 22% versus 42%, respectively). This is likely a consequence of the lower incidence of overweight and obesity in Japan compared with Europe.30
Regarding the 3 ELN2017 risk groups, weight loss was associated with higher NRM and shorter OS in patients with favorable and intermediate ELN2017 risk, but not in the adverse-risk group (Figure 5); most likely due to the aggressive phenotype with high relapse incidences of the underlying AML. We also observed a strong prognostic impact of weight loss in patients transplanted in morphologic remission (Supplementary Figure S4, http://links.lww.com/HS/A131). In the group of patients transplanted with active disease—that usually show dismal outcomes due to disease progression—we observed a lower prognostic impact of ΔBMI, but still a trend for shorter OS in patients suffering weight loss (Supplementary Figure S4D, http://links.lww.com/HS/A131). When we took the BMI at diagnosis into account, we observed that weight loss prior to HSCT lead to higher NRM and shorter OS mostly in under-/normal- and overweight but not in obese patients at diagnosis (Supplementary Figure S5, http://links.lww.com/HS/A131). This highlights the importance of preventing weight loss, especially in nonobese AML patients. Finally, also in separate analyses for younger and older AML patients (< 50 and ≥ 50 y at HSCT), ΔBMI > –2 showed similar results (Supplementary Figure S6, http://links.lww.com/HS/A131).
Our study has some limitations as we cannot clarify retrospectively whether the weight reduction was caused by the underlying malignancy, the applied treatment or was intended by the patient. We also lack information on protein, lipid, and glycemic profiles or body composition and other parameters like the lean BMI might help to better distinguish between the different proportions of muscle and fatty tissues.31 However, the number and severity of comorbidities reflected by the HCT-CI score, the number of applied chemotherapies, the time between diagnosis and HSCT, the ECOG performance status prior to HSCT, as well as the causes of NRM (considering GvHD, infection, or others) did not differ between patients who suffered weight loss and patients who did not. This suggests that weight loss, in general, irrespective of the contributing reasons, should be regarded as an adverse prognostic factor in AML patients prior to HSCT. Whether weight loss during therapy remains a relevant clinical problem in recently approved new treatment approaches such as liposomal cytarabine/daunorubicin32—for which compared with standard 7 + 3 a lower incidence of colitis and diarrhea has been noticed—or azacitidine/venetoclax combinations have to be subject of future studies.33
In conclusion, obesity at AML diagnosis associated with higher NRM and shorter OS following HSCT. Weight loss (BMI > –2) during treatment cycles until HSCT—especially in ELN2017 favorable- and intermediate-risk patients—represents a strong prognosticator for inferior outcomes after HSCT. BMI monitoring and intervention by, for example, diet adjustments or nutritional supplementation during AML treatment could improve patients’ outcomes. Prospective interventional studies should be implemented to evaluate which dietary and physical supportive care strategies may be most effective and beneficial and should be implemented into national and international guidelines.34 This seems of particular clinical relevance, as weight loss impacted outcomes in the majority of analyzed AML patients irrespective of comorbidities or disease risk.
Sources of funding
This study was supported by the Verein Zusammen gegen den Krebs e.V. and the Deutsche Gesellschaft für Innere Medizin (Clinician Scientist Program, MJ).
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgments
The authors would like to thank Christel Müller, Daniela Bretschneider, Evelin Hennig, Janet Bogardt, Annette Jilo, and Dagmar Cron for their help in determining cytogenetic and morphologic analyses, and Julia Schulz, Christine Günther, Scarlett Schwabe, Ines Kovacs, and Kathrin Wildenberger for their help in sample processing.
Supplementary Material
Footnotes
SS and MJ contributed equally to this article.
Supplemental digital content is available for this article.
References
- 1.World Health Organization. Obesity and Overweight. 2020. Available at: https//www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight. Accessed August 24, 2020.
- 2.Di Angelantonio E, Bhupathiraju SN, Wormser D, et al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016; 388:776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdelaal M, le Roux CW, Docherty NG. Morbidity and mortality associated with obesity. Ann Transl Med. 2017; 5:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taghizadeh N, Boezen HM, Schouten JP, et al. BMI and lifetime changes in BMI and cancer mortality risk. PLoS One. 2015; 10:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reeves GK, Pirie K, Beral V, et al. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. Br Med J. 2007; 335:1134–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005; 106:2912–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hadjibabaie M, Tabeefar H, Alimoghaddam K, et al. The relationship between body mass index and outcomes in leukemic patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Transplant. 2012; 26:149–155. [DOI] [PubMed] [Google Scholar]
- 8.Voshtina E, Szabo A, Hamadani M, et al. Impact of obesity on clinical outcomes of elderly patients undergoing allogeneic hematopoietic cell transplantation for myeloid malignancies. Biol Blood Marrow Transplant. 2019; 25:e33–e38. [DOI] [PubMed] [Google Scholar]
- 9.Le Blanc K, Ringdén O, Remberger M. A low body mass index is correlated with poor survival after allogeneic stem cell transplantation. Haematologica. 2003; 88:1044–1052. [PubMed] [Google Scholar]
- 10.Deeg HJ, Seidel K, Bruemmer B, et al. Impact of patient weight on non-relapse mortality after marrow transplantation. Bone Marrow Transplant. 1995; 15:461–468. [PubMed] [Google Scholar]
- 11.Navarro WH, Agovi MA, Logan BR, et al. Obesity does not preclude safe and effective myeloablative hematopoietic cell transplantation (HCT) for acute myelogenous leukemia (AML) in adults. Biol Blood Marrow Transplant. 2010; 16:1442–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baumgartner A, Zueger N, Bargetzi A, et al. Association of nutritional parameters with clinical outcomes in patients with acute myeloid leukemia undergoing haematopoietic stem cell transplantation. Ann Nutr Metab. 2016; 69:89–98. [DOI] [PubMed] [Google Scholar]
- 13.Ando T, Fujisawa S, Teshigawara H, et al. Impact of treatment-related weight changes from diagnosis to hematopoietic stem-cell transplantation on clinical outcome of acute myeloid leukemia. Int J Hematol. 2019; 109:673–683. [DOI] [PubMed] [Google Scholar]
- 14.Medeiros BC, Othus M, Estey EH, et al. Impact of body-mass index on the outcome of adult patients with acute myeloid leukemia. Haematologica. 2012; 97:1401–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee HJ, Licht AS, Hyland AJ, et al. Is obesity a prognostic factor for acute myeloid leukemia outcome? Ann Hematol. 2012; 91:359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castillo JJ, Mulkey F, Geyer S, et al. Relationship between obesity and clinical outcome in adults with acute myeloid leukemia: a pooled analysis from four CALGB (alliance) clinical trials. Am J Hematol. 2016; 91:199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017; 129:424–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016; 127:2391–2405. [DOI] [PubMed] [Google Scholar]
- 19.Bill M, Jentzsch M, Grimm J, et al. Prognostic impact of the European LeukemiaNet standardized reporting system in older AML patients receiving stem cell transplantation after non-myeloablative conditioning. Bone Marrow Transplant. 2017; 52:932–935. [DOI] [PubMed] [Google Scholar]
- 20.Jentzsch M, Bill M, Grimm J, et al. High expression of the stem cell marker GPR56 at diagnosis identifies acute myeloid leukemia patients at higher relapse risk after allogeneic stem cell transplantation in context with the CD34+/CD38- population. Haematologica. 2020; 105:e507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grimm J, Bill M, Jentzsch M, et al. Clinical impact of clonal hematopoiesis in acute myeloid leukemia patients receiving allogeneic transplantation. Bone Marrow Transplant. 2019; 54:1189–1197. [DOI] [PubMed] [Google Scholar]
- 22.Jentzsch M, Bill M, Grimm J, et al. Prognostic impact of blood MN1 copy numbers before allogeneic stem cell transplantation in patients with acute myeloid leukemia. HemaSphere. 2019; 3:e167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jentzsch M, Bill M, Grimm J, et al. High BAALC copy numbers in peripheral blood prior to allogeneic transplantation predict early relapse in acute myeloid leukemia patients. Oncotarget. 2017; 8:87944–87954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bill M, Grimm J, Jentzsch M, et al. Digital droplet PCR-based absolute quantification of pre-transplant NPM1 mutation burden predicts relapse in acute myeloid leukemia patients. Ann Hematol. 2018; 97:1757–1765. [DOI] [PubMed] [Google Scholar]
- 25.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 26.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988; 16:1141–1154. [Google Scholar]
- 27.Ando T, Yamazaki E, Ogusa E, et al. Body mass index is a prognostic factor in adult patients with acute myeloid leukemia. Int J Hematol. 2017; 105:623–630. [DOI] [PubMed] [Google Scholar]
- 28.Griggs JJ, Mangu PB, Temin S, et al. Appropriate chemotherapy dosing for obese adult patients with cancer: American Society of Clinical Oncology Clinical Practice guideline. J Oncol Pract. 2012; 8:e59–e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radujkovic A, Becker N, Benner A, et al. Pre-transplant weight loss predicts inferior outcome after allogeneic stem cell transplantation in patients with myelodysplastic syndrome. Oncotarget. 2015; 6:35095–35106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Organisation for Economic Co-operation and Development. Health at a Glance 2017. Paris: OECD Indicators, OECD Publishing; 2017. [Google Scholar]
- 31.Heber D, Ingles S, Ashley JM, et al. Clinical detection of sarcopenic obesity by bioelectrical impedance analysis. Am J Clin Nutr. 1996; 64(3 suppl):472S–477S. [DOI] [PubMed] [Google Scholar]
- 32.Lancet JE, Uy GL, Cortes JE, et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J Clin Oncol. 2018; 36:2684–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020; 383:617–629. [DOI] [PubMed] [Google Scholar]
- 34.van Lieshout R, Tick LW, de Laat D, et al. Adherence to guidelines on nutrition support during intensive treatment of acute myeloid leukemia patients: a nationwide comparison. Clin Nutr ESPEN. 2020; 39:242–250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


