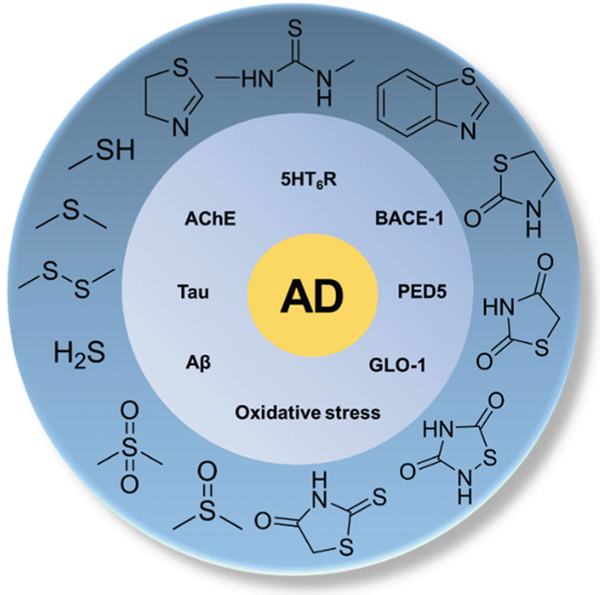
Abstract
Sulfur is widely existent in natural products and synthetic organic compounds as organosulfur, which are often associated with a multitude of biological activities. OBenzothiazole, in which benzene ring is fused to the 4,5-positions of the thiazolerganosulfur compounds continue to garner increasing amounts of attention in the field of medicinal chemistry, especially in the development of therapeutic agents for Alzheimer’s disease (AD). AD is a fatal neurodegenerative disease and the primary cause of age-related dementia posing severe societal and economic burdens. Unfortunately, there is no cure for AD. A lot of research has been conducted on sulfur-containing compounds in the context of AD due to their innate antioxidant potential and some are currently being evaluated in clinical trials. In this review, we have described emerging trends in the field, particularly the concept of multi-targeting and formulation of disease-modifying strategies. SAR, pharmacological targets, in vitro/vivo ADMET, efficacy in AD animal models, and applications in clinical trials of such sulfur compounds have also been discussed. This article provides a comprehensive review of organosulfur-based AD therapeutic agents and provides insights into their future development.
Keywords: AD, SAR, ADMET, Clinical trials
Graphical Abstract
Introduction
Sulfur-containing compounds [1] are often associated with foul odors but are widely used in various biological applications. The oxidation state of sulfur can range from −2 to +6, so sulfur-containing compounds exist in different forms [2]. Common classes of such compounds include chemical element sulfur, sulfides, thiols, disulfides, thioesters, thioketones, thioureas, sulfur-containing heterocyclic compounds with −2 oxidation state; sulfoxides and thiocarboxylic acids with +4 oxidation state; while sulfones and sulfonamides with +6 oxidation state. Sulfur is one of the core chemical elements and an elemental nutrient needed for biochemical functions in all living organisms [3]. For example, several amino acids (cysteine, cystine, and methionine) and vitamins (biotin and thiamine) are organosulfur compounds. Many bioactive sulfur-containing compounds, such as glutathione, hydrogen sulfide, and taurine also play an important role in the living organisms by maintaining cellular redox homeostasis. In addition, organosulfur compounds are also used in pharmaceuticals, dyes, and agrochemicals. For example, antibacterial sulfonamides [4] is one such well-known sulfur-containing class of drugs. Most β-lactam antibiotics [5], including penicillin, cephalosporin, and monobactams also contain sulfur.
Alzheimer’s disease (AD) is a chronic, fatal neurodegenerative disease that usually manifests slowly and gradually worsens over time [6]. It is prominently characterized by progressive cognitive dysfunction, which includes difficulty remembering, problems with language, disorientation, mood swings, loss of motivation, and other behavioral issues [7]. As dementia worsens, patients often withdraw from family and society. This cognitive dysfunction further leads to biological functions loss and, ultimately to death. More than 5 million Americans were living with AD in 2010 and the number is increasing annually, expected to reach about 15 million by 2050 [8]. It is estimated that the cost of medical care for AD will increase to $1.1 trillion by 2050 [9]. Unfortunately, despite numerous efforts and investments, there is currently no cure for this fatal disease. This is because the pathophysiology and precise molecular mechanisms of AD are still poorly understood, while advanced age is the main risk factor [10]. It is reported that 1 in 10 adults over the age of 65 has Alzheimer’s dementia. The only clinically approved drugs for AD focus on increasing neurotransmission to improve cognition, but unfortunately, these drugs only offer temporary relief from worsening dementia symptoms [11].
Over the past two decades, efforts in understanding the biology and clinical pathophysiology and therapeutic drug discovery have yielded some important insights to AD therapy [12]. AD has a complex pathophysiology that includes aggregation of pathological proteins, impaired neurotransmission, increased oxidative stress, or microglia-mediated neuroinflammation. Hundreds of candidate drugs targeting these processes are advanced to clinical trials, which include γ-secretase inhibitors, β-secretase (BACE) inhibitors, immunotherapy for amyloid beta (Aβ) clearance, Tau aggregation inhibitors, serotonin 5-hydroxytryptamine receptor 6 (5HT6R) antagonists, acetylcholinesterase (AChE) inhibitors, but with limited success [11]. Many sulfur compounds are part of these promising drug candidates targeting key enzymes involved in AD pathophysiology and have demonstrated neuroprotection in preclinical models of AD. The advantages of sulfur are multipronged. First, the importance of thiol redox homeostasis is well documented in AD onset and progression [13]. Sulfur-containing endogenous compounds such as glutathione (GSH) are considered a biomarker for AD [14]. Many thiol-containing compounds display antioxidant activity to balance the increased oxidative stress observed in AD pathology [15]. Furthermore, sulfur is incorporated as a crucial pharmacophore in the design of bioactive compounds targeted toward enzymes involved in AD pathogenesis. For example, sulfonamide is the key pharmacophore utilized in the development of BACE-1 inhibitors and 5HT6R antagonists [16]. Additionally, sulfur is a bioisostere of oxygen and widely used to improve lipophilicity and metabolism stability [17].
In this review, we focus mainly on sulfur-containing compounds as therapy options for AD. The design and structure-activity relationships, pharmacological targets, in vitro/vivo ADMET, efficacy in AD animal models, and applications in clinical trials of such sulfur compounds are described based on the chemical class. Table 1 comprehensively summarizes all the sulfur therapeutics subjected to clinical testing.
Table 1.
Sulfur-containing therapeutics in clinical trials
| Target (reference) | Drug | Trial status | Outcome | Clinical trial identifier |
|---|---|---|---|---|
| Cysteine based thiols | NAC | Phase II terminated | No benefit over placebo | NCT00903695 |
| Phase I | NA | NCT03493178 | ||
| Recruiting | ||||
| Cerefolin NAC | Observational | Unknown | NCT01370954 | |
| NAC/vitamin supplement | Phase II completed | Improved cognition and baseline performance | NCT01320527 | |
| Glutathione | GSH | Nutritional intervention | Recruiting | NCT03448055 |
| Completed; observational study | NCT01713816 | |||
| Alpha lipoic acid [84, 85] | Fish oil/APA | Phase I/Phase II | Completed; no significant benefit | NCT00090402 |
| NCT01058941 | ||||
| Lipoic acid/vitamin E/vitamin C | Phase I | No change in Tau pathology | NCT00117403 | |
| 5HT6 receptor antagonist [224] | Interpirdine (SB-742457) | Phase III | Terminated due to lack of efficacy | NCT02586909 |
| NCT02585934 | ||||
| SAM-315 | Phase II | Failed to show benefit over donepezil | NCT00710684 | |
| NCT00708552 | ||||
| NCT00348192 | ||||
| NCT00224497 | ||||
| Phase I | Completed | NCT00551772 | ||
| Phase I | Discontinued | NCT00480467 | ||
| NCT00479440 | ||||
| NCT00474552 | ||||
| Sulfonic acid | Homotaurine (Tampiprostate, ALZ-801, 3APS, Alzhemed) | Phase III | Discontinued, failed to improve cognition | NCT00088673 |
| NCT00217763 | ||||
| NCT00314912 | ||||
| Phase II completed | No significant benefit over placebo | NCT04422743 | ||
| Phase I | Completed; well tolerated in healthy subjects | NCT04157712 | ||
| NCT04585347 | ||||
| Sulfonamide, BACE-1 inhibitors [286, 305] | MK-8931 (Verubecestat) | Phase III terminated | Failed to show efficacy | NCT01953601 |
| MK-8931 (Verubecestat) [286] | ||||
| Phase II/ | Reduced Aβ levels in MCI patients, discontinued due to adverse events | NCT01739348 | ||
| Phase III | ||||
| Phase II discontinued | ||||
| Phase I | Completed; well tolerated in subjects | NCT02910739 | ||
| NCT01537757 | ||||
| NCT01496170 | ||||
| Lanabecestat (LY3314814) | Phase III | Terminated, no efficacy over placebo | NCT02783573 | |
| NCT02972658 | ||||
| Phase II/ | Terminated, no efficacy over placebo | NCT02245737 | ||
| Phase III | ||||
| Phase I | Completed; well tolerated, no interactions with other drugs | NCT02406261 | ||
| NCT02540668 | ||||
| LY2886721 | Phase I | Completed; well tolerated, lowered plasma Aβ levels in AD patients | NCT01534273 | |
| NCT01807026 | ||||
| NCT01227252 | ||||
| NCT01133405 | ||||
| PhaseI/Phase II | Phase II terminated due to hepatotoxicity | NCT01561430 | ||
| SUVN-502 | Phase IIa | Completed; no significant benefit terminated, no significant benefit | NCT02580305 | |
| SAM-760 [287] | Phase II | NCT01712074 | ||
| Phase I | Completed; well tolerated in healthy subjects and | NCT01213355 | ||
| AD patients | NCT02005991 | |||
| NCT01159496 | ||||
| NCT00948662 | ||||
| γ-Secretase inhibitor | Avagacestat [306] | Phase II | Terminated due to gastrointestinal effects | NCT00890890 |
| NCT00810147 | ||||
| Phase I | Completed; well tolerated in healthy subjects | NCT01454115 | ||
| NCT01039194 | ||||
| NCT01042314 | ||||
| NCT00901498 | ||||
| NCT01057030 | ||||
| NCT00828646 | ||||
| Begacestat | Phase I | Completed; results unknown | NCT00959881 | |
| Thiazole, glutamate receptor inhibition | Riluzole | Phase II | Completed | NCT01703117 |
| Troriluzole (BHV-4157) | Phase II/Phase III | Active, ongoing | NCT03605667 | |
| Phenothiazine, tau aggregation inhibition | TRX 0237 (LMTM) | Phase III | Completed; failed to show efficacy | NCT01689233 |
| NCT01689246 | ||||
| NCT01626378 | ||||
| NCT02245568 | ||||
| Phase III | ||||
| Phase III | Ongoing | NCT03446001 | ||
| Phase II | Terminated for administrative reasons | NCT01626391 | ||
| TDZD | Rosiglitazone, Rosiglitazone XR | Phase III | terminated; no effects on cognition or memory | NCT00550420 |
| function | NCT00348140 | |||
| NCT00490568 | ||||
| NCT00428090 | ||||
| Phase II | Completed | NCT00381238 | ||
| NCT00334568 | ||||
| NCT00242593 | ||||
| Phase I | Completed; well tolerated in healthy and AD subjects | NCT00733785 | ||
| NCT00688207 | ||||
| NCT00468897 | ||||
| Thiourea | LY2811376 | Phase I | Completed; renal toxicity and | NCT00838084 |
| neurodegeneration found | ||||
| LY3202626 | Phase II | Terminated; low likelihood of statistical significance | NCT02791191 | |
| Phase I | Completed; well tolerated in AD patients and | NCT02555449 | ||
| reduced Aβ in CSF samples | NCT02323334 | |||
| NCT03023826 | ||||
| Atabecestat (JNJ-54861911) | Phase II/ | Terminated due to changes in liver enzyme levels | NCT02569398 | |
| PhaseIII | ||||
| Phase II | Completed; liver related adverse events | NCT02260674 | ||
| Phase I | Completed; well-tolerated in healthy and AD | NCT01827982 | ||
| patients | NCT01887535 | |||
| NCT01978548 | ||||
| Elenbecestat | Phase III | Active | NCT03036280 | |
| Thiourea, BACE-1 inhibitor | PF-06751979 | Phase I | Completed; well tolerated and reduced Aβ levels in blood | NCT03126721 |
| NCT02509117 | ||||
| NCT02793232 |
Organic thiols, disulfides, and prodrug compounds
Design rationale and pharmacological target
Cysteine based thiols
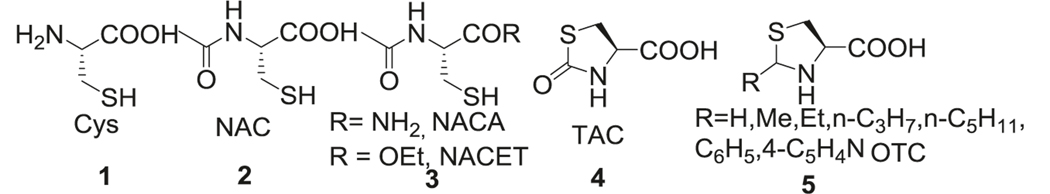
Cellular availability of Cysteine (Cys, 1) is the rate-limiting factor for the synthesis of antioxidant GSH and maintenance of cellular redox potential. Supplementation with Cys and its prodrugs have been used as GSH precursors. Among Cys based prodrugs, N-acetyl cysteine (NAC, 2) has been extensively studied. NAC reduces reactive oxygen species (ROS) and apoptosis by replenishing and maintaining GSH stores in the brain [18]. NAC also has been shown to suppress the inflammatory nuclear factor-kappa B, which is involved in AD pathogenesis [19]. NAC activated the extracellular signal-regulated kinase (Ras-ERK) pathway in PC12 cells preventing neuronal death by a non-antioxidant mechanisms [20]. Additionally, NAC enabled neurogenesis and differentiation of neuronal stem cells [21]. However, poor bioavailability and low membrane penetration has limited its clinical application [22, 23]. N-acetylcysteine amide (NACA) and N-Acetylcysteine ethyl ester (NACET) derivatives (3) of NAC were synthesized to increase GSH content and have been reported to possess antioxidant and anti-inflammatory activities [24] (Fig. 1).
Fig. 1.
Cysteine based thiol derivatives
Similar to GSH, Cys is easily oxidized. Hence strategies to mask thiol in the form of thiazolidine-4-carboxylic acids were applied to prodrug design. Among these, L-thiazolidine-4-carboxylic acid (TCA, 4) [25, 26] and L-2-oxothiazolidine-4-carboxylate (OTC, 5) [27, 28] are the most studied prodrugs. These prodrugs are hydrolyzed by enzyme prolinase to release Cys and show improved half-life (t1/2) in plasma. Naturally occurring thiol protected cysteine analogs from Allium plants such as S-allyl cysteine (SAC), S-methyl cysteine (SMC), S-ethyl cysteine (SEC), and S-propyl cysteine (SPC) [29] also enhanced plasma and tissue GSH levels (Fig. 1).
GSH based thiols
GSH is the most abundant and important endogenous antioxidant that counteracts ROS and nucleophilic compounds such as 4-hydroxynonenal (HNE) and acrolein in the brain. Mandal et al. reported reduced GSH levels in the hippocampi and frontal cortices of mild cognitive impairment (MCI) and AD patients, which is directly associated with cognitive function [30]. Hence, maintaining or restoring cellular GSH levels and inhibiting its degradation is considered a beneficial pharmacological strategy for preventing or halting AD progression.
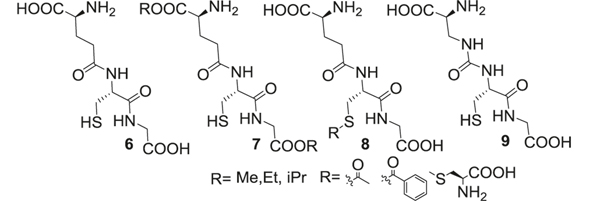
GSH (6) can react with ROS or reduce lipid peroxides through glutathione peroxidase (GPx) to protect cells against oxidative stress injury. GSH can be regenerated from glutathione disulfide (GSSG) by glutathione reductase (GR) using equivalents of NADPH. Under normal physiological conditions, cellular biosynthesis of GSH is homeostatically controlled through ATP-dependent steps. Enzymes such as γ-glutamylcysteine synthetase (GCS) and GSH synthetase are important for the synthesis of GSH, deficiency of which has been implicated in AD progression. In extracellular spaces of cells, GSH is degraded exclusively by expressed γ-glutamyl transpeptidase (GGT) to form cysteinylglycine and glutamate. Levels of cysteine/cystine and GSH/glutathione disulfide (GSSG) redox pairs are maintained by NADPH dependent enzyme systems such as thioredoxin and glutaredoxin, GR, and GPx (Fig. 2).
Fig. 2.
GSH based thiol derivatives
The challenge in the application of GSH (6) is its poor stability and bioavailability in biological systems. GSH has a short t1/2 in plasma (<3 min) and poor cell membrane permeability, administration of high doses is necessary to reach therapeutic concentrations. Researchers have synthesized several carboxylic ester and thiol modified GSH prodrugs (7–8) (monomethyl, monoethyl (GEE), diethyl, isopropyl esters, S-acetyl GSH), which effectively increased intracellular GSH levels in human lymphoid cells, fibroblasts, endothelial cells, and rat hepatocytes in vitro compared to native GSH [31–34]. GEE significantly elevated intracellular GSH levels in the neuronal cells and provided neuroprotection against H2O2-induced oxidative stress [35]. L-cysteine-glutathione (L-CySSG, 8), linked L-Cys with thiol of GSH through a disulfide link [36]. The disulfide bond was reduced by GR to release a molecule of GSH and Cys. L-CySSG improved the stability of GSH toward oxidation and the released Cys enabled the synthesis of GSH (Fig. 2).
We and others have an active interest in the role of the glyoxalase (GLO) enzyme system in the progression of AD and developing substrates of GLO-1 to restore its function. GLO is involved in detoxification of toxic dicarbonyls such as methylglyoxal using GSH as a cofactor and has implications in glycation induced toxicity of Aβ1–42. Supplementation of GSH, however, is unable to restore GLO-1 activity due to its hydrolysis by ubiquitous GGT resulting in loss of activity. We have synthesized a GLO-1 substrate, Ψ-GSH (9) [37], by replacing the γ- glutamyl-cysteinyl amide bond in GSH with a ureide linkage. Ψ-GSH was found to be stable against GGT-mediated breakdown and was able to substitute for GSH in enzymatic reactions, a property crucial for preventing and halting damage caused by AD pathology (Fig. 2).
Alpha-lipoic acid
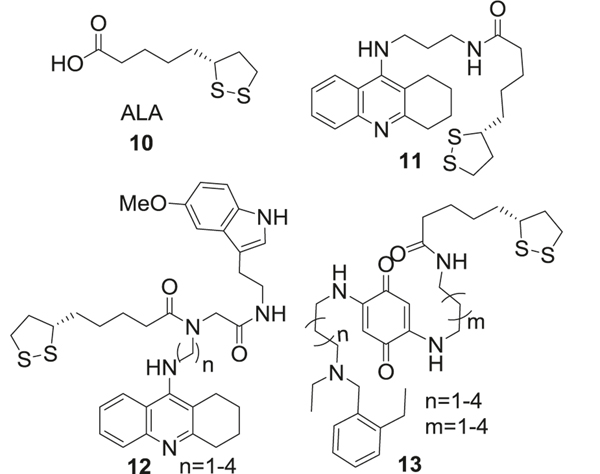
The anti-AD effect of lipoic acid (ALA, 10) is attributed to multitude of properties. ALA activated AChE and increased glucose uptake, thus providing more acetyl-CoA to generate acetylcholine (ACh). ACh is a neurotransmitter used by all cholinergic neurons and is transported into synaptic vesicles by vesicular ACh transporter [38, 39], where it plays an important role in the peripheral and central nervous systems. A variety of studies have shown that ACh modulates the memory process, such as acquisition, encoding, consolidation, reconsolidation, extinction, and retrieval [40] (Fig. 3).
Fig. 3.
Alpha-lipoic acid and its analogs
ALA increased intracellular GSH levels by chelating redox-active transition metals, thus inhibiting the formation of hydroxyl radicals and Aβ aggregation. Levels of several antioxidant enzymes including catalase, GR, glutathione-S-transferase (GST), NADPH, and quinone oxidoreductase-1 (NQO1) were enhanced by ALA [41]. Also, ALA prevented the induction of iNOS, inhibited TNFα-induced activation of NF-κB [42], levels of which are increased in AD. Furthermore, ALA reduced the levels of lipid peroxidation products responsible for mitochondrial and cell signaling disruption by GPx-mediated inactivation of hydrogen peroxide and by scavenging generated HNE and acrolein [43] (Fig. 3).
The chemical activity of ALA is mainly due to the dithionate ring, whose sulfur atoms confer high electron density to ALA making it an efficient antioxidant. The long carbon chain and ring system make it more lipophilic than other natural antioxidants. ALA could easily cross the blood–brain barrier (BBB) and keep a uniform uptake profile throughout the central nervous system (CNS), which is beneficial against AD. Modifications of ALA mainly focused on linking the free carboxylic acid to other AD therapeutic agents. A hybrid of ALA with a derivative of tacrine, an AChE inhibitor (AChEI) approved for AD treatment, was synthesized [44]. The ALA-tacrine hybrid (11) could bind to both, catalytic and peripheral sites of AChE (IC50 = 0.25 nM), and reduced Aβ aggregation (IC50 = 45 μM). Further studies found that (R)-LA-taurine enantiomer was twice as potent as (S)-enantiomer (IC50 = 0.23 nM vs. 0.47 nM, respectively) as an AChEI, which is consistent with better inhibitory potential of R-LA isomer than L-LA [45]. In other research, a melatonin-ALA-tacrine hybrid was synthesized and tested (12) [46]. The length of the linker between the ALA and tacrine was vital for its potency. The analog with a 6-carbon linker was the most active with IC50 of 1.25 nM for butyrylcholinesterase (BChE), IC50 of 3.62 nM for AChE and exhibited antioxidant potential. A similar design rationale was adapted for synthesis of ALA hybrid with benzoquinone moiety of memoquin by using a polyamine linker (13), which showed both AChE and BChE inhibitory activities [47]. Compared to memoquin, all the hybrid molecules with different linker lengths showed moderate inhibition of AChE, however, were more potent inhibitors of Aβ aggregation. The hybrid with the shortest linker (n = 1, m = 1) was the most active inhibitor of these enzymes (Fig. 3).
In vitro/in vivo ADMET
Cys based thiols
NAC (2) can enter cells without active transport and is rapidly hydrolyzed to yield Cys. However, because NAC is negatively charged at physiological pH, it displays poor membrane permeability, and bioavailability [22]. Low bioavailability of NAC is one of the major limitations for its application in oxidative stress-related diseases such as AD. The Cmax and t1/2 were 554 mg/L and 5.7 h, respectively, after intravenous administration in patients at 150 mg/kg [48]. NAC has a relatively small volume of distribution (536 ml/kg). Oral NAC is rapidly absorbed, but its bioavailability is low (9.1%) because of significant first-pass metabolism [49]. The Tmax of NAC was 1.4 ± 0.7 h and the mean elimination t1/2 was 2.5 ± 0.6 h after a single daily dose [50]. Oral NAC causes nausea, vomiting, diarrhea, flatus, and gastroesophageal reflux, while intravenously NAC may cause anaphylactic reactions [51].
The NAC esters were hydrolyzed within 1 h in plasma to NAC, whereas thiol protected prodrugs offered extended stability (t1/2 of 18 h for rats and over 100 h for humans) [52]. Oral NAC and NACET administered at equivalent dosages reached comparable Cmax (69 ± 10 μM vs. 96 ± 15 μM) but different Tmax (10 min vs. 120 min) in rats [53]. Intravenous administration of both compounds yielded relatively low concentrations of NACET in plasma (Cmax = 75 ± 12 μM, t1/2 = 0.36 ± 0.06 h), while NAC reached high plasma concentrations (Cmax = 1250 ± 220 μM) with longer half-life (t1/2 = 4.35 ± 0.57 h). These studies demonstrated that oral bioavailability of NACET is 58.5 ± 8.8%, while that of NAC is below 4.8 ± 1.2% [52–54].
GSH based thiols
GSH (6) has a very short half-life (t1/2 = 10 min) and a high dose is needed to maintain its therapeutic concentrations[55]. Moreover, GSH synthesis is hampered in AD brain. Therefore, GSH analogs and prodrugs could potentially substitute for GSH while possessing a favorable pharmacokinetics (PK) profile. Intraduodenal administration of GEE at 0.5 and 5 mmol/kg dose in rats resulted in a Cmax of 3.6 ± 0.9 and 46.3 ± 3.5 nmol/mL, with a corresponding Tmax of about 45 min. However, no significant increase in GSH and Cys plasma levels were observed after administration of 0.15 and 0.5 mmol/kg of GSH [56].
Ψ-GSH (9), a ureide analog of GSH, is stable toward GGT mediated breakdown [37]. The elimination half-life of ψ-GSH (t1/2 = 1.227 h) was 3 times longer than GSH (t1/2 = 0.495 h) in mice after i.p. administration in mice (500 mg/kg). The maximum serum concentration of ψ-GSH (Cmax = 109.7 μg/mL) was much higher than that of GSH (Cmax = 43.97 μg/mL) and it was able to cross the BBB. This compound was also orally bioavailable [37]. No appreciable toxicity of ψ-GSH was observed at doses up to 2000 mg/kg.
ALA and derivatives
Cell permeability assessment using the CACO-2 transwell model showed that ALA is rapidly traversed through cell monolayer in a pH-dependent manner [57]. PK analysis of oral ALA at 8.25 mg/kg dose displayed serum Cmax of 16.03 μg/mL (range: 10.6–33.8 mcg/mL) with rapid renal clearance and plasma t1/2 of 30 min in mice [58]. There are no literature reports on the maximum allowable exposure limit for ALA in humans. In mice, a LD50 of 400–500 mg/kg has been reported [59]. A dose of 2400 mg/day ALA was used to assess adverse health effects in human clinical trials, however, this study failed to find any adverse effects of the treatment [60].
Efficacy in animal models
Although there are reservations for the use of NAC (2) in human AD trials, the administration of NAC showed beneficial effects against oxidative damage in AD murine models [61]. The ability of in vivo NAC to reduce protein carbonyls levels, lipid peroxidation, and protein nitration was demonstrated in the APP (Amyloid Precursor Protein)/Presenilin-1 (PS1) mice. It also enhanced the activities of GPx and GR [62]. NAC-treatment improved cognition reduced neuronal loss, and tau expression in specific regions of the brain in mice [63, 64]. There are reports in the literature on the significant anti-Aβ efficacy of NAC in TgCRND8 transgenic mouse model [65] and alleviation of oxidative damage in ApoE−/− mice [61].
Among various GSH analogs, Ψ-GSH (9) was shown to mitigate the buildup of AD indicators in the APP/PS1 mouse model [66]. Aβ deposition and oxidative stress indicators (lipid peroxidation, protein carbonyl, and ROS) were also dramatically reduced in the ψ-GSH-treated group. Transgenic AD mice treated with ψ-GSH showed significant learned behavior and long term cognitive/reference memory improvement. In vivo engagement of GLO-1 enzyme by ψ-GSH was demonstrated by reduction levels of methylglyoxal in brain. Further advancement of this class of compounds is currently underway in our laboratories.
Biological evaluation of ALA (10) has been extensively reviewed elsewhere [60, 67, 68]. In relation to AD, the effects of ALA supplements on hippocampus-dependent memory were investigated in aged Tg2576 mice [69]. The study demonstrated significant cognitive benefits of ALA treatment by restoration of spatial learning and memory in the Morris water maze. However, ALA treatment did not have an apparent effect on Aβ plaque burden [69]. In another study, young and aged Tg2576 mice were fed with the R-LA diet for 10 months [70]. The study showed significant improvement in oxidative stress markers, however, no cognitive improvement was noticed in the Y-maze assay [70]. Another study evaluated the neuroprotective effect of ALA in the P301s tau transgenic mice [71]. ALA treatment significantly improved the spatial memory and cognition capacity of the mice in the Morris water maze and novel object recognition test. The protective effect against tau-induced neurotoxicity and attenuation of cognitive dysfunction were attributed to antioxidant and anti-inflammatory activities of ALA [71].
Clinical trials
Cysteine based thiols
In a placebo-controlled trial (n = 34), 6-month treatment of NAC (2) (600 mg) showed improvement in cognitive function. However, NAC was given as a nutraceutical formulation containing other antioxidants including alpha-tocopherol, vitamin B12, and S-adenosylmethionine [72]. Interestingly a similar study in a larger group of AD subjects (n = 106) also showed a significant improvement over the placebo group [73]. A nutraceutical Memory XL supplement containing 600 mg of NAC (2 pills/day for 6 months) was administered to patients (n = 8) with MCI in phase II clinical trial that was terminated due to lack of beneficial outcomes (NCT00903695). In another randomized double-blind phase I clinical trial, 60 participants (NCT03493178) were treated with a combination of NAC and glycine for 12 weeks, and the improvement in cognitive function was measured for another 12 weeks post treatment. The primary outcome of cognitive function was measured using free and cued selective reminding tests as well as delayed recall scores. In addition, blood concentrations of GSH, Cys, and glycine were measured. NAC, given in combination has shown improvements in cognition assessment tests and this benefit declined upon cessation of the supplementation.
GSH based thiols
Dietary nutritional assessment in patients with MCI and AD displayed reduced plasma levels of antioxidant micronutrients like vitamin C, vitamin A, and vitamin E. Besides, GSH levels were found to be depleted in patients compared to healthy subjects [74]. However, studies using oral supplementation of GSH have shown poor results. In a randomized double-blind study involving healthy adult subjects, oral administration of GSH (500 mg, twice daily) did not significantly improve plasma GSH concentration (total, oxidized, or ratio levels) and failed to show a reduction in oxidative stress markers. Another study involving 7 healthy volunteers, showed that even a high dose (3 g, oral administration), failed to improve plasma levels of GSH, Cys, and glutamate after 4 h. This has been mainly attributed to intestinal degradation by trans-peptidases [75, 76]. In contrast, a 6-month randomized double-blind study of GSH supplementation showed increased plasma levels of GSH after oral administration at doses 250 and 1000 mg, and this increase was reversed 1 month after stopping the GSH supplementation [77]. In a randomized crossover study with healthy volunteers, the sublingual form of GSH (450 mg) showed improved bioavailability when compared with oral GSH administration [78].
Lipoic acid
Preclinical studies in animal models have shown that ALA (10) can ameliorate cognitive impairment in mild to moderate AD. In a pilot study with nine probable AD patients, treatment for 1 year with 600 mg of ALA stabilized cognitive function as measured by mini-mental state examination (MMSE) and cognitive scale using ADAS-Cog scores [79]. In a slightly larger phase II clinical trial involving 39 patients with MCI, 600 mg of ALA was used alone or in combination with fish-oil containing omega-3 fatty acids. In this study, oxidative stress as measured by peripheral F2-isoprostane did not show any significant reduction after 12-month treatment. However, MMSE-based cognitive decline showed slower progression in ALA-treated groups compared to placebo [80]. In another double-blind randomized study with 78 subjects with MCI, treatment with 900 mg/day of ALA for 16 weeks in combination with antioxidants like vitamin C, vitamin E did not change the levels of Aβ or tau-phosphorylation. No changes in peripheral F2-isoprostane were observed either. Cognitive decline measurement by MMSE and ADAS-Cog did not show any significant change in the ALA-treated group over placebo [81].
Hydrogen sulfide donors and elemental sulfur
Design Rationale and pharmacological target
For a long time, hydrogen sulfide (H2S, 14), like other gas transmitters NO or CO, was considered a poisonous gas with a pungent odor. However, Abe and Kimura found that H2S selectively improved the N-methyl-D-aspartate receptor (NMDAR) mediated function, and its role in the CNS was discovered [82]. Since then, H2S is recognized as generated endogenously by living organisms and has an important role in the regulation of physiological and pathological processes [83]. H2S has been reported to be produced mainly through cystathionine-β-synthase (CBS), cystathionine-γ-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase (3-MST). Recently, researchers discovered relatively high concentrations of H2S (47–166 μmol/L) in the brains of mammals [84] and that changes in its levels are relevant to AD pathogenesis [85, 86].
Under physiological conditions, H2S exists in three forms: the neutral molecular form (H2S) and two different ionic forms (HS− and S2−), but the bioactive form among these, is still unknown. Increasing evidence from both in vivo and in vitro studies suggest that H2S has potential therapeutic value for the treatment of AD [87]. H2S could protect against AD-related oxidative stress [88], Aβ release [89], apoptosis [90], and inflammation [91]. It has been reported to reduce Aβ plaques by decreasing β-and γ-secretase enzyme expressions, which has a protective effect on neurons of AD mice [92]. H2S has also shown neuroprotective effects by increasing the levels of Bcl2, an antiapoptotic protein, and by decreasing the expression of apoptotic proteins like Bax [93, 94]. As inflammation plays a key role in neurodegeneration, the anti-inflammatory action of H2S-releasing agents could play a beneficial role in treating AD
Although H2S has good solubility in water, it is still very unstable and is easily oxidized in air. Also, as a gas, it is difficult to measure its precise concentration, needs a special equipment for use, and has an unpleasant odor [95]. Hence several different forms of sulfur-containing precursors or prodrugs of H2S were developed [96, 97].
Elemental sulfur
Biological evaluation of elemental Sulfur (15) as a source of H2S has been a topic of recent investigations. Oral administration of sublimed sulfur formulation was studied as a potential AD therapeutic due to its effect on β-galactosidase mediated autophagy and homocysteine (Hcy)/H2S signaling pathway [98]. Besides, sulfurous water has been reported to protect peripheral mononuclear blood cells in AD patients by preventing H2O2 induced oxidative DNA damage while also preventing homocysteine-induced cytotoxicity [99].
Inorganic sulfide salts
NaSH (16) and Na2S (17) are two widely used inorganic H2S donors that are reported to show antioxidant effects primarily through modulation of GSH levels in the brain. GSH levels in rat brains measured by HPLC revealed an increased transport of GSH into mitochondria by H2S-releasing NaSH [88, 100, 101]. Protein expression studies using western blot showed NaSH-induced H2S release, downregulation of APP, and BACE-1 [102]. Reduced lipopolysaccharide (LPS)-induced inflammation as measured by TNF-α levels, was observed in murine microglial cells upon addition of NaSH [103]. NaSH has also been reported to reduce Aβ-induced pro-inflammatory cytokines like IL-1 and IL-6 [104].
Upon hydrolysis, both compounds generate H2S quickly in the buffer. Under physiological conditions, HS−/H2S ratio is about 3:1 [105]. Compared to H2S gas, these salts are readily available and do not need special equipment to produce or use. Similar to H2S, its salts are also unstable, for example, the t1/2 of Na2S•9H2O solutions (10–100 μM concentrations) range from 0.5 min to 5 min [106]. Additionally, the release of H2S from these salts cannot be controlled, and a sudden spike in H2S concentration may cause problems such as blood pressure change or imbalance in redox potential [107]. Lastly, all sulfide salts have unpleasant odor, which prevent their use in the clinic.
Allyl sulfide
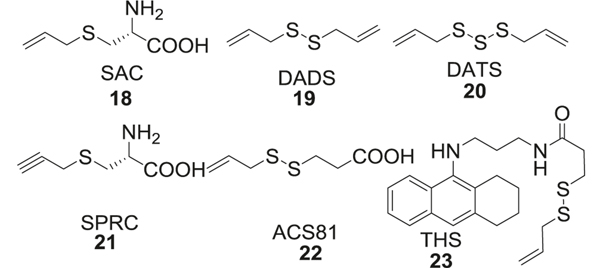
S-Allylcysteine (SAC, 18), diallyl disulfide (DADS, 19), and diallyl trisulfide (DATS, 20) are the main bioactive components in garlic [108]. SAC showed cytoprotective effects by preventing Aβ-induced cell death in PC12 cells [109]. Thioflavin fluorescence studies and size exclusion HPLC studies showed prevention of Aβ aggregation in vitro by allyl sulfides [110]. S-Propargyl-cysteine (SPRC, 21) which is a SAC structural analog, can be used to modulate endogenous H2S levels [111]. One of the benefits of these allyl sulfides compared to the salts is that the release of H2S can be controlled. It is reported that the release of H2S from DATS occurs at a steady rate over a prolonged time in relatively low concentrations, while the same concentration of Na2S causes immediate release of H2S at 10 times higher concentration than DATS [112] (Fig. 4).
Fig. 4.
Allyl sulfide derivatives
Recently Cheng et al. linked H2S-releasing allyl sulfide moiety (ACS81, 22) with tacrine, later being a potent acetylcholinesterase (AChE) inhibitor, to form a tacrine-H2S donor hybrid (THS, 23) [93] (Fig. 4).
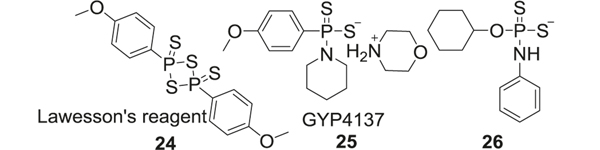
Lawesson’s reagent and analogs
Lawesson’s reagent (24), which is used as a sulfurization agent in organic synthesis [113], also releases H2S upon hydrolysis and could be used as an H2S donor [114, 115]. Compared to inorganic sulfides, the H2S release rate from Lawesson’s regent is slower. However, it is spontaneous enough to cause an immediate H2S spike and the reagent also suffers from poor water solubility limiting its further applications [116]. Attempts have been made to improve solubility by modifing Lawesson’s reagent. GYY4137 (25), a water-soluble derivative of Lawesson’s reagent, is one such successfully modified H2S donor [117]. SAR studies have shown that replacement of all sulfur groups of GYY4137 with oxygen eliminated its cytotoxicity against different types of cells. GYY4137 inhibited LPS-induced pro-inflammatory ROS production in human THP-1 and SH-SY5Y cells after 24 h [118]. GYY4137 generated H2S at a slower rate when compared to parent Lawesson’s reagent and the sulfide salts. Further modifications of GYY4137 to tune H2S release rate included substitution of the C–P bond with C–O bond, and substitution of morphine with phenylamine (26). These analogs stabilized GYY4137 and further decreased the releasing rate of H2S [119] (Fig. 5).
Fig. 5.
Lawesson’s reagent and its analogs
1,2-dithiole-3-thiones (DTT)
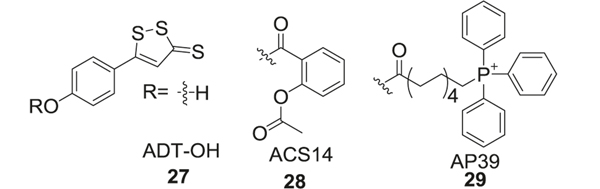
1,2-Dithiole-3-thiones (DTT) has been widely considered as a H2S donor [120, 121]. Although its H2S-release mechanism is still not fully understood, some research has indicated hydrolysis of DTT forming H2S and 1,2-dithiol-3-one [114].
To reduce the side effects of nonsteroidal anti-inflammatory drugs (NSAID), DTT was introduced as a DTT-NSAID hybrid. These compounds reduced the side effects of NSAIDs such as gastrointestinal ulceration and bleeding and enhanced their anti-inflammatory effect [122–124]. Several of these hybrids have been evaluated as AD therapeutics[125]. For such hybrids, phenol was introduced at the 5-position of the heterocycle forming 5-(4-hydroxyphenyl)-3H-1,2-dithiole-3-thione (ADT-OH, 27), and the –OH group of the phenol was esterified with a carboxylic acid of the NSAIDs. One such example is direct linking of aspirin with ADT-OH (ACS14, 28). Other modifications included the inclusion of mitochondria-targeted triphenylphosphine (TPP), which modified cellular bioenergetics and demonstrated protection against neuronal damage observed in AD (AP39, 29) [126]. Although DTT-NSAID hybrids showed promising bioactivities related to H2S release both in vitro and in vivo, some issues remained that hindered their further development. First, the H2S release mechanism of DTT was still unclear and high reactivity of DTT was a major concern for undesired side effects. Also, the unpleasant odor of these compounds was also an obstacle for clinical use (Fig. 6).
Fig. 6.
1,2-Dithiole-3-thiones (DTT) and its analogs
Organic isothiocyanates
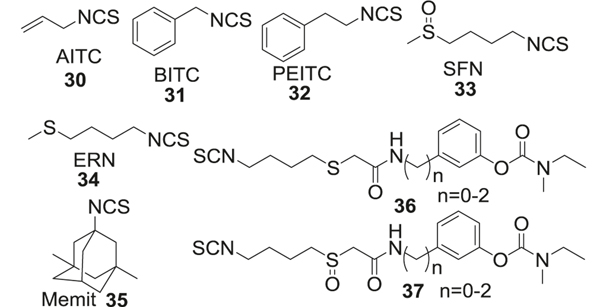
Organic isothiocyanates (ITCs) isolated from some botanical species such as cabbage, broccoli, rocket salad, and cauliflower, have been proposed as a new H2S-donor family in a recent discovery. ITCs are formed by enzymatic hydrolysis of natural glucosinolates (GLS) by myrosinase [127]. Interestingly, it was found that the ITCs not only share the pharmacological profile of H2S such as antioxidant [128] and anti-inflammatory [129] properties, but demonstrate impressively overlapping molecular mechanisms. In 2014, it was first discovered that certain natural ITCs in Brassicaceae exhibited H2S-releasing properties [130]. Follow-up studies indicated the chemotherapeutic potential of various ITCs [131], such as allyl isothiocyanate (AITC, 30), benzyl isothiocyanate (BITC, 31), phenethyl isothiocyanate (PEITC, 32), sulforaphane (SFN, 33), and erucin (ERN, 34). It is reported that almost all selected ITCs have Cys-dependent H2S release mechanism and demonstrate absence or decreased H2S release [132] without Cys. This is proposed to be due to the nucleophilic reaction of cysteine thiol with isothiocyanates resulting in the formation of dithiocarbamic adducts, followed by subsequent intramolecular cyclization to obtain dihydrothiazole derivatives and H2S release [133]. The cyclization is the rate-limiting step and the electron-withdrawing nature of the isothiocyanate dictates the rate of H2S release. The advantages of these ITCs are good water solubility, low molecular weights [134], and easily amenable to further modifications [135]. The challenge however is that ITCs like H2S are highly volatile and possess high reactivity, posing problems in their pharmaceutical applications [136] (Fig. 7).
Fig. 7.
Organic isothiocyanates (ITCs)
A strategy was recently developed to overcome the limitations of ITCs by their incorporation in drug-like compounds forming multifunctional drugs [127, 137]. Similar to fragment-based design, these hybrid drugs take advantage of the H2S-releasing property of the ITC moiety, and improved ADMET properties and pharmacological effects of the drug-like entity. One such recent example is the development of Memit (35), an isothiocyanate of memantine resulting from the replacement of its primary amine functional group [138]. Memantine is a well-known neuroprotective drug that was approved in 2003 by the FDA as a treatment option for moderate to severe AD [139]. It is an uncompetitive antagonist of the voltage-dependent NMDA receptors and targets NMDAR pathological hyperfunction. Memit (35) decreased the ROS levels and also showed cytoprotective effects by reducing Aβ aggregation in rat microglial cells. In vitro aggregation studies using thioflavin fluorescence showed reduced Aβ1–42 self-aggregation in the presence of Memit [140]. The compound displays slow and prolonged H2S releasing property (Fig. 7).
To further expand this class of H2S-releasing ITCs, Rapposelli [135] developed hybrids of rivastigmine (36, 37), an FDA approved AChE inhibitor for AD, with SFN and ERN [141]. SFN activated Nrf2 pathway, demonstrating cytoprotective effects by reduction of oxidative damage in astrocytes [128]. Furthermore, the increased expression of Nrf2 by SFN has been reported to protect the BBB after cerebral insult in rat brain [128, 142]. The hybrids were comparable in the H2S release efficacy and produced H2S levels similar to those achieved by DADS in a murine microglial cell line (BV-2) (Fig. 7).
In vitro/in vivo ADMET
The rate constant (k) for H2S volatilization is 0.13 min−1 and the half-life is 5 min (t½ = 0.693/k) in buffer solutions. H2S is highly diffusible and most endogenous H2S is released along with CO2 through exhalation. The release of H2S from donors occurs under acidic conditions (pH of 5.4–7.4) and the normal distribution of H2S in plasma and other tissues is at 30–100 μM [143]. It therefore is usually stored in mitochondria as bound sulfur. H2S is primarily oxidized in mitochondria to thiosulfate and further oxidized to sulfate in the kidneys. Exogenously administered NaSH (16) and Na2S (17) directly release H2S and most of the released H2S is eliminated from the body as thiosulfate, sulfite metabolites through urine. In plasma, H2S forms sulfhemoglobin and is metabolized by cytochrome C oxidase in the liver [93, 144, 145]. The toxicity of H2S is mostly concentration-dependent as it provides therapeutic benefits at low concentrations (100–160 μM), however, induces acute toxicity at higher doses mediated through its CNS and pulmonary actions.
Efficacy in animal models
Many H2S donors have demonstrated therapeutic potential in AD models. NaSH has been most studied amongst all. Cognitive and behavioral studies demonstrated beneficial effects of NaSH pretreatment against Aβ-induced memory impairment in a rat model on Morris water maze. Treatment with NaSH lowered the levels of APP, PS1, and Aβ proteins and improved the cognitive functions in 3 × Tg-AD mice [140]. Anti-inflammatory effects of NaSH treatment were also observed in the LPS injected rat model by reduced expression of pro-inflammatory cytokines (IL-1β) and TNFα [146]. Additionally, NaSH treatment attenuated neurodegeneration and neuroinflammation in homocysteine treated mice [147]. H2S donors in garlic extract showed increased expression of presynaptic proteins like synaptophysin and SNAP25 in the transgenic mouse model with APP mutation [148]. Another H2S releasing compound, a conjugate of tacrine with a H2S releasing moiety (ACS81), was tested in an aluminum chloride-induced AD model. The compound improved cognitive and locomotor functions in these mice. Besides, such conjugation with H2S donor improved the safety profile of tacrine while retaining the AChE inhibition in the hippocampus of rat brains [93].
Clinical trials
Although there is promising in vitro and in vivo animal data on the therapeutic potential of H2S, so far there have been no conclusive clinical trials studying the cognitive benefits of H2S or its donors. A phase I clinical trial involving H2S donor SG1002 showed good tolerance by healthy subjects at oral doses of 200–800 mg/kg. At 800 mg/kg, SG1002 showed elevated levels of H2S for up to 12 h [149]. SG1002 is an inorganic mixture, containing S8, Na2SO4, Na2S2O3, Na2S3O6, Na2S4O6, and Na2S5O6. In a clinical trial involving AD patients, sulfurous water demonstrated protection against cellular H2O2 and homocysteine-induced oxidative damage. In this study, peripheral mononuclear blood cells of AD patients were analyzed for oxidative damage to the DNA [99]. An exploratory study in healthy volunteers (n = 16) showed decreased homocysteine levels after the administration of elemental sulfur formulation at 200 mg/day for a 30-day treatment period. Increased homocysteine accumulation is usually associated with lowered GSH production, a hallmark of AD.
Alkyl sulfide derivatives
Design rationale and pharmacological target
Organic sulfides are compounds with the connectivity C–S–C formula. This substitution is considered a bioisostere for ether linkage by replacing the oxygen atom of the ether with sulfur and is widely used in drug development. Compared to ethers, sulfides are less volatile, higher melting, and less hydrophilic [150]. These properties arise from the polarizability of the sulfur center, which is greater than that for oxygen in ethers. Several sulfide-containing compounds are widely used in AD therapy.
MMP inhibitors
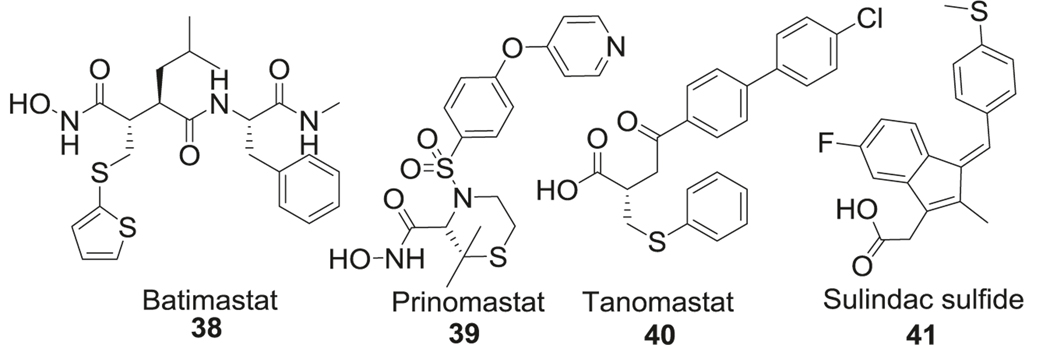
Nerve growth factor-dependent cholinergic neurons undergo degeneration during the prodromal and advanced stages of AD. One of the contributing factors is the degradation of mature NGF by matrix metalloproteinases (MMP). Postmortem brain analysis of patients diagnosed with MCI and mild AD showed higher MMP-9 activity compared to healthy volunteers [151]. Batimastat (38) was the first sulfide-containing MMP inhibitor that was tested in patients [152]. MMPs are a superfamily of endopeptidases which are capable of degrading components of the extracellular matrix, remodeling tissues, shedding cell surface receptors, and processing various signaling molecules. Recently, the role of MMPs (matrixes) in AD has gained interest. The capacity of several MMPs (MMP-2, −3, −9, and MT1-MMP) in degrading APP leading to generation and aggregation of Aβ was discovered [153]. Increased mRNA levels of certain gelatinases (MMP-2 and MMP-9) have been found in the hippocampus of the 5xFAD model, which leads to degradation of tight junction proteins at the BBB, leading to BBB leakage and thus exacerbating chronic inflammation in AD [154]. Moreover, MMP-9 has been reported to present neurotoxicity in the hippocampus due to its interactions with nitric oxide, leukocyte infiltration in the damaged CNS, and other indirect mechanisms [155, 156] (Fig. 8).
Fig. 8.
Alkyl sulfide-containing AD therapeutics
In addition to the effect of MMPs on APP metabolism and neuroinflammation in AD, increasing evidence has reported functional links between MMPs and tau protein. Tau is reported to be a substrate for both MMP-3 and MMP-9, but only MMP-9 caused the increase in toxic forms of tau [157, 158]. Another study found that MMP-3 and MMP-10 levels in CSF is related to tau or p-tau levels in AD patients [159, 160]. Batimastat (BB-94, 38) is a relatively nonspecific MMP inhibitor; inhibits MMP-1, −2, −3, −7, and −9 with IC50 in the range of 3–20 nM, respectively [161, 162]. Compared to batimastat, next-generation compounds such as prinomastat (39) and tanomastat (40) demonstrated improvement in vivo PK profiles [152]. Tanomastat (Bay12–9566, 40) is an orally bioavailable potent inhibitor of MMP −2, −3, and −9 with IC50 of 11, 134, 301 nM, respectively [163]. Prinomastat (AG-3340, 39) inhibits MMP-2, 9, 13, and 14 (IC50: MMP-3, 6.3 nM; MMP-9, 5.0 nM) [164]. These inhibitors were designed to fit the binding cavity of MMPs and chelate the catalytic zinc atom by a zinc binding group. The presence of sulfide groups helped to improve BBB penetration of these inhibitors (Fig. 8).
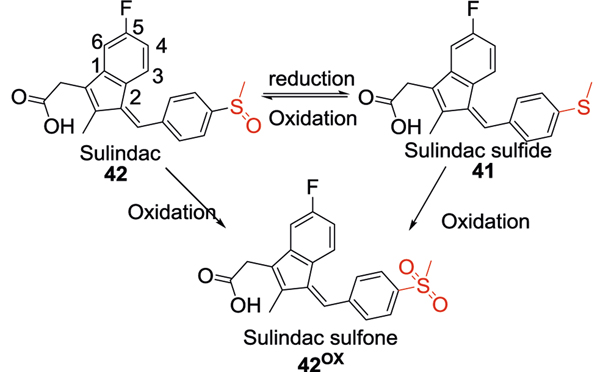
Sulindac sulfide
Similar to the MMP inhibitors, the sulfide group was also utilized in the development of γ-secretase modulators [165]. Under physiological conditions, around 90% of APP is cleaved by α-secretase. If APP is cleaved by β-secretase under disease conditions, the amino acid fragment is further cleaved by γ-secretase generating different species, of which Aβ40 and Aβ42 are the most abundant species in the brain and CSF. Sulindac sulfide (41), an NSAIDs, can reduce the production of Aβ42 through modulation of γ-secretase [166]. Such γ-secretase modulators exhibit better pharmacological profiles than γ-secretase inhibitors by selectively decreasing the Aβ42 levels without any effect on other functions of γ-secretase. In addition to γ-secretase modulatory activity, sulindac sulfide attenuated Aβ neurotoxicity by acceleration of Aβ fibrils formation, thus reducing exposure to toxic soluble aggregates of Aβ [167]. However, higher doses of the compound were required to achieve the therapeutic drug concentration in the CNS due to poor brain penetration and aqueous solubility (Fig. 8).
In vitro/in vivo ADMET
Because of poor aqueous solubility, the bioavailability of batimastat (38) was low when administered by oral or parenteral routes. The peak plasma concentrations were reached 1 h after dosing. The t½ of batimastat was 19.1 days after intraperitoneal injection in patients with malignant ascites [164, 168]. Fever, hepatotoxicity, and acute bowel toxicity were reported in clinical trials [169, 170].
The bioavailability of tanomastat (40) was 70–98% after oral administration in mice, rats, guinea pigs [171]. The peak plasma concentration was reached within 0.5–2 h. In rats and dogs, tanomastat was mainly metabolized in the liver and then excreted by the biliary mechanism. In healthy human volunteers, PK profile of tanomastat was linear at doses up to 100 mg/day. Hepatotoxicity was a major concern with in rodents and dogs. Additionally, depressed erythropoiesis and tubular nephropathy were reported in female rats [171].
Prinomastat (39) is a lipophilic MMP inhibitor that crosses the BBB. The t½ of prinomastat was 2–5 h after oral administration in patients [162, 172] and the Tmax was around 1 h. It has been reported that prinomastat causes moderate but reversible arthralgia, myalgia, muscoloskeletel syndrome at high doses [173].
No data on the PK of sulindac sulfide is available.
Efficacy in animal models
Batimastat (38), tanomastat (40), and prinomastat (39) have shown anticancer activities in numerous animal models, but there is no data available of their efficacy in an AD animal models. One study reported a reversible, short-term memory impairment after intracortical administration of batimastat at a dose of 0.0001M and led to longer-lasting memory impairments after administration into the brains of neonatal rats [174].
The efficacy of sulindac sulfide (41) has been investigated in the LPS-induced mouse model. Oral administration of sulindac sulfide (3.75 or 7.5 mg/kg) was initiated 3 weeks before the injection of LPS. The pretreatment mitigated memory impairment in this model as assessed by the Morris water maze test and passive avoidance test. Sulindac sulfide at the tested doses also significantly reduced Aβ42 levels in the brain and suppressed the activation of astrocytes by LPS. Moreover, the compound significantly decreased the number of apoptotic cells (3.75 mg/kg, 11.4 ± 2.8%; 7.5 mg/kg. 6.1 ± 1.8%) [104].
Clinical trials
MMP inhibitors
Analysis of plasma and CSF samples in AD patients (n = 14) by gelatin zymography showed significantly increased MMP-3 activity, whereas MMP-2 activity was significantly decreased in CSF. Interestingly this study showed a reduction in MMP-9 levels in plasma. A similar study in a group of 56 patients with AD-related dementia and vascular dementia showed a correlation between total tau protein and MMP-3, MMP-10 levels in CSF of both AD and vascular dementia group, whereas MMP-9 was significantly increased in the vascular dementia group. Both groups showed an increase in phosphorylated tau levels [160]. Significant issues with bioavailability and dose-limiting toxicities have been noted in clinical trials for MMP inhibitors. For example, phase III clinical trial of batimastat (38) was terminated due to peritonitis from intraperitoneal injections as the drug was not suitable for oral administration [175, 176]. Prinomasat (39) and tanomastat (40) exhibited relatively poor selectivity and their clinical evaluation was halted due to their nonspecific side effects. In phase I clinical trials, patients on prinomastat (1–100 mg twice daily oral dose) exhibited joint and muscle pains which were dose-related but the mechanism is unknown [172]. Plasma analysis of volunteers in phase I studies of tanomastat showed asymptomatic reversible effects on platelets and mild anemia [174, 177].
Sulindac sulfide
Analysis of six NSAID clinical trials with more than 13,000 dementia-free patients showed that the use of NSAIDS lowered AD risk by 23%. However, only some NSAIDS like sulindac sulfide have reported Aβ lowering potential in preclinical studies. A pooled study analysis of clinical trials comparing Aβ lowering NSAIDS like sulindac sulfide (41) found that any NSAID use regardless of its reported Aβ lowering effect, reduced the AD risk in dementia-free patients. The risk of AD in these patients was analyzed by reported outcomes such as neurocognitive tests, neuroimaging, and clinical evaluations [178]. Chronic NSAID use has been associated with increased adverse effects including gastrointestinal, renal, and cardiovascular effects. Chronic NSAID use increases the risk of peptic ulcers in older patients (>65 years of age). In addition, monitoring of renal function is recommended in patients with long half-life NSAIDS due to the risk of acute renal failure. Nonselective NSAIDS also increased the risk of heart failure in older adults [179].
Sulfoxide, sulfone, and sulfonic acid derivatives
Design rationale and pharmacological target
Sulindac
Sulindac (42) is an FDA approved NSAID for the treatment of acute or chronic inflammatory conditions. It is a prodrug and the active metabolite is sulindac sulfide. In the liver, the oxidized form sulindac is reduced to sulindac sulfide, which helps to maintain constant blood level and reduce gastrointestinal side effects. We have already discussed sulindac sulfide in the alkyl sulfide. Sulindac is also a nonselective cyclooxygenase (COX) inhibitor and has been applied for the treatment of AD. Sulindac and its sulfide could be oxidized to the inactive sulfone form through metabolic processes. Generally, the presence of the indene ring resulted in decreased CNS and gastrointestinal side effects but also reduced aqueous solubility. Substitution of 5-fluorine with methoxy group resulted in increased analgesic activity and the Z-isomer was more potent than E-isomer [180–183]. Sulindac (42) has COX inhibition activity at lower concentrations but shows Aβ lowering effect at higher concentrations (>50 μM). Several studies have reported that NSAIDs like sulindac target γ-secretase activity and generated variants of amyloid peptides with lower neurotoxicity compared to Aβ1–42. Protein expression studies in CHO cells overexpressing APP and PS1 showed Aβ42 lowering effect of sulindac [184]. ELISA assays in this cell model showed that this decrease in Aβ levels occurs at 40–60 μM concentrations. Fluorescence studies further showed interactions of sulindac with the lipid bilayer of the APP protein, thereby altering the membrane structure. However direct action on Aβ peptides is much debated. Molecular modeling studies using NMR reported direct interaction of sulindac with amyloid fibrils [185], but atomic force microscopy studies revealed this interaction to be weak [186]. Aggregation studies in neuronal cell cultures showed increased formation of large oligomeric aggregates with sulindac that have reduced toxicity [187] (Fig. 9).
Fig. 9.
Sulindac and its metabolites
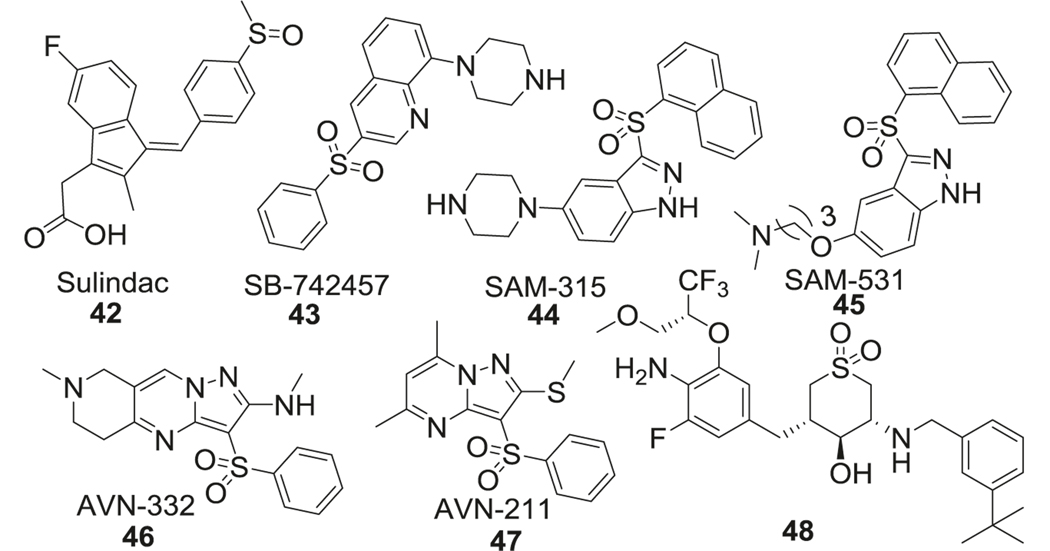
Sulfones
A sulfone is another important sulfur-containing group which is widely used in pharmacology. Compared to sulfonamide, sulfone needs more harsh conditions for synthesis, which limits its application in drug design [188]. In the design of 5HT6R antagonists, the sulfonamide group was substituted with the sulfone group to enhance stability [189]. Details of the sulfonamide SAR studies are discussed in the sulfonamide section. Here we focus on sulfone containing 5HT6R antagonists with promising applications in AD. SB-742457 (43) [190] is a sulfone containing quinolone derivative, which demonstrated 5HT6R antagonistic potency along with high selectivity (>100-fold vs. 84 other similar receptors) and excellent oral bioavailability in phase III clinical trials for AD. Intepirdine (SB-742457, 43) has been used in combination with AChEIs like donepezil and has shown beneficial effects including improved cognition in early phase I and phase II clinical trials [191]. SAM315 (44) and SAM 531 (45), both contain an indazole ring linked to a naphthalene ring through a sulfonyl group and an electron-rich scaffold attached at the 3 positions of indazole [192]. AVN-322 (46) and AVN-211 (47) [193–196] are pyrazolopyrimidine derivatives that exhibited excellent selectivity (>2500-fold) within a panel of more than 65 relevant therapeutic targets and are currently being evaluated in phase II clinical trials for AD (Fig. 10).
Fig. 10.
Analogs containing sulfoxide and sulfone functional groups
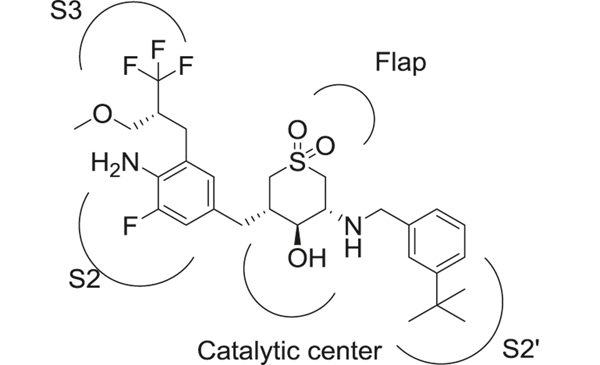
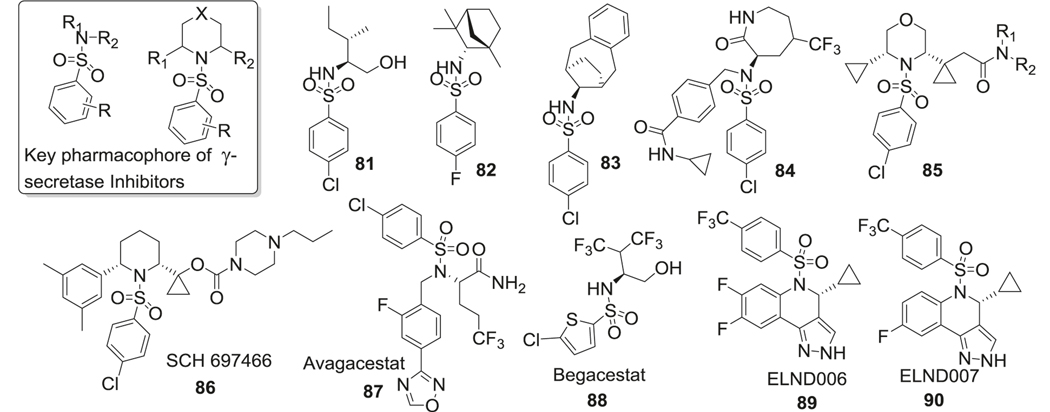
Cyclic sulfone hydroxyethylamine (Fig. 11) is an important scaffold utilized in the development of BACE-1 inhibitors. This class of compounds displayed improved BBB permeability due to the presence of a secondary amine in the scaffold [197]. The sulfone hydroxyethylamines retained essential H-bond interactions with the BACE-1 catalytic diad and the flap region but reduced flexibility compared to sulfonamide-containing compounds described in the sulfonamide section. The best compound (48) reported in this series displayed high potency against BACE-1 (enzymatic IC50 = 2 nM) and >200-fold selectivity over cathepsin D [197]. Pharmacodynamic studies in transgenic AD animal models also demonstrated significant effects on AD pathology by reduction of brain Aβ levels.
Fig. 11.
Interactions of cyclic sulfone hydroxyethylamine 48 with BACE-1
Sulfonic acids
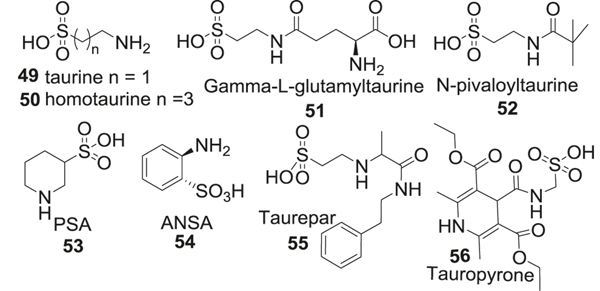
Taurine (49) is a sulfur-containing amino acid with neuroprotective and neuromodulatory effects and plays an important role in addressing pathophysiological changes in AD [198, 199]. Taurine displays an antioxidant mechanism by inhibiting ROS production in mitochondria. Taurine and its analog homotaurine have been reported to reduce Aβ-induced neurotoxicity in neuronal cell cultures by activating GABA receptors. It does not seem to inhibit glutamate-induced neurotoxicity [200, 201]. IHC studies in mice brains showed no effect of taurine treatment on Aβ deposition [202]. Taurine has been reported to modulate synaptic activity by influencing the levels of proteins like synapsin [198]. The sulfonic acid group in taurine presents unique physical properties, which include very low solubility in water (10.48 g/100 mL at 25 °C), low pKa value, and difficulty crossing the BBB. Its cyclical conformation with intramolecular hydrogen bonding also presents passive diffusion. The concentrations of taurine in the CNS are thus dependent on a complex taurine-specific transporter at the BBB, expressions of which are reported to decline under disease conditions or oxidative stress. These properties limit the utility of taurine as an AD therapeutic. Several other taurine-based analogs were developed to address these problems. Critical reviews on taurine and its analogs [199, 203] have been published, herein we focus on the application of these compounds in AD therapy. Compared to taurine, its analog homotaurine (50) with 4 carbon aliphatic chain shows preferential binding to soluble monomeric Aβ peptide and maintains Aβ in a non-fibrillar form, thereby reducing the oligomer-induced aggregation and plaque formation [203]. Also, the neuroprotective activity of homotaurine is reported partly due to its inhibition of Aβ-induced caspase activity in neuronal cell cultures [201]. N-protected taurine analogs, γ-L-glutamyltaurine (51) [204], or N-pivaloyltaurine (52) improved stability of the parent compound along with enhanced lipophilicity [205]. Another modification involved introduction of sulfonic acid group and cyclic amine in the form of piperidine in piperidine 3-sulfinic acid (PSA, 53) and aniline 2-sulfinic acid (ANSA, 54), both of which displayed neuroprotective effects [206]. Additionally, linking taurine to another pharmacophore resulted in the formation of hybrid compounds, such as taurepar (55) and tauropyrone (56), which displayed increased lipophilicity and improved neuroprotective activities [207, 208] (Fig. 12).
Fig. 12.
Analogs containing sulfonic acid functional group
In vitro/in vivo ADMET
Sulindac
Sulindac (42) is orally bioavailable (90%) and reaches peak plasma concentration in 1 h after a single oral dose of 400 mg. Sulindac metabolites, sulfide, and sulfone reach peak plasma concentrations after 2 h. The plasma concentration of sulindac and its metabolites after a single dose or a 2-week treatment appears to be two times higher in older subjects compared to younger subjects. At 100 mg dose, sulfone metabolite is more prominent in the plasma whereas, at 400 mg, sulfide metabolite is the highest. Sulindac and its metabolites are extensively bound to albumin in the plasma (>90%). Its conversion to sulindac sulfide is reversible, whereas the formation of sulfone metabolite by oxidation is irreversible. The liver and kidneys are the major sites of metabolism for sulindac and it is excreted primarily in the urine. However, the sulfide metabolite is reoxidized to sulindac before elimination in the urine. The main adverse effect related to sulindac is hepatotoxicity; however, this is very rarely observed (0.1% patients). The mechanism of hepatotoxicity seems to be through hypersensitivity-induced hepatitis [180].
Sulfone
5HT6R are almost exclusively expressed in the brain tissue with no reported expression in peripheral tissues. Antagonists that are highly selective to 5HT6R therefore show minimal adverse effects related to peripheral distribution [209]. Some sulfone-based 5HT6R antagonists like AVN-322 (46) showed good oral bioavailability and BBB penetration in animal models including BALB/c mice and Wistar rats [191]. Intepirdine (SB-742457, 43) showed 76% oral bioavailability in rats at 0.3 mg/kg dose.
Sulfonic acids
In humans, endogenous taurine is predominantly intracellular and has very little presence in the plasma. However, oral supplementation of taurine markedly increased plasma taurine levels for up to 8 h. In one study, oral administration of 4 g of taurine increased plasma concentrations in healthy volunteers from 40 to 690 μM in 1.5 h after administration. Taurine (49) is widely distributed across the body including skeletal muscle. Physical activity seems to increase the concentration of taurine in muscle tissue where it is converted to acetyl taurine. Taurine is mainly transported by tauT and PAT1 transporters. However, the transporters have low capacity and therefore saturation occurs even after 10 mg of supplementation, resulting in excretion of excess taurine. Taurine is mainly excreted by urine in healthy humans and excretion is reduced during starvation or kidney failure. It is interesting to note that de novo synthesis of taurine in the brain is very low and is highest in hepatocytes. It has been reported that taurine can be transported across the BBB by a sodium-dependent carrier mechanism in neuronal culture and this uptake can be blocked by β-alanine [210].
Efficacy in animal models
Sulindac
Several animal studies have reported the therapeutic benefit of sulindac (42) in AD models. In the LPS-induced AD model, pretreatment with oral sulindac for 3 weeks at 3–7 mg/kg dose reduced Aβ production and inhibited memory impairment in male ICR mice [104]. In aged Fischer 344 rats, behavioral studies using radial arm water maze and contextual fear conditioning showed improved learning and memory in animals upon pretreatment with sulindac. Interestingly, HPLC data showed aged mice with higher levels of sulfone metabolite in the blood [181]. In Sprague-dawley rats, intraperitoneal infusion of sulindac increased GSH levels and showed neuroprotective effects after ischemic reperfusion injury [211].
Sulfonic acids
Oral administration of 1 g/kg/day taurine (49) in double transgenic mice (APPswe/PSdE9) ameliorated cognitive deficits. In an oligomeric Aβ-infusion model, mice treated with 250 mg/kg/day taurine showed improved performance on the Y-maze test [212]. Treatment with taurine in 5 × FAD transgenic mice showed increased glutamate uptake by the brain. Several preclinical studies also showed that chronic administration of taurine was well tolerated by the animals [202, 213]. In TgCRND8 mice, 30–100 mg/kg/day treatment with homotaurine caused a significant reduction in Aβ plaque burden and dose-dependent reduction in plasma Aβ levels [214]. Oral pretreatment with taurine (50 mg/kg/day) for 15 days also inhibited streptozotocin-induced cognitive impairment and neurotoxicity due to GSH depletion in aged Wistar rats [215]. Passive avoidance tasks in aged FVB/NJ mice upon chronic taurine treatment (0.05% in water) showed marked improvement, indicating the beneficial effect of taurine on age-related cognitive decline [216]. Taurine also protected mice from chemical-induced memory impairment, without affecting motor coordination and locomotor activity. In aged dogs, taurine supplementation (500 mg/kg) improved T-maze performance at 8 and 12 months [217].
Clinical trials
Sulindac
Sulindac sulfone, also known as Exisulind is approved for use as an antineoplastic agent. Early clinical studies showed that Exisulind was well tolerated by patients without any serious adverse effects. This drug has not been tested for AD, although preclinical data shows therapeutic potential of sulindac and its metabolites in AD [218].
Sulfones
Inhibition of the 5-HT6 receptor improves cholinergic and glutamatergic neurotransmission and has been hypothesized to improve AD-related cognitive impairment, learning, and memory deficits in preclinical models. However, small-molecule inhibitors of 5HT6 have failed to provide safety profile and therapeutic benefit in phase II and phase III clinical trials in healthy and AD patients. In two randomized clinical trials for SB-742457 (43) (Intepirdine) in patients with mild to moderate AD, treatment (15 and 35 mg daily) failed to show any cognitive benefits over placebo. The first study involved 574 patients on intepirdine alone and in the second one, patients (n = 684) were also given donepezil. Cognitive assessment was done using the ADAS-Cog score [219]. A phase I clinical study exploring tolerability and PK properties of SAM-315 (44) in 56 healthy volunteers was discontinued (NCT00479440). AVN-322 has shown good tolerability in healthy and MCI subjects; however, a phase II trial of this drug has been discontinued by the sponsoring companies. Similar compounds like intepirdine and latrepirdine (dimebon) also failed to provide therapeutic benefit in combination or as monotherapy in AD patients when compared to donepezil alone in phase III randomized clinical trials [220].
Sulfonic acids
A randomized, placebo-controlled, double-blind phase II clinical study in 58 patients with MCI showed that homotaurine (50) supplementation for 3 months at different doses was well tolerated. However, MMSE and ADAS-Cog tests showed no significant benefits over the placebo group [203]. In a larger phase III clinical trial in 1052 patients with mild to moderate AD, homotaurine supplementation with AChEI for 18 months failed to show significant therapeutic benefit [201]. However, in a phase I study involving 127 healthy volunteers ALZ-801, a valine-conjugated prodrug of homotaurine showed good tolerability after single-dose and 14-day treatment. A phase III clinical study with 300 participants in being planned for 2021 [221]. In 20 patients with MCI and carrying the ApoE4 gene, supplementation of taurine (49) for 1 year slightly improved episodic short-term memory. Serum analysis in these patients showed no significant change in the levels of pro-inflammatory cytokines over time [222]. In another study involving 245 patients with MCI, homotaurine (100 mg/day) showed significant improvement in the MMSE score after 8–12 months of treatment [217].
Sulfonamide derivatives
Design rationale and pharmacological target
The sulfonamide group is an important sulfur-containing motif used in medicinal chemistry. Since the discovery of sulfonamide-based antibacterial drugs, more and more compounds in this category are found to have a wide range of biological activities such as antibacterial, antifungal, anti-inflammatory, antioxidant, anticancer, antiviral, and anti-malarial [223]. Sulfonamide group which is considered a bioisostere of acrylamide has unique features such as stability against hydrolysis, resistance to reduction at sulfur [224]. Because of these unique properties, sulfonamide compounds were explored for their varied effects on key enzymes like BACE-1 and AChE in AD pathology [225]. Some of the well-studied targets of sulfonamide compounds are discussed here.
BACE-1 inhibitors
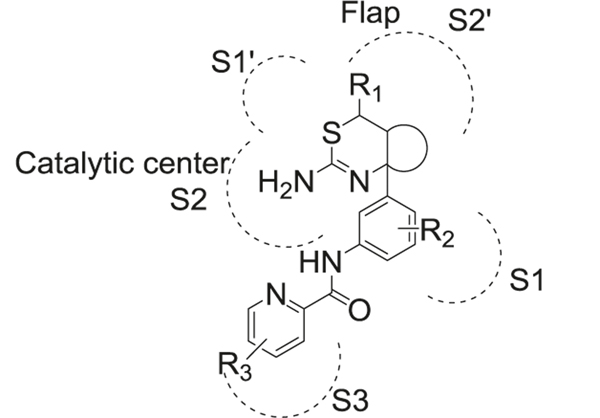
Sulfonamide is a part of originally designed BACE-1 inhibitors. BACE-1 plays an important role in the formation of neurotoxic Aβ [226]. The inhibition of BACE-1 was considered a promising strategy to target AD. It has been shown that in transgenic mice, BACE-1 knockout reduced Aβ1–42 production and subsequent plaque formation in the brain [227]. The catalytic site of BACE-1 consists of several sub-pockets with flexibility toward binding to a ligand. Through X-ray crystallography of sulfonamide-based inhibitors binding to BACE-1, tight binding interactions of sulfonamide to S2 sub-pocket of active sites were discovered [228].
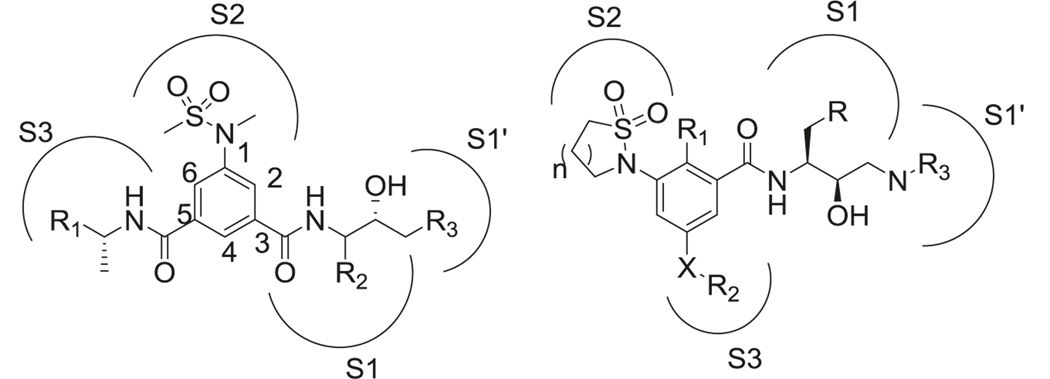
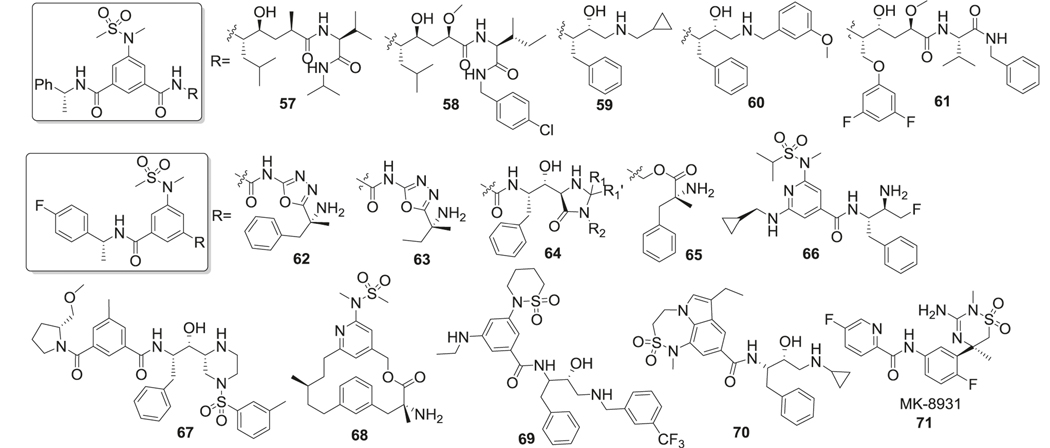
First-generation peptidomimetic BACE-1 inhibitors contained phenyl methanesulfonamide motif to mimic the peptide bond (Fig. 13 left). The sulfone oxygen of sulfonamide interacted with Asn233 and Ser325 by hydrogen bond, but also made ionic interactions with Arg235 [229]. The second sulfonamide oxygen formed hydrogen bonds with Thr232 and Asn233. Van der Waal interactions with Thr231, 232, Asn233, and Arg235 were also found in the S2 unit. Amide binding to S3 unit could be replaced by different hydrophobic groups (57, 64, 65, 66, 67) while still maintaining high levels of enzyme inhibition [230]. The methylbenzylamide in this position displayed the highest binding affinity. Interestingly, in one of the studies, the (R)-methylbenzylamide substitution in the S3 unit showed better potency than (S)-methylbenzylamide [231]. Substitution at the S1 site was generally considered to be a Leu-Ala isostere to mimic the transition state. R2 could be hydrophobic groups such as isobutene (57, 58) or benzyl (59, 60, 61), which indicated that the S1 pocket is tolerant of bulky substitutions [232]. The amide in the S1 site was critical for BACE-1 inhibition. The introduction of 1,3,4-oxadiazole (62, 63) as an ester mimic to the amide group resulted in a non-transition‐state inhibitor [233]. Compared to traditional transition state ligands for BACE-1, both the inhibition efficacy and the stability of these compounds were improved. R3 group could be a variety of motifs such as another amino acid (57), amine (59), benzylamine (60) to occupy the S1’ pocket, which helped in the improvement of the potency, selectivity, and metabolic stability. Another modification of this category was linking the 3 and 5 positions of the phenyl ring in phenylmethanesulfonamide motif to form macrocyclic inhibitors (68) [234]. These macrocyclic inhibitors displayed low nanomolar inhibition of BACE-1 (IC50 as low as 2 nM) and some of them showed better brain penetration and stability compared to noncyclic inhibitors [235] (Fig. 15).
Fig. 13.
Interactions of methanesulfonamide (left) and cyclic sulfonamide (right) analogs with BACE-1 active site
Fig. 15.
Structures of sulfonamide-containing BACE-1 inhibitors
Another important modification was involving the sulfonamide in a ring system (69) (Fig. 13 right). Different ring sizes of cyclic sulfonamides were synthesized and tested, with the most potent inhibition presented by a six-membered ring analog [236]. These compounds also showed good selectivity for BACE-1 over BACE-2 and cathepsin D. Interactions of cyclic sulfonamides with the binding pocket are different from the noncyclic compounds and the sulfone oxygen formed H-bond with Asn-294. Substituted aryl group was preferred as the R group, while substitution of fluorine at R1 site improved both inhibition and selectivity against BACE-1. Amine derivatives in X position were more potent than ether or alkyl derivatives and the ethyl substituent in R2 position was optimal for potency and selectivity. Different benzylic rings and their meta-substituted analogs were preferred at the R3 position for BACE-1 inhibitory activity (Fig. 15).
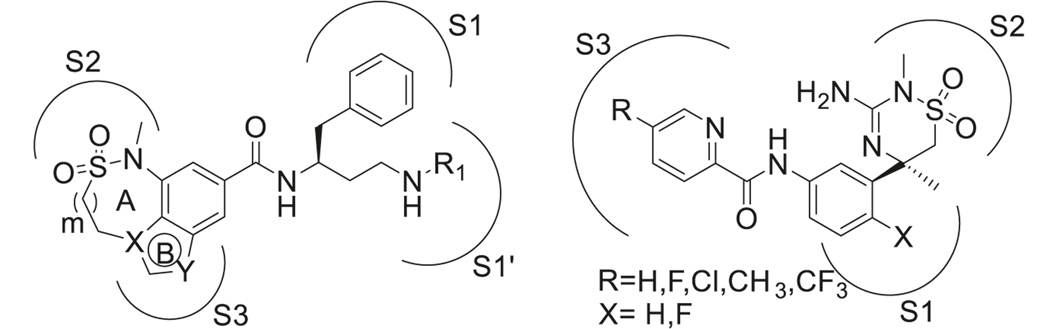
To improve the PK properties of the above mentioned compounds, tricyclic structures (Fig. 14 left) were introduced by linking nitrogen in 1 and 3 positions of the benzylic core, which are the major metabolic sites of these compounds (70) [237]. The SAR studies found that a seven-membered ring A was well-tolerated and displayed the highest potency. A five-membered ring B and nitrogen substitution at X, Y sites rather than carbon were more favorable for inhibitory activity [238]. These compounds displayed drastically improved PK properties compared to the compounds discussed before, with better oral bioavailability and brain penetration [239].
Fig. 14.
Active site interactions of tricyclic (left) and guanidine (right) sulfonamides with BACE-1 active site
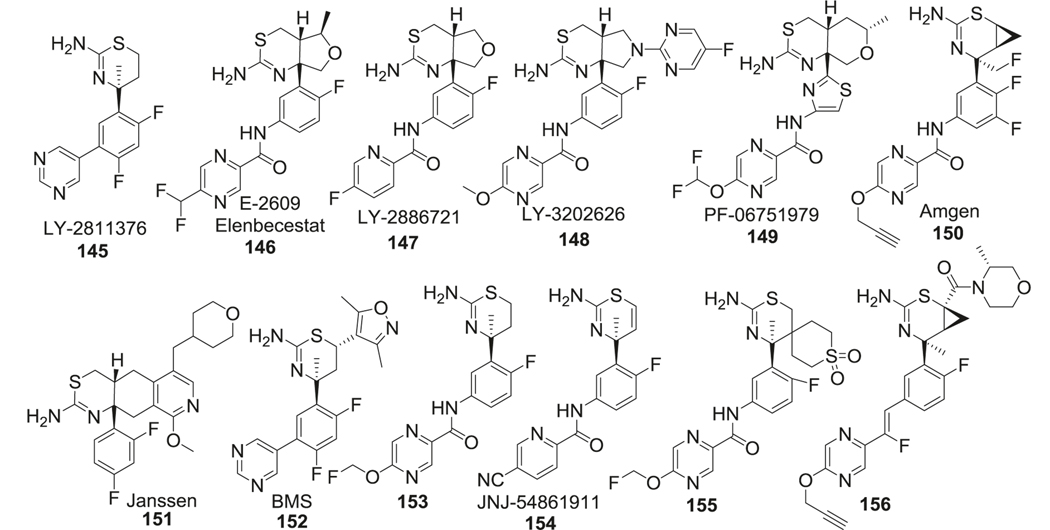
By combining sulfonamide and guanidine scaffolds (Fig. 14 right), MK-8931 (71) (verubecestat/Merck) was synthesized and tested in AD models. It is the first BACE-1 inhibitor to reach phase III clinical trials [240]. MK-8931 (71) has been reported to inhibit BACE-1 and BACE-2 enzymes, but no other aspartyl proteases. Compared to other sulfonamide inhibitors, it has better oral bioavailability, cellular penetration, and brain permeability. It is a nanomolar inhibitor of BACE-1 with IC50 as low as 2.2 nM and 45,000-fold selectivity over cathepsin D. The strong hydrogen bond interactions between the amidine moiety of MK-8931 and the BACE-1 catalytic dyad of aspartic acids were obvious in their co-crystal structure, which contributed to its high potency. Further studies showed that substitution of fluorine at X was vital for BACE-1 inhibitory activity and R groups occupying the S3 site were quite tolerant to electron-donating or electron-withdrawing groups (Fig. 15).
5HT6R antagonists
5-HT6R, or serotonin receptors, which are a member of the G protein-coupled receptor (GPCR) family and ligand-gated ion channels, almost exclusively expressed in the central and peripheral nervous systems and neurotransmitter serotonin acts as a natural ligand. Interestingly, more than 80% of 5-HT6R antagonists contain a sulfonyl group and a heterocyclic ring.
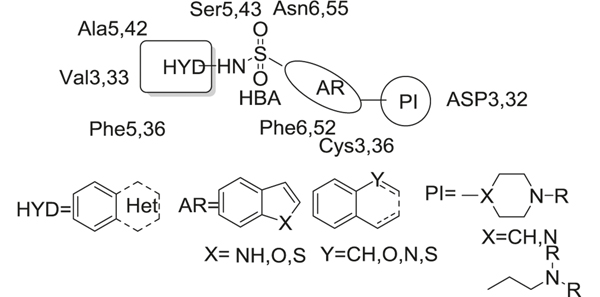
Further SAR studies showed four common key structural elements of 5-HT6R antagonists (Fig. 16): a positive ionizable atom (PI), an aromatic ring-hydrophobic site (AR), a hydrogen bond acceptor group (HBA), and a hydrophobic site (HYD). The PI was generally represented by a positively charged amine such as piperazine and (dimethylamino)-ethyl fragment, which could form electrostatic interactions with the carboxylic acids of Asp3, 32, and the thiol groups of Cys3, 36. The AR region was mainly occupied by indole or indole-like π–electron donor systems, which formed π–π stacking interactions with Phe6, 52. The HBA was either a sulfonamide or sulfone group, which formed hydrogen bonds with Ser5, 43, and Asn6, 55. The HBA also linked the HYD and AR parts together. The HYD was open to diverse aromatic or heteroaromatic rings, which allowed insertion into a hydrophobic pocket formed by Ala5, 42, Val3, 33, and Phe5, 36. A report recently described significant developments in the field of medicinal chemistry and pharmacology of 5-HT6R antagonists as potential therapies for AD [189]. Here we focus on the clinical findings and some recent developments in sulfonamide-based compounds.
Fig. 16.
Active site interactions of sulfonamide-containing 5HT6R antagonists
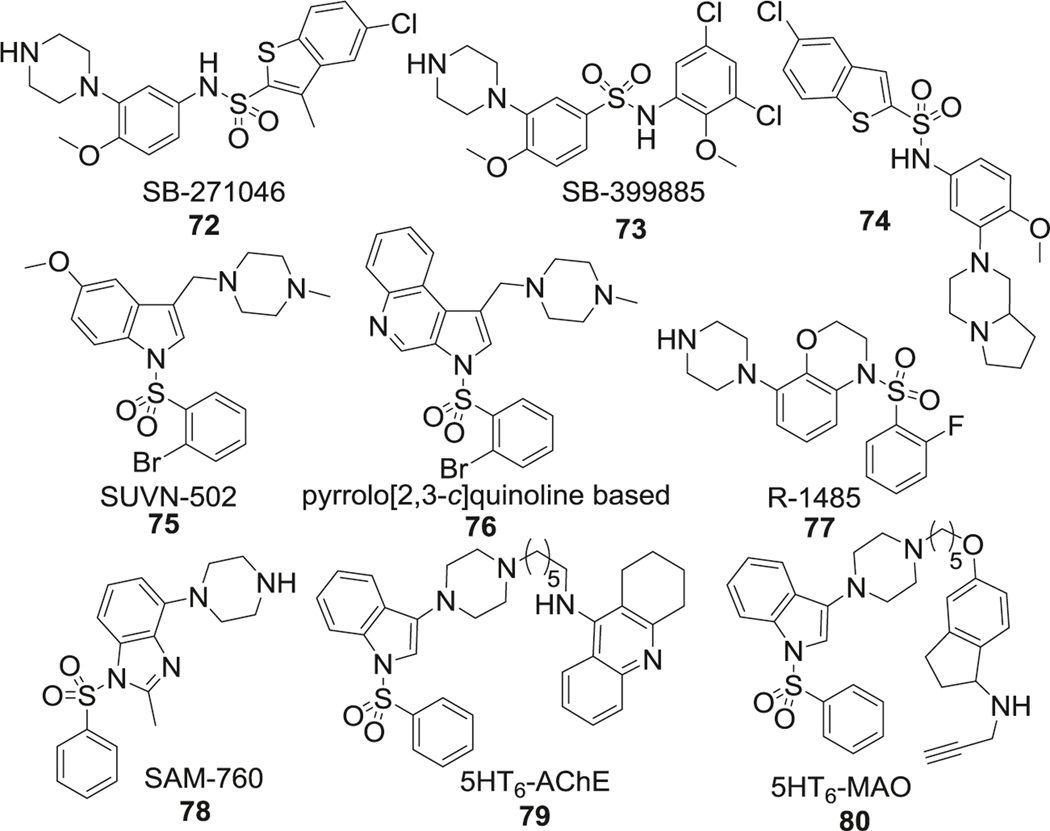
SB-271046 (72) was the first 5-HT6R antagonist reported to enter into phase I clinical trials [241]. SAR study of SB-271046 showed that lipophilic aryl sulfonyl moieties, such as halogen-substituted aromatic rings (73), were beneficial for 5-HT6R affinity. Although SB-271046 provided excellent in vitro antagonistic potency against 5-HT6R (IC50 = 2.0 nM), moderate half-life (t1/2 = 4.8 h), and excellent oral bioavailability (F > 80%) in rat, but further development efforts were halted due to its insufficient BBB permeability. Additional modifications within this family of compounds focused on improving the PK profile. One such modification was protection of the free amine in piperazine (74) to improve logP. In addition, further studies demonstrated that reversed sulfonamides retained the 5-HT6R antagonistic potency of the parent molecule while improved the brain permeability (Fig. 17).
Fig. 17.
Sulfonamide-based 5HT6 receptor antagonists
Another lead compound in this class is SUVN-502 (75) [242]. SAR studies found that inclusion of 5-substituted indole rings was important for the antagonistic potency and electron-donating group such as the methoxy group was favored at this position. Substitution of small alkyl groups like methyl or ethyl were tolerated on the piperazine nitrogen, and bulky substitutions here caused significant loss of potency. Halogen, especially bromine substitution at the 2 position of sulfonamide aromatic ring, improved the 5-HT6R antagonistic potency, while substitution at any other position or multi-substitution was not favored. SUVN-502 displayed high selectivity (>1000-fold) for 5-HT6R over 5-HT2A receptors and showed no affinity for 5-HT1A, 5- HT2C, 5-HT4B, and 5-HT7 receptors. Furthermore, by changing the indole ring in SUVN-502 to pyrrolo[3,2‑c]quinolone (76), a novel class of 5-HT6R antagonists was obtained and these analogs reversed phencyclidine-induced memory deficits and displayed distinct procognitive properties in AD mouse models [243] (Fig. 17).
Other sulfonamide derivatives such as R1485 (77) with benzoxazine ring or SAM760 (78) with tertiary sulfonamide using the amine of the indole ring improved brain penetration, bioavailability, and reduced cardiotoxicity compared to earlier analogs (Fig. 17).
Recently multitarget-directed ligands combining 5-HT6R antagonists with other key AD-related enzyme inhibitors were developed. For example, free amine in the 4-piperazinyl sulfonamide structure of 5-HT6R antagonists was successfully conjugated with MAO inhibitor (79) [244] and AChE inhibitor (80) [245] (Fig. 17).
γ-Secretase inhibitors
γ-Secretase enzymes are involved in Aβ production during sequential proteolysis of APP. Inhibition of γ-secretase by small-molecule γ-secretase inhibitors is considered a promising treatment strategy for AD. Most of the compounds targeting the γ-secretase enzyme in AD either lowered the production of Aβ through enzyme inhibition or modulated enzyme activity resulting in shorter amyloid fragments with lower aggregation potential [246]. Aryl sulfonamide-containing inhibitors were developed to avoid undesired cleavage of Notch protein and the resultant downstream signaling that cause adverse gastrointestinal and dermatological events and deterioration of cognition [247]. Some reviews on the identification of aryl sulfonamide inhibitors and further advances have been published [246, 247]. Here we focus on the clinical applications and some recent development of these compounds in the context of AD.
SAR studies of sulfonamide-based inhibitors found that aryl sulfonamide was essential for inhibitory activity. The preferred aryl group was mainly phenylsulfonamide, but other aryl groups such as thiophene displayed a significant increase in potency [246]. It was also found that the aryl sulfonamide group was sensitive to modifications and the 4-chlorophenyl group was the optimal substituent. Both mono and di substitution of the sulfonamide nitrogen were attempted. For mono substitution of the sulphonamide nitrogen, the reported groups were, methylpentan-1-ol (81), cyclohexane (82), or cycloheptane (83) [247]. For di substitution, different substituted aryl groups and side chains containing branched allyl group or bulky ring systems such as azepan-2-one (84) were tolerated. These modifications improved the potency, brain/plasma ratio, and Notch-sparing selectivity. Besides, sulfonamide nitrogen was also a part of a ring system in the form of morpholine (85) or piperidine (86), which offered better potency and stability [248] (Fig. 18).
Fig. 18.
Sulfonamide-containing γ-secretase inhibitors
The lead resulting from above mentioned SAR is avagacestat (87) containing the key 4-chlorophenyl-sulfonamide pharmacophore, which was the first γ-secretase inhibitor to be tested in a clinical trial for AD but discontinued after phase I [249]. Further SAR studies focused on improving stability and brain penetration [250]. Begacestat (88) containing 5-chlorothiophene moiety displayed improved potency [251], while the trifluoromethyl group offered improved stability [252] (Fig. 18).
Other compounds like ELND006 (89), ELND007(90) [253], or SCH 697466 (86) [254], which introduced piperidine rings in the arylsulfonamide scaffold improved Notch-sparing selectivity. However, this scaffold displayed poor PK properties. Hence further modifications still focused on improving bioavailability and stability. Compared to avagacestat and begacestat, these advanced compounds not only showed better Notch-sparing selectivity but also enhanced bioavailability and metabolic stability (Fig. 18).
AChE inhibitors
Loss of cholinergic neurons and the subsequent reduction of ACh levels have been associated with loss of synapse, cognitive impairment and dementia observed in AD patients [40, 255]. AChE and BuChE negatively affect the levels of neurotransmitters and contribute to the initiation of Aβ self-assembly [256]. AChE or BuChE inhibitors could increase neurotransmitters concentration, thus supporting cognitive improvement [257]. However, modulation of ACh levels through enzyme inhibitors has only shown to be effective for a short duration as AD therapy. In addition, although cholinesterase inhibitors like donepezil, memantine, rivastigmine improved cognition and improved management of AD, they were unable to reverse the disease progression [258]. Despite this, recent studies have shown that modulation of the cholinergic system could still provide therapeutic benefit in AD by influencing the cholinergic loss, cognitive impairment, and amyloid-related pathologies. Sulfonamide was developed as a new scaffold to improve the potency of AChE or BuChE inhibitors. It was reported that the oxygen in the sulfonamide group formed H-bond interactions with residues in the peripheral anionic site (PAS) and the amide moiety of the sulfonamide interacted with the catalytic or acetylation site (CAS) of AChE [259]. The first developed sulfonamide-based inhibitor was dimethyl piperidine aryl sulfonamides (91) [260]. Further SAR studies found that the 2,6-dichloro substituted aryl sulfonamides presented the best potency and improvement in memory function in animal studies. Replacement of piperidine with N-substituted piperazine or different substituted aryl groups (92) [261], bi (93) [262], or tri (94) [263] sulfonamide compounds were designed. However, these compounds failed to show any improvement over the parent mono-sulfonamide. Introduction of the sulfonamide to existent AChEIs was another useful strategy to improve the potency [264]. The hybrid tacrine-sulfonamide derivatives (95) showed ~sixfold improvement in potency compared to tacrine alone. An AChEI was also conjugated with an MAO-B inhibitor by sulfonamide linker (96) [265] (Fig. 19).
Fig. 19.
Sulfonamide-containing inhibitors of AChE
NLR family pyrin domain containing 3 (NLRP3) inhibitors
NLRP3 inflammasome is recognized as a novel target for the treatment of AD. NLRP3 or crypyrin, is a sensor molecule, predominantly expressed in macrophages. Its complex with adapter proteins such as ASC and procaspase-1 is known as NLRP3 inflammasome, which plays a crucial role in the immune response. Activation of NLRP3 inflammasome negatively influences Aβ aggregation and tau phosphorylation, leading to the progression of AD pathology and cognitive deficits.
Sulfonamide is one of the most used motifs in the development of NLRP3 inhibitors [266]. SAR studies indicated that the presence of sulfonyl and benzamide moieties in the inhibitor structure is necessary for NLRP3 inhibitory activity [267]. Small-molecule inhibitors like MCC950 (97), CRID1 (99), JC121 (102), and JC124 (103) that prevented activation and formation of caspases and interleukins via NLRP3 have been studied. CP424174 (98) selectively inhibited posttranslational processing of IL-1β and presented a potent anti-inflammatory response [268]. Also, immunofluorescence staining showed that TAK242 (100), a specific inhibitor of toll-like receptor (TLR4) decreased Aβ-induced NLRP3 activation in microglial cells of APP/PS1 transgenic mice [269]. These compounds have been reviewed by Fulp et al. (97–103) [267]. However, none of them have been studied clinically as potential AD therapeutics (Fig. 20).
Fig. 20.
Sulfonamide-containing NLRP3 inhibitors
Phosphodiesterase 5 (PDE5) inhibitors
Phosphodiesterases (PDEs), which interfere with signaling pathways of secondary messengers such as NO, cGMP, PKG, CREB, and histone deacetylases (HDACs), have gained much attention as drug targets for neurodegenerative diseases [270]. Some marketed PDE5 inhibitors for the treatment of erectile dysfunction and arterial pulmonary hypertension, including sildenafil (104), and vardenafil (105), have been tested as treatment options for AD [271, 272]. These PDE5 inhibitors showed restoration of cognitive function in AD mouse models [273]. In addition to enzyme inhibition, sildenafil (104) has also been reported to improve cerebral blood flow and suppress neuronal apoptosis and neuroinflammation [274] (Fig. 21).
Fig. 21.
Sulfonamide-containing PDE5 inhibitors
Generally, guanine or guanine-like groups were critical for binding to the active site of PDE5 and their inhibitory activities [275]. The sulfonamide group was chosen due to lower lipophilicity and increased solubility [276]. Vardenafil is a better selective PDE5 inhibitor than sildenafil due to the arrangement of the nitrogen atom in the heterocyclic core, which offers a higher affinity toward PDE5 [277]. The alkyl substituent on the piperazine ring in vardenafil does not interfere with binding but enhances its permeability. New generation of PDE5 inhibitors lodenafil (106), udenafil (107), and mirodenafil (108) offer more selectivity, but none of them have been approved by the FDA (Fig. 21).
In vitro/in vivo ADMET
BACE-1 inhibitors
Although many studies report the high efficacy of BACE-1 inhibitors in vitro, very few of them retain their potency in vivo. This is due to difficulty of peptidomimetics like piperazine-sulfonamides in accessing BACE-1 enzymes across the BBB. In addition, these drugs are substrates for efflux pumps like P-gp which limit their availability for effective brain penetration to show efficacy in the clinical setting [278]. However, with recent advances using high-throughput screening and X-ray crystallography, several small-molecule inhibitors were developed that can effectively cross the BBB [279]. Mass spectrometry analysis of APP mice treated with isonicotinamide sulfonamide (66) showed brain concentrations closer to its IC50. However, PK studies showed high clearance due to susceptibility to efflux pumps like P-gp. PK studies involving rats and monkeys showed good oral availability of verbucestat at 3 mg/kg and chronic toxicology studies showed a good safety profile while retaining its pharmacological action [280, 281].
5HT6R antagonist
5HT6R antagonist SB-271046 (72) showed good oral bioavailability with a minimum oral effective dose of 0.1 mg/kg and Tmax of 4 h. It showed a plasma half-life of 4.8h in rats with very low brain penetration (10%). SB-399885 (73), however, had higher brain penetration. SB-271046 presented a very low clearance rate (7.7 ml/min/kg), but it was found to have no effect on P450 enzymes indicating low hepatotoxicity. SAM760 is orally bioavailable and predominantly metabolized in the liver by CYP3A (85%). The primary metabolite of SAM760 (78) was found to be benzene sulfinic acid formed by thiol reductive cleavage. It has an average half-life of 27–34 h [282].
γ-Secretase inhibitors and modulators
γ -Secretase inhibitors pose a similar PK challenge due to poor substrate specificity and bioavailability. Sulfonamide pyrazole (89,90) compounds showed well in vitro inhibition of γ-secretase (IC50 = 4 nM), but poor oral bioavailability in Sprague-Dawley rats at 100 mg/kg dose. Additionally, the compound showed a very high clearance rate [283, 284]. Notch-sparing inhibitor BMS-708163 (87), a diazolylbenzyl sulfonamide, showed good selectivity toward APP-cleavage and was well tolerated in healthy subjects upon single oral administration of 400 mg and a daily dose of 150 mg for 4 weeks. It also showed a Tmax of 1–2 h in blood with a plasma half-life of 40 h [249]. Another sulfonamide ELND-006 (89) showed good brain penetration in PDAPP mice at 0.3 mg/kg as measured by LC–MS [285]. In vivo toxicity studies in these animal models also showed a good safety profile for MK-8931 [281, 283].
AChE inhibitors
Well-known cholinesterase inhibitors like tacrine are generally well absorbed orally and achieve peak concentration in <2 h. Tacrine is highly protein-bound and metabolized extensively by CYP450. Hepatotoxicity is one of the main adverse effects of these compounds. Computational methods showed that piperazine-sulfonamides (92), inhibitors of BuAChE showed good solubility and absorption parameters, while also showing moderate BBB penetration. However, while AMES toxicity prediction showed no DNA damage, the compounds showed mild hepatotoxic effects indicated by their inhibition of CYP450 [278, 279].
NLRP3 inhibitors
As NLRP3 mediates ATP sensitive potassium channels, one of the main adverse effects of NLRP3 inhibition is cardiotoxicity. Microsomal stability studies showed that MCC950 (97) was very stable in the liver with over 70% remaining after 1 h. The compound showed very less binding to CYP enzymes indicating a lack of significant hepatotoxicity. At the therapeutic IC50 of 10 nM, the compound did not show any cardiotoxicity as measured by hERG safety assay. PK studies in C57BL/6 mice using 3 mg/kg iv dose and an oral dose of 20 mg/kg showed that MCC950 has good oral bioavailability (68%) and a plasma half-life of 3 h [286]. Another well-studied NLRP3 inhibitor glyburide, an antidiabetic medication. It is almost completely absorbed from the gastrointestinal tract and single-dose administration in normal subjects showed peak absorption in 4 h with a terminal half-life of about 10h. It is extensively bound to serum proteins (99%). Liver enzyme CYP2C9 is thought to be the main metabolizing enzyme for glyburide. Glyburide and its metabolites (trans and cis hydroxyl derivatives) are excreted by both bile and urine [287].
PDE5 inhibitors
PDE5 inhibitors like sildenafil (104) and vardenafil (105) are orally bioavailable and their PK and metabolism have been well studied [288]. Oral sildenafil dose has a bioavailability of 41% with a plasma half-life of 4.5 h and a Tmax of 0.5–2.5 h. It is rapidly cleared from the body with concentration barely detectable after 24 h due to first-pass metabolism.
Efficacy in animal models
BACE-1 inhibitors
BACE-1 inhibitors like MK-8931 (71) showed a reduction in Aβ accumulation in brains of Sprague-Dawley rats and cynomolgus monkeys. MK-8931 also reduced the plasma and brain cortex levels of Aβ40 in the animals. In addition, MK-8931 reduced Aβ production in the spinal cord and cortex of monkeys [280, 281].
CRND8 mice treated with piperazinyl sulfonamides (67) showed lower plasma levels of Aβ40, but not in the cortex of the treated animals [278]. Piperazine derivatives also prevented the loss of synapse by reducing calcium stores in APP knock-in mice [279].
5HT6R antagonist
In aged mice inhibition of 5HT6R by SB-271046 (72) and SB-399885 (73) reversed the deficits in non-spatial recognition memory and working memory [289]. Animals treated with 3–30 mg/kg of SB-271046 and SB-399885 showed a dose-dependent improvement in working memory assessed by spontaneous alternation in T-maze. In Wistar rats, repeated oral administration of SB-271046 and SB-399885 at 30 and 10 mg/kg dose, respectively, reversed scopolamine-induced amnesia [290]. However, these compounds had no significant effect on spatial learning. Preclinical studies in AD rats showed significant cognitive improvements on radial arm maze and water maze tests after SUVN 502 treatment (75) by modulating glutamate levels [274].
γ-Secretase inhibitors and modulators
Preclinical studies in rodent models showed that γ-secretase inhibition reduced Aβ accumulation in the brain and also improved cognition [291]. Begacestat (88) caused a robust decrease in Aβ levels in the brain, plasma, and CSF and also improved the performance on contextual fear conditioning tests [292, 293].
NLRP3 inflammasome inhibitor
NLRP3 deficient mice have been reported to show lowered levels of inflammatory cytokines as well as reduced brain caspase levels [294]. Benzene sulfonamides such as JC124 (103) lowered oxidative stress levels in CRND8 mice. Treatment with JC124 also provided neuroprotective effects by preserving synaptic functions and lowering brain levels of Aβ in mice [295]. In the LPS-induced AD mouse model, sulfonamide MCC950 (97) inhibited caspase activation and also improved cognitive function as measured by spontaneous T maze [286]. Sulfonamides CP-424174 (98), showed selective inhibition of IL-1 but not of IL-6 or TNFα [296]. TLR4 inhibitor TAK242 (100) provided neuroprotection by inhibiting inflammatory cytokine production and decreasing Aβ-induced NLRP3 activation in microglial cells in APP/PS1 mice [269].
PDE5 inhibitors
Several PDE5 inhibitors have shown promise in preclinical AD models. Treatment of female Tg2576 mice with sildenafil (104) (15 mg/kg, i.p) for 5 days caused an increase in transcription of memory-related genes and improved cognitive performance on Morris water maze. Besides, chronic treatment with sildenafil for 5 weeks caused reduction of tau phosphorylation in mice brains [297]. In APP/PS1 mice, sildenafil treatment (3 mg/kg, i.p) showed improvement in behavioral tasks like fear conditioning test and radial arm water maze test. Sildenafil treatment improved contextual learning and working memory in the transgenic animals [298].
Clinical trials
Several sulfonamide derivatives have shown efficacy in preclinical models of AD. However, very few have been developed for clinical trials owing to their poor bioavailability and efficacy issues. BACE-1 inhibitor MK-8931 (71) was developed by Merck and entered phase III clinical trial, however, it failed to show efficacy over the placebo. Plasma levels of Aβ showed no significant change over placebo. Similarly, no improvement in cognitive assessment was observed. A phase III randomized, placebo-controlled, parallel-group, double-blind clinical trial was conducted to study the efficacy of MK-8931 (oral, 12 mg/dose) in prodromal AD patients. MK-8931 showed a reduction in Aβ40 levels in healthy and AD patients over 36 h [281]. In a phase III study with 40 participants, treatment with MK-8931 (40 mg/day) showed decreased cognitive performance on ADAS−Cog compared with the placebo group. In addition, psychiatric problems like anxiety, depression, and nightmares worsened with MK-8931 [299]. Other BACE-1 antagonists like LY3314814, CNP520 similarly failed to show efficacy over the placebo in clinical trials. Difficulty in developing secretase inhibitors is due to off target effects of these compounds. For example, phase II clinical trial of BACE-1 inhibitor LY2886721 developed by Lily, was terminated due to hepatotoxicity observed in patients.
Phase I clinical trial for SUVN-502 (75) showed that it is well tolerated for up to 200 mg of single oral dose in healthy young and old adult volunteers. A phase IIa clinical trial (NCT02580305) for SUVN-502 in combination with donepezil and memantine was done in 563 patients with mild to moderate AD. The placebo-controlled trial compared SUVN-502 at doses 50 and 100 mg for 26 weeks with cognition assessment using ADAS-Cog as the primary outcome. The treatment however failed to produce significant benefit over placebo treatment. SAM760 (78) developed by Pfizer underwent multiple phase I clinical trials. These trials included single and multiple ascending doses and evaluated the safety and tolerability of the drug. In phase II placebo-controlled study, 30 mg oral dose of SAM-760 given as capsule showed no added benefit over donepezil [282].
Several clinical trials studied the safety and tolerability of γ-secretase inhibitor avagacestat (87). In a phase II study (n = 200), 25–125 mg/day oral dose of avagacestat showed gastrointestinal and dermatological side effects. In addition, higher doses of worsened cognitive performance on ADAS-Cog score in a 24-week study [300]. A phase I study involving coadministration of begacestat (88) and donepezil in 47 randomized healthy subjects was recently completed (NCT00959881).
AChE inhibitors like tacrine, donepezil, and galantamine have traditionally been used for symptomatic relief of AD-related cognitive impairment. These inhibitors have also been reported for side effects involving the peripheral cholinergic system such as dyspepsia and appetite loss. Most of these side effects seem to be dose dependent. Another serious side effect observed involves syncope and fall caused by cholinergic bradycardia [301].
NLRP3 containing inflammasome activation offered promising results in preclinical models and has been studied as a therapeutic target for AD. Inzomelid, an inhibitor structurally related to compound, has completed phase I clinical trial in healthy adults for the cryopyrin-associated periodic syndrome. The trial included 80 participants and safety and tolerability was measured as primary endpoints. NLRP3 inhibition in plasma was measured as a secondary outcome but no data was provided (NCT04015076). NLRP3 inhibitors like glyburide (glibenclamide) and gli-pizide have been studied for their effects on memory and cognition in patients with diabetes [302].
PDE inhibitor sildenafil (104) is given as a single dose, decreased cerebral vascular reactivity while improving cerebral metabolic oxygen and blood flow in a small number of AD patients (N = 14) [303]. However, in cannabis-induced memory impairment, pretreatment with vardenafil at 20 mg/dose failed to preserve cognitive function. This was a randomized double-blind study of 15 patients diagnosed with cannabis-induced cognitive deficits [304]. In patients with erectile dysfunction, a pilot study (n = 27) found that treatment with 100 mg of udenafil at 3-day intervals for 2 months improved cognitive function on MMSE assessment battery and verbal learning tests [305].
Thiazole, benzothiazole, and thiadiazolidinone derivatives
Design rationale and pharmacological target/mechanism
The thiazole and thiazole derivatives are present as a part of many natural and synthetic compounds with a wide range of biological activities, such as antioxidant, antibacterial, antifungal, antitubercular, diuretic, anti-inflammatory, anti-virus, and anticancer activities [306]. The thiazole ring is highly reactive due to the presence of an acidic proton, an electron-donating group (–S–) and an electron-accepting group (C–N) in the ring system. Thiazole fused with other ring systems offers some useful scaffolds such as benzothiazole, phenolic thiazole. Several reviews already describe the synthesis, bioactivity, and therapeutic application of such compounds [307, 308]. Herein, we focus on the recent developments and applications of such scaffolds in the development of AD therapeutics.
Thiazole
Thiazole is a widely used motif in the development of AChEIs. For example, Acotiamide (109) is a thiazole ring containing drugs presenting potent AChE inhibitory activity for the treatment of postprandial fullness, upper abdominal bloating, and early satiation due to functional dyspepsia [309]. It is reported that the planar structure of thiazole in these compounds led to the important electrostatic interactions with the catalytic residues Phe330 and Trp84 present at the anionic site (CAS) of AChE [310]. Modification of C-2 of thiazole is widely explored in the design of AChEIs due to its high reactivity [311]. Substitution at this position was either an aromatic ring or a linker attached to an aromatic system, which enhanced the interaction of the scaffold with the catalytic pocket of AChE [312]. The linker was mostly an amine (110) [313], imine (111, 112) [314, 315], amide (113) [316], or other groups with H-bond donors. For example, by linking benzyl rings to the C-2 position of thiazole through an amide bond (114), new thiazole-based AChEI was synthesized [310]. Further SAR studies found that benzyl substitution in the C-4 position was also necessary for inhibitory activity. The substitution of an electron-withdrawing group in the benzoyl ring at the C-2 position further improved the potency. These compounds in addition to be potent AChEIs, also exhibited inhibition of Aβ aggregation and BACE-1 activity, thus making them prospective lead molecules for AD. Other modifications of the thiazole was coupling molecules with distinct biological activity to form multi-targeted AChEIs. For example, by fusing the pharmacophoric features of AchEIs, donepezil and diarylthiazole, benzylpiperidine-linked diarylthiazoles were designed and evaluated as potential multitarget directed ligands (115) for treatment of AD [317]. Docking studies proved binding of the diaryltriazine and benzylpiperidine portions to the peripheral anionic site (PAS) and the catalytic active site (CAS) of AChE, respectively. Thiazole containing inhibitors displayed significant in vivo anti-AChE, antioxidant and antiapoptotic properties, while some of them also exhibited moderate to high inhibition of AChE-induced Aβ1–42 aggregation [318] (Fig. 22).
Fig. 22.
Structures of thiazole containing AChE inhibitors
Thiazoles were also used in the development of cyclin-dependent kinase 5 (Cdk5) inhibitors. Cdk5 belongs to the serine/threonine-protein kinase family and plays an important role in tau phosphorylation contributing to AD pathogenesis [319]. Like other Cdks, the activity of Cdk5 depends on its interaction with activators, such as p35, p25, and p39. By using high-throughput screening with Cdk5/p25, N-(5-isopropyl-thiazol-2-yl)isobutyramide was found as a leading compound (116), which showed potent inhibition of cdk5 and cdk2/cyclin E (IC50 = ca. 320 nM) [320]. In the presence of increasing concentrations of ATP, the thiazole-based compounds were less effective at inhibiting Cdk5/p25, suggesting their competitive binding at the ATP site [321]. Further SAR studies found that the cyclobutane (117) was the best substitution for 5-position of the thiazole ring, and too bulky or small substituents resulted in reduction of inhibitory potency [322]. The C-2 position could be attached with a linker to an aromatic ring system. Further modifications included linking thiazole rings to another pharmacophore to enhance the binding affinity (118) [321] (Fig. 22).
Benzothiazoles
Benzothiazole, in which benzene ring is fused to the 4,5-positions of the thiazole ring, is one of the most important thiazole derivatives. Benzothiazole is also a widely used scaffold in AD therapeutic development [323] as a part of tau or Aβ aggregation inhibitors due to its high affinities for β-sheet structures [324]. It is reported that the tau aggregation process correlates with cognitive decline and neurodegeneration in AD, thus inhibitors of such aggregation are beneficial against neuronal dysfunction in AD [325]. Benzothiazole inhibitory scaffolds capable of tau aggregation antagonistic activity in vitro have been identified for this purpose. Most of them are polymethine members of the broad π-delocalized lipophilic cation (DLC) family that passively cross cell membranes and accumulate intracellularly depending in part on membrane polarization [326] (Fig. 23).
Fig. 23.
Benzothiazole containing AD therapeutics
Cyanine dyes, which are identified by a polymethine chain linking two benzothiazoles, have shown Aβ and tau aggregation inhibitory properties. For example, N744 (119) [327] showed tau aggregation inhibition with IC50 of 300nM. SAR studies found that modifications of the benzothiazole heterocycle or the polymethine bridge drastically influenced the tau aggregation inhibitory potency [326]. Although at high concentrations self-assembly of N744 was observed which caused the loss of inhibitory activity [328]. Another example of cyanine dye is riluzole (120), a medication used to treat amyotrophic lateral sclerosis [329]. A phase II clinical trial was initiated to evaluate riluzole as a treatment option for mild AD [330]. A prodrug of riluzole, troriluzole (121), was also evaluated in phase II clinical trial for AD [331]. Riluzole is a multitargeted agent that showed neuronal protection by reducing both tau and Aβ aggregation [332]. Another target for riluzole included NMDA receptors and glutamate receptors, which play an important role in AD [333] (Fig. 23).
There are many synthetic dual-specificity tyrosine phosphorylation-regulated kinase 1A (DYRK1A) inhibitors with benzothiazole as their core structure [334]. DYRK1A, a member of eukaryotic serine-threonine protein kinase, belongs to the CMGC group of kinases [335]. Inhibition of DYRK1A has beneficial effects on cognitive dysfunctions observed in Down syndrome and AD [336]. Like most tyrosine kinase inhibitors, DYRK1A inhibitors compete with the ATP-binding pocket at the catalytic site of the kinase [337]. Researchers have found that DYRK1A phosphorylates tau protein and APP in human neuroblastoma cells [338]. Consequently, overexpression of DYRK1A was reported to enhance the levels of phospho-APP and Aβ in the brain [339]. TG003 (122) is the lead DYRK1A inhibitor that showed moderate inhibition of DYRK1A (IC50 = 930 nM) [340]. Further SAR studies found that the hydroxyl group at the five position of the benzothiazole ring is vital for potency as it forms crucial hydrogen bond interactions with Leu241 in the active site of DYRK1A. The carbonyl group is also indispensable for the interaction, but could be replaced by an amide group (123). Further modifications were focused on the fusion of the benzothiazole ring with other ring systems such as pyrimidine (124, 125) [341, 342] or dihydroisobenzofuran (126) [343] to improve inhibitory activity (Fig. 23).
Benzothiazole scaffold has also been incorporated as a part of Aβ binding alcohol dehydrogenase (ABAD) inhibitors. ABAD is a mitochondrial protein and overexpression of ABAD in neuroblastoma cells enhances the cytotoxic effects of Aβ. The binding of Aβ to ABAD has been shown to disrupt the enzymatic activity of ABAD toward specific substrates [344], such as those known to possess neuroprotective effects. For example, the Aβ–ABAD interaction disrupts the neuronal balance of estradiol/estrone, which leads to a reduction in the levels of estradiol [345, 346]. Reduced estradiol levels enhance the formation of Aβ leading to tau hyperphosphorylation and affecting glycogen synthase kinase-3β (GSK-3β) activity [347–349]. Targeting the Aβ–ABAD interaction has emerged as a novel therapeutic strategy for AD. Frentizole (127), an FDA approved immunosuppressant drug, was identified as an Aβ–ABAD interaction inhibitor (IC50 = 200 μM) and considered a potential treatment option for AD [350]. SAR studies identified that the urea pharmacophore is essential for the inhibitory activity. The presence of a benzothiazole ring with a halogen group also enhances activity. Inclusion of a terminal phenyl ring with polar substituents enhanced ABAD inhibition activity (128). Frentizole-based compounds (127, 128) were tested for cellular cytotoxicity and potency in HEK293 mts17β-HSD10 cells. These compounds showed 10–30% cytotoxicity at 25 μM by lactate dehydrogenase assay, however, the measured IC50 values for ABAD inhibition ranged from 2 to 10 μM [351]. Further studies on the frentizole chemotype were aimed at changes to the linker moiety, resulting in the identification of a benzothiazole amine phosphonate analog (129) [352] and a guanidine analog (130) [353] with improved PK profiles (Fig. 23).
Phenothiazine
Phenothiazine is a well-studied structure, which shows tau and Aβ aggregation inhibitory properties. The first reported inhibitor in this category is methylene blue [354] (MB, 131), which has both reduced (Leuco methylene blue, 132) and oxidized forms while the oxidized form is the active form with the inhibitory activities. SAR studies found that redox cycling was important for its potency and any substitution on the nitrogen of thiazine resulted in the loss of activities [355]. MB (131) is also an MAO and NO-synthase inhibitor and promotes cell survi-val. TRx0237 (methylene blue, 131) was evaluated in clinical trials for AD. Other phenothiazine derivatives, for example, toluidine blue O (TBO, 133) and thionine (TH, 134) were investigated and showed tau and Aβ aggregation inhibitory activities [356]. TBO (133) and TH (134) also showed AChE and BChE inhibition [357, 358] and effects on secreted Aβ40, Aβ1–42, sAPPα, and intracellular APP levels in PS70 cells [356]. Future studies focused on introduction of phenothiazine into other pharmacophores forming multitargeted drugs [359–361] (Fig. 24).
Fig. 24.
Phenothiazine containing AD theapeutics
Thiadiazolidinone derivatives
Thiadiazolidinone is another widely used motif with a favorable ADMET profile, which is introduced in the design of AD therapeutics. These derivatives have been shown to engage multiple biological targets and present neuroprotective effects [362–364].
Rhodanine core is the crucial part of the most effective tau aggregation inhibitors [365] and it belongs to the thiazolidine family (Fig. 25). The inhibition mechanism of rhodanine-based compounds is still poorly understood. It is reported that the rhodamine core, which serves as a carboxylic acid bioisostere with low electronegativity, can engage in hydrogen bond and Van der Waals interactions with the tau protein. Further SAR [366] studies of these compounds found that carboxylic acid (135) substituent at the N5 position of rhodanine core was preferred and the optimal linker length between the rhodanine to the carboxylic acid was two carbons. At 3-position, an electron-rich heteroaromatic substitution (136) was deemed necessary. The presence of an aromatic substitution on such heteroaromatic side chain was also important and bulky substituents like naphthalene (137) were tolerated suggesting importance of hydrophobic and/or π–stacking interactions (Fig. 28).
Fig. 25.
SAR for rhodanine derivatives as tau aggregation inhibitors
Fig. 28.
Structures of PPARγ agonists containing thiadiazolidinone ring
Thiadiazolidine (TDZD) itself was first reported as a non-ATP competitive GSK-3β Inhibitor [367]. It is found that over-activity or/and expression of GSK3 in AD patients accounts for memory impairment, tau hyper-phosphorylation, Aβ production, and other inflammatory responses [368]. Consequently, many structural analogs of TDZD such as triazoles, hydantoins, dithiazolidindiones, rhodanines, maleimides, and its derivatives were synthesized and evaluated in GSK-3β inhibition assay [369, 370].
These SAR studies concluded that the nature of the heteroaromatic ring and particularly, the presence of the sulfur atom in the TDZD scaffold is essential for the inhibitory activity (Fig. 26 left). One of the reasons is that the sulfur atom of TDZDs interacted with the thiol group of Cys199 of GSK-3β, which was close to the heteroaromatic ring of TDZD in the ATP-binding site. The aromaticity of the N4 substituent was found to be important, indicating the presence of hydrophobic interactions with a non-polar residue of the binding site, for example, Tyr216. The size of the N2 substituent of the TDZD ring enhanced the inhibitory potency because of additional cation–π interactions between the N2-phenyl ring and the positively charged arginine residues [371, 372]. In this category, compounds such as tideglusib (138) (R = naphthyl, Ar = Ph) and TDZD-8 (139) (R = Me, Ar = Ph) were considered potential AD therapeutics (Fig. 28).
Fig. 26.
Interactions of TZD derivatives with GSK-3β active site, left: TDZD, right: ITDZ
Another class of compounds incorporating this moiety is imino-thiadiazoles (ITDZ, 140) [373]. By changing the carbonyl groups in positions 3 and 5 of thiadiazole with different imino and aryl/alkyl moieties, the stability and cell permeability of the ITDZ scaffold was improved (Fig. 26 right). While TDZD-based compounds are non-competitive inhibitors of GSK-3β, ITDZs are competitive inhibitors in the low micromolar range. SAR studies showed hydrogen bond interactions of the imino hydrogen with Phe67, while the thiadiazole formed S–π interaction with Phe67. Aromatic substitution at the 3-position formed π–π stacking interactions with Phe93 and was vital for the inhibitory activity. Hydrogen bond donor groups such as amine were preferred on the imino nitrogen due to its interaction with charged Asp 90. Aromatic substitution at the N-2 position was tolerant of various sizes and electronic character.
Structural similarity between tau aggregation inhibitors and GSK-3 inhibitors resulted in the generation of thiadiazole with dual GSK-3β and tau-aggregation inhibitory activities [374]. Among the thiadiazoles heterocycle, 2,4-thiazolidinedione (TZD, 141) scaffold was chosen for further modifications. The SAR studies found that an electron-rich heteroaromatic ring such as indole in 5 position, resulted in low IC50 against GSK-3β. Such compounds were also selective to GSK-3β and showed minimal interactions with homologous kinases, such as CK1δ, CK1ε, and Cdc7. The tested compounds also showed anti-aggregation effects on tau fibrils due to the maintenance of the thiazolidine structure and planarity of the molecule.
The chemical structure of TZD derivatives is also shared by agonists of the peroxisome proliferator-activated receptor (PPARγ) [375] such as rosiglitazone (142), pioglitazone (143) [376], and troglitazone (144) [377, 378]. Functionally, PPARγ is involved in the regulation of glucose metabolism, lipid uptake/oxidation, inflammation, and the expression of immunoregulatory genes. In AD, PPARγ plays multiple roles in influencing the pathology of this disease.
The full agonists of PPARγ present some common pharmacophores: polar acidic head groups and a hydrophobic tail separated by an aromatic or aliphatic linker [379]. Acidic hydrogen on the thiazolidinedione ring of these compounds formed a hydrogen bond with Tyr473 and this interaction leads to significant stabilization of the helix of PPARγ near the active site, thus stabilizing active conformation of the receptor leading to gene transcription [380, 381]. Other important residues, His449, His323, and Ser289 formed a polar pocket to further stabilize the active site of PPARγ (Fig. 27). TZD based PPARγ agonists inhibited neuroinflammation through inactivation of NFκB–dependent promoter [382, 383]. Pioglitazone upregulated neuronal expression of PPARγ as evaluated by RT-PCR and western blot analysis [384]. Elevated PPARγ levels blocked BACE expression, thus inhibiting the generation of intracellular Aβ peptide as determined by luciferase assay [385]. One of the disadvantages of these compounds is that they not only influence the trans-expression function of PPARγ but also its transactivation which is responsible for their clinical adverse effects such as heart failure [379, 386, 387]. Further modification focused on the hydrophobic tail to eliminate these treatment-related side effects [388] (Fig. 28).
Fig. 27.
Interactions of TZD derivatives with PPAR-γ active site
In vitro/in vivo ADMET
Thiazoles
PK of Acotiamide (109), the lead thiazole-based AChE inhibitor, was evaluated in preclinical animal models and human subjects. In one of the studies, acotiamide (10 mg/kg) was administered to Sprague-Dawley rats (n = 6) after overnight fasting (12 h), and mean values for Cmax, Tmax, and t½ were 327.80 ± 44.63 μg/mL, 0.25 h, and 3.67 ± 0.95 respectively [389]. A single oral dose of acotiamide (100 mg) was rapidly absorbed in healthy volunteers with mean values for Cmax, Tmax, t½ were 30.82 ng/mL, 2.42 h, and 13.31 h, respectively [390]. Acotiamide is predominantly metabolized by uridyl diphosphate-glucosyltransferase 1 family polypeptide A9 (UGP1A9) and UGT1A8 [391]. The drug is not associated with significant drug–drug interactions with cytochrome P450 (CYP) substrates at clinically effective doses [391]. No specific clinically significant safety concerns are documented for chronic administration of acotiamide (>50 weeks) [392]. In other studies, the hybrid AChEI containing benzylpiperidine-linked diarylthiazoles (115) after single oral administration of 2000 mg/kg dose displayed mean values for Cmax, Tmax, and t½ as 21.20 ± 1.99 ng/mL, 3.66 ± 0.57 h, and 19.42 ± 4.69 h, respectively. No apparent heart, liver, and kidney toxicity was observed after 14 days of treatment in rats [317].
Benzothiazoles
Benzothiazole containing tau aggregation inhibitor, riluzole (120) presented a satisfactory 60% bioavailability after oral ingestion with Tmax of 2 h peak, and the plasma t½ of 12 h, indicating well in vivo stability. The BBB permeability was confirmed by analysis of mouse brain homogenates; however, the drug was also found to be a substrate for P-gp efflux transporters [393, 394]. Riluzole was found to be metabolized mostly in the liver by CYP450-dependent hydroxylation and glucuronidation [395].
PK analysis of benzothiazole phosphonate (129) was conducted by intravenous injection to mice at 10 mg/kg dose. Mean values for Cmax, Tmax, and t½ were 4.8 ng/mL, 0.03 h, and 8.3 h in the brain and 2129.4 ng/mL, 0.03 h, and 5.5 h in plasma. Detectable levels in the brain suggested the ability of benzothiazole-based ABAD inhibitors to cross the BBB [396].
Benzothiazole containing DYRK1A and CDK5 inhibitors are relatively new, and no ADMET studies are reported in the literature.
Phenothiazine
Oral administration of 10mg of MB (131) to adult male volunteers showed high oral bioavailability (72%) and was recovered mainly in urine as leuco methylene blue (132) [397]. When administered as prophylaxis for ifosfamide-associated encephalopathy, MB exhibited a 5 h half-life, a high-volume distribution, and good brain permeability [398]. It is reported that bioavailability correlates with significant liver accumulation after oral administration, which is also found in the cyanine dyes-based tau aggregation inhibitors described before [399].
Among TDZD derivatives, the stability of tideglusib (144) was tested in mouse liver microsomes and found to be highly susceptible to metabolism (t½ = 16 min). The plasma t½ was 5.12 h and Tmax was 0.25 h [400]. In preclinical studies, no significant toxicity was observed after tideglusib treatment (200 mg/kg, p.o.) [401].
PK profiles of PPAR agonists have been extensively studied. Pioglitazone (143), rosiglitazone (142) and troglitazone (144) are absorbed within 2–3 h after oral administration [402, 403]. These drugs are mainly metabolized by CYP 450 in the liver. CYP2C8 and CYP3A4 are the principal enzymes for biotransformation of pioglitazone and troglitazone, while CYP2C9 and CYP2C8 are responsible for the metabolism of rosiglitazone [402–404]. The plasma t½ of pioglitazone and rosiglitazone (pioglitazone: 3–7 h; rosiglitazone: 3–4 h) is shorter than that of troglitazone (7.6–24 h). The adverse effects of pioglitazone included ventricular hypertrophy with congestion of liver and kidneys [405]. Rosiglitazone has been related to cardiovascular risk, especially heart failure [406], while troglitazone is associated with severe hepatotoxicity [407].
Efficacy in animal models
Thiazole derivatives have been studied for therapeutic potential in AD due to their reported anti-inflammatory and neuroprotective activities in addition to their enzyme inhibitory activity described above. Phenyl-butadienyl-benzothiazole derivatives (PBB) have been used as ligands for visualizing tau-related pathologies by PET imaging in transgenic mouse models [408].
In TauP301L mice expressing hereditary tauopathy, riluzole (120) treatment (12 mg/kg/day in drinking water) improved the performance of the mice on radial arm maze and Morris water maze. Immunoblotting of tissue samples showed attenuation of tau hyperphosphorylation by riluzole in the hippocampus region of P301 mice [409]. In transgenic 5 × FAD mice, treatment with riluzole (12 mg/kg, oral) for 5 months improved hippocampus-dependent memory. ELISA assays of brain tissue samples showed a reduction in the toxic Aβ oligomers. However, no change in the mRNA level of APP was observed [410]. Riluzole also attenuated memory defects in rat models at 10 mg/kg oral dose and improved spatial memory in Morris water maze and passive avoidance tests [332].
TDZD-based compound, TDZD-8 (139), has been one of the most useful pharmacological tools used to study GSK-3β function. TDZD-8 showed protective effects against Aβ1–42 neurotoxicity in vivo [411]. The okadaic acid (OKA)-induced AD-like zebrafish model has been used to study the effects of TDZD-8 on AD phenotype [412]. TDZD-8 (1 μM) treatment reduced the ratio of active/inactive GSK-3β in OKA-induced zebrafish model, along with the reduction in the levels of phosphorylated tau. TDZD-8 treatment also restored memory dysfunction in this model as assessed by the spatial alternation paradigm [412].
Another GSK 3β inhibitor, tideglusib (138) was tested in a double transgenic APP/tau mouse model. The treatment reduced Aβ burden by up to 59% and also successfully prevented spatial memory impairment in the Morris water maze test [413]. Interestingly, the treatment also caused upregulation of brain insulin growth factor 1 (IGF-1) in both APP/PS1 transgenic and wild type mice, promoting hippocampal neurogenesis in vitro and in vivo [414, 415]. Kainic acid-induced excitotoxic injury to hippocampal neurons in adult rats was prevented by tideglusib treatment through activation of the PPARγ nuclear receptor [416].
PPARγ agonists offered promising results in mouse models of AD. When tested in APPV717I-transgenic mice, pioglitazone at a dose of 40 mg/kg/day reduced Aβ1–42 deposition by ~25 and 33% in the hippocampus and frontal cortex of the brain compared to non-treated transgenic mice [417]. In this study, pioglitazone (143) also reduced glial activation and BACE-1 expression. Rosiglitazone (142) reversed insulin abnormalities and normalized stress responses in Tg2576 mice [418]. In another study, rosiglitazone treatment attenuated learning and memory deficits in Tg2576 mice [419]. This study demonstrated that rosiglitazone reduced brain Aβ1–42, but did not change Aβ1–42 deposition [419]. Troglitazone (144) treatment reduced Aβ1–42 burden in the transgenic J20 mice [420]. The study also demonstrated enhancement of phagocytic pathology after troglitazone treatment, while reducing the expression of pro-inflammatory cytokines.
Clinical trials
Thiazole and benzo thiazole
Acotiamide (Z-338, 109), has been approved for use in functional dyspepsia. In multiple clinical trials, it was shown to be well-tolerated and safe at doses of 100 mg for 52 weeks (NCT01973790). Riluzole (120) has been initially approved for use in lateral sclerosis. In preclinical studies, it inhibited glutamate receptor activity and showed neuroprotective effects. A randomized double-blind phase II study (NCT01703117) in 42 patients taking donepezil started in 2013. In this study, the effect of riluzole on neuronal marker N-acetyl aspartate was measured using PET scan after 6 months of treatment. In addition, changes in cognition were measured between 36 months. This study is expected to conclude in November 2020. Troriluzole (121), a prodrug of riluzole, is currently in phase II/III clinical trials (NCT03605667). In this randomized open-label study, 350 elderly volunteers with mild AD will be given 280 mg drug or placebo for 48 weeks. Changes in ADAS-Cog score and clinical dementia rating (CDR-sum of boxes) will be evaluated.
TRx0237 (131), a second-generation phenothiazine derivative is a tau aggregation inhibitor. A phase II clinical trial evaluating the safety of oral TRx0237 at 250 mg/day dose was discontinued. A phase III study with more than 800 participants failed to show cognitive improvement in MCI subjects (NCT01689246). Another phase III study with 180 participants, comparing the effect of 200 mg drug in frontotemporal dementia failed to show improvement in cognitive score measured by ADAS-Cog. Another phase II/III trial in 180 patients with all-cause dementia is currently underway.
TDZD derivatives
Thiazolidinediones like rosiglitazone (142), in a 6-month clinical trial in patients with AD and MCI, showed preservation of memory function, but blood Aβ levels remained unchanged [421]. At 8 mg dose, rosiglitazone improved cognitive function in mild to moderate AD patients. However, a phase III clinical trial of rosiglitazone as monotherapy in patients with mild to moderate AD failed to show the therapeutic benefit over that of placebo after 24 weeks [422]. Rosiglitazone treatment showed an adverse effect of peripheral edema (15% compared with 7% of placebo). A double-blind, placebo-controlled randomized controlled trial was done in 25 nondiabetic patients with AD to explore the tolerability of the pioglitazone. At 45 mg dose pioglitazone was well tolerated however, peripheral edema was also observed in these patients. Secondary outcomes involving cognitive impairment showed no significant improvement in drug-treated group over placebo [423]. Another adverse event associated with thiazolidinediones like pioglitazone (143) has been the risk of heart failure. A meta-analysis of as many as 19 clinical trials in more than 16,000 patients showed that treatment of pioglitazone did not increase death due to myocardial infarction, but it did increase the risk of serious heart failure when compared to placebo treatment [424].
Thiourea derivatives
Design rationale and pharmacological target
Dihydrothiazine
Dihydrothiazine is another widely used motif in the development of BACE-1 inhibitors. Dihydrothiazine was independently discovered using fragment-based drug discovery or high-throughput screening methods [425–429]. The SAR [430, 431] studies found that the amidine group formed a strong salt bridge with catalytic aspartates Asp32 and Asp228 in the S2 subunit, and sulfur in the thiazine ring improved the potency and stability compared to the corresponding N or O analog [432]. The substituted aromatic group within the S1 pocket led to important π interactions, which could be either phenyl or thiazole. Substitution of fluorine on this aromatic ring improved the inhibitory potency. This scaffold was used in the development of the next generation of BACE-1 inhibitors and some of the compounds were tested in clinical trials [433]. Here we focus on the recent progress in the inhibitor design and their applications in AD clinical trials (Fig. 29).
Fig. 29.
Interactions of dihydrothiazine derivatives with BACE-1 active site
The aminothiazine LY2811376 (145) developed by Eli Lilly, is one of the lead compounds in this category [427]. LY2811376 showed modest potency (IC50 = 0.24 μM) with tenfold selectivity toward BACE-1 over BACE-2. In vitro cell culture experiments in human kidney cells overexpressing APP and in primary neuronal cell culture of PDAPP transgenic model showed reduction in amyloid production after LY2811376 treatment [427]. Besides, it showed little activity toward other aspartyl proteases like cathepsin D. In addition to dihydrothiazine, dihydrothiazine derivatives with fused tetrahydrofuran (THF), tetrahydropyran (THP), cyclopropane, cyclohexyl- and cyclopentyl-rings, were also used in the development of the BACE-1 inhibitors to improve potency and selectivity [433]. Amongst these compounds, E-2609(elenbecestat, 146), LY-3202626 (147), LY-2886721 (148), PF06751919 (149), Amgen (150), and Janssen (151) [434] were evaluated or planned to be evaluated in clinic trials. LY2886721, another BACE-1 inhibitor showed a similar reduction in Aβ levels in primary neuronal cell culture, albeit at much lower EC50 levels compared to LY2811376 [435]. LY3202626, the last of these classes of compounds developed by Eli Lilly, cleaves APP protein to release C99 fragments. This upstream inhibition lowers Aβ levels in AD patients for a much longer term [436] (Fig. 30).
Fig. 30.
Dihydrothiazine containing BACE-1 inhibitors
One of the strategies to enhance the selectivity was to target the flap region, a flexible antiparallel β-hairpin which controls substrate access in the S2′ pocket [437]. This flap is one of the regions where residue differences exist between BACE-1 and BACE-2. Introduction of the bulky group into the dihydrothiazine backbone or fused ring with dihydrothiazine, such as methyl, fluoromethyl [438], isoxazole (152) [439], pyrazole [438], stabilized the flap region of BACE-1, and improved the selectivity [440]. Further SAR demonstrated that incorporation of different spirocycles to the dihydrothiazine backbone (153, 155) improved the BACE-1 selectivity by 550-fold over BACE-2 [441] (Fig. 30).
The introduction of amide linkage on phenyl substitution of the dihydrothiazine scaffold improved the BACE-1 inhibitory activity. Such an amide linker was generally installed between two aromatic rings allowing the compounds to enter the deep S3 pocket, thus achieving selectivity over cathepsin D [439]. For example, compared to LY2811376 (147), JNJ-54861911 (154) [442] (atabecestat) containing the amide linker between two aromatic rings displayed better potency (IC50 = 9.8 nM). BACE-1 inhibitors like elenbecstat, atabecestat (JNJ-54861911 (154)), and PF-06751979 (149) were reported to lower Aβ levels in CSF and plasma samples in mice through BACE inhibition. Further modifications were focused on improving selectivity over cathepsin D and BACE-2, enhancing the BACE-1 inhibitory activity, improving BBB permeability and metabolic stability. Further modifications involved substitution of the aromatic ring with CN, OCHF2, OCH3, CHF2, allowing deeper insertion into the S3 pocket [443]. One of the problems of amide linker, however, was its metabolic instability. Other linkers such as substituted ethane groups [156] were used to avoid this metabolic liability [444] (Fig. 30).
Thione backbone is used in the development of ABAD inhibitors, which is a relatively new target for AD [445]. A potent ABAD inhibitor, AG18051 (157), containing a pyrazolo[3,4-d]pyrimidine-4(1H)-thione backbone (IC50 = 92 nM) has been reported [446]. SAR studies have shown that the pyrazole ring is important for the ABAD inhibitory activity and substitution of phenyl ring attached to pyrazole, especially with a hydroxyl group (158), improved the potency. The bulky amide group (159) is tolerated and did not significantly influence the activity (Fig. 31).
Fig. 31.
Thiamide containing ABAD inhibitors
Thiourea
Pyrimidinylthiourea derivatives were designed as potential multifunctional anti-AD agents [447]. The SAR studies found the thiourea moiety to be an indispensable scaffold for AChE inhibitory activity. The 4,6-disubstituted pyrimidine was also critical for AChE inhibition and the imidazole ring was the most preferred substituent at the C-6 position of the pyrimidine ring (160). The imidazole ring could be substituted at the 4 positions with aminomethyl moieties to modulate BBB permeability. These compounds were potent AChEIs (AChE, IC50 = 0.067 μM, SI > 597), and displayed specific metal-chelating ability, and significant antioxidant effects. Another modification involved incorporation of MAO-B inhibitory scaffold to the four positions of imidazole ring to target both AChE and MAO-B (161) [448]. Thiourea scaffold has also been used as a metal-chelating motif to link AChE inhibitor pharmacophore (162) with Aβ40 aggregation inhibitor motif (163) [449], M1 inhibitor with AChE inhibitor (164) [450], and MAO-A inhibitor with ABAD inhibitor (165) [451] (Fig. 32).
Fig. 32.
Thiourea containing AChE inhibitors
Thiourea is also a key pharmacophore of somatostatin receptor-4(sst4) agonists. It is reported that sst4 not only plays an important role in learning and memory processes, but also modulates formation of soluble Aβ1–42 [452]. NNC 26–9100 (166) was the first non-peptide agonist that displayed high affinity and selectivity for sst4 [453, 454]. SAR [454] studies found that the thiourea group was critical for sst4 activity and an imidazole substitution on thiourea was preferred. The bulky group was also needed as a secondary substituent on thiourea, for example, an indole group increased the selectivity for sst4. Other modifications, for example, incorporation of thiourea in a ring system forming 2-thiohydantoin (167), could not improve the potency [455]. Fluorescent probe assays showed marked antioxidant potential of these compounds in vitro by inhibition of ROS production. MTT assays in neuronal cells SH-SY5Y, showed low cytotoxicity for thiourea derivatives even at concentrations of 100 μM (Fig. 33).
Fig. 33.
sst4 agonists containing thiourea scafflold
Thiourea has also been used in the development of glutaminyl cyclase (QC) inhibitors. QC, a metalloenzyme upregulated in the brains of AD patients, is responsible for the generation of pyroglutamate Aβ. This modified form of Aβ is considered more toxic, aggregation-prone and forms a major part of Aβ plaques in humans. The SAR studies showed the importance of thiourea functionality for potency [456]. Replacing thiourea moiety with other groups decreased the activity dramatically. Imidazole ring and an aromatic substituent on each side of the thiourea were essential for QC inhibition (168). The imidazole could engage the active site Zn2+ [457]. The aromatic substitution of amine in thiourea is needed. It is reported that the addition of an electron-rich ring system to the four positions of aromatic substituent through a flexible ring enabled insertion into the active site (169), thus enhancing the inhibitory activity [458] (Fig. 34).
Fig. 34.
QC inhibitors containing thiourea scaffold
In vitro/in vivo ADMET
Dihydrothiazine
PK studies of LY2811376 (145) in humans using an oral dose of 90 mg achieved peak plasma concentration at 2 h, while the maximum concentration in CSF was reached at 5 h and was threefold higher than the plasma level. The mean plasma half-life was 40 h. In vivo studies in APP, transgenic mice showed high brain penetration of LY2811376 after an oral dose of 100 mg/kg, achieving brain exposure more than the IC50 levels needed for the Aβ lowering activity. This study also showed its rapid clearance. A 3-month toxicology study in rats showed that LY2811376 at oral doses of more than 30 mg/kg has increased the risk of photogeneration in eyes and neurodegeneration in brain glial cells. However, a follow-up study in BACE-1 knockout mice found it to be unrelated to BACE inhibition [427]. For LY3202626 (148) and LY2886721 (147), oral administration of 10–70 mg dose in healthy volunteers resulted in a plasma Tmax of 4–5 h and a mean half-life of 18–24 h. Similar to LY2811376, this drug also showed increased accumulation in CSF compared to plasma. Metabolite profiling studies of plasma samples showed active metabolism of LY3202626 by demethylation and amide hydrolysis. In vitro studies in hepatocytes also showed oxidation and formation of sulfenic acid intermediates by LY3202626 [435, 459]. PK analysis of PF-06751979 after oral administration at 50–130 mg in human subjects showed plasma Tmax of 4 h and half-life of 29–33 h. The Tmax however increased to 6 h in fed state compared to fasting subjects and the half-life was more than 40 h in older subjects. About 10% of the drug was excreted unchanged in the urine. Long term studies in dogs for up to 9 months showed that PF-06751979 (149) is well tolerated without BACE-2 inhibition related side effects [434]. In TgrasH2 mice, treatment with elenbecestat for 26 weeks at an oral dose of up to 300 mg/kg was well tolerated and did not show any adverse effects [460]. Elenbecestat (E2609, 146) showed a plasma t½ of 12–16 h after single oral dosing of 30 mg/kg in nonhuman primates [461]. Oral administration of JNJ-54861911 (154) in healthy volunteers offered plasma Tmax of up to 2 h after a single dose and 4 h after multiple dosing, indicating rapid absorption. In addition, single dosing showed a half-life of 9–16 h, which increased to 18 h after multiple dosing for 14 days. JNJ-54861911 showed more plasma binding and less CSF accumulation [462].
Thiourea
In vitro membrane permeation assays displayed good BBB permeability of pyrimidine thiourea derivatives (160). In vivo concentrations in plasma were 3 times higher than that in the brain, indicating plasma protein binding of the drug [447]. QC inhibitor PDB150 (168) was reported to have poor brain penetration. PET imaging in animals (mice and rats) treated with radiolabeled PDB150 confirmed poor BBB permeability. Additional experiments with liver microsome from Sprague-Dawley rats showed that PDB150 has a half-life of 1 h [463].
Efficacy in animal models
Dihydrothiazine
Preclinical studies of LY series of BACE-1 inhibitors showed Aβ lowering action in AD mouse models. In female PDAPP transgenic mice, oral administration of LY2811376 (145) and LY2886721 (147) at doses 10–100 mg/kg showed a dose-dependent decrease in Aβ. ELISA studies of the brain cortex showed a similar dose-dependent decrease in C99 levels, a product of APP proteolysis. Similar studies in beagle dogs showed lowered plasma Aβ levels for up to 14 h by 5 mg/kg LY2811376. CSF levels of Aβ also remained low for up to 9 h [427]. ELISA assay of brain samples from male 129/sve treated with subcutaneous PF-06751979 (149) for 5 days at 50 mg/kg showed reduced Aβ42 levels. Dogs treated with JNJ-54861911 (154) for 14 days at a dose of 0.3 mg/kg/day orally, showed a 50% reduction in CSF and brain Aβ levels. In a similar study in monkeys at doses of 0.3–3 mg/kg, JNJ-54861911 significantly and dose-dependently reduced Aβ in CSF and plasma over 6 h. IHC analysis showed that repeated oral administration of JNJ-54861911 reduced the number and area of the Aβ plaques in the APP/PS1 mice [462].
Thiourea
In the Scopolamine-induced amyloid AD model, oral administration of 200 mg/kg pyrimidinethiourea derivatives (160) improved cognitive performance on Morris water maze [447]. IHC analysis of brains from humans and animal models of AD showed early deposition of pyroglutamate Aβ in humans and nonhuman primates whereas it occurs later in transgenic murine models [464]. Several QC inhibitors have shown promise in different transgenic animal models of AD [465, 466].
Clinical trials
Dihydrothiazine
Although BACE-1 inhibitors showed amyloid lowering action in vitro and preclinical models, most of them failed in clinical trials due to risks of toxicity. For example, a safety study of a single oral dose of LY2811376 (145) by Eli Lilly in 30 healthy volunteers was discontinued after phase I due to the risk of retinal toxicity and neurodegeneration. In this study, LY2811376 (500 mg/day) was given as an oral capsule (NCT00838084). A similar compound, LY2886721 (147) reached phase 2 after showing good tolerability and safety in phase I study with 170 healthy volunteers and AD patients treated with an oral dose of 15–70 mg/day. Single ascending dose and multiple ascending dose studies for up to 26 weeks were conducted to determine the tolerability safety and efficacy of the drug. Treatment showed lower levels of Aβ in CSF samples of patients, however, the study was terminated at phase II due to abnormal liver biochemical results in some patients (NCT01561430). It is not clear if this is related to BACE-1 inhibition. For LY3202626 (148), two phase I trials were conducted to evaluate the PK parameters and a third trial to understand the effect of food and dosage form in 30 healthy volunteers. Phase I study in 136 healthy and AD patients showed that the drug was well tolerated at doses 0.1–45 mg and the treatment showed dose-dependent lowering of Aβ40 in CSF with a 90% reduction at 26 mg/day. A phase II trial with 316 volunteers with mild AD was terminated due to the lack of efficacy attributed to test parameters (NCT02791191). The patients received LY3202626 at doses 3–12 mg/day orally for 52 weeks. Tau PET scan to measure neurofibrillary pathology burden was used as the primary outcome and plasma Aβ levels were measured as a secondary outcome in this study. A second phase II trial involved the combination of LY3202626 with donanemab, an antibody that targets amyloid aggregates. However, in 2018, the BACE-1 investigation arm in this clinical trial was discontinued.
Phase I trials for Atabecestat (JNJ-54861911, 154), in healthy volunteers, showed well tolerability, safety, and lowered CSF amyloid levels. Three similar phase I randomized placebo-controlled studies were done in 15, 30, and 45 volunteers at an oral dose of 10–50 mg for 4 weeks [467]. Phase II study in 90 patients with early AD treated with JNJ-54861911 at 5–25 mg/day for 6–12 months day was terminated following elevated liver enzymes in 12 patients [468]. Some patients also reported worsening cognitive defects upon long term treatment. Another phase IIb/3 study was terminated due to liver enzyme related adverse events (NCT02569398). In this double-blind, placebo-controlled study (n = 557), patients were amyloid positive and asymptomatic for AD (at risk of developing dementia) and were receiving 5–25 mg/day for up to 54 months.
Phase I study of elenbecestat (146) in 500 healthy volunteers showed well tolerability and safety. Single and multiple dosing at 5–800 mg showed dose-dependent reduction in CSF Aβ levels after 14 days. A phase II study in 70 volunteers with MCI showed a 50% reduction in CSF amyloid levels in patients treated with elenbcestat at 5–50 mg/day. At 50 mg/day, elenbecestat showed positive cognitive outcomes as measured by ADAS-Cog score. However, some subjects reported nightmares as a possible side effect (NCT02322021). A phase III clinical trial is currently active (n = 1181) in patients with early AD treated with 50 mg oral elenbecestat, for 24 months with a 12-month extension. The study is expected to be completed in 2023 (NCT02956486).
A phase I trial for PF-06751979 (149) developed by Pfizer, was done in 46 healthy volunteers to study the safety and PK PD parameters. Doses of 100–700 mg/day were given in single and multiple ascending studies (NCT02793232). Results showed a reduction in CSF Aβ levels. Two other phase I studies in smaller groups found similar safety and dose-dependent reduction in plasma Aβ levels [460]. However, in 2018, Pfizer discontinued research involving neuroscience research including PF-06751979.
Conclusion
In summary, major classes of sulfur-containing compounds with multitude of pharmacological targets and their preclinical and clinical applications in the development of AD therapeutics are presented. A number of these sulfur-containing compounds that were advanced to human clinical trials have offered limited success. In this review, pharmacological targets, ADMET, and clinical applications of various sulfur-containing compounds with respect to AD are discussed. Due to the complex underlying pathophysiology of AD, therapeutics targeting single pathological events in AD have not been successful, and thus emerging need for multipronged treatment strategies to tackle this devastating disease [469]. Many sulfur-containing compounds belong to disease-modifying drugs [470], which act on multiple targets involved in the pathogenesis of the disease [11]. These compounds are used alone as multifunctional and multitarget therapies [471] or as a part of combination treatments [471, 472] and are expected to provide more efficient therapeutic approaches. Incorporation of sulfur pharmacophore offers improved pharmacokinetic properties along with engagement of multiple targets involved in the complex pathophysiology of AD when fused or merged with other pharmacophores resulting in multipronged approach toward AD therapy. For example, lipoic acid linked with AChE inhibitors formed complexes which targeted both neuroinflammation and oxidative stress in AD pathogenesis (section Organic Thiols, disulfides, and prodrug compounds.) Some the thiol compounds, on other hand, were themselves used as a linker to link or fuse different pharmacological ligands to produce multitargeted therapies. For example, thiourea linker has been used as a part of AChE inhibitor-MAO or Aβ aggregation inhibitor complex, MAO inhibitor-ABAD inhibitor complex, which are discussed in section Thiourea derivatives. Additionally, repurposing of some of the clinically approved sulfur-containing drugs such as riluzole for AD, presents an extremely efficient strategy due to superior PK and known safety profiles of these medications [473].
Organosulfur is essential for vital functions in the human body and generally presents low toxicity. Pharmacological limitations of sulfur based compounds and their applications as AD therapeutics are either due to instability of thiol pharmacophore or metabolism of these compounds in physiological systems. For example, biothiols, thiol based drugs and H2S releasing compounds (sections organic thiols and hydrogen sulfide donors), are known for their efficient antioxidant property. However, in presence of oxygen, these drugs can simultaneously lead to formation of thiyl radicals and superoxides. Inactivation of these harmful thiyl radicals could be impaired under disease conditions. A sustained and controlled release of H2S is necessary for pharmacological action of H2S donors and prevention of any potential toxicity. Also, sulfur usually in the form of thiol, imparts an unpleasant odor to these compounds and this requires formulation as prodrugs [107].
Sulfonamides that form a major class of these sulfur-containing drugs, are well known for the “sulfa-allergy” presumably caused by hypersensitivity of patients to sulfonamides. Sulfonamides also inhibit the folate biosynthesis and some metabolites cause renal insufficiency. However, newer generation of more soluble sulfonamides have been developed to address these issues. Apart from hypersensitivity, drugs like alkyl sulfides have been investigated for hepatotoxicity due to their interaction with cytochrome P450 enzyme system. Sulfur containing drugs like sulindac are given as sulfoxide prodrugs due to their increased solubility and absorption characteristics (section alkyl sulfides). Sulfonic acid derivatives such as taurine and its analogs suffer from limited BBB permeability and require much higher doses to elicit pharmacological response in AD.
Protocols addressing the shortcomings of organic sulfur compounds are constantly evolving and the advantages of such therapeutics as mono- and combination AD therapy are increasingly evident. With recent focus on conducting AD clinical trials in prodromal or preclinical populations with positive diagnostic markers, treatment options such as multitarget oriented sulfur-containing therapeutics hold promise and warrant further investigations.
Acknowledgements
This work was supported in part by the Research Endowment funds from the Center for Drug Design (CDD) at the University of Minnesota, Minneapolis, and by the NIH grant # R01AG062469.
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Meyer B. Elemental sulfur. Chem Rev. 1976;76:367–88. [Google Scholar]
- 2.Oae S. Organic chemistry of sulfur: Springer Science & Business Media; 2012. pp. 33–67. [Google Scholar]
- 3.Huxtable RJ. Biochemistry of sulfur: Springer Science & Business Media; 2013. pp. 121–257. [Google Scholar]
- 4.Maren TH. Relations between structure and biological activity of sulfonamides. Annu Rev Pharmacol Toxicol. 1976;16:309–27. [DOI] [PubMed] [Google Scholar]
- 5.Wilke MS, Lovering AL, Strynadka NC. β-Lactam antibiotic resistance: a current structural perspective. Curr Opin Microbiol. 2005;8:525–33. [DOI] [PubMed] [Google Scholar]
- 6.Association As. 2018 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2018;14:367–429. [Google Scholar]
- 7.Lanctôt KL, Amatniek J, Ancoli-Israel S, Arnold SE, Ballard C, Cohen-Mansfield J, et al. Neuropsychiatric signs and symptoms of Alzheimer’s disease: new treatment paradigms. Alzheimer’s Dement Transl Res Clin Interv. 2017;3:440–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matthews KA, Xu W, Gaglioti AH, Holt JB, Croft JB, Mack D, et al. Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015–2060) in adults aged ≥65 years. Alzheimer’s Dement. 2019;15:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jia J, Wei C, Chen S, Li F, Tang Y, Qin W, et al. The cost of Alzheimer’s disease in China and re‐estimation of costs world-wide. Alzheimer’s Dement. 2018;14:483–91. [DOI] [PubMed] [Google Scholar]
- 10.Tobin MK, Musaraca K, Disouky A, Shetti A, Bheri A, Honer WG, et al. Human hippocampal neurogenesis persists in aged adults and Alzheimer’s disease patients. Cell Stem Cell. 2019;24:974–82.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cummings J, Lee G, Ritter A, Zhong K. Alzheimer’s disease drug development pipeline: 2018. Alzheimer’s Dement Transl Res Clin Interv. 2018;4:195–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham WV, Bonito-Oliva A, Sakmar TP. Update on Alzheimer’s disease therapy and prevention strategies. Annu Rev Med. 2017;68:413–30. [DOI] [PubMed] [Google Scholar]
- 13.Paul BD, Sbodio JI, Snyder SH. Cysteine metabolism in neuronal redox homeostasis. Trends Pharmacol Sci. 2018;39:513–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rani P, Krishnan S, Rani, Cathrine C. Study on analysis of peripheral biomarkers for Alzheimer’s disease diagnosis. Front Neurol. 2017;8:328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butterfield DA, Halliwell B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat Rev Neurosci. 2019;20:148–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hauser AS, Attwood MM, Rask-Andersen M, Schiöth HB, Gloriam DE. Trends in GPCR drug discovery: new agents, targets and indications. Nat Rev Drug Discov. 2017;16:829–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown N. Bioisosterism in medicinal chemistry. Wiley Online Library; 2012. pp. 15–29. [Google Scholar]
- 18.Medved I, Brown MJ, Bjorksten AR, Murphy KT, Petersen AC, Sostaric S, et al. N-acetylcysteine enhances muscle cysteine and glutathione availability and attenuates fatigue during prolonged exercise in endurance-trained individuals. J Appl Physiol. 1985;97:1477–85. [DOI] [PubMed] [Google Scholar]
- 19.Jones SV, Kounatidis I. Nuclear Factor-Kappa B and Alzheimer disease, unifying genetic and environmental risk factors from cell to humans. Front Immunol. 2017;8:1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan CY, Greene LA. Prevention of PC12 cell death by N-acetylcysteine requires activation of the Ras pathway. J Neurosci. 1998;18:4042–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qian HR, Yang Y. Neuron differentiation and neuritogenesis stimulated by N-acetylcysteine (NAC). Acta Pharmacol Sin. 2009;30:907–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ates B, Abraham L, Ercal N. Antioxidant and free radical scavenging properties of N-acetylcysteine amide (NACA) and comparison with N-acetylcysteine (NAC). Free Radic Res. 2008;42:372–7. [DOI] [PubMed] [Google Scholar]
- 23.Kahns AH, Bundgaard H. Prodrugs as drug delivery systems. 107. Synthesis and chemical and enzymatic hydrolysis kinetics of various mono-and diester prodrugs of N-acetylcysteine. Int J Pharm. 1990;62:193–205. [Google Scholar]
- 24.Lee KS, Kim SR, Park HS, Park SJ, Min KH, Lee KY, et al. A novel thiol compound, N-acetylcysteine amide, attenuates allergic airway disease by regulating activation of NF-kappaB and hypoxia-inducible factor-1alpha. Exp Mol Med. 2007;39: 756–68. [DOI] [PubMed] [Google Scholar]
- 25.Ham Y-H, Jason Chan K, Chan W. Thioproline serves as an efficient antioxidant protecting human cells from oxidative stress and improves cell viability. Chem Res Toxicol. 2020;33:1815–21. [DOI] [PubMed] [Google Scholar]
- 26.Liu J, Chan KJ, Chan W. Identification of protein thiazolidination as a novel molecular signature for oxidative stress and formaldehyde exposure. Chem Res Toxicol. 2016;29:1865–71. [DOI] [PubMed] [Google Scholar]
- 27.Porta P, Aebi S, Summer K, Lauterburg BH. L-2-oxothiazolidine-4-carboxylic acid, a cysteine prodrug: pharmacokinetics and effects on thiols in plasma and lymphocytes in human. J Pharmacol Exp Ther. 1991;257:331–4. [PubMed] [Google Scholar]
- 28.Roberts JC, Nagasawa HT, Zera RT, Fricke RF, Goon DJ. Prodrugs of L-cysteine as protective agents against acetaminophen-induced hepatotoxicity. 2-(Polyhydroxyalkyl)-and 2-(polyacetoxyalkyl) thiazolidine-4 (R)-carboxylic acids. J Med Chem. 1987;30:1891–6. [DOI] [PubMed] [Google Scholar]
- 29.Jones MG, Hughes J, Tregova A, Milne J, Tomsett AB, Collin HA. Biosynthesis of the flavour precursors of onion and garlic. J Exp Bot. 2004;55:1903–18. [DOI] [PubMed] [Google Scholar]
- 30.Mandal PK, Saharan S, Tripathi M, Murari G. Brain glutathione levels-a novel biomarker for mild cognitive impairment and Alzheimer’s disease. Biol Psychiatry. 2015;78:702–10. [DOI] [PubMed] [Google Scholar]
- 31.Fernandez-Checa JC, Garcia-Ruiz C, Ookhtens M, Kaplowitz N. Impaired uptake of glutathione by hepatic mitochondria from chronic ethanol-fed rats. Tracer kinetic studies in vitro and in vivo and susceptibility to oxidant stress. J Clin Investig. 1991;87:397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Puri RN, Meister A. Transport of glutathione, as gamma-glutamylcysteinylglycyl ester, into liver and kidney. Proc Natl Acad Sci USA. 1983;80:5258–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsan MF, White JE, Rosano CL. Modulation of endothelial GSH concentrations: effect of exogenous GSH and GSH monoethyl ester. J Appl Physiol. 1989;66:1029–34. [DOI] [PubMed] [Google Scholar]
- 34.Wellner VP, Anderson ME, Puri RN, Jensen GL, Meister A. Radioprotection by glutathione ester: transport of glutathione ester into human lymphoid cells and fibroblasts. Proc Natl Acad Sci USA. 1984;81:4732–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zeevalk GD, Manzino L, Sonsalla PK, Bernard LP. Characterization of intracellular elevation of glutathione (GSH) with glutathione monoethyl ester and GSH in brain and neuronal cultures: relevance to Parkinson’s disease. Exp Neurol. 2007;203:512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berkeley LI, Cohen JF, Crankshaw DL, Shirota FN, Nagasawa HT. Hepatoprotection by L‐cysteine‐glutathione mixed disulfide, a sulfhydryl‐modified prodrug of glutathione. J Biochem Mol Toxicol. 2003;17:95–7. [DOI] [PubMed] [Google Scholar]
- 37.More SS, Vince R. Potential of a γ-glutamyl-transpeptidasestable glutathione analogue against amyloid-β toxicity. ACS Chem Neurosci. 2012;3:204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Potter LT. Synthesis, storage and release of [14C]acetylcholine in isolated rat diaphragm muscles. J Physiol. 1970;206:145–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nachmansohn D, Machado A. THE FORMATION OF ACET-YLCHOLINE. A NEW ENZYME:“CHOLINE ACETYLASE”. J Neurophysiol. 1943;6:397–403. [Google Scholar]
- 40.Ferreira-Vieira TH, Guimaraes IM, Silva FR, Ribeiro FM. Alzheimer’s disease: targeting the cholinergic system. Curr Neuropharmacol. 2016;14:101–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cao Z, Tsang M, Zhao H, Li Y. Induction of endogenous antioxidants and phase 2 enzymes by alpha-lipoic acid in rat cardiac H9C2 cells: protection against oxidative injury. Biochem Biophys Res Commun. 2003;310:979–85. [DOI] [PubMed] [Google Scholar]
- 42.ZHANG W-J, FREI B. α-Lipoic acid inhibits TNF-a-induced NF-κB activation and adhesion molecule expression in human aortic endothelial cells. FASEB J. 2001;15:2423–32. [DOI] [PubMed] [Google Scholar]
- 43.Holmquist L, Stuchbury G, Berbaum K, Muscat S, Young S, Hager K, et al. Lipoic acid as a novel treatment for Alzheimer’s disease and related dementias. Pharmacol Ther. 2007;113:154–64. [DOI] [PubMed] [Google Scholar]
- 44.Rosini M, Andrisano V, Bartolini M, Bolognesi ML, Hrelia P, Minarini A, et al. Rational approach to discover multipotent anti-Alzheimer drugs. J Med Chem. 2005;48:360–3. [DOI] [PubMed] [Google Scholar]
- 45.Rosini M, Simoni E, Bartolini M, Tarozzi A, Matera R, Milelli A, et al. Exploiting the lipoic acid structure in the search for novel multitarget ligands against Alzheimer’s disease. Eur J Med Chem. 2011;46:5435–42. [DOI] [PubMed] [Google Scholar]
- 46.Benchekroun M, Romero A, Egea J, Leon R, Michalska P, Buendia I, et al. The antioxidant additive approach for Alzheimer’s disease therapy: new ferulic (lipoic) acid plus melatonin modified tacrines as cholinesterases inhibitors, direct antioxidants, and nuclear factor (erythroid-derived 2)-like 2 activators. J Med Chem. 2016;59:9967–73. [DOI] [PubMed] [Google Scholar]
- 47.Bolognesi ML, Cavalli A, Bergamini C, Fato R, Lenaz G, Rosini M, et al. Toward a rational design of multitarget-directed antioxidants: merging memoquin and lipoic acid molecular frame-works. J Med Chem. 2009;52:7883–6. [DOI] [PubMed] [Google Scholar]
- 48.Prescott LF, Donovan JW, Jarvie DR, Proudfoot AT. The disposition and kinetics of intravenous N-acetylcysteine in patients with paracetamol overdosage. Eur J Clin Pharmacol. 1989;37:501–6. [DOI] [PubMed] [Google Scholar]
- 49.Olsson B, Johansson M, Gabrielsson J, Bolme P. Pharmacokinetics and bioavailability of reduced and oxidized N-acetylcysteine. Eur J Clin Pharmacol. 1988;34:77–82. [DOI] [PubMed] [Google Scholar]
- 50.Pendyala L, Creaven PJ. Pharmacokinetic and pharmacodynamic studies of N-acetylcysteine, a potential chemopreventive agent during a phase I trial. Cancer Epidemiol Biomark Prev. 1995;4:245–51. [PubMed] [Google Scholar]
- 51.Kerr F, Dawson A, Whyte IM, Buckley N, Murray L, Graudins A, et al. The Australasian Clinical Toxicology Investigators Collaboration randomized trial of different loading infusion rates of N-acetylcysteine. Ann Emerg Med. 2005;45:402–8. [DOI] [PubMed] [Google Scholar]
- 52.Santangelo F. Intracellular thiol concentration modulating inflammatory response: influence on the regulation of cell functions through cysteine prodrug approach. Curr Med Chem. 2003;10:2599–610. [DOI] [PubMed] [Google Scholar]
- 53.Giustarini D, Milzani A, Dalle-Donne I, Tsikas D, Rossi R. N-Acetylcysteine ethyl ester (NACET): a novel lipophilic cell-permeable cysteine derivative with an unusual pharmacokinetic feature and remarkable antioxidant potential. Biochem Pharmacol. 2012;84:1522–33. [DOI] [PubMed] [Google Scholar]
- 54.Holdiness MR. Clinical Pharmacokinetics of N-Acetylcysteine. Clin Pharmacokinet. 1991;20:123–34. [DOI] [PubMed] [Google Scholar]
- 55.Hong SY, Gil HW, Yang JO, Lee EY, Kim HK, Kim SH, et al. Pharmacokinetics of glutathione and its metabolites in normal subjects. J Korean Med Sci. 2005;20:721–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grattagliano I, Wieland P, Schranz C, Lauterburg BH. Effect of oral glutathione monoethyl ester and glutathione on circulating and hepatic sulfhydrils in the rat. Pharmacol Toxicol. 1994;75:343–7. [DOI] [PubMed] [Google Scholar]
- 57.Takaishi N, Yoshida K, Satsu H, Shimizu M. Transepithelial transport of alpha-lipoic acid across human intestinal Caco-2 cell monolayers. J Agric Food Chem. 2007;55:5253–9. [DOI] [PubMed] [Google Scholar]
- 58.Carlson DA, Smith AR, Fischer SJ, Young KL, Packer L. The plasma pharmacokinetics of R-(+)-lipoic acid administered as sodium R-(+)-lipoate to healthy human subjects. Alter Med Rev. 2007;12:343–51. [PubMed] [Google Scholar]
- 59.Biewenga GP, Haenen GR, Bast A. The pharmacology of the antioxidant lipoic acid. Gen Pharmacol. 1997;29:315–31. [DOI] [PubMed] [Google Scholar]
- 60.Shay KP, Moreau RF, Smith EJ, Smith AR, Hagen TM. Alpha-lipoic acid as a dietary supplement: molecular mechanisms and therapeutic potential. Biochim Biophys Acta. 2009;1790:1149–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tchantchou F, Graves M, Rogers E, Ortiz D, Shea TB. N-acteyl cysteine alleviates oxidative damage to central nervous system of ApoE-deficient mice following folate and vitamin E-deficiency. J Alzheimers Dis. 2005;7:135–8. [DOI] [PubMed] [Google Scholar]
- 62.Huang Q, Aluise CD, Joshi G, Sultana R, St Clair DK, Markesbery WR, et al. Potential in vivo amelioration by N-acetyl-L-cysteine of oxidative stress in brain in human double mutant APP/PS-1 knock-in mice: toward therapeutic modulation of mild cognitive impairment. J Neurosci Res. 2010;88:2618–29. [DOI] [PubMed] [Google Scholar]
- 63.Farr SA, Poon HF, Dogrukol-Ak D, Drake J, Banks WA, Eyerman E, et al. The antioxidants alpha-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. J Neurochem. 2003;84:1173–83. [DOI] [PubMed] [Google Scholar]
- 64.Joy T, Rao MS, Madhyastha S. N-Acetyl cysteine supplement minimize tau expression and neuronal loss in animal model of Alzheimer’s disease. Brain Sci. 2018;8:185–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tucker S, Ahl M, Cho HH, Bandyopadhyay S, Cuny GD, Bush AI, et al. RNA therapeutics directed to the non coding regions of APP mRNA, in vivo anti-amyloid efficacy of paroxetine, erythromycin, and N-acetyl cysteine. Curr Alzheimer Res. 2006;3:221–7. [DOI] [PubMed] [Google Scholar]
- 66.More SS, Vartak AP, Vince R. Restoration of glyoxalase enzyme activity precludes cognitive dysfunction in a mouse model of Alzheimer’s disease. ACS Chem Neurosci. 2013;4:330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gorąca A, Huk-Kolega H, Piechota A, Kleniewska P, Ciejka E, Skibska B. Lipoic acid—biological activity and therapeutic potential. Pharmacol Rep. 2011;63:849–58. [DOI] [PubMed] [Google Scholar]
- 68.Salehi B, Berkay Yılmaz Y, Antika G, Boyunegmez Tumer T, Fawzi Mahomoodally M, Lobine D, et al. Insights on the use of α-lipoic acid for therapeutic purposes. Biomolecules. 2019;9:356–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Quinn JF, Bussiere JR, Hammond RS, Montine TJ, Henson E, Jones RE, et al. Chronic dietary alpha-lipoic acid reduces deficits in hippocampal memory of aged Tg2576 mice. Neurobiol Aging. 2007;28:213–25. [DOI] [PubMed] [Google Scholar]
- 70.Siedlak SL, Casadesus G, Webber KM, Pappolla MA, Atwood CS, Smith MA, et al. Chronic antioxidant therapy reduces oxidative stress in a mouse model of Alzheimer’s disease. Free Radic Res. 2009;43:156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang YH, Wang DW, Xu SF, Zhang S, Fan YG, Yang YY, et al. alpha-Lipoic acid improves abnormal behavior by mitigation of oxidative stress, inflammation, ferroptosis, and tauopathy in P301S Tau transgenic mice. Redox Biol. 2018;14:535–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Remington R, Lortie JJ, Hoffmann H, Page R, Morrell C, Shea TB. A Nutritional formulation for cognitive performance in mild cognitive impairment: a placebo-controlled trial with an open-label extension. J Alzheimers Dis. 2015;48:591–5. [DOI] [PubMed] [Google Scholar]
- 73.Remington R, Bechtel C, Larsen D, Samar A, Doshanjh L, Fishman P, et al. A Phase II randomized clinical trial of a nutritional formulation for cognition and mood in Alzheimer’s disease. J Alzheimers Dis. 2015;45:395–405. [DOI] [PubMed] [Google Scholar]
- 74.Rinaldi P, Polidori MC, Metastasio A, Mariani E, Mattioli P, Cherubini A, et al. Plasma antioxidants are similarly depleted in mild cognitive impairment and in Alzheimer’s disease. Neurobiol Aging. 2003;24:915–9. [DOI] [PubMed] [Google Scholar]
- 75.Allen J, Bradley RD. Effects of oral glutathione supplementation on systemic oxidative stress biomarkers in human volunteers. J Alter Complement Med. 2011;17:827–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Witschi A, Reddy S, Stofer B, Lauterburg BH. The systemic availability of oral glutathione. Eur J Clin Pharmacol. 1992;43:667–9. [DOI] [PubMed] [Google Scholar]
- 77.Richie JP Jr., Nichenametla S, Neidig W, Calcagnotto A, Haley JS, Schell TD, et al. Randomized controlled trial of oral glutathione supplementation on body stores of glutathione. Eur J Nutr. 2015;54:251–63. [DOI] [PubMed] [Google Scholar]
- 78.Schmitt B, Vicenzi M, Garrel C, Denis FM. Effects of N-acetylcysteine, oral glutathione (GSH) and a novel sublingual form of GSH on oxidative stress markers: a comparative crossover study. Redox Biol. 2015;6:198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hager K, Marahrens A, Kenklies M, Riederer P, Münch G. Alpha-lipoic acid as a new treatment option for Alzheimer [corrected] type dementia. Arch Gerontol Geriatr. 2001;32: 275–82. [DOI] [PubMed] [Google Scholar]
- 80.Shinto L, Quinn J, Montine T, Dodge HH, Woodward W, Baldauf-Wagner S, et al. A randomized placebo-controlled pilot trial of omega-3 fatty acids and alpha lipoic acid in Alzheimer’s disease. J Alzheimers Dis. 2014;38:111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Galasko DR, Peskind E, Clark CM, Quinn JF, Ringman JM, Jicha GA, et al. Antioxidants for Alzheimer disease: a randomized clinical trial with cerebrospinal fluid biomarker measures. Arch Neurol. 2012;69:836–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci. 1996;16:1066–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wallace JL, Wang R. Hydrogen sulfide-based therapeutics: exploiting a unique but ubiquitous gasotransmitter. Nat Rev Drug Discov. 2015;14:329–45. [DOI] [PubMed] [Google Scholar]
- 84.Savage JC, Gould DH. Determination of sulfide in brain tissue and rumen fluid by ion-interaction reversed-phase high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 1990;526:540–5. [DOI] [PubMed] [Google Scholar]
- 85.Tang XQ, Shen XT, Huang YE, Chen RQ, Ren YK, Fang HR, et al. Inhibition of endogenous hydrogen sulfide generation is associated with homocysteine-induced neurotoxicity: role of ERK1/2 activation. J Mol Neurosci. 2011;45:60–7. [DOI] [PubMed] [Google Scholar]
- 86.Liu XQ, Liu XQ, Jiang P, Huang H, Yan Y. Plasma levels of endogenous hydrogen sulfide and homocysteine in patients with Alzheimer’s disease and vascular dementia and the significance thereof. Zhonghua Yi Xue Za Zhi. 2008;88:2246–9. [PubMed] [Google Scholar]
- 87.Wei H-J, Li X, Tang X-Q. Therapeutic benefits of H2S in Alzheimer’s disease. J Clin Neurosci. 2014;21:1665–9. [DOI] [PubMed] [Google Scholar]
- 88.Whiteman M, Cheung NS, Zhu Y-Z, Chu SH, Siau JL, Wong BS, et al. Hydrogen sulphide: a novel inhibitor of hypochlorous acid-mediated oxidative damage in the brain? Biochem Biophys Res Commun. 2005;326:794–8. [DOI] [PubMed] [Google Scholar]
- 89.Xuan A, Long D, Li J, Ji W, Zhang M, Hong L, et al. Hydrogen sulfide attenuates spatial memory impairment and hippocampal neuroinflammation in beta-amyloid rat model of Alzheimer’s disease. J Neuroinflammation. 2012;9:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tang X-Q, Yang C-T, Chen J, Yin W-L, Tian S-W, Hu B, et al. Effect of hydrogen sulphide on beta-amyloid-induced damage in PC12 cells. Clin Exp Pharmacol Physiol. 2008;35:180–6. [DOI] [PubMed] [Google Scholar]
- 91.Hu LF, Wong PT, Moore PK, Bian JS. Hydrogen sulfide attenuates lipopolysaccharide-induced inflammation by inhibition of p38 mitogen-activated protein kinase in microglia. J Neurochem. 2007;100:1121–8. [DOI] [PubMed] [Google Scholar]
- 92.Zhao FL, Qiao PF, Yan N, Gao D, Liu MJ, Yan Y. Hydrogen sulfide selectively inhibits γ-secretase activity and decreases mitochondrial Aβ production in neurons from APP/PS1 transgenic mice. Neurochem Res. 2016;41:1145–59. [DOI] [PubMed] [Google Scholar]
- 93.Cheng X-j, Gu J-x, Pang Y-p, Liu J, Xu T, Li X-r, et al. Tacrine–hydrogen sulfide donor hybrid ameliorates cognitive impairment in the aluminum chloride mouse model of Alzheimer’s disease. ACS Chem Neurosci. 2019;10:3500–9. [DOI] [PubMed] [Google Scholar]
- 94.Tang XQ, Ren YK, Zhou CF, Yang CT, Gu HF, He JQ, et al. Hydrogen sulfide prevents formaldehyde-induced neurotoxicity to PC12 cells by attenuation of mitochondrial dysfunction and pro-apoptotic potential. Neurochem Int. 2012;61:16–24. [DOI] [PubMed] [Google Scholar]
- 95.Zheng Y, Ji X, Ji K, Wang B. Hydrogen sulfide prodrugs—a review. Acta Pharm Sin B. 2015;5:367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Powell CR, Dillon KM, Matson JB. A review of hydrogen sulfide (H2S) donors: chemistry and potential therapeutic applications. Biochemical Pharmacol. 2018;149:110–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Song ZJ, Ng MY, Lee Z-W, Dai W, Hagen T, Moore PK, et al. Hydrogen sulfide donors in research and drug development. MedChemComm. 2014;5:557–70. [Google Scholar]
- 98.Chiu S, Khan AS, Terpstra KJ, Elias H, Khazaeipool Z, Carriere A, et al. editors. Repurposing elemental sulfur for alzheimer dementia (AD) therapeutics: role of hydrogen sulfide and Î-Galactosidase. J Nerv Syst. 2017;1:6–7. [Google Scholar]
- 99.Guzmán R, Campos C, Yuguero R, Masegù C, Gil P, Moragón ÁC. Protective effect of sulfurous water in peripheral blood mononuclear cells of Alzheimer’s disease patients. Life Sci. 2015;132:61–7. [DOI] [PubMed] [Google Scholar]
- 100.Kimura Y, Goto Y-I, Kimura H. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid Redox Signal. 2009;12:1–13. [DOI] [PubMed] [Google Scholar]
- 101.Kimura Y, Kimura H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 2004;18:1165–7. [DOI] [PubMed] [Google Scholar]
- 102.Liu Y, Deng Y, Liu H, Yin C, Li X, Gong Q. Hydrogen sulfide ameliorates learning memory impairment in APP/PS1 transgenic mice: a novel mechanism mediated by the activation of Nrf2. Pharmacol Biochem Behav. 2016;150–151:207–16. [DOI] [PubMed] [Google Scholar]
- 103.Hu LF, Lu M, Hon Wong PT, Bian JS. Hydrogen sulfide: neurophysiology and neuropathology. Antioxid Redox Signal. 2011;15:405–19. [DOI] [PubMed] [Google Scholar]
- 104.Lee JW, Lee YK, Yuk DY, Choi DY, Ban SB, Oh KW, et al. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J Neuroinflammation. 2008;5:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Giggenbach W. Optical spectra of highly alkaline sulfide solutions and the second dissociation constant of hydrogen sulfide. Inorg Chem. 1971;10:1333–8. [Google Scholar]
- 106.DeLeon ER, Stoy GF, Olson KR. Passive loss of hydrogen sulfide in biological experiments. Anal Biochem. 2012;421:203–7. [DOI] [PubMed] [Google Scholar]
- 107.Caliendo G, Cirino G, Santagada V, Wallace JL. Synthesis and biological effects of hydrogen sulfide (H2S): development of H2S-releasing drugs as pharmaceuticals. J Medicinal Chem. 2010;53:6275–86. [DOI] [PubMed] [Google Scholar]
- 108.Benavides GA, Squadrito GL, Mills RW, Patel HD, Isbell TS, Patel RP, et al. Hydrogen sulfide mediates the vasoactivity of garlic. Proc Natl Acad Sci USA. 2007;104:17977–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Griffin B, Selassie M, Gwebu ET. Effect of aged garlic extract on the cytotoxicity of alzheimer β-amyloid peptide in neuronal PC12 Cells. Nutritional Neurosci. 2000;3:139–42. [DOI] [PubMed] [Google Scholar]
- 110.Gupta VB, Rao KSJ. Anti-amyloidogenic activity of S-allyl-L-cysteine and its activity to destabilize Alzheimer’s beta-amyloid fibrils in vitro. Neurosci Lett. 2007;429:75–80. [DOI] [PubMed] [Google Scholar]
- 111.Pan LL, Liu XH, Gong QH, Zhu YZ. S-Propargyl-cysteine (SPRC) attenuated lipopolysaccharide-induced inflammatory response in H9c2 cells involved in a hydrogen sulfide-dependent mechanism. Amino Acids. 2011;41:205–15. [DOI] [PubMed] [Google Scholar]
- 112.Predmore BL, Kondo K, Bhushan S, Zlatopolsky MA, King AL, Aragon JP, et al. The polysulfide diallyl trisulfide protects the ischemic myocardium by preservation of endogenous hydrogen sulfide and increasing nitric oxide bioavailability. Am J Physiol Heart Circ Physiol. 2012;302:H2410–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ozturk T, Ertas E, Mert O. Use of Lawesson’s reagent in organic syntheses. Chem Rev. 2007;107:5210–78. [DOI] [PubMed] [Google Scholar]
- 114.Li L, Rossoni G, Sparatore A, Lee LC, Del Soldato P, Moore PK. Anti-inflammatory and gastrointestinal effects of a novel diclofenac derivative. Free Radic Biol Med. 2007;42:706–19. [DOI] [PubMed] [Google Scholar]
- 115.Nicolau LAD, Silva RO, Damasceno SRB, Carvalho NS, Costa NRD, Aragão KS, et al. The hydrogen sulfide donor, Lawesson’s reagent, prevents alendronate-induced gastric damage in rats. Braz J Med Biol Res. 2013;46:708–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Medeiros JV, Bezerra VH, Gomes AS, Barbosa AL, Lima-Júnior RC, Soares PM, et al. Hydrogen sulfide prevents ethanol-induced gastric damage in mice: role of ATP-sensitive potassium channels and capsaicin-sensitive primary afferent neurons. J Pharmacol Exp Thera. 2009;330:764–70. [DOI] [PubMed] [Google Scholar]
- 117.Li L, Whiteman M, Guan YY, Neo KL, Cheng Y, Lee SW, et al. Characterization of a novel, water-soluble hydrogen sulphide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation. 2008;117:2351–60. [DOI] [PubMed] [Google Scholar]
- 118.Yurinskaya MM, Krasnov GS, Kulikova DA, Zatsepina OG, Vinokurov MG, Chuvakova LN, et al. H2S counteracts pro-inflammatory effects of LPS through modulation of multiple pathways in human cells. Inflamm Res. 2020;69:481–95. [DOI] [PubMed] [Google Scholar]
- 119.Park C-M, Zhao Y, Zhu Z, Pacheco A, Peng B, Devarie-Baez NO, et al. Synthesis and evaluation of phosphorodithioate-based hydrogen sulfide donors. Mol Biosyst. 2013;9:2430–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Qandil AM. Prodrugs of nonsteroidal anti-inflammatory drugs (NSAIDs), more than meets the eye: a critical review. Int J Mol Sci. 2012;13:17244–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sehajpal S, Prasad DN, Singh RK. Prodrugs of non-steroidal anti-inflammatory drugs (NSAIDs): a long march towards synthesis of safer NSAIDs. Mini Rev Med Chem. 2018;18:1199–219. [DOI] [PubMed] [Google Scholar]
- 122.Sparatore A, Santus G, Giustarini D, Rossi R, Del Soldato P. Therapeutic potential of new hydrogen sulfide-releasing hybrids. Expert Rev Clin Pharmacol. 2011;4:109–21. [DOI] [PubMed] [Google Scholar]
- 123.Wallace JL, Caliendo G, Santagada V, Cirino G, Fiorucci S. Gastrointestinal safety and anti-inflammatory effects of a hydrogen sulfide-releasing diclofenac derivative in the rat. Gastroenterology. 2007;132:261–71. [DOI] [PubMed] [Google Scholar]
- 124.Chattopadhyay M, Kodela R, Nath N, Dastagirzada YM, Velázquez-Martínez CA, Boring D, et al. Hydrogen sulfide-releasing NSAIDs inhibit the growth of human cancer cells: a general property and evidence of a tissue type-independent effect. Biochem Pharmacol. 2012;83:715–22. [DOI] [PubMed] [Google Scholar]
- 125.Liu YY, Sparatore A, Del Soldato P, Bian JS. H2S releasing aspirin protects amyloid beta induced cell toxicity in BV-2 microglial cells. Neuroscience. 2011;193:80–8. [DOI] [PubMed] [Google Scholar]
- 126.Zhao F-L, Fang F, Qiao P-f, Yan N, Gao D, Yan Y. AP39, a mitochondria-targeted hydrogen sulfide donor, supports cellular bioenergetics and protects against Alzheimer’s disease by preserving mitochondrial function in APP/PS1 mice and neurons. Oxid Med Cell Longev. 2016;2016:8360738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nesi G, Sestito S, Digiacomo M, Rapposelli S. Oxidative stress, mitochondrial abnormalities and proteins deposition: multitarget approaches in Alzheimer’s disease. Curr Top Med Chem. 2017;17:3062–79. [DOI] [PubMed] [Google Scholar]
- 128.Huang CS, Lin AH, Liu CT, Tsai CW, Chang IS, Chen HW, et al. Isothiocyanates protect against oxidized LDL-induced endothelial dysfunction by upregulating Nrf2-dependent antioxidation and suppressing NFκB activation. Mol Nutr Food Res. 2013;57:1918–30. [DOI] [PubMed] [Google Scholar]
- 129.Shehatou GS, Suddek GM. Sulforaphane attenuates the development of atherosclerosis and improves endothelial dysfunction in hypercholesterolemic rabbits. Exp Biol Med. 2016;241: 426–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Citi V, Martelli A, Testai L, Marino A, Breschi MC, Calderone V. Hydrogen sulfide releasing capacity of natural isothiocyanates: is it a reliable explanation for the multiple biological effects of Brassicaceae? Planta Med. 2014;80:610–3. [DOI] [PubMed] [Google Scholar]
- 131.Martelli A, Citi V, Testai L, Brogi S, Calderone V. Organic isothiocyanates as hydrogen sulfide donors. Antioxid Redox Signal. 2020;32:110–44. [DOI] [PubMed] [Google Scholar]
- 132.Levinn CM, Cerda MM, Pluth MD. Activatable small-molecule hydrogen sulfide donors. Antioxid Redox Signal. 2020;32: 96–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lin Y, Yang X, Lu Y, Liang D, Huang D. Isothiocyanates as H (2)S donors triggered by cysteine: reaction mechanism and structure and activity relationship. Org Lett. 2019;21:5977–80. [DOI] [PubMed] [Google Scholar]
- 134.Bricker GV, Riedl KM, Ralston RA, Tober KL, Oberyszyn TM, Schwartz SJ. Isothiocyanate metabolism, distribution, and interconversion in mice following consumption of thermally processed broccoli sprouts or purified sulforaphane. Mol Nutr food Res. 2014;58:1991–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rapposelli S, Gambari L, Digiacomo M, Citi V, Lisignoli G, Manferdini C, et al. A Novel H2S-releasing Amino-Bisphosphonate which combines bone anti-catabolic and anabolic functions. Sci Rep. 2017;7:11940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zheng Y, Yu B, De La Cruz LK, Roy Choudhury M, Anifowose A, Wang B. Toward hydrogen sulfide based therapeutics: critical drug delivery and developability issues. Med Res Rev. 2018;38:57–100. [DOI] [PubMed] [Google Scholar]
- 137.Bolognesi ML, Matera R, Minarini A, Rosini M, Melchiorre C. Alzheimer’s disease: new approaches to drug discovery. Curr Opin Chem Biol. 2009;13:303–8. [DOI] [PubMed] [Google Scholar]
- 138.Sestito S, Daniele S, Pietrobono D, Citi V, Bellusci L, Chiellini G, et al. Memantine prodrug as a new agent for Alzheimer’s Disease. Sci Rep. 2019;9:4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Alam S, Lingenfelter KS, Bender AM, Lindsley CW. Classics in chemical neuroscience: memantine. ACS Chem Neurosci. 2017;8:1823–9. [DOI] [PubMed] [Google Scholar]
- 140.Giuliani D, Ottani A, Zaffe D, Galantucci M, Strinati F, Lodi R, et al. Hydrogen sulfide slows down progression of experimental Alzheimer’s disease by targeting multiple pathophysiological mechanisms. Neurobiol Learn Mem. 2013;104:82–91. [DOI] [PubMed] [Google Scholar]
- 141.Sestito S, Pruccoli L, Runfola M, Citi V, Martelli A, Saccomanni G, et al. Design and synthesis of H(2)S-donor hybrids: a new treatment for Alzheimer’s disease? Eur J Med Chem. 2019;184:111745. [DOI] [PubMed] [Google Scholar]
- 142.Zhao J, Moore AN, Redell JB, Dash PK. Enhancing expression of Nrf2-driven genes protects the blood brain barrier after brain injury. J Neurosci. 2007;27:10240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Furne J, Saeed A, Levitt M. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1479–85. [DOI] [PubMed] [Google Scholar]
- 144.Ritter JM. Human pharmacology of hydrogen sulfide, putative gaseous mediator. Br J Clin Pharmacol. 2010;69:573–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Saeedi A, Najibi A, Mohammadi-Bardbori A. Effects of long-term exposure to hydrogen sulfide on human red blood cells. Int J Occup Environ Med. 2015;6:20–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gong QH, Wang Q, Pan LL, Liu XH, Huang H, Zhu YZ. Hydrogen sulfide attenuates lipopolysaccharide-induced cognitive impairment: a pro-inflammatory pathway in rats. Pharmacol Biochem Behav. 2010;96:52–8. [DOI] [PubMed] [Google Scholar]
- 147.Kamat PK, Kalani A, Givvimani S, Sathnur PB, Tyagi SC, Tyagi N. Hydrogen sulfide attenuates neurodegeneration and neuro-vascular dysfunction induced by intracerebral-administered homocysteine in mice. Neuroscience. 2013;252:302–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ray B, Chauhan NB, Lahiri DK. Oxidative insults to neurons and synapse are prevented by aged garlic extract and S-allyl-L-cysteine treatment in the neuronal culture and APP-Tg mouse model. J Neurochem. 2011;117:388–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Polhemus DJ, Li Z, Pattillo CB, Gojon G Sr., Gojon G Jr., Giordano T, et al. A novel hydrogen sulfide prodrug, SG1002, promotes hydrogen sulfide and nitric oxide bioavailability in heart failure patients. Cardiovasc Ther. 2015;33:216–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cremlyn RJW, Cremlyn RJ. An introduction to organosulfur chemistry. New York: Wiley; 1996. [Google Scholar]
- 151.Bruno MA, Mufson EJ, Wuu J, Cuello AC. Increased matrix metalloproteinase 9 activity in mild cognitive impairment. J Neuropathol Exp Neurol. 2009;68:1309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Whittaker M, Floyd CD, Brown P, Gearing AJ. Design and therapeutic application of matrix metalloproteinase inhibitors. Chem Rev. 1999;99:2735–76. [DOI] [PubMed] [Google Scholar]
- 153.De Strooper B. Proteases and proteolysis in Alzheimer disease: a multifactorial view on the disease process. Physiol Rev 2010;90:465–94. [DOI] [PubMed] [Google Scholar]
- 154.Py NA, Bonnet AE, Bernard A, Marchalant Y, Charrat E, Checler F, et al. Differential spatio-temporal regulation of MMPs in the 5xFAD mouse model of Alzheimer’s disease: evidence for a pro-amyloidogenic role of MT1-MMP. Front Aging Neurosci. 2014;6:247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kauppinen TM, Swanson RA. Poly(ADP-ribose) polymerase-1 promotes microglial activation, proliferation, and matrix metalloproteinase-9-mediated neuron death. J Immunol. 2005;174:2288–96. [DOI] [PubMed] [Google Scholar]
- 156.Jourquin J, Tremblay E, Decanis N, Charton G, Hanessian S, Chollet AM, et al. Neuronal activity-dependent increase of net matrix metalloproteinase activity is associated with MMP-9 neurotoxicity after kainate. Eur J Neurosci. 2003;18:1507–17. [DOI] [PubMed] [Google Scholar]
- 157.Nubling G, Levin J, Bader B, Israel L, Botzel K, Lorenzl S, et al. Limited cleavage of tau with matrix-metalloproteinase MMP-9, but not MMP-3, enhances tau oligomer formation. Exp Neurol. 2012;237:470–6. [DOI] [PubMed] [Google Scholar]
- 158.Terni B, Ferrer I. Abnormal expression and distribution of MMP2 at initial stages of Alzheimer’s disease-related pathology.J Alzheimers Dis. 2015;46:461–9. [DOI] [PubMed] [Google Scholar]
- 159.Hanzel CE, Iulita MF, Eyjolfsdottir H, Hjorth E, Schultzberg M, Eriksdotter M, et al. Analysis of matrix metallo-proteases and the plasminogen system in mild cognitive impairment and Alzheimer’s disease cerebrospinal fluid. J Alzheimers Dis. 2014;40:667–78. [DOI] [PubMed] [Google Scholar]
- 160.Bjerke M, Zetterberg H, Edman Å, Blennow K, Wallin A, Andreasson U. Cerebrospinal fluid matrix metalloproteinases and tissue inhibitor of metalloproteinases in combination with subcortical and cortical biomarkers in vascular dementia and Alzheimer’s disease. J Alzheimer’s Dis. 2011;27:665–76. [DOI] [PubMed] [Google Scholar]
- 161.Rasmussen HS, McCann PP. Matrix metalloproteinase inhibition as a novel anticancer strategy: a review with special focus on batimastat and marimastat. Pharmacol Ther. 1997;75:69–75. [DOI] [PubMed] [Google Scholar]
- 162.Hidalgo M, Eckhardt SG. Development of matrix metalloproteinase inhibitors in cancer therapy. J Natl Cancer Inst. 2001;93:178–93. [DOI] [PubMed] [Google Scholar]
- 163.Rothenberg ML, Nelson AR, Hande KR. New drugs on the horizon: matrix metalloproteinase inhibitors. Oncologist. 1998;3: 271–4. [PubMed] [Google Scholar]
- 164.Sorensen MD, Blaehr LK, Christensen MK, Hoyer T, Latini S, Hjarnaa PJ, et al. Cyclic phosphinamides and phosphonamides, novel series of potent matrix metalloproteinase inhibitors with antitumour activity. Bioorg Med Chem. 2003;11:5461–84. [DOI] [PubMed] [Google Scholar]
- 165.Bursavich MG, Harrison BA. Blain J-Fo. Gamma secretase modulators: new Alzheimer’s drugs on the horizon? J Med. Chem. 2016;59:7389–409. [DOI] [PubMed] [Google Scholar]
- 166.Weggen S, Eriksen JL, Das P, Sagi SA, Wang R, Pietrzik CU, et al. A subset of NSAIDs lower amyloidogenic Aβ42 independently of cyclooxygenase activity. Nature. 2001;414:212–6. [DOI] [PubMed] [Google Scholar]
- 167.Prade E, Barucker C, Sarkar R, Althoff-Ospelt G, Lopez del Amo JM, Hossain S, et al. Sulindac sulfide induces the formation of large oligomeric aggregates of the Alzheimer’s disease amyloid-β peptide which exhibit reduced neurotoxicity. Biochemistry. 2016;55:1839–49. [DOI] [PubMed] [Google Scholar]
- 168.Beattie GJ, Smyth JF. Phase I study of intraperitoneal metalloproteinase inhibitor BB94 in patients with malignant ascites. Clin Cancer Res. 1998;4:1899–902. [PubMed] [Google Scholar]
- 169.Macaulay VM, O’Byrne KJ, Saunders MP, Braybrooke JP, Long L, Gleeson F, et al. Phase I study of intrapleural batimastat (BB-94), a matrix metalloproteinase inhibitor, in the treatment of malignant pleural effusions. Clin Cancer Res. 1999;5:513–20. [PubMed] [Google Scholar]
- 170.Doherty AM, Bristol JA. Annual reports in medicinal chemistry. Academic press; 1998. pp.131–40. [Google Scholar]
- 171.Rowinsky EK, Humphrey R, Hammond LA, Aylesworth C, Smetzer L, Hidalgo M, et al. Phase I and pharmacologic study of the specific matrix metalloproteinase inhibitor BAY 12–9566 on a protracted oral daily dosing schedule in patients with solid malignancies. J Clin Oncol. 2000;18:178–86. [DOI] [PubMed] [Google Scholar]
- 172.Hande KR, Collier M, Paradiso L, Stuart-Smith J, Dixon M, Clendeninn N, et al. Phase I and pharmacokinetic study of prinomastat, a matrix metalloprotease inhibitor. Clin Cancer Res. 2004;10:909–15. [DOI] [PubMed] [Google Scholar]
- 173.Weber GF. Molecular therapies of cancer. Springer; 2015. pp. 246–51. [Google Scholar]
- 174.Dubrovskaya NM, Nalivaeva NN, Turner AJ, Zhuravin IA. Effects of an inhibitor of alpha-secretase, which metabolizes the amyloid peptide precursor, on memory formation in rats. Neurosci Behav Physiol. 2006;36:911–3. [DOI] [PubMed] [Google Scholar]
- 175.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer—trials and tribulations. Science. 2002;295:2387–92. [DOI] [PubMed] [Google Scholar]
- 176.Talbot DC, Brown PD. Experimental and clinical studies on the use of matrix metalloproteinase inhibitors for the treatment of cancer. Eur J Cancer. 1996;32A:2528–33. [DOI] [PubMed] [Google Scholar]
- 177.Hirte H, Goel R, Major P, Seymour L, Huan S, Stewart D, et al. A phase I dose escalation study of the matrix metalloproteinase inhibitor BAY 12–9566 administered orally in patients with advanced solid tumours. Ann Oncol. 2000;11:1579–84. [DOI] [PubMed] [Google Scholar]
- 178.Szekely C, Green R, Breitner JC, Østbye T, Beiser A, Corrada M, et al. No advantage of Aβ42-lowering NSAIDs for prevention of Alzheimer dementia in six pooled cohort studies. Neurology. 2008;70:2291–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Marcum ZA, Hanlon JT. Recognizing the risks of chronic non-steroidal anti-inflammatory drug use in older adults. Ann Longterm Care. 2010;18:24–7. [PMC free article] [PubMed] [Google Scholar]
- 180.Davies NM, Watson MS. Clinical pharmacokinetics of sulindac. Clin Pharmacokinet. 1997;32:437–59. [DOI] [PubMed] [Google Scholar]
- 181.Mesches MH, Gemma C, Veng LM, Allgeier C, Young DA, Browning MD, et al. Sulindac improves memory and increases NMDA receptor subunits in aged Fischer 344 rats. Neurobiol Aging. 2004;25:315–24. [DOI] [PubMed] [Google Scholar]
- 182.Duggan DE. Sulindac: therapeutic implications of the prodrug/pharmacophore equilibrium. Drug Metab Rev. 1981;12:325–37. [DOI] [PubMed] [Google Scholar]
- 183.Walters MJ, Blobaum AL, Kingsley PJ, Felts AS, Sulikowski GA, Marnett LJ. The influence of double bond geometry in the inhibition of cyclooxygenases by sulindac derivatives. Bioorg Med. Chem Lett. 2009;19:3271–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Weggen S, Eriksen JL, Sagi SA, Pietrzik CU, Ozols V, Fauq A, et al. Evidence that nonsteroidal anti-inflammatory drugs decrease amyloid beta 42 production by direct modulation of gamma-secretase activity. J Biol Chem. 2003;278:31831–7. [DOI] [PubMed] [Google Scholar]
- 185.Prade E, Bittner HJ, Sarkar R, Lopez Del Amo JM, Althoff-Ospelt G, Multhaup G, et al. Structural mechanism of the interaction of Alzheimer disease Aβ fibrils with the non-steroidal anti-inflammatory drug (NSAID) sulindac sulfide. J Biol Chem. 2015;290:28737–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Fu Z, Aucoin D, Ahmed M, Ziliox M, Van Nostrand WE, Smith SO. Capping of aβ42 oligomers by small molecule inhibitors. Biochemistry. 2014;53:7893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Prade E, Barucker C, Sarkar R, Althoff-Ospelt G, Lopez del Amo JM, Hossain S, et al. Sulindac sulfide induces the formation of large oligomeric aggregates of the Alzheimer’s disease amyloid-β peptide which exhibit reduced neurotoxicity. Biochemistry. 2016;55:1839–49. [DOI] [PubMed] [Google Scholar]
- 188.Feng M, Tang B, Liang SH, Jiang X. Sulfur containing scaffolds in drugs: synthesis and application in medicinal chemistry. Curr Top Med Chem. 2016;16:1200–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Benhamú B, Martín-Fontecha M, Vázquez-Villa H, Pardo L, López-Rodríguez ML. Serotonin 5-HT6 receptor antagonists for the treatment of cognitive deficiency in Alzheimer’s disease. J Med Chem. 2014;57:7160–81. [DOI] [PubMed] [Google Scholar]
- 190.Maher-Edwards G, Zvartau-Hind M, Hunter AJ, Gold M, Hopton G, Jacobs G, et al. Double-blind, controlled phase II study of a 5-HT6 receptor antagonist, SB-742457, in Alzheimer’s disease. Curr Alzheimer Res. 2010;7:374–85. [DOI] [PubMed] [Google Scholar]
- 191.Ivachtchenko AV, Ivanenkov YA, Veselov MS, Okun IM. AVN-322 is a safe orally bio-available potent and highly selective antagonist of 5-HT6R with demonstrated ability to improve impaired memory in animal models. Curr Alzheimer Res. 2017;14:268–94. [DOI] [PubMed] [Google Scholar]
- 192.Liu KG, Robichaud AJ, Bernotas RC, Yan Y, Lo JR, Zhang MY, et al. 5-Piperazinyl-3-sulfonylindazoles as potent and selective 5-hydroxytryptamine-6 antagonists. J Med Chem. 2010;53: 7639–46. [DOI] [PubMed] [Google Scholar]
- 193.Ivachtchenko AV, Dmitriev DE, Golovina ES, Kadieva MG, Koryakova AG, Kysil VM, et al. (3-Phenylsulfonylcycloalkano [e and d]pyrazolo[1,5-a]pyrimidin-2-yl)amines: potent and selective antagonists of the serotonin 5-HT6 receptor. J Med Chem. 2010;53:5186–96. [DOI] [PubMed] [Google Scholar]
- 194.Ivachtchenko AV, Golovina ES, Kadieva MG, Koryakova AG, Mitkin OD, Tkachenko SE, et al. 2-Substituted 5,6-dimethyl-3-phenylsulfonyl-pyrazolo[1,5-a]pyrimidines: New series of highly potent and specific serotonin 5-HT6 receptor antagonists. Eur J Med Chem. 2011;46:1189–97. [DOI] [PubMed] [Google Scholar]
- 195.Ivachtchenko AV, Golovina ES, Kadieva MG, Kysil VM, Mitkin OD, Tkachenko SE, et al. Synthesis and SAR of 3-arylsulfonyl-pyrazolo[1,5-a]pyrimidines as potent serotonin 5-HT6 receptor antagonists. Bioorg Med Chem. 2011;19:1482–91. [DOI] [PubMed] [Google Scholar]
- 196.Ivachtchenko AV, Golovina ES, Kadieva MG, Kysil VM, Mitkin OD, Tkachenko SE, et al. Synthesis and structure–activity relationship (SAR) of (5,7-Disubstituted 3-phenylsulfonyl-pyrazolo [1,5-a]pyrimidin-2-yl)-methylamines as potent serotonin 5-HT6 Receptor (5-HT6R) antagonists. J Med Chem. 2011;54:8161–73. [DOI] [PubMed] [Google Scholar]
- 197.Rueeger H, Lueoend R, Rogel O, Rondeau J-M, Möbitz H, Machauer R, et al. Discovery of cyclic sulfone hydroxyethylamines as potent and selective β-Site APP-cleaving enzyme 1 (BACE1) inhibitors: structure-based design and in vivo reduction of amyloid β-peptides. J Med Chem. 2012;55:3364–86. [DOI] [PubMed] [Google Scholar]
- 198.Jakaria M, Azam S, Haque ME, Jo S-H, Uddin MS, Kim I-S, et al. Taurine and its analogs in neurological disorders: Focus on therapeutic potential and molecular mechanisms. Redox Biol. 2019;24:101223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Chung MC, Malatesta P, Bosquesi PL, Yamasaki PR, Santos JLD, Vizioli EO. Advances in drug design based on the amino Acid approach: taurine analogues for the treatment of CNS diseases. Pharmaceuticals. 2012;5:1128–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Louzada PR, Paula Lima AC, Mendonca-Silva DL, Noël F, De Mello FG, Ferreira ST. Taurine prevents the neurotoxicity of beta-amyloid and glutamate receptor agonists: activation of GABA receptors and possible implications for Alzheimer’s disease and other neurological disorders. FASEB J. 2004;18:511–8. [DOI] [PubMed] [Google Scholar]
- 201.Manzano S, Agüera L, Aguilar M, Olazarán J. A review on tramiprosate (Homotaurine) in Alzheimer’s disease and other neurocognitive disorders. Front Neurol. 2020;11:614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Oh SJ, Lee H-J, Jeong YJ, Nam KR, Kang KJ, Han SJ, et al. Evaluation of the neuroprotective effect of taurine in Alzheimer’s disease using functional molecular imaging. Sci Rep. 2020;10:15551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Aisen PS, Saumier D, Briand R, Laurin J, Gervais F, Tremblay P, et al. A Phase II study targeting amyloid-beta with 3APS in mild-to-moderate Alzheimer disease. Neurology. 2006;67: 1757–63. [DOI] [PubMed] [Google Scholar]
- 204.Tóth S, Csaba G. γ-l-Glutamyl-taurine (Litoralon®) prevents the micronucleus formation induced by mitomycin C. Mutat Res Lett. 1988;209:85–9. [DOI] [PubMed] [Google Scholar]
- 205.Salimäki J, Scriba G, Piepponen TP, Rautolahti N, Ahtee L. The effects of systemically administered taurine and N-pivaloyltaurine on striatal extracellular dopamine and taurine in freely moving rats. Naunyn-Schmiedebergs Arch Pharmacol. 2003;368:134–41. [DOI] [PubMed] [Google Scholar]
- 206.Ricci L, Frosini M, Gaggelli N, Valensin G, Machetti F, Sgaragli G, et al. Inhibition of rabbit brain 4-aminobutyrate transaminase by some taurine analogues: a kinetic analysis. Biochemical Pharmacol. 2006;71:1510–9. [DOI] [PubMed] [Google Scholar]
- 207.Ward R, Cirkovic-Vellichovia T, Ledeque F, Tirizitis G, Dubars G, Datla K, et al. Neuroprotection by Taurine and Taurine Analogues. Springer; 2006. pp. 299–306. [DOI] [PubMed] [Google Scholar]
- 208.Klusa V, Klimaviciusa L, Duburs G, Poikans J, Zharkovsky A. Anti-neurotoxic effects of tauropyrone, a taurine analogue. Adv Exp Med Biol. 2006;583:499–508. [DOI] [PubMed] [Google Scholar]
- 209.Mancinelli A. Pharmacokinetics of 5-HT6 receptor ligands. Int Rev Neurobiol. 2010;94:153–72. [DOI] [PubMed] [Google Scholar]
- 210.O’Flaherty L, Stapleton PP, Redmond HP, Bouchier-Hayes DJ. Intestinal taurine transport: a review. Eur J Clin Investig. 1997;27:873–80. [DOI] [PubMed] [Google Scholar]
- 211.Cosar M, Kaner T, Sahin O, Topaloglu N, Guven M, Aras AB, et al. The neuroprotective effect of Sulindac after ischemia-reperfusion injury in rats. Acta Cir Bras. 2014;29:268–73. [DOI] [PubMed] [Google Scholar]
- 212.Jang H, Lee S, Choi SL, Kim HY, Baek S, Kim Y. Taurine directly binds to oligomeric amyloid-β and recovers cognitive deficits in Alzheimer model mice. Adv Exp Med Biol. 2017;975:233–41. [DOI] [PubMed] [Google Scholar]
- 213.Murakami S, Kondo Y, Nagate T. Effects of long-term treatment with taurine in mice fed a high-fat diet: improvement in cholesterol metabolism and vascular lipid accumulation by taurine. Adv Exp Med Biol. 2000;483:177–86. [DOI] [PubMed] [Google Scholar]
- 214.Gervais F, Paquette J, Morissette C, Krzywkowski P, Yu M, Azzi M, et al. Targeting soluble Abeta peptide with Tramiprosate for the treatment of brain amyloidosis. Neurobiol Aging. 2007;28:537–47. [DOI] [PubMed] [Google Scholar]
- 215.Javed H, Khan A, Vaibhav K, Moshahid Khan M, Ahmad A, Ejaz Ahmad M, et al. Taurine ameliorates neurobehavioral, neurochemical and immunohistochemical changes in sporadic dementia of Alzheimer’s type (SDAT) caused by intracerebroventricular streptozotocin in rats. Neurological Sci. 2013;34:2181–92. [DOI] [PubMed] [Google Scholar]
- 216.El Idrissi A. Taurine improves learning and retention in aged mice. Neurosci Lett. 2008;436:19–22. [DOI] [PubMed] [Google Scholar]
- 217.Benedetti R, Marchegiani A, Tambella AM, Fruganti A, Serri E, Malfatti A, et al. Effects of chronic supplementation of homotaurine on cognitive processes and spatial cognition in aged dogs: preliminary results. J Vet Behav. 2019;33:90–5. [Google Scholar]
- 218.Goluboff ET. Exisulind, a selective apoptotic antineoplastic drug. Expert Opin Investig Drugs. 2001;10:1875–82. [DOI] [PubMed] [Google Scholar]
- 219.Maher-Edwards G, Watson C, Ascher J, Barnett C, Boswell D, Davies J, et al. Two randomized controlled trials of SB742457 in mild-to-moderate Alzheimer’s disease. Alzheimers Dement. 2015;1:23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Andrews M, Tousi B, Sabbagh MN. 5HT6 antagonists in the treatment of Alzheimer’s dementia: current progress. Neurol Ther. 2018;7:51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hey JA, Yu JY, Versavel M, Abushakra S, Kocis P, Power A, et al. Clinical pharmacokinetics and safety of ALZ-801, a novel prodrug of tramiprosate in development for the treatment of Alzheimer’s disease. Clin Pharmacokinet. 2018;57:315–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Bossù P, Salani F, Ciaramella A, Sacchinelli E, Mosca A, Banaj N, et al. Anti-inflammatory effects of homotaurine in patients with amnestic mild cognitive impairment. Front Aging Neurosci. 2018;10:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Zhao C, Rakesh KP, Ravidar L, Fang W-Y, Qin H-L. Pharmaceutical and medicinal significance of sulfur (SVI)-containing motifs for drug discovery: a critical review. Eur J Med Chem. 2019;162:679–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Grygorenko OO, Biitseva AV, Zhersh S. Amino sulfonic acids, peptidosulfonamides and other related compounds. Tetrahedron. 2018;74:1355–421. [Google Scholar]
- 225.Bag S, Tulsan R, Sood A, Cho H, Redjeb H, Zhou W, et al. Sulfonamides as multifunctional agents for Alzheimer’s disease. Bioorg Med Chem Lett. 2015;25:626–30. [DOI] [PubMed] [Google Scholar]
- 226.Moussa-Pacha NM, Abdin SM, Omar HA, Alniss H, Al-Tel TH. BACE1 inhibitors: current status and future directions in treating Alzheimer’s disease. Med Res Rev. 2020;40:339–84. [DOI] [PubMed] [Google Scholar]
- 227.Luo Y, Bolon B, Kahn S, Bennett BD, Babu-Khan S, Denis P, et al. Mice deficient in BACE1, the Alzheimer’s β-secretase, have normal phenotype and abolished β-amyloid generation. Nat Neurosci. 2001;4:231–2. [DOI] [PubMed] [Google Scholar]
- 228.Yuan J, Venkatraman S, Zheng Y, McKeever BM, Dillard LW, Singh SB. Structure-based design of β-site APP cleaving enzyme 1 (BACE1) inhibitors for the treatment of Alzheimer’s disease. J Med Chem. 2013;56:4156–80. [DOI] [PubMed] [Google Scholar]
- 229.Specker E, Böttcher J, Heine A, Sotriffer CA, Lilie H, Schoop A, et al. Hydroxyethylene sulfones as a new scaffold to address aspartic proteases: design, synthesis, and structural characterization. J Med Chem. 2005;48:6607–19. [DOI] [PubMed] [Google Scholar]
- 230.Stachel SJ, Coburn CA, Steele TG, Crouthamel MC, Pietrak BL, Lai MT, et al. Conformationally biased P3 amide replacements of beta-secretase inhibitors. Bioorg Med Chem Lett. 2006;16:641–4. [DOI] [PubMed] [Google Scholar]
- 231.Ghosh AK, Kumaragurubaran N, Hong L, Kulkarni SS, Xu X, Chang W, et al. Design, synthesis, and X-ray structure of potent memapsin 2 (β-Secretase) inhibitors with isophthalamide derivatives as the P2-P3-ligands. J Med Chem. 2007;50:2399–407. [DOI] [PubMed] [Google Scholar]
- 232.Sandgren V, Bäck M, Kvarnström I, Dahlgren A. Design and synthesis of hydroxyethylene-based BACE-1 inhibitors incorporating extended P1 substituents. Open Med Chem J. 2013;7:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Rajapakse HA, Nantermet PG, Selnick HG, Munshi S, McGaughey GB, Lindsley SR, et al. Discovery of oxadiazoyl tertiary carbinamine inhibitors of β-secretase (BACE-1). J Med Chem. 2006;49:7270–3. [DOI] [PubMed] [Google Scholar]
- 234.Lindsley SR, Moore KP, Rajapakse HA, Selnick HG, Young MB, Zhu H, et al. Design, synthesis, and SAR of macrocyclic tertiary carbinamine BACE-1 inhibitors. Bioorg Med Chem Lett. 2007;17:4057–61. [DOI] [PubMed] [Google Scholar]
- 235.Moore KP, Zhu H, Rajapakse HA, McGaughey GB, Colussi D, Price EA, et al. Strategies toward improving the brain penetration of macrocyclic tertiary carbinamine BACE-1 inhibitors. Bioorg Med Chem Lett. 2007;17:5831–5. [DOI] [PubMed] [Google Scholar]
- 236.Beswick P, Charrier N, Clarke B, Demont E, Dingwall C, Dunsdon R, et al. BACE-1 inhibitors part 3: identification of hydroxy ethylamines (HEAs) with nanomolar potency in cells. Bioorg Med Chem Lett. 2008;18:1022–6. [DOI] [PubMed] [Google Scholar]
- 237.Charrier N, Clarke B, Cutler L, Demont E, Dingwall C, Dunsdon R, et al. Second generation of hydroxyethylamine BACE-1 inhibitors: optimizing potency and oral bioavailability. J Med Chem. 2008;51:3313–7. [DOI] [PubMed] [Google Scholar]
- 238.Charrier N, Clarke B, Demont E, Dingwall C, Dunsdon R, Hawkins J, et al. Second generation of BACE-1 inhibitors part 2: optimisation of the non-prime side substituent. Bioorg Med Chem Lett. 2009;19:3669–73. [DOI] [PubMed] [Google Scholar]
- 239.Charrier N, Clarke B, Cutler L, Demont E, Dingwall C, Dunsdon R, et al. Second generation of BACE-1 inhibitors. Part 1: the need for improved pharmacokinetics. Bioorg Med Chem Lett. 2009;19:3664–8. [DOI] [PubMed] [Google Scholar]
- 240.Scott JD, Li SW, Brunskill AP, Chen X, Cox K, Cumming JN, et al. Discovery of the 3-Imino-1,2,4-thiadiazinane 1,1-dioxide derivative verubecestat (MK-8931)-A β-site amyloid precursor protein cleaving enzyme 1 inhibitor for the treatment of Alzheimer’s disease. J Med Chem. 2016;59:10435–50. [DOI] [PubMed] [Google Scholar]
- 241.Zhao SH, Berger J, Clark RD, Sethofer SG, Krauss NE, Brothers JM, et al. 3,4-Dihydro-2H-benzo[1,4]oxazine derivatives as 5-HT6 receptor antagonists. Bioorg Med Chem Lett. 2007;17:3504–7. [DOI] [PubMed] [Google Scholar]
- 242.Nirogi R, Abraham R, Benade V, Medapati RB, Jayarajan P, Bhyrapuneni G, et al. SUVN-502, a novel, potent, pure, and orally active 5-HT6 receptor antagonist: pharmacological, behavioral, and neurochemical characterization. Behav Pharmacol. 2019;30:16–35. [DOI] [PubMed] [Google Scholar]
- 243.Grychowska K, Satała G, Kos T, Partyka A, Colacino E, Chaumont-Dubel S, et al. Novel 1H-Pyrrolo[3,2-c]quinoline based 5-HT6 receptor antagonists with potential application for the treatment of cognitive disorders associated with Alzheimer’s disease. ACS Chem Neurosci. 2016;7:972–83. [DOI] [PubMed] [Google Scholar]
- 244.Canale V, Grychowska K, Kurczab R, Ryng M, Keeri AR, Satała G, et al. A dual-acting 5-HT6 receptor inverse agonist/MAO-B inhibitor displays glioprotective and pro-cognitive properties. Eur J Med Chem. 2020;208:112765. [DOI] [PubMed] [Google Scholar]
- 245.Więckowska A, Kołaczkowski M, Bucki A, Godyń J, Marcinkowska M, Więckowski K, et al. Novel multi-target-directed ligands for Alzheimer’s disease: combining cholinesterase inhibitors and 5-HT(6) receptor antagonists. Design, synthesis and biological evaluation. Eur J Med Chem. 2016;124:63–81. [DOI] [PubMed] [Google Scholar]
- 246.Kreft AF, Martone R, Porte A. Recent advances in the identification of γ-secretase inhibitors to clinically test the Aβ oligomer hypothesis of Alzheimer’s disease. J Med Chem. 2009;52:6169–88. [DOI] [PubMed] [Google Scholar]
- 247.Strooper BD, Gutiérrez LC. Learning by failing: ideas and concepts to tackle γ-secretases in Alzheimer’s disease and beyond. Annu Rev Pharmacol Toxicol. 2015;55:419–37. [DOI] [PubMed] [Google Scholar]
- 248.Zhao Z, Pissarnitski DA, Josien HB, Bara TA, Clader JW, Li H, et al. Substituted 4-morpholine N-arylsulfonamides as γ-secretase inhibitors. Eur J Med Chem. 2016;124:36–48. [DOI] [PubMed] [Google Scholar]
- 249.Gillman KW, Starrett JE, Parker MF, Xie K, Bronson JJ, Marcin LR, et al. Discovery and evaluation of BMS-708163, a potent, selective and orally bioavailable γ-secretase inhibitor. ACS Med Chem Lett. 2010;1:120–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Stepan AF, Karki K, McDonald WS, Dorff PH, Dutra JK, DiRico KJ, et al. Metabolism-directed design of oxetane-containing arylsulfonamide derivatives as γ-secretase inhibitors. J Med Chem. 2011;54:7772–83. [DOI] [PubMed] [Google Scholar]
- 251.Comery TA, Martone RL, Aschmies S, Atchison KP, Diamantidis G, Gong X, et al. Acute gamma-secretase inhibition improves contextual fear conditioning in the Tg2576 mouse model of Alzheimer’s disease. J Neurosci. 2005;25:8898–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Zhang M, Porte A, Diamantidis G, Sogi K, Kubrak D, Resnick L, et al. Asymmetric synthesis of novel α-amino acids with β-branched side chains. Bioorg Med Chem Lett. 2007;17:2401–3. [DOI] [PubMed] [Google Scholar]
- 253.Probst G, Aubele DL, Bowers S, Dressen D, Garofalo AW, Hom RK, et al. Discovery of (R)-4-Cyclopropyl-7,8-difluoro-5-(4-(trifluoromethyl)phenylsulfonyl)-4,5-dihydro-1H-pyrazolo[4,3-c]quinoline (ELND006) and (R)-4-Cyclopropyl-8-fluoro-5-(6-(trifluoromethyl)pyridin-3-ylsulfonyl)-4,5-dihydro-2H-pyrazolo [4,3-c]quinoline (ELND007): metabolically stable γ-secretase inhibitors that selectively inhibit the production of amyloid-β over Notch. J Med Chem. 2013;56:5261–74. [DOI] [PubMed] [Google Scholar]
- 254.Hyde LA, Zhang Q, Del Vecchio RA, Leach PT, Cohen-Williams ME, Chen L, et al. In vivo characterization of a novel γ-secretase inhibitor SCH 697466 in Rodents and investigation of strategies for managing notch-related side effects. Int J Alzheimer’s Dis. 2013;2013:823528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann Neurol. 1990;27:457–64. [DOI] [PubMed] [Google Scholar]
- 256.Diamant S, Podoly E, Friedler A, Ligumsky H, Livnah O, Soreq H. Butyrylcholinesterase attenuates amyloid fibril formation in vitro. Proc Natl Acad Sci USA. 2006;103:8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Sharma K. Cholinesterase inhibitors as Alzheimer’s therapeutics (Review). Mol Med Rep. 2019;20:1479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Raina P, Santaguida P, Ismaila A, Patterson C, Cowan D, Levine M, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med. 2008;148:379–97. [DOI] [PubMed] [Google Scholar]
- 259.Apaydın S, Török M. Sulfonamide derivatives as multi-target agents for complex diseases. Bioorg Med Chem Lett. 2019;29:2042–50. [DOI] [PubMed] [Google Scholar]
- 260.Girisha HR, Narendra Sharath Chandra JN, Boppana S, Malviya M, Sadashiva CT, Rangappa KS. Active site directed docking studies: Synthesis and pharmacological evaluation of cis-2,6-dimethyl piperidine sulfonamides as inhibitors of acetylcholinesterase. Eur J Med Chem. 2009;44:4057–62. [DOI] [PubMed] [Google Scholar]
- 261.Hassan M, Abbasi MA, Aziz ur R, Siddiqui SZ, Shahzadi S, Raza H, et al. Designing of promising medicinal scaffolds for Alzheimer’s disease through enzyme inhibition, lead optimization, molecular docking and dynamic simulation approaches. Bioorg Chem. 2019;91:103138. [DOI] [PubMed] [Google Scholar]
- 262.Mutahir S, Jończyk J, Bajda M, Khan IU, Khan MA, Ullah N, et al. Novel biphenyl bis-sulfonamides as acetyl and butyrylcholinesterase inhibitors: Synthesis, biological evaluation and molecular modeling studies. Bioorg Chem. 2016;64:13–20. [DOI] [PubMed] [Google Scholar]
- 263.Riaz S, Khan IU, Bajda M, Ashraf M, Qurat ul A, Shaukat A, et al. Pyridine sulfonamide as a small key organic molecule for the potential treatment of type-II diabetes mellitus and Alzheimer’s disease: in vitro studies against yeast α-glucosidase, acetylcholinesterase and butyrylcholinesterase. Bioorg Chem. 2015;63:64–71. [DOI] [PubMed] [Google Scholar]
- 264.Ulus R, Zengin Kurt B, Gazioğlu I, Kaya M. Microwave assisted synthesis of novel hybrid tacrine-sulfonamide derivatives and investigation of their antioxidant and anticholinesterase activities. Bioorg Chem. 2017;70:245–55. [DOI] [PubMed] [Google Scholar]
- 265.Košak U, Knez D, Coquelle N, Brus B, Pišlar A, Nachon F, et al. N-Propargylpiperidines with naphthalene-2-carboxamide or naphthalene-2-sulfonamide moieties: potential multifunctional anti-Alzheimer’s agents. Bioorg Med Chem. 2017;25:633–45. [DOI] [PubMed] [Google Scholar]
- 266.Baldwin AG, Brough D, Freeman S. Inhibiting the inflammasome: a chemical perspective. J Med Chem. 2016;59:1691–710. [DOI] [PubMed] [Google Scholar]
- 267.Fulp J, He L, Toldo S, Jiang Y, Boice A, Guo C, et al. Structural insights of benzenesulfonamide analogues as NLRP3 inflammasome inhibitors: design, synthesis, and biological characterization. J Med Chem. 2018;61:5412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Zhang Y, Zhao Y, Zhang J, Yang G. Mechanisms of NLRP3 inflammasome activation: its role in the treatment of Alzheimer’s disease. Neurochem Res. 2020;45:2560–72. [DOI] [PubMed] [Google Scholar]
- 269.Cui W, Sun C, Ma Y, Wang S, Wang X, Zhang Y. Inhibition of TLR4 Induces M2 Microglial Polarization and Provides Neuroprotection via the NLRP3 Inflammasome in Alzheimer’s disease. Front Neurosci. 2020;14:444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Rotella DP. Phosphodiesterase type 5 inhibitors: discovery and therapeutic utility. Drugs Future. 2001;26:153–62. [Google Scholar]
- 271.Zuccarello E, Acquarone E, Calcagno E, Argyrousi EK, Deng SX, Landry DW, et al. Development of novel phosphodiesterase 5 inhibitors for the therapy of Alzheimer’s disease. Biochem Pharmacol. 2020;176:113818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Prickaerts J, Heckman PRA, Blokland A. Investigational phosphodiesterase inhibitors in phase I and phase II clinical trials for Alzheimer’s disease. Expert Opin Investig Drugs. 2017;26:1033–48. [DOI] [PubMed] [Google Scholar]
- 273.García-Osta A, Cuadrado-Tejedor M, García-Barroso C, Oyarzábal J, Franco R. Phosphodiesterases as therapeutic targets for Alzheimer’s disease. ACS Chem Neurosci. 2012;3:832–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Sanders O. Sildenafil for the treatment of Alzheimer’s disease: a systematic review. J Alzheimers Dis Rep. 2020;4:91–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Eros D, Cs S-K, Kiss R, Gy K, Hegymegi-Barakonyi B, Kovesdi I, et al. Structure—activity relationships of PDE5 inhibitors (supporting material). Curr Med Chem. 2008;15:1570–85. [DOI] [PubMed] [Google Scholar]
- 276.Bischoff E. Potency, selectivity, and consequences of non-selectivity of PDE inhibition. Int J Impot Res. 2004;16:S11–4. [DOI] [PubMed] [Google Scholar]
- 277.Bischoff E. Vardenafil preclinical trial data: potency, pharmacodynamics, pharmacokinetics, and adverse events. Int J Impot Res. 2004;16:S34–7. [DOI] [PubMed] [Google Scholar]
- 278.Cumming J, Babu S, Huang Y, Carrol C, Chen X, Favreau L, et al. Piperazine sulfonamide BACE1 inhibitors: design, synthesis, and in vivo characterization. Bioorg Med Chem Lett. 2010;20:2837–42. [DOI] [PubMed] [Google Scholar]
- 279.Popugaeva E, Cherniuk D, Zhang H, Postnikova T, Pac K, Fedorova E, et al. Derivatives of piperazines as potential therapeutic agents for Alzheimers disease. Mol Pharmacol. 2019. 10.1124/mol.118.114348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Blume T, Filser S, Jaworska A, Blain JF, Koenig G, Moschke K, et al. BACE1 inhibitor MK-8931 alters formation but not stability of dendritic spines. Front Aging Neurosci. 2018;10:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Kennedy ME, Stamford AW, Chen X, Cox K, Cumming JN, Dockendorf MF, et al. The BACE1 inhibitor verubecestat (MK-8931) reduces CNS β-amyloid in animal models and in Alzheimer’s disease patients. Sci Transl Med. 2016;8:363ra150. [DOI] [PubMed] [Google Scholar]
- 282.Fullerton T, Binneman B, David W, Delnomdedieu M, Kupiec J, Lockwood P, et al. A Phase 2 clinical trial of PF-05212377 (SAM-760) in subjects with mild to moderate Alzheimer’s disease with existing neuropsychiatric symptoms on a stable daily dose of donepezil. Alzheimer’s Res Ther. 2018;10:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Truong AP, Aubele DL, Probst GD, Neitzel ML, Semko CM, Bowers S, et al. Design, synthesis, and structure-activity relationship of novel orally efficacious pyrazole/sulfonamide based dihydroquinoline gamma-secretase inhibitors. Bioorg Med Chem Lett. 2009;19:4920–3. [DOI] [PubMed] [Google Scholar]
- 284.Ye XM, Konradi AW, Sun M, Yuan S, Aubele DL, Dappen M, et al. Discovery of a novel [3.2.1] benzo fused bicyclic sulfonamide-pyrazoles as potent, selective and efficacious γ-secretase inhibitors. Bioorg Med Chem Lett. 2013;23:996–1000. [DOI] [PubMed] [Google Scholar]
- 285.Wolfe MS. γ-Secretase inhibitors and modulators for Alzheimer’s disease. J Neurochem. 2012;120(Suppl 1):89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Dempsey C, Rubio Araiz A, Bryson KJ, Finucane O, Larkin C, Mills EL, et al. Inhibiting the NLRP3 inflammasome with MCC950 promotes non-phlogistic clearance of amyloid-β and cognitive function in APP/PS1 mice. Brain Behav Immun. 2017;61:306–16. [DOI] [PubMed] [Google Scholar]
- 287.Areosa Sastre A, Vernooij RW, González-Colaço Harmand M, Martínez G. Effect of the treatment of Type 2 diabetes mellitus on the development of cognitive impairment and dementia. Cochrane Database Syst Rev. 2017;6:CD003804-CD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Nichols DJ, Muirhead GJ, Harness JA. Pharmacokinetics of sildenafil after single oral doses in healthy male subjects: absolute bioavailability, food effects and dose proportionality. Br J Clin Pharmacol. 2002;53 Suppl 1:5S–12S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Foley AG, Murphy KJ, Hirst WD, Gallagher HC, Hagan JJ, Upton N, et al. The 5-HT6 receptor antagonist SB-271046 reverses scopolamine-disrupted consolidation of a passive avoidance task and ameliorates spatial task deficits in aged rats. Neuropsychopharmacology. 2004;29:93–100. [DOI] [PubMed] [Google Scholar]
- 290.Da Silva Costa-Aze V, Quiedeville A, Boulouard M, Dauphin F. 5-HT6 receptor blockade differentially affects scopolamine-induced deficits of working memory, recognition memory and aversive learning in mice. Psychopharmacology. 2012;222:99–115. [DOI] [PubMed] [Google Scholar]
- 291.Barten DM, Meredith JE Jr., Zaczek R, Houston JG, Albright CF. Gamma-secretase inhibitors for Alzheimer’s disease: balancing efficacy and toxicity. Drugs R D 2006;7:87–97. [DOI] [PubMed] [Google Scholar]
- 292.Mayer SC, Kreft AF, Harrison B, Abou-Gharbia M, Antane M, Aschmies S, et al. Discovery of begacestat, a Notch-1-sparing gamma-secretase inhibitor for the treatment of Alzheimer’s disease. J Med Chem. 2008;51:7348–51. [DOI] [PubMed] [Google Scholar]
- 293.Martone RL, Zhou H, Atchison K, Comery T, Xu JZ, Huang X, et al. Begacestat (GSI-953): a novel, selective thiophene sulfonamide inhibitor of amyloid precursor protein gamma-secretase for the treatment of Alzheimer’s disease. J Pharmacol Exp Ther. 2009;331:598–608. [DOI] [PubMed] [Google Scholar]
- 294.Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493:674–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Yin J, Zhao F, Chojnacki JE, Fulp J, Klein WL, Zhang S, et al. NLRP3 inflammasome inhibitor ameliorates amyloid pathology in a mouse model of Alzheimer’s disease. Mol Neurobiol. 2018;55:1977–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Perregaux DG, McNiff P, Laliberte R, Hawryluk N, Peurano H, Stam E, et al. Identification and Characterization of a Novel Class of Interleukin-1 Post-Translational Processing Inhibitors. J Pharmacol Exp Ther. 2001;299:187. [PubMed] [Google Scholar]
- 297.Cuadrado-Tejedor M, Hervias I, Ricobaraza A, Puerta E, Pérez-Roldán JM, García-Barroso C, et al. Sildenafil restores cognitive function without affecting β-amyloid burden in a mouse model of Alzheimer’s disease. Br J Pharmacol. 2011;164:2029–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Puzzo D, Staniszewski A, Deng SX, Privitera L, Leznik E, Liu S, et al. Phosphodiesterase 5 inhibition improves synaptic function, memory, and amyloid-beta load in an Alzheimer’s disease mouse model. J Neurosci. 2009;29:8075–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Egan MF, Kost J, Voss T, Mukai Y, Aisen PS, Cummings JL, et al. Randomized trial of verubecestat for prodromal Alzheimer’s disease. N Engl J Med. 2019;380:1408–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Coric V, van Dyck CH, Salloway S, Andreasen N, Brody M, Richter RW, et al. Safety and tolerability of the γ-secretase inhibitor avagacestat in a phase 2 study of mild to moderate Alzheimer disease. Arch Neurol. 2012;69:1430–40. [DOI] [PubMed] [Google Scholar]
- 301.Schneider LS. A critical review of cholinesterase inhibitors as a treatment modality in Alzheimer’s disease. Dialogues Clin Neurosci. 2000;2:111–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Alagiakrishnan K, Sankaralingam S, Ghosh M, Mereu L, Senior P. Antidiabetic drugs and their potential role in treating mild cognitive impairment and Alzheimer’s disease. Discov Med. 2013;16:277–86. [PubMed] [Google Scholar]
- 303.Sheng M, Lu H, Liu P, Li Y, Ravi H, Peng S-L, et al. Sildenafil improves vascular and metabolic function in patients with Alzheimer’s disease. J Alzheimers Dis. 2017;60:1351–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Theunissen EL, Heckman P, de Sousa Fernandes Perna EB, Kuypers KP, Sambeth A, Blokland A, et al. Rivastigmine but not vardenafil reverses cannabis-induced impairment of verbal memory in healthy humans. Psychopharmacology. 2015;232:343–53. [DOI] [PubMed] [Google Scholar]
- 305.Shim YS, Pae CU, Kim SW, Kim HW, Kim JC, Koh JS. Effects of repeated dosing with Udenafil (Zydena) on cognition, somatization and erection in patients with erectile dysfunction: a pilot study. Int J Impot Res. 2011;23:109–14. [DOI] [PubMed] [Google Scholar]
- 306.Rouf A, Tanyeli C. Bioactive thiazole and benzothiazole derivatives. Eur J Med Chem. 2015;97:911–27. [DOI] [PubMed] [Google Scholar]
- 307.Ayati A, Emami S, Asadipour A, Shafiee A, Foroumadi A. Recent applications of 1, 3-thiazole core structure in the identification of new lead compounds and drug discovery. Eur J Med Chem. 2015;97:699–718. [DOI] [PubMed] [Google Scholar]
- 308.Mishra CB, Kumari S, Tiwari M. Thiazole: a promising heterocycle for the development of potent CNS active agents. Eur J Med Chem. 2015;92:1–34. [DOI] [PubMed] [Google Scholar]
- 309.Nagahama K, Matsunaga Y, Kawachi M, Ito K, Tanaka T, Hori Y, et al. Acotiamide, a new orally active acetylcholinesterase inhibitor, stimulates gastrointestinal motor activity in conscious dogs. Neurogastroenterol Motil. 2012;24:566–74.e256. [DOI] [PubMed] [Google Scholar]
- 310.Sun Z-Q, Tu L-X, Zhuo F-J, Liu S-X. Design and discovery of Novel Thiazole acetamide derivatives as anticholinesterase agent for possible role in the management of Alzheimer’s. Bioorg Med Chem Lett. 2016;26:747–50. [DOI] [PubMed] [Google Scholar]
- 311.Nayak S, Gaonkar SL. A review on recent synthetic strategies and pharmacological importance of 1, 3-thiazole derivatives. Mini Rev Med Chem. 2019;19:215–38. [DOI] [PubMed] [Google Scholar]
- 312.Shi DH, Tang ZM, Liu YW, Harjani JR, Zhu HL, Ma XD, et al. Design, synthesis and biological evaluation of novel 2‐phenylthiazole derivatives for the treatment of Alzheimer’s disease. ChemistrySelect. 2017;2:10572–9. [Google Scholar]
- 313.Sahin Z, Ertas M, Bender C, Bülbül EF, Berk B, Biltekin SN, et al. Thiazole‐substituted benzoylpiperazine derivatives as acetylcholinesterase inhibitors. Drug Dev Res. 2018;79:406–25. [DOI] [PubMed] [Google Scholar]
- 314.Rahim F, Javed MT, Ullah H, Wadood A, Taha M, Ashraf M, et al. Synthesis, molecular docking, acetylcholinesterase and butyrylcholinesterase inhibitory potential of thiazole analogs as new inhibitors for Alzheimer disease. Bioorg Chem. 2015;62:106–16. [DOI] [PubMed] [Google Scholar]
- 315.Sağlık BN, Levent S, Osmaniye D, Acar Çevik U, Kaya Çavuşoğlu B, Özkay Y, et al. Design, synthesis, and biological activity evaluation of new donepezil-like compounds bearing thiazole ring for the treatment of Alzheimer’s disease. Crystals. 2020;10:637. [Google Scholar]
- 316.Mumtaz A, Shoaib M, Zaib S, Shah MS, Bhatti HA, Saeed A, et al. Synthesis, molecular modelling and biological evaluation of tetrasubstituted thiazoles towards cholinesterase enzymes and cytotoxicity studies. Bioorg Chem. 2018;78:141–8. [DOI] [PubMed] [Google Scholar]
- 317.Shidore M, Machhi J, Shingala K, Murumkar P, Sharma MK, Agrawal N, et al. Benzylpiperidine-linked diarylthiazoles as potential anti-Alzheimer’s agents: synthesis and biological evaluation. J Med Chem. 2016;59:5823–46. [DOI] [PubMed] [Google Scholar]
- 318.Kaduszkiewicz H, Zimmermann T, Beck-Bornholdt H-P, van den Bussche H. Cholinesterase inhibitors for patients with Alzheimer’s disease: systematic review of randomised clinical trials. Bmj. 2005;331:321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Bhounsule AS, Bhatt LK, Prabhavalkar KS, Oza M. Cyclin dependent kinase 5: a novel avenue for Alzheimer’s disease. Brain Res Bull. 2017;132:28–38. [DOI] [PubMed] [Google Scholar]
- 320.Nugiel DA, Vidwans A, Etzkorn A-M, Rossi KA, Benfield PA, Burton CR, et al. Synthesis and evaluation of indenopyrazoles as cyclin-dependent kinase inhibitors. 2. Probing the indeno ring substituent pattern. J Med Chem. 2002;45:5224–32. [DOI] [PubMed] [Google Scholar]
- 321.Shiradkar MR, Akula KC, Dasari V, Baru V, Chiningiri B, Gandhi S, et al. Clubbed thiazoles by MAOS: a novel approach to cyclin-dependent kinase 5/p25 inhibitors as a potential treatment for Alzheimer’s disease. Bioorg Med Chem. 2007;15:2601–10. [DOI] [PubMed] [Google Scholar]
- 322.Helal CJ, Sanner MA, Cooper CB, Gant T, Adam M, Lucas JC, et al. Discovery and SAR of 2-aminothiazole inhibitors of cyclin-dependent kinase 5/p25 as a potential treatment for Alzheimer’s disease. Bioorg Med Chem Lett. 2004;14:5521–5. [DOI] [PubMed] [Google Scholar]
- 323.Keri RS, Patil MR, Patil SA, Budagumpi S. A comprehensive review in current developments of benzothiazole-based molecules in medicinal chemistry. Eur J Med Chem. 2015;89:207–51. [DOI] [PubMed] [Google Scholar]
- 324.Klunk WE, Wang Y, Huang G-f, Debnath ML, Holt DP, Mathis CA. Uncharged thioflavin-T derivatives bind to amyloid-beta protein with high affinity and readily enter the brain. Life Sci. 2001;69:1471–84. [DOI] [PubMed] [Google Scholar]
- 325.Al Mamun A, Uddin MS, Mathew B, Ashraf GM. Toxic tau: structural origins of tau aggregation in Alzheimer’s disease. Neural Regen Res. 2020;15:1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Chang E, Congdon EE, Honson NS, Duff KE, Kuret J. Structure – activity relationship of cyanine tau aggregation inhibitors. J Med Chem. 2009;52:3539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Necula M, Chirita CN, Kuret J. Cyanine dye N744 inhibits tau fibrillization by blocking filament extension: implications for the treatment of tauopathic neurodegenerative diseases. Biochemistry. 2005;44:10227–37. [DOI] [PubMed] [Google Scholar]
- 328.Schafer KN, Murale DP, Kim K, Cisek K, Kuret J, Churchill DG. Structure–activity relationship of cyclic thiacarbocyanine tau aggregation inhibitors. Bioorg Med Chem Lett. 2011;21:3273–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Van Kan H, Groeneveld G, Kalmijn S, Spieksma M, Van Den Berg L, Guchelaar H. Association between CYP1A2 activity and riluzole clearance in patients with amyotrophic lateral sclerosis. Br J Clin Pharmacol. 2005;59:310–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Whitcomb DJ, Molnár E. Is riluzole a new drug for Alzheimer’s disease? J Neurochem. 2015;135:207–9. [DOI] [PubMed] [Google Scholar]
- 331.Cummings J, Lee G, Ritter A, Sabbagh M, Zhong K. Alzheimer’s disease drug development pipeline: 2019. Alzheimer’s Dement. 2019;5:272–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Mokhtari Z, Baluchnejadmojarad T, Nikbakht F, Mansouri M, Roghani M. Riluzole ameliorates learning and memory deficits in Aβ25–35-induced rat model of Alzheimer’s disease and is independent of cholinoceptor activation. Biomed Pharmacother. 2017;87:135–44. [DOI] [PubMed] [Google Scholar]
- 333.Pereira AC, Gray JD, Kogan JF, Davidson RL, Rubin TG, Okamoto M, et al. Age and Alzheimer’s disease gene expression profiles reversed by the glutamate modulator riluzole. Mol Psychiatry. 2017;22:296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Jarhad DB, Mashelkar KK, Kim H-R, Noh M, Jeong LS. Dual-specificity tyrosine phosphorylation-regulated kinase 1A (DYRK1A) inhibitors as potential therapeutics. J Med Chem. 2018;61:9791–810. [DOI] [PubMed] [Google Scholar]
- 335.Soppa U, Becker W. DYRK protein kinases. Curr Biol. 2015;25: R488–9. [DOI] [PubMed] [Google Scholar]
- 336.Abbassi R, Johns TG, Kassiou M, Munoz L. DYRK1A in neurodegeneration and cancer: molecular basis and clinical implications. Pharmacol Ther. 2015;151:87–98. [DOI] [PubMed] [Google Scholar]
- 337.Kimura R, Kamino K, Yamamoto M, Nuripa A, Kida T, Kazui H, et al. The DYRK1A gene, encoded in chromosome 21 Down syndrome critical region, bridges between β-amyloid production and tau phosphorylation in Alzheimer disease. Hum Mol Genet. 2007;16:15–23. [DOI] [PubMed] [Google Scholar]
- 338.Ryoo SR, Cho HJ, Lee HW, Jeong HK, Radnaabazar C, Kim YS, et al. Dual‐specificity tyrosine (Y)‐phosphorylation regulated kinase 1A‐mediated phosphorylation of amyloid precursor protein: evidence for a functional link between Down syndrome and Alzheimer’s disease. J Neurochem. 2008;104:1333–44. [DOI] [PubMed] [Google Scholar]
- 339.Coutadeur S, Benyamine H, Delalonde L, de Oliveira C, Leblond B, Foucourt A, et al. A novel DYRK1A (dual specificity tyrosine phosphorylation‐regulated kinase 1A) inhibitor for the treatment of Alzheimer’s disease: effect on Tau and amyloid pathologies in vitro. J Neurochemistr. 2015;133:440–51. [DOI] [PubMed] [Google Scholar]
- 340.Ogawa Y, Nonaka Y, Goto T, Ohnishi E, Hiramatsu T, Kii I, et al. Development of a novel selective inhibitor of the Down syndrome-related kinase Dyrk1A. Nat Commun. 2010;1:1–9. [DOI] [PubMed] [Google Scholar]
- 341.Chaikuad A, Diharce J, Schröder M, Foucourt A, Leblond B, Casagrande A-S, et al. An unusual binding model of the methyl 9-anilinothiazolo [5, 4-f] quinazoline-2-carbimidates (EHT 1610 and EHT 5372) confers high selectivity for dual-specificity tyrosine phosphorylation-regulated kinases. J Med Chem. 2016;59:10315–21. [DOI] [PubMed] [Google Scholar]
- 342.Rosse G. Tricyclic pyrimidines as inhibitors of DYRK1A/DYRK1B as potential treatment for Down’s syndrome or Alzheimer’s disease. ACS Med Chem Lett. 2013;4:502–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Masaki S, Kii I, Sumida Y, Kato-Sumida T, Ogawa Y, Ito N, et al. Design and synthesis of a potent inhibitor of class 1 DYRK kinases as a suppressor of adipogenesis. Bioorg Med Chem. 2015;23:4434–41. [DOI] [PubMed] [Google Scholar]
- 344.Morsy A, Trippier PC. Amyloid-binding alcohol dehydrogenase (ABAD) inhibitors for the treatment of Alzheimer’s disease: miniperspective. J Med Chem. 2018;62:4252–64. [DOI] [PubMed] [Google Scholar]
- 345.Grimm A, Lim Y-A, Mensah-Nyagan AG, Götz J, Eckert A. Alzheimer’s disease, oestrogen and mitochondria: an ambiguous relationship. Mol Neurobiol. 2012;46:151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Xu H, Wang R, ZHANG YW, Zhang X. Estrogen, β‐amyloid metabolism/trafficking, and Alzheimer’s disease. Ann NY Acad Sci. 2006;1089:324–42. [DOI] [PubMed] [Google Scholar]
- 347.ALVAREZ‐DE‐LA‐ROSA M, Silva I, Nilsen J, Perez M, GARCÍA‐SEGURA LM, Ávila J, et al. Estradiol prevents neural tau hyperphosphorylation characteristic of Alzheimer’s disease. Ann NY Acad Sci. 2005;1052:210–24. [DOI] [PubMed] [Google Scholar]
- 348.Goodenough S, Schleusner D, Pietrzik C, Skutella T, Behl C. Glycogen synthase kinase 3β links neuroprotection by 17β-estradiol to key Alzheimer processes. Neuroscience. 2005;132:581–9. [DOI] [PubMed] [Google Scholar]
- 349.Pike CJ, Carroll JC, Rosario ER, Barron AM. Protective actions of sex steroid hormones in Alzheimer’s disease. Front Neuroendocrinol. 2009;30:239–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Xie Y, Deng S, Chen Z, Yan S, Landry DW. Identification of small-molecule inhibitors of the Aβ–ABAD interaction. Bioorg Med Chem Lett. 2006;16:4657–60. [DOI] [PubMed] [Google Scholar]
- 351.Aitken L, Benek O, McKelvie BE, Hughes RE, Hroch L, Schmidt M, et al. Novel benzothiazole-based ureas as 17β-HSD10 inhibitors, a potential alzheimer’s disease treatment. Molecules. 2019;24:2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Valasani KR, Hu G, Chaney MO, Yan SS. Structure‐based design and synthesis of benzothiazole phosphonate analogues with inhibitors of human ABAD‐Aβ for treatment of Alzheimer’s disease. Chem Biol drug Des. 2013;81:238–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Benek O, Hroch L, Aitken L, Dolezal R, Guest P, Benkova M, et al. 6-Benzothiazolyl ureas, thioureas and guanidines are potent inhibitors of ABAD/17β-HSD10 and potential drugs for Alzheimer. Med Chem. 2017;13:345–58. [PubMed] [Google Scholar]
- 354.Oz M, Lorke DE, Petroianu GA. Methylene blue and Alzheimer’s disease. Biochem Pharmacol. 2009;78:927–32. [DOI] [PubMed] [Google Scholar]
- 355.Akoury E, Pickhardt M, Gajda M, Biernat J, Mandelkow E, Zweckstetter M. Mechanistic basis of phenothiazine‐driven inhibition of Tau aggregation. Angew Chem Int Ed. 2013;52:3511–5. [DOI] [PubMed] [Google Scholar]
- 356.Yuksel M, Biberoglu K, Onder S, Akbulut KG, Tacal O. Effects of phenothiazine-structured compounds on APP processing in Alzheimer’s disease cellular model. Biochimie. 2017;138:82–9. [DOI] [PubMed] [Google Scholar]
- 357.Bruchey AK, Gonzalez-Lima F. Behavioral, physiological and biochemical hormetic responses to the autoxidizable dye methylene blue. Am J Pharmacol Toxicol. 2008;3:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Atamna H, Nguyen A, Schultz C, Boyle K, Newberry J, Kato H, et al. Methylene blue delays cellular senescence and enhances key mitochondrial biochemical pathways. FASEB J. 2008;22:703–12. [DOI] [PubMed] [Google Scholar]
- 359.Tin G, Mohamed T, Gondora N, Beazely MA, Rao PP. Tricyclic phenothiazine and phenoselenazine derivatives as potential multi-targeting agents to treat Alzheimer’s disease. MedChemComm. 2015;6:1930–41. [Google Scholar]
- 360.Makhaeva GF, Shevtsova EF, Boltneva NP, Lushchekina SV, Kovaleva NV, Rudakova EV, et al. Overview of novel multifunctional agents based on conjugates of γ-carbolines, carbazoles, tetrahydrocarbazoles, phenothiazines, and aminoadamantanes for treatment of Alzheimer’s disease. Chem-Biol Interact. 2019;308: 224–34. [DOI] [PubMed] [Google Scholar]
- 361.Makhaeva GF, Lushchekina SV, Boltneva NP, Sokolov VB, Grigoriev VV, Serebryakova OG, et al. Conjugates of γ-Carbolines and Phenothiazine as new selective inhibitors of butyrylcholinesterase and blockers of NMDA receptors for Alzheimer Disease. Sci Rep. 2015;5:13164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Jain AK, Vaidya A, Ravichandran V, Kashaw SK, Agrawal RK. Recent developments and biological activities of thiazolidinone derivatives: a review. Bioorg Med Chem. 2012;20:3378–95. [DOI] [PubMed] [Google Scholar]
- 363.Tripathi AC, Gupta SJ, Fatima GN, Sonar PK, Verma A, Saraf SK. 4-Thiazolidinones: the advances continue…. Eur J Med Chem. 2014;72:52–77. [DOI] [PubMed] [Google Scholar]
- 364.Verma A, Saraf SK. 4-Thiazolidinone—a biologically active scaffold. Eur J Med Chem. 2008;43:897–905. [DOI] [PubMed] [Google Scholar]
- 365.Bulic B, Pickhardt M, Khlistunova I, Biernat J, Mandelkow EM, Mandelkow E, et al. Rhodanine‐based tau aggregation inhibitors in cell models of tauopathy. Angew Chem Int Ed. 2007;46:9215–9. [DOI] [PubMed] [Google Scholar]
- 366.Bulic B, Pickhardt M, Mandelkow E. Progress and developments in tau aggregation inhibitors for Alzheimer disease. J Med Chem. 2013;56:4135–55. [DOI] [PubMed] [Google Scholar]
- 367.Maqbool M, Mobashir M, Hoda N. Pivotal role of glycogen synthase kinase-3: A therapeutic target for Alzheimer’s disease. Eur J Med Chem. 2016;107:63–81. [DOI] [PubMed] [Google Scholar]
- 368.Hooper C, Killick R, Lovestone S. The GSK3 hypothesis of Alzheimer’s disease. J Neurochem. 2008;104:1433–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Castro A, Encinas A, Gil C, Bräse S, Porcal W, Pérez C, et al. Non-ATP competitive glycogen synthase kinase 3β (GSK-3β) inhibitors: study of structural requirements for thiadiazolidinone derivatives. Bioorg Med Chem. 2008;16:495–510. [DOI] [PubMed] [Google Scholar]
- 370.Martinez A, Alonso M, Castro A, Dorronsoro I, Gelpí JL, Luque FJ, et al. SAR and 3D-QSAR studies on thiadiazolidinone derivatives: exploration of structural requirements for glycogen synthase kinase 3 inhibitors. J Med Chem. 2005;48:7103–12. [DOI] [PubMed] [Google Scholar]
- 371.Martinez A, Alonso M, Castro A, Pérez C, Moreno FJ. First non-ATP competitive glycogen synthase kinase 3 β (GSK-3β) inhibitors: thiadiazolidinones (TDZD) as potential drugs for the treatment of Alzheimer’s disease. J Med Chem. 2002;45:1292–9. [DOI] [PubMed] [Google Scholar]
- 372.Xu M, Wang S, Zhu L, Wu P, Dai W, Rakesh K. Structure-activity relationship (SAR) studies of synthetic glycogen synthase kinase-3β inhibitors: a critical review. Eur J Med Chem. 2019;164:448–70. [DOI] [PubMed] [Google Scholar]
- 373.Palomo V, Perez DI, Perez C, Morales-Garcia JA, Soteras I, Alonso-Gil S, et al. 5-imino-1, 2, 4-thiadiazoles: first small molecules as substrate competitive inhibitors of glycogen synthase kinase 3. J Med Chem. 2012;55:1645–61. [DOI] [PubMed] [Google Scholar]
- 374.Gandini A, Bartolini M, Tedesco D, Martinez-Gonzalez L, Roca C, Campillo NE, et al. Tau-centric multitarget approach for Alzheimer’s disease: development of first-in-class dual glycogen synthase kinase 3β and tau-aggregation inhibitors. J Med Chem. 2018;61:7640–56. [DOI] [PubMed] [Google Scholar]
- 375.Dixit VA, Bharatam PV. SAR and computer-aided drug design approaches in the discovery of peroxisome proliferator-activated receptor γ activators: a perspective. J Comput Med. 2013;2013:1–38. [Google Scholar]
- 376.Galimberti D, Scarpini E. Pioglitazone for the treatment of Alzheimer’s disease. Expert Opin Investig Drugs. 2017;26:97–101. [DOI] [PubMed] [Google Scholar]
- 377.Rosen CJ. The rosiglitazone story—lessons from an FDA Advisory Committee meeting. N Engl J Med. 2007;357:844–6. [DOI] [PubMed] [Google Scholar]
- 378.Smith MT. Mechanisms of troglitazone hepatotoxicity. Chem Res Toxicol. 2003;16:679–87. [DOI] [PubMed] [Google Scholar]
- 379.Garcia-Vallvé S, Guasch L, Tomas-Hernández S, del Bas JM, Ollendorff V, Arola L, et al. Peroxisome proliferator-activated receptor γ (PPARγ) and ligand choreography: Newcomers Take the Stage: Miniperspective. J Med Chem. 2015;58:5381–94. [DOI] [PubMed] [Google Scholar]
- 380.Ji C, Zhang J. Protein polarization is critical to stabilizing AF-2 and helix-2′ domains in ligand binding to PPAR-γ. J Am Chem Soc. 2008;130:17129–33. [DOI] [PubMed] [Google Scholar]
- 381.Yamagishi K, Yamamoto K, Mochizuki Y, Nakano T, Yamada S, Tokiwa H. Flexible ligand recognition of peroxisome proliferator-activated receptor-γ (PPARγ). Bioorg Med Chem Lett. 2010;20:3344–7. [DOI] [PubMed] [Google Scholar]
- 382.Daynes RA, Jones DC. Emerging roles of PPARs in inflammation and immunity. Nat Rev Immunol. 2002;2:748–59. [DOI] [PubMed] [Google Scholar]
- 383.Deeks ED, Keam SJ. Rosiglitazone : a review of its use in type 2 diabetes mellitus. Drugs. 2007;67:2747–79. [DOI] [PubMed] [Google Scholar]
- 384.Gray E, Ginty M, Kemp K, Scolding N, Wilkins A. The PPARgamma agonist pioglitazone protects cortical neurons from inflammatory mediators via improvement in peroxisomal function. J Neuroinflammation. 2012;9:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Sastre M, Dewachter I, Rossner S, Bogdanovic N, Rosen E, Borghgraef P, et al. Nonsteroidal anti-inflammatory drugs repress beta-secretase gene promoter activity by the activation of PPARgamma. Proc Natl Acad Sci USA. 2006;103:443–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Grey A. Skeletal consequences of thiazolidinedione therapy. Osteoporos Int. 2008;19:129–37. [DOI] [PubMed] [Google Scholar]
- 387.Guan Y, Hao C, Cha DR, Rao R, Lu W, Kohan DE, et al. Thiazolidinediones expand body fluid volume through PPARγ stimulation of ENaC-mediated renal salt absorption. Nat Med. 2005;11:861–6. [DOI] [PubMed] [Google Scholar]
- 388.Toyota Y, Nomura S, Makishima M, Hashimoto Y, Ishikawa M. Structure-activity relationships of rosiglitazone for peroxisome proliferator-activated receptor gamma transrepression. Bioorg Med Chem Lett. 2017;27:2776–80. [DOI] [PubMed] [Google Scholar]
- 389.Patel PN, Kalariya PD, Swamy CV, Gananadhamu S, Srinivas R. Quantitation of acotiamide in rat plasma by UHPLC‐Q‐TOF‐MS: method development, validation and application to pharmacokinetics. Biomed Chromatogr. 2016;30:363–8. [DOI] [PubMed] [Google Scholar]
- 390.Nolan ML, Scott LJ. Acotiamide: first global approval. Drugs. 2013;73:1377–83. [DOI] [PubMed] [Google Scholar]
- 391.Furuta S, Kamada E, Omata T, Sugimoto T, Kawabata Y, Yonezawa K, et al. Drug–drug interactions of Z-338, a novel gastroprokinetic agent, with terfenadine, comparison with cisapride, and involvement of UGT1A9 and 1A8 in the human metabolism of Z-338. Eur J Pharmacol. 2004;497:223–31. [DOI] [PubMed] [Google Scholar]
- 392.Tack J, Pokrotnieks J, Urbonas G, Banciu C, Yakusevich V, Bunganic I, et al. Long‐term safety and efficacy of acotiamide in functional dyspepsia (postprandial distress syndrome)—results from the European phase 3 open‐label safety trial. Neurogastroenterol Motil. 2018;30:e13284. [DOI] [PubMed] [Google Scholar]
- 393.Milane A, Fernandez C, Vautier S, Bensimon G, Meininger V, Farinotti R. Minocycline and riluzole brain disposition: interactions with p‐glycoprotein at the blood–brain barrier. J Neurochem. 2007;103:164–73. [DOI] [PubMed] [Google Scholar]
- 394.Toklu HZ, Uysal MK, Kabasakal L, Sirvanci S, Ercan F, Kaya M. The effects of riluzole on neurological, brain biochemical, and histological changes in early and late term of sepsis in rats. J Surg Res. 2009;152:238–48. [DOI] [PubMed] [Google Scholar]
- 395.Bryson HM, Fulton B, Benfield P. Riluzole. Drugs. 1996;52:549–63. [DOI] [PubMed] [Google Scholar]
- 396.Vangavaragu JR, Valasani KR, Fang D, Williams TD, Yan SS. Determination of small molecule ABAD inhibitors crossing blood-brain barrier and pharmacokinetics. J Alzheimer’s Dis. 2014;42:333–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Disanto AR, Wagner JG. Pharmacokinetics of highly ionized drugs II: methylene blue—absorption, metabolism, and excretion in man and dog after oral administration. J Pharm Sci. 1972;61:1086–90. [DOI] [PubMed] [Google Scholar]
- 398.Walter-Sack I, Rengelshausen J, Oberwittler H, Burhenne J, Mueller O, Meissner P, et al. High absolute bioavailability of methylene blue given as an aqueous oral formulation. Eur J Clin Pharmacol. 2009;65:179–89. [DOI] [PubMed] [Google Scholar]
- 399.Peter C, Hongwan D, Küpfer A, Lauterburg B. Pharmacokinetics and organ distribution of intravenous and oral methylene blue. Eur J Clin Pharmacol. 2000;56:247–50. [DOI] [PubMed] [Google Scholar]
- 400.Saini NK, Suresh PS, Lella M, Bhamidipati RK, Rajagopal S, Mullangi R. LC-MS/MS determination of tideglusib, a novel GSK-3β inhibitor in mice plasma and its application to a pharmacokinetic study in mice. J Pharm Biomed Anal. 2018;148:100–7. [DOI] [PubMed] [Google Scholar]
- 401.Bharathy N, Svalina MN, Settelmeyer TP, Cleary MM, Berlow NE, Airhart SD, et al. Preclinical testing of the glycogen synthase kinase-3β inhibitor tideglusib for rhabdomyosarcoma. Oncotarget. 2017;8:62976–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Al-Majed A, Bakheit AH, Abdel Aziz HA, Alharbi H, Al-Jenoobi FI. Pioglitazone. Profiles Drug Subst Excip Relat Methodol. 2016;41:379–438. [DOI] [PubMed] [Google Scholar]
- 403.Eckland D, Danhof M. Clinical pharmacokinetics of pioglitazone. Exp Clin Endocrinol Diabetes. 2000;108:234–42. [Google Scholar]
- 404.Baldwin SJ, Clarke SE, Chenery RJ. Characterization of the cytochrome P450 enzymes involved in the in vitro metabolism of rosiglitazone. Br J Clin Pharmacol. 1999;48:424–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Chinnam P, Mohsin M, Shafee LM. Evaluation of acute toxicity of pioglitazone in mice. Toxicol Int. 2012;19:250–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Wallach JD, Wang K, Zhang AD, Cheng D, Grossetta Nardini HK, Lin H, et al. Updating insights into rosiglitazone and cardiovascular risk through shared data: individual patient and summary level meta-analyses. BMJ. 2020;368:l7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Stamford AW, Scott JD, Li SW, Babu S, Tadesse D, Hunter R, et al. Discovery of an orally available, brain penetrant BACE1 inhibitor that affords robust CNS Aβ reduction. ACS Med Chem Lett. 2012;3:897–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Maruyama M, Shimada H, Suhara T, Shinotoh H, Ji B, Maeda J, et al. Imaging of Tau pathology in a tauopathy mouse model and in Alzheimer patients compared to normal controls. Neuron. 2013;79:1094–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Hunsberger HC, Weitzner DS, Rudy CC, Hickman JE, Libell EM, Speer RR, et al. Riluzole rescues glutamate alterations, cognitive deficits, and tau pathology associated with P301L tau expression. J Neurochem. 2015;135:381–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Okamoto M, Gray JD, Larson CS, Kazim SF, Soya H, McEwen BS, et al. Riluzole reduces amyloid beta pathology, improves memory, and restores gene expression changes in a transgenic mouse model of early-onset Alzheimer’s disease. Transl Psychiatry. 2018;8:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Kerr F, Sofola-Adesakin O, Ivanov DK, Gatliff J, Gomez Perez-Nievas B, Bertrand HC, et al. Direct Keap1-Nrf2 disruption as a potential therapeutic target for Alzheimer’s disease. PLoS Genet. 2017;13:e1006593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Koehler D, Shah ZA, Williams FE. The GSK3beta inhibitor, TDZD-8, rescues cognition in a zebrafish model of okadaic acid-induced Alzheimer’s disease. Neurochemistry Int. 2019;122:31–7. [DOI] [PubMed] [Google Scholar]
- 413.Sereno L, Coma M, Rodriguez M, Sanchez-Ferrer P, Sanchez MB, Gich I, et al. A novel GSK-3beta inhibitor reduces Alzheimer’s pathology and rescues neuronal loss in vivo. Neurobiol Dis. 2009;35:359–67. [DOI] [PubMed] [Google Scholar]
- 414.Bolos M, Fernandez S, Torres-Aleman I. Oral administration of a GSK3 inhibitor increases brain insulin-like growth factor I levels. J Biol Chem. 2010;285:17693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Morales-Garcia JA, Luna-Medina R, Alonso-Gil S, Sanz-Sancristobal M, Palomo V, Gil C, et al. Glycogen synthase kinase 3 inhibition promotes adult hippocampal neurogenesis in vitro and in vivo. ACS Chem Neurosci. 2012;3:963–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Luna-Medina R, Cortes-Canteli M, Sanchez-Galiano S, Morales-Garcia JA, Martinez A, Santos A, et al. NP031112, a thiadiazolidinone compound, prevents inflammation and neurodegeneration under excitotoxic conditions: potential therapeutic role in brain disorders. J Neurosci. 2007;27:5766–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Heneka MT, Sastre M, Dumitrescu-Ozimek L, Hanke A, Dewachter I, Kuiperi C, et al. Acute treatment with the PPARgamma agonist pioglitazone and ibuprofen reduces glial inflammation and Abeta1–42 levels in APPV717I transgenic mice. Brain. 2005;128:1442–53. [DOI] [PubMed] [Google Scholar]
- 418.Pedersen WA, Flynn ER. Insulin resistance contributes to aberrant stress responses in the Tg2576 mouse model of Alzheimer’s disease. Neurobiol Dis. 2004;17:500–6. [DOI] [PubMed] [Google Scholar]
- 419.Pedersen WA, McMillan PJ, Kulstad JJ, Leverenz JB, Craft S, Haynatzki GR. Rosiglitazone attenuates learning and memory deficits in Tg2576 Alzheimer mice. Exp Neurol. 2006;199:265–73. [DOI] [PubMed] [Google Scholar]
- 420.Escribano L, Simon AM, Gimeno E, Cuadrado-Tejedor M, Lopez de Maturana R, Garcia-Osta A, et al. Rosiglitazone rescues memory impairment in Alzheimer’s transgenic mice: mechanisms involving a reduced amyloid and tau pathology. Neuropsychopharmacology. 2010;35:1593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Watson GS, Cholerton BA, Reger MA, Baker LD, Plymate SR, Asthana S, et al. Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am J Geriatr Psychiatry. 2005;13:950–8. [DOI] [PubMed] [Google Scholar]
- 422.Gold M, Alderton C, Zvartau-Hind M, Egginton S, Saunders AM, Irizarry M, et al. Rosiglitazone monotherapy in mild-to-moderate Alzheimer’s disease: results from a randomized, double-blind, placebo-controlled phase III study. Dement Geriatr Cogn Disord. 2010;30:131–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Geldmacher DS, Fritsch T, McClendon MJ, Landreth G. A randomized pilot clinical trial of the safety of pioglitazone in treatment of patients with Alzheimer disease. Arch Neurol. 2011;68:45–50. [DOI] [PubMed] [Google Scholar]
- 424.Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. Jama. 2007;298:1180–8. [DOI] [PubMed] [Google Scholar]
- 425.Edwards PD, Albert JS, Sylvester M, Aharony D, Andisik D, Callaghan O, et al. Application of fragment-based lead generation to the discovery of novel, cyclic amidine beta-secretase inhibitors with nanomolar potency, cellular activity, and high ligand efficiency. J Med Chem. 2007;50:5912–25. [DOI] [PubMed] [Google Scholar]
- 426.Zhu Z, Sun Z-Y, Ye Y, Voigt J, Strickland C, Smith EM, et al. Discovery of cyclic acylguanidines as highly potent and selective β-site amyloid cleaving enzyme (BACE) inhibitors: part I—inhibitor design and validation. J Med Chem. 2010;53:951–65. [DOI] [PubMed] [Google Scholar]
- 427.May PC, Dean RA, Lowe SL, Martenyi F, Sheehan SM, Boggs LN, et al. Robust central reduction of amyloid-β in humans with an orally available, non-peptidic β-secretase inhibitor. J Neurosci. 2011;31:16507–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Stamford AW, Scott JD, Li SW, Babu S, Tadesse D, Hunter R, et al. Discovery of an orally available, brain penetrant BACE1 inhibitor that affords robust CNS Aβ reduction. ACS Med Chem Lett. 2012;3:897–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Nowak P, Cole DC, Aulabaugh A, Bard J, Chopra R, Cowling R, et al. Discovery and initial optimization of 5,5′-disubstituted aminohydantoins as potent β-secretase (BACE1) inhibitors. Bioorg Med Chem Lett. 2010;20:632–5. [DOI] [PubMed] [Google Scholar]
- 430.Prati F, Bottegoni G, Bolognesi ML, Cavalli A. BACE-1 inhibitors: from recent single-target molecules to multitarget compounds for Alzheimer’s disease. J Med Chem. 2018;61:619–37. [DOI] [PubMed] [Google Scholar]
- 431.Stamford A, Strickland C. Inhibitors of BACE for treating Alzheimer’s disease: a fragment-based drug discovery story. Curr Opin Chem Biol. 2013;17:320–8. [DOI] [PubMed] [Google Scholar]
- 432.Tadano G, Komano K, Yoshida S, Suzuki S, Nakahara K, Fuchino K, et al. Discovery of an extremely potent thiazine-based β-secretase inhibitor with reduced cardiovascular and liver toxicity at a low projected human dose. J Med Chem. 2019;62:9331–7. [DOI] [PubMed] [Google Scholar]
- 433.Hsiao C-C, Rombouts F, Gijsen HJM. New evolutions in the BACE1 inhibitor field from 2014 to 2018. Bioorg Med Chem Lett. 2019;29:761–77. [DOI] [PubMed] [Google Scholar]
- 434.O’Neill BT, Beck EM, Butler CR, Nolan CE, Gonzales C, Zhang L, et al. Design and synthesis of clinical candidate PF-06751979: a potent, brain penetrant, β-site amyloid precursor protein cleaving enzyme 1 (BACE1) inhibitor lacking hypopigmentation. J Med Chem. 2018;61:4476–504. [DOI] [PubMed] [Google Scholar]
- 435.May PC, Willis BA, Lowe SL, Dean RA, Monk SA, Cocke PJ, et al. The potent BACE1 inhibitor LY2886721 elicits robust central Aβ pharmacodynamic responses in mice, dogs, and humans. J Neurosci. 2015;35:1199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.McKinzie DL, May PC, Boggs LN, Yang Z, Brier RA, Monk SA, et al. P1–080: nonclinical pharmacological characterization of the Bace1 inhibitor LY3202626. Alzheimer’s Dement. 2016;12:P432–3. [Google Scholar]
- 437.Johansson P, Kaspersson K, Gurrell IK, Bäck E, Eketjäll S, Scott CW, et al. Toward β-secretase-1 inhibitors with improved isoform selectivity. J Med Chem. 2018;61:3491–502. [DOI] [PubMed] [Google Scholar]
- 438.Brodney MA, Beck EM, Butler CR, Barreiro G, Johnson EF, Riddell D, et al. Utilizing structures of CYP2D6 and BACE1 complexes to reduce risk of drug-drug interactions with a novel series of centrally efficacious BACE1 inhibitors. J Med Chem. 2015;58:3223–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Wu Y-J, Guernon J, Yang F, Snyder L, Shi J, McClure A, et al. Targeting the BACE1 active site flap leads to a potent inhibitor that elicits robust brain Aβ reduction in rodents. ACS Med Chem Lett. 2016;7:271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Butler CR, Brodney MA, Beck EM, Barreiro G, Nolan CE, Pan F, et al. Discovery of a series of efficient, centrally efficacious BACE1 inhibitors through structure-based drug design. J Med Chem. 2015;58:2678–702. [DOI] [PubMed] [Google Scholar]
- 441.Fujimoto K, Matsuoka E, Asada N, Tadano G, Yamamoto T, Nakahara K, et al. Structure-based design of selective β-site amyloid precursor protein cleaving enzyme 1 (BACE1) inhibitors: targeting the flap to gain selectivity over BACE2. J Med Chem. 2019;62:5080–95. [DOI] [PubMed] [Google Scholar]
- 442.Henley D, Raghavan N, Sperling R, Aisen P, Raman R, Romano G. Preliminary results of a trial of atabecestat in preclinical Alzheimer’s disease. N Engl J Med. 2019;380:1483–5. [DOI] [PubMed] [Google Scholar]
- 443.Wu YJ, Guernon J, Rajamani R, Toyn JH, Ahlijanian MK, Albright CF, et al. Discovery of furo[2,3-d][1,3]thiazinamines as beta amyloid cleaving enzyme-1 (BACE1) inhibitors. Bioorg Med Chem Lett. 2016;26:5729–31. [DOI] [PubMed] [Google Scholar]
- 444.Pettus LH, Bourbeau MP, Bradley J, Bartberger MD, Chen K, Hickman D, et al. Discovery of AM-6494: a potent and orally efficacious β-site amyloid precursor protein cleaving enzyme 1 (BACE1) inhibitor with in vivo selectivity over BACE2. J Med Chem. 2020;63:2263–81. [DOI] [PubMed] [Google Scholar]
- 445.Morsy A, Trippier PC. Amyloid-binding alcohol dehydrogenase (ABAD) inhibitors for the treatment of Alzheimer’s disease. J Med Chem. 2019;62:4252–64. [DOI] [PubMed] [Google Scholar]
- 446.Kissinger CR, Rejto PA, Pelletier LA, Thomson JA, Showalter RE, Abreo MA, et al. Crystal structure of human ABAD/HSD10 with a bound inhibitor: implications for design of Alzheimer’s disease therapeutics. J Mol Biol. 2004;342:943–52. [DOI] [PubMed] [Google Scholar]
- 447.Li X, Wang H, Lu Z, Zheng X, Ni W, Zhu J, et al. Development of multifunctional pyrimidinylthiourea derivatives as potential anti-Alzheimer agents. J Med Chem. 2016;59:8326–44. [DOI] [PubMed] [Google Scholar]
- 448.Xu Y-X, Wang H, Li X-K, Dong S-N, Liu W-W, Gong Q, et al. Discovery of novel propargylamine-modified 4-aminoalkyl imidazole substituted pyrimidinylthiourea derivatives as multifunctional agents for the treatment of Alzheimer’s disease. Eur J Med Chem. 2018;143:33–47. [DOI] [PubMed] [Google Scholar]
- 449.Park J-E, Elkamhawy A, Hassan AHE, Pae AN, Lee J, Paik S, et al. Synthesis and evaluation of new pyridyl/pyrazinyl thiourea derivatives: neuroprotection against amyloid-β-induced toxicity. Eur J Med Chem. 2017;141:322–34. [DOI] [PubMed] [Google Scholar]
- 450.Gazova Z, Soukup O, Sepsova V, Siposova K, Drtinova L, Jost P, et al. Multi-target-directed therapeutic potential of 7-methoxytacrine-adamantylamine heterodimers in the Alzheimer’s disease treatment. Biochim Biophys Acta Mol Basis Dis. 2017;1863:607–19. [DOI] [PubMed] [Google Scholar]
- 451.Hroch L, Guest P, Benek O, Soukup O, Janockova J, Dolezal R, et al. Synthesis and evaluation of frentizole-based indolyl thiourea analogues as MAO/ABAD inhibitors for Alzheimer’s disease treatment. Bioorg Med Chem. 2017;25:1143–52. [DOI] [PubMed] [Google Scholar]
- 452.Crider AM, Witt KA. Somatostatin sst4 ligands: chemistry and pharmacology. Mini Rev Med Chem. 2007;7:213–20. [DOI] [PubMed] [Google Scholar]
- 453.Ankersen M, Crider M, Liu S, Ho B, Andersen HS, Stidsen C. Discovery of a novel non-peptide somatostatin agonist with SST4 selectivity. J Am Chem Soc. 1998;120:1368–73. [Google Scholar]
- 454.Crider AM, Liu S, Li T, Mahajan S, Ankersen M, Stidsen CE. Somatostatin receptor subtype 4 (sst4) ligands: synthesis and evaluation of Indol-3-yl- and 2-Pyridyl-Thioureas. Lett Drug Des Discov. 2004;1:84–7. [Google Scholar]
- 455.Sandoval KE, Farr SA, Banks WA, Crider AM, Morley JE, Witt KA. Somatostatin receptor subtype-4 agonist NNC 26–9100 decreases extracellular and intracellular Aβ1–42 trimers. Eur J Pharmacol. 2012;683:116–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Buchholz M, Hamann A, Aust S, Brandt W, Böhme L, Hoffmann T, et al. Inhibitors for human glutaminyl cyclase by structure based design and bioisosteric replacement. J Med Chem. 2009;52:7069–80. [DOI] [PubMed] [Google Scholar]
- 457.Buchholz M, Heiser U, Schilling S, Niestroj AJ, Zunkel K, Demuth H-U. The first potent inhibitors for human glutaminyl cyclase: synthesis and structure–activity relationship. J Med Chem. 2006;49:664–77. [DOI] [PubMed] [Google Scholar]
- 458.Hoang VH, Tran PT, Cui M, Ngo VT, Ann J, Park J, et al. Discovery of potent human glutaminyl cyclase inhibitors as anti-Alzheimer’s agents based on rational design. J Med Chem. 2017;60:2573–90. [DOI] [PubMed] [Google Scholar]
- 459.Katyayan K, Yi P, Monk S, Cassidy K. Excretion, mass balance, and metabolism of [(14)C]LY3202626 in humans: an interplay of microbial reduction, reabsorption, and aldehyde oxidase oxidation that leads to an extended excretion profile. Drug Metab Dispos. 2020;48:698–707. [DOI] [PubMed] [Google Scholar]
- 460.Qiu R, Ahn JE, Alexander R, Brodney MA, He P, Leurent C, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamic effects of PF-06751979, a potent and selective oral BACE1 inhibitor: results from phase I studies in healthy adults and healthy older subjects. J Alzheimers Dis. 2019;71:581–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Matijevic M, Watanabe H, Sato Y, Bernier F, McGrath S, Burns L, et al. P4–163: a single dose of the beta-secretase inhibitor, e2609, decreases CSF bace1 enzymatic activity in cynomolgus monkeys. Alzheimers Dement. 2015;11:P841. [Google Scholar]
- 462.Timmers M, Van Broeck B, Ramael S, Slemmon J, De Waepenaert K, Russu A, et al. Profiling the dynamics of CSF and plasma Aβ reduction after treatment with JNJ-54861911, a potent oral BACE inhibitor. Alzheimers Dement. 2016;2:202–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Brooks AF, Jackson IM, Shao X, Kropog GW, Sherman P, Quesada CA, et al. Synthesis and evaluation of [11C]PBD150, a radiolabeled glutaminyl cyclase inhibitor for the potential detection of Alzheimer’s disease prior to amyloid β aggregation. MedChemComm. 2015;6:1065–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Frost JL, Le KX, Cynis H, Ekpo E, Kleinschmidt M, Palmour RM, et al. Pyroglutamate-3 amyloid-β deposition in the brains of humans, non-human primates, canines, and Alzheimer disease-like transgenic mouse models. Am J Pathol. 2013;183:369–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Schilling S, Zeitschel U, Hoffmann T, Heiser U, Francke M, Kehlen A, et al. Glutaminyl cyclase inhibition attenuates pyroglutamate Aβ and Alzheimer’s disease–like pathology. Nat Med. 2008;14:1106–11. [DOI] [PubMed] [Google Scholar]
- 466.Morawski M, Schilling S, Kreuzberger M, Waniek A, Jäger C, Koch B, et al. Glutaminyl cyclase in human cortex: correlation with (pGlu)-amyloid-β load and cognitive decline in Alzheimer’s disease. J Alzheimers Dis. 2014;39:385–400. [DOI] [PubMed] [Google Scholar]
- 467.Timmers M, Streffer JR, Russu A, Tominaga Y, Shimizu H, Shiraishi A, et al. Pharmacodynamics of atabecestat (JNJ-54861911), an oral BACE1 inhibitor in patients with early Alzheimer’s disease: randomized, double-blind, placebo-controlled study. Alzheimers Res Ther. 2018;10:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Novak G, Streffer JR, Timmers M, Henley D, Brashear HR, Bogert J, et al. Long-term safety and tolerability of atabecestat (JNJ-54861911), an oral BACE1 inhibitor, in early Alzheimer’s disease spectrum patients: a randomized, double-blind, placebo-controlled study and a two-period extension study. Alzheimers Res Ther. 2020;12:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.Benek O, Korabecny J, Soukup O. A perspective on multi-target drugs for Alzheimer’s disease. Trends Pharmacol Sci. 2020; 41:434–45. [DOI] [PubMed] [Google Scholar]
- 470.Cummings J, Ritter A, Zhong K. Clinical trials for disease-modifying therapies in Alzheimer’s disease: a primer, lessons learned, and a blueprint for the future. J Alzheimers Dis. 2018;64:S3–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Savelieff MG, Nam G, Kang J, Lee HJ, Lee M, Lim MH. Development of multifunctional molecules as potential therapeutic candidates for Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis in the last decade. Chem Rev. 2018;119:1221–322. [DOI] [PubMed] [Google Scholar]
- 472.Cummings JL, Tong G, Ballard C. Treatment combinations for Alzheimer’s disease: current and future pharmacotherapy options. J Alzheimers Dis. 2019;67:779–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 473.Bachurin SO, Bovina EV, Ustyugov AA. Drugs in clinical trials for Alzheimer’s disease: the major trends. Med Res Rev. 2017;37:1186–225. [DOI] [PubMed] [Google Scholar]