Like other microbes that live on or in the human body, the bacteria that inhabit the upper respiratory tract, in particular the nasal cavity, have evolved to survive in an environment that presents a number of physical and chemical challenges; these microbes are constantly bombarded with nutritional fluctuations, changes in humidity, the presence of inhaled particulate matter (odorants and allergens), and competition with other microbes. Indeed, only a specialized set of species is able to colonize this niche and successfully contend with the host’s immune system and the constant threat from competitors.
KEYWORDS: commensal bacteria, nasal microbiome, polymicrobial interactions
ABSTRACT
Like other microbes that live on or in the human body, the bacteria that inhabit the upper respiratory tract, in particular the nasal cavity, have evolved to survive in an environment that presents a number of physical and chemical challenges; these microbes are constantly bombarded with nutritional fluctuations, changes in humidity, the presence of inhaled particulate matter (odorants and allergens), and competition with other microbes. Indeed, only a specialized set of species is able to colonize this niche and successfully contend with the host’s immune system and the constant threat from competitors. To this end, bacteria that live in the nasal cavity have evolved a variety of approaches to outcompete contenders for the limited nutrients and space; broadly speaking, these strategies may be considered a type of “bacterial warfare.” A greater molecular understanding of bacterial warfare has the potential to reveal new approaches or molecules that can be developed as novel therapeutics. As such, there are many studies within the last decade that have sought to understand the complex polymicrobial interactions that occur in various environments. Here, we review what is currently known about the age-dependent structure and interbacterial relationships within the nasal microbiota and summarize the molecular mechanisms that are predicted to dictate bacterial warfare in this niche. Although the currently described interactions are complex, in reality, we have likely only scratched the surface in terms of a true understanding of the types of interbacterial competition and cooperation that are thought to take place in and on the human body.
INTRODUCTION
THE COMPOSITION OF THE NASAL MICROBIOTA
The advent of high-throughput sequencing technology set the stage for researchers to challenge the dogma that most human body sites are sterile or have a low microbial burden. In reality, many human anatomical sites are home to microbial communities that represent billions of microbes; these communities are often incredibly genetically diverse and are collectively termed the microbiota (1, 2). While, overall, the specific roles for various species of a particular community are poorly understood, a subset of specific genera/species has been shown to aid the host with digestion, to synthesize important dietary compounds, and to act as the host’s primary defense against incoming pathogens (3, 4). While these phenomena and the associated microbes that live there have been best studied within the gastrointestinal tract, there is growing interest in the development of a greater understanding of the microbes that live in the nasal cavity.
This interest is partially driven by the understanding that the microbes that live within this niche must cope with a variety of stressors to survive and persist in this location. For example, the nasal cavity is a nutrient-poor, acidic, and salty environment (5, 6). Importantly, the microenvironment of the nasal cavity differs based on the exact anatomical location within the nose (Fig. 1a). For example, the anterior nares (nostrils), which lead to the nasal vestibule, are the most acidic and high-salinity portion of the nasal cavity, and therefore, this area is the most difficult for microbes to colonize within the nose (6, 7). Adjacent to the anterior nares lies the middle meatus, which makes up, by surface area, the largest portion of the nose (6). The middle meatus comprises a network of bone and mucosal folds and is also home to mucin-secreting goblet cells that are responsible for the production of the mucus that lines the nasal cavity. Nasal mucus is highly growth restrictive as a microbial food source since it contains only meager amounts of essential nutrients and metabolites such as glucose, iron, essential amino acids, and nucleotides (5, 6). Furthermore, mass spectrometric analysis of human nasal secretions indicates that the nasal mucus contains high concentrations of organic acids and inorganic salts, both of which can restrict microbial growth (5).
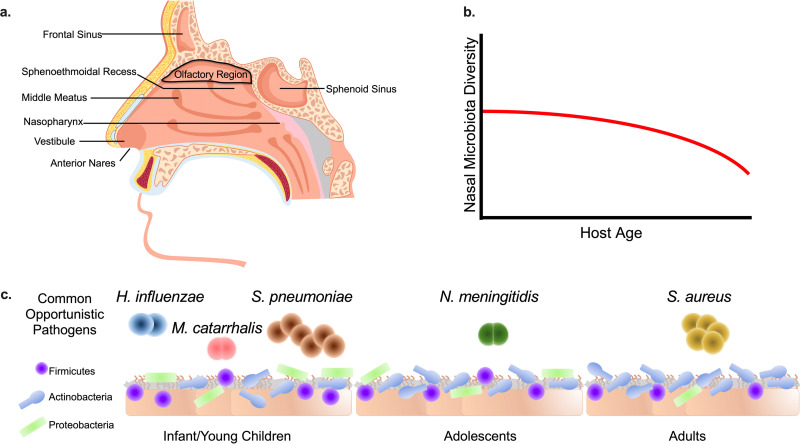
FIG 1.
Nasal microbiota composition and structure change throughout life. (a) Nasal cavity anatomy and typical microbiota sampling sites (anterior nares, middle meatus, sphenoethmoidal recess, and nasopharynx) (6, 7). The anterior nares are the entrance to the nasal cavity and lead directly to the vestibule. The middle meatus is adjacent to the vestibule, and the nasopharynx connects to the throat. The sphenoethmoidal recess is located within the posterior portion of the nasal cavity. The olfactory region is located at the ceiling of the nasal cavity. The frontal and sphenoid sinuses are located within the facial skeleton. (b) The diversity of the nasal microbiota is variable between individuals but tends to decrease and stabilize within an individual’s lifetime, especially toward late adulthood (9). (c) The bacterial component of the nasal cavity is primarily composed of members of the Actinobacteria, Firmicutes, and Proteobacteria phyla (13). The abundance of each phylum changes over time (9, 10). During infancy, the nasal cavity typically resembles the mother’s vaginal/skin microbiota (11), with the Actinobacteria, Firmicutes, and Proteobacteria phyla predominating (70). Upon entering adolescence and adulthood, Proteobacteria are typically displaced by the Actinobacteria and Firmicutes phyla, especially in the anterior nares (13). Finally, in adulthood, the nasal microbiota structure remains relatively stable, and Actinobacteria predominate (7, 13). Infants and young children are often nasally colonized by the opportunistic pathogens S. pneumoniae, H. influenzae, and M. catarrhalis (21). Similarly, N. meningitidis often asymptomatically nasally colonizes adolescences and young adults (22). Approximately 25% of adults are colonized by S. aureus (31, 71).
The remaining portion of the nasal cavity contains classical upper airway ciliated pseudostratified and columnar epithelial cells that aid in the movements of odorants and other matter through the nasal passage (6). This posterior region of the nasal cavity also includes the sphenoethmoidal recess, which allows drainage of the sphenoidal and ethmoid sinuses. The majority of nasal immune cells are located within the most posterior portion of the nasal cavity. Therein, resident macrophages and dendritic cells are responsible for the innate immune functions of the nasal mucosal barrier. Furthermore, the nasal-associated lymphoid tissue contains naive T and B cells, which are responsible for the adaptive component of the nasal immune system (8). Due to the complex physiological conditions found in the nasal cavity, only particular microbial species have adapted to survive, thrive, and outcompete other microbes in this microenvironment.
In terms of typical bacterial components of the nasal microbiota, 16S rRNA-based high-throughput sequencing indicates that the microbial community in the nasal cavity changes throughout an individual’s life and based on spatial location but tends to stabilize with time (Fig. 1b); this topic has been recently and expertly reviewed by Kumpitsch et al. (9) and is briefly summarized here to provide context for the remainder of the review. Although complex, the nasal microbiota is mostly compromised of the Actinobacteria, Firmicutes, and Proteobacteria phyla; the abundance of each phylum varies based on spatial location within the nasal cavity and shifts throughout life (Fig. 1c) (9, 10). For example, in infants, the initial nasal microbiota closely resembles the mother’s vaginal and skin microbiota (11). In adults, differences in microbial communities are linked to spatial location within the nasal cavity. Sampling of the anterior nares, middle meatus, and sphenoethmoidal recess of healthy adult volunteers revealed distinct microbial communities in each site (7). The anterior nares were found to be home to the least diverse microbial community; likely due to the harsh physiological conditions found in this niche, this community is primarily composed of the Gram-positive bacterial phyla Actinobacteria and Firmicutes (7, 10, 12). In contrast, similar microbial communities, with respect to species richness and evenness, were observed in the middle meatus and the sphenoethmoidal recess and contained an enriched amount of Proteobacteria compared to the anterior nares (7).
Among the phyla present at all typically sampled nasal cavity sites, the phylum Actinobacteria is the most predominant and is strikingly present throughout all stages of life (9, 10). Corynebacterium and Cutibacterium, many species of which are symbionts, tend to be the most abundant and prevalent Actinobacteria genera found in the nasal cavity (10, 12, 13). In particular, a high abundance of Corynebacterium in the nasal cavity is associated with increased nasal microbiota stability and a decreased risk for respiratory infections and associated complications, especially early in life (14). Analysis of the nasal microbiota of newborns revealed that Corynebacterium abundance is highly enriched in the anterior nares within the first 3 to 6 weeks of life and is correlated with a decreased risk of rhinitis (14). In contrast, Cutibacterium tends to be enriched in the nasal cavity of adolescents and is correlated with the onset of acne; some Cutibacterium acnes (formerly Propionibacterium acnes) phylogenetic groups have been found to be causative agents of acne (15, 16). After the Actinobacteria, the Firmicutes are generally the next most abundant phylum found as a part of the nasal microbiota (Fig. 1c) (9). As predicted by microbiota-based studies, some Firmicutes species are more adept at persistence in the nasal cavity than others, in particular the Staphylococcus, Streptococcus, and Dolosigranulum genera. For example, the commensal species Staphylococcus epidermidis colonizes nearly 100% of individuals early in life, persists within the nasal cavity and surrounding skin, and is associated with a stable nasal microbiota (17). Finally, Proteobacteria genera such as Moraxella and Haemophilus can colonize the nasal cavity at levels similar to those of Actinobacteria and Firmicutes in early childhood but then decrease over time toward adulthood (18, 19); they then remain at a constant albeit low abundance (9, 10, 13), especially in the anterior nares (Fig. 1c).
While commensal bacteria make up the overwhelming majority of the bacteria present in the nasal cavity, culture-dependent and -independent microbiota-based studies indicate that opportunistic pathogens, which are microbes that maintain the ability to act as either pathogens or commensals, are less prevalent in the nasal cavity (7, 20, 21). Moreover, their presence is often linked to poor clinical outcomes. For example, Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis, which are primarily found in the nasal microbiota of young children, are associated with respiratory disease and ear infections (21) (Fig. 1c). Similarly, Neisseria meningitidis asymptomatically colonizes some children and adolescents but can cause severe invasive disease upon breaching the bloodstream (22) (Fig. 1c). Finally, at any given time, Staphylococcus aureus nasally colonizes approximately 25% of individuals, predominantly adults (23); a small subset of individuals are persistently colonized (7, 23). While all of the factors that contribute to the likelihood of S. aureus colonization are not clear, certain Toll-like receptor 2 polymorphisms have been shown to be associated with higher rates of colonization (24). Moreover, individuals who spend a significant amount of time in congregate settings (i.e., military populations, prison populations, or sports teams) are the most at risk for S. aureus nasal colonization (12, 25, 26). Colonized individuals are at an increased risk for respiratory infections and invasive S. aureus-mediated disease over their lifetime (23, 26, 27). Thus, nasal colonization by several opportunistic pathogens can result in severe and sometimes fatal disease.
In terms of microbial colonization, cross-sectional studies demonstrate that the resident nasal microbiota are critical to help control colonization by opportunistic pathogens (7, 12, 28–31). These studies suggest that the identification of strategies that help to maximize the stability of the commensal nasal flora could have the potential to decrease opportunistic pathogen colonization and subsequent disease incidence. Furthermore, untangling the specific molecular mechanisms that commensal bacteria weaponize against particular opportunistic pathogens has the potential to identify novel molecular targets or antimicrobial compounds. This information can then potentially be harnessed as a means to develop novel therapeutics.
POLYMICROBIAL INTERACTIONS/COMPETITION IN THE NASAL CAVITY
In niches that are hostile to microbial growth, like the nasal cavity, competition is common among microbes since they must compete for a limited supply of nutrients and space. This competition is mediated through a diverse repertoire of molecular weaponry, the use of which is ultimately shaped by nutrient availability, cell density, and the genetic relatedness of the competitors (32). While these molecular polymicrobial interactions take many forms, a simple classification strategy that we use here asks whether or not the downstream effects require physical contact between the microbes. These are broadly defined as either contact-dependent (CD) or contact-independent (CI) effects; sometimes, more complex polymicrobial interactions contain aspects of both CD and CI interactions (33). Finally, it is worth noting that antagonism may occur due to the basic superiority of particular traits of specific bacteria within the polymicrobial interactome. For example, if a bacterium is better at nutrient acquisition, it may inhibit the growth of a competitor by indirectly starving that competitor for a particular nutrient; a classic example is competition for iron (34). In the following sections, we review the most recent advancements in examining the roles that both CD and CI interactions play in the natural evolution of interbacterial competition among the bacterial communities in the nasal cavity.
CROSS-PHYLUM COMPETITION IN THE NASAL CAVITY
Actinobacteria-Firmicutes interactions.
Our current knowledge of the molecular interactions that occur among particular members of the nasal microbiota is summarized in Fig. 2. The distinct physiological conditions of the nasal cavity prime the bacterial species that live there to be well adapted for “bacterial warfare”; these resident bacterial species are adept in their ability to contend and compete with other microbes. In this warfare, bacteria have evolved multiple mechanisms to block colonization, inhibit growth, or directly kill competitors; these strategies directly govern the structure of the nasal microbial community that is observed in 16 rRNA sequencing-based epidemiological studies. For instance, the nasal cavity of most adults is primarily colonized by bacteria from the Actinobacteria and Firmicutes phyla. Furthermore, high-throughput 16S rRNA gene sequencing studies actually show that a distinct inverse relationship exists between these phyla in this niche (7, 10) and that this inverse relationship can be extrapolated to certain genera that likely participate in direct competition with one another. Importantly, this phenomenon has been observed in a variety of cohorts, which include those in hospital and community settings and individuals found in confined settings (10, 12, 25). Thus, because of the potential to reveal novel antimicrobial compounds and/or previously uncharacterized molecular targets, there has been an increased interest in determining the underlying mechanisms that dictate this inverse relationship.
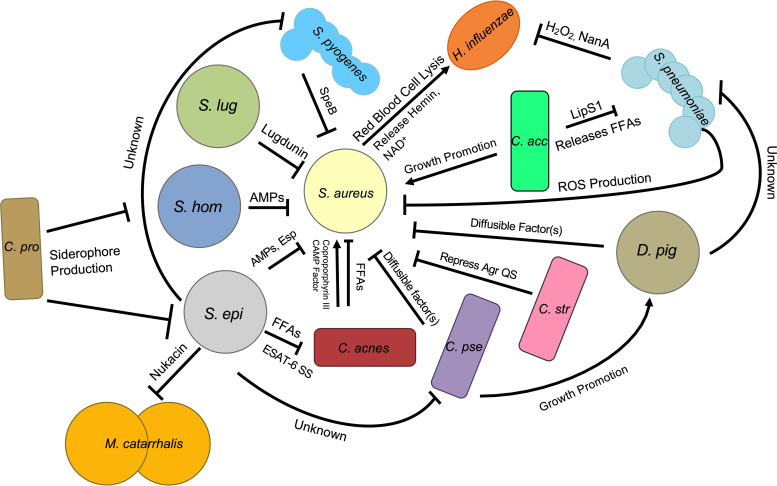
FIG 2.
Staphylococcus aureus and the ecology of the nasal microbiota. Interbacterial interactions in the nasal cavity are complex and dynamic and include both competitive and cooperative strategies. Coagulase-negative Staphylococcus (CoNS) species secrete antimicrobial peptides (AMPs) (S. epidermidis [S. epi] and S. hominis [S. hom]) (58), the Esp serine protease (S. epidermidis) (57), or the small compound lugdunin (S. lugdunensis [S. lug]) (29) to inhibit S. aureus nasal colonization. Corynebacterium striatum (C. str) and C. pseudodiphtheriticum (C. pse) repress Agr QS via a compound that is present in the extracellular space (35), and C. pseudodiphtheriticum actively kills S. aureus using a diffusible compound (37). S. pneumoniae inhibits S. aureus as well as H. influenzae by reactive oxygen species (ROS) and hydrogen peroxide production, respectively (50, 52); H. influenzae is also inhibited via the activity of the neuraminidase NanA (51). S. pyogenes inhibits S. aureus biofilms with the protease SpeB. C. acnes releases coproporphyrin III and CAMP factor, which promote S. aureus biofilm production and virulence, respectively (40, 41). Cutibacterium acnes ferments glycerol into free fatty acids (FFAs) that inhibit S. aureus growth. S. epidermidis inhibits C. pseudodiphtheriticum and S. pyogenes by an unknown mechanism(s) (38). S. epidermidis ferments triacylglycerides to FFAs that inhibit C. acnes. S. epidermidis may utilize an ESAT-6 secretion system (SS) to inhibit C. acnes. S. epidermidis kills M. catarrhalis with nukacin. C. propinquum (C. pro) inhibits some CoNS species via siderophore production (34). C. accolens (C. acc) may indirectly promote S. aureus colonization by releasing FFAs from the surface of nasal epithelial cells; these FFAs kill S. pneumoniae (28). D. pigrum (D. pig) inhibits S. aureus and S. pneumoniae growth by an unknown mechanism (61). C. pseudodiphtheriticum can support D. pigrum growth (61). S. aureus promotes H. influenzae colonization by lysing red blood cells, which release hemin and NAD+, both of which support H. influenzae growth (72).
At a molecular level, it is clear that species-specific interactions drive the inverse correlation between Actinobacteria and Firmicutes in the nasal cavity; the currently characterized interactions often involve specific interactions between members of the Streptococcus, Staphylococcus, Corynebacterium, and Cutibacterium genera. For example, Yan et al. used linear discriminant analysis to demonstrate that Corynebacterium accolens nasal colonization was a strong predictor of simultaneous human nasal S. aureus colonization. Furthermore, C. accolens supports S. aureus growth in vitro (7). An additional study also indicated that C. accolens nasal colonization is inversely correlated with S. pneumoniae and positively correlated with S. aureus presence in the nasal cavity (28). Investigation of the mechanism that promotes C. accolens antagonism toward S. pneumoniae revealed that, in a CI manner, C. accolens secretes a lipase, LipS1, that converts triacylglycerols found on the surface of nasal epithelial cells, triolein and trilinolein, into the free fatty (FFAs) acids oleic and linoleic acids; these FFAs act to support the growth of C. accolens and also display potent specific bactericidal activity against S. pneumoniae (28). Thus, although this is yet to be conclusively proven, it is possible that the ability of C. accolens to antagonize S. pneumoniae, combined with the ability to promote the growth of S. aureus, may act to enhance S. aureus colonization in the nasal cavity specifically via the elimination of S. pneumoniae.
While C. accolens may cooperate with and even promote S. aureus nasal colonization, several other Corynebacterium species inhibit S. aureus to some degree. For example, several Corynebacterium species have been found to induce S. aureus to enter what has been termed a “commensal state” via the action of a small diffusible compound (35). While the identity of this compound is unknown at the time of this review, the production of this compound does not require physical contact between these two species. Specifically, treatment of S. aureus with cell-free conditioned medium derived from Corynebacterium striatum led to global changes in S. aureus transcription; these changes included the downregulation of the important virulence-associated genes agrBDCA and psm, which encode the Agr quorum sensing (QS) system and the phenol-soluble modulins (PSMs), respectively (35). Conversely, the transcription of genes that are likely required for the colonization of the nasal cavity, such as spa (protein A) and metI (cysteine and methionine synthesis), is upregulated (35). Thus, some Corynebacterium species appear to be able to modulate and reduce the virulence potential of S. aureus in the context of nasal colonization.
In addition to the modulation of gene expression of S. aureus, at least one Corynebacterium species, Corynebacterium pseudodiphtheriticum, appears to have the ability to directly prevent colonization by and/or eradicate S. aureus from the nasal cavity. Indeed, Yan et al. demonstrated that individuals colonized with C. pseudodiphtheriticum had a very low probability of S. aureus nasal colonization (7). Furthermore, they found that C. pseudodiphtheriticum inhibits S. aureus growth in vitro. Consistent with this, simple C. pseudodiphtheriticum inoculation into the nasal cavity leads to the eradication of S. aureus in healthy volunteers (36). More recently, it was demonstrated that C. pseudodiphtheriticum releases a diffusible compound in a CI manner that mediates potent bactericidal activity against S. aureus; the activity of this compound also takes advantage of S. aureus-specific virulence factors: Agr QS and the PSMs (37). Interestingly, while C. pseudodiphtheriticum negatively impacts S. aureus viability, C. pseudodiphtheriticum does not impact the viability of S. epidermidis, which is closely related to S. aureus. Instead, at least one study demonstrated that S. epidermidis inhibits the growth of C. pseudodiphtheriticum in vitro by an unknown mechanism (38). Although the exact reason why particular Corynebacterium species appear to have evolved to specifically target select opportunistic pathogens but also spare closely related commensal species remains to be determined, it is clear that this antagonism can have positive impacts on the host via the elimination of a possible disease-causing microbe.
Small compounds that are released into the extracellular milieu that directly inhibit or induce global changes in gene transcription in competitor bacteria or that provide other types of advantages for the producing species are a common theme in antibiosis interactions. However, the production of these compounds can be energetically expensive. Thus, the provided advantage may be dependent on the overall conditions and microenvironment. As such, various bacterial species have evolved alternative molecular mechanisms that allow for efficient competition. For example, exploitation competition, where a bacterial species prevents competitors from acquiring resources via the rapid consumption or sequestration of important nutrients, is an indirect mechanism used by some bacteria as a means to compete against unrelated microbes (34). For example, Corynebacterium propinquum, a common nasal commensal bacterium that is closely related to C. pseudodiphtheriticum, releases siderophores that are able to sequester iron from the environment (34). This sequestration indirectly inhibits the growth of competing coagulase-negative Staphylococcus (CoNS) species that need iron for growth (34, 39). There appear to be levels of species specificity to this interaction since the growth of S. aureus is unaffected in the presence of the C. propinquum-produced siderophores (34). Although it remains to be proven, this may be due to the use of cognate siderophores by S. aureus.
Similar to Corynebacterium, Cutibacterium is predicted to participate in both antagonistic and symbiotic interactions with some Firmicutes species. For example, coproporphyrin III, which is released by some Cutibacterium species in a CI manner, induces S. aureus biofilm formation, which may then promote S. aureus colonization (40). Moreover, C. acnes (formerly P. acnes) synthesizes and releases Christie-Atkins-Munch-Petersen (CAMP) factor, which increases the hemolytic activity of the S. aureus toxin beta-hemolysin; this may also help S. aureus establish itself within the host and promote invasive disease (41, 42). It is unclear if physical contact between S. aureus and C. acnes is necessary for this increased hemolytic activity to occur. Furthermore, while the exact molecular mechanisms leading to this augmentation of S. aureus hemolytic activity remain unknown, the increase in hemolytic activity does not appear to involve active growth inhibition; CFU of S. aureus grown alone or in coculture with C. acnes were similar. It should be noted that C. acnes has also been found to antagonize S. aureus. For example, Shu et al. demonstrated that in a murine ear infection model, C. acnes ferments glycerol into short-chain FFAs that maintain potent inhibitory activity against S. aureus, including methicillin-resistant S. aureus (MRSA) (43). A similar scenario may also play out in the nasal cavity, where triacylglycerides are plentiful on the surface of nasal epithelial cells.
In contrast, C. acnes is directly antagonized by S. epidermidis, another common inhabitant of the nasal cavity, via multiple mechanisms. For example, nasal cavity isolates of S. epidermidis ferment glycerol, a common carbon source on the surface of human epithelial cells, into succinic acid, which is able to effectively limit the growth of C. acnes (44). The conversion of triacylglycerides and glycerol into toxic FFAs appears to be a common CI tactic that some bacteria utilize to limit the growth of competitors (28, 43). Although the origin of this mechanism of competition is unclear, we hypothesize that the use of triacylglycerides and glycerol as energy sources by certain microbes evolved due to the lack of more readily available carbon sources; the antimicrobial properties of the breakdown products (FFAs) were an advantageous indirect bonus. Overall, this suggests that this mechanism of competition may have evolved early in the evolution of interbacterial competition in the human nasal passages and on skin. In addition to indirect mechanisms of inhibition, S. epidermidis may also inhibit C. acnes using mechanisms that require physical contact. For example, S. epidermidis strain FS1 possesses potent CD antimicrobial activity against C. acnes (45). An analysis of the S. epidermidis FS1 accessory genome revealed the presence of a putative lactococcin 972 superfamily bacteriocin and cognate immunity protein in addition to an epidermin-like peptide precursor that may be directly responsible for this killing activity (45). Similarly, S. epidermidis strain 14.1.R1, which possess specific antimicrobial activity against C. acnes, possesses an ESAT-6 secretion system that may mediate the anti-C. acnes phenotype of this strain (45). Overall, 16S rRNA-based sequencing studies of the nasal microbiota and mechanistic studies of molecular interactions between competing species make it clear that interactions between Actinobacteria and various Firmicutes species are multifaceted. Moreover, some of these commensal species may have the potential to serve as next-generation probiotics. Moreover, it is intriguing to speculate that some of the specific small molecules/proteins produced by these species may have untapped therapeutic potential.
Proteobacteria-Firmicutes interactions.
Similar to the Actinobacteria, a distinct inverse relationship exists in the nasal cavity between the Proteobacteria and Firmicutes phyla; this inverse relationship has been observed in multiple cohort studies and may impart some benefit to the host. For example, a study of the nasal microbiota of military trainees who presented with skin and soft tissue infections (SSTIs) revealed that Proteobacteria abundance was decreased in the anterior nares of those with SSTIs compared to those without an SSTI (12). In this particular study, differences in Proteobacteria abundances were largely driven by an overall increase in numerous genera within the Proteobacteria phylum instead of a particular genus or species (12). These results support the interesting possibility that the presence of Proteobacteria may provide some form of protection against the development of SSTI. However, if this is the case, the presence of Proteobacteria may not protect against other types of disease in younger cohorts. For example, a comparison of the microbiota of the anterior nares of infants suffering from rhinitis with wheeze to those of controls without rhinitis revealed a higher abundance of Proteobacteria, in particular the Oxalobacteraceae family, and decreased Staphylococcaceae abundance (Firmicutes) compared to controls (14). Therefore, depending on the associated types of disease, and perhaps the age of the individual, a high abundance of Proteobacteria in the nasal cavity may be associated with either favorable or detrimental outcomes.
While broad correlations between the Proteobacteria and Firmicutes phyla have been uncovered in 16S rRNA sequencing-based epidemiological studies, very few studies have explored the complex interbacterial molecular mechanisms that govern these relationships. This being said, there are a few instances where interactions between specific species are starting to be examined. For example, complex associations between S. aureus, S. pneumoniae, and Haemophilus influenzae have been observed in the nasal cavity and oropharynx. These relationships are highly dependent upon the age and location of the sampled cohort (21, 46, 47). One molecular mechanism that may partially explain some of the complicated interactions among these species in the nasal cavity and oropharynx involves the ability of S. aureus to lyse red blood cells at a low level; the lysed cells release hemin and NAD+ into the extracellular milieu (48). These compounds can then support H. influenzae colonization; nasal H. influenzae bacterial densities are higher in the presence of S. aureus (47, 49). In contrast, S. pneumoniae negatively impacts H. influenzae using a variety of CI mechanisms, including the production of hydrogen peroxide, which may ultimately inhibit H. influenzae colonization (50). Furthermore, S. pneumoniae secretion of the neuraminidase NanA removes sialic groups from H. influenzae lipooligosaccharides (LOSs), which may negatively impact H. influenzae attachment to epithelial cells and thus reduce colonization potential (51). Beyond these particular species, competitive interactions have also been characterized between S. epidermidis and the opportunistic pathogen Moraxella catarrhalis; some S. epidermidis strains synthesize and excrete the compound nukacin that directly kills M. catarrhalis (25). Overall, other interactions that drive broad correlations between various Proteobacteria and Firmicutes species remain to be characterized.
INTRAPHYLUM COMPETITION
Staphylococcus and Streptococcus.
While cross-phylum antagonistic interactions are common in the nasal cavity, competition between various genera or species within a phylum also influences the composition of the nasal microbiota. For example, one of the most pronounced inverse correlations that has been documented in various nasal microbiota studies occurs between S. aureus and S. pneumoniae. Indeed, multiple studies suggest that reductions in S. pneumoniae burdens in the nasal cavity as an individual ages may create a niche for S. aureus colonization (20, 28). Furthermore, direct competition between these two species has been demonstrated (28, 52–54). For example, Khan et al. demonstrated that S. pneumoniae is able to disrupt S. aureus biofilms using a CD mechanism in a manner independent of hydrogen peroxide production (53). In an additional mechanism, physical contact between S. pneumoniae and S. aureus can induce the production of S. pneumoniae-generated hydrogen peroxide, which is subsequently converted to hydroxyl radicals. These hydroxy radicals diffuse throughout the extracellular milieu and kill S. aureus (52). Hydrogen peroxide production is dependent on the S. pneumoniae SpxB and LctO enzymes (52). The S. aureus-specific surface components that are sensed by S. pneumoniae prior to the initiation of hydrogen peroxide/hydroxy radical production remain unknown (52). In some instances, S. aureus is able to overcome these strategies and displace S. pneumoniae in the nasal cavity. Although the exact mechanisms by which this may occur are unclear, the production of enzymes such as catalase may ultimately protect S. aureus from reactive oxygen species (55).
While not as frequent a colonizer of the nasal cavity as S. pneumoniae, Streptococcus pyogenes mediates CI antibiofilm activity against S. aureus. SpeB, an S. pyogenes-secreted protease, is able to disrupt S. aureus biofilms via cleavage of the surface-exposed S. aureus protein SdrC. In contrast, select S. epidermidis nasal isolates were found to strongly inhibit some strains of S. pyogenes in vitro by an unknown mechanism (38). It is unknown if physical contact is necessary for this S. epidermidis inhibitory activity. It is clear that, as with many different types of interbacterial competition, interactions between Streptococcus and Staphylococcus are species and, in some cases, strain specific.
Coagulase-negative Staphylococcus and S. aureus.
Although closely related genetically, competition for similar environmental niches leads to well-documented competition between various members of the Staphylococcus genus; this competition in turn contributes to the overall structure of the nasal microbiota (13, 31). Multiple studies have demonstrated that various coagulase-negative Staphylococcus (CoNS) species have evolved multiple CI strategies to inhibit S. aureus nasal colonization. Of these species, S. epidermidis, in particular, appears to possess an arsenal of weapons that it uses to inhibit S. aureus colonization (31, 56). For example, the S. epidermidis-secreted serine protease Esp displays potent antibiofilm and bacteriostatic properties against S. aureus. Inoculation of Esp-secreting S. epidermidis or purified Esp into the nasal cavity of S. aureus carriers results in the clearance of S. aureus (57). Moreover, both S. epidermidis and Staphylococcus hominis secrete antimicrobial peptides (AMPs) that exhibit strain-specific anti-S. aureus activity and synergize with the human AMP LL-37 (58). Application of the AMP secreted by S. hominis to pigskin that had been colonized with S. aureus led to a significant reduction in the S. aureus burden (58). In addition to AMPs and secreted enzymes, at least one staphylococcal species secretes a small molecule that displays high therapeutic potential. A Staphylococcus lugdunensis strain isolated from the human nasal cavity was found to produce a novel small molecule (lugdunin) that kills S. aureus and other Gram-positive pathogens. Excitingly, resistance to lugdunin was not observed over several generations (29). Furthermore, the application of lugdunin-producing S. lugdunensis strains to the nasal cavity of cotton rats led to the clearance of S. aureus (29). Although the exact mechanism by which lugdunin inhibits S. aureus remains unknown at the time of this review, it is hypothesized that it may interfere with the electron transport chain (29).
Dolosigranulum pigrum, S. pneumoniae, and S. aureus.
More recently, Dolosigranulum pigrum has been found to participate in competitive molecular interactions with both S. pneumoniae and S. aureus; D. pigrum may be protective against colonization by these microorganisms. Several studies have shown that D. pigrum and S. aureus are inversely correlated in the nasal cavity (7, 17, 59). Furthermore, S. pneumoniae is absent from the nasal microbiota of young individuals when high abundances of D. pigrum and some Corynebacterium species are present (60). To this end, at least one study has demonstrated that D. pigrum directly inhibits S. aureus growth in vitro through the action of an unknown diffusible compound(s) (61). Intriguingly, D. pigrum also inhibits the growth of S. pneumoniae but only in the presence of C. pseudodiphtheriticum. Moreover, those authors showed that C. pseudodiphtheriticum supports the growth of D. pigrum (61). Analysis of the D. pigrum accessory genome revealed several biosynthetic gene clusters that are predicted to encode antimicrobial compounds that are likely responsible for D. pigrum inhibitory activity against S. aureus and S. pneumoniae (61). Combined, these data suggest that molecular interactions of the nasal microbiota are far more complex than initially observed.
FUTURE OF NASAL MICROBIOTA MOLECULAR RESEARCH
While there are many interesting areas that remain to be explored in terms of the role of the nasal microbiota in health and disease, we note that there are numerous possibilities by which gained knowledge could be used as a way to ultimately block or treat disease. For example, as implied by the research highlighted here, an enticing path forward could be the identification of approaches that help to boost the presence and stability of specific commensal nasal flora. Millennia of competition and coevolution suggest that these particular microbes are likely best suited to block colonization by incoming opportunistic pathogens, which ultimately would lead to a reduction in associated disease occurrence. The direct use of commensal nasal microbes as probiotics that eliminate the presence of opportunistic pathogens in a manner that parallels the use of fecal transplants to restore the colonic microbiota is a particularly intriguing approach. For example, C. pseudodiphtheriticum and S. epidermidis are two exciting candidates that have already been demonstrated to reduce either S. aureus or S. pneumoniae nasal burdens, respectively (36, 57). Moreover, the beneficial properties of C. pseudodiphtheriticum may extend outside competition with S. aureus. Specifically, the application of C. pseudodiphtheriticum to the nasal cavity of infant mice improved clinical outcomes of infection with respiratory syncytial virus and S. pneumoniae via modulation of the host immune system, including an increased CD4+ T-cell response (62). Thus, it is intriguing to speculate that probiotic-like application of some of these commensal microorganisms could be developed as a means to prevent disease.
Despite this possibility, the importance of looking beyond the simple identification of specific species that can counteract a pathogen to clearly define the molecular mechanism by which the various probiotic species are effective cannot be overstated. This is largely due to the fact that even “harmless” commensal bacteria have the potential to cause significant disease in immunocompromised individuals, who are the most likely to be colonized by opportunistic pathogens (63–65). Thus, perhaps the purification of commensal-derived secondary metabolites, AMPs, and other compounds represents the best way to combat pathogen colonization on a macroscale. Since these molecules have evolved their antimicrobial properties in vivo, it is possible that this approach could avoid some of the inherent complications encountered when screening synthetic chemicals for compounds that often are unable to bypass the bacterial permeability barrier (66). Alternatively, high-throughput metagenomic mining of genomes with software programs such as antiSMASH could be used as a means to aid in the bioinformatic identification and characterization of the genetic signatures of secondary metabolic synthesis, bacteriocin/AMP production, and other small molecules (67). Importantly, these approaches could even be applied to sequence data from currently unculturable nasal microbes. This approach has already been used successfully; although first identified via a transposon mutant strain screen, antiSMASH bioinformatic analysis was used to further identify and characterize the operon that encodes a nonribosomal peptide synthase that synthesizes the S. lugdunensis-derived antimicrobial compound lugdunin (29).
While the identification of compounds that directly kill pathogens may seem most ideal, the discovery and use of commensal-derived antivirulence compounds could be another strategy to mitigate the disease potential of these pathogens. Importantly, since many of these compounds target specific pathways, this strategy could simultaneously preserve the structure and diversity of the overall nasal microbiome. Furthermore, disarming pathogens by targeting their virulence factors appears to induce significantly less evolutionary pressure in terms of resistance (68). While this area remains to be broadly explored, the anti-Agr QS compounds secreted by some Corynebacterium species and also Bacillus species are prime examples of molecules that could be of interest (35, 37, 69).
In summary, this review highlights some of the intricate polymicrobial interactions that occur in the nasal cavity and highlights the importance of defining the underlying molecular mechanisms that govern the broad correlations that are often observed in nasal microbiota-based epidemiological studies. We strongly believe that a greater understanding of the strategies that bacteria use to compete with one another has the potential to be harnessed for the elucidation of new therapeutic targets as well as actual therapeutics. Clearly, we have only just scratched the surface in terms of a true understanding of the various types of interbacterial competition and cooperation that take place in/on the human body. Thus, it is time to refocus our efforts beyond simple correlation studies so that we can truly define and understand the arsenal of weapons utilized by various microbes during their constant struggle to defend their environmental niches.
ACKNOWLEDGMENTS
This work was supported by a U.S. Department of Defense Program project grant (HT9404-12-1-0019 to D. S. Merrell) and a Military Infectious Diseases Research Program award (HU0001-15-2-0031 to D. S. Merrell).
Biographies

Britney L. Hardy was born in Washington, DC, and was raised in neighboring Landover, MD. Despite living right down the street from FedEx Field, she has never attended a Washington football game. After high school, she attended the University of Maryland, College Park (UMD), and obtained a B.S. in Biological Sciences: Microbiology. While at UMD, she developed a passion for science outreach and began volunteering at various events throughout the Washington, DC, region with ASM and AAAS. She did not stray too far for graduate school, as she attended the Uniformed Services University of the Health Sciences in Bethesda, MD, and obtained a Ph.D. in Emerging Infectious Diseases. She received several awards for her research on interbacterial competition from ASM on the branch and national levels. She looks forward to continuing her studies of interbacterial competition as a postdoc. Her nonresearch interests are simple, a Washington, DC, classic, brunch!

D. Scott Merrell (Scotty), born and raised in rural Bald Knob, AR, left the south to complete his Ph.D. studies at Tufts Medical School and conduct postdoctoral training at Stanford University. He is currently a Professor in the Department of Microbiology and Immunology and Program Director of the Emerging Infectious Diseases graduate program at the Uniformed Services University in Bethesda, MD. Intrigued by the stress response and how bacteria manage to colonize in inhospitable environments, his research group studies colonization and virulence factors of several pathogens. He is a former “Applied Genomics of Infectious Diseases” ID Training Fellow and Damon Runyon-Walter Winchell Cancer Fund Postdoctoral Fellow and received a Merck Irving S. Sigal Memorial Award for excellence in basic research in medical microbiology and infectious diseases. Despite his many years away from Arkansas, he still confesses a secret passion for Southern-fried catfish, hush puppies, pickled green tomatoes, and the use of “y’all” in polite conversation.
REFERENCES
- 1.Sender R, Fuchs S, Milo R. 2016. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol 14:e1002533. doi: 10.1371/journal.pbio.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Human Microbiome Project Consortium. 2012. Structure, function and diversity of the healthy human microbiome. Nature 486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vivarelli S, Salemi R, Candido S, Falzone L, Santagati M, Stefani S, Torino F, Banna GL, Tonini G, Libra M. 2019. Gut microbiota and cancer: from pathogenesis to therapy. Cancers 11:38. doi: 10.3390/cancers11010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parfrey LW, Walters WA, Knight R. 2011. Microbial eukaryotes in the human microbiome: ecology, evolution, and future directions. Front Microbiol 2:153. doi: 10.3389/fmicb.2011.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krismer B, Liebeke M, Janek D, Nega M, Rautenberg M, Hornig G, Unger C, Weidenmaier C, Lalk M, Peschel A. 2014. Nutrient limitation governs Staphylococcus aureus metabolism and niche adaptation in the human nose. PLoS Pathog 10:e1003862. doi: 10.1371/journal.ppat.1003862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geurkink N 1983. Nasal anatomy, physiology, and function. J Allergy Clin Immunol 72:123–128. doi: 10.1016/0091-6749(83)90518-3. [DOI] [PubMed] [Google Scholar]
- 7.Yan M, Pamp SJ, Fukuyama J, Hwang PH, Cho D-Y, Holmes S, Relman DA. 2013. Nasal microenvironments and interspecific interactions influence nasal microbiota complexity and S. aureus carriage. Cell Host Microbe 14:631–640. doi: 10.1016/j.chom.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Debertin AS, Tschernig T, Tönjes H, Kleemann WJ, Tröger HD, Pabst R. 2003. Nasal-associated lymphoid tissue (NALT): frequency and localization in young children. Clin Exp Immunol 134:503–507. doi: 10.1111/j.1365-2249.2003.02311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumpitsch C, Koskinen K, Schöpf V, Moissl-Eichinger C. 2019. The microbiome of the upper respiratory tract in health and disease. BMC Biol 17:87. doi: 10.1186/s12915-019-0703-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lemon KP, Klepac-Ceraj V, Schiffer HK, Brodie EL, Lynch SV, Kolter R. 2010. Comparative analyses of the bacterial microbiota of the human nostril and oropharynx. mBio 1:e00129-10. doi: 10.1128/mBio.00129-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biesbroek G, Tsivtsivadze E, Sanders EA, Montijn R, Veenhoven RH, Keijser BJ, Bogaert D. 2014. Early respiratory microbiota composition determines bacterial succession patterns and respiratory health in children. Am J Respir Crit Care Med 190:1283–1292. doi: 10.1164/rccm.201407-1240OC. [DOI] [PubMed] [Google Scholar]
- 12.Johnson RC, Ellis MW, Lanier JB, Schlett CD, Cui T, Merrell DS. 2015. Correlation between nasal microbiome composition and remote purulent skin and soft tissue infections. Infect Immun 83:802–811. doi: 10.1128/IAI.02664-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bassis CM, Tang AL, Young VB, Pynnonen MA. 2014. The nasal cavity microbiota of healthy adults. Microbiome 2:27. doi: 10.1186/2049-2618-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ta LDH, Yap GC, Tay CJX, Lim ASM, Huang C-H, Chu CW, De Sessions PF, Shek LP, Goh A, Van Bever HPS, Teoh OH, Soh JY, Thomas B, Ramamurthy MB, Goh DYT, Lay C, Soh S-E, Chan YH, Saw S-M, Kwek K, Chong Y-S, Godfrey KM, Hibberd ML, Lee BW. 2018. Establishment of the nasal microbiota in the first 18 months of life: correlation with early-onset rhinitis and wheezing. J Allergy Clin Immunol 142:86–95. doi: 10.1016/j.jaci.2018.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahluwalia J, Borok J, Haddock ES, Ahluwalia RS, Schwartz EW, Hosseini D, Amini S, Eichenfield LF. 2019. The microbiome in preadolescent acne: assessment and prospective analysis of the influence of benzoyl peroxide. Pediatr Dermatol 36:200–206. doi: 10.1111/pde.13741. [DOI] [PubMed] [Google Scholar]
- 16.Lomholt HB, Kilian M. 2010. Population genetic analysis of Propionibacterium acnes identifies a subpopulation and epidemic clones associated with acne. PLoS One 5:e12277. doi: 10.1371/journal.pone.0012277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu CM, Price LB, Hungate BA, Abraham AG, Larsen LA, Christensen K, Stegger M, Skov R, Andersen PS. 2015. Staphylococcus aureus and the ecology of the nasal microbiome. Sci Adv 1:e1400216. doi: 10.1126/sciadv.1400216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teo SM, Mok D, Pham K, Kusel M, Serralha M, Troy N, Holt BJ, Hales BJ, Walker ML, Hollams E, Bochkov YA, Grindle K, Johnston SL, Gern JE, Sly PD, Holt PG, Holt KE, Inouye M. 2015. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe 17:704–715. doi: 10.1016/j.chom.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shilts MH, Rosas-Salazar C, Tovchigrechko A, Larkin EK, Torralba M, Akopov A, Halpin R, Peebles RS, Moore ML, Anderson LJ, Nelson KE, Hartert TV, Das SR. 2016. Minimally invasive sampling method identifies differences in taxonomic richness of nasal microbiomes in young infants associated with mode of delivery. Microb Ecol 71:233–242. doi: 10.1007/s00248-015-0663-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bogaert D, van Belkum A, Sluijter M, Luijendijk A, de Groot R, Rümke HC, Verbrugh HA, Hermans PWM. 2004. Colonisation by Streptococcus pneumoniae and Staphylococcus aureus in healthy children. Lancet 363:1871–1872. doi: 10.1016/S0140-6736(04)16357-5. [DOI] [PubMed] [Google Scholar]
- 21.Pettigrew MM, Gent JF, Revai K, Patel JA, Chonmaitree T. 2008. Microbial interactions during upper respiratory tract infections. Emerg Infect Dis 14:1584–1591. doi: 10.3201/eid1410.080119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bakir M, Yagci A, Ulger N, Akbenlioglu C, Ilki A, Soyletir G. 2001. Asymtomatic [sic] carriage of Neisseria meningitidis and Neisseria lactamica in relation to Streptococcus pneumoniae and Haemophilus influenzae colonization in healthy children: apropos of 1400 children sampled. Eur J Epidemiol 17:1015–1018. doi: 10.1023/a:1020021109462. [DOI] [PubMed] [Google Scholar]
- 23.Kluytmans JAJW, Wertheim HFL. 2005. Nasal carriage of Staphylococcus aureus and prevention of nosocomial infections. Infection 33:3–8. doi: 10.1007/s15010-005-4012-9. [DOI] [PubMed] [Google Scholar]
- 24.Vuononvirta J, Toivonen L, Gröndahl-Yli-Hannuksela K, Barkoff A-M, Lindholm L, Mertsola J, Peltola V, He Q. 2011. Nasopharyngeal bacterial colonization and gene polymorphisms of mannose-binding lectin and Toll-like receptors 2 and 4 in infants. PLoS One 6:e26198. doi: 10.1371/journal.pone.0026198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh J, Johnson RC, Schlett CD, Elassal EM, Crawford KB, Mor D, Lanier JB, Law NN, Walters WA, Teneza-Mora N, Bennett JW, Hall ER, Millar EV, Ellis MW, Merrell DS. 2016. Multi-body-site microbiome and culture profiling of military trainees suffering from skin and soft tissue infections at Fort Benning, Georgia. mSphere 1:e00232-16. doi: 10.1128/mSphere.00232-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weidenmaier C, Goerke C, Wolz C. 2012. Staphylococcus aureus determinants for nasal colonization. Trends Microbiol 20:243–250. doi: 10.1016/j.tim.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 27.von Eiff C, Becker K, Machka K, Stammer H, Peters G. 2001. Nasal carriage as a source of Staphylococcus aureus bacteremia. N Engl J Med 344:11–16. doi: 10.1056/NEJM200101043440102. [DOI] [PubMed] [Google Scholar]
- 28.Bomar L, Brugger SD, Yost BH, Davies SS, Lemon KP. 2016. Corynebacterium accolens releases antipneumococcal free fatty acids from human nostril and skin surface triacylglycerols. mBio 7:e01725-15. doi: 10.1128/mBio.01725-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zipperer A, Konnerth MC, Laux C, Berscheid A, Janek D, Weidenmaier C, Burian M, Schilling NA, Slavetinsky C, Marschal M, Willmann M, Kalbacher H, Schittek B, Brötz-Oesterhelt H, Grond S, Peschel A, Krismer B. 2016. Human commensals producing a novel antibiotic impair pathogen colonization. Nature 535:511–516. doi: 10.1038/nature18634. [DOI] [PubMed] [Google Scholar]
- 30.Wos-Oxley ML, Plumeier I, von Eiff C, Taudien S, Platzer M, Vilchez-Vargas R, Becker K, Pieper DH. 2010. A poke into the diversity and associations within human anterior nare microbial communities. ISME J 4:839–851. doi: 10.1038/ismej.2010.15. [DOI] [PubMed] [Google Scholar]
- 31.Frank DN, Feazel LM, Bessesen MT, Price CS, Janoff EN, Pace NR. 2010. The human nasal microbiota and Staphylococcus aureus carriage. PLoS One 5:e10598. doi: 10.1371/journal.pone.0010598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Granato ET, Meiller-Legrand TA, Foster KR. 2019. The evolution and ecology of bacterial warfare. Curr Biol 29:R521–R537. doi: 10.1016/j.cub.2019.04.024. [DOI] [PubMed] [Google Scholar]
- 33.Peters BM, Jabra-Rizk MA, O’May GA, Costerton JW, Shirtliff ME. 2012. Polymicrobial interactions: impact on pathogenesis and human disease. Clin Microbiol Rev 25:193–213. doi: 10.1128/CMR.00013-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stubbendieck RM, May DS, Chevrette MG, Temkin MI, Wendt-Pienkowski E, Cagnazzo J, Carlson CM, Gern JE, Currie CR. 2019. Competition among nasal bacteria suggests a role for siderophore-mediated interactions in shaping the human nasal microbiota. Appl Environ Microbiol 85:e02406-18. doi: 10.1128/AEM.02406-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramsey MM, Freire MO, Gabrilska RA, Rumbaugh KP, Lemon KP. 2016. Staphylococcus aureus shifts toward commensalism in response to Corynebacterium species. Front Microbiol 7:1230. doi: 10.3389/fmicb.2016.01230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kiryukhina NV, Melnikov VG, Suvorov AV, Morozova YA, Ilyin VK. 2013. Use of Corynebacterium pseudodiphtheriticum for elimination of Staphylococcus aureus from the nasal cavity in volunteers exposed to abnormal microclimate and altered gaseous environment. Probiotics Antimicrob Proteins 5:233–238. doi: 10.1007/s12602-013-9147-x. [DOI] [PubMed] [Google Scholar]
- 37.Hardy BL, Dickey SW, Plaut RD, Riggins DP, Stibitz S, Otto M, Merrell DS. 2019. Corynebacterium pseudodiphtheriticum exploits Staphylococcus aureus virulence components in a novel polymicrobial defense strategy. mBio 10:e02491-18. doi: 10.1128/mBio.02491-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janek D, Zipperer A, Kulik A, Krismer B, Peschel A. 2016. High frequency and diversity of antimicrobial activities produced by nasal Staphylococcus strains against bacterial competitors. PLoS Pathog 12:e1005812. doi: 10.1371/journal.ppat.1005812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lindsay JA, Riley TV. 1994. Staphylococcal iron requirements, siderophore production, and iron-regulated protein expression. Infect Immun 62:2309–2314. doi: 10.1128/IAI.62.6.2309-2314.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wollenberg MS, Claesen J, Escapa IF, Aldridge KL, Fischbach MA, Lemon KP. 2014. Propionibacterium-produced coproporphyrin III induces Staphylococcus aureus aggregation and biofilm formation. mBio 5:e01286-14. doi: 10.1128/mBio.01286-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lo C-W, Lai Y-K, Liu Y-T, Gallo RL, Huang C-M. 2011. Staphylococcus aureus hijacks a skin commensal to intensify its virulence: immunization targeting β-hemolysin and CAMP factor. J Invest Dermatol 131:401–409. doi: 10.1038/jid.2010.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scholz CFP, Kilian M. 2016. The natural history of cutaneous propionibacteria, and reclassification of selected species within the genus Propionibacterium to the proposed novel genera Acidipropionibacterium gen. nov., Cutibacterium gen. nov. and Pseudopropionibacterium gen. nov. Int J Syst Evol Microbiol 66:4422–4432. doi: 10.1099/ijsem.0.001367. [DOI] [PubMed] [Google Scholar]
- 43.Shu M, Wang Y, Yu J, Kuo S, Coda A, Jiang Y, Gallo RL, Huang C-M. 2013. Fermentation of Propionibacterium acnes, a commensal bacterium in the human skin microbiome, as skin probiotics against methicillin-resistant Staphylococcus aureus. PLoS One 8:e55380. doi: 10.1371/journal.pone.0055380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Kuo S, Shu M, Yu J, Huang S, Dai A, Two A, Gallo RL, Huang C-M. 2014. Staphylococcus epidermidis in the human skin microbiome mediates fermentation to inhibit the growth of Propionibacterium acnes: implications of probiotics in acne vulgaris. Appl Microbiol Biotechnol 98:411–424. doi: 10.1007/s00253-013-5394-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Christensen GJM, Scholz CFP, Enghild J, Rohde H, Kilian M, Thürmer A, Brzuszkiewicz E, Lomholt HB, Brüggemann H. 2016. Antagonism between Staphylococcus epidermidis and Propionibacterium acnes and its genomic basis. BMC Genomics 17:152. doi: 10.1186/s12864-016-2489-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shiri T, Nunes MC, Adrian PV, Van Niekerk N, Klugman KP, Madhi SA. 2013. Interrelationship of Streptococcus pneumoniae, Haemophilus influenzae and Staphylococcus aureus colonization within and between pneumococcal-vaccine naïve mother-child dyads. BMC Infect Dis 13:483. doi: 10.1186/1471-2334-13-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Margolis E, Yates A, Levin BR. 2010. The ecology of nasal colonization of Streptococcus pneumoniae, Haemophilus influenzae and Staphylococcus aureus: the role of competition and interactions with host’s immune response. BMC Microbiol 10:59. doi: 10.1186/1471-2180-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nilsson IM, Hartford O, Foster T, Tarkowski A. 1999. Alpha-toxin and gamma-toxin jointly promote Staphylococcus aureus virulence in murine septic arthritis. Infect Immun 67:1045–1049. doi: 10.1128/IAI.67.3.1045-1049.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Artman M, Domenech E, Weiner M. 1983. Growth of Haemophilus influenzae in simulated blood cultures supplemented with hemin and NAD. J Clin Microbiol 18:376–379. doi: 10.1128/JCM.18.2.376-379.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pericone CD, Overweg K, Hermans PW, Weiser JN. 2000. Inhibitory and bactericidal effects of hydrogen peroxide production by Streptococcus pneumoniae on other inhabitants of the upper respiratory tract. Infect Immun 68:3990–3997. doi: 10.1128/iai.68.7.3990-3997.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shakhnovich EA, King SJ, Weiser JN. 2002. Neuraminidase expressed by Streptococcus pneumoniae desialylates the lipopolysaccharide of Neisseria meningitidis and Haemophilus influenzae: a paradigm for interbacterial competition among pathogens of the human respiratory tract. Infect Immun 70:7161–7164. doi: 10.1128/iai.70.12.7161-7164.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu X, Gordon O, Jiang W, Antezana BS, Angulo-Zamudio UA, del Rio C, Moller A, Brissac T, Tierney ARP, Warncke K, Orihuela CJ, Read TD, Vidal JE. 2019. Interaction between Streptococcus pneumoniae and Staphylococcus aureus generates OH radicals that rapidly kill Staphylococcus aureus strains. J Bacteriol 201:e00474-19. doi: 10.1128/JB.00474-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khan F, Wu X, Matzkin GL, Khan MA, Sakai F, Vidal JE. 2016. Streptococcus pneumoniae eradicates preformed Staphylococcus aureus biofilms through a mechanism requiring physical contact. Front Cell Infect Microbiol 6:104. doi: 10.3389/fcimb.2016.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Slupsky CM, Cheypesh A, Chao DV, Fu H, Rankin KN, Marrie TJ, Lacy P. 2009. Streptococcus pneumoniae and Staphylococcus aureus pneumonia induce distinct metabolic responses. J Proteome Res 8:3029–3036. doi: 10.1021/pr900103y. [DOI] [PubMed] [Google Scholar]
- 55.Park B, Nizet V, Liu GY. 2008. Role of Staphylococcus aureus catalase in niche competition against Streptococcus pneumoniae. J Bacteriol 190:2275–2278. doi: 10.1128/JB.00006-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sullivan SB, Kamath S, McConville TH, Gray BT, Lowy FD, Gordon PG, Uhlemann A-C. 2016. Staphylococcus epidermidis protection against Staphylococcus aureus colonization in people living with human immunodeficiency virus in an inner-city outpatient population: a cross-sectional study. Open Forum Infect Dis 3:ofw234. doi: 10.1093/ofid/ofw234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iwase T, Uehara Y, Shinji H, Tajima A, Seo H, Takada K, Agata T, Mizunoe Y. 2010. Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature 465:346–349. doi: 10.1038/nature09074. [DOI] [PubMed] [Google Scholar]
- 58.Nakatsuji T, Chen TH, Narala S, Chun KA, Two AM, Yun T, Shafiq F, Kotol PF, Bouslimani A, Melnik AV, Latif H, Kim J-N, Lockhart A, Artis K, David G, Taylor P, Streib J, Dorrestein PC, Grier A, Gill SR, Zengler K, Hata TR, Leung DYM, Gallo RL. 2017. Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci Transl Med 9:eaah4680. doi: 10.1126/scitranslmed.aah4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Escapa IF, Chen T, Huang Y, Gajare P, Dewhirst FE, Lemon KP. 2018. New insights into human nostril microbiome from the Expanded Human Oral Microbiome Database (eHOMD): a resource for the microbiome of the human aerodigestive tract. mSystems 3:e00187-18. doi: 10.1128/mSystems.00187-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Laufer AS, Metlay JP, Gent JF, Fennie KP, Kong Y, Pettigrew MM. 2011. Microbial communities of the upper respiratory tract and otitis media in children. mBio 2:e00245-10. doi: 10.1128/mBio.00245-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brugger SD, Eslami SM, Pettigrew MM, Escapa IF, Henke MT, Kong Y, Lemon KP. 2020. Dolosigranulum pigrum cooperation and competition in human nasal microbiota. mSphere 5:e00852-20. doi: 10.1128/mSphere.00852-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kanmani P, Clua P, Vizoso-Pinto MG, Rodriguez C, Alvarez S, Melnikov V, Takahashi H, Kitazawa H, Villena J. 2017. Respiratory commensal bacteria Corynebacterium pseudodiphtheriticum improves resistance of infant mice to respiratory syncytial virus and Streptococcus pneumoniae superinfection. Front Microbiol 8:1613. doi: 10.3389/fmicb.2017.01613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Camello TCF, Souza MC, Martins CAS, Damasco PV, Marques EA, Pimenta FP, Pereira GA, Hirata JR, Mattos-Guaraldi AL. 2009. Corynebacterium pseudodiphtheriticum isolated from relevant clinical sites of infection: a human pathogen overlooked in emerging countries. Lett Appl Microbiol 48:458–464. doi: 10.1111/j.1472-765X.2009.02553.x. [DOI] [PubMed] [Google Scholar]
- 64.Indumathi V, Shikha R, Suryaprakash D. 2014. Diphtheria-like illness in a fully immunised child caused by Corynebacterium pseudodiphtheriticum. Indian J Med Microbiol 32:443–445. doi: 10.4103/0255-0857.142250. [DOI] [PubMed] [Google Scholar]
- 65.Otto M 2009. Staphylococcus epidermidis—the ‘accidental’ pathogen. Nat Rev Microbiol 7:555–567. doi: 10.1038/nrmicro2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lewis K 2017. New approaches to antimicrobial discovery. Biochem Pharmacol 134:87–98. doi: 10.1016/j.bcp.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 67.Medema MH, Blin K, Cimermancic P, de Jager V, Zakrzewski P, Fischbach MA, Weber T, Takano E, Breitling R. 2011. antiSMASH: rapid identification, annotation and analysis of secondary metabolite biosynthesis gene clusters in bacterial and fungal genome sequences. Nucleic Acids Res 39:W339–W346. doi: 10.1093/nar/gkr466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Totsika M 2017. Disarming pathogens: benefits and challenges of antimicrobials that target bacterial virulence instead of growth and viability. Future Med Chem 9:267–269. doi: 10.4155/fmc-2016-0227. [DOI] [PubMed] [Google Scholar]
- 69.Piewngam P, Zheng Y, Nguyen TH, Dickey SW, Joo H-S, Villaruz AE, Glose KA, Fisher EL, Hunt RL, Li B, Chiou J, Pharkjaksu S, Khongthong S, Cheung GYC, Kiratisin P, Otto M. 2018. Pathogen elimination by probiotic Bacillus via signalling interference. Nature 562:532–537. doi: 10.1038/s41586-018-0616-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peterson SW, Knox NC, Golding GR, Tyler SD, Tyler AD, Mabon P, Embree JE, Fleming F, Fanella S, Van Domselaar G, Mulvey MR, Graham MR. 2016. A study of the infant nasal microbiome development over the first year of life and in relation to their primary adult caregivers using cpn60 universal target (UT) as a phylogenetic marker. PLoS One 11:e0152493. doi: 10.1371/journal.pone.0152493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sakr A, Brégeon F, Mège J-L, Rolain J-M, Blin O. 2018. Staphylococcus aureus nasal colonization: an update on mechanisms, epidemiology, risk factors, and subsequent infections. Front Microbiol 9:2419. doi: 10.3389/fmicb.2018.02419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nair N, Biswas R, Götz F, Biswas L. 2014. Impact of Staphylococcus aureus on pathogenesis in polymicrobial infections. Infect Immun 82:2162–2169. doi: 10.1128/IAI.00059-14. [DOI] [PMC free article] [PubMed] [Google Scholar]