Abstract
Background
Obesity is a risk factor for complications from SARS-CoV-2 infection, increasing the need for effective weight management measures in primary care. However, in the UK, COVID-19 restrictions have hampered primary care weight management referral and delivery, and COVID-19 related weight gain has been reported. The present study evaluated outcomes from a multicomponent weight loss and health promotion programme in UK primary care, delivered remotely due to COVID-19 restrictions.
Method
Patients with obesity, type 2 diabetes or pre-diabetes attended six 90 min sessions over 10 weeks on Zoom. The dietary component comprised a low-carbohydrate ‘real food’ approach, augmented with education on physical activity, intermittent fasting, gut health, stress management, sleep and behaviour change. Anthropometric and cardiometabolic data were self-reported. Mental well-being was assessed with the Warwick Edinburgh Mental Wellbeing Scale. Subjective outcomes and participant feedback about the programme were collected with an anonymous online survey.
Results
Twenty participants completed the programme. Weight loss and improvements in body mass index, waist circumference, systolic and diastolic blood pressure and mental well-being achieved statistical and clinical significance. Mean weight loss (5.8 kg) represented a 6.5% weight loss. Participants’ subjective outcomes included weight loss without hunger (67%) and increased confidence in their ability to improve health (83%). All participants reported the usage of Zoom to access the programme as acceptable with 83% reporting it worked well.
Conclusion
A multicomponent weight loss and health promotion programme with a low-carbohydrate dietary component, clinically and statistically significantly improved health outcomes including weight status, blood pressure and mental well-being in a group of primary care patients when delivered remotely. Further research is warranted.
Keywords: weight management, blood pressure lowering, dietary patterns, mental health, metabolic syndrome
Introduction
Increasing prevalence of obesity and related metabolic dysfunctions such as type 2 diabetes (T2D), hypertension and cardiovascular disease are an ongoing problem globally.1 In England, 63% of adults are overweight or obese,2 which is a risk factor for COVID-19 complications.3 4 Furthermore, UK surveys found the first COVID-19 lockdown resulted in weight gain for up to 48%5 6 of respondents.
Primary care practitioners are in a unique position to address weight management with patients. In the UK, options for primary care weight management include digital applications although more commonly involve community-based, group lifestyle and weight management services delivered by the National Health Service (NHS), commercial providers or the voluntary sector.7 Usually, these services are based on UK National Institute for Health and Care Excellence (NICE) guidelines, which recommend that weight loss efforts focus on calorie deficit creation through reduced energy intake and increased physical activity.8 However, evidence indicates that carbohydrate restriction is also effective both via digital applications and in primary care and community settings to address excess weight, cardiometabolic risk and glycaemic control.9–15
One proposed mechanism for the effectiveness of carbohydrate restriction for weight loss is that it reduces insulin secretion, reducing its anabolic, fat-storing effects and therefore facilitating oxidation of fatty acids from adipose tissue.16 Furthermore, because insulin stimulates glucose uptake, suppresses fatty acid oxidation and promotes fat and glycogen deposition, hyperinsulinaemia effectively removes metabolic fuels from the circulation, potentially driving hunger and overeating.16 This could partially explain the extended satiety often experienced with carbohydrate restriction that can lead to spontaneous intermittent fasting by missing a meal, extending gaps between meals or snacking cessation. A recent review of intermittent fasting protocols such as 5:2, alternate day fasting and time-restricted eating found that although weight loss can occur due to energy restriction, cardiometabolic health benefits such as increased insulin sensitivity can occur independent of weight loss.17
Carbohydrate restriction has also been associated with reduced blood pressure in primary care patients.11 15 Rather than hypertension being addressed by weight loss per se, it has been speculated that dietary changes may be responsible,18 and there is evidence that hyperinsulinaemia increases sodium retention in people with T2D and hyperglycaemia.19 20 Reducing circulating insulin levels with carbohydrate restriction could contribute to blood pressure improvement.
With carbohydrate restriction, serum glucose can drop rapidly and substantially, and blood pressure can improve; therefore, some hypoglycaemic and antihypertensive medications may need to be adjusted or discontinued.11 21 Medication review is therefore an important consideration for patients following a carbohydrate-restricted eating pattern.11 21
Anecdotally, carbohydrate restriction can improve mental well-being although the evidence base is weak.22 Certain dietary patterns can affect glycaemia, immune activity and the gut microbiome to influence mood and mental well-being,23 and poor diet quality has been linked to depression and other severe mental illness mediated by dietary inflammation.24 25 The SMILES (Supporting the Modification of lifestyle In Lowered Emotional States) randomised controlled trial (RCT) found dietary improvement to be an effective treatment for major depression.26
There is no accepted definition of a low-carbohydrate diet, a situation that has hampered synthesis of research evidence. However, it has been suggested that <130 g/day (26% daily energy intake (DEI)) denotes ‘low carbohydrate’27 28 ranging down to ≤20–50 g/day (<10% DEI) a ‘very low carbohydrate’ or ketogenic diet.27 Concern that sufficient dietary carbohydrate is required to supply glucose for brain function can be addressed with recognition that the brain's energy requirement can be met with the products of gluconeogenesis, glycogenolysis and with very low carbohydrate intake, ketogenesis.29 While not advocating carbohydrate restriction, NICE advises a low-glycaemic index diet for T2D management,30 and some national diabetes organisations recognise carbohydrate restriction as a therapeutic dietary option to improve glycaemic control and weight loss.29 31–33 The long-term sustainability and safety of carbohydrate restriction is debated,34 although a recent primary care service evaluation reported successful compliance with concomitant weight and cardiometabolic improvements over 6 years.15
In 2020 in the UK, COVID-19 restrictions disrupted opportunities in primary care for brief interventions to address excess weight and referral to weight management services. Face-to-face community-based interventions were not possible, although some commercial services were delivered remotely.35 36 Remotely delivered primary care consultations are increasingly available37 and are acceptable and beneficial to patients and clinicians.38 However, the authors are unaware of studies that have explored the efficacy of remotely delivered, community-based group weight loss interventions in primary care. The present service evaluation appraises outcomes from a weight loss and health promotion programme delivered as part of an ongoing initiative by the registered UK charity the Public Health Collaboration (PHC) (www.phcuk.org). The PHC delivers group programmes free of charge, including within primary care, with the aim of improving T2D management and weight status through carbohydrate restriction. Clinically significant weight loss and metabolic improvements have been achieved.39 PHC interventions vary in content, duration and structure but typically involve six to eight 60–90 min sessions over 6–12 weeks for up to 20 people. The purpose of the present study was to evaluate outcomes from a six-session, 10-week programme which, due to COVID-19 restrictions, was delivered on Zoom rather than face to face as originally intended. Because evidence suggests the combined effects of several healthy lifestyle behaviours reduces risk of mortality,40 the programme included education on several lifestyle factors associated with health improvement in addition to diet. Participants were patients from a group of general practices in Hampshire, UK. Primary outcomes were improvements in weight status and mental well-being. Secondary outcomes were improvements in blood pressure and HbA1c. Subjective outcomes and participant feedback about the programme was assessed with an online questionnaire.
Method
Study design
A before–after without control design was used to evaluate outcomes from a six-session, 10-week, multicomponent, group-based weight loss intervention delivered on Zoom. Primary outcomes were weight loss (kg) and changes in body mass index (BMI) in kg/m2, waist circumference (cm) and mental well-being. Mental well-being was assessed using the Warwick Edinburgh Mental Wellbeing Scale (WEMWBS)41 (online supplemental file 1), which is validated for measuring mental well-being in populations and is sensitive to change over time.42 Secondary outcomes were changes in systolic and diastolic blood pressure (mm Hg) and glycated haemoglobin (HbA1c) (mmol/mol).
bmjnph-2020-000219supp001.pdf (195.7KB, pdf)
Recruitment
In June 2020, patients from a four-practice, 32 000-patient primary care network in Hampshire, UK, were invited to a 60 min information session on Zoom about the Low Carb Real Food Lifestyle Programme (‘the programme’). Each practice used their own recruitment methods, which included email, text and promotion via website and social media. Eligible participants were those aged ≥18 years with T2D, pre-diabetes or who had been advised to lose weight, plus those living with or caring for someone in one of these categories. Following the information session, interested patients registered online using a Google Form, supplying contact information, reason for applying, general practice (GP) surgery, age group, sex and General Data Protection Regulation consent. On the same form, all gave optional consent to their data being anonymously analysed and reported. All also gave optional consent to their general practitioner being informed of their registration.
Mechanism
Six 90 min sessions were conducted on Zoom fortnightly with participants divided into five groups. Each group had two facilitators to ensure adequate technical and administrative support for both facilitators and participants in what was an unfamiliar medium for most people involved. Between sessions, participants could access optional extra support through private social media groups. Details of group structure, facilitators and programme fidelity control are outlined in online supplemental file 2. The programme used a low-carbohydrate dietary component augmented with sessions covering physical activity, sleep, stress management, intermittent fasting, gut health and behaviour change. Programme content is outlined in box 1.
Box 1. Programme content for the Low Carb Real Food Lifestyle Programme, July–September 2020.
Information session (6 July): introduction to a low carb/real food lifestyle, how/why it is helpful, what the course involves, medication adjustment guidance and registration administration.
Session 1 (13 July): administration regarding data collection, goal setting, hormonal model of obesity and T2D, recognising carbohydrates, insulin resistance and hyperinsulinaemia, getting started with low carb/real food, sample meal plans and food swaps.
Session 2 (27 July): avoiding processed food, food labels and shopping, further familiarisation with the low carb/real food approach and goal setting.
Session 3 (10 August): habit/behaviour change, lapse and relapse, how to deal with eating out, travelling, pressure from friends and colleagues and goal setting.
Sessions 4, 5 and 6 (24 August and 7 and 21 September): physical activity, intermittent fasting, stress management, sleep, gut health, and goal setting. (Facilitators covered these topics in whichever order suited their group’s needs.)
Session 6 (21 September): review, celebration and next steps/looking to the future.
The programme was designed to provide enough information and physiology education to help participants understand, engage in and feel some control over their health. For the dietary component, there was no calorie restriction, carbohydrate counting or set meal plans. Instead, participants were encouraged to restrict sugar, processed foods and starchy carbohydrates such as bread, pasta, rice and potatoes and to focus on eating minimally processed foods to satiety. They were encouraged to experiment to discover what suited their preferences and lifestyle and to make changes at their own pace. Cooking from scratch was encouraged. Resources provided included a one-page guide to low-carbohydrate eating previously used in general practice,11 lists of foods to enjoy and avoid and various online resources and recipe suggestions (online supplemental file 3).
In the information session, participants were informed of guidance to consult their medical practitioner if they were on medications, which could be affected by carbohydrate restriction.11 21 This information was delivered by a general practitioner. It was emphasised in every session that the programme constituted information not medical advice.
Data collection
Data were collected before programme start and after the final session. Anthropometric and cardiometabolic data were self-reported using a personal progress sheet (online supplemental file 4). Specifically, participants were encouraged to monitor and record weight and waist circumference regularly and to record other data such as blood pressure, HbA1c and serum lipids as available, plus medications and any dose adjustments. Participants were encouraged to download the NHS app43 to access their medical records or to request most recent test results from their general practitioner. COVID-19 restrictions prevented participants accessing surgery blood pressure machines, but they were encouraged to buy their own. Plans to test for serum lipids and HbA1c at programme start and end were abandoned, although participants supplied data if available. Test results from within one calendar month of the last session were included. Participants emailed their completed progress sheets to the lead author (LW) at programme end. Mental well-being was measured at programme start and end using the WEMWBS, a self-administered questionnaire. Answer sheets were emailed to LW. An anonymous post-programme online survey was developed to collect participant feedback about their experience (online supplemental file 5).
Statistical analysis
Statistical analyses were performed with R V.4.0.2. Summaries of data at baseline and 10 weeks are shown as mean, median and IQR (25th percentile, 75th percentile) for non-normally distributed continuous variables (weight, BMI, waist circumference, mental well-being, blood pressure and HbA1c). Comparisons between data at baseline and 10 weeks of continuous variables were made using the Wilcoxon signed-rank test for paired samples. A p value of <0.05 was considered statistically significant. Only data for which there were matched pairs were analysed.
Results
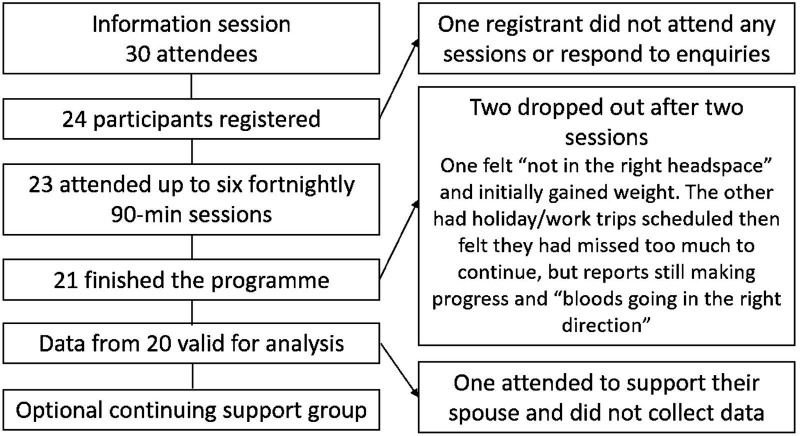
Due to the range of recruitment methods employed by the practices, the number of patients exposed to promotional material cannot be ascertained. Data were collected from 20 participants; participants attended a mean of five sessions. Figure 1 shows the flow of participants through the programme. Table 1 shows participant characteristics at baseline and reasons for registration.
Figure 1.
Flow of participants through the programme.
Table 1.
Participant characteristics at baseline and reasons for registration
| N (%) | |
| Participants | 20 (100) |
| Female | 17 (85) |
| Male | 3 (15) |
| Glycaemic status | |
| T2D (HbA1c ≥48.0 mmol/mol) | 10 (50) |
| Pre-diabetes (HbA1c 42.0–47.9 mmol/mol) | 1 (5) |
| Normal/unmeasured | 9 (45) |
| Weight status | |
| Obese (BMI ≥30.0 kg/m2) | 12 (60) |
| Overweight (BMI 25.0–29.9 kg/m2) | 5 (25) |
| Normal weight (BMI 18.5–24.9 kg/m2) | 3 (15) |
| Age group in years | |
| 40–49 | 4 (20) |
| 50–59 | 4 (20) |
| 60–69 | 5 (25) |
| ≥70 | 7 (35) |
| Reason(s) for registration | |
| Weight loss | 20 (100) |
| Improved glycaemic control | 12 (60) |
| Reversal of pre-diabetes (only one was pre-diabetic) | 2 (10) |
| To support a family member | 3 (15) |
BMI, body mass index; T2D, type 2 diabetes.
Table 2 summarises outcomes. Insufficient data were available for analysis of serum lipids.
Table 2.
Outcomes for weight status, blood pressure and well-being
| Matched pairs, n | Baseline mean | 10 weeks mean | Change mean | Change median (IQR) | P value | |
| Body weight (kg) | 20 | 90.7 | 84.9 | −5.8 | −5.4 (−6.9 to −4.4) | <0.001 |
| Percent weight loss (%) | 20 | – | 6.5 | – | 6.5 (4.5 to 8.2) | – |
| BMI (kg/m2) | 20 | 32.1 | 30.0 | −2.0 | −2.0 (−2.5 to −1.5) | <0.001 |
| Waist circumference (cm) | 11 | 101.3 | 96.1 | −5.2 | −5.1 (−7.3 to −3.8) | 0.006 |
| HbA1c (mmol/mol) | 7 | 54.6 | 45.4 | −9.1 | −11.0 (−13.0 to −4.5) | 0.059 |
| Blood pressure | ||||||
| Systolic (mm Hg) | 7 | 130.6 | 117.4 | 13.1 | −14.0 (−19.5 to −9.5) | 0.035 |
| Diastolic (mm Hg) | 7 | 77.9 | 72.9 | 5.0 | 4.0 (−6.5 to −2.0) | 0.042 |
| Mental well-being | ||||||
| WEMWBS score | 17 | 45.2 | 51.7 | 6.5 | 7.0 (3.0 to 10.0) | 0.001 |
Data are only shown for participants who supplied data at baseline and 10 weeks (matched pairs).
BMI, body mass index; IQR, interquartile range (25th percentile, 75th percentile); WEMWBS, Warwick Edinburgh Mental Wellbeing Scale.
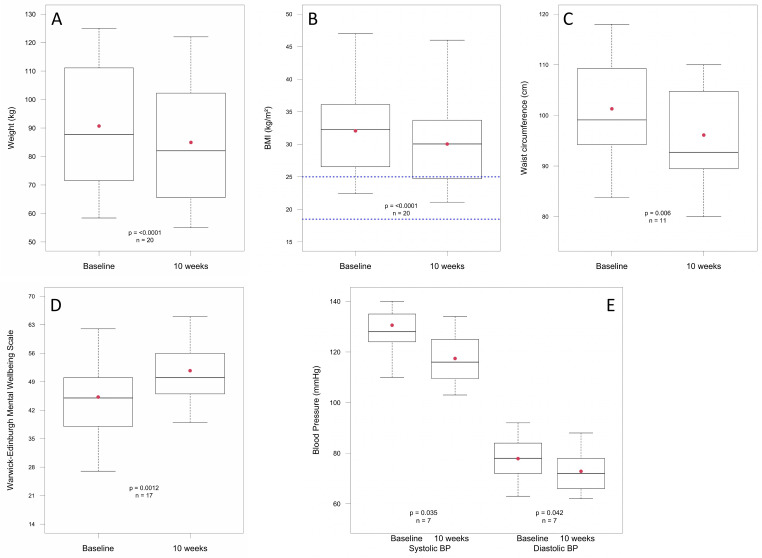
All primary outcomes improved significantly (figure 2A–E). Mean weight loss was 5.8 kg (IQR 4.4–6.9), p<0.001, representing a mean weight loss of 6.5% (IQR 4.5–8.2); mean BMI reduced: 2.0 kg/m2 (IQR 1.5–2.5), p<0.001; mean waist circumference reduced: 5.2 cm (IQR 3.8–7.3), p=0.006. Mean mental well-being score improved by a significant (p=0.001) 6.5 units (IQR 3.0–10.0). A change of three units represents a change likely to be noticeable and important to an individual.42
Figure 2.
Box and whisker charts showing baseline and 10-week distributions of patient data. The box represents the median value and the IQR, the red dot indicates the mean value and the upper and lower whiskers indicate either the minimum/maximum value or 1.5 times the IQR (outliers are not shown). Blue dotted line in figure part B denotes BMI range 18.5–25.0 kg/m2. BMI, body mass index.
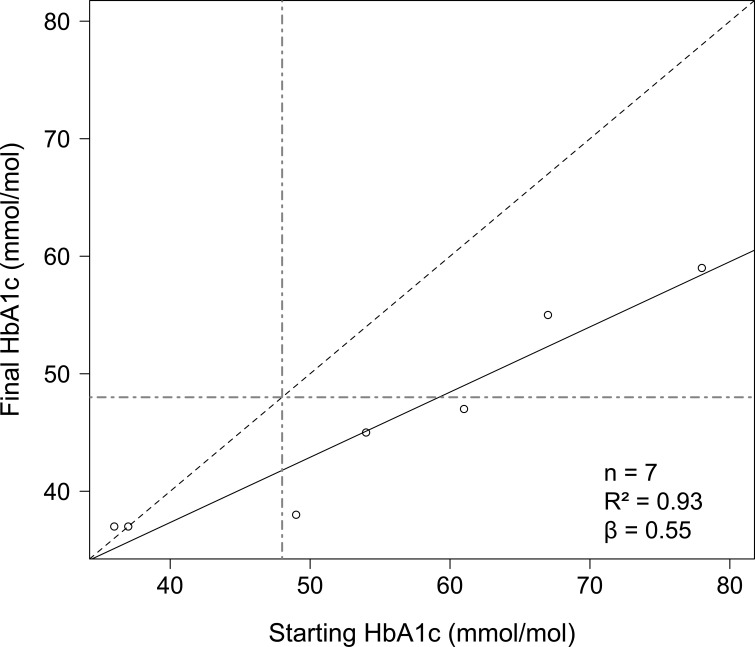
Regarding secondary outcomes, blood pressure improved significantly: mean systolic blood pressure reduced by 13.1 mm Hg (IQR 9.5–19.5), p=0.035, and mean diastolic blood pressure reduced by 5.0 mm Hg (IQR 2.0–6.5), p=0.042. Mean HbA1c improved by 9.1 mmol/mol (p=0.059). All participants for whom HbA1c data were available for analysis who did not start with a healthy HbA1c had a reduced HbA1c after the intervention, with the highest starting values showing the greatest reduction (figure 3).
Figure 3.
Initial HbA1c versus HbA1c within 1 month of programme end. The blue dashed line represents no change in HbA1c. Points above this line represent participants with an increased HbA1c; points on this line represent those with no change; and points below this line represent participants with reductions in HbA1c. The black solid line is the line of best fit for linear regression of final HbA1c with respect to starting HbA1c. The green dot-dashed lines represent an HbA1c of 48 mmol/mol.
Both of the two participants on insulin reduced their dosage, one by 100 units/day to 20. One patient had their gliclazide dose reduced after 1 month.
Participant feedback
The feedback survey elicited 18 responses (online supplemental file 5). A range of subjective health improvements were reported, as summarised in table 3, box 2.
Table 3.
Summary of subjective results from participant feedback survey
| N (%) | ||
| ‘Yes’ | ‘Somewhat’ | |
| Lost weight without hunger | 12 (67) | 5 (28) |
| Health and well-being had improved | 16 (89) | 2 (11) |
| Skin had improved | 5 (28) | 5 (28) |
| Food cravings had reduced | 12 (67) | 5 (28) |
| Better energy and vitality | 4 (22) | 13 (72) |
| Less ‘brain fog’/clearer thinking | 2 (11) | 5 (28) |
| Better sleep | 4 (22) | 6 (33) |
| Lower stress levels | 2 (11) | 9 (50) |
| Gained confidence in making good decisions about their health | 12 (67) | 6 (33) |
| Gained confidence and/or hope that they can improve their health | 15 (83) | 2 (11) |
Box 2. Examples of free-form comments from participant feedback survey.
‘Feel better physically’ (two participants said this).
‘My confidence has improved’.
‘Feel positive about my health – have learned how to improve things’.
‘My resting heart rate has decreased’.
‘Don’t feel bloated after a meal’.
‘How easy it was to lose weight – I didn’t think I could lose my spare tyre and I mostly have’.
‘Better energy overall – plus feeling healthier’.
Asked how confident they were that they would be able to maintain the changes they had made, 78% of participants responded 7 out of 10 or above. Asked about their experience of taking part via Zoom, 83% selected ‘worked well’; the remainder (17%) selected ‘not ideal but generally ok’.
Discussion
The present study evaluated outcomes from a six-session, 10-week multicomponent weight loss and health promotion programme delivered by the PHC to primary care patients on Zoom. The programme resulted in significant weight loss and significantly improved BMI, waist circumference, blood pressure and mental well-being. A number of subjective health improvements were also reported including weight loss without hunger, decreased food cravings and increased health-related confidence. Participants found Zoom an acceptable way to access the programme. To the authors’ knowledge, this is the first evaluation of a remotely delivered group-based weight loss or health promotion programme in primary care. These outcomes were achieved during the COVID-19 pandemic when weight gain and increased anxiety and mental illness were reported.5 6 44
The programme encouraged participants to address several lifestyle factors that could have contributed to the significant outcomes. Relating to diet, notwithstanding the unknown carbohydrate restriction compliance, the anthropometric and cardiometabolic outcomes align with meta-analyses of RCTs comparing low-carbohydrate diets with low-fat or low-calorie diets that demonstrate comparable or greater efficacy with carbohydrate restriction for weight loss,45 T2D management46 and cardiometabolic risk reduction.45 47 Most UK primary care weight loss programmes are based on government dietary recommendations that recommend adult daily intake of at least 300 g carbohydrate representing 50%–55% DEI.48 Although participants did not count carbohydrates, their daily intake could be reasonably estimated as approximately 100 g carbohydrate or approximately 20%–25% DEI. Aligned with other primary care studies describing carbohydrate restriction,11 15 our outcomes suggest that carbohydrate counting and strict carbohydrate restriction might not be necessary to improve metabolic health and weight status in all weight loss candidates. Instead, advice to eliminate sugar and restrict starches and ultra-processed foods could provide a realistic, acceptable alternative. Blood pressure outcomes were consistent with findings from studies of 2-year and 6-year carbohydrate restriction among UK primary care patients11 15 and support the concept that addressing hyperinsulinaemia could improve hypertension.19 20
Additional to carbohydrate restriction, intermittent fasting could have contributed to reduction of insulin levels and increased insulin sensitivity, even if only applied at the level of not snacking between meals. The extent of participants’ engagement with intermittent fasting was not explored further than with discussion during Zoom sessions. However, facilitators reported participants expressing interest—and sometimes relief—when discovering that, unless food was required for medication purposes, it was possible to delay or forego a meal, should they not feel hungry. Two-thirds of feedback survey respondents reported losing weight without hunger and with reduced food cravings. Along with the absence of calorie restriction and the flexibility to adapt the eating pattern to personal preferences and goals, this could have contributed to the significant weight loss outcomes. This has been reported previously with carbohydrate restriction49 50 with one proposed mechanism being the effect of higher fat and protein intake on the physiological drivers of feeding behaviour.51 52
The benefits of physical activity for health promotion are well established. However, helping participants understand that physical activity can improve insulin sensitivity, and the inter-related effects of physical activity, sleep and stress reduction could have been important. The stress reduction and sleep components were likely relevant because sleep deprivation and high stress levels have been shown to contribute to weight gain and inhibition of weight loss.53 54
The present evaluation cannot infer which components contributed to mental well-being improvements. Participants could have improved one or more factors associated with improved mental health such as increased diet quality, gut health, physical activity and sleep quality and reduced stress and dietary inflammation.24–26 Confidence and self-efficacy are associated with mental well-being so it was encouraging that the majority of feedback survey respondents reported increased health-related confidence and hope, and confidence in their ability to maintain changes. This indicates that participants improved self-efficacy, which systematic review and meta-analysis have found promotes health behaviour change.55–57
The programme could be considered to use a group consultation modality. Group consultations have been shown to be effective for several conditions both to manage new cases in monthly sessions and for annual review in stable cases.58 Shared patient experience and peer group support are additional benefits.55 59 The present evaluation supports the literature and adds to it by providing preliminary information about remotely delivered sessions.
Strengths and limitations
The programme was delivered remotely in a pragmatic response to the unusual circumstances brought about by COVID-19. That an effective health promotion programme can be delivered to a range of participants without the need for premises or meeting in person provides promise for addressing obesity and related metabolic conditions in novel ways. While remote delivery may reduce accessibility for some, it could increase accessibility and convenience for others, providing an element of patient choice. The outcomes were encouraging but must be interpreted with caution in light of methodological limitations. The sample was small and self-selected so selection bias was possible. However, an alternative view is that the programme offered an alternative to usual care that appealed to potential participants, with the information session helping them decide whether to participate. This aligns with the concepts of patient choice and individualised care. The sample included participants across a range of health statuses that reflect the ‘real-world’ nature of primary care. In contrast to one major T2D and weight loss RCT,60 the sample included participants aged ≥65 years (≥35% were ≥70 years), those with a T2D diagnosis ≥6 years (25%), two (10%) on insulin and three (15%) who attended to support a family member as recommended by NICE.61 Data were self-reported, which introduces the possibility of reporting bias. For example, the blood pressure improvement was based on a sample of just seven, mainly limited to participants who owned blood pressure monitors. Conversely, that changes were significant despite a small sample suggests the intervention was effective. There are no reliable data indicating the extent to which participants complied with the suggested carbohydrate restriction; however, significant improvements in weight status imply efficacy. Additionally, all participants lost weight, including the three with a healthy BMI at baseline, which has implications for weight gain prevention. The lack of control group prevents comparison with usual care. However, mean weight loss of 5.8 kg compares favourably with results from 12-week commercial weight loss services where programme completers or ‘high attenders’ lost between 4.25 kg and 5.29 kg.62–64 Relatedly, a study that compared 12-week weight loss from a range of commercial and NHS interventions found that between 15% and 46% of participants achieved ≥5% weight loss,64 while in the present evaluation, 70% achieved ≥5% weight loss. Greater 12-week weight loss was achieved with carbohydrate restriction in primary care in a recent RCT, although the protocol involved energy restriction to 825–1000 kcal/day and four 15–20 min one-to-one consultations with a practice nurse.65 It is therefore encouraging that clinically and statistically significant outcomes such as those from the present evaluation can be achieved with a light-touch group consultation approach without energy restriction.
The possible effects of confounding factors should be acknowledged. These could include specific participant characteristics or behaviours, medications, interventions or circumstances that could have affected outcomes and were not taken into account. The COVID-19 pandemic itself could have been a confounder with both potentially positive effects, such as more time to exercise, cook from scratch, eat at home and fewer social occasions to challenge compliance, and potentially negative effects such as increased stress and reduced access to preferred food retailers or exercise venues. Furthermore, the participants were predominantly female, all Caucasian and from an area with low deprivation levels,66 which reduces generalisability to other populations.
Longer term follow-up is needed to ascertain whether outcomes are maintained or improved after this type of intervention. Larger scale exploration of the relative effects, including on serum lipids, of different components is warranted as is exploration of the relationships between mental well-being and lifestyle change. It would also be valuable to further investigate the efficacy of remote group consultations for weight loss and health promotion. Qualitative exploration of the patient experience with carbohydrate restriction would be valuable.
Conclusion
The present evaluation of outcomes from a light-touch, remotely delivered, multicomponent weight loss and health promotion programme showed clinically and statistically significant outcomes for weight loss, cardiometabolic risk and mental well-being for a small group of primary care patients. It provides preliminary indication that remotely delivered interventions could bean effective and relevant therapeutic option while the COVID-19 pandemic continues to impede primary care weight management services. Further research is warranted.
Acknowledgments
The authors would like to thank the participating patients, and staff and partners of the A31 Group Primary Care Network. We are grateful to Dr Campbell Murdoch, Dr Jen Unwin and Dr David Unwin for reviewing drafts of the manuscript, and to Sam Feltham for his help throughout the project. Finally, our thanks go to the Public Health Collaboration ambassadors who facilitated the group sessions and online support groups.
Footnotes
Twitter: @RethinkCake
Contributors: LW conceived and designed the programme, led the programme roll-out, collected the data and drafted the manuscript. NS contributed to the programme design, facilitated patient access and recruitment and edited early drafts of the manuscript. CD analysed and interpreted the data, produced the figures and edited final versions of the manuscript.
Funding: All the work relating to the programme was performed by volunteers from the charity the Public Health Collaboration (registered number: 1171887).
Competing interests: LW and CD are volunteer ambassadors for the Public Health Collaboration charity (registration number 1171887).
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. For raw data or any other queries, please contact the corresponding author, LW: lou@louwalker.com.
Ethics statements
Patient consent for publication
Not required.
References
- 1. Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol 2019;15:288–98. 10.1038/s41574-019-0176-8 [DOI] [PubMed] [Google Scholar]
- 2. NHS Digital . Statistics on obesity, physical activity and diet, England, 2020: NHS digital 2020.
- 3. Sattar N, McInnes IB, McMurray JJV. Obesity is a risk factor for severe COVID-19 infection: multiple potential mechanisms. Circulation 2020;142:4–6. 10.1161/CIRCULATIONAHA.120.047659 [DOI] [PubMed] [Google Scholar]
- 4. Public Health England . Excess weight and COVID-19. insights from new evidence 2020.
- 5. Allington D, Beaver K, Duffy B. Getting used to life under lockdown? coronavirus in the UK, 2020. Available: https://www.ipsos.com/sites/default/files/ct/news/documents/2020-05/kings_charts_28.5.20.pdf [Accessed 10 Oct 2020].
- 6. Covid Symptom Study . Has lockdown influenced our eating habits? the silent pandemic: how lockdown is affecting our future health. 2020, 2020. Available: https://covid.joinzoe.com/post/lockdown-weight-gain#:~:text=The%20factors%20that%20may%20have,less%20healthy%20diet%20(19%25) [Accessed 11 Oct 2020].
- 7. National Institute for Clinical and Care Excellence . Weight management: lifestyle services for overweight or obese adults. Public health guideline [PH53] 2014.
- 8. National Institute for Clinical and Care Excellence . Obesity: Idenfication, assessment and management. Clinical guideline 2014. [Google Scholar]
- 9. Athinarayanan SJ, Adams RN, Hallberg SJ, et al. Long-Term effects of a novel continuous remote care intervention including nutritional ketosis for the management of type 2 diabetes: a 2-year Non-randomized clinical trial. Front Endocrinol 2019;10:348. 10.3389/fendo.2019.00348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Saslow LR, Summers C, Aikens JE, et al. Outcomes of a digitally delivered low-carbohydrate type 2 diabetes self-management program: 1-year results of a single-arm longitudinal study. JMIR Diabetes 2018;3:e12. 10.2196/diabetes.9333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Unwin DJ, Tobin SD, Murray SW, et al. Substantial and sustained improvements in blood pressure, weight and lipid profiles from a carbohydrate restricted diet: an observational study of insulin resistant patients in primary care. Int J Environ Res Public Health 2019;16. 10.3390/ijerph16152680. [Epub ahead of print: 26 Jul 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ahmed SR, Bellamkonda S, Zilbermint M, et al. Effects of the low carbohydrate, high fat diet on glycemic control and body weight in patients with type 2 diabetes: experience from a community-based cohort. BMJ Open Diabetes Res Care 2020;8:e000980. 10.1136/bmjdrc-2019-000980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Idris I, Hampton J, Moncrieff F, et al. Effectiveness of a digital lifestyle change program in obese and type 2 diabetes populations: service evaluation of real-world data. JMIR Diabetes 2020;5:e15189. 10.2196/15189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Unwin D, Unwin J. Low carbohydrate diet to achieve weight loss and improve HbA 1c in type 2 diabetes and pre-diabetes: experience from one general practice. Practical Diabetes 2014;31:76–9. 10.1002/pdi.1835 [DOI] [Google Scholar]
- 15. Unwin D, Khalid AA, Unwin J, et al. Insights from a general practice service evaluation supporting a lower carbohydrate diet in patients with type 2 diabetes mellitus and prediabetes: a secondary analysis of routine clinic data including HbA1c, weight and prescribing over 6 years. BMJ Nutr Prev Health 2020;3:285-294. 10.1136/bmjnph-2020-000072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ludwig DS, Ebbeling CB. The Carbohydrate-Insulin Model of Obesity: Beyond "Calories In, Calories Out". JAMA Intern Med 2018;178:1098–103. 10.1001/jamainternmed.2018.2933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hoddy KK, Marlatt KL, Çetinkaya H, et al. Intermittent fasting and metabolic health: from religious fast to Time-Restricted feeding. Obesity 2020;28 Suppl 1:S29–37. 10.1002/oby.22829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harsha DW, Bray GA. Weight loss and blood pressure control (pro). Hypertension 2008;51:1420–5. 10.1161/HYPERTENSIONAHA.107.094011 [DOI] [PubMed] [Google Scholar]
- 19. Quiñones-Galvan A, Ferrannini E. Renal effects of insulin in man. J Nephrol 1997;10:188–91. [PubMed] [Google Scholar]
- 20. Brands MW, Manhiani MM. Sodium-Retaining effect of insulin in diabetes. Am J Physiol Regul Integr Comp Physiol 2012;303:R1101–9. 10.1152/ajpregu.00390.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Murdoch C, Unwin D, Cavan D, et al. Adapting diabetes medication for low carbohydrate management of type 2 diabetes: a practical guide. Br J Gen Pract 2019;69:360–1. 10.3399/bjgp19X704525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mullens A, Scher B. Ketogenic diet for mental health: come for the weight loss, stay for the mental health benefits? 2020. Available: https://www.dietdoctor.com/low-carb/mental-health [Accessed 9 Oct 2020].
- 23. Firth J, Gangwisch JE, Borisini A, et al. Food and mood: how do diet and nutrition affect mental wellbeing? BMJ 2020;369:m2382. 10.1136/bmj.m2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Berk M, Williams LJ, Jacka FN, et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med 2013;11:200. 10.1186/1741-7015-11-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Firth J, Veronese N, Cotter J, et al. What is the role of dietary inflammation in severe mental illness? A review of observational and experimental findings. Front Psychiatry 2019;10:350–50. 10.3389/fpsyt.2019.00350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jacka FN, O'Neil A, Opie R, et al. A randomised controlled trial of dietary improvement for adults with major depression (the 'SMILES' trial). BMC Med 2017;15:23. 10.1186/s12916-017-0791-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Feinman RD, Pogozelski WK, Astrup A, et al. Dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base. Nutrition 2015;31:1–13. 10.1016/j.nut.2014.06.011 [DOI] [PubMed] [Google Scholar]
- 28. Accurso A, Bernstein RK, Dahlqvist A, et al. Dietary carbohydrate restriction in type 2 diabetes mellitus and metabolic syndrome: time for a critical appraisal. Nutr Metab 2008;5:9 10.1186/1743-7075-5-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care 2019;42:731–54. 10.2337/dci19-0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. National Institute for Clinical and Care Excellence . Type 2 diabetes in adults: management. NICE guideline (NG28) 2015. [PubMed]
- 31. Diabetes UK . Position statement: low-carb diets for people with diabetes, 2017. Available: https://www.diabetes.org.uk/resources-s3/2017-09/low-carb-diets-position-statement-May-2017.pdf [Accessed 23 Oct 2020].
- 32. Diabetes Australia . Position statement: low carbohydrate eating for people with diabetes, 2018. Available: https://www.diabetesaustralia.com.au/wp-content/uploads/Diabetes-Australia-Position-Statement-Low-Carb-Eating.pdf [Accessed 23 Oct 2020].
- 33. Davies MJ, D’Alessio DA, Fradkin J. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American diabetes association (ADA) and the European association for the study of diabetes (EASD). Diabetes Care 2018:dci180033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. British Dietetic Association . BDA policy statement: low carbohydrate diets for the management of type 2 diabetes in adults, 2018. Available: https://www.bda.uk.com/resource/low-carbohydrate-diets-for-the-management-of-type-2-diabetes-in-adults.html [Accessed 7 Jan 2021].
- 35. Slimming World . Slimming World’s Lockdown Commitment. Secondary Slimming World’s Lockdown Commitment, 2020. Available: https://www.slimmingworld.co.uk/press/slimming-worlds-lockdown-commitment [Accessed 10 Nov 2020].
- 36. Weight Watchers . Important workshop update. secondary important workshop update, 2020. Available: https://www.weightwatchers.com/uk/find-a-workshop/ [Accessed 10 Nov 2020].
- 37. NHS . Gp online consultations 2020.
- 38. Donaghy E, Atherton H, Hammersley V, et al. Acceptability, benefits, and challenges of video consulting: a qualitative study in primary care. Br J Gen Pract 2019;69:e586–94. 10.3399/bjgp19X704141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. The Public Health Collaboration . Public Health Collaboration Ambassador & Healthcare Professionals Case Study Book, 2020. Available: https://phcuk.org/wp-content/uploads/2020/12/Public-Health-Collaboration-Ambassador-Healthcare-Professionals-Case-Study-Book-22.12.2020.pdf [Accessed 5 Jan 2021].
- 40. Loef M, Walach H. The combined effects of healthy lifestyle behaviors on all cause mortality: a systematic review and meta-analysis. Prev Med 2012;55:163–70. 10.1016/j.ypmed.2012.06.017 [DOI] [PubMed] [Google Scholar]
- 41. Tennant R, Hiller L, Fishwick R, et al. The Warwick-Edinburgh mental well-being scale (WEMWBS): development and UK validation. Health Qual Life Outcomes 2007;5:63 10.1186/1477-7525-5-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Maheswaran H, Weich S, Powell J, et al. Evaluating the responsiveness of the Warwick Edinburgh mental well-being scale (WEMWBS): group and individual level analysis. Health Qual Life Outcomes 2012;10:156. 10.1186/1477-7525-10-156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. National Health Service . Nhs APP. secondary NHS APP. Available: https://www.nhs.uk/nhs-services/online-services/nhs-app/ [Accessed 18 Jan].
- 44. O'Connor RC, Wetherall K, Cleare S, et al. Mental health and well-being during the COVID-19 pandemic: longitudinal analyses of adults in the UK COVID-19 Mental Health & Wellbeing study. Br J Psychiatry 2020:1–17. 10.1192/bjp.2020.212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sackner-Bernstein J, Kanter D, Kaul S. Dietary intervention for overweight and obese adults: comparison of low-carbohydrate and low-fat diets. A meta-analysis. PLoS One 2015;10:e0139817–e17. 10.1371/journal.pone.0139817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Huntriss R, Campbell M, Bedwell C. The interpretation and effect of a low-carbohydrate diet in the management of type 2 diabetes: a systematic review and meta-analysis of randomised controlled trials. Eur J Clin Nutr 2018;72:311–25. 10.1038/s41430-017-0019-4 [DOI] [PubMed] [Google Scholar]
- 47. Choi YJ, Jeon SM, Shin S. Impact of a ketogenic diet on metabolic parameters in patients with obesity or overweight and with or without type 2 diabetes: a meta-analysis of randomized controlled trials. Nutrients 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Public Health England . Government dietary recommendations 2016.
- 49. Martin CK, Rosenbaum D, Han H, et al. Change in food cravings, food preferences, and appetite during a low-carbohydrate and low-fat diet. Obesity 2011;19:1963–70. 10.1038/oby.2011.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kelly T, Unwin D, Finucane F. Low-Carbohydrate diets in the management of obesity and type 2 diabetes: a review from clinicians using the approach in practice. Int J Environ Res Public Health 2020;17:2557. 10.3390/ijerph17072557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hu T, Yao L, Reynolds K, et al. The effects of a low-carbohydrate diet on appetite: a randomized controlled trial. Nutr Metab Cardiovasc Dis 2016;26:476–88. 10.1016/j.numecd.2015.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Adam-Perrot A, Clifton P, Brouns F. Low-Carbohydrate diets: nutritional and physiological aspects. Obes Rev 2006;7:49–58. 10.1111/j.1467-789X.2006.00222.x [DOI] [PubMed] [Google Scholar]
- 53. Elder CR, Gullion CM, Funk KL, et al. Impact of sleep, screen time, depression and stress on weight change in the intensive weight loss phase of the life study. Int J Obes 2012;36:86–92. 10.1038/ijo.2011.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Geiker NRW, Astrup A, Hjorth MF, et al. Does stress influence sleep patterns, food intake, weight gain, abdominal obesity and weight loss interventions and vice versa? Obes Rev 2018;19:81–97. 10.1111/obr.12603 [DOI] [PubMed] [Google Scholar]
- 55. Deakin T, McShane CE, Cade JE, et al. Group based training for self-management strategies in people with type 2 diabetes mellitus. Cochrane Database Syst Rev 2005;2:Cd003417. 10.1002/14651858.CD003417.pub2 [DOI] [PubMed] [Google Scholar]
- 56. Náfrádi L, Nakamoto K, Schulz PJ. Is patient empowerment the key to promote adherence? A systematic review of the relationship between self-efficacy, health locus of control and medication adherence. PLoS One 2017;12:e0186458. 10.1371/journal.pone.0186458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sheeran P, Maki A, Montanaro E, et al. The impact of changing attitudes, norms, and self-efficacy on health-related intentions and behavior: a meta-analysis. Health Psychol 2016;35:1178–88. 10.1037/hea0000387 [DOI] [PubMed] [Google Scholar]
- 58. Russell-Westhead M, O'Brien N, Goff I, et al. Mixed methods study of a new model of care for chronic disease: co-design and sustainable implementation of group consultations into clinical practice. Rheumatol Adv Pract 2020;4:rkaa003. 10.1093/rap/rkaa003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jones T, Darzi A, Egger G, et al. Process and systems: a systems approach to embedding group consultations in the NHS. Future Healthc J 2019;6:8–16. 10.7861/futurehosp.6-1-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lean ME, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (direct): an open-label, cluster-randomised trial. Lancet 2018;391:541–51. 10.1016/S0140-6736(17)33102-1 [DOI] [PubMed] [Google Scholar]
- 61. National Institute for Clinical and Care Excellence . Obesity: identification, assessment and management. Clinical guideline [CG189] 2014. [Google Scholar]
- 62. Ahern AL, Wheeler GM, Aveyard P, et al. Extended and standard duration weight-loss programme referrals for adults in primary care (wrap): a randomised controlled trial. Lancet 2017;389:2214–25. 10.1016/S0140-6736(17)30647-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hartmann-Boyce J, Johns DJ, Jebb SA, et al. Behavioural weight management programmes for adults assessed by trials conducted in everyday contexts: systematic review and meta-analysis. Obes Rev 2014;15:920–32. 10.1111/obr.12220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jolly K, Lewis A, Beach J, et al. Comparison of range of commercial or primary care led weight reduction programmes with minimal intervention control for weight loss in obesity: Lighten up randomised controlled trial. BMJ 2011;343:d6500. 10.1136/bmj.d6500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Morris E, Aveyard P, Dyson P, et al. A food-based, low-energy, low-carbohydrate diet for people with type 2 diabetes in primary care: a randomized controlled feasibility trial. Diabetes Obes Metab 2020;22:512–20. 10.1111/dom.13915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. National Statistics. English Indices of Deprivation 2019 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjnph-2020-000219supp001.pdf (195.7KB, pdf)
Data Availability Statement
Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. For raw data or any other queries, please contact the corresponding author, LW: lou@louwalker.com.