Abstract
SARS–CoV-2 (COVID-19) infection can cause a severe respiratory distress syndrome. The risk of severe manifestations and mortality characteristically increase in the elderly and in the presence of non-COVID-19 comorbidity. We and others previously demonstrated that the low molecular weight (LMW) and protein thiol/disulfide ratio declines in human plasma with age and such decline is even more rapid in the case of inflammatory and premature aging diseases, which are also associated with the most severe complications of COVID-19 infection. The same decline with age of the LMW thiol/disulfide ratio observed in plasma appears to occur in the extracellular fluids of the respiratory tract and in association with many pulmonary diseases that characteristically reduce the concentrations and adaptive stress response of the lung glutathione. Early evidence in literature suggests that the thiol to disulfide balance of critical Cys residues of the COVID-19 spike protein and the ACE-2 receptor may influence the risk of infection and the severity of the disease, with a more oxidizing environment producing the worst prognosis.
With this hypothesis paper we propose that the age-dependent decline of LMW thiol/disulfide ratio of the extracellular fluids, could play a role in promoting the physical (protein-protein) interaction of CoV-2 and the host cell in the airways. Therefore, this redox-dependent interaction is expected to affect the risk of severe infection in an age-dependent manner. The hypothesis can be verified in experimental models of in vitro CoV-2 infection and at the clinical level in that LMW thiols and protein thiolation can now be investigated with standardized, reliable and versatile laboratory protocols. Presenting the verification strategy of our hypothesis, we also discuss available nutritional and ancillary pharmacological strategies to intervene on the thiol/disulfide ratio of extracellular fluids of subjects at risk of infection and COVID-19 patients.
Keywords: SARS-CoV-2, COVID-19, Spike S protein, ACE-2 receptor, Aging, Oxidative stress, Low molecular mass thiols, Protein thiols, S-thiolation, Glutathione, Cysteine, Inflammation, Lung diseases
Graphical abstract
Highlights
-
•
Age-dependent disparities characterize clinical manifestations and lethality rate of SARS-CoV-2 disease.
-
•
Low molecular weight and protein thiol to disulfide ratio declines with age in extracellular fluids.
-
•
We hypothesize that these age-dependent changes might influence the virus-host cell interaction and the risk of severe manifestations.
-
•
LMW thiols and protein thiolation can now be investigated with standardized laboratory protocols.
-
•
The thiol/disulfide ratio of extracellular fluids represents a clinically relevant therapeutic target.
1. Introduction
SARS–CoV-2 (COVID-19 or CoV-2) infection can cause a severe respiratory distress syndrome with high risk of mortality and disability. Alarming epidemiology projections are reported in many regions with more than 2.3 millions of deaths and 107 millions of confirmed cases registered worldwide at the time of the preparation of this manuscript (https://covid19.who.int; last consultation done on Feb 11, 2021).
Since the beginning of this pandemic, the scientific community made huge efforts to identify host factors that sustain the infection, its cytopathic effects in the lung tissue, and the inflammatory and vascular complications. Notwithstanding, there is still a lack of knowledge on these factors that are of main importance to predict the risk of disease at the individual level and to develop more efficient therapies and measures of prevention.
Susceptibility to infection, and, more characteristically, severity of symptoms, mortality and disability, are all age-dependent aspects of this pandemic disease [1]. Indeed, only a small percentage of people under 30 develops severe illness and most of them presents as asymptomatic or paucisymptomatic. Moreover, mortality is extremely low in the youngest [1,2]. It has also been reported that under 20 years of age the susceptibility to infection is approximately half that of adults over 25 years. More important, clinical symptoms are observed in 20% of infected subjects in the age category between 10 and 25 years, rising to 70% in the over-70 in association with a dramatic increase in the risk of death [1].
The reason(s) standing behind these age-related aspects that differentiate COVID-19 from other pandemic viruses, such as the 1918 pandemic influenza [3], remain(s) elusive.
An increased susceptibility to oxidative stress with age has been speculated to play a role in this context and several studies in the last months pointed out that a decline of the antioxidant defence systems of the lung, including the glutathione (GSH) and thioredoxin (Trx) systems, act as a possible player in both the ACE-2 receptor-dependent mechanism of the viral infection and the host’ capability to respond to the pathogen and its cytopathic and inflammatory effects (for an extensive overview of the literature on these aspects see Refs. [[4], [5], [6]] and references therein). However, the mechanism(s) that stand(s) behind the redox paradigm of COVID-19 infection and its increased virulence with age, remain(s) elusive.
In this hypothesis paper, we suggest that the decline with age of homeostatic mechanisms that control the redox of extracellular low molecular weight (eLMW) thiols could represent the actual causal event and a molecular indicator of the increased risk of infection and development of severe disease.
2. Protein thiols are involved in the receptor-dependent mechanism of SARS-CoV-2 infection
To depict the molecular background of this hypothesis, we recapitulate the receptor-dependent process of the viral infection and the main aspects that characterize its sensitivity to redox processes.
Coronaviruses’ tropism is primarily determined by the ability of the spike (S) entry glycoprotein to bind to a cell surface receptor. SARS–CoV-2 binding to the angiotensin-converting enzyme 2 (ACE 2) receptor is identified as the main mechanism of infection to human cells [7]. SARS-CoV and SARS -CoV-2 both possess a trimeric spike S protein with a receptor-binding domain (RBD), which is the actual epitope for the recognition and high-affinity interaction with ACE-2. Subsequently, the S protein is cleaved by the transmembrane protease serine 2 [8,9]. One of the reasons for the faster human-to-human diffusion of SARS-CoV-2 with respect to SARS-CoV, lies in the fact that its spike S protein has a 20-fold higher affinity for human ACE2, with a reported Kd of ~15 nM [10]. Biochemical studies show that the infectivity of different SARS–CoV-2 strains in host cells correlates with the binding free energy of the interaction between the respective RBDs and the ACE2 receptor [11].
The number of ACE2 receptors available for the molecular engagement with the RBDs is another factor that might influence the virus's ability to spread within the host and replicate. However, it is not clear if ACE2 expression directly represents either the infectivity or pathogenicity of the virus [12,13]. ACE2 is present in lung cells whereas it is rarely expressed in immune cells where other receptors (such as CD147 and CD26) may have a role in mediating virus entry [14]. Worth of note, ACE2 expression in most tissues appears to decline with the host’ age [15] and the same is for CD147 in peripheral blood mononuclear cells [14], which is unexpected from the virus's age-dependant virulence. As a consequence, it is reasonable to hypothesize that other age-dependent factors have to intervene in this context.
Aging is associated with progressive decline of homeostatic systems that control the redox balance of all tissues, including the lung tissue, which may explain an increased susceptibility to infections and to develop pulmonary diseases by a defective host response and poor control of inflammatory and fibrotic pathways. These alterations ultimately interfere with tissue repair and regeneration processes, also increasing the risk of viral infections, including SARS-CoV infection. At the same time, a specific disulfide-thiol balance at the viral surface (i.e. of envelope proteins) and at the cell-surface of the host is required for the binding of the virus and for its entry into the cell [4]. Accordingly, reducing or alkylating agents are reported to disrupt the natural disulfide-thiol balance at the surface of a mature viral envelope of some of these viruses, which may interfere with the infectious potential [reviewed in Ref. [16]]. This suggests that a pro-oxidant environment at the host cell surface may favor the virus-host cell interaction regardless of the receptor mechanism, thus representing a conserved and efficient mechanism of infection common to different types of infections.
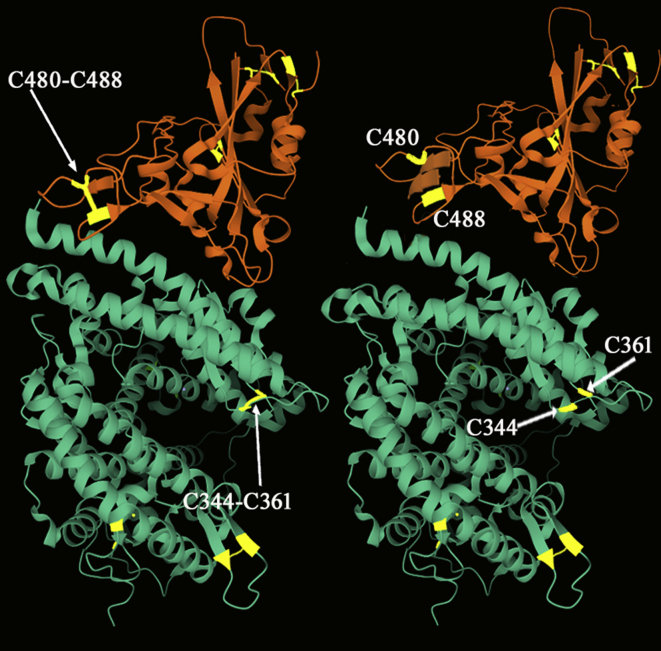
Recent evidence in literature indicated that such thiol to disulfide balance may also influence the protein-protein interaction mechanism of SARS-Cov-2 spike S protein and ACE2 receptor of the host cell [17,18]. Four disulfide bridges (C391–C525; C336–C361; C379–C432; C480–C488) are present in the RBD of SARS-Cov-2 and three are present on the ACE-2 receptor (C344–C361; C133–C141; C530–C542). This receptor also shows one reduced Cys residue in position 261. Molecular dynamics simulation studies suggest that the reduction of disulfides in ACE2 and the RBD can impact CoV-2 binding [18] (Fig. 1). Specifically, the cleavage of the C344–C361 disulfide bridge impacts on the conformational change of the two α helices that fit into the concave-shaped loop of the SARS-CoV-2 spike RBD. Likewise, the reduction of C480–C488 disulfide in RBD of the SARS CoV-2 spike modifies the β-sheet loop motif of the domain. Moreover, in these simulation studies, the reduction of the two disulfides on SARS-CoV-2 spike protein and ACE2 appears to produce synergistic effects of decrease on their binding affinity. No information is currently present in literature on the possible effect of Cys oxidation in position C261 of ACE2. Even if we assume that this Cys residue cannot form intramolecular disulfides, it shouldn't be ruled out that its oxidation to a mixed disulfide might occur during the reaction with eLMW thiols to modify receptor conformation and allosterism as it is demonstrated for several proteins involved in virus-cell interaction [16,19].
Fig. 1.
Schematic representation of the contact zone between SARS-CoV-2 spike receptor-binding domain (orange) and ACE2 (green). Disulfide bridges critical for binding affinity are present at position C480–C488 and C344–C361 (shown on the left); their reduction at the protein-protein interface is proposed to produce conformational changes that decrease binding affinity [17,18]. The figure was drawn using PDB entry 6M0J. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The fact that some intra- and inter-molecular disulfides in both ACE2 and the SARS-CoV-2 spike RBD seem to have an important role for the infection process [14] let to consider that the binding properties of spike S protein and ACE2 receptor could be regulated by the balance between thiols and disulfides of the extracellular environment. We here discuss how the age-dependent changes in the physiological balance between extracellular thiols and disulfides may have an effect on this process. Our observations can offer a mechanistic explanation for the observed age-related differences in terms of susceptibility to the infection and severity of clinical manifestations in COVID-19 [1].
3. Low molecular weight thiols are key redox players of the extracellular environment
To shed light on host’ factors that affect the redox interaction of proteins involved in COVID-19 infection, extracellular systems able to control the balance of reduced (R–SH) and oxidized (R–SS) forms of solvent-exposed Cys have to be taken into consideration. These include as main variable the levels and redox status of LMW thiols [20]. These compounds are key players in the redox buffering of protein Cys being involved in thiol-disulfide exchange reactions. LMW-SH react with protein Cys by nucleophilic attack to a pre-existing disulfide bond [21] following this reaction scheme:
-
a)
PS-SP + RSH ←→ PS-SR + PSH
-
b)
PS-SR + RSH ←→ PSH + RS-SR
thus, resulting in:
-
c)
2RSH + PS-SP←→ 2PSH + RS-SR
where RSH is a generic LMW-SH, PSH is a protein –SH group, PSSP are inter(intra)-chain protein disulfides and PS-SR are mixed disulfides formed between the protein and LMW thiols. The thiolate form of these species are the actual reacting forms in these equations and the increase or decrease of RSH or RS-SR levels, shifts the equilibrium of reaction (c) towards the right or the left, respectively.
The kinetics and the equilibrium constants of these reactions vary according to the thiols and disulfides involved and the pKa of their Cys residues, i.e. that of the nucleophilic thiol and that of the leaving thiol group. In the case of PSH and inter-chain or intra-chain PSSP, other variables should also be considered, such as entropic barriers and steric hindrance, as well as their dynamics in the reaction milieu [reviewed in Ref. [21]].
LMW thiols/disulfides found in extracellular fluids include as the most abundant and redox-active couples cysteine/cystine (Cys/CySS), cysteinilglycine/cystinilglycine (CysCgly/CysGlySS) and glutathione/glutathione disulfide (GSH/GSSG) (Fig. 2). In human plasma, the average ratio for these couples is 0.2 and average concentrations of total Cys and total GSH are ~200 μM and <10 μM, respectively [[22], [23], [24]]. In contrast, GSH is by far the main LMW thiol in cells with concentrations ranging in the different cell types from 1 to 10 mM [25,26], while GSH/GSSG ratios range between 200 and 800 [27]. Micromolar concentrations of Cys and other LMW species are present in the cells, and also in these thiols the reduced forms predominate over the oxidized counterparts [28].
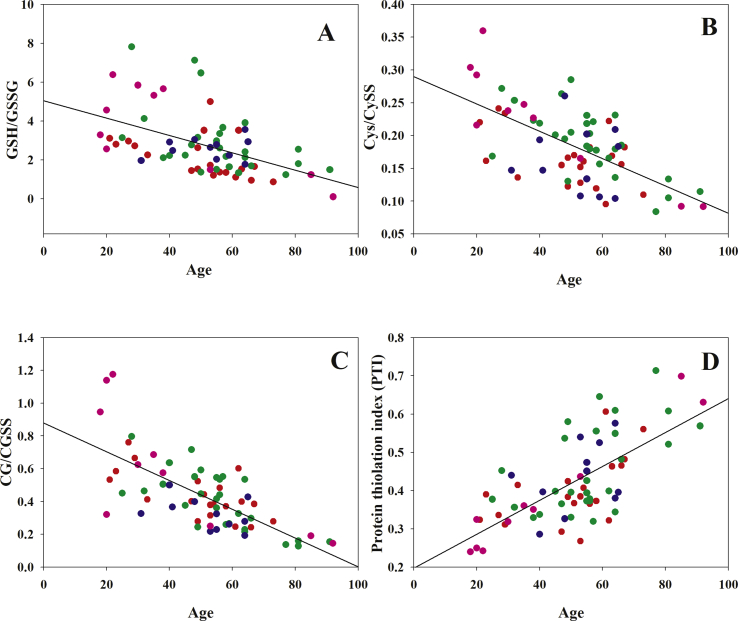
Fig. 2.
Age-dependence of thiol/disulfide ratios in plasma. Panel A: Age vs GSH/GSSG, r = −0.5, two-tailed p values < 0.001; panel B: Age vs cysteine/cystine, r = −0.315, two-tailed p values < 0.01 panel C: Age vs cysteinylglycine/cystinylglycine, r = −0.708, two-tailed p values < 0.0001; panel D: Age vs PTI, r = 0.686, two-tailed p values < 0.0001. Data from Refs. [22] (red symbols), [58] (green), [61] (blue), [62] (pink). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
These differences indicate that the cells maintain markedly reducing conditions whereas a more oxidizing environment is encountered extracellularly. Important enough, cellular stressors such as alkylating agents and reactive oxygen species modulate the metabolism of cellular LMW thiols and, consequently, the composition of their extracellular counterparts, which is present in tissues as a ubiquitous and dynamic equilibrium with cell type and disease-specific differences (reviewed in Ref. [29]). Bidirectional transport systems control the levels of Cys and GSH at the two sides of the plasmalemma; a net influx of both Cys and CySS provides the cells of the Cys needed for the biosynthesis of GSH, whereas the efflux of Cys, GSH and other LMW thiols redistribute these sulfur-containing species in the medium ensuring their availability for other cells and ultimately for the interorgan metabolism of Cys and GSH. Under conditions of cellular stress, a battery of adaptive response genes intervene to control these fluxes and the redox homeostasis of the cell. These genes affect the synthesis and reduction potential of GSH, and consequently the composition of extracellular LMW thiols, in a compensatory and adaptive manner [28]. Such stress response process is expression of the metabolic and homeostatic competence of tissues, which characteristically decrease with age thus increasing the risk of chronic diseases [30], as well as of virial infections [31].
Also, homeostatic processes that maintain the redox state of extracellular thiols decline as a function of the subject’ age [22,32]. Jones at al. described some differences for the redox decline of Cys/CySS and GSH/GSSG couples in plasma, with a linear oxidation of cysteine/cystine redox state over the entire age span, and a linear oxidation of GSH/GSSG that started after approx. 45 years [32], indicating the capacity of the GSH antioxidant system to compensate for oxidations thanks to metabolic processes that are more efficient in the first part of human lifespan. We also found that the age-dependent decrease of some eLMW SH/SS ratios in the human plasma of healthy subjects, is associated with an increased oxidation of PSH to PS-SR (also referred as to “Protein Thiolation Index” or PTI) (Fig. 2, panel D). These differences in some LMW thiol/disulfide redox couples and their control systems can be explained by the fact that GSH/GSSG predominates and provides control mechanisms within the cells, whereas Cys/CySS predominates in the extracellular fluid [33]. The redox states of these two thiol couples are not in equilibrium with each other and distinct regulatory functions have been proposed, including the direct participation in the interactions with proteins and other LMW thiols to produce opposite situations as far as the balance between thiol and disulfide species is concerned at the two sides of the cell membrane [33].
In the cells the glycolytic activity of the pentose phosphate pathway (PPP) provides reducing equivalents under the form of NADPH to support the enzymatic activity of glutathione reductase (GR, reaction a) that with other physiological oxidoreductases, such as thioredoxin reductase, maintain the intracellular LMW in the reduced form [29,34]:
-
d)
GSSG + 2NADPH → 2GSH + 2NADP+ + 2H+ (the GR reaction process);
As a result of this reducing environment, intracellularly only a minor fraction of protein Cys (less than 10%) [35] are present as disulfides, with reaction (c) that is shifted to the right being coupled and subordinate to reaction (d). Therefore, disulfide formation is infrequent and almost all the solvent-exposed Cys residues of intracellular proteins are kept reduced [35].
Conversely, the extracellular environment lacks of redox-homeostasis systems as efficient as those found in cells and thiol-disulfide exchange reactions are sustained by the activity extracellular oxidoreductases [36]. In the case of plasma, NADPH and GR are present in traces possibly deriving from unspecific release of cellular components during blood drawing and processing of sample for the isolation of plasma; other enzymatic antioxidants such as catalase and glutathione peroxidase, are also present in traces in plasma and other biological fluids [37]. Also the Trx reductase system that plays a major role control of protein Cys redox in cells, does not appear to affect the redox processes of extracellular fluids that present decreased Cys/CySS and GSH/GSSG, and hypothetical protein dithiol-disulfide ratios (PrSH/PrSS) compared with the cytosolic counterpart [33].
These aspects make disulfide crosslink formation as a more likely process to occur for proteins exposed to the extracellular environment than for intracellular proteins. As an example of this, human serum albumin (HSA), which is by far the most characterized thiol-containing extracellular protein, form mixed disulfides with eLMW thiols (essentially eLMW-SS) that react with Cys34, i.e. the PTI. Cys34 is the only solvent-exposed Cys residue of HSA that also contains 17 buried disulfides. For example, plasma homocysteine (Hcy) is more than 80% bound to HSA [38] and also Cys - the main eLMW thiol - forms mixed disulfides with HSA and other eLMW thiols, including the same Hcy [39]. Cys34 cannot form disulfides with other HSA molecules or other proteins, being located in a partially protected crevice that hampers its reactivity by steric hindrance [40], but it is known that the thiol-disulfide equilibrium of cellular receptors regulates the binding of HSA [36], and the same redox mechanism is involved in coronavirus’ interaction with the host cell, including the interaction of SARS-CoV-2 with ACE2 receptors in the lung epithelia (discussed earlier).
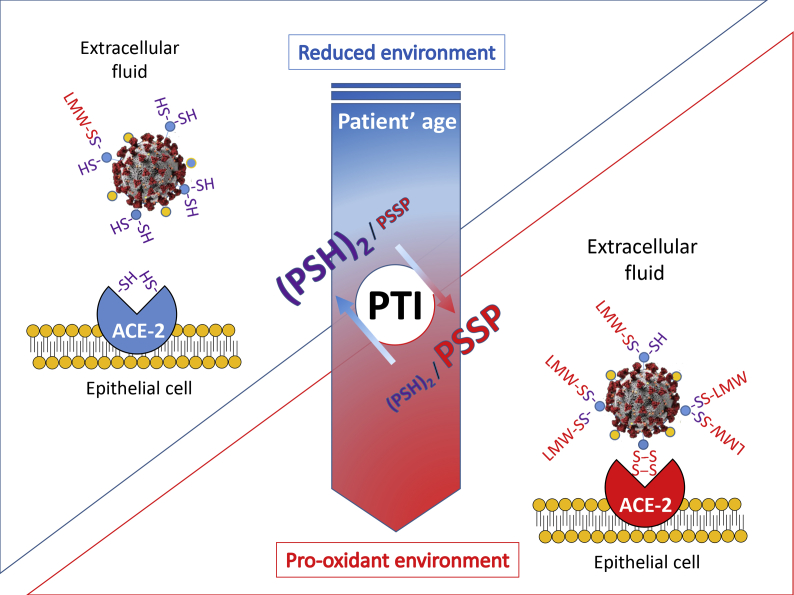
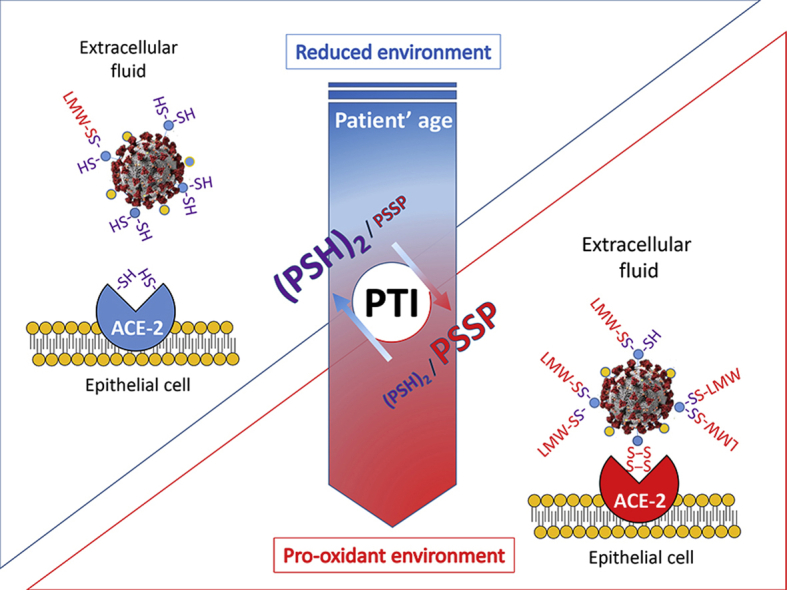
Therefore, the available data appear to support the notion that to a more oxidized extracellular environment corresponds a higher probability that solvent-exposed Cys residues of proteins result in the formation of intramolecular or intermolecular disulfides with other proteins or eLMW thiols (also referred as to mixed disulfides) and that these redox processes directly influence the SARS-CoV-2 interaction with the host cell (Fig. 3).
Fig. 3.
(Graphical Abstract) The Protein Thiolation Index (PTI), an indicator of the thiol/disulfide balance of extracellular fluids, increases its levels with age, thus mirroring the decline of redox homeostasis systems of tissues. In this study we propose that PTI and extracellular thiol analysis can be utilized to predict the risk of more severe infections and clinical manifestations by higher oxidation of interaction domain Cys that are critical for CoV-2 spike protein and ACE-2 receptor binding. Pro-oxidant conditions of the extracellular environment have been described to promote the physical (protein-protein) interaction of viral proteins and host cell receptors in many other viruses [[16], [19]], thus representing an age-dependent molecular process of increased susceptibility to viral infections and their complications.
4. LMW thiols of lung lining fluids and plasma in the age-dependent risk of pulmonary diseases
The epithelial surface of the lung varies in adults from 70 to 140 m2 depending on sex and anthropometric characteristics, and at the same time it contains the largest endothelial surface of any organ. These aspects make this tissue at risk of oxidant damage by the exposure to high oxygen tension during respiration and to a number of environmental oxidants and xenobiotics. Thiols provide an important first line of defence against this pro-oxidant environment; in fact the lung tissue is a major storage area for GSH (6.1–17.5 nmol/mg of tissue) [41]. GSH is released in the lung lining fluid or epithelial lining fluid (LLF or ELF, respectively). It is present in this heterogenous class of extracellular fluids together with other non-enzymatic antioxidants, including Cys and other LMW thiols. Although GSH levels vary in the different areas of the respiratory tract, being lower in nasal than in alveolar lining fluid [42], its concentrations in these extracellular fluids range between 100 and 400 μM [43,44], which are much (from 10 to 100 fold) higher compared with those found in blood plasma (<10 μM, commonly between 1 and 6 μM). Moreover, these concentrations have been described to increase by the effect of smoking in the earliest phased of COPD, indicating the presence of an adaptive response of the lung to environmental stressors (tobacco smoke) and the pro-oxidant effects of inflammation (reviewed elsewhere in Ref. [45]). Such response is critical for the antioxidant protection of the lung tissue, and important enough, its efficacy declines with patients’ age. In fact, both the levels of GSH in the ELF and the GSH adaptive response to cigarette smoking have been reported to decline in older compared to younger cigarette smokers, with concomitant increase in exhaled nitric oxide [46]; furthermore, when this age-related decline of the GSH system was investigated in mice, mixed disulfide formation was demonstrated in ELF and lung proteins in association with indices of inflammation and lung emphysema.
Animal studies demonstrate that the lung GSH decreases with age [47]. Moreover, changes in the levels and detoxification function of lung GSH have been reported in several pulmonary diseases and have been associated with pathogenic mechanisms and severity of clinical manifestations that are all age-dependent [45,48]. Along with the decline of GSH in the ELF of COPD patients with age [46,49], changes in the thiol/disulfide balance of extracellular fluids have been described to play a pathogenic role in idiopathic pulmonary fibrosis (IPF), an age-related aliment of the lung caused by the activation of lung fibrinogenesis and oxidative stress [50]. Again, blood plasma thiol/disulphide redox couples decreased with age [22,32] and smoking [51], two known risk factors for IPF. This pulmonary disease is characterized by a marked reduction of GSH in blood plasma coupled with increased levels of GSSG, and this reduction is also observed in LLF and induced sputum [reviewed in Ref. [45]. Together, these pieces of information prompt the concept that both aging and oxidative stress impair the GSH system of the lung tissue thus interfering with its defense role against the oxidant excess of pulmonary diseases [46,52,53].
The synthesis of GSH depends on the availability of Cys that together with its disulfide CySS, constitute the most abundant LMW thiol/disulfide redox couple of plasma. Unluckily, this redox couple and those of other eLMW thiols have not been investigated in the human lung; however, in the bleomycin model of pulmonary fibrosis, GSH/GSSG and Cys/CySS decreased in plasma during the inflammatory and fibrotic phases of the lung injury, respectively, and a decreased Cys/CySS was also observed in LLF possibly by the decreased food intake that limits the availability of the extracellular Cys [54]. Such experimental data indicate that Cys availability and oxidation contribute to the Cys/CySS redox of extracellular fluids. These aspects of Cys metabolism are also expected to influence the lung metabolism of GSH and the GSH/GSSG ratio of LLF. Accordingly, the administration of N-acetylcysteine (NAC) and other Cys analogues, stimulates the metabolism of GSH in the lung increasing the levels of this tripeptide in LLF and its antioxidant protection function [55].
The availability of Cys and its redox state in the LLF are also reported to directly influence mucolytic processes. In fact, pharmacological interventions with NAC or other Cys analogues, such as erdosteine and carbocysteine, have been reported modulating Cys oxidation and cross-link formation in mucin polymers thus increasing their solubility [56]. This is not surprising since eLMW thiols participate in mixed disulfides formation with extracellular proteins to eventually interfere with their intra- and inter-molecular interactions (this applies for mucin crosslinks in the LLF as well as for HSA in plasma that was discussed earlier in the previous section). Different from the thiolation processes of lung cells (these are investigated as S-glutathionylation reactions), which can occur both spontaneously and by means of specific enzymatic reactions in the different subcellular compartments [26,45,57], extracellular S-thiolations only occur spontaneously by the activity of nucleophilic eLMW thiols following the same biochemical principles that govern protein thiolation in plasma and other extracellular fluids [25,58]. In this respect, it is conceivable to assume that similarly to human plasma, solvent-exposed Cys of LLF proteins and extracellular epitopes of lung epithelial cell receptors (including ACE2 receptor) may undergo mixed disulfide formation with eLMW thiols or other proteins (eventually the spike S protein of CoV-2), which depend on LMW thiol availability (essentially Cys intake through the diet) and thiol-disulfide balance of this biological fluid [54]. The dietary intake of Cys, its endogenous formation in the transulfuration pathway, and the redox of LMW thiol couples and PTI in human plasma are all age-dependent aspects [59]. These aspects are also reported to affect the thiol redox of LLF and that of extracellular domains of proteins on the lung epithelia.
5. Hypothesis formulation
Here we hypothesize that an age-depended decline of levels and redox balance of LMW thiols in LLF directly promotes protein disulfide formation that is a proposed prerequisite for SARS-CoV-2 virus to bind ACE receptors and infect the host. We base this hypothesis on data in literature that demonstrate close similarities in the reduction of the LMW thiols to disulfides ratio in plasma and LLF. Considering these similarities, oxidation of solvent-exposed Cys residues of both the spike S protein and ACE2 receptor (Fig. 1) of the lung epithelia, and possibly in other tissues, are more probable as the host’ age increases (Fig. 3). In this respect, we suggest that plasma LMW thiols and PTI could be used as surrogate indicators of the age-dependent risk of the host to get infected and to develop severe clinical symptoms. The PTI is robust indicator of impaired redox homeostasis in aging studies first described in our laboratories and now available for extensive application in screening protocols of the general population and high-risk groups, as well as in clinical trials to assess the efficacy of therapies and measures of prevention (vide infra). Such extensive application of the test will also provide the means to verify the present hypothesis and plan for specific interventions.
6. Verification strategy: assessing eLMW thiols and their redox interactions with proteins in experimental models of CoV-2 infection and clinical trials
The verification of our hypothesis is essentially based on thiol analytics and its application to experimental and clinical models. Measuring the redox couples of LMW thiols remains problematic being difficult to avoid pre-analytical oxidations and unspecific formation of mixed disulfides by mobilization of protein-bound thiols and trans-thiolation reactions [60]. Standardized protocols of sample collection and manipulation during the preparation to the analysis have been produced and are routinely utilized in specialized laboratories to prevent such pre-analytical biases; main critical variables are the timing of these steps and the accuracy of sample derivatization to block reactive R–SH of LMW and protein components [22,23,27,61,62]. Much easier is the analysis of total LMW or protein thiols (reduced and oxidized forms are not distinguished), which are measured after reduction of the analytical sample without preliminary steps of derivatization.
These protocols have been implemented in different types of samples, including biological fluids, cells and tissues (reviewed in Ref. [25]), and for specific applications in large cohorts of patients of clinical trials and screening campaigns of the general population [61,62].
6.1. In vitro experimental verifications
In principle, the verification strategy for our hypothesis could be based on in vitro experiments in which changes in the composition and redox of eLMW thiols are introduced by means of different strategies, while the binding of spike S and ACE-2 is investigated in cell-free experiments or in host cells exposed to CoV2 infection. A first series of these studies has already been planned and is now in progress in our laboratories.
6.2. Clinical verifications
The determination of the fraction of Cys34 of HSA that is S-thiolated at the steady-state (i.e. involved in the formation of mixed disulfides with eLMW-SS), also referred as to PTI (Fig. 2, Fig. 3), could be utilized as a useful verification tool of this hypothesis in clinical trials. This is a reliable index of the redox equilibrium between LMW and protein thiols of extracellular fluids, and an indicator of oxidative stress [58,61,62]. Its levels linearly increase with the subject age mirroring the increased susceptibility of plasma proteins to undergo S-thiolation during aging by the impaired thiol/disulfide status of eLMW species [63] (Fig. 2). Because sufficient evidence is available in literature to assert that the age- and lung disease-dependent modifications of thiol/disulphide redox can be evaluated with the PTI, this laboratory test could directly be applied in clinical trials to test with an “a priori” approach if increased levels of this indicator correlate with the risk of infection and severity of its manifestations. This type of study could easily be planned in collaboration with CoV-2 diagnostic centers.
In verifying this hypothesis, it is important to consider that thiol concentrations and oxidation state of extracellular fluids depend on a rather complex series of factors that are all independently affected by the aging process, the main ones include: i) the dietary intake of cysteine [59], ii) the transmembrane flux of cellular thiols that is important to sustain the biosynthesis and efflux of GSH in the lung [45] and also to control signal transduction throughout adaptive stress response and programmed cell death pathways [28], iii) the metabolism of circulating GSH by the γ-glutamyltranspeptidase (γ-GT) activity of tissues and especially of the kidney [64,65].
All these aspects (the quality of nutrition, the efficacy of the adaptive stress response and kidney function) and their decline with the age are among the risk factors for severe manifestations and risk of death in CoV-2 patients. Therefore, it is not surprising that these aspects are among the main variables that one should consider to design and implement rationalized strategies of prevention and protection from COVID-19 in the elderly [66]. Their clinical relevance in relationship with thiol-disulfide balance of extracellular fluids can be inferred by the study of different age-related ailments [64], including chronic kidney disease (CKD) that has previously been investigated in our laboratories. This condition causes a very severe form of premature aging and secondary immune dysfunction [67], and therefore it is not surprising that these patients have a markedly higher risk of severe complications and mortality once exposed to CoV-2 infection [68]. Along with an impaired renal metabolism of thiols, these patients present severe protein-caloric malnutrition and increased protein catabolism, and chronic exposure to retention solutes and pro-inflammatory mediators that ultimately interfere with the stress adaption response of tissues and thiol-disulfide balance of blood plasma, and increased levels of PTI [24,69,70]. A reduced or absent function of tubular epithelia cells that express high levels of γ−GT enzyme protein is observed in the late stages of the diseases, and recent studies by our group suggest that the activity of this enzyme is important to control both the renal metabolism and extracellular levels of LMW thiols [65].
A trend toward a decrease of circulating S-thiolated proteins in healthy subjects during spring and summer was also observed in our laboratories [62], which is another factor to consider in the verification strategy to assess host’ susceptibility to CoV-2 infection in relationship with factors that may influence the extracellular redox.
Gender differences should also be investigated being significantly higher the age-dependent risk of severe complications and mortality in male compared to female CoV-2 patients [71]. Although the thiol to disulphide balance of plasma does not appear to be under the influence of gender [22,32], the decline with age of GSH/GSSG, but not that of Cys/CySS, appears to differ in male compared with female [32], and the same has been reported for the blood levels of total GSH in some studies on age-related diseases (recently reviewed in Refs. [72,73]).
6.3. Pharmacological and nutritional verifications
Another aspect to consider for the verification of the hypothesis proposed in this study, is the role of pharmacological and/or nutritional treatments that are supposed to target extracellular LMW thiols. This type of verification could be performed in ongoing clinical trials and among the registered studies, and consultable at www.clinicaltrials.org, some are based on treatments with LMW-SH (NAC, GSH or bucillamine). All these studies are essentially aimed at limiting the damages of acute inflammation (cytokine storm) rather than preventing the virus-host cell interaction and thus the infection in the respiratory tract. This is quite surprising to us because LMW-SH are not very efficient agents in acute inflammation. Nevertheless, a bounce of studies reports these treatments may result in better clinical outcomes [74,75], thus suggesting more complex effects than simple anti-inflammatory.
Timing of the intervention with chemo-protection and prevention agents is crucial as it is for antiviral agents. Although the time-course of virus infection and the extent of the viral load differ between patients, the available evidence suggests that the viral load peaks around symptom onset and decreases from one to three weeks after this step of the infectious disease [76]. In this context, a protection or even early-phase prevention treatment with LMW-SH is difficult to plan and in fact it has never been implemented in clinical trials so far for subjects at risk of infection or disease complications. However, in the future, several currently FDA-approved drugs could be taken into consideration for this purpose, including NAC, GSH, sodium methanethiolate (Mesna), thiopronine, bucillamine, anethole dithiolethione [55,75,[77], [78], [79]]. The established safety profile of these drugs that are already in use to treat other pathologies, suggest that these could be utilized even at high doses and for long-lasting treatments [[80], [81], [82], [83], [84], [85]].
Increasing thiol/disulfide ratio in the extracellular milieu by a higher intake of antioxidants (not only of sulfur-containing molecules), is another option to consider for the verification of our hypothesis. Dietary or pharmacological antioxidants, are assumed to counteract the toxic effect of electrophiles that are often identified with the generic definition of reactive oxygen species. Such definition suggests that this class of compounds could indirectly increase the thiol/disulfide ratio of extracellular fluids. However, a very small number of studies, have explored the effects of antioxidants on extracellular thiols with unbiased analytical procedures, which are essential to accurately determine their concentrations and redox [86,87]. This is a major lack of knowledge that leaves open-up to further verification the opportunity to use the different groups of molecules that fall in this wide category of active ingredients in chemo-protection or prevention measures.
A more direct approach can be represented by the treatment with physiological sulfur-containing amino acids. Jones et al. [59,88] reported an increased Cys/CySS ratio after a normal meal supplemented with methionine and cysteine. Also, whey proteins appear to produce the same results, being rich in cysteine [89]. Food items reach in thiol compounds, such as GSH and cysteine (e.g. asparagus, avocado, and spinach are rich in GSH, and red pepper and asparagus are rich in cysteine) can be utilized to increase the serum levels of Cys [90]. Moreover, there is general agreement on the fact that a higher intake of fruit and vegetables rich in phenolics and other electrophilic bioactives can increase the antioxidant levels in plasma, also affecting extracellular thiols, possibly by the stimulation of hepatic detoxification genes that include those of GSH biosynthesis and GSH-dependent phase II enzymes [91]. For example, soy isoflavons utilized as functional ingredients in food have been demonstrated to increase the plasma levels of free thiols while improving insulin function in type 2 diabetic patients [92]. Nrf2 activators, such as selenium-containing agents [28], and sulforaphane, resveratrol and many others (reviewed in Ref. [31]), are also reported to increase the cellular biosynthesis of GSH and sustain its reduction and extracellular availability by induction of membrane transport genes that control its efflux as part of the detoxification response found in virtually all cells. The effects of these food-derived antioxidants and detoxification compounds on the levels and redox status of extracellular LMW thiols and PTI remain unexplored.
7. Conclusions
In summary, the take home message of the present hypothesis paper is that, if the binding of the spike S protein to ACE2 receptor of the host cell depends on conformational changes of these proteins by the formation of protein disulfide (Fig. 1), the thiol-disulfide ratio of LMW thiols in LLF must be a direct player of the process. In fact, the formation of mixed and intramolecular disulfides on solvent-exposed Cys of these proteins will be the results of the presence of sufficient oxidizing conditions that lower this ratio in eLMW thiols (Fig. 3). A pro-oxidant environment has been proposed to promote the virus-host cell interaction also in the case of other viral infections [[16], [19]]. Despite the widely recognized role of the GSH system in the redox homeostasis of the respiratory tissues, the role of eLMW thiols in COVID-19 infection has been ignored so far. The age-dependent decline of the GSH system of the lung and other tissues, and the impaired control of the thiol to disulfide balance of the extracellular fluids, including the LLF, offer a convincing mechanism for an increased risk of severe clinical manifestations in this viral infection. Herein we propose to assess such mechanistic interpretation in experimental models of COVID-19 infection (this is to assess the redox principles of the hypothesis) and in clinical trials (this is to confirm the age-dependence and the clinical relevance of the hypothesis). To provide a realistic verification strategy for our hypothesis, a standardized laboratory procedure for the microanalysis of PTI in human blood, has recently been developed [62] and it is now available for the screening of the general population and groups at risk of severe infection, as well as for clinical applications in the study of therapeutic agents and measures of prevention.
Declaration of competing interest
The authors have no conflicts of interest to declare.
Acknowledgments
F. G. is recipient of the following grant programs:
-“Fondazione Cassa di Risparmio di Perugia”, Perugia, Italy; call “Settore Ricerca Scientifica e Tecnologica 2019: Invecchiamento. Cura e prevenzione della fragilità: aspetti biologici, fisiopatologici e problematiche cliniche”; project ID # 10435 (2019.0320).
-“Ricerca di base”, University of Perugia, Italy.
-JPI-HDHL (Joint Programming Initiative - A Healthy Diet for a Healthy Life), ERA-NET program Horizon 2020 cofounded by the Italian Ministry of University and Research. “PREVNUT - Development of targeted nutrition for prevention of undernutrition for older adults” (GA N° 696295).
D.B. is a fellow of research of the grant program 2019 of the “Fondazione Cassa di Risparmio di Perugia”, Perugia, Italy; Project ID # 10435 (2019.0320).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2021.101902.
Contributor Information
Daniela Giustarini, Email: daniela.giustarini@unisi.it.
Francesco Galli, Email: francesco.galli@unipg.it.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Davies N.G. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat. Med. 2020;26(8):1205–1211. doi: 10.1038/s41591-020-0962-9. [DOI] [PubMed] [Google Scholar]
- 2.Omori R., Matsuyama R., Nakata Y. The age distribution of mortality from novel coronavirus disease (COVID-19) suggests no large difference of susceptibility by age. Sci. Rep. 2020;10(1):16642. doi: 10.1038/s41598-020-73777-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersen E. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect. Dis. 2020;20(9):e238–e244. doi: 10.1016/S1473-3099(20)30484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suhail S. Role of oxidative stress on SARS-CoV (SARS) and SARS-CoV-2 (COVID-19) infection: a review. Protein J. 2020;39(6):644–656. doi: 10.1007/s10930-020-09935-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Derouiche S. Oxidative stress associated with SARS-cov-2 (COVID-19) increases the severity of the lung disease - a systematic review. J Infect Dis Epidemiol. 2020;6(121) [Google Scholar]
- 6.Laforge M. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat. Rev. Immunol. 2020;20(9):515–516. doi: 10.1038/s41577-020-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou P. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmann M. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mercurio I. Protein structure analysis of the interactions between SARS-CoV-2 spike protein and the human ACE2 receptor: from conformational changes to novel neutralizing antibodies. Cell. Mol. Life Sci. 2020 doi: 10.1007/s00018-020-03580-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan R. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J. Mutations strengthened SARS-CoV-2 infectivity. J. Mol. Biol. 2020;432(19):5212–5226. doi: 10.1016/j.jmb.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaudhry F. Manipulation of ACE2 expression in COVID-19. Open Heart. 2020;7(2):e001424. doi: 10.1136/openhrt-2020-001424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nawijn M.C., Timens W. Can ACE2 expression explain SARS-CoV-2 infection of the respiratory epithelia in COVID-19? Mol. Syst. Biol. 2020;16(7) doi: 10.15252/msb.20209841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radzikowska U. Distribution of ACE2, CD147, CD26, and other SARS-CoV-2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID-19 risk factors. Allergy. 2020;75(11):2829–2845. doi: 10.1111/all.14429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.AlGhatrif M., Cingolani O., Lakatta E.G. The dilemma of coronavirus disease 2019, aging, and cardiovascular disease: insights from cardiovascular aging science. JAMA Cardiol. 2020;5(7):747–748. doi: 10.1001/jamacardio.2020.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fenouillet E., Barbouche R., Jones I.M. Cell entry by enveloped viruses: redox considerations for HIV and SARS-coronavirus. Antioxidants Redox Signal. 2007;9(8):1009–1034. doi: 10.1089/ars.2007.1639. [DOI] [PubMed] [Google Scholar]
- 17.Debnath U. Chemrxiv; 2020. Conformational Perturbation of SARS-CoV-2 Spike Protein Using N-Acetyl Cysteine, a Molecular Scissor: A Probable Strategy to Combat COVID-19. [DOI] [PubMed] [Google Scholar]
- 18.Hati S., Bhattacharyya S. Impact of thiol-disulfide balance on the binding of covid-19 spike protein with angiotensin-converting enzyme 2 receptor. ACS Omega. 2020;5(26):16292–16298. doi: 10.1021/acsomega.0c02125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lavillette D. Significant redox insensitivity of the functions of the SARS-CoV spike glycoprotein: comparison with HIV envelope. J. Biol. Chem. 2006;281(14):9200–9204. doi: 10.1074/jbc.M512529200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ulrich K., Jakob U. The role of thiols in antioxidant systems. Free Radic. Biol. Med. 2019;140:14–27. doi: 10.1016/j.freeradbiomed.2019.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bechtel T.J., Weerapana E. From structure to redox: the diverse functional roles of disulfides and implications in disease. Proteomics. 2017;17(6) doi: 10.1002/pmic.201600391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giustarini D. Age-related influence on thiol, disulfide, and protein-mixed disulfide levels in human plasma. J. Gerontol. A Biol. Sci. Med. Sci. 2006;61(10):1030–1038. doi: 10.1093/gerona/61.10.1030. [DOI] [PubMed] [Google Scholar]
- 23.Jones D.P., Liang Y. Measuring the poise of thiol/disulfide couples in vivo. Free Radic. Biol. Med. 2009;47(10):1329–1338. doi: 10.1016/j.freeradbiomed.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galli F. Blood thiol status and erythrocyte glutathione-S-transferase in chronic kidney disease patients on treatment with frequent (daily) hemodialysis. Free Radic. Res. 2014;48(3):273–281. doi: 10.3109/10715762.2013.861901. [DOI] [PubMed] [Google Scholar]
- 25.Giustarini D. Assessment of glutathione/glutathione disulphide ratio and S-glutathionylated proteins in human blood, solid tissues, and cultured cells. Free Radic. Biol. Med. 2017;112:360–375. doi: 10.1016/j.freeradbiomed.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Scirè A. Glutathione compartmentalization and its role in glutathionylation and other regulatory processes of cellular pathways. Biofactors. 2019;45(2):152–168. doi: 10.1002/biof.1476. [DOI] [PubMed] [Google Scholar]
- 27.Giustarini D. Glutathione, glutathione disulfide, and S-glutathionylated proteins in cell cultures. Free Radic. Biol. Med. 2015;89:972–981. doi: 10.1016/j.freeradbiomed.2015.10.410. [DOI] [PubMed] [Google Scholar]
- 28.Bartolini D. Glutathione S-transferase P influences the Nrf2-dependent response of cellular thiols to seleno-compounds. Cell Biol. Toxicol. 2020;36(4):379–386. doi: 10.1007/s10565-020-09517-5. [DOI] [PubMed] [Google Scholar]
- 29.Bartolini D. Targeting glutathione S-transferase P and its interactome with selenium compounds in cancer therapy. Biochim. Biophys. Acta Gen. Subj. 2019;1863(1):130–143. doi: 10.1016/j.bbagen.2018.09.023. [DOI] [PubMed] [Google Scholar]
- 30.Pomatto L.C.D., Davies K.J.A. The role of declining adaptive homeostasis in ageing. J. Physiol. 2017;595(24):7275–7309. doi: 10.1113/JP275072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cuadrado A. Can activation of NRF2 Be a strategy against COVID-19? Trends Pharmacol. Sci. 2020;41(9):598–610. doi: 10.1016/j.tips.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones D.P. Redox analysis of human plasma allows separation of pro-oxidant events of aging from decline in antioxidant defenses. Free Radic. Biol. Med. 2002;33(9):1290–1300. doi: 10.1016/s0891-5849(02)01040-7. [DOI] [PubMed] [Google Scholar]
- 33.Jones D.P. Radical-free biology of oxidative stress. Am. J. Physiol. Cell Physiol. 2008;295(4):C849–C868. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leichert L.I., Jakob U. Global methods to monitor the thiol-disulfide state of proteins in vivo. Antioxidants Redox Signal. 2006;8(5–6):763–772. doi: 10.1089/ars.2006.8.763. [DOI] [PubMed] [Google Scholar]
- 35.Hansen R.E., Roth D., Winther J.R. Quantifying the global cellular thiol–disulfide status. Proc. Natl. Acad. Sci. U. S. A. 2009;106:422–427. doi: 10.1073/pnas.0812149106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yi M.C., Khosla C. Thiol-disulfide exchange reactions in the mammalian extracellular environment. Annu. Rev. Chem. Biomol. Eng. 2016;7:197–222. doi: 10.1146/annurev-chembioeng-080615-033553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strålin P. The interstitium of the human arterial wall contains very large amounts of extracellular superoxide dismutase. Arterioscler. Thromb. Vasc. Biol. 1995;15(11):2032–2036. doi: 10.1161/01.atv.15.11.2032. [DOI] [PubMed] [Google Scholar]
- 38.Galli F. The effect of PMMA-based protein-leaking dialyzers on plasma homocysteine levels. Kidney Int. 2003;64(2):748–755. doi: 10.1046/j.1523-1755.2003.00134.x. [DOI] [PubMed] [Google Scholar]
- 39.Sengupta S. Relative roles of albumin and ceruloplasmin in the formation of homocystine, homocysteine-cysteine-mixed disulfide, and cystine in circulation. J. Biol. Chem. 2001;276(50):46896–46904. doi: 10.1074/jbc.M108451200. [DOI] [PubMed] [Google Scholar]
- 40.Narazaki R. Covalent binding between bucillamine derivatives and human serum albumin. Pharm. Res. (N. Y.) 1996;13(9):1317–1321. doi: 10.1023/a:1016057513490. [DOI] [PubMed] [Google Scholar]
- 41.Rahman I., MacNee W. Lung glutathione and oxidative stress: implications in cigarette smoke-induced airway disease. Am. J. Physiol. 1999;277(6):L1067–L1088. doi: 10.1152/ajplung.1999.277.6.L1067. [DOI] [PubMed] [Google Scholar]
- 42.Cross C.E. Oxidants, antioxidants, and respiratory tract lining fluids. Environ. Health Perspect. 1994;102(Suppl 10):185–191. doi: 10.1289/ehp.94102s10185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cantin A.M. Normal alveolar epithelial lining fluid contains high levels of glutathione. J. Appl. Physiol. 1985;63(1):152–157. doi: 10.1152/jappl.1987.63.1.152. 1987. [DOI] [PubMed] [Google Scholar]
- 44.van der Vliet A. Determination of low-molecular-mass antioxidant concentrations in human respiratory tract lining fluids. Am. J. Physiol. 1999;276(2):L289–L296. doi: 10.1152/ajplung.1999.276.2.L289. [DOI] [PubMed] [Google Scholar]
- 45.Janssen-Heininger Y. Endoplasmic reticulum stress and glutathione therapeutics in chronic lung diseases. Redox Biol. 2020;33:101516. doi: 10.1016/j.redox.2020.101516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gould N.S. Glutathione depletion accelerates cigarette smoke-induced inflammation and airspace enlargement. Toxicol. Sci. 2015;147(2):466–474. doi: 10.1093/toxsci/kfv143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giustarini D. Low molecular mass thiols, disulfides and protein mixed disulfides in rat tissues: influence of sample manipulation, oxidative stress and ageing. Mech. Ageing Dev. 2011;132(4):141–148. doi: 10.1016/j.mad.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 48.Galli F. Oxidative stress and antioxidant therapy in cystic fibrosis. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2012;1822(5):690–713. doi: 10.1016/j.bbadis.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 49.Gould N.S. Aging adversely affects the cigarette smoke-induced glutathione adaptive response in the lung. Am. J. Respir. Crit. Care Med. 2010;182(9):1114–1122. doi: 10.1164/rccm.201003-0442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Psathakis K. Exhaled markers of oxidative stress in idiopathic pulmonary fibrosis. Eur. J. Clin. Invest. 2006;36(5):362–367. doi: 10.1111/j.1365-2362.2006.01636.x. [DOI] [PubMed] [Google Scholar]
- 51.Moriarty S.E. Oxidation of glutathione and cysteine in human plasma associated with smoking. Free Radic. Biol. Med. 2003;35(12):1582–1588. doi: 10.1016/j.freeradbiomed.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 52.Elko E.A. Age-dependent dysregulation of redox genes may contribute to fibrotic pulmonary disease susceptibility. Free Radic. Biol. Med. 2019;141:438–446. doi: 10.1016/j.freeradbiomed.2019.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou L. Aging-related decline in the induction of Nrf2-regulated antioxidant genes in human bronchial epithelial cells. Redox Biol. 2018;14:35–40. doi: 10.1016/j.redox.2017.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iyer S.S. Oxidation of extracellular cysteine/cystine redox state in bleomycin-induced lung fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2009;296(1):L37–L45. doi: 10.1152/ajplung.90401.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Flora S., Balansky R., La Maestra S. Rationale for the use of N-acetylcysteine in both prevention and adjuvant therapy of COVID-19. Faseb. J. 2020;34(10):13185–13193. doi: 10.1096/fj.202001807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yuan S. Oxidation increases mucin polymer cross-links to stiffen airway mucus gels. Sci. Transl. Med. 2015;7(276):276ra27. doi: 10.1126/scitranslmed.3010525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Townsend D.M. Novel role for glutathione S-transferase pi. Regulator of protein S-Glutathionylation following oxidative and nitrosative stress. J. Biol. Chem. 2009;284(1):436–445. doi: 10.1074/jbc.M805586200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giustarini D. Protein thiolation index (PTI) as a biomarker of oxidative stress. Free Radic. Biol. Med. 2012;53(4):907–915. doi: 10.1016/j.freeradbiomed.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 59.Jones D.P. Dietary sulfur amino acid effects on fasting plasma cysteine/cystine redox potential in humans. Nutrition. 2011;27(2):199–205. doi: 10.1016/j.nut.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Giustarini D. Pitfalls in the analysis of the physiological antioxidant glutathione (GSH) and its disulfide (GSSG) in biological samples: an elephant in the room. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016;1019:21–28. doi: 10.1016/j.jchromb.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Giustarini D. Determination of protein thiolation index (PTI) as a biomarker of oxidative stress in human serum. Anal. Biochem. 2017;538:38–41. doi: 10.1016/j.ab.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 62.Giustarini D. Protein thiolation index in microvolumes of plasma. Anal. Biochem. 2021;618:114125. doi: 10.1016/j.ab.2021.114125. [DOI] [PubMed] [Google Scholar]
- 63.Jones D.P. Extracellular redox state: refining the definition of oxidative stress in aging. Rejuvenation Res. 2006;9(2):169–181. doi: 10.1089/rej.2006.9.169. [DOI] [PubMed] [Google Scholar]
- 64.Moriarty-Craige S.E., Jones D.P. Extracellular thiols and thiol/disulfide redox in metabolism. Annu. Rev. Nutr. 2004;24:481–509. doi: 10.1146/annurev.nutr.24.012003.132208. [DOI] [PubMed] [Google Scholar]
- 65.Giustarini D. Anethole dithiolethione increases glutathione in kidney by inhibiting. Oxid. Med. Cell. Longev. 2020;2020:3562972. doi: 10.1155/2020/3562972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Galli F. Better prepare for the next one. Lifestyle lessons from the COVID-19 pandemic. Pharma. Nutr. 2020;12:100193. doi: 10.1016/j.phanu.2020.100193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ebert T. Inflammation and premature ageing in chronic kidney disease. Toxins. 2020;12(4):227. doi: 10.3390/toxins12040227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Benedetti C. COVID-19 and the kidneys: an update. Front. Med. 2020;7:423. doi: 10.3389/fmed.2020.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fanti P. Dietary intake of proteins and calories is inversely associated with the oxidation state of plasma thiols in end-stage renal disease patients. J. Ren. Nutr. 2015;25(6):494–503. doi: 10.1053/j.jrn.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Colombo G. Plasma protein thiolation index (PTI) as a biomarker of thiol-specific oxidative stress in haemodialyzed patients. Free Radic. Biol. Med. 2015;89:443–451. doi: 10.1016/j.freeradbiomed.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 71.Borges do Nascimento I.J. Novel coronavirus infection (COVID-19) in humans: a scoping review and meta-analysis. J. Clin. Med. 2020;9(4):941. doi: 10.3390/jcm9040941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cruz-Topete D., Dominic P., Stokes K.Y. Uncovering sex-specific mechanisms of action of testosterone and redox balance. Redox Biol. 2020;31:101490. doi: 10.1016/j.redox.2020.101490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang L., Ahn Y.J., Asmis R. Sexual dimorphism in glutathione metabolism and glutathione-dependent responses. Redox Biol. 2020;31:101410. doi: 10.1016/j.redox.2019.101410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Polonikov A. Endogenous deficiency of glutathione as the most likely cause of serious manifestations and death in COVID-19 patients. ACS Infect. Dis. 2020;6(7):1558–1562. doi: 10.1021/acsinfecdis.0c00288. [DOI] [PubMed] [Google Scholar]
- 75.Horowitz R.I., Freeman P.R., Bruzzese J. Efficacy of glutathione therapy in relieving dyspnea associated with COVID-19 pneumonia: a report of 2 cases. Respir. Med. Case Rep. 2020;30:101063. doi: 10.1016/j.rmcr.2020.101063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Walsh K.A. SARS-CoV-2 detection, viral load and infectivity over the course of an infection. J. Infect. 2020;81(3):357–371. doi: 10.1016/j.jinf.2020.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Giustarini D. Anethole dithiolethione lowers the homocysteine and raises the glutathione levels in solid tissues and plasma of rats: a novel non-vitamin homocysteine-lowering agent. Biochem. Pharmacol. 2014;89(2):246–254. doi: 10.1016/j.bcp.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang Y., Munday R. Dithiolethiones for cancer chemoprevention: where do we stand? Mol. Canc. Therapeut. 2008;7(11):3470–3479. doi: 10.1158/1535-7163.MCT-08-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Revive Therapeutics L. 2020. Bucillamine in Treatment of Patients with COVID-19. [Google Scholar]
- 80.Holdiness M.R. Clinical pharmacokinetics of N-acetylcysteine. Clin. Pharmacokinet. 1991;20(2):123–134. doi: 10.2165/00003088-199120020-00004. [DOI] [PubMed] [Google Scholar]
- 81.Tam J. Nebulized and oral thiol derivatives for pulmonary disease in cystic fibrosis. Cochrane Database Syst. Rev. 2013;(7):CD007168. doi: 10.1002/14651858.CD007168.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Smith P.F. Pharmacokinetics and pharmacodynamics of mesna-mediated plasma cysteine depletion. J. Clin. Pharmacol. 2003;43(12):1324–1328. doi: 10.1177/0091270003260332. [DOI] [PubMed] [Google Scholar]
- 83.Carlsson S.M. Pharmacokinetics of intravenous 2-mercaptopropionylglycine in man. Eur. J. Clin. Pharmacol. 1990;38(5):499–503. doi: 10.1007/BF02336691. [DOI] [PubMed] [Google Scholar]
- 84.Suda A. The efficacy and safety of bucillamine as a second-line DMARD in the treatment of rheumatoid arthritis: a retrospective cohort study. Mod. Rheumatol. 2008;18(6):609–614. doi: 10.1007/s10165-008-0103-7. [DOI] [PubMed] [Google Scholar]
- 85.Lam S. A randomized phase IIb trial of anethole dithiolethione in smokers with bronchial dysplasia. J. Natl. Cancer Inst. 2002;94(13):1001–1009. doi: 10.1093/jnci/94.13.1001. [DOI] [PubMed] [Google Scholar]
- 86.D'Agostino L.A. Comprehensive plasma thiol redox status determination for metabolomics. J. Proteome Res. 2011;10(2):592–603. doi: 10.1021/pr100771g. [DOI] [PubMed] [Google Scholar]
- 87.Andersson A., Lindgren A., Hultberg B. Effect of thiol oxidation and thiol export from erythrocytes on determination of redox status of homocysteine and other thiols in plasma from healthy subjects and patients with cerebral infarction. Clin. Chem. 1995;41(3):361–366. [PubMed] [Google Scholar]
- 88.Park Y. Postprandial cysteine/cystine redox potential in human plasma varies with meal content of sulfur amino acids. J. Nutr. 2010;140(4):760–765. doi: 10.3945/jn.109.116764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tosukhowong P. Biochemical and clinical effects of Whey protein supplementation in Parkinson's disease: a pilot study. J. Neurol. Sci. 2016;367:162–170. doi: 10.1016/j.jns.2016.05.056. [DOI] [PubMed] [Google Scholar]
- 90.Minich D.M., Brown B.I. A review of dietary (Phyto)Nutrients for glutathione support. Nutrients. 2019;11(9):2073. doi: 10.3390/nu11092073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Galli F. Interactions of polyphenolic compounds with drug disposition and metabolism. Curr. Drug Metabol. 2007;8(8):830–838. doi: 10.2174/138920007782798180. [DOI] [PubMed] [Google Scholar]
- 92.Clerici C. Novel soy germ pasta improves endothelial function, blood pressure, and oxidative stress in patients with type 2 diabetes. Diabetes Care. 2011;34(9):1946–1948. doi: 10.2337/dc11-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.