Abstract
Background:
The heart undergoes physiological hypertrophy during pregnancy in healthy individuals. Metabolic syndrome (MetS) is now prevalent in women of child-bearing age and might add risks of adverse cardiovascular events during pregnancy. The present study asks if cardiac remodeling during pregnancy in obese individuals with MetS is abnormal and does this predispose them to higher risk for cardiovascular disorders?
Methods:
The idea that MetS induces pathological cardiac remodeling during pregnancy was studied in a long-term (15 weeks) Western diet (WD) feeding animal model that recapitulated features of human MetS. Pregnant female mice with WD (45% kcal fat)-induced MetS were compared to pregnant and nonpregnant females fed a control diet (10% kcal fat, CD).
Results:
Pregnant mice fed a WD had increased heart mass and exhibited key features of pathological hypertrophy, including fibrosis and upregulation of fetal genes associated with pathological hypertrophy. Hearts from pregnant animals with WD-induced MetS had a distinct gene expression profile that could underlie their pathological remodeling. Concurrently, pregnant female mice with MetS showed more severe cardiac hypertrophy and exacerbated cardiac dysfunction when challenged with angiotensin II/phenylephrine infusion after delivery.
Conclusions:
These results suggest that preexisting MetS could disrupt physiological hypertrophy during pregnancy to produce pathological cardiac remodeling that could predispose the heart to chronic disorders.
Keywords: Pregnancy, Metabolic Syndrome, Cardiac Remodeling, Cardiac Hypertrophy, Fibrosis
Summary:
Preexisting metabolic syndrome induces pathological cardiac remodeling during pregnancy.
Introduction
Pregnancy causes physiological changes in the cardiovascular system to meet the increased demands of both the mother and the developing fetus1. The most significant changes in the cardiovascular system include increased blood volume, increased cardiac output and decreased systemic vascular resistance, accompanied by physiological cardiac hypertrophy1–4. This physiological cardiac remodeling is distinct from the remodeling that occurs in the heart with pathological stressors5. Cardiovascular disease remains the leading cause of maternal death in North America6, 7, suggesting that aspects of physiological cardiovascular remodeling in pregnancy could be disrupted by the underlying disease state8.
Obesity, as a potential risk factor for cardiovascular diseases, is now a major global health issue9–11. Among obese people, a large percentage of the population subsequently becomes metabolically unhealthy, which has been defined as Metabolic syndrome (MetS)12. There is a strong link between obesity, MetS and cardiovascular diseases13–17. There has been a significant increase in the prevalence of obesity and MetS in women of childbearing age, with more than 30% of females in their reproductive years being obese and many have developed MetS as a comorbidity18–22. If and how obesity and/or MetS disrupt cardiac remodeling during pregnancy is not well-understood and is the focus of the present research. A Western diet induced MetS animal model was employed to test the hypothesis that preexisting MetS disrupts physiological cardiac remodeling during pregnancy to induce pathological remodeling.
Materials and Methods
All animal procedures were approved by the Temple University Lewis Katz School of Medicine Institutional Animal Care and Use Committee. Animals were fed a control diet (CD) or a Western diet (WD). Formula of the diets are provided in Supplementary Table I. The human study was approved by the local institutional review committee (2020-001-01(Y)) and all participants provided written consent. See the extended Materials and Methods section in the Online Data Supplement for details.
Data Availability
The data, methods used in the analysis, and materials used to conduct the research will be made available to any researcher for purposes of reproducing the results. Data of standard RNA-sequencing will be uploaded to the GEO database.
Statistical Analysis
Data are represented as mean±SD. The distributions of all continuous variables were tested for normality assumptions using the D’Agostino & Pearson normality test using GraphPad Prism. For human case-control study and parameters with a single measurement in the animal study, group comparisons were performed using the 2-sample t-test or the Mann-Whitney U-test depending on the distribution of the data. For body weights data and echocardiography parameters with repeated measures over time, linear mixed-effects models were used to estimate mean values at each assessment time point and to test treatment group differences at each time point as well as change vs. baseline over time within each treatment group while at the same time taking into account in the analyses the correlation among longitudinal measurements within each animal. In each linear mixed-effects model, time and treatment group were included as fixed effects along with its time-by-treatment group interaction term. Comparisons under these mixed-effects models were performed using Tukey or Dunnett-Hsu post-hoc multiple comparison tests. For in vivo data between two groups (CD versus WD) under multiple conditions (Non-pregnant versus Post-partum), the analysis was performed by two-way ANOVA, followed by Sidak’s or Tukey’s multiple comparisons test. For the in vitro study with cultured fibroblasts in Figure 5, the difference between multiple treatments was evaluated using one-way ANOVA followed by Tukey’s post-hoc multiple comparisons test. Two-sided testing was used for all statistical tests. A p-value of ≤0.05 was considered statistically significant. Data analyses were performed using either the GraphPad Prism software (Version 7.0d, GraphPad Inc., La Jolla, CA) or SAS version 9.4 (SAS Institute Inc., Cary, NC).
Figure 5. Angiopoietin like protein 4 (Angptl4) promotes cardiac fibroblast proliferation and transformation in vitro.

A. Expression level of Angptl4 in heart tissues by RT-PCR and the top 25 most upregulated genes in the WD-PP group determined by RNA-sequencing. **p<0.01, significantly different vs. CD; #<0.05, significantly different vs. non-pregnant. (CD: Control diet, WD: Western diet, PP: Post-partum; NP: Non-pregnant). B. Representative images and quantification of rat primary cardiac fibroblasts (CFs) migration in culture with vehicle, 5μg/ml Angptl4 and 10μg/ml Angptl4 treatment (n=3). C. EdU and CellMask™ Orange staining and quantification of rat CFs after 48h treatment with vehicle, 5μg/ml Angptl4, 10μg/ml Angptl4, and 10ng/mL transforming growth factor beta (TGFβ) (n=4). D. Immunoblotting for Vimentin, α-smooth muscle actin (αSMA) and GAPDH in rat CFs treated with vehicle, 5μg/ml Angptl4, 10μg/ml Angptl4, and 10ng/ml TGFβ and quantification of protein expression (n=3). Data are presented as mean±SD. *p<0.05, **p<0.01, *** p<0.005, ****p<0.001 vs. Control.
Results
Western diet induces an early-stage MetS phenotype in female C57BL/6 mice
A mouse model of early-stage MetS phenotype was established using long-term (15 weeks) feeding with a WD (45% kcal fat) in female C57BL/6J mice. Mice fed a CD (10% kcal fat) served as controls (Supplementary Figure I. A). WD mice gained significantly more weight compared with CD mice (Supplementary Figure I. B). After 15 weeks of feeding there were no differences in hemodynamic parameters between groups, including maximum developed left ventricular (LV) pressure, maximum dP/dT, and minimum dP/dT (Supplementary Figure I. C). WD mice had impaired glucose tolerance tests (Supplementary Figure I. D) and significantly increased plasma levels of cholesterol (p=0.0006), high-density lipoprotein (HDL) (p=0.0182) and low-density lipoprotein (LDL) (p=0.0109) (Supplementary Figure I. E). Heart weight to tibia length ratio (HW/TL) and cardiomyocyte cross-sectional area (CSA) were not significantly different in WD mice compared with CD mice after 15 weeks of feeding (Supplementary Figure I. F). Overall, these data suggest that 15 weeks of WD feeding in female mice induces a phenotype that is similar to early-stage human MetS, with features of increased body weight, impaired glucose tolerance and dyslipidemia, without overt cardiac remodeling.
Diet induced early-stage MetS induces signs of pathological cardiac hypertrophy after pregnancy
Pregnancy was induced in both CD and WD mice that were kept on their respective diet for the entire duration of the study and terminal studies were performed 1-day post-partum (Post-partum). Female mice that were not mated were kept on CD or WD for the same amount of time as their pregnant counterparts, therefore serving as age and diet matched controls (Non-pregnant) (Fig.1A). WD mice had significantly greater body weights compared with CD mice in both non-pregnant and pregnant post-partum groups (Fig.1B). Post-partum HW/TL was significantly increased in both WD and CD mice compared with the non-pregnant counterparts, however, WD mice exhibited a significantly greater HW/TL compared with CD mice post-partum (7.202±0.503 mg/mm versus 6.412±0.47 mg/mm, p=0.0028) (Fig.1B). HW/TL regressed to pre-pregnancy levels within 1-week post-partum in CD mice (5.733±0.352 mg vs. 5.387±0.474 mg, p=0.7157). However, HW/TL was still significantly above pre-pregnancy levels in WD mice at 1-week and 6-week post-partum (6.599±0.411 mg/6.959±0.481 mg vs. 5.892±0.723 mg, p=0.0214/p=0.0007). In addition, HW/TL in WD mice was significantly higher than in CD mice at this time (Fig.1B). Cardiomyocyte CSA in WD mice (275.9±40.429 μm2) was significantly greater than in CD mice (222.9±12.2 μm2) 1-day post-partum (Fig.1C). The gene expression level of atrial natriuretic peptide (ANP), a so-called fetal gene associated with pathological hypertrophy, was significantly increased with WD feeding (2.9-fold) and was further elevated post-partum. The expression level of beta-myosin heavy chain (βMHC) in WD mice post-partum was also significantly increased (1.7-fold) compared with CD mice (Fig.1D). There was an increase of collagen deposition in non-pregnant WD mice compared with CD mice, as determined by Picro-Sirius Red staining (1.838±0.163% vs. 0.943±0.286%), and the trend was greater in post-partum WD mice (2.519±0.192%) (Fig.1E). Overall, these findings suggest that pregnancy in animals with MetS can induce pathological rather than physiological cardiac hypertrophy.
Figure 1. Western diet induced early-stage MetS leads to pathological cardiac hypertrophy after pregnancy.
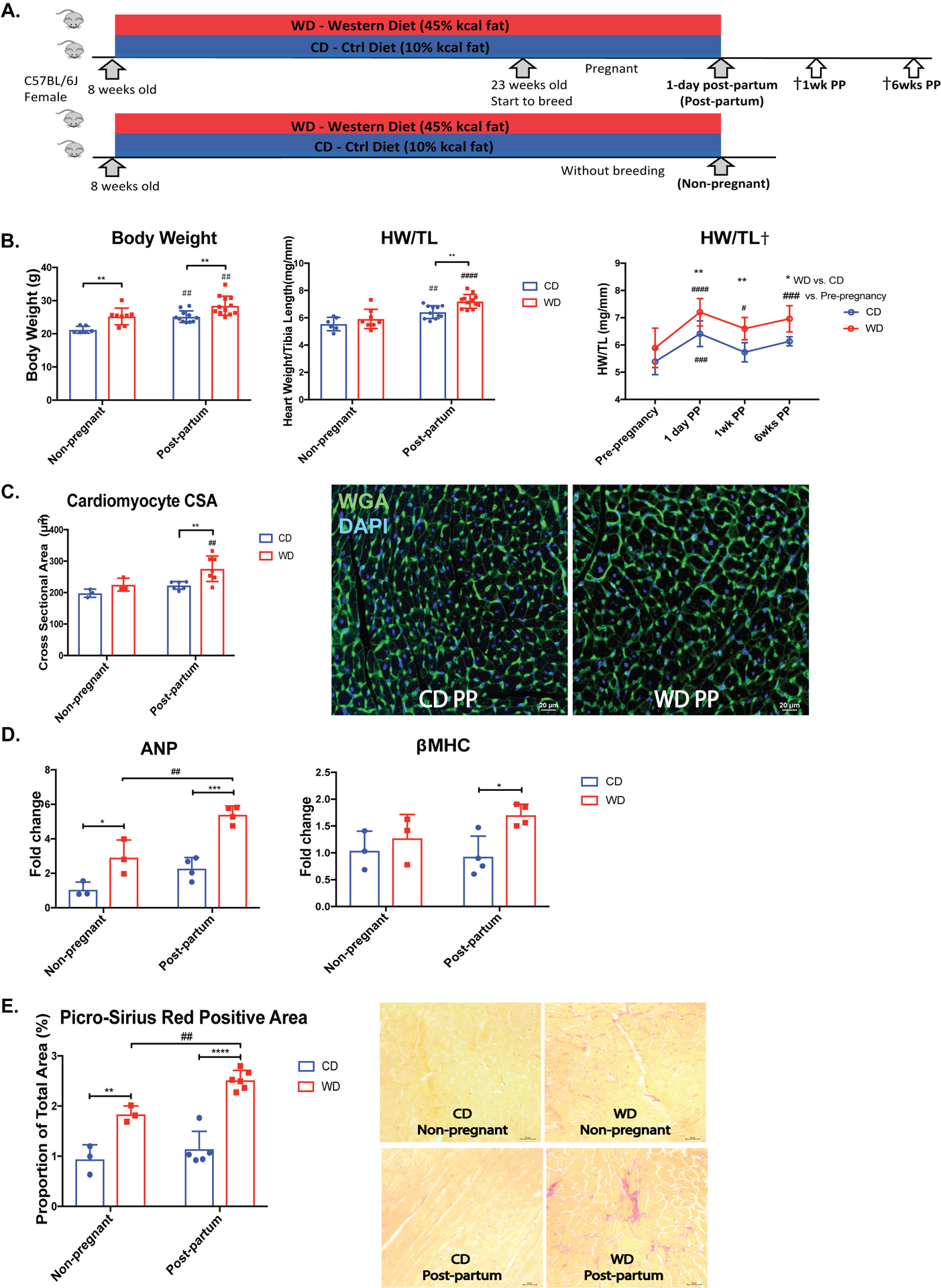
A. 8-week old female mice were fed a Control diet (CD) or Western diet (WD). After 15 weeks mice were bred and became pregnant while staying on their respective diet (CD or WD) and were euthanized 1-day post-partum (Post-partum). †A small group of mice were euthanized 1-week (1wk PP) or 6-week post-partum (6wks PP) to determine heart weight to tibia length ratio (HW/TL). Another group of mice were not bred and kept on the diet for 18–20 weeks in total (Non-pregnant). B. Body weight and HW/TL at the time of sacrifice (CD: Non-pregnant n=6; Post-partum n=11; 1wk PP n=6; 6wks PP n=4; WD: Non-pregnant n=8; Post-partum n=12; 1wk PP n=9; 6wks PP n=6). C. Quantification of cardiomyocyte cross sectional area (CSA) and representative images of wheat germ agglutinin (WGA) stained hearts. (CD: Non-pregnant n=3; Post-partum n=6; WD: Non-pregnant n=3; Post-partum n=7). D. Gene expression levels of atrial natriuretic peptide (ANP) and beta-myosin heavy chain (βMHC) in heart tissues by RT-PCR. E. Representative images of hearts from CD and WD groups stained with Picro-Sirius red, with quantification of the percentage of Picro-Sirius red positive area. Data are presented as mean±SD. *p<0.05, **p<0.01, ***p<0.005, ****p<0.0001, significantly different between WD and CD. #p<0.05, ##p<0.01, ###p<0.005, ####p<0.0001, significantly different vs. non-pregnant in the same diet treated group.
The myocardial triglyceride levels were significantly greater in non-pregnant WD mice compared with CD mice (p=0.023), but this difference was not found post-partum (Supplementary Figure II). Protein carbonylation and lipid peroxidation were increased in WD mice post-partum (Supplementary Figure III. A–B), accompanied with elevated protein levels of β-tubulin (Supplementary Figure III. C–D). These data suggest that oxidative stress might be greater in WD mice after pregnancy.
Despite the signs of early-stage pathological hypertrophy, there was no difference in the average litter weight and litter size between groups (Supplementary Figure IV. A), but WD mice showed a higher incidence of dystocia23 and maternal post-partum deaths compared with CD mice (Supplementary Figure IV. B–C).
Diet-induced early-stage MetS leads to concentric cardiac remodeling during pregnancy without significantly affecting cardiac function
Echocardiography (ECHO) was performed at baseline (before special diet feeding), before breeding (after 15 weeks of CD or WD), mid-pregnancy, late-pregnancy and 1-day post-partum. The heart rate was comparable at different time points between groups (Fig.2A). There was no difference in ejection fraction (EF) between groups after long-term feeding and throughout pregnancy (Fig.2B). ECHO-derived LV mass was significantly increased in WD mice from mid-pregnancy until 1-day post-partum compared with CD mice (Fig.2C), accompanied with increased relative wall thickness (Fig.2D). All mice had increased cardiac output, stroke volume and LV end-diastolic volume at late-pregnancy, consistent with increased blood volume present at later stages of pregnancy (Fig.2E–G). CD mice had decreased E/A during pregnancy that was not found in WD mice, suggesting differences in diastolic properties (Fig.2H). E/E’, isovolumic relaxation time (IVRT), mitral valve pressure half-time (MV PHT), and left ventricle Myocardial Performance Index (LV MPI) were significantly different in WD mice post-partum compared with CD mice (Supplementary Table II). These findings suggest that in parallel with increased heart weight and cardiomyocyte hypertrophy, pregnancy with preexisting MetS induces concentric cardiac remodeling with changes in diastolic properties without significantly affecting systolic function.
Figure 2. Western diet induced early-stage MetS leads to concentric cardiac remodeling during pregnancy.

Parasternal echocardiography was performed at Baseline (8wks old), Before Breeding (after 15 weeks of CD or WD, 23wks old), Mid-pregnancy (Day 9.5–10.5), Late-pregnancy (Day 17.5–19.5) and 1-day Post-partum. A. Heart Rate B. Ejection Fraction (EF) C. Left ventricular (LV) Mass D. Relative Wall Thickness E. Stroke Volume F. Cardiac Output G. Left ventricular (LV) end-diastolic volume H. E/A were measured. (CD: Baseline n=10; Before Breeding n=8; Mid-pregnancy n=5; Late-Pregnancy n=10; Post-partum n=11; WD: Baseline n=8; Before Breeding n=10; Mid-pregnancy n=6; Late-Pregnancy n=14; Post-partum n=15). Data are presented as mean±SD. *p<0.05, **p<0.01, ****p<0.0001 between WD and CD. #p<0.05, significantly different vs. Baseline in the same diet treated group. (CD: Control diet, WD: Western diet).
Excess weight women exhibit significant LV hypertrophy during pregnancy
To explore the idea that MetS could influence pregnancy-induced cardiac remodeling, we performed a case-control study during pregnancy in women with excess weight. Primipara with singleton pregnancy that have no pregnancy complications or past medical history of cardiovascular disease were included in the study. Women were divided into two groups based on pre-pregnancy body mass index (BMI). Females were clustered as Normal Weight group (18.5≤BMI≤24.9, n=20) and Excess Weight group (BMI≥25, n=18). Echocardiography was performed in the third trimester. There were no differences in age, gestational weeks or body weight gain during pregnancy between groups (Supplementary Table III). Although no difference in EF was found by ECHO, the Excess Weight group exhibited a significantly greater LV mass (133 ±5.364 g versus 99.14±3.769 g) and LV mass index (=LV mass/ body surface area) (66.79 ±2.45 g/m2 versus 55.69±1.899 g/m2). The Excess Weight group also showed increases in end-diastolic LV volume, end-systolic LV volume, and stroke volume (Supplementary Table III). There was a linear correlation between LV mass at later stages of pregnancy and pre-pregnancy BMI in the Excess Weight group (r=0.5963, p=0.009), but not in the Normal Weight group (r=0.347, p=0.1338) (Supplementary Figure V). Lipid profile such as triglycerides, total cholesterol, HDL and LDL were measured from some participants (n=8 in Normal Weight group, n=9 in Excess Weight group) and showed no significant difference between groups (Supplementary Table IV). These findings demonstrate a significant correlation between pre-pregnancy BMI and increased cardiac hypertrophy during pregnancy in this excess weight human population.
Pregnancy exacerbates dyslipidemia and leads to changes of plasma cytokine levels in WD mice
WD induced dyslipidemia with increased levels of plasma cholesterol, LDL, and HDL. Pregnancy led to further increases of plasma cholesterol in WD mice (129.75±20.87 mg/dl in WD post-partum vs. 93.00±16.45 mg/dl in WD non-pregnant). Plasma LDL levels were increased, but not significantly in WD post-partum mice compared with non-pregnant mice (p=0.0758) (Fig. 3A). Meanwhile, WD mice had increased plasma levels of tumor necrosis factor alpha (TNFα) (p=0.112) and reduced plasma levels of interleukin (IL)-10 (p=0.1645) compared with CD mice post-partum, suggesting systemic inflammation in WD mice after pregnancy.
Figure 3. Pregnancy exacerbates dyslipidemia and lead to changes of plasma cytokine level.

A. Plasma lipid profile of total cholesterol, low-density lipoprotein (LDL) and high-density lipoprotein (HDL) at time of sacrifice. (CD: Non-pregnant n=6, Post-partum n=8; WD: Non-pregnant n=8, Post-partum n=8) B. Plasma levels of leptin receptor (Leptin R), tumor necrosis factor alpha (TNFα) and interleukin (IL)-10. (n=3). Data are presented as mean±SD. *p<0.05, **p<0.01, ****p<0.0001, significantly different between WD and CD. ###p<0.005, significantly different vs. non-pregnant in the same diet treated group. (CD: Control diet, WD: Western diet).
Pregnancy with preexisting MetS leads to a unique gene expression profile
Standard RNA-sequencing was performed using heart tissues from both Post-partum (PP) and Non-pregnant (NP) mice from CD and WD treated animals. To investigate the effect of normal pregnancy and pregnancy with WD-induced preexisting MetS on cardiac gene expression, the following comparisons were made: CD-PP vs. CD-NP, WD-PP vs. WD-NP. Volcano plots depict the fold change and p value, representing differentially expressed genes (DEGs) between groups by a log fold-change threshold of 1.5. The total number of up- and down-regulated genes in each comparison and the number of genes overlapped are presented in the Venn diagram. Normal pregnancy induced 177 differentially expressed genes (CD-PP vs. CD-NP), pregnancy in WD mice induced 341 differentially expressed genes (WD-PP vs. WD-NP). Only 20 differentially expressed genes were common to these groups (Fig.4A). Gene ontology (GO) was conducted with these DEGs datasets; extracellular structure organization (GO:0043062) and muscle structure development (GO: 0061061) were the leading GO terms due to pregnancy in CD and WD mice respectively (Fig.4B). GO terms related to developmental processes, metabolic processes (Fig.4C), cellular processes and response to stimulus (Supplementary Figure VI. A) also present distinct profiles between CD and WD mice. Gene Set Enrichment Analysis (GSEA) based on Hallmark gene sets found significant gene enrichment in pathways related to cell cycle regulation during pregnancy in both CD and WD mice (Supplementary Figure VI. B). In WD mice, pregnancy induced significant gene enrichment in biological processes including oxidative phosphorylation, transforming growth factor beta (TGFβ) signaling, TNFα signaling, adipogenesis and hypoxia, which were not observed in CD mice (Fig.4D). Hierarchical clustering analysis of the genes that are known to be involved in pathological cardiac hypertrophy, fibrosis, and oxidative stress were upregulated in the hearts of WD versus CD mice post-partum (Fig.4E). We also performed hierarchical clustering of genes in collagen catabolic process (GO: 0030574) and extracellular matrix structural constituent conferring tensile strength (GO: 0030020). There was an upregulation of both collagens and a series of collagen catabolic genes in CD mice post-partum, with a different pattern in WD mice (Supplementary Figure VI. C). Together, RNA-sequencing data suggests a significant alteration of the gene expression profile in pregnant WD mice.
Figure 4. Western diet induced early-stage MetS leads to a unique gene expression profile with pregnancy.

A. Volcano plots and Venn diagram representing the total number of differentially expressed genes (DEGs), number of up- or down-regulated genes, and number of overlapped genes of CD-PP vs. CD-NP and WD-PP vs. WD-NP. B. Top GO terms of DEGs in CD-PP vs. CD-NP and WD-PP vs. WD-NP respectively. C. GO terms related to the developmental process and metabolic process of DEGs. D. Gene Set Enrichment Analysis of DEGs in WD and CD mice respectively, with enrichment score (ES), normalized enrichment score (NES), normalized (NOM) p-value, and false discover rate (FDR). E. Hierarchical clustering of the expression level of genes that are involved in the regulation of cardiac hypertrophy, fibrosis, and oxidative stress process. (n=3 in each group). (CD: Control diet, WD: Western diet, NP: Non-pregnant, PP: Post-partum).
Angiopoietin like protein-4 mediates the crosstalk between metabolism disorders and cardiac fibrosis
Angiopoietin like protein 4 (Angptl4), which governs lipid metabolism, is a downstream factor of the TNFα signaling pathway24. The expression levels of Angptl4 were significantly upregulated in WD mice post-partum as determined by RT-PCR. Meanwhile, Angptl4 ranks second of the most upregulated genes in WD-PP group determined by RNA-sequencing (Fig.5A). To investigate the potential contribution of Angptl4 to the pathological fibrotic remodeling observed in WD mice, we performed a scratch assay with rat primary cardiac fibroblasts (CFs) that were treated with vehicle, 5μg/ml and 10μg/ml Angptl4. Angptl4 significantly promoted the migration of the fibroblasts (Fig.5B). Cultured rat CFs treated with Angptl4 were stained with EdU and CellMask™ Orange, and CFs treated with 10ng/ml TGFβ served as a positive control. Angptl4 caused a dose-dependent increase in fibroblast proliferation (by cell number/field and EdU incorporation). 10μg/ml Angptl4 and TGFβ increased cell proliferation by 3.2-fold compared with vehicle (Fig. 5C). Protein levels of vimentin were increased in high dose Angptl4 and TGFβ treatments. Protein levels of α-smooth muscle actin (αSMA) were not different between treatments; however, the ratio of vimentin to αSMA showed a dose-dependent increase with Angptl4 treatment (Fig. 5D). These findings show that Angptl4 activates primary CFs in vitro, suggesting a role for Angptl4 in the fibrosis observed in WD-PP hearts.
Post-partum mice with preexisting MetS are more susceptible to future pathological stimuli
To investigate if mice with preexisting MetS-induced post-partum remodeling are prone to abnormal responses to pathological stressors, WD and CD mice were exposed to angiotensin II and phenylephrine (AngII/PE) infusion immediately after delivery for 7 days. Non-pregnant WD and CD counterparts served as controls. Mice were maintained on the same maintenance chow during AngII/PE treatment (Fig. 6A). Body weight was not different after 7-day AngII/PE treatment between groups. Post-partum mice in both CD and WD groups showed significantly increased HW/TL compared with non-pregnant counterparts after AngII/PE treatment (1.2-fold and 1.4-fold respectively), while post-partum WD mice had greater HW/TL compared with CD mice (9.826±0.806 mg/mm vs. 8.282±0.621 mg/mm, p=0.0002) (Fig. 6B). In parallel, post-partum WD mice treated with AngII/PE had increased cardiomyocyte CSA compared with CD mice (337.287±5.732 μm2 vs. 270.793±26.381 μm2, p=0.0057) and non-pregnant counterparts (337.287±5.732 μm2 vs. 246.454±9.758 μm2, p=0.0013) (Fig. 6C). Post-partum WD mice treated with AngII/PE showed a trend for a reduction in EF (p=0.0797) and a significant reduction in global longitudinal strain (GLS) (p=0.0005) (speckle-tracking strain analysis). Moreover, GLS of post-partum WD mice was lower than in post-partum CD mice with AngII/PE treatment (p=0.0559), suggesting an early stage of systolic dysfunction in post-partum WD mice after challenge with pathological stimuli. Post-partum WD mice also had increased LV mass and reduced stroke volume after AngII/PE treatment compared with CD mice (Fig. 6D).
Figure 6. Post-partum mice with preexisting MetS are more susceptible to future pathological stimuli.

A. Control diet (CD) and Western diet (WD) mice were subjected to 7 days of Angiotensin II/phenylephrine (AngII/PE) infusion starting 1-day post-partum (PP). CD and WD non-pregnant (NP) counterparts were also treated with 7 days of AngII/PE infusion as control (Angiotensin II 1.5μg/day/kg, phenylephrine 50μg/day/kg). B. Body weight (BW) and heart weight to tibia length ratio (HW/TL) at time of sacrifice (CD: NP–AngII/PE n=9; PP–AngII/PE n=6; NP +AngII/PE n=5; PP+AngII/PE n=5; WD: NP–AngII/PE n=9; PP–AngII/PE n=9; NP+AngII/PE n=4; PP+AngII/PE n=4). C. Quantification and representative images of cardiomyocyte cross-sectional area (CSA) in WGA-stained hearts (3–7 mice per group). D. Parasternal Echocardiography was performed at the terminal study time point (CD: NP–AngII/PE n=6; PP–AngII/PE n=4; NP +AngII/PE n=5; PP+AngII/PE n=5; WD: NP–AngII/PE n=6; PP–AngII/PE n=5; NP+AngII/PE n=4; PP+AngII/PE n=4). Representative images of speckle tracking strain analysis are shown. Ejection Fraction (EF) and global longitudinal strain (GLS) were obtained from speckle tracking strain analysis. Left ventricular (LV) Mass and Stroke Volume were obtained from conventional M-mode image analysis. Data are presented as mean±SD. *p<0.05, **p<0.01, ***p<0.005, ****p<0.0001, significantly different between WD and CD. #p<0.05, ##p<0.01, ###p<0.005, ####p<0.0001, significantly different vs. non-pregnant or vs. –AngII/PE in the same diet treated group.
Discussion
Pregnancy requires an increased cardiac output to support the developing fetus. This physiological stressor induces physiological cardiovascular remodeling in normal females. This study explored the idea that females with underlying health conditions could have abnormal remodeling of the heart during pregnancy1. Current statistics show that the top cause of pregnancy-related death in the United States is cardiovascular disease7, 25. Obesity and associated MetS are linked to cardiovascular diseases26, and are now affecting a larger population of women of reproductive age18–21. Obese women have a 50% higher mortality rate than non-obese women during pregnancy27. It is also known that maternal obesity has a strong link to a higher incidence of future cardiovascular diseases28. These observations suggest that there is a potential disruptive effect of obesity and/or MetS on the normal cardiac response to pregnancy. Cardiovascular health in obese women during pregnancy, especially within asymptomatic populations, has not been well studied. In particular, the effects of obesity and/or MetS on cardiac remodeling during pregnancy is not well established, and defining these effects was the objective of this study.
Diet-induced MetS leads to pathological cardiac hypertrophy with pregnancy in a mouse model
In CD mice, pregnancy induced physiological hypertrophy that regressed to baseline by 7 days post-partum. Cardiomyocyte CSA was unchanged, suggesting an increase in cell length in this form of hypertrophy, with no fetal gene upregulation or fibrosis accumulation (Fig.1). Echocardiography showed the heart developed a normal physiological response to pregnancy, with increased cardiac output and proportional chamber enlargement4 (Fig.2). The E/A ratio, a measure of diastolic function, showed a reduction during pregnancy (Fig.2H), which is consistent with previous findings in human patients29, 30. Collectively these findings document physiological structural remodeling during normal pregnancy that rapidly regresses after delivery, consistent with previous findings1, 8, 31–33 (Fig.7).
Figure 7. Mechanisms of pregnancy induced cardiac remodeling in mice with preexisting MetS.

Normal pregnancy induced physiological remodeling which largely returned to baseline shortly after delivery (Fig.1). However, mice with preexisting MetS showed signs of pathological remodeling after pregnancy (Fig.1). Increased metabolic processes led to an activation of TNFα signaling pathway (Fig.4C–D). As a downstream factor of the TNFα pathway, Angptl4 gene expression level was upregulated (Fig.5A). Angptl4 activated cultured cardiac primary fibroblast migration, proliferation, and activation (Fig.5B–D), which may interface dysregulated metabolism in mice with MetS with pathological cardiac remodeling during pregnancy in mice with MetS. The aberrant cardiac remodeling could lead to long-term abnormalities after pregnancy in mice with MetS (Fig.1B) and predispose those mice to increased risks of future adverse cardiac remodeling when challenged with pathological stimuli (Fig. 6). (CSA: cross-sectional area, TNFα: Tumor necrosis factor alpha, Angptl4: Angiopoietin like protein 4).
WD mice had an early-stage MetS phenotype without an overt pathological cardiac phenotype after 15 weeks of WD feeding. However, these WD mice had a greater amount of hypertrophy during pregnancy. This increased heart mass was accompanied by increased cardiomyocyte CSA, activation of fetal gene expression and accumulation of cardiac fibrosis (Fig.1B–E). A concentric remodeling phenotype was determined by ECHO (Fig. 2). Post-delivery regression of hypertrophy was impaired in WD mice, as evidenced by the sustained increase in HW/TL 1 week and even 6 weeks post-partum (Fig.1B). Collectively, these data suggest that preexisting MetS induces pathological hypertrophy during pregnancy. The structural changes and excessive cardiac hypertrophy demonstrated in the WD mouse model during pregnancy were similar to those in our human study.
Cardiac hypertrophy is increased in women with excess weight during pregnancy
Our human subject case-control study showed that excess weight females exhibited increased LV mass and chamber dilation during the 3rd trimester compared with normal weight females. These findings complement a recently published study which found significant LV hypertrophy in extremely obese pregnant women (BMI≥35 kg/m2) that was accompanied by diastolic dysfunction and impaired deformation34. Our findings showed an abnormal relationship between LV mass and preconception BMI in excess weight populations, which may explain why extremely obese women with BMI≥35 kg/m2 have a more severe phenotype than the excess weight women we recruited in this study.
Pregnancy exacerbates metabolism disorders and induces systemic inflammation in WD mice
WD per se induced dyslipidemia (Supplementary Figure I. E) that was further exacerbated after pregnancy (Fig. 3A). WD mice post-partum also had increased plasma levels of the pro-inflammatory cytokine TNFα and reduced levels of the anti-inflammatory cytokine IL-1035, suggesting systemic inflammation after pregnancy in mice with preexisting MetS. The effects of maternal obesity on their offspring have been widely studied10, 36–40 and were not a focus of our study. However, maternal obesity is known to be associated with a higher incidence of dysfunctional labor in humans41. Along these lines, our studies showed more frequent dystocia and subsequent post-partum deaths in the WD group (Supplementary Figure II. B–C).
Diet-induced MetS leads to dysregulated gene expression that could explain aberrant cardiac remodeling after pregnancy
Pregnancy with preexisting MetS induced a highly distinct gene expression profile compared with normal pregnancy. Gene ontology analysis in WD mice showed the most significant GO term was muscle structure development during pregnancy, which is the leading GO term of the human heart during early embryonic development42, that was not identified in CD mice. However, the top GO term induced in normal pregnancy was extracellular structure organization which was absent in WD mice (Fig.4B). A distinct gene expression pattern between CD and WD mice post-partum in extracellular matrix (ECM) structural constituents and collagen catabolic process was also observed (Supplementary Figure VI. C). Previous studies have shown that pregnancy can lead to upregulation of ECM related genes within 12 hours post-delivery without inducing actual fibrosis43, 44. These data suggest an on-going extracellular structural re-organization at the transcriptome level in the myocardium shortly after delivery, which is likely a response to the rapid blood volume change after delivery45 and results in rapid reverse remodeling without fibrosis accumulation. In the WD mice this reverse remodeling appears to be disrupted.
With Gene Set Enrichment Analysis, we determined WD mice post-partum showed significant gene enrichment in biological processes including oxidative phosphorylation, TGFβ signaling, and TNFα signaling (Fig.4D) that are usually coupled with generation of reactive oxygen species, deposition of extracellular matrix and cell damage46–48. Along these lines, the expression pattern of cardiac hypertrophy, fibrosis and oxidative stress related genes showed an augmentation specifically in WD mice post-partum (Fig.4E). Collectively, the transcriptome analysis suggests that pregnancy with preexisting MetS induces a distinct gene expression profile in the myocardium that is consistent with a pathological hypertrophy reprogramming profile.
In WD mice, GO analysis on metabolic processes presented significant GO terms of fatty acid, monosaccharide and ketone metabolic process (Fig.4C). TNFα is known as a multi-functional cytokine that regulates different biological processes including lipid metabolism. Previous research showed that TNFα activation may lead to increased transcription levels of Angptl4, which plays a role in regulating lipoprotein lipase (LPL) activity to control lipoprotein catabolism24, 49. Our data showed that the TNFα pathway was significantly enriched (Fig. 4D), with an upregulation of Angptl4 expression in post-partum WD mice (Fig.5A). Angptl4 expression in the heart is upregulated in diabetic animal models50, 51 and Angptl4 protein localizes specifically to cardiomyocytes52. Thus, myocyte derived Angptl4 may influence other cell types, particularly fibroblasts in the myocardium to contribute to the aberrant remodeling we observed in WD pregnant mice. Our in vitro data revealed that Angptl4 induced the transformation of CFs by promoting migration, proliferation and activation (Fig.5). Collectively, our results suggest that WD and pregnancy induces dysregulated metabolism and activation of the TNFα signaling pathway. Angptl4, as a downstream factor in the TNFα pathway, may interface dysregulated metabolism with cardiac fibrosis (Fig.7).
Post-partum mice with preexisting MetS are more susceptible to pathological stimuli
Previous research suggests that maternal obesity has a strong link to a higher incidence of future cardiovascular diseases28. Our results support this idea. Our study showed that post-partum mice with preexisting MetS were more susceptible to equivalent pathological stimuli with AngII/PE, compared with normal mice, with increased pathological hypertrophy, aggravated cardiac dysfunction, and structural remodeling (Fig. 6). After a normal pregnancy, cardiac hypertrophy quickly regresses post-partum (Fig. 1B). However, the present results showed that mice with preexisting MetS had preserved cardiac hypertrophy for at least 6 weeks after delivery (Fig. 1B). These results suggest the aberrant cardiac remodeling and gene reprogramming in WD mice can persist after pregnancy and predispose those individuals to a higher risk for future cardiovascular disorders (Fig.7). These observations may explain why obese females are at a higher risk of peripartum cardiomyopathy (PPCM)53 and show greater cardiac remodeling and diminished cardiac recovery when they develop PPCM54.
Limitations
Pregnancy is a complex biological process involving functional changes of multiple organs and systems. The pathological cardiac remodeling we observed in WD mice post-partum is likely to be caused by multiple factors. A limitation of the animal model is that female C57BL/6J mice are less susceptible to diet-induced morbid obesity55, 56 and hypertension57, 58 without genetic manipulation. Therefore, it is interesting that we were able to find evidence of abnormal cardiac remodeling during pregnancy in animals with a modest MetS phenotype, suggesting that even early-stage MetS may induce adverse cardiac responses during and after pregnancy. Detailed studies of the underlying mechanisms for this adverse remodeling, including but not limited to oxidative stress, metabolism disorders and cardiomyocyte ultrastructural disorganization will be required to define cause and effect relationships.
Conclusions
In summary, the present study shows that pregnancy in mice with preexisting MetS induces pathological remodeling, including concentric hypertrophy, fibrosis accumulation and dysregulated gene expression. This adverse remodeling adds risk during pregnancy and predisposes affected individuals to future cardiovascular complications.
Supplementary Material
Clinical Perspective.
What is new?
This study shows that pregnancy with preexisting cardiovascular risk factors can induce pathological rather than physiological cardiac remodeling.
Pathological remodeling during pregnancy with MetS can lead to greater cardiac dysfunction when challenged with pathological stimuli after pregnancy.
What are the clinical implications?
The current findings emphasize the importance for monitoring cardiovascular health in women with preexisting risk factors during and after pregnancy.
Women with MetS during pregnancy could be at risk for premature cardiac dysfunction after delivery.
Acknowledgments:
We thank M.K., F.Y. and T.A.M. for their collaboration.
Funding: Yijun Yang was supported by China Scholarship Council (201506230186). S.R.H. and T.A.M. were supported by a grant from the National Institutes of Health (HL147558). T.A.M. was also supported by the National Institute of Health by grants HL116848, DK119594, HL127240, HL150225. J.G.T was supported by the National Institutes of Health by grant HL147463. T.A.M. and J.G.T. were supported by the American Heart Association (16SFRN31400013).
Non-standard Abbreviations and Acronyms:
- MetS
Metabolic Syndrome
- WD
Western diet
- CD
Control diet
- HW/TL
Heart weight to tibia length ratio
- LV
Left ventricular
- CSA
Cross-sectional area
- HDL
High-density lipoprotein
- LDL
Low-density lipoprotein
- ANP
Atrial natriuretic peptide
- βMHC
beta-myosin heavy chain
- EF
Ejection fraction
- IVRT
Isovolumic relaxation time
- MV PHT
Mitral valve pressure half-time
- LV MPI
Left ventricle myocardial performance index
- BMI
Body mass index
- PP
Post-partum
- NP
Non-pregnant
- DEGs
Differentially expressed genes
- GO
Gene ontology
- GSEA
Gene set enrichment analysis
- TGFβ
Transforming growth factor beta
- TNFα
Tumor necrosis factor alpha
- Angptl4
Angiopoietin like protein 4
- CFs
Cardiac fibroblasts
- αSMA
α-smooth muscle actin
- AngII
Angiotensin II
- PE
Phenylephrine
- GLS
Global longitudinal strain
- ECM
Extracellular matrix
- LPL
Lipoprotein lipase
- PPCM
Peripartum cardiomyopathy
- WGA
Wheat germ agglutinin
Footnotes
Conflict of Interest Disclosures
T.A.M. received support from Italfarmaco for an unrelated project. All other authors declare that they have no conflict of interests.
Supplemental Materials
Expanded Materials and Methods
Supplemental Figures I – VI
Supplemental Tables I – IV
References and Notes:
- 1.Sanghavi M and Rutherford JD. Cardiovascular physiology of pregnancy. Circulation. 2014;130:1003–1008. doi: 10.1161/CIRCULATIONAHA.114.009029 [DOI] [PubMed] [Google Scholar]
- 2.Bader RA, Bader ME, Rose DF and Braunwald E. Hemodynamics at rest and during exercise in normal pregnancy as studies by cardiac catheterization. J Clin Invest. 1955;34:1524–1536. doi: 10.1172/JCI103205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robson SC, Hunter S, Boys RJ and Dunlop W. Serial study of factors influencing changes in cardiac output during human pregnancy. Am J Physiol. 1989;256:H1060–5.doi: 10.1152/ajpheart.1989.256.4.H1060 [DOI] [PubMed] [Google Scholar]
- 4.Hemodynamic Fu Q. and Electrocardiographic Aspects of Uncomplicated Singleton Pregnancy. Adv Exp Med Biol. 2018;1065:413–431. doi: 10.1007/978-3-319-77932-4_26 [DOI] [PubMed] [Google Scholar]
- 5.Nakamura M and Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol. 2018;15:387–407. doi: 10.1038/s41569-018-0007-y [DOI] [PubMed] [Google Scholar]
- 6.Creanga AA, Syverson C, Seed K and Callaghan WM. Pregnancy-Related Mortality in the United States, 2011–2013. Obstet Gynecol. 2017;130:366–373. doi: 10.1097/AOG.0000000000002114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petersen EE, Davis NL, Goodman D, Cox S, Syverson C, Seed K, Shapiro-Mendoza C, Callaghan WM and Barfield W. Racial/Ethnic Disparities in Pregnancy-Related Deaths - United States, 2007–2016. MMWR Morb Mortal Wkly Rep. 2019;68:762–765. doi: 10.15585/mmwr.mm6835a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung E and Leinwand LA. Pregnancy as a cardiac stress model. Cardiovasc Res. 2014;101:561–570. doi: 10.1093/cvr/cvu013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agha M and Agha R. The rising prevalence of obesity: part B-public health policy solutions. Int J Surg Oncol (N Y). 2017;2:e19. doi: 10.1097/IJ9.0000000000000019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Callahan EA. Current Status and Response to the Global Obesity Pandemic: Proceedings of a Workshop. Washington (DC): National Academies Press (US); 2019. doi: 10.17226/25273 [DOI] [PubMed] [Google Scholar]
- 11.Gregg EW and Shaw JE. Global Health Effects of Overweight and Obesity. N Engl J Med. 2017;377:80–81. doi: 10.1056/NEJMe1706095 [DOI] [PubMed] [Google Scholar]
- 12.Engin A The Definition and Prevalence of Obesity and Metabolic Syndrome. Adv Exp Med Biol. 2017;960:1–17. doi: 10.1007/978-3-319-48382-5_1 [DOI] [PubMed] [Google Scholar]
- 13.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J and Salonen JT. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709 [DOI] [PubMed] [Google Scholar]
- 14.Girman CJ, Rhodes T, Mercuri M, Pyorala K, Kjekshus J, Pedersen TR, Beere PA, Gotto AM, Clearfield M, Group S and the ATRG. The metabolic syndrome and risk of major coronary events in the Scandinavian Simvastatin Survival Study (4S) and the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS). Am J Cardiol. 2004;93:136–141. doi: 10.1016/j.amjcard.2003.09.028 [DOI] [PubMed] [Google Scholar]
- 15.Ninomiya JK, L’Italien G, Criqui MH, Whyte JL, Gamst A and Chen RS. Association of the metabolic syndrome with history of myocardial infarction and stroke in the Third National Health and Nutrition Examination Survey. Circulation. 2004;109:42–46. doi: 10.1161/01.CIR.0000108926.04022.0C [DOI] [PubMed] [Google Scholar]
- 16.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH, American Heart A, Obesity Committee of the Council on Nutrition PA and Metabolism. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016 [DOI] [PubMed] [Google Scholar]
- 17.Robertson J, Lindgren M, Schaufelberger M, Adiels M, Bjorck L, Lundberg CE, Sattar N, Rosengren A and Aberg M. Body Mass Index in Young Women and Risk of Cardiomyopathy: A Long-Term Follow-Up Study in Sweden. Circulation. 2020;141:520–529. doi: 10.1161/CIRCULATIONAHA.119.044056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramos RG and Olden K. The prevalence of metabolic syndrome among US women of childbearing age. Am J Public Health. 2008;98:1122–1127. doi: 10.2105/AJPH.2007.120055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simmons D Diabetes and obesity in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25:25–36. doi: 10.1016/j.bpobgyn.2010.10.006 [DOI] [PubMed] [Google Scholar]
- 20.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flegal KM, Carroll MD, Ogden CL and Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014 [DOI] [PubMed] [Google Scholar]
- 22.Moore JX, Chaudhary N and Akinyemiju T. Metabolic Syndrome Prevalence by Race/Ethnicity and Sex in the United States, National Health and Nutrition Examination Survey, 1988–2012. Prev Chronic Dis. 2017;14:E24. doi: 10.5888/pcd14.160287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burkholder T, Foltz C, Karlsson E, Linton CG and Smith JM. Health Evaluation of Experimental Laboratory Mice. Curr Protoc Mouse Biol. 2012;2:145–165. doi: 10.1002/9780470942390.mo110217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makoveichuk E, Vorrsjo E, Olivecrona T and Olivecrona G. TNF-alpha decreases lipoprotein lipase activity in 3T3-L1 adipocytes by up-regulation of angiopoietin-like protein 4. Biochim Biophys Acta Mol Cell Biol Lipids. 2017;1862:533–540. doi: 10.1016/j.bbalip.2017.02.005 [DOI] [PubMed] [Google Scholar]
- 25.Wilson PW, D’Agostino RB, Parise H, Sullivan L and Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066–3072. doi: 10.1161/CIRCULATIONAHA.105.539528 [DOI] [PubMed] [Google Scholar]
- 26.Lavie CJ, Alpert MA, Arena R, Mehra MR, Milani RV and Ventura HO. Impact of obesity and the obesity paradox on prevalence and prognosis in heart failure. JACC Heart Fail. 2013;1:93–102. doi: 10.1016/j.jchf.2013.01.006 [DOI] [PubMed] [Google Scholar]
- 27.Buschur E and Kim C. Guidelines and interventions for obesity during pregnancy. Int J Gynaecol Obstet. 2012;119:6–10. doi: 10.1016/j.ijgo.2012.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Staff AC, Redman CW, Williams D, Leeson P, Moe K, Thilaganathan B, Magnus P, Steegers EA, Tsigas EZ, Ness RB, et al. Pregnancy and Long-Term Maternal Cardiovascular Health: Progress Through Harmonization of Research Cohorts and Biobanks. Hypertension. 2016;67:251–260. doi: 10.1161/HYPERTENSIONAHA.115.06357 [DOI] [PubMed] [Google Scholar]
- 29.Fok WY, Chan LY, Wong JT, Yu CM and Lau TK. Left ventricular diastolic function during normal pregnancy: assessment by spectral tissue Doppler imaging. Ultrasound Obstet Gynecol. 2006;28:789–793. doi: 10.1002/uog.3849 [DOI] [PubMed] [Google Scholar]
- 30.Melchiorre K, Sharma R, Khalil A and Thilaganathan B. Maternal Cardiovascular Function in Normal Pregnancy: Evidence of Maladaptation to Chronic Volume Overload. Hypertension. 2016;67:754–762. doi: 10.1161/HYPERTENSIONAHA.115.06667 [DOI] [PubMed] [Google Scholar]
- 31.De Haas S, Ghossein-Doha C, Geerts L, van Kuijk SMJ, van Drongelen J and Spaanderman MEA. Cardiac remodeling in normotensive pregnancy and in pregnancy complicated by hypertension: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2017;50:683–696. doi: 10.1002/uog.17410 [DOI] [PubMed] [Google Scholar]
- 32.Eghbali M, Deva R, Alioua A, Minosyan TY, Ruan H, Wang Y, Toro L and Stefani E. Molecular and functional signature of heart hypertrophy during pregnancy. Circ Res. 2005;96:1208–1216. doi: 10.1161/01.RES.0000170652.71414.16 [DOI] [PubMed] [Google Scholar]
- 33.Chung E, Yeung F and Leinwand LA. Akt and MAPK signaling mediate pregnancy-induced cardiac adaptation. J Appl Physiol (1985). 2012;112:1564–1575. doi: 10.1152/japplphysiol.00027.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buddeberg BS, Sharma R, O’Driscoll JM, Kaelin Agten A, Khalil A and Thilaganathan B. Cardiac maladaptation in obese pregnant women at term. Ultrasound Obstet Gynecol. 2019;54:344–349. doi: 10.1002/uog.20170 [DOI] [PubMed] [Google Scholar]
- 35.Murray DR and Freeman GL. Proinflammatory cytokines: predictors of a failing heart? Circulation. 2003;107:1460–1462. doi: 10.1161/01.cir.0000060808.79274.0c [DOI] [PubMed] [Google Scholar]
- 36.Chang E, Hafner H, Varghese M, Griffin C, Clemente J, Islam M, Carlson Z, Zhu A, Hak L, Abrishami S, et al. Programming effects of maternal and gestational obesity on offspring metabolism and metabolic inflammation. Sci Rep. 2019;9:16027. doi: 10.1038/s41598-019-52583-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guzzardi MA, Liistro T, Gargani L, Ait Ali L, D’Angelo G, Rocchiccioli S, La Rosa F, Kemeny A, Sanguinetti E, Ucciferri N, et al. Maternal Obesity and Cardiac Development in the Offspring: Study in Human Neonates and Minipigs. JACC Cardiovasc Imaging. 2018;11:1750–1755. doi: 10.1016/j.jcmg.2017.08.024 [DOI] [PubMed] [Google Scholar]
- 38.Gaillard R Maternal obesity during pregnancy and cardiovascular development and disease in the offspring. Eur J Epidemiol. 2015;30:1141–1152. doi: 10.1007/s10654-015-0085-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Q, Zhu C, Sun M, Maimaiti R, Ford SP, Nathanielsz PW, Ren J and Guo W. Maternal obesity impairs fetal cardiomyocyte contractile function in sheep. FASEB J. 2019;33:2587–2598. doi: 10.1096/fj.201800988R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dong M, Zheng Q, Ford SP, Nathanielsz PW and Ren J. Maternal obesity, lipotoxicity and cardiovascular diseases in offspring. J Mol Cell Cardiol. 2013;55:111–116. doi: 10.1016/j.yjmcc.2012.08.023 [DOI] [PubMed] [Google Scholar]
- 41.Leddy MA, Power ML and Schulkin J. The impact of maternal obesity on maternal and fetal health. Rev Obstet Gynecol. 2008;1:170–178. [PMC free article] [PubMed] [Google Scholar]
- 42.Pervolaraki E, Dachtler J, Anderson RA and Holden AV. The developmental transcriptome of the human heart. Sci Rep. 2018;8:15362. doi: 10.1038/s41598-018-33837-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chung E, Heimiller J and Leinwand LA. Distinct cardiac transcriptional profiles defining pregnancy and exercise. PLoS One. 2012;7:e42297. doi: 10.1371/journal.pone.0042297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parrott ME, Aljrbi E, Biederman DL, Montalvo RN, Barth JL and LaVoie HA. Feature Article: Maternal cardiac messenger RNA expression of extracellular matrix proteins in mice during pregnancy and the postpartum period. Exp Biol Med (Maywood). 2018;243:1220–1232. doi: 10.1177/1535370218818457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dolgilevich SM, Siri FM, Atlas SA and Eng C. Changes in collagenase and collagen gene expression after induction of aortocaval fistula in rats. Am J Physiol Heart Circ Physiol. 2001;281:H207–14. doi: 10.1152/ajpheart.2001.281.1.H207 [DOI] [PubMed] [Google Scholar]
- 46.Zhou B and Tian R. Mitochondrial dysfunction in pathophysiology of heart failure. J Clin Invest. 2018;128:3716–3726. doi: 10.1172/JCI120849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leask A Getting to the heart of the matter: new insights into cardiac fibrosis. Circ Res. 2015;116:1269–1276. doi: 10.1161/CIRCRESAHA.116.305381 [DOI] [PubMed] [Google Scholar]
- 48.Hori M and Yamaguchi O. Is tumor necrosis factor-alpha friend or foe for chronic heart failure? Circ Res. 2013;113:492–494. doi: 10.1161/CIRCRESAHA.113.302024 [DOI] [PubMed] [Google Scholar]
- 49.Aryal B, Price NL, Suarez Y and Fernandez-Hernando C. ANGPTL4 in Metabolic and Cardiovascular Disease. Trends Mol Med. 2019;25:723–734. doi: 10.1016/j.molmed.2019.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kesherwani V, Shahshahan HR and Mishra PK. Cardiac transcriptome profiling of diabetic Akita mice using microarray and next generation sequencing. PLoS One. 2017;12:e0182828. doi: 10.1371/journal.pone.0182828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sarkozy M, Zvara A, Gyemant N, Fekete V, Kocsis GF, Pipis J, Szucs G, Csonka C, Puskas LG, Ferdinandy P, et al. Metabolic syndrome influences cardiac gene expression pattern at the transcript level in male ZDF rats. Cardiovasc Diabetol. 2013;12:16. doi: 10.1186/1475-2840-12-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Georgiadi A, Lichtenstein L, Degenhardt T, Boekschoten MV, van Bilsen M, Desvergne B, Muller M and Kersten S. Induction of cardiac Angptl4 by dietary fatty acids is mediated by peroxisome proliferator-activated receptor beta/delta and protects against fatty acid-induced oxidative stress. Circ Res. 2010;106:1712–1721. doi: 10.1161/CIRCRESAHA.110.217380 [DOI] [PubMed] [Google Scholar]
- 53.Kao DP, Hsich E and Lindenfeld J. Characteristics, adverse events, and racial differences among delivering mothers with peripartum cardiomyopathy. JACC Heart Fail. 2013;1:409–416. doi: 10.1016/j.jchf.2013.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Davis EM, Ewald G, Givertz MM, Rajagopalan N, Cooper LT, Briller J, Felker GM, Bozkurt B, Drazner MH, Hanley-Yanez K, et al. Maternal Obesity Affects Cardiac Remodeling and Recovery in Women with Peripartum Cardiomyopathy. Am J Perinat. 2019;36:476–483. doi: 10.1055/s-0038-1669439 [DOI] [PubMed] [Google Scholar]
- 55.Chu DT, Malinowska E, Jura M and Kozak LP. C57BL/6J mice as a polygenic developmental model of diet-induced obesity. Physiol Rep. 2017;5: e13093. doi: 10.14814/phy2.13093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang Y, Smith DL Jr., Keating KD, Allison DB and Nagy TR. Variations in body weight, food intake and body composition after long-term high-fat diet feeding in C57BL/6J mice. Obesity (Silver Spring). 2014;22:2147–2155. doi: 10.1002/oby.20811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lerman LO, Kurtz TW, Touyz RM, Ellison DH, Chade AR, Crowley SD, Mattson DL, Mullins JJ, Osborn J, Eirin A, et al. Animal Models of Hypertension: A Scientific Statement From the American Heart Association. Hypertension. 2019;73:e87–e120. doi: 10.1161/HYP.0000000000000090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bruder-Nascimento T, Ekeledo OJ, Anderson R, Le HB and Belin de Chantemele EJ. Long Term High Fat Diet Treatment: An Appropriate Approach to Study the Sex-Specificity of the Autonomic and Cardiovascular Responses to Obesity in Mice. Front Physiol. 2017;8:32. doi: 10.3389/fphys.2017.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nuttall FQ. Body Mass Index: Obesity, BMI, and Health: A Critical Review. Nutr Today. 2015;50:117–128. doi: 10.1097/NT.0000000000000092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Christ A, Lauterbach M and Latz E. Western Diet and the Immune System: An Inflammatory Connection. Immunity. 2019;51:794–811. doi: 10.1016/j.immuni.2019.09.020 [DOI] [PubMed] [Google Scholar]
- 61.Duran JM, Makarewich CA, Sharp TE, Starosta T, Zhu F, Hoffman NE, Chiba Y, Madesh M, Berretta RM, Kubo H, et al. Bone-derived stem cells repair the heart after myocardial infarction through transdifferentiation and paracrine signaling mechanisms. Circ Res. 2013;113:539–552. doi: 10.1161/CIRCRESAHA.113.301202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harper SC, Johnson J, Borghetti G, Zhao H, Wang T, Wallner M, Kubo H, Feldsott EA, Yang Y, Joo Y, et al. GDF11 Decreases Pressure Overload-Induced Hypertrophy, but Can Cause Severe Cachexia and Premature Death. Circ Res. 2018;123:1220–1231. doi: 10.1161/CIRCRESAHA.118.312955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wallner M, Duran JM, Mohsin S, Troupes CD, Vanhoutte D, Borghetti G, Vagnozzi RJ, Gross P, Yu D, Trappanese DM, et al. Acute Catecholamine Exposure Causes Reversible Myocyte Injury Without Cardiac Regeneration. Circ Res. 2016;119:865–879. doi: 10.1161/CIRCRESAHA.116.308687 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data, methods used in the analysis, and materials used to conduct the research will be made available to any researcher for purposes of reproducing the results. Data of standard RNA-sequencing will be uploaded to the GEO database.


