Abstract
Background: This study aimed to clarify the relationship between F. nucleatum levels and the prognosis of CRC, which is still controversial.
Methods: Relevant articles were searched on PubMed, Web of Science, PMC and Embase up to April 7, 2020. Outcomes of interest included clinical characteristics, molecular characteristic and survival analysis. HR (OR), odds ratios (OR) and 95% confidence interval (CI) were calculated to explore the prognostic value and relationship of clinical characteristics of Fusobacterium nucleatum in CRC.
Results: A total of 3626 CRC patients from 13 eligible studies were included. High levels of F. nucleatum were associated with worse prognosis, as such parameters as overall survival (OS) (hazard ratio [HR] = 1.40, 95% confidence interval [CI]: 1.40 - 1.63, P < 0.0001), disease-free survival (DFS) (HR = 1.71, 95% CI: 1.29-2.26, P = 0.0002), and cancer-specific survival (OR= 1.93, 95% CI: 1.42-2.62, P <0.0001). F. nucleatum levels were related with T3-T4 stage (OR = 2.20, 95% CI: 1.66-2.91, P < 0.00001), M1 stage (OR = 2.11, 95% CI: 1.25-3.56, P = 0.005), poor tumor differentiation (OR = 1.83, 95% CI: 1.11-3.03, P =0.02), microsatellite instability-high (OR = 2.53, 95% CI: 1.53-4.20, P = 0.0003), and KRAS mutation (OR =1.27, 95% CI: 1.00-1.61, P=0.05) showed.
Conclusions: High levels of F. nucleatum suggest a poor prognosis and are associated with tumor growth, distant metastasis, poor differentiation, MSI-high, and KRAS mutation in CRC patients.
Keywords: Colorectal cancer, Fusobacterium nucleatum
Introduction
Colorectal cancer (CRC) is the third most frequent malignant tumor with 1.85 million new cases per year around the world based on the statistics in 2019 of the International Agency for Research on Cancer (IARC) of the World Health Organization (WHO) 1. Although great progress has been made in the comprehensive treatment of CRC such as surgery, chemoradiotherapy, anti-angiogenesis, and immunotherapy, the 5-year survival rate is only 10%-20% for CRC patients with distant metastases 2. Therefore, to discover risk factors related to the prognosis is important in improving the survival of CRC patients. Previous studies have reported that epigenetic changes, genetic mutations, chronic inflammation, lifestyle and diet are important factors for the survival of CRC patients 2. Recently, some studies found that intestinal microecological balance is also tight related to the prognosis of CRC 3. A study of Johnson. C et al. showed that colonic mucosal biofilms produced by intestinal flora may affect the development and progression of cancer as a regulator of cellular proliferation and colon cancer growth 4. However, the role of gut microbiota in CRC is still not entirely clear due to its complexity.
Fusobacterium nucleatum (F. nucleatum) is a kind of gram-negative bacilli in the gastrointestinal tract with the ability to express important biofilm tissue behaviors and interactions with host cells through the expression of numerous adhesins 5. Previous studies have proven that it may have a close relationship with occurrence and metastasis of CRC 5-7. A former clinical cohort study demonstrated that high levels of F. nucleatum could be CRC biomarker for prognosis and is associated with a higher CRC-specific mortality 7. On the contrary, some of them indicated that there is no significant correlation between F. nucleatum levels and the prognosis of CRC, especially in the analysis of the clinical survival rate 8. Overall, the correlation between high abundance of F. nucleatum and the clinical and prognosis characteristics of CRC is still controversial.
In view of the above controversial statements, here we investigated this study with an integrated large sample size to clear the prognostic role of F. nucleatum infection in patients with CRC.
Methods
Data sources
According to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA Statement) 9, we searched and accessed relevant articles published up to April 7, 2020 through PubMed, Web of Science, Medline, PMC and Embase using MeSH. Key terms included the following: “Fusobacterium nucleatum”, “Fusobacterium spp”, “Fn”, “Colorectal Neoplasm(s)”, “Colorectal Tumor(s)”, “Colorectal Carcinoma(s)”, “Colorectal Cancer(s)”, “prognosis”, “Prognoses” and “Prognostic Factor(s)”.
Inclusion criteria
In this meta-analysis, the criteria were showed as following for included eligible studies: (1) The articles were original articles, (2) All included studies were controlled clinical studies of CRC patients with complete data, (3) The diagnosis of CRC was based on histology, (4) Subjects of studies were human, and the experimental samples were tumor tissues and surrounding tissues after surgical resection, (5) In the included studies, the DNA content of F. nucleatum in tissues was detected by quantitative polymerase chain reaction (PCR) or 16S ribosomal RNA (16S rRNA) sequencing or other detection methods, and cases were divided into low level and high level for the study according to the median cut-off point amount(2-ΔCt) of detectable fusobacteria DNA. The review articles, meeting minutes, letters, and only abstracts were excluded in this meta-analysis to ensure that original data was obtained. For studies with the same research team or with overlapping subjects, we selected the articles with the most comprehensive data.
Data Extraction
Data for each study were extracted by two independent reviewers, H.F and J. P, and verified using predefined standards. When there were disagreements between two reviewers, it was decided by the third reviewer. The data of the included articles collected were as follows: the first author's name, the year of publication, patient ethnicity, date of birth, sample size for different types (F. nucleatum-high/ F. nucleatum-low), sample type, diagnostic techniques of F. nucleatum, the tumor-node-metastasis (TNM) stage, tumor-associated genes type ( KRAS mutation and BRAF mutation) and microsatellite instability (MSI), survival analysis, and follow-up time. The Engauge Digitizer 4.1 (http://digitizer.sourceforge.net/) was used to extract survival data from Kaplan-Meier curves if the detailed odds ratios (OR) and 95% confidence intervals (CIs) for survival were not directly stated in studies. 10 Multivariate analysis were used if both a univariate and multivariate analysis stated in the same comparison. Any discrepancies were discussed and resolved by consensus. The Newcastle-Ottawa scale (NOS) was used to grade articles and ensure the quality of the included studies 10. The included domains were as follows: the adequacy of case definition, representativeness of the cases, number of cases, ascertainment of exposure, detection method and cutoff, assessment of outcome, and adequate follow-up. A higher score indicates a better methodological quality.
Statistical Analysis
The meta-analysis was performed by means of Review Manager 5.3 (Cochrane Collaboration, Oxford, UK). As for dichotomous variables, the ORs were calculated, reporting 95% CI. Survival outcomes were summarized by using the generic inverse variance method. Overall survival (OS) was defined as the time from diagnosis until death. Disease-free survival (DFS) was defined as the interval between the initial primary diagnosis of CRC and the first relapse or death. Hazard ratio (HR) and 95% CI were calculated to assess the association between high level of F. nucleatum and survival. A fixed model was performed in aggregating and analyzing for results when I2< 50%. If I2 >50%, the random-effects analysis was utilized. The pooled effects were determined by conducting a Z test, and the statistical significance was defined as the two-sided P < 0.05.
Results
Flow Diagram of the Studies Retrieved for the Review
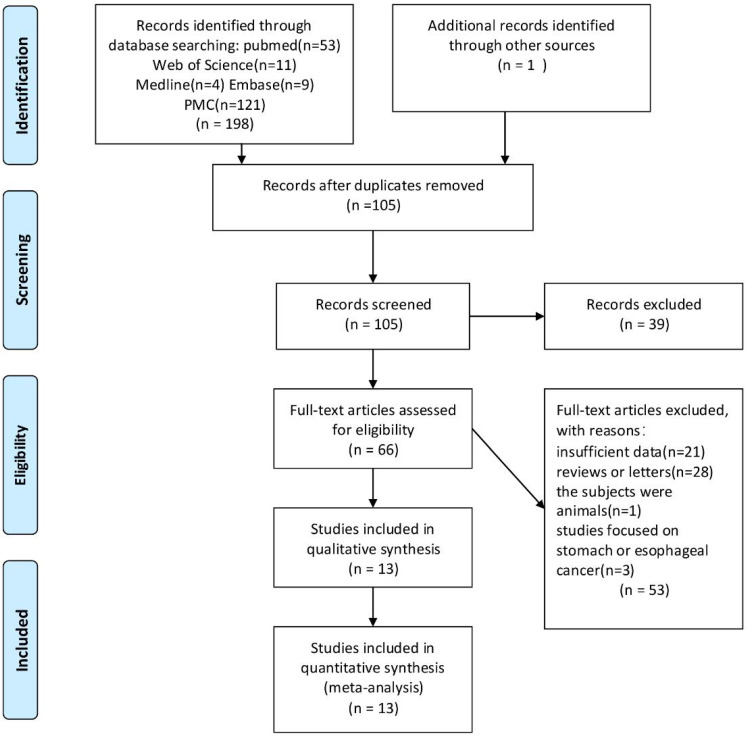
All 198 articles were identified through PubMed, Web of Science, Medline, PMC and Embase searching after filtration. Among them, 94 articles were duplicated, 39 articles were excluded because of unmatched titles and abstracts, 53 articles were excluded from reading full texts (because of inappropriate objects and incomplete data). The resultant 13 articles 7-8, 11-21 involved 3,690 CRC patients and were included into the meta-analysis. Figure 1 reveals the flowchart of study selection.
Figure 1.
Flow diagram of study selection process.
Baseline Characteristics of Included Studies
Table 1 summarizes the main characteristics of the included studies. The included cases originated from 8 countries among North America, Asia, and Europe. All the specimens were tumor tissues after surgical resection. The most commonly used test method for F. nucleatum was the quantitative polymerase chain reaction (qPCR) 7, 12-15, 18-21, and the 16S rRNA method was used in three studies 8, 11, 16 and the droplet digital PCR in one study 17. All cases described in the retrieved articles were divided into two groupsbased on expression level of F. nucleatum DNA. There were 1, 796 in the high-level group and 1, 894 in the low-level group.
Table 1.
Characteristics of the included studies
| Author | Year | Country | No.of patients | Age(years) | Data collection | Specimens | Test methods |
Cut-off value | Stage | Analysis index | Follow-up months | Analysis methods | NOS score | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (mean or median) | |||||||||||||||
| Fn-high | Fn-low | Fn-high | Fn-low | ||||||||||||
| Yanglong Chen | 2019 | China | 35 | 66 | 58.72±12.638 | 58.52±10.421 | Retro | tissues | qPCR | 2-ΔCt | II~III | OS | 120 | MA | 5 |
| Kosuke Mima | 2016 | America | 67 | 917 | 68.8 ± 8.3 | 69.2 ± 8.9 | Pro | tissues | qPCR | 2-ΔCt | I~IV | OS, CSS | 120 | MA, UA | 7 |
| Andrew T. Kunzmann | 2019 | CzechRepublic | 61 | 129 | NR | NR | Retro | tissues | qPCR | 2-ΔCt | I~IV | OS | 72 | UA | 5 |
| Katsuhiko Nosho | 2016 | Japan | 44 | 463 | 65.0 ± 12.1 | 67.3 ± 11.7 | Retro | tissues | qPCR | NR | I~IV | NR | NR | NR | 4 |
| Hyeon Jeong Oh | 2019 | South Korea | 204 | 389 | >18 | >18 | Retro | tissues | qPCR | 2-ΔCt | II~III | DFS | 120 | MA | 6 |
| Yuan Sun | 2016 | China | 118 | 34 | 28-84 | 28-84 | Retro | tissues | qPCR | 2-ΔCt | I~IV | OS | 60 | UA | 5 |
| Zhiliang Wei | 2016 | China | 90 | 90 | NR | NR | Retro | tissues | 16sRNA | 0.52% | I~IV | OS, DFS | 36 | MA | 5 |
| Yuko Yamaoka | 2017 | Japan | 50 | 50 | 62.5 ± 9.8 | 63.9 ± 14.5 | Retro | tissues | droplet digital PCR | NR | I~IV | OS | 120 | UA | 6 |
| Xuebing Yan | 2017 | China | 187 | 93 | NR | NR | Retro | tissues | qPCR | 2-ΔCt | I~IV | CSS, DFS | 60 | MA, UA | 6 |
| Yongyu Chen | 2017 | China | 61 | 27 | 58 | 59.4 | Retro | tissues | 16sRNA, FISH | NR | I~IV | NR | NR | NR | 4 |
| Bundgaard-Nielsen | 2019 | Denmark | 29 | 60 | 71 ±10.1 | 71 ±10.1 | Retro | tissues | 16sRNA | NR | I~IV | OS, DFS | 96 | UA | 5 |
| Dae-Won Lee | 2018 | Korea | 64 | 64 | NR | NR | Retro | tissues | qPCR | 2-ΔCt | II~III | OS, DFS | 96 | MA | 6 |
| Ana Carolina de Carvalho | 2019 | Brasil | 18 | 134 | 60.63 ± 13.7 | 60.63 ± 13.7 | Retro | tissues | qPCR | 2-ΔCt | I~IV | OS, CSS | 96 | UA | 5 |
Pro prospective, Retro retrospective. CRC colorectal cancer. qPCR quantitative polymerase chain reaction, 16sRNA 16S ribosomal RNA sequencing, droplet digital PCR polymerase chain reaction polymerase chain reaction, qrT-PCR quantitative reverse transcription polymerase chain reaction, FISH fluorescence in situ hybridization. NR not report. OS overall survival, DFS disease-free survival, MA multivariate analysis, UA univariate analysis.
Association between F. nucleatum levels and prognosis of CRC patients
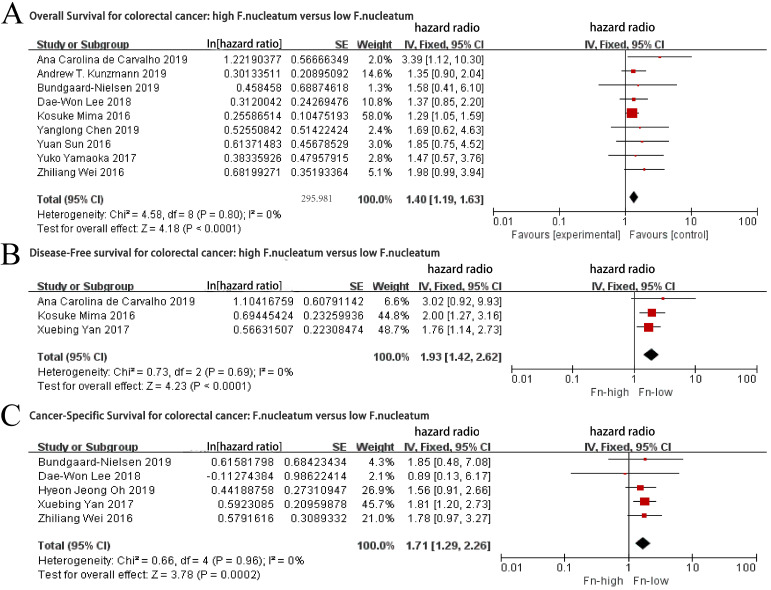
As the role of F. nucleatum in the prognosis of CRC patients is still controversial, we first analyzed the relationship between F. nucleatum levels and the prognosis of patients with CRC. Nine studies 7-8, 12, 15-17, 19-21 reporting on a total of 2158 patients were pooled for analysis of the association between the F. nucleatum levels and OS. The fixed-effects model was performed as a consequence of low heterogeneity. As shown in Figure 2A, worse OS were revealed in the CRC patients with high F. nucleatum levels (HR= 1.40, 95% CI: 1.40-1.63, P < 0.0001), without significant inter-study heterogeneity (I2 = 0%, P = 0.80).
Figure 2.
Forest plot for: a. Overall Survival outcomes for colorectal cancer with high level of F.nuleatum versus low level of F.nuleatum; b. Disease-Free Survival outcomes for colorectal cancer with high level of F.nuleatum versus low level of F.nuleatum; c. Cancer-Specific Survival outcomes for colorectal cancer with high level of F.nuleatum versus low level of F.nuleatum.
Data from five articles 8, 14, 16, 18, 20 with a total of 1270 patients were pooled for analysis of the relationship between the levels of F. nucleatum and DFS. The fixed-effects model was applied as a consequence of low heterogeneity (I2= 0%, P = 0.96). Our results demonstrated that the CRC patients with high levels of F. nucleatum had a worse DFS than those with low levels of F. nucleatum (HR = 1.71, 95% CI: 1.29-2.26, P = 0.0002, Figure 2B).
A total of 1498 patients in three studies 7, 18, 21 were included to examine the association between the levels of F. nucleatum and cancer-specific survival (CSS). In this analysis, there was a significant association between high levels of F. nucleatum and poor CSS (HR = 1.93, 95% CI: 1.42-2.62 P <0.0001), with a low heterogeneity (I2= 0%, P = 0.69, Figure 2C) through the application of the fixed-effects model.
Association between high levels of F. nucleatum and CRC Clinical Characteristics
As listed in Table 2, data from eleven articles 7, 11-19, 21 included 3413 patients were pooled for analysis of the correlation between the levels of F. nucleatum and primary tumor site in a random-effects model (I2 = 60%, P=0.005). The results suggested that there was no correlation between F. nucleatum infection and tumor site (OR = 1.26, 95% CI: 0.91-1.75, P = 0.17, supplemental Figure 1A).
Table 2.
Association between high F.nuleatum abundance and Clinical characteristics of patients with CRC
| Factors | No. of studies | No. of patients | Pooled OR (95%CI) | P-value | Heterogenelty | Statistical method | |
|---|---|---|---|---|---|---|---|
| I2 (%) | P-value | ||||||
| Tumor side | 11 | 3413 | 1.26 (0.91, 1.75) | 0.17 | 60% | 0.005 | Random |
| TNM Stage | 9 | 3758 | 1.20 (0.96, 1.51) | 0.11 | 25% | 0.22 | Fixed |
| T stage | 8 | 2528 | 2.20 (1.66, 2.91) | <0.00001 | 0% | 0.65 | Fixed |
| N stage | 8 | 2445 | 1.27 (0.98, 1.64) | 0.07 | 37% | 0.14 | Fixed |
| M stage | 3 | 560 | 2.11 (1.25, 3.56) | 0.005 | 0% | 0.95 | Fixed |
| Differentation | 8 | 2118 | 1.83 (1.11, 3.03) | 0.02 | 60% | 0.01 | Random |
A total of 3758 patients in nine studies 7, 11-17, 21 were pooled to examine the association between the levels of F. nucleatum and TNM stage (supplemental Figure 1B). High abundance of F. nucleatum were not associated with the overall TNM stage of CRC (OR= 1.20, 95% CI: 0.96-1.51, P = 0.11), with low heterogeneity (I2 = 25%, p = 0.22). However, high levels of F. nucleatum were correlated with high T stages (T3-T4) (OR = 2.20, 95% CI: 1.66-2.91, P < 0.00001) and M (M1) (OR = 2.11, 95% CI: 1.25-3.56, P = 0.005) stage, without heterogeneity (I2 = 0%). Eight studies reporting on a total of 1445 patients revealed that high F. nucleatum levels were not correlated with N stage (OR = 1.27, 95% CI: 0.98-1.64, P = 0.07), with low heterogeneity (I2 = 37%) (supplemental Figure 1C-E). Therefore, our results revealed that there was a relationship between high level of F. nucleatum and large tumor size and distant metastases in CRC.
Furthermore, eight studies 7, 11, 15-19, 21 with a total of 2118 patients reported the association between the levels of F. nucleatum and tumor differentiation (supplemental Figure 1F). As shown in Table 2, high levels of F. nucleatum were significantly associated with poor tumor differentiation (OR = 1.83, 95% CI: 1.11-3.03, P = 0.02) in CRC patients, with high heterogeneity (I2 = 60%).
Association Between high levels of F. nucleatum and molecular characteristics in CRC
In order to further reveal the association between F. nucleatum infection and CRC progression, we analyzed the association between F. nucleatum levels and tumor-specific molecular characteristics. As shown in Table 3, data from six studies 7, 11-14, 21 with 2520 patients demonstrated that high levels of F. nucleatum were significantly associated with MSI-high type CRC (OR = 2.53, 95% CI: 1.53-4.20, P = 0.0003), although with a high heterogeneity (I2 = 83%, P < 0.0001) (supplemental Figure 2A). The correlation between high F. nucleatum levels and KRAS mutation was also found in the fixed-effects model with low heterogeneity (I2 = 28%, P = 0.23) in six studies 7,11-14, 17 with a total of 2404 patients. The OR was 1.27 with a 95% CI of 1.00-1.61 (P = 0.05) (supplemental Figure 2B).
Table 3.
Association between high F.nuleatum abundance and molecular characteristic of patients with CRC
| Factors | No. of studies | No. of patients | Pooled OR (95%CI) | P-value | Heterogenelty | Statistical method | |
|---|---|---|---|---|---|---|---|
| I2 (%) | P-value | ||||||
| MSI | 6 | 2520 | 2.53 (1.53, 4.20) | 0.0003 | 83% | <0.0001 | Random |
| KRAS | 6 | 2404 | 1.27 (1.00, 1.61) | 0.05 | 28% | 0.23 | Fixed |
| BRAF | 6 | 2499 | 1.93 (0.91, 4.11) | 0.09 | 65% | 0.01 | Random |
| MLH1 | 3 | 1211 | 0.78 (0.06, 9.39) | 0.84 | 94% | <0.0001 | Random |
| PIK2CA | 3 | 1603 | 1.21 (0.74, 1.97) | 0.45 | 0% | 0.86 | Fixed |
MSI microsatellite instability, KRAS k-ras gene mutation, BRAF b-raf gene mutation, MLH1 MLH1 hypermethylation, PIK2CA PIK3CA gene mutation.
Six studies 7, 11-14, 21 with a total of 2499 patients were pooled for analysis of the association between the levels of F. nucleatum and BRAF mutation. Our results demonstrated that high levels of F. nucleatum were not associated with BRAF mutation in CRC patients (OR = 1.93, 95% CI: 0.91-4.11, P = 0.09) (supplemental Figure 2C). In addition, our pool results with three studies7, 17, 21 with 1085 patients found that high F. nucleatum levels in CRC tissue had no correlation with MLH1 hypermethylation (OR= 0.78, 95% CI: 0.06-9.93, P = 0.84) (supplemental Figure 2D). A total of 1603 patients in three studies 7, 12, 13 were pooled for analysis of the association between high F. nucleatum levels and PIK3CA mutation through the fixed-effects model, and there was no correlation between and PIK3CA mutation in CRC (OR = 1.21, 95% CI: 0.74-1.97, P = 0.45) (supplemental Figure 2E).
Sensitivity Analysis
To assess the impact of a single study on the overall meta-analysis, included studies detecting F. nucleatum by quantitative reverse transcription polymerase chain reaction (qPCR) were selected to perform sensitivity analysis (Table 4). The results of the sensitivity analysis are summarized in Table 4. The similar results of those all studies together were revealed in this analyze by using qPCR, including the relationship between F. nucleatum and tumor side, TNM Stage, T stage, N stage, KRAS mutation, OS and DFS in CRC.
Table 4.
Sensitivity analysis of studies evaluated F.nuleatum on clinicopathological characteristics of CRC
| Factors | No. of studies | No. of patients | Pooled OR (95%CI) | P-value | Heterogenelty | Statistical method | |
|---|---|---|---|---|---|---|---|
| I2 (%) | P-value | ||||||
| Tumor side | 8 | 3035 | 1.17 (0.77, 1.77) | 0.47 | 67% | 0.003 | Random |
| TNM Stage | 7 | 2662 | 1.05 (0.80~1.36) | 0.74 | 2% | 0.41 | Fixed |
| T stage | 6 | 2249 | 2.14 (1.56, 2.93) | <0.00001 | 0% | 0.46 | Fixed |
| N stage | 5 | 2067 | 1.01 (0.74, 1.38) | 0.94 | 0% | 0.43 | Fixed |
| OS | 5 | 1789 | 1.36 (1.16, 1.61) | 0.0002 | 0% | 0.63 | Fixed |
| DFS | 3 | 1119 | 1.68 (1.22, 2.32) | 0.002 | 0% | 0.74 | Fixed |
OS overall survival, DFS disease-free survival.
Risk of Bias
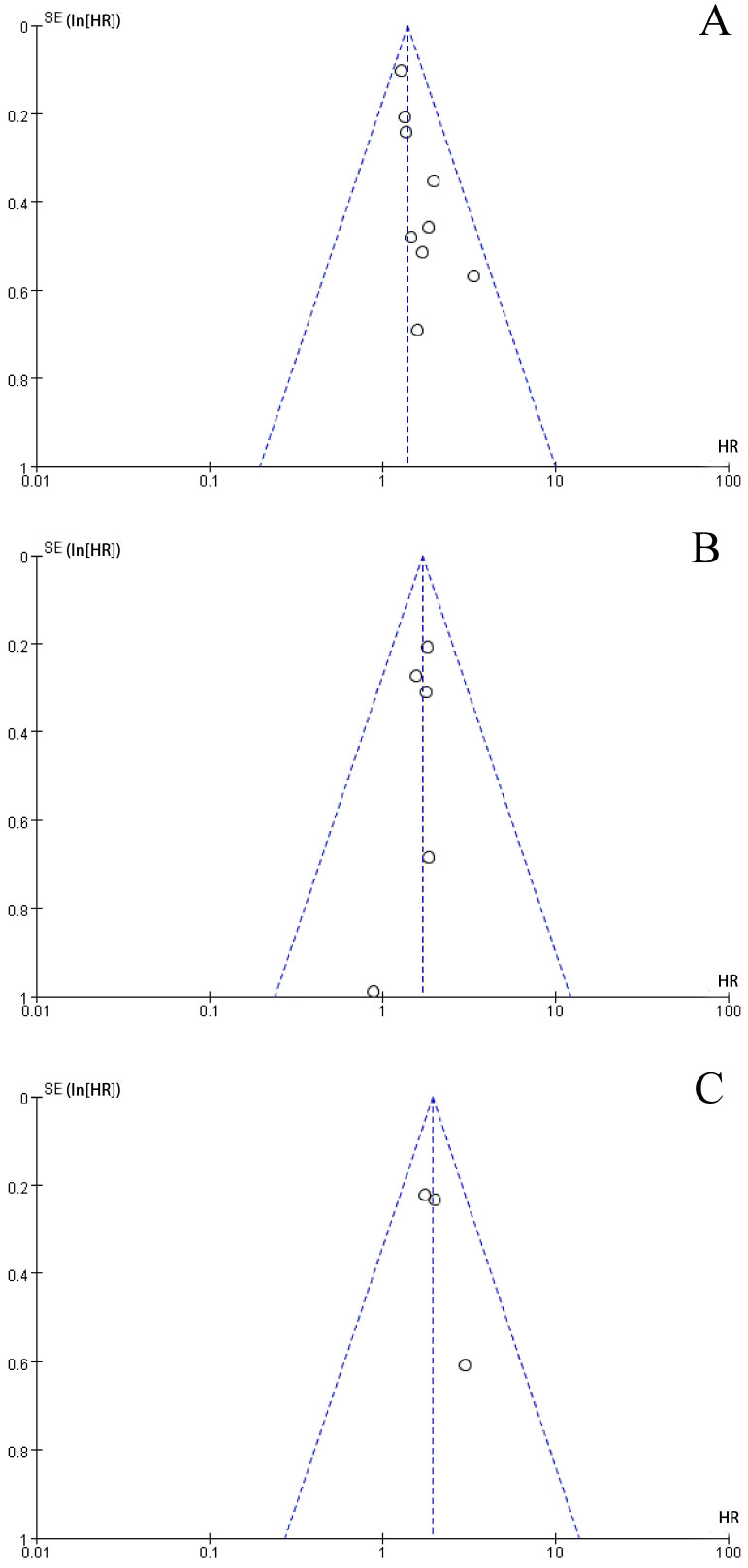
The funnel plot was performed to assess publication bias. As shown in Figure 3, the shape of the funnel plots of the main results were roughly symmetrical, without obvious evidence of asymmetry. The funnel plots for main outcomes including OS, DFS, and CSS demonstrated no evidence of publication bias in our study.
Figure 3.
Funnel plots for: a. Overall Survival outcomes for colorectal cancer with high level of F.nuleatum versus low level of F.nuleatum; b. Disease-Free Survival outcomes for colorectal cancer with high level of F.nuleatum versus low level of F.nuleatum; c. Cancer-Specific Survival outcomes for colorectal cancer with high level of F.nuleatum versus low level of F.nuleatum.
Discussion
This meta-analysis demonstrated that high levels of F. nucleatum are closely related to poor prognosis of CRC patients including OS, DFS, and CSS. Additionally, the correlation between the clinicopathological features of CRC such as tumor site, clinical stage, and tumor differentiation were also observed. We also analyzed the correlation between high levels of F. nucleatum and molecular characteristics of CRC such as MSI and KRAS, BRAF, and PIK2CA mutations, as well as MLH1 hypermethylation. Our results confirmed that high levels of F. nucleatum were significantly associated with MSI-high type and KRAS mutation of CRC. Although previous meta-analyses have reported the carcinogenesis and diagnostic value of F. nucleatum for CRC, studies on the prognosis of F. nucleatum in CRC are few 22,23,24. To the best of our knowledge, this is the first meta-analysis demonstrating the association between F. nucleatum and the clinical and molecular characteristics of CRC, and it is the most comprehensive meta-analysis clarifying the prognostic role of F. nucleatum levels in CRC.
Our results showed a correlation between abundance of F. nucleatum and poor OS in CRC patients. This is similar with a previous study 23, which reports that high level of F. nucleatum in the tumor tissue was associated with poorer OS in CRC patients. However, they found that infection of F. nucleatum was not associated with the DFS and CSS in CRC patients. This was contrary to our study findings. Many evidences have shown that F. nucleatum is associated with CRC development. As a conditional pathogen, F. nucleatum has a high detection rate in metastatic CRC lesions 24.
This is the first meta-analysis demonstrating that high levels of F. nucleatum in CRC tissues were not associated with the tumor side. This contradicts a previous study 25 which reported that the proportion of F. nucleatum-high colorectal cancers gradually increases from the rectum to the cecum. However, another study 14 reported that high F. nucleatum levels had no correlation with the tumor side in CRC patients. Therefore, as F. nucleatum is a critical cancer-promoting factor, our results provided a more convincing evidence to confirm this.
Our results revealed that F. nucleatum had no correlation with the overall TNM stage, but high levels of F. nucleatum were associated with high T and M stages of CRC. Previous experiments had shown that F. nucleatum can activate autophagy pathway-1 by up-regulating CARD3 expression, leading to distant metastasis of tumors 26. Moreover, F. nucleatum can accomplish a series of pathogenic effects by changing the permeability of vascular endothelium 27. This means that F. nucleatum promotes CRC proliferation and distant metastasis via hematogenous metastasis. For more mechanisms, FadA protein, an adhesion molecule of F. nucleatum, can bind to wnt7b E-cadherin on CRC cells and promote F. nucleatum adhesion and invasion of host epithelial cells. Then, F. nucleatum activates β-catenin signaling that regulates expression of related oncogenes and promotes colorectal cancer cell proliferation 28. Moreover, F. nucleatum promotes the expression of several cytokines, such cytokines as IL-6, IL-8, and IL-18, and lead to a proinflammatory microenvironment in CRC which accelerates CRC growth and metastasis 28. Although our meta-analysis results showed a marginal association of high F. nucleatum levels with the higher N stage of CRC, more evidence is needed to clarify the role of F. nucleatum in lymphatic metastasis of CRC. We also found that high levels of F. nucleatum were associated with poorly differentiated of CRC. This was also a result confirmed that F. nucleatum is not only associated with carcinogenesis, but also with poor CRC differentiation.
The accumulation of genetic and epigenetic alterations, influenced from microbial and other environmental exposures and host responses to these exposures, these are all important factors affecting the development of CRC 29. The current study have revealed that high levels of F. nucleatum were related with key tumor molecular features of CRC, including MSI-high and KRAS mutation which have been associated with clinical outcome in advanced CRC. The present data indicate a significant correlation between high F. nucleatum levels and MSI-high from six studies of 2520 patients. MSI status has been proven as a critical predictor for prognosis, and response to chemotherapy or immunotherapy in CRC patients 30-32. A previous study reported the relationship between F. nucleatum and the immune response to CRC by different MSI statuses 33, suggesting that high F. nucleatum levels correlated with MSI status and regulated the antitumor immune response in CRC. Moreover, KRAS mutation also is an important molecular feature for chemotherapy resistance in CRC 34 and high F. nucleatum levels in CRC tissues will cause chemoresistance. Therefore, our results further confirmed this correlation implying that F. nucleatum is another underlying biomarker for the response to immunotherapy and chemotherapy. There was no significant association between high levels of F. nucleatum and mutations of BRAF and PIK2CA, as well as MLH1 hypermethylation in CRC tissues. These results agree with previous studies conducted in single populations, suggesting a role of F. nucleatum mainly with specific mutations in CRC. A more in-depth study of the association between the F. nucleatum levels and other mutations of CRC such as TP53, AKT1, PTEN, and so on, can reveal more biological roles of F. nucleatum in CRC.
Our meta-analysis had some limitations. Firstly, the number of the included studies was relatively small and they were only English studies. This could have resulted in publication bias. Secondly, although sensitivity analysis had been conducted and our results were further confirmed, most of the included studies used qPCR to measure F. nucleatum levels and few used 16S rRNA sequencing. The different methods and cut-off values may cause heterogeneity in some results. Lastly, there were some included studies without reported HRs and 95% CIs in the prognostic outcomes. We attempted to extract survival data from Kaplan-Meier curves according to the previous reported method 10. This may have impacted the precision of the prognostic outcomes of F. nucleatum in CRC.
Despite these shortcomings, there is sufficient evidence to suggest that CRC with high F. nucleatum levels are at high risk of poor prognosis, including OS, DFS, and CSS. Our results also suggest that high F. nucleatum levels are correlated with tumor growth, distant metastasis, poor differentiation, MSI-high, and KRAS mutation in CRC. Future research on the relationship between F. nucleatum and other clinical and molecular characteristics in CRC should be assessed. In addition, understanding more mechanisms of F. nucleatum in the progression of CRC, and whether antibiotic therapy targeting F. nucleatum will help to prolong the prognosis of patients with CRC, will facilitate the identification of more treatment strategies in CRC.
Supplementary Material
Supplementary materials.
Acknowledgments
Funding
This work was supported by the funding of Guangdong Basic and Applied Basic Research Fund Project (2019A1515110543) and Guangdong Medical Science and Technology Research Fund Project (20171026169569).
Authors' contributions
SH participated in data analysis and wrote the manuscript. HJ and JP reviewed the manuscript. All authors read and approved the manuscript.
Consent for publication
Written informed consent to publish the clinical data was obtained from the patient before the initiation of the study.
Abbreviations
- F. nucleatum
Fusobacterium. nucleatum
- CRC
colorectal cancer
- qPCR
quantitative polymerase chain reaction
- 16sRNA
16S ribosomal RNA sequencing
- qrT-PCR
quantitative reverse transcription polymerase chain reaction
- OS
overall survival
- DFS
disease-free survival
- CSS
cancer-specific survival
- MSI
microsatellite instability
- KRAS
k-ras gene mutation
- BRAF
b-raf gene mutation
- MLH1
MLH1 hypermethylation
- PIK2CA
PIK3CA gene mutation
References
- 1.Rindi G, Klimstra DS, Abedi-Ardekani B, Asa SL, Bosman FT, Brambilla E. et al. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 2018;31:1770–86. doi: 10.1038/s41379-018-0110-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: a cancer journal for clinicians. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 3.Sears CL, Garrett WS. Microbes, microbiota, and colon cancer. Cell host & microbe. 2014;15:317–28. doi: 10.1016/j.chom.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo L, Shokeen B, He X, Shi W, Lux R. Streptococcus mutans SpaP binds to RadD of Fusobacterium nucleatum ssp. polymorphum. Molecular oral microbiology. 2017;32:355–64. doi: 10.1111/omi.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kostic AD, Gevers D, Pedamallu CS, Michaud M, Duke F, Earl AM. et al. Genomic analysis identifies association of Fusobacterium wth colorectal carcinoma. Genome research. 2012;22:292–8. doi: 10.1101/gr.126573.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bullman S, Pedamallu CS, Sicinska E, Clancy TE, Zhang X, Cai D. et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science (New York, NY) 2017;358:1443–8. doi: 10.1126/science.aal5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mima K, Nishihara R, Qian ZR, Cao Y, Sukawa Y, Nowak JA. et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut. 2016;65:1973–80. doi: 10.1136/gutjnl-2015-310101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bundgaard-Nielsen C, Baandrup UT, Nielsen LP, Sørensen S. The presence of bacteria varies between colorectal adenocarcinomas, precursor lesions and non-malignant tissue. BMC cancer. 2019;19:399. doi: 10.1186/s12885-019-5571-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y, Lu Y, Ke Y, Li Y. Prognostic impact of the Fusobacterium nucleatum status in colorectal cancers. Medicine. 2019;98:e17221. doi: 10.1097/MD.0000000000017221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kunzmann AT, Proença MA, Jordao HW, Jiraskova K, Schneiderova M, Levy M. et al. Fusobacterium nucleatum tumor DNA levels are associated with survival in colorectal cancer patients. European journal of clinical microbiology & infectious diseases: official publication of the European Society of Clinical Microbiology. 2019;38:1891–9. doi: 10.1007/s10096-019-03649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nosho K, Sukawa Y, Adachi Y, Ito M, Mitsuhashi K, Kurihara H. et al. Association of Fusobacterium nucleatum with immunity and molecular alterations in colorectal cancer. World journal of gastroenterology. 2016;22:557–66. doi: 10.3748/wjg.v22.i2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oh HJ, Kim JH, Bae JM, Kim HJ, Cho NY, Kang GH. Prognostic Impact of Fusobacterium nucleatum Depends on Combined Tumor Location and Microsatellite Instability Status in Stage II/III Colorectal Cancers Treated with Adjuvant Chemotherapy. Journal of pathology and translational medicine. 2019;53:40–9. doi: 10.4132/jptm.2018.11.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun Y, An QM, Tian XY, Wang ZL, Hao CY. Fusobacterium nucleatum infection is correlated with tumor metastasis and postoperative survival of colorectal cancer patients in China. Translational Cancer Research. 2016;5:579–88. [Google Scholar]
- 16.Wei Z, Cao S, Liu S, Yao Z, Sun T, Li Y. et al. Could gut microbiota serve as prognostic biomarker associated with colorectal cancer patients' survival? A pilot study on relevant mechanism. Oncotarget. 2016;7:46158–72. doi: 10.18632/oncotarget.10064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamaoka Y, Suehiro Y, Hashimoto S, Hoshida T, Fujimoto M, Watanabe M. et al. Fusobacterium nucleatum as a prognostic marker of colorectal cancer in a Japanese population. Journal of gastroenterology. 2018;53:517–24. doi: 10.1007/s00535-017-1382-6. [DOI] [PubMed] [Google Scholar]
- 18.Yan X, Liu L, Li H, Qin H, Sun Z. Clinical significance of Fusobacterium nucleatum, epithelial-mesenchymal transition, and cancer stem cell markers in stage III/IV colorectal cancer patients. OncoTargets and therapy. 2017;10:5031–46. doi: 10.2147/OTT.S145949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, Peng Y, Yu J, Chen T, Wu Y, Shi L. et al. Invasive Fusobacterium nucleatum activates beta-catenin signaling in colorectal cancer via a TLR4/P-PAK1 cascade. Oncotarget. 2017;8:31802–14. doi: 10.18632/oncotarget.15992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee DW, Han SW, Kang JK, Bae JM, Kim HP, Won JK. et al. Association Between Fusobacterium nucleatum, Pathway Mutation, and Patient Prognosis in Colorectal Cancer. Annals of surgical oncology. 2018;25:3389–95. doi: 10.1245/s10434-018-6681-5. [DOI] [PubMed] [Google Scholar]
- 21.de Carvalho AC, de Mattos Pereira L, Datorre JG, Dos Santos W, Berardinelli GN, Matsushita MM. et al. Microbiota Profile and Impact of Fusobacterium nucleatum in Colorectal Cancer Patients of Barretos Cancer Hospital. Frontiers in oncology. 2019;9:813. doi: 10.3389/fonc.2019.00813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nosho K, Sukawa Y, Adachi Y, Ito M, Mitsuhashi K, Kurihara H. et al. Association of Fusobacterium nucleatum with immunity and molecular alterations in colorectal cancer. World journal of gastroenterology. 2016;22:557–66. doi: 10.3748/wjg.v22.i2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gethings-Behncke C, Coleman HG, Jordao HWT, Longley DB, Crawford N, Murray LJ. et al. Fusobacterium nucleatum in the Colorectum and Its Association with Cancer Risk and Survival: A Systematic Review and Meta-analysis. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2020;29:539–48. doi: 10.1158/1055-9965.EPI-18-1295. [DOI] [PubMed] [Google Scholar]
- 24.Colov EP, Degett TH, Raskov H, Gögenur I. The impact of the gut microbiota on prognosis after surgery for colorectal cancer - a systematic review and meta-analysis. APMIS: acta pathologica, microbiologica, et immunologica Scandinavica. 2020;128:162–76. doi: 10.1111/apm.13032. [DOI] [PubMed] [Google Scholar]
- 25.Mima K, Cao Y, Chan AT, Qian ZR, Nowak JA, Masugi Y. et al. Fusobacterium nucleatum in Colorectal Carcinoma Tissue According to Tumor Location. Clinical and translational gastroenterology. 2016;7:e200. doi: 10.1038/ctg.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y, Chen Y, Zhang J, Cao P, Su W, Deng Y. et al. Fusobacterium nucleatum Promotes Metastasis in Colorectal Cancer by Activating Autophagy Signaling via the Upregulation of CARD3 Expression. Theranostics. 2020;10:323–39. doi: 10.7150/thno.38870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fardini Y, Wang X, Témoin S, Nithianantham S, Lee D, Shoham M. et al. Fusobacterium nucleatum adhesin FadA binds vascular endothelial cadherin and alters endothelial integrity. Molecular microbiology. 2011;82:1468–80. doi: 10.1111/j.1365-2958.2011.07905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell host & microbe. 2013;14:195–206. doi: 10.1016/j.chom.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawakami H, Zaanan A, Sinicrope FA. Microsatellite instability testing and its role in the management of colorectal cancer. Current treatment options in oncology. 2015;16:30. doi: 10.1007/s11864-015-0348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gavin PG, Colangelo LH, Fumagalli D, Tanaka N, Remillard MY, Yothers G. et al. Mutation profiling and microsatellite instability in stage II and III colon cancer: an assessment of their prognostic and oxaliplatin predictive value. Clinical cancer research: an official journal of the American Association for Cancer Research. 2012;18:6531–41. doi: 10.1158/1078-0432.CCR-12-0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang G, Zheng RY, Jin ZS. Correlations between microsatellite instability and the biological behaviour of tumours. Journal of cancer research and clinical oncology. 2019;145:2891–9. doi: 10.1007/s00432-019-03053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamada T, Zhang X, Mima K, Bullman S, Sukawa Y, Nowak JA. et al. Fusobacterium nucleatum in Colorectal Cancer Relates to Immune Response Differentially by Tumor Microsatellite Instability Status. Cancer immunology research. 2018;6:1327–36. doi: 10.1158/2326-6066.CIR-18-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu T, Guo F, Yu Y, Sun T, Ma D, Han J. et al. Fusobacterium nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell. 2017;170:548–63.e16. doi: 10.1016/j.cell.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taieb J, Balogoun R, Le Malicot K, Tabernero J, Mini E, Folprecht G. et al. Adjuvant FOLFOX +/- cetuximab in full RAS and BRAF wildtype stage III colon cancer patients. Annals of oncology: official journal of the European Society for Medical Oncology. 2017;28:824–30. doi: 10.1093/annonc/mdw687. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials.