ABSTRACT
Calcium (Ca2+) is a universal signalling molecule of life. The Ca2+ signalling is an evolutionarily conserved process from prokaryotes to eukaryotes. Ca2+ at high concentration is deleterious to the cell; therefore, cell maintains a low resting level of intracellular free Ca2+ concentration ([Ca2+]c). The resting [Ca2+]c is tightly regulated, and a transient increase of the [Ca2+]c initiates a signalling cascade in the cell. Ca2+ signalling plays an essential role in various processes, including growth, development, reproduction, tolerance to stress conditions, and virulence in fungi. In this review, we describe the evolutionary aspects of Ca2+ signalling and cell functions of major Ca2+ signalling proteins in different fungi.
KEYWORDS: Calcium signalling, calcineurin-Crz1 pathway, Ca2+ transporter, calmodulin and NCS-1, fungi, phospholipase
Evolution of calcium as a unique signalling molecule
Since the evolution of life on earth, where water covers three-fourth of the surface, both calcium (Ca2+) and magnesium (Mg2+) have evolved as divalent cations with a difference in the ability to form a complex with water molecules. Ca2+ has formed when oxygen and neon fused with successive particles (Clapham 2007). Ca2+ has a high degree of hydration and can accommodate 6–8 water molecules (Williams 2006). Ca2+ binds less tightly to water than Mg2+ and can precipitate phosphate, which may be lethal to the cell (Clapham 2007). Ca2+ can easily interact with molecules of complex geometry like proteins due to its unique properties such as charge, ionic radius, polarisability, and hydration energy (Brini et al. 2013a, 2013b). Therefore, cell efficiently controls the Ca2+ level for its survival and signalling. Thus, Ca2+ has evolved as a universal signalling molecule, signifying its importance in the evolution of life that started about 3.5 billion years ago (Plattner and Verkhratsky 2013). From the time life evolved, ATP has emerged as the central molecule, which was responsible for the formation of DNA/RNA subsequently (Ponnamperuma et al. 1963; Galimov 2009); and ATP synthesis was dependent on low Ca2+ concentration (Verkhratsky and Parpura 2014). It is also possible that in the process of evolution, Ca2+ assisted in the stability of DNA or RNA molecules, which exist as primitive stable molecules for the evolution of life (Jaiswal 2001). Since the early days of bacteria and protozoan evolution, Ca2+ was considered as a molecule of cell signalling, much before it got established as a ubiquitous secondary messenger molecule in the eukaryotic system (Shemarova and Nesterov 2005; Case et al. 2007), and the evolution of proteins allows the messenger function (Williams 2006). Thus, Ca2+ has evolved as an essential signaling ion across the different forms of life (Plattner and Verkhratsky 2013). Due to its versatile role in cell signaling, Ca2+ is also considered as a molecule for life and death (Berridge 1998; Berridge et al. 1998).
Ca2+ concentration gradient is the main switch behind the Ca2+ signalling machinery
The Ca2+ concentration outside the cell is as high as 10−3 M (Chin and Means 2000), the cytosolic free Ca2+ concentration ([Ca2+]c) at resting state is maintained at ∼100 nM; therefore, cells maintain a more than 10,000-fold gradient across the plasma membrane (Berridge et al. 2003). Cells store excess Ca2+ in various intracellular stores, including endoplasmic reticulum (ER), mitochondria, and vacuoles (Cornelius and Nakashima 1987). In the endoplasmic reticulum, the Ca2+ concentration ([Ca2+]ER) is maintained at several hundred µM. Besides, cells also need to maintain intracellular Ca2+ homoeostasis to avoid severe Ca2+ fluctuations and their effects (Berridge et al. 2003). Specific receptors and channels mediate the entry of Ca2+ across the plasma membrane in response to stimuli, including membrane depolarisation, mechanical stretch, and external agonists. The inositol 1,4,5-triphosphates receptors (IP3R) and the ryanodine receptors (RyR) induces Ca2+ release from the internal stores (Mikoshiba and Hattori 2000; Zeng et al. 2003; Hamilton 2005). Several proteins, including the plasma membrane Ca2+-ATPase (PMCA), the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA), the Na+/Ca2+ exchanger, and the mitochondrial uniporter are responsible for sequestering the excess Ca2+ from the cytosol by transporting Ca2+ either to external medium or into different cellular compartments (Berridge et al. 2003). Thus, in response to various stimuli, the Ca2+ signalling pathway is activated and causes expression of the specific target genes in the nucleus (Figure 1). In this review, we briefly describe major families of Ca2+ signalling proteins (Table 1) and their roles in cellular processes in different fungi.
Figure 1.
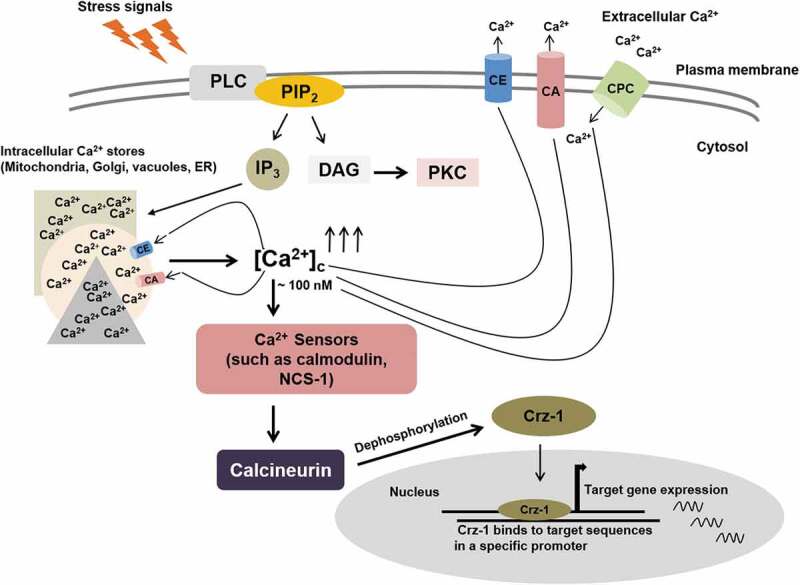
Overview of calcium signalling machinery in fungi. The membrane-bound phosphoinositide-specific phospholipase C (PLC) hydrolyzes phosphatidylinositol-4, 5-bisphosphate (PIP2) to produce two important second messenger molecules inositol 1,4,5-triphosphates (IP3) and diacylglycerol (DAG). IP3 induces Ca2+ release from intracellular stores, including mitochondria, Golgi, vacuoles, and endoplasmic reticulum (ER). DAG activates protein kinase C (PKC) that is involved in various signalling processes (Clapham 2007). PLCs also response to different stress signals (Barman et al. 2018). The resting intracellular free Ca2+ concentration ([Ca2+]c) is about 100 nM, an increase (shown using arrows pointing upward) of the [Ca2+]c is detected by various Ca2+ sensing proteins such as calmodulin (CaM) and NCS-1, which activate specific downstream signalling cascade. Calcineurin-Crz-1 pathway is shown here as an instance of a downstream signalling cascade. Ca2+ and CaM activate the serine/threonine phosphatase calcineurin that dephosphorylates the transcription factor Crz-1 for its nuclear location and subsequent expression of target genes in responses to stimuli. Excess Ca2+ is removed from the cytosol by Ca2+ exchangers (CE) and Ca2+ -ATPases (CA) proteins, whereas Ca2+ permeable channels (CPC) are required for the influx of extracellular Ca2+; and these processes are required to maintain Ca2+ homoeostasis in the cell (Tamuli et al. 2013)
Table 1.
Calcium signalling genes in different yeast and fungal species
| Organism |
Genome Size (Mb) |
Number of protein coding genes |
Number of Calcium signaling genes |
Subfamily |
References |
| Saccharomyces cerevisiae | 12.1 | 5, 596 | 40 | Ca2+ permeable channel (3), Ca2+/cation ATPases (5), Ca2+ exchanger (4), phospholipase C (2), CaM (1), Ca2+/CaM regulated proteins (25) | Zelter et al. 2004; Otero et al. 2010; Engel et al. 2014 |
| Schizosaccharomyces pombe | 13.8 | 4,940 | 18 | Ca2+ permeable channel (3), CaM (2), Ca2+/cation ATPases (8), Phospholipase (1), Ca2+/CaM regulated proteins (4) | Takeda and Yamamoto 1987; Toda et al. 1993; Yoshida et al. 1994; Okorokova-Facanha et al. 2003; Wood et al. 2002; Cortés et al. 2004 |
| Aspergillus fumigatus | 29.4 | 9,926 | 13 | Ca2+ permeable channel (3), Ca2+ exchanger (3), Ca2+/cation ATPases (3), Phospholipase (3), CaM (1) | Birch et al. 1996; Nierman et al. 2005; Dinamarco et al. 2012; de Castro et al. 2014 |
| Beauveria bassiana | 33.7 | 10,366 | 21 | Ca2+ permeable channel (3), Ca2+/cation ATPases (6), Ca2+ exchanger (5), CaM (1), Ca2+/CaMK (2)Ca2+/CaM regulated proteins (4) | Xiao et al. 2012; Fan et al. 2012; Li et al. 2015 |
| Candida albicans | 14 | 6,100 | 11 | Ca2+/cation ATPases (1), Ca2+ permeable channel (4), CaMK (1), CaM (1), Ca2+/H+ exchanger (1), Phospholipase (3) | Bennett et al. 1998; Andaluz et al. 2001; Jones et al. 2004; Luna-Tapia et al. 2019 |
| Cryptococcus neoformans | 19 | 6,572 | 40 | Ca2+ permeable channel (3), Ca2+/cation ATPases (6), Ca2+ exchanger (3), phospholipase C (2), CaM (1), Ca2+/CaM regulated proteins (21), Ca2+/CaMK (4) | Odom et al. 1997; Kmetzsch et al. 2011; Bahn and Jung 2013; Lee et al. 2016 |
| Fusarium oxysporum | 59.9 | 17,735 | 4 | Ca2+ Permeable channel (3), Phospholipase (1) | Ma et al. 2010; Su et al. 2017 |
| Magnaporthe grisea | 40.3 | 11,109 | 42 | Ca2+ Permeable channel (3), Ca2+/cation ATPases (12), Ca2+ exchanger (6), phospholipase C (4), CaM (1), Ca2+/CaM regulated proteins (16) | Zelter et al. 2004; Dean et al. 2005 |
| Neurospora crassa | 40 | 10,082 | 48 | Ca2+ Permeable channel (3), Ca2+/cation ATPases (9), Ca2+ exchanger (8), phospholipase C (4), CaM (1), Ca2+/CaM regulated proteins (23) | Galagan et al. 2003; Zelter et al. 2004; Tamuli et al. 2013 |
Phospholipases and their significance in Ca2+ signalling pathway in fungi
Phospholipases are a diverse class of enzymes involved in hydrolysing membrane phospholipids, mainly glycerophospholipids (Köhler et al. 2006; Hong et al. 2016). Phospholipases act on membrane phospholipids to produce small lipophilic signalling molecules like free fatty acids (FFAs), diacylglycerol (DAG), phosphatidic acid (PA), and lysophospholipids (LPLs) (Köhler et al. 2006; Hong et al. 2016). Phospholipases are of two types, acyl hydrolases, and phosphodiesterases. Phospholipases are classified, based on the specific ester linkage they cleave within phospholipid molecule, into four broad classes such as phospholipase A (PLA1 and PLA2), phospholipase B (PLB), phospholipase C (PLC), and phospholipase D (PLD) (Köhler et al. 2006). Both PLA and PLB are acyl hydrolases, while PLC and PLD belong to the phosphodiesterase class of phospholipases (Richmond and Smith 2011). The membrane-bound phosphoinositide-specific PLC cleaves phosphatidylinositol-4, 5-bisphosphate (PIP2) into two important second messengers, inositol 1,4,5-triphosphates (IP3) that induces Ca2+ release from internal stores and diacylglycerol (DAG) that activates protein kinase C (PKC), and triggers a range of cellular activities (Cornelius and Nakashima 1987; Chae et al. 2007; Clapham 2007). The IP3 causes release of Ca2+ to activate calmodulin (CaM)-dependent enzymes (Berridge 1993), IP3 also acts as the precursor of many inositol polyphosphates like IP5 or IP6 (York et al. 1999). Majority of the eukaryotic PLCs contain five conserved domains, comprising of two catalytic domains X and Y, an N-terminal pleckstrin homology (PH) domain for interaction with the membrane phospholipid, a C-terminal Ca2+ dependent C2 domain for binding to phospholipids, and an EF-hand motif for Ca2+ binding and interaction of the PH domain with phospholipid (Sutton et al. 1995; Yamamoto et al. 1999). PLC is important for various cellular processes and pathogenicity in several organisms, including filamentous fungi. The budding yeast Saccharomyces cerevisiae contains Plc1p, a phosphatidylinositol-specific phospholipase C (PI-PLC), which shows sequence homology to mammalian PI-PLC-δ isoforms (Flick and Thorner 1993). The Plc1p is necessary for growth at nonpermissive temperatures (above 35°C), survival under osmotic stress, and utilisation of alternative carbon sources like galactose, raffinose, or glycerol at permissive temperatures (23 to 30°C) (Flick and Thorner 1993). In the fission yeast Schizosaccharomyces pombe, plc1-1 is a homolog of plc1, mutation of plc1-1 induced by nitrosoguanidine caused sensitivity to high amounts of phosphate in the medium
(Fankhauser et al. 1995). The growth defect of the plc1-1 deletion mutant was partially restored in low inositol and low phosphate minimal media containing a high concentration of nitrogen, which suggests a potential role of plc1-1 in ammonium sensing (Fankhauser et al. 1995). In the grey mould fungus Botrytis cinerea, Plc1 homolog BcPLC1 is required for vegetative growth, conidial formation, germination, and virulence (Schumacher et al. 2008). In the human-pathogenic fungus Candida albicans, the PLC1 homolog CaPLC1 is an essential gene and its conditional mutant showed increased sensitivity to a high concentration of sorbitol or NaCl, increased sensitivity to lower (18°C) or higher temperatures (43°C), and reduced growth in medium containing galactose, but not glucose, as the sole carbon source (Kunze et al. 2005). The CaPLC1 conditional mutant was also sensitive to nocodazole that inhibits chromosome segregation, and showed reduced growth in filamentous growth-inducing conditions and on media containing only arginine as the sole nitrogen source (Kunze et al. 2005). However, two additional PLC genes in CaPLC2 and CaPLC3 genes were non-essential for growth as loss of Caplc2 and Caplc3 did not show any visible growth defects in C. albicans (Knechtle et al. 2005; Kunze et al. 2005). Moreover, in a mouse systemic infection model, the CaPLC2 and CaPLC3 mutants showed survival rate similar to the wild type, suggesting that both CaPLC2 and CaPLC3 are not essential for virulence (Knechtle et al. 2005; Kunze et al. 2005). In the citrus fungal pathogen, Alternaria alternata, the PLC1 homolog is important for vegetative growth, conidial formation, Ca2+ homoeostasis, and virulence (Tsai and Chung 2014). In encapsulated yeast and human pathogen Cryptococcus neoformans, CnPlc1, a homolog of the mammalian PI-PLC-δ is essential for cellular homoeostasis and virulence (Lev et al. 2013). The model filamentous fungus Neurospora crassa possesses four novel PLC-δ proteins, including PLC-1, which is highly divergent among the natural isolates (Galagan et al. 2003; Borkovich et al. 2004; Gavric et al. 2007; Barman and Tamuli 2015, 2017; Barman et al. 2018). The plc-1 mutant, generated using repeat-induced point mutation (RIP; Selker et al. 1987), is viable, but showed reduced growth, abnormal hyphal morphology, lower turgor, increased sensitivity to low extracellular and increase intracellular Ca2+ concentrations, and responded differently to PLC inhibitor 3-nitrocoumarin (Gavric et al. 2007). In another study, phenotypes of the RIP-generated and knockout mutants of plc-1 were different, and analysis of the mutant phenotypes suggested a role for plc-1 in hyphal tip growth in N. crassa (Lew et al. 2015). Moreover, the plc-1 knock out mutant displays aberrant hyphal morphology in the presence of Ca2+ ionophore A23187, accumulates an increased amount of carotenoid, and shows reduced survival during oxidative and thermal stress (Barman and Tamuli 2015; Barman et al. 2018). In the rice-blast fungus Magnaporthe oryzae, PI-PLC-δ isoform MoPLC1 regulates intracellular Ca2+ fluxes and plays an essential role in fungal development, appressorium formation, and pathogenicity (Rho et al. 2009).
Ca2+ transporters and their involvement in cell processes in fungi
In fungi, six major types of Ca2+ transporters, including Ca2+ pumps, Ca2+/H+ exchangers (Lange and Peiter 2020), high-affinity calcium system (HACS), low-affinity calcium system (LACS), TRP-like Ca2+ channels, and mitochondrial Ca2+ uniporter (MCU) have been identified (Tisi et al. 2016). The HACS and LACS are two critical Ca2+ uptake systems that are conserved across different fungi and mediate the entry of Ca2+ under different cellular conditions (Martin et al. 2011). In fungi, Ca2+ release from internal stores such as Golgi bodies, endoplasmic reticulum, vacuole, and mitochondria are carried out by P-type Ca2+ ATPases driving the energy from the synthesis of ATP to transfer Ca2+ against the ion gradient (Li et al. 2019). In A. fumigatus, McuA is a Ca2+ uniporter localised to the mitochondrial membrane, and the deletion of mcuA results in disruption of the mitochondrial Ca2+ homoeostasis, suggesting its role in Ca2+ uptake (Song et al. 2016). In addition, the deletion of mcuA also causes resistance to azoles and oxidative stress; however, overexpression of mcuA restores the azole sensitivity phenotype in a deletion mutant of agcA that encodes for a mitochondrial carrier protein in A. fumigatus (Song et al. 2016). The knockouts of cchA, midA, and yvcA, which are the homologues of the S. cerevisiae genes encoding for voltage-gated CCH1, stretch-activated MID1, and vacuolar YVC1 Ca2+ channels, respectively, were not virulent in mice model of invasive aspergillosis, suggesting that these transporters contribute to virulence in A. fumigatus (de Castro et al. 2014). In fungi, vacuoles are one of the major Ca2+ stores, the Ca2+ ATPases and the Ca2+/H+ exchangers are important transporters that guide the entry of Ca2+ into the vacuoles (Pittman 2011). The pmcA, pmcB, and pmcC type Ca2+ transporters in A. fumigatus are homologues of the S. cerevisiae plasma membrane Ca2+-ATPase PMC1 (Cunningham and Fink 1994), transcribed by the calcineurin A-CrzA pathway, and necessary for growth and survival under the Ca2+ stress condition (Dinamarco et al. 2012). In A. fumigatus, the pmcA conditional mutant was not virulent in the mice model of invasive aspergillosis, suggesting that pmcA has a role in conferring virulence and pathogenicity to the fungi; pmcA also regulates the metabolism of Ca2+ and Mn2+ (Dinamarco et al. 2012). There are different classes of P-type Ca2+ ATPases such as plasma membrane Ca2+-ATPase (PMCA), sarco (endo) plasmic reticulum Ca2+-ATPase (SERCA) and secretory pathway Ca2+-ATPase (SPCA) are ATP-driven pumps involved in the active transport and homoeostasis of Ca2+ (Sze et al. 2000; Carafoli 2002; Ton and Rao 2004). In S. cerevisiae, deletion of the Ca2+-ATPase Pmr1p results in the lack of adequate amount of Ca2+ within the Golgi, an increase in the cytosolic Ca2+ concentration causing vacuolar fragmentation possibly to sequester excess Ca2+ (Kellermayer et al. 2003). In S. pombe, Cps5p is the Pmr1p homolog, which plays a role in the cell wall formation, protein glycosylation, and maintains intracellular Ca2+ homoeostasis by depletion of the excessive cytosolic Ca2+ via the interaction with a vacuolar Ca2+-ATPase homolog, Pmc1p (Cortés et al. 2004). In C.albicans, the deletion of a Ca2+-ATPase gene CaPMR1, the homolog of PMR1 in S. cerevisiae, results in altered glycosylation, causing a defect in the cell wall formation and loss of virulence (Bates et al. 2005). In N. crassa, the deletion of a PMCA family of Ca2+ ATPase NCA-2 causes restricted growth, sensitivity to increasing concentration of Ca2+ in the media, and female sterile phenotype (Bowman et al. 2011; Deka and Tamuli 2013). In addition, the nca-2 gene plays a role in carotenoid accumulation, regulation of the circadian clock with a Ca2+ sensor ncs-1, and thermotolerance with a cation-ATPase trm-9 in N. crassa (Deka and Tamuli 2013; Laxmi and Tamuli 2015).
Calmodulin is a major calcium-binding protein involved diverse cell functions in fungi
The change in the Ca2+ concentration inside the cell is detected by an array of Ca2+ sensors belonging to the EF-hand family of proteins. CaM is a primary Ca2+ sensor containing EF-hand (Lewit-Bentley and Réty 2000) motifs, and conserved from lower to higher eukaryotes (Chin and Means 2000). Because of its evolutionary conservation, CaM transduces about 300 effectors for the activation of the downstream signalling cascade in the eukaryotes (Halling et al. 2016). The S. cerevisiae CaM has three functional EF-hands; disruption of this gene has a lethal phenotype, and therefore, essential for viability (Davis et al. 1986). In S. pombe, disruption of cam1 causes growth defects and abolishes cell division (Takeda and Yamamoto 1987). The cam1 disruption results in improper chromosomal segregation, hyper condensation, uneven distribution of the chromosomal material, and absence of the spindle body proteins responsible for spore formation in S. pombe (Itadani et al. 2010). Immunofluorescence assay of the GFP-tagged cam1 protein in S. pombe indicated the localisation of the Cam1 to the spindle body fibres and also to the sites responsible for polarised cell growth (Moser et al. 1997; Itadani et al. 2010). Calmodulin also mediates protein phosphorylation via calmodulin-dependent kinase in the presence of N-acetyl-D-glucosamine (GlcNAc), which is involved in germ tube formation causing morphogenesis of C. albicans; however, calmodulin inhibitor trifluoperazine (TFP) inhibits the phosphorylation and germ tube formation (Roy and Datta 1987; Paranjape et al. 1990). In addition, calmodulin binds to an integral membrane protein Dfi1p that activates mitogen-activated protein kinase (MAPK) Cek1p, which is required for invasive filamentation in C. albicans (Davis et al. 2013). In C. neoformans, CAM1 is required for the infestation in the human body at 37°C (Kraus et al. 2005). In the presence of calcineurin inhibitor FK506, the temperature-sensitive cam1-ts mutant of C. neoformans showed defects in growth and bud formation at 25°C (Kraus et al. 2005). The cam1-ts mutant also showed reduced CAM1 expression and growth defect at 37°C, which was not complemented when transformed with a calmodulin independent calcineurin A allele (CNA1-AIΔ) lacking the coding region for the C-terminal calmodulin-binding site and the autoinhibitory domain (Kraus et al. 2005). Therefore, CAM1 has a role in both calcineurin-dependent and independent developmental pathways in C. neoformans (Kraus et al. 2005). In M. grisea, CaM is required for early-stage appressorium formation and conidial germination (Lee and Lee 1998; Liu and Kolattukudy 1999). The cmd is an essential gene in N. crassa; therefore, knockout of the cmd gene is not viable (Tamuli et al. 2013). CaM antagonists trifluoperazine (TFP) and chlorpromazine (CPZ) caused defects in hyphae formation, reduced growth, and impaired sexual development in N. crassa (Laxmi and Tamuli 2015). In addition, the RIP-generated cmd mutants showed reduced growth, less carotenoid accumulation, decreased survival in exposure to ultraviolet (UV) irradiation, and defect in the sexual development causing female sterility (Laxmi and Tamuli 2017). CaM also interacts with Ca2+/CaM-dependent kinases, including Ca2+/CaMK-2 that is required for full fertility in N. crassa (Kumar and Tamuli 2014; Laxmi and Tamuli 2017).
Neuronal calcium sensor-1 regulates growth, sexual development, and Ca2+ homoeostasis in fungi
Neuronal calcium sensor-1 (NCS-1), which belongs to the family of EF-hand containing protein, binds to Ca2+ and senses the change in the concentration of the Ca2+ inside the cell (Burgoyne 2007; Tamuli et al. 2011). The NCS-1 was identified as frequenin (Frq1), enriched in the synapses of the Drosophila melanogaster nervous system, which facilitates the frequency-dependent release of neurotransmitter (Pongs et al. 1993). Additionally, Frq2, paralogue of Frq1, was evolved via an unusual frequenin gene duplication in D. melanogaster (Sánchez-Gracia et al. 2010). In S. cerevisiae, the NCS-1 orthologue FRQ1 plays a role in cell growth, and regulates a phosphatidylinositol-4-OH kinase (PIK-1) that is essential for cell survival (Hendricks et al. 1999). In S. pombe, Ncs1p has a role in the sexual development by regulation of spore formation and conjugation via Ca2+ dependent manner (Hamasaki-Katagiri et al. 2004). In A. fumigatus, the knockout mutantion of ncs-1 homologue ncsA causes polarity defects, confers Ca2+ resistance, and increased sensitivity to EGTA, sodium dodecyl sulphate (SDS), ergosterol-depletion agents voriconazole and itraconazole, and the ergosterol intercalating agent amphotericin B; however, did not affect the virulence (Mota Júnior et al. 2008). NcsA also promotes expression of the S. cerevisiae Pmc1 homologs pmcA, a P-type Ca2+-ATPase, and pmcB, a Ca2+-translocating P-type ATPase in A. fumigatus (Mota Júnior et al. 2008; Soriani et al. 2008). In M. grisea, null-mutant of the FRQ1/NCS-1-like gene Mg-NCS-1, showed restricted growth at high Ca2+ concentrations or acidic conditions (Saitoh et al. 2003). The knockout mutant of ncs-1 in N. crassa shows reduced growth and increased sensitivity to high concentrations of Ca2+ and UV irradiations (Tamuli et al. 2011; Deka et al. 2011). Besides, in response to the high concentrations of Ca2+, transcription of ncs-1 is upregulated by the Crz-1 transcription factor in N. crassa (Gohain and Tamuli 2019). Furthermore, NCS-1 interacts with the Ca2+ permeable channel MID-1 in the plasma membrane, possibly to block Ca2+ influx, which might be critical for survival under the high concentration of Ca2+ (Gohain and Tamuli 2019).
Calcineurin plays important roles in asexual and sexual developments, stress responses, and pathogenicity in fungi
Calcineurin was first identified as an inhibitor of CaM-dependent cyclic nucleotide phosphodiesterase in a column fraction (Wang and Desai 1976). Calcineurin is the only serine/threonine phosphatase that requires both Ca2+ and CaM for its activity (Klee et al. 1979, 1998). The heterodimeric enzyme calcineurin has two subunits, a catalytic subunit and a regulatory subunit (Klee and Krinks 1978). Calcineurin has a critical role in fungal development, stress responses, and virulence in pathogenic fungi (Rusnak and Mertz 2000; Juvvadi et al. 2014). The S. cerevisiae MATa strains containing null-mutations in the calcineurin or protein phosphatase 2B (PP2B) subunits encoding genes CNA1 and CNA2 showed an increased sensitivity to growth arrest induced by the mating pheromone α-factor (Cyert et al. 1991). In S. cerevisiae, null mutations in both the calcineurin catalytic subunits (cmp1cmp2 or cna1cna2), and the regulatory (cnb1) subunit, cause sensitivity to high concentrations of Mn2+ (MnCl2), because the mutants were not able to block the entry of Mn2+ into the cell (Farcasanu et al. 1995). In N. crassa, the RIP-generated cnb-1 mutant showed a defect in hyphal growth and differentiation (Kothe and Free 1998). Inhibition of cna-1 using antisense RNA and inhibitor of calcineurin FK506, results in a loss of steep Ca2+ gradient at the hyphal tip in N. crassa (Prokisch et al. 1997). Furthermore, the RIP-generated Cna1 and cnb-1 mutants displayed reduced thermotolerance, increased sensitivity to osmotic stress, and defect in the asexual and sexual developments in N. crassa (Kumar et al. 2019). In M. oryzae, application of antisense RNA against the catalytic subunit of calcineurin MCNA exhibited lessening in mycelial formation, conidiation, and appressorium formation, resulting in the reduction of the fungal pathogenicity (Choi et al. 2009a). In C. neoformans, the calcineurin catalytic subunit CNA1 is required for growth at elevated temperature, survival in increased levels of CO2 and alkaline pH conditions, cation homoeostasis, and virulence (Odom et al. 1997). In the entomopathogenic fungus Beauveria bassiana, the cnA1 and cnA2 genes encode for two calcineurin catalytic subunit paralogues CnA1 and CnA2, and the cnB gene encodes the calcineurin regulatory subunit B (CnB) (Li et al. 2015). The B. bassiana ∆cnA1, ∆cnA2, and ∆cnB deletion mutants showed reduced growth and conidiation, decreased virulence, sensitivity to stress-inducing chemicals and the fungicide carbendazim, and reduced survival in response to heat-shock, UV-B irradiation (Li et al. 2015). In addition, the ∆cnA1 and ∆cnA2 mutants showed altered cell wall composition, and the ΔcnB mutant showed sensitivity to osmotic stress induced by NaCl and KCl (Li et al. 2015). In A. fumigatus, calcineurin regulates hyphal growth, septation, and virulence (Lamoth et al. 2013; Juvvadi et al. 2014). In S. pombe, analysis of a null mutant of calcineurin-like gene ppb1+ showed its involvement in cytokinesis, pole body positioning, cell shape and polarity, and mating (Yoshida et al. 1994).
In addition, calcineurin plays a critical role in fungal pathogenicity. A. fumigatus causes a common life-threatening disease called aspergillosis in humans. In the Aspergillus and Candida species, the antifungal paradoxical effect is a phenomenon, where reversal of growth inhibition occurs at higher concentrations of the antifungal drug echinocandins, usually caspofungin, which inhibit β-1,3-glucan synthase (FKS1 in C. albicans, and FksA in A. fumigatus) causing damage of the fungal cell walls (Sanglard et al. 2003; Steinbach et al. 2015). The key players in the paradoxical effect are calcineurin catalytic subunit (CnaA) and the heat shock protein 90 (Hsp90) in A. fumigatus and C. albicans (Steinbach et al. 2015). Furthermore, the A. fumigatus calcineurin upregulates chitin biosynthesis, which causes increased chitin content in the cell wall, by transcriptional regulation of chitin synthase genes during paradoxical effect in response to high concentrations of caspofungin (Fortwendel et al. 2010). In A. fumigatus, caspofungin transiently increases [Ca2+]c concentration, and activates CaM-calcineurin signalling, which causes paradoxical effect (Juvvadi et al. 2015). The A. fumigatus calcineurin catalytic A subunit (CnaA) contains a serine-proline-rich-region (SPRR) unique to the filamentous fungi, but absent in the human calcineurin α-catalytic subunit (Juvvadi et al. 2013). In the SPRR, phosphorylation of S406, S408, S410, and S413 residues are required for the function of calcineurin in hyphal growth and virulence of the A. fumigatus (Juvvadi et al. 2013). Identification of this critical SPRR region unique to filamentous fungi, but absent in human, is a significant step towards the development of new antifungal drugs for invasive aspergillosis (Juvvadi et al. 2013, 2014). Moreover, at paradoxical-growth concentrations of caspofugin (4 μg/ml), phosphorylation of S406, S410, and S413 residues in the SPRR of CnaA activates calcineurin, which may cause nuclear localisation of the transcription factor calcineurin responsive zinc finger 1 homologue CrzA for transcriptional activation to regulate paradoxical growth (Juvvadi et al. 2015). CrzA also has a role in chitin synthase expression in caspofungin paradoxical effect (CPE) in A. fumigatus (Ries et al. 2017). The pathways mediated by the CrzA and ZipD, which is a basic leucine zipper transcription factor and another target of calcineurin, genetically interact during Ca2+ stress (de Castro et al. 2019). Furthermore, in A. fumigatus, ZipD regulates cell wall composition and organisation, tolerance to osmotic stress, pathogenesis, and resistance to echinocandin antifungals, including caspofungin (de Castro et al. 2019). Therefore, targeting calcineurin CrzA and ZipD transcription factors may be potential drug targets against A. fumigatus. In addition, the role of CrzA has been investigated for differentiation and mycotoxin production in aflatoxin producing fungi A. flavus and A. parasiticus (Chang 2008; Lim et al. 2019). In M. oryzae, the calcineurin catalytic subunit A (MCNA) plays a role in mycelial growth, conidiation, formation of specialised infection structure called appressorium, and pathogenicity (Choi et al. 2009a). Moreover, in M. oryzae, the knock-down of catalytic subunit A-like gene moderately reduced appressorium formation, but the knock-down of regulatory subunit B-like gene causes a complete loss of pathogenicity against the host plants, suggesting that the catalytic and regulatory subunits play a distinct role in pathogenicity (Nguyen et al. 2008). In C. neoformans, Δcna1 deletion mutants were unable to survive at a body temperature of 37°C, alkaline pH, and CO2 at high levels (Odom et al. 1997). The C. neoformans virulence was studied using an immunosuppressed rabbit, an animal model of cryptococcal meningitis (Odom et al. 1997). The virulence was examined by removal of cerebral blood spinal fluid (CSF) and counting of the colony-forming units (CFU), which revealed a low number CFU in the Δcna1 mutant of C. neoformans, indicating that calcineurin is required for growth in the mammalian host (Odom et al. 1997). Similarly, disruption of cnb1 in C. neoformans results in temperature-sensitivity and reduced virulence in the murine model of cryptococcosis (Fox et al. 2001). Calcineurin in C. neoformans was identified as a novel antifungal drug target (Cruz et al. 2000). C. neoformans is sensitive to FK506 and CsA at physiological temperature (Cruz et al. 2000). A mutational analysis using a novel 6 bp duplication in the calcineurin B gene (CNB1) inhibits the immunophilins FKBP12 – FK506 binding to Cnb1, which revealed the mechanism of drug action in C. neoformans (Fox et al. 2001). Calcineurin A is also vital for providing tolerance to antifungal agents along with some metabolic inhibitors in C. albicans (Cruz et al. 2002). In C. albicans, disruption of cna-1 results in the loss of viability in the presence of antifungal agents fluconazole, amorolfine, itraconazole, terbinafine, and voriconazole (Sanglard et al. 2003). In addition to calcineurin, Hsp90 has a role in providing resistance to echinocandins in C. albicans (Singh et al. 2009). Calcineurin inhibitors, cyclosporine and fluconazole have a synergistic effect in the prevention of biofilm formation and increase susceptibility to fluconazole in C. albicans (Jia et al. 2016).
Calcineurin responsive zinc finger 1 (Crz1) plays a critical role in regulating cellular functions, tolerance to stress conditions, and virulence
There are different target proteins of calcineurin found across different organisms (Li et al. 2011). The best-known target is a transcription factor called calcineurin-responsive zinc finger 1 (Crz1) in lower eukaryotes and the nuclear factor of activated T cells (NFAT) in mammals (Thewes 2014). In S. cerevisiae, the calcineurin target Crz1p, also known as Tcn1p, drives the expressions of PMC1, PMR1, PMR2A, and FKS2 genes, which play a vital role in the tolerance to high Ca2+, Mn2+, Na+, and cell wall damage, respectively (Matheos et al. 1997; Stathopoulos and Cyert 1997). In S. pombe, the null mutant of prz1, the crz1 homologue, did not show any defects in morphology and sexual development; however, the Δprz1 mutant was hypersensitive to Ca2+ (Hirayama et al. 2003). In M. grisea, the CRZ1 homologue MgCRZ1 partially complemented the S. cerevisiae Δcrz1 mutant and suppressed the Li+ sensitivity (Zhang et al. 2009). The MgCRZ1 is required for growth, development, Ca2+ stress tolerance, and full virulence (Zhang et al. 2009). In C. neoformans, crz1 drives the expression of the chitin synthase gene chs6, and crz1 deletion mutant showed a defect in cell wall synthesis (Lev et al. 2012). In C. albicans, the CRZ1 deletion mutant was more sensitive to Ca2+, Li+, and Mn2+ cations, hypersensitive to anionic detergents SDS and antifungal azoles, including fluconazole and miconazole (Santos and de Larrinoa 2005). In Aspergillus parasiticus, crzA is required for vegetative growth, asexual development, and aflatoxin production under the Ca2+ stress condition (Chang 2008). In A. fumigatus, knock out of crzA results in a defect in germination and polarised hyphal growth, and reduced sporulation (Cramer et al. 2008). The A. fumigatus ΔcrzA mutants also displayed sensitivity to heat shock conditions (Soriani et al. 2008). Studies in A. flavus, which produces aflatoxin, the deletion of crzA renders the strain more vulnerable to cell wall stress and osmotic pressure (Lim et al. 2019). In the A. flavus ΔcrzA mutants, conidiophore production was reduced as the head of the conidiophore become short, along with a decrease in the number of conidia (Lim et al. 2019).
Crz 1 also plays a critical role in fungal virulence. In M. grisea, when infected to onion surface or rice leaves, Δmocrz1 showed a significant reduction in the appressorial penetration rate (Choi et al. 2009b). In M. grisea, lipid droplets are transported from conidia to nascent appressorium, which fully melanised within 12–24 h (Zhang et al. 2009). However, in the M. grisea Δcrz1 mutant, the lipid droplets were undegraded even after 48 h (Zhang et al. 2009). Thus, targeting calcineurin-CRZ1 signalling cascade results in a lack of functional appressorium that failed to penetrate the host cuticle, causing loss of full virulence in M. grisea (Zhang et al. 2009). In B. bassiana, Δcrz1 mutant showed defects in growth and conidiation, suppressed growth in the presence of osmotic salts, sensitivity to stress-inducing chemicals and carbendazim and osmotic salts, decreased thermotolerance, reduced resistance to UV-B irradiation, changed cell wall composition, and longer virulence period (Li et al. 2015).
Conclusions
Ca2+ signalling regulates multiple cell functions ranging from growth, development, fertility, stress tolerance, and virulence in fungi. Phospholipases are enzymes that act on membrane phospholipids and classified into four types. In response to specific signals, PLC produces IP3 and DAG, which mediate Ca2+ release and activation of PKC, respectively. There are also six major types of Ca2+ transporters that are required for Ca2+ homoeostasis and signalling. The increase in [Ca2+]c activates various Ca2+ sensors such as CaM and NCS-1 that interact with specific downstream proteins as a response to a signal. Calcineurin, which consists of catalytic A and regulatory B subunits, is one of the major downstream Ca2+ signalling proteins required for multiple cell functions, including growth, stress tolerance, and virulence in fungi. Calcineurin is also identified as a target for antifungal drugs. Calcineurin activates the Crz 1 transcription factor to activate expressions of target genes required for growth, developments, tolerance to stress conditions, and pathogenicity. Further research on the Ca2+ signalling machinery will unravel its complex molecular network under different cellular conditions.
Acknowledgements
We are thankful to all researchers in the relevant field for their contributions that helped us to write this review article. We regret our inability to cite all the references, which is beyond the scope of this review. AR, AK, and DB were supported by Research Fellowships from the Ministry of Human Resource Development, Government of India. We thank IIT Guwahati for partial financial support.
Funding Statement
This work was supported by the Ministry of Human Resource Development [Research Fellowships to AR, AK, and DB].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Andaluz E, Coque JR, Cueva R, Larriba G.. 2001. Sequencing of a 4.3 kbp region of chromosome 2 of Candida albicans reveals the presence of homologues of SHE9 from Saccharomyces cerevisiae and of bacterial phosphatidylinositol‐phospholipase C. Yeast. 18(8):711–721. doi: 10.1002/yea.716. [DOI] [PubMed] [Google Scholar]
- Bahn Y-S, Jung K-W. 2013. Stress signaling pathways for the pathogenicity of Cryptococcus. Eukaryot Cell. 12(12):1564–1577. doi: 10.1128/EC.00218-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barman A, Gohain D, Bora U, Tamuli R. 2018. Phospholipases play multiple cellular roles including growth, stress tolerance, sexual development, and virulence in fungi. Microbiol Res. 209:55–69. doi: 10.1016/j.micres.2017.12.012 [DOI] [PubMed] [Google Scholar]
- Barman A, Tamuli R. 2015. Multiple cellular roles of Neurospora crassa plc-1, splA2, and cpe-1 in regulation of cytosolic free calcium, carotenoid accumulation, stress responses, and acquisition of thermotolerance. J Microbiol. 53(4):226–235. doi: 10.1007/s12275-015-4465-1. [DOI] [PubMed] [Google Scholar]
- Barman A, Tamuli R. 2017. The pleiotropic vegetative and sexual development phenotypes of Neurospora crassa arise from double mutants of the calcium signaling genes plc-1, splA2, and cpe-1. Curr Genet. 63(5):861–875. doi: 10.1007/s00294-017-0682-y. [DOI] [PubMed] [Google Scholar]
- Bates S, Maccallum DM, Bertram G, Munro CA, Hughes HB, Buurman ET, Brown AJP, Odds FC, Gow NAR. 2005. Candida albicans Pmr1p, a secretory pathway P-type Ca2+/Mn2+ - ATPase, is required for glycosylation and virulence. J Biol Chem. 280(24):23408–23415. doi: 10.1074/jbc.M502162200. [DOI] [PubMed] [Google Scholar]
- Bennett DE, McCreary CE, Coleman DC. 1998. Genetic characterization of a phospholipase C gene from Candida albicans: presence of homologous sequences in Candida species other than Candida albicans. Microbiol. 144(1):55–72. doi: 10.1099/00221287-144-1-55. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. 1993. Inositol trisphosphate and calcium signalling. Nature. 361(6410):315. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. 1998. Neuronal calcium signaling. Neuron. 21(1):13–26. doi: 10.1016/S0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Lipp P. 1998. Calcium-a life and death signal. Nature. 395(6703):645–648. doi: 10.1038/27094. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL. 2003. Calcium: calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 4(7):517. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- Birch M, Robson G, Law D, Denning DW. 1996. Evidence of multiple extracellular phospholipase activities of Aspergillus fumigatus. Infect Immun. 64(3):751–755. doi: 10.1128/IAI.64.3.751-755.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkovich KA, Alex LA, Yarden O, Freitag M, Turner GE, Read ND, Seiler S, Bell-Pedersen D, Paietta J, Plesofsky N, et al. 2004. Lessons from the genome sequence of Neurospora crassa: tracing the path from genomic blueprint to multicellular organism. Microbiol Mol Biol Rev. 68(1):1–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman BJ, Abreu S, Margolles-clark E, Draskovic M, Bowman EJ. 2011. Role of four calcium transport proteins, encoded by nca-1, nca-2, nca-3, and cax, in maintaining intracellular calcium levels in Neurospora crassa. Eukaryot Cell. 10(5):654–661. doi: 10.1128/EC.00239-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brini M, Calì T, Ottolini D, Carafoli E. 2013a. Intracellular calcium homeostasis and signaling. In: Banci L, editor. Met Ions Life Sci. Vol. 12. Dordrecht: Springer; p. 119–168. [DOI] [PubMed] [Google Scholar]
- Brini M, Calì T, Ottolini D, Carafoli E. 2013b. The plasma membrane calcium pump in health and disease. Febs J. 280(21):5385–5397. doi: 10.1111/febs.12193. [DOI] [PubMed] [Google Scholar]
- Burgoyne RD. 2007. Neuronal calcium sensor proteins: generating diversity in neuronal Ca2+ signalling. Nat Rev Neurosci. 8(3):182. doi: 10.1038/nrn2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli E. 2002. Calcium signaling: a tale for all seasons. Proc Natl Acad Sci. 99(3):1115–1122. doi: 10.1073/pnas.032427999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case RM, Eisner D, Gurney A, Jones O, Muallem S, Verkhratsky A. 2007. Evolution of calcium homeostasis: from birth of the first cell to an omnipresent signalling system. Cell Calcium. 42(4–5):345–350. doi: 10.1016/j.ceca.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Chae SW, Kim J-M, Yun YP, Lee WK, Kim J-S, Kim Y-H, Lee K-S, Ko YJ, Lee K-H, Rha HK. 2007. Identification and analysis of the promoter region of the human PLC-δ4 gene. Mol Biol Rep. 34(2):69–77. doi: 10.1007/s11033-006-9014-x. [DOI] [PubMed] [Google Scholar]
- Chang P-K. 2008. Aspergillus parasiticus crzA, which encodes calcineurin response zinc-finger protein, is required for aflatoxin production under calcium stress. Int J Mol Sci. 9(10):2027–2043. doi: 10.3390/ijms9102027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin D, Means AR. 2000. Calmodulin: a prototypical calcium sensor. Trends Cell Biol. 10(8):322–328. doi: 10.1016/S0962-8924(00)01800-6. [DOI] [PubMed] [Google Scholar]
- Choi J, Kim Y, Kim S, Park J, Lee Y-H. 2009b. MoCRZ1, a gene encoding a calcineurin-responsive transcription factor, regulates fungal growth and pathogenicity of Magnaporthe oryzae. Fungal Genet Biol. 46(3):243–254. doi: 10.1016/j.fgb.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Choi J-H, Kim Y-S, Lee Y-H. 2009a. Functional analysis of MCNA, a gene encoding a catalytic subunit of calcineurin, in the rice blast fungus Magnaporthe oryzae. J Microbiol Biotechnol. 19(1):11–16. [PubMed] [Google Scholar]
- Clapham DE. 2007. Calcium signaling. Cell. 131(6):1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Cornelius G, Nakashima H. 1987. Vacuoles play a decisive role in calcium homeostasis Neurospora crassa . Microbio. 133(8):2341–2347. [Google Scholar]
- Cortés JCG, Katoh-Fukui R, Moto K, Ribas JC, Ishiguro J. 2004. Schizosaccharomyces pombe Pmr1p is essential for cell wall integrity and is required for polarized cell growth and cytokinesis. Eukaryot Cell. 3(5):1124–1135. doi: 10.1128/EC.3.5.1124-1135.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer RA, Perfect BZ, Pinchai N, Park S, Perlin DS, Asfaw YG, Heitman J, Perfect JR, Steinbach WJ. 2008. Calcineurin target CrzA regulates conidial germination, hyphal growth, and pathogenesis of Aspergillus fumigatus. Eukaryot Cell. 7(7):1085–1097. doi: 10.1128/EC.00086-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz MC, Del Poeta M, Wang P, Wenger R, Zenke G, Quesniaux VFJ, Movva NR, Perfect JR, Cardenas ME, Heitman J. 2000. Immunosuppressive and nonimmunosuppressive cyclosporine analogs are toxic to the opportunistic fungal pathogen Cryptococcus neoformans via cyclophilin-dependent inhibition of calcineurin. Antimicrob Agents Chemother. 44(1):143–149. doi: 10.1128/AAC.44.1.143-149.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz MC, Goldstein AL, Blankenship JR, Del Poeta M, Davis D, Cardenas ME, Perfect JR, McCusker JH, Heitman J. 2002. Calcineurin is essential for survival during membrane stress in Candida albicans. Embo J. 21(4):546–559. doi: 10.1093/emboj/21.4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KW, Fink GR. 1994. Calcineurin-dependent growth control in Saccharomyces cerevisiae mutants lacking PMC1, a homolog of plasma membrane Ca2+ ATPases. J Cell Biol. 124(3):351–363. doi: 10.1083/jcb.124.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyert MS, Kunisawa R, Kaim D, Thorner J. 1991. Yeast has homologs (CNA1 and CNA2 gene products) of mammalian calcineurin, a calmodulin-regulated phosphoprotein phosphatase. Proc Natl Acad Sci. 88(16):7376–7380. doi: 10.1073/pnas.88.16.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TN, Urdea MS, Masiarz FR, Thorner J. 1986. Isolation of the yeast calmodulin gene: calmodulin is an essential protein. Cell. 47(3):423–431. doi: 10.1016/0092-8674(86)90599-4. [DOI] [PubMed] [Google Scholar]
- Davis TR, Zucchi PC, Kumamoto CA. 2013. Calmodulin binding to Dfi1p promotes invasiveness of Candida albicans. PLoS One. 8(10):e76239. doi: 10.1371/journal.pone.0076239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castro PA, Chiaratto J, Winkelströter LK, Bom VLP, Ramalho LNZ, Goldman MHS, Brown NA, Goldman GH. 2014. The involvement of the Mid1/Cch1/Yvc1 calcium channels in Aspergillus fumigatus virulence. PLoS One. 9(8):e103957. doi: 10.1371/journal.pone.0103957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castro PA, Colabardini AC, Manfiolli AO, Chiaratto J, Silva LP, Mattos EC, Palmisano G, Almeida F, Persinoti GF, Ries LNA. 2019. Aspergillus fumigatus calcium-responsive transcription factors regulate cell wall architecture promoting stress tolerance, virulence and caspofungin resistance. PLoS Genet. 15(12):12. doi: 10.1371/journal.pgen.1008551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean RA, Talbot NJ, Ebbole DJ, Farman ML, Mitchell TK, Orbach MJ, Thon M, Kulkarni R, Xu J-R, Pan H. 2005. The genome sequence of the rice blast fungus Magnaporthe grisea. Nature. 434(7036):980. doi: 10.1038/nature03449. [DOI] [PubMed] [Google Scholar]
- Deka R, Kumar R, Tamuli R. 2011. Neurospora crassa homologue of neuronal calcium sensor-1 has a role in growth, calcium stress tolerance, and ultraviolet survival. Genetica. 139(7):885–894. doi: 10.1007/s10709-011-9592-y. [DOI] [PubMed] [Google Scholar]
- Deka R, Tamuli R. 2013. Neurospora crassa ncs-1, mid-1 and nca-2 double-mutant phenotypes suggest diverse interaction among three Ca2+-regulating gene products. J Genet. 92(3):559–563. doi: 10.1007/s12041-013-0270-y. [DOI] [PubMed] [Google Scholar]
- Dinamarco TM, Freitas FZ, Almeida RS, Brown NA, Dos Reis TF, Ramalho LNZ, Savoldi M, Goldman MHS, Bertolini MC, Goldman GH. 2012. Functional characterization of an Aspergillus fumigatus calcium transporter (PmcA) that is essential for fungal infection. PLoS One. 7(5):e37591. doi: 10.1371/journal.pone.0037591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SR, Dietrich FS, Fisk DG, Binkley G, Balakrishnan R, Costanzo MC, Dwight SS, Hitz BC, Karra K, Nash RS. 2014. The reference genome sequence of Saccharomyces cerevisiae: then and now. G3 genes, genomes. Genet. 4(3):389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Ortiz-Urquiza A, Kudia RA, Keyhani NO. 2012. A fungal homologue of neuronal calcium sensor-1, Bbcsa1, regulates extracellular acidification and contributes to virulence in the entomopathogenic fungus Beauveria bassiana. Microbiol. 158(7):1843–1851. doi: 10.1099/mic.0.058867-0. [DOI] [PubMed] [Google Scholar]
- Fankhauser H, Schweingruber AM, Edenharter E, Schweingruber ME. 1995. Growth of a mutant defective in a putative phosphoinositide-specific phospholipase C of Schizosaccharomyces pombe is restored by low concentrations of phosphate and inositol. Curr Genet. 28(2):199–203. doi: 10.1007/BF00315789. [DOI] [PubMed] [Google Scholar]
- Farcasanu IC, Hirata D, Tsuchiya E, Nishiyama F, Miyakawa T. 1995. Protein phosphatase 2B of Saccharomyces cerevisiae is required for tolerance to manganese, in blocking the entry of ions into the cells. Eur J Biochem. 232(3):712–717. doi: 10.1111/j.1432-1033.1995.tb20865.x. [DOI] [PubMed] [Google Scholar]
- Flick JS, Thorner J. 1993. Genetic and biochemical characterization of a phosphatidylinositol-specific phospholipase C in Saccharomyces cerevisiae. Mol Cell Biol. 13(9):5861–5876. doi: 10.1128/MCB.13.9.5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortwendel JR, Juvvadi PR, Perfect BZ, Rogg LE, Perfect JR, Steinbach WJ. 2010. Transcriptional regulation of chitin synthases by calcineurin controls paradoxical growth of Aspergillus fumigatus in response to caspofungin. Antimicrob Agents Chemother. 54(4):1555–1563. doi: 10.1128/AAC.00854-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox DS, Cruz MC, Sia RAL, Ke H, Cox GM, Cardenas ME, Heitman J. 2001. Calcineurin regulatory subunit is essential for virulence and mediates interactions with FKBP12–FK506 in Cryptococcus neoformans. Mol Microbiol. 39(4):835–849. doi: 10.1046/j.1365-2958.2001.02295.x. [DOI] [PubMed] [Google Scholar]
- Galagan JE, Calvo SE, Borkovich KA, Selker EU, Read ND, Jaffe D, FitzHugh W, Ma L-J, Smirnov S, Purcell S, et al. 2003. The genome sequence of the filamentous fungus Neurospora crassa. Nature. 422(6934):859–868. doi: 10.1038/nature01554 [DOI] [PubMed] [Google Scholar]
- Galimov E. 2009. Concept of sustained ordering and an ATP-related mechanism of life’s origin. Int J Mol Sci. 10(5):2019–2030. doi: 10.3390/ijms10052019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavric O, Dos Santos DB, Griffiths A. 2007. Mutation and divergence of the phospholipase C gene in Neurospora crassa. Fungal Genet Biol. 44(4):242–249. doi: 10.1016/j.fgb.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Gohain D, Tamuli R. 2019. Calcineurin responsive zinc‐finger‐1 binds to a unique promoter sequence to upregulate neuronal calcium sensor‐1, whose interaction with MID‐1 increases tolerance to calcium stress in Neurospora crassa. Mol Microbiol. 111(6):1510–1528. doi: 10.1111/mmi.14234. [DOI] [PubMed] [Google Scholar]
- Halling DB, Liebeskind BJ, Hall AW, Aldrich RW. 2016. Conserved properties of individual Ca2+-binding sites in calmodulin. Proc Natl Acad Sci. 113(9):E1216–E1225. doi: 10.1073/pnas.1600385113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamasaki-Katagiri N, Molchanova T, Takeda K, Ames JB. 2004. Fission yeast homolog of neuronal calcium sensor-1 (Ncs1p) regulates sporulation and confers calcium tolerance. J Biol Chem. 279(13):12744–12754. doi: 10.1074/jbc.M311895200. [DOI] [PubMed] [Google Scholar]
- Hamilton SL. 2005. Ryanodine receptors. Cell Calcium. 38(3–4):253–260. doi: 10.1016/j.ceca.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Hendricks KB, Wang BQ, Schnieders EA, Thorner J. 1999. Yeast homologue of neuronal frequenin is a regulator of phosphatidylinositol-4-OH kinase. Nat Cell Biol. 1(4):234–241. doi: 10.1038/12058. [DOI] [PubMed] [Google Scholar]
- Hirayama S, Sugiura R, Lu Y, Maeda T, Kawagishi K, Yokoyama M, Tohda H, Giga-Hama Y, Shuntoh H, Kuno T. 2003. Zinc finger protein prz1 regulates Ca2+ but not Cl− homeostasis in fission yeast. Identification of distinct branches of calcineurin signaling pathway in fission yeast. J Biol Chem. 278(20):18078–18084. doi: 10.1074/jbc.M212900200. [DOI] [PubMed] [Google Scholar]
- Hong Y, Zhao J, Guo L, Kim S-C, Deng X, Wang G, Zhang G, Li M, Wang X. 2016. Plant phospholipases D and C and their diverse functions in stress responses. Prog Lipid Res. 62:55–74. doi: 10.1016/j.plipres.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Itadani A, Nakamura T, Hirata A, Shimoda C. 2010. Schizosaccharomyces pombe calmodulin, Cam1, plays a crucial role in sporulation by recruiting and stabilizing the spindle pole body components responsible for assembly of the forespore membrane. Eukaryot Cell. 9(12):1925–1935. doi: 10.1128/EC.00022-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal JK. 2001. Calcium—how and why? J Biosci. 26(3):357–363. doi: 10.1007/BF02703745. [DOI] [PubMed] [Google Scholar]
- Jia W, Zhang H, Li C, Li G, Liu X, Wei J. 2016. The calcineruin inhibitor cyclosporine a synergistically enhances the susceptibility of Candida albicans biofilms to fluconazole by multiple mechanisms. BMC Microbiol. 16(1):113. doi: 10.1186/s12866-016-0728-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T, Federspiel NA, Chibana H, Dungan J, Kalman S, Magee BB, Newport G, Thorstenson YR, Agabian N, Magee PT. 2004. The diploid genome sequence of Candida albicans. Proc Natl Acad Sci. 101(19):7329–7334. doi: 10.1073/pnas.0401648101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juvvadi PR, Gehrke C, Fortwendel JR, Lamoth F, Soderblom EJ, Cook EC, Hast MA, Asfaw YG, Moseley MA, Creamer TP. 2013. Phosphorylation of calcineurin at a novel serine-proline rich region orchestrates hyphal growth and virulence in Aspergillus fumigatus. PLoS Pathog. 9(8):e1003564. doi: 10.1371/journal.ppat.1003564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juvvadi PR, Lamoth F, Steinbach WJ. 2014. Calcineurin as a multifunctional regulator: unraveling novel functions in fungal stress responses, hyphal growth, drug resistance, and pathogenesis. Fungal Biol Rev. 28(2–3):56–69. doi: 10.1016/j.fbr.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juvvadi PR, Muñoz A, Lamoth F, Soderblom EJ, Moseley MA, Read ND, Steinbach WJ. 2015. Calcium-mediated induction of paradoxical growth following caspofungin treatment is associated with calcineurin activation and phosphorylation in Aspergillus fumigatus. Antimicrob Agents Chemother. 59(8):4946–4955. doi: 10.1128/AAC.00263-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellermayer R, Aiello DP, Miseta A, Bedwell DM. 2003. Extracellular Ca2+ sensing contributes to excess Ca2+ accumulation and vacuolar fragmentation in a pmr1∆ mutant of S. cerevisiae. J Cell Sci. 15;116(8):1637–1646. doi: 10.1242/jcs.00372. [DOI] [PubMed] [Google Scholar]
- Klee CB, Crouch TH, Krinks MH. 1979. Calcineurin: A calcium- and calmodulin-binding protein of the nervous system. Proc Natl Acad Sci U S A. 76(12):6270–6273. doi: 10.1073/pnas.76.12.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee CB, Krinks MH. 1978. Purification of cyclic 3ʹ, 5ʹ-nucleotide phosphodiesterase inhibitory protein by affinity chromatography on activator protein coupled to sepharose. Biochemistry. 17(1):120–126. doi: 10.1021/bi00594a017. [DOI] [PubMed] [Google Scholar]
- Klee CB, Ren H, Wang X. 1998. Regulation of the calmodulin-stimulated protein phosphatase, calcineurin. J Biol Chem. 273(22):13367–13370. doi: 10.1074/jbc.273.22.13367. [DOI] [PubMed] [Google Scholar]
- Kmetzsch L, Staats CC, Rodrigues ML, Schrank A, Vainstein MH. 2011. Calcium signaling components in the human pathogen Cryptococcus neoformans: Cryptococcus neoformans. Commun Integr Biol. 4(2):186–187. doi: 10.4161/cib.4.2.14271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knechtle P, Goyard S, Brachat S, Ibrahim-Granet O, D’Enfert C. 2005. Phosphatidylinositol-dependent phospholipases C Plc2 and Plc3 of Candida albicans are dispensable for morphogenesis and host–pathogen interaction. Res Microbiol. 156(7):822–829. doi: 10.1016/j.resmic.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Köhler GA, Brenot A, Haas-Stapleton E, Agabian N, Deva R, Nigam S. 2006. Phospholipase A2 and phospholipase B activities in fungi. Biochim Biophys Acta (BBA)-Mol Cell Biol Lipids. 1761(11):1391–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothe GO, Free SJ. 1998. Calcineurin subunit B is required for normal vegetative growth in Neurospora crassa. Fungal Genet Biol. 23(3):248–258. doi: 10.1006/fgbi.1998.1037. [DOI] [PubMed] [Google Scholar]
- Kraus PR, Nichols CB, Heitman J. 2005. Calcium- and calcineurin-independent roles for calmodulin in Cryptococcus neoformans morphogenesis and high-temperature growth. Eukaryot Cell. 4(6):1079–1087. doi: 10.1128/EC.4.6.1079-1087.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Roy A, Deshmukh MV, Tamuli R. 2019. Dominant mutants of the calcineurin catalytic subunit (CNA-1) showed developmental defects, increased sensitivity to stress conditions, and CNA-1 interacts with CaM and CRZ-1 in Neurospora crassa. Arch Microbiol. 202(4):921–934. doi: 10.1007/s00203-019-01768-z. [DOI] [PubMed] [Google Scholar]
- Kumar R, Tamuli R. 2014. Calcium/calmodulin-dependent kinases are involved in growth, thermotolerance, oxidative stress survival, and fertility in Neurospora crassa. Arch Microbiol. 196(4):295–305. doi: 10.1007/s00203-014-0966-2. [DOI] [PubMed] [Google Scholar]
- Kunze D, Melzer I, Bennett D, Sanglard D, MacCallum D, Nörskau J, Coleman DC, Odds FC, Schäfer W, Hube B. 2005. Functional analysis of the phospholipase C gene CaPLC1 and two unusual phospholipase C genes, CaPLC2 and CaPLC3, of Candida albicans. Microbiol. 151(10):3381–3394. doi: 10.1099/mic.0.28353-0. [DOI] [PubMed] [Google Scholar]
- Lamoth F, Juvvadi PR, Gehrke C, Steinbach WJ. 2013. vitro activity of calcineurin and heat shock protein 90 inhibitors against Aspergillus fumigatus azole-and echinocandin-resistant strains. Antimicrob Agents Chemother. 57(2):1035–1039. doi: 10.1128/AAC.01857-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange M, Peiter E. 2020. Calcium transport proteins in fungi: the phylogenetic diversity of their relevance for growth, virulence, and stress resistance. Front Microbiol. 10:3100. doi: 10.3389/fmicb.2019.03100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxmi V, Tamuli R. 2015. The Neurospora crassa cmd, trm-9, and nca-2 genes play a role in growth, development, and survival in stress conditions. Gen Appl Biol. 6(7):1–8. doi: 10.5376/gab.2015.06.0007. [DOI] [Google Scholar]
- Laxmi V, Tamuli R. 2017. The calmodulin gene in Neurospora crassa is required for normal vegetative growth, ultraviolet survival, and sexual development. Arch Microbiol. 199(4):531–542. doi: 10.1007/s00203-016-1319-0. [DOI] [PubMed] [Google Scholar]
- Lee K-T, So Y-S, Yang D-H, Jung K-W, Choi J, Lee D-G, Kwon H, Jang J, Wang LL, Cha S. 2016. Systematic functional analysis of kinases in the fungal pathogen Cryptococcus neoformans. Nat Commun. 7(1):12766. doi: 10.1038/ncomms12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Lee YH. 1998. Calcium/calmodulin-dependent signaling for appressorium formation in the plant pathogenic fungus Magnaporthe grisea. Mol Cells. 8(6):698–704. [PubMed] [Google Scholar]
- Lev S, Desmarini D, Chayakulkeeree M, Sorrell TC, Djordjevic JT. 2012. The Crz1/Sp1 transcription factor of Cryptococcus neoformans is activated by calcineurin and regulates cell wall integrity. PLoS One. 7(12):e51403. doi: 10.1371/journal.pone.0051403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev S, Desmarini D, Li C, Chayakulkeeree M, Traven A, Sorrell TC, Djordjevic JT. 2013. Phospholipase C of Cryptococcus neoformans regulates homeostasis and virulence by providing inositol trisphosphate as a substrate for Arg1 kinase. Infect Immun. 81(4):1245–1255. doi: 10.1128/IAI.01421-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew RR, Giblon RE, Lorenti MSH. 2015. The phenotype of a phospholipase C (plc-1) mutant in a filamentous fungus, Neurospora crassa. Fungal Genet Biol. 82:158–167. doi: 10.1016/j.fgb.2015.07.007. [DOI] [PubMed] [Google Scholar]
- Lewit-Bentley A, Réty S. 2000. EF-hand calcium-binding proteins. Curr Opin Struct Biol. 10(6):637–643. doi: 10.1016/S0959-440X(00)00142-1. [DOI] [PubMed] [Google Scholar]
- Li F, Wang Z-L, Zhang L-B, Ying S-H, Feng M-G. 2015. The role of three calcineurin subunits and a related transcription factor (Crz1) in conidiation, multistress tolerance and virulence in Beauveria bassiana. Appl Microbiol Biotechnol. 99(2):827–840. doi: 10.1007/s00253-014-6124-6. [DOI] [PubMed] [Google Scholar]
- Li H, Rao A, Hogan PG. 2011. Interaction of calcineurin with substrates and targeting proteins. Trends Cell Biol. 21(2):91–103. doi: 10.1016/j.tcb.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang Y, Lu L. 2019. Calcium signaling pathway is involved in non-CYP51 azole resistance in Aspergillus fumigatus. Med Mycol. 57(Supplement_2):S233–S238. doi: 10.1093/mmy/myy075. [DOI] [PubMed] [Google Scholar]
- Lim S-Y, Son Y-E, Lee D-H, Eom T-J, Kim M-J, Park H-S. 2019. Function of crzA in fungal development and aflatoxin production in Aspergillus flavus. Toxins (Basel). 11(10):567. doi: 10.3390/toxins11100567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z-M, Kolattukudy PE. 1999. Early expression of the calmodulin gene, which precedes appressorium formation in Magnaporthe grisea, is inhibited by self-inhibitors and requires surface attachment. J Bacteriol. 181(11):3571–3577. doi: 10.1128/JB.181.11.3571-3577.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna-Tapia A, DeJarnette C, Sansevere E, Reitler P, Butts A, Hevener KE, Palmer GE. 2019. The vacuolar Ca2+ ATPase Pump Pmc1p Is required for Candida albicans pathogenesis. mSphere. 4(1):e00715–18. doi: 10.1128/mSphere.00715-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L-J, Van Der Does HC, Borkovich KA, Coleman JJ, Daboussi M-J, Di Pietro A, Dufresne M, Freitag M, Grabherr M, Henrissat B. 2010. Comparative genomics reveals mobile pathogenicity chromosomes in fusarium. Nature. 464(7287):367. doi: 10.1038/nature08850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DC, Kim H, Mackin NA, Maldonado-Báez L, Evangelista CC, Beaudry VG, Dudgeon DD, Naiman DQ, Erdman SE, Cunningham KW. 2011. New regulators of a high affinity Ca2+ influx system revealed through a genome-wide screen in yeast. J Biol Chem. 286(12):10744–10754. doi: 10.1074/jbc.M110.177451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheos DP, Kingsbury TJ, Ahsan US, Cunningham KW. 1997. Tcn1p/Crz1p, a calcineurin-dependent transcription factor that differentially regulates gene expression in Saccharomyces cerevisiae. Genes Dev. 11(24):3445–3458. doi: 10.1101/gad.11.24.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikoshiba K, Hattori M. 2000. IP3 receptor-operated calcium entry. Sci STKE. 2000(51):–pe1. doi: 10.1126/stke.2000.51.pe1. [DOI] [PubMed] [Google Scholar]
- Moser MJ, Flory MR, Davis TN. 1997. Calmodulin localizes to the spindle pole body of Schizosaccharomyces pombe and performs an essential function in chromosome segregation. J Cell Sci. 110(15):1805–1812. [DOI] [PubMed] [Google Scholar]
- Mota Júnior AO, Malavazi I, Soriani FM, Heinekamp T, Jacobsen I, Brakhage AA, Savoldi M, Goldman MHS, da Silva Ferreira ME, Goldman GH. 2008. Molecular characterization of the Aspergillus fumigatus NCS-1 homologue, NcsA. Mol Genet Genomics. 280(6):483‐495. doi: 10.1007/s00438-008-0381-y. [DOI] [PubMed] [Google Scholar]
- Nguyen QB, Kadotani N, Kasahara S, Tosa Y, Mayama S, Nakayashiki H. 2008. Systematic functional analysis of calcium‐signalling proteins in the genome of the rice‐blast fungus, Magnaporthe oryzae, using a high‐throughput RNA‐silencing system. Mol Microbiol. 68(6):1348–1365. doi: 10.1111/j.1365-2958.2008.06242.x. [DOI] [PubMed] [Google Scholar]
- Nierman WC, Pain A, Anderson MJ, Wortman JR, Kim HS, Arroyo J, Berriman M, Abe K, Archer DB, Bermejo C. 2005. Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus. Nature. 438(7071):1151. doi: 10.1038/nature04332. [DOI] [PubMed] [Google Scholar]
- Odom A, Muir S, Lim E, Toffaletti DL, Perfect J, Heitman J. 1997. Calcineurin is required for virulence of Cryptococcus neoformans. Embo J. 16(10):2576–2589. doi: 10.1093/emboj/16.10.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okorokova-Facanha AL, Okorokov LA, Ekwall K. 2003. An inventory of the P-type ATPases in the fission yeast Schizosaccharomyces pombe. Curr Genet. 43(4):273–280. doi: 10.1007/s00294-003-0395-2. [DOI] [PubMed] [Google Scholar]
- Otero JM, Vongsangnak W, Asadollahi MA, Olivares-Hernandes R, Maury J, Farinelli L, Barlocher L, Østerås M, Schalk M, Clark A. 2010. Whole genome sequencing of Saccharomyces cerevisiae: from genotype to phenotype for improved metabolic engineering applications. BMC Genomics. 11(1):723. doi: 10.1186/1471-2164-11-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranjape V, Roy BG, Datta A. 1990. Involvement of calcium, calmodulin and protein phosphorylation in morphogenesis of Candida albicans. Microbiol. 136(11):2149–2154. [DOI] [PubMed] [Google Scholar]
- Pittman JK. 2011. Vacuolar Ca2+ uptake. Cell Calcium. 50(2):139–146. doi: 10.1016/j.ceca.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Plattner H, Verkhratsky A. 2013. Ca2+ signalling early in evolution–all but primitive. J Cell Sci. 126(10):2141–2150. doi: 10.1242/jcs.127449. [DOI] [PubMed] [Google Scholar]
- Pongs O, Lindemeier J, Zhu XR, Theil T, Engelkamp D, Krah-Jentgens I, Lambrecht H, Koch KW, Schwemer J, Rivosecchi R, et al. 1993. Frequenin–a novel calcium-binding protein that modulates synaptic efficacy in the drosophila nervous system. Neuron. 11(1):15–28. doi: 10.1016/0896-6273(93)90267-u. [DOI] [PubMed] [Google Scholar]
- Ponnamperuma C, Sagan C, Mariner R. 1963. Synthesis of adenosine triphosphate under possible primitive earth conditions. Nature. 199(4890):222–226. doi: 10.1038/199222a0. [DOI] [PubMed] [Google Scholar]
- Prokisch H, Yarden O, Dieminger M, Tropschug M, Barthelmess IB. 1997. Impairment of calcineurin function in Neurospora crassa reveals its essential role in hyphal growth, morphology and maintenance of the apical Ca2+ gradient. Mol Gen Genet MGG. 256(2):104–114. doi: 10.1007/s004380050551. [DOI] [PubMed] [Google Scholar]
- Rho H, Jeon J, Lee Y. 2009. Phospholipase C‐mediated calcium signalling is required for fungal development and pathogenicity in Magnaporthe oryzae. Mol Plant Pathol. 10(3):337–346. doi: 10.1111/j.1364-3703.2009.00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond GS, Smith TK. 2011. Phospholipases A1. Int J Mol Sci. 12(1):588–612. doi: 10.3390/ijms12010588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries LNA, Rocha MC, de Castro PA, Silva-Rocha R, Silva RN, Freitas FZ, de Assis LJ, Bertolini MC, Malavazi I, Goldman GH. 2017. The Aspergillus fumigatus CrzA transcription factor activates chitin synthase gene expression during the caspofungin paradoxical effect. MBio. 8(3):e00705–17. doi: 10.1128/mBio.00705-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy BG, Datta A. 1987. A calmodulin inhibitor blocks morphogenesis in Candida albicans. FEMS Microbiol Lett. 41(3):327–329. doi: 10.1111/j.1574-6968.1987.tb02221.x. [DOI] [Google Scholar]
- Rusnak F, Mertz P. 2000. Calcineurin: form and function. Physiol Rev. 80(4):1483–1521. doi: 10.1152/physrev.2000.80.4.1483. [DOI] [PubMed] [Google Scholar]
- Saitoh K, Arie T, Teraoka T, Yamaguchi I, Kamakura T. 2003. Targeted gene disruption of the neuronal calcium sensor 1 homologue in rice blast fungus, Magnaporthe grisea. Biosci Biotechnol Biochem. 67(3):651–653. doi: 10.1271/bbb.67.651. [DOI] [PubMed] [Google Scholar]
- Sánchez-Gracia A, Romero-Pozuelo J, Ferrús A. 2010. Two frequenins in Drosophila: unveiling the evolutionary history of an unusual Neuronal Calcium Sensor (NCS) duplication. BMC Evol Biol. 10(1):54. doi: 10.1186/1471-2148-10-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanglard D, Ischer F, Marchetti O, Entenza J, Bille J. 2003. Calcineurin A of Candida albicans: involvement in antifungal tolerance, cell morphogenesis and virulence. Mol Microbiol. 48(4):959–976. doi: 10.1046/j.1365-2958.2003.03495.x. [DOI] [PubMed] [Google Scholar]
- Santos M, de Larrinoa IF. 2005. Functional characterization of the Candida albicans CRZ1 gene encoding a calcineurin-regulated transcription factor. Curr Genet. 48(2):88–100. doi: 10.1007/s00294-005-0003-8. [DOI] [PubMed] [Google Scholar]
- Schumacher J, Viaud M, Simon A, Tudzynski B. 2008. The Gα subunit BCG1, the phospholipase C (BcPLC1) and the calcineurin phosphatase co‐ordinately regulate gene expression in the grey mould fungus Botrytis cinerea. Mol Microbiol. 67(5):1027–1050. doi: 10.1111/j.1365-2958.2008.06105.x. [DOI] [PubMed] [Google Scholar]
- Selker EU, Cambareri EB, Jensen BC, Haack KR. 1987. Rearrangement of duplicated DNA in specialized cells of Neurospora. Cell. 51(5):741–752. doi: 10.1016/0092-8674(87)90097-3. [DOI] [PubMed] [Google Scholar]
- Shemarova IV, Nesterov VP. 2005. Evolution of mechanisms of Ca2+-signaling: role of calcium ions in signal transduction in prokaryotes. J Evol Biochem Physiol. 41(1):12–19. doi: 10.1007/s10893-005-0029-z. [DOI] [PubMed] [Google Scholar]
- Singh SD, Robbins N, Zaas AK, Schell WA, Perfect JR, Cowen LE. 2009. Hsp90 governs echinocandin resistance in the pathogenic yeast Candida albicans via calcineurin. PLoS Pathog. 5(7):e1000532. doi: 10.1371/journal.ppat.1000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Liu X, Zhai P, Huang J, Lu L. 2016. A putative mitochondrial calcium uniporter in A. fumigatus contributes to mitochondrial Ca2+ homeostasis and stress responses. Fungal Genet Biol. 94:15–22. doi: 10.1016/j.fgb.2016.07.001. [DOI] [PubMed] [Google Scholar]
- Soriani FM, Malavazi I, da Silva Ferreira ME, Savoldi M, Von Zeska Kress MR, de Souza Goldman MH, Loss O, Bignell E, Goldman GH. 2008. Functional characterization of the Aspergillus fumigatus CRZ1 homologue, CrzA. Mol Microbiol. 67(6):1274–1291. doi: 10.1111/j.1365-2958.2008.06122.x. [DOI] [PubMed] [Google Scholar]
- Stathopoulos AM, Cyert MS. 1997. Calcineurin acts through the CRZ1/TCN1-encoded transcription factor to regulate gene expression in yeast. Genes Dev. 11(24):3432–3444. doi: 10.1101/gad.11.24.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach WJ, Lamoth F, Juvvadi PR. 2015. Potential microbiological effects of higher dosing of echinocandins. Clin Infect Dis. 61(suppl_6):S669–S677. doi: 10.1093/cid/civ725. [DOI] [PubMed] [Google Scholar]
- Su L, Ji D, Tao X, Yu L, Wu J, Xia Y. 2017. Recombinant expression, characterization, and application of a phospholipase B from Fusarium oxysporum. J Biotechnol. 242:92–100. doi: 10.1016/j.jbiotec.2016.12.009. [DOI] [PubMed] [Google Scholar]
- Sutton RB, Davletov BA, Berghuis AM, Sudhof TC, Sprang SR. 1995. Structure of the first C2 domain of synaptotagmin I: a novel Ca2+/phospholipid-binding fold. Cell. 80(6):929–938. doi: 10.1016/0092-8674(95)90296-1. [DOI] [PubMed] [Google Scholar]
- Sze H, Liang F, Hwang I, Curran AC, Harper JF. 2000. Diversity and regulation of plant Ca2+ pumps: insights from expression in yeast. Annu Rev Plant Biol. 51(1):433–462. doi: 10.1146/annurev.arplant.51.1.433. [DOI] [PubMed] [Google Scholar]
- Takeda T, Yamamoto M. 1987. Analysis and in vivo disruption of the gene coding for calmodulin in Schizosaccharomyces pombe. Proc Natl Acad Sci. 84(11):3580–3584. doi: 10.1073/pnas.84.11.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamuli R, Kumar R, Deka R. 2011. Cellular roles of neuronal calcium sensor‐1 and calcium/calmodulin‐dependent kinases in fungi. J Basic Microbiol. 51(2):120–128. doi: 10.1002/jobm.201000184. [DOI] [PubMed] [Google Scholar]
- Tamuli R, Kumar R, Srivastava DA, Deka R. 2013. Calcium Signalling. In: Kasbekar DP, McCluskey K, editors. Neurospora Geno Mol Biol. First ed. Norfolk: Caister Academic Press; p. 209–226. [Google Scholar]
- Thewes S. 2014. Calcineurin-Crz1 signaling in lower eukaryotes. Eukaryot Cell. 13(6):694–705. doi: 10.1128/EC.00038-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisi R, Rigamonti M, Groppi S, Belotti F. 2016. Calcium homeostasis and signaling in fungi and their relevance for pathogenicity of yeasts and filamentous fungi. AIMS Mol Sci. 3(4):505–549. doi: 10.3934/molsci.2016.4.505. [DOI] [Google Scholar]
- Toda T, Shimanuki M, Yanagida M. 1993. Two novel protein kinase C‐related genes of fission yeast are essential for cell viability and implicated in cell shape control. Embo J. 12(5):1987–1995. doi: 10.1002/j.1460-2075.1993.tb05848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ton V-K, Rao R. 2004. Functional expression of heterologous proteins in yeast: insights into Ca2+ signaling and Ca2+-transporting ATPases. Am J Physiol Physiol. 287(3):C580–C589. doi: 10.1152/ajpcell.00135.2004. [DOI] [PubMed] [Google Scholar]
- Tsai H-C, Chung K-R. 2014. Calcineurin phosphatase and phospholipase C are required for developmental and pathological functions in the citrus fungal pathogen Alternaria alternata. Microbiol. 160(7):1453–1465. doi: 10.1099/mic.0.077818-0. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Parpura V. 2014. Calcium signalling and calcium channels: evolution and general principles. Eur J Pharmacol. 739:1–3. doi: 10.1016/j.ejphar.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JH, Desai R. 1976. A brain protein and its effect on the Ca2+-and protein modulator-activated cyclic nucleotide phosphodiesterase. Biochem Biophys Res Commun. 72(3):926–932. doi: 10.1016/S0006-291X(76)80220-3. [DOI] [PubMed] [Google Scholar]
- Williams RJP. 2006. The evolution of calcium biochemistry. Biochim Biophys Acta (BBA)-Mol Cell Res. 1763(11):1139–1146. doi: 10.1016/j.bbamcr.2006.08.042. [DOI] [PubMed] [Google Scholar]
- Wood V, Gwilliam R, Rajandream M-A, Lyne M, Lyne R, Stewart A, Sgouros J, Peat N, Hayles J, Baker S. 2002. The genome sequence of Schizosaccharomyces pombe. Nature. 415(6874):871. doi: 10.1038/nature724. [DOI] [PubMed] [Google Scholar]
- Xiao G, Ying S-H, Zheng P, Wang Z-L, Zhang S, Xie X-Q, Shang Y, Leger RJS, Zhao G-P, Wang C. 2012. Genomic perspectives on the evolution of fungal entomopathogenicity in Beauveria bassiana. Sci Rep. 2(1):483. doi: 10.1038/srep00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Takeuchi H, Kanematsu T, Allen V, Yagisawa H, Kikkawa U, Watanabe Y, Nakasima A, Katan M, Hirata M. 1999. Involvement of EF hand motifs in the Ca2+‐dependent binding of the pleckstrin homology domain to phosphoinositides. Eur J Biochem. 265(1):481–490. doi: 10.1046/j.1432-1327.1999.00786.x. [DOI] [PubMed] [Google Scholar]
- York JD, Odom AR, Murphy R, Ives EB, Wente SR. 1999. A phospholipase C-dependent inositol polyphosphate kinase pathway required for efficient messenger RNA export. Sci. 285(5424):96–100. doi: 10.1126/science.285.5424.96. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Toda T, Yanagida M. 1994. A calcineurin-like gene ppb1+ in fission yeast: mutant defects in cytokinesis, cell polarity, mating and spindle pole body positioning. J Cell Sci. 107(7):1725–1735. [DOI] [PubMed] [Google Scholar]
- Zelter A, Bencina M, Bowman BJ, Yarden O, Read ND. 2004. A comparative genomic analysis of the calcium signaling machinery in Neurospora crassa, Magnaporthe grisea, and Saccharomyces cerevisiae. Fungal Genet Biol. 41(9):827–841. doi: 10.1016/j.fgb.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Zeng W, Mak D-OD, Li Q, Shin DM, Foskett JK, Muallem S. 2003. A new mode of Ca2+ signaling by G protein-coupled receptors: gating of IP3 receptor Ca2+ release channels by Gβγ. Curr Biol. 13(10):872–876. doi: 10.1016/S0960-9822(03)00330-0. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhao Q, Liu K, Zhang Z, Wang Y, Zheng X. 2009. MgCRZ1, a transcription factor of Magnaporthe grisea, controls growth, development and is involved in full virulence. FEMS Microbiol Lett. 293(2):160–169. doi: 10.1111/j.1574-6968.2009.01524.x. [DOI] [PubMed] [Google Scholar]


