Abstract
Purpose of Review:
Robust evidence is emerging regarding the contribution of sex-specific risk factors to a woman’s unique risk of atherosclerotic cardiovascular disease (ASCVD). This review summarizes the available literature regarding the association of sex-specific risk factors and ASCVD in women.
Recent Findings:
The American College of Cardiology and American Heart Association Guidelines recommend estimation of 10-year risk of a first ASCVD event using the 2013 Pooled-Cohort Equations. This can be further personalized by identifying sex-specific risk factors present in a woman’s history. There are multiple vulnerable periods across a woman’s life course that are associated with increased risk of ASCVD. Risk factors across the reproductive life course that have been shown to correlate with higher risk for future ASCVD include early menarche, adverse pregnancy outcomes (such as pre-eclampsia or pre-term birth), early natural or surgical menopause. In addition, certain conditions that are more common among women, including autoimmune diseases, history of chest irradiation and certain chemotherapies, among others, also need to be considered. Finally, risk assessment can be refined with subclinical disease imaging (coronary calcium score) if there remains uncertainty about clinical management with lipid lowering therapies for primary prevention after inclusion of these risk enhancers.
Summary:
Risk assessment for ASCVD in women requires a personalized approach that incorporates sex-specific risk factors to guide primary prevention measures, such as lipid lowering therapies. Coronary calcium score imaging may also help further refine risk assessment, but no clinical trials conducted to date have addressed this question.
Keywords: sex-specific risk factors, risk prediction, primary prevention, atherosclerotic cardiovascular disease, adverse pregnancy outcomes
INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of death in men and women in the United States (US) and worldwide.[1] Though CVD is often thought to be a disease predominantly of males, it is nearly equally as common in women in the US and worldwide. In the year 2017, heart disease accounted for 24.2% of deaths in males in the US, and 21.8% of deaths in U.S. females.[1] Furthermore, the World Health Organization reports 9.4 million deaths worldwide from ischemic heart disease in 2016: 4.9 million of these were in men, and 4.8 million were in women. The worldwide rate of death from stroke was also equal among sexes – with 2.9 million deaths in both men and women.[2] Though a large portion of the atherosclerotic CVD (ASCVD) burden can be attributed to traditional risk factors such as hyperlipidemia, hypertension, diabetes, and obesity, there is emerging evidence of sex-specific risk factors that also contribute to a woman’s unique risk of developing ASCVD. In this article, the data on the role of these sex-specific risk factors, and how to incorporate them into personalized ASCVD risk assessment, will be reviewed, and strategies to optimize prevention for women will be discussed.
SEX-SPECIFIC RISK PREDICTION OF ATHEROSCLEROSIS
The American College of Cardiology (ACC) and American Heart Association (AHA) recommend prediction of 10-year risk of a first hard ASCVD event using the 2013 Pooled-Cohort Equations, which were derived from logitudinal data in non-Hispanic Black and non-Hispanic white men and women 40-79 years of age.[3] These equations have been validated broadly and recommendations for their use in the general population and specific subgroups have been updated recently.[4**-6]
Numerous studies have demonstrated higher rates of ASCVD among women with a history of certain sex-specific risk factors (such as adverse pregnancy outcomes and early onset menopause). However, parity and hypertensive disorders of pregnancy including pre-eclampsia have not demonstrated additional clinical utility for risk prediction, as measured by net reclassification index, beyond currently existing risk prediction tools. Data are limited in that available studies, such as one conducted in the Nurses’ Health Study, are limited by use of self-report of a history of hypertensive disorder of pregnancy.[7] There is a need for prospective, well-adjudicated data to examine whether sex-specific risk factors may enhance the personalization of 10-year and lifetime risk ASCVD risk prediction models.
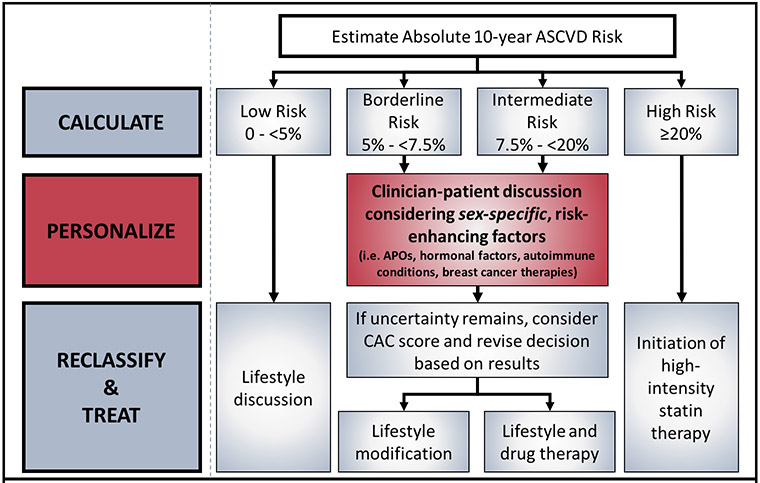
We propose that the clinical workflow for assessment of ASCVD risk for primary prevention in women should begin with quantitative risk calculation, followed by personalization based on sex-specific risk enhancers. Finally, in cases where uncertainty remains, the use of subclinical disease imaging such as a coronary artery calcium (CAC) score should be considered.[4, 5] (Figure 1)
Figure 1.
Personalization of ASCVD Risk Prediction to include sex-specific risk enhancing factors (as suggested in 2018 Prevention Guidelines)
Race/Ethnicity
An important advance in the 2013 ACC/AHA Cholesterol guidelines was the recognition with the Pooled Cohort Equations (PCE) of greater 10-year ASCVD risk for black women than for their white counterparts at the same levels of risk factors.[8] For example, a 55-year-old non-smoking, non-diabetic, but hypertensive woman on anti-hypertensive therapy with systolic BP of 135 mm Hg, total cholesterol of 220 mg/dL and an HDL-C of 45 mg/dL has a 10 year risk of 4% if she is white and a risk of 7.7% if she is black. Distribution of estimated 10-year risk of a first hard ASCVD event in the CVD-free, nonpregnant U.S. population, 40 to 79 years of age, indicated that 67.5% of white women had a low predicted 10-year ASCVD risk of less than 5%, whereas only 55.2% of black women had low risk.[8]
VULNERABLE PERIODS OF CARDIOVASCULAR RISK ACROSS A WOMAN’S LIFE COURSE
Early Life/Adolescence
Timing of Menarche
Early age at menarche (defined as prior to 12 years of age) has been associated with a higher future risk of CVD.[9-11] In a large cohort of greater than 1.2 million women in the United Kingdom without underlying CVD, the average age of menarche was reported to be 13 years of age. Compared with women who experienced menarche at 13 years, those who had menarche at 10 years or younger had a significantly higher relative risk of coronary heart disease (CHD) (relative risk 1.27; 95% confidence interval 1.22-1.31; p<0.0001).[11] In a US cohort, the Women’s Ischemia Syndrome Evaluation (WISE) study, of 648 middle-aged women, those with early menarche (≤10 years) were found to have a 4.53-fold higher risk (95% CI 2.13, 9.63) of cardiovascular disease as well as those with late menarche (≥15 years, HR 2.58; 1.28, 5.21).[12]
Premenstrual Syndrome
Premenstrual syndrome (PMS) is a constellation of physical and emotional symptoms that can occur in the window of time between ovulation and menses, and is associated with development of subsequent hypertension. In the Nurses’ Health Study II, 1257 women with clinically significant PMS symptoms were compared to 2463 age-matched controls and followed for 6-20 years for incident hypertension. Women with PMS were more likely to have incident hypertension (adjusted hazard ratio 1.4; 95% CI 1.2-1.6), and especially more likely to develop hypertension before the age of 40 years (adjusted hazard ratio 3.3, 95% CI 1.7-6.5).[13]
Reproductive Years
Pregnancy-Related Factors
Parity
In a meta-analysis of ten cohort studies, with over 3 million women included in the analysis, ever parity is associated with higher CVD risk compared with nulliparous status, with an even higher risk of CVD with greater number of pregnancies.[14] In an analysis of 8,583 white and black women from the Atherosclerosis Risk in Communities Study, a history of 5+ live births was associated with higher CHD risk, specifically hospitalized myocardial infarctions (MI), compared with women with 1-2 prior births.[15] A cohort study of 100,387 women found that women with twin pregnancies do not have increased CVD risk when compared to women with singleton pregnancies.[16]
Lactation
Adverse metabolic changes occur during pregnancy, including accumulation of fat mass, hyperinsulinemia, insulin resistance, and hyperlipidemia. Breastfeeding is thought to reverse the metabolic syndrome-like changes faster and more completely than no breastfeeding. In an analysis of 63,260 Danish women from the Danish National Birth Cohort, breastfeeding for greater than 4 months was associated with a 30% and 20% lower risk of hypertension and CVD, respectively, when compared with breastfeeding for less than 4 months or not at all.[17]
Adverse Pregnancy Outcomes (APOs)
Hypertensive disorders of pregnancy (Pre-eclampsia, gestational HTN)
Hypertensive disorders of pregnancy (HDP) are defined as either pre-eclampsia or gestational hypertension (GH). Women with HDP have a nearly two-fold higher risk of CVD than women without HDP.[18-20] HDP are associated with cardiovascular aging, and contribute to a more diverse group of cardiovascular conditions than previously recognized, including coronary artery disease, heart failure, aortic stenosis, and mitral regurgitation – all partially mediated by chronic hypertension.[21**]
The more severe the HDP, the worse the risk for CVD, as seen in one study where nearly half of women with early-onset preeclampsia went on to develop hypertension with higher average blood pressures, compared to 25% of women with late-onset preeclampsia.[22] Furthermore, risk of future CVD is higher when women have recurrent pre-eclampsia compared with a single episode.[23, 24] Interestingly though, women with pre-eclampsia in a multiple pregnancy do not appear to have a higher CVD risk compared with women in a singleton pregnancy, suggesting that their pre-eclampsia may be related to the larger burden of pregnancy on the cardiovascular system rather than the woman’s underlying cardiovascular phenotype.[25]
A portion of the excess CVD risk in women with HDP is associated with conventional cardiovascular risk factors, such as hypertension, diabetes, obesity and hypercholesterolemia, indicating that these are important targets for prevention.[26*, 27] However, the portion of CVD risk not attributable to these traditional risk factors in post-HDP women is not well understood and deserves further study.[28] Given the known higher risk for long-term ASCVD, the American Heart Association (AHA) and American Stroke Association (ASA) recommend that women with pre-eclampsia should have 6 month and 1 year post-partum follow-up visits for blood pressure and CVD risk monitoring. The uptake of this recommendation appears to be poor, however, as shown in a study examining the discharge planning for women with pre-eclampsia at an urban academic women’s hospital – out of 561 women, only 39% had documented follow-up visits specifically addressing blood pressure within the first year following delivery.[29]
Several studies have tested adding HDP to the already existing ASCVD risk prediction models – in some, this has not been shown to improve discrimination or reclassification[7, 30*] – and in others, the addition of HDP to these models only mildly improved CVD prediction.[31] However, these models relied on self-report of HDP and one study that manually adjudicated self-report and birth records of HDP identified a very poor sensitivity and specificity for self-report. This degree of misclassification of HDP status undermines its utility as a risk predictor. It is not known if pregnancy complications will enhance prediction of ASCVD risk in women, but newer risk prediction models in young women should be considered in more contemporary cohorts with more prospective, well-adjudicated pregnancy health and complication data.[32] However, it is unlikely that addition of APO will improve risk prediction in middle-aged women once risk factors, such as hypertension and diabetes have already developed.
Gestational Diabetes
The rate of gestational diabetes (GDM) has significantly increased over the past several decades, from 0.3% in 1979-1980 to 5.8% in 2008-2010.[33] Women with GDM have a 7- to 13-fold higher risk of developing type 2 diabetes mellitus (T2DM) later in life than women without GDM.[34*] An analysis of 89,000 women in the Nurses’ Health Study II showed that women with history of GDM have a modestly elevated risk for CVD, particularly MI events, when compared to parous women without GDM. Much of the increased CVD risk in this analysis was attenuated by subsequent weight gain and healthy lifestyle behaviors, with a small remaining absolute rate increase of 0.3 CVD events per 1000 person-years.[35] Other studies have found that the risk of CVD is in greater part attributable to GDM, citing that women with GDM have a two-fold higher risk for CVD even when corrected for the subsequent development of T2DM or metabolic syndrome.[36, 37] Furthermore, women with GDM and high pre-pregnancy maternal weight (>200lb) have a compounded risk of CVD compared to women with GDM alone.[38] Finally, in a Canadian study, even women who did not have GDM but had an abnormal glucose challenge test result had higher risk for CVD.[39]
Low offspring birth weight
Women who deliver a low birth weight (LBW) infant may be at higher risk for future cardiovascular disease. In an analysis from the Health, Aging, and Body Composition Study, women who had a history of a pregnancy complicated by LBW (defined as <2500 grams) were more likely to have risk factors of ASCVD such as hypertension and insulin resistance.[40] However, conflicting reports have been published with a recent meta-analysis examining 4 studies that did not identify a different risk of ASCVD for women with or without a LBW infant.[41]
Preterm delivery
Women who give birth before 37 weeks gestation have a greater risk of CVD when compared with women who give birth at term. In a study of 47,908 Israeli women who gave birth between 1988-1999 and were followed for more than a decade, preterm delivery (PTD) was present in 12.5% of women, and was found to be independently associated with a higher risk of future cardiovascular hospitalizations.[42] Furthermore, a systematic review of 10 studies including over 2 million women found that women with history of PTD were significantly more likely to develop future CVD.[43] The severity and number of PTDs cause a dose-dependent increase on maternal CVD risk.[44] In an analysis of nearly 200,000 women with a history of PTD who were followed for three decades, approximately 25% of the association between PTD and future maternal cardiovascular hospitalization could be attributed to vascular disorders of pregnancy, particularly pre-eclampsia.[45] Though the association of PTD and future maternal CVD is well-documented, many studies have been unable to adjust for the common link of maternal smoking that is a risk factor for both. In an Australian study of >700,000 women, association of preterm birth and maternal CVD risk was found to be independent of smoking status during pregnancy.[46]
Spontaneous Pregnancy Loss
Early spontaneous abortion (<12wks), late pregnancy loss (12-19weeks), and stillbirth (>20 weeks) are all associated with later maternal development of hypertension, diabetes mellitus, and hyperlipidemia.[47*, 48] Recurrent miscarriage (>3) was associated with five times higher risk of MI, and the history of a stillbirth increased risk for MI by 2.3 times, after adjusting for other ASCVD risk factors.[49] This risk factor is especially important given how common it is—in data from 79,121 women from the Women’s Health Initiative (WHI), approximately 35% experienced a history of a pregnancy loss.[50]
Hormonal Factors
Oral Contraceptive Use
The use of combined estrogen-progestin oral contraceptives has been associated with a two-fold higher rate of MI and thrombotic stroke, with even greater risk at higher estrogen doses.[51] However, given that MI and stroke in healthy reproductive age women is so rare, even a doubling of risk in this group still results in a very low absolute risk. The use of these agents should be carefully considered and likely avoided for reproductive age women with multiple other risk factors that put them at higher risk for ASCVD, such as smoking, obesity, diabetes, hyperlipidemia, and hypertension.
Post-menopausal Hormonal Therapy
Over the past two decades, there has been significant controversy regarding the role of hormonal therapy (HT) in post-menopausal women in the primary prevention of ASCVD. In the 1980’s, suggestions of benefit based on observational data supporting the antiatherogenic effects of estrogen (e.g. favorable lipid profiles, insulin sensitivity, and endothelial function) led to widespread use. However, subsequent large clinical trials have suggested minimal benefit or harm, depending on their design. A series of publications from the WHI suggested that HT after menopause is not beneficial for primary prevention, and in fact demonstrated an increased risk of stroke in the estrogen therapy arm compared with placebo among 10,000 healthy postmenopausal women aged 50-79 years, with prior hysterectomy, randomized to estrogen versus placebo.[52] Furthermore, a Cochrane review including 19 trials and greater than 40,000 post-menopausal women found high quality evidence that women who started hormone treatment more than 10 years after the menopause showed similar findings – that treatment with HT for primary or secondary prevention of CVD is not effective, and causes increased risk of stroke and venous thromboembolism. Therefore, HT should be used in caution for those with predisposing risk factors for CVD events seeking relief from menopausal symptoms.[53]
Polycystic ovarian syndrome (PCOS)
Women with polycystic ovarian syndrome (PCOS) have a higher prevalence of concomitant CVD risk factors: insulin resistance, T2DM, and metabolic syndrome.[54, 55] Pre- and peri-menopausal women with PCOS have been shown to have higher rates of CVD,[56] which is largely thought to be caused by their underlying risk factor burden. Given that women with PCOS have a high prevalence of metabolic syndrome and exhibit adverse cardiometabolic risk factors, they warrant early screening and regular monitoring throughout their reproductive lifespan.
Menopause
The association between menopause and CVD has been studied for many years. Premature natural and surgical menopause (<40 years) is associated with higher risk of for a variety of CVD events in the long term, even when adjusted for HT use after menopause. This risk has been demonstrated repeatedly to be even higher in those who have premature surgical menopause compared to premature natural menopause. [57**-59] Furthermore, even premenopausal women with low ovarian reserve (expressed by unmeasurable serum AMH levels) may have a greater risk of CVD.[60] Conversely, women with a very early CVD event (<35 years) had twice as high of likelihood to go on to experience early menopause (<45years).[61] Some women experience a distinctive increase in lipids in the year before and after their final menstrual period, so monitoring of lipids should be performed diligently in peri-menopausal women to enhance primary prevention targets for ASCVD.[62]
DISEASE-SPECIFIC RISK ENHANCERS IN WOMEN
Autoimmune Conditions
Systemic lupus erythematosus
Women are 9 times more likely to develop systemic lupus erythematosus (SLE) than their male counterparts. Patients with SLE have been shown to have higher rates of subclinical atherosclerosis, in terms of both coronary plaque[63] and carotid plaque[64], which appear to be independent of traditional risk factors. In addition, women with SLE are more likely to have HDP (20%) than women without SLE (7%). In this population, HDP is associated with a two-fold higher rate of CVD and three-fold higher rate of incident hypertension.[65]
Rheumatoid Arthritis
Women are 2-3 times more likely to develop rheumatoid arthritis (RA) than men. Given that the inflammatory mileu is thought to play a role in atherosclerotic plaque development and progression in this population, those with RA who have frequent inflammatory flares have a greater burden of ASCVD than those who are in remission for longer.[66]
In women with autoimmune conditions, aggressive control of inflammation along with identification and treatment of other traditional cardiovascular risk factors should be implemented to reduce ASCVD. Further studies are needed to understand if there is an increased ASCVD risk in less common autoimmune conditions such as systemic sclerosis, Sjogren’s, polymyalgia rheumatic, antiphospholipid syndrome, and giant cell arteritis, all of which are autoimmune inflammatory states that are more prevalent in women than men.[67]
Breast Cancer
Chemotherapy and Radiation
Though breast cancer can affect both sexes, this disease is female-predominant, with a 100 times higher likelihood of occurring in women than in men.[68] Cancer survivorship has improved dramatically in recent decades with improvement in understanding of disease pathophysiology and mechanisms as well as emergence of targeted therapies. With cancer survivorship however, comes other long-term medical conditions. There are greater than 3.8 million breast cancer survivors in the US, and the number one cause of death in this group is CVD. Given the shared risk factor burden and various short-term and long-term cardiometabolic toxic effects known to result from breast cancer treatment, the American Heart Association recently issued a Scientific Statement highlighting the need to focus on cardiovascular prevention[69**] in all breast cancer survivors and to pursue appropriate diagnostics and/or cardiology consultation to address the high rates of coronary artery disease, congestive heart failure, and thromboembolic disease.
Anthracycline-based chemotherapies and trastuzumab have been repeatedly demonstrated to lead to left ventricular dysfunction and heart failure. In addition, these chemotherapeutic agents can also contribute to a decline in exercise tolerance and cardio-pulmonary reserve despite preserved ejection fraction.[70]
Radiation therapy administered as adjuvant therapy or breast cancer elevates the risk of CVD.[71] In particular, radiation therapy for left-sided breast cancer has been associated with higher rates of ischemic heart disease.[70]
Familial Hypercholesterolemia (FH)
FH is an autosomal co-dominant monogenic condition. It results in lifelong elevated total and low-density lipoprotein (LDL) cholesterol, associated often with corneal arcus and tendon xanthomas in adults before age 50, a family history of premature CHD, and if untreated, an increased risk for premature CHD and death.[72] An analysis of 116 kindreds, whose probands were diagnosed at the National Heart, Lung, Blood institute (NHLBI) in the pre-statin era, showed an increase in CHD occurring in women more than a decade after affected men. Affected women had a risk of nonfatal or fatal CHD by age 60 of 32.8% compared with only 9.1% in non-affected women. Perak et al[73] showed substantially elevated 30-year CHD risks in those with an FH phenotype defined as LDL-C ≥ 190 mg/dL with hazard ratios up to 5.0 (95% CI 1.1-21.7). Moreover, CHD risk was accelerated across index ages in those with the FH phenotype by 10 to 20 years in men and 20 to 30 years in women. The EOMI (Early-Onset Myocardial Infarction) study found that approximately 2% of cases of early MI in women ≤60 years and men ≤ 50 years had a pathogenic variant in one of the three main genes associated with FH, the LDL Receptor (LDLR) gene.[74] Genetic testing is recommended in those with a clinical diagnosis of FH, given that a pathogenic variant in LDLR, apo B or PCSK9 confirms monogenic FH that is associated with a higher risk of premature CAD than polygenic FH.[75] Diagnosis at a young age is crucial, since effective lipid lowering therapy is associated with reduced rates of CAD and improved survival.[76] Statin therapy should not be deferred in women until a threshold 10-year risk of 7.5% is achieved as this algorithm does not apply in FH.[77] Pregnancy poses a specific hazard for women with FH as cholesterol rises with each trimester. Statin therapy is recommended to be stopped before pregnancy to avoid the potential for teratogenic effects,[4] and can be resumed after cessation of breast feeding.
TRANSGENDER MEDICINE
Female hormonal supplementation in men
Clinicians are more frequently encountering transgender people in their practice, however they often lack formal training or experience in the assessment and management of the potential complications of transgender therapies. Data on the effects of estrogen therapy on rates of myocardial infarction are mixed[78]. However, there is clear evidence of increased rates of venous thromboembolic disease and possible increase in stroke in cisgender and transgender women taking oral supplemental estrogen therapy[78, 79]. Therefore, it is prudent to ensure other cardiovascular risk factors – such as smoking, hypertension, hyperlipidemia, and obesity – are well-managed prior to initiation of oral estrogen therapy in transgender women. Clinicians who counsel both cis- and transgender women 60 years and older must communicate the documented higher CVD risks from estrogen therapy in this age group. Thus, a decision regarding estrogen usage should consider benefits/all harms (some not cardiovascular) and be individualized in the context of a clinician-patient risk discussion.
ROLE OF PREVENTIVE TESTING TO RISK STRATIFY WOMEN
For those with borderline to intermediate 10-year ASCVD risk, the 2018 AHA/ACC/Multi-Society Cholesterol Guidelines[4] recommend a clinician-patient risk discussion based on quantitative risk assessment and consideration of personal risk-enhancing factors, and for those in whom risk status remains uncertain, the use of coronary artery calcium (CAC) scoring to help facilitate decision making.
A consideration of enhancing factors (see Figure 2) is especially pertinent to women since they often present with multiple such factors that allow the clinician to personalize both their short and long-term ASCVD risk. Single enhancing factors as noted above may not increase 10-year ASCVD risk substantially over the risk determined by the PCE for women, but when multiple enhancing factors are present, they have the potential to reclassify risk. For example, a 62 year old non-diabetic, non-hypertensive, non-smoker black woman with a total cholesterol of 172mg/dL, HDL of 55mg/DL, and systolic blood pressure of 112mmHg who would ordinarily have a 10-year ASCVD risk score of 3.7%, should have a very different clinician-patient discussion than her counterpart with all of the same demographic and comorbidity profile, but with the addition of a prior pregnancy complicated by pre-eclampsia, followed by early menopause at age 39, and a history of chest irradiation for breast cancer. At a minimum, incorporating sex-specific risk enhancing factors into the clinician-patient discussion allows the patient and clinician to see clearly the stable elements of risk that characterize her propensity to ASCVD. In addition, bringing sex-specific risk enhancing factors to the forefront of the primary prevention discussion also has the potential to improve patient adherence to lipid-lowering therapy when recommended, as this allows the patient to better understand her personalized risk, as contrasted with simply being given a risk percentage and told to start a therapy based on a number alone. We await studies that are needed to see if this improvement in adherence to therapy is, in fact, the case.
Figure 2.
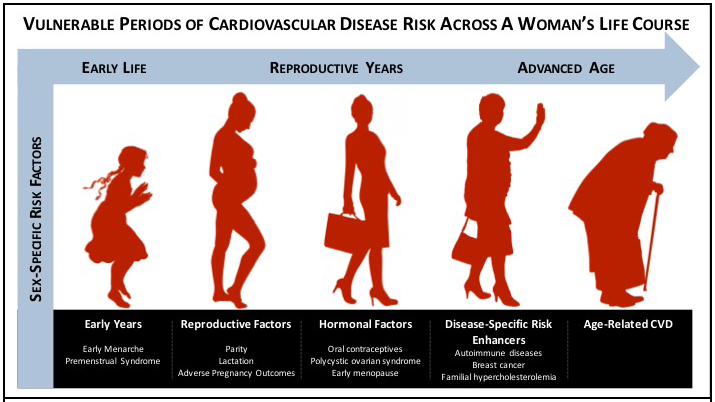
Vulnerable periods of cardiovascular disease risk across a woman’s life course
Coronary artery calcium scoring
The Multi-Ethic Study of Atherosclerosis (MESA), a prospective cohort study of 6814 participants (51% women), found CAC to be robustly associated with fatal and non fatal MI and stroke over a 10-year period.[80*] Multiple studies have shown that although men have higher CAC burden, women have a larger proportion of mixed and non-calcified plaques, which portend higher acute and long-term cardiovascular mortality.[81, 82] Using MESA data, a validated ASCVD risk score is available for those patients who have undergone a CAC test to estimate 10- year CHD risk using traditional risk factors and CAC.[83] Furthermore, the absence of coronary calcium, or a CAC score = 0, is clinically very meaningful in reclassifying risk. In a study of more than 4500 MESA participants, approximately half of those who would have traditionally been recommended a statin based on their PCE 10-year ASCVD risk score had a CAC = 0 and an extremely low ASCVD event risk (1.5 per 1000 patient years).[84] Thus, these patients would be reclassified to not be recommended to initiate statin therapy. The significant improvement in risk prediction obtained could be especially helpful in women who prefer not to take a statin despite having a 10-year risk of ≥ 7.5% and enhance the clinician-patient risk discussion.
Breast Arterial Calcification
Breast arterial calcification (BAC) is another surrogate marker that may demonstrate utility for ASCVD screening for women. BAC can be assessed from mammograms and reflects calcification of the breast arteries in the media of the artery (Mönckeberg medial calcific sclerosis). While BAC is in earlier stages of development and discovery and limited data on clinical applications are available, early studies, albeit small in size and mostly retrospective, demonstrate a strong association between BAC and CAC [85-87]. BAC can be obtained without additional cost or radiation to women during mammograms that are already routinely performed starting at age 40 for breast cancer screening, and therefore are an appealing potential tool to leverage. Currently, limitations of the translation of BAC into routine clinical practice are hampered by the lack of reproducible and quantitative assessment as well as prospective studies for validation.
CONCLUSIONS
Women at every stage of the life-cycle after menarche have characteristics that require a woman-centered determination of their risk of ASCVD. Moreover, even among women, race and ethnicity factors further affect absolute global cardiovascular risk. Recent guidelines have recommended a consideration of risk-enhancing factors in primary prevention clinician-patient risk discussions. These factors should be included in a detailed obstetric and gynecological history in order to personalize the assessment of ASCVD risk for each individual woman. The most powerful factor, however, to allow for reclassification of ASCVD risk is a CAC score. This may be especially useful in older women who do not want to take cholesterol lowering medications such as statins to determine more precisely if they would, in fact, benefit. Many such patients with a calcium score of 0 have a lower overall risk that puts them beneath the treatment threshold. This review of sex-specific factors should be considered a first step on the route to the precision medicine that is eagerly awaited in the future.
Table.
Key Research Questions/Gaps in Evidence
| Question | Current Evidence | Gaps in Evidence/Future Directions |
|---|---|---|
| Does inclusion of sex-specific risk factors in short-term and long-term ASCVD risk prediction have clinical utility and inform initiation of preventive therapies? | Multiple sex-specific risk factors (premature menarche, adverse pregnancy outcomes, premature menopause) are associated with higher risk of ASCVD, independent of traditional risk factors. | Prospective, adjudicated, epidemiological studies incorporating pregnancy and non-pregnancy cohorts and electronic health record data are needed to understand the potential additional predictive utility of these risk factors |
| What are the pathways and mechanisms that underlie the transition from adverse pregnancy outcomes to ASCVD? | Adverse pregnancy outcomes constitute a spectrum of placental vascular disorders that are associated with higher risk of ASCVD risk factors in follow-up. | Dissemination and implementation of collaborative care beginning in the early post-partum period focusing on lifestyle behavior modification and optimization of CVH to assess best strategies to assess, modify, and prevent a woman’s risk of ASCVD needs to be studied. Discovery of unique biomarkers that may predict risk or be targeted to prevent ASCVD may enhance personalized care. |
| Should subclinical imaging tools (CAC) be employed in women with a sex-specific risk factor to identify women at risk earlier in life and inform guideline-recommended initiation of evidence-based therapy? | Sequential screening with CAC can reclassify risk for ASCVD in intermediate and high predicted risk middle-aged women. | Randomized controlled trial evidence to assess the impact of CAC on ASCVD events may complement the available robust epidemiological data to guide decisions on statin therapy initiation when there is uncertainty. |
Acknowledgments
Funding
This research was supported by grants from the National Institutes of Health/National Heart, Lung, and Blood Institute (KL2TR001424) and the American Heart Association (AHA#19TPA34890060) to Dr. Khan. Research reported in this publication was supported, in part, by the National Institutes of Health's National Center for Advancing Translational Sciences, Grant Number KL2TR001424 (SSK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Conflict of Interest
Dr. Mehta has nothing to disclose.
Dr. Khan reports grants from NIH/NCATS, and grants from American Heart association, which are outside the submitted work.
Dr. Lloyd-Jones has nothing to disclose.
Dr. Stone has nothing to disclose.
REFERENCES
(Bulleted references as important (*) or very important (**) with brief explanations of importance)
- 1.Heron M. Deaths: Leading Causes for 2017. National Vital Statistics Report. 2019;68(6). [PubMed] [Google Scholar]
- 2.World health statistics 2019: monitoring health for the SDGs, sustainable development goals. World Health Organization; 2019. [Google Scholar]
- 3.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S1–45. [DOI] [PubMed] [Google Scholar]
- 4.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. Journal of the American College of Cardiology. 2019;73(24):e285–e350.** Guideline document that highlights importance of incorporating risk enhancing factors and sex-specific risk factors into primary prevention risk assessment.
- 5.Lloyd-Jones DM, Braun LT, Ndumele CE, Smith SC Jr., Sperling LS, Virani SS, et al. Use of Risk Assessment Tools to Guide Decision-Making in the Primary Prevention of Atherosclerotic Cardiovascular Disease: A Special Report From the American Heart Association and American College of Cardiology. J Am Coll Cardiol. 2019;73(24):3153–67. [DOI] [PubMed] [Google Scholar]
- 6.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596–e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stuart JJ, Tanz LJ, Cook NR, Spiegelman D, Missmer SA, Rimm EB, et al. Hypertensive Disorders of Pregnancy and 10-Year Cardiovascular Risk Prediction. J Am Coll Cardiol. 2018;72(11):1252–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goff DC Jr., Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB Sr., Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2935–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ley SH, Li Y, Tobias DK, Manson JE, Rosner B, Hu FB, et al. Duration of Reproductive Life Span, Age at Menarche, and Age at Menopause Are Associated With Risk of Cardiovascular Disease in Women. J Am Heart Assoc. 2017;6(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peters SAE, Woodward M. Women's reproductive factors and incident cardiovascular disease in the UK Biobank. Heart. 2018;104(13):1069–75. [DOI] [PubMed] [Google Scholar]
- 11.Canoy D, Beral V, Balkwill A, Wright FL, Kroll ME, Reeves GK, et al. Age at menarche and risks of coronary heart and other vascular diseases in a large UK cohort. Circulation. 2015;131(3):237–44. [DOI] [PubMed] [Google Scholar]
- 12.Lee JJ, Cook-Wiens G, Johnson BD, Braunstein GD, Berga SL, Stanczyk FZ, et al. Age at Menarche and Risk of Cardiovascular Disease Outcomes: Findings From the National Heart Lung and Blood Institute-Sponsored Women's Ischemia Syndrome Evaluation. J Am Heart Assoc. 2019;8(12):e012406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bertone-Johnson ER, Whitcomb BW, Rich-Edwards JW, Hankinson SE, Manson JE. Premenstrual Syndrome and Subsequent Risk of Hypertension in a Prospective Study. Am J Epidemiol. 2015;182(12):1000–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li W, Ruan W, Lu Z, Wang D. Parity and risk of maternal cardiovascular disease: A dose–response meta-analysis of cohort studies. European Journal of Preventive Cardiology. 2019;26(6):592–602. [DOI] [PubMed] [Google Scholar]
- 15.Oliver-Williams C, Vladutiu CJ, Loehr LR, Rosamond WD, Stuebe AM. The Association between Parity and Subsequent Cardiovascular Disease in Women: The Atherosclerosis Risk in Communities Study. Journal of Women's Health. 2019;28(5):721–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okby R, Shoham-Vardi I, Sergienko R, Sheiner E. Twin pregnancy: Is it a risk factor for long-term cardiovascular disease? Journal of Maternal-Fetal and Neonatal Medicine. 2016;29(10):1626–30. [DOI] [PubMed] [Google Scholar]
- 17.Kirkegaard H, Bliddal M, Stovring H, Rasmussen KM, Gunderson EP, Kober L, et al. Breastfeeding and later maternal risk of hypertension and cardiovascular disease - The role of overall and abdominal obesity. Prev Med. 2018;114:140–8. [DOI] [PubMed] [Google Scholar]
- 18.Brown MC, Best KE, Pearce MS, Waugh J, Robson SC, Bell R. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. European Journal of Epidemiology. 2013;28(1):1–19. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed R, Dunford J, Mehran R, Robson S, Kunadian V. Pre-eclampsia and future cardiovascular risk among women: a review. J Am Coll Cardiol. 2014;63(18):1815–22. [DOI] [PubMed] [Google Scholar]
- 20.Leon LJ, McCarthy FP, Direk K, Gonzalez-Izquierdo A, Prieto-Merino D, Casas JP, et al. Preeclampsia and Cardiovascular Disease in a Large UK Pregnancy Cohort of Linked Electronic Health Records: A CALIBER Study. Circulation. 2019;140(13):1050–60. [DOI] [PubMed] [Google Scholar]
- 21.Honigberg MC, Zekavat SM, Aragam K, Klarin D, Bhatt DL, Scott N, et al. Hypertensive disorders of pregnancy are associated with long-term risk of diverse cardiovascular disease. Circulation. 2019;140.** Women who have a hypertensive disorder of pregnancy are at higher long-term risk for a diverse group of cardiovascular disease.
- 22.Veerbeek JHW, Hermes W, Breimer AY, Van Rijn BB, Koenen SV, Mol BW, et al. Cardiovascular disease risk factors after early-onset preeclampsia, late-onset preeclampsia, and pregnancy-induced hypertension. Hypertension. 2015;65(3):600–6. [DOI] [PubMed] [Google Scholar]
- 23.Brouwers L, van der Meiden-van Roest AJ, Savelkoul C, Vogelvang TE, Lely AT, Franx A, et al. Recurrence of pre-eclampsia and the risk of future hypertension and cardiovascular disease: a systematic review and meta-analysis. BJOG: An International Journal of Obstetrics and Gynaecology. 2018;125(13):1642–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kessous R, Shoham-Vardi I, Pariente G, Sergienko R, Sheiner E. Long-term maternal atherosclerotic morbidity in women with pre-eclampsia. Heart. 2015;101(6):442–6. [DOI] [PubMed] [Google Scholar]
- 25.Bergman L, Callbo PN, Hesselman S, Wikström AK, Edstedt Bonamy AK, Sandström A. Women with preeclampsia in a multiple pregnancy have no association with increased risk of future cardiovascular disease; a national register-based cohort study. Pregnancy Hypertension. 2019;17:S7. [Google Scholar]
- 26.Haug EB, Horn J, Markovitz AR, Fraser A, Klykken B, Dalen H, et al. Association of Conventional Cardiovascular Risk Factors with Cardiovascular Disease after Hypertensive Disorders of Pregnancy: Analysis of the Nord-Trøndelag Health Study. JAMA Cardiology. 2019;4(7):628–35.*The elevated risk of cardiovascular disease risk in women with hypertensive disorders of pregnancy can be in part attributed to traditional ASCVD risk factors, which should be targeted for prevention.
- 27.Stuart JJ, Tanz LJ, Missmer SA, Rimm EB, Spiegelman D, James-Todd TM, et al. Hypertensive disorders of pregnancy and maternal cardiovascular disease risk factor development: An observational cohort study. Annals of Internal Medicine. 2018;169(4):224–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riise HKR, Sulo G, Tell GS, Igland J, Egeland G, Nygard O, et al. Hypertensive pregnancy disorders increase the risk of maternal cardiovascular disease after adjustment for cardiovascular risk factors. International Journal of Cardiology. 2019;282:81–7. [DOI] [PubMed] [Google Scholar]
- 29.Huang J, Eisaman D, Berlacher K, Suyama J. Follow-up for cardiovascular disease risk in women with preeclampsia one year after delivery. Circulation. 2018;138. [Google Scholar]
- 30.Timpka S, Fraser A, Schyman T, Stuart JJ, Åsvold BO, Mogren I, et al. The value of pregnancy complication history for 10-year cardiovascular disease risk prediction in middle-aged women. European Journal of Epidemiology. 2018;33(10):1003–10.*The addition of adverse pregnancy outcomes to already existing ASCVD risk prediction models has not been shown to improve discrimination or reclassification.
- 31.Markovitz AR, Stuart JJ, Horn J, Williams PL, Rimm EB, Missmer SA, et al. Does pregnancy complication history improve cardiovascular disease risk prediction? Findings from the HUNT study in Norway. European Heart Journal. 2019;40(14):1113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Catov JM, Countouris M, Hauspurg A. Hypertensive Disorders of Pregnancy and CVD Prediction: Accounting for Risk Accrual During the Reproductive Years. J Am Coll Cardiol. 2018;72(11):1264–6. [DOI] [PubMed] [Google Scholar]
- 33.Lavery JA, Friedman AM, Keyes KM, Wright JD, Ananth CV. Gestational diabetes in the United States: temporal changes in prevalence rates between 1979 and 2010. BJOG. 2017;124(5):804–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hopmans TEJP, van Houten C, Kasius A, Kouznetsova OI, Nguyen LA, Rooijmans SV, et al. Increased risk of type II diabetes mellitus and cardiovascular disease after gestational diabetes mellitus: a systematic review. Ned Tijdschr Geneeskd. 2015;159:A8043.*Women with gestational diabetes have a 7- to 13-fold higher risk of developing type 2 diabetes mellitus than women without gestational diabetes.
- 35.Tobias DK, Stuart JJ, Li S, Chavarro J, Rimm EB, Rich-Edwards J, et al. Association of History of Gestational Diabetes With Long-term Cardiovascular Disease Risk in a Large Prospective Cohort of US Women. JAMA Intern Med. 2017;177(12):1735–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kramer CK, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia. 2019;62(6):905–14. [DOI] [PubMed] [Google Scholar]
- 37.Gunderson EP, Chiang V, Pletcher MJ, Jacobs DR, Quesenberry CP, Sidney S, et al. History of gestational diabetes mellitus and future risk of atherosclerosis in mid-life: the Coronary Artery Risk Development in Young Adults study. J Am Heart Assoc. 2014;3(2):e000490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaul P, Savu A, Nerenberg KA, Donovan LE, Chik CL, Ryan EA, et al. Impact of gestational diabetes mellitus and high maternal weight on the development of diabetes, hypertension and cardiovascular disease: A population-level analysis. Diabetic Medicine. 2015;32(2):164–73. [DOI] [PubMed] [Google Scholar]
- 39.Retnakaran R, Shah BR. Glucose screening in pregnancy and future risk of cardiovascular disease in women: a retrospective, population-based cohort study. The Lancet Diabetes and Endocrinology. 2019;7(5):378–84. [DOI] [PubMed] [Google Scholar]
- 40.Catov JM, Newman AB, Roberts JM, Sutton–Tyrrell KC, Kelsey SF, Harris T, et al. Association Between Infant Birth Weight and Maternal Cardiovascular Risk Factors in the Health, Aging, and Body Composition Study. Annals of Epidemiology. 2007;17(1):36–43. [DOI] [PubMed] [Google Scholar]
- 41.Grandi SM, Filion KB, Yoon S, Ayele HT, Doyle CM, Hutcheon JA, et al. Cardiovascular Disease-Related Morbidity and Mortality in Women with a History of Pregnancy Complications: Systematic Review and Meta-Analysis. Circulation. 2019;139(8):1069–79. [DOI] [PubMed] [Google Scholar]
- 42.Kessous R, Shoham-Vardi I, Pariente G, Holcberg G, Sheiner E. An association between preterm delivery and long-term maternal cardiovascular morbidity. Am J Obstet Gynecol. 2013;209(4):368 e1–8. [DOI] [PubMed] [Google Scholar]
- 43.Robbins CL, Hutchings Y, Dietz PM, Kuklina EV, Callaghan WM. History of preterm birth and subsequent cardiovascular disease: A systematic review. American Journal of Obstetrics and Gynecology. 2014;210(4):285–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ngo AD, Roberts CL, Chen JS, Figtree G. Interaction of maternal smoking and preterm birth on future risk of maternal cardiovascular disease: A population-based record linkage study. European Journal of Preventive Cardiology. 2016;23(6):613–20. [DOI] [PubMed] [Google Scholar]
- 45.Auger N, Potter BJ, He S, Healy-Profitos J, Schnitzer ME, Paradis G. Maternal Cardiovascular Disease 3 Decades After Preterm Birth: Longitudinal Cohort Study of Pregnancy Vascular Disorders. Hypertension. 2020:HYPERTENSIONAHA11914221. [DOI] [PubMed] [Google Scholar]
- 46.Ngo AD, Chen JS, Figtree G, Morris JM, Roberts CL. Preterm birth and future risk of maternal cardiovascular disease - is the association independent of smoking during pregnancy? BMC Pregnancy and Childbirth. 2015;15(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Horn J, Tanz LJ, Stuart JJ, Markovitz AR, Skurnik G, Rimm EB, et al. Early or late pregnancy loss and development of clinical cardiovascular disease risk factors: a prospective cohort study. BJOG: An International Journal of Obstetrics and Gynaecology. 2019;126(1):33–42.*Spontaneous pregnancy loss is associated with increased risk of hypertension, type 2 diabetes mellitus, and hyperlipidemia.
- 48.Ranthe MF, Andersen EAW, Wohlfahrt J, Bundgaard H, Melbye M, Boyd HA. Pregnancy loss and later risk of Atherosclerotic disease. Circulation. 2013;127(17):1775–82. [DOI] [PubMed] [Google Scholar]
- 49.Kharazmi E, Dossus L, Rohrmann S, Kaaks R. Pregnancy loss and risk of cardiovascular disease: A prospective population-based cohort study (EPIC-Heidelberg). Heart. 2011;97(1):49–54. [DOI] [PubMed] [Google Scholar]
- 50.Hall PS, Nah G, Vittinghoff E, Parker DR, Manson JE, Howard BV, et al. Relation of Pregnancy Loss to Risk of Cardiovascular Disease in Parous Postmenopausal Women (From the Women's Health Initiative). Am J Cardiol. 2019;123(10):1620–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lidegaard O, Lokkegaard E, Jensen A, Skovlund CW, Keiding N. Thrombotic Stroke and Myocardial Infarction with Hormonal Contraception. N Engl J Med. 2012;366:2257–66. [DOI] [PubMed] [Google Scholar]
- 52.Anderson GL, Limacher M, A.R. A. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–12. [DOI] [PubMed] [Google Scholar]
- 53.Main C, Knight B, Moxham T, Gabriel Sanchez R, Sanchez Gomez LM, Roquéi Figuls M, et al. Hormone therapy for preventing cardiovascular disease in post-menopausal women. Cochrane Database of Systematic Reviews. 2013;2013(4). [DOI] [PubMed] [Google Scholar]
- 54.Moran LJ, Misso ML, Wild RA, Norman RJ. Impaired glucose tolerance, type 2 diabetes and metabolic syndrome in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2010;16(4):347–63. [DOI] [PubMed] [Google Scholar]
- 55.Kazemi M, Pierson RA, Lujan ME, Chilibeck PD, McBreairty LE, Gordon JJ, et al. Comprehensive Evaluation of Type 2 Diabetes and Cardiovascular Disease Risk Profiles in Reproductive-Age Women with Polycystic Ovary Syndrome: A Large Canadian Cohort. Journal of Obstetrics and Gynaecology Canada. 2019;41(10):1453–60. [DOI] [PubMed] [Google Scholar]
- 56.Wild S, Pierpoint T, McKeigue P, Jacobs H. Cardiovascular disease in women with polycystic ovary syndrome at long-term follow-up: a restrospective cohort study. Clinical Endocrinology 2000;52:595–600. [DOI] [PubMed] [Google Scholar]
- 57.Honigberg MC, Zekavat SM, Aragam K, Finneran P, Klarin D, Bhatt DL, et al. Association of Premature Natural and Surgical Menopause with Incident Cardiovascular Disease. JAMA - Journal of the American Medical Association. 2019;322(24):2411–21.**Natural or surgical menopause before the age of 40 is associated with increased risk of cardiovascular disease.
- 58.Honigberg M, Zekavat SM, Aragam K, Klarin D, Scott N, Natarajan P. Natural and surgical menopause before age 40 are associated with long-term incident cardiovascular disease risk. Circulation. 2019;140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Atsma F, Bartelink MLEL, Grobbee DE, Van Der Schouw YT. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: A meta-analysis. Menopause. 2006;13(2):265–79. [DOI] [PubMed] [Google Scholar]
- 60.Van Der Schouw YT, De Kat AC, Verschuren WM, Eijkemans MJ, Broekmans FJ. Low ovarian reserve is associated with cardiovascular disease risk. Circulation. 2016;133. [DOI] [PubMed] [Google Scholar]
- 61.Zhu D, Chung HF, Pandeya N, Dobson AJ, Hardy R, Kuh D, et al. Premenopausal cardiovascular disease and age at natural menopause: a pooled analysis of over 170,000 women. European Journal of Epidemiology. 2019;34(3):235–46. [DOI] [PubMed] [Google Scholar]
- 62.Matthews KA, Crawford SL, Chae CU, Everson-Rose SA, Sowers MF, Sternfeld B, et al. Are Changes in Cardiovascular Disease Risk Factors in Midlife Women Due to Chronological Aging or to the Menopausal Transition? Journal of the American College of Cardiology. 2009;54(25):2366–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Asanuma Y, Oeser A, Shintani A, Turner E, Olsen N, Fazio S, et al. Premature coronary-artery atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349:2407–15. [DOI] [PubMed] [Google Scholar]
- 64.Roman MJ, Shanker B, Davis A, Lockshin MD, Sammaritano L, Simantov R, et al. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349:2399–406. [DOI] [PubMed] [Google Scholar]
- 65.Simard JF, Rossides M, Arkema EV, Svenungsson E, Wikstrom AK, Mittleman MA, et al. Maternal hypertensive disorders in SLE pregnancy and future cardiovascular outcomes. Arthritis Care Res (Hoboken). 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Myasoedova E, Chandran A, Ilhan B, Major BT, Michet CJ, Matteson EL, et al. The role of rheumatoid arthritis (RA) flare and cumulative burden of RA severity in the risk of cardiovascular disease. Ann Rheum Dis. 2016;75(3):560–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kurmann RD, Mankad R. Atherosclerotic vascular disease in the autoimmune rheumatologic woman. Clinical Cardiology. 2018;41(2):258–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Giordano SH, Longo DL. Breast Cancer in Men. New England Journal of Medicine. 2018;378(24):2311–20. [DOI] [PubMed] [Google Scholar]
- 69.Mehta LS, Watson KE, Barac A, Beckie TM, Bittner V, Cruz-Flores S, et al. Cardiovascular Disease and Breast Cancer: Where These Entities Intersect: A Scientific Statement From the American Heart Association. Circulation. 2018;137(8):e30–e66.**Scientific statement highlighting the need to focus on cardiovascular disease prevention in all breast cancer survivors.
- 70.Xie Y, Collins WJ, Audeh MW, Shiao SL, Gottlieb RA, Goodman MT, et al. Breast Cancer Survivorship and Cardiovascular Disease: Emerging Approaches in Cardio-Oncology. Curr Treat Options Cardiovasc Med. 2015;17(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hamood R, Hamood H, Merhasin I, Keinan-Boker L. Risk of cardiovascular disease after radiotherapy in survivors of breast cancer: A case-cohort study. Journal of Cardiology. 2019;73(4):280–91. [DOI] [PubMed] [Google Scholar]
- 72.Gidding SS, Champagne MA, de Ferranti SD, Defesche J, Ito MK, Knowles JW, et al. The Agenda for Familial Hypercholesterolemia: A Scientific Statement From the American Heart Association. Circulation. 2015;132(22):2167–92. [DOI] [PubMed] [Google Scholar]
- 73.Perak AM, Ning H, de Ferranti SD, Gooding HC, Wilkins JT, Lloyd-Jones DM. Long-Term Risk of Atherosclerotic Cardiovascular Disease in US Adults With the Familial Hypercholesterolemia Phenotype. Circulation. 2016;134(1):9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Do R, Stitziel NO, Won HH, Jorgensen AB, Duga S, Angelica Merlini P, et al. Exome sequencing identifies rare LDLR and APOA5 alleles conferring risk for myocardial infarction. Nature. 2015;518(7537):102–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Trinder M, Li X, DeCastro ML, Cermakova L, Sadananda S, Jackson LM, et al. Risk of Premature Atherosclerotic Disease in Patients With Monogenic Versus Polygenic Familial Hypercholesterolemia. J Am Coll Cardiol. 2019;74(4):512–22. [DOI] [PubMed] [Google Scholar]
- 76.Sniderman AD, Tsimikas S, Fazio S. The severe hypercholesterolemia phenotype: clinical diagnosis, management, and emerging therapies. J Am Coll Cardiol. 2014;63(19):1935–47. [DOI] [PubMed] [Google Scholar]
- 77.Knowles JW, Stone NJ, Ballantyne CM. Familial Hypercholesterolemia and the 2013 American College of Cardiology/American Heart Association Guidelines: Myths, Oversimplification, and Misinterpretation Versus Facts. Am J Cardiol. 2015;116(3):481–4. [DOI] [PubMed] [Google Scholar]
- 78.Shatzel JJ, Connelly KJ, DeLoughery TG. Thrombotic issues in transgender medicine: A review. Am J Hematol. 2017;92(2):204–8. [DOI] [PubMed] [Google Scholar]
- 79.den Heijer M, Bakker A, Gooren L. Long term hormonal treatment for transgender people. BMJ. 2017;359:j5027. [DOI] [PubMed] [Google Scholar]
- 80.Budoff MJ, Young R, Burke G, Jeffrey Carr J, Detrano RC, Folsom AR, et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA). Eur Heart J. 2018;39(25):2401–8.*Coronary artery calcium is robustly associated with fatal and non-fatal myocardial infarction and stroke over a 10-year period.
- 81.Plank F, Beyer C, Friedrich G, Wildauer M, Feuchtner G. Sex differences in coronary artery plaque composition detected by coronary computed tomography: quantitative and qualitative analysis. Netherlands Heart Journal. 2019;27(5):272–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bigeh A, Shekar C, Gulati M. Sex Differences in Coronary Artery Calcium and Long-term CV Mortality. Curr Cardiol Rep. 2020;22(4):21. [DOI] [PubMed] [Google Scholar]
- 83.McClelland RL, Jorgensen NW, Budoff M, Blaha MJ, Post WS, Kronmal RA, et al. 10-Year Coronary Heart Disease Risk Prediction Using Coronary Artery Calcium and Traditional Risk Factors: Derivation in the MESA (Multi-Ethnic Study of Atherosclerosis) With Validation in the HNR (Heinz Nixdorf Recall) Study and the DHS (Dallas Heart Study). J Am Coll Cardiol. 2015;66(15):1643–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nasir K, Bittencourt MS, Blaha MJ, Blankstein R, Agatson AS, Rivera JJ, et al. Implications of Coronary Artery Calcium Testing Among Statin Candidates According to American College of Cardiology/American Heart Association Cholesterol Management Guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2015;66(15):1657–68. [DOI] [PubMed] [Google Scholar]
- 85.Yoon YE, Kim KM, Han JS, Kang SH, Chun EJ, Ahn S, et al. Prediction of Subclinical Coronary Artery Disease With Breast Arterial Calcification and Low Bone Mass in Asymptomatic Women: Registry for the Women Health Cohort for the BBC Study. JACC Cardiovasc Imaging. 2019;12(7 Pt 1):1202–11. [DOI] [PubMed] [Google Scholar]
- 86.Bui QM, Daniels LB. A Review of the Role of Breast Arterial Calcification for Cardiovascular Risk Stratification in Women. Circulation. 2019;139(8):1094–101. [DOI] [PubMed] [Google Scholar]
- 87.Newallo D, Meinel FG, Schoepf UJ, Baumann S, De Cecco CN, Leddy RJ, et al. Mammographic detection of breast arterial calcification as an independent predictor of coronary atherosclerotic disease in a single ethnic cohort of African American women. Atherosclerosis. 2015;242(1):218–21. [DOI] [PubMed] [Google Scholar]