Abstract
The CD40 receptor and its ligand CD40L is one of the most critical molecular pairs of the stimulatory immune checkpoints. Both CD40 and CD40 L have a membrane form and a soluble form generated by proteolytic cleavage or alternative splicing. CD40 and CD40L are widely expressed in various types of cells, among which B cells and myeloid cells constitutively express high levels of CD40, and T cells and platelets express high levels of CD40L upon activation. CD40L self-assembles into functional trimers which induces CD40 trimerization and downstream signaling. The canonical CD40/CD40L signaling is mediated by recruitment of TRAFs and NF-κB activation, which is supplemented by signal pathways such as PI3K/AKT, MAPKs and JAK3/STATs. CD40/CD40L immune checkpoint leads to activation of both innate and adaptive immune cells via two-way signaling. CD40/CD40L interaction also participates in regulating thrombosis, tissue inflammation, hematopoiesis and tumor cell fate. Because of its essential role in immune activation, CD40/CD40L interaction has been regarded as an attractive immunotherapy target. In recent years, significant advance has been made in CD40/CD40L-targeted therapy. Various types of agents, including agonistic/antagonistic monoclonal antibodies, cellular vaccines, adenoviral vectors and protein antagonist, have been developed and evaluated in early-stage clinical trials for treating malignancies, autoimmune diseases and allograft rejection. In general, these agents have demonstrated favorable safety and some of them show promising clinical efficacy. The mechanisms of benefits include immune cell activation and tumor cell lysis/apoptosis in malignancies, or immune cell inactivation in autoimmune diseases and allograft rejection. This review provides a comprehensive overview of the structure, processing, cellular expression pattern, signaling and effector function of CD40/CD40L checkpoint molecules. In addition, we summarize the progress, targeted diseases and outcomes of current ongoing and completed clinical trials of CD40/CD40L-targeted therapy.
Keywords: CD40/CD40L, immune checkpoint, molecular basis, effector function, therapeutic implications
Introduction
Since the development of expression cloning in the late 1980s, a number of stimulatory and inhibitory immunoreceptors with their natural ligands, have been identified as gatekeepers of immune responses known as “immune checkpoints”. Stimulatory immune checkpoints serve to boost the magnitude of cell activation initiated by T cell receptor (TCR)/ B cell receptor (BCR) engagements, which are necessary in generating effective immune responses against infections and tumors. On the contrary, inhibitory immune checkpoints negatively regulate immune cell activation, which limit excessive immune-mediated tissue damage and play a major role in immune tolerance.(1–2) In the past decades, immune checkpoint-based therapy has emerged as a promising immunotherapeutic in the clinic. Blockade of stimulatory immune checkpoints or activation of inhibitory immune checkpoints has been used to ameliorate immune responses in autoimmune and inflammatory diseases and to prevent rejection after allogeneic transplantation. In contrast, blockade of inhibitory immune checkpoints or activation of stimulatory immune checkpoints has been used to boost immune responses against tumors. Recently, the most successful immune checkpoint-based therapy is targeting on inhibitory immune checkpoint programmed cell death protein 1(PD1)/programmed cell death ligand 1 (PDL1). Anti-PD1/PDL1 therapy has been approved to treat a wide variety of malignancies, including melanoma, lung cancer, liver cancer, gastric cancer, kidney cancer, bladder cancer, head and neck tumor, Hodgkin’s lymphoma, etc.(3) However, the response rate of anti-PD1/PDL1 therapy alone or in combination, is only ranging from 10% to 50% in most malignancies. Thus, other immune checkpoints are under active investigation for their therapeutic potential.
CD40 and CD40 ligand (CD40L) have been well-documented as stimulatory immune checkpoint and play a broad role in various immunological processes contributing to both humoral and cell-mediated immune responses. CD40 belongs to the tumor necrosis factor receptor superfamily (TNFRSF). CD40L is a member of TNF superfamily (TNFSF). They are under intense investigation as an attractive target for immunotherapy, with several drugs enhancing or blocking their activities in clinical trials. In this review, we focus on describing the molecular basis and therapeutic implications of CD40/CD40L checkpoint molecules. We summarize the understanding regarding to structure, interaction, cell-type expression profile and signaling of CD40/CD40L checkpoint molecules. CD40/CD40L interaction in various cells and consequential effector functions are categorized. We also discuss the current development of therapeutic strategies targeting CD40/CD40L interaction.
CD40/CD40L checkpoint molecular structure
We summarize CD40/CD40L checkpoint molecular structure, processing and interaction in Figure 1. CD40, also known as Bp50 and TNFRSF5, is a type I membrane glycoprotein with a 62 amino acid (aa) cytoplasmic tail, a 22 aa transmembrane domain and a 173 aa extracellular domain in human. It was first discovered in 1987 as a 50-kDa protein expressed on B cells which regulates B cell proliferation.(4) CD40 locus is on human chromosome 20 and on mouse chromosome 2. Similar to other members of TNFRSF, the extracellular domain of CD40 is characterized by the presence of 4 cysteine-rich domains (CRDs) engaged in oligomerization and ligand binding.(5) There are 2 forms of CD40: membrane CD40 (mCD40) and soluble CD40 (sCD40). A fraction of mCD40 and sCD40 self-assemble into noncovalent homodimers through CRD1 in the extracellular domain.(6) sCD40 can be generated by alternative splicing in the cytoplasm (7) or from a proteolytic process in which mCD40 is cleaved via tumor necrosis factor-α-converting enzyme on the cell surface after its ligation with CD40L.(8) sCD40 acts as a natural antagonist of CD40 by shedding CD40/CD40L interaction.(9) In addition to its natural ligand CD40L, microbial proteins heat shock protein 70 and borrelial OspA have been reported to bind to CD40 as well.(10–11)
Figure 1. CD40/CD40L checkpoint molecules processing and interaction.
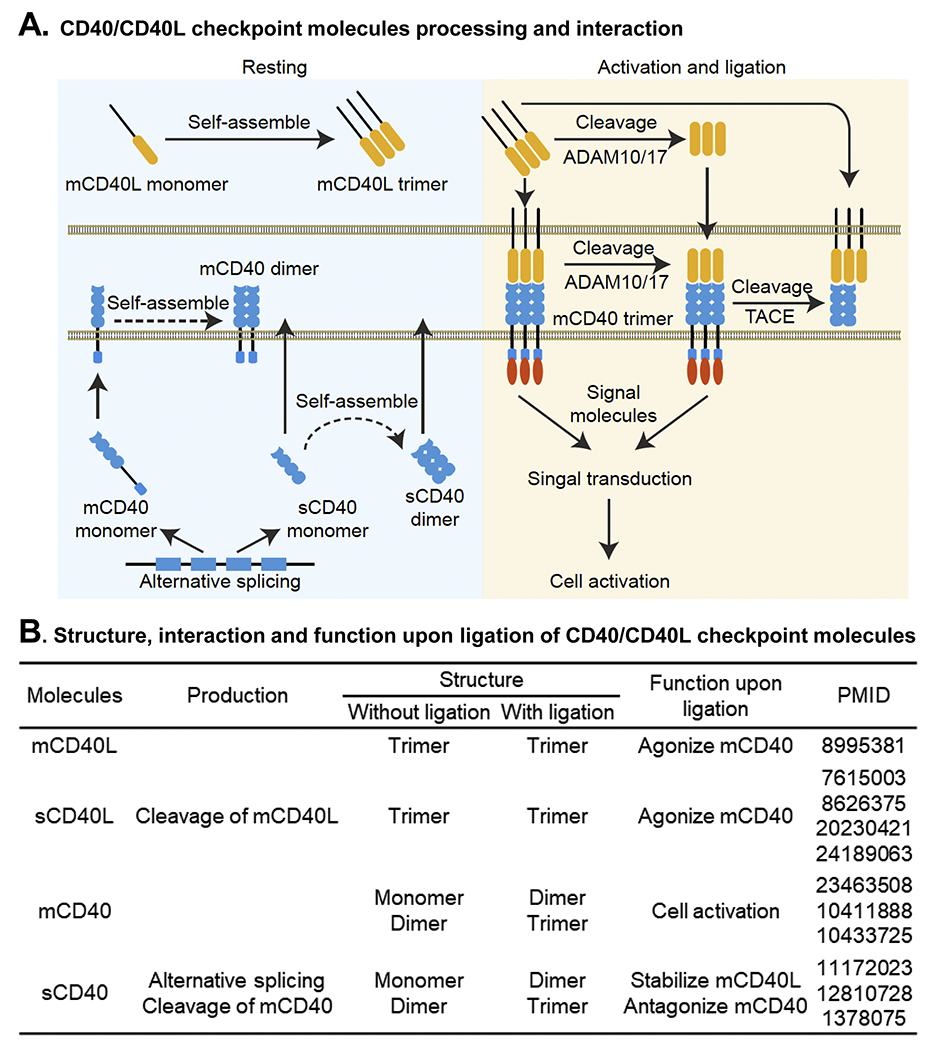
A. CD40/CD40L checkpoint molecules processing and interaction. In resting condition, CD40L is expressed as a membrane monomer (mCD40L) which self-assembles into noncovalent trimers and is stored intracellularly. CD40 is expressed as a membrane monomer (mCD40) and a soluble monomer (sCD40) by alternative splicing. A small portion of mCD40 and sCD40 self-assemble into noncovalent dimers. After cell activation, mCD40 translocates to the cell surface and undergoes proteolytic cleavage by ADAMs to generate trimeric sCD40L. Binding of both mCD40L and sCD40L to CD40 induces mCD40 trimerization, recruitment of signal molecules and subsequent signal transduction, leading to cell activation. After ligation, mCD40 can be cleaved into sCD40 by TACE on the cell surface. sCD40 antagonizes mCD40 by shedding CD40/CD40L interaction. B. Structure, interaction and function upon ligation of CD40/CD40L checkpoint molecules. Abbreviations: ADAM, a disintegrin and metalloprotease; CD40L, CD40 ligand; mCD40, membrane CD40; mCD40L, membrane CD40L; sCD40, soluble CD40; sCD40L, soluble CD40L; TACE, tumor necrosis factor-α converting enzyme.
CD40L, also known as gp39, CD154 and TNFSF5, is a type II membrane glycoprotein with a 22 aa cytoplasmic tail a 24 aa transmembrane domain and a 215 aa extracellular domain in human. It was firstly recognized in 1992 on murine activated T helper (Th) cell clone for its capacity to bind to CD40.(12) CD40L locus is on both human and mouse chromosome X. Similar to other members of the TNFSF, CD40L contains a conserved TNF-homology domain in extracellular region that enables trimerization and receptor binding.(13) CD40L assembles as noncovalent homotrimers or heterotrimers consisting of full-length and truncated forms of CD40L. Upon cell activation, CD40L translocates to the cell surface known as membrane CD40L (mCD40L).(14) A soluble form of CD40L (sCD40L) co-exists with mCD40L. Several mechanisms have been shown to contribute to the generation of sCD40L. sCD40L can be generated by enzymatic cleavage of mCD40L on the cell surface via a disintegrin and metalloprotease (ADMA) 10 and ADAM17(15) or via matrix metalloproteinase-2 (MMP-2).(16) sCD40L can also result from enzymatic cleavage of full-length CD40L intracellularly.(17) sCD40L is trimeric and biologically active.(18) In addition to its classical receptor CD40, CD40L also binds to αIIbβ3, α5β1, αMβ2 and αvβ3 integrals.(19–20)
CD40/CD40L interaction has been analyzed through molecular modeling, mutagenesis and X-ray crystallography. The binding of trimeric mCD40L or sCD40L to mCD40 on the cell surface triggers the trimerization of the receptor which is necessary for downstream signaling.(21) The ligand binding sites are located in the inter-subunit grooves of CD40L trimer and associate with CDR1, CDR2 and CDR3 regions of CD40.(22) The interface of CD40/CD40L interaction shows charge complementarity, with the CD40L chain being positively charged and the CD40 chain being negatively charged. Several charged residues in the interface of CD40L chain (Y82, D84, N86, K143, R203, R207, Y146, R203 and Q220) and CD40 chain (K143, Y145, D84, E114, E117, E74 and E117) have been reported to contribute to the stability of the complex.(23–25)
CD40/CD40L checkpoint expression profiles and regulation
To date, most of the CD40/CD40L studies have been focused on a specific cell type without addressing their systemic expression profiles. By virtue of bioinformatic analysis and database mining, we profiled the cell-type expression of CD40L and CD40 genes in various types of primary human cells. In a normalized transcriptional dataset gathering 714 RNA-seq studies,(26) we selected data from human primary cells without risk factor challenges and found that mRNA levels of CD40L are relatively higher in T cells and platelets, whereas that of CD40 are relatively higher in B cells and myeloid cell lineages (Figure 2A). Analysis of a single cell RNA-seq dataset of human lung(27) also demonstrated high levels of CD40 in myeloid cells and B cells, and CD40L in T cells (Figure 2B). Therefore, CD40/CD40L checkpoint molecules may be especially important in mediating T cell, B cell, myeloid, and platelet interactions. The current understanding for biological function of the CD40/CD40L interaction emphasizes its crucial roles in T cell-dependent B cell differentiation and activation which are indispensable for humoral response, and in mediating a bi-directional dialogue between T cells and monocytes (MCs)/macrophage (MΦ) which provides an amplification loop in cellular immunity, as well as in T cell-mediated platelet activation.
Figure 2. CD40 and CD40L expression profiles in primary human cells.
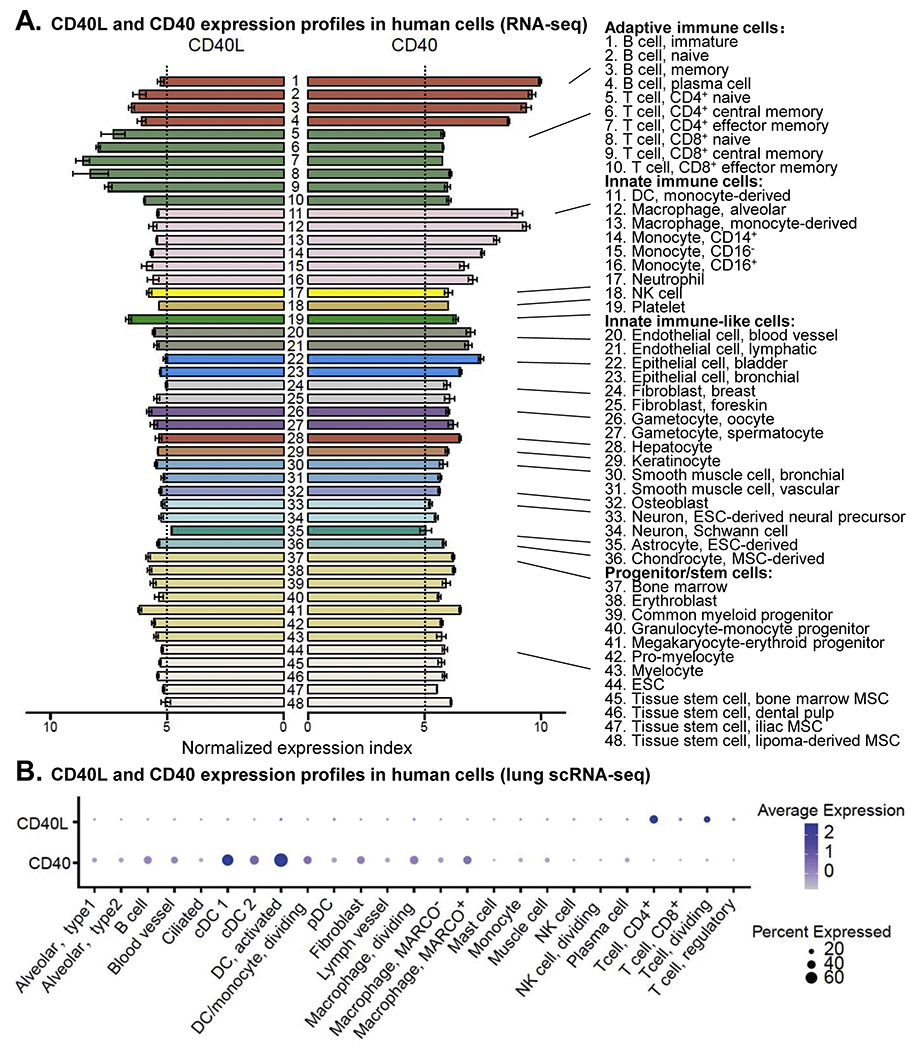
A. CD40 and CD40L expression profiles in human cells (RNA-seq). Expression levels of CD40 and CD40 genes in various types of primary human cells, without risk factor challenge, were extracted from a normalized transcriptional dataset gathering 714 RNA-seq studies (PMID 24053356). Both CD40 and CD40L are expressed in all primary human cells presented in this dataset. CD40L gene is expressed relatively higher in T cells and platelets, whereas CD40 gene is higher in B cells and myeloid cell lineages. B. CD40 and CD40L expression profiles in human cells (lung scRNA-seq). Expression levels of CD40 and CD40L genes in human lung across various cell types were extracted from a scRNA-seq datasets (PMID 31892341). Abbreviations: CD40L, CD40 ligand; cDC, conventional DC; DC, dendritic cell; ESC, embryonic stem cell; MSC, mesenchymal stem cell; NK cell, natural killer cell; pDC, plasmacytoid DC.
mCD40 is constitutively expressed on B cells, and appears to be reduced on end-stage plasma cells.(28) In MCs, MΦ and dendritic cells (DCs), the expression of mCD40 increase with cell development and maturation.(29–31) The expression of mCD40 has found induced by “danger” signals including pathogen-associated molecular patterns (PAMP), such as lipopolysaccharide.(31) and damage-associated molecular patterns (DAMP), such as high mobility group box protein 1.(32) We and others reported that metabolite-associated danger signal (MADS) homocysteine,(33–34) induces the expression of mCD40 on MCs. Inflammatory cytokines such as granulocyte/macrophage colony-stimulating factor (GM-CSF), interleukin-3 (IL-3) and interferon-γ (IFN-γ)(35) can increase mCD40 expression.
CD40L is highly expressed in activated CD4+ T cells. Resting CD4+ T cells have little or no detectable mCD40L. Activation-induced mCD40L expression has been reported in Th1, Th2, Th17 and follicular helper T (Tfh) cells, as well as regulatory T cells, albeit at much lower levels compared to Th cells.(36–37) The rapid and transit expression of mCD40L on CD4+ T cells is tightly regulated by 4 mechanisms. First, preformed CD40L in secretory lysosomes translocates to the cell surface in effector and memory CD4+ T cells within a few minutes upon activation.(38) Second, CD40L mRNA expression is regulated at both the transcriptional and posttranscriptional levels.(39) Third, mCD40L is proteolytically cleaved into sCD40L after cell activation. Lastly, CD40 ligation induces internalization of mCD40L followed by lysosomal degradation.(40) Release of sCD40L and induction of mCD40L occur in concert but were found to differ in kinetics following cell activation.(41) Various stimuli can promote CD40L expression in CD4+ T cells, including signals through TCR,(42) co-stimulatory molecules(43) and inflammatory cytokines such as IL-12.(44)
Platelets are another important source of CD40L. Resting platelets have no detectable mCD40L. After stimulation with a wide range of platelet activators, such as thrombin, collagen or adenosine di-phosphate (ADP) plus adrenaline, mCD40L is rapidly and transiently expressed due to the translocation of CD40L from intraplatelet stores.(45) Meanwhile, sCD40L is released by proteolytical cleavage after platelet activation, resulting in the down-regulation of mCD40L.(46) Furthermore, mCD40L expression is inducible on other immune cells, such as CD8+ T cells, mast cells, basophils, natural killer (NK) cells, eosinophils and innate lymphoid cells (ILCs), as well as conditional innate immune cells such as endothelial cells (ECs) and vascular smooth muscle cells (VSMCs).(47–52)
CD40/CD40L intracellular signaling
CD40 signaling is primarily mediated through different members of adapter protein TNF receptor associated factor (TRAF) on a cell type and stimuli dependent manner (Figure 3 and Table 1). TRAF family proteins have 7 recognized members which include TRAF1-7. All TRAF family members, with the exception of TRAF7, have a TRAF domain located at the C terminus, contributing to the formation of TRAF homotrimers or heterooligomers and the interaction with cytoplasmic domain of CD40 and downstream effectors. TRAF1, TRAF2, TRAF3, TRAF5 and TRAF6 have been reported to bind to CD40. Trimeric TRAFs are recruited to CD40 trimer.(53) Trimeric TRAF2/3 bind strongly, whereas TRAF1/5/6 bind weakly to CD40.(24,54) TRAF2/3 have been reported to translocate to lipid rafts by CD40 ligation, which is essential for downstream signaling.(55) There are two nonoverlapping TRAF binding sites in CD40 cytoplasmic domain.(56–57) One is located at the membrane distal region and interacts with TRAF 1/2/3/5. The other is located at the membrane proximal region and binds to TRAF6. Both binding sites have been reported to regulate signaling activity in concert or independently. Germinal center (GC) formation in response to immunization is arrested only in mutagenesis of both the two binding sites.(58) Disrupting CD40-TRAF6 but not CD40-TRAF2/3/5 interaction attenuates atherosclerosis and promotes plaque fibrosis in apolipoprotein E (ApoE)−/− mice,(59) and inhibiting CD40-TRAF6 signaling in MΦ reduces atherosclerosis.(60)
Figure 3. CD40/CD40L signal pathways.
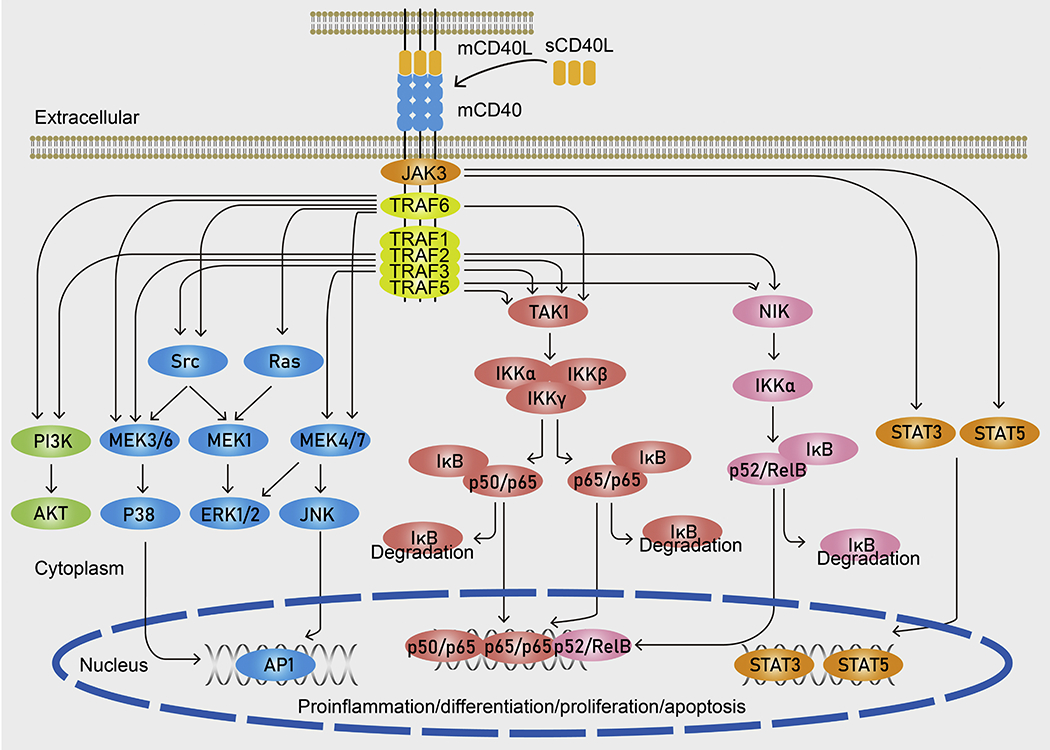
The ligation of trimeric mCD40 with mCD40L or sCD40L results in the recruitment of signal molecules TRAFs to CD40 cytoplasmic domain. CD40 cytoplasmic domain contains two distinct TRAF binding sites. TRAF1, TRAF2, TRAF3 and TRAF5 bind competitively to one site and TRAF6 binds to another. CD40/CD40L ligation activates the canonical NF-κB pathway (IKKα, IKKβ and IKKγ dependent) and non-canonical NF-κB pathway (IKKα dependent), leading to nuclear translocation of p50/p65, p65/p65 and p52/RelB dimers and their DNA binding. Other well-documented downstream pathways of CD40-TRAF interaction include the three MAPK pathways (p38, ERK1/2 and JNK) and PI3K/AKT pathway, leading to AP1 nuclear translocation and DNA binding. In addition to TRAF-mediated signaling, CD40 can activate the JAK3/STATs pathways. Abbreviations: Ab, antibody; AKT, protein kinase B; AP1, activator protein 1; CD40L, CD40 ligand; ERK1/2, extracellular signal-regulated kinases 1/2; IKK, inhibitor of NF-κB kinase; IκB, NF-κB inhibitor; JNK, c-Jun N-terminal kinase; MEK1, MAPK/ERK kinase; NF-κB, nuclear factor kappa-B; NIK, NF-κB kinase inducing kinase; JAK3, janus kinase 3; PI3K, phosphoinositide-3-kinase; STAT, signal transducer and activator of transcription; TAK1, TGFβ-activated kinase 1; TRAF, tumor necrosis factor receptor-associated factor.
Table 1.
CD40/CD40L signal pathways in cellular immune response
| Cell | Signal pathway | PMID |
|---|---|---|
| B cell | TRAF2↑ → canonical NF-κB (p65:p65)↑ & noncanonical NF-κB (p52:Rc1B)↓ → cell proliferation & activation↑ | 15539150 |
| B cell | TRAF2↑ → MEKK1↑ → JNK & p38↑ → AP1↑ → cell proliferation & Ab production↑ | 17143273 |
| B cell | TRAF6↑ → JNK & p38↑ → cell proliferation, activation & Ab isotype switching↑ | 12354380 |
| WEHI 231 B cell | TRAF3↑ → PI3K↑ → NADPH oxidase↑ → ROS production↑ | 14688330 |
| A20 B cell | TRAF6↑ → PI3K↑ → AKT↑ → apoptosis↓ | 12637493 |
| B cell | JAK3↑ → STAT3↑ → CD23, ICAM-1 & lymphotoxin-α↑ | 9133417 |
| DC | TRAF2 & 3↑ → Lyn↑ → ERK1/2↑ → IL-1α/β & IL-1Ra↑ TRAF2 & 3↑ → Lyn↑ → p38↑ → IL12p40↑ TRAF2 & 3↑ → p38↑ → IL12p40↑ |
10880443 |
| MC/MΦ | TRAF6↑ → Src↑ → ERK1/2↑ → TNF-α IL-6 & IL-1β↑ | 15634933 |
| MC | JAK3↑ → STAT5↑ | 10395671 |
| Endothelial cell | TRAF6↑ → c-Cb1↑ → PI3K↑ → AKT ↑ → proliferation, motility, angiogenesis↑ & apoptosis↓ | 12637493 |
| Lung fibroblast | TRAF6↑ → canonical NF-κB (p65) & JNK↑ → IL-8 & IL-6↑ | 16143987 |
| Airway epithelial cell | TRAF3↑ → canonical NF-κB (p65:p65)↑ → RANTES↑ | 12122011 |
| 293T cell | TRAF6↑ → canonical NF-κB (p50:p65)↑ TRAF2 & 5↑ → canonical NF-κB (p50:p65)↑ TRAF2 & 5 noncanonical NF-κB (p52:RelB)↑ |
15708970 |
| 293T cell | Cytoplasmic tail-N → TRAF2, 3 & 5 ↑; Cytoplasmic tail-C → TRAF6 ↑ | 9990007 |
| Spodoptera frugiperda (Sf21) cell | PVQET sequence of CD40 cytoplasmic domain → TRAF1, 2 & 3↑ QEPQEINF sequence of CD40 cytoplasmic domain → TRAF6↑ |
9718306 |
| HEK 293 cell | Affinity to trimeric CD40: trimeric TRAF2 > TRAF3 >> TRAF1 & 6 | 10433725 |
| HEK 293 cell | Affinity to CD40: TRAF2 & 3 > TRAF5 & 6 | 9990039 |
| HEK 293 cell | TRAF6↑ → Ras↑ → Raf↑ → MEKl↑ → ERK1/2↑; TRAF6↑ → ERK1/2↑ | 9432981 |
| Malignant urothelial cell | TRAF3↑ → JNK↑ → APl↑ → caspase-9↑ → caspase-3↑ → apoptosis ↑ | 16429118 |
Abbreviations: Ab, antibody; AKT, protein kinase B; AP1, activator protein 1; CD40L, CD40 ligand; DC, dendritic cell; ERK1/2, extracellular signal-regulated kinases 1/2; ICAM-1, intercellular adhesion molecule-1; IL, interleukin; JAK3, janus kinase 3; JNK, c-Jun N-terminal kinase; Lyn, LYN proto-oncogene, Src family tyrosine kinase; MC, monocyte; MΦ, macrophage; MEK1, MAPK/ERK kinasel; MEKK1, MAPK kinase kinase 1; NF-κΒ, nuclear factor kappa-B; PI3K, phosphoinositide-3-kinase; RANTES, regulated upon activation normal T cell expressed and secreted factor; ROS, reactive oxygen species; STAT, signal transducer and activator of transcription; TNF-α, tumor necrosis factor-α; TRAF, tumor necrosis factor receptor-associated factor.
Depending on cell types, CD40-TRAF complexes regulate a wide range of signal pathways. The most known signal pathway initiated by CD40-TRAF complexes is the nuclear factor kappa-B (NF-κB) pathway. The canonical NF-κB pathway involves activation of trimeric inhibitor of NF-κB kinase (IKK) complex (IKKα/β/γ) by TGFβ-activated kinase 1 (TAK1) and subsequent IKK complex-mediated NF-κB inhibitor (IκB) α degradation, resulting in rapid and transient translocation of NF-κB hetero- or homo-dimers to the nucleus. The non-canonical NF-κB pathway has been elucidated by NF-κB inducing kinase (NIK)-mediated activation of IKKα and subsequent proteolysis of p100 into p52, which associates with RelB and then translocates into the nucleus.(61) In 293T cells, TRAF2/5 activates both pathways after recruitment to CD40, whereas TRAF6 only activates the canonical NF-κB signaling and TRAF3 acts as a negative regulator of the noncanonical NF-κB pathway.(62) In mouse mature B cells, TRAF2 mediates activation of the canonical NF-κB pathway but serves as an inhibitor of the noncanonical pathway.(63) In airway epithelial cells, TRAF3-mediated enhancement of the canonical NF-κB signaling is essential for CD40-induced RANTES (regulated on activation, normal T cell expressed and secreted) expression.(64)
Other well-documented effector events downstream of CD40-TRAF engagement include the three mitogen-activated protein kinase (MAPK) pathways (extracellular-signal-regulated kinase (ERK) pathway, JUN N-terminal kinase (JNK) pathway and p38 pathway), the Src tyrosine kinase (represented by Lyn) pathway and the phosphoinositide 3-kinase (PI3K)/protein kinase B (PKB, also known as AKT) pathway. In mouse B cells, MAPK kinase kinase 1 is recruited to CD40-TRAF2 complex, followed by JNK and p38 activation as well as activator protein 1 (AP1) translocation, ultimately resulting in cell proliferation and antibody production.(65) In TRAF2 deficient mice, CD40-medated activation of JNK is severely impaired in B cells.(66) In mice lacking CD40 binding motif for TRAF6, CD40-mediated isotype switching, proliferation and activation of p38 and JNK are severely impaired in B cells.(67) In WEHI 231 B cells, CD40-induced reactive oxygen species production requires TRAF3 recruitment and activation of PI3K.(68) In A20 B cells, CD40-TRAF6 engagement activates the PI3K/AKT pathway and rescues cells from CD95-induced apoptosis.(69) In MΦ, a cell-permeable peptide that inhibits CD40-TRAF6 interaction impairs activation of Src/ERK1/2 and inflammatory cytokines production.(70) In human DCs, CD40-TRAF2/3 engagement trigger Lyn-dependent ERK1/2 activation and Lyn-dependent/independent p38 activation, leading to IL-1α/β, IL-1Ra and IL12p40 expression, respectively.(71) In ECs, CD40-TRAF6 engagement activates the PI3K/AKT pathway and promotes proliferation, survival and migration, as well as vessel-like structure formation.(72) In lung fibroblasts, CD40-TRAF6 engagement activates the canonical NF-κB and JNK pathways, leading to IL-8 and IL-6 production.(73) In 293 cell line, both Ras-dependent and independent activation of ERK1/2 pathway involve CD40-TRAF6 engagement.(74) In malignant human urothelial cells, mCD40L but not sCD40L, triggers cell apoptosis via recruitment of TRAF3, followed by activation of JNK and translocation of AP1, as well as induction of the caspase-9/3-associated intrinsic apoptotic pathway.(75)
In addition to TRAF-mediated signaling, CD40 can also activate the Janus kinase 3 (JAK3)/signal transducer and activator of transcription (STAT) pathway. In mouse primary B cells, CD40-induced proliferation and production of antibodies occur even though all TRAF binding sites are disrupted.(58) In human MCs, phosphorylation of JAK3 is associated with CD40 upon ligation and leads to STAT5 nuclear translocation.(76) In mouse B cells, JAK3 binds to PTNKAPHP motif in the membrane-proximal region of CD40 cytoplasmic domain and activates STAT3, which is essential for CD40-induced expression of CD23, intercellular adhesion molecule-1 (ICAM-1) and lymphotoxin-α.(77)
Effector function of CD40/CD40L interaction
Over the past decades, emerging evidence demonstrates that CD40/CD40L interaction regulates many biological processes including humoral immunity, cellular immunity, tissue inflammation, thrombosis, hematopoiesis and tumor cells fate. We list the details of CD40/C40L interaction and function in Table 2 and present models to illustrate CD40/CD40L effector function in different cell types in Figure 4.
Table 2.
CD40/CD40L checkpoint molecules interaction, effector function and immunological process
| CD40L source | CD40 source | Effector function | Immunological process | PMID |
|---|---|---|---|---|
| TC | BC | BC proliferation, survival, differentiation, Ab production, IgD/M to IgA/G/E switching, memory response & costimulatory activity ↑ | TC-dependent humoral immunity; Cellular immunity | 27573866 29669250 7533092 |
| Mast cell ILC Neutrophil NK cell |
BC | BC proliferation, differentiation, Ab production, IgD/M to IgA/G/E switching & memory response↑ | TC-independent humoral immunity | 20101023 24562309 22197976 17630353 |
| TC Platelet |
MC/MΦ | MC/MΦ differentiation, costimulatory activity, cytokine production & anti-microbial activity ↑; MC apoptosis↓; TC Th1 response, CD8+ CTL response & memory CTL maintenance↑ | Cellular immunity | 15100268, 7594496 7843250, 8929557 27554817,8627184 10810999 |
| TC Platelet |
DC | DC maturation, proliferation, costimulatory activity & cytokine production↑; DC apoptosis↓; TC Th1 response, CD8+ CTL response & memory CTL maintenance↑ | Cellular immunity | 12893749 22154528 32453421 20811042 |
| TC Platelet |
Neutrophil | Oxidative burst & anti-microbial activity ↑ | Cellular immunity | 29518426 23785403 |
| TC | Platelet | Platelet activation & release of RANTES↑ | Thrombosis | 14764664 |
| TC Platelet |
EC | EC IL-8, MCP-1 & adhesion molecules↑ | Tissue inflammation | 7500031 9468137 |
| TC Platelet |
VSMC | VSMC proliferation, MMPs & adhesion molecules↑ | Tissue inflammation | 9285647 28717419 |
| TC | Fibroblast | Fibroblast IL6/8, RANTES & adhesion molecules↑; TC extravasation↑ | Tissue inflammation | 9144479 |
| TC | KC | KC IL8, TNF-α & adhesion molecules↑ | Tissue inflammation | 8898941 8977185 |
| TC | Epithelia | Epithelia IL6/8/15 & RANTES↑ | Tissue inflammation | 11053480, 11134253 |
| TC Platelet |
HPC | HPC proliferation, myeloid lineage differentiation & megakaryocytopoiesis↑ | Myelopoiesis | 9022082 9016882 |
| Platelet | Platelet | Platelet activation↑ | Thrombosis | 12676820 |
| Platelet | Leukocyte | Platelet-MC & platelet-neutrophil aggregates↑ Leukocyte extravasation↑ | Thrombosis Tissue inflammation |
12676820 27152726 |
| TC | Tumor cell | Proliferation (malignant BC), IL-6, TNF-α costimulatory activity & apoptosis↑ | Tumor cell fate | 18497318,10068672 12070030, 9182676 |
Abbreviations: Ab, antibody; BC, B cell; CD40L, CD40 ligand; CTL, cytotoxic T lymphocyte; DC, dendritic cell; EC, endothelial cell; HPC, hemopoietic progenitor cell; IL, interleukin; ILC, innate lymphoid cell; MC, monocyte; MCP-1, monocyte chemotactic protein-1; MΦ, macrophage; MMP, matrix metalloprotease; NK cell, natural killer cell; RANTES, regulated upon activation normal T cell expressed and secreted factor; TC, T cell; Th1, T helper1; TNF-α tumor necrosis factor-α; VSMC, vascular smooth muscle cell.
Figure 4. Effector function of CD40/CD40L interaction.
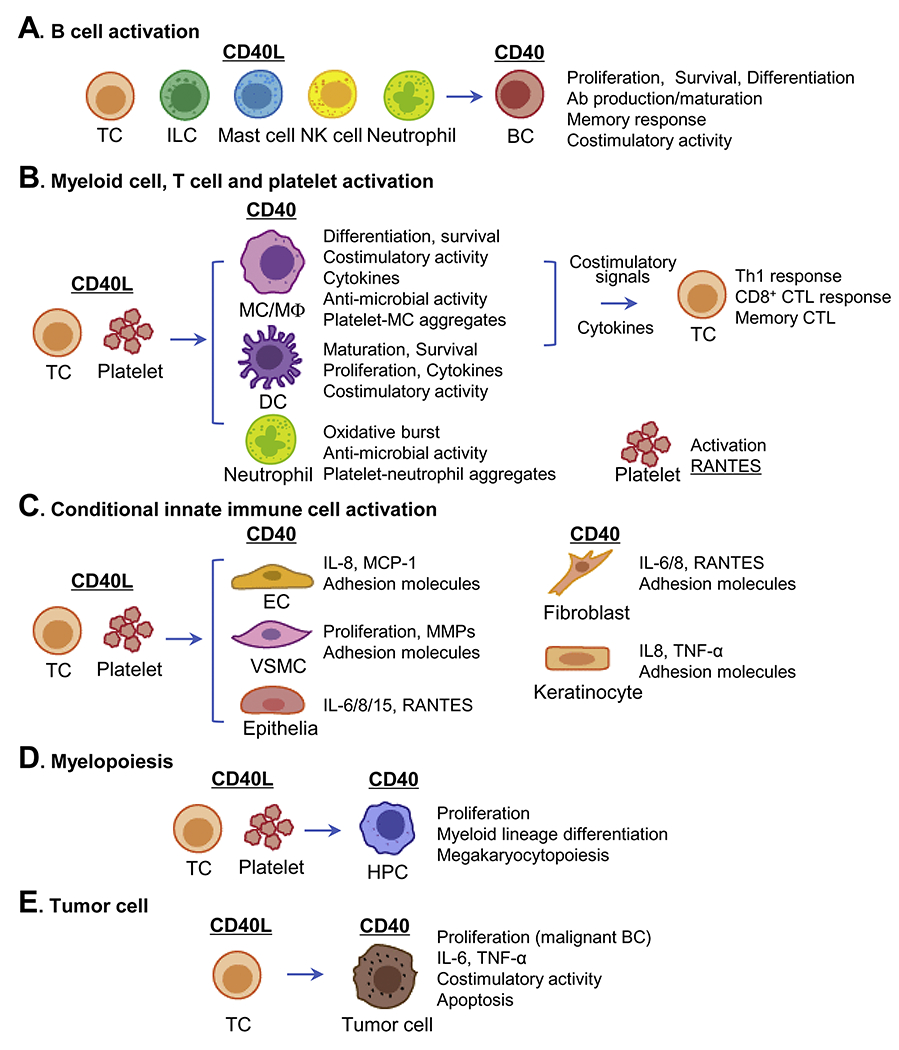
CD40L, expressed by several types of immune cells, binds to its receptor CD40 which is expressed by both immune cells and non-immune cells, participating in B cell activation (A), myeloid cell, T cell and platelet activation (B), conditional innate immune cell activation (C), myelopoiesis (D) and regulation of tumor cells (E). Abbreviations: Ab, antibody; BC, B cell; CD40L, CD40 ligand; CTL, cytotoxic T lymphocyte; DC, dendritic cell; EC, endothelial cell; HPC, hemopoietic progenitor cell; IL, interleukin; ILC, innate lymphoid cell; MC, monocyte; MCP-1, monocyte chemotactic protein-1; MΦ, macrophage; MMP, matrix metalloprotease; NK cell, natural killer cell; RANTES, regulated upon activation normal T cell expressed and secreted factor; TC, T cell; Th1, T helper 1; TNF-α, tumor necrosis factor-α; VSMC, vascular smooth muscle cell.
CD40/CD40L in B cell activation —
B cells constitutively express high levels of CD40. The engagement of CD40L with CD40 is essential for B cell proliferation, differentiation, high-affinity antibody production, isotype switching, memory response and costimulatory activity (Figure 4A).
Mature B cells have three major populations: follicular (FO) B cells, marginal zone (MZ) B cells and B1 cells.(78) FO B cells are the dominant population which present in lymphoid follicles and in the periphery. Upon encountering with antigen and interaction with follicular DCs and Tfh cells, FO B cells undergo proliferation and differentiate into germinal center (GC) B cells, accompanied by immunoglobin (Ig) class-switch recombination (CSR) and somatic hypermutation (SHM) which result in the production of IgG/E/A antibodies with high affinity to specific antigens. Furthermore, GC B cells with high-affinity BCR exit the GC and differentiate into long-lived memory B cells or plasma cells. The most known effector function of CD40/CD40L checkpoint is to regulate FO B cell division, survival, differentiation. CSR and SHM in the GC response. Individuals with CD40 or CD40L deficiency (hyper-IgM syndrome, HIGM) and CD40−/− or CD40L−/− transgenic mice lack GC in lymphoid organ follicles and have abnormally low levels of circulating IgG/E/A and memory B cells.(79–81) CD4+ Tfh cells are identified as the primary CD40L-bearing cells that regulate FO B cell differentiation in the GC.(82) Furthermore, CD4+ Tfh cells exist as discrete subsets to fine-tune GC response, and progressively differentiate with sequential IL-21 and IL-4 production and enhanced CD40L expression.(83) A strong CD40/CD40L signal leads to differentiation of plasma cells. CD40 promotes the differentiation of Bcl6loCD69hi GC B cells, which have a more stable contact with CD4+ Tfh cells and favor a plasma cell fate.(84) A relatively strong CD40 signal provided by CD4+ Tfh cells results in differentiation of CD80hi memory B cells toward plasma cells.(85) A population of CD8+ Tfh cells was recently identified to regulate GC B cell response and autoantibody production through CD40/CD40L interaction.(86) Mast cells, which are often co-localized with B cells in lymphoid organs, can drive B cell proliferation and differentiation toward IgA-secreting plasma cells.(49) MZ B and B1 cells are innate-like B cells, which are mainly located in marginal sinus of the spleen and serous cavities, respectively. They are highly responsive to T cell-independent antigens. Upon encountering with antigens, MZ B cells and B1 cells rapidly differentiate into short-lived plasmablasts that are the dominant source of early protective antibodies. This response may undergo Ig CSR and exhibit reduced SHM. Compared with GC B cells, the role of CD40/CD40L checkpoint in MZ B cells and B1 cells is poorly understood. ILCs, neutrophils and NK cells have been found to activate MZ B cells via CD40/CD40L interaction and induce antibody production.(51, 87–88) In cultured B1 cells, CD40L induces proliferation and CSR.(89)
CD40/CD40L interaction between T cells and B cells also promotes the costimulatory activity of B cells which then act as competent antigen presenting cells (APCs) to prime T cells (Figure 4A). Expression of inducible T cell costimulator ligand on GC B cells is enhanced by CD40 activation which may be involved in maintenance of the Tfh cell phenotype.(90) In cultured B cells, the addition of CD40L-expressed T cells results in the upregulation of costimulatory molecules B7-1/2 which could be blocked by anti-CD40L.(91) The costimulatory activity of B cells is reduced upon interaction with T cells from CD40L−/− mice.(92)
CD40/CD40L in myeloid cell activation —
Among the myeloid cells, CD40/CD40L checkpoint molecules are often related to the activation of MCs, MΦ, DCs and neutrophils. It is well-recognized that CD40/CD40L interaction regulates many aspects of MC/MΦ/DC/neutrophil biology including maturation, survival, cytokine production, costimulatory and anti-microbial activities (Figure 4B).
CD40/CD40L interaction between MCs and T cells/platelets is important in promoting MC differentiation into DCs,(93–94) stimulating expression of costimulatory molecules, such as CD86, inflammatory cytokines (such as IL-1β, TNF-α, IL-6 and IL-8),(95) MMPs,(96) pro-coagulate factors (such as collagenase, stromelysin and tissue factor),(97) and preventing apoptosis.(98) We recently found that serum concentration of sCD40L and peripheral levels of CD40+ MC are significantly elevated in chronic kidney disease (CKD) patients.(33) As CD40+ MC population is positively correlated with the severity of CKD in human, we proposed CD40+ MC as a biomarker for CKD severity. Uremic toxin, homocysteine and sCD40L induce human peripheral mononuclear cells differentiation into CD40+ MCs. Compared with the traditional human proinflammatory intermediate MCs (CD14++CD16+). CD40+ MCs express higher levels of inflammatory markers. We proposed that CD40+ MC represent stronger proinflammatory features than CD14++CD16+ intermediate MCs in human.
MΦ from CD40L deficient HIGM patients exhibits defective function in fungicidal killing and reduced oxidative burst.(99) CD40L produced by activated CD4+ T cells is important for MΦ activation which leads to IFN-γ and nitrite production.(100) Consistently, MΦ stimulated with CD4+ T cells from CD40L−/− mice produce less TNF-α and reactive nitrogen intermediates.(101) Engagement of CD40 reroutes Toxoplasma gondii, an intracellular pathogen, to the lysosomal compartment in MΦ and mediates an autophagy-dependent anti-microbial activity.(102)
In general, CD40 has been considered as a marker of maturation in DCs. MC-derived DCs from HIGM patients with CD40 deficiency exhibit low expression of HLA-DR and IL-12, as well as reduced costimulatory capacity to activate allogeneic T cells.(103) MC-derived DCs from X-HIGM patients with CD40L deficiency show reduced expression of CD80/CD86/HLA-DR and defective capacity to induce IFN-γ production in T cells, as well as impaired immune responses.(104) Plasmacytoid DCs from HIGM patients with CD40 deficiency show decreased IFN-α secretion in response to herpes simplex virus 1 infection.(103) In a murine model of pancreatic cancer, CD40 agonist restores conventional DC1(cDC1) abundance, inhibits cDC1 apoptosis, and promotes cDC1 maturation.(105) In SLE patients, activated platelets enhance IFN-α secretion by plasmacytoid DCs through CD40/CD40L interaction.(106)
CD40/CD40L interaction is important for anti-microbial activity of neutrophils by regulating oxidative burst. CD40L deficient neutrophils from X-HIGM patients exhibit defective oxidative burst in response to P brasiliensis. Neutrophils from CD40L−/− mice also display reduced oxidative burst and inability to control bacteria proliferation after peritoneal cavity infection.(107) Ligation of CD40 by sCD40L strongly stimulates oxidative burst in cultured neutrophils.(108)
CD40/CD40L in T cell activation —
As proposed in our recent publications,(109–110) CD40/CD40L interaction between T cells and APCs triggers two-way signaling including forward and reverse signal. The reverse signal leads to activation and differentiation of APCs, and the forward signal results in T cell/B cell activation and differentiation. CD40/CD40L interaction regulates Th1 differentiation. CD8+ cytotoxic T lymphocyte (CTL) activation and memory CTL maintenance through a bi-directional dialogue between T cells and APCs, providing an amplification loop in the immune response (Figure 4B).
CD40/CD40L ligation induces APCs to secret IL-12, which promotes Th1 polarization.(111) CD40/CD40L deficient HIGM patients are susceptible to opportunistic infections caused by various bacteria, whose clearance depend on effective pathogen-specific Th1 responses.(112–113) In response to Leishmania major challenge, CD40L−/− mice develop uncontrolled infection and fail to mount a vigorous Th1-like response.(114) Immunization with T-cell dependent antigen KLH induces a defective Th1 response in CD40L−/− mice.(115) In Th1 cell-mediated colitis,(116) blocking CD40/CD40L inhibits pathogenic Th1 response and attenuates tissue inflammation.
CD40/CD40L ligation is required for CD8+ CTL activation and memory CTL maintenance.(117) CD40L−/− mice produce impaired primary CD8+ CTL response to vesicular stomatitis virus, and fail to mount an efficient memory CD8+ CTL response to lymphocytic choriomeningitis(118) Intra-tumoral administration of an adenovirus encoding a chimeric m40L induces tumor-specific CD8+ CTL response and suppresses tumor growth.(119) It has been demonstrated that CD40L-bearing CD4+ T cells licenses APCs via CD40 signaling which in turn activate CD8+ T cells.(120) CD40L-bearing CD4+ T cells can also directly activate CD40-bearing CD8+ T cells.(121) Furthermore, it has been reported that 30-50% of stimulated effector CD8+ T cells express surface CD40L and could directly license DCs, which in turn promote proliferation and differentiation of CD8+ T cells.(122) In line with this finding, 20-30% memory CD8+ T cells were found to express CD40L after viral infection, and have the capacity to activate APCs.(123) These studies raise the possibility that CD40L-bearing CD8+ T cells and CD40-bearing APCs directly cooperate to maximize CD8+ T cell responses, and support the theory of two-way signaling of immune checkpoint, by which the reverse signal leading to activation and differentiation of APCs, and the forward signal results in T cell/B cell activation and differentiation.(109–110)
CD40/CD40L in platelet activation —
Activated platelets express high levels of CD40 and CD40L. Over 90% circulating sCD40L derive from activated platelets.(124) CD40/CD40L checkpoint molecules have been well elucidated in regulating platelet activation and platelet-leukocyte aggregation (Figure 4C).
CD40/CD40L interaction regulates platelet activation. A wide range of platelet activators such as thrombin, collagen and ADP induce formation of platelet aggregates and promote rapid and transient expression of mCD40L on the cell surface and release of sCD40L.(45) Ligation of CD40 on the platelet by sCD40L further enhances platelet activation as evidenced by increased expression of CD62P, release of α-granule and dense granule, and classical morphological changes.(125–126) CD40L-bearing activated T cells, which are often co-localized with platelets in microcirculation,(127) induce platelet activation through a CD40-dependent pathway, leading to enhanced expression of CD62P and release of RANTES.(128) In addition, it has been suggested that CD40L regulates platelet activation through its non-classic receptor integrin αIIbβ3 rather than CD40. Exposure of platelets to sCD40L but not sCD40L containing a mutated αIIbβ3-binding domain, results in platelet activation.(129) In FeCl3-induced injury of mesenteric arterioles, CD40L−/−ApoE−/− mice but not CD40−/−ApoE−/− mice, show instability of arterial thrombi which is reversed by infusion of sCD40L rather than sCD40L lacking the integrin αIIbβ3-recognition sequence.(130)
CD40/CD40L interaction is involved in the formation of platelet-leukocyte aggregates (PLAs), especially platelet-MC and platelet-neutrophil aggregates, which are linked to several inflammatory and thrombotic conditions such as cardiovascular diseases, rheumatoid arthritis and inflammatory bowel disease.(131) Reduced numbers of PLAs were detected with both CD40L−/− platelets and CD40−/− leukocytes.(132–133) sCD40L promotes formation of platelet-MC and platelet-neutrophil aggregates, and this action is abrogated by the addition of anti-CD40.(126) At the site of inflammation, intravascularly adherent platelets capture leukocytes through CD40/CD40L interaction, leading to leukocyte infiltration.(134)
CD40/CD40L in conditional innate immune cell activation —
Conditional innate immune cells refer to tissue parenchymal cells which can be transformed to innate immune-like cells under inflammatory stimuli. These include ECs, VSMCs, fibroblasts, epithelia, keratinocytes, etc.(135) CD40/CD40L interaction promotes conditional innate immune cells toward a proinflammatory phenotype (Figure 4C).
CD40L-bearing Jurkat T cells induce ICAM-1, vascular cell adhesion molecule-1 (VCAM-1) and E-selectin expression in human umbilical vein endothelial cells (HUVECs).(136) CD40L-bearing activated T cells induce the expression of MMP1/2/3/9 in human VSMCs by CD40 ligation.(137) sCD40L stimulates lung fibroblast activation, as evidenced by production of IL-6 and IL-8.(138) Ligation of CD40 on human intestinal fibroblasts by CD40L-bearing T cells or sCD40L, increases their T-cell binding capacity and promotes T cell migration through endothelial monolayers by upregulating ICAM-1 and VCAM-1 expression as well as interleukin-8 and RANTES production.(139) CD40 ligation by CD40L enhances ICAM-1 and IL-8 expression in IFN-γ stimulated keratinocytes.(140) In addition. CD40L induces TNF-α secretion in keratinocytes.(141) CD40L, in combination with IL-17, synergistically enhances production of IL-6, IL-8 and RANTES in human tubular epithelia.(142) CD40L-transfected fibroblasts induce human tubular epithelia to secrete IL-15, which could be blocked by anti-CD40L.(143)
Similarly, CD40L-bearing platelets induce ICAM-1, VCAM-1 and E-selectin expression, and IL-8 and MC chemotactic protein-1 secretion in HUVECs,(45) VSMC proliferation and MC-VSMC adhesion.(144) Collectively, CD40L-bearing T cells and platelets induce inflammatory features in tissue parenchymal cells and their transformation to conditional innate immune cells.
CD40/CD40L in myelopoiesis —
CD40/CD40L interaction supports the proliferation and differentiation of CD34+ hematopoietic progenitor cells (HPCs) toward the myeloid lineages (Figure 4D). In a murine model of bone marrow (BM) transplantation. sCD40L treatment promotes the recovery of hematopoietic precursors, primarily affecting myeloid lineages.(145) In response to TNF-α stimulation. CD34+CD40+ HPCs are highly enriched in monocytic, dendritic, granulocytic precursors, whereas CD34+ CD40− HPCs contain granulocytic and erythroid precursors.(146) Supportively, CD40 ligation induces proliferation of human cord blood CD34+ HPCs and their differentiation into functional DCs.(147) In addition, CD40/CD40L interaction has been suggested to indirectly promote myelopoiesis including megakaryocytopoiesis by inducing Flt3-ligand and thrombopoietin in BM stromal cells.(148)
CD40/CD40L in tumor cells —
CD40 is constitutively expressed in several types of malignant cells. CD40/CD40L interaction is associated with diverse functional changes in tumor cells, such as survival and proliferation, and sometimes apoptosis and growth arrest, based on tumor cell types and immune microenvironment (Figure 4E).
CD40 is highly expressed in B-cell malignancies including non-Hodgkin’s lymphoma (NHL), chronic lymphocytic leukemia (CLL) and multiple myeloma (MM). As in normal B cells, engagement of CD40 and CD40L promotes proliferation and inhibits apoptosis in malignant B cells.(149–151) CD40/CD40L interaction upregulates pro-inflammatory cytokines such as TNF-α and IL-6, and costimulatory molecules B7-1/2 in CLL cells, which in turn potentiate priming of T cells.(150,152)
Paradoxically, engagement of CD40 and CD40L has also been reported to induce growth arrest and apoptosis in malignant B cells.(153–155) CD40-mediated apoptosis in malignant B cells may be regulated by p53, which is mutated in over 50% of human malignancies.(156) Loss of p53 function in normal urothelial cells renders them more susceptible to CD40-mediated apoptosis.(157) In addition, CD40/CD40L interaction induces malignant B cells to express death receptors CD95, DR5 and TNF-R1, which in turn confer susceptibility to apoptosis in inflammatory microenvironment.(158–159) CD40/CD40L interaction has also been reported to promote apoptosis in other malignancies such as breast cancer(160), renal cell carcinoma, etc.(161)
Strategies and mechanisms of CD40/CD40L-targeted therapy
To date, several agents targeting CD40/CD40L interaction have been developed and evaluated in early-phase clinical trials. Strategies of CD40/CD40L agonist therapy include agonistic CD40 monoclonal antibody (mAb), adenovirus or plasmid or mRNA electroporation-mediated CD40/CD40L expression (Ad/plasmid/mRNA-CD40/CD40L), and trimeric sCD40L (Figure 5A). The agents of CD40/CD40L agonist therapy have been tested in various types of hematopoietic and solid tumor malignancies.
Figure 5. Strategies and mechanisms of CD40/CD40L-targeted therapy.
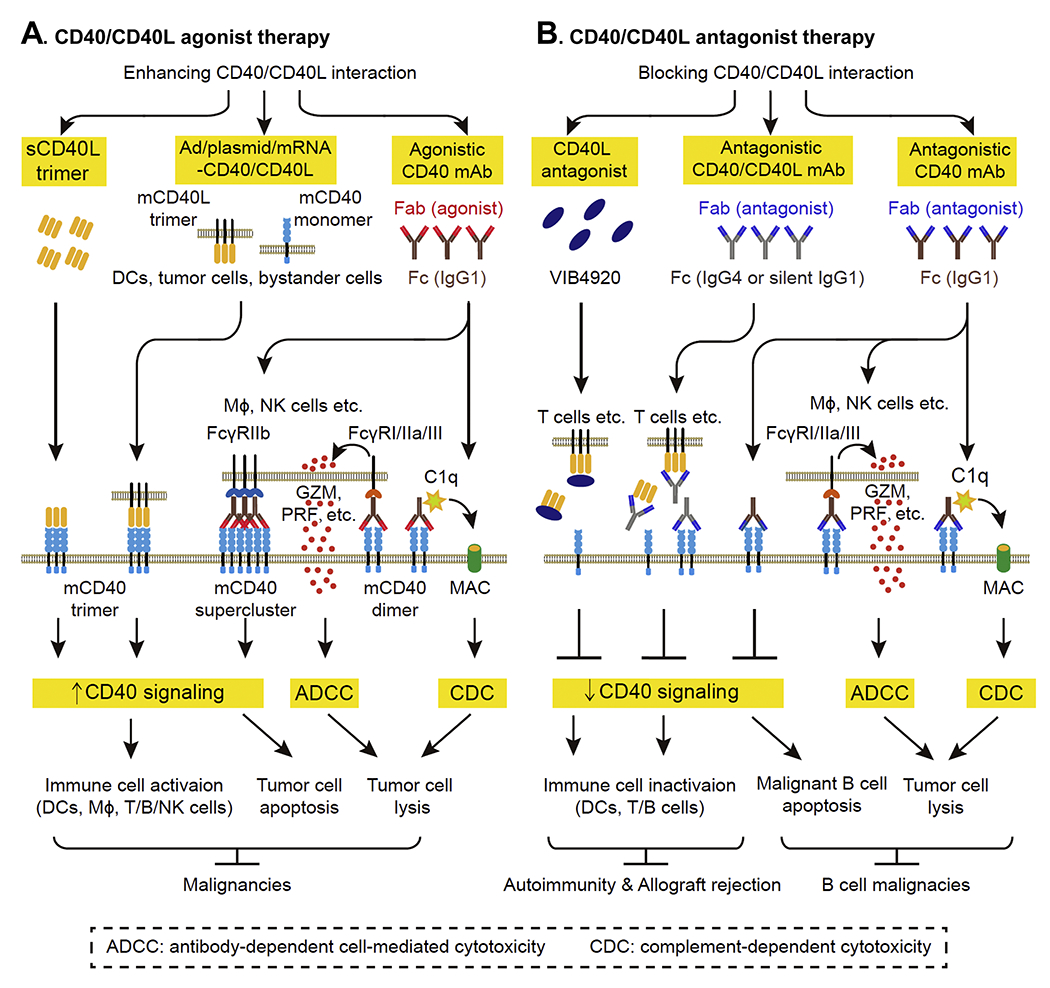
A. CD40/CD40L agonist therapy enhancing CD40/CD40L interaction by sCD40L trimer, adenovirus or plasmid or mRNA electroporation-mediated CD40/CD40L expression (Adv/plasmid/mRNA-CD40/CD40L) and agonistic CD40 mAb has been investigated in malignancies. B. CD40/CD40L antagonist therapy blocking CD40/CD40L interaction by CD40L antagonist and antagonistic CD40/CD40L mAb has been evaluated in autoimmune diseases, allograft rejection and B-cell malignancies. Abbreviations: CD40L, CD40 ligand; DC, dendritic cell; mAb, monoclonal antibody; Fab, antigen-binding fragment; Fc, constant fragment; FcγR, Fc γ receptor; GZM, granzyme; MAC: membrane attack complex; mCD40, membrane CD40; mCD40L, membrane CD40L; MΦ, macrophage; NK cell, natural killer cell; PRF, perforin; sCD40L, soluble CD40L.
The primary mechanism underlying anti-tumor action of these agents is enhancing CD40/CD40L signaling. The constant fragment (Fc) of agonistic CD40 mAb can bind to Fc γ receptor (FcγRIIb) on immune cells (DCs, MΦ, T cell, B cells and NK cells). This leads to mAb accumulation, CD40 superclustering and a subsequent strong CD40 signaling.(162–163) In the cases of Ad/plasmid/mRNA-CD40/CD40L and trimeric sCD40L, the engagement of trimeric CD40L and mCD40 is enhanced, leading to enhancement of CD40 signaling as well. Enhanced CD40 signaling further results in immune cell activation and CD40-expressing tumor cell apoptosis. Other mechanisms underlying anti-tumor action of CD40 agonistic CD40 mAb involve complement-dependent cytotoxicity (CDC) and antibody-dependent cell-mediated cytotoxicity (ADCC). CDC is induced by binding of complement 1q to the Fc region of CD40 IgG1 mAb, which initiates complement cascade and formation of membrane attack complex on mAb-coated tumor cells. ADCC is induced by the binding of CD40 IgG1 mAb to FcγRI/IIa/III on immune cells, which causes immune cell to release lysis granules (e.g. granzyme and perforin) and lyse mAb-coated tumor cells.(164)
In contrast to agonist therapy, CD40/CD40L antagonist therapy blocks CD40/CD40L interaction using antagonistic CD40/CD40L mAb and CD40L antagonist strategies (Figure 5B). Antagonistic CD40/CD40L mAb and CD40L antagonist switch off immune responses by inhibiting CD40 signaling and inactivating immune cells, which are beneficial for autoimmune diseases and allograft transplantation. Antagonistic CD40/CD40L mAb is either human IgG4 or engineered human IgG1 with minimal FcγR affinity to abrogate Fc/FcγR-mediated immune activation. As discussed above, CD40 signaling promotes growth and survival in malignant B cells.(150–152) Therefore, antagonistic CD40 IgG1 mAb is used to treat B cell malignancies because of its ability to abrogate CD40 signaling-mediated tumor growth and induce Fc-dependent CDC or ADCC.
Clinical trials of CD40-targeted agonist therapy —
We summarize clinical trials for CD40-targeted agonist therapy in Table 3A. All agents were well tolerated.
Table 3A.
Clinical trials of CD40 agonist therapy
| Drug | Feature | Phase | Disease | Outcome | PMID/Identifier |
|---|---|---|---|---|---|
| CP-870,893 | Human mAb | 1 | Solid tumors | Safe; Effective | 17327609 |
| 1 | Advanced solid tumor | Safe; Little efficiency | 20855968 | ||
| 1 | Advanced pancreatic ductal adenocarcinoma | Safe a; Effective | 23983255 21436454 |
||
| 1b | Malignant pleural mesothelioma | Safeb | 26386124 | ||
| 1 | Metastatic melanoma | Safe c; Effective | 30288340 | ||
| Dacetuzumab | Humanized mAb | 1 | Refractory NHL | Safe; Effective | 19636010 |
| 1 | CLL | Safe; Little efficiency | 20038235 | ||
| 1 | Advanced MM | Safe; Little efficiency | 20133895 | ||
| Relapsed DLBCL | Safe d; Effective | 22775314 | |||
| 2 | Relapsed DLBCL | Safe; Effective | 24919462 | ||
| 2b | Relapsed DLBCL | Safe e; Not effective | 25651427 | ||
| ChiLob 7/4 | Chimeric mAb | 1 | CD40-expressing solid tumors and DLBCL | Safe; Little efficiency | 25589626 |
| ADC-1013 | Human mAb | 1 | Advanced solid tumors | Safe | 30664811 |
| Selicrelumab | Human mAb | 1 | Solid tumors | Unpublished | NCT02665416 |
| 1 | Refractory B-cell NHL | Ongoing | NCT03892525 | ||
| APX005M | Humanized mAb | 1 | Solid tumors | Not reported | NCT02482168 |
| 1/2 | NSCLC& melanoma | Ongoing | NCT03123783 | ||
| 2 | Rectal adenocarcinoma | Ongoing | NCT04130854 | ||
| 2 | Soft tissue sarcoma | Ongoing | NCT03719430 | ||
| 2 | Melanoma | Ongoing |
NCT04337931 NCT02706353 |
||
| 1 | Pediatric central nervous system tumors | Ongoing | NCT03389802 | ||
| SEA-CD40 | Humanized mAb | 1 | Carcinoma | Ongoing | NCT02376699 |
| CDX-1140 | Human mAb | 1 | Carcinoma | Ongoing | NCT04364230 |
| 1 | Advanced malignancies | Ongoing | NCT03329950 | ||
| 2141-V11 | Fc-engineered human mAb | 1 | Solid tumor & cancer of skin | Ongoing | NCT04059588 |
| NG-350A | Ad-human CD40 mAb | 1 | Metastatic cancer & epithelial Tumor | Ongoing | NCT03852511 |
| BPX101 | Autologous DC transduced with Ad-human CD40 | 1 | Metastatic castration-resistant prostate cancer | Safe; Effective | 28608115 |
Abbreviations: Ad-human mAb, adenovirus carrying agonistic human CD40 mAb gene; Ad-human CD40, adenovirus carrying human CD40 gene; CLL, chronic lymphocytic leukemia; DLBCL, diffuse large B-cell lymphoma; Fc, constant fragment; mAb, monoclonal antibody; MM, multiple myeloma; NHL, non-Hodgkin lymphoma; NSCLC, non-small cell lung cancer.
in combination with gemcitabine;
in combination with cisplatin & pemetrexed;
in combination with tremelimumab;
in combination with rituximab & gemcitabine;
in combination with R-ICE (rituximab, ifosfamide, carboplatin and etoposide).
CP-870,893 is the first human IgG2 mAb with strong agonistic activity. Fc of IgG2 binds to complement or FcR with lower infinity than other isotypes. Thus, CP-870,893 induces minimal CDC or ADCC.(165) However, the hinge region of human IgG2 (h2) could adopt a h2B conformation, which promotes the clustering of mCD40 and imparts the strong agonistic property to CP-870,893.(166) In multiple phase 1 trials, CP-870,893 promoted activation of DCs, MΦ, B cells and T cells and showed single-agent anti-tumor efficacy(167) or synergic effects in combination with other anti-tumor drugs (gemcitabine, cisplatin, pemetrexed and tremelimumab), as demonstrated by partial or complete tumor remission in patients with metastatic melanoma, advanced pancreatic ductal adenocarcinoma and malignant pleural mesothelioma.(168–170) However, in a phase 1 trial with 27 advanced solid tumor patients where melanoma was also represented, weekly CP-870,893 failed to induce tumor remission.(171) The most common adverse events of CP-870,893 was transient grade 1 to 2 cytokine response syndrome (CRS).
Dacetuzumab is a humanized IgG1 mAb with weak agonistic activity. It induces growth inhibition, apoptosis and ADCC in human malignant B cell lines.(172) In phase 1 trials, tumor remission was not observed with dacetuzumab monotherapy in CLL(173) and MM.(174) Dacetuzumab induced tumor remission as monotherapy in patients of NHL in a phase 1 trial(175) or had synergic anti-tumor activity in combination with rituximab and gemcitabine in patients with relapsed diffuse large B cell lymphoma (DLBCL) in a phase 2 trial.(176) In a phase 2b trial targeting patients with relapsed DLBCL after R-CHOP treatment (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone), dacetuzumab plus R-ICE (rituximab, ifosfamide, carboplatin and etoposide) led to more frequent cytopenias, cough and infection, but failed to demonstrate higher tumor remission rates when compared with placebo plus R-ICE.(177) Adverse events often related to dacetuzumab included grade 1 to 2 CRS and increased hepatic enzymes.
ChiLob7/4 is a chimeric IgG1 mAb with modest agonistic activity. ChiLob7/4 causes growth inhibition, CDC and ADCC in a variety of CD40-expressing human malignant cell lines,(178) and induces activation of DCs, B cells and NK cells.(179) In a phase 1 trial with patients of CD40-expressing solid tumors and DLBCL, ChiLob7/4 did not induce tumor remission.(180)
ADC-1013 is a human IgG1 mAb with strong agonistic activity. It induces ADCC and DC activation which in turn promotes tumor-specific T-cell proliferation. ADC-1013 demonstrated significant antitumor effects and induced anti-tumor T cell response in humanized bladder cancer model.(181) In a phase 1 trial conducted in advanced solid malignancies, intra-tumoral administration of ADC-1013 induced B cell activation and was well-tolerated in superficial lesions.(182)
Currently, several clinical trials with CD40 agonistic mAbs are ongoing. The ongoing trials show progresses as following: 1) combinatory therapy with new drugs such as antiangiogenic mAbs (NCT02665416) or anti-PDL1 (NCT03892525); 2) next generation mAbs with enhanced anti-tumor activity after Fab (CDX-1140: NCT04364230/NCT03329950) or Fc engineering (APX005M: NCT02482168/NCT03123783/NCT04130854/ NCT03719430/NCT04337931/NCT02706353/NCT03389802, SEA-CD40: NCT02376699 and 2141-V11: NCT04059588 ); and 3) expansion in targeted diseases including non-small cell lung cancer, urothelial carcinoma, head and neck cancer, rectal adenocarcinoma, soft tissue sarcoma, pediatric central nervous system tumors, etc. A clinical trial utilizing intra-tumoral injected adenoviral vector expressing CD40 agonistic mAb is also ongoing (NCT03852511), which may circumvent detrimental effects caused by systemic immune activation.
BPX101 is a novel strategy using autologous DCs expressing CD40 and tumor antigen to treat metastatic castration-resistant prostate cancer in a phase 1 trail.(183) To manufacture BPX101, autologous DCs are transduced with adenoviral vector Ad5f35 encoding inducible human CD40, incubated with human prostate-specific membrane antigen and then pre-activated with rimiducid and LPS to enhance maturation and antigen presentation. Enhancing CD40 signaling in tumor-antigen loaded DCs could license them as competent APCs, which in turn induce tumor-specific T cell response to eliminate tumors. BPX101 activated T cells and showed anti-tumor activity as demonstrated by prostate specific antigen decline and objective tumor remission.
Clinical trials of CD40-targeted antagonist therapy —
We summarize clinical trials for CD40-targeted agonist therapy in Table 3B. All agents were well tolerated.
Table 3B.
Clinical trials of CD40 antagonist therapy
| Drug | Feature | Phase | Disease | Outcome | PMID/Identifier |
|---|---|---|---|---|---|
| Ch5D12 | Chimeric mAb | 1/2a | Crohn’s disease | Safe; Effective | 16011669 |
| Bleselumab | Human mAb | 1 | Moderate-severe psoriasis | Safe; Not effective | 29679478 |
| 1b, 2 | Kidney Transplantation | Safe; Noninferiority | 31509331 31397943 |
||
| BI 655064 | Humanized mAb | I | Healthy subject | Safe | 29127458 30113724 |
| 2a | Rheumatoid arthritis | Safe; Not effective | 30902820 | ||
| Iscalimab | Human mAb | 1 | Rheumatoid arthritis Healthy subject | Safe | 31647605 |
| 2 | Grave’s disease | Safe; Effective | 31512728 | ||
| 1/2 | Kidney Transplantation | Unpublished | NCT02217410 | ||
| 2 | Kidney transplantation Liver transplantation | Ongoing |
NCT03663335 NCT03781414 |
||
| 2 | Lupus nephritis | Ongoing | NCT03610516 | ||
| 2 | Systemic lupus erythematosus | Ongoing | NCT03656562 | ||
| 2 | Sjögren syndrome | Ongoing | NCT03905525 | ||
| Lucatumumab | Human mAb | 1 | CLL | Safe; Minimal efficiency | 22475052 |
| 1 | Relapsed MM | Safe; Moderate efficiency | 22861192 | ||
| 1a/2 | Advanced lymphoma | Safe; Moderate efficiency | 24219359 |
Abbreviations: CLL, chronic lymphocytic leukemia; mAb: monoclonal antibody; MM, multiple myeloma.
Ch5D12 is a chimeric IgG4 mAb with antagonistic activity against CD40. It reduces B cell activation and proliferation and GC formation in non-human primates.(184–185) In a phase 1/2a trial with moderate to severe Crohn’s disease patients, ch5D12 reduced mucosal inflammation and induced disease remission.(186)
Bleselumab is a human IgG4 mAb. It inhibits sCD40L stimulated proliferation of human peripheral blood mononuclear cells, and does not induce CDC and ADCC or destabilize platelet thrombi.(187) In a phase 1 trial with moderate-severe psoriasis patients, bleselumab failed to demonstrate clinical efficacy.(188) In a phase lb trial with kidney transplantation patients, bleselumab displayed dose-dependent prolonged B cell CD40 occupancy.(189) Recently, in a phase 2 trial with kidney transplantation patients, bleselumab plus tacrolimus demonstrated noninferiority to standard of care and had a favorable benefit-risk ratio.(190)
BI 655064 is humanized IgG1 mAb with mutated Fc. It blocks CD40L-induced proliferation of B cells and activation of ECs and DCs.(191) In two phase 1 trials with healthy subjects, BI 655064 induced dose-dependent CD40 occupancy.(192–193) In a phase 2a trial with active rheumatoid arthritis patients, BI 655064 reduced B cell activation, rheumatoid factor concentration and inflammatory markers levels, but did not demonstrate clinical efficacy.(194)
Iscalimab is human IgG1 mAb with a mutated Fc region. It inhibits CD40L-induced activation of cultured human leukocytes but does not lead to ADCC or CDC. It also blocks T cell-dependent antibody response and abrogates GC formation in nonhuman primates.(195) In a phase 1 trial with healthy subjects and rheumatoid patients, iscalimab showed antagonistic activity by inhibiting T-cell dependent antibody response and CD40L-induced B cell activation.(196) In a phase 2 trial, 47% of Grave’s disease patients showed clinical response and decreased serum concentrations of autoantibodies.(197) Currently, multiple phase 1 to 2 trails with iscalimab are ongoing in various diseases including kidney transplantation (NCT02217410/NCT03663335), liver transplantation (NCT03781414), lupus nephritis (NCT03610516), systemic lupus erythematosus (SLE, NCT03656562) and Sjögren syndrome (NCT03905525).
Lucatumumab is a human IgG1 antagonistic mAb. It inhibits CD40L-induced proliferation and survival of CLL cells and mediates ADCC.(150) In a phase 1 trial with patients of relapsed CLL, lucatumumab demonstrated minimal anti-tumor activity.(198) In a phase 1 trial with patients of relapsed MM, lucatumumab showed modest anti-tumor activity.(199) In a phase la/2 trial with patients of advanced lymphoma, lucatumumab demonstrated modest anti-tumor activity, especially in patients with follicular lymphoma and marginal zone lymphoma of mucosa-associated lymphatic tissue.(200)
Clinical trials of CD40L-targeted agonist therapy —
We summarize current CD40L-targeted agonist therapy in Table 4A. All agents were well tolerated.
Table 4A.
Clinical trials of CD40L agonist therapy
| Drug | Feature | Phase | Disease | Outcome | PMID/Identifier |
|---|---|---|---|---|---|
| Avrend | Trimeric soluble human CD40L | 1 | Advanced solid tumors and NHL | Safe; Effective | 11432896 |
| Ad-CD40L | Adenovirus carrying human CD40L gene | 1, 2 | Melanoma | Safe; Effective | 27031851 28427434 |
| 1/2 | Bladder cancer | Safe; Effective | 20448220 | ||
| Ad-CD40L-CLL cells | Autologous CLL cells transduced with Ad-murine or humanized CD40L | 1 | CLL | Safe; Effective | 11049967 20882050 |
| GM.CD40L | Bystander cells expressing liuman GM-CSF/CD40L | 2 | Refectory lung adenocarcinoma | Safe | 23994887 |
| 1/2 | Advanced lung adenocarcinoma | Safe | 30209589 | ||
| TriMixDC | Autologous DC electroporated with human CD40L/TLR4/ CD70 mRNA | 1 | Advanced melanoma | Safe | 21577140 |
| TriMixDC-MEL | Autologous DC electroporated with human CD40L/TLR4/ CD70/tumor antigen mRNA | 1 | Advanced melanoma | Safe | 23904461 |
| 2 | Melanoma | Unpublished |
NCT00101166 |
Abbreviations: CD40L, CD40L ligand; CLL, chronic lymphocytic leukemia; DC, dendritic cell; GM-CSF, granulocyte-macrophage colony-stimulating factor; NHL, non-Hodgkin lymphoma; TLR4, toll like receptor 4.
Avrend is a trimeric recombinant protein of human sCD40L. In preclinical studies, it activated anti-tumor immune responses by enhancing antigen presentation in APCs and triggering T cell response, which led to tumor remission in human breast cancer and lymphoma-bearing immune-deficient SCID mice.(201–202) In a phase 1 trial with patients of advanced solid tumors or NHL, Avrend showed anti-tumor efficacy.(203)
In a phase 1 trial with CLL patients, infusion of autologous CLL cells transduced with adenovirus encoding murine mCD40L, led to induction of tumor-specific T cells and antibody response, as well as tumor remission.(204) However, some patients developed antibodies against murine CD40L. To mitigate the problem, the same investigators further conducted a phase 1 trial with CLL cells transduced with adenovirus encoding ISF35 (humanized mCD40L).(205) ISF35-transduced CLL cells demonstrated anti-tumor activity in CLL patients. In two trails with metastatic melanoma patients, adenovirus carrying CD40L gene (Ad-CD40L) induced T cell activation correlated to prolonged survival.(206–207) In a trail with bladder cancer patients, Ad-CD40L promoted T cell infiltration into bladder and showed antitumor activity.(208)
In addition. CD40L co-expression with other immunostimulatory molecules by plasmid transfection or mRNA electroporation in bystander cells or autologous DCs were used to induce a tumor-specific immune response. For example, CD40L co-expressed with GM-CSF in bystander cells, and with toll-like receptor 4 (TLR4) /CD70 or TLR4/CD70/tumor antigen in autologous DCs were used to treat advanced lung adenocarcinoma and advanced melanoma. In phase 1 to 2 trials, these agents promoted lymphocytes infiltration in tumors.(209–212)
Clinical trials of CD40L-targeted antagonist therapy —
Current CD40L-targeted antagonist therapy is summarized in Table 4B. The majority of these agents were relatively well tolerated, although trials of some CD40L antagonistic mAbs were discontinued because of thrombosis.
Table 4B.
Clinical trials of CD40L antagonist therapy
| Drug | Feature | Phase | Disease | Outcome | PMID/Identifier |
|---|---|---|---|---|---|
| BG9588 | Humanized mAb | 1 | Proliferative lupus nephritis | Thromboembolic events; Effective | 12632425 |
| 1 | Systemic lupus erythematosus | Thromboembolic events; Effective | 14617752 | ||
| IDEC-131 | Humanized mAb | 1, 2 | Systemic lupus erythematosus | Safe; Noneffective | 11196549 12483729 |
| 1 | Refractory immune thrombocytopenic purpura | Safe; Effective | 14551140 18341638 |
||
| Dapirolizumab | Humanized mAb Fab | 1 | Systemic lupus erythematosus | Safe; Effective | 28780512 |
| AT-1501 | Fc-engineered humanized mAb | 2a | Amyotrophic lateral sclerosis | Unpublished | NCT04322149 |
| BMS-986004 | Fc-engineered humanized mAb | 1/2 | Acute graft-versus-host disease | Unpublished | NCT03605927 |
| VIB4920 | Engineered human protein | 1 | Rheumatoid arthritis | Safe; Effective | 31019027 |
Abbreviations: CD40L, CD40L ligand; Fab, antigen-binding fragment; Fc, constant fragment; mAb: monoclonal antibody.
BG9588 is a humanized CD40L antagonistic IgG1 mAb. Although BG9588 showed potential efficacy by inhibiting abnormal B cell response and production of autoantibodies in patients with lupus nephritis and SLE, two phase 1 trials with this agent were terminated prematurely due to increased thrombotic events (TEs).(213–214) IDEC-131 is also a humanized CD40L antagonistic IgG1 mAb and has been evaluated in several phase 1 to 2 trails with patients of SLE, thrombocytopenic purpura, multiple sclerosis and Crolm’s disease.(215–218) In these trials, no definite TEs were reported in patients with SLE and thrombocytopenic purpura, but trials regarding multiple sclerosis and Crolm’s disease were stopped because of TEs.(219) The TEs in these trails may be caused by Fc fragment of mAb crosslinking to FcγRIIa on platelets, leading to subsequent platelet activation and aggregation.(220)
Dapirolizumab is the Fab fragment of a humanized, monovalent pegylated CD40L antagonistic mAb. In a phase 1 trial with patients of SLE, Dapirolizumab showed potential efficacy in patients with high disease activity.(221)
Currently, phase 1 to 2 trials with Fc-engineered CD40L antagonistic mAbs (AT-1501 and BMS-986004), are ongoing in patients with amyotrophic lateral sclerosis (NCT04322149) or acute graft-versus-host disease (NCT03605927).
VIB4920 is an engineered human tenascin C protein which binds to human CD40L and has antagonistic activity.(222) In a phase 1 trial with healthy volunteers, VIB4920 suppressed T cell-dependent antibody response and B cell activation/memory response. In addition. VIB4920 reduces serum levels of rheumatoid factor autoantibody and showed efficacy in patients with rheumatoid arthritis. (223)
Summary of clinical efficacy of CD40/CD40L-targeted therapy —
So far, clinical evaluation of CD40/CD40L-targeted therapy only involved early-stage clinical trials enrolling limited numbers of patients. In general, the current completed trials of CD40/CD40L-targeted therapy demonstrated clinical safety and favorable immune responses as single-agent or combinatory therapy in malignancies and autoimmune diseases. In CD40/CD40L anti-tumor therapy, clinical efficacy is relatively low with complete response rate ranging from 2% to 20% and partial response rate from 4% to 28.6%. In contrast, CD40/CD40L anti-autoimmune disease therapy is promising with clinical response rate ranging from 46% to 75% (Table 5). Improvement in clinical efficacy is expected from the ongoing trails with advanced CD40/CD40L anti-tumor therapy because of the following: 1) CD40 agonistic mAbs have enhanced agonistic capacity (e.g. APX005M) and Fc-dependent ant-tumor activity (e.g. CDX-1140, SEA-CD40 and 2141-V11); 2) combinatory therapy with antiangiogenic mAbs or anti-PDL1 is syngenetic. Furthermore, recent preclinical studies have shown even greater promise in tumor control if CD40 agonists were co-administrated with other immunostimulatory therapies, such as Flt3 ligand which promotes DC maturation and survival,(224) with anti-VEGFA/Ang2 mAbs which induce regression of the tumor microvasculature and APC activation,(225) and with anti-PD-1 and anti-CTLA4 mAbs which work together with CD40 agonist to enhance anti-tumor responses.(226)
Table 5.
Summary of clinical efficacy of CD40/CD40L-targeted therapy
| Drug | Feature | Disease | Efficacy, n (%) | PMID |
|---|---|---|---|---|
| Malignancy (single agent) | ||||
| CP-870,893 | CD40 agonistic human mAb | Solid tumors | PR: 4 (27%) in melanoma | 17327609 |
| Dacetuzumab | CD40 agonistic humanized mAb | Refractory NHL | CR: 1 (2%); PR: 5 (10%); SD:13 (26%) | 19636010 |
| Relapsed DLBCL | CR: 2 (4%); PR: 2 (4%); SD:13 (28%) | 24919462 | ||
| Avrend | Trimeric soluble human CD40L | Advanced solid tumors and NHL | CR: 1 (3%); PR: 1 (3%); SD: 4 (12%) | 11432896 |
| Lucatumumab | CD40 antagonistic human mAb | Relapsed MM | PR: 1 (4%); SD: 12 (43%) | 22861192 |
| Advanced lymphoma | FL: CR: 1 (4.8%); PR: 6 (28.6%); SD:11 (52.4%); DLBCL: CR: 2 (5.9%); PR: 2 (5.9%); SD:11 (32.4%); MALT: CR: 1 (14.3%); PR: 2 (28.6%); SD:1 (14.3%); MCL: SD: 5 (41.7%); HL: PR: 5 (13.5%); SD:12 (32.4%) | 24219359 | ||
| Malignancy (combinatory therapy) | ||||
| CP-870,893+gemcitabine | +chemotherapy | Advanced pancreatic ductal adenocarcinoma | PR: 4 (18.2%) | 23983255 |
| CP-870,893+gemcitabine | +chemotherapy | Advanced pancreatic ductal adenocarcinoma | PR: 4 (19%); SD: 11 (52.4%) | 2143645 |
| CP-870,893+tremelimumab | +CTLA4 antagonistic mAb | Metastatic melanoma | CR: 2 (9.1%); PR: 4 (18.2) | 30288340 |
| Dacetuzumab+rituximab+gemcitabine | + CD20 antagonistic mAb+chemotherapy | Relapsed DLBCL | CR: 6 (20%); PR: 8 (27%); SD:13 (28%) | 22775314 |
| Autoimmunity | ||||
| ch5D12 | CD40 antagonistic chimeric mAb | Crohn’s disease | CRR: 13 (72%) | 16011669 |
| Iscalimab | CD40 antagonistic human mAb | Grave’s disease | CRR: 7 (47%) | 31512728 |
| Dapirolizumab | CD40L antagonistic humanized mAb Fab | Systemic lupus erythematosus | CRR: 11 (46%) | 28780512 |
| VIB4920 | CD40L antagonist human protein | Rheumatoid arthritis | CRR: 8 (75%) in the 1500-mg group and 6 (50%) in 1000-mg | 31019027 |
Abbreviations: CD40L, CD40L ligand; CR: complete response; CRR: clinical response rate; CTLA4: cytotoxic T-Lymphocyte associated protein 4; DLBCL: diffuse large B-cell lymphoma; Fab, antigen-binding fragment; FL: follicular lymphoma; HL: Hodgkin lymphoma; mAb: monoclonal antibody; MALT: mucosa associated lymphatic tissue lymphoma; MCL: mantle cell lymphoma; PR: partial response; SD: stable disease.
Conclusion and future perspectives
In the past decades, mounting studies have been made in elucidating the molecular basis of CD40/CD40 immune checkpoint. Both CD40 and CD40L have a membrane form and a soluble form generated by proteolytic cleavage or alternative splicing. Initiation of CD40/CD40L signaling requires trimerization or multimerization of the receptor. CD40 are widely expressed in various types of immune cells and non-immune cells. B cells, MCs, MΦ and DCs are the primary cells constitutively expressing high levels of CD40 which can be further enhanced by “danger” signals including PAMP, DAMP and MADS. CD40+ MCs represent a population of MCs with strong inflammatory features in human. CD40/CD40L interaction plays indispensable roles in humoral and cellular immunity by regulating B cell, T cell and APC activation and immune memory. CD40/CD40L molecular pair mediates a two-way signaling between T cells and APCs, by which the reverse signal leads to activation and differentiation of APCs and the forward signal results in T cell/B cell activation and differentiation. The role of CD40 expression in most immune cell subsets has not been tested and will require future experimental investigation.
Recent advances from recently accomplished early phase CD40/CD40L-targeted clinical trials demonstrate promising benefits in the treatments of malignancies, autoimmunity and allograft rejection. Considering its critical roles in the regulation of platelet and conditional innate immune cell activation, CD40/CD40L-targeted strategy could be beneficial for chronical disease involving abnormal activation of these cells, such as cardiovascular diseases and chronic kidney disease. Further investigation in molecular mechanisms underlying CD40/CD40L regulation in these cells would provide important insight and novel therapeutic targets.
Acknowledgments
Source of Funding
This work is supported in part by the National Institutes of Health (NIH) grants HL82774, HL110764, HL130233, HL131460, DK104114, DK113775 and HL131460 to HW.
Abbreviations
- ADMA
a disintegrin and metalloprotease
- ADP
adenosine di-phosphate
- AKT
protein kinase B
- AP1
activator protein 1
- ApoE
apolipoprotein E
- ADCC
antibody-dependent cell-mediated cytotoxicity
- APC
antigen presenting cell
- BM
bone marrow
- BCR
B cell receptor
- CRD
cysteine-rich domain
- CD40L
CD40 ligand
- CSR
class-switch recombination
- CKD
chronic kidney disease
- CDC
complement-dependent cytotoxicity
- cDC1
conventional DC1
- CTL
cytotoxic T lymphocyte
- CRS
cytokine response syndrome
- CLL
chronic lymphocytic leukemia
- DC
dendritic cell
- DLBCL
diffuse large B cell lymphoma
- ERK
extracellular-signal-regulated kinase
- EC
endothelial cell
- FO B cell
follicular B cell
- Fc
constant fragment
- FcγR
Fc γ receptor
- GC
germinal center
- GM-CSF
granulocyte/macrophage colony-stimulating factor
- HIGM
hyper-IgM syndrome
- HLA-DR
leukocyte antigen-DR
- HUVEC
human umbilical vein endothelial cell
- HPC
hematopoietic progenitor cell
- ICAM-1
intercellular adhesion molecule-1
- Ig
immunoglobin
- IKK
inhibitor of NF-κB kinase
- IκB
NF-κB inhibitor
- IL
interleukin
- ILC
innate lymphoid cell
- IFN
interferon
- JNK
JUN N-terminal kinase
- JAK3
janus kinase 3
- mCD40
membrane CD40
- mCD40L
membrane CD40 ligand
- MC
monocytes
- MΦ
macrophage
- MMP
matrix metalloproteinase
- MAPK
mitogen-activated protein kinase
- MZ B cell
marginal zone B cell
- MM
multiple myeloma
- mAb
monoclonal antibody
- NHL
non-Hodgkin’s lymphoma
- NF-κB
nuclear factor kappa-B
- NK cell
natural killer cell
- PI3K
phosphoinositide 3-kinase
- PD1
programmed cell death protein 1
- PDL1
prograimned cell death ligand 1
- PLA
platelet-leukocyte aggregate
- RANTES
regulated on activation, normal T cell expressed and secreted
- sCD40
soluble CD40
- sCD40L
soluble CD40 ligand
- STAT
signal transducer and activator of transcription
- SHM
somatic hypermutation
- SLE
systemic lupus erythematosus
- TLR4
toll-like receptor 4
- TEs
thrombotic events
- TCR
T cell receptor
- Tfh cell
follicular helper T cell
- Th cell
T helper cell
- TNFRSF
tumor necrosis factor receptor superfamily
- TNFSF
tumor necrosis factor superfamily
- TRAF
TNF receptor associated factor
- VCAM-1
vascular cell adhesion molecule-1
- VSMC
vascular smooth muscle cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures
The authors have none to declare.
References
- 1.Zhang Q, Vignali DA. Co-stimulatory and Co-inhibitory Pathways in Autoimmunity. Immunity. 2016; 44:1034–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He X, Xu C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020;30(8):660–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun C, Mezzadra R, Schumacher TN. Regulation and Function of the PD-L1 Checkpoint. Immunity. 2018; 48:434–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ledbetter JA, Shu G, Gallagher M, Clark EA. Augmentation of normal and malignant B cell proliferation by monoclonal antibody to the B cell-specific antigen BP50 (CDW40). J Immunol. 1987; 138:788–94. [PubMed] [Google Scholar]
- 5.Mallett S, Fossum S, Barclay AN. Characterization of the MRC OX40 antigen of activated CD4 positive T lymphocytes--a molecule related to nerve growth factor receptor. EMBO J. 1990; 9:1063–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smulski CR, Beyrath J, …, Foumel S Cysteine-rich domain 1 of CD40 mediates receptor self-assembly. J Biol Chem. 2013; 288:10914–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tone M, Tone Y, Fairchild PJ, Wykes M, Waldmann H. Regulation of CD40 function by its isoforms generated through alternative splicing. Proc Natl Acad Sci U S A. 2001; 98: 1751–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Contin C, Pitard V, Itai T, Nagata S, Moreau JF, Déchanet-Merville J. Membrane-anchored CD40 is processed by the tumor necrosis factor-alpha-converting enzyme. Implications for CD40 signaling. J Biol Chem. 2003; 278:32801–9. [DOI] [PubMed] [Google Scholar]
- 9.Fanslow WC, Anderson DM, Grabstein KH, Clark EA, Cosman D, Annitage RJ. Soluble forms of CD40 inhibit biologic responses of human B cells. J Immunol. 1992; 149:655–60. [PubMed] [Google Scholar]
- 10.Wang Y, Kelly CG, …, Lelmer T CD40 is a cellular receptor mediating mycobacterial heat shock protein 70 stimulation of CC-chemokines. Immunity. 2001; 15:971–83. [DOI] [PubMed] [Google Scholar]
- 11.Mlynarcik P, Pulzova L, …, Bliide MR. Deciphering the interface between a CD40 receptor and borrelial ligand OspA. Microbiol Res. 2015; 170:51–60. [DOI] [PubMed] [Google Scholar]
- 12.Armitage RJ, Fanslow WC, …, Maliszewski CR. Molecular and biological characterization of a murine ligand for CD40. Nature. 1992; 357:80–2. [DOI] [PubMed] [Google Scholar]
- 13.Peitsch MC, Jongeneel CV. A 3-D model for the CD40 ligand predicts that it is a compact trimer similar to the tumor necrosis factors. Int Immunol. 1993; 5:233–8. [DOI] [PubMed] [Google Scholar]
- 14.Hsu YM, Lucci J, Su L, Ehrenfels B, Garber E, Thomas D. Heteromultimeric complexes of CD40 ligand are present on the cell surface of human T lymphocytes. J Biol Chem. 1997; 272:911–5. [DOI] [PubMed] [Google Scholar]
- 15.Yacoub D, Benslimane N, Al-Zoobi L, Hassan G, Nadiri A, Mourad W. CD 154 is released from T-cells by a disintegrin and metalloproteinase domain-containing protein 10 (ADAM10) and ADAM17 in a CD40 protein-dependent manner. J Biol Chem. 2013; 288:36083–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi WS, Jeon OH, Kim DS. CD40 ligand shedding is regulated by interaction between matrix metalloproteinase-2 and platelet integrin alpha(IIb)beta(3). J Thromb Haemost. 2010; 8:1364–71. [DOI] [PubMed] [Google Scholar]
- 17.Pietravalle F, Lecoanet-Henchoz S, …, Gauchat JF. Human native soluble CD40L is a biologically active trimer, processed inside microsomes. J Biol Chem. 1996; 271:5965–7. [DOI] [PubMed] [Google Scholar]
- 18.Graf D, Müller S, Korthöuer U, van Kooten C, Weise C, Kroczek RA. A soluble form of TRAP (CD40 ligand) is rapidly released after T cell activation. Em J Immunol. 1995; 25:1749–54. [DOI] [PubMed] [Google Scholar]
- 19.Alturaihi H, Hassan GS, …, Mourad W. Interaction of CD154 with different receptors and its role in bidirectional signals. Eur J Immunol. 2015; 45:592–602. [DOI] [PubMed] [Google Scholar]
- 20.Takada YK, Yu J, Shimoda M, Takada Y. Integrin Binding to the Trimeric Interface of CD40L Plays a Critical Role in CD40/CD40L Signaling. J Immunol. 2019; 203:1383–91. [DOI] [PubMed] [Google Scholar]
- 21.Pullen SS, Labadia ME, …, Kehry MR. High-affinity interactions of tumor necrosis factor receptor-associated factors (TRAFs) and CD40 require TRAF trimerization and CD40 multimerization. Biochemistry. 1999; 38:10168–77. [DOI] [PubMed] [Google Scholar]
- 22.An HJ, Kim YJ, …, Lee H. Crystallographic and mutational analysis of the CD40-CD154 complex and its implications for receptor activation. J Biol Chem. 2011; 286:11226–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bajorath J, Chalupny NJ, …, Aruffo A. Identification of residues on CD40 and its ligand which are critical for the receptor-ligand interaction. Biochemistry. 1995; 34:1833–44. [DOI] [PubMed] [Google Scholar]
- 24.Singh J, Garber E, …, Thomas D. The role of polar interactions in the molecular recognition of CD40L with its receptor CD40. Protein Sci. 1998; 7:1124–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bajorath J, Marken JS, …, Aruffo A. Analysis of gp39/CD40 interactions using molecular models and site-directed mutagenesis. Biochemistry. 1995; 34:9884–92. [DOI] [PubMed] [Google Scholar]
- 26.Mabbott NA, Baillie JK, Brown H, Freeman TC, Hume DA. An expression atlas of human primary cells: inference of gene function from coexpression networks. BMC Genomics. 2013; 14:632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madissoon E, Wilbrey-Clark A, …, Meyer KB. scRNA-seq assessment of the human lung, spleen, and esophagus tissue stability after cold preservation. Genome Biol. 2019; 21:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quiding-Järbrink M, …, Czerkinsky C. Human circulating specific antibody-forming cells after systemic and mucosal immunizations: differential homing commitments and cell surface differentiation markers. Eur J Immunol 1995; 25:322–7. [DOI] [PubMed] [Google Scholar]
- 29.Pons AR, Noguera A, Blanquer D, Sauleda J, Pons J, Agustí AG. Phenotypic characterisation of alveolar macrophages and peripheral blood monocytes in COPD. Eur Respir J. 2005; 25:647–52. [DOI] [PubMed] [Google Scholar]
- 30.Velazquez VM, Hon H, Ibegbu C, Knechtle SJ, Kirk AD, Grakoui A. Hepatic enrichment and activation of myeloid dendritic cells during chronic hepatitis C virus infection. Hepatology. 2012; 56:2071–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D’Antò V, Eckhardt A, …, Schweikl H. The influence of Ni(II) on surface antigen expression in murine macrophages. Biomaterials. 2009; 30:1492–501. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi H, Sadamori H, …, Nishibori M. Histamine inhibits high mobility group box 1-induced adhesion molecule expression on human monocytes. Eur J Pharmacol. 2013; 718:305–13. [DOI] [PubMed] [Google Scholar]
- 33.Yang J, Fang P, …, Wang H. Chronic Kidney Disease Induces Inflammatory CD40+ Monocyte Differentiation via Homocysteine Elevation and DNA Hypomethylation. Circ Res. 2016; 119:1226–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prontera C, Martelli N, …, Romano M. Homocysteine modulates the CD40/CD40L system. J Am Coll Cardiol. 2007; 49:2182–90. [DOI] [PubMed] [Google Scholar]
- 35.Alderson MR, Annitage RJ, Tough TW, Strockbine L, Fanslow WC, Spriggs MK. CD40 expression by human monocytes: regulation by cytokines and activation of monocytes by the ligand for CD40. J Exp Med. 1993; 178:669–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koguchi Y, Buenafe AC, …, Parker DC. Preformed CD40L is stored in Th1, Th2, Th17, and T follicular helper cells as well as CD4+ 8- thymocytes and invariant NKT cells but not in Treg cells. PLoS One. 2012; 7:e31296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li W, Carlson TL, Green WR. Stimulation-dependent induction of CD154 on a subset of CD4+ FoxP3+ T-regulatory cells. Int Immunopharmacol. 2011; 1:1205–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koguchi Y, Thauland TJ, Slifka MK, Parker DC. Preformed CD40 ligand exists in secretory lysosomes in effector and memory CD4+ T cells and is quickly expressed on the cell surface in an antigen-specific manner. Blood. 2007; 110:2520–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vavassori S, Covey LR. Post-transcriptional regulation in lymphocytes: the case of CD 154. RNA Biol. 2009; 6:259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yellin MJ, Sippel K, …, Ledennan S CD40 molecules induce down-modulation and endocytosis of T cell surface T cell-B cell activating molecule/CD40-L. Potential role in regulating helper effector function. J Immunol. 1994; 152:598–608. [PubMed] [Google Scholar]
- 41.Ludewig B, Henn V, Schröder JM, Graf D, Kroczek RA. Induction, regulation, and function of soluble TRAP (CD40 ligand) during interaction of primary CD4+ CD45RA+ T cells with dendritic cells. Em J Immunol 1996;26:3137–43. [DOI] [PubMed] [Google Scholar]
- 42.Jaiswal AI, Dubey C, Swain SL, Croft M. Regulation of CD40 ligand expression on naive CD4 T cells: a role for TCR but not co-stimulatory signals. Int Immunol. 1996; 8:275–85. [DOI] [PubMed] [Google Scholar]
- 43.Chakrabarty S, Snyder JT, …, Ragheb JA. Human CD14hi monocytes and myeloid dendritic cells provide a cell contact-dependent costimulatory signal for early CD40 ligand expression. Blood. 2011; 117:1585–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peng X, Remade JE, Kasran A, Huylebroeck D, Ceuppens JL. IL-12 up-regulates CD40 ligand (CD154) expression on human T cells. J Immunol. 1998; 160:1166–72. [PubMed] [Google Scholar]
- 45.Henn V, Slupsky JR, …, Kroczek RA. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998; 391:591–4. [DOI] [PubMed] [Google Scholar]
- 46.Henn V, Steinbach S, Büchner K, Presek P, Kroczek RA. The inflammatory action of CD40 ligand (CD154) expressed on activated human platelets is temporally limited by coexpressed CD40. Blood. 2001; 98:1047–54. [DOI] [PubMed] [Google Scholar]
- 47.Tay NQ, Lee DCP, Chua YL, Prabhu N, Gascoigne NRJ, Kemeny DM. CD40L Expression Allows CD8+ T Cells to Promote Their Own Expansion and Differentiation through Dendritic Cells. Front Immunol. 2017; 8:1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Papa I, Saliba D, …, Vinuesa CG. TFH-derived dopamine accelerates productive synapses in germinal centres. Nature. 2017; 547:318–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Merluzzi S, Frossi B, Gri G, Parusso S, Tripodo C, Pucillo C. Mast cells enhance proliferation of B lymphocytes and drive their differentiation toward IgA-secreting plasma cells. Blood. 2010; 115:2810–7. [DOI] [PubMed] [Google Scholar]
- 50.Zhang AL, Colmenero P, …, Söderström K. Natural killer cells trigger differentiation of monocytes into dendritic cells. Blood. 2007; 110:2484–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Magri G, Miyajima M, …, Cerutti A. Innate lymphoid cells integrate stromal and immunological signals to enhance antibody production by splenic marginal zone B cells. Nat Immunol. 2014; 15:354–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schönbeck U, Libby P. The CD40/CD154 receptor/ligand dyad. Cell Mol Life Sci. 2001; 58:4–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McWhirter SM, Pullen SS, Holton JM, Crate JJ, Kehry MR, Alber T. Crystallographic analysis of CD40 recognition and signaling by human TRAF2. Proc Natl Acad Sci U S A. 1999; 96:8408–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee HH, Dempsey PW, Parks TP, Zhu X, Baltimore D, Cheng G. Specificities of CD40 signaling: involvement of TRAF2 in CD40-induced NF-kappaB activation and intercellular adhesion molecule-1 up-regulation. Proc Natl Acad Sci U S A. 1999; 96:1421–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hostager BS, Catlett IM, Bishop GA. Recruitment of CD40 and tumor necrosis factor receptor-associated factors 2 and 3 to membrane microdomains during CD40 signaling. J Biol Chem. 2000; 275:15392–8. [DOI] [PubMed] [Google Scholar]
- 56.Pullen SS, Miller HG, Everdeen DS, Dang TT, Crate JJ, Kehry MR. CD40-tumor necrosis factor receptor-associated factor (TRAF) interactions: regulation of CD40 signaling through multiple TRAF binding sites and TRAF hetero-oligomerization. Biochemistry. 1998; 37:11836–45. [DOI] [PubMed] [Google Scholar]
- 57.Tsukamoto N, Kobayashi N, Azuma S, Yamamoto T, Inoue J. Two differently regulated nuclear factor kappaB activation pathways triggered by the cytoplasmic tail of CD40. Proc Natl Acad Sci U S A. 1999; 96:1234–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ahonen C, Manning E, …, Noelle RJ. The CD40-TRAF6 axis controls affinity maturation and the generation of long-lived plasma cells. Nat Immunol. 2002; 3:451–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lutgens E, Lievens D, …, Weber C. Deficient CD40-TRAF6 signaling in leukocytes prevents atherosclerosis by skewing the immune response toward an antiinflammatory profile. J Exp Med. 2010; 207:391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seijkens TTP, van Tiel CM, …, Lutgens E Targeting CD40-Induced TRAF6 Signaling in Macrophages Reduces Atherosclerosis. J Am Coll Cardiol. 2018; 71:527–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun SC. The non-canonical NF-κB pathway in immunity and inflammation. Nat Rev Immunol. 2017; 17:545–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hauer J, Püschner S, …, Engelmann H. TNF receptor (TNFR)-associated factor (TRAF) 3 serves as an inhibitor of TRAF2/5-mediated activation of the noncanonical NF-kappaB pathway by TRAF-binding TNFRs. Proc Natl Acad Sci U S A. 2005; 102:2874–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grech AP, Amesbury M, Chan T, Gardam S, Basten A, Brink R. TRAF2 differentially regulates the canonical and noncanonical pathways of NF-kappaB activation in mature B cells. Immunity. 2004; 21:629–42. [DOI] [PubMed] [Google Scholar]
- 64.Propst SM, Estell K, Schwiebert LM. CD40-mediated activation of NF-kappa B in airway epithelial cells. J Biol Chem. 2002; 277:37054–63. [DOI] [PubMed] [Google Scholar]
- 65.Gallagher E, Enzler T, …, Karin M Kinase MEKK1 is required for CD40-dependent activation of the kinases Jnk and p38, germinal center formation, B cell proliferation and antibody production. Nat Immunol. 2007; 8:57–63. [DOI] [PubMed] [Google Scholar]
- 66.Lee SY, Reichlin A, Santana A, Sokol KA, Nussenzweig MC, Choi Y. TRAF2 is essential for JNK but not NF-kappaB activation and regulates lymphocyte proliferation and survival. Immunity. 1997; 7:703–13. [DOI] [PubMed] [Google Scholar]
- 67.Jabara H, Laouini D, …, Gelia R The binding site for TRAF2 and TRAF3 but not for TRAF6 is essential for CD40-mediated immunoglobulin class switching. Immunity. 2002; 17:265–76. [DOI] [PubMed] [Google Scholar]
- 68.Ha YJ, Lee JR. Role of TNF receptor-associated factor 3 in the CD40 signaling by production of reactive oxygen species through association with p40phox, a cytosolic subunit of nicotinamide adenine dinucleotide phosphate oxidase. J Immunol. 2004; 172:231–9. [DOI] [PubMed] [Google Scholar]
- 69.Benson RJ, Hostager BS, Bishop GA. Rapid CD40-mediated rescue from CD95-induced apoptosis requires TNFR-associated factor-6 and PI3K. Eur J Immunol. 2006; 36:2535–43. [DOI] [PubMed] [Google Scholar]
- 70.Mukundan L, Bishop GA, Head KZ, Zhang L, Wahl LM, Suttles J. TNF receptor-associated factor 6 is an essential mediator of CD40-activated proinflammatory pathways in monocytes and macrophages. J Immunol 2005; 174:1081–90. [DOI] [PubMed] [Google Scholar]
- 71.Vidalain PO, Azocar O, Servet-Delprat C, Rabourdin-Combe C, Gerlier D, Manié S. CD40 signaling in human dendritic cells is initiated within membrane rafts. EMBO J. 2000; 19:3304–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Deregibus MC, Buttiglieri S, Russo S, Bussolati B, Camussi G. CD40-dependent activation of phosphatidylinositol 3-kinase/Akt pathway mediates endothelial cell survival and in vitro angiogenesis. J Biol Chem. 2003; 278:18008–14. [DOI] [PubMed] [Google Scholar]
- 73.Purkerson JM, Smith RS, Pollock SJ, Phipps RP. The TRAF6, but not the TRAF2/3, binding domain of CD40 is required for cytokine production in human lung fibroblasts. Eur J Immunol. 2005; 35:2920–8. [DOI] [PubMed] [Google Scholar]
- 74.Kashiwada M, Shirakata Y, …, Takemori T. Tumor necrosis factor receptor-associated factor 6 (TRAF6) stimulates extracellular signal-regulated kinase (ERK) activity in CD40 signaling along a ras-independent pathway. J Exp Med. 1998; 187:237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Georgopoulos NT, Steele LP, Thomson MJ, Selby PJ, Southgate J, Trejdosiewicz LK. A novel mechanism of CD40-induced apoptosis of carcinoma cells involving TRAF3 and JNK/AP-1 activation. Cell Death Differ. 2006; 13:1789–801. [DOI] [PubMed] [Google Scholar]
- 76.Revy P, Hivroz C, Andreu G, Graber P, Martinache C, Fischer A, Durandy A. Activation of the Janus kinase 3-STAT5a pathway after CD40 triggering of human monocytes but not of resting B cells. J Immunol. 1999; 163:787–93. [PubMed] [Google Scholar]
- 77.Hanissian SH, Gelia RS. Jak3 is associated with CD40 and is critical for CD40 induction of gene expression in B cells. Immunity. 1997; 6:379–87. [DOI] [PubMed] [Google Scholar]
- 78.Nutt SL, Hodgkin PD, Tarlinton DM, Corcoran LM. The generation of antibody-secreting plasma cells. Nat Rev Immunol. 2015; 15:160–71. [DOI] [PubMed] [Google Scholar]
- 79.Qamar N, Fuleihan RL. The hyper IgM syndromes. Clin Rev Allergy Immunol. 2014; 46:120–30. [DOI] [PubMed] [Google Scholar]
- 80.Xu J, Foy TM, …, Flavell RA. Mice deficient for the CD40 ligand. Immunity. 1994; 1:423–31. [DOI] [PubMed] [Google Scholar]
- 81.Bergqvist P, Gärdby E, Stensson A, Bemark M, Lycke NY. Gut IgA class switch recombination in the absence of CD40 does not occur in the lamina propria and is independent of germinal centers. J Immunol. 2006; 177:7772–83. [DOI] [PubMed] [Google Scholar]
- 82.Breitfeld D, Ohl L, …, Förster R. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000; 192:1545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Weinstein JS, Herman EI. …, Craft J. TFH cells progressively differentiate to regulate the germinal center response. Nat Immunol. 2016; 17:1197–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ise W, Fujii K, …, Kurosaki T. T Follicular Helper Cell-Germinal Center B Cell Interaction Strength Regulates Entry into Plasma Cell or Recycling Germinal Center Cell Fate. Immunity. 2018; 48:702–15. [DOI] [PubMed] [Google Scholar]
- 85.Koike T, Harada K, Horiuchi S, Kitamura D. The quantity of CD40 signaling determines the differentiation of B cells into functionally distinct memory cell subsets. Elife. 2019; 8:e44245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen Y, Yu M, …, Wang D. CXCR5+PD-1+ follicular helper CD8 T cells control B cell tolerance. Nat Commun. 2019; 10:4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Puga I, Cols M, …, Cerutti A. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat Immunol. 2011; 13:170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li S, Yan Y, …, Billiau AD. Rapidly induced, T-cell independent xenoantibody production is mediated by marginal zone B cells and requires help from NK cells. Blood. 2007; 110:3926–35. [DOI] [PubMed] [Google Scholar]
- 89.Martin RK, Damle SR, …, Conrad DH. B1 Cell IgE Impedes Mast Cell-Mediated Enhancement of Parasite Expulsion through B2 IgE Blockade. Cell Rep. 2018; 22:1824–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu D, Xu H, …, Qi H. T-B-cell entanglement and ICOSL-driven feed-forward regulation of germinal centre reaction. Nature. 2015; 517:214–8. [DOI] [PubMed] [Google Scholar]
- 91.Roy M, Aruffo A, Ledbetter J, Linsley P, Keliry M, Noelle R. Studies on the interdependence of gp39 and B7 expression and function during antigen-specific immune responses. Eur J Immunol. 1995; 25:596–603. [DOI] [PubMed] [Google Scholar]
- 92.Wu Y, Xu J, …, Liu Y. Rapid induction of a novel costimulatory activity on B cells by CD40 ligand. Curr Biol. 1995; 5:1303–11. [DOI] [PubMed] [Google Scholar]
- 93.Brossart P, Grünebach F, …, Brugger W. Generation of functional human dendritic cells from adherent peripheral blood monocytes by CD40 ligation in the absence of granulocyte-macropliage colony-stimulating factor. Blood. 1998; 92:4238–47. [PubMed] [Google Scholar]
- 94.Hagihara M, Higuchi A, …, Goto S. Platelets, after exposure to a high shear stress, induce IL-10-producing, mature dendritic cells in vitro. J Immunol. 2004; 172:5297–303. [DOI] [PubMed] [Google Scholar]
- 95.Kiener PA, Moran-Davis P, Rankin BM, Wahl AF, Aruffo A, Hollenbaugh D. Stimulation of CD40 with purified soluble gp39 induces proinflammatory responses in human monocytes. J Immunol. 1995; 155:4917–25. [PubMed] [Google Scholar]
- 96.Malik N, Greenfield BW, Wahl AF, Kiener PA. Activation of human monocytes through CD40 induces matrix metalloproteinases. J Immunol. 1996; 156:3952–60. [PubMed] [Google Scholar]
- 97.Mach F, Schönbeck U, Bonnefoy TY, Pober JS, Libby P. Activation of monocyte/macrophage functions related to acute atheroma complication by ligation of CD40: induction of collagenase, stromclysin. and tissue factor. Circulation. 1997; 96:396–9. [DOI] [PubMed] [Google Scholar]
- 98.Suttles J, Evans M, Miller RW, Poe JC, Stout RD, Walil LM. T cell rescue of monocytes from apoptosis: role of the CD40-CD40L interaction and requirement for CD40-mediated induction of protein tyrosine kinase activity. J Leukoc Biol. 1996; 60:651–7. [DOI] [PubMed] [Google Scholar]
- 99.Cabral-Marques O, Ramos RN, …, Condino-Neto A. Human CD40 ligand deficiency dysregulates the macrophage transcriptome causing functional defects that are improved by exogenous IFN-γ. J Allergy Clin Immunol. 2017; 139:900–12. [DOI] [PubMed] [Google Scholar]
- 100.Tian L, Noelle RJ, Lawrence DA. Activated T cells enhance nitric oxide production by murine splenic macrophages through gp39 and LFA-1. Eur J Immunol. 1995; 25:306–9. [DOI] [PubMed] [Google Scholar]
- 101.Stout RD, Suttles J, Xu J, Grewal IS, Flavell RA. Impaired T cell-mediated macrophage activation in CD40 ligand-deficient mice. J Immunol. 1996; 156:8–11. [PubMed] [Google Scholar]
- 102.Andrade RM, Wessendarp M, Gubbels MJ, Striepen B, Subauste CS. CD40 induces macrophage anti-Toxoplasma gondii activity by triggering autophagy-dependent fusion of pathogen-containing vacuoles and lysosomes. J Clin Invest. 2006; 116:2366–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fontana S, Moratto D, …, Badolato R. Functional defects of dendritic cells in patients with CD40 deficiency. Blood. 2003; 102:4099–106. [DOI] [PubMed] [Google Scholar]
- 104.Cabral-Marques O, Arslanian C, …, Condino-Neto A. Dendritic cells from X-linked hyper-IgM patients present impaired responses to Candida albicans and Paracoccidioides brasiliensis. J Allergy Clin Immunol. 2012; 129:778–86. [DOI] [PubMed] [Google Scholar]
- 105.Lin JH, Huffman AP, …, Vonderheide RH. Type 1 conventional dendritic cells are systemically dysregulated early in pancreatic carcinogenesis. J Exp Med. 2020; 217:e20190673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Duffau P, Seneschal J, …, Blanco P. Platelet CD154 potentiates interferon-alpha secretion by plasmacytoid dendritic cells in systemic lupus erythematosus. Sci Transl Med. 2010; 2:47ra63. [DOI] [PubMed] [Google Scholar]
- 107.Cabral-Marques O, França TT, …, Condino-Neto A. CD40 ligand deficiency causes functional defects of peripheral neutrophils that are improved by exogenous IFN-γ. J Allergy Clin Immunol. 2018; 142:1571–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jin R, Yu S, …, Li G. Soluble CD40 ligand stimulates CD40-dependent activation of the β2 integral Mac-1 and protein kinase C zeda (PKCζ) in neutrophils: implications for neutrophil-platelet interactions and neutrophil oxidative burst. PLoS One. 2013;8:e64631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dai J, Fang P, …, Wang H. Metabolism-associated danger signal-induced immune response and reverse immune checkpoint-activated CD40+ monocyte differentiation. J Hematol Oncol. 2017; 10:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sun L, Yang X, Yuan Z, Wang H. Metabolic Reprogramming in Immune Response and Tissue Inflammation. Arterioscler Thromb Vase Biol. 2020. July 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stuber E, Strober W, Neurath M. Blocking the CD40L-CD40 interaction in vivo specifically prevents the priming of T helper 1 cells through the inhibition of interleukin 12 secretion. J Exp Med. 1996; 183:693–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.de la Morena MT. Clinical Phenotypes of Hyper-IgM Syndromes. J Allergy Clin Immunol Pract. 2016; 4:1023–36. [DOI] [PubMed] [Google Scholar]
- 113.Subauste CS, Wessendarp M, Sorensen RU, Leiva LE. CD40-CD40 ligand interaction is central to cell-mediated immunity against Toxoplasma gondii: patients with hyper IgM syndrome have a defective type 1 immune response that can be restored by soluble CD40 ligand trimer. J Immunol. 1999; 162:6690–700. [PubMed] [Google Scholar]
- 114.Campbell KA, Ovendale PJ, Kennedy MK, Fanslow WC, Reed SG, Maliszewski CR. CD40 ligand is required for protective cell-mediated immunity to Leishmania major. Immunity. 1996; 4:283–9. [DOI] [PubMed] [Google Scholar]
- 115.Grewal IS, Xu J, Flavell RA. Impairment of antigen-specific T-cell priming in mice lacking CD40 ligand. Nature. 1995; 378:617–20. [DOI] [PubMed] [Google Scholar]
- 116.De Jong YP, Comiskey M, …, Terhorst C. Chronic murine colitis is dependent on the CD154/CD40 pathway and can be attenuated by anti-CD154 administration. Gastroenterology. 2000; 119:715–23. [DOI] [PubMed] [Google Scholar]
- 117.Clarke SR. The critical role of CD40/CD40L in the CD4-dependent generation of CD8+ T cell immunity. J Leukoc Biol. 2000;67:607–14. [DOI] [PubMed] [Google Scholar]
- 118.Andreasen SO, Christensen JE, Marker O, Thomsen AR. Role of CD40 ligand and CD28 in induction and maintenance of antiviral CD8+ effector T cell responses. J Immunol. 2000; 164:3689–97. [DOI] [PubMed] [Google Scholar]
- 119.Singh M, Vianden C, …, Overwijk WW. Intratumoral CD40 activation and checkpoint blockade induces T cell-mediated eradication of melanoma in the brain. Nat Commun. 2017; 8:1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lee BO, Hartson L, Randall TD. CD40-deficient, influenza-specific CD8 memory T cells develop and function normally in a CD40-sufficient enviromnent. J Exp Med. 2003; 198:1759–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bourgeois C, Rocha B, Tanchot C. A role for CD40 expression on CD8+ T cells in the generation of CD8+ T cell memory. Science. 2002; 297:2060–3. [DOI] [PubMed] [Google Scholar]
- 122.Tay NQ, Lee DCP, Chua YL, Prabhu N, Gascoigne NRJ, Kemeny DM. CD40L Expression Allows CD8+ T Cells to Promote Their Own Expansion and Differentiation through Dendritic Cells. Front Immunol. 2017; 8:1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Frentsch M, Stark R, …, Thiel A. CD40L expression permits CD8+ T cells to execute immunologic helper functions. Blood. 2013; 122:405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.André P, Nannizzi-Alaimo L, Prasad SK, Phillips DR. Platelet-derived CD40L: the switch-hitting player of cardiovascular disease. Circulation. 2002; 106:896–9. [DOI] [PubMed] [Google Scholar]
- 125.Langer F, Ingersoll SB, …, Francis JL. The role of CD40 in CD40L- and antibody-mediated platelet activation. Thromb Haemost. 2005; 93:1137–46. [DOI] [PubMed] [Google Scholar]
- 126.Inwald DP, McDowall A, Peters MJ, Callard RE, Klein NJ. CD40 is constitutively expressed on platelets and provides a novel mechanism for platelet activation. Circ Res. 2003; 92:1041–8. [DOI] [PubMed] [Google Scholar]
- 127.Khandoga A, Hanschen M, Kessler JS, Krombach F. CD4+ T cells contribute to postischemic liver injury in mice by interacting with sinusoidal endothelium and platelets. Hepatology. 2006; 43:306–15. [DOI] [PubMed] [Google Scholar]
- 128.Danese S, de la Motte C, Reyes BM, Sans M, Levine AD, Fiocchi C. Cutting edge: T cells trigger CD40-dependent platelet activation and granular RANTES release: a novel pathway for immune response amplification. J Immunol. 2004; 172:2011–5. [DOI] [PubMed] [Google Scholar]
- 129.Prasad KS, Andre P, He M, Bao M, Manganello J, Phillips DR. Soluble CD40 ligand induces beta3 integrin tyrosine phosphorylation and triggers platelet activation by outside-in signaling. Proc Natl Acad Sci U S A. 2003; 100:12367–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.André P, Prasad KS, …, Wagner DD. CD40L stabilizes arterial thrombi by a beta3 integrin--dependent mechanism. Nat Med. 2002; 8:247–52. [DOI] [PubMed] [Google Scholar]
- 131.Finsterbusch M, Schrottmaier WC, Kral-Pointner JB, Salzmann M, Assinger A. Measuring and interpreting platelet-leukocyte aggregates. Platelets. 2018; 29:677–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lievens D, Zemecke A, …, Biessen EA, Daemen MJ, Heemskerk JW, Weber C, Lutgens E. Platelet CD40L mediates thrombotic and inflammatory processes in atherosclerosis. Blood. 2010; 116:4317–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gerdes N, Seijkens T, …, Lutgens E. Platelet CD40 Exacerbates Atherosclerosis by Transcellular Activation of Endothelial Cells and Leukocytes. Arterioscler Thromb Vase Biol. 2016; 36:482–90. [DOI] [PubMed] [Google Scholar]
- 134.Zuchtriegel G, Uhl B, …, Reichel CA. Platelets Guide Leukocytes to Their Sites of Extravasation. PLoS Biol. 2016; 14:e1002459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mai J, Virtue A, Shen J, Wang H, Yang XF. An evolving new paradigm: endothelial cells--conditional innate immune cells. J Hematol Oncol. 2013; 6:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yellin MJ, Brett J, …, Chess L. Functional interactions of T cells with endothelial cells: the role of CD40L-CD40-mediated signals. J Exp Med. 1995; 182:1857–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schönbeck U, Mach F, …, Libby P. Regulation of matrix metalloproteinase expression in human vascular smooth muscle cells by T lymphocytes: a role for CD40 signaling in plaque rapture? Circ Res. 1997; 81:448–54. [DOI] [PubMed] [Google Scholar]
- 138.Sempowski GD, Chess PR, Phipps RP. CD40 is a functional activation antigen and B7-independent T cell costimulatory molecule on normal human lung fibroblasts. J Immunol. 1997; 158:4670–7. [PubMed] [Google Scholar]
- 139.Vogel JD, West GA, …, Fiocchi C. CD40-mediated immune-nonimmune cell interactions induce mucosal fibroblast chemokines leading to T-cell transmigration. Gastroenterology. 2004; 126:63–80. [DOI] [PubMed] [Google Scholar]
- 140.Denfeld RW, Hollenbaugh D, …, Simon JC. CD40 is functionally expressed on human keratinocytes. Eur J Immunol. 1996; 26:2329–34. [DOI] [PubMed] [Google Scholar]
- 141.Péguet-Navarro J, Dalbiez-Gauthier C, …, Schmitt D. CD40 ligation of human keratinocytes inhibits their proliferation and induces their differentiation. J Immunol. 1997; 158:144–52. [PubMed] [Google Scholar]
- 142.Woltman AM, de Haij S, Boonstra JG, Gobin SJ, Dalia MR, van Kooten C. Interleukin-17 and CD40-ligand synergistically enhance cytokine and chemokine production by renal epithelial cells. J Am Soc Nephrol. 2000; 11:2044–55. [DOI] [PubMed] [Google Scholar]
- 143.Weiler M, Kacliko L, Chaimovitz C, Van Kooten C, Douvdevani A. CD40 ligation enhances IL-15 production by tubular epithelial cells. J Am Soc Nephrol. 2001; 12:80–7. [DOI] [PubMed] [Google Scholar]
- 144.Vajen T, Benedikter BJ, …, Koenen RR. Platelet extracellular vesicles induce a pro-inflammatory smooth muscle cell phenotype. J Extracell Vesicles. 2017; 6:1322454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Funakoshi S, Taub DD, …, Murphy WJ. Immunologic and hematopoietic effects of CD40 stimulation after syngeneic bone marrow transplantation in mice. J Clin Invest. 1997; 99:484–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rondelli D, Lemoli RM, …, Tura S. Rapid induction of CD40 on a subset of granulocyte colony-stimulating factor-mobilized CD34(+) blood cells identifies myeloid committed progenitors and permits selection of nonimmunogenic CD40(−) progenitor cells. Blood. 1999; 94:2293–300. [PubMed] [Google Scholar]
- 147.Flores-Romo L, Björck P, Duvert V, van Kooten C, Saeland S, Banchereau J. CD40 ligation on human cord blood CD34+ hematopoietic progenitors induces their proliferation and differentiation into functional dendritic cells. J Exp Med. 1997; 185:341–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Solanilla A, Déchanet J, …, Ripoche J. CD40-ligand stimulates myelopoiesis by regulating flt3-ligand and thrombopoietin production in bone marrow stromal cells. Blood. 2000; 95:3758–64. [PubMed] [Google Scholar]
- 149.Voorzanger-Rousselot N, Favrot M, Blay JY. Resistance to cytotoxic chemotherapy induced by CD40 ligand in lymphoma cells. Blood. 1998; 92:3381–7. [PubMed] [Google Scholar]
- 150.Luqman M, Klabunde S, …, Younes A. The antileukemia activity of a human anti-CD40 antagonist antibody, HCD122, on human chronic lymphocytic leukemia cells. Blood. 2008; 112:711–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Teoh G, Tai YT, …, Anderson KC. CD40 activation mediates p53-dependent cell cycle regulation in human multiple myeloma cell lines. Blood. 2000; 95:1039–46. [PubMed] [Google Scholar]
- 152.Buhmann R, Nolte A, Westhaus D, Emmerich B, Hallek M. CD40-activated B-cell chronic lymphocytic leukemia cells for tumor immunotherapy: stimulation of allogeneic versus autologous T cells generates different types of effector cells. Blood. 1999; 93:1992–2002. [PubMed] [Google Scholar]
- 153.Pellat-Deceunynck C, Amiot M, Robillard N, Wijdenes J, Bataille R. CD11a-CD18 and CD102 interactions mediate human myeloma cell growth arrest induced by CD40 stimulation. Cancer Res. 1996; 56:1909–16. [PubMed] [Google Scholar]
- 154.Szocinski JL, Khaled AR, …, Murphy WJ. Activation-induced cell death of aggressive histology lymphomas by CD40 stimulation: induction of bax. Blood. 2002; 100:217–23. [DOI] [PubMed] [Google Scholar]
- 155.de Totero D, Tazzari PL, …, Gobbi M. CD40 triggering enhances fludarabine-induced apoptosis of chronic lymphocytic leukemia B-cells through autocrine release of tumor necrosis factor-alpha and interferon-gama and tumor necrosis factor receptor-I-II upregulation. Haematologica. 2003; 88:148–58. [PubMed] [Google Scholar]
- 156.Ozaki T, Nakagawara A. Role of p53 in Cell Death and Human Cancers. Cancers (Basel). 2011; 3:994–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bugajska U, Georgopoulos NT, …, Trejdosiewicz LK. The effects of malignant transformation on susceptibility of human urothelial cells to CD40-mediated apoptosis. J Natl Cancer Inst. 2002; 94:1381–95. [DOI] [PubMed] [Google Scholar]
- 158.Dicker F, Kater AP, …, Kipps TJ. CD154 induces p73 to overcome the resistance to apoptosis of chronic lymphocytic leukemia cells lacking functional p53. Blood. 2006; 108:3450–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Dicker F, Kater AP, Fukuda T, Kipps TJ. Fas-ligand (CD178) and TRAIL synergistically induce apoptosis of CD40-activated chronic lymphocytic leukemia B cells. Blood. 2005; 105:3193–8. [DOI] [PubMed] [Google Scholar]
- 160.Gomes EM, Rodrigues MS, …, Tong AW. Antitumor activity of an oncolytic adenoviral-CD40 ligand (CD 154) transgene construct in human breast cancer cells. Clin Cancer Res. 2009; 15:1317–25. [DOI] [PubMed] [Google Scholar]
- 161.Ibraheem K, Yluned AMA, Qayyum T, Bryan NP, Georgopoulos NT. CD40 induces renal cell carcinoma-specific differential regulation of TRAF proteins, ASK1 activation and JNK/p3 8-mediated, ROS-dependent mitochondrial apoptosis. Cell Death Discov. 2019; 5:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dalian R, Barnhart BC, Li F, Yamniuk AP, Korman AJ, Ravetch JV. Therapeutic Activity of Agonistic, Human Anti-CD40 Monoclonal Antibodies Requires Selective FcγR Engagement. Cancer Cell. 2016; 29:820–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Haswell LE, Glennie MJ, Al-Shamkhani A. Analysis of the oligomeric requirement for signaling by CD40 using soluble multimeric forms of its ligand, CD154. Em J Immunol. 2001; 31(10):3094–100. [DOI] [PubMed] [Google Scholar]
- 164.Nimmerjahn F, Gordan S, Lux A. FcγR dependent mechanisms of cytotoxic, agonistic, and neutralizing antibody activities. Trends Immunol. 2015; 36:325–36. [DOI] [PubMed] [Google Scholar]
- 165.Vonderheide RH, Flaherty KT, …, Antonia SJ. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J Clin Oncol. 2007; 25:876–83. [DOI] [PubMed] [Google Scholar]
- 166.White AL, Chan HT, …, Glennie MJ. Conformation of the human immunoglobulin G2 hinge imparts superagonistic properties to immunostimulatory anticancer antibodies. Cancer Cell. 2015; 27:138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Beatty GL, Torigian DA, …, O’Dwyer PJ. A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clin Cancer Res. 2013; 19:6286–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Beatty GL, Chiorean EG, …, Vonderheide RH. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011; 331:1612–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Nowak AK, Cook AM, …, Lake RA. A phase lb clinical trial of the CD40-activating antibody CP-870,893 in combination with cisplatin and pemetrexed in malignant pleural mesothelioma. Ann Oncol. 2015; 26:2483–90. [DOI] [PubMed] [Google Scholar]
- 170.Bajor DL, Mick R, …, Vonderheide RH. Long-term outcomes of a phase I study of agonist CD40 antibody and CTLA-4 blockade in patients with metastatic melanoma. Oncoimmunology. 2018; 7:e1468956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rüter J, Antonia SJ, Burris HA, Huhn RD, Vonderheide RH. Immune modulation with weekly dosing of an agonist CD40 antibody in a phase I study of patients with advanced solid tumors. Cancer Biol Ther. 2010; 10:983–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Khubchandani S, Czuczman MS, Hemandez-Ilizaliturri FJ. Dacetuzumab, a humanized mAb against CD40 for the treatment of hematological malignancies. Curr Opin Investig Drugs. 2009; 10:579–87. [PubMed] [Google Scholar]
- 173.Furman RR, Forero-Torres A, Shustov A, Drachman JG. A phase I study of dacetuzumab (SGN-40, a humanized anti-CD40 monoclonal antibody) in patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2010; 51:228–35. [DOI] [PubMed] [Google Scholar]
- 174.Hussein M, Berenson JR, …, Whiting N. A phase I multidose study of dacetuzumab (SGN-40; humanized anti-CD40 monoclonal antibody) in patients with multiple myeloma. Haematologica. 2010; 95:845–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Advani R, Forero-Tones A, …, Draclunan JG. Phase I study of the humanized anti-CD40 monoclonal antibody dacetuzumab in refractory or recurrent non-Hodgkin’s lymphoma. J Clin Oncol. 2009; 27:4371–7. [DOI] [PubMed] [Google Scholar]
- 176.de Vos S, Forero-Tones A, …, Advani R. A phase II study of dacetuzumab (SGN-40) in patients with relapsed diffuse large B-cell lymphoma (DLBCL) and correlative analyses of patient-specific factors. J Hematol Oncol. 2014; 7:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Fayad L, Ansell SM, …, Draclunan JG. Dacetuzumab plus rituximab, ifosfamide, carboplatin and etoposide as salvage therapy for patients with diffuse large B-cell lymphoma relapsing after rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone: a randomized, double-blind, placebo-controlled phase 2b trial. Leuk Lymphoma. 2015; 56:2569–78. [DOI] [PubMed] [Google Scholar]
- 178.Geldart TR, Harvey M, Carr N, Glennie M, Johnson R Cancer immunotherapy with a chimeric anti-CD40 monoclonal antibody: evidence of preclinical efficacy. Proc ASCO. 2004; 14:2577. [Google Scholar]
- 179.Chowdhury F, Johnson PW, Glennie MJ, Williams AP. Ex vivo assays of dendritic cell activation and cytokine profiles as predictors of in vivo effects in an anti-human CD40 monoclonal antibody ChiLob 7/4 phase I trial. Cancer Immunol Res. 2014; 2:229–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Johnson P, Challis R, …, Williams A. Clinical and biological effects of an agonist anti-CD40 antibody: a Cancer Research UK phase I study. Clin Cancer Res. 2015; 21:1321–8. [DOI] [PubMed] [Google Scholar]
- 181.Mangsbo SM, Broos S, …, Ellmark P The human agonistic CD40 antibody ADC-1013 eradicates bladder tumors and generates T-cell-dependent tumor immunity. Clin Cancer Res. 2015; 21:1115–26. [DOI] [PubMed] [Google Scholar]
- 182.Irenaeus SMM, Nielsen D, …, Ullenhag GJ. First-in-human study with intratumoral administration of a CD40 agonistic antibody, ADC-1013, in advanced solid malignancies. Int J Cancer. 2019; 145:1189–99. [DOI] [PubMed] [Google Scholar]
- 183.Sonpavde G, McMannis JD, …, Slawin KM. Phase I trial of antigen-targeted autologous dendritic cell-based vaccine with in vivo activation of inducible CD40 for advanced prostate cancer. Cancer Immunol Immunother. 2017; 66:1345–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Boon L, Laman JD, …, de Boer M. Preclinical assessment of anti-CD40 Mab 5D12 in cynomolgus monkeys. Toxicology. 2002; 174:53–65. [DOI] [PubMed] [Google Scholar]
- 185.de Vos AF, Melief MJ, …, Laman JD. Antagonist anti-human CD40 antibody inhibits germinal center formation in cynomolgus monkeys. Eur J Immunol. 2004; 34:3446–55. [DOI] [PubMed] [Google Scholar]
- 186.Kasran A, Boon L, …, Ceuppens JL. Safety and tolerability of antagonist anti-human CD40 Mab cli5D12 in patients with moderate to severe Crolm’s disease. Aliment Pharmacol Ther. 2005; 22:111–22. [DOI] [PubMed] [Google Scholar]
- 187.Okimura K, Maeta K, …, Mima T. Characterization of ASKP1240, a fully human antibody targeting human CD40 with potent immunosuppressive effects. Am J Transplant. 2014; 14:1290–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Anil Kumar MS, Papp K, Tainaka R, Valluri U, Wang X, Zhu T, Schwabe C. Randomized, controlled study of bleselumab (ASKP1240) pharmacokinetics and safety in patients with moderate-to-severe plaque psoriasis. Biopharm Drug Dispos. 2018; 39:245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Vincenti F, Klintmalm G, …, Holman J. A randomized, phase 1b study of the pharmacokinetics, pharmacodynamics, safety, and tolerability of bleselumab, a fully human, anti-CD40 monoclonal antibody, in kidney transplantation. Am J Transplant. 2020; 20:172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Harland RC, Klintmalm G, …, Wang X. Efficacy and safety of bleselumab in kidney transplant recipients: A phase 2, randomized, open-label, noninferiority study. Am J Transplant. 2020; 20:159–71. [DOI] [PubMed] [Google Scholar]
- 191.Ralph K, Nicoletti A, …, Grimaldi C. THU0407 Preclinical Characterization of a Highly Selective and Potent Antagonistic Anti-CD40 mAb. Annals of the Rheumatic Diseases. 2015; 74:344. [Google Scholar]
- 192.Albach FN, Wagner F, …, Steffgen J. Safety, pharmacokinetics and pharmacodynamics of single rising doses of BI 655064, an antagonistic anti-CD40 antibody in healthy subjects: a potential novel treatment for autoimmune diseases. Eur J Clin Pharmacol. 2018; 74:161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Schwabe C, Rosenstock B, …, Steffgen J. Safety, Pharmacokinetics, and Pharmacodynamics of Multiple Rising Doses of BI 655064, an Antagonistic Anti-CD40 Antibody, in Healthy Subjects: A Potential Novel Treatment for Autoimmune Diseases. J Clin Pharmacol. 2018; 58:1566–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Visvanathan S, Daniluk S, …, Steffgen J. Effects of BI 655064, an antagonistic anti-CD40 antibody, on clinical and biomarker variables in patients with active rheumatoid arthritis: a randomised, double-blind, placebo-controlled, phase Ha study. Ann Rheum Dis. 2019; 78:754–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ristov J, Espié P, …, Rush JS. Characterization of the in vitro and in vivo properties of CFZ533, a blocking and non-depleting anti-CD40 monoclonal antibody. Am J Transplant. 2018; 18:2895–904. [DOI] [PubMed] [Google Scholar]
- 196.Espié P, He Y, …, Gergely P. First-in-human clinical trial to assess pharmacokinetics, pharmacodynamics, safety, and tolerability of iscalimab, an anti-CD40 monoclonal antibody. Am J Transplant. 2020; 20:463–73. [DOI] [PubMed] [Google Scholar]
- 197.Kahaly GJ, Stan MN, …, He Y. A Novel Anti-CD40 Monoclonal Antibody, Iscalimab, for Control of Graves Hyperthyroidism-A Proof-of-Concept Trial. J Clin Endocrinol Metab. 2020; 105:dgz013. [DOI] [PubMed] [Google Scholar]
- 198.Byrd JC, Kipps TJ, …, O’Brien S. Phase I study of the anti-CD40 humanized monoclonal antibody lucatmnumab (HCD122) in relapsed chronic lymphocytic leukemia. Leuk Lymphoma. 2012; 53:2136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Bensinger W, Maziarz RT, …, Stadtmauer EA. A phase 1 study of lucatmnumab, a fully human anti-CD40 antagonist monoclonal antibody administered intravenously to patients with relapsed or refractory multiple myeloma. Br J Haematol. 2012; 159:58–66. [DOI] [PubMed] [Google Scholar]
- 200.Fanale M, Assouline S, …, Freedman AS. Phase IA/II, multicentre, open-label study of the CD40 antagonistic monoclonal antibody lucatmnumab in adult patients with advanced non-Hodgkin or Hodgkin lymphoma. BrJ Haematol. 2014; 164:258–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hirano A, Longo DL, …, Murphy WJ. Inhibition of human breast carcinoma growth by a soluble recombinant human CD40 ligand. Blood. 1999; 93:2999–3007. [PubMed] [Google Scholar]
- 202.Funakoshi S, Longo DL, …, Murphy WJ. Inhibition of human B-cell lymphoma growth by CD40 stimulation. Blood. 1994; 83:2787–94. [PubMed] [Google Scholar]
- 203.Vonderheide RH, Dutcher JP, …, Gribben JG. Phase I study of recombinant human CD40 ligand in cancer patients. J Clin Oncol. 2001; 19:3280–7. [DOI] [PubMed] [Google Scholar]
- 204.Wierda WG, Cantwell MJ, Woods SJ, Rassenti LZ, Prussak CE, Kipps TJ. CD40-ligand (CD154) gene therapy for chronic lymphocytic leukemia. Blood. 2000; 96:2917–24. [PubMed] [Google Scholar]
- 205.Wierda WG, Castro JE, …, Kipps TJ. Aphase I study of immune gene therapy for patients with CLL using a membrane-stable, humanized CD154. Leukemia. 2010; 24:1893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Loskog A, Maleka A, …, Ullenliag G. Immunostimulatory AdCD40L gene therapy combined with low-dose cyclophosphamide in metastatic melanoma patients. Br J Cancer. 2016; 114:872–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Scliiza A, Wenthe J, …, Ullenliag G. Adenovirus-mediated CD40L gene transfer increases Teffector/Tregulatory cell ratio and upregulates death receptors in metastatic melanoma patients. J Transl Med. 2017; 15:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Malmström PU, Loskog AS, …, Tötterman TH. AdCD40L immunogene therapy for bladder carcinoma--the first phase I/IIa trial. Clin Cancer Res. 2010; 16:3279–87. [DOI] [PubMed] [Google Scholar]
- 209.Creelan BC, Antonia S, …, Chiappori A. Phase II trial of a GM-CSF-producing and CD40L-expressing bystander cell line combined with an allogeneic tumor cell-based vaccine for refractory lung adenocarcinoma. J Immunother. 2013; 36:442–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Gray JE, Chiappori A, …, Antonia SJ. A phase I/randomized phase II study of GM.CD40L vaccine in combination with CCL21 in patients with advanced lung adenocarcinoma. Cancer Immunol Immunother. 2018;67:1853–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wilgenhof S, Van Nulfel AM, …, Neyns B. Therapeutic vaccination with an autologous in RNA electroporated dendritic cell vaccine in patients with advanced melanoma. J Immunother. 2011; 34:448–56. [DOI] [PubMed] [Google Scholar]
- 212.Wilgenhof S, Van Nuffel AM, …, Neyns B. A phase IB study on intravenous synthetic mRNA electroporated dendritic cell immunotherapy in pretreated advanced melanoma patients. Ann Oncol. 2013; 24:2686–93. [DOI] [PubMed] [Google Scholar]
- 213.Bomnpas DT, Furie R, …, Vaislmaw A; BG9588 Lupus Nephritis Trial Group. A short course of BG9588 (anti-CD40 ligand antibody) improves serologic activity and decreases hematuria in patients with proliferative lupus glomerulonephritis. Arthritis Rheum. 2003; 48:719–27. [DOI] [PubMed] [Google Scholar]
- 214.Graimner AC, Slota R, …, Lipsky PE. Abnormal germinal center reactions in systemic lupus erythematosus demonstrated by blockade of CD154-CD40 interactions. J Clin Invest. 2003; 112:1506–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Davis JC Jr, Totoritis MC, Rosenberg J, Sklenar TA, Wofsy D. Phase I clinical trial of a monoclonal antibody against CD40-ligand (IDEC-131) in patients with systemic lupus erythematosus. J Rheumatol. 2001:28:95–101. [PubMed] [Google Scholar]
- 216.Kalunian KC, Davis JC Jr, Merrill JT, Totoritis MC, Wofsy D; IDEC-131 Lupus Study Group. Treatment of systemic lupus erythematosus by inhibition of T cell costimulation with anti-CD 154: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002; 46:3251–8. [DOI] [PubMed] [Google Scholar]
- 217.Kuwana M, Nomura S, …, Ikeda Y. Effect of a single injection of humanized anti-CD154 monoclonal antibody on the platelet-specific autoimmune response in patients with immune thrombocytopenic purpura. Blood. 2004; 103:1229–36. [DOI] [PubMed] [Google Scholar]
- 218.Patel VL, Schwartz J, Bussel JB. The effect of anti-CD40 ligand in immune thrombocytopenic purpura. Br J Haematol. 2008; 141:545–8. [DOI] [PubMed] [Google Scholar]
- 219.Luebke Robert W; House Robert V; Kimber Ian. Immunotoxicology and immunopharmacology. Target Organ Toxicology Series (3 ed.). Boca Raton, Florida: CRC Press, Taylor & Francis Group; 2007. p. 131. [Google Scholar]
- 220.Robles-Carrillo L, Meyer T, …, Amirkhosravi A. Anti-CD40L immune complexes potently activate platelets in vitro and cause thrombosis in FCGR2 A transgenic mice. J Immunol. 2010; 185:1577–83. [DOI] [PubMed] [Google Scholar]
- 221.Chamberlain C, Colman PJ, …, Hiepe F. Repeated administration of dapirolizumab pegol in a randomised phase I study is well tolerated and accompanied by improvements in several composite measures of systemic lupus erythematosus disease activity and changes in whole blood transcriptomic profiles. Ann Rheum Dis. 2017; 76:1837–44. [DOI] [PubMed] [Google Scholar]
- 222.Nicholson SM, Casey KA, …, Ryan PC. The enhanced immunopharmacology of VIB4920, a novel Tn3 fusion protein and CD40L antagonist, and assessment of its safety profile in cynomolgus monkeys. Br J Pharmacol. 2020; 177:1061–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kamell JL, Albulescu M, …, Drappa J. A CD40L-targeting protein reduces autoantibodies and improves disease activity in patients with autoimmunity. Sci Transl Med. 2019; 11:eaar6584. [DOI] [PubMed] [Google Scholar]
- 224.Lin JH, Huffman AP, …, Vonderheide RH. Type 1 conventional dendritic cells are systemically dysregulated early in pancreatic carcinogenesis. J Exp Med. 2020; 217:e20190673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kashyap AS, Schmittnaegel M, …, Zippelius A. Optimized antiangiogenic reprogramming of the tumor microenvironment potentiates CD40 immunotherapy. Proc Natl Acad Sci U S A. 2020; 117:541–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Morrison AH, Diamond MS, Hay CA, Byrne KT, Vonderheide RH. Sufficiency of CD40 activation and immune checkpoint blockade for T cell priming and tumor immunity. Proc Natl Acad Sci U S A. 2020; 117:8022–31. [DOI] [PMC free article] [PubMed] [Google Scholar]


