Abstract
Background
Given the prevalence of gait dysfunction following stroke, walking recovery is a primary goal of rehabilitation. However, current gait rehabilitation approaches fail to demonstrate consistent benefits. Gait asymmetry, prevalent among stroke survivors who regain the ability to walk, is associated with an increased energy cost of walking and is a significant predictor of falls post-stroke. Furthermore, differential patterns of gait asymmetry may respond differently to gait training parameters.
Research question
The purpose of this study was to determine whether differential responses to locomotor task condition occur on the basis of step length asymmetry pattern (Symmetrical, NPshort, Pshort) observed during overground walking.
Methods
Participants first walked overground at their self-selected walking speed. Overground data were compared against three task conditions all tested during treadmill walking: self-selected speed with 0% body weight support (TM); self-selected speed with 30% body weight support (BWS); and fastest comfortable speed with 30% body weight support and nonparetic leg guidance (GuidanceNP). Our primary metrics were: symmetry indices of step length, stride length, and single limb support duration.
Results
We identified differences in the response to locomotor task conditions for each step length asymmetry subgroup. GuidanceNP induced an acute spatial symmetry only in the NPshort group and temporal symmetry in the Symmetrical and Pshort groups. Importantly, we found the TM and BWS conditions were insufficient to impact either spatial or temporal gait symmetry.
Significance
Task conditions consistent with locomotor training do not produce uniform effects across subpatterns of gait asymmetry. We identified differential responses to locomotor task conditions between groups with distinct asymmetry patterns, suggesting these subgroups may require unique intervention strategies. Despite group differences in asymmetry characteristics, improvements in symmetry noted in each group were driven by changes in both the paretic and nonparetic limbs.
Keywords: Stroke, gait, non-paretic training, symmetry, locomotor training, spatiotemporal parameters
Introduction
Walking recovery is among the top rehabilitation goals of stroke survivors.1 Regrettably, only 50% of stroke survivors undergoing gait rehabilitation demonstrate walking improvements.2 While nearly 80% of stroke survivors regain walking ability3, roughly half experience persistent gait asymmetry4 which is associated with increased energy cost and is a significant predictor of post-stroke falls.5,6 Gait asymmetries can be quantified in numerous ways, generally falling into two broad categories: spatial and temporal.4,7-9 Spatial asymmetries are commonly quantified by relating step length of the paretic leg to the nonparetic, whereas temporal asymmetries quantify the relationship of swing or single-limb-support time (SLS) between legs.4,10
While gait asymmetry following stroke is broadly recognized, the best approach for its remediation remains unknown.8,11,12 Compounding this issue, different patterns of gait asymmetry exist among stroke survivors (e.g., shorter paretic step length/time, shorter nonparetic step length/time, symmetrical step lengths/time).4,7,10,13 Yet, the majority of work arguing for interventions to reduce gait asymmetry ignores the asymmetry pattern, reporting instead only asymmetry magnitude4,14 which limits the interpretative power of a finding of reduced asymmetry.
Task-specific interventions have become the favored means to facilitate neuromotor recovery following stroke.15,16 Locomotor training (LT) is one example of a task-specific intervention founded in the rationale that applying appropriate sensory inputs* drives motor recovery. These sensory inputs are arguably facilitated using a permissive environment† which typically includes: a treadmill, partial body weight support (BWS), and manual assistance.15 However, speed-based study results regarding the benefits of LT are equivocal.2,14,17
Failure to demonstrate consistent effects of LT may result because the paradigm was not explicitly designed to remediate gait asymmetry. Indeed, recent work demonstrating meaningful improvements in gait symmetry has occurred through error-augmentation, that is by forcing participants to adjust to a split-belt treadmill environment that exacerbates asymmetry.18 However, error-augmentation was no better than error-minimizing techniques in restoring gait symmetry during overground walking.19 LT, arguably an error-minimizing technique, aims to normalize gait parameters throughout training. While the intent of the guidance provided during LT is to normalize the gait pattern, it is delivered through coordinated effort between patient and trainer(s) and consequently may fall short of the intended goal. Furthermore, the biomechanical effect during implementation of LT may not be straightforward. Without detailed measurements, the actual biomechanical effects of LT remain unknown.
Studies that categorize the pattern of gait asymmetry are often dominated by one asymmetry pattern, a shorter nonparetic step.12,18 However, shorter paretic, relative to nonparetic, step lengths are also noted, as are minimally detectable asymmetries. Grouped together these variations can complicate interpretation of data from a heterogenous sample.7 Importantly, individuals with different gait asymmetry patterns might respond differently to treatment, thus positive, or desired, effects can be obscured when combined with absent or negative responses. Therefore, there is a need to understand how people with different asymmetry patterns respond acutely to LT parameters.
Here we studied how the three key components of LT influence the biomechanics of gait post-stroke and whether these differ by spatial asymmetry pattern. We aimed to determine how task condition including: treadmill walking (TM), BWS, and manual guidance of the nonparetic limb (GuidanceNP) influences the spatiotemporal parameters of walking post-stroke. To determine the effects of task condition on spatial and temporal asymmetry, we investigated these changes relative to three spatial asymmetry patterns: 1) symmetrical step lengths (Symmetrical), 2) shorter paretic step length than nonparetic (Pshort), and 3) shorter nonparetic step length than paretic (NPshort). We hypothesized: i) the TM and BWS conditions would be insufficient to influence spatial or temporal symmetry and ii) the GuidanceNP condition would induce spatial and temporal symmetry in the NPshort group, yet would be unable to induce symmetry improvements in the Pshort group.
Methods
Participants
We studied 39 individuals with chronic (>6mo), post-stroke hemiparesis, able to walk independently at least 10 meters with an ankle foot orthosis (AFO) or assistive device. We excluded participants who demonstrated: severe perceptual or cognitive deficits, significant lower extremity contractures or joint pain, cardiovascular impairments contraindicative of walking, body weight >300 pounds, pathological fracture, or profound sensory deficits. The University of Florida Institutional Review Board (IRB-01 #160-2008) approved all procedures described herein; all participants provided written informed consent prior to participation.
Session 1
Clinical metrics
To quantify motor impairment, gait, and balance function we assessed the: synergy subscale of the Lower Extremity Fugl-Meyer (LE FM),20 Dynamic Gait Index (DGI),21 and Berg Balance Score (BBS),22 respectively.
Overground Walking
During overground walking (OG) all participants wore walking shoes and were permitted to use an assistive device if needed (n= 3), but not an AFO. If needed, an aircast (DJO, Vista, CA) was used for medial-lateral ankle stability (n=5). Participants were instructed to walk at their comfortable speed, “as if they were taking a walk through the park,” and walked twice over a GAITRite® Electronic Walkway (CIR Systems Inc., Sparta, NJ); data were averaged to obtain self-selected walking speed. The following measures were extracted: step length, stride length, SLS percent (SLS%), and gait speed.
Step Length Asymmetry Categorization
To determine the presence and pattern of step length asymmetry during overground walking, we calculated a paretic step ratio (PSR) which quantifies the proportional contribution of the paretic step to the stride length.13 We categorized our sample according to overground PSR values as follows: 1) symmetrical step lengths (Symmetrical; 0.475 ≤ PSR ≤ 0.525), 2) paretic step length shorter than nonparetic (Pshort; PSR < 0.475), and 3) nonparetic step length shorter than paretic (NPshort; PSR > 0.525).7
Temporal Asymmetry Quantification
To investigate the effects of task condition on temporal asymmetry, we calculated a temporal symmetry index (TSI) similar to our PSR calculation used for spatial symmetry with the following equation: TSI =SLS%paretic / (SLS%paretic + SLS%nonparetic), where SLS%paretic and SLS%nonparetic are the portions of the gait cycle spent in SLS on the paretic and nonparetic limbs, respectively. Consistent with our spatial asymmetry designation, we used a TSI range of 0.475-0.525 to represent temporal symmetry.7
Familiarization: Locomotor Task Conditions
To become familiar with the LT paradigm, each participant walked on the treadmill for three conditions: self-selected speed with 0% BWS (TM; Therastride, St. Louis MO); self-selected speed with 30% BWS; and fastest comfortable speed with 30% BWS and nonparetic leg guidance (GuidanceNP). We provided nonparetic foot guidance‡ during swing to promote increased step length, normalize step timing, and mitigate the exaggerated influence of the nonparetic over the paretic leg during bilateral locomotor-related tasks.16,23 Each participant performed up to three 5-minute bouts, resting between bouts. Blood pressure and heart rate were assessed prior to activity initiation and monitored throughout the session if the participant became symptomatic.
Session 2
Instrumented gait data including kinematics and kinetics were acquired using 12 infrared cameras (Vicon MX, Vicon Motion Systems Ltd., Oxford, UK; sf: 200Hz) and a modified Helen Hayes marker set (41 single markers, 11 rigid clusters) as participants walked on an instrumented split-belt treadmill (Bertec, Columbus, OH) while wearing a modified climbing harness (Robertson Mountaineering, Henderson, NV).
Experimental Testing
The three treadmill walking conditions, TM, BWS, and GuidanceNP, were tested in random order. Data were collected for as long as the participant could tolerate walking, up to a maximum of 40 seconds. To isolate effects of TM, BWS, and GuidanceNP, neither handrail hold nor AD/AFO’s were permitted during data collection. As with overground walking, an aircast was provided to control ankle instability if necessary.
Data Processing
Marker data were reduced using Vicon Nexus (Vicon Motion Systems Ltd., Version 1.6.1, Oxford, UK), modeled and filtered in Visual 3D (C-Motion, Version 4.82.0, Germantown, MD), and processed with custom Matlab (The MathWorks, Version 7.7.0 R2008b, Natick, MA) scripts to extract parameters for comparison with those obtained overground. We calculated spatial and temporal measures using marker and vertical ground reaction force data, respectively. Heel marker and ground reaction force data were filtered with a 4th order bi-directional Butterworth lowpass filter (6Hz and 10Hz cutoff, respectively).
Statistical Analysis
Participant characteristics are detailed in Table 1. Demographics were analyzed for differences between step length asymmetry groups (i.e., Symmetrical, Pshort, NPshort) using α= 0.05. We used the Chi-Square test to determine if differences in sex or side of paresis existed among groups. Since data were not normally-distributed, we used separate Kruskal-Wallis tests to assess for differences in age and stroke chronicity.
Table 1. Demographics.
Data for age, chronicity, and gait speeds are Mean ± SD. Data for LE Fugl-Meyer Synergy, Berg Balance Score, and Dynamic Gait Index are Median (Min, Max). Abbreviations: Symmetrical: paretic and nonparetic step lengths are equivalent; Pshort: paretic step length shorter than nonparetic; NPshort: nonparetic step length shorter than paretic; yr: years; m/f: male/female; r/l: right/left; mo: months; m/s: meters per second GuidanceNP: fastest comfortable walking speed, with 30% BWS, and nonparetic limb guidance; LE: lower extremity.
| All | Symmetrical | Pshort | NPshort | p-value | |
|---|---|---|---|---|---|
| n | 39 | 17 | 11 | 11 | |
| age (yr) | 61.3 ± 11.4 | 63.4 ± 9 | 65.5 ± 8 | 53.9 ± 14.6 | 0.09 |
| sex (m/f) | 29/10 | 13/4 | 8/3 | 8/3 | 0.97 |
| paretic side (r/l) | 21/18 | 8/9 | 5/6 | 8/3 | 0.33 |
| chronicity (mo) | 68.4 ± 61.7 | 71.8 ± 68.5 | 48.3 ± 43.6 | 83.5 ± 65.9 | 0.38 |
| gait speed (m/s) | |||||
| Overground (OG) | 0.63 ± 0.2 | 0.69 ± 0.2 | 0.59 ± 0.2 | 0.55 ± 0.16 | |
| Treadmill (TM) | 0.44 ± 0.14 | 0.48 ± 0.16 | 0.41 ± 0.12 | 0.4 ± 0.11 | |
| Body weight support (BWS) | 0.52 ± 0.2 | 0.59 ± 0.25 | 0.47 ± 0.14 | 0.47 ± 0.13 | |
| GuidanceNP | 0.72 ± 0.25 | 0.78 ± 0.26 | 0.64 ± 0.18 | 0.72 ± 0.29 | |
| LE Fugl-Meyer Synergy (/22) | 16 (5, 22) | 16 (8, 22) | 20 (5, 22) | 13 (8, 20) | 0.1 |
| Berg Balance Score (/56) | 46 (32, 55) | 47 (40, 55) | 48 (41, 55) | 45 (32, 55) | 0.61 |
| Dynamic Gait Index (/24) | 15 (7, 22) | 15 (9, 22) | 12 (7, 21) | 15 (10, 19) | 0.81 |
The spatial symmetry index, PSR, was the primary outcome. Secondary outcomes included: stride length and TSI. Each variable was tested for normality. Separate 1-way ANCOVAs were performed on PSR, stride length, and TSI to determine the effect of experimental condition (OG, TM, BWS, GuidanceNP) for each asymmetry category. Gait speed was used as a covariate and retained in the respective model when significant. In total we performed 9 ANCOVAs. To correct for multiple comparisons, we utilized the Holm-Bonferroni method with a target α=0.05 to adjust α-levels for all variables.24 We used Tukey’s HSD to isolate differences when effects were detected. The α-level applied for each model was carried through and used for the respective post hoc analyses. All statistical tests were performed with JMP® Pro (SAS Institute Inc. Version 14.0.0, Cary, NC) software.
Results
Overview
All participants (age: 61.3±11.4 yrs; 29 male; chronicity: 68.4±61.7 mo) experienced a single, monohemispheric stroke (confirmed with neuroimaging). The three asymmetry groups did not differ in demographic characteristics or clinical assessment of functional status (Table 1; all p’s>0.05). However, patterns of response to the experimental conditions differed for each asymmetry subgroup (Table 2).
Table 2. Individual leg data for each asymmetry group, across experimental conditions.
Data are mean ± SD. Reference values for single limb support duration (sec) are 0.33±0.04 sec for the overground walking speeds recorded for the Pshort and NPshort groups, and 0.3±0.03 sec for the Symmetrical group (laboratory reference data, unpublished). Statistical analyses investigated spatial (step length) and temporal (single limb support, %) symmetry metrics; here we present individual leg data to aid interpretation of changes in symmetry. Abbreviations: Symmetrical: paretic and nonparetic step lengths are equivalent; Pshort: paretic step length shorter than nonparetic; NPshort: nonparetic step length shorter than paretic; OG: overground; TM: treadmill condition at self-selected walking speed, with 0% BWS; BWS: body weight support condition at self-selected walking speed, with 30% BWS; GuidanceNP: fastest comfortable walking speed, with 30% BWS, and nonparetic limb guidance.
| Symmetrical | Pshort | NPshort | ||||
|---|---|---|---|---|---|---|
| paretic | nonparetic | paretic | nonparetic | paretic | nonparetic | |
| Step length (m) | ||||||
| OG | 0.5 ± 0.09 | 0.5 ± 0.08 | 0.34 ± 0.12 | 0.47 ± 0.11 | 0.49 ± 0.08 | 0.37 ± 0.1 |
| TM | 0.35 ± 0.09 | 0.34 ± 0.09 | 0.2 ± 0.08 | 0.33 ± 0.09 | 0.35 ± 0.08 | 0.27 ± 0.13 |
| BWS | 0.41 ± 0.13 | 0.38 ± 0.12 | 0.28 ± 0.08 | 0.38 ± 0.09 | 0.4 ± 0.09 | 0.31 ± 0.11 |
| GuidanceNP | 0.43 ± 0.15 | 0.52 ± 0.1 | 0.28 ± 0.13 | 0.48 ± 0.09 | 0.47 ± 0.13 | 0.48 ± 0.12 |
| Single limb support (%) | ||||||
| OG | 0.26 ± 0.06 | 0.37 ± 0.05 | 0.25 ± 0.06 | 0.32 ± 0.05 | 0.23 ± 0.04 | 0.38 ± 0.06 |
| TM | 0.23 ± 0.06 | 0.29 ± 0.03 | 0.22 ± 0.05 | 0.26 ± 0.04 | 0.19 ± 0.06 | 0.33 ± 0.04 |
| BWS | 0.26 ± 0.05 | 0.3 ± 0.04 | 0.25 ± 0.04 | 0.27 ± 0.05 | 0.22 ± 0.05 | 0.34 ± 0.05 |
| GuidanceNP | 0.31 ± 0.04 | 0.32 ± 0.05 | 0.3 ± 0.05 | 0.27 ± 0.05 | 0.28 ± 0.06 | 0.36 ± 0.03 |
| Single limb support (sec) | ||||||
| OG | 0.38 ± 0.06 | 0.55 ± 0.12 | 0.35 ± 0.06 | 0.44 ± 0.07 | 0.37 ± 0.09 | 0.61 ± 0.09 |
| TM | 0.33 ± 0.1 | 0.43 ± 0.11 | 0.29 ± 0.09 | 0.34 ± 0.06 | 0.29 ± 0.11 | 0.50 ± 0.06 |
| BWS | 0.36 ± 0.08 | 0.43 ± 0.1 | 0.34 ± 0.03 | 0.37 ± 0.09 | 0.34 ± 0.12 | 0.52 ± 0.09 |
| GuidanceNP | 0.41 ± 0.07 | 0.43 ± 0.08 | 0.38 ± 0.05 | 0.35 ± 0.08 | 0.39 ± 0.09 | 0.51 ± 0.09 |
| Stride length (m) | ||||||
| OG | 0.99 ± 0.17 | 0.81 ± 0.22 | 0.87 ± 0.17 | |||
| TM | 0.68 ± 0.17 | 0.54 ± 0.15 | 0.62 ± 0.19 | |||
| BWS | 0.79 ± 0.24 | 0.66 ± 0.13 | 0.71 ± 0.19 | |||
| GuidanceNP | 0.96 ± 0.23 | 0.76 ± 0.18 | 0.95 ± 0.25 | |||
For spatial symmetry, gait speed was not a significant covariate for any group (all p’s>0.006). For temporal symmetry, gait speed was a significant covariate for the Symmetrical (p=0.0002), but not the Pshort (p=0.28) or NPshort (p=0.0061) groups. Stride length co-varied with gait speed for all groups (all p’s<0.0001).
Symmetrical step lengths (Symmetrical)
The Symmetrical group (n=17) was characterized by equivalent paretic (0.50±0.09m) and nonparetic (0.50±0.08) step lengths (PSR: 0.50 ± 0.01) while walking overground. We identified significant effects of Experimental Condition for spatial (p=0.0019) and temporal symmetry (p=0.0012). Step lengths were similar for the OG, TM, and BWS conditions while the nonparetic step length was longer during the GuidanceNP condition (Figure 1a,b). During GuidanceNP relatively longer nonparetic step length (0.52±0.1m) was achieved simultaneously with a reduction of paretic (0.43±0.15m) step length (Figure 1b). Although the Symmetrical group participants exhibited spatial symmetry when walking overground, they revealed temporal asymmetry with a significantly reduced paretic (26±6%) relative to nonparetic (37±5%) SLS%. Temporal asymmetry was noted during walking in the TM and BWS conditions (Figure 1c); however, GuidanceNP induced temporal symmetry by increasing paretic (∆: 5%) and decreasing nonparetic (∆: 5%) SLS% concurrently (Figure 1d). Stride lengths achieved OG were reduced in the BWS and TM conditions but restored with the GuidanceNP condition (p=0.003; Figure 2).
Figure 1. Symmetrical step lengths (Symmetrical).
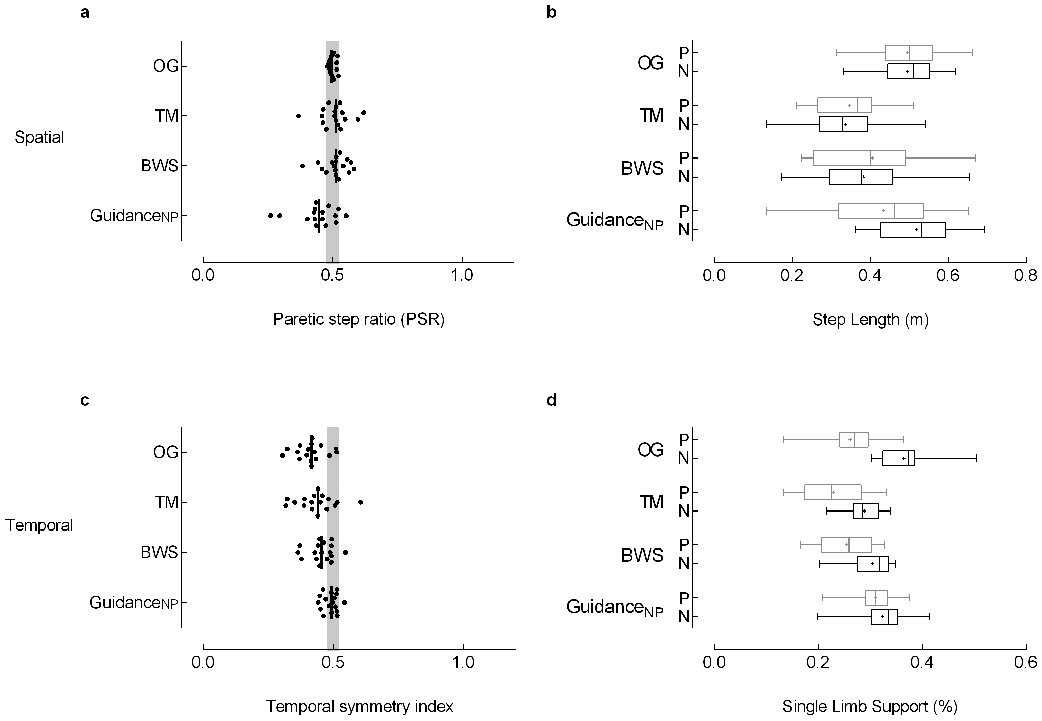
The Symmetrical group (n=17) was characterized by equivalent paretic and nonparetic step lengths while walking overground. (a) Spatial and (c) temporal symmetry were calculated with a symmetry index (SI) with the general equation SI =Xp/(Xp+Xnp), where Xp and Xnp are the paretic and nonparetic values for the variable of interest, respectively. Step length and percent of the gait cycle spent in single limb support were used to assess spatial and temporal symmetry. The symmetry index calculated for step length results in the paretic step ratio (PSR) used to categorize asymmetry groups (see Methods). Individual data are illustrated; the vertical black line represents the group median. The vertical gray shaded areas denote the SI values that represent symmetry (0.475 ≤ SI ≤ 0.525).7 Box-and-whisker plots for (b) step length and (d) single limb support duration (SLS%) illustrate the distribution of the individual leg data. The whiskers illustrate the 5th and 95th percentiles. Group means are depicted with “+”. Paretic and nonparetic leg data are illustrated in grey and black, respectively. Of note, the temporal symmetry achieved in the Symmetrical group with GuidanceNP results from a concurrent nonparetic reduction and paretic increase in SLS%. Abbreviations: OG: overground condition at self-selected walking speed; TM: treadmill condition at self-selected walking speed, with 0% BWS; BWS: body weight support condition at self-selected walking speed, with 30% BWS; GuidanceNP: fastest comfortable walking speed, with 30% BWS, and nonparetic limb guidance.
Figure 2. Stride length.
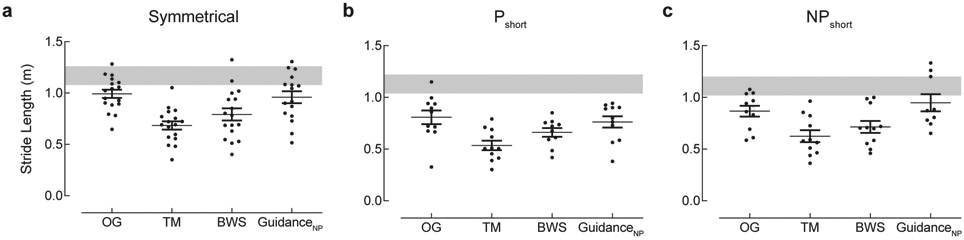
Stride length depicts the combined length of the paretic and nonparetic steps. The shaded gray regions represent reference values (± 1 standard error) for overground stride length calculated from a known regression equation relating stride length and gait speed.30 Data are mean ± SEM. Abbreviations: Symmetrical: paretic and nonparetic step lengths are equivalent; Pshort: paretic step length shorter than nonparetic; NPshort: nonparetic step length shorter than paretic; OG: overground condition at self-selected walking speed; TM: treadmill condition at self-selected walking speed, with 0% BWS; BWS: body weight support condition at self-selected walking speed, with 30% BWS; GuidanceNP: fastest comfortable walking speed, with 30% BWS and nonparetic limb guidance.
Paretic step length shorter than nonparetic (Pshort)
The Pshort group (n=11) was characterized by a shorter paretic (0.34±0.12m) than nonparetic (0.47±0.11m) step length (PSR: 0.41 ± 0.05) while walking OG. For spatial symmetry no significant effect of Experimental Condition (p=0.27) was revealed; the shorter paretic step was consistent across conditions (Figure 3a,b). We identified a significant Experimental Condition effect for stride length (p=0.005); BWS produced stride lengths similar to OG while the TM and GuidanceNP conditions produced shorter stride lengths than OG. Experimental Condition tended to affect temporal symmetry (p=0.012) though this effect failed to reach statistical significance. Of note, the median symmetry index for SLS% fell within the range of symmetry for the GuidanceNP condition (Figure 3c). During GuidanceNP, we observed a concurrent decrease in nonparetic (∆: 5%) and increase in paretic (∆: 5%) SLS% (Figure 3d).
Figure 3. Paretic step length shorter than nonparetic (Pshort).
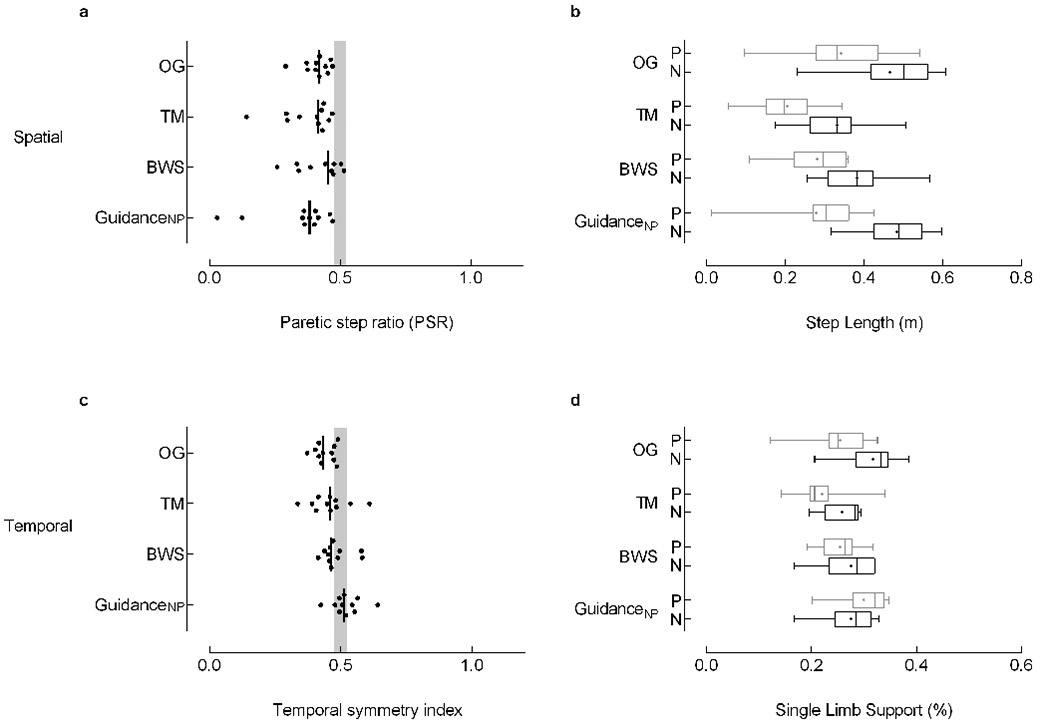
The Pshort group (n=11) was characterized by a shorter paretic step length than nonparetic step length while walking overground. Spatial (top, left) and temporal (bottom, left) symmetry were calculated with a symmetry index (SI) with the general equation SI =Xp/(Xp+Xnp), where Xp and Xnp are the paretic and nonparetic values for the variable of interest, respectively. Individual data are illustrated; the vertical black line represents the group median. The vertical gray shaded areas denote the SI values that represent symmetry (0.475 ≤ SI ≤ 0.525).7 Re-analysis of data without the extreme values noted in GuidanceNP condition of (a) do not influence symmetry findings or interpretation. Box-and-whisker plots for step length (top, right) and single limb support duration (SLS%; bottom, right) illustrate the distribution of the individual leg data. The whiskers illustrate the 5th and 95th percentiles. Group means are depicted with “+”. Paretic and nonparetic leg data are illustrated in grey and black, respectively. Note, a concurrent decrease in nonparetic SLS% (∆: 5%) and increase in paretic SLS% (∆: 5%) between the overground and GuidanceNP conditions (c). While these changes resulted in temporal symmetry, they failed to reach statistical significance. Abbreviations: OG: overground condition at self-selected walking speed; TM: treadmill condition at self-selected walking speed, with 0% BWS; BWS: body weight support condition at self-selected walking speed, with 30% BWS; GuidanceNP: fastest comfortable walking speed, with 30% BWS, and nonparetic limb guidance; P: paretic; N: nonparetic.
Nonparetic step length shorter than paretic (NPshort)
The NPshort group (n=11) walked with shorter nonparetic (0.37±0.01m) than paretic step (0.49±0.08m) lengths (PSR: 0.58 ± 0.05) overground. Experimental Condition tended to affect spatial symmetry (p=0.024). Spatial asymmetry noted OG persisted in the TM and BWS conditions, but GuidanceNP produced symmetric step lengths in the NPshort group (Figure 4a) by increasing the nonparetic step length (∆: 0.11m; Figure 4b), though these changes failed to reach statistical significance given the adjusted α-level. We did not identify a significant effect of Experimental Condition on temporal symmetry (p=0.12); paretic SLS% was consistently less than nonparetic across all walking conditions (Figure 4 c,d). Importantly, paretic SLS% increased from 23% to 28% between OG and GuidanceNP conditions.
Figure 4. Nonparetic step length shorter than paretic (NPshort).
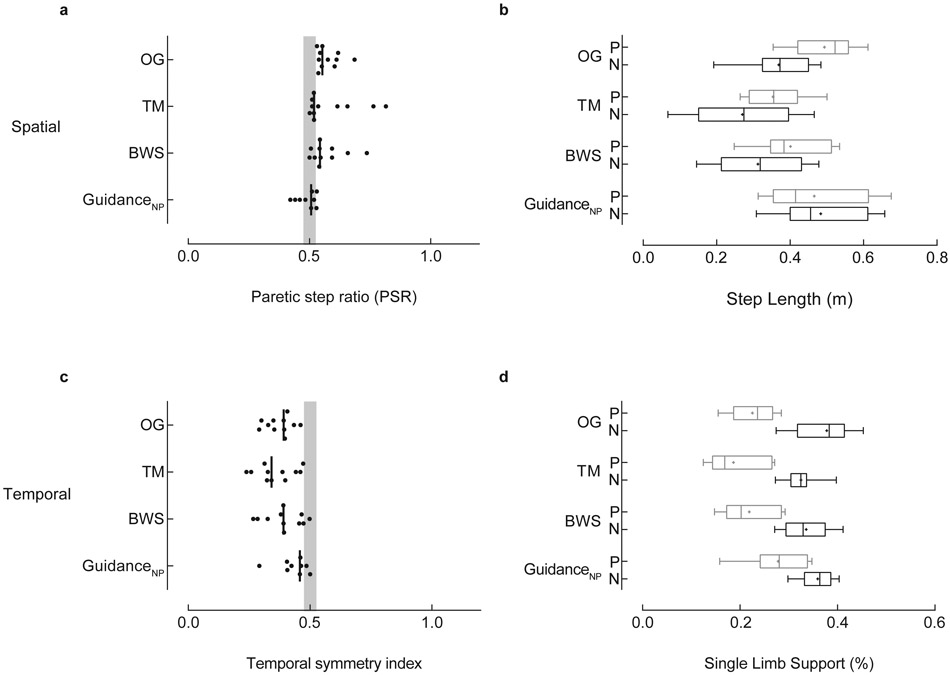
The NPshort group (n=11) walked with shorter nonparetic than paretic step lengths overground. Spatial (top, left) and temporal (bottom, left) symmetry were calculated with a symmetry index (SI) with the general equation SI =Xp/(Xp+Xnp), where Xp and Xnp are the paretic and nonparetic values for the variable of interest, respectively. Individual data are illustrated; the vertical black line represents the group median. The vertical gray shaded areas denote the SI values that represent symmetry (0.475 ≤ SI ≤ 0.525).7 Box-and-whisker plots for step length (top, right) and single limb support duration (SLS%; bottom, right) illustrate the distribution of the individual leg data. The whiskers illustrate the 5th and 95th percentiles. Group means are depicted with “+”. Paretic and nonparetic leg data are illustrated in grey and black, respectively. Note, the GuidanceNP condition produced symmetrical step lengths (a) by increasing the nonparetic step length (b; ∆: 0.11m). Importantly, P SLS% increased from 23% to 28% of the gait cycle between OG and GuidanceNP conditions (d), though this increase did not achieve statistical significance. Abbreviations: OG: overground condition at self-selected walking speed; TM: treadmill condition at self-selected walking speed, with 0% BWS; BWS: body weight support condition at self-selected walking speed, with 30% BWS; GuidanceNP: fastest comfortable walking speed, with 30% BWS, and nonparetic limb guidance; P: paretic; N: nonparetic.
Discussion
Here we investigated whether groups with different spatial asymmetry patterns responded differently to task conditions representing components of LT. Across groups we found responses in spatial and temporal symmetry differed by task condition. As hypothesized, TM and BWS failed to influence spatial or temporal symmetry. Further, we expected the GuidanceNP condition would: 1) improve spatial and temporal symmetry in the NPshort group, and 2) have no effect on symmetry in the Pshort group. While the GuidanceNP condition induced a somewhat positive effect on spatial symmetry in the NPshort group, temporal asymmetry remained unchanged. In contrast, the Pshort group showed subtle improvements in temporal, but not spatial symmetry, in response to GuidanceNP. Furthermore, while GuidanceNP induced temporal symmetry for the Symmetrical group, it simultaneously induced spatial asymmetry.
Effects induced by LT parameters
Each seemingly simple decision regarding LT parameters can influence the gait pattern. Indeed, prior work reported improved SLS symmetry simply by walking on a treadmill or using BWS.11,25,26 Our results contrast with these findings. However, the improved symmetry previously noted during treadmill walking occurred with simultaneous use of handrails11,25 and may, therefore, be an artefact of increased postural support available through the upper extremity rather than a direct response to a treadmill-induced perturbation.26 Generally, we found the treadmill or BWS alone were insufficient to induce either spatial or temporal symmetry; GuidanceNP was more successful in inducing symmetry, although responses differed by asymmetry subgroup.
Effects in the context of asymmetry subgroups
The underlying premise that a single task-specific training approach would positively benefit a group with heterogeneous gait deficits limits opportunity to better understand the interaction between subgroup characteristics and treatment effects. Interestingly, in relatively homogenous samples – intentionally selected for short nonparetic relative to paretic step length, improved spatial symmetry was noted in response to each of two different training paradigms (i.e., split-belt training, unilateral step training).12,18 Similarly, GuidanceNP induced spatial symmetry in the NPshort group, despite failing to reach statistical significance. However, spatial symmetry was not achieved in other subgroups during the GuidanceNP condition.
Notably, achieving improved temporal symmetry appears more elusive.12,18 For example, Lewek reported improved temporal symmetry in an individual with spatial symmetry but temporal asymmetry; however, in an individual with both spatial and temporal asymmetries (NPshort per our definition), temporal asymmetry remained unchanged despite improvements in spatial symmetry.27 Though we did not investigate treatment response (i.e., repeated session exposure), our findings align with prior work.12,18,27 However, GuidanceNP was, able to induce temporal symmetry acutely in the Symmetrical group.
Consistent with prior work, our data reveal it is possible to increase symmetry in one domain while simultaneously decreasing it in another.8 At present, we are unable to determine whether targeting improvements in spatial or temporal symmetry would lead to greater benefit. However, our data support the recommendation that interventions should be designed to address individual patient needs. Additional work is needed to understand the interaction between spatial and temporal gait symmetry following stroke.
Proposed mechanism of nonparetic guidance effect
Paretic limb motor impairments can be exaggerated by a counter-productive influence induced through volitional movement of the nonparetic leg during bilateral locomotor-related tasks.23 Additionally, passive movement of the nonparetic limb can elicit task-appropriate rhythmic activation pattern in the paretic limb.28 We therefore expected external nonparetic limb guidance to provide a more appropriate sensorimotor state and facilitate positive expression of paretic limb function. Indeed, we noted acute increases in both relative and absolute durations of paretic SLS in each of the three asymmetry groups (Figures 1,3,4; Table 2) during GuidanceNP.
Is targeting improved symmetry sufficient?
Improved gait symmetry is reported as an acute effect11,25 or a treatment outcome of gait-related interventions.12,17,18,27 While an improved symmetry ratio might be interpreted as a positive effect, our results illustrate that symmetry ratios can be misleading. A change in symmetry ratio alone cannot elucidate the source of change, specifically, whether improvements result from changes in the paretic, nonparetic, or both limbs.17,25,27 Improved symmetry ratios alone are therefore insufficient to conclude a beneficial outcome has occurred. Indeed, individual leg data illustrate improved symmetry ratios are often achieved through a non-physiologic reduction from the nonparetic limb with minimal or no improvement noted in the paretic limb.11,12 Consistent with prior work, we observed a decrease in nonparetic SLS% on the TM (not tested explicitly; Table 2) across all groups.11 While it could be argued this change produced improved symmetry through reduction of the between-leg difference11, changes induced on the TM did not achieve our definition of symmetry, neither did they approach physiologic durations of SLS in the paretic limb. Of note, our externally-guided condition, GuidanceNP, was the only experimental condition that restored SLS symmetry between legs. Restored temporal symmetry was observed only in the spatially symmetrical group. Furthermore, the change in SLS% symmetry during GuidanceNP was driven by concurrent changes in both legs: increased paretic and reduced nonparetic SLS. Notably, these changes were consistent with the gait pattern used by healthy controls walking on a treadmill, characterized by SLS duration of ~31-32% of the gait cycle.29 We emphasize these observations were made acutely, in response to an experimental condition. The possibility that repeated sessions could produce larger and sustained changes in gait and the differential response by asymmetry group are worthy of further investigation.
Conclusions
Commonly used rehabilitation interventions for gait dysfunction following stroke do not produce uniform effects. We identified differential acute responses to LT conditions between groups with disparate asymmetry patterns, suggesting these subgroups may benefit from distinct intervention strategies. Improvements in temporal symmetry in the Symmetrical group were noted to result from both limbs. Similarly, improvements in spatial symmetry noted in the NPshort group were driven by bilateral improvements, namely increased nonparetic step length combined with increased paretic SLS. By investigating individual limb effects, we were able to determine these changes in spatial and temporal symmetry resulted from desirable effects rather than compensatory mechanisms.
Acknowledgements
This research was conducted at the VA Brain Rehabilitation Research Center, Gainesville, FL and supported by the Department of Veterans Affairs, Rehabilitation Research & Development Service, Project #A6365B (SAK & CP), Research Career Scientist Awards #F7823S (CP) and #A9272S (SAK), and the VA Brain Rehabilitation Research Center (VA RRD #B6793C). Dr. Little received support from the Foundation for Physical Therapy and NIH T32 Neuromuscular Plasticity Training Grant (No. 5 T32 HD043730-08, K Vandenborne, PI). Dr. Mercado received support from the University Scholars’ Program at the University of Florida. Dr. Kautz also received support from NIH P20 GM109040.
This material is the result of work supported with resources and use of facilities at the NF/SG Veterans Administration Health Care System, Gainesville, FL, USA. The contents do not represent the views of the Department of Veterans Affairs, the NIH, or the United States Government. The funding source played no role in either writing this manuscript or the decision to submit for publication. The corresponding author retains full access to all data in the study and assumes final responsibility for the decision to submit for publication.
We thank: Helen Emery for her assistance with data collection and Yueh-Yun Chi, PhD for her assistance with the statistical analysis.
Footnotes
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
The sensory inputs considered requisite to locomotor training efficacy include: 1) afferent stimuli from limb loading, 2) proper trunk alignment and upright orientation relative to gravity, 3) hip extension, 4) appropriate walking speed, and 5) phasic timing of loading/unloading cycles during walking.16
Prior work revealed adequate body weight support can facilitate locomotor pattern expression in individuals with neurologic injuries, manifested as normalization of muscle activity timing, improved trunk and knee kinematics during loading, and improved spatiotemporal characteristics.
Hand placement was on the dorsum of the nonparetic foot. All trainers were taught the guidance procedure by a licensed physical therapist proficient in locomotor training prior to the initiation of the study. Manual assistance was provided by the same trainer for all trials of each participant.
References
- 1.Pollock A, St George B, Fenton M, Firkins L. Top 10 research priorities relating to life after stroke--consensus from stroke survivors, caregivers, and health professionals. Int J Stroke. 2014;9:313–320. doi: 10.1111/j.1747-4949.2012.00942.x [DOI] [PubMed] [Google Scholar]
- 2.Duncan PW, Sullivan KJ, Behrman AL, et al. Body-weight-supported treadmill rehabilitation after stroke. N Engl J Med. 2011;364:2026–2036. doi: 10.1056/NEJMoa1010790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jorgensen HS, Nakayama H, Raaschou HO, Olsen TS. Recovery of walking function in stroke patients: the Copenhagen Stroke Study. Arch Phys Med Rehabil. 1995;76:27–32. http://www.ncbi.nlm.nih.gov/pubmed/7811170. [DOI] [PubMed] [Google Scholar]
- 4.Patterson KK, Gage WH, Brooks D, Black SE, McIlroy WE. Evaluation of gait symmetry after stroke: a comparison of current methods and recommendations for standardization. Gait Posture. 2010;31:241–246. doi: 10.1016/j.gaitpost.2009.10.014 [DOI] [PubMed] [Google Scholar]
- 5.Sánchez N, Finley J. Individual Differences in Locomotor Function Predict the Capacity to Reduce Asymmetry and Modify the Energetic Cost of Walking Poststroke. Neurorehabil Neural Repair. 2018;32(8):701–713. doi: 10.1177/1545968318787913 [DOI] [PubMed] [Google Scholar]
- 6.Bower K, Thilarajah S, Pua Y-H, et al. Dynamic balance and instrumented gait variables are independent predictors of falls following stroke. doi: 10.1186/s12984-018-0478-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balasubramanian CK, Neptune RR, Kautz SA. Variability in spatiotemporal step characteristics and its relationship to walking performance post-stroke. Gait Posture. 2009;29(3):408–414. doi: 10.1016/j.gaitpost.2008.10.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malone LA, Bastian AJ. Spatial and temporal asymmetries in gait predict split-belt adaptation behavior in stroke. Neurorehabil Neural Repair. 2014;28:230–240. doi: 10.1177/1545968313505912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wonsetler EC, Bowden MG. A systematic review of mechanisms of gait speed change post-stroke. Part 1: spatiotemporal parameters and asymmetry ratios. Top Stroke Rehabil. 2017;24(6):435–446. doi: 10.1080/10749357.2017.1285746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim CM, Eng JJ. Symmetry in vertical ground reaction force is accompanied by symmetry in temporal but not distance variables of gait in persons with stroke. Gait Posture. 2003;18(1):23–28. doi: 10.1016/S0966-6362(02)00122-4 [DOI] [PubMed] [Google Scholar]
- 11.Harris-Love ML, Forrester LW, Macko RF, Silver KH, Smith G V. Hemiparetic gait parameters in overground versus treadmill walking. Neurorehabil Neural Repair. 2001;15:105–112. http://www.ncbi.nlm.nih.gov/pubmed/11811252. [DOI] [PubMed] [Google Scholar]
- 12.Kahn JH, Hornby TG. Rapid and long-term adaptations in gait symmetry following unilateral step training in people with hemiparesis. Phys Ther. 2009;89:474–483. doi: 10.2522/ptj.20080237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowden MG, Balasubramanian CK, Behrman AL, Kautz SA. Validation of a speed-based classification system using quantitative measures of walking performance poststroke. Neurorehabil Neural Repair. 2008;22:672–675. doi: 10.1177/1545968308318837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Westlake KP, Patten C. Pilot study of Lokomat versus manual-assisted treadmill training for locomotor recovery post-stroke. J Neuroeng Rehabil. 2009;6:18. doi: 10.1186/1743-0003-6-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barbeau H Locomotor training in neurorehabilitation: emerging rehabilitation concepts. Neurorehabil Neural Repair. 2003;17:3–11. [DOI] [PubMed] [Google Scholar]
- 16.Visintin M, Barbeau H, Korner-Bitensky N, Mayo NE. A new approach to retrain gait in stroke patients through body weight support and treadmill stimulation. Stroke. 1998;29(6):1122–1128. http://www.ncbi.nlm.nih.gov/pubmed/9626282. [DOI] [PubMed] [Google Scholar]
- 17.Hornby TG, Campbell DD, Kahn JH, Demott T, Moore JL, Roth HR. Enhanced gait-related improvements after therapist- versus robotic-assisted locomotor training in subjects with chronic stroke: a randomized controlled study. Stroke. 2008;39:1786–1792. doi: 10.1161/strokeaha.107.504779 [DOI] [PubMed] [Google Scholar]
- 18.Reisman DS, McLean H, Keller J, Danks KA, Bastian AJ. Repeated split-belt treadmill training improves poststroke step length asymmetry. Neurorehabil Neural Repair. 2013;27:460–468. doi: 10.1177/1545968312474118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewek MD, Braun CH, Wutzke C, Giuliani C. The Role of Movement Errors in Modifying Spatiotemporal Gait Asymmetry Post-Stroke: A Randomized Controlled Trial. Clin Rehabil. 2018;32(2):161–172. doi: 10.1177/0269215517723056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. http://www.ncbi.nlm.nih.gov/pubmed/1135616. [PubMed] [Google Scholar]
- 21.Jonsdottir J, Cattaneo D. Reliability and validity of the dynamic gait index in persons with chronic stroke. Arch Phys Med Rehabil. 2007;88:1410–1415. doi: 10.1016/j.apmr.2007.08.109 [DOI] [PubMed] [Google Scholar]
- 22.Berg KO, Wood-Dauphinee SL, Williams JI, Maki B. Measuring balance in the elderly: validation of an instrument. Can J Public Heal. 1992;83 Suppl 2:S7–11. http://www.ncbi.nlm.nih.gov/pubmed/1468055. [PubMed] [Google Scholar]
- 23.Kautz SA, Patten C. Interlimb influences on paretic leg function in poststroke hemiparesis. J Neurophysiol. 2005;93:2460–2473. doi: 10.1152/jn.00963.2004 [DOI] [PubMed] [Google Scholar]
- 24.Holm S A Simple Sequentially Rejective Multiple Test Procedure. Vol 6; 1979. https://www.jstor.org/stable/pdf/4615733.pdf?refreqid=excelsior%3Afc012354f031b28835f401a3b77eb259. Accessed May 20, 2019. [Google Scholar]
- 25.Hassid E, Rose D, Commisarow J, Guttry M, Dobkin BH. Improved Gait Symmetry in Hemiparetic Stroke Patients Induced During Body Weight-Supported Treadmill Stepping. J Neuro Rehab. 1997;11:21–26. http://journals.sagepub.com/doi/pdf/10.1177/154596839701100104. Accessed October 9, 2018. [Google Scholar]
- 26.Chen G, Patten C, Kothari DH, Zajac FE. Gait deviations associated with post-stroke hemiparesis: improvement during treadmill walking using weight support, speed, support stiffness, and handrail hold. Gait Posture. 2005;22:57–62. doi: 10.1016/j.gaitpost.2004.06.008 [DOI] [PubMed] [Google Scholar]
- 27.Lewek MD, Feasel J, Wentz E, Brooks FP Jr., Whitton MC. Use of visual and proprioceptive feedback to improve gait speed and spatiotemporal symmetry following chronic stroke: a case series. Phys Ther. 2012;92:748–756. doi: 10.2522/ptj.20110206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kautz SA, Patten C, Neptune RR. Does unilateral pedaling activate a rhythmic locomotor pattern in the nonpedaling leg in post-stroke hemiparesis? J Neurophysiol. 2006;95:3154–3163. doi: 10.1152/jn.00951.2005 [DOI] [PubMed] [Google Scholar]
- 29.Kautz SA, Bowden MG, Clark DJ, Neptune RR. Comparison of motor control deficits during treadmill and overground walking poststroke. Neurorehabil Neural Repair. 2011;25:756–765. doi: 10.1177/1545968311407515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirtley C, Whittle MW, Jefferson RJ. Influence of walking speed on gait parameters. J Biomed Eng. 1985;7:282–288. http://www.ncbi.nlm.nih.gov/pubmed/4057987. [DOI] [PubMed] [Google Scholar]


