Abstract
Akt kinases translate various external cues into intracellular signals that control cell survival, proliferation, metabolism and differentiation. This review discusses the requirement for Akt and its targets in determining the fate and function of T cells. We discuss the importance of Akt at various stages of T cell development including β-selection during which Akt fulfills the energy requirements of highly proliferative DN3 cells. Akt also plays an integral role in CD8 T cell biology where its regulation of Foxo transcription factors and mTORC1 metabolic activity controls effector versus memory CD8 T cell differentiation. Finally, Akt promotes the differentiation of naïve CD4 T cells into Th1, Th17 and Tfh cells but inhibits the development of Treg cells. We also highlight how modulating Akt in T cells is a promising avenue for enhancing cell-based cancer immunotherapy.
Keywords: Akt, T cell differentiation, mTOR, Foxo, thymocytes, CD8 T cells, Th1, Th17, Tfh, Treg
INTRODUCTION
Akt kinases, also known as PKB kinases are members of the AGC superfamily of serine/threonine protein kinases and have three isoforms (Akt1, Akt2 and Akt3). All isoforms have high structural similarity, possessing an N-terminal pleckstrin homology (PH) domain, a kinase domain and a C-terminal hydrophobic motif (HM) [1]. Akt1 is ubiquitously expressed [2] while Akt2 is highly expressed in insulin responsive organs such as the liver and skeletal muscles [3], and Akt3 is mainly expressed in the testis and the brain [4]. Although Akt isoforms are largely functionally redundant, distinct isoform-specific functions can be observed in certain circumstances (reviewed in [5]).
In T cells, TCR and CD28 co-stimulation triggers activation of class I phosphatidylinositol 3-kinases (PI3Ks) leading to the phosphorylation of the 3-hydroxyl group of the inositol ring in the plasma membrane lipid phosphatidylinositol 4,5-bisphosphate (PIP2) to generate phosphatidylinositol 3,4,5-trisphosphate (PIP3) [6]. PIP3 acts as a plasma membrane docking site for the PH domains of Akt [7] and its upstream activator PDK1 [8]. Akt and PDK1 co-localization results in PDK1-mediated phosphorylation of Akt at Threonine (T)308 in its activation T loop [9,10]. For maximum activation, Akt1 requires additional phosphorylation at Serine (S)473 in its hydrophobic motif by mammalian/mechanistic target of rapamycin complex (mTORC)2 [11]. Once activated, Akt phosphorylates multiple downstream targets to regulate cell survival, proliferation, metabolism, and the fate and function of T cells (Figures 1 and 2). Known targets of Akt signaling in T cells include the kinases Gsk3-β and mTORC1, and the transcription factors CREB, Foxo and NF-ĸB (reviewed in [12,13]). However, more recently it has been reported that Akt has differential targets and in turn promotes distinct CD4 T cell fates when phosphorylated only at T308 or at both T308 and S473. Akt activity is countered by the phosphoinositide phosphatase PTEN, which removes the 3-phosphate group of PIP3 [14], and the protein phosphatases PP2A and PHLPP1/2, which dephosphorylate Akt at T308 and S473, respectively [15,16].
Figure 1.
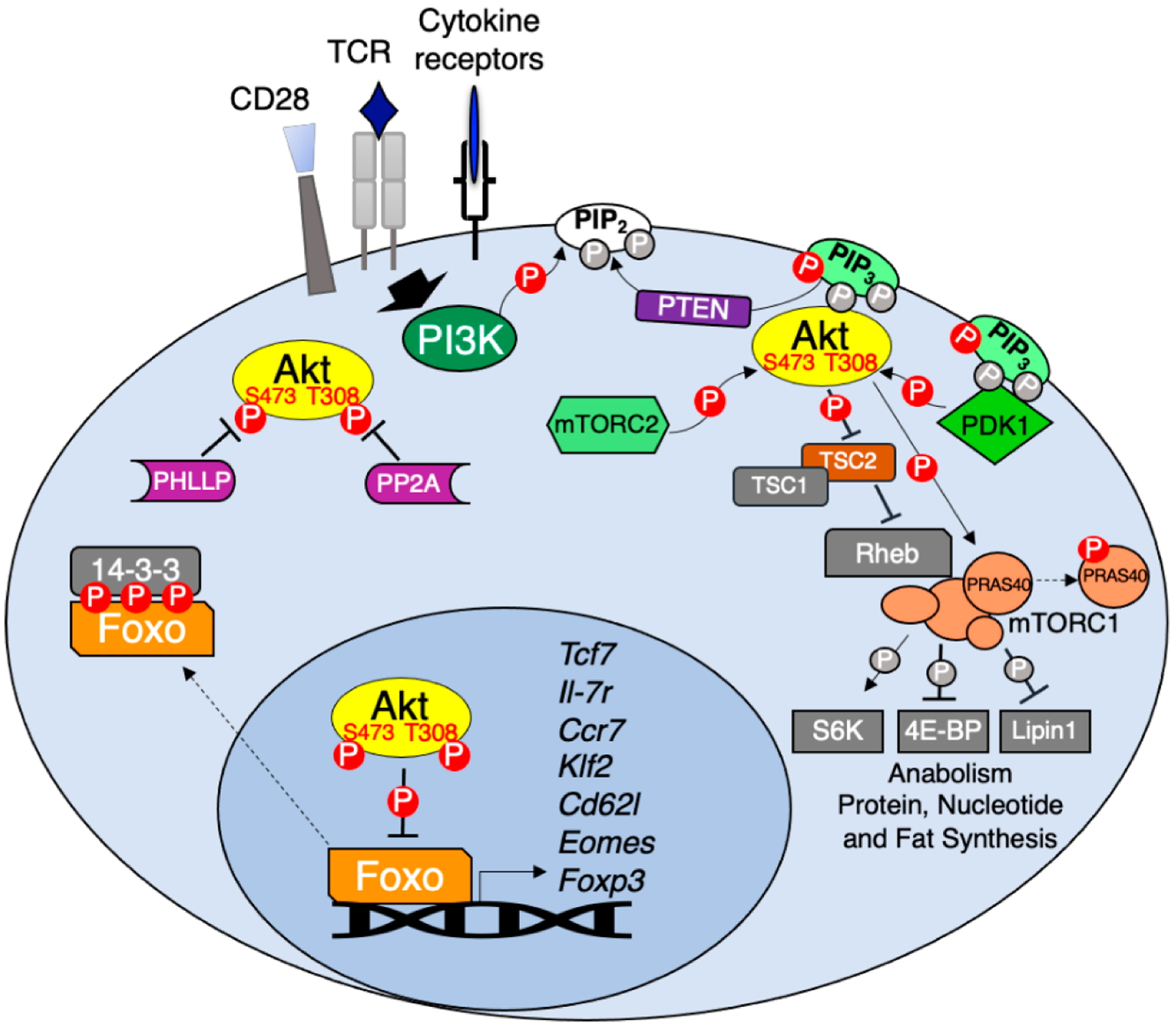
Akt regulation and downstream signaling. TCR, CD28 co-stimulation and various cytokines activate PI3K which in turn phosphorylates membrane PIP2 to generate PIP3. PIP3 acts as the docking site for both Akt and its upstream kinase PDK1 leading to Akt T308 phosphorylation. Full Akt activation occurs following mTORC2 phosphorylation of Akt at S473. Active Akt phosphorylates multiple substrates including the mTORC1 inhibitor TSC2 and PRAS40, resulting in mTORC1-dependent activation of anabolic metabolism. Akt phosphorylation of Foxo transcription factors promotes their association with 14-3-3 adapters leading to Foxo cytoplasmic retention. In the absence of Akt signaling Foxo regulates the expression of genes important for memory CD8 T cell differentiation (Tcf7, Il7r, Ccr7, Klf2, Sell and Eomes) and Treg development (Foxp3). Negative regulators of Akt include PTEN, which dephosphorylates PIP3 back to PIP2 and protein phosphatases PP2A and PHLPP1/2, which dephosphorylate Akt at T308 and S473, respectively.
Figure 2.
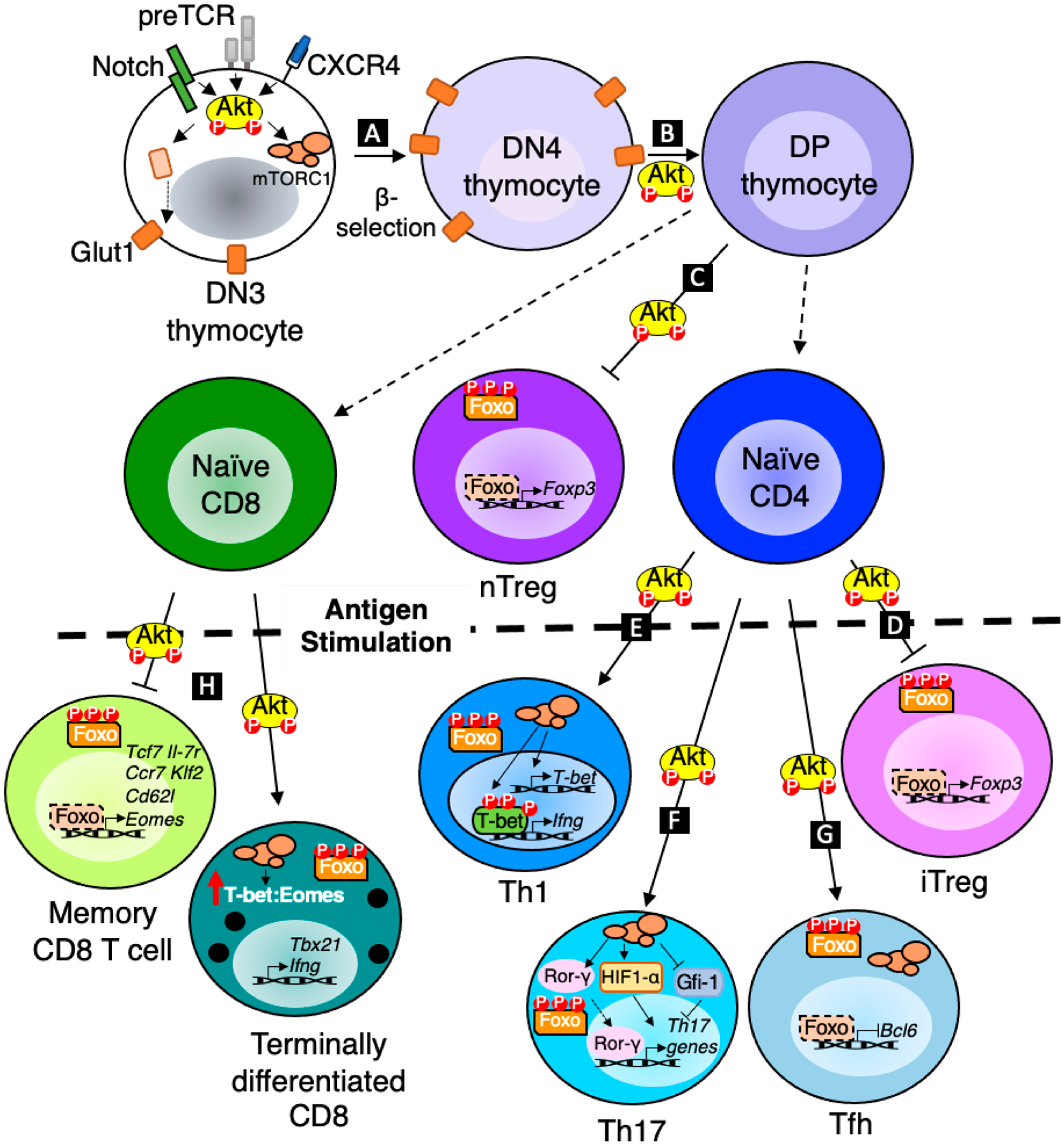
Akt regulation of T cell development, differentiation and function. (A) Akt promotes glucose metabolism through upregulation of Glut1 to fulfil energy requirements of proliferating DN3 cells post β-selection. (B) Akt also promotes DN4 to DP transition in the thymus. (C,D) Akt inhibits Foxo1/3 dependent expression of Foxp3 to prevent nTreg and iTreg development. (E) Akt dependent mTORC1 activation increases T-bet expression which in turn promotes the transcription of Th1 effector molecules. (F) Akt dependent mTORC1 activation positively regulates Rorγ and HIF1α and represses Gfi-1 to promote the transcription of Th17 related genes. (G) Akt inhibits Foxo1 to promote Bcl6 expression, and Akt/mTORC1 activity is also required for Tfh development. (H) Akt dependent mTORC1 activation and Foxo nuclear exclusion promote effector vs memory CD8 T cell differentiation.
In this review, we highlight how the signals discussed above (Figure 1) position Akt as a critical regulator of T cell development, differentiation, metabolism, and effector function (Figure 2). In addition, we discuss how the multifaceted roles of Akt in T cells make this kinase an attractive target for improving cancer immunotherapy.
CONTROL OF EARLY T CELL DEVELOPMENT IN THE THYMUS BY AKT
T cells engage several signaling pathways as they progress through developmental stages in the thymus. Thymocytes in the earliest stages are termed double negative (DN) cells because they lack expression of both CD4 and CD8 co-receptors. DN cells are further subdivided by differential expression of CD44 and CD25 receptors. Multipotent early thymic DN1 cells (CD44+CD25−) commit to the T cell fate following Notch signaling and differentiate into DN2 cells (CD44+CD25+) [17]. DN2 cells mature into DN3 cells (CD44−CD25+). DN3 cells that successfully rearrange and express a unique TCR β chain paired with surrogate pre-Tα chain signal through the pre-T cell receptors (preTCR) to progress through the first checkpoint in thymic development referred to as β-selection [18]. Akt activity is crucial at this checkpoint and is required for the survival, proliferation, metabolism, and differentiation of DN3 thymocytes into DN4 cells (CD44−CD25−) [19,20]. DN4 cells mature into DP cells (CD4+CD8+) which in turn can mature into SP cells (CD4+ or CD8+) following positive and negative selection [21].
All three isoforms of Akt are expressed in thymocytes, but Akt1 and Akt2 are more abundant [22,23]. Loss of any one isoform does not drastically affect thymic development indicating that Akt isoforms have redundant roles in the thymus [22,24]. However, Akt1 deficient mice, which are smaller in body size, do have smaller thymii and thymocytes that are more susceptible to apoptosis [24,25]. Double deficiency in Akt1 and Akt2 leads to a developmental arrest at the DN3 stage where normally cells undergo β-selection, resulting in significantly lower proportions of DN4 and DP cells which become highly susceptible to apoptosis due to insufficient glucose uptake [23]. Ectopic expression of Bcl-xL, which is associated with Akt dependent survival [26], however, is not sufficient to rescue thymic cellularity in the absence of Akt1 and Akt2 [19]. These studies indicate that Akt promotes thymocyte cell survival beyond its control of pro- and anti-apoptotic proteins including Bcl-xL and Bim in thymocytes [26,27].
Akt activity in DN3 thymocytes is not only regulated by preTCR but also by CXCR4 [28,29] and Notch [30–32]. In addition to directing thymocyte localization, the chemokine receptor CXCR4 physically associates with preTCR and acts as a co-activator of preTCR signaling [33]. Antagonism of CXCR4 signaling decreases Akt phosphorylation and results in a developmental block at the DN3 stage [28,29,33]. DN3 cell size, survival, Glut1 expression and glycolysis are also decreased when removed from Notch ligands but can be rescued by ectopic expression of constitutively active Akt [31], suggesting that endogenous Notch signaling activates Akt to support DN3 cell proliferation and metabolism post β-selection. However, in contrast to the traditional PI3K/Akt pathway activated by preTCR and CXCR4, Notch activates Akt indirectly by inducing HES1, which acts to transcriptionally repress expression of PTEN [34]. Together, preTCR, CXCR4 and Notch act cooperatively to control PI3K and Akt kinases during early thymocyte development to promote cell survival by fulfilling the energy requirement of proliferating DN3 cells post β-selection through upregulation of Glut1 receptors.
The effect of Akt on post β-selection thymocytes can be difficult to discern due to developmental defects in DN3 cells. Nevertheless, loss of Akt1 and Akt2 not only led to a block at the DN3 stage but also decreased proliferation of DN4 cells [22], indicating that (pre)TCR-induced Akt activation is required even after β-selection. The ability of constitutively active Akt1 to rescue DN3 cell development to the DP stage in Rag2-deficient cells that are incapable of generating functional TCR chains indicates that Akt also has a prominent role in promoting differentiation of early DN to DP thymocytes [22]. The inability of active Akt1 to promote further maturation to the SP stage [22], however, suggests that Akt is either not required or is insufficient for this developmental process. This notion is supported by observations made when PI3K/Akt activation is antagonized by PTEN over-expression beginning at the DP stage [35]. While DP, SP and peripheral T cell numbers were significantly reduced due to a transitional block before the DP stage, PTEN over-expression did not affect DP cell survival [35]. The inability to detect Akt phosphorylation in normal DP cells along with their high expression of PTEN further suggests that Akt activity is dispensable in DP thymocyte survival [35]. Progression from DP to SP thymocytes requires thymocytes to undergo positive selection, a process that evaluates the ability of newly generated TCRs to appropriately interact with self-peptide MHC complexes, and avoid negative selection of potentially auto-reactive TCRs. While little is known about the role of endogenous Akt activity during selection of DP thymocytes, constitutive expression of active Akt1 enhances positive selection of CD4 T cell through increased MAPK and Lck activity [36]. Further analyses to determine whether Akt contributes in part to DP to SP thymocyte maturation would require conditional ablation of Akt isoforms in DP thymocytes since use of PI3K or PTEN genetic models are complicated by the important contributions of Tec kinases, which are also activated by PI3K and antagonized by PTEN activity (reviewed in [37]).
REGULATION OF EFFECTOR AND MEMORY CD8 T CELL DIFFERENTIATION BY AKT
After successful development in the thymus, naïve CD8 T cells primed by mature antigen presenting cells undergo activation and clonal expansion and give rise to short-lived effector cells (SLECs) and memory precursor effector cells (MPECs) in the periphery [38]. SLECs provide immediate protection against pathogens but are prone to apoptosis following antigen clearance. MPECs serve as the primary progenitors of long-lived memory cells that produce enhanced secondary responses upon antigen re-encounter. The development of SLECs and MPECs is controlled by integrating signals received through the TCR and various co-receptors and cytokine receptors. Many of these receptors differentially activate Akt kinases to influence the fate decisions made by cytotoxic CD8 T lymphocytes (CTLs) [39].
In contrast to early thymocytes in which Akt promotes global proliferation, survival, or glucose uptake, the major role of Akt in peripheral CD8 T cells is promoting terminal differentiation of activated T cells into SLECs at the expense of memory T cells [40]. These effects are largely due to Akt regulation of two key families of transcription factors: T-box transcription factors and forkhead transcription factors. How Akt regulates these transcription factors in CD8 T cells is described in more detail below.
The T-box transcription factors T-bet and Eomesodermin (Eomes) were initially reported to be interchangeable in CD8 T cells [41]. However, genetic replacement of T-bet with Eomes demonstrated that T-bet is uniquely required for SLEC development [42]. Expression of constitutively active Akt in CD8 T cells increases the ratio of T-bet to Eomes and favors CD8 effector function and terminal differentiation [43]. Akt increases T-bet activity in part by inhibiting the TSC complex to allow Rheb-dependent mTORC1 activation [44]. mTOR kinase increases the T-bet to Eomes ratio and in turn promotes the effector over memory cell fate in activated CD8 T cells [45]. mTOR kinase mainly acts through the mTORC1 complex to regulate CD8 T cell differentiation and loss of mTORC1 activity results in higher proportion of memory precursor CD8 T cells [46]. mTORC1 also independently favors CD8 effector activity by activating ribosomal protein S6 kinase (S6K) and inhibiting eukaryotic translation initiation factor 4E–binding protein (4E-BP) to promote anabolic metabolism to generate lipids, proteins and nucleic acids [47]. Upon activation, CD8 T cells undergo asymmetric division including differential inheritance of mTORC1 activity where the daughter cell proximal to the APC has higher mTORC1 activity and is more glycolytic while the distal daughter cell has lower mTORC1 activity, is less glycolytic, and gives rise to memory CD8 T cells [48].
Akt also skews CD8 responses towards effector T cell differentiation and function by inhibiting the forkhead transcription factors Foxo1 and Foxo3. Phosphorylation of Foxo proteins by Akt triggers Foxo association with 14-3-3 adaptor proteins resulting in their cytoplasmic retention (reviewed in [49]). Foxo1 positively regulates genes required for memory CD8 T cell differentiation, survival, lymphatic trafficking and homeostasis such as TCF1, IL-7R, CCR7, KLF2 and CD62L [50–53] and represses genes important for effector cell differentiation and activity such as Tbet [54] and GzmB [53]. Consequently, Akt activity diminishes the expression of target genes important for memory T cell function and increases genes important for effector T cells. Overexpression of IL-7R is sufficient to rescue MPEC differentiation in CD8 T cells expressing constitutively active Akt [43]. In contrast, expression of an Akt-insensitive Foxo3 is sufficient to abrogate increased IFNg expression in antigen stimulated CD8 T cells that have high Akt activity [40]. Together, these observations demonstrate that Akt-dependent inhibition of Foxo prevents memory T cell differentiation, in part, by preventing IL-7R expression while it simultaneously promotes expression of CD8 T cell effector proteins.
Foxo1 also promotes CD8 memory T cell differentiation and persistence by regulating the expression of TCF1 (Tcf7) [53,55]. TCF1 expression levels vary among memory T cell subsets with high TCF1 observed in long-lived central memory T cells [56]. Foxo1 deficiency results in reduced expression of TCF1 [52], which is required for the optimal generation of central memory T cells [57]. In contrast, effector memory T cells, which traffic through peripheral tissues and quickly differentiate into effector cells upon re-activation, express low to intermediate levels of TCF1 [56]. Enforced Foxo1 expression in CD8 memory cells stabilizes TCF1 expression by preventing PRC2-dependent H2K27me3 silencing marks at the Tcf7 locus [58]. Memory T cell reactivation and expansion during recall responses is also Foxo1-dependent [52,53,55], indicating that Foxo1 activity not only directs the differentiation of memory CD8 T cells, but its continued activity maintains memory T cell identity, longevity and re-activation potential [59–61]. Thus, Akt-inhibition of Foxo1 activity has the potential to impact CD8 memory T cell formation and function at multiple stages of the T cell response. Accordingly, complete loss of Akt activity due to Akt1 and Akt2 deficiency increases central memory T cell differentiation as well as the proliferative capacity of CD8 T cells even following repeat stimulations [62]. However, disrupting PI3K-dependent Akt phosphorylation at Thr308 through expression of a mutant PDK1 hinders the survival of effector T cells as they transition from effector to effector memory T cells [63], indicating that modest levels of Akt activity are required for effector memory T cell differentiation. In contrast, constitutive Akt activity drastically lowers the proportion of MPECs and memory cells, but subsequent pharmacological inhibition of Akt can selectively rescue effector memory cells in vivo [43]. Collectively, these studies reveal the importance of Akt in regulating multiple distinct phases of CD8 effector and memory responses through the control of Tbet, Eomes and Foxo transcription factors whose gene targets promote cell survival, expression of cytokines and cytolytic enzymes and effector or memory T cell differentiation.
REGULATION OF DIFFERENTIATION OF TH1, TH2, TH17 AND TFH CELLS BY AKT
CD4 T helper 1 (Th1), Th2 and Th17 cells regulate defense against intracellular pathogens, parasites and extracellular pathogens, respectively [64] while T follicular helper cells (Tfh) are specialized in helping B cells undergo immunoglobulin affinity maturation, class switch recombination and differentiation into memory B cells within germinal centers (GC) [65]. The differentiation of naïve CD4 T cells into these T helper subsets is controlled by environmental cues. Specific cytokines trigger distinct signaling pathways to activate lineage-specific transcription factors including Tbet, Gata3, RORγt and Bcl6 to promote Th1, Th2, Th17 and Tfh differentiation, respectively, and is influenced by TCR-induced PI3K and Akt pathways [66–68]. Akt activity promotes Th1, Th17 and Tfh lineages through indirect regulation of Tbet, RORγt and Bcl6 expression but has limited effects on Th2 differentiation.
The ability of Akt to influence CD4 differentiation was first reported in Akt overexpression studies, which showed that Akt promoted IFNg expression in Th1 cells but did not increase Th2 cell specific genes [69]. Akt promotes expression of T-bet via mTORC1 [70]. mTORC1 activity leads to phosphorylation of T-bet at 4 residues that, when partially disrupted, decreases T-bet dependent permissive epigenetic regulation of the IFNg locus and lowers IFNg production [71]. While mTORC1 is a downstream effector of Akt, mTORC2 lies upstream and is responsible for phosphorylating Akt at Serine 473 for full catalytic activity [11]. Genetic ablation of Rictor disrupts mTORC2 and Akt activation, resulting in a defect in both Th1 and Th2 cell differentiation [72]. However, expression of constitutively active Akt only rescues Th1 differentiation [72] suggesting that Rictor/mTORC2-dependent Akt activation is critical for Th1 differentiation. Direct comparison of models that disrupt Rictor (mTORC2) or Rheb (mTORC1) demonstrated that mTORC1 is proximally required for inducing Tbet and RORγt for Th1 and Th17 cell differentiation, respectively [70]. In contrast, disruption of mTORC2 behaves like an mTOR deficient model and demonstrates the importance of mTORC2 in separately promoting Th2 differentiation and in fully activating Akt for Th1 and Th17 differentiation [70,72,73].
Akt regulates Th17 cell differentiation in multiple ways. Akt-induced mTORC1 activation induces transcription factors important for Th17 differentiation and function, HIF1a and RORγt, and inhibits expression of Gfi1, a transcriptional suppressor of Th17 gene targets [74]. mTORC1 promotes HIF1a expression [75], which in turn induces RORγt expression [76]. mTORC1 dependent S6K1 kinase activity is required to inhibit Gfi1 expression while mTORC1 dependent S6K2 kinase binds to RORγ to facilitate nuclear translocation [77]. Together, HIF1a and RORγ promote transcription of Th17 cell specific genes including IL-17 [76] and various glycolytic proteins to help establish Th17 cell identity [75]. Th17 and T regulatory (Treg) cells share common pathways important for their differentiation; however, key signals that favor one fate inhibit the other. Akt is a proximal signal that favors differentiation of Th17 cells at the expense of Treg cells. Casein Kinase 2 (CK2) is a positive regulator of Akt signaling that is important for Th17 differentiation [78,79]. Treatment with CX4945 a pharmacological CK2 inhibitor decreases Akt phosphorylation at both T308 and S473 and favors Treg over Th17 cell differentiation [80,81]. T cells deficient in the CK2 catalytic subunit CK2α have reduced Akt-dependent Foxo1 phosphorylation and consequently higher expression Foxp3 [82]. Moreover, Foxo1 knockdown rescues IL-17A expression and inhibits Foxp3 expression in CK2α-deficient T cells [82]. Thus, CK2 acts to increase Akt phosphorylation of Foxo1 to sway CD4 differentiation towards Th17 cells and away from Tregs.
Tfh differentiation and activity is regulated by graded Akt activity. In comparison to Th1 cells, Tfh cells exhibit decreased Akt T308 and S473 phosphorylation and downstream mTORC1 activity [83]. Further increasing Akt or mTORC1 activity either through IL-2 stimulation or expression of constitutively active Akt favors Th1 differentiation at the expense of Tfh cells in the LCMV viral infection model [83]. Yet, Raptor- (mTORC1), Rictor- (mTORC2) or mTOR-deficient mice exhibit to varying degrees reduced basal and antigen-induced Tfh cells, GC B cell populations and antibody responses, indicating a Tfh requirement for both Akt-dependent mTORC1 and Akt-activating mTORC2 activity [84]. mTORC1 and mTORC2 participate at distinct stages during Tfh differentiation with mTORC1 promoting CD4 T cell proliferation and glycolysis and mTORC2 acting in part through phosphorylation of Akt at S473 [84,85]. Akt activity promotes Tfh development through inhibition of Foxo1, which negatively regulates Bcl6 expression [86]. Ectopic expression of an Akt-insensitive Foxo1 reduces Tfh cell differentiation [86,87], thereby demonstrating that while too much Akt activity diverts CD4 T cells towards Th1 cells the appropriate Akt activity is required for Bcl6 induction during Tfh cell differentiation.
INHIBITION OF TREG DEVELOPMENT BY AKT
T regulatory cells are a specialized subset of CD4 T cells that express the transcription factor Foxp3 and function to maintain peripheral self-tolerance [88]. Tregs can be subdivided based on their developmental origin; natural Tregs (nTregs) develop in the thymus while induced Tregs (iTregs), differentiate from naïve CD4 T cells in peripheral lymph nodes [89]. The development and function of both nTregs and iTregs are negatively impacted by PI3K/Akt signaling.
As previously described, Akt inhibits nuclear localization of Foxo and transcription of its targets including Foxp3, a necessary transcription factor for Treg identity and function [90]. Foxo-dependent Foxp3 transcription is required during both thymic nTreg development and TCR and TGFβ-induced iTreg differentiation [91,92]. The importance of Tregs was first appreciated in studies linking Foxp3 mutations with the multi-organ autoimmune inflammatory disease observed in scurfy mice and IPEX patients [93–95]. Mice with Treg-specific Foxo1 deficiency phenocopy scurfy mice, highlighting the pivotal role of Foxo1 in Treg function [96]. Surprisingly, Foxo1-deficient Tregs are increased in proportion compared to conventional WT CD4 T cells and retain their ability to suppress T cell proliferation in vitro [96]. However, Foxo1-deficient Tregs are defective in preventing experimental colitis mediated by the transfer of conventional T cells into Rag-deficient mice [96]. This defect can be attributed to aberrant IFNg expression in Foxo1-deficient Tregs since secondary deletion of IFNg rescues Treg-dependent colitis prevention [96]. In contrast, deficiency in both Foxo1 and Foxo3 results in a profound Treg defect including reduced Treg proportions and numbers and an inability to suppress conventional T cells in vitro [91]. This indicates that Foxo transcription factors have partially redundant functions but are collectively required for Treg development and function.
Ectopic expression of constitutively active Akt reduces Foxp3 expression, nTreg development and iTreg differentiation [97]. In contrast, treatment of activated CD4 T cells with Akt or PI3K inhibitors results in higher proportions and levels of Foxp3 expression [98]. Moreover, treatment with PI3K/mTOR inhibitors induces a Treg-like transcriptional profile marked by upregulation of Ctla4, Foxp3 and down regulation of Il2 and Ifng [98]. An Akt insensitive variant of Foxo3a promotes Foxp3 expression in stimulated CD4 T cells in the presence of TGF-β and increases the percentage of Foxp3+ cells [92,99]. In vivo, PTEN expression in Tregs is responsible for physiologically countering PI3K/Akt to maintain Treg stability and suppressive activity [100,101]. Additional studies identified an important upstream requirement for Roquin proteins in maintaining Treg identity through inhibition of the Akt/Foxo axis by regulating PTEN expression and preventing Foxo degradation by Itch [101]. Together, these studies highlight the importance of Akt-mediated regulation of Foxo proteins in the development and function of nTregs and iTregs.
Akt isoforms may have distinct roles in Treg development and function. Akt1 appears to be the predominant isoform that limits Treg function in the setting of autoimmunity. Genetic ablation of Akt1 improves suppression of T cell-mediated CNS disease in an experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis [102]. In contrast, Akt2 deficiency results in defective Treg suppressive activity and EAE exacerbation [102], indicating that Akt1 and Akt2 isoforms may act in opposition in this context. Unlike mouse Tregs, human Tregs can gain effector activity such as production of the Th1 cytokine IFNg [103]. The generation of Th1-Treg cells requires Akt1-dependent Foxo regulation [104]. Akt1 deficiency allows Th1-Tregs to regain suppressive capacity; however, Akt3 deficiency has the opposite effect [104]. This suggests that while Akt1 checks Treg suppressive activity in both mouse and human, Treg activity is enhanced by Akt2 in mouse and Akt3 in human. One contradictory study, however, identified Akt2 and not Akt1 as the isoform that limits Foxo-dependent Foxp3 induction during iTreg differentiation [105]. Whether or not Akt isoforms are differentially engaged during nTreg and iTreg development or whether distinct Akt isoforms result in a different magnitude or kinetics of downstream signaling remains unresolved.
However, it has recently been suggested that low levels of Akt activity are important for Treg development. Weak TCR signaling that results in phosphorylation of Akt at T308 and to a much lower extent at S473 can promote commitment to the Treg lineage over conventional CD4 subsets [106–108]. Ex vivo stimulated human Tregs show Akt phosphorylation predominantly at T308 [109]. Increasing Akt activity through expression of constitutively activate Akt in human Treg cells causes them to lose their suppressive capacity and instead produce effector cytokines TNFα and IFNg [109]. Proteomic analyses of Akt substrates using mass spectrometry revealed differential target phosphorylation in response to weak vs strong TCR signaling [108]. Following weak TCR signaling, Akt phosphorylates heterogeneous nuclear ribonucleoproteins hnRNP L and hnRNP A1 [108]. Knockdown of both of these ribonucleoproteins diminishes Treg proportions [108]. Akt also differentially regulates metabolites to control Treg fate [110]. In response to weak TCR signaling, Akt selectively phosphorylates and inhibits Citrate synthase (CS), a component of the TCA cycle to promote higher proportions of Foxp3+ Tregs [110]. Further analysis revealed that inhibition of CS allows its substrate acetyl-CoA to be used for H3K27 acetylation at the Foxp3 promoter in CD4 T cells [110]. Thus, Akt activity induced by weak TCR signaling can favor differential targets through select phosphorylation of Akt T308 while additional phosphorylation at S473 in response to strong TCR signaling promotes alternative CD4 T cell fates. Further increasing our understanding of the receptors that control the kinetics and magnitude of Akt activity in naïve T cells and how their engagement results in phosphorylation of distinct subsets of Akt substrates to control T cell differentiation will be important to reveal additional mechanistic insight on the regulation of T cell responses.
PERSPECTIVE
Altogether, it has become apparent that the requirements for Akt activity in T cells depends on its maturation stage, its lineage and its environmental context (Figure 2). The dominant effects of Akt kinase activity on T cells make Akt an attractive target to manipulate in T cell-based immunotherapies. In particular, the importance of generating antitumor CD8 memory T cells has recently been recognized as an important goal for adoptive cell transfer therapy to provide durable protection against tumor recurrence. The ability of Akt to limit memory CD8 T cell formation suggests that pharmacological treatment of CD8 T cells prior to therapy may increase memory T cell differentiation. Preclinical studies using a melanoma mouse model support this strategy, demonstrating that CTLs treated with an Akt inhibitor provided better tumor control and survival [111]. Similarly, pharmacological inhibition of Akt reprogrammed tumor-infiltrating lymphocytes (TILs) isolated from cancer patients to adopt a memory transcriptional and metabolic profile, which improved their longevity when adoptively transferred into NOD scid gamma (NSG) mice [111]. Moreover, CAR T cells generated in the presence of Akt inhibition provided better tumor control and survival when adoptively transferred into mice [112,113]. Similarly, CD8 T cells treated with a variety of Akt inhibitors had a similar transcriptional profile to stem cell memory T cells, high expansion capacity and higher polyfunctionality upon antigen recall [114]. Preventing Akt regulation of Foxo1 may allow TCF1 expression and intratumoral accumulation of stem-like CD8 T cells that are responsive to PD-1 blockade. Thus, combining Akt inhibition with PD-1/PDL-1 blockade may further enhance antitumor responses [115–117]. Several Akt inhibitors are already in clinical trials to inhibit cancer cell survival and proliferation (reviewed in [118]), but here we propose that Akt inhibition combined with cell-based therapies will equip the immune system for better tumor control. However, the complexity of upstream signals that activate Akt coupled with our limited understanding of Akt’s numerous targets highlight the importance for further investigation into the temporal and spatial control of Akt in different T cell subsets to guide the design of personalized therapies.
FUNDING
This work was supported by the National Institutes of Health, grant numbers R01AI089805 to YHH, T32-AI007363 to LBH and the Burroughs Wellcome Fund, Big Data in the Life Sciences Training Program to LA.
ABBREVIATIONS
- CCR7
C-C chemokine receptor 7
- CS
Citrate synthase
- CD62L
L-Selectin
- CK2
Casein Kinase 2
- CTLs
Cytotoxic CD8 T lymphocytes
- DN
double negative (CD4−CD8−)
- DP
double positive (CD4+CD8+)
- Eomes
Eomesodermin
- GC
germinal center
- IFNg
Interferon gamma
- IL-7R
Interleukin-7 receptor
- iTregs
induced Tregs
- KLF2
Kruppel-like factor 2
- MPECs
memory precursor effector cells
- mTORC
mammalian/mechanistic target of rapamycin complex
- nTregs
natural Tregs
- PDK1
Phosphoinositide-dependent protein kinase-1
- PH
pleckstrin homology
- PI3K
Class I phosphatidylinositol 3-kinases
- PIP2
phosphatidylinositol 4,5-bisphosphate
- PIP3
phosphatidylinositol 3,4,5-trisphosphate
- preTCR
pre-T cell receptor
- PTEN
Phosphatase and tensin homolog
- SLECs
short-lived effector cells
- SP
single positive (CD4+ or CD8+)
- TCF1
T cell factor 1
- TCR
T cell receptor
- Tfh
T follicular helper
- Th
T helper
- Treg
T regulatory
Footnotes
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Hanada M, Feng J, Hemmings BA. Structure, regulation and function of PKB/AKT—a major therapeutic target. Biochim Biophys Acta. 2004;1697(1–2):3–16. [DOI] [PubMed] [Google Scholar]
- 2.Yang ZZ, Tschopp O, Hemmings-Mieszczak M, Feng J, Brodbeck D, Perentes E, et al. Protein kinase B alpha/Akt1 regulates placental development and fetal growth. J Biol Chem. 2003;278(34):32124–31. [DOI] [PubMed] [Google Scholar]
- 3.Altomare DA, Lyons GE, Mitsuuchi Y, Cheng JQ, Testa JR. Akt2 mRNA is highly expressed in embryonic brown fat and the AKT2 kinase is activated by insulin. Oncogene. 1998;16(18):2407–11. [DOI] [PubMed] [Google Scholar]
- 4.Konishi H, Kuroda S, Tanaka M, Matsuzaki H, Ono Y, Kameyama K, et al. Molecular cloning and characterization of a new member of the RAC protein kinase family: association of the pleckstrin homology domain of three types of RAC protein kinase with protein kinase C subspecies and beta gamma subunits of G proteins. Biochem Biophys Res Commun. 1995;216(2):526–34. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez E, McGraw TE. The Akt kinases: isoform specificity in metabolism and cancer. Cell Cycle. 2009;8(16):2502–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan TO, Rittenhouse SE, Tsichlis PN. AKT/PKB and Other D3 Phosphoinositide-Regulated Kinases: Kinase Activation by Phosphoinositide-Dependent Phosphorylation. Annu Rev Biochem. 1999;68(1):965–1014. [DOI] [PubMed] [Google Scholar]
- 7.Bellacosa A, Chan TO, Ahmed NN, Datta K, Malstrom S, Stokoe D, et al. Akt activation by growth factors is a multiple-step process: the role of the PH domain. Oncogene. 1998;17(3):313–25. [DOI] [PubMed] [Google Scholar]
- 8.Currie RA, Walker KS, Gray A, Deak M, Casamayor A, Downes CP, et al. Role of phosphatidylinositol 3,4,5-trisphosphate in regulating the activity and localization of 3-phosphoinositide-dependent protein kinase-1. Biochem J. 1999;337(Pt 3):575–83. [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson KE, Coadwell J, Stephens LR, Hawkins PT. Translocation of PDK-1 to the plasma membrane is important in allowing PDK-1 to activate protein kinase B. Curr Biol. 1998;8(12):684–91. [DOI] [PubMed] [Google Scholar]
- 10.Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7(4):261–9. [DOI] [PubMed] [Google Scholar]
- 11.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307(5712):1098–101. [DOI] [PubMed] [Google Scholar]
- 12.Kane LP, Weiss A. The PI-3 kinase/Akt pathway and T cell activation: pleiotropic pathways downstream of PIP3. Immunol Rev. 2003;192:7–20. [DOI] [PubMed] [Google Scholar]
- 13.Manning BD, Toker A. AKT/PKB Signaling: Navigating the Network. Cell. 2017;169(3):381–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273(22):13375–8. [DOI] [PubMed] [Google Scholar]
- 15.Gao T, Furnari F, Newton AC. PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Mol Cell. 2005;18(1):13–24. [DOI] [PubMed] [Google Scholar]
- 16.Kuo Y-C, Huang K-Y, Yang C-H, Yang Y-S, Lee W-Y, Chiang C-W. Regulation of phosphorylation of Thr-308 of Akt, cell proliferation, and survival by the B55alpha regulatory subunit targeting of the protein phosphatase 2A holoenzyme to Akt. J Biol Chem. 2008;283(4):1882–92. [DOI] [PubMed] [Google Scholar]
- 17.Germar K, Dose M, Konstantinou T, Zhang J, Wang H, Lobry C, et al. T-cell factor 1 is a gatekeeper for T-cell specification in response to Notch signaling. Proc Natl Acad Sci U S A. 2011;108(50):20060–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michie AM, Zuniga-Pflucker JC. Regulation of thymocyte differentiation: pre-TCR signals and beta-selection. Semin Immunol. 2002;14(5):311–23. [DOI] [PubMed] [Google Scholar]
- 19.Juntilla MM, Koretzky GA. Critical roles of the PI3K/Akt signaling pathway in T cell development. Immunol Lett. 2008;116(2):104–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fayard E, Moncayo G, Hemmings BA, Holländer GA. Phosphatidylinositol 3- kinase signaling in thymocytes: the need for stringent control. Sci Signal. 2010;3(135):re5. [DOI] [PubMed] [Google Scholar]
- 21.Carpenter AC, Bosselut R. Decision checkpoints in the thymus. Nat Immunol. 2010;11(8):666–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mao C, Tili EG, Dose M, Haks MC, Bear SE, Maroulakou I, et al. Unequal contribution of Akt isoforms in the double-negative to double-positive thymocyte transition. J Immunol. 2007;178(9):5443–53. [DOI] [PubMed] [Google Scholar]
- 23.Juntilla MM, Wofford JA, Birnbaum MJ, Rathmell JC, Koretzky GA. Akt1 and Akt2 are required for alphabeta thymocyte survival and differentiation. Proc Natl Acad Sci U S A. 2007;104(29):12105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen WS, Xu PZ, Gottlob K, Chen ML, Sokol K, Shiyanova T, et al. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 2001;15(17):2203–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fayard E, Gill J, Paolino M, Hynx D, Holländer GA, Hemmings BA, et al. Deletion of PKBalpha/Akt1 affects thymic development. PLoS One. 2007;2(10):e992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones RG, Parsons M, Bonnard M, Chan VS, Yeh WC, Woodgett JR, et al. Protein kinase B regulates T lymphocyte survival, nuclear factor kappaB activation, and Bcl-X(L) levels in vivo. J Exp Med. 2000;191(10):1721–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mandal M, Crusio KM, Meng F, Liu S, Kinsella M, Clark MR, et al. Regulation of lymphocyte progenitor survival by the proapoptotic activities of Bim and Bid. Proc Natl Acad Sci U S A. 2008;105(52):20840–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahamed JA, Madhivadhani P. Costimulatory role of CXCR4 with pre-TCR and its crosstalk with PI3K in beta-selection of thymocytes. Sci Signal. 2010;3(119):jc4. [DOI] [PubMed] [Google Scholar]
- 29.Janas ML, Varano G, Gudmundsson K, Noda M, Nagasawa T, Turner M. Thymic development beyond beta-selection requires phosphatidylinositol 3- kinase activation by CXCR4. J Exp Med. 2010;207(1):247–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Westernberg L, Conche C, Huang YH, Rigaud S, Deng Y, Siegemund S, et al. Non-canonical antagonism of PI3K by the kinase Itpkb delays thymocyte beta-selection and renders it Notch-dependent. Elife. 2016;5:e10786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ciofani M, Zuniga-Pflucker JC. Notch promotes survival of pre-T cells at the beta-selection checkpoint by regulating cellular metabolism. Nat Immunol. 2005;6(9):881–8. [DOI] [PubMed] [Google Scholar]
- 32.Ciofani M, Schmitt TM, Ciofani A, Michie AM, Cuburu N, Aublin A, et al. Obligatory role for cooperative signaling by pre-TCR and Notch during thymocyte differentiation. J Immunol. 2004;172(9):5230–9. [DOI] [PubMed] [Google Scholar]
- 33.Trampont PC, Tosello-Trampont A-C, Shen Y, Duley AK, Sutherland AE, Bender TP, et al. CXCR4 acts as a costimulator during thymic beta-selection. Nat Immunol. 2010;11(2):162–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong GW, Knowles GC, Mak TW, Ferrando AA, Zúñiga-Pflücker JC. HES1 opposes a PTEN-dependent check on survival, differentiation, and proliferation of TCRbeta-selected mouse thymocytes. Blood. 2012;120(7):1439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xue L, Chiang L, Kang C, Winoto A. The role of the PI3K-AKT kinase pathway in T-cell development beyond the beta checkpoint. Eur J Immunol. 2008;38(11):3200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Na S-Y, Patra A, Scheuring Y, Marx A, Tolaini M, Kioussis D, et al. Constitutively active protein kinase B enhances Lck and Erk activities and influences thymocyte selection and activation. J Immunol. 2003;171(3):1285–96. [DOI] [PubMed] [Google Scholar]
- 37.Berg LJ, Finkelstein LD, Lucas JA, Schwartzberg PL. TEC FAMILY KINASES IN T LYMPHOCYTE DEVELOPMENT AND FUNCTION. Annu Rev Immunol. 2005;23(1):549–600. [DOI] [PubMed] [Google Scholar]
- 38.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27(2):281–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim EH, Suresh M. Role of PI3K/Akt signaling in memory CD8 T cell differentiation. Front Immunol. 2013;4:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Macintyre AN, Finlay D, Preston G, Sinclair LV, Waugh CM, Tamas P, et al. Protein kinase B controls transcriptional programs that direct cytotoxic T cell fate but is dispensable for T cell metabolism. Immunity. 2011;34(2):224–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302(5647):1041–3. [DOI] [PubMed] [Google Scholar]
- 42.Fixemer J, Hummel JF, Arnold F, Klose CSN, Hofherr A, Weissert K, et al. Eomes cannot replace its paralog T-bet during expansion and differentiation of CD8 effector T cells. PLoS Pathog. 2020;16(9):e1008870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim EH, Sullivan JA, Plisch EH, Tejera MM, Jatzek A, Choi KY, et al. Signal integration by Akt regulates CD8 T cell effector and memory differentiation. J Immunol. 2012;188(9):4305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pollizzi KN, Powell JD. Regulation of T cells by mTOR: the known knowns and the known unknowns. Trends Immunol. 2015;36(1):13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 2010;32(1):67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460(7251):108–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.MacIver NJ, Michalek RD, Rathmell JC. Metabolic regulation of T lymphocytes. Annu Rev Immunol. 2013;31:259–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pollizzi KN, Sun I-H, Patel CH, Lo Y-C, Oh M-H, Waickman AT, et al. Asymmetric inheritance of mTORC1 kinase activity during division dictates CD8(+) T cell differentiation. Nat Immunol. 2016;17(6):704–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tzivion G, Dobson M, Ramakrishnan G. FoxO transcription factors; Regulation by AKT and 14-3-3 proteins. Biochim Biophys Acta. 2011;1813(11):1938–45. [DOI] [PubMed] [Google Scholar]
- 50.Kerdiles YM, Beisner DR, Tinoco R, Dejean AS, Castrillon DH, DePinho RA, et al. Foxo1 links homing and survival of naive T cells by regulating L-selectin, CCR7 and interleukin 7 receptor. Nat Immunol. 2009;10(2):176–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ouyang W, Beckett O, Flavell RA, Li MO. An essential role of the Forkhead-box transcription factor Foxo1 in control of T cell homeostasis and tolerance. Immunity. 2009;30(3):358–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim MV, Ouyang W, Liao W, Zhang MQ, Li MO. The transcription factor Foxo1 controls central-memory CD8+ T cell responses to infection. Immunity. 2013;39(2):286–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tejera MM, Kim EH, Sullivan JA, Plisch EH, Suresh M. FoxO1 controls effector-to-memory transition and maintenance of functional CD8 T cell memory. J Immunol. 2013;191(1):187–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rao RR, Li Q, Bupp MRG, Shrikant PA. Transcription factor Foxo1 represses T-bet-mediated effector functions and promotes memory CD8(+) T cell differentiation. Immunity. 2012;36(3):374–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hess Michelini R, Doedens AL, Goldrath AW, Hedrick SM. Differentiation of CD8 memory T cells depends on Foxo1. J Exp Med. 2013;210(6):1189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kratchmarov R, Magun AM, Reiner SL. TCF1 expression marks self-renewing human CD8(+) T cells. Blood Adv. 2018;2(14):1685–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou X, Yu S, Zhao D-M, Harty JT, Badovinac VP, Xue H-H. Differentiation and persistence of memory CD8(+) T cells depend on T cell factor 1. Immunity. 2010;33(2):229–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gray SM, Amezquita RA, Guan T, Kleinstein SH, Kaech SM. Polycomb Repressive Complex 2-Mediated Chromatin Repression Guides Effector CD8(+) T Cell Terminal Differentiation and Loss of Multipotency. Immunity. 2017;46(4):596–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Utzschneider DT, Delpoux A, Wieland D, Huang X, Lai C-Y, Hofmann M, et al. Active Maintenance of T Cell Memory in Acute and Chronic Viral Infection Depends on Continuous Expression of FOXO1. Cell Rep. 2018;22(13):3454–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Delpoux A, Michelini RH, Verma S, Lai CY, Omilusik KD, Utzschneider DT, et al. Continuous activity of Foxo1 is required to prevent anergy and maintain the memory state of CD8(+) T cells. J Exp Med. 2018;215(2):575–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Delpoux A, Lai C-Y, Hedrick SM, Doedens AL. FOXO1 opposition of CD8(+) T cell effector programming confers early memory properties and phenotypic diversity. Proc Natl Acad Sci U S A. 2017;114(42):E8865–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abu Eid R, Friedman KM, Mkrtichyan M, Walens A, King W, Janik J, et al. Akt1 and −2 inhibition diminishes terminal differentiation and enhances central memory CD8(+) T-cell proliferation and survival. Oncoimmunology. 2015;4(5):e1005448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rogel A, Willoughby JE, Buchan SL, Leonard HJ, Thirdborough SM, Al-Shamkhani A, et al. Akt signaling is critical for memory CD8(+) T-cell development and tumor immune surveillance. Proc Natl Acad Sci U S A. 2017;114(7):E1178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luckheeram RV, Zhou R, Verma AD, Xia B. CD4(+)T cells: differentiation and functions. Clin Dev Immunol. 2012;2012:925135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Crotty S T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41(4):529–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhu J, Paul WE. Peripheral CD4+ T-cell differentiation regulated by networks of cytokines and transcription factors. Immunol Rev. 2010;238(1):247–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Han JM, Patterson SJ, Levings MK. The Role of the PI3K Signaling Pathway in CD4(+) T Cell Differentiation and Function. Front Immunol. 2012;3:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Preite S, Huang B, Cannons JL, McGavern DB, Schwartzberg PL. PI3K Orchestrates T Follicular Helper Cell Differentiation in a Context Dependent Manner: Implications for Autoimmunity. Front Immunol. 2018;9:3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kane LP, Andres PG, Howland KC, Abbas AK, Weiss A. Akt provides the CD28 costimulatory signal for up-regulation of IL-2 and IFN-gamma but not TH2 cytokines. Nat Immunol. 2001;2(1):37–44. [DOI] [PubMed] [Google Scholar]
- 70.Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12(4):295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chornoguz O, Hagan RS, Haile A, Arwood ML, Gamper CJ, Banerjee A, et al. mTORC1 Promotes T-bet Phosphorylation To Regulate Th1 Differentiation. J Immunol. 2017;198(10):3939–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee K, Gudapati P, Dragovic S, Spencer C, Joyce S, Killeen N, et al. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity. 2010;32(6):743–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, et al. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30(6):832–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ichiyama K, Hashimoto M, Sekiya T, Nakagawa R, Wakabayashi Y, Sugiyama Y, et al. Gfi1 negatively regulates T(h)17 differentiation by inhibiting RORgammat activity. Int Immunol. 2009;21(7):881–9. [DOI] [PubMed] [Google Scholar]
- 75.Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, et al. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. 2011;208(7):1367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dang EV, Barbi J, Yang H-Y, Jinasena D, Yu H, Zheng Y, et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146(5):772–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kurebayashi Y, Nagai S, Ikejiri A, Ohtani M, Ichiyama K, Baba Y, et al. PI3K-Akt-mTORC1-S6K1/2 axis controls Th17 differentiation by regulating Gfi1 expression and nuclear translocation of RORgamma. Cell Rep. 2012;1(4):360–73. [DOI] [PubMed] [Google Scholar]
- 78.Di Maira G, Salvi M, Arrigoni G, Marin O, Sarno S, Brustolon F, et al. Protein kinase CK2 phosphorylates and upregulates Akt/PKB. Cell Death Differ. 2005;12(6):668–77. [DOI] [PubMed] [Google Scholar]
- 79.Silva A, Yunes JA, Cardoso BA, Martins LR, Jotta PY, Abecasis M, et al. PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability. J Clin Invest. 2008;118(11):3762–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jang SW, Hwang SS, Kim HS, Lee KO, Kim MK, Lee W, et al. Casein kinase 2 is a critical determinant of the balance of Th17 and Treg cell differentiation. Exp Mol Med. 2017;49(9):e375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gibson SA, Yang W, Yan Z, Liu Y, Rowse AL, Weinmann AS, et al. Protein Kinase CK2 Controls the Fate between Th17 Cell and Regulatory T Cell Differentiation. J Immunol. 2017;198(11):4244–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gibson SA, Yang W, Yan Z, Qin H, Benveniste EN. CK2 Controls Th17 and Regulatory T Cell Differentiation Through Inhibition of FoxO1. J Immunol. 2018;201(2):383–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ray JP, Staron MM, Shyer JA, Ho P-C, Marshall HD, Gray SM, et al. The Interleukin-2-mTORC1 Kinase Axis Defines the Signaling, Differentiation, and Metabolism of T Helper 1 and Follicular B Helper T Cells. Immunity. 2015;43(4):690–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang J, Lin X, Pan Y, Wang J, Chen P, Huang H, et al. Critical roles of mTOR Complex 1 and 2 for T follicular helper cell differentiation and germinal center responses. Elife. 2016;5:e17936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zeng H, Cohen S, Guy C, Shrestha S, Neale G, Brown SA, et al. mTORC1 and mTORC2 Kinase Signaling and Glucose Metabolism Drive Follicular Helper T Cell Differentiation. Immunity. 2016;45(3):540–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stone EL, Pepper M, Katayama CD, Kerdiles YM, Lai C-Y, Emslie E, et al. ICOS coreceptor signaling inactivates the transcription factor FOXO1 to promote Tfh cell differentiation. Immunity. 2015;42(2):239–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Preite S, Cannons JL, Radtke AJ, Vujkovic-Cvijin I, Gomez-Rodriguez J, Volpi S, et al. Hyperactivated PI3Kdelta promotes self and commensal reactivity at the expense of optimal humoral immunity. Nat Immunol. 2018;19(9):986–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fontenot JD, Gavin MA, Rudensky AY, Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330–6. [DOI] [PubMed] [Google Scholar]
- 89.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30(5):626–35. [DOI] [PubMed] [Google Scholar]
- 90.Merkenschlager M, von Boehmer H. PI3 kinase signalling blocks Foxp3 expression by sequestering Foxo factors. J Exp Med. 2010;207(7):1347–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ouyang W, Beckett O, Ma Q, Paik J-h, DePinho RA, Li MO, et al. Foxo proteins cooperatively control the differentiation of Foxp3+ regulatory T cells. Nat Immunol. 2010;11(7):618–27. [DOI] [PubMed] [Google Scholar]
- 92.Harada Y, Harada Y, Elly C, Ying G, Paik J-h, DePinho RA, et al. Transcription factors Foxo3a and Foxo1 couple the E3 ligase Cbl-b to the induction of Foxp3 expression in induced regulatory T cells. J Exp Med. 2010;207(7):1381–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27(1):20–1. [DOI] [PubMed] [Google Scholar]
- 94.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27(1):18–20. [DOI] [PubMed] [Google Scholar]
- 95.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27(1):68–73. [DOI] [PubMed] [Google Scholar]
- 96.Ouyang W, Liao W, Luo CT, Yin N, Huse M, Kim MV, et al. Novel Foxo1-dependent transcriptional programs control T(reg) cell function. Nature. 2012;491(7425):554–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205(3):565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci U S A. 2008;105(22):7797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ma J, Ding Y, Fang X, Wang R, Sun Z. Protein kinase C-theta inhibits inducible regulatory T cell differentiation via an AKT-Foxo1/3a-dependent pathway. J Immunol. 2012;188(11):5337–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shrestha S, Yang K, Guy C, Vogel P, Neale G, Chi H, et al. Treg cells require the phosphatase PTEN to restrain TH1 and TFH cell responses. Nat Immunol. 2015;16(2):178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Essig K, Hu D, Guimaraes JC, Alterauge D, Edelmann S, Raj T, et al. Roquin Suppresses the PI3K-mTOR Signaling Pathway to Inhibit T Helper Cell Differentiation and Conversion of Treg to Tfr Cells. Immunity. 2017;47(6):1067–82.e12. [DOI] [PubMed] [Google Scholar]
- 102.Ouyang S, Zeng Q, Tang N, Guo H, Tang R, Yin W, Wang A, et al. Akt-1 and Akt2 Differentially Regulate the Development of Experimental Autoimmune Encephalomyelitis by Controlling Proliferation of Thymus-Derived Regulatory T Cells. J Immunol. 2019;202(5):1441–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dominguez-Villar M, Baecher-Allan CM, Hafler DA. Identification of T helper type 1-like, Foxp3+ regulatory T cells in human autoimmune disease. Nat Med. 2011;17(6):673–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kitz A, de Marcken M, Gautron AS, Mitrovic M, Hafler DA, Dominguez-Villar M. AKT isoforms modulate Th1-like Treg generation and function in human autoimmune disease. EMBO Rep. 2016;17(8):1169–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Qiao G, Zhao Y, Li Z, Tang PQ, Langdon WY, Yang T, et al. T cell activation threshold regulated by E3 ubiquitin ligase Cbl-b determines fate of inducible regulatory T cells. J Immunol. 2013;191(2):632–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Turner MS, Kane LP, Morel PA. Dominant role of antigen dose in CD4+Foxp3+ regulatory T cell induction and expansion. J Immunol. 2009;183(8):4895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Etemire E, Krull M, Hasenberg M, Reichardt P, Gunzer M. Transiently reduced PI3K/Akt activity drives the development of regulatory function in antigen-stimulated Naive T-cells. PLoS One. 2013;8(7):e68378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hawse WF, Boggess WC, Morel PA. TCR Signal Strength Regulates Akt Substrate Specificity To Induce Alternate Murine Th and T Regulatory Cell Differentiation Programs. J Immunol. 2017;199(2):589–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Crellin NK, Garcia RV, Levings MK. Altered activation of AKT is required for the suppressive function of human CD4+CD25+ T regulatory cells. Blood. 2007;109(5):2014–22. [DOI] [PubMed] [Google Scholar]
- 110.Hawse WF, Cattley RT, Wendell SG. Cutting Edge: TCR Signal Strength Regulates Acetyl-CoA Metabolism via AKT. J Immunol. 2019;203(11):2771–5. [DOI] [PubMed] [Google Scholar]
- 111.Crompton JG, Sukumar M, Roychoudhuri R, Clever D, Gros A, Eil RL, et al. Akt inhibition enhances expansion of potent tumor-specific lymphocytes with memory cell characteristics. Cancer Res. 2015;75(2):296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Klebanoff CA, Scott CD, Leonardi AJ, Yamamoto TN, Cruz AC, Ouyang C, et al. Inhibition of AKT signaling uncouples T cell differentiation from expansion for receptor-engineered adoptive immunotherapy. JCI Insight. 2017;2(23):e95103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang Q, Ding J, Sun S, Liu H, Lu M, Wei X, et al. Akt inhibition at the initial stage of CAR-T preparation enhances the CAR-positive expression rate, memory phenotype and in vivo efficacy. Am J Cancer Res. 2019;9(11):2379–96. [PMC free article] [PubMed] [Google Scholar]
- 114.Mousset CM, Hobo W, Ji Y, Fredrix H, De Giorgi V, Allison RD, et al. Ex vivo AKT-inhibition facilitates generation of polyfunctional stem cell memory-like CD8(+) T cells for adoptive immunotherapy. Oncoimmunology. 2018;7(10):e1488565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Miller BC, Sen DR, Al Abosy R, Bi K, Virkud YV, LaFleur MW, et al. Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat Immunol. 2019;20(3):326–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Siddiqui I, Schaeuble K, Chennupati V, Marraco SAF, Calderon-Copete S, Ferreira DP, et al. Intratumoral Tcf1(+)PD-1(+)CD8(+) T Cells with Stem-like Properties Promote Tumor Control in Response to Vaccination and Checkpoint Blockade Immunotherapy. Immunity. 2019;50(1):195–211.e10. [DOI] [PubMed] [Google Scholar]
- 117.Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016;537(7620):417–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shariati M, Meric-Bernstam F. Targeting AKT for cancer therapy. Expert Opin Investig Drugs. 2019;28(11):977–88. [DOI] [PMC free article] [PubMed] [Google Scholar]


