Abstract
Objectives
Coronavirus disease 2019 (COVID-19) is a novel infectious disease, with significant morbidity and mortality. This meta-analysis is to evaluate the prevalence of disseminated intravascular coagulation (DIC) in COVID-19 patients and to determine the association of DIC with the severity and prognosis of COVID-19.
Methods
We searched the PubMed, EMBASE, and China National Knowledge Infrastructure (CNKI) database until August 12, 2020. The meta-analysis was performed using Stata 16.0 software.
Results
14 studies were included in our meta-analysis. The pooled analysis revealed that the incidence of COVID-19 patients developing DIC was 3% (95%: 1%–5%, P < 0.001). In addition, deaths were more likely to be associated with DIC (Log OR = 2.46, 95% CI: 0.94–3.99, P < 0.001) with statistical significance.
Conclusions
DIC is associated with the severity and poor prognosis of COVID-19 patients. Therefore, attention should be paid to coagulation dysfunction in COVID-19 patients. Monitoring of coagulation indicators may improve the prognosis of COVID-19 inpatients.
Abbreviations: DIC, disseminated intravascular coagulation; COVID-19, coronavirus disease 2019; APTT, activated partial thromboplastin time; PT, prothrombin time; FDP, fibrin degradation products; CRP, C-reactive protein
Keywords: Coronavirus disease 2019 (COVID-19), SARS-CoV-2, Disseminated intravascular coagulation
1. Introduction
At the end of 2019, hospitals reported a cluster of cases with pneumonia of unknown cause in Wuhan, Hubei, China, attracting great attention nationally and worldwide [1]. researchers rapidly isolated a novel coronavirus (SARS-CoV-2, also referred to as 2019-nCoV) from confirmed infected pneumonia patients. Phylogenetic analysis shows that SARS-CoV-2 is a new member of the coronaviridae but is distinct from SARS-CoV and MERS-CoV. The World Health Organization (WHO) declared that COVID-19 has become a global health concern, causing severe respiratory tract infections in humans [2,3]. As of 22 Aug 2020, 22,812,491 laboratory-confirmed cases and 795,132 deaths in 216 countries, regions, or territories have been documented [https://www.who.int (accessed 14 August 2020)].
Coronavirus disease 2019 (COVID-19) is a viral infection that can result in cytokine storm, systemic inflammatory response and coagulopathy that is prognostic of poor outcomes. [4] In previous studies, SARS-CoV-1 were reported to be associated with thrombocytopenia, thrombocytosis, and prolonged activated partial thromboplastin time (APTT) [5]. Currently, accumulated evidence reveal that a coagulation disorder is often seen in COVID-19, and the incidence is higher in severe cases [6]. A broad range of laboratory coagulation parameter abnormalities was reported in patients with COVID-19 including alterations in D-dimer, prothrombin time (PT), fibrinogen, Fibrin degradation products (FDP), platelet count and Antithrombin III activity [7]. In addition, thrombotic complications in patients diagnosed with COVID-19 are emerging as important result that contribute to significant mortality [8].
Though accumulated evidence reveal that a coagulation disorder is often seen in COVID-19, the detailed incidence of DIC are not so common reported. As presented in different articles, the incidence of DIC varied widely among articles. Guan, W. J.'s analysis focusing on abnormal coagulation parameters revealed that 0.09% of patients with COVID-19 met DIC [9]. However, Chen, T. and his colleagues found that 7.7% of patients with COVID-19 had DIC [10]. In addition, There are many diagnostic criteria for DIC, including The Japanese Association for Acute Medicine JAAM-DIC 2016 score [11], ISTH overt-DIC score [12] and SIC score [13]. When comparing the main scoring systems, the JAAM-DIC criteria were suggested to be more sensitive than the ISTH ones and allow an earlier diagnosis compared to the ISTH score [14]. For sepsis, the JAAM-DIC score does not use the fibrinogen decrease as a criterion, and it takes into account the kinetics of platelet decrease. For the SIC score, previous recommendation highlighted it to predict mortality. SIC score associates “readily available” biological values (platelets and INR) and SOFA score. SIC would allow the detection of patients with “coagulopathy” who are at high-risk of developing DIC. In addition,SIC score was not supposed to be used alone, but combined with ISTH overt-DIC score if SIC score was positive [15]. For the JAAM-DIC score, In a retrospective analysis, it showed that a SIC score of 4 points or more had a higher predictive value for 28-day mortality than the JAAM-DIC score [16]. In general, several scoring systems may be used by physicians, but they all have advantages and disadvantages. The epidemiology of DIC among COVID-19 patients is currently based on small case series and retrospective studies. The current meta-analysis was also limited to China. As the epidemic is now raging in hundreds of countries around the world, the research results of cases limited to China cannot be applied to the global scope. This systematic review and meta-analysis focus on this gap in knowledge, helping first-line healthcare providers' understanding of DIC incidence and mortality in COVID-19.Furthermore, increasing evidence to support DIC, a devastating systemic disorder is linked with severe COVID-19, prompting considerable concern [17]. However, the OR value of DIC for disease risk stratification and prognosis assessment was still unclear, so another purpose was to evaluate the relationship between DIC and disease stratification and prognosis.
2. Materials and methods
2.1. Search strategy
Our current systematic review and meta-analysis was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement. The PROSPERO database registration number: CRD42020206596.We selected relevant studies published between Jan 1, 2020 and Aug 12, 2020, by searching them in PubMed, Embase, and CNKI. The terms for the literature search were combinations of “COVID-19”, “2019-nCoV”, “SARS-CoV-2”, “2019 novel coronavirus”, “Novel coronavirus 2019”, and “severe acute respiratory syndrome coronavirus 2” with “Consumption Coagulopathy”, “Disseminated Intravascular Coagulation”, and “DIC”. In conformity with the quality standards for reporting systematic reviews and meta-analyses of observational studies, two independent researchers (Zhou XH and Cheng ZP) screened retrieved articles. The researchers independently assessed full texts of articles deemed eligible for inclusion. All disagreements were resolved by discussion with a third reviewer (Hu Yu). The reference list of all identified documents was scrutinized to identify eligible studies.
2.2. Selection criteria and data extraction
The inclusion criteria were as follows: (1) patients should be confirmed to have been infected with SARS-CoV-2 by laboratory detection or clinical diagnosis; (2) the full text of each article should be available; and (3) outcome should include the incidence of DIC. Meanwhile, the following selection criteria were used to exclude the studies: (1) duplicated studies, (2) studies with sample sizes smaller than 10, (3) studies that focus only on children or infants or pregnant woman, and (4) case reports, clinical guidelines, consensus documents, reviews, and systematic reviews.
Two authors (Zhou XH and Cheng ZP) independently extracted relevant information, including first author, published journal, inclusion period, country, the number of COVID-19 patients, the mean or median age of patients, gender ratio, incidence of DIC. We also separate patients into groups of severe and non-severe patients or groups of survivor and non-survivors for further analysis. The degree of severity of COVID-19 were admitted according to American Thoracic Society guidelines for community-acquired pneumonia by clinicians [18].
2.3. Data analysis
All data analyses were performed using STATA 16.0 software. The Log OR, and relevant 95% CIs were used to estimate pooled results from studies. In case of no obvious heterogeneity (I2 < 50% and P > 0.1 in the Q test), the fixed-effects model was applied. Otherwise, the random-effects model was used. All P-values of ≤0.05 were considered to be significant statistically. In addition, we performed the Egger's regression test to analyze the publication bias. and we used the trim-and-fill method to eliminate the impact of the publication bias. Furthermore, we conducted a subgroup analysis of the incidence of DIC according to the diagnostic criteria of DIC.
The quality of each study was independently assessed by two participants using the Newcastle Ottawa Scale (NOS) [19]. NOS scores of at least six were considered high-quality literature, and those with higher NOS scores showed higher literature quality.
3. Results
3.1. Literature search and screening
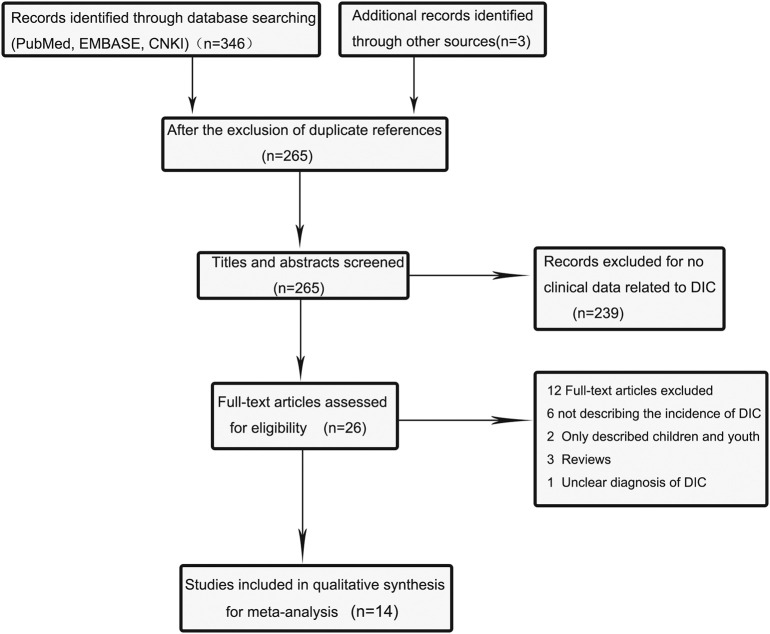
The database searches identified a total of 346 potentially relevant articles, including 137 in PubMed, 96 in EMBASE, and 113 in CNKI. Three articles were added after we read the literature we have searched. Of these articles, 84 were excluded due to duplication. After screening titles and abstracts, we further excluded 239 due to non-relevance. After full texts were carefully reviewed, 12 articles were removed for not reporting clinical features of COVID-19 or describing the incidence of DIC. Finally, the meta-analysis included 14 eligible articles [7,9,[20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31]]. The flow diagram (Fig. 1 ) illustrates the detailed procedure of literature search.
Fig. 1.
Flow diagram of the literature search and selection process in the meta-analysis.
3.2. Characteristics of studies and demographic features
Ultimately, our analysis included 14 articles, mostly from China. Other studies came from European countries, such as Spain, Italy and France. and we summarize their demographic data in Table 1 . The sample size of groups varied from 32 to 1099, and the median age was between 46.7 and 68.6 years old. The overall proportion of male ranged from 50.3% to 81.3%. Moreover, four studies have described in detail the occurrence of DIC in survivors and deaths. [21,25,27,31] Two studies described ICU patients and non-ICU patients [9,24], and two study described the incidence of DIC in patients with severe and non-severe disease [27,30]. All articles are of high quality because of NOS score no less than six. Detailed descriptions of the studies included are shown in Table 1.
Table 1.
Main characteristics of the included studies in our-analysis.
| Study | Date | Region | Period | Sample size |
Male:N(%) |
Age:mean ± SD |
Newcastle-Ottawa Scale(NOS) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Non-survivor | Survivor | Total | Non-survivor | Survivor | Total | Non-survivor | Survivor | |||||
| Liu,X. | 2020/4/24 | China | -2020/3/11 | 47 | 47 | 0 | 32(68%) | 32(68%) | – | – | – | – | 6 |
| Lu, J. | 2020/4/15 | China | 2020/1–2020/3 | 73 | 73 | 0 | 40(54.8%) | 40(54.8%) | – | 58.4 ± 11.5 | 58.4 ± 11.5 | – | 6 |
| Qin,W. | 2020/8/11 | China | 2019/12–2020/2 | 582 | 63 | 519 | 293(50.3%) | 45(71.4%) | 248(47.8%) | 63 ± 12.6 | 71 ± 10.6 | 61 ± 12.6 | 8 |
| Bao, C.Q. | 2020/7/23 | China | 2020/2/10–2020/3/15 | 178 | 7 | 171 | 106(60.0%) | – | – | 62.7 ± 12.0 | – | – | 7 |
| Deng, Y. | 2020/3/27 | China | 2020/1/1–2020/2/21 | 225 | 109 | 116 | 124(55.1%) | 73 (67.0%) | 51 (44.0%) | 55.3 ± 13.4 | 68.3 ± 9.0 | 43.3 ± 18.0 | 8 |
| Helms, J. | 2020/5/6 | France | 2020/3/3–2020/3/31 | 150 | – | – | 122(81.3%) | – | – | 62.3 ± 13.5 | – | – | 7 |
| Liao, D.Y. | 2020/7/14 | China | 2020/1/23–2020/2/23 | 231 | 55 | 176 | 137(59.3%) | 40(72.7%) | 97(55.1%) | 68 ± 13.4 | 70 ± 11.4 | 66.8 ± 14.0 | 8 |
| Lodigiani, C. | 2020/5/1 | Italy | 2020/2/13–2020/4/10 | 388 | – | – | 264(68.0%) | – | – | 65.3 ± 14.9 | – | – | 7 |
| Martín-Rojas, R. M. | 2020/8/5 | Spain | 2020/4/3–2020/5/3 | 206 | 18 | 188 | 131(63.6%) | 11(61.1%) | 120(63.8%) | 63.6 ± 13.4 | 76.0 ± 12.3 | 62.4 ± 12.9 | 8 |
| Mazzaccaro, D. | 2020/6/12 | Italy | 2020/3/18–2020/4/20 | 32 | – | – | 23(71.9%) | – | – | 68.6 ± 12 | – | – | 7 |
| Tang, N. | 2020/2/20 | China | 2020/1/1–2020/2/3 | 183 | 21 | 162 | 98(53.6%) | 16(76.2%) | 82(50.6%) | 54.1 ± 16.2 | 64.0 ± 20.7 | 52.4 ± 15.6 | 8 |
| Nowak, B. | 2020/5/19 | Poland | 2020/3/16–2020/4/7 | 169 | 46 | 123 | 87(51.5%) | 30 (65.2%) | 57 (46.3%) | 63.7 ± 19.6 | 75.3 ± 11.9 | 59.3 ± 20.1 | 8 |
| Fogarty, H. | 2020/6/1 | Ireland | 2020/3/13–2020/4/10 | 83 | 33 | 50 | 55(66.3%) | 22(66.7%) | 33(66%) | 62 ± 16.3 | 67.9 ± 11.9 | 60.5 ± 17.7 | 8 |
| Guan, W. J. | 2020/2/29 | China | 2019/12/11–2020/1/29 | 1099 | 926 | 173 | 637(58.0%) | – | – | 46.7 ± 17.1 | – | – | 8 |
3.3. Meta-analysis
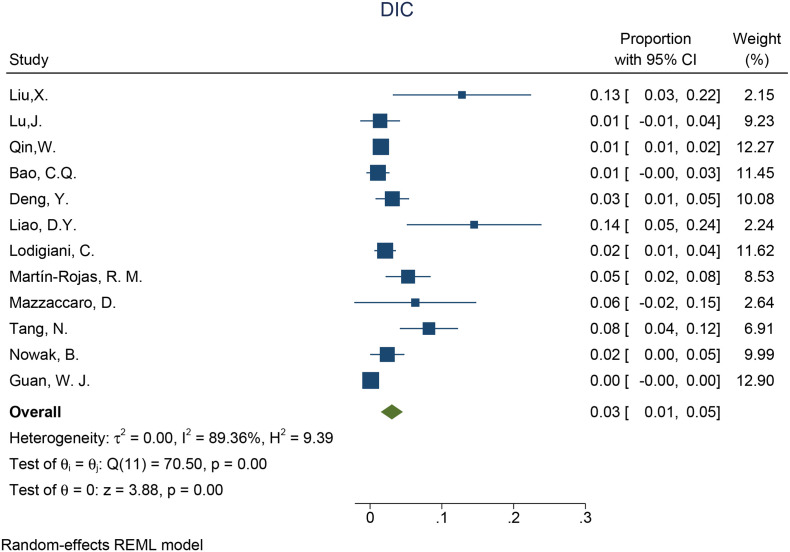
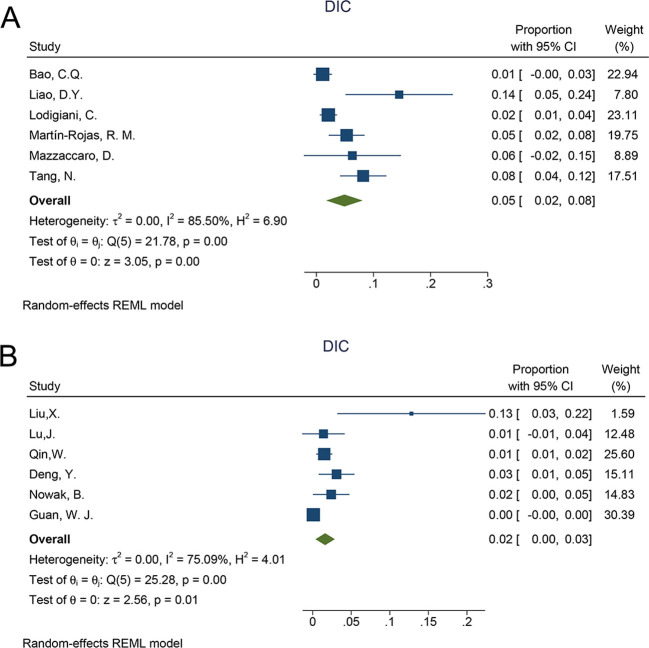
3237 patients were analyzed for the incidence of DIC in the whole COVID-19 patients. Given the high statistical heterogeneity among the 12 articles ((I2 = 89.36% and P < 0.1 in the Q test)), a random-effects model was chosen. (Fig. 2) the incidence of DIC in whole COVID-19 patients was 3% (95%: 1%–5%, P < 0.001). In order to find possible sources of heterogeneity, subgroup analysis was conducted according to different DIC diagnostic standards. Of the twelve articles, six studies used the ISTH Overt-DIC criteria, which indicated that DIC was considered present if the score was 5 or greater [12]. The heterogeneity of the subgroup using ISTH criteria was lower than the original analysis (I2 = 85.5% vs I2 = 89.36%), indicating that the differences in diagnostic methods is at least due to the high heterogeneity. The incidence of DIC in the subgroup using ISTH criteria was higher than the subgroup using non-ISTH criteria (proportion:5%; 95% CI 2%–8%, p < 0.001 vs proportion:2%; 95% CI 0%–3%, p = 0.01) (Supplementary Fig. 1).
Fig. 2.
Forest plot of incidence of DIC.
The following is the supplementary data related to this article.Supplementary Fig. 1.
The subgroup analysis of incidence ofDIC,A: ISTH criteria; B:non-ISTH criteria.
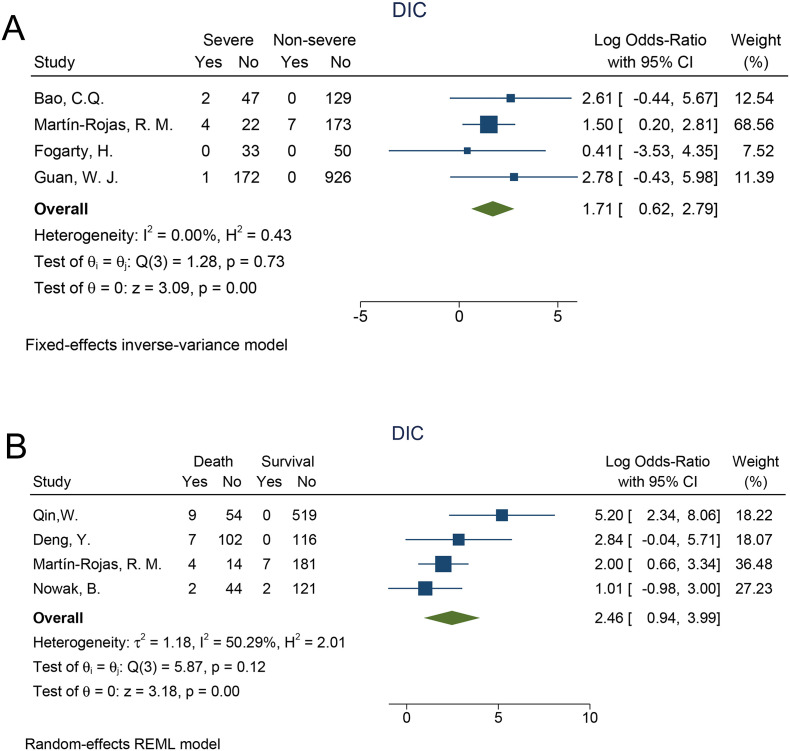
To exploring whether the occurrence of DIC predict risk stratification and prognosis, subgroup analysis based on disease severity and outcome were also conducted. We found that DIC correlated with disease severity in patients with COVID-19 (Log OR = 1.71, 95% CI: 0.62–2.79, P < 0.001) with low heterogeneity (I2 = 0% and P = 0.73 in the Q test), suggesting that the incidence of DIC was significantly elevated in severe patients/ICU patients compared with Non-severe patients/Non-ICU patients. (Fig. 3A) The analysis of the occurrence of DIC in survivors and deaths is shown in Fig. 3B. Deaths were more likely to be associated with DIC (Log OR = 2.46, 95% CI: 0.94–3.99, P < 0.001) with statistical significance. The heterogeneity test result (I2 = 50.29% and P = 0.12 in the Q test) indicated that the heterogeneity was low and the result was reliable.
Fig. 3.
The Log risk ratio of DIC in survivors compared with deaths and severepatients compared with Non-severe patients.
3.4. Risk of publication bias
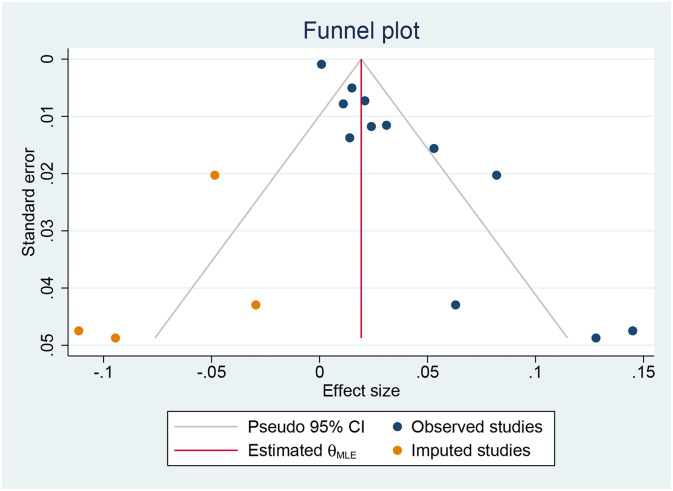
Egger's test indicates statistical significance (p < 0.001). Therefore, we addressed the potential publication bias by the trim-and-fill method. The result, which included 12 observational studies and 4 imputed studies, showed that the incidence of COVID-19 patients developing DIC was 2% (95%: 0%–4%). The funnel plot after applying the trim-and-fill method is shown in Fig. 4 .
Fig. 4.
Results of publication bias after trim and fill method.
4. Discussion
COVID-19, caused by SARS-CoV-2, is rapidly spreading to many countries around the world, posing a critical threat to global health [32]. In this comprehensive meta-analysis, we combined the outcomes of multiple centers and illuminate the incidence DIC in COVID-19 patients, as well as in different subgroups.
Currently, studies have provided evidence that COVID-19 is commonly accompanied with excessive inflammation [9]. One of the most important clinical features of the infection is a profound coagulopathy. In clinical practice, some thrombotic complications described in patients with increasing frequency, including strokes, deep vein thrombosis, myocardial infarction, pulmonary embolism, as well as Disseminated intravascular coagulation (DIC) [20,33,34]. Currently, many clinical studies have analyzed COVID-19 patients with coagulation disorders. Papanellas' research showed that patients with COVID-19 display marked alterations of the coagulation system, which, however, are not compatible with a consumption coagulopathy typical of DIC [35]. Nevertheless, in a recent cohort study, 71.4% of non-survivors and 0.6% survivors met the ISTH criteria of disseminated intravascular coagulation [33]. Therefore, the relationship between coagulation dysfunction and prognosis in patients infected with COVID-19 was complex and might vary in different clinical study. The reason for the discrepancy between the different studies is unknown and might partly be due to patient selection, associated comorbidities and pharmacologic treatments. Since some studies have shown that coagulation dysfunction may be a major cause of death in severe COVID-19 patients, so monitoring coagulation and anticoagulation biomarkers, such as D-dimer,fibrin degradation product (FDP) levels, and prothrombin time, is necessary and helpful for the early diagnosis and a timely intervention of DIC [36]. But how to properly apply these indicators remained unknown for that elucidation of the pathophysiology of COVID-19 from the perspective of laboratory hematology is ongoing. For example, D-dimer is an excellent marker, but shows limitations in assessing the pathophysiology of the coagulation abnormalities seen in COVID-19. Similarly, the treatment of COVID-19 should be used according to the pathophysiological condition [37]. Recently, evidence has shown the application of heparin sodium and LMWH inhibits blood coagulation, reduces inflammation, and inhibits platelet aggregation, thereby preventing thrombosis and delaying coagulopathy progression to DIC in high-risk patients [13,20,30]. However, when and how to applying preventive anticoagulation therapy remains unclear.
In this study, our meta-analysis showed that 3% of the COVID-19 patients were complicated by DIC, and the incidence was higher in non-survivors than in survivors, which indicated that complication with DIC tends to be associated with enhanced risk of severe COVID-19, even the mortality. In addition, the clinical classification of patient severity is often associated with ICU management. We integrated ICU/non-ICU patients and severe/non-severe patients for meta-analysis, further verifying that DIC was a risk factor for aggravation of the disease. As discussed, the development of coagulation test abnormalities seen in SARS-CoV-2–infected patients is most likely a result of the profound inflammatory response [38]. Therefore, we can combine inflammatory markers (interleukin-6, C-reactive protein (CRP) and procalcitonin) with coagulation indicators to evaluate the coagulation function of patients. Moreover, clinical heterogeneity between studies is noteworthy, our study was limited by variable diagnostic criteria for DIC, bias of which we mitigated through subgroup analysis. However, the heterogeneity that still exists is determined by many factors, for example, the included studies apply different criteria for inclusion of cases, which may in the future enable more detailed analyses.
4.1. Study limitations
The number of studies included was limited in terms of sample size, data availability, and methodologic quality. Given that the included studies are all from China and European countries, factors such as virus strain types, medical levels, races, etc., may affect the results. It will be better to include more studies with a broad geographic scope, to get a more comprehensive understanding of COVID-19-associated DIC. Most published literatures are observational studies, making it difficult to confirm causality between COVID-19 and DIC. In addition, patient overlap is possible between a few of the studies. As such, as more data from more regions becomes available. This should be further evaluated in future studies.
5. Conclusion
In the current pandemic, prevention and control of COVID-19 remains paramount. This meta-analysis showed DIC was associated with increased mortality in COVID-19 pneumonia. Therefore, assessment and optimal management of DIC biomarkers may significantly avoid further disease progression in patients with COVID-19.
Funding
This study was supported by grant from the National Natural Science Foundation of China (81800134 to Cheng Zhipeng), grant from the Key Special Project of Ministry of Science and Technology, China (No. 2020YFC0845700 to Hu Yu) and grant from Scientific Research Projects of Chinese Academy of Engineering (NO. 2020-KYGG-01-07 to Chen Wei).
Ethical approval
Not applicable as we utilized publicly available data.
Declaration of competing interest
The author reports no conflicts of interest in this work.
Acknowledgments
Acknowledgements
The PROSPERO database registration number: CRD42020206596.
CRediT authorship contribution statement
Author contributions: Xianghui Zhou made the design of the work; Xianghui Zhou, Zhipeng Cheng, Lili Luo,Ying Zhu, Wenyi Lin, Zhangyin Ming searched, selected materials and extracted data; Xianghui Zhou and Zhipeng Cheng wrote this manuscript; Yu Hu and Wei Chen revised the paper carefully. All authors have read and approved the final manuscript.
References
- 1.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnes G.D., Burnett A., Allen A., Blumenstein M., Clark N.P., Cuker A., Dager W.E., Deitelzweig S.B., Ellsworth S., Garcia D., Kaatz S., Minichiello T. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. J. Thromb. Thrombolysis. 2020;50(1):72–81. doi: 10.1007/s11239-020-02138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong R.S., Wu A., K.F. To, Lee N., Lam C.W., Wong C.K., Chan P.K., Ng M.H., Yu L.M., Hui D.S., Tam J.S., Cheng G., Sung J.J. Haematological manifestations in patients with severe acute respiratory syndrome: retrospective analysis. Bmj. 2003;326(7403):1358–1362. doi: 10.1136/bmj.326.7403.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., Zhang Y., Song J., Wang S., Chao Y., Yang Z., Xu J., Zhou X., Chen D., Xiong W., Xu L., Zhou F., Jiang J., Bai C., Zheng J., Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):1–11. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liao D., Zhou F., Luo L., Xu M., Wang H., Xia J., Gao Y., Cai L., Wang Z., Yin P., Wang Y., Tang L., Deng J., Mei H., Hu Y. Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: a retrospective cohort study. Lancet Haematol. 2020;7(9):e671–e678. doi: 10.1016/S2352-3026(20)30217-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E., Nigoghossian C., Ageno W., Madjid M., Guo Y., Tang L.V., Hu Y., Giri J., Cushman M., Quéré I., Dimakakos E.P., Gibson C.M., Lippi G., Favaloro E.J., Fareed J., Caprini J.A., Tafur A.J., Burton J.R., Francese D.P., Wang E.Y., Falanga A., McLintock C., Hunt B.J., Spyropoulos A.C., Barnes G.D., Eikelboom J.W., Weinberg I., Schulman S., Carrier M., Piazza G., Beckman J.A., Steg P.G., Stone G.W., Rosenkranz S., Goldhaber S.Z., Parikh S.A., Monreal M., Krumholz H.M., Konstantinides S.V., Weitz J.I., Lip G.Y.H. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., Ma K., Xu D., Yu H., Wang H., Wang T., Guo W., Chen J., Ding C., Zhang X., Huang J., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. Bmj. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iba T., Di Nisio M., Thachil J., Wada H., Asakura H., Sato K., Kitamura N., Saitoh D. Revision of the Japanese Association for Acute Medicine (JAAM) disseminated intravascular coagulation (DIC) diagnostic criteria using antithrombin activity. Crit. Care. 2016;20:287. doi: 10.1186/s13054-016-1468-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor F.B., Jr., Toh C.H., Hoots W.K., Wada H., Levi M. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb. Haemost. 2001;86(5):1327–1330. [PubMed] [Google Scholar]
- 13.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iba T., Levy J.H., Warkentin T.E., Thachil J., van der Poll T., Levi M. Diagnosis and management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J. Thromb. Haemost. 2019;17(11):1989–1994. doi: 10.1111/jth.14578. [DOI] [PubMed] [Google Scholar]
- 15.Iba T., Levy J.H., Yamakawa K., Thachil J., Warkentin T.E., Levi M. Proposal of a two-step process for the diagnosis of sepsis-induced disseminated intravascular coagulation. J. Thromb. Haemost. 2019;17(8):1265–1268. doi: 10.1111/jth.14482. [DOI] [PubMed] [Google Scholar]
- 16.Iba T., Nisio M.D., Levy J.H., Kitamura N., Thachil J. New criteria for sepsis-induced coagulopathy (SIC) following the revised sepsis definition: a retrospective analysis of a nationwide survey. BMJ Open. 2017;7(9) doi: 10.1136/bmjopen-2017-017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh P., Schwartz R.A. Disseminated intravascular coagulation: a devastating systemic disorder of special concern with COVID-19. Dermatol. Ther. 2020;33(6) doi: 10.1111/dth.14053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Metlay J.P., Waterer G.W., Long A.C., Anzueto A., Brozek J., Crothers K., Cooley L.A., Dean N.C., Fine M.J., Flanders S.A., Griffin M.R., Metersky M.L., Musher D.M., Restrepo M.I., Whitney C.G. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 2019;200(7):e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 20.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X., Merdji H., Clere-Jehl R., Schenck M., Fagot Gandet F., Fafi-Kremer S., Castelain V., Schneider F., Grunebaum L., Anglés-Cano E., Sattler L., Mertes P.M., Meziani F. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Xi Xu Yu, Hu Ming-Dong, et al. Epidemiological and clinical characteristics of 47 corona virus disease 2019 non-survivors in Huoshenshan Hospital. Medical Journal of Chinese People's Liberation Army. 2020;45(05):475–480. [Google Scholar]
- 22.Lu Jing, Yu Lei, Jia-ying Gu, Qiao-fa Lu. Clinical feature analysis on death cases of the COVID-19. Tianjin Medical Journal. 2020;48(06):465–469. [Google Scholar]
- 23.Tan Wei, Hu Bingzhu, Zhu Zhang, et al. Clinical characteristics and death risk factors of severe COVID-19. Chinese Journal of Tuberculosis and Respiratory Diseases. 2020;43(08):648–653. doi: 10.3760/cma.j.cn112147-20200320-00380. [DOI] [PubMed] [Google Scholar]
- 24.Bao C., Tao X., Cui W., Yi B., Pan T., Young K.H., Qian W. SARS-CoV-2 induced thrombocytopenia as an important biomarker significantly correlated with abnormal coagulation function, increased intravascular blood clot risk and mortality in COVID-19 patients. Exp Hematol Oncol. 2020;9:16. doi: 10.1186/s40164-020-00172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng Y., Liu W., Liu K., Fang Y.Y., Shang J., Zhou L., Wang K., Leng F., Wei S., Chen L., Liu H.G. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 in Wuhan, China: a retrospective study. Chin. Med. J. 2020;133(11):1261–1267. doi: 10.1097/CM9.0000000000000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lodigiani C., Iapichino G., Carenzo L., Cecconi M., Ferrazzi P., Sebastian T., Kucher N., Studt J.D., Sacco C., Alexia B., Sandri M.T., Barco S. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan. Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martín-Rojas R.M., Pérez-Rus G., Delgado-Pinos V.E., Domingo-González A., Regalado-Artamendi I., Alba-Urdiales N., Demelo-Rodríguez P., Monsalvo S., Rodríguez-Macías G., Ballesteros M., Osorio-Prendes S., Díez-Martín J.L., Pascual C. COVID-19 coagulopathy: an in-depth analysis of the coagulation system. Eur. J. Haematol. 2020;105(6):741–750. doi: 10.1111/ejh.13501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazzaccaro D., Giacomazzi F., Giannetta M., Varriale A., Scaramuzzo R., Modafferi A., Malacrida G., Righini P., Marrocco-Trischitta M.M., Nano G. Non-overt coagulopathy in Non-ICU patients with mild to moderate COVID-19 pneumonia. J. Clin. Med. 2020:9(6). doi: 10.3390/jcm9061781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fogarty H., Townsend L., Ni Cheallaigh C., Bergin C., Martin-Loeches I., Browne P., Bacon C.L., Gaule R., Gillett A., Byrne M., Ryan K., O’Connell N., O’Sullivan J.M., Conlon N., O’Donnell J.S. COVID19 coagulopathy in Caucasian patients. Br. J. Haematol. 2020;189(6):1044–1049. doi: 10.1111/bjh.16749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nowak B., Szymański P., Pańkowski I., Szarowska A., Życińska K., Rogowski W., Gil R., Furmanek M., Tatur J., Zaczyński A., Król Z., Wierzba W. Clinical characteristics and short-term outcomes of patients with coronavirus disease 2019: a retrospective single-center experience of a designated hospital in Poland. Pol Arch Intern Med. 2020;130(5):407–411. doi: 10.20452/pamw.15361. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J.J., Dong X., Cao Y.Y., Yuan Y.D., Yang Y.B., Yan Y.Q., Akdis C.A., Gao Y.D. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan. China. Allergy. 2020;75(7):1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 33.Arachchillage D.R.J., Laffan M. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18(5):1233–1234. doi: 10.1111/jth.14820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klok F.A., Kruip M., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M., Kaptein F.H.J., van Paassen J., Stals M.A.M., Huisman M.V., Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paparella D., Colucci M., Squiccimarro E., Raimondo P., De Palma F., Ranieri P., Mariggiò M.A., Grasso S. Clotting abnormalities in critically ill COVID-19 patients are inconsistent with overt disseminated intravascular coagulation. Thromb. Res. 2020;196:272–275. doi: 10.1016/j.thromres.2020.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iba T., Di Nisio M., Thachil J., Wada H., Asakura H., Sato K., Saitoh D. A proposal of the modification of Japanese society on thrombosis and hemostasis (JSTH) disseminated intravascular coagulation (DIC) diagnostic criteria for Sepsis-associated DIC. Clin. Appl. Thromb. Hemost. 2018;24(3):439–445. doi: 10.1177/1076029617720069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Asakura H., Ogawa H. COVID-19-associated coagulopathy and disseminated intravascular coagulation. Int. J. Hematol. 2021;113(1):45–57. doi: 10.1007/s12185-020-03029-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]