Abstract
Background
Several recent studies have stated that glycyrrhizin and licorice extract are present in most traditional Chinese medicine formulas used against SARS-CoV-2 in China. Significant data are showing that glycyrrhizin and licorice extract have multiple beneficial activities in combating most features of SARS-CoV-2.
Purpose
The aim of current review was to highlight recent progresses in research that showed the evidence of the potential use of glycyrrhizin and licorice extract against COVID-19.
Methodology
We have reviewed the information published from 1979 to October 2020. These studies demonstrated the effects , use and safety of glycyrrhizin and icorice extract against viral infections,bacterial infections, inflammatory disorders of lung ( in vitro and in vivo). These studies were collated through online electronic databases research (Academic libraries as PubMed, Scopus, Web of Science and Egyptian Knowledge Bank).
Results
Pooled effect size of articles provides information about the rationale for using glycyrrhizin and licorice extract to treat COVID-19. Fifty studies demonstrate antiviral activity of glycyrrhizin and licorice extract. The most frequent mechanism of the antiviral activity is due to disrupting viral uptake into the host cells and disrupting the interaction between receptor- binding domain (RBD) of SARS-COV2 and ACE2 in recent articles. Fifty studies indicate that glycyrrhizin and licorice extract have significant antioxidant, anti-inflammatory and immunomodulatory effects. Twenty five studies provide evidence for the protective effect of glycyrrhizin and licorice extract against inflammation-induced acute lung injury and cardiovascular disorders.
Conclusion
The current study showed several evidence regarding the beneficial effects of glycyrrhizin and licorice extract in combating COVID-19. More randomized clinical trials are needed to obtain a precise conclusion.
Keywords: COVID-19, Glycyrrhizin and licorice extract;Antiviral and antimicrobial, Anti-inflammatory and antioxidant, Immunododulator, Acute lung injury protector
Abbreviations: : ACE2, angiotensin-converting enzyme 2; ALI, acute lung injury; ARDS, acute Respiratory Distress Syndrome; DCs, dendritic cells; COVID-19, Coronavirus disease 2019; COX-2, cyclooxygenase-2; 18β-GA, 18β-glycyrrhetinic acid; Gl, glycyrrhizin; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; HMGB1, high-mobility group box 1; h, hour; IL, interleukin; iNOS, inducible nitric oxide synthase; licorice extract, LE; MAPKs, mitogen-activated protein kinases; MERS, Middle East respiratory syndrome; MR, mineralocorticoid receptor; MRSA, Methicillin-resistant Staphylococcus aureus; NO, nitric oxide; RBD, receptor-binding domain; ROS, reactive oxygen species; S, Spike; SARS, severe acute respiratory syndrome; TCM, traditional Chinese medicine; TLR, toll-like receptor; TNF-α, tumor necrosis factor alpha; TMPRSS2, type 2 transmembrane serine protease
Graphical abstract
Introduction
Coronavirus disease 2019 (COVID-19), is a kind of viral pneumonia caused by a novel coronavirus named Severe Acute Respiratory Syndrome. The pathogen that causes COVID-19 disease is a SARS-CoV2 or new coronavirus that has similar genetic structures with the other coronavirus as SARS-CoV . The SARS-CoV-2 shares 79.5% of genetic sequence and the same cell entry receptor, angiotensin-converting enzyme II (ACE2), with SARS-CoV (Zhou et al., 2020). It has an envelope spike (S) protein that is important for receptor binding and membrane fusion of coronavirus. Angiotensin-converting enzyme II (ACE2) is the cell receptor for SARS-CoV2 similar to SARS-CoV (Xu et al., 2020).
The S protein of SARS-CoV-2 has an affinity property to binds ACE2 10- to 20-fold greater than S protein of SARS-CoV. This high affinity of S protein for human ACE2 probably the reason for the rapid spread of SARS-CoV-2 (Wan et al., 2020). Hirano and Murakami (2020) documented that ACE2 as the SARS-CoV-2 receptor for cellular is critical for the virus entry. The targeting ACE2 has a promise for the prevention of SARS-CoV-2 infection during the initial phase of disease (Letko and Munster, 2020). In the later stages, a reduction of ACE2 enzyme, which leads to an increased angiotensin II concentration. This increase leads to an increased inflammatory reactions and cytokine storm as shown in COVID-19 patients. Targeting of pathways of cytokine release, especially the IL-6-STAT3 axis may be needed (Hirano and Murakami, 2020). The cytokine storm will lead to a violent attack by the body's immune system, causing Acute Respiratory Distress Syndrome(ARDS), multiple organ failure, and ultimately death in severe cases of SARS-CoV- 2 infections (Xu et al., 2020; Huang et al., 2020). SARS-CoV2 can activate the clotting chain through various mechanisms, leading to severe hypercoagulability, and ischemic changes such as ecchymosis of the fingers and toes (Li et al., 2020).
Recently, a high percentage of patients show high interest in natural medicines. This is mainly due to the general feeling that natural medicines is safer than synthetic drugs. Glycyrrhiza glabra L. (Fabaceae) (licorice) root is used as food and as a medicinal plant. It is native to Mediterranean areas, but it now grows in Russia, India, and China (Pastorino et al., 2018). It contains a lot of phytochemicals including more than 300 flavonoids (of them 42 chalcones) and 20 triterpenoids (Li et al., 2000). The chalcones play a crucial role in licorice's pharmacological effects (Maria Pia et al., 2019). The pharmacological values of licorice has been reported since ancient times.. The active ingredients, glycyrrhizic acid (also known as glycyrrhizin, Gl),18β‐glycyrrhetinic acid(the major metaboliteof Gl), glabrin A and B, isoflavones and others have been demonstrated to have different pharmacological activities. Glycyrrhizin, an abundant bioactive component of the medicinal licorice root is rapidly metabolized by gut commensal bacteria into 18β-glycyrrhetinic acid. Licorice contains leading natural active agents, promising as a basis for developing novel antiviral agents.The most common way to extract the active ingredients of licorice root is by hot water or an ethanol / water mixture, however, other solvents are used to prepare licorice roots extracts (Tian et al., 2008). Licorice extract or its constituents suppress many RNA and DNA viruses (Fiore et al., 2008; Batiha et al., 2020). Moreover, it has been shown that a small dose of licorice extract has anti-aging activity (Reigada et al., 2020),therefore, it can eliminate the accumulation of senescent cells. Accumulation of senescent cells is the main cause of exaggeration of cytokine load or cytokine storm in elderly and obese infected by COVID-19 and an increased number of deaths. This assumption is supported by the recently published study suggesting that senotherapeutics such as azithromycin can reduce RNA virus replication in senescent cells and may have potential therapeutic activity against COVID-19 (Malavolta et al., 2020).
Traditional Chinese Medicine (TCM) has played a crucial role in treating SARS, influenza, and other acute respiratory infectious diseases (Hsu et al., 2006) . The clinical evidence has shown a good therapeutic effect for TCM in treating SARS coronaviral infections and because of the similarities in genomics, and clinical features of the COVID-19 and SARS-CoV, TCM widely used in the therapy of COVID-19. More than 85% of patients infected by SARS-CoV-2 in China have received TCM (Yang et al., 2020). Previously, treatment with TCM in SARS-CoV patients in a controlled clinical study showed a significant improvement of symptoms and shortening of the disease course (Hsu et al., 2006). The laboratory studies supported the positive clinical effect of TCM. Glycyrrhizin or licorice extract is frequently used in many preparations of TCM (Ang et al., 2020). It significantly decreased the replication of the SARS virus isolated from patients (Cinatl et al., 2003; Chen et al., 2004). However, determining its use depends on "syndrome differentiation", in which individual plans are produced for each case.
The therapeutic potential of glycyrrhizin and licorice extract for COVID-19 will be discussed in this review. This article focuses and summarizes the benefits of glycyrrhizin and licorice extract in the attenuation of COVID-19. In this review, we present preliminary data regarding the potential activity of glycyrrhizin and licorice extract against SARS-CoV-2. Potential therapeutic effects of glycyrrhizin and licorice extract and boswellia serrate gum will be evaluated in a pilot clinical study as complementary intervension for COVID-19 in Egyptian patients (ClinicalTrials.gov Identifier: NCT04487964).
Therapeutic basis of the potential use of glycyrrhizin and licorice extract against SARS-CoV-2 .
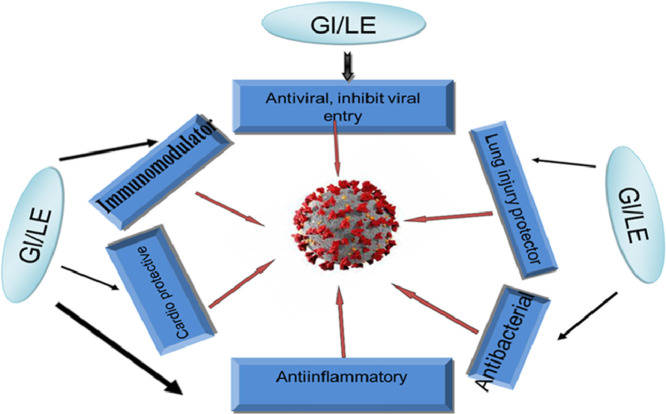
Glycyrrhizin and licorice extract have been deeply and widely studied for their various activities. According to these research studies, glycyrrhizin and licorice extract have a wide range of pharmacological activities such as anti-inflammatory, antioxidative, antiviral, anticancer, antimicrobial, antidiabetic, immunomodulatory, acute lung injury prevention, cardioprotective and hepatoprotective activities (Wang et al., 2020; Editorial: Nat Plants., 2020). We will discuss and evaluate the different effects of glycyrrhizin and licorice extract that can be used to combat SARS-CoV-2 and neutralize any tissue destructive effects of the virus.
Antiviral effect of glycyrrhizin and licorice extract
Several review articles concluded that the antiviral activity of licorice extract and glycyrrhizin has been reported against various viruses including SARS-CoV and SARS-CoV2 (Wang et al., 2015a; Pastorino et al., 2018; Sun et al., 2019; Bailly and Vergoten, 2020). Licorice extract has been documented to inhibit the growth of viruses and exhibit potent inhibitory activity against virus entry. In past years, Pompei et al. (1979) published the first study demonstrating the efficacy of licorice extract against viruses. It reported that components of Glycyrrhiza Glabra root extract inhibit the growth and cellular diseases of many unrelated RNA viruses. Water extract from licorice shows antiviral activity against several viruses such as the human respiratory syncytial virus (HRSV) (Feng et al., 2013), and Enterovirus 71 in a human foreskin fibroblast cell line (Kuo et al., 2009). It reduced HRSV infection to a large extent by inhibiting viral attachment, uptake and stimulation of IFN secretion. However, the alkaline extract of licorice root shows higher anti-HIV activity than the aqueous extract (Ohno et al., 2014; Fukuchi et al., 2016). Also, methanol extract from licorice root was found to have more anti-hepatitis C virus activity than glycyrrhizin (Adianti et al., 2014), While ethanol extract of licorice has shown an important property to inhibit RANTES secretion by H1N1-infected A549 bronchial epithelial cells (Ko et al., 2006). Moreover, randomized controlled trials confirmed that licorice extract reduces liver cell damage in chronic hepatitis B and C (Fiore et al., 2008). Recently, it was found that licorice extract can be a strong inhibitor of the Main Protease of SARS-CoV2, but glycyrrhizin has a high binding affinity and good ADMET(Absorption, Distribution, Metabolism, Excretion, and Toxicity) properties than other ingredients of licorice (Srivastava et al., 2020).
The active ingredients of licorice extract that have antiviral activity include certain triterpenoids, flavonoids, and oleanane-type triterpene saponins (Wei et al., 2014). Glycyrrhizin, glycyrrhetinic acid, and its derivatives are the main triterpenoid active components of licorice extract. These compounds have broad-spectrum antiviral activity against many RNA and DNA viruses such as SARS coronavirus, herpes virus, HIV, hepatitis virus, influenza viruses, cytomegaloviruses, and respiratory syncytial virus (Pu et al., 2013; Wang et al., 2015a; Pastorino et al., 2018; Batiha et al., 2020) (Tables 1 and 2 ). Different studies have suggested that two triterpenoids of licorice are mainly responsible for the antiviral activity reported: glycyrrhizin and 18β‐glycyrrhetinic acid (Wang et al., 2015a; Pastorino et al., 2018). Glycyrrhizin suppresses many RNA and DNA viruses (Baltina et al., 2009). These compounds were found to be effectively preventing the early stage of virus infection by affecting viral attachment and penetration. Glycyrrhizin may act through affecting many cellular factors, as casein kinase II, protein kinase II and transcription factors (activating protein I and nuclear factor-kB) (Baltina et al., 2009).
Table 1.
Antiviral activity and mechanism of action of licorice extract in articles published from 2006 to 2020.
| Extract | Method of research | Major finding | Mechanism of actions | References |
|---|---|---|---|---|
| Licorice extract 95% ethanol extract of Glycyrrhiza ‘uralensis’ |
H1N1infected human bronchial epithelial cells (A549). | Inhibition of influenza A virus (H1N1). | Inhibit RANTES secretion. | Ko et al., 2006. |
| Licorice extract | Randomized controlled trials | Reduced hepatocellular damage in chronic hepatitis B and C. | Reduced transport to the membrane. | Fiore et al., 2008. |
| Aqueous extract of Glycyrrhiza uralensis. | Human foreskin fibroblast cell line. | Inhibited enterovirus 71. | By preventing viral attachment and penetration. | Kuo et al., 2009. |
| Hot water extracts of licorice | Human Respiratory Tract Cell Lines. | Anti-Viral Activity Against Human Respiratory Syncytial Virus. Aquous ext., are highly effective against HRSV infection on airway epithelial cells. | By preventing viral attachment, internalization, and by stimulating IFN secretion. | Feng et al., 2013 |
| Licorice extract | HCV cell culture system. | It has anti-HCV more than glycyrrhizin. | Unknown | Adianti et al., 2014 |
| Licorice extract | Cell line | Superiority of alkaline extraction over water extraction as anti-HIV. | Unknown | Ohno et al., 2014 |
| Licorice extract rich Oleanane-Type Triterpene Saponins | MDCK cells | Inhibit many virus. | Inhibition of neuraminidase. | Wei et al., 2014 |
| Licorice extract rich oleanane-type triterpenoid saponins. | Cell line | In vitro anti-influenza virus activity comparable to and even higher than that of oseltamivir. | Suppression of virus release by GL treatment may be due to its inhibitory effect on PLA2G1B. | Song et al., 2014 |
| Alkanine extract & water extract of licorice root | Cells line | Alkaline extract was highly effective against HIV and more than aquous extract. While aquous extract was more effective against HSV-infected cells. |
Unknown | Fukuchi et al., 2016 |
| Licorice extract and bioactive ingredients | Molecular Docking and ADMET Study | Inhibitor SARS-CoV2 while Gl better ADMET. | Potential to be strong inhibitors for Main protease of SARS-CoV2. | Srivastava et al., 2020 |
Table 2.
The antiviral activity and mechanism of action of glycyrrhizin (Glycyrrhizic Acid) and its various derivatives in articles published from 1979 to 2020.
| Glycyrrhizic Acid and its derivatives | Method of research | Major finding | Mechanism of actions | References |
|---|---|---|---|---|
| Glycyrrhizic acid Glycyrrhizic acid Glycyrrhizin Ribavirin, 6-azauridine, pyrazofurin, mycophenolic acid, and glycyrrhizin Glycyrrhizic Acid Derivatives Glycyrrhizin Glycyrrhizin Chalcones isolated by bioassay-guided fractionation of acetone extract of Glycyrrhiza ‘inflata.’ . Glycyrrhizin 18β-glycyrrhetinic acid (GRA) and Glycyrrhizin (GA) Glycyrrhizin Pentacyclic triterpenes,glycyrr. Licorice triterpene glycyrrhizic acid (GRA Glycyrrhizin Gycyrrhizic acid derivatives Glycyrrhizic acid Triterpenoids Water-Soluble β-Cyclodextrin-glycyrrhetinic Acid Conjugates Glycyrrhizic acid (GL) derivatives Glycyrrhizin Glycyrrhizic‐Acid Based Carbon Dots Glycyrrhizin |
Cell culture Cell culture Cell line MT-4 and MOLT-4 cells Two clinical isolates of coronavirus (FFM-1 and FFM-2) from patients with SARS Human Respiratory Tract Cell Lines Lung epithelial (A549) cells Porcine reproductive and respiratory syndrome virus (PRRSV). HCV infected liver cells MDCK cells A/WSN/33 (H1N1) virus using the cytopathic effect assay. Cell culture-produced HCV (HCVcc. Balb/C mice Cultured human cells MARC-145 cells infected with porcine reproductive and respiratory syndrome virus ( PRRSV). Porcine kidney (PK‐15) cells, African green monkey kidney (Vero) cells, Sprague–Dawley Cell lines Many celllines as Human embryonic kidney (293T cells). Cell lines Infected DENV type 2 (DENV2) in Vero E6 cells. Cell line MOCK infected cells Molecular docking study |
Inhibits growth and cytopathology of several unrelated DNA and RNA viruses, while not affecting cell activity and ability to replicate. Inhibits the growth of several DNA and RNA viruses in cell cultures and inactivates Herpes simplex 1 virus irreversibly. Suppress hepatitis B virus. glycyrrhizin administered intravenously might bind to hepatocytes at the concentration at which glycyrrhizin could modify the expression of HBV-related antigens on the hepatocyte . Of all the compounds, glycyrrhizin was the most active in inhibiting replication of the SARS-associated virus. Modified glycyrrhizin has 70-fold increased activity against SARS-CoV but also increased cytotoxicity. It has antiviral effect and this inhibitory effect was abolished by treatment 1 h after virus infection. It inhibited H5N1-induced expression of the pro-inflammatory molecules CXCL10, interleukin 6, CCL2, and CCL5 and interfered with H5N1 replication. Strong inhibitory effects on influenza viral strains, H1N1, H9N2, novel H1N1 (WT), and oseltamivir-resistant novel H1N1and synergistic effect with oseltamivir. GL inhibit HCV full length and function in a dose dependent manner and had synergistic effect with interferon. GRA, but not GA, has significant antiviral activity against rotavirus replication in vitro, Treatment of HCV-infected Huh7 cells caused a reduction of infectious HCV production. combination treatment with GL augmented IFN-induced reduction of virus in the HCVcc system. Exhibited good inhibitory activities against the influenza virus A/WSN/33 (H1N1) in MDCK cells and showed anti-HIV activities. GRA demonstrated a strong antiherpes simplex virus type 1, (HSV1) activity, resistance typewhereas rapamycin had no activity. Glycyrrhizin significantly reduced PRRSV proliferation and PRRSV-encoded protein expression in a dose-dependent manner. GL derivatives are potent as anti-influenza A/H1N1 agents. Entecavir and glycyrrhizic acid combination but not Glycyrrhizin produce synergistic anti-HBV activity. Effective against Ebola, Marburg, HIV, and influenza A, triterpenoids are viral fusion inhibitors. Findings suggested that GA could be used as a lead compound for the development of potential anti-influenza virus agents. GL conjugates were found as potent anti- Dengue virus. Glycyrrhizin (GLY) inhibited porcine epidemic diarrhea virus (PEDV) infection, Gly‐CDs possess extraordinary antiviral activity, providing a promising candidate for treatment of respiratory syndrome virus infection. Has potential anti-2019-nCoV and may prevent the 2019-nCoV infection. |
Unknown Unknown Inhibit RANTES secretion. Unknown The mechanism of glycyrrhizin's activity against SARS-CV may be through Glycyrrhizin affects cellular signaling pathways such as protein kinase C; casein kinase II; and transcription factors such as activator protein 1 and nuclear factor κB. The antiviral activity is mediated by an interaction with the cell membrane which most likely results in reduced endocytotic activity and hence reduced virus uptake. Inhibition of H5N1-induced formation of reactive oxygen species and (in turn) reduced activation of NFκB, JNK, and p38, redox-sensitive signaling events known to be relevant for influenza A. Neuraminidase inhibitors GL dose dependently inhibit the expression of HCV 3a core gene both at mRNA and protein levels. Inhibitory effects on various neuraminidases. Due to its inhibitory effect on PLA2G1B( phospholipase A2 of group 1B PLA2 Triterpenoids bind tightly to the viral envelope hemagglutinin (HA), disrupting the interaction of HA with the sialic acid receptor and thus the attachment of viruses to host cells. GRA induced a Beclin 1 production that was more than twofold higher than that produced by rapamycin, GRA is a strong inducer of the autophagy activator Beclin 1, which establishes a resistance state to HSV1 replication. Gycyrrhizin mainly inhibits the penetration stage, and has little effect on the steps of adsorption or release of PRRSV in its life cycle. With multisite inhibition mechanisms. Synergistic actions were primarily due to the inhibitory effect of glycyrrhizic acid on MRP4 and BCRP, which transport Entecavir out of hepatocytes. Triterpenoid block the entry of many viruses by capturing the HR2 domain prevalent in viral envelopes. Unknown May through interaction with DENV2 targets like NS2B-NS3 protease, NS3 helicase, and NS5 RNA-dependent RNA polymerase. Glycyrrhizin inhibited PEDV infection and decreased proinflammatory cytokine secretion via the HMGB1/TLR4-mitogen-activated protein kinase (MAPK) p38 pathway. Host receptor for 2019-nCoV Angiotensinconverting enzyme 2 (ACE2), is the same as the host receptor for SARS-CoV. Gycyrrhizin. Unknown |
Pompei et al., 1979 Pompei et al., 1980 Sato et al., 1996 Cinatl et al., 2003 Hoever et al., 2005 Wolkerstorfer et al., 2009 Michaelis et al., 2011 Dao et al., 2011 Ashfaq et al., 2017 Hardy et al., 2012 Matsumoto et al., 2013 Yu et al., 2014 Laconi et al., 2014 Duan et al., 2015 Baltina et al., 2015 Chen et al., 2017b Si et al., 2018 Liang et al., 2019 Baltina et al., 2019 Gao et al., 2020 Tong et al.,(2020) Chen and Du, 2020 |
Glycyrrhizin was found to be effective against viral hepatitis. It prevents the early stage of HCV- infection through affecting viral attachment and penetration. Glycyrrhizin is used clinically in the therapy of chronic viral hepatitis B in Japan and China for about 40 years (Sato et al., 1996; Sun et al., 2019). An anti-HBV mechanism of glycyrrhizin is through affecting the hepatitis B surface antigen (HBsAg). It reduced transport to the membrane and sialylation of the hepatitis B virus surface antigen (Sun et al., 2019). Glycyrrhizin has been used also, by I .V. injection to treat hepatitis C in Japan. Few adverse reactions and significant inhibition in the progression of cirrhosis and hepatocarcinoma were observed after the use of glycyrrhizin (Matsumoto et al., 2013; Pastorino et al., 2018). The mechanism of glycyrrhizin against hepatitis C virus (HCV) was through targeting the release step in which hepatitis C viral particles infect cells (Matsumoto et al., 2013).
Regarding respiratory syncytial and influenza virus, Michaelis et al. (2011) observed that glycyrrhizin induces antioxidative activity in H5N1 influenza a virus-infected cell and therefore, it inhibits virus replication. Other investigators (Wolkerstorfer et al., 2009; Baltina et al., 2015) confirmed that glycyrrhizin inhibits influenza A/H1N1 by the prevention of virus uptake into the cell. The antiviral activities of glycyrrhizin against SARS-associated coronavirus and influenza virus have also been demonstrated (Hoever et al., 2005; Baltina et al., 2015; Ang et al., 2020; Ahmad et al., 2020). The antiviral mechanisms of glycyrrhizin are through inhibiting the uptake and penetration of the virus in the early stage of the life cycle. Glycyrrhizin was highly effective when given during and after the uptake period of the virus; it exhibits the highest antiviral activity (Ang et al., 2020). Additionally, it has been shown that glycyrrhizin induced the production of a higher amount of Beclin 1 and showed an improved antiviral effect in resistance virus strain, therefore, the prophylactic activity of glycyrrhizin and licorice extract could perhaps also be extended to important human pathogenic viruses (Laconi et al., 2014).
Recently, some studies suggested that glycyrrhizin and licorice extract have the potential benefits against novel coronavirus through binding with ACE2 then inhibiting the virus absorption and penetration. Glycyrrhizin is one of the new treatments used for COVID-19 in China (Zhang and Liu, 2020). Additionally, based on the similarities between SARS-CoV with SARS-CoV-2 and the benefit of glycyrrhizin use against SARS-CoV, Many investigators suggested that glycyrrhizin has therapeutic potential against COVID-19 by binding to ACE2, and preventing the 2019-nCoV bind to ACE2 and virus absorption into the cell (Luo et al., 2020). Moreover, Murck (2020) proposed that glycyrrhizin and its metabolites have two mechanisms in combating COVID-19 through direct inhibition of expression of type 2 transmembrane serine protease (TMPRSS2), which is necessary for virus entry and activation of MR (mineralocorticoid receptor), thus reducing the expression of ACE2 protects members from being linked to COVID-19. Interestingly, It was reported that glycyrrhizic acid (glycyrrhizin) has the highest activity in disrupting the interaction between receptor- binding domain (RBD) of SARS-COV2 and ACE2, therefore, it has broad spectrum anti-coronavirus (Yu et al., 2020).
Certain derivatives of the glycyrrhizic acid (glycyrrhizin) have been shown to have a tenfold increase in the antiviral activity (Xiao et al., 2018). The anti-SARS-CoV activity of glycyrrhizic acid (glycyrrhizin) was shown to be increased up to 70-fold by conjugation of glycyrrhizin with an amide or two amino acid residues, however, the cytotoxicity also increased resulting in a decrease in the selectivity index (Hoever et al., 2005). Recently, it has been observed that glycyrrhizic acid derivatives were observed as Dengue virus inhibitors (Baltina et al., 2019). More recently, Tong et al.,(2020) observed that glycyrrhizic-Acid-Based Carbon Dots (Gly-CDs), semisynthetic derivatives of glycyrrhizic acid, possess multisite viral inhibition and unusual antiviral activity, providing a promising antiviral agent for the treatment of respiratory syndrome virus infection. Also, other studies reported that glycyrrhizin could be used as a parent compound for the development of new anti-influenza virus agents (Liang et al., 2019). More recently, Ding et al. (2020) reported an interesting clinical investigation of a patient who was suffering from severe COVID-19 recovered after treatment with diammonium glycyrrhizinate (DG), a derivative of glycyrrhetinic acid. They suggested that combining DG with vitamin C might be a promising an alternative treatment for severe symptoms of COVID- 19 during quarantine.
Prevention of COVID-19-induced secondary bacterial infection by glycyrrhizin and licorice extract
Viral respiratory infections often lead to bacterial pneumonia.Therefore, antibacterial agents have become a popular treatment for COVID-19 patients in combination with antiviral agents (Hendaus et al., 2015). In uncontrolled studies that appeared to show the combination of hydroxychloroquine, azithromycin was effective in COVID 19 (Gautret et al., 2020).
Many studies observed that flavonoids/triterpenoid of licorice extract has bacteriostatic at low concentration or bactericidal at high concentration for many gram-positive and gram-negative bacteria in vitro and in vivo. It has been observed that ethanol and methanol extracts of licorice have potential antibacterial activity in vitro against many gram-positive and gram-negative bacterial strains (Salmonella tophi, Staphylococcus aureus, Escherichia coli, Vibrio cholera, Bacillus cereus, and Bacillus subtilis strains). The components detected in the ethanol and methanol extracts responsible for the antibacterial activity are flavonoid as chlorogenic acid, caffeic acid, quercitin, myricitin, kaempferol (Sedighinia et al., 2012). Licorice ethanol extract significant inhibited Escherichia coli, Proteus mirabilis, Staphylococcus aureus, Streptococcus pyogenes, Pseudomonas aeruginosa, Klebsiella pneumonia, and Bacillus. The highest inhibition zone is observed on Streptococcus pyogenes (Alwan et al., 2015). The aqueous extract was less effective than ethanol extract (Shirazi et al., 2007; Zhou et al., 2019). Importantly, microbial resistance to antimicrobial drugs is a matter of great clinical importance; therefore, we will evaluate the antibacterial activity of licorice extract, flavonoids and triterpenoid content against resistant microorganisms such as Streptococcus, Staphylococcus aureus and Pseudomonas aeruginosa
Streptococcus pyogenes is a common cause of upper respiratory infections. Ethanol or aqueous extracts of licorice showed marked antibacterial activities against S. pyogenes isolates from the throat of infected patients., however, ethanol extract was found to be two-fold more effective than aqueous extracts (Kazia et al., 2014). .Many components of licorice were tested separately against S. pyogenes. Licoricidin, a flavonoid constituent was found to have the highest antibacterial activity against the bacterial of the upper respiratory tract such as Streptococcus pyogenes, Moraxella catarrhalis, and Haemophilus influenza. The coumarin components such as glycycoumarin, glycerol, and glycerin exhibit moderate antibacterial activity against bacteria of the upper respiratory airway tract (Tanaka et al., 2001). Recently, Wijesundara and Rupasinghe (2019) assessed many hot aqueous infusions from different herbs (13 herbs) against Streptococcal pharyngitis. Aqueous infusion of licorice root showed the highest activity and the lowest minimum inhibitory concentrations. Additionally, the hot aqueous infusions of licorice showed inhibitory activity on biofilm formation resulted from Streptococcus mutants that cause dental caries.
The antiadherence and antimicrobial property of licorice extract on Streptococcus mutants in vitro was reported by many studies. Moreover, the anticarcinogenic activity of the extract of licorice has been well documented (Bhadoria et al., 2019). Alcohol licorice extract was shown to have an inhibitory effect on Streptococcus mutants superior to that of licorice aqueous extract or Chlorhexidine (Ajagannanavar et al., 2014). In comparison between the efficacy of licorice and propolis extract used as anticavity against Streptococcus mutants, licorice extract exhibited better antibacterial efficacy (Godbole et al., 2019). Licorice-containing lollipops were effective in reducing significantly salivary S. mutants in high caries-risk children aged 3–6 and decrease significantly the risk of dental caries in children (Chen et al., 2019). Also, in twenty patients aged between 18 and 21 years, licorice lollipop was effective in suppressing S. mutans and dental caries (Krishnakumar et al., 2018).
Increasing antibiotic resistance to staphylococcus aurous strains is considered the main cause of antibiotic failure. MRSA(Methicillin-resistant Staphylococcus aureus) has become a major source of infection in hospitals and the community. Many studies observed that licorice extract and its components have antibacterial potential against MRSA. The extract of licorice by 80% methanol exhibited potent antibacterial activity against standard S. aureus as well as MRSA (Lee et al., 2009). 18β-Glycyrrhetinic acid, triterpenoid of licorice, and its derivative disodium succinoyl glycyrrhetinate suppress MRSA survival and inhibit virulence gene expression but they are bactericidal at high concentrations in vitro and in vivo (Long et al., 2013; Oyama et al., 2016). Flavonoids from licorice such as glabrol, licochalcone a, licochalcone C, and licochalcone, exhibit high antibacterial activity against MRSA. Glabrol exhibited rapid bactericidal action with low levels of resistance development in vitro, so, it is a promising agent that can be used as a drug for the treatment of MRSA (Wu et al., 2019). Some studies demonstrated the effect of licorice extract or its components on the susceptibility of MRSA strains to antibiotics. Licorice flavonoids were shown to enhance the susceptibility of MRSA strains to oxacillin, a β-lactam antibiotic (Hatano et al., 2005). Screening of a 350 compound revealed that 18β-glycyrrhetinic acid (18β-GA) was the most effective component in potentiation of the antibacterial activity of certain antibiotics against Staphylococcus aureus and increases the bactericidal activity of tobramycin and polymyxin B against the MRSA strain (de Breij et al., 2016).
Regarding the effect of glycyrrhizin and licorice extract against Pseudomonas aeruginosa, the most resistant bacteria. It was documented that glycyrrhizin disturbed the permeability of bacterial membrane and efflux pump activity leading to a reduction of viability of multi-drug resistant Pseudomonas aeruginosa. It also reduces HMGB1 and oxidative damage. Moreover, it potentiates the effect of antibiotics like Ciprofloxacin and tobramycin against multi-drug resistant Pseudomonas aeruginosa. It may be used as a therapeutic for Pseudomonas aeruginosa Keratitis (Ekanayaka et al., 2016, 2018; Hazlett et al., 2019).
From the aforementioned studies, we suggest that the use of licorice extract or glycyrrhizin as a complementary treatment for COVID-19 can protect patients from a bacterial infection that usually occurs after a viral infection which is the main cause for severe pneumonia
Prevention of COVID-19- induced autoimmune diseases by immunomodulatory activity of glycyrrhizin and licorice extract
Licorice contains various bioactive compounds such as polysaccharides, triterpenes, and flavonoids that could enhance immunity through the activation of different targets. Early, an increase of interferon-gamma production was observed in glycyrrhizin-treated human peripheral lymphocytes in response to the surface antigen of the hepatitis B virus (Shinada et al., 1986). Also, glycyrrhizin treatment enhances the lymphocytic proliferation in response to viral infection after 4 days postinoculation (Soufy et al., 2012). Licorice extract revealed a dose-dependent cell-mediated and humeral immunomodulatory activity against mixed Eimeria infection (Hussain et al., 2017). Aqueous licorice extract increased significantly leukocyte count and phagocytic index, but the use of aqueous licorice extract with zinc displayed a more marked rise of leukocyte count and phagocytic index compared to control (Mazumder et al., 2012). 18β-glycyrrhetinic acid reduced the duration of viral antigen shedding and increases the serum antibody titers in 18β-glycyrrhetinic acid-treated animals (Hendricks et al., 2012). The mechanism of lymphoid follicles induction by 18β-glycyrrhetinic acid in the gut may through the increase of chemokine and chemokine receptor genes expression that modulates B and T cell recruitment to lymphoid follicles (Hendricks et al., 2014). Glycyrrhizin attenuates Salmonella enterica Serovar Typhimurium infection in mice by promoting the release of immune factors (Xu et al., 2018a). Licorice extract, or the two components, isoliquiritigenin, and naringenin, could promote Regulatory T cells induction both in vitro and in vivo. Also, they enhanced immune suppression of Regulatory T cells. Therefore they are useful against inflammatory and autoimmune diseases (Guo et al., 2015).
Polysaccharides derived from many plants have shown to have immunostimulatory activity (Kikete et al., 2018). The polysaccharide can promote dendritic cell maturation and induce the upregulated expression of co-stimulatory molecules (Aipire et al., 2017). The low molecular weight, licorice polysaccharide displays immunomodulatory and anticancer activities. Treatment of murine bone marrow-derived dendritic cells (DCs) with licorice polysaccharide resulted in the maturation of DCs, increased the cell surface molecules expression CD80, CD86, and enhanced the expression of IL-12 p70, antitumor cytokine, by dendritic cells in a time-dependent manner (Li et al., 2012). They also increase the T lymphocytes count and thymus/spleen index. Furthermore, they decrease the pro-tumor cytokine, TNFα, and increase the levels of serum antitumor cytokines, (IL 2, IL 6, and IL 7) (Ayeka et al., 2017; Aipire et al., 2020a). Many fractions of licorice polysaccharide have been tested and only those of low molecular weight showed significant enhancement of the maturation of DCs. These fractions may be used with cancer immunotherapy to enhance the therapeutic effect (Aipire et al., 2020b). The most important action for licorice polysaccharides is the ability of the polysaccharides to enhance the anticancer cytokine IL-7, which is essential for the maturation and proliferation of immune cells and it is shown with good prognosis of cancer (Ayeka et al., 2016). Moreover, three purified licorice polysaccharides with lower molecular weight exhibited at the same concentration higher antioxidant activities (Zhang et al., 2015; Chen et al., 2017a).This antioxidant activity was highly enhanced by the selenylation modification of licorice polysaccharide (Lian et al., 2018).
Reduction of COVID-19-induced oxidative stress and inflammation by glycyrrhizin and licorice extract
A lot of studies reported that licorice extract and its constituent's triterpenes and flavonoids show evidence of anti-inflammatory activity via inhibition of TNF, MMPs, PGE2, and free radicals (Yang et al., 2017). Total flavonoids isolated from licorice and licorice extract have been observed to have anti-inflammatory effects in vitro through inhibiting iNOS, COX-2 gene, and signals of mitogen-activated protein kinases (MAPKs) (Yang et al., 2013; Vasanth et al., 2020). Total flavonoids also displayed an anti-inflammatory effect by inhibition of iNOS expression in LPS/IFN- stimulated RAW264.7 macrophages without cytotoxicity and regulation of ERK/NF-B/miR-155 signaling (Jiang et al., 2018; Frattaruolo et al., 2019). The inhibitory effect of flavonoid on inflammation depends on the multi-pathway integrated mechanism. Therefore licorice flavonoid action may have high potent anti-inflammatory activity with low side effects in patients (Yu et al., 2019). Glycyrrhizin, a triterpene of licorice shows marked analgesic and anti-inflammatory effects through decreasing the expression levels of TNF-α, IL-6, iNOS, and COX-2. (Wang et al., 2015b). Due to its ability to bind the COX/mPGEs pathway for a long time, ammonium glycyrrhizinate exhibited antinociceptive and anti-inflammatory activity until 24–48 h after a single administration (Maione et al., 2019).
Flavonoids from licorice extract show marked anti-inflammatory effects in acute inflammatory models. It markedly decreased the expression of IL-1β and iNOS and reduced levels of NO and MDA at the site of inflammation (Yin et al., 2018). In the model of pancreatitis, Isoliquiritigenin suppresses oxidative stress and alleviates acute pancreatitis through modulation of the Nrf2/HO-1 pathway (Liu et al., 2018; Zhang et al., 2018a). Licorice extract and its constituents have been shown to exert therapeutic potential activity against many inflammatory diseases such as acute kidney injury. Isoliquiritigenin alleviated LPS-induced acute kidney injury by inhibition of the NF-κB pathway and TNF-alpha-induced release of HMGB (Chi et al., 2017; Tang et al., 2018). Moreover, Isoliquiritigenin ameliorates renal Inflammation and fibrosis induced by unilateral ureteral obstruction (Liao et al., 2020). It also, suppressed the Ang II-induced hypertensive renal injury via inhibiting inflammation cytokines, excessive deposition, and Nrf2 and NF-κB pathways (Xiong et al., 2018). Licorice extract suppresses inflammation that plays a crucial role in the induction of many complications to diabetes such as diabetic retina complications. Glycyrrhizin could protect the diabetic retina from vascular damage by anti-Inflammatory mechanisms through binding to an HMGB1 and inhibiting the cytokine activities (Mollica et al., 2007; Shah et al., 2018; Liu et al., 2019a, 2019b).
The antioxidant activity of glycyrrhizin/licorice extract has been documented by many investigators. Licorice extract has been used as a good source of natural antioxidants for health benefits (Velvizhiv and Annapurani, 2018). Many studies indicated that glycyrrhizin and other active components of licorice extract were found to possess excellent antioxidant activities. However, other studies found that the high content of the phenolic component in the extract of licorice is responsible for its powerful antioxidant activity (Ju et al., 1989; Visavadiya et al., 2009; Li et al., 2011; Kim et al., 2012; Zhang et al., 2019). Glycyrrhizin and licorice extract inhibit the generation of reactive oxygen species (ROS) by neutrophils at the site of inflammation that is responsible for tissue damage (Maksoud et al., 2019). The mechanism of the antiviral effect of glycyrrhizin may be through suppression of formation of reactive oxygen species induced by H5N1 and in turn, inhibits activation of p38, JNK, and NFkB in lung cells and inhibit H5N1 replication and pro-inflammatory gene expression induced by H5N1 (Michaelis et al., 2011). The correlation between doses of glycyrrhizin and licorice extract and antioxidant capacity is inconsistent. Khattab et al. (2018) suggested that the antioxidant of licorice extract at the lowest dose is more effective than the larger doses in murine model of bronchial asthma
These anti-inflammatory and antioxidant data of glycyrrhizin and licorice extract support the use of them at the early stage of infection by COVID-19 to prevent the progress of inflammation and induction of a state of hyperinflammation or cytokine storm syndrome.
The potential therapeutic effect of glycyrrhizin and licorice extract against COVID-19 induced acute lung injury(ALI)
Many active constituents of licorice root have been shown to have a therapeutic effect against acute lung injury which is the main cause of the rapid onset of acute respiratory failure (Table 3 ). They can be used as a novel therapeutic strategy for pulmonary inflammation (Lee et al., 2019; Shen et al., 2020). Glycyrrhizin inhibited LPS-induced ALI, the changes in lung histopathology, alveolar bleeding, and neutrophil infiltration (Ni et al., 2011). The anti-oxidative and anti-inflammatory effects of isoliquiritigenin in the lung made it able to protect the lung from injury induced by LPS. It significantly inhibited lung histopathological changes, lung inflammation, and lung injury by activating PPAR-γ and inhibiting NF-κB activation (Liu et al., 2017; Zhang et al., 2018b).
Table 3.
Protective effect of glycyrrhizin and licorice extract against acute lung injury that may occur due to COVID-19 or others in articles published from 2011 to 2020.
| Active substance used | Method of research | Major finding | Mechanism of actions | References |
|---|---|---|---|---|
| Glycyrrhizin Isoliquiritigenin (ISL), a flavonoid isolated from licorice. Isoliquiritigenin ILG Glycyrrhizin intraperitoneally administered Glycyrrhizin Glycyrrhizin Glycyrrhizic acid (GA) Ethanolic extract of Licorice( G. glabra ) Glycyrrhizic acid (GL), aqueous extract . Glycyrrhetinic acid (GA) is a major bioactive hydrolysis product of GL. Glycyrrhizin(GL) Glycyrrhizic acid (GA), and tilianin (TN). Approved SKBHT, berbal combination contain licorice. |
Lipopolysaccharide (LPS)-induced acute lung injury (ALI) in mice. LPS in RAW 264.7 cells. & mice. Animal model of LPS-induced ALI. LPS-induced ALI in a mouse model. Mice + LPS LPS induce ALI in a mouse model. LPS-induced ALI in mice. ALI murine models were established by intratracheal instillation of bacterial LPS. Murine hepatitis virus (MHV) infection model. Human alveolar epithelial cell line A549 and normal human bronchial epithelial cell line BEAS-2B Mouse model of Chronic obstructive pulmonary disease COPD. Mouse model of ALI |
GL potently protected against LPS-induced ALI. ISL significantly alleviated ALI in mice, inhibited reactive oxygen species (ROS) generation and cytotoxicity induced by t-BHP and pro-inflammatory enzymes production. ILG significantly inhibited LPS-induced lung histopathological changes. ILG inhibited the inflammatory of LPS-induced lung injury. GL can be used as a novel therapeutic strategy for pulmonary inflammation. Against ALI and acute respiratory distress syndrome (ARDS). GL reduce lipopolysaccharide-induced acute lung injury. Significantly alleviated lung injury in LPS-induced ALI mice. GA significantly attenuated lung injury and decreased the production of inflammatory factors TNF-α, IL-1β, and high-mobility group box 1 HMGB1. Has protective effect on ALI in mice, inhibited pro-inflammatory mRNA expression levels, and the tissue injury. GA has strong hepatoprotective activity agent in hepatic infectious disease. GL suppress Epithelial-mesenchymal transition (EMT) that plays an important role in fibrosis, chronic inflammation of lung. The histolopathological lung injury was alleviated by combinational more effectively inhibited neutrophilic airway inflammation . SKBHT suppresses inflammation in the lung. |
The protective effects of GL may attribute partly to the suppression of COX-2 and iNOS expression. ISL activated AMPK/Nrf2/ARE signaling (survival pathway that alleviates oxidative injury) and inhibited LPS-induced NLRP3 and NF-κB activation in the lung(pro-inflammatory pathways that cause damage to cells). By activating PPAR-γ and inhibiting NF-κB activation. GL inhibited proinflammatory cytokines playing a key role in the initial phase of inflammatory response, through inhibition of the TLR-4/NF-κB signal pathway Glycyrrhizin inactivates toll-like receptor (TLR) signaling pathway and NF-κB pathway-related or it inhibiting TLR2 which essential for TLR activation. GL inhibited proinflammatory cytokines playing a key role in the initial phase of inflammatory response, through inhibition of the TLR-4/NF-κB signal pathway GA inhibited the production of inflammatory factors and regulates the PI3K/AKT/mTOR pathway related autophagy. Antiinflammatory and antioxidative stress. Not only by suppressing HMGB1 release and blocking HMGB1 cytokine activity, but also via block an underlying viral-induced HMGB1-TLR4 immunological regulation axis that occurs during the cytokine storm. Gl act by block Smad2/3 signaling pathway through inhibiting high-mobility group box1 (HMGB1). By regulating the expression of inflammatory cytokines and CXCL-2 by blocking the IL-17/STAT3 pathway. SKBHT suppressed NF-κB activity and activating Nrf2 and TNFAIP3. |
Ni et al., 2011 Liu et al., 2017 Zhang et al., 2018a Lee et al., 2019 Kong et al., 2019 Lee et al., 2019 Qu et al., 2019 Shen et al., 2020 Shi et al., 2020 Gui et al., 2020. Kim et al., 2020a Kim et al., 2020a |
The protective effect of ethanol extract of licorice against LPS-induced acute lung injury was confirmed in mice. It reduced the level of pro-inflammatory cytokines tumor necrosis factor (TNF)-α in bronchoalveolar lavage, IL-1β, and NO in lung tissues. Furthermore, it decreased the expression of iNOS and COX-2 (Ni et al., 2011). Recent mechanism of inhibition of viral-induced inflammation and injury by licorice extract and its constituents suggested that glycyrrhetinic acid, a major bioactive hydrolysis product of glycyrrhizic acid prevents viral inflammatory injury through blocking HMGB1 cytokine activity and underlying viral-induced HMGB1-TLR4 immunological regulation axis that develops during the cytokine storm (Shi et al., 2020).
The mechanism of the protective effect of glycyrrhizin maybe also through the inactivation of the toll-like receptor (TLR) signaling pathway by suppressing TLR2 that is needed for overexpression-activated TLR signaling pathway to promote acute lung injury (Lee et al., 2019; Kong et al., 2019). Furthermore, glycyrrhizic acid can alleviate acute lung injury induced by LPS through modulating autophagy via the PI3K/AKT/mTOR pathway (Qu et al., 2019). Additionally, glycyrrhizin suppresses the epithelial-mesenchymal transition by decreasing high-mobility group box1 via the TGF-β1/Smad2/3 pathway in lung epithelial cells thereby it protects the lung from acute inflammation, fibrosis, and chronic inflammation (Gui et al., 2020).
Chinese traditional medicine as Sikyungbanha-Tang that contains many herbs in addition to licorice extract popularly is common to patients with respiratory inflammatory symptoms in China and Korea. It increases the anti-inflammatory factor through increasing the expression of genes regulated by Nrf2,therefore, it suppresses ALI in mice (Kim et al., 2020a). A herbal combinational mixture of Glycyrrhiza glabra and, Agastache rugosa extract was found to have a high inhibitory effect on neutrophilic airway inflammation by antagonizing the IL-17/STAT3 pathway. Therefore, this mixture may be used as a therapeutic agent to treat COPD(chronic obstructive pulmonary disease) (Kim et al., 2020b).
Licorice extract or its constituent popularly prescribed to patients with bronchial asthma. Glycyrrhizic acid and its dervitives were shown to have an anti-asthmatic effect in allergic asthma through the inhibition of inflammatory mediators (Fouladi et al., 2019). In a recent study randomized controlled trial, licorice extract, at a dose equivalent to 200 mg of glycyrrhizin/daily for 4 weeks, improved the pulmonary function without significant change in systolic and diastolic blood pressure or reduction in serum potassium level (Sadek et al., 2020). Licorice extract also,prevents the production of the cytokines and free radicals induced by ova albumin in a murine model of bronchial asthma (Khattab et al., 2018).
Lung injury in COVID-19 is the bad progress of the infection that leads to serious complications. Licorice in many studies was efficient to combat the developing of acute lung injury, therefore, it may be a good candidate for the prevention of acute lung injury associated with COVID-19. Moreover, it has been shown that glycyrrhizic acid and dervitives decrease mucus production by reducing MUC5AC mRNA expression in vitro and in vivo (Nishimoto et al., 2010).
The potential therapeutic effect of glycyrrhizin and licorice extract against COVID-19 induced cardiovascular disorders
Many studies demonstrated the antithrombotic effect of some ingredients of licorice extract. Isoliquiritigenin inhibits the aggregation of platelets in vitro and in vivo. The antiplatelet effect of isoliquiritigenin in vitro was similar to that of aspirin. It showed an inhibitory effect on aldose reductase in vivo with a marked inhibitory effect on platelet aggregation (Tawata et al., 1992). Glycyrrhizin was observed to have an antithrombotic effect in two models of inducing thrombosis in rats (Mendes-Silva et al., 2003). Glycyrrhizin administration preoperative could prevent venous thrombosis during the initial phase of thrombus formation by inhibition of neutrophil adhesion to venous endothelium (Nakata et al., 2008; Nakata and Kira, 2016). In a recent report, Shin and his colleagues observed that licorice root at large doses could induce intracranial hemorrhagic stroke and cerebral microbleeds due to direct inhibitors of blood coagulation factor Xa as well as of thrombin by glycyrrhizin and glycyrrhetinic acid (Shin et al., 2019).
The cardiac protective effect of licorice and its constituents were documented by several studies. Glycyrrhizic acid exhibited cardioprotective effects through the reduction of inflammation and oxidative status and modulating regulating NF-κB or Nrf2 signaling pathway (Haleagrahara et al., 2011; Xu et al., 2018b). Glycyrrhizic acid protects the myocardia from isoproterenol (ISO)-induced myocardial ischemia injury in rats through inhibition of calcium influx via L-type calcium channels (Li et al., 2019). However, Upadhyay and his colleagues suggested that the antioxidant property of licorice extract play a crucial role in the cardioprotective effect and preserving the cardiomyocytes health by licorice extract against doxorubicin-induced cardiotoxicity (Upadhyay et al., 2020). The inhibiting of oxidative stress and regulating Ca2+ homeostasis by L-Type Calcium Channels are responsible for the cardioprotective activity of monoammonium glycyrrhizinate injection against ISO-induced myocardial ischemia (Zhao et al., 2020).
It is clear that licorice extract and glycyrrhizin can protect COVID −19 patients from thrombus formation, microthrombus, severe hypercoagulability, and myocardial ischemia injury induced by SARS-CoV-2
Adverse reactions and contraindications of glycyrrhizin and licorice extract
Administration of high doses of licorice extract or its constituents as glycyrrhizin inhibits the 11-β-hydrogenase type II enzyme (11β-HSD2) that oxidizing cortisol to cortisone and causes pseudohyperaldosteronism. Also, Continuous inhibition of 11β-HSD2 due to excess licorice or its constituent's consumption will cause a state of hypernatremia, hypokalemia, and increased fluid volume due to water retention (Nazari et al., 2017; Deutch et al., 2019). However, The LD50 of glycyrrhizin in mice after oral administration was documented to be 14.2–18.0 g/kg (Vispute and Khopade, 2011). This figure of LD50 confirms the high safety margin of licorice. The European Union suggested a 100 mg/day as the maximum limit for consumption of glycyrrhizin (equal 60–70 g licorice) (Murphy et al., 2009). The daily doses of licorice root needed for treatment of ulcer and gastritis have been suggested to be a range between 1 and 15 g. However, higher doses for long periods may enhance the risk of hypokalemia and hypertension (Al-Snafi, 2018; Batiha et al., 2020). Moreover, Isbrucker, and Burdock (2006) proposed that the acceptable safe daily consumption of glycyrrhizin is 0.015–0.229 mg/kg body weight/day. People with kidney impairment, hypertension, and heart failure are more sensitive to the side effects of licorice and glycyrrhizin, therefore high doses and chronic use of licorice extract or glycyrrhizin are contraindicated in pregnancy, patients with hypertension, heart failure and kidney impairment, Moreover, administration of oral contraceptives, hydrocortisone, and prednisolone are contraindicated in patients used large dose of glycyrrhizin (Vispute and Khopade, 2011).
We proposed the use of a small doses of licorice extract contain 10–50 mg glycyrrhizin as a daily prophylactic dose against COVID-19. Also, a large doses of licorice extract contain 50–100 mg glycyrrhizin can be used three daily during the initial phase of disease to prevent the progress of disease and eradicate the virus. This recommendation based on FDA statement of GRAS about the safety of the chronic use of glycyrrhizin(Cosmetic Ingredient Review Expert Panel 2007). However, many investigations recommended higher doses of glycyrrhizin for inhibiting virus replication(Chen et al., 2004). In a recent report, a patient suffered from severe COVID-19 recovered after treatment with 150 mg of dimmonium glycyrrhizinate three times daily (Ding et al., 2020). Also, there are clinical trials with a dose of 300 mg orally glycyrrhizin / day, were registered on the WHO website to register clinical trials, an open-label randomized trial (ChiCTR2000029768) and a case series (ChiCTR2000030490).
Conclusions and perspectives
In the current review, we conducted a comprehensive review of recent progress in studies of the beneficial therapeutic potential, safety, and mechanism of action of glycyrrhizin and licorice extract against COVID-19. We have reviewed the information published from 1979 to October 2020. These studies demonstrated that glycyrrhizin and licorice extract has broad antiviral activity against various viruses including SARS-CoV-2 by disrupting entry of virus into host cells (ACE). Such observations have documented the efficacy of glycyrrhizin and licorice against COVID-19-induced secondary bacterial infection, autoimmune aggressive response , oxidative stress,inflammation, acute lung injury, and cardiovascular disorders. Together, this review presents important data showing that glycyrrhizin and licorice extract have multiple beneficial activities in combating SARS-CoV-2 and most of the features of COVID-19 disease. More randomized clinical trials are needed to obtain a precise conclusion about our proposal.
CRediT authorship contribution statement
Adel A. Gomaa: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing. Yasmin A. Abdel-Wadood: Conceptualization, Data curation, Writing - review & editing.
Declaration of Competing Interest
All authors declare that they have no conflict of interest.
Funding
This study was not funded.
References
- Adianti M., Aoki C., Komoto M., Deng L., Shoji I., et al. Anti-hepatitis C virus compounds obtained from Glycyrrhiza uralensis and other Glycyrrhiza species. Microbiol. Immunol. 2014;58:180–187. doi: 10.1111/1348-0421.12127. PMID: 24397541; PMCID: PMC7168410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad A., Rehman M.U., Alkharfy K.M. An alternative approach to minimize the risk of coronavirus (Covid-19) and similar infections. Eur. Rev. Med. Pharmacol. Sci. 2020;24:4030–4034. doi: 10.26355/eurrev_202004_20873. [DOI] [PubMed] [Google Scholar]
- Aipire A., Li J., Yuan P., He J., Hu Y., Liu L., Feng X., Li Y., Zhang F., Yang J., Li J. Glycyrrhiza uralensis water extract enhances dendritic cell maturation and antitumor efficacy of HPV dendritic cell-based vaccine. Sci. Rep. 2017;7:43796. doi: 10.1038/srep43796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aipire A., Mahabati M., Cai S., et al. The immunostimulatory activity of polysaccharides from Glycyrrhiza uralensis. PeerJ. 2020;8:e8294. doi: 10.7717/peerj.8294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aipire A., Yuan P., Aimaier A., et al. Preparation, characterization, and immuno-enhancing activity of polysaccharides from Glycyrrhiza uralensis. Biomolecules. 2020;10:159. doi: 10.3390/biom10010159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajagannanavar S.L., Battur H., Shamarao S., Sivakumar V., Patil P.U., Shanavas P. Effect of aqueous and alcoholic licorice (Glycyrrhiza glabra) root extract against streptococcus mutans and lactobacillus acidophilus in comparison to chlorhexidine: an in vitro study. J. Int. Oral. Health. 2014;6:29–34. [PMC free article] [PubMed] [Google Scholar]
- Al-Snafi A.E. Glycyrrhiza glabra: a phytochemical and pharmacological review. J. Pharm. 2018;8:1–17. doi: 10.1002/ptr.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alwan A., Nesrullah Z., Faraj E. Study the effect of ethanolic extract of Glycyrrhiza glabra on pathogenic bacteria. Int. J. Curr. Microbiol. App. Sci. 2015;4(5):473–484. [Google Scholar]
- Ang L., Lee H.W., Choi J.Y., Zhang J., Soo. Lee M. Herbal medicine and pattern identification for treating COVID-19: a rapid review of guidelines. Integr. Med. Res. 2020;9 doi: 10.1016/j.imr.2020.100407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashfaq U.A., Masoud M.S., Nawaz Z., et al. glycyrrhizin as antiviral agent against hepatitis C virus. J. Transl. Med. 2017;9:112. doi: 10.1186/1479-5876-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayeka P.A., Bian Y., Githaiga P.M., Zhao Y. The immunomodulatory activities of licorice polysaccharides (Glycyrrhiza uralensis Fisch.) in CT 26 tumor-bearing mice. BMC Complement. Altern. Med. 2017;17:536. doi: 10.1186/s12906-017-2030-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayeka P.A., Bian Y., Mwitari P.G., et al. Immunomodulatory and anticancer potential of Gan cao (Glycyrrhiza uralensis Fisch.) polysaccharides by CT-26 colon carcinoma cell growth inhibition and cytokine IL-7 upregulation in vitro. BMC Complement. Altern. Med. 2016;16:206. doi: 10.1186/s12906-016-1171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly C., Vergoten G. Glycyrrhizin: an alternative drug for the treatment of COVID-19 infection and the associated respiratory syndrome? Pharmacol. Ther. 2020;214 doi: 10.1016/j.pharmthera.2020.107618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltina L.A., Zarubaev V.V., Baltina L.A., Orshanskaya I.A., Fairushina A.I., Kiselev O.I., Yunusov M.S. Glycyrrhizic acid derivatives as influenza A/H1N1 virus inhibitors. Bioorg. Med. Chem. Lett. 2015;25 doi: 10.1016/j.bmcl.2015.02.074. 1742e1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltina L.A., Kondratenko R.M., Baltina J.r., Plyasunova O.A., Pokrovskyi A.G., Tolstikov G.A. Prospects for the creation of new antiviral drugs based on Glycyrrhizic acid and its derivatives. Pharm. Chem. J. 2009;43:539–548. doi: 10.1007/s11094-010-0348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltina L.A., Tasi Y.T., Huang S.H., Lai H.C., Baltina L.A., Petrova S.F., Yunusov M.S., Lin C.W. Glycyrrhizic acid derivatives as Dengue virus inhibitors. Bioorg. Med. Chem. Lett. 2019;29(20) doi: 10.1016/j.bmcl.2019.126645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batiha G., Beshbishy A.M., El-Mleeh A., Abdel-Daim M.M., Prasad Devkota H. Traditional uses, bioactive chemical constituents, and pharmacological and toxicological activities of Glycyrrhiza glabra L. (Fabaceae) Biomolecules. 2020;10:352. doi: 10.3390/biom10030352. Published 2020 Feb 25. doi:10.3390/biom10030352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhadoria N., Gunwal M.K., Suryawanshi H., Sonarkar S.S. Antiadherence and antimicrobial property of herbal extracts (Glycyrrhiza glabra and Terminalia chebula) on Streptococcus mutans: an in vitro experimental study. J. Oral. Maxillofac. Pathol. 2019;23:73–77. doi: 10.4103/jomfp.JOMFP_103_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Chan K.H., Jiang Y., Kao R.Y., Lu H.T., Fan K.W., et al. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J. Clin. Virol. 2004;31:69–75. doi: 10.1016/j.jcv.2004.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Li W.C., Gu X.L. Optimized extraction, preliminary characterization, and in vitro antioxidant activity of polysaccharides from Glycyrrhiza uralensis fisch. Med. Sci. Monit. 2017;23:1783–1791. doi: 10.12659/MSM.900471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Chen H., Wang W., et al. Glycyrrhetic acid, but not glycyrrhizic acid, strengthened entecavir activity by promoting its subcellular distribution in the liver via efflux inhibition. Eur. J. Pharm. Sci. 2017;106:313–327. doi: 10.1016/j.ejps.2017.06.015. [DOI] [PubMed] [Google Scholar]
- Chen Y., Agnello M., Dinis M., et al. Lollipop containing Glycyrrhiza uralensis extract reduces Streptococcus mutans colonization and maintains oral microbial diversity in Chinese preschool children. PLoS One. 2019;14 doi: 10.1371/journal.pone.0221756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi J.H., Seo G.S., Cheon J.H., Lee S.H. Isoliquiritigenin inhibits TNF-alpha-induced re¬lease of high-mobility group box 1 through acti¬vation of HDAC in human intestinal epithelial HT-29 cells. Eur. J. Pharmacol. 2017;796:101–109. doi: 10.1016/j.ejphar.2016.12.026. [DOI] [PubMed] [Google Scholar]
- Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H.W. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet N. Am. Ed. 2003;361:2045–2046. doi: 10.1016/S0140-6736(03)13615-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosmetic Ingredient Review Expert Panel Final report on the safety assessment of glycyrrhetinic acid, potassium glycyrrhetinate, disodium succinoyl glycyrrhetinate, glyceryl glycyrrhetinate, glycyrrhetinyl stearate, stearyl glycyrrhetinate, glycyrrhizic acid, ammonium glycyrrhizate, dipotassium glycyrrhizate, disodium glycyrrhizate, trisodium glycyrrhizate, methyl glycyrrhizate, and potassium glycyrrhizinate. Int. J. Toxicol. 2007;26(Suppl 2):79–112. doi: 10.1080/10915810701351228. PMID: 17613133. [DOI] [PubMed] [Google Scholar]
- Dao T.T., Nguyen P.H., Lee H.S., Kim E., Park J., Lim S.I., Oh W.K. Chalcones as novel influenza A (H1N1) neuraminidase inhibitors from Glycyrrhiza inflata. Bioorg. Med. Chem. Lett. 2011;21:294–298. doi: 10.1016/j.bmcl.2010.11.016. [DOI] [PubMed] [Google Scholar]
- de Breij A., Karnaoukh T.G., Schrumpf J., et al. The licorice pentacyclic triterpenoid component 18β-glycyrrhetinic acid enhances the activity of antibiotics against strains of methicillin-resistant Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis. 2016;35:555–562. doi: 10.1007/s10096-015-2570-z. [DOI] [PubMed] [Google Scholar]
- Deutch M.R., Grimm D., Wehland M., Infanger M., Krüger M. Bioactive candy: effects of licorice on the cardiovascular system. Foods. 2019;8:495. doi: 10.3390/foods8100495. Published 2019 Oct 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding H., Deng W., Ding L., Ye X., Yin S., Huang W. Glycyrrhetinic acid and its derivatives as potential alternative medicine to relieve symptoms in nonhospitalized COVID-19 patients. J. Med. Virol. 2020;27 doi: 10.1002/jmv.26064. 10. 1002/jmv.26064Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan E., Wang D., Fang L., et al. Suppression of porcine reproductive and respiratory syndrome virus proliferation by glycyrrhizin. Antiviral Res. 2015;120:122–125. doi: 10.1016/j.antiviral.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Editorial Redeploying plant defences. Nat. Plants. 2020;6:177. doi: 10.1038/s41477-020-0628. .doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekanayaka S.A., McClellan S.A., Barrett R.P., Hazlett L.D. Topical glycyrrhizin is therapeutic for pseudomonas aeruginosa keratitis. J. Ocul. Pharmacol. Ther. 2018;34:239–249. doi: 10.1089/jop.2017.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekanayaka S.A., McClellan S.A., Barrett R.P., Kharotia S., Hazlett L.D. Glycyrrhizin reduces HMGB1 and bacterial load in Pseudomonas aeruginosa keratitis. Invest. Ophthalmol. Vis. Sci. 2016;57:5799–5809. doi: 10.1167/iovs.16-20103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y.C., Wang K.C., Chiang L.C., Shieh D.E., Yen M.H., Chang S.J. Water extract of licorice had anti-viral activity against human respiratory syncytial virus in human respiratory tract cell lines. J. Ethnopharmacol. 2013;148:466–473. doi: 10.1016/j.jep.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore C., Eisenhut M., Krausse R., et al. Antiviral effects of Glycyrrhiza species. Phytother. Res. 2008;22:141–148. doi: 10.1002/ptr.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouladi S., Masjedi M., Ganjalikhani M., Eskandari N. The review of in vitro and in vivo studies over the Glycyrrhizic acid as natural remedy option for treatment of allergic asthma. Iran. J. Allergy Asthma Immunol. 2019;18:1–11. [PubMed] [Google Scholar]
- Frattaruolo L., Carullo G., Brindisi M., et al. Antioxidant and anti-inflammatory activities of flavanones from Glycyrrhiza glabra L. (licorice) leaf phytocomplexes: identification of licoflavanone as a modulator of NF-kB/MAPK pathway. Antioxidants (Basel). 2019;8:186. doi: 10.3390/antiox8060186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuchi K., Okudaira N., Adachi K., et al. Antiviral and antitumor activity of licorice root extracts. In Vivo. 2016;30:777–785. doi: 10.21873/invivo.10994. [DOI] [PubMed] [Google Scholar]
- Gao R., Zhang Y., Kang Y., et al. Glycyrrhizin inhibits PEDV infection and proinflammatory cytokine secretion via the HMGB1/TLR4-MAPK p38 pathway. Int. J. Mol. Sci. 2020;21:2961. doi: 10.3390/ijms21082961. Published 2020 Apr 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautret P., Lagier J.C., Parola P., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label nonrandomized clinical trial. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. 105949. [PMID: 32205204] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Godbole E., Tyagi S., Kulkarni P., Singla S., Mali S., Helge S. Efficacy of liquorice and propolis extract used as cavity cleaning agents against streptococcus mutans in deciduous molars using confocal microscopy: an in vitro study. Int. J. Clin. Pediatr. Dent. 2019;12:194–200. doi: 10.5005/jp-journals-10005-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui Y., Sun J., You W., Wei Y., Tian H., Jiang S. Glycyrrhizin suppresses epithelial-mesenchymal transition by inhibiting high-mobility group box1 via the TGF-β1/Smad2/3 pathway in lung epithelial cells. PeerJ. 2020;8:8514. doi: 10.7717/peerj.8514. Published 2020 Feb 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo A., He D., Xu H., et al. Promotion of regulatory T cell induction by immunomodulatory herbal medicine licorice and its two constituents. Sci. Rep. 2015;5:14046. doi: 10.1038/srep14046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haleagrahara N., Varkkey J., Chakravarthi S. Cardioprotective effects of Glycyrrhizic acid against isoproterenol-induced myocardial ischemia in rats. Int. J. Mol. Sci. 2011;12:7100–7113. doi: 10.3390/ijms12107100. 10. 3390/ijms12107100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy M.E., Hendricks J.M., Paulson J.M., Faunce N.R. 18β-glycyrrhetinic acid inhibits rotavirus replication in culture. Virol. J. 2012;9:96. doi: 10.1186/1743-422X-9-96. Published 2012 May 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatano T., Kusuda M., Inada K., et al. Effects of tannins and related polyphenols on methicillin-resistant Staphylococcus aureus. Phytochemistry. 2005;66:2047–2055. doi: 10.1016/j.phytochem.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Hazlett L.D., Ekanayaka S.A., McClellan S.A., Francis R. Glycyrrhizin use for multi-drug resistant Pseudomonas aeruginosa: in vitro and in vivo studies. Invest. Ophthalmol. Vis. Sci. 2019;60:2978–2989. doi: 10.1167/iovs.19-27200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendaus M.A., Jomha F.A., Alhammadi A.H. Virus-induced secondary bacterial infection: a concise review. Ther. Clin. Risk Manag. 2015;11:1265–1271. doi: 10.2147/TCRM.S87789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks J.M., Hoffman C., Pascual D.W., Hardy M.E. 18β-glycyrrhetinic acid delivered orally induces isolated lymphoid follicle maturation at the intestinal mucosa and attenuates rotavirus shedding. PLoS One. 2012;7:e49491. doi: 10.1371/journal.pone.0049491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks J.M., DC, Hardy M.E. Differential induction of isolated lymphoid follicles in the gut by 18β-glycyrrhetinic acid. PLoS One. 2014;9 doi: 10.1371/journal.pone.0100878. Published 2014 Jul 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T., Murakami M. COVID-19: a new virus, but a familiar receptor and cytokine release syndrome. Immunity. 2020;19(52):731–733. doi: 10.1016/j.immuni.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoever G., Baltina L., Michaelis M., et al. Antiviral activity of glycyrrhizic acid derivatives against SARS-coronavirus. J. Med. Chem. 2005;48:1256–1259. doi: 10.1021/jm0493008. [DOI] [PubMed] [Google Scholar]
- Hsu C.H., Hwang K.C., Chao C.L., Chang S.G., Ho M.S., Chou P. Can herbal medicine assist against avian flu? Learning from the experience of using supplementary treatment with Chinese medicine on SARS or SARS-like infectious disease in 2003. J. Altern. Complement. Med. 2006;12:505–506. doi: 10.1089/acm.2006.12.505. [DOI] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain K., Iqbal Z., Abbas R., Khan M., Saleemi M.K. Immunomodulatory activity of Glycyrrhiza glabra extract against mixed Eimeria infection in chickens. Int. J. Agric. Biol. 2017;19:928–932. [Google Scholar]
- Isbrucker R.A., Burdock G.A. Risk and safety assessment on the consumption of Licorice root (Glycyrrhizasp.), its extract and powder as a food ingredient, with emphasis on the pharmacology and toxicology of glycyrrhizin. Regul. Toxicol. Pharmacol. 2006;4:167–192. doi: 10.1016/j.yrtph.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Jiang Y.X., Dai Y.Y., Pan Y.F., et al. Total flavonoids from radix Glycyrrhiza exert anti-inflammatory and antitumorigenic effects by inactivating iNOS signaling pathways. Evid. Based Complement. Altern. Med. 2018 doi: 10.1155/2018/6714282. 2018:6714282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju H.S., Li X.J., Zhao B.L., Han Z.W., Xin W.J. Effects of Glycyrrhiza Flavonoids on lipid peroxidation and active oxygen radicals. Acta Pharm. Sin. B. 1989;24:807–812. [PubMed] [Google Scholar]
- Kazia Y., Khatoona S., Kumara P., Qazib N. Antibacterial effect of ethanol and aqueous root extracts from Glycyrrhiza glabra (Licorice) against Streptococcus pyogenes isolated from throat infection. J. Ear Nose Throat Photon. 2014;105:140–147. [Google Scholar]
- Khattab H., Abdel-Dayem U., Jambi H., Abba A., Abdul-Jawad M., El-Shitany N. Licorice (Glycyrrhizza glabra) extract prevents production of Th2 cytokines and free radicals induced by ova albumin in mice. Int. J. Pharmacol. 2018;14 1072-10. [Google Scholar]
- Kikete S., Luo L., Jia B., Wang L., Ondieki G., Bian Y. Plant-derived polysaccharides activate dendritic cell-based anti-cancer immunity. Cytotechnology. 2018;70 doi: 10.1007/s10616-018-0202-z. 1097_1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.J., Seo J.Y., Suh H.J., Lim S.S., Kim J.S. Antioxidant activities of licorice-derived prenylflavonoids. Nutr. Res. Pract. 2012;6:491–498. doi: 10.4162/nrp.2012.6.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.H., Ahn S., Won R. Suppressing acute lung injury in mice is related to the activation of Nrf2 and TNFAIP3. Evid. Based Complement. Altern. Med. 2020 doi: 10.1155/2020/8125758. 2020Published 2020 Mar 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.H., Hong J.H., Yang W.K., et al. Herbal combinational medication of Glycyrrhiza glabra, agastache rugosa containing glycyrrhizic acid, tilianin inhibits neutrophilic lung inflammation by affecting CXCL2, interleukin-17/STAT3 signal pathways in a murine model of COPD. Nutrients. 2020;12:E926. doi: 10.3390/nu12040926. Published 2020 Mar 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko H.C., Wei B.L., Chiou W.F. The effect of medicinal plants used in Chinese folk medicine on RANTES secretion by virus-infected human epithelial cells. J. Ethnopharmacol. 2006;19:205–210. doi: 10.1016/j.jep.2006.03.004. Epub 2006 Mar 17. PMID: 16621378. [DOI] [PubMed] [Google Scholar]
- Kong D., Wang Z., Tian J., Liu T., Zhou H. Glycyrrhizin inactivates toll-like receptor (TLR) signaling pathway to reduce lipopolysaccharide-induced acute lung injury by inhibiting TLR2. J. Cell. Physiol. 2019;234:4597–4607. doi: 10.1002/jcp.27242. [DOI] [PubMed] [Google Scholar]
- Krishnakumar G., Gaviappa D., Guruswamy S. Anticaries efficacy of liquorice lollipop: an ex vivo study. J. Contemp. Dent. Pract. 2018;19:937–942. [PubMed] [Google Scholar]
- Kuo K.K., Chang J.S., Wang K.C., Chiang L.C. Water extract of Glycyrrhiza uralensis inhibited enterovirus 71 in a human foreskin fibroblast cell line. Am. J. Chin. Med. 2009;37:383–394. doi: 10.1142/S0192415X09006904. [DOI] [PubMed] [Google Scholar]
- Laconi S., Madeddu M.A., Pompei R. Autophagy activation and antiviral activity by a licorice triterpene. Phytother. Res. 2014;28:1890–1892. doi: 10.1002/ptr.5189. [DOI] [PubMed] [Google Scholar]
- Lee J.W., Ji Y.J., Yu M.H., et al. Antimicrobial effect and resistant regulation of Glycyrrhiza uralensis on methicillin-resistant Staphylococcus aureus. Nat. Prod. Res. 2009;23:101–111. doi: 10.1080/14786410801886757. [DOI] [PubMed] [Google Scholar]
- Lee S.A., Lee S.H., Kim J.Y., Lee W.S. Effects of glycyrrhizin on lipopolysaccharide-induced acute lung injury in a mouse model. J. Thorac. Dis. 2019;11:1287–1302. doi: 10.21037/jtd.2019.04.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letko M., Munster V. Functional assessment of cell entry and receptor usage for lineage B β-coronaviruses, including 2019-nCoV. bioRxiv. 2020 doi: 10.1101/2020.01.22.915660. 2020.2001.2022. 915660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Wen Z., Xue Y., et al. Cardioprotective effects of glycyrrhizic acid involve inhibition of calcium influx via L-type calcium channels and myocardial contraction in rats [published online ahead of print, 2019 Dec 6] Naunyn Schmiedebergs Arch. Pharmacol. 2019 doi: 10.1007/s00210-019-01767-3. doi:10.1007/s00210-019-01767-3. [DOI] [PubMed] [Google Scholar]
- Li T., Lu H., Zhang W. Clinical observation and management of COVID-19 patients. Emerg. Microbes Infect. 2020;9:687–690. doi: 10.1080/22221751.2020.1741327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., He X., Liu B., et al. Maturation of murine bone marrow-derived dendritic cells induced by radix Glycyrrhizae polysaccharide. Molecules. 2012;17:6557–6568. doi: 10.3390/molecules17066557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.L., Zhou A.G., Zhang L., Chen W.J. Antioxidant status and immune activity of glycyrrhizin in allergic rhinitis mice. Int. J. Mol. Sci. 2011;12:905–916. doi: 10.3390/ijms12020905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Asada Y., Yoshikawa T. Flavonoid constituents from Glycyrrhiza glabra hairy root cultures. Phytochemistry. 2000;55:447–456. doi: 10.1016/s0031-9422(00)00337-x. [DOI] [PubMed] [Google Scholar]
- Lian K.X., Zhu X.Q., Chen J., Liu G., Gu X.L. Selenylation modification: enhancement of the antioxidant activity of a Glycyrrhiza uralensis polysaccharide. Glycoconj. J. 2018;35:243–253. doi: 10.1007/s10719-018-9817-8. [DOI] [PubMed] [Google Scholar]
- Liang S., Li M., Yu X., et al. Synthesis and structure-activity relationship studies of water-soluble β-cyclodextrin-glycyrrhetinic acid conjugates as potential anti-influenza virus agents. Eur. J. Med. Chem. 2019;166:328–338. doi: 10.1016/j.ejmech.2019.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y., Tan R.Z., Li J.C., et al. Isoliquiritigenin attenuates UUO-induced renal inflammation and fibrosis by inhibiting mincle/Syk/NF-Kappa B signaling pathway. Drug Des. Dev. Ther. 2020;14:1455–1468. doi: 10.2147/DDDT.S243420. Published 2020 Apr 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Jiang Y., Steinle J.J. Epac1 and Glycyrrhizin both inhibit HMGB1 levels to reduce diabetes-induced neuronal and vascular damage in the mouse retina. J. Clin. Med. 2019;8:772. doi: 10.3390/jcm8060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Jiang Y., Steinle J.J. Glycyrrhizin protects the diabetic retina against permeability, neuronal, and vascular damage through anti-inflammatory mechanisms. J. Clin. Med. 2019;8:957. doi: 10.3390/jcm8070957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Lv H., Wen Z., Ci X., Peng L. Isoliquiritigenin activates nuclear factor erythroid-2 related factor 2 to suppress the NOD-like receptor protein 3 inflammasome and inhibits the NF-κB pathway in macrophages and in acute lung injury. Front. Immunol. 2017;8:1518. doi: 10.3389/fimmu.2017.01518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Zhu Q., Zhang M., et al. Isoliquiritigenin ameliorates acute pancreatitis in mice via inhibition of oxidative stress and modulation of the Nrf2/HO-1 pathway. Oxid. Med. Cell. Longev. 2018 doi: 10.1155/2018/7161592. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long D.R., Mead J., Hendricks J.M., Hardy M.E., Voyich J.M. 18β-Glycyrrhetinic acid inhibits methicillin-resistant Staphylococcus aureus survival and attenuates virulence gene expression. Antimicrob. Agents Chemother. 2013;57:241–247. doi: 10.1128/AAC.01023-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Liu D., Li J. Pharmacologic perspective: glycyrrhizin may be an efficacious therapeutic agent for COVID-19 [published online ahead of print, 2020 Apr 23] Int. J. Antimicrob. Agents. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maione F., Minosi P., Di Giannuario A., et al. Long-lasting anti-inflammatory and antinociceptive effects of acute ammonium glycyrrhizinate administration: pharmacological, biochemical, and docking studies. Molecules. 2019;24:2453. doi: 10.3390/molecules24132453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksoud H.A., Abdel Magid A., Mostafa Y.M., Elharrif M., Mohamed S.R. Ameliorative effect of liquorice extract versus silymarin in experimentally induced chronic hepatitis: a biochemical and genetical study. Clin. Nutr. Exp. 2019;23:69–79. doi: 10.1016/j.yclnex.2018.10.005. DO - [DOI] [Google Scholar]
- Malavolta M., Giacconi R., Brunetti D., Provinciali M., Maggi F. Exploring the relevance of senotherapeutics for the current SARS-CoV-2 emergency and similar future global health threats. Cells. 2020;9:909. doi: 10.3390/cells9040909. Published 2020 Apr 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maria Pia G.D., Sara F., Mario F., Lorenza S. Biological effects of licochalcones. Mini Rev. Med. Chem. 2019;19:647–656. doi: 10.2174/1389557518666180601095420. PMID: 30049263. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Matsuura T., Aoyagi H., et al. Antiviral activity of glycyrrhizin against hepatitis C virus in vitro. PLoS One. 2013;8:e68992. doi: 10.1371/journal.pone.0068992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumder P.M., Pattnayak S., Parvani H., Sasmal D., Rathinavelusamy P. Evaluation of immunomodulatory activity of Glycyrhiza glabra L roots in combination with zing. Asian Pac. J. Trop. Biomed. 2012;2:144. S15-S20, 2012. [Google Scholar]
- Mendes-Silva W., Assafim M., Ruta B., Monteiro R.Q., Guimarães J.A., Zingali R.B. Antithrombotic effect of glycyrrhizin, a plant-derived thrombin inhibitor. Thromb. Res. 2003;112:93–98. doi: 10.1016/j.thromres.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Michaelis M., Geiler J., Naczk P., Sithisarn P., Leutz A., Doerr H.W., et al. Glycyrrhizin exerts antioxidative effects in H5N1 influenza A virus-infected cells and inhibits virus replication and pro-inflammatory gene expression. PLoS One. 2011;6:e19705. doi: 10.1371/journal.pone.0019705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollica L., De Marchis F., Spitaleri A., Dallacosta C., Pennacchini D., Zamai M., Agresti A., Trisciuoglio L., Musco G., Bianchi M.E. Glycyrrhizin binds to high-mobility group box 1 protein and inhibits its cytokine activities. Chem. Biol. 2007;14:431–441. doi: 10.1016/j.chembiol.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Murck H. Symptomatic protective action of glycyrrhizin (Licorice) in COVID-19 infection? Front. Immunol. 2020;11:1239. doi: 10.3389/fimmu.2020.01239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy S.C., Agger S., Rainey P.M. Too much of a good thing: a woman with hypertension and hypokalemia. Clin. Chem. 2009;55:2093–2096. doi: 10.1373/clinchem.2009.127506. [DOI] [PubMed] [Google Scholar]
- Nakata N., Kira Y., Yabunaka Y., Takaoka K. Prevention of venous thrombosis by preoperative glycyrrhizin infusion in a rat model. J. Orthop. Sci. 2008;13:456–462. doi: 10.1007/s00776-008-1259. [DOI] [PubMed] [Google Scholar]
- Nakata N., Kira Y. Effects of preoperative glycyrrhizin infusion for the prevention of venous thrombosis on the tissue expression of antithrombin in a rat model. Ann. Vasc. Dis. 2016;9:95–101. doi: 10.3400/avd.oa.16-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazari S., Rameshrad M., Hosseinzadeh H. Toxicological effects of Glycyrrhiza glabra (Licorice): a review. Phytother. Res. 2017;31:1635–1650. doi: 10.1002/ptr.5893. [DOI] [PubMed] [Google Scholar]
- Ni Y.F., Kuai J.K., Lu Z.F., et al. Glycyrrhizin treatment is associated with attenuation of lipopolysaccharide-induced acute lung injury by inhibiting cyclooxygenase-2 and inducible nitric oxide synthase expression. J. Surg. Res. 2011;165:e29–e35. doi: 10.1016/j.jss.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Nishimoto Y., Hisatsune A., Katsuki H., Miyata T., Yokomizo K., Isohama Y. Glycyrrhizin attenuates mucus production by inhibition of MUC5AC mRNA expression in vivo and in vitro. J. Pharmacol. Sci. 2010;113:76–83. doi: 10.1254/jphs.09344fp. [DOI] [PubMed] [Google Scholar]
- Ohno H., Miyoshi S., Araho D., Kanamoato T., Terakubo S., et al. Efficient utilization of licorice root by alkaline extraction. In Vivo (Brooklyn) 2014;28:785–794. [PubMed] [Google Scholar]
- Oyama K., Kawada-Matsuo M., Oogai Y., Hayashi T., Nakamura N., Komatsuzawa H. Antibacterial effects of Glycyrrhetinic acid and its derivatives on staphylococcus aureus. PLoS One. 2016;11 doi: 10.1371/journal.pone.0165831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorino G., Cornara L., Soares S., Rodrigues F., Oliveira M.P. Liquorice (Glycyrrhiza glabra): a phytochemical and pharmacological review. Phytother. Res. 2018;32:2323–2339. doi: 10.1002/ptr.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompei R., Flore O., Marcialis M.A., Pani A., Loddo B. Glycyrrhizic acid inhibits virus growth and inactivates virus particles. Nature. 1979;281:689–690. doi: 10.1038/281689a0. [DOI] [PubMed] [Google Scholar]
- Pompei R., Pani A., Flore O., Marcialis M.A., Loddo B. Antiviral activity of glycyrrhizic acid. Experientia. 1980;36:304. doi: 10.1007/BF01952290. [DOI] [PubMed] [Google Scholar]
- Pu J.Y., He L., Wu S.Y., Zhang P., Huang X. Anti-virus research of triterpenoids in licorice. Bing Du Xue Bao. 2013;29:673–679. [PubMed] [Google Scholar]
- Qu L., Chen C., He W., et al. Glycyrrhizic acid ameliorates LPS-induced acute lung injury by regulating autophagy through the PI3K/AKT/mTOR pathway. Am. J. Transl. Res. 2019;11:2042–2055. [PMC free article] [PubMed] [Google Scholar]
- Reigada I., Moliner C., Valero M.S., Weinkove D., Langa E., Gómez Rincón C. Antioxidant and antiaging effects of licorice on the caenorhabditis elegans model. J. Med. Food. 2020;23:72–78. doi: 10.1089/jmf.2019.0081. [DOI] [PubMed] [Google Scholar]
- Sadek E., Tawfik N., Hussein A., Abdelhakeem M. Efficacy and safety of liquorice extract in patients with bronchial asthma: a randomized controlled trial. Indian J. Public Health Res. Dev. 2020;11:585–590. doi: 10.37506/ijphrd.v11i4.5397. [DOI] [Google Scholar]
- Sato H., Goto W., Yamamura J., et al. Therapeutic basis of glycyrrhizin in chronic hepatitis B. Antivir. Res. 1996;30:171–177. doi: 10.1016/0166-3542(96)00942-4. [DOI] [PubMed] [Google Scholar]
- Sedighinia F., Safipour Afshar A., Soleimanpour S., Zarif R., Asili J., Ghazvini K. Antibacterial activity of Glycyrrhiza glabra against oral pathogens: an in vitro study. Avicenna J. Phytomed. 2012;2:118–124. [PMC free article] [PubMed] [Google Scholar]
- Shah S., Wahid F., Khan N., Farooq U., Shah J., et al. Inhibitory effects of Glycyrrhiza glabra and its major constituent glycyrrhizin on inflammation-associated corneal neovascularization. Evid. Based Complement. Altern. Med. 2018 doi: 10.1155/2018/8438101. 2018, Article ID 8438101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Han N., Chen H., Zhang M., Cai W. Evaluation of lipopolysaccharide-induced acute lung injury attenuation in mice by Glycyrrhiza glabra. Pharmacogn. Mag. 2020;16:92–98. http://www.phcog.com/text.asp?2020/16/67/92/277997 [Google Scholar]
- Shi X., Yu L., Zhang Y., Liu Z., et al. Glycyrrhetinic acid alleviates hepatic inflammation injury in viral hepatitis disease via a HMGB1-TLR4 signaling pathway. Int. Immunopharmacol. 2020;84 doi: 10.1016/j.intimp.2020.106578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H., Chung M., Rose D.Z. Licorice root associated with intracranial hemorrhagic stroke and cerebral microbleeds. Neurohospitalist. 2019;9:169–171. doi: 10.1177/1941874418805332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinada M., Azuma M., Kawai H., et al. Enhancement of interferon-gamma production in glycyrrhizin-treated human peripheral lymphocytes in response to concanavalin A and to surface antigen of hepatitis B virus. Proc. Soc. Exp. Biol. Med. 1986;181:205–210. doi: 10.3181/00379727-181-42241. [DOI] [PubMed] [Google Scholar]
- Shirazi M.H., Ranjbar R., Eshraghi S., Sadeghi G., et al. An evaluation of antibacterial activity of Glycyrrhiza glabra extract on the growth of Salmonella, Shigella and ETEC E. coli. J. Biol. Sci. 2007;7:827–829. [Google Scholar]
- Si L., Meng K., Tian Z., Sun J., Li H., et al. Triterpenoids manipulate a broad range of virus-host fusion via wrapping the HR2 domain prevalent in viral envelopes. Sci. Adv. 2018;21:eaau8408. doi: 10.1126/sciadv.aau8408. et al. PMID: 30474060; PMCID: PMC6248931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W., Si L., Ji S., Uralsaponins M.Y. antiviral triterpenoid saponins from the roots of Glycyrrhiza uralensis. J. Nat. Prod. 2014;77:1632–1643. doi: 10.1021/np500253m. [DOI] [PubMed] [Google Scholar]
- Soufy H., Yassein S., Ahmed A.R., et al. Antiviral and immune stimulant activities of glycyrrhizin against duck hepatitis virus. Afr. J. Tradit. Complement. Altern. Med. 2012;9:389–395. doi: 10.4314/ajtcam.v9i3.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava V., Yadav A., Sarkar P. Molecular docking and ADMET study of bioactive compounds of Glycyrrhiza glabra against main protease of SARS-CoV2. Mater. Today Proc. 2020 doi: 10.1016/j.matpr.2020.10.055. Epub ahead of print. PMID: 33078096; PMCID: PMC7556787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z.G., Zhao T.T., Lu N., Yang Y.A., Zhu H.L. Research progress of Glycyrrhizic acid on antiviral activity. Mini Rev. Med. Chem. 2019;19:826–832. doi: 10.2174/1389557519666190119111125. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Kikuzaki H., Fukuda S., Nakatani N. Antibacterial compounds of licorice against upper airway respiratory tract pathogens. J. Nutr. Sci. Vitaminol. 2001;47:270–273. doi: 10.3177/jnsv.47.270. (Tokyo) [DOI] [PubMed] [Google Scholar]
- Tang Y., Wang C., Wang Y., et al. Isoliquiritigenin attenuates LPS-induced AKI by suppression of inflammation involving NF-κB pathway. Am. J. Transl. Res. 2018;10:4141–4151. [PMC free article] [PubMed] [Google Scholar]
- Tawata M., Aida K., Noguchi T., et al. Anti-platelet action of isoliquiritigenin, an aldose reductase inhibitor in licorice. Eur. J. Pharmacol. 1992;212:87–92. doi: 10.1016/0014-2999(92)90076-g. [DOI] [PubMed] [Google Scholar]
- Tian M., Yan H., Row K.H. Extraction of glycyrrhizic acid and glabridin from licorice. Int. J. Mol. Sci. 2008;9:571–577. doi: 10.3390/ijms9040571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong T., Hu H., Zhou J., Deng S., Zhang X., et al. Glycyrrhizic-acid-based carbon dots with high antiviral activity by multisite inhibition mechanisms. Small. 2020;16 doi: 10.1002/smll.201906206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay S., Mantha A.K., Dhiman M. Glycyrrhiza glabra (Licorice) root extract attenuates doxorubicin-induced cardiotoxicity via alleviating oxidative stress and stabilising the cardiac health in H9c2 cardiomyocytes [published online ahead of print, 2020 Feb 24] J. Ethnopharmacol. 2020 doi: 10.1016/j.jep.2020.112690. [DOI] [PubMed] [Google Scholar]
- Vasanth M.P., Purushotham K.G., Sathish M., Vimal R.D., Venkatesh M. In-vitro anti-inflammatory activity of liquorice (Glycyrrhiza glabra) using aqueous extract. Int. J. Pharm. Sci. Res. 2020;11:657–662. doi: 10.26452/ijrps.v11i1.1872. [DOI] [Google Scholar]
- Velvizhiv S., Annapurani S. Estimation of total flavonoid, phenolic content, and free radical scavenging potential of Glycyrrhiza glabra root extract. Asian J. Pharm. Clin. Res. 2018;11(231) doi: 10.22159/ajpcr.2018.v11i4.23711. 2018 DO - [DOI] [Google Scholar]
- Visavadiya N.P., Soni B., Dalwadi N. Evaluation of antioxidant and anti-atherogenic properties of Glycyrrhiza glabra root using in vitro models. Int. J. Food Sci. Nutr. 2009;60:135e49. doi: 10.1080/09637480902877998. [DOI] [PubMed] [Google Scholar]
- Vispute S., Khopade A. Glycyrrhiza glabra Linn–Klitaka: a review. Int. J. Pharm. Bio Sci. 2011;2:42–51. [Google Scholar]
- Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS. J. Virol. 2020;94 doi: 10.1128/JVI.00127-20. e00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Liang J., Zhang J., Wang Y., Chai X. Natural chalcones in Chinese materia medica: licorice. Evid. Based Complement. Altern. Med. 2020 doi: 10.1155/2020/3821248. 3821248. Published 2020 Mar 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.L., Li Y.X., Niu Y.T., et al. Observing anti-inflammatory and anti-nociceptive activities of glycyrrhizin through regulating COX-2 and pro-inflammatory cytokines expressions in mice. Inflammation. 2015;38:2269–2278. doi: 10.1007/s10753-015-0212-3. [DOI] [PubMed] [Google Scholar]
- Wang L., Yang R., Yuan B., Liu Y., Liu C. The antiviral and antimicrobial activities of licorice, a widely-used Chinese herb. Acta Pharm. Sin. B. 2015;5:310–315. doi: 10.1016/j.apsb.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J.H., Zheng Y.F., Li C.Y., Tang Y.P., Peng G.P. Bioactive constituents of oleanane-type triterpene saponins from the roots of Glycyrrhiza glabra. J. Asian Nat. Prod. Res. 2014;16:1044–1053. doi: 10.1080/10286020.2014.960857. [DOI] [PubMed] [Google Scholar]
- Wijesundara N.M., Rupasinghe H.P.V. Herbal tea for the management of pharyngitis: inhibition of streptococcus pyogenes growth and biofilm formation by herbal infusions. Biomedicines. 2019;7:63. doi: 10.3390/biomedicines7030063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkerstorfer A., Kurz H., Bachhofner N., Szolar O.H.J. Glycyrrhizin inhibits influenza A virus uptake into the cell. Antivir. Res. 2009;83:171–178. doi: 10.1016/j.antiviral.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S.C., Yang Z.Q., Liu F., et al. Antibacterial effect and mode of action of flavonoids from licorice against methicillin-resistant staphylococcus aureus. Front. Microbiol. 2019;10:2489. doi: 10.3389/fmicb.2019.02489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S., Tian Z., Wang Y., Si L., Zhang L., Zhou D. Recent progress in the antiviral activity and mechanism study of pentacyclic triterpenoids and their derivatives. Med. Res. Rev. 2018;38:951–976. doi: 10.1002/med.21484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong D., Hu W., Ye S.T., Tan Y.S. Isoliquiritigenin alleviated the Ang II-induced hypertensive renal injury through suppressing inflammation cytokines and oxidative stress-induced apoptosis via Nrf2 and NF-κB pathways. Biochem. Biophys. Res. Commun. 2018;506:161–168. doi: 10.1016/j.bbrc.2018.09.013. [DOI] [PubMed] [Google Scholar]
- Xu C., Liang C., Sun W., Chen J., Chen X. Glycyrrhizic acid ameliorates myocardial ischemic injury by the regulation of inflammation and oxidative state. Drug Des. Dev. Ther. 2018;12:1311–1319. doi: 10.2147/DDDT.S165225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X., Li T., Chen Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral. Sci. 2020;12:8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Gong L., Wang B., et al. Glycyrrhizin attenuates Salmonella enterica serovar typhimurium infection: new insights into its protective mechanism. Front. Immunol. 2018;9:2321. doi: 10.3389/fimmu.2018.02321. Published 2018 Oct 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R., Yuan B.C., Ma Y.S., Zhou S., Liu Y. The anti-inflammatory activity of licorice, a widely used Chinese herb. Pharm. Biol. 2017;55:5–18. doi: 10.1080/13880209.2016.1225775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.L., Liu D., Bian K., Zhang D.D. Study on in vitro anti-inflammatory activity of total flavonoids from Glycyrrhizae radix Et rhizoma and its ingredients. Zhongguo Zhong Yao Za Zhi. 2013;38:99–104. [PubMed] [Google Scholar]
- Yang Y., Islam M.S., Wang J., Li Y., Chen X. Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): a review and perspective. Int. J. Biol. Sci. 2020;16:1708–1717. doi: 10.7150/ijbs.45538. Published 2020 Mar 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L., Guan E., Zhang Y., et al. Chemical profile and anti-inflammatory activity of total flavonoids from Glycyrrhiza uralensis fisch. Iran. J. Pharm. Res. 2018;17:726–734. [PMC free article] [PubMed] [Google Scholar]
- Yu M.R., Si L.L., Wang Y.F., Wu Y.M., Yu F., et al. Discovery of pentacyclic triterpenoids as potential entry inhibitors of influenza viruses. J. Med. Chem. 2014;57:10058–10071. doi: 10.1021/jm5014067. [DOI] [PubMed] [Google Scholar]
- Yu S., Zhu Y., Xu J., et al. Glycyrrhizic acid exerts inhibitory activity against the spike protein of SARS-CoV-2. Phytomedicine. 2020 doi: 10.1016/j.phymed.2020.153364. Available online 2 October 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Bao Y., Meng X., et al. Multi-pathway integrated adjustment mechanism of licorice flavonoids presenting anti-inflammatory activity. Oncol. Lett. 2019;18:4956–4963. doi: 10.3892/ol.2019.10793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C.H., Yu Y., Liang Y.Z., Chen X.Q. Purification, partial characterization and antioxidant activity of polysaccharides from Glycyrrhiza uralensis. Int. J. Biol. Macromol. 2015;79:681–686. doi: 10.1016/j.ijbiomac.2015.05.060. [DOI] [PubMed] [Google Scholar]
- Zhang L., Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J. Med. Virol. 2020;92:479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Wu Y.Q., Xie L., et al. Isoliquiritigenin protects against pancreatic injury and intestinal dysfunction after severe acute pancreatitis via Nrf2 signaling [published correction appears in Front Pharmacol. 2019 Jul 12;10:788] Front. Pharmacol. 2018;9:936. doi: 10.3389/fphar.2018.00936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Wang G., Zhou S. Protective effects of isoliquiritigenin on LPS-induced acute lung injury by activating PPAR-γ. Inflammation. 2018;41:1290–1296. doi: 10.1007/s10753-018-0777-8. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wang C., Yang F., et al. A strategy for qualitative and quantitative profiling of Glycyrrhiza extract and discovery of potential markers by fingerprint-activity relationship modeling. Sci. Rep. 2019;9:1309. doi: 10.1038/s41598-019-38601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z., Liu M., Zhang Y., et al. Cardioprotective effect of monoammonium glycyrrhizinate injection against myocardial ischemic injury in vivo and in vitro: involvement of inhibiting oxidative stress and regulating Ca2+ homeostasis by L-type calcium channels. Drug Des. Dev. Ther. 2020;14:331–346. doi: 10.2147/DDDT.S232130. Published 2020 Jan 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J.X., Braun M.S., Wetterauer P., Wetterauer B., Wink M. Antioxidant, cytotoxic, and antimicrobial activities of Glycyrrhiza glabra L., Paeonia lactiflora Pall., and Eriobotrya japonica (Thunb.) Lindl. Extracts. Medicines (Basel). 2019;2016:43. doi: 10.3390/medicines6020043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]